User login
FDA approves mirabegron to treat pediatric NDO
The Food and Drug Administration has expanded the indication for mirabegron (Myrbetriq/Myrbetriq Granules) to treat neurogenic detrusor overactivity (NDO), a bladder dysfunction related to neurologic impairment, in children aged 3 years and older.
This comes 1 year after the FDA approved solifenacin succinate, the first treatment of NDO in pediatric patients aged 2 years and older.
The approval of the drug for these new indications is a “positive step” for the treatment of NDO in young patients, Christine P. Nguyen, MD, director of the FDA’s Division of Urology, Obstetrics, and Gynecology, said in an FDA statement.
“Mirabegron, the active ingredient in Myrbetriq and Myrbetriq Granules, works by a different mechanism of action from the currently approved treatments, providing a new treatment option for these young patients. We remain committed to facilitating the development and approval of safe and effective therapies for pediatric NDO patients,” Dr. Nguyen said.
NDO is a bladder dysfunction that frequently occurs in patients with congenital conditions, such as spina bifida. It also occurs in people who suffer from other diseases or injuries of the nervous system, such as multiple sclerosis and spinal cord injury. Symptoms of the condition include urinary frequency and incontinence.
The condition is characterized by the overactivity of the bladder wall muscle, which is normally relaxed to allow storage of urine. Irregular bladder muscle contraction increases storage pressure and decreases the amount of urine the bladder can hold. This can also put the upper urinary tract at risk for deterioration and cause permanent damage to the kidneys.
The effectiveness of Myrbetriq and Myrbetriq Granules for pediatric NDO was determined in a study of 86 children and adolescents aged 3-17 years. The researchers found that after 24 weeks of treatment, the drug improved the patients’ bladder capacity, reduced the number of bladder wall muscle contractions, and improved the volume of urine that could be held. It also reduced the daily number of episodes of leakage.
Side effects of Myrbetriq and Myrbetriq Granules include urinary tract infection, cold symptoms, angioedema, constipation, and headache. The FDA said the drug may also increase blood pressure and may worsen blood pressure in patients who have a history of hypertension.
The FDA approved mirabegron in 2012 to treat overactive bladder in adults.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the indication for mirabegron (Myrbetriq/Myrbetriq Granules) to treat neurogenic detrusor overactivity (NDO), a bladder dysfunction related to neurologic impairment, in children aged 3 years and older.
This comes 1 year after the FDA approved solifenacin succinate, the first treatment of NDO in pediatric patients aged 2 years and older.
The approval of the drug for these new indications is a “positive step” for the treatment of NDO in young patients, Christine P. Nguyen, MD, director of the FDA’s Division of Urology, Obstetrics, and Gynecology, said in an FDA statement.
“Mirabegron, the active ingredient in Myrbetriq and Myrbetriq Granules, works by a different mechanism of action from the currently approved treatments, providing a new treatment option for these young patients. We remain committed to facilitating the development and approval of safe and effective therapies for pediatric NDO patients,” Dr. Nguyen said.
NDO is a bladder dysfunction that frequently occurs in patients with congenital conditions, such as spina bifida. It also occurs in people who suffer from other diseases or injuries of the nervous system, such as multiple sclerosis and spinal cord injury. Symptoms of the condition include urinary frequency and incontinence.
The condition is characterized by the overactivity of the bladder wall muscle, which is normally relaxed to allow storage of urine. Irregular bladder muscle contraction increases storage pressure and decreases the amount of urine the bladder can hold. This can also put the upper urinary tract at risk for deterioration and cause permanent damage to the kidneys.
The effectiveness of Myrbetriq and Myrbetriq Granules for pediatric NDO was determined in a study of 86 children and adolescents aged 3-17 years. The researchers found that after 24 weeks of treatment, the drug improved the patients’ bladder capacity, reduced the number of bladder wall muscle contractions, and improved the volume of urine that could be held. It also reduced the daily number of episodes of leakage.
Side effects of Myrbetriq and Myrbetriq Granules include urinary tract infection, cold symptoms, angioedema, constipation, and headache. The FDA said the drug may also increase blood pressure and may worsen blood pressure in patients who have a history of hypertension.
The FDA approved mirabegron in 2012 to treat overactive bladder in adults.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the indication for mirabegron (Myrbetriq/Myrbetriq Granules) to treat neurogenic detrusor overactivity (NDO), a bladder dysfunction related to neurologic impairment, in children aged 3 years and older.
This comes 1 year after the FDA approved solifenacin succinate, the first treatment of NDO in pediatric patients aged 2 years and older.
The approval of the drug for these new indications is a “positive step” for the treatment of NDO in young patients, Christine P. Nguyen, MD, director of the FDA’s Division of Urology, Obstetrics, and Gynecology, said in an FDA statement.
“Mirabegron, the active ingredient in Myrbetriq and Myrbetriq Granules, works by a different mechanism of action from the currently approved treatments, providing a new treatment option for these young patients. We remain committed to facilitating the development and approval of safe and effective therapies for pediatric NDO patients,” Dr. Nguyen said.
NDO is a bladder dysfunction that frequently occurs in patients with congenital conditions, such as spina bifida. It also occurs in people who suffer from other diseases or injuries of the nervous system, such as multiple sclerosis and spinal cord injury. Symptoms of the condition include urinary frequency and incontinence.
The condition is characterized by the overactivity of the bladder wall muscle, which is normally relaxed to allow storage of urine. Irregular bladder muscle contraction increases storage pressure and decreases the amount of urine the bladder can hold. This can also put the upper urinary tract at risk for deterioration and cause permanent damage to the kidneys.
The effectiveness of Myrbetriq and Myrbetriq Granules for pediatric NDO was determined in a study of 86 children and adolescents aged 3-17 years. The researchers found that after 24 weeks of treatment, the drug improved the patients’ bladder capacity, reduced the number of bladder wall muscle contractions, and improved the volume of urine that could be held. It also reduced the daily number of episodes of leakage.
Side effects of Myrbetriq and Myrbetriq Granules include urinary tract infection, cold symptoms, angioedema, constipation, and headache. The FDA said the drug may also increase blood pressure and may worsen blood pressure in patients who have a history of hypertension.
The FDA approved mirabegron in 2012 to treat overactive bladder in adults.
A version of this article first appeared on Medscape.com.
Success in achondroplasia spurs testing vosoritide in more growth disorders
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
FROM ENDO 2021
Conservative or surgical management for that shoulder dislocation?
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
PRACTICE RECOMMENDATIONS
› Start with conservative management of shoulder dislocation in patients older than 30 years and those with uncomplicated injuries. B
› Discourage strict immobilization; its utility is debated and it may not change outcomes. B
› Recommend a progressive rehabilitative program after the initial acute shoulder injury. B
› Consider surgical management for patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability or for the highly physically active individual. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Helping parents and children deal with a child’s limb deformity
After 15 years of limping and a gradual downhill slide in mobility, recreational walking had become uncomfortable enough that I’ve decided to shed my proudly worn cloak of denial and seek help. Even I could see that the x-ray made a total knee replacement the only option for some return to near normalcy. Scheduling a total knee replacement became a no-brainer.
My decision to accept the risks to reap the benefits of surgery is small potatoes compared with the decisions that the parents of a child born with a deformed lower extremity must face. In the Family Partnerships section of the February 2021 issue of Pediatrics you will find a heart-wrenching story of a family who embarked on what turned out to be painful and frustrating journey to lengthen their daughter’s congenitally deficient leg. In their own words, the mother and daughter describe how neither of them were prepared for the pain and life-altering complications the daughter has endured. Influenced by the optimism exuded by surgeons, the family gave little thought to the magnitude of the decision they were being asked to make. One has to wonder in retrospect if a well-timed amputation and prosthesis might have been a better decision. However, the thought of removing an extremity, even one that isn’t fully functional, is not one that most of us like to consider.
