User login
The Power of a Multidisciplinary Tumor Board: Managing Unresectable and/or High-Risk Skin Cancers
Multidisciplinary tumor boards are composed of providers from many fields who deliver coordinated care for patients with unresectable and high-risk skin cancers. Providers who comprise the tumor board often are radiation oncologists, hematologists/oncologists, general surgeons, dermatologists, dermatologic surgeons, and pathologists. The benefit of having a tumor board is that each patient is evaluated simultaneously by a group of physicians from various specialties who bring diverse perspectives that will contribute to the overall treatment plan. The cases often encompass high-risk tumors including unresectable basal cell carcinomas or invasive melanomas. By combining knowledge from each specialty in a team approach, the tumor board can effectively and holistically develop a care plan for each patient.
For the tumor board at the Warren Alpert Medical School of Brown University (Providence, Rhode Island), we often prepare a presentation with comprehensive details about the patient and tumor. During the presentation, we also propose a treatment plan prior to describing each patient at the weekly conference and amend the plans during the discussion. Tumor boards also provide a consulting role to the community and hospital providers in which patients are being referred by their primary provider and are seeking a second opinion or guidance.
In many ways, the tumor board is a multidisciplinary approach for patient advocacy in the form of treatment. These physicians meet on a regular basis to check on the patient’s progress and continually reevaluate how to have discussions about the patient’s care. There are many reasons why it is important to refer patients to a multidisciplinary tumor board.
Improved Workup and Diagnosis
One of the values of a tumor board is that it allows for patient data to be collected and assembled in a way that tells a story. The specialist from each field can then discuss and weigh the benefits and risks for each diagnostic test that should be performed for the workup in each patient. Physicians who refer their patients to the tumor board use their recommendations to both confirm the diagnosis and shift their treatment plans, depending on the information presented during the meeting.1 There may be a change in the tumor type, decision to refer for surgery, cancer staging, and list of viable options, especially after reviewing pathology and imaging.2 The discussion of the treatment plan may consider not only surgical considerations but also the patient’s quality of life. At times, noninvasive interventions are more appropriate and align with the patient’s goals of care. In addition, during the tumor board clinic there may be new tumors that are identified and biopsied, providing increased diagnosis and surveillance for patients who may have a higher risk for developing skin cancer.
Education for Residents and Providers
The multidisciplinary tumor board not only helps patients but also educates both residents and providers on the evidence-based therapeutic management of high-risk tumors.2 Research literature on cutaneous oncology is dynamic, and the weekly tumor board meetings help providers stay informed about the best and most effective treatments for their patients.3 In addition to the attending specialists, participants of the tumor board also may include residents, medical students, medical assistance staff, nurses, physician assistants, and fellows. Furthermore, the recommendations given by the tumor board serve to educate both the patient and the provider who referred them to the tumor board. Although we have access to excellent dermatology textbooks as residents, the most impactful educational experience is seeing the patients in tumor board clinic and participating in the immensely educational discussions at the weekly conferences. Through this experience, I have learned that treatment plans should be personalized to the patient. There are many factors to take into consideration when deciphering what the best course of treatment will be for a patient. Sometimes the best option is Mohs micrographic surgery, while other times it may be scheduling several sessions of palliative radiation oncology. Treatment depends on the individual patient and their condition.
Coordination of Care
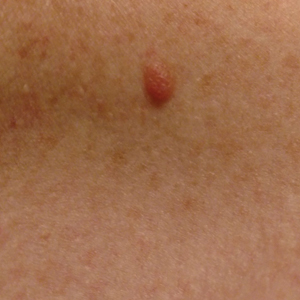
During a week that I was on call, I was consulted to biopsy a patient with a giant hemorrhagic basal cell carcinoma that caused substantial cheek and nose distortion as well as anemia secondary to acute blood loss. The patient not only did not have a dermatologist but also did not have a primary care physician given he had not had contact with the health care system in more than 30 years. The reason for him not seeking care was multifactorial, but the approach to his care became multidisciplinary. We sought to connect him with the right providers to help him in any way that we could. We presented him at our multidisciplinary tumor board and started him on sonedigib, a medication that binds to and inhibits the smoothened protein.4 Through the tumor board, we were able to establish sustained contact with the patient. The tumor board created effective communication between providers to get him the referrals that he needed for dermatology, pathology, radiation oncology, hematology/oncology, and otolaryngology. The discussions centered around being cognizant of the patient’s apprehension with the health care system as well as providing medical and surgical treatment that would help his quality of life. We built a consensus on what the best plan was for the patient and his family. This consensus would have been more difficult had it not been for the combined specialties of the tumor board. In general, studies have shown that weekly tumor boards have resulted in decreased mortality rates for patients with advanced cancers.5
Final Thoughts
The multidisciplinary tumor board is a powerful resource for hospitals and the greater medical community. At these weekly conferences you realize there may still be hope that begins at the line where your expertise ends. It represents a team of providers who compassionately refuse to give up on patients when they are the last refuge.
- Foster TJ, Bouchard-Fortier A, Olivotto IA, et al. Effect of multidisciplinary case conferences on physician decision making: breast diagnostic rounds. Cureus. 2016;8:E895.
- El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology international survey. J Glob Oncol. 2015;1:57-64.
- Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16:1209-1215.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11:E267-E278.
Multidisciplinary tumor boards are composed of providers from many fields who deliver coordinated care for patients with unresectable and high-risk skin cancers. Providers who comprise the tumor board often are radiation oncologists, hematologists/oncologists, general surgeons, dermatologists, dermatologic surgeons, and pathologists. The benefit of having a tumor board is that each patient is evaluated simultaneously by a group of physicians from various specialties who bring diverse perspectives that will contribute to the overall treatment plan. The cases often encompass high-risk tumors including unresectable basal cell carcinomas or invasive melanomas. By combining knowledge from each specialty in a team approach, the tumor board can effectively and holistically develop a care plan for each patient.
For the tumor board at the Warren Alpert Medical School of Brown University (Providence, Rhode Island), we often prepare a presentation with comprehensive details about the patient and tumor. During the presentation, we also propose a treatment plan prior to describing each patient at the weekly conference and amend the plans during the discussion. Tumor boards also provide a consulting role to the community and hospital providers in which patients are being referred by their primary provider and are seeking a second opinion or guidance.
In many ways, the tumor board is a multidisciplinary approach for patient advocacy in the form of treatment. These physicians meet on a regular basis to check on the patient’s progress and continually reevaluate how to have discussions about the patient’s care. There are many reasons why it is important to refer patients to a multidisciplinary tumor board.
Improved Workup and Diagnosis
One of the values of a tumor board is that it allows for patient data to be collected and assembled in a way that tells a story. The specialist from each field can then discuss and weigh the benefits and risks for each diagnostic test that should be performed for the workup in each patient. Physicians who refer their patients to the tumor board use their recommendations to both confirm the diagnosis and shift their treatment plans, depending on the information presented during the meeting.1 There may be a change in the tumor type, decision to refer for surgery, cancer staging, and list of viable options, especially after reviewing pathology and imaging.2 The discussion of the treatment plan may consider not only surgical considerations but also the patient’s quality of life. At times, noninvasive interventions are more appropriate and align with the patient’s goals of care. In addition, during the tumor board clinic there may be new tumors that are identified and biopsied, providing increased diagnosis and surveillance for patients who may have a higher risk for developing skin cancer.
Education for Residents and Providers
The multidisciplinary tumor board not only helps patients but also educates both residents and providers on the evidence-based therapeutic management of high-risk tumors.2 Research literature on cutaneous oncology is dynamic, and the weekly tumor board meetings help providers stay informed about the best and most effective treatments for their patients.3 In addition to the attending specialists, participants of the tumor board also may include residents, medical students, medical assistance staff, nurses, physician assistants, and fellows. Furthermore, the recommendations given by the tumor board serve to educate both the patient and the provider who referred them to the tumor board. Although we have access to excellent dermatology textbooks as residents, the most impactful educational experience is seeing the patients in tumor board clinic and participating in the immensely educational discussions at the weekly conferences. Through this experience, I have learned that treatment plans should be personalized to the patient. There are many factors to take into consideration when deciphering what the best course of treatment will be for a patient. Sometimes the best option is Mohs micrographic surgery, while other times it may be scheduling several sessions of palliative radiation oncology. Treatment depends on the individual patient and their condition.
Coordination of Care
During a week that I was on call, I was consulted to biopsy a patient with a giant hemorrhagic basal cell carcinoma that caused substantial cheek and nose distortion as well as anemia secondary to acute blood loss. The patient not only did not have a dermatologist but also did not have a primary care physician given he had not had contact with the health care system in more than 30 years. The reason for him not seeking care was multifactorial, but the approach to his care became multidisciplinary. We sought to connect him with the right providers to help him in any way that we could. We presented him at our multidisciplinary tumor board and started him on sonedigib, a medication that binds to and inhibits the smoothened protein.4 Through the tumor board, we were able to establish sustained contact with the patient. The tumor board created effective communication between providers to get him the referrals that he needed for dermatology, pathology, radiation oncology, hematology/oncology, and otolaryngology. The discussions centered around being cognizant of the patient’s apprehension with the health care system as well as providing medical and surgical treatment that would help his quality of life. We built a consensus on what the best plan was for the patient and his family. This consensus would have been more difficult had it not been for the combined specialties of the tumor board. In general, studies have shown that weekly tumor boards have resulted in decreased mortality rates for patients with advanced cancers.5
Final Thoughts
The multidisciplinary tumor board is a powerful resource for hospitals and the greater medical community. At these weekly conferences you realize there may still be hope that begins at the line where your expertise ends. It represents a team of providers who compassionately refuse to give up on patients when they are the last refuge.
Multidisciplinary tumor boards are composed of providers from many fields who deliver coordinated care for patients with unresectable and high-risk skin cancers. Providers who comprise the tumor board often are radiation oncologists, hematologists/oncologists, general surgeons, dermatologists, dermatologic surgeons, and pathologists. The benefit of having a tumor board is that each patient is evaluated simultaneously by a group of physicians from various specialties who bring diverse perspectives that will contribute to the overall treatment plan. The cases often encompass high-risk tumors including unresectable basal cell carcinomas or invasive melanomas. By combining knowledge from each specialty in a team approach, the tumor board can effectively and holistically develop a care plan for each patient.
For the tumor board at the Warren Alpert Medical School of Brown University (Providence, Rhode Island), we often prepare a presentation with comprehensive details about the patient and tumor. During the presentation, we also propose a treatment plan prior to describing each patient at the weekly conference and amend the plans during the discussion. Tumor boards also provide a consulting role to the community and hospital providers in which patients are being referred by their primary provider and are seeking a second opinion or guidance.
In many ways, the tumor board is a multidisciplinary approach for patient advocacy in the form of treatment. These physicians meet on a regular basis to check on the patient’s progress and continually reevaluate how to have discussions about the patient’s care. There are many reasons why it is important to refer patients to a multidisciplinary tumor board.
Improved Workup and Diagnosis
One of the values of a tumor board is that it allows for patient data to be collected and assembled in a way that tells a story. The specialist from each field can then discuss and weigh the benefits and risks for each diagnostic test that should be performed for the workup in each patient. Physicians who refer their patients to the tumor board use their recommendations to both confirm the diagnosis and shift their treatment plans, depending on the information presented during the meeting.1 There may be a change in the tumor type, decision to refer for surgery, cancer staging, and list of viable options, especially after reviewing pathology and imaging.2 The discussion of the treatment plan may consider not only surgical considerations but also the patient’s quality of life. At times, noninvasive interventions are more appropriate and align with the patient’s goals of care. In addition, during the tumor board clinic there may be new tumors that are identified and biopsied, providing increased diagnosis and surveillance for patients who may have a higher risk for developing skin cancer.
Education for Residents and Providers
The multidisciplinary tumor board not only helps patients but also educates both residents and providers on the evidence-based therapeutic management of high-risk tumors.2 Research literature on cutaneous oncology is dynamic, and the weekly tumor board meetings help providers stay informed about the best and most effective treatments for their patients.3 In addition to the attending specialists, participants of the tumor board also may include residents, medical students, medical assistance staff, nurses, physician assistants, and fellows. Furthermore, the recommendations given by the tumor board serve to educate both the patient and the provider who referred them to the tumor board. Although we have access to excellent dermatology textbooks as residents, the most impactful educational experience is seeing the patients in tumor board clinic and participating in the immensely educational discussions at the weekly conferences. Through this experience, I have learned that treatment plans should be personalized to the patient. There are many factors to take into consideration when deciphering what the best course of treatment will be for a patient. Sometimes the best option is Mohs micrographic surgery, while other times it may be scheduling several sessions of palliative radiation oncology. Treatment depends on the individual patient and their condition.
Coordination of Care
During a week that I was on call, I was consulted to biopsy a patient with a giant hemorrhagic basal cell carcinoma that caused substantial cheek and nose distortion as well as anemia secondary to acute blood loss. The patient not only did not have a dermatologist but also did not have a primary care physician given he had not had contact with the health care system in more than 30 years. The reason for him not seeking care was multifactorial, but the approach to his care became multidisciplinary. We sought to connect him with the right providers to help him in any way that we could. We presented him at our multidisciplinary tumor board and started him on sonedigib, a medication that binds to and inhibits the smoothened protein.4 Through the tumor board, we were able to establish sustained contact with the patient. The tumor board created effective communication between providers to get him the referrals that he needed for dermatology, pathology, radiation oncology, hematology/oncology, and otolaryngology. The discussions centered around being cognizant of the patient’s apprehension with the health care system as well as providing medical and surgical treatment that would help his quality of life. We built a consensus on what the best plan was for the patient and his family. This consensus would have been more difficult had it not been for the combined specialties of the tumor board. In general, studies have shown that weekly tumor boards have resulted in decreased mortality rates for patients with advanced cancers.5
Final Thoughts
The multidisciplinary tumor board is a powerful resource for hospitals and the greater medical community. At these weekly conferences you realize there may still be hope that begins at the line where your expertise ends. It represents a team of providers who compassionately refuse to give up on patients when they are the last refuge.
- Foster TJ, Bouchard-Fortier A, Olivotto IA, et al. Effect of multidisciplinary case conferences on physician decision making: breast diagnostic rounds. Cureus. 2016;8:E895.
- El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology international survey. J Glob Oncol. 2015;1:57-64.
- Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16:1209-1215.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11:E267-E278.
- Foster TJ, Bouchard-Fortier A, Olivotto IA, et al. Effect of multidisciplinary case conferences on physician decision making: breast diagnostic rounds. Cureus. 2016;8:E895.
- El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology international survey. J Glob Oncol. 2015;1:57-64.
- Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16:1209-1215.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11:E267-E278.
Resident Pearl
- Participating in a multidisciplinary tumor board allows residents to learn more about how to manage and treat high-risk skin cancers. The multidisciplinary team approach provides high-quality care for challenging patients.
Benzene found in some sunscreen products, online pharmacy says
Valisure, an online pharmacy known for testing every batch of medication it sells, announced that it has
The company tested 294 batches from 69 companies and found benzene in 27% – many in major national brands like Neutrogena and Banana Boat. Some batches contained as much as three times the emergency FDA limit of 2 parts per million.
Long-term exposure to benzene is known to cause cancer in humans.
“This is especially concerning with sunscreen because multiple FDA studies have shown that sunscreen ingredients absorb through the skin and end up in the blood at high levels,” said David Light, CEO of Valisure.
The FDA is seeking more information about the potential risks from common sunscreen ingredients.
“There is not a safe level of benzene that can exist in sunscreen products,” Christopher Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in Valisure’s FDA petition. “The total mass of sunscreen required to cover and protect the human body, in single daily application or repeated applications daily, means that even benzene at 0.1 ppm in a sunscreen could expose people to excessively high nanogram amounts of benzene.”
Valisure’s testing previously led to FDA recalls of heartburn medications and hand sanitizers.
Examining sunscreen’s environmental impact
Chemicals in sunscreen may be harmful to other forms of life, too. For years, scientists have been examining whether certain chemicals in sunscreen could be causing damage to marine life, in particular the world’s coral reefs. Specific ingredients, including oxybenzone, benzophenone-1, benzophenone-8, OD-PABA, 4-methylbenzylidene camphor, 3-benzylidene camphor, nano-titanium dioxide, nano-zinc oxide, octinoxate, and octocrylene, have been identified as potential risks.
Earlier this year, the National Academies of Sciences, Engineering, and Medicine created a committee to review the existing science about the potential environmental hazards. Over the next 2 years, they’ll also consider the public health implications if people stopped using sunscreen.
Valisure’s announcement included this message: “It is important to note that not all sunscreen products contain benzene and that uncontaminated products are available, should continue to be used, and are important for protecting against potentially harmful solar radiation.”
Using sunscreen with SPF 15 every day can lower risk of squamous cell carcinoma by around 40% and melanoma by 50%. The American Academy of Dermatology recommends a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher.
A version of this article first appeared on WebMD.com.
Valisure, an online pharmacy known for testing every batch of medication it sells, announced that it has
The company tested 294 batches from 69 companies and found benzene in 27% – many in major national brands like Neutrogena and Banana Boat. Some batches contained as much as three times the emergency FDA limit of 2 parts per million.
Long-term exposure to benzene is known to cause cancer in humans.
“This is especially concerning with sunscreen because multiple FDA studies have shown that sunscreen ingredients absorb through the skin and end up in the blood at high levels,” said David Light, CEO of Valisure.
The FDA is seeking more information about the potential risks from common sunscreen ingredients.
“There is not a safe level of benzene that can exist in sunscreen products,” Christopher Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in Valisure’s FDA petition. “The total mass of sunscreen required to cover and protect the human body, in single daily application or repeated applications daily, means that even benzene at 0.1 ppm in a sunscreen could expose people to excessively high nanogram amounts of benzene.”
Valisure’s testing previously led to FDA recalls of heartburn medications and hand sanitizers.
Examining sunscreen’s environmental impact
Chemicals in sunscreen may be harmful to other forms of life, too. For years, scientists have been examining whether certain chemicals in sunscreen could be causing damage to marine life, in particular the world’s coral reefs. Specific ingredients, including oxybenzone, benzophenone-1, benzophenone-8, OD-PABA, 4-methylbenzylidene camphor, 3-benzylidene camphor, nano-titanium dioxide, nano-zinc oxide, octinoxate, and octocrylene, have been identified as potential risks.
Earlier this year, the National Academies of Sciences, Engineering, and Medicine created a committee to review the existing science about the potential environmental hazards. Over the next 2 years, they’ll also consider the public health implications if people stopped using sunscreen.
Valisure’s announcement included this message: “It is important to note that not all sunscreen products contain benzene and that uncontaminated products are available, should continue to be used, and are important for protecting against potentially harmful solar radiation.”
Using sunscreen with SPF 15 every day can lower risk of squamous cell carcinoma by around 40% and melanoma by 50%. The American Academy of Dermatology recommends a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher.
A version of this article first appeared on WebMD.com.
Valisure, an online pharmacy known for testing every batch of medication it sells, announced that it has
The company tested 294 batches from 69 companies and found benzene in 27% – many in major national brands like Neutrogena and Banana Boat. Some batches contained as much as three times the emergency FDA limit of 2 parts per million.
Long-term exposure to benzene is known to cause cancer in humans.
“This is especially concerning with sunscreen because multiple FDA studies have shown that sunscreen ingredients absorb through the skin and end up in the blood at high levels,” said David Light, CEO of Valisure.
The FDA is seeking more information about the potential risks from common sunscreen ingredients.
“There is not a safe level of benzene that can exist in sunscreen products,” Christopher Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in Valisure’s FDA petition. “The total mass of sunscreen required to cover and protect the human body, in single daily application or repeated applications daily, means that even benzene at 0.1 ppm in a sunscreen could expose people to excessively high nanogram amounts of benzene.”
Valisure’s testing previously led to FDA recalls of heartburn medications and hand sanitizers.
Examining sunscreen’s environmental impact
Chemicals in sunscreen may be harmful to other forms of life, too. For years, scientists have been examining whether certain chemicals in sunscreen could be causing damage to marine life, in particular the world’s coral reefs. Specific ingredients, including oxybenzone, benzophenone-1, benzophenone-8, OD-PABA, 4-methylbenzylidene camphor, 3-benzylidene camphor, nano-titanium dioxide, nano-zinc oxide, octinoxate, and octocrylene, have been identified as potential risks.
Earlier this year, the National Academies of Sciences, Engineering, and Medicine created a committee to review the existing science about the potential environmental hazards. Over the next 2 years, they’ll also consider the public health implications if people stopped using sunscreen.
Valisure’s announcement included this message: “It is important to note that not all sunscreen products contain benzene and that uncontaminated products are available, should continue to be used, and are important for protecting against potentially harmful solar radiation.”
Using sunscreen with SPF 15 every day can lower risk of squamous cell carcinoma by around 40% and melanoma by 50%. The American Academy of Dermatology recommends a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher.
A version of this article first appeared on WebMD.com.
Survey: Many Mohs surgeons are struggling on the job
.
In a measurement of well-being, 40% of members of the American College of Mohs
Surgery (ACMS) who responded to the survey – and 52% of women – scored at a level considered “at-risk” for adverse outcomes, such as poor quality of life.
“I didn’t think the numbers were going to be that high,” said study author Kemi O. Awe, MD, PhD, a dermatology resident at the University of Alabama at Birmingham, especially in light of Mohs surgery’s reputation as being an especially desirable field in dermatology. She presented the findings at the annual meeting of the ACMS.
Dr. Awe, who hopes to become a Mohs surgeon herself, said in an interview that she launched the study in part to understand how colleagues are faring. “Dermatology is known as a specialty that has a good lifestyle and less stress, but the rate of burnout is actually going up.”
