User login
Anakinra treatment for pediatric ‘cytokine storms’: Does one size fit all?
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
FROM ARTHRITIS & RHEUMATOLOGY
Does this patient have bacterial conjunctivitis?
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
Emapalumab produces major responses in macrophage activation syndrome
MADRID – Initial results of an ongoing small pilot study of the anti-interferon gamma (IFN-gamma) monoclonal antibody emapalumab has demonstrated efficacy in the treatment of glucocorticoid-refractory macrophage activation syndrome (MAS) in patients with systemic juvenile idiopathic arthritis (SJIA).
“By week 4 there was a complete response in all six patients treated. All of them had failed conventional therapy,” Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology at IRCCS Ospedale Pediatrico Bambino Gesù, Rome, reported at the European Congress of Rheumatology.
The results are the first of a multicenter, pilot study with twin protocols in Europe and North America. Emapalumab (Gamifant) is already approved by the Food and Drug Administration for the treatment of primary hemophagocytic lymphohistiocytosis (HLH) unresponsive to conventional therapy.
The study is enrolling children with MAS complicating SJIA that is unresponsive to high-dose intravenous glucocorticoids. Emapalumab, which has been shown to neutralize IFN-gamma in animal models, is being administered in an initial dose of 6 mg/kg followed by doses of 3 mg/kg every 3 days for 4 weeks.
MAS is a common complication of rheumatic diseases, particularly SJIA, according to Dr. De Benedetti. It has been characterized as a secondary form of HLH involving an excessive activation and expansion of macrophages as well as T cells. It can produce a wide variety of complications, including hepatosplenomegaly, liver dysfunction, and coagulation abnormalities. If uncontrolled, it can lead to organ failure and death.
Among the first six patients, four had confirmed SJIA and two had presumptive SJIA. The average age was 11 years with a range of 2 to 25 years. Four of the patients were female.
Many of the patients had failed therapies in addition to glucocorticoids, such as cyclosporine and anakinra. A diagnosis of HLH and prior treatment with a biologic therapy were exclusion criteria.
By 8 weeks, six had a complete response, which included the resolution of symptoms by normalization of ferritin, liver enzymes, and D-dimers. In three of the six patients, a complete response was achieved by week 4.
“Steroid tapering by investigator discretion was permitted, and four of the six patients had a meaningful tapering of steroids within 8 weeks,” Dr. De Benedetti reported.
Of the three serious adverse events recorded so far, only reactivation of cytomegalovirus (CMV) infection was attributed to emapalumab. This infection resolved with treatment. Several other infections observed over the course of the study were not thought to be related to treatment.
The initial results have encouraged an expansion of the study protocol in Europe where several treatment centers are expected to begin enrolling patients shortly. A second parallel study protocol will begin soon in North America, but no patient had been treated at the time that Dr. De Benedetti presented these initial findings.
Based on evidence that IFN-gamma drives hyperinflammation and hypercytokinemia in MAS, the initial results with emapalumab are encouraging, according to Dr. De Benedetti. He said the results not only provide evidence that emapalumab is active in MAS but support the pathogenic role of IFN-gamma in this disease.
Dr. De Benedetti reported financial relationships with multiple pharmaceutical companies, including SOBI, the sponsor of this study.
SOURCE: De Benedetti F et al. Ann Rheum Dis. Jun 2019;78(Suppl2):178. Abstract OPO204, doi: 10.1136/annrheumdis-2019-eular.3341.
MADRID – Initial results of an ongoing small pilot study of the anti-interferon gamma (IFN-gamma) monoclonal antibody emapalumab has demonstrated efficacy in the treatment of glucocorticoid-refractory macrophage activation syndrome (MAS) in patients with systemic juvenile idiopathic arthritis (SJIA).
“By week 4 there was a complete response in all six patients treated. All of them had failed conventional therapy,” Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology at IRCCS Ospedale Pediatrico Bambino Gesù, Rome, reported at the European Congress of Rheumatology.
The results are the first of a multicenter, pilot study with twin protocols in Europe and North America. Emapalumab (Gamifant) is already approved by the Food and Drug Administration for the treatment of primary hemophagocytic lymphohistiocytosis (HLH) unresponsive to conventional therapy.
The study is enrolling children with MAS complicating SJIA that is unresponsive to high-dose intravenous glucocorticoids. Emapalumab, which has been shown to neutralize IFN-gamma in animal models, is being administered in an initial dose of 6 mg/kg followed by doses of 3 mg/kg every 3 days for 4 weeks.
MAS is a common complication of rheumatic diseases, particularly SJIA, according to Dr. De Benedetti. It has been characterized as a secondary form of HLH involving an excessive activation and expansion of macrophages as well as T cells. It can produce a wide variety of complications, including hepatosplenomegaly, liver dysfunction, and coagulation abnormalities. If uncontrolled, it can lead to organ failure and death.
Among the first six patients, four had confirmed SJIA and two had presumptive SJIA. The average age was 11 years with a range of 2 to 25 years. Four of the patients were female.
Many of the patients had failed therapies in addition to glucocorticoids, such as cyclosporine and anakinra. A diagnosis of HLH and prior treatment with a biologic therapy were exclusion criteria.
By 8 weeks, six had a complete response, which included the resolution of symptoms by normalization of ferritin, liver enzymes, and D-dimers. In three of the six patients, a complete response was achieved by week 4.
“Steroid tapering by investigator discretion was permitted, and four of the six patients had a meaningful tapering of steroids within 8 weeks,” Dr. De Benedetti reported.
Of the three serious adverse events recorded so far, only reactivation of cytomegalovirus (CMV) infection was attributed to emapalumab. This infection resolved with treatment. Several other infections observed over the course of the study were not thought to be related to treatment.
The initial results have encouraged an expansion of the study protocol in Europe where several treatment centers are expected to begin enrolling patients shortly. A second parallel study protocol will begin soon in North America, but no patient had been treated at the time that Dr. De Benedetti presented these initial findings.
Based on evidence that IFN-gamma drives hyperinflammation and hypercytokinemia in MAS, the initial results with emapalumab are encouraging, according to Dr. De Benedetti. He said the results not only provide evidence that emapalumab is active in MAS but support the pathogenic role of IFN-gamma in this disease.
Dr. De Benedetti reported financial relationships with multiple pharmaceutical companies, including SOBI, the sponsor of this study.
SOURCE: De Benedetti F et al. Ann Rheum Dis. Jun 2019;78(Suppl2):178. Abstract OPO204, doi: 10.1136/annrheumdis-2019-eular.3341.
