User login
Home visits: A practical approach
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
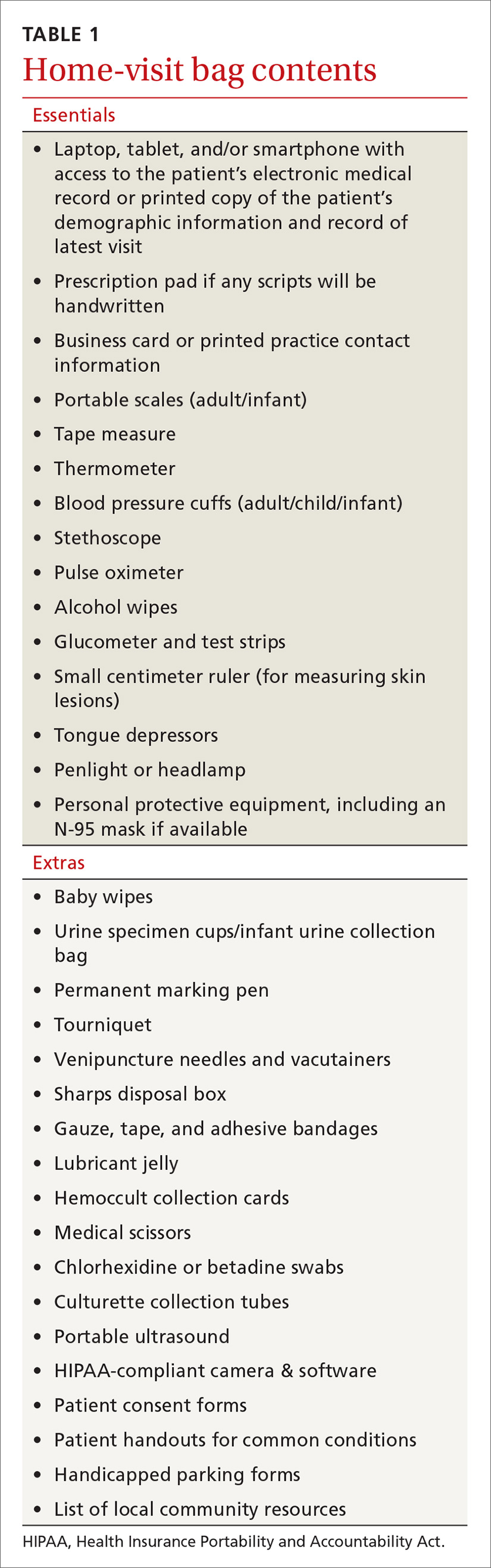
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.
Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
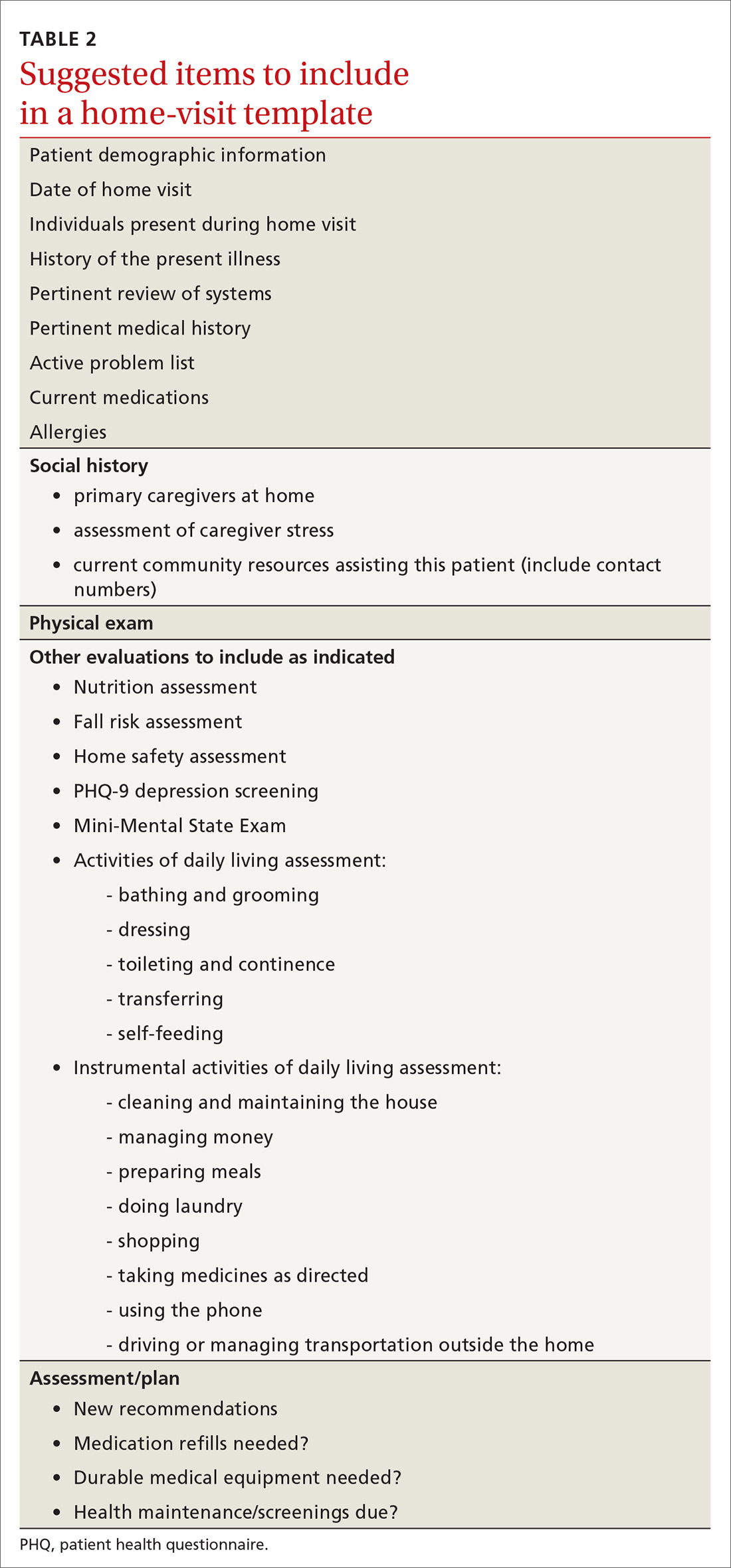
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.
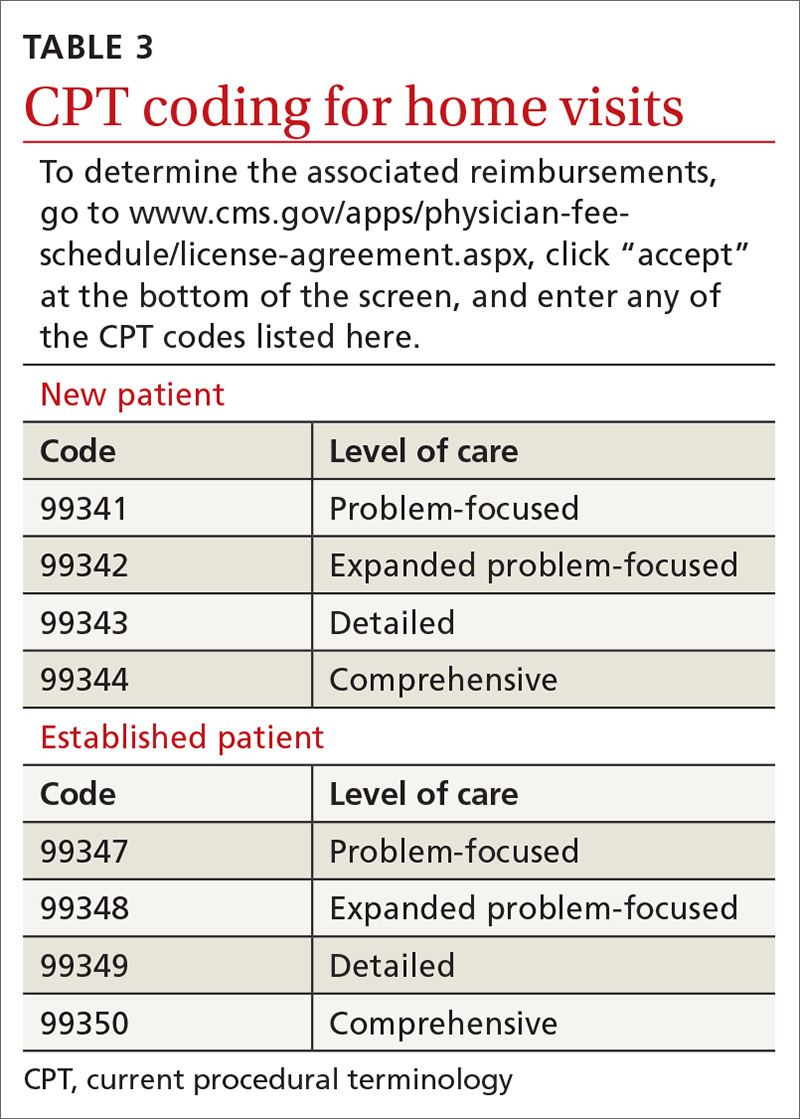
Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34
For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
PRACTICE RECOMMENDATIONS
❯ Consider incorporating home visits into the primary care of select vulnerable patients because doing so improves clinical outcomes, including mortality rates in neonates and elders. A
❯ Employ team-based home care and include community health workers, nurses, pharmacists, social workers, chaplains, and others. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
COVID-19 vaccines: Preparing for patient questions
With U.S. approval of one coronavirus vaccine likely imminent and approval of a second one expected soon after, physicians will likely be deluged with questions. Public attitudes about the vaccines vary by demographics, with a recent poll showing that men and older adults are more likely to choose vaccination, and women and people of color evincing more wariness.
Although the reasons for reluctance may vary, questions from patient will likely be similar. Some are related to the “warp speed” language about the vaccines. Other concerns arise from the fact that the platform – mRNA – has not been used in human vaccines before. And as with any vaccine, there are rumors and false claims making the rounds on social media.
In anticipation of the most common questions physicians may encounter, two experts, Krutika Kuppalli, MD, assistant professor of medicine in the division of infectious diseases at the Medical University of South Carolina, Charleston, and Angela Rasmussen, PhD, virologist and nonresident affiliate at Georgetown University’s Center for Global Health Science and Security, Washington, talked in an interview about what clinicians can expect and what evidence-based – as well as compassionate – answers might look like.
Q: Will this vaccine give me COVID-19?
“There is not an intact virus in there,” Dr. Rasmussen said. The mRNA-based vaccines cannot cause COVID-19 because they don’t use any part of the coronavirus itself. Instead, the Moderna and Pfizer vaccines contain manufactured mRNA molecules that carry the instructions for building the virus’ spike protein. After vaccine administration, the recipient’s own cells take up this mRNA, use it to build this bit of protein, and display it on their surfaces. The foreign protein flag triggers the immune system response.
The mRNA does not enter the cell nucleus or interact with the recipient’s DNA. And because it’s so fragile, it degrades quite quickly. To keep that from happening before cell entry, the mRNAs are cushioned in protective fats.
Q: Was this vaccine made too quickly?
“People have been working on this platform for 30 years, so it’s not that this is brand new,” Dr. Kuppalli said.
Researchers began working on mRNA vaccines in the 1990s. Technological developments in the last decade have meant that their use has become feasible, and they have been tested in animals against many viral diseases. The mRNA vaccines are attractive because they’re expected to be safe and easily manufactured from common materials. That’s what we’ve seen in the COVID-19 pandemic, the Centers for Disease Control and Prevention says on its website. Design of the spike protein mRNA component began as soon as the viral genome became available in January.
Usually, rolling out a vaccine takes years, so less than a year under a program called Operation Warp Speed can seem like moving too fast, Dr. Rasmussen acknowledged. “The name has given people the impression that by going at warp speed, we’re cutting all the corners. [But] the reality is that Operation Warp Speed is mostly for manufacturing and distribution.”
What underlies the speed is a restructuring of the normal vaccine development process, Dr. Kuppalli said. The same phases of development – animal testing, a small initial human phase, a second for safety testing, a third large phase for efficacy – were all conducted as for any vaccine. But in this case, some phases were completed in parallel, rather than sequentially. This approach has proved so successful that there is already talk about making it the model for developing future vaccines.
Two other factors contributed to the speed, said Dr. Kuppalli and Dr. Rasmussen. First, gearing up production can slow a rollout, but with these vaccines, companies ramped up production even before anyone knew if the vaccines would work – the “warp speed” part. The second factor has been the large number of cases, making exposures more likely and thus accelerating the results of the efficacy trials. “There is so much COVID being transmitted everywhere in the United States that it did not take long to hit the threshold of events to read out phase 3,” Dr. Rasmussen said.
Q: This vaccine has never been used in humans. How do we know it’s safe?
The Pfizer phase 3 trial included more than 43,000 people, and Moderna’s had more than 30,000. The first humans received mRNA-based COVID-19 vaccines in March. The most common adverse events emerge right after a vaccination, Dr. Kuppalli said.
As with any vaccine that gains approval, monitoring will continue.
UK health officials have reported that two health care workers vaccinated in the initial rollout of the Pfizer vaccine had what seems to have been a severe allergic response. Both recipients had a history of anaphylactic allergic responses and carried EpiPens, and both recovered. During the trial, allergic reaction rates were 0.63% in the vaccine group and 0.51% in the placebo group.
As a result of the two reactions, UK regulators are now recommending that patients with a history of severe allergies not receive the vaccine at the current time.
Q: What are the likely side effects?
So far, the most common side effects are pain at the injection site and an achy, flu-like feeling, Dr. Kuppalli said. More severe reactions have been reported, but were not common in the trials.
Dr. Rasmussen noted that the common side effects are a good sign, and signal that the recipient is generating “a robust immune response.”
“Everybody I’ve talked to who’s had the response has said they would go through it again,” Dr. Kruppalli said. “I definitely plan on lining up and being one of the first people to get the vaccine.”
Q: I already had COVID-19 or had a positive antibody test. Do I still need to get the vaccine?
Dr. Rasmussen said that there are “too many unknowns” to say if a history of COVID-19 would make a difference. “We don’t know how long neutralizing antibodies last” after infection, she said. “What we know is that the vaccine tends to produce antibody titers towards the higher end of the spectrum,” suggesting better immunity with vaccination than after natural infection.
