User login
Give patients can’ts but also can do’s
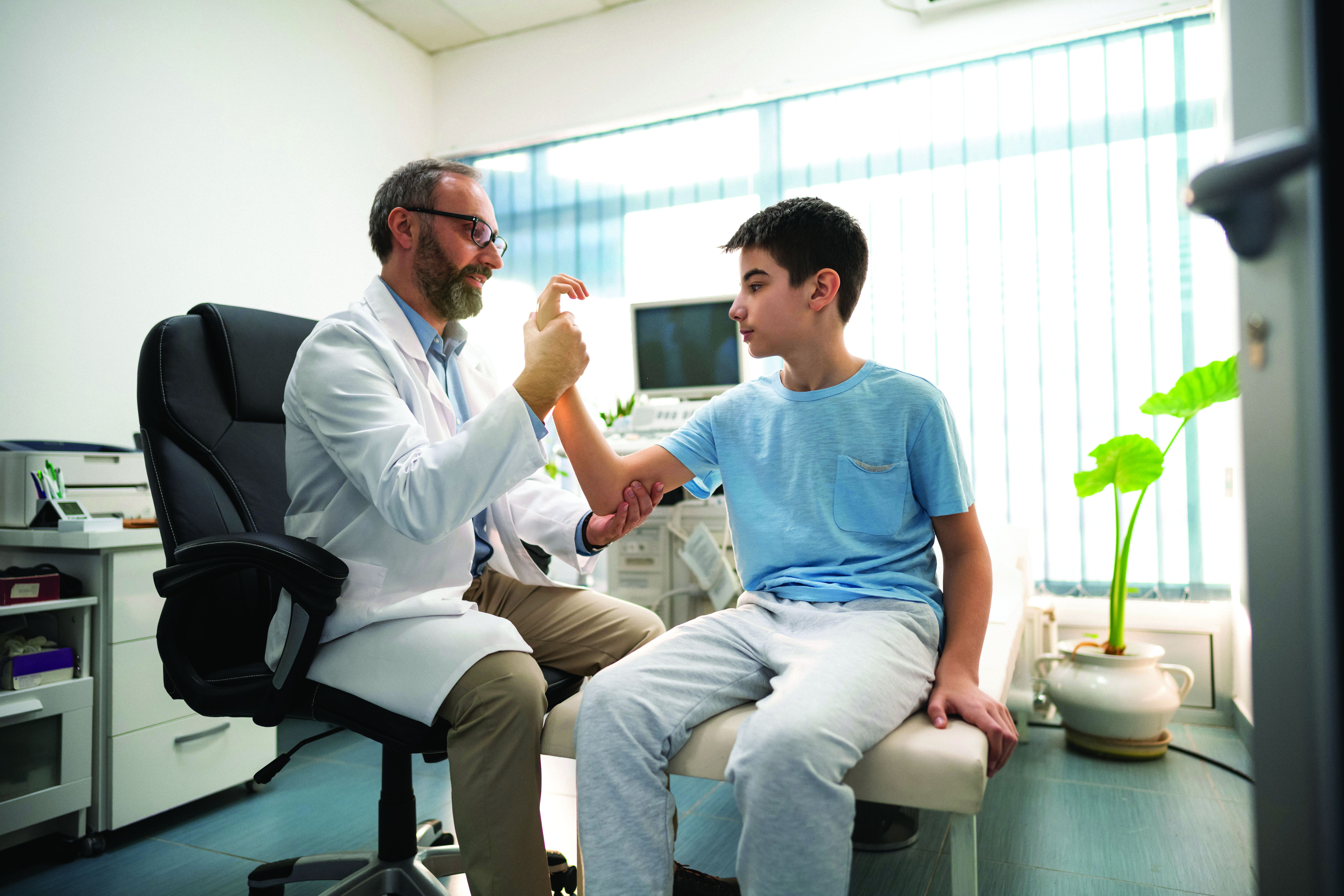
On his last shift in the last hockey game of the regular season, our 14-year-old grandson broke his arm. Although this was his first fracture, the rest of the nuclear family has had ample experience with orthopedic trauma over the last year, both planned and unplanned.
As I drove Peter and my daughter-in-law to his first postsetting and casting appointment I told him how sorry I was that he had been told “no contact sports for the next 3 months.” This was a tough pill for a kid eager to begin his first high school lacrosse season. Then I asked him what the doctor had told him he could do in the way of activity.
Based on personal and professional experience I was not surprised when he told me that no one had suggested things he could be doing. In fact, being a cautious and thoughtful kid, he was concerned about what he should be doing around the house let alone any athletic activities. It turns out he wasn’t even lifting his laptop computer with two hands because some nurse had told him not to lift anything over 2 pounds.
I told him “Peter, even some of the most experienced doctors focus on the ‘can’ts’ and forget to tell you the ‘cans’ and ‘shoulds.’ While you’re in the waiting room make up a mental list of what you would like to be doing that you aren’t.”
As he climbed back in the car for the ride home I asked how the visit went. The x-ray showed good alignment and the doctor was pleased. But, as I predicted, they were already on the launch pad to the receptionist to make a follow-up appointment without the physician uttering a single word about what activities he could resume. Always a very coachable kid, Peter piped up with the list he had created in the waiting room and was relieved to hear that he could do anything as long as it didn’t hurt. In fact, the doctor encouraged him to use his fingers because it might speed the healing.
Not every patient, regardless of age, is as cautious as my grandson and in some circumstances the physician must err on the side of emphasizing the “don’ts.” However, in my experience, too many physicians forget to include a generous list of “can do’s” in their visit closing discussions. This oversight is a mistake for several reasons.
First, and maybe most importantly, even a brief discussion of “can do’s” can soften the depressing message that the patient will not be able to do things he or she enjoys. I can’t quote the references but I am sure there is plenty of evidence that depression slows the healing process.
Second, and this is particularly true in older patients with orthopedic problems – failure to include a plan for return to activity can hinder recovery. I can recall more than a few patients who were seen in the emergency department and diagnosed with sprains but not given even the simplest instructions on how to begin moving the injured joint. When they finally returned to see me we had to begin the painful and unnecessary project of thawing a frozen joint.
Fortunately, we have evolved past the era when best rest was near the top of the list of our recommended remedies. However, there still remains a bias against activity in some situations. The most recent example is the evolving strategies for the management of concussion. There is some evidence that involving the patient in a return to activity plan may shorten the time to recovery. The myth about brain rest has been slow to die.
Finally, providing the patient with a personalized list of “can do’s” makes good business sense because it can head off those time-gobbling call backs that tie up you and your office staff. As an experienced physician, you have probably learned the most frequently asked “Can Jason do ... ?” questions. Make your own list and give the patient your answers. An ounce of anticipatory guidance is worth hours on the telephone or sorting through the email inbox.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
On his last shift in the last hockey game of the regular season, our 14-year-old grandson broke his arm. Although this was his first fracture, the rest of the nuclear family has had ample experience with orthopedic trauma over the last year, both planned and unplanned.
As I drove Peter and my daughter-in-law to his first postsetting and casting appointment I told him how sorry I was that he had been told “no contact sports for the next 3 months.” This was a tough pill for a kid eager to begin his first high school lacrosse season. Then I asked him what the doctor had told him he could do in the way of activity.
Based on personal and professional experience I was not surprised when he told me that no one had suggested things he could be doing. In fact, being a cautious and thoughtful kid, he was concerned about what he should be doing around the house let alone any athletic activities. It turns out he wasn’t even lifting his laptop computer with two hands because some nurse had told him not to lift anything over 2 pounds.
I told him “Peter, even some of the most experienced doctors focus on the ‘can’ts’ and forget to tell you the ‘cans’ and ‘shoulds.’ While you’re in the waiting room make up a mental list of what you would like to be doing that you aren’t.”
As he climbed back in the car for the ride home I asked how the visit went. The x-ray showed good alignment and the doctor was pleased. But, as I predicted, they were already on the launch pad to the receptionist to make a follow-up appointment without the physician uttering a single word about what activities he could resume. Always a very coachable kid, Peter piped up with the list he had created in the waiting room and was relieved to hear that he could do anything as long as it didn’t hurt. In fact, the doctor encouraged him to use his fingers because it might speed the healing.
Not every patient, regardless of age, is as cautious as my grandson and in some circumstances the physician must err on the side of emphasizing the “don’ts.” However, in my experience, too many physicians forget to include a generous list of “can do’s” in their visit closing discussions. This oversight is a mistake for several reasons.
First, and maybe most importantly, even a brief discussion of “can do’s” can soften the depressing message that the patient will not be able to do things he or she enjoys. I can’t quote the references but I am sure there is plenty of evidence that depression slows the healing process.
Second, and this is particularly true in older patients with orthopedic problems – failure to include a plan for return to activity can hinder recovery. I can recall more than a few patients who were seen in the emergency department and diagnosed with sprains but not given even the simplest instructions on how to begin moving the injured joint. When they finally returned to see me we had to begin the painful and unnecessary project of thawing a frozen joint.
Fortunately, we have evolved past the era when best rest was near the top of the list of our recommended remedies. However, there still remains a bias against activity in some situations. The most recent example is the evolving strategies for the management of concussion. There is some evidence that involving the patient in a return to activity plan may shorten the time to recovery. The myth about brain rest has been slow to die.
Finally, providing the patient with a personalized list of “can do’s” makes good business sense because it can head off those time-gobbling call backs that tie up you and your office staff. As an experienced physician, you have probably learned the most frequently asked “Can Jason do ... ?” questions. Make your own list and give the patient your answers. An ounce of anticipatory guidance is worth hours on the telephone or sorting through the email inbox.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
On his last shift in the last hockey game of the regular season, our 14-year-old grandson broke his arm. Although this was his first fracture, the rest of the nuclear family has had ample experience with orthopedic trauma over the last year, both planned and unplanned.
As I drove Peter and my daughter-in-law to his first postsetting and casting appointment I told him how sorry I was that he had been told “no contact sports for the next 3 months.” This was a tough pill for a kid eager to begin his first high school lacrosse season. Then I asked him what the doctor had told him he could do in the way of activity.
Based on personal and professional experience I was not surprised when he told me that no one had suggested things he could be doing. In fact, being a cautious and thoughtful kid, he was concerned about what he should be doing around the house let alone any athletic activities. It turns out he wasn’t even lifting his laptop computer with two hands because some nurse had told him not to lift anything over 2 pounds.
I told him “Peter, even some of the most experienced doctors focus on the ‘can’ts’ and forget to tell you the ‘cans’ and ‘shoulds.’ While you’re in the waiting room make up a mental list of what you would like to be doing that you aren’t.”
As he climbed back in the car for the ride home I asked how the visit went. The x-ray showed good alignment and the doctor was pleased. But, as I predicted, they were already on the launch pad to the receptionist to make a follow-up appointment without the physician uttering a single word about what activities he could resume. Always a very coachable kid, Peter piped up with the list he had created in the waiting room and was relieved to hear that he could do anything as long as it didn’t hurt. In fact, the doctor encouraged him to use his fingers because it might speed the healing.
Not every patient, regardless of age, is as cautious as my grandson and in some circumstances the physician must err on the side of emphasizing the “don’ts.” However, in my experience, too many physicians forget to include a generous list of “can do’s” in their visit closing discussions. This oversight is a mistake for several reasons.
First, and maybe most importantly, even a brief discussion of “can do’s” can soften the depressing message that the patient will not be able to do things he or she enjoys. I can’t quote the references but I am sure there is plenty of evidence that depression slows the healing process.
Second, and this is particularly true in older patients with orthopedic problems – failure to include a plan for return to activity can hinder recovery. I can recall more than a few patients who were seen in the emergency department and diagnosed with sprains but not given even the simplest instructions on how to begin moving the injured joint. When they finally returned to see me we had to begin the painful and unnecessary project of thawing a frozen joint.
Fortunately, we have evolved past the era when best rest was near the top of the list of our recommended remedies. However, there still remains a bias against activity in some situations. The most recent example is the evolving strategies for the management of concussion. There is some evidence that involving the patient in a return to activity plan may shorten the time to recovery. The myth about brain rest has been slow to die.
Finally, providing the patient with a personalized list of “can do’s” makes good business sense because it can head off those time-gobbling call backs that tie up you and your office staff. As an experienced physician, you have probably learned the most frequently asked “Can Jason do ... ?” questions. Make your own list and give the patient your answers. An ounce of anticipatory guidance is worth hours on the telephone or sorting through the email inbox.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Virtual and in-person pediatric visits get similar family ratings
CHICAGO – Satisfaction ratings for virtual outpatient visits for pediatric orthopedic patients were similar to those for in-person office visits across most categories in an analysis of postencounter surveys completed by patients at the Cleveland Clinic.
Satisfaction ratings for both virtual and office visits were consistently higher than 85% across all measured parameters, according to the data presented at the annual meeting of the American Academy of Orthopaedic Surgeons.
Ahmed Emara, MD, a clinical research fellow in adult joint reconstruction at the Cleveland Clinic, led the study, which included data from all patients or guardians at the clinic who experienced such visits from March 2020 to March 2021.
A total of 1,686 responses were received, of which 226 (13.4%) involved virtual visits and 1,460 (86.6%) involved in-office visits. The primary endpoint was a patient-reported satisfaction score of good or excellent.
Analysis included ratings for access, care provider, telemedicine technology, and overall assessment/perception of satisfaction.
Target areas for improvement
In some areas, the virtual visits were less satisfactory than the in-office visits.
Patients had lower odds of reporting good/excellent satisfaction regarding their ability to schedule at a particularly convenient time (odds ratio, 0.1; 95% confidence interval, 0.08-0.18; P < .001). The study authors said scheduling more virtual time slots may help increase satisfaction in that area.