Over the last several decades I have read stories about people – usually athletes – born with short or deformed lower extremities who have faced the decision of amputation. I recall one college-age young man who despite his deformity and with the help of a prosthesis was a competitive multisport athlete. However, it became clear that his deformed foot was preventing him from accessing the most advanced prosthetic technology. Although he was highly motivated, he described his struggle with the decision to part with a portion of his body that despite its appearance and dysfunction had been with him since birth. On the other hand, I have read stories of young people who had become so frustrated by their deformity that they were more than eager to undergo amputation despite the concerns of their parents.
Early in my career I encountered a 3-year-old with phocomelia whose family was visiting from out of town and had come to our clinic because his older sibling was sick. The youngster, as I recall, had only one complete extremity, an arm. Like most 3-year-olds, he was driven to explore at breakneck speed. I will never forget watching him streak back and forth the length of our linoleum covered hallway like a crab skittering along the beach. His mother described how she and his well-meaning physicians were struggling unsuccessfully to get him to accept prostheses. Later I learned that his resistance is shared by many of the survivors of the thalidomide disaster who felt that the most frustrating period in their lives came when, again well-meaning, caregivers had tried to make them look and function more normally by fitting them with prostheses.
These anecdotal observations make clear a philosophy that we should have already internalized. In most clinic decisions the patient, pretty much regardless of age, should be a full participant in the process. And, to do this the patient and his or her family must be as informed as possible. Managing the aftermath of a traumatic amputation presents it own special set of challenges, but when it comes to elective amputation or prosthetic application for a congenital deficiency it is dangerous for us to insert our personal bias into the decision making.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
After 15 years of limping and a gradual downhill slide in mobility, recreational walking had become uncomfortable enough that I’ve decided to shed my proudly worn cloak of denial and seek help. Even I could see that the x-ray made a total knee replacement the only option for some return to near normalcy. Scheduling a total knee replacement became a no-brainer.
My decision to accept the risks to reap the benefits of surgery is small potatoes compared with the decisions that the parents of a child born with a deformed lower extremity must face. In the Family Partnerships section of the February 2021 issue of Pediatrics you will find a heart-wrenching story of a family who embarked on what turned out to be painful and frustrating journey to lengthen their daughter’s congenitally deficient leg. In their own words, the mother and daughter describe how neither of them were prepared for the pain and life-altering complications the daughter has endured. Influenced by the optimism exuded by surgeons, the family gave little thought to the magnitude of the decision they were being asked to make. One has to wonder in retrospect if a well-timed amputation and prosthesis might have been a better decision. However, the thought of removing an extremity, even one that isn’t fully functional, is not one that most of us like to consider.
Over the last several decades I have read stories about people – usually athletes – born with short or deformed lower extremities who have faced the decision of amputation. I recall one college-age young man who despite his deformity and with the help of a prosthesis was a competitive multisport athlete. However, it became clear that his deformed foot was preventing him from accessing the most advanced prosthetic technology. Although he was highly motivated, he described his struggle with the decision to part with a portion of his body that despite its appearance and dysfunction had been with him since birth. On the other hand, I have read stories of young people who had become so frustrated by their deformity that they were more than eager to undergo amputation despite the concerns of their parents.
Early in my career I encountered a 3-year-old with phocomelia whose family was visiting from out of town and had come to our clinic because his older sibling was sick. The youngster, as I recall, had only one complete extremity, an arm. Like most 3-year-olds, he was driven to explore at breakneck speed. I will never forget watching him streak back and forth the length of our linoleum covered hallway like a crab skittering along the beach. His mother described how she and his well-meaning physicians were struggling unsuccessfully to get him to accept prostheses. Later I learned that his resistance is shared by many of the survivors of the thalidomide disaster who felt that the most frustrating period in their lives came when, again well-meaning, caregivers had tried to make them look and function more normally by fitting them with prostheses.
These anecdotal observations make clear a philosophy that we should have already internalized. In most clinic decisions the patient, pretty much regardless of age, should be a full participant in the process. And, to do this the patient and his or her family must be as informed as possible. Managing the aftermath of a traumatic amputation presents it own special set of challenges, but when it comes to elective amputation or prosthetic application for a congenital deficiency it is dangerous for us to insert our personal bias into the decision making.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
After 15 years of limping and a gradual downhill slide in mobility, recreational walking had become uncomfortable enough that I’ve decided to shed my proudly worn cloak of denial and seek help. Even I could see that the x-ray made a total knee replacement the only option for some return to near normalcy. Scheduling a total knee replacement became a no-brainer.
My decision to accept the risks to reap the benefits of surgery is small potatoes compared with the decisions that the parents of a child born with a deformed lower extremity must face. In the Family Partnerships section of the February 2021 issue of Pediatrics you will find a heart-wrenching story of a family who embarked on what turned out to be painful and frustrating journey to lengthen their daughter’s congenitally deficient leg. In their own words, the mother and daughter describe how neither of them were prepared for the pain and life-altering complications the daughter has endured. Influenced by the optimism exuded by surgeons, the family gave little thought to the magnitude of the decision they were being asked to make. One has to wonder in retrospect if a well-timed amputation and prosthesis might have been a better decision. However, the thought of removing an extremity, even one that isn’t fully functional, is not one that most of us like to consider.
Over the last several decades I have read stories about people – usually athletes – born with short or deformed lower extremities who have faced the decision of amputation. I recall one college-age young man who despite his deformity and with the help of a prosthesis was a competitive multisport athlete. However, it became clear that his deformed foot was preventing him from accessing the most advanced prosthetic technology. Although he was highly motivated, he described his struggle with the decision to part with a portion of his body that despite its appearance and dysfunction had been with him since birth. On the other hand, I have read stories of young people who had become so frustrated by their deformity that they were more than eager to undergo amputation despite the concerns of their parents.
Early in my career I encountered a 3-year-old with phocomelia whose family was visiting from out of town and had come to our clinic because his older sibling was sick. The youngster, as I recall, had only one complete extremity, an arm. Like most 3-year-olds, he was driven to explore at breakneck speed. I will never forget watching him streak back and forth the length of our linoleum covered hallway like a crab skittering along the beach. His mother described how she and his well-meaning physicians were struggling unsuccessfully to get him to accept prostheses. Later I learned that his resistance is shared by many of the survivors of the thalidomide disaster who felt that the most frustrating period in their lives came when, again well-meaning, caregivers had tried to make them look and function more normally by fitting them with prostheses.
These anecdotal observations make clear a philosophy that we should have already internalized. In most clinic decisions the patient, pretty much regardless of age, should be a full participant in the process. And, to do this the patient and his or her family must be as informed as possible. Managing the aftermath of a traumatic amputation presents it own special set of challenges, but when it comes to elective amputation or prosthetic application for a congenital deficiency it is dangerous for us to insert our personal bias into the decision making.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Dan Kastner wins Crafoord Prize in Polyarthritis
“for establishing the concept of autoinflammatory diseases.” The prize, named after the donor Holger Crafoord because of his bout with severe rheumatoid arthritis toward the end of his life, is for 6 million Swedish kronor (approximately USD $700,000).
Dr. Kastner, scientific director at the U.S. National Human Genome Research Institute’s division of intramural research, received the award for identifying the mechanisms responsible for familial Mediterranean fever, tumor necrosis factor receptor–associated periodic syndrome, and other diagnoses within the group of autoinflammatory diseases.