Q: Can patients of color feel safe getting the vaccine?
“People of color might be understandably reluctant to take a vaccine that was developed in a way that appears to be faster [than past development],” said Dr. Rasmussen. She said physicians should acknowledge and understand the history that has led them to feel that way, “everything from Tuskegee to Henrietta Lacks to today.”
Empathy is key, and “providers should meet patients where they are and not condescend to them.”
Dr. Kuppalli agreed. “Clinicians really need to work on trying to strip away their biases.”
Thus far there are no safety signals that differ by race or ethnicity, according to the companies. The Pfizer phase 3 trial enrolled just over 9% Black participants, 0.5% Native American/Alaska Native, 0.2% Native Hawaiian/Pacific Islander, 2.3% multiracial participants, and 28% Hispanic/Latinx. For its part, Moderna says that approximately 37% of participants in its phase 3 trial come from communities of color.
Q: What about children and pregnant women?
Although the trials included participants from many different age groups and backgrounds, children and pregnant or lactating women were not among them. Pfizer gained approval in October to include participants as young as age 12 years, and a Moderna spokesperson said in an interview that the company planned pediatric inclusion at the end of 2020, pending approval.
“Unfortunately, we don’t have data on pregnant and lactating women,” Dr. Kuppalli said. She said she hopes that public health organizations such as the CDC will address that in the coming weeks. Dr. Rasmussen called the lack of data in pregnant women and children “a big oversight.”
Dr. Rasmussen has disclosed no relevant financial relationships. Dr. Kuppalli is a consultant with GlaxoSmithKline.
A version of this article originally appeared on Medscape.com.
With U.S. approval of one coronavirus vaccine likely imminent and approval of a second one expected soon after, physicians will likely be deluged with questions. Public attitudes about the vaccines vary by demographics, with a recent poll showing that men and older adults are more likely to choose vaccination, and women and people of color evincing more wariness.
Although the reasons for reluctance may vary, questions from patient will likely be similar. Some are related to the “warp speed” language about the vaccines. Other concerns arise from the fact that the platform – mRNA – has not been used in human vaccines before. And as with any vaccine, there are rumors and false claims making the rounds on social media.
In anticipation of the most common questions physicians may encounter, two experts, Krutika Kuppalli, MD, assistant professor of medicine in the division of infectious diseases at the Medical University of South Carolina, Charleston, and Angela Rasmussen, PhD, virologist and nonresident affiliate at Georgetown University’s Center for Global Health Science and Security, Washington, talked in an interview about what clinicians can expect and what evidence-based – as well as compassionate – answers might look like.
Q: Will this vaccine give me COVID-19?
“There is not an intact virus in there,” Dr. Rasmussen said. The mRNA-based vaccines cannot cause COVID-19 because they don’t use any part of the coronavirus itself. Instead, the Moderna and Pfizer vaccines contain manufactured mRNA molecules that carry the instructions for building the virus’ spike protein. After vaccine administration, the recipient’s own cells take up this mRNA, use it to build this bit of protein, and display it on their surfaces. The foreign protein flag triggers the immune system response.
The mRNA does not enter the cell nucleus or interact with the recipient’s DNA. And because it’s so fragile, it degrades quite quickly. To keep that from happening before cell entry, the mRNAs are cushioned in protective fats.
Q: Was this vaccine made too quickly?
“People have been working on this platform for 30 years, so it’s not that this is brand new,” Dr. Kuppalli said.
Researchers began working on mRNA vaccines in the 1990s. Technological developments in the last decade have meant that their use has become feasible, and they have been tested in animals against many viral diseases. The mRNA vaccines are attractive because they’re expected to be safe and easily manufactured from common materials. That’s what we’ve seen in the COVID-19 pandemic, the Centers for Disease Control and Prevention says on its website. Design of the spike protein mRNA component began as soon as the viral genome became available in January.
Usually, rolling out a vaccine takes years, so less than a year under a program called Operation Warp Speed can seem like moving too fast, Dr. Rasmussen acknowledged. “The name has given people the impression that by going at warp speed, we’re cutting all the corners. [But] the reality is that Operation Warp Speed is mostly for manufacturing and distribution.”
What underlies the speed is a restructuring of the normal vaccine development process, Dr. Kuppalli said. The same phases of development – animal testing, a small initial human phase, a second for safety testing, a third large phase for efficacy – were all conducted as for any vaccine. But in this case, some phases were completed in parallel, rather than sequentially. This approach has proved so successful that there is already talk about making it the model for developing future vaccines.
Two other factors contributed to the speed, said Dr. Kuppalli and Dr. Rasmussen. First, gearing up production can slow a rollout, but with these vaccines, companies ramped up production even before anyone knew if the vaccines would work – the “warp speed” part. The second factor has been the large number of cases, making exposures more likely and thus accelerating the results of the efficacy trials. “There is so much COVID being transmitted everywhere in the United States that it did not take long to hit the threshold of events to read out phase 3,” Dr. Rasmussen said.
Q: This vaccine has never been used in humans. How do we know it’s safe?
The Pfizer phase 3 trial included more than 43,000 people, and Moderna’s had more than 30,000. The first humans received mRNA-based COVID-19 vaccines in March. The most common adverse events emerge right after a vaccination, Dr. Kuppalli said.
As with any vaccine that gains approval, monitoring will continue.
UK health officials have reported that two health care workers vaccinated in the initial rollout of the Pfizer vaccine had what seems to have been a severe allergic response. Both recipients had a history of anaphylactic allergic responses and carried EpiPens, and both recovered. During the trial, allergic reaction rates were 0.63% in the vaccine group and 0.51% in the placebo group.
As a result of the two reactions, UK regulators are now recommending that patients with a history of severe allergies not receive the vaccine at the current time.
Q: What are the likely side effects?
So far, the most common side effects are pain at the injection site and an achy, flu-like feeling, Dr. Kuppalli said. More severe reactions have been reported, but were not common in the trials.
Dr. Rasmussen noted that the common side effects are a good sign, and signal that the recipient is generating “a robust immune response.”
“Everybody I’ve talked to who’s had the response has said they would go through it again,” Dr. Kruppalli said. “I definitely plan on lining up and being one of the first people to get the vaccine.”
Q: I already had COVID-19 or had a positive antibody test. Do I still need to get the vaccine?
Dr. Rasmussen said that there are “too many unknowns” to say if a history of COVID-19 would make a difference. “We don’t know how long neutralizing antibodies last” after infection, she said. “What we know is that the vaccine tends to produce antibody titers towards the higher end of the spectrum,” suggesting better immunity with vaccination than after natural infection.
Q: Can patients of color feel safe getting the vaccine?
“People of color might be understandably reluctant to take a vaccine that was developed in a way that appears to be faster [than past development],” said Dr. Rasmussen. She said physicians should acknowledge and understand the history that has led them to feel that way, “everything from Tuskegee to Henrietta Lacks to today.”
Empathy is key, and “providers should meet patients where they are and not condescend to them.”
Dr. Kuppalli agreed. “Clinicians really need to work on trying to strip away their biases.”
Thus far there are no safety signals that differ by race or ethnicity, according to the companies. The Pfizer phase 3 trial enrolled just over 9% Black participants, 0.5% Native American/Alaska Native, 0.2% Native Hawaiian/Pacific Islander, 2.3% multiracial participants, and 28% Hispanic/Latinx. For its part, Moderna says that approximately 37% of participants in its phase 3 trial come from communities of color.
Q: What about children and pregnant women?
Although the trials included participants from many different age groups and backgrounds, children and pregnant or lactating women were not among them. Pfizer gained approval in October to include participants as young as age 12 years, and a Moderna spokesperson said in an interview that the company planned pediatric inclusion at the end of 2020, pending approval.
“Unfortunately, we don’t have data on pregnant and lactating women,” Dr. Kuppalli said. She said she hopes that public health organizations such as the CDC will address that in the coming weeks. Dr. Rasmussen called the lack of data in pregnant women and children “a big oversight.”
Dr. Rasmussen has disclosed no relevant financial relationships. Dr. Kuppalli is a consultant with GlaxoSmithKline.
A version of this article originally appeared on Medscape.com.
With U.S. approval of one coronavirus vaccine likely imminent and approval of a second one expected soon after, physicians will likely be deluged with questions. Public attitudes about the vaccines vary by demographics, with a recent poll showing that men and older adults are more likely to choose vaccination, and women and people of color evincing more wariness.
Although the reasons for reluctance may vary, questions from patient will likely be similar. Some are related to the “warp speed” language about the vaccines. Other concerns arise from the fact that the platform – mRNA – has not been used in human vaccines before. And as with any vaccine, there are rumors and false claims making the rounds on social media.
In anticipation of the most common questions physicians may encounter, two experts, Krutika Kuppalli, MD, assistant professor of medicine in the division of infectious diseases at the Medical University of South Carolina, Charleston, and Angela Rasmussen, PhD, virologist and nonresident affiliate at Georgetown University’s Center for Global Health Science and Security, Washington, talked in an interview about what clinicians can expect and what evidence-based – as well as compassionate – answers might look like.
Q: Will this vaccine give me COVID-19?
“There is not an intact virus in there,” Dr. Rasmussen said. The mRNA-based vaccines cannot cause COVID-19 because they don’t use any part of the coronavirus itself. Instead, the Moderna and Pfizer vaccines contain manufactured mRNA molecules that carry the instructions for building the virus’ spike protein. After vaccine administration, the recipient’s own cells take up this mRNA, use it to build this bit of protein, and display it on their surfaces. The foreign protein flag triggers the immune system response.
The mRNA does not enter the cell nucleus or interact with the recipient’s DNA. And because it’s so fragile, it degrades quite quickly. To keep that from happening before cell entry, the mRNAs are cushioned in protective fats.
Q: Was this vaccine made too quickly?
“People have been working on this platform for 30 years, so it’s not that this is brand new,” Dr. Kuppalli said.
Researchers began working on mRNA vaccines in the 1990s. Technological developments in the last decade have meant that their use has become feasible, and they have been tested in animals against many viral diseases. The mRNA vaccines are attractive because they’re expected to be safe and easily manufactured from common materials. That’s what we’ve seen in the COVID-19 pandemic, the Centers for Disease Control and Prevention says on its website. Design of the spike protein mRNA component began as soon as the viral genome became available in January.
Usually, rolling out a vaccine takes years, so less than a year under a program called Operation Warp Speed can seem like moving too fast, Dr. Rasmussen acknowledged. “The name has given people the impression that by going at warp speed, we’re cutting all the corners. [But] the reality is that Operation Warp Speed is mostly for manufacturing and distribution.”
What underlies the speed is a restructuring of the normal vaccine development process, Dr. Kuppalli said. The same phases of development – animal testing, a small initial human phase, a second for safety testing, a third large phase for efficacy – were all conducted as for any vaccine. But in this case, some phases were completed in parallel, rather than sequentially. This approach has proved so successful that there is already talk about making it the model for developing future vaccines.
Two other factors contributed to the speed, said Dr. Kuppalli and Dr. Rasmussen. First, gearing up production can slow a rollout, but with these vaccines, companies ramped up production even before anyone knew if the vaccines would work – the “warp speed” part. The second factor has been the large number of cases, making exposures more likely and thus accelerating the results of the efficacy trials. “There is so much COVID being transmitted everywhere in the United States that it did not take long to hit the threshold of events to read out phase 3,” Dr. Rasmussen said.
Q: This vaccine has never been used in humans. How do we know it’s safe?
The Pfizer phase 3 trial included more than 43,000 people, and Moderna’s had more than 30,000. The first humans received mRNA-based COVID-19 vaccines in March. The most common adverse events emerge right after a vaccination, Dr. Kuppalli said.
As with any vaccine that gains approval, monitoring will continue.
UK health officials have reported that two health care workers vaccinated in the initial rollout of the Pfizer vaccine had what seems to have been a severe allergic response. Both recipients had a history of anaphylactic allergic responses and carried EpiPens, and both recovered. During the trial, allergic reaction rates were 0.63% in the vaccine group and 0.51% in the placebo group.
As a result of the two reactions, UK regulators are now recommending that patients with a history of severe allergies not receive the vaccine at the current time.
Q: What are the likely side effects?
So far, the most common side effects are pain at the injection site and an achy, flu-like feeling, Dr. Kuppalli said. More severe reactions have been reported, but were not common in the trials.
Dr. Rasmussen noted that the common side effects are a good sign, and signal that the recipient is generating “a robust immune response.”
“Everybody I’ve talked to who’s had the response has said they would go through it again,” Dr. Kruppalli said. “I definitely plan on lining up and being one of the first people to get the vaccine.”
Q: I already had COVID-19 or had a positive antibody test. Do I still need to get the vaccine?
Dr. Rasmussen said that there are “too many unknowns” to say if a history of COVID-19 would make a difference. “We don’t know how long neutralizing antibodies last” after infection, she said. “What we know is that the vaccine tends to produce antibody titers towards the higher end of the spectrum,” suggesting better immunity with vaccination than after natural infection.
Q: Can patients of color feel safe getting the vaccine?
“People of color might be understandably reluctant to take a vaccine that was developed in a way that appears to be faster [than past development],” said Dr. Rasmussen. She said physicians should acknowledge and understand the history that has led them to feel that way, “everything from Tuskegee to Henrietta Lacks to today.”
Empathy is key, and “providers should meet patients where they are and not condescend to them.”
Dr. Kuppalli agreed. “Clinicians really need to work on trying to strip away their biases.”
Thus far there are no safety signals that differ by race or ethnicity, according to the companies. The Pfizer phase 3 trial enrolled just over 9% Black participants, 0.5% Native American/Alaska Native, 0.2% Native Hawaiian/Pacific Islander, 2.3% multiracial participants, and 28% Hispanic/Latinx. For its part, Moderna says that approximately 37% of participants in its phase 3 trial come from communities of color.
Q: What about children and pregnant women?
Although the trials included participants from many different age groups and backgrounds, children and pregnant or lactating women were not among them. Pfizer gained approval in October to include participants as young as age 12 years, and a Moderna spokesperson said in an interview that the company planned pediatric inclusion at the end of 2020, pending approval.