Satisfaction was also lower than with in-office visits with respect to providers’ explanations of patients’ conditions (OR, 0.4; 95% CI, 0.17-0.91; P = .03). Providers may need to find ways to better provide educational material in addition to the virtual consultation, the authors wrote.
No significant differences in categories of satisfaction
The researchers accounted for age, sex, traumatic etiology, and anatomic location of the complaint in multivariate regression analysis and found no significant differences between the two types of visits in the odds of getting a good/excellent rating for the following areas: patient inclusion in treatment decision (P = .562), discussion of proposed treatment (P = .222), concern by the provider (P = .189), degree of care for the patient as a person (P = .208), adequacy of teamwork in care provision (P = .053), likelihood of recommending the practice to others (P = .108), ease of receiving care at a particular practice (P = .109), ease of contacting the clinic (P = .177), and likelihood of recommending a particular provider (P = .218).
Anna Dimitriovna Vergun, MD, a pediatric orthopedist at the University of North Carolina at Chapel Hill, who was not involved in the study, said in an interview she had been conducting virtual visits even before the pandemic, when she worked for several years at a Shriner’s children’s hospital in Los Angeles, before coming to UNC. The virtual visits were necessary because the hospital offered charity care and covered an area that included several states.
She said that during the height of the pandemic, 80% of her visits at UNC were virtual; it is down to about 5% now.
Some consultations don’t need physical visits at all, Dr. Vergun noted. For example, UNC is starting a clinic for prenatal counseling in cases in which ultrasound detects a limb deformity. Without a virtual option, she said, pregnant mothers in all parts of the state may have to drive long distances when no physical exam is necessary.
And sometimes, a visit simply involves checking in with families to see whether pain is being controlled, which is done well virtually.
“Those are particularly useful for telemedicine,” Dr. Vergun said. “There’s a lot of space for this to be useful. You sometimes don’t realize it until you start doing it and getting feedback from the families that they like it.”
Other exams may be better suited to office visits, she said. These include spine and hip exams and exams in which providers need to check reflexes.
She said she sees many cases of club feet, for which an in-person exam is needed to determine flexibility.
Expert says virtual misses nuances
Ryan Fitzgerald, MD, an orthopedic expert with Children’s Orthopaedic and Scoliosis Surgery Associates in St. Petersburg, Fla., who also was not involved in the study, said in an interview he doesn’t offer the virtual option now because he thinks those visits usually miss too much.
COSSA is a private practice that provides orthopedic services for Johns Hopkins All Children’s Hospital.
“I think physicians’ perspective versus the families’ perspective may be quite a bit different,” he said.
While families like the convenience, “a lot of what we do is watching the patient walk, looking at their hip range of motion, and virtually, that’s a really difficult thing to do,” he said.
You can instruct a family on how to turn a camera on the patient, but “it doesn’t always translate,” he said.
He said virtual visits also highlight disparities in access, because many families don’t own the hardware needed for such visits, and internet connections can be spotty or images pixelated.
Dr. Fitzgerald said virtual visits were helpful during the pandemic and would be beneficial for yearly checkups “if you know [the patient] well and it’s a fairly run-of-the-mill thing.”
However, he said, “everything we do is about human interaction, and I think that’s a downfall of the virtual platform right now. While it is helpful in situations like COVID and where it is a very basic follow-up, it still has a ways to go.”
Dr. Fitzgerald is a consultant for OrthoPediatrics, Medtronic, and Depuy Synthes. Dr. Vergun disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Satisfaction ratings for virtual outpatient visits for pediatric orthopedic patients were similar to those for in-person office visits across most categories in an analysis of postencounter surveys completed by patients at the Cleveland Clinic.
Satisfaction ratings for both virtual and office visits were consistently higher than 85% across all measured parameters, according to the data presented at the annual meeting of the American Academy of Orthopaedic Surgeons.
Ahmed Emara, MD, a clinical research fellow in adult joint reconstruction at the Cleveland Clinic, led the study, which included data from all patients or guardians at the clinic who experienced such visits from March 2020 to March 2021.
A total of 1,686 responses were received, of which 226 (13.4%) involved virtual visits and 1,460 (86.6%) involved in-office visits. The primary endpoint was a patient-reported satisfaction score of good or excellent.
Analysis included ratings for access, care provider, telemedicine technology, and overall assessment/perception of satisfaction.
Target areas for improvement
In some areas, the virtual visits were less satisfactory than the in-office visits.
Patients had lower odds of reporting good/excellent satisfaction regarding their ability to schedule at a particularly convenient time (odds ratio, 0.1; 95% confidence interval, 0.08-0.18; P < .001). The study authors said scheduling more virtual time slots may help increase satisfaction in that area.
Satisfaction was also lower than with in-office visits with respect to providers’ explanations of patients’ conditions (OR, 0.4; 95% CI, 0.17-0.91; P = .03). Providers may need to find ways to better provide educational material in addition to the virtual consultation, the authors wrote.
No significant differences in categories of satisfaction
The researchers accounted for age, sex, traumatic etiology, and anatomic location of the complaint in multivariate regression analysis and found no significant differences between the two types of visits in the odds of getting a good/excellent rating for the following areas: patient inclusion in treatment decision (P = .562), discussion of proposed treatment (P = .222), concern by the provider (P = .189), degree of care for the patient as a person (P = .208), adequacy of teamwork in care provision (P = .053), likelihood of recommending the practice to others (P = .108), ease of receiving care at a particular practice (P = .109), ease of contacting the clinic (P = .177), and likelihood of recommending a particular provider (P = .218).
Anna Dimitriovna Vergun, MD, a pediatric orthopedist at the University of North Carolina at Chapel Hill, who was not involved in the study, said in an interview she had been conducting virtual visits even before the pandemic, when she worked for several years at a Shriner’s children’s hospital in Los Angeles, before coming to UNC. The virtual visits were necessary because the hospital offered charity care and covered an area that included several states.
She said that during the height of the pandemic, 80% of her visits at UNC were virtual; it is down to about 5% now.
Some consultations don’t need physical visits at all, Dr. Vergun noted. For example, UNC is starting a clinic for prenatal counseling in cases in which ultrasound detects a limb deformity. Without a virtual option, she said, pregnant mothers in all parts of the state may have to drive long distances when no physical exam is necessary.
And sometimes, a visit simply involves checking in with families to see whether pain is being controlled, which is done well virtually.
“Those are particularly useful for telemedicine,” Dr. Vergun said. “There’s a lot of space for this to be useful. You sometimes don’t realize it until you start doing it and getting feedback from the families that they like it.”
Other exams may be better suited to office visits, she said. These include spine and hip exams and exams in which providers need to check reflexes.
She said she sees many cases of club feet, for which an in-person exam is needed to determine flexibility.
Expert says virtual misses nuances
Ryan Fitzgerald, MD, an orthopedic expert with Children’s Orthopaedic and Scoliosis Surgery Associates in St. Petersburg, Fla., who also was not involved in the study, said in an interview he doesn’t offer the virtual option now because he thinks those visits usually miss too much.
COSSA is a private practice that provides orthopedic services for Johns Hopkins All Children’s Hospital.
“I think physicians’ perspective versus the families’ perspective may be quite a bit different,” he said.
While families like the convenience, “a lot of what we do is watching the patient walk, looking at their hip range of motion, and virtually, that’s a really difficult thing to do,” he said.
You can instruct a family on how to turn a camera on the patient, but “it doesn’t always translate,” he said.
He said virtual visits also highlight disparities in access, because many families don’t own the hardware needed for such visits, and internet connections can be spotty or images pixelated.
Dr. Fitzgerald said virtual visits were helpful during the pandemic and would be beneficial for yearly checkups “if you know [the patient] well and it’s a fairly run-of-the-mill thing.”
However, he said, “everything we do is about human interaction, and I think that’s a downfall of the virtual platform right now. While it is helpful in situations like COVID and where it is a very basic follow-up, it still has a ways to go.”
Dr. Fitzgerald is a consultant for OrthoPediatrics, Medtronic, and Depuy Synthes. Dr. Vergun disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Satisfaction ratings for virtual outpatient visits for pediatric orthopedic patients were similar to those for in-person office visits across most categories in an analysis of postencounter surveys completed by patients at the Cleveland Clinic.
Satisfaction ratings for both virtual and office visits were consistently higher than 85% across all measured parameters, according to the data presented at the annual meeting of the American Academy of Orthopaedic Surgeons.
Ahmed Emara, MD, a clinical research fellow in adult joint reconstruction at the Cleveland Clinic, led the study, which included data from all patients or guardians at the clinic who experienced such visits from March 2020 to March 2021.
A total of 1,686 responses were received, of which 226 (13.4%) involved virtual visits and 1,460 (86.6%) involved in-office visits. The primary endpoint was a patient-reported satisfaction score of good or excellent.
Analysis included ratings for access, care provider, telemedicine technology, and overall assessment/perception of satisfaction.
Target areas for improvement
In some areas, the virtual visits were less satisfactory than the in-office visits.
Patients had lower odds of reporting good/excellent satisfaction regarding their ability to schedule at a particularly convenient time (odds ratio, 0.1; 95% confidence interval, 0.08-0.18; P < .001). The study authors said scheduling more virtual time slots may help increase satisfaction in that area.
Satisfaction was also lower than with in-office visits with respect to providers’ explanations of patients’ conditions (OR, 0.4; 95% CI, 0.17-0.91; P = .03). Providers may need to find ways to better provide educational material in addition to the virtual consultation, the authors wrote.
No significant differences in categories of satisfaction
The researchers accounted for age, sex, traumatic etiology, and anatomic location of the complaint in multivariate regression analysis and found no significant differences between the two types of visits in the odds of getting a good/excellent rating for the following areas: patient inclusion in treatment decision (P = .562), discussion of proposed treatment (P = .222), concern by the provider (P = .189), degree of care for the patient as a person (P = .208), adequacy of teamwork in care provision (P = .053), likelihood of recommending the practice to others (P = .108), ease of receiving care at a particular practice (P = .109), ease of contacting the clinic (P = .177), and likelihood of recommending a particular provider (P = .218).
Anna Dimitriovna Vergun, MD, a pediatric orthopedist at the University of North Carolina at Chapel Hill, who was not involved in the study, said in an interview she had been conducting virtual visits even before the pandemic, when she worked for several years at a Shriner’s children’s hospital in Los Angeles, before coming to UNC. The virtual visits were necessary because the hospital offered charity care and covered an area that included several states.
She said that during the height of the pandemic, 80% of her visits at UNC were virtual; it is down to about 5% now.
Some consultations don’t need physical visits at all, Dr. Vergun noted. For example, UNC is starting a clinic for prenatal counseling in cases in which ultrasound detects a limb deformity. Without a virtual option, she said, pregnant mothers in all parts of the state may have to drive long distances when no physical exam is necessary.
And sometimes, a visit simply involves checking in with families to see whether pain is being controlled, which is done well virtually.
“Those are particularly useful for telemedicine,” Dr. Vergun said. “There’s a lot of space for this to be useful. You sometimes don’t realize it until you start doing it and getting feedback from the families that they like it.”
Other exams may be better suited to office visits, she said. These include spine and hip exams and exams in which providers need to check reflexes.
She said she sees many cases of club feet, for which an in-person exam is needed to determine flexibility.
Expert says virtual misses nuances
Ryan Fitzgerald, MD, an orthopedic expert with Children’s Orthopaedic and Scoliosis Surgery Associates in St. Petersburg, Fla., who also was not involved in the study, said in an interview he doesn’t offer the virtual option now because he thinks those visits usually miss too much.
COSSA is a private practice that provides orthopedic services for Johns Hopkins All Children’s Hospital.
“I think physicians’ perspective versus the families’ perspective may be quite a bit different,” he said.
While families like the convenience, “a lot of what we do is watching the patient walk, looking at their hip range of motion, and virtually, that’s a really difficult thing to do,” he said.
You can instruct a family on how to turn a camera on the patient, but “it doesn’t always translate,” he said.
He said virtual visits also highlight disparities in access, because many families don’t own the hardware needed for such visits, and internet connections can be spotty or images pixelated.
Dr. Fitzgerald said virtual visits were helpful during the pandemic and would be beneficial for yearly checkups “if you know [the patient] well and it’s a fairly run-of-the-mill thing.”
However, he said, “everything we do is about human interaction, and I think that’s a downfall of the virtual platform right now. While it is helpful in situations like COVID and where it is a very basic follow-up, it still has a ways to go.”
Dr. Fitzgerald is a consultant for OrthoPediatrics, Medtronic, and Depuy Synthes. Dr. Vergun disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAOS 2022
Doctors treat osteoporosis with hormone therapy against guidelines
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
New JIA guidelines emphasize earlier DMARD use
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Eighteen-year study shows inconsistencies in treating, classifying JIA
“Children are not little adults” is a common refrain in pediatric medicine, but when it comes to a condition like juvenile idiopathic arthritis (JIA), rheumatologists might be better off treating pediatric and adult rheumatic disease more similarly.
A recent study published in Arthritis Care & Research followed children diagnosed with JIA for 18 years. Although not the first long-term study to examine children with JIA, it is unique in that it took place “during a time where biologic DMARDs [disease-modifying antirheumatic drugs] were emerging as a fundamental therapy in the management of children with JIA,” said Dawn M. Wahezi, MD, chief of the division of pediatric rheumatology at the Children’s Hospital at Montefiore in New York, who was not involved with the study.
Additionally, the study highlights the International League of Associations for Rheumatology (ILAR) consensus-based classification criteria as an imperfect method to categorize patients with JIA.