“Dan Kastner is often called the father of autoinflammatory diseases, a title that he thoroughly deserves. His discoveries have taught us a great deal about the immune system and its functions, contributing to effective treatments that reduce the symptoms of diseases from which patients previously suffered enormously, sometimes leading to premature death,” Olle Kämpe, chair of the prize committee, said in a press announcement.
While the Crafoord Prize normally is awarded on a 3-year rotating basis for achievements in mathematics and astronomy, geosciences, and biosciences, the prize in polyarthritis is “only awarded when there has been scientific progress that motivates a prize,” according to the press release.
“for establishing the concept of autoinflammatory diseases.” The prize, named after the donor Holger Crafoord because of his bout with severe rheumatoid arthritis toward the end of his life, is for 6 million Swedish kronor (approximately USD $700,000).
Dr. Kastner, scientific director at the U.S. National Human Genome Research Institute’s division of intramural research, received the award for identifying the mechanisms responsible for familial Mediterranean fever, tumor necrosis factor receptor–associated periodic syndrome, and other diagnoses within the group of autoinflammatory diseases.
“Dan Kastner is often called the father of autoinflammatory diseases, a title that he thoroughly deserves. His discoveries have taught us a great deal about the immune system and its functions, contributing to effective treatments that reduce the symptoms of diseases from which patients previously suffered enormously, sometimes leading to premature death,” Olle Kämpe, chair of the prize committee, said in a press announcement.
While the Crafoord Prize normally is awarded on a 3-year rotating basis for achievements in mathematics and astronomy, geosciences, and biosciences, the prize in polyarthritis is “only awarded when there has been scientific progress that motivates a prize,” according to the press release.
“for establishing the concept of autoinflammatory diseases.” The prize, named after the donor Holger Crafoord because of his bout with severe rheumatoid arthritis toward the end of his life, is for 6 million Swedish kronor (approximately USD $700,000).
Dr. Kastner, scientific director at the U.S. National Human Genome Research Institute’s division of intramural research, received the award for identifying the mechanisms responsible for familial Mediterranean fever, tumor necrosis factor receptor–associated periodic syndrome, and other diagnoses within the group of autoinflammatory diseases.
“Dan Kastner is often called the father of autoinflammatory diseases, a title that he thoroughly deserves. His discoveries have taught us a great deal about the immune system and its functions, contributing to effective treatments that reduce the symptoms of diseases from which patients previously suffered enormously, sometimes leading to premature death,” Olle Kämpe, chair of the prize committee, said in a press announcement.
While the Crafoord Prize normally is awarded on a 3-year rotating basis for achievements in mathematics and astronomy, geosciences, and biosciences, the prize in polyarthritis is “only awarded when there has been scientific progress that motivates a prize,” according to the press release.
Greater reductions in knee OA pain seen with supportive rather than flexible shoes
according to a randomized trial that included more than 160 patients.
“Contrary to our hypothesis, flat flexible shoes were not superior to stable supportive shoes,” reported Kade L. Paterson, PhD, of the University of Melbourne, and colleagues. Their study was published Jan. 12 in Annals of Internal Medicine.
Research gap
Abnormal knee joint loading has been implicated in the pathogenesis of knee OA. Guidelines recommend that patients wear appropriate footwear, but research has not established which shoes are best.
The 2019 American College of Rheumatology clinical guidelines note that “optimal footwear is likely to be of considerable importance for those with knee and/or hip OA,” but “the available studies do not define the best type of footwear to improve specific outcomes for knee or hip OA.”
Some doctors call for thick, shock-absorbing soles and arch supports, based on expert opinion. On the other hand, studies have found that knee loading is lower with flat flexible shoes, and preliminary evidence has suggested that flat flexible shoes may improve OA symptoms, the investigators said.
To study this question, they enrolled in their trial 164 patients aged 50 years and older who had radiographic medial knee OA. Participants had knee pain on most days of the previous month, tibiofemoral osteophytes, and moderate to severe tibiofemoral OA.
The researchers randomly assigned 82 participants to flat flexible shoes and 82 participants to stable supportive shoes, worn for at least 6 hours a day for 6 months.
In the trial, flat flexible shoes included Merrell Bare Access (men’s and women’s), Vivobarefoot Primus Lite (men’s and women’s), Vivobarefoot Mata Canvas (men’s), Converse Dainty Low (women’s), and Lacoste Marice (men’s).
Stable supportive shoes included ASICS Kayano (men’s and women’s), Merrell Jungle Moc (men’s), Nike Air Max 90 Ultra (women’s), Rockport Edge Hill (men’s), and New Balance 624 (women’s).
After participants were randomly assigned to a group, they chose two different pairs of shoes from their assigned footwear group.
“Participants were not told that the purpose of the study was to compare flat flexible with stable supportive shoes,” the researchers noted. “Instead, they were informed only that the trial was comparing the effects of ‘different shoes’ on knee OA symptoms.”
The primary outcomes were changes in walking pain on a 0-10 scale and physical function as assessed by the Western Ontario and McMaster Universities Osteoarthritis Index subscale at 6 months. The researchers also assessed other measures of pain and function, physical activity, and quality of life.
In all, 161 participants reported 6-month primary outcomes. The between-group difference in change in pain favored stable supportive shoes (mean difference, 1.1 units). In the flat flexible shoe group, overall average knee pain while walking decreased from 6.3 at baseline to 5.2 at 6 months. In the stable supportive shoe group, knee pain while walking decreased from 6.1 to 4.
In addition, improvements in knee-related quality of life and ipsilateral hip pain favored stable supportive shoes.
Participants who wore stable supportive shoes also were less likely to report adverse events, compared with those who wore flat flexible shoes (15% vs. 32%). Knee pain, ankle or foot pain, and shin or calf pain were among the adverse events reported.
‘Important work’
“This study suggests that more supportive shoes may help some patients with knee osteoarthritis feel better,” Constance R. Chu, MD, professor of orthopedic surgery at Stanford (Calif.) University, said in an interview. “Shoes, insoles, wedges, and high heels have been shown to change loading of the knee related to knee pain and osteoarthritis ... This is important work toward providing more specific information on the optimum shoes for people with different patterns and types of arthritis to reduce pain and disability from early knee OA.”
The reported changes in pain may be clinically meaningful for many but not all patients, the authors wrote. “Despite biomechanical evidence showing that flat flexible shoes reduce medial knee load compared with stable supportive shoes, our findings show that this does not translate to improved knee osteoarthritis symptoms,” they said. “This may be because relationships between knee loading and symptoms are not as strong as previously thought, or because the small reductions in medial knee load with flat flexible shoes are insufficient to substantively improve pain and function.”
The trial did not include a control group of patients who wore their usual shoes, and it focused on a select subgroup of patients with knee OA, which may limit the study’s generalizability, the authors noted. The study excluded people with lateral joint space narrowing greater than or equal to medial, those with recent or planned knee surgery, and those who were using shoe orthoses or customized shoes.
The study was supported by grants from the National Health and Medical Research Council. Dr. Chu had no relevant disclosures.
according to a randomized trial that included more than 160 patients.
“Contrary to our hypothesis, flat flexible shoes were not superior to stable supportive shoes,” reported Kade L. Paterson, PhD, of the University of Melbourne, and colleagues. Their study was published Jan. 12 in Annals of Internal Medicine.
Research gap
Abnormal knee joint loading has been implicated in the pathogenesis of knee OA. Guidelines recommend that patients wear appropriate footwear, but research has not established which shoes are best.
The 2019 American College of Rheumatology clinical guidelines note that “optimal footwear is likely to be of considerable importance for those with knee and/or hip OA,” but “the available studies do not define the best type of footwear to improve specific outcomes for knee or hip OA.”
