User login
Tebentafusp improves OS: A first in metastatic uveal melanoma
Tebentafusp is the first investigational therapy in a phase 3 trial to improve OS in metastatic uveal melanoma, said Jessica Hassel, MD, of University Hospital Heidelberg in Germany, when presenting the results at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT002).
Dr. Hassel explained that tebentafusp is a bispecific fusion protein designed to target gp100 through a high affinity T-cell receptor binding domain and an anti-CD3 T-cell engaging domain, which redirects T cells to kill gp100-expressing tumor cells. Because the T-cell receptor binding domain only recognizes a specific gp100-derived peptide presented on HLA-A*02:01, tebentafusp can only be used to treat patients with this HLA type.
In the phase 3 trial, investigators enrolled 378 treatment-naive HLA-A*02:01-positive patients with metastatic uveal melanoma. Their median age was 65 years, and 50% were men.
Patients were assigned 2:1 to receive tebentafusp (n = 252) or investigator’s choice of pembrolizumab (n = 103), ipilimumab (n = 16), or dacarbazine (n = 7).
Prolonged OS despite low response rate
At a median follow-up of 14.1 months, patients receiving tebentafusp had significantly longer OS than that of patients in the investigator’s choice arm – 21.7 months and 16.0 months, respectively. The estimated 1-year OS rate was 73.2% in the tebentafusp arm and 58.5% in the standard therapy arm (hazard ratio, 0.51; 95% confidence interval, 0.37-0.71; P < .0001). The OS benefit was consistent across subgroups, Dr. Hassel said.
At a median follow-up of 11.4 months, the median progression-free survival was 3.3 months in the tebentafusp arm and 2.9 months in the investigator’s choice arm (HR, 0.73; 95% CI, 0.58-0.94; P = .0139).
The objective response rate was 9% in the tebentafusp arm and 5% in the investigator’s choice arm. There was only one complete response, and it was in the tebentafusp arm.
The disease control rate, defined as response or stable disease for 12 or more weeks, was 46% in the tebentafusp arm and 27% in the investigator’s choice arm. Rates of progressive disease were 52% and 62%, respectively.
Dr. Hassel pointed out that a landmark analysis of OS in patients with a best response of progressive disease, with patients continuing to receive treatment after progression, showed a hazard ratio of 0.4 (95% CI, 0.248-0.642) for those receiving tebentafusp vs. investigator’s choice. The OS benefit, despite low response rates, suggests that patients progress but are then stabilized with tebentafusp treatment.
“So this drug is slowing down developing disease,” she said.
‘Manageable’ adverse events
Target-mediated or cytokine-mediated adverse events were the most common side effects with tebentafusp. These included pyrexia (76%), pruritus (69%), and rash (83%), which decreased in frequency and severity after the first three to four doses.
While cytokine release syndrome was common (89%), the rate of grade 3-4 cytokine release syndrome was very low (1%). Adverse events were generally manageable with standard interventions, Dr. Hassel said.
The discontinuation rate was lower in the tebentafusp arm than in the investigator’s choice arm – 2% and 4.5%, respectively. There were no tebentafusp-related deaths.
‘Practice-changing’ results
“This is the first randomized controlled trial to be positive for overall survival in uveal melanoma. These are seminal and practice-changing results,” said AACR discussant Caroline Robert, MD, PhD, of Gustave Roussy and Paris-Saclay University in France.
She observed that the biology of uveal melanoma is distinct from that of cutaneous melanoma, and future research will have to address why tebentafusp doesn’t work as well in cutaneous melanoma. Tebentafusp will be evaluated in combination with immune checkpoint inhibitors as well, she added.
The major limitation of tebentafusp, Dr. Hassel observed, is that it can be used only in HLA-A*02:01-positive patients. “There still remains an unmet need for patients who do not have this particular surface protein,” she said.
The study was sponsored by Immunocore. Dr. Hassel disclosed relationships with Immunocore and other companies. Dr. Robert disclosed relationships with Bristol Myers Squibb, Pierre Fabre, Novartis, and other companies.
Tebentafusp is the first investigational therapy in a phase 3 trial to improve OS in metastatic uveal melanoma, said Jessica Hassel, MD, of University Hospital Heidelberg in Germany, when presenting the results at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT002).
Dr. Hassel explained that tebentafusp is a bispecific fusion protein designed to target gp100 through a high affinity T-cell receptor binding domain and an anti-CD3 T-cell engaging domain, which redirects T cells to kill gp100-expressing tumor cells. Because the T-cell receptor binding domain only recognizes a specific gp100-derived peptide presented on HLA-A*02:01, tebentafusp can only be used to treat patients with this HLA type.
In the phase 3 trial, investigators enrolled 378 treatment-naive HLA-A*02:01-positive patients with metastatic uveal melanoma. Their median age was 65 years, and 50% were men.
Patients were assigned 2:1 to receive tebentafusp (n = 252) or investigator’s choice of pembrolizumab (n = 103), ipilimumab (n = 16), or dacarbazine (n = 7).
Prolonged OS despite low response rate
At a median follow-up of 14.1 months, patients receiving tebentafusp had significantly longer OS than that of patients in the investigator’s choice arm – 21.7 months and 16.0 months, respectively. The estimated 1-year OS rate was 73.2% in the tebentafusp arm and 58.5% in the standard therapy arm (hazard ratio, 0.51; 95% confidence interval, 0.37-0.71; P < .0001). The OS benefit was consistent across subgroups, Dr. Hassel said.
At a median follow-up of 11.4 months, the median progression-free survival was 3.3 months in the tebentafusp arm and 2.9 months in the investigator’s choice arm (HR, 0.73; 95% CI, 0.58-0.94; P = .0139).
The objective response rate was 9% in the tebentafusp arm and 5% in the investigator’s choice arm. There was only one complete response, and it was in the tebentafusp arm.
The disease control rate, defined as response or stable disease for 12 or more weeks, was 46% in the tebentafusp arm and 27% in the investigator’s choice arm. Rates of progressive disease were 52% and 62%, respectively.
Dr. Hassel pointed out that a landmark analysis of OS in patients with a best response of progressive disease, with patients continuing to receive treatment after progression, showed a hazard ratio of 0.4 (95% CI, 0.248-0.642) for those receiving tebentafusp vs. investigator’s choice. The OS benefit, despite low response rates, suggests that patients progress but are then stabilized with tebentafusp treatment.
“So this drug is slowing down developing disease,” she said.
‘Manageable’ adverse events
Target-mediated or cytokine-mediated adverse events were the most common side effects with tebentafusp. These included pyrexia (76%), pruritus (69%), and rash (83%), which decreased in frequency and severity after the first three to four doses.
While cytokine release syndrome was common (89%), the rate of grade 3-4 cytokine release syndrome was very low (1%). Adverse events were generally manageable with standard interventions, Dr. Hassel said.
The discontinuation rate was lower in the tebentafusp arm than in the investigator’s choice arm – 2% and 4.5%, respectively. There were no tebentafusp-related deaths.
‘Practice-changing’ results
“This is the first randomized controlled trial to be positive for overall survival in uveal melanoma. These are seminal and practice-changing results,” said AACR discussant Caroline Robert, MD, PhD, of Gustave Roussy and Paris-Saclay University in France.
She observed that the biology of uveal melanoma is distinct from that of cutaneous melanoma, and future research will have to address why tebentafusp doesn’t work as well in cutaneous melanoma. Tebentafusp will be evaluated in combination with immune checkpoint inhibitors as well, she added.
The major limitation of tebentafusp, Dr. Hassel observed, is that it can be used only in HLA-A*02:01-positive patients. “There still remains an unmet need for patients who do not have this particular surface protein,” she said.
The study was sponsored by Immunocore. Dr. Hassel disclosed relationships with Immunocore and other companies. Dr. Robert disclosed relationships with Bristol Myers Squibb, Pierre Fabre, Novartis, and other companies.
Tebentafusp is the first investigational therapy in a phase 3 trial to improve OS in metastatic uveal melanoma, said Jessica Hassel, MD, of University Hospital Heidelberg in Germany, when presenting the results at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT002).
Dr. Hassel explained that tebentafusp is a bispecific fusion protein designed to target gp100 through a high affinity T-cell receptor binding domain and an anti-CD3 T-cell engaging domain, which redirects T cells to kill gp100-expressing tumor cells. Because the T-cell receptor binding domain only recognizes a specific gp100-derived peptide presented on HLA-A*02:01, tebentafusp can only be used to treat patients with this HLA type.
In the phase 3 trial, investigators enrolled 378 treatment-naive HLA-A*02:01-positive patients with metastatic uveal melanoma. Their median age was 65 years, and 50% were men.
Patients were assigned 2:1 to receive tebentafusp (n = 252) or investigator’s choice of pembrolizumab (n = 103), ipilimumab (n = 16), or dacarbazine (n = 7).
Prolonged OS despite low response rate
At a median follow-up of 14.1 months, patients receiving tebentafusp had significantly longer OS than that of patients in the investigator’s choice arm – 21.7 months and 16.0 months, respectively. The estimated 1-year OS rate was 73.2% in the tebentafusp arm and 58.5% in the standard therapy arm (hazard ratio, 0.51; 95% confidence interval, 0.37-0.71; P < .0001). The OS benefit was consistent across subgroups, Dr. Hassel said.
At a median follow-up of 11.4 months, the median progression-free survival was 3.3 months in the tebentafusp arm and 2.9 months in the investigator’s choice arm (HR, 0.73; 95% CI, 0.58-0.94; P = .0139).
The objective response rate was 9% in the tebentafusp arm and 5% in the investigator’s choice arm. There was only one complete response, and it was in the tebentafusp arm.
The disease control rate, defined as response or stable disease for 12 or more weeks, was 46% in the tebentafusp arm and 27% in the investigator’s choice arm. Rates of progressive disease were 52% and 62%, respectively.
Dr. Hassel pointed out that a landmark analysis of OS in patients with a best response of progressive disease, with patients continuing to receive treatment after progression, showed a hazard ratio of 0.4 (95% CI, 0.248-0.642) for those receiving tebentafusp vs. investigator’s choice. The OS benefit, despite low response rates, suggests that patients progress but are then stabilized with tebentafusp treatment.
“So this drug is slowing down developing disease,” she said.
‘Manageable’ adverse events
Target-mediated or cytokine-mediated adverse events were the most common side effects with tebentafusp. These included pyrexia (76%), pruritus (69%), and rash (83%), which decreased in frequency and severity after the first three to four doses.
