User login
Benlysta approved for children with SLE
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
Childhood-onset SLE rate doubles in children born in winter
SAN FRANCISCO – Simone Appenzeller, MD, PhD, reported at an international congress on systemic lupus erythematosus.
She presented a cross-sectional study of 760 consecutive SLE patients seen in a university lupus clinic and 700 healthy controls. Ninety-eight of the lupus patients had childhood-onset disease that began no later than age 16 years, while 662 had adult-onset SLE.
Southeastern Brazil, where the study was conducted, features a mostly subtropical climate with relatively mild winters. Because it is in the southern hemisphere, winter lasts from June 21 to September 21, while spring runs from September 22 to December 20.
Forty-six percent of subjects with childhood-onset SLE were born in winter, 17% in spring, another 17% in summer, and just over 19% in autumn, according to Dr. Appenzeller, a rheumatologist at the University of Campinas (Brazil).
In contrast, the occurrence of adult-onset SLE showed no variation by birth month or season.
The birth disparity between childhood- and adult-onset SLE was greatest in August, the depth of Brazilian winter, when 15.3% of all subjects with childhood-onset SLE were born, compared with 9% of adult-onset SLE patients and 8% of healthy controls.
The explanation for the increased likelihood of patients with childhood-onset SLE to be born in the winter months probably involves a gene-environment interaction, Dr. Appenzeller said. The most likely environmental factors are low seasonal maternal vitamin D levels and/or exposure to winter respiratory infections. The existence of a genetic component to the birth month disparity is suggested by another study by the Brazilian investigators in which they determined that the prevalence of SLE in both the first- and second-degree relatives of individuals with childhood-onset SLE was significantly higher than in patients with adult-onset disease.
Dr. Appenzeller reported having no financial conflicts regarding her study, funded by the Brazilian National Council for Scientific and Technological Development and other noncommercial research organizations.
SAN FRANCISCO – Simone Appenzeller, MD, PhD, reported at an international congress on systemic lupus erythematosus.
She presented a cross-sectional study of 760 consecutive SLE patients seen in a university lupus clinic and 700 healthy controls. Ninety-eight of the lupus patients had childhood-onset disease that began no later than age 16 years, while 662 had adult-onset SLE.
Southeastern Brazil, where the study was conducted, features a mostly subtropical climate with relatively mild winters. Because it is in the southern hemisphere, winter lasts from June 21 to September 21, while spring runs from September 22 to December 20.
Forty-six percent of subjects with childhood-onset SLE were born in winter, 17% in spring, another 17% in summer, and just over 19% in autumn, according to Dr. Appenzeller, a rheumatologist at the University of Campinas (Brazil).
In contrast, the occurrence of adult-onset SLE showed no variation by birth month or season.
The birth disparity between childhood- and adult-onset SLE was greatest in August, the depth of Brazilian winter, when 15.3% of all subjects with childhood-onset SLE were born, compared with 9% of adult-onset SLE patients and 8% of healthy controls.
The explanation for the increased likelihood of patients with childhood-onset SLE to be born in the winter months probably involves a gene-environment interaction, Dr. Appenzeller said. The most likely environmental factors are low seasonal maternal vitamin D levels and/or exposure to winter respiratory infections. The existence of a genetic component to the birth month disparity is suggested by another study by the Brazilian investigators in which they determined that the prevalence of SLE in both the first- and second-degree relatives of individuals with childhood-onset SLE was significantly higher than in patients with adult-onset disease.
Dr. Appenzeller reported having no financial conflicts regarding her study, funded by the Brazilian National Council for Scientific and Technological Development and other noncommercial research organizations.
SAN FRANCISCO – Simone Appenzeller, MD, PhD, reported at an international congress on systemic lupus erythematosus.
She presented a cross-sectional study of 760 consecutive SLE patients seen in a university lupus clinic and 700 healthy controls. Ninety-eight of the lupus patients had childhood-onset disease that began no later than age 16 years, while 662 had adult-onset SLE.
Southeastern Brazil, where the study was conducted, features a mostly subtropical climate with relatively mild winters. Because it is in the southern hemisphere, winter lasts from June 21 to September 21, while spring runs from September 22 to December 20.
Forty-six percent of subjects with childhood-onset SLE were born in winter, 17% in spring, another 17% in summer, and just over 19% in autumn, according to Dr. Appenzeller, a rheumatologist at the University of Campinas (Brazil).
In contrast, the occurrence of adult-onset SLE showed no variation by birth month or season.
The birth disparity between childhood- and adult-onset SLE was greatest in August, the depth of Brazilian winter, when 15.3% of all subjects with childhood-onset SLE were born, compared with 9% of adult-onset SLE patients and 8% of healthy controls.
The explanation for the increased likelihood of patients with childhood-onset SLE to be born in the winter months probably involves a gene-environment interaction, Dr. Appenzeller said. The most likely environmental factors are low seasonal maternal vitamin D levels and/or exposure to winter respiratory infections. The existence of a genetic component to the birth month disparity is suggested by another study by the Brazilian investigators in which they determined that the prevalence of SLE in both the first- and second-degree relatives of individuals with childhood-onset SLE was significantly higher than in patients with adult-onset disease.
Dr. Appenzeller reported having no financial conflicts regarding her study, funded by the Brazilian National Council for Scientific and Technological Development and other noncommercial research organizations.
REPORTING FROM LUPUS 2019
Young lupus patients need more than medications
SAN FRANCISCO – – and therein lies the importance of introducing interventions beyond simply prescribing appropriate medications, Hermine I. Brunner, MD, asserted at an international congress on systemic lupus erythematosus.
Pilot studies conducted by her research group as well as others suggest that brief cognitive-behavioral interventions, web-based patient and caregiver education, and social media interactions significantly improve the fatigue and depression, poor quality of life, and lack of adherence to medication that are pervasive in young patients with SLE, according to Dr. Brunner, director of the division of rheumatology and professor of pediatrics at the University of Cincinnati and scientific director of the Pediatric Rheumatology Collaborative Study Group.
“Don’t misunderstand: I don’t think we can treat lupus simply with a psychological intervention at the bedside. However, I think doctors would be well advised to offer both psychological interventions and medication when they see young lupus patients, because without the psychological intervention the patients may not feel sufficiently at ease to take their medication. They will not get the benefit of the medications you’ve prescribed,” she said.
Patients with SLE take an average of eight medications daily. Their medication adherence rate is comparable to that of patients with diabetes or many other chronic diseases: that is to say, lousy. When investigators at the University of Texas MD Anderson Cancer Center, Houston, utilized an electronic monitoring system to chart adherence to prescribed oral medications in adults with SLE, they found that over the course of 2 years of follow-up only one-fourth of them had an adherence rate of 80% or better, which is the standard definition of adherence (Lupus. 2012 Oct;21[11]:1158-65).
Treatment adherence is particularly problematic in adolescents and young adults with SLE. They often have great difficulty in mastering the self-management skills required to stay on top of their disease when they have so much else going on during what is a vulnerable and challenging period of development, even for healthy youths.
The texting intervention
Dr. Brunner and her colleagues at Cincinnati Children’s Hospital Medical Center recognized the scope of the nonadherence problem early on. Years ago they started sending text messaging reminders of pending clinic visits to their patients who had a poor track record of showing up for appointments.
“We texted patients 2 weeks before their scheduled visit, 1 week before, and then again the day before the visit,” she explained.
This simple intervention resulted in a 47% reduction in missed appointments, compared with a control group. Also, text recipients were more likely to cancel appointments instead of simply not showing up, an important benefit from a practice management and scheduling standpoint (J Rheumatol. 2012 Jan;39[1]:174-9). Disappointingly, however, the text messaging intervention had no impact on adherence to prescribed use of hydroxychloroquine. This led the investigators to conduct a deeper dive into the roots of the nonadherence problem in childhood-onset lupus.
Disease control, quality of life
Dr. Brunner and her coworkers conducted an in-depth assessment of health-related quality of life in 50 patients with childhood-onset SLE over the course of 6 months. The results were surprising.
“When we looked at the correlation between disease control and quality of life, actually there was none,” according to the pediatric rheumatologist.
Instead, the investigators found that young patients with persistently low quality of life despite objectively measured good disease control scored high for fatigue and depressive symptoms (Lupus. 2018 Jan;27[1]:124-33). This led Dr. Brunner and her coinvestigators to consider developing a practical behavioral intervention to address these potentially modifiable predictors of impaired health-related quality of life in their patient population.
The need for novel approaches was highlighted in focus groups conducted by the investigators, in which patients and their primary caregivers emphasized that current therapeutic strategies don’t adequately address key problems of living with lupus, especially the prominent fatigue, pain, and depressed mood that hamper daily function and personal relationships. Patients said they don’t feel an immediate benefit from taking their medications, so why bother? And parents expressed frustration about how difficult it is to get their teenagers to understand the consequences of nonadherence when they’re at an age when they don’t yet even grasp the concept of their own mortality (Lupus. 2019 Mar. doi: 10.1177/0961203319839478. These observations spurred the Cincinnati investigators to develop a modified cognitive-behavioral therapy (CBT) protocol, known as TEACH, which they believe is the first CBT intervention to specifically target psychological problems in young people with childhood-onset SLE.
The TEACH program
TEACH (Treatment and Education Approach for Childhood-Onset Lupus) is a six-session program that teaches patients and caregivers self-advocacy, relaxation techniques, how to improve sleep hygiene, the importance of engaging in planned pleasant activities, and why taking medications matters. The program content differs depending upon whether the patient is an adolescent or young adult.
Results of a recently published small feasibility study were highly encouraging, showing that 83% of people who enrolled in the program completed it. Posttreatment assessment showed that patients had a marked decrease in depressive symptoms as measured by both the Children’s Depression Inventory and the Beck Depression Inventory. They also showed a significant reduction in fatigue. However, while favorable trends in terms of reduced pain and anxiety symptoms were noted, they didn’t achieve statistical significance (Pediatr Rheumatol Online J. 2019 Feb 18. doi: 10.1186/s12969-019-0307-8). The next step in this project is a planned controlled randomized trial.
A web-based medication adherence program
Researchers at Pennsylvania State University took a different approach. They created a publicly available educational website, www.facinglupustogether.com, aimed at improving self-management skills – and especially medication adherence – in teens and young adults with SLE.
The website contains eight modules: Making the transition and taking charge of my medications, Learning about lupus, Learning about lupus medications, Managing symptoms of lupus, How do I handle lupus and my family, How do I handle lupus and my friends, Lupus and stress, and My personal goals and how I will achieve them. Each takes about 10 minutes to complete.
