User login
A guide to diagnosing and managing ascites in cirrhosis
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
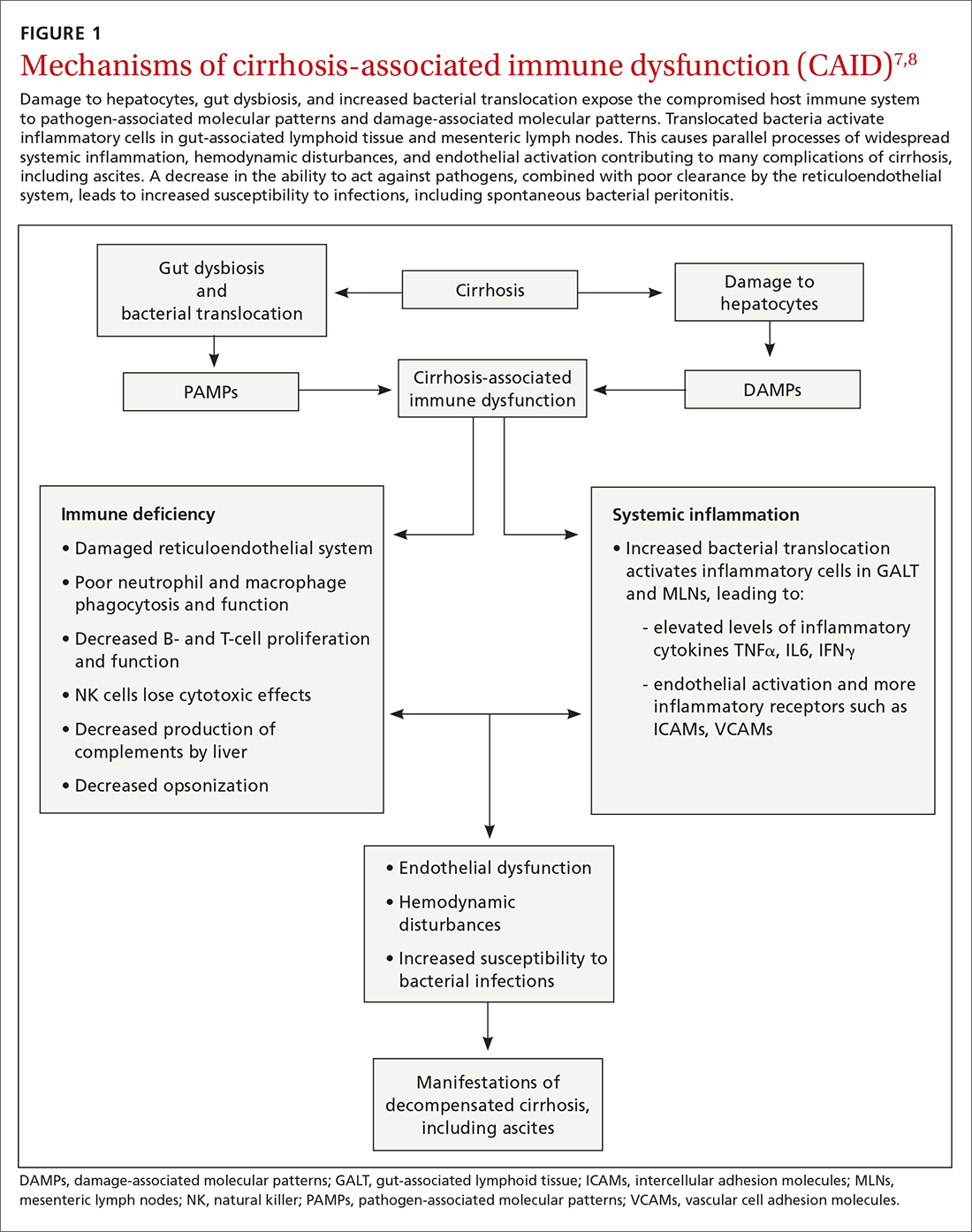
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8
Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).

History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
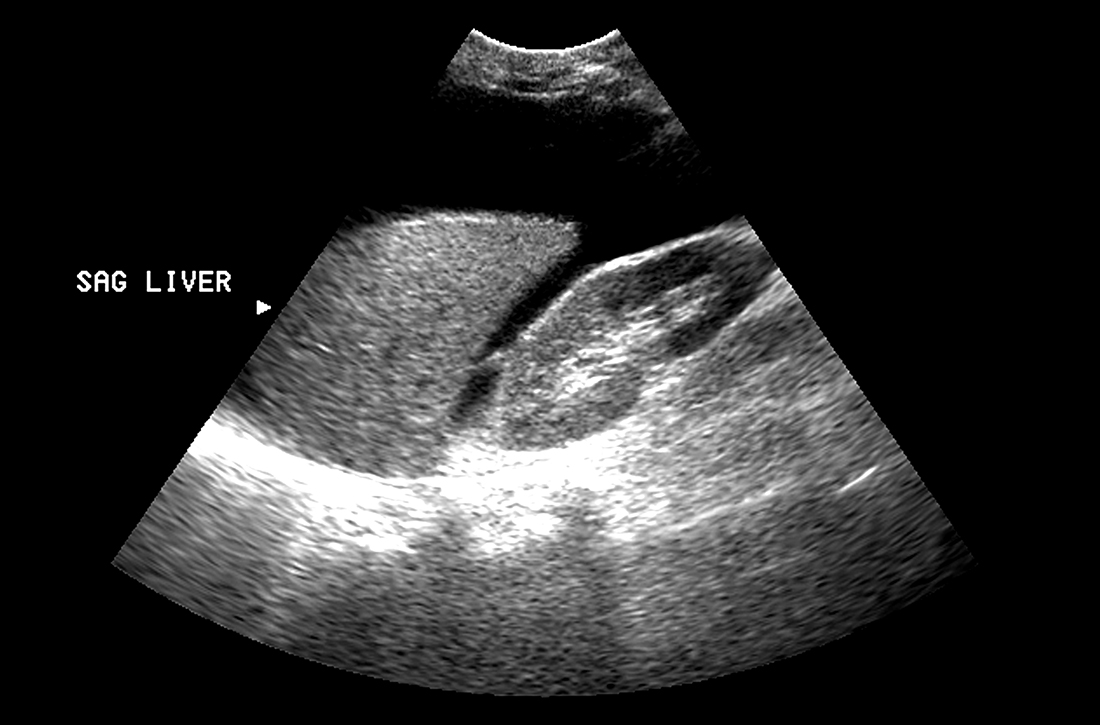
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
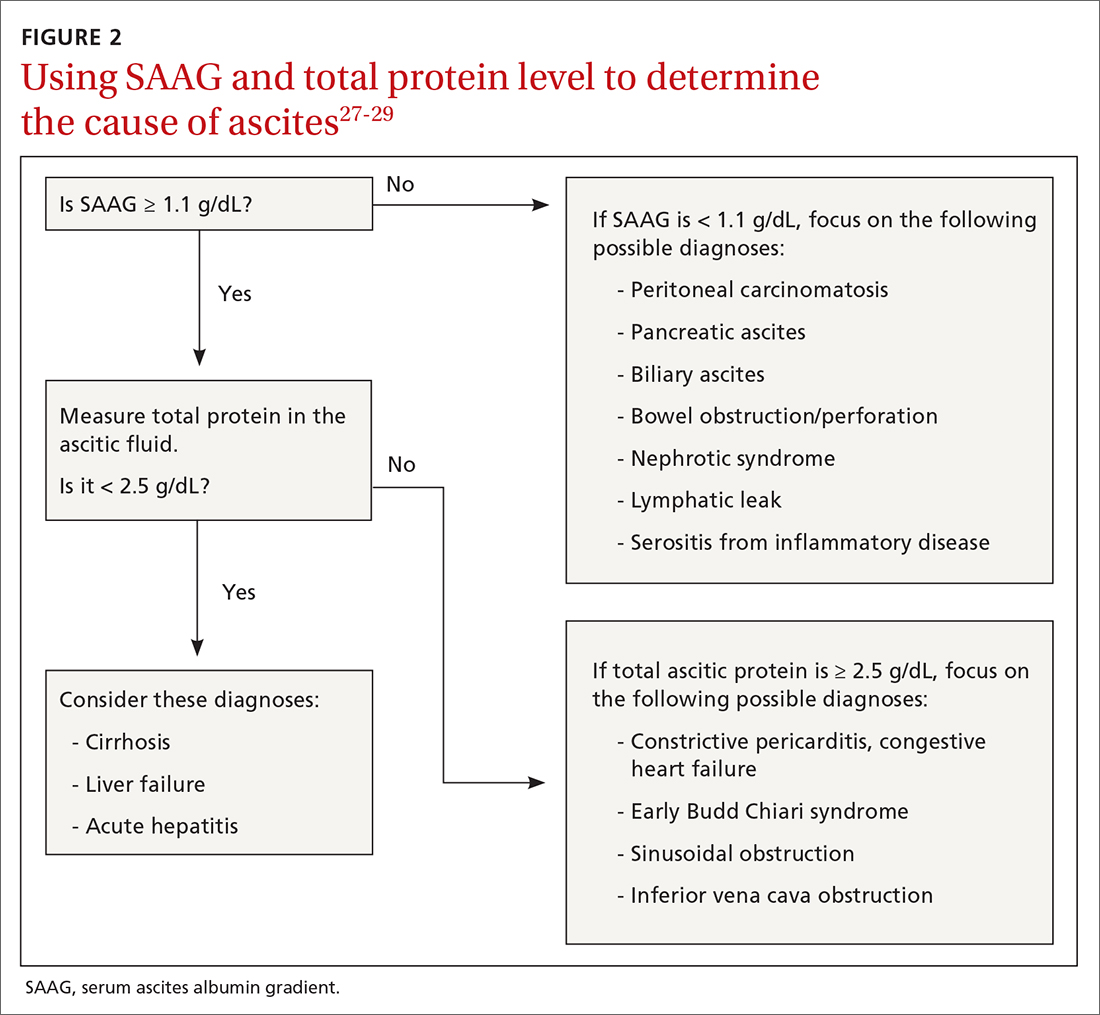
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29
Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8

Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).

History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29

Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8

Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).

History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29

Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
PRACTICE RECOMMENDATIONS
› Calculate the serum ascites albumin gradient and measure the total ascites protein level to distinguish cirrhotic ascites from that caused by heart failure or other disorders. C
› Recommend sodium restriction of 4.9-6.9 g for patients with established ascites secondary to cirrhosis. C
› Avoid giving angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs in cirrhosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most labeled penicillin-allergic are no longer intolerant
The mislabeling has implications for patient outcomes and efforts to fight antibiotic resistance, said Olajumoke Fadugba, MD, program director for the allergy and immunology fellowship at University of Pennsylvania Health System, Philadelphia.
About 10% of the general population reports a history of penicillin allergy (up to 15% of hospitalized patients), but up to 90% of patients with that label are able to tolerate penicillin, Dr. Fadugba said. The mislabeling comes either because reactions were improperly characterized early on or people have outgrown the allergy.
“There are data that tell us penicillin IgE-mediated wanes over time and that after 10 years of avoidance of a drug, greater than 80% of patients have a resolution of their penicillin IgE.”
Data also show patients outgrow their aminopenicillin reactions (including those from amoxicillin and Ampicillin) faster than parenteral penicillin reactions, she noted.
Josune Iglesias, MD, assistant professor of internal medicine at Rush University Medical Center in Chicago, said in an interview that she often sees patients who said their parents told them when they were kids that they were allergic to penicillin and that information just keeps getting entered into their records.
She said physicians are aware the penicillin-allergic label is not always accurate, but there is hesitancy to challenge those labels.
“We are cautious because of the potential side effects and the harm that we could cause if we unlabel the patient,” she said. “I think having this information will help us unlabel those patients well so we don’t cause harm.”
Also, the threat to antibiotic resistance is real, she said, when penicillin is eliminated as an option unnecessarily.
When a person is labeled allergic to penicillin, the treatment choices often go to broad-spectrum antibiotics that are more costly, have potentially worse side effects, and may contribute to resistance.
“It’s really important, especially with older people, patients sicker with chronic conditions to really make sure we unlabel those patients [who are not truly penicillin allergic],” Dr. Iglesias said.
The label can also cause harm in the hospital setting and worsen outcomes, according to Dr. Fadugba.
She noted that the penicillin allergy label has been linked with longer hospital length of stay, higher rate of readmission, acute kidney injury, multidrug-resistant organisms such as MRSA, and nosocomial infections including Clostridioides difficile.