For the study, Dr. Awe and colleagues sent a survey to ACMS members between October and December 2020. The 91 respondents had an average age of 46, and 58% were male. Most practiced in academic facilities (56%), while the rest worked in private practice (39%) or multispecialty (4%) practices. Almost all (89%) were married or in partnerships.
The survey calculated scores on the expanded Physician Well Being Index, a validated tool for measuring physician distress. Forty percent of 68 respondents to this part of the survey got a score of 3 or higher, which the study describes as “a threshold for respondents who are ‘at-risk’ of adverse outcomes such as poor quality of life, depression, and a high level of fatigue.”
Women were more likely to be considered at risk (52%) than men (28%). “This isn’t different than what’s already out there: Female physicians are more likely to be burned out compared to men,” Dr. Awe said.
Compared with their male counterparts, female Mohs surgeons were more likely to say that time at work, malpractice concerns, insurance reimbursement, and compensation structure negatively affected their well-being (P ≤ .05).
It’s unclear whether there’s a well-being gender gap among dermatologists overall, however. Dr. Awe highlighted a 2019 survey of 108 dermatologists that found no significant difference in overall burnout between men and women – about 42% of both genders reported symptoms. But the survey did find that “dermatologists with children living at home had significantly higher levels of burnout,” with a P value of .03.
Dr. Awe said the findings offer insight into what to look out for when pursuing a career as a Mohs surgeon. “There’s potentially excess stress about being a Mohs surgeon,” she said, although the field also has a reputation as being fulfilling and rewarding.
In an interview, Stanford (Calif.) University dermatologist Zakia Rahman, MD, praised the study and said it “certainly provides a framework to address professional fulfillment amongst Mohs surgeons.”
It was especially surprising, she said, that female surgeons didn’t rate their compensation structure as positively as did their male colleagues. “It is possible that there is still a significant amount of gender-based difference in compensation between male and female Mohs surgeons. This is an area that can be further explored.”
Moving forward, she said, “our professional dermatology societies must examine the increase in burnout within our specialty. Further funding and research in this area is needed.”
For now, dermatologists can focus on strategies that can reduce burnout in the field, Sailesh Konda, MD, a Mohs surgeon at the Univeristy of Florida, Gainesville, said in an interview. Dr. Konda highlighted a report published in 2020 that, he said, "recommended focusing on incremental changes that help restore autonomy and control over work, connecting with colleagues within dermatology and the broader medical community, developing self-awareness and recognition of a perfectionist mindset, and restoring meaning and joy to patient care.”*
No funding is reported for the study. Dr. Awe, Dr. Rahman, and Dr. Konda have no relevant disclosures.
*This story was updated on June 2 for clarity.
.
In a measurement of well-being, 40% of members of the American College of Mohs
Surgery (ACMS) who responded to the survey – and 52% of women – scored at a level considered “at-risk” for adverse outcomes, such as poor quality of life.
“I didn’t think the numbers were going to be that high,” said study author Kemi O. Awe, MD, PhD, a dermatology resident at the University of Alabama at Birmingham, especially in light of Mohs surgery’s reputation as being an especially desirable field in dermatology. She presented the findings at the annual meeting of the ACMS.
Dr. Awe, who hopes to become a Mohs surgeon herself, said in an interview that she launched the study in part to understand how colleagues are faring. “Dermatology is known as a specialty that has a good lifestyle and less stress, but the rate of burnout is actually going up.”
For the study, Dr. Awe and colleagues sent a survey to ACMS members between October and December 2020. The 91 respondents had an average age of 46, and 58% were male. Most practiced in academic facilities (56%), while the rest worked in private practice (39%) or multispecialty (4%) practices. Almost all (89%) were married or in partnerships.
The survey calculated scores on the expanded Physician Well Being Index, a validated tool for measuring physician distress. Forty percent of 68 respondents to this part of the survey got a score of 3 or higher, which the study describes as “a threshold for respondents who are ‘at-risk’ of adverse outcomes such as poor quality of life, depression, and a high level of fatigue.”
Women were more likely to be considered at risk (52%) than men (28%). “This isn’t different than what’s already out there: Female physicians are more likely to be burned out compared to men,” Dr. Awe said.
Compared with their male counterparts, female Mohs surgeons were more likely to say that time at work, malpractice concerns, insurance reimbursement, and compensation structure negatively affected their well-being (P ≤ .05).
It’s unclear whether there’s a well-being gender gap among dermatologists overall, however. Dr. Awe highlighted a 2019 survey of 108 dermatologists that found no significant difference in overall burnout between men and women – about 42% of both genders reported symptoms. But the survey did find that “dermatologists with children living at home had significantly higher levels of burnout,” with a P value of .03.
Dr. Awe said the findings offer insight into what to look out for when pursuing a career as a Mohs surgeon. “There’s potentially excess stress about being a Mohs surgeon,” she said, although the field also has a reputation as being fulfilling and rewarding.
In an interview, Stanford (Calif.) University dermatologist Zakia Rahman, MD, praised the study and said it “certainly provides a framework to address professional fulfillment amongst Mohs surgeons.”
It was especially surprising, she said, that female surgeons didn’t rate their compensation structure as positively as did their male colleagues. “It is possible that there is still a significant amount of gender-based difference in compensation between male and female Mohs surgeons. This is an area that can be further explored.”
Moving forward, she said, “our professional dermatology societies must examine the increase in burnout within our specialty. Further funding and research in this area is needed.”
For now, dermatologists can focus on strategies that can reduce burnout in the field, Sailesh Konda, MD, a Mohs surgeon at the Univeristy of Florida, Gainesville, said in an interview. Dr. Konda highlighted a report published in 2020 that, he said, "recommended focusing on incremental changes that help restore autonomy and control over work, connecting with colleagues within dermatology and the broader medical community, developing self-awareness and recognition of a perfectionist mindset, and restoring meaning and joy to patient care.”*
No funding is reported for the study. Dr. Awe, Dr. Rahman, and Dr. Konda have no relevant disclosures.
*This story was updated on June 2 for clarity.
.
In a measurement of well-being, 40% of members of the American College of Mohs
Surgery (ACMS) who responded to the survey – and 52% of women – scored at a level considered “at-risk” for adverse outcomes, such as poor quality of life.
“I didn’t think the numbers were going to be that high,” said study author Kemi O. Awe, MD, PhD, a dermatology resident at the University of Alabama at Birmingham, especially in light of Mohs surgery’s reputation as being an especially desirable field in dermatology. She presented the findings at the annual meeting of the ACMS.
Dr. Awe, who hopes to become a Mohs surgeon herself, said in an interview that she launched the study in part to understand how colleagues are faring. “Dermatology is known as a specialty that has a good lifestyle and less stress, but the rate of burnout is actually going up.”
For the study, Dr. Awe and colleagues sent a survey to ACMS members between October and December 2020. The 91 respondents had an average age of 46, and 58% were male. Most practiced in academic facilities (56%), while the rest worked in private practice (39%) or multispecialty (4%) practices. Almost all (89%) were married or in partnerships.
The survey calculated scores on the expanded Physician Well Being Index, a validated tool for measuring physician distress. Forty percent of 68 respondents to this part of the survey got a score of 3 or higher, which the study describes as “a threshold for respondents who are ‘at-risk’ of adverse outcomes such as poor quality of life, depression, and a high level of fatigue.”
Women were more likely to be considered at risk (52%) than men (28%). “This isn’t different than what’s already out there: Female physicians are more likely to be burned out compared to men,” Dr. Awe said.
Compared with their male counterparts, female Mohs surgeons were more likely to say that time at work, malpractice concerns, insurance reimbursement, and compensation structure negatively affected their well-being (P ≤ .05).
It’s unclear whether there’s a well-being gender gap among dermatologists overall, however. Dr. Awe highlighted a 2019 survey of 108 dermatologists that found no significant difference in overall burnout between men and women – about 42% of both genders reported symptoms. But the survey did find that “dermatologists with children living at home had significantly higher levels of burnout,” with a P value of .03.
Dr. Awe said the findings offer insight into what to look out for when pursuing a career as a Mohs surgeon. “There’s potentially excess stress about being a Mohs surgeon,” she said, although the field also has a reputation as being fulfilling and rewarding.
In an interview, Stanford (Calif.) University dermatologist Zakia Rahman, MD, praised the study and said it “certainly provides a framework to address professional fulfillment amongst Mohs surgeons.”
It was especially surprising, she said, that female surgeons didn’t rate their compensation structure as positively as did their male colleagues. “It is possible that there is still a significant amount of gender-based difference in compensation between male and female Mohs surgeons. This is an area that can be further explored.”
Moving forward, she said, “our professional dermatology societies must examine the increase in burnout within our specialty. Further funding and research in this area is needed.”
For now, dermatologists can focus on strategies that can reduce burnout in the field, Sailesh Konda, MD, a Mohs surgeon at the Univeristy of Florida, Gainesville, said in an interview. Dr. Konda highlighted a report published in 2020 that, he said, "recommended focusing on incremental changes that help restore autonomy and control over work, connecting with colleagues within dermatology and the broader medical community, developing self-awareness and recognition of a perfectionist mindset, and restoring meaning and joy to patient care.”*
No funding is reported for the study. Dr. Awe, Dr. Rahman, and Dr. Konda have no relevant disclosures.
*This story was updated on June 2 for clarity.
FROM THE ACMS ANNUAL MEETING
Novel immunotherapy relatlimab in advanced melanoma
Adding the novel immune checkpoint inhibitor relatlimab to the more established nivolumab (Opdivo) significantly extended the progression-free survival (PFS) of patients with previously untreated advanced melanoma in comparison with nivolumab alone in the phase 3 RELATIVITY-047 trial.
Both drugs are from Bristol-Myers Squibb, which funded the study.
“Our findings demonstrate that relatlimab plus nivolumab is a potential novel treatment option for this patient population,” said lead researcher Evan J. Lipson, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Relatlimab has a different mechanism of action from currently available immune checkpoint inhibitors, such as nivolumab and similar agents, which act as inhibitors of the programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1). In contrast, relatlimab acts as an antibody that targets lymphocyte-activation gene 3 (LAG-3), which inhibits T cells and thus helps cancer cells evade immune attack.
“This is the first phase 3 study to validate inhibition of the LAG-3 immune checkpoint as a therapeutic strategy for patients with cancer, and it establishes the LAG-3 pathway as the third immune checkpoint pathway in history, after CLTA-4 and PD-1, for which blockade appears to have clinical benefit,” Dr. Lipson said at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology (ASCO), where this study will be presented (abstract 9503).
Commenting for ASCO, Julie R. Gralow, MD, chief medical officer and executive vice president, agreed that “these results provide validation of the LAG-3 immune checkpoint as a therapeutic target ... and they also support combination treatment with immunotherapies that act on different parts of the immune system.”
When Dr. Lipson was asked whether he would recommend the combination of relatlimab plus nivolumab as a first-line treatment for this patient population, he said that “for many patients,” the first-line treatment choice is made on a “case-by-case” basis.
“We are fortunate in melanoma that we have an ever-expanding list of seemingly effective options, and I think we’ll find at some point this will be added to that list,” he said. “Whether this is the first-line choice for any given patient really depends on a lot of factors,” he added.
Dr. Gralow added a note of caution. “The combination was clearly more toxic, and so I think there will be a lot of discussion” as to when it would be used and for which patients, she said.
In the absence of head-to-head comparisons, “I’m not sure that we have one answer” as to which treatment to choose, she added. With the ever-increasing number of options available in melanoma, the individual treatment choice is “getting more complicated,” she said.
Study details
The global RELATIVITY-047 study was conducted in 714 patients with previously untreated unresectable or metastatic melanoma. The participants were randomly assigned to receive either relatlimab plus nivolumab or nivolumab alone.
Dr. Lipson explained that the treatments were given as a fixed-dosed combination, meaning the preparation of relatlimab and nivolumab was given in the “same medication phial and administered as a single intravenous infusion in order to reduce preparation and infusion times and minimize the risk of administration errors.”
PFS, as determined on blinded independent central review, was significantly longer with the combination therapy than with nivolumab alone, at a median of 10.12 months vs. 4.63 months (hazard ratio, 0.75; P = .0055).
At 12 months, the PFS rate among patients given relatlimab plus nivolumab was 47.7%, versus 36.0% among those given nivolumab alone.
“This significant improvement meant that the study met its primary endpoint,” Dr. Lipson said, adding that the PFS benefit “appeared relatively early in the course of therapy.” The curves separated at 12 weeks, and benefit was “sustained” over the course of follow-up.
He added that the performance of nivolumab alone was “in the range” of that seen in previous studies, although he underlined that cross-trial comparison is difficult, given the differences in study design.
“In general, treatment-related adverse events” associated with the combination therapy were “manageable and reflected the safety profile that we typically see with immune checkpoint inhibitors,” he noted.
The results showed that 40.3% of patients who received the combination therapy experienced a grade 3-4 adverse event, compared with 33.4% of those given nivolumab alone. Grade 3-4 treatment-related adverse events leading to discontinuation occurred in 8.5% and 3.1% of patients, respectively.
Three treatment-related deaths occurred in the relatlimab and nivolumab arm. Two such deaths occurred in the nivolumab-alone group.
The study was funded by Bristol Myers Squibb. Dr. Lipson has relationships with Array BioPharma, Bristol Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and Sysmex (inst). Dr. Gralow has relationships with AstraZeneca, Genentech, Sandoz, and Immunomedics.
A version of this article first appeared on Medscape.com.
Adding the novel immune checkpoint inhibitor relatlimab to the more established nivolumab (Opdivo) significantly extended the progression-free survival (PFS) of patients with previously untreated advanced melanoma in comparison with nivolumab alone in the phase 3 RELATIVITY-047 trial.
Both drugs are from Bristol-Myers Squibb, which funded the study.
“Our findings demonstrate that relatlimab plus nivolumab is a potential novel treatment option for this patient population,” said lead researcher Evan J. Lipson, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Relatlimab has a different mechanism of action from currently available immune checkpoint inhibitors, such as nivolumab and similar agents, which act as inhibitors of the programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1). In contrast, relatlimab acts as an antibody that targets lymphocyte-activation gene 3 (LAG-3), which inhibits T cells and thus helps cancer cells evade immune attack.
“This is the first phase 3 study to validate inhibition of the LAG-3 immune checkpoint as a therapeutic strategy for patients with cancer, and it establishes the LAG-3 pathway as the third immune checkpoint pathway in history, after CLTA-4 and PD-1, for which blockade appears to have clinical benefit,” Dr. Lipson said at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology (ASCO), where this study will be presented (abstract 9503).
Commenting for ASCO, Julie R. Gralow, MD, chief medical officer and executive vice president, agreed that “these results provide validation of the LAG-3 immune checkpoint as a therapeutic target ... and they also support combination treatment with immunotherapies that act on different parts of the immune system.”
When Dr. Lipson was asked whether he would recommend the combination of relatlimab plus nivolumab as a first-line treatment for this patient population, he said that “for many patients,” the first-line treatment choice is made on a “case-by-case” basis.
“We are fortunate in melanoma that we have an ever-expanding list of seemingly effective options, and I think we’ll find at some point this will be added to that list,” he said. “Whether this is the first-line choice for any given patient really depends on a lot of factors,” he added.
Dr. Gralow added a note of caution. “The combination was clearly more toxic, and so I think there will be a lot of discussion” as to when it would be used and for which patients, she said.
In the absence of head-to-head comparisons, “I’m not sure that we have one answer” as to which treatment to choose, she added. With the ever-increasing number of options available in melanoma, the individual treatment choice is “getting more complicated,” she said.
Study details
The global RELATIVITY-047 study was conducted in 714 patients with previously untreated unresectable or metastatic melanoma. The participants were randomly assigned to receive either relatlimab plus nivolumab or nivolumab alone.
Dr. Lipson explained that the treatments were given as a fixed-dosed combination, meaning the preparation of relatlimab and nivolumab was given in the “same medication phial and administered as a single intravenous infusion in order to reduce preparation and infusion times and minimize the risk of administration errors.”
PFS, as determined on blinded independent central review, was significantly longer with the combination therapy than with nivolumab alone, at a median of 10.12 months vs. 4.63 months (hazard ratio, 0.75; P = .0055).
At 12 months, the PFS rate among patients given relatlimab plus nivolumab was 47.7%, versus 36.0% among those given nivolumab alone.
“This significant improvement meant that the study met its primary endpoint,” Dr. Lipson said, adding that the PFS benefit “appeared relatively early in the course of therapy.” The curves separated at 12 weeks, and benefit was “sustained” over the course of follow-up.
He added that the performance of nivolumab alone was “in the range” of that seen in previous studies, although he underlined that cross-trial comparison is difficult, given the differences in study design.
“In general, treatment-related adverse events” associated with the combination therapy were “manageable and reflected the safety profile that we typically see with immune checkpoint inhibitors,” he noted.
The results showed that 40.3% of patients who received the combination therapy experienced a grade 3-4 adverse event, compared with 33.4% of those given nivolumab alone. Grade 3-4 treatment-related adverse events leading to discontinuation occurred in 8.5% and 3.1% of patients, respectively.
Three treatment-related deaths occurred in the relatlimab and nivolumab arm. Two such deaths occurred in the nivolumab-alone group.
The study was funded by Bristol Myers Squibb. Dr. Lipson has relationships with Array BioPharma, Bristol Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and Sysmex (inst). Dr. Gralow has relationships with AstraZeneca, Genentech, Sandoz, and Immunomedics.
A version of this article first appeared on Medscape.com.
Adding the novel immune checkpoint inhibitor relatlimab to the more established nivolumab (Opdivo) significantly extended the progression-free survival (PFS) of patients with previously untreated advanced melanoma in comparison with nivolumab alone in the phase 3 RELATIVITY-047 trial.
Both drugs are from Bristol-Myers Squibb, which funded the study.
“Our findings demonstrate that relatlimab plus nivolumab is a potential novel treatment option for this patient population,” said lead researcher Evan J. Lipson, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Relatlimab has a different mechanism of action from currently available immune checkpoint inhibitors, such as nivolumab and similar agents, which act as inhibitors of the programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1). In contrast, relatlimab acts as an antibody that targets lymphocyte-activation gene 3 (LAG-3), which inhibits T cells and thus helps cancer cells evade immune attack.
“This is the first phase 3 study to validate inhibition of the LAG-3 immune checkpoint as a therapeutic strategy for patients with cancer, and it establishes the LAG-3 pathway as the third immune checkpoint pathway in history, after CLTA-4 and PD-1, for which blockade appears to have clinical benefit,” Dr. Lipson said at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology (ASCO), where this study will be presented (abstract 9503).
Commenting for ASCO, Julie R. Gralow, MD, chief medical officer and executive vice president, agreed that “these results provide validation of the LAG-3 immune checkpoint as a therapeutic target ... and they also support combination treatment with immunotherapies that act on different parts of the immune system.”
When Dr. Lipson was asked whether he would recommend the combination of relatlimab plus nivolumab as a first-line treatment for this patient population, he said that “for many patients,” the first-line treatment choice is made on a “case-by-case” basis.
“We are fortunate in melanoma that we have an ever-expanding list of seemingly effective options, and I think we’ll find at some point this will be added to that list,” he said. “Whether this is the first-line choice for any given patient really depends on a lot of factors,” he added.
Dr. Gralow added a note of caution. “The combination was clearly more toxic, and so I think there will be a lot of discussion” as to when it would be used and for which patients, she said.
In the absence of head-to-head comparisons, “I’m not sure that we have one answer” as to which treatment to choose, she added. With the ever-increasing number of options available in melanoma, the individual treatment choice is “getting more complicated,” she said.
Study details
The global RELATIVITY-047 study was conducted in 714 patients with previously untreated unresectable or metastatic melanoma. The participants were randomly assigned to receive either relatlimab plus nivolumab or nivolumab alone.
Dr. Lipson explained that the treatments were given as a fixed-dosed combination, meaning the preparation of relatlimab and nivolumab was given in the “same medication phial and administered as a single intravenous infusion in order to reduce preparation and infusion times and minimize the risk of administration errors.”
PFS, as determined on blinded independent central review, was significantly longer with the combination therapy than with nivolumab alone, at a median of 10.12 months vs. 4.63 months (hazard ratio, 0.75; P = .0055).
At 12 months, the PFS rate among patients given relatlimab plus nivolumab was 47.7%, versus 36.0% among those given nivolumab alone.
“This significant improvement meant that the study met its primary endpoint,” Dr. Lipson said, adding that the PFS benefit “appeared relatively early in the course of therapy.” The curves separated at 12 weeks, and benefit was “sustained” over the course of follow-up.
He added that the performance of nivolumab alone was “in the range” of that seen in previous studies, although he underlined that cross-trial comparison is difficult, given the differences in study design.
“In general, treatment-related adverse events” associated with the combination therapy were “manageable and reflected the safety profile that we typically see with immune checkpoint inhibitors,” he noted.
The results showed that 40.3% of patients who received the combination therapy experienced a grade 3-4 adverse event, compared with 33.4% of those given nivolumab alone. Grade 3-4 treatment-related adverse events leading to discontinuation occurred in 8.5% and 3.1% of patients, respectively.
Three treatment-related deaths occurred in the relatlimab and nivolumab arm. Two such deaths occurred in the nivolumab-alone group.
The study was funded by Bristol Myers Squibb. Dr. Lipson has relationships with Array BioPharma, Bristol Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and Sysmex (inst). Dr. Gralow has relationships with AstraZeneca, Genentech, Sandoz, and Immunomedics.
A version of this article first appeared on Medscape.com.
Telemedicine is popular among Mohs surgeons – for now
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
FROM THE ACMS ANNUAL MEETING
New guideline provides recommendations on reconstruction after skin cancer resection
You’ve successfully resected a skin cancer lesion, leaving clear margins. Now what?