While cytokine release syndrome was common (89%), the rate of grade 3-4 cytokine release syndrome was very low (1%). Adverse events were generally manageable with standard interventions, Dr. Hassel said.
The discontinuation rate was lower in the tebentafusp arm than in the investigator’s choice arm – 2% and 4.5%, respectively. There were no tebentafusp-related deaths.
‘Practice-changing’ results
“This is the first randomized controlled trial to be positive for overall survival in uveal melanoma. These are seminal and practice-changing results,” said AACR discussant Caroline Robert, MD, PhD, of Gustave Roussy and Paris-Saclay University in France.
She observed that the biology of uveal melanoma is distinct from that of cutaneous melanoma, and future research will have to address why tebentafusp doesn’t work as well in cutaneous melanoma. Tebentafusp will be evaluated in combination with immune checkpoint inhibitors as well, she added.
The major limitation of tebentafusp, Dr. Hassel observed, is that it can be used only in HLA-A*02:01-positive patients. “There still remains an unmet need for patients who do not have this particular surface protein,” she said.
The study was sponsored by Immunocore. Dr. Hassel disclosed relationships with Immunocore and other companies. Dr. Robert disclosed relationships with Bristol Myers Squibb, Pierre Fabre, Novartis, and other companies.
FROM AACR 2021
Adverse reactions to immunotherapy can appear after a year
Clinicians should be on the lookout for immune-related adverse events (irAEs) even after patients have been receiving anti-PD-1 immunotherapy for a year or longer, according to team of international investigators.
They reported that, among melanoma patients, the incidence of new-onset reactions that occurred 1 year or longer after anti-PD-1 treatment was 5.3%.
In a review of 118 patients, the investigators found that irAEs are often “high grade, difficult to manage, and can lead to death.”
Reactions are more likely to occur in those for whom treatment with an anti-PD-1 checkpoint inhibitor – primarily pembrolizumab and nivolumab – continued for longer than a year, and patients can present “long after stopping” the treatment, the investigators noted.
The findings were published online in Annals of Oncology.
“We do not yet understand why some patients have no side effects for months or years, then develop toxicities so late in their course,” said one of the coauthors, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn.
“Physicians should continue to monitor patients for side effects, even if they have been on anti-PD-1 therapy for some time, since delayed side effects may cause morbidity and even death,” Dr. Johnson said.
Patients and clinicians need “to be aware of these risks when making decisions regarding therapy continuation” and need “to consider irAE as a possible diagnosis in any presentation where there is a history of checkpoint inhibitor treatment, regardless of the time frame, to enable early recognition and appropriate treatment,” Dr. Johnson and colleagues concluded.
Largest series to document delayed reactions
Immunotherapies have revolutionized cancer treatment of many types of tumors, but they carry a well known risk for autoimmune toxicity, which typically occurs within the first 4-6 months, the authors wrote.
Delayed reactions have been reported but are not as well described. The new study is the largest to date on this question, and Dr. Johnson said the findings likely apply across indications, not simply in regard to melanoma patients.
An expert not involved in the study agrees.
“We are definitely seeing delayed reactions to immunotherapy in our practice” in several organ systems, including the skin, said Jennifer Choi, MD, chief of oncodermatology at Northwestern University’s Comprehensive Cancer Center, Chicago.
“Some of these side effects can take months to resolve and may require systemic treatment, such as steroids, nonsteroidal immunosuppressants, or biologics. Clinicians must be on high alert of any possible side effect for a patient on immunotherapy throughout their entire course, and even after they have completed treatment,” Dr. Choi said in an interview.
Anti-PD-1 therapy doesn’t “follow the typical drug hypersensitivity laws and rules with respect to timing,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
Median onset was 16 months
The investigators reported in detail on 118 patients. A total of 140 delayed irAEs that occurred 1 year or longer after treatment were identified in 20 centers around the world.
The median onset of delayed irAE was 16 months after start of treatment. Most occurred in conjunction with stand-alone anti-PD-1 therapy, but in the case of 20 patients, a combination of an anti-PD-1 drug and the anti-CTLA-4 drug ipilimumab was used.
In 39% of patients (n = 55), the adverse reaction was of grade 3 or worse. These included two deaths: one case of fatal encephalitis with concurrent anti-PD-1 use, and a death from immune-related multiple organ failure 11 months after anti-PD-1 discontinuation.
Most of the patients (n = 87; 74%) were receiving anti-PD-1 therapy at the time of onset of the adverse reaction; 15 patients (12%) were within 3 months of their last dose, and 16 (14%) were 3 months past their last dose.
Among the subgroup who developed an irAE after discontinuation of treatment was a patient with grade 4 colitis that required colectomy 26 months afterward, although Dr. Johnson noted it’s difficult to be sure that the colitis was related to the immunotherapy, because it occurred so long after treatment had ended.
An early warning system
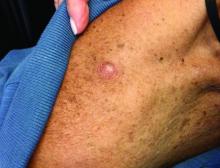
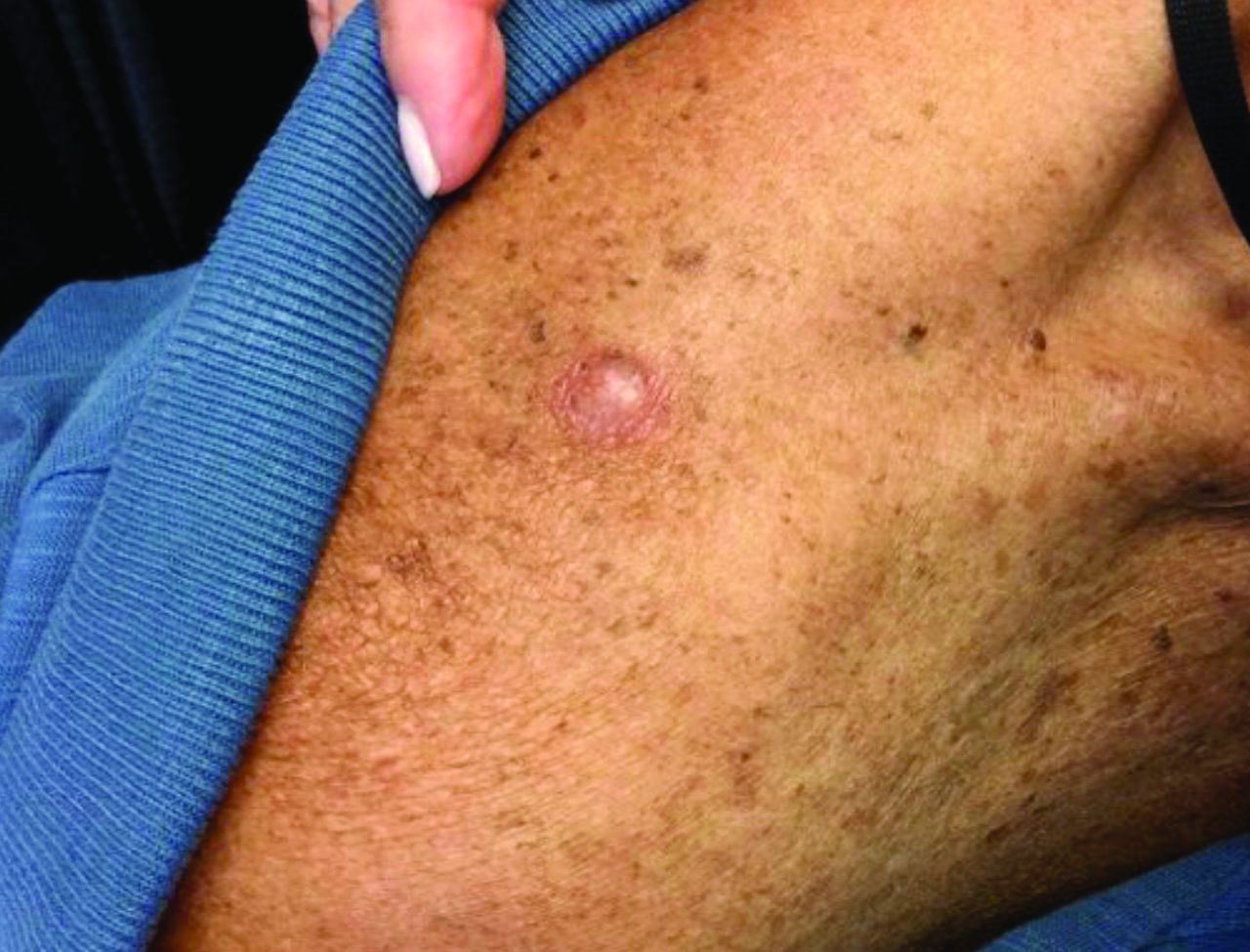
The most common reactions were colitis, pneumonitis, and rash.
The reactions were often tough to manage, the authors reported. Eighty patients (68%) required steroids, and 27 (23%) required steroids plus additional immunosuppressives, such as tumor necrosis factor blockers, particularly for colitis and renal, rheumatologic, and neurologic complications. Rheumatologic events required a median corticosteroid course of 15 months plus additional immunosuppression in half of cases and often left patients with ongoing morbidity.
“Often, the skin is one of the first and most easily visible immune-related adverse event that develops,” said Bernice Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, who was not involved in the study and was approached for comment.
Presentations can range from small itchy plaques to total body dermatitis. It is something to be aware of, because the skin can act as an early warning system to catch internal organ damage earlier, she said.
On a positive note, the investigators found no indication that the effect of immunotherapy was diminished by delayed reactions and their treatment.
Managing events “gets a little complicated” when anti-PD-1 drugs are still being administered, but “we have successfully utilized systemic steroid pulses for several weeks without impeding the efficacy of the therapy. For the lichenoid and psoriasiform dermatitis, topical steroids and oral retinoids have been useful and can be used concurrently with immunotherapy,” Dr. Friedman said.
Question on treatment duration
No obvious factors were predictive of delayed events, including previous autoimmune disease or earlier reactions, which usually affected different organs, the authors said.
The findings raise a question about the appropriate duration of anti-PD-1 therapy, at least for melanoma.
The standard duration of adjuvant therapy was empirically determined to be 1 year for melanoma, and trials support anti-PD-1 therapy for up to 2 years for metastatic disease.