MADRID – Initial results of an ongoing small pilot study of the anti-interferon gamma (IFN-gamma) monoclonal antibody emapalumab has demonstrated efficacy in the treatment of glucocorticoid-refractory macrophage activation syndrome (MAS) in patients with systemic juvenile idiopathic arthritis (SJIA).
“By week 4 there was a complete response in all six patients treated. All of them had failed conventional therapy,” Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology at IRCCS Ospedale Pediatrico Bambino Gesù, Rome, reported at the European Congress of Rheumatology.
The results are the first of a multicenter, pilot study with twin protocols in Europe and North America. Emapalumab (Gamifant) is already approved by the Food and Drug Administration for the treatment of primary hemophagocytic lymphohistiocytosis (HLH) unresponsive to conventional therapy.
The study is enrolling children with MAS complicating SJIA that is unresponsive to high-dose intravenous glucocorticoids. Emapalumab, which has been shown to neutralize IFN-gamma in animal models, is being administered in an initial dose of 6 mg/kg followed by doses of 3 mg/kg every 3 days for 4 weeks.
MAS is a common complication of rheumatic diseases, particularly SJIA, according to Dr. De Benedetti. It has been characterized as a secondary form of HLH involving an excessive activation and expansion of macrophages as well as T cells. It can produce a wide variety of complications, including hepatosplenomegaly, liver dysfunction, and coagulation abnormalities. If uncontrolled, it can lead to organ failure and death.
Among the first six patients, four had confirmed SJIA and two had presumptive SJIA. The average age was 11 years with a range of 2 to 25 years. Four of the patients were female.
Many of the patients had failed therapies in addition to glucocorticoids, such as cyclosporine and anakinra. A diagnosis of HLH and prior treatment with a biologic therapy were exclusion criteria.
By 8 weeks, six had a complete response, which included the resolution of symptoms by normalization of ferritin, liver enzymes, and D-dimers. In three of the six patients, a complete response was achieved by week 4.
“Steroid tapering by investigator discretion was permitted, and four of the six patients had a meaningful tapering of steroids within 8 weeks,” Dr. De Benedetti reported.
Of the three serious adverse events recorded so far, only reactivation of cytomegalovirus (CMV) infection was attributed to emapalumab. This infection resolved with treatment. Several other infections observed over the course of the study were not thought to be related to treatment.
The initial results have encouraged an expansion of the study protocol in Europe where several treatment centers are expected to begin enrolling patients shortly. A second parallel study protocol will begin soon in North America, but no patient had been treated at the time that Dr. De Benedetti presented these initial findings.
Based on evidence that IFN-gamma drives hyperinflammation and hypercytokinemia in MAS, the initial results with emapalumab are encouraging, according to Dr. De Benedetti. He said the results not only provide evidence that emapalumab is active in MAS but support the pathogenic role of IFN-gamma in this disease.
Dr. De Benedetti reported financial relationships with multiple pharmaceutical companies, including SOBI, the sponsor of this study.
SOURCE: De Benedetti F et al. Ann Rheum Dis. Jun 2019;78(Suppl2):178. Abstract OPO204, doi: 10.1136/annrheumdis-2019-eular.3341.
REPORTING FROM EULAR 2019 CONGRESS
Recombinant vaccine cut herpes zoster rate in immunocompromised patients
Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT), results of a randomized, placebo-controlled trial indicate.
The incidence of herpes zoster was 30 per 1,000 person-years for patients who received the adjuvanted recombinant zoster vaccine (Shingrix) versus 94 per 1,000 person-years for those who received placebo, according to study results.
Recombinant zoster vaccine induced humoral and cellular responses that were strong and occurring at a rate higher than what was seen in the placebo group, said senior author Keith M. Sullivan, MD, of Duke University Medical Center, Durham, N.C., and coauthors, who reported findings on behalf of the Zoster Efficacy Study in Patients Undergoing HSCT (ZOE-HSCT) Study Group.
“The vaccinations were generally well tolerated, and most symptoms were mild and transient and did not substantially deter participants from receiving their second dose,” Dr. Sullivan and colleagues wrote in JAMA.
The risk of herpes zoster is increased for 2-3 years after autologous HSCT because of diminished T-cell immunity, according to the authors.
“Antiviral prophylaxis is commonly administered to patients after HSCT to prevent such complications, but the efficacy depends on adherence to treatment,” they said.
While vaccines could provide long-term protection, immunocompromised individuals receiving live attenuated vaccine would be at increased risk of varicella caused by spread of the vaccine strain, they added.
There have been a few encouraging recent studies of non-live vaccines in this setting, including one large phase 3 trial of a heat-inactivated varicella-zoster virus vaccine that showed patients undergoing autologous HSCT had a 63.8% estimated efficacy in preventing herpes zoster, investigators from that study said in The Lancet (2018 May 26;391[10135]:2116-27).
A phase 1/2a study of the adjuvanted recombinant zoster vaccine in patients undergoing HSCT demonstrated strong humoral and cell-mediated immunity responses, which provided the rationale for studying the vaccine further in the randomized ZOE-HSCT study, according to Dr. Sullivan and coauthors.
Their study included a total of 1,846 adults who had undergone autologous HSCT. They were randomized to receive two doses of the recombinant zoster vaccine, the first at 50-70 days after the procedure and the second 1-2 months later.
Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, which resulted in overall incidences of 30 and 94 per 1,000 person-years.
The incidence rate ratio of a first episode of herpes zoster was 0.36 for individuals receiving at least one dose, which authors said was equivalent to a vaccine efficacy of 63.7%.
That efficacy rate is “very similar” to the estimated efficacy reported for the heat-inactivated varicella-zoster virus vaccine reported in The Lancet, said Dr. Sullivan and coauthors.
However, the heat-inactivated vaccine achieved that level of protection with a four-dose schedule, including one dose given prior to autologous HSCT.
“An advantage of the short 2-dose posttransplantation schedule is that more patients might complete the vaccination program,” they said in a discussion of the results, noting that 94.7% of the recombinant zoster vaccine recipients completed two doses, compared with 81.9% of recipients who received the heat-inactivated herpes zoster vaccine in the previous report.
The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Dr. Sullivan reported disclosures related to GlaxoSmithKline (GSK), Kiadis Pharmaceutical, Roche Genentech, and the National Institute of Allergy and Infectious Diseases. Coauthors provided disclosures related to GSK, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
SOURCE: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT), results of a randomized, placebo-controlled trial indicate.
The incidence of herpes zoster was 30 per 1,000 person-years for patients who received the adjuvanted recombinant zoster vaccine (Shingrix) versus 94 per 1,000 person-years for those who received placebo, according to study results.