Some doctors call for thick, shock-absorbing soles and arch supports, based on expert opinion. On the other hand, studies have found that knee loading is lower with flat flexible shoes, and preliminary evidence has suggested that flat flexible shoes may improve OA symptoms, the investigators said.
To study this question, they enrolled in their trial 164 patients aged 50 years and older who had radiographic medial knee OA. Participants had knee pain on most days of the previous month, tibiofemoral osteophytes, and moderate to severe tibiofemoral OA.
The researchers randomly assigned 82 participants to flat flexible shoes and 82 participants to stable supportive shoes, worn for at least 6 hours a day for 6 months.
In the trial, flat flexible shoes included Merrell Bare Access (men’s and women’s), Vivobarefoot Primus Lite (men’s and women’s), Vivobarefoot Mata Canvas (men’s), Converse Dainty Low (women’s), and Lacoste Marice (men’s).
Stable supportive shoes included ASICS Kayano (men’s and women’s), Merrell Jungle Moc (men’s), Nike Air Max 90 Ultra (women’s), Rockport Edge Hill (men’s), and New Balance 624 (women’s).
After participants were randomly assigned to a group, they chose two different pairs of shoes from their assigned footwear group.
“Participants were not told that the purpose of the study was to compare flat flexible with stable supportive shoes,” the researchers noted. “Instead, they were informed only that the trial was comparing the effects of ‘different shoes’ on knee OA symptoms.”
The primary outcomes were changes in walking pain on a 0-10 scale and physical function as assessed by the Western Ontario and McMaster Universities Osteoarthritis Index subscale at 6 months. The researchers also assessed other measures of pain and function, physical activity, and quality of life.
In all, 161 participants reported 6-month primary outcomes. The between-group difference in change in pain favored stable supportive shoes (mean difference, 1.1 units). In the flat flexible shoe group, overall average knee pain while walking decreased from 6.3 at baseline to 5.2 at 6 months. In the stable supportive shoe group, knee pain while walking decreased from 6.1 to 4.
In addition, improvements in knee-related quality of life and ipsilateral hip pain favored stable supportive shoes.
Participants who wore stable supportive shoes also were less likely to report adverse events, compared with those who wore flat flexible shoes (15% vs. 32%). Knee pain, ankle or foot pain, and shin or calf pain were among the adverse events reported.
‘Important work’
“This study suggests that more supportive shoes may help some patients with knee osteoarthritis feel better,” Constance R. Chu, MD, professor of orthopedic surgery at Stanford (Calif.) University, said in an interview. “Shoes, insoles, wedges, and high heels have been shown to change loading of the knee related to knee pain and osteoarthritis ... This is important work toward providing more specific information on the optimum shoes for people with different patterns and types of arthritis to reduce pain and disability from early knee OA.”
The reported changes in pain may be clinically meaningful for many but not all patients, the authors wrote. “Despite biomechanical evidence showing that flat flexible shoes reduce medial knee load compared with stable supportive shoes, our findings show that this does not translate to improved knee osteoarthritis symptoms,” they said. “This may be because relationships between knee loading and symptoms are not as strong as previously thought, or because the small reductions in medial knee load with flat flexible shoes are insufficient to substantively improve pain and function.”
The trial did not include a control group of patients who wore their usual shoes, and it focused on a select subgroup of patients with knee OA, which may limit the study’s generalizability, the authors noted. The study excluded people with lateral joint space narrowing greater than or equal to medial, those with recent or planned knee surgery, and those who were using shoe orthoses or customized shoes.
The study was supported by grants from the National Health and Medical Research Council. Dr. Chu had no relevant disclosures.
according to a randomized trial that included more than 160 patients.
“Contrary to our hypothesis, flat flexible shoes were not superior to stable supportive shoes,” reported Kade L. Paterson, PhD, of the University of Melbourne, and colleagues. Their study was published Jan. 12 in Annals of Internal Medicine.
Research gap
Abnormal knee joint loading has been implicated in the pathogenesis of knee OA. Guidelines recommend that patients wear appropriate footwear, but research has not established which shoes are best.
The 2019 American College of Rheumatology clinical guidelines note that “optimal footwear is likely to be of considerable importance for those with knee and/or hip OA,” but “the available studies do not define the best type of footwear to improve specific outcomes for knee or hip OA.”
Some doctors call for thick, shock-absorbing soles and arch supports, based on expert opinion. On the other hand, studies have found that knee loading is lower with flat flexible shoes, and preliminary evidence has suggested that flat flexible shoes may improve OA symptoms, the investigators said.
To study this question, they enrolled in their trial 164 patients aged 50 years and older who had radiographic medial knee OA. Participants had knee pain on most days of the previous month, tibiofemoral osteophytes, and moderate to severe tibiofemoral OA.
The researchers randomly assigned 82 participants to flat flexible shoes and 82 participants to stable supportive shoes, worn for at least 6 hours a day for 6 months.
In the trial, flat flexible shoes included Merrell Bare Access (men’s and women’s), Vivobarefoot Primus Lite (men’s and women’s), Vivobarefoot Mata Canvas (men’s), Converse Dainty Low (women’s), and Lacoste Marice (men’s).
Stable supportive shoes included ASICS Kayano (men’s and women’s), Merrell Jungle Moc (men’s), Nike Air Max 90 Ultra (women’s), Rockport Edge Hill (men’s), and New Balance 624 (women’s).
After participants were randomly assigned to a group, they chose two different pairs of shoes from their assigned footwear group.
“Participants were not told that the purpose of the study was to compare flat flexible with stable supportive shoes,” the researchers noted. “Instead, they were informed only that the trial was comparing the effects of ‘different shoes’ on knee OA symptoms.”
The primary outcomes were changes in walking pain on a 0-10 scale and physical function as assessed by the Western Ontario and McMaster Universities Osteoarthritis Index subscale at 6 months. The researchers also assessed other measures of pain and function, physical activity, and quality of life.
In all, 161 participants reported 6-month primary outcomes. The between-group difference in change in pain favored stable supportive shoes (mean difference, 1.1 units). In the flat flexible shoe group, overall average knee pain while walking decreased from 6.3 at baseline to 5.2 at 6 months. In the stable supportive shoe group, knee pain while walking decreased from 6.1 to 4.
In addition, improvements in knee-related quality of life and ipsilateral hip pain favored stable supportive shoes.
Participants who wore stable supportive shoes also were less likely to report adverse events, compared with those who wore flat flexible shoes (15% vs. 32%). Knee pain, ankle or foot pain, and shin or calf pain were among the adverse events reported.
‘Important work’
“This study suggests that more supportive shoes may help some patients with knee osteoarthritis feel better,” Constance R. Chu, MD, professor of orthopedic surgery at Stanford (Calif.) University, said in an interview. “Shoes, insoles, wedges, and high heels have been shown to change loading of the knee related to knee pain and osteoarthritis ... This is important work toward providing more specific information on the optimum shoes for people with different patterns and types of arthritis to reduce pain and disability from early knee OA.”
The reported changes in pain may be clinically meaningful for many but not all patients, the authors wrote. “Despite biomechanical evidence showing that flat flexible shoes reduce medial knee load compared with stable supportive shoes, our findings show that this does not translate to improved knee osteoarthritis symptoms,” they said. “This may be because relationships between knee loading and symptoms are not as strong as previously thought, or because the small reductions in medial knee load with flat flexible shoes are insufficient to substantively improve pain and function.”
The trial did not include a control group of patients who wore their usual shoes, and it focused on a select subgroup of patients with knee OA, which may limit the study’s generalizability, the authors noted. The study excluded people with lateral joint space narrowing greater than or equal to medial, those with recent or planned knee surgery, and those who were using shoe orthoses or customized shoes.