However, the authors suggest that “shorter treatment duration may reduce the risk of delayed irAE” and may be sufficient for patients who have a complete response.
“This should be considered when making decisions regarding therapy continuation in responding patients,” they wrote.
Ongoing clinical trials are investigating the optimal duration of therapy, they wrote.
No outside funding was reported. Dr. Johnson has been an adviser for Array Biopharma, BMS, Iovance, Jansen, Merck, and Novartis and has received research funding from BMS and Incyte. Other investigators reported similar ties. Dr. Choi, Dr. Kwong, and Dr. Friedman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should be on the lookout for immune-related adverse events (irAEs) even after patients have been receiving anti-PD-1 immunotherapy for a year or longer, according to team of international investigators.
They reported that, among melanoma patients, the incidence of new-onset reactions that occurred 1 year or longer after anti-PD-1 treatment was 5.3%.
In a review of 118 patients, the investigators found that irAEs are often “high grade, difficult to manage, and can lead to death.”
Reactions are more likely to occur in those for whom treatment with an anti-PD-1 checkpoint inhibitor – primarily pembrolizumab and nivolumab – continued for longer than a year, and patients can present “long after stopping” the treatment, the investigators noted.
The findings were published online in Annals of Oncology.
“We do not yet understand why some patients have no side effects for months or years, then develop toxicities so late in their course,” said one of the coauthors, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn.
“Physicians should continue to monitor patients for side effects, even if they have been on anti-PD-1 therapy for some time, since delayed side effects may cause morbidity and even death,” Dr. Johnson said.
Patients and clinicians need “to be aware of these risks when making decisions regarding therapy continuation” and need “to consider irAE as a possible diagnosis in any presentation where there is a history of checkpoint inhibitor treatment, regardless of the time frame, to enable early recognition and appropriate treatment,” Dr. Johnson and colleagues concluded.
Largest series to document delayed reactions
Immunotherapies have revolutionized cancer treatment of many types of tumors, but they carry a well known risk for autoimmune toxicity, which typically occurs within the first 4-6 months, the authors wrote.
Delayed reactions have been reported but are not as well described. The new study is the largest to date on this question, and Dr. Johnson said the findings likely apply across indications, not simply in regard to melanoma patients.
An expert not involved in the study agrees.
“We are definitely seeing delayed reactions to immunotherapy in our practice” in several organ systems, including the skin, said Jennifer Choi, MD, chief of oncodermatology at Northwestern University’s Comprehensive Cancer Center, Chicago.
“Some of these side effects can take months to resolve and may require systemic treatment, such as steroids, nonsteroidal immunosuppressants, or biologics. Clinicians must be on high alert of any possible side effect for a patient on immunotherapy throughout their entire course, and even after they have completed treatment,” Dr. Choi said in an interview.
Anti-PD-1 therapy doesn’t “follow the typical drug hypersensitivity laws and rules with respect to timing,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
Median onset was 16 months
The investigators reported in detail on 118 patients. A total of 140 delayed irAEs that occurred 1 year or longer after treatment were identified in 20 centers around the world.
The median onset of delayed irAE was 16 months after start of treatment. Most occurred in conjunction with stand-alone anti-PD-1 therapy, but in the case of 20 patients, a combination of an anti-PD-1 drug and the anti-CTLA-4 drug ipilimumab was used.
In 39% of patients (n = 55), the adverse reaction was of grade 3 or worse. These included two deaths: one case of fatal encephalitis with concurrent anti-PD-1 use, and a death from immune-related multiple organ failure 11 months after anti-PD-1 discontinuation.
Most of the patients (n = 87; 74%) were receiving anti-PD-1 therapy at the time of onset of the adverse reaction; 15 patients (12%) were within 3 months of their last dose, and 16 (14%) were 3 months past their last dose.
Among the subgroup who developed an irAE after discontinuation of treatment was a patient with grade 4 colitis that required colectomy 26 months afterward, although Dr. Johnson noted it’s difficult to be sure that the colitis was related to the immunotherapy, because it occurred so long after treatment had ended.
An early warning system
The most common reactions were colitis, pneumonitis, and rash.
The reactions were often tough to manage, the authors reported. Eighty patients (68%) required steroids, and 27 (23%) required steroids plus additional immunosuppressives, such as tumor necrosis factor blockers, particularly for colitis and renal, rheumatologic, and neurologic complications. Rheumatologic events required a median corticosteroid course of 15 months plus additional immunosuppression in half of cases and often left patients with ongoing morbidity.
“Often, the skin is one of the first and most easily visible immune-related adverse event that develops,” said Bernice Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, who was not involved in the study and was approached for comment.
Presentations can range from small itchy plaques to total body dermatitis. It is something to be aware of, because the skin can act as an early warning system to catch internal organ damage earlier, she said.
On a positive note, the investigators found no indication that the effect of immunotherapy was diminished by delayed reactions and their treatment.
Managing events “gets a little complicated” when anti-PD-1 drugs are still being administered, but “we have successfully utilized systemic steroid pulses for several weeks without impeding the efficacy of the therapy. For the lichenoid and psoriasiform dermatitis, topical steroids and oral retinoids have been useful and can be used concurrently with immunotherapy,” Dr. Friedman said.
Question on treatment duration
No obvious factors were predictive of delayed events, including previous autoimmune disease or earlier reactions, which usually affected different organs, the authors said.
The findings raise a question about the appropriate duration of anti-PD-1 therapy, at least for melanoma.
The standard duration of adjuvant therapy was empirically determined to be 1 year for melanoma, and trials support anti-PD-1 therapy for up to 2 years for metastatic disease.
However, the authors suggest that “shorter treatment duration may reduce the risk of delayed irAE” and may be sufficient for patients who have a complete response.
“This should be considered when making decisions regarding therapy continuation in responding patients,” they wrote.
Ongoing clinical trials are investigating the optimal duration of therapy, they wrote.
No outside funding was reported. Dr. Johnson has been an adviser for Array Biopharma, BMS, Iovance, Jansen, Merck, and Novartis and has received research funding from BMS and Incyte. Other investigators reported similar ties. Dr. Choi, Dr. Kwong, and Dr. Friedman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should be on the lookout for immune-related adverse events (irAEs) even after patients have been receiving anti-PD-1 immunotherapy for a year or longer, according to team of international investigators.
They reported that, among melanoma patients, the incidence of new-onset reactions that occurred 1 year or longer after anti-PD-1 treatment was 5.3%.
In a review of 118 patients, the investigators found that irAEs are often “high grade, difficult to manage, and can lead to death.”
Reactions are more likely to occur in those for whom treatment with an anti-PD-1 checkpoint inhibitor – primarily pembrolizumab and nivolumab – continued for longer than a year, and patients can present “long after stopping” the treatment, the investigators noted.
The findings were published online in Annals of Oncology.
“We do not yet understand why some patients have no side effects for months or years, then develop toxicities so late in their course,” said one of the coauthors, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn.
“Physicians should continue to monitor patients for side effects, even if they have been on anti-PD-1 therapy for some time, since delayed side effects may cause morbidity and even death,” Dr. Johnson said.
Patients and clinicians need “to be aware of these risks when making decisions regarding therapy continuation” and need “to consider irAE as a possible diagnosis in any presentation where there is a history of checkpoint inhibitor treatment, regardless of the time frame, to enable early recognition and appropriate treatment,” Dr. Johnson and colleagues concluded.
Largest series to document delayed reactions
Immunotherapies have revolutionized cancer treatment of many types of tumors, but they carry a well known risk for autoimmune toxicity, which typically occurs within the first 4-6 months, the authors wrote.
Delayed reactions have been reported but are not as well described. The new study is the largest to date on this question, and Dr. Johnson said the findings likely apply across indications, not simply in regard to melanoma patients.
An expert not involved in the study agrees.
“We are definitely seeing delayed reactions to immunotherapy in our practice” in several organ systems, including the skin, said Jennifer Choi, MD, chief of oncodermatology at Northwestern University’s Comprehensive Cancer Center, Chicago.
“Some of these side effects can take months to resolve and may require systemic treatment, such as steroids, nonsteroidal immunosuppressants, or biologics. Clinicians must be on high alert of any possible side effect for a patient on immunotherapy throughout their entire course, and even after they have completed treatment,” Dr. Choi said in an interview.
Anti-PD-1 therapy doesn’t “follow the typical drug hypersensitivity laws and rules with respect to timing,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
Median onset was 16 months
The investigators reported in detail on 118 patients. A total of 140 delayed irAEs that occurred 1 year or longer after treatment were identified in 20 centers around the world.
The median onset of delayed irAE was 16 months after start of treatment. Most occurred in conjunction with stand-alone anti-PD-1 therapy, but in the case of 20 patients, a combination of an anti-PD-1 drug and the anti-CTLA-4 drug ipilimumab was used.
In 39% of patients (n = 55), the adverse reaction was of grade 3 or worse. These included two deaths: one case of fatal encephalitis with concurrent anti-PD-1 use, and a death from immune-related multiple organ failure 11 months after anti-PD-1 discontinuation.
Most of the patients (n = 87; 74%) were receiving anti-PD-1 therapy at the time of onset of the adverse reaction; 15 patients (12%) were within 3 months of their last dose, and 16 (14%) were 3 months past their last dose.
Among the subgroup who developed an irAE after discontinuation of treatment was a patient with grade 4 colitis that required colectomy 26 months afterward, although Dr. Johnson noted it’s difficult to be sure that the colitis was related to the immunotherapy, because it occurred so long after treatment had ended.
An early warning system
The most common reactions were colitis, pneumonitis, and rash.
The reactions were often tough to manage, the authors reported. Eighty patients (68%) required steroids, and 27 (23%) required steroids plus additional immunosuppressives, such as tumor necrosis factor blockers, particularly for colitis and renal, rheumatologic, and neurologic complications. Rheumatologic events required a median corticosteroid course of 15 months plus additional immunosuppression in half of cases and often left patients with ongoing morbidity.
“Often, the skin is one of the first and most easily visible immune-related adverse event that develops,” said Bernice Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, who was not involved in the study and was approached for comment.
Presentations can range from small itchy plaques to total body dermatitis. It is something to be aware of, because the skin can act as an early warning system to catch internal organ damage earlier, she said.
On a positive note, the investigators found no indication that the effect of immunotherapy was diminished by delayed reactions and their treatment.
