User login
Is exercise therapy effective treatment for low back pain?
EVIDENCE SUMMARY
General exercise offers benefit …at least for chronic LBP
A 2017 systematic review of 4 systematic reviews and 50 RCTs (122 total trials) evaluated general exercise vs usual care for acute (< 4 weeks), subacute (4 to 12 weeks), or chronic (≥ 12 weeks) LBP with or without radiculopathy in adults.1 Exercise was not consistently associated with decreased pain in acute or subacute LBP. For chronic LBP, 3 RCTs (n = 200) associated exercise with decreased pain (weighted mean difference [WMD] = –9.2 on a 0-100 point visual acuity scale; 95% CI, –16.0 to –2.4) and improved function (WMD = –12.4 on the Oswestry Disability Index; 95% CI, –23.0 to –1.7) at short-term follow-up (≤ 3 months). This effect was found to decrease at long-term (≥ 1 year) follow-up (WMD for pain = –4.9; 95% CI, –10.5 to 0.6 and WMD for function = –3.2; 95% CI, 6.0 to –0.4). In a meta-analysis of 10 studies (n = 1992) included in this systematic review, exercise was associated with a lower likelihood of work disability (odds ratio, 0.66; CI, 0.48 to 0.92) at 12 months.1
Yoga, Pilates, and motor control exercise: Your results may vary
Several reviews have explored the effects of specific exercise modalities on LBP. A 2017 meta-analysis of 9 RCTs in the United States, United Kingdom, and India of nonpregnant adults (≥ 18 years old) with chronic LBP (N = 810) found that yoga (any tradition of yoga with a physical component) vs no exercise demonstrated a statistically, but not clinically, significant decrease in pain at 3 to 4 months (mean difference [MD] = –4.6 on a 0-100 point scale; 95% CI, –7.0 to –2.1), 6 months (MD = –7.8; 95% CI, –13.4 to –2.3), and 12 months (MD = –5.4; 95% CI, –14.5 to –3.7). Clinically significant pain benefit was considered a change of 15 or more points.2
A 2015 meta-analysis of RCTs (10 trials; N = 510) comparing the effects of Pilates (a form of body conditioning involving isometric contractions and core exercises focusing on stability) vs minimal intervention on chronic (> 12 weeks) LBP in nonpregnant adults (≥ 16 years old) found low-quality evidence for decreased pain at short-term follow-up (≤ 3 months; MD = –14.1 on a 0-100 point scale; 95% CI, –18.9 to –9.2). There was moderate-quality evidence for decreased pain at intermediate follow-up (3-12 months; MD = –10.5; 95% CI, –18.5 to –2.6).3
A 2016 systematic review evaluated motor control exercise (MCE; a form of exercise that focuses on trunk muscle control and coordination) in adults (≥ 16 years old) with chronic LBP (≥ 12 weeks). There was low- to moderate-quality evidence that, compared to minimal intervention, MCE decreases pain at short-term (≤ 6 months; 4 RCTs; MD = –10.0 on a 0-100 point scale; 95% CI, –15.7 to –4.4), intermediate (6-12 months; 4 RCTs; MD = –12.6; 95% CI, –20.5 to –4.7), and long-term follow-up (> 12 months; 3 RCTs; MD = –13.0; 95% CI, –18.5 to –7.4). When comparing MCE to general exercise, there were no clinically significant differences in pain or disability at intermediate and long-term follow-up.4Common limitations included heterogeneity of intervention methodology, inability to blind results, inability to assess cointerventions, and in some cases, small sample sizes of trials.
Recommendations from others
The 2017 American College of Physicians (ACP) clinical practice guideline on noninvasive treatments for LBP does not recommend exercise therapy in acute or subacute LBP; recommended therapies include superficial heat, massage, acupuncture, or spinal manipulation.5 The ACP recommends general exercise, yoga, tai chi, or MCE for chronic LBP, in addition to multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, progressive relaxation, biofeedback, laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
The 2017 US Department of Veterans Affairs and US Department of Defense clinical practice guideline on treatment of LBP notes insufficient evidence for benefit of clinician-guided exercise therapy in acute LBP.6 For chronic LBP, clinician-directed exercise, yoga, tai chi, or Pilates is recommended.
Editor’s takeaway
Convincing evidence demonstrates that exercise modestly improves chronic LBP—but only modestly (4% to 15%), and not in acute LBP. This small magnitude of effect may disappoint expectations, but exercise remains among our better interventions for this common chronic problem. Few—if any—interventions have proven better, and exercise has beneficial side effects, a low cost, and widespread availability.
1. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-506. doi: 10.7326/M16-2459
2. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain (review). Cochrane Database Syst Rev. 2017;1:CD010671. doi: 10.1002/14651858.CD010671.pub2
3. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;7:CD010265. doi: 10.1002/14651858.CD010265.pub2
4. Saragiotto BT, Maher CG, Yamato TP, et. al. Motor control exercise for chronic non‐specific low‐back pain. Cochrane Database Syst Rev. 2016;1:CD012004. doi: 10.1002/14651858.CD012004
5. Qaseem A, Wilt TJ, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530. doi: 10.7326/M16-2367
6. Pangarkar SS, Kang DG, Sandbrink F, et al. VA/DoD clinical practice guideline: diagnosis and treatment of low back pain. J Gen Intern Med. 2019;34:2620-2629. doi: 10.1007/s11606-019-05086-4
EVIDENCE SUMMARY
General exercise offers benefit …at least for chronic LBP
A 2017 systematic review of 4 systematic reviews and 50 RCTs (122 total trials) evaluated general exercise vs usual care for acute (< 4 weeks), subacute (4 to 12 weeks), or chronic (≥ 12 weeks) LBP with or without radiculopathy in adults.1 Exercise was not consistently associated with decreased pain in acute or subacute LBP. For chronic LBP, 3 RCTs (n = 200) associated exercise with decreased pain (weighted mean difference [WMD] = –9.2 on a 0-100 point visual acuity scale; 95% CI, –16.0 to –2.4) and improved function (WMD = –12.4 on the Oswestry Disability Index; 95% CI, –23.0 to –1.7) at short-term follow-up (≤ 3 months). This effect was found to decrease at long-term (≥ 1 year) follow-up (WMD for pain = –4.9; 95% CI, –10.5 to 0.6 and WMD for function = –3.2; 95% CI, 6.0 to –0.4). In a meta-analysis of 10 studies (n = 1992) included in this systematic review, exercise was associated with a lower likelihood of work disability (odds ratio, 0.66; CI, 0.48 to 0.92) at 12 months.1
Yoga, Pilates, and motor control exercise: Your results may vary
Several reviews have explored the effects of specific exercise modalities on LBP. A 2017 meta-analysis of 9 RCTs in the United States, United Kingdom, and India of nonpregnant adults (≥ 18 years old) with chronic LBP (N = 810) found that yoga (any tradition of yoga with a physical component) vs no exercise demonstrated a statistically, but not clinically, significant decrease in pain at 3 to 4 months (mean difference [MD] = –4.6 on a 0-100 point scale; 95% CI, –7.0 to –2.1), 6 months (MD = –7.8; 95% CI, –13.4 to –2.3), and 12 months (MD = –5.4; 95% CI, –14.5 to –3.7). Clinically significant pain benefit was considered a change of 15 or more points.2
A 2015 meta-analysis of RCTs (10 trials; N = 510) comparing the effects of Pilates (a form of body conditioning involving isometric contractions and core exercises focusing on stability) vs minimal intervention on chronic (> 12 weeks) LBP in nonpregnant adults (≥ 16 years old) found low-quality evidence for decreased pain at short-term follow-up (≤ 3 months; MD = –14.1 on a 0-100 point scale; 95% CI, –18.9 to –9.2). There was moderate-quality evidence for decreased pain at intermediate follow-up (3-12 months; MD = –10.5; 95% CI, –18.5 to –2.6).3
A 2016 systematic review evaluated motor control exercise (MCE; a form of exercise that focuses on trunk muscle control and coordination) in adults (≥ 16 years old) with chronic LBP (≥ 12 weeks). There was low- to moderate-quality evidence that, compared to minimal intervention, MCE decreases pain at short-term (≤ 6 months; 4 RCTs; MD = –10.0 on a 0-100 point scale; 95% CI, –15.7 to –4.4), intermediate (6-12 months; 4 RCTs; MD = –12.6; 95% CI, –20.5 to –4.7), and long-term follow-up (> 12 months; 3 RCTs; MD = –13.0; 95% CI, –18.5 to –7.4). When comparing MCE to general exercise, there were no clinically significant differences in pain or disability at intermediate and long-term follow-up.4Common limitations included heterogeneity of intervention methodology, inability to blind results, inability to assess cointerventions, and in some cases, small sample sizes of trials.
Recommendations from others
The 2017 American College of Physicians (ACP) clinical practice guideline on noninvasive treatments for LBP does not recommend exercise therapy in acute or subacute LBP; recommended therapies include superficial heat, massage, acupuncture, or spinal manipulation.5 The ACP recommends general exercise, yoga, tai chi, or MCE for chronic LBP, in addition to multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, progressive relaxation, biofeedback, laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
The 2017 US Department of Veterans Affairs and US Department of Defense clinical practice guideline on treatment of LBP notes insufficient evidence for benefit of clinician-guided exercise therapy in acute LBP.6 For chronic LBP, clinician-directed exercise, yoga, tai chi, or Pilates is recommended.
Editor’s takeaway
Convincing evidence demonstrates that exercise modestly improves chronic LBP—but only modestly (4% to 15%), and not in acute LBP. This small magnitude of effect may disappoint expectations, but exercise remains among our better interventions for this common chronic problem. Few—if any—interventions have proven better, and exercise has beneficial side effects, a low cost, and widespread availability.
EVIDENCE SUMMARY
General exercise offers benefit …at least for chronic LBP
A 2017 systematic review of 4 systematic reviews and 50 RCTs (122 total trials) evaluated general exercise vs usual care for acute (< 4 weeks), subacute (4 to 12 weeks), or chronic (≥ 12 weeks) LBP with or without radiculopathy in adults.1 Exercise was not consistently associated with decreased pain in acute or subacute LBP. For chronic LBP, 3 RCTs (n = 200) associated exercise with decreased pain (weighted mean difference [WMD] = –9.2 on a 0-100 point visual acuity scale; 95% CI, –16.0 to –2.4) and improved function (WMD = –12.4 on the Oswestry Disability Index; 95% CI, –23.0 to –1.7) at short-term follow-up (≤ 3 months). This effect was found to decrease at long-term (≥ 1 year) follow-up (WMD for pain = –4.9; 95% CI, –10.5 to 0.6 and WMD for function = –3.2; 95% CI, 6.0 to –0.4). In a meta-analysis of 10 studies (n = 1992) included in this systematic review, exercise was associated with a lower likelihood of work disability (odds ratio, 0.66; CI, 0.48 to 0.92) at 12 months.1
Yoga, Pilates, and motor control exercise: Your results may vary
Several reviews have explored the effects of specific exercise modalities on LBP. A 2017 meta-analysis of 9 RCTs in the United States, United Kingdom, and India of nonpregnant adults (≥ 18 years old) with chronic LBP (N = 810) found that yoga (any tradition of yoga with a physical component) vs no exercise demonstrated a statistically, but not clinically, significant decrease in pain at 3 to 4 months (mean difference [MD] = –4.6 on a 0-100 point scale; 95% CI, –7.0 to –2.1), 6 months (MD = –7.8; 95% CI, –13.4 to –2.3), and 12 months (MD = –5.4; 95% CI, –14.5 to –3.7). Clinically significant pain benefit was considered a change of 15 or more points.2
A 2015 meta-analysis of RCTs (10 trials; N = 510) comparing the effects of Pilates (a form of body conditioning involving isometric contractions and core exercises focusing on stability) vs minimal intervention on chronic (> 12 weeks) LBP in nonpregnant adults (≥ 16 years old) found low-quality evidence for decreased pain at short-term follow-up (≤ 3 months; MD = –14.1 on a 0-100 point scale; 95% CI, –18.9 to –9.2). There was moderate-quality evidence for decreased pain at intermediate follow-up (3-12 months; MD = –10.5; 95% CI, –18.5 to –2.6).3
A 2016 systematic review evaluated motor control exercise (MCE; a form of exercise that focuses on trunk muscle control and coordination) in adults (≥ 16 years old) with chronic LBP (≥ 12 weeks). There was low- to moderate-quality evidence that, compared to minimal intervention, MCE decreases pain at short-term (≤ 6 months; 4 RCTs; MD = –10.0 on a 0-100 point scale; 95% CI, –15.7 to –4.4), intermediate (6-12 months; 4 RCTs; MD = –12.6; 95% CI, –20.5 to –4.7), and long-term follow-up (> 12 months; 3 RCTs; MD = –13.0; 95% CI, –18.5 to –7.4). When comparing MCE to general exercise, there were no clinically significant differences in pain or disability at intermediate and long-term follow-up.4Common limitations included heterogeneity of intervention methodology, inability to blind results, inability to assess cointerventions, and in some cases, small sample sizes of trials.
Recommendations from others
The 2017 American College of Physicians (ACP) clinical practice guideline on noninvasive treatments for LBP does not recommend exercise therapy in acute or subacute LBP; recommended therapies include superficial heat, massage, acupuncture, or spinal manipulation.5 The ACP recommends general exercise, yoga, tai chi, or MCE for chronic LBP, in addition to multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, progressive relaxation, biofeedback, laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
The 2017 US Department of Veterans Affairs and US Department of Defense clinical practice guideline on treatment of LBP notes insufficient evidence for benefit of clinician-guided exercise therapy in acute LBP.6 For chronic LBP, clinician-directed exercise, yoga, tai chi, or Pilates is recommended.
Editor’s takeaway
Convincing evidence demonstrates that exercise modestly improves chronic LBP—but only modestly (4% to 15%), and not in acute LBP. This small magnitude of effect may disappoint expectations, but exercise remains among our better interventions for this common chronic problem. Few—if any—interventions have proven better, and exercise has beneficial side effects, a low cost, and widespread availability.
1. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-506. doi: 10.7326/M16-2459
2. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain (review). Cochrane Database Syst Rev. 2017;1:CD010671. doi: 10.1002/14651858.CD010671.pub2
3. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;7:CD010265. doi: 10.1002/14651858.CD010265.pub2
4. Saragiotto BT, Maher CG, Yamato TP, et. al. Motor control exercise for chronic non‐specific low‐back pain. Cochrane Database Syst Rev. 2016;1:CD012004. doi: 10.1002/14651858.CD012004
5. Qaseem A, Wilt TJ, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530. doi: 10.7326/M16-2367
6. Pangarkar SS, Kang DG, Sandbrink F, et al. VA/DoD clinical practice guideline: diagnosis and treatment of low back pain. J Gen Intern Med. 2019;34:2620-2629. doi: 10.1007/s11606-019-05086-4
1. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-506. doi: 10.7326/M16-2459
2. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain (review). Cochrane Database Syst Rev. 2017;1:CD010671. doi: 10.1002/14651858.CD010671.pub2
3. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;7:CD010265. doi: 10.1002/14651858.CD010265.pub2
4. Saragiotto BT, Maher CG, Yamato TP, et. al. Motor control exercise for chronic non‐specific low‐back pain. Cochrane Database Syst Rev. 2016;1:CD012004. doi: 10.1002/14651858.CD012004
5. Qaseem A, Wilt TJ, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530. doi: 10.7326/M16-2367
6. Pangarkar SS, Kang DG, Sandbrink F, et al. VA/DoD clinical practice guideline: diagnosis and treatment of low back pain. J Gen Intern Med. 2019;34:2620-2629. doi: 10.1007/s11606-019-05086-4
EVIDENCE-BASED ANSWER:
Yes, it is somewhat effective. Exercise therapy—including general exercise, yoga, Pilates, and motor control exercise—has been shown to modestly decrease pain in chronic low back pain (LBP); levels of benefit in short- (≤ 3 months) and long- (≥ 1 year) term follow-up range from 4% to 15% improvement (strength of recommendation [SOR] A, based on a systematic review of randomized controlled trials [RCTs]).
Exercise therapy may improve function and decrease work disability in subacute and chronic LBP, respectively (SOR A, based on a meta-analysis of RCTs). Exercise therapy has not been associated with improvement in acute LBP (SOR A, based on a meta-analysis of RCTs).
Bone risk: Is time since menopause a better predictor than age?
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Step-by-step evaluation and treatment of shoulder dislocation
The architecture of the glenohumeral joint makes it the most common large joint to become dislocated, accounting for approximately 45% of all dislocations. Anterior dislocation constitutes more than 95% of glenohumeral joint dislocations; posterior dislocation, only 2% to 5%.1,2
For the family physician, determining appropriate follow-up after emergent reduction depends on several distinct variables, which we review here; subsequent treatment might involve, as we outline, physical therapy, immobilization, surgical intervention, or a combination of several modalities. Treatment decisions can make the difference between successful rehabilitation and potential disability, particularly in typically young and active patients.
Numerous mechanisms of injury
Anterior shoulder dislocations typically occur with the affected shoulder in a position of abduction and external rotation; 90% of patients are 21 to 30 years of age, and men are affected 3 times more often than women.2 Unsurprisingly, athletes are affected most frequently, with the common sports-related mechanism of injury being either sudden pressure exerted on the abducted and externally rotated arm or a fall onto an outstretched hand with the arm elevated. Repetitive microtrauma from such sports as swimming, baseball, and volleyball can also lead to instability.
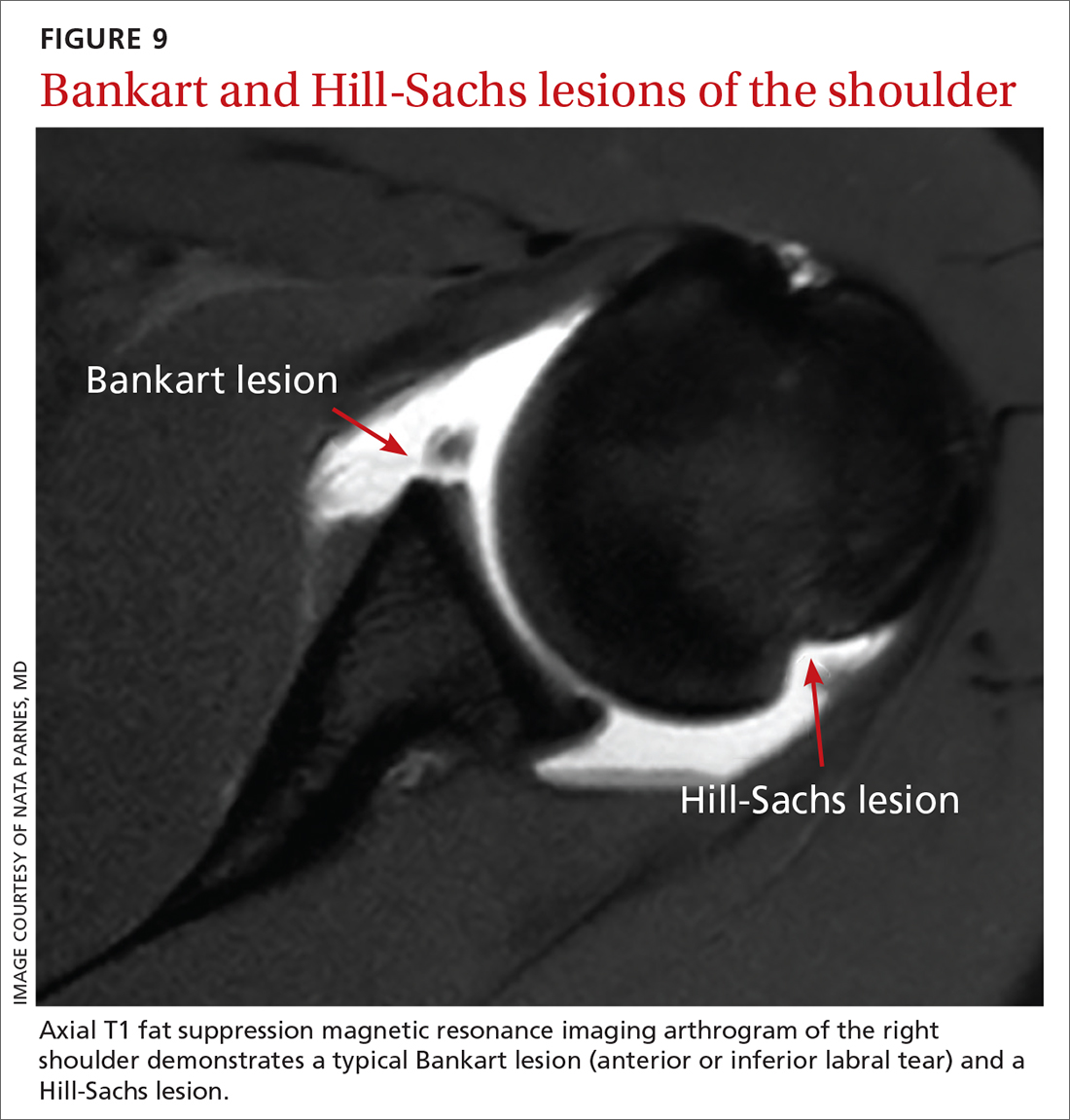
Bankart lesion. This tear of the anterior or inferior section of the labrum is the most characteristic lesion noted in anterior dislocations, found in 73% of first-time dislocations and 100% of recurrent dislocations.3,4
Hills-Sachs lesion is often associated with a Bankart lesion. The Hills-Sachs lesion is an impaction fracture of the posterolateral aspect of the humeral head resulting from its displacement over the anterior lip of the glenoid. Hill-Sachs lesions are seen in 71% of first-time and recurrent dislocations.3
Less common concomitant injuries during anterior shoulder dislocation include rupture of the rotator-cuff tendons (particularly in patients older than 40 years), glenoid and proximal humerus fractures, a tear of the superior labrum (known as a “SLAP lesion”), cartilage injury, and neurovascular injury.
Posterior instability typically occurs as a result of a strong muscle contraction, as seen in electrocution or seizure; however, it can be caused by athletic trauma, particularly in football.5 Repetitive forces exerted on the forward-flexed and internally rotated shoulder position during blocking puts football players at increased risk of posterior instability.5
Continue to: Multidirectional instability
Multidirectional instability is more frequently attributable to congenital hyperlaxity of the glenohumeral joint capsule, rather than to acute injury. However, athletes can also develop capsular laxity from repetitive microtrauma to the shoulder.5
Emergent reduction: Prompt action needed
Acute dislocation of the shoulder should be reduced as soon as possible to minimize neurovascular injury and patient discomfort. (Typically, but not always, this is done in the emergency department.) It is crucial to have effective muscle relaxation before any attempt at reduction, to minimize the risk of iatrogenic injury to bone, cartilage, tendons, and neurovascular structures.
Muscle relaxation can be facilitated with intravenous midazolam or other agents, as specified by institutional protocol. Intra-articular lidocaine injection or intravenous fentanyl is often utilized in conjunction with the sedating agent to reduce pain and further accommodate relaxation.
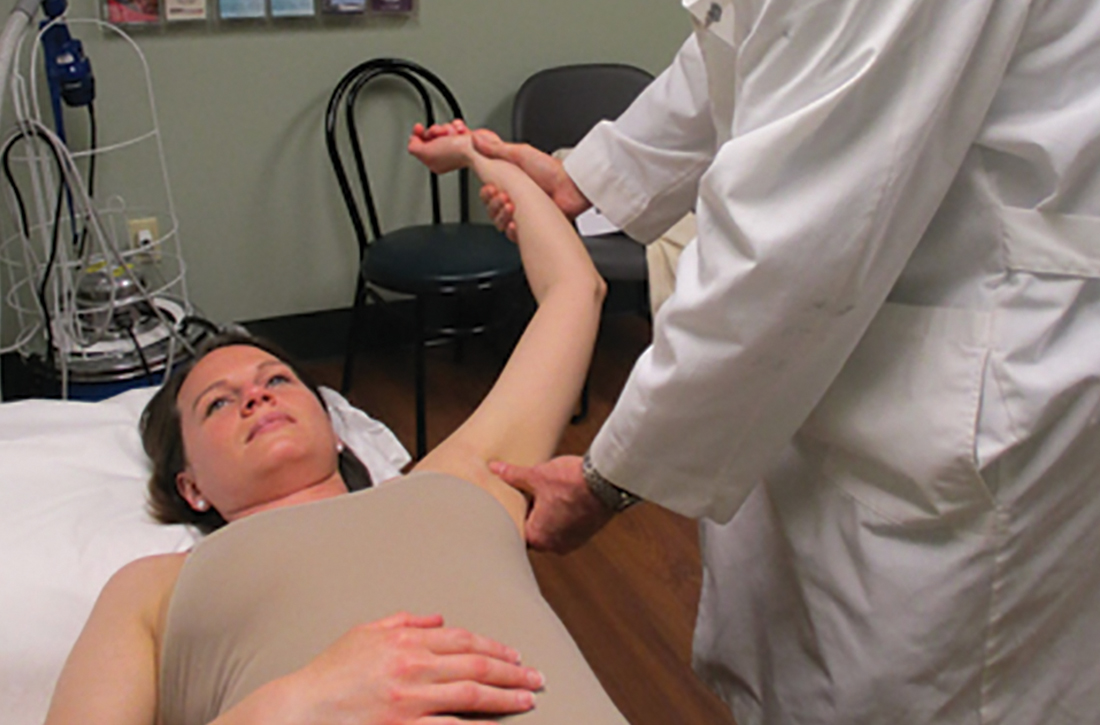
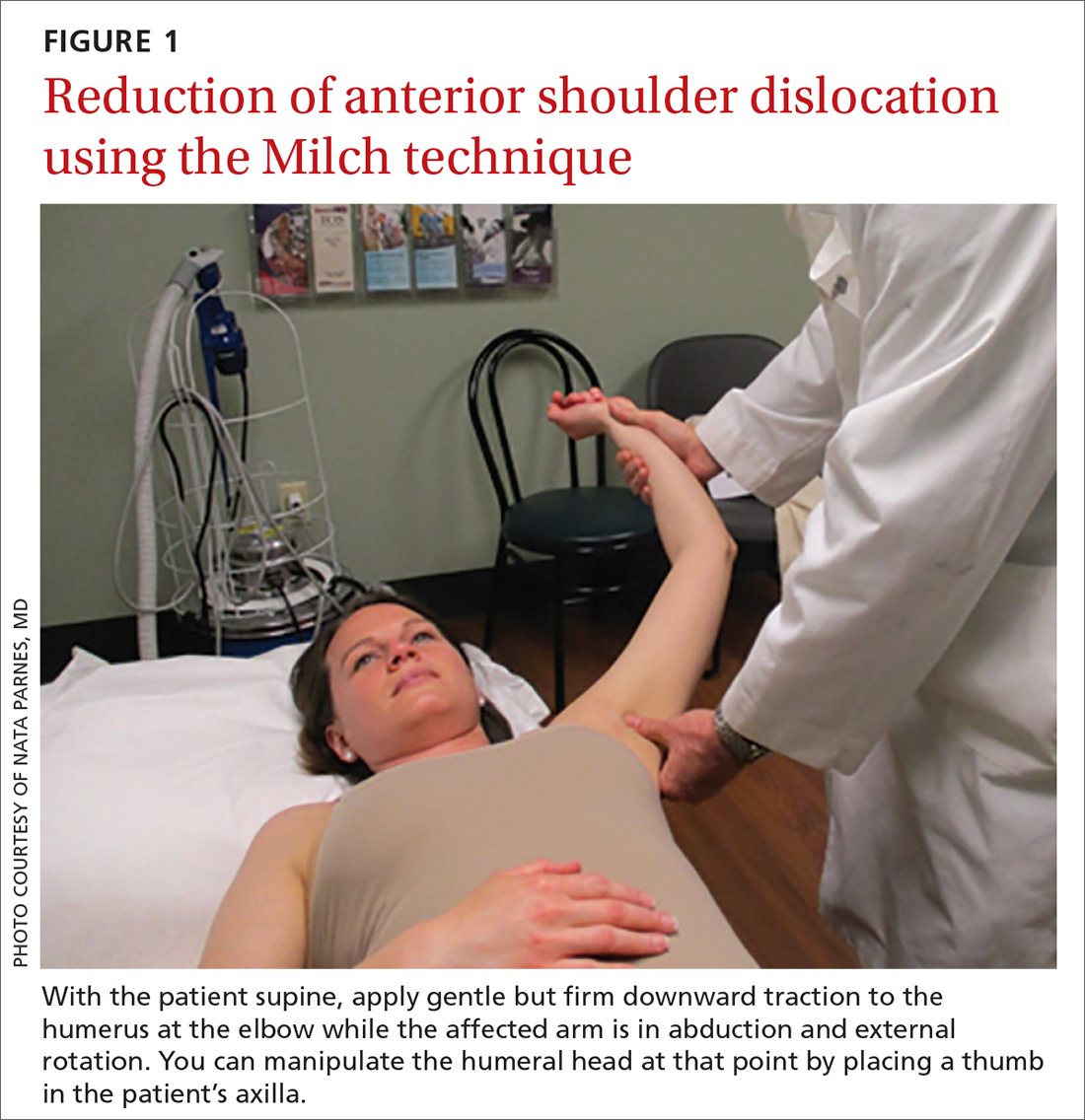
Anterior reduction. Any one of several techniques can be used to perform emergent reduction of anterior shoulder dislocations, all of which have demonstrated success. The Milch technique is among the least traumatic for effective reduction.6 In this technique (FIGURE 1), the patient is supine; gentle but firm downward traction is applied to the humerus at the elbow of the affected arm while the arm is in abduction and external rotation. The provider can manipulate the humeral head at that point by placing a thumb in the patient’s axilla; the arm can also be further internally rotated and adducted until reduction is achieved.
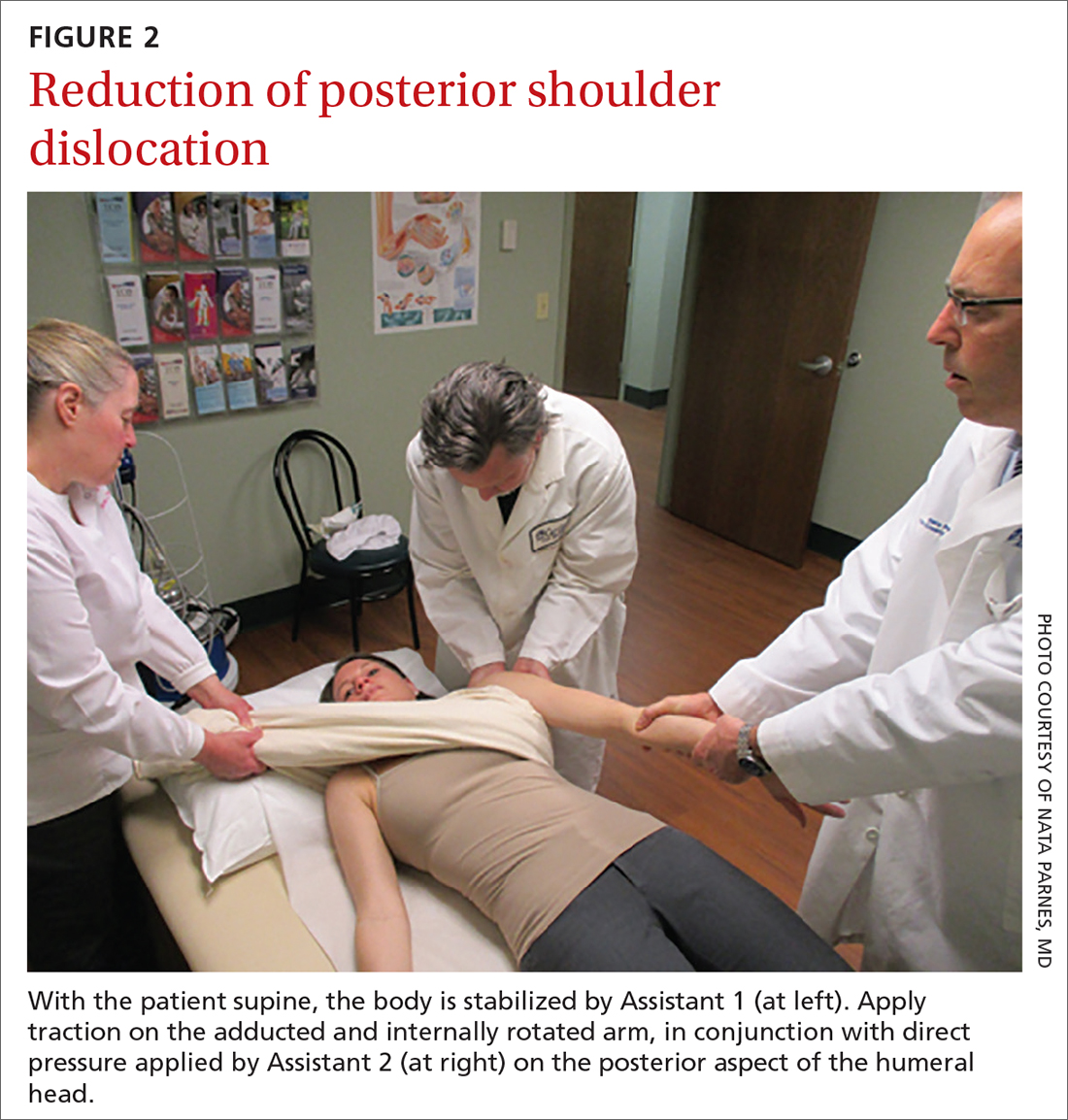
Posterior reduction of a dislocation is performed while the patient is supine, with the body stabilized. Traction is applied on the adducted and internally rotated arm in conjunction with direct pressure on the posterior aspect of the humeral head (FIGURE 2).
Continue to: Follow-up actions
Follow-up actions. Before discharging the patient after reduction of a dislocation, it is essential to:
- perform post-reduction evaluation of shoulder stability at different levels of abduction
- perform a thorough neurovascular assessment
- obtain an anteroposterior (AP) radiograph to ensure proper positioning of the glenohumeral joint.
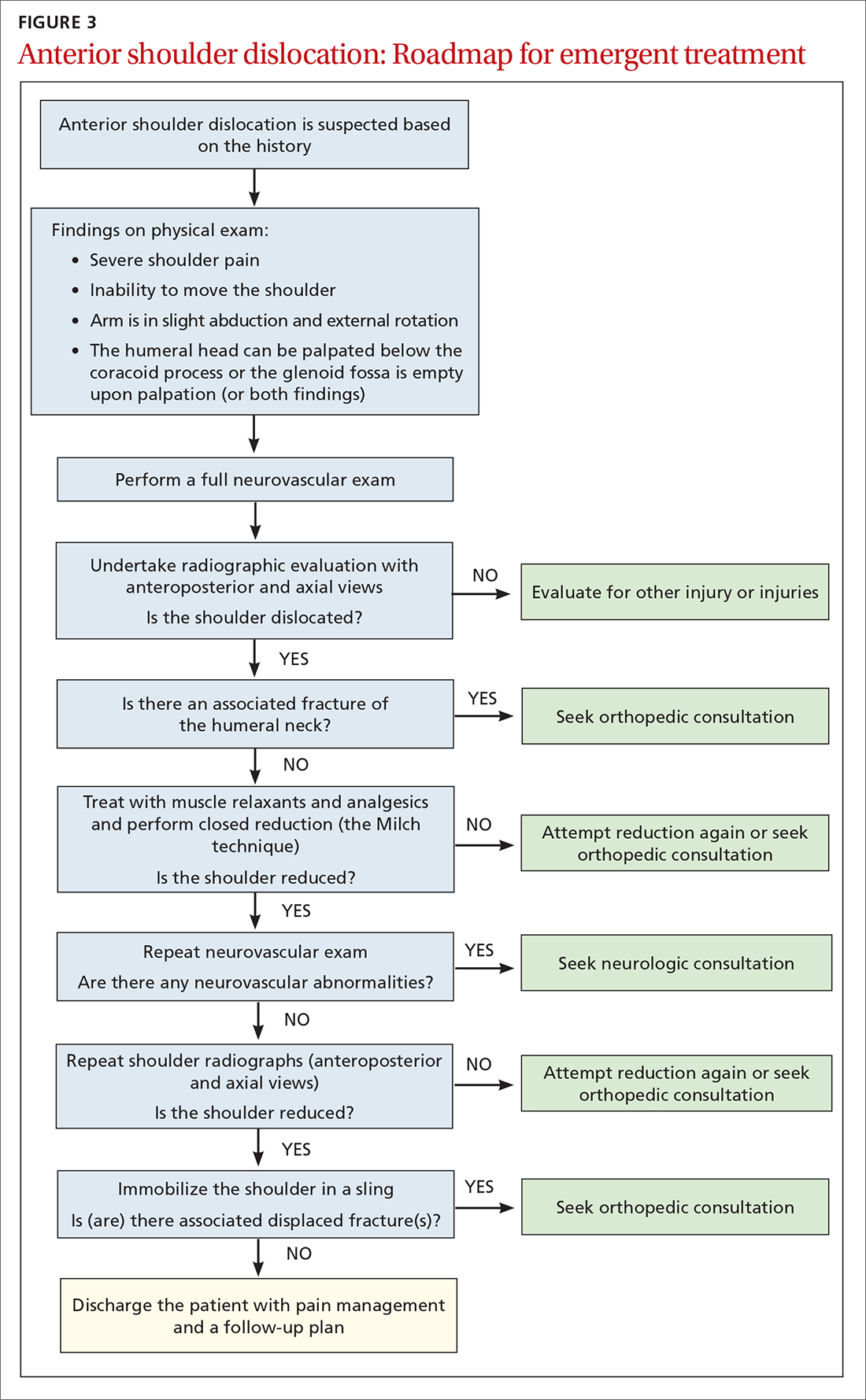
The reduced shoulder should be immobilized in a sling. The discharge plan should include pain management for several days and a follow-up appointment in 5 to 8 days with the primary care provider2 (FIGURE 3).
Follow-up evaluation by the primary care provider
History. Prior to the initial examination at follow-up, obtain a comprehensive history that includes the nature of the injury and the direction of force that was placed on the shoulder. Determine whether the shoulder was reduced spontaneously or required manual reduction in the field or an emergency department. Note any associated injury sustained concurrently and the presence (or absence) of neck pain, numbness, tingling, or weakness in the affected arm.
Physical exam starts with thorough inspection of the affected shoulder, with comparison to the contralateral side, at rest and during shoulder motion. Palpation to reveal points of tenderness should include the anterior joint line, acromioclavicular joint, bicipital groove, subacromial space, acromion, and greater tuberosity.
Following inspection and palpation, assess active and passive range of motion in forward elevation, abduction, internal and external rotation at the side of the body, and internal and external rotation in shoulder abduction. Assessment might be limited by pain and apprehension, and should be performed within the patient’s comfortable range of motion.
Continue to: Once range of motion...
Once range of motion is determined, assess7:
- muscle power of the rotator cuff in abduction (for the supraspinatus muscle)
- resisted external rotation at the side of the body (the infraspinatus)
- resisted external rotation in abduction > 60° (the teres minor)
- resisted internal rotation (the subscapularis).
Specific tests for shoulder laxity and stability
It is important during the primary care follow-up examination to differentiate true instability and shoulder hyperlaxity, particularly in young, flexible patients (TABLE). Many of these patients present with painless hypermobility of the shoulder without true injury to the labrum or ligamentous structures. It might appear to the patient, or to family, that the shoulder is subluxating; however, the humeral head returns to a centered position on the glenoid in a hypermobile state—typically, without pain. Actual shoulder instability is defined as loss of the ability of the humeral head to re-center, accompanied by pain—pathology that is frequently associated with damage to the capsulolabral complex.
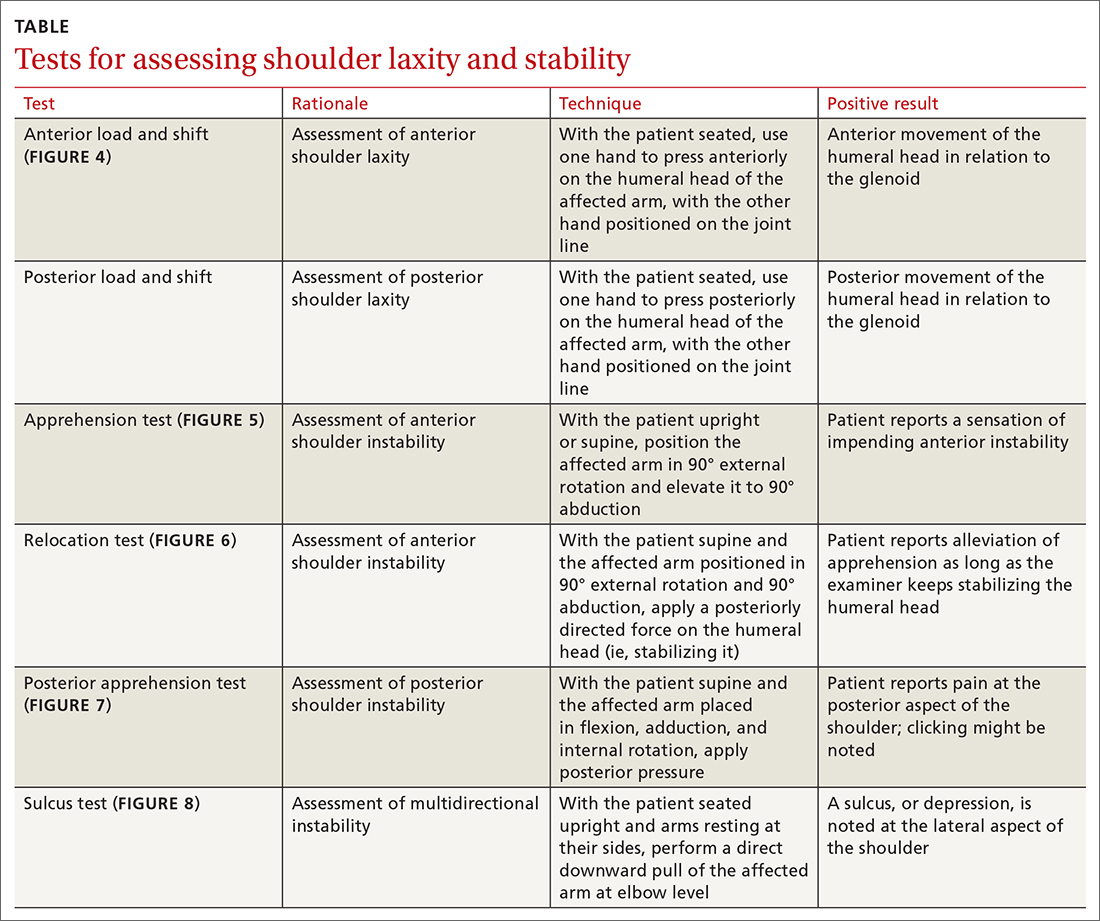
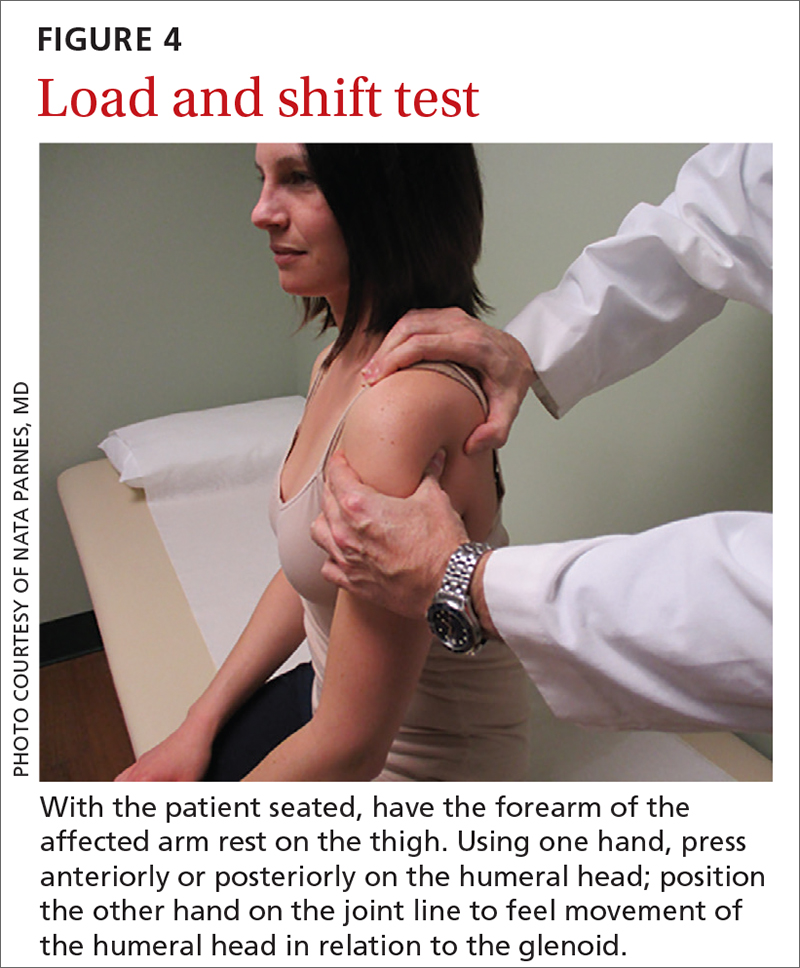
The load and shift test is used to assess anterior and posterior laxity. The patient is seated, and the forearm is allowed to rest on the thigh. Examination is performed using 1 hand to press anteriorly or posteriorly on the humeral head; the other hand is simultaneously positioned on the joint line to feel movement of the humeral head in relation to the glenoid (FIGURE 4).
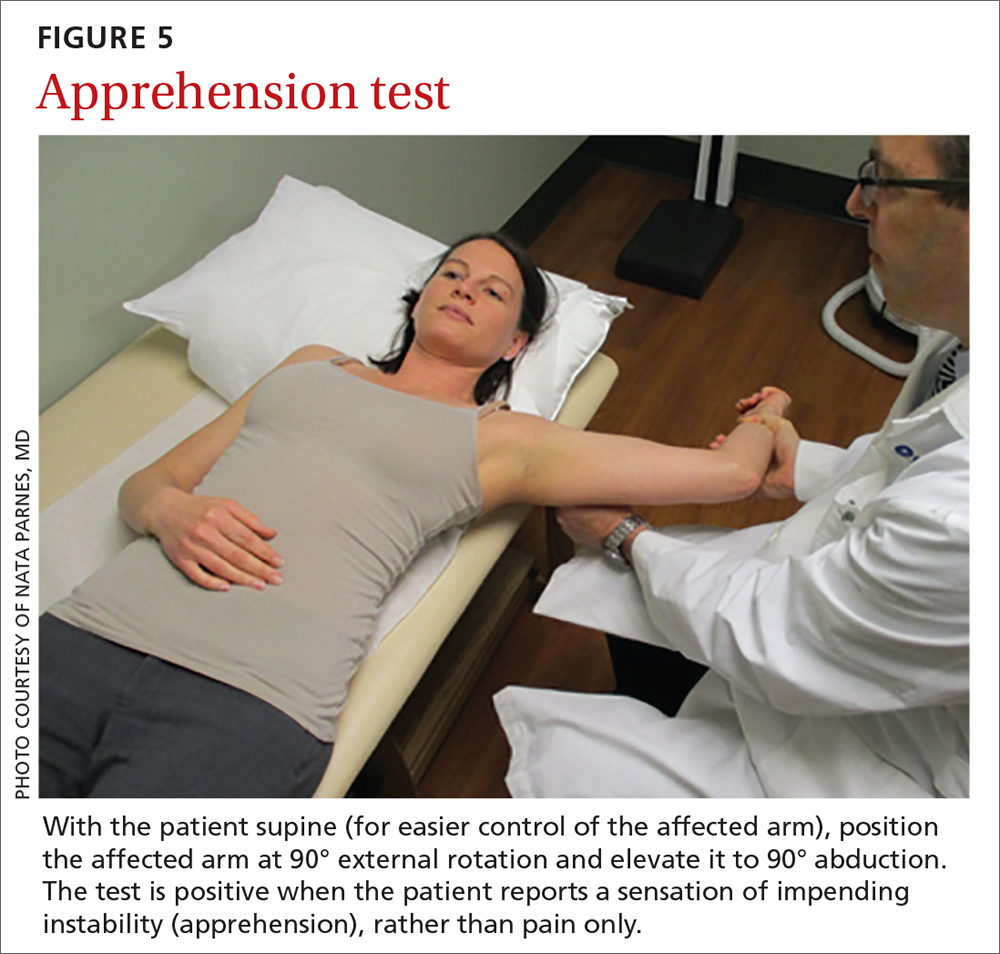
The apprehension test is a common maneuver used to assess anterior shoulder instability. It is performed by positioning the affected arm to 90° external rotation and then elevating it to 90° abduction. Although this maneuver can be performed with the patient upright, it is beneficial to have them supine, to more easily control the arm (FIGURE 5). A positive test is noted when the patient reports a sensation of impending instability (apprehension), rather than pain alone.
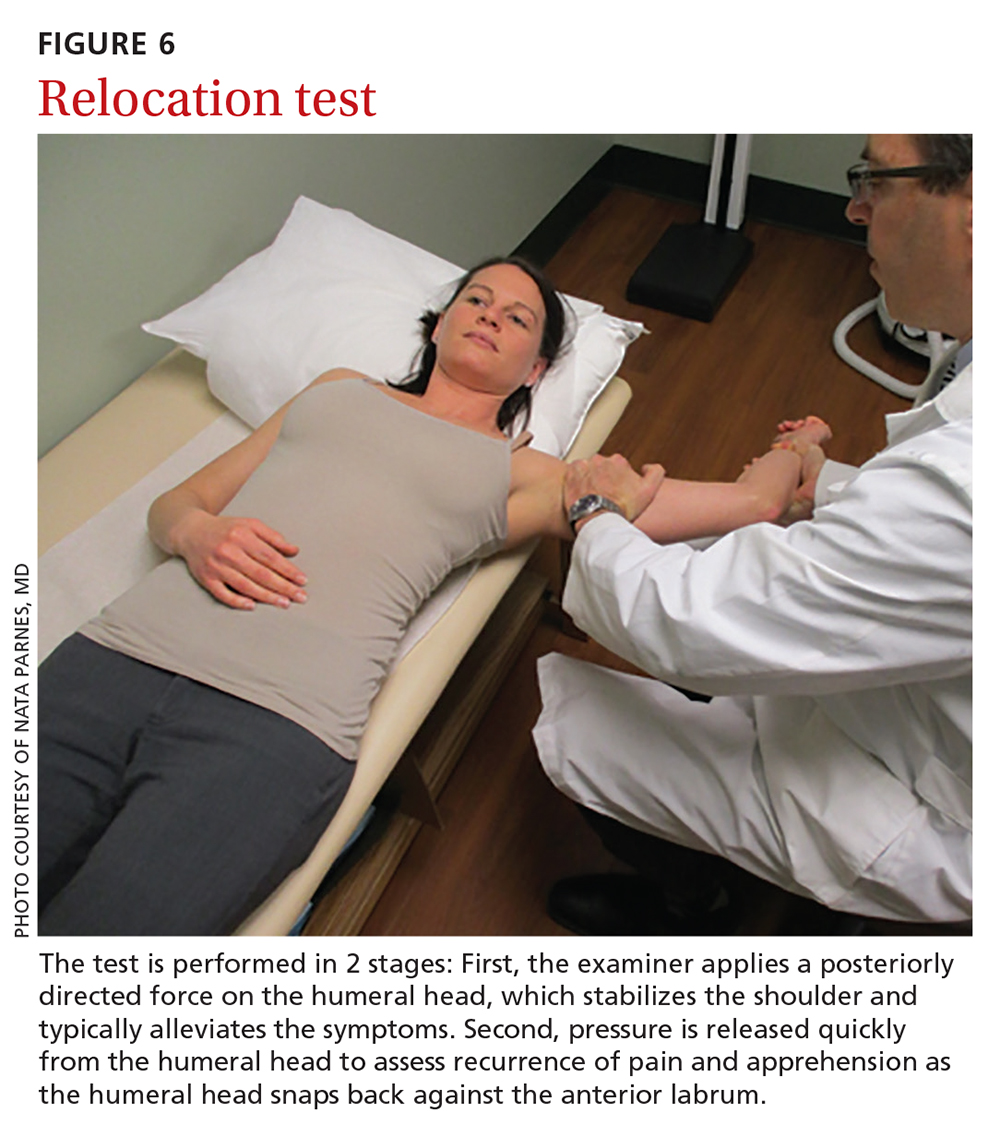
Relocation test. When the apprehension test is positive, the supine position can be exploited to further perform the relocation test, in 2 stages (FIGURE 6):
- Apply a posteriorly directed force on the humeral head, which stabilizes the shoulder and typically alleviates symptoms.
- Release pressure quickly from the humeral head to assess recurrence of pain and apprehension as the humeral head snaps back against the anterior labrum.
Continue to: Combined, apprehension and relocation...
Combined, apprehension and relocation tests to identify anterior shoulder instability have been shown to significantly improve specificity while maintaining sensitivity.8
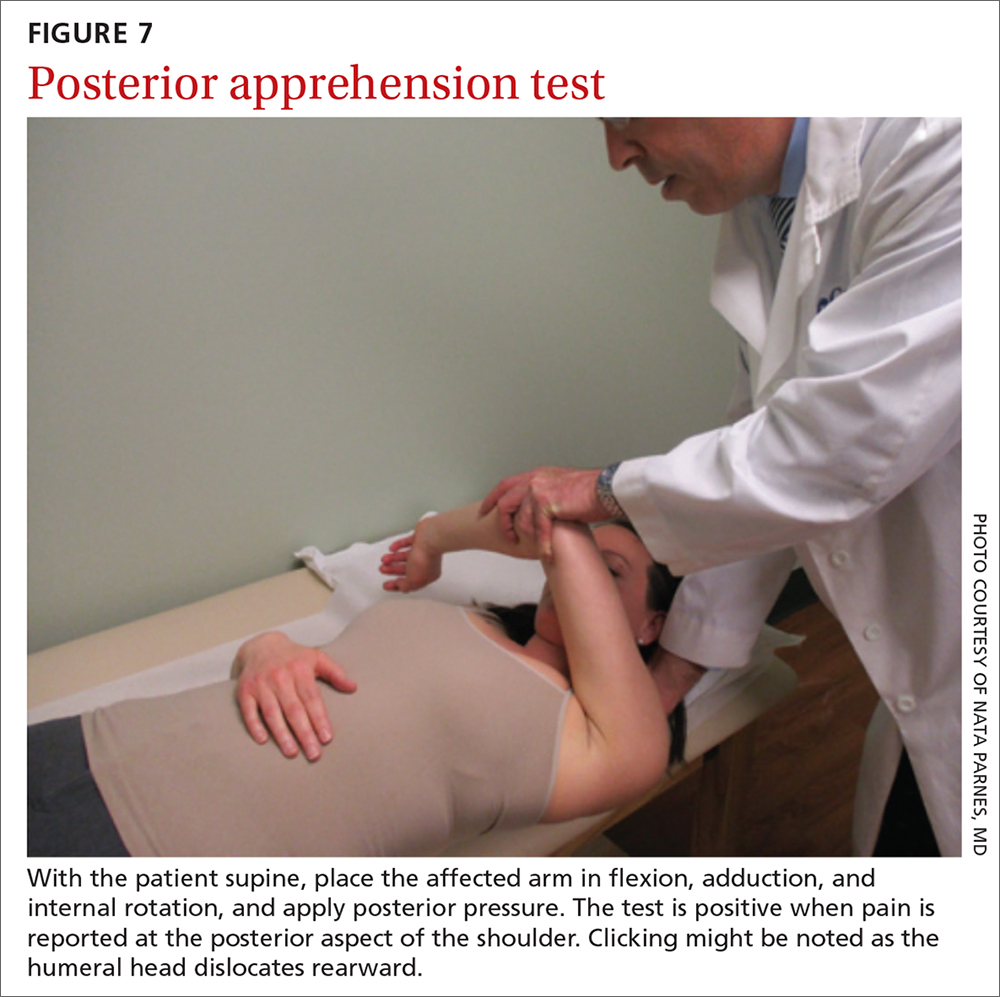
The posterior apprehension test is used to assess posterior instability. The patient is supine; the affected arm is placed in flexion, adduction, and internal rotation; and posterior pressure is applied (FIGURE 7). A positive test is noted when pain is reported at the posterior aspect of the shoulder. Clicking might be noted as the humeral head dislocates rearward.1
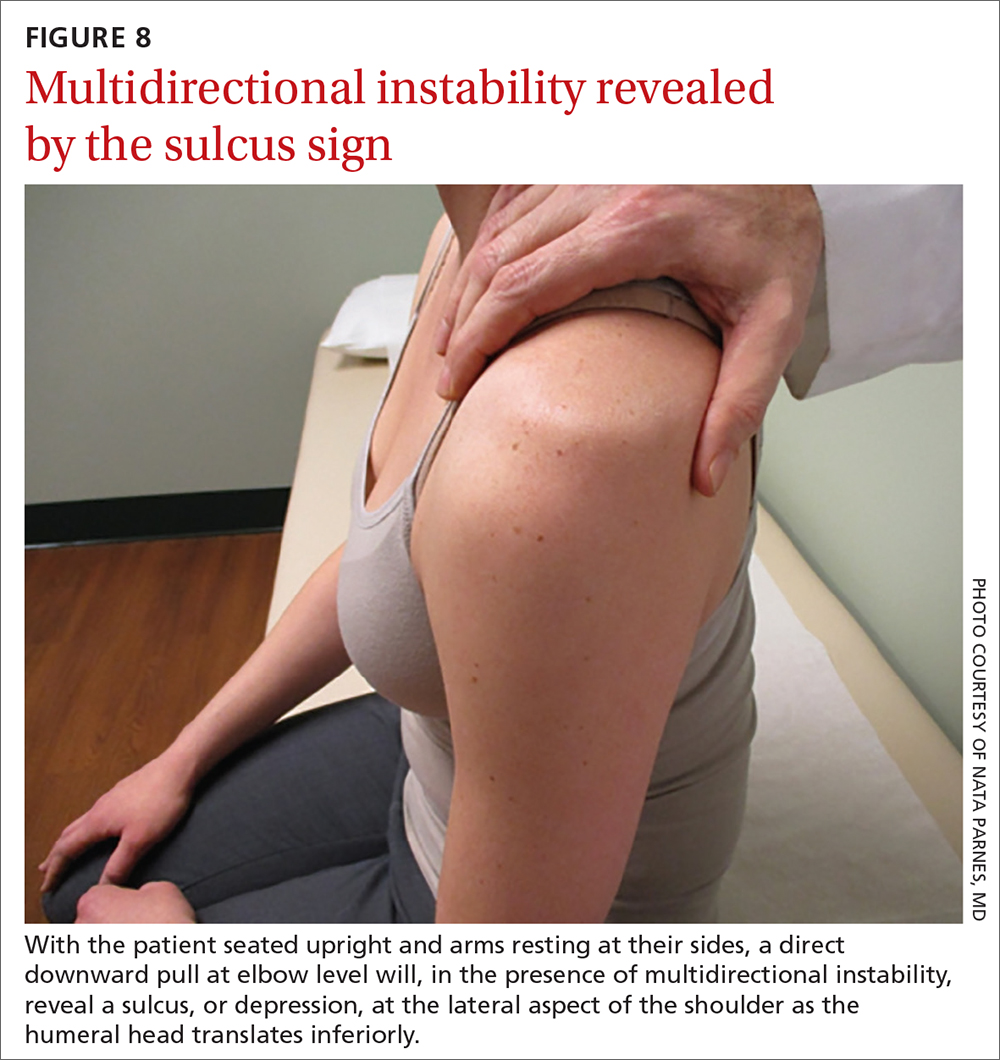
Sulcus sign. Multidirectional instability is elicited using the sulcus sign. While the patient is seated upright, arms resting at their sides, a direct downward pull at elbow level will, when positive, reveal a depression (sulcus) at the lateral aspect of the affected shoulder as the humeral head translates inferiorly (FIGURE 8). A positive sulcus sign is documented in 3 grades, according to the amount of translation1:
- Grade I: < 1 cm
- Grade II: 1-2 cm
- Grade III: > 2 cm.
Neurovascular status should be verified at every physical evaluation, with motor and sensory function tested in the axillary, musculocutaneous, median, radial, and ulnar nerve distributions. If nerve injury is suspected, electromyography and nerve-conduction testing is indicated.9-13 Vascular compromise is much less common but equally important to assess.11
Use of imaging
Post-reduction radiographs, including internal and external AP—and especially axillary—views are invaluable. Not only do they help to ensure reduction, but they also help to assess for fracture. A magnetic resonance imaging (MRI) arthrogram is the preferred imaging modality if a labral tear is suspected (FIGURE 9). Other concomitant shoulder injuries, such as subtle bone fracture, rotator cuff tear, and biceps pathology can also be reliably diagnosed with noncontrast MRI.
Continue to: Roadmap for treatment
Roadmap for treatment
The rate of recurrence after a first anterior shoulder dislocation is strongly associated with a person’s age and level of activity. Active patients younger than 20 years have a 92% to 96% recurrence rate14; patients 20 to 40 years, 25% to 48%; and patients older than 40 years, < 10%.15
Young, athletic patients who are treated nonoperatively are left at an unacceptably high risk of recurrence, leading to progressive damage to bony and soft-tissue structures.16,17 Surgical labral repair after a first-time anterior dislocation produced improved outcomes in terms of recurrent dislocation (7.9%), compared to outcomes after nonsurgical treatment (52.9%),14 and has been associated with a lower incidence of future glenohumeral osteoarthritis.18 For those reasons, we recommend referral to an orthopedic surgeon for all patients younger than 20 years who sustain an anterior shoulder dislocation.
Patients older than 20 years who do not have concomitant shoulder injury, and who demonstrate full strength in abduction, external rotation, and internal rotation of the shoulder on clinical examination, have a low probability of associated rotator-cuff tear. They can be immobilized in a sling for 1 to 3 weeks, followed by a 6 to 12–week regimen of physical therapy.
Concomitant tear of the rotator cuff. Weakness on examination requires MRI or a magnetic resonance arthrogram for evaluation of associated rotator-cuff tear. A tear identified on MRI should be referred to an orthopedic surgeon because timely repair can be crucial to attaining best outcomes. Conservative treatment of traumatic full-tendon rotator-cuff tear is associated with poor results, progression in the size of the tear, and advancement of muscle atrophy.19,20 For patients younger than 40 years, arthroscopic rotator-cuff repair, with or without labral repair, produces excellent clinical outcomes, carries a low risk of complications, and results in a > 95% rate of return to a preoperative level of recreational and job activities.21
Patients who demonstrate weakness of the rotator-cuff muscles on examination, but who do not have a tear noted on MRI, should be evaluated by electromyography and nerve-conduction testing to assess nerve injury as an alternative cause of weakness.10,11 If a neurologic deficit is found on nerve-conduction testing, the patient should be referred for neurologic evaluation.10
Continue to: Patients with negative findings...
Patients with negative findings on MRI and nerve-conduction studies should be offered physical therapy. Patients with recurrent anterior shoulder dislocation should be referred to an orthopedic surgeon for surgical repair. Frequently, improper or delayed treatment with chronic instability results in degenerative arthropathy of the joint22 (FIGURE 10).