In a pilot study, 37 patients tackled one module per week and were randomized to respond to questions about the weekly topic either in a journal or by discussing the key points in an online social media forum with other young people with SLE. The idea was to create an intervention that capitalizes on the excellent social media skills possessed by today’s youth. And indeed, incorporation of social media proved to be a winning strategy. Medication adherence for hydroxychloroquine in the group randomized to social media participation jumped from 50% in the 3 months prior to starting the program to 92% in the first 3 months post completion, whereas medication adherence didn’t change significantly in the other study arm. The social media group also experienced significant improvements in self-efficacy, sense of community, acceptance of illness, optimism and control over the future, and other measures of empowerment. The control group did not show significant change in any of these domains (Pediatr Rheumatol Online J. 2018 Mar 14. doi: 10.1186/s12969-018-0232-2).
The TEACH study was sponsored by the National Institutes of Health. The web-based medication adherence program pilot study was supported by the Lupus Foundation of America. What the two approaches share in common is a conviction that, when it comes to addressing pain, fatigue, diminished quality of life, and poor medication adherence in young patients with SLE: “Our medication prescription alone doesn’t do it,” Dr. Brunner said.
She reported having no financial conflicts regarding her presentation.
SAN FRANCISCO – – and therein lies the importance of introducing interventions beyond simply prescribing appropriate medications, Hermine I. Brunner, MD, asserted at an international congress on systemic lupus erythematosus.
Pilot studies conducted by her research group as well as others suggest that brief cognitive-behavioral interventions, web-based patient and caregiver education, and social media interactions significantly improve the fatigue and depression, poor quality of life, and lack of adherence to medication that are pervasive in young patients with SLE, according to Dr. Brunner, director of the division of rheumatology and professor of pediatrics at the University of Cincinnati and scientific director of the Pediatric Rheumatology Collaborative Study Group.
“Don’t misunderstand: I don’t think we can treat lupus simply with a psychological intervention at the bedside. However, I think doctors would be well advised to offer both psychological interventions and medication when they see young lupus patients, because without the psychological intervention the patients may not feel sufficiently at ease to take their medication. They will not get the benefit of the medications you’ve prescribed,” she said.
Patients with SLE take an average of eight medications daily. Their medication adherence rate is comparable to that of patients with diabetes or many other chronic diseases: that is to say, lousy. When investigators at the University of Texas MD Anderson Cancer Center, Houston, utilized an electronic monitoring system to chart adherence to prescribed oral medications in adults with SLE, they found that over the course of 2 years of follow-up only one-fourth of them had an adherence rate of 80% or better, which is the standard definition of adherence (Lupus. 2012 Oct;21[11]:1158-65).
Treatment adherence is particularly problematic in adolescents and young adults with SLE. They often have great difficulty in mastering the self-management skills required to stay on top of their disease when they have so much else going on during what is a vulnerable and challenging period of development, even for healthy youths.
The texting intervention
Dr. Brunner and her colleagues at Cincinnati Children’s Hospital Medical Center recognized the scope of the nonadherence problem early on. Years ago they started sending text messaging reminders of pending clinic visits to their patients who had a poor track record of showing up for appointments.
“We texted patients 2 weeks before their scheduled visit, 1 week before, and then again the day before the visit,” she explained.
This simple intervention resulted in a 47% reduction in missed appointments, compared with a control group. Also, text recipients were more likely to cancel appointments instead of simply not showing up, an important benefit from a practice management and scheduling standpoint (J Rheumatol. 2012 Jan;39[1]:174-9). Disappointingly, however, the text messaging intervention had no impact on adherence to prescribed use of hydroxychloroquine. This led the investigators to conduct a deeper dive into the roots of the nonadherence problem in childhood-onset lupus.
Disease control, quality of life
Dr. Brunner and her coworkers conducted an in-depth assessment of health-related quality of life in 50 patients with childhood-onset SLE over the course of 6 months. The results were surprising.
“When we looked at the correlation between disease control and quality of life, actually there was none,” according to the pediatric rheumatologist.
Instead, the investigators found that young patients with persistently low quality of life despite objectively measured good disease control scored high for fatigue and depressive symptoms (Lupus. 2018 Jan;27[1]:124-33). This led Dr. Brunner and her coinvestigators to consider developing a practical behavioral intervention to address these potentially modifiable predictors of impaired health-related quality of life in their patient population.
The need for novel approaches was highlighted in focus groups conducted by the investigators, in which patients and their primary caregivers emphasized that current therapeutic strategies don’t adequately address key problems of living with lupus, especially the prominent fatigue, pain, and depressed mood that hamper daily function and personal relationships. Patients said they don’t feel an immediate benefit from taking their medications, so why bother? And parents expressed frustration about how difficult it is to get their teenagers to understand the consequences of nonadherence when they’re at an age when they don’t yet even grasp the concept of their own mortality (Lupus. 2019 Mar. doi: 10.1177/0961203319839478. These observations spurred the Cincinnati investigators to develop a modified cognitive-behavioral therapy (CBT) protocol, known as TEACH, which they believe is the first CBT intervention to specifically target psychological problems in young people with childhood-onset SLE.
The TEACH program
TEACH (Treatment and Education Approach for Childhood-Onset Lupus) is a six-session program that teaches patients and caregivers self-advocacy, relaxation techniques, how to improve sleep hygiene, the importance of engaging in planned pleasant activities, and why taking medications matters. The program content differs depending upon whether the patient is an adolescent or young adult.
Results of a recently published small feasibility study were highly encouraging, showing that 83% of people who enrolled in the program completed it. Posttreatment assessment showed that patients had a marked decrease in depressive symptoms as measured by both the Children’s Depression Inventory and the Beck Depression Inventory. They also showed a significant reduction in fatigue. However, while favorable trends in terms of reduced pain and anxiety symptoms were noted, they didn’t achieve statistical significance (Pediatr Rheumatol Online J. 2019 Feb 18. doi: 10.1186/s12969-019-0307-8). The next step in this project is a planned controlled randomized trial.
A web-based medication adherence program
Researchers at Pennsylvania State University took a different approach. They created a publicly available educational website, www.facinglupustogether.com, aimed at improving self-management skills – and especially medication adherence – in teens and young adults with SLE.
The website contains eight modules: Making the transition and taking charge of my medications, Learning about lupus, Learning about lupus medications, Managing symptoms of lupus, How do I handle lupus and my family, How do I handle lupus and my friends, Lupus and stress, and My personal goals and how I will achieve them. Each takes about 10 minutes to complete.
In a pilot study, 37 patients tackled one module per week and were randomized to respond to questions about the weekly topic either in a journal or by discussing the key points in an online social media forum with other young people with SLE. The idea was to create an intervention that capitalizes on the excellent social media skills possessed by today’s youth. And indeed, incorporation of social media proved to be a winning strategy. Medication adherence for hydroxychloroquine in the group randomized to social media participation jumped from 50% in the 3 months prior to starting the program to 92% in the first 3 months post completion, whereas medication adherence didn’t change significantly in the other study arm. The social media group also experienced significant improvements in self-efficacy, sense of community, acceptance of illness, optimism and control over the future, and other measures of empowerment. The control group did not show significant change in any of these domains (Pediatr Rheumatol Online J. 2018 Mar 14. doi: 10.1186/s12969-018-0232-2).
The TEACH study was sponsored by the National Institutes of Health. The web-based medication adherence program pilot study was supported by the Lupus Foundation of America. What the two approaches share in common is a conviction that, when it comes to addressing pain, fatigue, diminished quality of life, and poor medication adherence in young patients with SLE: “Our medication prescription alone doesn’t do it,” Dr. Brunner said.
She reported having no financial conflicts regarding her presentation.
SAN FRANCISCO – – and therein lies the importance of introducing interventions beyond simply prescribing appropriate medications, Hermine I. Brunner, MD, asserted at an international congress on systemic lupus erythematosus.
Pilot studies conducted by her research group as well as others suggest that brief cognitive-behavioral interventions, web-based patient and caregiver education, and social media interactions significantly improve the fatigue and depression, poor quality of life, and lack of adherence to medication that are pervasive in young patients with SLE, according to Dr. Brunner, director of the division of rheumatology and professor of pediatrics at the University of Cincinnati and scientific director of the Pediatric Rheumatology Collaborative Study Group.
“Don’t misunderstand: I don’t think we can treat lupus simply with a psychological intervention at the bedside. However, I think doctors would be well advised to offer both psychological interventions and medication when they see young lupus patients, because without the psychological intervention the patients may not feel sufficiently at ease to take their medication. They will not get the benefit of the medications you’ve prescribed,” she said.
Patients with SLE take an average of eight medications daily. Their medication adherence rate is comparable to that of patients with diabetes or many other chronic diseases: that is to say, lousy. When investigators at the University of Texas MD Anderson Cancer Center, Houston, utilized an electronic monitoring system to chart adherence to prescribed oral medications in adults with SLE, they found that over the course of 2 years of follow-up only one-fourth of them had an adherence rate of 80% or better, which is the standard definition of adherence (Lupus. 2012 Oct;21[11]:1158-65).
Treatment adherence is particularly problematic in adolescents and young adults with SLE. They often have great difficulty in mastering the self-management skills required to stay on top of their disease when they have so much else going on during what is a vulnerable and challenging period of development, even for healthy youths.
The texting intervention
Dr. Brunner and her colleagues at Cincinnati Children’s Hospital Medical Center recognized the scope of the nonadherence problem early on. Years ago they started sending text messaging reminders of pending clinic visits to their patients who had a poor track record of showing up for appointments.
“We texted patients 2 weeks before their scheduled visit, 1 week before, and then again the day before the visit,” she explained.
This simple intervention resulted in a 47% reduction in missed appointments, compared with a control group. Also, text recipients were more likely to cancel appointments instead of simply not showing up, an important benefit from a practice management and scheduling standpoint (J Rheumatol. 2012 Jan;39[1]:174-9). Disappointingly, however, the text messaging intervention had no impact on adherence to prescribed use of hydroxychloroquine. This led the investigators to conduct a deeper dive into the roots of the nonadherence problem in childhood-onset lupus.
Disease control, quality of life
Dr. Brunner and her coworkers conducted an in-depth assessment of health-related quality of life in 50 patients with childhood-onset SLE over the course of 6 months. The results were surprising.
“When we looked at the correlation between disease control and quality of life, actually there was none,” according to the pediatric rheumatologist.
Instead, the investigators found that young patients with persistently low quality of life despite objectively measured good disease control scored high for fatigue and depressive symptoms (Lupus. 2018 Jan;27[1]:124-33). This led Dr. Brunner and her coinvestigators to consider developing a practical behavioral intervention to address these potentially modifiable predictors of impaired health-related quality of life in their patient population.
