User login
Hearing loss tied to decline in physical functioning
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
AAP updates guidance for return to sports and physical activities
As pandemic restrictions ease and young athletes once again take to fields, courts, tracks, and rinks, doctors are sharing ways to help them get back to sports safely.
That means taking steps to prevent COVID-19.
It also means trying to avoid sports-related injuries, which may be more likely if young athletes didn’t move around so much during the pandemic.
For adolescents who are eligible, getting a COVID-19 vaccine may be the most important thing they can do, according to the American Academy of Pediatrics.
“The AAP encourages all people who are eligible to receive the COVID-19 vaccine as soon as it is available,” the organization wrote in updated guidance on returning to sports and physical activity.
“I don’t think it can be overemphasized how important these vaccines are, both for the individual and at the community level,” says Aaron L. Baggish, MD, an associate professor of medicine at Harvard Medical School, Boston, and director of the Cardiovascular Performance Program at Massachusetts General Hospital in Boston.
Dr. Baggish, team cardiologist for the New England Patriots, the Boston Bruins, the New England Revolution, U.S. Men’s and Women’s Soccer, and U.S. Rowing, as well as medical director for the Boston Marathon, has studied the effects of COVID-19 on the heart in college athletes and written return-to-play recommendations for athletes of high school age and older.
“Millions of people have received these vaccines from age 12 up,” Dr. Baggish says. “The efficacy continues to look very durable and near complete, and the risk associated with vaccination is incredibly low, to the point where the risk-benefit ratio across the age spectrum, whether you’re athletic or not, strongly favors getting vaccinated. There is really no reason to hold off at this point.”
While outdoor activities are lower-risk for spreading COVID-19 and many people have been vaccinated, masks still should be worn in certain settings, the AAP notes.
“Indoor spaces that are crowded are still high-risk for COVID-19 transmission. And we recognize that not everyone in these settings may be vaccinated,” says Susannah Briskin, MD, lead author of the AAP guidance.
“So for indoor sporting events with spectators, in locker rooms or other small spaces such as a training room, and during shared car rides or school transportation to and from events, individuals should continue to mask,” adds Dr. Briskin, a pediatrician in the Division of Sports Medicine and fellowship director for the Primary Care Sports Medicine program at University Hospitals Rainbow Babies & Children’s Hospital.
For outdoor sports, athletes who are not fully vaccinated should be encouraged to wear masks on the sidelines and during group training and competition when they are within 3 feet of others for sustained amounts of time, according to the AAP.
Get back into exercise gradually
In general, athletes who have not been active for more than a month should resume exercise gradually, Dr. Briskin says. Starting at 25% of normal volume and increasing slowly over time – with 10% increases each week – is one rule of thumb.
“Those who have taken a prolonged break from sports are at a higher risk of injury when they return,” she notes. “Families should also be aware of an increased risk for heat-related illness if they are not acclimated.”
Caitlyn Mooney, MD, a team doctor for the University of Texas, San Antonio, has heard reports of doctors seeing more injuries like stress fractures. Some cases may relate to people going from “months of doing nothing to all of a sudden going back to sports,” says Dr. Mooney, who is also a clinical assistant professor of pediatrics and orthopedics at UT Health San Antonio.
“The coaches, the parents, and the athletes themselves really need to keep in mind that it’s not like a regular season,” Dr. Mooney says. She suggests gradually ramping up activity and paying attention to any pain. “That’s a good indicator that maybe you’re going too fast,” she adds.
Athletes should be mindful of other symptoms too when restarting exercise, especially after illness.
It is “very important that any athlete with recent COVID-19 monitor for new symptoms when they return to exercise,” says Jonathan Drezner, MD, a professor of family medicine at the University of Washington, Seattle. “A little fatigue from detraining may be expected, but exertional chest pain deserves more evaluation.”
Dr. Drezner – editor-in-chief of the British Journal of Sports Medicine and team doctor for the Seattle Seahawks – along with Dr. Baggish and colleagues, found a low prevalence of cardiac involvement in a study of more than 3,000 college athletes with prior SARS-CoV-2 infection.
“Any athlete, despite their initial symptom course, who has cardiopulmonary symptoms on return to exercise, particularly chest pain, should see their physician for a comprehensive cardiac evaluation,” Dr. Drezner says. “Cardiac MRI should be reserved for athletes with abnormal testing or when clinical suspicion of myocardial involvement is high.”
If an athlete had COVID-19 with moderate symptoms (such as fever, chills, or a flu-like syndrome) or cardiopulmonary symptoms (such as chest pain or shortness of breath), cardiac testing should be considered, he notes.
These symptoms “were associated with a higher prevalence of cardiac involvement,” Dr. Drezner said in an email. “Testing may include an ECG, echocardiogram (ultrasound), and troponin (blood test).”
For kids who test positive for SARS-CoV-2 but do not have symptoms, or their symptoms last less than 4 days, a phone call or telemedicine visit with their doctor may be enough to clear them to play, says Dr. Briskin, who’s also an assistant professor of pediatrics at Case Western Reserve University, Cleveland.
“This will allow the physician an opportunity to screen for any concerning cardiac signs or symptoms, update the patient’s electronic medical record with the recent COVID-19 infection, and provide appropriate guidance back to exercise,” she adds.
Dr. Baggish, Dr. Briskin, Dr. Mooney, and Dr. Drezner had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
As pandemic restrictions ease and young athletes once again take to fields, courts, tracks, and rinks, doctors are sharing ways to help them get back to sports safely.
That means taking steps to prevent COVID-19.
It also means trying to avoid sports-related injuries, which may be more likely if young athletes didn’t move around so much during the pandemic.
For adolescents who are eligible, getting a COVID-19 vaccine may be the most important thing they can do, according to the American Academy of Pediatrics.
“The AAP encourages all people who are eligible to receive the COVID-19 vaccine as soon as it is available,” the organization wrote in updated guidance on returning to sports and physical activity.
“I don’t think it can be overemphasized how important these vaccines are, both for the individual and at the community level,” says Aaron L. Baggish, MD, an associate professor of medicine at Harvard Medical School, Boston, and director of the Cardiovascular Performance Program at Massachusetts General Hospital in Boston.
Dr. Baggish, team cardiologist for the New England Patriots, the Boston Bruins, the New England Revolution, U.S. Men’s and Women’s Soccer, and U.S. Rowing, as well as medical director for the Boston Marathon, has studied the effects of COVID-19 on the heart in college athletes and written return-to-play recommendations for athletes of high school age and older.
“Millions of people have received these vaccines from age 12 up,” Dr. Baggish says. “The efficacy continues to look very durable and near complete, and the risk associated with vaccination is incredibly low, to the point where the risk-benefit ratio across the age spectrum, whether you’re athletic or not, strongly favors getting vaccinated. There is really no reason to hold off at this point.”
While outdoor activities are lower-risk for spreading COVID-19 and many people have been vaccinated, masks still should be worn in certain settings, the AAP notes.
“Indoor spaces that are crowded are still high-risk for COVID-19 transmission. And we recognize that not everyone in these settings may be vaccinated,” says Susannah Briskin, MD, lead author of the AAP guidance.
“So for indoor sporting events with spectators, in locker rooms or other small spaces such as a training room, and during shared car rides or school transportation to and from events, individuals should continue to mask,” adds Dr. Briskin, a pediatrician in the Division of Sports Medicine and fellowship director for the Primary Care Sports Medicine program at University Hospitals Rainbow Babies & Children’s Hospital.
For outdoor sports, athletes who are not fully vaccinated should be encouraged to wear masks on the sidelines and during group training and competition when they are within 3 feet of others for sustained amounts of time, according to the AAP.
Get back into exercise gradually
In general, athletes who have not been active for more than a month should resume exercise gradually, Dr. Briskin says. Starting at 25% of normal volume and increasing slowly over time – with 10% increases each week – is one rule of thumb.
“Those who have taken a prolonged break from sports are at a higher risk of injury when they return,” she notes. “Families should also be aware of an increased risk for heat-related illness if they are not acclimated.”
Caitlyn Mooney, MD, a team doctor for the University of Texas, San Antonio, has heard reports of doctors seeing more injuries like stress fractures. Some cases may relate to people going from “months of doing nothing to all of a sudden going back to sports,” says Dr. Mooney, who is also a clinical assistant professor of pediatrics and orthopedics at UT Health San Antonio.
“The coaches, the parents, and the athletes themselves really need to keep in mind that it’s not like a regular season,” Dr. Mooney says. She suggests gradually ramping up activity and paying attention to any pain. “That’s a good indicator that maybe you’re going too fast,” she adds.
Athletes should be mindful of other symptoms too when restarting exercise, especially after illness.
It is “very important that any athlete with recent COVID-19 monitor for new symptoms when they return to exercise,” says Jonathan Drezner, MD, a professor of family medicine at the University of Washington, Seattle. “A little fatigue from detraining may be expected, but exertional chest pain deserves more evaluation.”
Dr. Drezner – editor-in-chief of the British Journal of Sports Medicine and team doctor for the Seattle Seahawks – along with Dr. Baggish and colleagues, found a low prevalence of cardiac involvement in a study of more than 3,000 college athletes with prior SARS-CoV-2 infection.
“Any athlete, despite their initial symptom course, who has cardiopulmonary symptoms on return to exercise, particularly chest pain, should see their physician for a comprehensive cardiac evaluation,” Dr. Drezner says. “Cardiac MRI should be reserved for athletes with abnormal testing or when clinical suspicion of myocardial involvement is high.”
If an athlete had COVID-19 with moderate symptoms (such as fever, chills, or a flu-like syndrome) or cardiopulmonary symptoms (such as chest pain or shortness of breath), cardiac testing should be considered, he notes.
These symptoms “were associated with a higher prevalence of cardiac involvement,” Dr. Drezner said in an email. “Testing may include an ECG, echocardiogram (ultrasound), and troponin (blood test).”
For kids who test positive for SARS-CoV-2 but do not have symptoms, or their symptoms last less than 4 days, a phone call or telemedicine visit with their doctor may be enough to clear them to play, says Dr. Briskin, who’s also an assistant professor of pediatrics at Case Western Reserve University, Cleveland.
“This will allow the physician an opportunity to screen for any concerning cardiac signs or symptoms, update the patient’s electronic medical record with the recent COVID-19 infection, and provide appropriate guidance back to exercise,” she adds.
Dr. Baggish, Dr. Briskin, Dr. Mooney, and Dr. Drezner had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
As pandemic restrictions ease and young athletes once again take to fields, courts, tracks, and rinks, doctors are sharing ways to help them get back to sports safely.
That means taking steps to prevent COVID-19.
It also means trying to avoid sports-related injuries, which may be more likely if young athletes didn’t move around so much during the pandemic.
For adolescents who are eligible, getting a COVID-19 vaccine may be the most important thing they can do, according to the American Academy of Pediatrics.
“The AAP encourages all people who are eligible to receive the COVID-19 vaccine as soon as it is available,” the organization wrote in updated guidance on returning to sports and physical activity.
“I don’t think it can be overemphasized how important these vaccines are, both for the individual and at the community level,” says Aaron L. Baggish, MD, an associate professor of medicine at Harvard Medical School, Boston, and director of the Cardiovascular Performance Program at Massachusetts General Hospital in Boston.
Dr. Baggish, team cardiologist for the New England Patriots, the Boston Bruins, the New England Revolution, U.S. Men’s and Women’s Soccer, and U.S. Rowing, as well as medical director for the Boston Marathon, has studied the effects of COVID-19 on the heart in college athletes and written return-to-play recommendations for athletes of high school age and older.
“Millions of people have received these vaccines from age 12 up,” Dr. Baggish says. “The efficacy continues to look very durable and near complete, and the risk associated with vaccination is incredibly low, to the point where the risk-benefit ratio across the age spectrum, whether you’re athletic or not, strongly favors getting vaccinated. There is really no reason to hold off at this point.”
While outdoor activities are lower-risk for spreading COVID-19 and many people have been vaccinated, masks still should be worn in certain settings, the AAP notes.
“Indoor spaces that are crowded are still high-risk for COVID-19 transmission. And we recognize that not everyone in these settings may be vaccinated,” says Susannah Briskin, MD, lead author of the AAP guidance.
“So for indoor sporting events with spectators, in locker rooms or other small spaces such as a training room, and during shared car rides or school transportation to and from events, individuals should continue to mask,” adds Dr. Briskin, a pediatrician in the Division of Sports Medicine and fellowship director for the Primary Care Sports Medicine program at University Hospitals Rainbow Babies & Children’s Hospital.
For outdoor sports, athletes who are not fully vaccinated should be encouraged to wear masks on the sidelines and during group training and competition when they are within 3 feet of others for sustained amounts of time, according to the AAP.
Get back into exercise gradually
In general, athletes who have not been active for more than a month should resume exercise gradually, Dr. Briskin says. Starting at 25% of normal volume and increasing slowly over time – with 10% increases each week – is one rule of thumb.
“Those who have taken a prolonged break from sports are at a higher risk of injury when they return,” she notes. “Families should also be aware of an increased risk for heat-related illness if they are not acclimated.”
Caitlyn Mooney, MD, a team doctor for the University of Texas, San Antonio, has heard reports of doctors seeing more injuries like stress fractures. Some cases may relate to people going from “months of doing nothing to all of a sudden going back to sports,” says Dr. Mooney, who is also a clinical assistant professor of pediatrics and orthopedics at UT Health San Antonio.
