User login
Approach to asymptomatic creatine kinase elevation
Measuring serum creatine kinase (CK) is an important part of the evaluation of patients with muscle weakness or myalgia, and of assessing patients with myopathies or rhabdomyolysis. But elevated CK sometimes is an incidental finding in a patient without muscle-related symptoms or with only minimal nonspecific muscle symptoms (eg, cramps, spasms, fatigue) that do not significantly interfere with activities of daily living. This condition is sometimes referred to as “asymptomatic hyper-CK-emia.” Four other muscle enzymes that may also be elevated are aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and aldolase.
This review focuses on the evaluation of patients with elevated CK without significant muscle-related symptoms and proposes an algorithm for this purpose (Figure 1).
CURRENT THRESHOLDS MAY BE LOW
What appears to be an elevated CK level may in fact be normal, and it is important to determine in the initial assessment whether a CK value is truly abnormal.
Most laboratories use the central 95% of observations in white people as a reference range for serum CK, assuming that levels have a gaussian (bell-shaped) distribution, which is usually about 0 to 200 IU/L. Using these parameters, an abnormal CK level was observed in 19% of men and 5% of women in a study of nearly 1,000 healthy young people,1 leading to overdiagnosis.
The actual distribution of serum CK levels in a healthy population is markedly skewed toward higher values and is nongaussian.1–3 A 97.5% normal threshold is associated with a much lower false-positive rate and is recommended by the European Federation of Neurological Societies (now the European Academy of Neurology).4 This group also recommends pursuing further investigation only for patients whose level is at least 1.5 times the upper limit of normal; this threshold results in only a small reduction in sensitivity.
CK levels vary significantly by sex and race.5 Possible reasons include differences in muscle mass or total body mass and inherited differences in the permeability of the sarcolemma to CK.6 There is also a small reduction in CK levels as people age.2
The European Federation of Neurological Societies suggests redefining elevated CK as values 1.5 times beyond the upper limit of normal. Based on a 97.5% threshold and normal values determined by Brewster et al3 for black and white men and women, the following thresholds can be used to help decide whether to pursue further evaluation4:
- White women—325 IU/L
- White men—504 IU/L
- Black women—621 IU/L
- Black men—1,200 IU/L
PHYSICAL ACTIVITY RAISES CK
CK levels transiently rise after exercise or heavy manual labor. Serum CK levels may increase to as much as 30 times the upper limit of normal within 24 hours of strenuous physical activity, then slowly decline over the next 7 days. The degree of CK elevation depends on the type and duration of exercise, with greater elevation in those who are untrained.2,4
In assessing asymptomatic or minimally symptomatic CK elevation, the test should be repeated after 7 days without exercise. A large community study in Norway found that repeat CK levels in people with incidentally discovered elevated CK were normal after 3 days of rest in 70% of cases.2
NONNEUROMUSCULAR CAUSES
NEED TO BE INVESTIGATED
Asymptomatic or minimally symptomatic elevated CK can be due to a primary neuromuscular disease or a variety of nonneuromuscular causes.
Patients who still have elevated CK after taking into account the 97.5% threshold, repeat testing after a week of rest, and a level more than 1.5 times the upper limit of normal for sex and race should first be evaluated for the many nonneuromuscular conditions that can cause elevated CK (Table 1).7–9
Cardiac causes should be evaluated by history and physical examination, electrocardiography, and possibly testing for cardiac troponins.
Drugs commonly elevate CK
Prescription drugs and supplements are an important and common cause of CK elevation, so it is important to carefully review medications the patient is taking.
Statins can cause myalgia, muscle weakness, and rhabdomyolysis. Up to 5% of users develop CK elevation, typically 2 to 10 times the upper limit of normal.10 CK usually drops after stopping statins but may require weeks to months to normalize. Rarely, statin users develop a serious immune-mediated necrotizing myopathy.11–13
The diversity of response to statin therapy appears to have a genetic basis. The SEARCH Collaborative Group14 conducted a genome-wide association study of 300,000 markers in 85 patients with definite or incipient myopathy and in 90 controls, all of whom were taking simvastatin 80 mg daily. They identified a single-nucleotide polymorphism in the SLCO1B1 gene on chromosome 12 that was strongly associated with a higher risk of statin-induced myopathy.
Patients with statin-related myopathy seem to have a higher frequency of occult metabolic muscle disease than the general population, also suggesting genetic susceptibility, although ascertainment bias could be a factor.14
Mechanisms of CK elevation in response to statins include increased muscle membrane fragility due to decreased cholesterol content, inhibition of isoprenoid production (a necessary step in the synthesis of membrane proteins), and depletion of ubiquinone, leading to mitochondrial dysfunction.
Macro CK: An abnormal enzyme complex
About 4% of patients with asymptomatic or minimally symptomatic elevated CK have “macro CK,” an enzyme complex with an atypically high molecular mass and reduced clearance, resulting in abnormally high blood levels of CK. Macro CK type 1 is more common and is found in up to 1.2% of the general population: complexes are composed of CK and immunoglobulin and are associated with autoimmune diseases.9,15 Macro CK type 2 complexes consist of CK and an undetermined protein and are associated with malignancies.
CK electrophoresis is required to detect macro CK. Types 1 and 2 can be distinguished by protein G affinity chromatography.9,15
Endocrine disorders
Muscle involvement in endocrine disorders often presents with muscle weakness in addition to muscle enzyme abnormalities.
Hypothyroidism often causes weakness, cramps, myalgia, and a mild to moderate serum CK elevation.16 Severe CK elevation has been reported to occur after vigorous exercise.17 Thyroid replacement usually results in normalization of serum CK levels in 1 to 2 months.18
Hyperthyroidism is typically associated with normal serum CK concentrations, but in rare cases it can cause rhabdomyolysis.19
NEUROMUSCULAR CAUSES ARE NOT ALWAYS WORTH PURSUING
Only after the nonneuromuscular causes of elevated CK have been ruled out should neuromuscular disorders be considered (Table 2). Evaluation with electromyography, nerve conduction studies, and muscle biopsy may lead to the diagnosis of a specific neuromuscular disorder: patients may be in the presymptomatic stage of disease and may or may not eventually develop muscle weakness or other symptoms.20,21
Is testing needed?
Most adult dystrophies and metabolic myopathies have no available treatment and their course is often benign, particularly if they present only with asymptomatic elevated CK. The value of a potentially extensive, expensive, and invasive evaluation for a specific neuromuscular cause should be weighed against the limited yield and treatment options. Moreover, specialized testing such as biochemical muscle enzyme analysis, sarcolemmal protein staining, and genetic testing are not available at all centers.
The European Federation of Neurological Societies guidelines recommend biopsy for patients with asymptomatic elevated CK who also have any of the following:
- Abnormal (myopathic) findings on electromyography
- CK more than three times the upper limit of normal
- Age less than 25
- Exercise intolerance.4
Idiopathic inflammatory myopathies rarely present with asymptomatic elevated CK.22–26 In one study,27 they were found in just 5% of patients with asymptomatic elevated CK.
Hypomyopathic dermatomyositis and inclusion body myositis can present with mild CK elevations with normal muscle strength, especially early in the disease course. A myositis subset of antisynthetase syndrome can present with mildly elevated CK and interstitial lung disease.27 Many of the inflammatory myopathies respond to treatment so are worth investigating.
In view of complexities in diagnosis of these conditions, one should proceed with testing only after discussing it with patients. Referral to a rheumatology specialist is preferred.
MUSCLE BIOPSY, ELECTROMYOGRAPHY, AND NERVE CONDUCTION STUDIES
Electromyography, nerve conduction studies, or muscle biopsy, or a combination of these tests, is usually needed to investigate neuromuscular causes of elevated CK.
Muscle biopsy abnormalities are found in about two-thirds of cases of asymptomatic elevated CK, but most abnormalities include nonspecific myopathic changes that are not diagnostic. A muscle biopsy that may include special stains for sarcolemmal proteins for muscular dystrophy and biochemical muscle enzyme analysis for metabolic myopathies is diagnostic in only 20% to 25% cases of asymptomatic elevated CK on average, with a variation between different series of 0% to 79%.7,21,27–33
Electromyography and nerve conduction studies alone add little to the workup of asymptomatic elevated CK apart from a modest negative predictive value and as a guide for muscle biopsy. For a very few neuromuscular disorders causing an elevated CK (eg, motor neuron disease, Charcot-Marie-Tooth disease, myotonic dystrophy), electromyography and nerve conduction studies could suffice to make the diagnosis.
Electromyography and nerve conduction studies detect abnormalities in nearly half of cases of asymptomatic CK elevation,7,21,27,28,30,31,33 but, as with biopsy, most changes are nonspecific. Although electromyography and nerve conduction studies can help distinguish primary neuropathic from myopathic disorders, the sensitivity and specificity are low for diagnosis. Normal studies do not rule out a condition, and abnormal studies are not diagnostic of a particular condition, although completely normal studies provide strong evidence against a severe neuromuscular disorder.
Combined testing
Using combined muscle biopsy, electromyography, and nerve conduction studies, the likelihood of making a diagnosis in patients with asymptomatic elevated CK is 28% on average (range of studies 4%–79%),2,7,21,26–28,30–32 and findings are nonspecific in 30% to 40% of cases. Findings are normal in about 30% to 40% of cases, which are thus diagnosed as idiopathic asymptomatic elevated CK.28–31,34
Prelle et al31 retrospectively reviewed the cases of 114 patients, ages 3 to 70, with incidentally discovered elevated CK and few or no symptoms, who underwent muscle biopsy, electromyography, and nerve conduction studies after nonneuromuscular causes were ruled out. Although muscle biopsy findings were abnormal in 39% of cases, a diagnosis was established in only 18% of cases after an extensive workup: the diagnosis was definitive in only 10% and included dystrophinopathies, metabolic myopathies, and rare noninflammatory myopathies. For the remaining 8%, the diagnosis was probable and included four cases of partial carnitine palmitoyl transferase deficiency, three cases of malignant hyperthermia, and two rare inherited disorders.
DNA testing
In women with a serum CK less than three times the upper limit of normal who have a family history of Duchenne or Becker muscular dystrophy, DNA analysis of blood lymphocytes identifies 70% of carriers.4
IDIOPATHIC ELEVATED SERUM CK
Rowland et al35 first coined the term “idiopathic hyper-CK-emia” and defined it as persistent elevation of serum CK despite a normal neurologic examination and testing, including electromyography, nerve conduction studies, and muscle biopsy.35,36 To receive this diagnosis, patients must also have no family history or clinical evidence of neuromuscular disease.
Idiopathic elevated serum CK is sometimes familial. In one study,37 elevated CK was found in family members of 13 of 28 unrelated probands. In the 13 families, 41 individuals had elevated CK. Genetic studies revealed that the condition is genetically heterogeneous and autosomal dominant in at least 60% of cases, with higher penetrance in men.
D’Adda et al26 followed 55 people with idiopathic elevated CK for 7 years. Ten percent were eventually diagnosed with a neuromuscular disorder, 10% developed malignancy, and the remaining 80% developed no new condition. The CK level normalized or decreased in many patients, but most continued to have persistent CK elevations with minimal or no symptoms.
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279:107–115.
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21:494–500.
- Brewster LM, Mairuhu G, Sturk A, van Montfrans GA. Distribution of creatine kinase in the general population: implications for statin therapy. Am Heart J 2007; 154:655–661.
- Kyriakides T, Angelini C, Schaefer J, et al; European Federation of Neurological Societies. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol 2010; 17:767–773.
- Prisant LM, Downton M, Watkins LO, et al. Efficacy and tolerability of lovastatin in 459 African-Americans with hypercholesterolemia. Am J Cardiol 1996; 78:420–444.
- Wong ET, Cobb C, Umehara MK, et al. Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol 1983; 79:582–586.
- Brewster LM, de Visser M. Persistent hyperCKemia: fourteen patients studied in retrospect. Acta Neurol Scand 1988; 77:60–63.
- Weglinski MR, Wedel DJ, Engel AG. Malignant hyperthermia testing in patients with persistently increased serum creatine kinase levels. Anesth Analg 1997; 84:1038–1041.
- Galarraga B, Sinclair D, Fahie-Wilson MN, McCrae FC, Hull RG, Ledingham JM. A rare but important cause for a raised serum creatine kinase concentration: two case reports and a literature review. Rheumatology (Oxford) 2003; 42:186–188.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013; 29:1553–1568.
- Arora R, Liebo M, Maldonado F. Statin-induced myopathy: the two faces of Janus. J Cardiovasc Pharmacol Ther 2006; 11:105–112.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc (2003) 2006; 46:479–490.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Wyness SP, Hunsaker JJ, La’ulu SL, Rao LV, Roberts WL. Detection of macro-creatine kinase and macroamylase by polyethylene glycol precipitation and ultrafiltration methods. Clin Chim Acta 2011; 412:2052–2057.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Riggs JE. Acute exertional rhabdomyolysis in hypothyroidism: the result of a reversible defect in glycogenolysis? Mil Med 1990; 155:171–172.
- Mastaglia FL, Ojeda VJ, Sarnat HB, Kakulas BA. Myopathies associated with hypothyroidism: a review based upon 13 cases. Aust N Z J Med 1988; 18:799–806.
- Alshanti M, Eledrisi MS, Jones E. Rhabdomyolysis associated with hyperthyroidism. Am J Emerg Med 2001; 19:317.
- Rosalki SB. Serum enzymes in disease of skeletal muscle. Clin Lab Med 1989; 9:767–781.
- Joy JL, Oh SJ. Asymptomatic hyper-CK-emia: an electrophysiologic and histopathologic study. Muscle Nerve 1989; 12:206–209.
- Merlini L, Sabatelli P, Columbaro M, et al. Hyper-CK-emia as the sole manifestation of myotonic dystrophy type 2. Muscle Nerve 2005; 31:764–767.
- Eeg-Olofsson O, Kalimo H, Eeg-Olofsson KE, et al. Duchenne muscular dystrophy and idiopathic hyperCKemia in the same family. Eur J Paediatr Neurol 2008; 12:404–407.
- Dwianingsih EK, Takeshima Y, Itoh K, et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol Genet Metab 2010; 101:233–237.
- Carbone I, Bruno C, Sotgia F, et al. Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology 2000; 54:1373–1376.
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253:1399–1403.
- Fernandez C, de Paula AM, Figarella-Branger D, et al. Diagnostic evaluation of clinically normal subjects with chronic hyperCKemia. Neurology 2006; 66:1585–1587.
- Simmons Z, Peterlin BL, Boyer PJ, Towfighi J. Muscle biopsy in the evaluation of patients with modestly elevated creatine kinase levels. Muscle Nerve 2003; 27:242–244.
- Filosto M, Tonin P, Vattemi G, et al. The role of muscle biopsy in investigating isolated muscle pain. Neurology 2007; 68:181–186.
- Malandrini A, Orrico A, Gaudiano C, et al. Muscle biopsy and in vitro contracture test in subjects with idiopathic hyperCKemia. Anesthesiology 2008; 109:625–628.
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249:305–311.
- Dabby R, Sadeh M, Herman O, et al. Asymptomatic or minimally symptomatic hyperCKemia: histopathologic correlates. Isr Med Assoc J 2006; 8:110–113.
- Reijneveld JC, Notermans NC, Linssen WH, Wokke JH. Benign prognosis in idiopathic hyper-CK-emia. Muscle Nerve 2000; 23:575–579.
- Restivo DA, Pavone V, Nicotra A. Single-fiber electromyography in hyperCKemia: the value of fiber density. Neurol Sci 2012; 33:819–824.
- Rowland LP, Willner J, Cerri C, DiMauro S, Miranda A. Approaches to the membrane theory of Duchenne muscular dystrophy. In: Angelini C, Danielli GA, Fontanari D, editors. Muscular Dystrophy Research: Advances and New Trends, Amsterdam: Excerpta Medica; 1980:3–13.
- Reijneveld JC, Notermans NC, Linssen WH, Bär PR, Wokke JH. Hyper-CK-aemia revisited. Neuromuscul Disord 2001; 11:163–164.
- Capasso M, De Angelis MV, Di Muzio A, et al. Familial idiopathic hyper-CK-emia: an underrecognized condition. Muscle Nerve 2006; 33:760–765.
Measuring serum creatine kinase (CK) is an important part of the evaluation of patients with muscle weakness or myalgia, and of assessing patients with myopathies or rhabdomyolysis. But elevated CK sometimes is an incidental finding in a patient without muscle-related symptoms or with only minimal nonspecific muscle symptoms (eg, cramps, spasms, fatigue) that do not significantly interfere with activities of daily living. This condition is sometimes referred to as “asymptomatic hyper-CK-emia.” Four other muscle enzymes that may also be elevated are aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and aldolase.
This review focuses on the evaluation of patients with elevated CK without significant muscle-related symptoms and proposes an algorithm for this purpose (Figure 1).
CURRENT THRESHOLDS MAY BE LOW
What appears to be an elevated CK level may in fact be normal, and it is important to determine in the initial assessment whether a CK value is truly abnormal.
Most laboratories use the central 95% of observations in white people as a reference range for serum CK, assuming that levels have a gaussian (bell-shaped) distribution, which is usually about 0 to 200 IU/L. Using these parameters, an abnormal CK level was observed in 19% of men and 5% of women in a study of nearly 1,000 healthy young people,1 leading to overdiagnosis.
The actual distribution of serum CK levels in a healthy population is markedly skewed toward higher values and is nongaussian.1–3 A 97.5% normal threshold is associated with a much lower false-positive rate and is recommended by the European Federation of Neurological Societies (now the European Academy of Neurology).4 This group also recommends pursuing further investigation only for patients whose level is at least 1.5 times the upper limit of normal; this threshold results in only a small reduction in sensitivity.
CK levels vary significantly by sex and race.5 Possible reasons include differences in muscle mass or total body mass and inherited differences in the permeability of the sarcolemma to CK.6 There is also a small reduction in CK levels as people age.2
The European Federation of Neurological Societies suggests redefining elevated CK as values 1.5 times beyond the upper limit of normal. Based on a 97.5% threshold and normal values determined by Brewster et al3 for black and white men and women, the following thresholds can be used to help decide whether to pursue further evaluation4:
- White women—325 IU/L
- White men—504 IU/L
- Black women—621 IU/L
- Black men—1,200 IU/L
PHYSICAL ACTIVITY RAISES CK
CK levels transiently rise after exercise or heavy manual labor. Serum CK levels may increase to as much as 30 times the upper limit of normal within 24 hours of strenuous physical activity, then slowly decline over the next 7 days. The degree of CK elevation depends on the type and duration of exercise, with greater elevation in those who are untrained.2,4
In assessing asymptomatic or minimally symptomatic CK elevation, the test should be repeated after 7 days without exercise. A large community study in Norway found that repeat CK levels in people with incidentally discovered elevated CK were normal after 3 days of rest in 70% of cases.2
NONNEUROMUSCULAR CAUSES
NEED TO BE INVESTIGATED
Asymptomatic or minimally symptomatic elevated CK can be due to a primary neuromuscular disease or a variety of nonneuromuscular causes.
Patients who still have elevated CK after taking into account the 97.5% threshold, repeat testing after a week of rest, and a level more than 1.5 times the upper limit of normal for sex and race should first be evaluated for the many nonneuromuscular conditions that can cause elevated CK (Table 1).7–9
Cardiac causes should be evaluated by history and physical examination, electrocardiography, and possibly testing for cardiac troponins.
Drugs commonly elevate CK
Prescription drugs and supplements are an important and common cause of CK elevation, so it is important to carefully review medications the patient is taking.
Statins can cause myalgia, muscle weakness, and rhabdomyolysis. Up to 5% of users develop CK elevation, typically 2 to 10 times the upper limit of normal.10 CK usually drops after stopping statins but may require weeks to months to normalize. Rarely, statin users develop a serious immune-mediated necrotizing myopathy.11–13
The diversity of response to statin therapy appears to have a genetic basis. The SEARCH Collaborative Group14 conducted a genome-wide association study of 300,000 markers in 85 patients with definite or incipient myopathy and in 90 controls, all of whom were taking simvastatin 80 mg daily. They identified a single-nucleotide polymorphism in the SLCO1B1 gene on chromosome 12 that was strongly associated with a higher risk of statin-induced myopathy.
Patients with statin-related myopathy seem to have a higher frequency of occult metabolic muscle disease than the general population, also suggesting genetic susceptibility, although ascertainment bias could be a factor.14
Mechanisms of CK elevation in response to statins include increased muscle membrane fragility due to decreased cholesterol content, inhibition of isoprenoid production (a necessary step in the synthesis of membrane proteins), and depletion of ubiquinone, leading to mitochondrial dysfunction.
Macro CK: An abnormal enzyme complex
About 4% of patients with asymptomatic or minimally symptomatic elevated CK have “macro CK,” an enzyme complex with an atypically high molecular mass and reduced clearance, resulting in abnormally high blood levels of CK. Macro CK type 1 is more common and is found in up to 1.2% of the general population: complexes are composed of CK and immunoglobulin and are associated with autoimmune diseases.9,15 Macro CK type 2 complexes consist of CK and an undetermined protein and are associated with malignancies.
CK electrophoresis is required to detect macro CK. Types 1 and 2 can be distinguished by protein G affinity chromatography.9,15
Endocrine disorders
Muscle involvement in endocrine disorders often presents with muscle weakness in addition to muscle enzyme abnormalities.
Hypothyroidism often causes weakness, cramps, myalgia, and a mild to moderate serum CK elevation.16 Severe CK elevation has been reported to occur after vigorous exercise.17 Thyroid replacement usually results in normalization of serum CK levels in 1 to 2 months.18
Hyperthyroidism is typically associated with normal serum CK concentrations, but in rare cases it can cause rhabdomyolysis.19
NEUROMUSCULAR CAUSES ARE NOT ALWAYS WORTH PURSUING
Only after the nonneuromuscular causes of elevated CK have been ruled out should neuromuscular disorders be considered (Table 2). Evaluation with electromyography, nerve conduction studies, and muscle biopsy may lead to the diagnosis of a specific neuromuscular disorder: patients may be in the presymptomatic stage of disease and may or may not eventually develop muscle weakness or other symptoms.20,21
Is testing needed?
Most adult dystrophies and metabolic myopathies have no available treatment and their course is often benign, particularly if they present only with asymptomatic elevated CK. The value of a potentially extensive, expensive, and invasive evaluation for a specific neuromuscular cause should be weighed against the limited yield and treatment options. Moreover, specialized testing such as biochemical muscle enzyme analysis, sarcolemmal protein staining, and genetic testing are not available at all centers.
The European Federation of Neurological Societies guidelines recommend biopsy for patients with asymptomatic elevated CK who also have any of the following:
- Abnormal (myopathic) findings on electromyography
- CK more than three times the upper limit of normal
- Age less than 25
- Exercise intolerance.4
Idiopathic inflammatory myopathies rarely present with asymptomatic elevated CK.22–26 In one study,27 they were found in just 5% of patients with asymptomatic elevated CK.
Hypomyopathic dermatomyositis and inclusion body myositis can present with mild CK elevations with normal muscle strength, especially early in the disease course. A myositis subset of antisynthetase syndrome can present with mildly elevated CK and interstitial lung disease.27 Many of the inflammatory myopathies respond to treatment so are worth investigating.
In view of complexities in diagnosis of these conditions, one should proceed with testing only after discussing it with patients. Referral to a rheumatology specialist is preferred.
MUSCLE BIOPSY, ELECTROMYOGRAPHY, AND NERVE CONDUCTION STUDIES
Electromyography, nerve conduction studies, or muscle biopsy, or a combination of these tests, is usually needed to investigate neuromuscular causes of elevated CK.
Muscle biopsy abnormalities are found in about two-thirds of cases of asymptomatic elevated CK, but most abnormalities include nonspecific myopathic changes that are not diagnostic. A muscle biopsy that may include special stains for sarcolemmal proteins for muscular dystrophy and biochemical muscle enzyme analysis for metabolic myopathies is diagnostic in only 20% to 25% cases of asymptomatic elevated CK on average, with a variation between different series of 0% to 79%.7,21,27–33
Electromyography and nerve conduction studies alone add little to the workup of asymptomatic elevated CK apart from a modest negative predictive value and as a guide for muscle biopsy. For a very few neuromuscular disorders causing an elevated CK (eg, motor neuron disease, Charcot-Marie-Tooth disease, myotonic dystrophy), electromyography and nerve conduction studies could suffice to make the diagnosis.
Electromyography and nerve conduction studies detect abnormalities in nearly half of cases of asymptomatic CK elevation,7,21,27,28,30,31,33 but, as with biopsy, most changes are nonspecific. Although electromyography and nerve conduction studies can help distinguish primary neuropathic from myopathic disorders, the sensitivity and specificity are low for diagnosis. Normal studies do not rule out a condition, and abnormal studies are not diagnostic of a particular condition, although completely normal studies provide strong evidence against a severe neuromuscular disorder.
Combined testing
Using combined muscle biopsy, electromyography, and nerve conduction studies, the likelihood of making a diagnosis in patients with asymptomatic elevated CK is 28% on average (range of studies 4%–79%),2,7,21,26–28,30–32 and findings are nonspecific in 30% to 40% of cases. Findings are normal in about 30% to 40% of cases, which are thus diagnosed as idiopathic asymptomatic elevated CK.28–31,34
Prelle et al31 retrospectively reviewed the cases of 114 patients, ages 3 to 70, with incidentally discovered elevated CK and few or no symptoms, who underwent muscle biopsy, electromyography, and nerve conduction studies after nonneuromuscular causes were ruled out. Although muscle biopsy findings were abnormal in 39% of cases, a diagnosis was established in only 18% of cases after an extensive workup: the diagnosis was definitive in only 10% and included dystrophinopathies, metabolic myopathies, and rare noninflammatory myopathies. For the remaining 8%, the diagnosis was probable and included four cases of partial carnitine palmitoyl transferase deficiency, three cases of malignant hyperthermia, and two rare inherited disorders.
DNA testing
In women with a serum CK less than three times the upper limit of normal who have a family history of Duchenne or Becker muscular dystrophy, DNA analysis of blood lymphocytes identifies 70% of carriers.4
IDIOPATHIC ELEVATED SERUM CK
Rowland et al35 first coined the term “idiopathic hyper-CK-emia” and defined it as persistent elevation of serum CK despite a normal neurologic examination and testing, including electromyography, nerve conduction studies, and muscle biopsy.35,36 To receive this diagnosis, patients must also have no family history or clinical evidence of neuromuscular disease.
Idiopathic elevated serum CK is sometimes familial. In one study,37 elevated CK was found in family members of 13 of 28 unrelated probands. In the 13 families, 41 individuals had elevated CK. Genetic studies revealed that the condition is genetically heterogeneous and autosomal dominant in at least 60% of cases, with higher penetrance in men.
D’Adda et al26 followed 55 people with idiopathic elevated CK for 7 years. Ten percent were eventually diagnosed with a neuromuscular disorder, 10% developed malignancy, and the remaining 80% developed no new condition. The CK level normalized or decreased in many patients, but most continued to have persistent CK elevations with minimal or no symptoms.
Measuring serum creatine kinase (CK) is an important part of the evaluation of patients with muscle weakness or myalgia, and of assessing patients with myopathies or rhabdomyolysis. But elevated CK sometimes is an incidental finding in a patient without muscle-related symptoms or with only minimal nonspecific muscle symptoms (eg, cramps, spasms, fatigue) that do not significantly interfere with activities of daily living. This condition is sometimes referred to as “asymptomatic hyper-CK-emia.” Four other muscle enzymes that may also be elevated are aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and aldolase.
This review focuses on the evaluation of patients with elevated CK without significant muscle-related symptoms and proposes an algorithm for this purpose (Figure 1).
CURRENT THRESHOLDS MAY BE LOW
What appears to be an elevated CK level may in fact be normal, and it is important to determine in the initial assessment whether a CK value is truly abnormal.
Most laboratories use the central 95% of observations in white people as a reference range for serum CK, assuming that levels have a gaussian (bell-shaped) distribution, which is usually about 0 to 200 IU/L. Using these parameters, an abnormal CK level was observed in 19% of men and 5% of women in a study of nearly 1,000 healthy young people,1 leading to overdiagnosis.
The actual distribution of serum CK levels in a healthy population is markedly skewed toward higher values and is nongaussian.1–3 A 97.5% normal threshold is associated with a much lower false-positive rate and is recommended by the European Federation of Neurological Societies (now the European Academy of Neurology).4 This group also recommends pursuing further investigation only for patients whose level is at least 1.5 times the upper limit of normal; this threshold results in only a small reduction in sensitivity.
CK levels vary significantly by sex and race.5 Possible reasons include differences in muscle mass or total body mass and inherited differences in the permeability of the sarcolemma to CK.6 There is also a small reduction in CK levels as people age.2
The European Federation of Neurological Societies suggests redefining elevated CK as values 1.5 times beyond the upper limit of normal. Based on a 97.5% threshold and normal values determined by Brewster et al3 for black and white men and women, the following thresholds can be used to help decide whether to pursue further evaluation4:
- White women—325 IU/L
- White men—504 IU/L
- Black women—621 IU/L
- Black men—1,200 IU/L
PHYSICAL ACTIVITY RAISES CK
CK levels transiently rise after exercise or heavy manual labor. Serum CK levels may increase to as much as 30 times the upper limit of normal within 24 hours of strenuous physical activity, then slowly decline over the next 7 days. The degree of CK elevation depends on the type and duration of exercise, with greater elevation in those who are untrained.2,4
In assessing asymptomatic or minimally symptomatic CK elevation, the test should be repeated after 7 days without exercise. A large community study in Norway found that repeat CK levels in people with incidentally discovered elevated CK were normal after 3 days of rest in 70% of cases.2
NONNEUROMUSCULAR CAUSES
NEED TO BE INVESTIGATED
Asymptomatic or minimally symptomatic elevated CK can be due to a primary neuromuscular disease or a variety of nonneuromuscular causes.