Recombinant zoster vaccine induced humoral and cellular responses that were strong and occurring at a rate higher than what was seen in the placebo group, said senior author Keith M. Sullivan, MD, of Duke University Medical Center, Durham, N.C., and coauthors, who reported findings on behalf of the Zoster Efficacy Study in Patients Undergoing HSCT (ZOE-HSCT) Study Group.
“The vaccinations were generally well tolerated, and most symptoms were mild and transient and did not substantially deter participants from receiving their second dose,” Dr. Sullivan and colleagues wrote in JAMA.
The risk of herpes zoster is increased for 2-3 years after autologous HSCT because of diminished T-cell immunity, according to the authors.
“Antiviral prophylaxis is commonly administered to patients after HSCT to prevent such complications, but the efficacy depends on adherence to treatment,” they said.
While vaccines could provide long-term protection, immunocompromised individuals receiving live attenuated vaccine would be at increased risk of varicella caused by spread of the vaccine strain, they added.
There have been a few encouraging recent studies of non-live vaccines in this setting, including one large phase 3 trial of a heat-inactivated varicella-zoster virus vaccine that showed patients undergoing autologous HSCT had a 63.8% estimated efficacy in preventing herpes zoster, investigators from that study said in The Lancet (2018 May 26;391[10135]:2116-27).
A phase 1/2a study of the adjuvanted recombinant zoster vaccine in patients undergoing HSCT demonstrated strong humoral and cell-mediated immunity responses, which provided the rationale for studying the vaccine further in the randomized ZOE-HSCT study, according to Dr. Sullivan and coauthors.
Their study included a total of 1,846 adults who had undergone autologous HSCT. They were randomized to receive two doses of the recombinant zoster vaccine, the first at 50-70 days after the procedure and the second 1-2 months later.
Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, which resulted in overall incidences of 30 and 94 per 1,000 person-years.
The incidence rate ratio of a first episode of herpes zoster was 0.36 for individuals receiving at least one dose, which authors said was equivalent to a vaccine efficacy of 63.7%.
That efficacy rate is “very similar” to the estimated efficacy reported for the heat-inactivated varicella-zoster virus vaccine reported in The Lancet, said Dr. Sullivan and coauthors.
However, the heat-inactivated vaccine achieved that level of protection with a four-dose schedule, including one dose given prior to autologous HSCT.
“An advantage of the short 2-dose posttransplantation schedule is that more patients might complete the vaccination program,” they said in a discussion of the results, noting that 94.7% of the recombinant zoster vaccine recipients completed two doses, compared with 81.9% of recipients who received the heat-inactivated herpes zoster vaccine in the previous report.
The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Dr. Sullivan reported disclosures related to GlaxoSmithKline (GSK), Kiadis Pharmaceutical, Roche Genentech, and the National Institute of Allergy and Infectious Diseases. Coauthors provided disclosures related to GSK, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
SOURCE: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT), results of a randomized, placebo-controlled trial indicate.
The incidence of herpes zoster was 30 per 1,000 person-years for patients who received the adjuvanted recombinant zoster vaccine (Shingrix) versus 94 per 1,000 person-years for those who received placebo, according to study results.
Recombinant zoster vaccine induced humoral and cellular responses that were strong and occurring at a rate higher than what was seen in the placebo group, said senior author Keith M. Sullivan, MD, of Duke University Medical Center, Durham, N.C., and coauthors, who reported findings on behalf of the Zoster Efficacy Study in Patients Undergoing HSCT (ZOE-HSCT) Study Group.
“The vaccinations were generally well tolerated, and most symptoms were mild and transient and did not substantially deter participants from receiving their second dose,” Dr. Sullivan and colleagues wrote in JAMA.
The risk of herpes zoster is increased for 2-3 years after autologous HSCT because of diminished T-cell immunity, according to the authors.
“Antiviral prophylaxis is commonly administered to patients after HSCT to prevent such complications, but the efficacy depends on adherence to treatment,” they said.
While vaccines could provide long-term protection, immunocompromised individuals receiving live attenuated vaccine would be at increased risk of varicella caused by spread of the vaccine strain, they added.
There have been a few encouraging recent studies of non-live vaccines in this setting, including one large phase 3 trial of a heat-inactivated varicella-zoster virus vaccine that showed patients undergoing autologous HSCT had a 63.8% estimated efficacy in preventing herpes zoster, investigators from that study said in The Lancet (2018 May 26;391[10135]:2116-27).
A phase 1/2a study of the adjuvanted recombinant zoster vaccine in patients undergoing HSCT demonstrated strong humoral and cell-mediated immunity responses, which provided the rationale for studying the vaccine further in the randomized ZOE-HSCT study, according to Dr. Sullivan and coauthors.
Their study included a total of 1,846 adults who had undergone autologous HSCT. They were randomized to receive two doses of the recombinant zoster vaccine, the first at 50-70 days after the procedure and the second 1-2 months later.
Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, which resulted in overall incidences of 30 and 94 per 1,000 person-years.
The incidence rate ratio of a first episode of herpes zoster was 0.36 for individuals receiving at least one dose, which authors said was equivalent to a vaccine efficacy of 63.7%.
That efficacy rate is “very similar” to the estimated efficacy reported for the heat-inactivated varicella-zoster virus vaccine reported in The Lancet, said Dr. Sullivan and coauthors.
However, the heat-inactivated vaccine achieved that level of protection with a four-dose schedule, including one dose given prior to autologous HSCT.
“An advantage of the short 2-dose posttransplantation schedule is that more patients might complete the vaccination program,” they said in a discussion of the results, noting that 94.7% of the recombinant zoster vaccine recipients completed two doses, compared with 81.9% of recipients who received the heat-inactivated herpes zoster vaccine in the previous report.
The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Dr. Sullivan reported disclosures related to GlaxoSmithKline (GSK), Kiadis Pharmaceutical, Roche Genentech, and the National Institute of Allergy and Infectious Diseases. Coauthors provided disclosures related to GSK, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
SOURCE: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
FROM JAMA
Key clinical point: Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster versus placebo in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT).
Major finding: Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, resulting in overall incidences of 30 and 94 per 1,000 person-years.
Study details: A randomized clinical trial (ZOE-HSCT) including 1,846 adults who had undergone autologous HSCT.
Disclosures: The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Study authors reported disclosures related to GlaxoSmithKline, Kiadis Pharmaceutical, Roche Genentech, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
Source: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
Giant cell arteritis: An updated review of an old disease
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
KEY POINTS
- Giant cell arteritis can present with cranial symptoms, extracranial large-vessel involvement, or polymyalgia rheumatica.
- Temporal artery biopsy is the standard for diagnosis.