Mia Glerup, MD, PhD, of the department of pediatrics at Aarhus (Denmark) University Hospital and colleagues prospectively analyzed 373 patients from Denmark, Norway, Sweden, and Finland with new-onset JIA between 1997 and 2000 and evaluated them at baseline, 8 years, and 18 years. At each visit, the researchers collected data on demographics, disease activity, ILAR category, treatment, and blood samples.
Patients in the cohort were mostly girls (66.7%) with a median age of 5.9 years at onset. Approximately one-third (34.8%) of patients were antinuclear antibody (ANA) positive and 21.6% were HLA-B27 positive. The most common JIA categories at baseline were persistent oligoarthritis (53.9%), polyarticular rheumatoid factor (RF) negative (21.1%), and undifferentiated arthritis (10.2%).
Dr. Glerup and colleagues found that the proportion of patients not receiving DMARDs declined from 73.2% at baseline to 59.7% at 8 years, and then rose again to 70% at 18 years (risk ratio, 1.3; P = .003). The group of 103 patients who used conventional DMARDs (cDMARDs) either as monotherapy or in combination with a biologic DMARD (bDMARD) at 8 years dwindled to 44 (42.7%) at 18 years (RR, 0.4; P < .001), whereas 32 of 52 patients (61.5%) using bDMARDs at 8 years were still taking them at 18 years (RR, 0.6; P = .02). Across the whole study, 14.7% of patients never received any JIA treatment, and 33 of 85 patients (38.8%) on continuous DMARDs developed uveitis during the study period.
Overall, 62.7% of patients received DMARDs at least once, including 89.7% with polyarticular RF negative, 77.3% with oligoarticular extended, 76.9% with systemic, 75.7% with juvenile enthesitis-related arthritis (ERA), 66.7% with polyarticular RF-positive, 65.2% with juvenile psoriatic arthritis (JPsA), 58.9% with undifferentiated JIA, and 27.6% of patients with persistent oligoarticular disease.
The median number of active joints dropped from 3 (range, 1-30) at baseline to 0 at 8 years (range, 0-13), whereas the median cumulative number of affected joints rose from 3 at baseline (range, 1-30) to 6 at 8 years (range, 1-41). At last follow-up, the median number of active joints was 0 (range, 0-5) and median cumulative number of affected joints was 7 (range, 1-47). The percentage of patients in remission barely changed from 52% at 8 years to 51% at 18.
Some patients also changed ILAR categories during the study period, with 7% shifting between baseline and 8 years, and 11% shifting between 8-year and 18-year follow-up. Compared with baseline, by the 18-year follow-up time point there was a significant decrease in the number of patients categorized as oligoarticular (230 vs. 197 patients; P = .02), a significant increase in patients in the psoriatic ILAR category (8 vs. 28 patients; P < .001), and a nonsignificant increase in the number of patients in the undifferentiated category (45 vs. 63 patients; P = .06).
“Almost half of the changes in the distribution between the ILAR categories were caused by updated information on heredity in a first-degree relative obtained at the follow-up visits,” Dr. Glerup and colleagues write.
The results of the long-term study show that patients are “likely to remain in remission – with the converse also evident, as patients still with evidence of disease activity at 8 years after disease onset were more likely to have refractory disease,” Dr. Wahezi said.
Commenting on the study’s findings, Lisa F. Imundo, MD, director of adolescent rheumatology at Columbia University Medical Center in New York, said they are “great news to be able to give parents of young kids with arthritis.” However, she questioned whether the results are generalizable to populations of patients “who are in the worst prognostic group.”
For example, a substantial proportion of patients were classified under the oligoarticular category. “That’s already a group that we know from experience tends to have a better outcome than some of the other groups of JIA,” she said.
“That kind of weaves its way through the whole study, because then they show a lot of patients have come off their medication. Patients who had more severe disease in more joints would be less likely, I think, to just stop their medication and stop going to doctors,” Dr. Imundo explained.
Although the study is valuable for its long-term follow-up, there is also a question of generalizability across a more diverse ethnic and racial group. The authors do not elaborate on the racial breakdown of their patients, Dr. Imundo said, “so we’re going to have to assume that the vast majority are going to [have] Caucasian Nordic ethnic background, and that goes along with them having this high percentage of HLA-B27 positivity, which is a gene that’s more prevalent in northern European populations.”
Jonathan Hausmann, MD, a pediatric and adult rheumatologist at Boston Children’s Hospital, Boston,, told this news organization that he believes the overall conclusions from the study – that JIA persists over time and that ILAR classification is a somewhat imprecise measure of assessing JIA types in children – would be generalizable to other groups.
However, long-term registries evaluating JIA in more diverse populations, such as the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, could confirm these results, said Dr. Hausmann, who is a registry informatics associate with CARRA and was not associated with the research.
Long-term management of JIA
In an accompanying editorial, Jaime Guzman, MD, MSc, and Ross E. Petty, MD, PhD, of British Columbia Children’s Hospital and the University of British Columbia, Vancouver, said a rheumatologist’s interpretation of the study would be tied to what they learned about children with arthritis in medical school. They would see the glass as “half full” if children who achieved remission stayed in remission if they learned that a child might end up outgrowing JIA but potentially develop lifelong disability, whereas others may focus on the outcome of approximately half of patients not achieving remission.
“When I was going through medical school, I remember learning that JIA is a disease of children, and typically, they outgrow it as they become adults,” Dr. Hausmann said. “I think this study and many other studies have shown that that’s actually not the case – that, in fact, it may be a majority of kids continue having active disease even through adulthood.”
If a rheumatologist knows JIA is likely to continue into adulthood, “that’s huge,” Dr. Hausmann said. “That means when we first diagnose patients with JIA as kids, we need to set expectations with the families that this may not just go away; this may be something that could be more lifelong.”
Education on the part of the patient, their parents, and their clinician on the expected trajectory of the disease is critical so that children can continue their own care as they transition to adulthood, Dr. Hausmann explained. “The earlier the kids develop the skills to discuss their medicines, their side effects, the better they’ll be able to transition to adult medicine,” he said.
For the patients who go into remission and stay in remission, the message is also important. “To have the reassurance that a lot of those kids won’t be having active joint symptoms or need to be on medication, that’s a huge positive message that can get out there, so I think that’s great,” Dr. Imundo said.
Time to move on from ILAR classification?
Another big takeaway from the study was how patients’ ILAR classification changed across the 18-year follow-up. First proposed in 1995, the JIA ILAR classification has been revised several times for clarification purposes. In its current form, the ILAR classification considers a patient’s history when categorizing JIA types but also includes factors such as immediate family history. This system of assessing JIA has been criticized and there are initiatives to create a new JIA classification system to replace it.
“The ILAR criteria were designed to classify patients 6 months after disease onset in an attempt to find some commonality in clinical phenotypes, prognosis, and suggested management,” Dr. Wahezi said. “While there continues to be debate as to whether we can improve our classification of JIA patients, it is not surprising that phenotypes may evolve over time as new clinical features develop. As pediatric rheumatologists, we are well accustomed to having to modify management plans as children manifest with new clinical features over time.”
Although the percentage of patients who switched ILAR classifications over the study period was “much higher” than she would have thought, Dr. Imundo said it was the reasons provided in the study that seemed odd to her. “The classification scheme relies on your family history, like someone else in your family now has psoriasis, so your arthritis classification changes,” she explained.
“We want to head toward a much more unified classification scheme, a simpler one. We now understand that some of the diseases that we see in pediatrics are really the equivalent or same disease in adults,” she said.
“Most of the pediatric categories of JIA have distinct adult correlates,” Dr. Hausmann agreed. RF-positive polyarthritis in children and rheumatoid arthritis in adults are correlated, as are systemic JIA and adult-onset Still’s disease, he explained. “That has been borne out also by genetic susceptibility studies that the genetic predispositions to systemic arthritis in children is the same as the genetic predisposition to adult-onset Still’s disease in adults. By and large, there are a lot of similarities between the two.
“I think we need to incorporate some of that knowledge in better classifying kids with JIA so that we can find the best treatments and the best outcomes, and we can provide information to families about the expected course of the disease over time so that can inform our discussions.”
Some pediatric rheumatologists accept the classification system is flawed, but not all concur with the degree to which these problems impact patient care. “While the ILAR classification criteria may be subject to criticism, it does provide general context and prognostic implications for patients and families,” Dr. Wahezi said.
“The medicines certainly are very similar across the JIA categories, so the implications are not as broad” when classification changes,” Dr. Hausmann said. “But it certainly shows that there are things that we still don’t know. I think classification is actually pretty important because it might give you a sense of how persistent the disease will be.”
Dr. Imundo said the ILAR classification’s “time is limited,” and rheumatologists may soon need to adopt a new way of classifying children with rheumatic disease – “a more data-driven, genetics-driven scheme.”
“These categories are so imperfect, and the patients are changing. I feel like that says to me, let’s find something that’s more predictive that really helps us a little better than what we have now,” she said.
The study had no specific funding. The authors of the study and the editorial have disclosed no relevant financial relationships. Dr. Hausmann reports receiving salary support from CARRA. Dr. Imundo and Dr. Wahezi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Children are not little adults” is a common refrain in pediatric medicine, but when it comes to a condition like juvenile idiopathic arthritis (JIA), rheumatologists might be better off treating pediatric and adult rheumatic disease more similarly.
A recent study published in Arthritis Care & Research followed children diagnosed with JIA for 18 years. Although not the first long-term study to examine children with JIA, it is unique in that it took place “during a time where biologic DMARDs [disease-modifying antirheumatic drugs] were emerging as a fundamental therapy in the management of children with JIA,” said Dawn M. Wahezi, MD, chief of the division of pediatric rheumatology at the Children’s Hospital at Montefiore in New York, who was not involved with the study.
Additionally, the study highlights the International League of Associations for Rheumatology (ILAR) consensus-based classification criteria as an imperfect method to categorize patients with JIA.
Mia Glerup, MD, PhD, of the department of pediatrics at Aarhus (Denmark) University Hospital and colleagues prospectively analyzed 373 patients from Denmark, Norway, Sweden, and Finland with new-onset JIA between 1997 and 2000 and evaluated them at baseline, 8 years, and 18 years. At each visit, the researchers collected data on demographics, disease activity, ILAR category, treatment, and blood samples.
Patients in the cohort were mostly girls (66.7%) with a median age of 5.9 years at onset. Approximately one-third (34.8%) of patients were antinuclear antibody (ANA) positive and 21.6% were HLA-B27 positive. The most common JIA categories at baseline were persistent oligoarthritis (53.9%), polyarticular rheumatoid factor (RF) negative (21.1%), and undifferentiated arthritis (10.2%).
Dr. Glerup and colleagues found that the proportion of patients not receiving DMARDs declined from 73.2% at baseline to 59.7% at 8 years, and then rose again to 70% at 18 years (risk ratio, 1.3; P = .003). The group of 103 patients who used conventional DMARDs (cDMARDs) either as monotherapy or in combination with a biologic DMARD (bDMARD) at 8 years dwindled to 44 (42.7%) at 18 years (RR, 0.4; P < .001), whereas 32 of 52 patients (61.5%) using bDMARDs at 8 years were still taking them at 18 years (RR, 0.6; P = .02). Across the whole study, 14.7% of patients never received any JIA treatment, and 33 of 85 patients (38.8%) on continuous DMARDs developed uveitis during the study period.
Overall, 62.7% of patients received DMARDs at least once, including 89.7% with polyarticular RF negative, 77.3% with oligoarticular extended, 76.9% with systemic, 75.7% with juvenile enthesitis-related arthritis (ERA), 66.7% with polyarticular RF-positive, 65.2% with juvenile psoriatic arthritis (JPsA), 58.9% with undifferentiated JIA, and 27.6% of patients with persistent oligoarticular disease.
The median number of active joints dropped from 3 (range, 1-30) at baseline to 0 at 8 years (range, 0-13), whereas the median cumulative number of affected joints rose from 3 at baseline (range, 1-30) to 6 at 8 years (range, 1-41). At last follow-up, the median number of active joints was 0 (range, 0-5) and median cumulative number of affected joints was 7 (range, 1-47). The percentage of patients in remission barely changed from 52% at 8 years to 51% at 18.
Some patients also changed ILAR categories during the study period, with 7% shifting between baseline and 8 years, and 11% shifting between 8-year and 18-year follow-up. Compared with baseline, by the 18-year follow-up time point there was a significant decrease in the number of patients categorized as oligoarticular (230 vs. 197 patients; P = .02), a significant increase in patients in the psoriatic ILAR category (8 vs. 28 patients; P < .001), and a nonsignificant increase in the number of patients in the undifferentiated category (45 vs. 63 patients; P = .06).
“Almost half of the changes in the distribution between the ILAR categories were caused by updated information on heredity in a first-degree relative obtained at the follow-up visits,” Dr. Glerup and colleagues write.
The results of the long-term study show that patients are “likely to remain in remission – with the converse also evident, as patients still with evidence of disease activity at 8 years after disease onset were more likely to have refractory disease,” Dr. Wahezi said.
Commenting on the study’s findings, Lisa F. Imundo, MD, director of adolescent rheumatology at Columbia University Medical Center in New York, said they are “great news to be able to give parents of young kids with arthritis.” However, she questioned whether the results are generalizable to populations of patients “who are in the worst prognostic group.”
For example, a substantial proportion of patients were classified under the oligoarticular category. “That’s already a group that we know from experience tends to have a better outcome than some of the other groups of JIA,” she said.
“That kind of weaves its way through the whole study, because then they show a lot of patients have come off their medication. Patients who had more severe disease in more joints would be less likely, I think, to just stop their medication and stop going to doctors,” Dr. Imundo explained.