That’s
The guideline – a joint effort of the American Society of Plastic Surgeons, American Society for Dermatologic Surgery, American Academy of Dermatology, American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology – Head and Neck Surgery Foundation, American College of Mohs Surgery, American Society for Mohs Surgery, and American Society of Ophthalmic Plastic and Reconstructive Surgery – was published online in the Journal of the American Academy of Dermatology.
From the outset, the panel members realized that to keep the guideline manageable they had to limit recommendations to the practice of reconstruction defined as “cutaneous closure that requires a flap, graft, or tissue rearrangement.”
Other wound closure methods, such as secondary intention healing; simple closures; and complex closures that do not involve flaps, grafts, muscle, or bone, were not covered in the recommendations.
As with similar guidelines, the developers selected seven clinical questions to be addressed, and attempted to find consensus through literature searches, appraisal of the evidence, grading of recommendations, peer review, and public comment.
“We had a very heterogeneous set of things that we were trying to comment on, so we had to keep things somewhat generic,” lead author Andrew Chen, MD, chief of the division of plastic surgery, at the University of Connecticut Health Center, Farmington, said in an interview.
“Skin cancer and reconstruction affect different body areas and areas of different sizes. When we were creating the guidelines, we had to tailor the questions we could ask based on things that would make sense to answer, because obviously we couldn’t ask a question such as: ‘What’s better, a skin graft or a flap?’ Well, there are some things you can’t put a skin graft on – it won’t last, so we couldn’t ask that kind of question,” Dr. Chen said.
Curtis Cetrulo, MD, a plastic and reconstructive surgeon at Massachusetts General Hospital, Boston, who was not involved in the guideline process, said in an interview that the broad recommendations are in keeping with his practice and experience. He also acknowledged, however, the difficulty in creating a guideline that covers the complexity and heterogeneity of reconstructive surgery.
“These are generally good recommendations, but they’re recommendations only, with generally weak levels of evidence. What we really need are clinical trials that can give us definitive answers to some of these questions,” he said.
Recommendations
The seven key recommendations, based on the clinical questions raised, are summarized below:
- Delayed (asynchronous) reconstruction is acceptable. Although the quality of the evidence is low and the recommendations are listed as an option, the guideline authors said that depending on the situation, reconstruction can be performed either immediately after resection or delayed by days, weeks, “or even months.”
- Systemic antibiotics should not be routinely prescribed in the interim between resection and reconstruction in adults. Here too, the evidence is low and the recommendation strength is weak, but in “the absence of data showing convincing benefits, systemic antibiotic therapy does not appear necessary or desirable in most cases when there is an interval between cancer resection and reconstruction,” the work group wrote.
- Clinicians may administer perioperative systemic antibiotics in a facility-based setting for adults undergoing reconstruction (3a), but antibiotics should not be routinely prescribed in an office-based setting (3b). The rationale for these recommendations, supported by a moderate level of evidence, is that the risk of surgical-site infection is generally higher in facilities, compared with an office-based setting. Patients who undergo reconstruction in hospitals or surgical centers are more likely to have complex reconstructions or have risks that may make them suitable candidates for antibiotics, but patients in office-based setting may often be spared from the additional costs, side effects, and possible drug interactions from antibiotic use. “There is no evidence in either setting that long-term antibiotic prophylaxis provides infection risk reduction, compared with short-term prophylaxis,” the guideline working group wrote.
- Continue anticoagulant, antithrombotic, and antiplatelet medications for adult patients undergoing reconstruction after skin cancer resection in the office-based setting (4a), and in the facility-based setting should coordinate with the physician managing anticoagulation before modifying the medication prior to surgery (4b). Evidence quality and recommendation strength are both moderate.
- The guideline authors recommend against routine prescription of narcotics as first-line treatment for pain in adults undergoing skin reconstruction (5a), favoring instead acetaminophen and NSAIDs as first-line therapy (5b). Evidence quality and recommendation strength are both moderate.
- In the absence of standardized protocols for the management of pain medications, oral antibiotics, and/or anticoagulants in the perioperative period, clinicians should discuss possible approaches with adult patients. “Educating patients about their perioperative treatment through discussion of treatment strategies may help alleviate anxiety, improve communication, increase patient satisfaction, and maximize patient compliance with the postoperative orders,” the guideline authors wrote.
- The authors suggest that adult patients may be offered follow-up assessments to discuss functional and cosmetic outcomes. “The return of the patient for follow-up visits is an excellent opportunity to better understand and measure these outcomes, improve patient-physician communication, and foster quality improvement. Postoperative follow-up can lead to increased communication between the patient and physician, thereby empowering patients to comment on satisfaction and other important outcomes measures,” they wrote.
What’s next
The guideline developers acknowledged that data are limited regarding reconstructive surgery following skin cancer resection, and that higher-quality studies would help to improve future guidelines. Dr. Chen said that greater use of prospective surgical databases and more systematic collection of patient-reported outcomes could inform further efforts.
The guideline development process was supported by the various groups represented. Dr. Chen and Dr. Cetrulo reported no relevant disclosures.
You’ve successfully resected a skin cancer lesion, leaving clear margins. Now what?
That’s
The guideline – a joint effort of the American Society of Plastic Surgeons, American Society for Dermatologic Surgery, American Academy of Dermatology, American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology – Head and Neck Surgery Foundation, American College of Mohs Surgery, American Society for Mohs Surgery, and American Society of Ophthalmic Plastic and Reconstructive Surgery – was published online in the Journal of the American Academy of Dermatology.
From the outset, the panel members realized that to keep the guideline manageable they had to limit recommendations to the practice of reconstruction defined as “cutaneous closure that requires a flap, graft, or tissue rearrangement.”
Other wound closure methods, such as secondary intention healing; simple closures; and complex closures that do not involve flaps, grafts, muscle, or bone, were not covered in the recommendations.
As with similar guidelines, the developers selected seven clinical questions to be addressed, and attempted to find consensus through literature searches, appraisal of the evidence, grading of recommendations, peer review, and public comment.
“We had a very heterogeneous set of things that we were trying to comment on, so we had to keep things somewhat generic,” lead author Andrew Chen, MD, chief of the division of plastic surgery, at the University of Connecticut Health Center, Farmington, said in an interview.
“Skin cancer and reconstruction affect different body areas and areas of different sizes. When we were creating the guidelines, we had to tailor the questions we could ask based on things that would make sense to answer, because obviously we couldn’t ask a question such as: ‘What’s better, a skin graft or a flap?’ Well, there are some things you can’t put a skin graft on – it won’t last, so we couldn’t ask that kind of question,” Dr. Chen said.
Curtis Cetrulo, MD, a plastic and reconstructive surgeon at Massachusetts General Hospital, Boston, who was not involved in the guideline process, said in an interview that the broad recommendations are in keeping with his practice and experience. He also acknowledged, however, the difficulty in creating a guideline that covers the complexity and heterogeneity of reconstructive surgery.
“These are generally good recommendations, but they’re recommendations only, with generally weak levels of evidence. What we really need are clinical trials that can give us definitive answers to some of these questions,” he said.
Recommendations
The seven key recommendations, based on the clinical questions raised, are summarized below:
- Delayed (asynchronous) reconstruction is acceptable. Although the quality of the evidence is low and the recommendations are listed as an option, the guideline authors said that depending on the situation, reconstruction can be performed either immediately after resection or delayed by days, weeks, “or even months.”
- Systemic antibiotics should not be routinely prescribed in the interim between resection and reconstruction in adults. Here too, the evidence is low and the recommendation strength is weak, but in “the absence of data showing convincing benefits, systemic antibiotic therapy does not appear necessary or desirable in most cases when there is an interval between cancer resection and reconstruction,” the work group wrote.
- Clinicians may administer perioperative systemic antibiotics in a facility-based setting for adults undergoing reconstruction (3a), but antibiotics should not be routinely prescribed in an office-based setting (3b). The rationale for these recommendations, supported by a moderate level of evidence, is that the risk of surgical-site infection is generally higher in facilities, compared with an office-based setting. Patients who undergo reconstruction in hospitals or surgical centers are more likely to have complex reconstructions or have risks that may make them suitable candidates for antibiotics, but patients in office-based setting may often be spared from the additional costs, side effects, and possible drug interactions from antibiotic use. “There is no evidence in either setting that long-term antibiotic prophylaxis provides infection risk reduction, compared with short-term prophylaxis,” the guideline working group wrote.
- Continue anticoagulant, antithrombotic, and antiplatelet medications for adult patients undergoing reconstruction after skin cancer resection in the office-based setting (4a), and in the facility-based setting should coordinate with the physician managing anticoagulation before modifying the medication prior to surgery (4b). Evidence quality and recommendation strength are both moderate.
- The guideline authors recommend against routine prescription of narcotics as first-line treatment for pain in adults undergoing skin reconstruction (5a), favoring instead acetaminophen and NSAIDs as first-line therapy (5b). Evidence quality and recommendation strength are both moderate.
- In the absence of standardized protocols for the management of pain medications, oral antibiotics, and/or anticoagulants in the perioperative period, clinicians should discuss possible approaches with adult patients. “Educating patients about their perioperative treatment through discussion of treatment strategies may help alleviate anxiety, improve communication, increase patient satisfaction, and maximize patient compliance with the postoperative orders,” the guideline authors wrote.
- The authors suggest that adult patients may be offered follow-up assessments to discuss functional and cosmetic outcomes. “The return of the patient for follow-up visits is an excellent opportunity to better understand and measure these outcomes, improve patient-physician communication, and foster quality improvement. Postoperative follow-up can lead to increased communication between the patient and physician, thereby empowering patients to comment on satisfaction and other important outcomes measures,” they wrote.
What’s next
The guideline developers acknowledged that data are limited regarding reconstructive surgery following skin cancer resection, and that higher-quality studies would help to improve future guidelines. Dr. Chen said that greater use of prospective surgical databases and more systematic collection of patient-reported outcomes could inform further efforts.
The guideline development process was supported by the various groups represented. Dr. Chen and Dr. Cetrulo reported no relevant disclosures.
You’ve successfully resected a skin cancer lesion, leaving clear margins. Now what?
That’s
The guideline – a joint effort of the American Society of Plastic Surgeons, American Society for Dermatologic Surgery, American Academy of Dermatology, American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology – Head and Neck Surgery Foundation, American College of Mohs Surgery, American Society for Mohs Surgery, and American Society of Ophthalmic Plastic and Reconstructive Surgery – was published online in the Journal of the American Academy of Dermatology.
From the outset, the panel members realized that to keep the guideline manageable they had to limit recommendations to the practice of reconstruction defined as “cutaneous closure that requires a flap, graft, or tissue rearrangement.”
Other wound closure methods, such as secondary intention healing; simple closures; and complex closures that do not involve flaps, grafts, muscle, or bone, were not covered in the recommendations.
As with similar guidelines, the developers selected seven clinical questions to be addressed, and attempted to find consensus through literature searches, appraisal of the evidence, grading of recommendations, peer review, and public comment.
“We had a very heterogeneous set of things that we were trying to comment on, so we had to keep things somewhat generic,” lead author Andrew Chen, MD, chief of the division of plastic surgery, at the University of Connecticut Health Center, Farmington, said in an interview.
“Skin cancer and reconstruction affect different body areas and areas of different sizes. When we were creating the guidelines, we had to tailor the questions we could ask based on things that would make sense to answer, because obviously we couldn’t ask a question such as: ‘What’s better, a skin graft or a flap?’ Well, there are some things you can’t put a skin graft on – it won’t last, so we couldn’t ask that kind of question,” Dr. Chen said.
Curtis Cetrulo, MD, a plastic and reconstructive surgeon at Massachusetts General Hospital, Boston, who was not involved in the guideline process, said in an interview that the broad recommendations are in keeping with his practice and experience. He also acknowledged, however, the difficulty in creating a guideline that covers the complexity and heterogeneity of reconstructive surgery.
“These are generally good recommendations, but they’re recommendations only, with generally weak levels of evidence. What we really need are clinical trials that can give us definitive answers to some of these questions,” he said.
Recommendations
The seven key recommendations, based on the clinical questions raised, are summarized below:
- Delayed (asynchronous) reconstruction is acceptable. Although the quality of the evidence is low and the recommendations are listed as an option, the guideline authors said that depending on the situation, reconstruction can be performed either immediately after resection or delayed by days, weeks, “or even months.”
- Systemic antibiotics should not be routinely prescribed in the interim between resection and reconstruction in adults. Here too, the evidence is low and the recommendation strength is weak, but in “the absence of data showing convincing benefits, systemic antibiotic therapy does not appear necessary or desirable in most cases when there is an interval between cancer resection and reconstruction,” the work group wrote.
- Clinicians may administer perioperative systemic antibiotics in a facility-based setting for adults undergoing reconstruction (3a), but antibiotics should not be routinely prescribed in an office-based setting (3b). The rationale for these recommendations, supported by a moderate level of evidence, is that the risk of surgical-site infection is generally higher in facilities, compared with an office-based setting. Patients who undergo reconstruction in hospitals or surgical centers are more likely to have complex reconstructions or have risks that may make them suitable candidates for antibiotics, but patients in office-based setting may often be spared from the additional costs, side effects, and possible drug interactions from antibiotic use. “There is no evidence in either setting that long-term antibiotic prophylaxis provides infection risk reduction, compared with short-term prophylaxis,” the guideline working group wrote.
- Continue anticoagulant, antithrombotic, and antiplatelet medications for adult patients undergoing reconstruction after skin cancer resection in the office-based setting (4a), and in the facility-based setting should coordinate with the physician managing anticoagulation before modifying the medication prior to surgery (4b). Evidence quality and recommendation strength are both moderate.
- The guideline authors recommend against routine prescription of narcotics as first-line treatment for pain in adults undergoing skin reconstruction (5a), favoring instead acetaminophen and NSAIDs as first-line therapy (5b). Evidence quality and recommendation strength are both moderate.
- In the absence of standardized protocols for the management of pain medications, oral antibiotics, and/or anticoagulants in the perioperative period, clinicians should discuss possible approaches with adult patients. “Educating patients about their perioperative treatment through discussion of treatment strategies may help alleviate anxiety, improve communication, increase patient satisfaction, and maximize patient compliance with the postoperative orders,” the guideline authors wrote.
- The authors suggest that adult patients may be offered follow-up assessments to discuss functional and cosmetic outcomes. “The return of the patient for follow-up visits is an excellent opportunity to better understand and measure these outcomes, improve patient-physician communication, and foster quality improvement. Postoperative follow-up can lead to increased communication between the patient and physician, thereby empowering patients to comment on satisfaction and other important outcomes measures,” they wrote.
What’s next
The guideline developers acknowledged that data are limited regarding reconstructive surgery following skin cancer resection, and that higher-quality studies would help to improve future guidelines. Dr. Chen said that greater use of prospective surgical databases and more systematic collection of patient-reported outcomes could inform further efforts.
The guideline development process was supported by the various groups represented. Dr. Chen and Dr. Cetrulo reported no relevant disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Desmoplastic Melanoma Masquerading as Neurofibroma
Desmoplastic melanoma (DMM) is a rare variant of melanoma that presents major challenges to both clinicians and pathologists.1 Clinically, the lesions may appear as subtle bland papules, nodules, or plaques. They can be easily mistaken for benign growths, leading to a delayed diagnosis. Consequently, most DMMs at the time of diagnosis tend to be thick, with a mean Breslow depth ranging from 2.0 to 6.5 mm.2 Histopathologic evaluation has its difficulties. At scanning magnification, these tumors may show low cellularity, mimicking a benign proliferation. It is well recognized that S-100 and other tumor markers lack specificity for DMM, which can be positive in a range of neural tumors and other cell types.2 In some amelanotic tumors, DMM becomes virtually indistinguishable from benign peripheral sheath tumors such as neurofribroma.3
Desmoplastic melanoma is exceedingly uncommon in the United States, with an estimated annual incidence rate of 2.0 cases per million.2 Typical locations of presentation include sun-exposed skin, with the head and neck regions representing more than half of reported cases.2 Desmoplastic melanoma largely is a disease of fair-skinned patients, with 95.5% of cases in the United States occurring in white non-Hispanic individuals. Advancing age, male gender, and head and neck location are associated with an increased risk for DMM-specific death.2 It is important that new or changing lesions in the correct cohort and location are biopsied promptly. We present this case to highlight the ongoing challenges of diagnosing DMM both clinically and histologically and to review the salient features of this often benign-appearing tumor.
Case Report
A 51-year-old White man with a history of prostate cancer, a personal and family history of melanoma, and benign neurofibromas presented with a 6-mm, pink, well-demarcated, soft papule on the left lateral neck (Figure 1). The lesion had been stable for many years but began growing more rapidly 1 to 2 years prior to presentation. The lesion was asymptomatic, and he denied changes in color or texture. There also was no bleeding or ulceration. A review of systems was unremarkable. A shave biopsy of the lesion revealed a nodular spindle cell tumor in the dermis resembling a neurofibroma on low power (Figure 2). However, overlying the tumor was a confluent proliferation positive for MART-1 and S-100, which was consistent with a diagnosis of melanoma in situ (Figure 3). Higher-power evaluation of the dermal proliferation showed both bland and hyperchromatic spindled and epithelioid cells (Figure 4), with rare mitotic figures highlighted by PHH3, an uncommon finding in neurofibromas (Figure 5). The dermal spindle cells were positive for S-100 and p75 and negative for Melan-A. Epithelial membrane antigen highlighted a faint sheath surrounding the dermal component. Ki-67 revealed a mildly increased proliferative index in the dermal component. The diagnosis of DMM was made after outside dermatopathology consultation was in agreement. However, the possibility of a melanoma in situ growing in association with an underlying neurofibroma remained a diagnostic consideration histologically. The lesion was widely excised.