- Adverse effects of glucocorticoid treatment, particularly bone loss, need to be managed.
- In patients treated with glucocorticoids alone, the relapse rate is high when the drugs are tapered; thus, prolonged treatment is required.
Clinical trials: More to learn than the results
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
Allergy immunotherapy: Who, what, when … and how safe?
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).
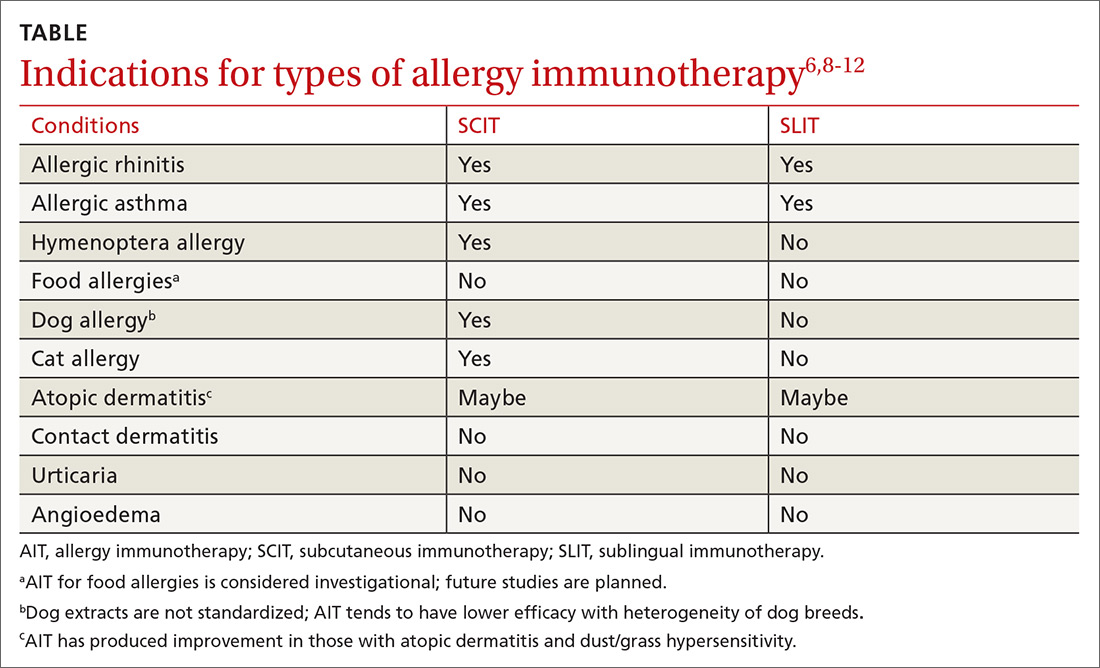
AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).

AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).

AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
PRACTICE RECOMMENDATIONS
› Diagnose allergies that are amenable to allergy immunotherapy (AIT) using skin prick/puncture allergy testing in conjunction with clinical symptoms, triggers, and exposure. A
› Do not use AIT for urticaria, angioedema, drug hypersensitivity, or latex allergy. A
› Do not initiate AIT during pregnancy or in patients with acquired immune deficiency syndrome or severe asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Correction: Subclinical hypothyroidism
In Azim S, Nasr C, “Subclinical hypothyroidism: When to treat,” Cleve Clin J Med 2019; 86(2):101–110, on page 103, in the section “Subclinical hypothyroidism can resolve or progress,” the sentence “The rate of progression to overt hypothyroidism is estimated to be 33% to 35% over 10 to 20 years of follow-up” contained an error. The correct rate of progression is 33% to 55%. This error has been corrected online.
In Azim S, Nasr C, “Subclinical hypothyroidism: When to treat,” Cleve Clin J Med 2019; 86(2):101–110, on page 103, in the section “Subclinical hypothyroidism can resolve or progress,” the sentence “The rate of progression to overt hypothyroidism is estimated to be 33% to 35% over 10 to 20 years of follow-up” contained an error. The correct rate of progression is 33% to 55%. This error has been corrected online.
In Azim S, Nasr C, “Subclinical hypothyroidism: When to treat,” Cleve Clin J Med 2019; 86(2):101–110, on page 103, in the section “Subclinical hypothyroidism can resolve or progress,” the sentence “The rate of progression to overt hypothyroidism is estimated to be 33% to 35% over 10 to 20 years of follow-up” contained an error. The correct rate of progression is 33% to 55%. This error has been corrected online.
Erythematous swollen ear
A 25-year-old woman presented with an exceedingly tender right ear. She’d had the helix of her ear pierced 3 days prior to presentation and 2 days after that, the ear had become tender. The tenderness was progressively worsening and associated with throbbing pain. The patient, who’d had her ears pierced before, was otherwise in good health and denied fever, chills, or travel outside of the country. She had been going to the gym regularly and took frequent showers. Physical examination revealed an erythematous swollen ear that was tender to the touch (FIGURE). The entire auricle was swollen except for the earlobe. The patient also reported purulent material draining from the helical piercing site.
