User login
Afternoon napping associated with better cognition in elderly, study shows
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
Dan Kastner wins Crafoord Prize in Polyarthritis
“for establishing the concept of autoinflammatory diseases.” The prize, named after the donor Holger Crafoord because of his bout with severe rheumatoid arthritis toward the end of his life, is for 6 million Swedish kronor (approximately USD $700,000).
Dr. Kastner, scientific director at the U.S. National Human Genome Research Institute’s division of intramural research, received the award for identifying the mechanisms responsible for familial Mediterranean fever, tumor necrosis factor receptor–associated periodic syndrome, and other diagnoses within the group of autoinflammatory diseases.
“Dan Kastner is often called the father of autoinflammatory diseases, a title that he thoroughly deserves. His discoveries have taught us a great deal about the immune system and its functions, contributing to effective treatments that reduce the symptoms of diseases from which patients previously suffered enormously, sometimes leading to premature death,” Olle Kämpe, chair of the prize committee, said in a press announcement.
While the Crafoord Prize normally is awarded on a 3-year rotating basis for achievements in mathematics and astronomy, geosciences, and biosciences, the prize in polyarthritis is “only awarded when there has been scientific progress that motivates a prize,” according to the press release.
“for establishing the concept of autoinflammatory diseases.” The prize, named after the donor Holger Crafoord because of his bout with severe rheumatoid arthritis toward the end of his life, is for 6 million Swedish kronor (approximately USD $700,000).
Dr. Kastner, scientific director at the U.S. National Human Genome Research Institute’s division of intramural research, received the award for identifying the mechanisms responsible for familial Mediterranean fever, tumor necrosis factor receptor–associated periodic syndrome, and other diagnoses within the group of autoinflammatory diseases.
“Dan Kastner is often called the father of autoinflammatory diseases, a title that he thoroughly deserves. His discoveries have taught us a great deal about the immune system and its functions, contributing to effective treatments that reduce the symptoms of diseases from which patients previously suffered enormously, sometimes leading to premature death,” Olle Kämpe, chair of the prize committee, said in a press announcement.
While the Crafoord Prize normally is awarded on a 3-year rotating basis for achievements in mathematics and astronomy, geosciences, and biosciences, the prize in polyarthritis is “only awarded when there has been scientific progress that motivates a prize,” according to the press release.
“for establishing the concept of autoinflammatory diseases.” The prize, named after the donor Holger Crafoord because of his bout with severe rheumatoid arthritis toward the end of his life, is for 6 million Swedish kronor (approximately USD $700,000).
Dr. Kastner, scientific director at the U.S. National Human Genome Research Institute’s division of intramural research, received the award for identifying the mechanisms responsible for familial Mediterranean fever, tumor necrosis factor receptor–associated periodic syndrome, and other diagnoses within the group of autoinflammatory diseases.
“Dan Kastner is often called the father of autoinflammatory diseases, a title that he thoroughly deserves. His discoveries have taught us a great deal about the immune system and its functions, contributing to effective treatments that reduce the symptoms of diseases from which patients previously suffered enormously, sometimes leading to premature death,” Olle Kämpe, chair of the prize committee, said in a press announcement.
While the Crafoord Prize normally is awarded on a 3-year rotating basis for achievements in mathematics and astronomy, geosciences, and biosciences, the prize in polyarthritis is “only awarded when there has been scientific progress that motivates a prize,” according to the press release.
60-year-old man • chronic cough • history of GERD & dyslipidemia • throat tickle • Dx?
THE CASE
A 60-year-old man with a past medical history of gastroesophageal reflux disease (GERD) and dyslipidemia presented to his family physician for evaluation of chronic cough. Five years prior, the patient had developed a high fever and respiratory symptoms, including a cough, and was believed to have had severe otitis media. He was treated with multiple courses of antibiotics and corticosteroids for persistent otitis media. Although the condition eventually resolved, his cough continued.
The persistent cough prompted the patient to consult a succession of specialists. First, he saw a gastroenterologist; following an esophagogastroduodenoscopy, he was prescribed pantoprazole. Despite the proton-pump inhibitor (PPI) therapy, the cough remained. Next, he had multiple visits with an otolaryngologist but that yielded no specific diagnosis for the cough. He also saw an allergist-immunologist, who identified a ragweed allergy, gave him a diagnosis of cough-variant asthma, and prescribed antihistamines and mometasone furoate and formoterol fumarate dihydrate. Neither was helpful.
After 5 years of frustration, the patient complained to his family physician that he still had a cough and “a tickle” in his throat that was worsened by speaking and drinking cold beverages. He denied fever, shortness of breath, nausea, vomiting, or any other associated symptoms.
THE DIAGNOSIS
The failed treatment attempts with antihistamines, corticosteroids, bronchodilators, and PPI therapy excluded multiple etiologies for the cough. The throat discomfort and feeling of a “tickle” prompted us to consider a nerve-related disorder on the differential. The diagnosis of laryngeal sensory neuropathy (LSN) was considered.
DISCUSSION
LSN is a relatively uncommon cause of chronic refractory cough that can also manifest with throat discomfort, dysphagia, and dysphonia.1 It is thought to result from some type of insult to the recurrent laryngeal nerve or superior laryngeal nerve via viral infections, metabolic changes, or mechanical trauma, leading to a change in the firing threshold.2 The hypothesis of nerve damage is supported by the increased incidence of LSN in patients with goiters and those with type 2 diabetes.3,4 When there is a decrease in the laryngeal sensory threshold, dysfunctional laryngeal behavior results, leading to symptoms such as persistent cough and throat clearing.
Diagnosis. LSN is often diagnosed clinically, after GERD, allergies, asthma, angiotensin-converting enzyme inhibitor intake, and psychogenic disorders have been ruled out.1 Our patient had a prior diagnosis or investigation of nearly all of these conditions. Other clues pointing to an LSN diagnosis include a cough lasting 8 weeks or more, recurrent sensory disturbances (such as a tickle) of instantaneous onset before each cough episode, triggers that can include talking or a change in air temperature, daily coughing episodes numbering in the 10s to 100s, and a nonproductive cough.5,6
Beyond clinical clues, laryngeal electromyography, which evaluates the neuromuscular system in the larynx by recording action potentials generated in the laryngeal muscles during contraction, can be used for diagnosis.4 Videostroboscopy, which allows for an enlarged and slow motion view of the vocal cords, can also be used.
Continue to: Treatment
Treatment. To both confirm the diagnosis and treat the patient in a rapid, practical fashion, a trial of a neuromodulating agent such as pregabalin or gabapentin can be employed.6-9 A study identifying 28 LSN patients found symptomatic relief in 68% of patients taking gabapentin 100 to 900 mg/d.2 In another study, 12 LSN patients given pregabalin found relief after a 1-month regimen.1 Another study of 12 patients showed amitriptyline hydrochloride and gabapentin provided a positive response in 2 months, and the addition of reflux precautions and acid-suppression therapy was helpful.9 Finally, a group of 32 patients trialed on 3 different medications (amitriptyline, desipramine, and gabapentin) found similar efficacy among the 3.6
Another option. Aside from medications, botulinum toxin type A has been shown in a case series to directly decrease laryngeal hypertonicity and possibly reduce neurogenic inflammation and neuropeptide-mediated cough.10 Another study found that 18 patients with neurogenic cough who received superior laryngeal nerve blocks had cough severity index scores decrease from an average of 26.8 pretreatment to 14.6 posttreatment (P < .0001).11
Our patient agreed to a trial of gabapentin 300 mg once a day, with titration up to a maximum of 900 mg tid. When the patient returned to the clinic 4 months later, he reported that when he reached 300 mg bid, the cough completely resolved.
THE TAKEAWAY
A persistent cough with minimal identifiable triggers is a huge disruption to a patient’s life; having to visit multiple specialists before receiving a diagnosis compounds that. In our patient’s case, the process took 5 years, which underscores how important it is that LSN be considered in the differential diagnosis. Since this is generally a diagnosis of exclusion, it is important to take a careful history of a patient with a chronic cough. If LSN seems likely, trialing a patient on neuromodulating medication is the next best step, with dose titration if necessary.
Selena R. Pasadyn, 675 West 130th Street, Hinckley, OH, 44233; [email protected]
1. Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope. 2009;119:1844-1847.
2. Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol. 2005;114:253-257.
3. Hamdan AL, Jabour J, Azar ST. Goiter and laryngeal sensory neuropathy. Int J Otolaryngol. 2013;2013:765265.
4. Hamdan AL, Dowli A, Barazi R, et al. Laryngeal sensory neuropathy in patients with diabetes mellitus. J Laryngol Otol. 2014;128:725-729.
5. Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg. 2006;135:17-21.
6. Bastian ZJ, Bastian RW. The use of neuralgia medications to treat sensory neuropathic cough: our experience in a retrospective cohort of thirty-two patients. PeerJ. 2015;3:e816.
7. Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, et al. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough. 2012;8:9.
8. Mishriki YY. Laryngeal neuropathy as a cause of chronic intractable cough. Am J Med. 2007;120:e5.
9. Norris BK, Schweinfurth JM. Management of recurrent laryngeal sensory neuropathic symptoms. Ann Otol Rhinol Laryngol. 2010;119:188-191.
10. Chu MW, Lieser JD, Sinacori JT. Use of botulinum toxin type a for chronic cough: a neuropathic model. Arch Otolaryngol Head Neck Surg. 2010;136:447.
11. Simpson CB, Tibbetts KM, Loochtan MJ, et al. Treatment of chronic neurogenic cough with in-office superior laryngeal nerve block. Laryngoscope. 2018;128:1898-1903.
THE CASE
A 60-year-old man with a past medical history of gastroesophageal reflux disease (GERD) and dyslipidemia presented to his family physician for evaluation of chronic cough. Five years prior, the patient had developed a high fever and respiratory symptoms, including a cough, and was believed to have had severe otitis media. He was treated with multiple courses of antibiotics and corticosteroids for persistent otitis media. Although the condition eventually resolved, his cough continued.
The persistent cough prompted the patient to consult a succession of specialists. First, he saw a gastroenterologist; following an esophagogastroduodenoscopy, he was prescribed pantoprazole. Despite the proton-pump inhibitor (PPI) therapy, the cough remained. Next, he had multiple visits with an otolaryngologist but that yielded no specific diagnosis for the cough. He also saw an allergist-immunologist, who identified a ragweed allergy, gave him a diagnosis of cough-variant asthma, and prescribed antihistamines and mometasone furoate and formoterol fumarate dihydrate. Neither was helpful.
After 5 years of frustration, the patient complained to his family physician that he still had a cough and “a tickle” in his throat that was worsened by speaking and drinking cold beverages. He denied fever, shortness of breath, nausea, vomiting, or any other associated symptoms.
THE DIAGNOSIS
The failed treatment attempts with antihistamines, corticosteroids, bronchodilators, and PPI therapy excluded multiple etiologies for the cough. The throat discomfort and feeling of a “tickle” prompted us to consider a nerve-related disorder on the differential. The diagnosis of laryngeal sensory neuropathy (LSN) was considered.
DISCUSSION
LSN is a relatively uncommon cause of chronic refractory cough that can also manifest with throat discomfort, dysphagia, and dysphonia.1 It is thought to result from some type of insult to the recurrent laryngeal nerve or superior laryngeal nerve via viral infections, metabolic changes, or mechanical trauma, leading to a change in the firing threshold.2 The hypothesis of nerve damage is supported by the increased incidence of LSN in patients with goiters and those with type 2 diabetes.3,4 When there is a decrease in the laryngeal sensory threshold, dysfunctional laryngeal behavior results, leading to symptoms such as persistent cough and throat clearing.
Diagnosis. LSN is often diagnosed clinically, after GERD, allergies, asthma, angiotensin-converting enzyme inhibitor intake, and psychogenic disorders have been ruled out.1 Our patient had a prior diagnosis or investigation of nearly all of these conditions. Other clues pointing to an LSN diagnosis include a cough lasting 8 weeks or more, recurrent sensory disturbances (such as a tickle) of instantaneous onset before each cough episode, triggers that can include talking or a change in air temperature, daily coughing episodes numbering in the 10s to 100s, and a nonproductive cough.5,6
Beyond clinical clues, laryngeal electromyography, which evaluates the neuromuscular system in the larynx by recording action potentials generated in the laryngeal muscles during contraction, can be used for diagnosis.4 Videostroboscopy, which allows for an enlarged and slow motion view of the vocal cords, can also be used.
Continue to: Treatment
Treatment. To both confirm the diagnosis and treat the patient in a rapid, practical fashion, a trial of a neuromodulating agent such as pregabalin or gabapentin can be employed.6-9 A study identifying 28 LSN patients found symptomatic relief in 68% of patients taking gabapentin 100 to 900 mg/d.2 In another study, 12 LSN patients given pregabalin found relief after a 1-month regimen.1 Another study of 12 patients showed amitriptyline hydrochloride and gabapentin provided a positive response in 2 months, and the addition of reflux precautions and acid-suppression therapy was helpful.9 Finally, a group of 32 patients trialed on 3 different medications (amitriptyline, desipramine, and gabapentin) found similar efficacy among the 3.6
Another option. Aside from medications, botulinum toxin type A has been shown in a case series to directly decrease laryngeal hypertonicity and possibly reduce neurogenic inflammation and neuropeptide-mediated cough.10 Another study found that 18 patients with neurogenic cough who received superior laryngeal nerve blocks had cough severity index scores decrease from an average of 26.8 pretreatment to 14.6 posttreatment (P < .0001).11