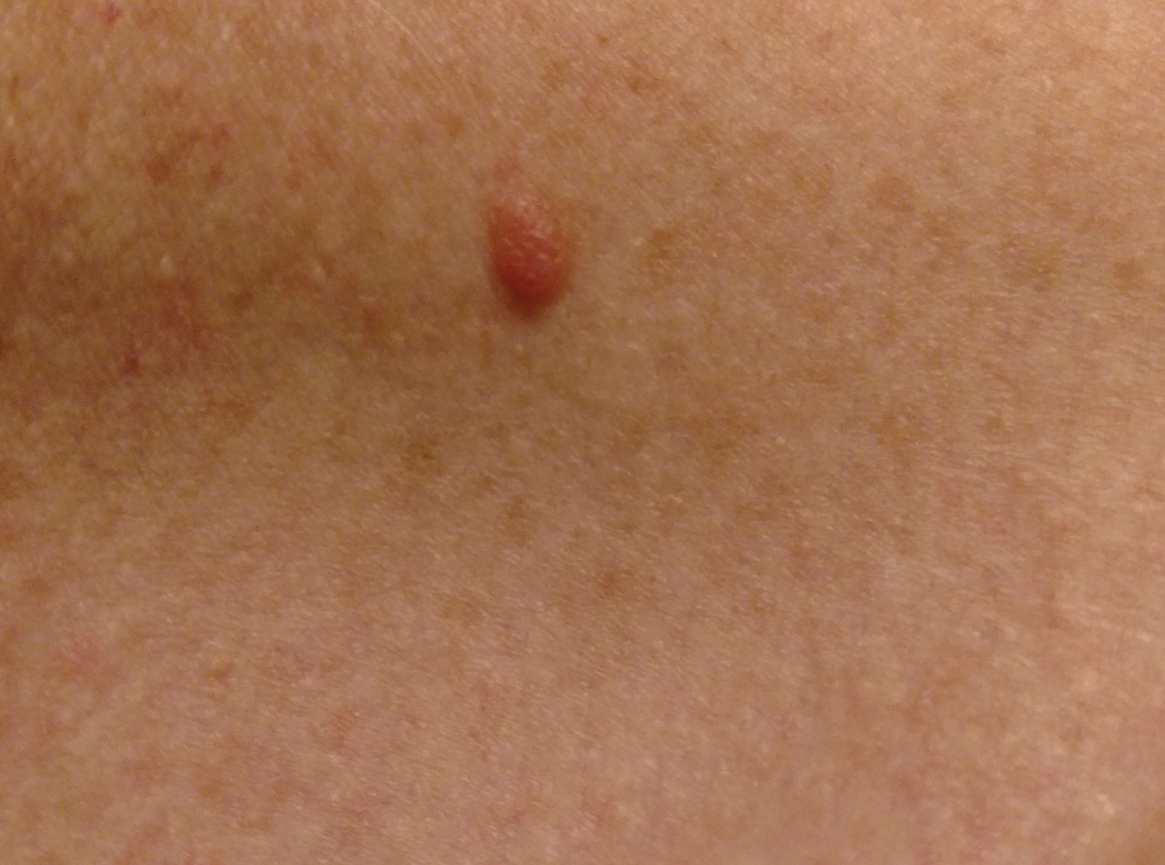
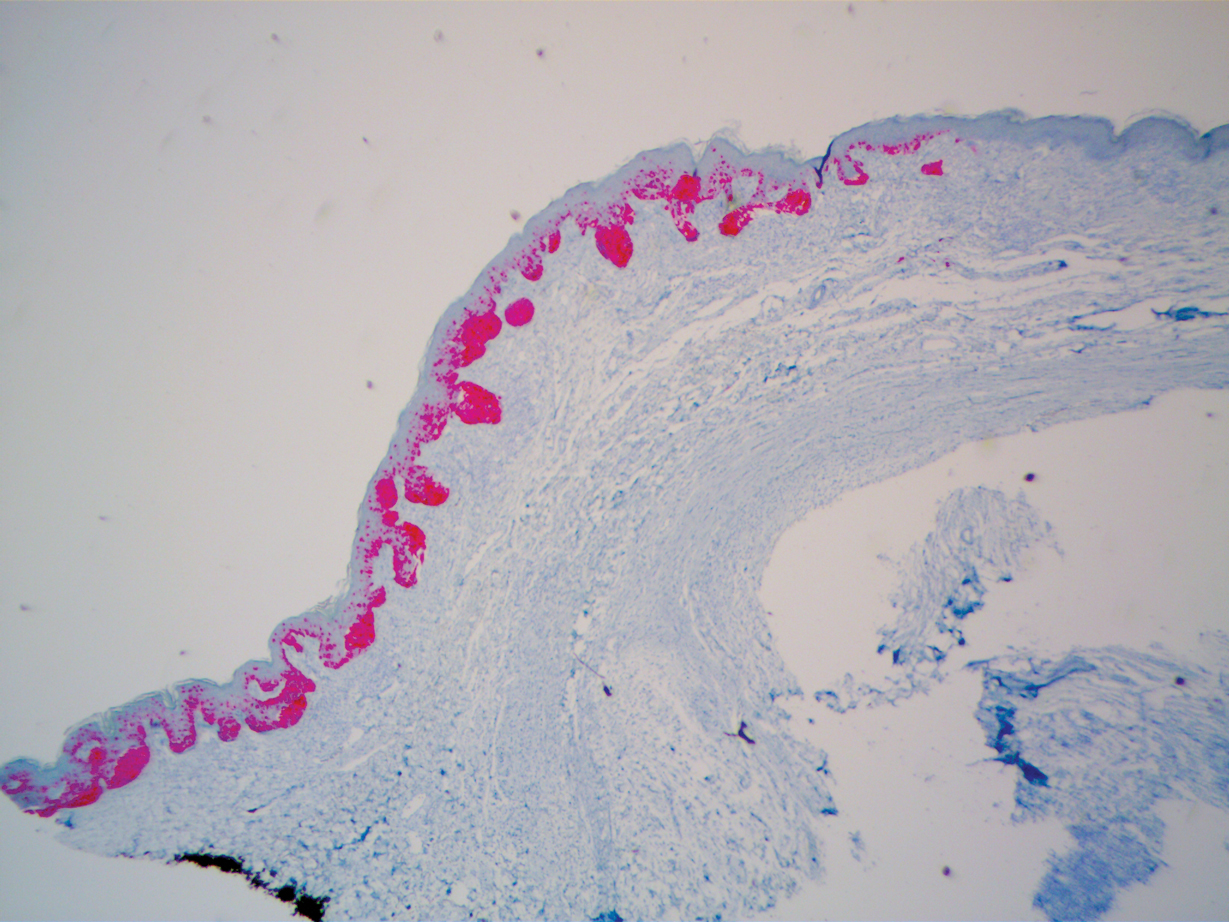
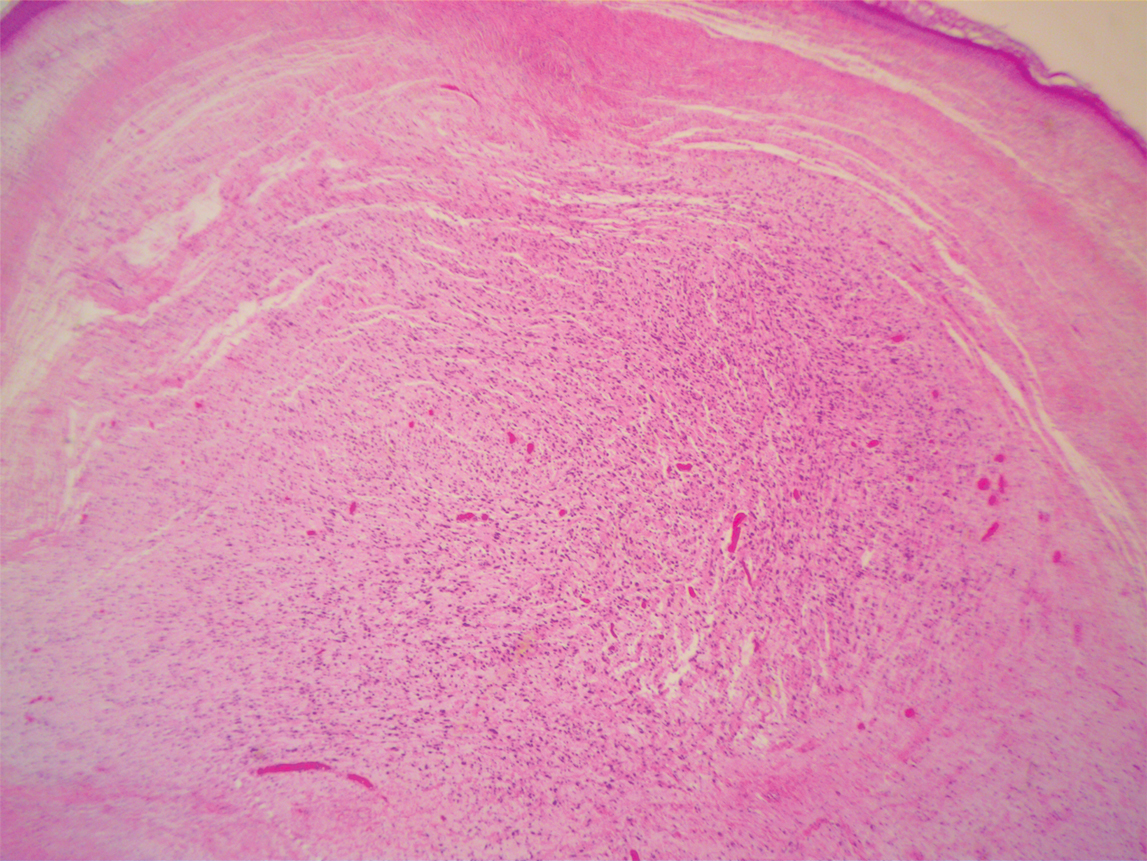
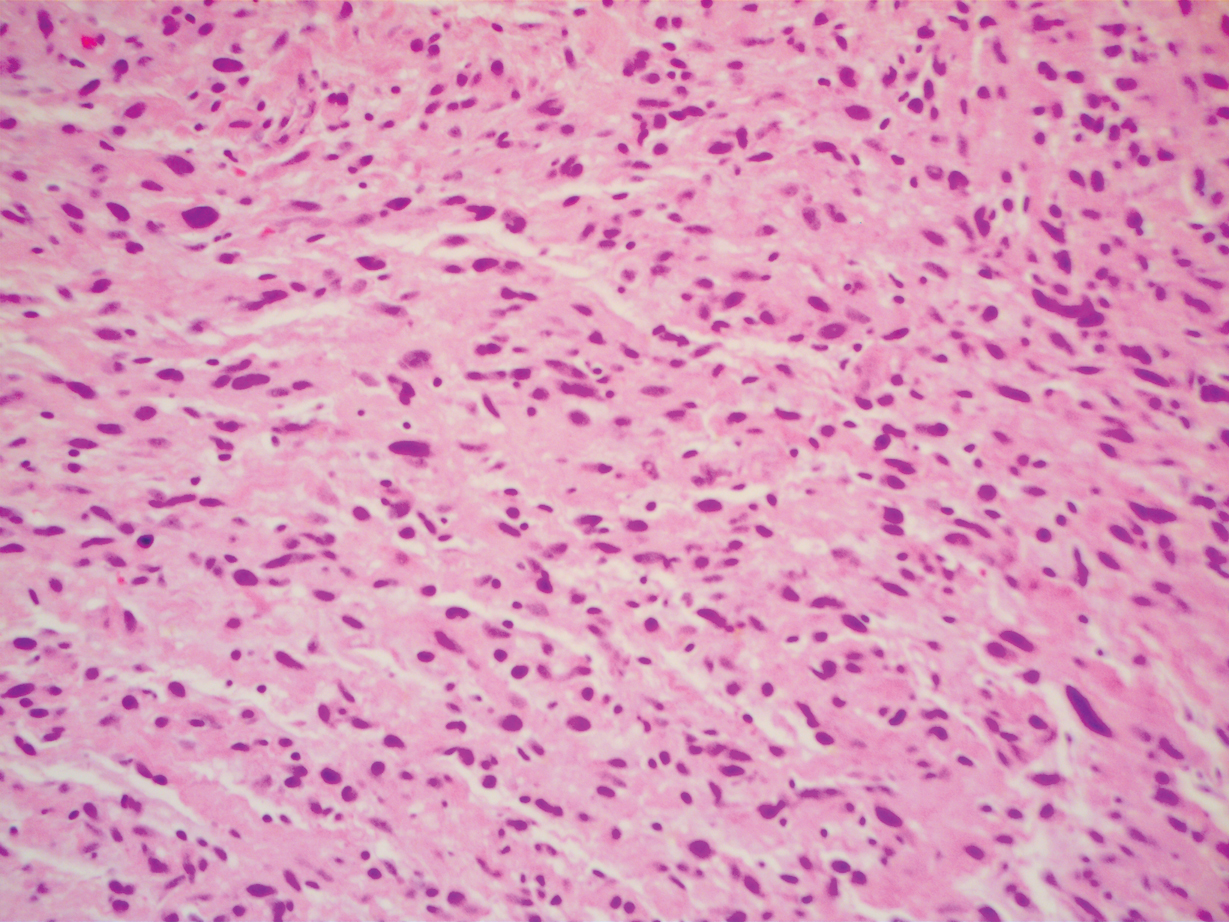
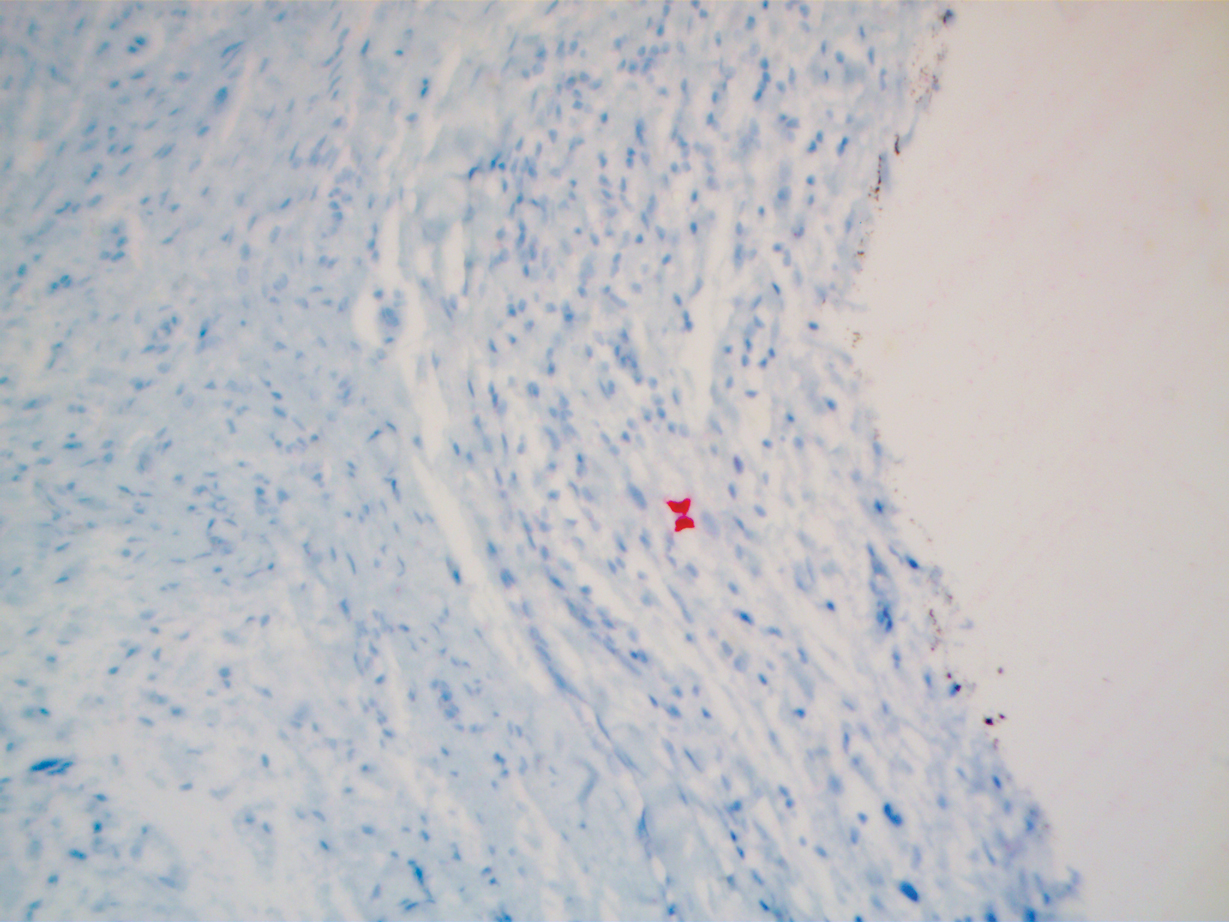
Comment
Differential for DMM
Early DMMs may not show sufficient cytologic atypia to permit obvious distinction from neurofibromas, which becomes problematic when encountering a spindle cell proliferation within severely sun-damaged skin, or even more so when an intraepidermal population of melanocytes is situated above a dermal population of slender, spindled, S-100–positive cells, as seen in our patient.4 For these challenging scenarios, Yeh and McCalmont4 have proposed evaluating for a CD34 “fingerprint” pattern. This pattern typically is widespread in neurofibroma but absent or limited in DMM, and it is a useful adjunct in the differential diagnosis when conventional immunohistochemistry has little contribution.
There are several case reports in the literature of DMM mimicking other benign or malignant proliferations. In 2012, Jou et al5 described a case of a 62-year-old White man who presented with an oral nodule consistent with fibrous inflammatory hyperplasia clinically. Incisional biopsy later confirmed the diagnosis of amelanotic DMM.5 Similar case reports have been described in which the diagnosis of DMM was later found to resemble a sarcoma and malignant peripheral nerve sheath tumor.6,7
Diagnosis of DMM
The prototypical DMM is an asymmetrical and deeply infiltrative spindle cell lesion in severely sun-damaged skin. By definition, the individual melanocytes are separated by connective tissue components, giving the tumor a paucicellular appearance.1 Although the low cellularity can give a deceptively bland scanning aspect, on high-power examination there usually are identifiable atypical spindled cells with enlarged, elongated, and hyperchromatic nuclei. S-100 typically is diffusely positive in DMM, though occasional cases show more limited staining.8 Other commonly used and more specific markers of melanocytic differentiation, including HMB45 and Melan-A, typically are negative in the paucicellular spindle cell components.9 Desmoplastic melanoma can be further categorized by the degree of fibrosis within a particular tumor. If fibrosis is prominent throughout the entire tumor, it is named pure DMM. On the other hand, fibrosis may only represent a portion of an otherwise nondesmoplastic melanoma, which is known as combined DMM.10
Conclusion
We present this case to highlight the ongoing challenges of diagnosing DMM both clinically and histologically. Although a bland-appearing lesion, key clinical features prompting a biopsy in our patient included recent growth of the lesion, a personal history of melanoma, the patient’s fair skin type, a history of heavy sun exposure, and the location of the lesion. According to Busam,11 an associated melanoma in situ component is identified in 80% to 85% of DMM cases. Detection of a melanoma in situ component associated with a malignant spindle cell tumor can help establish the diagnosis of DMM. In the absence of melanoma in situ, a strong diffuse immunoreactivity for S-100 and lack of epithelial markers support the diagnosis.11 After review of the literature, our case likely represents DMM as opposed to a melanoma in situ developing within a neurofibroma.
- Wood BA. Desmoplastic melanoma: recent advances and persisting challenges. Pathology. 2013;45:453-463.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Machado I, Llombart B, Cruz J, et al. Desmoplastic melanoma may mimic a cutaneous peripheral nerve sheath tumor: report of 3 challenging cases. J Cutan Pathol. 2017;4:632-638.
- Yeh I, McCalmont, TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Jou A, Miranda FV, Oliveira MG, et al. Oral desmoplastic melanoma mimicking inflammatory hyperplasia. Gerodontology. 2012;29:E1163-E1167.
- Ishikura H, Kojo T, Ichimura H, et al. Desmoplastic malignant melanoma of the uterine cervix: a rare primary malignancy in the uterus mimicking a sarcoma. Histopathology. 1998;33:93-94.
- Barnett SL, Wells MJ, Mickey B, et al. Perineural extension of cutaneous desmoplastic melanoma mimicking an intracranial malignant peripheral nerve sheath tumor. case report. J Neurosurg. 2011;115:273-277.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- Skelton HG, Maceira J, Smith KJ, et al. HMB45 negative spindle cell malignant melanoma. Am J Dermatopathol. 1997;19:580-584.
- George E, McClain SE, Slingluff CL, et al. Subclassification of desmoplastic melanoma: pure and mixed variants have significantly different capacities for lymph node metastasis. J Cutan Pathol. 2009;36:425-432.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
Desmoplastic melanoma (DMM) is a rare variant of melanoma that presents major challenges to both clinicians and pathologists.1 Clinically, the lesions may appear as subtle bland papules, nodules, or plaques. They can be easily mistaken for benign growths, leading to a delayed diagnosis. Consequently, most DMMs at the time of diagnosis tend to be thick, with a mean Breslow depth ranging from 2.0 to 6.5 mm.2 Histopathologic evaluation has its difficulties. At scanning magnification, these tumors may show low cellularity, mimicking a benign proliferation. It is well recognized that S-100 and other tumor markers lack specificity for DMM, which can be positive in a range of neural tumors and other cell types.2 In some amelanotic tumors, DMM becomes virtually indistinguishable from benign peripheral sheath tumors such as neurofribroma.3
Desmoplastic melanoma is exceedingly uncommon in the United States, with an estimated annual incidence rate of 2.0 cases per million.2 Typical locations of presentation include sun-exposed skin, with the head and neck regions representing more than half of reported cases.2 Desmoplastic melanoma largely is a disease of fair-skinned patients, with 95.5% of cases in the United States occurring in white non-Hispanic individuals. Advancing age, male gender, and head and neck location are associated with an increased risk for DMM-specific death.2 It is important that new or changing lesions in the correct cohort and location are biopsied promptly. We present this case to highlight the ongoing challenges of diagnosing DMM both clinically and histologically and to review the salient features of this often benign-appearing tumor.
Case Report
A 51-year-old White man with a history of prostate cancer, a personal and family history of melanoma, and benign neurofibromas presented with a 6-mm, pink, well-demarcated, soft papule on the left lateral neck (Figure 1). The lesion had been stable for many years but began growing more rapidly 1 to 2 years prior to presentation. The lesion was asymptomatic, and he denied changes in color or texture. There also was no bleeding or ulceration. A review of systems was unremarkable. A shave biopsy of the lesion revealed a nodular spindle cell tumor in the dermis resembling a neurofibroma on low power (Figure 2). However, overlying the tumor was a confluent proliferation positive for MART-1 and S-100, which was consistent with a diagnosis of melanoma in situ (Figure 3). Higher-power evaluation of the dermal proliferation showed both bland and hyperchromatic spindled and epithelioid cells (Figure 4), with rare mitotic figures highlighted by PHH3, an uncommon finding in neurofibromas (Figure 5). The dermal spindle cells were positive for S-100 and p75 and negative for Melan-A. Epithelial membrane antigen highlighted a faint sheath surrounding the dermal component. Ki-67 revealed a mildly increased proliferative index in the dermal component. The diagnosis of DMM was made after outside dermatopathology consultation was in agreement. However, the possibility of a melanoma in situ growing in association with an underlying neurofibroma remained a diagnostic consideration histologically. The lesion was widely excised.





Comment
Differential for DMM
Early DMMs may not show sufficient cytologic atypia to permit obvious distinction from neurofibromas, which becomes problematic when encountering a spindle cell proliferation within severely sun-damaged skin, or even more so when an intraepidermal population of melanocytes is situated above a dermal population of slender, spindled, S-100–positive cells, as seen in our patient.4 For these challenging scenarios, Yeh and McCalmont4 have proposed evaluating for a CD34 “fingerprint” pattern. This pattern typically is widespread in neurofibroma but absent or limited in DMM, and it is a useful adjunct in the differential diagnosis when conventional immunohistochemistry has little contribution.
There are several case reports in the literature of DMM mimicking other benign or malignant proliferations. In 2012, Jou et al5 described a case of a 62-year-old White man who presented with an oral nodule consistent with fibrous inflammatory hyperplasia clinically. Incisional biopsy later confirmed the diagnosis of amelanotic DMM.5 Similar case reports have been described in which the diagnosis of DMM was later found to resemble a sarcoma and malignant peripheral nerve sheath tumor.6,7
Diagnosis of DMM
The prototypical DMM is an asymmetrical and deeply infiltrative spindle cell lesion in severely sun-damaged skin. By definition, the individual melanocytes are separated by connective tissue components, giving the tumor a paucicellular appearance.1 Although the low cellularity can give a deceptively bland scanning aspect, on high-power examination there usually are identifiable atypical spindled cells with enlarged, elongated, and hyperchromatic nuclei. S-100 typically is diffusely positive in DMM, though occasional cases show more limited staining.8 Other commonly used and more specific markers of melanocytic differentiation, including HMB45 and Melan-A, typically are negative in the paucicellular spindle cell components.9 Desmoplastic melanoma can be further categorized by the degree of fibrosis within a particular tumor. If fibrosis is prominent throughout the entire tumor, it is named pure DMM. On the other hand, fibrosis may only represent a portion of an otherwise nondesmoplastic melanoma, which is known as combined DMM.10
Conclusion
We present this case to highlight the ongoing challenges of diagnosing DMM both clinically and histologically. Although a bland-appearing lesion, key clinical features prompting a biopsy in our patient included recent growth of the lesion, a personal history of melanoma, the patient’s fair skin type, a history of heavy sun exposure, and the location of the lesion. According to Busam,11 an associated melanoma in situ component is identified in 80% to 85% of DMM cases. Detection of a melanoma in situ component associated with a malignant spindle cell tumor can help establish the diagnosis of DMM. In the absence of melanoma in situ, a strong diffuse immunoreactivity for S-100 and lack of epithelial markers support the diagnosis.11 After review of the literature, our case likely represents DMM as opposed to a melanoma in situ developing within a neurofibroma.