Although the study is valuable for its long-term follow-up, there is also a question of generalizability across a more diverse ethnic and racial group. The authors do not elaborate on the racial breakdown of their patients, Dr. Imundo said, “so we’re going to have to assume that the vast majority are going to [have] Caucasian Nordic ethnic background, and that goes along with them having this high percentage of HLA-B27 positivity, which is a gene that’s more prevalent in northern European populations.”
Jonathan Hausmann, MD, a pediatric and adult rheumatologist at Boston Children’s Hospital, Boston,, told this news organization that he believes the overall conclusions from the study – that JIA persists over time and that ILAR classification is a somewhat imprecise measure of assessing JIA types in children – would be generalizable to other groups.
However, long-term registries evaluating JIA in more diverse populations, such as the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, could confirm these results, said Dr. Hausmann, who is a registry informatics associate with CARRA and was not associated with the research.
Long-term management of JIA
In an accompanying editorial, Jaime Guzman, MD, MSc, and Ross E. Petty, MD, PhD, of British Columbia Children’s Hospital and the University of British Columbia, Vancouver, said a rheumatologist’s interpretation of the study would be tied to what they learned about children with arthritis in medical school. They would see the glass as “half full” if children who achieved remission stayed in remission if they learned that a child might end up outgrowing JIA but potentially develop lifelong disability, whereas others may focus on the outcome of approximately half of patients not achieving remission.
“When I was going through medical school, I remember learning that JIA is a disease of children, and typically, they outgrow it as they become adults,” Dr. Hausmann said. “I think this study and many other studies have shown that that’s actually not the case – that, in fact, it may be a majority of kids continue having active disease even through adulthood.”
If a rheumatologist knows JIA is likely to continue into adulthood, “that’s huge,” Dr. Hausmann said. “That means when we first diagnose patients with JIA as kids, we need to set expectations with the families that this may not just go away; this may be something that could be more lifelong.”
Education on the part of the patient, their parents, and their clinician on the expected trajectory of the disease is critical so that children can continue their own care as they transition to adulthood, Dr. Hausmann explained. “The earlier the kids develop the skills to discuss their medicines, their side effects, the better they’ll be able to transition to adult medicine,” he said.
For the patients who go into remission and stay in remission, the message is also important. “To have the reassurance that a lot of those kids won’t be having active joint symptoms or need to be on medication, that’s a huge positive message that can get out there, so I think that’s great,” Dr. Imundo said.
Time to move on from ILAR classification?
Another big takeaway from the study was how patients’ ILAR classification changed across the 18-year follow-up. First proposed in 1995, the JIA ILAR classification has been revised several times for clarification purposes. In its current form, the ILAR classification considers a patient’s history when categorizing JIA types but also includes factors such as immediate family history. This system of assessing JIA has been criticized and there are initiatives to create a new JIA classification system to replace it.
“The ILAR criteria were designed to classify patients 6 months after disease onset in an attempt to find some commonality in clinical phenotypes, prognosis, and suggested management,” Dr. Wahezi said. “While there continues to be debate as to whether we can improve our classification of JIA patients, it is not surprising that phenotypes may evolve over time as new clinical features develop. As pediatric rheumatologists, we are well accustomed to having to modify management plans as children manifest with new clinical features over time.”
Although the percentage of patients who switched ILAR classifications over the study period was “much higher” than she would have thought, Dr. Imundo said it was the reasons provided in the study that seemed odd to her. “The classification scheme relies on your family history, like someone else in your family now has psoriasis, so your arthritis classification changes,” she explained.
“We want to head toward a much more unified classification scheme, a simpler one. We now understand that some of the diseases that we see in pediatrics are really the equivalent or same disease in adults,” she said.
“Most of the pediatric categories of JIA have distinct adult correlates,” Dr. Hausmann agreed. RF-positive polyarthritis in children and rheumatoid arthritis in adults are correlated, as are systemic JIA and adult-onset Still’s disease, he explained. “That has been borne out also by genetic susceptibility studies that the genetic predispositions to systemic arthritis in children is the same as the genetic predisposition to adult-onset Still’s disease in adults. By and large, there are a lot of similarities between the two.
“I think we need to incorporate some of that knowledge in better classifying kids with JIA so that we can find the best treatments and the best outcomes, and we can provide information to families about the expected course of the disease over time so that can inform our discussions.”
Some pediatric rheumatologists accept the classification system is flawed, but not all concur with the degree to which these problems impact patient care. “While the ILAR classification criteria may be subject to criticism, it does provide general context and prognostic implications for patients and families,” Dr. Wahezi said.
“The medicines certainly are very similar across the JIA categories, so the implications are not as broad” when classification changes,” Dr. Hausmann said. “But it certainly shows that there are things that we still don’t know. I think classification is actually pretty important because it might give you a sense of how persistent the disease will be.”
Dr. Imundo said the ILAR classification’s “time is limited,” and rheumatologists may soon need to adopt a new way of classifying children with rheumatic disease – “a more data-driven, genetics-driven scheme.”
“These categories are so imperfect, and the patients are changing. I feel like that says to me, let’s find something that’s more predictive that really helps us a little better than what we have now,” she said.
The study had no specific funding. The authors of the study and the editorial have disclosed no relevant financial relationships. Dr. Hausmann reports receiving salary support from CARRA. Dr. Imundo and Dr. Wahezi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Children are not little adults” is a common refrain in pediatric medicine, but when it comes to a condition like juvenile idiopathic arthritis (JIA), rheumatologists might be better off treating pediatric and adult rheumatic disease more similarly.
A recent study published in Arthritis Care & Research followed children diagnosed with JIA for 18 years. Although not the first long-term study to examine children with JIA, it is unique in that it took place “during a time where biologic DMARDs [disease-modifying antirheumatic drugs] were emerging as a fundamental therapy in the management of children with JIA,” said Dawn M. Wahezi, MD, chief of the division of pediatric rheumatology at the Children’s Hospital at Montefiore in New York, who was not involved with the study.
Additionally, the study highlights the International League of Associations for Rheumatology (ILAR) consensus-based classification criteria as an imperfect method to categorize patients with JIA.
Mia Glerup, MD, PhD, of the department of pediatrics at Aarhus (Denmark) University Hospital and colleagues prospectively analyzed 373 patients from Denmark, Norway, Sweden, and Finland with new-onset JIA between 1997 and 2000 and evaluated them at baseline, 8 years, and 18 years. At each visit, the researchers collected data on demographics, disease activity, ILAR category, treatment, and blood samples.
Patients in the cohort were mostly girls (66.7%) with a median age of 5.9 years at onset. Approximately one-third (34.8%) of patients were antinuclear antibody (ANA) positive and 21.6% were HLA-B27 positive. The most common JIA categories at baseline were persistent oligoarthritis (53.9%), polyarticular rheumatoid factor (RF) negative (21.1%), and undifferentiated arthritis (10.2%).
Dr. Glerup and colleagues found that the proportion of patients not receiving DMARDs declined from 73.2% at baseline to 59.7% at 8 years, and then rose again to 70% at 18 years (risk ratio, 1.3; P = .003). The group of 103 patients who used conventional DMARDs (cDMARDs) either as monotherapy or in combination with a biologic DMARD (bDMARD) at 8 years dwindled to 44 (42.7%) at 18 years (RR, 0.4; P < .001), whereas 32 of 52 patients (61.5%) using bDMARDs at 8 years were still taking them at 18 years (RR, 0.6; P = .02). Across the whole study, 14.7% of patients never received any JIA treatment, and 33 of 85 patients (38.8%) on continuous DMARDs developed uveitis during the study period.
Overall, 62.7% of patients received DMARDs at least once, including 89.7% with polyarticular RF negative, 77.3% with oligoarticular extended, 76.9% with systemic, 75.7% with juvenile enthesitis-related arthritis (ERA), 66.7% with polyarticular RF-positive, 65.2% with juvenile psoriatic arthritis (JPsA), 58.9% with undifferentiated JIA, and 27.6% of patients with persistent oligoarticular disease.
The median number of active joints dropped from 3 (range, 1-30) at baseline to 0 at 8 years (range, 0-13), whereas the median cumulative number of affected joints rose from 3 at baseline (range, 1-30) to 6 at 8 years (range, 1-41). At last follow-up, the median number of active joints was 0 (range, 0-5) and median cumulative number of affected joints was 7 (range, 1-47). The percentage of patients in remission barely changed from 52% at 8 years to 51% at 18.
Some patients also changed ILAR categories during the study period, with 7% shifting between baseline and 8 years, and 11% shifting between 8-year and 18-year follow-up. Compared with baseline, by the 18-year follow-up time point there was a significant decrease in the number of patients categorized as oligoarticular (230 vs. 197 patients; P = .02), a significant increase in patients in the psoriatic ILAR category (8 vs. 28 patients; P < .001), and a nonsignificant increase in the number of patients in the undifferentiated category (45 vs. 63 patients; P = .06).
“Almost half of the changes in the distribution between the ILAR categories were caused by updated information on heredity in a first-degree relative obtained at the follow-up visits,” Dr. Glerup and colleagues write.
The results of the long-term study show that patients are “likely to remain in remission – with the converse also evident, as patients still with evidence of disease activity at 8 years after disease onset were more likely to have refractory disease,” Dr. Wahezi said.
Commenting on the study’s findings, Lisa F. Imundo, MD, director of adolescent rheumatology at Columbia University Medical Center in New York, said they are “great news to be able to give parents of young kids with arthritis.” However, she questioned whether the results are generalizable to populations of patients “who are in the worst prognostic group.”
For example, a substantial proportion of patients were classified under the oligoarticular category. “That’s already a group that we know from experience tends to have a better outcome than some of the other groups of JIA,” she said.
“That kind of weaves its way through the whole study, because then they show a lot of patients have come off their medication. Patients who had more severe disease in more joints would be less likely, I think, to just stop their medication and stop going to doctors,” Dr. Imundo explained.
Although the study is valuable for its long-term follow-up, there is also a question of generalizability across a more diverse ethnic and racial group. The authors do not elaborate on the racial breakdown of their patients, Dr. Imundo said, “so we’re going to have to assume that the vast majority are going to [have] Caucasian Nordic ethnic background, and that goes along with them having this high percentage of HLA-B27 positivity, which is a gene that’s more prevalent in northern European populations.”
Jonathan Hausmann, MD, a pediatric and adult rheumatologist at Boston Children’s Hospital, Boston,, told this news organization that he believes the overall conclusions from the study – that JIA persists over time and that ILAR classification is a somewhat imprecise measure of assessing JIA types in children – would be generalizable to other groups.
However, long-term registries evaluating JIA in more diverse populations, such as the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, could confirm these results, said Dr. Hausmann, who is a registry informatics associate with CARRA and was not associated with the research.
Long-term management of JIA
In an accompanying editorial, Jaime Guzman, MD, MSc, and Ross E. Petty, MD, PhD, of British Columbia Children’s Hospital and the University of British Columbia, Vancouver, said a rheumatologist’s interpretation of the study would be tied to what they learned about children with arthritis in medical school. They would see the glass as “half full” if children who achieved remission stayed in remission if they learned that a child might end up outgrowing JIA but potentially develop lifelong disability, whereas others may focus on the outcome of approximately half of patients not achieving remission.
“When I was going through medical school, I remember learning that JIA is a disease of children, and typically, they outgrow it as they become adults,” Dr. Hausmann said. “I think this study and many other studies have shown that that’s actually not the case – that, in fact, it may be a majority of kids continue having active disease even through adulthood.”
If a rheumatologist knows JIA is likely to continue into adulthood, “that’s huge,” Dr. Hausmann said. “That means when we first diagnose patients with JIA as kids, we need to set expectations with the families that this may not just go away; this may be something that could be more lifelong.”
Education on the part of the patient, their parents, and their clinician on the expected trajectory of the disease is critical so that children can continue their own care as they transition to adulthood, Dr. Hausmann explained. “The earlier the kids develop the skills to discuss their medicines, their side effects, the better they’ll be able to transition to adult medicine,” he said.
For the patients who go into remission and stay in remission, the message is also important. “To have the reassurance that a lot of those kids won’t be having active joint symptoms or need to be on medication, that’s a huge positive message that can get out there, so I think that’s great,” Dr. Imundo said.
Time to move on from ILAR classification?
Another big takeaway from the study was how patients’ ILAR classification changed across the 18-year follow-up. First proposed in 1995, the JIA ILAR classification has been revised several times for clarification purposes. In its current form, the ILAR classification considers a patient’s history when categorizing JIA types but also includes factors such as immediate family history. This system of assessing JIA has been criticized and there are initiatives to create a new JIA classification system to replace it.
“The ILAR criteria were designed to classify patients 6 months after disease onset in an attempt to find some commonality in clinical phenotypes, prognosis, and suggested management,” Dr. Wahezi said. “While there continues to be debate as to whether we can improve our classification of JIA patients, it is not surprising that phenotypes may evolve over time as new clinical features develop. As pediatric rheumatologists, we are well accustomed to having to modify management plans as children manifest with new clinical features over time.”
Although the percentage of patients who switched ILAR classifications over the study period was “much higher” than she would have thought, Dr. Imundo said it was the reasons provided in the study that seemed odd to her. “The classification scheme relies on your family history, like someone else in your family now has psoriasis, so your arthritis classification changes,” she explained.
“We want to head toward a much more unified classification scheme, a simpler one. We now understand that some of the diseases that we see in pediatrics are really the equivalent or same disease in adults,” she said.
