User login
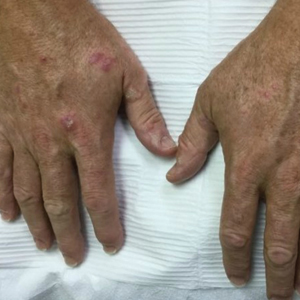
AI detects ugly-duckling skin lesions for melanoma follow-up
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
How has the pandemic affected rural and urban cancer patients?
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR: COVID-19 AND CANCER 2021
Checkpoint inhibitors’ ‘big picture’ safety shown with preexisting autoimmune diseases
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
FROM ANNALS OF INTERNAL MEDICINE
Neoadjuvant immunotherapy shows promise in stage III melanoma
The next dramatic , John M. Kirkwood, MD, predicted at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
These agents have already demonstrated profound efficacy, first in stage IV metastatic disease and more recently as adjuvant therapy for resected stage III melanoma. Now, there is a great interest in learning whether by prescribing them preoperatively, patients might reduce their risk of advancing to metastatic disease. And neoadjuvant therapy offers an extremely attractive feature: It yields results in an accelerated fashion.
“The major problem with postoperative adjuvant trials in melanoma since 1984 is the long time to maturity. Many of us don’t want to wait the full 9 or 10 years for a full-bore, phase 3 adjuvant trial in stage III melanoma to mature,” explained Dr. Kirkwood, professor of medicine, dermatology, and translational science and coleader of the melanoma and skin cancer program at the University of Pittsburgh. “The opportunity to treat a patient who presents with a bulky lymph node, has a biopsy, and then can be treated for 3 or 6 weeks or sometimes even longer periods with a therapy that’s promising allows us to ask what’s going on in the tumor tissue, what’s going on in the clinical response at 3 or 6 weeks, and if there’s pathological complete or near-complete response under the microscope.”
Because pathological complete response is a strong predictor of relapse-free survival, this neoadjuvant-forward therapeutic strategy has the potential to provide patients and their physicians with an early forecast of likely clinical outcome only 4-6 weeks into treatment. Also, there is both preclinical and clinical evidence that neoadjuvant therapy may offer a survival advantage over adjuvant therapy, perhaps as a result of early treatment of micrometastatic disease. Another benefit of neoadjuvant therapy for melanoma is the resultant tumor shrinkage, which can permit less extensive surgery.
Dr. Kirkwood highlighted a phase 2 clinical trial conducted at the University of Pittsburgh to illustrate the potential of neoadjuvant therapy in melanoma. The ongoing single-arm study includes 32 patients with stage IIIB or IIIC resectable melanoma along with accessible tumor for biopsy and intratumoral injections of CMP-001, a toll-like receptor 9 agonist. According to the Eighth Edition of the American Joint Committee on Cancer staging manual, stage IIIB melanoma has a 10-year mortality of 23%, and stage IIIC disease has 40%.
CMP-001 triggers type 1 interferon production through activation of plasmacytoid dendritic cells. The resultant inflammatory response draws T cells into the tumor to enhance the response to immunotherapy, which in this study was nivolumab (Opdivo), a human programmed death ligand 1 (PD-L1)–blocking antibody. The neoadjuvant regimen consisted of seven once-weekly intratumoral injections of CMP-001, plus three 240-mg doses of nivolumab given at 2-week intervals. This was followed by resection, then 1 year of adjuvant therapy with nivolumab at 480 mg every 4 weeks and intratumoral CMP-001 every 4 weeks.
In an interim analysis, a major pathologic response occurred in an impressive 15 of 21 patients (71%) after 6 weeks of neoadjuvant therapy. Thirteen of the 15 had a pathologic complete response. Encouragingly, no one with a pathologic complete or near-complete response has relapsed to date.
“A pathologic complete response or near-complete response with neoadjuvant therapy appears to be a biomarker of durable disease control and is associated with excellent outcomes,” Dr. Kirkwood observed, adding that the Pittsburgh experience has been mirrored in reports from the Netherlands, Australia, and University of Texas M.D. Anderson Cancer Center, Houston, involving other neoadjuvant agents.
Other potential early biomarkers of favorable outcome with neoadjuvant therapy include CD8+ T cells in the tumor at baseline, tumor mutational burden, T-cell clonality, and a T-cell–inflamed gene-expression profile.
There were no dose-limiting toxicities or delays in surgery related to the neoadjuvant treatment.
Of note, imaging often inaccurately showed only a partial response in patients who actually had a pathologic complete response, meaning totally devoid of tumor, Dr. Kirkwood said.
Corroboration of these findings is planned in the national multicenter ECOG-ACRIN neoadjuvant trial EA6194.
“Consider referring to this trial any patients who present with bulky nodal disease for whom a treatment assessment at 4-6 weeks is desired in order to predict what the outcome may be,” he suggested.
Dr. Kirkwood reported receiving research grants from Amgen, BMS, Castle Biosciences, Checkmate, Immunocore, Iovance, and Novartis and serving as a consultant to a handful of companies.
Global Academy for Medical Education and this news organization are owned by the same company.
The next dramatic , John M. Kirkwood, MD, predicted at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
These agents have already demonstrated profound efficacy, first in stage IV metastatic disease and more recently as adjuvant therapy for resected stage III melanoma. Now, there is a great interest in learning whether by prescribing them preoperatively, patients might reduce their risk of advancing to metastatic disease. And neoadjuvant therapy offers an extremely attractive feature: It yields results in an accelerated fashion.
“The major problem with postoperative adjuvant trials in melanoma since 1984 is the long time to maturity. Many of us don’t want to wait the full 9 or 10 years for a full-bore, phase 3 adjuvant trial in stage III melanoma to mature,” explained Dr. Kirkwood, professor of medicine, dermatology, and translational science and coleader of the melanoma and skin cancer program at the University of Pittsburgh. “The opportunity to treat a patient who presents with a bulky lymph node, has a biopsy, and then can be treated for 3 or 6 weeks or sometimes even longer periods with a therapy that’s promising allows us to ask what’s going on in the tumor tissue, what’s going on in the clinical response at 3 or 6 weeks, and if there’s pathological complete or near-complete response under the microscope.”
Because pathological complete response is a strong predictor of relapse-free survival, this neoadjuvant-forward therapeutic strategy has the potential to provide patients and their physicians with an early forecast of likely clinical outcome only 4-6 weeks into treatment. Also, there is both preclinical and clinical evidence that neoadjuvant therapy may offer a survival advantage over adjuvant therapy, perhaps as a result of early treatment of micrometastatic disease. Another benefit of neoadjuvant therapy for melanoma is the resultant tumor shrinkage, which can permit less extensive surgery.
Dr. Kirkwood highlighted a phase 2 clinical trial conducted at the University of Pittsburgh to illustrate the potential of neoadjuvant therapy in melanoma. The ongoing single-arm study includes 32 patients with stage IIIB or IIIC resectable melanoma along with accessible tumor for biopsy and intratumoral injections of CMP-001, a toll-like receptor 9 agonist. According to the Eighth Edition of the American Joint Committee on Cancer staging manual, stage IIIB melanoma has a 10-year mortality of 23%, and stage IIIC disease has 40%.
CMP-001 triggers type 1 interferon production through activation of plasmacytoid dendritic cells. The resultant inflammatory response draws T cells into the tumor to enhance the response to immunotherapy, which in this study was nivolumab (Opdivo), a human programmed death ligand 1 (PD-L1)–blocking antibody. The neoadjuvant regimen consisted of seven once-weekly intratumoral injections of CMP-001, plus three 240-mg doses of nivolumab given at 2-week intervals. This was followed by resection, then 1 year of adjuvant therapy with nivolumab at 480 mg every 4 weeks and intratumoral CMP-001 every 4 weeks.
In an interim analysis, a major pathologic response occurred in an impressive 15 of 21 patients (71%) after 6 weeks of neoadjuvant therapy. Thirteen of the 15 had a pathologic complete response. Encouragingly, no one with a pathologic complete or near-complete response has relapsed to date.
“A pathologic complete response or near-complete response with neoadjuvant therapy appears to be a biomarker of durable disease control and is associated with excellent outcomes,” Dr. Kirkwood observed, adding that the Pittsburgh experience has been mirrored in reports from the Netherlands, Australia, and University of Texas M.D. Anderson Cancer Center, Houston, involving other neoadjuvant agents.
Other potential early biomarkers of favorable outcome with neoadjuvant therapy include CD8+ T cells in the tumor at baseline, tumor mutational burden, T-cell clonality, and a T-cell–inflamed gene-expression profile.
There were no dose-limiting toxicities or delays in surgery related to the neoadjuvant treatment.
Of note, imaging often inaccurately showed only a partial response in patients who actually had a pathologic complete response, meaning totally devoid of tumor, Dr. Kirkwood said.
Corroboration of these findings is planned in the national multicenter ECOG-ACRIN neoadjuvant trial EA6194.
“Consider referring to this trial any patients who present with bulky nodal disease for whom a treatment assessment at 4-6 weeks is desired in order to predict what the outcome may be,” he suggested.
Dr. Kirkwood reported receiving research grants from Amgen, BMS, Castle Biosciences, Checkmate, Immunocore, Iovance, and Novartis and serving as a consultant to a handful of companies.
Global Academy for Medical Education and this news organization are owned by the same company.
The next dramatic , John M. Kirkwood, MD, predicted at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
These agents have already demonstrated profound efficacy, first in stage IV metastatic disease and more recently as adjuvant therapy for resected stage III melanoma. Now, there is a great interest in learning whether by prescribing them preoperatively, patients might reduce their risk of advancing to metastatic disease. And neoadjuvant therapy offers an extremely attractive feature: It yields results in an accelerated fashion.
“The major problem with postoperative adjuvant trials in melanoma since 1984 is the long time to maturity. Many of us don’t want to wait the full 9 or 10 years for a full-bore, phase 3 adjuvant trial in stage III melanoma to mature,” explained Dr. Kirkwood, professor of medicine, dermatology, and translational science and coleader of the melanoma and skin cancer program at the University of Pittsburgh. “The opportunity to treat a patient who presents with a bulky lymph node, has a biopsy, and then can be treated for 3 or 6 weeks or sometimes even longer periods with a therapy that’s promising allows us to ask what’s going on in the tumor tissue, what’s going on in the clinical response at 3 or 6 weeks, and if there’s pathological complete or near-complete response under the microscope.”
Because pathological complete response is a strong predictor of relapse-free survival, this neoadjuvant-forward therapeutic strategy has the potential to provide patients and their physicians with an early forecast of likely clinical outcome only 4-6 weeks into treatment. Also, there is both preclinical and clinical evidence that neoadjuvant therapy may offer a survival advantage over adjuvant therapy, perhaps as a result of early treatment of micrometastatic disease. Another benefit of neoadjuvant therapy for melanoma is the resultant tumor shrinkage, which can permit less extensive surgery.
Dr. Kirkwood highlighted a phase 2 clinical trial conducted at the University of Pittsburgh to illustrate the potential of neoadjuvant therapy in melanoma. The ongoing single-arm study includes 32 patients with stage IIIB or IIIC resectable melanoma along with accessible tumor for biopsy and intratumoral injections of CMP-001, a toll-like receptor 9 agonist. According to the Eighth Edition of the American Joint Committee on Cancer staging manual, stage IIIB melanoma has a 10-year mortality of 23%, and stage IIIC disease has 40%.
CMP-001 triggers type 1 interferon production through activation of plasmacytoid dendritic cells. The resultant inflammatory response draws T cells into the tumor to enhance the response to immunotherapy, which in this study was nivolumab (Opdivo), a human programmed death ligand 1 (PD-L1)–blocking antibody. The neoadjuvant regimen consisted of seven once-weekly intratumoral injections of CMP-001, plus three 240-mg doses of nivolumab given at 2-week intervals. This was followed by resection, then 1 year of adjuvant therapy with nivolumab at 480 mg every 4 weeks and intratumoral CMP-001 every 4 weeks.
