User login
‘Goodie bag’ pill mill doctor sentenced to 2 decades in prison
A Pennsylvania-based internist was sentenced to 20 years in prison by a federal judge on May 10 for running a prescription “pill mill” from his medical practice.
Since May 2005, Andrew Berkowitz, MD, 62, of Huntington Valley, Pa., was president and CEO of A+ Pain Management, a clinic in the Philadelphia area, according to his LinkedIn profile.
Prosecutors said patients, no matter their complaint, would leave Dr. Berkowitz’s offices with “goodie bags” filled with a selection of drugs. A typical haul included topical analgesics, such as Relyyt and/or lidocaine; muscle relaxants, including chlorzoxazone and/or cyclobenzaprine; anti-inflammatories, such as celecoxib and/or fenoprofen; and schedule IV substances, including tramadol, eszopiclone, and quazepam.
The practice was registered in Pennsylvania as a nonpharmacy dispensing site, allowing Dr. Berkowitz to bill insurers for the drugs, according to The Pennsylvania Record, a journal covering Pennsylvania’s legal system. Dr. Berkowitz also prescribed oxycodone for “pill seeking” patients, who gave him their tacit approval of submitting claims to their insurance providers, which included Medicare, Aetna, and others, for the items in the goodie bag.
In addition, Dr. Berkowitz fraudulently billed insurers for medically unnecessary physical therapy, acupuncture, and chiropractic adjustments, as well as for treatments that were never provided, according to federal officials.
According to the Department of Justice, Dr. Berkowitz collected more than $4,000 per bag from insurers. From 2015 to 2018, prosecutors estimate that Dr. Berkowitz took in more than $4 million in fraudulent proceeds from his scheme.
The pill mill came to the attention of federal authorities after Blue Cross investigators forwarded to the FBI several complaints it had received about Dr. Berkowitz. In 2017, the FBI sent a cooperating witness to Dr. Berkowitz’s clinic. The undercover patient received a prescription for oxycodone, Motrin, and Flexeril and paid $185, according to The Record.
After being indicted in 2019, Dr. Berkowitz pleaded guilty in January 2020 to 19 counts of health care fraud and to 23 counts of distributing oxycodone outside the course of professional practice and without a legitimate medical purpose.
On May 10, he was sentenced to 20 years in prison, followed by 5 years of supervised release. In addition, he was ordered to pay a $40,000 fine and almost $4 million in restitution. As a result of civil False Claims Act liability for false claims submitted to Medicare, he is also obligated to pay approximately $1.8 million and is subject to a permanent prohibition on prescribing, distributing, or dispensing controlled substances.
Dr. Berkowitz’s actions were deemed especially egregious in light of the opioid epidemic.
“Doctors are supposed to treat illness, not feed it,” said Jacqueline Maguire, special agent in charge of the FBI’s Philadelphia division. “Andrew Berkowitz prescribed patients unnecessary pills and handed out opioids to addicts.” Jennifer Arbittier Williams, acting U.S. Attorney, added upon announcing the sentence, “Doctors who dare engage in health care fraud and drug diversion, two drivers of the opioid epidemic ravaging our communities, should heed this sentence as a warning that they will be held responsible, criminally and financially.”
A version of this article first appeared on Medscape.com.
A Pennsylvania-based internist was sentenced to 20 years in prison by a federal judge on May 10 for running a prescription “pill mill” from his medical practice.
Since May 2005, Andrew Berkowitz, MD, 62, of Huntington Valley, Pa., was president and CEO of A+ Pain Management, a clinic in the Philadelphia area, according to his LinkedIn profile.
Prosecutors said patients, no matter their complaint, would leave Dr. Berkowitz’s offices with “goodie bags” filled with a selection of drugs. A typical haul included topical analgesics, such as Relyyt and/or lidocaine; muscle relaxants, including chlorzoxazone and/or cyclobenzaprine; anti-inflammatories, such as celecoxib and/or fenoprofen; and schedule IV substances, including tramadol, eszopiclone, and quazepam.
The practice was registered in Pennsylvania as a nonpharmacy dispensing site, allowing Dr. Berkowitz to bill insurers for the drugs, according to The Pennsylvania Record, a journal covering Pennsylvania’s legal system. Dr. Berkowitz also prescribed oxycodone for “pill seeking” patients, who gave him their tacit approval of submitting claims to their insurance providers, which included Medicare, Aetna, and others, for the items in the goodie bag.
In addition, Dr. Berkowitz fraudulently billed insurers for medically unnecessary physical therapy, acupuncture, and chiropractic adjustments, as well as for treatments that were never provided, according to federal officials.
According to the Department of Justice, Dr. Berkowitz collected more than $4,000 per bag from insurers. From 2015 to 2018, prosecutors estimate that Dr. Berkowitz took in more than $4 million in fraudulent proceeds from his scheme.
The pill mill came to the attention of federal authorities after Blue Cross investigators forwarded to the FBI several complaints it had received about Dr. Berkowitz. In 2017, the FBI sent a cooperating witness to Dr. Berkowitz’s clinic. The undercover patient received a prescription for oxycodone, Motrin, and Flexeril and paid $185, according to The Record.
After being indicted in 2019, Dr. Berkowitz pleaded guilty in January 2020 to 19 counts of health care fraud and to 23 counts of distributing oxycodone outside the course of professional practice and without a legitimate medical purpose.
On May 10, he was sentenced to 20 years in prison, followed by 5 years of supervised release. In addition, he was ordered to pay a $40,000 fine and almost $4 million in restitution. As a result of civil False Claims Act liability for false claims submitted to Medicare, he is also obligated to pay approximately $1.8 million and is subject to a permanent prohibition on prescribing, distributing, or dispensing controlled substances.
Dr. Berkowitz’s actions were deemed especially egregious in light of the opioid epidemic.
“Doctors are supposed to treat illness, not feed it,” said Jacqueline Maguire, special agent in charge of the FBI’s Philadelphia division. “Andrew Berkowitz prescribed patients unnecessary pills and handed out opioids to addicts.” Jennifer Arbittier Williams, acting U.S. Attorney, added upon announcing the sentence, “Doctors who dare engage in health care fraud and drug diversion, two drivers of the opioid epidemic ravaging our communities, should heed this sentence as a warning that they will be held responsible, criminally and financially.”
A version of this article first appeared on Medscape.com.
A Pennsylvania-based internist was sentenced to 20 years in prison by a federal judge on May 10 for running a prescription “pill mill” from his medical practice.
Since May 2005, Andrew Berkowitz, MD, 62, of Huntington Valley, Pa., was president and CEO of A+ Pain Management, a clinic in the Philadelphia area, according to his LinkedIn profile.
Prosecutors said patients, no matter their complaint, would leave Dr. Berkowitz’s offices with “goodie bags” filled with a selection of drugs. A typical haul included topical analgesics, such as Relyyt and/or lidocaine; muscle relaxants, including chlorzoxazone and/or cyclobenzaprine; anti-inflammatories, such as celecoxib and/or fenoprofen; and schedule IV substances, including tramadol, eszopiclone, and quazepam.
The practice was registered in Pennsylvania as a nonpharmacy dispensing site, allowing Dr. Berkowitz to bill insurers for the drugs, according to The Pennsylvania Record, a journal covering Pennsylvania’s legal system. Dr. Berkowitz also prescribed oxycodone for “pill seeking” patients, who gave him their tacit approval of submitting claims to their insurance providers, which included Medicare, Aetna, and others, for the items in the goodie bag.
In addition, Dr. Berkowitz fraudulently billed insurers for medically unnecessary physical therapy, acupuncture, and chiropractic adjustments, as well as for treatments that were never provided, according to federal officials.
According to the Department of Justice, Dr. Berkowitz collected more than $4,000 per bag from insurers. From 2015 to 2018, prosecutors estimate that Dr. Berkowitz took in more than $4 million in fraudulent proceeds from his scheme.
The pill mill came to the attention of federal authorities after Blue Cross investigators forwarded to the FBI several complaints it had received about Dr. Berkowitz. In 2017, the FBI sent a cooperating witness to Dr. Berkowitz’s clinic. The undercover patient received a prescription for oxycodone, Motrin, and Flexeril and paid $185, according to The Record.
After being indicted in 2019, Dr. Berkowitz pleaded guilty in January 2020 to 19 counts of health care fraud and to 23 counts of distributing oxycodone outside the course of professional practice and without a legitimate medical purpose.
On May 10, he was sentenced to 20 years in prison, followed by 5 years of supervised release. In addition, he was ordered to pay a $40,000 fine and almost $4 million in restitution. As a result of civil False Claims Act liability for false claims submitted to Medicare, he is also obligated to pay approximately $1.8 million and is subject to a permanent prohibition on prescribing, distributing, or dispensing controlled substances.
Dr. Berkowitz’s actions were deemed especially egregious in light of the opioid epidemic.
“Doctors are supposed to treat illness, not feed it,” said Jacqueline Maguire, special agent in charge of the FBI’s Philadelphia division. “Andrew Berkowitz prescribed patients unnecessary pills and handed out opioids to addicts.” Jennifer Arbittier Williams, acting U.S. Attorney, added upon announcing the sentence, “Doctors who dare engage in health care fraud and drug diversion, two drivers of the opioid epidemic ravaging our communities, should heed this sentence as a warning that they will be held responsible, criminally and financially.”
A version of this article first appeared on Medscape.com.
Prior authorizations delay TNF inhibitors for children with JIA
Children with juvenile idiopathic arthritis (JIA) who need a tumor necrosis factor (TNF) inhibitor after failing conventional disease-modifying antirheumatic drug (DMARD) treatment often experience insurance delays before beginning the new drug because of prior authorization denials, according to research presented at the 2022 annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The findings were also published as a research letter in JAMA Network Open.
“Prompt escalation to TNF inhibitors is recommended for children with JIA refractory to DMARDs,” author Jordan Roberts, MD, a clinical fellow of the Harvard Medical School Rheumatology Program, Boston, told CARRA attendees. TNF inhibitors are increasingly used as first-line treatment in JIA since growing evidence suggests better outcomes from early treatment with biologics. “Prior authorization requirements that delay TNF inhibitor initiation among children with JIA are common in clinical practice,” Dr. Roberts said, but little evidence exists to understand the extent of this problem and its causes.