“Most of the pediatric categories of JIA have distinct adult correlates,” Dr. Hausmann agreed. RF-positive polyarthritis in children and rheumatoid arthritis in adults are correlated, as are systemic JIA and adult-onset Still’s disease, he explained. “That has been borne out also by genetic susceptibility studies that the genetic predispositions to systemic arthritis in children is the same as the genetic predisposition to adult-onset Still’s disease in adults. By and large, there are a lot of similarities between the two.
“I think we need to incorporate some of that knowledge in better classifying kids with JIA so that we can find the best treatments and the best outcomes, and we can provide information to families about the expected course of the disease over time so that can inform our discussions.”
Some pediatric rheumatologists accept the classification system is flawed, but not all concur with the degree to which these problems impact patient care. “While the ILAR classification criteria may be subject to criticism, it does provide general context and prognostic implications for patients and families,” Dr. Wahezi said.
“The medicines certainly are very similar across the JIA categories, so the implications are not as broad” when classification changes,” Dr. Hausmann said. “But it certainly shows that there are things that we still don’t know. I think classification is actually pretty important because it might give you a sense of how persistent the disease will be.”
Dr. Imundo said the ILAR classification’s “time is limited,” and rheumatologists may soon need to adopt a new way of classifying children with rheumatic disease – “a more data-driven, genetics-driven scheme.”
“These categories are so imperfect, and the patients are changing. I feel like that says to me, let’s find something that’s more predictive that really helps us a little better than what we have now,” she said.
The study had no specific funding. The authors of the study and the editorial have disclosed no relevant financial relationships. Dr. Hausmann reports receiving salary support from CARRA. Dr. Imundo and Dr. Wahezi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE & RESEARCH
CDC, AAP issues new guidelines to better define developmental milestones
The Centers for Disease Control and Prevention and the American Academy of Pediatrics recently issued revised milestone guidelines for their developmental surveillance campaign, Learn the Signs, Act Early (LTSAE).
The new guidelines, published in Pediatrics, were drafted in “easy-to-understand” language and identify the behaviors that 75% or more of children should exhibit at certain ages based on developmental resources, existing data, and clinician experience. The previous milestone checklists, developed in 2004, used 50th percentile or average-age milestones.
The CDC, in collaboration with the AAP, convened a group of eight subject matter experts in various fields of child development, including a developmental pediatrician and researcher from Kennedy Krieger Institute, to develop new and clearer guidelines.
“The goals of the group were to identify evidence-informed milestones to include in CDC checklists, clarify when most children can be expected to reach a milestone (to discourage a wait-and-see approach), and support clinical judgment regarding screening between recommended ages,” wrote lead author Jennifer M. Zubler, MD, of the National Center on Birth Defects and Developmental Disabilities in Atlanta, and colleagues.
Key changes
The experts established 11 criteria for CDC surveillance milestones and tools, including milestones most children (75% or more) would be expected to reach by defined health supervision visit ages and those that are easily recognized in natural settings.
Criteria for developmental milestones and surveillance tools:
- Milestones are included at the age most (≥75%) children would be expected to demonstrate the milestone.
- Eliminate “warning signs.”
- Are easy for families of different social, cultural, and ethnic backgrounds to observe and use.
- Are able to be answered with yes, not yet, or not sure.
- Use plain language, avoiding vague terms like may, can, and begins.
- Are organized in developmental domains.
- Show progression of skills with age, when possible.
- Milestones are not repeated across checklists.
- Include open-ended questions.
- Include information for developmental promotion.
- Include information on how to act early if there are concerns.
The previous guidelines were critiqued by some clinicians as being “not helpful to individual families who had concerns about their child’s development,” and in some cases, led to delays in diagnoses as decision-makers opted for a “wait-and-see approach.”
“The earlier a child is identified with a developmental delay the better, as treatment as well as learning interventions can begin,” Paul Lipkin, MD, an associate professor of pediatrics at the Johns Hopkins University, Baltimore, said in an accompanying press release. “Revising the guidelines with expertise and data from clinicians in the field accomplishes these goals.”
Additional changes included new checklists for children between the ages of 15 and 30 months, additional social and emotional milestones, as well as the removal of complex language and duplicate milestones. The experts also developed new, open-ended questions to aid discussions with families.
“Review of a child’s development with these milestones opens up a continuous dialogue between a parent and the health care provider about their child’s present and future development,” said Dr. Lipkin.
Originally pioneered in 2005, the LTSAE awareness campaign provides free resources to clinicians and families to support early detection of children with developmental delays and disabilities. After the new guidelines were drafted, they were presented to parents of various racial groups, income levels, and educational backgrounds to confirm ease of use and understandability.
“These criteria and revised checklists can be used to support developmental surveillance, clinical judgment regarding additional developmental screening, and research in developmental surveillance processes,” wrote Dr. Zubler.
Expert perspective
“These new guidelines will allow us to catch more children with developmental delays as they raise the threshold to 75% of children achieving those milestones at that particular age,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella added that the new guidelines simplify the milestones and reduce redundancy across different developmental domains. “Most importantly, it gave me the opportunity to see just how great the CDC milestone tracker app is – I think parents would really like it.”
This project was supported by the CDC and Prevention of the Department of Health & Human Services. One author is a developer of the Ages & Stages Questionnaires and receives royalties from Brookes Publishing, the company that publishes this tool; the other authors have indicated they have no relevant conflicts of interest to disclose.
The Centers for Disease Control and Prevention and the American Academy of Pediatrics recently issued revised milestone guidelines for their developmental surveillance campaign, Learn the Signs, Act Early (LTSAE).
The new guidelines, published in Pediatrics, were drafted in “easy-to-understand” language and identify the behaviors that 75% or more of children should exhibit at certain ages based on developmental resources, existing data, and clinician experience. The previous milestone checklists, developed in 2004, used 50th percentile or average-age milestones.
The CDC, in collaboration with the AAP, convened a group of eight subject matter experts in various fields of child development, including a developmental pediatrician and researcher from Kennedy Krieger Institute, to develop new and clearer guidelines.
“The goals of the group were to identify evidence-informed milestones to include in CDC checklists, clarify when most children can be expected to reach a milestone (to discourage a wait-and-see approach), and support clinical judgment regarding screening between recommended ages,” wrote lead author Jennifer M. Zubler, MD, of the National Center on Birth Defects and Developmental Disabilities in Atlanta, and colleagues.
Key changes
The experts established 11 criteria for CDC surveillance milestones and tools, including milestones most children (75% or more) would be expected to reach by defined health supervision visit ages and those that are easily recognized in natural settings.
Criteria for developmental milestones and surveillance tools:
- Milestones are included at the age most (≥75%) children would be expected to demonstrate the milestone.
- Eliminate “warning signs.”
- Are easy for families of different social, cultural, and ethnic backgrounds to observe and use.
- Are able to be answered with yes, not yet, or not sure.
- Use plain language, avoiding vague terms like may, can, and begins.
- Are organized in developmental domains.
- Show progression of skills with age, when possible.
- Milestones are not repeated across checklists.
- Include open-ended questions.
- Include information for developmental promotion.
- Include information on how to act early if there are concerns.
The previous guidelines were critiqued by some clinicians as being “not helpful to individual families who had concerns about their child’s development,” and in some cases, led to delays in diagnoses as decision-makers opted for a “wait-and-see approach.”
“The earlier a child is identified with a developmental delay the better, as treatment as well as learning interventions can begin,” Paul Lipkin, MD, an associate professor of pediatrics at the Johns Hopkins University, Baltimore, said in an accompanying press release. “Revising the guidelines with expertise and data from clinicians in the field accomplishes these goals.”
Additional changes included new checklists for children between the ages of 15 and 30 months, additional social and emotional milestones, as well as the removal of complex language and duplicate milestones. The experts also developed new, open-ended questions to aid discussions with families.
“Review of a child’s development with these milestones opens up a continuous dialogue between a parent and the health care provider about their child’s present and future development,” said Dr. Lipkin.
Originally pioneered in 2005, the LTSAE awareness campaign provides free resources to clinicians and families to support early detection of children with developmental delays and disabilities. After the new guidelines were drafted, they were presented to parents of various racial groups, income levels, and educational backgrounds to confirm ease of use and understandability.
“These criteria and revised checklists can be used to support developmental surveillance, clinical judgment regarding additional developmental screening, and research in developmental surveillance processes,” wrote Dr. Zubler.
Expert perspective
“These new guidelines will allow us to catch more children with developmental delays as they raise the threshold to 75% of children achieving those milestones at that particular age,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella added that the new guidelines simplify the milestones and reduce redundancy across different developmental domains. “Most importantly, it gave me the opportunity to see just how great the CDC milestone tracker app is – I think parents would really like it.”
This project was supported by the CDC and Prevention of the Department of Health & Human Services. One author is a developer of the Ages & Stages Questionnaires and receives royalties from Brookes Publishing, the company that publishes this tool; the other authors have indicated they have no relevant conflicts of interest to disclose.
The Centers for Disease Control and Prevention and the American Academy of Pediatrics recently issued revised milestone guidelines for their developmental surveillance campaign, Learn the Signs, Act Early (LTSAE).
The new guidelines, published in Pediatrics, were drafted in “easy-to-understand” language and identify the behaviors that 75% or more of children should exhibit at certain ages based on developmental resources, existing data, and clinician experience. The previous milestone checklists, developed in 2004, used 50th percentile or average-age milestones.
The CDC, in collaboration with the AAP, convened a group of eight subject matter experts in various fields of child development, including a developmental pediatrician and researcher from Kennedy Krieger Institute, to develop new and clearer guidelines.
“The goals of the group were to identify evidence-informed milestones to include in CDC checklists, clarify when most children can be expected to reach a milestone (to discourage a wait-and-see approach), and support clinical judgment regarding screening between recommended ages,” wrote lead author Jennifer M. Zubler, MD, of the National Center on Birth Defects and Developmental Disabilities in Atlanta, and colleagues.
Key changes
The experts established 11 criteria for CDC surveillance milestones and tools, including milestones most children (75% or more) would be expected to reach by defined health supervision visit ages and those that are easily recognized in natural settings.
Criteria for developmental milestones and surveillance tools:
- Milestones are included at the age most (≥75%) children would be expected to demonstrate the milestone.
- Eliminate “warning signs.”
- Are easy for families of different social, cultural, and ethnic backgrounds to observe and use.
- Are able to be answered with yes, not yet, or not sure.
- Use plain language, avoiding vague terms like may, can, and begins.
- Are organized in developmental domains.
- Show progression of skills with age, when possible.
- Milestones are not repeated across checklists.
- Include open-ended questions.
- Include information for developmental promotion.
- Include information on how to act early if there are concerns.
The previous guidelines were critiqued by some clinicians as being “not helpful to individual families who had concerns about their child’s development,” and in some cases, led to delays in diagnoses as decision-makers opted for a “wait-and-see approach.”
“The earlier a child is identified with a developmental delay the better, as treatment as well as learning interventions can begin,” Paul Lipkin, MD, an associate professor of pediatrics at the Johns Hopkins University, Baltimore, said in an accompanying press release. “Revising the guidelines with expertise and data from clinicians in the field accomplishes these goals.”
Additional changes included new checklists for children between the ages of 15 and 30 months, additional social and emotional milestones, as well as the removal of complex language and duplicate milestones. The experts also developed new, open-ended questions to aid discussions with families.
“Review of a child’s development with these milestones opens up a continuous dialogue between a parent and the health care provider about their child’s present and future development,” said Dr. Lipkin.
Originally pioneered in 2005, the LTSAE awareness campaign provides free resources to clinicians and families to support early detection of children with developmental delays and disabilities. After the new guidelines were drafted, they were presented to parents of various racial groups, income levels, and educational backgrounds to confirm ease of use and understandability.
“These criteria and revised checklists can be used to support developmental surveillance, clinical judgment regarding additional developmental screening, and research in developmental surveillance processes,” wrote Dr. Zubler.
Expert perspective
“These new guidelines will allow us to catch more children with developmental delays as they raise the threshold to 75% of children achieving those milestones at that particular age,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella added that the new guidelines simplify the milestones and reduce redundancy across different developmental domains. “Most importantly, it gave me the opportunity to see just how great the CDC milestone tracker app is – I think parents would really like it.”
This project was supported by the CDC and Prevention of the Department of Health & Human Services. One author is a developer of the Ages & Stages Questionnaires and receives royalties from Brookes Publishing, the company that publishes this tool; the other authors have indicated they have no relevant conflicts of interest to disclose.
FROM PEDIATRICS
Which injections are effective for lateral epicondylitis?