The study was supported by grants from the National Health and Medical Research Council. Dr. Chu had no relevant disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Data call for biologics trials in undertreated juvenile arthritis subtype
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
FROM ARTHRITIS CARE & RESEARCH
At-home exercises for 4 common musculoskeletal complaints
The mainstay of treatment for many musculoskeletal (MSK) complaints is physical or occupational therapy. But often an individual’s underlying biomechanical issue is one that can be easily addressed with a home exercise plan, and, in light of the COVID-19 pandemic, patients may wish to avoid in-person physical therapy. This article describes the rationale for, and methods of providing, home exercises for several MSK conditions commonly seen in the primary care setting.
General rehabilitation principles: First things first
With basic MSK complaints, focus on controlling pain and swelling before undertaking restoration of function. Tailor pharmacologic and nonpharmacologic options to the patient’s needs, using first-line modalities such as ice and compression to reduce inflammation, and prescribing scheduled doses of an anti-inflammatory medication to help with both pain and inflammation.
Once pain is sufficiently controlled, have patients begin basic rehabilitation with simple range-of-motion exercises that move the injured region through normal patterns, as tolerated. Later, the patient can progress through more specific exercises to return the injured region to full functional capacity.
Explain to patients that it takes about 7 to 10 days of consistent care to decrease inflammation, but that they should begin prescribed exercises once they are able to tolerate them. Plan a follow-up visit in 2 to 3 weeks to check on the patient’s response to prescribed care.
Which is better, ice or heat?
Ice and heat are both commonly used to treat MSK injuries and pain, although scrutiny of the use of either intervention has increased. Despite the widespread use of these modalities, there is little evidence to support their effect on patient outcomes. The historical consensus has been that ice decreases pain, inflammation, and edema,while heat can facilitate movement in rehabilitation by improving blood flow and decreasing stiffness.1-3 In our practice, we encourage use of both topical modalities as a way to start exercise therapy when pain from the acute injury limits participation. Patients often ask which modality they should use. Ice is generally applied in the acute injury phase (48-72 hours after injury), while heat has been thought to be more beneficial in the chronic stages.
Ccontinue to: When and how to apply ice
When and how to apply ice. Applying an ice pack or a bag of frozen vegetables directly to the affected area will help control pain and swelling. Ice should be applied for 15 to 20 minutes at a time, once an hour. If a patient has sensitivity to cold or if the ice pack is a gel-type, have the patient place a layer (eg, towel) between the ice and skin to avoid injury to the skin. Additional caution should be exercised in patients with peripheral vascular disease, cryoglobulinemia, Raynaud disease, or a history of frostbite at the site.4
An alternative method we sometimes recommend is ice-cup massage. The patient can fill a small paper cup with water and freeze it. The cup is then used to massage the injured area, providing a more active method of icing whereby the cold can penetrate more quickly. Ice-cup massage should be done for 5 to 10 minutes, 3 to 4 times a day.
When and how to apply heat. Heat will help relax and loosen muscles and is a preferred treatment for older injuries, chronic pain, muscle tension, and spasms.5 Because heat can increase blood flow and, likely, inflammation, it should not be used in the acute injury phase. A heating pad or a warm, wet towel can be applied for up to 20 minutes at a time to help relieve pain and tension. Heat is also beneficial before participating in rehab activities as a method of “warming up” a recently injured area.6 However, ice should still be used following activity to prevent any new inflammation.
Anti-inflammatory medications
For an acute injury, nonsteroidal anti-inflammatory drugs (NSAIDs) not only can decrease inflammation and aid in healing but can alleviate pain. We typically start with over-the-counter (OTC) NSAIDs taken on a schedule. A good suggestion is to have the patient take the scheduled NSAID with food for 7 to 10 days or until symptoms subside.
Topical analgesics
