User login
When is an allergic reaction to raw plant food due to tree pollen?
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
Improving Inpatient COVID-19 Vaccination Rates Among Adult Patients at a Tertiary Academic Medical Center
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.
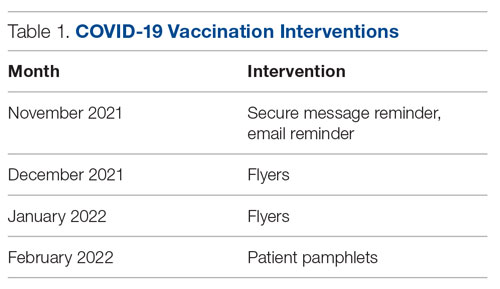
In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
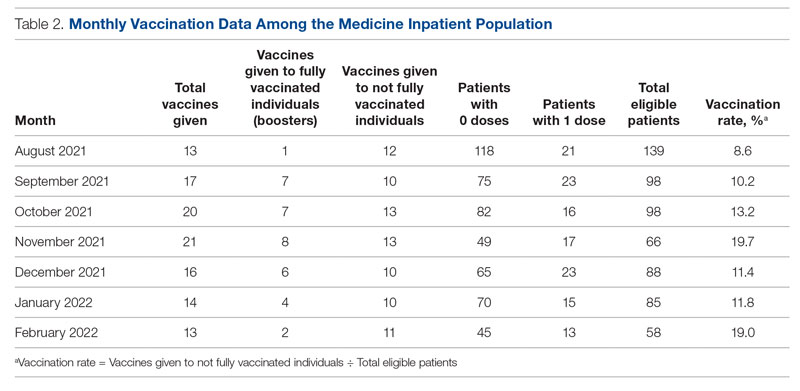
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.
Results
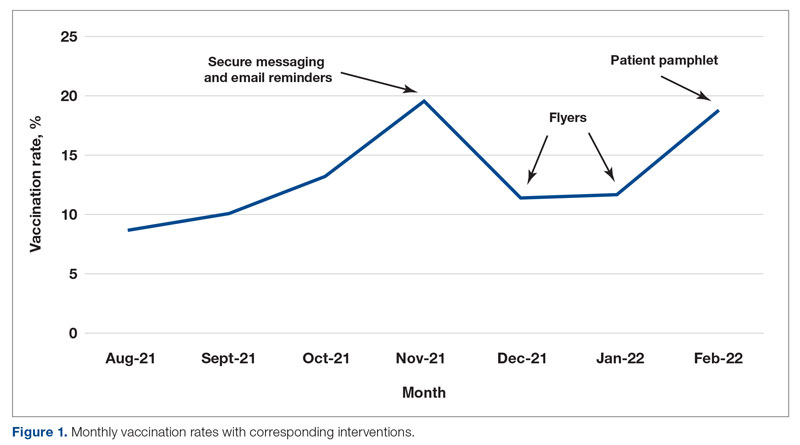
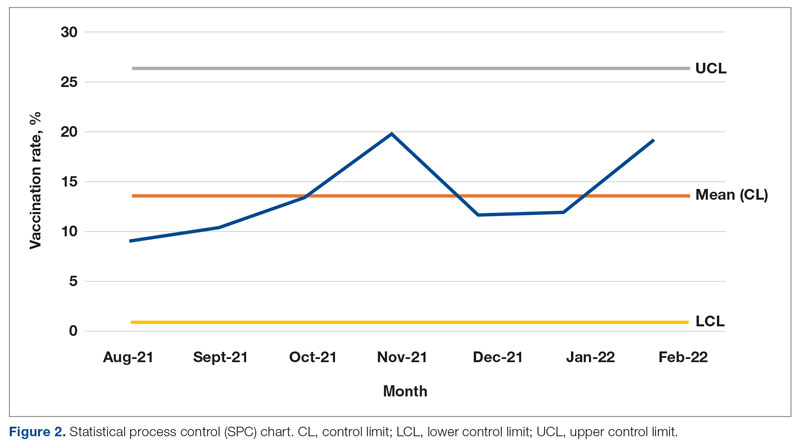
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).
Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
Many young kids with COVID may show no symptoms
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
FROM JAMA NETWORK OPEN
Author Q&A: Intravenous Immunoglobulin for Treatment of COVID-19 in Select Patients
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Evusheld for COVID-19: Lifesaving and free, but still few takers
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Fourth patient cleared of HIV after stem cell transplant for blood cancer
MONTREAL – from a naturally HIV-resistant donor, U.S. researchers announced at a meeting of the International AIDS Society.
The man received the transplant nearly 3.5 years ago. Since discontinuation of antiretroviral therapy (ART) more than 17 months ago, he has shown no evidence of HIV-1 RNA rebound and no detectable HIV-1 DNA, reported lead investigator Jana K. Dickter, MD, associate clinical professor in the division of infectious diseases at City of Hope, a Duarte, Calif.–based stem cell transplantation center for patients with blood cancers and patients with HIV/blood cancer.
Known as the City of Hope (COH) patient, he is different from the three previously reported patients in that “he was the oldest person to successfully undergo a stem cell transplant with HIV and leukemia and then achieve remission from both conditions,” Dr. Dickter said during a press briefing for the meeting. “He has been living with HIV the longest of any of the patients to date – more than 31 years prior to transplant – and he had also received the least immunosuppressive preparative regimen prior to transplant,” she added.
She said that, like the three previous patients, known as the Berlin, London, and New York patients, the COH patient received a transplant from a donor with natural resistance to HIV because of a rare CCR5-delta 32 mutation.
Dr. Dickter and her coinvestigators used the term “remission” but went further, suggesting that an “HIV cure is feasible” after transplant, given this and the previous cases.
“It’s a bit early to say the patient is cured, but they are clearly in remission,” said Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the meeting. Nevertheless, Dr. Lewin, professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity, in Melbourne, acknowledged that cure is “very likely.”
“Two of the previously reported patients have been off ART for long periods of time – Berlin, 12 years (until Timothy’s death in 2020); London, 4 years – and both had far more extensive investigations to try and find intact virus, including very large blood draws, tissue biopsies, etc. For the New York and now this COH patient, the duration off ART has been much shorter. ... But given the prior cases, it is very likely that the New York and COH patients are indeed cured. But I think it’s too early to make that call, hence my preference to use the word, ‘remission,’ “ she told this news organization.
“Although a transplant is not an option for most people with HIV, these cases are still interesting, still inspiring, and help illuminate the search for a cure,” she added.