“The coaches, the parents, and the athletes themselves really need to keep in mind that it’s not like a regular season,” Dr. Mooney says. She suggests gradually ramping up activity and paying attention to any pain. “That’s a good indicator that maybe you’re going too fast,” she adds.
Athletes should be mindful of other symptoms too when restarting exercise, especially after illness.
It is “very important that any athlete with recent COVID-19 monitor for new symptoms when they return to exercise,” says Jonathan Drezner, MD, a professor of family medicine at the University of Washington, Seattle. “A little fatigue from detraining may be expected, but exertional chest pain deserves more evaluation.”
Dr. Drezner – editor-in-chief of the British Journal of Sports Medicine and team doctor for the Seattle Seahawks – along with Dr. Baggish and colleagues, found a low prevalence of cardiac involvement in a study of more than 3,000 college athletes with prior SARS-CoV-2 infection.
“Any athlete, despite their initial symptom course, who has cardiopulmonary symptoms on return to exercise, particularly chest pain, should see their physician for a comprehensive cardiac evaluation,” Dr. Drezner says. “Cardiac MRI should be reserved for athletes with abnormal testing or when clinical suspicion of myocardial involvement is high.”
If an athlete had COVID-19 with moderate symptoms (such as fever, chills, or a flu-like syndrome) or cardiopulmonary symptoms (such as chest pain or shortness of breath), cardiac testing should be considered, he notes.
These symptoms “were associated with a higher prevalence of cardiac involvement,” Dr. Drezner said in an email. “Testing may include an ECG, echocardiogram (ultrasound), and troponin (blood test).”
For kids who test positive for SARS-CoV-2 but do not have symptoms, or their symptoms last less than 4 days, a phone call or telemedicine visit with their doctor may be enough to clear them to play, says Dr. Briskin, who’s also an assistant professor of pediatrics at Case Western Reserve University, Cleveland.
“This will allow the physician an opportunity to screen for any concerning cardiac signs or symptoms, update the patient’s electronic medical record with the recent COVID-19 infection, and provide appropriate guidance back to exercise,” she adds.
Dr. Baggish, Dr. Briskin, Dr. Mooney, and Dr. Drezner had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
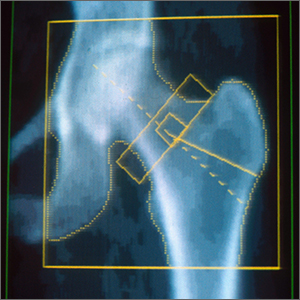
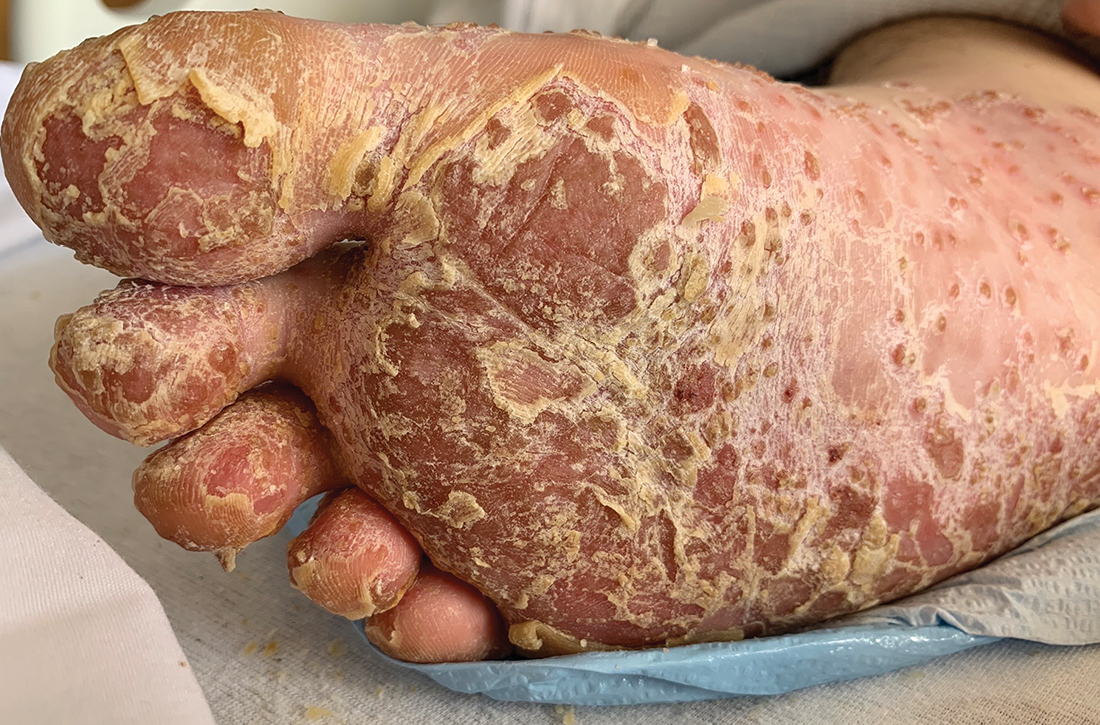
5-year-old boy • calf pain • fever • cough & rhinitis • Dx?
THE CASE
A 5-year-old previously healthy white boy presented to clinic with bilateral calf pain and refusal to bear weight since awakening that morning. Associated symptoms included a 3-day history of generalized fatigue, subjective fevers, cough, congestion, and rhinitis. The night prior to presentation, he showed no symptoms of gait abnormalities, muscle pain, or weakness. There was no history of similar symptoms, trauma, overexertion, foreign travel, or family history of musculoskeletal disease. He was fully immunized, except for the annual influenza vaccine. He was not taking any medications. This case occurred before the onset of the COVID-19 pandemic.
Objective findings included fever of 101 °F, refusal to bear weight, and symmetrical bilateral tenderness to palpation of the gastrocnemius-soleus complex. Pain was elicited with passive dorsiflexion. There was no erythema, edema, or sensory deficits, and the distal leg compartments were soft. There was normal range of motion of the hips, knees, and ankles. Dorsalis pedis pulses were 2+, and patella reflexes were 2/4 bilaterally.
Lab results included a white blood cell count of 2500/μL (normal range, 4500 to 11,000/μL);absolute neutrophil count, 900/μL (1500 to 8000/μL); platelet count, 131,000/μL (150,000 to 450,000/μL); creatine kinase level, 869 IU/L (22 to 198 U/L); and aspartate aminotransferase level, 116 U/L (8 to 33 U/L). A rapid influenza swab was positive for influenza B. Plain films of the bilateral hips and lower extremities were unremarkable. C-reactive protein (CRP) level, urinalysis, and renal function tests were within normal limits. Creatine kinase (CK) level peaked (1935 U/L; normal range, 22 to 198 U/L) within the first 24 hours of presentation and then trended down.
The Diagnosis
The patient’s sudden onset of symmetrical bilateral calf pain in the setting of an upper respiratory tract infection was extremely suspicious for benign acute childhood myositis (BACM). Lab work and radiologic evaluation were performed to rule out more ominous causes of refusal to bear weight. The suspicion of BACM was further validated by influenza B serology, an elevated CK, and a normal CRP.
Discussion
BACM was first described by Lundberg in 1957.1 The overall incidence and prevalence are unclear.2 A viral prodrome involving rhinorrhea, low-grade fever, sore throat, cough, and malaise typically precedes bilateral calf pain by 3 days.2-4 Myositis symptoms typically last for 4 days.3 While several infectious etiologies have been linked to this condition, influenza B has the greatest association.5,6
❚ Patient population. BACM occurs predominately in school-aged children (6-8 years old) and has a male-to-female ratio of 2:1.3,5,6 In a retrospective study of 219 children, BACM was strongly associated with male gender and ages 6 to 9 years.3 In another retrospective study of 54 children,80% of patients were male, and the mean age was 7.3 years.5
❚ Key symptoms and differential. The distinguishing feature of BACM is bilateral symmetric gastrocnemius-soleus tenderness.2,4 Additionally, the lack of neurologic symptoms is an important differentiator, as long as refusal to bear weight is not mistaken for weakness.6 These features help to distinguish BACM from other items in the differential, including trauma, Guillain-Barre syndrome, osteomyelitis, malignancy, deep vein thrombosis, and inherited musculoskeletal disorders.2
Continue to: Labratory evaluation...
❚ Laboratory evaluation will often show mild neutropenia, thrombocytopenia, and mild elevation in CK.7,8 CRP is typically normal.4,7,9 In a retrospective study of 28 admissions for BACM from 2001 to 2012, common findings included leukopenia (35%), neutropenia (25%), and thrombocytopenia (21%). The median CK value was 4181 U/L.4 In another analysis of BACM cases, mean CK was 1872 U/L.5
❚ Biopsy is unnecessary; however, calf muscle samples from 11 of 12 children with suspected BACM due to influenza B infection were consistent with patchy necrosis without significant myositis.10
❚ Complications. Rhabdomyolysis, although rare, has been reported with BACM. In 1 analysis, 10 of 316 patients with influenza-associated myositis developed rhabdomyolysis; 8 experienced renal failure. Rhabdomyolysis was 4 times more likely to occur in girls, and 86% of cases were associated with influenza A.6 Common manifestations of rhabdomyolysis associated with influenza include diffuse myopathy, gross hematuria, and myoglobinuria.6
❚ Treatment is mainly supportive.4,8,9 Antivirals typically are not indicated, as the bilateral calf pain manifests during the recovery phase of the illness.4,9,11 BACM is self-limited and should resolve within 3 days of myositis manifestation.2 Patients should follow up in 2 to 3 weeks to verify symptom resolution.2
If muscle pain, swelling, and tenderness worsen, further work-up is indicated. In more severe cases, including those involving renal failure, intensive care management and even dialysis may be necessary.4,6
❚ Our patient was hospitalized due to fever in the setting of neutropenia. Ultimately, he was treated with acetaminophen and intravenous fluids for mild dehydration and elevated CK levels. He was discharged home after 3 days, at which time he had complete resolution of pain and was able to resume normal activities.
The Takeaway
Benign acute childhood myositis is a self-limited disorder with an excellent prognosis. It has a typical presentation and therefore should be a clinical diagnosis; however, investigative studies may be warranted to rule out more ominous causes. Reassurance to family that the condition should self-resolve in a few days is important. Close follow-up should be scheduled to ensure resolution of symptoms.
CORRESPONDENCE
Nicholas A. Rathjen, DO, William Beaumont Army Medical Center, Department of Soldier and Family Care, 11335 SSG Sims Street, Fort Bliss, TX 79918; nicholas.a.rathjen@gmail. com
- Lundberg A. Myalgia cruris epidemica. Acta Paediatr. 1957;46:18-31. doi: 10.1111/j.1651-2227.1957.tb08627.x
- Magee H, Goldman RD. Viral myositis in children. Can Fam Physician. 2017;63:365-368.
- Mall S, Buchholz U, Tibussek D, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30:e142-e146. doi: 10.1097/INF.0b013e318217e356
- Santos JA, Albuquerque C, Lito D, et al. Benign acute childhood myositis: an alarming condition with an excellent prognosis! Am J Emerg Med. 2014;32:1418-1419. doi: 10.1016/j.ajem.2014.08.022
- Rosenberg T, Heitner S, Scolnik D, et al. Outcome of benign acute childhood myositis: the experience of 2 large tertiary care pediatric hospitals. Pediatr Emerg Care. 2018;34:400-402. doi: 10.1097/PEC.0000000000000830
- Agyeman P, Duppenthaler A, Heininger U, et al. Influenza-associated myositis in children. Infection. 2004;32:199-203. doi: 10.1007/s15010-004-4003-2
- Mackay MT, Kornberg AJ, Shield LK, et al. Benign acute childhood myositis: laboratory and clinical features. Neurology. 1999;53:2127-2131. doi: 10.1212/wnl.53.9.2127
- Neocleous C, Spanou C, Mpampalis E, et al. Unnecessary diagnostic investigations in benign acute childhood myositis: a case series report. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012023
- Felipe Cavagnaro SM, Alejandra Aird G, Ingrid Harwardt R, et al. Benign acute childhood myositis: clinical series and literature review. Rev Chil Pediatr. 2017;88:268-274. doi: 10.1016/j.rchipe.2016.07.002
- Bove KE, Hilton PK, Partin J, et al. Morphology of acute myopathy associated with influenza B infection. Pediatric Pathology. 1983;1:51-66. https://doi.org/10.3109/15513818309048284
- Koliou M, Hadjiloizou S, Ourani S, et al. A case of benign acute childhood myositis associated with influenza A (HINI) virus infection. Clin Microbiol Infect. 2010;16:193-195. doi: 10.1111/j.1469-0691.2009.03064.x
THE CASE
A 5-year-old previously healthy white boy presented to clinic with bilateral calf pain and refusal to bear weight since awakening that morning. Associated symptoms included a 3-day history of generalized fatigue, subjective fevers, cough, congestion, and rhinitis. The night prior to presentation, he showed no symptoms of gait abnormalities, muscle pain, or weakness. There was no history of similar symptoms, trauma, overexertion, foreign travel, or family history of musculoskeletal disease. He was fully immunized, except for the annual influenza vaccine. He was not taking any medications. This case occurred before the onset of the COVID-19 pandemic.
