User login
Ustekinumab becomes second biologic approved for PsA in kids
The Food and Drug Administration has approved the dual interleukin-12 and IL-23 inhibitor ustekinumab (Stelara) for the treatment of juvenile psoriatic arthritis (jPsA) in patients aged 6 years and older, according to an Aug. 1 announcement from its manufacturer, Janssen.
The approval makes jPsA the sixth approved indication for ustekinumab, which include active psoriatic arthritis in adults, moderate to severe plaque psoriasis in both adults and children aged 6 years or older who are candidates for phototherapy or systemic therapy, moderately to severely active Crohn’s disease in adults, and moderately to severely active ulcerative colitis in adults.
In addition, ustekinumab is now the second biologic to be approved for jPsA, following the agency’s December 2021 approval of secukinumab (Cosentyx) to treat jPsA in children and adolescents aged 2 years and older as well as enthesitis-related arthritis in children and adolescents aged 4 years and older.
In pediatric patients, ustekinumab is administered as a subcutaneous injection dosed four times per year after two starter doses.
Ustekinumab’s approval is based on “an extrapolation of the established data and existing safety profile” of ustekinumab in multiple phase 3 studies in adult and pediatric patients with moderate to severe plaque psoriasis and adult patients with active PsA, according to Janssen.
“With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population,” according to the October 2021 announcement from the company that the Biologics License Application had been submitted to the FDA.
Juvenile arthritis occurs in an estimated 20-45 children per 100,000 in the United States, with about 5% of those children having jPsA, according to the National Psoriasis Foundation.
The prescribing information for ustekinumab includes specific warnings and areas of concern. The drug should not be administered to individuals with known hypersensitivity to ustekinumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, sometimes serious, and a test for tuberculosis infection should be given before administration.
Patients taking ustekinumab should not be given a live vaccine, and their doctors should be informed if anyone in their household needs a live vaccine. They also should not receive the BCG vaccine during the 1 year before receiving the drug or 1 year after they stop taking it, according to Johnson & Johnson.
The most common adverse effects include nasal congestion, sore throat, runny nose, upper respiratory infections, fever, headache, tiredness, itching, nausea and vomiting, redness at the injection site, vaginal yeast infections, urinary tract infections, sinus infection, bronchitis, diarrhea, stomach pain, and joint pain.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the dual interleukin-12 and IL-23 inhibitor ustekinumab (Stelara) for the treatment of juvenile psoriatic arthritis (jPsA) in patients aged 6 years and older, according to an Aug. 1 announcement from its manufacturer, Janssen.
The approval makes jPsA the sixth approved indication for ustekinumab, which include active psoriatic arthritis in adults, moderate to severe plaque psoriasis in both adults and children aged 6 years or older who are candidates for phototherapy or systemic therapy, moderately to severely active Crohn’s disease in adults, and moderately to severely active ulcerative colitis in adults.
In addition, ustekinumab is now the second biologic to be approved for jPsA, following the agency’s December 2021 approval of secukinumab (Cosentyx) to treat jPsA in children and adolescents aged 2 years and older as well as enthesitis-related arthritis in children and adolescents aged 4 years and older.
In pediatric patients, ustekinumab is administered as a subcutaneous injection dosed four times per year after two starter doses.
Ustekinumab’s approval is based on “an extrapolation of the established data and existing safety profile” of ustekinumab in multiple phase 3 studies in adult and pediatric patients with moderate to severe plaque psoriasis and adult patients with active PsA, according to Janssen.
“With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population,” according to the October 2021 announcement from the company that the Biologics License Application had been submitted to the FDA.
Juvenile arthritis occurs in an estimated 20-45 children per 100,000 in the United States, with about 5% of those children having jPsA, according to the National Psoriasis Foundation.
The prescribing information for ustekinumab includes specific warnings and areas of concern. The drug should not be administered to individuals with known hypersensitivity to ustekinumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, sometimes serious, and a test for tuberculosis infection should be given before administration.
Patients taking ustekinumab should not be given a live vaccine, and their doctors should be informed if anyone in their household needs a live vaccine. They also should not receive the BCG vaccine during the 1 year before receiving the drug or 1 year after they stop taking it, according to Johnson & Johnson.
The most common adverse effects include nasal congestion, sore throat, runny nose, upper respiratory infections, fever, headache, tiredness, itching, nausea and vomiting, redness at the injection site, vaginal yeast infections, urinary tract infections, sinus infection, bronchitis, diarrhea, stomach pain, and joint pain.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the dual interleukin-12 and IL-23 inhibitor ustekinumab (Stelara) for the treatment of juvenile psoriatic arthritis (jPsA) in patients aged 6 years and older, according to an Aug. 1 announcement from its manufacturer, Janssen.
The approval makes jPsA the sixth approved indication for ustekinumab, which include active psoriatic arthritis in adults, moderate to severe plaque psoriasis in both adults and children aged 6 years or older who are candidates for phototherapy or systemic therapy, moderately to severely active Crohn’s disease in adults, and moderately to severely active ulcerative colitis in adults.
In addition, ustekinumab is now the second biologic to be approved for jPsA, following the agency’s December 2021 approval of secukinumab (Cosentyx) to treat jPsA in children and adolescents aged 2 years and older as well as enthesitis-related arthritis in children and adolescents aged 4 years and older.
In pediatric patients, ustekinumab is administered as a subcutaneous injection dosed four times per year after two starter doses.
Ustekinumab’s approval is based on “an extrapolation of the established data and existing safety profile” of ustekinumab in multiple phase 3 studies in adult and pediatric patients with moderate to severe plaque psoriasis and adult patients with active PsA, according to Janssen.
“With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population,” according to the October 2021 announcement from the company that the Biologics License Application had been submitted to the FDA.
Juvenile arthritis occurs in an estimated 20-45 children per 100,000 in the United States, with about 5% of those children having jPsA, according to the National Psoriasis Foundation.
The prescribing information for ustekinumab includes specific warnings and areas of concern. The drug should not be administered to individuals with known hypersensitivity to ustekinumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, sometimes serious, and a test for tuberculosis infection should be given before administration.
Patients taking ustekinumab should not be given a live vaccine, and their doctors should be informed if anyone in their household needs a live vaccine. They also should not receive the BCG vaccine during the 1 year before receiving the drug or 1 year after they stop taking it, according to Johnson & Johnson.
The most common adverse effects include nasal congestion, sore throat, runny nose, upper respiratory infections, fever, headache, tiredness, itching, nausea and vomiting, redness at the injection site, vaginal yeast infections, urinary tract infections, sinus infection, bronchitis, diarrhea, stomach pain, and joint pain.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not lower risk of fractures
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Uveitis in juvenile arthritis patients persists into midlife
Active uveitis remained in 43.4% of juvenile idiopathic arthritis (JIA) patients up to 40 years after a diagnosis, based on data from 30 individuals.
Uveitis occurs in approximately 10%-20% of patients with JIA, but data on the long-term activity and prevalence are limited, although previous studies suggest that uveitis can persist into adulthood, wrote Dr. Angelika Skarin of Skåne University in Lund, Sweden, and colleagues.
In a study published in Pediatric Rheumatology, the researchers reviewed ophthalmic records from 30 JIA patients at a mean of 40.7 years after uveitis onset. They compared these records to data collected from the same patient population at a mean of 7.2 and 24.0 years after onset. In the previous follow-up studies, 49% of the patients had active uveitis at 24 years, and the prevalence of cataracts and glaucoma increased between the 7-year and 24-year assessments.
In the current study, 43.4% of the population had active uveitis at the 40-year follow-up, which corresponded to 23.6% of the original study cohort. The mean age of the participants overall was 46.9 years, the mean duration of joint disease was 42.99 years, and the mean time from onset of uveitis was 40.7 years.
In addition, 66.6% of the patients in the current study had cataracts or had undergone cataract surgery in one or both eyes, and 40.0% had glaucoma.
By the time of the current study, of the original cohort of 55 individuals, 11 were deceased; rheumatic disease was declared the main cause in four patients and a contributing factor in three others.
Potential drivers of the earliest cases of glaucoma and ocular hypertension (G/OH) include increased intraocular pressure as a result of topical corticosteroid treatment, the researchers noted in their discussion. However, G/OH occurring later than the 7-year follow-up was “more likely to be the type observed in many patients with long-standing chronic uveitis, where a gradual increase in intraocular pressure is assumed to be caused by impaired aqueous outflow,” they said.
Only 4 of the 30 patients did not have regular ophthalmology visits, which suggests a study population with ocular symptoms or concerns about their eyesight, the researchers wrote. “The fact that 13% of our original cohort were reported to have severe visual impairment or worse in both eyes at any of the three follow-ups is noteworthy,” compared to reports of visual impairment of less than 0.5% in a German study in the general population for similar ages.
The findings were limited by several factors, including the retrospective design, small study population, and lack of data on 25 of the original 55-member study cohort, which may reduce the reliability of the current study, the researchers noted. However, the results reflect data from previous studies and support the need for JIA patients to continue regular ophthalmic checkups throughout life, they concluded.
The study was supported by Stiftelsen för Synskadade i f.d. Malmöhus län, Sweden, Skånes Universitetssjukhus Stiftelser och Donationer, Ögonfonden, and the Swedish Society of Medicine. The researchers had no financial conflicts to disclose.
Active uveitis remained in 43.4% of juvenile idiopathic arthritis (JIA) patients up to 40 years after a diagnosis, based on data from 30 individuals.