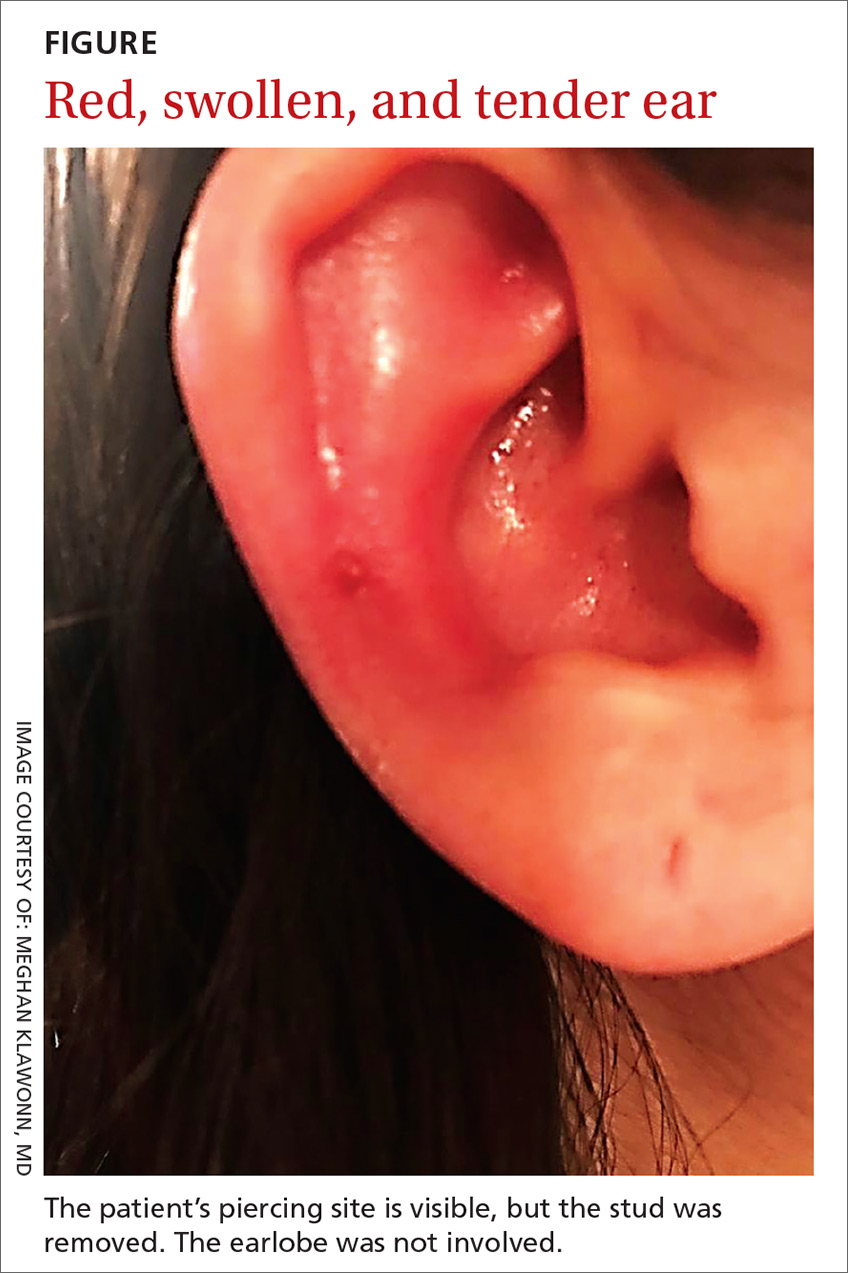
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Auricular perichondritis
Auricular perichondritis is an inflammation of the connective tissue surrounding the cartilage of the ear. Infectious and autoimmune factors may play a role. The underlying cartilage also may become involved. A useful clinical clue to the diagnosis of auricular perichondritis is sparing of the earlobe, which does not contain cartilage. Autoimmune causes typically have bilateral involvement. Infectious causes are usually associated with trauma and purulent drainage at the wound site. Ear piercings are an increasingly common cause, but perichondritis due to minor trauma, as a surgical complication, or in the absence of an obvious inciting trigger can occur. A careful history usually will reveal the cause.
In this case, the patient indicated that an open piercing gun at a shopping mall kiosk had been used to pierce her ear. Piercing with a sterile straight needle would have been preferable and less likely to be associated with secondary infection, as the shearing trauma to the perichondrium experienced with a piercing gun is thought to predispose to infection.1 Exposure to fresh water from the shower could have been a source for Pseudomonas infection.1
Differential: Pinpointing the diagnosis early is vital
A red and tender ear can raise a differential diagnosis that includes erysipelas, relapsing polychondritis, and auricular perichondritis. Erysipelas is a bacterial infection that spreads through the lymphatic system and is associated with intense and well-demarcated erythema. Erysipelas typically involves the face or lower legs. Infection after piercing or traumatic injury should raise suspicion of pseudomonal infection.2-5 Untreated infection can spread quickly and lead to permanent ear deformity. Although the same pattern of inflammation can be seen in relapsing polychondritis, relapsing polychondritis typically involves both ears as well as the eyes and joints.
Prompt treatment is necessary to avoid cosmetic disfigurement
The timing of the reaction in our patient made infection obvious because Pseudomonas aeruginosa seems to have a particular affinity for damaged cartilage.2
Ciprofloxacin 500 mg twice daily is the treatment of choice. Although many skin infections can be empirically treated with oral cephalosporin, penicillin, or erythromycin, it is important to recognize that infected piercing sites and auricular perichondritis due to pseudomonal infection will not respond to these agents. That’s because these agents do not provide as good coverage for Pseudomonas as they do for Staphylococci or other bacteria more often associated with skin infection. Treatment with an agent such as amoxicillin and clavulanic acid or oral cephalexin can mean the loss of valuable time and subsequent cosmetic disfigurement.6
Continue to: When fluctuance is present...
When fluctuance is present, incision and drainage, or even debridement, may be necessary. When extensive infection leads to cartilage necrosis and liquefaction, treatment is difficult and may result in lasting disfigurement. Prompt empiric treatment currently is considered the best option.6
Our patient was prescribed a course of ciprofloxacin 500 mg every 12 hours for 10 days. She noted improvement within 2 days, and the infection resolved without complication.
CORRESPONDENCE
Matthew F. Helm, MD, Penn State Health Hershey Medical Center, 500 University Dr, HU14, Hershey, PA 17033; [email protected]
1. Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health. 2007;98:74-77.
2. Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121:530-534.
3. Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204-209.
4. Lee TC, Gold WL. Necrotizing Pseudomonas chondritis after piercing of the upper ear. CMAJ. 2011;183:819-821.
5. Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by “high ear piercing”: a case report and review of the literature. J Oral Maxillofac Surg. 2008;66:543-546.
6. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127:505-508.
A 25-year-old woman presented with an exceedingly tender right ear. She’d had the helix of her ear pierced 3 days prior to presentation and 2 days after that, the ear had become tender. The tenderness was progressively worsening and associated with throbbing pain. The patient, who’d had her ears pierced before, was otherwise in good health and denied fever, chills, or travel outside of the country. She had been going to the gym regularly and took frequent showers. Physical examination revealed an erythematous swollen ear that was tender to the touch (FIGURE). The entire auricle was swollen except for the earlobe. The patient also reported purulent material draining from the helical piercing site.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Auricular perichondritis
Auricular perichondritis is an inflammation of the connective tissue surrounding the cartilage of the ear. Infectious and autoimmune factors may play a role. The underlying cartilage also may become involved. A useful clinical clue to the diagnosis of auricular perichondritis is sparing of the earlobe, which does not contain cartilage. Autoimmune causes typically have bilateral involvement. Infectious causes are usually associated with trauma and purulent drainage at the wound site. Ear piercings are an increasingly common cause, but perichondritis due to minor trauma, as a surgical complication, or in the absence of an obvious inciting trigger can occur. A careful history usually will reveal the cause.