“Unfortunately, we don’t have data on pregnant and lactating women,” Dr. Kuppalli said. She said she hopes that public health organizations such as the CDC will address that in the coming weeks. Dr. Rasmussen called the lack of data in pregnant women and children “a big oversight.”
Dr. Rasmussen has disclosed no relevant financial relationships. Dr. Kuppalli is a consultant with GlaxoSmithKline.
A version of this article originally appeared on Medscape.com.
Widespread Purple Plaques
The Diagnosis: Kaposi Sarcoma
On initial presentation, the differential diagnosis included secondary syphilis, Kaposi sarcoma (KS), lichen planus pigmentosus, sarcoidosis, and psoriasis. A laboratory workup was ordered, which included complete blood cell count, comprehensive metabolic panel, antinuclear antibodies, anti-Ro/Sjögren syndrome antigen A and anti-La/Sjögren syndrome antigen B autoantibodies, angiotensin-converting enzyme, rapid plasma reagin, and human immunodeficiency virus (HIV) antibodies. A 4-mm punch biopsy of the rash also was performed from the right upper back. Histology revealed a vascular proliferation that was diffusely positive for human herpesvirus 8 (HHV-8)(Figure 1). The patient was informed of the diagnosis, at which time he revealed he had a history of homosexual relationships, with his last sexual contact being more than 1 year prior to presentation. The laboratory workup confirmed a diagnosis of HIV, and the remainder of the tests were unremarkable.
He was referred to our university's HIV clinic where he was started on highly active antiretroviral therapy (HAART). His facial swelling worsened, leading to hospital admission. Computed tomography (CT) of the chest, abdomen, and pelvis showed diffuse lymphadenopathy and lung nodules concerning for visceral involvement of KS. Hematology and oncology was consulted for further evaluation, and he was treated with 6 cycles of doxorubicin 20 mg/m2, which led to resolution of the lung nodules on CT and improvement of the rash burden. He was then started on alitretinoin gel 0.1% twice daily, which led to continued slow improvement (Figure 2).

Kaposi sarcoma is a vascular neoplasm that occurs from infection with HHV-8. It typically presents as painless, reddish to violaceous macules or patches involving the skin and mucosa that often progress to plaques or nodules with possible visceral involvement. Kaposi sarcoma is classified into 4 subtypes based on epidemiology and clinical presentation: classic, endemic, iatrogenic, and AIDS associated.1,2
Classic KS primarily affects elderly males of Mediterranean or Eastern European descent, with a mean age of 64.1 years and a male to female ratio of 3 to 1. It has an indolent course and a strong predilection for the skin of the lower extremities. The endemic form occurs mainly in Africa and has a more aggressive course, especially the lymphadenopathic type that affects children younger than 10 years.3 Iatrogenic KS develops in immunosuppressed patients, such as transplant recipients, and may regress if the immunosuppressive agent is stopped.1 Kaposi sarcoma is an AIDS-defining illness and is the most common malignancy in AIDS patients. It is strongly associated with a low CD4 count, which accounts for the notable decline in its incidence after the widespread introduction of HAART.1 Among HIV patients, KS has the highest incidence in men who have sex with men. This population has a higher seroprevalence of HHV-8, which suggests possible sexual transmission of HHV-8. AIDS-associated KS most commonly involves the lower extremities, face, and oral mucosa. It may have visceral involvement, particularly of the gastrointestinal and respiratory systems, which carries a poor prognosis.4,5
Approximately 40% of patients presenting with KS have gastrointestinal tract involvement.6 Of these patients, up to 80% are asymptomatic, with diagnosis usually being made on endoscopy.7 In contrast, pulmonary KS is less common and typically is symptomatic. It can involve the lung parenchyma, airways, or pleura and is diagnosed by chest radiography or CT scans. Glucocorticoid therapy is a known trigger for pulmonary KS exacerbation.8
All 4 subtypes share the same histopathologic findings consisting of spindled endothelial cell proliferation, inflammation, and angiogenesis. Immunohistochemistry reveals tumor cells that are CD34 and CD31 positive but are factor VIII negative. Staining for HHV-8 antigen is used to confirm the diagnosis. The inflammatory infiltrate predominantly is lymphocytic with scattered plasma cells.9
The laboratory results and histopathologic findings clearly indicated a diagnosis of KS in our patient. Other entities in the clinical differential would have shown notably different histopathologic findings and laboratory results. Lichen planus pigmentosus displays a lichenoid infiltrate and pigment dropout on histology. Histologic findings of psoriasis include psoriasiform acanthosis, dilated vessels in the dermal papillae, thinning of suprapapillary plates, and neutrophilic microabscesses. Sarcoidosis would demonstrate naked granulomas on histopathology. Syphilis displays variable but often psoriasiform or lichenoid findings on histology, and a positive rapid plasma reagin also would be noted.
First-line treatment of AIDS-related KS is HAART. For patients with severe and rapidly progressive KS or with visceral involvement, cytotoxic chemotherapy with doxorubicin or taxanes often is required. Additional therapies include radiotherapy, topical alitretinoin, and cryotherapy.1,10
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206; quiz 207-208.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:239-250.
- Beral V, Peterman TA, Berkelman RL, et al. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
- Smith NA, Sabin CA, Gopal R, et al. Serologic evidence of human herpesvirus 8 transmission by homosexual but not heterosexual sex. J Infect Dis. 1999;180:600-606.
- Arora M, Goldberg EM. Kaposi sarcoma involving the gastrointestinal tract. Gastroenterol Hepatol (N Y). 2010;6:459-462.
- Parente F, Cernuschi M, Orlando G, et al. Kaposi’s sarcoma and AIDS: frequency of gastrointestinal involvement and its effect on survival. a prospective study in a heterogeneous population. Scand J Gastroenterol. 1991;26:1007-1012.
- Gasparetto TD, Marchiori E, Lourenco S, et al. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis. 2009;4:18.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Regnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
The Diagnosis: Kaposi Sarcoma
On initial presentation, the differential diagnosis included secondary syphilis, Kaposi sarcoma (KS), lichen planus pigmentosus, sarcoidosis, and psoriasis. A laboratory workup was ordered, which included complete blood cell count, comprehensive metabolic panel, antinuclear antibodies, anti-Ro/Sjögren syndrome antigen A and anti-La/Sjögren syndrome antigen B autoantibodies, angiotensin-converting enzyme, rapid plasma reagin, and human immunodeficiency virus (HIV) antibodies. A 4-mm punch biopsy of the rash also was performed from the right upper back. Histology revealed a vascular proliferation that was diffusely positive for human herpesvirus 8 (HHV-8)(Figure 1). The patient was informed of the diagnosis, at which time he revealed he had a history of homosexual relationships, with his last sexual contact being more than 1 year prior to presentation. The laboratory workup confirmed a diagnosis of HIV, and the remainder of the tests were unremarkable.

He was referred to our university's HIV clinic where he was started on highly active antiretroviral therapy (HAART). His facial swelling worsened, leading to hospital admission. Computed tomography (CT) of the chest, abdomen, and pelvis showed diffuse lymphadenopathy and lung nodules concerning for visceral involvement of KS. Hematology and oncology was consulted for further evaluation, and he was treated with 6 cycles of doxorubicin 20 mg/m2, which led to resolution of the lung nodules on CT and improvement of the rash burden. He was then started on alitretinoin gel 0.1% twice daily, which led to continued slow improvement (Figure 2).

Kaposi sarcoma is a vascular neoplasm that occurs from infection with HHV-8. It typically presents as painless, reddish to violaceous macules or patches involving the skin and mucosa that often progress to plaques or nodules with possible visceral involvement. Kaposi sarcoma is classified into 4 subtypes based on epidemiology and clinical presentation: classic, endemic, iatrogenic, and AIDS associated.1,2
Classic KS primarily affects elderly males of Mediterranean or Eastern European descent, with a mean age of 64.1 years and a male to female ratio of 3 to 1. It has an indolent course and a strong predilection for the skin of the lower extremities. The endemic form occurs mainly in Africa and has a more aggressive course, especially the lymphadenopathic type that affects children younger than 10 years.3 Iatrogenic KS develops in immunosuppressed patients, such as transplant recipients, and may regress if the immunosuppressive agent is stopped.1 Kaposi sarcoma is an AIDS-defining illness and is the most common malignancy in AIDS patients. It is strongly associated with a low CD4 count, which accounts for the notable decline in its incidence after the widespread introduction of HAART.1 Among HIV patients, KS has the highest incidence in men who have sex with men. This population has a higher seroprevalence of HHV-8, which suggests possible sexual transmission of HHV-8. AIDS-associated KS most commonly involves the lower extremities, face, and oral mucosa. It may have visceral involvement, particularly of the gastrointestinal and respiratory systems, which carries a poor prognosis.4,5
Approximately 40% of patients presenting with KS have gastrointestinal tract involvement.6 Of these patients, up to 80% are asymptomatic, with diagnosis usually being made on endoscopy.7 In contrast, pulmonary KS is less common and typically is symptomatic. It can involve the lung parenchyma, airways, or pleura and is diagnosed by chest radiography or CT scans. Glucocorticoid therapy is a known trigger for pulmonary KS exacerbation.8
All 4 subtypes share the same histopathologic findings consisting of spindled endothelial cell proliferation, inflammation, and angiogenesis. Immunohistochemistry reveals tumor cells that are CD34 and CD31 positive but are factor VIII negative. Staining for HHV-8 antigen is used to confirm the diagnosis. The inflammatory infiltrate predominantly is lymphocytic with scattered plasma cells.9
The laboratory results and histopathologic findings clearly indicated a diagnosis of KS in our patient. Other entities in the clinical differential would have shown notably different histopathologic findings and laboratory results. Lichen planus pigmentosus displays a lichenoid infiltrate and pigment dropout on histology. Histologic findings of psoriasis include psoriasiform acanthosis, dilated vessels in the dermal papillae, thinning of suprapapillary plates, and neutrophilic microabscesses. Sarcoidosis would demonstrate naked granulomas on histopathology. Syphilis displays variable but often psoriasiform or lichenoid findings on histology, and a positive rapid plasma reagin also would be noted.
First-line treatment of AIDS-related KS is HAART. For patients with severe and rapidly progressive KS or with visceral involvement, cytotoxic chemotherapy with doxorubicin or taxanes often is required. Additional therapies include radiotherapy, topical alitretinoin, and cryotherapy.1,10
The Diagnosis: Kaposi Sarcoma
On initial presentation, the differential diagnosis included secondary syphilis, Kaposi sarcoma (KS), lichen planus pigmentosus, sarcoidosis, and psoriasis. A laboratory workup was ordered, which included complete blood cell count, comprehensive metabolic panel, antinuclear antibodies, anti-Ro/Sjögren syndrome antigen A and anti-La/Sjögren syndrome antigen B autoantibodies, angiotensin-converting enzyme, rapid plasma reagin, and human immunodeficiency virus (HIV) antibodies. A 4-mm punch biopsy of the rash also was performed from the right upper back. Histology revealed a vascular proliferation that was diffusely positive for human herpesvirus 8 (HHV-8)(Figure 1). The patient was informed of the diagnosis, at which time he revealed he had a history of homosexual relationships, with his last sexual contact being more than 1 year prior to presentation. The laboratory workup confirmed a diagnosis of HIV, and the remainder of the tests were unremarkable.

He was referred to our university's HIV clinic where he was started on highly active antiretroviral therapy (HAART). His facial swelling worsened, leading to hospital admission. Computed tomography (CT) of the chest, abdomen, and pelvis showed diffuse lymphadenopathy and lung nodules concerning for visceral involvement of KS. Hematology and oncology was consulted for further evaluation, and he was treated with 6 cycles of doxorubicin 20 mg/m2, which led to resolution of the lung nodules on CT and improvement of the rash burden. He was then started on alitretinoin gel 0.1% twice daily, which led to continued slow improvement (Figure 2).

Kaposi sarcoma is a vascular neoplasm that occurs from infection with HHV-8. It typically presents as painless, reddish to violaceous macules or patches involving the skin and mucosa that often progress to plaques or nodules with possible visceral involvement. Kaposi sarcoma is classified into 4 subtypes based on epidemiology and clinical presentation: classic, endemic, iatrogenic, and AIDS associated.1,2
Classic KS primarily affects elderly males of Mediterranean or Eastern European descent, with a mean age of 64.1 years and a male to female ratio of 3 to 1. It has an indolent course and a strong predilection for the skin of the lower extremities. The endemic form occurs mainly in Africa and has a more aggressive course, especially the lymphadenopathic type that affects children younger than 10 years.3 Iatrogenic KS develops in immunosuppressed patients, such as transplant recipients, and may regress if the immunosuppressive agent is stopped.1 Kaposi sarcoma is an AIDS-defining illness and is the most common malignancy in AIDS patients. It is strongly associated with a low CD4 count, which accounts for the notable decline in its incidence after the widespread introduction of HAART.1 Among HIV patients, KS has the highest incidence in men who have sex with men. This population has a higher seroprevalence of HHV-8, which suggests possible sexual transmission of HHV-8. AIDS-associated KS most commonly involves the lower extremities, face, and oral mucosa. It may have visceral involvement, particularly of the gastrointestinal and respiratory systems, which carries a poor prognosis.4,5
Approximately 40% of patients presenting with KS have gastrointestinal tract involvement.6 Of these patients, up to 80% are asymptomatic, with diagnosis usually being made on endoscopy.7 In contrast, pulmonary KS is less common and typically is symptomatic. It can involve the lung parenchyma, airways, or pleura and is diagnosed by chest radiography or CT scans. Glucocorticoid therapy is a known trigger for pulmonary KS exacerbation.8
All 4 subtypes share the same histopathologic findings consisting of spindled endothelial cell proliferation, inflammation, and angiogenesis. Immunohistochemistry reveals tumor cells that are CD34 and CD31 positive but are factor VIII negative. Staining for HHV-8 antigen is used to confirm the diagnosis. The inflammatory infiltrate predominantly is lymphocytic with scattered plasma cells.9
The laboratory results and histopathologic findings clearly indicated a diagnosis of KS in our patient. Other entities in the clinical differential would have shown notably different histopathologic findings and laboratory results. Lichen planus pigmentosus displays a lichenoid infiltrate and pigment dropout on histology. Histologic findings of psoriasis include psoriasiform acanthosis, dilated vessels in the dermal papillae, thinning of suprapapillary plates, and neutrophilic microabscesses. Sarcoidosis would demonstrate naked granulomas on histopathology. Syphilis displays variable but often psoriasiform or lichenoid findings on histology, and a positive rapid plasma reagin also would be noted.