Objective findings included fever of 101 °F, refusal to bear weight, and symmetrical bilateral tenderness to palpation of the gastrocnemius-soleus complex. Pain was elicited with passive dorsiflexion. There was no erythema, edema, or sensory deficits, and the distal leg compartments were soft. There was normal range of motion of the hips, knees, and ankles. Dorsalis pedis pulses were 2+, and patella reflexes were 2/4 bilaterally.
Lab results included a white blood cell count of 2500/μL (normal range, 4500 to 11,000/μL);absolute neutrophil count, 900/μL (1500 to 8000/μL); platelet count, 131,000/μL (150,000 to 450,000/μL); creatine kinase level, 869 IU/L (22 to 198 U/L); and aspartate aminotransferase level, 116 U/L (8 to 33 U/L). A rapid influenza swab was positive for influenza B. Plain films of the bilateral hips and lower extremities were unremarkable. C-reactive protein (CRP) level, urinalysis, and renal function tests were within normal limits. Creatine kinase (CK) level peaked (1935 U/L; normal range, 22 to 198 U/L) within the first 24 hours of presentation and then trended down.
The Diagnosis
The patient’s sudden onset of symmetrical bilateral calf pain in the setting of an upper respiratory tract infection was extremely suspicious for benign acute childhood myositis (BACM). Lab work and radiologic evaluation were performed to rule out more ominous causes of refusal to bear weight. The suspicion of BACM was further validated by influenza B serology, an elevated CK, and a normal CRP.
Discussion
BACM was first described by Lundberg in 1957.1 The overall incidence and prevalence are unclear.2 A viral prodrome involving rhinorrhea, low-grade fever, sore throat, cough, and malaise typically precedes bilateral calf pain by 3 days.2-4 Myositis symptoms typically last for 4 days.3 While several infectious etiologies have been linked to this condition, influenza B has the greatest association.5,6
❚ Patient population. BACM occurs predominately in school-aged children (6-8 years old) and has a male-to-female ratio of 2:1.3,5,6 In a retrospective study of 219 children, BACM was strongly associated with male gender and ages 6 to 9 years.3 In another retrospective study of 54 children,80% of patients were male, and the mean age was 7.3 years.5
❚ Key symptoms and differential. The distinguishing feature of BACM is bilateral symmetric gastrocnemius-soleus tenderness.2,4 Additionally, the lack of neurologic symptoms is an important differentiator, as long as refusal to bear weight is not mistaken for weakness.6 These features help to distinguish BACM from other items in the differential, including trauma, Guillain-Barre syndrome, osteomyelitis, malignancy, deep vein thrombosis, and inherited musculoskeletal disorders.2
Continue to: Labratory evaluation...
❚ Laboratory evaluation will often show mild neutropenia, thrombocytopenia, and mild elevation in CK.7,8 CRP is typically normal.4,7,9 In a retrospective study of 28 admissions for BACM from 2001 to 2012, common findings included leukopenia (35%), neutropenia (25%), and thrombocytopenia (21%). The median CK value was 4181 U/L.4 In another analysis of BACM cases, mean CK was 1872 U/L.5
❚ Biopsy is unnecessary; however, calf muscle samples from 11 of 12 children with suspected BACM due to influenza B infection were consistent with patchy necrosis without significant myositis.10
❚ Complications. Rhabdomyolysis, although rare, has been reported with BACM. In 1 analysis, 10 of 316 patients with influenza-associated myositis developed rhabdomyolysis; 8 experienced renal failure. Rhabdomyolysis was 4 times more likely to occur in girls, and 86% of cases were associated with influenza A.6 Common manifestations of rhabdomyolysis associated with influenza include diffuse myopathy, gross hematuria, and myoglobinuria.6
❚ Treatment is mainly supportive.4,8,9 Antivirals typically are not indicated, as the bilateral calf pain manifests during the recovery phase of the illness.4,9,11 BACM is self-limited and should resolve within 3 days of myositis manifestation.2 Patients should follow up in 2 to 3 weeks to verify symptom resolution.2
If muscle pain, swelling, and tenderness worsen, further work-up is indicated. In more severe cases, including those involving renal failure, intensive care management and even dialysis may be necessary.4,6
❚ Our patient was hospitalized due to fever in the setting of neutropenia. Ultimately, he was treated with acetaminophen and intravenous fluids for mild dehydration and elevated CK levels. He was discharged home after 3 days, at which time he had complete resolution of pain and was able to resume normal activities.
The Takeaway
Benign acute childhood myositis is a self-limited disorder with an excellent prognosis. It has a typical presentation and therefore should be a clinical diagnosis; however, investigative studies may be warranted to rule out more ominous causes. Reassurance to family that the condition should self-resolve in a few days is important. Close follow-up should be scheduled to ensure resolution of symptoms.
CORRESPONDENCE
Nicholas A. Rathjen, DO, William Beaumont Army Medical Center, Department of Soldier and Family Care, 11335 SSG Sims Street, Fort Bliss, TX 79918; nicholas.a.rathjen@gmail. com
THE CASE
A 5-year-old previously healthy white boy presented to clinic with bilateral calf pain and refusal to bear weight since awakening that morning. Associated symptoms included a 3-day history of generalized fatigue, subjective fevers, cough, congestion, and rhinitis. The night prior to presentation, he showed no symptoms of gait abnormalities, muscle pain, or weakness. There was no history of similar symptoms, trauma, overexertion, foreign travel, or family history of musculoskeletal disease. He was fully immunized, except for the annual influenza vaccine. He was not taking any medications. This case occurred before the onset of the COVID-19 pandemic.
Objective findings included fever of 101 °F, refusal to bear weight, and symmetrical bilateral tenderness to palpation of the gastrocnemius-soleus complex. Pain was elicited with passive dorsiflexion. There was no erythema, edema, or sensory deficits, and the distal leg compartments were soft. There was normal range of motion of the hips, knees, and ankles. Dorsalis pedis pulses were 2+, and patella reflexes were 2/4 bilaterally.
Lab results included a white blood cell count of 2500/μL (normal range, 4500 to 11,000/μL);absolute neutrophil count, 900/μL (1500 to 8000/μL); platelet count, 131,000/μL (150,000 to 450,000/μL); creatine kinase level, 869 IU/L (22 to 198 U/L); and aspartate aminotransferase level, 116 U/L (8 to 33 U/L). A rapid influenza swab was positive for influenza B. Plain films of the bilateral hips and lower extremities were unremarkable. C-reactive protein (CRP) level, urinalysis, and renal function tests were within normal limits. Creatine kinase (CK) level peaked (1935 U/L; normal range, 22 to 198 U/L) within the first 24 hours of presentation and then trended down.
The Diagnosis
The patient’s sudden onset of symmetrical bilateral calf pain in the setting of an upper respiratory tract infection was extremely suspicious for benign acute childhood myositis (BACM). Lab work and radiologic evaluation were performed to rule out more ominous causes of refusal to bear weight. The suspicion of BACM was further validated by influenza B serology, an elevated CK, and a normal CRP.
Discussion
BACM was first described by Lundberg in 1957.1 The overall incidence and prevalence are unclear.2 A viral prodrome involving rhinorrhea, low-grade fever, sore throat, cough, and malaise typically precedes bilateral calf pain by 3 days.2-4 Myositis symptoms typically last for 4 days.3 While several infectious etiologies have been linked to this condition, influenza B has the greatest association.5,6
❚ Patient population. BACM occurs predominately in school-aged children (6-8 years old) and has a male-to-female ratio of 2:1.3,5,6 In a retrospective study of 219 children, BACM was strongly associated with male gender and ages 6 to 9 years.3 In another retrospective study of 54 children,80% of patients were male, and the mean age was 7.3 years.5
❚ Key symptoms and differential. The distinguishing feature of BACM is bilateral symmetric gastrocnemius-soleus tenderness.2,4 Additionally, the lack of neurologic symptoms is an important differentiator, as long as refusal to bear weight is not mistaken for weakness.6 These features help to distinguish BACM from other items in the differential, including trauma, Guillain-Barre syndrome, osteomyelitis, malignancy, deep vein thrombosis, and inherited musculoskeletal disorders.2
Continue to: Labratory evaluation...
❚ Laboratory evaluation will often show mild neutropenia, thrombocytopenia, and mild elevation in CK.7,8 CRP is typically normal.4,7,9 In a retrospective study of 28 admissions for BACM from 2001 to 2012, common findings included leukopenia (35%), neutropenia (25%), and thrombocytopenia (21%). The median CK value was 4181 U/L.4 In another analysis of BACM cases, mean CK was 1872 U/L.5
❚ Biopsy is unnecessary; however, calf muscle samples from 11 of 12 children with suspected BACM due to influenza B infection were consistent with patchy necrosis without significant myositis.10
❚ Complications. Rhabdomyolysis, although rare, has been reported with BACM. In 1 analysis, 10 of 316 patients with influenza-associated myositis developed rhabdomyolysis; 8 experienced renal failure. Rhabdomyolysis was 4 times more likely to occur in girls, and 86% of cases were associated with influenza A.6 Common manifestations of rhabdomyolysis associated with influenza include diffuse myopathy, gross hematuria, and myoglobinuria.6
❚ Treatment is mainly supportive.4,8,9 Antivirals typically are not indicated, as the bilateral calf pain manifests during the recovery phase of the illness.4,9,11 BACM is self-limited and should resolve within 3 days of myositis manifestation.2 Patients should follow up in 2 to 3 weeks to verify symptom resolution.2
If muscle pain, swelling, and tenderness worsen, further work-up is indicated. In more severe cases, including those involving renal failure, intensive care management and even dialysis may be necessary.4,6
❚ Our patient was hospitalized due to fever in the setting of neutropenia. Ultimately, he was treated with acetaminophen and intravenous fluids for mild dehydration and elevated CK levels. He was discharged home after 3 days, at which time he had complete resolution of pain and was able to resume normal activities.
The Takeaway
Benign acute childhood myositis is a self-limited disorder with an excellent prognosis. It has a typical presentation and therefore should be a clinical diagnosis; however, investigative studies may be warranted to rule out more ominous causes. Reassurance to family that the condition should self-resolve in a few days is important. Close follow-up should be scheduled to ensure resolution of symptoms.
CORRESPONDENCE
Nicholas A. Rathjen, DO, William Beaumont Army Medical Center, Department of Soldier and Family Care, 11335 SSG Sims Street, Fort Bliss, TX 79918; nicholas.a.rathjen@gmail. com
- Lundberg A. Myalgia cruris epidemica. Acta Paediatr. 1957;46:18-31. doi: 10.1111/j.1651-2227.1957.tb08627.x
- Magee H, Goldman RD. Viral myositis in children. Can Fam Physician. 2017;63:365-368.
- Mall S, Buchholz U, Tibussek D, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30:e142-e146. doi: 10.1097/INF.0b013e318217e356
- Santos JA, Albuquerque C, Lito D, et al. Benign acute childhood myositis: an alarming condition with an excellent prognosis! Am J Emerg Med. 2014;32:1418-1419. doi: 10.1016/j.ajem.2014.08.022
- Rosenberg T, Heitner S, Scolnik D, et al. Outcome of benign acute childhood myositis: the experience of 2 large tertiary care pediatric hospitals. Pediatr Emerg Care. 2018;34:400-402. doi: 10.1097/PEC.0000000000000830
- Agyeman P, Duppenthaler A, Heininger U, et al. Influenza-associated myositis in children. Infection. 2004;32:199-203. doi: 10.1007/s15010-004-4003-2
- Mackay MT, Kornberg AJ, Shield LK, et al. Benign acute childhood myositis: laboratory and clinical features. Neurology. 1999;53:2127-2131. doi: 10.1212/wnl.53.9.2127
- Neocleous C, Spanou C, Mpampalis E, et al. Unnecessary diagnostic investigations in benign acute childhood myositis: a case series report. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012023
- Felipe Cavagnaro SM, Alejandra Aird G, Ingrid Harwardt R, et al. Benign acute childhood myositis: clinical series and literature review. Rev Chil Pediatr. 2017;88:268-274. doi: 10.1016/j.rchipe.2016.07.002
- Bove KE, Hilton PK, Partin J, et al. Morphology of acute myopathy associated with influenza B infection. Pediatric Pathology. 1983;1:51-66. https://doi.org/10.3109/15513818309048284
- Koliou M, Hadjiloizou S, Ourani S, et al. A case of benign acute childhood myositis associated with influenza A (HINI) virus infection. Clin Microbiol Infect. 2010;16:193-195. doi: 10.1111/j.1469-0691.2009.03064.x
- Lundberg A. Myalgia cruris epidemica. Acta Paediatr. 1957;46:18-31. doi: 10.1111/j.1651-2227.1957.tb08627.x
- Magee H, Goldman RD. Viral myositis in children. Can Fam Physician. 2017;63:365-368.