The need for novel approaches was highlighted in focus groups conducted by the investigators, in which patients and their primary caregivers emphasized that current therapeutic strategies don’t adequately address key problems of living with lupus, especially the prominent fatigue, pain, and depressed mood that hamper daily function and personal relationships. Patients said they don’t feel an immediate benefit from taking their medications, so why bother? And parents expressed frustration about how difficult it is to get their teenagers to understand the consequences of nonadherence when they’re at an age when they don’t yet even grasp the concept of their own mortality (Lupus. 2019 Mar. doi: 10.1177/0961203319839478. These observations spurred the Cincinnati investigators to develop a modified cognitive-behavioral therapy (CBT) protocol, known as TEACH, which they believe is the first CBT intervention to specifically target psychological problems in young people with childhood-onset SLE.
The TEACH program
TEACH (Treatment and Education Approach for Childhood-Onset Lupus) is a six-session program that teaches patients and caregivers self-advocacy, relaxation techniques, how to improve sleep hygiene, the importance of engaging in planned pleasant activities, and why taking medications matters. The program content differs depending upon whether the patient is an adolescent or young adult.
Results of a recently published small feasibility study were highly encouraging, showing that 83% of people who enrolled in the program completed it. Posttreatment assessment showed that patients had a marked decrease in depressive symptoms as measured by both the Children’s Depression Inventory and the Beck Depression Inventory. They also showed a significant reduction in fatigue. However, while favorable trends in terms of reduced pain and anxiety symptoms were noted, they didn’t achieve statistical significance (Pediatr Rheumatol Online J. 2019 Feb 18. doi: 10.1186/s12969-019-0307-8). The next step in this project is a planned controlled randomized trial.
A web-based medication adherence program
Researchers at Pennsylvania State University took a different approach. They created a publicly available educational website, www.facinglupustogether.com, aimed at improving self-management skills – and especially medication adherence – in teens and young adults with SLE.
The website contains eight modules: Making the transition and taking charge of my medications, Learning about lupus, Learning about lupus medications, Managing symptoms of lupus, How do I handle lupus and my family, How do I handle lupus and my friends, Lupus and stress, and My personal goals and how I will achieve them. Each takes about 10 minutes to complete.
In a pilot study, 37 patients tackled one module per week and were randomized to respond to questions about the weekly topic either in a journal or by discussing the key points in an online social media forum with other young people with SLE. The idea was to create an intervention that capitalizes on the excellent social media skills possessed by today’s youth. And indeed, incorporation of social media proved to be a winning strategy. Medication adherence for hydroxychloroquine in the group randomized to social media participation jumped from 50% in the 3 months prior to starting the program to 92% in the first 3 months post completion, whereas medication adherence didn’t change significantly in the other study arm. The social media group also experienced significant improvements in self-efficacy, sense of community, acceptance of illness, optimism and control over the future, and other measures of empowerment. The control group did not show significant change in any of these domains (Pediatr Rheumatol Online J. 2018 Mar 14. doi: 10.1186/s12969-018-0232-2).
The TEACH study was sponsored by the National Institutes of Health. The web-based medication adherence program pilot study was supported by the Lupus Foundation of America. What the two approaches share in common is a conviction that, when it comes to addressing pain, fatigue, diminished quality of life, and poor medication adherence in young patients with SLE: “Our medication prescription alone doesn’t do it,” Dr. Brunner said.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM LUPUS 2019
Dr. Douglas Paauw: Consider rechallenging patients with penicillin allergy
PHILADELPHIA – As fluoroquinolone warnings stack up, internists seeking treatment alternatives should consider rechallenging patients with penicillin allergy or referring those patients for testing, said Douglas S. Paauw, MD, during a presentation.
This was one of the pieces of advice provided by Dr. Paauw, professor of medicine in the division of general internal medicine at the University of Washington, Seattle, at the annual meeting of the American College of Physicians.
“The FDA [Food and Drug Administration] has been just killing trees, sending us letters over the last 5-10 years, with fluoroquinolone warnings,” said Dr. Paauw, referencing previous warnings describing risks of tendon rupture, peripheral neuropathy, hypoglycemia, mental health side effects, and more.
“I think the buzz in 2019 is that we should not overreact to a history of penicillin allergy,” he said.
As many as 98% of patients who have reported penicillin allergy don’t have true allergy and can safely receive penicillin, he explained.
“If they don’t have an allergy, make sure you get it out of the electronic record,” Dr. Paauw also advised.
The latest warning on fluoroquinolones from the FDA, issued in Dec. 20, 2018, said that clinicians should avoid prescribing these antibiotics in patients who have, or are at risk of, aortic aneurysm. This comprises a very large proportion of patients in an internist’s practice, Dr. Paauw noted. The warning specifically singled out elderly patients as being in the at-risk population, along with patients who have peripheral atherosclerotic vascular diseases, hypertension, or genetic conditions such as Marfan syndrome or Ehlers-Danlos syndrome, he added.
Dr. Paauw further supported his suggestions by describing two relevant studies.
In one of those studies, which was published this year in an allergy and asthma journal, 20 subjects with a history of penicillin allergy agreed to direct oral amoxicillin rechallenge by an allergist, he said. None of those 20 patients were observed to have developed immediate or delayed hypersensitivity reactions, investigators reported. That study included a total of 50 adults with a penicillin allergy label, of whom 24 (48%) had the label removed from their medical records.
In another recent and reassuring study, penicillin allergy testing was conducted in 100 children with parent-reported penicillin allergy that was considered low risk based on reported symptoms, Dr. Paauw said. Of that group, all 100 children were found to be negative for true penicillin allergy.
Dr. Paauw had no relevant disclosures.
SOURCE: Paauw DS. Annual Meeting of the American College of Physicians, Presentation MTP 013.
PHILADELPHIA – As fluoroquinolone warnings stack up, internists seeking treatment alternatives should consider rechallenging patients with penicillin allergy or referring those patients for testing, said Douglas S. Paauw, MD, during a presentation.
This was one of the pieces of advice provided by Dr. Paauw, professor of medicine in the division of general internal medicine at the University of Washington, Seattle, at the annual meeting of the American College of Physicians.
“The FDA [Food and Drug Administration] has been just killing trees, sending us letters over the last 5-10 years, with fluoroquinolone warnings,” said Dr. Paauw, referencing previous warnings describing risks of tendon rupture, peripheral neuropathy, hypoglycemia, mental health side effects, and more.
“I think the buzz in 2019 is that we should not overreact to a history of penicillin allergy,” he said.
As many as 98% of patients who have reported penicillin allergy don’t have true allergy and can safely receive penicillin, he explained.
“If they don’t have an allergy, make sure you get it out of the electronic record,” Dr. Paauw also advised.
The latest warning on fluoroquinolones from the FDA, issued in Dec. 20, 2018, said that clinicians should avoid prescribing these antibiotics in patients who have, or are at risk of, aortic aneurysm. This comprises a very large proportion of patients in an internist’s practice, Dr. Paauw noted. The warning specifically singled out elderly patients as being in the at-risk population, along with patients who have peripheral atherosclerotic vascular diseases, hypertension, or genetic conditions such as Marfan syndrome or Ehlers-Danlos syndrome, he added.
Dr. Paauw further supported his suggestions by describing two relevant studies.
In one of those studies, which was published this year in an allergy and asthma journal, 20 subjects with a history of penicillin allergy agreed to direct oral amoxicillin rechallenge by an allergist, he said. None of those 20 patients were observed to have developed immediate or delayed hypersensitivity reactions, investigators reported. That study included a total of 50 adults with a penicillin allergy label, of whom 24 (48%) had the label removed from their medical records.
In another recent and reassuring study, penicillin allergy testing was conducted in 100 children with parent-reported penicillin allergy that was considered low risk based on reported symptoms, Dr. Paauw said. Of that group, all 100 children were found to be negative for true penicillin allergy.
Dr. Paauw had no relevant disclosures.
SOURCE: Paauw DS. Annual Meeting of the American College of Physicians, Presentation MTP 013.
PHILADELPHIA – As fluoroquinolone warnings stack up, internists seeking treatment alternatives should consider rechallenging patients with penicillin allergy or referring those patients for testing, said Douglas S. Paauw, MD, during a presentation.
This was one of the pieces of advice provided by Dr. Paauw, professor of medicine in the division of general internal medicine at the University of Washington, Seattle, at the annual meeting of the American College of Physicians.
“The FDA [Food and Drug Administration] has been just killing trees, sending us letters over the last 5-10 years, with fluoroquinolone warnings,” said Dr. Paauw, referencing previous warnings describing risks of tendon rupture, peripheral neuropathy, hypoglycemia, mental health side effects, and more.
“I think the buzz in 2019 is that we should not overreact to a history of penicillin allergy,” he said.
As many as 98% of patients who have reported penicillin allergy don’t have true allergy and can safely receive penicillin, he explained.
“If they don’t have an allergy, make sure you get it out of the electronic record,” Dr. Paauw also advised.
The latest warning on fluoroquinolones from the FDA, issued in Dec. 20, 2018, said that clinicians should avoid prescribing these antibiotics in patients who have, or are at risk of, aortic aneurysm. This comprises a very large proportion of patients in an internist’s practice, Dr. Paauw noted. The warning specifically singled out elderly patients as being in the at-risk population, along with patients who have peripheral atherosclerotic vascular diseases, hypertension, or genetic conditions such as Marfan syndrome or Ehlers-Danlos syndrome, he added.
Dr. Paauw further supported his suggestions by describing two relevant studies.
In one of those studies, which was published this year in an allergy and asthma journal, 20 subjects with a history of penicillin allergy agreed to direct oral amoxicillin rechallenge by an allergist, he said. None of those 20 patients were observed to have developed immediate or delayed hypersensitivity reactions, investigators reported. That study included a total of 50 adults with a penicillin allergy label, of whom 24 (48%) had the label removed from their medical records.
In another recent and reassuring study, penicillin allergy testing was conducted in 100 children with parent-reported penicillin allergy that was considered low risk based on reported symptoms, Dr. Paauw said. Of that group, all 100 children were found to be negative for true penicillin allergy.
Dr. Paauw had no relevant disclosures.
SOURCE: Paauw DS. Annual Meeting of the American College of Physicians, Presentation MTP 013.
REPORTING FROM INTERNAL MEDICINE 2019
Ligelizumab maintains urticaria control for up to 1 year
WASHINGTON – in an open-label extension study, Diane Baker, MD, said at the annual meeting of the American Academy of Dermatology.
About 75% of the cohort experienced complete disease control at least once during the study. Novartis is developing ligelizumab (QGE031) as a treatment option for patients with spontaneous chronic urticaria (CSU) whose symptoms are inadequately controlled by H1-antihistamines. Like omalizumab (Xolair), which is approved in the United States and Europe for treatment of CSU, ligelizumab is a humanized anti-IgE monoclonal antibody. But the investigational agent binds to IgE with greater affinity than omalizumab, said Dr. Baker, a dermatologist who practices in Portland, Ore.