Dr. Dickter acknowledged that the complexity of stem cell transplant procedures and their potential for significant side effects make them unsuitable as treatment options for most people with HIV, although she said the COH case is evidence that some HIV patients with blood cancers may not need such intensive pretransplant conditioning regimens.
The COH patient received a reduced-intensity fludarabine and melphalan regimen that had been designed at Dr. Dickter’s center “for older and less fit patients to make transplantation more tolerable,” she said. In addition, the graft-vs.-host disease prophylaxis that the COH patient received included only tacrolimus and sirolimus, whereas the previous patients received additional immunosuppressive therapies, and some also had undergone total body irradiation.
Dr. Dickter has disclosed no relevant financial relationships. Dr. Lewin has relationships with AbbVie, BMS, Esfam, Genentech, Gilead, Immunocore, Merck, Vaxxinity, and Viiv.
A version of this article first appeared on Medscape.com.
MONTREAL – from a naturally HIV-resistant donor, U.S. researchers announced at a meeting of the International AIDS Society.
The man received the transplant nearly 3.5 years ago. Since discontinuation of antiretroviral therapy (ART) more than 17 months ago, he has shown no evidence of HIV-1 RNA rebound and no detectable HIV-1 DNA, reported lead investigator Jana K. Dickter, MD, associate clinical professor in the division of infectious diseases at City of Hope, a Duarte, Calif.–based stem cell transplantation center for patients with blood cancers and patients with HIV/blood cancer.
Known as the City of Hope (COH) patient, he is different from the three previously reported patients in that “he was the oldest person to successfully undergo a stem cell transplant with HIV and leukemia and then achieve remission from both conditions,” Dr. Dickter said during a press briefing for the meeting. “He has been living with HIV the longest of any of the patients to date – more than 31 years prior to transplant – and he had also received the least immunosuppressive preparative regimen prior to transplant,” she added.
She said that, like the three previous patients, known as the Berlin, London, and New York patients, the COH patient received a transplant from a donor with natural resistance to HIV because of a rare CCR5-delta 32 mutation.
Dr. Dickter and her coinvestigators used the term “remission” but went further, suggesting that an “HIV cure is feasible” after transplant, given this and the previous cases.
“It’s a bit early to say the patient is cured, but they are clearly in remission,” said Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the meeting. Nevertheless, Dr. Lewin, professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity, in Melbourne, acknowledged that cure is “very likely.”
“Two of the previously reported patients have been off ART for long periods of time – Berlin, 12 years (until Timothy’s death in 2020); London, 4 years – and both had far more extensive investigations to try and find intact virus, including very large blood draws, tissue biopsies, etc. For the New York and now this COH patient, the duration off ART has been much shorter. ... But given the prior cases, it is very likely that the New York and COH patients are indeed cured. But I think it’s too early to make that call, hence my preference to use the word, ‘remission,’ “ she told this news organization.
“Although a transplant is not an option for most people with HIV, these cases are still interesting, still inspiring, and help illuminate the search for a cure,” she added.
Dr. Dickter acknowledged that the complexity of stem cell transplant procedures and their potential for significant side effects make them unsuitable as treatment options for most people with HIV, although she said the COH case is evidence that some HIV patients with blood cancers may not need such intensive pretransplant conditioning regimens.
The COH patient received a reduced-intensity fludarabine and melphalan regimen that had been designed at Dr. Dickter’s center “for older and less fit patients to make transplantation more tolerable,” she said. In addition, the graft-vs.-host disease prophylaxis that the COH patient received included only tacrolimus and sirolimus, whereas the previous patients received additional immunosuppressive therapies, and some also had undergone total body irradiation.
Dr. Dickter has disclosed no relevant financial relationships. Dr. Lewin has relationships with AbbVie, BMS, Esfam, Genentech, Gilead, Immunocore, Merck, Vaxxinity, and Viiv.
A version of this article first appeared on Medscape.com.
MONTREAL – from a naturally HIV-resistant donor, U.S. researchers announced at a meeting of the International AIDS Society.
The man received the transplant nearly 3.5 years ago. Since discontinuation of antiretroviral therapy (ART) more than 17 months ago, he has shown no evidence of HIV-1 RNA rebound and no detectable HIV-1 DNA, reported lead investigator Jana K. Dickter, MD, associate clinical professor in the division of infectious diseases at City of Hope, a Duarte, Calif.–based stem cell transplantation center for patients with blood cancers and patients with HIV/blood cancer.
Known as the City of Hope (COH) patient, he is different from the three previously reported patients in that “he was the oldest person to successfully undergo a stem cell transplant with HIV and leukemia and then achieve remission from both conditions,” Dr. Dickter said during a press briefing for the meeting. “He has been living with HIV the longest of any of the patients to date – more than 31 years prior to transplant – and he had also received the least immunosuppressive preparative regimen prior to transplant,” she added.
She said that, like the three previous patients, known as the Berlin, London, and New York patients, the COH patient received a transplant from a donor with natural resistance to HIV because of a rare CCR5-delta 32 mutation.
Dr. Dickter and her coinvestigators used the term “remission” but went further, suggesting that an “HIV cure is feasible” after transplant, given this and the previous cases.
“It’s a bit early to say the patient is cured, but they are clearly in remission,” said Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the meeting. Nevertheless, Dr. Lewin, professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity, in Melbourne, acknowledged that cure is “very likely.”
“Two of the previously reported patients have been off ART for long periods of time – Berlin, 12 years (until Timothy’s death in 2020); London, 4 years – and both had far more extensive investigations to try and find intact virus, including very large blood draws, tissue biopsies, etc. For the New York and now this COH patient, the duration off ART has been much shorter. ... But given the prior cases, it is very likely that the New York and COH patients are indeed cured. But I think it’s too early to make that call, hence my preference to use the word, ‘remission,’ “ she told this news organization.
“Although a transplant is not an option for most people with HIV, these cases are still interesting, still inspiring, and help illuminate the search for a cure,” she added.
Dr. Dickter acknowledged that the complexity of stem cell transplant procedures and their potential for significant side effects make them unsuitable as treatment options for most people with HIV, although she said the COH case is evidence that some HIV patients with blood cancers may not need such intensive pretransplant conditioning regimens.
The COH patient received a reduced-intensity fludarabine and melphalan regimen that had been designed at Dr. Dickter’s center “for older and less fit patients to make transplantation more tolerable,” she said. In addition, the graft-vs.-host disease prophylaxis that the COH patient received included only tacrolimus and sirolimus, whereas the previous patients received additional immunosuppressive therapies, and some also had undergone total body irradiation.