- Mall S, Buchholz U, Tibussek D, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30:e142-e146. doi: 10.1097/INF.0b013e318217e356
- Santos JA, Albuquerque C, Lito D, et al. Benign acute childhood myositis: an alarming condition with an excellent prognosis! Am J Emerg Med. 2014;32:1418-1419. doi: 10.1016/j.ajem.2014.08.022
- Rosenberg T, Heitner S, Scolnik D, et al. Outcome of benign acute childhood myositis: the experience of 2 large tertiary care pediatric hospitals. Pediatr Emerg Care. 2018;34:400-402. doi: 10.1097/PEC.0000000000000830
- Agyeman P, Duppenthaler A, Heininger U, et al. Influenza-associated myositis in children. Infection. 2004;32:199-203. doi: 10.1007/s15010-004-4003-2
- Mackay MT, Kornberg AJ, Shield LK, et al. Benign acute childhood myositis: laboratory and clinical features. Neurology. 1999;53:2127-2131. doi: 10.1212/wnl.53.9.2127
- Neocleous C, Spanou C, Mpampalis E, et al. Unnecessary diagnostic investigations in benign acute childhood myositis: a case series report. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012023
- Felipe Cavagnaro SM, Alejandra Aird G, Ingrid Harwardt R, et al. Benign acute childhood myositis: clinical series and literature review. Rev Chil Pediatr. 2017;88:268-274. doi: 10.1016/j.rchipe.2016.07.002
- Bove KE, Hilton PK, Partin J, et al. Morphology of acute myopathy associated with influenza B infection. Pediatric Pathology. 1983;1:51-66. https://doi.org/10.3109/15513818309048284
- Koliou M, Hadjiloizou S, Ourani S, et al. A case of benign acute childhood myositis associated with influenza A (HINI) virus infection. Clin Microbiol Infect. 2010;16:193-195. doi: 10.1111/j.1469-0691.2009.03064.x
Osteoporosis management: Use a goal-oriented, individualized approach
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10
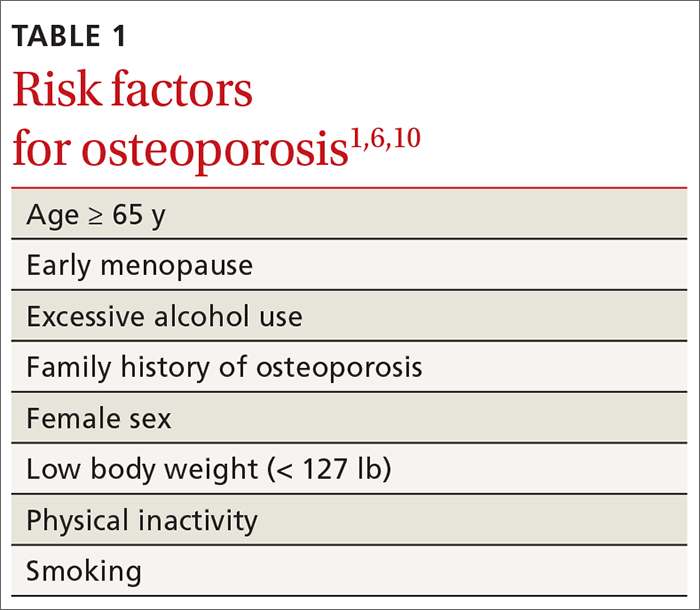
Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
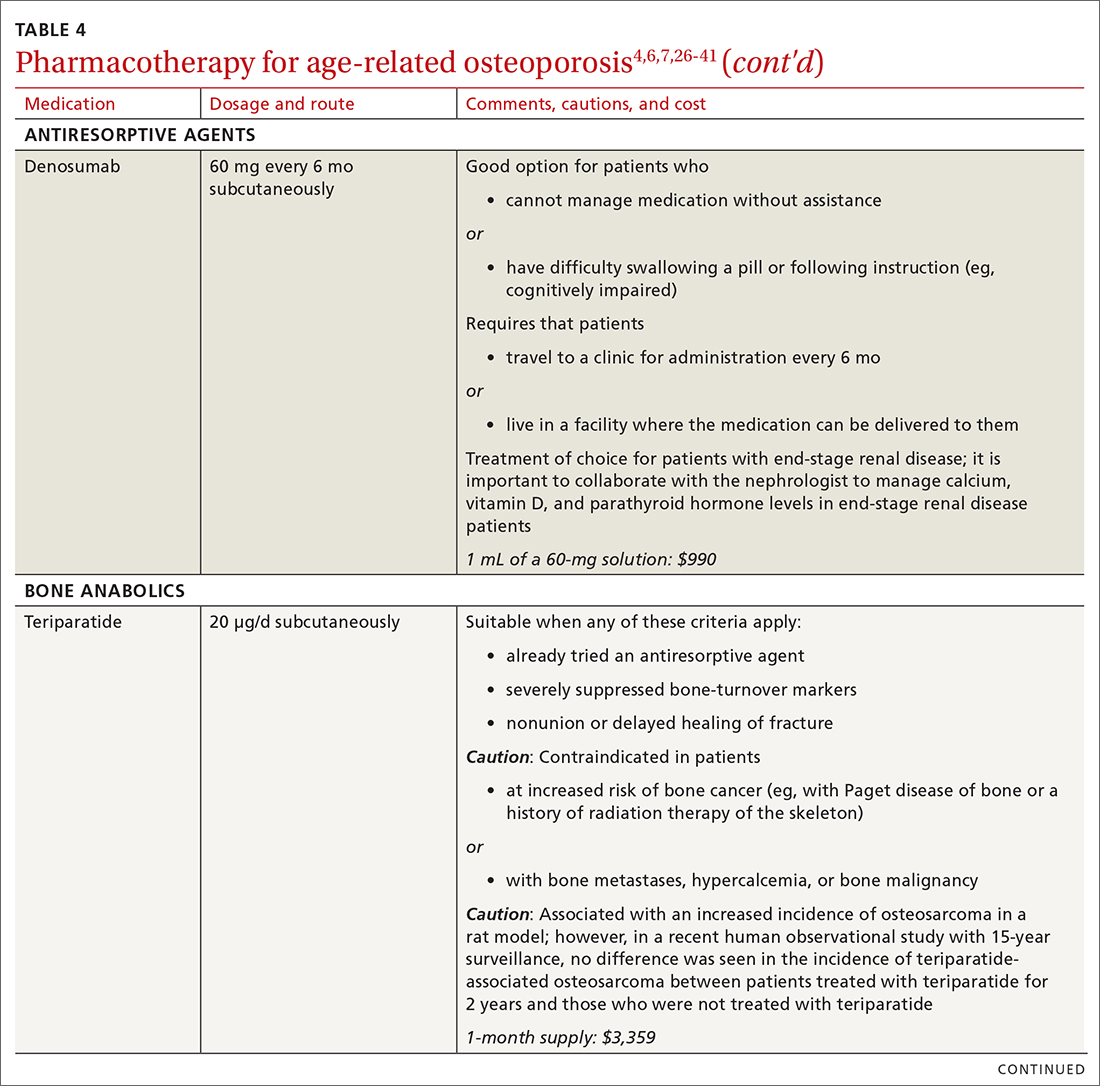
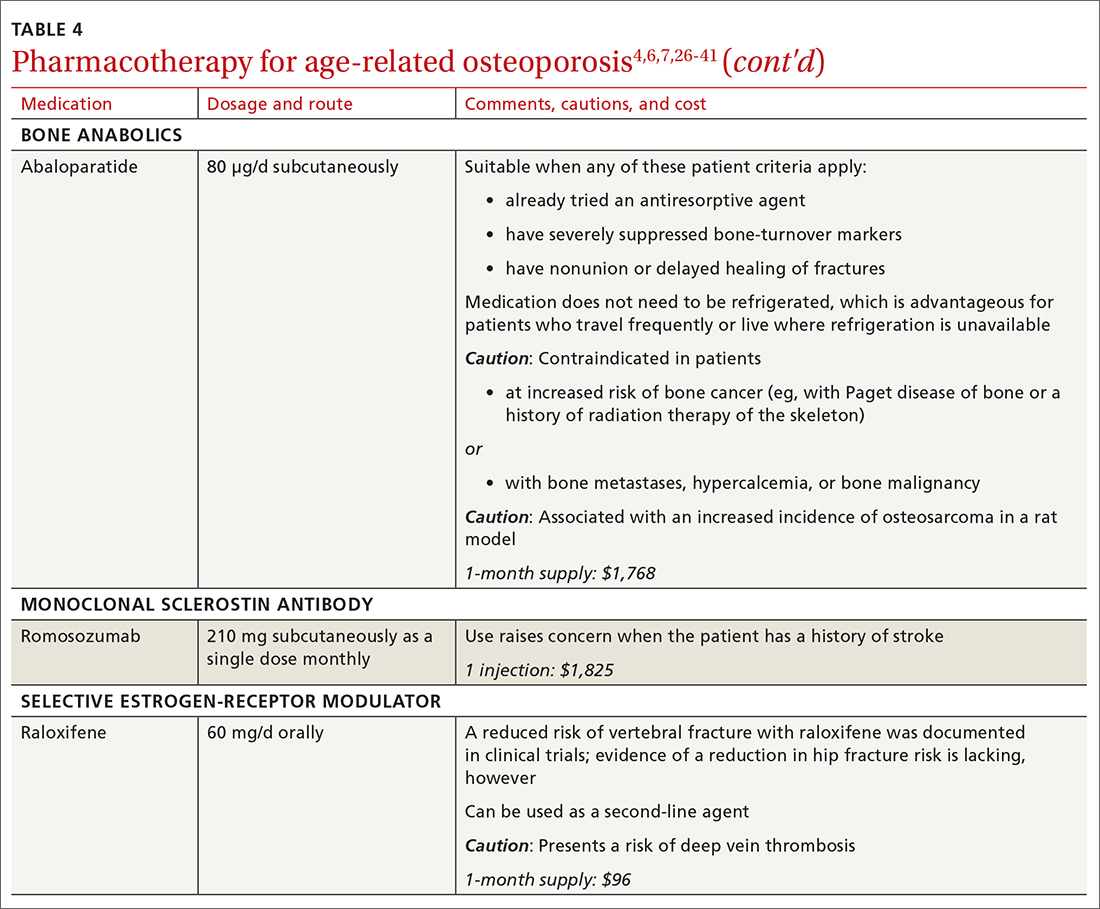
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)

❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
PRACTICE RECOMMENDATIONS
❯ Consider screening for osteoporosis, using bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA), in all postmenopausal women ≥ 65 years and in women < 65 years at high risk of osteoporosis.
❯ Consider screening in men ≥ 70 years and in younger men at high risk of fracture.
❯ Use the trabecular bone score with DXA BMD to screen patients at high risk of fracture who have a normal BMD—eg, patients with type 2 diabetes or ankylosing spondylitis.
❯ Offer individualized pharmacotherapy to older patients with a diagnosis of osteoporosis and to those at high risk of fracture.
Foot rash and joint pain
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.

An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.

An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.

An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
Secukinumab provides clinical benefit in phase 3 juvenile arthritis trial
Favorable safety sustained at 104 weeks
Secukinumab (Cosentyx), an interleukin-17A inhibitor, is effective and reasonably well tolerated for treatment of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) in children and adolescents, according to a phase 3 trial presented at a late breaking abstracts session of the annual European Congress of Rheumatology.
On the primary outcome of time to flare, the curves for secukinumab and placebo separated almost immediately, with fewer than half the number of flares occurring in the experimental arm over the course of the study, according to Nicolino Ruperto, MD, senior research scientist at IRCCS Istituto Giannina Gaslini in Genoa, Italy.
The trial, called JUNIPERA, was conducted over 2 years and included an open-label treatment period (TP1) and then a randomized, placebo-controlled comparison (TP2). In TP1, 86 children were initiated on open-label secukinumab administered subcutaneously on weeks 1, 2, 3, 4, 8, and 12. The dose was 75 mg for children less than 50 kg and 150 kg for those heavier.
Average patient age was 13.1 years
Of these 86 children, 52 had ERA and 34 had JPsA. Disease duration of at least 6 months was required for entry. Patients up to the age of 18 years were permitted to enroll. The average age was 13.1 years. Most patients, two-thirds of whom were male, had received an immunomodulator prior to study entry.
At the end of TP1, 69.9% of patients had achieved 70% improvement in the Juvenile Idiopathic Arthritis American College of Rheumatology joint score (JIA ACR70). The 90.4% of patients who achieved JIA ACR30 were invited to enroll in TP2. A total of 75 patients did so.
At the end of TP2, response rates strongly favored secukinumab over placebo for JIA ACR30 (89.2% vs. 64.9%; P = .014) and JIA ACR70 (67.7% vs. 43.2%; P = .042). Higher but not statistically significant differences in response rates were seen for secukinumab over placebo for JIA ACR50 (78.4% vs. 62.2%; P = .152), JIA ACR90 (51.4% vs. 40.5%; P = .431) and JIA ACR100 (43.2% vs. 37.8%; P = .755).
During TP2, there were 10 flares in the group randomized to secukinumab versus 21 flares in the placebo group, translating by hazard ratio (HR) into a 72% risk reduction (HR, 0.28; P < .001).
Side effects similar to those in adults
The types and rates of serious adverse events were similar to those reported previously in adult patients, according to Dr. Ruperto. Although the rate of serious adverse events (14.6% vs. 10.6%) was only moderately higher in the experimental arm, more patients randomized to secukinumab than placebo discontinued therapy (13.2% vs. 6.3%) before the end of follow-up.
The side effects that occurred more commonly on secukinumab included gastrointestinal complaints, such as diarrhea (22.9% vs. 15.8%). Other adverse events occurring in more than 10% of patients included headache and nasopharyngitis, but most side effects were mild and resolved.