Desmoplastic melanoma (DMM) is a rare variant of melanoma that presents major challenges to both clinicians and pathologists.1 Clinically, the lesions may appear as subtle bland papules, nodules, or plaques. They can be easily mistaken for benign growths, leading to a delayed diagnosis. Consequently, most DMMs at the time of diagnosis tend to be thick, with a mean Breslow depth ranging from 2.0 to 6.5 mm.2 Histopathologic evaluation has its difficulties. At scanning magnification, these tumors may show low cellularity, mimicking a benign proliferation. It is well recognized that S-100 and other tumor markers lack specificity for DMM, which can be positive in a range of neural tumors and other cell types.2 In some amelanotic tumors, DMM becomes virtually indistinguishable from benign peripheral sheath tumors such as neurofribroma.3
Desmoplastic melanoma is exceedingly uncommon in the United States, with an estimated annual incidence rate of 2.0 cases per million.2 Typical locations of presentation include sun-exposed skin, with the head and neck regions representing more than half of reported cases.2 Desmoplastic melanoma largely is a disease of fair-skinned patients, with 95.5% of cases in the United States occurring in white non-Hispanic individuals. Advancing age, male gender, and head and neck location are associated with an increased risk for DMM-specific death.2 It is important that new or changing lesions in the correct cohort and location are biopsied promptly. We present this case to highlight the ongoing challenges of diagnosing DMM both clinically and histologically and to review the salient features of this often benign-appearing tumor.
Case Report
A 51-year-old White man with a history of prostate cancer, a personal and family history of melanoma, and benign neurofibromas presented with a 6-mm, pink, well-demarcated, soft papule on the left lateral neck (Figure 1). The lesion had been stable for many years but began growing more rapidly 1 to 2 years prior to presentation. The lesion was asymptomatic, and he denied changes in color or texture. There also was no bleeding or ulceration. A review of systems was unremarkable. A shave biopsy of the lesion revealed a nodular spindle cell tumor in the dermis resembling a neurofibroma on low power (Figure 2). However, overlying the tumor was a confluent proliferation positive for MART-1 and S-100, which was consistent with a diagnosis of melanoma in situ (Figure 3). Higher-power evaluation of the dermal proliferation showed both bland and hyperchromatic spindled and epithelioid cells (Figure 4), with rare mitotic figures highlighted by PHH3, an uncommon finding in neurofibromas (Figure 5). The dermal spindle cells were positive for S-100 and p75 and negative for Melan-A. Epithelial membrane antigen highlighted a faint sheath surrounding the dermal component. Ki-67 revealed a mildly increased proliferative index in the dermal component. The diagnosis of DMM was made after outside dermatopathology consultation was in agreement. However, the possibility of a melanoma in situ growing in association with an underlying neurofibroma remained a diagnostic consideration histologically. The lesion was widely excised.





Comment
Differential for DMM
Early DMMs may not show sufficient cytologic atypia to permit obvious distinction from neurofibromas, which becomes problematic when encountering a spindle cell proliferation within severely sun-damaged skin, or even more so when an intraepidermal population of melanocytes is situated above a dermal population of slender, spindled, S-100–positive cells, as seen in our patient.4 For these challenging scenarios, Yeh and McCalmont4 have proposed evaluating for a CD34 “fingerprint” pattern. This pattern typically is widespread in neurofibroma but absent or limited in DMM, and it is a useful adjunct in the differential diagnosis when conventional immunohistochemistry has little contribution.
There are several case reports in the literature of DMM mimicking other benign or malignant proliferations. In 2012, Jou et al5 described a case of a 62-year-old White man who presented with an oral nodule consistent with fibrous inflammatory hyperplasia clinically. Incisional biopsy later confirmed the diagnosis of amelanotic DMM.5 Similar case reports have been described in which the diagnosis of DMM was later found to resemble a sarcoma and malignant peripheral nerve sheath tumor.6,7
Diagnosis of DMM
The prototypical DMM is an asymmetrical and deeply infiltrative spindle cell lesion in severely sun-damaged skin. By definition, the individual melanocytes are separated by connective tissue components, giving the tumor a paucicellular appearance.1 Although the low cellularity can give a deceptively bland scanning aspect, on high-power examination there usually are identifiable atypical spindled cells with enlarged, elongated, and hyperchromatic nuclei. S-100 typically is diffusely positive in DMM, though occasional cases show more limited staining.8 Other commonly used and more specific markers of melanocytic differentiation, including HMB45 and Melan-A, typically are negative in the paucicellular spindle cell components.9 Desmoplastic melanoma can be further categorized by the degree of fibrosis within a particular tumor. If fibrosis is prominent throughout the entire tumor, it is named pure DMM. On the other hand, fibrosis may only represent a portion of an otherwise nondesmoplastic melanoma, which is known as combined DMM.10
Conclusion
We present this case to highlight the ongoing challenges of diagnosing DMM both clinically and histologically. Although a bland-appearing lesion, key clinical features prompting a biopsy in our patient included recent growth of the lesion, a personal history of melanoma, the patient’s fair skin type, a history of heavy sun exposure, and the location of the lesion. According to Busam,11 an associated melanoma in situ component is identified in 80% to 85% of DMM cases. Detection of a melanoma in situ component associated with a malignant spindle cell tumor can help establish the diagnosis of DMM. In the absence of melanoma in situ, a strong diffuse immunoreactivity for S-100 and lack of epithelial markers support the diagnosis.11 After review of the literature, our case likely represents DMM as opposed to a melanoma in situ developing within a neurofibroma.
- Wood BA. Desmoplastic melanoma: recent advances and persisting challenges. Pathology. 2013;45:453-463.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Machado I, Llombart B, Cruz J, et al. Desmoplastic melanoma may mimic a cutaneous peripheral nerve sheath tumor: report of 3 challenging cases. J Cutan Pathol. 2017;4:632-638.
- Yeh I, McCalmont, TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Jou A, Miranda FV, Oliveira MG, et al. Oral desmoplastic melanoma mimicking inflammatory hyperplasia. Gerodontology. 2012;29:E1163-E1167.
- Ishikura H, Kojo T, Ichimura H, et al. Desmoplastic malignant melanoma of the uterine cervix: a rare primary malignancy in the uterus mimicking a sarcoma. Histopathology. 1998;33:93-94.
- Barnett SL, Wells MJ, Mickey B, et al. Perineural extension of cutaneous desmoplastic melanoma mimicking an intracranial malignant peripheral nerve sheath tumor. case report. J Neurosurg. 2011;115:273-277.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- Skelton HG, Maceira J, Smith KJ, et al. HMB45 negative spindle cell malignant melanoma. Am J Dermatopathol. 1997;19:580-584.
- George E, McClain SE, Slingluff CL, et al. Subclassification of desmoplastic melanoma: pure and mixed variants have significantly different capacities for lymph node metastasis. J Cutan Pathol. 2009;36:425-432.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Wood BA. Desmoplastic melanoma: recent advances and persisting challenges. Pathology. 2013;45:453-463.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Machado I, Llombart B, Cruz J, et al. Desmoplastic melanoma may mimic a cutaneous peripheral nerve sheath tumor: report of 3 challenging cases. J Cutan Pathol. 2017;4:632-638.
- Yeh I, McCalmont, TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Jou A, Miranda FV, Oliveira MG, et al. Oral desmoplastic melanoma mimicking inflammatory hyperplasia. Gerodontology. 2012;29:E1163-E1167.
- Ishikura H, Kojo T, Ichimura H, et al. Desmoplastic malignant melanoma of the uterine cervix: a rare primary malignancy in the uterus mimicking a sarcoma. Histopathology. 1998;33:93-94.
- Barnett SL, Wells MJ, Mickey B, et al. Perineural extension of cutaneous desmoplastic melanoma mimicking an intracranial malignant peripheral nerve sheath tumor. case report. J Neurosurg. 2011;115:273-277.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- Skelton HG, Maceira J, Smith KJ, et al. HMB45 negative spindle cell malignant melanoma. Am J Dermatopathol. 1997;19:580-584.
- George E, McClain SE, Slingluff CL, et al. Subclassification of desmoplastic melanoma: pure and mixed variants have significantly different capacities for lymph node metastasis. J Cutan Pathol. 2009;36:425-432.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
Practice Points
- Desmoplastic melanoma remains a diagnostic challenge both clinically and histologically.
- New or changing lesions on sun-exposed sites of elderly patients with fair skin types should have a low threshold for biopsy.
- Consensus between more than one dermatopathologist is sometimes required to make the diagnosis histologically.
Mohs Micrographic Surgery During the COVID-19 Pandemic: Considering the Patient Perspective
Guidelines on Skin Cancer Surgeries During the COVID-19 Pandemic
At the start of the COVID-19 pandemic, the Centers for Disease Control and Prevention issued recommendations to decrease the spread of SARS-CoV-2 and optimize the use of personal protective equipment (PPE) for frontline workers.1 In the field of dermatologic surgery, the American College of Mohs Surgery, the National Comprehensive Cancer Network, the American Society for Dermatologic Surgery, and the American Academy of Dermatology made recommendations to postpone nonessential and nonurgent procedures.2-4 The initial guidelines of the American College of Mohs Surgery advised cancellation of all elective surgeries and deferred treatment of most cases of basal cell carcinoma for as long as 3 months; low-risk squamous cell carcinoma (SCC) and melanoma in situ treatment was deferred for as long as 2 or 3 months.3 Additional recommendations were made to reserve inpatient visits for suspicious lesions and high-risk cancers, postpone other nonessential and nonurgent appointments, and utilize telemedicine whenever possible.5
These recommendations led to great uncertainty and stress for patients and providers. Although numerous important variables, such as patient risk factors, severity of disease, availability of PPE and staff, and patient-to-provider transmission were considered when creating these guidelines, the patient’s experience likely was not a contributing factor.
COVID-19 Transmission During Mohs Surgery
There have been concerns that surgeons performing Mohs micrographic surgery (MMS) might be at an increased risk for COVID-19, given their close contact with high-risk sites (ie, nose, mouth) and cautery-generated aerosols; most of the estimated transmission risk associated with MMS has been based on head and neck surgery experience and publications.6-8 Tee and colleagues9 recently published their institution’s MMS COVID-19 preventive measures, which, to their knowledge, have prevented all intraoperative transmission of SARS-CoV-2, even in disease-positive patients. Currently, evidence is lacking to support a high risk for SARS-CoV-2 transmission during MMS when proper PPE and personal hygiene measures as well as strict infection control protocols—presurgical COVID-19 testing in high-risk cases, COVID-19 screening optimization, visitor restrictions, and appropriate disinfection between patients—are in place.
The Impact of Postponing Treatment on Patients
Although studies have focused on the effects of the COVID-19 pandemic on physicians practicing MMS,10 little is known about the effects of delays in skin cancer treatment on patients. A survey conducted in the United Kingdom investigating the patient’s perspective found that patients expressed worry and concern about the possibility that their MMS would be postponed and greatly appreciated continuation of treatment during the pandemic.11
Other medical specialties have reported their patient experiences during the pandemic. In a study examining patient perception of postponed surgical treatment of pelvic floor disorders due to COVID-19, nearly half of survey respondents were unhappy with the delay in receiving care. Furthermore, patients who reported being unhappy were more likely to report feelings of isolation and anxiety because their surgery was postponed.12 In another study involving patients with lung cancer, 9.1% (N=15) of patients postponed their treatment during the COVID-19 pandemic because of pandemic-related anxiety.13
With the goal of improving care at our institution, we conducted a brief institutional review board–approved survey to evaluate how postponing MMS treatment due to the COVID-19 pandemic affected patients. All MMS patients undergoing surgery in June 2020 and July 2020 (N=99) were asked to complete our voluntary and anonymous 23-question survey in person during their procedure. We obtained 88 responses (response rate, 89%). Twenty percent of surveyed patients (n=18) reported that their MMS had been postponed; 78% of those whose MMS was postponed (n=14) indicated some level of anxiety during the waiting period. It was unclear which patients had their treatment postponed based on national guidelines and which ones elected to postpone surgery.
Tips for Health Care Providers
Patient-provider communication highlighting specific skin cancer risk and the risk vs benefit of postponing treatment might reduce anxiety and stress during the waiting period.14 A study found that COVID-19 posed a bigger threat than most noninvasive skin cancers; therefore, the authors of that study concluded that treatment for most skin cancers could be safely postponed.15 Specifically, those authors recommended prioritizing treatment for Merkel cell carcinoma, invasive SCC, and melanoma with positive margins or macroscopic residual disease. They proposed that all other skin cancers, including basal cell carcinoma, SCC in situ, and melanoma with negative margins and no macroscopic residual disease, could be safely delayed for as long as 3 months.15
For patients with multiple risk factors for COVID-19–related morbidity or mortality, delaying skin cancer treatment likely has less risk than contracting the virus.15 This information should be communicated with patients. Investigation of specific patient concerns is warranted, and case-by-case evaluation of patients’ risk factors and skin cancer risk should be considered.
Based on the current, though limited, literature, delaying medical treatment can have a negative impact on the patient experience. Furthermore, proper precautions have been shown to limit intraoperative transmission of SARS-CoV-2 during MMS, but research is lacking. Practitioners should utilize shared decision-making and evaluate a given patient’s risk factors and concerns when deciding whether to postpone treatment. We encourage other institutions to evaluate the effects that delaying MMS has had on their patients, as further studies would improve understanding of patients’ experiences during a pandemic and potentially influence future dermatology guidelines.
- Center for Disease Control and Prevention. COVID-19. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- American College of Mohs Surgery. Mohs surgery ambulatory protocol during COVID pandemic (version 6-3-20). June 4, 2020. Accessed April 20, 2021. http://staging.mohscollege.org/UserFiles/AM20/Member%20Alert/MohsSurgeryAmbulatoryProtocolDuringCOVIDPandemicFinal.pdf
- COVID-19 resources. National Comprehensive Cancer Network website. Accessed April 20, 2021. https://www.nccn.org/covid-19
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed April 10, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic.J Am Acad Dermatol. 2020;83:295-296. doi:10.1016/j.jaad.2020.05.002
- Yuan JT, Jiang SIB. Urgent safety considerations for dermatologic surgeons in the COVID-19 pandemic. Dermatol Online J. 2020;26:1. Accessed April 20, 2021. http://escholarship.org/uc/item/2qr3w771
- Otolaryngologists may contract COVID-19 during surgery. ENTtoday. March 20, 2020. Accessed April 20, 2021. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/
- Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg. 2020;163:98-103. doi:10.1177/0194599820927335
- Tee MW, Stewart C, Aliessa S, et al. Dermatological surgery during the COVID-19 pandemic: experience of a large academic center. J Am Acad Dermatol. 2021;84:1094-1096. doi:10.1016/j.jaad.2020.12.003
- Hooper J, Feng H. The impact of COVID-19 on micrographic surgery and dermatologic oncology fellows. Dermatol Surg. 2020;46:1762-1763. doi:10.1097/DSS.0000000000002766
- Nicholson P, Ali FR, Patalay R, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180. doi:10.1111/ced.14423
- Mou T, Brown O, Gillingham A, et al. Patients’ perceptions on surgical care suspension for pelvic floor disorders during the COVID-19 pandemic. Female Pelvic Med Reconstr Surg. 2020;26:477-482. doi:10.1097/SPV.0000000000000918
- Fujita K, Ito T, Saito Z, et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983-2986. doi:10.1111/1759-7714.13615
- Nikumb VB, Banerjee A, Kaur G, et al. Impact of doctor-patient communication on preoperative anxiety: study at industrial township, Pimpri, Pune. Ind Psychiatry J. 2009;18:19-21. doi:10.4103/0972-6748.57852
- Baumann BC, MacArthur KM, Brewer JD, et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900-3906. doi:10.1002/cncr.32969
Guidelines on Skin Cancer Surgeries During the COVID-19 Pandemic
At the start of the COVID-19 pandemic, the Centers for Disease Control and Prevention issued recommendations to decrease the spread of SARS-CoV-2 and optimize the use of personal protective equipment (PPE) for frontline workers.1 In the field of dermatologic surgery, the American College of Mohs Surgery, the National Comprehensive Cancer Network, the American Society for Dermatologic Surgery, and the American Academy of Dermatology made recommendations to postpone nonessential and nonurgent procedures.2-4 The initial guidelines of the American College of Mohs Surgery advised cancellation of all elective surgeries and deferred treatment of most cases of basal cell carcinoma for as long as 3 months; low-risk squamous cell carcinoma (SCC) and melanoma in situ treatment was deferred for as long as 2 or 3 months.3 Additional recommendations were made to reserve inpatient visits for suspicious lesions and high-risk cancers, postpone other nonessential and nonurgent appointments, and utilize telemedicine whenever possible.5
These recommendations led to great uncertainty and stress for patients and providers. Although numerous important variables, such as patient risk factors, severity of disease, availability of PPE and staff, and patient-to-provider transmission were considered when creating these guidelines, the patient’s experience likely was not a contributing factor.
COVID-19 Transmission During Mohs Surgery
There have been concerns that surgeons performing Mohs micrographic surgery (MMS) might be at an increased risk for COVID-19, given their close contact with high-risk sites (ie, nose, mouth) and cautery-generated aerosols; most of the estimated transmission risk associated with MMS has been based on head and neck surgery experience and publications.6-8 Tee and colleagues9 recently published their institution’s MMS COVID-19 preventive measures, which, to their knowledge, have prevented all intraoperative transmission of SARS-CoV-2, even in disease-positive patients. Currently, evidence is lacking to support a high risk for SARS-CoV-2 transmission during MMS when proper PPE and personal hygiene measures as well as strict infection control protocols—presurgical COVID-19 testing in high-risk cases, COVID-19 screening optimization, visitor restrictions, and appropriate disinfection between patients—are in place.
The Impact of Postponing Treatment on Patients
Although studies have focused on the effects of the COVID-19 pandemic on physicians practicing MMS,10 little is known about the effects of delays in skin cancer treatment on patients. A survey conducted in the United Kingdom investigating the patient’s perspective found that patients expressed worry and concern about the possibility that their MMS would be postponed and greatly appreciated continuation of treatment during the pandemic.11
Other medical specialties have reported their patient experiences during the pandemic. In a study examining patient perception of postponed surgical treatment of pelvic floor disorders due to COVID-19, nearly half of survey respondents were unhappy with the delay in receiving care. Furthermore, patients who reported being unhappy were more likely to report feelings of isolation and anxiety because their surgery was postponed.12 In another study involving patients with lung cancer, 9.1% (N=15) of patients postponed their treatment during the COVID-19 pandemic because of pandemic-related anxiety.13
With the goal of improving care at our institution, we conducted a brief institutional review board–approved survey to evaluate how postponing MMS treatment due to the COVID-19 pandemic affected patients. All MMS patients undergoing surgery in June 2020 and July 2020 (N=99) were asked to complete our voluntary and anonymous 23-question survey in person during their procedure. We obtained 88 responses (response rate, 89%). Twenty percent of surveyed patients (n=18) reported that their MMS had been postponed; 78% of those whose MMS was postponed (n=14) indicated some level of anxiety during the waiting period. It was unclear which patients had their treatment postponed based on national guidelines and which ones elected to postpone surgery.
Tips for Health Care Providers
Patient-provider communication highlighting specific skin cancer risk and the risk vs benefit of postponing treatment might reduce anxiety and stress during the waiting period.14 A study found that COVID-19 posed a bigger threat than most noninvasive skin cancers; therefore, the authors of that study concluded that treatment for most skin cancers could be safely postponed.15 Specifically, those authors recommended prioritizing treatment for Merkel cell carcinoma, invasive SCC, and melanoma with positive margins or macroscopic residual disease. They proposed that all other skin cancers, including basal cell carcinoma, SCC in situ, and melanoma with negative margins and no macroscopic residual disease, could be safely delayed for as long as 3 months.15
For patients with multiple risk factors for COVID-19–related morbidity or mortality, delaying skin cancer treatment likely has less risk than contracting the virus.15 This information should be communicated with patients. Investigation of specific patient concerns is warranted, and case-by-case evaluation of patients’ risk factors and skin cancer risk should be considered.
Based on the current, though limited, literature, delaying medical treatment can have a negative impact on the patient experience. Furthermore, proper precautions have been shown to limit intraoperative transmission of SARS-CoV-2 during MMS, but research is lacking. Practitioners should utilize shared decision-making and evaluate a given patient’s risk factors and concerns when deciding whether to postpone treatment. We encourage other institutions to evaluate the effects that delaying MMS has had on their patients, as further studies would improve understanding of patients’ experiences during a pandemic and potentially influence future dermatology guidelines.