EVIDENCE SUMMARY
Neither corticosteroids nor platelet-rich plasma are superior to placebo
A 2014 systematic review of RCTs of nonsurgical treatments for lateral epicondylitis identified 4 studies comparing corticosteroid injections to saline or anesthetic injections.1 In the first study, investigators followed 64 patients for 6 months. Both groups significantly improved from baseline, but there were no differences in pain or function at 1 or 6 months. Skin discoloration occurred in 2 patients who received lidocaine injection and 1 who received dexamethasone.2
In a second RCT of patients with symptoms for > 4 weeks, 39 participants were randomized to either betamethasone/bupivacaine or bupivacaine-only injections. In-person follow-up occurred at 4 and 8 weeks and telephone follow-up at 6 months. Both groups statistically improved from baseline to 6 months. No differences were seen between groups in pain or functional improvement at 4, 8, or 26 weeks, but the betamethasone group showed statistically greater improvement on the Visual Analog Scale (VAS) from 8 weeks to the final 6-month telephone follow-up. No functional assessments were reported at 6 months.3
The third RCT of 165 patients with lateral epicondylitis for > 6 weeks evaluated 4 intervention groups: corticosteroid injection with/without physiotherapy and placebo (small-volume saline) injection with/without physiotherapy. At the end of 1 year, the corticosteroid injection groups had less complete recovery (83% vs 96%; relative risk [RR] = 0.86; 99% CI, 0.75-0.99) and more recurrences (54% vs 12%; RR = 0.23; 99% CI, 0.10-0.51) than the placebo groups.4
The fourth RCT randomized 120 patients to either 2 mL lidocaine or 1 mL lidocaine plus 1 mL of triamcinolone. At 1-year follow-up, 57 of 60 lidocaine-injected patients had an excellent recovery and 56 of 60 triamcinolone plus lidocaine patients had an excellent recovery.5
Platelet-rich plasma. A meta-analysis6 of RCTs of PRP vs saline injections included 5 trials and 276 patients with a mean age of 48 years; duration of follow-up was 2 to 12 months. No significant differences were found between the groups for pain score—measured by VAS or the Patient-Rated Tennis Elbow Evaluation (PRTEE)—(standardized mean difference [SMD] = –0.51; 95% CI, –1.32 to –0.30) nor for functional score (SMD = 0.07; 95% CI, –0.46 to 0.33). Two of the trials reported adverse reactions of pain around the injection site: 16% to 20% in the PRP group vs 8% to 15% in the saline group.
Corticosteroids and PRP. A 2013 3-armed RCT7 (n = 60) compared 1-time injections of PRP, corticosteroid, and saline for treatment of lateral epicondylitis. Pain was evaluated at 1 and 3 months using the PRTEE. Compared to saline, corticosteroid showed a statistically significant, but not a minimum clinically important, reduction (8% greater improvement) at 1 month but not at 3 months. PRP pain reduction at both 1 and 3 months was not significantly different from placebo. Importantly, a small sample size combined with a high dropout rate (> 70%) limit validity of this study.
Botulinum toxin shows modest pain improvement, but …
A 2017 meta-analysis8 of 4 RCTs (n = 278) compared the effectiveness of botulinum toxin vs saline injection and other nonsurgical treatments for lateral epicondylitis. The studies compared the mean differences in pain relief and hand grip strength in adult patients with lateral epicondylitis symptoms for at least 3 months. Compared with saline injection, botulinum toxin injection significantly reduced pain to a small or medium SMD, at 2 to 4 weeks post injection (SMD = –0.73; 95% CI, –1.29 to –0.17); 8 to 12 weeks post injection (SMD = –0.45; 95% CI, –0.74 to –0.15); and 16+ weeks post injection (SMD = –0.54; 95% CI, –0.98 to –0.11). Harm from botulinum toxin was greater than from saline or corticosteroid, with a significant reduction in grip strength at 2 to 4 weeks (SMD = –0.33; 95% CI, –0.59 to –0.08).
Continue to: Prolotherapy needs further study
Prolotherapy needs further study
A 2008 RCT9 of 20 adults with at least 6 months of lateral epicondylitis received either prolotherapy (1 part 5% sodium morrhuate, 1.5 parts 50% dextrose, 0.5 parts 4% lidocaine, 0.5 parts 0.5% bupivacaine HCl, and 3.5 parts normal saline) injections or 0.9% saline injections at baseline, 4 weeks, and 8 weeks. On a 10-point Likert scale, the prolotherapy group had a lower mean pain score at 16 weeks than the saline injection group (0.5 vs 3.5), but not at 8 weeks (3.3 vs 3.6). This pilot study’s results are limited by its small sample size.
Hyaluronic acid improves pain, but not enough
A 2010 double-blind RCT10 (n = 331) compared hyaluronic acid injection vs saline injection in treatment of lateral epicondylitis in adults with > 3 months of symptoms. Two injections were performed 1 week apart, with follow-up at 30 days and at 1 year after the first injection. VAS score in the hyaluronic acid group, at rest and after grip testing, was significantly different (statistically) than in the placebo group but did not meet criteria for minimum clinically important improvement. Review of the literature showed limited follow-up studies on hyaluronic acid for lateral epicondylitis to confirm this RCT.
Autologous blood has no advantage over placebo
The only RCT of autologous blood compared to saline injections11 included patients with lateral epicondylitis for < 6 months: 10 saline injections vs 9 autologous blood injections. Patient scores on the Disabilities of the Arm, Shoulder, and Hand scale (which measures symptoms from 0 to 100; lower is better) showed no difference but favored the saline injections at 2-month (28 vs 20) and 6-month (20 vs 10) follow-up.
Editor’s takeaway
Limiting the evidence review to studies with a placebo comparator clarifies the lack of effectiveness of lateral epicondylitis injections. Neither corticosteroid, platelet-rich plasma, botulinum toxin, prolotherapy, hyaluronic acid, or autologous blood injections have proven superior to saline or anesthetic injections. However, all injections that contained “placebo” significantly improved lateralepicondylitis.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Lindenhovius A, Henket M, Gilligan BP, et al. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg Am. 2008;33:909-919. doi: 10.1016/j.jhsa.2008.02.004
3. Newcomer KL, Laskowski ER, Idank DM, et al. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11:214-222. doi: 10.1097/00042752-200110000-00002
4. Coombes BK, Bisset L, Brooks P, et al. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309:461-469. doi: 10.1001/jama.2013.129
5. Altay T, Gunal I, Ozturk H. Local injection treatment for lateral epicondylitis. Clin Orthop Relat Res. 2002;398:127-130.
6. Simental-Mendía M, Vilchez-Cavazos F, Álvarez-Villalobos N, et al. Clinical efficacy of platelet-rich plasma in the treatment of lateral epicondylitis: a systematic review and meta-analysis of randomized placebo-controlled clinical trials. Clin Rheumatol. 2020;39:2255-2265. doi: 10.1007/s10067-020-05000-y
7. Krogh T, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich-plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635. doi:10.1177/0363546512472975
8. Lin Y, Wu W, Hsu Y, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2017;32:131-145. doi:10.1177/0269215517702517
9. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sports Med. 2008;18:248-254. doi: 10.1097/JSM.0b013e318170fc87
10. Petrella R, Cogliano A, Decaria J, et al. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:4. doi: 10.1186/1758-2555-2-4
11. Wolf JM, Ozer K, Scott F, et al. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am. 2011;36:1269-1272. doi: 10.1016/j.jhsa.2011.05.014
EVIDENCE SUMMARY
Neither corticosteroids nor platelet-rich plasma are superior to placebo
A 2014 systematic review of RCTs of nonsurgical treatments for lateral epicondylitis identified 4 studies comparing corticosteroid injections to saline or anesthetic injections.1 In the first study, investigators followed 64 patients for 6 months. Both groups significantly improved from baseline, but there were no differences in pain or function at 1 or 6 months. Skin discoloration occurred in 2 patients who received lidocaine injection and 1 who received dexamethasone.2
In a second RCT of patients with symptoms for > 4 weeks, 39 participants were randomized to either betamethasone/bupivacaine or bupivacaine-only injections. In-person follow-up occurred at 4 and 8 weeks and telephone follow-up at 6 months. Both groups statistically improved from baseline to 6 months. No differences were seen between groups in pain or functional improvement at 4, 8, or 26 weeks, but the betamethasone group showed statistically greater improvement on the Visual Analog Scale (VAS) from 8 weeks to the final 6-month telephone follow-up. No functional assessments were reported at 6 months.3
The third RCT of 165 patients with lateral epicondylitis for > 6 weeks evaluated 4 intervention groups: corticosteroid injection with/without physiotherapy and placebo (small-volume saline) injection with/without physiotherapy. At the end of 1 year, the corticosteroid injection groups had less complete recovery (83% vs 96%; relative risk [RR] = 0.86; 99% CI, 0.75-0.99) and more recurrences (54% vs 12%; RR = 0.23; 99% CI, 0.10-0.51) than the placebo groups.4
The fourth RCT randomized 120 patients to either 2 mL lidocaine or 1 mL lidocaine plus 1 mL of triamcinolone. At 1-year follow-up, 57 of 60 lidocaine-injected patients had an excellent recovery and 56 of 60 triamcinolone plus lidocaine patients had an excellent recovery.5
Platelet-rich plasma. A meta-analysis6 of RCTs of PRP vs saline injections included 5 trials and 276 patients with a mean age of 48 years; duration of follow-up was 2 to 12 months. No significant differences were found between the groups for pain score—measured by VAS or the Patient-Rated Tennis Elbow Evaluation (PRTEE)—(standardized mean difference [SMD] = –0.51; 95% CI, –1.32 to –0.30) nor for functional score (SMD = 0.07; 95% CI, –0.46 to 0.33). Two of the trials reported adverse reactions of pain around the injection site: 16% to 20% in the PRP group vs 8% to 15% in the saline group.
Corticosteroids and PRP. A 2013 3-armed RCT7 (n = 60) compared 1-time injections of PRP, corticosteroid, and saline for treatment of lateral epicondylitis. Pain was evaluated at 1 and 3 months using the PRTEE. Compared to saline, corticosteroid showed a statistically significant, but not a minimum clinically important, reduction (8% greater improvement) at 1 month but not at 3 months. PRP pain reduction at both 1 and 3 months was not significantly different from placebo. Importantly, a small sample size combined with a high dropout rate (> 70%) limit validity of this study.
Botulinum toxin shows modest pain improvement, but …
A 2017 meta-analysis8 of 4 RCTs (n = 278) compared the effectiveness of botulinum toxin vs saline injection and other nonsurgical treatments for lateral epicondylitis. The studies compared the mean differences in pain relief and hand grip strength in adult patients with lateral epicondylitis symptoms for at least 3 months. Compared with saline injection, botulinum toxin injection significantly reduced pain to a small or medium SMD, at 2 to 4 weeks post injection (SMD = –0.73; 95% CI, –1.29 to –0.17); 8 to 12 weeks post injection (SMD = –0.45; 95% CI, –0.74 to –0.15); and 16+ weeks post injection (SMD = –0.54; 95% CI, –0.98 to –0.11). Harm from botulinum toxin was greater than from saline or corticosteroid, with a significant reduction in grip strength at 2 to 4 weeks (SMD = –0.33; 95% CI, –0.59 to –0.08).
Continue to: Prolotherapy needs further study
Prolotherapy needs further study
A 2008 RCT9 of 20 adults with at least 6 months of lateral epicondylitis received either prolotherapy (1 part 5% sodium morrhuate, 1.5 parts 50% dextrose, 0.5 parts 4% lidocaine, 0.5 parts 0.5% bupivacaine HCl, and 3.5 parts normal saline) injections or 0.9% saline injections at baseline, 4 weeks, and 8 weeks. On a 10-point Likert scale, the prolotherapy group had a lower mean pain score at 16 weeks than the saline injection group (0.5 vs 3.5), but not at 8 weeks (3.3 vs 3.6). This pilot study’s results are limited by its small sample size.
Hyaluronic acid improves pain, but not enough
A 2010 double-blind RCT10 (n = 331) compared hyaluronic acid injection vs saline injection in treatment of lateral epicondylitis in adults with > 3 months of symptoms. Two injections were performed 1 week apart, with follow-up at 30 days and at 1 year after the first injection. VAS score in the hyaluronic acid group, at rest and after grip testing, was significantly different (statistically) than in the placebo group but did not meet criteria for minimum clinically important improvement. Review of the literature showed limited follow-up studies on hyaluronic acid for lateral epicondylitis to confirm this RCT.
Autologous blood has no advantage over placebo
The only RCT of autologous blood compared to saline injections11 included patients with lateral epicondylitis for < 6 months: 10 saline injections vs 9 autologous blood injections. Patient scores on the Disabilities of the Arm, Shoulder, and Hand scale (which measures symptoms from 0 to 100; lower is better) showed no difference but favored the saline injections at 2-month (28 vs 20) and 6-month (20 vs 10) follow-up.
Editor’s takeaway
Limiting the evidence review to studies with a placebo comparator clarifies the lack of effectiveness of lateral epicondylitis injections. Neither corticosteroid, platelet-rich plasma, botulinum toxin, prolotherapy, hyaluronic acid, or autologous blood injections have proven superior to saline or anesthetic injections. However, all injections that contained “placebo” significantly improved lateralepicondylitis.
EVIDENCE SUMMARY
Neither corticosteroids nor platelet-rich plasma are superior to placebo
A 2014 systematic review of RCTs of nonsurgical treatments for lateral epicondylitis identified 4 studies comparing corticosteroid injections to saline or anesthetic injections.1 In the first study, investigators followed 64 patients for 6 months. Both groups significantly improved from baseline, but there were no differences in pain or function at 1 or 6 months. Skin discoloration occurred in 2 patients who received lidocaine injection and 1 who received dexamethasone.2
In a second RCT of patients with symptoms for > 4 weeks, 39 participants were randomized to either betamethasone/bupivacaine or bupivacaine-only injections. In-person follow-up occurred at 4 and 8 weeks and telephone follow-up at 6 months. Both groups statistically improved from baseline to 6 months. No differences were seen between groups in pain or functional improvement at 4, 8, or 26 weeks, but the betamethasone group showed statistically greater improvement on the Visual Analog Scale (VAS) from 8 weeks to the final 6-month telephone follow-up. No functional assessments were reported at 6 months.3
The third RCT of 165 patients with lateral epicondylitis for > 6 weeks evaluated 4 intervention groups: corticosteroid injection with/without physiotherapy and placebo (small-volume saline) injection with/without physiotherapy. At the end of 1 year, the corticosteroid injection groups had less complete recovery (83% vs 96%; relative risk [RR] = 0.86; 99% CI, 0.75-0.99) and more recurrences (54% vs 12%; RR = 0.23; 99% CI, 0.10-0.51) than the placebo groups.4
The fourth RCT randomized 120 patients to either 2 mL lidocaine or 1 mL lidocaine plus 1 mL of triamcinolone. At 1-year follow-up, 57 of 60 lidocaine-injected patients had an excellent recovery and 56 of 60 triamcinolone plus lidocaine patients had an excellent recovery.5
Platelet-rich plasma. A meta-analysis6 of RCTs of PRP vs saline injections included 5 trials and 276 patients with a mean age of 48 years; duration of follow-up was 2 to 12 months. No significant differences were found between the groups for pain score—measured by VAS or the Patient-Rated Tennis Elbow Evaluation (PRTEE)—(standardized mean difference [SMD] = –0.51; 95% CI, –1.32 to –0.30) nor for functional score (SMD = 0.07; 95% CI, –0.46 to 0.33). Two of the trials reported adverse reactions of pain around the injection site: 16% to 20% in the PRP group vs 8% to 15% in the saline group.