The extension study was a follow-up to a 12-week, phase 2, dose-finding trial of 382 CSU patients. In the study, which was not powered for efficacy endpoints, 51% of those who received 72 mg subcutaneously every 4 weeks had a Hives Severity Score of 0 by week 12, compared with 42% of those who received 240 mg every 4 weeks and 26% of those taking the omalizumab comparator. Additionally, 47% of those in the 72-mg group and 46% of the 240-mg group achieved a score of 0 on another indicator, the Urticaria Activity Score, which measures symptoms over 7 days (UAS7).
The extension study, which evaluated the 240-mg dose, showed the durability of that response, with 52% of those in the 240-mg group maintained a UAS7 of 0 at 1 year, according to Dr. Baker. By the end of the year, most patients (75.8%) had experienced at least one period of complete symptom control, and 84.0% experienced a UAS of 6 or lower at least once.
Adverse events were common in the cohort, with 84% experiencing at least one. But most (78%) were mild or moderate, and there was no clear side effect pattern, Dr. Baker said. Eight patients discontinued treatment because of an adverse event, and another eight dropped out because of lack of efficacy. Other reasons for discontinuation were pregnancy, protocol deviation, and physician or patient decision.
Novartis has launched two 1-year, phase 3 trials randomizing patients to 72 mg or 240 mg of ligelizumab or 300 mg of omalizumab every 4 weeks in a similar patient population, Dr. Baker said. PEARL 1 and PEARL 2, the largest pivotal trials to date in CSU, will enroll more than 2,000 patients, according to a company press release.
Dr. Baker is a clinical trials investigator for Novartis.
SOURCE: Baker D et al. AAD 2019, Session S034.
WASHINGTON – in an open-label extension study, Diane Baker, MD, said at the annual meeting of the American Academy of Dermatology.
About 75% of the cohort experienced complete disease control at least once during the study. Novartis is developing ligelizumab (QGE031) as a treatment option for patients with spontaneous chronic urticaria (CSU) whose symptoms are inadequately controlled by H1-antihistamines. Like omalizumab (Xolair), which is approved in the United States and Europe for treatment of CSU, ligelizumab is a humanized anti-IgE monoclonal antibody. But the investigational agent binds to IgE with greater affinity than omalizumab, said Dr. Baker, a dermatologist who practices in Portland, Ore.
The extension study was a follow-up to a 12-week, phase 2, dose-finding trial of 382 CSU patients. In the study, which was not powered for efficacy endpoints, 51% of those who received 72 mg subcutaneously every 4 weeks had a Hives Severity Score of 0 by week 12, compared with 42% of those who received 240 mg every 4 weeks and 26% of those taking the omalizumab comparator. Additionally, 47% of those in the 72-mg group and 46% of the 240-mg group achieved a score of 0 on another indicator, the Urticaria Activity Score, which measures symptoms over 7 days (UAS7).
The extension study, which evaluated the 240-mg dose, showed the durability of that response, with 52% of those in the 240-mg group maintained a UAS7 of 0 at 1 year, according to Dr. Baker. By the end of the year, most patients (75.8%) had experienced at least one period of complete symptom control, and 84.0% experienced a UAS of 6 or lower at least once.
Adverse events were common in the cohort, with 84% experiencing at least one. But most (78%) were mild or moderate, and there was no clear side effect pattern, Dr. Baker said. Eight patients discontinued treatment because of an adverse event, and another eight dropped out because of lack of efficacy. Other reasons for discontinuation were pregnancy, protocol deviation, and physician or patient decision.
Novartis has launched two 1-year, phase 3 trials randomizing patients to 72 mg or 240 mg of ligelizumab or 300 mg of omalizumab every 4 weeks in a similar patient population, Dr. Baker said. PEARL 1 and PEARL 2, the largest pivotal trials to date in CSU, will enroll more than 2,000 patients, according to a company press release.
Dr. Baker is a clinical trials investigator for Novartis.
SOURCE: Baker D et al. AAD 2019, Session S034.
WASHINGTON – in an open-label extension study, Diane Baker, MD, said at the annual meeting of the American Academy of Dermatology.
About 75% of the cohort experienced complete disease control at least once during the study. Novartis is developing ligelizumab (QGE031) as a treatment option for patients with spontaneous chronic urticaria (CSU) whose symptoms are inadequately controlled by H1-antihistamines. Like omalizumab (Xolair), which is approved in the United States and Europe for treatment of CSU, ligelizumab is a humanized anti-IgE monoclonal antibody. But the investigational agent binds to IgE with greater affinity than omalizumab, said Dr. Baker, a dermatologist who practices in Portland, Ore.
The extension study was a follow-up to a 12-week, phase 2, dose-finding trial of 382 CSU patients. In the study, which was not powered for efficacy endpoints, 51% of those who received 72 mg subcutaneously every 4 weeks had a Hives Severity Score of 0 by week 12, compared with 42% of those who received 240 mg every 4 weeks and 26% of those taking the omalizumab comparator. Additionally, 47% of those in the 72-mg group and 46% of the 240-mg group achieved a score of 0 on another indicator, the Urticaria Activity Score, which measures symptoms over 7 days (UAS7).
The extension study, which evaluated the 240-mg dose, showed the durability of that response, with 52% of those in the 240-mg group maintained a UAS7 of 0 at 1 year, according to Dr. Baker. By the end of the year, most patients (75.8%) had experienced at least one period of complete symptom control, and 84.0% experienced a UAS of 6 or lower at least once.
Adverse events were common in the cohort, with 84% experiencing at least one. But most (78%) were mild or moderate, and there was no clear side effect pattern, Dr. Baker said. Eight patients discontinued treatment because of an adverse event, and another eight dropped out because of lack of efficacy. Other reasons for discontinuation were pregnancy, protocol deviation, and physician or patient decision.
Novartis has launched two 1-year, phase 3 trials randomizing patients to 72 mg or 240 mg of ligelizumab or 300 mg of omalizumab every 4 weeks in a similar patient population, Dr. Baker said. PEARL 1 and PEARL 2, the largest pivotal trials to date in CSU, will enroll more than 2,000 patients, according to a company press release.
Dr. Baker is a clinical trials investigator for Novartis.
SOURCE: Baker D et al. AAD 2019, Session S034.
REPORTING FROM AAD 2019
FDA panel calls for changes to breast implant rupture screening
A Food and Drug Administration advisory panel urged the agency to switch its recommended screening method for silent breast implant ruptures from MRI to ultrasound and to push the first screening examination back from the current 3 years post implant to 5 years.
Members of the FDA’s General and Plastic Surgery Advisory Panel also made suggestions to the FDA regarding how it might improve communication about the risks of breast implants to the public in general and to people considering implants in particular.
The panel also discussed the sort of safety and efficacy assessments the FDA should require for acellular dermal matrix (ADM), also known as mesh, to add the material’s label for use during breast reconstruction or implant augmentation. Surgeons have used mesh routinely as a surgical aid at other body sites, such as the abdomen. Although ADM is now also widely used during breast surgery, it has never undergone testing or labeling for use in that setting.
The FDA convened the advisory committee meeting largely to assess and discuss data and concerns about two recently appreciated complications of breast implant placement – breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) and a still poorly defined and described constellation of autoimmune and rheumatoid-like symptoms reported anecdotally by some breast implant recipients called Breast Implant Illness (BII). But agency officials asked the panel to also address these other issues related to the safety of breast implants and implant surgery.
The revised screening recommendations were primarily a response to a lack of compliance with current FDA recommendations to screen for breast implant rupture with MRI starting 3 years after placement and then every 2 years.
The problem is that a screening MRI costs about $1,500-$2,000 and is generally not covered by insurance when done for this purpose, although it is often covered when used to investigate a suspected rupture. The result is that less than 5% of implanted patients comply with the recommended screening schedule, noted committee chair Frank R. Lewis Jr., MD, executive director, emeritus, of the American Board of Surgery in Philadelphia.
“Effectively it’s a useless recommendation,” he said. “Ultrasound is far easier, quicker, and cheaper” and seems effective for screening.
The advisory panel recommended starting ultrasound screening 5 years after implantation, based on MRI screening data showing that virtually all ruptures don’t occur until after 5 years, and then following with ultrasound screening every 3 years after that. The panel recommended using MRI when the ultrasound result is equivocal or when the patient has symptoms suggesting rupture.
After hearing testimony during the sessions from several dozen women who told horror stories of the complications they experienced from breast implants, panel member Karen E. Burke, MD, PhD, spoke for many on the panel when she said “no doubt patients feel that the informed consent process failed them, that they were not aware of the risks.”
Dr. Burke suggested that patients must be informed so that they realize that breast implants are not static objects that will always sit unchanged in their body for the rest of their lives, that certain factors such as allergy or family history of tissue disease might predispose them to autoimmune-type reactions and that the diverse symptoms described for BII are possible sequelae.
A black box warning for the potential of developing anaplastic large-cell lymphoma should also go into the label, said Dr. Burke, a dermatologist who practices in New York City.
Dr. Lewis ridiculed the information booklets that implant manufacturers currently provide for patients as too long and dense. “They were not constructed to inform patients in the best way; they were constructed to provide legal protection.” He called for creating a two- or three-page list of potential adverse effects and points to consider.
Other panel members suggested public service advertisements similar to what is used to inform consumers about the risk from cigarettes. Dr. Burke recommended getting the word out about BII to other medical specialties that are more likely to see affected patients first, such as rheumatologists, immunologists, and dermatologists. She vowed to speak about these complications at an upcoming meeting of the American Academy of Dermatology. But other panel members noted that BII right now remains without any official medical definition nor clear causal link to breast implants.
The question of exactly what safety and efficacy data the FDA might require from manufacturers seeking a breast surgery indication for ADM was less clear.
Binita Ashar, MD, director of the FDA’s Division of Surgical Devices, highlighted the agency’s dilemma about considering data for a breast surgery indication. “The challenge for us is that we can’t expect a control arm because everyone today is using” mesh, she explained. “We’re looking for guidance on how to understand the risk-to-benefit profile” of ADM.
A plastic surgeon on the advisory panel, Pierre M. Chevray, MD, PhD, from Houston Methodist Hospital summarized the way ADM mesh reached its current niche in routine, U.S. breast surgery.
About 20 years ago, plastic surgeons began using mesh during implant surgery to improve eventual breast cosmesis. Surgeons began to wrap the implant in mesh and then attached the mesh to the pectoral muscle so that the implant could go on top of the muscle and not beneath it. It greatly diminished capsular contraction around the implant over time, reduced the risk for implant movement, and allowed for more natural positioning of the breast with the implant inside, he said.
Another factor in the growing use of mesh was heavy promotion by manufacturers to a generation of plastic surgeons, Dr. Chevray said. But use of ADM may also lead to a slightly increased rate of seromas and infections.