In an interim analysis, a major pathologic response occurred in an impressive 15 of 21 patients (71%) after 6 weeks of neoadjuvant therapy. Thirteen of the 15 had a pathologic complete response. Encouragingly, no one with a pathologic complete or near-complete response has relapsed to date.
“A pathologic complete response or near-complete response with neoadjuvant therapy appears to be a biomarker of durable disease control and is associated with excellent outcomes,” Dr. Kirkwood observed, adding that the Pittsburgh experience has been mirrored in reports from the Netherlands, Australia, and University of Texas M.D. Anderson Cancer Center, Houston, involving other neoadjuvant agents.
Other potential early biomarkers of favorable outcome with neoadjuvant therapy include CD8+ T cells in the tumor at baseline, tumor mutational burden, T-cell clonality, and a T-cell–inflamed gene-expression profile.
There were no dose-limiting toxicities or delays in surgery related to the neoadjuvant treatment.
Of note, imaging often inaccurately showed only a partial response in patients who actually had a pathologic complete response, meaning totally devoid of tumor, Dr. Kirkwood said.
Corroboration of these findings is planned in the national multicenter ECOG-ACRIN neoadjuvant trial EA6194.
“Consider referring to this trial any patients who present with bulky nodal disease for whom a treatment assessment at 4-6 weeks is desired in order to predict what the outcome may be,” he suggested.
Dr. Kirkwood reported receiving research grants from Amgen, BMS, Castle Biosciences, Checkmate, Immunocore, Iovance, and Novartis and serving as a consultant to a handful of companies.
Global Academy for Medical Education and this news organization are owned by the same company.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Managing cancer outpatients during the pandemic: Tips from MSKCC
“We’ve tried a lot of new things to ensure optimal care for our patients,” said Tiffany A. Traina, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York. “We need to effectively utilize all resources at our disposal to keep in touch with our patients during this time.”
Dr. Traina described the approach to outpatient management used at MSKCC during a presentation at the AACR Virtual Meeting: COVID-19 and Cancer.
Four guiding principles
MSKCC has established four guiding principles on how to manage cancer patients during the pandemic: openness, safety, technology, and staffing.
Openness ensures that decisions are guided by clinical priorities to provide optimal patient care and allow for prioritization of clinical research and education, Dr. Traina said.
The safety of patients and staff is of the utmost importance, she added. To ensure safety in the context of outpatient care, several operational levers were developed, including COVID surge planning, universal masking and personal protective equipment guidelines, remote work, clinical levers, and new dashboards and communications.
Dr. Traina said data analytics and dashboards have been key technological tools used to support evidence-based decision-making and deliver care remotely for patients during the pandemic.
Staffing resources have also shifted to support demand at different health system locations.
Screening, cohorting, and telemedicine
One measure MSKCC adopted is the MSK Engage Questionnaire, a COVID-19 screening questionnaire assigned to every patient with a scheduled outpatient visit. After completing the questionnaire, patients receive a response denoting whether they need to come into the outpatient setting.
On the staffing side, clinic coordinators prepare appointments accordingly, based on the risk level for each patient.
“We also try to cohort COVID-positive patients into particular areas within the outpatient setting,” Dr. Traina explained. “In addition, we control flow through ambulatory care locations by having separate patient entrances and use other tools to make flow as efficient as possible.”
On the technology side, interactive dashboards are being used to model traffic through different buildings.
“These data and analytics are useful for operational engineering, answering questions such as (1) Are there backups in chemotherapy? and (2) Are patients seeing one particular physician?” Dr. Traina explained. “One important key takeaway is the importance of frequently communicating simple messages through multiple mechanisms, including signage, websites, and dedicated resources.”
Other key technological measures are leveraging telemedicine to convert inpatient appointments to a virtual setting, as well as developing and deploying a system for centralized outpatient follow-up of COVID-19-positive patients.
“We saw a 3,000% increase in telemedicine utilization from February 2020 to June 2020,” Dr. Traina reported. “In a given month, we have approximately 230,000 outpatient visits, and a substantial proportion of these are now done via telemedicine.”
Dr. Traina also noted that multiple organizations have released guidelines addressing when to resume anticancer therapy in patients who have been COVID-19 positive. Adherence is important, as unnecessary COVID-19 testing may delay cancer therapy and is not recommended.
During a live discussion, Louis P. Voigt, MD, of MSKCC, said Dr. Traina’s presentation provided “a lot of good ideas for other institutions who may be facing similar challenges.”
Dr. Traina and Dr. Voigt disclosed no conflicts of interest. No funding sources were reported.
“We’ve tried a lot of new things to ensure optimal care for our patients,” said Tiffany A. Traina, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York. “We need to effectively utilize all resources at our disposal to keep in touch with our patients during this time.”
Dr. Traina described the approach to outpatient management used at MSKCC during a presentation at the AACR Virtual Meeting: COVID-19 and Cancer.
Four guiding principles
MSKCC has established four guiding principles on how to manage cancer patients during the pandemic: openness, safety, technology, and staffing.
Openness ensures that decisions are guided by clinical priorities to provide optimal patient care and allow for prioritization of clinical research and education, Dr. Traina said.
The safety of patients and staff is of the utmost importance, she added. To ensure safety in the context of outpatient care, several operational levers were developed, including COVID surge planning, universal masking and personal protective equipment guidelines, remote work, clinical levers, and new dashboards and communications.
Dr. Traina said data analytics and dashboards have been key technological tools used to support evidence-based decision-making and deliver care remotely for patients during the pandemic.
Staffing resources have also shifted to support demand at different health system locations.
Screening, cohorting, and telemedicine
One measure MSKCC adopted is the MSK Engage Questionnaire, a COVID-19 screening questionnaire assigned to every patient with a scheduled outpatient visit. After completing the questionnaire, patients receive a response denoting whether they need to come into the outpatient setting.
On the staffing side, clinic coordinators prepare appointments accordingly, based on the risk level for each patient.
“We also try to cohort COVID-positive patients into particular areas within the outpatient setting,” Dr. Traina explained. “In addition, we control flow through ambulatory care locations by having separate patient entrances and use other tools to make flow as efficient as possible.”
On the technology side, interactive dashboards are being used to model traffic through different buildings.
“These data and analytics are useful for operational engineering, answering questions such as (1) Are there backups in chemotherapy? and (2) Are patients seeing one particular physician?” Dr. Traina explained. “One important key takeaway is the importance of frequently communicating simple messages through multiple mechanisms, including signage, websites, and dedicated resources.”
Other key technological measures are leveraging telemedicine to convert inpatient appointments to a virtual setting, as well as developing and deploying a system for centralized outpatient follow-up of COVID-19-positive patients.
“We saw a 3,000% increase in telemedicine utilization from February 2020 to June 2020,” Dr. Traina reported. “In a given month, we have approximately 230,000 outpatient visits, and a substantial proportion of these are now done via telemedicine.”
Dr. Traina also noted that multiple organizations have released guidelines addressing when to resume anticancer therapy in patients who have been COVID-19 positive. Adherence is important, as unnecessary COVID-19 testing may delay cancer therapy and is not recommended.
During a live discussion, Louis P. Voigt, MD, of MSKCC, said Dr. Traina’s presentation provided “a lot of good ideas for other institutions who may be facing similar challenges.”
Dr. Traina and Dr. Voigt disclosed no conflicts of interest. No funding sources were reported.
“We’ve tried a lot of new things to ensure optimal care for our patients,” said Tiffany A. Traina, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York. “We need to effectively utilize all resources at our disposal to keep in touch with our patients during this time.”
Dr. Traina described the approach to outpatient management used at MSKCC during a presentation at the AACR Virtual Meeting: COVID-19 and Cancer.
Four guiding principles
MSKCC has established four guiding principles on how to manage cancer patients during the pandemic: openness, safety, technology, and staffing.
Openness ensures that decisions are guided by clinical priorities to provide optimal patient care and allow for prioritization of clinical research and education, Dr. Traina said.
The safety of patients and staff is of the utmost importance, she added. To ensure safety in the context of outpatient care, several operational levers were developed, including COVID surge planning, universal masking and personal protective equipment guidelines, remote work, clinical levers, and new dashboards and communications.
Dr. Traina said data analytics and dashboards have been key technological tools used to support evidence-based decision-making and deliver care remotely for patients during the pandemic.
Staffing resources have also shifted to support demand at different health system locations.
Screening, cohorting, and telemedicine
One measure MSKCC adopted is the MSK Engage Questionnaire, a COVID-19 screening questionnaire assigned to every patient with a scheduled outpatient visit. After completing the questionnaire, patients receive a response denoting whether they need to come into the outpatient setting.
On the staffing side, clinic coordinators prepare appointments accordingly, based on the risk level for each patient.
“We also try to cohort COVID-positive patients into particular areas within the outpatient setting,” Dr. Traina explained. “In addition, we control flow through ambulatory care locations by having separate patient entrances and use other tools to make flow as efficient as possible.”
On the technology side, interactive dashboards are being used to model traffic through different buildings.
“These data and analytics are useful for operational engineering, answering questions such as (1) Are there backups in chemotherapy? and (2) Are patients seeing one particular physician?” Dr. Traina explained. “One important key takeaway is the importance of frequently communicating simple messages through multiple mechanisms, including signage, websites, and dedicated resources.”
Other key technological measures are leveraging telemedicine to convert inpatient appointments to a virtual setting, as well as developing and deploying a system for centralized outpatient follow-up of COVID-19-positive patients.
“We saw a 3,000% increase in telemedicine utilization from February 2020 to June 2020,” Dr. Traina reported. “In a given month, we have approximately 230,000 outpatient visits, and a substantial proportion of these are now done via telemedicine.”
Dr. Traina also noted that multiple organizations have released guidelines addressing when to resume anticancer therapy in patients who have been COVID-19 positive. Adherence is important, as unnecessary COVID-19 testing may delay cancer therapy and is not recommended.
During a live discussion, Louis P. Voigt, MD, of MSKCC, said Dr. Traina’s presentation provided “a lot of good ideas for other institutions who may be facing similar challenges.”
Dr. Traina and Dr. Voigt disclosed no conflicts of interest. No funding sources were reported.
FROM AACR: COVID-19 AND CANCER 2021
COVID-19 vaccination in cancer patients: NCCN outlines priorities
Vaccination timing considerations vary based on factors such as cancer and treatment type, and reasons for delaying vaccination in the general public also apply to cancer patients (recent COVID-19 exposure, for example).
In general, however, patients with cancer should be assigned to Centers for Disease Control and Prevention priority group 1 b/c and immunized when vaccination is available to them, the guidelines state. Exceptions to this recommendation include:
- Patients undergoing hematopoietic stem cell transplant or receiving engineered cellular therapy such as chimeric antigen receptor T-cell therapy. Vaccination should be delayed for at least 3 months in these patients to maximize vaccine efficacy. Caregivers of these patients, however, should be immunized when possible.
- Patients with hematologic malignancies who are receiving intensive cytotoxic chemotherapy, such as cytarabine- or anthracycline-based regimens for acute myeloid leukemia. Vaccination in these patients should be delayed until absolute neutrophil count recovery.
- Patients undergoing major surgery. Vaccination should occur at least a few days before or after surgery.
- Patients who have experienced a severe or immediate adverse reaction to any of the ingredients in the mRNA COVID-19 vaccines.
Conversely, vaccination should occur when available in patients with hematologic malignancies and marrow failure who are expected to have limited or no recovery, patients with hematologic malignancies who are on long-term maintenance therapy, and patients with solid tumors who are receiving cytotoxic chemotherapy, targeted therapy, checkpoint inhibitors and other immunotherapy, or radiotherapy.
Caregivers, household contacts, and other close contacts who are 16 years of age and older should be vaccinated whenever they are eligible.