Because oral medications can occasionally cause adverse effects or be contraindicated in some patients, topical analgesics can be a good substitute due to their minimal adverse effects. Acceptable topical medications include NSAIDs, lidocaine, menthol, and arnica. Other than prescribed topical NSAIDs, these products can be applied directly to the painful area on an as-needed basis. Often, a topical patch is a nice option to recommend for use during work or school, and a topical cream or ointment can be used at bedtime.
Continue to: Graduated rehabilitation
Graduated rehabilitation
The following 4 common MSK injuries are ones that can benefit from a graduated approach to rehabilitation at home.
Lateral ankle sprain
Lateral ankle sprain, usually resulting from an inversion mechanism, is the most common type of acute ankle sprain seen in primary care and sports medicine settings.7-9 The injury causes lateral ankle pain and swelling, decreased range of motion and strength, and pain with weight-bearing activities.
Treatment and rehabilitation after this type of injury are critical to restoring normal function and increasing the likelihood of returning to pre-injury levels of activity.9,10 Goals for an acute ankle sprain include controlling swelling, regaining full range of motion, increasing muscle strength and power, and improving balance.
Phase 1: Immediately following injury, have the patient protect the injured area with rest, ice, compression, and elevation (RICE). This will help to decrease swelling and pain. Exercises to regain range of motion, such as stretching and doing ankle “ABCs,” should begin within 48 to 72 hours of the initial injury (TABLE 1).9-11
Continue to: Phase 2
Phase 2: Once the patient has achieved full range of motion and pain is controlled, begin the process of regaining strength. The 4-way ankle exercise program (with elastic tubing) is an easy at-home exercise that has been shown to improve strength in plantar flexion, dorsiflexion, eversion, and inversion (TABLE 1).9-11
Phase 3: Once your patient is able to bear full weight with little to no pain, begin a balance program (TABLE 19-11). This is the most frequently neglected component of rehabilitation and the most common reason patients return with chronic ankle pain or repeat ankle injuries. Deficits in postural stability and balance have been reported in unstable ankles following acute ankle sprains,10,12-15 and studies have shown that individuals with poor stability are at a greater risk of injury.13-16
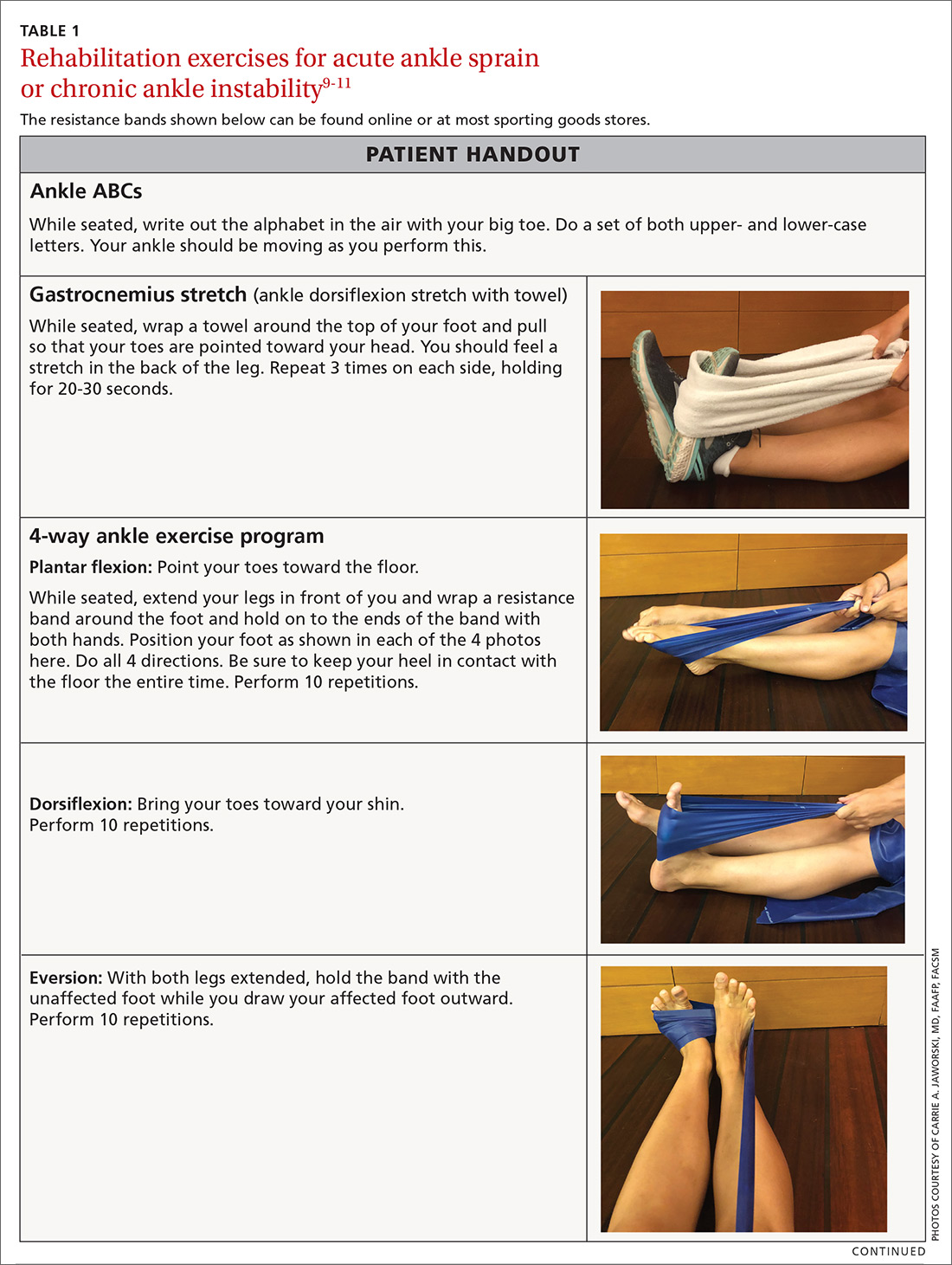
For most lateral ankle sprains, patients can expect time to recovery to range from 2 to 8 weeks. Longer recoveries are associated with more severe injuries or those that involve the syndesmosis.
Plantar fasciitis
Plantar fasciitis (PF) of the foot can be frustrating for a patient due to its chronic nature. Most patients will present with pain in the heel that is aggravated by weight-bearing activities. A conservative management program that focuses on reducing pain and inflammation, reducing tissue stress, and restoring strength and flexibility has been shown to be effective for this type of injury.17,18
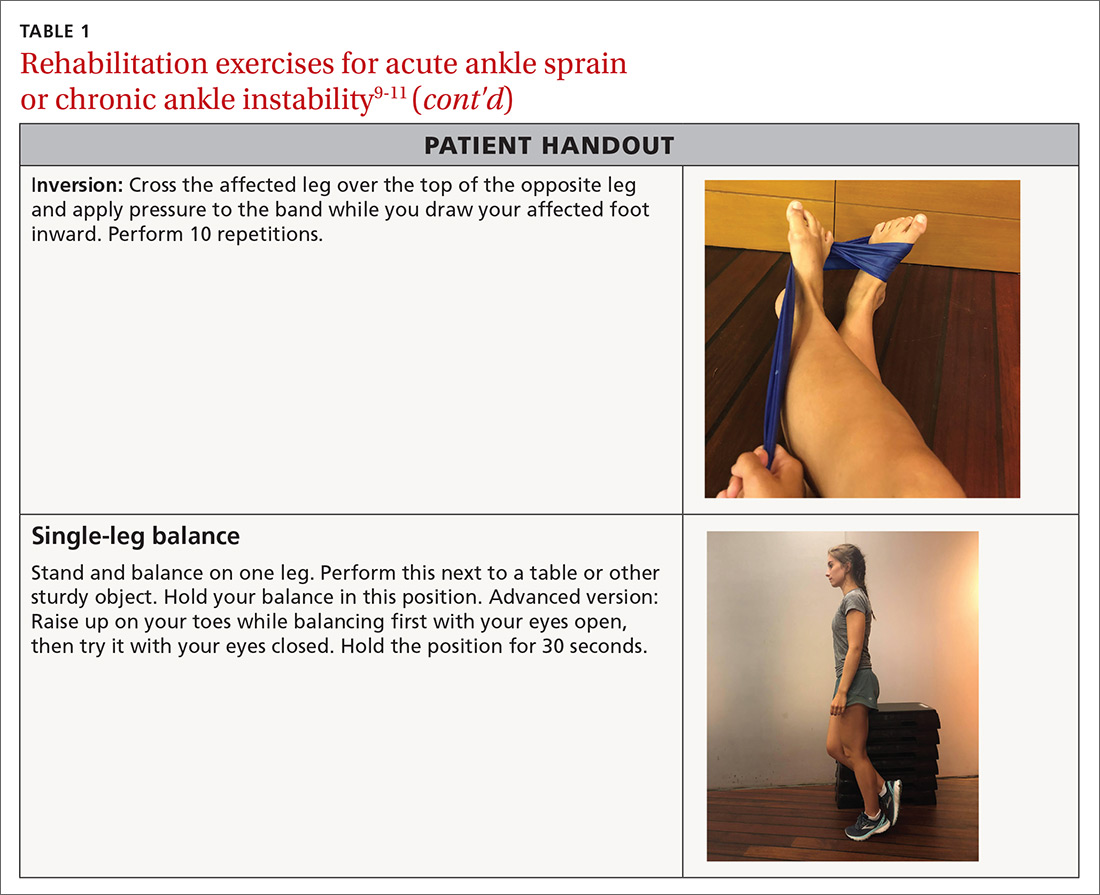
Step 1: Reduce pain and inflammation. Deep-tissue massage and cryotherapy are easy ways to help with pain and inflammation. Deep-tissue massage can be accomplished by rolling the bottom of the foot on a golf or lacrosse ball. A favorite recommendation of ours to reduce inflammation is to use the ice-cup massage, mentioned earlier, for 5 minutes. Or rolling the bottom of the foot on a frozen water bottle will accomplish both tasks at once (TABLE 217,18).
Step 2: Reduce tissue stress. Management tools commonly used to reduce tissue stress are OTC orthotics and night splints. The night splint has been shown to improve symptoms,but patients often stop using it due to discomfort.19 Many kinds of night splints are available, but we have found that the sock variety with a strap to keep the foot in dorsiflexion is best tolerated, and it should be covered by most care plans.
Continue to: Step 3
Step 3: Restore muscle strength and flexibility. Restoring flexibility of the gastrocnemius and soleus is most frequently recommended for treating PF. Strengthening exercises that involve intrinsic and extrinsic muscles of the foot and ankle are also essential.17,18 Helpful exercises include those listed in TABLE 1.9-11 Additionally, an eccentric heel stretch can help to alleviate PF symptoms (TABLE 217,18).
A reasonable timeline for follow-up on newly diagnosed PF is 4 to 6 weeks. While many patients will not have recovered in that time, the goal is to document progress in recovery. If no progress is made, consider other treatment modalities.