“The benefit from mesh is hard to prove and is questionable” because it largely depends on a subjective assessment by a surgeon or patient, Dr. Chevray said. “The cost [of ADM] is substantial, but no data have shown that outcomes are better” with its use. Despite that, “nearly every surgeon uses mesh” these days, he noted.
A Food and Drug Administration advisory panel urged the agency to switch its recommended screening method for silent breast implant ruptures from MRI to ultrasound and to push the first screening examination back from the current 3 years post implant to 5 years.
Members of the FDA’s General and Plastic Surgery Advisory Panel also made suggestions to the FDA regarding how it might improve communication about the risks of breast implants to the public in general and to people considering implants in particular.
The panel also discussed the sort of safety and efficacy assessments the FDA should require for acellular dermal matrix (ADM), also known as mesh, to add the material’s label for use during breast reconstruction or implant augmentation. Surgeons have used mesh routinely as a surgical aid at other body sites, such as the abdomen. Although ADM is now also widely used during breast surgery, it has never undergone testing or labeling for use in that setting.
The FDA convened the advisory committee meeting largely to assess and discuss data and concerns about two recently appreciated complications of breast implant placement – breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) and a still poorly defined and described constellation of autoimmune and rheumatoid-like symptoms reported anecdotally by some breast implant recipients called Breast Implant Illness (BII). But agency officials asked the panel to also address these other issues related to the safety of breast implants and implant surgery.
The revised screening recommendations were primarily a response to a lack of compliance with current FDA recommendations to screen for breast implant rupture with MRI starting 3 years after placement and then every 2 years.
The problem is that a screening MRI costs about $1,500-$2,000 and is generally not covered by insurance when done for this purpose, although it is often covered when used to investigate a suspected rupture. The result is that less than 5% of implanted patients comply with the recommended screening schedule, noted committee chair Frank R. Lewis Jr., MD, executive director, emeritus, of the American Board of Surgery in Philadelphia.
“Effectively it’s a useless recommendation,” he said. “Ultrasound is far easier, quicker, and cheaper” and seems effective for screening.
The advisory panel recommended starting ultrasound screening 5 years after implantation, based on MRI screening data showing that virtually all ruptures don’t occur until after 5 years, and then following with ultrasound screening every 3 years after that. The panel recommended using MRI when the ultrasound result is equivocal or when the patient has symptoms suggesting rupture.
After hearing testimony during the sessions from several dozen women who told horror stories of the complications they experienced from breast implants, panel member Karen E. Burke, MD, PhD, spoke for many on the panel when she said “no doubt patients feel that the informed consent process failed them, that they were not aware of the risks.”
Dr. Burke suggested that patients must be informed so that they realize that breast implants are not static objects that will always sit unchanged in their body for the rest of their lives, that certain factors such as allergy or family history of tissue disease might predispose them to autoimmune-type reactions and that the diverse symptoms described for BII are possible sequelae.
A black box warning for the potential of developing anaplastic large-cell lymphoma should also go into the label, said Dr. Burke, a dermatologist who practices in New York City.
Dr. Lewis ridiculed the information booklets that implant manufacturers currently provide for patients as too long and dense. “They were not constructed to inform patients in the best way; they were constructed to provide legal protection.” He called for creating a two- or three-page list of potential adverse effects and points to consider.
Other panel members suggested public service advertisements similar to what is used to inform consumers about the risk from cigarettes. Dr. Burke recommended getting the word out about BII to other medical specialties that are more likely to see affected patients first, such as rheumatologists, immunologists, and dermatologists. She vowed to speak about these complications at an upcoming meeting of the American Academy of Dermatology. But other panel members noted that BII right now remains without any official medical definition nor clear causal link to breast implants.
The question of exactly what safety and efficacy data the FDA might require from manufacturers seeking a breast surgery indication for ADM was less clear.
Binita Ashar, MD, director of the FDA’s Division of Surgical Devices, highlighted the agency’s dilemma about considering data for a breast surgery indication. “The challenge for us is that we can’t expect a control arm because everyone today is using” mesh, she explained. “We’re looking for guidance on how to understand the risk-to-benefit profile” of ADM.
A plastic surgeon on the advisory panel, Pierre M. Chevray, MD, PhD, from Houston Methodist Hospital summarized the way ADM mesh reached its current niche in routine, U.S. breast surgery.
About 20 years ago, plastic surgeons began using mesh during implant surgery to improve eventual breast cosmesis. Surgeons began to wrap the implant in mesh and then attached the mesh to the pectoral muscle so that the implant could go on top of the muscle and not beneath it. It greatly diminished capsular contraction around the implant over time, reduced the risk for implant movement, and allowed for more natural positioning of the breast with the implant inside, he said.
Another factor in the growing use of mesh was heavy promotion by manufacturers to a generation of plastic surgeons, Dr. Chevray said. But use of ADM may also lead to a slightly increased rate of seromas and infections.
“The benefit from mesh is hard to prove and is questionable” because it largely depends on a subjective assessment by a surgeon or patient, Dr. Chevray said. “The cost [of ADM] is substantial, but no data have shown that outcomes are better” with its use. Despite that, “nearly every surgeon uses mesh” these days, he noted.
A Food and Drug Administration advisory panel urged the agency to switch its recommended screening method for silent breast implant ruptures from MRI to ultrasound and to push the first screening examination back from the current 3 years post implant to 5 years.
Members of the FDA’s General and Plastic Surgery Advisory Panel also made suggestions to the FDA regarding how it might improve communication about the risks of breast implants to the public in general and to people considering implants in particular.
The panel also discussed the sort of safety and efficacy assessments the FDA should require for acellular dermal matrix (ADM), also known as mesh, to add the material’s label for use during breast reconstruction or implant augmentation. Surgeons have used mesh routinely as a surgical aid at other body sites, such as the abdomen. Although ADM is now also widely used during breast surgery, it has never undergone testing or labeling for use in that setting.
The FDA convened the advisory committee meeting largely to assess and discuss data and concerns about two recently appreciated complications of breast implant placement – breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) and a still poorly defined and described constellation of autoimmune and rheumatoid-like symptoms reported anecdotally by some breast implant recipients called Breast Implant Illness (BII). But agency officials asked the panel to also address these other issues related to the safety of breast implants and implant surgery.
The revised screening recommendations were primarily a response to a lack of compliance with current FDA recommendations to screen for breast implant rupture with MRI starting 3 years after placement and then every 2 years.
The problem is that a screening MRI costs about $1,500-$2,000 and is generally not covered by insurance when done for this purpose, although it is often covered when used to investigate a suspected rupture. The result is that less than 5% of implanted patients comply with the recommended screening schedule, noted committee chair Frank R. Lewis Jr., MD, executive director, emeritus, of the American Board of Surgery in Philadelphia.
“Effectively it’s a useless recommendation,” he said. “Ultrasound is far easier, quicker, and cheaper” and seems effective for screening.
The advisory panel recommended starting ultrasound screening 5 years after implantation, based on MRI screening data showing that virtually all ruptures don’t occur until after 5 years, and then following with ultrasound screening every 3 years after that. The panel recommended using MRI when the ultrasound result is equivocal or when the patient has symptoms suggesting rupture.
After hearing testimony during the sessions from several dozen women who told horror stories of the complications they experienced from breast implants, panel member Karen E. Burke, MD, PhD, spoke for many on the panel when she said “no doubt patients feel that the informed consent process failed them, that they were not aware of the risks.”
Dr. Burke suggested that patients must be informed so that they realize that breast implants are not static objects that will always sit unchanged in their body for the rest of their lives, that certain factors such as allergy or family history of tissue disease might predispose them to autoimmune-type reactions and that the diverse symptoms described for BII are possible sequelae.
A black box warning for the potential of developing anaplastic large-cell lymphoma should also go into the label, said Dr. Burke, a dermatologist who practices in New York City.
Dr. Lewis ridiculed the information booklets that implant manufacturers currently provide for patients as too long and dense. “They were not constructed to inform patients in the best way; they were constructed to provide legal protection.” He called for creating a two- or three-page list of potential adverse effects and points to consider.
Other panel members suggested public service advertisements similar to what is used to inform consumers about the risk from cigarettes. Dr. Burke recommended getting the word out about BII to other medical specialties that are more likely to see affected patients first, such as rheumatologists, immunologists, and dermatologists. She vowed to speak about these complications at an upcoming meeting of the American Academy of Dermatology. But other panel members noted that BII right now remains without any official medical definition nor clear causal link to breast implants.
The question of exactly what safety and efficacy data the FDA might require from manufacturers seeking a breast surgery indication for ADM was less clear.
Binita Ashar, MD, director of the FDA’s Division of Surgical Devices, highlighted the agency’s dilemma about considering data for a breast surgery indication. “The challenge for us is that we can’t expect a control arm because everyone today is using” mesh, she explained. “We’re looking for guidance on how to understand the risk-to-benefit profile” of ADM.
A plastic surgeon on the advisory panel, Pierre M. Chevray, MD, PhD, from Houston Methodist Hospital summarized the way ADM mesh reached its current niche in routine, U.S. breast surgery.
About 20 years ago, plastic surgeons began using mesh during implant surgery to improve eventual breast cosmesis. Surgeons began to wrap the implant in mesh and then attached the mesh to the pectoral muscle so that the implant could go on top of the muscle and not beneath it. It greatly diminished capsular contraction around the implant over time, reduced the risk for implant movement, and allowed for more natural positioning of the breast with the implant inside, he said.
Another factor in the growing use of mesh was heavy promotion by manufacturers to a generation of plastic surgeons, Dr. Chevray said. But use of ADM may also lead to a slightly increased rate of seromas and infections.
“The benefit from mesh is hard to prove and is questionable” because it largely depends on a subjective assessment by a surgeon or patient, Dr. Chevray said. “The cost [of ADM] is substantial, but no data have shown that outcomes are better” with its use. Despite that, “nearly every surgeon uses mesh” these days, he noted.
AT AN FDA ADVISORY PANEL MEETING
There’s No Doubt: Blame the Drug
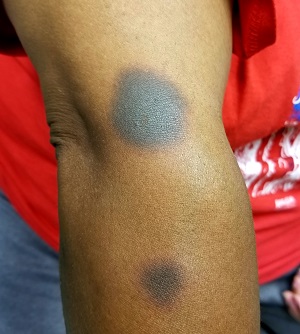
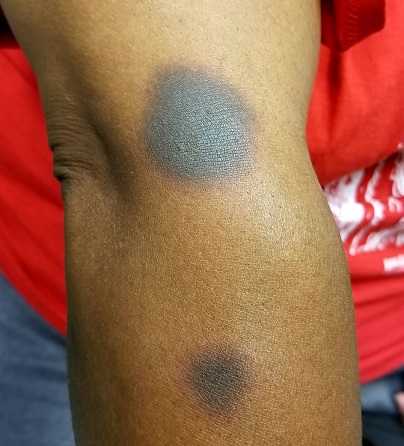
It’s taken 2 years for family members to convince this 50-year-old black woman to consult dermatology about dark circles on her arms and trunk. The asymptomatic lesions were only faintly discolored at manifestation but have darkened with each recurrence. The 6 lesions she currently has are in the exact locations they originally manifested in.