The researchers therefore conducted a retrospective cohort study using a search of electronic health records from January 2018 to December 2019 to find all children at a single center with a new diagnosis of nonsystemic JIA. Then the authors pulled the timing of prior authorization requests, approvals, denials, and first TNF inhibitor dose from the medical notes. They also sought out any children who had been recommended a TNF inhibitor but never started one.
The total population included 54 children with an average age of 10 years, about two-thirds of whom had private insurance (63%). The group was predominantly White (63%), although 13% declined to provide race, and 7% were Hispanic. Most subtypes of disease were represented: oligoarticular persistent (28%), oligoarticular extended (2%), polyarticular rheumatoid factor-negative (15%), polyarticular rheumatoid factor-positive (15%), psoriatic arthritis (26%), enthesitis-related arthritis (12%), and undifferentiated arthritis (2%).
The 44 participants with private insurance had an average of two joints with active disease, while the 10 patients with public insurance had an average of four involved joints. Nearly all the patients (91%) had previously taken or were currently taking DMARDs when the prior authorization was submitted, and 61% had received NSAIDs.
All but one of the patients’ insurance plans required a prior authorization. The first prior authorization was denied for about one-third of the public insurance patients (30%) and a quarter of the private insurance patients. About 1 in 5 patients overall (22%) required a written appeal to override the denial, and 4% required peer-to-peer review. Meanwhile, 7% of patients began another medication because of the denial.
It took a median of 3 days for prior authorizations to be approved and a median of 24 days from the time the TNF inhibitor was recommended to the patient receiving the first dose. However, 22% of patients waited at least 2 weeks before the prior authorization was approved, and more than a quarter of the requests took over 30 days before the patient could begin the medication. In the public insurance group in particular, a quarter of children waited at least 19 days for approval and at least 44 days before starting the medication.
In fact, when the researchers looked at the difference in approval time between those who did and did not receive an initial denial, the difference was stark. Median approval time was 16 days when the prior authorization was denied, compared with a median of 5 days when the first prior authorization was approved. Similarly, time to initiation of the drug after recommendation was a median of 35 days for those whose prior authorization was first denied and 17 days for those with an initial approval.
The most common reason for an initial denial was the insurance company requiring a different TNF inhibitor than the one the rheumatologist wanted to prescribe. “These were all children whose rheumatologist has recommended either infliximab or etanercept that were required to use adalimumab instead,” Dr. Roberts said.
The other reasons for initial denial were similarly familiar ones:
- Required submission to another insurer
- Additional documentation required
- Lack of medical necessity
- Prescription was for an indication not approved by the Food and Drug Administration
- Age of patient
- Nonbiologic DMARD required
- NSAID required for step therapy
Only three children who were advised to begin a TNF inhibitor did not do so, including one who was lost to follow-up, one who had injection-related anxiety, and one who had safety concerns about the medication.
“Several children were required to use alternative TNF inhibitors than the one that was recommended due to restricted formularies, which may reduce shared decisionmaking between physicians and families and may not be the optimal clinical choice for an individual child,” Dr. Roberts said in her conclusion. Most children, however, were able to get approval for the TNF inhibitor originally requested, “suggesting that utilization management strategies present barriers to timely care despite appropriate specialty medication requests,” she said. “Therefore, it’s important for us to advocate for access to medications for children with JIA.”
Findings are not surprising
“I have these same experiences at my institution – often insurance will dictate clinical practice, and step therapy is the only option, causing a delay to initiation of TNFi even if we think, as the pediatric rheumatologist, that a child needs this medicine to be initiated on presentation to our clinic,” Nayimisha Balmuri, MD, assistant professor of pediatrics in the division of allergy, immunology, and rheumatology at the Johns Hopkins School of Medicine, Baltimore, told this news organization.
Dr. Balmuri, who was not involved in the study, noted that in her clinic at Johns Hopkins, it is hit or miss if an appeal to insurance companies or to the state (if it is Medicaid coverage) will be successful. “Unfortunately, [we are] mostly unsuccessful, and we have to try another DMARD for 8 to 12 weeks first before trying to get TNFi,” she said.
Dr. Balmuri called for bringing these issues to the attention of state and federal legislators. “It’s so important for us to continue to advocate for our patients at the state and national level! We are the advocates for our patients, and we are uniquely trained to know the best medications to initiate to help patients maximize their chance to reach remission of arthritis. Insurance companies need to hear our voices!”
Dr. Roberts reported grants from CARRA, the Lupus Foundation of America, and the National Institute of Allergy and Infectious Diseases during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children with juvenile idiopathic arthritis (JIA) who need a tumor necrosis factor (TNF) inhibitor after failing conventional disease-modifying antirheumatic drug (DMARD) treatment often experience insurance delays before beginning the new drug because of prior authorization denials, according to research presented at the 2022 annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The findings were also published as a research letter in JAMA Network Open.
“Prompt escalation to TNF inhibitors is recommended for children with JIA refractory to DMARDs,” author Jordan Roberts, MD, a clinical fellow of the Harvard Medical School Rheumatology Program, Boston, told CARRA attendees. TNF inhibitors are increasingly used as first-line treatment in JIA since growing evidence suggests better outcomes from early treatment with biologics. “Prior authorization requirements that delay TNF inhibitor initiation among children with JIA are common in clinical practice,” Dr. Roberts said, but little evidence exists to understand the extent of this problem and its causes.
The researchers therefore conducted a retrospective cohort study using a search of electronic health records from January 2018 to December 2019 to find all children at a single center with a new diagnosis of nonsystemic JIA. Then the authors pulled the timing of prior authorization requests, approvals, denials, and first TNF inhibitor dose from the medical notes. They also sought out any children who had been recommended a TNF inhibitor but never started one.
The total population included 54 children with an average age of 10 years, about two-thirds of whom had private insurance (63%). The group was predominantly White (63%), although 13% declined to provide race, and 7% were Hispanic. Most subtypes of disease were represented: oligoarticular persistent (28%), oligoarticular extended (2%), polyarticular rheumatoid factor-negative (15%), polyarticular rheumatoid factor-positive (15%), psoriatic arthritis (26%), enthesitis-related arthritis (12%), and undifferentiated arthritis (2%).
The 44 participants with private insurance had an average of two joints with active disease, while the 10 patients with public insurance had an average of four involved joints. Nearly all the patients (91%) had previously taken or were currently taking DMARDs when the prior authorization was submitted, and 61% had received NSAIDs.
All but one of the patients’ insurance plans required a prior authorization. The first prior authorization was denied for about one-third of the public insurance patients (30%) and a quarter of the private insurance patients. About 1 in 5 patients overall (22%) required a written appeal to override the denial, and 4% required peer-to-peer review. Meanwhile, 7% of patients began another medication because of the denial.
It took a median of 3 days for prior authorizations to be approved and a median of 24 days from the time the TNF inhibitor was recommended to the patient receiving the first dose. However, 22% of patients waited at least 2 weeks before the prior authorization was approved, and more than a quarter of the requests took over 30 days before the patient could begin the medication. In the public insurance group in particular, a quarter of children waited at least 19 days for approval and at least 44 days before starting the medication.
In fact, when the researchers looked at the difference in approval time between those who did and did not receive an initial denial, the difference was stark. Median approval time was 16 days when the prior authorization was denied, compared with a median of 5 days when the first prior authorization was approved. Similarly, time to initiation of the drug after recommendation was a median of 35 days for those whose prior authorization was first denied and 17 days for those with an initial approval.
The most common reason for an initial denial was the insurance company requiring a different TNF inhibitor than the one the rheumatologist wanted to prescribe. “These were all children whose rheumatologist has recommended either infliximab or etanercept that were required to use adalimumab instead,” Dr. Roberts said.
The other reasons for initial denial were similarly familiar ones:
- Required submission to another insurer
- Additional documentation required
- Lack of medical necessity
- Prescription was for an indication not approved by the Food and Drug Administration
- Age of patient
- Nonbiologic DMARD required
- NSAID required for step therapy
Only three children who were advised to begin a TNF inhibitor did not do so, including one who was lost to follow-up, one who had injection-related anxiety, and one who had safety concerns about the medication.
“Several children were required to use alternative TNF inhibitors than the one that was recommended due to restricted formularies, which may reduce shared decisionmaking between physicians and families and may not be the optimal clinical choice for an individual child,” Dr. Roberts said in her conclusion. Most children, however, were able to get approval for the TNF inhibitor originally requested, “suggesting that utilization management strategies present barriers to timely care despite appropriate specialty medication requests,” she said. “Therefore, it’s important for us to advocate for access to medications for children with JIA.”
Findings are not surprising
“I have these same experiences at my institution – often insurance will dictate clinical practice, and step therapy is the only option, causing a delay to initiation of TNFi even if we think, as the pediatric rheumatologist, that a child needs this medicine to be initiated on presentation to our clinic,” Nayimisha Balmuri, MD, assistant professor of pediatrics in the division of allergy, immunology, and rheumatology at the Johns Hopkins School of Medicine, Baltimore, told this news organization.
Dr. Balmuri, who was not involved in the study, noted that in her clinic at Johns Hopkins, it is hit or miss if an appeal to insurance companies or to the state (if it is Medicaid coverage) will be successful. “Unfortunately, [we are] mostly unsuccessful, and we have to try another DMARD for 8 to 12 weeks first before trying to get TNFi,” she said.
Dr. Balmuri called for bringing these issues to the attention of state and federal legislators. “It’s so important for us to continue to advocate for our patients at the state and national level! We are the advocates for our patients, and we are uniquely trained to know the best medications to initiate to help patients maximize their chance to reach remission of arthritis. Insurance companies need to hear our voices!”