In this case, the patient indicated that an open piercing gun at a shopping mall kiosk had been used to pierce her ear. Piercing with a sterile straight needle would have been preferable and less likely to be associated with secondary infection, as the shearing trauma to the perichondrium experienced with a piercing gun is thought to predispose to infection.1 Exposure to fresh water from the shower could have been a source for Pseudomonas infection.1
Differential: Pinpointing the diagnosis early is vital
A red and tender ear can raise a differential diagnosis that includes erysipelas, relapsing polychondritis, and auricular perichondritis. Erysipelas is a bacterial infection that spreads through the lymphatic system and is associated with intense and well-demarcated erythema. Erysipelas typically involves the face or lower legs. Infection after piercing or traumatic injury should raise suspicion of pseudomonal infection.2-5 Untreated infection can spread quickly and lead to permanent ear deformity. Although the same pattern of inflammation can be seen in relapsing polychondritis, relapsing polychondritis typically involves both ears as well as the eyes and joints.
Prompt treatment is necessary to avoid cosmetic disfigurement
The timing of the reaction in our patient made infection obvious because Pseudomonas aeruginosa seems to have a particular affinity for damaged cartilage.2
Ciprofloxacin 500 mg twice daily is the treatment of choice. Although many skin infections can be empirically treated with oral cephalosporin, penicillin, or erythromycin, it is important to recognize that infected piercing sites and auricular perichondritis due to pseudomonal infection will not respond to these agents. That’s because these agents do not provide as good coverage for Pseudomonas as they do for Staphylococci or other bacteria more often associated with skin infection. Treatment with an agent such as amoxicillin and clavulanic acid or oral cephalexin can mean the loss of valuable time and subsequent cosmetic disfigurement.6
Continue to: When fluctuance is present...
When fluctuance is present, incision and drainage, or even debridement, may be necessary. When extensive infection leads to cartilage necrosis and liquefaction, treatment is difficult and may result in lasting disfigurement. Prompt empiric treatment currently is considered the best option.6
Our patient was prescribed a course of ciprofloxacin 500 mg every 12 hours for 10 days. She noted improvement within 2 days, and the infection resolved without complication.
CORRESPONDENCE
Matthew F. Helm, MD, Penn State Health Hershey Medical Center, 500 University Dr, HU14, Hershey, PA 17033; [email protected]
A 25-year-old woman presented with an exceedingly tender right ear. She’d had the helix of her ear pierced 3 days prior to presentation and 2 days after that, the ear had become tender. The tenderness was progressively worsening and associated with throbbing pain. The patient, who’d had her ears pierced before, was otherwise in good health and denied fever, chills, or travel outside of the country. She had been going to the gym regularly and took frequent showers. Physical examination revealed an erythematous swollen ear that was tender to the touch (FIGURE). The entire auricle was swollen except for the earlobe. The patient also reported purulent material draining from the helical piercing site.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Auricular perichondritis
Auricular perichondritis is an inflammation of the connective tissue surrounding the cartilage of the ear. Infectious and autoimmune factors may play a role. The underlying cartilage also may become involved. A useful clinical clue to the diagnosis of auricular perichondritis is sparing of the earlobe, which does not contain cartilage. Autoimmune causes typically have bilateral involvement. Infectious causes are usually associated with trauma and purulent drainage at the wound site. Ear piercings are an increasingly common cause, but perichondritis due to minor trauma, as a surgical complication, or in the absence of an obvious inciting trigger can occur. A careful history usually will reveal the cause.
In this case, the patient indicated that an open piercing gun at a shopping mall kiosk had been used to pierce her ear. Piercing with a sterile straight needle would have been preferable and less likely to be associated with secondary infection, as the shearing trauma to the perichondrium experienced with a piercing gun is thought to predispose to infection.1 Exposure to fresh water from the shower could have been a source for Pseudomonas infection.1
Differential: Pinpointing the diagnosis early is vital
A red and tender ear can raise a differential diagnosis that includes erysipelas, relapsing polychondritis, and auricular perichondritis. Erysipelas is a bacterial infection that spreads through the lymphatic system and is associated with intense and well-demarcated erythema. Erysipelas typically involves the face or lower legs. Infection after piercing or traumatic injury should raise suspicion of pseudomonal infection.2-5 Untreated infection can spread quickly and lead to permanent ear deformity. Although the same pattern of inflammation can be seen in relapsing polychondritis, relapsing polychondritis typically involves both ears as well as the eyes and joints.
Prompt treatment is necessary to avoid cosmetic disfigurement
The timing of the reaction in our patient made infection obvious because Pseudomonas aeruginosa seems to have a particular affinity for damaged cartilage.2
Ciprofloxacin 500 mg twice daily is the treatment of choice. Although many skin infections can be empirically treated with oral cephalosporin, penicillin, or erythromycin, it is important to recognize that infected piercing sites and auricular perichondritis due to pseudomonal infection will not respond to these agents. That’s because these agents do not provide as good coverage for Pseudomonas as they do for Staphylococci or other bacteria more often associated with skin infection. Treatment with an agent such as amoxicillin and clavulanic acid or oral cephalexin can mean the loss of valuable time and subsequent cosmetic disfigurement.6
Continue to: When fluctuance is present...
When fluctuance is present, incision and drainage, or even debridement, may be necessary. When extensive infection leads to cartilage necrosis and liquefaction, treatment is difficult and may result in lasting disfigurement. Prompt empiric treatment currently is considered the best option.6
Our patient was prescribed a course of ciprofloxacin 500 mg every 12 hours for 10 days. She noted improvement within 2 days, and the infection resolved without complication.
CORRESPONDENCE
Matthew F. Helm, MD, Penn State Health Hershey Medical Center, 500 University Dr, HU14, Hershey, PA 17033; [email protected]
1. Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health. 2007;98:74-77.
2. Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121:530-534.
3. Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204-209.
4. Lee TC, Gold WL. Necrotizing Pseudomonas chondritis after piercing of the upper ear. CMAJ. 2011;183:819-821.
5. Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by “high ear piercing”: a case report and review of the literature. J Oral Maxillofac Surg. 2008;66:543-546.
6. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127:505-508.
1. Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health. 2007;98:74-77.
2. Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121:530-534.
3. Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204-209.
4. Lee TC, Gold WL. Necrotizing Pseudomonas chondritis after piercing of the upper ear. CMAJ. 2011;183:819-821.
5. Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by “high ear piercing”: a case report and review of the literature. J Oral Maxillofac Surg. 2008;66:543-546.
6. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127:505-508.
Pyoderma gangrenosum mistaken for diabetic ulcer
A 55-year-old man with type 2 diabetes mellitus, hypertension, anemia, and ulcerative colitis presented to the emergency department with an ulcer on his left leg (Figure 1). He said the lesion had started as a “large pimple” that ruptured one night while he was sleeping and then became drastically worse over the past week. He said the lesion was painful and was “oozing blood.”