Convinced she had “ringworm,” the patient tried several different antifungal creams, to no avail. Then her primary care provider prescribed a 2-month course of oral antifungal medication (terbinafine)—again, with no change to the lesions.
The patient is otherwise healthy except for osteoarthritis, for which she takes ibuprofen (800 mg 2 or 3 times a day). She denies any history of seizure, chronic urinary tract infection, or other chronic infection.
EXAMINATION
The 6 perfectly round, uniformly pigmented, dark brown macular lesions on the patient’s arms and trunk range from 2 to 5 cm in diameter. A faint, rusty brown halo can be seen around the periphery of the lesions, giving them a targetoid look. The surfaces of the lesions are completely smooth.
What’s the diagnosis?
DISCUSSION
The list of dark brown, round macules that come and go in the exact same locations is a short one—in fact, it only includes fixed drug eruption (FDE). This is a unique adverse reaction to one of several medications; common culprits are ibuprofen or other NSAIDs, aspirin, members of the sulfa family, penicillin, and several antiseizure medications.
The exact mechanism for this reaction is unknown, but it does appear to involve the localized production of cytokines. There is a range of morphologic presentations for FDE: some lesions are more targetoid than others; some are darker (especially in patients with darker skin) while others are more pink.
Furthermore, many patients develop additional lesions over time. A few, on the other hand, will reach a point at which they stop reacting to the offending product.
Of all the drugs causing FDE, sulfa-based products are among the more consistent offenders. Thus, we always ask about chronic urinary tract infections.
TAKE-HOME LEARNING POINTS
- Fixed drug eruptions (FDE) usually manifest with round, targetoid macules that are occasionally blistery.
- The lesions recur or darken with each “challenge” from the drug, which can be one of several potential offenders.
- The fact that the lesions keep recurring in the same location (ie, fixed) is pathognomic for FDE.
- FDE lesions can occur almost anywhere on the body—even on palms or soles.
It’s taken 2 years for family members to convince this 50-year-old black woman to consult dermatology about dark circles on her arms and trunk. The asymptomatic lesions were only faintly discolored at manifestation but have darkened with each recurrence. The 6 lesions she currently has are in the exact locations they originally manifested in.
Convinced she had “ringworm,” the patient tried several different antifungal creams, to no avail. Then her primary care provider prescribed a 2-month course of oral antifungal medication (terbinafine)—again, with no change to the lesions.
The patient is otherwise healthy except for osteoarthritis, for which she takes ibuprofen (800 mg 2 or 3 times a day). She denies any history of seizure, chronic urinary tract infection, or other chronic infection.

EXAMINATION
The 6 perfectly round, uniformly pigmented, dark brown macular lesions on the patient’s arms and trunk range from 2 to 5 cm in diameter. A faint, rusty brown halo can be seen around the periphery of the lesions, giving them a targetoid look. The surfaces of the lesions are completely smooth.
What’s the diagnosis?
DISCUSSION
The list of dark brown, round macules that come and go in the exact same locations is a short one—in fact, it only includes fixed drug eruption (FDE). This is a unique adverse reaction to one of several medications; common culprits are ibuprofen or other NSAIDs, aspirin, members of the sulfa family, penicillin, and several antiseizure medications.
The exact mechanism for this reaction is unknown, but it does appear to involve the localized production of cytokines. There is a range of morphologic presentations for FDE: some lesions are more targetoid than others; some are darker (especially in patients with darker skin) while others are more pink.
Furthermore, many patients develop additional lesions over time. A few, on the other hand, will reach a point at which they stop reacting to the offending product.
Of all the drugs causing FDE, sulfa-based products are among the more consistent offenders. Thus, we always ask about chronic urinary tract infections.
TAKE-HOME LEARNING POINTS
- Fixed drug eruptions (FDE) usually manifest with round, targetoid macules that are occasionally blistery.
- The lesions recur or darken with each “challenge” from the drug, which can be one of several potential offenders.
- The fact that the lesions keep recurring in the same location (ie, fixed) is pathognomic for FDE.
- FDE lesions can occur almost anywhere on the body—even on palms or soles.
It’s taken 2 years for family members to convince this 50-year-old black woman to consult dermatology about dark circles on her arms and trunk. The asymptomatic lesions were only faintly discolored at manifestation but have darkened with each recurrence. The 6 lesions she currently has are in the exact locations they originally manifested in.
Convinced she had “ringworm,” the patient tried several different antifungal creams, to no avail. Then her primary care provider prescribed a 2-month course of oral antifungal medication (terbinafine)—again, with no change to the lesions.
The patient is otherwise healthy except for osteoarthritis, for which she takes ibuprofen (800 mg 2 or 3 times a day). She denies any history of seizure, chronic urinary tract infection, or other chronic infection.

EXAMINATION
The 6 perfectly round, uniformly pigmented, dark brown macular lesions on the patient’s arms and trunk range from 2 to 5 cm in diameter. A faint, rusty brown halo can be seen around the periphery of the lesions, giving them a targetoid look. The surfaces of the lesions are completely smooth.
What’s the diagnosis?
DISCUSSION
The list of dark brown, round macules that come and go in the exact same locations is a short one—in fact, it only includes fixed drug eruption (FDE). This is a unique adverse reaction to one of several medications; common culprits are ibuprofen or other NSAIDs, aspirin, members of the sulfa family, penicillin, and several antiseizure medications.
The exact mechanism for this reaction is unknown, but it does appear to involve the localized production of cytokines. There is a range of morphologic presentations for FDE: some lesions are more targetoid than others; some are darker (especially in patients with darker skin) while others are more pink.
Furthermore, many patients develop additional lesions over time. A few, on the other hand, will reach a point at which they stop reacting to the offending product.
Of all the drugs causing FDE, sulfa-based products are among the more consistent offenders. Thus, we always ask about chronic urinary tract infections.
TAKE-HOME LEARNING POINTS
- Fixed drug eruptions (FDE) usually manifest with round, targetoid macules that are occasionally blistery.
- The lesions recur or darken with each “challenge” from the drug, which can be one of several potential offenders.
- The fact that the lesions keep recurring in the same location (ie, fixed) is pathognomic for FDE.
- FDE lesions can occur almost anywhere on the body—even on palms or soles.
Lentiviral gene therapy appears effective in X-CGD
HOUSTON – , said Donald B. Kohn, MD, of the University of California, Los Angeles.
Seven of nine patients treated were “alive and well” at 12 months’ follow-up after receiving lentiviral vector transduced CD34+ cells, Dr. Kohn reported in a late-breaking clinical trial session at the Transplantation & Cellular Therapy Meetings.
Most patients were able to discontinue antibiotic prophylaxis for this disease, which is associated with severe, recurrent, and prolonged life-threatening infections, he said.
Results of the small study provide “proof of concept” for use of the gene therapy in the disease, though additional studies are needed to formally assess the clinical safety and efficacy of the approach, he said.
The estimated incidence of chronic granulomatous disease is 1 in 200,000 births in the United States, and the X-linked form is most common, occurring in about 60% of patients, Dr. Kohn told attendees of the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Most of these patients are treated with antibacterial or antifungal prophylaxis. While allogeneic hematopoietic stem cell transplantation is also an option, according to Dr. Kohn, the approach is limited by a lack of matched donors and graft-versus-host disease.
Dr. Kohn reported results for nine patients in the United States and the United Kingdom who were treated with the same G1XCGD lentiviral vector. The patients, who ranged in age from 2 to 27 years, underwent CD34+ cell mobilization or bone marrow isolation, transduction with the lentiviral vector, busulfan conditioning, and autologous transplantation.
All patients had confirmed X-linked chronic granulomatous disease, and had had at least one severe infection or inflammatory complication requiring hospitalization.
There were no infusion-related adverse events, and one serious adverse event, which was an inflammatory syndrome that resolved with steroids. Two patients died from complications unrelated to gene therapy, Dr. Kohn reported.
“The other patients are basically doing quite well,” he said.
Of the seven patients alive at the 12-month follow up, six were reported as “clinically well” and off antibiotic prophylaxis, according to Dr. Kohn, while the seventh patient was clinically well and receiving antimicrobial support.
Dr. Kohn is a scientific advisory board member for Orchard Therapeutics, which licensed the lentiviral gene therapy for X-CGD discussed in his presentation. He is also an inventor of intellectual property related to the therapy that UCLA has licensed to Orchard.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Kohn DB et al. TCT 2019, Abstract LBA1.
HOUSTON – , said Donald B. Kohn, MD, of the University of California, Los Angeles.
Seven of nine patients treated were “alive and well” at 12 months’ follow-up after receiving lentiviral vector transduced CD34+ cells, Dr. Kohn reported in a late-breaking clinical trial session at the Transplantation & Cellular Therapy Meetings.
Most patients were able to discontinue antibiotic prophylaxis for this disease, which is associated with severe, recurrent, and prolonged life-threatening infections, he said.
Results of the small study provide “proof of concept” for use of the gene therapy in the disease, though additional studies are needed to formally assess the clinical safety and efficacy of the approach, he said.
The estimated incidence of chronic granulomatous disease is 1 in 200,000 births in the United States, and the X-linked form is most common, occurring in about 60% of patients, Dr. Kohn told attendees of the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Most of these patients are treated with antibacterial or antifungal prophylaxis. While allogeneic hematopoietic stem cell transplantation is also an option, according to Dr. Kohn, the approach is limited by a lack of matched donors and graft-versus-host disease.
Dr. Kohn reported results for nine patients in the United States and the United Kingdom who were treated with the same G1XCGD lentiviral vector. The patients, who ranged in age from 2 to 27 years, underwent CD34+ cell mobilization or bone marrow isolation, transduction with the lentiviral vector, busulfan conditioning, and autologous transplantation.
All patients had confirmed X-linked chronic granulomatous disease, and had had at least one severe infection or inflammatory complication requiring hospitalization.
There were no infusion-related adverse events, and one serious adverse event, which was an inflammatory syndrome that resolved with steroids. Two patients died from complications unrelated to gene therapy, Dr. Kohn reported.
“The other patients are basically doing quite well,” he said.
Of the seven patients alive at the 12-month follow up, six were reported as “clinically well” and off antibiotic prophylaxis, according to Dr. Kohn, while the seventh patient was clinically well and receiving antimicrobial support.
Dr. Kohn is a scientific advisory board member for Orchard Therapeutics, which licensed the lentiviral gene therapy for X-CGD discussed in his presentation. He is also an inventor of intellectual property related to the therapy that UCLA has licensed to Orchard.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Kohn DB et al. TCT 2019, Abstract LBA1.