Managing events “gets a little complicated” when anti-PD-1 drugs are still being administered, but “we have successfully utilized systemic steroid pulses for several weeks without impeding the efficacy of the therapy. For the lichenoid and psoriasiform dermatitis, topical steroids and oral retinoids have been useful and can be used concurrently with immunotherapy,” Dr. Friedman said.
Question on treatment duration
No obvious factors were predictive of delayed events, including previous autoimmune disease or earlier reactions, which usually affected different organs, the authors said.
The findings raise a question about the appropriate duration of anti-PD-1 therapy, at least for melanoma.
The standard duration of adjuvant therapy was empirically determined to be 1 year for melanoma, and trials support anti-PD-1 therapy for up to 2 years for metastatic disease.
However, the authors suggest that “shorter treatment duration may reduce the risk of delayed irAE” and may be sufficient for patients who have a complete response.
“This should be considered when making decisions regarding therapy continuation in responding patients,” they wrote.
Ongoing clinical trials are investigating the optimal duration of therapy, they wrote.
No outside funding was reported. Dr. Johnson has been an adviser for Array Biopharma, BMS, Iovance, Jansen, Merck, and Novartis and has received research funding from BMS and Incyte. Other investigators reported similar ties. Dr. Choi, Dr. Kwong, and Dr. Friedman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rankings of most common cancers to shift over next 20 years
The next 20 years will see a big shift in cancer type rankings, researchers predict.
At the moment, the most common cancers in the United States are breast, lung, prostate, colorectal, and melanoma.
the study authors predicted. Breast cancer will remain the top cancer to be diagnosed, lung cancer will drop from second to third, and colorectal cancer will remain at fourth.
These predicted rankings of cancer types by their total number of annual cases were published online April 7, 2021, in JAMA Network Open.
The authors also rank cancer type by mortality. Currently, most cancer deaths are caused by lung cancer, followed by colorectal, pancreatic, and breast. By 2040, the most notable change in cancer deaths is that liver and intrahepatic bile duct cancer, currently at sixth, will jump up to third.
Two decades from now, the ranking in terms of cancer deaths will be lung, pancreatic, liver and intrahepatic bile duct, and colorectal.
“Our findings reflect the shifting dynamics of cancer screening and treatment,” lead author Lola Rahib, PhD, a pancreatic cancer scientist at Cancer Commons, the advocacy nonprofit, commented in a press statement.
The new analysis used population-growth projections (based on 2010 U.S. Census data) and current population-based cancer incidence and death rates (from Surveillance, Epidemiology, and End Results 2014-2016) to calculate the changes in incidences and deaths to the year 2040.
The projected, estimated numbers are not ironclad, the researchers acknowledged.
“Our projections assume that the observed rates and trends [from recent years] don’t change over time,” Dr. Rahib said in an interview, but she pointed out that change may indeed happen.
“Any long-term projections should be considered with a grain of salt,” said Kim Miller, MPH, a surveillance research scientist at the American Cancer Society, who was approached for comment.
Dr. Miller explained that “cancer trends can sometimes rapidly change within a few years.” Projections just 2-4 years ahead are “extremely difficult” and those 20 years ahead are even more so, she added in an interview.
“We’re encouraged to see the projected decreases in deaths from lung, colorectal, and breast cancer in the coming years,” said coauthor Lynn Matrisian, PhD, MBA, chief science officer at the Pancreatic Cancer Action Network. “It’s time to shift focus to some of the less commonly diagnosed cancers with the lowest survival rates, like pancreatic and liver cancer.”
Difference in opinion on prostate cancer
The huge fall in the incidence of prostate cancer that the authors predict will come about as a result of changes in prostate-specific antigen (PSA)–screening recommendations over the last 15 years, they suggested.
“The most recent change in 2018 recommends that men aged 55-69 can make their own decisions regarding screening, but previous changes recommended against PSA screening,” said Dr. Rahib.
“These changes in screening guidelines have influenced the number of diagnoses of prostate cancer in recent years and will continue to do so to 2040,” Dr. Rahib commented.
Dr. Miller casts doubt on this prediction.
Using data through 2017, “we have seen that the patterns in prostate cancer incidence are already shifting from the steep declines we saw in the early 2010s,” she said. “I would use caution when interpreting the overall trends for prostate, because this cancer in particular is dramatically affected by changes in recommendations for screening with the PSA test.”
Screening has also influenced colorectal cancer incidence, the authors pointed out, saying that the uptake of colorectal cancer screening is associated with a decrease in the number of colorectal cancers and deaths out to 2040, as a result of effectiveness of screening.
For breast cancer, the authors highlighted the fact that, although the number of breast cancers will continue to increase, the number of breast cancer deaths will decrease. That ongoing trend is most likely attributable to increased screening and advancements in treatment.
The study was supported by the National Institutes of Health, National Cancer Institute, the Cancer Prevention and Research Institute of Texas, Cancer Commons and the Pancreatic Cancer Action Network. The study authors and Dr. Miller disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The next 20 years will see a big shift in cancer type rankings, researchers predict.
At the moment, the most common cancers in the United States are breast, lung, prostate, colorectal, and melanoma.
the study authors predicted. Breast cancer will remain the top cancer to be diagnosed, lung cancer will drop from second to third, and colorectal cancer will remain at fourth.
These predicted rankings of cancer types by their total number of annual cases were published online April 7, 2021, in JAMA Network Open.
The authors also rank cancer type by mortality. Currently, most cancer deaths are caused by lung cancer, followed by colorectal, pancreatic, and breast. By 2040, the most notable change in cancer deaths is that liver and intrahepatic bile duct cancer, currently at sixth, will jump up to third.
Two decades from now, the ranking in terms of cancer deaths will be lung, pancreatic, liver and intrahepatic bile duct, and colorectal.
“Our findings reflect the shifting dynamics of cancer screening and treatment,” lead author Lola Rahib, PhD, a pancreatic cancer scientist at Cancer Commons, the advocacy nonprofit, commented in a press statement.
The new analysis used population-growth projections (based on 2010 U.S. Census data) and current population-based cancer incidence and death rates (from Surveillance, Epidemiology, and End Results 2014-2016) to calculate the changes in incidences and deaths to the year 2040.
The projected, estimated numbers are not ironclad, the researchers acknowledged.
“Our projections assume that the observed rates and trends [from recent years] don’t change over time,” Dr. Rahib said in an interview, but she pointed out that change may indeed happen.
“Any long-term projections should be considered with a grain of salt,” said Kim Miller, MPH, a surveillance research scientist at the American Cancer Society, who was approached for comment.
Dr. Miller explained that “cancer trends can sometimes rapidly change within a few years.” Projections just 2-4 years ahead are “extremely difficult” and those 20 years ahead are even more so, she added in an interview.
“We’re encouraged to see the projected decreases in deaths from lung, colorectal, and breast cancer in the coming years,” said coauthor Lynn Matrisian, PhD, MBA, chief science officer at the Pancreatic Cancer Action Network. “It’s time to shift focus to some of the less commonly diagnosed cancers with the lowest survival rates, like pancreatic and liver cancer.”
Difference in opinion on prostate cancer
The huge fall in the incidence of prostate cancer that the authors predict will come about as a result of changes in prostate-specific antigen (PSA)–screening recommendations over the last 15 years, they suggested.
“The most recent change in 2018 recommends that men aged 55-69 can make their own decisions regarding screening, but previous changes recommended against PSA screening,” said Dr. Rahib.
“These changes in screening guidelines have influenced the number of diagnoses of prostate cancer in recent years and will continue to do so to 2040,” Dr. Rahib commented.
Dr. Miller casts doubt on this prediction.
Using data through 2017, “we have seen that the patterns in prostate cancer incidence are already shifting from the steep declines we saw in the early 2010s,” she said. “I would use caution when interpreting the overall trends for prostate, because this cancer in particular is dramatically affected by changes in recommendations for screening with the PSA test.”
Screening has also influenced colorectal cancer incidence, the authors pointed out, saying that the uptake of colorectal cancer screening is associated with a decrease in the number of colorectal cancers and deaths out to 2040, as a result of effectiveness of screening.
For breast cancer, the authors highlighted the fact that, although the number of breast cancers will continue to increase, the number of breast cancer deaths will decrease. That ongoing trend is most likely attributable to increased screening and advancements in treatment.
The study was supported by the National Institutes of Health, National Cancer Institute, the Cancer Prevention and Research Institute of Texas, Cancer Commons and the Pancreatic Cancer Action Network. The study authors and Dr. Miller disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The next 20 years will see a big shift in cancer type rankings, researchers predict.
At the moment, the most common cancers in the United States are breast, lung, prostate, colorectal, and melanoma.
the study authors predicted. Breast cancer will remain the top cancer to be diagnosed, lung cancer will drop from second to third, and colorectal cancer will remain at fourth.
These predicted rankings of cancer types by their total number of annual cases were published online April 7, 2021, in JAMA Network Open.
The authors also rank cancer type by mortality. Currently, most cancer deaths are caused by lung cancer, followed by colorectal, pancreatic, and breast. By 2040, the most notable change in cancer deaths is that liver and intrahepatic bile duct cancer, currently at sixth, will jump up to third.
Two decades from now, the ranking in terms of cancer deaths will be lung, pancreatic, liver and intrahepatic bile duct, and colorectal.
“Our findings reflect the shifting dynamics of cancer screening and treatment,” lead author Lola Rahib, PhD, a pancreatic cancer scientist at Cancer Commons, the advocacy nonprofit, commented in a press statement.
The new analysis used population-growth projections (based on 2010 U.S. Census data) and current population-based cancer incidence and death rates (from Surveillance, Epidemiology, and End Results 2014-2016) to calculate the changes in incidences and deaths to the year 2040.
The projected, estimated numbers are not ironclad, the researchers acknowledged.
“Our projections assume that the observed rates and trends [from recent years] don’t change over time,” Dr. Rahib said in an interview, but she pointed out that change may indeed happen.
“Any long-term projections should be considered with a grain of salt,” said Kim Miller, MPH, a surveillance research scientist at the American Cancer Society, who was approached for comment.
Dr. Miller explained that “cancer trends can sometimes rapidly change within a few years.” Projections just 2-4 years ahead are “extremely difficult” and those 20 years ahead are even more so, she added in an interview.
“We’re encouraged to see the projected decreases in deaths from lung, colorectal, and breast cancer in the coming years,” said coauthor Lynn Matrisian, PhD, MBA, chief science officer at the Pancreatic Cancer Action Network. “It’s time to shift focus to some of the less commonly diagnosed cancers with the lowest survival rates, like pancreatic and liver cancer.”