Our patient agreed to a trial of gabapentin 300 mg once a day, with titration up to a maximum of 900 mg tid. When the patient returned to the clinic 4 months later, he reported that when he reached 300 mg bid, the cough completely resolved.
THE TAKEAWAY
A persistent cough with minimal identifiable triggers is a huge disruption to a patient’s life; having to visit multiple specialists before receiving a diagnosis compounds that. In our patient’s case, the process took 5 years, which underscores how important it is that LSN be considered in the differential diagnosis. Since this is generally a diagnosis of exclusion, it is important to take a careful history of a patient with a chronic cough. If LSN seems likely, trialing a patient on neuromodulating medication is the next best step, with dose titration if necessary.
Selena R. Pasadyn, 675 West 130th Street, Hinckley, OH, 44233; [email protected]
THE CASE
A 60-year-old man with a past medical history of gastroesophageal reflux disease (GERD) and dyslipidemia presented to his family physician for evaluation of chronic cough. Five years prior, the patient had developed a high fever and respiratory symptoms, including a cough, and was believed to have had severe otitis media. He was treated with multiple courses of antibiotics and corticosteroids for persistent otitis media. Although the condition eventually resolved, his cough continued.
The persistent cough prompted the patient to consult a succession of specialists. First, he saw a gastroenterologist; following an esophagogastroduodenoscopy, he was prescribed pantoprazole. Despite the proton-pump inhibitor (PPI) therapy, the cough remained. Next, he had multiple visits with an otolaryngologist but that yielded no specific diagnosis for the cough. He also saw an allergist-immunologist, who identified a ragweed allergy, gave him a diagnosis of cough-variant asthma, and prescribed antihistamines and mometasone furoate and formoterol fumarate dihydrate. Neither was helpful.
After 5 years of frustration, the patient complained to his family physician that he still had a cough and “a tickle” in his throat that was worsened by speaking and drinking cold beverages. He denied fever, shortness of breath, nausea, vomiting, or any other associated symptoms.
THE DIAGNOSIS
The failed treatment attempts with antihistamines, corticosteroids, bronchodilators, and PPI therapy excluded multiple etiologies for the cough. The throat discomfort and feeling of a “tickle” prompted us to consider a nerve-related disorder on the differential. The diagnosis of laryngeal sensory neuropathy (LSN) was considered.
DISCUSSION
LSN is a relatively uncommon cause of chronic refractory cough that can also manifest with throat discomfort, dysphagia, and dysphonia.1 It is thought to result from some type of insult to the recurrent laryngeal nerve or superior laryngeal nerve via viral infections, metabolic changes, or mechanical trauma, leading to a change in the firing threshold.2 The hypothesis of nerve damage is supported by the increased incidence of LSN in patients with goiters and those with type 2 diabetes.3,4 When there is a decrease in the laryngeal sensory threshold, dysfunctional laryngeal behavior results, leading to symptoms such as persistent cough and throat clearing.
Diagnosis. LSN is often diagnosed clinically, after GERD, allergies, asthma, angiotensin-converting enzyme inhibitor intake, and psychogenic disorders have been ruled out.1 Our patient had a prior diagnosis or investigation of nearly all of these conditions. Other clues pointing to an LSN diagnosis include a cough lasting 8 weeks or more, recurrent sensory disturbances (such as a tickle) of instantaneous onset before each cough episode, triggers that can include talking or a change in air temperature, daily coughing episodes numbering in the 10s to 100s, and a nonproductive cough.5,6
Beyond clinical clues, laryngeal electromyography, which evaluates the neuromuscular system in the larynx by recording action potentials generated in the laryngeal muscles during contraction, can be used for diagnosis.4 Videostroboscopy, which allows for an enlarged and slow motion view of the vocal cords, can also be used.
Continue to: Treatment
Treatment. To both confirm the diagnosis and treat the patient in a rapid, practical fashion, a trial of a neuromodulating agent such as pregabalin or gabapentin can be employed.6-9 A study identifying 28 LSN patients found symptomatic relief in 68% of patients taking gabapentin 100 to 900 mg/d.2 In another study, 12 LSN patients given pregabalin found relief after a 1-month regimen.1 Another study of 12 patients showed amitriptyline hydrochloride and gabapentin provided a positive response in 2 months, and the addition of reflux precautions and acid-suppression therapy was helpful.9 Finally, a group of 32 patients trialed on 3 different medications (amitriptyline, desipramine, and gabapentin) found similar efficacy among the 3.6
Another option. Aside from medications, botulinum toxin type A has been shown in a case series to directly decrease laryngeal hypertonicity and possibly reduce neurogenic inflammation and neuropeptide-mediated cough.10 Another study found that 18 patients with neurogenic cough who received superior laryngeal nerve blocks had cough severity index scores decrease from an average of 26.8 pretreatment to 14.6 posttreatment (P < .0001).11
Our patient agreed to a trial of gabapentin 300 mg once a day, with titration up to a maximum of 900 mg tid. When the patient returned to the clinic 4 months later, he reported that when he reached 300 mg bid, the cough completely resolved.
THE TAKEAWAY
A persistent cough with minimal identifiable triggers is a huge disruption to a patient’s life; having to visit multiple specialists before receiving a diagnosis compounds that. In our patient’s case, the process took 5 years, which underscores how important it is that LSN be considered in the differential diagnosis. Since this is generally a diagnosis of exclusion, it is important to take a careful history of a patient with a chronic cough. If LSN seems likely, trialing a patient on neuromodulating medication is the next best step, with dose titration if necessary.
Selena R. Pasadyn, 675 West 130th Street, Hinckley, OH, 44233; [email protected]
1. Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope. 2009;119:1844-1847.
2. Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol. 2005;114:253-257.
3. Hamdan AL, Jabour J, Azar ST. Goiter and laryngeal sensory neuropathy. Int J Otolaryngol. 2013;2013:765265.
4. Hamdan AL, Dowli A, Barazi R, et al. Laryngeal sensory neuropathy in patients with diabetes mellitus. J Laryngol Otol. 2014;128:725-729.
5. Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg. 2006;135:17-21.
6. Bastian ZJ, Bastian RW. The use of neuralgia medications to treat sensory neuropathic cough: our experience in a retrospective cohort of thirty-two patients. PeerJ. 2015;3:e816.
7. Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, et al. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough. 2012;8:9.
8. Mishriki YY. Laryngeal neuropathy as a cause of chronic intractable cough. Am J Med. 2007;120:e5.
9. Norris BK, Schweinfurth JM. Management of recurrent laryngeal sensory neuropathic symptoms. Ann Otol Rhinol Laryngol. 2010;119:188-191.
10. Chu MW, Lieser JD, Sinacori JT. Use of botulinum toxin type a for chronic cough: a neuropathic model. Arch Otolaryngol Head Neck Surg. 2010;136:447.
11. Simpson CB, Tibbetts KM, Loochtan MJ, et al. Treatment of chronic neurogenic cough with in-office superior laryngeal nerve block. Laryngoscope. 2018;128:1898-1903.
1. Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope. 2009;119:1844-1847.
2. Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol. 2005;114:253-257.
3. Hamdan AL, Jabour J, Azar ST. Goiter and laryngeal sensory neuropathy. Int J Otolaryngol. 2013;2013:765265.
4. Hamdan AL, Dowli A, Barazi R, et al. Laryngeal sensory neuropathy in patients with diabetes mellitus. J Laryngol Otol. 2014;128:725-729.
5. Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg. 2006;135:17-21.
6. Bastian ZJ, Bastian RW. The use of neuralgia medications to treat sensory neuropathic cough: our experience in a retrospective cohort of thirty-two patients. PeerJ. 2015;3:e816.
7. Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, et al. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough. 2012;8:9.
8. Mishriki YY. Laryngeal neuropathy as a cause of chronic intractable cough. Am J Med. 2007;120:e5.
9. Norris BK, Schweinfurth JM. Management of recurrent laryngeal sensory neuropathic symptoms. Ann Otol Rhinol Laryngol. 2010;119:188-191.
10. Chu MW, Lieser JD, Sinacori JT. Use of botulinum toxin type a for chronic cough: a neuropathic model. Arch Otolaryngol Head Neck Surg. 2010;136:447.
11. Simpson CB, Tibbetts KM, Loochtan MJ, et al. Treatment of chronic neurogenic cough with in-office superior laryngeal nerve block. Laryngoscope. 2018;128:1898-1903.
Maternal autoimmune disease raises children’s risk of ADHD
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
FROM JAMA PEDIATRICS
Atopic dermatitis: More than just a rash
Atopic dermatitis (AD), also known as eczema, is a chronic inflammatory skin condition that is well known for its relapsing, pruritic rash in children and adults. Less recognized are its associated conditions—allergic rhinitis, asthma, food allergies, attention-deficit/hyperactivity disorder (ADHD), depression, and anxiety—and its burden on patients and their families. In fact, families that have children with AD report lower overall quality of life than those with otherwise healthy children.1 Given AD’s prevalence across age groups and its effect on the family, family physicians are uniquely positioned to diagnose, care for, and counsel patients with AD and its associated maladies.
The prevalence and pathogenesis of AD
AD affects up to 20% of children and 5% of adults in the United States.2 AD typically manifests before a child reaches age 5 (often in the first 6 months of life), and it is slightly more common in females (1.3:1). A family history of atopy (eczema, asthma, allergic rhinitis) is common. In fact, children with one atopic parent have a 2- to 3-fold increased risk of atopic dermatitis; those with 2 atopic parents have a 3- to 5-fold increased risk.3
The pathophysiology of AD is complex, culminating in impaired barrier function of the skin and transepidermal water loss resulting in dry and inflamed skin. Additionally, alterations in a cell-mediated immune response leading to an immunoglobulin (Ig) E-mediated hypersensitivity is also theorized to play a role in the development of AD.
Signs and symptoms
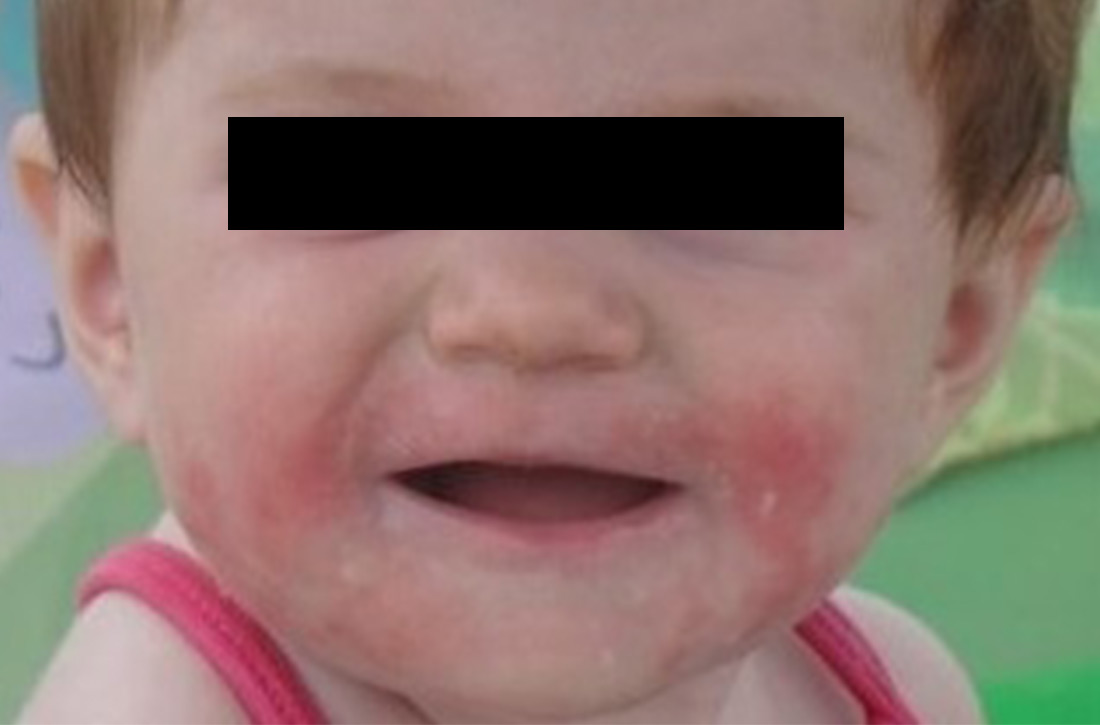
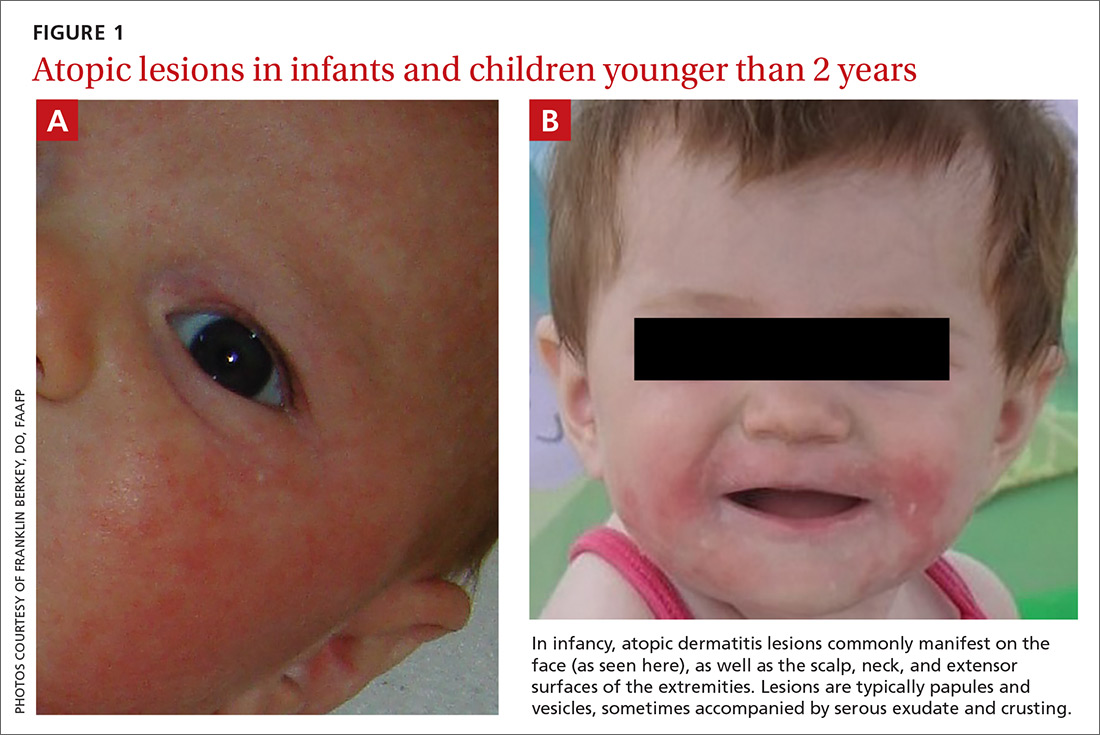
Signs at birth. Physical signs of atopic dermatitis typically appear between birth and 6 months. In infancy, lesions generally occur on the scalp, face (FIGURES 1A and 1B), neck, and extensor surfaces of the extremities. Lesions are typically papules and vesicles, sometimes accompanied by serous exudate and crusting. Eczematous lesions typically spare the groin and diaper area, and their presence in this area should raise suspicion for an alternative diagnosis.
Beginning at age 2 years, eczematous lesions are more commonly limited to the folds of the flexor surfaces. Instead of the weeping and crusting lesions seen in infancy, eczema in older children manifests as dry, lichenified papules and plaques in areas that are typically affected in adults: the wrist, hands, ankles, and popliteal and antecubital fossa.2
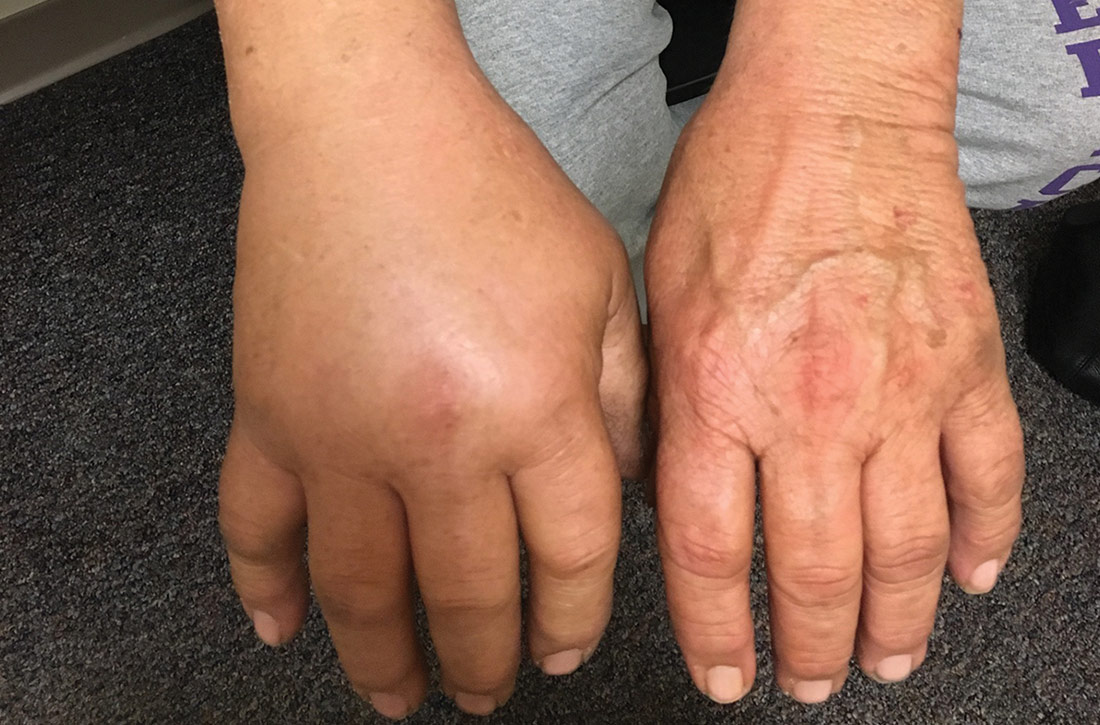
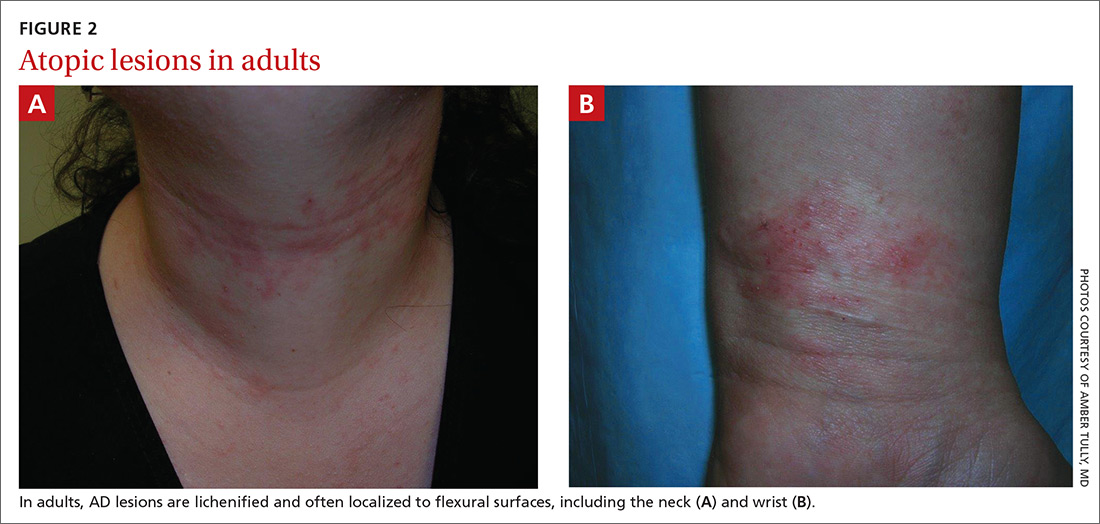
Although lesions in adults are similar to those of childhood, they may manifest in a more localized area (hand or eyelid, for example). As is the case in childhood, the lesions are dry, sometimes lichenified, and found on the flexural surfaces (FIGURES 2A and 2B).2
Symptom triggers are unproven
While anecdotal reports cite various triggers for AD flares, a systematic review found little scientific evidence to substantiate identifiable triggers.4 Triggers often cited and studied are foods, dust mite exposure, airborne allergens, detergents, sunlight, fabrics, bacterial infections, and stress. While as many as one-third of people with AD who also have confirmed dust mite allergy report worsening of symptoms when exposed to dust, a Cochrane review of 7 randomized controlled trials totaling 324 adults and children with eczema found that efforts at dust mite mitigation (laundering of bed covers, increased vacuuming, spraying for mites) were not effective in reducing symptoms.5
Continue to: How quality of life diminishes with AD
How quality of life diminishes with AD
AD substantially lessens quality of life. For children, the most distressing physical symptoms include itching that inhibits sleep and provokes scratching, pain, and bleeding. Emotional distress can cause irritability, crying, and uncooperativeness with treatments. Parents also report that they frequently restrict their children from activities, such as playing in the heat or swimming, that may lead to worsening of their eczema.6
The loss of sleep associated with AD is not completely understood but is likely multifactorial. Pruritus and scratching leading to sleeplessness is the most obvious culprit, but an altered circadian rhythm, immune system response, and changes in skin physiology are also likely factors.7 Whatever the cause, sleep disturbance is reported in as many as 60% of patients with AD, and the degree of sleep disturbance is proportional to increases in disease severity and worsening of quality-of-life scores.8 Lost sleep is not limited to patients; parents of children with AD also report significant loss of sleep and subsequent decreased work productivity and quality of life.9
Children with AD are often the target of bullying.10 A 2015 survey by the National Eczema Association indicates that 1 in 5 children reported being bullied due to their AD.11
Associated conditions and comorbidities
AD increases patients’ risks for other illnesses, due either to their underlying atopy or to the effects of
Atopic march
Atopic march—the clinical succession of AD, allergic rhinitis, and asthma—is a well-established clinical progression. The presence of all 3 conditions appears to be more common in children diagnosed with AD before 2 years of age.12 Typically, allergic rhinitis manifests at around age 4, and asthma develops between ages 6 and 8. The severity of AD predicts progression. Compared with an 8% chance of asthma developing among the general population, children with mild AD have a 20% to 30% chance of developing asthma, and those with severe AD have about a 70% chance.12
Continue to: Food allergies
Food allergies
Patients with AD are at higher risk for food-induced anaphylaxis, with up to one-third of AD patients having an IgE-mediated food allergy.13 While it is theorized that the impaired skin barrier of an atopic child may allow for early sensitization and allergy development, a landmark 2015 study demonstrated that early allergen introduction (specifically, peanuts) may serve as a preventive strategy in those at high risk of food allergies.14 Current guidelines recommend that physicians be aware of the increased possibility of food allergies in those with AD, and consider evaluating a child for milk, egg, peanut, wheat, and soy allergy if the child is younger than 5 years and has eczema that does not resolve with treatment, or has eczema and a history of an allergic reaction to a specific food.15