On examination, the lesion was 7 cm by 6.5 cm, with fibrinous, necrotic tissue, purulence, and a violaceous tint at the borders. The patient’s body temperature was 100.5°F (38.1°C) and the white blood cell count was 8.1 x 109/L (reference range 4.0–11.0).
Based on the patient’s medical history, the lesion was initially diagnosed as an infected diabetic ulcer. He was admitted to the hospital and intravenous (IV) vancomycin and clindamycin were started. During this time, the lesion expanded in size, and a second lesion appeared on the right anterior thigh, in similar fashion to how the original lesion had started. The original lesion expanded to 8 cm by 8.5 cm by hospital day 2. The patient continued to have episodes of low-grade fever without leukocytosis.
Cultures of blood and tissue from the lesions were negative, ruling out bacterial infection. Magnetic resonance imaging of the left tibia was negative for osteomyelitis. Punch biopsy of the ulcer border was done on day 3 to evaluate for pyoderma gangrenosum.
On hospital day 5, the patient developed acute kidney injury, with a creatinine increase to 2.17 mg/dL over 24 hours from a baseline value of 0.82 mg/dL. The IV antibiotics were discontinued, and IV fluid hydration was started. At this time, diabetic ulcer secondary to infection and osteomyelitis were ruled out. The lesions were diagnosed as pyoderma gangrenosum.
The patient was started on prednisone 30 mg twice daily. After 2 days, the low-grade fevers resolved, both lesions began to heal, and his creatinine level returned to baseline (Figure 2). He was discharged on hospital day 10. The prednisone was tapered over 1 month, with wet-to-dry dressing changes for wound care.
After discharge, he remained adherent to his steroid regimen. At a follow-up visit to his dermatologist, the ulcers had fully closed, and the skin had begun to heal. Results of the punch biopsy study came back 2 days after the patient was discharged and further confirmed the diagnosis, with a mixed lymphocytic composition composed primarily of neutrophils.
APPROACH TO DIAGNOSIS
Pyoderma gangrenosum is rare, with an incidence of 3 to 10 cases per million people per year.1 It is a rapidly progressive ulcerative condition typically associated with inflammatory bowel disease.2 Despite its name, the condition involves neither gangrene nor infection. The ulcer typically appears on the legs and is rapidly growing, painful, and purulent, with tissue necrosis and a violaceous border.3
Pyoderma gangrenosum is often misdiagnosed as infective ulcer and inappropriately treated with antibiotics.2 It can also be mistreated with surgical debridement, which can result in severe complications such as pathergy.1
The differential diagnosis includes diabetic ulcer, peripheral vascular disease, vasculitis, bacterial infection, osteomyelitis, and malignancy. Because it presents as an open, necrotic ulcer, ruling out infection is a top priority.3 However, an initial workup to rule out infection or other conditions can delay diagnosis and treatment,1 and treatment with broad-spectrum antibiotics poses the risk of nephrotoxicity and new complications during the hospital stay.
Diagnosis requires meeting 2 major criteria—ie, presence of the characteristic ulcerous lesion, and exclusion of other causes of skin ulceration—and at least 2 minor criteria including histologic confirmation of neutrophil infiltrate at the ulcer border, the presence of a systemic disease associated with pyoderma gangrenosum, and a rapid response to steroid treatment.4,5
Our patient was at high risk for an infected diabetic ulcer. After infection was ruled out, clinical suspicion for pyoderma gangrenosum was high, given the patient’s presentation and his history of ulcerative colitis.
TREATMENT
Treatment of pyoderma gangrenosum begins with systemic corticosteroids, as was done in this patient. Additional measures depend on whether the disease is localized or extensive and can include wound care, topical treatments, immunosuppressants, and immunomodulators.1
- Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J 2012; 3(1):7–13. doi:10.4103/2229-5178.93482
- Marinopoulos S, Theofanakis C, Zacharouli T, Sotiropoulou M, Dimitrakakis C. Pyoderma gangrenosum of the breast: a case report study. Int J Surg Case Rep 2017; 31:203–205. doi:10.1016/j.ijscr.2017.01.036
- Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015; 8:285–293. doi:10.2147/CCID.S61202
- Su WP, David MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137(6):1000–1005. pmid:9470924
A 55-year-old man with type 2 diabetes mellitus, hypertension, anemia, and ulcerative colitis presented to the emergency department with an ulcer on his left leg (Figure 1). He said the lesion had started as a “large pimple” that ruptured one night while he was sleeping and then became drastically worse over the past week. He said the lesion was painful and was “oozing blood.”
On examination, the lesion was 7 cm by 6.5 cm, with fibrinous, necrotic tissue, purulence, and a violaceous tint at the borders. The patient’s body temperature was 100.5°F (38.1°C) and the white blood cell count was 8.1 x 109/L (reference range 4.0–11.0).
Based on the patient’s medical history, the lesion was initially diagnosed as an infected diabetic ulcer. He was admitted to the hospital and intravenous (IV) vancomycin and clindamycin were started. During this time, the lesion expanded in size, and a second lesion appeared on the right anterior thigh, in similar fashion to how the original lesion had started. The original lesion expanded to 8 cm by 8.5 cm by hospital day 2. The patient continued to have episodes of low-grade fever without leukocytosis.
Cultures of blood and tissue from the lesions were negative, ruling out bacterial infection. Magnetic resonance imaging of the left tibia was negative for osteomyelitis. Punch biopsy of the ulcer border was done on day 3 to evaluate for pyoderma gangrenosum.
On hospital day 5, the patient developed acute kidney injury, with a creatinine increase to 2.17 mg/dL over 24 hours from a baseline value of 0.82 mg/dL. The IV antibiotics were discontinued, and IV fluid hydration was started. At this time, diabetic ulcer secondary to infection and osteomyelitis were ruled out. The lesions were diagnosed as pyoderma gangrenosum.
The patient was started on prednisone 30 mg twice daily. After 2 days, the low-grade fevers resolved, both lesions began to heal, and his creatinine level returned to baseline (Figure 2). He was discharged on hospital day 10. The prednisone was tapered over 1 month, with wet-to-dry dressing changes for wound care.
After discharge, he remained adherent to his steroid regimen. At a follow-up visit to his dermatologist, the ulcers had fully closed, and the skin had begun to heal. Results of the punch biopsy study came back 2 days after the patient was discharged and further confirmed the diagnosis, with a mixed lymphocytic composition composed primarily of neutrophils.