Patients who still have elevated CK after taking into account the 97.5% threshold, repeat testing after a week of rest, and a level more than 1.5 times the upper limit of normal for sex and race should first be evaluated for the many nonneuromuscular conditions that can cause elevated CK (Table 1).7–9
Cardiac causes should be evaluated by history and physical examination, electrocardiography, and possibly testing for cardiac troponins.
Drugs commonly elevate CK
Prescription drugs and supplements are an important and common cause of CK elevation, so it is important to carefully review medications the patient is taking.
Statins can cause myalgia, muscle weakness, and rhabdomyolysis. Up to 5% of users develop CK elevation, typically 2 to 10 times the upper limit of normal.10 CK usually drops after stopping statins but may require weeks to months to normalize. Rarely, statin users develop a serious immune-mediated necrotizing myopathy.11–13
The diversity of response to statin therapy appears to have a genetic basis. The SEARCH Collaborative Group14 conducted a genome-wide association study of 300,000 markers in 85 patients with definite or incipient myopathy and in 90 controls, all of whom were taking simvastatin 80 mg daily. They identified a single-nucleotide polymorphism in the SLCO1B1 gene on chromosome 12 that was strongly associated with a higher risk of statin-induced myopathy.
Patients with statin-related myopathy seem to have a higher frequency of occult metabolic muscle disease than the general population, also suggesting genetic susceptibility, although ascertainment bias could be a factor.14
Mechanisms of CK elevation in response to statins include increased muscle membrane fragility due to decreased cholesterol content, inhibition of isoprenoid production (a necessary step in the synthesis of membrane proteins), and depletion of ubiquinone, leading to mitochondrial dysfunction.
Macro CK: An abnormal enzyme complex
About 4% of patients with asymptomatic or minimally symptomatic elevated CK have “macro CK,” an enzyme complex with an atypically high molecular mass and reduced clearance, resulting in abnormally high blood levels of CK. Macro CK type 1 is more common and is found in up to 1.2% of the general population: complexes are composed of CK and immunoglobulin and are associated with autoimmune diseases.9,15 Macro CK type 2 complexes consist of CK and an undetermined protein and are associated with malignancies.
CK electrophoresis is required to detect macro CK. Types 1 and 2 can be distinguished by protein G affinity chromatography.9,15
Endocrine disorders
Muscle involvement in endocrine disorders often presents with muscle weakness in addition to muscle enzyme abnormalities.
Hypothyroidism often causes weakness, cramps, myalgia, and a mild to moderate serum CK elevation.16 Severe CK elevation has been reported to occur after vigorous exercise.17 Thyroid replacement usually results in normalization of serum CK levels in 1 to 2 months.18
Hyperthyroidism is typically associated with normal serum CK concentrations, but in rare cases it can cause rhabdomyolysis.19
NEUROMUSCULAR CAUSES ARE NOT ALWAYS WORTH PURSUING
Only after the nonneuromuscular causes of elevated CK have been ruled out should neuromuscular disorders be considered (Table 2). Evaluation with electromyography, nerve conduction studies, and muscle biopsy may lead to the diagnosis of a specific neuromuscular disorder: patients may be in the presymptomatic stage of disease and may or may not eventually develop muscle weakness or other symptoms.20,21
Is testing needed?
Most adult dystrophies and metabolic myopathies have no available treatment and their course is often benign, particularly if they present only with asymptomatic elevated CK. The value of a potentially extensive, expensive, and invasive evaluation for a specific neuromuscular cause should be weighed against the limited yield and treatment options. Moreover, specialized testing such as biochemical muscle enzyme analysis, sarcolemmal protein staining, and genetic testing are not available at all centers.
The European Federation of Neurological Societies guidelines recommend biopsy for patients with asymptomatic elevated CK who also have any of the following:
- Abnormal (myopathic) findings on electromyography
- CK more than three times the upper limit of normal
- Age less than 25
- Exercise intolerance.4
Idiopathic inflammatory myopathies rarely present with asymptomatic elevated CK.22–26 In one study,27 they were found in just 5% of patients with asymptomatic elevated CK.
Hypomyopathic dermatomyositis and inclusion body myositis can present with mild CK elevations with normal muscle strength, especially early in the disease course. A myositis subset of antisynthetase syndrome can present with mildly elevated CK and interstitial lung disease.27 Many of the inflammatory myopathies respond to treatment so are worth investigating.
In view of complexities in diagnosis of these conditions, one should proceed with testing only after discussing it with patients. Referral to a rheumatology specialist is preferred.
MUSCLE BIOPSY, ELECTROMYOGRAPHY, AND NERVE CONDUCTION STUDIES
Electromyography, nerve conduction studies, or muscle biopsy, or a combination of these tests, is usually needed to investigate neuromuscular causes of elevated CK.
Muscle biopsy abnormalities are found in about two-thirds of cases of asymptomatic elevated CK, but most abnormalities include nonspecific myopathic changes that are not diagnostic. A muscle biopsy that may include special stains for sarcolemmal proteins for muscular dystrophy and biochemical muscle enzyme analysis for metabolic myopathies is diagnostic in only 20% to 25% cases of asymptomatic elevated CK on average, with a variation between different series of 0% to 79%.7,21,27–33
Electromyography and nerve conduction studies alone add little to the workup of asymptomatic elevated CK apart from a modest negative predictive value and as a guide for muscle biopsy. For a very few neuromuscular disorders causing an elevated CK (eg, motor neuron disease, Charcot-Marie-Tooth disease, myotonic dystrophy), electromyography and nerve conduction studies could suffice to make the diagnosis.
Electromyography and nerve conduction studies detect abnormalities in nearly half of cases of asymptomatic CK elevation,7,21,27,28,30,31,33 but, as with biopsy, most changes are nonspecific. Although electromyography and nerve conduction studies can help distinguish primary neuropathic from myopathic disorders, the sensitivity and specificity are low for diagnosis. Normal studies do not rule out a condition, and abnormal studies are not diagnostic of a particular condition, although completely normal studies provide strong evidence against a severe neuromuscular disorder.
Combined testing
Using combined muscle biopsy, electromyography, and nerve conduction studies, the likelihood of making a diagnosis in patients with asymptomatic elevated CK is 28% on average (range of studies 4%–79%),2,7,21,26–28,30–32 and findings are nonspecific in 30% to 40% of cases. Findings are normal in about 30% to 40% of cases, which are thus diagnosed as idiopathic asymptomatic elevated CK.28–31,34
Prelle et al31 retrospectively reviewed the cases of 114 patients, ages 3 to 70, with incidentally discovered elevated CK and few or no symptoms, who underwent muscle biopsy, electromyography, and nerve conduction studies after nonneuromuscular causes were ruled out. Although muscle biopsy findings were abnormal in 39% of cases, a diagnosis was established in only 18% of cases after an extensive workup: the diagnosis was definitive in only 10% and included dystrophinopathies, metabolic myopathies, and rare noninflammatory myopathies. For the remaining 8%, the diagnosis was probable and included four cases of partial carnitine palmitoyl transferase deficiency, three cases of malignant hyperthermia, and two rare inherited disorders.
DNA testing
In women with a serum CK less than three times the upper limit of normal who have a family history of Duchenne or Becker muscular dystrophy, DNA analysis of blood lymphocytes identifies 70% of carriers.4
IDIOPATHIC ELEVATED SERUM CK
Rowland et al35 first coined the term “idiopathic hyper-CK-emia” and defined it as persistent elevation of serum CK despite a normal neurologic examination and testing, including electromyography, nerve conduction studies, and muscle biopsy.35,36 To receive this diagnosis, patients must also have no family history or clinical evidence of neuromuscular disease.
Idiopathic elevated serum CK is sometimes familial. In one study,37 elevated CK was found in family members of 13 of 28 unrelated probands. In the 13 families, 41 individuals had elevated CK. Genetic studies revealed that the condition is genetically heterogeneous and autosomal dominant in at least 60% of cases, with higher penetrance in men.
D’Adda et al26 followed 55 people with idiopathic elevated CK for 7 years. Ten percent were eventually diagnosed with a neuromuscular disorder, 10% developed malignancy, and the remaining 80% developed no new condition. The CK level normalized or decreased in many patients, but most continued to have persistent CK elevations with minimal or no symptoms.
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279:107–115.
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21:494–500.
- Brewster LM, Mairuhu G, Sturk A, van Montfrans GA. Distribution of creatine kinase in the general population: implications for statin therapy. Am Heart J 2007; 154:655–661.
- Kyriakides T, Angelini C, Schaefer J, et al; European Federation of Neurological Societies. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol 2010; 17:767–773.
- Prisant LM, Downton M, Watkins LO, et al. Efficacy and tolerability of lovastatin in 459 African-Americans with hypercholesterolemia. Am J Cardiol 1996; 78:420–444.
- Wong ET, Cobb C, Umehara MK, et al. Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol 1983; 79:582–586.
- Brewster LM, de Visser M. Persistent hyperCKemia: fourteen patients studied in retrospect. Acta Neurol Scand 1988; 77:60–63.
- Weglinski MR, Wedel DJ, Engel AG. Malignant hyperthermia testing in patients with persistently increased serum creatine kinase levels. Anesth Analg 1997; 84:1038–1041.
- Galarraga B, Sinclair D, Fahie-Wilson MN, McCrae FC, Hull RG, Ledingham JM. A rare but important cause for a raised serum creatine kinase concentration: two case reports and a literature review. Rheumatology (Oxford) 2003; 42:186–188.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013; 29:1553–1568.
- Arora R, Liebo M, Maldonado F. Statin-induced myopathy: the two faces of Janus. J Cardiovasc Pharmacol Ther 2006; 11:105–112.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc (2003) 2006; 46:479–490.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Wyness SP, Hunsaker JJ, La’ulu SL, Rao LV, Roberts WL. Detection of macro-creatine kinase and macroamylase by polyethylene glycol precipitation and ultrafiltration methods. Clin Chim Acta 2011; 412:2052–2057.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Riggs JE. Acute exertional rhabdomyolysis in hypothyroidism: the result of a reversible defect in glycogenolysis? Mil Med 1990; 155:171–172.
- Mastaglia FL, Ojeda VJ, Sarnat HB, Kakulas BA. Myopathies associated with hypothyroidism: a review based upon 13 cases. Aust N Z J Med 1988; 18:799–806.
- Alshanti M, Eledrisi MS, Jones E. Rhabdomyolysis associated with hyperthyroidism. Am J Emerg Med 2001; 19:317.
- Rosalki SB. Serum enzymes in disease of skeletal muscle. Clin Lab Med 1989; 9:767–781.
- Joy JL, Oh SJ. Asymptomatic hyper-CK-emia: an electrophysiologic and histopathologic study. Muscle Nerve 1989; 12:206–209.
- Merlini L, Sabatelli P, Columbaro M, et al. Hyper-CK-emia as the sole manifestation of myotonic dystrophy type 2. Muscle Nerve 2005; 31:764–767.
- Eeg-Olofsson O, Kalimo H, Eeg-Olofsson KE, et al. Duchenne muscular dystrophy and idiopathic hyperCKemia in the same family. Eur J Paediatr Neurol 2008; 12:404–407.
- Dwianingsih EK, Takeshima Y, Itoh K, et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol Genet Metab 2010; 101:233–237.
- Carbone I, Bruno C, Sotgia F, et al. Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology 2000; 54:1373–1376.
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253:1399–1403.
- Fernandez C, de Paula AM, Figarella-Branger D, et al. Diagnostic evaluation of clinically normal subjects with chronic hyperCKemia. Neurology 2006; 66:1585–1587.
- Simmons Z, Peterlin BL, Boyer PJ, Towfighi J. Muscle biopsy in the evaluation of patients with modestly elevated creatine kinase levels. Muscle Nerve 2003; 27:242–244.
- Filosto M, Tonin P, Vattemi G, et al. The role of muscle biopsy in investigating isolated muscle pain. Neurology 2007; 68:181–186.
- Malandrini A, Orrico A, Gaudiano C, et al. Muscle biopsy and in vitro contracture test in subjects with idiopathic hyperCKemia. Anesthesiology 2008; 109:625–628.
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249:305–311.
- Dabby R, Sadeh M, Herman O, et al. Asymptomatic or minimally symptomatic hyperCKemia: histopathologic correlates. Isr Med Assoc J 2006; 8:110–113.
- Reijneveld JC, Notermans NC, Linssen WH, Wokke JH. Benign prognosis in idiopathic hyper-CK-emia. Muscle Nerve 2000; 23:575–579.
- Restivo DA, Pavone V, Nicotra A. Single-fiber electromyography in hyperCKemia: the value of fiber density. Neurol Sci 2012; 33:819–824.
- Rowland LP, Willner J, Cerri C, DiMauro S, Miranda A. Approaches to the membrane theory of Duchenne muscular dystrophy. In: Angelini C, Danielli GA, Fontanari D, editors. Muscular Dystrophy Research: Advances and New Trends, Amsterdam: Excerpta Medica; 1980:3–13.
- Reijneveld JC, Notermans NC, Linssen WH, Bär PR, Wokke JH. Hyper-CK-aemia revisited. Neuromuscul Disord 2001; 11:163–164.
- Capasso M, De Angelis MV, Di Muzio A, et al. Familial idiopathic hyper-CK-emia: an underrecognized condition. Muscle Nerve 2006; 33:760–765.
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279:107–115.
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21:494–500.
- Brewster LM, Mairuhu G, Sturk A, van Montfrans GA. Distribution of creatine kinase in the general population: implications for statin therapy. Am Heart J 2007; 154:655–661.
- Kyriakides T, Angelini C, Schaefer J, et al; European Federation of Neurological Societies. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol 2010; 17:767–773.
- Prisant LM, Downton M, Watkins LO, et al. Efficacy and tolerability of lovastatin in 459 African-Americans with hypercholesterolemia. Am J Cardiol 1996; 78:420–444.
- Wong ET, Cobb C, Umehara MK, et al. Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol 1983; 79:582–586.
- Brewster LM, de Visser M. Persistent hyperCKemia: fourteen patients studied in retrospect. Acta Neurol Scand 1988; 77:60–63.
- Weglinski MR, Wedel DJ, Engel AG. Malignant hyperthermia testing in patients with persistently increased serum creatine kinase levels. Anesth Analg 1997; 84:1038–1041.
- Galarraga B, Sinclair D, Fahie-Wilson MN, McCrae FC, Hull RG, Ledingham JM. A rare but important cause for a raised serum creatine kinase concentration: two case reports and a literature review. Rheumatology (Oxford) 2003; 42:186–188.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013; 29:1553–1568.
- Arora R, Liebo M, Maldonado F. Statin-induced myopathy: the two faces of Janus. J Cardiovasc Pharmacol Ther 2006; 11:105–112.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc (2003) 2006; 46:479–490.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Wyness SP, Hunsaker JJ, La’ulu SL, Rao LV, Roberts WL. Detection of macro-creatine kinase and macroamylase by polyethylene glycol precipitation and ultrafiltration methods. Clin Chim Acta 2011; 412:2052–2057.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Riggs JE. Acute exertional rhabdomyolysis in hypothyroidism: the result of a reversible defect in glycogenolysis? Mil Med 1990; 155:171–172.
- Mastaglia FL, Ojeda VJ, Sarnat HB, Kakulas BA. Myopathies associated with hypothyroidism: a review based upon 13 cases. Aust N Z J Med 1988; 18:799–806.
- Alshanti M, Eledrisi MS, Jones E. Rhabdomyolysis associated with hyperthyroidism. Am J Emerg Med 2001; 19:317.
- Rosalki SB. Serum enzymes in disease of skeletal muscle. Clin Lab Med 1989; 9:767–781.
- Joy JL, Oh SJ. Asymptomatic hyper-CK-emia: an electrophysiologic and histopathologic study. Muscle Nerve 1989; 12:206–209.
- Merlini L, Sabatelli P, Columbaro M, et al. Hyper-CK-emia as the sole manifestation of myotonic dystrophy type 2. Muscle Nerve 2005; 31:764–767.
- Eeg-Olofsson O, Kalimo H, Eeg-Olofsson KE, et al. Duchenne muscular dystrophy and idiopathic hyperCKemia in the same family. Eur J Paediatr Neurol 2008; 12:404–407.
- Dwianingsih EK, Takeshima Y, Itoh K, et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol Genet Metab 2010; 101:233–237.
- Carbone I, Bruno C, Sotgia F, et al. Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology 2000; 54:1373–1376.
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253:1399–1403.
- Fernandez C, de Paula AM, Figarella-Branger D, et al. Diagnostic evaluation of clinically normal subjects with chronic hyperCKemia. Neurology 2006; 66:1585–1587.
- Simmons Z, Peterlin BL, Boyer PJ, Towfighi J. Muscle biopsy in the evaluation of patients with modestly elevated creatine kinase levels. Muscle Nerve 2003; 27:242–244.
- Filosto M, Tonin P, Vattemi G, et al. The role of muscle biopsy in investigating isolated muscle pain. Neurology 2007; 68:181–186.
- Malandrini A, Orrico A, Gaudiano C, et al. Muscle biopsy and in vitro contracture test in subjects with idiopathic hyperCKemia. Anesthesiology 2008; 109:625–628.
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249:305–311.
- Dabby R, Sadeh M, Herman O, et al. Asymptomatic or minimally symptomatic hyperCKemia: histopathologic correlates. Isr Med Assoc J 2006; 8:110–113.
- Reijneveld JC, Notermans NC, Linssen WH, Wokke JH. Benign prognosis in idiopathic hyper-CK-emia. Muscle Nerve 2000; 23:575–579.
- Restivo DA, Pavone V, Nicotra A. Single-fiber electromyography in hyperCKemia: the value of fiber density. Neurol Sci 2012; 33:819–824.
- Rowland LP, Willner J, Cerri C, DiMauro S, Miranda A. Approaches to the membrane theory of Duchenne muscular dystrophy. In: Angelini C, Danielli GA, Fontanari D, editors. Muscular Dystrophy Research: Advances and New Trends, Amsterdam: Excerpta Medica; 1980:3–13.
- Reijneveld JC, Notermans NC, Linssen WH, Bär PR, Wokke JH. Hyper-CK-aemia revisited. Neuromuscul Disord 2001; 11:163–164.
- Capasso M, De Angelis MV, Di Muzio A, et al. Familial idiopathic hyper-CK-emia: an underrecognized condition. Muscle Nerve 2006; 33:760–765.
KEY POINTS
- Standard reference ranges for serum CK levels used by most laboratories are too low and lead to overdiagnosis of abnormal values.
- Serum CK levels are strongly affected by race, sex, and physical activity.
- A patient with truly elevated levels should be evaluated for a variety of nonneuromuscular causes, including endocrine disorders, metabolic disturbances, drug effects, and malignancy.
- Neuromuscular causes should be investigated only after ruling out nonneuromuscular causes and after considering whether potential benefits of a diagnosis outweigh the risks and expense of extensive testing.
Autoantibody-mediated encephalitis: Not just paraneoplastic, not just limbic, and not untreatable
A 79-year-old woman with a history of breast cancer in remission and hypertension presented to a local emergency department because of subacute memory loss and compulsive shopping. Her serum sodium concentration was 127 mmol/L (reference range 132–148). Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain were normal, and she was sent home.
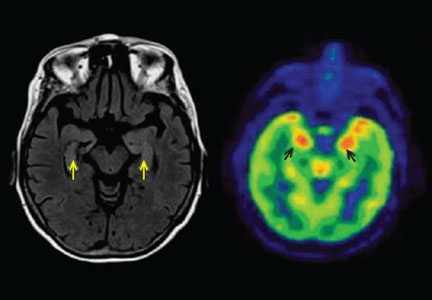
Three days later, she experienced a generalized tonic-clonic seizure that evolved into status epilepticus. She was intubated and admitted to the intensive care unit. Cerebrospinal fluid analysis was normal, and infectious causes of encephalitis were ruled out. MRI showed increased signal in both hippocampi (Figure 1). Her seizures were refractory to treatment, and she was given pentobarbital to induce a coma.
Serum evaluation of neuronal antibodies revealed elevated titers of the voltage-gated potassium channel (VGKC) complex antibody, with subsequent subtyping confirming the leucine-rich glioma-inactivated protein 1 (LGI1) protein as the antigenic target.
She received a 5-day course of intravenous immunoglobulin and methylprednisolone, pentobarbital was withdrawn, and the seizures did not recur, but weeks later she remained comatose. Positron emission tomography (PET) of the brain revealed hypermetabolism in the medial and anterior aspects of both temporal lobes. She underwent five sessions of plasma exchange, after which she began to improve and follow commands. She was ultimately discharged to an acute rehabilitation facility after a 4-week hospital stay.
She received infusions of intravenous immunoglobulin twice a month for 6 months. At her last follow-up visit, she was seizure-free and neurologically intact except for mild inattention.
NEWLY RECOGNIZED DISEASES
Although autoantibody-mediated encephalitic syndromes were first described more than 50 years ago,1,2 their autoimmune basis was not recognized until the early 1980s.3 In the past 10 years, a flood of novel clinical syndromes associated with neuronal autoantibodies has been described that may be markedly improved or even completely resolved with immunotherapy. In cases of unexplained seizure, encephalitis, or acute-onset psychiatric syndromes, suspecting these syndromes can lead to diagnosis, treatment, and a good outcome.
This review describes the key clinical autoantibody-mediated encephalitic syndromes, explains the better-characterized antibody associations, and discusses their diagnosis and treatment.
CLASSIFIED ANATOMICALLY, IMMUNOLOGICALLY, OR EPONYMOUSLY
Autoantibody-mediated encephalitis is also known as autoimmune-mediated encephalitis, autoimmune-mediated limbic encephalitis, and autoimmune synaptic encephalitis.
How to categorize these syndromes is still in flux: they can be listed by the area of the brain affected, the antibody involved, or the name of the discoverer (eg, Morvan syndrome).
Autoantibodies identified in autoimmune encephalitis fall under two broad categories:
- Those targeting intracellular (intranuclear or intracytoplasmic) antigens; the syndromes they cause are more likely to be paraneoplastic and less responsive to immunotherapy
- Those targeting antigens on the neuronal surface: the syndromes they cause are less likely to be paraneoplastic and are more responsive to immunotherapy.4
SYNDROMES DEFINED BY BRAIN AREA AFFECTED
Below, we provide examples of neurologic syndromes of autoantibody-mediated encephalitis according to the region of the brain most affected, ie, the limbic system, the brainstem, or the cerebellum (Figure 2).
LIMBIC ENCEPHALITIS
Memory loss, behavioral changes, seizures
Patients with limbic encephalitis (such as the patient described in the vignette above) present with symptoms attributed to dysfunction of mesial temporal lobe structures, most notably the hippocampus. Prominent symptoms include short-term memory loss, behavioral disturbances such as agitation and confusion, and psychiatric problems such as depression and psychosis. Recurrent seizures are a salient feature and, not uncommonly, progress to status epilepticus.
Antibodies are not all cancer-associated
Cerebrospinal fluid analysis can be normal or show abnormalities suggesting immune activation, eg, slight pleocytosis, elevated protein, increased immunoglobulin G synthesis, and oligoclonal banding.5
In many cases, an autoantibody is found in the blood or in the cerebrospinal fluid. Some patients may express more than one autoantibody, so the traditional view of “one antibody, one syndrome” is incorrect.
Although initially identified as a rare paraneoplastic disorder, limbic encephalitis sometimes occurs in the absence of malignancy.
Multiple antibodies have been linked to the syndrome (Table 1).6–9 The “classic” antibodies initially found in paraneoplastic forms are now generally viewed as nonpathogenic, in part because they are directed against intracellular antigens. Neuronal injury in paraneoplastic limbic encephalitis is believed to be mediated by cytotoxic T lymphocytes, with neuronal autoantibodies being produced after the injury.4 Recently defined antibodies, such as those targeting the N-methyl-d-aspartate (NMDA) receptor6 and the LGI1 protein,7 are now understood to be common causes of limbic encephalitis.
Imaging usually shows limbic focal changes
Structural MRI or functional fluorodeoxyglucose (FDG)-PET imaging may show focal changes in limbic system structures, such as the mesial temporal lobes. It is now recognized that other cortical areas may be involved, and the term “limbic encephalitis” may give way to “cortical” or “focal encephalitis.”
In about 60% of patients, MRI shows hyperintense fluid-attenuated inversion recovery (FLAIR) or T2 signal changes in the mesial temporal lobes, likely reflecting inflammatory changes.4,10,11 On FDG-PET, hypermetabolism may be observed in the mesial temporal lobes early in the disease despite normal findings on MRI.12 Hypometabolism, either diffuse or localized to the mesial temporal lobes, eventually sets in, likely reflecting cytotoxic injury in the aftermath of prolonged inflammation or seizures.
Consider other causes
Before diagnosing limbic encephalitis, it is essential to evaluate for infectious meningoencephalitis, especially herpes simplex viral encephalitis. Thiamine deficiency (Wernicke encephalopathy), drug intoxication, prion disease, Hashimoto encephalopathy, tumor, and subclinical status epilepticus should also be considered. Some of these conditions are associated with the same neuronal autoantibodies detected in limbic encephalitis. Further complicating the picture, case reports have shown the presence of serum neuronal autoantibodies—VGKC complex13–15 and NMDA-receptor antibodies16,17—in confirmed cases of prion disease. In addition, adequately treated herpes simplex viral encephalitis can precipitate the production of NMDA-receptor antibodies and their characteristic syndrome.18–20
BRAINSTEM ENCEPHALITIS
The brainstem—the midbrain, pons, and medulla—can be affected, either in isolation or more commonly as part of a more widespread autoantibody-mediated encephalitis. Symptoms and signs include eye movement abnormalities, ptosis, dysphagia, dysarthria, ataxia, facial palsy, vertigo, hearing impairment, reduced consciousness, and hypoventilation.21
Anti-Hu, anti-Ri, and anti-Ma2 antibodies are most commonly associated with brainstem encephalitis (Table 2). Anti-Ma2-associated encephalitis may improve after a combination of immunotherapy and tumor removal21; the others have a poor prognosis.
Neuromyelitis optica spectrum disorders
Neuromyelitis optica spectrum disorders most commonly involve demyelination affecting the optic nerves and spinal cord, leading to unilateral or bilateral optic neuritis and transverse myelitis spanning three or more vertebral segments.22 The initial clinical manifestation may be an encephalitic pattern, affecting predominantly the brainstem in a restricted fashion,22 or the central nervous system in a more diffuse pattern, mimicking either acute disseminated encephalomyelitis or, in less severe cases, posterior reversible encephalopathy syndrome.23
Testing for antiaquaporin-4 antibody, also known as neuromyelitis optica immunoglobulin G, is the single most decisive laboratory test for diagnosing neuromyelitis optica spectrum disorders, so serum and cerebrospinal fluid evaluation for this autoantibody should be considered when caring for a patient whose clinical picture suggests brainstem encephalitis.22
Bickerstaff brainstem encephalitis
Bickerstaff brainstem encephalitis was first described more than half a century ago in patients with postinfectious ataxia, ophthalmoparesis, and altered consciousness. This rare disease was later found to be associated with antiganglioside GQ1b (anti-GQ1b) autoantibody. MRI is normal in about 90% of cases, so recognizing the clinical presentation and analyzing anti-GQ1b serum titers are critical to diagnosis.
Recovery is usually spontaneous and complete and can be hastened by immunotherapy, especially intravenous immunoglobulin.24
Other causes of brainstem encephalitis
The differential diagnosis of a presentation of brainstem encephalitis includes:
- Infectious causes, the most common being Listeria species followed by enterovirus 71 and herpes simplex virus.25 Tuberculosis, brucellosis, and Whipple disease should also be considered.
- Primary central nervous system inflammatory and demyelinating conditions, eg, multiple sclerosis and acute disseminated encephalomyelitis.
- Systemic inflammatory conditions, eg, Behçet disease, systemic lupus erythematosus, and sarcoidosis.
- Direct brainstem neoplastic involvement, as might occur in primary central nervous system lymphoma or leptomeningeal carcinomatosis.
CEREBELLAR SYNDROME
Patients with autoantibody-mediated encephalitis localized predominantly to the cerebellum typically present with dizziness, vertigo, and unsteady gait, progressing eventually to limb and gait ataxia.4 Symptoms are often subacute, progressing over weeks.