Interestingly, despite the strong association between AD and food allergies, it is not clear that food allergies trigger atopic flares; as such, elimination diets are not universally recommended in those without a proven food allergy.
Psychiatric diagnoses
Children with AD have an increased prevalence of several psychiatric conditions, including ADHD, depression, anxiety, conduct disorder, and autism when compared with peers who do not have AD, and the probability correlates with the severity of AD
What we do know is that one of the strongest associations between AD and a psychiatric condition is with ADHD, with a recent pooled meta-analysis showing a 46% increase in risk.17 The incidence of depression among children with AD appears to correlate with the severity of AD symptoms: estimated at 5% with mild AD, 7% with moderate disease, and 14% with severe disease (compared with 3% without AD). Similar incremental increases are seen when correlating AD and anxiety.16
Nonpharmacologic care
Bathing
Bathing habits are critical to controlling AD. While bathing serves to both hydrate the skin and remove allergens, the water’s evaporation off the skin surface can lead to increased transepidermal water loss. Combining bathing and immediate application of a moisturizer improves skin hydration in patients with AD vs bathing alone.18 Thus, consensus guidelines recommend once-daily bathing (bath or shower) to remove scale and crust, followed by immediate application of a moisturizing emollient.19
Continue to: Emollients
Emollients
Application of moisturizing emollients is the mainstay of nonpharmacologic care of AD, and there is strong evidence that their regimented use reduces disease burden and the need for prescription treatment.19 Emollient creams and ointments help retain moisture and improve the skin’s barrier. While ointments may provide a better barrier, patients tend to prefer creams as they are less greasy than ointments.
Emollient therapy may also help prevent development of AD, especially in those infants thought to be at high risk with a family history of atopy. In a multinational randomized controlled trial, infants who received daily full-body application of emollient beginning at 3 weeks of life were significantly less likely than controls to develop AD by 6 months.20 While the mechanism of action is not clearly understood, it is believed that early emollient use prevents skin dehydration and maintains the skin’s barrier integrity, thus decreasing allergen epidermal penetration and subsequent inflammation.
Bleach bath
A bleach bath, prepared by adding 1/2 cup of unconcentrated bleach (5.25% sodium hypochlorite) to a standard 40-gallon bathtub, produces a chlorine mixture equivalent to an average swimming pool. Soaking in a bleach bath for 10 minutes once or twice weekly is thought to reduce inflammation and bacteria on the skin, but studies of its efficacy in improving atopic symptoms are mixed.
In a pooled analysis of 5 studies evaluating bleach baths vs standard baths, there was no significant difference in disease severity at 4 weeks.21 Thus, while bleach baths were effective in decreasing disease severity, they appeared to be no more effective than a standard water bath.21 Bleach baths may be helpful, however, in cases of moderate-to-severe disease with frequent bacterial infections.19
Pharmacologic therapy
Steroids
For symptoms refractory to nonpharmacologic skin care, topical steroids are the initial pharmacologic treatment for AD.19 Choose steroid potency based on symptom severity and disease location. Low- to medium-potency is appropriate for mild disease, and medium- to high-potency is useful for moderate-to-severe symptoms. High-potency steroids are generally avoided on the face and skin folds; however, they can be used for short periods in these areas to induce remission. They must then be quickly tapered and discontinued.
Continue to: Frequency
Frequency. Topical corticosteroids are typically applied twice daily, although recent studies indicate that once-daily application is just as efficacious.22 In addition to treatment of an acute flare, topical steroids are useful as maintenance therapy for patients with recurrent outbreaks in the same anatomical site. Guidelines suggest once- or twice-weekly application of a medium-potency steroid to prolong time between flares.19
For children, a practical guide is for caregivers to apply the amount of steroid covering 1 adult fingertip to an area of the child’s skin equal to that of 2 adult palms.23 Topical steroids are generally well tolerated and have a good safety profile. Adverse effects are proportional to the amount and duration of use and include purpura, telangiectasias, striae, and skin atrophy. The risk of skin atrophy increases with higher potency steroids, occlusion (covering affected area after steroid application), use on thin-skinned areas, and older patient age.24
Reassure patients/parents about the safety of topical steroids, as fears regarding the potential adverse effects can limit compliance. In one study of 200 patients with AD, 72.5% of respondents expressed fear of using steroids on their own skin or that of their child, and 24% admitted being noncompliant with therapy based on these concerns.25
Treating flares. Oral steroids are sometimes needed to abort or control an AD flare in older children and adults. A tapering course of prednisone over 5 to 7 days, transitioning to medium- to high-dose topical steroids, may be needed to achieve symptom control.
Topical calcineurin inhibitors
Topical calcineurin inhibitors, including tacrolimus and pimecrolimus, are generally second-line therapy to topical corticosteroids. However, as nonsteroidal agents, topical calcineurin inhibitors do not cause skin atrophy and can be a first-line option in areas where atrophy is more common (face, eyelids, neck, and skin folds).26
Continue to: A Cochrane review found...
A Cochrane review found tacrolimus 0.1% to be better than low‐potency topical corticosteroids on the face and neck areas, while results were equivocal when compared with moderate‐potency topical corticosteroids on the trunk and extremities (no difference based on physician assessment, but marginal benefit favoring tacrolimus based on participant scoring).27 When compared head-to-head, tacrolimus was more effective than pimecrolimus, although tacrolimus has a higher rate of local irritation. The most common adverse effects are stinging and burning at the application site, although these adverse effects generally improve with repeated application.
There have been long-term safety concerns with topical calcineurin inhibitors—chiefly a 2006 Food and Drug Administration (FDA) black box warning regarding a possible link between topical calcineurin inhibitors and cancer. However, while there may be a slight increased risk of lymphoma in AD patients, a recent meta-analysis did not find an association between topical calcineurin inhibitors use and lymphoma.28 Given the initial concern—and pending additional data—the FDA currently recommends reserving topical calcineurin inhibitors for second-line therapy and only for the minimum amount of time to induce improvement. It also recommends avoiding their use in patients younger than 2 years and in those with compromised immune systems.
Cisaborole
Cisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor, received FDA approval in 2016 for mild-to-moderate AD. By inhibiting PDE4, the drug limits inflammation. In a multicenter randomized trial, patients applying cisaborole 2% twice a day noted reductions in pruritus, inflammation, excoriation, and lichenification.29 Adverse effects are minimal and limited to application site irritation.
Systemic treatments
While beyond the care of a family physician, symptoms refractory to conservative, nonpharmacologic measures and combinations of topical pharmaceuticals can be treated with systemic immunomodulators such as cyclosporine, azathioprine, and methotrexate. Phototherapy is also effective in patients with more widespread skin involvement. Dupilumab, an injectable monoclonal antibody that binds to interleukin-4 receptor and inhibits inflammation, is approved to treat moderate-to-severe AD in adults.30
Ineffective therapies: Oral montelukast and probiotics
While oral antihistamines are frequently prescribed and used, there are no studies evaluating the use of antihistamines (H1) as monotherapy for AD.31 Nonetheless, while not altering the disease process, the sedative effect of antihistamines may palliate the nocturnal pruritus frequently associated with AD. Although nonsedating antihistamines may still have a role for atopic patients with concurrent seasonal and environmental allergies, there is no evidence to support their use in the treatment of AD.
Continue to: Data are limited...
Data are limited on the effectiveness of leukotriene receptor antagonists for AD, and all studies meeting inclusion for a Cochrane review assessed oral montelukast. The review found no benefit with the use of montelukast 10 mg in terms of severity of disease, pruritus, or need for topical steroids.32
A systematic review investigating the benefit of probiotics for the treatment of AD found no improvement in patient-rated eczema scores for quality of life.33 Additionally, a review of 11 randomized controlled trials including 596 participants found no evidence to suggest efficacy of fish oil, zinc, selenium, vitamin D, vitamin E, pyridoxine, sea buckthorn oil, hempseed oil, or sunflower oil in the treatment of AD.34
Education can reduce AD severity
Family physicians can be a source of education and support for patients and families of patients with AD. Support programs for adults with AD—including education, relaxation techniques, and cognitive behavioral therapy—have been shown to decrease disease severity.35 Comparable improvement in disease severity has been demonstrated in children with AD when similar education is provided to them and their families.
CORRESPONDENCE
Franklin Berkey, DO, Penn State Health, 1850 East Park Avenue, Suite 207, State College, PA 16803; fberkey@ pennstatehealth.psu.edu.
1. Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
2. Ahn C, Huang W. Clinical presentation of atopic dermatitis. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:38-46.
3. Eichenfield LF, Tom WL, Chamblin SL, et al. Guidelines of care for the management of atopic dermatitis. Part 1: diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
4. Langan SM, Williams HC. What causes worsening of eczema? A systematic review. Br J Dermatol. 2006;155:504-514.
5. Nankervis H, Pynn EV, Boyle RJ, et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev. 2015:CD008426.
6. Chamlin SL, Frieden IJ, Williams ML, et al. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114:607-611.
7. Chang Y-S, Chiang B-L. Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. Int J Mol Sci. 2016;17:462.
8. Camfferman D, Kennedy JD, Gold M, et al. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev. 2010;14:359-369.
9. Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
10. Drucker AM, Wang AR, Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:P26-P30.
11. National Eczema Association. Tools for school: addressing school bullying for kids with eczema. Accessed January 5, 2021. https://nationaleczema.org/children-with-eczema-experience-bullying/
12. Bantz SK, Zhu Z, Zhen T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202
13. Laird M, Sicco KL. Defining and measuring the scope of atopic dermatitis. Adv Exp Med Biol. 2017;1027:93-104.
14. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
15. Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58.
16. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
17. Strom MA, Fishbein AB, Paller AS, et al. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol. 2016;175:920-929.
18. Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
19. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
20. Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
21. Chopra R, Vakharia PP, Sacotte R, et al. Efficacy of bleach baths in reducing severity of atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119:435-440.
22. Williams HC. Established corticosteroid creams should be applied only once daily in patients with atopic eczema. BMJ. 2007;334:1272.
23. Long CC, Mills CM, Finlay AY. A practical guide to topical therapy in children. Br J Dermatol. 1998;138:293-296.
24. Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156:203-221.
25. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142:931-936.
26. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: a meta-analysis of randomised controlled trials. BMJ. 2005;330:516.
27. Cury Martins J, Martins C, Aoki V, et al. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015:CD009864.
28. Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
29. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.
30. Dupilumab [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals Inc; 2017.
31. van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25.
32. Ferguson L, Futamura M, Vakirlis E, et al. Leukotriene receptor antagonists for eczema. Cochrane Database Syst Rev. 2018:CD011224.
33. Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018:CD006135.
34. Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
35. Sy W, Lamb AJ. Atopic dermatitis disease education. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:179-184.
Atopic dermatitis (AD), also known as eczema, is a chronic inflammatory skin condition that is well known for its relapsing, pruritic rash in children and adults. Less recognized are its associated conditions—allergic rhinitis, asthma, food allergies, attention-deficit/hyperactivity disorder (ADHD), depression, and anxiety—and its burden on patients and their families. In fact, families that have children with AD report lower overall quality of life than those with otherwise healthy children.1 Given AD’s prevalence across age groups and its effect on the family, family physicians are uniquely positioned to diagnose, care for, and counsel patients with AD and its associated maladies.
The prevalence and pathogenesis of AD
AD affects up to 20% of children and 5% of adults in the United States.2 AD typically manifests before a child reaches age 5 (often in the first 6 months of life), and it is slightly more common in females (1.3:1). A family history of atopy (eczema, asthma, allergic rhinitis) is common. In fact, children with one atopic parent have a 2- to 3-fold increased risk of atopic dermatitis; those with 2 atopic parents have a 3- to 5-fold increased risk.3
The pathophysiology of AD is complex, culminating in impaired barrier function of the skin and transepidermal water loss resulting in dry and inflamed skin. Additionally, alterations in a cell-mediated immune response leading to an immunoglobulin (Ig) E-mediated hypersensitivity is also theorized to play a role in the development of AD.
Signs and symptoms
Signs at birth. Physical signs of atopic dermatitis typically appear between birth and 6 months. In infancy, lesions generally occur on the scalp, face (FIGURES 1A and 1B), neck, and extensor surfaces of the extremities. Lesions are typically papules and vesicles, sometimes accompanied by serous exudate and crusting. Eczematous lesions typically spare the groin and diaper area, and their presence in this area should raise suspicion for an alternative diagnosis.