First-line treatment of AIDS-related KS is HAART. For patients with severe and rapidly progressive KS or with visceral involvement, cytotoxic chemotherapy with doxorubicin or taxanes often is required. Additional therapies include radiotherapy, topical alitretinoin, and cryotherapy.1,10
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206; quiz 207-208.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:239-250.
- Beral V, Peterman TA, Berkelman RL, et al. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
- Smith NA, Sabin CA, Gopal R, et al. Serologic evidence of human herpesvirus 8 transmission by homosexual but not heterosexual sex. J Infect Dis. 1999;180:600-606.
- Arora M, Goldberg EM. Kaposi sarcoma involving the gastrointestinal tract. Gastroenterol Hepatol (N Y). 2010;6:459-462.
- Parente F, Cernuschi M, Orlando G, et al. Kaposi’s sarcoma and AIDS: frequency of gastrointestinal involvement and its effect on survival. a prospective study in a heterogeneous population. Scand J Gastroenterol. 1991;26:1007-1012.
- Gasparetto TD, Marchiori E, Lourenco S, et al. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis. 2009;4:18.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Regnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206; quiz 207-208.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:239-250.
- Beral V, Peterman TA, Berkelman RL, et al. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
- Smith NA, Sabin CA, Gopal R, et al. Serologic evidence of human herpesvirus 8 transmission by homosexual but not heterosexual sex. J Infect Dis. 1999;180:600-606.
- Arora M, Goldberg EM. Kaposi sarcoma involving the gastrointestinal tract. Gastroenterol Hepatol (N Y). 2010;6:459-462.
- Parente F, Cernuschi M, Orlando G, et al. Kaposi’s sarcoma and AIDS: frequency of gastrointestinal involvement and its effect on survival. a prospective study in a heterogeneous population. Scand J Gastroenterol. 1991;26:1007-1012.
- Gasparetto TD, Marchiori E, Lourenco S, et al. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis. 2009;4:18.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Regnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
A 24-year-old Black man presented for evaluation of an asymptomatic rash on the face, chest, back, and arms that had been progressively spreading over the course of 3 months. He had some swelling of the lips prior to the onset of the rash and was prescribed prednisone 10 mg daily by an outside physician. He had no known medical problems and was taking no medications. Physical examination revealed numerous violaceous plaques scattered symmetrically on the trunk, arms, legs, and face. His family history was negative for autoimmune disease, and a review of systems was unremarkable. He denied any recent sexual contacts.
Can a health care worker refuse the COVID-19 vaccine?
As hospitals across the country develop their plans to vaccinate their health care employees against COVID-19, a key question has come to the fore: What if an employee – whether nurse, physician, or other health care worker – refuses to receive the vaccine? Can hospitals require their employees to be vaccinated against COVID-19? And what consequences could an employee face for refusing the vaccine?
My answer needs to be based, in part, on the law related to previous vaccines – influenza, for example – because at the time of this writing (early December 2020), no vaccine for COVID-19 has been approved, although approval of at least one vaccine is expected within a week. So there have been no offers of vaccine and refusals yet, nor are there any cases to date involving an employee who refused a COVID-19 vaccine. As of December 2020, there are no state or federal laws that either require an employee to be vaccinated against COVID-19 or that protect an employee who refuses vaccination against COVID-19. It will take a while after the vaccine is approved and distributed before refusals, reactions, policies, cases, and laws begin to emerge.
If we look at the law related to health care workers refusing to be vaccinated against the closest relative to COVID-19 – influenza – then the answer would be yes, employers can require employees to be vaccinated.
An employer can fire an employee who refuses influenza vaccination. If an employee who refused and was fired sues the employer for wrongful termination, the employee has more or less chance of success depending on the reason for refusal. Some courts and the Equal Employment Opportunity Commission have held that a refusal on religious grounds is protected by the U.S. Constitution, as in this recent case. The Constitution protects freedom to practice one’s religion. Specific religions may have a range of tenets that support refusal to be vaccinated.
A refusal on medical grounds has been successful if the medical grounds fall under the protections of the Americans with Disabilities Act but may fail when the medical grounds for the claim are not covered by the ADA.
Refusal for secular, nonmedical reasons, such as a health care worker’s policy of treating their body as their temple, has not gone over well with employers or courts. However, in at least one case, a nurse who refused vaccination on secular, nonmedical grounds won her case against her employer, on appeal. The appeals court found that the hospital violated her First Amendment rights.
Employees who refuse vaccination for religious or medical reasons still will need to take measures to protect patients and other employees from infection. An employer such as a hospital can, rather than fire the employee, offer the employee an accommodation, such as requiring that the employee wear a mask or quarantine. There are no cases that have upheld an employee’s right to refuse to wear a mask or quarantine.
The situation with the COVID-19 vaccine is different from the situation surrounding influenza vaccines. There are plenty of data on effectiveness and side effects of influenza vaccines, but there is very little evidence of short- or long-term effects of the COVID-19 vaccines currently being tested and/or considered for approval. One could argue that the process of vaccine development is the same for all virus vaccines. However, public confidence in the vaccine vetting process is not what it once was. It has been widely publicized that the COVID-19 vaccine trials have been rushed. As of December 2020, only 60% of the general population say they would take the vaccine, although researchers say confidence is increasing.
The Centers for Disease Control and Prevention has designated health care workers as first in line to get the vaccine, but some health care workers may not want to be the first to try it. A CDC survey found that 63% of health care workers polled in recent months said they would get a COVID-19 vaccine.
Unions have entered the conversation. A coalition of unions that represent health care workers said, “we need a transparent, evidence-based federal vaccine strategy based on principles of equity, safety, and priority, as well as robust efforts to address a high degree of skepticism about safety of an authorized vaccine.” The organization declined to promote a vaccine until more is known.
As of publication date, the EEOC guidance for employers responding to COVID-19 does not address vaccines.
The CDC’s Interim Guidance for Businesses and Employers Responding to Coronavirus Disease 2019, May 2020, updated Dec. 4, 2020, does not address vaccines. The CDC’s page on COVID-19 vaccination for health care workers does not address a health care worker’s refusal. The site does assure health care workers that the vaccine development process is sound: “The current vaccine safety system is strong and robust, with the capacity to effectively monitor COVID-19 vaccine safety. Existing data systems have validated analytic methods that can rapidly detect statistical signals for possible vaccine safety problems. These systems are being scaled up to fully meet the needs of the nation. Additional systems and data sources are also being developed to further enhance safety monitoring capabilities. CDC is committed to ensuring that COVID-19 vaccines are safe.”
In the coming months, government officials and vaccine manufacturers will be working to reassure the public of the safety of the vaccine and the rigor of the vaccine development process. In November 2020, National Institute of Allergy and Infectious Diseases Director Anthony Fauci, MD, told Kaiser Health News: “The company looks at the data. I look at the data. Then the company puts the data to the FDA. The FDA will make the decision to do an emergency-use authorization or a license application approval. And they have career scientists who are really independent. They’re not beholden to anybody. Then there’s another independent group, the Vaccines and Related Biological Products Advisory Committee. The FDA commissioner has vowed publicly that he will go according to the opinion of the career scientists and the advisory board.” President-elect Joe Biden said he would get a vaccine when Dr. Fauci thinks it is safe.
An employee who, after researching the vaccine and the process, still wants to refuse when offered the vaccine is not likely to be fired for that reason right away, as long as the employee takes other precautions, such as wearing a mask. If the employer does fire the employee and the employee sues the employer, it is impossible to predict how a court would decide the case.
Related legal questions may arise in the coming months. For example:
- Is an employer exempt from paying workers’ compensation to an employee who refuses to be vaccinated and then contracts the virus while on the job?
- Can a prospective employer require COVID-19 vaccination as a precondition of employment?
- Is it within a patient’s rights to receive an answer to the question: Has my health care worker been vaccinated against COVID-19?
- If a hospital allows employees to refuse vaccination and keep working, and an outbreak occurs, and it is suggested through contact tracing that unvaccinated workers infected patients, will a court hold the hospital liable for patients’ damages?
Answers to these questions are yet to be determined.
Carolyn Buppert (www.buppert.com) is an attorney and former nurse practitioner who focuses on the legal issues affecting nurse practitioners.
A version of this article originally appeared on Medscape.com.
As hospitals across the country develop their plans to vaccinate their health care employees against COVID-19, a key question has come to the fore: What if an employee – whether nurse, physician, or other health care worker – refuses to receive the vaccine? Can hospitals require their employees to be vaccinated against COVID-19? And what consequences could an employee face for refusing the vaccine?
My answer needs to be based, in part, on the law related to previous vaccines – influenza, for example – because at the time of this writing (early December 2020), no vaccine for COVID-19 has been approved, although approval of at least one vaccine is expected within a week. So there have been no offers of vaccine and refusals yet, nor are there any cases to date involving an employee who refused a COVID-19 vaccine. As of December 2020, there are no state or federal laws that either require an employee to be vaccinated against COVID-19 or that protect an employee who refuses vaccination against COVID-19. It will take a while after the vaccine is approved and distributed before refusals, reactions, policies, cases, and laws begin to emerge.
If we look at the law related to health care workers refusing to be vaccinated against the closest relative to COVID-19 – influenza – then the answer would be yes, employers can require employees to be vaccinated.
An employer can fire an employee who refuses influenza vaccination. If an employee who refused and was fired sues the employer for wrongful termination, the employee has more or less chance of success depending on the reason for refusal. Some courts and the Equal Employment Opportunity Commission have held that a refusal on religious grounds is protected by the U.S. Constitution, as in this recent case. The Constitution protects freedom to practice one’s religion. Specific religions may have a range of tenets that support refusal to be vaccinated.
A refusal on medical grounds has been successful if the medical grounds fall under the protections of the Americans with Disabilities Act but may fail when the medical grounds for the claim are not covered by the ADA.
Refusal for secular, nonmedical reasons, such as a health care worker’s policy of treating their body as their temple, has not gone over well with employers or courts. However, in at least one case, a nurse who refused vaccination on secular, nonmedical grounds won her case against her employer, on appeal. The appeals court found that the hospital violated her First Amendment rights.
Employees who refuse vaccination for religious or medical reasons still will need to take measures to protect patients and other employees from infection. An employer such as a hospital can, rather than fire the employee, offer the employee an accommodation, such as requiring that the employee wear a mask or quarantine. There are no cases that have upheld an employee’s right to refuse to wear a mask or quarantine.
The situation with the COVID-19 vaccine is different from the situation surrounding influenza vaccines. There are plenty of data on effectiveness and side effects of influenza vaccines, but there is very little evidence of short- or long-term effects of the COVID-19 vaccines currently being tested and/or considered for approval. One could argue that the process of vaccine development is the same for all virus vaccines. However, public confidence in the vaccine vetting process is not what it once was. It has been widely publicized that the COVID-19 vaccine trials have been rushed. As of December 2020, only 60% of the general population say they would take the vaccine, although researchers say confidence is increasing.
The Centers for Disease Control and Prevention has designated health care workers as first in line to get the vaccine, but some health care workers may not want to be the first to try it. A CDC survey found that 63% of health care workers polled in recent months said they would get a COVID-19 vaccine.
Unions have entered the conversation. A coalition of unions that represent health care workers said, “we need a transparent, evidence-based federal vaccine strategy based on principles of equity, safety, and priority, as well as robust efforts to address a high degree of skepticism about safety of an authorized vaccine.” The organization declined to promote a vaccine until more is known.
As of publication date, the EEOC guidance for employers responding to COVID-19 does not address vaccines.
The CDC’s Interim Guidance for Businesses and Employers Responding to Coronavirus Disease 2019, May 2020, updated Dec. 4, 2020, does not address vaccines. The CDC’s page on COVID-19 vaccination for health care workers does not address a health care worker’s refusal. The site does assure health care workers that the vaccine development process is sound: “The current vaccine safety system is strong and robust, with the capacity to effectively monitor COVID-19 vaccine safety. Existing data systems have validated analytic methods that can rapidly detect statistical signals for possible vaccine safety problems. These systems are being scaled up to fully meet the needs of the nation. Additional systems and data sources are also being developed to further enhance safety monitoring capabilities. CDC is committed to ensuring that COVID-19 vaccines are safe.”
In the coming months, government officials and vaccine manufacturers will be working to reassure the public of the safety of the vaccine and the rigor of the vaccine development process. In November 2020, National Institute of Allergy and Infectious Diseases Director Anthony Fauci, MD, told Kaiser Health News: “The company looks at the data. I look at the data. Then the company puts the data to the FDA. The FDA will make the decision to do an emergency-use authorization or a license application approval. And they have career scientists who are really independent. They’re not beholden to anybody. Then there’s another independent group, the Vaccines and Related Biological Products Advisory Committee. The FDA commissioner has vowed publicly that he will go according to the opinion of the career scientists and the advisory board.” President-elect Joe Biden said he would get a vaccine when Dr. Fauci thinks it is safe.
An employee who, after researching the vaccine and the process, still wants to refuse when offered the vaccine is not likely to be fired for that reason right away, as long as the employee takes other precautions, such as wearing a mask. If the employer does fire the employee and the employee sues the employer, it is impossible to predict how a court would decide the case.
Related legal questions may arise in the coming months. For example:
- Is an employer exempt from paying workers’ compensation to an employee who refuses to be vaccinated and then contracts the virus while on the job?
- Can a prospective employer require COVID-19 vaccination as a precondition of employment?
- Is it within a patient’s rights to receive an answer to the question: Has my health care worker been vaccinated against COVID-19?
- If a hospital allows employees to refuse vaccination and keep working, and an outbreak occurs, and it is suggested through contact tracing that unvaccinated workers infected patients, will a court hold the hospital liable for patients’ damages?
Answers to these questions are yet to be determined.
Carolyn Buppert (www.buppert.com) is an attorney and former nurse practitioner who focuses on the legal issues affecting nurse practitioners.