Dr. Dickter has disclosed no relevant financial relationships. Dr. Lewin has relationships with AbbVie, BMS, Esfam, Genentech, Gilead, Immunocore, Merck, Vaxxinity, and Viiv.
A version of this article first appeared on Medscape.com.
AT AIDS 2022
How to overcome hesitancy for COVID-19 and other vaccines
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.
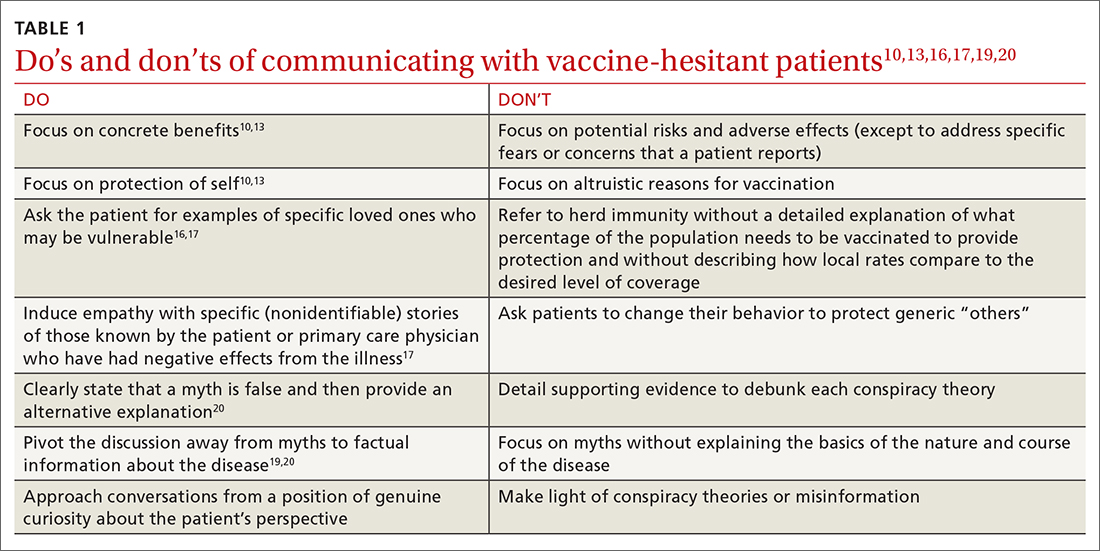
Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.

Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; [email protected]
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.

Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.

Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; [email protected]
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.

Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.

Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; [email protected]
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
PRACTICE RECOMMENDATIONS
› Focus on personal benefits of vaccination with patients who express strong hesitancy and endorse vaccine myths; refocus the conversation away from myths and back to disease facts. C
› Emphasize personal and collective benefit to patients who are uncertain about vaccination; provide education about herd immunity and local vaccine coverage. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Deaths rare in tonsillectomy, but some children at more risk
It’s rare for a child to die after a tonsillectomy, but children who die are more likely to have a complex chronic condition such as cerebral palsy or Down syndrome, according to a retrospective cohort study published in JAMA.
“Among children undergoing tonsillectomy, the rate of postoperative death was 7 per 100,000 operations overall, [but] among children with complex chronic conditions, the rate of postoperative death was 117 per 100,000 operations, representing 44% of overall deaths,” write researchers at the University of Wisconsin–Madison. “These findings may inform decisionmaking for pediatric tonsillectomy.”
The rate of death in children after tonsillectomy has been uncertain, the authors write. Specific mortality rates for children at increased risk for complications, including those under 3 years old and those with sleep-disordered breathing or complex chronic conditions, have not been available.
To learn how likely children undergoing tonsillectomy are to die after their surgery, as well as which children are most at risk, lead study author M. Bruce Edmonson, MD, MPH, department of pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, and his colleagues drew data from five states, including ambulatory surgery, inpatient, and emergency department discharge data sets provided by the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality for California, Florida, Maryland, New York, and Wisconsin.
Participants included 504,262 patients under 21 years of age whose discharge records linked their inpatient or outpatient tonsillectomy, with or without adenoidectomy, with at least 90 days of follow-up.
In a longitudinal analysis, the research team investigated postoperative death within 30 days or during a surgical stay lasting over 30 days. They calculated postoperative mortality per 100,000 operations, both overall and classified by age group, sleep-disordered breathing, and complex chronic conditions.
The 504,262 children ranged in age from 0 to 20 years and underwent a total of 505,182 tonsillectomies. Of these, 10.1% were performed in children aged under 3 years, 28.9% in children with sleep-disordered breathing, and 2.8% in those with complex chronic conditions.
The 36 linked postoperative deaths occurred between 2 and 20.5 days after surgical admission, and 19 (53%) of the deaths occurred after surgical discharge.
The unadjusted mortality rate was 7.04 (95% confidence interval, 4.97-9.98) deaths per 100,000 procedures. In multivariable models, children younger than 3 years and children with sleep-disordered breathing were not significantly more likely to die.
But children with complex chronic conditions were significantly more likely to die than were children without those conditions (117.22 vs. 3.87 deaths per 100,000 procedures, respectively).
Children with complex chronic conditions underwent only 2.8% of all tonsillectomies, but they accounted for 44% of postoperative deaths. Most deaths linked with complex chronic conditions occurred among children with neurologic, neuromuscular, congenital, or genetic disorders.
Findings can help providers advise patients and their families about tonsillectomy risks
Kavita Dedhia, MD, MSHP, attending otolaryngologist, Division of Otolaryngology, Children’s Hospital of Philadelphia, Pennsylvania, told this news organization that she was not surprised by the findings.
“This study suggests that mortality is an extremely rare complication of tonsillectomy, and that children with complex medical conditions are at highest risk,” Dr. Dedhia, who was not involved in the study, said in an email.
“Due to their underlying comorbidities, medically fragile children are considered to be at higher risk while undergoing anesthesia and surgical procedures,” she added.
Dr. Dedhia noted that nonpatient factors the study did not explore may have affected the mortality rates, including each hospital’s experience with managing children with complex medical conditions, as well as whether the hospitals were tertiary care facilities, and pediatric or adult hospitals.
She would like to know what hospital or practice characteristics may have contributed to the mortality risk and whether increased mortality in these patients is limited to tonsillectomy or is also found with other surgical procedures.
“The strength of this study is that it is large and multi-regional and that it informs providers about patient factors impacting mortality in pediatric tonsillectomy,” Dr. Dedhia said. “This study arms surgeons with data to discuss mortality risk with the families of medically complex children undergoing tonsillectomy.”
