User login
When is MRI useful in the management of congenital melanocytic nevi?
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
FROM SPD 2021
Recent trend: Melanoma mortality declining rapidly
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.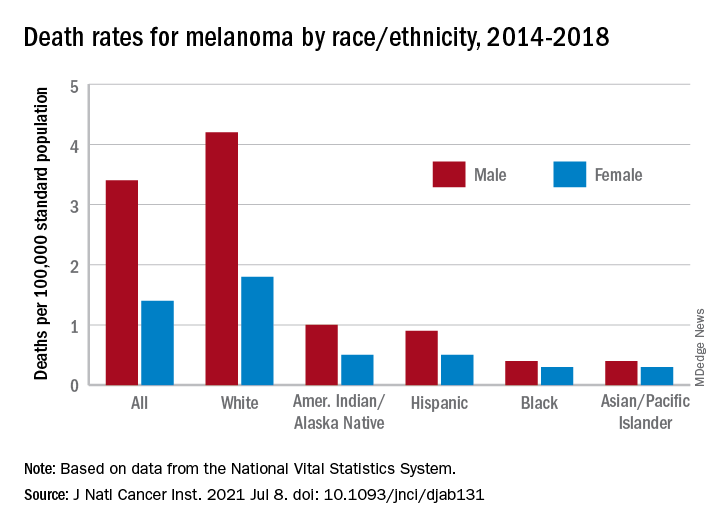
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Stop using Neutrogena and Aveeno spray sunscreen, J&J warns
Benzene is not an ingredient of sunscreen, and should not be present in these products. The levels detected were low and would not be expected to have an adverse effect on health, but the company says it is recalling the products anyway “out of an abundance of caution.”
The sunscreen products that have been recalled are:
- NEUTROGENA® Beach Defense® aerosol sunscreen.
- NEUTROGENA® Cool Dry Sport aerosol sunscreen.
- NEUTROGENA® Invisible Daily™ defense aerosol sunscreen.
- NEUTROGENA® Ultra Sheer® aerosol sunscreen.
- AVEENO® Protect + Refresh aerosol sunscreen.
These products were distributed nationwide through a variety of retail stores. Consumers should stop using these products and throw them away, the company said.
At the same time, it emphasized the importance of using alternative sunscreen products to protect the skin from excessive sun exposure, which can lead to skin cancer including melanoma.
Johnson & Johnson has launched an investigation into how benzene got into these products.
One of the company’s other spray sunscreen products, Neutrogena Wet Skin, was not included in the recall.
Recently, benzene was found in 78 widely-used sunscreen products in tests conducted by the online pharmacy and laboratory Valisure. Most of the products were aerosol sprays, and the company called on the Food and Drug Administration to recall them all.
That petition suggested that the finding of benzene was the result of contamination somewhere in the manufacturing process.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University. “We don’t want those things to be blurred.”
There is a risk that people take away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview.
He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, he said.
A version of this article first appeared on WebMD.com.
Benzene is not an ingredient of sunscreen, and should not be present in these products. The levels detected were low and would not be expected to have an adverse effect on health, but the company says it is recalling the products anyway “out of an abundance of caution.”
The sunscreen products that have been recalled are:
- NEUTROGENA® Beach Defense® aerosol sunscreen.
- NEUTROGENA® Cool Dry Sport aerosol sunscreen.
- NEUTROGENA® Invisible Daily™ defense aerosol sunscreen.
- NEUTROGENA® Ultra Sheer® aerosol sunscreen.
- AVEENO® Protect + Refresh aerosol sunscreen.
These products were distributed nationwide through a variety of retail stores. Consumers should stop using these products and throw them away, the company said.
At the same time, it emphasized the importance of using alternative sunscreen products to protect the skin from excessive sun exposure, which can lead to skin cancer including melanoma.
Johnson & Johnson has launched an investigation into how benzene got into these products.
One of the company’s other spray sunscreen products, Neutrogena Wet Skin, was not included in the recall.
Recently, benzene was found in 78 widely-used sunscreen products in tests conducted by the online pharmacy and laboratory Valisure. Most of the products were aerosol sprays, and the company called on the Food and Drug Administration to recall them all.
That petition suggested that the finding of benzene was the result of contamination somewhere in the manufacturing process.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University. “We don’t want those things to be blurred.”
There is a risk that people take away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview.
He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, he said.
A version of this article first appeared on WebMD.com.
Benzene is not an ingredient of sunscreen, and should not be present in these products. The levels detected were low and would not be expected to have an adverse effect on health, but the company says it is recalling the products anyway “out of an abundance of caution.”
The sunscreen products that have been recalled are:
- NEUTROGENA® Beach Defense® aerosol sunscreen.
- NEUTROGENA® Cool Dry Sport aerosol sunscreen.
- NEUTROGENA® Invisible Daily™ defense aerosol sunscreen.
- NEUTROGENA® Ultra Sheer® aerosol sunscreen.
- AVEENO® Protect + Refresh aerosol sunscreen.