HOUSTON – , said Donald B. Kohn, MD, of the University of California, Los Angeles.
Seven of nine patients treated were “alive and well” at 12 months’ follow-up after receiving lentiviral vector transduced CD34+ cells, Dr. Kohn reported in a late-breaking clinical trial session at the Transplantation & Cellular Therapy Meetings.
Most patients were able to discontinue antibiotic prophylaxis for this disease, which is associated with severe, recurrent, and prolonged life-threatening infections, he said.
Results of the small study provide “proof of concept” for use of the gene therapy in the disease, though additional studies are needed to formally assess the clinical safety and efficacy of the approach, he said.
The estimated incidence of chronic granulomatous disease is 1 in 200,000 births in the United States, and the X-linked form is most common, occurring in about 60% of patients, Dr. Kohn told attendees of the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Most of these patients are treated with antibacterial or antifungal prophylaxis. While allogeneic hematopoietic stem cell transplantation is also an option, according to Dr. Kohn, the approach is limited by a lack of matched donors and graft-versus-host disease.
Dr. Kohn reported results for nine patients in the United States and the United Kingdom who were treated with the same G1XCGD lentiviral vector. The patients, who ranged in age from 2 to 27 years, underwent CD34+ cell mobilization or bone marrow isolation, transduction with the lentiviral vector, busulfan conditioning, and autologous transplantation.
All patients had confirmed X-linked chronic granulomatous disease, and had had at least one severe infection or inflammatory complication requiring hospitalization.
There were no infusion-related adverse events, and one serious adverse event, which was an inflammatory syndrome that resolved with steroids. Two patients died from complications unrelated to gene therapy, Dr. Kohn reported.
“The other patients are basically doing quite well,” he said.
Of the seven patients alive at the 12-month follow up, six were reported as “clinically well” and off antibiotic prophylaxis, according to Dr. Kohn, while the seventh patient was clinically well and receiving antimicrobial support.
Dr. Kohn is a scientific advisory board member for Orchard Therapeutics, which licensed the lentiviral gene therapy for X-CGD discussed in his presentation. He is also an inventor of intellectual property related to the therapy that UCLA has licensed to Orchard.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Kohn DB et al. TCT 2019, Abstract LBA1.
REPORTING FROM TCT 2019
Novel transplant regimen improves survival in primary immunodeficiency
HOUSTON – Allogeneic hematopoietic stem cell transplantation (allo-HCT) following a novel reduced-intensity conditioning regimen was largely successful in a heterogeneous cohort of 29 adults and children with primary immunodeficiency in a prospective clinical trial.
At 1 year after transplant, overall survival was 98% and the estimated graft failure–free and graft-versus-host disease (GVHD)–free survival was 82% among the participants, who had various underlying primary immunodeficiencies (PIDs), Dimana Dimitrova, MD, reported at the Transplantation and Cellular Therapy Meetings.
GVHD-free survival was defined in this National Institutes of Health study as the absence of steroid-refractory grade 3-4 acute GVHD and chronic GVHD, noted Dr. Dimitrova of the NIH.
All patients, including 19 adults and 10 children (median age, 25 years), received a serotherapy-free, radiation-free, reduced-intensity conditioning regimen designed to optimize immune reconstitution, minimize toxicity and GVHD, reduce the risk of infectious complications, and enable successful use of alternative donors.
The conditioning platform included pentostatin on day –11 and day –7 at 4 mg/m2 along with 8 days of low-dose cyclophosphamide and 2 days of pharmacokinetically dosed busulfan at 4,600 mmol/min. GVHD prophylaxis included posttransplantation cyclophosphamide, mycophenolate mofetil (MMF), and sirolimus.
All patients received T cell–replete bone marrow or peripheral blood stem cell allografts; 72% received alternative donor grafts, Dr. Dimitrova said.
Two patients died, including one with bacterial sepsis and invasive aspergillosis who died on day +44 and one with presumed viral encephalitis who died on day +110. The patients were high risk overall (median HCT–comorbidity index score of 3, with a range of 0-11), and the two who died had HCT-CI scores of 6 and 8, respectively.
An additional accidental death occurred at 18 months after transplant “in the setting of continued remission, good graft function, and no transplant-related complications,” she said.
Neutrophil recovery occurred at a median of 17 days after transplant; three patients experienced graft failure, including one primary failure with autologous recovery on day +14 and two secondary graft failures.
“Two patients with known underlying difficult-to-engraft diseases required second transplants using different nonmyeloabalative platforms, and nevertheless required donor lymphocyte infusions to avoid threatened secondary graft failure,” she said. “The third patient actually had sufficiently improved infectious disease control and has not needed a second transplant to date.”
Overall GVHD incidence using the novel platform has been extremely low, she said, noting that 14% of patients had grade 2-4 GVHD and 3% had grade 3-4 acute GVHD. There was no steroid-refractory GVHD or chronic GVHD.
Among the infectious complications, other than those that led to the two deaths, were cytomegalovirus reactivation in 7 of 16 patients at risk, BK virus–associated hemorrhagic cystitis in 19 of 22 patients at risk, and a suspected case of viral cardiomyopathy that ultimately resolved.
“Importantly, although many patients had Epstein-Barr virus [EBV] control issues prior to transplant, no patients received preemptive EBV-directed therapy, and no patients had EBV-PTLD [posttransplant lymphoproliferative disorder],” she said.
Additionally, blood stream infections were detected in five patients, there were two cases of confirmed aspergillosis, and one child developed cutaneous candidiasis. Other complications and toxicities appeared to relate to underlying pretransplant issues in the affected organ or exuberant immune responses to existing infection.
“Phenotype reversal was evident to some degree in all evaluable patients, even in those with mixed chimerism or unknown underlying genetic defect,” Dr. Dimitrova said.
All 10 patients with malignancy or lymphoproliferative disease as an additional indication for allo-HCT remain in remission, and most patients who required immunoglobulin replacement therapy prior to transplant have been able to discontinue it, she noted.
The findings of this study are of note, because while it has been known for decades that allo-HCT is a potentially curative therapy for patients with PIDs that arise from defects in cells of hematopoietic origin, it frequently fails because of complicating factors or is not an option, Dr. Dimitrova said.
“These patients will often enter transplant with multiple comorbidities and disease sequelae, particularly as diagnosis of PIDs increases in older children and adults following years of illness,” she explained, adding that related donor options may be limited if family members are also affected.
For this reason, and with the goal of improving access to allo-HCT to all who require it, the novel conditioning platform used in this study was developed.
The platform was well tolerated overall, Dr. Dimitrova said, emphasizing the “notably low” GVHD rates.
“Currently we are investigating reduced MMF with the goal of promoting earlier immune reconstitution, and a separate protocol has opened that includes several modifications to this platform aimed at patients with increased risk of graft failure who may not tolerate mixed chimerism early on,” she said, noting that both protocols are currently enrolling.
The meeting was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Dimitrova reported having no financial disclosures.
SOURCE: Dimitrova D et al. TCT 2019, Abstract 54.
HOUSTON – Allogeneic hematopoietic stem cell transplantation (allo-HCT) following a novel reduced-intensity conditioning regimen was largely successful in a heterogeneous cohort of 29 adults and children with primary immunodeficiency in a prospective clinical trial.
At 1 year after transplant, overall survival was 98% and the estimated graft failure–free and graft-versus-host disease (GVHD)–free survival was 82% among the participants, who had various underlying primary immunodeficiencies (PIDs), Dimana Dimitrova, MD, reported at the Transplantation and Cellular Therapy Meetings.
GVHD-free survival was defined in this National Institutes of Health study as the absence of steroid-refractory grade 3-4 acute GVHD and chronic GVHD, noted Dr. Dimitrova of the NIH.
All patients, including 19 adults and 10 children (median age, 25 years), received a serotherapy-free, radiation-free, reduced-intensity conditioning regimen designed to optimize immune reconstitution, minimize toxicity and GVHD, reduce the risk of infectious complications, and enable successful use of alternative donors.
The conditioning platform included pentostatin on day –11 and day –7 at 4 mg/m2 along with 8 days of low-dose cyclophosphamide and 2 days of pharmacokinetically dosed busulfan at 4,600 mmol/min. GVHD prophylaxis included posttransplantation cyclophosphamide, mycophenolate mofetil (MMF), and sirolimus.
All patients received T cell–replete bone marrow or peripheral blood stem cell allografts; 72% received alternative donor grafts, Dr. Dimitrova said.
Two patients died, including one with bacterial sepsis and invasive aspergillosis who died on day +44 and one with presumed viral encephalitis who died on day +110. The patients were high risk overall (median HCT–comorbidity index score of 3, with a range of 0-11), and the two who died had HCT-CI scores of 6 and 8, respectively.
An additional accidental death occurred at 18 months after transplant “in the setting of continued remission, good graft function, and no transplant-related complications,” she said.
Neutrophil recovery occurred at a median of 17 days after transplant; three patients experienced graft failure, including one primary failure with autologous recovery on day +14 and two secondary graft failures.
“Two patients with known underlying difficult-to-engraft diseases required second transplants using different nonmyeloabalative platforms, and nevertheless required donor lymphocyte infusions to avoid threatened secondary graft failure,” she said. “The third patient actually had sufficiently improved infectious disease control and has not needed a second transplant to date.”
Overall GVHD incidence using the novel platform has been extremely low, she said, noting that 14% of patients had grade 2-4 GVHD and 3% had grade 3-4 acute GVHD. There was no steroid-refractory GVHD or chronic GVHD.
Among the infectious complications, other than those that led to the two deaths, were cytomegalovirus reactivation in 7 of 16 patients at risk, BK virus–associated hemorrhagic cystitis in 19 of 22 patients at risk, and a suspected case of viral cardiomyopathy that ultimately resolved.
“Importantly, although many patients had Epstein-Barr virus [EBV] control issues prior to transplant, no patients received preemptive EBV-directed therapy, and no patients had EBV-PTLD [posttransplant lymphoproliferative disorder],” she said.
Additionally, blood stream infections were detected in five patients, there were two cases of confirmed aspergillosis, and one child developed cutaneous candidiasis. Other complications and toxicities appeared to relate to underlying pretransplant issues in the affected organ or exuberant immune responses to existing infection.
“Phenotype reversal was evident to some degree in all evaluable patients, even in those with mixed chimerism or unknown underlying genetic defect,” Dr. Dimitrova said.
All 10 patients with malignancy or lymphoproliferative disease as an additional indication for allo-HCT remain in remission, and most patients who required immunoglobulin replacement therapy prior to transplant have been able to discontinue it, she noted.
The findings of this study are of note, because while it has been known for decades that allo-HCT is a potentially curative therapy for patients with PIDs that arise from defects in cells of hematopoietic origin, it frequently fails because of complicating factors or is not an option, Dr. Dimitrova said.