Difference in opinion on prostate cancer
The huge fall in the incidence of prostate cancer that the authors predict will come about as a result of changes in prostate-specific antigen (PSA)–screening recommendations over the last 15 years, they suggested.
“The most recent change in 2018 recommends that men aged 55-69 can make their own decisions regarding screening, but previous changes recommended against PSA screening,” said Dr. Rahib.
“These changes in screening guidelines have influenced the number of diagnoses of prostate cancer in recent years and will continue to do so to 2040,” Dr. Rahib commented.
Dr. Miller casts doubt on this prediction.
Using data through 2017, “we have seen that the patterns in prostate cancer incidence are already shifting from the steep declines we saw in the early 2010s,” she said. “I would use caution when interpreting the overall trends for prostate, because this cancer in particular is dramatically affected by changes in recommendations for screening with the PSA test.”
Screening has also influenced colorectal cancer incidence, the authors pointed out, saying that the uptake of colorectal cancer screening is associated with a decrease in the number of colorectal cancers and deaths out to 2040, as a result of effectiveness of screening.
For breast cancer, the authors highlighted the fact that, although the number of breast cancers will continue to increase, the number of breast cancer deaths will decrease. That ongoing trend is most likely attributable to increased screening and advancements in treatment.
The study was supported by the National Institutes of Health, National Cancer Institute, the Cancer Prevention and Research Institute of Texas, Cancer Commons and the Pancreatic Cancer Action Network. The study authors and Dr. Miller disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Melanoma presents at later stages, but at an earlier age in Asian Americans
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
FROM SOC SOCIETY 2021
Steroid-refractory pneumonitis from ICIs: Experience at major centers
Pneumonitis is an uncommon and potentially life-threatening complication of immune checkpoint inhibitor (ICI) therapy. A fraction of patients with ICI-related pneumonitis fail to respond to initial therapy with high-dose systemic steroids.
The recently published experiences at two major cancer centers shed light on the outcomes from treatment and can provide some advice to clinicians for dealing with affected patients.
The Johns Hopkins experience
Because ICI-related pneumonitis typically improves within 48-72 hours of steroid therapy, at Johns Hopkins University, Baltimore, steroid-refractory pneumonitis is defined as pneumonitis that demonstrates no clinical improvement after high-dose corticosteroids for 2-14 days. If the immune toxicity–specialized, multidisciplinary management team implements additional immunosuppressive therapy, that is regarded as confirmatory evidence.
Aanika Balaji, a medical student at Johns Hopkins University, and colleagues retrospectively summarized the clinical course of 12 patients with ICI-related pneumonitis between 2011 and 2020. Clinical improvement with subsequent treatment was evidenced by reduction in either level of care or oxygen requirements.
Three-quarters of the patients were current or former smokers, and the same proportion had lung cancer. Most patients (91.6%) had received chemotherapy, 58.3% had prior chest radiotherapy, and 58.3% had achieved partial response or stable disease with an ICI.
Steroid-refractory ICI-related pneumonitis developed between 40 and 127 days (median, 85 days) after the first dose of ICI therapy. Subsequent immunosuppressive management included IVIg, infliximab, or the combination, in addition to ICU-level supportive care.
Among the seven patients who received IVIg alone, two patients (29%) achieved clinical improvement and hospital discharge. The remainder died.
The two patients treated with infliximab and the three patients treated with sequential IVIg and infliximab died. All deaths were attributed to ICI-related pneumonitis or infectious complications.
Overall, clinically relevant findings were:
- Steroid-refractory ICI-related pneumonitis was seen in 18.5% of patients referred for multidisciplinary care.
- Steroid-refractory ICI-related pneumonitis occurred at a median of 85 days into a patient’s ICI treatment.
- Some patients improved clinically after IVIg therapy, but mortality was high overall.
- Infliximab therapy, alone or in combination with IVIg, was ineffective.
The Memorial Sloan Kettering experience
Jason Beattie, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues performed a retrospective study of patients who had pneumonitis after ICI therapy and/or received immune modulator therapy after corticosteroids in the setting of ICI cancer treatment.
Manual record review was performed to exclude cases of pneumonitis from other causes. The period reviewed was roughly contemporaneous with the Johns Hopkins series.
Patients with ICI-related pneumonitis were divided into “steroid refractory” (i.e., no response to high-dose corticosteroids) or “steroid resistant” (i.e., initial response, followed by worsening) categories.
The researchers identified 26 patients with ICI-related pneumonitis, all of whom had advanced malignancy (8 lung cancer, 4 malignant melanoma, 4 renal cell cancer, and 10 “other” cancers).
A majority of patients (85%) were current or former smokers, 73% had received ICI monotherapy, 35% had received prior chest radiation at a median interval of 4.9 months prior to pneumonitis onset, and 27% had preexisting pulmonary disease.
Twelve patients (46%) had steroid-refractory ICI-related pneumonitis, and 14 (54%) had steroid-resistant ICI-related pneumonitis.
The two groups differed in time to pneumonitis onset (a median of 68 days in the refractory group and 182 days in the resistant group) and time to immune modulator therapy after beginning steroids (median 7 days and 2.9 months, respectively). In the steroid-refractory cases, pneumonitis was more severe.
In addition to corticosteroids, most patients received infliximab monotherapy or infliximab with mycophenolate mofetil. In contrast to the Johns Hopkins series, IVIg was not used in the Memorial Sloan Kettering cases.
Outcomes from immune modulators were graded based on clinical evidence (progress notes, oxygen requirements, level of care, radiologic information, etc.) of resolution of pneumonitis on imaging at least 8 weeks after cessation of steroids and immune modulator therapy, durable improvement for at least 8 weeks after immune modulator therapy, transient improvement followed by pneumonitis relapse or inadequate follow-up because of death or hospice referral, or no improvement.
Ten patients (38%) had durable improvement of ICI-related pneumonitis, of whom three (12%) had complete resolution. Two of the patients with complete resolution had steroid-refractory pneumonitis, both of whom had received infliximab followed by mycophenolate mofetil.
Among the seven patients with durable improvement, four remained alive on immune modulators. Time to resolution of pneumonitis was protracted, ranging from 2.3 months to 8.4 months in the steroid-refractory patients.
Durable response was less common with steroid-refractory (25%) than steroid-resistant (50%) disease, with a significant difference in 90-day survival of 25% and 71%, respectively.
Among the 13 (50%) patients with transient improvement in ICI-related pneumonitis, 8 ultimately died, either because of recurrent ICI-related pneumonitis or infection. All three patients with no improvement from immune modulators died.
The 90-day all-cause mortality was 50%, with durable pneumonitis improvement and freedom from severe infectious complications occurring in only about a third of patients.
Lessons for clinicians
The National Comprehensive Cancer Network, the Society for Immunotherapy of Cancer, and the European Society of Medical Oncology have all published guidelines and recommendations for immunosuppression for steroid-refractory adverse events from ICIs.
Unfortunately, there is little experience with steroid-unresponsive ICI-related pneumonitis. The ideal sequence, dose, and duration of additional immune modulator therapy for ICI-related pneumonitis are unclear and may differ from the approaches to other immune-related toxicities.
This is important because, as suggested in an editorial by Margaret Gatti-Mays, MD, and James L. Gulley, MD, PhD, it is likely that ICI-related pneumonitis will be seen more in routine practice than in clinical trial populations. In addition, across all tumor types, ICI-related pneumonitis is the most common cause of ICI-associated death from toxicity.
The retrospective studies from Johns Hopkins and Memorial Sloan Kettering constitute the largest published experience with ICI-related pneumonitis and yield important clinical insights.
Uniform definitions of potentially important patient subgroups (e.g., steroid refractory vs. steroid resistant) are needed. The steroid-refractory and steroid-resistant subgroups have distinctly different clinical features and outcomes. Uniformity in the subgroup definitions would be a useful starting point from both clinical and research perspectives.
Preferred treatment choices need to be tested systematically in multi-institutional studies. Any potential impact of treatment for ICI-related pneumonitis on antitumor immune control should be identified.
Endpoints of interest need to be defined and measured prospectively. All-cause mortality after 90 days is important, but, as the authors of both reviews noted, there are vitally important narratives and differences in functionality that are completely concealed by restricting the focus to mortality.
Potential causal relationships with antecedent exposure to tobacco, radiation, intrathoracic tumor burden, or other factors need to be defined.
Clinicians need predictive biomarkers for ICI-related pneumonitis (e.g., in peripheral blood, pulmonary function testing, or bronchoscopy specimens). At-risk patients may benefit from early intervention.
The limitations of single-institution record reviews in guiding real-world patient management notwithstanding, these reviews illustrate the value of registries and prospective studies to guide the path forward. Taking these next steps will ensure for our patients that the success of immune-targeted therapy against their cancer never becomes a Pyrrhic victory.
The Johns Hopkins investigators and the editorialists reported having no disclosures. The Memorial Sloan Kettering investigators disclosed relationships with Targeted Oncology, Merck, Array BioPharma, Novartis, and many other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Pneumonitis is an uncommon and potentially life-threatening complication of immune checkpoint inhibitor (ICI) therapy. A fraction of patients with ICI-related pneumonitis fail to respond to initial therapy with high-dose systemic steroids.
The recently published experiences at two major cancer centers shed light on the outcomes from treatment and can provide some advice to clinicians for dealing with affected patients.
The Johns Hopkins experience
Because ICI-related pneumonitis typically improves within 48-72 hours of steroid therapy, at Johns Hopkins University, Baltimore, steroid-refractory pneumonitis is defined as pneumonitis that demonstrates no clinical improvement after high-dose corticosteroids for 2-14 days. If the immune toxicity–specialized, multidisciplinary management team implements additional immunosuppressive therapy, that is regarded as confirmatory evidence.
Aanika Balaji, a medical student at Johns Hopkins University, and colleagues retrospectively summarized the clinical course of 12 patients with ICI-related pneumonitis between 2011 and 2020. Clinical improvement with subsequent treatment was evidenced by reduction in either level of care or oxygen requirements.
Three-quarters of the patients were current or former smokers, and the same proportion had lung cancer. Most patients (91.6%) had received chemotherapy, 58.3% had prior chest radiotherapy, and 58.3% had achieved partial response or stable disease with an ICI.