Unique concerns in patients with cancer
The NCCN recommendations were developed to address the unique issues and concerns with respect to patients with cancer, who have an increased risk of severe illness from SARS-CoV-2 infection. But the guidelines come with a caveat: “[t]here are limited safety and efficacy data in these patients,” the NCCN emphasized in a press statement.
“Right now, there is urgent need and limited data,” Steven Pergam, MD, co-leader of the NCCN COVID-19 Vaccination Committee, said in the statement.
“Our number one goal is helping to get the vaccine to as many people as we can,” Dr. Pergam said. “That means following existing national and regional directions for prioritizing people who are more likely to face death or severe illness from COVID-19.”
Dr. Pergam, associate professor at Fred Hutchinson Cancer Research Center in Seattle, further explained that “people receiving active cancer treatment are at greater risk for worse outcomes from COVID-19, particularly if they are older and have additional comorbidities, like immunosuppression.”
NCCN’s recommendations couldn’t have come at a better time for patients with cancer, according to Nora Disis, MD, a professor at the University of Washington in Seattle.
“The NCCN’s recommendations to prioritize COVID vaccinations for cancer patients on active treatment is an important step forward in protecting our patients from the infection,” Dr. Disis said in an interview.
“Cancer patients may be at higher risk for the complications seen with infection. In addition, cancer is a disease of older people, and a good number of our patients have the comorbidities that would predict a poorer outcome if they should become sick,” Dr. Disis added. “With the correct treatment, many patients with cancer will be long-term survivors. It is important that they be protected from infection with COVID to realize their best outcome.”
Additional vaccine considerations
The NCCN recommendations also address several other issues of importance for cancer patients, including:
- Deprioritizing other vaccines. COVID-19 vaccines should take precedence over other vaccines because data on dual vaccination are lacking. The NCCN recommends waiting 14 days after COVID-19 vaccination to deliver other vaccines.
- Vaccinating clinical trial participants. Trial leads should be consulted to prevent protocol violations or exclusions.
- Decision-making in the setting of limited vaccine availability. The NCCN noted that decisions on allocation must be made in accordance with state and local vaccine guidance but suggests prioritizing appropriate patients on active treatment, those planning to start treatment, and those who have just completed treatment. Additional risk factors for these patients, as well as other factors associated with risk for adverse COVID-19 outcomes, should also be considered. These include advanced age, comorbidities, and adverse social and demographic factors such as poverty and limited health care access.
- The need for ongoing prevention measures. Vaccines have been shown to decrease the incidence of COVID-19 and related complications, but it remains unclear whether vaccines prevent infection and subsequent transmission. This means everyone should continue following prevention recommendations, such as wearing masks and avoiding crowds.
The NCCN stressed that these recommendations are “intended to be a living document that is constantly evolving – it will be updated rapidly whenever new data comes out, as well as any potential new vaccines that may get approved in the future.” The NCCN also noted that the advisory committee will meet regularly to refine the recommendations as needed.
Dr. Pergam disclosed relationships with Chimerix Inc., Merck & Co., Global Life Technologies Inc., and Sanofi-Aventis. Dr. Disis disclosed grants from Pfizer, Bavarian Nordisk, Janssen, and Precigen. She is the founder of EpiThany and editor-in-chief of JAMA Oncology.
Vaccination timing considerations vary based on factors such as cancer and treatment type, and reasons for delaying vaccination in the general public also apply to cancer patients (recent COVID-19 exposure, for example).
In general, however, patients with cancer should be assigned to Centers for Disease Control and Prevention priority group 1 b/c and immunized when vaccination is available to them, the guidelines state. Exceptions to this recommendation include:
- Patients undergoing hematopoietic stem cell transplant or receiving engineered cellular therapy such as chimeric antigen receptor T-cell therapy. Vaccination should be delayed for at least 3 months in these patients to maximize vaccine efficacy. Caregivers of these patients, however, should be immunized when possible.
- Patients with hematologic malignancies who are receiving intensive cytotoxic chemotherapy, such as cytarabine- or anthracycline-based regimens for acute myeloid leukemia. Vaccination in these patients should be delayed until absolute neutrophil count recovery.
- Patients undergoing major surgery. Vaccination should occur at least a few days before or after surgery.
- Patients who have experienced a severe or immediate adverse reaction to any of the ingredients in the mRNA COVID-19 vaccines.
Conversely, vaccination should occur when available in patients with hematologic malignancies and marrow failure who are expected to have limited or no recovery, patients with hematologic malignancies who are on long-term maintenance therapy, and patients with solid tumors who are receiving cytotoxic chemotherapy, targeted therapy, checkpoint inhibitors and other immunotherapy, or radiotherapy.
Caregivers, household contacts, and other close contacts who are 16 years of age and older should be vaccinated whenever they are eligible.
Unique concerns in patients with cancer
The NCCN recommendations were developed to address the unique issues and concerns with respect to patients with cancer, who have an increased risk of severe illness from SARS-CoV-2 infection. But the guidelines come with a caveat: “[t]here are limited safety and efficacy data in these patients,” the NCCN emphasized in a press statement.
“Right now, there is urgent need and limited data,” Steven Pergam, MD, co-leader of the NCCN COVID-19 Vaccination Committee, said in the statement.
“Our number one goal is helping to get the vaccine to as many people as we can,” Dr. Pergam said. “That means following existing national and regional directions for prioritizing people who are more likely to face death or severe illness from COVID-19.”
Dr. Pergam, associate professor at Fred Hutchinson Cancer Research Center in Seattle, further explained that “people receiving active cancer treatment are at greater risk for worse outcomes from COVID-19, particularly if they are older and have additional comorbidities, like immunosuppression.”
NCCN’s recommendations couldn’t have come at a better time for patients with cancer, according to Nora Disis, MD, a professor at the University of Washington in Seattle.
“The NCCN’s recommendations to prioritize COVID vaccinations for cancer patients on active treatment is an important step forward in protecting our patients from the infection,” Dr. Disis said in an interview.
“Cancer patients may be at higher risk for the complications seen with infection. In addition, cancer is a disease of older people, and a good number of our patients have the comorbidities that would predict a poorer outcome if they should become sick,” Dr. Disis added. “With the correct treatment, many patients with cancer will be long-term survivors. It is important that they be protected from infection with COVID to realize their best outcome.”
Additional vaccine considerations
The NCCN recommendations also address several other issues of importance for cancer patients, including:
- Deprioritizing other vaccines. COVID-19 vaccines should take precedence over other vaccines because data on dual vaccination are lacking. The NCCN recommends waiting 14 days after COVID-19 vaccination to deliver other vaccines.
- Vaccinating clinical trial participants. Trial leads should be consulted to prevent protocol violations or exclusions.
- Decision-making in the setting of limited vaccine availability. The NCCN noted that decisions on allocation must be made in accordance with state and local vaccine guidance but suggests prioritizing appropriate patients on active treatment, those planning to start treatment, and those who have just completed treatment. Additional risk factors for these patients, as well as other factors associated with risk for adverse COVID-19 outcomes, should also be considered. These include advanced age, comorbidities, and adverse social and demographic factors such as poverty and limited health care access.
- The need for ongoing prevention measures. Vaccines have been shown to decrease the incidence of COVID-19 and related complications, but it remains unclear whether vaccines prevent infection and subsequent transmission. This means everyone should continue following prevention recommendations, such as wearing masks and avoiding crowds.
The NCCN stressed that these recommendations are “intended to be a living document that is constantly evolving – it will be updated rapidly whenever new data comes out, as well as any potential new vaccines that may get approved in the future.” The NCCN also noted that the advisory committee will meet regularly to refine the recommendations as needed.
Dr. Pergam disclosed relationships with Chimerix Inc., Merck & Co., Global Life Technologies Inc., and Sanofi-Aventis. Dr. Disis disclosed grants from Pfizer, Bavarian Nordisk, Janssen, and Precigen. She is the founder of EpiThany and editor-in-chief of JAMA Oncology.
Vaccination timing considerations vary based on factors such as cancer and treatment type, and reasons for delaying vaccination in the general public also apply to cancer patients (recent COVID-19 exposure, for example).
In general, however, patients with cancer should be assigned to Centers for Disease Control and Prevention priority group 1 b/c and immunized when vaccination is available to them, the guidelines state. Exceptions to this recommendation include:
- Patients undergoing hematopoietic stem cell transplant or receiving engineered cellular therapy such as chimeric antigen receptor T-cell therapy. Vaccination should be delayed for at least 3 months in these patients to maximize vaccine efficacy. Caregivers of these patients, however, should be immunized when possible.
- Patients with hematologic malignancies who are receiving intensive cytotoxic chemotherapy, such as cytarabine- or anthracycline-based regimens for acute myeloid leukemia. Vaccination in these patients should be delayed until absolute neutrophil count recovery.
- Patients undergoing major surgery. Vaccination should occur at least a few days before or after surgery.
- Patients who have experienced a severe or immediate adverse reaction to any of the ingredients in the mRNA COVID-19 vaccines.
Conversely, vaccination should occur when available in patients with hematologic malignancies and marrow failure who are expected to have limited or no recovery, patients with hematologic malignancies who are on long-term maintenance therapy, and patients with solid tumors who are receiving cytotoxic chemotherapy, targeted therapy, checkpoint inhibitors and other immunotherapy, or radiotherapy.
Caregivers, household contacts, and other close contacts who are 16 years of age and older should be vaccinated whenever they are eligible.
Unique concerns in patients with cancer
The NCCN recommendations were developed to address the unique issues and concerns with respect to patients with cancer, who have an increased risk of severe illness from SARS-CoV-2 infection. But the guidelines come with a caveat: “[t]here are limited safety and efficacy data in these patients,” the NCCN emphasized in a press statement.
“Right now, there is urgent need and limited data,” Steven Pergam, MD, co-leader of the NCCN COVID-19 Vaccination Committee, said in the statement.
“Our number one goal is helping to get the vaccine to as many people as we can,” Dr. Pergam said. “That means following existing national and regional directions for prioritizing people who are more likely to face death or severe illness from COVID-19.”
Dr. Pergam, associate professor at Fred Hutchinson Cancer Research Center in Seattle, further explained that “people receiving active cancer treatment are at greater risk for worse outcomes from COVID-19, particularly if they are older and have additional comorbidities, like immunosuppression.”
NCCN’s recommendations couldn’t have come at a better time for patients with cancer, according to Nora Disis, MD, a professor at the University of Washington in Seattle.
“The NCCN’s recommendations to prioritize COVID vaccinations for cancer patients on active treatment is an important step forward in protecting our patients from the infection,” Dr. Disis said in an interview.
“Cancer patients may be at higher risk for the complications seen with infection. In addition, cancer is a disease of older people, and a good number of our patients have the comorbidities that would predict a poorer outcome if they should become sick,” Dr. Disis added. “With the correct treatment, many patients with cancer will be long-term survivors. It is important that they be protected from infection with COVID to realize their best outcome.”
Additional vaccine considerations
The NCCN recommendations also address several other issues of importance for cancer patients, including:
- Deprioritizing other vaccines. COVID-19 vaccines should take precedence over other vaccines because data on dual vaccination are lacking. The NCCN recommends waiting 14 days after COVID-19 vaccination to deliver other vaccines.
- Vaccinating clinical trial participants. Trial leads should be consulted to prevent protocol violations or exclusions.
- Decision-making in the setting of limited vaccine availability. The NCCN noted that decisions on allocation must be made in accordance with state and local vaccine guidance but suggests prioritizing appropriate patients on active treatment, those planning to start treatment, and those who have just completed treatment. Additional risk factors for these patients, as well as other factors associated with risk for adverse COVID-19 outcomes, should also be considered. These include advanced age, comorbidities, and adverse social and demographic factors such as poverty and limited health care access.
- The need for ongoing prevention measures. Vaccines have been shown to decrease the incidence of COVID-19 and related complications, but it remains unclear whether vaccines prevent infection and subsequent transmission. This means everyone should continue following prevention recommendations, such as wearing masks and avoiding crowds.