Getting an effective drug history is an important part of determining who really has a penicillin allergy.
A questionnaire should ask whether the patient was likely to have had an immediate hypersensitivity to penicillin, such as hives or anaphylaxis, which would be more worrisome than a delayed rash.
Knowing the time frame of the reaction helps determine how likely or unlikely people are to still have the allergy, Dr. Fadugba said. “We also want to ask, have they received a penicillin antibiotic since that initial reaction and have they tolerated it?”
She continued: “If a patient received amoxicillin 2 weeks ago, and they tolerated it, you can essentially remove the allergy label and essentially change that patient’s potential hospital course – that immediate course or future outcomes.”
After obtaining the history, there are choices to make.
If a patient is not allergic, she said, the next step is removing the label and documenting why so that in the future another clinician doesn’t see the deleted label and put it back. If a person is deemed allergic by history, clinicians should document the nature of the reaction and if the patient needs a beta-lactam during a hospitalization or in clinic, make a decision based on what kind of beta-lactam they need.
“Generally, for a fourth-generation cephalosporin, for a distant history of penicillin allergy, you can probably give the full dose or – if you’re conservative – give it cautiously, perhaps 10% initially and then monitor because cross-reactivity is known to be low, about 2%,” Dr. Fadugba said.
If the patient needs a penicillin antibiotic specifically, options are guided by the resources.
If a clinician has personnel or an allergy specialist available, skin testing may be an option and “if negative, you can rule out the allergy,” Dr. Fadugba said.
“If that’s not available and the patient really needs a penicillin, you can consider desensitization,” she said.
However, she said, “If the patient is very high risk, then you have no choice but to use an alternative, especially if you can’t desensitize.”
Dr. Fadugba is a consultant for the Health Resources & Services Administration. Dr. Iglesias disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The mislabeling has implications for patient outcomes and efforts to fight antibiotic resistance, said Olajumoke Fadugba, MD, program director for the allergy and immunology fellowship at University of Pennsylvania Health System, Philadelphia.
About 10% of the general population reports a history of penicillin allergy (up to 15% of hospitalized patients), but up to 90% of patients with that label are able to tolerate penicillin, Dr. Fadugba said. The mislabeling comes either because reactions were improperly characterized early on or people have outgrown the allergy.
“There are data that tell us penicillin IgE-mediated wanes over time and that after 10 years of avoidance of a drug, greater than 80% of patients have a resolution of their penicillin IgE.”
Data also show patients outgrow their aminopenicillin reactions (including those from amoxicillin and Ampicillin) faster than parenteral penicillin reactions, she noted.
Josune Iglesias, MD, assistant professor of internal medicine at Rush University Medical Center in Chicago, said in an interview that she often sees patients who said their parents told them when they were kids that they were allergic to penicillin and that information just keeps getting entered into their records.
She said physicians are aware the penicillin-allergic label is not always accurate, but there is hesitancy to challenge those labels.
“We are cautious because of the potential side effects and the harm that we could cause if we unlabel the patient,” she said. “I think having this information will help us unlabel those patients well so we don’t cause harm.”
Also, the threat to antibiotic resistance is real, she said, when penicillin is eliminated as an option unnecessarily.
When a person is labeled allergic to penicillin, the treatment choices often go to broad-spectrum antibiotics that are more costly, have potentially worse side effects, and may contribute to resistance.
“It’s really important, especially with older people, patients sicker with chronic conditions to really make sure we unlabel those patients [who are not truly penicillin allergic],” Dr. Iglesias said.
The label can also cause harm in the hospital setting and worsen outcomes, according to Dr. Fadugba.
She noted that the penicillin allergy label has been linked with longer hospital length of stay, higher rate of readmission, acute kidney injury, multidrug-resistant organisms such as MRSA, and nosocomial infections including Clostridioides difficile.
Getting an effective drug history is an important part of determining who really has a penicillin allergy.
A questionnaire should ask whether the patient was likely to have had an immediate hypersensitivity to penicillin, such as hives or anaphylaxis, which would be more worrisome than a delayed rash.
Knowing the time frame of the reaction helps determine how likely or unlikely people are to still have the allergy, Dr. Fadugba said. “We also want to ask, have they received a penicillin antibiotic since that initial reaction and have they tolerated it?”
She continued: “If a patient received amoxicillin 2 weeks ago, and they tolerated it, you can essentially remove the allergy label and essentially change that patient’s potential hospital course – that immediate course or future outcomes.”
After obtaining the history, there are choices to make.
If a patient is not allergic, she said, the next step is removing the label and documenting why so that in the future another clinician doesn’t see the deleted label and put it back. If a person is deemed allergic by history, clinicians should document the nature of the reaction and if the patient needs a beta-lactam during a hospitalization or in clinic, make a decision based on what kind of beta-lactam they need.
“Generally, for a fourth-generation cephalosporin, for a distant history of penicillin allergy, you can probably give the full dose or – if you’re conservative – give it cautiously, perhaps 10% initially and then monitor because cross-reactivity is known to be low, about 2%,” Dr. Fadugba said.
If the patient needs a penicillin antibiotic specifically, options are guided by the resources.
If a clinician has personnel or an allergy specialist available, skin testing may be an option and “if negative, you can rule out the allergy,” Dr. Fadugba said.
“If that’s not available and the patient really needs a penicillin, you can consider desensitization,” she said.
However, she said, “If the patient is very high risk, then you have no choice but to use an alternative, especially if you can’t desensitize.”
Dr. Fadugba is a consultant for the Health Resources & Services Administration. Dr. Iglesias disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The mislabeling has implications for patient outcomes and efforts to fight antibiotic resistance, said Olajumoke Fadugba, MD, program director for the allergy and immunology fellowship at University of Pennsylvania Health System, Philadelphia.
About 10% of the general population reports a history of penicillin allergy (up to 15% of hospitalized patients), but up to 90% of patients with that label are able to tolerate penicillin, Dr. Fadugba said. The mislabeling comes either because reactions were improperly characterized early on or people have outgrown the allergy.
“There are data that tell us penicillin IgE-mediated wanes over time and that after 10 years of avoidance of a drug, greater than 80% of patients have a resolution of their penicillin IgE.”
Data also show patients outgrow their aminopenicillin reactions (including those from amoxicillin and Ampicillin) faster than parenteral penicillin reactions, she noted.
Josune Iglesias, MD, assistant professor of internal medicine at Rush University Medical Center in Chicago, said in an interview that she often sees patients who said their parents told them when they were kids that they were allergic to penicillin and that information just keeps getting entered into their records.
She said physicians are aware the penicillin-allergic label is not always accurate, but there is hesitancy to challenge those labels.
“We are cautious because of the potential side effects and the harm that we could cause if we unlabel the patient,” she said. “I think having this information will help us unlabel those patients well so we don’t cause harm.”
Also, the threat to antibiotic resistance is real, she said, when penicillin is eliminated as an option unnecessarily.
When a person is labeled allergic to penicillin, the treatment choices often go to broad-spectrum antibiotics that are more costly, have potentially worse side effects, and may contribute to resistance.
“It’s really important, especially with older people, patients sicker with chronic conditions to really make sure we unlabel those patients [who are not truly penicillin allergic],” Dr. Iglesias said.
The label can also cause harm in the hospital setting and worsen outcomes, according to Dr. Fadugba.
She noted that the penicillin allergy label has been linked with longer hospital length of stay, higher rate of readmission, acute kidney injury, multidrug-resistant organisms such as MRSA, and nosocomial infections including Clostridioides difficile.
Getting an effective drug history is an important part of determining who really has a penicillin allergy.
A questionnaire should ask whether the patient was likely to have had an immediate hypersensitivity to penicillin, such as hives or anaphylaxis, which would be more worrisome than a delayed rash.
Knowing the time frame of the reaction helps determine how likely or unlikely people are to still have the allergy, Dr. Fadugba said. “We also want to ask, have they received a penicillin antibiotic since that initial reaction and have they tolerated it?”
She continued: “If a patient received amoxicillin 2 weeks ago, and they tolerated it, you can essentially remove the allergy label and essentially change that patient’s potential hospital course – that immediate course or future outcomes.”
After obtaining the history, there are choices to make.
If a patient is not allergic, she said, the next step is removing the label and documenting why so that in the future another clinician doesn’t see the deleted label and put it back. If a person is deemed allergic by history, clinicians should document the nature of the reaction and if the patient needs a beta-lactam during a hospitalization or in clinic, make a decision based on what kind of beta-lactam they need.
“Generally, for a fourth-generation cephalosporin, for a distant history of penicillin allergy, you can probably give the full dose or – if you’re conservative – give it cautiously, perhaps 10% initially and then monitor because cross-reactivity is known to be low, about 2%,” Dr. Fadugba said.
If the patient needs a penicillin antibiotic specifically, options are guided by the resources.
If a clinician has personnel or an allergy specialist available, skin testing may be an option and “if negative, you can rule out the allergy,” Dr. Fadugba said.
“If that’s not available and the patient really needs a penicillin, you can consider desensitization,” she said.
However, she said, “If the patient is very high risk, then you have no choice but to use an alternative, especially if you can’t desensitize.”
Dr. Fadugba is a consultant for the Health Resources & Services Administration. Dr. Iglesias disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Corticosteroid bursts may increase risk of sepsis, GI bleeding in children
The adverse events are rare, and the risk attenuates in subsequent months, the analysis shows. Still, the study “provides evidence that corticosteroid bursts are not innocuous but may pose potentially serious health risks,” study author Tsung-Chieh Yao, MD, PhD, and colleagues said. “Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events.”
The study, which was published online in JAMA Pediatrics, indicates that oral corticosteroids are “not a benign medication, which is something that we should have all along known,” commented Harold J. Farber, MD, MSPH, professor of pediatrics at Baylor College of Medicine and a pediatric pulmonologist at Texas Children’s Hospital, both in Houston.
While oral corticosteroids may be important for the treatment of asthma, inflammatory bowel disease, and rheumatoid arthritis, they often are overprescribed – a phenomenon that Dr. Farber and collaborators saw when they analyzed data from children with public health insurance in Texas.
The medication is “not uncommonly used for minor asthma exacerbations or minor respiratory symptoms, which do not require oral steroids,” said Dr. Farber, who was not involved with the study. “What this study tells us is to save it for when they are really needed,” such as to treat a severe asthma exacerbation.
Despite the risk of adverse events, oral corticosteroids remain an important medication, and clinicians should aim to strike “the right balance,” Dr. Farber said.
Prior research has shown that the long-term use of oral corticosteroids is associated with adverse events such as infections, glaucoma, hyperglycemia, cardiovascular diseases, and osteoporosis. In addition, data indicate that corticosteroid bursts are associated with GI bleeding and sepsis in adults. But few studies have looked at the risk of corticosteroid bursts in children, the researchers said.
To evaluate associations of corticosteroid bursts – defined as the use of oral corticosteroids for 14 days or less – with GI bleeding, sepsis, pneumonia, and glaucoma in children, Dr. Yao and colleagues analyzed data from the National Health Insurance Research Database in Taiwan between 2013 and 2017. Dr. Yao is affiliated with the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan City, Taiwan.
Of more than 4.5 million children in the database, 42% received at least one corticosteroid burst, typically for acute respiratory tract infections and allergic diseases. The researchers focused on 1,064,587 children who received a single corticosteroid burst, and compared the incidence of adverse events before and after treatment using a self-controlled case series design. “Corticosteroid bursts were significantly associated with a 1.4- to 2.2-fold increase of GI bleeding, sepsis, and pneumonia, but not glaucoma, within the first month after initiation of corticosteroid therapy,” the investigators reported.
Incidence rate ratios in the 5-30 days after starting corticosteroid bursts were 1.41 for GI bleeding, 2.02 for sepsis, 2.19 for pneumonia, and 0.98 for glaucoma, compared with a pretreatment reference period.
The incidence rate per 1,000 person-years for GI bleeding was 2.48 with corticosteroid bursts, compared with 1.88 without corticosteroids. For sepsis, the rates with and without corticosteroids were 0.37 and 0.34, respectively. And for pneumonia, the rates were 25.74 versus 16.39.
Further research is needed to assess the validity of these findings, the authors noted. Because many children receive corticosteroid bursts worldwide, however, the “findings call for a careful reevaluation regarding the prudent use” of this treatment.
The study was supported by grants from the National Health Research Institutes; Ministry of Science and Technology of Taiwan; National Cheng Kung University, Tainan, Taiwan; Chang Gung Medical Foundation; and the National Institutes of Health. A coauthor disclosed grants from GlaxoSmithKline outside of the study.