Dr. Roberts reported grants from CARRA, the Lupus Foundation of America, and the National Institute of Allergy and Infectious Diseases during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children with juvenile idiopathic arthritis (JIA) who need a tumor necrosis factor (TNF) inhibitor after failing conventional disease-modifying antirheumatic drug (DMARD) treatment often experience insurance delays before beginning the new drug because of prior authorization denials, according to research presented at the 2022 annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The findings were also published as a research letter in JAMA Network Open.
“Prompt escalation to TNF inhibitors is recommended for children with JIA refractory to DMARDs,” author Jordan Roberts, MD, a clinical fellow of the Harvard Medical School Rheumatology Program, Boston, told CARRA attendees. TNF inhibitors are increasingly used as first-line treatment in JIA since growing evidence suggests better outcomes from early treatment with biologics. “Prior authorization requirements that delay TNF inhibitor initiation among children with JIA are common in clinical practice,” Dr. Roberts said, but little evidence exists to understand the extent of this problem and its causes.
The researchers therefore conducted a retrospective cohort study using a search of electronic health records from January 2018 to December 2019 to find all children at a single center with a new diagnosis of nonsystemic JIA. Then the authors pulled the timing of prior authorization requests, approvals, denials, and first TNF inhibitor dose from the medical notes. They also sought out any children who had been recommended a TNF inhibitor but never started one.
The total population included 54 children with an average age of 10 years, about two-thirds of whom had private insurance (63%). The group was predominantly White (63%), although 13% declined to provide race, and 7% were Hispanic. Most subtypes of disease were represented: oligoarticular persistent (28%), oligoarticular extended (2%), polyarticular rheumatoid factor-negative (15%), polyarticular rheumatoid factor-positive (15%), psoriatic arthritis (26%), enthesitis-related arthritis (12%), and undifferentiated arthritis (2%).
The 44 participants with private insurance had an average of two joints with active disease, while the 10 patients with public insurance had an average of four involved joints. Nearly all the patients (91%) had previously taken or were currently taking DMARDs when the prior authorization was submitted, and 61% had received NSAIDs.
All but one of the patients’ insurance plans required a prior authorization. The first prior authorization was denied for about one-third of the public insurance patients (30%) and a quarter of the private insurance patients. About 1 in 5 patients overall (22%) required a written appeal to override the denial, and 4% required peer-to-peer review. Meanwhile, 7% of patients began another medication because of the denial.
It took a median of 3 days for prior authorizations to be approved and a median of 24 days from the time the TNF inhibitor was recommended to the patient receiving the first dose. However, 22% of patients waited at least 2 weeks before the prior authorization was approved, and more than a quarter of the requests took over 30 days before the patient could begin the medication. In the public insurance group in particular, a quarter of children waited at least 19 days for approval and at least 44 days before starting the medication.
In fact, when the researchers looked at the difference in approval time between those who did and did not receive an initial denial, the difference was stark. Median approval time was 16 days when the prior authorization was denied, compared with a median of 5 days when the first prior authorization was approved. Similarly, time to initiation of the drug after recommendation was a median of 35 days for those whose prior authorization was first denied and 17 days for those with an initial approval.
The most common reason for an initial denial was the insurance company requiring a different TNF inhibitor than the one the rheumatologist wanted to prescribe. “These were all children whose rheumatologist has recommended either infliximab or etanercept that were required to use adalimumab instead,” Dr. Roberts said.
The other reasons for initial denial were similarly familiar ones:
- Required submission to another insurer
- Additional documentation required
- Lack of medical necessity
- Prescription was for an indication not approved by the Food and Drug Administration
- Age of patient
- Nonbiologic DMARD required
- NSAID required for step therapy
Only three children who were advised to begin a TNF inhibitor did not do so, including one who was lost to follow-up, one who had injection-related anxiety, and one who had safety concerns about the medication.
“Several children were required to use alternative TNF inhibitors than the one that was recommended due to restricted formularies, which may reduce shared decisionmaking between physicians and families and may not be the optimal clinical choice for an individual child,” Dr. Roberts said in her conclusion. Most children, however, were able to get approval for the TNF inhibitor originally requested, “suggesting that utilization management strategies present barriers to timely care despite appropriate specialty medication requests,” she said. “Therefore, it’s important for us to advocate for access to medications for children with JIA.”
Findings are not surprising
“I have these same experiences at my institution – often insurance will dictate clinical practice, and step therapy is the only option, causing a delay to initiation of TNFi even if we think, as the pediatric rheumatologist, that a child needs this medicine to be initiated on presentation to our clinic,” Nayimisha Balmuri, MD, assistant professor of pediatrics in the division of allergy, immunology, and rheumatology at the Johns Hopkins School of Medicine, Baltimore, told this news organization.
Dr. Balmuri, who was not involved in the study, noted that in her clinic at Johns Hopkins, it is hit or miss if an appeal to insurance companies or to the state (if it is Medicaid coverage) will be successful. “Unfortunately, [we are] mostly unsuccessful, and we have to try another DMARD for 8 to 12 weeks first before trying to get TNFi,” she said.
Dr. Balmuri called for bringing these issues to the attention of state and federal legislators. “It’s so important for us to continue to advocate for our patients at the state and national level! We are the advocates for our patients, and we are uniquely trained to know the best medications to initiate to help patients maximize their chance to reach remission of arthritis. Insurance companies need to hear our voices!”
Dr. Roberts reported grants from CARRA, the Lupus Foundation of America, and the National Institute of Allergy and Infectious Diseases during the conduct of the study.
A version of this article first appeared on Medscape.com.
Does platelet-rich plasma improve patellar tendinopathy symptoms?
Evidence summary
Symptoms improve with PRP—but not significantly
A 2014 double-blind RCT (n = 23) explored recovery outcomes in patients with patellar tendinopathy who received either 1 injection of leukocyte-rich PRP or ultrasound-guided dry needling.1 Both groups also completed standardized eccentric exercises. Participants were predominantly men, ages ≥ 18 years. Symptomatic improvement was assessed using the Victorian Institute of Sport Assessment–Patella (VISA-P), an 8-item subjective questionnaire of functionality with a range of 0 to 100, with 100 as the maximum score for an asymptomatic individual.
At 12 weeks posttreatment, VISA-P scores improved in both groups. However, the improvement in the dry needling group was not statistically significant (5.2 points; 95% CI, –2.2 to 12.6; P = .20), while in the PRP group it was statistically significant (25.4 points; 95% CI, 10.3 to 40.6; P = .01). At ≥ 26 weeks, statistically significant improvement was observed in both treatment groups: scores improved by 33.2 points (95% CI, 24.1 to 42.4; P = .001) in the dry needling group and by 28.9 points (95% CI, 11.4 to 46.3; P = .01) in the PRP group. However, the difference between the groups’ VISA-P scores at ≥ 26 weeks was not significant (P = .66).1
No significant differences observed for PRP vs placebo or physical therapy
A 2019 single-blind RCT (n = 57) involved patients who were treated with 1 injection of either leukocyte-rich PRP, leukocyte-poor PRP, or saline, all in combination with 6 weeks of physical therapy.2 Participants were predominantly men, ages 18 to 50 years, and engaged in recreational sporting activities. There was no statistically significant difference in mean change in VISA-P score at any timepoint of the 2-year study period. P values were not reported.2
A 2010 RCT (n = 31) compared PRP (unspecified whether leukocyte-rich or -poor) in combination with physical therapy to physical therapy alone.3 Groups were matched for sex, age, and sports activity level; patients in the PRP group were required to have failed previous treatment, while control subjects must not have received any treatment for at least 2 months. Subjects were evaluated pretreatment, immediately posttreatment, and 6 months posttreatment. Clinical evaluation was aided by use of the Tegner activity score, a 1-item score that grades activity level on a scale of 0 to 10; the EuroQol-visual analog scale (EQ-VAS), which evaluates subjective rating of overall health; and pain level scores.
At 6 months posttreatment, no statistically significant differences were observed between groups in EQ-VAS and pain level scores. However, Tegner activity scores among PRP recipients showed significant percent improvement over controls at 6 months posttreatment (39% vs 20%; P = .048).3
Recommendations from others
Currently, national orthopedic and professional athletic medical associations have recommended that further research be conducted in order to make a strong statement in favor of or against PRP.4,5
Editor’s takeaway
Existing data regarding PRP fails, again, to show consistent benefits. These small sample sizes, inconsistent comparators, and heterogeneous results limit our certainty. This lack of quality evidence does not prove a lack of effect, but it raises serious doubts.
1. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416
2. Scott A, LaPrade R, Harmon K, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
3. Filardo G, Kon E, Villa S Della, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909. doi: 10.1007/s00264-009-0845-7
4. LaPrade R, Dragoo J, Koh J, et al. AAOS Research Symposium updates and consensus: biologic treatment of orthopaedic injuries. J Am Acad Orthop Surg. 2016;24:e62-e78. doi: 10.5435/JAAOS-D-16-00086
5. Rodeo SA, Bedi A. 2019-2020 NFL and NFL Physician Society orthobiologics consensus statement. Sports Health. 2020;12:58-60. doi: 10.1177/1941738119889013
Evidence summary
Symptoms improve with PRP—but not significantly
A 2014 double-blind RCT (n = 23) explored recovery outcomes in patients with patellar tendinopathy who received either 1 injection of leukocyte-rich PRP or ultrasound-guided dry needling.1 Both groups also completed standardized eccentric exercises. Participants were predominantly men, ages ≥ 18 years. Symptomatic improvement was assessed using the Victorian Institute of Sport Assessment–Patella (VISA-P), an 8-item subjective questionnaire of functionality with a range of 0 to 100, with 100 as the maximum score for an asymptomatic individual.