The study authors and Dr. Dedhia report no relevant financial relationships. Funding information was not provided.
A version of this article first appeared on Medscape.com.
It’s rare for a child to die after a tonsillectomy, but children who die are more likely to have a complex chronic condition such as cerebral palsy or Down syndrome, according to a retrospective cohort study published in JAMA.
“Among children undergoing tonsillectomy, the rate of postoperative death was 7 per 100,000 operations overall, [but] among children with complex chronic conditions, the rate of postoperative death was 117 per 100,000 operations, representing 44% of overall deaths,” write researchers at the University of Wisconsin–Madison. “These findings may inform decisionmaking for pediatric tonsillectomy.”
The rate of death in children after tonsillectomy has been uncertain, the authors write. Specific mortality rates for children at increased risk for complications, including those under 3 years old and those with sleep-disordered breathing or complex chronic conditions, have not been available.
To learn how likely children undergoing tonsillectomy are to die after their surgery, as well as which children are most at risk, lead study author M. Bruce Edmonson, MD, MPH, department of pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, and his colleagues drew data from five states, including ambulatory surgery, inpatient, and emergency department discharge data sets provided by the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality for California, Florida, Maryland, New York, and Wisconsin.
Participants included 504,262 patients under 21 years of age whose discharge records linked their inpatient or outpatient tonsillectomy, with or without adenoidectomy, with at least 90 days of follow-up.
In a longitudinal analysis, the research team investigated postoperative death within 30 days or during a surgical stay lasting over 30 days. They calculated postoperative mortality per 100,000 operations, both overall and classified by age group, sleep-disordered breathing, and complex chronic conditions.
The 504,262 children ranged in age from 0 to 20 years and underwent a total of 505,182 tonsillectomies. Of these, 10.1% were performed in children aged under 3 years, 28.9% in children with sleep-disordered breathing, and 2.8% in those with complex chronic conditions.
The 36 linked postoperative deaths occurred between 2 and 20.5 days after surgical admission, and 19 (53%) of the deaths occurred after surgical discharge.
The unadjusted mortality rate was 7.04 (95% confidence interval, 4.97-9.98) deaths per 100,000 procedures. In multivariable models, children younger than 3 years and children with sleep-disordered breathing were not significantly more likely to die.
But children with complex chronic conditions were significantly more likely to die than were children without those conditions (117.22 vs. 3.87 deaths per 100,000 procedures, respectively).
Children with complex chronic conditions underwent only 2.8% of all tonsillectomies, but they accounted for 44% of postoperative deaths. Most deaths linked with complex chronic conditions occurred among children with neurologic, neuromuscular, congenital, or genetic disorders.
Findings can help providers advise patients and their families about tonsillectomy risks
Kavita Dedhia, MD, MSHP, attending otolaryngologist, Division of Otolaryngology, Children’s Hospital of Philadelphia, Pennsylvania, told this news organization that she was not surprised by the findings.
“This study suggests that mortality is an extremely rare complication of tonsillectomy, and that children with complex medical conditions are at highest risk,” Dr. Dedhia, who was not involved in the study, said in an email.
“Due to their underlying comorbidities, medically fragile children are considered to be at higher risk while undergoing anesthesia and surgical procedures,” she added.
Dr. Dedhia noted that nonpatient factors the study did not explore may have affected the mortality rates, including each hospital’s experience with managing children with complex medical conditions, as well as whether the hospitals were tertiary care facilities, and pediatric or adult hospitals.
She would like to know what hospital or practice characteristics may have contributed to the mortality risk and whether increased mortality in these patients is limited to tonsillectomy or is also found with other surgical procedures.
“The strength of this study is that it is large and multi-regional and that it informs providers about patient factors impacting mortality in pediatric tonsillectomy,” Dr. Dedhia said. “This study arms surgeons with data to discuss mortality risk with the families of medically complex children undergoing tonsillectomy.”
The study authors and Dr. Dedhia report no relevant financial relationships. Funding information was not provided.
A version of this article first appeared on Medscape.com.
It’s rare for a child to die after a tonsillectomy, but children who die are more likely to have a complex chronic condition such as cerebral palsy or Down syndrome, according to a retrospective cohort study published in JAMA.
“Among children undergoing tonsillectomy, the rate of postoperative death was 7 per 100,000 operations overall, [but] among children with complex chronic conditions, the rate of postoperative death was 117 per 100,000 operations, representing 44% of overall deaths,” write researchers at the University of Wisconsin–Madison. “These findings may inform decisionmaking for pediatric tonsillectomy.”
The rate of death in children after tonsillectomy has been uncertain, the authors write. Specific mortality rates for children at increased risk for complications, including those under 3 years old and those with sleep-disordered breathing or complex chronic conditions, have not been available.
To learn how likely children undergoing tonsillectomy are to die after their surgery, as well as which children are most at risk, lead study author M. Bruce Edmonson, MD, MPH, department of pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, and his colleagues drew data from five states, including ambulatory surgery, inpatient, and emergency department discharge data sets provided by the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality for California, Florida, Maryland, New York, and Wisconsin.
Participants included 504,262 patients under 21 years of age whose discharge records linked their inpatient or outpatient tonsillectomy, with or without adenoidectomy, with at least 90 days of follow-up.
In a longitudinal analysis, the research team investigated postoperative death within 30 days or during a surgical stay lasting over 30 days. They calculated postoperative mortality per 100,000 operations, both overall and classified by age group, sleep-disordered breathing, and complex chronic conditions.
The 504,262 children ranged in age from 0 to 20 years and underwent a total of 505,182 tonsillectomies. Of these, 10.1% were performed in children aged under 3 years, 28.9% in children with sleep-disordered breathing, and 2.8% in those with complex chronic conditions.
The 36 linked postoperative deaths occurred between 2 and 20.5 days after surgical admission, and 19 (53%) of the deaths occurred after surgical discharge.
The unadjusted mortality rate was 7.04 (95% confidence interval, 4.97-9.98) deaths per 100,000 procedures. In multivariable models, children younger than 3 years and children with sleep-disordered breathing were not significantly more likely to die.
But children with complex chronic conditions were significantly more likely to die than were children without those conditions (117.22 vs. 3.87 deaths per 100,000 procedures, respectively).
Children with complex chronic conditions underwent only 2.8% of all tonsillectomies, but they accounted for 44% of postoperative deaths. Most deaths linked with complex chronic conditions occurred among children with neurologic, neuromuscular, congenital, or genetic disorders.