These products were distributed nationwide through a variety of retail stores. Consumers should stop using these products and throw them away, the company said.
At the same time, it emphasized the importance of using alternative sunscreen products to protect the skin from excessive sun exposure, which can lead to skin cancer including melanoma.
Johnson & Johnson has launched an investigation into how benzene got into these products.
One of the company’s other spray sunscreen products, Neutrogena Wet Skin, was not included in the recall.
Recently, benzene was found in 78 widely-used sunscreen products in tests conducted by the online pharmacy and laboratory Valisure. Most of the products were aerosol sprays, and the company called on the Food and Drug Administration to recall them all.
That petition suggested that the finding of benzene was the result of contamination somewhere in the manufacturing process.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University. “We don’t want those things to be blurred.”
There is a risk that people take away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview.
He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, he said.
A version of this article first appeared on WebMD.com.
Cancer mortality continues to drop in females as breast cancer reversal looms
Overall cancer mortality in females continues to decrease in the United States, but “previous declining trends in death rates slowed” for breast cancer in recent years, according to an annual report by several national organizations.
The analysis of long-term trends in cancer death rates shows that a decline of 1.4% per year from 2001 to 2016 accelerated to 2.1% per year in 2016-2018, the American Cancer Society, Centers for Disease Control and Prevention, National Cancer Institute, and the North American Association of Central Cancer Registries said.
Decreases in overall cancer mortality were seen in females of all races and ethnic groups over the most recent 5-year period included in the report, 2014-2018, varying from –1.6% per year in both non-Hispanic Blacks and Whites to –0.9% for non-Hispanic American Indians/Alaska Natives (AI/ANs), Farhad Islami, MD, PhD, of the American Cancer Society, Atlanta, and associates said in the Journal of the National Cancer Institute.
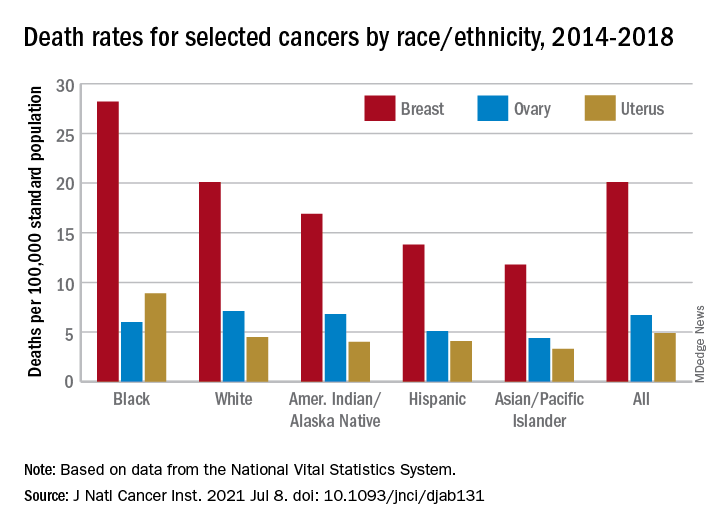
Over those 5 years, death rates fell for 14 of the 20 most common cancers in females; increased for liver, uterus, brain, pancreas, and soft tissue including heart; and remained stable for cancers of the oral cavity/pharynx, they reported.
Breast cancer was among those that declined, but the rate of that decline has been slowing. Mortality declined by an average of 2.3% per year in 2003-2007, by 1.6% a year in 2007-2014, and by just 1.0% annually during 2014-2018, based on data from the National Center for Health Statistics’ National Vital Statistics System.
Mortality from all cancers in 2014-2018 was 133.5 deaths per 100,000 standard population, with the racial/ethnic gap ranging from 85.4 per 100,000 (non-Hispanic Asian/Pacific Islander) to 154.9 (non-Hispanic Black), Dr. Islami and associates said.
Melanoma had the largest decline in mortality over that period among the 20 most common cancers in females, falling by an average of 4.4% per year, with lung cancer next at 4.3%. Among those with increased death rates, uterine cancer saw the largest rise at 2.0% a year, the research team said.
The deaths caused by cancer of the uterus were most common in non-Hispanic Black females, 8.9 per 100,000 population, followed by non-Hispanic White (4.5), Hispanic (4.1), non-Hispanic AI/AN (4.0), and non-Hispanic Asian/Pacific Islander (3.3), they reported.
“Long-term increasing trends in uterine cancer death rates parallel trends in incidence, although death rates are increasing at a somewhat faster rate. Increasing uterine cancer incidence has been attributed to increasing obesity prevalence and decreased use of combined hormone replacement therapy,” Dr. Islami and associates pointed out.
Breast cancer deaths also were most common among Blacks in 2014-2018, occurring at a rate of 28.2 per 100,000, as were deaths from cancer of the cervix (3.4 per 100,000), while ovarian cancers deaths were highest in White females (7.1 per 100,000), the researchers noted.