Posterior and multidirectional instability can typically be treated conservatively; however, whereas posterior dislocation typically must be immobilized for 3 to 6 weeks post reduction, multidirectional instability does not require immobilization. Instead, physical therapy should start as soon as possible. In these cases, recurrent dislocation or subluxation that persists after conservative treatment should be referred for possible surgical intervention.5
Instability with associated fracture
Fracture concomitant with dislocation most commonly involves the humeral neck, humeral head, greater tuberosity, or the glenoid itself.2 Clinical variables that predict a fracture associated with shoulder dislocation include23:
- first episode of dislocation
- age ≥ 40 years
- fall from higher than 1 flight of stairs
- fight or assault
- motor vehicle crash.
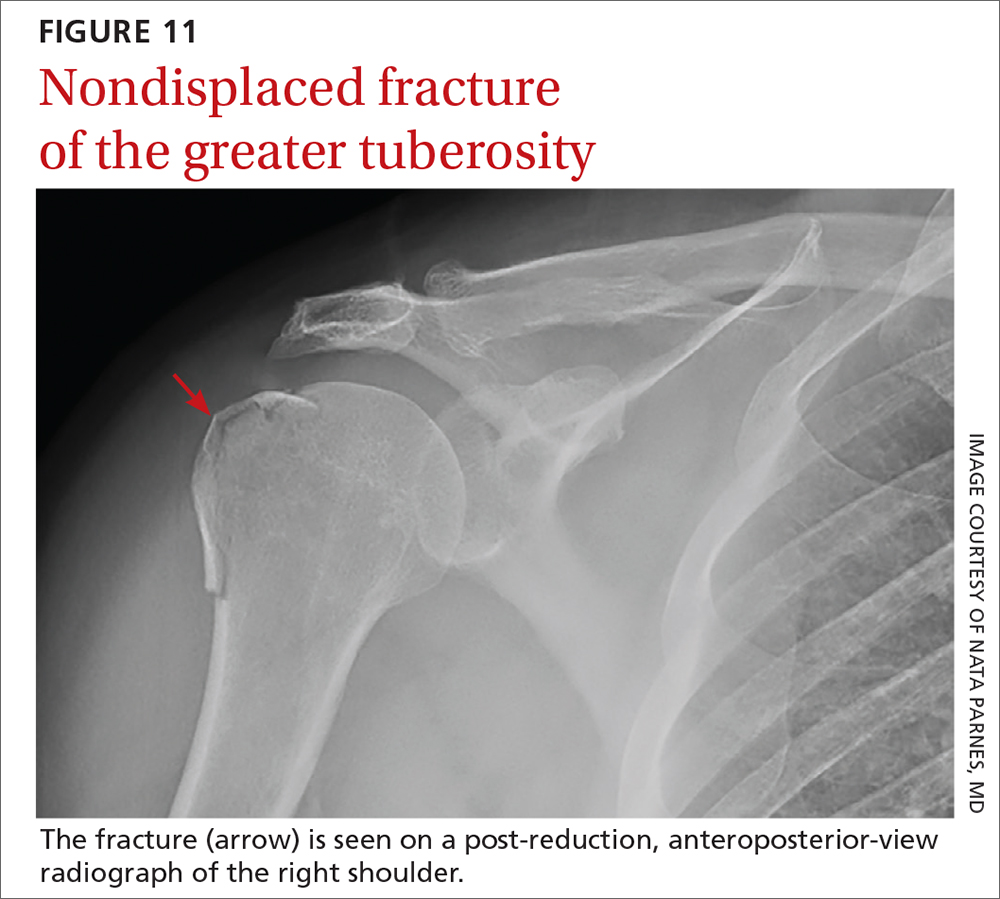
A computed tomography scan with 3-dimensional reconstruction can help characterize associated fracture accurately—including location, size, and displacement—and can play an important role in treatment planning and prognosis in these complicated injuries. Displaced fracture should be referred to an orthopedic surgeon. Nondisplaced fracture of the humeral head or greater tuberosity (FIGURE 11) poses less risk of complications and can be treated conservatively with 6 weeks in an arm sling, followed by physical therapy.24
Summing up
Management of shoulder dislocation must, first, be tailored to the individual and, second, account for several interactive factors—including age, direction of instability, functional demands, risk of recurrence, and associated injuries. In many patients, conservative treatment produces a favorable long-term outcome. Particularly in young, active patients with anterior shoulder instability, most surgeons consider open or arthroscopic reconstruction to be the treatment of choice.2,18
Continue to: Pre-reduction and post-reduction...
Pre-reduction and post-reduction imaging should be carefully examined for the presence of concomitant injury, which might change the preferred treatment modality appreciably.
Last, communication among emergency department providers, the primary care provider, orthopedist, radiologist, and neurologist is crucial for determining an appropriate patient-centered approach to initial and long-term management.
CORRESPONDENCE
Nata Parnes, MD, Carthage Area Hospital, 3 Bridge Street, Carthage, NY; [email protected]
1. Valencia Mora M, Ruiz Ibán MA, Heredia JD, et al. Physical exam and evaluation of the unstable shoulder. Open Orthop J. 2017;11(suppl 6, M12):946-956. doi: 10.2174/1874325001711010946
2. Khiami F, A, Loriaut P. Management of recent first-time anterior shoulder dislocation. Orthop Traumatol Surg Res. 2015;101(1 suppl):S51-S57. doi: 10.1016/j.otsr.2014.06.027
3. Antonio GE, Griffith JF, Yu AB, et al. First-time shoulder dislocation: high prevalence of labral injury and age-related differences revealed by MR arthrography. J Magn Reson Imaging. 2007;26:983-991. doi: 10.1002/jmri.21092
4. Carrazzone OL, Tamaoki MJS, Ambra LFM, et al. Prevalence of lesions associated with traumatic recurrent shoulder dislocation. Rev Bras Ortop. 2015;46:281-287. doi: 10.1016/S2255-4971(15)30196-8
5. Mahaffey BL, Smith PA. Shoulder instability in young athletes. Am Fam Physician. 1999;59:2773-2787.
6. Amar E, Maman E, Khashan M, et al. Milch versus Stimson technique for nonsedated reduction of anterior shoulder dislocation: a prospective randomized trial and analysis of factors affecting success. J Shoulder Elbow Surg. 2012;21:1443-1449. doi: 10.1016/j.jse.2012.01.004
7. Jain NB, Wilcox RB 3rd, Katz JN, et al. Clinical examination of the rotator cuff. PM R. 2013;5:45-56. doi: 10.1016/j.pmrj.2012.08.019
8. Lizzio VA, Meta F, Fidai M, et al. Clinical evaluation and physical exam findings in patients with anterior shoulder instability. Curr Rev Musculoskelet Med. 2017;10:434-441. doi: 10.1007/s12178-017-9434-3
9. Farber AJ, Castillo R, Clough M, et al. Clinical assessment of three common tests for traumatic anterior shoulder instability. J Bone Joint Surg Am. 2006;88:1467-1474. doi: 10.2106/JBJS.E.00594
10. Robinson CM, Shur N, Sharpe T, et al. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94:18-26. doi: 10.2106/JBJS.J.01795
11. de Laat EA, Visser CP, Coene LN, et al. Nerve lesions in primary shoulder dislocations and humeral neck fractures. A prospective clinical and EMG study. J Bone Joint Surg Br. 1994;76:381-383.
12. Avis D, Power D. Axillary nerve injury associated with glenohumeral dislocation: a review and algorithm for management. EFORT Open Rev. 2018;3:70-77. doi: 10.1302/2058-5241.3.170003
13. Drury JK, Scullion JE. Vascular complications of anterior dislocation of the shoulder. Br J Surg. 1980;67:579-581. doi: 10.1002/bjs.1800670817
14. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010. doi: 10.2174/1874325001711011001
15. te Slaa RL, Brand R, Marti RK. A prospective arthroscopic study of acute first-time anterior shoulder dislocation in the young: a five-year follow-up study. J Shoulder Elbow Surg. 2003;12:529-534. doi: 10.1016/s1058-2746(03)00218-0
16. Kavaja L, T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506. doi: 10.1136/bjsports-2017-098539
17. Krych AJ, Sousa PL, King AH, et al. The effect of cartilage injury after arthroscopic stabilization for shoulder instability. Orthopedics. 2015;38:e965-e969. doi: 10.3928/01477447-20151020-03
18. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
19. Maman E, Harris C, White L, et al. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am. 2009;91:1898-1906. doi: 10.2106/JBJS.G.01335
20. Safran O, Schroeder J, Bloom R, et al. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39:710-714. doi: 10.1177/0363546510393944
21. Parnes N, Bartoszewski NR, Defranco MJ. Arthroscopic repair of full-thickness rotator cuff tears in active patients younger than 40 years: 2- to 5-year clinical outcomes. Orthopedics 2018;41:e52-e57. doi: 10.3928/01477447-20171114-02
22. Sofu H, Gürsu S, Koçkara N, et al. Recurrent anterior shoulder instability: review of the literature and current concepts. World J Clin Cases. 2014;2:676-682. doi: 10.12998/wjcc.v2.i11.676
23. Emond M, Le Sage N, Lavoie A, et al. Clinical factors predicting fractures associated with an anterior shoulder dislocation. Acad Emerg Med. 2004;11:853-858. doi: 10.1111/j.1553-2712.2004.tb00768.x
24. Parnes N, Jupiter JB. Fixed-angle locking plating of displaced proximal humerus fractures. Instr Course Lect. 2010;59:539-552.
The architecture of the glenohumeral joint makes it the most common large joint to become dislocated, accounting for approximately 45% of all dislocations. Anterior dislocation constitutes more than 95% of glenohumeral joint dislocations; posterior dislocation, only 2% to 5%.1,2
For the family physician, determining appropriate follow-up after emergent reduction depends on several distinct variables, which we review here; subsequent treatment might involve, as we outline, physical therapy, immobilization, surgical intervention, or a combination of several modalities. Treatment decisions can make the difference between successful rehabilitation and potential disability, particularly in typically young and active patients.
Numerous mechanisms of injury
Anterior shoulder dislocations typically occur with the affected shoulder in a position of abduction and external rotation; 90% of patients are 21 to 30 years of age, and men are affected 3 times more often than women.2 Unsurprisingly, athletes are affected most frequently, with the common sports-related mechanism of injury being either sudden pressure exerted on the abducted and externally rotated arm or a fall onto an outstretched hand with the arm elevated. Repetitive microtrauma from such sports as swimming, baseball, and volleyball can also lead to instability.
Bankart lesion. This tear of the anterior or inferior section of the labrum is the most characteristic lesion noted in anterior dislocations, found in 73% of first-time dislocations and 100% of recurrent dislocations.3,4
Hills-Sachs lesion is often associated with a Bankart lesion. The Hills-Sachs lesion is an impaction fracture of the posterolateral aspect of the humeral head resulting from its displacement over the anterior lip of the glenoid. Hill-Sachs lesions are seen in 71% of first-time and recurrent dislocations.3
Less common concomitant injuries during anterior shoulder dislocation include rupture of the rotator-cuff tendons (particularly in patients older than 40 years), glenoid and proximal humerus fractures, a tear of the superior labrum (known as a “SLAP lesion”), cartilage injury, and neurovascular injury.
Posterior instability typically occurs as a result of a strong muscle contraction, as seen in electrocution or seizure; however, it can be caused by athletic trauma, particularly in football.5 Repetitive forces exerted on the forward-flexed and internally rotated shoulder position during blocking puts football players at increased risk of posterior instability.5
Continue to: Multidirectional instability
Multidirectional instability is more frequently attributable to congenital hyperlaxity of the glenohumeral joint capsule, rather than to acute injury. However, athletes can also develop capsular laxity from repetitive microtrauma to the shoulder.5
Emergent reduction: Prompt action needed
Acute dislocation of the shoulder should be reduced as soon as possible to minimize neurovascular injury and patient discomfort. (Typically, but not always, this is done in the emergency department.) It is crucial to have effective muscle relaxation before any attempt at reduction, to minimize the risk of iatrogenic injury to bone, cartilage, tendons, and neurovascular structures.
Muscle relaxation can be facilitated with intravenous midazolam or other agents, as specified by institutional protocol. Intra-articular lidocaine injection or intravenous fentanyl is often utilized in conjunction with the sedating agent to reduce pain and further accommodate relaxation.
Anterior reduction. Any one of several techniques can be used to perform emergent reduction of anterior shoulder dislocations, all of which have demonstrated success. The Milch technique is among the least traumatic for effective reduction.6 In this technique (FIGURE 1), the patient is supine; gentle but firm downward traction is applied to the humerus at the elbow of the affected arm while the arm is in abduction and external rotation. The provider can manipulate the humeral head at that point by placing a thumb in the patient’s axilla; the arm can also be further internally rotated and adducted until reduction is achieved.