Although the proportion of patients with flare increased over time in both groups, Dr. Ruperto reported that protection against flares and relative improvement in clinical markers of disease activity relative to placebo “were sustained out to 2 years of follow-up.”
The submission of these data to regulatory agencies is anticipated. If secukinumab is given an indication for these forms of arthritis, it will join an indication for plaque psoriasis in children that was granted just a few days before these data were presented. The psoriasis indication is the only current use approved for children in the United States.
More biologics needed for JPsA
Additional biologics will be helpful for children with arthritis who are poorly controlled on available treatments, according to Natasha M. Ruth, MD, director of the division of pediatric rheumatology at the Medical University of South Carolina, Charleston. Dr. Ruth was senior author of a case study published 2 years ago in which secukinumab was used to control psoriatic arthritis and nail manifestations of psoriasis.
“It was a girl who had already failed to improve adequately to TNF inhibitors,” reported Dr. Ruth, who had said the child and her parent were very concerned about the nail appearance.
“The nail involvement completely resolved, so it was a very good result in a difficult situation,” Dr. Ruth explained. She said that the decision to try secukinumab was made collaboratively in a clinic in which dermatologists and rheumatologists at her institution work together on difficult cases.
“There is a need for more biologics with different mechanisms of action,” Dr. Ruth said. Based on her experience, secukinumab could be an important addition to treatment options.
Dr. Ruperto reported having financial relationships with more than 20 pharmaceutical companies, including Novartis, which provided financial support for this trial. Many coauthors had financial relationships with multiple companies, including Novartis, and some were employees of the company. Dr. Ruth reported having no potential conflicts of interest.
Favorable safety sustained at 104 weeks
Favorable safety sustained at 104 weeks
Secukinumab (Cosentyx), an interleukin-17A inhibitor, is effective and reasonably well tolerated for treatment of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) in children and adolescents, according to a phase 3 trial presented at a late breaking abstracts session of the annual European Congress of Rheumatology.
On the primary outcome of time to flare, the curves for secukinumab and placebo separated almost immediately, with fewer than half the number of flares occurring in the experimental arm over the course of the study, according to Nicolino Ruperto, MD, senior research scientist at IRCCS Istituto Giannina Gaslini in Genoa, Italy.
The trial, called JUNIPERA, was conducted over 2 years and included an open-label treatment period (TP1) and then a randomized, placebo-controlled comparison (TP2). In TP1, 86 children were initiated on open-label secukinumab administered subcutaneously on weeks 1, 2, 3, 4, 8, and 12. The dose was 75 mg for children less than 50 kg and 150 kg for those heavier.
Average patient age was 13.1 years
Of these 86 children, 52 had ERA and 34 had JPsA. Disease duration of at least 6 months was required for entry. Patients up to the age of 18 years were permitted to enroll. The average age was 13.1 years. Most patients, two-thirds of whom were male, had received an immunomodulator prior to study entry.
At the end of TP1, 69.9% of patients had achieved 70% improvement in the Juvenile Idiopathic Arthritis American College of Rheumatology joint score (JIA ACR70). The 90.4% of patients who achieved JIA ACR30 were invited to enroll in TP2. A total of 75 patients did so.
At the end of TP2, response rates strongly favored secukinumab over placebo for JIA ACR30 (89.2% vs. 64.9%; P = .014) and JIA ACR70 (67.7% vs. 43.2%; P = .042). Higher but not statistically significant differences in response rates were seen for secukinumab over placebo for JIA ACR50 (78.4% vs. 62.2%; P = .152), JIA ACR90 (51.4% vs. 40.5%; P = .431) and JIA ACR100 (43.2% vs. 37.8%; P = .755).
During TP2, there were 10 flares in the group randomized to secukinumab versus 21 flares in the placebo group, translating by hazard ratio (HR) into a 72% risk reduction (HR, 0.28; P < .001).
Side effects similar to those in adults
The types and rates of serious adverse events were similar to those reported previously in adult patients, according to Dr. Ruperto. Although the rate of serious adverse events (14.6% vs. 10.6%) was only moderately higher in the experimental arm, more patients randomized to secukinumab than placebo discontinued therapy (13.2% vs. 6.3%) before the end of follow-up.
The side effects that occurred more commonly on secukinumab included gastrointestinal complaints, such as diarrhea (22.9% vs. 15.8%). Other adverse events occurring in more than 10% of patients included headache and nasopharyngitis, but most side effects were mild and resolved.
Although the proportion of patients with flare increased over time in both groups, Dr. Ruperto reported that protection against flares and relative improvement in clinical markers of disease activity relative to placebo “were sustained out to 2 years of follow-up.”
The submission of these data to regulatory agencies is anticipated. If secukinumab is given an indication for these forms of arthritis, it will join an indication for plaque psoriasis in children that was granted just a few days before these data were presented. The psoriasis indication is the only current use approved for children in the United States.
More biologics needed for JPsA
Additional biologics will be helpful for children with arthritis who are poorly controlled on available treatments, according to Natasha M. Ruth, MD, director of the division of pediatric rheumatology at the Medical University of South Carolina, Charleston. Dr. Ruth was senior author of a case study published 2 years ago in which secukinumab was used to control psoriatic arthritis and nail manifestations of psoriasis.
“It was a girl who had already failed to improve adequately to TNF inhibitors,” reported Dr. Ruth, who had said the child and her parent were very concerned about the nail appearance.
“The nail involvement completely resolved, so it was a very good result in a difficult situation,” Dr. Ruth explained. She said that the decision to try secukinumab was made collaboratively in a clinic in which dermatologists and rheumatologists at her institution work together on difficult cases.
“There is a need for more biologics with different mechanisms of action,” Dr. Ruth said. Based on her experience, secukinumab could be an important addition to treatment options.
Dr. Ruperto reported having financial relationships with more than 20 pharmaceutical companies, including Novartis, which provided financial support for this trial. Many coauthors had financial relationships with multiple companies, including Novartis, and some were employees of the company. Dr. Ruth reported having no potential conflicts of interest.
Secukinumab (Cosentyx), an interleukin-17A inhibitor, is effective and reasonably well tolerated for treatment of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) in children and adolescents, according to a phase 3 trial presented at a late breaking abstracts session of the annual European Congress of Rheumatology.
On the primary outcome of time to flare, the curves for secukinumab and placebo separated almost immediately, with fewer than half the number of flares occurring in the experimental arm over the course of the study, according to Nicolino Ruperto, MD, senior research scientist at IRCCS Istituto Giannina Gaslini in Genoa, Italy.
The trial, called JUNIPERA, was conducted over 2 years and included an open-label treatment period (TP1) and then a randomized, placebo-controlled comparison (TP2). In TP1, 86 children were initiated on open-label secukinumab administered subcutaneously on weeks 1, 2, 3, 4, 8, and 12. The dose was 75 mg for children less than 50 kg and 150 kg for those heavier.
Average patient age was 13.1 years
Of these 86 children, 52 had ERA and 34 had JPsA. Disease duration of at least 6 months was required for entry. Patients up to the age of 18 years were permitted to enroll. The average age was 13.1 years. Most patients, two-thirds of whom were male, had received an immunomodulator prior to study entry.
At the end of TP1, 69.9% of patients had achieved 70% improvement in the Juvenile Idiopathic Arthritis American College of Rheumatology joint score (JIA ACR70). The 90.4% of patients who achieved JIA ACR30 were invited to enroll in TP2. A total of 75 patients did so.
At the end of TP2, response rates strongly favored secukinumab over placebo for JIA ACR30 (89.2% vs. 64.9%; P = .014) and JIA ACR70 (67.7% vs. 43.2%; P = .042). Higher but not statistically significant differences in response rates were seen for secukinumab over placebo for JIA ACR50 (78.4% vs. 62.2%; P = .152), JIA ACR90 (51.4% vs. 40.5%; P = .431) and JIA ACR100 (43.2% vs. 37.8%; P = .755).
During TP2, there were 10 flares in the group randomized to secukinumab versus 21 flares in the placebo group, translating by hazard ratio (HR) into a 72% risk reduction (HR, 0.28; P < .001).
Side effects similar to those in adults
The types and rates of serious adverse events were similar to those reported previously in adult patients, according to Dr. Ruperto. Although the rate of serious adverse events (14.6% vs. 10.6%) was only moderately higher in the experimental arm, more patients randomized to secukinumab than placebo discontinued therapy (13.2% vs. 6.3%) before the end of follow-up.
The side effects that occurred more commonly on secukinumab included gastrointestinal complaints, such as diarrhea (22.9% vs. 15.8%). Other adverse events occurring in more than 10% of patients included headache and nasopharyngitis, but most side effects were mild and resolved.
Although the proportion of patients with flare increased over time in both groups, Dr. Ruperto reported that protection against flares and relative improvement in clinical markers of disease activity relative to placebo “were sustained out to 2 years of follow-up.”
The submission of these data to regulatory agencies is anticipated. If secukinumab is given an indication for these forms of arthritis, it will join an indication for plaque psoriasis in children that was granted just a few days before these data were presented. The psoriasis indication is the only current use approved for children in the United States.
More biologics needed for JPsA
Additional biologics will be helpful for children with arthritis who are poorly controlled on available treatments, according to Natasha M. Ruth, MD, director of the division of pediatric rheumatology at the Medical University of South Carolina, Charleston. Dr. Ruth was senior author of a case study published 2 years ago in which secukinumab was used to control psoriatic arthritis and nail manifestations of psoriasis.
“It was a girl who had already failed to improve adequately to TNF inhibitors,” reported Dr. Ruth, who had said the child and her parent were very concerned about the nail appearance.
“The nail involvement completely resolved, so it was a very good result in a difficult situation,” Dr. Ruth explained. She said that the decision to try secukinumab was made collaboratively in a clinic in which dermatologists and rheumatologists at her institution work together on difficult cases.
“There is a need for more biologics with different mechanisms of action,” Dr. Ruth said. Based on her experience, secukinumab could be an important addition to treatment options.
Dr. Ruperto reported having financial relationships with more than 20 pharmaceutical companies, including Novartis, which provided financial support for this trial. Many coauthors had financial relationships with multiple companies, including Novartis, and some were employees of the company. Dr. Ruth reported having no potential conflicts of interest.
FROM THE EULAR 2021 CONGRESS
Fall prevention advice for patients with Parkinson’s
A 75-year-old man with Parkinson’s disease has had three falls over the past 4 weeks. He has been compliant with his Parkinson’s treatment. Which of the following options would most help decrease his fall risk?
A. Vitamin D supplementation
B. Vitamin B12 supplementation
C. Calcium supplementation
D. Tai chi
There has been recent evidence that vitamin D supplementation is not helpful in preventing falls in most community-dwelling older adults. Bolland and colleagues performed a meta-analysis of 81 randomized, controlled trials and found that vitamin D supplementation does not prevent fractures or falls.1 They found no difference or benefit in high-dose versus low-dose vitamin D supplementation.
The U.S. Preventive Services Task Force recommends against vitamin D supplementation for the purpose of preventing falls in community-dwelling adults over the age of 65.2 The same USPSTF report recommends exercise intervention, as having the strongest evidence for fall prevention in community-dwelling adults age 65 or older who are at risk for falls.
The benefits of tai chi
Tai chi with it’s emphasis on balance, strength training as well as stress reduction is an excellent option for older adults.
Lui and colleagues performed a meta-analyses of five randomized, controlled trials (355 patients) of tai chi in patients with Parkinson disease.3 Tai chi significantly decreased fall rates (odds ratio, 0.47; 95% confidence interval, 0.30-0.74; P = .001) and significantly improved balance and functional mobility (P < .001) in people with Parkinson disease, compared with no training.
Tai chi can also help prevent falls in a more general population of elderly patients. Lomas-Vega and colleagues performed a meta-analysis of 10 high-quality studies that met inclusion criteria evaluating tai chi for fall prevention.4 Fall risk was reduced over short-term follow-up (incident rate ratio, 0.57; 95% CI, 0.46-0.70) and a small protective effect was seen over long-term follow-up (IRR, 0.87; 95% CI, 0.77-0.98).
Pearl: Consider tai chi in your elderly patients with fall risk to increase their balance and reduce risks of falls.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Bolland MJ et al. Lancet Diabetes Endocrinol. 2018;6(11):847.
2. U.S. Preventive Services Task Force. JAMA. 2018;319(16):1696.
3. Liu HH et al. Parkinsons Dis. 2019 Feb 21;2019:9626934
4. Lomas-Vega R et al. J Am Geriatr Soc. 2017;65(9):2037.
A 75-year-old man with Parkinson’s disease has had three falls over the past 4 weeks. He has been compliant with his Parkinson’s treatment. Which of the following options would most help decrease his fall risk?
A. Vitamin D supplementation
B. Vitamin B12 supplementation
C. Calcium supplementation
D. Tai chi
There has been recent evidence that vitamin D supplementation is not helpful in preventing falls in most community-dwelling older adults. Bolland and colleagues performed a meta-analysis of 81 randomized, controlled trials and found that vitamin D supplementation does not prevent fractures or falls.1 They found no difference or benefit in high-dose versus low-dose vitamin D supplementation.
The U.S. Preventive Services Task Force recommends against vitamin D supplementation for the purpose of preventing falls in community-dwelling adults over the age of 65.2 The same USPSTF report recommends exercise intervention, as having the strongest evidence for fall prevention in community-dwelling adults age 65 or older who are at risk for falls.