The continuing racial and ethnic disparity “largely reflects a combination of multiple intertwined factors” of tumor biology, diagnosis, treatment, and systemic discrimination, they wrote, adding that Black persons “are more likely to have a higher exposure to some cancer risk factors and limited access to healthy food, safe places for physical activity, and evidence-based cancer preventive services.”
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
Overall cancer mortality in females continues to decrease in the United States, but “previous declining trends in death rates slowed” for breast cancer in recent years, according to an annual report by several national organizations.

The analysis of long-term trends in cancer death rates shows that a decline of 1.4% per year from 2001 to 2016 accelerated to 2.1% per year in 2016-2018, the American Cancer Society, Centers for Disease Control and Prevention, National Cancer Institute, and the North American Association of Central Cancer Registries said.
Decreases in overall cancer mortality were seen in females of all races and ethnic groups over the most recent 5-year period included in the report, 2014-2018, varying from –1.6% per year in both non-Hispanic Blacks and Whites to –0.9% for non-Hispanic American Indians/Alaska Natives (AI/ANs), Farhad Islami, MD, PhD, of the American Cancer Society, Atlanta, and associates said in the Journal of the National Cancer Institute.
Over those 5 years, death rates fell for 14 of the 20 most common cancers in females; increased for liver, uterus, brain, pancreas, and soft tissue including heart; and remained stable for cancers of the oral cavity/pharynx, they reported.
Breast cancer was among those that declined, but the rate of that decline has been slowing. Mortality declined by an average of 2.3% per year in 2003-2007, by 1.6% a year in 2007-2014, and by just 1.0% annually during 2014-2018, based on data from the National Center for Health Statistics’ National Vital Statistics System.
Mortality from all cancers in 2014-2018 was 133.5 deaths per 100,000 standard population, with the racial/ethnic gap ranging from 85.4 per 100,000 (non-Hispanic Asian/Pacific Islander) to 154.9 (non-Hispanic Black), Dr. Islami and associates said.
Melanoma had the largest decline in mortality over that period among the 20 most common cancers in females, falling by an average of 4.4% per year, with lung cancer next at 4.3%. Among those with increased death rates, uterine cancer saw the largest rise at 2.0% a year, the research team said.
The deaths caused by cancer of the uterus were most common in non-Hispanic Black females, 8.9 per 100,000 population, followed by non-Hispanic White (4.5), Hispanic (4.1), non-Hispanic AI/AN (4.0), and non-Hispanic Asian/Pacific Islander (3.3), they reported.
“Long-term increasing trends in uterine cancer death rates parallel trends in incidence, although death rates are increasing at a somewhat faster rate. Increasing uterine cancer incidence has been attributed to increasing obesity prevalence and decreased use of combined hormone replacement therapy,” Dr. Islami and associates pointed out.
Breast cancer deaths also were most common among Blacks in 2014-2018, occurring at a rate of 28.2 per 100,000, as were deaths from cancer of the cervix (3.4 per 100,000), while ovarian cancers deaths were highest in White females (7.1 per 100,000), the researchers noted.
The continuing racial and ethnic disparity “largely reflects a combination of multiple intertwined factors” of tumor biology, diagnosis, treatment, and systemic discrimination, they wrote, adding that Black persons “are more likely to have a higher exposure to some cancer risk factors and limited access to healthy food, safe places for physical activity, and evidence-based cancer preventive services.”
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
Overall cancer mortality in females continues to decrease in the United States, but “previous declining trends in death rates slowed” for breast cancer in recent years, according to an annual report by several national organizations.

The analysis of long-term trends in cancer death rates shows that a decline of 1.4% per year from 2001 to 2016 accelerated to 2.1% per year in 2016-2018, the American Cancer Society, Centers for Disease Control and Prevention, National Cancer Institute, and the North American Association of Central Cancer Registries said.
Decreases in overall cancer mortality were seen in females of all races and ethnic groups over the most recent 5-year period included in the report, 2014-2018, varying from –1.6% per year in both non-Hispanic Blacks and Whites to –0.9% for non-Hispanic American Indians/Alaska Natives (AI/ANs), Farhad Islami, MD, PhD, of the American Cancer Society, Atlanta, and associates said in the Journal of the National Cancer Institute.
Over those 5 years, death rates fell for 14 of the 20 most common cancers in females; increased for liver, uterus, brain, pancreas, and soft tissue including heart; and remained stable for cancers of the oral cavity/pharynx, they reported.
Breast cancer was among those that declined, but the rate of that decline has been slowing. Mortality declined by an average of 2.3% per year in 2003-2007, by 1.6% a year in 2007-2014, and by just 1.0% annually during 2014-2018, based on data from the National Center for Health Statistics’ National Vital Statistics System.
Mortality from all cancers in 2014-2018 was 133.5 deaths per 100,000 standard population, with the racial/ethnic gap ranging from 85.4 per 100,000 (non-Hispanic Asian/Pacific Islander) to 154.9 (non-Hispanic Black), Dr. Islami and associates said.