Posterior reduction of a dislocation is performed while the patient is supine, with the body stabilized. Traction is applied on the adducted and internally rotated arm in conjunction with direct pressure on the posterior aspect of the humeral head (FIGURE 2).

Continue to: Follow-up actions
Follow-up actions. Before discharging the patient after reduction of a dislocation, it is essential to:
- perform post-reduction evaluation of shoulder stability at different levels of abduction
- perform a thorough neurovascular assessment
- obtain an anteroposterior (AP) radiograph to ensure proper positioning of the glenohumeral joint.
The reduced shoulder should be immobilized in a sling. The discharge plan should include pain management for several days and a follow-up appointment in 5 to 8 days with the primary care provider2 (FIGURE 3).

Follow-up evaluation by the primary care provider
History. Prior to the initial examination at follow-up, obtain a comprehensive history that includes the nature of the injury and the direction of force that was placed on the shoulder. Determine whether the shoulder was reduced spontaneously or required manual reduction in the field or an emergency department. Note any associated injury sustained concurrently and the presence (or absence) of neck pain, numbness, tingling, or weakness in the affected arm.
Physical exam starts with thorough inspection of the affected shoulder, with comparison to the contralateral side, at rest and during shoulder motion. Palpation to reveal points of tenderness should include the anterior joint line, acromioclavicular joint, bicipital groove, subacromial space, acromion, and greater tuberosity.
Following inspection and palpation, assess active and passive range of motion in forward elevation, abduction, internal and external rotation at the side of the body, and internal and external rotation in shoulder abduction. Assessment might be limited by pain and apprehension, and should be performed within the patient’s comfortable range of motion.
Continue to: Once range of motion...
Once range of motion is determined, assess7:
- muscle power of the rotator cuff in abduction (for the supraspinatus muscle)
- resisted external rotation at the side of the body (the infraspinatus)
- resisted external rotation in abduction > 60° (the teres minor)
- resisted internal rotation (the subscapularis).
Specific tests for shoulder laxity and stability
It is important during the primary care follow-up examination to differentiate true instability and shoulder hyperlaxity, particularly in young, flexible patients (TABLE). Many of these patients present with painless hypermobility of the shoulder without true injury to the labrum or ligamentous structures. It might appear to the patient, or to family, that the shoulder is subluxating; however, the humeral head returns to a centered position on the glenoid in a hypermobile state—typically, without pain. Actual shoulder instability is defined as loss of the ability of the humeral head to re-center, accompanied by pain—pathology that is frequently associated with damage to the capsulolabral complex.

The load and shift test is used to assess anterior and posterior laxity. The patient is seated, and the forearm is allowed to rest on the thigh. Examination is performed using 1 hand to press anteriorly or posteriorly on the humeral head; the other hand is simultaneously positioned on the joint line to feel movement of the humeral head in relation to the glenoid (FIGURE 4).

The apprehension test is a common maneuver used to assess anterior shoulder instability. It is performed by positioning the affected arm to 90° external rotation and then elevating it to 90° abduction. Although this maneuver can be performed with the patient upright, it is beneficial to have them supine, to more easily control the arm (FIGURE 5). A positive test is noted when the patient reports a sensation of impending instability (apprehension), rather than pain alone.

Relocation test. When the apprehension test is positive, the supine position can be exploited to further perform the relocation test, in 2 stages (FIGURE 6):
- Apply a posteriorly directed force on the humeral head, which stabilizes the shoulder and typically alleviates symptoms.
- Release pressure quickly from the humeral head to assess recurrence of pain and apprehension as the humeral head snaps back against the anterior labrum.

Continue to: Combined, apprehension and relocation...
Combined, apprehension and relocation tests to identify anterior shoulder instability have been shown to significantly improve specificity while maintaining sensitivity.8
The posterior apprehension test is used to assess posterior instability. The patient is supine; the affected arm is placed in flexion, adduction, and internal rotation; and posterior pressure is applied (FIGURE 7). A positive test is noted when pain is reported at the posterior aspect of the shoulder. Clicking might be noted as the humeral head dislocates rearward.1

Sulcus sign. Multidirectional instability is elicited using the sulcus sign. While the patient is seated upright, arms resting at their sides, a direct downward pull at elbow level will, when positive, reveal a depression (sulcus) at the lateral aspect of the affected shoulder as the humeral head translates inferiorly (FIGURE 8). A positive sulcus sign is documented in 3 grades, according to the amount of translation1:
- Grade I: < 1 cm
- Grade II: 1-2 cm
- Grade III: > 2 cm.

Neurovascular status should be verified at every physical evaluation, with motor and sensory function tested in the axillary, musculocutaneous, median, radial, and ulnar nerve distributions. If nerve injury is suspected, electromyography and nerve-conduction testing is indicated.9-13 Vascular compromise is much less common but equally important to assess.11
Use of imaging
Post-reduction radiographs, including internal and external AP—and especially axillary—views are invaluable. Not only do they help to ensure reduction, but they also help to assess for fracture. A magnetic resonance imaging (MRI) arthrogram is the preferred imaging modality if a labral tear is suspected (FIGURE 9). Other concomitant shoulder injuries, such as subtle bone fracture, rotator cuff tear, and biceps pathology can also be reliably diagnosed with noncontrast MRI.

Continue to: Roadmap for treatment
Roadmap for treatment
The rate of recurrence after a first anterior shoulder dislocation is strongly associated with a person’s age and level of activity. Active patients younger than 20 years have a 92% to 96% recurrence rate14; patients 20 to 40 years, 25% to 48%; and patients older than 40 years, < 10%.15
Young, athletic patients who are treated nonoperatively are left at an unacceptably high risk of recurrence, leading to progressive damage to bony and soft-tissue structures.16,17 Surgical labral repair after a first-time anterior dislocation produced improved outcomes in terms of recurrent dislocation (7.9%), compared to outcomes after nonsurgical treatment (52.9%),14 and has been associated with a lower incidence of future glenohumeral osteoarthritis.18 For those reasons, we recommend referral to an orthopedic surgeon for all patients younger than 20 years who sustain an anterior shoulder dislocation.
Patients older than 20 years who do not have concomitant shoulder injury, and who demonstrate full strength in abduction, external rotation, and internal rotation of the shoulder on clinical examination, have a low probability of associated rotator-cuff tear. They can be immobilized in a sling for 1 to 3 weeks, followed by a 6 to 12–week regimen of physical therapy.
Concomitant tear of the rotator cuff. Weakness on examination requires MRI or a magnetic resonance arthrogram for evaluation of associated rotator-cuff tear. A tear identified on MRI should be referred to an orthopedic surgeon because timely repair can be crucial to attaining best outcomes. Conservative treatment of traumatic full-tendon rotator-cuff tear is associated with poor results, progression in the size of the tear, and advancement of muscle atrophy.19,20 For patients younger than 40 years, arthroscopic rotator-cuff repair, with or without labral repair, produces excellent clinical outcomes, carries a low risk of complications, and results in a > 95% rate of return to a preoperative level of recreational and job activities.21
Patients who demonstrate weakness of the rotator-cuff muscles on examination, but who do not have a tear noted on MRI, should be evaluated by electromyography and nerve-conduction testing to assess nerve injury as an alternative cause of weakness.10,11 If a neurologic deficit is found on nerve-conduction testing, the patient should be referred for neurologic evaluation.10
Continue to: Patients with negative findings...
Patients with negative findings on MRI and nerve-conduction studies should be offered physical therapy. Patients with recurrent anterior shoulder dislocation should be referred to an orthopedic surgeon for surgical repair. Frequently, improper or delayed treatment with chronic instability results in degenerative arthropathy of the joint22 (FIGURE 10).