The NCCN stressed that these recommendations are “intended to be a living document that is constantly evolving – it will be updated rapidly whenever new data comes out, as well as any potential new vaccines that may get approved in the future.” The NCCN also noted that the advisory committee will meet regularly to refine the recommendations as needed.
Dr. Pergam disclosed relationships with Chimerix Inc., Merck & Co., Global Life Technologies Inc., and Sanofi-Aventis. Dr. Disis disclosed grants from Pfizer, Bavarian Nordisk, Janssen, and Precigen. She is the founder of EpiThany and editor-in-chief of JAMA Oncology.
Expert offers tips for sorting out pink lesions on dermoscopy
Even in the most experienced hands, .
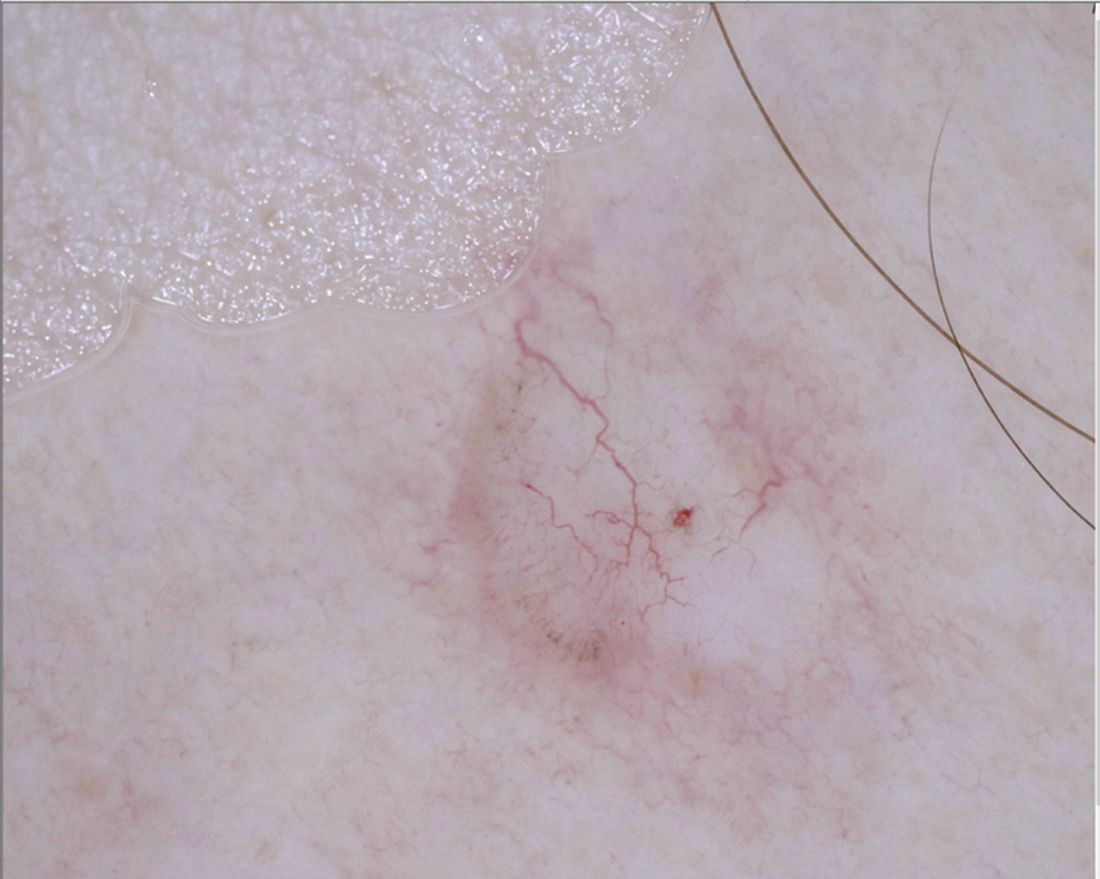
“For me, pink lesions are challenging,” Jennifer A. Stein, MD, PhD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference. “How can dermoscopy help us distinguish between Spitz nevus, melanoma, clear cell acanthoma, psoriasis, basal cell carcinoma, and squamous cell carcinoma?”
Dr. Stein, professor of dermatology at New York University, offered four tips. First, look for the shiny white perpendicular lines, otherwise known as the chrysalis or crystalline pattern. “You can only see this feature when you’re looking with polarized light,” she said. “This is why you want a dermatoscope that has polarized light, and better yet, one that you’re able to turn on and off, the hybrid kind, because then you can convince yourself that you’re looking at this feature, because it blinks on and off.”
The differential diagnosis for white shiny perpendicular lines includes dermatofibroma/scars (which is most common), Spitz and atypical genital nevi, BCC, and melanoma. “Dermatofibromas sometimes have white circles or rings in the center,” Dr. Stein said. “In BCC, the lines aren’t always perpendicular. Sometimes it’s more of a blotch or strands.”
A second tip for managing a pink lesion on dermoscopy is to look for any brown color. “When you see that combo together you have to worry,” she said. “When you see pigment network on dermoscopy, you have to put melanoma in your differential. If you see shiny white lines in something that is melanocytic, there’s a 98% specificity for melanoma.”
A third tip she offered for managing pink lesions is to check the blood vessels for clues. “For years, I was just naming the vessels based on making the diagnosis and then deciding, ‘that’s a basal cell carcinoma; those must be branching vessels,’ ” said Dr. Stein, who manages NYU’s medical dermatology faculty group practice.
However, blood vessel patterns differ. For example, branching or arborizing vessels are suggestive of BCC. “These vessels are very crisp-looking on dermoscopy,” she said. “They’re all in the same plane of focus and they look like they were drawn in with a fine point marker. That’s different from other blood vessel patterns.” She also pointed out that superficial basal cells have short, fine telangiectasias. “When you put on the polarized light, the clue is the white, shiny structures,” she said.
Dotted vessels, meanwhile, appear on dermoscopy as small red dots aligned perpendicular to the skin surface. The differential includes inflammatory lesions like psoriasis, stasis, and trauma; clear cell acanthoma (characterized by a “string of pearls” arrangement), nevi, and melanoma. “I find dermoscopy most useful in diagnosing SCC – especially squamous cell in situ,” she said. “Important clinical clues suggestive of SCC or melanoma include a solitary lesion, it’s new, it’s growing, and it’s not going away with a topical steroid.”
An additional pattern to be aware of are hairpin vessels, which are looped and feature a sharp bend at one end. These are often seen in seborrheic keratoses. “You can’t count on the hairpin vessels alone, because you can see this in anything keratotic, such as in keratoacanthoma (at the periphery with a yellow keratotic center), warts, SCC, BCC, as well as in dermal nevi and Spitz nevi,” said Dr. Stein, who recommended dermoscopedia.org as resource.
Comma vessels, meanwhile, appear in dermal or compound nevi. She described these as “slightly curved vessels that are much less in focus than branched vessels, because they come in and out of the plane of focus,” she said. “If you put your dermatoscope on top of the nevus and wobble it around you can appreciate the curve. If you look at it from the side, it looks like a curve. If you look at it straight on it will look more like a line. If you look at from the end it will look like a dot.”
Another vessel type she discussed are linear irregular and polymorphous vessels, which she described as “any combination of different types of vessels. We get most worried when we see dotted and linear irregular vessels together. In that case, you worry about melanoma. These can also be seen in nevi and other tumors, such as BCC.”
Dr. Stein’s fourth tip of the presentation was a reminder to consider dermoscopy as one piece of the clinical exam. “Always think about the lesion in context of the rest of the clinical picture and history,” she said. “Don’t get discouraged if it’s hard; just keep practicing. Look for any brown and use your clinical clues to put together to make the right decision.”
She disclosed that NYU receives compensation from MoleSafe for her telemedicine dermoscopic diagnoses.
Even in the most experienced hands, .

“For me, pink lesions are challenging,” Jennifer A. Stein, MD, PhD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference. “How can dermoscopy help us distinguish between Spitz nevus, melanoma, clear cell acanthoma, psoriasis, basal cell carcinoma, and squamous cell carcinoma?”
Dr. Stein, professor of dermatology at New York University, offered four tips. First, look for the shiny white perpendicular lines, otherwise known as the chrysalis or crystalline pattern. “You can only see this feature when you’re looking with polarized light,” she said. “This is why you want a dermatoscope that has polarized light, and better yet, one that you’re able to turn on and off, the hybrid kind, because then you can convince yourself that you’re looking at this feature, because it blinks on and off.”
The differential diagnosis for white shiny perpendicular lines includes dermatofibroma/scars (which is most common), Spitz and atypical genital nevi, BCC, and melanoma. “Dermatofibromas sometimes have white circles or rings in the center,” Dr. Stein said. “In BCC, the lines aren’t always perpendicular. Sometimes it’s more of a blotch or strands.”
A second tip for managing a pink lesion on dermoscopy is to look for any brown color. “When you see that combo together you have to worry,” she said. “When you see pigment network on dermoscopy, you have to put melanoma in your differential. If you see shiny white lines in something that is melanocytic, there’s a 98% specificity for melanoma.”
A third tip she offered for managing pink lesions is to check the blood vessels for clues. “For years, I was just naming the vessels based on making the diagnosis and then deciding, ‘that’s a basal cell carcinoma; those must be branching vessels,’ ” said Dr. Stein, who manages NYU’s medical dermatology faculty group practice.
However, blood vessel patterns differ. For example, branching or arborizing vessels are suggestive of BCC. “These vessels are very crisp-looking on dermoscopy,” she said. “They’re all in the same plane of focus and they look like they were drawn in with a fine point marker. That’s different from other blood vessel patterns.” She also pointed out that superficial basal cells have short, fine telangiectasias. “When you put on the polarized light, the clue is the white, shiny structures,” she said.
Dotted vessels, meanwhile, appear on dermoscopy as small red dots aligned perpendicular to the skin surface. The differential includes inflammatory lesions like psoriasis, stasis, and trauma; clear cell acanthoma (characterized by a “string of pearls” arrangement), nevi, and melanoma. “I find dermoscopy most useful in diagnosing SCC – especially squamous cell in situ,” she said. “Important clinical clues suggestive of SCC or melanoma include a solitary lesion, it’s new, it’s growing, and it’s not going away with a topical steroid.”
An additional pattern to be aware of are hairpin vessels, which are looped and feature a sharp bend at one end. These are often seen in seborrheic keratoses. “You can’t count on the hairpin vessels alone, because you can see this in anything keratotic, such as in keratoacanthoma (at the periphery with a yellow keratotic center), warts, SCC, BCC, as well as in dermal nevi and Spitz nevi,” said Dr. Stein, who recommended dermoscopedia.org as resource.
Comma vessels, meanwhile, appear in dermal or compound nevi. She described these as “slightly curved vessels that are much less in focus than branched vessels, because they come in and out of the plane of focus,” she said. “If you put your dermatoscope on top of the nevus and wobble it around you can appreciate the curve. If you look at it from the side, it looks like a curve. If you look at it straight on it will look more like a line. If you look at from the end it will look like a dot.”
Another vessel type she discussed are linear irregular and polymorphous vessels, which she described as “any combination of different types of vessels. We get most worried when we see dotted and linear irregular vessels together. In that case, you worry about melanoma. These can also be seen in nevi and other tumors, such as BCC.”
Dr. Stein’s fourth tip of the presentation was a reminder to consider dermoscopy as one piece of the clinical exam. “Always think about the lesion in context of the rest of the clinical picture and history,” she said. “Don’t get discouraged if it’s hard; just keep practicing. Look for any brown and use your clinical clues to put together to make the right decision.”
She disclosed that NYU receives compensation from MoleSafe for her telemedicine dermoscopic diagnoses.
Even in the most experienced hands, .

“For me, pink lesions are challenging,” Jennifer A. Stein, MD, PhD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference. “How can dermoscopy help us distinguish between Spitz nevus, melanoma, clear cell acanthoma, psoriasis, basal cell carcinoma, and squamous cell carcinoma?”