Melanoma had the largest decline in mortality over that period among the 20 most common cancers in females, falling by an average of 4.4% per year, with lung cancer next at 4.3%. Among those with increased death rates, uterine cancer saw the largest rise at 2.0% a year, the research team said.
The deaths caused by cancer of the uterus were most common in non-Hispanic Black females, 8.9 per 100,000 population, followed by non-Hispanic White (4.5), Hispanic (4.1), non-Hispanic AI/AN (4.0), and non-Hispanic Asian/Pacific Islander (3.3), they reported.
“Long-term increasing trends in uterine cancer death rates parallel trends in incidence, although death rates are increasing at a somewhat faster rate. Increasing uterine cancer incidence has been attributed to increasing obesity prevalence and decreased use of combined hormone replacement therapy,” Dr. Islami and associates pointed out.
Breast cancer deaths also were most common among Blacks in 2014-2018, occurring at a rate of 28.2 per 100,000, as were deaths from cancer of the cervix (3.4 per 100,000), while ovarian cancers deaths were highest in White females (7.1 per 100,000), the researchers noted.
The continuing racial and ethnic disparity “largely reflects a combination of multiple intertwined factors” of tumor biology, diagnosis, treatment, and systemic discrimination, they wrote, adding that Black persons “are more likely to have a higher exposure to some cancer risk factors and limited access to healthy food, safe places for physical activity, and evidence-based cancer preventive services.”
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Indoor tanning ICD-10 codes may be underused, study finds
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
FROM SID 2021
Rate of cutaneous toxicities from ICIs may be lower than previously reported
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
FROM SID 2021
Americans’ sun protection practices fall short of intentions
commissioned by the American Academy of Dermatology.
With the pandemic seemingly behind it, the United States enters the summer months facing the paradox of sun protection. Four out of five adults know that sunscreen should be reapplied every 2 hours when they’re outdoors, but only one in three make the actual effort, and 77% are likely to use sunscreen at the beach or a pool, compared with 41% when they’re gardening or working outside on their homes, the AAD reported.
“These findings are surprising and seem to suggest that many people do not take skin cancer seriously or perhaps believe skin cancer won’t happen to them,” Robert T. Brodell, MD, professor of dermatology at the University of Mississippi Medical Center, Jackson, said in a written statement from the AAD, adding that “unprotected exposure to ultraviolet rays is the most preventable risk factor for skin cancer, including melanoma.”
A quarter of all survey respondents reported getting sunburned in 2020, with the youngest adults most likely to feel the wrath of the sun. Sunburn was reported by 43% of those aged 18-23 years, 37% of those aged 24-39, 25% of the 40- to 55-year-olds, 12% of the 56- to 74-year-olds, and 7% of those aged 75 and older. More than a quarter of those who got sunburned said that it was bad enough to make their clothes feel uncomfortable, the academy said.
“Americans see the damaging effects of the sun on their skin as they get older, and two out of three look back and wish they had been more careful. But when it comes to cancer, specifically, most feel unconcerned in spite of their own risk,” according to a statement from Versta Research, which conducted the poll on behalf of the AAD. The survey was conducted from Feb. 22 to March 10, 2021, and involved 1,056 respondents, with a ±3% margin of error.
The lack of concern for skin cancer looks like this: More than two-thirds of the respondents (69%) have at least one possible risk factor – lighter skin tone, blue or green eyes, more than 50 moles, family history – but only 36% expressed concern about developing it. “Indeed, half of survey respondents (49%) say they are more worried about avoiding sunburn than they are about preventing skin cancer, and a third (32%) are more worried about avoiding premature wrinkles than they are about preventing cancer,” the AAD said.
The AAD is considering the creation of a social media quiz or interactive tool, and if the results of this survey were recast as a potential “Knowledge and Awareness Quiz” and graded with a traditional scheme (A = 90%-100%, B = 80%-89%, etc.), then 34% of the respondents would have failed, 15% would have gotten a D, and only 5% would have earned an A, the academy noted.
commissioned by the American Academy of Dermatology.

With the pandemic seemingly behind it, the United States enters the summer months facing the paradox of sun protection. Four out of five adults know that sunscreen should be reapplied every 2 hours when they’re outdoors, but only one in three make the actual effort, and 77% are likely to use sunscreen at the beach or a pool, compared with 41% when they’re gardening or working outside on their homes, the AAD reported.
“These findings are surprising and seem to suggest that many people do not take skin cancer seriously or perhaps believe skin cancer won’t happen to them,” Robert T. Brodell, MD, professor of dermatology at the University of Mississippi Medical Center, Jackson, said in a written statement from the AAD, adding that “unprotected exposure to ultraviolet rays is the most preventable risk factor for skin cancer, including melanoma.”
A quarter of all survey respondents reported getting sunburned in 2020, with the youngest adults most likely to feel the wrath of the sun. Sunburn was reported by 43% of those aged 18-23 years, 37% of those aged 24-39, 25% of the 40- to 55-year-olds, 12% of the 56- to 74-year-olds, and 7% of those aged 75 and older. More than a quarter of those who got sunburned said that it was bad enough to make their clothes feel uncomfortable, the academy said.