Multiple neuronal autoantibodies have been found to occur with cerebellar encephalitis (Table 2). In most cases, they are paraneoplastic and considered not to be pathogenic, given the intracellular location of their target antigen.4 In such cases, the syndrome is more accurately described as autoantibody-associated rather than autoantibody-mediated. Only in a minority of cases have neuronal autoantibodies been demonstrated to be directly pathogenic, ie, antimetabotropic glutamate receptor type 1 (anti-mGluR1) antibody-associated cerebellitis26 and antiglutamic acid decarboxylase (anti-GAD)-associated cerebellar ataxia.27
Differential diagnosis of cerebellar syndromes
The differential diagnosis of autoantibody-associated cerebellar syndromes is broad and includes:
- Alcohol-induced atrophy
- Drug-induced cerebellar atrophy (eg, from lithium, phenytoin, gabapentin, metronidazole, amiodarone, carbamazepine)
- Vitamin B1 and E deficiency
- Hypothyroidism, hypoparathyroidism
- Neurodegenerative disease (eg, prion disease, multiple system atrophy)
- Parainfectious causes (eg, after infection with Epstein-Barr virus)
- Immune-mediated diseases (Miller-Fisher syndrome, associated with anti-GQ1b antibodies, and antigliadin-associated ataxia, which can occur in isolation or as part of celiac disease).4
SYNDROMES ASSOCIATED WITH SPECIFIC ANTIBODIES
A few of the autoantibody-mediated encephalitic syndromes have specific antibody associations and characteristic clinical presentations. The most prominent of these syndromes are VGKC complex antibody encephalitis (as in the patient described at the beginning of this article) and anti-NMDA receptor encephalitis.
VGKC COMPLEX ANTIBODY-MEDIATED LIMBIC ENCEPHALITIS
VGKC complex antibodies, initially reported to be associated with the peripheral nerve hyperexcitability disorder neuromyotonia, were subsequently found in Morvan syndrome.28,29 Patients with this syndrome often present with autonomic dysfunction and peripheral nerve hyperexcitability but also develop insomnia, confusion, hallucinations, and memory loss. Drawing on the clinical overlap between Morvan syndrome and limbic encephalitis, Buckley et al30 were the first to report VGKC complex antibodies in two cases of limbic encephalitis.
VGKC complex antibodies are now understood to be associated with a wide variety of neurologic conditions, including chronic idiopathic pain, epilepsy,31 movement disorders, cranial nerve abnormalities, autonomic dysfunction,32 and gut dysmotility.33 In contrast, these antibodies are rare in healthy people.34 Limbic encephalitis associated with VGKC complex antibody usually lacks cerebellar and brainstem dysfunction, which may help distinguish it from other types of autoantibody-mediated limbic encephalitis.12
VGKC complex antibody does not bind to the potassium channel itself. Instead it recognizes other constituents of the channel complex, most notably LGI1 and contactin-associated protein 2 (CASPR2). LGI1 antibody is more commonly associated with limbic encephalitis—as illustrated in our case study—in addition to a distinctive type of seizure affecting the arm and face (faciobrachial dystonic seizure).34 The CASPR2 antibody, on the other hand, more often correlates with peripheral nerve manifestations and Morvan syndrome.29 Hyponatremia is commonly seen on serum chemical analysis and provides a clue that these syndromes are present.12
Good response to immunotherapy
A critical change in therapy came as clinicians realized that seizures were often refractory to standard antiepileptic drugs but responded well to immunotherapies. On the basis of these observations, sera of patients with long-standing epilepsy have been reanalyzed to look for neuronal autoantibodies.31 These antibodies should be checked in cases of new-onset refractory status epilepticus of unknown origin that does not respond to antiepileptic medications.
About half of patients with VGKC complex antibody-mediated limbic encephalitis have normal findings on brain MRI.5 Seven of 10 patients who were prospectively followed for VGKC complex antibody-mediated faciobrachial dystonic seizures had normal brain MRIs.35
VGKC complex antibody-mediated limbic encephalitis does not usually recur.36 Most cases are nonparaneoplastic, as evidenced by failure to detect a single active tumor in 64 patients after a median follow-up of 3 years. The prognosis is generally favorable except in cases with coexisting tumors.12
ANTI-NMDA RECEPTOR ENCEPHALITIS
Often associated with ovarian teratoma
Anti-NMDA receptor encephalitis typically affects women in their 20s and 30s, and about half of patients have an ovarian teratoma. It can also occur in younger patients and in men, in whom it is less likely to be associated with a neoplasm.37
Typical initial symptoms include striking and often stereotyped neuropsychiatric disturbances manifesting as psychosis, confusion, seizures, and amnesia. After 1 to 2 weeks, new symptoms set in, including reduced consciousness, movement disorders (ranging from orolingualfacial dyskinesia to rigidity and choreoathetosis), autonomic dysfunction, and hypoventilation, often prompting admission to the intensive care unit.38
Although the outcome is favorable in most cases, recovery, in contrast to VGKC complex antibody-mediated limbic encephalitis, is slow and may take longer than 1 year. Up to a quarter of patients have a relapse, underscoring the importance of maintenance immunotherapy.
It is important to undertake an intensive search for possible ovarian and extraovarian teratomas in young women with this syndrome—including CT of the pelvis, vaginal ultrasonography, and PET imaging—as removal of the teratoma may be curative.37
DIAGNOSIS OF AUTOANTIBODY-MEDIATED ENCEPHALITIS
Critical to diagnosing autoantibody-mediated encephalitis is awareness of these disorders. Since antibody testing may be very specific and is not usually part of the standard batteries of tests, a high level of suspicion is needed. Patients may present to different specialists in different settings; therefore, clinicians in pediatrics, rheumatology, psychiatry, and intensive care medicine need to be aware of these syndromes to avoid delay and misdiagnosis.
Clinical features suggesting autoantibody-mediated encephalitis include:
- Acute or subacute onset of a neurologic syndrome
- New-onset refractory status epilepticus of unknown etiology
- Acute or subacute psychiatric illness with unexpected progression to neurologic symptoms or delirium
- Unusual movement disorders not conforming to standard syndromes
- Cognitive impairment, psychosis, or behavioral or language disorders with atypical findings on imaging or cerebrospinal fluid analysis.
Imaging. Diagnosis of autoantibody-mediated encephalitis focuses on evidence suggesting an inflammatory central nervous system syndrome. MRI may show hyperintense signals on T2, FLAIR, or diffusion-weighted imaging changes in various brain regions. In many cases, however, MRI is negative despite severe clinical symptoms. In a study of 72 patients suspected of having autoimmune dementia of various etiologies, including but not restricted to antineuronal surface antibody-mediated causes, Flanagan et al39 identified atypical neuroimaging findings in only 29%. PET imaging may show hypermetabolism in certain brain areas correlating to clinical syndromes but is often difficult to obtain in a timely fashion.
Cerebrospinal fluid is often abnormal, showing elevated protein, increased immunoglobulin G synthesis, or oligoclonal banding. As with imaging studies, the cerebrospinal fluid may be normal despite severe clinical manifestations.
Electroencephalography may show focal slowing or seizure activity. Neuropsychologic testing may show different patterns of abnormalities.
Antibody testing. None of these tests can be used in isolation, and the diagnosis of autoantibody-mediated encephalitis hinges on recognizing a clinical syndrome and ordering supportive testing. Specific antibodies are more likely in different clinical syndromes and should be sought (Table 3).
Patients who have autoantibody-mediated encephalitis may test negative for autoantibodies for many possible reasons:
- Blood testing for antibodies may be less sensitive than cerebrospinal fluid testing
- Antibody titers may vary in the course of the disease
- The patient may be expressing an antibody that is less often tested for (eg, anti-AMPA receptor or antigamma-aminobutyric acid B) or one that has not yet been isolated.
Evaluating for malignancy is recommended in all cases of autoantibody-mediated encephalitis. The initial workup may involve CT of the chest, abdomen, and pelvis, as well as mammography in women and serum prostate-specific antigen testing and testicular ultrasonography in men. Ordering FDG-PET in cases in which CT is negative or inconclusive increases cancer detection.40 If no cancer is found, close tumor surveillance—every 3 to 6 months—is recommended for at least 2 years.41
TREATMENT
Owing in large part to the rarity of autoantibody-mediated encephalitides, no randomized trials of therapy have been performed. Treatment at present is guided mostly by case series and expert consensus, which suggest first-line therapy with intravenous immunoglobulin, high-dose corticosteroids, plasmapheresis, or a combination.
Different syndromes and antibody-related disorders respond differently to therapy. Syndromes associated with antibodies against intracellular antigens tend to be more resistant to immune therapy than cell surface antigen-related syndromes.4
Tiered approach
Combined treatment with intravenous immunoglobulin and high-dose corticosteroids may be superior to treatment with steroids alone for LGI1-antibody mediated limbic encephalitis.42
In cases refractory to first-line (“tier 1”) therapy, second-line immunotherapy with drugs affecting B-cell populations (eg, rituximab, cyclophosphamide, and mycophenolate mofetil) has been used.
A tiered approach has been most extensively studied for anti-NMDA-receptor encephalitis, with better outcomes found using second-line therapy.43
Treatment strategies for these disorders will likely evolve over time with additional experience.
Outpatient management
Once the patient is discharged from the hospital, a multidisciplinary approach to care is recommended, including physical rehabilitation, speech therapy, neuropsychiatric and neuroimmunologic follow-up, and annual surveillance for malignancies.
- Brierley JB, Corsellis JAN, Hierons R, Nevin S. Subacute encephalitis of later adult life mainly affecting the limbic areas. Brain 1960; 83:357–368.
- Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain 1968; 91:481–496.
- Greenlee JE, Brashear HR. Antibodies to cerebellar Purkinje cells in patients with paraneoplastic cerebellar degeneration and ovarian carcinoma. Ann Neurol 1983; 14:609–613.
- Rosenfeld MR, Dalmau JO. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum (Minneap Minn) 2012; 18:366–383.
- Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-surface central nervous system autoantibodies: clinical relevance and emerging paradigms. Ann Neurol 2014; 76:168–184.
- Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61:25–36.
- Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010; 133:2734–2748.
- Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010; 9:67–76.
- Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009; 65:424–434.
- Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry 2012; 83:638–645.
- Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 2005; 128:1764–1777.
- Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004; 127:701–712.
- Jammoul A, Lederman RJ, Tavee J, Li Y. Presence of voltage-gated potassium channel complex antibody in a case of genetic prion disease. BMJ Case Rep 2014; pii:bcr2013201622.
- Angus-Leppan H, Rudge P, Mead S, Collinge J, Vincent A. Autoantibodies in sporadic Creutzfeldt-Jakob disease. JAMA Neurol 2013; 70:919–922.
- Fujita K, Yuasa T, Watanabe O, et al. Voltage-gated potassium channel complex antibodies in Creutzfeldt-Jakob disease. J Neurol 2012; 259:2249–2250.
- Fujita K, Yuasa T, Takahashi Y, et al. Antibodies to N-methyl-D-aspartate glutamate receptors in Creutzfeldt–Jakob disease patients. J Neuroimmunol 2012; 251:90–93.
- Mackay G, Ahmad K, Stone J, et al. NMDA receptor autoantibodies in sporadic Creutzfeldt-Jakob disease. J Neurol 2012; 259:1979–1981.
- Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus–1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013; 81:1637–1639.
- Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol 2014; 71:344–346.
- Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014; 75:317–323.
- Blaes F. Paraneoplastic brain stem encephalitis. Curr Treat Options Neurol 2013; 15:201–209.
- Wildemann B, Jarius S. The expanding range of autoimmune disorders of the nervous system. Lancet Neurol 2013; 12:22–24.
- Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler 2011; 17:1107–1112.
- Shahrizaila N, Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J Neurol Neurosurg Psychiatry 2013; 84:576–583.
- Jubelt B, Mihai C, Li MT, Veerapaneni P. Rhombencephalitis/brainstem encephalitis. Curr Neurol Neurosci Rep 2011; 11:543–552.
- Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 2000; 342:21–27.
- Ishida K, Mitoma H, Son SY, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol 1999; 46:263–267.
- Hart IK, Waters C, Vincent A, et al. Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol 1997; 41:238–246.
- Barber P, Anderson NE, Vincent A. Morvan’s syndrome associated with voltage-gated K+ channel antibodies. Neurology 2000; 54:771–772.
- Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 2001; 50:73–78.
- Majoie HJ, de Baets M, Renier W, Lang B, Vincent A. Antibodies to voltage-gated potassium and calcium channels in epilepsy. Epilepsy Res 2006; 71:135–141.
- Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology 2008; 70:1883–1890.
- Knowles CH, Lang B, Clover L, et al. A role for autoantibodies in some cases of acquired non-paraneoplastic gut dysmotility. Scand J Gastroenterol 2002; 37:166–170.
- Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011; 69:892–900.
- Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013: 136:3151–3162.
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol 2011; 10:759–772.
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74.
- Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133:1655–1667.
- Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc 2010; 85:881–897.
- Younes-Mhenni S, Janier MF, Cinotti L, et al. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain 2004; 127:2331–2338.
- Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 2011; 77:179–189.
- Shin YW, Lee ST, Shin JW, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 2013; 265:75–81.
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165.
A 79-year-old woman with a history of breast cancer in remission and hypertension presented to a local emergency department because of subacute memory loss and compulsive shopping. Her serum sodium concentration was 127 mmol/L (reference range 132–148). Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain were normal, and she was sent home.
Three days later, she experienced a generalized tonic-clonic seizure that evolved into status epilepticus. She was intubated and admitted to the intensive care unit. Cerebrospinal fluid analysis was normal, and infectious causes of encephalitis were ruled out. MRI showed increased signal in both hippocampi (Figure 1). Her seizures were refractory to treatment, and she was given pentobarbital to induce a coma.
Serum evaluation of neuronal antibodies revealed elevated titers of the voltage-gated potassium channel (VGKC) complex antibody, with subsequent subtyping confirming the leucine-rich glioma-inactivated protein 1 (LGI1) protein as the antigenic target.
She received a 5-day course of intravenous immunoglobulin and methylprednisolone, pentobarbital was withdrawn, and the seizures did not recur, but weeks later she remained comatose. Positron emission tomography (PET) of the brain revealed hypermetabolism in the medial and anterior aspects of both temporal lobes. She underwent five sessions of plasma exchange, after which she began to improve and follow commands. She was ultimately discharged to an acute rehabilitation facility after a 4-week hospital stay.
She received infusions of intravenous immunoglobulin twice a month for 6 months. At her last follow-up visit, she was seizure-free and neurologically intact except for mild inattention.
NEWLY RECOGNIZED DISEASES
Although autoantibody-mediated encephalitic syndromes were first described more than 50 years ago,1,2 their autoimmune basis was not recognized until the early 1980s.3 In the past 10 years, a flood of novel clinical syndromes associated with neuronal autoantibodies has been described that may be markedly improved or even completely resolved with immunotherapy. In cases of unexplained seizure, encephalitis, or acute-onset psychiatric syndromes, suspecting these syndromes can lead to diagnosis, treatment, and a good outcome.
This review describes the key clinical autoantibody-mediated encephalitic syndromes, explains the better-characterized antibody associations, and discusses their diagnosis and treatment.
CLASSIFIED ANATOMICALLY, IMMUNOLOGICALLY, OR EPONYMOUSLY
Autoantibody-mediated encephalitis is also known as autoimmune-mediated encephalitis, autoimmune-mediated limbic encephalitis, and autoimmune synaptic encephalitis.
How to categorize these syndromes is still in flux: they can be listed by the area of the brain affected, the antibody involved, or the name of the discoverer (eg, Morvan syndrome).
Autoantibodies identified in autoimmune encephalitis fall under two broad categories:
- Those targeting intracellular (intranuclear or intracytoplasmic) antigens; the syndromes they cause are more likely to be paraneoplastic and less responsive to immunotherapy
- Those targeting antigens on the neuronal surface: the syndromes they cause are less likely to be paraneoplastic and are more responsive to immunotherapy.4
SYNDROMES DEFINED BY BRAIN AREA AFFECTED
Below, we provide examples of neurologic syndromes of autoantibody-mediated encephalitis according to the region of the brain most affected, ie, the limbic system, the brainstem, or the cerebellum (Figure 2).
LIMBIC ENCEPHALITIS
Memory loss, behavioral changes, seizures
Patients with limbic encephalitis (such as the patient described in the vignette above) present with symptoms attributed to dysfunction of mesial temporal lobe structures, most notably the hippocampus. Prominent symptoms include short-term memory loss, behavioral disturbances such as agitation and confusion, and psychiatric problems such as depression and psychosis. Recurrent seizures are a salient feature and, not uncommonly, progress to status epilepticus.
Antibodies are not all cancer-associated
Cerebrospinal fluid analysis can be normal or show abnormalities suggesting immune activation, eg, slight pleocytosis, elevated protein, increased immunoglobulin G synthesis, and oligoclonal banding.5
In many cases, an autoantibody is found in the blood or in the cerebrospinal fluid. Some patients may express more than one autoantibody, so the traditional view of “one antibody, one syndrome” is incorrect.
Although initially identified as a rare paraneoplastic disorder, limbic encephalitis sometimes occurs in the absence of malignancy.
Multiple antibodies have been linked to the syndrome (Table 1).6–9 The “classic” antibodies initially found in paraneoplastic forms are now generally viewed as nonpathogenic, in part because they are directed against intracellular antigens. Neuronal injury in paraneoplastic limbic encephalitis is believed to be mediated by cytotoxic T lymphocytes, with neuronal autoantibodies being produced after the injury.4 Recently defined antibodies, such as those targeting the N-methyl-d-aspartate (NMDA) receptor6 and the LGI1 protein,7 are now understood to be common causes of limbic encephalitis.
Imaging usually shows limbic focal changes
Structural MRI or functional fluorodeoxyglucose (FDG)-PET imaging may show focal changes in limbic system structures, such as the mesial temporal lobes. It is now recognized that other cortical areas may be involved, and the term “limbic encephalitis” may give way to “cortical” or “focal encephalitis.”
In about 60% of patients, MRI shows hyperintense fluid-attenuated inversion recovery (FLAIR) or T2 signal changes in the mesial temporal lobes, likely reflecting inflammatory changes.4,10,11 On FDG-PET, hypermetabolism may be observed in the mesial temporal lobes early in the disease despite normal findings on MRI.12 Hypometabolism, either diffuse or localized to the mesial temporal lobes, eventually sets in, likely reflecting cytotoxic injury in the aftermath of prolonged inflammation or seizures.
Consider other causes
Before diagnosing limbic encephalitis, it is essential to evaluate for infectious meningoencephalitis, especially herpes simplex viral encephalitis. Thiamine deficiency (Wernicke encephalopathy), drug intoxication, prion disease, Hashimoto encephalopathy, tumor, and subclinical status epilepticus should also be considered. Some of these conditions are associated with the same neuronal autoantibodies detected in limbic encephalitis. Further complicating the picture, case reports have shown the presence of serum neuronal autoantibodies—VGKC complex13–15 and NMDA-receptor antibodies16,17—in confirmed cases of prion disease. In addition, adequately treated herpes simplex viral encephalitis can precipitate the production of NMDA-receptor antibodies and their characteristic syndrome.18–20
BRAINSTEM ENCEPHALITIS
The brainstem—the midbrain, pons, and medulla—can be affected, either in isolation or more commonly as part of a more widespread autoantibody-mediated encephalitis. Symptoms and signs include eye movement abnormalities, ptosis, dysphagia, dysarthria, ataxia, facial palsy, vertigo, hearing impairment, reduced consciousness, and hypoventilation.21
Anti-Hu, anti-Ri, and anti-Ma2 antibodies are most commonly associated with brainstem encephalitis (Table 2). Anti-Ma2-associated encephalitis may improve after a combination of immunotherapy and tumor removal21; the others have a poor prognosis.
Neuromyelitis optica spectrum disorders
Neuromyelitis optica spectrum disorders most commonly involve demyelination affecting the optic nerves and spinal cord, leading to unilateral or bilateral optic neuritis and transverse myelitis spanning three or more vertebral segments.22 The initial clinical manifestation may be an encephalitic pattern, affecting predominantly the brainstem in a restricted fashion,22 or the central nervous system in a more diffuse pattern, mimicking either acute disseminated encephalomyelitis or, in less severe cases, posterior reversible encephalopathy syndrome.23
Testing for antiaquaporin-4 antibody, also known as neuromyelitis optica immunoglobulin G, is the single most decisive laboratory test for diagnosing neuromyelitis optica spectrum disorders, so serum and cerebrospinal fluid evaluation for this autoantibody should be considered when caring for a patient whose clinical picture suggests brainstem encephalitis.22
Bickerstaff brainstem encephalitis
Bickerstaff brainstem encephalitis was first described more than half a century ago in patients with postinfectious ataxia, ophthalmoparesis, and altered consciousness. This rare disease was later found to be associated with antiganglioside GQ1b (anti-GQ1b) autoantibody. MRI is normal in about 90% of cases, so recognizing the clinical presentation and analyzing anti-GQ1b serum titers are critical to diagnosis.
Recovery is usually spontaneous and complete and can be hastened by immunotherapy, especially intravenous immunoglobulin.24
Other causes of brainstem encephalitis
The differential diagnosis of a presentation of brainstem encephalitis includes:
- Infectious causes, the most common being Listeria species followed by enterovirus 71 and herpes simplex virus.25 Tuberculosis, brucellosis, and Whipple disease should also be considered.
- Primary central nervous system inflammatory and demyelinating conditions, eg, multiple sclerosis and acute disseminated encephalomyelitis.
- Systemic inflammatory conditions, eg, Behçet disease, systemic lupus erythematosus, and sarcoidosis.
- Direct brainstem neoplastic involvement, as might occur in primary central nervous system lymphoma or leptomeningeal carcinomatosis.
CEREBELLAR SYNDROME
Patients with autoantibody-mediated encephalitis localized predominantly to the cerebellum typically present with dizziness, vertigo, and unsteady gait, progressing eventually to limb and gait ataxia.4 Symptoms are often subacute, progressing over weeks.
Multiple neuronal autoantibodies have been found to occur with cerebellar encephalitis (Table 2). In most cases, they are paraneoplastic and considered not to be pathogenic, given the intracellular location of their target antigen.4 In such cases, the syndrome is more accurately described as autoantibody-associated rather than autoantibody-mediated. Only in a minority of cases have neuronal autoantibodies been demonstrated to be directly pathogenic, ie, antimetabotropic glutamate receptor type 1 (anti-mGluR1) antibody-associated cerebellitis26 and antiglutamic acid decarboxylase (anti-GAD)-associated cerebellar ataxia.27
Differential diagnosis of cerebellar syndromes
The differential diagnosis of autoantibody-associated cerebellar syndromes is broad and includes:
- Alcohol-induced atrophy
- Drug-induced cerebellar atrophy (eg, from lithium, phenytoin, gabapentin, metronidazole, amiodarone, carbamazepine)
- Vitamin B1 and E deficiency
- Hypothyroidism, hypoparathyroidism
- Neurodegenerative disease (eg, prion disease, multiple system atrophy)
- Parainfectious causes (eg, after infection with Epstein-Barr virus)
- Immune-mediated diseases (Miller-Fisher syndrome, associated with anti-GQ1b antibodies, and antigliadin-associated ataxia, which can occur in isolation or as part of celiac disease).4
SYNDROMES ASSOCIATED WITH SPECIFIC ANTIBODIES
A few of the autoantibody-mediated encephalitic syndromes have specific antibody associations and characteristic clinical presentations. The most prominent of these syndromes are VGKC complex antibody encephalitis (as in the patient described at the beginning of this article) and anti-NMDA receptor encephalitis.
VGKC COMPLEX ANTIBODY-MEDIATED LIMBIC ENCEPHALITIS
VGKC complex antibodies, initially reported to be associated with the peripheral nerve hyperexcitability disorder neuromyotonia, were subsequently found in Morvan syndrome.28,29 Patients with this syndrome often present with autonomic dysfunction and peripheral nerve hyperexcitability but also develop insomnia, confusion, hallucinations, and memory loss. Drawing on the clinical overlap between Morvan syndrome and limbic encephalitis, Buckley et al30 were the first to report VGKC complex antibodies in two cases of limbic encephalitis.
VGKC complex antibodies are now understood to be associated with a wide variety of neurologic conditions, including chronic idiopathic pain, epilepsy,31 movement disorders, cranial nerve abnormalities, autonomic dysfunction,32 and gut dysmotility.33 In contrast, these antibodies are rare in healthy people.34 Limbic encephalitis associated with VGKC complex antibody usually lacks cerebellar and brainstem dysfunction, which may help distinguish it from other types of autoantibody-mediated limbic encephalitis.12
VGKC complex antibody does not bind to the potassium channel itself. Instead it recognizes other constituents of the channel complex, most notably LGI1 and contactin-associated protein 2 (CASPR2). LGI1 antibody is more commonly associated with limbic encephalitis—as illustrated in our case study—in addition to a distinctive type of seizure affecting the arm and face (faciobrachial dystonic seizure).34 The CASPR2 antibody, on the other hand, more often correlates with peripheral nerve manifestations and Morvan syndrome.29 Hyponatremia is commonly seen on serum chemical analysis and provides a clue that these syndromes are present.12
Good response to immunotherapy
A critical change in therapy came as clinicians realized that seizures were often refractory to standard antiepileptic drugs but responded well to immunotherapies. On the basis of these observations, sera of patients with long-standing epilepsy have been reanalyzed to look for neuronal autoantibodies.31 These antibodies should be checked in cases of new-onset refractory status epilepticus of unknown origin that does not respond to antiepileptic medications.
About half of patients with VGKC complex antibody-mediated limbic encephalitis have normal findings on brain MRI.5 Seven of 10 patients who were prospectively followed for VGKC complex antibody-mediated faciobrachial dystonic seizures had normal brain MRIs.35
VGKC complex antibody-mediated limbic encephalitis does not usually recur.36 Most cases are nonparaneoplastic, as evidenced by failure to detect a single active tumor in 64 patients after a median follow-up of 3 years. The prognosis is generally favorable except in cases with coexisting tumors.12
ANTI-NMDA RECEPTOR ENCEPHALITIS
Often associated with ovarian teratoma
Anti-NMDA receptor encephalitis typically affects women in their 20s and 30s, and about half of patients have an ovarian teratoma. It can also occur in younger patients and in men, in whom it is less likely to be associated with a neoplasm.37
Typical initial symptoms include striking and often stereotyped neuropsychiatric disturbances manifesting as psychosis, confusion, seizures, and amnesia. After 1 to 2 weeks, new symptoms set in, including reduced consciousness, movement disorders (ranging from orolingualfacial dyskinesia to rigidity and choreoathetosis), autonomic dysfunction, and hypoventilation, often prompting admission to the intensive care unit.38
Although the outcome is favorable in most cases, recovery, in contrast to VGKC complex antibody-mediated limbic encephalitis, is slow and may take longer than 1 year. Up to a quarter of patients have a relapse, underscoring the importance of maintenance immunotherapy.
It is important to undertake an intensive search for possible ovarian and extraovarian teratomas in young women with this syndrome—including CT of the pelvis, vaginal ultrasonography, and PET imaging—as removal of the teratoma may be curative.37
DIAGNOSIS OF AUTOANTIBODY-MEDIATED ENCEPHALITIS
Critical to diagnosing autoantibody-mediated encephalitis is awareness of these disorders. Since antibody testing may be very specific and is not usually part of the standard batteries of tests, a high level of suspicion is needed. Patients may present to different specialists in different settings; therefore, clinicians in pediatrics, rheumatology, psychiatry, and intensive care medicine need to be aware of these syndromes to avoid delay and misdiagnosis.
Clinical features suggesting autoantibody-mediated encephalitis include:
- Acute or subacute onset of a neurologic syndrome
- New-onset refractory status epilepticus of unknown etiology
- Acute or subacute psychiatric illness with unexpected progression to neurologic symptoms or delirium
- Unusual movement disorders not conforming to standard syndromes
- Cognitive impairment, psychosis, or behavioral or language disorders with atypical findings on imaging or cerebrospinal fluid analysis.
Imaging. Diagnosis of autoantibody-mediated encephalitis focuses on evidence suggesting an inflammatory central nervous system syndrome. MRI may show hyperintense signals on T2, FLAIR, or diffusion-weighted imaging changes in various brain regions. In many cases, however, MRI is negative despite severe clinical symptoms. In a study of 72 patients suspected of having autoimmune dementia of various etiologies, including but not restricted to antineuronal surface antibody-mediated causes, Flanagan et al39 identified atypical neuroimaging findings in only 29%. PET imaging may show hypermetabolism in certain brain areas correlating to clinical syndromes but is often difficult to obtain in a timely fashion.
Cerebrospinal fluid is often abnormal, showing elevated protein, increased immunoglobulin G synthesis, or oligoclonal banding. As with imaging studies, the cerebrospinal fluid may be normal despite severe clinical manifestations.
Electroencephalography may show focal slowing or seizure activity. Neuropsychologic testing may show different patterns of abnormalities.
Antibody testing. None of these tests can be used in isolation, and the diagnosis of autoantibody-mediated encephalitis hinges on recognizing a clinical syndrome and ordering supportive testing. Specific antibodies are more likely in different clinical syndromes and should be sought (Table 3).
Patients who have autoantibody-mediated encephalitis may test negative for autoantibodies for many possible reasons:
- Blood testing for antibodies may be less sensitive than cerebrospinal fluid testing
- Antibody titers may vary in the course of the disease
- The patient may be expressing an antibody that is less often tested for (eg, anti-AMPA receptor or antigamma-aminobutyric acid B) or one that has not yet been isolated.