Posterior and multidirectional instability can typically be treated conservatively; however, whereas posterior dislocation typically must be immobilized for 3 to 6 weeks post reduction, multidirectional instability does not require immobilization. Instead, physical therapy should start as soon as possible. In these cases, recurrent dislocation or subluxation that persists after conservative treatment should be referred for possible surgical intervention.5
Instability with associated fracture
Fracture concomitant with dislocation most commonly involves the humeral neck, humeral head, greater tuberosity, or the glenoid itself.2 Clinical variables that predict a fracture associated with shoulder dislocation include23:
- first episode of dislocation
- age ≥ 40 years
- fall from higher than 1 flight of stairs
- fight or assault
- motor vehicle crash.
A computed tomography scan with 3-dimensional reconstruction can help characterize associated fracture accurately—including location, size, and displacement—and can play an important role in treatment planning and prognosis in these complicated injuries. Displaced fracture should be referred to an orthopedic surgeon. Nondisplaced fracture of the humeral head or greater tuberosity (FIGURE 11) poses less risk of complications and can be treated conservatively with 6 weeks in an arm sling, followed by physical therapy.24

Summing up
Management of shoulder dislocation must, first, be tailored to the individual and, second, account for several interactive factors—including age, direction of instability, functional demands, risk of recurrence, and associated injuries. In many patients, conservative treatment produces a favorable long-term outcome. Particularly in young, active patients with anterior shoulder instability, most surgeons consider open or arthroscopic reconstruction to be the treatment of choice.2,18
Continue to: Pre-reduction and post-reduction...
Pre-reduction and post-reduction imaging should be carefully examined for the presence of concomitant injury, which might change the preferred treatment modality appreciably.
Last, communication among emergency department providers, the primary care provider, orthopedist, radiologist, and neurologist is crucial for determining an appropriate patient-centered approach to initial and long-term management.
CORRESPONDENCE
Nata Parnes, MD, Carthage Area Hospital, 3 Bridge Street, Carthage, NY; [email protected]
The architecture of the glenohumeral joint makes it the most common large joint to become dislocated, accounting for approximately 45% of all dislocations. Anterior dislocation constitutes more than 95% of glenohumeral joint dislocations; posterior dislocation, only 2% to 5%.1,2
For the family physician, determining appropriate follow-up after emergent reduction depends on several distinct variables, which we review here; subsequent treatment might involve, as we outline, physical therapy, immobilization, surgical intervention, or a combination of several modalities. Treatment decisions can make the difference between successful rehabilitation and potential disability, particularly in typically young and active patients.
Numerous mechanisms of injury
Anterior shoulder dislocations typically occur with the affected shoulder in a position of abduction and external rotation; 90% of patients are 21 to 30 years of age, and men are affected 3 times more often than women.2 Unsurprisingly, athletes are affected most frequently, with the common sports-related mechanism of injury being either sudden pressure exerted on the abducted and externally rotated arm or a fall onto an outstretched hand with the arm elevated. Repetitive microtrauma from such sports as swimming, baseball, and volleyball can also lead to instability.
Bankart lesion. This tear of the anterior or inferior section of the labrum is the most characteristic lesion noted in anterior dislocations, found in 73% of first-time dislocations and 100% of recurrent dislocations.3,4
Hills-Sachs lesion is often associated with a Bankart lesion. The Hills-Sachs lesion is an impaction fracture of the posterolateral aspect of the humeral head resulting from its displacement over the anterior lip of the glenoid. Hill-Sachs lesions are seen in 71% of first-time and recurrent dislocations.3
Less common concomitant injuries during anterior shoulder dislocation include rupture of the rotator-cuff tendons (particularly in patients older than 40 years), glenoid and proximal humerus fractures, a tear of the superior labrum (known as a “SLAP lesion”), cartilage injury, and neurovascular injury.
Posterior instability typically occurs as a result of a strong muscle contraction, as seen in electrocution or seizure; however, it can be caused by athletic trauma, particularly in football.5 Repetitive forces exerted on the forward-flexed and internally rotated shoulder position during blocking puts football players at increased risk of posterior instability.5
Continue to: Multidirectional instability
Multidirectional instability is more frequently attributable to congenital hyperlaxity of the glenohumeral joint capsule, rather than to acute injury. However, athletes can also develop capsular laxity from repetitive microtrauma to the shoulder.5
Emergent reduction: Prompt action needed
Acute dislocation of the shoulder should be reduced as soon as possible to minimize neurovascular injury and patient discomfort. (Typically, but not always, this is done in the emergency department.) It is crucial to have effective muscle relaxation before any attempt at reduction, to minimize the risk of iatrogenic injury to bone, cartilage, tendons, and neurovascular structures.
Muscle relaxation can be facilitated with intravenous midazolam or other agents, as specified by institutional protocol. Intra-articular lidocaine injection or intravenous fentanyl is often utilized in conjunction with the sedating agent to reduce pain and further accommodate relaxation.
Anterior reduction. Any one of several techniques can be used to perform emergent reduction of anterior shoulder dislocations, all of which have demonstrated success. The Milch technique is among the least traumatic for effective reduction.6 In this technique (FIGURE 1), the patient is supine; gentle but firm downward traction is applied to the humerus at the elbow of the affected arm while the arm is in abduction and external rotation. The provider can manipulate the humeral head at that point by placing a thumb in the patient’s axilla; the arm can also be further internally rotated and adducted until reduction is achieved.

Posterior reduction of a dislocation is performed while the patient is supine, with the body stabilized. Traction is applied on the adducted and internally rotated arm in conjunction with direct pressure on the posterior aspect of the humeral head (FIGURE 2).

Continue to: Follow-up actions
Follow-up actions. Before discharging the patient after reduction of a dislocation, it is essential to:
- perform post-reduction evaluation of shoulder stability at different levels of abduction
- perform a thorough neurovascular assessment
- obtain an anteroposterior (AP) radiograph to ensure proper positioning of the glenohumeral joint.
The reduced shoulder should be immobilized in a sling. The discharge plan should include pain management for several days and a follow-up appointment in 5 to 8 days with the primary care provider2 (FIGURE 3).

Follow-up evaluation by the primary care provider
History. Prior to the initial examination at follow-up, obtain a comprehensive history that includes the nature of the injury and the direction of force that was placed on the shoulder. Determine whether the shoulder was reduced spontaneously or required manual reduction in the field or an emergency department. Note any associated injury sustained concurrently and the presence (or absence) of neck pain, numbness, tingling, or weakness in the affected arm.
Physical exam starts with thorough inspection of the affected shoulder, with comparison to the contralateral side, at rest and during shoulder motion. Palpation to reveal points of tenderness should include the anterior joint line, acromioclavicular joint, bicipital groove, subacromial space, acromion, and greater tuberosity.
Following inspection and palpation, assess active and passive range of motion in forward elevation, abduction, internal and external rotation at the side of the body, and internal and external rotation in shoulder abduction. Assessment might be limited by pain and apprehension, and should be performed within the patient’s comfortable range of motion.
Continue to: Once range of motion...
Once range of motion is determined, assess7:
- muscle power of the rotator cuff in abduction (for the supraspinatus muscle)
- resisted external rotation at the side of the body (the infraspinatus)
- resisted external rotation in abduction > 60° (the teres minor)
- resisted internal rotation (the subscapularis).
Specific tests for shoulder laxity and stability
It is important during the primary care follow-up examination to differentiate true instability and shoulder hyperlaxity, particularly in young, flexible patients (TABLE). Many of these patients present with painless hypermobility of the shoulder without true injury to the labrum or ligamentous structures. It might appear to the patient, or to family, that the shoulder is subluxating; however, the humeral head returns to a centered position on the glenoid in a hypermobile state—typically, without pain. Actual shoulder instability is defined as loss of the ability of the humeral head to re-center, accompanied by pain—pathology that is frequently associated with damage to the capsulolabral complex.

The load and shift test is used to assess anterior and posterior laxity. The patient is seated, and the forearm is allowed to rest on the thigh. Examination is performed using 1 hand to press anteriorly or posteriorly on the humeral head; the other hand is simultaneously positioned on the joint line to feel movement of the humeral head in relation to the glenoid (FIGURE 4).

The apprehension test is a common maneuver used to assess anterior shoulder instability. It is performed by positioning the affected arm to 90° external rotation and then elevating it to 90° abduction. Although this maneuver can be performed with the patient upright, it is beneficial to have them supine, to more easily control the arm (FIGURE 5). A positive test is noted when the patient reports a sensation of impending instability (apprehension), rather than pain alone.

Relocation test. When the apprehension test is positive, the supine position can be exploited to further perform the relocation test, in 2 stages (FIGURE 6):
- Apply a posteriorly directed force on the humeral head, which stabilizes the shoulder and typically alleviates symptoms.
- Release pressure quickly from the humeral head to assess recurrence of pain and apprehension as the humeral head snaps back against the anterior labrum.

Continue to: Combined, apprehension and relocation...
Combined, apprehension and relocation tests to identify anterior shoulder instability have been shown to significantly improve specificity while maintaining sensitivity.8
The posterior apprehension test is used to assess posterior instability. The patient is supine; the affected arm is placed in flexion, adduction, and internal rotation; and posterior pressure is applied (FIGURE 7). A positive test is noted when pain is reported at the posterior aspect of the shoulder. Clicking might be noted as the humeral head dislocates rearward.1

Sulcus sign. Multidirectional instability is elicited using the sulcus sign. While the patient is seated upright, arms resting at their sides, a direct downward pull at elbow level will, when positive, reveal a depression (sulcus) at the lateral aspect of the affected shoulder as the humeral head translates inferiorly (FIGURE 8). A positive sulcus sign is documented in 3 grades, according to the amount of translation1:
- Grade I: < 1 cm
- Grade II: 1-2 cm
- Grade III: > 2 cm.

Neurovascular status should be verified at every physical evaluation, with motor and sensory function tested in the axillary, musculocutaneous, median, radial, and ulnar nerve distributions. If nerve injury is suspected, electromyography and nerve-conduction testing is indicated.9-13 Vascular compromise is much less common but equally important to assess.11
Use of imaging
Post-reduction radiographs, including internal and external AP—and especially axillary—views are invaluable. Not only do they help to ensure reduction, but they also help to assess for fracture. A magnetic resonance imaging (MRI) arthrogram is the preferred imaging modality if a labral tear is suspected (FIGURE 9). Other concomitant shoulder injuries, such as subtle bone fracture, rotator cuff tear, and biceps pathology can also be reliably diagnosed with noncontrast MRI.

Continue to: Roadmap for treatment
Roadmap for treatment
The rate of recurrence after a first anterior shoulder dislocation is strongly associated with a person’s age and level of activity. Active patients younger than 20 years have a 92% to 96% recurrence rate14; patients 20 to 40 years, 25% to 48%; and patients older than 40 years, < 10%.15
Young, athletic patients who are treated nonoperatively are left at an unacceptably high risk of recurrence, leading to progressive damage to bony and soft-tissue structures.16,17 Surgical labral repair after a first-time anterior dislocation produced improved outcomes in terms of recurrent dislocation (7.9%), compared to outcomes after nonsurgical treatment (52.9%),14 and has been associated with a lower incidence of future glenohumeral osteoarthritis.18 For those reasons, we recommend referral to an orthopedic surgeon for all patients younger than 20 years who sustain an anterior shoulder dislocation.
Patients older than 20 years who do not have concomitant shoulder injury, and who demonstrate full strength in abduction, external rotation, and internal rotation of the shoulder on clinical examination, have a low probability of associated rotator-cuff tear. They can be immobilized in a sling for 1 to 3 weeks, followed by a 6 to 12–week regimen of physical therapy.
Concomitant tear of the rotator cuff. Weakness on examination requires MRI or a magnetic resonance arthrogram for evaluation of associated rotator-cuff tear. A tear identified on MRI should be referred to an orthopedic surgeon because timely repair can be crucial to attaining best outcomes. Conservative treatment of traumatic full-tendon rotator-cuff tear is associated with poor results, progression in the size of the tear, and advancement of muscle atrophy.19,20 For patients younger than 40 years, arthroscopic rotator-cuff repair, with or without labral repair, produces excellent clinical outcomes, carries a low risk of complications, and results in a > 95% rate of return to a preoperative level of recreational and job activities.21
Patients who demonstrate weakness of the rotator-cuff muscles on examination, but who do not have a tear noted on MRI, should be evaluated by electromyography and nerve-conduction testing to assess nerve injury as an alternative cause of weakness.10,11 If a neurologic deficit is found on nerve-conduction testing, the patient should be referred for neurologic evaluation.10
Continue to: Patients with negative findings...
Patients with negative findings on MRI and nerve-conduction studies should be offered physical therapy. Patients with recurrent anterior shoulder dislocation should be referred to an orthopedic surgeon for surgical repair. Frequently, improper or delayed treatment with chronic instability results in degenerative arthropathy of the joint22 (FIGURE 10).

Posterior and multidirectional instability can typically be treated conservatively; however, whereas posterior dislocation typically must be immobilized for 3 to 6 weeks post reduction, multidirectional instability does not require immobilization. Instead, physical therapy should start as soon as possible. In these cases, recurrent dislocation or subluxation that persists after conservative treatment should be referred for possible surgical intervention.5
Instability with associated fracture
Fracture concomitant with dislocation most commonly involves the humeral neck, humeral head, greater tuberosity, or the glenoid itself.2 Clinical variables that predict a fracture associated with shoulder dislocation include23:
- first episode of dislocation
- age ≥ 40 years
- fall from higher than 1 flight of stairs
- fight or assault
- motor vehicle crash.
A computed tomography scan with 3-dimensional reconstruction can help characterize associated fracture accurately—including location, size, and displacement—and can play an important role in treatment planning and prognosis in these complicated injuries. Displaced fracture should be referred to an orthopedic surgeon. Nondisplaced fracture of the humeral head or greater tuberosity (FIGURE 11) poses less risk of complications and can be treated conservatively with 6 weeks in an arm sling, followed by physical therapy.24