The adverse events are rare, and the risk attenuates in subsequent months, the analysis shows. Still, the study “provides evidence that corticosteroid bursts are not innocuous but may pose potentially serious health risks,” study author Tsung-Chieh Yao, MD, PhD, and colleagues said. “Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events.”
The study, which was published online in JAMA Pediatrics, indicates that oral corticosteroids are “not a benign medication, which is something that we should have all along known,” commented Harold J. Farber, MD, MSPH, professor of pediatrics at Baylor College of Medicine and a pediatric pulmonologist at Texas Children’s Hospital, both in Houston.
While oral corticosteroids may be important for the treatment of asthma, inflammatory bowel disease, and rheumatoid arthritis, they often are overprescribed – a phenomenon that Dr. Farber and collaborators saw when they analyzed data from children with public health insurance in Texas.
The medication is “not uncommonly used for minor asthma exacerbations or minor respiratory symptoms, which do not require oral steroids,” said Dr. Farber, who was not involved with the study. “What this study tells us is to save it for when they are really needed,” such as to treat a severe asthma exacerbation.
Despite the risk of adverse events, oral corticosteroids remain an important medication, and clinicians should aim to strike “the right balance,” Dr. Farber said.
Prior research has shown that the long-term use of oral corticosteroids is associated with adverse events such as infections, glaucoma, hyperglycemia, cardiovascular diseases, and osteoporosis. In addition, data indicate that corticosteroid bursts are associated with GI bleeding and sepsis in adults. But few studies have looked at the risk of corticosteroid bursts in children, the researchers said.
To evaluate associations of corticosteroid bursts – defined as the use of oral corticosteroids for 14 days or less – with GI bleeding, sepsis, pneumonia, and glaucoma in children, Dr. Yao and colleagues analyzed data from the National Health Insurance Research Database in Taiwan between 2013 and 2017. Dr. Yao is affiliated with the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan City, Taiwan.
Of more than 4.5 million children in the database, 42% received at least one corticosteroid burst, typically for acute respiratory tract infections and allergic diseases. The researchers focused on 1,064,587 children who received a single corticosteroid burst, and compared the incidence of adverse events before and after treatment using a self-controlled case series design. “Corticosteroid bursts were significantly associated with a 1.4- to 2.2-fold increase of GI bleeding, sepsis, and pneumonia, but not glaucoma, within the first month after initiation of corticosteroid therapy,” the investigators reported.
Incidence rate ratios in the 5-30 days after starting corticosteroid bursts were 1.41 for GI bleeding, 2.02 for sepsis, 2.19 for pneumonia, and 0.98 for glaucoma, compared with a pretreatment reference period.
The incidence rate per 1,000 person-years for GI bleeding was 2.48 with corticosteroid bursts, compared with 1.88 without corticosteroids. For sepsis, the rates with and without corticosteroids were 0.37 and 0.34, respectively. And for pneumonia, the rates were 25.74 versus 16.39.
Further research is needed to assess the validity of these findings, the authors noted. Because many children receive corticosteroid bursts worldwide, however, the “findings call for a careful reevaluation regarding the prudent use” of this treatment.
The study was supported by grants from the National Health Research Institutes; Ministry of Science and Technology of Taiwan; National Cheng Kung University, Tainan, Taiwan; Chang Gung Medical Foundation; and the National Institutes of Health. A coauthor disclosed grants from GlaxoSmithKline outside of the study.
The adverse events are rare, and the risk attenuates in subsequent months, the analysis shows. Still, the study “provides evidence that corticosteroid bursts are not innocuous but may pose potentially serious health risks,” study author Tsung-Chieh Yao, MD, PhD, and colleagues said. “Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events.”
The study, which was published online in JAMA Pediatrics, indicates that oral corticosteroids are “not a benign medication, which is something that we should have all along known,” commented Harold J. Farber, MD, MSPH, professor of pediatrics at Baylor College of Medicine and a pediatric pulmonologist at Texas Children’s Hospital, both in Houston.
While oral corticosteroids may be important for the treatment of asthma, inflammatory bowel disease, and rheumatoid arthritis, they often are overprescribed – a phenomenon that Dr. Farber and collaborators saw when they analyzed data from children with public health insurance in Texas.
The medication is “not uncommonly used for minor asthma exacerbations or minor respiratory symptoms, which do not require oral steroids,” said Dr. Farber, who was not involved with the study. “What this study tells us is to save it for when they are really needed,” such as to treat a severe asthma exacerbation.
Despite the risk of adverse events, oral corticosteroids remain an important medication, and clinicians should aim to strike “the right balance,” Dr. Farber said.
Prior research has shown that the long-term use of oral corticosteroids is associated with adverse events such as infections, glaucoma, hyperglycemia, cardiovascular diseases, and osteoporosis. In addition, data indicate that corticosteroid bursts are associated with GI bleeding and sepsis in adults. But few studies have looked at the risk of corticosteroid bursts in children, the researchers said.
To evaluate associations of corticosteroid bursts – defined as the use of oral corticosteroids for 14 days or less – with GI bleeding, sepsis, pneumonia, and glaucoma in children, Dr. Yao and colleagues analyzed data from the National Health Insurance Research Database in Taiwan between 2013 and 2017. Dr. Yao is affiliated with the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan City, Taiwan.
Of more than 4.5 million children in the database, 42% received at least one corticosteroid burst, typically for acute respiratory tract infections and allergic diseases. The researchers focused on 1,064,587 children who received a single corticosteroid burst, and compared the incidence of adverse events before and after treatment using a self-controlled case series design. “Corticosteroid bursts were significantly associated with a 1.4- to 2.2-fold increase of GI bleeding, sepsis, and pneumonia, but not glaucoma, within the first month after initiation of corticosteroid therapy,” the investigators reported.
Incidence rate ratios in the 5-30 days after starting corticosteroid bursts were 1.41 for GI bleeding, 2.02 for sepsis, 2.19 for pneumonia, and 0.98 for glaucoma, compared with a pretreatment reference period.
The incidence rate per 1,000 person-years for GI bleeding was 2.48 with corticosteroid bursts, compared with 1.88 without corticosteroids. For sepsis, the rates with and without corticosteroids were 0.37 and 0.34, respectively. And for pneumonia, the rates were 25.74 versus 16.39.
Further research is needed to assess the validity of these findings, the authors noted. Because many children receive corticosteroid bursts worldwide, however, the “findings call for a careful reevaluation regarding the prudent use” of this treatment.
The study was supported by grants from the National Health Research Institutes; Ministry of Science and Technology of Taiwan; National Cheng Kung University, Tainan, Taiwan; Chang Gung Medical Foundation; and the National Institutes of Health. A coauthor disclosed grants from GlaxoSmithKline outside of the study.
FROM JAMA PEDIATRICS
FDA expands use of SLIT pollen allergy treatment to children
The Food and Drug Administration has approved a new indication for ALK’s under-the-tongue immunotherapy tablet Ragwitek (Ambrosia artemisiifolia) to treat ragweed pollen–induced hay fever in children aged 5-17 years.
Ragwitek received FDA approval in 2014 to treat short ragweed pollen–induced hay fever, with or without allergic rhinoconjunctivitis, in adults aged 18-65 years.
The approval for Ragwitek comes with a boxed warning regarding a risk for life-threatening allergic reactions associated with the immunotherapy treatment, including anaphylaxis and severe laryngopharyngeal restriction. The package insert specifies that physicians should prescribe autoinjectable epinephrine with the drug.
“Ragwitek tablets provide a new immunotherapy treatment option for children and adolescents with seasonal ragweed allergies which often causes uncomfortable nasal symptoms and red, itchy eyes during the late summer and early fall,” David I. Bernstein, MD, University of Cincinnati, Bernstein Clinical Research, said in a company press release.
Short ragweed pollen is one of the most common weed allergies. Allergic rhinitis, or hay fever, affects 10%-30% of the population worldwide, according to the American Academy of Allergy Asthma & Immunology. In the United States, approximately 7.7% of adults and 7.2% of children were diagnosed with it annually, according to the Centers for Disease Control and Prevention.
The new indication was based partly on data from a phase 3 clinical trial in children with short ragweed–induced allergic rhinitis, or hay fever, published in the Journal of Allergy and Clinical Immunology. In the study, researchers evaluated the efficacy and safety of the treatment in 1,022 participants aged 5-17 years with a history of ragweed-induced rhinoconjunctivitis and sensitivity to ragweed over a 20- to 28-week treatment period.
Researchers found that Ragwitek improved symptoms in children and adolescents and decreased their use of symptom-relieving medication, compared with placebo.
Among children and adolescents aged 5-17 years, the most common adverse reactions reported were throat irritation/tickle (48.3% in the Ragwitek group vs. 17.7% in the placebo group), itching in the mouth (47.8% vs. 11.2%), itching in the ear (33.9% vs. 6.3%), mouth pain (18.9% vs. 4.5%), swelling of the lips (13.8% vs. 1.2%), nausea (11.5% vs. 3.3%), swelling of the tongue (11.3% vs. 0.8%), throat swelling (10.7% vs. 1.6%), and stomach pain (10.1% vs. 4.5%).
The FDA also recommends that Ragwitek not be prescribed to people with severe, unstable, or uncontrolled asthma, those with a history of severe systemic allergic reactions, and those with a history of eosinophilic esophagitis. The immunotherapy treatment also may not be suitable for people who are unresponsive to epinephrine or inhaled bronchodilators.
In addition, the treatment is not approved for the immediate relief of allergic symptoms in children or adults. The once-daily treatment, which contains an extract from short ragweed pollen, should begin 12 weeks before the start of ragweed pollen season and continue throughout the season, according to the FDA.
Dr. Bernstein said that the under-the-tongue immunotherapy works by targeting the specific allergy trigger and reducing allergy symptoms by “stimulating the immune system.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new indication for ALK’s under-the-tongue immunotherapy tablet Ragwitek (Ambrosia artemisiifolia) to treat ragweed pollen–induced hay fever in children aged 5-17 years.
Ragwitek received FDA approval in 2014 to treat short ragweed pollen–induced hay fever, with or without allergic rhinoconjunctivitis, in adults aged 18-65 years.
The approval for Ragwitek comes with a boxed warning regarding a risk for life-threatening allergic reactions associated with the immunotherapy treatment, including anaphylaxis and severe laryngopharyngeal restriction. The package insert specifies that physicians should prescribe autoinjectable epinephrine with the drug.
“Ragwitek tablets provide a new immunotherapy treatment option for children and adolescents with seasonal ragweed allergies which often causes uncomfortable nasal symptoms and red, itchy eyes during the late summer and early fall,” David I. Bernstein, MD, University of Cincinnati, Bernstein Clinical Research, said in a company press release.
Short ragweed pollen is one of the most common weed allergies. Allergic rhinitis, or hay fever, affects 10%-30% of the population worldwide, according to the American Academy of Allergy Asthma & Immunology. In the United States, approximately 7.7% of adults and 7.2% of children were diagnosed with it annually, according to the Centers for Disease Control and Prevention.
The new indication was based partly on data from a phase 3 clinical trial in children with short ragweed–induced allergic rhinitis, or hay fever, published in the Journal of Allergy and Clinical Immunology. In the study, researchers evaluated the efficacy and safety of the treatment in 1,022 participants aged 5-17 years with a history of ragweed-induced rhinoconjunctivitis and sensitivity to ragweed over a 20- to 28-week treatment period.
Researchers found that Ragwitek improved symptoms in children and adolescents and decreased their use of symptom-relieving medication, compared with placebo.
Among children and adolescents aged 5-17 years, the most common adverse reactions reported were throat irritation/tickle (48.3% in the Ragwitek group vs. 17.7% in the placebo group), itching in the mouth (47.8% vs. 11.2%), itching in the ear (33.9% vs. 6.3%), mouth pain (18.9% vs. 4.5%), swelling of the lips (13.8% vs. 1.2%), nausea (11.5% vs. 3.3%), swelling of the tongue (11.3% vs. 0.8%), throat swelling (10.7% vs. 1.6%), and stomach pain (10.1% vs. 4.5%).
The FDA also recommends that Ragwitek not be prescribed to people with severe, unstable, or uncontrolled asthma, those with a history of severe systemic allergic reactions, and those with a history of eosinophilic esophagitis. The immunotherapy treatment also may not be suitable for people who are unresponsive to epinephrine or inhaled bronchodilators.
In addition, the treatment is not approved for the immediate relief of allergic symptoms in children or adults. The once-daily treatment, which contains an extract from short ragweed pollen, should begin 12 weeks before the start of ragweed pollen season and continue throughout the season, according to the FDA.