Evaluating for malignancy is recommended in all cases of autoantibody-mediated encephalitis. The initial workup may involve CT of the chest, abdomen, and pelvis, as well as mammography in women and serum prostate-specific antigen testing and testicular ultrasonography in men. Ordering FDG-PET in cases in which CT is negative or inconclusive increases cancer detection.40 If no cancer is found, close tumor surveillance—every 3 to 6 months—is recommended for at least 2 years.41
TREATMENT
Owing in large part to the rarity of autoantibody-mediated encephalitides, no randomized trials of therapy have been performed. Treatment at present is guided mostly by case series and expert consensus, which suggest first-line therapy with intravenous immunoglobulin, high-dose corticosteroids, plasmapheresis, or a combination.
Different syndromes and antibody-related disorders respond differently to therapy. Syndromes associated with antibodies against intracellular antigens tend to be more resistant to immune therapy than cell surface antigen-related syndromes.4
Tiered approach
Combined treatment with intravenous immunoglobulin and high-dose corticosteroids may be superior to treatment with steroids alone for LGI1-antibody mediated limbic encephalitis.42
In cases refractory to first-line (“tier 1”) therapy, second-line immunotherapy with drugs affecting B-cell populations (eg, rituximab, cyclophosphamide, and mycophenolate mofetil) has been used.
A tiered approach has been most extensively studied for anti-NMDA-receptor encephalitis, with better outcomes found using second-line therapy.43
Treatment strategies for these disorders will likely evolve over time with additional experience.
Outpatient management
Once the patient is discharged from the hospital, a multidisciplinary approach to care is recommended, including physical rehabilitation, speech therapy, neuropsychiatric and neuroimmunologic follow-up, and annual surveillance for malignancies.
A 79-year-old woman with a history of breast cancer in remission and hypertension presented to a local emergency department because of subacute memory loss and compulsive shopping. Her serum sodium concentration was 127 mmol/L (reference range 132–148). Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain were normal, and she was sent home.
Three days later, she experienced a generalized tonic-clonic seizure that evolved into status epilepticus. She was intubated and admitted to the intensive care unit. Cerebrospinal fluid analysis was normal, and infectious causes of encephalitis were ruled out. MRI showed increased signal in both hippocampi (Figure 1). Her seizures were refractory to treatment, and she was given pentobarbital to induce a coma.
Serum evaluation of neuronal antibodies revealed elevated titers of the voltage-gated potassium channel (VGKC) complex antibody, with subsequent subtyping confirming the leucine-rich glioma-inactivated protein 1 (LGI1) protein as the antigenic target.
She received a 5-day course of intravenous immunoglobulin and methylprednisolone, pentobarbital was withdrawn, and the seizures did not recur, but weeks later she remained comatose. Positron emission tomography (PET) of the brain revealed hypermetabolism in the medial and anterior aspects of both temporal lobes. She underwent five sessions of plasma exchange, after which she began to improve and follow commands. She was ultimately discharged to an acute rehabilitation facility after a 4-week hospital stay.
She received infusions of intravenous immunoglobulin twice a month for 6 months. At her last follow-up visit, she was seizure-free and neurologically intact except for mild inattention.
NEWLY RECOGNIZED DISEASES
Although autoantibody-mediated encephalitic syndromes were first described more than 50 years ago,1,2 their autoimmune basis was not recognized until the early 1980s.3 In the past 10 years, a flood of novel clinical syndromes associated with neuronal autoantibodies has been described that may be markedly improved or even completely resolved with immunotherapy. In cases of unexplained seizure, encephalitis, or acute-onset psychiatric syndromes, suspecting these syndromes can lead to diagnosis, treatment, and a good outcome.
This review describes the key clinical autoantibody-mediated encephalitic syndromes, explains the better-characterized antibody associations, and discusses their diagnosis and treatment.
CLASSIFIED ANATOMICALLY, IMMUNOLOGICALLY, OR EPONYMOUSLY
Autoantibody-mediated encephalitis is also known as autoimmune-mediated encephalitis, autoimmune-mediated limbic encephalitis, and autoimmune synaptic encephalitis.
How to categorize these syndromes is still in flux: they can be listed by the area of the brain affected, the antibody involved, or the name of the discoverer (eg, Morvan syndrome).
Autoantibodies identified in autoimmune encephalitis fall under two broad categories:
- Those targeting intracellular (intranuclear or intracytoplasmic) antigens; the syndromes they cause are more likely to be paraneoplastic and less responsive to immunotherapy
- Those targeting antigens on the neuronal surface: the syndromes they cause are less likely to be paraneoplastic and are more responsive to immunotherapy.4
SYNDROMES DEFINED BY BRAIN AREA AFFECTED
Below, we provide examples of neurologic syndromes of autoantibody-mediated encephalitis according to the region of the brain most affected, ie, the limbic system, the brainstem, or the cerebellum (Figure 2).
LIMBIC ENCEPHALITIS
Memory loss, behavioral changes, seizures
Patients with limbic encephalitis (such as the patient described in the vignette above) present with symptoms attributed to dysfunction of mesial temporal lobe structures, most notably the hippocampus. Prominent symptoms include short-term memory loss, behavioral disturbances such as agitation and confusion, and psychiatric problems such as depression and psychosis. Recurrent seizures are a salient feature and, not uncommonly, progress to status epilepticus.
Antibodies are not all cancer-associated
Cerebrospinal fluid analysis can be normal or show abnormalities suggesting immune activation, eg, slight pleocytosis, elevated protein, increased immunoglobulin G synthesis, and oligoclonal banding.5
In many cases, an autoantibody is found in the blood or in the cerebrospinal fluid. Some patients may express more than one autoantibody, so the traditional view of “one antibody, one syndrome” is incorrect.
Although initially identified as a rare paraneoplastic disorder, limbic encephalitis sometimes occurs in the absence of malignancy.
Multiple antibodies have been linked to the syndrome (Table 1).6–9 The “classic” antibodies initially found in paraneoplastic forms are now generally viewed as nonpathogenic, in part because they are directed against intracellular antigens. Neuronal injury in paraneoplastic limbic encephalitis is believed to be mediated by cytotoxic T lymphocytes, with neuronal autoantibodies being produced after the injury.4 Recently defined antibodies, such as those targeting the N-methyl-d-aspartate (NMDA) receptor6 and the LGI1 protein,7 are now understood to be common causes of limbic encephalitis.
Imaging usually shows limbic focal changes
Structural MRI or functional fluorodeoxyglucose (FDG)-PET imaging may show focal changes in limbic system structures, such as the mesial temporal lobes. It is now recognized that other cortical areas may be involved, and the term “limbic encephalitis” may give way to “cortical” or “focal encephalitis.”
In about 60% of patients, MRI shows hyperintense fluid-attenuated inversion recovery (FLAIR) or T2 signal changes in the mesial temporal lobes, likely reflecting inflammatory changes.4,10,11 On FDG-PET, hypermetabolism may be observed in the mesial temporal lobes early in the disease despite normal findings on MRI.12 Hypometabolism, either diffuse or localized to the mesial temporal lobes, eventually sets in, likely reflecting cytotoxic injury in the aftermath of prolonged inflammation or seizures.
Consider other causes
Before diagnosing limbic encephalitis, it is essential to evaluate for infectious meningoencephalitis, especially herpes simplex viral encephalitis. Thiamine deficiency (Wernicke encephalopathy), drug intoxication, prion disease, Hashimoto encephalopathy, tumor, and subclinical status epilepticus should also be considered. Some of these conditions are associated with the same neuronal autoantibodies detected in limbic encephalitis. Further complicating the picture, case reports have shown the presence of serum neuronal autoantibodies—VGKC complex13–15 and NMDA-receptor antibodies16,17—in confirmed cases of prion disease. In addition, adequately treated herpes simplex viral encephalitis can precipitate the production of NMDA-receptor antibodies and their characteristic syndrome.18–20
BRAINSTEM ENCEPHALITIS
The brainstem—the midbrain, pons, and medulla—can be affected, either in isolation or more commonly as part of a more widespread autoantibody-mediated encephalitis. Symptoms and signs include eye movement abnormalities, ptosis, dysphagia, dysarthria, ataxia, facial palsy, vertigo, hearing impairment, reduced consciousness, and hypoventilation.21
Anti-Hu, anti-Ri, and anti-Ma2 antibodies are most commonly associated with brainstem encephalitis (Table 2). Anti-Ma2-associated encephalitis may improve after a combination of immunotherapy and tumor removal21; the others have a poor prognosis.
Neuromyelitis optica spectrum disorders
Neuromyelitis optica spectrum disorders most commonly involve demyelination affecting the optic nerves and spinal cord, leading to unilateral or bilateral optic neuritis and transverse myelitis spanning three or more vertebral segments.22 The initial clinical manifestation may be an encephalitic pattern, affecting predominantly the brainstem in a restricted fashion,22 or the central nervous system in a more diffuse pattern, mimicking either acute disseminated encephalomyelitis or, in less severe cases, posterior reversible encephalopathy syndrome.23
Testing for antiaquaporin-4 antibody, also known as neuromyelitis optica immunoglobulin G, is the single most decisive laboratory test for diagnosing neuromyelitis optica spectrum disorders, so serum and cerebrospinal fluid evaluation for this autoantibody should be considered when caring for a patient whose clinical picture suggests brainstem encephalitis.22
Bickerstaff brainstem encephalitis
Bickerstaff brainstem encephalitis was first described more than half a century ago in patients with postinfectious ataxia, ophthalmoparesis, and altered consciousness. This rare disease was later found to be associated with antiganglioside GQ1b (anti-GQ1b) autoantibody. MRI is normal in about 90% of cases, so recognizing the clinical presentation and analyzing anti-GQ1b serum titers are critical to diagnosis.
Recovery is usually spontaneous and complete and can be hastened by immunotherapy, especially intravenous immunoglobulin.24
Other causes of brainstem encephalitis
The differential diagnosis of a presentation of brainstem encephalitis includes:
- Infectious causes, the most common being Listeria species followed by enterovirus 71 and herpes simplex virus.25 Tuberculosis, brucellosis, and Whipple disease should also be considered.
- Primary central nervous system inflammatory and demyelinating conditions, eg, multiple sclerosis and acute disseminated encephalomyelitis.
- Systemic inflammatory conditions, eg, Behçet disease, systemic lupus erythematosus, and sarcoidosis.
- Direct brainstem neoplastic involvement, as might occur in primary central nervous system lymphoma or leptomeningeal carcinomatosis.
CEREBELLAR SYNDROME
Patients with autoantibody-mediated encephalitis localized predominantly to the cerebellum typically present with dizziness, vertigo, and unsteady gait, progressing eventually to limb and gait ataxia.4 Symptoms are often subacute, progressing over weeks.
Multiple neuronal autoantibodies have been found to occur with cerebellar encephalitis (Table 2). In most cases, they are paraneoplastic and considered not to be pathogenic, given the intracellular location of their target antigen.4 In such cases, the syndrome is more accurately described as autoantibody-associated rather than autoantibody-mediated. Only in a minority of cases have neuronal autoantibodies been demonstrated to be directly pathogenic, ie, antimetabotropic glutamate receptor type 1 (anti-mGluR1) antibody-associated cerebellitis26 and antiglutamic acid decarboxylase (anti-GAD)-associated cerebellar ataxia.27
Differential diagnosis of cerebellar syndromes
The differential diagnosis of autoantibody-associated cerebellar syndromes is broad and includes:
- Alcohol-induced atrophy
- Drug-induced cerebellar atrophy (eg, from lithium, phenytoin, gabapentin, metronidazole, amiodarone, carbamazepine)
- Vitamin B1 and E deficiency
- Hypothyroidism, hypoparathyroidism
- Neurodegenerative disease (eg, prion disease, multiple system atrophy)
- Parainfectious causes (eg, after infection with Epstein-Barr virus)
- Immune-mediated diseases (Miller-Fisher syndrome, associated with anti-GQ1b antibodies, and antigliadin-associated ataxia, which can occur in isolation or as part of celiac disease).4
SYNDROMES ASSOCIATED WITH SPECIFIC ANTIBODIES
A few of the autoantibody-mediated encephalitic syndromes have specific antibody associations and characteristic clinical presentations. The most prominent of these syndromes are VGKC complex antibody encephalitis (as in the patient described at the beginning of this article) and anti-NMDA receptor encephalitis.
VGKC COMPLEX ANTIBODY-MEDIATED LIMBIC ENCEPHALITIS
VGKC complex antibodies, initially reported to be associated with the peripheral nerve hyperexcitability disorder neuromyotonia, were subsequently found in Morvan syndrome.28,29 Patients with this syndrome often present with autonomic dysfunction and peripheral nerve hyperexcitability but also develop insomnia, confusion, hallucinations, and memory loss. Drawing on the clinical overlap between Morvan syndrome and limbic encephalitis, Buckley et al30 were the first to report VGKC complex antibodies in two cases of limbic encephalitis.
VGKC complex antibodies are now understood to be associated with a wide variety of neurologic conditions, including chronic idiopathic pain, epilepsy,31 movement disorders, cranial nerve abnormalities, autonomic dysfunction,32 and gut dysmotility.33 In contrast, these antibodies are rare in healthy people.34 Limbic encephalitis associated with VGKC complex antibody usually lacks cerebellar and brainstem dysfunction, which may help distinguish it from other types of autoantibody-mediated limbic encephalitis.12
VGKC complex antibody does not bind to the potassium channel itself. Instead it recognizes other constituents of the channel complex, most notably LGI1 and contactin-associated protein 2 (CASPR2). LGI1 antibody is more commonly associated with limbic encephalitis—as illustrated in our case study—in addition to a distinctive type of seizure affecting the arm and face (faciobrachial dystonic seizure).34 The CASPR2 antibody, on the other hand, more often correlates with peripheral nerve manifestations and Morvan syndrome.29 Hyponatremia is commonly seen on serum chemical analysis and provides a clue that these syndromes are present.12
Good response to immunotherapy
A critical change in therapy came as clinicians realized that seizures were often refractory to standard antiepileptic drugs but responded well to immunotherapies. On the basis of these observations, sera of patients with long-standing epilepsy have been reanalyzed to look for neuronal autoantibodies.31 These antibodies should be checked in cases of new-onset refractory status epilepticus of unknown origin that does not respond to antiepileptic medications.
About half of patients with VGKC complex antibody-mediated limbic encephalitis have normal findings on brain MRI.5 Seven of 10 patients who were prospectively followed for VGKC complex antibody-mediated faciobrachial dystonic seizures had normal brain MRIs.35
VGKC complex antibody-mediated limbic encephalitis does not usually recur.36 Most cases are nonparaneoplastic, as evidenced by failure to detect a single active tumor in 64 patients after a median follow-up of 3 years. The prognosis is generally favorable except in cases with coexisting tumors.12
ANTI-NMDA RECEPTOR ENCEPHALITIS
Often associated with ovarian teratoma
Anti-NMDA receptor encephalitis typically affects women in their 20s and 30s, and about half of patients have an ovarian teratoma. It can also occur in younger patients and in men, in whom it is less likely to be associated with a neoplasm.37
Typical initial symptoms include striking and often stereotyped neuropsychiatric disturbances manifesting as psychosis, confusion, seizures, and amnesia. After 1 to 2 weeks, new symptoms set in, including reduced consciousness, movement disorders (ranging from orolingualfacial dyskinesia to rigidity and choreoathetosis), autonomic dysfunction, and hypoventilation, often prompting admission to the intensive care unit.38
Although the outcome is favorable in most cases, recovery, in contrast to VGKC complex antibody-mediated limbic encephalitis, is slow and may take longer than 1 year. Up to a quarter of patients have a relapse, underscoring the importance of maintenance immunotherapy.
It is important to undertake an intensive search for possible ovarian and extraovarian teratomas in young women with this syndrome—including CT of the pelvis, vaginal ultrasonography, and PET imaging—as removal of the teratoma may be curative.37
DIAGNOSIS OF AUTOANTIBODY-MEDIATED ENCEPHALITIS
Critical to diagnosing autoantibody-mediated encephalitis is awareness of these disorders. Since antibody testing may be very specific and is not usually part of the standard batteries of tests, a high level of suspicion is needed. Patients may present to different specialists in different settings; therefore, clinicians in pediatrics, rheumatology, psychiatry, and intensive care medicine need to be aware of these syndromes to avoid delay and misdiagnosis.
Clinical features suggesting autoantibody-mediated encephalitis include:
- Acute or subacute onset of a neurologic syndrome
- New-onset refractory status epilepticus of unknown etiology
- Acute or subacute psychiatric illness with unexpected progression to neurologic symptoms or delirium
- Unusual movement disorders not conforming to standard syndromes
- Cognitive impairment, psychosis, or behavioral or language disorders with atypical findings on imaging or cerebrospinal fluid analysis.
Imaging. Diagnosis of autoantibody-mediated encephalitis focuses on evidence suggesting an inflammatory central nervous system syndrome. MRI may show hyperintense signals on T2, FLAIR, or diffusion-weighted imaging changes in various brain regions. In many cases, however, MRI is negative despite severe clinical symptoms. In a study of 72 patients suspected of having autoimmune dementia of various etiologies, including but not restricted to antineuronal surface antibody-mediated causes, Flanagan et al39 identified atypical neuroimaging findings in only 29%. PET imaging may show hypermetabolism in certain brain areas correlating to clinical syndromes but is often difficult to obtain in a timely fashion.
Cerebrospinal fluid is often abnormal, showing elevated protein, increased immunoglobulin G synthesis, or oligoclonal banding. As with imaging studies, the cerebrospinal fluid may be normal despite severe clinical manifestations.
Electroencephalography may show focal slowing or seizure activity. Neuropsychologic testing may show different patterns of abnormalities.
Antibody testing. None of these tests can be used in isolation, and the diagnosis of autoantibody-mediated encephalitis hinges on recognizing a clinical syndrome and ordering supportive testing. Specific antibodies are more likely in different clinical syndromes and should be sought (Table 3).
Patients who have autoantibody-mediated encephalitis may test negative for autoantibodies for many possible reasons:
- Blood testing for antibodies may be less sensitive than cerebrospinal fluid testing
- Antibody titers may vary in the course of the disease
- The patient may be expressing an antibody that is less often tested for (eg, anti-AMPA receptor or antigamma-aminobutyric acid B) or one that has not yet been isolated.
Evaluating for malignancy is recommended in all cases of autoantibody-mediated encephalitis. The initial workup may involve CT of the chest, abdomen, and pelvis, as well as mammography in women and serum prostate-specific antigen testing and testicular ultrasonography in men. Ordering FDG-PET in cases in which CT is negative or inconclusive increases cancer detection.40 If no cancer is found, close tumor surveillance—every 3 to 6 months—is recommended for at least 2 years.41
TREATMENT
Owing in large part to the rarity of autoantibody-mediated encephalitides, no randomized trials of therapy have been performed. Treatment at present is guided mostly by case series and expert consensus, which suggest first-line therapy with intravenous immunoglobulin, high-dose corticosteroids, plasmapheresis, or a combination.
Different syndromes and antibody-related disorders respond differently to therapy. Syndromes associated with antibodies against intracellular antigens tend to be more resistant to immune therapy than cell surface antigen-related syndromes.4
Tiered approach
Combined treatment with intravenous immunoglobulin and high-dose corticosteroids may be superior to treatment with steroids alone for LGI1-antibody mediated limbic encephalitis.42
In cases refractory to first-line (“tier 1”) therapy, second-line immunotherapy with drugs affecting B-cell populations (eg, rituximab, cyclophosphamide, and mycophenolate mofetil) has been used.
A tiered approach has been most extensively studied for anti-NMDA-receptor encephalitis, with better outcomes found using second-line therapy.43
Treatment strategies for these disorders will likely evolve over time with additional experience.
Outpatient management
Once the patient is discharged from the hospital, a multidisciplinary approach to care is recommended, including physical rehabilitation, speech therapy, neuropsychiatric and neuroimmunologic follow-up, and annual surveillance for malignancies.
- Brierley JB, Corsellis JAN, Hierons R, Nevin S. Subacute encephalitis of later adult life mainly affecting the limbic areas. Brain 1960; 83:357–368.
- Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain 1968; 91:481–496.
- Greenlee JE, Brashear HR. Antibodies to cerebellar Purkinje cells in patients with paraneoplastic cerebellar degeneration and ovarian carcinoma. Ann Neurol 1983; 14:609–613.
- Rosenfeld MR, Dalmau JO. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum (Minneap Minn) 2012; 18:366–383.
- Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-surface central nervous system autoantibodies: clinical relevance and emerging paradigms. Ann Neurol 2014; 76:168–184.
- Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61:25–36.
- Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010; 133:2734–2748.
- Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010; 9:67–76.
- Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009; 65:424–434.
- Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry 2012; 83:638–645.
- Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 2005; 128:1764–1777.
- Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004; 127:701–712.
- Jammoul A, Lederman RJ, Tavee J, Li Y. Presence of voltage-gated potassium channel complex antibody in a case of genetic prion disease. BMJ Case Rep 2014; pii:bcr2013201622.
- Angus-Leppan H, Rudge P, Mead S, Collinge J, Vincent A. Autoantibodies in sporadic Creutzfeldt-Jakob disease. JAMA Neurol 2013; 70:919–922.
- Fujita K, Yuasa T, Watanabe O, et al. Voltage-gated potassium channel complex antibodies in Creutzfeldt-Jakob disease. J Neurol 2012; 259:2249–2250.
- Fujita K, Yuasa T, Takahashi Y, et al. Antibodies to N-methyl-D-aspartate glutamate receptors in Creutzfeldt–Jakob disease patients. J Neuroimmunol 2012; 251:90–93.
- Mackay G, Ahmad K, Stone J, et al. NMDA receptor autoantibodies in sporadic Creutzfeldt-Jakob disease. J Neurol 2012; 259:1979–1981.
- Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus–1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013; 81:1637–1639.
- Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol 2014; 71:344–346.
- Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014; 75:317–323.
- Blaes F. Paraneoplastic brain stem encephalitis. Curr Treat Options Neurol 2013; 15:201–209.
- Wildemann B, Jarius S. The expanding range of autoimmune disorders of the nervous system. Lancet Neurol 2013; 12:22–24.
- Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler 2011; 17:1107–1112.
- Shahrizaila N, Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J Neurol Neurosurg Psychiatry 2013; 84:576–583.
- Jubelt B, Mihai C, Li MT, Veerapaneni P. Rhombencephalitis/brainstem encephalitis. Curr Neurol Neurosci Rep 2011; 11:543–552.
- Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 2000; 342:21–27.
- Ishida K, Mitoma H, Son SY, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol 1999; 46:263–267.
- Hart IK, Waters C, Vincent A, et al. Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol 1997; 41:238–246.
- Barber P, Anderson NE, Vincent A. Morvan’s syndrome associated with voltage-gated K+ channel antibodies. Neurology 2000; 54:771–772.
- Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 2001; 50:73–78.
- Majoie HJ, de Baets M, Renier W, Lang B, Vincent A. Antibodies to voltage-gated potassium and calcium channels in epilepsy. Epilepsy Res 2006; 71:135–141.
- Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology 2008; 70:1883–1890.
- Knowles CH, Lang B, Clover L, et al. A role for autoantibodies in some cases of acquired non-paraneoplastic gut dysmotility. Scand J Gastroenterol 2002; 37:166–170.
- Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011; 69:892–900.
- Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013: 136:3151–3162.
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol 2011; 10:759–772.
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74.
- Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133:1655–1667.
- Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc 2010; 85:881–897.
- Younes-Mhenni S, Janier MF, Cinotti L, et al. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain 2004; 127:2331–2338.
- Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 2011; 77:179–189.
- Shin YW, Lee ST, Shin JW, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 2013; 265:75–81.
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165.
- Brierley JB, Corsellis JAN, Hierons R, Nevin S. Subacute encephalitis of later adult life mainly affecting the limbic areas. Brain 1960; 83:357–368.
- Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain 1968; 91:481–496.
- Greenlee JE, Brashear HR. Antibodies to cerebellar Purkinje cells in patients with paraneoplastic cerebellar degeneration and ovarian carcinoma. Ann Neurol 1983; 14:609–613.
- Rosenfeld MR, Dalmau JO. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum (Minneap Minn) 2012; 18:366–383.
- Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-surface central nervous system autoantibodies: clinical relevance and emerging paradigms. Ann Neurol 2014; 76:168–184.
- Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61:25–36.
- Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010; 133:2734–2748.
- Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010; 9:67–76.
- Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009; 65:424–434.
- Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry 2012; 83:638–645.
- Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 2005; 128:1764–1777.
- Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004; 127:701–712.
- Jammoul A, Lederman RJ, Tavee J, Li Y. Presence of voltage-gated potassium channel complex antibody in a case of genetic prion disease. BMJ Case Rep 2014; pii:bcr2013201622.
- Angus-Leppan H, Rudge P, Mead S, Collinge J, Vincent A. Autoantibodies in sporadic Creutzfeldt-Jakob disease. JAMA Neurol 2013; 70:919–922.
- Fujita K, Yuasa T, Watanabe O, et al. Voltage-gated potassium channel complex antibodies in Creutzfeldt-Jakob disease. J Neurol 2012; 259:2249–2250.
- Fujita K, Yuasa T, Takahashi Y, et al. Antibodies to N-methyl-D-aspartate glutamate receptors in Creutzfeldt–Jakob disease patients. J Neuroimmunol 2012; 251:90–93.
- Mackay G, Ahmad K, Stone J, et al. NMDA receptor autoantibodies in sporadic Creutzfeldt-Jakob disease. J Neurol 2012; 259:1979–1981.
- Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus–1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013; 81:1637–1639.
- Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol 2014; 71:344–346.
- Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014; 75:317–323.
- Blaes F. Paraneoplastic brain stem encephalitis. Curr Treat Options Neurol 2013; 15:201–209.
- Wildemann B, Jarius S. The expanding range of autoimmune disorders of the nervous system. Lancet Neurol 2013; 12:22–24.
- Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler 2011; 17:1107–1112.
- Shahrizaila N, Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J Neurol Neurosurg Psychiatry 2013; 84:576–583.
- Jubelt B, Mihai C, Li MT, Veerapaneni P. Rhombencephalitis/brainstem encephalitis. Curr Neurol Neurosci Rep 2011; 11:543–552.
- Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 2000; 342:21–27.
- Ishida K, Mitoma H, Son SY, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol 1999; 46:263–267.
- Hart IK, Waters C, Vincent A, et al. Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol 1997; 41:238–246.
- Barber P, Anderson NE, Vincent A. Morvan’s syndrome associated with voltage-gated K+ channel antibodies. Neurology 2000; 54:771–772.
- Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 2001; 50:73–78.
- Majoie HJ, de Baets M, Renier W, Lang B, Vincent A. Antibodies to voltage-gated potassium and calcium channels in epilepsy. Epilepsy Res 2006; 71:135–141.
- Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology 2008; 70:1883–1890.
- Knowles CH, Lang B, Clover L, et al. A role for autoantibodies in some cases of acquired non-paraneoplastic gut dysmotility. Scand J Gastroenterol 2002; 37:166–170.
- Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011; 69:892–900.
- Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013: 136:3151–3162.
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol 2011; 10:759–772.
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74.
- Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133:1655–1667.
- Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc 2010; 85:881–897.
- Younes-Mhenni S, Janier MF, Cinotti L, et al. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain 2004; 127:2331–2338.
- Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 2011; 77:179–189.
- Shin YW, Lee ST, Shin JW, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 2013; 265:75–81.
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165.
KEY POINTS
- Autoantibody-mediated encephalitis accounts for a portion of cases of unexplained status epilepticus, encephalitis, and acute-onset psychiatric symptoms.
- Magnetic resonance imaging and cerebrospinal fluid analysis may be normal early in the disease course.
- Patients can express more than one autoantibody and present with more than one neuronal syndrome.
- Syndromes in which antibodies attack antigens on the surface of neurons are more likely to respond to immunotherapy than those involving intracellular antigens.
- Anti-N-methyl-d-aspartate receptor encephalitis typically presents with psychosis, seizures, and movement disorders in young women and is often associated with an ovarian teratoma.
- Limbic encephalitis, mediated by antibody to the voltage-gated potassium channel complex, is typically nonneoplastic and responds well to immunotherapy.
PPIs caused remission in about half of esophageal eosinophilia cases
About half of patients with symptomatic esophageal eosinophilia achieved complete clinical and histologic remission on proton pump inhibitors (PPIs), according to a systematic review and meta-analysis of 33 studies.
“Our results support the concept of PPIs as first-line therapy in both children and adults,” Dr. Alfredo Lucendo of the Servicio de Salud de Castillo–La Mancha, Hospital General de Tomelloso, Albacete, Spain, and his associates wrote in the January issue of Clinical Gastroenterology and Hepatology. “Other effective alternatives, such as dietary or topical steroid therapy, likely might be set aside as second-line treatment, owing to long-term safety concerns (topical steroid therapy) and impairment of quality of life and nutritional inadequacy (dietary interventions).”