“Americans see the damaging effects of the sun on their skin as they get older, and two out of three look back and wish they had been more careful. But when it comes to cancer, specifically, most feel unconcerned in spite of their own risk,” according to a statement from Versta Research, which conducted the poll on behalf of the AAD. The survey was conducted from Feb. 22 to March 10, 2021, and involved 1,056 respondents, with a ±3% margin of error.
The lack of concern for skin cancer looks like this: More than two-thirds of the respondents (69%) have at least one possible risk factor – lighter skin tone, blue or green eyes, more than 50 moles, family history – but only 36% expressed concern about developing it. “Indeed, half of survey respondents (49%) say they are more worried about avoiding sunburn than they are about preventing skin cancer, and a third (32%) are more worried about avoiding premature wrinkles than they are about preventing cancer,” the AAD said.
The AAD is considering the creation of a social media quiz or interactive tool, and if the results of this survey were recast as a potential “Knowledge and Awareness Quiz” and graded with a traditional scheme (A = 90%-100%, B = 80%-89%, etc.), then 34% of the respondents would have failed, 15% would have gotten a D, and only 5% would have earned an A, the academy noted.
commissioned by the American Academy of Dermatology.

With the pandemic seemingly behind it, the United States enters the summer months facing the paradox of sun protection. Four out of five adults know that sunscreen should be reapplied every 2 hours when they’re outdoors, but only one in three make the actual effort, and 77% are likely to use sunscreen at the beach or a pool, compared with 41% when they’re gardening or working outside on their homes, the AAD reported.
“These findings are surprising and seem to suggest that many people do not take skin cancer seriously or perhaps believe skin cancer won’t happen to them,” Robert T. Brodell, MD, professor of dermatology at the University of Mississippi Medical Center, Jackson, said in a written statement from the AAD, adding that “unprotected exposure to ultraviolet rays is the most preventable risk factor for skin cancer, including melanoma.”
A quarter of all survey respondents reported getting sunburned in 2020, with the youngest adults most likely to feel the wrath of the sun. Sunburn was reported by 43% of those aged 18-23 years, 37% of those aged 24-39, 25% of the 40- to 55-year-olds, 12% of the 56- to 74-year-olds, and 7% of those aged 75 and older. More than a quarter of those who got sunburned said that it was bad enough to make their clothes feel uncomfortable, the academy said.
“Americans see the damaging effects of the sun on their skin as they get older, and two out of three look back and wish they had been more careful. But when it comes to cancer, specifically, most feel unconcerned in spite of their own risk,” according to a statement from Versta Research, which conducted the poll on behalf of the AAD. The survey was conducted from Feb. 22 to March 10, 2021, and involved 1,056 respondents, with a ±3% margin of error.
The lack of concern for skin cancer looks like this: More than two-thirds of the respondents (69%) have at least one possible risk factor – lighter skin tone, blue or green eyes, more than 50 moles, family history – but only 36% expressed concern about developing it. “Indeed, half of survey respondents (49%) say they are more worried about avoiding sunburn than they are about preventing skin cancer, and a third (32%) are more worried about avoiding premature wrinkles than they are about preventing cancer,” the AAD said.
The AAD is considering the creation of a social media quiz or interactive tool, and if the results of this survey were recast as a potential “Knowledge and Awareness Quiz” and graded with a traditional scheme (A = 90%-100%, B = 80%-89%, etc.), then 34% of the respondents would have failed, 15% would have gotten a D, and only 5% would have earned an A, the academy noted.
Nivolumab-Induced Granuloma Annulare
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.