A version of this article originally appeared on Medscape.com.
As hospitals across the country develop their plans to vaccinate their health care employees against COVID-19, a key question has come to the fore: What if an employee – whether nurse, physician, or other health care worker – refuses to receive the vaccine? Can hospitals require their employees to be vaccinated against COVID-19? And what consequences could an employee face for refusing the vaccine?
My answer needs to be based, in part, on the law related to previous vaccines – influenza, for example – because at the time of this writing (early December 2020), no vaccine for COVID-19 has been approved, although approval of at least one vaccine is expected within a week. So there have been no offers of vaccine and refusals yet, nor are there any cases to date involving an employee who refused a COVID-19 vaccine. As of December 2020, there are no state or federal laws that either require an employee to be vaccinated against COVID-19 or that protect an employee who refuses vaccination against COVID-19. It will take a while after the vaccine is approved and distributed before refusals, reactions, policies, cases, and laws begin to emerge.
If we look at the law related to health care workers refusing to be vaccinated against the closest relative to COVID-19 – influenza – then the answer would be yes, employers can require employees to be vaccinated.
An employer can fire an employee who refuses influenza vaccination. If an employee who refused and was fired sues the employer for wrongful termination, the employee has more or less chance of success depending on the reason for refusal. Some courts and the Equal Employment Opportunity Commission have held that a refusal on religious grounds is protected by the U.S. Constitution, as in this recent case. The Constitution protects freedom to practice one’s religion. Specific religions may have a range of tenets that support refusal to be vaccinated.
A refusal on medical grounds has been successful if the medical grounds fall under the protections of the Americans with Disabilities Act but may fail when the medical grounds for the claim are not covered by the ADA.
Refusal for secular, nonmedical reasons, such as a health care worker’s policy of treating their body as their temple, has not gone over well with employers or courts. However, in at least one case, a nurse who refused vaccination on secular, nonmedical grounds won her case against her employer, on appeal. The appeals court found that the hospital violated her First Amendment rights.
Employees who refuse vaccination for religious or medical reasons still will need to take measures to protect patients and other employees from infection. An employer such as a hospital can, rather than fire the employee, offer the employee an accommodation, such as requiring that the employee wear a mask or quarantine. There are no cases that have upheld an employee’s right to refuse to wear a mask or quarantine.
The situation with the COVID-19 vaccine is different from the situation surrounding influenza vaccines. There are plenty of data on effectiveness and side effects of influenza vaccines, but there is very little evidence of short- or long-term effects of the COVID-19 vaccines currently being tested and/or considered for approval. One could argue that the process of vaccine development is the same for all virus vaccines. However, public confidence in the vaccine vetting process is not what it once was. It has been widely publicized that the COVID-19 vaccine trials have been rushed. As of December 2020, only 60% of the general population say they would take the vaccine, although researchers say confidence is increasing.
The Centers for Disease Control and Prevention has designated health care workers as first in line to get the vaccine, but some health care workers may not want to be the first to try it. A CDC survey found that 63% of health care workers polled in recent months said they would get a COVID-19 vaccine.
Unions have entered the conversation. A coalition of unions that represent health care workers said, “we need a transparent, evidence-based federal vaccine strategy based on principles of equity, safety, and priority, as well as robust efforts to address a high degree of skepticism about safety of an authorized vaccine.” The organization declined to promote a vaccine until more is known.
As of publication date, the EEOC guidance for employers responding to COVID-19 does not address vaccines.
The CDC’s Interim Guidance for Businesses and Employers Responding to Coronavirus Disease 2019, May 2020, updated Dec. 4, 2020, does not address vaccines. The CDC’s page on COVID-19 vaccination for health care workers does not address a health care worker’s refusal. The site does assure health care workers that the vaccine development process is sound: “The current vaccine safety system is strong and robust, with the capacity to effectively monitor COVID-19 vaccine safety. Existing data systems have validated analytic methods that can rapidly detect statistical signals for possible vaccine safety problems. These systems are being scaled up to fully meet the needs of the nation. Additional systems and data sources are also being developed to further enhance safety monitoring capabilities. CDC is committed to ensuring that COVID-19 vaccines are safe.”
In the coming months, government officials and vaccine manufacturers will be working to reassure the public of the safety of the vaccine and the rigor of the vaccine development process. In November 2020, National Institute of Allergy and Infectious Diseases Director Anthony Fauci, MD, told Kaiser Health News: “The company looks at the data. I look at the data. Then the company puts the data to the FDA. The FDA will make the decision to do an emergency-use authorization or a license application approval. And they have career scientists who are really independent. They’re not beholden to anybody. Then there’s another independent group, the Vaccines and Related Biological Products Advisory Committee. The FDA commissioner has vowed publicly that he will go according to the opinion of the career scientists and the advisory board.” President-elect Joe Biden said he would get a vaccine when Dr. Fauci thinks it is safe.
An employee who, after researching the vaccine and the process, still wants to refuse when offered the vaccine is not likely to be fired for that reason right away, as long as the employee takes other precautions, such as wearing a mask. If the employer does fire the employee and the employee sues the employer, it is impossible to predict how a court would decide the case.
Related legal questions may arise in the coming months. For example:
- Is an employer exempt from paying workers’ compensation to an employee who refuses to be vaccinated and then contracts the virus while on the job?
- Can a prospective employer require COVID-19 vaccination as a precondition of employment?
- Is it within a patient’s rights to receive an answer to the question: Has my health care worker been vaccinated against COVID-19?
- If a hospital allows employees to refuse vaccination and keep working, and an outbreak occurs, and it is suggested through contact tracing that unvaccinated workers infected patients, will a court hold the hospital liable for patients’ damages?
Answers to these questions are yet to be determined.
Carolyn Buppert (www.buppert.com) is an attorney and former nurse practitioner who focuses on the legal issues affecting nurse practitioners.
A version of this article originally appeared on Medscape.com.
A 70-year-old presented with a 3-week history of asymptomatic violaceous papules on his feet
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
New tool may provide point-of-care differentiation between bacterial, viral infections
The World Health Organization estimates that 14.9 million of 57 million annual deaths worldwide (25%) are related directly to diseases caused by bacterial and/or viral infections.
The first crucial step in order to build a successful surveillance system is to accurately identify and diagnose disease, Ivana Pennisi reminded the audience at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. A problem, particularly in primary care, is differentiating between patients with bacterial infections who might benefit from antibiotics and those with viral infections where supportive treatment is generally required. One solution might a rapid point-of-care tool.
Ms. Pennisi described early experiences of using microchip technology to detect RNA biomarkers in the blood rather than look for the pathogen itself. Early results suggest high diagnostic accuracy at low cost.
It is known that when a bacteria or virus enters the body, it stimulates the immune system in a unique way leading to the expression of different genes in the host blood. As part of the Personalized Management of Febrile Illnesses study, researchers have demonstrated a number of high correlated transcripts. Of current interest are two genes which are upregulated in childhood febrile illnesses.
Ms. Pennisi, a PhD student working as part of a multidisciplinary at the department of infectious disease and Centre for Bioinspired Technology at Imperial College, London, developed loop-mediated isothermal amplification (LAMP) assays to detect for the first time host RNA signatures on a nucleic acid–based point-of-care handheld system to discriminate bacterial from viral infection. The amplification reaction is then combined with microchip technology in the well of a portable point-of-care device named Lacewing. It translates the nucleic acid amplification signal into a quantitative electrochemical signal without the need for a thermal cycler.
The combination of genomic expertise in the section of paediatrics lead by Michael Levin, PhD, and microchip-based technologies in the department of electrical and electronic engineering under the guidance of Pantelis Georgiou, PhD, enabled the team overcome many clinical challenges.
Ms. Pennisi presented her team’s early experiences with clinical samples from 455 febrile children. First, transcription isothermal amplification techniques were employed to confirm bacterial and viral infections. Results were then validated using standard fluorescent-based quantitative polymerase chain reaction (PCR) instruments. In order to define a decision boundary between bacterial and viral patients, cutoff levels were determined using multivariate logistic regression analysis. Results then were evaluated using microarrays, reverse transcriptase PCR (RT-PCR), and the eLAMP to confirm comparability with preferred techniques.
In conclusion, Ms. Pennisi reported that the two-gene signature combined with the use of eLAMP technology in She outlined her vision for the future: “The patient sample and reagent are loaded into a disposable cartridge. This is then placed into a device to monitor in real time the reaction and share all the data via a Bluetooth to a dedicated app on a smart phone. All data and location of the outbreak are then stored in [the] cloud, making it easier for epidemiological studies and tracking of new outbreaks. We hope that by enhancing the capability of our platform, we contribute to better patient care.”
“Distinguishing between bacterial and viral infections remains one of the key questions in the daily pediatric acute care,” commented Lauri Ivaska, MD, from the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital. “One of the most promising laboratory methods to do this is by measuring quantities of two specific host RNA transcripts from a blood sample. It would be of great importance if this could be done reliably by using a fast and cheap method as presented here by Ivana Pennisi.”
Ms. Pennisi had no relevant financial disclosures.
The World Health Organization estimates that 14.9 million of 57 million annual deaths worldwide (25%) are related directly to diseases caused by bacterial and/or viral infections.
The first crucial step in order to build a successful surveillance system is to accurately identify and diagnose disease, Ivana Pennisi reminded the audience at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. A problem, particularly in primary care, is differentiating between patients with bacterial infections who might benefit from antibiotics and those with viral infections where supportive treatment is generally required. One solution might a rapid point-of-care tool.
Ms. Pennisi described early experiences of using microchip technology to detect RNA biomarkers in the blood rather than look for the pathogen itself. Early results suggest high diagnostic accuracy at low cost.
It is known that when a bacteria or virus enters the body, it stimulates the immune system in a unique way leading to the expression of different genes in the host blood. As part of the Personalized Management of Febrile Illnesses study, researchers have demonstrated a number of high correlated transcripts. Of current interest are two genes which are upregulated in childhood febrile illnesses.
Ms. Pennisi, a PhD student working as part of a multidisciplinary at the department of infectious disease and Centre for Bioinspired Technology at Imperial College, London, developed loop-mediated isothermal amplification (LAMP) assays to detect for the first time host RNA signatures on a nucleic acid–based point-of-care handheld system to discriminate bacterial from viral infection. The amplification reaction is then combined with microchip technology in the well of a portable point-of-care device named Lacewing. It translates the nucleic acid amplification signal into a quantitative electrochemical signal without the need for a thermal cycler.
The combination of genomic expertise in the section of paediatrics lead by Michael Levin, PhD, and microchip-based technologies in the department of electrical and electronic engineering under the guidance of Pantelis Georgiou, PhD, enabled the team overcome many clinical challenges.
Ms. Pennisi presented her team’s early experiences with clinical samples from 455 febrile children. First, transcription isothermal amplification techniques were employed to confirm bacterial and viral infections. Results were then validated using standard fluorescent-based quantitative polymerase chain reaction (PCR) instruments. In order to define a decision boundary between bacterial and viral patients, cutoff levels were determined using multivariate logistic regression analysis. Results then were evaluated using microarrays, reverse transcriptase PCR (RT-PCR), and the eLAMP to confirm comparability with preferred techniques.
In conclusion, Ms. Pennisi reported that the two-gene signature combined with the use of eLAMP technology in She outlined her vision for the future: “The patient sample and reagent are loaded into a disposable cartridge. This is then placed into a device to monitor in real time the reaction and share all the data via a Bluetooth to a dedicated app on a smart phone. All data and location of the outbreak are then stored in [the] cloud, making it easier for epidemiological studies and tracking of new outbreaks. We hope that by enhancing the capability of our platform, we contribute to better patient care.”
“Distinguishing between bacterial and viral infections remains one of the key questions in the daily pediatric acute care,” commented Lauri Ivaska, MD, from the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital. “One of the most promising laboratory methods to do this is by measuring quantities of two specific host RNA transcripts from a blood sample. It would be of great importance if this could be done reliably by using a fast and cheap method as presented here by Ivana Pennisi.”
Ms. Pennisi had no relevant financial disclosures.
The World Health Organization estimates that 14.9 million of 57 million annual deaths worldwide (25%) are related directly to diseases caused by bacterial and/or viral infections.
The first crucial step in order to build a successful surveillance system is to accurately identify and diagnose disease, Ivana Pennisi reminded the audience at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. A problem, particularly in primary care, is differentiating between patients with bacterial infections who might benefit from antibiotics and those with viral infections where supportive treatment is generally required. One solution might a rapid point-of-care tool.
Ms. Pennisi described early experiences of using microchip technology to detect RNA biomarkers in the blood rather than look for the pathogen itself. Early results suggest high diagnostic accuracy at low cost.
It is known that when a bacteria or virus enters the body, it stimulates the immune system in a unique way leading to the expression of different genes in the host blood. As part of the Personalized Management of Febrile Illnesses study, researchers have demonstrated a number of high correlated transcripts. Of current interest are two genes which are upregulated in childhood febrile illnesses.
Ms. Pennisi, a PhD student working as part of a multidisciplinary at the department of infectious disease and Centre for Bioinspired Technology at Imperial College, London, developed loop-mediated isothermal amplification (LAMP) assays to detect for the first time host RNA signatures on a nucleic acid–based point-of-care handheld system to discriminate bacterial from viral infection. The amplification reaction is then combined with microchip technology in the well of a portable point-of-care device named Lacewing. It translates the nucleic acid amplification signal into a quantitative electrochemical signal without the need for a thermal cycler.
The combination of genomic expertise in the section of paediatrics lead by Michael Levin, PhD, and microchip-based technologies in the department of electrical and electronic engineering under the guidance of Pantelis Georgiou, PhD, enabled the team overcome many clinical challenges.
Ms. Pennisi presented her team’s early experiences with clinical samples from 455 febrile children. First, transcription isothermal amplification techniques were employed to confirm bacterial and viral infections. Results were then validated using standard fluorescent-based quantitative polymerase chain reaction (PCR) instruments. In order to define a decision boundary between bacterial and viral patients, cutoff levels were determined using multivariate logistic regression analysis. Results then were evaluated using microarrays, reverse transcriptase PCR (RT-PCR), and the eLAMP to confirm comparability with preferred techniques.