Steroid-refractory ICI-related pneumonitis developed between 40 and 127 days (median, 85 days) after the first dose of ICI therapy. Subsequent immunosuppressive management included IVIg, infliximab, or the combination, in addition to ICU-level supportive care.
Among the seven patients who received IVIg alone, two patients (29%) achieved clinical improvement and hospital discharge. The remainder died.
The two patients treated with infliximab and the three patients treated with sequential IVIg and infliximab died. All deaths were attributed to ICI-related pneumonitis or infectious complications.
Overall, clinically relevant findings were:
- Steroid-refractory ICI-related pneumonitis was seen in 18.5% of patients referred for multidisciplinary care.
- Steroid-refractory ICI-related pneumonitis occurred at a median of 85 days into a patient’s ICI treatment.
- Some patients improved clinically after IVIg therapy, but mortality was high overall.
- Infliximab therapy, alone or in combination with IVIg, was ineffective.
The Memorial Sloan Kettering experience
Jason Beattie, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues performed a retrospective study of patients who had pneumonitis after ICI therapy and/or received immune modulator therapy after corticosteroids in the setting of ICI cancer treatment.
Manual record review was performed to exclude cases of pneumonitis from other causes. The period reviewed was roughly contemporaneous with the Johns Hopkins series.
Patients with ICI-related pneumonitis were divided into “steroid refractory” (i.e., no response to high-dose corticosteroids) or “steroid resistant” (i.e., initial response, followed by worsening) categories.
The researchers identified 26 patients with ICI-related pneumonitis, all of whom had advanced malignancy (8 lung cancer, 4 malignant melanoma, 4 renal cell cancer, and 10 “other” cancers).
A majority of patients (85%) were current or former smokers, 73% had received ICI monotherapy, 35% had received prior chest radiation at a median interval of 4.9 months prior to pneumonitis onset, and 27% had preexisting pulmonary disease.
Twelve patients (46%) had steroid-refractory ICI-related pneumonitis, and 14 (54%) had steroid-resistant ICI-related pneumonitis.
The two groups differed in time to pneumonitis onset (a median of 68 days in the refractory group and 182 days in the resistant group) and time to immune modulator therapy after beginning steroids (median 7 days and 2.9 months, respectively). In the steroid-refractory cases, pneumonitis was more severe.
In addition to corticosteroids, most patients received infliximab monotherapy or infliximab with mycophenolate mofetil. In contrast to the Johns Hopkins series, IVIg was not used in the Memorial Sloan Kettering cases.
Outcomes from immune modulators were graded based on clinical evidence (progress notes, oxygen requirements, level of care, radiologic information, etc.) of resolution of pneumonitis on imaging at least 8 weeks after cessation of steroids and immune modulator therapy, durable improvement for at least 8 weeks after immune modulator therapy, transient improvement followed by pneumonitis relapse or inadequate follow-up because of death or hospice referral, or no improvement.
Ten patients (38%) had durable improvement of ICI-related pneumonitis, of whom three (12%) had complete resolution. Two of the patients with complete resolution had steroid-refractory pneumonitis, both of whom had received infliximab followed by mycophenolate mofetil.
Among the seven patients with durable improvement, four remained alive on immune modulators. Time to resolution of pneumonitis was protracted, ranging from 2.3 months to 8.4 months in the steroid-refractory patients.
Durable response was less common with steroid-refractory (25%) than steroid-resistant (50%) disease, with a significant difference in 90-day survival of 25% and 71%, respectively.
Among the 13 (50%) patients with transient improvement in ICI-related pneumonitis, 8 ultimately died, either because of recurrent ICI-related pneumonitis or infection. All three patients with no improvement from immune modulators died.
The 90-day all-cause mortality was 50%, with durable pneumonitis improvement and freedom from severe infectious complications occurring in only about a third of patients.
Lessons for clinicians
The National Comprehensive Cancer Network, the Society for Immunotherapy of Cancer, and the European Society of Medical Oncology have all published guidelines and recommendations for immunosuppression for steroid-refractory adverse events from ICIs.
Unfortunately, there is little experience with steroid-unresponsive ICI-related pneumonitis. The ideal sequence, dose, and duration of additional immune modulator therapy for ICI-related pneumonitis are unclear and may differ from the approaches to other immune-related toxicities.
This is important because, as suggested in an editorial by Margaret Gatti-Mays, MD, and James L. Gulley, MD, PhD, it is likely that ICI-related pneumonitis will be seen more in routine practice than in clinical trial populations. In addition, across all tumor types, ICI-related pneumonitis is the most common cause of ICI-associated death from toxicity.
The retrospective studies from Johns Hopkins and Memorial Sloan Kettering constitute the largest published experience with ICI-related pneumonitis and yield important clinical insights.
Uniform definitions of potentially important patient subgroups (e.g., steroid refractory vs. steroid resistant) are needed. The steroid-refractory and steroid-resistant subgroups have distinctly different clinical features and outcomes. Uniformity in the subgroup definitions would be a useful starting point from both clinical and research perspectives.
Preferred treatment choices need to be tested systematically in multi-institutional studies. Any potential impact of treatment for ICI-related pneumonitis on antitumor immune control should be identified.
Endpoints of interest need to be defined and measured prospectively. All-cause mortality after 90 days is important, but, as the authors of both reviews noted, there are vitally important narratives and differences in functionality that are completely concealed by restricting the focus to mortality.
Potential causal relationships with antecedent exposure to tobacco, radiation, intrathoracic tumor burden, or other factors need to be defined.
Clinicians need predictive biomarkers for ICI-related pneumonitis (e.g., in peripheral blood, pulmonary function testing, or bronchoscopy specimens). At-risk patients may benefit from early intervention.
The limitations of single-institution record reviews in guiding real-world patient management notwithstanding, these reviews illustrate the value of registries and prospective studies to guide the path forward. Taking these next steps will ensure for our patients that the success of immune-targeted therapy against their cancer never becomes a Pyrrhic victory.
The Johns Hopkins investigators and the editorialists reported having no disclosures. The Memorial Sloan Kettering investigators disclosed relationships with Targeted Oncology, Merck, Array BioPharma, Novartis, and many other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Pneumonitis is an uncommon and potentially life-threatening complication of immune checkpoint inhibitor (ICI) therapy. A fraction of patients with ICI-related pneumonitis fail to respond to initial therapy with high-dose systemic steroids.
The recently published experiences at two major cancer centers shed light on the outcomes from treatment and can provide some advice to clinicians for dealing with affected patients.
The Johns Hopkins experience
Because ICI-related pneumonitis typically improves within 48-72 hours of steroid therapy, at Johns Hopkins University, Baltimore, steroid-refractory pneumonitis is defined as pneumonitis that demonstrates no clinical improvement after high-dose corticosteroids for 2-14 days. If the immune toxicity–specialized, multidisciplinary management team implements additional immunosuppressive therapy, that is regarded as confirmatory evidence.
Aanika Balaji, a medical student at Johns Hopkins University, and colleagues retrospectively summarized the clinical course of 12 patients with ICI-related pneumonitis between 2011 and 2020. Clinical improvement with subsequent treatment was evidenced by reduction in either level of care or oxygen requirements.
Three-quarters of the patients were current or former smokers, and the same proportion had lung cancer. Most patients (91.6%) had received chemotherapy, 58.3% had prior chest radiotherapy, and 58.3% had achieved partial response or stable disease with an ICI.
Steroid-refractory ICI-related pneumonitis developed between 40 and 127 days (median, 85 days) after the first dose of ICI therapy. Subsequent immunosuppressive management included IVIg, infliximab, or the combination, in addition to ICU-level supportive care.
Among the seven patients who received IVIg alone, two patients (29%) achieved clinical improvement and hospital discharge. The remainder died.
The two patients treated with infliximab and the three patients treated with sequential IVIg and infliximab died. All deaths were attributed to ICI-related pneumonitis or infectious complications.
Overall, clinically relevant findings were:
- Steroid-refractory ICI-related pneumonitis was seen in 18.5% of patients referred for multidisciplinary care.
- Steroid-refractory ICI-related pneumonitis occurred at a median of 85 days into a patient’s ICI treatment.
- Some patients improved clinically after IVIg therapy, but mortality was high overall.
- Infliximab therapy, alone or in combination with IVIg, was ineffective.
The Memorial Sloan Kettering experience
Jason Beattie, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues performed a retrospective study of patients who had pneumonitis after ICI therapy and/or received immune modulator therapy after corticosteroids in the setting of ICI cancer treatment.
Manual record review was performed to exclude cases of pneumonitis from other causes. The period reviewed was roughly contemporaneous with the Johns Hopkins series.
Patients with ICI-related pneumonitis were divided into “steroid refractory” (i.e., no response to high-dose corticosteroids) or “steroid resistant” (i.e., initial response, followed by worsening) categories.
The researchers identified 26 patients with ICI-related pneumonitis, all of whom had advanced malignancy (8 lung cancer, 4 malignant melanoma, 4 renal cell cancer, and 10 “other” cancers).
A majority of patients (85%) were current or former smokers, 73% had received ICI monotherapy, 35% had received prior chest radiation at a median interval of 4.9 months prior to pneumonitis onset, and 27% had preexisting pulmonary disease.
Twelve patients (46%) had steroid-refractory ICI-related pneumonitis, and 14 (54%) had steroid-resistant ICI-related pneumonitis.
The two groups differed in time to pneumonitis onset (a median of 68 days in the refractory group and 182 days in the resistant group) and time to immune modulator therapy after beginning steroids (median 7 days and 2.9 months, respectively). In the steroid-refractory cases, pneumonitis was more severe.
In addition to corticosteroids, most patients received infliximab monotherapy or infliximab with mycophenolate mofetil. In contrast to the Johns Hopkins series, IVIg was not used in the Memorial Sloan Kettering cases.
Outcomes from immune modulators were graded based on clinical evidence (progress notes, oxygen requirements, level of care, radiologic information, etc.) of resolution of pneumonitis on imaging at least 8 weeks after cessation of steroids and immune modulator therapy, durable improvement for at least 8 weeks after immune modulator therapy, transient improvement followed by pneumonitis relapse or inadequate follow-up because of death or hospice referral, or no improvement.
Ten patients (38%) had durable improvement of ICI-related pneumonitis, of whom three (12%) had complete resolution. Two of the patients with complete resolution had steroid-refractory pneumonitis, both of whom had received infliximab followed by mycophenolate mofetil.