Uveitis occurs in approximately 10%-20% of patients with JIA, but data on the long-term activity and prevalence are limited, although previous studies suggest that uveitis can persist into adulthood, wrote Dr. Angelika Skarin of Skåne University in Lund, Sweden, and colleagues.
In a study published in Pediatric Rheumatology, the researchers reviewed ophthalmic records from 30 JIA patients at a mean of 40.7 years after uveitis onset. They compared these records to data collected from the same patient population at a mean of 7.2 and 24.0 years after onset. In the previous follow-up studies, 49% of the patients had active uveitis at 24 years, and the prevalence of cataracts and glaucoma increased between the 7-year and 24-year assessments.
In the current study, 43.4% of the population had active uveitis at the 40-year follow-up, which corresponded to 23.6% of the original study cohort. The mean age of the participants overall was 46.9 years, the mean duration of joint disease was 42.99 years, and the mean time from onset of uveitis was 40.7 years.
In addition, 66.6% of the patients in the current study had cataracts or had undergone cataract surgery in one or both eyes, and 40.0% had glaucoma.
By the time of the current study, of the original cohort of 55 individuals, 11 were deceased; rheumatic disease was declared the main cause in four patients and a contributing factor in three others.
Potential drivers of the earliest cases of glaucoma and ocular hypertension (G/OH) include increased intraocular pressure as a result of topical corticosteroid treatment, the researchers noted in their discussion. However, G/OH occurring later than the 7-year follow-up was “more likely to be the type observed in many patients with long-standing chronic uveitis, where a gradual increase in intraocular pressure is assumed to be caused by impaired aqueous outflow,” they said.
Only 4 of the 30 patients did not have regular ophthalmology visits, which suggests a study population with ocular symptoms or concerns about their eyesight, the researchers wrote. “The fact that 13% of our original cohort were reported to have severe visual impairment or worse in both eyes at any of the three follow-ups is noteworthy,” compared to reports of visual impairment of less than 0.5% in a German study in the general population for similar ages.
The findings were limited by several factors, including the retrospective design, small study population, and lack of data on 25 of the original 55-member study cohort, which may reduce the reliability of the current study, the researchers noted. However, the results reflect data from previous studies and support the need for JIA patients to continue regular ophthalmic checkups throughout life, they concluded.
The study was supported by Stiftelsen för Synskadade i f.d. Malmöhus län, Sweden, Skånes Universitetssjukhus Stiftelser och Donationer, Ögonfonden, and the Swedish Society of Medicine. The researchers had no financial conflicts to disclose.
Active uveitis remained in 43.4% of juvenile idiopathic arthritis (JIA) patients up to 40 years after a diagnosis, based on data from 30 individuals.
Uveitis occurs in approximately 10%-20% of patients with JIA, but data on the long-term activity and prevalence are limited, although previous studies suggest that uveitis can persist into adulthood, wrote Dr. Angelika Skarin of Skåne University in Lund, Sweden, and colleagues.
In a study published in Pediatric Rheumatology, the researchers reviewed ophthalmic records from 30 JIA patients at a mean of 40.7 years after uveitis onset. They compared these records to data collected from the same patient population at a mean of 7.2 and 24.0 years after onset. In the previous follow-up studies, 49% of the patients had active uveitis at 24 years, and the prevalence of cataracts and glaucoma increased between the 7-year and 24-year assessments.
In the current study, 43.4% of the population had active uveitis at the 40-year follow-up, which corresponded to 23.6% of the original study cohort. The mean age of the participants overall was 46.9 years, the mean duration of joint disease was 42.99 years, and the mean time from onset of uveitis was 40.7 years.
In addition, 66.6% of the patients in the current study had cataracts or had undergone cataract surgery in one or both eyes, and 40.0% had glaucoma.
By the time of the current study, of the original cohort of 55 individuals, 11 were deceased; rheumatic disease was declared the main cause in four patients and a contributing factor in three others.
Potential drivers of the earliest cases of glaucoma and ocular hypertension (G/OH) include increased intraocular pressure as a result of topical corticosteroid treatment, the researchers noted in their discussion. However, G/OH occurring later than the 7-year follow-up was “more likely to be the type observed in many patients with long-standing chronic uveitis, where a gradual increase in intraocular pressure is assumed to be caused by impaired aqueous outflow,” they said.
Only 4 of the 30 patients did not have regular ophthalmology visits, which suggests a study population with ocular symptoms or concerns about their eyesight, the researchers wrote. “The fact that 13% of our original cohort were reported to have severe visual impairment or worse in both eyes at any of the three follow-ups is noteworthy,” compared to reports of visual impairment of less than 0.5% in a German study in the general population for similar ages.
The findings were limited by several factors, including the retrospective design, small study population, and lack of data on 25 of the original 55-member study cohort, which may reduce the reliability of the current study, the researchers noted. However, the results reflect data from previous studies and support the need for JIA patients to continue regular ophthalmic checkups throughout life, they concluded.
The study was supported by Stiftelsen för Synskadade i f.d. Malmöhus län, Sweden, Skånes Universitetssjukhus Stiftelser och Donationer, Ögonfonden, and the Swedish Society of Medicine. The researchers had no financial conflicts to disclose.
FROM PEDIATRIC RHEUMATOLOGY
No more ‘escape hatch’: Post Roe, new worries about meds linked to birth defects
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Biomarkers may help to predict persistent oligoarticular JIA
Ongoing research in patients with oligoarticular juvenile idiopathic arthritis (JIA) so far suggests that a set of biomarkers in synovial fluid may help to predict which patients may be more likely to stay with persistent oligoarticular disease rather than progress to polyarticular disease, according to new research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year. Identifying biomarkers in synovial fluid or possibly serum could aid families and physicians in being more proactive in treatment protocols, said AnneMarie C. Brescia, MD, of Nemours Children’s Hospital in Wilmington, Del.
“JIA carries the risk of permanent joint damage and disability, which can result when joint involvement evolves from oligoarticular into a polyarticular course, termed extended oligoarticular disease,” Dr. Brescia told attendees. “Since disease progression increases the risk for disability, early prediction of this course is essential.”
This group – those whose oligoarticular disease will begin recruiting joints and ultimately become extended oligoarticular JIA – is “very important because they have been shown to have worse health-related quality of life and greater risk of needing a joint replacement than even polyarticular [JIA],” Dr. Brescia said. “So, our lab has really focused on trying to predict who will fall in this group.”
Melissa Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University in Indianapolis, was not involved in the study but agreed that having highly sensitive and specific biomarkers could be particularly helpful in clinical care.
“Biomarkers can help guide treatment decisions and help physicians and their patients share the decision-making about next choices and when to change,” Dr. Oliver told this news organization. “If a provider and parent know that their child has these markers in their serum or synovial fluid that may predict extension of their disease, then they may be more aggressive upfront with therapy.”
The study aimed to determine whether differential levels of synovial fluid proteins could be used to predict whether JIA would evolve into an extended course before it became clinically evident. Although early aggressive treatment is common with rheumatoid arthritis and can lead to remission, JIA treatment paradigms tend to be more reactive, Dr. Brescia said.
“It would be better to switch to proactive, that if we’re able to predict that this patient may have a more difficult course with extension to polyarticular, we could be prepared, we could inform the parents, and it would just help us have a more proactive approach,” she said.
The researchers used antibody arrays to detect the following inflammatory mediators in blinded samples: CD14, interleukin (IL)-1-alpha, IL-3, IL-5, IL-6, vascular endothelial growth factor (VEGF), and angiogenin. They analyzed 37 samples with persistent disease and 32 samples from disease that had not yet extended but would become extended in that patient. The samples came from patients who were taking no medicines or only NSAIDs. The researchers assessed the sensitivity and specificity of each biomarker. Sensitivity referred the biomarker’s ability to correctly indicate that the sample would extend, and specificity referred to the biomarker’s accuracy in determining that the disease in the sample would remain persistent.
Combining samples from cohorts at Nemours Children’s Health (14 persistent and 7 extended-to-be) and Cincinnati Children’s Hospital (23 persistent and 25 extended-to-be) yielded the following results: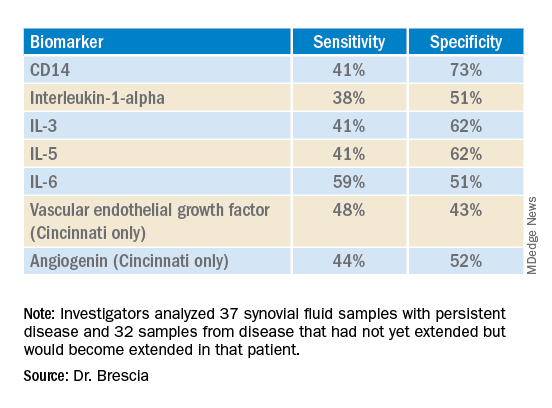
The findings revealed that the selected biomarkers were more accurate at predicting whose disease would remain persistent than predicting those that would extend, Dr. Brescia said. CD14 was the most specific biomarker, and IL-6 was the most sensitive biomarker in both groups.
When the researchers translated the findings from ELISA to the Luminex platform, positive results in synovial fluid for all these biomarkers were also positive in serum samples. Although the differences between persistent and extended-to-be samples did not reach statistical significance using Luminex, the pattern was the same for each biomarker.