Dr. Bernstein said that the under-the-tongue immunotherapy works by targeting the specific allergy trigger and reducing allergy symptoms by “stimulating the immune system.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new indication for ALK’s under-the-tongue immunotherapy tablet Ragwitek (Ambrosia artemisiifolia) to treat ragweed pollen–induced hay fever in children aged 5-17 years.
Ragwitek received FDA approval in 2014 to treat short ragweed pollen–induced hay fever, with or without allergic rhinoconjunctivitis, in adults aged 18-65 years.
The approval for Ragwitek comes with a boxed warning regarding a risk for life-threatening allergic reactions associated with the immunotherapy treatment, including anaphylaxis and severe laryngopharyngeal restriction. The package insert specifies that physicians should prescribe autoinjectable epinephrine with the drug.
“Ragwitek tablets provide a new immunotherapy treatment option for children and adolescents with seasonal ragweed allergies which often causes uncomfortable nasal symptoms and red, itchy eyes during the late summer and early fall,” David I. Bernstein, MD, University of Cincinnati, Bernstein Clinical Research, said in a company press release.
Short ragweed pollen is one of the most common weed allergies. Allergic rhinitis, or hay fever, affects 10%-30% of the population worldwide, according to the American Academy of Allergy Asthma & Immunology. In the United States, approximately 7.7% of adults and 7.2% of children were diagnosed with it annually, according to the Centers for Disease Control and Prevention.
The new indication was based partly on data from a phase 3 clinical trial in children with short ragweed–induced allergic rhinitis, or hay fever, published in the Journal of Allergy and Clinical Immunology. In the study, researchers evaluated the efficacy and safety of the treatment in 1,022 participants aged 5-17 years with a history of ragweed-induced rhinoconjunctivitis and sensitivity to ragweed over a 20- to 28-week treatment period.
Researchers found that Ragwitek improved symptoms in children and adolescents and decreased their use of symptom-relieving medication, compared with placebo.
Among children and adolescents aged 5-17 years, the most common adverse reactions reported were throat irritation/tickle (48.3% in the Ragwitek group vs. 17.7% in the placebo group), itching in the mouth (47.8% vs. 11.2%), itching in the ear (33.9% vs. 6.3%), mouth pain (18.9% vs. 4.5%), swelling of the lips (13.8% vs. 1.2%), nausea (11.5% vs. 3.3%), swelling of the tongue (11.3% vs. 0.8%), throat swelling (10.7% vs. 1.6%), and stomach pain (10.1% vs. 4.5%).
The FDA also recommends that Ragwitek not be prescribed to people with severe, unstable, or uncontrolled asthma, those with a history of severe systemic allergic reactions, and those with a history of eosinophilic esophagitis. The immunotherapy treatment also may not be suitable for people who are unresponsive to epinephrine or inhaled bronchodilators.
In addition, the treatment is not approved for the immediate relief of allergic symptoms in children or adults. The once-daily treatment, which contains an extract from short ragweed pollen, should begin 12 weeks before the start of ragweed pollen season and continue throughout the season, according to the FDA.
Dr. Bernstein said that the under-the-tongue immunotherapy works by targeting the specific allergy trigger and reducing allergy symptoms by “stimulating the immune system.”
A version of this article first appeared on Medscape.com.
Risk of hypogammaglobulinemia, infections with rituximab increased in pediatric patients
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
FROM CARRA 2021
Milk is overtaking nuts as top food allergy threat
When Lesley Solomon’s son was 10 years old, he was standing in an unlucky spot on the playground when a schoolmate kicked over a cup of hot chocolate, sending droplets flying into the air. For the young boy with a severe milk allergy, the hot liquid splattering was less of a hazard for him than the dairy stirred into the drink.
Ms. Solomon’s son quickly washed the fluids off his clothes and skin, took some Benadryl, and called his parents. But on the car ride home, his throat began to close and his pulse raced. It was one of about a dozen times he has needed an epinephrine injection, which increases blood flow, reduces swelling, and reverses anaphylaxis.
“Until you see a child going through that anaphylaxis and not being able to breathe, or throwing up so much that they can’t breathe, you don’t understand” how serious food allergies can be, said Ms. Solomon, who is senior vice president and chief innovation officer of the Dana-Farber Cancer Institute in Boston and cofounder of the Food Allergy Science Initiative, an independent nonprofit that funds food allergy research.
The rate of children hospitalized for food-induced anaphylaxis rose by 25% from 2006 to 2012 – from 1.2 to 1.5 per 100,000 – according to a 2019 analysis of data from pediatric hospitals in the United States. And severe symptoms were more often linked to milk than to peanuts or tree nuts, the study showed.
Cow’s milk is the most common food allergy in children aged younger than 5 years, and accounts for about half of all food allergies in children younger than 1. Most children grow out of it, but when milk allergy persists into the teenage years and adulthood, it is more likely to cause severe reactions.
A dangerous allergy
“Cow’s milk allergy is the most distressing of the food allergies. Many people are unaware that it can cause anaphylaxis that is so severe,” said Carla Davis, MD, director of the food allergy program at the Texas Children’s Hospital in Houston. “People do not think about how much of this is in our food.”
And cow’s milk was shown to be the food allergy most likely to lead to death in school-aged children in the United Kingdom, according to an analysis of national data reported by this news organization.
Lack of awareness is what makes milk allergy so dangerous, said Paul Turner, BMBCh, PhD, a pediatric allergist and immunologist from Imperial College London, who was involved in the British analysis. “We need to get that information out to the public and businesses so they take the same level of care that they have with nuts, and when someone says they have milk allergy, they take it seriously.
In food allergy, the body treats certain proteins, such as the casein and whey in milk, as invaders, mounting an immune response. Antibodies known as IgE – which normally protect against bacteria, viruses, and parasites – trigger inflammation, the release of histamine, and can lead to symptoms, typically within minutes, ranging from rash and swelling to vomiting, difficulty swallowing, and difficulty breathing.
So, the very thing that makes milk a healthy choice for kids – its high protein content – can cause serious reactions in a small portion of children and adults. “You don’t need much milk to get a decent dose” of the allergen, Dr. Turner pointed out.
The mechanisms of milk allergy are complex, even compared with other food allergies. The IgE antibody can be detected with a skin-prick test or IgE blood test, but some people have positive results even though they are not allergic. To complicate things further, people can also have non–IgE-mediated milk allergy, which cannot be detected with testing and can lead to symptoms that emerge hours or even days after exposure.
More serious than lactose intolerance
Unfortunately, milk allergy is often confused with a milk-related digestive problem. Globally, about 70% of people lack the enzyme to break down the sugar in milk; the condition, known as lactose intolerance, can cause bloating, abdominal cramps, and diarrhea but is not life-threatening.
“Because lactose intolerance is so common, people don’t think of milk allergy as something that can be significant or severe,” said Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at the Northwestern University, Chicago.
In babies, colic, the regurgitation of milk-based formula, and rash are sometimes misinterpreted as a milk allergy, leading parents to buy expensive, specialized formula unnecessarily.
Frustrated by a lack of data about food allergies, Dr. Gupta and colleagues launched a nationally representative survey of 38,408 American parents in 2009, which was updated in 2015 and 2016.
On average, children with milk allergy had their first reaction before the age of 2, most commonly vomiting, diarrhea, hives, and eczema; this is a younger age of onset than for other food allergies. And children with milk allergy were twice as likely as children with other allergies to grow out of it.
Yet about one-third of milk-allergic children in the updated study were 11 years and older. And in a similar survey of adults who self-reported symptoms, milk allergy was as common as peanut allergy (1.9% vs 1.8%). “We don’t know why milk allergy is becoming more persistent,” Dr. Gupta said. And, she warned, only one in four children with a milk allergy had a current prescription for an epinephrine autoinjector, compared with about 70% of children with peanut allergy.
Food allergy can’t be caused by genetics alone, said Christine Olsen, MD, cofounder and CEO of the Food Allergy Science Initiative at the Broad Institute in Cambridge, Mass. “There may be a genetic predisposition, but there must be something environmental” that has influenced the development of food allergies.
One theory is that the body’s natural defense against noxious substances has been disrupted in the modern world by processed foods, chemical additives, and hygienic surroundings.
Dr. Olson’s own son vomited when he had his first small taste of hummus as a baby; he is severely allergic to sesame. The immediacy of his bodily reaction made Dr. Olsen think that the response involved neurons, not just a misguided immune system.
Researchers are currently looking for drug targets that could shut off the immune response as quickly as it starts. If you think of the fact that some kids outgrow their allergies and some adults get allergies, that suggests there’s some lever that you can turn on and off,” said Dr. Olsen, who is also a radiation oncologist.
Preventing allergy
The approach to food allergy prevention has already been transformed by the Learning Early About Peanut Allergy (LEAP) study conducted in the United Kingdom. LEAP investigators randomly assigned 640 infants to ingest regular amounts of peanut snacks or peanut butter or to avoid peanut products until they reached 5 years of age. The babies who had regular exposure to peanut from an early age were much less likely to develop a peanut allergy than those who avoided peanuts.
The National Institute of Allergy and Infectious Diseases revised its guidelines and now recommends that all babies be exposed to peanut-containing food at around 6 months of age; for high-risk babies, that can start as early as 4 months.
Allergy experts are planning to study that concept again with other foods, including cow’s milk. The 5-year iREACH study, launched by the Center for Food Allergy & Asthma Research at Northwestern and Lurie Children’s Hospital in Chicago, is currently enrolling 10,500 infants to test early exposure to peanuts, milk, egg, and cashew. A portion of the infants will have severe eczema, putting them at high risk for food allergies, and others will be low risk, said Dr. Gupta, who is the principal iREACH investigator.
“Hopefully in the next 5 years we will have data showing whether this prevention technique will work for other common food allergens, in addition to peanuts,” she said.
Introducing foods early “promotes tolerance rather than early sensitization,” explained Stephanie Leeds, MD, an allergist and immunologist at Yale University, New Haven, Conn. In the future, rather than just diagnosing and treating food allergies, allergists might work with pediatricians to help prevent them from ever happening.
A version of this article first appeared on Medscape.com.
When Lesley Solomon’s son was 10 years old, he was standing in an unlucky spot on the playground when a schoolmate kicked over a cup of hot chocolate, sending droplets flying into the air. For the young boy with a severe milk allergy, the hot liquid splattering was less of a hazard for him than the dairy stirred into the drink.
Ms. Solomon’s son quickly washed the fluids off his clothes and skin, took some Benadryl, and called his parents. But on the car ride home, his throat began to close and his pulse raced. It was one of about a dozen times he has needed an epinephrine injection, which increases blood flow, reduces swelling, and reverses anaphylaxis.
“Until you see a child going through that anaphylaxis and not being able to breathe, or throwing up so much that they can’t breathe, you don’t understand” how serious food allergies can be, said Ms. Solomon, who is senior vice president and chief innovation officer of the Dana-Farber Cancer Institute in Boston and cofounder of the Food Allergy Science Initiative, an independent nonprofit that funds food allergy research.
The rate of children hospitalized for food-induced anaphylaxis rose by 25% from 2006 to 2012 – from 1.2 to 1.5 per 100,000 – according to a 2019 analysis of data from pediatric hospitals in the United States. And severe symptoms were more often linked to milk than to peanuts or tree nuts, the study showed.
Cow’s milk is the most common food allergy in children aged younger than 5 years, and accounts for about half of all food allergies in children younger than 1. Most children grow out of it, but when milk allergy persists into the teenage years and adulthood, it is more likely to cause severe reactions.
A dangerous allergy
“Cow’s milk allergy is the most distressing of the food allergies. Many people are unaware that it can cause anaphylaxis that is so severe,” said Carla Davis, MD, director of the food allergy program at the Texas Children’s Hospital in Houston. “People do not think about how much of this is in our food.”
And cow’s milk was shown to be the food allergy most likely to lead to death in school-aged children in the United Kingdom, according to an analysis of national data reported by this news organization.
Lack of awareness is what makes milk allergy so dangerous, said Paul Turner, BMBCh, PhD, a pediatric allergist and immunologist from Imperial College London, who was involved in the British analysis. “We need to get that information out to the public and businesses so they take the same level of care that they have with nuts, and when someone says they have milk allergy, they take it seriously.
In food allergy, the body treats certain proteins, such as the casein and whey in milk, as invaders, mounting an immune response. Antibodies known as IgE – which normally protect against bacteria, viruses, and parasites – trigger inflammation, the release of histamine, and can lead to symptoms, typically within minutes, ranging from rash and swelling to vomiting, difficulty swallowing, and difficulty breathing.