The benefits of tai chi
Tai chi with it’s emphasis on balance, strength training as well as stress reduction is an excellent option for older adults.
Lui and colleagues performed a meta-analyses of five randomized, controlled trials (355 patients) of tai chi in patients with Parkinson disease.3 Tai chi significantly decreased fall rates (odds ratio, 0.47; 95% confidence interval, 0.30-0.74; P = .001) and significantly improved balance and functional mobility (P < .001) in people with Parkinson disease, compared with no training.
Tai chi can also help prevent falls in a more general population of elderly patients. Lomas-Vega and colleagues performed a meta-analysis of 10 high-quality studies that met inclusion criteria evaluating tai chi for fall prevention.4 Fall risk was reduced over short-term follow-up (incident rate ratio, 0.57; 95% CI, 0.46-0.70) and a small protective effect was seen over long-term follow-up (IRR, 0.87; 95% CI, 0.77-0.98).
Pearl: Consider tai chi in your elderly patients with fall risk to increase their balance and reduce risks of falls.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Bolland MJ et al. Lancet Diabetes Endocrinol. 2018;6(11):847.
2. U.S. Preventive Services Task Force. JAMA. 2018;319(16):1696.
3. Liu HH et al. Parkinsons Dis. 2019 Feb 21;2019:9626934
4. Lomas-Vega R et al. J Am Geriatr Soc. 2017;65(9):2037.
A 75-year-old man with Parkinson’s disease has had three falls over the past 4 weeks. He has been compliant with his Parkinson’s treatment. Which of the following options would most help decrease his fall risk?
A. Vitamin D supplementation
B. Vitamin B12 supplementation
C. Calcium supplementation
D. Tai chi
There has been recent evidence that vitamin D supplementation is not helpful in preventing falls in most community-dwelling older adults. Bolland and colleagues performed a meta-analysis of 81 randomized, controlled trials and found that vitamin D supplementation does not prevent fractures or falls.1 They found no difference or benefit in high-dose versus low-dose vitamin D supplementation.
The U.S. Preventive Services Task Force recommends against vitamin D supplementation for the purpose of preventing falls in community-dwelling adults over the age of 65.2 The same USPSTF report recommends exercise intervention, as having the strongest evidence for fall prevention in community-dwelling adults age 65 or older who are at risk for falls.
The benefits of tai chi
Tai chi with it’s emphasis on balance, strength training as well as stress reduction is an excellent option for older adults.
Lui and colleagues performed a meta-analyses of five randomized, controlled trials (355 patients) of tai chi in patients with Parkinson disease.3 Tai chi significantly decreased fall rates (odds ratio, 0.47; 95% confidence interval, 0.30-0.74; P = .001) and significantly improved balance and functional mobility (P < .001) in people with Parkinson disease, compared with no training.
Tai chi can also help prevent falls in a more general population of elderly patients. Lomas-Vega and colleagues performed a meta-analysis of 10 high-quality studies that met inclusion criteria evaluating tai chi for fall prevention.4 Fall risk was reduced over short-term follow-up (incident rate ratio, 0.57; 95% CI, 0.46-0.70) and a small protective effect was seen over long-term follow-up (IRR, 0.87; 95% CI, 0.77-0.98).
Pearl: Consider tai chi in your elderly patients with fall risk to increase their balance and reduce risks of falls.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Bolland MJ et al. Lancet Diabetes Endocrinol. 2018;6(11):847.
2. U.S. Preventive Services Task Force. JAMA. 2018;319(16):1696.
3. Liu HH et al. Parkinsons Dis. 2019 Feb 21;2019:9626934
4. Lomas-Vega R et al. J Am Geriatr Soc. 2017;65(9):2037.
Quinolones and tendon health: Third-generation drugs may be safer
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
FROM ANNALS OF FAMILY MEDICINE
Challenges persist in adolescents with rheumatic disease transitioning to adult care
The inadequacies and challenges of transitioning pediatric rheumatology patients to adult care were highlighted in several research studies shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
“Not surprisingly, these studies demonstrate that transition challenges remain pervasive,” Rebecca Sadun, MD, PhD, who was not involved in any of the research, said in an interview. Nevertheless, she pointed out that one of the studies showed that eight of nine sites participating in one of the studies had at least developed a formal transition policy, and three were able to fully integrate that policy into their health care system despite the ongoing pandemic.
In that study, Joyce Chang, MD, of the Children’s Hospital of Philadelphia and colleagues used structured interviews and then quantitative research to explore processes for transition polices across nine rheumatology sites. Aside from the three that had already implemented their policies, three others were preparing implementation. The other three withdrew because of COVID-19. None of the sites had reached sustainment phase. Six of the sites had access to a social work network, and two sites had fewer than four providers.
The authors found that a higher level of change efficacy or change commitment using the Organizational Readiness for Implementing Change framework did not correspond with reaching implementation.
“The first sites to reach implementation had access to [information technology] support and involved nursing, though this was not sufficient or necessary,” the authors wrote. They noted the need for strategies to reduce the burden of data collection to improve the resilience of implementation efforts against health care system stress.
Who more often transitions to adult care?
Effective transition policies can help reduce the likelihood of young patients falling through the cracks as they grow from adolescence into young adulthood, especially those at highest risk for losing continuity of care.
“Young adults who are both medically and socially complex are at highest risk,” said Dr. Sadun, an assistant professor of adult and pediatric rheumatology at Duke University, Durham, N.C. “This is especially true for patients with systemic illnesses, patients requiring biologic medications, and patients with custody, transportation, and financial barriers.”
Research led by Emily A. Smitherman, MD, an assistant professor of pediatric rheumatology at Children’s of Alabama in Birmingham, looked more closely at who is and is not transitioning their care. The researchers analyzed retrospective data from the CARRA Registry, including the Long-Term Follow-Up Call Registry, through December 2019. Among 1,311 patients with inactive status, 537 of these patients had juvenile idiopathic arthritis and were aged at least 18 years. Only 186 of those patients, however, had data in the Long-Term Follow-Up Registry. Patients who were Black or had lower income were less likely to have data in the Long-Term Follow-Up Registry.
Just over half the patients in the long-term registry had transferred their care to an adult rheumatologist, and 83% overall were under the care of any physician. Patients who transferred their care were significantly more likely to have private insurance (87% vs. 70%; P = .009) and were more likely to be full-time students (74% vs. 58%; P = .036).
The researchers found no association between patients’ disease status at their last CARRA Registry visit and a successful transition to adult care. However, those who had transferred care to an adult rheumatologist tended to have a higher median level of pain (4 vs. 2 on a scale of 0-10) and more disease activity (3 vs. 1 on 0-10 scale) than did those who had not transferred care (P = .022 and P = .011, respectively). A higher proportion of those who transferred care had also experienced morning stiffness over the past week (49% vs. 30%; P = .015).
How young adults prefer to learn transition skills
The third study aimed to better understand the experience and preferences of young adults themselves as they transitioned from pediatric to adult care. Kristine Carandang, PhD, a postdoctoral scholar at the University of California, San Diego, and colleagues first conducted focus groups with 39 adolescents and young adults, ages 16-28 years, who had rheumatic conditions. Using the qualitative data from the focus groups, they designed a survey to capture quantitative data on young patients’ experiences.
“What we’re always trying to work on is, how do we bring that youth voice more clearly into the research literature?” Courtney K. Wells, PhD, MSW, an assistant professor of social work at the University of Wisconsin–River Falls, said in an interview. She noted that both she and Dr. Carandang were patients with rheumatic diseases, so they had lived and grown up with disease themselves and then become researchers.
“We have the information that’s in the literature, but then we also both work with youth in a couple different ways, and what we hear from youth is what we heard in our paper, but it isn’t all represented in the literature,” Dr. Wells said. That disconnect is why they also included two young adults as coauthors in the study.
“As much as we appreciate the model of the six components [of health care transition], we recognize that the youth voice isn’t represented very well,” Dr. Wells said. “The way it’s written is more for doctors and policy makers and targeted for the health care system rather than the young people themselves.”
Their research bore that out. Among 137 survey respondents, aged 18-28 years, the vast majority (89%) were women and most (75%) were White. Half the patients (50%) had a diagnosis of lupus.
“For 9 out of 11 self-management and self-advocacy skills examined, there was a significant difference between how adolescent and young adult patients experienced learning self- management skills versus how they would have preferred to learn the skills,” the researchers concluded. “Overall, adolescent and young adult patients most frequently learned about transition skills from their parents. Most participants would have preferred to learn these skills from their rheumatology team.”
For example, 46.7% of the respondents learned how to communicate their medical history from their parents, but 48.5% would have preferred to learn that from their rheumatology team. Only a quarter (24.8%) had learned that skill from their health care team.
“For most of these skills, they were getting that information from their parents, which is concerning because their parents don’t necessarily have information that is accurate,” Dr. Wells said. “Their parents managed their health care, and they taught them to do it the way they were doing it.”
Just over one-third of respondents said they learned from their parents how to track their symptoms so they could answer the rheumatologists’ questions. Only one in five respondents (20.4%) had learned this skill from their rheumatology team, but 41.2% would have preferred hearing it from their health care team, compared with 22.1% who preferred learning it from their parents.
Nearly half the respondents reported learning from their parents how to advocate for themselves when dissatisfied with their care or symptoms (49.6%) and how to talk to the office staff to make appointments, fill out paperwork, and access health records (47.4%). Just over half (51.5%) would have preferred to learn about office communication from their health care team. Preferences on self-advocacy were split between learning from parents (36.8%) and learning from their health care team (31.6%).
An opportunity for other organizations to support transition
The researchers noted that education did not necessarily need to come only from rheumatologists. Other health care professionals, including nurses and social workers, could help young patients develop skills as well.
“They said they’re also open to talking to other people, but they want their rheumatologist to lead the whole process,” Dr. Wells said. While reimbursement gaps may have presented a barrier in the past, Dr. Wells said that current billing codes have removed that obstacle, allowing physicians to bill for discussing transition skills and care.
“Largely, it’s a time issue,” Dr. Wells said. “Rheumatologists are going to tell patients first about their disease, ask how their medication is working and how their health is. Then, if we have time, we’ll cover the transition pieces, and what it boils down to is that they just don’t have time.”
The respondents indicated an interest in technology that can help their education and transition, such as patient portals, telehealth, and smartphone apps.
While 66.4% of respondents said they would attend an in-person health care appointment to learn skills for transitioning to adult care, 74.5% would attend a telehealth appointment, and 77.2% would complete a structured program within a patient portal.
Dr. Wells said she doesn’t see many of the health care system pressures easing up to allow rheumatologists more time for transition care, but she sees an opportunity for organizations, such as the Arthritis Foundation or Lupus Foundation of America, to step in and help.
“It is a matter of being creative and that, ultimately, is the barrier: Whose job is it to make it happen?” she said. “That’s where some other groups are going to need to be advocates.”
Another notable set of findings from this research was the need for young patients’ access to mental health and sexual/reproductive health services. Just over two-thirds of respondents preferred to discuss these topics with their rheumatology team, but only 59.1% felt comfortable starting the conversation about mental health, and only 47.4% felt comfortable broaching the topic of reproductive/sexual health. Even more patients preferred discussing use of drugs and alcohol with their health care team (71.5%), but more patients also felt comfortable initiating that discussion (72.2%).
“It may be that somebody who’s trained to address those issues, especially the mental health piece, may be more appropriate to have that role, and that’s part of the transition, too, these other larger life issues,” Dr. Wells said. “One of the benefits of going to an adult rheumatologist is that they are the most knowledgeable and prepared to help you with those topics.”
None of the individuals quoted in this story had any disclosures to report.
The inadequacies and challenges of transitioning pediatric rheumatology patients to adult care were highlighted in several research studies shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
“Not surprisingly, these studies demonstrate that transition challenges remain pervasive,” Rebecca Sadun, MD, PhD, who was not involved in any of the research, said in an interview. Nevertheless, she pointed out that one of the studies showed that eight of nine sites participating in one of the studies had at least developed a formal transition policy, and three were able to fully integrate that policy into their health care system despite the ongoing pandemic.
In that study, Joyce Chang, MD, of the Children’s Hospital of Philadelphia and colleagues used structured interviews and then quantitative research to explore processes for transition polices across nine rheumatology sites. Aside from the three that had already implemented their policies, three others were preparing implementation. The other three withdrew because of COVID-19. None of the sites had reached sustainment phase. Six of the sites had access to a social work network, and two sites had fewer than four providers.
The authors found that a higher level of change efficacy or change commitment using the Organizational Readiness for Implementing Change framework did not correspond with reaching implementation.
“The first sites to reach implementation had access to [information technology] support and involved nursing, though this was not sufficient or necessary,” the authors wrote. They noted the need for strategies to reduce the burden of data collection to improve the resilience of implementation efforts against health care system stress.
Who more often transitions to adult care?
Effective transition policies can help reduce the likelihood of young patients falling through the cracks as they grow from adolescence into young adulthood, especially those at highest risk for losing continuity of care.
“Young adults who are both medically and socially complex are at highest risk,” said Dr. Sadun, an assistant professor of adult and pediatric rheumatology at Duke University, Durham, N.C. “This is especially true for patients with systemic illnesses, patients requiring biologic medications, and patients with custody, transportation, and financial barriers.”