“Luminex is more sensitive than ELISA. We believe that conducting an LDA [linear discriminant analysis] using these Luminex measurements will allow us to determine new cutoffs or new protein levels that are appropriate for Luminex to predict who will extend,” Dr. Brescia said. “It’s also our goal to develop a serum panel because ... being able to detect these markers in serum would expand the applicability of these markers to more patients.”
Dr. Brescia then described the group’s work in defining clinically relevant subpopulations of patients based on fibroblast-like synoviocytes (FLS) cells in the synovial intimal lining that produce inflammatory cytokines.
“Our compelling, single-cell, RNA sequencing preliminary data revealing multiple subpopulations within the total FLS population supports our hypothesis that distinct FLS subpopulations correlate with clinical outcome,” said Dr. Brescia. They looked at the percentage of chondrocyte-like, fibroblast-like, and smooth muscle-like subpopulations in samples from patients with oligoarticular JIA, extended-to-be JIA, and polyarticular JIA. Chondrocytes occurred in the largest proportion, and polyarticular JIA FLS had the largest percentage of chondrocytes, compared with the other two subpopulation groups.
“This is a work in progress,” Dr. Brescia said, “so hopefully you’ll hear about it next year.” In response to an attendee’s question, she said she believes identifying reliable biomarkers will eventually lead to refining treatment paradigms.
“I think it will at least change the guidance we can provide parents about making next choices and how quickly to accelerate to those next choices,” Dr. Brescia said. For example, if a child’s serum or synovial fluid has markers that show a very high likelihood of extension, the parent may decide to proceed to the next level medication sooner. “I do think it will push both parents and doctors to be a little more proactive instead of reactive when the poor patient comes back with 13 joints involved when they had just been an oligo for years.”
Dr. Oliver noted the promise of CD14 and IL-6 in potentially predicting which patients’ disease will stay persistent but cautioned that it’s still early in evaluating these biomarkers, especially with the limited patient samples in this study.
“I think these results are promising, and it’s great that there are groups out there working on this,” Dr. Oliver said. “Once we have a reliable, highly sensitive and specific biomarker, that will definitely help providers, parents, and patients be more informed.”
The research was supported by the Open Net Foundation, the Arthritis Foundation, Delaware Community Foundation, the Delaware Clinical and Translational Research (DE-CTR) ACCEL Program, the Nancy Taylor Foundation for Chronic Diseases, and CARRA. Dr. Brescia and Dr. Oliver have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ongoing research in patients with oligoarticular juvenile idiopathic arthritis (JIA) so far suggests that a set of biomarkers in synovial fluid may help to predict which patients may be more likely to stay with persistent oligoarticular disease rather than progress to polyarticular disease, according to new research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year. Identifying biomarkers in synovial fluid or possibly serum could aid families and physicians in being more proactive in treatment protocols, said AnneMarie C. Brescia, MD, of Nemours Children’s Hospital in Wilmington, Del.
“JIA carries the risk of permanent joint damage and disability, which can result when joint involvement evolves from oligoarticular into a polyarticular course, termed extended oligoarticular disease,” Dr. Brescia told attendees. “Since disease progression increases the risk for disability, early prediction of this course is essential.”
This group – those whose oligoarticular disease will begin recruiting joints and ultimately become extended oligoarticular JIA – is “very important because they have been shown to have worse health-related quality of life and greater risk of needing a joint replacement than even polyarticular [JIA],” Dr. Brescia said. “So, our lab has really focused on trying to predict who will fall in this group.”
Melissa Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University in Indianapolis, was not involved in the study but agreed that having highly sensitive and specific biomarkers could be particularly helpful in clinical care.
“Biomarkers can help guide treatment decisions and help physicians and their patients share the decision-making about next choices and when to change,” Dr. Oliver told this news organization. “If a provider and parent know that their child has these markers in their serum or synovial fluid that may predict extension of their disease, then they may be more aggressive upfront with therapy.”
The study aimed to determine whether differential levels of synovial fluid proteins could be used to predict whether JIA would evolve into an extended course before it became clinically evident. Although early aggressive treatment is common with rheumatoid arthritis and can lead to remission, JIA treatment paradigms tend to be more reactive, Dr. Brescia said.
“It would be better to switch to proactive, that if we’re able to predict that this patient may have a more difficult course with extension to polyarticular, we could be prepared, we could inform the parents, and it would just help us have a more proactive approach,” she said.
The researchers used antibody arrays to detect the following inflammatory mediators in blinded samples: CD14, interleukin (IL)-1-alpha, IL-3, IL-5, IL-6, vascular endothelial growth factor (VEGF), and angiogenin. They analyzed 37 samples with persistent disease and 32 samples from disease that had not yet extended but would become extended in that patient. The samples came from patients who were taking no medicines or only NSAIDs. The researchers assessed the sensitivity and specificity of each biomarker. Sensitivity referred the biomarker’s ability to correctly indicate that the sample would extend, and specificity referred to the biomarker’s accuracy in determining that the disease in the sample would remain persistent.
Combining samples from cohorts at Nemours Children’s Health (14 persistent and 7 extended-to-be) and Cincinnati Children’s Hospital (23 persistent and 25 extended-to-be) yielded the following results:
The findings revealed that the selected biomarkers were more accurate at predicting whose disease would remain persistent than predicting those that would extend, Dr. Brescia said. CD14 was the most specific biomarker, and IL-6 was the most sensitive biomarker in both groups.
When the researchers translated the findings from ELISA to the Luminex platform, positive results in synovial fluid for all these biomarkers were also positive in serum samples. Although the differences between persistent and extended-to-be samples did not reach statistical significance using Luminex, the pattern was the same for each biomarker.
“Luminex is more sensitive than ELISA. We believe that conducting an LDA [linear discriminant analysis] using these Luminex measurements will allow us to determine new cutoffs or new protein levels that are appropriate for Luminex to predict who will extend,” Dr. Brescia said. “It’s also our goal to develop a serum panel because ... being able to detect these markers in serum would expand the applicability of these markers to more patients.”
Dr. Brescia then described the group’s work in defining clinically relevant subpopulations of patients based on fibroblast-like synoviocytes (FLS) cells in the synovial intimal lining that produce inflammatory cytokines.
“Our compelling, single-cell, RNA sequencing preliminary data revealing multiple subpopulations within the total FLS population supports our hypothesis that distinct FLS subpopulations correlate with clinical outcome,” said Dr. Brescia. They looked at the percentage of chondrocyte-like, fibroblast-like, and smooth muscle-like subpopulations in samples from patients with oligoarticular JIA, extended-to-be JIA, and polyarticular JIA. Chondrocytes occurred in the largest proportion, and polyarticular JIA FLS had the largest percentage of chondrocytes, compared with the other two subpopulation groups.
“This is a work in progress,” Dr. Brescia said, “so hopefully you’ll hear about it next year.” In response to an attendee’s question, she said she believes identifying reliable biomarkers will eventually lead to refining treatment paradigms.
“I think it will at least change the guidance we can provide parents about making next choices and how quickly to accelerate to those next choices,” Dr. Brescia said. For example, if a child’s serum or synovial fluid has markers that show a very high likelihood of extension, the parent may decide to proceed to the next level medication sooner. “I do think it will push both parents and doctors to be a little more proactive instead of reactive when the poor patient comes back with 13 joints involved when they had just been an oligo for years.”
Dr. Oliver noted the promise of CD14 and IL-6 in potentially predicting which patients’ disease will stay persistent but cautioned that it’s still early in evaluating these biomarkers, especially with the limited patient samples in this study.
“I think these results are promising, and it’s great that there are groups out there working on this,” Dr. Oliver said. “Once we have a reliable, highly sensitive and specific biomarker, that will definitely help providers, parents, and patients be more informed.”
The research was supported by the Open Net Foundation, the Arthritis Foundation, Delaware Community Foundation, the Delaware Clinical and Translational Research (DE-CTR) ACCEL Program, the Nancy Taylor Foundation for Chronic Diseases, and CARRA. Dr. Brescia and Dr. Oliver have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ongoing research in patients with oligoarticular juvenile idiopathic arthritis (JIA) so far suggests that a set of biomarkers in synovial fluid may help to predict which patients may be more likely to stay with persistent oligoarticular disease rather than progress to polyarticular disease, according to new research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year. Identifying biomarkers in synovial fluid or possibly serum could aid families and physicians in being more proactive in treatment protocols, said AnneMarie C. Brescia, MD, of Nemours Children’s Hospital in Wilmington, Del.
“JIA carries the risk of permanent joint damage and disability, which can result when joint involvement evolves from oligoarticular into a polyarticular course, termed extended oligoarticular disease,” Dr. Brescia told attendees. “Since disease progression increases the risk for disability, early prediction of this course is essential.”
This group – those whose oligoarticular disease will begin recruiting joints and ultimately become extended oligoarticular JIA – is “very important because they have been shown to have worse health-related quality of life and greater risk of needing a joint replacement than even polyarticular [JIA],” Dr. Brescia said. “So, our lab has really focused on trying to predict who will fall in this group.”
Melissa Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University in Indianapolis, was not involved in the study but agreed that having highly sensitive and specific biomarkers could be particularly helpful in clinical care.
“Biomarkers can help guide treatment decisions and help physicians and their patients share the decision-making about next choices and when to change,” Dr. Oliver told this news organization. “If a provider and parent know that their child has these markers in their serum or synovial fluid that may predict extension of their disease, then they may be more aggressive upfront with therapy.”