Corticosteroids and PRP. A 2013 3-armed RCT7 (n = 60) compared 1-time injections of PRP, corticosteroid, and saline for treatment of lateral epicondylitis. Pain was evaluated at 1 and 3 months using the PRTEE. Compared to saline, corticosteroid showed a statistically significant, but not a minimum clinically important, reduction (8% greater improvement) at 1 month but not at 3 months. PRP pain reduction at both 1 and 3 months was not significantly different from placebo. Importantly, a small sample size combined with a high dropout rate (> 70%) limit validity of this study.
Botulinum toxin shows modest pain improvement, but …
A 2017 meta-analysis8 of 4 RCTs (n = 278) compared the effectiveness of botulinum toxin vs saline injection and other nonsurgical treatments for lateral epicondylitis. The studies compared the mean differences in pain relief and hand grip strength in adult patients with lateral epicondylitis symptoms for at least 3 months. Compared with saline injection, botulinum toxin injection significantly reduced pain to a small or medium SMD, at 2 to 4 weeks post injection (SMD = –0.73; 95% CI, –1.29 to –0.17); 8 to 12 weeks post injection (SMD = –0.45; 95% CI, –0.74 to –0.15); and 16+ weeks post injection (SMD = –0.54; 95% CI, –0.98 to –0.11). Harm from botulinum toxin was greater than from saline or corticosteroid, with a significant reduction in grip strength at 2 to 4 weeks (SMD = –0.33; 95% CI, –0.59 to –0.08).
Continue to: Prolotherapy needs further study
Prolotherapy needs further study
A 2008 RCT9 of 20 adults with at least 6 months of lateral epicondylitis received either prolotherapy (1 part 5% sodium morrhuate, 1.5 parts 50% dextrose, 0.5 parts 4% lidocaine, 0.5 parts 0.5% bupivacaine HCl, and 3.5 parts normal saline) injections or 0.9% saline injections at baseline, 4 weeks, and 8 weeks. On a 10-point Likert scale, the prolotherapy group had a lower mean pain score at 16 weeks than the saline injection group (0.5 vs 3.5), but not at 8 weeks (3.3 vs 3.6). This pilot study’s results are limited by its small sample size.
Hyaluronic acid improves pain, but not enough
A 2010 double-blind RCT10 (n = 331) compared hyaluronic acid injection vs saline injection in treatment of lateral epicondylitis in adults with > 3 months of symptoms. Two injections were performed 1 week apart, with follow-up at 30 days and at 1 year after the first injection. VAS score in the hyaluronic acid group, at rest and after grip testing, was significantly different (statistically) than in the placebo group but did not meet criteria for minimum clinically important improvement. Review of the literature showed limited follow-up studies on hyaluronic acid for lateral epicondylitis to confirm this RCT.
Autologous blood has no advantage over placebo
The only RCT of autologous blood compared to saline injections11 included patients with lateral epicondylitis for < 6 months: 10 saline injections vs 9 autologous blood injections. Patient scores on the Disabilities of the Arm, Shoulder, and Hand scale (which measures symptoms from 0 to 100; lower is better) showed no difference but favored the saline injections at 2-month (28 vs 20) and 6-month (20 vs 10) follow-up.
Editor’s takeaway
Limiting the evidence review to studies with a placebo comparator clarifies the lack of effectiveness of lateral epicondylitis injections. Neither corticosteroid, platelet-rich plasma, botulinum toxin, prolotherapy, hyaluronic acid, or autologous blood injections have proven superior to saline or anesthetic injections. However, all injections that contained “placebo” significantly improved lateralepicondylitis.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Lindenhovius A, Henket M, Gilligan BP, et al. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg Am. 2008;33:909-919. doi: 10.1016/j.jhsa.2008.02.004
3. Newcomer KL, Laskowski ER, Idank DM, et al. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11:214-222. doi: 10.1097/00042752-200110000-00002
4. Coombes BK, Bisset L, Brooks P, et al. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309:461-469. doi: 10.1001/jama.2013.129
5. Altay T, Gunal I, Ozturk H. Local injection treatment for lateral epicondylitis. Clin Orthop Relat Res. 2002;398:127-130.
6. Simental-Mendía M, Vilchez-Cavazos F, Álvarez-Villalobos N, et al. Clinical efficacy of platelet-rich plasma in the treatment of lateral epicondylitis: a systematic review and meta-analysis of randomized placebo-controlled clinical trials. Clin Rheumatol. 2020;39:2255-2265. doi: 10.1007/s10067-020-05000-y
7. Krogh T, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich-plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635. doi:10.1177/0363546512472975
8. Lin Y, Wu W, Hsu Y, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2017;32:131-145. doi:10.1177/0269215517702517
9. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sports Med. 2008;18:248-254. doi: 10.1097/JSM.0b013e318170fc87
10. Petrella R, Cogliano A, Decaria J, et al. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:4. doi: 10.1186/1758-2555-2-4
11. Wolf JM, Ozer K, Scott F, et al. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am. 2011;36:1269-1272. doi: 10.1016/j.jhsa.2011.05.014
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Lindenhovius A, Henket M, Gilligan BP, et al. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg Am. 2008;33:909-919. doi: 10.1016/j.jhsa.2008.02.004
3. Newcomer KL, Laskowski ER, Idank DM, et al. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11:214-222. doi: 10.1097/00042752-200110000-00002
4. Coombes BK, Bisset L, Brooks P, et al. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309:461-469. doi: 10.1001/jama.2013.129
5. Altay T, Gunal I, Ozturk H. Local injection treatment for lateral epicondylitis. Clin Orthop Relat Res. 2002;398:127-130.
6. Simental-Mendía M, Vilchez-Cavazos F, Álvarez-Villalobos N, et al. Clinical efficacy of platelet-rich plasma in the treatment of lateral epicondylitis: a systematic review and meta-analysis of randomized placebo-controlled clinical trials. Clin Rheumatol. 2020;39:2255-2265. doi: 10.1007/s10067-020-05000-y
7. Krogh T, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich-plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635. doi:10.1177/0363546512472975
8. Lin Y, Wu W, Hsu Y, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2017;32:131-145. doi:10.1177/0269215517702517
9. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sports Med. 2008;18:248-254. doi: 10.1097/JSM.0b013e318170fc87
10. Petrella R, Cogliano A, Decaria J, et al. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:4. doi: 10.1186/1758-2555-2-4
11. Wolf JM, Ozer K, Scott F, et al. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am. 2011;36:1269-1272. doi: 10.1016/j.jhsa.2011.05.014
EVIDENCE-BASED ANSWER:
Placebo injections actually improve lateral epicondylitis at high rates. No other injections convincingly improve it better than placebo.
Corticosteroid injection is not superior to saline or anesthetic injection (strength of recommendation [SOR] A, systematic review of randomized controlled trials [RCTs]). Platelet-rich plasma (PRP) injection is not superior to saline injection (SOR A, meta-analysis of RCTs).
Botulinum toxin injection, compared to saline injection, modestly improved pain in lateral epicondylitis, but with short-term grip-strength weakness (SOR A, meta-analysis of RCTs). Prolotherapy injection, compared to saline injection, improved pain at 16-week, but not at 8-week, follow-up (SOR B, one small pilot RCT).
Hyaluronic acid injection, compared to saline injection, resulted in a statistically significant pain reduction (6%) but did not achieve the minimum clinically important difference (SOR B, single RCT). Autologous blood injection, compared to saline injection, did not improve disability ratings (SOR B, one small RCT).
When the evidence suggests that placebo is best
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
Botulinum toxin for chronic pain: What's on the horizon?
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.
The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.
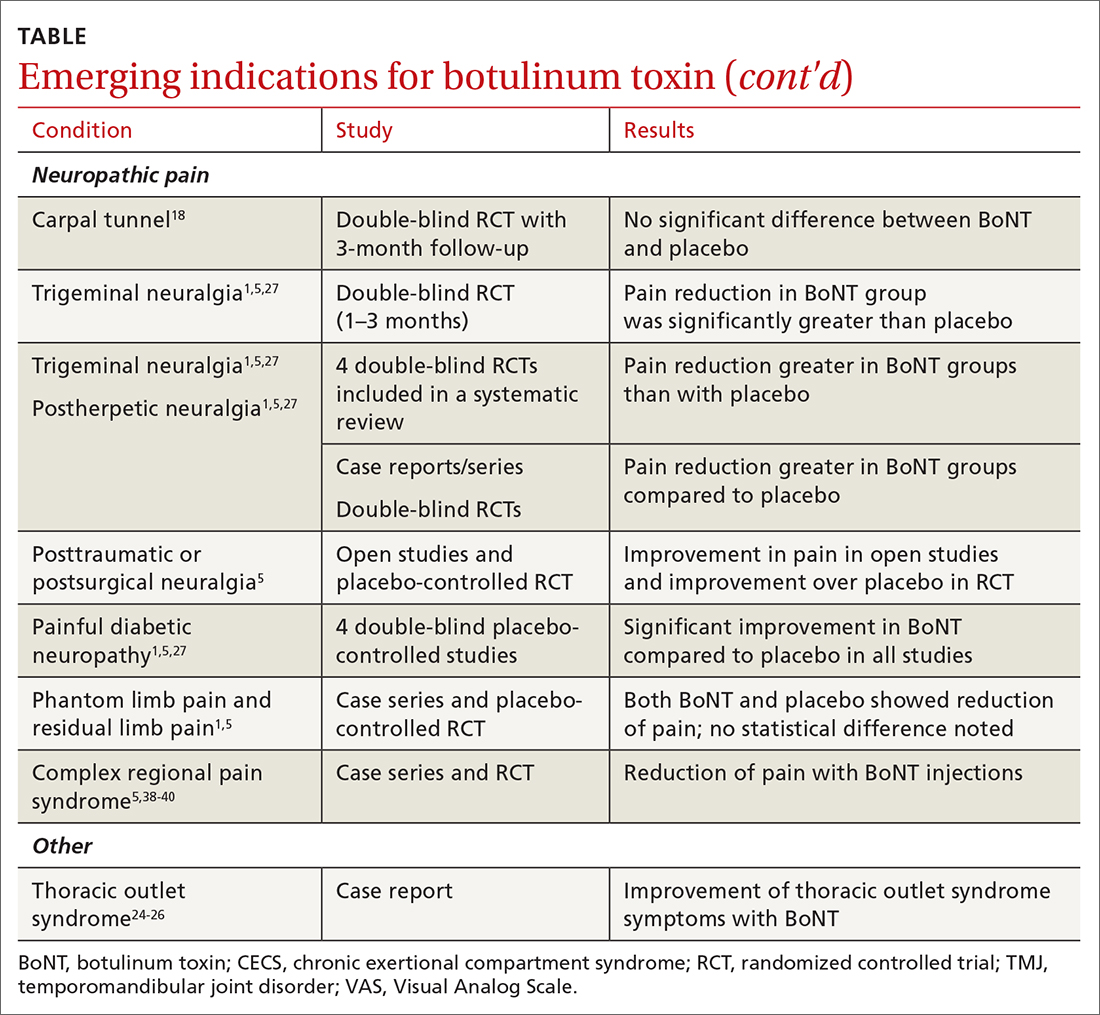
Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
PRACTICE RECOMMENDATIONS
› Consider botulinum toxin (BoNT) for patients with headache, spasticity, or cervical dystonia, as the FDA has approved BoNT for pain relief in these conditions. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Drug combo at outset of polyarticular JIA benefits patients most
Initiating treatment of polyarticular juvenile idiopathic arthritis (polyJIA) with both a conventional synthetic disease-modifying antirheumatic drug and a biologic DMARD resulted in more patients achieving clinical inactive disease 2 years later than did starting with only a csDMARD and stepping up to a biologic, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“The 24-month results support the 12-month primary results that suggested that the early-combination group was superior and that, at 24 months, more early combination CTP [consensus treatment plan] patients achieve CID [clinical inactive disease], compared to step up,” Yukiko Kimura, MD, division chief of pediatric rheumatology at HMH Hackensack (N.J.) University Medical Center, told attendees. “This suggests that starting biologics early in polyJIA may lead to better long-term outcomes in many patients.”
Dr. Kimura noted that polyarticular JIA patients are already at risk for poor outcomes, and initial therapy can especially impact outcomes. Further, little evidence exists to suggest when the best time is to start biologics, a gap this study aimed to address.
Diane Brown, MD, PhD, a pediatric rheumatologist at Children’s Hospital Los Angeles who was not involved in the study, was pleased to see the results, which she said support her own preferences and practice patterns.