Findings can help providers advise patients and their families about tonsillectomy risks
Kavita Dedhia, MD, MSHP, attending otolaryngologist, Division of Otolaryngology, Children’s Hospital of Philadelphia, Pennsylvania, told this news organization that she was not surprised by the findings.
“This study suggests that mortality is an extremely rare complication of tonsillectomy, and that children with complex medical conditions are at highest risk,” Dr. Dedhia, who was not involved in the study, said in an email.
“Due to their underlying comorbidities, medically fragile children are considered to be at higher risk while undergoing anesthesia and surgical procedures,” she added.
Dr. Dedhia noted that nonpatient factors the study did not explore may have affected the mortality rates, including each hospital’s experience with managing children with complex medical conditions, as well as whether the hospitals were tertiary care facilities, and pediatric or adult hospitals.
She would like to know what hospital or practice characteristics may have contributed to the mortality risk and whether increased mortality in these patients is limited to tonsillectomy or is also found with other surgical procedures.
“The strength of this study is that it is large and multi-regional and that it informs providers about patient factors impacting mortality in pediatric tonsillectomy,” Dr. Dedhia said. “This study arms surgeons with data to discuss mortality risk with the families of medically complex children undergoing tonsillectomy.”
The study authors and Dr. Dedhia report no relevant financial relationships. Funding information was not provided.
A version of this article first appeared on Medscape.com.
FROM JAMA
Introduce allergens early, say French allergists
Although in many cases, food-allergen tolerance can be achieved with oral immunotherapy, primary prevention of food allergies remains crucial, according to the French Society of Allergology. In new recommendations that were presented at a session of the Congress of French Pediatric Societies, the academic society advocated early introduction of allergens for all children, starting at 4 months of age.
The latest prevention data from two major studies, LEAP and EAT, have prompted European and French experts to rethink their stance on food diversification. The new French proposals were recently published under the coordination of Dominique Sabouraud-Leclerc, MD, pediatrics department, Reims (France) University Hospital, on behalf of the Food Allergy Working Group of the French Society of Allergology.
For all newborns, regardless of whether they have a history of atopic or nonatopic dermatitis, food diversification is now recommended from 4 months of age instead of 6 months, as was previously recommended. If the child does not develop atopic dermatitis or develops only a mild form, peanuts, eggs, and nuts may be introduced at home.
However, if the child experiences severe atopic dermatitis, an allergy testing panel for peanuts, nuts, eggs, and cow’s milk proteins should be performed. An oral food challenge may be conducted at the allergist’s discretion.
Regarding peanuts, the working group proposed introducing a purée in the form of either a mixture of peanuts/hazelnuts/cashew nuts (1 level teaspoon five times a week; 2 g of protein/food per week) or a 100% peanut paste (1 scant teaspoon four times a week; 2 g of peanut protein/week). If the family is worried, the allergist can suggest monitoring the child in the clinic waiting room for 30 minutes after the first dose.
“We shouldn’t delay the introduction of the primary allergens anymore, regardless of whether children are at risk for a food allergy, and particularly a peanut allergy,” explained Stéphanie Lejeune, MD, pediatric pulmonologist and allergist at Lille (France) Regional University Hospital, who presented these new findings at the congress. “In fact, if we only target at-risk children, we overlook children with no family history who will nevertheless develop food allergies. The idea is to introduce everything, especially peanuts, between 4 and 6 months of age and to no longer do so gradually, one food after another, as was being done until now, beginning at 6 months and over. We must give priority to regularity over quantity.”
Although this approach is based on clinical trials, no real-life data are currently available.
LEAP and EAT studies support early introduction of peanuts
A study from 2021 summed up the risk factors for peanut allergy. About 61% of infants (4-11 months) had atopic dermatitis, 18% had a food allergy, 62% had a first-degree relative with a peanut allergy, and 11% had a confirmed peanut allergy. The risk of peanut allergy increased with age and severe eczema.
In 2015, the LEAP study, which was conducted in the United Kingdom with 640 infants aged 4-11 months who had risk factors for peanut allergy, revolutionized peanut-allergy primary prevention. Regardless of whether the children were sensitized or not, the number of children who developed a peanut allergy was systematically lower in the group that ingested the allergen in comparison with the “avoidance” group.
Additionally, the LEAP-ON study showed that protection against peanut allergy persisted for 12 months after cessation of consumption between ages 5 and 6 years among children who had consumed peanuts previously.
Early diversification in the general population was investigated in the EAT study, which involved 1303 breastfed infants. Of these infants, 24% had atopic dermatitis (median SCORAD score, 7.5). They were divided into two arms: avoidance and breast feeding until 6 months (standard introduction) or early introduction at 3 months (boiled egg, milk, peanuts, sesame, white fish, wheat, 2 g of protein twice a week). In the per-protocol analysis, there were 13 cases of peanut allergy in the standard introduction group; there were no cases in the early introduction group.
A version of this article first appeared on Medscape.com.
Although in many cases, food-allergen tolerance can be achieved with oral immunotherapy, primary prevention of food allergies remains crucial, according to the French Society of Allergology. In new recommendations that were presented at a session of the Congress of French Pediatric Societies, the academic society advocated early introduction of allergens for all children, starting at 4 months of age.
The latest prevention data from two major studies, LEAP and EAT, have prompted European and French experts to rethink their stance on food diversification. The new French proposals were recently published under the coordination of Dominique Sabouraud-Leclerc, MD, pediatrics department, Reims (France) University Hospital, on behalf of the Food Allergy Working Group of the French Society of Allergology.
For all newborns, regardless of whether they have a history of atopic or nonatopic dermatitis, food diversification is now recommended from 4 months of age instead of 6 months, as was previously recommended. If the child does not develop atopic dermatitis or develops only a mild form, peanuts, eggs, and nuts may be introduced at home.
However, if the child experiences severe atopic dermatitis, an allergy testing panel for peanuts, nuts, eggs, and cow’s milk proteins should be performed. An oral food challenge may be conducted at the allergist’s discretion.
Regarding peanuts, the working group proposed introducing a purée in the form of either a mixture of peanuts/hazelnuts/cashew nuts (1 level teaspoon five times a week; 2 g of protein/food per week) or a 100% peanut paste (1 scant teaspoon four times a week; 2 g of peanut protein/week). If the family is worried, the allergist can suggest monitoring the child in the clinic waiting room for 30 minutes after the first dose.