Dr. Stein, professor of dermatology at New York University, offered four tips. First, look for the shiny white perpendicular lines, otherwise known as the chrysalis or crystalline pattern. “You can only see this feature when you’re looking with polarized light,” she said. “This is why you want a dermatoscope that has polarized light, and better yet, one that you’re able to turn on and off, the hybrid kind, because then you can convince yourself that you’re looking at this feature, because it blinks on and off.”
The differential diagnosis for white shiny perpendicular lines includes dermatofibroma/scars (which is most common), Spitz and atypical genital nevi, BCC, and melanoma. “Dermatofibromas sometimes have white circles or rings in the center,” Dr. Stein said. “In BCC, the lines aren’t always perpendicular. Sometimes it’s more of a blotch or strands.”
A second tip for managing a pink lesion on dermoscopy is to look for any brown color. “When you see that combo together you have to worry,” she said. “When you see pigment network on dermoscopy, you have to put melanoma in your differential. If you see shiny white lines in something that is melanocytic, there’s a 98% specificity for melanoma.”
A third tip she offered for managing pink lesions is to check the blood vessels for clues. “For years, I was just naming the vessels based on making the diagnosis and then deciding, ‘that’s a basal cell carcinoma; those must be branching vessels,’ ” said Dr. Stein, who manages NYU’s medical dermatology faculty group practice.
However, blood vessel patterns differ. For example, branching or arborizing vessels are suggestive of BCC. “These vessels are very crisp-looking on dermoscopy,” she said. “They’re all in the same plane of focus and they look like they were drawn in with a fine point marker. That’s different from other blood vessel patterns.” She also pointed out that superficial basal cells have short, fine telangiectasias. “When you put on the polarized light, the clue is the white, shiny structures,” she said.
Dotted vessels, meanwhile, appear on dermoscopy as small red dots aligned perpendicular to the skin surface. The differential includes inflammatory lesions like psoriasis, stasis, and trauma; clear cell acanthoma (characterized by a “string of pearls” arrangement), nevi, and melanoma. “I find dermoscopy most useful in diagnosing SCC – especially squamous cell in situ,” she said. “Important clinical clues suggestive of SCC or melanoma include a solitary lesion, it’s new, it’s growing, and it’s not going away with a topical steroid.”
An additional pattern to be aware of are hairpin vessels, which are looped and feature a sharp bend at one end. These are often seen in seborrheic keratoses. “You can’t count on the hairpin vessels alone, because you can see this in anything keratotic, such as in keratoacanthoma (at the periphery with a yellow keratotic center), warts, SCC, BCC, as well as in dermal nevi and Spitz nevi,” said Dr. Stein, who recommended dermoscopedia.org as resource.
Comma vessels, meanwhile, appear in dermal or compound nevi. She described these as “slightly curved vessels that are much less in focus than branched vessels, because they come in and out of the plane of focus,” she said. “If you put your dermatoscope on top of the nevus and wobble it around you can appreciate the curve. If you look at it from the side, it looks like a curve. If you look at it straight on it will look more like a line. If you look at from the end it will look like a dot.”
Another vessel type she discussed are linear irregular and polymorphous vessels, which she described as “any combination of different types of vessels. We get most worried when we see dotted and linear irregular vessels together. In that case, you worry about melanoma. These can also be seen in nevi and other tumors, such as BCC.”
Dr. Stein’s fourth tip of the presentation was a reminder to consider dermoscopy as one piece of the clinical exam. “Always think about the lesion in context of the rest of the clinical picture and history,” she said. “Don’t get discouraged if it’s hard; just keep practicing. Look for any brown and use your clinical clues to put together to make the right decision.”
She disclosed that NYU receives compensation from MoleSafe for her telemedicine dermoscopic diagnoses.
FROM ODAC 2021
Male Genital Examinations: Special Considerations and Pearls for Dermatologists
Men have unique dermatologic needs yet are significantly less likely than women to visit a dermatologist’s office.1 Male patients might have preconceived notions about the nature of dermatology visits and necessary areas of the body to be examined: For example, male patients might associate the genital examination with a urologist and not expect a dermatologist to complete such a seemingly private examination.2
Genital examinations are currently underperformed: Only one-quarter of dermatologists report examining a male patient’s genitals at most or all visits.3 In this commentary, we discuss the importance of genital examinations in men’s dermatology, specific issues that can arise, and strategies to enhance the quality and frequency of genital examinations in male patients.
Invaluable Aspect of Care
Thorough inspection of a male patient’s genital region is an important part of conducting a total-body skin examination (TBSE) for routine surveillance and evaluation of genital dermatoses. Sexually transmitted infections, warts, and other common lesions can be missed in diagnosis without careful inspection of the genital region. Additionally, scrotal malignancies, such as primary and metastatic melanoma and basal cell carcinoma, though rare, might be overlooked until symptoms become severe.4,5
There is no substitute for a physical examination but, in certain circumstances, it might be appropriate for a dermatologist to ask a patient if he has concerning lesions on his genitals. However, patients often are unsure of worrisome signs, and areas of the perineum might not be easily visible to a patient. Genital inspection during the physical examination allows for a teachable moment, during which the dermatologist can educate the patient about benign lesions and variants, such as pearly penile papules, seborrheic keratoses, and sebaceous cysts.6 These lesions might not require intervention but should be monitored for atypical features or infection.6
Also, the dermatologist might incidentally discover transmissible lesions, such as condylomata caused by human papillomavirus, which has been shown to be present in approximately 50% of men in the United States7—many of whom are unaware. Inflammatory dermatoses, such as psoriasis, often affect the genitals and go unnoticed; prompt intervention can decrease the likelihood of complications.6
Protocol for Genital Examinations
To examine the genitals, all surfaces of the penis, scrotum, and perineum should be evaluated, with anatomic and pathologic variants noted. The patient or physician should stretch the penis, maneuvering it in multiple directions so that all aspects can be examined. In uncircumcised men, the foreskin should be retracted so that the head of the penis can be examined, followed by replacement of the foreskin by the patient.8 The scrotum also should be examined and lifted to fully view the perineum.
Providers should not grasp the penis with the whole hand but use the thumb and first finger to hold the head of the penis to maneuver it.8 Similarly, using the back of the hand and fingers to manipulate the genitals establishes boundaries and sets a clinical tone for the examination.
Unintentional Erection
Unique to the male dermatologic examination is the unintentional patient erection; a physician might be unsure of how to approach such a potentially awkward situation. An erection is not always an indication of sexual arousal; rather, it can reflect an autonomic reflex in response to physical stimulation. Erections occur commonly in health care settings, especially if the genitals are being manipulated.9
Generally, the course of action here depends on the patient’s response.10 For patients who appear unbothered, it might be appropriate to ignore the erection and proceed with the examination, especially if the physician is not actively examining the genital region. If the patient appears embarrassed, the physician can say “This is completely normal” or “Random erections are common” to normalize the situation. Joking or laughing should be avoided. For a patient who appears upset, the physician can step outside the room for a brief period to give the patient privacy, then re-enter and ask him if he is comfortable continuing with the examination.
When a patient develops an erection, the physician might become uncomfortable and, consciously or subconsciously, increase the pace of the examination, which is a natural tendency, but expediency at the expense of comprehensive care is inappropriate.
Examiner’s Body Language and Tone
Throughout the genital examination, the physician should be mindful of their comments and body language to avoid exacerbating patient vulnerability. Using anatomic terms, rather than colloquial ones, to describe the genitalia is advised to prevent misunderstanding and maintain a professional clinical environment. Providers should be prepared to explain anatomic terms because some patients are not familiar with medical terminology.
Presence of a Chaperone
Involving a chaperone, as recommended by the American Medical Association, might make a patient more comfortable and alleviate potential misunderstanding. Still, physicians should be aware that some patients might feel uncomfortable with a chaperone, interpreting their presence as an expectation of impropriety.11 Universal offering of a chaperone to all patients, regardless of the gender of the physician, as well as appropriate signage in the clinical environment, normalizes chaperone invitation and use.
Other Helpful Considerations
Various strategies in the male genital examination can increase patient and physician comfort and improve care:
- The patient should be offered a gown before a TBSE or any skin examination during which the genitals will be examined.
- The patient should be allowed to keep his shorts or underwear on to avoid the feeling of being naked, which can provoke anxiety. Prior to beginning the examination, disclose that it will include “under the covered areas.”
- Ask the patient for permission to conduct the examination, enumerate the steps, and provide a rationale for a genital examination. These steps help gain cooperation, alleviate anticipation, and prevent surprise.
- To increase the patient’s comfort level, he can be asked whether he prefers to be examined supine or standing.
- Consider allowing the patient, himself, to expose and manipulate his genitals during the examination to increase his involvement and sense of autonomy.
- For genital examinations, patients often prefer that the examiner be a physician of the same gender. Accommodating a patient’s request regarding the examiner’s gender might not always be possible, but the medical practice should make an honest attempt to oblige.
Lastly, providers should be cognizant of the needs of male sexual and gender minority populations (ie, gay, bisexual, transgender/gender diverse, queer or questioning, intersex, and asexual persons). For example, transgender women might retain male anatomy or have surgical alteration of the genital region that also requires evaluation. In such patient populations, the genital examination is equally important to evaluate for dermatologic conditions that require treatment.
Final Thoughts
The male genital examination is an important component of the TBSE, as dermatologists can recognize lesions before symptoms present. Robust educational methods for trainees and practitioners in dermatology are lacking, and development of curricula might be beneficial to increase comfort in performing the genital examination. Still, use of the procedures described in this commentary can normalize the men’s genital examination, optimize the physical examination, and improve men’s overall dermatologic health.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Brezinski EA, Harskamp CT, Ledo L, et al. Public perception of dermatologists and comparison with other medical specialties: results from a national survey. J Am Acad Dermatol. 2014;71:875-881.
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Gonzalez CD, Hawkes JE, Bowles TL. Malignant melanoma scrotal metastasis: the importance of the genital examination. JAAD Case Rep. 2017;3:10-12.
- Solimani F, Juratli H, Hoch M, et al. Basal cell carcinoma of the scrotum: an important but easily overlooked entity. J Eur Acad Dermatol Venereol. 2018;32:E254-E255.
- Gabrielson AT, Le TV, Fontenot C, et al. Male genital dermatology: a primer for the sexual medicine physician. Sex Med Rev. 2019;7:71-83.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- Albaugh JA, Kellogg-Spadt S. Genital and dermatologic examination. part II: the male patient. Urol Nurs. 2003;23:366-367.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379-395.
- Norwick P, Weston GK, Grant-Kels JM. Erection ethics. J Am Acad Dermatol. 2019;81:1225.
- Vogel L. Chaperones: friend or foe, and to whom? CMAJ. 2012;184:642-643.
Men have unique dermatologic needs yet are significantly less likely than women to visit a dermatologist’s office.1 Male patients might have preconceived notions about the nature of dermatology visits and necessary areas of the body to be examined: For example, male patients might associate the genital examination with a urologist and not expect a dermatologist to complete such a seemingly private examination.2
Genital examinations are currently underperformed: Only one-quarter of dermatologists report examining a male patient’s genitals at most or all visits.3 In this commentary, we discuss the importance of genital examinations in men’s dermatology, specific issues that can arise, and strategies to enhance the quality and frequency of genital examinations in male patients.