“Starting sooner with combination therapy, taking advantage of the advances with biologics and our long history with methotrexate at the same time, gives better outcomes for the long run,” Dr. Brown said in an interview. “Having studies like this to back up my own recommendations can be very powerful when talking to families, and it is absolutely invaluable when battling with insurance companies who always want you to take the cheapest road.”
Study details
The findings were an update of 12-month results in the CARRA STOP-JIA study that enrolled 400 untreated patients with polyJIA and compared three Childhood Arthritis and Rheumatology Research Alliance (CARRA) CTPs. Overall, 49.5% of participants received biologics within 3 months of starting the study. For these updated results, 275 participants had complete data at 24 months for the three CTPs:
- A step-up group of 177 patients who started therapy with a csDMARD and added a biologic if needed at least 3 months later
- An early-combination group of 73 patients who started therapy with a csDMARD and biologic together
- A biologic-first group of 25 patients who started with biologic monotherapy, adding a csDMARD only if needed at least 3 months later.
The primary outcome was the percentage of participants who reached CID without taking glucocorticoids at 24 months. Since the participants were not randomized, the researchers made adjustments to account for baseline differences between the groups, including differences in JIA categories, number of active joints, physician global assessment of disease activity, and the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10).
At 24 months in an intention to treat analysis, 59.4% of the early-combination group had achieved CID, compared with 48% of the biologic-first group and 40.1% of the step-up group (P = .009 for early combination vs. step up). All three groups had improved since the 12-month time point, when 37% of the early-combination group, 24% of the biologic-first group, and 32% of the step-up group had reached CID.
There were no significant differences between the groups in secondary outcomes of achieving cJADAS10 inactive disease of 2.5 or less or 70% improvement in pediatric ACR response criteria at 24 months. All groups improved in PROMIS pain interference or mobility measures from baseline. Most of the 17 severe adverse events were infections.
Moving from step-up therapy to early-combination treatment
Dr. Brown said that she spent many years in her practice using the step-up therapy because it was difficult to get insurance companies to pay for biologics without first showing that methotrexate was insufficient.
”But methotrexate takes so long to control the disease that you need a lot of steroids, with all of their side effects, at least temporarily, or you must simply accept a longer period of active and symptomatic disease before you get to that desired state of clinically inactive disease,” Dr. Brown said. “And during that time, you can be accumulating what may be permanent damage to joints, as well as increase in risk of contractures and deconditioning for that child who is too uncomfortable to move and exercise and play normally.”
Dr. Brown is also wary of using a biologic as an initial therapy by itself because the actions of biologics are so specific. ”I like to back up the powerful, rapid, and specific actions of a biologic with the broader, if slower, action of methotrexate to minimize chances that the immune system is going to find a way around blockade of a single cytokine by your biologic,” she said.
While patient preference will also play a role in what CTP patients with polyJIA start with, Dr. Brown said that she believes more medication upfront can result in less medication and better outcomes in the long run, as the findings of this study suggest. The results here are helpful when speaking with families who are anxious about “so much medicine” or “such powerful medicines,” she said. ”I hope it will also help ease the fears of other providers who share the same concerns about ‘so much medicine.’ ”
The study’s biggest limitation is not being a randomized, controlled trial, but Dr. Brown said the researchers demonstrated effectively that the disease burden remains similar across the groups at baseline.
”It would also be useful to have a clear breakdown of adverse events and opportunistic infections because an excess of opportunistic infections would be a key concern with early combination therapy,” she said, although she added that the study overall was a ”beautiful example of the value of registry data.”
Dr. Kimura emphasized that polyJIA remains a challenging disease to treat, with 40%-60% of participants not reaching CID at 24 months. The registry follow-up will continue for up to 10 years to hopefully provide more information about longer-term outcomes from different treatments.
The research was funded by a grant from Genentech to CARRA. Dr. Kimura reported royalties from UpToDate and salary support from CARRA. Dr. Brown had no disclosures.
Initiating treatment of polyarticular juvenile idiopathic arthritis (polyJIA) with both a conventional synthetic disease-modifying antirheumatic drug and a biologic DMARD resulted in more patients achieving clinical inactive disease 2 years later than did starting with only a csDMARD and stepping up to a biologic, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“The 24-month results support the 12-month primary results that suggested that the early-combination group was superior and that, at 24 months, more early combination CTP [consensus treatment plan] patients achieve CID [clinical inactive disease], compared to step up,” Yukiko Kimura, MD, division chief of pediatric rheumatology at HMH Hackensack (N.J.) University Medical Center, told attendees. “This suggests that starting biologics early in polyJIA may lead to better long-term outcomes in many patients.”
Dr. Kimura noted that polyarticular JIA patients are already at risk for poor outcomes, and initial therapy can especially impact outcomes. Further, little evidence exists to suggest when the best time is to start biologics, a gap this study aimed to address.
Diane Brown, MD, PhD, a pediatric rheumatologist at Children’s Hospital Los Angeles who was not involved in the study, was pleased to see the results, which she said support her own preferences and practice patterns.
“Starting sooner with combination therapy, taking advantage of the advances with biologics and our long history with methotrexate at the same time, gives better outcomes for the long run,” Dr. Brown said in an interview. “Having studies like this to back up my own recommendations can be very powerful when talking to families, and it is absolutely invaluable when battling with insurance companies who always want you to take the cheapest road.”
Study details
The findings were an update of 12-month results in the CARRA STOP-JIA study that enrolled 400 untreated patients with polyJIA and compared three Childhood Arthritis and Rheumatology Research Alliance (CARRA) CTPs. Overall, 49.5% of participants received biologics within 3 months of starting the study. For these updated results, 275 participants had complete data at 24 months for the three CTPs:
- A step-up group of 177 patients who started therapy with a csDMARD and added a biologic if needed at least 3 months later
- An early-combination group of 73 patients who started therapy with a csDMARD and biologic together
- A biologic-first group of 25 patients who started with biologic monotherapy, adding a csDMARD only if needed at least 3 months later.
The primary outcome was the percentage of participants who reached CID without taking glucocorticoids at 24 months. Since the participants were not randomized, the researchers made adjustments to account for baseline differences between the groups, including differences in JIA categories, number of active joints, physician global assessment of disease activity, and the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10).
At 24 months in an intention to treat analysis, 59.4% of the early-combination group had achieved CID, compared with 48% of the biologic-first group and 40.1% of the step-up group (P = .009 for early combination vs. step up). All three groups had improved since the 12-month time point, when 37% of the early-combination group, 24% of the biologic-first group, and 32% of the step-up group had reached CID.
There were no significant differences between the groups in secondary outcomes of achieving cJADAS10 inactive disease of 2.5 or less or 70% improvement in pediatric ACR response criteria at 24 months. All groups improved in PROMIS pain interference or mobility measures from baseline. Most of the 17 severe adverse events were infections.
Moving from step-up therapy to early-combination treatment
Dr. Brown said that she spent many years in her practice using the step-up therapy because it was difficult to get insurance companies to pay for biologics without first showing that methotrexate was insufficient.
”But methotrexate takes so long to control the disease that you need a lot of steroids, with all of their side effects, at least temporarily, or you must simply accept a longer period of active and symptomatic disease before you get to that desired state of clinically inactive disease,” Dr. Brown said. “And during that time, you can be accumulating what may be permanent damage to joints, as well as increase in risk of contractures and deconditioning for that child who is too uncomfortable to move and exercise and play normally.”
Dr. Brown is also wary of using a biologic as an initial therapy by itself because the actions of biologics are so specific. ”I like to back up the powerful, rapid, and specific actions of a biologic with the broader, if slower, action of methotrexate to minimize chances that the immune system is going to find a way around blockade of a single cytokine by your biologic,” she said.
While patient preference will also play a role in what CTP patients with polyJIA start with, Dr. Brown said that she believes more medication upfront can result in less medication and better outcomes in the long run, as the findings of this study suggest. The results here are helpful when speaking with families who are anxious about “so much medicine” or “such powerful medicines,” she said. ”I hope it will also help ease the fears of other providers who share the same concerns about ‘so much medicine.’ ”
The study’s biggest limitation is not being a randomized, controlled trial, but Dr. Brown said the researchers demonstrated effectively that the disease burden remains similar across the groups at baseline.
”It would also be useful to have a clear breakdown of adverse events and opportunistic infections because an excess of opportunistic infections would be a key concern with early combination therapy,” she said, although she added that the study overall was a ”beautiful example of the value of registry data.”
Dr. Kimura emphasized that polyJIA remains a challenging disease to treat, with 40%-60% of participants not reaching CID at 24 months. The registry follow-up will continue for up to 10 years to hopefully provide more information about longer-term outcomes from different treatments.
The research was funded by a grant from Genentech to CARRA. Dr. Kimura reported royalties from UpToDate and salary support from CARRA. Dr. Brown had no disclosures.
Initiating treatment of polyarticular juvenile idiopathic arthritis (polyJIA) with both a conventional synthetic disease-modifying antirheumatic drug and a biologic DMARD resulted in more patients achieving clinical inactive disease 2 years later than did starting with only a csDMARD and stepping up to a biologic, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“The 24-month results support the 12-month primary results that suggested that the early-combination group was superior and that, at 24 months, more early combination CTP [consensus treatment plan] patients achieve CID [clinical inactive disease], compared to step up,” Yukiko Kimura, MD, division chief of pediatric rheumatology at HMH Hackensack (N.J.) University Medical Center, told attendees. “This suggests that starting biologics early in polyJIA may lead to better long-term outcomes in many patients.”
Dr. Kimura noted that polyarticular JIA patients are already at risk for poor outcomes, and initial therapy can especially impact outcomes. Further, little evidence exists to suggest when the best time is to start biologics, a gap this study aimed to address.
Diane Brown, MD, PhD, a pediatric rheumatologist at Children’s Hospital Los Angeles who was not involved in the study, was pleased to see the results, which she said support her own preferences and practice patterns.
“Starting sooner with combination therapy, taking advantage of the advances with biologics and our long history with methotrexate at the same time, gives better outcomes for the long run,” Dr. Brown said in an interview. “Having studies like this to back up my own recommendations can be very powerful when talking to families, and it is absolutely invaluable when battling with insurance companies who always want you to take the cheapest road.”
Study details
The findings were an update of 12-month results in the CARRA STOP-JIA study that enrolled 400 untreated patients with polyJIA and compared three Childhood Arthritis and Rheumatology Research Alliance (CARRA) CTPs. Overall, 49.5% of participants received biologics within 3 months of starting the study. For these updated results, 275 participants had complete data at 24 months for the three CTPs:
- A step-up group of 177 patients who started therapy with a csDMARD and added a biologic if needed at least 3 months later
- An early-combination group of 73 patients who started therapy with a csDMARD and biologic together
- A biologic-first group of 25 patients who started with biologic monotherapy, adding a csDMARD only if needed at least 3 months later.
The primary outcome was the percentage of participants who reached CID without taking glucocorticoids at 24 months. Since the participants were not randomized, the researchers made adjustments to account for baseline differences between the groups, including differences in JIA categories, number of active joints, physician global assessment of disease activity, and the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10).
At 24 months in an intention to treat analysis, 59.4% of the early-combination group had achieved CID, compared with 48% of the biologic-first group and 40.1% of the step-up group (P = .009 for early combination vs. step up). All three groups had improved since the 12-month time point, when 37% of the early-combination group, 24% of the biologic-first group, and 32% of the step-up group had reached CID.
There were no significant differences between the groups in secondary outcomes of achieving cJADAS10 inactive disease of 2.5 or less or 70% improvement in pediatric ACR response criteria at 24 months. All groups improved in PROMIS pain interference or mobility measures from baseline. Most of the 17 severe adverse events were infections.
Moving from step-up therapy to early-combination treatment
Dr. Brown said that she spent many years in her practice using the step-up therapy because it was difficult to get insurance companies to pay for biologics without first showing that methotrexate was insufficient.
”But methotrexate takes so long to control the disease that you need a lot of steroids, with all of their side effects, at least temporarily, or you must simply accept a longer period of active and symptomatic disease before you get to that desired state of clinically inactive disease,” Dr. Brown said. “And during that time, you can be accumulating what may be permanent damage to joints, as well as increase in risk of contractures and deconditioning for that child who is too uncomfortable to move and exercise and play normally.”
Dr. Brown is also wary of using a biologic as an initial therapy by itself because the actions of biologics are so specific. ”I like to back up the powerful, rapid, and specific actions of a biologic with the broader, if slower, action of methotrexate to minimize chances that the immune system is going to find a way around blockade of a single cytokine by your biologic,” she said.
While patient preference will also play a role in what CTP patients with polyJIA start with, Dr. Brown said that she believes more medication upfront can result in less medication and better outcomes in the long run, as the findings of this study suggest. The results here are helpful when speaking with families who are anxious about “so much medicine” or “such powerful medicines,” she said. ”I hope it will also help ease the fears of other providers who share the same concerns about ‘so much medicine.’ ”
The study’s biggest limitation is not being a randomized, controlled trial, but Dr. Brown said the researchers demonstrated effectively that the disease burden remains similar across the groups at baseline.
”It would also be useful to have a clear breakdown of adverse events and opportunistic infections because an excess of opportunistic infections would be a key concern with early combination therapy,” she said, although she added that the study overall was a ”beautiful example of the value of registry data.”
Dr. Kimura emphasized that polyJIA remains a challenging disease to treat, with 40%-60% of participants not reaching CID at 24 months. The registry follow-up will continue for up to 10 years to hopefully provide more information about longer-term outcomes from different treatments.
The research was funded by a grant from Genentech to CARRA. Dr. Kimura reported royalties from UpToDate and salary support from CARRA. Dr. Brown had no disclosures.
FROM ACR 2021