So, the very thing that makes milk a healthy choice for kids – its high protein content – can cause serious reactions in a small portion of children and adults. “You don’t need much milk to get a decent dose” of the allergen, Dr. Turner pointed out.
The mechanisms of milk allergy are complex, even compared with other food allergies. The IgE antibody can be detected with a skin-prick test or IgE blood test, but some people have positive results even though they are not allergic. To complicate things further, people can also have non–IgE-mediated milk allergy, which cannot be detected with testing and can lead to symptoms that emerge hours or even days after exposure.
More serious than lactose intolerance
Unfortunately, milk allergy is often confused with a milk-related digestive problem. Globally, about 70% of people lack the enzyme to break down the sugar in milk; the condition, known as lactose intolerance, can cause bloating, abdominal cramps, and diarrhea but is not life-threatening.
“Because lactose intolerance is so common, people don’t think of milk allergy as something that can be significant or severe,” said Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at the Northwestern University, Chicago.
In babies, colic, the regurgitation of milk-based formula, and rash are sometimes misinterpreted as a milk allergy, leading parents to buy expensive, specialized formula unnecessarily.
Frustrated by a lack of data about food allergies, Dr. Gupta and colleagues launched a nationally representative survey of 38,408 American parents in 2009, which was updated in 2015 and 2016.
On average, children with milk allergy had their first reaction before the age of 2, most commonly vomiting, diarrhea, hives, and eczema; this is a younger age of onset than for other food allergies. And children with milk allergy were twice as likely as children with other allergies to grow out of it.
Yet about one-third of milk-allergic children in the updated study were 11 years and older. And in a similar survey of adults who self-reported symptoms, milk allergy was as common as peanut allergy (1.9% vs 1.8%). “We don’t know why milk allergy is becoming more persistent,” Dr. Gupta said. And, she warned, only one in four children with a milk allergy had a current prescription for an epinephrine autoinjector, compared with about 70% of children with peanut allergy.
Food allergy can’t be caused by genetics alone, said Christine Olsen, MD, cofounder and CEO of the Food Allergy Science Initiative at the Broad Institute in Cambridge, Mass. “There may be a genetic predisposition, but there must be something environmental” that has influenced the development of food allergies.
One theory is that the body’s natural defense against noxious substances has been disrupted in the modern world by processed foods, chemical additives, and hygienic surroundings.
Dr. Olson’s own son vomited when he had his first small taste of hummus as a baby; he is severely allergic to sesame. The immediacy of his bodily reaction made Dr. Olsen think that the response involved neurons, not just a misguided immune system.
Researchers are currently looking for drug targets that could shut off the immune response as quickly as it starts. If you think of the fact that some kids outgrow their allergies and some adults get allergies, that suggests there’s some lever that you can turn on and off,” said Dr. Olsen, who is also a radiation oncologist.
Preventing allergy
The approach to food allergy prevention has already been transformed by the Learning Early About Peanut Allergy (LEAP) study conducted in the United Kingdom. LEAP investigators randomly assigned 640 infants to ingest regular amounts of peanut snacks or peanut butter or to avoid peanut products until they reached 5 years of age. The babies who had regular exposure to peanut from an early age were much less likely to develop a peanut allergy than those who avoided peanuts.
The National Institute of Allergy and Infectious Diseases revised its guidelines and now recommends that all babies be exposed to peanut-containing food at around 6 months of age; for high-risk babies, that can start as early as 4 months.
Allergy experts are planning to study that concept again with other foods, including cow’s milk. The 5-year iREACH study, launched by the Center for Food Allergy & Asthma Research at Northwestern and Lurie Children’s Hospital in Chicago, is currently enrolling 10,500 infants to test early exposure to peanuts, milk, egg, and cashew. A portion of the infants will have severe eczema, putting them at high risk for food allergies, and others will be low risk, said Dr. Gupta, who is the principal iREACH investigator.
“Hopefully in the next 5 years we will have data showing whether this prevention technique will work for other common food allergens, in addition to peanuts,” she said.
Introducing foods early “promotes tolerance rather than early sensitization,” explained Stephanie Leeds, MD, an allergist and immunologist at Yale University, New Haven, Conn. In the future, rather than just diagnosing and treating food allergies, allergists might work with pediatricians to help prevent them from ever happening.
A version of this article first appeared on Medscape.com.
When Lesley Solomon’s son was 10 years old, he was standing in an unlucky spot on the playground when a schoolmate kicked over a cup of hot chocolate, sending droplets flying into the air. For the young boy with a severe milk allergy, the hot liquid splattering was less of a hazard for him than the dairy stirred into the drink.
Ms. Solomon’s son quickly washed the fluids off his clothes and skin, took some Benadryl, and called his parents. But on the car ride home, his throat began to close and his pulse raced. It was one of about a dozen times he has needed an epinephrine injection, which increases blood flow, reduces swelling, and reverses anaphylaxis.
“Until you see a child going through that anaphylaxis and not being able to breathe, or throwing up so much that they can’t breathe, you don’t understand” how serious food allergies can be, said Ms. Solomon, who is senior vice president and chief innovation officer of the Dana-Farber Cancer Institute in Boston and cofounder of the Food Allergy Science Initiative, an independent nonprofit that funds food allergy research.
The rate of children hospitalized for food-induced anaphylaxis rose by 25% from 2006 to 2012 – from 1.2 to 1.5 per 100,000 – according to a 2019 analysis of data from pediatric hospitals in the United States. And severe symptoms were more often linked to milk than to peanuts or tree nuts, the study showed.
Cow’s milk is the most common food allergy in children aged younger than 5 years, and accounts for about half of all food allergies in children younger than 1. Most children grow out of it, but when milk allergy persists into the teenage years and adulthood, it is more likely to cause severe reactions.
A dangerous allergy
“Cow’s milk allergy is the most distressing of the food allergies. Many people are unaware that it can cause anaphylaxis that is so severe,” said Carla Davis, MD, director of the food allergy program at the Texas Children’s Hospital in Houston. “People do not think about how much of this is in our food.”
And cow’s milk was shown to be the food allergy most likely to lead to death in school-aged children in the United Kingdom, according to an analysis of national data reported by this news organization.
Lack of awareness is what makes milk allergy so dangerous, said Paul Turner, BMBCh, PhD, a pediatric allergist and immunologist from Imperial College London, who was involved in the British analysis. “We need to get that information out to the public and businesses so they take the same level of care that they have with nuts, and when someone says they have milk allergy, they take it seriously.
In food allergy, the body treats certain proteins, such as the casein and whey in milk, as invaders, mounting an immune response. Antibodies known as IgE – which normally protect against bacteria, viruses, and parasites – trigger inflammation, the release of histamine, and can lead to symptoms, typically within minutes, ranging from rash and swelling to vomiting, difficulty swallowing, and difficulty breathing.
So, the very thing that makes milk a healthy choice for kids – its high protein content – can cause serious reactions in a small portion of children and adults. “You don’t need much milk to get a decent dose” of the allergen, Dr. Turner pointed out.
The mechanisms of milk allergy are complex, even compared with other food allergies. The IgE antibody can be detected with a skin-prick test or IgE blood test, but some people have positive results even though they are not allergic. To complicate things further, people can also have non–IgE-mediated milk allergy, which cannot be detected with testing and can lead to symptoms that emerge hours or even days after exposure.
More serious than lactose intolerance
Unfortunately, milk allergy is often confused with a milk-related digestive problem. Globally, about 70% of people lack the enzyme to break down the sugar in milk; the condition, known as lactose intolerance, can cause bloating, abdominal cramps, and diarrhea but is not life-threatening.
“Because lactose intolerance is so common, people don’t think of milk allergy as something that can be significant or severe,” said Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at the Northwestern University, Chicago.
In babies, colic, the regurgitation of milk-based formula, and rash are sometimes misinterpreted as a milk allergy, leading parents to buy expensive, specialized formula unnecessarily.
Frustrated by a lack of data about food allergies, Dr. Gupta and colleagues launched a nationally representative survey of 38,408 American parents in 2009, which was updated in 2015 and 2016.
On average, children with milk allergy had their first reaction before the age of 2, most commonly vomiting, diarrhea, hives, and eczema; this is a younger age of onset than for other food allergies. And children with milk allergy were twice as likely as children with other allergies to grow out of it.
Yet about one-third of milk-allergic children in the updated study were 11 years and older. And in a similar survey of adults who self-reported symptoms, milk allergy was as common as peanut allergy (1.9% vs 1.8%). “We don’t know why milk allergy is becoming more persistent,” Dr. Gupta said. And, she warned, only one in four children with a milk allergy had a current prescription for an epinephrine autoinjector, compared with about 70% of children with peanut allergy.
Food allergy can’t be caused by genetics alone, said Christine Olsen, MD, cofounder and CEO of the Food Allergy Science Initiative at the Broad Institute in Cambridge, Mass. “There may be a genetic predisposition, but there must be something environmental” that has influenced the development of food allergies.
One theory is that the body’s natural defense against noxious substances has been disrupted in the modern world by processed foods, chemical additives, and hygienic surroundings.
Dr. Olson’s own son vomited when he had his first small taste of hummus as a baby; he is severely allergic to sesame. The immediacy of his bodily reaction made Dr. Olsen think that the response involved neurons, not just a misguided immune system.
Researchers are currently looking for drug targets that could shut off the immune response as quickly as it starts. If you think of the fact that some kids outgrow their allergies and some adults get allergies, that suggests there’s some lever that you can turn on and off,” said Dr. Olsen, who is also a radiation oncologist.
Preventing allergy
The approach to food allergy prevention has already been transformed by the Learning Early About Peanut Allergy (LEAP) study conducted in the United Kingdom. LEAP investigators randomly assigned 640 infants to ingest regular amounts of peanut snacks or peanut butter or to avoid peanut products until they reached 5 years of age. The babies who had regular exposure to peanut from an early age were much less likely to develop a peanut allergy than those who avoided peanuts.
The National Institute of Allergy and Infectious Diseases revised its guidelines and now recommends that all babies be exposed to peanut-containing food at around 6 months of age; for high-risk babies, that can start as early as 4 months.
Allergy experts are planning to study that concept again with other foods, including cow’s milk. The 5-year iREACH study, launched by the Center for Food Allergy & Asthma Research at Northwestern and Lurie Children’s Hospital in Chicago, is currently enrolling 10,500 infants to test early exposure to peanuts, milk, egg, and cashew. A portion of the infants will have severe eczema, putting them at high risk for food allergies, and others will be low risk, said Dr. Gupta, who is the principal iREACH investigator.
“Hopefully in the next 5 years we will have data showing whether this prevention technique will work for other common food allergens, in addition to peanuts,” she said.
Introducing foods early “promotes tolerance rather than early sensitization,” explained Stephanie Leeds, MD, an allergist and immunologist at Yale University, New Haven, Conn. In the future, rather than just diagnosing and treating food allergies, allergists might work with pediatricians to help prevent them from ever happening.
A version of this article first appeared on Medscape.com.
Lenvatinib Plus Pembrolizumab Improves Outcomes in Previously Untreated Advanced Clear Cell Renal Cell Carcinoma
Study Overview
Objective. To evaluate the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab compared with sunitinib alone for the treatment of newly diagnosed advanced clear cell renal cell carcinoma (ccRCC).
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 1:1:1 ratio to receive treatment with 1 of 3 regimens: lenvatinib 20 mg daily plus pembrolizumab 200 mg on day 1 of each 21-day cycle; lenvatinib 18 mg daily plus everolimus 5 mg once daily for each 21-day cycle; or sunitinib 50 mg daily for 4 weeks followed by 2 weeks off. Patients were stratified according to geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group.
Setting and participants. A total of 1417 patients were screened, and 1069 patients underwent randomization between October 2016 and July 2019: 355 patients were randomized to the lenvatinib plus pembrolizumab group, 357 were randomized to the lenvatinib plus everolimus group, and 357 were randomized to the sunitinib alone group. The patients must have had a diagnosis of previously untreated advanced renal cell carcinoma with a clear-cell component. All the patients need to have a Karnofsky performance status of at least 70, adequate renal function, and controlled blood pressure with or without antihypertensive medications.
Main outcome measures. The primary endpoint assessed the progression-free survival (PFS) as evaluated by independent review committee using RECIST, version 1.1. Imaging was performed at the time of screening and every 8 weeks thereafter. Secondary endpoints were safety, overall survival (OS), and objective response rate as well as investigator-assessed PFS. Also, they assessed the duration of response. During the treatment period, the safety and adverse events were assessed up to 30 days from the last dose of the trial drug.