The study also confirmed that esophageal pH monitoring does not accurately predict therapeutic response to PPI therapy. “The performance of this test before histologic reevaluation on PPI therapy should be discouraged,” according to the researchers (Clin Gastroenterol Hepatol. 2015. [doi:10.1016/j.cgh.2015.07.041]). Eosinophilic esophagitis was first described as a distinct disorder about 20 years ago, but only recently was understood to be the most common cause of chronic esophageal symptoms among children and young adults. Some cases are now known to respond to PPI therapy, but reported remission rates have varied depending on study design and patient population, the investigators said. In addition, no one had systematically reviewed studies of PPI-responsive esophageal eosinophilia for quality or to determine the optimal type of PPI, dose, or treatment duration.
Therefore, the investigators searched MEDLINE, EMBASE, SCOPUS, and abstracts from the annual meetings of the American Gastroenterological Association, the American College of Gastroenterology, and United European Gastroenterology, identifying 33 studies of 619 patients with symptomatic esophageal eosinophilia. Eleven of the studies were prospective, of which only two were randomized controlled trials. The researchers defined a histologic response as less than 15 eosinophils per high-powered frame after PPI therapy. “Missing data regarding PPI therapy were common and prevented us from drawing conclusions on the most effective PPI drug and doses,” they said. In addition, most studies lacked structured or objective survey tools or other measures of clinical improvement, making it impossible to rule out self-adapted coping strategies as a main cause of improvement over time.
With those caveats in mind, about 61% of patients in the pooled analysis had a clinical response to PPI therapy (95% confidence interval, 48%-72%), and half achieved clinical and histologic remission (95% CI, 42%-59%), the investigators reported. Therapy was somewhat more effective when administered twice a day instead of once daily, when clinicians used esophageal pH monitoring, and when studies were prospective instead of retrospective, but the differences were not significant. Nor did therapeutic response significantly differ based on the age of patients, type of report, or quality of the study.
The overall findings “should be interpreted with caution because of poor-quality evidence, heterogeneity, and publication bias,” the researchers said. Prospective studies are needed to examine the best PPI, dose, and dosing interval to use in an initial trial in the clinic; to clarify long-term effects and dosing strategies; to assess the ability of PPIs to reverse fibrotic esophageal remodeling; and to examine the effects of the CYP2C19 genotype on clinical and histologic response, they added. “More quality evidence on pediatric PPI-responsive eosinophilic esophagitis is needed urgently,” they emphasized.
The authors reported no funding sources and had no disclosures.
Proton pump inhibitor–responsive esophageal eosinophilia (PPI-REE) is a condition in which patients have symptoms of esophageal dysfunction (often dysphagia or heartburn), biopsies with at least 15 eosinophils per high-power field (eos/hpf), and symptomatic and histologic resolution after a PPI trial, typically at twice-daily dosing. Currently, PPI-REE and eosinophilic esophagitis (EoE) overlap substantially, but in the most recent guidelines, they are still considered to be distinct entities. PPI-REE was first reported almost 10 years ago, and since then multiple prospective and retrospective studies in both children and adults have further characterized it. The study by Dr. Lucendo and colleagues, a comprehensive and rigorously conducted systematic review and meta-analysis of 33 studies accounting for 619 patients, found that just over 50% of patients with esophageal eosinophilia had histologic remission (less than 15 eos/hpf) and just over 60% had symptomatic improvement after PPI use. Moreover, similar responses were seen whether or not there was pathologic acid exposure on pH testing.

|
| Dr. Evan S. Dellon |
While there was heterogeneity between studies on meta-analysis, there are several important messages from this study. First, PPI-REE is commonly seen in patients with esophageal eosinophilia, and is not always simply due to reflux. Second, PPIs have a potent antieosinophil effect in these patients. Interestingly, novel acid-independent mechanisms for this anti-inflammatory action recently have been described in other studies. Third, a PPI trial remains important prior to confirming the diagnosis of EoE, and PPIs should be considered the first-line treatment when esophageal eosinophilia is identified. However, it bears emphasizing that all esophageal eosinophilia is not due to EoE. If a patient responds to the PPI trial, there is no clear need to move toward topical steroid or dietary elimination therapy specifically for EoE, and starting multiple antieosinophil treatments concomitantly precludes determining which is most effective. In the future, understanding which patients with esophageal eosinophilia will most benefit from a PPI trial will be important, as we are currently unable to predict this from clinical, endoscopic, and histologic factors. Future studies and guidelines will also need to address whether EoE and PPI-REE are distinct diseases or manifestations of the same underlying process.
Dr. Evan S. Dellon, MPH, is associate professor of medicine and epidemiology at the Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine at Chapel Hill. He has received research funding from Meritage, Miraca, Receptos, and Regeneron and consulted for Aptalis, Banner, Novartis, Receptos, Regeneron, and Roche.
Proton pump inhibitor–responsive esophageal eosinophilia (PPI-REE) is a condition in which patients have symptoms of esophageal dysfunction (often dysphagia or heartburn), biopsies with at least 15 eosinophils per high-power field (eos/hpf), and symptomatic and histologic resolution after a PPI trial, typically at twice-daily dosing. Currently, PPI-REE and eosinophilic esophagitis (EoE) overlap substantially, but in the most recent guidelines, they are still considered to be distinct entities. PPI-REE was first reported almost 10 years ago, and since then multiple prospective and retrospective studies in both children and adults have further characterized it. The study by Dr. Lucendo and colleagues, a comprehensive and rigorously conducted systematic review and meta-analysis of 33 studies accounting for 619 patients, found that just over 50% of patients with esophageal eosinophilia had histologic remission (less than 15 eos/hpf) and just over 60% had symptomatic improvement after PPI use. Moreover, similar responses were seen whether or not there was pathologic acid exposure on pH testing.

|
| Dr. Evan S. Dellon |
While there was heterogeneity between studies on meta-analysis, there are several important messages from this study. First, PPI-REE is commonly seen in patients with esophageal eosinophilia, and is not always simply due to reflux. Second, PPIs have a potent antieosinophil effect in these patients. Interestingly, novel acid-independent mechanisms for this anti-inflammatory action recently have been described in other studies. Third, a PPI trial remains important prior to confirming the diagnosis of EoE, and PPIs should be considered the first-line treatment when esophageal eosinophilia is identified. However, it bears emphasizing that all esophageal eosinophilia is not due to EoE. If a patient responds to the PPI trial, there is no clear need to move toward topical steroid or dietary elimination therapy specifically for EoE, and starting multiple antieosinophil treatments concomitantly precludes determining which is most effective. In the future, understanding which patients with esophageal eosinophilia will most benefit from a PPI trial will be important, as we are currently unable to predict this from clinical, endoscopic, and histologic factors. Future studies and guidelines will also need to address whether EoE and PPI-REE are distinct diseases or manifestations of the same underlying process.
Dr. Evan S. Dellon, MPH, is associate professor of medicine and epidemiology at the Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine at Chapel Hill. He has received research funding from Meritage, Miraca, Receptos, and Regeneron and consulted for Aptalis, Banner, Novartis, Receptos, Regeneron, and Roche.
Proton pump inhibitor–responsive esophageal eosinophilia (PPI-REE) is a condition in which patients have symptoms of esophageal dysfunction (often dysphagia or heartburn), biopsies with at least 15 eosinophils per high-power field (eos/hpf), and symptomatic and histologic resolution after a PPI trial, typically at twice-daily dosing. Currently, PPI-REE and eosinophilic esophagitis (EoE) overlap substantially, but in the most recent guidelines, they are still considered to be distinct entities. PPI-REE was first reported almost 10 years ago, and since then multiple prospective and retrospective studies in both children and adults have further characterized it. The study by Dr. Lucendo and colleagues, a comprehensive and rigorously conducted systematic review and meta-analysis of 33 studies accounting for 619 patients, found that just over 50% of patients with esophageal eosinophilia had histologic remission (less than 15 eos/hpf) and just over 60% had symptomatic improvement after PPI use. Moreover, similar responses were seen whether or not there was pathologic acid exposure on pH testing.

|
| Dr. Evan S. Dellon |
While there was heterogeneity between studies on meta-analysis, there are several important messages from this study. First, PPI-REE is commonly seen in patients with esophageal eosinophilia, and is not always simply due to reflux. Second, PPIs have a potent antieosinophil effect in these patients. Interestingly, novel acid-independent mechanisms for this anti-inflammatory action recently have been described in other studies. Third, a PPI trial remains important prior to confirming the diagnosis of EoE, and PPIs should be considered the first-line treatment when esophageal eosinophilia is identified. However, it bears emphasizing that all esophageal eosinophilia is not due to EoE. If a patient responds to the PPI trial, there is no clear need to move toward topical steroid or dietary elimination therapy specifically for EoE, and starting multiple antieosinophil treatments concomitantly precludes determining which is most effective. In the future, understanding which patients with esophageal eosinophilia will most benefit from a PPI trial will be important, as we are currently unable to predict this from clinical, endoscopic, and histologic factors. Future studies and guidelines will also need to address whether EoE and PPI-REE are distinct diseases or manifestations of the same underlying process.
Dr. Evan S. Dellon, MPH, is associate professor of medicine and epidemiology at the Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine at Chapel Hill. He has received research funding from Meritage, Miraca, Receptos, and Regeneron and consulted for Aptalis, Banner, Novartis, Receptos, Regeneron, and Roche.
About half of patients with symptomatic esophageal eosinophilia achieved complete clinical and histologic remission on proton pump inhibitors (PPIs), according to a systematic review and meta-analysis of 33 studies.
“Our results support the concept of PPIs as first-line therapy in both children and adults,” Dr. Alfredo Lucendo of the Servicio de Salud de Castillo–La Mancha, Hospital General de Tomelloso, Albacete, Spain, and his associates wrote in the January issue of Clinical Gastroenterology and Hepatology. “Other effective alternatives, such as dietary or topical steroid therapy, likely might be set aside as second-line treatment, owing to long-term safety concerns (topical steroid therapy) and impairment of quality of life and nutritional inadequacy (dietary interventions).”
The study also confirmed that esophageal pH monitoring does not accurately predict therapeutic response to PPI therapy. “The performance of this test before histologic reevaluation on PPI therapy should be discouraged,” according to the researchers (Clin Gastroenterol Hepatol. 2015. [doi:10.1016/j.cgh.2015.07.041]). Eosinophilic esophagitis was first described as a distinct disorder about 20 years ago, but only recently was understood to be the most common cause of chronic esophageal symptoms among children and young adults. Some cases are now known to respond to PPI therapy, but reported remission rates have varied depending on study design and patient population, the investigators said. In addition, no one had systematically reviewed studies of PPI-responsive esophageal eosinophilia for quality or to determine the optimal type of PPI, dose, or treatment duration.
Therefore, the investigators searched MEDLINE, EMBASE, SCOPUS, and abstracts from the annual meetings of the American Gastroenterological Association, the American College of Gastroenterology, and United European Gastroenterology, identifying 33 studies of 619 patients with symptomatic esophageal eosinophilia. Eleven of the studies were prospective, of which only two were randomized controlled trials. The researchers defined a histologic response as less than 15 eosinophils per high-powered frame after PPI therapy. “Missing data regarding PPI therapy were common and prevented us from drawing conclusions on the most effective PPI drug and doses,” they said. In addition, most studies lacked structured or objective survey tools or other measures of clinical improvement, making it impossible to rule out self-adapted coping strategies as a main cause of improvement over time.
With those caveats in mind, about 61% of patients in the pooled analysis had a clinical response to PPI therapy (95% confidence interval, 48%-72%), and half achieved clinical and histologic remission (95% CI, 42%-59%), the investigators reported. Therapy was somewhat more effective when administered twice a day instead of once daily, when clinicians used esophageal pH monitoring, and when studies were prospective instead of retrospective, but the differences were not significant. Nor did therapeutic response significantly differ based on the age of patients, type of report, or quality of the study.
The overall findings “should be interpreted with caution because of poor-quality evidence, heterogeneity, and publication bias,” the researchers said. Prospective studies are needed to examine the best PPI, dose, and dosing interval to use in an initial trial in the clinic; to clarify long-term effects and dosing strategies; to assess the ability of PPIs to reverse fibrotic esophageal remodeling; and to examine the effects of the CYP2C19 genotype on clinical and histologic response, they added. “More quality evidence on pediatric PPI-responsive eosinophilic esophagitis is needed urgently,” they emphasized.
The authors reported no funding sources and had no disclosures.
About half of patients with symptomatic esophageal eosinophilia achieved complete clinical and histologic remission on proton pump inhibitors (PPIs), according to a systematic review and meta-analysis of 33 studies.
“Our results support the concept of PPIs as first-line therapy in both children and adults,” Dr. Alfredo Lucendo of the Servicio de Salud de Castillo–La Mancha, Hospital General de Tomelloso, Albacete, Spain, and his associates wrote in the January issue of Clinical Gastroenterology and Hepatology. “Other effective alternatives, such as dietary or topical steroid therapy, likely might be set aside as second-line treatment, owing to long-term safety concerns (topical steroid therapy) and impairment of quality of life and nutritional inadequacy (dietary interventions).”
The study also confirmed that esophageal pH monitoring does not accurately predict therapeutic response to PPI therapy. “The performance of this test before histologic reevaluation on PPI therapy should be discouraged,” according to the researchers (Clin Gastroenterol Hepatol. 2015. [doi:10.1016/j.cgh.2015.07.041]). Eosinophilic esophagitis was first described as a distinct disorder about 20 years ago, but only recently was understood to be the most common cause of chronic esophageal symptoms among children and young adults. Some cases are now known to respond to PPI therapy, but reported remission rates have varied depending on study design and patient population, the investigators said. In addition, no one had systematically reviewed studies of PPI-responsive esophageal eosinophilia for quality or to determine the optimal type of PPI, dose, or treatment duration.
Therefore, the investigators searched MEDLINE, EMBASE, SCOPUS, and abstracts from the annual meetings of the American Gastroenterological Association, the American College of Gastroenterology, and United European Gastroenterology, identifying 33 studies of 619 patients with symptomatic esophageal eosinophilia. Eleven of the studies were prospective, of which only two were randomized controlled trials. The researchers defined a histologic response as less than 15 eosinophils per high-powered frame after PPI therapy. “Missing data regarding PPI therapy were common and prevented us from drawing conclusions on the most effective PPI drug and doses,” they said. In addition, most studies lacked structured or objective survey tools or other measures of clinical improvement, making it impossible to rule out self-adapted coping strategies as a main cause of improvement over time.
With those caveats in mind, about 61% of patients in the pooled analysis had a clinical response to PPI therapy (95% confidence interval, 48%-72%), and half achieved clinical and histologic remission (95% CI, 42%-59%), the investigators reported. Therapy was somewhat more effective when administered twice a day instead of once daily, when clinicians used esophageal pH monitoring, and when studies were prospective instead of retrospective, but the differences were not significant. Nor did therapeutic response significantly differ based on the age of patients, type of report, or quality of the study.
The overall findings “should be interpreted with caution because of poor-quality evidence, heterogeneity, and publication bias,” the researchers said. Prospective studies are needed to examine the best PPI, dose, and dosing interval to use in an initial trial in the clinic; to clarify long-term effects and dosing strategies; to assess the ability of PPIs to reverse fibrotic esophageal remodeling; and to examine the effects of the CYP2C19 genotype on clinical and histologic response, they added. “More quality evidence on pediatric PPI-responsive eosinophilic esophagitis is needed urgently,” they emphasized.
The authors reported no funding sources and had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Proton pump inhibitors should be considered in the first-line treatment of esophageal eosinophilia.
Major finding: Half of patients achieved clinical and histologic remission after a trial of PPIs.
Data source: Meta-analysis of 33 studies of 619 patients with symptomatic esophageal eosinophilia indicative of eosinophilic esophagitis.
Disclosures: The authors reported no funding sources and had no disclosures.
Updates in Pediatrics
TALKING OUT CHILDHOOD OBESITY
Resnicow K, McMaster F, Bocian A, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649-657.
Counseling parents of overweight children using motivational interviewing from both health care providers and registered dietitians can significantly improve BMI, according to a study of 42 practices in the Pediatric Research in Office Settings Network of the American Academy of Pediatrics.
Researchers randomly assigned parents of overweight children, ages 2 through 8, to one of three groups: (1) usual care, (2) four provider-delivered motivational interviewing sessions over two years, or (3) four provider-delivered motivational interviewing sessions plus six sessions with a registered dietitian over two years. At study end, BMI percentile and change in BMI for the different groups were as follows:
COMMENTARY
The results of this study are exciting. Motivational interviewing is a technique in which the practitioner asks questions of a patient and allows the patient to discover his/her own conclusions about the topic. By so doing, the patient is more engaged in the discussion and is less resistant to input. This technique, with excellent evidence of effectiveness in the area of drug and alcohol abuse, has been shown to facilitate effective behavioral change in many areas and is recommended by the American Heart Association for behavioral change in adults.1,2 This is an exciting paper demonstrating evidence-based efficacy in addressing childhood obesity—a critical health issue—and is worth trying in the office.
1. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
2. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128(19):2169-2176. doi: 10.1161/01.cir.0000435173.25936.e1.
Continue for long-acting reversible contraception among teens >>
LONG-ACTING REVERSIBLE CONTRACEPTION AMONG TEENS
Romero L, Pazol K, Warner L, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15-19 years seeking contraceptive services – United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2015;64(13):363-369.
Efforts to improve teen access to long-acting reversible contraception (LARC) have increased use of these methods, according to a CDC review of services provided at Title X National Family Planning Program centers. The report found
• LARC rates among teen patients increased from 0.4% in 2005 to 7.2% in 2013.
• In 2013, 2.8% of those seeking contraception used IUDs and 4.3% used implants.
• Among Title X patients, 7.6% of 18- and 19-year-olds used LARC, compared with 6.5% of 15- to 17-year-olds.
• Rates of LARC were lowest in Mississippi (0.7%) and highest in Colorado (25.8%).
COMMENTARY
LARCs, which include IUD and implantable hormonal contraceptive devices, require no effort for adherence on the part of the user; once in place, they are effective without further action. Current CDC guidelines on contraceptive use clearly recommend LARC for teenagers based on the efficacy and safety.1 LARCs are favored for teenagers because poor compliance has yielded suboptimal effectiveness of oral contraceptives and condoms in teenagers, who often forget to take their birth control pills or don’t use condoms when they should. Many clinicians have been slow to recommend LARCs in teenagers based on safety concerns related to adverse experience with IUDs 20 to 30 years ago. According to CDC guidelines, IUDs and implantable contraceptive devices now have robust safety data, and this article shows that they are being increasingly made available to teenagers who need them.
1. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. US Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62(RR-05):1-60.
Continue to testing for celiac in pediatric rheumatology patients >>
TESTING FOR CELIAC IN PEDIATRIC RHEUMATOLOGY PATIENTS
Sherman Y, Karanicolas R, DiMarco B, et al. Unrecognized celiac disease in children presenting for rheumatology evaluation. Pediatrics. 2015; [Epub ahead of print].
Children presenting for rheumatology evaluation should be screened for celiac disease, according to a review of 2,125 pediatric patients who were screened for celiac as part of the standard initial serologic evaluation.
Researchers identified 36 new cases of celiac disease (2.0% prevalence rate). The most common presenting complaints among these patients were myalgia, arthralgia, and rash. Less frequent complaints included gastrointestinal complaints of abdominal pain, nausea, and diarrhea.
After initiating a gluten-free diet, all of the patients with celiac disease reported improvement or complete resolution of musculoskeletal symptoms.
COMMENTARY
Celiac disease has a prevalence of 0.5% to 1% in the US and can present with a range of symptoms that include diarrhea, abdominal discomfort, and weight loss. In addition to these typical symptoms, celiac disease can cause a wide range of systemic symptoms, including osteopenia, abnormal liver function tests, anemia, neurologic symptoms, and general malaise and fatigue.1 This study suggests that celiac testing be considered in children presenting with rheumatologic symptoms, including myalgia, arthralgia, and rash.
1. Presutti RJ, Cangemi JR, Cassidy HD, et al. Celiac disease. Am Fam Physician. 2007;76(12):1795-1802, 1809-1810.
Continue for educating parents about antibiotic use >>
EDUCATING PARENTS ABOUT ANTIBIOTIC USE
Vaz LE, Kleinman KP, Lakoma MD, et al. Prevalence of parental misconceptions about antibiotic use. Pediatrics. 2015;136:221-231.
Misperceptions about antibiotic use persist and continue to be more prevalent among parents of Medicaid-insured children, according to a study of 1,500 Massachusetts parents.
Investigators examined antibiotic-related knowledge and attitudes among both Medicaid-insured and private-insured parents and found
• Fewer Medicaid parents answered questions correctly, except for one regarding bronchitis.
• Medicaid patients were more likely to request unnecessary antibiotics.
• More parents in 2013 understood that green nasal discharge did not require antibiotics than in 2000.
• Medicaid-insured parents were younger, less likely to be white, and had less education than those commercially insured.
COMMENTARY
Decreasing the unnecessary use of antibiotics for viral infections is an important component of decreasing the development of antibiotic-resistant organisms. An important driver of clinician use of antibiotics is patients’ expectations for antibiotics. This study shows that much work remains to be done in changing patient expectations, which is not surprising to any practicing clinician. This study also suggests that the expectation for antibiotics is greater among those individuals with Medicaid insurance, which suggests that an opportunity exists for Medicaid insurance plans to do targeted patient education on this issue—which should improve patient outcomes, decrease cost from use of unneeded antibiotics, and decrease the development of antibiotic-resistant organisms.
Continue for newborn pulmonary hypertension and maternal antidepressant use >>
NEWBORN PULMONARY HYPERTENSION AND MATERNAL ANTIDEPRESSANT USE
Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313(21):2142-2151.
Taking antidepressants during late pregnancy may increase the risk for persistent pulmonary hypertension of the newborn (PPHN), according to a nested cohort study of more than 3.7 million pregnant women in the 2000-2010 Medicaid Analytic eXtract.
Investigators compared offspring of mothers who used selective serotonin reuptake inhibitors (SSRIs) or non-SSRI monotherapy in the last 90 days of pregnancy to those who did not and found 3.4% of women filled at least one prescription for antidepressants late in pregnancy, primarily SSRIs.
Rates and odds ratios (ORs) of PPHN stratified by use and type of antidepressant were as follows:
The study authors note the absolute risk is small, and the increased risk is more modest than previous studies found.
COMMENTARY
Depression affects more than 12% of pregnancies and has important consequences, including increased risk for suicide, preterm birth, poor fetal growth, and impaired fetal and infant development.1 PPHN is a serious condition that can require intubation and can be fatal in 10% to 20% of cases. This study adds to the conflicting data suggesting that SSRI use in pregnancy can lead to an increase in this rare condition. The decision to use an antidepressant during pregnancy is a difficult one, because depression has serious consequences but so, potentially, does treatment. The treatment of depression during pregnancy requires thoughtful, informed discussion between patient and provider.
1. Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605-1611. doi:10.1056/NEJMcp1102730.
Continue for new infant vaccine treats 6 diseases >>
NEW INFANT VACCINE TREATS 6 DISEASES
Marshall GS, Adams GL, Leonardi ML, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015:136(2):323-332.
The safety and immunogenicity of DTaP5-IPV-Hib-HepB fully liquid investigational hexavalent vaccine are comparable with the analogous licensed component vaccines and provide a new combination vaccine option aligned with the recommended US infant immunization schedule, according to a phase III study of 1,465 participants. Overall, 981 healthy infants were vaccinated in group 1 with the hexavalent vaccine and 484 in group 2 with the analogous licensed component vaccines.
The study found
• Immune responses in group 1 to all antigens contained in the vaccine one month after dose 3 were essentially noninferior to those in group 2.
• Adverse event rates after any dose were similar in both groups.
COMMENTARY
Vaccine administration has led to many diseases, including Haemophilus influenzae type b, polio, and measles, becoming quite rare. As we have recently seen with the resurgence of measles, continued vigilance and high immunization rates are important in ensuring that these diseases remain rare. The development of a hexavalent vaccine is another step in making immunization easier for clinicians to administer and for patients to accept.
Continue for treating infants with bronchiolitis >>
TREATING INFANTS WITH BRONCHIOLITIS
Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136:1036-1043.
Infants hospitalized with bronchiolitis saw no difference in length of stay or seven-day readmission rates when treated with nebulized 3% hypertonic saline (HS) compared with nebulized normal saline (NS), according to a randomized, controlled study of 227 infants who were younger than 12 months when admitted. Patients received either 4 mL nebulized 3% HS (113 infants) or 4 mL 0.9% NS (114 infants) every four hours from enrollment until hospital discharge. Researchers found
• Median length of stay of HS and NS groups was 2.1 days vs 2.1 days, respectively.
• Seven-day readmission rates for HS and NS groups were 4.3% vs 3.1%, respectively.
• Clinical worsening events were similar between groups.
COMMENTARY
Bronchiolitis, the most common lower respiratory tract infection in infants, is usually due to a viral infection, most often respiratory syncytial virus, and can cause disease that ranges in severity from mild to life-threatening. Infants with bronchiolitis typically present with rhinitis, tachypnea, wheezing, and cough, and occasionally crackles and use of accessory muscles. While many medications are used, supportive care and monitoring are the mainstays of therapy. Maintaining pulse above 90% is important, using supplemental oxygen when needed to achieve this. Alpha-adrenergic and beta-adrenergic bronchodilators are often used; though the evidence suggests that they are not usually helpful, they can be tried and continued if they appear to help. Systemic steroids are often used, but they too lack evidence of efficacy.1 This study shows that hypertonic saline can now join the list of interventions used with evidence of a lack of efficacy.
1. Diagnosis and management of bronchiolitis. Subcommittee on diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. doi:10.1542/peds.2006-2223.
Continue for azithromycin and preschool children >>
AZITHROMYCIN AND PRESCHOOL CHILDREN: CAN ANTIBIOTICS LESSEN ONSET OF SEVERE LRTIs?
Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034-2044.
The use of azithromycin early during an apparent respiratory tract illness (RTI) reduced the likelihood of severe lower RTI (LRTI) among young children with a history of recurrent severe LRTI, compared with placebo, according to a study of 607 children ages 12 to 71 months. Participants were randomized in a 1:1 ratio to receive either azithromycin (12 mg/kg/d for 5 d) or matching placebo at the start of an RTI. Researchers found
• A total of 937 treated RTIs were experienced by 443 children, including 92 severe LRTIs (azithromycin group, 35; placebo group, 57).
• Azithromycin significantly reduced the risk for progression to severe LRTI relative to placebo (HR, 0.64).
• Induction of azithromycin-resistant organisms and adverse events were infrequent.
COMMENTARY
Recurrent episodes of severe wheezing with RTI are an important and common occurrence, affecting up to 15% to 20% of children prior to age 6.1 LRTI was defined in this study as RTI that required the use of additional rescue medication. The current approach to RTI is to try to minimize the use of antibiotics unless an infection is clearly bacterial in origin, and to treat severe LRTI when it occurs. The results of this trial suggest that in children at high risk for severe LRTI, identified by their episodes of recurrent wheezing, early treatment of RTI with azithromycin may decrease the development of severe LRTI by more than 35%—an important result. It is important to recognize that this study does not suggest treating all RTIs with antibiotics, but rather that the use of a macrolide antibiotic may be considered, perhaps, in the select group of children similar to those studied, with a history of recurrent wheezing with previous RTIs.
1. Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
TALKING OUT CHILDHOOD OBESITY
Resnicow K, McMaster F, Bocian A, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649-657.
Counseling parents of overweight children using motivational interviewing from both health care providers and registered dietitians can significantly improve BMI, according to a study of 42 practices in the Pediatric Research in Office Settings Network of the American Academy of Pediatrics.
Researchers randomly assigned parents of overweight children, ages 2 through 8, to one of three groups: (1) usual care, (2) four provider-delivered motivational interviewing sessions over two years, or (3) four provider-delivered motivational interviewing sessions plus six sessions with a registered dietitian over two years. At study end, BMI percentile and change in BMI for the different groups were as follows:
COMMENTARY
The results of this study are exciting. Motivational interviewing is a technique in which the practitioner asks questions of a patient and allows the patient to discover his/her own conclusions about the topic. By so doing, the patient is more engaged in the discussion and is less resistant to input. This technique, with excellent evidence of effectiveness in the area of drug and alcohol abuse, has been shown to facilitate effective behavioral change in many areas and is recommended by the American Heart Association for behavioral change in adults.1,2 This is an exciting paper demonstrating evidence-based efficacy in addressing childhood obesity—a critical health issue—and is worth trying in the office.
1. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
2. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128(19):2169-2176. doi: 10.1161/01.cir.0000435173.25936.e1.
Continue for long-acting reversible contraception among teens >>
LONG-ACTING REVERSIBLE CONTRACEPTION AMONG TEENS
Romero L, Pazol K, Warner L, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15-19 years seeking contraceptive services – United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2015;64(13):363-369.
Efforts to improve teen access to long-acting reversible contraception (LARC) have increased use of these methods, according to a CDC review of services provided at Title X National Family Planning Program centers. The report found
• LARC rates among teen patients increased from 0.4% in 2005 to 7.2% in 2013.
• In 2013, 2.8% of those seeking contraception used IUDs and 4.3% used implants.
• Among Title X patients, 7.6% of 18- and 19-year-olds used LARC, compared with 6.5% of 15- to 17-year-olds.
• Rates of LARC were lowest in Mississippi (0.7%) and highest in Colorado (25.8%).