At 12 weeks posttreatment, VISA-P scores improved in both groups. However, the improvement in the dry needling group was not statistically significant (5.2 points; 95% CI, –2.2 to 12.6; P = .20), while in the PRP group it was statistically significant (25.4 points; 95% CI, 10.3 to 40.6; P = .01). At ≥ 26 weeks, statistically significant improvement was observed in both treatment groups: scores improved by 33.2 points (95% CI, 24.1 to 42.4; P = .001) in the dry needling group and by 28.9 points (95% CI, 11.4 to 46.3; P = .01) in the PRP group. However, the difference between the groups’ VISA-P scores at ≥ 26 weeks was not significant (P = .66).1
No significant differences observed for PRP vs placebo or physical therapy
A 2019 single-blind RCT (n = 57) involved patients who were treated with 1 injection of either leukocyte-rich PRP, leukocyte-poor PRP, or saline, all in combination with 6 weeks of physical therapy.2 Participants were predominantly men, ages 18 to 50 years, and engaged in recreational sporting activities. There was no statistically significant difference in mean change in VISA-P score at any timepoint of the 2-year study period. P values were not reported.2
A 2010 RCT (n = 31) compared PRP (unspecified whether leukocyte-rich or -poor) in combination with physical therapy to physical therapy alone.3 Groups were matched for sex, age, and sports activity level; patients in the PRP group were required to have failed previous treatment, while control subjects must not have received any treatment for at least 2 months. Subjects were evaluated pretreatment, immediately posttreatment, and 6 months posttreatment. Clinical evaluation was aided by use of the Tegner activity score, a 1-item score that grades activity level on a scale of 0 to 10; the EuroQol-visual analog scale (EQ-VAS), which evaluates subjective rating of overall health; and pain level scores.
At 6 months posttreatment, no statistically significant differences were observed between groups in EQ-VAS and pain level scores. However, Tegner activity scores among PRP recipients showed significant percent improvement over controls at 6 months posttreatment (39% vs 20%; P = .048).3
Recommendations from others
Currently, national orthopedic and professional athletic medical associations have recommended that further research be conducted in order to make a strong statement in favor of or against PRP.4,5
Editor’s takeaway
Existing data regarding PRP fails, again, to show consistent benefits. These small sample sizes, inconsistent comparators, and heterogeneous results limit our certainty. This lack of quality evidence does not prove a lack of effect, but it raises serious doubts.
Evidence summary
Symptoms improve with PRP—but not significantly
A 2014 double-blind RCT (n = 23) explored recovery outcomes in patients with patellar tendinopathy who received either 1 injection of leukocyte-rich PRP or ultrasound-guided dry needling.1 Both groups also completed standardized eccentric exercises. Participants were predominantly men, ages ≥ 18 years. Symptomatic improvement was assessed using the Victorian Institute of Sport Assessment–Patella (VISA-P), an 8-item subjective questionnaire of functionality with a range of 0 to 100, with 100 as the maximum score for an asymptomatic individual.
At 12 weeks posttreatment, VISA-P scores improved in both groups. However, the improvement in the dry needling group was not statistically significant (5.2 points; 95% CI, –2.2 to 12.6; P = .20), while in the PRP group it was statistically significant (25.4 points; 95% CI, 10.3 to 40.6; P = .01). At ≥ 26 weeks, statistically significant improvement was observed in both treatment groups: scores improved by 33.2 points (95% CI, 24.1 to 42.4; P = .001) in the dry needling group and by 28.9 points (95% CI, 11.4 to 46.3; P = .01) in the PRP group. However, the difference between the groups’ VISA-P scores at ≥ 26 weeks was not significant (P = .66).1
No significant differences observed for PRP vs placebo or physical therapy
A 2019 single-blind RCT (n = 57) involved patients who were treated with 1 injection of either leukocyte-rich PRP, leukocyte-poor PRP, or saline, all in combination with 6 weeks of physical therapy.2 Participants were predominantly men, ages 18 to 50 years, and engaged in recreational sporting activities. There was no statistically significant difference in mean change in VISA-P score at any timepoint of the 2-year study period. P values were not reported.2