The study aimed to determine whether differential levels of synovial fluid proteins could be used to predict whether JIA would evolve into an extended course before it became clinically evident. Although early aggressive treatment is common with rheumatoid arthritis and can lead to remission, JIA treatment paradigms tend to be more reactive, Dr. Brescia said.
“It would be better to switch to proactive, that if we’re able to predict that this patient may have a more difficult course with extension to polyarticular, we could be prepared, we could inform the parents, and it would just help us have a more proactive approach,” she said.
The researchers used antibody arrays to detect the following inflammatory mediators in blinded samples: CD14, interleukin (IL)-1-alpha, IL-3, IL-5, IL-6, vascular endothelial growth factor (VEGF), and angiogenin. They analyzed 37 samples with persistent disease and 32 samples from disease that had not yet extended but would become extended in that patient. The samples came from patients who were taking no medicines or only NSAIDs. The researchers assessed the sensitivity and specificity of each biomarker. Sensitivity referred the biomarker’s ability to correctly indicate that the sample would extend, and specificity referred to the biomarker’s accuracy in determining that the disease in the sample would remain persistent.
Combining samples from cohorts at Nemours Children’s Health (14 persistent and 7 extended-to-be) and Cincinnati Children’s Hospital (23 persistent and 25 extended-to-be) yielded the following results:
The findings revealed that the selected biomarkers were more accurate at predicting whose disease would remain persistent than predicting those that would extend, Dr. Brescia said. CD14 was the most specific biomarker, and IL-6 was the most sensitive biomarker in both groups.
When the researchers translated the findings from ELISA to the Luminex platform, positive results in synovial fluid for all these biomarkers were also positive in serum samples. Although the differences between persistent and extended-to-be samples did not reach statistical significance using Luminex, the pattern was the same for each biomarker.
“Luminex is more sensitive than ELISA. We believe that conducting an LDA [linear discriminant analysis] using these Luminex measurements will allow us to determine new cutoffs or new protein levels that are appropriate for Luminex to predict who will extend,” Dr. Brescia said. “It’s also our goal to develop a serum panel because ... being able to detect these markers in serum would expand the applicability of these markers to more patients.”
Dr. Brescia then described the group’s work in defining clinically relevant subpopulations of patients based on fibroblast-like synoviocytes (FLS) cells in the synovial intimal lining that produce inflammatory cytokines.
“Our compelling, single-cell, RNA sequencing preliminary data revealing multiple subpopulations within the total FLS population supports our hypothesis that distinct FLS subpopulations correlate with clinical outcome,” said Dr. Brescia. They looked at the percentage of chondrocyte-like, fibroblast-like, and smooth muscle-like subpopulations in samples from patients with oligoarticular JIA, extended-to-be JIA, and polyarticular JIA. Chondrocytes occurred in the largest proportion, and polyarticular JIA FLS had the largest percentage of chondrocytes, compared with the other two subpopulation groups.
“This is a work in progress,” Dr. Brescia said, “so hopefully you’ll hear about it next year.” In response to an attendee’s question, she said she believes identifying reliable biomarkers will eventually lead to refining treatment paradigms.
“I think it will at least change the guidance we can provide parents about making next choices and how quickly to accelerate to those next choices,” Dr. Brescia said. For example, if a child’s serum or synovial fluid has markers that show a very high likelihood of extension, the parent may decide to proceed to the next level medication sooner. “I do think it will push both parents and doctors to be a little more proactive instead of reactive when the poor patient comes back with 13 joints involved when they had just been an oligo for years.”
Dr. Oliver noted the promise of CD14 and IL-6 in potentially predicting which patients’ disease will stay persistent but cautioned that it’s still early in evaluating these biomarkers, especially with the limited patient samples in this study.
“I think these results are promising, and it’s great that there are groups out there working on this,” Dr. Oliver said. “Once we have a reliable, highly sensitive and specific biomarker, that will definitely help providers, parents, and patients be more informed.”
The research was supported by the Open Net Foundation, the Arthritis Foundation, Delaware Community Foundation, the Delaware Clinical and Translational Research (DE-CTR) ACCEL Program, the Nancy Taylor Foundation for Chronic Diseases, and CARRA. Dr. Brescia and Dr. Oliver have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CARRA 2022
Is nonoperative treatment effective for acute Achilles tendon rupture?
Evidence summary
Surgical repair: Re-injury risk goes down, complications risk goes up
A 2021 network meta-analysis including 38 RCTs (N = 2480) reported outcomes in patients ages 18 and older with acute Achilles tendon rupture (AATR) and 3 or more months of follow-up.1 A significant increase in re-rupture rate was shown in patients who underwent nonoperative vs open repair (risk ratio [RR] = 2.41; 95% CI, 1.12-5.18). There was a significant decrease in wound-related complications in nonoperative vs open-repair patients (RR = 0.23; 95% CI, 0.06-0.88). There was also a significant difference in incidence of sural nerve injury in nonoperative vs open repair (RR = 0.27; 95% CI, 0.08-0.94). There were no significant differences in return to sport between open repair and nonoperative repair (RR = 0.62; 95% CI, 0.22-1.77). Insufficient data were reported to calculate the number needed to treat (NNT) and number needed to harm (NNH) for these outcomes.
Additionally, the authors looked at traditional standard rehabilitation and accelerated functional rehabilitation in both the operative and the nonoperative setting. The type of rehabilitation program did not have a significant impact on complications of re-rupture, wound, or sural nerve injury.
The included studies had an overall low risk of publication bias based on Begg’s funnel plot test (Pr > |z| = 0.86). The highest risk was performance bias, as neither the participants nor personnel were blinded to treatment in 71% of the studies.
Functional outcomes are similar for surgical vs nonoperative repair
In a 2019 meta-analysis of 9 RCTs (N = 822), adults ages 18 and older with AATR and a minimum of 12 months’ follow-up were randomized to either operative or nonoperative repair. There was a decreased rate of rupture with surgical repair and an associated increased rate of complications (ie, superficial wound infections and nerve injury). However, there was no significant difference in Physical Activity Scale (PAS) score between the 2 groups (mean difference, –0.05; 95% CI, –0.37 to 0.27).2 With surgical intervention, the NNT for Achilles tendon re-rupture was 15, and the NNH for superficial wound infection and nerve injury, respectively, were 22 and 28. Limitations of the study included different operative techniques and rehab protocols, which may have affected the results of the included studies.
A third meta-analysis consisted of 10 RCTs and 19 observational studies (N = 15,862) with patients ages 16 years and older treated operatively vs nonoperatively. Function and return-to-activity rates in both the short term (≤ 1 year) and long term (> 1 year) were evaluated using the Achilles tendon Total Rupture Score (ATRS).3 Surgical management was associated with decreased re-rupture rates but increased complication rates. However, when the analysis was limited to studies using accelerated functional rehabilitation programs, there was no significant difference in re-rupture rate (RR = 0.26 to 1.37; P = .23). Only 1 observational study found a statistically significant difference in short-term functional outcomes favoring operative management, and no studies found a significant difference in long-term functional outcomes. These functional outcomes were not pooled for statistical analysis due to high interrater variability of the ATRS.
An RCT showed equal “customer satisfaction”
One RCT randomized 61 patients to either surgical or nonsurgical management and followed them for a mean of 15.7 years.4 Patient-reported outcomes of function, symptoms, and impact on daily life were measured using various surveys. There was no statistically significant difference in the function and impact on daily life after treatment according to the Short Musculoskeletal Function Assessment or the ATRS (P = .289 and .313, respectively). When assessed using the Net Promoter Score (a single-question metric used in consumer industry to assess whether an individual would recommend the product to others), there was no statistical significance for the patients to recommend one treatment over another: 79% of operatively managed patients vs 87% of nonoperatively managed patients would recommend their treatment to others (P = .225).
Recommendations from others
The American College of Foot and Ankle Surgeons consensus statement finds no difference between operative and nonoperative management with regard to complications, functional outcome, and return to activity long term, when looking at available Level 1 evidence.5 They do acknowledge that although some Level III studies suggest operative intervention will return high-functioning patients to full activity sooner, there should be discussion regarding the risks and complications of both operative and nonoperative management. Patients with increased risk factors for postoperative complications (diabetes, obesity, cigarette smoking) should have special considerations regarding the decision to operate.
Editor’s takeaway
Large data sets with consistent results show that nonoperative treatment of Achilles tendon rupture is an excellent option. However, we cannot say if it is better or worse than operative treatment, because both options have advantages and disadvantages. One must weigh the alternatives with individual patient preferences and circumstances.