“These patients will often enter transplant with multiple comorbidities and disease sequelae, particularly as diagnosis of PIDs increases in older children and adults following years of illness,” she explained, adding that related donor options may be limited if family members are also affected.
For this reason, and with the goal of improving access to allo-HCT to all who require it, the novel conditioning platform used in this study was developed.
The platform was well tolerated overall, Dr. Dimitrova said, emphasizing the “notably low” GVHD rates.
“Currently we are investigating reduced MMF with the goal of promoting earlier immune reconstitution, and a separate protocol has opened that includes several modifications to this platform aimed at patients with increased risk of graft failure who may not tolerate mixed chimerism early on,” she said, noting that both protocols are currently enrolling.
The meeting was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Dimitrova reported having no financial disclosures.
SOURCE: Dimitrova D et al. TCT 2019, Abstract 54.
HOUSTON – Allogeneic hematopoietic stem cell transplantation (allo-HCT) following a novel reduced-intensity conditioning regimen was largely successful in a heterogeneous cohort of 29 adults and children with primary immunodeficiency in a prospective clinical trial.
At 1 year after transplant, overall survival was 98% and the estimated graft failure–free and graft-versus-host disease (GVHD)–free survival was 82% among the participants, who had various underlying primary immunodeficiencies (PIDs), Dimana Dimitrova, MD, reported at the Transplantation and Cellular Therapy Meetings.
GVHD-free survival was defined in this National Institutes of Health study as the absence of steroid-refractory grade 3-4 acute GVHD and chronic GVHD, noted Dr. Dimitrova of the NIH.
All patients, including 19 adults and 10 children (median age, 25 years), received a serotherapy-free, radiation-free, reduced-intensity conditioning regimen designed to optimize immune reconstitution, minimize toxicity and GVHD, reduce the risk of infectious complications, and enable successful use of alternative donors.
The conditioning platform included pentostatin on day –11 and day –7 at 4 mg/m2 along with 8 days of low-dose cyclophosphamide and 2 days of pharmacokinetically dosed busulfan at 4,600 mmol/min. GVHD prophylaxis included posttransplantation cyclophosphamide, mycophenolate mofetil (MMF), and sirolimus.
All patients received T cell–replete bone marrow or peripheral blood stem cell allografts; 72% received alternative donor grafts, Dr. Dimitrova said.
Two patients died, including one with bacterial sepsis and invasive aspergillosis who died on day +44 and one with presumed viral encephalitis who died on day +110. The patients were high risk overall (median HCT–comorbidity index score of 3, with a range of 0-11), and the two who died had HCT-CI scores of 6 and 8, respectively.
An additional accidental death occurred at 18 months after transplant “in the setting of continued remission, good graft function, and no transplant-related complications,” she said.
Neutrophil recovery occurred at a median of 17 days after transplant; three patients experienced graft failure, including one primary failure with autologous recovery on day +14 and two secondary graft failures.
“Two patients with known underlying difficult-to-engraft diseases required second transplants using different nonmyeloabalative platforms, and nevertheless required donor lymphocyte infusions to avoid threatened secondary graft failure,” she said. “The third patient actually had sufficiently improved infectious disease control and has not needed a second transplant to date.”
Overall GVHD incidence using the novel platform has been extremely low, she said, noting that 14% of patients had grade 2-4 GVHD and 3% had grade 3-4 acute GVHD. There was no steroid-refractory GVHD or chronic GVHD.
Among the infectious complications, other than those that led to the two deaths, were cytomegalovirus reactivation in 7 of 16 patients at risk, BK virus–associated hemorrhagic cystitis in 19 of 22 patients at risk, and a suspected case of viral cardiomyopathy that ultimately resolved.
“Importantly, although many patients had Epstein-Barr virus [EBV] control issues prior to transplant, no patients received preemptive EBV-directed therapy, and no patients had EBV-PTLD [posttransplant lymphoproliferative disorder],” she said.
Additionally, blood stream infections were detected in five patients, there were two cases of confirmed aspergillosis, and one child developed cutaneous candidiasis. Other complications and toxicities appeared to relate to underlying pretransplant issues in the affected organ or exuberant immune responses to existing infection.
“Phenotype reversal was evident to some degree in all evaluable patients, even in those with mixed chimerism or unknown underlying genetic defect,” Dr. Dimitrova said.
All 10 patients with malignancy or lymphoproliferative disease as an additional indication for allo-HCT remain in remission, and most patients who required immunoglobulin replacement therapy prior to transplant have been able to discontinue it, she noted.
The findings of this study are of note, because while it has been known for decades that allo-HCT is a potentially curative therapy for patients with PIDs that arise from defects in cells of hematopoietic origin, it frequently fails because of complicating factors or is not an option, Dr. Dimitrova said.
“These patients will often enter transplant with multiple comorbidities and disease sequelae, particularly as diagnosis of PIDs increases in older children and adults following years of illness,” she explained, adding that related donor options may be limited if family members are also affected.
For this reason, and with the goal of improving access to allo-HCT to all who require it, the novel conditioning platform used in this study was developed.
The platform was well tolerated overall, Dr. Dimitrova said, emphasizing the “notably low” GVHD rates.
“Currently we are investigating reduced MMF with the goal of promoting earlier immune reconstitution, and a separate protocol has opened that includes several modifications to this platform aimed at patients with increased risk of graft failure who may not tolerate mixed chimerism early on,” she said, noting that both protocols are currently enrolling.
The meeting was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Dimitrova reported having no financial disclosures.
SOURCE: Dimitrova D et al. TCT 2019, Abstract 54.
REPORTING FROM TCT 2019
Age 1 food allergies often disappear by age 6
SAN FRANCISCO –
Among 131 infants diagnosed with a peanut allergy when they were 1 year old and then followed with repeat testing 5 years later, 41 (31%) had complete resolution of their peanut allergy, while the allergy persisted in the other 90 children, Rachel L. Peters, PhD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The study also followed 404 infants diagnosed with an egg allergy at 1 year of age and found that by age 6 the allergy had resolved in 368 (91%), while persisting in 36 children, said Dr. Peters, an epidemiologist at Murdoch Children’s Research Institute in Parkville, Australia.
The analysis also identified risk factors that linked with an increased rate of allergy persistence. For peanut allergy persistence beyond the first year, the correlating factors were early-onset eczema, tree nut allergy, and a stronger peanut allergy identified by a greater than 4-mm reaction to a peanut skin-prick test. Factors that linked with an increased rate of persistent egg allergy were eczema, peanut allergy, gastrointestinal or respiratory reaction symptoms to milk, and reaction on an oral food challenge elicited by a low dose (less than 0.5 mL) of milk.
A consequence of the frequent resolution of these food allergies was that a positive skin-prick test reaction to either peanut or egg at 1 year old was poorly predictive of allergy status at age 6, while skin-prick tests at age 6 worked well for identifying a persistent food allergy at that age.
The analyses that Dr. Peters and her associates ran used data collected in the HealthNuts study, a comprehensive, prospective, population-based study of food allergies in children that enrolled 5,276 infants at 1 year old. The HealthNuts researchers enrolled infants at immunization clinics in the Melbourne area, with enrollment stratified to represent the people who live in that region (Clin Exp Allergy. 2010 Oct;40[10]:1516-22).
[email protected]
On Twitter @mitchelzoler
SOURCE: Peters R et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB421.
SAN FRANCISCO –
Among 131 infants diagnosed with a peanut allergy when they were 1 year old and then followed with repeat testing 5 years later, 41 (31%) had complete resolution of their peanut allergy, while the allergy persisted in the other 90 children, Rachel L. Peters, PhD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The study also followed 404 infants diagnosed with an egg allergy at 1 year of age and found that by age 6 the allergy had resolved in 368 (91%), while persisting in 36 children, said Dr. Peters, an epidemiologist at Murdoch Children’s Research Institute in Parkville, Australia.
The analysis also identified risk factors that linked with an increased rate of allergy persistence. For peanut allergy persistence beyond the first year, the correlating factors were early-onset eczema, tree nut allergy, and a stronger peanut allergy identified by a greater than 4-mm reaction to a peanut skin-prick test. Factors that linked with an increased rate of persistent egg allergy were eczema, peanut allergy, gastrointestinal or respiratory reaction symptoms to milk, and reaction on an oral food challenge elicited by a low dose (less than 0.5 mL) of milk.
A consequence of the frequent resolution of these food allergies was that a positive skin-prick test reaction to either peanut or egg at 1 year old was poorly predictive of allergy status at age 6, while skin-prick tests at age 6 worked well for identifying a persistent food allergy at that age.
The analyses that Dr. Peters and her associates ran used data collected in the HealthNuts study, a comprehensive, prospective, population-based study of food allergies in children that enrolled 5,276 infants at 1 year old. The HealthNuts researchers enrolled infants at immunization clinics in the Melbourne area, with enrollment stratified to represent the people who live in that region (Clin Exp Allergy. 2010 Oct;40[10]:1516-22).
[email protected]
On Twitter @mitchelzoler
SOURCE: Peters R et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB421.
SAN FRANCISCO –
Among 131 infants diagnosed with a peanut allergy when they were 1 year old and then followed with repeat testing 5 years later, 41 (31%) had complete resolution of their peanut allergy, while the allergy persisted in the other 90 children, Rachel L. Peters, PhD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The study also followed 404 infants diagnosed with an egg allergy at 1 year of age and found that by age 6 the allergy had resolved in 368 (91%), while persisting in 36 children, said Dr. Peters, an epidemiologist at Murdoch Children’s Research Institute in Parkville, Australia.
The analysis also identified risk factors that linked with an increased rate of allergy persistence. For peanut allergy persistence beyond the first year, the correlating factors were early-onset eczema, tree nut allergy, and a stronger peanut allergy identified by a greater than 4-mm reaction to a peanut skin-prick test. Factors that linked with an increased rate of persistent egg allergy were eczema, peanut allergy, gastrointestinal or respiratory reaction symptoms to milk, and reaction on an oral food challenge elicited by a low dose (less than 0.5 mL) of milk.
A consequence of the frequent resolution of these food allergies was that a positive skin-prick test reaction to either peanut or egg at 1 year old was poorly predictive of allergy status at age 6, while skin-prick tests at age 6 worked well for identifying a persistent food allergy at that age.
The analyses that Dr. Peters and her associates ran used data collected in the HealthNuts study, a comprehensive, prospective, population-based study of food allergies in children that enrolled 5,276 infants at 1 year old. The HealthNuts researchers enrolled infants at immunization clinics in the Melbourne area, with enrollment stratified to represent the people who live in that region (Clin Exp Allergy. 2010 Oct;40[10]:1516-22).
[email protected]
On Twitter @mitchelzoler
SOURCE: Peters R et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB421.
REPORTING FROM AAAAI