COMMENTARY
LARCs, which include IUD and implantable hormonal contraceptive devices, require no effort for adherence on the part of the user; once in place, they are effective without further action. Current CDC guidelines on contraceptive use clearly recommend LARC for teenagers based on the efficacy and safety.1 LARCs are favored for teenagers because poor compliance has yielded suboptimal effectiveness of oral contraceptives and condoms in teenagers, who often forget to take their birth control pills or don’t use condoms when they should. Many clinicians have been slow to recommend LARCs in teenagers based on safety concerns related to adverse experience with IUDs 20 to 30 years ago. According to CDC guidelines, IUDs and implantable contraceptive devices now have robust safety data, and this article shows that they are being increasingly made available to teenagers who need them.
1. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. US Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62(RR-05):1-60.
Continue to testing for celiac in pediatric rheumatology patients >>
TESTING FOR CELIAC IN PEDIATRIC RHEUMATOLOGY PATIENTS
Sherman Y, Karanicolas R, DiMarco B, et al. Unrecognized celiac disease in children presenting for rheumatology evaluation. Pediatrics. 2015; [Epub ahead of print].
Children presenting for rheumatology evaluation should be screened for celiac disease, according to a review of 2,125 pediatric patients who were screened for celiac as part of the standard initial serologic evaluation.
Researchers identified 36 new cases of celiac disease (2.0% prevalence rate). The most common presenting complaints among these patients were myalgia, arthralgia, and rash. Less frequent complaints included gastrointestinal complaints of abdominal pain, nausea, and diarrhea.
After initiating a gluten-free diet, all of the patients with celiac disease reported improvement or complete resolution of musculoskeletal symptoms.
COMMENTARY
Celiac disease has a prevalence of 0.5% to 1% in the US and can present with a range of symptoms that include diarrhea, abdominal discomfort, and weight loss. In addition to these typical symptoms, celiac disease can cause a wide range of systemic symptoms, including osteopenia, abnormal liver function tests, anemia, neurologic symptoms, and general malaise and fatigue.1 This study suggests that celiac testing be considered in children presenting with rheumatologic symptoms, including myalgia, arthralgia, and rash.
1. Presutti RJ, Cangemi JR, Cassidy HD, et al. Celiac disease. Am Fam Physician. 2007;76(12):1795-1802, 1809-1810.
Continue for educating parents about antibiotic use >>
EDUCATING PARENTS ABOUT ANTIBIOTIC USE
Vaz LE, Kleinman KP, Lakoma MD, et al. Prevalence of parental misconceptions about antibiotic use. Pediatrics. 2015;136:221-231.
Misperceptions about antibiotic use persist and continue to be more prevalent among parents of Medicaid-insured children, according to a study of 1,500 Massachusetts parents.
Investigators examined antibiotic-related knowledge and attitudes among both Medicaid-insured and private-insured parents and found
• Fewer Medicaid parents answered questions correctly, except for one regarding bronchitis.
• Medicaid patients were more likely to request unnecessary antibiotics.
• More parents in 2013 understood that green nasal discharge did not require antibiotics than in 2000.
• Medicaid-insured parents were younger, less likely to be white, and had less education than those commercially insured.
COMMENTARY
Decreasing the unnecessary use of antibiotics for viral infections is an important component of decreasing the development of antibiotic-resistant organisms. An important driver of clinician use of antibiotics is patients’ expectations for antibiotics. This study shows that much work remains to be done in changing patient expectations, which is not surprising to any practicing clinician. This study also suggests that the expectation for antibiotics is greater among those individuals with Medicaid insurance, which suggests that an opportunity exists for Medicaid insurance plans to do targeted patient education on this issue—which should improve patient outcomes, decrease cost from use of unneeded antibiotics, and decrease the development of antibiotic-resistant organisms.
Continue for newborn pulmonary hypertension and maternal antidepressant use >>
NEWBORN PULMONARY HYPERTENSION AND MATERNAL ANTIDEPRESSANT USE
Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313(21):2142-2151.
Taking antidepressants during late pregnancy may increase the risk for persistent pulmonary hypertension of the newborn (PPHN), according to a nested cohort study of more than 3.7 million pregnant women in the 2000-2010 Medicaid Analytic eXtract.
Investigators compared offspring of mothers who used selective serotonin reuptake inhibitors (SSRIs) or non-SSRI monotherapy in the last 90 days of pregnancy to those who did not and found 3.4% of women filled at least one prescription for antidepressants late in pregnancy, primarily SSRIs.
Rates and odds ratios (ORs) of PPHN stratified by use and type of antidepressant were as follows:
The study authors note the absolute risk is small, and the increased risk is more modest than previous studies found.
COMMENTARY
Depression affects more than 12% of pregnancies and has important consequences, including increased risk for suicide, preterm birth, poor fetal growth, and impaired fetal and infant development.1 PPHN is a serious condition that can require intubation and can be fatal in 10% to 20% of cases. This study adds to the conflicting data suggesting that SSRI use in pregnancy can lead to an increase in this rare condition. The decision to use an antidepressant during pregnancy is a difficult one, because depression has serious consequences but so, potentially, does treatment. The treatment of depression during pregnancy requires thoughtful, informed discussion between patient and provider.
1. Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605-1611. doi:10.1056/NEJMcp1102730.
Continue for new infant vaccine treats 6 diseases >>
NEW INFANT VACCINE TREATS 6 DISEASES
Marshall GS, Adams GL, Leonardi ML, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015:136(2):323-332.
The safety and immunogenicity of DTaP5-IPV-Hib-HepB fully liquid investigational hexavalent vaccine are comparable with the analogous licensed component vaccines and provide a new combination vaccine option aligned with the recommended US infant immunization schedule, according to a phase III study of 1,465 participants. Overall, 981 healthy infants were vaccinated in group 1 with the hexavalent vaccine and 484 in group 2 with the analogous licensed component vaccines.
The study found
• Immune responses in group 1 to all antigens contained in the vaccine one month after dose 3 were essentially noninferior to those in group 2.
• Adverse event rates after any dose were similar in both groups.
COMMENTARY
Vaccine administration has led to many diseases, including Haemophilus influenzae type b, polio, and measles, becoming quite rare. As we have recently seen with the resurgence of measles, continued vigilance and high immunization rates are important in ensuring that these diseases remain rare. The development of a hexavalent vaccine is another step in making immunization easier for clinicians to administer and for patients to accept.
Continue for treating infants with bronchiolitis >>
TREATING INFANTS WITH BRONCHIOLITIS
Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136:1036-1043.
Infants hospitalized with bronchiolitis saw no difference in length of stay or seven-day readmission rates when treated with nebulized 3% hypertonic saline (HS) compared with nebulized normal saline (NS), according to a randomized, controlled study of 227 infants who were younger than 12 months when admitted. Patients received either 4 mL nebulized 3% HS (113 infants) or 4 mL 0.9% NS (114 infants) every four hours from enrollment until hospital discharge. Researchers found
• Median length of stay of HS and NS groups was 2.1 days vs 2.1 days, respectively.
• Seven-day readmission rates for HS and NS groups were 4.3% vs 3.1%, respectively.
• Clinical worsening events were similar between groups.
COMMENTARY
Bronchiolitis, the most common lower respiratory tract infection in infants, is usually due to a viral infection, most often respiratory syncytial virus, and can cause disease that ranges in severity from mild to life-threatening. Infants with bronchiolitis typically present with rhinitis, tachypnea, wheezing, and cough, and occasionally crackles and use of accessory muscles. While many medications are used, supportive care and monitoring are the mainstays of therapy. Maintaining pulse above 90% is important, using supplemental oxygen when needed to achieve this. Alpha-adrenergic and beta-adrenergic bronchodilators are often used; though the evidence suggests that they are not usually helpful, they can be tried and continued if they appear to help. Systemic steroids are often used, but they too lack evidence of efficacy.1 This study shows that hypertonic saline can now join the list of interventions used with evidence of a lack of efficacy.
1. Diagnosis and management of bronchiolitis. Subcommittee on diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. doi:10.1542/peds.2006-2223.
Continue for azithromycin and preschool children >>
AZITHROMYCIN AND PRESCHOOL CHILDREN: CAN ANTIBIOTICS LESSEN ONSET OF SEVERE LRTIs?
Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034-2044.
The use of azithromycin early during an apparent respiratory tract illness (RTI) reduced the likelihood of severe lower RTI (LRTI) among young children with a history of recurrent severe LRTI, compared with placebo, according to a study of 607 children ages 12 to 71 months. Participants were randomized in a 1:1 ratio to receive either azithromycin (12 mg/kg/d for 5 d) or matching placebo at the start of an RTI. Researchers found
• A total of 937 treated RTIs were experienced by 443 children, including 92 severe LRTIs (azithromycin group, 35; placebo group, 57).
• Azithromycin significantly reduced the risk for progression to severe LRTI relative to placebo (HR, 0.64).
• Induction of azithromycin-resistant organisms and adverse events were infrequent.
COMMENTARY
Recurrent episodes of severe wheezing with RTI are an important and common occurrence, affecting up to 15% to 20% of children prior to age 6.1 LRTI was defined in this study as RTI that required the use of additional rescue medication. The current approach to RTI is to try to minimize the use of antibiotics unless an infection is clearly bacterial in origin, and to treat severe LRTI when it occurs. The results of this trial suggest that in children at high risk for severe LRTI, identified by their episodes of recurrent wheezing, early treatment of RTI with azithromycin may decrease the development of severe LRTI by more than 35%—an important result. It is important to recognize that this study does not suggest treating all RTIs with antibiotics, but rather that the use of a macrolide antibiotic may be considered, perhaps, in the select group of children similar to those studied, with a history of recurrent wheezing with previous RTIs.
1. Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
TALKING OUT CHILDHOOD OBESITY
Resnicow K, McMaster F, Bocian A, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649-657.
Counseling parents of overweight children using motivational interviewing from both health care providers and registered dietitians can significantly improve BMI, according to a study of 42 practices in the Pediatric Research in Office Settings Network of the American Academy of Pediatrics.
Researchers randomly assigned parents of overweight children, ages 2 through 8, to one of three groups: (1) usual care, (2) four provider-delivered motivational interviewing sessions over two years, or (3) four provider-delivered motivational interviewing sessions plus six sessions with a registered dietitian over two years. At study end, BMI percentile and change in BMI for the different groups were as follows:
COMMENTARY
The results of this study are exciting. Motivational interviewing is a technique in which the practitioner asks questions of a patient and allows the patient to discover his/her own conclusions about the topic. By so doing, the patient is more engaged in the discussion and is less resistant to input. This technique, with excellent evidence of effectiveness in the area of drug and alcohol abuse, has been shown to facilitate effective behavioral change in many areas and is recommended by the American Heart Association for behavioral change in adults.1,2 This is an exciting paper demonstrating evidence-based efficacy in addressing childhood obesity—a critical health issue—and is worth trying in the office.
1. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
2. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128(19):2169-2176. doi: 10.1161/01.cir.0000435173.25936.e1.
Continue for long-acting reversible contraception among teens >>
LONG-ACTING REVERSIBLE CONTRACEPTION AMONG TEENS
Romero L, Pazol K, Warner L, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15-19 years seeking contraceptive services – United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2015;64(13):363-369.
Efforts to improve teen access to long-acting reversible contraception (LARC) have increased use of these methods, according to a CDC review of services provided at Title X National Family Planning Program centers. The report found
• LARC rates among teen patients increased from 0.4% in 2005 to 7.2% in 2013.
• In 2013, 2.8% of those seeking contraception used IUDs and 4.3% used implants.
• Among Title X patients, 7.6% of 18- and 19-year-olds used LARC, compared with 6.5% of 15- to 17-year-olds.
• Rates of LARC were lowest in Mississippi (0.7%) and highest in Colorado (25.8%).
COMMENTARY
LARCs, which include IUD and implantable hormonal contraceptive devices, require no effort for adherence on the part of the user; once in place, they are effective without further action. Current CDC guidelines on contraceptive use clearly recommend LARC for teenagers based on the efficacy and safety.1 LARCs are favored for teenagers because poor compliance has yielded suboptimal effectiveness of oral contraceptives and condoms in teenagers, who often forget to take their birth control pills or don’t use condoms when they should. Many clinicians have been slow to recommend LARCs in teenagers based on safety concerns related to adverse experience with IUDs 20 to 30 years ago. According to CDC guidelines, IUDs and implantable contraceptive devices now have robust safety data, and this article shows that they are being increasingly made available to teenagers who need them.
1. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. US Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62(RR-05):1-60.
Continue to testing for celiac in pediatric rheumatology patients >>
TESTING FOR CELIAC IN PEDIATRIC RHEUMATOLOGY PATIENTS
Sherman Y, Karanicolas R, DiMarco B, et al. Unrecognized celiac disease in children presenting for rheumatology evaluation. Pediatrics. 2015; [Epub ahead of print].
Children presenting for rheumatology evaluation should be screened for celiac disease, according to a review of 2,125 pediatric patients who were screened for celiac as part of the standard initial serologic evaluation.
Researchers identified 36 new cases of celiac disease (2.0% prevalence rate). The most common presenting complaints among these patients were myalgia, arthralgia, and rash. Less frequent complaints included gastrointestinal complaints of abdominal pain, nausea, and diarrhea.
After initiating a gluten-free diet, all of the patients with celiac disease reported improvement or complete resolution of musculoskeletal symptoms.
COMMENTARY
Celiac disease has a prevalence of 0.5% to 1% in the US and can present with a range of symptoms that include diarrhea, abdominal discomfort, and weight loss. In addition to these typical symptoms, celiac disease can cause a wide range of systemic symptoms, including osteopenia, abnormal liver function tests, anemia, neurologic symptoms, and general malaise and fatigue.1 This study suggests that celiac testing be considered in children presenting with rheumatologic symptoms, including myalgia, arthralgia, and rash.
1. Presutti RJ, Cangemi JR, Cassidy HD, et al. Celiac disease. Am Fam Physician. 2007;76(12):1795-1802, 1809-1810.
Continue for educating parents about antibiotic use >>
EDUCATING PARENTS ABOUT ANTIBIOTIC USE
Vaz LE, Kleinman KP, Lakoma MD, et al. Prevalence of parental misconceptions about antibiotic use. Pediatrics. 2015;136:221-231.
Misperceptions about antibiotic use persist and continue to be more prevalent among parents of Medicaid-insured children, according to a study of 1,500 Massachusetts parents.
Investigators examined antibiotic-related knowledge and attitudes among both Medicaid-insured and private-insured parents and found
• Fewer Medicaid parents answered questions correctly, except for one regarding bronchitis.
• Medicaid patients were more likely to request unnecessary antibiotics.
• More parents in 2013 understood that green nasal discharge did not require antibiotics than in 2000.
• Medicaid-insured parents were younger, less likely to be white, and had less education than those commercially insured.
COMMENTARY
Decreasing the unnecessary use of antibiotics for viral infections is an important component of decreasing the development of antibiotic-resistant organisms. An important driver of clinician use of antibiotics is patients’ expectations for antibiotics. This study shows that much work remains to be done in changing patient expectations, which is not surprising to any practicing clinician. This study also suggests that the expectation for antibiotics is greater among those individuals with Medicaid insurance, which suggests that an opportunity exists for Medicaid insurance plans to do targeted patient education on this issue—which should improve patient outcomes, decrease cost from use of unneeded antibiotics, and decrease the development of antibiotic-resistant organisms.
Continue for newborn pulmonary hypertension and maternal antidepressant use >>
NEWBORN PULMONARY HYPERTENSION AND MATERNAL ANTIDEPRESSANT USE
Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313(21):2142-2151.
Taking antidepressants during late pregnancy may increase the risk for persistent pulmonary hypertension of the newborn (PPHN), according to a nested cohort study of more than 3.7 million pregnant women in the 2000-2010 Medicaid Analytic eXtract.
Investigators compared offspring of mothers who used selective serotonin reuptake inhibitors (SSRIs) or non-SSRI monotherapy in the last 90 days of pregnancy to those who did not and found 3.4% of women filled at least one prescription for antidepressants late in pregnancy, primarily SSRIs.
Rates and odds ratios (ORs) of PPHN stratified by use and type of antidepressant were as follows:
The study authors note the absolute risk is small, and the increased risk is more modest than previous studies found.
COMMENTARY
Depression affects more than 12% of pregnancies and has important consequences, including increased risk for suicide, preterm birth, poor fetal growth, and impaired fetal and infant development.1 PPHN is a serious condition that can require intubation and can be fatal in 10% to 20% of cases. This study adds to the conflicting data suggesting that SSRI use in pregnancy can lead to an increase in this rare condition. The decision to use an antidepressant during pregnancy is a difficult one, because depression has serious consequences but so, potentially, does treatment. The treatment of depression during pregnancy requires thoughtful, informed discussion between patient and provider.
1. Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605-1611. doi:10.1056/NEJMcp1102730.
Continue for new infant vaccine treats 6 diseases >>
NEW INFANT VACCINE TREATS 6 DISEASES
Marshall GS, Adams GL, Leonardi ML, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015:136(2):323-332.
The safety and immunogenicity of DTaP5-IPV-Hib-HepB fully liquid investigational hexavalent vaccine are comparable with the analogous licensed component vaccines and provide a new combination vaccine option aligned with the recommended US infant immunization schedule, according to a phase III study of 1,465 participants. Overall, 981 healthy infants were vaccinated in group 1 with the hexavalent vaccine and 484 in group 2 with the analogous licensed component vaccines.
The study found
• Immune responses in group 1 to all antigens contained in the vaccine one month after dose 3 were essentially noninferior to those in group 2.
• Adverse event rates after any dose were similar in both groups.
COMMENTARY
Vaccine administration has led to many diseases, including Haemophilus influenzae type b, polio, and measles, becoming quite rare. As we have recently seen with the resurgence of measles, continued vigilance and high immunization rates are important in ensuring that these diseases remain rare. The development of a hexavalent vaccine is another step in making immunization easier for clinicians to administer and for patients to accept.
Continue for treating infants with bronchiolitis >>
TREATING INFANTS WITH BRONCHIOLITIS
Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136:1036-1043.
Infants hospitalized with bronchiolitis saw no difference in length of stay or seven-day readmission rates when treated with nebulized 3% hypertonic saline (HS) compared with nebulized normal saline (NS), according to a randomized, controlled study of 227 infants who were younger than 12 months when admitted. Patients received either 4 mL nebulized 3% HS (113 infants) or 4 mL 0.9% NS (114 infants) every four hours from enrollment until hospital discharge. Researchers found
• Median length of stay of HS and NS groups was 2.1 days vs 2.1 days, respectively.
• Seven-day readmission rates for HS and NS groups were 4.3% vs 3.1%, respectively.
• Clinical worsening events were similar between groups.
COMMENTARY
Bronchiolitis, the most common lower respiratory tract infection in infants, is usually due to a viral infection, most often respiratory syncytial virus, and can cause disease that ranges in severity from mild to life-threatening. Infants with bronchiolitis typically present with rhinitis, tachypnea, wheezing, and cough, and occasionally crackles and use of accessory muscles. While many medications are used, supportive care and monitoring are the mainstays of therapy. Maintaining pulse above 90% is important, using supplemental oxygen when needed to achieve this. Alpha-adrenergic and beta-adrenergic bronchodilators are often used; though the evidence suggests that they are not usually helpful, they can be tried and continued if they appear to help. Systemic steroids are often used, but they too lack evidence of efficacy.1 This study shows that hypertonic saline can now join the list of interventions used with evidence of a lack of efficacy.
1. Diagnosis and management of bronchiolitis. Subcommittee on diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. doi:10.1542/peds.2006-2223.
Continue for azithromycin and preschool children >>
AZITHROMYCIN AND PRESCHOOL CHILDREN: CAN ANTIBIOTICS LESSEN ONSET OF SEVERE LRTIs?
Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034-2044.
The use of azithromycin early during an apparent respiratory tract illness (RTI) reduced the likelihood of severe lower RTI (LRTI) among young children with a history of recurrent severe LRTI, compared with placebo, according to a study of 607 children ages 12 to 71 months. Participants were randomized in a 1:1 ratio to receive either azithromycin (12 mg/kg/d for 5 d) or matching placebo at the start of an RTI. Researchers found
• A total of 937 treated RTIs were experienced by 443 children, including 92 severe LRTIs (azithromycin group, 35; placebo group, 57).
• Azithromycin significantly reduced the risk for progression to severe LRTI relative to placebo (HR, 0.64).
• Induction of azithromycin-resistant organisms and adverse events were infrequent.
COMMENTARY
Recurrent episodes of severe wheezing with RTI are an important and common occurrence, affecting up to 15% to 20% of children prior to age 6.1 LRTI was defined in this study as RTI that required the use of additional rescue medication. The current approach to RTI is to try to minimize the use of antibiotics unless an infection is clearly bacterial in origin, and to treat severe LRTI when it occurs. The results of this trial suggest that in children at high risk for severe LRTI, identified by their episodes of recurrent wheezing, early treatment of RTI with azithromycin may decrease the development of severe LRTI by more than 35%—an important result. It is important to recognize that this study does not suggest treating all RTIs with antibiotics, but rather that the use of a macrolide antibiotic may be considered, perhaps, in the select group of children similar to those studied, with a history of recurrent wheezing with previous RTIs.
1. Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
Reslizumab: FDA panel recommends approval for adults, but not children
SILVER SPRING, MD. Members of the FDA Pulmonary-Allergy Drugs Advisory Committee voted 11-3 on Dec. 9 to recommend the approval of reslizumab, a humanized monoclonal antibody, for the treatment of asthma and elevated blood eosinophils in patients aged 18 years and older but did not recommend approval for children aged 12-17 years.
The advisers were tasked to consider a dosage of 3 mg/kg of reslizumab, administered intravenously once every 4 weeks for the management of severe asthma. The lack of a recommendation for the pediatric population is because of what panel members considered to be insufficient data culled from a small sample population (19 patients), along with results that did not provide enough evidence that the treatment was of significant benefit to adolescents.
There were “limited data” presented to support use of reslizumab in pediatrics, and “all the evidence was going in the wrong direction,” according to panel member Erica H. Brittain, Ph.D., of the National Institute of Allergy and Infectious Diseases, Bethesda, Md.
Panelist Dr. Thomas A.E. Platts-Mills, professor of medicine at the University of Virginia, Charlottesville, added that a larger study is needed in order to more accurately assess the drug’s efficacy, and should also include patients younger than the age of 12 years.
Both of the questions posited to the panel by the FDA regarding reslizumab approval for pediatric patients, “Do the efficacy data provide substantial evidence of a clinically meaningful benefit?” and “Do the available efficacy and safety data support approval of reslizumab?” received unanimous “No” votes from the 14-member voting panel.
The advisory panel members considered data from five separate trials evaluating the safety and efficacy of reslizumab to be marketed as Cinqair by Teva Pharmaceuticals. Those trials included two 16-week lung-function studies examining forced expiratory volume in 1 second (FEV1), two year-long asthma exacerbation studies, and an open-label safety extension study.
Advisers generally agreed that reslizumab demonstrated substantial improvement in FEV1 and asthma exacerbation in the adult population. Specifically, in the two exacerbation studies, clinical asthma exacerbations did not occur at all over the 12-month study period in 61% and 73% of subjects on reslizumab, vs. 44% and 52% of subjects in the control cohort, respectively, with FEV1 dropping.
However, panelists voiced their concern about the risk of muscle toxicity and, especially, anaphylaxis. In a presentation regarding the treatment’s immunogenicity issues, João A. Pedras-Vasconcelos, Ph.D., of the FDA Office of Pharmaceutical Quality, cautioned that enough work was not done by the sponsors to “thoroughly investigate [the] root causes of anaphylaxis.”
Ultimately, the advisory committee largely agreed that the unmet need for reslizumab outweighed the potential risks associated with it.
In casting his “yes” vote regarding the adequacy of reslizumab’s safety profile, panel chair Dr. Dennis R. Ownby, professor of pediatrics at Georgia Regents University in Augusta, admitted that he was “reluctant” to endorse reslizumab, but said that he believes “this is a drug that clinicians will use very cautiously, [so] I’m placing faith on our practicing physicians” to prescribe the drug responsibly.
Advisers voted 11-3 to recommend approval of reslizumab as a safe and effective treatment of severe asthma and elevated blood eosinophils. Approval of reslizumab would make it the third monoclonal antibody to be FDA approved to treat asthma.
The FDA is not required to follow the advice of its advisory panels, but often does. No members of the panel reported any relevant financial conflicts of interest.
SILVER SPRING, MD. Members of the FDA Pulmonary-Allergy Drugs Advisory Committee voted 11-3 on Dec. 9 to recommend the approval of reslizumab, a humanized monoclonal antibody, for the treatment of asthma and elevated blood eosinophils in patients aged 18 years and older but did not recommend approval for children aged 12-17 years.
The advisers were tasked to consider a dosage of 3 mg/kg of reslizumab, administered intravenously once every 4 weeks for the management of severe asthma. The lack of a recommendation for the pediatric population is because of what panel members considered to be insufficient data culled from a small sample population (19 patients), along with results that did not provide enough evidence that the treatment was of significant benefit to adolescents.
There were “limited data” presented to support use of reslizumab in pediatrics, and “all the evidence was going in the wrong direction,” according to panel member Erica H. Brittain, Ph.D., of the National Institute of Allergy and Infectious Diseases, Bethesda, Md.
Panelist Dr. Thomas A.E. Platts-Mills, professor of medicine at the University of Virginia, Charlottesville, added that a larger study is needed in order to more accurately assess the drug’s efficacy, and should also include patients younger than the age of 12 years.
Both of the questions posited to the panel by the FDA regarding reslizumab approval for pediatric patients, “Do the efficacy data provide substantial evidence of a clinically meaningful benefit?” and “Do the available efficacy and safety data support approval of reslizumab?” received unanimous “No” votes from the 14-member voting panel.
The advisory panel members considered data from five separate trials evaluating the safety and efficacy of reslizumab to be marketed as Cinqair by Teva Pharmaceuticals. Those trials included two 16-week lung-function studies examining forced expiratory volume in 1 second (FEV1), two year-long asthma exacerbation studies, and an open-label safety extension study.
Advisers generally agreed that reslizumab demonstrated substantial improvement in FEV1 and asthma exacerbation in the adult population. Specifically, in the two exacerbation studies, clinical asthma exacerbations did not occur at all over the 12-month study period in 61% and 73% of subjects on reslizumab, vs. 44% and 52% of subjects in the control cohort, respectively, with FEV1 dropping.
However, panelists voiced their concern about the risk of muscle toxicity and, especially, anaphylaxis. In a presentation regarding the treatment’s immunogenicity issues, João A. Pedras-Vasconcelos, Ph.D., of the FDA Office of Pharmaceutical Quality, cautioned that enough work was not done by the sponsors to “thoroughly investigate [the] root causes of anaphylaxis.”
Ultimately, the advisory committee largely agreed that the unmet need for reslizumab outweighed the potential risks associated with it.
In casting his “yes” vote regarding the adequacy of reslizumab’s safety profile, panel chair Dr. Dennis R. Ownby, professor of pediatrics at Georgia Regents University in Augusta, admitted that he was “reluctant” to endorse reslizumab, but said that he believes “this is a drug that clinicians will use very cautiously, [so] I’m placing faith on our practicing physicians” to prescribe the drug responsibly.
Advisers voted 11-3 to recommend approval of reslizumab as a safe and effective treatment of severe asthma and elevated blood eosinophils. Approval of reslizumab would make it the third monoclonal antibody to be FDA approved to treat asthma.
The FDA is not required to follow the advice of its advisory panels, but often does. No members of the panel reported any relevant financial conflicts of interest.
SILVER SPRING, MD. Members of the FDA Pulmonary-Allergy Drugs Advisory Committee voted 11-3 on Dec. 9 to recommend the approval of reslizumab, a humanized monoclonal antibody, for the treatment of asthma and elevated blood eosinophils in patients aged 18 years and older but did not recommend approval for children aged 12-17 years.
The advisers were tasked to consider a dosage of 3 mg/kg of reslizumab, administered intravenously once every 4 weeks for the management of severe asthma. The lack of a recommendation for the pediatric population is because of what panel members considered to be insufficient data culled from a small sample population (19 patients), along with results that did not provide enough evidence that the treatment was of significant benefit to adolescents.
There were “limited data” presented to support use of reslizumab in pediatrics, and “all the evidence was going in the wrong direction,” according to panel member Erica H. Brittain, Ph.D., of the National Institute of Allergy and Infectious Diseases, Bethesda, Md.
Panelist Dr. Thomas A.E. Platts-Mills, professor of medicine at the University of Virginia, Charlottesville, added that a larger study is needed in order to more accurately assess the drug’s efficacy, and should also include patients younger than the age of 12 years.
Both of the questions posited to the panel by the FDA regarding reslizumab approval for pediatric patients, “Do the efficacy data provide substantial evidence of a clinically meaningful benefit?” and “Do the available efficacy and safety data support approval of reslizumab?” received unanimous “No” votes from the 14-member voting panel.
The advisory panel members considered data from five separate trials evaluating the safety and efficacy of reslizumab to be marketed as Cinqair by Teva Pharmaceuticals. Those trials included two 16-week lung-function studies examining forced expiratory volume in 1 second (FEV1), two year-long asthma exacerbation studies, and an open-label safety extension study.