A 2010 RCT (n = 31) compared PRP (unspecified whether leukocyte-rich or -poor) in combination with physical therapy to physical therapy alone.3 Groups were matched for sex, age, and sports activity level; patients in the PRP group were required to have failed previous treatment, while control subjects must not have received any treatment for at least 2 months. Subjects were evaluated pretreatment, immediately posttreatment, and 6 months posttreatment. Clinical evaluation was aided by use of the Tegner activity score, a 1-item score that grades activity level on a scale of 0 to 10; the EuroQol-visual analog scale (EQ-VAS), which evaluates subjective rating of overall health; and pain level scores.
At 6 months posttreatment, no statistically significant differences were observed between groups in EQ-VAS and pain level scores. However, Tegner activity scores among PRP recipients showed significant percent improvement over controls at 6 months posttreatment (39% vs 20%; P = .048).3
Recommendations from others
Currently, national orthopedic and professional athletic medical associations have recommended that further research be conducted in order to make a strong statement in favor of or against PRP.4,5
Editor’s takeaway
Existing data regarding PRP fails, again, to show consistent benefits. These small sample sizes, inconsistent comparators, and heterogeneous results limit our certainty. This lack of quality evidence does not prove a lack of effect, but it raises serious doubts.
1. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416
2. Scott A, LaPrade R, Harmon K, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
3. Filardo G, Kon E, Villa S Della, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909. doi: 10.1007/s00264-009-0845-7
4. LaPrade R, Dragoo J, Koh J, et al. AAOS Research Symposium updates and consensus: biologic treatment of orthopaedic injuries. J Am Acad Orthop Surg. 2016;24:e62-e78. doi: 10.5435/JAAOS-D-16-00086
5. Rodeo SA, Bedi A. 2019-2020 NFL and NFL Physician Society orthobiologics consensus statement. Sports Health. 2020;12:58-60. doi: 10.1177/1941738119889013
1. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416
2. Scott A, LaPrade R, Harmon K, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
3. Filardo G, Kon E, Villa S Della, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909. doi: 10.1007/s00264-009-0845-7
4. LaPrade R, Dragoo J, Koh J, et al. AAOS Research Symposium updates and consensus: biologic treatment of orthopaedic injuries. J Am Acad Orthop Surg. 2016;24:e62-e78. doi: 10.5435/JAAOS-D-16-00086
5. Rodeo SA, Bedi A. 2019-2020 NFL and NFL Physician Society orthobiologics consensus statement. Sports Health. 2020;12:58-60. doi: 10.1177/1941738119889013
EVIDENCE-BASED ANSWER:
IT’S UNCLEAR. High-quality data have not consistently established the effectiveness of platelet-rich plasma (PRP) injections to improve symptomatic recovery in patellar tendinopathy, compared to placebo (strength of recommendation [SOR]: A, based on 3 small randomized controlled trials [RCTs]). The 3 small RCTs included only 111 patients, total. One found no evidence of significant improvement with PRP compared to controls. The other 2 studies showed mixed results, with different outcome measures favoring different treatment groups and heterogeneous results depending on follow-up duration.
43-year-old male • fatigue • unintentional weight loss • pancytopenia • Dx?
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.
Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
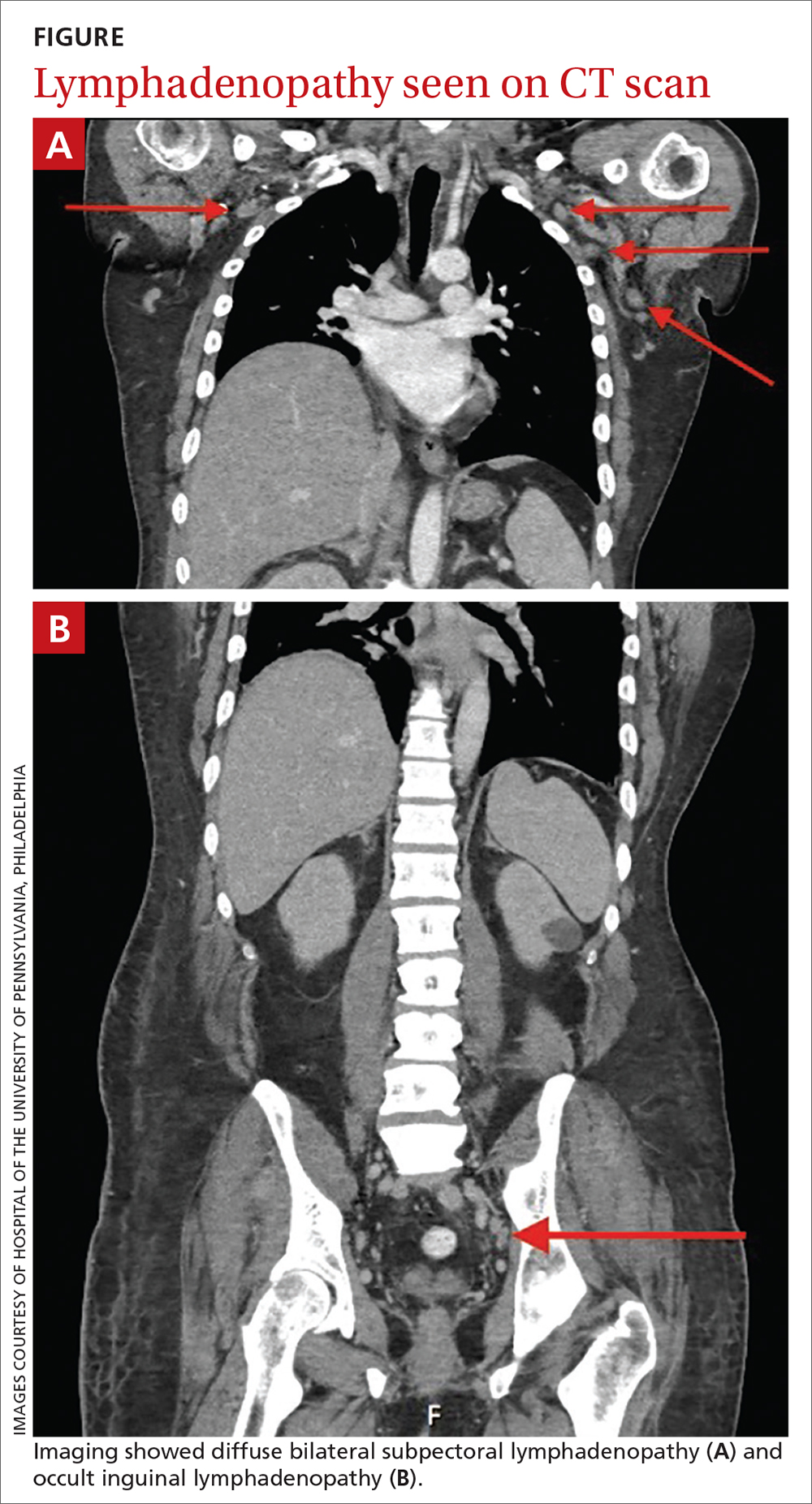
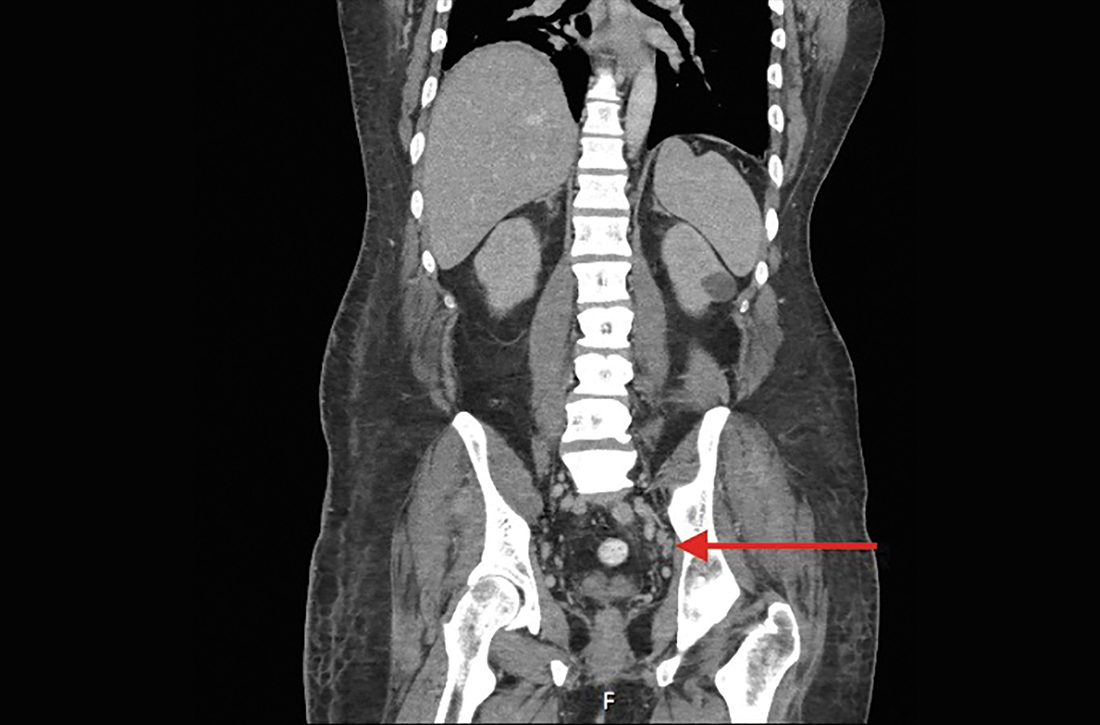
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.
Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.
Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
Atypical knee pain
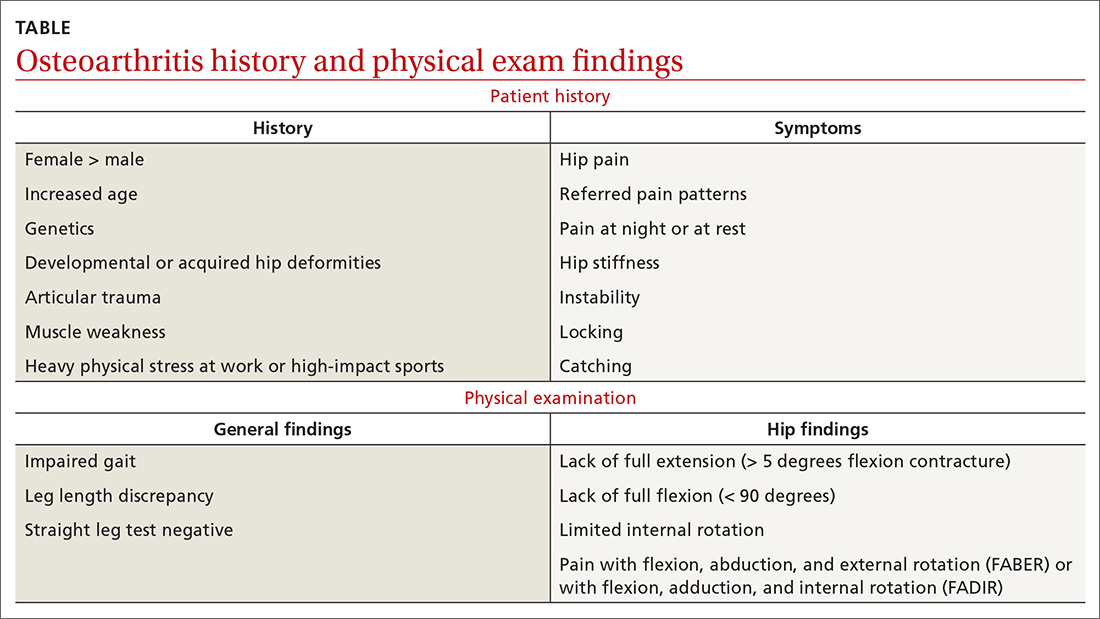
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
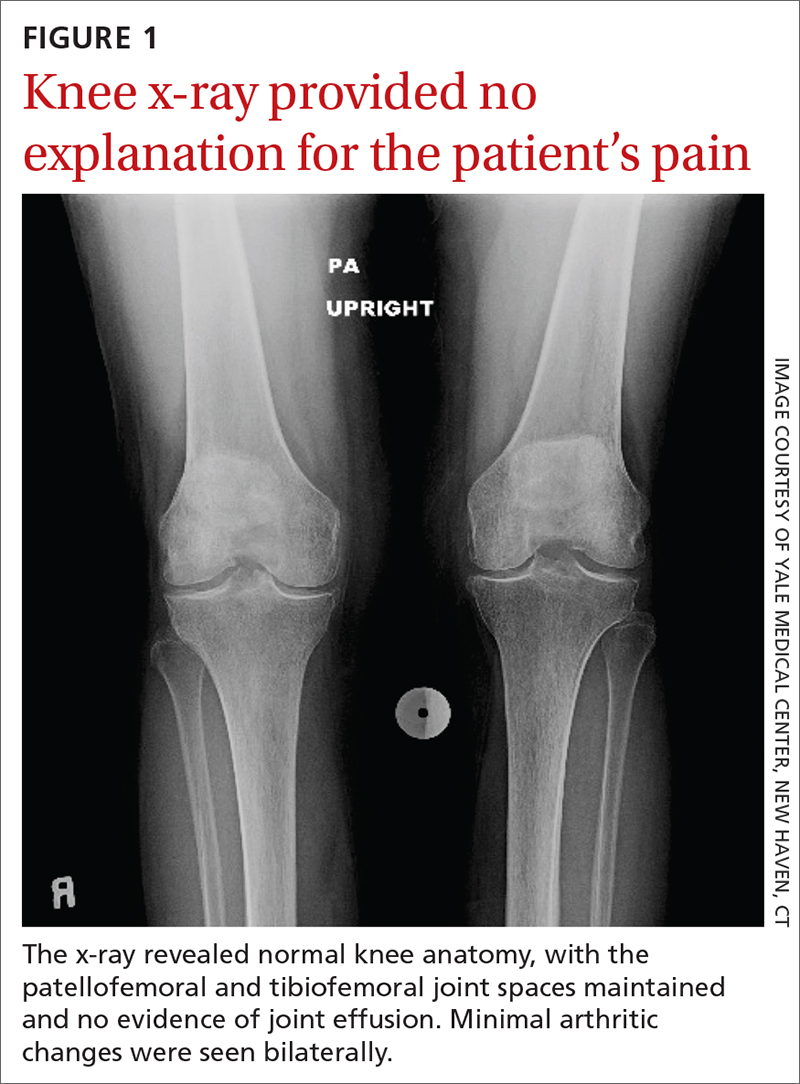
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
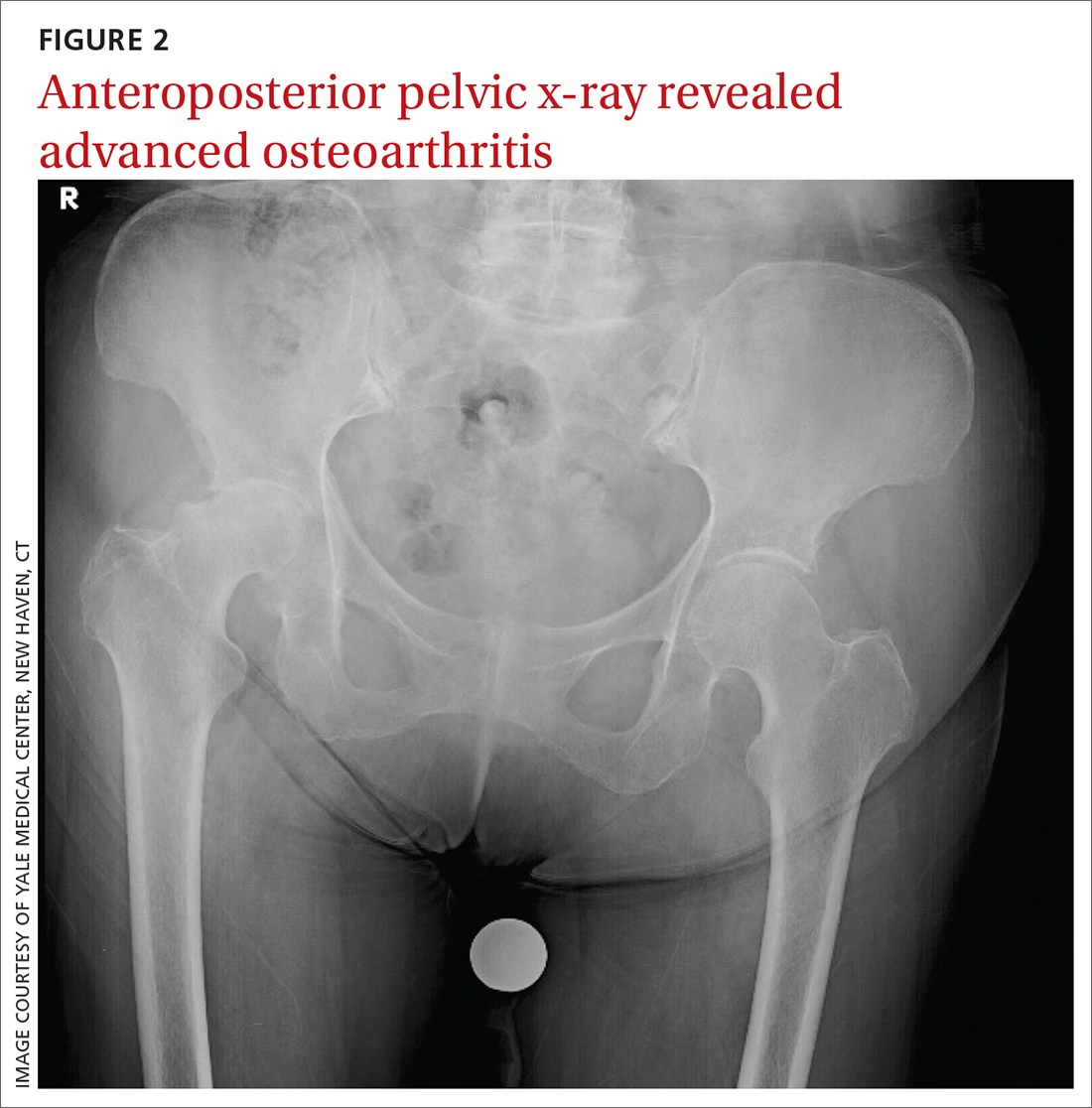
Dx: Right hip OA with referred knee pain
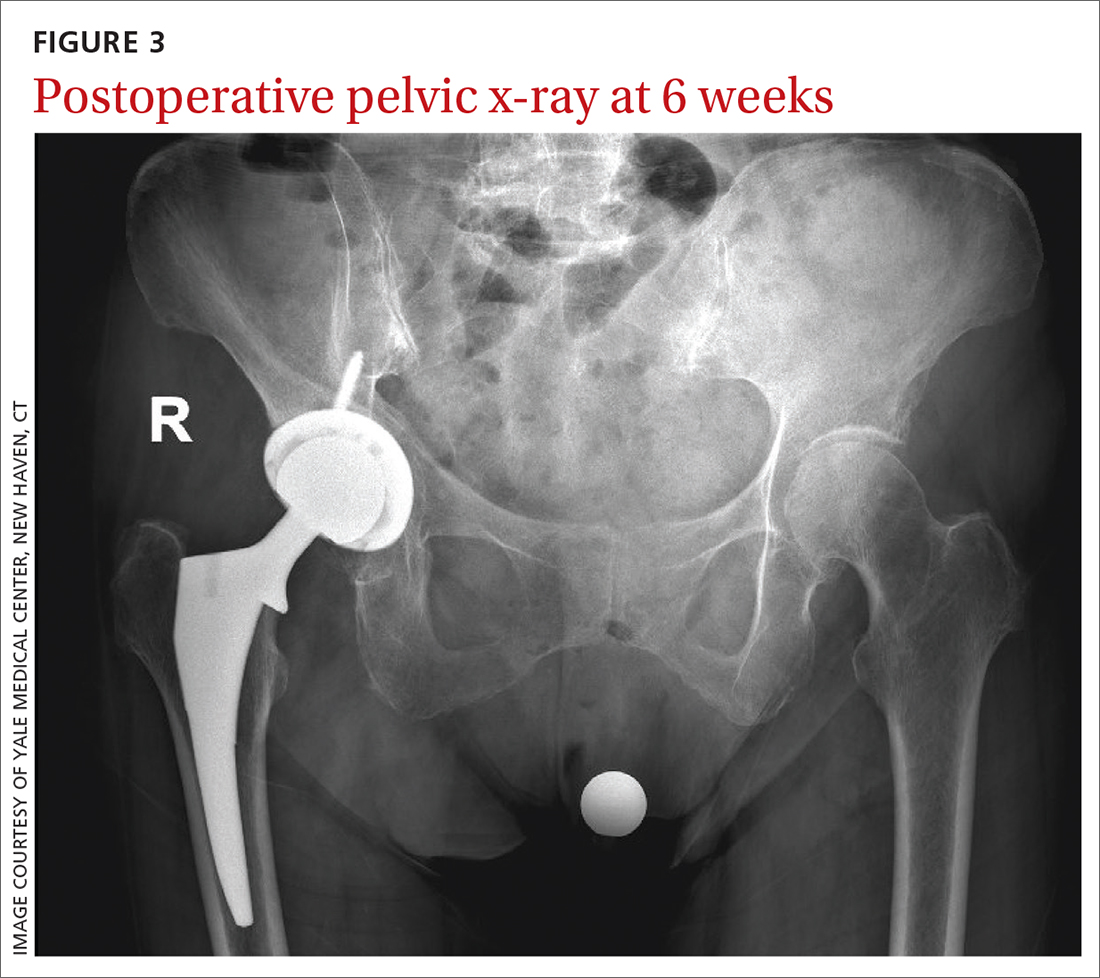
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.
Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.
The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.
Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.
The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.
Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.
The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
‘Where does it hurt?’: Primary care tips for common ortho problems
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022
Surgery shows no survival, morbidity benefit for mild hyperparathyroidism
Patients who receive parathyroidectomy for mild primary hyperparathyroidism show no benefits in survival or morbidity, including fractures, cancer, or cardiovascular outcomes over more than 10 years, compared with those not receiving the surgery, results from a randomized, prospective trial show.