Beginning at age 2 years, eczematous lesions are more commonly limited to the folds of the flexor surfaces. Instead of the weeping and crusting lesions seen in infancy, eczema in older children manifests as dry, lichenified papules and plaques in areas that are typically affected in adults: the wrist, hands, ankles, and popliteal and antecubital fossa.2
Although lesions in adults are similar to those of childhood, they may manifest in a more localized area (hand or eyelid, for example). As is the case in childhood, the lesions are dry, sometimes lichenified, and found on the flexural surfaces (FIGURES 2A and 2B).2

Symptom triggers are unproven
While anecdotal reports cite various triggers for AD flares, a systematic review found little scientific evidence to substantiate identifiable triggers.4 Triggers often cited and studied are foods, dust mite exposure, airborne allergens, detergents, sunlight, fabrics, bacterial infections, and stress. While as many as one-third of people with AD who also have confirmed dust mite allergy report worsening of symptoms when exposed to dust, a Cochrane review of 7 randomized controlled trials totaling 324 adults and children with eczema found that efforts at dust mite mitigation (laundering of bed covers, increased vacuuming, spraying for mites) were not effective in reducing symptoms.5
Continue to: How quality of life diminishes with AD
How quality of life diminishes with AD
AD substantially lessens quality of life. For children, the most distressing physical symptoms include itching that inhibits sleep and provokes scratching, pain, and bleeding. Emotional distress can cause irritability, crying, and uncooperativeness with treatments. Parents also report that they frequently restrict their children from activities, such as playing in the heat or swimming, that may lead to worsening of their eczema.6
The loss of sleep associated with AD is not completely understood but is likely multifactorial. Pruritus and scratching leading to sleeplessness is the most obvious culprit, but an altered circadian rhythm, immune system response, and changes in skin physiology are also likely factors.7 Whatever the cause, sleep disturbance is reported in as many as 60% of patients with AD, and the degree of sleep disturbance is proportional to increases in disease severity and worsening of quality-of-life scores.8 Lost sleep is not limited to patients; parents of children with AD also report significant loss of sleep and subsequent decreased work productivity and quality of life.9
Children with AD are often the target of bullying.10 A 2015 survey by the National Eczema Association indicates that 1 in 5 children reported being bullied due to their AD.11
Associated conditions and comorbidities
AD increases patients’ risks for other illnesses, due either to their underlying atopy or to the effects of