Advisers generally agreed that reslizumab demonstrated substantial improvement in FEV1 and asthma exacerbation in the adult population. Specifically, in the two exacerbation studies, clinical asthma exacerbations did not occur at all over the 12-month study period in 61% and 73% of subjects on reslizumab, vs. 44% and 52% of subjects in the control cohort, respectively, with FEV1 dropping.
However, panelists voiced their concern about the risk of muscle toxicity and, especially, anaphylaxis. In a presentation regarding the treatment’s immunogenicity issues, João A. Pedras-Vasconcelos, Ph.D., of the FDA Office of Pharmaceutical Quality, cautioned that enough work was not done by the sponsors to “thoroughly investigate [the] root causes of anaphylaxis.”
Ultimately, the advisory committee largely agreed that the unmet need for reslizumab outweighed the potential risks associated with it.
In casting his “yes” vote regarding the adequacy of reslizumab’s safety profile, panel chair Dr. Dennis R. Ownby, professor of pediatrics at Georgia Regents University in Augusta, admitted that he was “reluctant” to endorse reslizumab, but said that he believes “this is a drug that clinicians will use very cautiously, [so] I’m placing faith on our practicing physicians” to prescribe the drug responsibly.
Advisers voted 11-3 to recommend approval of reslizumab as a safe and effective treatment of severe asthma and elevated blood eosinophils. Approval of reslizumab would make it the third monoclonal antibody to be FDA approved to treat asthma.
The FDA is not required to follow the advice of its advisory panels, but often does. No members of the panel reported any relevant financial conflicts of interest.
AT AN FDA ADVISORY COMMITTEE MEETING
Gut Grief: The Truth About Gluten Sensitivity
IN THIS ARTICLE
• So what is gluten?
• Selected symptoms of celiac disease
• Selected foods and products containing gluten
Gluten has become a dietary pariah (see “So What Is Gluten?"). The American public’s enthusiasm for a gluten-free diet has spurred a gluten-free food industry that has grown, on average, 34% per year since 2009, with annual sales predicted to reach an impressive $15.5 billion by 2016.1 This trend coincides with the national media’s intense focus on gluten sensitivity (GS), as well as best-selling books such as Wheat Belly and Grain Brain.2,3
Gluten-free food products, once relegated to boutique food shops and limited shelf space, now fill sections in large grocery and drugstore chains. Many restaurants have added gluten-free items to their menus (although gluten-free Big Macs have been available in Finland for more than 20 years).4 Only celiac disease (CD), which affects approximately 1% of the American population, requires strict gluten avoidance; yet more than 30% of US adults report having reduced their gluten intake, most claiming they did so to promote a “healthier” diet or support weight loss.1
PREVALENCE AND PATHOLOGY OF GS DISORDERS
Gluten sensitivity, once used to denote CD alone, now includes a group of gluten-intolerant conditions unrelated to CD—primarily nonceliac gluten sensitivity (NCGS) and wheat allergy (WA)—although the nomenclature is likely to change. While these disorders differ in underlying pathogenesis, each demonstrates a resolution of symptoms when the patient is placed on a gluten-free diet. Of these GS disorders, only NCGS lacks clarity with regard to incidence, diagnosis, and pathology.5
Celiac Disease
Celiac disease is an autoimmune, T-cell–activated disease that manifests in genetically susceptible individuals (with gene variants HLA-DQ2 and HLA-DQ8); it can occur at any age. The incidence of CD in the US has increased from 1 in 500 in 1974 to a current estimate of 1 in 100, although many with CD are believed to be undiagnosed.4,6
CD is the only autoimmune disease for which a trigger is known: gluten. Suspicion for CD should be heightened if the patient or a family member has a history of autoimmune disease. Nearly one-quarter of patients with CD will develop an autoimmune thyroid disorder.7
In CD, a significant enteropathy occurs in response to gluten intake, characterized by inflammation of the proximal small intestine. Individuals with CD produce tissue transglutaminase (tTG) or transglutaminase 2 (TG2) autoantibodies, resulting in gluten-specific CD4+ Th1 T-cell activation and an immune response that causes an upregulation of zonulin.8 Zonulin, a protein that modulates the permeability of the intestinal mucosal wall, is believed to play a role in “leaky gut syndrome” and autoimmune disease. The upregulation of zonulin in CD creates a disruption of the intestinal mucosal lining, causing villous mucosal atrophy and impairment of intestinal permeability and absorption.9
Nonceliac Gluten Sensitivity
NCGS is a poorly understood condition first described in the 1980s and recently “rediscovered” as a gluten-related disorder.10 Its actual prevalence is unknown because of unclear diagnostic criteria but is likely much higher than that of CD.1,4 Unlike CD, there does not appear to be a genetic predisposition for NCGS, nor is it believed to be an autoimmune disorder. However, research does suggest that NCGS may increase the risk for autoimmune diseases, such as Hashimoto thyroiditis. NCGS can occur at any age but appears more commonly in adults than children, and in women than men.4
A small but meticulous 2013 study raised doubt about NCGS as a specific gluten-related disorder.11 The results suggested that NCGS should be viewed as a variant of irritable bowel syndrome (IBS), not triggered by gluten but by poorly absorbed carbohydrates found in wheat known as fructans and galactans, and perhaps by other foods containing fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPS). It is believed those with diarrhea-prone IBS are particularly sensitive to gluten.11 As a result, ardent claims of NCGS and improved health with gluten-free diets in those without CD are often discounted.
More recent research findings refute this conjecture, suggesting that NCGS is likely a reaction to other proteins within the gluten family, such as beta-, gamma-, or omega-gliadin, glutenin, wheat germ agglutinin, gluteomorphin, and deamidated gliadin. Development of GS is believed to be triggered by such factors as intestinal infections, altered microbiota, or food additives.4
In any event, the pathogenesis of NCGS remains unclear, and it does not present with the diagnostic antibodies or inflammatory enteropathy seen in patients with CD. Despite this, NCGS does present with gastrointestinal (GI) and extra-intestinal symptoms similar to those of CD.
Wheat Allergies
Wheat is frequently implicated in food allergies, especially in infants and children. The incidence of WA is not known, although up to 4% of adults and 6% of children are estimated to have food allergies. In WA, there is an IgE antibody–mediated reaction to one or more of the wheat proteins (albumin, gliadin, globulin, gluten) that occurs within minutes to hours after exposure to the offending food. Many children with IgE-mediated allergies may “outgrow” them with time.12
Continue for the clinical presentation of GS >>
CLINICAL PRESENTATION OF GS
Although CD is a disorder associated with the GI system, the “classic” GI symptoms of bloating, flatulence, diarrhea, and/or constipation are often absent (silent CD), especially in older individuals. It is for this reason that the diagnosis of CD is easily missed.
Delaying diagnosis can have serious health consequences, as CD is associated with significant morbidities, such as malnutrition (worse in children), iron-deficiency anemia, neuropsychiatric aberrations (depression, anxiety, attention-deficit, and cerebral ataxia), osteoporosis, lymphoma, and death (see Table 1).4,13 CD may also present with dermatitis herpetiformis, a chronic vesicular rash, seen most often in adult males.
The role of gluten in the development of autism spectrum disorders or schizophrenia, though not proven, remains hotly debated, especially as close biochemical links are now recognized between the gut and the brain. It is clear, however, that gluten intake in severely gluten-sensitive individuals can directly affect mood and brain function. Most CD-associated morbidities will resolve after one year of complete gluten avoidance.1,13
Prominent symptoms of NCGS occur soon after gluten ingestion and disappear within days to weeks of gluten avoidance. The classic NCGS presentation combines IBS-like symptoms, such as abdominal cramps, bloating, diarrhea, and constipation, with systemic manifestations that include “brain fog,” fatigue, headache, joint and muscle pain, peripheral numbness, skin rash, aphthous stomatitis, anemia, and depression or anxiety. As with CD, GI symptoms usually predominate in children and abate with gluten avoidance.14,15
Allergic reactions to wheat will present within minutes to two hours of wheat exposure and may manifest with pruritic rash, hives, swelling of the lips or tongue, rhinitis, abdominal cramps, vomiting, diarrhea, constipation, and/or anaphylaxis. Subtle reactions may make diagnosis difficult.12
DIAGNOSTIC STUDIES FOR GS
The effectiveness of diagnostic testing for CD has been well established. Testing for antitissue transglutaminase antibodies (tTG-IgA) is the preferred laboratory test for CD, with a sensitivity of 93%, specificity of 98%, and few false-negative results. The endomysial antibody (EMA-IgA) test, though highly specific for CD, lacks the sensitivity of tTG-IgA. Newer antibody tests, such as deamidated gliadin peptide IgA and IgG, have not proven superior in detecting CD. Genetic testing for HLA-DQ2 and HLA-DQ8 may also be performed, but many people carry the gene without ever developing CD.13
To improve the reliability of CD antibody tests, the patient should have consumed gluten regularly for at least one month prior to testing. If the patient has been on a gluten-free diet for several weeks, then a gluten challenge should be done: The patient would be instructed to consume at least 3 g/d of gluten (two slices of bread) for a minimum of two weeks (versus eight weeks in previous protocols), after which the celiac antibody tests would be repeated.16
If these antibody test results are negative but the suspicion for CD remains high, an endoscopy with a duodenal biopsy should be performed. The appearance of villous atrophy would confirm the diagnosis of CD.1,13,16
Unlike CD, there are currently no reliable diagnostic tests for NCGS, although some researchers suggest testing for IgG antigliadin antibodies (AGA); NCGS is currently a diagnosis of exclusion.7 In NCGS, celiac antibodies will be negative and the duodenal biopsy will demonstrate only mild inflammation without the mucosal atrophy of CD. As with CD, patients affected by NCGS will also test negative for the wheat allergy IgE response.
Another option is a gluten challenge. The patient is instructed to follow a gluten-free diet for six weeks and monitor for NCGS symptoms. If symptoms abate, a gluten-containing diet is then reintroduced and the patient is evaluated for the reemergence of NCGS symptoms. If symptoms are not reduced with a gluten-free diet, NCGS may be excluded. Newer GS laboratory tests will emerge that can assay more forms of gliadin antibodies, possibly aiding in NCGS diagnosis.4,14
To make a diagnosis of WA, skin prick tests and allergen-specific IgE testing are used, along with a medical history, clinical presentation, and possibly a food challenge.
Continue for management of GS >>
MANAGEMENT OF GS
The hallmark treatment for GS, regardless of its causative factor, is a strict gluten-free diet (GFD). For patients with CD, a 100% GFD is recommended for life. It is not yet known whether this lifelong duration is necessary for those with NCGS and WA, or if there is a safe threshold in these patients for gluten consumption. It is helpful for patients to keep a food diary, noting what they eat and how that affects the appearance or attenuation of symptoms.
Transitioning to a gluten-free lifestyle can be confusing, frustrating, and expensive for patients. Removing gluten from the diet is also challenging, as wheat is the predominant grain consumed in this country. Barley and rye (less so oats) also contain gluten, leaving limited alternatives, like amaranth, corn, quinoa, rice, and tapioca. Unlike CD and NCGS, WA requires only elimination of wheat-containing products; thus, it may not be necessary for affected patients to avoid barley and rye.1,4
Extensive patient education is important for success. Referral to a knowledgeable nutritionist is ideal but not always practical. Lists of foods to avoid on a gluten-free diet are readily available, but important points should be stressed, including how to read food labels. For example, the term wheat-free does not mean gluten-free (see Table 2).1,17 As of August 2014, the food industry, by law, can only claim a product is “gluten-free” if it contains no more than 20 parts per million (ppm) of gluten.1
Due to malabsorption issues, it is recommended that patients with CD be monitored for micronutrient deficiencies (ie, iron, B1, B6, B12, and zinc), and osteopenia/osteoporosis (dual-energy x-ray absorptiometry [DEXA] at the time of diagnosis) and be offered fertility counseling. What patients with GS need most of all are informed, caring providers to help guide them through diagnosis and treatment.6,13
Continue for the conclusion >>
CONCLUSION
Gluten-free diets are increasing in popularity, and many people who do not have CD claim improved health and vitality when they avoid gluten. Much is known about the incidence and pathogenesis of the gluten-associated disorders of CD and WA. Far less is known about the controversial disorder of NCGS. The symptoms and morbidities associated with NCGS have been well documented and present a curious mix of CD and IBS, yet neither condition fully accounts for the pathogenesis of NCGS. While CD is linked to more serious morbidities (including death if the disease is not readily diagnosed), NCGS and WA do produce significant manifestations and risks.
Research into NCGS remains limited and conflicting, and biomarkers for the disorder are not yet known. Unsupported or not, many patients attribute mood disorders, pain, and chronic ills to gluten intake and seek input from their health care providers. Rather than dismiss their claims, clinicians can provide pertinent instructions on a gluten-free lifestyle and healthy diet, and encourage the use of food diaries to document food-symptom associations. Gluten sensitivities are not benign and “going gluten-free” may be of great benefit for many patients with GS. That’s a fact.
REFERENCES
1. Capili B, Chang M, Anastasi JK. Nonceliac gluten sensitivity—is it really the gluten? J Nurs Pract. 2014;10(9):666-673.
2. Davis W. Wheat Belly: Lose the Wheat, Lose the Weight, and Find Your Path Back to Health. Emmaus, PA: Rodale Books; 2011.
3. Perlmutter D. Grain Brain: The Surprising Truth about Wheat, Carbs, and Sugar—Your Brain’s Silent Killers. New York, NY: Little, Brown and Company; 2013.
4. Brown AC. Gluten sensitivity: problems of an emerging condition separate from celiac disease. Expert Rev Gastroenterol Hepatol. 2012;6(1): 43-55.
5. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012 Feb 7;10:13.
6. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656-676.
7. Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Non-celiac gluten sensitivity: literature review. J Am Coll Nutr. 2014;33(1):39-54.
8. Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):728-736.
9. Fasano A, Sapone A, Zevallos V, Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148(6):1195-1204.
10. Catassi C, Bai JC, Bonaz B, Boouma G. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5(10):3839-3853.
11. Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320-328.
12. Guandalini S, Newland C. Differentiating food allergies from food intolerances. Curr Gastroenterol Rep. 2011;13(5):426-434.
13. Scanlon SA, Murray JA. Update on celiac disease—etiology, differential diagnosis, drug targets, and management devices. Clin Exp Gastroenterol. 2011;4:297-311.
14. Catassi C, Elli L, Bonaz B, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno Experts’ Criteria. Nutrients. 2015;7(6):4966-4977.
15. Peters SL, Biesiekierski JR, Yelland GW,et al. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity—an exploratory clinical study. Aliment Pharmacol Ther. 2014:39(10): 1104–1112.
16. Leffler D, Schuppen D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62(7):996-1004.
17. Celiac Disease Foundation. Sources of gluten (2015). https://celiac.org/live-gluten-free/glutenfreediet/sources-of-gluten. Accessed November 24, 2015.
IN THIS ARTICLE
• So what is gluten?
• Selected symptoms of celiac disease
• Selected foods and products containing gluten
Gluten has become a dietary pariah (see “So What Is Gluten?"). The American public’s enthusiasm for a gluten-free diet has spurred a gluten-free food industry that has grown, on average, 34% per year since 2009, with annual sales predicted to reach an impressive $15.5 billion by 2016.1 This trend coincides with the national media’s intense focus on gluten sensitivity (GS), as well as best-selling books such as Wheat Belly and Grain Brain.2,3
Gluten-free food products, once relegated to boutique food shops and limited shelf space, now fill sections in large grocery and drugstore chains. Many restaurants have added gluten-free items to their menus (although gluten-free Big Macs have been available in Finland for more than 20 years).4 Only celiac disease (CD), which affects approximately 1% of the American population, requires strict gluten avoidance; yet more than 30% of US adults report having reduced their gluten intake, most claiming they did so to promote a “healthier” diet or support weight loss.1
PREVALENCE AND PATHOLOGY OF GS DISORDERS
Gluten sensitivity, once used to denote CD alone, now includes a group of gluten-intolerant conditions unrelated to CD—primarily nonceliac gluten sensitivity (NCGS) and wheat allergy (WA)—although the nomenclature is likely to change. While these disorders differ in underlying pathogenesis, each demonstrates a resolution of symptoms when the patient is placed on a gluten-free diet. Of these GS disorders, only NCGS lacks clarity with regard to incidence, diagnosis, and pathology.5
Celiac Disease
Celiac disease is an autoimmune, T-cell–activated disease that manifests in genetically susceptible individuals (with gene variants HLA-DQ2 and HLA-DQ8); it can occur at any age. The incidence of CD in the US has increased from 1 in 500 in 1974 to a current estimate of 1 in 100, although many with CD are believed to be undiagnosed.4,6
CD is the only autoimmune disease for which a trigger is known: gluten. Suspicion for CD should be heightened if the patient or a family member has a history of autoimmune disease. Nearly one-quarter of patients with CD will develop an autoimmune thyroid disorder.7
In CD, a significant enteropathy occurs in response to gluten intake, characterized by inflammation of the proximal small intestine. Individuals with CD produce tissue transglutaminase (tTG) or transglutaminase 2 (TG2) autoantibodies, resulting in gluten-specific CD4+ Th1 T-cell activation and an immune response that causes an upregulation of zonulin.8 Zonulin, a protein that modulates the permeability of the intestinal mucosal wall, is believed to play a role in “leaky gut syndrome” and autoimmune disease. The upregulation of zonulin in CD creates a disruption of the intestinal mucosal lining, causing villous mucosal atrophy and impairment of intestinal permeability and absorption.9
Nonceliac Gluten Sensitivity
NCGS is a poorly understood condition first described in the 1980s and recently “rediscovered” as a gluten-related disorder.10 Its actual prevalence is unknown because of unclear diagnostic criteria but is likely much higher than that of CD.1,4 Unlike CD, there does not appear to be a genetic predisposition for NCGS, nor is it believed to be an autoimmune disorder. However, research does suggest that NCGS may increase the risk for autoimmune diseases, such as Hashimoto thyroiditis. NCGS can occur at any age but appears more commonly in adults than children, and in women than men.4
A small but meticulous 2013 study raised doubt about NCGS as a specific gluten-related disorder.11 The results suggested that NCGS should be viewed as a variant of irritable bowel syndrome (IBS), not triggered by gluten but by poorly absorbed carbohydrates found in wheat known as fructans and galactans, and perhaps by other foods containing fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPS). It is believed those with diarrhea-prone IBS are particularly sensitive to gluten.11 As a result, ardent claims of NCGS and improved health with gluten-free diets in those without CD are often discounted.
More recent research findings refute this conjecture, suggesting that NCGS is likely a reaction to other proteins within the gluten family, such as beta-, gamma-, or omega-gliadin, glutenin, wheat germ agglutinin, gluteomorphin, and deamidated gliadin. Development of GS is believed to be triggered by such factors as intestinal infections, altered microbiota, or food additives.4
In any event, the pathogenesis of NCGS remains unclear, and it does not present with the diagnostic antibodies or inflammatory enteropathy seen in patients with CD. Despite this, NCGS does present with gastrointestinal (GI) and extra-intestinal symptoms similar to those of CD.
Wheat Allergies
Wheat is frequently implicated in food allergies, especially in infants and children. The incidence of WA is not known, although up to 4% of adults and 6% of children are estimated to have food allergies. In WA, there is an IgE antibody–mediated reaction to one or more of the wheat proteins (albumin, gliadin, globulin, gluten) that occurs within minutes to hours after exposure to the offending food. Many children with IgE-mediated allergies may “outgrow” them with time.12
Continue for the clinical presentation of GS >>
CLINICAL PRESENTATION OF GS
Although CD is a disorder associated with the GI system, the “classic” GI symptoms of bloating, flatulence, diarrhea, and/or constipation are often absent (silent CD), especially in older individuals. It is for this reason that the diagnosis of CD is easily missed.
Delaying diagnosis can have serious health consequences, as CD is associated with significant morbidities, such as malnutrition (worse in children), iron-deficiency anemia, neuropsychiatric aberrations (depression, anxiety, attention-deficit, and cerebral ataxia), osteoporosis, lymphoma, and death (see Table 1).4,13 CD may also present with dermatitis herpetiformis, a chronic vesicular rash, seen most often in adult males.
The role of gluten in the development of autism spectrum disorders or schizophrenia, though not proven, remains hotly debated, especially as close biochemical links are now recognized between the gut and the brain. It is clear, however, that gluten intake in severely gluten-sensitive individuals can directly affect mood and brain function. Most CD-associated morbidities will resolve after one year of complete gluten avoidance.1,13
Prominent symptoms of NCGS occur soon after gluten ingestion and disappear within days to weeks of gluten avoidance. The classic NCGS presentation combines IBS-like symptoms, such as abdominal cramps, bloating, diarrhea, and constipation, with systemic manifestations that include “brain fog,” fatigue, headache, joint and muscle pain, peripheral numbness, skin rash, aphthous stomatitis, anemia, and depression or anxiety. As with CD, GI symptoms usually predominate in children and abate with gluten avoidance.14,15
Allergic reactions to wheat will present within minutes to two hours of wheat exposure and may manifest with pruritic rash, hives, swelling of the lips or tongue, rhinitis, abdominal cramps, vomiting, diarrhea, constipation, and/or anaphylaxis. Subtle reactions may make diagnosis difficult.12
DIAGNOSTIC STUDIES FOR GS
The effectiveness of diagnostic testing for CD has been well established. Testing for antitissue transglutaminase antibodies (tTG-IgA) is the preferred laboratory test for CD, with a sensitivity of 93%, specificity of 98%, and few false-negative results. The endomysial antibody (EMA-IgA) test, though highly specific for CD, lacks the sensitivity of tTG-IgA. Newer antibody tests, such as deamidated gliadin peptide IgA and IgG, have not proven superior in detecting CD. Genetic testing for HLA-DQ2 and HLA-DQ8 may also be performed, but many people carry the gene without ever developing CD.13
To improve the reliability of CD antibody tests, the patient should have consumed gluten regularly for at least one month prior to testing. If the patient has been on a gluten-free diet for several weeks, then a gluten challenge should be done: The patient would be instructed to consume at least 3 g/d of gluten (two slices of bread) for a minimum of two weeks (versus eight weeks in previous protocols), after which the celiac antibody tests would be repeated.16
If these antibody test results are negative but the suspicion for CD remains high, an endoscopy with a duodenal biopsy should be performed. The appearance of villous atrophy would confirm the diagnosis of CD.1,13,16
Unlike CD, there are currently no reliable diagnostic tests for NCGS, although some researchers suggest testing for IgG antigliadin antibodies (AGA); NCGS is currently a diagnosis of exclusion.7 In NCGS, celiac antibodies will be negative and the duodenal biopsy will demonstrate only mild inflammation without the mucosal atrophy of CD. As with CD, patients affected by NCGS will also test negative for the wheat allergy IgE response.
Another option is a gluten challenge. The patient is instructed to follow a gluten-free diet for six weeks and monitor for NCGS symptoms. If symptoms abate, a gluten-containing diet is then reintroduced and the patient is evaluated for the reemergence of NCGS symptoms. If symptoms are not reduced with a gluten-free diet, NCGS may be excluded. Newer GS laboratory tests will emerge that can assay more forms of gliadin antibodies, possibly aiding in NCGS diagnosis.4,14
To make a diagnosis of WA, skin prick tests and allergen-specific IgE testing are used, along with a medical history, clinical presentation, and possibly a food challenge.
Continue for management of GS >>
MANAGEMENT OF GS
The hallmark treatment for GS, regardless of its causative factor, is a strict gluten-free diet (GFD). For patients with CD, a 100% GFD is recommended for life. It is not yet known whether this lifelong duration is necessary for those with NCGS and WA, or if there is a safe threshold in these patients for gluten consumption. It is helpful for patients to keep a food diary, noting what they eat and how that affects the appearance or attenuation of symptoms.
Transitioning to a gluten-free lifestyle can be confusing, frustrating, and expensive for patients. Removing gluten from the diet is also challenging, as wheat is the predominant grain consumed in this country. Barley and rye (less so oats) also contain gluten, leaving limited alternatives, like amaranth, corn, quinoa, rice, and tapioca. Unlike CD and NCGS, WA requires only elimination of wheat-containing products; thus, it may not be necessary for affected patients to avoid barley and rye.1,4
Extensive patient education is important for success. Referral to a knowledgeable nutritionist is ideal but not always practical. Lists of foods to avoid on a gluten-free diet are readily available, but important points should be stressed, including how to read food labels. For example, the term wheat-free does not mean gluten-free (see Table 2).1,17 As of August 2014, the food industry, by law, can only claim a product is “gluten-free” if it contains no more than 20 parts per million (ppm) of gluten.1
Due to malabsorption issues, it is recommended that patients with CD be monitored for micronutrient deficiencies (ie, iron, B1, B6, B12, and zinc), and osteopenia/osteoporosis (dual-energy x-ray absorptiometry [DEXA] at the time of diagnosis) and be offered fertility counseling. What patients with GS need most of all are informed, caring providers to help guide them through diagnosis and treatment.6,13
Continue for the conclusion >>
CONCLUSION
Gluten-free diets are increasing in popularity, and many people who do not have CD claim improved health and vitality when they avoid gluten. Much is known about the incidence and pathogenesis of the gluten-associated disorders of CD and WA. Far less is known about the controversial disorder of NCGS. The symptoms and morbidities associated with NCGS have been well documented and present a curious mix of CD and IBS, yet neither condition fully accounts for the pathogenesis of NCGS. While CD is linked to more serious morbidities (including death if the disease is not readily diagnosed), NCGS and WA do produce significant manifestations and risks.
Research into NCGS remains limited and conflicting, and biomarkers for the disorder are not yet known. Unsupported or not, many patients attribute mood disorders, pain, and chronic ills to gluten intake and seek input from their health care providers. Rather than dismiss their claims, clinicians can provide pertinent instructions on a gluten-free lifestyle and healthy diet, and encourage the use of food diaries to document food-symptom associations. Gluten sensitivities are not benign and “going gluten-free” may be of great benefit for many patients with GS. That’s a fact.
REFERENCES
1. Capili B, Chang M, Anastasi JK. Nonceliac gluten sensitivity—is it really the gluten? J Nurs Pract. 2014;10(9):666-673.
2. Davis W. Wheat Belly: Lose the Wheat, Lose the Weight, and Find Your Path Back to Health. Emmaus, PA: Rodale Books; 2011.
3. Perlmutter D. Grain Brain: The Surprising Truth about Wheat, Carbs, and Sugar—Your Brain’s Silent Killers. New York, NY: Little, Brown and Company; 2013.
4. Brown AC. Gluten sensitivity: problems of an emerging condition separate from celiac disease. Expert Rev Gastroenterol Hepatol. 2012;6(1): 43-55.
5. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012 Feb 7;10:13.
6. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656-676.
7. Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Non-celiac gluten sensitivity: literature review. J Am Coll Nutr. 2014;33(1):39-54.
8. Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):728-736.
9. Fasano A, Sapone A, Zevallos V, Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148(6):1195-1204.
10. Catassi C, Bai JC, Bonaz B, Boouma G. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5(10):3839-3853.
11. Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320-328.
12. Guandalini S, Newland C. Differentiating food allergies from food intolerances. Curr Gastroenterol Rep. 2011;13(5):426-434.
13. Scanlon SA, Murray JA. Update on celiac disease—etiology, differential diagnosis, drug targets, and management devices. Clin Exp Gastroenterol. 2011;4:297-311.
14. Catassi C, Elli L, Bonaz B, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno Experts’ Criteria. Nutrients. 2015;7(6):4966-4977.
15. Peters SL, Biesiekierski JR, Yelland GW,et al. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity—an exploratory clinical study. Aliment Pharmacol Ther. 2014:39(10): 1104–1112.
16. Leffler D, Schuppen D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62(7):996-1004.
17. Celiac Disease Foundation. Sources of gluten (2015). https://celiac.org/live-gluten-free/glutenfreediet/sources-of-gluten. Accessed November 24, 2015.
IN THIS ARTICLE
• So what is gluten?