In conclusion, Ms. Pennisi reported that the two-gene signature combined with the use of eLAMP technology in She outlined her vision for the future: “The patient sample and reagent are loaded into a disposable cartridge. This is then placed into a device to monitor in real time the reaction and share all the data via a Bluetooth to a dedicated app on a smart phone. All data and location of the outbreak are then stored in [the] cloud, making it easier for epidemiological studies and tracking of new outbreaks. We hope that by enhancing the capability of our platform, we contribute to better patient care.”
“Distinguishing between bacterial and viral infections remains one of the key questions in the daily pediatric acute care,” commented Lauri Ivaska, MD, from the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital. “One of the most promising laboratory methods to do this is by measuring quantities of two specific host RNA transcripts from a blood sample. It would be of great importance if this could be done reliably by using a fast and cheap method as presented here by Ivana Pennisi.”
Ms. Pennisi had no relevant financial disclosures.
FROM ESPID 2020
Three genes could predict congenital Zika infection susceptibility
Dr. Irene Rivero-Calle, MD, shared at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
ZIKV, an emerging flavivirus, is responsible for one the most critical pandemic emergencies of the last decade and has been associated with severe neonatal brain disabilities, declared Dr. Rivero-Calle, of the Hospital Clínico Universitario de Santiago de Compostela in Santiago de Compostela, Spain. “We think that understanding the genomic background could explain some of the most relevant symptoms of congenital Zika syndrome (CZS) and could be essential to better comprehend this disease.”
To achieve this understanding, Dr. Rivero-Calle and her colleagues conducted a study aiming to analyze any genetic factors that could explain the variation in phenotypes in newborns from mothers who had a Zika infection during their pregnancy. Additionally, they strove to “elucidate if the possible genetic association is specific to mothers or their newborns, and to check if this genomic background or any genomic ancestry pattern could be related with the phenotype,” she explained.
In their study, Dr. Rivero-Calle and her team analyzed 80 samples, comprising 40 samples from mothers who had been infected by ZIKV during their pregnancy and 40 from their newborns. Of those descendants, 20 were asymptomatic and 20 were symptomatic (13 had CZS, 3 had microcephaly, 2 had a pathologic MRI, 1 had hearing loss, and 1 was born preterm).
Population stratification, which Dr. Rivero-Calle explained “lets us know if the population is African, European, or Native American looking at the genes,” did not show any relation with the phenotype. We had a mixture of population genomics among all samples.”
Dr. Rivero-Calle and her team then performed three analyses: genotype analysis, an allelic test, and gene analysis. The allelic test and gene-collapsing method highlighted three genes (PANO1, PIDD1, and SLC25A22) as potential determinants of the varying phenotypes in the newborns from ZIKV-infected mothers. Overrepresentation analysis of gene ontology terms shows that PIDD1 and PANO1 are related to apoptosis and cell death, which is closely related to early infantile epilepsy. This could explain the most severe complications of CZS: seizures, brain damage, microcephaly, and detrimental neurodevelopmental growth. Regarding reactome and KEGG analysis, gene PIID1 is related with p53 pathway, which correlates with cell’s death and apoptosis, and with microcephaly, a typical phenotypic feature of CZS.
“So, in conclusion, we found three genes which could predict susceptibility to congenital Zika infection; we saw that the functionality of these genes seems to be deeply related with mechanisms which could explain the different phenotypes; and we saw that these three genes only appear in the children’s cohort, so there is no candidate gene in the mother’s genomic background which can help predict the phenotype of the newborn,” Dr. Rivero-Calle declared. “Finally, there is no ancestry pattern associated with disabilities caused by Zika infection.”
Dr. Rivero-Calle reported that this project (ZikAction) has received funding from the European Union’s Horizon 2020 research and innovation program, under grant agreement 734857.
Dr. Irene Rivero-Calle, MD, shared at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
ZIKV, an emerging flavivirus, is responsible for one the most critical pandemic emergencies of the last decade and has been associated with severe neonatal brain disabilities, declared Dr. Rivero-Calle, of the Hospital Clínico Universitario de Santiago de Compostela in Santiago de Compostela, Spain. “We think that understanding the genomic background could explain some of the most relevant symptoms of congenital Zika syndrome (CZS) and could be essential to better comprehend this disease.”
To achieve this understanding, Dr. Rivero-Calle and her colleagues conducted a study aiming to analyze any genetic factors that could explain the variation in phenotypes in newborns from mothers who had a Zika infection during their pregnancy. Additionally, they strove to “elucidate if the possible genetic association is specific to mothers or their newborns, and to check if this genomic background or any genomic ancestry pattern could be related with the phenotype,” she explained.
In their study, Dr. Rivero-Calle and her team analyzed 80 samples, comprising 40 samples from mothers who had been infected by ZIKV during their pregnancy and 40 from their newborns. Of those descendants, 20 were asymptomatic and 20 were symptomatic (13 had CZS, 3 had microcephaly, 2 had a pathologic MRI, 1 had hearing loss, and 1 was born preterm).
Population stratification, which Dr. Rivero-Calle explained “lets us know if the population is African, European, or Native American looking at the genes,” did not show any relation with the phenotype. We had a mixture of population genomics among all samples.”
Dr. Rivero-Calle and her team then performed three analyses: genotype analysis, an allelic test, and gene analysis. The allelic test and gene-collapsing method highlighted three genes (PANO1, PIDD1, and SLC25A22) as potential determinants of the varying phenotypes in the newborns from ZIKV-infected mothers. Overrepresentation analysis of gene ontology terms shows that PIDD1 and PANO1 are related to apoptosis and cell death, which is closely related to early infantile epilepsy. This could explain the most severe complications of CZS: seizures, brain damage, microcephaly, and detrimental neurodevelopmental growth. Regarding reactome and KEGG analysis, gene PIID1 is related with p53 pathway, which correlates with cell’s death and apoptosis, and with microcephaly, a typical phenotypic feature of CZS.
“So, in conclusion, we found three genes which could predict susceptibility to congenital Zika infection; we saw that the functionality of these genes seems to be deeply related with mechanisms which could explain the different phenotypes; and we saw that these three genes only appear in the children’s cohort, so there is no candidate gene in the mother’s genomic background which can help predict the phenotype of the newborn,” Dr. Rivero-Calle declared. “Finally, there is no ancestry pattern associated with disabilities caused by Zika infection.”
Dr. Rivero-Calle reported that this project (ZikAction) has received funding from the European Union’s Horizon 2020 research and innovation program, under grant agreement 734857.
Dr. Irene Rivero-Calle, MD, shared at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
ZIKV, an emerging flavivirus, is responsible for one the most critical pandemic emergencies of the last decade and has been associated with severe neonatal brain disabilities, declared Dr. Rivero-Calle, of the Hospital Clínico Universitario de Santiago de Compostela in Santiago de Compostela, Spain. “We think that understanding the genomic background could explain some of the most relevant symptoms of congenital Zika syndrome (CZS) and could be essential to better comprehend this disease.”
To achieve this understanding, Dr. Rivero-Calle and her colleagues conducted a study aiming to analyze any genetic factors that could explain the variation in phenotypes in newborns from mothers who had a Zika infection during their pregnancy. Additionally, they strove to “elucidate if the possible genetic association is specific to mothers or their newborns, and to check if this genomic background or any genomic ancestry pattern could be related with the phenotype,” she explained.
In their study, Dr. Rivero-Calle and her team analyzed 80 samples, comprising 40 samples from mothers who had been infected by ZIKV during their pregnancy and 40 from their newborns. Of those descendants, 20 were asymptomatic and 20 were symptomatic (13 had CZS, 3 had microcephaly, 2 had a pathologic MRI, 1 had hearing loss, and 1 was born preterm).
Population stratification, which Dr. Rivero-Calle explained “lets us know if the population is African, European, or Native American looking at the genes,” did not show any relation with the phenotype. We had a mixture of population genomics among all samples.”
Dr. Rivero-Calle and her team then performed three analyses: genotype analysis, an allelic test, and gene analysis. The allelic test and gene-collapsing method highlighted three genes (PANO1, PIDD1, and SLC25A22) as potential determinants of the varying phenotypes in the newborns from ZIKV-infected mothers. Overrepresentation analysis of gene ontology terms shows that PIDD1 and PANO1 are related to apoptosis and cell death, which is closely related to early infantile epilepsy. This could explain the most severe complications of CZS: seizures, brain damage, microcephaly, and detrimental neurodevelopmental growth. Regarding reactome and KEGG analysis, gene PIID1 is related with p53 pathway, which correlates with cell’s death and apoptosis, and with microcephaly, a typical phenotypic feature of CZS.
“So, in conclusion, we found three genes which could predict susceptibility to congenital Zika infection; we saw that the functionality of these genes seems to be deeply related with mechanisms which could explain the different phenotypes; and we saw that these three genes only appear in the children’s cohort, so there is no candidate gene in the mother’s genomic background which can help predict the phenotype of the newborn,” Dr. Rivero-Calle declared. “Finally, there is no ancestry pattern associated with disabilities caused by Zika infection.”
Dr. Rivero-Calle reported that this project (ZikAction) has received funding from the European Union’s Horizon 2020 research and innovation program, under grant agreement 734857.
FROM ESPID 2020
C. difficile control could require integrated approach
Clostridioides difficile (C. diff) infection (CDI) is a pathogen of both humans and animals, and to control it will require an integrated approach that encompasses human health care, veterinary health care, environmental regulation, and public policy. That is the conclusion of a group led by Su-Chen Lim, MD, and Tom Riley, MD, of Edith Cowan University in Australia, who published a review in Clinical Microbiology and Infection.
CDI was generally considered a nuisance infection until the early 21st century, when a hypervirulent fluoroquinolone-resistant strain emerged in North America. The strain is now documented In the United States, Canada, and most countries in Europe.
Another new feature of CDI is increased evidence of community transmission, which was previously rare. This is defined as cases where the patient experienced symptom onset outside the hospital, and had no history of hospitalization in the previous 12 weeks or symptom onset within 48 hours of hospital admission. Community-associated CDI now accounts for 41% of U.S. cases, nearly 30% of Australian cases, and about 14% in Europe, according to recent studies.
Several features of CDI suggest a need for an integrated management plan. The preferred habitat of C. diff is the gastrointestinal track of mammals, and likely colonizes all mammalian neonates. Over time, colonization by other microbes likely crowd it out and prevent overgrowth. But widespread use of antimicrobials in animal production can lead to the creation of an environment resembling that of the neonate, allowing C. diff to expand. That has led to food animals becoming a major C. diff reservoir, and whole-genome studies showed that strains found in humans, food, animals, and the environment are closely related and sometimes genetically indistinguishable, suggesting transmission between humans and animals that may be attributable to contaminated food and environments.
The authors suggest that C. diff infection control should be guided by the One Health initiative, which seeks cooperation between physicians, osteopathic physicians, veterinarians, dentists, nurses, and other scientific and environmental disciplines. The goal is to enhance surveillance and interdisciplinary communication, as well as integrated policies. The authors note that C. diff is often thought of by physicians as primarily a hospital problem, who may be unaware of the increased prevalence of community-acquired disease. It is also a significant problem in agriculture, since as many as 50% of piglets succumb to the disease. Other studies have recently shown that asymptomatic carriers of toxigenic strains are likely to transmit the bacteria to C. diff-negative patients. Asymptomatic carriers cluster with symptomatic patients. In one Cleveland hospital, more than 25% of hospital-associated CDI cases were found to have been colonized prior to admission, suggesting that these were not true hospital-associated cases.
C. diff has been isolated from a wide range of sources, including food animals, meat, seafood, vegetables, household environments, and natural environments like rivers, lakes, and soil. About 20% of calves and 70% of piglets are colonized with C. diff. It has a high prevalence in meat products in the United States, but lower in the Europe, possibly because of different slaughtering practices.
The authors suggest that zoonotic C. diff spread is unlikely to be confined to any geographic region or population, and that widespread C. diff contamination is occurring through food or the environment. This could be occurring because spores can withstand cooking temperatures and disseminate through the air, and even through manure from food animals made into compost or fertilizer.
Veterinary efforts mimicking hospital measures have reduced animal CDI, but there are no rapid diagnostic tests for CDI in animals, making it challenging to control its spread in this context.
The authors call for enhanced antimicrobial stewardship in both human and animal settings, including banning of antimicrobial agents as growth promoters. This has been done in the United States and Europe, but not in Brazil, China, Canada, India, and Australia. They also call for research on inactivation of C. diff spores during waste treatment.
Even better, the authors suggest that vaccines should be developed and employed in both animals and humans. No such vaccine exists in animals, but Pfizer has one for humans in a phase 3 clinical trial, but it does not prevent colonization. Others are in development.
The epidemiology of CDI is an ongoing challenge, with emerging new strains and changing social and environmental conditions. “However, it is with the collaborative efforts of industry partners, policymakers, veterinarians, clinicians, and researchers that CDI needs to be approached, a perfect example of One Health. Opening an interdisciplinary dialogue to address CDI and One Health issues has to be the focus of future studies,” the authors concluded.
SOURCE: SC Lim et al. Clinical Microbiology and Infection. 2020;26:85-863.
Clostridioides difficile (C. diff) infection (CDI) is a pathogen of both humans and animals, and to control it will require an integrated approach that encompasses human health care, veterinary health care, environmental regulation, and public policy. That is the conclusion of a group led by Su-Chen Lim, MD, and Tom Riley, MD, of Edith Cowan University in Australia, who published a review in Clinical Microbiology and Infection.
CDI was generally considered a nuisance infection until the early 21st century, when a hypervirulent fluoroquinolone-resistant strain emerged in North America. The strain is now documented In the United States, Canada, and most countries in Europe.
Another new feature of CDI is increased evidence of community transmission, which was previously rare. This is defined as cases where the patient experienced symptom onset outside the hospital, and had no history of hospitalization in the previous 12 weeks or symptom onset within 48 hours of hospital admission. Community-associated CDI now accounts for 41% of U.S. cases, nearly 30% of Australian cases, and about 14% in Europe, according to recent studies.