1. Shi F, Wu S, Cai W, et al. Multiple comparisons of the efficacy and safety for six treatments in acute Achilles tendon rupture patients: a systematic review and network meta-analysis. Foot Ankle Surg. 2021;27:468-479. doi: 10.1016/j.fas.2020.07.004
2. Reda Y, Farouk A, Abdelmonem I, et al. Surgical versus non-surgical treatment for acute Achilles tendon rupture. A systematic review of literature and meta-analysis. Foot Ankle Surg. 2020;26:280-288. doi: 10.1016/j.fas.2019.03.010
3. Ochen Y, Beks RB, van Heijl M, et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019;364:k5120. doi: 10.1136/bmj.k5120
4. Maempel JF, Clement ND, Wickramasinghe NR, et al. Operative repair of acute Achilles tendon rupture does not give superior patient-reported outcomes to nonoperative management. Bone Joint J. 2020;102-B:933-940. doi: 10.1302/0301-620x.102b7.bjj-2019-0783.r3
5. Naldo J, Agnew P, Brucato M, et al. ACFAS clinical consensus statement: acute Achilles tendon pathology. J Foot Ankle Surg. 2021;60:93-101. doi: 10.1053/j.jfas.2020.02.006
Evidence summary
Surgical repair: Re-injury risk goes down, complications risk goes up
A 2021 network meta-analysis including 38 RCTs (N = 2480) reported outcomes in patients ages 18 and older with acute Achilles tendon rupture (AATR) and 3 or more months of follow-up.1 A significant increase in re-rupture rate was shown in patients who underwent nonoperative vs open repair (risk ratio [RR] = 2.41; 95% CI, 1.12-5.18). There was a significant decrease in wound-related complications in nonoperative vs open-repair patients (RR = 0.23; 95% CI, 0.06-0.88). There was also a significant difference in incidence of sural nerve injury in nonoperative vs open repair (RR = 0.27; 95% CI, 0.08-0.94). There were no significant differences in return to sport between open repair and nonoperative repair (RR = 0.62; 95% CI, 0.22-1.77). Insufficient data were reported to calculate the number needed to treat (NNT) and number needed to harm (NNH) for these outcomes.
Additionally, the authors looked at traditional standard rehabilitation and accelerated functional rehabilitation in both the operative and the nonoperative setting. The type of rehabilitation program did not have a significant impact on complications of re-rupture, wound, or sural nerve injury.
The included studies had an overall low risk of publication bias based on Begg’s funnel plot test (Pr > |z| = 0.86). The highest risk was performance bias, as neither the participants nor personnel were blinded to treatment in 71% of the studies.
Functional outcomes are similar for surgical vs nonoperative repair
In a 2019 meta-analysis of 9 RCTs (N = 822), adults ages 18 and older with AATR and a minimum of 12 months’ follow-up were randomized to either operative or nonoperative repair. There was a decreased rate of rupture with surgical repair and an associated increased rate of complications (ie, superficial wound infections and nerve injury). However, there was no significant difference in Physical Activity Scale (PAS) score between the 2 groups (mean difference, –0.05; 95% CI, –0.37 to 0.27).2 With surgical intervention, the NNT for Achilles tendon re-rupture was 15, and the NNH for superficial wound infection and nerve injury, respectively, were 22 and 28. Limitations of the study included different operative techniques and rehab protocols, which may have affected the results of the included studies.
A third meta-analysis consisted of 10 RCTs and 19 observational studies (N = 15,862) with patients ages 16 years and older treated operatively vs nonoperatively. Function and return-to-activity rates in both the short term (≤ 1 year) and long term (> 1 year) were evaluated using the Achilles tendon Total Rupture Score (ATRS).3 Surgical management was associated with decreased re-rupture rates but increased complication rates. However, when the analysis was limited to studies using accelerated functional rehabilitation programs, there was no significant difference in re-rupture rate (RR = 0.26 to 1.37; P = .23). Only 1 observational study found a statistically significant difference in short-term functional outcomes favoring operative management, and no studies found a significant difference in long-term functional outcomes. These functional outcomes were not pooled for statistical analysis due to high interrater variability of the ATRS.
An RCT showed equal “customer satisfaction”
One RCT randomized 61 patients to either surgical or nonsurgical management and followed them for a mean of 15.7 years.4 Patient-reported outcomes of function, symptoms, and impact on daily life were measured using various surveys. There was no statistically significant difference in the function and impact on daily life after treatment according to the Short Musculoskeletal Function Assessment or the ATRS (P = .289 and .313, respectively). When assessed using the Net Promoter Score (a single-question metric used in consumer industry to assess whether an individual would recommend the product to others), there was no statistical significance for the patients to recommend one treatment over another: 79% of operatively managed patients vs 87% of nonoperatively managed patients would recommend their treatment to others (P = .225).
Recommendations from others
The American College of Foot and Ankle Surgeons consensus statement finds no difference between operative and nonoperative management with regard to complications, functional outcome, and return to activity long term, when looking at available Level 1 evidence.5 They do acknowledge that although some Level III studies suggest operative intervention will return high-functioning patients to full activity sooner, there should be discussion regarding the risks and complications of both operative and nonoperative management. Patients with increased risk factors for postoperative complications (diabetes, obesity, cigarette smoking) should have special considerations regarding the decision to operate.
Editor’s takeaway
Large data sets with consistent results show that nonoperative treatment of Achilles tendon rupture is an excellent option. However, we cannot say if it is better or worse than operative treatment, because both options have advantages and disadvantages. One must weigh the alternatives with individual patient preferences and circumstances.
Evidence summary
Surgical repair: Re-injury risk goes down, complications risk goes up
A 2021 network meta-analysis including 38 RCTs (N = 2480) reported outcomes in patients ages 18 and older with acute Achilles tendon rupture (AATR) and 3 or more months of follow-up.1 A significant increase in re-rupture rate was shown in patients who underwent nonoperative vs open repair (risk ratio [RR] = 2.41; 95% CI, 1.12-5.18). There was a significant decrease in wound-related complications in nonoperative vs open-repair patients (RR = 0.23; 95% CI, 0.06-0.88). There was also a significant difference in incidence of sural nerve injury in nonoperative vs open repair (RR = 0.27; 95% CI, 0.08-0.94). There were no significant differences in return to sport between open repair and nonoperative repair (RR = 0.62; 95% CI, 0.22-1.77). Insufficient data were reported to calculate the number needed to treat (NNT) and number needed to harm (NNH) for these outcomes.
Additionally, the authors looked at traditional standard rehabilitation and accelerated functional rehabilitation in both the operative and the nonoperative setting. The type of rehabilitation program did not have a significant impact on complications of re-rupture, wound, or sural nerve injury.
The included studies had an overall low risk of publication bias based on Begg’s funnel plot test (Pr > |z| = 0.86). The highest risk was performance bias, as neither the participants nor personnel were blinded to treatment in 71% of the studies.
Functional outcomes are similar for surgical vs nonoperative repair
In a 2019 meta-analysis of 9 RCTs (N = 822), adults ages 18 and older with AATR and a minimum of 12 months’ follow-up were randomized to either operative or nonoperative repair. There was a decreased rate of rupture with surgical repair and an associated increased rate of complications (ie, superficial wound infections and nerve injury). However, there was no significant difference in Physical Activity Scale (PAS) score between the 2 groups (mean difference, –0.05; 95% CI, –0.37 to 0.27).2 With surgical intervention, the NNT for Achilles tendon re-rupture was 15, and the NNH for superficial wound infection and nerve injury, respectively, were 22 and 28. Limitations of the study included different operative techniques and rehab protocols, which may have affected the results of the included studies.
A third meta-analysis consisted of 10 RCTs and 19 observational studies (N = 15,862) with patients ages 16 years and older treated operatively vs nonoperatively. Function and return-to-activity rates in both the short term (≤ 1 year) and long term (> 1 year) were evaluated using the Achilles tendon Total Rupture Score (ATRS).3 Surgical management was associated with decreased re-rupture rates but increased complication rates. However, when the analysis was limited to studies using accelerated functional rehabilitation programs, there was no significant difference in re-rupture rate (RR = 0.26 to 1.37; P = .23). Only 1 observational study found a statistically significant difference in short-term functional outcomes favoring operative management, and no studies found a significant difference in long-term functional outcomes. These functional outcomes were not pooled for statistical analysis due to high interrater variability of the ATRS.
An RCT showed equal “customer satisfaction”
One RCT randomized 61 patients to either surgical or nonsurgical management and followed them for a mean of 15.7 years.4 Patient-reported outcomes of function, symptoms, and impact on daily life were measured using various surveys. There was no statistically significant difference in the function and impact on daily life after treatment according to the Short Musculoskeletal Function Assessment or the ATRS (P = .289 and .313, respectively). When assessed using the Net Promoter Score (a single-question metric used in consumer industry to assess whether an individual would recommend the product to others), there was no statistical significance for the patients to recommend one treatment over another: 79% of operatively managed patients vs 87% of nonoperatively managed patients would recommend their treatment to others (P = .225).
Recommendations from others
The American College of Foot and Ankle Surgeons consensus statement finds no difference between operative and nonoperative management with regard to complications, functional outcome, and return to activity long term, when looking at available Level 1 evidence.5 They do acknowledge that although some Level III studies suggest operative intervention will return high-functioning patients to full activity sooner, there should be discussion regarding the risks and complications of both operative and nonoperative management. Patients with increased risk factors for postoperative complications (diabetes, obesity, cigarette smoking) should have special considerations regarding the decision to operate.
Editor’s takeaway
Large data sets with consistent results show that nonoperative treatment of Achilles tendon rupture is an excellent option. However, we cannot say if it is better or worse than operative treatment, because both options have advantages and disadvantages. One must weigh the alternatives with individual patient preferences and circumstances.