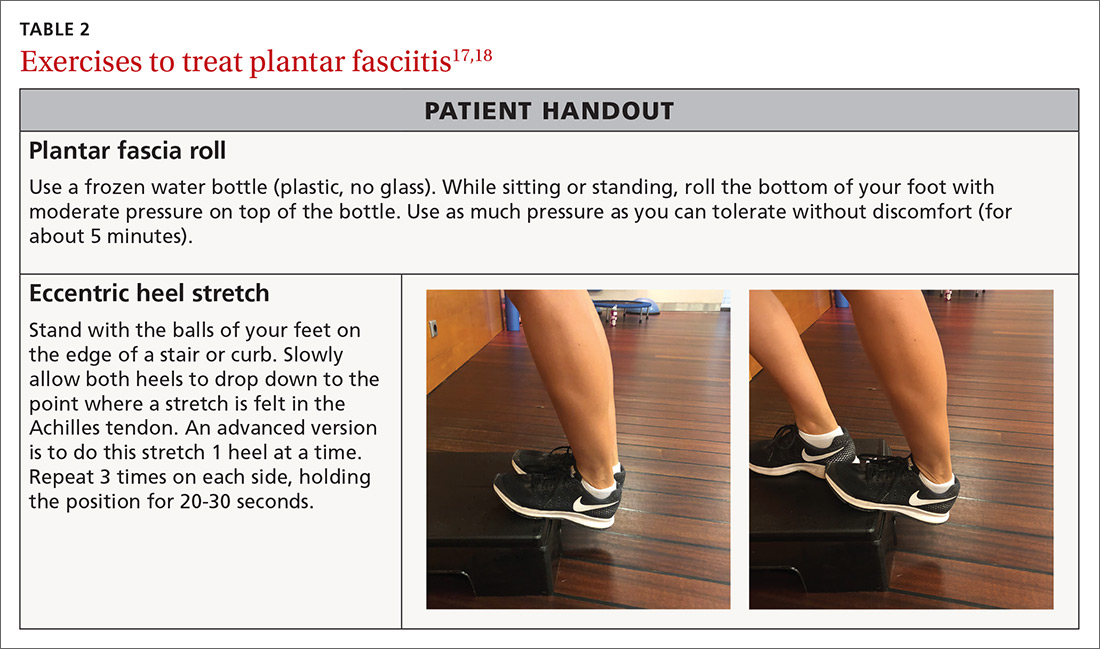
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS) is one of the most common orthopedic complaints, estimated to comprise 7.3% of all orthopedic visits.20 Commonly called “runner’s knee,” PFPS is the leading cause of anterior knee pain in active individuals. Studies suggest a gender bias, with PFPS being diagnosed more frequently in females than in males, particularly between the ages of 10 and 19.20 Often, there is vague anterior knee pain, or pain that worsens with activities such as climbing hills or stairs, or with long sitting or when fatigued.
In general, unbalanced patellar tracking within the trochlear groove likely leads to this pain. Multiple contributory factors have been described; however, evidence increasingly has shown that deficiencies in hip strength may contribute significantly to maltracking of the patella with resultant pain. Specifically, weakness in hip external rotators and abductors is associated with abnormal lower extremity mechanics.21 One randomized controlled trial by Ferber et al found that therapy protocols directed at hip and core strength showed earlier resolution of pain and greater strength when compared with knee protocols alone.22
We routinely talk to patients about how the knee is the “victim” caught between weak hips and/or flat feet. It is prudent to look for both in the office visit. This can be done with one simple maneuver: Ask your patient to do a squat followed by 3 or 4 single-leg squats on each side. This will often reveal dysfunction at the foot/ankle or weakness in the hips/core as demonstrated by pronated feet (along with valgus tracking of the knees inward) or loss of balance upon squatting.
There is general consensus that a nonsurgical approach is the mainstay of treatment for PFPS.23 Pelvic stabilization and hip strengthening are standard components along with treatment protocols of exercises tailored to one’s individual weaknesses.
Numerous types of exercises do not require specialized equipment and can be taught in the office (TABLE 324). Explain to patients that the recovery process may take several months. Monthly follow-up to document progress is essential and helps to ensure compliance with one’s home program.


Continue to : Neck pain
Neck pain
The annual prevalence of nonspecific neck pain ranges from 27% to 48%, with 70% of individuals being afflicted at some time in their lives.25 First rule out any neurologic factors that might suggest cervical disc disease or spinal stenosis. If a patient describes weakness or sensory changes along one or both upper extremities, obtain imaging and consider more formalized therapy with a physical therapist.
In patients without any red flags, investigate possible biomechanical causes. It is essential to review the patient’s work and home habits, particularly in light of COVID-19, to determine if adjustments may be needed. Factors to consider are desk and computer setups at work or home, reading or laptop use in bed, sleep habits, and frequency of cellular phone calls/texting.26 A formal ergonomic assessment of the patient’s workplace may be helpful.
A mainstay in treating mechanical neck pain is alleviating trapezial tightness or spasm. Manipulative therapies such as osteopathic manipulation, massage, and chiropractic care can provide pain relief in the acute setting as well as help with control of chronic symptoms.27 A simple self-care tool is using a tennis ball to massage the trapezial muscles. This can be accomplished by having the patient position the tennis ball along the upper trapezial muscles, holding it in place by leaning against a wall, and initiating self-massage. Another method of self-massage is to put 2 tennis balls in an athletic tube sock and tie off the end, place the sock on the floor, and lie on it in the supine position.
There is also evidence that exercise of any kind can help control neck pain.28,29 The easiest exercises one can offer a patient with neck stiffness, or even mild cervical strains, is self-directed stretching through gentle pressure applied in all 4 directions on the neck. This technique can be repeated hourly both at work and at home (TABLE 4).
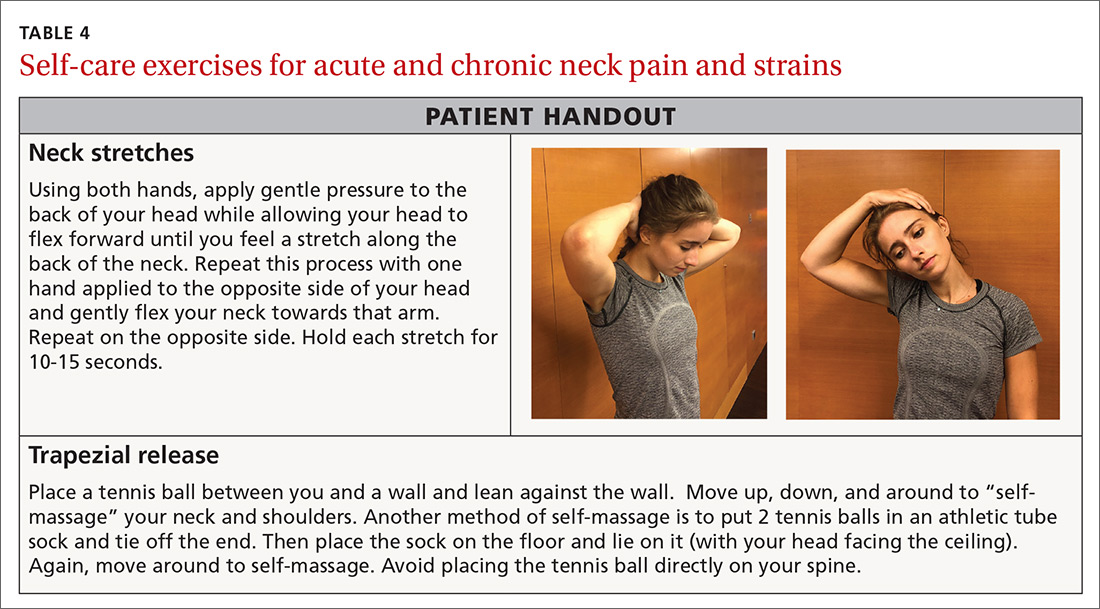
Reminders that can help ensure success
You can use the approaches described here for numerous other MSK conditions in helping patients on the road to recovery.