Several features of CDI suggest a need for an integrated management plan. The preferred habitat of C. diff is the gastrointestinal track of mammals, and likely colonizes all mammalian neonates. Over time, colonization by other microbes likely crowd it out and prevent overgrowth. But widespread use of antimicrobials in animal production can lead to the creation of an environment resembling that of the neonate, allowing C. diff to expand. That has led to food animals becoming a major C. diff reservoir, and whole-genome studies showed that strains found in humans, food, animals, and the environment are closely related and sometimes genetically indistinguishable, suggesting transmission between humans and animals that may be attributable to contaminated food and environments.
The authors suggest that C. diff infection control should be guided by the One Health initiative, which seeks cooperation between physicians, osteopathic physicians, veterinarians, dentists, nurses, and other scientific and environmental disciplines. The goal is to enhance surveillance and interdisciplinary communication, as well as integrated policies. The authors note that C. diff is often thought of by physicians as primarily a hospital problem, who may be unaware of the increased prevalence of community-acquired disease. It is also a significant problem in agriculture, since as many as 50% of piglets succumb to the disease. Other studies have recently shown that asymptomatic carriers of toxigenic strains are likely to transmit the bacteria to C. diff-negative patients. Asymptomatic carriers cluster with symptomatic patients. In one Cleveland hospital, more than 25% of hospital-associated CDI cases were found to have been colonized prior to admission, suggesting that these were not true hospital-associated cases.
C. diff has been isolated from a wide range of sources, including food animals, meat, seafood, vegetables, household environments, and natural environments like rivers, lakes, and soil. About 20% of calves and 70% of piglets are colonized with C. diff. It has a high prevalence in meat products in the United States, but lower in the Europe, possibly because of different slaughtering practices.
The authors suggest that zoonotic C. diff spread is unlikely to be confined to any geographic region or population, and that widespread C. diff contamination is occurring through food or the environment. This could be occurring because spores can withstand cooking temperatures and disseminate through the air, and even through manure from food animals made into compost or fertilizer.
Veterinary efforts mimicking hospital measures have reduced animal CDI, but there are no rapid diagnostic tests for CDI in animals, making it challenging to control its spread in this context.
The authors call for enhanced antimicrobial stewardship in both human and animal settings, including banning of antimicrobial agents as growth promoters. This has been done in the United States and Europe, but not in Brazil, China, Canada, India, and Australia. They also call for research on inactivation of C. diff spores during waste treatment.
Even better, the authors suggest that vaccines should be developed and employed in both animals and humans. No such vaccine exists in animals, but Pfizer has one for humans in a phase 3 clinical trial, but it does not prevent colonization. Others are in development.
The epidemiology of CDI is an ongoing challenge, with emerging new strains and changing social and environmental conditions. “However, it is with the collaborative efforts of industry partners, policymakers, veterinarians, clinicians, and researchers that CDI needs to be approached, a perfect example of One Health. Opening an interdisciplinary dialogue to address CDI and One Health issues has to be the focus of future studies,” the authors concluded.
SOURCE: SC Lim et al. Clinical Microbiology and Infection. 2020;26:85-863.
Clostridioides difficile (C. diff) infection (CDI) is a pathogen of both humans and animals, and to control it will require an integrated approach that encompasses human health care, veterinary health care, environmental regulation, and public policy. That is the conclusion of a group led by Su-Chen Lim, MD, and Tom Riley, MD, of Edith Cowan University in Australia, who published a review in Clinical Microbiology and Infection.
CDI was generally considered a nuisance infection until the early 21st century, when a hypervirulent fluoroquinolone-resistant strain emerged in North America. The strain is now documented In the United States, Canada, and most countries in Europe.
Another new feature of CDI is increased evidence of community transmission, which was previously rare. This is defined as cases where the patient experienced symptom onset outside the hospital, and had no history of hospitalization in the previous 12 weeks or symptom onset within 48 hours of hospital admission. Community-associated CDI now accounts for 41% of U.S. cases, nearly 30% of Australian cases, and about 14% in Europe, according to recent studies.
Several features of CDI suggest a need for an integrated management plan. The preferred habitat of C. diff is the gastrointestinal track of mammals, and likely colonizes all mammalian neonates. Over time, colonization by other microbes likely crowd it out and prevent overgrowth. But widespread use of antimicrobials in animal production can lead to the creation of an environment resembling that of the neonate, allowing C. diff to expand. That has led to food animals becoming a major C. diff reservoir, and whole-genome studies showed that strains found in humans, food, animals, and the environment are closely related and sometimes genetically indistinguishable, suggesting transmission between humans and animals that may be attributable to contaminated food and environments.
The authors suggest that C. diff infection control should be guided by the One Health initiative, which seeks cooperation between physicians, osteopathic physicians, veterinarians, dentists, nurses, and other scientific and environmental disciplines. The goal is to enhance surveillance and interdisciplinary communication, as well as integrated policies. The authors note that C. diff is often thought of by physicians as primarily a hospital problem, who may be unaware of the increased prevalence of community-acquired disease. It is also a significant problem in agriculture, since as many as 50% of piglets succumb to the disease. Other studies have recently shown that asymptomatic carriers of toxigenic strains are likely to transmit the bacteria to C. diff-negative patients. Asymptomatic carriers cluster with symptomatic patients. In one Cleveland hospital, more than 25% of hospital-associated CDI cases were found to have been colonized prior to admission, suggesting that these were not true hospital-associated cases.
C. diff has been isolated from a wide range of sources, including food animals, meat, seafood, vegetables, household environments, and natural environments like rivers, lakes, and soil. About 20% of calves and 70% of piglets are colonized with C. diff. It has a high prevalence in meat products in the United States, but lower in the Europe, possibly because of different slaughtering practices.
The authors suggest that zoonotic C. diff spread is unlikely to be confined to any geographic region or population, and that widespread C. diff contamination is occurring through food or the environment. This could be occurring because spores can withstand cooking temperatures and disseminate through the air, and even through manure from food animals made into compost or fertilizer.
Veterinary efforts mimicking hospital measures have reduced animal CDI, but there are no rapid diagnostic tests for CDI in animals, making it challenging to control its spread in this context.
The authors call for enhanced antimicrobial stewardship in both human and animal settings, including banning of antimicrobial agents as growth promoters. This has been done in the United States and Europe, but not in Brazil, China, Canada, India, and Australia. They also call for research on inactivation of C. diff spores during waste treatment.
Even better, the authors suggest that vaccines should be developed and employed in both animals and humans. No such vaccine exists in animals, but Pfizer has one for humans in a phase 3 clinical trial, but it does not prevent colonization. Others are in development.
The epidemiology of CDI is an ongoing challenge, with emerging new strains and changing social and environmental conditions. “However, it is with the collaborative efforts of industry partners, policymakers, veterinarians, clinicians, and researchers that CDI needs to be approached, a perfect example of One Health. Opening an interdisciplinary dialogue to address CDI and One Health issues has to be the focus of future studies,” the authors concluded.
SOURCE: SC Lim et al. Clinical Microbiology and Infection. 2020;26:85-863.
FROM CLINICAL MICROBIOLOGY AND INFECTION
Meningococcal transmission risk appears low among pediatric health care professionals
at a university – lower than expected for all age groups, Lisa-Maria Steurer, MD, said regarding study findings reported at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“This implicates that the risk of horizontal meningococcal transmission via this health care professional cohort seems to be low,” said Dr. Steurer, of the Medical University of Vienna.
Her data were based on a survey conducted between April and October 2018 at the department of paediatrics and adolescent medicine at the tertiary university pediatric hospital. The study aimed to determine colonization rates of Neisseria meningitidis and the serogroup distribution of carried meningococcal isolates in asymptomatic health care professionals employed there, reported Dr. Steurer. Her research team also sought to identify what factors increased risk of N. meningitidis carriage.
“We who work in pediatrics and adolescent medicine are exposed to those patient cohorts with the highest risk for meningococcal carriage, but also to those patients who have the highest risk for serious, invasive meningococcal disease, which peaks at the extremities of age,” declared Dr. Steurer. “But currently, there is no surveillance of asymptomatic carriers in this health care professional cohort.”
A total of 437 oropharyngeal swabs were collected from enrolled nurses, pediatricians, and medical students working in the department and immediately plated onto selective agar plates. Conventional culture was used to identify bacteria, and meningococcal isolates were characterized further through whole-genome sequencing. Sociodemographic data and information on participants’ vaccination status were collected via questionnaire.
The main finding was an overall meningococcal prevalence of 1.14%. Among the participants, the median age was 33 years, and the highest rate of carriage, 4.4%, was observed in those aged 18-25 years. None of the carriers were older than 35 years. There was a negative association found between carriage and participants’ age and time employed in the field, Dr. Steurer said.
“Risk-factor analysis found an inverse correlation with meningococcal carriage for age and timespan working in pediatrics. On the contrary, no correlations with carriage could be found for all other factors evaluated,” she said. These factors included recent contact with an immunodeficient patient, respiratory tract infection, smoking, vaccination against any meningococcal serogroup, different professions, main work settings, month of swab collection, and living with children or adolescents in the same household.
Of the study population, 29% reported that they had been vaccinated against at least one meningococcal serogroup. “Interestingly, while more than 50% of doctors and medical students had a vaccination against at least one meningococcal serogroup, only 17% of nurses were vaccinated,” Dr. Steurer remarked.
The study was financially supported by Pfizer. Dr. Steurer had no other relevant financial disclosures.
at a university – lower than expected for all age groups, Lisa-Maria Steurer, MD, said regarding study findings reported at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“This implicates that the risk of horizontal meningococcal transmission via this health care professional cohort seems to be low,” said Dr. Steurer, of the Medical University of Vienna.
Her data were based on a survey conducted between April and October 2018 at the department of paediatrics and adolescent medicine at the tertiary university pediatric hospital. The study aimed to determine colonization rates of Neisseria meningitidis and the serogroup distribution of carried meningococcal isolates in asymptomatic health care professionals employed there, reported Dr. Steurer. Her research team also sought to identify what factors increased risk of N. meningitidis carriage.
“We who work in pediatrics and adolescent medicine are exposed to those patient cohorts with the highest risk for meningococcal carriage, but also to those patients who have the highest risk for serious, invasive meningococcal disease, which peaks at the extremities of age,” declared Dr. Steurer. “But currently, there is no surveillance of asymptomatic carriers in this health care professional cohort.”
A total of 437 oropharyngeal swabs were collected from enrolled nurses, pediatricians, and medical students working in the department and immediately plated onto selective agar plates. Conventional culture was used to identify bacteria, and meningococcal isolates were characterized further through whole-genome sequencing. Sociodemographic data and information on participants’ vaccination status were collected via questionnaire.
The main finding was an overall meningococcal prevalence of 1.14%. Among the participants, the median age was 33 years, and the highest rate of carriage, 4.4%, was observed in those aged 18-25 years. None of the carriers were older than 35 years. There was a negative association found between carriage and participants’ age and time employed in the field, Dr. Steurer said.
“Risk-factor analysis found an inverse correlation with meningococcal carriage for age and timespan working in pediatrics. On the contrary, no correlations with carriage could be found for all other factors evaluated,” she said. These factors included recent contact with an immunodeficient patient, respiratory tract infection, smoking, vaccination against any meningococcal serogroup, different professions, main work settings, month of swab collection, and living with children or adolescents in the same household.
Of the study population, 29% reported that they had been vaccinated against at least one meningococcal serogroup. “Interestingly, while more than 50% of doctors and medical students had a vaccination against at least one meningococcal serogroup, only 17% of nurses were vaccinated,” Dr. Steurer remarked.
The study was financially supported by Pfizer. Dr. Steurer had no other relevant financial disclosures.
at a university – lower than expected for all age groups, Lisa-Maria Steurer, MD, said regarding study findings reported at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“This implicates that the risk of horizontal meningococcal transmission via this health care professional cohort seems to be low,” said Dr. Steurer, of the Medical University of Vienna.
Her data were based on a survey conducted between April and October 2018 at the department of paediatrics and adolescent medicine at the tertiary university pediatric hospital. The study aimed to determine colonization rates of Neisseria meningitidis and the serogroup distribution of carried meningococcal isolates in asymptomatic health care professionals employed there, reported Dr. Steurer. Her research team also sought to identify what factors increased risk of N. meningitidis carriage.
“We who work in pediatrics and adolescent medicine are exposed to those patient cohorts with the highest risk for meningococcal carriage, but also to those patients who have the highest risk for serious, invasive meningococcal disease, which peaks at the extremities of age,” declared Dr. Steurer. “But currently, there is no surveillance of asymptomatic carriers in this health care professional cohort.”
A total of 437 oropharyngeal swabs were collected from enrolled nurses, pediatricians, and medical students working in the department and immediately plated onto selective agar plates. Conventional culture was used to identify bacteria, and meningococcal isolates were characterized further through whole-genome sequencing. Sociodemographic data and information on participants’ vaccination status were collected via questionnaire.
The main finding was an overall meningococcal prevalence of 1.14%. Among the participants, the median age was 33 years, and the highest rate of carriage, 4.4%, was observed in those aged 18-25 years. None of the carriers were older than 35 years. There was a negative association found between carriage and participants’ age and time employed in the field, Dr. Steurer said.
“Risk-factor analysis found an inverse correlation with meningococcal carriage for age and timespan working in pediatrics. On the contrary, no correlations with carriage could be found for all other factors evaluated,” she said. These factors included recent contact with an immunodeficient patient, respiratory tract infection, smoking, vaccination against any meningococcal serogroup, different professions, main work settings, month of swab collection, and living with children or adolescents in the same household.
Of the study population, 29% reported that they had been vaccinated against at least one meningococcal serogroup. “Interestingly, while more than 50% of doctors and medical students had a vaccination against at least one meningococcal serogroup, only 17% of nurses were vaccinated,” Dr. Steurer remarked.
The study was financially supported by Pfizer. Dr. Steurer had no other relevant financial disclosures.
FROM ESPID 2020
Children and school during the pandemic: What’s the answer?
Countries across the world are in the process of closing and reopening schools to contain the spread of COVID-19. Should there be universal testing and quarantining of sick school children and their classmates?
In a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Andreea M. Panciu, MD, from the National Institute of Infectious Diseases in Bucharest, argued for routine testing and quarantining of all school children. Her opposite number, Danilo Buonsenso, MD, from the Centre for Global Health Research and Studies, Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico, Rome, made the case for a more selective approach.