Summing up
Management of shoulder dislocation must, first, be tailored to the individual and, second, account for several interactive factors—including age, direction of instability, functional demands, risk of recurrence, and associated injuries. In many patients, conservative treatment produces a favorable long-term outcome. Particularly in young, active patients with anterior shoulder instability, most surgeons consider open or arthroscopic reconstruction to be the treatment of choice.2,18
Continue to: Pre-reduction and post-reduction...
Pre-reduction and post-reduction imaging should be carefully examined for the presence of concomitant injury, which might change the preferred treatment modality appreciably.
Last, communication among emergency department providers, the primary care provider, orthopedist, radiologist, and neurologist is crucial for determining an appropriate patient-centered approach to initial and long-term management.
CORRESPONDENCE
Nata Parnes, MD, Carthage Area Hospital, 3 Bridge Street, Carthage, NY; [email protected]
1. Valencia Mora M, Ruiz Ibán MA, Heredia JD, et al. Physical exam and evaluation of the unstable shoulder. Open Orthop J. 2017;11(suppl 6, M12):946-956. doi: 10.2174/1874325001711010946
2. Khiami F, A, Loriaut P. Management of recent first-time anterior shoulder dislocation. Orthop Traumatol Surg Res. 2015;101(1 suppl):S51-S57. doi: 10.1016/j.otsr.2014.06.027
3. Antonio GE, Griffith JF, Yu AB, et al. First-time shoulder dislocation: high prevalence of labral injury and age-related differences revealed by MR arthrography. J Magn Reson Imaging. 2007;26:983-991. doi: 10.1002/jmri.21092
4. Carrazzone OL, Tamaoki MJS, Ambra LFM, et al. Prevalence of lesions associated with traumatic recurrent shoulder dislocation. Rev Bras Ortop. 2015;46:281-287. doi: 10.1016/S2255-4971(15)30196-8
5. Mahaffey BL, Smith PA. Shoulder instability in young athletes. Am Fam Physician. 1999;59:2773-2787.
6. Amar E, Maman E, Khashan M, et al. Milch versus Stimson technique for nonsedated reduction of anterior shoulder dislocation: a prospective randomized trial and analysis of factors affecting success. J Shoulder Elbow Surg. 2012;21:1443-1449. doi: 10.1016/j.jse.2012.01.004
7. Jain NB, Wilcox RB 3rd, Katz JN, et al. Clinical examination of the rotator cuff. PM R. 2013;5:45-56. doi: 10.1016/j.pmrj.2012.08.019
8. Lizzio VA, Meta F, Fidai M, et al. Clinical evaluation and physical exam findings in patients with anterior shoulder instability. Curr Rev Musculoskelet Med. 2017;10:434-441. doi: 10.1007/s12178-017-9434-3
9. Farber AJ, Castillo R, Clough M, et al. Clinical assessment of three common tests for traumatic anterior shoulder instability. J Bone Joint Surg Am. 2006;88:1467-1474. doi: 10.2106/JBJS.E.00594
10. Robinson CM, Shur N, Sharpe T, et al. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94:18-26. doi: 10.2106/JBJS.J.01795
11. de Laat EA, Visser CP, Coene LN, et al. Nerve lesions in primary shoulder dislocations and humeral neck fractures. A prospective clinical and EMG study. J Bone Joint Surg Br. 1994;76:381-383.
12. Avis D, Power D. Axillary nerve injury associated with glenohumeral dislocation: a review and algorithm for management. EFORT Open Rev. 2018;3:70-77. doi: 10.1302/2058-5241.3.170003
13. Drury JK, Scullion JE. Vascular complications of anterior dislocation of the shoulder. Br J Surg. 1980;67:579-581. doi: 10.1002/bjs.1800670817
14. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010. doi: 10.2174/1874325001711011001
15. te Slaa RL, Brand R, Marti RK. A prospective arthroscopic study of acute first-time anterior shoulder dislocation in the young: a five-year follow-up study. J Shoulder Elbow Surg. 2003;12:529-534. doi: 10.1016/s1058-2746(03)00218-0
16. Kavaja L, T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506. doi: 10.1136/bjsports-2017-098539
17. Krych AJ, Sousa PL, King AH, et al. The effect of cartilage injury after arthroscopic stabilization for shoulder instability. Orthopedics. 2015;38:e965-e969. doi: 10.3928/01477447-20151020-03
18. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
19. Maman E, Harris C, White L, et al. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am. 2009;91:1898-1906. doi: 10.2106/JBJS.G.01335
20. Safran O, Schroeder J, Bloom R, et al. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39:710-714. doi: 10.1177/0363546510393944
21. Parnes N, Bartoszewski NR, Defranco MJ. Arthroscopic repair of full-thickness rotator cuff tears in active patients younger than 40 years: 2- to 5-year clinical outcomes. Orthopedics 2018;41:e52-e57. doi: 10.3928/01477447-20171114-02
22. Sofu H, Gürsu S, Koçkara N, et al. Recurrent anterior shoulder instability: review of the literature and current concepts. World J Clin Cases. 2014;2:676-682. doi: 10.12998/wjcc.v2.i11.676
23. Emond M, Le Sage N, Lavoie A, et al. Clinical factors predicting fractures associated with an anterior shoulder dislocation. Acad Emerg Med. 2004;11:853-858. doi: 10.1111/j.1553-2712.2004.tb00768.x
24. Parnes N, Jupiter JB. Fixed-angle locking plating of displaced proximal humerus fractures. Instr Course Lect. 2010;59:539-552.
1. Valencia Mora M, Ruiz Ibán MA, Heredia JD, et al. Physical exam and evaluation of the unstable shoulder. Open Orthop J. 2017;11(suppl 6, M12):946-956. doi: 10.2174/1874325001711010946
2. Khiami F, A, Loriaut P. Management of recent first-time anterior shoulder dislocation. Orthop Traumatol Surg Res. 2015;101(1 suppl):S51-S57. doi: 10.1016/j.otsr.2014.06.027
3. Antonio GE, Griffith JF, Yu AB, et al. First-time shoulder dislocation: high prevalence of labral injury and age-related differences revealed by MR arthrography. J Magn Reson Imaging. 2007;26:983-991. doi: 10.1002/jmri.21092
4. Carrazzone OL, Tamaoki MJS, Ambra LFM, et al. Prevalence of lesions associated with traumatic recurrent shoulder dislocation. Rev Bras Ortop. 2015;46:281-287. doi: 10.1016/S2255-4971(15)30196-8
5. Mahaffey BL, Smith PA. Shoulder instability in young athletes. Am Fam Physician. 1999;59:2773-2787.
6. Amar E, Maman E, Khashan M, et al. Milch versus Stimson technique for nonsedated reduction of anterior shoulder dislocation: a prospective randomized trial and analysis of factors affecting success. J Shoulder Elbow Surg. 2012;21:1443-1449. doi: 10.1016/j.jse.2012.01.004
7. Jain NB, Wilcox RB 3rd, Katz JN, et al. Clinical examination of the rotator cuff. PM R. 2013;5:45-56. doi: 10.1016/j.pmrj.2012.08.019
8. Lizzio VA, Meta F, Fidai M, et al. Clinical evaluation and physical exam findings in patients with anterior shoulder instability. Curr Rev Musculoskelet Med. 2017;10:434-441. doi: 10.1007/s12178-017-9434-3
9. Farber AJ, Castillo R, Clough M, et al. Clinical assessment of three common tests for traumatic anterior shoulder instability. J Bone Joint Surg Am. 2006;88:1467-1474. doi: 10.2106/JBJS.E.00594
10. Robinson CM, Shur N, Sharpe T, et al. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94:18-26. doi: 10.2106/JBJS.J.01795
11. de Laat EA, Visser CP, Coene LN, et al. Nerve lesions in primary shoulder dislocations and humeral neck fractures. A prospective clinical and EMG study. J Bone Joint Surg Br. 1994;76:381-383.
12. Avis D, Power D. Axillary nerve injury associated with glenohumeral dislocation: a review and algorithm for management. EFORT Open Rev. 2018;3:70-77. doi: 10.1302/2058-5241.3.170003
13. Drury JK, Scullion JE. Vascular complications of anterior dislocation of the shoulder. Br J Surg. 1980;67:579-581. doi: 10.1002/bjs.1800670817
14. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010. doi: 10.2174/1874325001711011001
15. te Slaa RL, Brand R, Marti RK. A prospective arthroscopic study of acute first-time anterior shoulder dislocation in the young: a five-year follow-up study. J Shoulder Elbow Surg. 2003;12:529-534. doi: 10.1016/s1058-2746(03)00218-0
16. Kavaja L, T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506. doi: 10.1136/bjsports-2017-098539
17. Krych AJ, Sousa PL, King AH, et al. The effect of cartilage injury after arthroscopic stabilization for shoulder instability. Orthopedics. 2015;38:e965-e969. doi: 10.3928/01477447-20151020-03
18. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
19. Maman E, Harris C, White L, et al. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am. 2009;91:1898-1906. doi: 10.2106/JBJS.G.01335
20. Safran O, Schroeder J, Bloom R, et al. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39:710-714. doi: 10.1177/0363546510393944
21. Parnes N, Bartoszewski NR, Defranco MJ. Arthroscopic repair of full-thickness rotator cuff tears in active patients younger than 40 years: 2- to 5-year clinical outcomes. Orthopedics 2018;41:e52-e57. doi: 10.3928/01477447-20171114-02
22. Sofu H, Gürsu S, Koçkara N, et al. Recurrent anterior shoulder instability: review of the literature and current concepts. World J Clin Cases. 2014;2:676-682. doi: 10.12998/wjcc.v2.i11.676
23. Emond M, Le Sage N, Lavoie A, et al. Clinical factors predicting fractures associated with an anterior shoulder dislocation. Acad Emerg Med. 2004;11:853-858. doi: 10.1111/j.1553-2712.2004.tb00768.x
24. Parnes N, Jupiter JB. Fixed-angle locking plating of displaced proximal humerus fractures. Instr Course Lect. 2010;59:539-552.
PRACTICE RECOMMENDATIONS
› Refer first-time dislocation in patients younger than 20 years or who have a displaced fracture to an orthopedic surgeon. A
› Order magnetic resonance imaging (MRI) for all patients with a suspected rotator cuff tear. A
› Send patients with weakness of the rotator cuff—but no tear on MRI—for evaluation by electromyography and nerve-conduction studies. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Oral PTH shows promise for osteoporosis in early phase 2 study
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
Could the osteoporosis drug alendronate ward off diabetes?
A nationwide, retrospective, case-control study of older adults in Denmark suggests that the bisphosphonate alendronate that is widely used to treat osteoporosis may protect against new-onset type 2 diabetes. But these preliminary findings need to be confirmed in a randomized controlled trial, experts said.
The registry study showed that from 2008 to 2018, among individuals in Denmark age 50 and older (with a mean age of 67), those who were taking alendronate were 36% less likely to have new-onset type 2 diabetes than age- and sex-matched individuals who were not taking the drug, after controlling for multiple risk factors.
The results also suggest that longer alendronate use and higher compliance might be more protective.
Rikke Viggers, MD, a PhD student in the department of clinical medicine, Aalborg (Denmark) University, presented the findings during an oral session at the annual meeting of the European Association for the Study of Diabetes.
“Excitingly, our research suggests that alendronate, an inexpensive medicine widely used to treat osteoporosis, may also protect against type 2 diabetes,” Dr. Viggers summarized in a press release issued by the EASD.
“Type 2 diabetes is a serious lifelong condition that can lead to other serious health issues such as stroke, heart disease, blindness, and limb amputation,” she noted, “and anything that prevents or even delays it will also reduce a person’s risk of all these other conditions.”
“We believe that doctors should consider this when prescribing osteoporosis drugs to those with prediabetes or at high risk of type 2 diabetes,” she added.
Preliminary results, need for RCT
However, these are preliminary results, Dr. Viggers cautioned during the oral presentation and in an email. “This is a registry-based study,” she stressed, “and we cannot conclude causality.”
“We do not know if this effect [of decreased risk of developing diabetes among people taking alendronate] is ‘real’ and what the mechanisms are.”
“It could be a direct effect on peripheral tissues, for example, muscle and adipose tissue,” Dr. Viggers speculated, “or an indirect effect through bone metabolites that may impact glucose metabolism.”
The group is now conducting a randomized controlled trial in patients with diabetes and osteopenia or osteoporosis to examine the relationship between alendronate and insulin sensitivity, bone indices, and glycemic control.
They also aim to investigate whether alendronate is the optimal antiosteoporotic therapy for patients with type 2 diabetes. Preliminary results suggest that other bisphosphonates have similar effects.
“Alendronate decreases bone turnover and may not be beneficial in healthy bones,” Dr. Viggers noted. “However, as far as I know, potential other side effects have not been tested in healthy bones,” so further research is needed.
Invited to comment, Charles P. Vega, MD, who presented a case and a crowd-sourced opinion about deprescribing bisphosphonates, noted that type 2 diabetes is most often diagnosed between age 40 and 60, although a few cases are diagnosed after age 65, and the study by Dr. Viggers and colleagues suggests that alendronate might help lower the risk of diabetes onset in these older adults.
“This is an interesting retrospective analysis,” said Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, but like the study authors, he cautioned that “it should be verified with other data.”
“A meta-analysis from clinical trials of bisphosphonates which followed blood glucose levels would be helpful,” he said.
Current registry study findings
Glucose homeostasis has been linked to bone metabolism, Dr. Viggers said, and bisphosphonates were associated with increased insulin sensitivity and decreased risk of diabetes risk in two registry studies from Denmark and Taiwan.
The researchers aimed to investigate if the risk of developing type 2 diabetes was altered by previous use of alendronate.
Using data from the national Danish Patient Registry, they identified 163,588 individuals age 50 and older newly diagnosed with type 2 diabetes in 2008-2018.
They matched each patient with three individuals of the same gender and age range who did not have diabetes, for a total of 490,764 controls.
Roughly two-thirds of participants were in their 50s or 60s, a quarter were in their 70s, and 10% were 80 or older. About half of participants were women (45%).
Compared to the patients with new-onset type 2 diabetes, the control participants were healthier: they were less likely to have obesity (6% vs. 17%) and had a lower mean Charlson Comorbidity Index (0.38 vs. 0.88).
Using data from the national Danish Health Service Prescription Registry, the researchers identified individuals who filled prescriptions for alendronate in 2008-2018.
After controlling for heavy smoking, alcohol abuse, obesity, pancreatitis, hyperthyroidism, hypothyroidism, glucocorticoid use, marital status, household income, and Charlson Comorbidity Index, people taking alendronate were less likely to have new-onset diabetes than those not taking this drug (odds ratio, 0.64; 95% confidence interval, 0.62-0.66).
The odds of developing type 2 diabetes were even lower among those who took alendronate for 8 years or more versus never-users (OR, 0.47; 95% CI, 0.40-0.56), after controlling for the same variables.
Session Chair Zhila Semnani-Azad, a PhD student in nutritional science, University of Toronto, wanted to know if the researchers accounted for physical activity and vitamin D use. Dr. Viggers replied that the registries did not have this information.
The study was funded by a Steno Collaborative Project grant from the Novo Nordisk Foundation, Denmark. Dr. Viggers has disclosed receiving a grant from the foundation. Dr. Vega has disclosed serving as a consultant for Johnson & Johnson.
A version of this article first appeared on Medscape.com.
A nationwide, retrospective, case-control study of older adults in Denmark suggests that the bisphosphonate alendronate that is widely used to treat osteoporosis may protect against new-onset type 2 diabetes. But these preliminary findings need to be confirmed in a randomized controlled trial, experts said.
The registry study showed that from 2008 to 2018, among individuals in Denmark age 50 and older (with a mean age of 67), those who were taking alendronate were 36% less likely to have new-onset type 2 diabetes than age- and sex-matched individuals who were not taking the drug, after controlling for multiple risk factors.
The results also suggest that longer alendronate use and higher compliance might be more protective.
Rikke Viggers, MD, a PhD student in the department of clinical medicine, Aalborg (Denmark) University, presented the findings during an oral session at the annual meeting of the European Association for the Study of Diabetes.
“Excitingly, our research suggests that alendronate, an inexpensive medicine widely used to treat osteoporosis, may also protect against type 2 diabetes,” Dr. Viggers summarized in a press release issued by the EASD.
“Type 2 diabetes is a serious lifelong condition that can lead to other serious health issues such as stroke, heart disease, blindness, and limb amputation,” she noted, “and anything that prevents or even delays it will also reduce a person’s risk of all these other conditions.”
“We believe that doctors should consider this when prescribing osteoporosis drugs to those with prediabetes or at high risk of type 2 diabetes,” she added.
Preliminary results, need for RCT
However, these are preliminary results, Dr. Viggers cautioned during the oral presentation and in an email. “This is a registry-based study,” she stressed, “and we cannot conclude causality.”
“We do not know if this effect [of decreased risk of developing diabetes among people taking alendronate] is ‘real’ and what the mechanisms are.”
“It could be a direct effect on peripheral tissues, for example, muscle and adipose tissue,” Dr. Viggers speculated, “or an indirect effect through bone metabolites that may impact glucose metabolism.”
The group is now conducting a randomized controlled trial in patients with diabetes and osteopenia or osteoporosis to examine the relationship between alendronate and insulin sensitivity, bone indices, and glycemic control.
They also aim to investigate whether alendronate is the optimal antiosteoporotic therapy for patients with type 2 diabetes. Preliminary results suggest that other bisphosphonates have similar effects.
“Alendronate decreases bone turnover and may not be beneficial in healthy bones,” Dr. Viggers noted. “However, as far as I know, potential other side effects have not been tested in healthy bones,” so further research is needed.
Invited to comment, Charles P. Vega, MD, who presented a case and a crowd-sourced opinion about deprescribing bisphosphonates, noted that type 2 diabetes is most often diagnosed between age 40 and 60, although a few cases are diagnosed after age 65, and the study by Dr. Viggers and colleagues suggests that alendronate might help lower the risk of diabetes onset in these older adults.
“This is an interesting retrospective analysis,” said Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, but like the study authors, he cautioned that “it should be verified with other data.”
“A meta-analysis from clinical trials of bisphosphonates which followed blood glucose levels would be helpful,” he said.
Current registry study findings
Glucose homeostasis has been linked to bone metabolism, Dr. Viggers said, and bisphosphonates were associated with increased insulin sensitivity and decreased risk of diabetes risk in two registry studies from Denmark and Taiwan.
The researchers aimed to investigate if the risk of developing type 2 diabetes was altered by previous use of alendronate.
Using data from the national Danish Patient Registry, they identified 163,588 individuals age 50 and older newly diagnosed with type 2 diabetes in 2008-2018.
They matched each patient with three individuals of the same gender and age range who did not have diabetes, for a total of 490,764 controls.
Roughly two-thirds of participants were in their 50s or 60s, a quarter were in their 70s, and 10% were 80 or older. About half of participants were women (45%).
Compared to the patients with new-onset type 2 diabetes, the control participants were healthier: they were less likely to have obesity (6% vs. 17%) and had a lower mean Charlson Comorbidity Index (0.38 vs. 0.88).
Using data from the national Danish Health Service Prescription Registry, the researchers identified individuals who filled prescriptions for alendronate in 2008-2018.
After controlling for heavy smoking, alcohol abuse, obesity, pancreatitis, hyperthyroidism, hypothyroidism, glucocorticoid use, marital status, household income, and Charlson Comorbidity Index, people taking alendronate were less likely to have new-onset diabetes than those not taking this drug (odds ratio, 0.64; 95% confidence interval, 0.62-0.66).
The odds of developing type 2 diabetes were even lower among those who took alendronate for 8 years or more versus never-users (OR, 0.47; 95% CI, 0.40-0.56), after controlling for the same variables.
Session Chair Zhila Semnani-Azad, a PhD student in nutritional science, University of Toronto, wanted to know if the researchers accounted for physical activity and vitamin D use. Dr. Viggers replied that the registries did not have this information.
The study was funded by a Steno Collaborative Project grant from the Novo Nordisk Foundation, Denmark. Dr. Viggers has disclosed receiving a grant from the foundation. Dr. Vega has disclosed serving as a consultant for Johnson & Johnson.
A version of this article first appeared on Medscape.com.
A nationwide, retrospective, case-control study of older adults in Denmark suggests that the bisphosphonate alendronate that is widely used to treat osteoporosis may protect against new-onset type 2 diabetes. But these preliminary findings need to be confirmed in a randomized controlled trial, experts said.
The registry study showed that from 2008 to 2018, among individuals in Denmark age 50 and older (with a mean age of 67), those who were taking alendronate were 36% less likely to have new-onset type 2 diabetes than age- and sex-matched individuals who were not taking the drug, after controlling for multiple risk factors.
The results also suggest that longer alendronate use and higher compliance might be more protective.
Rikke Viggers, MD, a PhD student in the department of clinical medicine, Aalborg (Denmark) University, presented the findings during an oral session at the annual meeting of the European Association for the Study of Diabetes.
“Excitingly, our research suggests that alendronate, an inexpensive medicine widely used to treat osteoporosis, may also protect against type 2 diabetes,” Dr. Viggers summarized in a press release issued by the EASD.
“Type 2 diabetes is a serious lifelong condition that can lead to other serious health issues such as stroke, heart disease, blindness, and limb amputation,” she noted, “and anything that prevents or even delays it will also reduce a person’s risk of all these other conditions.”
“We believe that doctors should consider this when prescribing osteoporosis drugs to those with prediabetes or at high risk of type 2 diabetes,” she added.
Preliminary results, need for RCT
However, these are preliminary results, Dr. Viggers cautioned during the oral presentation and in an email. “This is a registry-based study,” she stressed, “and we cannot conclude causality.”
“We do not know if this effect [of decreased risk of developing diabetes among people taking alendronate] is ‘real’ and what the mechanisms are.”
“It could be a direct effect on peripheral tissues, for example, muscle and adipose tissue,” Dr. Viggers speculated, “or an indirect effect through bone metabolites that may impact glucose metabolism.”
The group is now conducting a randomized controlled trial in patients with diabetes and osteopenia or osteoporosis to examine the relationship between alendronate and insulin sensitivity, bone indices, and glycemic control.
They also aim to investigate whether alendronate is the optimal antiosteoporotic therapy for patients with type 2 diabetes. Preliminary results suggest that other bisphosphonates have similar effects.
“Alendronate decreases bone turnover and may not be beneficial in healthy bones,” Dr. Viggers noted. “However, as far as I know, potential other side effects have not been tested in healthy bones,” so further research is needed.
Invited to comment, Charles P. Vega, MD, who presented a case and a crowd-sourced opinion about deprescribing bisphosphonates, noted that type 2 diabetes is most often diagnosed between age 40 and 60, although a few cases are diagnosed after age 65, and the study by Dr. Viggers and colleagues suggests that alendronate might help lower the risk of diabetes onset in these older adults.
“This is an interesting retrospective analysis,” said Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, but like the study authors, he cautioned that “it should be verified with other data.”
“A meta-analysis from clinical trials of bisphosphonates which followed blood glucose levels would be helpful,” he said.
Current registry study findings
Glucose homeostasis has been linked to bone metabolism, Dr. Viggers said, and bisphosphonates were associated with increased insulin sensitivity and decreased risk of diabetes risk in two registry studies from Denmark and Taiwan.
The researchers aimed to investigate if the risk of developing type 2 diabetes was altered by previous use of alendronate.
Using data from the national Danish Patient Registry, they identified 163,588 individuals age 50 and older newly diagnosed with type 2 diabetes in 2008-2018.
They matched each patient with three individuals of the same gender and age range who did not have diabetes, for a total of 490,764 controls.
Roughly two-thirds of participants were in their 50s or 60s, a quarter were in their 70s, and 10% were 80 or older. About half of participants were women (45%).
Compared to the patients with new-onset type 2 diabetes, the control participants were healthier: they were less likely to have obesity (6% vs. 17%) and had a lower mean Charlson Comorbidity Index (0.38 vs. 0.88).
Using data from the national Danish Health Service Prescription Registry, the researchers identified individuals who filled prescriptions for alendronate in 2008-2018.
After controlling for heavy smoking, alcohol abuse, obesity, pancreatitis, hyperthyroidism, hypothyroidism, glucocorticoid use, marital status, household income, and Charlson Comorbidity Index, people taking alendronate were less likely to have new-onset diabetes than those not taking this drug (odds ratio, 0.64; 95% confidence interval, 0.62-0.66).
The odds of developing type 2 diabetes were even lower among those who took alendronate for 8 years or more versus never-users (OR, 0.47; 95% CI, 0.40-0.56), after controlling for the same variables.
Session Chair Zhila Semnani-Azad, a PhD student in nutritional science, University of Toronto, wanted to know if the researchers accounted for physical activity and vitamin D use. Dr. Viggers replied that the registries did not have this information.
The study was funded by a Steno Collaborative Project grant from the Novo Nordisk Foundation, Denmark. Dr. Viggers has disclosed receiving a grant from the foundation. Dr. Vega has disclosed serving as a consultant for Johnson & Johnson.