Main results. The baseline characteristics were well balanced between the treatment groups. More than 70% of enrolled participants were male. Approximately 60% of participants were MSKCC intermediate risk, 27% were favorable risk, and 9% were poor risk. Patients with a PD-L1 combined positive score of 1% or more represented 30% of the population. The remainder had a PD-L1 combined positive score of <1% (30%) or such data were not available (38%). Liver metastases were present in 17% of patients at baseline in each group, and 70% of patients had a prior nephrectomy. The data cutoff occurred in August 2020 for PFS and the median follow-up for OS was 26.6 months. Around 40% of the participants in the lenvatinib plus pembrolizumab group, 18.8% in the sunitinib group, and 31% in the lenvatinib plus everolimus group were still receiving trial treatment at data cutoff. The leading cause for discontinuing therapy was disease progression. Approximately 50% of patients in the lenvatinib/everolimus group and sunitinib group received subsequent checkpoint inhibitor therapy after progression.
The median PFS in the lenvatinib plus pembrolizumab group was significantly longer than in the sunitinib group, 23.9 months vs 9.2 months (hazard ratio [HR], 0.39; 95% CI, 0.32-0.49; P < 0.001). The median PFS was also significantly longer in the lenvatinib plus everolimus group compared with sunitinib, 14.7 vs 9.2 months (HR 0.65; 95% CI 0.53-0.80; P < 0.001). The PFS benefit favored the lenvatinib combination groups over sunitinib in all subgroups, including the MSKCC prognostic risk groups. The median OS was not reached with any treatment, with 79% of patients in the lenvatinib plus pembrolizumab group, 66% of patients in the lenvatinib plus everolimus group, and 70% in the sunitinib group still alive at 24 months. Survival was significantly longer in the lenvatinib plus pembrolizumab group compared with sunitinib (HR, 0.66; 95% CI, 0.49-0.88; P = 0.005). The OS favored lenvatinib/pembrolizumab over sunitinib in the PD-L1 positive or negative groups. The median duration of response in the lenvatinib plus pembrolizumab group was 25.8 months compared to 16.6 months and 14.6 months in the lenvatinib plus everolimus and sunitinib groups, respectively. Complete response rates were higher in the lenvatinib plus pembrolizumab group (16%) compared with lenvatinib/everolimus (9.8%) or sunitinib (4.2%). The median time to response was around 1.9 months in all 3 groups.
The most frequent adverse events seen in all groups were diarrhea, hypertension, fatigue, and nausea. Hypothyroidism was seen more frequently in the lenvatinib plus pembrolizumab group (47%). Grade 3 adverse events were seen in approximately 80% of patients in all groups. The most common grade 3 or higher adverse event was hypertension in all 3 groups. The median time for discontinuing treatment due to side effects was 8.97 months in the lenvatinib plus pembrolizumab arm, 5.49 months in the lenvatinib plus everolimus group, and 4.57 months in the sunitinib group. In the lenvatinib plus pembrolizumab group, 15 patients had grade 5 adverse events; 11 participants had fatal events not related to disease progression. In the lenvatinib plus everolimus group, there were 22 patients with grade 5 events, with 10 fatal events not related to disease progression. In the sunitinib group, 11 patients had grade 5 events, and only 2 fatal events were not linked to disease progression.
Conclusion. The combination of lenvatinib plus pembrolizumab significantly prolongs PFS and OS compared with sunitinib in patients with previously untreated and advanced ccRCC. The median OS has not yet been reached.
Commentary
The results of the current phase 3 CLEAR trial highlight the efficacy and safety of lenvatinib plus pembrolizumab as a first-line treatment in advanced ccRCC. This trial adds to the rapidly growing body of literature supporting the notion that the combination of anti-PD-1 based therapy with either CTLA-4 antibodies or VEGF receptor tyrosine kinase inhibitors (TKI) improves outcomes in previously untreated patients with advanced ccRCC. Previously presented data from Keynote-426 (pembrolizumab plus axitinib), Checkmate-214 (nivolumab plus ipilimumab), and Javelin Renal 101 (Avelumab plus axitinib) have also shown improved outcomes with combination therapy in the frontline setting.1-4 While the landscape of therapeutic options in the frontline setting continues to grow, there remains lack of clarity as to how to tailor our therapeutic decisions for specific patient populations. The exception would be nivolumab and ipilimumab, which are currently indicated for IMDC intermediate- or poor-risk patients.
The combination of VEGFR TKI therapy and PD-1 antibodies provides rapid disease control, with a median time to response in the current study of 1.9 months, and, generally speaking, a low risk of progression in the first 6 months of therapy. While cross-trial comparisons are always problematic, the PFS reported in this study and others with VEGFR TKI and PD-1 antibody combinations is quite impressive and surpasses that noted in Checkmate 214.3 While the median OS survival has not yet been reached, the long duration of PFS and complete response rate of 16% in this study certainly make this an attractive frontline option for newly diagnosed patients with advanced ccRCC. Longer follow-up is needed to confirm the survival benefit noted.
Applications for Clinical Practice
The current data support the use VEGFR TKI and anti-PD1 therapy in the frontline setting. How to choose between such combination regimens or combination immunotherapy remains unclear, and further biomarker-based assessments are needed to help guide therapeutic decisions for our patients.
1. Motzer, R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma [published online ahead of print, 2021 Feb 13]. N Engl J Med. 2021;10.1056/NEJMoa2035716. doi:10.1056/NEJMoa2035716
2. Rini, BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
3. Motzer, RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
4. Motzer, RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115.
Study Overview
Objective. To evaluate the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab compared with sunitinib alone for the treatment of newly diagnosed advanced clear cell renal cell carcinoma (ccRCC).
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 1:1:1 ratio to receive treatment with 1 of 3 regimens: lenvatinib 20 mg daily plus pembrolizumab 200 mg on day 1 of each 21-day cycle; lenvatinib 18 mg daily plus everolimus 5 mg once daily for each 21-day cycle; or sunitinib 50 mg daily for 4 weeks followed by 2 weeks off. Patients were stratified according to geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group.
Setting and participants. A total of 1417 patients were screened, and 1069 patients underwent randomization between October 2016 and July 2019: 355 patients were randomized to the lenvatinib plus pembrolizumab group, 357 were randomized to the lenvatinib plus everolimus group, and 357 were randomized to the sunitinib alone group. The patients must have had a diagnosis of previously untreated advanced renal cell carcinoma with a clear-cell component. All the patients need to have a Karnofsky performance status of at least 70, adequate renal function, and controlled blood pressure with or without antihypertensive medications.
Main outcome measures. The primary endpoint assessed the progression-free survival (PFS) as evaluated by independent review committee using RECIST, version 1.1. Imaging was performed at the time of screening and every 8 weeks thereafter. Secondary endpoints were safety, overall survival (OS), and objective response rate as well as investigator-assessed PFS. Also, they assessed the duration of response. During the treatment period, the safety and adverse events were assessed up to 30 days from the last dose of the trial drug.
Main results. The baseline characteristics were well balanced between the treatment groups. More than 70% of enrolled participants were male. Approximately 60% of participants were MSKCC intermediate risk, 27% were favorable risk, and 9% were poor risk. Patients with a PD-L1 combined positive score of 1% or more represented 30% of the population. The remainder had a PD-L1 combined positive score of <1% (30%) or such data were not available (38%). Liver metastases were present in 17% of patients at baseline in each group, and 70% of patients had a prior nephrectomy. The data cutoff occurred in August 2020 for PFS and the median follow-up for OS was 26.6 months. Around 40% of the participants in the lenvatinib plus pembrolizumab group, 18.8% in the sunitinib group, and 31% in the lenvatinib plus everolimus group were still receiving trial treatment at data cutoff. The leading cause for discontinuing therapy was disease progression. Approximately 50% of patients in the lenvatinib/everolimus group and sunitinib group received subsequent checkpoint inhibitor therapy after progression.
The median PFS in the lenvatinib plus pembrolizumab group was significantly longer than in the sunitinib group, 23.9 months vs 9.2 months (hazard ratio [HR], 0.39; 95% CI, 0.32-0.49; P < 0.001). The median PFS was also significantly longer in the lenvatinib plus everolimus group compared with sunitinib, 14.7 vs 9.2 months (HR 0.65; 95% CI 0.53-0.80; P < 0.001). The PFS benefit favored the lenvatinib combination groups over sunitinib in all subgroups, including the MSKCC prognostic risk groups. The median OS was not reached with any treatment, with 79% of patients in the lenvatinib plus pembrolizumab group, 66% of patients in the lenvatinib plus everolimus group, and 70% in the sunitinib group still alive at 24 months. Survival was significantly longer in the lenvatinib plus pembrolizumab group compared with sunitinib (HR, 0.66; 95% CI, 0.49-0.88; P = 0.005). The OS favored lenvatinib/pembrolizumab over sunitinib in the PD-L1 positive or negative groups. The median duration of response in the lenvatinib plus pembrolizumab group was 25.8 months compared to 16.6 months and 14.6 months in the lenvatinib plus everolimus and sunitinib groups, respectively. Complete response rates were higher in the lenvatinib plus pembrolizumab group (16%) compared with lenvatinib/everolimus (9.8%) or sunitinib (4.2%). The median time to response was around 1.9 months in all 3 groups.
The most frequent adverse events seen in all groups were diarrhea, hypertension, fatigue, and nausea. Hypothyroidism was seen more frequently in the lenvatinib plus pembrolizumab group (47%). Grade 3 adverse events were seen in approximately 80% of patients in all groups. The most common grade 3 or higher adverse event was hypertension in all 3 groups. The median time for discontinuing treatment due to side effects was 8.97 months in the lenvatinib plus pembrolizumab arm, 5.49 months in the lenvatinib plus everolimus group, and 4.57 months in the sunitinib group. In the lenvatinib plus pembrolizumab group, 15 patients had grade 5 adverse events; 11 participants had fatal events not related to disease progression. In the lenvatinib plus everolimus group, there were 22 patients with grade 5 events, with 10 fatal events not related to disease progression. In the sunitinib group, 11 patients had grade 5 events, and only 2 fatal events were not linked to disease progression.
Conclusion. The combination of lenvatinib plus pembrolizumab significantly prolongs PFS and OS compared with sunitinib in patients with previously untreated and advanced ccRCC. The median OS has not yet been reached.
Commentary
The results of the current phase 3 CLEAR trial highlight the efficacy and safety of lenvatinib plus pembrolizumab as a first-line treatment in advanced ccRCC. This trial adds to the rapidly growing body of literature supporting the notion that the combination of anti-PD-1 based therapy with either CTLA-4 antibodies or VEGF receptor tyrosine kinase inhibitors (TKI) improves outcomes in previously untreated patients with advanced ccRCC. Previously presented data from Keynote-426 (pembrolizumab plus axitinib), Checkmate-214 (nivolumab plus ipilimumab), and Javelin Renal 101 (Avelumab plus axitinib) have also shown improved outcomes with combination therapy in the frontline setting.1-4 While the landscape of therapeutic options in the frontline setting continues to grow, there remains lack of clarity as to how to tailor our therapeutic decisions for specific patient populations. The exception would be nivolumab and ipilimumab, which are currently indicated for IMDC intermediate- or poor-risk patients.
The combination of VEGFR TKI therapy and PD-1 antibodies provides rapid disease control, with a median time to response in the current study of 1.9 months, and, generally speaking, a low risk of progression in the first 6 months of therapy. While cross-trial comparisons are always problematic, the PFS reported in this study and others with VEGFR TKI and PD-1 antibody combinations is quite impressive and surpasses that noted in Checkmate 214.3 While the median OS survival has not yet been reached, the long duration of PFS and complete response rate of 16% in this study certainly make this an attractive frontline option for newly diagnosed patients with advanced ccRCC. Longer follow-up is needed to confirm the survival benefit noted.
Applications for Clinical Practice
The current data support the use VEGFR TKI and anti-PD1 therapy in the frontline setting. How to choose between such combination regimens or combination immunotherapy remains unclear, and further biomarker-based assessments are needed to help guide therapeutic decisions for our patients.
Study Overview
Objective. To evaluate the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab compared with sunitinib alone for the treatment of newly diagnosed advanced clear cell renal cell carcinoma (ccRCC).
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 1:1:1 ratio to receive treatment with 1 of 3 regimens: lenvatinib 20 mg daily plus pembrolizumab 200 mg on day 1 of each 21-day cycle; lenvatinib 18 mg daily plus everolimus 5 mg once daily for each 21-day cycle; or sunitinib 50 mg daily for 4 weeks followed by 2 weeks off. Patients were stratified according to geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group.