Should children be sent to school?
stated Dr. Panciu as she started the debate by explaining the challenges faced by schools in adhering to key mitigation strategies. The U.S. Centers for Disease Control and Prevention recommends that students keep 1.8 m (6 feet) distance from one another. “In many school settings this is not feasible without drastically limiting the number of students,” she explained. “This is a massive challenge for many schools that are already overcrowded.”
The use of facemasks also is a challenge in classrooms. Children have a lower tolerance or may not be able to use the mask properly. There also are concerns regarding impaired learning, speech development, social development, and facial recognition. “We need to look at the evidence; preventive measures work,” responded Dr. Buonsenso. If distance can be implemented, the more distance the lower the transmission of infection, with 1.5-2 meters having the best effects. “Distance can be difficult when school buildings do not allow it, however, governments have had time to plan, and this should not be a limitation to education for kids.”
A recent review clearly showed that children and adolescents aged under 20 years have a much lower risk of susceptibility to COVID-19 infection, compared with adults. This is especially the case for children younger than 14 years. “There is no excuse, let’s bring the children back to school,” argued Dr. Buonsenso.
Dr. Panciu responded with several studies that have tried to quantify the amount of SARS-CoV-2 virus that is carried by infected children. Viral load in the nasopharynx in children under 5 years with mild to moderate COVID-19 symptoms was higher than that of both children over 5 as well as adults. The viral load in young children did not seem to differ by age or symptom severity. “There doesn’t appear to be a significant difference in viral load between symptomatic children and symptomatic adults,” she stated.
“But the question is: ‘How infectious are children?’ ” reacted Dr. Buonsenso. Data from South Korea showed that, for children, particularly those under 10 years, the number of secondary cases of contacts was very low, suggesting that children are rarely spreading the virus.
Dr. Buonsenso and colleagues assessed 30 households containing children aged under 18 years where an adult had been infected with COVID-19 in Rome during the peak of the pandemic. In no cases was it found that a child was the index case. This was supported by data from China, also obtained during the peak of the pandemic, which showed that the number of children infected was very low, but more importantly the number of secondary attacks from contact with children was also very low.
What about children who are sick at school?
The debate moved to discussing what should be done when a child is sick at school. Dr. Panciu clarified recommendations by the CDC regarding what steps to take if a student displays signs of infection consistent with COVID-19: Should they test positive, they are to stay at home for 10 days from the time signs and symptoms first appeared. Further, any teachers or students identified as close contacts are advised to stay at home for 14 days. (Since the ESPID meeting, the CDC has made changes in quarantine times for COVID-19. People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.)
A significant problem is the overlap between COVID-19 symptoms and those associated with other common illnesses because of a range of viruses. This is particularly true in younger children who often suffer from viral infections. “It is common for children to have up to eight respiratory illnesses a year,” explained Dr. Panciu, “and some may have symptoms so mild that they don’t notice them.”
“We need to be a little bit more children focused, otherwise we are going to be isolating children all the time,” said Dr. Buonsenso. The Royal College of Paediatrics and Child Health state that a child with a simple runny nose or sporadic cough without a fever, who would have attended school in other times, should not be tested for COVID-19. He moved on to then cite several studies that show little or no evidence of COVID-19 transmission between school children. This included a prospective cohort study in Australia showing that child-to-child transmission occurred in 0.3%. “To date, the advantages from routine quarantine and over testing seem too low to balance the social consequences on children and families,” he concluded.
As the debate drew to a close, Dr. Panciu reported several studies that did demonstrate transmission between school-age children. Data from an overnight camp in Georgia where the median age was 12 years showed the attack rate was 44% for ages 11-17 years and 51% for ages 6-10 years. Similar conclusions were reached in an Israeli study looking at a large COVID-19 outbreak in a school. This occurred 10 days after reopening, in spite of preventive measures being in place. “Opening safely isn’t just about the adjustments a school makes,” she said, “it’s also about how much of the virus is circulating in the community, which affects the likelihood that students and staff will bring COVID-19 into their classrooms.”
Damian Roland, consultant and honorary associate professor in pediatric emergency medicine at the University of Leicester (England), commented: “Maximizing educational potential while reducing the spread of COVID19 is a challenge laden with scientific equipoise while simultaneously infused with emotion. The evidence of transmission between, and infectivity from, children is not complete, as this debate has demonstrated. It is important scientists, clinicians, educators, and policy makers make collaborative decisions, aware there is not one perfect answer, and willing to understand and incorporate others views and objectives rather than holding onto single beliefs or approaches.”
No financial conflicts of interest were declared.
Countries across the world are in the process of closing and reopening schools to contain the spread of COVID-19. Should there be universal testing and quarantining of sick school children and their classmates?
In a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Andreea M. Panciu, MD, from the National Institute of Infectious Diseases in Bucharest, argued for routine testing and quarantining of all school children. Her opposite number, Danilo Buonsenso, MD, from the Centre for Global Health Research and Studies, Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico, Rome, made the case for a more selective approach.
Should children be sent to school?
stated Dr. Panciu as she started the debate by explaining the challenges faced by schools in adhering to key mitigation strategies. The U.S. Centers for Disease Control and Prevention recommends that students keep 1.8 m (6 feet) distance from one another. “In many school settings this is not feasible without drastically limiting the number of students,” she explained. “This is a massive challenge for many schools that are already overcrowded.”
The use of facemasks also is a challenge in classrooms. Children have a lower tolerance or may not be able to use the mask properly. There also are concerns regarding impaired learning, speech development, social development, and facial recognition. “We need to look at the evidence; preventive measures work,” responded Dr. Buonsenso. If distance can be implemented, the more distance the lower the transmission of infection, with 1.5-2 meters having the best effects. “Distance can be difficult when school buildings do not allow it, however, governments have had time to plan, and this should not be a limitation to education for kids.”
A recent review clearly showed that children and adolescents aged under 20 years have a much lower risk of susceptibility to COVID-19 infection, compared with adults. This is especially the case for children younger than 14 years. “There is no excuse, let’s bring the children back to school,” argued Dr. Buonsenso.
Dr. Panciu responded with several studies that have tried to quantify the amount of SARS-CoV-2 virus that is carried by infected children. Viral load in the nasopharynx in children under 5 years with mild to moderate COVID-19 symptoms was higher than that of both children over 5 as well as adults. The viral load in young children did not seem to differ by age or symptom severity. “There doesn’t appear to be a significant difference in viral load between symptomatic children and symptomatic adults,” she stated.
“But the question is: ‘How infectious are children?’ ” reacted Dr. Buonsenso. Data from South Korea showed that, for children, particularly those under 10 years, the number of secondary cases of contacts was very low, suggesting that children are rarely spreading the virus.
Dr. Buonsenso and colleagues assessed 30 households containing children aged under 18 years where an adult had been infected with COVID-19 in Rome during the peak of the pandemic. In no cases was it found that a child was the index case. This was supported by data from China, also obtained during the peak of the pandemic, which showed that the number of children infected was very low, but more importantly the number of secondary attacks from contact with children was also very low.
What about children who are sick at school?
The debate moved to discussing what should be done when a child is sick at school. Dr. Panciu clarified recommendations by the CDC regarding what steps to take if a student displays signs of infection consistent with COVID-19: Should they test positive, they are to stay at home for 10 days from the time signs and symptoms first appeared. Further, any teachers or students identified as close contacts are advised to stay at home for 14 days. (Since the ESPID meeting, the CDC has made changes in quarantine times for COVID-19. People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.)
A significant problem is the overlap between COVID-19 symptoms and those associated with other common illnesses because of a range of viruses. This is particularly true in younger children who often suffer from viral infections. “It is common for children to have up to eight respiratory illnesses a year,” explained Dr. Panciu, “and some may have symptoms so mild that they don’t notice them.”
“We need to be a little bit more children focused, otherwise we are going to be isolating children all the time,” said Dr. Buonsenso. The Royal College of Paediatrics and Child Health state that a child with a simple runny nose or sporadic cough without a fever, who would have attended school in other times, should not be tested for COVID-19. He moved on to then cite several studies that show little or no evidence of COVID-19 transmission between school children. This included a prospective cohort study in Australia showing that child-to-child transmission occurred in 0.3%. “To date, the advantages from routine quarantine and over testing seem too low to balance the social consequences on children and families,” he concluded.
As the debate drew to a close, Dr. Panciu reported several studies that did demonstrate transmission between school-age children. Data from an overnight camp in Georgia where the median age was 12 years showed the attack rate was 44% for ages 11-17 years and 51% for ages 6-10 years. Similar conclusions were reached in an Israeli study looking at a large COVID-19 outbreak in a school. This occurred 10 days after reopening, in spite of preventive measures being in place. “Opening safely isn’t just about the adjustments a school makes,” she said, “it’s also about how much of the virus is circulating in the community, which affects the likelihood that students and staff will bring COVID-19 into their classrooms.”
Damian Roland, consultant and honorary associate professor in pediatric emergency medicine at the University of Leicester (England), commented: “Maximizing educational potential while reducing the spread of COVID19 is a challenge laden with scientific equipoise while simultaneously infused with emotion. The evidence of transmission between, and infectivity from, children is not complete, as this debate has demonstrated. It is important scientists, clinicians, educators, and policy makers make collaborative decisions, aware there is not one perfect answer, and willing to understand and incorporate others views and objectives rather than holding onto single beliefs or approaches.”
No financial conflicts of interest were declared.
Countries across the world are in the process of closing and reopening schools to contain the spread of COVID-19. Should there be universal testing and quarantining of sick school children and their classmates?
In a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Andreea M. Panciu, MD, from the National Institute of Infectious Diseases in Bucharest, argued for routine testing and quarantining of all school children. Her opposite number, Danilo Buonsenso, MD, from the Centre for Global Health Research and Studies, Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico, Rome, made the case for a more selective approach.
Should children be sent to school?
stated Dr. Panciu as she started the debate by explaining the challenges faced by schools in adhering to key mitigation strategies. The U.S. Centers for Disease Control and Prevention recommends that students keep 1.8 m (6 feet) distance from one another. “In many school settings this is not feasible without drastically limiting the number of students,” she explained. “This is a massive challenge for many schools that are already overcrowded.”
The use of facemasks also is a challenge in classrooms. Children have a lower tolerance or may not be able to use the mask properly. There also are concerns regarding impaired learning, speech development, social development, and facial recognition. “We need to look at the evidence; preventive measures work,” responded Dr. Buonsenso. If distance can be implemented, the more distance the lower the transmission of infection, with 1.5-2 meters having the best effects. “Distance can be difficult when school buildings do not allow it, however, governments have had time to plan, and this should not be a limitation to education for kids.”
A recent review clearly showed that children and adolescents aged under 20 years have a much lower risk of susceptibility to COVID-19 infection, compared with adults. This is especially the case for children younger than 14 years. “There is no excuse, let’s bring the children back to school,” argued Dr. Buonsenso.
Dr. Panciu responded with several studies that have tried to quantify the amount of SARS-CoV-2 virus that is carried by infected children. Viral load in the nasopharynx in children under 5 years with mild to moderate COVID-19 symptoms was higher than that of both children over 5 as well as adults. The viral load in young children did not seem to differ by age or symptom severity. “There doesn’t appear to be a significant difference in viral load between symptomatic children and symptomatic adults,” she stated.
“But the question is: ‘How infectious are children?’ ” reacted Dr. Buonsenso. Data from South Korea showed that, for children, particularly those under 10 years, the number of secondary cases of contacts was very low, suggesting that children are rarely spreading the virus.
Dr. Buonsenso and colleagues assessed 30 households containing children aged under 18 years where an adult had been infected with COVID-19 in Rome during the peak of the pandemic. In no cases was it found that a child was the index case. This was supported by data from China, also obtained during the peak of the pandemic, which showed that the number of children infected was very low, but more importantly the number of secondary attacks from contact with children was also very low.
What about children who are sick at school?
The debate moved to discussing what should be done when a child is sick at school. Dr. Panciu clarified recommendations by the CDC regarding what steps to take if a student displays signs of infection consistent with COVID-19: Should they test positive, they are to stay at home for 10 days from the time signs and symptoms first appeared. Further, any teachers or students identified as close contacts are advised to stay at home for 14 days. (Since the ESPID meeting, the CDC has made changes in quarantine times for COVID-19. People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.)
A significant problem is the overlap between COVID-19 symptoms and those associated with other common illnesses because of a range of viruses. This is particularly true in younger children who often suffer from viral infections. “It is common for children to have up to eight respiratory illnesses a year,” explained Dr. Panciu, “and some may have symptoms so mild that they don’t notice them.”
“We need to be a little bit more children focused, otherwise we are going to be isolating children all the time,” said Dr. Buonsenso. The Royal College of Paediatrics and Child Health state that a child with a simple runny nose or sporadic cough without a fever, who would have attended school in other times, should not be tested for COVID-19. He moved on to then cite several studies that show little or no evidence of COVID-19 transmission between school children. This included a prospective cohort study in Australia showing that child-to-child transmission occurred in 0.3%. “To date, the advantages from routine quarantine and over testing seem too low to balance the social consequences on children and families,” he concluded.
As the debate drew to a close, Dr. Panciu reported several studies that did demonstrate transmission between school-age children. Data from an overnight camp in Georgia where the median age was 12 years showed the attack rate was 44% for ages 11-17 years and 51% for ages 6-10 years. Similar conclusions were reached in an Israeli study looking at a large COVID-19 outbreak in a school. This occurred 10 days after reopening, in spite of preventive measures being in place. “Opening safely isn’t just about the adjustments a school makes,” she said, “it’s also about how much of the virus is circulating in the community, which affects the likelihood that students and staff will bring COVID-19 into their classrooms.”
Damian Roland, consultant and honorary associate professor in pediatric emergency medicine at the University of Leicester (England), commented: “Maximizing educational potential while reducing the spread of COVID19 is a challenge laden with scientific equipoise while simultaneously infused with emotion. The evidence of transmission between, and infectivity from, children is not complete, as this debate has demonstrated. It is important scientists, clinicians, educators, and policy makers make collaborative decisions, aware there is not one perfect answer, and willing to understand and incorporate others views and objectives rather than holding onto single beliefs or approaches.”
No financial conflicts of interest were declared.
FROM ESPID 2020