Invaluable Aspect of Care
Thorough inspection of a male patient’s genital region is an important part of conducting a total-body skin examination (TBSE) for routine surveillance and evaluation of genital dermatoses. Sexually transmitted infections, warts, and other common lesions can be missed in diagnosis without careful inspection of the genital region. Additionally, scrotal malignancies, such as primary and metastatic melanoma and basal cell carcinoma, though rare, might be overlooked until symptoms become severe.4,5
There is no substitute for a physical examination but, in certain circumstances, it might be appropriate for a dermatologist to ask a patient if he has concerning lesions on his genitals. However, patients often are unsure of worrisome signs, and areas of the perineum might not be easily visible to a patient. Genital inspection during the physical examination allows for a teachable moment, during which the dermatologist can educate the patient about benign lesions and variants, such as pearly penile papules, seborrheic keratoses, and sebaceous cysts.6 These lesions might not require intervention but should be monitored for atypical features or infection.6
Also, the dermatologist might incidentally discover transmissible lesions, such as condylomata caused by human papillomavirus, which has been shown to be present in approximately 50% of men in the United States7—many of whom are unaware. Inflammatory dermatoses, such as psoriasis, often affect the genitals and go unnoticed; prompt intervention can decrease the likelihood of complications.6
Protocol for Genital Examinations
To examine the genitals, all surfaces of the penis, scrotum, and perineum should be evaluated, with anatomic and pathologic variants noted. The patient or physician should stretch the penis, maneuvering it in multiple directions so that all aspects can be examined. In uncircumcised men, the foreskin should be retracted so that the head of the penis can be examined, followed by replacement of the foreskin by the patient.8 The scrotum also should be examined and lifted to fully view the perineum.
Providers should not grasp the penis with the whole hand but use the thumb and first finger to hold the head of the penis to maneuver it.8 Similarly, using the back of the hand and fingers to manipulate the genitals establishes boundaries and sets a clinical tone for the examination.
Unintentional Erection
Unique to the male dermatologic examination is the unintentional patient erection; a physician might be unsure of how to approach such a potentially awkward situation. An erection is not always an indication of sexual arousal; rather, it can reflect an autonomic reflex in response to physical stimulation. Erections occur commonly in health care settings, especially if the genitals are being manipulated.9
Generally, the course of action here depends on the patient’s response.10 For patients who appear unbothered, it might be appropriate to ignore the erection and proceed with the examination, especially if the physician is not actively examining the genital region. If the patient appears embarrassed, the physician can say “This is completely normal” or “Random erections are common” to normalize the situation. Joking or laughing should be avoided. For a patient who appears upset, the physician can step outside the room for a brief period to give the patient privacy, then re-enter and ask him if he is comfortable continuing with the examination.
When a patient develops an erection, the physician might become uncomfortable and, consciously or subconsciously, increase the pace of the examination, which is a natural tendency, but expediency at the expense of comprehensive care is inappropriate.
Examiner’s Body Language and Tone
Throughout the genital examination, the physician should be mindful of their comments and body language to avoid exacerbating patient vulnerability. Using anatomic terms, rather than colloquial ones, to describe the genitalia is advised to prevent misunderstanding and maintain a professional clinical environment. Providers should be prepared to explain anatomic terms because some patients are not familiar with medical terminology.
Presence of a Chaperone
Involving a chaperone, as recommended by the American Medical Association, might make a patient more comfortable and alleviate potential misunderstanding. Still, physicians should be aware that some patients might feel uncomfortable with a chaperone, interpreting their presence as an expectation of impropriety.11 Universal offering of a chaperone to all patients, regardless of the gender of the physician, as well as appropriate signage in the clinical environment, normalizes chaperone invitation and use.
Other Helpful Considerations
Various strategies in the male genital examination can increase patient and physician comfort and improve care:
- The patient should be offered a gown before a TBSE or any skin examination during which the genitals will be examined.
- The patient should be allowed to keep his shorts or underwear on to avoid the feeling of being naked, which can provoke anxiety. Prior to beginning the examination, disclose that it will include “under the covered areas.”
- Ask the patient for permission to conduct the examination, enumerate the steps, and provide a rationale for a genital examination. These steps help gain cooperation, alleviate anticipation, and prevent surprise.
- To increase the patient’s comfort level, he can be asked whether he prefers to be examined supine or standing.
- Consider allowing the patient, himself, to expose and manipulate his genitals during the examination to increase his involvement and sense of autonomy.
- For genital examinations, patients often prefer that the examiner be a physician of the same gender. Accommodating a patient’s request regarding the examiner’s gender might not always be possible, but the medical practice should make an honest attempt to oblige.
Lastly, providers should be cognizant of the needs of male sexual and gender minority populations (ie, gay, bisexual, transgender/gender diverse, queer or questioning, intersex, and asexual persons). For example, transgender women might retain male anatomy or have surgical alteration of the genital region that also requires evaluation. In such patient populations, the genital examination is equally important to evaluate for dermatologic conditions that require treatment.
Final Thoughts
The male genital examination is an important component of the TBSE, as dermatologists can recognize lesions before symptoms present. Robust educational methods for trainees and practitioners in dermatology are lacking, and development of curricula might be beneficial to increase comfort in performing the genital examination. Still, use of the procedures described in this commentary can normalize the men’s genital examination, optimize the physical examination, and improve men’s overall dermatologic health.
Men have unique dermatologic needs yet are significantly less likely than women to visit a dermatologist’s office.1 Male patients might have preconceived notions about the nature of dermatology visits and necessary areas of the body to be examined: For example, male patients might associate the genital examination with a urologist and not expect a dermatologist to complete such a seemingly private examination.2
Genital examinations are currently underperformed: Only one-quarter of dermatologists report examining a male patient’s genitals at most or all visits.3 In this commentary, we discuss the importance of genital examinations in men’s dermatology, specific issues that can arise, and strategies to enhance the quality and frequency of genital examinations in male patients.
Invaluable Aspect of Care
Thorough inspection of a male patient’s genital region is an important part of conducting a total-body skin examination (TBSE) for routine surveillance and evaluation of genital dermatoses. Sexually transmitted infections, warts, and other common lesions can be missed in diagnosis without careful inspection of the genital region. Additionally, scrotal malignancies, such as primary and metastatic melanoma and basal cell carcinoma, though rare, might be overlooked until symptoms become severe.4,5
There is no substitute for a physical examination but, in certain circumstances, it might be appropriate for a dermatologist to ask a patient if he has concerning lesions on his genitals. However, patients often are unsure of worrisome signs, and areas of the perineum might not be easily visible to a patient. Genital inspection during the physical examination allows for a teachable moment, during which the dermatologist can educate the patient about benign lesions and variants, such as pearly penile papules, seborrheic keratoses, and sebaceous cysts.6 These lesions might not require intervention but should be monitored for atypical features or infection.6
Also, the dermatologist might incidentally discover transmissible lesions, such as condylomata caused by human papillomavirus, which has been shown to be present in approximately 50% of men in the United States7—many of whom are unaware. Inflammatory dermatoses, such as psoriasis, often affect the genitals and go unnoticed; prompt intervention can decrease the likelihood of complications.6
Protocol for Genital Examinations
To examine the genitals, all surfaces of the penis, scrotum, and perineum should be evaluated, with anatomic and pathologic variants noted. The patient or physician should stretch the penis, maneuvering it in multiple directions so that all aspects can be examined. In uncircumcised men, the foreskin should be retracted so that the head of the penis can be examined, followed by replacement of the foreskin by the patient.8 The scrotum also should be examined and lifted to fully view the perineum.
Providers should not grasp the penis with the whole hand but use the thumb and first finger to hold the head of the penis to maneuver it.8 Similarly, using the back of the hand and fingers to manipulate the genitals establishes boundaries and sets a clinical tone for the examination.
Unintentional Erection
Unique to the male dermatologic examination is the unintentional patient erection; a physician might be unsure of how to approach such a potentially awkward situation. An erection is not always an indication of sexual arousal; rather, it can reflect an autonomic reflex in response to physical stimulation. Erections occur commonly in health care settings, especially if the genitals are being manipulated.9
Generally, the course of action here depends on the patient’s response.10 For patients who appear unbothered, it might be appropriate to ignore the erection and proceed with the examination, especially if the physician is not actively examining the genital region. If the patient appears embarrassed, the physician can say “This is completely normal” or “Random erections are common” to normalize the situation. Joking or laughing should be avoided. For a patient who appears upset, the physician can step outside the room for a brief period to give the patient privacy, then re-enter and ask him if he is comfortable continuing with the examination.
When a patient develops an erection, the physician might become uncomfortable and, consciously or subconsciously, increase the pace of the examination, which is a natural tendency, but expediency at the expense of comprehensive care is inappropriate.
Examiner’s Body Language and Tone
Throughout the genital examination, the physician should be mindful of their comments and body language to avoid exacerbating patient vulnerability. Using anatomic terms, rather than colloquial ones, to describe the genitalia is advised to prevent misunderstanding and maintain a professional clinical environment. Providers should be prepared to explain anatomic terms because some patients are not familiar with medical terminology.
Presence of a Chaperone
Involving a chaperone, as recommended by the American Medical Association, might make a patient more comfortable and alleviate potential misunderstanding. Still, physicians should be aware that some patients might feel uncomfortable with a chaperone, interpreting their presence as an expectation of impropriety.11 Universal offering of a chaperone to all patients, regardless of the gender of the physician, as well as appropriate signage in the clinical environment, normalizes chaperone invitation and use.
Other Helpful Considerations
Various strategies in the male genital examination can increase patient and physician comfort and improve care:
- The patient should be offered a gown before a TBSE or any skin examination during which the genitals will be examined.
- The patient should be allowed to keep his shorts or underwear on to avoid the feeling of being naked, which can provoke anxiety. Prior to beginning the examination, disclose that it will include “under the covered areas.”
- Ask the patient for permission to conduct the examination, enumerate the steps, and provide a rationale for a genital examination. These steps help gain cooperation, alleviate anticipation, and prevent surprise.
- To increase the patient’s comfort level, he can be asked whether he prefers to be examined supine or standing.
- Consider allowing the patient, himself, to expose and manipulate his genitals during the examination to increase his involvement and sense of autonomy.
- For genital examinations, patients often prefer that the examiner be a physician of the same gender. Accommodating a patient’s request regarding the examiner’s gender might not always be possible, but the medical practice should make an honest attempt to oblige.
Lastly, providers should be cognizant of the needs of male sexual and gender minority populations (ie, gay, bisexual, transgender/gender diverse, queer or questioning, intersex, and asexual persons). For example, transgender women might retain male anatomy or have surgical alteration of the genital region that also requires evaluation. In such patient populations, the genital examination is equally important to evaluate for dermatologic conditions that require treatment.
Final Thoughts
The male genital examination is an important component of the TBSE, as dermatologists can recognize lesions before symptoms present. Robust educational methods for trainees and practitioners in dermatology are lacking, and development of curricula might be beneficial to increase comfort in performing the genital examination. Still, use of the procedures described in this commentary can normalize the men’s genital examination, optimize the physical examination, and improve men’s overall dermatologic health.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Brezinski EA, Harskamp CT, Ledo L, et al. Public perception of dermatologists and comparison with other medical specialties: results from a national survey. J Am Acad Dermatol. 2014;71:875-881.
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Gonzalez CD, Hawkes JE, Bowles TL. Malignant melanoma scrotal metastasis: the importance of the genital examination. JAAD Case Rep. 2017;3:10-12.
- Solimani F, Juratli H, Hoch M, et al. Basal cell carcinoma of the scrotum: an important but easily overlooked entity. J Eur Acad Dermatol Venereol. 2018;32:E254-E255.
- Gabrielson AT, Le TV, Fontenot C, et al. Male genital dermatology: a primer for the sexual medicine physician. Sex Med Rev. 2019;7:71-83.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- Albaugh JA, Kellogg-Spadt S. Genital and dermatologic examination. part II: the male patient. Urol Nurs. 2003;23:366-367.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379-395.
- Norwick P, Weston GK, Grant-Kels JM. Erection ethics. J Am Acad Dermatol. 2019;81:1225.
- Vogel L. Chaperones: friend or foe, and to whom? CMAJ. 2012;184:642-643.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Brezinski EA, Harskamp CT, Ledo L, et al. Public perception of dermatologists and comparison with other medical specialties: results from a national survey. J Am Acad Dermatol. 2014;71:875-881.