Setting and participants. A total of 1417 patients were screened, and 1069 patients underwent randomization between October 2016 and July 2019: 355 patients were randomized to the lenvatinib plus pembrolizumab group, 357 were randomized to the lenvatinib plus everolimus group, and 357 were randomized to the sunitinib alone group. The patients must have had a diagnosis of previously untreated advanced renal cell carcinoma with a clear-cell component. All the patients need to have a Karnofsky performance status of at least 70, adequate renal function, and controlled blood pressure with or without antihypertensive medications.
Main outcome measures. The primary endpoint assessed the progression-free survival (PFS) as evaluated by independent review committee using RECIST, version 1.1. Imaging was performed at the time of screening and every 8 weeks thereafter. Secondary endpoints were safety, overall survival (OS), and objective response rate as well as investigator-assessed PFS. Also, they assessed the duration of response. During the treatment period, the safety and adverse events were assessed up to 30 days from the last dose of the trial drug.
Main results. The baseline characteristics were well balanced between the treatment groups. More than 70% of enrolled participants were male. Approximately 60% of participants were MSKCC intermediate risk, 27% were favorable risk, and 9% were poor risk. Patients with a PD-L1 combined positive score of 1% or more represented 30% of the population. The remainder had a PD-L1 combined positive score of <1% (30%) or such data were not available (38%). Liver metastases were present in 17% of patients at baseline in each group, and 70% of patients had a prior nephrectomy. The data cutoff occurred in August 2020 for PFS and the median follow-up for OS was 26.6 months. Around 40% of the participants in the lenvatinib plus pembrolizumab group, 18.8% in the sunitinib group, and 31% in the lenvatinib plus everolimus group were still receiving trial treatment at data cutoff. The leading cause for discontinuing therapy was disease progression. Approximately 50% of patients in the lenvatinib/everolimus group and sunitinib group received subsequent checkpoint inhibitor therapy after progression.
The median PFS in the lenvatinib plus pembrolizumab group was significantly longer than in the sunitinib group, 23.9 months vs 9.2 months (hazard ratio [HR], 0.39; 95% CI, 0.32-0.49; P < 0.001). The median PFS was also significantly longer in the lenvatinib plus everolimus group compared with sunitinib, 14.7 vs 9.2 months (HR 0.65; 95% CI 0.53-0.80; P < 0.001). The PFS benefit favored the lenvatinib combination groups over sunitinib in all subgroups, including the MSKCC prognostic risk groups. The median OS was not reached with any treatment, with 79% of patients in the lenvatinib plus pembrolizumab group, 66% of patients in the lenvatinib plus everolimus group, and 70% in the sunitinib group still alive at 24 months. Survival was significantly longer in the lenvatinib plus pembrolizumab group compared with sunitinib (HR, 0.66; 95% CI, 0.49-0.88; P = 0.005). The OS favored lenvatinib/pembrolizumab over sunitinib in the PD-L1 positive or negative groups. The median duration of response in the lenvatinib plus pembrolizumab group was 25.8 months compared to 16.6 months and 14.6 months in the lenvatinib plus everolimus and sunitinib groups, respectively. Complete response rates were higher in the lenvatinib plus pembrolizumab group (16%) compared with lenvatinib/everolimus (9.8%) or sunitinib (4.2%). The median time to response was around 1.9 months in all 3 groups.
The most frequent adverse events seen in all groups were diarrhea, hypertension, fatigue, and nausea. Hypothyroidism was seen more frequently in the lenvatinib plus pembrolizumab group (47%). Grade 3 adverse events were seen in approximately 80% of patients in all groups. The most common grade 3 or higher adverse event was hypertension in all 3 groups. The median time for discontinuing treatment due to side effects was 8.97 months in the lenvatinib plus pembrolizumab arm, 5.49 months in the lenvatinib plus everolimus group, and 4.57 months in the sunitinib group. In the lenvatinib plus pembrolizumab group, 15 patients had grade 5 adverse events; 11 participants had fatal events not related to disease progression. In the lenvatinib plus everolimus group, there were 22 patients with grade 5 events, with 10 fatal events not related to disease progression. In the sunitinib group, 11 patients had grade 5 events, and only 2 fatal events were not linked to disease progression.
Conclusion. The combination of lenvatinib plus pembrolizumab significantly prolongs PFS and OS compared with sunitinib in patients with previously untreated and advanced ccRCC. The median OS has not yet been reached.
Commentary
The results of the current phase 3 CLEAR trial highlight the efficacy and safety of lenvatinib plus pembrolizumab as a first-line treatment in advanced ccRCC. This trial adds to the rapidly growing body of literature supporting the notion that the combination of anti-PD-1 based therapy with either CTLA-4 antibodies or VEGF receptor tyrosine kinase inhibitors (TKI) improves outcomes in previously untreated patients with advanced ccRCC. Previously presented data from Keynote-426 (pembrolizumab plus axitinib), Checkmate-214 (nivolumab plus ipilimumab), and Javelin Renal 101 (Avelumab plus axitinib) have also shown improved outcomes with combination therapy in the frontline setting.1-4 While the landscape of therapeutic options in the frontline setting continues to grow, there remains lack of clarity as to how to tailor our therapeutic decisions for specific patient populations. The exception would be nivolumab and ipilimumab, which are currently indicated for IMDC intermediate- or poor-risk patients.
The combination of VEGFR TKI therapy and PD-1 antibodies provides rapid disease control, with a median time to response in the current study of 1.9 months, and, generally speaking, a low risk of progression in the first 6 months of therapy. While cross-trial comparisons are always problematic, the PFS reported in this study and others with VEGFR TKI and PD-1 antibody combinations is quite impressive and surpasses that noted in Checkmate 214.3 While the median OS survival has not yet been reached, the long duration of PFS and complete response rate of 16% in this study certainly make this an attractive frontline option for newly diagnosed patients with advanced ccRCC. Longer follow-up is needed to confirm the survival benefit noted.
Applications for Clinical Practice
The current data support the use VEGFR TKI and anti-PD1 therapy in the frontline setting. How to choose between such combination regimens or combination immunotherapy remains unclear, and further biomarker-based assessments are needed to help guide therapeutic decisions for our patients.
1. Motzer, R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma [published online ahead of print, 2021 Feb 13]. N Engl J Med. 2021;10.1056/NEJMoa2035716. doi:10.1056/NEJMoa2035716
2. Rini, BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
3. Motzer, RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
4. Motzer, RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115.
1. Motzer, R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma [published online ahead of print, 2021 Feb 13]. N Engl J Med. 2021;10.1056/NEJMoa2035716. doi:10.1056/NEJMoa2035716
2. Rini, BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
3. Motzer, RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
4. Motzer, RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115.
Use of Fecal Immunochemical Testing in Acute Patient Care in a Safety Net Hospital System
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.
Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).
A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).
Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.

Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).

A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).

Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.

Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).

A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).

Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
Let’s apply the lessons from the AIDS crisis to the COVID-19 pandemic
In 2020, COVID-19 disrupted our medical system, and life in general. In the 1980s, the AIDS epidemic devastated communities and overwhelmed hospitals. There were lessons learned from the AIDS epidemic that can be applied to the current situation.
Patients with HIV-spectrum illness faced stigmatization and societal indifference, including rejection by family members, increased rates of suicide, fears of sexual and/or intrauterine transmission, substance abuse issues, and alterations of body image for those with wasting syndromes and disfiguring Kaposi lesions. AIDS prevention strategies such as the provision of condoms and needle exchange programs were controversial, and many caregivers exposed to contaminated fluids had to endure months of antiretroviral treatment.
Similar to the AIDS epidemic, the COVID-19 pandemic has had significant psychological implications for patients and caregivers. Patients with COVID-19 infections also face feelings of guilt over potentially exposing a family member to the virus; devastating socioeconomic issues; restrictive hospital visitation policies for family members; disease news oversaturation; and feelings of hopelessness. People with AIDS in the 1980s faced the possibility of dying alone, and there was initial skepticism about medications to treat HIV—just as some individuals are now uneasy about recently introduced coronavirus vaccines.
The similarities of both diseases allow us some foresight on how to deal with current COVID-19 issues. Looking back on the AIDS epidemic should teach us to prioritize attending to the mental health of sufferers and caregivers, creating advocacy and support groups for when a patient’s family is unavailable, instilling public confidence in treatment options, maintaining staff morale, addressing substance abuse (due to COVID-related stress), and depoliticizing prevention strategies. Addressing these issues is especially critical for minority populations.
As respected medical care leaders, we can provide and draw extra attention to the needs of patients’ family members and health care personnel during this COVID-19 pandemic. Hopefully, the distribution of vaccines will shorten some of our communal and professional distress.
Robert Frierson, MD
Steven Lippmann, MD
Louisville, KY
In 2020, COVID-19 disrupted our medical system, and life in general. In the 1980s, the AIDS epidemic devastated communities and overwhelmed hospitals. There were lessons learned from the AIDS epidemic that can be applied to the current situation.
Patients with HIV-spectrum illness faced stigmatization and societal indifference, including rejection by family members, increased rates of suicide, fears of sexual and/or intrauterine transmission, substance abuse issues, and alterations of body image for those with wasting syndromes and disfiguring Kaposi lesions. AIDS prevention strategies such as the provision of condoms and needle exchange programs were controversial, and many caregivers exposed to contaminated fluids had to endure months of antiretroviral treatment.
Similar to the AIDS epidemic, the COVID-19 pandemic has had significant psychological implications for patients and caregivers. Patients with COVID-19 infections also face feelings of guilt over potentially exposing a family member to the virus; devastating socioeconomic issues; restrictive hospital visitation policies for family members; disease news oversaturation; and feelings of hopelessness. People with AIDS in the 1980s faced the possibility of dying alone, and there was initial skepticism about medications to treat HIV—just as some individuals are now uneasy about recently introduced coronavirus vaccines.
The similarities of both diseases allow us some foresight on how to deal with current COVID-19 issues. Looking back on the AIDS epidemic should teach us to prioritize attending to the mental health of sufferers and caregivers, creating advocacy and support groups for when a patient’s family is unavailable, instilling public confidence in treatment options, maintaining staff morale, addressing substance abuse (due to COVID-related stress), and depoliticizing prevention strategies. Addressing these issues is especially critical for minority populations.
As respected medical care leaders, we can provide and draw extra attention to the needs of patients’ family members and health care personnel during this COVID-19 pandemic. Hopefully, the distribution of vaccines will shorten some of our communal and professional distress.
Robert Frierson, MD
Steven Lippmann, MD
Louisville, KY
In 2020, COVID-19 disrupted our medical system, and life in general. In the 1980s, the AIDS epidemic devastated communities and overwhelmed hospitals. There were lessons learned from the AIDS epidemic that can be applied to the current situation.
Patients with HIV-spectrum illness faced stigmatization and societal indifference, including rejection by family members, increased rates of suicide, fears of sexual and/or intrauterine transmission, substance abuse issues, and alterations of body image for those with wasting syndromes and disfiguring Kaposi lesions. AIDS prevention strategies such as the provision of condoms and needle exchange programs were controversial, and many caregivers exposed to contaminated fluids had to endure months of antiretroviral treatment.
Similar to the AIDS epidemic, the COVID-19 pandemic has had significant psychological implications for patients and caregivers. Patients with COVID-19 infections also face feelings of guilt over potentially exposing a family member to the virus; devastating socioeconomic issues; restrictive hospital visitation policies for family members; disease news oversaturation; and feelings of hopelessness. People with AIDS in the 1980s faced the possibility of dying alone, and there was initial skepticism about medications to treat HIV—just as some individuals are now uneasy about recently introduced coronavirus vaccines.
The similarities of both diseases allow us some foresight on how to deal with current COVID-19 issues. Looking back on the AIDS epidemic should teach us to prioritize attending to the mental health of sufferers and caregivers, creating advocacy and support groups for when a patient’s family is unavailable, instilling public confidence in treatment options, maintaining staff morale, addressing substance abuse (due to COVID-related stress), and depoliticizing prevention strategies. Addressing these issues is especially critical for minority populations.
As respected medical care leaders, we can provide and draw extra attention to the needs of patients’ family members and health care personnel during this COVID-19 pandemic. Hopefully, the distribution of vaccines will shorten some of our communal and professional distress.
Robert Frierson, MD
Steven Lippmann, MD
Louisville, KY
Hospitalizations for food anaphylaxis triple, but deaths down in United Kingdom
The rate of hospital admissions in the United Kingdom for food-induced anaphylaxis more than tripled over the 20 years from 1998 to 2018, but the case fatality rate fell by more than half, researchers report in BMJ.