A version of this article first appeared on Medscape.com.
FROM EASD 2021
European agency recommends two new adalimumab biosimilars
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended marketing authorization this week for two new adalimumab biosimilars, Hukyndra and Libmyris.
The biosimilars, both developed by STADA Arzneimittel AG, will be available as a 40-mg solution for injection in a pre-filled syringe and pre-filled pen and 80-mg solution for injection in a pre-filled syringe. Both biosimilars will have 15 indications:
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- enthesitis-related arthritis
- ankylosing spondylitis
- axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- psoriatic arthritis
- chronic plaque psoriasis (adults and children)
- hidradenitis suppurativa
- Crohn’s disease (adults and children)
- ulcerative colitis (adults and children)
- uveitis (adults and children)
Data show that both Hukyndra and Libmyris are highly similar to the reference product Humira (adalimumab), a monoclonal antibody to tumor necrosis factor alpha, and have comparable quality, safety, and efficacy.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended marketing authorization this week for two new adalimumab biosimilars, Hukyndra and Libmyris.
The biosimilars, both developed by STADA Arzneimittel AG, will be available as a 40-mg solution for injection in a pre-filled syringe and pre-filled pen and 80-mg solution for injection in a pre-filled syringe. Both biosimilars will have 15 indications:
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- enthesitis-related arthritis
- ankylosing spondylitis
- axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- psoriatic arthritis
- chronic plaque psoriasis (adults and children)
- hidradenitis suppurativa
- Crohn’s disease (adults and children)
- ulcerative colitis (adults and children)
- uveitis (adults and children)
Data show that both Hukyndra and Libmyris are highly similar to the reference product Humira (adalimumab), a monoclonal antibody to tumor necrosis factor alpha, and have comparable quality, safety, and efficacy.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended marketing authorization this week for two new adalimumab biosimilars, Hukyndra and Libmyris.
The biosimilars, both developed by STADA Arzneimittel AG, will be available as a 40-mg solution for injection in a pre-filled syringe and pre-filled pen and 80-mg solution for injection in a pre-filled syringe. Both biosimilars will have 15 indications:
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- enthesitis-related arthritis
- ankylosing spondylitis
- axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- psoriatic arthritis
- chronic plaque psoriasis (adults and children)
- hidradenitis suppurativa
- Crohn’s disease (adults and children)
- ulcerative colitis (adults and children)
- uveitis (adults and children)
Data show that both Hukyndra and Libmyris are highly similar to the reference product Humira (adalimumab), a monoclonal antibody to tumor necrosis factor alpha, and have comparable quality, safety, and efficacy.
A version of this article first appeared on Medscape.com.
Three JAK inhibitors get boxed warnings, modified indications
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
Study informs about risks of discontinuing meds in JIA
Flares are modest in preliminary data.
Many but not all children with juvenile idiopathic arthritis (JIA) can regain remission after stopping and then restarting treatment, according to preliminary data from the ongoing Recapture-JIA study that were presented in a symposium sponsored by the Rheumatology Research Foundation.
The aim of this study is to evaluate the risks of discontinuing treatment after a period when JIA has been well controlled. Such data are of increasing interest to parents now that many children with JIA are achieving sustained periods of remission, according to Sarah Ringold, MD, a pediatric rheumatologist and associate professor of pediatrics at Seattle Children’s Hospital.
In follow-up so far, “recapture rates range from 50% to 76%” depending on type of JIA, reported Dr. Ringold, who said that patients with systemic JIA have so far been the most likely to achieve a good response when treatment is restarted.
The study is being conducted through the Childhood Arthritis and Rheumatology Research Alliance, which has 71 participating centers and has accrued data on more than 10,000 children with rheumatic diseases. For the study, the researchers identified 384 children with JIA who were already enrolled in the CARRA registry and had discontinued medications and then subsequently restarted them, and they also enrolled a prospective cohort of patients new to the registry who presented with flare after discontinuing their medication. Dr. Ringold reported on 64 of the patients in the prospective cohort.
Median time to flare: 219 days
Of findings so far, disease recurrence after discontinuation has been generally characterized by flares “of moderate activity” several months to more than a year after treatment discontinuation, according to Dr. Ringold, who emphasized repeatedly that these data are preliminary. The median time to a flare after treatment discontinuation was approximately 7 months (219 days).
In the combined cohorts, the median age at onset of JIA was 4 years. The median age at time of discontinuation was 9 years. More than half (55%) were taking a conventional disease-modifying antirheumatic drug (DMARD) and 35% were taking a tumor necrosis factor inhibitor at the time that their therapy was discontinued.
Most JIA types are represented. The most common form is rheumatoid factor–negative oligoarticular JIA. The main outcome looked the rate of clinically inactive disease at 6 months in children who had discontinued therapy after a period of remission. They defined clinically inactive disease as a Physician’s Global Assessment of less than 1 and an active joint count of 0.
Systemic JIA recapture rate at 6 months: 76%
At the time of disease flare after treatment discontinuation across both the retrospective and prospective cohorts, the median clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10; score range of 0-30) was 3.5. The recapture rate to clinically inactive disease at 6 months was 76% in those with systemic JIA and 50% in those with rheumatoid factor–positive polyarticular JIA. Other subtypes fell within this range. Rates of inactive disease at 6 months according to cJADAS10 score were lower, ranging from 26% with enthesitis-related arthritis/juvenile psoriatic arthritis to 57% with systemic JIA.
About 40% of those who restarted on therapy after a flare took the same medication again. About one-third of patients were restarted on glucocorticoids, mostly involving injections to inflamed joints, and data are not yet in about whether these were restarted alone or with other drugs, according to Dr. Ringold.
The final analysis of this study will explore clinical and laboratory variables associated with disease recapture. In the prospective cohort, which did not reach its planned enrollment of 150 children because the COVID pandemic, a broad array of these variables was evaluated at baseline.
Numerous studies have already looked at predictors of sustained remission after stopping medications of JIA, according to Dr. Ringold, but she said that there is relatively little information about outcomes in children who stop medications, flare, and are retreated. Other experts agree.
“We know little about how successfully DMARDs can be discontinued and used again after a disease flare,” reported Jens Klotsche, MD, a researcher at the German Rheumatism Research Center, which is part of the Leibniz Institute in Berlin. Dr. Klotsche, who is an author of a recent study that found etanercept effective for retreatment when children with JIA had discontinued therapy, agreed that “data from large cohort studies are necessary to support the treatment decisions by clinicians, parents, and patients.”
JIA recurrence risk is unclear
In a systematic review published 2 years ago, rates of flare following discontinuation of treatment for JIA were relatively high, but there were some limitations to this analysis, according to the lead author, Olha Halyabar, MD, a pediatric rheumatologist at Boston Children’s Hospital.
“The data in our systematic review showed that overall quality of evidence was low, with large variations and sometimes very different conclusions,” Dr. Halyabar said in an interview. She believes that the data generated by the CARRA analysis will be valuable, particularly in evaluating outcomes across subtypes.
“Even though, at this point, [previously published] reports indicate overall high rates of recurrence (>50% for some JIA subtypes), there are some encouraging studies from early treat-to-target strategies,” she said, adding that large datasets like those from CARRA offer an opportunity to gather data likely to be clinically useful.
Dr. Ringold cautioned that there are some limitations to the CARRA analysis, including some missing data from the retrospective cohort. She also pointed out that patients have been assessed at routine clinical visits rather than at standardized intervals, introducing a potential for bias.
For parents concerned about the costs, inconvenience, and side effects from sustained JIA treatment once remission is achieved, data from CARRA will allow clinicians to provide evidence-based counseling on balancing the risks of discontinuing therapy, including the likelihood of regaining remission when disease returns, against the goals of stopping treatment.
“Parents are having more conversations about when to stop medications,” Dr. Ringold said. She indicated that these data should be helpful for providing guidance.
Dr. Ringold, Dr. Klotsche, and Dr. Halyabar reported having no potential conflicts of interest.
Flares are modest in preliminary data.
Flares are modest in preliminary data.
Many but not all children with juvenile idiopathic arthritis (JIA) can regain remission after stopping and then restarting treatment, according to preliminary data from the ongoing Recapture-JIA study that were presented in a symposium sponsored by the Rheumatology Research Foundation.
The aim of this study is to evaluate the risks of discontinuing treatment after a period when JIA has been well controlled. Such data are of increasing interest to parents now that many children with JIA are achieving sustained periods of remission, according to Sarah Ringold, MD, a pediatric rheumatologist and associate professor of pediatrics at Seattle Children’s Hospital.
In follow-up so far, “recapture rates range from 50% to 76%” depending on type of JIA, reported Dr. Ringold, who said that patients with systemic JIA have so far been the most likely to achieve a good response when treatment is restarted.
The study is being conducted through the Childhood Arthritis and Rheumatology Research Alliance, which has 71 participating centers and has accrued data on more than 10,000 children with rheumatic diseases. For the study, the researchers identified 384 children with JIA who were already enrolled in the CARRA registry and had discontinued medications and then subsequently restarted them, and they also enrolled a prospective cohort of patients new to the registry who presented with flare after discontinuing their medication. Dr. Ringold reported on 64 of the patients in the prospective cohort.
Median time to flare: 219 days
Of findings so far, disease recurrence after discontinuation has been generally characterized by flares “of moderate activity” several months to more than a year after treatment discontinuation, according to Dr. Ringold, who emphasized repeatedly that these data are preliminary. The median time to a flare after treatment discontinuation was approximately 7 months (219 days).
In the combined cohorts, the median age at onset of JIA was 4 years. The median age at time of discontinuation was 9 years. More than half (55%) were taking a conventional disease-modifying antirheumatic drug (DMARD) and 35% were taking a tumor necrosis factor inhibitor at the time that their therapy was discontinued.
Most JIA types are represented. The most common form is rheumatoid factor–negative oligoarticular JIA. The main outcome looked the rate of clinically inactive disease at 6 months in children who had discontinued therapy after a period of remission. They defined clinically inactive disease as a Physician’s Global Assessment of less than 1 and an active joint count of 0.
Systemic JIA recapture rate at 6 months: 76%
At the time of disease flare after treatment discontinuation across both the retrospective and prospective cohorts, the median clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10; score range of 0-30) was 3.5. The recapture rate to clinically inactive disease at 6 months was 76% in those with systemic JIA and 50% in those with rheumatoid factor–positive polyarticular JIA. Other subtypes fell within this range. Rates of inactive disease at 6 months according to cJADAS10 score were lower, ranging from 26% with enthesitis-related arthritis/juvenile psoriatic arthritis to 57% with systemic JIA.
About 40% of those who restarted on therapy after a flare took the same medication again. About one-third of patients were restarted on glucocorticoids, mostly involving injections to inflamed joints, and data are not yet in about whether these were restarted alone or with other drugs, according to Dr. Ringold.
The final analysis of this study will explore clinical and laboratory variables associated with disease recapture. In the prospective cohort, which did not reach its planned enrollment of 150 children because the COVID pandemic, a broad array of these variables was evaluated at baseline.
Numerous studies have already looked at predictors of sustained remission after stopping medications of JIA, according to Dr. Ringold, but she said that there is relatively little information about outcomes in children who stop medications, flare, and are retreated. Other experts agree.
“We know little about how successfully DMARDs can be discontinued and used again after a disease flare,” reported Jens Klotsche, MD, a researcher at the German Rheumatism Research Center, which is part of the Leibniz Institute in Berlin. Dr. Klotsche, who is an author of a recent study that found etanercept effective for retreatment when children with JIA had discontinued therapy, agreed that “data from large cohort studies are necessary to support the treatment decisions by clinicians, parents, and patients.”
JIA recurrence risk is unclear
In a systematic review published 2 years ago, rates of flare following discontinuation of treatment for JIA were relatively high, but there were some limitations to this analysis, according to the lead author, Olha Halyabar, MD, a pediatric rheumatologist at Boston Children’s Hospital.
“The data in our systematic review showed that overall quality of evidence was low, with large variations and sometimes very different conclusions,” Dr. Halyabar said in an interview. She believes that the data generated by the CARRA analysis will be valuable, particularly in evaluating outcomes across subtypes.
“Even though, at this point, [previously published] reports indicate overall high rates of recurrence (>50% for some JIA subtypes), there are some encouraging studies from early treat-to-target strategies,” she said, adding that large datasets like those from CARRA offer an opportunity to gather data likely to be clinically useful.
Dr. Ringold cautioned that there are some limitations to the CARRA analysis, including some missing data from the retrospective cohort. She also pointed out that patients have been assessed at routine clinical visits rather than at standardized intervals, introducing a potential for bias.
For parents concerned about the costs, inconvenience, and side effects from sustained JIA treatment once remission is achieved, data from CARRA will allow clinicians to provide evidence-based counseling on balancing the risks of discontinuing therapy, including the likelihood of regaining remission when disease returns, against the goals of stopping treatment.
“Parents are having more conversations about when to stop medications,” Dr. Ringold said. She indicated that these data should be helpful for providing guidance.
Dr. Ringold, Dr. Klotsche, and Dr. Halyabar reported having no potential conflicts of interest.
Many but not all children with juvenile idiopathic arthritis (JIA) can regain remission after stopping and then restarting treatment, according to preliminary data from the ongoing Recapture-JIA study that were presented in a symposium sponsored by the Rheumatology Research Foundation.
The aim of this study is to evaluate the risks of discontinuing treatment after a period when JIA has been well controlled. Such data are of increasing interest to parents now that many children with JIA are achieving sustained periods of remission, according to Sarah Ringold, MD, a pediatric rheumatologist and associate professor of pediatrics at Seattle Children’s Hospital.
In follow-up so far, “recapture rates range from 50% to 76%” depending on type of JIA, reported Dr. Ringold, who said that patients with systemic JIA have so far been the most likely to achieve a good response when treatment is restarted.
The study is being conducted through the Childhood Arthritis and Rheumatology Research Alliance, which has 71 participating centers and has accrued data on more than 10,000 children with rheumatic diseases. For the study, the researchers identified 384 children with JIA who were already enrolled in the CARRA registry and had discontinued medications and then subsequently restarted them, and they also enrolled a prospective cohort of patients new to the registry who presented with flare after discontinuing their medication. Dr. Ringold reported on 64 of the patients in the prospective cohort.
Median time to flare: 219 days
Of findings so far, disease recurrence after discontinuation has been generally characterized by flares “of moderate activity” several months to more than a year after treatment discontinuation, according to Dr. Ringold, who emphasized repeatedly that these data are preliminary. The median time to a flare after treatment discontinuation was approximately 7 months (219 days).
In the combined cohorts, the median age at onset of JIA was 4 years. The median age at time of discontinuation was 9 years. More than half (55%) were taking a conventional disease-modifying antirheumatic drug (DMARD) and 35% were taking a tumor necrosis factor inhibitor at the time that their therapy was discontinued.
Most JIA types are represented. The most common form is rheumatoid factor–negative oligoarticular JIA. The main outcome looked the rate of clinically inactive disease at 6 months in children who had discontinued therapy after a period of remission. They defined clinically inactive disease as a Physician’s Global Assessment of less than 1 and an active joint count of 0.
Systemic JIA recapture rate at 6 months: 76%
At the time of disease flare after treatment discontinuation across both the retrospective and prospective cohorts, the median clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10; score range of 0-30) was 3.5. The recapture rate to clinically inactive disease at 6 months was 76% in those with systemic JIA and 50% in those with rheumatoid factor–positive polyarticular JIA. Other subtypes fell within this range. Rates of inactive disease at 6 months according to cJADAS10 score were lower, ranging from 26% with enthesitis-related arthritis/juvenile psoriatic arthritis to 57% with systemic JIA.
About 40% of those who restarted on therapy after a flare took the same medication again. About one-third of patients were restarted on glucocorticoids, mostly involving injections to inflamed joints, and data are not yet in about whether these were restarted alone or with other drugs, according to Dr. Ringold.
The final analysis of this study will explore clinical and laboratory variables associated with disease recapture. In the prospective cohort, which did not reach its planned enrollment of 150 children because the COVID pandemic, a broad array of these variables was evaluated at baseline.
Numerous studies have already looked at predictors of sustained remission after stopping medications of JIA, according to Dr. Ringold, but she said that there is relatively little information about outcomes in children who stop medications, flare, and are retreated. Other experts agree.
“We know little about how successfully DMARDs can be discontinued and used again after a disease flare,” reported Jens Klotsche, MD, a researcher at the German Rheumatism Research Center, which is part of the Leibniz Institute in Berlin. Dr. Klotsche, who is an author of a recent study that found etanercept effective for retreatment when children with JIA had discontinued therapy, agreed that “data from large cohort studies are necessary to support the treatment decisions by clinicians, parents, and patients.”
JIA recurrence risk is unclear
In a systematic review published 2 years ago, rates of flare following discontinuation of treatment for JIA were relatively high, but there were some limitations to this analysis, according to the lead author, Olha Halyabar, MD, a pediatric rheumatologist at Boston Children’s Hospital.
“The data in our systematic review showed that overall quality of evidence was low, with large variations and sometimes very different conclusions,” Dr. Halyabar said in an interview. She believes that the data generated by the CARRA analysis will be valuable, particularly in evaluating outcomes across subtypes.
“Even though, at this point, [previously published] reports indicate overall high rates of recurrence (>50% for some JIA subtypes), there are some encouraging studies from early treat-to-target strategies,” she said, adding that large datasets like those from CARRA offer an opportunity to gather data likely to be clinically useful.
Dr. Ringold cautioned that there are some limitations to the CARRA analysis, including some missing data from the retrospective cohort. She also pointed out that patients have been assessed at routine clinical visits rather than at standardized intervals, introducing a potential for bias.
For parents concerned about the costs, inconvenience, and side effects from sustained JIA treatment once remission is achieved, data from CARRA will allow clinicians to provide evidence-based counseling on balancing the risks of discontinuing therapy, including the likelihood of regaining remission when disease returns, against the goals of stopping treatment.
“Parents are having more conversations about when to stop medications,” Dr. Ringold said. She indicated that these data should be helpful for providing guidance.
Dr. Ringold, Dr. Klotsche, and Dr. Halyabar reported having no potential conflicts of interest.
FROM RHEUMATOLOGY RESEARCH FOUNDATION SUMMER SERIES
Traumatic Fractures Should Trigger Osteoporosis Assessment in Postmenopausal Women
Study Overview
Objective. To compare the risk of subsequent fractures after an initial traumatic or nontraumatic fracture in postmenopausal women.
Design. A prospective observational study utilizing data from the Women’s Health Initiative (WHI) Study, WHI Clinical Trials (WHI-CT), and WHI Bone Density Substudy to evaluate rates at which patients who suffered a traumatic fracture vs nontraumatic fracture develop a subsequent fracture.
Setting and participants. The WHI study, implemented at 40 United States clinical sites, enrolled 161 808 postmenopausal women aged 50 to 79 years at baseline between 1993 and 1998. The study cohort consisted of 75 335 patients who had self-reported fractures from September 1994 to December 1998 that were confirmed by the WHI Bone Density Substudy and WHI-CT. Of these participants, 253 (0.3%) were excluded because of a lack of follow-up information regarding incident fractures, and 8208 (10.9%) were excluded due to incomplete information on covariates, thus resulting in an analytic sample of 66 874 (88.8%) participants. Prospective fracture ascertainment with participants was conducted at least annually and the mechanism of fracture was assessed to differentiate traumatic vs nontraumatic incident fractures. Traumatic fractures were defined as fractures caused by motor vehicle collisions, falls from a height, falls downstairs, or sports injury. Nontraumatic fractures were defined as fractures caused by a trip and fall.
Main outcome measures. The primary outcome was an incident fracture at an anatomically distinct body part. Fractures were classified as upper extremity (carpal, elbow, lower or upper end of humerus, shaft of humerus, upper radius/ulna, or radius/ulna), lower extremity (ankle, hip, patella, pelvis, shaft of femur, tibia/fibula, or tibial plateau), or spine (lumbar and/or thoracic spine). Self-reported fractures were verified via medical chart review by WHI study physicians; hip fractures were confirmed by review of written reports of radiographic studies; and nonhip fractures were confirmed by review of radiography reports or clinical documentations.
Main results. In total, 66 874 women in the study (mean [SD] age) 63.1 (7.0) years without clinical fracture and 65.3 (7.2) years with clinical fracture at baseline were followed for 8.1 (1.6) years. Of these participants, 7142 (10.7%) experienced incident fracture during the study follow-up period (13.9 per 1000 person-years), and 721 (10.1%) of whom had a subsequent fracture. The adjusted hazard ratio (aHR) of subsequent fracture after an initial fracture was 1.49 (95% CI, 1.38-1.61, P < .001). Covariates adjusted were age, race, ethnicity, body mass index, treated diabetes, frequency of falls in the previous year, and physical function and activity. In women with initial traumatic fracture, the association between initial and subsequent fracture was increased (aHR, 1.25; 95% CI, 1.06-1.48, P = .01). Among women with initial nontraumatic fracture, the association between initial and subsequent fracture was also increased (aHR, 1.52; 95% CI, 1.37-1.68, P < .001). The confidence intervals for the 2 preceding associations for traumatic and nontraumatic initial fracture strata were overlapping.
Conclusion. Fractures, regardless of mechanism of injury, are similarly associated with an increased risk of subsequent fractures in postmenopausal women aged 50 years and older. Findings from this study provide evidence to support reevaluation of current clinical guidelines to include traumatic fracture as a trigger for osteoporosis screening.
Commentary
Osteoporosis is one of the most common age-associated disease that affects 1 in 4 women and 1 in 20 men over the age of 65.1 It increases the risk of fracture, and its clinical sequelae include reduced mobility, health decline, and increased all-cause mortality. The high prevalence of osteoporosis poses a clinical challenge as the global population continues to age. Pharmacological treatments such as bisphosphonates are highly effective in preventing or slowing bone mineral density (BMD) loss and reducing risk of fragility fractures (eg, nontraumatic fractures of the vertebra, hip, and femur) and are commonly used to mitigate adverse effects of degenerative bone changes secondary to osteoporosis.1