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Gonzalez CD, Hawkes JE, Bowles TL. Malignant melanoma scrotal metastasis: the importance of the genital examination. JAAD Case Rep. 2017;3:10-12.
- Solimani F, Juratli H, Hoch M, et al. Basal cell carcinoma of the scrotum: an important but easily overlooked entity. J Eur Acad Dermatol Venereol. 2018;32:E254-E255.
- Gabrielson AT, Le TV, Fontenot C, et al. Male genital dermatology: a primer for the sexual medicine physician. Sex Med Rev. 2019;7:71-83.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- Albaugh JA, Kellogg-Spadt S. Genital and dermatologic examination. part II: the male patient. Urol Nurs. 2003;23:366-367.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379-395.
- Norwick P, Weston GK, Grant-Kels JM. Erection ethics. J Am Acad Dermatol. 2019;81:1225.
- Vogel L. Chaperones: friend or foe, and to whom? CMAJ. 2012;184:642-643.
Practice Points
- Genital examinations are an important aspect of comprehensive dermatologic care for male patients.
- Unintentional patient erections are unique to male patients and should be addressed professionally, depending on the patient’s reaction.
- In addition to being mindful of body language and tone, dermatologists may consider involving a chaperone when performing genital examinations to optimize patient experience.
Hypertrophic Lichen Planus–like Eruption Following Pembrolizumab
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.
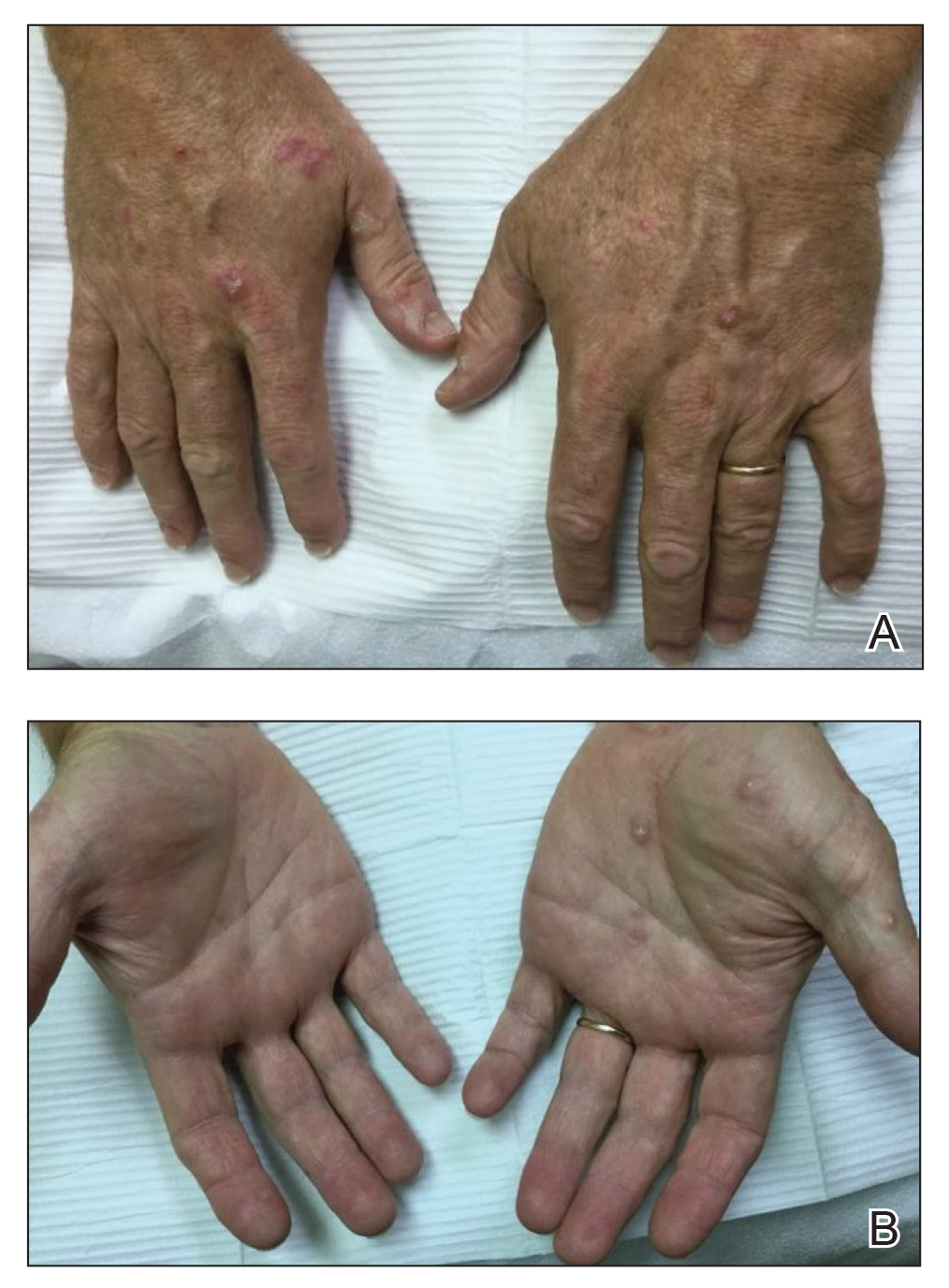
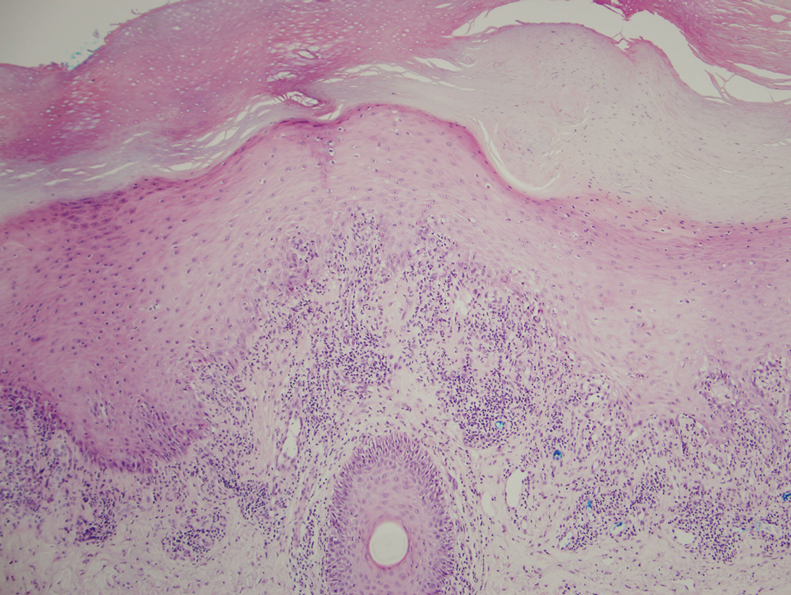
At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.


At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.


At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
Practice Points
- With an increased use of immunotherapy medications such as pembrolizumab for various cancers, it is important that dermatologists are aware of the wide range of adverse cutaneous reactions that can occur, including lichenoid reactions.
- Hypertrophic lichen planus should be considered in the differential diagnosis of patients with cutaneous lesions in addition to nail findings developing after starting programmed cell death protein 1 inhibitor therapy.
Does screening for skin cancer result in melanoma overdiagnosis?
When the COVID-19 pandemic first hit, cancer screening in the United States came to an abrupt halt. That experience, coupled with the financial fallout of the pandemic, has led some doctors to reassess business as usual.
In particular, a trio has taken aim at skin cancer screening – arguing that it should stop – in a ‘sounding board’ commentary published online Jan. 7 in the New England Journal of Medicine.
“The COVID-19 pandemic has functionally stopped skin cancer screening; what is important is not to restart it,” wrote the authors, led by H. Gilbert Welch, MD, MPH, at Brigham and Women’s Hospital, Boston, Massachusetts. Dr. Welch has often raised questions about cancer screening and highlighted the issue of overdiagnosis.
In this latest essay, Dr. Welch teamed up with pathologist Benjamin Mazer, MD, Yale University, New Haven, Conn., who writes commentaries for this news organization, and dermatologist Adewole S. Adamson, MD, University of Texas, Austin, to argue that screening for skin cancer has led to an overdiagnosis of melanoma.
However, two melanoma experts pointed out flaws in some of their arguments, and said the issue is more nuanced than they present.
Arguing that melanoma is overdiagnosed
The incidence of melanoma is six times as high as it was 40 years ago, making it the third most common cancer in the United States, the investigators pointed out. However, while case rates have skyrocketed, death rates from melanoma have remained about the same, which points to overdiagnosis.
They described a cycle of increased diagnostic scrutiny that is driving overdiagnosis of melanoma. This includes heightened awareness (perhaps overly) among patients, widespread skin screenings, lower clinical thresholds for biopsy, and lower thresholds among pathologists for diagnosis of melanoma. Fear of missing cancer, legal concerns, and financial incentives may all contribute.
“We view the rise in the incidence of melanoma as a sentinel event, a warning that an epidemic of inspection, surveillance, and biopsy of pigmented skin lesions is permeating through the general population,” they wrote.
Furthermore, overdiagnosis could contribute to unnecessary intervention.
Between 2004 and 2017, rates of biopsy among fee-for-service Medicare recipients almost doubled (from 5% to 8%), according to coding trends data cited in the article. Overdiagnosis and unnecessary intervention could cause psychological, financial, and physical harm to the patient, and the authors argued for interrupting the cycle.
“The most important step to break the cycle of melanoma overdiagnosis is to stop population-wide screening for skin cancer,” they wrote.
The U.S. Preventive Services Task Force currently states that there is insufficient evidence to weigh the balances versus the harms of skin cancer screening, leaving it open to interpretation.
“[T]he increase in melanoma diagnoses by a factor of 6, with at least an order of magnitude more persons undergoing a biopsy and no apparent effect on mortality, is more than enough to recommend against population-wide screening,” Dr. Welch and colleagues concluded.
But the issue may be more nuanced, argued a melanoma expert.
“Everyone agrees that screening high-risk groups has the greatest chance of reducing cancer mortality. In melanoma, the strongest risk factor is the number of moles and presence of clinically atypical moles,” David Polsky, MD, PhD, commented in an interview. Dr. Polsky is a professor of dermatologic oncology at the Perlmutter Cancer Center at New York University Langone Health.
However, population-based studies have shown that at least half of melanoma patients are not considered high risk based on the appearance of the mole, he explained.
“Studies to identify genetic risk factors for melanoma have not yet progressed to the point where these can be tested in the clinic. We clearly have a knowledge gap that needs to be addressed,” he said.
Moreover, it’s not easy to predict which early melanomas will metastasize, said dermatologist Jennifer Stein, MD, PhD, who specializes in treating patients at high risk for melanoma at NYU Langone.
“This paper suggests that it may not be important to detect and treat melanoma in situ, and that the increase in diagnosis of melanoma in situ has led to more harms than good,” she said. “There is evidence that most melanomas do originate as in situ lesions. Unfortunately, we cannot predict which ones will become more aggressive. For this reason, we treat melanoma in situ.”
Taking issue with some of the arguments
Both Dr. Polsky and Dr. Stein took issue with several of the arguments put forward by Dr. Welch and colleagues.
For instance, Dr. Welch and colleagues cited research suggesting that UV light is a weak risk factor for melanoma, but Dr. Polsky disagreed. “There are many lines of evidence ranging from epidemiological, clinical, and biological studies that prove the causative association between ultraviolet light and melanoma, while acknowledging that other factors, such as genetic predisposition, play an important role,” he said. “Since ultraviolet light in the form of outdoor sunburns or indoor tanning exposure are modifiable risk factors, it is important that we continue with our current public messaging on their causal role in the development of melanoma.”