“In contrast to existing data showing increased mortality and cardiovascular morbidity in mild primary hyperparathyroidism, we did not find any treatment effect of parathyroidectomy on these important clinical endpoints,” report the authors of the study, published in the Annals of Internal Medicine.
Reason to evaluate and revise current recommendations?
With mild primary hyperparathyroidism becoming the predominant form of hyperparathyroidism, the results suggest rethinking the current recommendations for the condition, the study authors note.
“Over the years, more active management of mild primary hyperparathyroidism has been recommended, with a widening of criteria for parathyroidectomy,” they write.
“With the low number of kidney stones (n = 5) and no effect of parathyroidectomy on fractures, there may be a need to evaluate and potentially revise the current recommendations.”
The authors of an accompanying editorial agree that “the [results] provide a strong rationale for nonoperative management of patients with mild primary hyperparathyroidism.”
“The findings suggest that most patients can be managed nonoperatively, with monitoring of serum calcium levels every 1 to 2 years or if symptoms occur,” write the editorial authors, Mark J. Bolland, PhD, and Andrew Grey, MD, of the department of medicine, University of Auckland, New Zealand.
Although parathyroidectomy is recommended for the treatment in patients with hyperparathyroidism with severe hypercalcemia or overt symptoms, there has been debate on the long-term benefits of surgery among those with milder cases.
Most previous studies that have shown benefits, such as reductions in the risk of fracture with parathyroidectomy, have importantly not distinguished between mild and more severe primary hyperparathyroidism, the authors note.
No significant differences in mortality between surgery, nonsurgery groups
For the Scandinavian Investigation of Primary Hyperparathyroidism (SIPH) trial, first author Mikkel Pretorius, MD, Oslo University Hospital and Faculty of Medicine, University of Oslo, and colleagues enrolled 191 patients between 1998 and 2005 in Sweden, Norway, and Denmark, who were aged 50-80 years and had mild primary hyperparathyroidism, defined as serum calcium levels of 10.42-11.22 mg/dL.
Participants were randomized to receive surgery (n = 95) or nonoperative observation without intervention (n = 96).
After a 10-year follow-up, 129 patients had completed the final visit. The overall death rate was 7.6%, and, with eight deaths in the surgery group and seven in the nonsurgery group, there were no significant differences between groups in terms of mortality (HR, 1.17; P = .76).
During an extended observation period that lasted until 2018, mortality rates increased by 23%, but with a relatively even distribution of 24 deaths in the surgery group and 20 among those with no surgery.
Chronic hypercalcemia related to primary hyperparathyroidism has been debated as being associated with an increased risk of cardiovascular disease or cancer, however, “the absolute numbers for these and the other disease-specific causes of death were nearly identical between groups,” the authors write, with 17 deaths from cardiovascular disease, eight from cancer, and eight from cerebrovascular disease.
In terms of morbidity, including cardiovascular events, cerebrovascular events, cancer, peripheral fractures, and renal stones, there were 101 events overall, with 52 in the parathyroidectomy group and 49 in the nonsurgery group, which again, was not a significant difference.
Sixteen vertebral fractures occurred overall in 14 patients, which were evenly split at seven patients in each group.
The authors note that “the incidence of peripheral fractures for women in our study was around 2,900 per 100,000 person-years, in the same range as for 70-year-old women in a study in Gothenburg, Sweden (about 2,600 per 100,000 person-years).”
There were no between-group differences in terms of time to death or first morbidity event for any of the prespecified events.
Of the 96 patients originally assigned to the nonsurgery group, 17 (18%) had surgery during follow-up, including three for serious hypercalcemia, three by their own choice, two for decreasing bone density, one for kidney stones, and the others for unclear or unrelated reasons.
Study limitations include that only 26 men (13 in each group) were included, and only 16 completed the study. “The external validity for men based on this study is therefore limited,” the authors note.
And although most people with primary hyperparathyroidism are adults, the older age of participants suggests the results should not be generalized to younger patients with benign parathyroid tumors.
The editorialists note that age should be one of the few factors that may, indeed, suggest appropriate candidates for parathyroidectomy.