APPROACH TO DIAGNOSIS
Pyoderma gangrenosum is rare, with an incidence of 3 to 10 cases per million people per year.1 It is a rapidly progressive ulcerative condition typically associated with inflammatory bowel disease.2 Despite its name, the condition involves neither gangrene nor infection. The ulcer typically appears on the legs and is rapidly growing, painful, and purulent, with tissue necrosis and a violaceous border.3
Pyoderma gangrenosum is often misdiagnosed as infective ulcer and inappropriately treated with antibiotics.2 It can also be mistreated with surgical debridement, which can result in severe complications such as pathergy.1
The differential diagnosis includes diabetic ulcer, peripheral vascular disease, vasculitis, bacterial infection, osteomyelitis, and malignancy. Because it presents as an open, necrotic ulcer, ruling out infection is a top priority.3 However, an initial workup to rule out infection or other conditions can delay diagnosis and treatment,1 and treatment with broad-spectrum antibiotics poses the risk of nephrotoxicity and new complications during the hospital stay.
Diagnosis requires meeting 2 major criteria—ie, presence of the characteristic ulcerous lesion, and exclusion of other causes of skin ulceration—and at least 2 minor criteria including histologic confirmation of neutrophil infiltrate at the ulcer border, the presence of a systemic disease associated with pyoderma gangrenosum, and a rapid response to steroid treatment.4,5
Our patient was at high risk for an infected diabetic ulcer. After infection was ruled out, clinical suspicion for pyoderma gangrenosum was high, given the patient’s presentation and his history of ulcerative colitis.
TREATMENT
Treatment of pyoderma gangrenosum begins with systemic corticosteroids, as was done in this patient. Additional measures depend on whether the disease is localized or extensive and can include wound care, topical treatments, immunosuppressants, and immunomodulators.1
A 55-year-old man with type 2 diabetes mellitus, hypertension, anemia, and ulcerative colitis presented to the emergency department with an ulcer on his left leg (Figure 1). He said the lesion had started as a “large pimple” that ruptured one night while he was sleeping and then became drastically worse over the past week. He said the lesion was painful and was “oozing blood.”
On examination, the lesion was 7 cm by 6.5 cm, with fibrinous, necrotic tissue, purulence, and a violaceous tint at the borders. The patient’s body temperature was 100.5°F (38.1°C) and the white blood cell count was 8.1 x 109/L (reference range 4.0–11.0).
Based on the patient’s medical history, the lesion was initially diagnosed as an infected diabetic ulcer. He was admitted to the hospital and intravenous (IV) vancomycin and clindamycin were started. During this time, the lesion expanded in size, and a second lesion appeared on the right anterior thigh, in similar fashion to how the original lesion had started. The original lesion expanded to 8 cm by 8.5 cm by hospital day 2. The patient continued to have episodes of low-grade fever without leukocytosis.
Cultures of blood and tissue from the lesions were negative, ruling out bacterial infection. Magnetic resonance imaging of the left tibia was negative for osteomyelitis. Punch biopsy of the ulcer border was done on day 3 to evaluate for pyoderma gangrenosum.
On hospital day 5, the patient developed acute kidney injury, with a creatinine increase to 2.17 mg/dL over 24 hours from a baseline value of 0.82 mg/dL. The IV antibiotics were discontinued, and IV fluid hydration was started. At this time, diabetic ulcer secondary to infection and osteomyelitis were ruled out. The lesions were diagnosed as pyoderma gangrenosum.
The patient was started on prednisone 30 mg twice daily. After 2 days, the low-grade fevers resolved, both lesions began to heal, and his creatinine level returned to baseline (Figure 2). He was discharged on hospital day 10. The prednisone was tapered over 1 month, with wet-to-dry dressing changes for wound care.
After discharge, he remained adherent to his steroid regimen. At a follow-up visit to his dermatologist, the ulcers had fully closed, and the skin had begun to heal. Results of the punch biopsy study came back 2 days after the patient was discharged and further confirmed the diagnosis, with a mixed lymphocytic composition composed primarily of neutrophils.
APPROACH TO DIAGNOSIS
Pyoderma gangrenosum is rare, with an incidence of 3 to 10 cases per million people per year.1 It is a rapidly progressive ulcerative condition typically associated with inflammatory bowel disease.2 Despite its name, the condition involves neither gangrene nor infection. The ulcer typically appears on the legs and is rapidly growing, painful, and purulent, with tissue necrosis and a violaceous border.3
Pyoderma gangrenosum is often misdiagnosed as infective ulcer and inappropriately treated with antibiotics.2 It can also be mistreated with surgical debridement, which can result in severe complications such as pathergy.1
The differential diagnosis includes diabetic ulcer, peripheral vascular disease, vasculitis, bacterial infection, osteomyelitis, and malignancy. Because it presents as an open, necrotic ulcer, ruling out infection is a top priority.3 However, an initial workup to rule out infection or other conditions can delay diagnosis and treatment,1 and treatment with broad-spectrum antibiotics poses the risk of nephrotoxicity and new complications during the hospital stay.
Diagnosis requires meeting 2 major criteria—ie, presence of the characteristic ulcerous lesion, and exclusion of other causes of skin ulceration—and at least 2 minor criteria including histologic confirmation of neutrophil infiltrate at the ulcer border, the presence of a systemic disease associated with pyoderma gangrenosum, and a rapid response to steroid treatment.4,5
Our patient was at high risk for an infected diabetic ulcer. After infection was ruled out, clinical suspicion for pyoderma gangrenosum was high, given the patient’s presentation and his history of ulcerative colitis.
TREATMENT
Treatment of pyoderma gangrenosum begins with systemic corticosteroids, as was done in this patient. Additional measures depend on whether the disease is localized or extensive and can include wound care, topical treatments, immunosuppressants, and immunomodulators.1
- Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J 2012; 3(1):7–13. doi:10.4103/2229-5178.93482
- Marinopoulos S, Theofanakis C, Zacharouli T, Sotiropoulou M, Dimitrakakis C. Pyoderma gangrenosum of the breast: a case report study. Int J Surg Case Rep 2017; 31:203–205. doi:10.1016/j.ijscr.2017.01.036
- Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015; 8:285–293. doi:10.2147/CCID.S61202
- Su WP, David MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137(6):1000–1005. pmid:9470924
- Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J 2012; 3(1):7–13. doi:10.4103/2229-5178.93482
- Marinopoulos S, Theofanakis C, Zacharouli T, Sotiropoulou M, Dimitrakakis C. Pyoderma gangrenosum of the breast: a case report study. Int J Surg Case Rep 2017; 31:203–205. doi:10.1016/j.ijscr.2017.01.036
- Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015; 8:285–293. doi:10.2147/CCID.S61202
- Su WP, David MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137(6):1000–1005. pmid:9470924