Furthermore, the 2012 study that the authors cited to support their argument that pathologists today are more likely to diagnose melanoma than in years past is flawed, according to Dr. Stein. The study was very small and included just nine contemporary pathologists. Unlike in real life, pathologists in the study could not diagnose lesions as “atypical,” and may have erred on the side of caution by calling them malignant.
“There were multiple limitations to this study that were acknowledged by its authors, who stated that it was a hypothesis-generating study and may not be generalizable,” Dr. Stein said.
In addition, Dr. Polsky took issue with the suggestion that awareness about melanoma among the general public is overly heightened.
“Reducing melanoma awareness would not be wise,” he said. “Studies have shown that awareness of melanoma is associated with the diagnosis of earlier-stage lesions that can be cured by simple skin surgery, without the need for more costly interventions utilized for more advanced melanomas.”
Dr. Mazer reported receiving travel compensation from Hillcrest Healthcare Systems, and is a commentator for this new organization. Dr. Welch has written three books on the subjects of overdiagnosis and testing for cancer. Dr. Adamson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When the COVID-19 pandemic first hit, cancer screening in the United States came to an abrupt halt. That experience, coupled with the financial fallout of the pandemic, has led some doctors to reassess business as usual.
In particular, a trio has taken aim at skin cancer screening – arguing that it should stop – in a ‘sounding board’ commentary published online Jan. 7 in the New England Journal of Medicine.
“The COVID-19 pandemic has functionally stopped skin cancer screening; what is important is not to restart it,” wrote the authors, led by H. Gilbert Welch, MD, MPH, at Brigham and Women’s Hospital, Boston, Massachusetts. Dr. Welch has often raised questions about cancer screening and highlighted the issue of overdiagnosis.
In this latest essay, Dr. Welch teamed up with pathologist Benjamin Mazer, MD, Yale University, New Haven, Conn., who writes commentaries for this news organization, and dermatologist Adewole S. Adamson, MD, University of Texas, Austin, to argue that screening for skin cancer has led to an overdiagnosis of melanoma.
However, two melanoma experts pointed out flaws in some of their arguments, and said the issue is more nuanced than they present.
Arguing that melanoma is overdiagnosed
The incidence of melanoma is six times as high as it was 40 years ago, making it the third most common cancer in the United States, the investigators pointed out. However, while case rates have skyrocketed, death rates from melanoma have remained about the same, which points to overdiagnosis.
They described a cycle of increased diagnostic scrutiny that is driving overdiagnosis of melanoma. This includes heightened awareness (perhaps overly) among patients, widespread skin screenings, lower clinical thresholds for biopsy, and lower thresholds among pathologists for diagnosis of melanoma. Fear of missing cancer, legal concerns, and financial incentives may all contribute.
“We view the rise in the incidence of melanoma as a sentinel event, a warning that an epidemic of inspection, surveillance, and biopsy of pigmented skin lesions is permeating through the general population,” they wrote.
Furthermore, overdiagnosis could contribute to unnecessary intervention.
Between 2004 and 2017, rates of biopsy among fee-for-service Medicare recipients almost doubled (from 5% to 8%), according to coding trends data cited in the article. Overdiagnosis and unnecessary intervention could cause psychological, financial, and physical harm to the patient, and the authors argued for interrupting the cycle.
“The most important step to break the cycle of melanoma overdiagnosis is to stop population-wide screening for skin cancer,” they wrote.
The U.S. Preventive Services Task Force currently states that there is insufficient evidence to weigh the balances versus the harms of skin cancer screening, leaving it open to interpretation.
“[T]he increase in melanoma diagnoses by a factor of 6, with at least an order of magnitude more persons undergoing a biopsy and no apparent effect on mortality, is more than enough to recommend against population-wide screening,” Dr. Welch and colleagues concluded.
But the issue may be more nuanced, argued a melanoma expert.
“Everyone agrees that screening high-risk groups has the greatest chance of reducing cancer mortality. In melanoma, the strongest risk factor is the number of moles and presence of clinically atypical moles,” David Polsky, MD, PhD, commented in an interview. Dr. Polsky is a professor of dermatologic oncology at the Perlmutter Cancer Center at New York University Langone Health.
However, population-based studies have shown that at least half of melanoma patients are not considered high risk based on the appearance of the mole, he explained.
“Studies to identify genetic risk factors for melanoma have not yet progressed to the point where these can be tested in the clinic. We clearly have a knowledge gap that needs to be addressed,” he said.
Moreover, it’s not easy to predict which early melanomas will metastasize, said dermatologist Jennifer Stein, MD, PhD, who specializes in treating patients at high risk for melanoma at NYU Langone.
“This paper suggests that it may not be important to detect and treat melanoma in situ, and that the increase in diagnosis of melanoma in situ has led to more harms than good,” she said. “There is evidence that most melanomas do originate as in situ lesions. Unfortunately, we cannot predict which ones will become more aggressive. For this reason, we treat melanoma in situ.”
Taking issue with some of the arguments
Both Dr. Polsky and Dr. Stein took issue with several of the arguments put forward by Dr. Welch and colleagues.
For instance, Dr. Welch and colleagues cited research suggesting that UV light is a weak risk factor for melanoma, but Dr. Polsky disagreed. “There are many lines of evidence ranging from epidemiological, clinical, and biological studies that prove the causative association between ultraviolet light and melanoma, while acknowledging that other factors, such as genetic predisposition, play an important role,” he said. “Since ultraviolet light in the form of outdoor sunburns or indoor tanning exposure are modifiable risk factors, it is important that we continue with our current public messaging on their causal role in the development of melanoma.”
Furthermore, the 2012 study that the authors cited to support their argument that pathologists today are more likely to diagnose melanoma than in years past is flawed, according to Dr. Stein. The study was very small and included just nine contemporary pathologists. Unlike in real life, pathologists in the study could not diagnose lesions as “atypical,” and may have erred on the side of caution by calling them malignant.
“There were multiple limitations to this study that were acknowledged by its authors, who stated that it was a hypothesis-generating study and may not be generalizable,” Dr. Stein said.
In addition, Dr. Polsky took issue with the suggestion that awareness about melanoma among the general public is overly heightened.
“Reducing melanoma awareness would not be wise,” he said. “Studies have shown that awareness of melanoma is associated with the diagnosis of earlier-stage lesions that can be cured by simple skin surgery, without the need for more costly interventions utilized for more advanced melanomas.”
Dr. Mazer reported receiving travel compensation from Hillcrest Healthcare Systems, and is a commentator for this new organization. Dr. Welch has written three books on the subjects of overdiagnosis and testing for cancer. Dr. Adamson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When the COVID-19 pandemic first hit, cancer screening in the United States came to an abrupt halt. That experience, coupled with the financial fallout of the pandemic, has led some doctors to reassess business as usual.
In particular, a trio has taken aim at skin cancer screening – arguing that it should stop – in a ‘sounding board’ commentary published online Jan. 7 in the New England Journal of Medicine.
“The COVID-19 pandemic has functionally stopped skin cancer screening; what is important is not to restart it,” wrote the authors, led by H. Gilbert Welch, MD, MPH, at Brigham and Women’s Hospital, Boston, Massachusetts. Dr. Welch has often raised questions about cancer screening and highlighted the issue of overdiagnosis.
In this latest essay, Dr. Welch teamed up with pathologist Benjamin Mazer, MD, Yale University, New Haven, Conn., who writes commentaries for this news organization, and dermatologist Adewole S. Adamson, MD, University of Texas, Austin, to argue that screening for skin cancer has led to an overdiagnosis of melanoma.
However, two melanoma experts pointed out flaws in some of their arguments, and said the issue is more nuanced than they present.
Arguing that melanoma is overdiagnosed
The incidence of melanoma is six times as high as it was 40 years ago, making it the third most common cancer in the United States, the investigators pointed out. However, while case rates have skyrocketed, death rates from melanoma have remained about the same, which points to overdiagnosis.
They described a cycle of increased diagnostic scrutiny that is driving overdiagnosis of melanoma. This includes heightened awareness (perhaps overly) among patients, widespread skin screenings, lower clinical thresholds for biopsy, and lower thresholds among pathologists for diagnosis of melanoma. Fear of missing cancer, legal concerns, and financial incentives may all contribute.
“We view the rise in the incidence of melanoma as a sentinel event, a warning that an epidemic of inspection, surveillance, and biopsy of pigmented skin lesions is permeating through the general population,” they wrote.
Furthermore, overdiagnosis could contribute to unnecessary intervention.
Between 2004 and 2017, rates of biopsy among fee-for-service Medicare recipients almost doubled (from 5% to 8%), according to coding trends data cited in the article. Overdiagnosis and unnecessary intervention could cause psychological, financial, and physical harm to the patient, and the authors argued for interrupting the cycle.
“The most important step to break the cycle of melanoma overdiagnosis is to stop population-wide screening for skin cancer,” they wrote.
The U.S. Preventive Services Task Force currently states that there is insufficient evidence to weigh the balances versus the harms of skin cancer screening, leaving it open to interpretation.
“[T]he increase in melanoma diagnoses by a factor of 6, with at least an order of magnitude more persons undergoing a biopsy and no apparent effect on mortality, is more than enough to recommend against population-wide screening,” Dr. Welch and colleagues concluded.
But the issue may be more nuanced, argued a melanoma expert.
“Everyone agrees that screening high-risk groups has the greatest chance of reducing cancer mortality. In melanoma, the strongest risk factor is the number of moles and presence of clinically atypical moles,” David Polsky, MD, PhD, commented in an interview. Dr. Polsky is a professor of dermatologic oncology at the Perlmutter Cancer Center at New York University Langone Health.
However, population-based studies have shown that at least half of melanoma patients are not considered high risk based on the appearance of the mole, he explained.
“Studies to identify genetic risk factors for melanoma have not yet progressed to the point where these can be tested in the clinic. We clearly have a knowledge gap that needs to be addressed,” he said.
Moreover, it’s not easy to predict which early melanomas will metastasize, said dermatologist Jennifer Stein, MD, PhD, who specializes in treating patients at high risk for melanoma at NYU Langone.
“This paper suggests that it may not be important to detect and treat melanoma in situ, and that the increase in diagnosis of melanoma in situ has led to more harms than good,” she said. “There is evidence that most melanomas do originate as in situ lesions. Unfortunately, we cannot predict which ones will become more aggressive. For this reason, we treat melanoma in situ.”
Taking issue with some of the arguments
Both Dr. Polsky and Dr. Stein took issue with several of the arguments put forward by Dr. Welch and colleagues.
For instance, Dr. Welch and colleagues cited research suggesting that UV light is a weak risk factor for melanoma, but Dr. Polsky disagreed. “There are many lines of evidence ranging from epidemiological, clinical, and biological studies that prove the causative association between ultraviolet light and melanoma, while acknowledging that other factors, such as genetic predisposition, play an important role,” he said. “Since ultraviolet light in the form of outdoor sunburns or indoor tanning exposure are modifiable risk factors, it is important that we continue with our current public messaging on their causal role in the development of melanoma.”
Furthermore, the 2012 study that the authors cited to support their argument that pathologists today are more likely to diagnose melanoma than in years past is flawed, according to Dr. Stein. The study was very small and included just nine contemporary pathologists. Unlike in real life, pathologists in the study could not diagnose lesions as “atypical,” and may have erred on the side of caution by calling them malignant.
“There were multiple limitations to this study that were acknowledged by its authors, who stated that it was a hypothesis-generating study and may not be generalizable,” Dr. Stein said.
In addition, Dr. Polsky took issue with the suggestion that awareness about melanoma among the general public is overly heightened.
“Reducing melanoma awareness would not be wise,” he said. “Studies have shown that awareness of melanoma is associated with the diagnosis of earlier-stage lesions that can be cured by simple skin surgery, without the need for more costly interventions utilized for more advanced melanomas.”
Dr. Mazer reported receiving travel compensation from Hillcrest Healthcare Systems, and is a commentator for this new organization. Dr. Welch has written three books on the subjects of overdiagnosis and testing for cancer. Dr. Adamson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.