Atopic march
Atopic march—the clinical succession of AD, allergic rhinitis, and asthma—is a well-established clinical progression. The presence of all 3 conditions appears to be more common in children diagnosed with AD before 2 years of age.12 Typically, allergic rhinitis manifests at around age 4, and asthma develops between ages 6 and 8. The severity of AD predicts progression. Compared with an 8% chance of asthma developing among the general population, children with mild AD have a 20% to 30% chance of developing asthma, and those with severe AD have about a 70% chance.12
Continue to: Food allergies
Food allergies
Patients with AD are at higher risk for food-induced anaphylaxis, with up to one-third of AD patients having an IgE-mediated food allergy.13 While it is theorized that the impaired skin barrier of an atopic child may allow for early sensitization and allergy development, a landmark 2015 study demonstrated that early allergen introduction (specifically, peanuts) may serve as a preventive strategy in those at high risk of food allergies.14 Current guidelines recommend that physicians be aware of the increased possibility of food allergies in those with AD, and consider evaluating a child for milk, egg, peanut, wheat, and soy allergy if the child is younger than 5 years and has eczema that does not resolve with treatment, or has eczema and a history of an allergic reaction to a specific food.15
Interestingly, despite the strong association between AD and food allergies, it is not clear that food allergies trigger atopic flares; as such, elimination diets are not universally recommended in those without a proven food allergy.
Psychiatric diagnoses
Children with AD have an increased prevalence of several psychiatric conditions, including ADHD, depression, anxiety, conduct disorder, and autism when compared with peers who do not have AD, and the probability correlates with the severity of AD
What we do know is that one of the strongest associations between AD and a psychiatric condition is with ADHD, with a recent pooled meta-analysis showing a 46% increase in risk.17 The incidence of depression among children with AD appears to correlate with the severity of AD symptoms: estimated at 5% with mild AD, 7% with moderate disease, and 14% with severe disease (compared with 3% without AD). Similar incremental increases are seen when correlating AD and anxiety.16
Nonpharmacologic care
Bathing
Bathing habits are critical to controlling AD. While bathing serves to both hydrate the skin and remove allergens, the water’s evaporation off the skin surface can lead to increased transepidermal water loss. Combining bathing and immediate application of a moisturizer improves skin hydration in patients with AD vs bathing alone.18 Thus, consensus guidelines recommend once-daily bathing (bath or shower) to remove scale and crust, followed by immediate application of a moisturizing emollient.19
Continue to: Emollients
Emollients
Application of moisturizing emollients is the mainstay of nonpharmacologic care of AD, and there is strong evidence that their regimented use reduces disease burden and the need for prescription treatment.19 Emollient creams and ointments help retain moisture and improve the skin’s barrier. While ointments may provide a better barrier, patients tend to prefer creams as they are less greasy than ointments.
Emollient therapy may also help prevent development of AD, especially in those infants thought to be at high risk with a family history of atopy. In a multinational randomized controlled trial, infants who received daily full-body application of emollient beginning at 3 weeks of life were significantly less likely than controls to develop AD by 6 months.20 While the mechanism of action is not clearly understood, it is believed that early emollient use prevents skin dehydration and maintains the skin’s barrier integrity, thus decreasing allergen epidermal penetration and subsequent inflammation.
Bleach bath
A bleach bath, prepared by adding 1/2 cup of unconcentrated bleach (5.25% sodium hypochlorite) to a standard 40-gallon bathtub, produces a chlorine mixture equivalent to an average swimming pool. Soaking in a bleach bath for 10 minutes once or twice weekly is thought to reduce inflammation and bacteria on the skin, but studies of its efficacy in improving atopic symptoms are mixed.
In a pooled analysis of 5 studies evaluating bleach baths vs standard baths, there was no significant difference in disease severity at 4 weeks.21 Thus, while bleach baths were effective in decreasing disease severity, they appeared to be no more effective than a standard water bath.21 Bleach baths may be helpful, however, in cases of moderate-to-severe disease with frequent bacterial infections.19
Pharmacologic therapy
Steroids
For symptoms refractory to nonpharmacologic skin care, topical steroids are the initial pharmacologic treatment for AD.19 Choose steroid potency based on symptom severity and disease location. Low- to medium-potency is appropriate for mild disease, and medium- to high-potency is useful for moderate-to-severe symptoms. High-potency steroids are generally avoided on the face and skin folds; however, they can be used for short periods in these areas to induce remission. They must then be quickly tapered and discontinued.
Continue to: Frequency
Frequency. Topical corticosteroids are typically applied twice daily, although recent studies indicate that once-daily application is just as efficacious.22 In addition to treatment of an acute flare, topical steroids are useful as maintenance therapy for patients with recurrent outbreaks in the same anatomical site. Guidelines suggest once- or twice-weekly application of a medium-potency steroid to prolong time between flares.19
For children, a practical guide is for caregivers to apply the amount of steroid covering 1 adult fingertip to an area of the child’s skin equal to that of 2 adult palms.23 Topical steroids are generally well tolerated and have a good safety profile. Adverse effects are proportional to the amount and duration of use and include purpura, telangiectasias, striae, and skin atrophy. The risk of skin atrophy increases with higher potency steroids, occlusion (covering affected area after steroid application), use on thin-skinned areas, and older patient age.24
Reassure patients/parents about the safety of topical steroids, as fears regarding the potential adverse effects can limit compliance. In one study of 200 patients with AD, 72.5% of respondents expressed fear of using steroids on their own skin or that of their child, and 24% admitted being noncompliant with therapy based on these concerns.25
Treating flares. Oral steroids are sometimes needed to abort or control an AD flare in older children and adults. A tapering course of prednisone over 5 to 7 days, transitioning to medium- to high-dose topical steroids, may be needed to achieve symptom control.
Topical calcineurin inhibitors
Topical calcineurin inhibitors, including tacrolimus and pimecrolimus, are generally second-line therapy to topical corticosteroids. However, as nonsteroidal agents, topical calcineurin inhibitors do not cause skin atrophy and can be a first-line option in areas where atrophy is more common (face, eyelids, neck, and skin folds).26
Continue to: A Cochrane review found...
A Cochrane review found tacrolimus 0.1% to be better than low‐potency topical corticosteroids on the face and neck areas, while results were equivocal when compared with moderate‐potency topical corticosteroids on the trunk and extremities (no difference based on physician assessment, but marginal benefit favoring tacrolimus based on participant scoring).27 When compared head-to-head, tacrolimus was more effective than pimecrolimus, although tacrolimus has a higher rate of local irritation. The most common adverse effects are stinging and burning at the application site, although these adverse effects generally improve with repeated application.
There have been long-term safety concerns with topical calcineurin inhibitors—chiefly a 2006 Food and Drug Administration (FDA) black box warning regarding a possible link between topical calcineurin inhibitors and cancer. However, while there may be a slight increased risk of lymphoma in AD patients, a recent meta-analysis did not find an association between topical calcineurin inhibitors use and lymphoma.28 Given the initial concern—and pending additional data—the FDA currently recommends reserving topical calcineurin inhibitors for second-line therapy and only for the minimum amount of time to induce improvement. It also recommends avoiding their use in patients younger than 2 years and in those with compromised immune systems.
Cisaborole
Cisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor, received FDA approval in 2016 for mild-to-moderate AD. By inhibiting PDE4, the drug limits inflammation. In a multicenter randomized trial, patients applying cisaborole 2% twice a day noted reductions in pruritus, inflammation, excoriation, and lichenification.29 Adverse effects are minimal and limited to application site irritation.
Systemic treatments
While beyond the care of a family physician, symptoms refractory to conservative, nonpharmacologic measures and combinations of topical pharmaceuticals can be treated with systemic immunomodulators such as cyclosporine, azathioprine, and methotrexate. Phototherapy is also effective in patients with more widespread skin involvement. Dupilumab, an injectable monoclonal antibody that binds to interleukin-4 receptor and inhibits inflammation, is approved to treat moderate-to-severe AD in adults.30
Ineffective therapies: Oral montelukast and probiotics
While oral antihistamines are frequently prescribed and used, there are no studies evaluating the use of antihistamines (H1) as monotherapy for AD.31 Nonetheless, while not altering the disease process, the sedative effect of antihistamines may palliate the nocturnal pruritus frequently associated with AD. Although nonsedating antihistamines may still have a role for atopic patients with concurrent seasonal and environmental allergies, there is no evidence to support their use in the treatment of AD.
Continue to: Data are limited...
Data are limited on the effectiveness of leukotriene receptor antagonists for AD, and all studies meeting inclusion for a Cochrane review assessed oral montelukast. The review found no benefit with the use of montelukast 10 mg in terms of severity of disease, pruritus, or need for topical steroids.32
A systematic review investigating the benefit of probiotics for the treatment of AD found no improvement in patient-rated eczema scores for quality of life.33 Additionally, a review of 11 randomized controlled trials including 596 participants found no evidence to suggest efficacy of fish oil, zinc, selenium, vitamin D, vitamin E, pyridoxine, sea buckthorn oil, hempseed oil, or sunflower oil in the treatment of AD.34
Education can reduce AD severity
Family physicians can be a source of education and support for patients and families of patients with AD. Support programs for adults with AD—including education, relaxation techniques, and cognitive behavioral therapy—have been shown to decrease disease severity.35 Comparable improvement in disease severity has been demonstrated in children with AD when similar education is provided to them and their families.
CORRESPONDENCE
Franklin Berkey, DO, Penn State Health, 1850 East Park Avenue, Suite 207, State College, PA 16803; fberkey@ pennstatehealth.psu.edu.
Atopic dermatitis (AD), also known as eczema, is a chronic inflammatory skin condition that is well known for its relapsing, pruritic rash in children and adults. Less recognized are its associated conditions—allergic rhinitis, asthma, food allergies, attention-deficit/hyperactivity disorder (ADHD), depression, and anxiety—and its burden on patients and their families. In fact, families that have children with AD report lower overall quality of life than those with otherwise healthy children.1 Given AD’s prevalence across age groups and its effect on the family, family physicians are uniquely positioned to diagnose, care for, and counsel patients with AD and its associated maladies.
The prevalence and pathogenesis of AD
AD affects up to 20% of children and 5% of adults in the United States.2 AD typically manifests before a child reaches age 5 (often in the first 6 months of life), and it is slightly more common in females (1.3:1). A family history of atopy (eczema, asthma, allergic rhinitis) is common. In fact, children with one atopic parent have a 2- to 3-fold increased risk of atopic dermatitis; those with 2 atopic parents have a 3- to 5-fold increased risk.3
The pathophysiology of AD is complex, culminating in impaired barrier function of the skin and transepidermal water loss resulting in dry and inflamed skin. Additionally, alterations in a cell-mediated immune response leading to an immunoglobulin (Ig) E-mediated hypersensitivity is also theorized to play a role in the development of AD.
Signs and symptoms
Signs at birth. Physical signs of atopic dermatitis typically appear between birth and 6 months. In infancy, lesions generally occur on the scalp, face (FIGURES 1A and 1B), neck, and extensor surfaces of the extremities. Lesions are typically papules and vesicles, sometimes accompanied by serous exudate and crusting. Eczematous lesions typically spare the groin and diaper area, and their presence in this area should raise suspicion for an alternative diagnosis.

Beginning at age 2 years, eczematous lesions are more commonly limited to the folds of the flexor surfaces. Instead of the weeping and crusting lesions seen in infancy, eczema in older children manifests as dry, lichenified papules and plaques in areas that are typically affected in adults: the wrist, hands, ankles, and popliteal and antecubital fossa.2
Although lesions in adults are similar to those of childhood, they may manifest in a more localized area (hand or eyelid, for example). As is the case in childhood, the lesions are dry, sometimes lichenified, and found on the flexural surfaces (FIGURES 2A and 2B).2

Symptom triggers are unproven
While anecdotal reports cite various triggers for AD flares, a systematic review found little scientific evidence to substantiate identifiable triggers.4 Triggers often cited and studied are foods, dust mite exposure, airborne allergens, detergents, sunlight, fabrics, bacterial infections, and stress. While as many as one-third of people with AD who also have confirmed dust mite allergy report worsening of symptoms when exposed to dust, a Cochrane review of 7 randomized controlled trials totaling 324 adults and children with eczema found that efforts at dust mite mitigation (laundering of bed covers, increased vacuuming, spraying for mites) were not effective in reducing symptoms.5
Continue to: How quality of life diminishes with AD
How quality of life diminishes with AD
AD substantially lessens quality of life. For children, the most distressing physical symptoms include itching that inhibits sleep and provokes scratching, pain, and bleeding. Emotional distress can cause irritability, crying, and uncooperativeness with treatments. Parents also report that they frequently restrict their children from activities, such as playing in the heat or swimming, that may lead to worsening of their eczema.6
The loss of sleep associated with AD is not completely understood but is likely multifactorial. Pruritus and scratching leading to sleeplessness is the most obvious culprit, but an altered circadian rhythm, immune system response, and changes in skin physiology are also likely factors.7 Whatever the cause, sleep disturbance is reported in as many as 60% of patients with AD, and the degree of sleep disturbance is proportional to increases in disease severity and worsening of quality-of-life scores.8 Lost sleep is not limited to patients; parents of children with AD also report significant loss of sleep and subsequent decreased work productivity and quality of life.9
Children with AD are often the target of bullying.10 A 2015 survey by the National Eczema Association indicates that 1 in 5 children reported being bullied due to their AD.11
Associated conditions and comorbidities
AD increases patients’ risks for other illnesses, due either to their underlying atopy or to the effects of