The high prevalence of osteoporosis and effectiveness of bisphosphonates raises the question of how to optimally identify adults at risk for osteoporosis so that pharmacologic therapy can be promptly initiated to prevent disease progression. Multiple osteoporosis screening guidelines, including those from the United States Preventive Services Task Force (USPSTF), American Association of Family Physicians, and National Osteoporosis Foundation, are widely used in the clinical setting to address this important clinical question. In general, the prevailing wisdom is to screen osteoporosis in postmenopausal women over the age of 65, women under the age of 65 who have a significant 10-year fracture risk, or women over the age of 50 who have experienced a fragility fracture.1 In the study reported by Crandall et al, it was shown that the risks of having subsequent fractures were similar after an initial traumatic or nontraumatic (fragility) fracture in postmenopausal women aged 50 years and older.2 This finding brings into question whether traumatic fractures should be viewed any differently than nontraumatic fractures in women over the age of 50 in light of evaluation for osteoporosis. Furthermore, these results suggest that most fractures in postmenopausal women may indicate decreased bone integrity, thus adding to the rationale that osteoporosis screening needs to be considered and expanded to include postmenopausal women under the age of 65 who endured a traumatic fracture.
Per current guidelines, a woman under the age of 65 is recommended for osteoporosis screening only if she has an increased 10-year fracture risk compared to women aged 65 years and older. This risk is calculated based on the World Health Organization fracture-risk algorithm (WHO FRAX) tool which uses multiple factors such as age, weight, and history of fragility fractures to predict whether an individual is at risk of developing a fracture in the next 10 years. The WHO FRAX tool does not include traumatic fractures in its risk calculation and current clinical guidelines do not account for traumatic fractures as a red flag to initiate osteoporosis screening. Therefore, postmenopausal women under the age of 65 are less likely to be screened for osteoporosis when they experience a traumatic fracture compared to a fragility fracture, despite being at a demonstrably higher risk for subsequent fracture. As an unintended consequence, this may lead to the under diagnosis of osteoporosis in postmenopausal women under the age of 65. Thus, Crandall et al conclude that a fracture due to any cause warrants follow up evaluation for osteoporosis including BMD testing in women older than 50 years of age.
Older men constitute another population who are commonly under screened for osteoporosis. The current USPSTF guidelines indicate that there is an insufficient body of evidence to screen men for osteoporosis given its lower prevalence.1 However, it is important to note that men have significantly increased mortality after a hip fracture, are less likely to be on pharmacological treatment for osteoporosis, and are under diagnosed for osteoporosis.3 Consistent with findings from the current study, Leslie et al showed that high-trauma and low-trauma fractures have similarly elevated subsequent fracture risk in both men and women over the age of 40 in a Canadian study.4 Moreover, in the same study, BMD was decreased in both men and women who suffered a fracture regardless of the injury mechanism. This finding further underscores a need to consider traumatic fractures as a risk factor for osteoporosis. Taken together, given that men are under screened and treated for osteoporosis but have increased mortality post-fracture, considerations to initiate osteoporosis evaluation should be similarly given to men who endured a traumatic fracture.
The study conducted by Crandall et al has several strengths. It is noteworthy for the large size of the WHI cohort with participants from across the United States which enables the capture of a wider range of age groups as women under the age of 65 are not common participants of osteoporosis studies. Additionally, data ascertainment and outcome adjudication utilizing medical records and physician review assure data quality. A limitation of the study is that the study cohort consists exclusively of women and therefore the findings are not generalizable to men. However, findings from this study echo those from other studies that investigate the relationship between fracture mechanisms and subsequent fracture risk in men and women.3,4 Collectively, these comparable findings highlight the need for additional research to validate traumatic fracture as a risk factor for osteoporosis and to incorporate it into clinical guidelines for osteoporosis screening.
Applications for Clinical Practice
The findings from the current study indicate that traumatic and fragility fractures may be more alike than previously recognized in regards to bone health and subsequent fracture prevention in postmenopausal women. If validated, these results may lead to changes in clinical practice whereby all fractures in postmenopausal women could trigger osteoporosis screening, assessment, and treatment if indicated for the secondary prevention of fractures.
1. US Preventive Services Task Force, Curry SJ, Krist Ah, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–2531. doi:10.1001/jama.2018.7498
2. Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern Med. Published online June 7, 2021. doi:10.1001/jamainternmed.2021.2617
3. Mackey DC, Lui L, Cawthon PM, et al. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA. 2007;298(20):2381–2388. doi:10.1001/jama.298.20.2381
4. Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31(6):1059–1067. doi:10.1007/s00198-019-05274-2
Study Overview
Objective. To compare the risk of subsequent fractures after an initial traumatic or nontraumatic fracture in postmenopausal women.
Design. A prospective observational study utilizing data from the Women’s Health Initiative (WHI) Study, WHI Clinical Trials (WHI-CT), and WHI Bone Density Substudy to evaluate rates at which patients who suffered a traumatic fracture vs nontraumatic fracture develop a subsequent fracture.
Setting and participants. The WHI study, implemented at 40 United States clinical sites, enrolled 161 808 postmenopausal women aged 50 to 79 years at baseline between 1993 and 1998. The study cohort consisted of 75 335 patients who had self-reported fractures from September 1994 to December 1998 that were confirmed by the WHI Bone Density Substudy and WHI-CT. Of these participants, 253 (0.3%) were excluded because of a lack of follow-up information regarding incident fractures, and 8208 (10.9%) were excluded due to incomplete information on covariates, thus resulting in an analytic sample of 66 874 (88.8%) participants. Prospective fracture ascertainment with participants was conducted at least annually and the mechanism of fracture was assessed to differentiate traumatic vs nontraumatic incident fractures. Traumatic fractures were defined as fractures caused by motor vehicle collisions, falls from a height, falls downstairs, or sports injury. Nontraumatic fractures were defined as fractures caused by a trip and fall.
Main outcome measures. The primary outcome was an incident fracture at an anatomically distinct body part. Fractures were classified as upper extremity (carpal, elbow, lower or upper end of humerus, shaft of humerus, upper radius/ulna, or radius/ulna), lower extremity (ankle, hip, patella, pelvis, shaft of femur, tibia/fibula, or tibial plateau), or spine (lumbar and/or thoracic spine). Self-reported fractures were verified via medical chart review by WHI study physicians; hip fractures were confirmed by review of written reports of radiographic studies; and nonhip fractures were confirmed by review of radiography reports or clinical documentations.
Main results. In total, 66 874 women in the study (mean [SD] age) 63.1 (7.0) years without clinical fracture and 65.3 (7.2) years with clinical fracture at baseline were followed for 8.1 (1.6) years. Of these participants, 7142 (10.7%) experienced incident fracture during the study follow-up period (13.9 per 1000 person-years), and 721 (10.1%) of whom had a subsequent fracture. The adjusted hazard ratio (aHR) of subsequent fracture after an initial fracture was 1.49 (95% CI, 1.38-1.61, P < .001). Covariates adjusted were age, race, ethnicity, body mass index, treated diabetes, frequency of falls in the previous year, and physical function and activity. In women with initial traumatic fracture, the association between initial and subsequent fracture was increased (aHR, 1.25; 95% CI, 1.06-1.48, P = .01). Among women with initial nontraumatic fracture, the association between initial and subsequent fracture was also increased (aHR, 1.52; 95% CI, 1.37-1.68, P < .001). The confidence intervals for the 2 preceding associations for traumatic and nontraumatic initial fracture strata were overlapping.
Conclusion. Fractures, regardless of mechanism of injury, are similarly associated with an increased risk of subsequent fractures in postmenopausal women aged 50 years and older. Findings from this study provide evidence to support reevaluation of current clinical guidelines to include traumatic fracture as a trigger for osteoporosis screening.
Commentary
Osteoporosis is one of the most common age-associated disease that affects 1 in 4 women and 1 in 20 men over the age of 65.1 It increases the risk of fracture, and its clinical sequelae include reduced mobility, health decline, and increased all-cause mortality. The high prevalence of osteoporosis poses a clinical challenge as the global population continues to age. Pharmacological treatments such as bisphosphonates are highly effective in preventing or slowing bone mineral density (BMD) loss and reducing risk of fragility fractures (eg, nontraumatic fractures of the vertebra, hip, and femur) and are commonly used to mitigate adverse effects of degenerative bone changes secondary to osteoporosis.1
The high prevalence of osteoporosis and effectiveness of bisphosphonates raises the question of how to optimally identify adults at risk for osteoporosis so that pharmacologic therapy can be promptly initiated to prevent disease progression. Multiple osteoporosis screening guidelines, including those from the United States Preventive Services Task Force (USPSTF), American Association of Family Physicians, and National Osteoporosis Foundation, are widely used in the clinical setting to address this important clinical question. In general, the prevailing wisdom is to screen osteoporosis in postmenopausal women over the age of 65, women under the age of 65 who have a significant 10-year fracture risk, or women over the age of 50 who have experienced a fragility fracture.1 In the study reported by Crandall et al, it was shown that the risks of having subsequent fractures were similar after an initial traumatic or nontraumatic (fragility) fracture in postmenopausal women aged 50 years and older.2 This finding brings into question whether traumatic fractures should be viewed any differently than nontraumatic fractures in women over the age of 50 in light of evaluation for osteoporosis. Furthermore, these results suggest that most fractures in postmenopausal women may indicate decreased bone integrity, thus adding to the rationale that osteoporosis screening needs to be considered and expanded to include postmenopausal women under the age of 65 who endured a traumatic fracture.
Per current guidelines, a woman under the age of 65 is recommended for osteoporosis screening only if she has an increased 10-year fracture risk compared to women aged 65 years and older. This risk is calculated based on the World Health Organization fracture-risk algorithm (WHO FRAX) tool which uses multiple factors such as age, weight, and history of fragility fractures to predict whether an individual is at risk of developing a fracture in the next 10 years. The WHO FRAX tool does not include traumatic fractures in its risk calculation and current clinical guidelines do not account for traumatic fractures as a red flag to initiate osteoporosis screening. Therefore, postmenopausal women under the age of 65 are less likely to be screened for osteoporosis when they experience a traumatic fracture compared to a fragility fracture, despite being at a demonstrably higher risk for subsequent fracture. As an unintended consequence, this may lead to the under diagnosis of osteoporosis in postmenopausal women under the age of 65. Thus, Crandall et al conclude that a fracture due to any cause warrants follow up evaluation for osteoporosis including BMD testing in women older than 50 years of age.
Older men constitute another population who are commonly under screened for osteoporosis. The current USPSTF guidelines indicate that there is an insufficient body of evidence to screen men for osteoporosis given its lower prevalence.1 However, it is important to note that men have significantly increased mortality after a hip fracture, are less likely to be on pharmacological treatment for osteoporosis, and are under diagnosed for osteoporosis.3 Consistent with findings from the current study, Leslie et al showed that high-trauma and low-trauma fractures have similarly elevated subsequent fracture risk in both men and women over the age of 40 in a Canadian study.4 Moreover, in the same study, BMD was decreased in both men and women who suffered a fracture regardless of the injury mechanism. This finding further underscores a need to consider traumatic fractures as a risk factor for osteoporosis. Taken together, given that men are under screened and treated for osteoporosis but have increased mortality post-fracture, considerations to initiate osteoporosis evaluation should be similarly given to men who endured a traumatic fracture.
The study conducted by Crandall et al has several strengths. It is noteworthy for the large size of the WHI cohort with participants from across the United States which enables the capture of a wider range of age groups as women under the age of 65 are not common participants of osteoporosis studies. Additionally, data ascertainment and outcome adjudication utilizing medical records and physician review assure data quality. A limitation of the study is that the study cohort consists exclusively of women and therefore the findings are not generalizable to men. However, findings from this study echo those from other studies that investigate the relationship between fracture mechanisms and subsequent fracture risk in men and women.3,4 Collectively, these comparable findings highlight the need for additional research to validate traumatic fracture as a risk factor for osteoporosis and to incorporate it into clinical guidelines for osteoporosis screening.
Applications for Clinical Practice
The findings from the current study indicate that traumatic and fragility fractures may be more alike than previously recognized in regards to bone health and subsequent fracture prevention in postmenopausal women. If validated, these results may lead to changes in clinical practice whereby all fractures in postmenopausal women could trigger osteoporosis screening, assessment, and treatment if indicated for the secondary prevention of fractures.
Study Overview
Objective. To compare the risk of subsequent fractures after an initial traumatic or nontraumatic fracture in postmenopausal women.
Design. A prospective observational study utilizing data from the Women’s Health Initiative (WHI) Study, WHI Clinical Trials (WHI-CT), and WHI Bone Density Substudy to evaluate rates at which patients who suffered a traumatic fracture vs nontraumatic fracture develop a subsequent fracture.
Setting and participants. The WHI study, implemented at 40 United States clinical sites, enrolled 161 808 postmenopausal women aged 50 to 79 years at baseline between 1993 and 1998. The study cohort consisted of 75 335 patients who had self-reported fractures from September 1994 to December 1998 that were confirmed by the WHI Bone Density Substudy and WHI-CT. Of these participants, 253 (0.3%) were excluded because of a lack of follow-up information regarding incident fractures, and 8208 (10.9%) were excluded due to incomplete information on covariates, thus resulting in an analytic sample of 66 874 (88.8%) participants. Prospective fracture ascertainment with participants was conducted at least annually and the mechanism of fracture was assessed to differentiate traumatic vs nontraumatic incident fractures. Traumatic fractures were defined as fractures caused by motor vehicle collisions, falls from a height, falls downstairs, or sports injury. Nontraumatic fractures were defined as fractures caused by a trip and fall.
Main outcome measures. The primary outcome was an incident fracture at an anatomically distinct body part. Fractures were classified as upper extremity (carpal, elbow, lower or upper end of humerus, shaft of humerus, upper radius/ulna, or radius/ulna), lower extremity (ankle, hip, patella, pelvis, shaft of femur, tibia/fibula, or tibial plateau), or spine (lumbar and/or thoracic spine). Self-reported fractures were verified via medical chart review by WHI study physicians; hip fractures were confirmed by review of written reports of radiographic studies; and nonhip fractures were confirmed by review of radiography reports or clinical documentations.
Main results. In total, 66 874 women in the study (mean [SD] age) 63.1 (7.0) years without clinical fracture and 65.3 (7.2) years with clinical fracture at baseline were followed for 8.1 (1.6) years. Of these participants, 7142 (10.7%) experienced incident fracture during the study follow-up period (13.9 per 1000 person-years), and 721 (10.1%) of whom had a subsequent fracture. The adjusted hazard ratio (aHR) of subsequent fracture after an initial fracture was 1.49 (95% CI, 1.38-1.61, P < .001). Covariates adjusted were age, race, ethnicity, body mass index, treated diabetes, frequency of falls in the previous year, and physical function and activity. In women with initial traumatic fracture, the association between initial and subsequent fracture was increased (aHR, 1.25; 95% CI, 1.06-1.48, P = .01). Among women with initial nontraumatic fracture, the association between initial and subsequent fracture was also increased (aHR, 1.52; 95% CI, 1.37-1.68, P < .001). The confidence intervals for the 2 preceding associations for traumatic and nontraumatic initial fracture strata were overlapping.
Conclusion. Fractures, regardless of mechanism of injury, are similarly associated with an increased risk of subsequent fractures in postmenopausal women aged 50 years and older. Findings from this study provide evidence to support reevaluation of current clinical guidelines to include traumatic fracture as a trigger for osteoporosis screening.
Commentary
Osteoporosis is one of the most common age-associated disease that affects 1 in 4 women and 1 in 20 men over the age of 65.1 It increases the risk of fracture, and its clinical sequelae include reduced mobility, health decline, and increased all-cause mortality. The high prevalence of osteoporosis poses a clinical challenge as the global population continues to age. Pharmacological treatments such as bisphosphonates are highly effective in preventing or slowing bone mineral density (BMD) loss and reducing risk of fragility fractures (eg, nontraumatic fractures of the vertebra, hip, and femur) and are commonly used to mitigate adverse effects of degenerative bone changes secondary to osteoporosis.1
The high prevalence of osteoporosis and effectiveness of bisphosphonates raises the question of how to optimally identify adults at risk for osteoporosis so that pharmacologic therapy can be promptly initiated to prevent disease progression. Multiple osteoporosis screening guidelines, including those from the United States Preventive Services Task Force (USPSTF), American Association of Family Physicians, and National Osteoporosis Foundation, are widely used in the clinical setting to address this important clinical question. In general, the prevailing wisdom is to screen osteoporosis in postmenopausal women over the age of 65, women under the age of 65 who have a significant 10-year fracture risk, or women over the age of 50 who have experienced a fragility fracture.1 In the study reported by Crandall et al, it was shown that the risks of having subsequent fractures were similar after an initial traumatic or nontraumatic (fragility) fracture in postmenopausal women aged 50 years and older.2 This finding brings into question whether traumatic fractures should be viewed any differently than nontraumatic fractures in women over the age of 50 in light of evaluation for osteoporosis. Furthermore, these results suggest that most fractures in postmenopausal women may indicate decreased bone integrity, thus adding to the rationale that osteoporosis screening needs to be considered and expanded to include postmenopausal women under the age of 65 who endured a traumatic fracture.
Per current guidelines, a woman under the age of 65 is recommended for osteoporosis screening only if she has an increased 10-year fracture risk compared to women aged 65 years and older. This risk is calculated based on the World Health Organization fracture-risk algorithm (WHO FRAX) tool which uses multiple factors such as age, weight, and history of fragility fractures to predict whether an individual is at risk of developing a fracture in the next 10 years. The WHO FRAX tool does not include traumatic fractures in its risk calculation and current clinical guidelines do not account for traumatic fractures as a red flag to initiate osteoporosis screening. Therefore, postmenopausal women under the age of 65 are less likely to be screened for osteoporosis when they experience a traumatic fracture compared to a fragility fracture, despite being at a demonstrably higher risk for subsequent fracture. As an unintended consequence, this may lead to the under diagnosis of osteoporosis in postmenopausal women under the age of 65. Thus, Crandall et al conclude that a fracture due to any cause warrants follow up evaluation for osteoporosis including BMD testing in women older than 50 years of age.
Older men constitute another population who are commonly under screened for osteoporosis. The current USPSTF guidelines indicate that there is an insufficient body of evidence to screen men for osteoporosis given its lower prevalence.1 However, it is important to note that men have significantly increased mortality after a hip fracture, are less likely to be on pharmacological treatment for osteoporosis, and are under diagnosed for osteoporosis.3 Consistent with findings from the current study, Leslie et al showed that high-trauma and low-trauma fractures have similarly elevated subsequent fracture risk in both men and women over the age of 40 in a Canadian study.4 Moreover, in the same study, BMD was decreased in both men and women who suffered a fracture regardless of the injury mechanism. This finding further underscores a need to consider traumatic fractures as a risk factor for osteoporosis. Taken together, given that men are under screened and treated for osteoporosis but have increased mortality post-fracture, considerations to initiate osteoporosis evaluation should be similarly given to men who endured a traumatic fracture.
The study conducted by Crandall et al has several strengths. It is noteworthy for the large size of the WHI cohort with participants from across the United States which enables the capture of a wider range of age groups as women under the age of 65 are not common participants of osteoporosis studies. Additionally, data ascertainment and outcome adjudication utilizing medical records and physician review assure data quality. A limitation of the study is that the study cohort consists exclusively of women and therefore the findings are not generalizable to men. However, findings from this study echo those from other studies that investigate the relationship between fracture mechanisms and subsequent fracture risk in men and women.3,4 Collectively, these comparable findings highlight the need for additional research to validate traumatic fracture as a risk factor for osteoporosis and to incorporate it into clinical guidelines for osteoporosis screening.
Applications for Clinical Practice
The findings from the current study indicate that traumatic and fragility fractures may be more alike than previously recognized in regards to bone health and subsequent fracture prevention in postmenopausal women. If validated, these results may lead to changes in clinical practice whereby all fractures in postmenopausal women could trigger osteoporosis screening, assessment, and treatment if indicated for the secondary prevention of fractures.
1. US Preventive Services Task Force, Curry SJ, Krist Ah, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–2531. doi:10.1001/jama.2018.7498
2. Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern Med. Published online June 7, 2021. doi:10.1001/jamainternmed.2021.2617
3. Mackey DC, Lui L, Cawthon PM, et al. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA. 2007;298(20):2381–2388. doi:10.1001/jama.298.20.2381
4. Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31(6):1059–1067. doi:10.1007/s00198-019-05274-2
1. US Preventive Services Task Force, Curry SJ, Krist Ah, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–2531. doi:10.1001/jama.2018.7498
2. Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern Med. Published online June 7, 2021. doi:10.1001/jamainternmed.2021.2617
3. Mackey DC, Lui L, Cawthon PM, et al. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA. 2007;298(20):2381–2388. doi:10.1001/jama.298.20.2381
4. Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31(6):1059–1067. doi:10.1007/s00198-019-05274-2
Managing work disability to help patients return to the job
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).
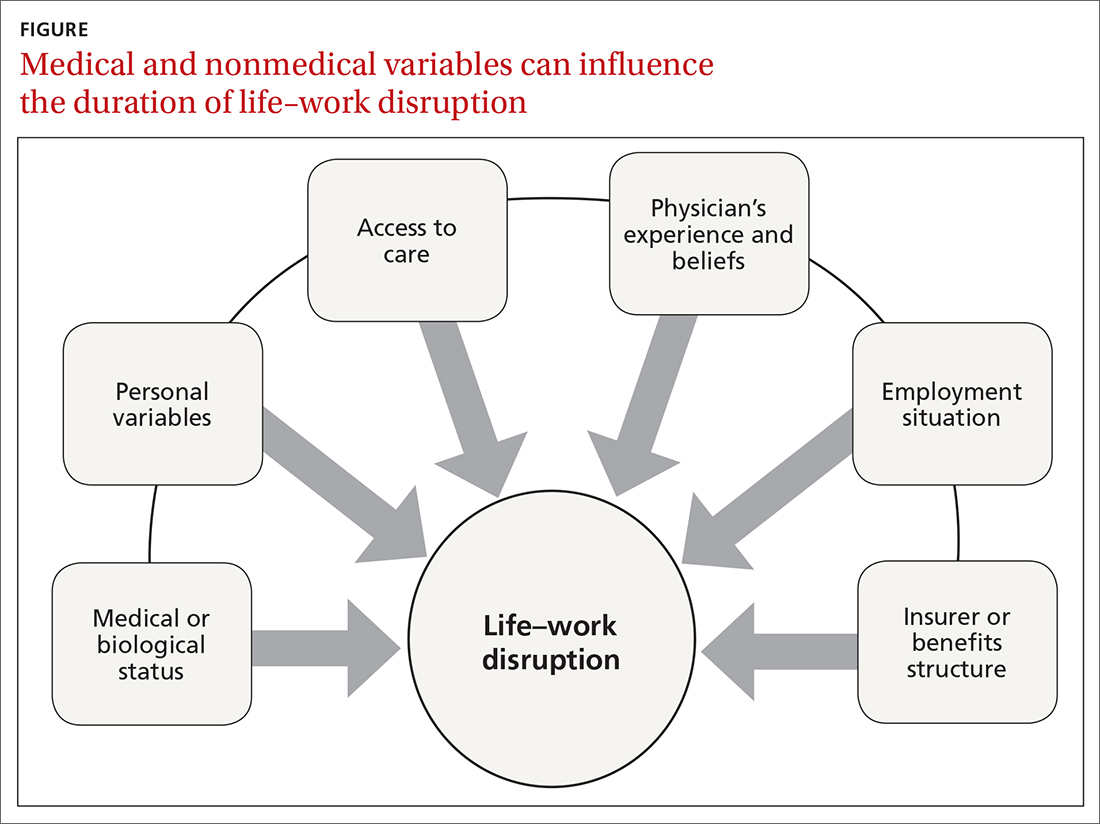
Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.
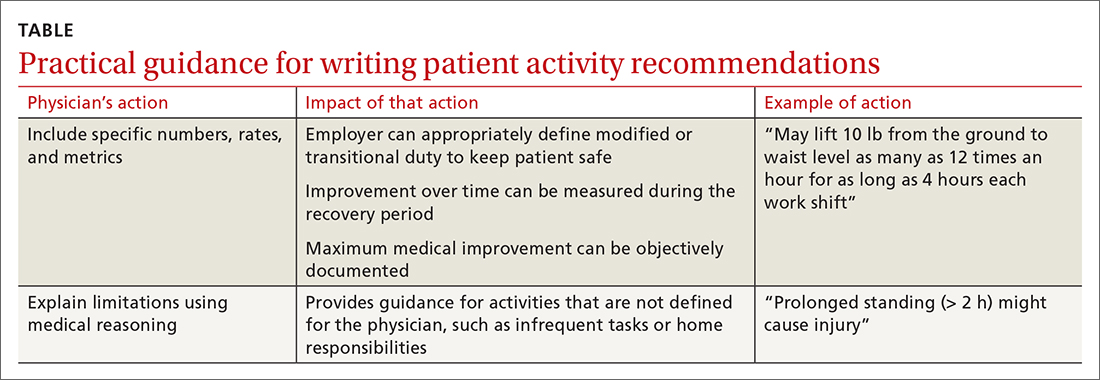
Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).

Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.

Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).

Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.

Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
PRACTICE RECOMMENDATIONS
› Set appropriate expectations for the patient at the start of any episode of work disability: Estimate the course of functional recovery over time and the total duration of life–work disruption. A
› Include detailed activity prescriptions in the treatment plan, with stepwise progression over time toward full recovery. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series