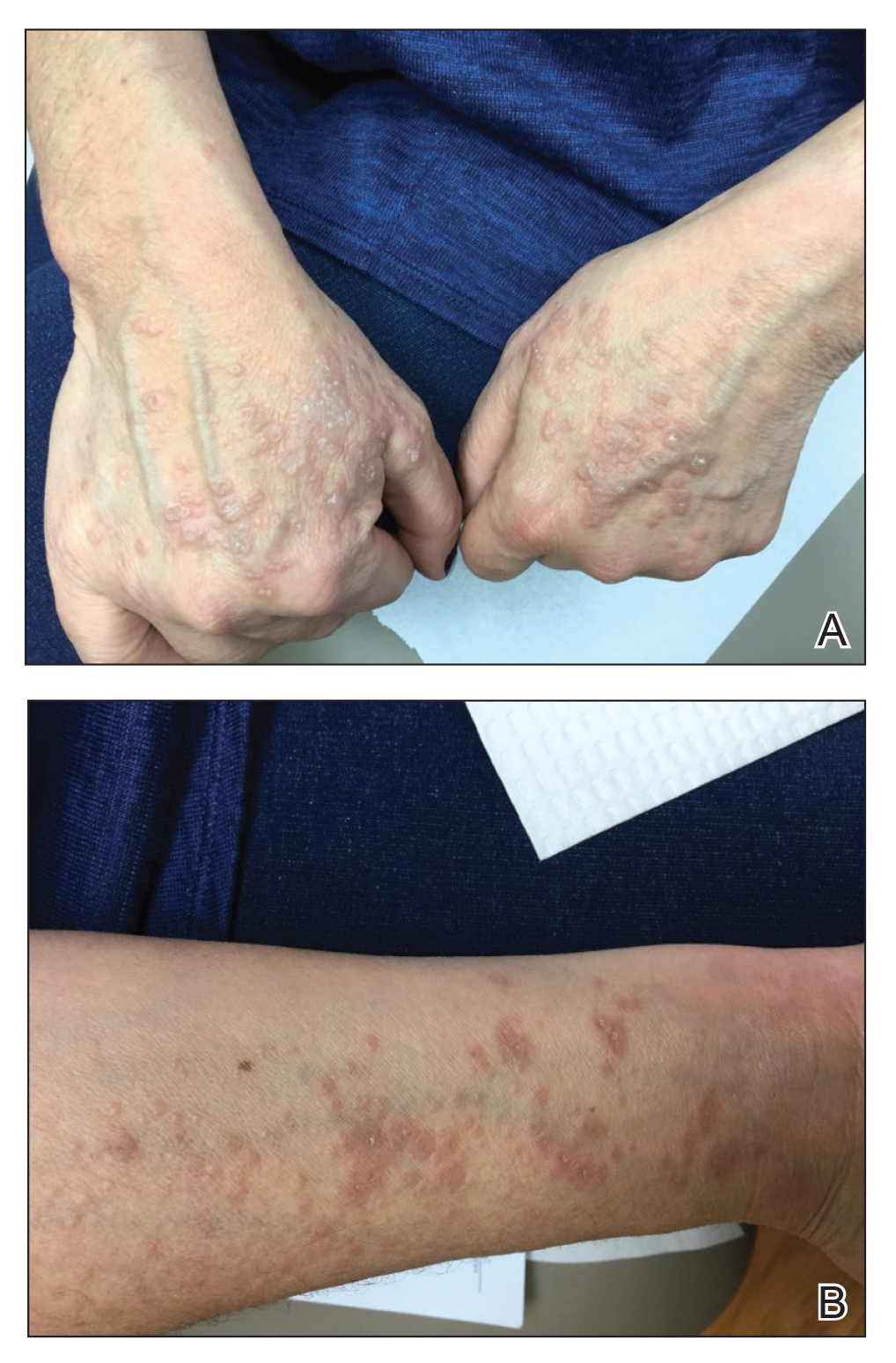
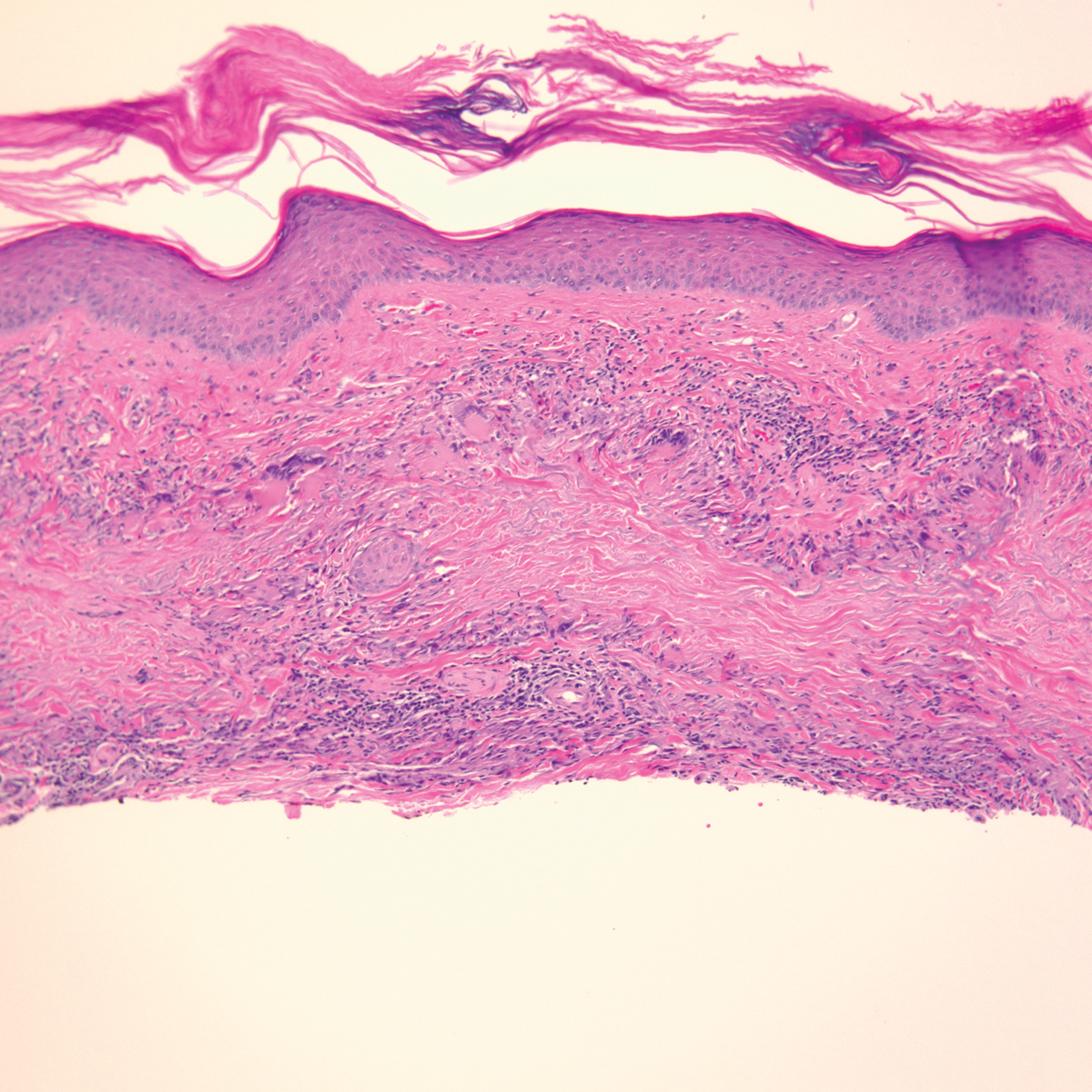
A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.