Among the seven patients with durable improvement, four remained alive on immune modulators. Time to resolution of pneumonitis was protracted, ranging from 2.3 months to 8.4 months in the steroid-refractory patients.
Durable response was less common with steroid-refractory (25%) than steroid-resistant (50%) disease, with a significant difference in 90-day survival of 25% and 71%, respectively.
Among the 13 (50%) patients with transient improvement in ICI-related pneumonitis, 8 ultimately died, either because of recurrent ICI-related pneumonitis or infection. All three patients with no improvement from immune modulators died.
The 90-day all-cause mortality was 50%, with durable pneumonitis improvement and freedom from severe infectious complications occurring in only about a third of patients.
Lessons for clinicians
The National Comprehensive Cancer Network, the Society for Immunotherapy of Cancer, and the European Society of Medical Oncology have all published guidelines and recommendations for immunosuppression for steroid-refractory adverse events from ICIs.
Unfortunately, there is little experience with steroid-unresponsive ICI-related pneumonitis. The ideal sequence, dose, and duration of additional immune modulator therapy for ICI-related pneumonitis are unclear and may differ from the approaches to other immune-related toxicities.
This is important because, as suggested in an editorial by Margaret Gatti-Mays, MD, and James L. Gulley, MD, PhD, it is likely that ICI-related pneumonitis will be seen more in routine practice than in clinical trial populations. In addition, across all tumor types, ICI-related pneumonitis is the most common cause of ICI-associated death from toxicity.
The retrospective studies from Johns Hopkins and Memorial Sloan Kettering constitute the largest published experience with ICI-related pneumonitis and yield important clinical insights.
Uniform definitions of potentially important patient subgroups (e.g., steroid refractory vs. steroid resistant) are needed. The steroid-refractory and steroid-resistant subgroups have distinctly different clinical features and outcomes. Uniformity in the subgroup definitions would be a useful starting point from both clinical and research perspectives.
Preferred treatment choices need to be tested systematically in multi-institutional studies. Any potential impact of treatment for ICI-related pneumonitis on antitumor immune control should be identified.
Endpoints of interest need to be defined and measured prospectively. All-cause mortality after 90 days is important, but, as the authors of both reviews noted, there are vitally important narratives and differences in functionality that are completely concealed by restricting the focus to mortality.
Potential causal relationships with antecedent exposure to tobacco, radiation, intrathoracic tumor burden, or other factors need to be defined.
Clinicians need predictive biomarkers for ICI-related pneumonitis (e.g., in peripheral blood, pulmonary function testing, or bronchoscopy specimens). At-risk patients may benefit from early intervention.
The limitations of single-institution record reviews in guiding real-world patient management notwithstanding, these reviews illustrate the value of registries and prospective studies to guide the path forward. Taking these next steps will ensure for our patients that the success of immune-targeted therapy against their cancer never becomes a Pyrrhic victory.
The Johns Hopkins investigators and the editorialists reported having no disclosures. The Memorial Sloan Kettering investigators disclosed relationships with Targeted Oncology, Merck, Array BioPharma, Novartis, and many other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Novel analysis quantifies the benefit of melanoma screening
Patients at very high risk for melanoma, including those with a family history or with inherited pathogenic variants of genes that increase the risk, likely benefit from routine whole-body screening for melanoma and education about UV protection.
Those are key findings from the first prospective cohort study to quantify the benefit of screening in melanoma-prone families, which was published online April 2 in Cancer Epidemiology, Biomarkers & Prevention.
“Whole-body screening for melanoma is currently routine for individuals at high risk for melanoma, which includes people from melanoma-prone families (at least two relatives who have had melanoma) and those with inherited pathogenic gene variants of the CDKN2A or CDK4 genes, which increase risk for melanoma,” lead author Michael R. Sargen, MD, said in an interview. “In our study, we investigated whether screening and educational interventions, including education about the appearance of melanoma and strategies for protecting skin from ultraviolet damage, contributed to early diagnosis of melanoma in individuals from melanoma-prone families.”
Of the 293 individuals who enrolled in the study between 1976 and 2014, 246 were diagnosed with melanoma before enrollment (the prestudy cohort) and 47 were diagnosed after enrollment (the prospective cohort). The researchers compared differences in melanoma thickness and tumor stage between participants in the prestudy and prospective cohorts, and compared tumor-thickness trends between participants in their study and cases in the general population using data from Surveillance, Epidemiology, and End Results (SEER) registries between 1973 and 2016. Because information on melanoma thickness was missing for 24% of melanoma cases in the NCI Familial Melanoma Study and 8.7% of melanoma cases found in the SEER registry, the researchers imputed the missing data.
After adjusting for gender and age, Dr. Sargen and his colleagues found that participants in the prospective cohort had significantly thinner melanomas, compared with those in the prestudy cohort (0.6 mm vs. 1.1 mm, respectively; P < .001). In addition, 83% of those in the prospective cohort were significantly more likely to be diagnosed at the early T1 stage, compared with 40% of those in the prestudy cohort (P < .001).
In their analysis, they also determined that after adjusting for gender and age, “all NCI family cases had systematically lower thickness than SEER cases during the study period.” The reductions in melanoma thickness and tumor stage, they concluded, “were not fully explained by calendar period effects of decreasing thickness in the general population and point to the potential benefit of skin cancer screening for patients with a family history of melanoma and those with pathogenic germline variants of melanoma-susceptibility genes.”
“Our data provide reassuring evidence that screening, alongside education about proper UV protection and the appearance of melanoma, is likely benefiting patients with a significantly elevated risk for melanoma,” Dr. Sargen said in the interview “Further studies are needed to determine whether individuals without a family history of melanoma may benefit from whole-body screening, and whether the benefits vary by ethnicity.”
He acknowledged certain limitations of the study, including the relatively small sample size of melanoma cases in the NCI Familial Melanoma Study and the imputation of missing melanoma-thickness data. “Additionally, since this was a prospective cohort study, we were not able to distinguish the independent effect of each intervention,” he said. “Randomized controlled studies are needed to understand the impact of each aspect of the intervention, such as whole-body screening, melanoma education, or strategies for skin protection.”
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard University, Boston, called the analysis “well done,” but commented on the potential role of selection bias impacting the findings. “People who have a strong family history of melanoma and who are opting to engage in an NCI study and come in for full-body skin checks and go through that education process may have very different health-seeking behaviors than individuals in the general population that would be reported to SEER,” she said.
She also raised the question of whether the results were driven by the early detection through the NCI’s program of provider screening or through the educational component that enables earlier self-detection. “If you’re an individual involved in a study and that brings attention to your moles and you have a strong family history of melanoma to begin with, it is not surprising that you are going to have heightened awareness of any changing mole and therefore are more likely to have melanoma detected at an earlier stage,” Dr. Asgari said.
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute. Dr. Sargen reported having no financial disclosures.
Dr. Asgari disclosed that she has received research support from the Melanoma Research Alliance.
Patients at very high risk for melanoma, including those with a family history or with inherited pathogenic variants of genes that increase the risk, likely benefit from routine whole-body screening for melanoma and education about UV protection.
Those are key findings from the first prospective cohort study to quantify the benefit of screening in melanoma-prone families, which was published online April 2 in Cancer Epidemiology, Biomarkers & Prevention.
“Whole-body screening for melanoma is currently routine for individuals at high risk for melanoma, which includes people from melanoma-prone families (at least two relatives who have had melanoma) and those with inherited pathogenic gene variants of the CDKN2A or CDK4 genes, which increase risk for melanoma,” lead author Michael R. Sargen, MD, said in an interview. “In our study, we investigated whether screening and educational interventions, including education about the appearance of melanoma and strategies for protecting skin from ultraviolet damage, contributed to early diagnosis of melanoma in individuals from melanoma-prone families.”
Of the 293 individuals who enrolled in the study between 1976 and 2014, 246 were diagnosed with melanoma before enrollment (the prestudy cohort) and 47 were diagnosed after enrollment (the prospective cohort). The researchers compared differences in melanoma thickness and tumor stage between participants in the prestudy and prospective cohorts, and compared tumor-thickness trends between participants in their study and cases in the general population using data from Surveillance, Epidemiology, and End Results (SEER) registries between 1973 and 2016. Because information on melanoma thickness was missing for 24% of melanoma cases in the NCI Familial Melanoma Study and 8.7% of melanoma cases found in the SEER registry, the researchers imputed the missing data.
After adjusting for gender and age, Dr. Sargen and his colleagues found that participants in the prospective cohort had significantly thinner melanomas, compared with those in the prestudy cohort (0.6 mm vs. 1.1 mm, respectively; P < .001). In addition, 83% of those in the prospective cohort were significantly more likely to be diagnosed at the early T1 stage, compared with 40% of those in the prestudy cohort (P < .001).
In their analysis, they also determined that after adjusting for gender and age, “all NCI family cases had systematically lower thickness than SEER cases during the study period.” The reductions in melanoma thickness and tumor stage, they concluded, “were not fully explained by calendar period effects of decreasing thickness in the general population and point to the potential benefit of skin cancer screening for patients with a family history of melanoma and those with pathogenic germline variants of melanoma-susceptibility genes.”
“Our data provide reassuring evidence that screening, alongside education about proper UV protection and the appearance of melanoma, is likely benefiting patients with a significantly elevated risk for melanoma,” Dr. Sargen said in the interview “Further studies are needed to determine whether individuals without a family history of melanoma may benefit from whole-body screening, and whether the benefits vary by ethnicity.”
He acknowledged certain limitations of the study, including the relatively small sample size of melanoma cases in the NCI Familial Melanoma Study and the imputation of missing melanoma-thickness data. “Additionally, since this was a prospective cohort study, we were not able to distinguish the independent effect of each intervention,” he said. “Randomized controlled studies are needed to understand the impact of each aspect of the intervention, such as whole-body screening, melanoma education, or strategies for skin protection.”
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard University, Boston, called the analysis “well done,” but commented on the potential role of selection bias impacting the findings. “People who have a strong family history of melanoma and who are opting to engage in an NCI study and come in for full-body skin checks and go through that education process may have very different health-seeking behaviors than individuals in the general population that would be reported to SEER,” she said.