“We shouldn’t delay the introduction of the primary allergens anymore, regardless of whether children are at risk for a food allergy, and particularly a peanut allergy,” explained Stéphanie Lejeune, MD, pediatric pulmonologist and allergist at Lille (France) Regional University Hospital, who presented these new findings at the congress. “In fact, if we only target at-risk children, we overlook children with no family history who will nevertheless develop food allergies. The idea is to introduce everything, especially peanuts, between 4 and 6 months of age and to no longer do so gradually, one food after another, as was being done until now, beginning at 6 months and over. We must give priority to regularity over quantity.”
Although this approach is based on clinical trials, no real-life data are currently available.
LEAP and EAT studies support early introduction of peanuts
A study from 2021 summed up the risk factors for peanut allergy. About 61% of infants (4-11 months) had atopic dermatitis, 18% had a food allergy, 62% had a first-degree relative with a peanut allergy, and 11% had a confirmed peanut allergy. The risk of peanut allergy increased with age and severe eczema.
In 2015, the LEAP study, which was conducted in the United Kingdom with 640 infants aged 4-11 months who had risk factors for peanut allergy, revolutionized peanut-allergy primary prevention. Regardless of whether the children were sensitized or not, the number of children who developed a peanut allergy was systematically lower in the group that ingested the allergen in comparison with the “avoidance” group.
Additionally, the LEAP-ON study showed that protection against peanut allergy persisted for 12 months after cessation of consumption between ages 5 and 6 years among children who had consumed peanuts previously.
Early diversification in the general population was investigated in the EAT study, which involved 1303 breastfed infants. Of these infants, 24% had atopic dermatitis (median SCORAD score, 7.5). They were divided into two arms: avoidance and breast feeding until 6 months (standard introduction) or early introduction at 3 months (boiled egg, milk, peanuts, sesame, white fish, wheat, 2 g of protein twice a week). In the per-protocol analysis, there were 13 cases of peanut allergy in the standard introduction group; there were no cases in the early introduction group.
A version of this article first appeared on Medscape.com.
Although in many cases, food-allergen tolerance can be achieved with oral immunotherapy, primary prevention of food allergies remains crucial, according to the French Society of Allergology. In new recommendations that were presented at a session of the Congress of French Pediatric Societies, the academic society advocated early introduction of allergens for all children, starting at 4 months of age.
The latest prevention data from two major studies, LEAP and EAT, have prompted European and French experts to rethink their stance on food diversification. The new French proposals were recently published under the coordination of Dominique Sabouraud-Leclerc, MD, pediatrics department, Reims (France) University Hospital, on behalf of the Food Allergy Working Group of the French Society of Allergology.
For all newborns, regardless of whether they have a history of atopic or nonatopic dermatitis, food diversification is now recommended from 4 months of age instead of 6 months, as was previously recommended. If the child does not develop atopic dermatitis or develops only a mild form, peanuts, eggs, and nuts may be introduced at home.
However, if the child experiences severe atopic dermatitis, an allergy testing panel for peanuts, nuts, eggs, and cow’s milk proteins should be performed. An oral food challenge may be conducted at the allergist’s discretion.
Regarding peanuts, the working group proposed introducing a purée in the form of either a mixture of peanuts/hazelnuts/cashew nuts (1 level teaspoon five times a week; 2 g of protein/food per week) or a 100% peanut paste (1 scant teaspoon four times a week; 2 g of peanut protein/week). If the family is worried, the allergist can suggest monitoring the child in the clinic waiting room for 30 minutes after the first dose.
“We shouldn’t delay the introduction of the primary allergens anymore, regardless of whether children are at risk for a food allergy, and particularly a peanut allergy,” explained Stéphanie Lejeune, MD, pediatric pulmonologist and allergist at Lille (France) Regional University Hospital, who presented these new findings at the congress. “In fact, if we only target at-risk children, we overlook children with no family history who will nevertheless develop food allergies. The idea is to introduce everything, especially peanuts, between 4 and 6 months of age and to no longer do so gradually, one food after another, as was being done until now, beginning at 6 months and over. We must give priority to regularity over quantity.”
Although this approach is based on clinical trials, no real-life data are currently available.
LEAP and EAT studies support early introduction of peanuts
A study from 2021 summed up the risk factors for peanut allergy. About 61% of infants (4-11 months) had atopic dermatitis, 18% had a food allergy, 62% had a first-degree relative with a peanut allergy, and 11% had a confirmed peanut allergy. The risk of peanut allergy increased with age and severe eczema.
In 2015, the LEAP study, which was conducted in the United Kingdom with 640 infants aged 4-11 months who had risk factors for peanut allergy, revolutionized peanut-allergy primary prevention. Regardless of whether the children were sensitized or not, the number of children who developed a peanut allergy was systematically lower in the group that ingested the allergen in comparison with the “avoidance” group.
Additionally, the LEAP-ON study showed that protection against peanut allergy persisted for 12 months after cessation of consumption between ages 5 and 6 years among children who had consumed peanuts previously.
Early diversification in the general population was investigated in the EAT study, which involved 1303 breastfed infants. Of these infants, 24% had atopic dermatitis (median SCORAD score, 7.5). They were divided into two arms: avoidance and breast feeding until 6 months (standard introduction) or early introduction at 3 months (boiled egg, milk, peanuts, sesame, white fish, wheat, 2 g of protein twice a week). In the per-protocol analysis, there were 13 cases of peanut allergy in the standard introduction group; there were no cases in the early introduction group.
A version of this article first appeared on Medscape.com.
Milk allergy frequently overdiagnosed
According to a consensus study, many infants in some countries are misdiagnosed with allergy to cow, sheep, or goat milk, and they’re prescribed specialized formulas they don’t need.
“Milk allergy overdiagnosis is common in some regions and can potentially harm mothers and infants,” the authors write in Clinical & Experimental Allergy. “These new consensus recommendations on the safe detection and management of milk allergy in children under 2 years aim to reduce harms associated with milk allergy overdiagnosis.”
“This guidance, developed by experts without commercial ties to the formula industry, aims to reduce milk allergy overdiagnosis and [to] support ... breastfeeding and less use of specialized formula, compared with current guidelines,” they add.
Up to 1% of European infants 2 years of age and younger are considered allergic to cow’s milk. Prescriptions for specialized formula for bottle-fed infants allergic to cow’s milk in Australia, England, and Norway have grown to over 10 times the expected volumes.
Lead study author Hilary I. Allen, National Heart and Lung Institute, Imperial College London, and her colleagues on several continents developed practical guidance for providers on safely detecting and managing milk allergy in infants.
Due to lack of high-certainty research evidence in this area, they used the Delphi consensus method.