A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.


A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
Practice Points
- Immune-related adverse events (irAEs) frequently occur in patients on immunotherapy, with the skin representing the most common site of involvement.
- Although rare, granulomatous reactions such as granuloma annulare increasingly are recognized as potential irAEs.
- Clinicians should be aware of this novel association to accurately diagnose and effectively treat adverse reactions in patients receiving immunotherapy.
Benzene was found in some sunscreens. Now what?
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Study findings support consideration of second biopsy for transected melanomas
in a review of cases at the university.
Had their true Breslow depths been known before definitive surgery, sentinel lymph node (SLN) biopsies and wider surgical margins would likely have been recommended.
The findings led the investigators to conclude that a second biopsy should be considered when the first one is transected to ensure surgical and other management decisions are based on an accurate Breslow depth.
A second biopsy is especially warranted for broadly transected biopsies and transected T1a tumors with gross residual tumor or pigment on preoperative exam; both scenarios significantly increased the risk of up-staging in the study, according to lead investigator James Duncan, MD, a Mohs surgery and dermatologic oncology fellow at the University of Alabama at Birmingham, who presented the findings at the annual meeting of the American College of Mohs Surgery.
“Accurate staging of malignancies, especially melanoma, is critical to determine prognosis and the best treatment approach,” said Vishal Patel, MD, director of cutaneous oncology at George Washington University, Washington, when asked for comment.
“This study identifies how transected biopsies can underestimate a melanoma’s true depth and thus impact treatment and outcomes. The authors highlight that when a biopsy is transected, or there is notable pigment at the base, attempts should be taken to sample the remaining tumor prior to surgery so the accurate tumor depth can be determined and treatment options be fully discussed with the patient,” Dr. Patel said.
The Birmingham team reviewed invasive melanoma cases at their university from 2017 to 2019.
Almost half (49.6%) of the 726 melanomas they identified were transected on biopsy, which is in line with prior reports. About 60% of the patients were men and 98% were White; the average age was 63 years.
Of the 360 transected tumors, 49 (13.6%) had up-staging at final excision that “would have prompted discussion of alternate surgical treatment such as SLN biopsy or wider surgical margins,” the team said.
Of the 89 transected pT1a melanomas identified, 47.1% with gross residual tumor or pigment on preoperative physical examination were up-staged following excision versus 6.9% with no remaining pigment or tumor prior to surgery (P < .01).
Broadly transected tumors were up-staged in 21.7% of cases versus 4.9% of focally transected tumors (P = .038). The average increase in Breslow depth for broadly transected tumors was 1.03 mm versus 0.03 mm for focally transected lesions (P = .04).
Shave biopsies, ulceration, and lack of concern for melanoma at the initial biopsy were among the factors associated with a higher risk of transection.
Superficial spreading melanoma was the most common subtype. Tumors were evenly distributed between the head, neck, and extremities. The average Breslow depth was 1.51 mm, and the majority of tumors were pT1a or pT2a.
The review excluded melanoma in situ, recurrences, metastases, noncutaneous melanomas, and biopsies where deep margin status was unknown.
There was no funding for the study, and Dr. Duncan and Dr. Patel had no relevant disclosures.
in a review of cases at the university.
Had their true Breslow depths been known before definitive surgery, sentinel lymph node (SLN) biopsies and wider surgical margins would likely have been recommended.
The findings led the investigators to conclude that a second biopsy should be considered when the first one is transected to ensure surgical and other management decisions are based on an accurate Breslow depth.