Research led by Emily A. Smitherman, MD, an assistant professor of pediatric rheumatology at Children’s of Alabama in Birmingham, looked more closely at who is and is not transitioning their care. The researchers analyzed retrospective data from the CARRA Registry, including the Long-Term Follow-Up Call Registry, through December 2019. Among 1,311 patients with inactive status, 537 of these patients had juvenile idiopathic arthritis and were aged at least 18 years. Only 186 of those patients, however, had data in the Long-Term Follow-Up Registry. Patients who were Black or had lower income were less likely to have data in the Long-Term Follow-Up Registry.
Just over half the patients in the long-term registry had transferred their care to an adult rheumatologist, and 83% overall were under the care of any physician. Patients who transferred their care were significantly more likely to have private insurance (87% vs. 70%; P = .009) and were more likely to be full-time students (74% vs. 58%; P = .036).
The researchers found no association between patients’ disease status at their last CARRA Registry visit and a successful transition to adult care. However, those who had transferred care to an adult rheumatologist tended to have a higher median level of pain (4 vs. 2 on a scale of 0-10) and more disease activity (3 vs. 1 on 0-10 scale) than did those who had not transferred care (P = .022 and P = .011, respectively). A higher proportion of those who transferred care had also experienced morning stiffness over the past week (49% vs. 30%; P = .015).
How young adults prefer to learn transition skills
The third study aimed to better understand the experience and preferences of young adults themselves as they transitioned from pediatric to adult care. Kristine Carandang, PhD, a postdoctoral scholar at the University of California, San Diego, and colleagues first conducted focus groups with 39 adolescents and young adults, ages 16-28 years, who had rheumatic conditions. Using the qualitative data from the focus groups, they designed a survey to capture quantitative data on young patients’ experiences.
“What we’re always trying to work on is, how do we bring that youth voice more clearly into the research literature?” Courtney K. Wells, PhD, MSW, an assistant professor of social work at the University of Wisconsin–River Falls, said in an interview. She noted that both she and Dr. Carandang were patients with rheumatic diseases, so they had lived and grown up with disease themselves and then become researchers.
“We have the information that’s in the literature, but then we also both work with youth in a couple different ways, and what we hear from youth is what we heard in our paper, but it isn’t all represented in the literature,” Dr. Wells said. That disconnect is why they also included two young adults as coauthors in the study.
“As much as we appreciate the model of the six components [of health care transition], we recognize that the youth voice isn’t represented very well,” Dr. Wells said. “The way it’s written is more for doctors and policy makers and targeted for the health care system rather than the young people themselves.”
Their research bore that out. Among 137 survey respondents, aged 18-28 years, the vast majority (89%) were women and most (75%) were White. Half the patients (50%) had a diagnosis of lupus.
“For 9 out of 11 self-management and self-advocacy skills examined, there was a significant difference between how adolescent and young adult patients experienced learning self- management skills versus how they would have preferred to learn the skills,” the researchers concluded. “Overall, adolescent and young adult patients most frequently learned about transition skills from their parents. Most participants would have preferred to learn these skills from their rheumatology team.”
For example, 46.7% of the respondents learned how to communicate their medical history from their parents, but 48.5% would have preferred to learn that from their rheumatology team. Only a quarter (24.8%) had learned that skill from their health care team.
“For most of these skills, they were getting that information from their parents, which is concerning because their parents don’t necessarily have information that is accurate,” Dr. Wells said. “Their parents managed their health care, and they taught them to do it the way they were doing it.”
Just over one-third of respondents said they learned from their parents how to track their symptoms so they could answer the rheumatologists’ questions. Only one in five respondents (20.4%) had learned this skill from their rheumatology team, but 41.2% would have preferred hearing it from their health care team, compared with 22.1% who preferred learning it from their parents.
Nearly half the respondents reported learning from their parents how to advocate for themselves when dissatisfied with their care or symptoms (49.6%) and how to talk to the office staff to make appointments, fill out paperwork, and access health records (47.4%). Just over half (51.5%) would have preferred to learn about office communication from their health care team. Preferences on self-advocacy were split between learning from parents (36.8%) and learning from their health care team (31.6%).
An opportunity for other organizations to support transition
The researchers noted that education did not necessarily need to come only from rheumatologists. Other health care professionals, including nurses and social workers, could help young patients develop skills as well.
“They said they’re also open to talking to other people, but they want their rheumatologist to lead the whole process,” Dr. Wells said. While reimbursement gaps may have presented a barrier in the past, Dr. Wells said that current billing codes have removed that obstacle, allowing physicians to bill for discussing transition skills and care.
“Largely, it’s a time issue,” Dr. Wells said. “Rheumatologists are going to tell patients first about their disease, ask how their medication is working and how their health is. Then, if we have time, we’ll cover the transition pieces, and what it boils down to is that they just don’t have time.”
The respondents indicated an interest in technology that can help their education and transition, such as patient portals, telehealth, and smartphone apps.
While 66.4% of respondents said they would attend an in-person health care appointment to learn skills for transitioning to adult care, 74.5% would attend a telehealth appointment, and 77.2% would complete a structured program within a patient portal.
Dr. Wells said she doesn’t see many of the health care system pressures easing up to allow rheumatologists more time for transition care, but she sees an opportunity for organizations, such as the Arthritis Foundation or Lupus Foundation of America, to step in and help.
“It is a matter of being creative and that, ultimately, is the barrier: Whose job is it to make it happen?” she said. “That’s where some other groups are going to need to be advocates.”
Another notable set of findings from this research was the need for young patients’ access to mental health and sexual/reproductive health services. Just over two-thirds of respondents preferred to discuss these topics with their rheumatology team, but only 59.1% felt comfortable starting the conversation about mental health, and only 47.4% felt comfortable broaching the topic of reproductive/sexual health. Even more patients preferred discussing use of drugs and alcohol with their health care team (71.5%), but more patients also felt comfortable initiating that discussion (72.2%).
“It may be that somebody who’s trained to address those issues, especially the mental health piece, may be more appropriate to have that role, and that’s part of the transition, too, these other larger life issues,” Dr. Wells said. “One of the benefits of going to an adult rheumatologist is that they are the most knowledgeable and prepared to help you with those topics.”
None of the individuals quoted in this story had any disclosures to report.
The inadequacies and challenges of transitioning pediatric rheumatology patients to adult care were highlighted in several research studies shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
“Not surprisingly, these studies demonstrate that transition challenges remain pervasive,” Rebecca Sadun, MD, PhD, who was not involved in any of the research, said in an interview. Nevertheless, she pointed out that one of the studies showed that eight of nine sites participating in one of the studies had at least developed a formal transition policy, and three were able to fully integrate that policy into their health care system despite the ongoing pandemic.
In that study, Joyce Chang, MD, of the Children’s Hospital of Philadelphia and colleagues used structured interviews and then quantitative research to explore processes for transition polices across nine rheumatology sites. Aside from the three that had already implemented their policies, three others were preparing implementation. The other three withdrew because of COVID-19. None of the sites had reached sustainment phase. Six of the sites had access to a social work network, and two sites had fewer than four providers.
The authors found that a higher level of change efficacy or change commitment using the Organizational Readiness for Implementing Change framework did not correspond with reaching implementation.
“The first sites to reach implementation had access to [information technology] support and involved nursing, though this was not sufficient or necessary,” the authors wrote. They noted the need for strategies to reduce the burden of data collection to improve the resilience of implementation efforts against health care system stress.
Who more often transitions to adult care?
Effective transition policies can help reduce the likelihood of young patients falling through the cracks as they grow from adolescence into young adulthood, especially those at highest risk for losing continuity of care.
“Young adults who are both medically and socially complex are at highest risk,” said Dr. Sadun, an assistant professor of adult and pediatric rheumatology at Duke University, Durham, N.C. “This is especially true for patients with systemic illnesses, patients requiring biologic medications, and patients with custody, transportation, and financial barriers.”
Research led by Emily A. Smitherman, MD, an assistant professor of pediatric rheumatology at Children’s of Alabama in Birmingham, looked more closely at who is and is not transitioning their care. The researchers analyzed retrospective data from the CARRA Registry, including the Long-Term Follow-Up Call Registry, through December 2019. Among 1,311 patients with inactive status, 537 of these patients had juvenile idiopathic arthritis and were aged at least 18 years. Only 186 of those patients, however, had data in the Long-Term Follow-Up Registry. Patients who were Black or had lower income were less likely to have data in the Long-Term Follow-Up Registry.
Just over half the patients in the long-term registry had transferred their care to an adult rheumatologist, and 83% overall were under the care of any physician. Patients who transferred their care were significantly more likely to have private insurance (87% vs. 70%; P = .009) and were more likely to be full-time students (74% vs. 58%; P = .036).
The researchers found no association between patients’ disease status at their last CARRA Registry visit and a successful transition to adult care. However, those who had transferred care to an adult rheumatologist tended to have a higher median level of pain (4 vs. 2 on a scale of 0-10) and more disease activity (3 vs. 1 on 0-10 scale) than did those who had not transferred care (P = .022 and P = .011, respectively). A higher proportion of those who transferred care had also experienced morning stiffness over the past week (49% vs. 30%; P = .015).
How young adults prefer to learn transition skills
The third study aimed to better understand the experience and preferences of young adults themselves as they transitioned from pediatric to adult care. Kristine Carandang, PhD, a postdoctoral scholar at the University of California, San Diego, and colleagues first conducted focus groups with 39 adolescents and young adults, ages 16-28 years, who had rheumatic conditions. Using the qualitative data from the focus groups, they designed a survey to capture quantitative data on young patients’ experiences.
“What we’re always trying to work on is, how do we bring that youth voice more clearly into the research literature?” Courtney K. Wells, PhD, MSW, an assistant professor of social work at the University of Wisconsin–River Falls, said in an interview. She noted that both she and Dr. Carandang were patients with rheumatic diseases, so they had lived and grown up with disease themselves and then become researchers.
“We have the information that’s in the literature, but then we also both work with youth in a couple different ways, and what we hear from youth is what we heard in our paper, but it isn’t all represented in the literature,” Dr. Wells said. That disconnect is why they also included two young adults as coauthors in the study.
“As much as we appreciate the model of the six components [of health care transition], we recognize that the youth voice isn’t represented very well,” Dr. Wells said. “The way it’s written is more for doctors and policy makers and targeted for the health care system rather than the young people themselves.”
Their research bore that out. Among 137 survey respondents, aged 18-28 years, the vast majority (89%) were women and most (75%) were White. Half the patients (50%) had a diagnosis of lupus.
“For 9 out of 11 self-management and self-advocacy skills examined, there was a significant difference between how adolescent and young adult patients experienced learning self- management skills versus how they would have preferred to learn the skills,” the researchers concluded. “Overall, adolescent and young adult patients most frequently learned about transition skills from their parents. Most participants would have preferred to learn these skills from their rheumatology team.”
For example, 46.7% of the respondents learned how to communicate their medical history from their parents, but 48.5% would have preferred to learn that from their rheumatology team. Only a quarter (24.8%) had learned that skill from their health care team.
“For most of these skills, they were getting that information from their parents, which is concerning because their parents don’t necessarily have information that is accurate,” Dr. Wells said. “Their parents managed their health care, and they taught them to do it the way they were doing it.”
Just over one-third of respondents said they learned from their parents how to track their symptoms so they could answer the rheumatologists’ questions. Only one in five respondents (20.4%) had learned this skill from their rheumatology team, but 41.2% would have preferred hearing it from their health care team, compared with 22.1% who preferred learning it from their parents.
Nearly half the respondents reported learning from their parents how to advocate for themselves when dissatisfied with their care or symptoms (49.6%) and how to talk to the office staff to make appointments, fill out paperwork, and access health records (47.4%). Just over half (51.5%) would have preferred to learn about office communication from their health care team. Preferences on self-advocacy were split between learning from parents (36.8%) and learning from their health care team (31.6%).
An opportunity for other organizations to support transition
The researchers noted that education did not necessarily need to come only from rheumatologists. Other health care professionals, including nurses and social workers, could help young patients develop skills as well.
“They said they’re also open to talking to other people, but they want their rheumatologist to lead the whole process,” Dr. Wells said. While reimbursement gaps may have presented a barrier in the past, Dr. Wells said that current billing codes have removed that obstacle, allowing physicians to bill for discussing transition skills and care.
“Largely, it’s a time issue,” Dr. Wells said. “Rheumatologists are going to tell patients first about their disease, ask how their medication is working and how their health is. Then, if we have time, we’ll cover the transition pieces, and what it boils down to is that they just don’t have time.”
The respondents indicated an interest in technology that can help their education and transition, such as patient portals, telehealth, and smartphone apps.
While 66.4% of respondents said they would attend an in-person health care appointment to learn skills for transitioning to adult care, 74.5% would attend a telehealth appointment, and 77.2% would complete a structured program within a patient portal.
Dr. Wells said she doesn’t see many of the health care system pressures easing up to allow rheumatologists more time for transition care, but she sees an opportunity for organizations, such as the Arthritis Foundation or Lupus Foundation of America, to step in and help.
“It is a matter of being creative and that, ultimately, is the barrier: Whose job is it to make it happen?” she said. “That’s where some other groups are going to need to be advocates.”
Another notable set of findings from this research was the need for young patients’ access to mental health and sexual/reproductive health services. Just over two-thirds of respondents preferred to discuss these topics with their rheumatology team, but only 59.1% felt comfortable starting the conversation about mental health, and only 47.4% felt comfortable broaching the topic of reproductive/sexual health. Even more patients preferred discussing use of drugs and alcohol with their health care team (71.5%), but more patients also felt comfortable initiating that discussion (72.2%).