Atopic march
Atopic march—the clinical succession of AD, allergic rhinitis, and asthma—is a well-established clinical progression. The presence of all 3 conditions appears to be more common in children diagnosed with AD before 2 years of age.12 Typically, allergic rhinitis manifests at around age 4, and asthma develops between ages 6 and 8. The severity of AD predicts progression. Compared with an 8% chance of asthma developing among the general population, children with mild AD have a 20% to 30% chance of developing asthma, and those with severe AD have about a 70% chance.12
Continue to: Food allergies
Food allergies
Patients with AD are at higher risk for food-induced anaphylaxis, with up to one-third of AD patients having an IgE-mediated food allergy.13 While it is theorized that the impaired skin barrier of an atopic child may allow for early sensitization and allergy development, a landmark 2015 study demonstrated that early allergen introduction (specifically, peanuts) may serve as a preventive strategy in those at high risk of food allergies.14 Current guidelines recommend that physicians be aware of the increased possibility of food allergies in those with AD, and consider evaluating a child for milk, egg, peanut, wheat, and soy allergy if the child is younger than 5 years and has eczema that does not resolve with treatment, or has eczema and a history of an allergic reaction to a specific food.15
Interestingly, despite the strong association between AD and food allergies, it is not clear that food allergies trigger atopic flares; as such, elimination diets are not universally recommended in those without a proven food allergy.
Psychiatric diagnoses
Children with AD have an increased prevalence of several psychiatric conditions, including ADHD, depression, anxiety, conduct disorder, and autism when compared with peers who do not have AD, and the probability correlates with the severity of AD
What we do know is that one of the strongest associations between AD and a psychiatric condition is with ADHD, with a recent pooled meta-analysis showing a 46% increase in risk.17 The incidence of depression among children with AD appears to correlate with the severity of AD symptoms: estimated at 5% with mild AD, 7% with moderate disease, and 14% with severe disease (compared with 3% without AD). Similar incremental increases are seen when correlating AD and anxiety.16
Nonpharmacologic care
Bathing
Bathing habits are critical to controlling AD. While bathing serves to both hydrate the skin and remove allergens, the water’s evaporation off the skin surface can lead to increased transepidermal water loss. Combining bathing and immediate application of a moisturizer improves skin hydration in patients with AD vs bathing alone.18 Thus, consensus guidelines recommend once-daily bathing (bath or shower) to remove scale and crust, followed by immediate application of a moisturizing emollient.19
Continue to: Emollients
Emollients
Application of moisturizing emollients is the mainstay of nonpharmacologic care of AD, and there is strong evidence that their regimented use reduces disease burden and the need for prescription treatment.19 Emollient creams and ointments help retain moisture and improve the skin’s barrier. While ointments may provide a better barrier, patients tend to prefer creams as they are less greasy than ointments.
Emollient therapy may also help prevent development of AD, especially in those infants thought to be at high risk with a family history of atopy. In a multinational randomized controlled trial, infants who received daily full-body application of emollient beginning at 3 weeks of life were significantly less likely than controls to develop AD by 6 months.20 While the mechanism of action is not clearly understood, it is believed that early emollient use prevents skin dehydration and maintains the skin’s barrier integrity, thus decreasing allergen epidermal penetration and subsequent inflammation.
Bleach bath
A bleach bath, prepared by adding 1/2 cup of unconcentrated bleach (5.25% sodium hypochlorite) to a standard 40-gallon bathtub, produces a chlorine mixture equivalent to an average swimming pool. Soaking in a bleach bath for 10 minutes once or twice weekly is thought to reduce inflammation and bacteria on the skin, but studies of its efficacy in improving atopic symptoms are mixed.
In a pooled analysis of 5 studies evaluating bleach baths vs standard baths, there was no significant difference in disease severity at 4 weeks.21 Thus, while bleach baths were effective in decreasing disease severity, they appeared to be no more effective than a standard water bath.21 Bleach baths may be helpful, however, in cases of moderate-to-severe disease with frequent bacterial infections.19
Pharmacologic therapy
Steroids
For symptoms refractory to nonpharmacologic skin care, topical steroids are the initial pharmacologic treatment for AD.19 Choose steroid potency based on symptom severity and disease location. Low- to medium-potency is appropriate for mild disease, and medium- to high-potency is useful for moderate-to-severe symptoms. High-potency steroids are generally avoided on the face and skin folds; however, they can be used for short periods in these areas to induce remission. They must then be quickly tapered and discontinued.
Continue to: Frequency
Frequency. Topical corticosteroids are typically applied twice daily, although recent studies indicate that once-daily application is just as efficacious.22 In addition to treatment of an acute flare, topical steroids are useful as maintenance therapy for patients with recurrent outbreaks in the same anatomical site. Guidelines suggest once- or twice-weekly application of a medium-potency steroid to prolong time between flares.19
For children, a practical guide is for caregivers to apply the amount of steroid covering 1 adult fingertip to an area of the child’s skin equal to that of 2 adult palms.23 Topical steroids are generally well tolerated and have a good safety profile. Adverse effects are proportional to the amount and duration of use and include purpura, telangiectasias, striae, and skin atrophy. The risk of skin atrophy increases with higher potency steroids, occlusion (covering affected area after steroid application), use on thin-skinned areas, and older patient age.24
Reassure patients/parents about the safety of topical steroids, as fears regarding the potential adverse effects can limit compliance. In one study of 200 patients with AD, 72.5% of respondents expressed fear of using steroids on their own skin or that of their child, and 24% admitted being noncompliant with therapy based on these concerns.25
Treating flares. Oral steroids are sometimes needed to abort or control an AD flare in older children and adults. A tapering course of prednisone over 5 to 7 days, transitioning to medium- to high-dose topical steroids, may be needed to achieve symptom control.
Topical calcineurin inhibitors
Topical calcineurin inhibitors, including tacrolimus and pimecrolimus, are generally second-line therapy to topical corticosteroids. However, as nonsteroidal agents, topical calcineurin inhibitors do not cause skin atrophy and can be a first-line option in areas where atrophy is more common (face, eyelids, neck, and skin folds).26
Continue to: A Cochrane review found...
A Cochrane review found tacrolimus 0.1% to be better than low‐potency topical corticosteroids on the face and neck areas, while results were equivocal when compared with moderate‐potency topical corticosteroids on the trunk and extremities (no difference based on physician assessment, but marginal benefit favoring tacrolimus based on participant scoring).27 When compared head-to-head, tacrolimus was more effective than pimecrolimus, although tacrolimus has a higher rate of local irritation. The most common adverse effects are stinging and burning at the application site, although these adverse effects generally improve with repeated application.
There have been long-term safety concerns with topical calcineurin inhibitors—chiefly a 2006 Food and Drug Administration (FDA) black box warning regarding a possible link between topical calcineurin inhibitors and cancer. However, while there may be a slight increased risk of lymphoma in AD patients, a recent meta-analysis did not find an association between topical calcineurin inhibitors use and lymphoma.28 Given the initial concern—and pending additional data—the FDA currently recommends reserving topical calcineurin inhibitors for second-line therapy and only for the minimum amount of time to induce improvement. It also recommends avoiding their use in patients younger than 2 years and in those with compromised immune systems.
Cisaborole
Cisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor, received FDA approval in 2016 for mild-to-moderate AD. By inhibiting PDE4, the drug limits inflammation. In a multicenter randomized trial, patients applying cisaborole 2% twice a day noted reductions in pruritus, inflammation, excoriation, and lichenification.29 Adverse effects are minimal and limited to application site irritation.
Systemic treatments
While beyond the care of a family physician, symptoms refractory to conservative, nonpharmacologic measures and combinations of topical pharmaceuticals can be treated with systemic immunomodulators such as cyclosporine, azathioprine, and methotrexate. Phototherapy is also effective in patients with more widespread skin involvement. Dupilumab, an injectable monoclonal antibody that binds to interleukin-4 receptor and inhibits inflammation, is approved to treat moderate-to-severe AD in adults.30
Ineffective therapies: Oral montelukast and probiotics
While oral antihistamines are frequently prescribed and used, there are no studies evaluating the use of antihistamines (H1) as monotherapy for AD.31 Nonetheless, while not altering the disease process, the sedative effect of antihistamines may palliate the nocturnal pruritus frequently associated with AD. Although nonsedating antihistamines may still have a role for atopic patients with concurrent seasonal and environmental allergies, there is no evidence to support their use in the treatment of AD.
Continue to: Data are limited...
Data are limited on the effectiveness of leukotriene receptor antagonists for AD, and all studies meeting inclusion for a Cochrane review assessed oral montelukast. The review found no benefit with the use of montelukast 10 mg in terms of severity of disease, pruritus, or need for topical steroids.32
A systematic review investigating the benefit of probiotics for the treatment of AD found no improvement in patient-rated eczema scores for quality of life.33 Additionally, a review of 11 randomized controlled trials including 596 participants found no evidence to suggest efficacy of fish oil, zinc, selenium, vitamin D, vitamin E, pyridoxine, sea buckthorn oil, hempseed oil, or sunflower oil in the treatment of AD.34
Education can reduce AD severity
Family physicians can be a source of education and support for patients and families of patients with AD. Support programs for adults with AD—including education, relaxation techniques, and cognitive behavioral therapy—have been shown to decrease disease severity.35 Comparable improvement in disease severity has been demonstrated in children with AD when similar education is provided to them and their families.
CORRESPONDENCE
Franklin Berkey, DO, Penn State Health, 1850 East Park Avenue, Suite 207, State College, PA 16803; fberkey@ pennstatehealth.psu.edu.
1. Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
2. Ahn C, Huang W. Clinical presentation of atopic dermatitis. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:38-46.
3. Eichenfield LF, Tom WL, Chamblin SL, et al. Guidelines of care for the management of atopic dermatitis. Part 1: diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
4. Langan SM, Williams HC. What causes worsening of eczema? A systematic review. Br J Dermatol. 2006;155:504-514.
5. Nankervis H, Pynn EV, Boyle RJ, et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev. 2015:CD008426.
6. Chamlin SL, Frieden IJ, Williams ML, et al. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114:607-611.
7. Chang Y-S, Chiang B-L. Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. Int J Mol Sci. 2016;17:462.
8. Camfferman D, Kennedy JD, Gold M, et al. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev. 2010;14:359-369.
9. Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
10. Drucker AM, Wang AR, Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:P26-P30.
11. National Eczema Association. Tools for school: addressing school bullying for kids with eczema. Accessed January 5, 2021. https://nationaleczema.org/children-with-eczema-experience-bullying/
12. Bantz SK, Zhu Z, Zhen T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202
13. Laird M, Sicco KL. Defining and measuring the scope of atopic dermatitis. Adv Exp Med Biol. 2017;1027:93-104.
14. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
15. Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58.
16. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
17. Strom MA, Fishbein AB, Paller AS, et al. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol. 2016;175:920-929.
18. Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
19. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
20. Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
21. Chopra R, Vakharia PP, Sacotte R, et al. Efficacy of bleach baths in reducing severity of atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119:435-440.
22. Williams HC. Established corticosteroid creams should be applied only once daily in patients with atopic eczema. BMJ. 2007;334:1272.
23. Long CC, Mills CM, Finlay AY. A practical guide to topical therapy in children. Br J Dermatol. 1998;138:293-296.
24. Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156:203-221.
25. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142:931-936.
26. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: a meta-analysis of randomised controlled trials. BMJ. 2005;330:516.
27. Cury Martins J, Martins C, Aoki V, et al. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015:CD009864.
28. Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
29. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.
30. Dupilumab [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals Inc; 2017.
31. van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25.
32. Ferguson L, Futamura M, Vakirlis E, et al. Leukotriene receptor antagonists for eczema. Cochrane Database Syst Rev. 2018:CD011224.
33. Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018:CD006135.
34. Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
35. Sy W, Lamb AJ. Atopic dermatitis disease education. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:179-184.
1. Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
2. Ahn C, Huang W. Clinical presentation of atopic dermatitis. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:38-46.
3. Eichenfield LF, Tom WL, Chamblin SL, et al. Guidelines of care for the management of atopic dermatitis. Part 1: diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
4. Langan SM, Williams HC. What causes worsening of eczema? A systematic review. Br J Dermatol. 2006;155:504-514.
5. Nankervis H, Pynn EV, Boyle RJ, et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev. 2015:CD008426.
6. Chamlin SL, Frieden IJ, Williams ML, et al. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114:607-611.
7. Chang Y-S, Chiang B-L. Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. Int J Mol Sci. 2016;17:462.
8. Camfferman D, Kennedy JD, Gold M, et al. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev. 2010;14:359-369.
9. Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
10. Drucker AM, Wang AR, Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:P26-P30.
11. National Eczema Association. Tools for school: addressing school bullying for kids with eczema. Accessed January 5, 2021. https://nationaleczema.org/children-with-eczema-experience-bullying/
12. Bantz SK, Zhu Z, Zhen T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202
13. Laird M, Sicco KL. Defining and measuring the scope of atopic dermatitis. Adv Exp Med Biol. 2017;1027:93-104.
14. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
15. Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58.
16. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
17. Strom MA, Fishbein AB, Paller AS, et al. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol. 2016;175:920-929.
18. Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
19. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
20. Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
21. Chopra R, Vakharia PP, Sacotte R, et al. Efficacy of bleach baths in reducing severity of atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119:435-440.
22. Williams HC. Established corticosteroid creams should be applied only once daily in patients with atopic eczema. BMJ. 2007;334:1272.
23. Long CC, Mills CM, Finlay AY. A practical guide to topical therapy in children. Br J Dermatol. 1998;138:293-296.
24. Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156:203-221.
25. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142:931-936.
26. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: a meta-analysis of randomised controlled trials. BMJ. 2005;330:516.
27. Cury Martins J, Martins C, Aoki V, et al. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015:CD009864.
28. Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
29. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.
30. Dupilumab [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals Inc; 2017.
31. van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25.
32. Ferguson L, Futamura M, Vakirlis E, et al. Leukotriene receptor antagonists for eczema. Cochrane Database Syst Rev. 2018:CD011224.
33. Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018:CD006135.
34. Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
35. Sy W, Lamb AJ. Atopic dermatitis disease education. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:179-184.
PRACTICE RECOMMENDATIONS
› Advise patients to regularly apply moisturizers, which reduces atopic dermatitis (AD) severity and may avert the need for pharmacologic intervention. A
› Assure patients that a topical corticosteroid is safe and effective as first-line treatment for AD symptoms refractory to nonpharmacologic recommendations. A
› Consider topical calcineurin inhibitors for both acute and chronic AD in adults and children, especially in areas more prone to topical corticosteroid adverse effects. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to identify and treat common bites and stings
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19
Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2
Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30
Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37
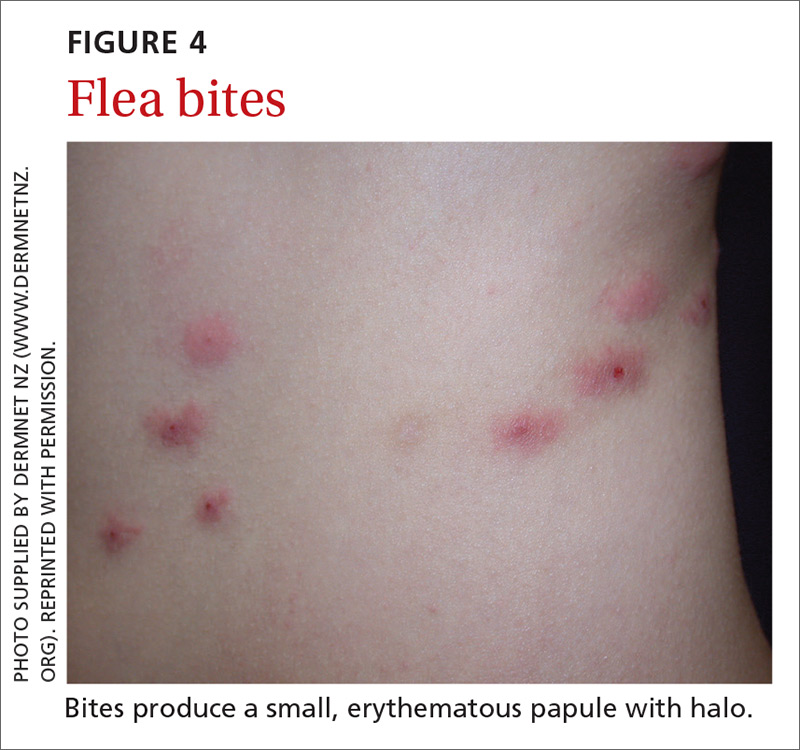
Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43
Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47
Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49
Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.
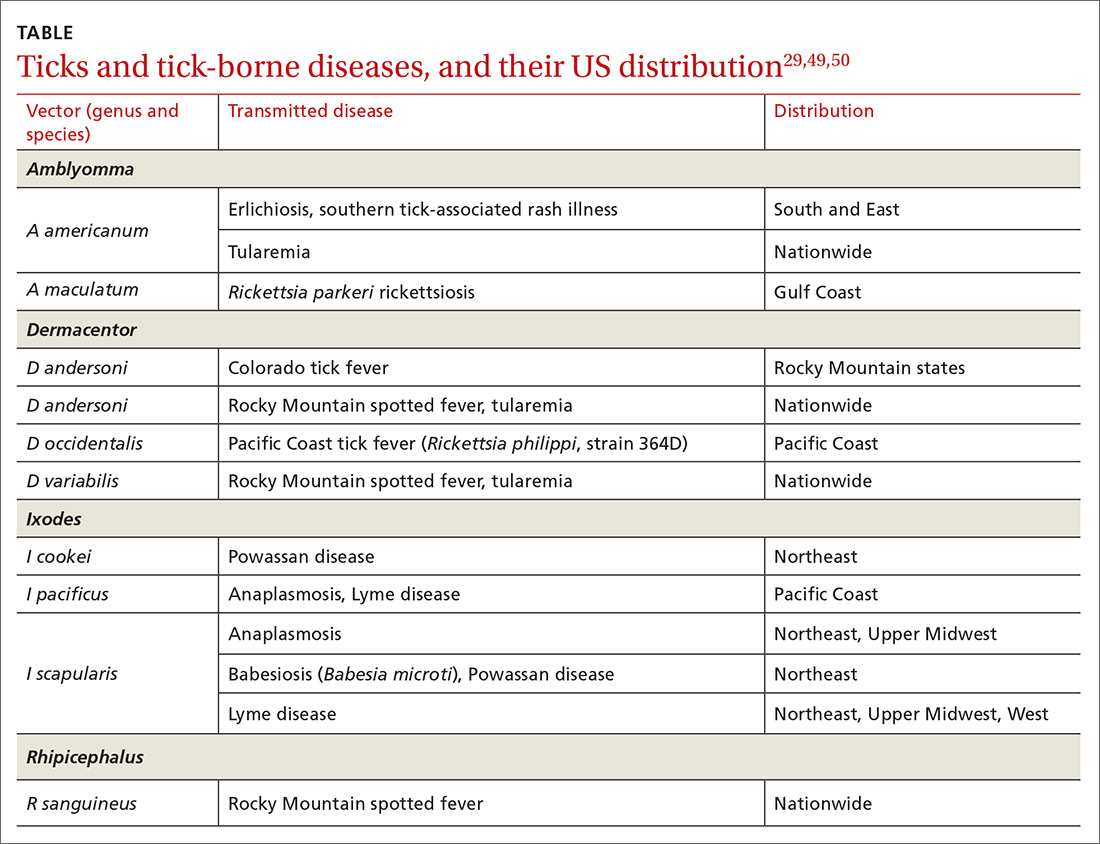
Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19

Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2

Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30

Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37

Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43

Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47

Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49

Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.

Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19

Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2

Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30

Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37

Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43

Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47

Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49

Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.

Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
PRACTICE RECOMMENDATIONS
❯ Recommend that patients use an insect repellent, such as an over-the-counter formulation that contains DEET, picaridin, or PMD (a chemical constituent of Eucalyptus citriodora oil) to prevent flea bites. C
❯ Prescribe nonsedating oral antihistamines as first-line symptomatic treatment of mild-to-moderate pruritus secondary to an insect bite. C
❯ When indicated, refer patients for venom immunotherapy, which is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis and death. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Immunodeficiency strongly linked to mental illness, suicidal behavior
Patients with a primary humoral immunodeficiency (PID) are 91% more likely to have a psychiatric disorder and 84% more likely to exhibit suicidal behavior, compared against those without the condition, new research shows.
Results showed that this association, which was stronger in women, could not be fully explained by comorbid autoimmune diseases or by familial confounding.
These findings have important clinical implications, study investigator Josef Isung, MD, PhD, Centre for Psychiatry Research, Karolinska Institute, Stockholm, Sweden, told Medscape Medical News.
Clinicians managing patients with PID “should be aware of this increased association with psychiatric disorders and perhaps screen for them,” said Isung.
The study was published in the November issue of JAMA Psychiatry.
Registry study
Mounting evidence suggests immune disruption plays a role in psychiatric disorders through a range of mechanisms, including altered neurodevelopment. However, little is known about the neuropsychiatric consequences resulting from the underproduction of homeostatic antibodies.
They’re associated with an increased risk for recurrent infections and of developing autoimmune diseases.
The immunodeficiency can be severe, even life threatening, but can also be relatively mild. One of the less severe PID types is selective IgA deficiency, which is linked to increased infections within the mucosa-associated lymphoid tissue (MALT), an important immune barrier.
Experts have long suspected that infections within the MALT are associated with certain forms of psychopathology in children, particularly obsessive-compulsive disorder and chronic tic disorders.
While patients with this selective IgA subtype may be at some increased risk for infection and autoimmune disease, their overall health otherwise is good, said Isung.
The prevalence of PIDs ranges from about 1:250 to 1:20,000, depending on the type of humoral immunodeficiency, although most would fall into the relatively rare category, he added.
Using several linked national Swedish registries, researchers identified individuals with any PID diagnosis affecting immunoglobulin levels, their full siblings, and those with a lifetime diagnosis of selective IgA deficiency. In addition, they collected data on autoimmune diseases.
The study outcome was a lifetime record of a psychiatric disorder, a suicide attempt, or death by suicide.
Strong link to autism
Researchers identified 8378 patients (59% women) with PID affecting immunoglobulin levels (median age at first diagnosis, 47.8 years). They compared this group with almost 14.3 million subjects without PID.
In those with PID, 27.6% had an autoimmune disease vs 6.8% of those without PID, a statistically significant difference (P < .001).
About 20.5% of those with PID and 10.7% of unexposed subjects had at least one diagnosis of a psychiatric disorder.
In a model adjusted for year of birth, sex, and history of autoimmune disease, subjects with PID had a 91% higher likelihood of any psychiatric disorder (adjusted odds ratio [AOR] 1.91; 95% CI, 1.81 - 2.01; P < .001) vs their counterparts without PID.
The AORs for individual psychiatric disorders ranged from 1.34 (95% CI, 1.17 - 1.54; P < .001) for schizophrenia and other psychotic disorders to 2.99 (95% CI, 2.42 - 3.70; P < .001) for autism spectrum disorders (ASDs)
It’s unclear why the association with PID was strongest for autism, “but being a neurodevelopmental disorder, maybe autism is logically more associated with this type of disruption,” said Isung.
Research suggests that immunologic disruption may play a role in ASD, either through altered maternal immune function in utero or through immune disruption after birth, the researchers note.
Compared to those without PID, individuals with it had a significantly increased likelihood of any suicidal behavior (AOR, 1.84; 95% CI, 1.66 - 2.04, P < .001) as well as individual outcomes of death by suicide and suicide attempts.
The association with psychiatric disorders and suicidal behavior was markedly stronger for exposure to both PID and autoimmune disease than for exposure to either of these alone, which suggest an additive effect for these immune-related conditions.
Sex differences
“It was unclear to us why women seemed particularly vulnerable,” said Isung. He noted that PIDs are generally about as common in women as in men, but women tend to have higher rates of psychiatric disorders.
The analysis of the sibling cohort also revealed an elevated risk for psychiatric disorders, including ASD and suicidal behavior, but to a lesser degree.
“From this we could infer that at least part of the associations would be genetic, but part would be related to the disruption in itself,” said Isung.
An analysis examining selective IgA subtype also revealed a link with psychiatric disorders and suicidal behavior, suggesting this link is not exclusive to severe PID cases.
“Our conclusion here was that it seems like PID itself, or the immune disruption in itself, could explain the association rather than the burden of illness,” said Isung.
However, he acknowledged that the long-term stress and mental health fallout of having a chronic illness like PID may also explain some of the increased risk for psychiatric disorders.
This study, he said, provides more evidence that immune disruptions affect neurodevelopment and the brain. However, he added, the underlying mechanism still isn’t fully understood.
The results highlight the need to raise awareness of the association between immunodeficiency and mental illness, including suicidality among clinicians, patients, and advocates.
These findings may also have implications in patients with other immune deficiencies, said Isung, noting, “it would be interesting to further explore associations with other immunocompromised populations.”
No surprises
Commenting on the findings for Medscape Medical News, Igor Galynker, MD, professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York City, said the study was “very well-done” and used “reliable and well-controlled” databases.
However, he added, the results “are neither particularly dramatic nor conclusive” as it makes sense that medical illnesses like PID would “increase risk of psychopathology,” said Galynker.
PID patients are much more likely to have contact with clinicians and to receive a psychiatric diagnosis, he said.
“People with a chronic illness are more stressed and generally have high incidences of depression, anxiety, and suicidal behavior. In addition to that, they may be more likely to be diagnosed with those conditions because they see a clinician more frequently.”
However, that reasoning doesn’t apply to autism, which manifests in early childhood and so is unlikely to be the result of stress, said Galynker, which is why he believes the finding that ASD is the psychiatric outcome most strongly associated with PID is “the most convincing.”
Galynker wasn’t surprised that the association between PID and psychiatric illnesses, and suicidal behaviors, was stronger among women.
“Women attempt suicide four times more often than men to begin with, so you would expect this to be more pronounced” in those with PID.
The study was supported by grants from the Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institute; Stockholm Care Services; the Soderstrom Konig Foundation; and the Fredrik & Ingrid Thurings Foundation. Isung and Galynker have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with a primary humoral immunodeficiency (PID) are 91% more likely to have a psychiatric disorder and 84% more likely to exhibit suicidal behavior, compared against those without the condition, new research shows.
Results showed that this association, which was stronger in women, could not be fully explained by comorbid autoimmune diseases or by familial confounding.
These findings have important clinical implications, study investigator Josef Isung, MD, PhD, Centre for Psychiatry Research, Karolinska Institute, Stockholm, Sweden, told Medscape Medical News.
Clinicians managing patients with PID “should be aware of this increased association with psychiatric disorders and perhaps screen for them,” said Isung.
The study was published in the November issue of JAMA Psychiatry.
Registry study
Mounting evidence suggests immune disruption plays a role in psychiatric disorders through a range of mechanisms, including altered neurodevelopment. However, little is known about the neuropsychiatric consequences resulting from the underproduction of homeostatic antibodies.
They’re associated with an increased risk for recurrent infections and of developing autoimmune diseases.
The immunodeficiency can be severe, even life threatening, but can also be relatively mild. One of the less severe PID types is selective IgA deficiency, which is linked to increased infections within the mucosa-associated lymphoid tissue (MALT), an important immune barrier.
Experts have long suspected that infections within the MALT are associated with certain forms of psychopathology in children, particularly obsessive-compulsive disorder and chronic tic disorders.
While patients with this selective IgA subtype may be at some increased risk for infection and autoimmune disease, their overall health otherwise is good, said Isung.
The prevalence of PIDs ranges from about 1:250 to 1:20,000, depending on the type of humoral immunodeficiency, although most would fall into the relatively rare category, he added.
Using several linked national Swedish registries, researchers identified individuals with any PID diagnosis affecting immunoglobulin levels, their full siblings, and those with a lifetime diagnosis of selective IgA deficiency. In addition, they collected data on autoimmune diseases.
The study outcome was a lifetime record of a psychiatric disorder, a suicide attempt, or death by suicide.
Strong link to autism
Researchers identified 8378 patients (59% women) with PID affecting immunoglobulin levels (median age at first diagnosis, 47.8 years). They compared this group with almost 14.3 million subjects without PID.
In those with PID, 27.6% had an autoimmune disease vs 6.8% of those without PID, a statistically significant difference (P < .001).
About 20.5% of those with PID and 10.7% of unexposed subjects had at least one diagnosis of a psychiatric disorder.
In a model adjusted for year of birth, sex, and history of autoimmune disease, subjects with PID had a 91% higher likelihood of any psychiatric disorder (adjusted odds ratio [AOR] 1.91; 95% CI, 1.81 - 2.01; P < .001) vs their counterparts without PID.
The AORs for individual psychiatric disorders ranged from 1.34 (95% CI, 1.17 - 1.54; P < .001) for schizophrenia and other psychotic disorders to 2.99 (95% CI, 2.42 - 3.70; P < .001) for autism spectrum disorders (ASDs)
It’s unclear why the association with PID was strongest for autism, “but being a neurodevelopmental disorder, maybe autism is logically more associated with this type of disruption,” said Isung.
Research suggests that immunologic disruption may play a role in ASD, either through altered maternal immune function in utero or through immune disruption after birth, the researchers note.
Compared to those without PID, individuals with it had a significantly increased likelihood of any suicidal behavior (AOR, 1.84; 95% CI, 1.66 - 2.04, P < .001) as well as individual outcomes of death by suicide and suicide attempts.
The association with psychiatric disorders and suicidal behavior was markedly stronger for exposure to both PID and autoimmune disease than for exposure to either of these alone, which suggest an additive effect for these immune-related conditions.
Sex differences
“It was unclear to us why women seemed particularly vulnerable,” said Isung. He noted that PIDs are generally about as common in women as in men, but women tend to have higher rates of psychiatric disorders.
The analysis of the sibling cohort also revealed an elevated risk for psychiatric disorders, including ASD and suicidal behavior, but to a lesser degree.
“From this we could infer that at least part of the associations would be genetic, but part would be related to the disruption in itself,” said Isung.
An analysis examining selective IgA subtype also revealed a link with psychiatric disorders and suicidal behavior, suggesting this link is not exclusive to severe PID cases.
“Our conclusion here was that it seems like PID itself, or the immune disruption in itself, could explain the association rather than the burden of illness,” said Isung.
However, he acknowledged that the long-term stress and mental health fallout of having a chronic illness like PID may also explain some of the increased risk for psychiatric disorders.
This study, he said, provides more evidence that immune disruptions affect neurodevelopment and the brain. However, he added, the underlying mechanism still isn’t fully understood.
The results highlight the need to raise awareness of the association between immunodeficiency and mental illness, including suicidality among clinicians, patients, and advocates.
These findings may also have implications in patients with other immune deficiencies, said Isung, noting, “it would be interesting to further explore associations with other immunocompromised populations.”
No surprises
Commenting on the findings for Medscape Medical News, Igor Galynker, MD, professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York City, said the study was “very well-done” and used “reliable and well-controlled” databases.
However, he added, the results “are neither particularly dramatic nor conclusive” as it makes sense that medical illnesses like PID would “increase risk of psychopathology,” said Galynker.
PID patients are much more likely to have contact with clinicians and to receive a psychiatric diagnosis, he said.
“People with a chronic illness are more stressed and generally have high incidences of depression, anxiety, and suicidal behavior. In addition to that, they may be more likely to be diagnosed with those conditions because they see a clinician more frequently.”
However, that reasoning doesn’t apply to autism, which manifests in early childhood and so is unlikely to be the result of stress, said Galynker, which is why he believes the finding that ASD is the psychiatric outcome most strongly associated with PID is “the most convincing.”
Galynker wasn’t surprised that the association between PID and psychiatric illnesses, and suicidal behaviors, was stronger among women.
“Women attempt suicide four times more often than men to begin with, so you would expect this to be more pronounced” in those with PID.
The study was supported by grants from the Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institute; Stockholm Care Services; the Soderstrom Konig Foundation; and the Fredrik & Ingrid Thurings Foundation. Isung and Galynker have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with a primary humoral immunodeficiency (PID) are 91% more likely to have a psychiatric disorder and 84% more likely to exhibit suicidal behavior, compared against those without the condition, new research shows.
Results showed that this association, which was stronger in women, could not be fully explained by comorbid autoimmune diseases or by familial confounding.
These findings have important clinical implications, study investigator Josef Isung, MD, PhD, Centre for Psychiatry Research, Karolinska Institute, Stockholm, Sweden, told Medscape Medical News.
Clinicians managing patients with PID “should be aware of this increased association with psychiatric disorders and perhaps screen for them,” said Isung.
The study was published in the November issue of JAMA Psychiatry.
Registry study
Mounting evidence suggests immune disruption plays a role in psychiatric disorders through a range of mechanisms, including altered neurodevelopment. However, little is known about the neuropsychiatric consequences resulting from the underproduction of homeostatic antibodies.
They’re associated with an increased risk for recurrent infections and of developing autoimmune diseases.
The immunodeficiency can be severe, even life threatening, but can also be relatively mild. One of the less severe PID types is selective IgA deficiency, which is linked to increased infections within the mucosa-associated lymphoid tissue (MALT), an important immune barrier.
Experts have long suspected that infections within the MALT are associated with certain forms of psychopathology in children, particularly obsessive-compulsive disorder and chronic tic disorders.
While patients with this selective IgA subtype may be at some increased risk for infection and autoimmune disease, their overall health otherwise is good, said Isung.
The prevalence of PIDs ranges from about 1:250 to 1:20,000, depending on the type of humoral immunodeficiency, although most would fall into the relatively rare category, he added.
Using several linked national Swedish registries, researchers identified individuals with any PID diagnosis affecting immunoglobulin levels, their full siblings, and those with a lifetime diagnosis of selective IgA deficiency. In addition, they collected data on autoimmune diseases.
The study outcome was a lifetime record of a psychiatric disorder, a suicide attempt, or death by suicide.
Strong link to autism
Researchers identified 8378 patients (59% women) with PID affecting immunoglobulin levels (median age at first diagnosis, 47.8 years). They compared this group with almost 14.3 million subjects without PID.
In those with PID, 27.6% had an autoimmune disease vs 6.8% of those without PID, a statistically significant difference (P < .001).
About 20.5% of those with PID and 10.7% of unexposed subjects had at least one diagnosis of a psychiatric disorder.
In a model adjusted for year of birth, sex, and history of autoimmune disease, subjects with PID had a 91% higher likelihood of any psychiatric disorder (adjusted odds ratio [AOR] 1.91; 95% CI, 1.81 - 2.01; P < .001) vs their counterparts without PID.
The AORs for individual psychiatric disorders ranged from 1.34 (95% CI, 1.17 - 1.54; P < .001) for schizophrenia and other psychotic disorders to 2.99 (95% CI, 2.42 - 3.70; P < .001) for autism spectrum disorders (ASDs)
It’s unclear why the association with PID was strongest for autism, “but being a neurodevelopmental disorder, maybe autism is logically more associated with this type of disruption,” said Isung.
Research suggests that immunologic disruption may play a role in ASD, either through altered maternal immune function in utero or through immune disruption after birth, the researchers note.
Compared to those without PID, individuals with it had a significantly increased likelihood of any suicidal behavior (AOR, 1.84; 95% CI, 1.66 - 2.04, P < .001) as well as individual outcomes of death by suicide and suicide attempts.
The association with psychiatric disorders and suicidal behavior was markedly stronger for exposure to both PID and autoimmune disease than for exposure to either of these alone, which suggest an additive effect for these immune-related conditions.
Sex differences
“It was unclear to us why women seemed particularly vulnerable,” said Isung. He noted that PIDs are generally about as common in women as in men, but women tend to have higher rates of psychiatric disorders.
The analysis of the sibling cohort also revealed an elevated risk for psychiatric disorders, including ASD and suicidal behavior, but to a lesser degree.
“From this we could infer that at least part of the associations would be genetic, but part would be related to the disruption in itself,” said Isung.
An analysis examining selective IgA subtype also revealed a link with psychiatric disorders and suicidal behavior, suggesting this link is not exclusive to severe PID cases.
“Our conclusion here was that it seems like PID itself, or the immune disruption in itself, could explain the association rather than the burden of illness,” said Isung.
However, he acknowledged that the long-term stress and mental health fallout of having a chronic illness like PID may also explain some of the increased risk for psychiatric disorders.
This study, he said, provides more evidence that immune disruptions affect neurodevelopment and the brain. However, he added, the underlying mechanism still isn’t fully understood.
The results highlight the need to raise awareness of the association between immunodeficiency and mental illness, including suicidality among clinicians, patients, and advocates.
These findings may also have implications in patients with other immune deficiencies, said Isung, noting, “it would be interesting to further explore associations with other immunocompromised populations.”
No surprises
Commenting on the findings for Medscape Medical News, Igor Galynker, MD, professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York City, said the study was “very well-done” and used “reliable and well-controlled” databases.
However, he added, the results “are neither particularly dramatic nor conclusive” as it makes sense that medical illnesses like PID would “increase risk of psychopathology,” said Galynker.
PID patients are much more likely to have contact with clinicians and to receive a psychiatric diagnosis, he said.
“People with a chronic illness are more stressed and generally have high incidences of depression, anxiety, and suicidal behavior. In addition to that, they may be more likely to be diagnosed with those conditions because they see a clinician more frequently.”
However, that reasoning doesn’t apply to autism, which manifests in early childhood and so is unlikely to be the result of stress, said Galynker, which is why he believes the finding that ASD is the psychiatric outcome most strongly associated with PID is “the most convincing.”
Galynker wasn’t surprised that the association between PID and psychiatric illnesses, and suicidal behaviors, was stronger among women.
“Women attempt suicide four times more often than men to begin with, so you would expect this to be more pronounced” in those with PID.
The study was supported by grants from the Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institute; Stockholm Care Services; the Soderstrom Konig Foundation; and the Fredrik & Ingrid Thurings Foundation. Isung and Galynker have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
67-year-old man • upper extremity pain & edema • recent diagnosis of heart failure • Dx?
THE CASE
A 67-year-old man with a history of gout, tobacco use, hypertension, hyperlipidemia, prediabetes, and newly diagnosed heart failure with reduced ejection fraction presented with a new concern for sudden-onset, atraumatic right upper extremity pain and swelling. The patient had awakened with these symptoms and on the following day went to the emergency department (ED) for evaluation. Review of the ED documentation highlighted that the patient was afebrile and was found to have a slight leukocytosis (11.7 x 103/µL) and an elevated C-reactive protein level (4 mg/dL; normal range, 0.3 to 1 mg/dL). A right upper extremity x-ray was unremarkable. The patient was treated with cephalexin and colchicine for cellulitis and possible acute gout.
Three days after the ED visit, the patient presented to his primary care clinic, reporting adherence to the prescribed therapies (cephalexin and colchicine) but no improvement in symptoms. He was again afebrile, and his blood pressure was controlled to goal (118/80 mm Hg). On exam, he had significant nonpitting, unilateral edema extending from the elbow through the fingers without erythema, warmth, or rash (FIGURE). A right upper extremity ultrasound was obtained; results were negative for deep vein thrombosis.
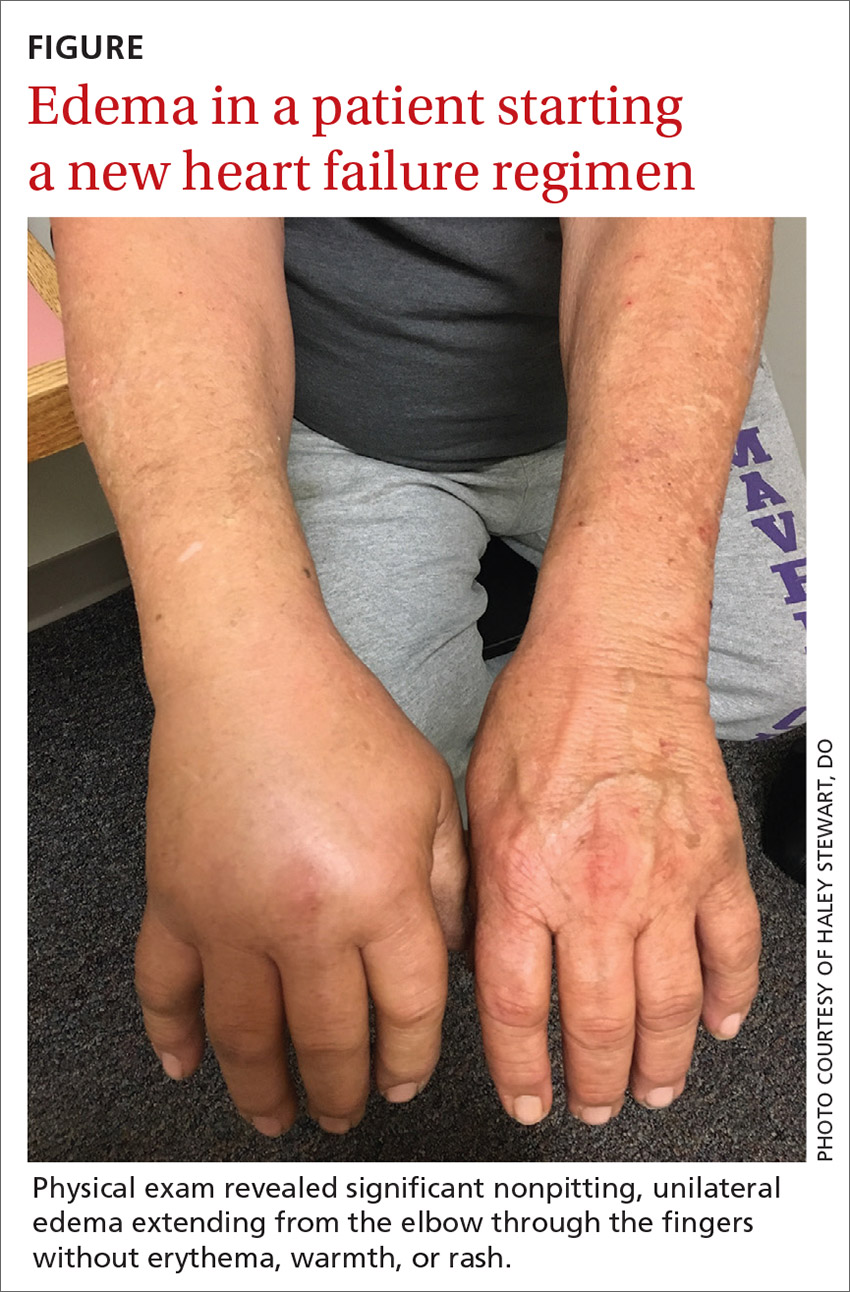
Medication reconciliation completed during the clinic visit revealed that the patient had started and continued to take newly prescribed medications for the treatment of heart failure, including metoprolol succinate, lisinopril, and furosemide. The patient confirmed that these were started 7 days prior to symptom onset.
THE DIAGNOSIS
Given the clinical resemblance to angioedema and the recent initiation of lisinopril, the patient was asked to hold this medication. He was also advised to discontinue the cephalexin and colchicine, given low suspicion for cellulitis and gout. Six days later, he returned to clinic and reported significantly improved pain and swelling.
DISCUSSION
Angioedema is a common condition in the United States, affecting approximately 15% of the general population.1 When associated with hypotension, respiratory compromise, and other end-organ dysfunction, it is treated as anaphylaxis. Angioedema without anaphylaxis can be categorized as either histaminergic or nonhistaminergic; the former is more common.2
Certain patient and disease characteristics are more prevalent in select subsets of angioedema, although there are no features that automatically identify an etiology. Here are some factors to consider:
Recent exposures. Within the histaminergic category, allergic angioedema has the longest list of potential causes, including medications (notably, antibiotics, nonsteroidal anti-inflammatory drugs, opiates, and perioperative medications), foods, latex, and insect stings and/or bites.2 Nonhistaminergic subtypes, which include hereditary and acquired angioedema, are caused by deficiencies or mutations in complement or coagulation pathways, which can be more challenging to diagnose.
Continue to: Acquired angioedema may also...
Acquired angioedema may also be associated with the use of angiotensin-converting enzyme (ACE) inhibitors. Risk factors for ACE inhibitor–induced angioedema include history of smoking, increasing age, and female gender.3 African-American race has been correlated with increased incidence of angioedema, with rates 4 to 5 times that of Whites,1 but race is now identified as a social and not a biological construct and should not be relied on to make medical decisions about prescribing.
The rate of occurrence for ACE inhibitor–induced angioedema is highest within the first 30 days of medication use2; however, it can occur anytime. The absolute risk has been estimated as 0.3% per year.4
Patient age. Histaminergic angioedema can occur at any age. The hereditary subtype of nonhistaminergic angioedema is more common in younger individuals, typically occurring in infancy to the second decade of life, and tends to run in families, while the acquired subtype often manifests in adults older than 40.2
Physical exam findings. The typical manifestation of nonhistaminergic angioedema is firm, nonpitting, nonpruritic swelling resulting from fluid shifts to the reticular dermis and subcutaneous or submucosal tissue. In comparison, histaminergic reactions commonly involve deeper dermal tissue.
Commonly affected anatomic sites also vary by angioedema type but do not directly distinguish a cause. Allergic and ACE inhibitor–induced subtypes more commonly involve the lips, tongue, larynx, and face, whereas hereditary and other acquired etiologies are more likely to affect the periphery, abdomen, face, larynx, and genitourinary systems.2 So the way that this patient presented was a bit unusual.
Continue to: Symptom history
Symptom history. Allergic angioedema often has a rapid onset and resolution, whereas hereditary and acquired subtypes appear more gradually.2 While the presence of urticaria distinguishes a histaminergic reaction, both histaminergic and nonhistaminergic angioedema may manifest without this symptom.
In our patient, the timeline of gradual symptom manifestation and the physical exam findings, as well as the patient’s age, tobacco history, and recent initiation of an ACE inhibitor, made acquired angioedema a more likely etiology.
Treatment for ACE inhibitor–induced angioedema, in addition to airway support, entails drug discontinuation. This typically leads to symptom resolution within 24 to 48 hours.2 Treatment with corticosteroids, antihistamines, and epinephrine is usually ineffective. Switching to an alternative ACE inhibitor is not recommended, as other members of the class carry the same risk. Instead, angiotensin receptor blockers (ARBs) are an appropriate substitute, as the incidence of cross-reactivity in ACE inhibitor–intolerant patients is estimated to be 10% or less,5 and the risk for recurrence has been shown to be no different than with placebo.3,4
Our patient was transitioned to losartan 25 mg/d without recurrence of his symptoms and with continued blood pressure control (125/60 mm Hg).
THE TAKEAWAY
Angioedema is a common condition. While many medications are associated with histaminergic angioedema, ACE inhibitors are a common cause of the acquired subtype of nonhistaminergic angioedema. Commonly affected sites include the lips, tongue, and face; however, this diagnosis is not dependent on location and may manifest at other sites, as seen in this case. Treatment involves medication discontinuation. When switching the patient’s medication, other members of the ACE inhibitor class should be avoided. ARBs are an appropriate alternative without increased risk for recurrence.
CORRESPONDENCE
Katherine Montag Schafer, University of Minnesota— Department of Family Medicine and Community Health, 1414 Maryland Avenue E, St Paul, MN 55106; [email protected]
1. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121:282-286.
2. Moellman JJ, Bernstein JA, Lindsell CA, et al; American College of Allergy, Asthma & Immunology (ACAAI), Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21:469-484.
3. Zuraw BL, Bernstein JA, Lang DM, et al; American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491-1493.
4. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383-391.
5. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45:520-524.
THE CASE
A 67-year-old man with a history of gout, tobacco use, hypertension, hyperlipidemia, prediabetes, and newly diagnosed heart failure with reduced ejection fraction presented with a new concern for sudden-onset, atraumatic right upper extremity pain and swelling. The patient had awakened with these symptoms and on the following day went to the emergency department (ED) for evaluation. Review of the ED documentation highlighted that the patient was afebrile and was found to have a slight leukocytosis (11.7 x 103/µL) and an elevated C-reactive protein level (4 mg/dL; normal range, 0.3 to 1 mg/dL). A right upper extremity x-ray was unremarkable. The patient was treated with cephalexin and colchicine for cellulitis and possible acute gout.
Three days after the ED visit, the patient presented to his primary care clinic, reporting adherence to the prescribed therapies (cephalexin and colchicine) but no improvement in symptoms. He was again afebrile, and his blood pressure was controlled to goal (118/80 mm Hg). On exam, he had significant nonpitting, unilateral edema extending from the elbow through the fingers without erythema, warmth, or rash (FIGURE). A right upper extremity ultrasound was obtained; results were negative for deep vein thrombosis.