“Younger patients (aged < 50 years) may have more aggressive disease,” they explain.
In addition, “patients with serum calcium levels above 3 mmol/L (> 12 mg/dL) are at greater risk for symptomatic hypercalcemia, and patients with a recent history of kidney stones may have fewer future stones after surgical cure.”
“Yet, such patients are a small minority of those with primary hyperparathyroidism,” they note.
The study authors underscore that “our data add evidence to guide the decisionmaking process in deliberative dialogue between clinicians and patients.”
The study received funding from Swedish government grants, the Norwegian Research Council, and the South-Eastern Norway Regional Health Authority.
A version of this article first appeared on Medscape.com.
Patients who receive parathyroidectomy for mild primary hyperparathyroidism show no benefits in survival or morbidity, including fractures, cancer, or cardiovascular outcomes over more than 10 years, compared with those not receiving the surgery, results from a randomized, prospective trial show.
“In contrast to existing data showing increased mortality and cardiovascular morbidity in mild primary hyperparathyroidism, we did not find any treatment effect of parathyroidectomy on these important clinical endpoints,” report the authors of the study, published in the Annals of Internal Medicine.
Reason to evaluate and revise current recommendations?
With mild primary hyperparathyroidism becoming the predominant form of hyperparathyroidism, the results suggest rethinking the current recommendations for the condition, the study authors note.
“Over the years, more active management of mild primary hyperparathyroidism has been recommended, with a widening of criteria for parathyroidectomy,” they write.
“With the low number of kidney stones (n = 5) and no effect of parathyroidectomy on fractures, there may be a need to evaluate and potentially revise the current recommendations.”
The authors of an accompanying editorial agree that “the [results] provide a strong rationale for nonoperative management of patients with mild primary hyperparathyroidism.”
“The findings suggest that most patients can be managed nonoperatively, with monitoring of serum calcium levels every 1 to 2 years or if symptoms occur,” write the editorial authors, Mark J. Bolland, PhD, and Andrew Grey, MD, of the department of medicine, University of Auckland, New Zealand.
Although parathyroidectomy is recommended for the treatment in patients with hyperparathyroidism with severe hypercalcemia or overt symptoms, there has been debate on the long-term benefits of surgery among those with milder cases.
Most previous studies that have shown benefits, such as reductions in the risk of fracture with parathyroidectomy, have importantly not distinguished between mild and more severe primary hyperparathyroidism, the authors note.
No significant differences in mortality between surgery, nonsurgery groups
For the Scandinavian Investigation of Primary Hyperparathyroidism (SIPH) trial, first author Mikkel Pretorius, MD, Oslo University Hospital and Faculty of Medicine, University of Oslo, and colleagues enrolled 191 patients between 1998 and 2005 in Sweden, Norway, and Denmark, who were aged 50-80 years and had mild primary hyperparathyroidism, defined as serum calcium levels of 10.42-11.22 mg/dL.
Participants were randomized to receive surgery (n = 95) or nonoperative observation without intervention (n = 96).
After a 10-year follow-up, 129 patients had completed the final visit. The overall death rate was 7.6%, and, with eight deaths in the surgery group and seven in the nonsurgery group, there were no significant differences between groups in terms of mortality (HR, 1.17; P = .76).
During an extended observation period that lasted until 2018, mortality rates increased by 23%, but with a relatively even distribution of 24 deaths in the surgery group and 20 among those with no surgery.
Chronic hypercalcemia related to primary hyperparathyroidism has been debated as being associated with an increased risk of cardiovascular disease or cancer, however, “the absolute numbers for these and the other disease-specific causes of death were nearly identical between groups,” the authors write, with 17 deaths from cardiovascular disease, eight from cancer, and eight from cerebrovascular disease.
In terms of morbidity, including cardiovascular events, cerebrovascular events, cancer, peripheral fractures, and renal stones, there were 101 events overall, with 52 in the parathyroidectomy group and 49 in the nonsurgery group, which again, was not a significant difference.
Sixteen vertebral fractures occurred overall in 14 patients, which were evenly split at seven patients in each group.
The authors note that “the incidence of peripheral fractures for women in our study was around 2,900 per 100,000 person-years, in the same range as for 70-year-old women in a study in Gothenburg, Sweden (about 2,600 per 100,000 person-years).”
There were no between-group differences in terms of time to death or first morbidity event for any of the prespecified events.
Of the 96 patients originally assigned to the nonsurgery group, 17 (18%) had surgery during follow-up, including three for serious hypercalcemia, three by their own choice, two for decreasing bone density, one for kidney stones, and the others for unclear or unrelated reasons.
Study limitations include that only 26 men (13 in each group) were included, and only 16 completed the study. “The external validity for men based on this study is therefore limited,” the authors note.
And although most people with primary hyperparathyroidism are adults, the older age of participants suggests the results should not be generalized to younger patients with benign parathyroid tumors.
The editorialists note that age should be one of the few factors that may, indeed, suggest appropriate candidates for parathyroidectomy.
“Younger patients (aged < 50 years) may have more aggressive disease,” they explain.
In addition, “patients with serum calcium levels above 3 mmol/L (> 12 mg/dL) are at greater risk for symptomatic hypercalcemia, and patients with a recent history of kidney stones may have fewer future stones after surgical cure.”
“Yet, such patients are a small minority of those with primary hyperparathyroidism,” they note.
The study authors underscore that “our data add evidence to guide the decisionmaking process in deliberative dialogue between clinicians and patients.”
The study received funding from Swedish government grants, the Norwegian Research Council, and the South-Eastern Norway Regional Health Authority.
A version of this article first appeared on Medscape.com.
Patients who receive parathyroidectomy for mild primary hyperparathyroidism show no benefits in survival or morbidity, including fractures, cancer, or cardiovascular outcomes over more than 10 years, compared with those not receiving the surgery, results from a randomized, prospective trial show.
“In contrast to existing data showing increased mortality and cardiovascular morbidity in mild primary hyperparathyroidism, we did not find any treatment effect of parathyroidectomy on these important clinical endpoints,” report the authors of the study, published in the Annals of Internal Medicine.
Reason to evaluate and revise current recommendations?
With mild primary hyperparathyroidism becoming the predominant form of hyperparathyroidism, the results suggest rethinking the current recommendations for the condition, the study authors note.
“Over the years, more active management of mild primary hyperparathyroidism has been recommended, with a widening of criteria for parathyroidectomy,” they write.
“With the low number of kidney stones (n = 5) and no effect of parathyroidectomy on fractures, there may be a need to evaluate and potentially revise the current recommendations.”
The authors of an accompanying editorial agree that “the [results] provide a strong rationale for nonoperative management of patients with mild primary hyperparathyroidism.”
“The findings suggest that most patients can be managed nonoperatively, with monitoring of serum calcium levels every 1 to 2 years or if symptoms occur,” write the editorial authors, Mark J. Bolland, PhD, and Andrew Grey, MD, of the department of medicine, University of Auckland, New Zealand.
Although parathyroidectomy is recommended for the treatment in patients with hyperparathyroidism with severe hypercalcemia or overt symptoms, there has been debate on the long-term benefits of surgery among those with milder cases.
Most previous studies that have shown benefits, such as reductions in the risk of fracture with parathyroidectomy, have importantly not distinguished between mild and more severe primary hyperparathyroidism, the authors note.
No significant differences in mortality between surgery, nonsurgery groups
For the Scandinavian Investigation of Primary Hyperparathyroidism (SIPH) trial, first author Mikkel Pretorius, MD, Oslo University Hospital and Faculty of Medicine, University of Oslo, and colleagues enrolled 191 patients between 1998 and 2005 in Sweden, Norway, and Denmark, who were aged 50-80 years and had mild primary hyperparathyroidism, defined as serum calcium levels of 10.42-11.22 mg/dL.
Participants were randomized to receive surgery (n = 95) or nonoperative observation without intervention (n = 96).
After a 10-year follow-up, 129 patients had completed the final visit. The overall death rate was 7.6%, and, with eight deaths in the surgery group and seven in the nonsurgery group, there were no significant differences between groups in terms of mortality (HR, 1.17; P = .76).
During an extended observation period that lasted until 2018, mortality rates increased by 23%, but with a relatively even distribution of 24 deaths in the surgery group and 20 among those with no surgery.
Chronic hypercalcemia related to primary hyperparathyroidism has been debated as being associated with an increased risk of cardiovascular disease or cancer, however, “the absolute numbers for these and the other disease-specific causes of death were nearly identical between groups,” the authors write, with 17 deaths from cardiovascular disease, eight from cancer, and eight from cerebrovascular disease.
In terms of morbidity, including cardiovascular events, cerebrovascular events, cancer, peripheral fractures, and renal stones, there were 101 events overall, with 52 in the parathyroidectomy group and 49 in the nonsurgery group, which again, was not a significant difference.
Sixteen vertebral fractures occurred overall in 14 patients, which were evenly split at seven patients in each group.
The authors note that “the incidence of peripheral fractures for women in our study was around 2,900 per 100,000 person-years, in the same range as for 70-year-old women in a study in Gothenburg, Sweden (about 2,600 per 100,000 person-years).”
There were no between-group differences in terms of time to death or first morbidity event for any of the prespecified events.
Of the 96 patients originally assigned to the nonsurgery group, 17 (18%) had surgery during follow-up, including three for serious hypercalcemia, three by their own choice, two for decreasing bone density, one for kidney stones, and the others for unclear or unrelated reasons.
Study limitations include that only 26 men (13 in each group) were included, and only 16 completed the study. “The external validity for men based on this study is therefore limited,” the authors note.
And although most people with primary hyperparathyroidism are adults, the older age of participants suggests the results should not be generalized to younger patients with benign parathyroid tumors.
The editorialists note that age should be one of the few factors that may, indeed, suggest appropriate candidates for parathyroidectomy.
“Younger patients (aged < 50 years) may have more aggressive disease,” they explain.
In addition, “patients with serum calcium levels above 3 mmol/L (> 12 mg/dL) are at greater risk for symptomatic hypercalcemia, and patients with a recent history of kidney stones may have fewer future stones after surgical cure.”