The study involved two rounds of anonymous consensus-building surveys and one formal meeting in 2021.
The team identified experts from diverse geographic and cultural settings by searching medical databases for the term “milk hypersensitivity.” They asked those experts to recommend colleagues. The researchers also contacted experts with ties to international professional organizations, such as the International Board of Lactation Consultant Examiners, as well as societies associated with the World Allergy Organization.
The 17 study participants included clinicians and researchers in general practice, health visiting, lactation support, midwifery, nutrition, and relevant areas of pediatrics from Africa, Asia, Australia, Europe, the Middle East, and North America. Experts with recent conflicts of interest with the breastmilk substitute (formula) industry were excluded from the study. Five authors of earlier milk allergy guidelines and seven parents contributed feedback.
In each survey round, participants used a nine-point scale to rank the importance of each proposed statement that addressed prevention of overdiagnosis or underdiagnosis, support of breastfeeding women, and the role of specialized formula products.
Based on the number of total points participants assigned, each statement was classified as “essential,” “recommended,” “no consensus,” or “excluded” due to lack of relevance.
The experts agreed on 38 essential statements in several categories, including:
- Maternal dietary restriction is often not necessary to manage milk allergy
- In infants with chronic symptoms who are exclusively breastfed, milk allergy diagnosis should be considered only in specific, rare circumstances
- Milk allergy diagnosis does not usually need to be considered for stool changes, aversive feeding, or occasional spots of blood in stool, if not related in time with milk protein ingestion
The consensus recommendations provide more restrictive criteria than earlier guidelines for detecting milk allergy, fewer maternal dietary exclusions, and less use of specialized formula.
During an infant formula shortage in the U.S., a timely study
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program, Children’s Mercy Kansas City, Missouri, welcomed the study’s engagement of specialists in various fields and avoidance of bias from formula companies.
“Food allergies have received a lot of attention, especially through websites and social media,” Ms. Shroba, who was not involved in the study, told this news organization in an email. “Unfortunately, a lot of that information is incorrect and can lead to misunderstanding and misdiagnosis.”
“This article helps guide practitioners through identifying the concerning symptoms of milk allergy versus normal infant symptoms,” she said. “It can help providers discern when testing, elimination diets, and changes in formula are warranted.
“This guidance emphasizes the reproducibility and specificity of symptoms, which are key elements of a food allergy diagnosis,” she explained. “By eliminating unnecessary milk allergy labeling, we can keep infants on appropriate diets for their age, such as breastfeeding or milk-based formulas. Proper diagnosis can also reduce unnecessary financial strain of specialty formulas, stress to the family regarding feedings, and a restrictive diet for the breastfeeding mother.”
The study will be useful to a wide range of health care providers, Jennifer Anne Dantzer, MD, assistant professor of pediatrics, Johns Hopkins Medicine, Baltimore, said in an email.
“With the current formula shortage, there has perhaps never been a more important time to do this study and provide additional guidance on who does or does not need special formula,” noted Dr. Dantzer, who also was not involved in the study. “A milk allergy diagnosis impacts the child and the family, so it is very important to avoid overdiagnosis and to support the breastfeeding mother.”
“These findings should provide reassurance that dietary exclusions for the breastfeeding mother are not needed for most children with milk allergy,” she said. “If a milk allergy is suspected, the child should be referred to an allergist.”
The authors recommend further related research into the safety and effectiveness of using the guidance in practice.
One coauthor reports financial relationships with a biotech company. Ms. Allen and her remaining coauthors, as well as Ms. Shroba and Dr. Dantzer, report no relevant financial relationships. The study was funded through fellowships.
A version of this article first appeared on Medscape.com.
According to a consensus study, many infants in some countries are misdiagnosed with allergy to cow, sheep, or goat milk, and they’re prescribed specialized formulas they don’t need.
“Milk allergy overdiagnosis is common in some regions and can potentially harm mothers and infants,” the authors write in Clinical & Experimental Allergy. “These new consensus recommendations on the safe detection and management of milk allergy in children under 2 years aim to reduce harms associated with milk allergy overdiagnosis.”
“This guidance, developed by experts without commercial ties to the formula industry, aims to reduce milk allergy overdiagnosis and [to] support ... breastfeeding and less use of specialized formula, compared with current guidelines,” they add.
Up to 1% of European infants 2 years of age and younger are considered allergic to cow’s milk. Prescriptions for specialized formula for bottle-fed infants allergic to cow’s milk in Australia, England, and Norway have grown to over 10 times the expected volumes.
Lead study author Hilary I. Allen, National Heart and Lung Institute, Imperial College London, and her colleagues on several continents developed practical guidance for providers on safely detecting and managing milk allergy in infants.
Due to lack of high-certainty research evidence in this area, they used the Delphi consensus method.
The study involved two rounds of anonymous consensus-building surveys and one formal meeting in 2021.
The team identified experts from diverse geographic and cultural settings by searching medical databases for the term “milk hypersensitivity.” They asked those experts to recommend colleagues. The researchers also contacted experts with ties to international professional organizations, such as the International Board of Lactation Consultant Examiners, as well as societies associated with the World Allergy Organization.
The 17 study participants included clinicians and researchers in general practice, health visiting, lactation support, midwifery, nutrition, and relevant areas of pediatrics from Africa, Asia, Australia, Europe, the Middle East, and North America. Experts with recent conflicts of interest with the breastmilk substitute (formula) industry were excluded from the study. Five authors of earlier milk allergy guidelines and seven parents contributed feedback.
In each survey round, participants used a nine-point scale to rank the importance of each proposed statement that addressed prevention of overdiagnosis or underdiagnosis, support of breastfeeding women, and the role of specialized formula products.
Based on the number of total points participants assigned, each statement was classified as “essential,” “recommended,” “no consensus,” or “excluded” due to lack of relevance.
The experts agreed on 38 essential statements in several categories, including:
- Maternal dietary restriction is often not necessary to manage milk allergy
- In infants with chronic symptoms who are exclusively breastfed, milk allergy diagnosis should be considered only in specific, rare circumstances
- Milk allergy diagnosis does not usually need to be considered for stool changes, aversive feeding, or occasional spots of blood in stool, if not related in time with milk protein ingestion
The consensus recommendations provide more restrictive criteria than earlier guidelines for detecting milk allergy, fewer maternal dietary exclusions, and less use of specialized formula.
During an infant formula shortage in the U.S., a timely study
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program, Children’s Mercy Kansas City, Missouri, welcomed the study’s engagement of specialists in various fields and avoidance of bias from formula companies.