Guidelines on Skin Cancer Surgeries During the COVID-19 Pandemic
At the start of the COVID-19 pandemic, the Centers for Disease Control and Prevention issued recommendations to decrease the spread of SARS-CoV-2 and optimize the use of personal protective equipment (PPE) for frontline workers.1 In the field of dermatologic surgery, the American College of Mohs Surgery, the National Comprehensive Cancer Network, the American Society for Dermatologic Surgery, and the American Academy of Dermatology made recommendations to postpone nonessential and nonurgent procedures.2-4 The initial guidelines of the American College of Mohs Surgery advised cancellation of all elective surgeries and deferred treatment of most cases of basal cell carcinoma for as long as 3 months; low-risk squamous cell carcinoma (SCC) and melanoma in situ treatment was deferred for as long as 2 or 3 months.3 Additional recommendations were made to reserve inpatient visits for suspicious lesions and high-risk cancers, postpone other nonessential and nonurgent appointments, and utilize telemedicine whenever possible.5
These recommendations led to great uncertainty and stress for patients and providers. Although numerous important variables, such as patient risk factors, severity of disease, availability of PPE and staff, and patient-to-provider transmission were considered when creating these guidelines, the patient’s experience likely was not a contributing factor.
COVID-19 Transmission During Mohs Surgery
There have been concerns that surgeons performing Mohs micrographic surgery (MMS) might be at an increased risk for COVID-19, given their close contact with high-risk sites (ie, nose, mouth) and cautery-generated aerosols; most of the estimated transmission risk associated with MMS has been based on head and neck surgery experience and publications.6-8 Tee and colleagues9 recently published their institution’s MMS COVID-19 preventive measures, which, to their knowledge, have prevented all intraoperative transmission of SARS-CoV-2, even in disease-positive patients. Currently, evidence is lacking to support a high risk for SARS-CoV-2 transmission during MMS when proper PPE and personal hygiene measures as well as strict infection control protocols—presurgical COVID-19 testing in high-risk cases, COVID-19 screening optimization, visitor restrictions, and appropriate disinfection between patients—are in place.
The Impact of Postponing Treatment on Patients
Although studies have focused on the effects of the COVID-19 pandemic on physicians practicing MMS,10 little is known about the effects of delays in skin cancer treatment on patients. A survey conducted in the United Kingdom investigating the patient’s perspective found that patients expressed worry and concern about the possibility that their MMS would be postponed and greatly appreciated continuation of treatment during the pandemic.11
Other medical specialties have reported their patient experiences during the pandemic. In a study examining patient perception of postponed surgical treatment of pelvic floor disorders due to COVID-19, nearly half of survey respondents were unhappy with the delay in receiving care. Furthermore, patients who reported being unhappy were more likely to report feelings of isolation and anxiety because their surgery was postponed.12 In another study involving patients with lung cancer, 9.1% (N=15) of patients postponed their treatment during the COVID-19 pandemic because of pandemic-related anxiety.13
With the goal of improving care at our institution, we conducted a brief institutional review board–approved survey to evaluate how postponing MMS treatment due to the COVID-19 pandemic affected patients. All MMS patients undergoing surgery in June 2020 and July 2020 (N=99) were asked to complete our voluntary and anonymous 23-question survey in person during their procedure. We obtained 88 responses (response rate, 89%). Twenty percent of surveyed patients (n=18) reported that their MMS had been postponed; 78% of those whose MMS was postponed (n=14) indicated some level of anxiety during the waiting period. It was unclear which patients had their treatment postponed based on national guidelines and which ones elected to postpone surgery.
Tips for Health Care Providers
Patient-provider communication highlighting specific skin cancer risk and the risk vs benefit of postponing treatment might reduce anxiety and stress during the waiting period.14 A study found that COVID-19 posed a bigger threat than most noninvasive skin cancers; therefore, the authors of that study concluded that treatment for most skin cancers could be safely postponed.15 Specifically, those authors recommended prioritizing treatment for Merkel cell carcinoma, invasive SCC, and melanoma with positive margins or macroscopic residual disease. They proposed that all other skin cancers, including basal cell carcinoma, SCC in situ, and melanoma with negative margins and no macroscopic residual disease, could be safely delayed for as long as 3 months.15
For patients with multiple risk factors for COVID-19–related morbidity or mortality, delaying skin cancer treatment likely has less risk than contracting the virus.15 This information should be communicated with patients. Investigation of specific patient concerns is warranted, and case-by-case evaluation of patients’ risk factors and skin cancer risk should be considered.
Based on the current, though limited, literature, delaying medical treatment can have a negative impact on the patient experience. Furthermore, proper precautions have been shown to limit intraoperative transmission of SARS-CoV-2 during MMS, but research is lacking. Practitioners should utilize shared decision-making and evaluate a given patient’s risk factors and concerns when deciding whether to postpone treatment. We encourage other institutions to evaluate the effects that delaying MMS has had on their patients, as further studies would improve understanding of patients’ experiences during a pandemic and potentially influence future dermatology guidelines.
- Center for Disease Control and Prevention. COVID-19. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- American College of Mohs Surgery. Mohs surgery ambulatory protocol during COVID pandemic (version 6-3-20). June 4, 2020. Accessed April 20, 2021. http://staging.mohscollege.org/UserFiles/AM20/Member%20Alert/MohsSurgeryAmbulatoryProtocolDuringCOVIDPandemicFinal.pdf
- COVID-19 resources. National Comprehensive Cancer Network website. Accessed April 20, 2021. https://www.nccn.org/covid-19
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed April 10, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic.J Am Acad Dermatol. 2020;83:295-296. doi:10.1016/j.jaad.2020.05.002
- Yuan JT, Jiang SIB. Urgent safety considerations for dermatologic surgeons in the COVID-19 pandemic. Dermatol Online J. 2020;26:1. Accessed April 20, 2021. http://escholarship.org/uc/item/2qr3w771
- Otolaryngologists may contract COVID-19 during surgery. ENTtoday. March 20, 2020. Accessed April 20, 2021. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/
- Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg. 2020;163:98-103. doi:10.1177/0194599820927335
- Tee MW, Stewart C, Aliessa S, et al. Dermatological surgery during the COVID-19 pandemic: experience of a large academic center. J Am Acad Dermatol. 2021;84:1094-1096. doi:10.1016/j.jaad.2020.12.003
- Hooper J, Feng H. The impact of COVID-19 on micrographic surgery and dermatologic oncology fellows. Dermatol Surg. 2020;46:1762-1763. doi:10.1097/DSS.0000000000002766
- Nicholson P, Ali FR, Patalay R, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180. doi:10.1111/ced.14423
- Mou T, Brown O, Gillingham A, et al. Patients’ perceptions on surgical care suspension for pelvic floor disorders during the COVID-19 pandemic. Female Pelvic Med Reconstr Surg. 2020;26:477-482. doi:10.1097/SPV.0000000000000918
- Fujita K, Ito T, Saito Z, et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983-2986. doi:10.1111/1759-7714.13615
- Nikumb VB, Banerjee A, Kaur G, et al. Impact of doctor-patient communication on preoperative anxiety: study at industrial township, Pimpri, Pune. Ind Psychiatry J. 2009;18:19-21. doi:10.4103/0972-6748.57852
- Baumann BC, MacArthur KM, Brewer JD, et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900-3906. doi:10.1002/cncr.32969
- Center for Disease Control and Prevention. COVID-19. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- American College of Mohs Surgery. Mohs surgery ambulatory protocol during COVID pandemic (version 6-3-20). June 4, 2020. Accessed April 20, 2021. http://staging.mohscollege.org/UserFiles/AM20/Member%20Alert/MohsSurgeryAmbulatoryProtocolDuringCOVIDPandemicFinal.pdf
- COVID-19 resources. National Comprehensive Cancer Network website. Accessed April 20, 2021. https://www.nccn.org/covid-19
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed April 10, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic.J Am Acad Dermatol. 2020;83:295-296. doi:10.1016/j.jaad.2020.05.002
- Yuan JT, Jiang SIB. Urgent safety considerations for dermatologic surgeons in the COVID-19 pandemic. Dermatol Online J. 2020;26:1. Accessed April 20, 2021. http://escholarship.org/uc/item/2qr3w771
- Otolaryngologists may contract COVID-19 during surgery. ENTtoday. March 20, 2020. Accessed April 20, 2021. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/
- Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg. 2020;163:98-103. doi:10.1177/0194599820927335
- Tee MW, Stewart C, Aliessa S, et al. Dermatological surgery during the COVID-19 pandemic: experience of a large academic center. J Am Acad Dermatol. 2021;84:1094-1096. doi:10.1016/j.jaad.2020.12.003
- Hooper J, Feng H. The impact of COVID-19 on micrographic surgery and dermatologic oncology fellows. Dermatol Surg. 2020;46:1762-1763. doi:10.1097/DSS.0000000000002766
- Nicholson P, Ali FR, Patalay R, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180. doi:10.1111/ced.14423
- Mou T, Brown O, Gillingham A, et al. Patients’ perceptions on surgical care suspension for pelvic floor disorders during the COVID-19 pandemic. Female Pelvic Med Reconstr Surg. 2020;26:477-482. doi:10.1097/SPV.0000000000000918
- Fujita K, Ito T, Saito Z, et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983-2986. doi:10.1111/1759-7714.13615
- Nikumb VB, Banerjee A, Kaur G, et al. Impact of doctor-patient communication on preoperative anxiety: study at industrial township, Pimpri, Pune. Ind Psychiatry J. 2009;18:19-21. doi:10.4103/0972-6748.57852
- Baumann BC, MacArthur KM, Brewer JD, et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900-3906. doi:10.1002/cncr.32969
Practice Points
- There is little evidence that supports a high risk for SARS-CoV-2 transmission during Mohs micrographic surgery when proper personal protective equipment and strict infection control protocols are in place.
- The effects of treatment delays due to COVID-19 on the patient experience have not been well studied, but the limited literature suggests a negative association.
- Shared decision-making and evaluation of individual patient risk factors and concerns should be considered when deciding whether to postpone skin cancer treatment.
Possible obesity effect detected in cancer death rates

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.
FROM JAMA NETWORK OPEN
Clinical Use of a Diagnostic Gene Expression Signature for Melanocytic Neoplasms
According to National Institutes of Health estimates, more than 90,000 new cases of melanoma were diagnosed in 2018.1 Overall 5-year survival for patients with melanoma exceeds 90%, but individual survival estimates are highly dependent on stage at diagnosis, and survival decreases markedly with metastasis. Therefore, early and accurate diagnosis is critical.
Diagnosis of melanocytic neoplasms usually is performed by dermatopathologists through microscopic examination of stained tissue biopsy sections, a technically simple and effective method that enables a definitive diagnosis of benign nevus or malignant melanoma to be made in most cases. However, approximately 15% of all biopsied melanocytic lesions will exhibit some degree of histopathologic ambiguity,2-4 meaning that some of their microscopic features will be characteristic of a benign nevus while others will suggest the possibility of malignant melanoma. Diagnostic interpretations often vary in these cases, even among experts, and a definitive diagnosis of benign or malignant may be difficult to achieve by microscopy alone.2-4 Because of the marked reduction in survival once a melanoma has metastasized, these diagnostically ambiguous lesions often are treated as possible malignant melanomas with complete surgical excision (or re-excision). However, some experts suggest that many histopathologically ambiguous melanocytic neoplasms are, in fact, benign,5 a notion supported by epidemiologic evidence.6,7 Therefore, excision of many ambiguous melanocytic neoplasms might be avoided if definitive diagnosis could be achieved.
A gene expression signature was developed and validated for use as an adjunct to traditional methods of differentiating malignant melanocytic neoplasms from their benign counterparts.8-11 This test quantifies the RNA transcripts produced by 14 genes known to be overexpressed in malignant melanomas by comparison to benign nevi. These values are then combined algorithmically with measurements of 9 reference genes to produce an objective numerical score that is classified as benign, malignant, or indeterminate. When used by board-certified dermatopathologists and dermatologists confronting ambiguous melanocytic lesions, the test produces substantial increases in definitive diagnoses and prompts changes in treatment recommendations.12,13 However, the long-term consequences of foregoing surgical excision of melanocytic neoplasms that are diagnostically ambiguous but classified as benign by this test have not yet been formally assessed. In the current study, prospectively tested patients whose ambiguous melanocytic neoplasms were classified as benign by the gene expression signature were followed for up to 4.5 years to evaluate the long-term safety of treatment decisions aligned with benign test results.
Methods
Study Population
As part of a prior study,12 US-based dermatopathologists submitted tissue sections from biopsied melanocytic neoplasms determined to be diagnostically ambiguous by histopathology for analysis with the gene expression signature (Myriad Genetics, Inc). Diagnostically ambiguous lesions were those lesions that were described as ambiguous, uncertain, equivocal, indeterminate, or other synonymous terms by the submitting dermatopathologist and therefore lacked a confident diagnosis of benign or malignant prior to testing. Patients initially were tested between May 2014 and August 2014, with samples submitted through a prospective clinical experience study designed to assess the impact of the test on diagnosis and treatment decisions. This study was performed under an institutional review board waiver of consent (Quorum #33403/1).
Patients were eligible for inclusion in the current study if their biopsy specimens (1) had an uncertain preliminary diagnosis according to the submitting dermatopathologist (pretest diagnosis of indeterminate); (2) received a negative (benign) score from the gene expression test; (3) were treated as benign by the dermatologist(s) involved in follow-up care; and (4) were submitted by a single site (St. Joseph Medical Center, Houston, Texas). Although a single dermatopathology site was used for this study, multiple dermatologists were involved in the final treatment of these patients. Patients with benign scores who received additional intervention were excluded, as they may have a lower rate of adverse events (ie, metastasis) than those who did not receive intervention and would therefore skew the analysis population. A total of 25 patients from the prior study met these inclusion criteria. The previously collected12 pretest and posttest de-identified data were compiled from the commercial laboratory databases, and the patients were followed from the time of testing via medical record review performed by the dermatology providers at participating sites. Clinical follow-up data were collected using study-specific case report forms (CRFs) that captured the following: (1) the dates and results of clinical follow-up visits; (2) the type(s) of treatment and interventions (if any) performed at those visits; (3) the specific indication for any intervention performed; (4) any evidence of persistent, locally recurrent, and/or distant melanocytic neoplasia (whether definitively attributable to the tested lesion or not); and (5) death from any cause. The CRF assigned interventions to 1 of 5 categories: excision, excision with sentinel lymph node biopsy, referral to dermatologic or other surgeon, examination only (without surgical intervention), and other. Selection of other required a free-text description of the treatment and indications. Pertinent information not otherwise captured by the CRF also was recordable as free text.
Gene Expression Testing
Gene expression testing was carried out at the time of specimen submission in the prior study12 as described previously.14 Briefly, formalin-fixed, paraffin-embedded, unstained tissue sections and/or tissue blocks were submitted for testing along with a single hematoxylin and eosin–stained slide used to identify and designate the representative portion(s) of the lesion to be tested. These areas were macrodissected from unstained tissue sections and pooled for RNA extraction. Expression of 14 biomarker genes and 9 reference genes was measured via
Statistical Analysis
Demographic and other baseline characteristics of the patient population were summarized. Follow-up time was calculated as the interval between the date a patient’s gene expression test result was first issued to the provider and the date of the patient’s last recorded visit during the study period. All patient dermatology office visits within the designated follow-up period were documented, with a nonstandard number of visits and follow-up time across all study patients. Statistical analyses were conducted using SAS software (SAS Institute Inc), R software version 3.5.0 (R Foundation for Statistical Computing), and IBM SPSS Statistics software (IBM SPSS Statistics for Windows, Version 25).
Results
Patient Sample
A total of 25 ambiguous melanocytic neoplasms from 25 patients met the study inclusion criteria of a benign gene expression result with subsequent treatment as a benign neoplasm during follow-up. The patient sample statistics are summarized in Table 1. Most patients were younger than 65 years, with an average age at the time of biopsy of 48.4 years. All 25 neoplasms produced negative (benign) gene expression signature scores, all were diagnosed as benign nevi posttest by the submitting dermatopathologist, and all patients were initially treated in accordance with the benign diagnosis by the dermatologist(s) involved in clinical follow-up care. Prior to testing with the gene expression signature, most of these histopathologically indeterminate lesions received differential diagnoses, the most common of which were dysplastic nevus (84%), melanoma arising from a nevus (72%), and superficial spreading melanoma (64%; eTable). After testing with the gene expression signature and receiving a benign score, most lesions received a single differential diagnosis of dysplastic nevus (88%).
Follow-up and Survival
Clinical follow-up time ranged from 0.6 to 53.3 months, with a mean duration (SD) of 38.5 (16.6) months, and patients attended an average of 4 postbiopsy dermatology appointments (mean [SD], 4.6 [3.6]). According to the participating dermatology care providers, none of the 25 patients developed any indication during follow-up that the diagnosis of benign nevus was inaccurate. No patient had evidence of locally recurrent or metastatic melanoma, and none died during the study period.
Treatment/Interventions
The treatment recorded in the CRF was examination only for 21 of 25 patients, excision for 3, and other for 1 (Table 2). Because the explanation for the selection of other in this case described an excision performed at the same anatomic location as the biopsy, this treatment also was considered an excision for purposes of the study analyses. The 3 excisions all occurred at the first postbiopsy dermatology encounter. Across all follow-up visits, no additional surgical interventions occurred (Table 2).
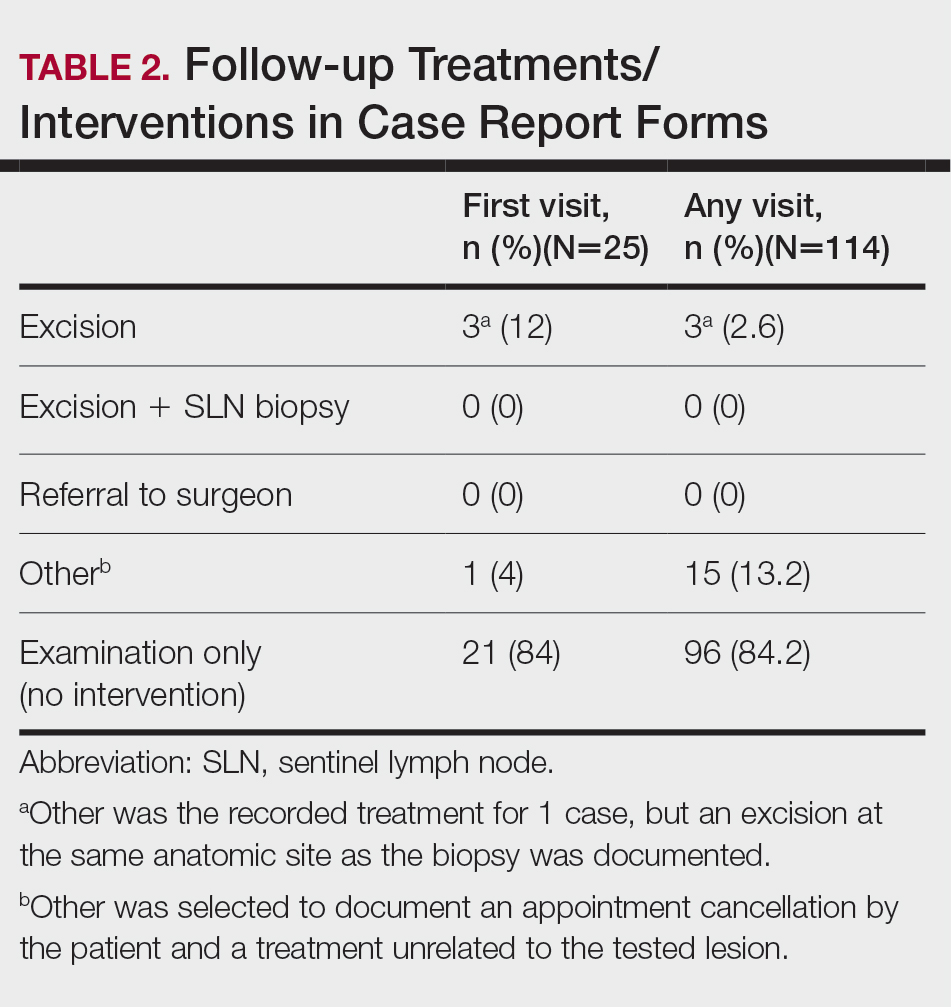
The first excision (case 1) involved a 67-year-old woman with a lesion on the mid pubic region described clinically as an atypical nevus that generated a pretest histopathologic differential diagnosis including dysplastic nevus, superficial spreading melanoma, and melanoma arising within a nevus (Table 3; Figure, A and B). The gene expression test result was benign (score, −5.4), and the final pathology report diagnosis was nevus with junctional dysplasia, moderate. Surgical excision was performed at the patient’s first return visit, 505 days after initial diagnosis, with moderately dysplastic nevus as the recorded indication for removal. No repigmentation or other evidence of local recurrence or progression was detected, and the treating dermatologist indicated no suspicion that the original diagnosis of benign nevus was incorrect during the 23-month follow-up period.