1. Shi F, Wu S, Cai W, et al. Multiple comparisons of the efficacy and safety for six treatments in acute Achilles tendon rupture patients: a systematic review and network meta-analysis. Foot Ankle Surg. 2021;27:468-479. doi: 10.1016/j.fas.2020.07.004
2. Reda Y, Farouk A, Abdelmonem I, et al. Surgical versus non-surgical treatment for acute Achilles tendon rupture. A systematic review of literature and meta-analysis. Foot Ankle Surg. 2020;26:280-288. doi: 10.1016/j.fas.2019.03.010
3. Ochen Y, Beks RB, van Heijl M, et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019;364:k5120. doi: 10.1136/bmj.k5120
4. Maempel JF, Clement ND, Wickramasinghe NR, et al. Operative repair of acute Achilles tendon rupture does not give superior patient-reported outcomes to nonoperative management. Bone Joint J. 2020;102-B:933-940. doi: 10.1302/0301-620x.102b7.bjj-2019-0783.r3
5. Naldo J, Agnew P, Brucato M, et al. ACFAS clinical consensus statement: acute Achilles tendon pathology. J Foot Ankle Surg. 2021;60:93-101. doi: 10.1053/j.jfas.2020.02.006
1. Shi F, Wu S, Cai W, et al. Multiple comparisons of the efficacy and safety for six treatments in acute Achilles tendon rupture patients: a systematic review and network meta-analysis. Foot Ankle Surg. 2021;27:468-479. doi: 10.1016/j.fas.2020.07.004
2. Reda Y, Farouk A, Abdelmonem I, et al. Surgical versus non-surgical treatment for acute Achilles tendon rupture. A systematic review of literature and meta-analysis. Foot Ankle Surg. 2020;26:280-288. doi: 10.1016/j.fas.2019.03.010
3. Ochen Y, Beks RB, van Heijl M, et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019;364:k5120. doi: 10.1136/bmj.k5120
4. Maempel JF, Clement ND, Wickramasinghe NR, et al. Operative repair of acute Achilles tendon rupture does not give superior patient-reported outcomes to nonoperative management. Bone Joint J. 2020;102-B:933-940. doi: 10.1302/0301-620x.102b7.bjj-2019-0783.r3
5. Naldo J, Agnew P, Brucato M, et al. ACFAS clinical consensus statement: acute Achilles tendon pathology. J Foot Ankle Surg. 2021;60:93-101. doi: 10.1053/j.jfas.2020.02.006
EVIDENCE-BASED ANSWER:
YES. Nonoperative and open sur- gical interventions provide equal long-term functional outcomes of the affected Achilles tendon and ankle (strength of recommendation [SOR], A; based on 2 meta-analyses and a separate randomized controlled trial [RCT]). Although nonoperative management is associated with increased risk of re-rupture, it confers lower risk for complications including wound infection and nerve injury (SOR, A; based on meta-analysis and separate RCT).
Select individuals—high-performing athletes or those who otherwise require near-baseline strength and function of their Achilles tendon—would likely benefit from surgical intervention (SOR, A; based on meta-analysis and consensus recommendations).
Patients with comorbid conditions that would put them at greater risk for postoperative complications should be advised to consider nonoperative treatment of acute Achilles tendon rupture (SOR, C; based on consensus opinion).
Tips for managing 4 common soft-tissue finger and thumb injuries
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).
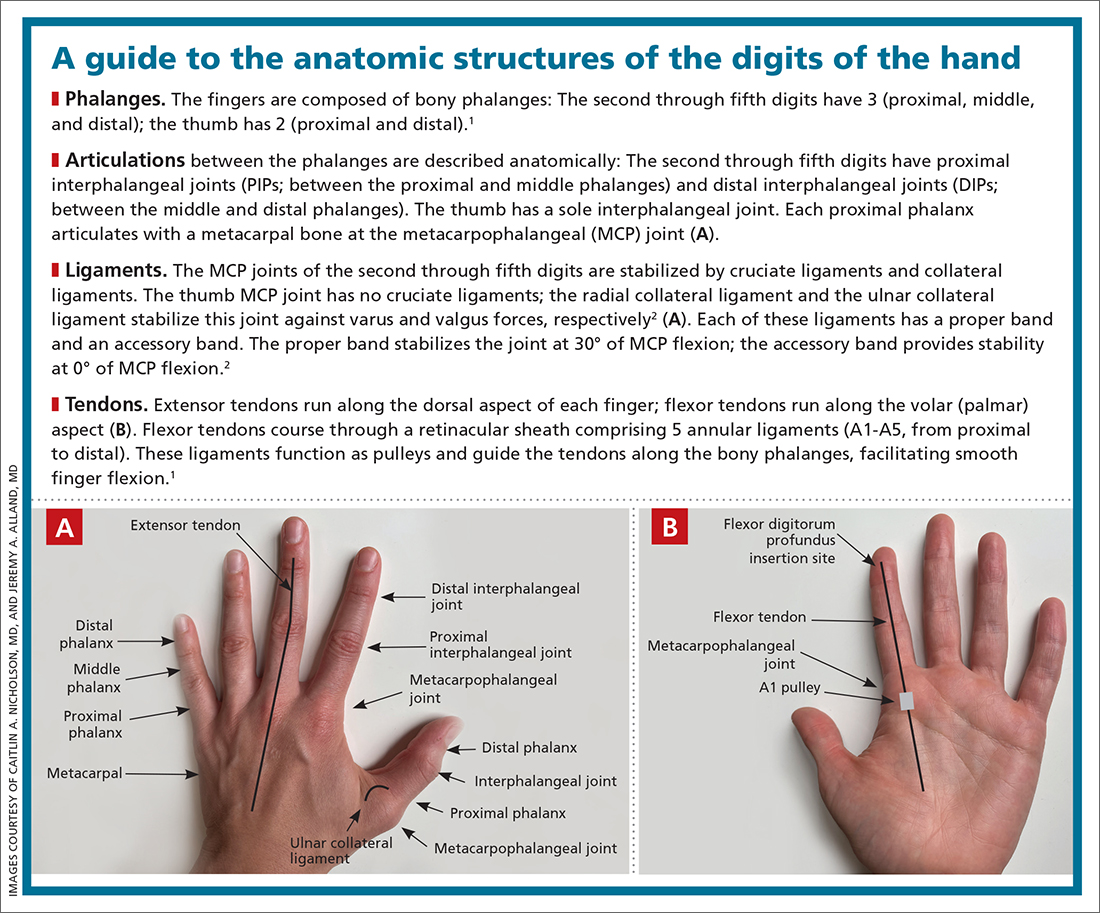
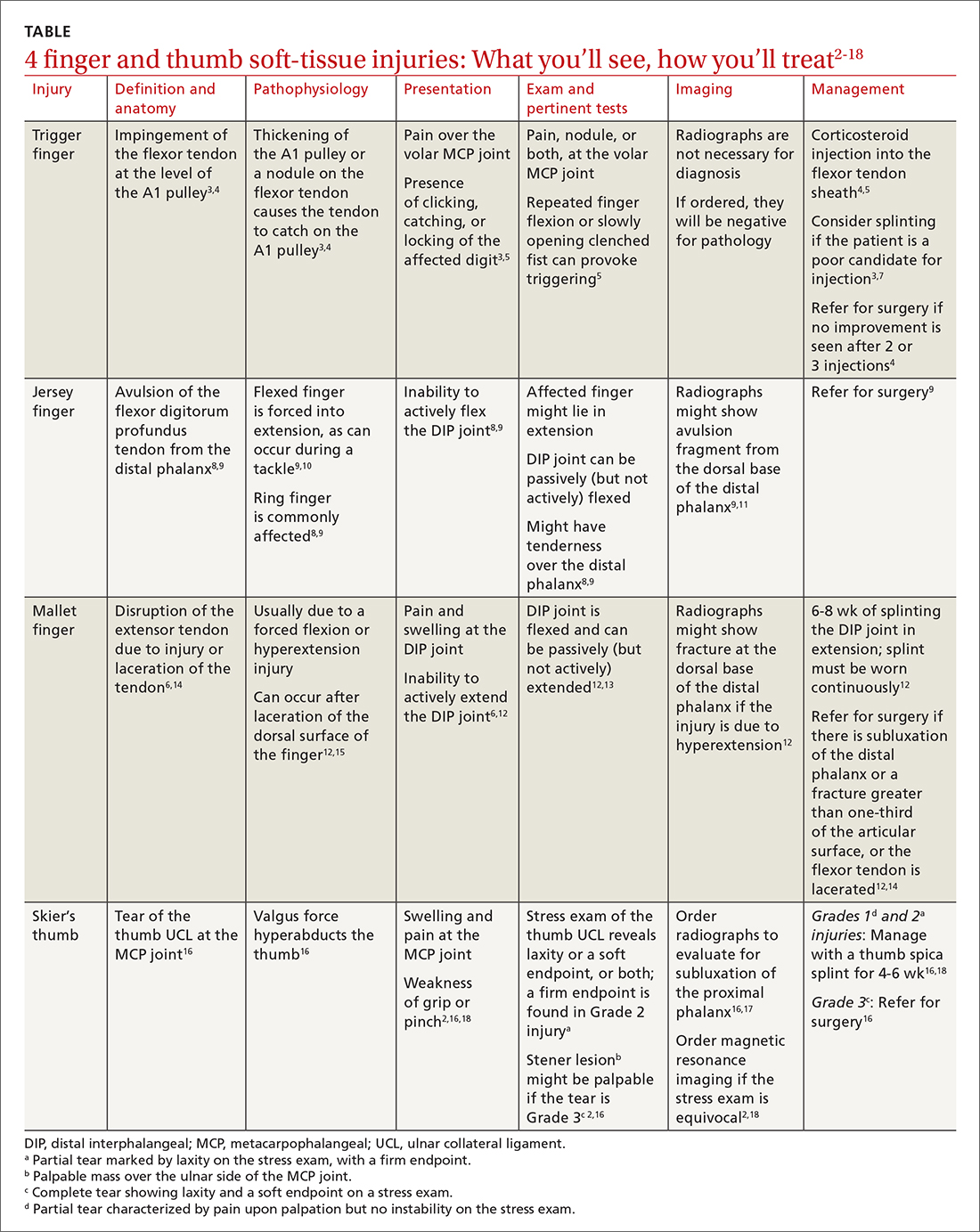
Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6
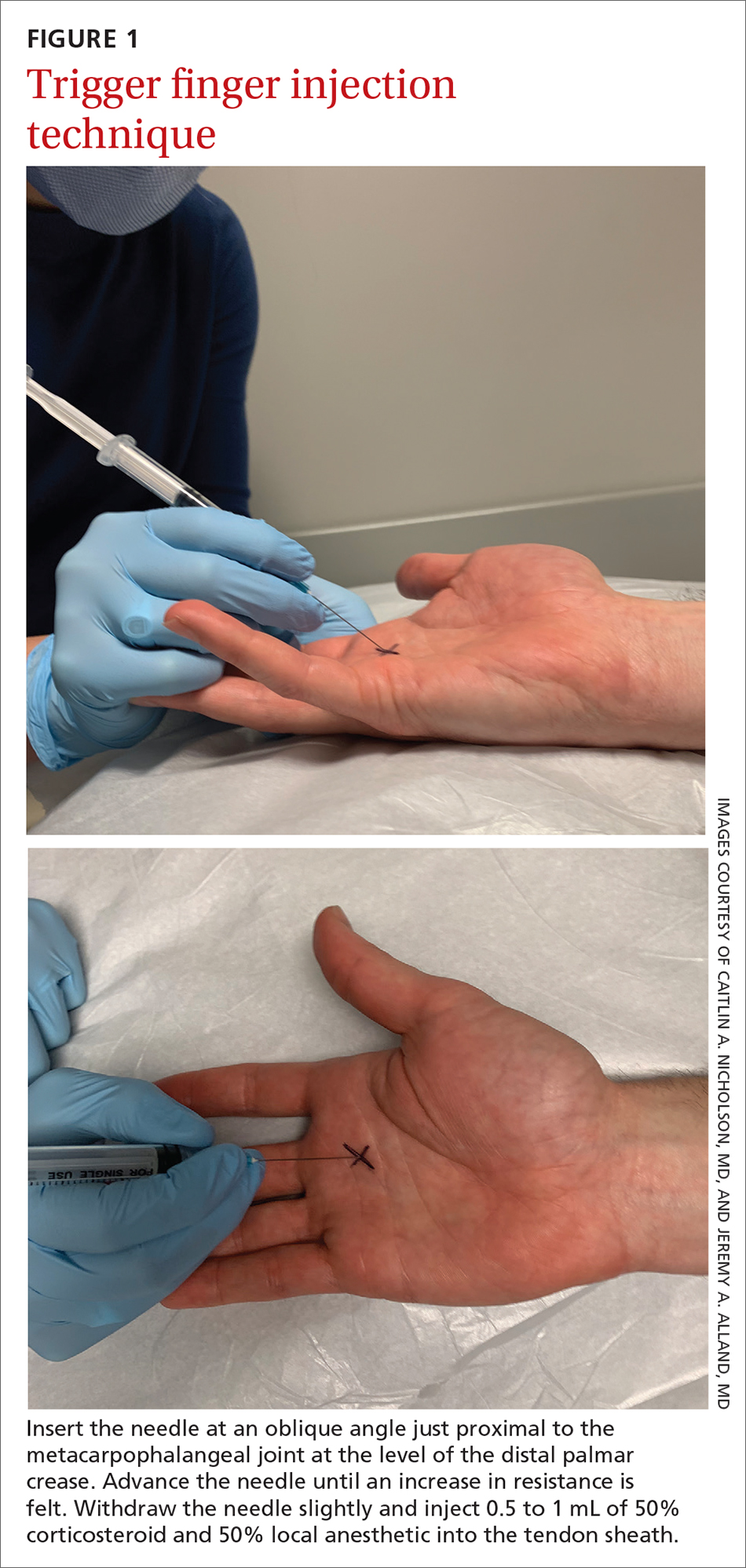
The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23
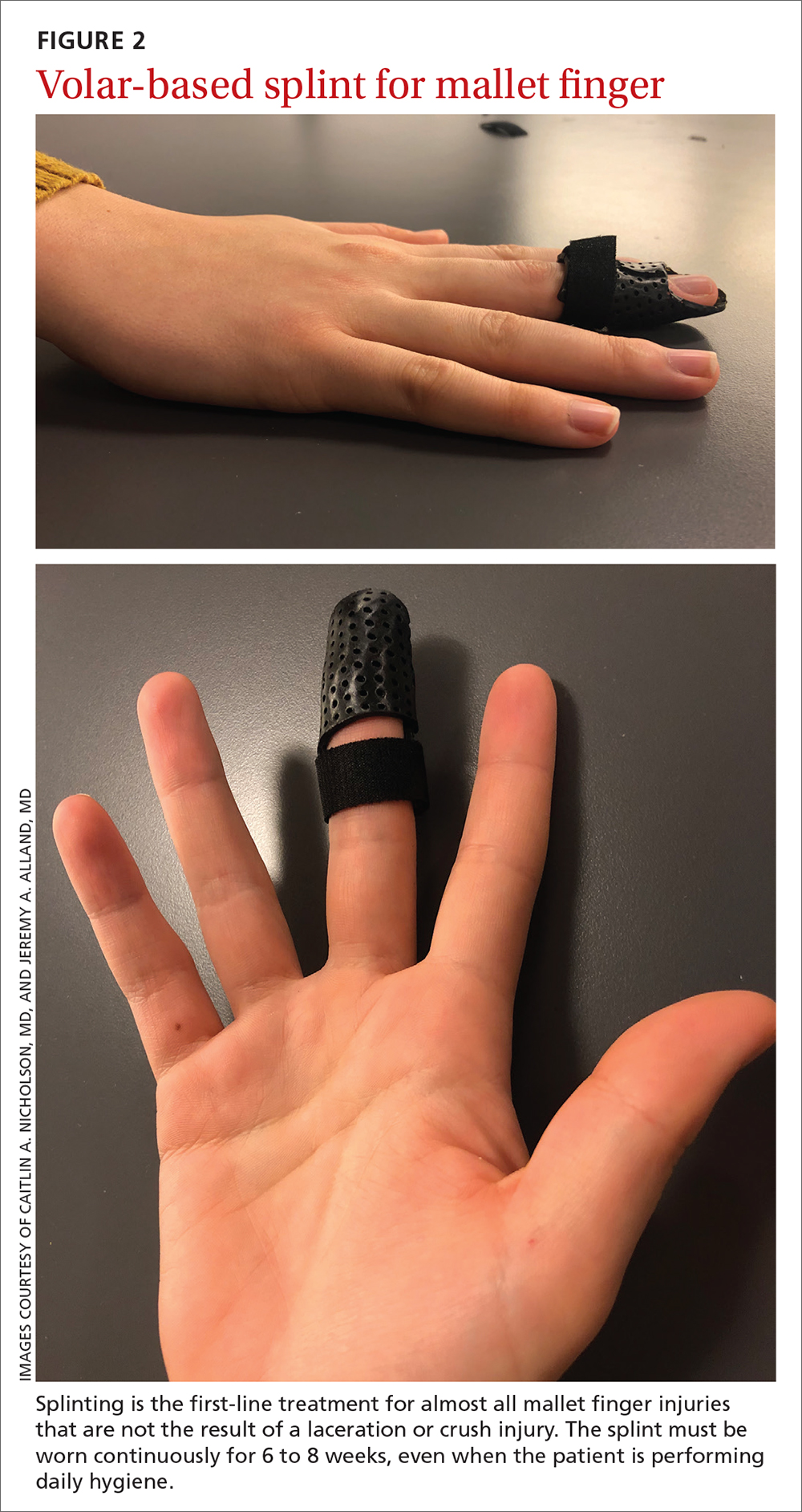
Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17
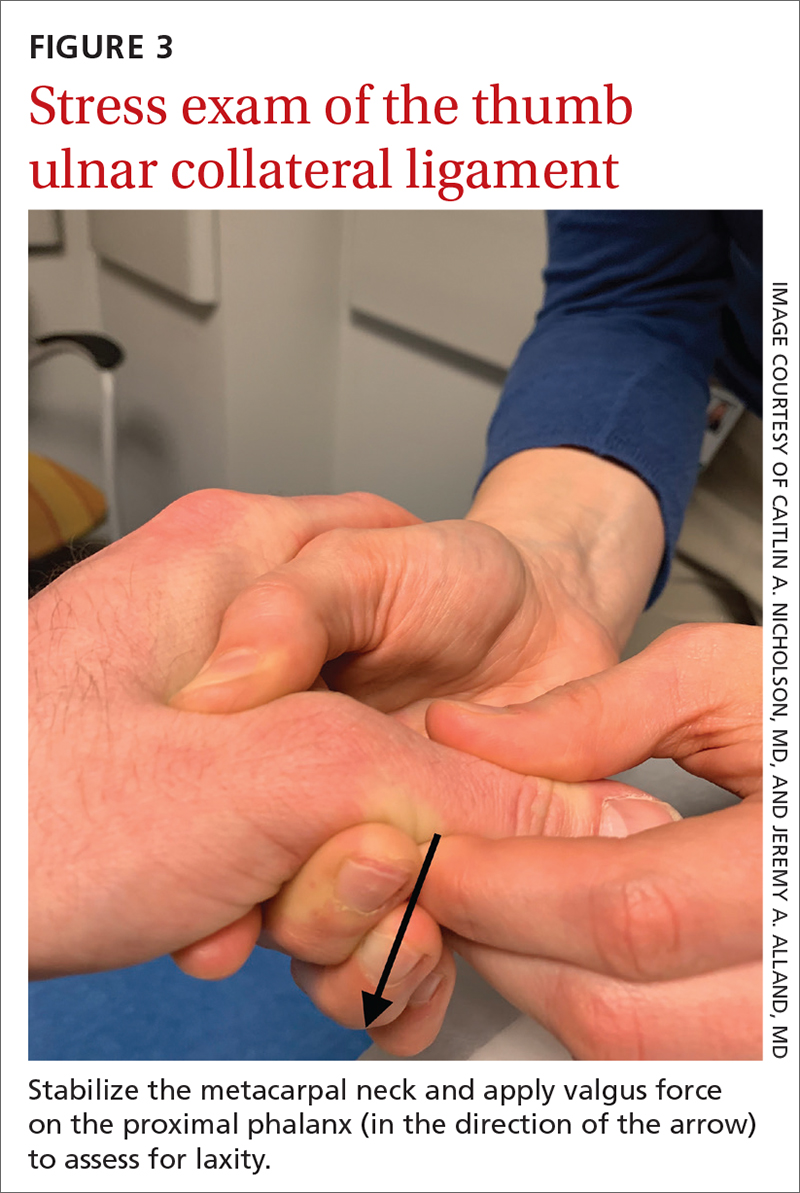
Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
PRACTICE RECOMMENDATIONS
› Treat trigger finger with a corticosteroid injection into the flexor tendon sheath. A
› Refer a case of jersey finger to a hand surgeon within 1 week after injury for flexor tendon repair. C
› Treat mallet finger with strict distal interphalangeal joint immobilization for 6 to 8 weeks. A
› Treat Grades 1 and 2 skier’s thumb with immobilization in a thumb spica splint or a cast for 4 to 6 weeks. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
FDA expands indication for spinal muscular atrophy drug
As previously reported, the FDA first approved oral risdiplam for SMA in children older than age 2 years in 2020.