She also raised the question of whether the results were driven by the early detection through the NCI’s program of provider screening or through the educational component that enables earlier self-detection. “If you’re an individual involved in a study and that brings attention to your moles and you have a strong family history of melanoma to begin with, it is not surprising that you are going to have heightened awareness of any changing mole and therefore are more likely to have melanoma detected at an earlier stage,” Dr. Asgari said.
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute. Dr. Sargen reported having no financial disclosures.
Dr. Asgari disclosed that she has received research support from the Melanoma Research Alliance.
Patients at very high risk for melanoma, including those with a family history or with inherited pathogenic variants of genes that increase the risk, likely benefit from routine whole-body screening for melanoma and education about UV protection.
Those are key findings from the first prospective cohort study to quantify the benefit of screening in melanoma-prone families, which was published online April 2 in Cancer Epidemiology, Biomarkers & Prevention.
“Whole-body screening for melanoma is currently routine for individuals at high risk for melanoma, which includes people from melanoma-prone families (at least two relatives who have had melanoma) and those with inherited pathogenic gene variants of the CDKN2A or CDK4 genes, which increase risk for melanoma,” lead author Michael R. Sargen, MD, said in an interview. “In our study, we investigated whether screening and educational interventions, including education about the appearance of melanoma and strategies for protecting skin from ultraviolet damage, contributed to early diagnosis of melanoma in individuals from melanoma-prone families.”
Of the 293 individuals who enrolled in the study between 1976 and 2014, 246 were diagnosed with melanoma before enrollment (the prestudy cohort) and 47 were diagnosed after enrollment (the prospective cohort). The researchers compared differences in melanoma thickness and tumor stage between participants in the prestudy and prospective cohorts, and compared tumor-thickness trends between participants in their study and cases in the general population using data from Surveillance, Epidemiology, and End Results (SEER) registries between 1973 and 2016. Because information on melanoma thickness was missing for 24% of melanoma cases in the NCI Familial Melanoma Study and 8.7% of melanoma cases found in the SEER registry, the researchers imputed the missing data.
After adjusting for gender and age, Dr. Sargen and his colleagues found that participants in the prospective cohort had significantly thinner melanomas, compared with those in the prestudy cohort (0.6 mm vs. 1.1 mm, respectively; P < .001). In addition, 83% of those in the prospective cohort were significantly more likely to be diagnosed at the early T1 stage, compared with 40% of those in the prestudy cohort (P < .001).
In their analysis, they also determined that after adjusting for gender and age, “all NCI family cases had systematically lower thickness than SEER cases during the study period.” The reductions in melanoma thickness and tumor stage, they concluded, “were not fully explained by calendar period effects of decreasing thickness in the general population and point to the potential benefit of skin cancer screening for patients with a family history of melanoma and those with pathogenic germline variants of melanoma-susceptibility genes.”
“Our data provide reassuring evidence that screening, alongside education about proper UV protection and the appearance of melanoma, is likely benefiting patients with a significantly elevated risk for melanoma,” Dr. Sargen said in the interview “Further studies are needed to determine whether individuals without a family history of melanoma may benefit from whole-body screening, and whether the benefits vary by ethnicity.”
He acknowledged certain limitations of the study, including the relatively small sample size of melanoma cases in the NCI Familial Melanoma Study and the imputation of missing melanoma-thickness data. “Additionally, since this was a prospective cohort study, we were not able to distinguish the independent effect of each intervention,” he said. “Randomized controlled studies are needed to understand the impact of each aspect of the intervention, such as whole-body screening, melanoma education, or strategies for skin protection.”
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard University, Boston, called the analysis “well done,” but commented on the potential role of selection bias impacting the findings. “People who have a strong family history of melanoma and who are opting to engage in an NCI study and come in for full-body skin checks and go through that education process may have very different health-seeking behaviors than individuals in the general population that would be reported to SEER,” she said.
She also raised the question of whether the results were driven by the early detection through the NCI’s program of provider screening or through the educational component that enables earlier self-detection. “If you’re an individual involved in a study and that brings attention to your moles and you have a strong family history of melanoma to begin with, it is not surprising that you are going to have heightened awareness of any changing mole and therefore are more likely to have melanoma detected at an earlier stage,” Dr. Asgari said.
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute. Dr. Sargen reported having no financial disclosures.
Dr. Asgari disclosed that she has received research support from the Melanoma Research Alliance.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS, AND PREVENTION
An 80-year-old patient presents with an asymptomatic firm pink plaque on his shoulder
Melanoma is a type of skin cancer that arises from melanocytes. According to the American Cancer Society, about 106,110 new melanomas will be diagnosed in the United States in 2021.The risk for developing melanoma increases with age. There are multiple clinical forms of cutaneous melanoma. The four main types are superficial spreading melanoma, nodular melanoma, melanoma in situ (lentigo maligna), and acral lentiginous melanoma. Rare variants include amelanotic melanoma, nevoid melanoma, spitzoid melanoma, and desmoplastic melanoma (DM). Melanoma can also rarely affect parts of the eye and mucosa.
, according to the Memorial Sloan Kettering Cancer Center. It typically presents as a subtle pigmented, pink, red, or skin colored patch, papule or plaque on sun-exposed skin (head and neck most frequently). Chronic UV exposure has been linked to DM. It may be mistaken for a scar or dermatofibroma. DM tends to grow locally and has less risk for nodal metastasis.1
Histologic diagnosis may be challenging. Two histologic variants in desmoplastic melanoma have been described: pure and mixed, depending on the degree of desmoplasia and cellularity present in the tumor.1 Pure DM tends to have a less aggressive course. Melanocytes can appear spindled in a fibrotic stroma. Patchy lymphocyte aggregates may be seen. Perineural invasion is more common in desmoplastic melanoma. Histologically, the differential includes spindle cell carcinoma and sarcoma. Immunostaining is helpful in differentiation.
Our patient had no lymphadenopathy on physical examination. Biopsy revealed a desmoplastic melanoma, 3.6 mm in depth, no ulceration, no regression, mitotic rate 1/mm2. He was referred to surgical oncology. The patient underwent wide excision. Sentinel lymph node biopsy was deferred.
It is imperative for dermatologists to be cognizant of this challenging subtype of melanoma when evaluating patients.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Chen L et al. J Am Acad Dermatol. 2013 May;68(5):825-33.
Melanoma is a type of skin cancer that arises from melanocytes. According to the American Cancer Society, about 106,110 new melanomas will be diagnosed in the United States in 2021.The risk for developing melanoma increases with age. There are multiple clinical forms of cutaneous melanoma. The four main types are superficial spreading melanoma, nodular melanoma, melanoma in situ (lentigo maligna), and acral lentiginous melanoma. Rare variants include amelanotic melanoma, nevoid melanoma, spitzoid melanoma, and desmoplastic melanoma (DM). Melanoma can also rarely affect parts of the eye and mucosa.
, according to the Memorial Sloan Kettering Cancer Center. It typically presents as a subtle pigmented, pink, red, or skin colored patch, papule or plaque on sun-exposed skin (head and neck most frequently). Chronic UV exposure has been linked to DM. It may be mistaken for a scar or dermatofibroma. DM tends to grow locally and has less risk for nodal metastasis.1
Histologic diagnosis may be challenging. Two histologic variants in desmoplastic melanoma have been described: pure and mixed, depending on the degree of desmoplasia and cellularity present in the tumor.1 Pure DM tends to have a less aggressive course. Melanocytes can appear spindled in a fibrotic stroma. Patchy lymphocyte aggregates may be seen. Perineural invasion is more common in desmoplastic melanoma. Histologically, the differential includes spindle cell carcinoma and sarcoma. Immunostaining is helpful in differentiation.
Our patient had no lymphadenopathy on physical examination. Biopsy revealed a desmoplastic melanoma, 3.6 mm in depth, no ulceration, no regression, mitotic rate 1/mm2. He was referred to surgical oncology. The patient underwent wide excision. Sentinel lymph node biopsy was deferred.
It is imperative for dermatologists to be cognizant of this challenging subtype of melanoma when evaluating patients.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Chen L et al. J Am Acad Dermatol. 2013 May;68(5):825-33.
Melanoma is a type of skin cancer that arises from melanocytes. According to the American Cancer Society, about 106,110 new melanomas will be diagnosed in the United States in 2021.The risk for developing melanoma increases with age. There are multiple clinical forms of cutaneous melanoma. The four main types are superficial spreading melanoma, nodular melanoma, melanoma in situ (lentigo maligna), and acral lentiginous melanoma. Rare variants include amelanotic melanoma, nevoid melanoma, spitzoid melanoma, and desmoplastic melanoma (DM). Melanoma can also rarely affect parts of the eye and mucosa.
, according to the Memorial Sloan Kettering Cancer Center. It typically presents as a subtle pigmented, pink, red, or skin colored patch, papule or plaque on sun-exposed skin (head and neck most frequently). Chronic UV exposure has been linked to DM. It may be mistaken for a scar or dermatofibroma. DM tends to grow locally and has less risk for nodal metastasis.1
Histologic diagnosis may be challenging. Two histologic variants in desmoplastic melanoma have been described: pure and mixed, depending on the degree of desmoplasia and cellularity present in the tumor.1 Pure DM tends to have a less aggressive course. Melanocytes can appear spindled in a fibrotic stroma. Patchy lymphocyte aggregates may be seen. Perineural invasion is more common in desmoplastic melanoma. Histologically, the differential includes spindle cell carcinoma and sarcoma. Immunostaining is helpful in differentiation.
Our patient had no lymphadenopathy on physical examination. Biopsy revealed a desmoplastic melanoma, 3.6 mm in depth, no ulceration, no regression, mitotic rate 1/mm2. He was referred to surgical oncology. The patient underwent wide excision. Sentinel lymph node biopsy was deferred.
It is imperative for dermatologists to be cognizant of this challenging subtype of melanoma when evaluating patients.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Chen L et al. J Am Acad Dermatol. 2013 May;68(5):825-33.
Don’t delay: Cancer patients need both doses of COVID vaccine
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
mCODE: Improving data sharing to enhance cancer care
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM JCO CLINICAL CANCER INFORMATICS
The Genital Examination in Dermatologic Practice
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
Resident Pearls
- Dermatologists should offer a genital examination to all patients who present for a routine total-body skin examination.
- It is critical to educate patients about the importance of examining the genital skin by discussing that skin diseases can arise in all areas of the body including the genital area. Encouraging genital self-examination also is helpful.
- If a patient declines, the dermatologist should strive to ensure that another provider is examining the genital skin.