A second biopsy is especially warranted for broadly transected biopsies and transected T1a tumors with gross residual tumor or pigment on preoperative exam; both scenarios significantly increased the risk of up-staging in the study, according to lead investigator James Duncan, MD, a Mohs surgery and dermatologic oncology fellow at the University of Alabama at Birmingham, who presented the findings at the annual meeting of the American College of Mohs Surgery.
“Accurate staging of malignancies, especially melanoma, is critical to determine prognosis and the best treatment approach,” said Vishal Patel, MD, director of cutaneous oncology at George Washington University, Washington, when asked for comment.
“This study identifies how transected biopsies can underestimate a melanoma’s true depth and thus impact treatment and outcomes. The authors highlight that when a biopsy is transected, or there is notable pigment at the base, attempts should be taken to sample the remaining tumor prior to surgery so the accurate tumor depth can be determined and treatment options be fully discussed with the patient,” Dr. Patel said.
The Birmingham team reviewed invasive melanoma cases at their university from 2017 to 2019.
Almost half (49.6%) of the 726 melanomas they identified were transected on biopsy, which is in line with prior reports. About 60% of the patients were men and 98% were White; the average age was 63 years.
Of the 360 transected tumors, 49 (13.6%) had up-staging at final excision that “would have prompted discussion of alternate surgical treatment such as SLN biopsy or wider surgical margins,” the team said.
Of the 89 transected pT1a melanomas identified, 47.1% with gross residual tumor or pigment on preoperative physical examination were up-staged following excision versus 6.9% with no remaining pigment or tumor prior to surgery (P < .01).
Broadly transected tumors were up-staged in 21.7% of cases versus 4.9% of focally transected tumors (P = .038). The average increase in Breslow depth for broadly transected tumors was 1.03 mm versus 0.03 mm for focally transected lesions (P = .04).
Shave biopsies, ulceration, and lack of concern for melanoma at the initial biopsy were among the factors associated with a higher risk of transection.
Superficial spreading melanoma was the most common subtype. Tumors were evenly distributed between the head, neck, and extremities. The average Breslow depth was 1.51 mm, and the majority of tumors were pT1a or pT2a.
The review excluded melanoma in situ, recurrences, metastases, noncutaneous melanomas, and biopsies where deep margin status was unknown.
There was no funding for the study, and Dr. Duncan and Dr. Patel had no relevant disclosures.
in a review of cases at the university.
Had their true Breslow depths been known before definitive surgery, sentinel lymph node (SLN) biopsies and wider surgical margins would likely have been recommended.
The findings led the investigators to conclude that a second biopsy should be considered when the first one is transected to ensure surgical and other management decisions are based on an accurate Breslow depth.
A second biopsy is especially warranted for broadly transected biopsies and transected T1a tumors with gross residual tumor or pigment on preoperative exam; both scenarios significantly increased the risk of up-staging in the study, according to lead investigator James Duncan, MD, a Mohs surgery and dermatologic oncology fellow at the University of Alabama at Birmingham, who presented the findings at the annual meeting of the American College of Mohs Surgery.
“Accurate staging of malignancies, especially melanoma, is critical to determine prognosis and the best treatment approach,” said Vishal Patel, MD, director of cutaneous oncology at George Washington University, Washington, when asked for comment.
“This study identifies how transected biopsies can underestimate a melanoma’s true depth and thus impact treatment and outcomes. The authors highlight that when a biopsy is transected, or there is notable pigment at the base, attempts should be taken to sample the remaining tumor prior to surgery so the accurate tumor depth can be determined and treatment options be fully discussed with the patient,” Dr. Patel said.
The Birmingham team reviewed invasive melanoma cases at their university from 2017 to 2019.
Almost half (49.6%) of the 726 melanomas they identified were transected on biopsy, which is in line with prior reports. About 60% of the patients were men and 98% were White; the average age was 63 years.
Of the 360 transected tumors, 49 (13.6%) had up-staging at final excision that “would have prompted discussion of alternate surgical treatment such as SLN biopsy or wider surgical margins,” the team said.
Of the 89 transected pT1a melanomas identified, 47.1% with gross residual tumor or pigment on preoperative physical examination were up-staged following excision versus 6.9% with no remaining pigment or tumor prior to surgery (P < .01).
Broadly transected tumors were up-staged in 21.7% of cases versus 4.9% of focally transected tumors (P = .038). The average increase in Breslow depth for broadly transected tumors was 1.03 mm versus 0.03 mm for focally transected lesions (P = .04).
Shave biopsies, ulceration, and lack of concern for melanoma at the initial biopsy were among the factors associated with a higher risk of transection.
Superficial spreading melanoma was the most common subtype. Tumors were evenly distributed between the head, neck, and extremities. The average Breslow depth was 1.51 mm, and the majority of tumors were pT1a or pT2a.
The review excluded melanoma in situ, recurrences, metastases, noncutaneous melanomas, and biopsies where deep margin status was unknown.
There was no funding for the study, and Dr. Duncan and Dr. Patel had no relevant disclosures.
FROM ACMS 2021