The FDA expanded the indication for risdiplam to include babies younger than 2 months old because of interim safety and efficacy data from the ongoing RAINBOWFISH study. It includes 25 babies from birth to 6 weeks of age at first dose, all of whom have genetically diagnosed SMA but are not yet presenting with symptoms.
After 12 months of risdiplam treatment, the majority of presymptomatic infants with SMA reached key motor milestones, Genentech said in a news release.
Of the six babies with two or three copies of the SMN2 gene, all were able to sit after 1 year of active treatment, roughly two-thirds could stand, and half could walk independently.
All babies were alive at 12 months without permanent ventilation.
“The approval of Evrysdi for presymptomatic babies is particularly important, as early treatment of SMA, before symptoms start to arise, can help babies to achieve motor milestones,” Richard Finkel, MD, principal investigator of the trial, said in the release.
“With the inclusion of SMA in newborn screening programs, this approval provides the opportunity to start treating at home with Evrysdi soon after the diagnosis is confirmed,” added Dr. Finkel, who is director of the experimental neuroscience program, St. Jude Children’s Research Hospital, Memphis.
From newborns to older adults?
SMA is a rare and often fatal genetic disease that causes muscle weakness and progressive loss of movement.
SMA, which affects about 1 in 10,000 babies, is caused by a mutation in the survival motor neuron 1 (SMN1) gene. The gene encodes the SMN protein, which is critical for the maintenance and function of motor neurons.
Risdiplam is an orally administered, centrally and peripherally distributed small molecule that modulates survival motor neuron 2 (SMN2) premessenger RNA splicing to increase SMN protein levels.
As part of the label extension, the prescribing information for risdiplam has also been updated to include 2-year pooled data from parts 1 and 2 of the FIREFISH study, which demonstrated long-term efficacy and safety in symptomatic infants with Type 1 SMA, the company noted.
“Because of its efficacy in multiple settings, Evrysdi is now available for people with SMA, from presymptomatic newborns to older adults,” Levi Garraway, MD, PhD, chief medical officer and head of global product development at Genentech, said in the release.
“We are proud of this achievement, which has the potential to make a real difference to those living with SMA and their caregivers,” Dr. Garraway added.
A version of this article first appeared on Medscape.com.
As previously reported, the FDA first approved oral risdiplam for SMA in children older than age 2 years in 2020.
The FDA expanded the indication for risdiplam to include babies younger than 2 months old because of interim safety and efficacy data from the ongoing RAINBOWFISH study. It includes 25 babies from birth to 6 weeks of age at first dose, all of whom have genetically diagnosed SMA but are not yet presenting with symptoms.
After 12 months of risdiplam treatment, the majority of presymptomatic infants with SMA reached key motor milestones, Genentech said in a news release.
Of the six babies with two or three copies of the SMN2 gene, all were able to sit after 1 year of active treatment, roughly two-thirds could stand, and half could walk independently.
All babies were alive at 12 months without permanent ventilation.
“The approval of Evrysdi for presymptomatic babies is particularly important, as early treatment of SMA, before symptoms start to arise, can help babies to achieve motor milestones,” Richard Finkel, MD, principal investigator of the trial, said in the release.
“With the inclusion of SMA in newborn screening programs, this approval provides the opportunity to start treating at home with Evrysdi soon after the diagnosis is confirmed,” added Dr. Finkel, who is director of the experimental neuroscience program, St. Jude Children’s Research Hospital, Memphis.
From newborns to older adults?
SMA is a rare and often fatal genetic disease that causes muscle weakness and progressive loss of movement.
SMA, which affects about 1 in 10,000 babies, is caused by a mutation in the survival motor neuron 1 (SMN1) gene. The gene encodes the SMN protein, which is critical for the maintenance and function of motor neurons.
Risdiplam is an orally administered, centrally and peripherally distributed small molecule that modulates survival motor neuron 2 (SMN2) premessenger RNA splicing to increase SMN protein levels.
As part of the label extension, the prescribing information for risdiplam has also been updated to include 2-year pooled data from parts 1 and 2 of the FIREFISH study, which demonstrated long-term efficacy and safety in symptomatic infants with Type 1 SMA, the company noted.
“Because of its efficacy in multiple settings, Evrysdi is now available for people with SMA, from presymptomatic newborns to older adults,” Levi Garraway, MD, PhD, chief medical officer and head of global product development at Genentech, said in the release.
“We are proud of this achievement, which has the potential to make a real difference to those living with SMA and their caregivers,” Dr. Garraway added.
A version of this article first appeared on Medscape.com.
As previously reported, the FDA first approved oral risdiplam for SMA in children older than age 2 years in 2020.
The FDA expanded the indication for risdiplam to include babies younger than 2 months old because of interim safety and efficacy data from the ongoing RAINBOWFISH study. It includes 25 babies from birth to 6 weeks of age at first dose, all of whom have genetically diagnosed SMA but are not yet presenting with symptoms.
After 12 months of risdiplam treatment, the majority of presymptomatic infants with SMA reached key motor milestones, Genentech said in a news release.
Of the six babies with two or three copies of the SMN2 gene, all were able to sit after 1 year of active treatment, roughly two-thirds could stand, and half could walk independently.
All babies were alive at 12 months without permanent ventilation.
“The approval of Evrysdi for presymptomatic babies is particularly important, as early treatment of SMA, before symptoms start to arise, can help babies to achieve motor milestones,” Richard Finkel, MD, principal investigator of the trial, said in the release.
“With the inclusion of SMA in newborn screening programs, this approval provides the opportunity to start treating at home with Evrysdi soon after the diagnosis is confirmed,” added Dr. Finkel, who is director of the experimental neuroscience program, St. Jude Children’s Research Hospital, Memphis.
From newborns to older adults?
SMA is a rare and often fatal genetic disease that causes muscle weakness and progressive loss of movement.
SMA, which affects about 1 in 10,000 babies, is caused by a mutation in the survival motor neuron 1 (SMN1) gene. The gene encodes the SMN protein, which is critical for the maintenance and function of motor neurons.
Risdiplam is an orally administered, centrally and peripherally distributed small molecule that modulates survival motor neuron 2 (SMN2) premessenger RNA splicing to increase SMN protein levels.
As part of the label extension, the prescribing information for risdiplam has also been updated to include 2-year pooled data from parts 1 and 2 of the FIREFISH study, which demonstrated long-term efficacy and safety in symptomatic infants with Type 1 SMA, the company noted.
“Because of its efficacy in multiple settings, Evrysdi is now available for people with SMA, from presymptomatic newborns to older adults,” Levi Garraway, MD, PhD, chief medical officer and head of global product development at Genentech, said in the release.
“We are proud of this achievement, which has the potential to make a real difference to those living with SMA and their caregivers,” Dr. Garraway added.
A version of this article first appeared on Medscape.com.
Pfizer COVID vaccine performs well in youth with rheumatic diseases
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CARRA 2022
Myositis guidelines aim to standardize adult and pediatric care
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
FROM BSR 2022