Medication reconciliation completed during the clinic visit revealed that the patient had started and continued to take newly prescribed medications for the treatment of heart failure, including metoprolol succinate, lisinopril, and furosemide. The patient confirmed that these were started 7 days prior to symptom onset.
THE DIAGNOSIS
Given the clinical resemblance to angioedema and the recent initiation of lisinopril, the patient was asked to hold this medication. He was also advised to discontinue the cephalexin and colchicine, given low suspicion for cellulitis and gout. Six days later, he returned to clinic and reported significantly improved pain and swelling.
DISCUSSION
Angioedema is a common condition in the United States, affecting approximately 15% of the general population.1 When associated with hypotension, respiratory compromise, and other end-organ dysfunction, it is treated as anaphylaxis. Angioedema without anaphylaxis can be categorized as either histaminergic or nonhistaminergic; the former is more common.2
Certain patient and disease characteristics are more prevalent in select subsets of angioedema, although there are no features that automatically identify an etiology. Here are some factors to consider:
Recent exposures. Within the histaminergic category, allergic angioedema has the longest list of potential causes, including medications (notably, antibiotics, nonsteroidal anti-inflammatory drugs, opiates, and perioperative medications), foods, latex, and insect stings and/or bites.2 Nonhistaminergic subtypes, which include hereditary and acquired angioedema, are caused by deficiencies or mutations in complement or coagulation pathways, which can be more challenging to diagnose.
Continue to: Acquired angioedema may also...
Acquired angioedema may also be associated with the use of angiotensin-converting enzyme (ACE) inhibitors. Risk factors for ACE inhibitor–induced angioedema include history of smoking, increasing age, and female gender.3 African-American race has been correlated with increased incidence of angioedema, with rates 4 to 5 times that of Whites,1 but race is now identified as a social and not a biological construct and should not be relied on to make medical decisions about prescribing.
The rate of occurrence for ACE inhibitor–induced angioedema is highest within the first 30 days of medication use2; however, it can occur anytime. The absolute risk has been estimated as 0.3% per year.4
Patient age. Histaminergic angioedema can occur at any age. The hereditary subtype of nonhistaminergic angioedema is more common in younger individuals, typically occurring in infancy to the second decade of life, and tends to run in families, while the acquired subtype often manifests in adults older than 40.2
Physical exam findings. The typical manifestation of nonhistaminergic angioedema is firm, nonpitting, nonpruritic swelling resulting from fluid shifts to the reticular dermis and subcutaneous or submucosal tissue. In comparison, histaminergic reactions commonly involve deeper dermal tissue.
Commonly affected anatomic sites also vary by angioedema type but do not directly distinguish a cause. Allergic and ACE inhibitor–induced subtypes more commonly involve the lips, tongue, larynx, and face, whereas hereditary and other acquired etiologies are more likely to affect the periphery, abdomen, face, larynx, and genitourinary systems.2 So the way that this patient presented was a bit unusual.
Continue to: Symptom history
Symptom history. Allergic angioedema often has a rapid onset and resolution, whereas hereditary and acquired subtypes appear more gradually.2 While the presence of urticaria distinguishes a histaminergic reaction, both histaminergic and nonhistaminergic angioedema may manifest without this symptom.
In our patient, the timeline of gradual symptom manifestation and the physical exam findings, as well as the patient’s age, tobacco history, and recent initiation of an ACE inhibitor, made acquired angioedema a more likely etiology.
Treatment for ACE inhibitor–induced angioedema, in addition to airway support, entails drug discontinuation. This typically leads to symptom resolution within 24 to 48 hours.2 Treatment with corticosteroids, antihistamines, and epinephrine is usually ineffective. Switching to an alternative ACE inhibitor is not recommended, as other members of the class carry the same risk. Instead, angiotensin receptor blockers (ARBs) are an appropriate substitute, as the incidence of cross-reactivity in ACE inhibitor–intolerant patients is estimated to be 10% or less,5 and the risk for recurrence has been shown to be no different than with placebo.3,4
Our patient was transitioned to losartan 25 mg/d without recurrence of his symptoms and with continued blood pressure control (125/60 mm Hg).
THE TAKEAWAY
Angioedema is a common condition. While many medications are associated with histaminergic angioedema, ACE inhibitors are a common cause of the acquired subtype of nonhistaminergic angioedema. Commonly affected sites include the lips, tongue, and face; however, this diagnosis is not dependent on location and may manifest at other sites, as seen in this case. Treatment involves medication discontinuation. When switching the patient’s medication, other members of the ACE inhibitor class should be avoided. ARBs are an appropriate alternative without increased risk for recurrence.
CORRESPONDENCE
Katherine Montag Schafer, University of Minnesota— Department of Family Medicine and Community Health, 1414 Maryland Avenue E, St Paul, MN 55106; [email protected]
THE CASE
A 67-year-old man with a history of gout, tobacco use, hypertension, hyperlipidemia, prediabetes, and newly diagnosed heart failure with reduced ejection fraction presented with a new concern for sudden-onset, atraumatic right upper extremity pain and swelling. The patient had awakened with these symptoms and on the following day went to the emergency department (ED) for evaluation. Review of the ED documentation highlighted that the patient was afebrile and was found to have a slight leukocytosis (11.7 x 103/µL) and an elevated C-reactive protein level (4 mg/dL; normal range, 0.3 to 1 mg/dL). A right upper extremity x-ray was unremarkable. The patient was treated with cephalexin and colchicine for cellulitis and possible acute gout.
Three days after the ED visit, the patient presented to his primary care clinic, reporting adherence to the prescribed therapies (cephalexin and colchicine) but no improvement in symptoms. He was again afebrile, and his blood pressure was controlled to goal (118/80 mm Hg). On exam, he had significant nonpitting, unilateral edema extending from the elbow through the fingers without erythema, warmth, or rash (FIGURE). A right upper extremity ultrasound was obtained; results were negative for deep vein thrombosis.

Medication reconciliation completed during the clinic visit revealed that the patient had started and continued to take newly prescribed medications for the treatment of heart failure, including metoprolol succinate, lisinopril, and furosemide. The patient confirmed that these were started 7 days prior to symptom onset.
THE DIAGNOSIS
Given the clinical resemblance to angioedema and the recent initiation of lisinopril, the patient was asked to hold this medication. He was also advised to discontinue the cephalexin and colchicine, given low suspicion for cellulitis and gout. Six days later, he returned to clinic and reported significantly improved pain and swelling.
DISCUSSION
Angioedema is a common condition in the United States, affecting approximately 15% of the general population.1 When associated with hypotension, respiratory compromise, and other end-organ dysfunction, it is treated as anaphylaxis. Angioedema without anaphylaxis can be categorized as either histaminergic or nonhistaminergic; the former is more common.2
Certain patient and disease characteristics are more prevalent in select subsets of angioedema, although there are no features that automatically identify an etiology. Here are some factors to consider:
Recent exposures. Within the histaminergic category, allergic angioedema has the longest list of potential causes, including medications (notably, antibiotics, nonsteroidal anti-inflammatory drugs, opiates, and perioperative medications), foods, latex, and insect stings and/or bites.2 Nonhistaminergic subtypes, which include hereditary and acquired angioedema, are caused by deficiencies or mutations in complement or coagulation pathways, which can be more challenging to diagnose.
Continue to: Acquired angioedema may also...
Acquired angioedema may also be associated with the use of angiotensin-converting enzyme (ACE) inhibitors. Risk factors for ACE inhibitor–induced angioedema include history of smoking, increasing age, and female gender.3 African-American race has been correlated with increased incidence of angioedema, with rates 4 to 5 times that of Whites,1 but race is now identified as a social and not a biological construct and should not be relied on to make medical decisions about prescribing.
The rate of occurrence for ACE inhibitor–induced angioedema is highest within the first 30 days of medication use2; however, it can occur anytime. The absolute risk has been estimated as 0.3% per year.4
Patient age. Histaminergic angioedema can occur at any age. The hereditary subtype of nonhistaminergic angioedema is more common in younger individuals, typically occurring in infancy to the second decade of life, and tends to run in families, while the acquired subtype often manifests in adults older than 40.2
Physical exam findings. The typical manifestation of nonhistaminergic angioedema is firm, nonpitting, nonpruritic swelling resulting from fluid shifts to the reticular dermis and subcutaneous or submucosal tissue. In comparison, histaminergic reactions commonly involve deeper dermal tissue.
Commonly affected anatomic sites also vary by angioedema type but do not directly distinguish a cause. Allergic and ACE inhibitor–induced subtypes more commonly involve the lips, tongue, larynx, and face, whereas hereditary and other acquired etiologies are more likely to affect the periphery, abdomen, face, larynx, and genitourinary systems.2 So the way that this patient presented was a bit unusual.
Continue to: Symptom history
Symptom history. Allergic angioedema often has a rapid onset and resolution, whereas hereditary and acquired subtypes appear more gradually.2 While the presence of urticaria distinguishes a histaminergic reaction, both histaminergic and nonhistaminergic angioedema may manifest without this symptom.
In our patient, the timeline of gradual symptom manifestation and the physical exam findings, as well as the patient’s age, tobacco history, and recent initiation of an ACE inhibitor, made acquired angioedema a more likely etiology.
Treatment for ACE inhibitor–induced angioedema, in addition to airway support, entails drug discontinuation. This typically leads to symptom resolution within 24 to 48 hours.2 Treatment with corticosteroids, antihistamines, and epinephrine is usually ineffective. Switching to an alternative ACE inhibitor is not recommended, as other members of the class carry the same risk. Instead, angiotensin receptor blockers (ARBs) are an appropriate substitute, as the incidence of cross-reactivity in ACE inhibitor–intolerant patients is estimated to be 10% or less,5 and the risk for recurrence has been shown to be no different than with placebo.3,4
Our patient was transitioned to losartan 25 mg/d without recurrence of his symptoms and with continued blood pressure control (125/60 mm Hg).
THE TAKEAWAY
Angioedema is a common condition. While many medications are associated with histaminergic angioedema, ACE inhibitors are a common cause of the acquired subtype of nonhistaminergic angioedema. Commonly affected sites include the lips, tongue, and face; however, this diagnosis is not dependent on location and may manifest at other sites, as seen in this case. Treatment involves medication discontinuation. When switching the patient’s medication, other members of the ACE inhibitor class should be avoided. ARBs are an appropriate alternative without increased risk for recurrence.
CORRESPONDENCE
Katherine Montag Schafer, University of Minnesota— Department of Family Medicine and Community Health, 1414 Maryland Avenue E, St Paul, MN 55106; [email protected]
1. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121:282-286.
2. Moellman JJ, Bernstein JA, Lindsell CA, et al; American College of Allergy, Asthma & Immunology (ACAAI), Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21:469-484.
3. Zuraw BL, Bernstein JA, Lang DM, et al; American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491-1493.
4. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383-391.
5. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45:520-524.
1. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121:282-286.
2. Moellman JJ, Bernstein JA, Lindsell CA, et al; American College of Allergy, Asthma & Immunology (ACAAI), Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21:469-484.
3. Zuraw BL, Bernstein JA, Lang DM, et al; American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491-1493.
4. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383-391.
5. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45:520-524.
EMA panel backs peanut allergy desensitizing powder Palforzia
The product is intended for desensitizing children and adolescents to peanut allergy.
Palforzia will be available as an oral powder in capsules (0.5, 1, 10, 20, and 100 mg) and as oral powder in sachet (300 mg). The active substance is defatted powder of Arachis hypogaea.
Through use of the product, children with a peanut allergy receive controlled exposure to precise, increasing amounts of peanut protein, mixed with soft food, every day. Over time, this may help to decrease their sensitivity to small amounts of peanuts.
According to the press release from the EMA, Palforzia can mitigate accidental exposure to small amounts of peanut protein. “[A] single dose of a least 1 gram of peanut protein would cause no more than mild allergy symptoms,” the EMA said.
The treatment is indicated for patients aged 4 to 17 years who have received a confirmed diagnosis of peanut allergy. Treatment may be continued for patients aged 18 years or older, according to the press release.
It should be administered under the supervision of a healthcare provider qualified in the diagnosis and treatment of allergic diseases and should be used in conjunction with a peanut-avoidant diet, the EMA notes.
The most common side effects that have been reported are abdominal pain, throat irritation, itch, nausea, vomiting, urticaria, and upper abdominal discomfort.
The next step in the approval process is to obtain market authorization from the European Commission. Detailed recommendations for use will be described in the summary of product characteristics, which will be published in the European public assessment report and will be made available throughout Europe.
“We are encouraged by the CHMP opinion, which recommends Palforzia as the first and only treatment option in the European Union for patients with peanut allergy and their families,” Andrew Oxtoby, president and chief executive officer of Aimmune Therapeutics, said in a statement. “Today’s decision underscores the strong and compelling data from our Palforzia clinical trials and follows the US FDA approval of Palforzia earlier this year. We look forward to the European Commission’s final decision for the marketing approval of Palforzia, which we expect later this year.”
The FDA said in granting its approval that patients, parents, or caregivers must be counseled on the need for always-available injectable epinephrine, the need for continued peanut avoidance, and on how to recognize signs of anaphylaxis.
This article originally appeared on Medscape.com.
The product is intended for desensitizing children and adolescents to peanut allergy.
Palforzia will be available as an oral powder in capsules (0.5, 1, 10, 20, and 100 mg) and as oral powder in sachet (300 mg). The active substance is defatted powder of Arachis hypogaea.
Through use of the product, children with a peanut allergy receive controlled exposure to precise, increasing amounts of peanut protein, mixed with soft food, every day. Over time, this may help to decrease their sensitivity to small amounts of peanuts.
According to the press release from the EMA, Palforzia can mitigate accidental exposure to small amounts of peanut protein. “[A] single dose of a least 1 gram of peanut protein would cause no more than mild allergy symptoms,” the EMA said.
The treatment is indicated for patients aged 4 to 17 years who have received a confirmed diagnosis of peanut allergy. Treatment may be continued for patients aged 18 years or older, according to the press release.
It should be administered under the supervision of a healthcare provider qualified in the diagnosis and treatment of allergic diseases and should be used in conjunction with a peanut-avoidant diet, the EMA notes.
The most common side effects that have been reported are abdominal pain, throat irritation, itch, nausea, vomiting, urticaria, and upper abdominal discomfort.
The next step in the approval process is to obtain market authorization from the European Commission. Detailed recommendations for use will be described in the summary of product characteristics, which will be published in the European public assessment report and will be made available throughout Europe.
“We are encouraged by the CHMP opinion, which recommends Palforzia as the first and only treatment option in the European Union for patients with peanut allergy and their families,” Andrew Oxtoby, president and chief executive officer of Aimmune Therapeutics, said in a statement. “Today’s decision underscores the strong and compelling data from our Palforzia clinical trials and follows the US FDA approval of Palforzia earlier this year. We look forward to the European Commission’s final decision for the marketing approval of Palforzia, which we expect later this year.”
The FDA said in granting its approval that patients, parents, or caregivers must be counseled on the need for always-available injectable epinephrine, the need for continued peanut avoidance, and on how to recognize signs of anaphylaxis.
This article originally appeared on Medscape.com.
The product is intended for desensitizing children and adolescents to peanut allergy.
Palforzia will be available as an oral powder in capsules (0.5, 1, 10, 20, and 100 mg) and as oral powder in sachet (300 mg). The active substance is defatted powder of Arachis hypogaea.
Through use of the product, children with a peanut allergy receive controlled exposure to precise, increasing amounts of peanut protein, mixed with soft food, every day. Over time, this may help to decrease their sensitivity to small amounts of peanuts.
According to the press release from the EMA, Palforzia can mitigate accidental exposure to small amounts of peanut protein. “[A] single dose of a least 1 gram of peanut protein would cause no more than mild allergy symptoms,” the EMA said.
The treatment is indicated for patients aged 4 to 17 years who have received a confirmed diagnosis of peanut allergy. Treatment may be continued for patients aged 18 years or older, according to the press release.
It should be administered under the supervision of a healthcare provider qualified in the diagnosis and treatment of allergic diseases and should be used in conjunction with a peanut-avoidant diet, the EMA notes.
The most common side effects that have been reported are abdominal pain, throat irritation, itch, nausea, vomiting, urticaria, and upper abdominal discomfort.
The next step in the approval process is to obtain market authorization from the European Commission. Detailed recommendations for use will be described in the summary of product characteristics, which will be published in the European public assessment report and will be made available throughout Europe.
“We are encouraged by the CHMP opinion, which recommends Palforzia as the first and only treatment option in the European Union for patients with peanut allergy and their families,” Andrew Oxtoby, president and chief executive officer of Aimmune Therapeutics, said in a statement. “Today’s decision underscores the strong and compelling data from our Palforzia clinical trials and follows the US FDA approval of Palforzia earlier this year. We look forward to the European Commission’s final decision for the marketing approval of Palforzia, which we expect later this year.”
The FDA said in granting its approval that patients, parents, or caregivers must be counseled on the need for always-available injectable epinephrine, the need for continued peanut avoidance, and on how to recognize signs of anaphylaxis.
This article originally appeared on Medscape.com.
Can this patient get IV contrast?
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.