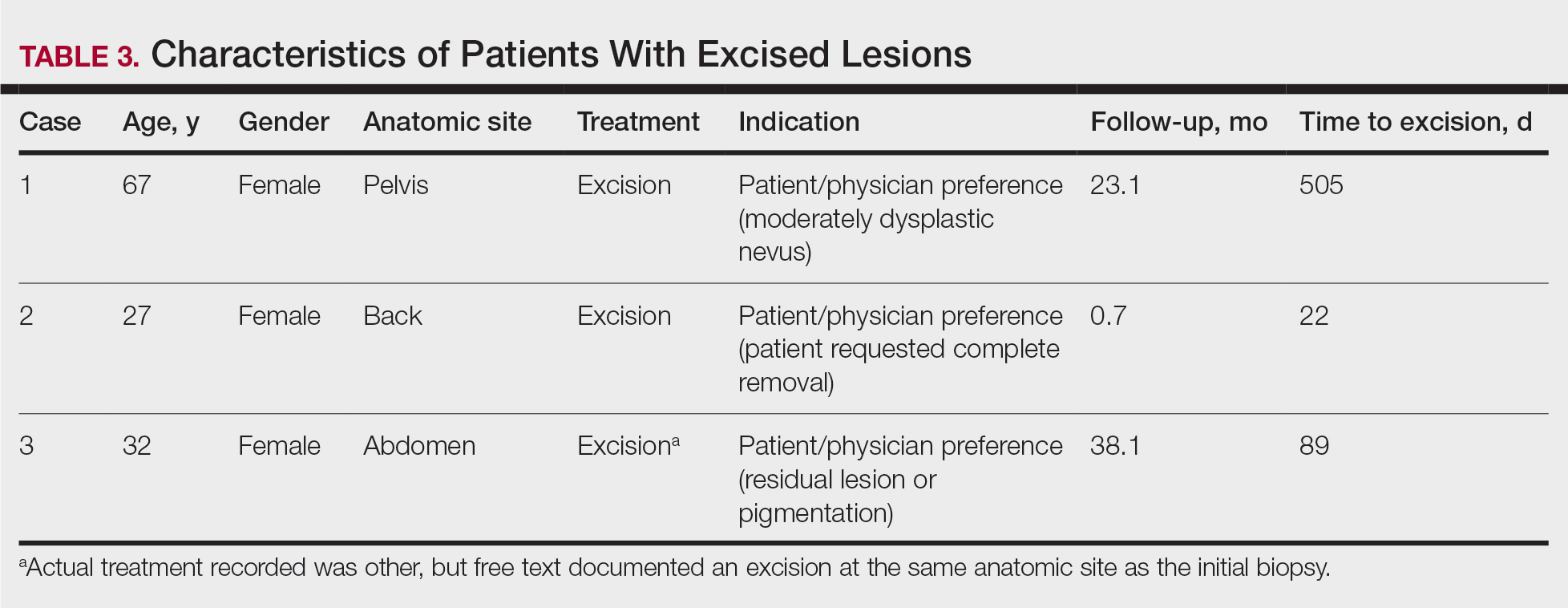
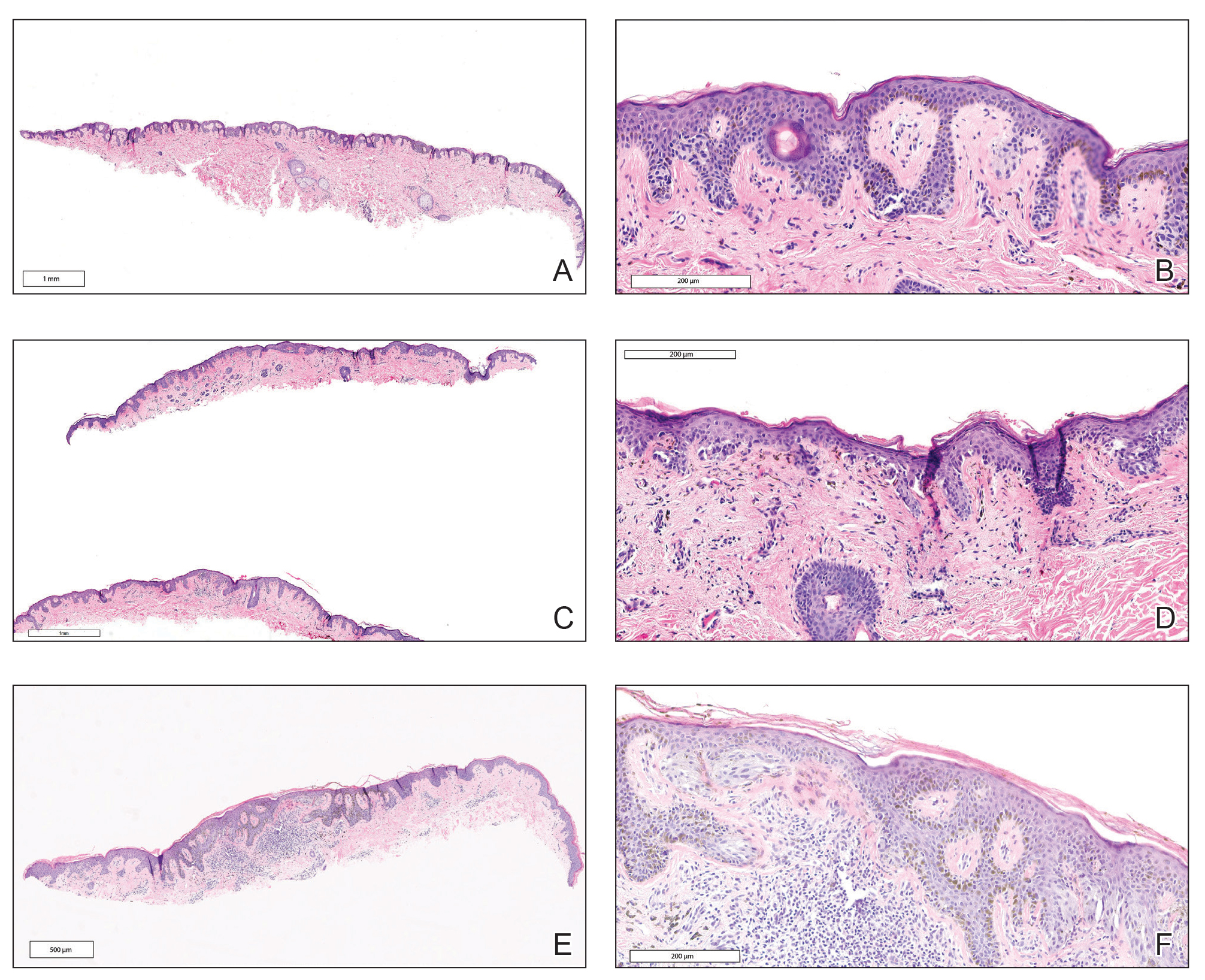
The second excision (case 2) involved a 27-year-old woman with a pigmented neoplasm on the mid upper back (Figure, C and D) biopsied to rule out dysplastic nevus that resulted in a pretest histopathologic differential diagnosis of dysplastic nevus vs superficial spreading melanoma or melanoma arising within a nevus. The gene expression test result classified the lesion as benign (score, −2.9), and the final pathology diagnosis was nevus, compound, with moderate dysplasia. Despite the benign diagnosis, residual neoplasm (or pigmentation) at the biopsy site prompted the patient to request excision at her first postbiopsy visit, 22 days after testing (Table 3). The CRF completed by the dermatologist reported no indication that the benign diagnosis was inaccurate, but the patient was subsequently lost to follow-up.
The third excision (case 3) involved a 32-year-old woman with a pigmented lesion on the abdomen (Table 3; Figure, E and F). The clinical description was irregular-appearing black papule, nevus with atypia, and the histopathologic differential diagnosis again included dysplastic nevus, superficial spreading melanoma, and melanoma arising within a preexisting nevus. The gene expression signature result was benign (score, −7.2), and the final diagnosis issued within the accompanying pathology report was nevus with moderate junctional dysplasia. Despite the benign diagnosis, excision was performed 89 days after test result availability, with apparent residual pigmentation as the specified indication. As with the other 2 cases, the treating dermatologist confirmed that neither clinical features nor follow-up events suggested malignancy.
Comment
This study followed a cohort of 25 patients with histopathologically ambiguous melanocytic neoplasms that were classified as benign by a diagnostic gene expression test with the intent of determining the outcomes of patients whose treatment aligned with their benign test result. All patients initially were managed according to their test result. During an average posttest clinical follow-up time of more than 3 years (38.5 months), the 25 biopsied lesions, most of which received a differential diagnosis of dysplastic nevus, were regarded as benign nevi by their dermatologists, and the vast majority (88%) received no further surgical intervention. Three patients underwent subsequent excision of the biopsied lesion, with patient or physician preference as the indication in each instance. None of the 25 patients developed evidence of local recurrence, metastasis, or other findings that prompted doubt of the benign diagnosis. The absence of adverse events during clinical follow-up, particularly given that most lesions were not subjected to further intervention, supports use of the gene expression test as a safe and effective adjunct to the diagnosis and treatment of ambiguous melanocytic neoplasms by dermatologists and dermatopathologists.
Ambiguous melanocytic neoplasms evaluated without the aid of molecular adjuncts often result in equivocal or less-than-definitive diagnoses, and further surgical intervention is commonly undertaken to mitigate against the possibility of a missed melanoma.13 In this study, treatment that was aligned with the benign test result allowed most patients to avoid further surgical intervention, which suggests that adjunctive use of the gene signature can contribute to reductions in the physical and economic burdens imposed by unnecessary surgical interventions.15,16 Moreover, any means of increasing accurate and definitive diagnoses may produce an immediate impact on health outcomes by reducing the anxiety that uncertainty often provokes in patients and health care providers alike.
Study Limitations
This study must be interpreted within the context of its limitations. Obtaining meaningful patient outcome data is a common challenge in health care research due to the requisite length of follow-up and sometimes the lack of definitive evidence of adverse events. This is particularly difficult for melanocytic neoplasms because of an apparent inclination for patients with benign diagnoses to abandon follow-up and an increasing tendency for even minimal diagnostic uncertainty to prompt complete excision. Additionally, the only definitive clinical outcome for melanocytic neoplasms is distant metastasis, which (fortunately for patients) is relatively rare. Not surprisingly, studies documenting clinical outcomes of patients with ambiguous melanocytic neoplasms tested prospectively with diagnostic adjuncts are scarce, and this study’s sample size and clinical follow-up compare favorably with the few that exist.17,18 Although most melanomas declare themselves through recurrence or metastasis within several years of initial biopsy,1,19 some are clinically dormant for as long as 10 years after initial detection.20,21 This may be particularly true for the small or early-stage lesions that now comprise the majority of biopsied neoplasms, and such events would go undetected by this study and many others. It also must be recognized that uneventful follow-up, regardless of duration, cannot prove that a biopsied melanocytic neoplasm was benign. Although only 5 patients had a follow-up time of less than 2 years (the time frame in which most recurrence or metastasis will occur), it cannot be definitively proven that a minimum of 2 years recurrence- or metastasis-free survival indicates a benign lesion. Many early-stage malignant melanomas are eradicated by complete excision or even by the initial biopsy if margins are uninvolved.
Because these limitations are intrinsic to melanocytic neoplasms and current management strategies, they pertain to all investigations seeking insights into biological potential through clinical outcomes. Similarly, all current diagnostic tools and procedures have the potential for sampling error, including histopathology. The rarity of adverse outcomes (recurrence and metastasis) in patients with benign test results within this cohort indicates that false-negative results are uncommon, which is further evidenced by a similar rarity of adverse events in prior studies of the gene expression signature.8-10,22 A particular strength of this study is that most of the ambiguous melanocytic neoplasms followed did not undergo excision after the initial biopsy, an increasingly uncommon situation that may increase their likelihood to be informative.
It must be emphasized that the gene expression test, similar to other diagnostic adjuncts, is neither a replacement for histopathologic interpretation nor a substitute for judgment. As with all tests, it can produce false-positive and false-negative results. Therefore, it should always be interpreted within the constellation of the many other data points that must be considered when making a distinction between benign nevus and malignant melanoma, including but not limited to patient age, family and personal history of melanoma, anatomic location, clinical features, and histopathologic findings. As is the case for many diseases, careful consideration of all relevant input is necessary to minimize the risk of misdiagnosis that might occur should any single data point prove inaccurate, including the results of adjunctive molecular tests.
Conclusion
Ancillary methods are emerging as useful tools for the diagnostic evaluation of melanocytic neoplasms that cannot be assigned definitive diagnoses using traditional techniques alone. This study suggests that patients with ambiguous melanocytic neoplasms may benefit from diagnoses and treatment decisions aligned with the results of a gene expression test, and that for those with a benign result, simple observation may be a safe alternative to surgical excision. This expands upon prior observations of the test’s influence on diagnoses and treatment decisions and supports its role as part of dermatopathologists’ and dermatologists’ decision-making process for histopathologically ambiguous melanocytic lesions.
- Noone AM, Howlander N, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute website. Updated September 10, 2018. Accessed April 21, 2021. https://seer.cancer.gov/archive/csr/1975_2015/
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751-756.
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997;182:266-272.
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi:10.1136/bmj.j2813
- Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol. 2011;38:264-267.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-1113.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Clarke LE, Warf BM, Flake DD 2nd, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244-252.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29:832-843.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95:e4887. doi:10.1097/MD.0000000000004887
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14:123-130.
- Warf MB, Flake DD 2nd, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9:407-416.
- Guy GP Jr, Ekwueme DU, Tangka FK, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-545.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Egnatios GL, Ferringer TC. Clinical follow-up of atypical spitzoid tumors analyzed by fluorescence in situ hybridization. Am J Dermatopathol. 2016;38:289-296.
- Fischer AS, High WA. The difficulty in interpreting gene expression profiling in BAP-negative melanocytic tumors. J Cutan Pathol. 2018;45:659-666. doi:10.1111/cup.13277
- Vollmer RT. The dynamics of death in melanoma. J Cutan Pathol. 2012;39:1075-1082.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136:2453-2457.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213-221.
According to National Institutes of Health estimates, more than 90,000 new cases of melanoma were diagnosed in 2018.1 Overall 5-year survival for patients with melanoma exceeds 90%, but individual survival estimates are highly dependent on stage at diagnosis, and survival decreases markedly with metastasis. Therefore, early and accurate diagnosis is critical.
Diagnosis of melanocytic neoplasms usually is performed by dermatopathologists through microscopic examination of stained tissue biopsy sections, a technically simple and effective method that enables a definitive diagnosis of benign nevus or malignant melanoma to be made in most cases. However, approximately 15% of all biopsied melanocytic lesions will exhibit some degree of histopathologic ambiguity,2-4 meaning that some of their microscopic features will be characteristic of a benign nevus while others will suggest the possibility of malignant melanoma. Diagnostic interpretations often vary in these cases, even among experts, and a definitive diagnosis of benign or malignant may be difficult to achieve by microscopy alone.2-4 Because of the marked reduction in survival once a melanoma has metastasized, these diagnostically ambiguous lesions often are treated as possible malignant melanomas with complete surgical excision (or re-excision). However, some experts suggest that many histopathologically ambiguous melanocytic neoplasms are, in fact, benign,5 a notion supported by epidemiologic evidence.6,7 Therefore, excision of many ambiguous melanocytic neoplasms might be avoided if definitive diagnosis could be achieved.
A gene expression signature was developed and validated for use as an adjunct to traditional methods of differentiating malignant melanocytic neoplasms from their benign counterparts.8-11 This test quantifies the RNA transcripts produced by 14 genes known to be overexpressed in malignant melanomas by comparison to benign nevi. These values are then combined algorithmically with measurements of 9 reference genes to produce an objective numerical score that is classified as benign, malignant, or indeterminate. When used by board-certified dermatopathologists and dermatologists confronting ambiguous melanocytic lesions, the test produces substantial increases in definitive diagnoses and prompts changes in treatment recommendations.12,13 However, the long-term consequences of foregoing surgical excision of melanocytic neoplasms that are diagnostically ambiguous but classified as benign by this test have not yet been formally assessed. In the current study, prospectively tested patients whose ambiguous melanocytic neoplasms were classified as benign by the gene expression signature were followed for up to 4.5 years to evaluate the long-term safety of treatment decisions aligned with benign test results.
Methods
Study Population
As part of a prior study,12 US-based dermatopathologists submitted tissue sections from biopsied melanocytic neoplasms determined to be diagnostically ambiguous by histopathology for analysis with the gene expression signature (Myriad Genetics, Inc). Diagnostically ambiguous lesions were those lesions that were described as ambiguous, uncertain, equivocal, indeterminate, or other synonymous terms by the submitting dermatopathologist and therefore lacked a confident diagnosis of benign or malignant prior to testing. Patients initially were tested between May 2014 and August 2014, with samples submitted through a prospective clinical experience study designed to assess the impact of the test on diagnosis and treatment decisions. This study was performed under an institutional review board waiver of consent (Quorum #33403/1).
Patients were eligible for inclusion in the current study if their biopsy specimens (1) had an uncertain preliminary diagnosis according to the submitting dermatopathologist (pretest diagnosis of indeterminate); (2) received a negative (benign) score from the gene expression test; (3) were treated as benign by the dermatologist(s) involved in follow-up care; and (4) were submitted by a single site (St. Joseph Medical Center, Houston, Texas). Although a single dermatopathology site was used for this study, multiple dermatologists were involved in the final treatment of these patients. Patients with benign scores who received additional intervention were excluded, as they may have a lower rate of adverse events (ie, metastasis) than those who did not receive intervention and would therefore skew the analysis population. A total of 25 patients from the prior study met these inclusion criteria. The previously collected12 pretest and posttest de-identified data were compiled from the commercial laboratory databases, and the patients were followed from the time of testing via medical record review performed by the dermatology providers at participating sites. Clinical follow-up data were collected using study-specific case report forms (CRFs) that captured the following: (1) the dates and results of clinical follow-up visits; (2) the type(s) of treatment and interventions (if any) performed at those visits; (3) the specific indication for any intervention performed; (4) any evidence of persistent, locally recurrent, and/or distant melanocytic neoplasia (whether definitively attributable to the tested lesion or not); and (5) death from any cause. The CRF assigned interventions to 1 of 5 categories: excision, excision with sentinel lymph node biopsy, referral to dermatologic or other surgeon, examination only (without surgical intervention), and other. Selection of other required a free-text description of the treatment and indications. Pertinent information not otherwise captured by the CRF also was recordable as free text.
Gene Expression Testing
Gene expression testing was carried out at the time of specimen submission in the prior study12 as described previously.14 Briefly, formalin-fixed, paraffin-embedded, unstained tissue sections and/or tissue blocks were submitted for testing along with a single hematoxylin and eosin–stained slide used to identify and designate the representative portion(s) of the lesion to be tested. These areas were macrodissected from unstained tissue sections and pooled for RNA extraction. Expression of 14 biomarker genes and 9 reference genes was measured via
Statistical Analysis
Demographic and other baseline characteristics of the patient population were summarized. Follow-up time was calculated as the interval between the date a patient’s gene expression test result was first issued to the provider and the date of the patient’s last recorded visit during the study period. All patient dermatology office visits within the designated follow-up period were documented, with a nonstandard number of visits and follow-up time across all study patients. Statistical analyses were conducted using SAS software (SAS Institute Inc), R software version 3.5.0 (R Foundation for Statistical Computing), and IBM SPSS Statistics software (IBM SPSS Statistics for Windows, Version 25).
Results
Patient Sample
A total of 25 ambiguous melanocytic neoplasms from 25 patients met the study inclusion criteria of a benign gene expression result with subsequent treatment as a benign neoplasm during follow-up. The patient sample statistics are summarized in Table 1. Most patients were younger than 65 years, with an average age at the time of biopsy of 48.4 years. All 25 neoplasms produced negative (benign) gene expression signature scores, all were diagnosed as benign nevi posttest by the submitting dermatopathologist, and all patients were initially treated in accordance with the benign diagnosis by the dermatologist(s) involved in clinical follow-up care. Prior to testing with the gene expression signature, most of these histopathologically indeterminate lesions received differential diagnoses, the most common of which were dysplastic nevus (84%), melanoma arising from a nevus (72%), and superficial spreading melanoma (64%; eTable). After testing with the gene expression signature and receiving a benign score, most lesions received a single differential diagnosis of dysplastic nevus (88%).


Follow-up and Survival
Clinical follow-up time ranged from 0.6 to 53.3 months, with a mean duration (SD) of 38.5 (16.6) months, and patients attended an average of 4 postbiopsy dermatology appointments (mean [SD], 4.6 [3.6]). According to the participating dermatology care providers, none of the 25 patients developed any indication during follow-up that the diagnosis of benign nevus was inaccurate. No patient had evidence of locally recurrent or metastatic melanoma, and none died during the study period.
Treatment/Interventions
The treatment recorded in the CRF was examination only for 21 of 25 patients, excision for 3, and other for 1 (Table 2). Because the explanation for the selection of other in this case described an excision performed at the same anatomic location as the biopsy, this treatment also was considered an excision for purposes of the study analyses. The 3 excisions all occurred at the first postbiopsy dermatology encounter. Across all follow-up visits, no additional surgical interventions occurred (Table 2).

The first excision (case 1) involved a 67-year-old woman with a lesion on the mid pubic region described clinically as an atypical nevus that generated a pretest histopathologic differential diagnosis including dysplastic nevus, superficial spreading melanoma, and melanoma arising within a nevus (Table 3; Figure, A and B). The gene expression test result was benign (score, −5.4), and the final pathology report diagnosis was nevus with junctional dysplasia, moderate. Surgical excision was performed at the patient’s first return visit, 505 days after initial diagnosis, with moderately dysplastic nevus as the recorded indication for removal. No repigmentation or other evidence of local recurrence or progression was detected, and the treating dermatologist indicated no suspicion that the original diagnosis of benign nevus was incorrect during the 23-month follow-up period.


The second excision (case 2) involved a 27-year-old woman with a pigmented neoplasm on the mid upper back (Figure, C and D) biopsied to rule out dysplastic nevus that resulted in a pretest histopathologic differential diagnosis of dysplastic nevus vs superficial spreading melanoma or melanoma arising within a nevus. The gene expression test result classified the lesion as benign (score, −2.9), and the final pathology diagnosis was nevus, compound, with moderate dysplasia. Despite the benign diagnosis, residual neoplasm (or pigmentation) at the biopsy site prompted the patient to request excision at her first postbiopsy visit, 22 days after testing (Table 3). The CRF completed by the dermatologist reported no indication that the benign diagnosis was inaccurate, but the patient was subsequently lost to follow-up.