• Selected symptoms of celiac disease
• Selected foods and products containing gluten
Gluten has become a dietary pariah (see “So What Is Gluten?"). The American public’s enthusiasm for a gluten-free diet has spurred a gluten-free food industry that has grown, on average, 34% per year since 2009, with annual sales predicted to reach an impressive $15.5 billion by 2016.1 This trend coincides with the national media’s intense focus on gluten sensitivity (GS), as well as best-selling books such as Wheat Belly and Grain Brain.2,3
Gluten-free food products, once relegated to boutique food shops and limited shelf space, now fill sections in large grocery and drugstore chains. Many restaurants have added gluten-free items to their menus (although gluten-free Big Macs have been available in Finland for more than 20 years).4 Only celiac disease (CD), which affects approximately 1% of the American population, requires strict gluten avoidance; yet more than 30% of US adults report having reduced their gluten intake, most claiming they did so to promote a “healthier” diet or support weight loss.1
PREVALENCE AND PATHOLOGY OF GS DISORDERS
Gluten sensitivity, once used to denote CD alone, now includes a group of gluten-intolerant conditions unrelated to CD—primarily nonceliac gluten sensitivity (NCGS) and wheat allergy (WA)—although the nomenclature is likely to change. While these disorders differ in underlying pathogenesis, each demonstrates a resolution of symptoms when the patient is placed on a gluten-free diet. Of these GS disorders, only NCGS lacks clarity with regard to incidence, diagnosis, and pathology.5
Celiac Disease
Celiac disease is an autoimmune, T-cell–activated disease that manifests in genetically susceptible individuals (with gene variants HLA-DQ2 and HLA-DQ8); it can occur at any age. The incidence of CD in the US has increased from 1 in 500 in 1974 to a current estimate of 1 in 100, although many with CD are believed to be undiagnosed.4,6
CD is the only autoimmune disease for which a trigger is known: gluten. Suspicion for CD should be heightened if the patient or a family member has a history of autoimmune disease. Nearly one-quarter of patients with CD will develop an autoimmune thyroid disorder.7
In CD, a significant enteropathy occurs in response to gluten intake, characterized by inflammation of the proximal small intestine. Individuals with CD produce tissue transglutaminase (tTG) or transglutaminase 2 (TG2) autoantibodies, resulting in gluten-specific CD4+ Th1 T-cell activation and an immune response that causes an upregulation of zonulin.8 Zonulin, a protein that modulates the permeability of the intestinal mucosal wall, is believed to play a role in “leaky gut syndrome” and autoimmune disease. The upregulation of zonulin in CD creates a disruption of the intestinal mucosal lining, causing villous mucosal atrophy and impairment of intestinal permeability and absorption.9
Nonceliac Gluten Sensitivity
NCGS is a poorly understood condition first described in the 1980s and recently “rediscovered” as a gluten-related disorder.10 Its actual prevalence is unknown because of unclear diagnostic criteria but is likely much higher than that of CD.1,4 Unlike CD, there does not appear to be a genetic predisposition for NCGS, nor is it believed to be an autoimmune disorder. However, research does suggest that NCGS may increase the risk for autoimmune diseases, such as Hashimoto thyroiditis. NCGS can occur at any age but appears more commonly in adults than children, and in women than men.4
A small but meticulous 2013 study raised doubt about NCGS as a specific gluten-related disorder.11 The results suggested that NCGS should be viewed as a variant of irritable bowel syndrome (IBS), not triggered by gluten but by poorly absorbed carbohydrates found in wheat known as fructans and galactans, and perhaps by other foods containing fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPS). It is believed those with diarrhea-prone IBS are particularly sensitive to gluten.11 As a result, ardent claims of NCGS and improved health with gluten-free diets in those without CD are often discounted.
More recent research findings refute this conjecture, suggesting that NCGS is likely a reaction to other proteins within the gluten family, such as beta-, gamma-, or omega-gliadin, glutenin, wheat germ agglutinin, gluteomorphin, and deamidated gliadin. Development of GS is believed to be triggered by such factors as intestinal infections, altered microbiota, or food additives.4
In any event, the pathogenesis of NCGS remains unclear, and it does not present with the diagnostic antibodies or inflammatory enteropathy seen in patients with CD. Despite this, NCGS does present with gastrointestinal (GI) and extra-intestinal symptoms similar to those of CD.
Wheat Allergies
Wheat is frequently implicated in food allergies, especially in infants and children. The incidence of WA is not known, although up to 4% of adults and 6% of children are estimated to have food allergies. In WA, there is an IgE antibody–mediated reaction to one or more of the wheat proteins (albumin, gliadin, globulin, gluten) that occurs within minutes to hours after exposure to the offending food. Many children with IgE-mediated allergies may “outgrow” them with time.12
Continue for the clinical presentation of GS >>
CLINICAL PRESENTATION OF GS
Although CD is a disorder associated with the GI system, the “classic” GI symptoms of bloating, flatulence, diarrhea, and/or constipation are often absent (silent CD), especially in older individuals. It is for this reason that the diagnosis of CD is easily missed.
Delaying diagnosis can have serious health consequences, as CD is associated with significant morbidities, such as malnutrition (worse in children), iron-deficiency anemia, neuropsychiatric aberrations (depression, anxiety, attention-deficit, and cerebral ataxia), osteoporosis, lymphoma, and death (see Table 1).4,13 CD may also present with dermatitis herpetiformis, a chronic vesicular rash, seen most often in adult males.
The role of gluten in the development of autism spectrum disorders or schizophrenia, though not proven, remains hotly debated, especially as close biochemical links are now recognized between the gut and the brain. It is clear, however, that gluten intake in severely gluten-sensitive individuals can directly affect mood and brain function. Most CD-associated morbidities will resolve after one year of complete gluten avoidance.1,13
Prominent symptoms of NCGS occur soon after gluten ingestion and disappear within days to weeks of gluten avoidance. The classic NCGS presentation combines IBS-like symptoms, such as abdominal cramps, bloating, diarrhea, and constipation, with systemic manifestations that include “brain fog,” fatigue, headache, joint and muscle pain, peripheral numbness, skin rash, aphthous stomatitis, anemia, and depression or anxiety. As with CD, GI symptoms usually predominate in children and abate with gluten avoidance.14,15
Allergic reactions to wheat will present within minutes to two hours of wheat exposure and may manifest with pruritic rash, hives, swelling of the lips or tongue, rhinitis, abdominal cramps, vomiting, diarrhea, constipation, and/or anaphylaxis. Subtle reactions may make diagnosis difficult.12
DIAGNOSTIC STUDIES FOR GS
The effectiveness of diagnostic testing for CD has been well established. Testing for antitissue transglutaminase antibodies (tTG-IgA) is the preferred laboratory test for CD, with a sensitivity of 93%, specificity of 98%, and few false-negative results. The endomysial antibody (EMA-IgA) test, though highly specific for CD, lacks the sensitivity of tTG-IgA. Newer antibody tests, such as deamidated gliadin peptide IgA and IgG, have not proven superior in detecting CD. Genetic testing for HLA-DQ2 and HLA-DQ8 may also be performed, but many people carry the gene without ever developing CD.13
To improve the reliability of CD antibody tests, the patient should have consumed gluten regularly for at least one month prior to testing. If the patient has been on a gluten-free diet for several weeks, then a gluten challenge should be done: The patient would be instructed to consume at least 3 g/d of gluten (two slices of bread) for a minimum of two weeks (versus eight weeks in previous protocols), after which the celiac antibody tests would be repeated.16
If these antibody test results are negative but the suspicion for CD remains high, an endoscopy with a duodenal biopsy should be performed. The appearance of villous atrophy would confirm the diagnosis of CD.1,13,16
Unlike CD, there are currently no reliable diagnostic tests for NCGS, although some researchers suggest testing for IgG antigliadin antibodies (AGA); NCGS is currently a diagnosis of exclusion.7 In NCGS, celiac antibodies will be negative and the duodenal biopsy will demonstrate only mild inflammation without the mucosal atrophy of CD. As with CD, patients affected by NCGS will also test negative for the wheat allergy IgE response.
Another option is a gluten challenge. The patient is instructed to follow a gluten-free diet for six weeks and monitor for NCGS symptoms. If symptoms abate, a gluten-containing diet is then reintroduced and the patient is evaluated for the reemergence of NCGS symptoms. If symptoms are not reduced with a gluten-free diet, NCGS may be excluded. Newer GS laboratory tests will emerge that can assay more forms of gliadin antibodies, possibly aiding in NCGS diagnosis.4,14
To make a diagnosis of WA, skin prick tests and allergen-specific IgE testing are used, along with a medical history, clinical presentation, and possibly a food challenge.
Continue for management of GS >>
MANAGEMENT OF GS
The hallmark treatment for GS, regardless of its causative factor, is a strict gluten-free diet (GFD). For patients with CD, a 100% GFD is recommended for life. It is not yet known whether this lifelong duration is necessary for those with NCGS and WA, or if there is a safe threshold in these patients for gluten consumption. It is helpful for patients to keep a food diary, noting what they eat and how that affects the appearance or attenuation of symptoms.
Transitioning to a gluten-free lifestyle can be confusing, frustrating, and expensive for patients. Removing gluten from the diet is also challenging, as wheat is the predominant grain consumed in this country. Barley and rye (less so oats) also contain gluten, leaving limited alternatives, like amaranth, corn, quinoa, rice, and tapioca. Unlike CD and NCGS, WA requires only elimination of wheat-containing products; thus, it may not be necessary for affected patients to avoid barley and rye.1,4
Extensive patient education is important for success. Referral to a knowledgeable nutritionist is ideal but not always practical. Lists of foods to avoid on a gluten-free diet are readily available, but important points should be stressed, including how to read food labels. For example, the term wheat-free does not mean gluten-free (see Table 2).1,17 As of August 2014, the food industry, by law, can only claim a product is “gluten-free” if it contains no more than 20 parts per million (ppm) of gluten.1
Due to malabsorption issues, it is recommended that patients with CD be monitored for micronutrient deficiencies (ie, iron, B1, B6, B12, and zinc), and osteopenia/osteoporosis (dual-energy x-ray absorptiometry [DEXA] at the time of diagnosis) and be offered fertility counseling. What patients with GS need most of all are informed, caring providers to help guide them through diagnosis and treatment.6,13
Continue for the conclusion >>
CONCLUSION
Gluten-free diets are increasing in popularity, and many people who do not have CD claim improved health and vitality when they avoid gluten. Much is known about the incidence and pathogenesis of the gluten-associated disorders of CD and WA. Far less is known about the controversial disorder of NCGS. The symptoms and morbidities associated with NCGS have been well documented and present a curious mix of CD and IBS, yet neither condition fully accounts for the pathogenesis of NCGS. While CD is linked to more serious morbidities (including death if the disease is not readily diagnosed), NCGS and WA do produce significant manifestations and risks.
Research into NCGS remains limited and conflicting, and biomarkers for the disorder are not yet known. Unsupported or not, many patients attribute mood disorders, pain, and chronic ills to gluten intake and seek input from their health care providers. Rather than dismiss their claims, clinicians can provide pertinent instructions on a gluten-free lifestyle and healthy diet, and encourage the use of food diaries to document food-symptom associations. Gluten sensitivities are not benign and “going gluten-free” may be of great benefit for many patients with GS. That’s a fact.
REFERENCES
1. Capili B, Chang M, Anastasi JK. Nonceliac gluten sensitivity—is it really the gluten? J Nurs Pract. 2014;10(9):666-673.
2. Davis W. Wheat Belly: Lose the Wheat, Lose the Weight, and Find Your Path Back to Health. Emmaus, PA: Rodale Books; 2011.
3. Perlmutter D. Grain Brain: The Surprising Truth about Wheat, Carbs, and Sugar—Your Brain’s Silent Killers. New York, NY: Little, Brown and Company; 2013.
4. Brown AC. Gluten sensitivity: problems of an emerging condition separate from celiac disease. Expert Rev Gastroenterol Hepatol. 2012;6(1): 43-55.
5. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012 Feb 7;10:13.
6. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656-676.
7. Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Non-celiac gluten sensitivity: literature review. J Am Coll Nutr. 2014;33(1):39-54.
8. Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):728-736.
9. Fasano A, Sapone A, Zevallos V, Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148(6):1195-1204.
10. Catassi C, Bai JC, Bonaz B, Boouma G. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5(10):3839-3853.
11. Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320-328.
12. Guandalini S, Newland C. Differentiating food allergies from food intolerances. Curr Gastroenterol Rep. 2011;13(5):426-434.
13. Scanlon SA, Murray JA. Update on celiac disease—etiology, differential diagnosis, drug targets, and management devices. Clin Exp Gastroenterol. 2011;4:297-311.
14. Catassi C, Elli L, Bonaz B, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno Experts’ Criteria. Nutrients. 2015;7(6):4966-4977.
15. Peters SL, Biesiekierski JR, Yelland GW,et al. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity—an exploratory clinical study. Aliment Pharmacol Ther. 2014:39(10): 1104–1112.
16. Leffler D, Schuppen D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62(7):996-1004.
17. Celiac Disease Foundation. Sources of gluten (2015). https://celiac.org/live-gluten-free/glutenfreediet/sources-of-gluten. Accessed November 24, 2015.
Pro-vaccine Community Outreach Campaign Appears Successful in Australia
A pro-vaccine community outreach campaign that uses local advocates and taps into common values can change attitudes among some people who traditionally doubt or refuse vaccines, according to a study published in Vaccine by Katie Attwell and Melanie Freeman of Murdoch University in Perth and of the Immunisation Alliance of Western Australia.
The “I immunise” campaign, conducted in 2014 in Freemantle, Western Australia, featured pro-vaccine testimonials from six community advocates on a website; posters uploaded to the campaign’s Facebook page and distributed to doctors’ offices, child health clinics, child care centers, playgroups, and private businesses; and billboards or large signs displayed on public buildings. Freemantle is one of the country’s lowest areas for vaccination coverage and has a prominent alternative lifestyle community.
In an online survey of 304 local residents (93 adhering to an alternative lifestyle and 211 following a traditional lifestyle) after the launch of the campaign, results showed that the campaign had polarized attitudes toward vaccination and led some (17%) to feel more negatively. However, it had an overall positive effect for at least 77%, including a third of responders who previously had refused or doubted vaccines. Some parents who responded positively said they would update their own vaccinations, share the campaign on their Facebook pages, or keep their children away from those who were not immunized.
The fact that the campaign had a positive impact on at least three-quarters of the target group “suggests that it has value as one type of influencing tool,” the authors wrote. But “more research is required into how [anti-vaccine] attitudes develop and how communities, governments, and health professionals can effectively challenge them.”
Read the article in Vaccine (2015 Nov 17;33[46]:6235-40).
A pro-vaccine community outreach campaign that uses local advocates and taps into common values can change attitudes among some people who traditionally doubt or refuse vaccines, according to a study published in Vaccine by Katie Attwell and Melanie Freeman of Murdoch University in Perth and of the Immunisation Alliance of Western Australia.
The “I immunise” campaign, conducted in 2014 in Freemantle, Western Australia, featured pro-vaccine testimonials from six community advocates on a website; posters uploaded to the campaign’s Facebook page and distributed to doctors’ offices, child health clinics, child care centers, playgroups, and private businesses; and billboards or large signs displayed on public buildings. Freemantle is one of the country’s lowest areas for vaccination coverage and has a prominent alternative lifestyle community.
In an online survey of 304 local residents (93 adhering to an alternative lifestyle and 211 following a traditional lifestyle) after the launch of the campaign, results showed that the campaign had polarized attitudes toward vaccination and led some (17%) to feel more negatively. However, it had an overall positive effect for at least 77%, including a third of responders who previously had refused or doubted vaccines. Some parents who responded positively said they would update their own vaccinations, share the campaign on their Facebook pages, or keep their children away from those who were not immunized.
The fact that the campaign had a positive impact on at least three-quarters of the target group “suggests that it has value as one type of influencing tool,” the authors wrote. But “more research is required into how [anti-vaccine] attitudes develop and how communities, governments, and health professionals can effectively challenge them.”
Read the article in Vaccine (2015 Nov 17;33[46]:6235-40).
A pro-vaccine community outreach campaign that uses local advocates and taps into common values can change attitudes among some people who traditionally doubt or refuse vaccines, according to a study published in Vaccine by Katie Attwell and Melanie Freeman of Murdoch University in Perth and of the Immunisation Alliance of Western Australia.
The “I immunise” campaign, conducted in 2014 in Freemantle, Western Australia, featured pro-vaccine testimonials from six community advocates on a website; posters uploaded to the campaign’s Facebook page and distributed to doctors’ offices, child health clinics, child care centers, playgroups, and private businesses; and billboards or large signs displayed on public buildings. Freemantle is one of the country’s lowest areas for vaccination coverage and has a prominent alternative lifestyle community.
In an online survey of 304 local residents (93 adhering to an alternative lifestyle and 211 following a traditional lifestyle) after the launch of the campaign, results showed that the campaign had polarized attitudes toward vaccination and led some (17%) to feel more negatively. However, it had an overall positive effect for at least 77%, including a third of responders who previously had refused or doubted vaccines. Some parents who responded positively said they would update their own vaccinations, share the campaign on their Facebook pages, or keep their children away from those who were not immunized.
The fact that the campaign had a positive impact on at least three-quarters of the target group “suggests that it has value as one type of influencing tool,” the authors wrote. But “more research is required into how [anti-vaccine] attitudes develop and how communities, governments, and health professionals can effectively challenge them.”
Read the article in Vaccine (2015 Nov 17;33[46]:6235-40).
FROM VACCINE
Study: Exposure History Critical to Design of Universal Flu Vaccine
In a study with implications for the development of new influenza vaccine strategies, researchers discovered that – among patients who received the 2009 H1N1 influenza vaccine – individuals with low levels of H1N1-specific antibodies prior to vaccination produced a more broadly protective immune response against the influenza virus than patients with high levels of H1N1-specific antibodies prior to vaccination.
A research team led by Patrick C. Wilson, Ph.D., of the Knapp Center for Lupus and Immunology Research at the University of Chicago, studied the B cell response in patients who received the pandemic 2009 H1N1 vaccine 2 years in a row and had varied histories of influenza exposure. All patients were 18 years or older, healthy, and had not received the yearly influenza vaccine prior to participating in the study. The researchers compared the patients’ “vaccine-induced plasmablast response upon first vaccination with the pandemic H1N1 strain in 2009-2010” with the patients’ plasmablast response upon revaccination with this same strain in 2010-2011 or 2011-2012. Each of the 21 study participants provided the researchers with at least four H1N1-specific plasmablasts.
The researchers “analyzed the immunoglobulin regions, strain specificity, and functional properties of the antibodies produced by this plasmablast population at the single-cell level across multiple years,” which allowed them to directly evaluate the effect of immune memory on the specificity of the current response to the virus.
Among the study’s findings was that “only individuals with low preexisting serological levels of pandemic H1N1 specific antibodies generated a broadly neutralizing plasmablast response directed toward the [hemagglutinin] stalk,” which is part of the hemagglutinin protein located on the surface of the influenza virus.
“[W]e demonstrate that the immune subdominance of the [hemagglutinin] stalk is a function of both the poor accessibility to the broadly protective epitopes and the inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains and determines the likelihood of generating a broadly protective response,” said Dr. Wilson and his coauthors. The authors reported no conflicts of interest.
Read the full study in Science Translational Medicine (doi: 10.1126/scitranslmed.aad0522).
In a study with implications for the development of new influenza vaccine strategies, researchers discovered that – among patients who received the 2009 H1N1 influenza vaccine – individuals with low levels of H1N1-specific antibodies prior to vaccination produced a more broadly protective immune response against the influenza virus than patients with high levels of H1N1-specific antibodies prior to vaccination.
A research team led by Patrick C. Wilson, Ph.D., of the Knapp Center for Lupus and Immunology Research at the University of Chicago, studied the B cell response in patients who received the pandemic 2009 H1N1 vaccine 2 years in a row and had varied histories of influenza exposure. All patients were 18 years or older, healthy, and had not received the yearly influenza vaccine prior to participating in the study. The researchers compared the patients’ “vaccine-induced plasmablast response upon first vaccination with the pandemic H1N1 strain in 2009-2010” with the patients’ plasmablast response upon revaccination with this same strain in 2010-2011 or 2011-2012. Each of the 21 study participants provided the researchers with at least four H1N1-specific plasmablasts.
The researchers “analyzed the immunoglobulin regions, strain specificity, and functional properties of the antibodies produced by this plasmablast population at the single-cell level across multiple years,” which allowed them to directly evaluate the effect of immune memory on the specificity of the current response to the virus.
Among the study’s findings was that “only individuals with low preexisting serological levels of pandemic H1N1 specific antibodies generated a broadly neutralizing plasmablast response directed toward the [hemagglutinin] stalk,” which is part of the hemagglutinin protein located on the surface of the influenza virus.
“[W]e demonstrate that the immune subdominance of the [hemagglutinin] stalk is a function of both the poor accessibility to the broadly protective epitopes and the inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains and determines the likelihood of generating a broadly protective response,” said Dr. Wilson and his coauthors. The authors reported no conflicts of interest.
Read the full study in Science Translational Medicine (doi: 10.1126/scitranslmed.aad0522).
In a study with implications for the development of new influenza vaccine strategies, researchers discovered that – among patients who received the 2009 H1N1 influenza vaccine – individuals with low levels of H1N1-specific antibodies prior to vaccination produced a more broadly protective immune response against the influenza virus than patients with high levels of H1N1-specific antibodies prior to vaccination.
A research team led by Patrick C. Wilson, Ph.D., of the Knapp Center for Lupus and Immunology Research at the University of Chicago, studied the B cell response in patients who received the pandemic 2009 H1N1 vaccine 2 years in a row and had varied histories of influenza exposure. All patients were 18 years or older, healthy, and had not received the yearly influenza vaccine prior to participating in the study. The researchers compared the patients’ “vaccine-induced plasmablast response upon first vaccination with the pandemic H1N1 strain in 2009-2010” with the patients’ plasmablast response upon revaccination with this same strain in 2010-2011 or 2011-2012. Each of the 21 study participants provided the researchers with at least four H1N1-specific plasmablasts.
The researchers “analyzed the immunoglobulin regions, strain specificity, and functional properties of the antibodies produced by this plasmablast population at the single-cell level across multiple years,” which allowed them to directly evaluate the effect of immune memory on the specificity of the current response to the virus.
Among the study’s findings was that “only individuals with low preexisting serological levels of pandemic H1N1 specific antibodies generated a broadly neutralizing plasmablast response directed toward the [hemagglutinin] stalk,” which is part of the hemagglutinin protein located on the surface of the influenza virus.
“[W]e demonstrate that the immune subdominance of the [hemagglutinin] stalk is a function of both the poor accessibility to the broadly protective epitopes and the inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains and determines the likelihood of generating a broadly protective response,” said Dr. Wilson and his coauthors. The authors reported no conflicts of interest.
Read the full study in Science Translational Medicine (doi: 10.1126/scitranslmed.aad0522).
FROM SCIENCE TRANSLATIONAL MEDICINE
Psoriasis Cohort Reveals High Arthritis Risk
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
FROM ARTHRITIS & RHEUMATOLOGY
EREFS value has diagnostic utility for eosinophilic esophagitis
The Eosinophilic Esophagitis Endoscopic Reference Score, or EREFS, is not only highly predictive of eosinophilic esophagitis (EoE) but also its responsiveness to treatment, which suggests it may be used as an outcome measure, researchers say.
A prospective study of 211 adults undergoing upper endoscopy to investigate symptoms of esophageal dysfunction compared the EREFS with consensus guidelines for diagnosis of eosinophilic esophagitis.
The guidelines approach identified 67 cases of eosinophilic esophagitis and 144 control subjects without eosinophilic esophagitis. When these patients were assessed via the EREFS, researchers found multiple, highly significant differences between the cases and controls, with a mean total EREFS of 3.88 for cases and 0.42 for controls, according to a paper published online in Clinical Gastroenterology and Hepatology.
“On ROC [receiver operator characteristic] analysis, a model that contained all 5 components of the EREFS system as categorical variables had an AUC [area under the curve] of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone,” wrote Dr. Evan S. Dellon and colleagues of the University of North Carolina at Chapel Hill.
In this model, a score of 2.0 or above showed a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, negative predictive value of 94%, and accuracy of 91%.
Most of the score’s predictive ability was attributed to its inflammatory component, and less from the fibrostenotic score, which the authors suggested was due to the high prevalence of strictures in the control group.
The EREFS also improved significantly after treatment, in conjunction with endoscopic findings.
Total EREFS significantly decreased from 3.88 to 2.01, the inflammatory score decreased from 2.41 to 1.22, and the fibrostenotic score decreased from 1.46 to 0.89.
Histologic responders to treatment showed much more significant decreases in EREFS compared with nonresponders (Clin Gastro Hepatol. 2015, Sept. 12 [http://dx.doi.org/10.1016/j.cgh.2015.08.040]).
Researchers also examined the impact of weighing the various features of EREFS differently.
“The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (less than 15 eosinophil/hpf) for response,” they reported.
Based on this finding, the researchers created a set of EREFS scores using these varied weights, and showed that doubling the exudates, rings, and edema scores achieved the score’s maximum responsiveness while still keeping the weighting system simple, although these changes did not alter the score’s overall predictive ability.
The EREFS score was developed as a way to standardize the description, recognition, and reporting of eosinophilic esophagitis, but its diagnostic utility and responsiveness to treatment were unknown, the authors said.
“This prospective study found that the EREFS classification has diagnostic utility for EoE,” they wrote. “Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure.”
The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
The Eosinophilic Esophagitis Endoscopic Reference Score, or EREFS, is not only highly predictive of eosinophilic esophagitis (EoE) but also its responsiveness to treatment, which suggests it may be used as an outcome measure, researchers say.
A prospective study of 211 adults undergoing upper endoscopy to investigate symptoms of esophageal dysfunction compared the EREFS with consensus guidelines for diagnosis of eosinophilic esophagitis.
The guidelines approach identified 67 cases of eosinophilic esophagitis and 144 control subjects without eosinophilic esophagitis. When these patients were assessed via the EREFS, researchers found multiple, highly significant differences between the cases and controls, with a mean total EREFS of 3.88 for cases and 0.42 for controls, according to a paper published online in Clinical Gastroenterology and Hepatology.
“On ROC [receiver operator characteristic] analysis, a model that contained all 5 components of the EREFS system as categorical variables had an AUC [area under the curve] of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone,” wrote Dr. Evan S. Dellon and colleagues of the University of North Carolina at Chapel Hill.
In this model, a score of 2.0 or above showed a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, negative predictive value of 94%, and accuracy of 91%.
Most of the score’s predictive ability was attributed to its inflammatory component, and less from the fibrostenotic score, which the authors suggested was due to the high prevalence of strictures in the control group.
The EREFS also improved significantly after treatment, in conjunction with endoscopic findings.
Total EREFS significantly decreased from 3.88 to 2.01, the inflammatory score decreased from 2.41 to 1.22, and the fibrostenotic score decreased from 1.46 to 0.89.
Histologic responders to treatment showed much more significant decreases in EREFS compared with nonresponders (Clin Gastro Hepatol. 2015, Sept. 12 [http://dx.doi.org/10.1016/j.cgh.2015.08.040]).
Researchers also examined the impact of weighing the various features of EREFS differently.
“The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (less than 15 eosinophil/hpf) for response,” they reported.
Based on this finding, the researchers created a set of EREFS scores using these varied weights, and showed that doubling the exudates, rings, and edema scores achieved the score’s maximum responsiveness while still keeping the weighting system simple, although these changes did not alter the score’s overall predictive ability.
The EREFS score was developed as a way to standardize the description, recognition, and reporting of eosinophilic esophagitis, but its diagnostic utility and responsiveness to treatment were unknown, the authors said.
“This prospective study found that the EREFS classification has diagnostic utility for EoE,” they wrote. “Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure.”
The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
The Eosinophilic Esophagitis Endoscopic Reference Score, or EREFS, is not only highly predictive of eosinophilic esophagitis (EoE) but also its responsiveness to treatment, which suggests it may be used as an outcome measure, researchers say.
A prospective study of 211 adults undergoing upper endoscopy to investigate symptoms of esophageal dysfunction compared the EREFS with consensus guidelines for diagnosis of eosinophilic esophagitis.
The guidelines approach identified 67 cases of eosinophilic esophagitis and 144 control subjects without eosinophilic esophagitis. When these patients were assessed via the EREFS, researchers found multiple, highly significant differences between the cases and controls, with a mean total EREFS of 3.88 for cases and 0.42 for controls, according to a paper published online in Clinical Gastroenterology and Hepatology.
“On ROC [receiver operator characteristic] analysis, a model that contained all 5 components of the EREFS system as categorical variables had an AUC [area under the curve] of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone,” wrote Dr. Evan S. Dellon and colleagues of the University of North Carolina at Chapel Hill.
In this model, a score of 2.0 or above showed a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, negative predictive value of 94%, and accuracy of 91%.
Most of the score’s predictive ability was attributed to its inflammatory component, and less from the fibrostenotic score, which the authors suggested was due to the high prevalence of strictures in the control group.
The EREFS also improved significantly after treatment, in conjunction with endoscopic findings.
Total EREFS significantly decreased from 3.88 to 2.01, the inflammatory score decreased from 2.41 to 1.22, and the fibrostenotic score decreased from 1.46 to 0.89.
Histologic responders to treatment showed much more significant decreases in EREFS compared with nonresponders (Clin Gastro Hepatol. 2015, Sept. 12 [http://dx.doi.org/10.1016/j.cgh.2015.08.040]).
Researchers also examined the impact of weighing the various features of EREFS differently.
“The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (less than 15 eosinophil/hpf) for response,” they reported.
Based on this finding, the researchers created a set of EREFS scores using these varied weights, and showed that doubling the exudates, rings, and edema scores achieved the score’s maximum responsiveness while still keeping the weighting system simple, although these changes did not alter the score’s overall predictive ability.
The EREFS score was developed as a way to standardize the description, recognition, and reporting of eosinophilic esophagitis, but its diagnostic utility and responsiveness to treatment were unknown, the authors said.
“This prospective study found that the EREFS classification has diagnostic utility for EoE,” they wrote. “Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure.”
The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The Eosinophilic Esophagitis Endoscopic Reference Score is highly predictive of eosinophilic esophagitis and responsiveness to treatment.
Major finding: A model containing all five components of the EREFS system as categorical variables had an AUC of 0.946.
Data source: A prospective study of 211 adults undergoing upper endoscopy to investigate esophageal dysfunction.
Disclosures: The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.