“Food allergies have received a lot of attention, especially through websites and social media,” Ms. Shroba, who was not involved in the study, told this news organization in an email. “Unfortunately, a lot of that information is incorrect and can lead to misunderstanding and misdiagnosis.”
“This article helps guide practitioners through identifying the concerning symptoms of milk allergy versus normal infant symptoms,” she said. “It can help providers discern when testing, elimination diets, and changes in formula are warranted.
“This guidance emphasizes the reproducibility and specificity of symptoms, which are key elements of a food allergy diagnosis,” she explained. “By eliminating unnecessary milk allergy labeling, we can keep infants on appropriate diets for their age, such as breastfeeding or milk-based formulas. Proper diagnosis can also reduce unnecessary financial strain of specialty formulas, stress to the family regarding feedings, and a restrictive diet for the breastfeeding mother.”
The study will be useful to a wide range of health care providers, Jennifer Anne Dantzer, MD, assistant professor of pediatrics, Johns Hopkins Medicine, Baltimore, said in an email.
“With the current formula shortage, there has perhaps never been a more important time to do this study and provide additional guidance on who does or does not need special formula,” noted Dr. Dantzer, who also was not involved in the study. “A milk allergy diagnosis impacts the child and the family, so it is very important to avoid overdiagnosis and to support the breastfeeding mother.”
“These findings should provide reassurance that dietary exclusions for the breastfeeding mother are not needed for most children with milk allergy,” she said. “If a milk allergy is suspected, the child should be referred to an allergist.”
The authors recommend further related research into the safety and effectiveness of using the guidance in practice.
One coauthor reports financial relationships with a biotech company. Ms. Allen and her remaining coauthors, as well as Ms. Shroba and Dr. Dantzer, report no relevant financial relationships. The study was funded through fellowships.
A version of this article first appeared on Medscape.com.
According to a consensus study, many infants in some countries are misdiagnosed with allergy to cow, sheep, or goat milk, and they’re prescribed specialized formulas they don’t need.
“Milk allergy overdiagnosis is common in some regions and can potentially harm mothers and infants,” the authors write in Clinical & Experimental Allergy. “These new consensus recommendations on the safe detection and management of milk allergy in children under 2 years aim to reduce harms associated with milk allergy overdiagnosis.”
“This guidance, developed by experts without commercial ties to the formula industry, aims to reduce milk allergy overdiagnosis and [to] support ... breastfeeding and less use of specialized formula, compared with current guidelines,” they add.
Up to 1% of European infants 2 years of age and younger are considered allergic to cow’s milk. Prescriptions for specialized formula for bottle-fed infants allergic to cow’s milk in Australia, England, and Norway have grown to over 10 times the expected volumes.
Lead study author Hilary I. Allen, National Heart and Lung Institute, Imperial College London, and her colleagues on several continents developed practical guidance for providers on safely detecting and managing milk allergy in infants.
Due to lack of high-certainty research evidence in this area, they used the Delphi consensus method.
The study involved two rounds of anonymous consensus-building surveys and one formal meeting in 2021.
The team identified experts from diverse geographic and cultural settings by searching medical databases for the term “milk hypersensitivity.” They asked those experts to recommend colleagues. The researchers also contacted experts with ties to international professional organizations, such as the International Board of Lactation Consultant Examiners, as well as societies associated with the World Allergy Organization.
The 17 study participants included clinicians and researchers in general practice, health visiting, lactation support, midwifery, nutrition, and relevant areas of pediatrics from Africa, Asia, Australia, Europe, the Middle East, and North America. Experts with recent conflicts of interest with the breastmilk substitute (formula) industry were excluded from the study. Five authors of earlier milk allergy guidelines and seven parents contributed feedback.
In each survey round, participants used a nine-point scale to rank the importance of each proposed statement that addressed prevention of overdiagnosis or underdiagnosis, support of breastfeeding women, and the role of specialized formula products.
Based on the number of total points participants assigned, each statement was classified as “essential,” “recommended,” “no consensus,” or “excluded” due to lack of relevance.
The experts agreed on 38 essential statements in several categories, including:
- Maternal dietary restriction is often not necessary to manage milk allergy
- In infants with chronic symptoms who are exclusively breastfed, milk allergy diagnosis should be considered only in specific, rare circumstances
- Milk allergy diagnosis does not usually need to be considered for stool changes, aversive feeding, or occasional spots of blood in stool, if not related in time with milk protein ingestion
The consensus recommendations provide more restrictive criteria than earlier guidelines for detecting milk allergy, fewer maternal dietary exclusions, and less use of specialized formula.
During an infant formula shortage in the U.S., a timely study
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program, Children’s Mercy Kansas City, Missouri, welcomed the study’s engagement of specialists in various fields and avoidance of bias from formula companies.
“Food allergies have received a lot of attention, especially through websites and social media,” Ms. Shroba, who was not involved in the study, told this news organization in an email. “Unfortunately, a lot of that information is incorrect and can lead to misunderstanding and misdiagnosis.”
“This article helps guide practitioners through identifying the concerning symptoms of milk allergy versus normal infant symptoms,” she said. “It can help providers discern when testing, elimination diets, and changes in formula are warranted.
“This guidance emphasizes the reproducibility and specificity of symptoms, which are key elements of a food allergy diagnosis,” she explained. “By eliminating unnecessary milk allergy labeling, we can keep infants on appropriate diets for their age, such as breastfeeding or milk-based formulas. Proper diagnosis can also reduce unnecessary financial strain of specialty formulas, stress to the family regarding feedings, and a restrictive diet for the breastfeeding mother.”
The study will be useful to a wide range of health care providers, Jennifer Anne Dantzer, MD, assistant professor of pediatrics, Johns Hopkins Medicine, Baltimore, said in an email.
“With the current formula shortage, there has perhaps never been a more important time to do this study and provide additional guidance on who does or does not need special formula,” noted Dr. Dantzer, who also was not involved in the study. “A milk allergy diagnosis impacts the child and the family, so it is very important to avoid overdiagnosis and to support the breastfeeding mother.”
“These findings should provide reassurance that dietary exclusions for the breastfeeding mother are not needed for most children with milk allergy,” she said. “If a milk allergy is suspected, the child should be referred to an allergist.”
The authors recommend further related research into the safety and effectiveness of using the guidance in practice.
One coauthor reports financial relationships with a biotech company. Ms. Allen and her remaining coauthors, as well as Ms. Shroba and Dr. Dantzer, report no relevant financial relationships. The study was funded through fellowships.
A version of this article first appeared on Medscape.com.