“Yet, such patients are a small minority of those with primary hyperparathyroidism,” they note.
The study authors underscore that “our data add evidence to guide the decisionmaking process in deliberative dialogue between clinicians and patients.”
The study received funding from Swedish government grants, the Norwegian Research Council, and the South-Eastern Norway Regional Health Authority.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Children with RMDs not at high risk for severe COVID-19, study finds
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Denosumab boosts bone strength in glucocorticoid users
Bone strength and microarchitecture remained stronger at 24 months after treatment with denosumab compared to risedronate, in a study of 110 adults using glucocorticoids.
Patients using glucocorticoids are at increased risk for vertebral and nonvertebral fractures at both the start of treatment or as treatment continues, wrote Piet Geusens, MD, of Maastricht University, the Netherlands, and colleagues.
Imaging data collected via high-resolution peripheral quantitative computed tomography (HR-pQCT) allow for the assessment of bone microarchitecture and strength, but specific data comparing the impact of bone treatment in patients using glucocorticoids are lacking, they said.
In a study published in the Journal of Bone and Mineral Research, the researchers identified a subset of 56 patients randomized to denosumab and 54 to risedronate patients out of a total of 590 patients who were enrolled in a phase 3 randomized, controlled trial of denosumab vs. risedronate for bone mineral density. The main results of the larger trial – presented at EULAR 2018 – showed greater increases in bone strength with denosumab over risedronate in patients receiving glucocorticoids.
In the current study, the researchers reviewed HR-pQCT scans of the distal radius and tibia at baseline, 12 months, and 24 months. Bone strength and microarchitecture were defined in terms of failure load (FL) as a primary outcome. Patients also were divided into subpopulations of those initiating glucocorticoid treatment (GC-I) and continuing treatment (GC-C).
Baseline characteristics were mainly balanced among the treatment groups within the GC-I and GC-C categories.
Among the GC-I patients, in the denosumab group, FL increased significantly from baseline to 12 months at the radius at tibia (1.8% and 1.7%, respectively) but did not change significantly in the risedronate group, which translated to a significant treatment difference between the drugs of 3.3% for radius and 2.5% for tibia.
At 24 months, the radius measure of FL was unchanged from baseline in denosumab patients but significantly decreased in risedronate patients, with a difference of –4.1%, which translated to a significant between-treatment difference at the radius of 5.6% (P < .001). Changes at the tibia were not significantly different between the groups at 24 months.
Among the GC-C patients, FL was unchanged from baseline to 12 months for both the denosumab and risedronate groups. However, FL significantly increased with denosumab (4.3%) and remained unchanged in the risedronate group.
The researchers also found significant differences between denosumab and risedronate in percentage changes in cortical bone mineral density, and less prominent changes and differences in trabecular bone mineral density.
The study findings were limited by several factors including the use of the HR-pQCT scanner, which limits the measurement of trabecular microarchitecture, and the use of only standard HR-pQCT parameters, which do not allow insight into endosteal changes, and the inability to correct for multiplicity of data, the researchers noted.
However, the results support the superiority of denosumab over risedronate for preventing FL and total bone mineral density loss at the radius and tibia in new glucocorticoid users, and for increasing FL and total bone mineral density at the radius in long-term glucocorticoid users, they said.
Denosumab therefore could be a useful therapeutic option and could inform decision-making in patients initiating GC-therapy or on long-term GC-therapy, they concluded.
The study was supported by Amgen. Dr. Geusens disclosed grants from Amgen, Celgene, Lilly, Merck, Pfizer, Roche, UCB, Fresenius, Mylan, and Sandoz, and grants and other funding from AbbVie, outside the current study.
Bone strength and microarchitecture remained stronger at 24 months after treatment with denosumab compared to risedronate, in a study of 110 adults using glucocorticoids.
Patients using glucocorticoids are at increased risk for vertebral and nonvertebral fractures at both the start of treatment or as treatment continues, wrote Piet Geusens, MD, of Maastricht University, the Netherlands, and colleagues.
Imaging data collected via high-resolution peripheral quantitative computed tomography (HR-pQCT) allow for the assessment of bone microarchitecture and strength, but specific data comparing the impact of bone treatment in patients using glucocorticoids are lacking, they said.
In a study published in the Journal of Bone and Mineral Research, the researchers identified a subset of 56 patients randomized to denosumab and 54 to risedronate patients out of a total of 590 patients who were enrolled in a phase 3 randomized, controlled trial of denosumab vs. risedronate for bone mineral density. The main results of the larger trial – presented at EULAR 2018 – showed greater increases in bone strength with denosumab over risedronate in patients receiving glucocorticoids.
In the current study, the researchers reviewed HR-pQCT scans of the distal radius and tibia at baseline, 12 months, and 24 months. Bone strength and microarchitecture were defined in terms of failure load (FL) as a primary outcome. Patients also were divided into subpopulations of those initiating glucocorticoid treatment (GC-I) and continuing treatment (GC-C).
Baseline characteristics were mainly balanced among the treatment groups within the GC-I and GC-C categories.
Among the GC-I patients, in the denosumab group, FL increased significantly from baseline to 12 months at the radius at tibia (1.8% and 1.7%, respectively) but did not change significantly in the risedronate group, which translated to a significant treatment difference between the drugs of 3.3% for radius and 2.5% for tibia.
At 24 months, the radius measure of FL was unchanged from baseline in denosumab patients but significantly decreased in risedronate patients, with a difference of –4.1%, which translated to a significant between-treatment difference at the radius of 5.6% (P < .001). Changes at the tibia were not significantly different between the groups at 24 months.
Among the GC-C patients, FL was unchanged from baseline to 12 months for both the denosumab and risedronate groups. However, FL significantly increased with denosumab (4.3%) and remained unchanged in the risedronate group.
The researchers also found significant differences between denosumab and risedronate in percentage changes in cortical bone mineral density, and less prominent changes and differences in trabecular bone mineral density.
The study findings were limited by several factors including the use of the HR-pQCT scanner, which limits the measurement of trabecular microarchitecture, and the use of only standard HR-pQCT parameters, which do not allow insight into endosteal changes, and the inability to correct for multiplicity of data, the researchers noted.
However, the results support the superiority of denosumab over risedronate for preventing FL and total bone mineral density loss at the radius and tibia in new glucocorticoid users, and for increasing FL and total bone mineral density at the radius in long-term glucocorticoid users, they said.
Denosumab therefore could be a useful therapeutic option and could inform decision-making in patients initiating GC-therapy or on long-term GC-therapy, they concluded.
The study was supported by Amgen. Dr. Geusens disclosed grants from Amgen, Celgene, Lilly, Merck, Pfizer, Roche, UCB, Fresenius, Mylan, and Sandoz, and grants and other funding from AbbVie, outside the current study.
Bone strength and microarchitecture remained stronger at 24 months after treatment with denosumab compared to risedronate, in a study of 110 adults using glucocorticoids.
Patients using glucocorticoids are at increased risk for vertebral and nonvertebral fractures at both the start of treatment or as treatment continues, wrote Piet Geusens, MD, of Maastricht University, the Netherlands, and colleagues.
Imaging data collected via high-resolution peripheral quantitative computed tomography (HR-pQCT) allow for the assessment of bone microarchitecture and strength, but specific data comparing the impact of bone treatment in patients using glucocorticoids are lacking, they said.
In a study published in the Journal of Bone and Mineral Research, the researchers identified a subset of 56 patients randomized to denosumab and 54 to risedronate patients out of a total of 590 patients who were enrolled in a phase 3 randomized, controlled trial of denosumab vs. risedronate for bone mineral density. The main results of the larger trial – presented at EULAR 2018 – showed greater increases in bone strength with denosumab over risedronate in patients receiving glucocorticoids.
In the current study, the researchers reviewed HR-pQCT scans of the distal radius and tibia at baseline, 12 months, and 24 months. Bone strength and microarchitecture were defined in terms of failure load (FL) as a primary outcome. Patients also were divided into subpopulations of those initiating glucocorticoid treatment (GC-I) and continuing treatment (GC-C).
Baseline characteristics were mainly balanced among the treatment groups within the GC-I and GC-C categories.
Among the GC-I patients, in the denosumab group, FL increased significantly from baseline to 12 months at the radius at tibia (1.8% and 1.7%, respectively) but did not change significantly in the risedronate group, which translated to a significant treatment difference between the drugs of 3.3% for radius and 2.5% for tibia.
At 24 months, the radius measure of FL was unchanged from baseline in denosumab patients but significantly decreased in risedronate patients, with a difference of –4.1%, which translated to a significant between-treatment difference at the radius of 5.6% (P < .001). Changes at the tibia were not significantly different between the groups at 24 months.
Among the GC-C patients, FL was unchanged from baseline to 12 months for both the denosumab and risedronate groups. However, FL significantly increased with denosumab (4.3%) and remained unchanged in the risedronate group.
The researchers also found significant differences between denosumab and risedronate in percentage changes in cortical bone mineral density, and less prominent changes and differences in trabecular bone mineral density.
The study findings were limited by several factors including the use of the HR-pQCT scanner, which limits the measurement of trabecular microarchitecture, and the use of only standard HR-pQCT parameters, which do not allow insight into endosteal changes, and the inability to correct for multiplicity of data, the researchers noted.
However, the results support the superiority of denosumab over risedronate for preventing FL and total bone mineral density loss at the radius and tibia in new glucocorticoid users, and for increasing FL and total bone mineral density at the radius in long-term glucocorticoid users, they said.
Denosumab therefore could be a useful therapeutic option and could inform decision-making in patients initiating GC-therapy or on long-term GC-therapy, they concluded.
The study was supported by Amgen. Dr. Geusens disclosed grants from Amgen, Celgene, Lilly, Merck, Pfizer, Roche, UCB, Fresenius, Mylan, and Sandoz, and grants and other funding from AbbVie, outside the current study.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
JIA disease activity, disability linked to social factors
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRIC RHEUMATOLOGY