The third excision (case 3) involved a 32-year-old woman with a pigmented lesion on the abdomen (Table 3; Figure, E and F). The clinical description was irregular-appearing black papule, nevus with atypia, and the histopathologic differential diagnosis again included dysplastic nevus, superficial spreading melanoma, and melanoma arising within a preexisting nevus. The gene expression signature result was benign (score, −7.2), and the final diagnosis issued within the accompanying pathology report was nevus with moderate junctional dysplasia. Despite the benign diagnosis, excision was performed 89 days after test result availability, with apparent residual pigmentation as the specified indication. As with the other 2 cases, the treating dermatologist confirmed that neither clinical features nor follow-up events suggested malignancy.
Comment
This study followed a cohort of 25 patients with histopathologically ambiguous melanocytic neoplasms that were classified as benign by a diagnostic gene expression test with the intent of determining the outcomes of patients whose treatment aligned with their benign test result. All patients initially were managed according to their test result. During an average posttest clinical follow-up time of more than 3 years (38.5 months), the 25 biopsied lesions, most of which received a differential diagnosis of dysplastic nevus, were regarded as benign nevi by their dermatologists, and the vast majority (88%) received no further surgical intervention. Three patients underwent subsequent excision of the biopsied lesion, with patient or physician preference as the indication in each instance. None of the 25 patients developed evidence of local recurrence, metastasis, or other findings that prompted doubt of the benign diagnosis. The absence of adverse events during clinical follow-up, particularly given that most lesions were not subjected to further intervention, supports use of the gene expression test as a safe and effective adjunct to the diagnosis and treatment of ambiguous melanocytic neoplasms by dermatologists and dermatopathologists.
Ambiguous melanocytic neoplasms evaluated without the aid of molecular adjuncts often result in equivocal or less-than-definitive diagnoses, and further surgical intervention is commonly undertaken to mitigate against the possibility of a missed melanoma.13 In this study, treatment that was aligned with the benign test result allowed most patients to avoid further surgical intervention, which suggests that adjunctive use of the gene signature can contribute to reductions in the physical and economic burdens imposed by unnecessary surgical interventions.15,16 Moreover, any means of increasing accurate and definitive diagnoses may produce an immediate impact on health outcomes by reducing the anxiety that uncertainty often provokes in patients and health care providers alike.
Study Limitations
This study must be interpreted within the context of its limitations. Obtaining meaningful patient outcome data is a common challenge in health care research due to the requisite length of follow-up and sometimes the lack of definitive evidence of adverse events. This is particularly difficult for melanocytic neoplasms because of an apparent inclination for patients with benign diagnoses to abandon follow-up and an increasing tendency for even minimal diagnostic uncertainty to prompt complete excision. Additionally, the only definitive clinical outcome for melanocytic neoplasms is distant metastasis, which (fortunately for patients) is relatively rare. Not surprisingly, studies documenting clinical outcomes of patients with ambiguous melanocytic neoplasms tested prospectively with diagnostic adjuncts are scarce, and this study’s sample size and clinical follow-up compare favorably with the few that exist.17,18 Although most melanomas declare themselves through recurrence or metastasis within several years of initial biopsy,1,19 some are clinically dormant for as long as 10 years after initial detection.20,21 This may be particularly true for the small or early-stage lesions that now comprise the majority of biopsied neoplasms, and such events would go undetected by this study and many others. It also must be recognized that uneventful follow-up, regardless of duration, cannot prove that a biopsied melanocytic neoplasm was benign. Although only 5 patients had a follow-up time of less than 2 years (the time frame in which most recurrence or metastasis will occur), it cannot be definitively proven that a minimum of 2 years recurrence- or metastasis-free survival indicates a benign lesion. Many early-stage malignant melanomas are eradicated by complete excision or even by the initial biopsy if margins are uninvolved.
Because these limitations are intrinsic to melanocytic neoplasms and current management strategies, they pertain to all investigations seeking insights into biological potential through clinical outcomes. Similarly, all current diagnostic tools and procedures have the potential for sampling error, including histopathology. The rarity of adverse outcomes (recurrence and metastasis) in patients with benign test results within this cohort indicates that false-negative results are uncommon, which is further evidenced by a similar rarity of adverse events in prior studies of the gene expression signature.8-10,22 A particular strength of this study is that most of the ambiguous melanocytic neoplasms followed did not undergo excision after the initial biopsy, an increasingly uncommon situation that may increase their likelihood to be informative.
It must be emphasized that the gene expression test, similar to other diagnostic adjuncts, is neither a replacement for histopathologic interpretation nor a substitute for judgment. As with all tests, it can produce false-positive and false-negative results. Therefore, it should always be interpreted within the constellation of the many other data points that must be considered when making a distinction between benign nevus and malignant melanoma, including but not limited to patient age, family and personal history of melanoma, anatomic location, clinical features, and histopathologic findings. As is the case for many diseases, careful consideration of all relevant input is necessary to minimize the risk of misdiagnosis that might occur should any single data point prove inaccurate, including the results of adjunctive molecular tests.
Conclusion
Ancillary methods are emerging as useful tools for the diagnostic evaluation of melanocytic neoplasms that cannot be assigned definitive diagnoses using traditional techniques alone. This study suggests that patients with ambiguous melanocytic neoplasms may benefit from diagnoses and treatment decisions aligned with the results of a gene expression test, and that for those with a benign result, simple observation may be a safe alternative to surgical excision. This expands upon prior observations of the test’s influence on diagnoses and treatment decisions and supports its role as part of dermatopathologists’ and dermatologists’ decision-making process for histopathologically ambiguous melanocytic lesions.
According to National Institutes of Health estimates, more than 90,000 new cases of melanoma were diagnosed in 2018.1 Overall 5-year survival for patients with melanoma exceeds 90%, but individual survival estimates are highly dependent on stage at diagnosis, and survival decreases markedly with metastasis. Therefore, early and accurate diagnosis is critical.
Diagnosis of melanocytic neoplasms usually is performed by dermatopathologists through microscopic examination of stained tissue biopsy sections, a technically simple and effective method that enables a definitive diagnosis of benign nevus or malignant melanoma to be made in most cases. However, approximately 15% of all biopsied melanocytic lesions will exhibit some degree of histopathologic ambiguity,2-4 meaning that some of their microscopic features will be characteristic of a benign nevus while others will suggest the possibility of malignant melanoma. Diagnostic interpretations often vary in these cases, even among experts, and a definitive diagnosis of benign or malignant may be difficult to achieve by microscopy alone.2-4 Because of the marked reduction in survival once a melanoma has metastasized, these diagnostically ambiguous lesions often are treated as possible malignant melanomas with complete surgical excision (or re-excision). However, some experts suggest that many histopathologically ambiguous melanocytic neoplasms are, in fact, benign,5 a notion supported by epidemiologic evidence.6,7 Therefore, excision of many ambiguous melanocytic neoplasms might be avoided if definitive diagnosis could be achieved.
A gene expression signature was developed and validated for use as an adjunct to traditional methods of differentiating malignant melanocytic neoplasms from their benign counterparts.8-11 This test quantifies the RNA transcripts produced by 14 genes known to be overexpressed in malignant melanomas by comparison to benign nevi. These values are then combined algorithmically with measurements of 9 reference genes to produce an objective numerical score that is classified as benign, malignant, or indeterminate. When used by board-certified dermatopathologists and dermatologists confronting ambiguous melanocytic lesions, the test produces substantial increases in definitive diagnoses and prompts changes in treatment recommendations.12,13 However, the long-term consequences of foregoing surgical excision of melanocytic neoplasms that are diagnostically ambiguous but classified as benign by this test have not yet been formally assessed. In the current study, prospectively tested patients whose ambiguous melanocytic neoplasms were classified as benign by the gene expression signature were followed for up to 4.5 years to evaluate the long-term safety of treatment decisions aligned with benign test results.
Methods
Study Population
As part of a prior study,12 US-based dermatopathologists submitted tissue sections from biopsied melanocytic neoplasms determined to be diagnostically ambiguous by histopathology for analysis with the gene expression signature (Myriad Genetics, Inc). Diagnostically ambiguous lesions were those lesions that were described as ambiguous, uncertain, equivocal, indeterminate, or other synonymous terms by the submitting dermatopathologist and therefore lacked a confident diagnosis of benign or malignant prior to testing. Patients initially were tested between May 2014 and August 2014, with samples submitted through a prospective clinical experience study designed to assess the impact of the test on diagnosis and treatment decisions. This study was performed under an institutional review board waiver of consent (Quorum #33403/1).
Patients were eligible for inclusion in the current study if their biopsy specimens (1) had an uncertain preliminary diagnosis according to the submitting dermatopathologist (pretest diagnosis of indeterminate); (2) received a negative (benign) score from the gene expression test; (3) were treated as benign by the dermatologist(s) involved in follow-up care; and (4) were submitted by a single site (St. Joseph Medical Center, Houston, Texas). Although a single dermatopathology site was used for this study, multiple dermatologists were involved in the final treatment of these patients. Patients with benign scores who received additional intervention were excluded, as they may have a lower rate of adverse events (ie, metastasis) than those who did not receive intervention and would therefore skew the analysis population. A total of 25 patients from the prior study met these inclusion criteria. The previously collected12 pretest and posttest de-identified data were compiled from the commercial laboratory databases, and the patients were followed from the time of testing via medical record review performed by the dermatology providers at participating sites. Clinical follow-up data were collected using study-specific case report forms (CRFs) that captured the following: (1) the dates and results of clinical follow-up visits; (2) the type(s) of treatment and interventions (if any) performed at those visits; (3) the specific indication for any intervention performed; (4) any evidence of persistent, locally recurrent, and/or distant melanocytic neoplasia (whether definitively attributable to the tested lesion or not); and (5) death from any cause. The CRF assigned interventions to 1 of 5 categories: excision, excision with sentinel lymph node biopsy, referral to dermatologic or other surgeon, examination only (without surgical intervention), and other. Selection of other required a free-text description of the treatment and indications. Pertinent information not otherwise captured by the CRF also was recordable as free text.
Gene Expression Testing
Gene expression testing was carried out at the time of specimen submission in the prior study12 as described previously.14 Briefly, formalin-fixed, paraffin-embedded, unstained tissue sections and/or tissue blocks were submitted for testing along with a single hematoxylin and eosin–stained slide used to identify and designate the representative portion(s) of the lesion to be tested. These areas were macrodissected from unstained tissue sections and pooled for RNA extraction. Expression of 14 biomarker genes and 9 reference genes was measured via
Statistical Analysis
Demographic and other baseline characteristics of the patient population were summarized. Follow-up time was calculated as the interval between the date a patient’s gene expression test result was first issued to the provider and the date of the patient’s last recorded visit during the study period. All patient dermatology office visits within the designated follow-up period were documented, with a nonstandard number of visits and follow-up time across all study patients. Statistical analyses were conducted using SAS software (SAS Institute Inc), R software version 3.5.0 (R Foundation for Statistical Computing), and IBM SPSS Statistics software (IBM SPSS Statistics for Windows, Version 25).
Results
Patient Sample
A total of 25 ambiguous melanocytic neoplasms from 25 patients met the study inclusion criteria of a benign gene expression result with subsequent treatment as a benign neoplasm during follow-up. The patient sample statistics are summarized in Table 1. Most patients were younger than 65 years, with an average age at the time of biopsy of 48.4 years. All 25 neoplasms produced negative (benign) gene expression signature scores, all were diagnosed as benign nevi posttest by the submitting dermatopathologist, and all patients were initially treated in accordance with the benign diagnosis by the dermatologist(s) involved in clinical follow-up care. Prior to testing with the gene expression signature, most of these histopathologically indeterminate lesions received differential diagnoses, the most common of which were dysplastic nevus (84%), melanoma arising from a nevus (72%), and superficial spreading melanoma (64%; eTable). After testing with the gene expression signature and receiving a benign score, most lesions received a single differential diagnosis of dysplastic nevus (88%).


Follow-up and Survival
Clinical follow-up time ranged from 0.6 to 53.3 months, with a mean duration (SD) of 38.5 (16.6) months, and patients attended an average of 4 postbiopsy dermatology appointments (mean [SD], 4.6 [3.6]). According to the participating dermatology care providers, none of the 25 patients developed any indication during follow-up that the diagnosis of benign nevus was inaccurate. No patient had evidence of locally recurrent or metastatic melanoma, and none died during the study period.
Treatment/Interventions
The treatment recorded in the CRF was examination only for 21 of 25 patients, excision for 3, and other for 1 (Table 2). Because the explanation for the selection of other in this case described an excision performed at the same anatomic location as the biopsy, this treatment also was considered an excision for purposes of the study analyses. The 3 excisions all occurred at the first postbiopsy dermatology encounter. Across all follow-up visits, no additional surgical interventions occurred (Table 2).

The first excision (case 1) involved a 67-year-old woman with a lesion on the mid pubic region described clinically as an atypical nevus that generated a pretest histopathologic differential diagnosis including dysplastic nevus, superficial spreading melanoma, and melanoma arising within a nevus (Table 3; Figure, A and B). The gene expression test result was benign (score, −5.4), and the final pathology report diagnosis was nevus with junctional dysplasia, moderate. Surgical excision was performed at the patient’s first return visit, 505 days after initial diagnosis, with moderately dysplastic nevus as the recorded indication for removal. No repigmentation or other evidence of local recurrence or progression was detected, and the treating dermatologist indicated no suspicion that the original diagnosis of benign nevus was incorrect during the 23-month follow-up period.


The second excision (case 2) involved a 27-year-old woman with a pigmented neoplasm on the mid upper back (Figure, C and D) biopsied to rule out dysplastic nevus that resulted in a pretest histopathologic differential diagnosis of dysplastic nevus vs superficial spreading melanoma or melanoma arising within a nevus. The gene expression test result classified the lesion as benign (score, −2.9), and the final pathology diagnosis was nevus, compound, with moderate dysplasia. Despite the benign diagnosis, residual neoplasm (or pigmentation) at the biopsy site prompted the patient to request excision at her first postbiopsy visit, 22 days after testing (Table 3). The CRF completed by the dermatologist reported no indication that the benign diagnosis was inaccurate, but the patient was subsequently lost to follow-up.
The third excision (case 3) involved a 32-year-old woman with a pigmented lesion on the abdomen (Table 3; Figure, E and F). The clinical description was irregular-appearing black papule, nevus with atypia, and the histopathologic differential diagnosis again included dysplastic nevus, superficial spreading melanoma, and melanoma arising within a preexisting nevus. The gene expression signature result was benign (score, −7.2), and the final diagnosis issued within the accompanying pathology report was nevus with moderate junctional dysplasia. Despite the benign diagnosis, excision was performed 89 days after test result availability, with apparent residual pigmentation as the specified indication. As with the other 2 cases, the treating dermatologist confirmed that neither clinical features nor follow-up events suggested malignancy.
Comment
This study followed a cohort of 25 patients with histopathologically ambiguous melanocytic neoplasms that were classified as benign by a diagnostic gene expression test with the intent of determining the outcomes of patients whose treatment aligned with their benign test result. All patients initially were managed according to their test result. During an average posttest clinical follow-up time of more than 3 years (38.5 months), the 25 biopsied lesions, most of which received a differential diagnosis of dysplastic nevus, were regarded as benign nevi by their dermatologists, and the vast majority (88%) received no further surgical intervention. Three patients underwent subsequent excision of the biopsied lesion, with patient or physician preference as the indication in each instance. None of the 25 patients developed evidence of local recurrence, metastasis, or other findings that prompted doubt of the benign diagnosis. The absence of adverse events during clinical follow-up, particularly given that most lesions were not subjected to further intervention, supports use of the gene expression test as a safe and effective adjunct to the diagnosis and treatment of ambiguous melanocytic neoplasms by dermatologists and dermatopathologists.
Ambiguous melanocytic neoplasms evaluated without the aid of molecular adjuncts often result in equivocal or less-than-definitive diagnoses, and further surgical intervention is commonly undertaken to mitigate against the possibility of a missed melanoma.13 In this study, treatment that was aligned with the benign test result allowed most patients to avoid further surgical intervention, which suggests that adjunctive use of the gene signature can contribute to reductions in the physical and economic burdens imposed by unnecessary surgical interventions.15,16 Moreover, any means of increasing accurate and definitive diagnoses may produce an immediate impact on health outcomes by reducing the anxiety that uncertainty often provokes in patients and health care providers alike.
Study Limitations
This study must be interpreted within the context of its limitations. Obtaining meaningful patient outcome data is a common challenge in health care research due to the requisite length of follow-up and sometimes the lack of definitive evidence of adverse events. This is particularly difficult for melanocytic neoplasms because of an apparent inclination for patients with benign diagnoses to abandon follow-up and an increasing tendency for even minimal diagnostic uncertainty to prompt complete excision. Additionally, the only definitive clinical outcome for melanocytic neoplasms is distant metastasis, which (fortunately for patients) is relatively rare. Not surprisingly, studies documenting clinical outcomes of patients with ambiguous melanocytic neoplasms tested prospectively with diagnostic adjuncts are scarce, and this study’s sample size and clinical follow-up compare favorably with the few that exist.17,18 Although most melanomas declare themselves through recurrence or metastasis within several years of initial biopsy,1,19 some are clinically dormant for as long as 10 years after initial detection.20,21 This may be particularly true for the small or early-stage lesions that now comprise the majority of biopsied neoplasms, and such events would go undetected by this study and many others. It also must be recognized that uneventful follow-up, regardless of duration, cannot prove that a biopsied melanocytic neoplasm was benign. Although only 5 patients had a follow-up time of less than 2 years (the time frame in which most recurrence or metastasis will occur), it cannot be definitively proven that a minimum of 2 years recurrence- or metastasis-free survival indicates a benign lesion. Many early-stage malignant melanomas are eradicated by complete excision or even by the initial biopsy if margins are uninvolved.
Because these limitations are intrinsic to melanocytic neoplasms and current management strategies, they pertain to all investigations seeking insights into biological potential through clinical outcomes. Similarly, all current diagnostic tools and procedures have the potential for sampling error, including histopathology. The rarity of adverse outcomes (recurrence and metastasis) in patients with benign test results within this cohort indicates that false-negative results are uncommon, which is further evidenced by a similar rarity of adverse events in prior studies of the gene expression signature.8-10,22 A particular strength of this study is that most of the ambiguous melanocytic neoplasms followed did not undergo excision after the initial biopsy, an increasingly uncommon situation that may increase their likelihood to be informative.
It must be emphasized that the gene expression test, similar to other diagnostic adjuncts, is neither a replacement for histopathologic interpretation nor a substitute for judgment. As with all tests, it can produce false-positive and false-negative results. Therefore, it should always be interpreted within the constellation of the many other data points that must be considered when making a distinction between benign nevus and malignant melanoma, including but not limited to patient age, family and personal history of melanoma, anatomic location, clinical features, and histopathologic findings. As is the case for many diseases, careful consideration of all relevant input is necessary to minimize the risk of misdiagnosis that might occur should any single data point prove inaccurate, including the results of adjunctive molecular tests.
Conclusion
Ancillary methods are emerging as useful tools for the diagnostic evaluation of melanocytic neoplasms that cannot be assigned definitive diagnoses using traditional techniques alone. This study suggests that patients with ambiguous melanocytic neoplasms may benefit from diagnoses and treatment decisions aligned with the results of a gene expression test, and that for those with a benign result, simple observation may be a safe alternative to surgical excision. This expands upon prior observations of the test’s influence on diagnoses and treatment decisions and supports its role as part of dermatopathologists’ and dermatologists’ decision-making process for histopathologically ambiguous melanocytic lesions.
- Noone AM, Howlander N, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute website. Updated September 10, 2018. Accessed April 21, 2021. https://seer.cancer.gov/archive/csr/1975_2015/
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751-756.
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997;182:266-272.
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi:10.1136/bmj.j2813
- Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol. 2011;38:264-267.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-1113.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Clarke LE, Warf BM, Flake DD 2nd, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244-252.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29:832-843.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95:e4887. doi:10.1097/MD.0000000000004887
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14:123-130.
- Warf MB, Flake DD 2nd, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9:407-416.
- Guy GP Jr, Ekwueme DU, Tangka FK, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-545.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Egnatios GL, Ferringer TC. Clinical follow-up of atypical spitzoid tumors analyzed by fluorescence in situ hybridization. Am J Dermatopathol. 2016;38:289-296.
- Fischer AS, High WA. The difficulty in interpreting gene expression profiling in BAP-negative melanocytic tumors. J Cutan Pathol. 2018;45:659-666. doi:10.1111/cup.13277
- Vollmer RT. The dynamics of death in melanoma. J Cutan Pathol. 2012;39:1075-1082.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136:2453-2457.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213-221.
- Noone AM, Howlander N, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute website. Updated September 10, 2018. Accessed April 21, 2021. https://seer.cancer.gov/archive/csr/1975_2015/
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751-756.
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997;182:266-272.
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi:10.1136/bmj.j2813
- Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol. 2011;38:264-267.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-1113.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Clarke LE, Warf BM, Flake DD 2nd, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244-252.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29:832-843.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95:e4887. doi:10.1097/MD.0000000000004887
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14:123-130.
- Warf MB, Flake DD 2nd, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9:407-416.
- Guy GP Jr, Ekwueme DU, Tangka FK, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-545.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Egnatios GL, Ferringer TC. Clinical follow-up of atypical spitzoid tumors analyzed by fluorescence in situ hybridization. Am J Dermatopathol. 2016;38:289-296.
- Fischer AS, High WA. The difficulty in interpreting gene expression profiling in BAP-negative melanocytic tumors. J Cutan Pathol. 2018;45:659-666. doi:10.1111/cup.13277
- Vollmer RT. The dynamics of death in melanoma. J Cutan Pathol. 2012;39:1075-1082.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136:2453-2457.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213-221.
Practice Point
- Implementation of a gene expression signature in the diagnosis of histopathologically ambiguous lesions can safely increase diagnostic accuracy and optimize treatment.