“It may be that somebody who’s trained to address those issues, especially the mental health piece, may be more appropriate to have that role, and that’s part of the transition, too, these other larger life issues,” Dr. Wells said. “One of the benefits of going to an adult rheumatologist is that they are the most knowledgeable and prepared to help you with those topics.”
None of the individuals quoted in this story had any disclosures to report.
FROM CARRA 2021
TNF inhibitors linked to threefold increased risk of psoriasis in JIA patients
Children with juvenile idiopathic arthritis (JIA) have nearly triple the risk of developing psoriasis after they begin therapy with tumor necrosis factor (TNF) inhibitors, according to preliminary research shared at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA).
Previous retrospective research at the Children’s Hospital of Philadelphia had found similar results, so the goal of this study was to look at prospectively collected data from the CARRA registry that represented a broader patient population than that of a single institution, lead author Yongdong (Dan) Zhao, MD, PhD, assistant professor of rheumatology at the University of Washington, Seattle, and pediatric rheumatologist at Seattle Children’s Hospital, said in an interview.
“The take-home message is that we confirmed this finding, and everyone who prescribed this should be aware [of the risk] and also make the family aware because often the family just thinks this is eczema and they self-manage without reporting it to the physician,” Dr. Zhao said. He advised that physicians look for evidence of psoriasis at visits and, depending on the severity, be prepared with a management plan if needed.
The researchers analyzed data from patients with JIA enrolled in the CARRA registry during June 2015–January 2020. They excluded patients with a diagnosis of inflammatory bowel disease, psoriasis at or before their JIA diagnosis, or missing data regarding the timing of psoriasis diagnosis or starting TNF inhibitors.
Among 8,222 children (29% of whom were male), just over half (54%) had ever used TNF inhibitors. Most of the patients (76%) were White, and their average age at the time of JIA diagnosis was 7 years. Compared to those with no exposure to the drugs, patients who had ever been prescribed a TNF inhibitor were three times more likely to receive a diagnosis of psoriasis afterward (unadjusted hazard ratio [HR] = 3.01; P < .01). The risk dropped only slightly (HR = 2.93; P < .01) after adjustment for gender, race, family history of psoriasis, initial International League of Associations for Rheumatology classification category, and ever having taken methotrexate.
Overall median follow-up time for the cohort was 46.7 months. The overall incidence of psoriasis in the cohort was 5.28 cases per 1,000 person-years, which split into 3.24 cases for those never exposed to TNF inhibitors and 8.49 for those ever exposed. The incidence was similar (8.31 cases per 1,000 person-years) after only the first course of TNF inhibitors.
The risk appeared greatest for adalimumab, with an incidence of 12.2 cases per 1,000 person-years after a first course in TNF inhibitor-naive patients, compared to etanercept (6.31 cases) and infliximab (9.04 cases), which did not reach statistical significance. Incidence for cumulative exposure was greater for adalimumab: 13.17 cases per 1,000 person-years, compared to 5.19 cases for etanercept and 8.77 cases for infliximab.
TNF inhibitors are first-line biologic treatment for JIA and have a longer track record for safety and effectiveness than that of newer drugs, Dr. Zhao said. They’re also commonly used for children with psoriasis, said Pamela Weiss, MD, associate professor of pediatrics and epidemiology, at the University of Pennsylvania, Philadelphia, and clinical research director of rheumatology at Children’s Hospital of Philadelphia. She was not involved in the study.
“TNF inhibitors are an incredibly useful class of medications for children with arthritis, including psoriatic arthritis,” Dr. Weiss said in an interview. “I don’t think these findings impact the risk-benefit profile of TNF inhibitors as paradoxical psoriasis is a known side effect of the medication and something most of us already counsel our families and patients about before starting a TNF inhibitor medication.”
Dr. Zhao likewise did not think the findings changed these drugs’ benefit-risk profile as long as people are aware of it. If the psoriasis is mild, he said, it’s often possible to continue the TNF inhibitor therapy along with a topical medication for the psoriasis, “but if it’s really severe, or by patient preference, you may have to switch to a different TNF inhibitor or stop it,” he said. Occasionally, he has added an additional biologic to treat the psoriasis because the underlying JIA disease in the patient couldn’t be controlled without the TNF inhibitor.
Dr. Weiss similarly said that management will depend on the severity and on shared decision-making between the physician, patient, and family.
“If it’s a small area, it can often be managed with topical corticosteroids,” Dr. Weiss said. “If it involves a large area of the body or severely affects the scalp, then stopping the TNF inhibitor therapy and starting another therapy that targets a different pathway might be considered.”
The research was funded by CARRA. Dr. Zhao has received research funding from Bristol-Myers Squibb and has consulted for Novartis. Dr. Weiss has received consulting fees from Pfizer and Lilly.
Children with juvenile idiopathic arthritis (JIA) have nearly triple the risk of developing psoriasis after they begin therapy with tumor necrosis factor (TNF) inhibitors, according to preliminary research shared at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA).
Previous retrospective research at the Children’s Hospital of Philadelphia had found similar results, so the goal of this study was to look at prospectively collected data from the CARRA registry that represented a broader patient population than that of a single institution, lead author Yongdong (Dan) Zhao, MD, PhD, assistant professor of rheumatology at the University of Washington, Seattle, and pediatric rheumatologist at Seattle Children’s Hospital, said in an interview.
“The take-home message is that we confirmed this finding, and everyone who prescribed this should be aware [of the risk] and also make the family aware because often the family just thinks this is eczema and they self-manage without reporting it to the physician,” Dr. Zhao said. He advised that physicians look for evidence of psoriasis at visits and, depending on the severity, be prepared with a management plan if needed.
The researchers analyzed data from patients with JIA enrolled in the CARRA registry during June 2015–January 2020. They excluded patients with a diagnosis of inflammatory bowel disease, psoriasis at or before their JIA diagnosis, or missing data regarding the timing of psoriasis diagnosis or starting TNF inhibitors.
Among 8,222 children (29% of whom were male), just over half (54%) had ever used TNF inhibitors. Most of the patients (76%) were White, and their average age at the time of JIA diagnosis was 7 years. Compared to those with no exposure to the drugs, patients who had ever been prescribed a TNF inhibitor were three times more likely to receive a diagnosis of psoriasis afterward (unadjusted hazard ratio [HR] = 3.01; P < .01). The risk dropped only slightly (HR = 2.93; P < .01) after adjustment for gender, race, family history of psoriasis, initial International League of Associations for Rheumatology classification category, and ever having taken methotrexate.
Overall median follow-up time for the cohort was 46.7 months. The overall incidence of psoriasis in the cohort was 5.28 cases per 1,000 person-years, which split into 3.24 cases for those never exposed to TNF inhibitors and 8.49 for those ever exposed. The incidence was similar (8.31 cases per 1,000 person-years) after only the first course of TNF inhibitors.
The risk appeared greatest for adalimumab, with an incidence of 12.2 cases per 1,000 person-years after a first course in TNF inhibitor-naive patients, compared to etanercept (6.31 cases) and infliximab (9.04 cases), which did not reach statistical significance. Incidence for cumulative exposure was greater for adalimumab: 13.17 cases per 1,000 person-years, compared to 5.19 cases for etanercept and 8.77 cases for infliximab.
TNF inhibitors are first-line biologic treatment for JIA and have a longer track record for safety and effectiveness than that of newer drugs, Dr. Zhao said. They’re also commonly used for children with psoriasis, said Pamela Weiss, MD, associate professor of pediatrics and epidemiology, at the University of Pennsylvania, Philadelphia, and clinical research director of rheumatology at Children’s Hospital of Philadelphia. She was not involved in the study.
“TNF inhibitors are an incredibly useful class of medications for children with arthritis, including psoriatic arthritis,” Dr. Weiss said in an interview. “I don’t think these findings impact the risk-benefit profile of TNF inhibitors as paradoxical psoriasis is a known side effect of the medication and something most of us already counsel our families and patients about before starting a TNF inhibitor medication.”
Dr. Zhao likewise did not think the findings changed these drugs’ benefit-risk profile as long as people are aware of it. If the psoriasis is mild, he said, it’s often possible to continue the TNF inhibitor therapy along with a topical medication for the psoriasis, “but if it’s really severe, or by patient preference, you may have to switch to a different TNF inhibitor or stop it,” he said. Occasionally, he has added an additional biologic to treat the psoriasis because the underlying JIA disease in the patient couldn’t be controlled without the TNF inhibitor.
Dr. Weiss similarly said that management will depend on the severity and on shared decision-making between the physician, patient, and family.
“If it’s a small area, it can often be managed with topical corticosteroids,” Dr. Weiss said. “If it involves a large area of the body or severely affects the scalp, then stopping the TNF inhibitor therapy and starting another therapy that targets a different pathway might be considered.”
The research was funded by CARRA. Dr. Zhao has received research funding from Bristol-Myers Squibb and has consulted for Novartis. Dr. Weiss has received consulting fees from Pfizer and Lilly.
Children with juvenile idiopathic arthritis (JIA) have nearly triple the risk of developing psoriasis after they begin therapy with tumor necrosis factor (TNF) inhibitors, according to preliminary research shared at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA).
Previous retrospective research at the Children’s Hospital of Philadelphia had found similar results, so the goal of this study was to look at prospectively collected data from the CARRA registry that represented a broader patient population than that of a single institution, lead author Yongdong (Dan) Zhao, MD, PhD, assistant professor of rheumatology at the University of Washington, Seattle, and pediatric rheumatologist at Seattle Children’s Hospital, said in an interview.
“The take-home message is that we confirmed this finding, and everyone who prescribed this should be aware [of the risk] and also make the family aware because often the family just thinks this is eczema and they self-manage without reporting it to the physician,” Dr. Zhao said. He advised that physicians look for evidence of psoriasis at visits and, depending on the severity, be prepared with a management plan if needed.
The researchers analyzed data from patients with JIA enrolled in the CARRA registry during June 2015–January 2020. They excluded patients with a diagnosis of inflammatory bowel disease, psoriasis at or before their JIA diagnosis, or missing data regarding the timing of psoriasis diagnosis or starting TNF inhibitors.
Among 8,222 children (29% of whom were male), just over half (54%) had ever used TNF inhibitors. Most of the patients (76%) were White, and their average age at the time of JIA diagnosis was 7 years. Compared to those with no exposure to the drugs, patients who had ever been prescribed a TNF inhibitor were three times more likely to receive a diagnosis of psoriasis afterward (unadjusted hazard ratio [HR] = 3.01; P < .01). The risk dropped only slightly (HR = 2.93; P < .01) after adjustment for gender, race, family history of psoriasis, initial International League of Associations for Rheumatology classification category, and ever having taken methotrexate.
Overall median follow-up time for the cohort was 46.7 months. The overall incidence of psoriasis in the cohort was 5.28 cases per 1,000 person-years, which split into 3.24 cases for those never exposed to TNF inhibitors and 8.49 for those ever exposed. The incidence was similar (8.31 cases per 1,000 person-years) after only the first course of TNF inhibitors.
The risk appeared greatest for adalimumab, with an incidence of 12.2 cases per 1,000 person-years after a first course in TNF inhibitor-naive patients, compared to etanercept (6.31 cases) and infliximab (9.04 cases), which did not reach statistical significance. Incidence for cumulative exposure was greater for adalimumab: 13.17 cases per 1,000 person-years, compared to 5.19 cases for etanercept and 8.77 cases for infliximab.
TNF inhibitors are first-line biologic treatment for JIA and have a longer track record for safety and effectiveness than that of newer drugs, Dr. Zhao said. They’re also commonly used for children with psoriasis, said Pamela Weiss, MD, associate professor of pediatrics and epidemiology, at the University of Pennsylvania, Philadelphia, and clinical research director of rheumatology at Children’s Hospital of Philadelphia. She was not involved in the study.
“TNF inhibitors are an incredibly useful class of medications for children with arthritis, including psoriatic arthritis,” Dr. Weiss said in an interview. “I don’t think these findings impact the risk-benefit profile of TNF inhibitors as paradoxical psoriasis is a known side effect of the medication and something most of us already counsel our families and patients about before starting a TNF inhibitor medication.”
Dr. Zhao likewise did not think the findings changed these drugs’ benefit-risk profile as long as people are aware of it. If the psoriasis is mild, he said, it’s often possible to continue the TNF inhibitor therapy along with a topical medication for the psoriasis, “but if it’s really severe, or by patient preference, you may have to switch to a different TNF inhibitor or stop it,” he said. Occasionally, he has added an additional biologic to treat the psoriasis because the underlying JIA disease in the patient couldn’t be controlled without the TNF inhibitor.
Dr. Weiss similarly said that management will depend on the severity and on shared decision-making between the physician, patient, and family.
“If it’s a small area, it can often be managed with topical corticosteroids,” Dr. Weiss said. “If it involves a large area of the body or severely affects the scalp, then stopping the TNF inhibitor therapy and starting another therapy that targets a different pathway might be considered.”
The research was funded by CARRA. Dr. Zhao has received research funding from Bristol-Myers Squibb and has consulted for Novartis. Dr. Weiss has received consulting fees from Pfizer and Lilly.
FROM CARRA 2021