“Cow’s milk is increasingly identified as the culprit allergen for fatal food reactions and is now the commonest cause of fatal anaphylaxis in children,” write Alessia Baseggio Conrado, PhD, a biochemist with the National Heart and Lung Institute at Imperial College London, and colleagues. “More education is needed to highlight the specific risks posed by cow’s milk to people who are allergic to increase awareness among food businesses.”
Whereas recognition of the risks posed by nut allergies has increased, people think milk allergy is mild, says senior author Paul. J. Turner, BMBCh, PhD, an allergist/immunologist at Imperial College. “This is often true in very young children, but school-aged children who still have milk allergy tend to have a more allergic profile, often with other allergies, including asthma,” Dr. Turner told this news organization. “Also, milk is very common in our diet, and you don’t need much milk to achieve a decent dose of allergen.”
During the study period, 101,891 people were hospitalized for anaphylaxis; 30,700 cases (30%) were coded as having been triggered by food.
These food-related admissions represent an increase from 1.23 to 4.04 per 100,000 population per year, for an annual increase of 5.7% (95% confidence interval, 5.5-5.9; P < .001), the authors write.
The largest jump occurred among children younger than 15 years, for whom admissions rose from 2.1 to 9.2 per 100,000 population per year, an annual increase of 6.6% (95% CI, 6.3-7.0). The annual increases were 5.9% (95% CI, 5.6-6.2) among persons aged 15 to 59 years and 2.1% (95% CI, 1.8-3.1) among those aged 60 years and older.
The investigators used data from England, Scotland, Wales, and Northern Ireland to track temporal trends and age and sex distributions for hospital admissions for which the primary diagnosis was anaphylaxis attributable to both food and nonfood triggers. These data were compared with nationally reported fatalities.
Over the 20-year period, 152 deaths were attributed to likely food-induced anaphylaxis. During that time, the case fatality rate for confirmed fatal food anaphylaxis fell from 0.7% to 0.19% (rate ratio, 0.931; 95% CI, 0.904-0.959; P < .001) and declined to 0.30% for suspected fatal food anaphylaxis (rate ratio, 0.970; 95% CI, 0.945-0.996; P = .024).
Between 1992 and 2018, at least 46% of all anaphylactic fatalities were deemed to be triggered by peanut or tree nut. Among school-aged children, 26% of anaphylactic fatalities were attributed to cow’s milk.
Not surprisingly, during the study period, there was an increase of 336% in prescriptions for adrenaline autoinjectors. Such prescriptions increased 11% per year.
Global trend
The data extend findings Dr. Turner and colleagues reported for England and Wales in 2014 regarding the entire United Kingdom population and align with epidemiologic trends in hospital admissions for anaphylaxis in the United States and Australia.
The researchers say better recognition and management of anaphylaxis could partly explain the decrease in fatalities, but the rise in hospitalizations remains puzzling. “Whether a true increase in the prevalence of anaphylaxis has occurred (rather than a reduction in the threshold to admit patients presenting with anaphylaxis) is unclear because evidence is lacking for an increase in prevalence of food allergy in the [United Kingdom] (and elsewhere) over the same time period,” they write.
Ronna L. Campbell, MD, PhD, an emergency physician at the Mayo Clinic in Rochester, Minn., has noted similar trends in the United States. “It may be that anaphylaxis recognition and diagnosis have improved, resulting in earlier administration of epinephrine,” Dr. Campbell said in an interview. “So while cases are increasing, earlier recognition and treatment result in decreased fatalities.” She is unaware of any new guidelines recommending increased hospitalization that would explain the puzzling rise in admissions.
According to the study authors, the clinical criteria used to diagnose anaphylaxis in the United Kingdom did not change during the study period. Although national guidance recommending the hospitalization of children younger than 16 who are suspected of having anaphylaxis was introduced in 2011 and may have boosted admissions, the year-on-year rate of increase has persisted since 2014. “Therefore the increase over the past 5 years cannot be attributed to the impact of the guidance,” they write.
The study was funded by grants from the U.K. Medical Research Council and U.K. Food Standards Agency. Two coauthors have disclosed financial relationships with industry outside of the submitted work. Dr. Conrado has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com
The rate of hospital admissions in the United Kingdom for food-induced anaphylaxis more than tripled over the 20 years from 1998 to 2018, but the case fatality rate fell by more than half, researchers report in BMJ.
“Cow’s milk is increasingly identified as the culprit allergen for fatal food reactions and is now the commonest cause of fatal anaphylaxis in children,” write Alessia Baseggio Conrado, PhD, a biochemist with the National Heart and Lung Institute at Imperial College London, and colleagues. “More education is needed to highlight the specific risks posed by cow’s milk to people who are allergic to increase awareness among food businesses.”
Whereas recognition of the risks posed by nut allergies has increased, people think milk allergy is mild, says senior author Paul. J. Turner, BMBCh, PhD, an allergist/immunologist at Imperial College. “This is often true in very young children, but school-aged children who still have milk allergy tend to have a more allergic profile, often with other allergies, including asthma,” Dr. Turner told this news organization. “Also, milk is very common in our diet, and you don’t need much milk to achieve a decent dose of allergen.”
During the study period, 101,891 people were hospitalized for anaphylaxis; 30,700 cases (30%) were coded as having been triggered by food.
These food-related admissions represent an increase from 1.23 to 4.04 per 100,000 population per year, for an annual increase of 5.7% (95% confidence interval, 5.5-5.9; P < .001), the authors write.
The largest jump occurred among children younger than 15 years, for whom admissions rose from 2.1 to 9.2 per 100,000 population per year, an annual increase of 6.6% (95% CI, 6.3-7.0). The annual increases were 5.9% (95% CI, 5.6-6.2) among persons aged 15 to 59 years and 2.1% (95% CI, 1.8-3.1) among those aged 60 years and older.
The investigators used data from England, Scotland, Wales, and Northern Ireland to track temporal trends and age and sex distributions for hospital admissions for which the primary diagnosis was anaphylaxis attributable to both food and nonfood triggers. These data were compared with nationally reported fatalities.
Over the 20-year period, 152 deaths were attributed to likely food-induced anaphylaxis. During that time, the case fatality rate for confirmed fatal food anaphylaxis fell from 0.7% to 0.19% (rate ratio, 0.931; 95% CI, 0.904-0.959; P < .001) and declined to 0.30% for suspected fatal food anaphylaxis (rate ratio, 0.970; 95% CI, 0.945-0.996; P = .024).
Between 1992 and 2018, at least 46% of all anaphylactic fatalities were deemed to be triggered by peanut or tree nut. Among school-aged children, 26% of anaphylactic fatalities were attributed to cow’s milk.
Not surprisingly, during the study period, there was an increase of 336% in prescriptions for adrenaline autoinjectors. Such prescriptions increased 11% per year.
Global trend
The data extend findings Dr. Turner and colleagues reported for England and Wales in 2014 regarding the entire United Kingdom population and align with epidemiologic trends in hospital admissions for anaphylaxis in the United States and Australia.
The researchers say better recognition and management of anaphylaxis could partly explain the decrease in fatalities, but the rise in hospitalizations remains puzzling. “Whether a true increase in the prevalence of anaphylaxis has occurred (rather than a reduction in the threshold to admit patients presenting with anaphylaxis) is unclear because evidence is lacking for an increase in prevalence of food allergy in the [United Kingdom] (and elsewhere) over the same time period,” they write.
Ronna L. Campbell, MD, PhD, an emergency physician at the Mayo Clinic in Rochester, Minn., has noted similar trends in the United States. “It may be that anaphylaxis recognition and diagnosis have improved, resulting in earlier administration of epinephrine,” Dr. Campbell said in an interview. “So while cases are increasing, earlier recognition and treatment result in decreased fatalities.” She is unaware of any new guidelines recommending increased hospitalization that would explain the puzzling rise in admissions.
According to the study authors, the clinical criteria used to diagnose anaphylaxis in the United Kingdom did not change during the study period. Although national guidance recommending the hospitalization of children younger than 16 who are suspected of having anaphylaxis was introduced in 2011 and may have boosted admissions, the year-on-year rate of increase has persisted since 2014. “Therefore the increase over the past 5 years cannot be attributed to the impact of the guidance,” they write.
The study was funded by grants from the U.K. Medical Research Council and U.K. Food Standards Agency. Two coauthors have disclosed financial relationships with industry outside of the submitted work. Dr. Conrado has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com
The rate of hospital admissions in the United Kingdom for food-induced anaphylaxis more than tripled over the 20 years from 1998 to 2018, but the case fatality rate fell by more than half, researchers report in BMJ.
“Cow’s milk is increasingly identified as the culprit allergen for fatal food reactions and is now the commonest cause of fatal anaphylaxis in children,” write Alessia Baseggio Conrado, PhD, a biochemist with the National Heart and Lung Institute at Imperial College London, and colleagues. “More education is needed to highlight the specific risks posed by cow’s milk to people who are allergic to increase awareness among food businesses.”
Whereas recognition of the risks posed by nut allergies has increased, people think milk allergy is mild, says senior author Paul. J. Turner, BMBCh, PhD, an allergist/immunologist at Imperial College. “This is often true in very young children, but school-aged children who still have milk allergy tend to have a more allergic profile, often with other allergies, including asthma,” Dr. Turner told this news organization. “Also, milk is very common in our diet, and you don’t need much milk to achieve a decent dose of allergen.”
During the study period, 101,891 people were hospitalized for anaphylaxis; 30,700 cases (30%) were coded as having been triggered by food.
These food-related admissions represent an increase from 1.23 to 4.04 per 100,000 population per year, for an annual increase of 5.7% (95% confidence interval, 5.5-5.9; P < .001), the authors write.
The largest jump occurred among children younger than 15 years, for whom admissions rose from 2.1 to 9.2 per 100,000 population per year, an annual increase of 6.6% (95% CI, 6.3-7.0). The annual increases were 5.9% (95% CI, 5.6-6.2) among persons aged 15 to 59 years and 2.1% (95% CI, 1.8-3.1) among those aged 60 years and older.
The investigators used data from England, Scotland, Wales, and Northern Ireland to track temporal trends and age and sex distributions for hospital admissions for which the primary diagnosis was anaphylaxis attributable to both food and nonfood triggers. These data were compared with nationally reported fatalities.
Over the 20-year period, 152 deaths were attributed to likely food-induced anaphylaxis. During that time, the case fatality rate for confirmed fatal food anaphylaxis fell from 0.7% to 0.19% (rate ratio, 0.931; 95% CI, 0.904-0.959; P < .001) and declined to 0.30% for suspected fatal food anaphylaxis (rate ratio, 0.970; 95% CI, 0.945-0.996; P = .024).
Between 1992 and 2018, at least 46% of all anaphylactic fatalities were deemed to be triggered by peanut or tree nut. Among school-aged children, 26% of anaphylactic fatalities were attributed to cow’s milk.
Not surprisingly, during the study period, there was an increase of 336% in prescriptions for adrenaline autoinjectors. Such prescriptions increased 11% per year.
Global trend
The data extend findings Dr. Turner and colleagues reported for England and Wales in 2014 regarding the entire United Kingdom population and align with epidemiologic trends in hospital admissions for anaphylaxis in the United States and Australia.
The researchers say better recognition and management of anaphylaxis could partly explain the decrease in fatalities, but the rise in hospitalizations remains puzzling. “Whether a true increase in the prevalence of anaphylaxis has occurred (rather than a reduction in the threshold to admit patients presenting with anaphylaxis) is unclear because evidence is lacking for an increase in prevalence of food allergy in the [United Kingdom] (and elsewhere) over the same time period,” they write.
Ronna L. Campbell, MD, PhD, an emergency physician at the Mayo Clinic in Rochester, Minn., has noted similar trends in the United States. “It may be that anaphylaxis recognition and diagnosis have improved, resulting in earlier administration of epinephrine,” Dr. Campbell said in an interview. “So while cases are increasing, earlier recognition and treatment result in decreased fatalities.” She is unaware of any new guidelines recommending increased hospitalization that would explain the puzzling rise in admissions.
According to the study authors, the clinical criteria used to diagnose anaphylaxis in the United Kingdom did not change during the study period. Although national guidance recommending the hospitalization of children younger than 16 who are suspected of having anaphylaxis was introduced in 2011 and may have boosted admissions, the year-on-year rate of increase has persisted since 2014. “Therefore the increase over the past 5 years cannot be attributed to the impact of the guidance,” they write.
The study was funded by grants from the U.K. Medical Research Council and U.K. Food Standards Agency. Two coauthors have disclosed financial relationships with industry outside of the submitted work. Dr. Conrado has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com