After the acute phase, advise patients to
• apply heat to the affected area before exercising. This can help bring blood flow to the region and promote ease of movement.
• continue icing the area following rehabilitation exercises in order to control exercise-induced inflammation.
• report any changing symptoms such as worsening pain, numbness, or weakness.
These techniques are one step in the recovery process. A home program can benefit the patient either alone or in combination with more advanced techniques that are best accomplished under the watchful eye of a physical or occupational therapist.
CORRESPONDENCE
Carrie A. Jaworski, MD, FAAFP, FACSM, 2180 Pfingsten Road, Suite 3100, Glenview, IL 60026; [email protected]
1. Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39:88-94.
2. Ho SS, Coel MN, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537-540.
3. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57-65.
4. Bleakley CM, O’Connor S, Tully MA, et al. The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain. BMC Musculoskelet Disord. 2007;8:125.
5. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395-403.
6. Cetin N, Aytar A, Atalay A, et al. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87:443-451.
7. Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284.
8. Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94.
9. Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002(3):CD003762.
10. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Ath Train. 2002;37:413-429.
11. Hü bscher M, Zech A, Pfeifer K, et al. Neuromuscular training for sports injury prevention: a systematic review. Med Sci Sports Exerc. 2010;42:413-421.
12. Emery CA, Meeuwisse WH. The effectiveness of a neuromuscular prevention strategy to reduce injuries in youth soccer: a cluster-randomised controlled trial. Br J Sports Med. 2010;44:555-562.
13. Tiemstra JD. Update on acute ankle sprains. Am Fam Physician. 2012;85:1170-1176.
14. Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Ath Train. 2002;37:376-380.
15. Schiftan GS, Ross LA, Hahne AJ. The effectiveness of proprioceptive training in preventing ankle sprains in sporting populations: a systematic review and meta-analysis. J Sci Med Sport. 2015;18:238–244.
16. Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684
17. Thompson JV, Saini SS, Reb CW, et al. Diagnosis and management of plantar fasciitis. J Am Osteopath Assoc. 2014;114:900-906.
18. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88:1775-1781.

19. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
20. Glaviano NR, Key M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. J Sports Phys Ther. 2015;10: 281-290.
21. Louden JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11: 820-830.
22. Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Ath Train. 2015;50: 366-377.
23. Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2013;41:31-49.
24. Bolgla LA. Hip strength and kinematics in patellofemoral syndrome. In: Brotzman SB, Manske RC eds. Clinical Orthopaedic Rehabilitation. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011:273-274.
25. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl 4):S39-S51.
26. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol. 2007; 21:447-463.
27. Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502.
28. Bronfort G, Evans R, Anderson A, et al. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1-10.
29. Evans R, Bronfort G, Bittell S, et al. A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients. J Manipulative Physiol Ther. 2003;26:403-411.
The mainstay of treatment for many musculoskeletal (MSK) complaints is physical or occupational therapy. But often an individual’s underlying biomechanical issue is one that can be easily addressed with a home exercise plan, and, in light of the COVID-19 pandemic, patients may wish to avoid in-person physical therapy. This article describes the rationale for, and methods of providing, home exercises for several MSK conditions commonly seen in the primary care setting.
General rehabilitation principles: First things first
With basic MSK complaints, focus on controlling pain and swelling before undertaking restoration of function. Tailor pharmacologic and nonpharmacologic options to the patient’s needs, using first-line modalities such as ice and compression to reduce inflammation, and prescribing scheduled doses of an anti-inflammatory medication to help with both pain and inflammation.
Once pain is sufficiently controlled, have patients begin basic rehabilitation with simple range-of-motion exercises that move the injured region through normal patterns, as tolerated. Later, the patient can progress through more specific exercises to return the injured region to full functional capacity.
Explain to patients that it takes about 7 to 10 days of consistent care to decrease inflammation, but that they should begin prescribed exercises once they are able to tolerate them. Plan a follow-up visit in 2 to 3 weeks to check on the patient’s response to prescribed care.
Which is better, ice or heat?
Ice and heat are both commonly used to treat MSK injuries and pain, although scrutiny of the use of either intervention has increased. Despite the widespread use of these modalities, there is little evidence to support their effect on patient outcomes. The historical consensus has been that ice decreases pain, inflammation, and edema,while heat can facilitate movement in rehabilitation by improving blood flow and decreasing stiffness.1-3 In our practice, we encourage use of both topical modalities as a way to start exercise therapy when pain from the acute injury limits participation. Patients often ask which modality they should use. Ice is generally applied in the acute injury phase (48-72 hours after injury), while heat has been thought to be more beneficial in the chronic stages.
Ccontinue to: When and how to apply ice
When and how to apply ice. Applying an ice pack or a bag of frozen vegetables directly to the affected area will help control pain and swelling. Ice should be applied for 15 to 20 minutes at a time, once an hour. If a patient has sensitivity to cold or if the ice pack is a gel-type, have the patient place a layer (eg, towel) between the ice and skin to avoid injury to the skin. Additional caution should be exercised in patients with peripheral vascular disease, cryoglobulinemia, Raynaud disease, or a history of frostbite at the site.4
An alternative method we sometimes recommend is ice-cup massage. The patient can fill a small paper cup with water and freeze it. The cup is then used to massage the injured area, providing a more active method of icing whereby the cold can penetrate more quickly. Ice-cup massage should be done for 5 to 10 minutes, 3 to 4 times a day.
When and how to apply heat. Heat will help relax and loosen muscles and is a preferred treatment for older injuries, chronic pain, muscle tension, and spasms.5 Because heat can increase blood flow and, likely, inflammation, it should not be used in the acute injury phase. A heating pad or a warm, wet towel can be applied for up to 20 minutes at a time to help relieve pain and tension. Heat is also beneficial before participating in rehab activities as a method of “warming up” a recently injured area.6 However, ice should still be used following activity to prevent any new inflammation.
Anti-inflammatory medications
For an acute injury, nonsteroidal anti-inflammatory drugs (NSAIDs) not only can decrease inflammation and aid in healing but can alleviate pain. We typically start with over-the-counter (OTC) NSAIDs taken on a schedule. A good suggestion is to have the patient take the scheduled NSAID with food for 7 to 10 days or until symptoms subside.
Topical analgesics
Because oral medications can occasionally cause adverse effects or be contraindicated in some patients, topical analgesics can be a good substitute due to their minimal adverse effects. Acceptable topical medications include NSAIDs, lidocaine, menthol, and arnica. Other than prescribed topical NSAIDs, these products can be applied directly to the painful area on an as-needed basis. Often, a topical patch is a nice option to recommend for use during work or school, and a topical cream or ointment can be used at bedtime.
Continue to: Graduated rehabilitation
Graduated rehabilitation
The following 4 common MSK injuries are ones that can benefit from a graduated approach to rehabilitation at home.
Lateral ankle sprain
Lateral ankle sprain, usually resulting from an inversion mechanism, is the most common type of acute ankle sprain seen in primary care and sports medicine settings.7-9 The injury causes lateral ankle pain and swelling, decreased range of motion and strength, and pain with weight-bearing activities.
Treatment and rehabilitation after this type of injury are critical to restoring normal function and increasing the likelihood of returning to pre-injury levels of activity.9,10 Goals for an acute ankle sprain include controlling swelling, regaining full range of motion, increasing muscle strength and power, and improving balance.
Phase 1: Immediately following injury, have the patient protect the injured area with rest, ice, compression, and elevation (RICE). This will help to decrease swelling and pain. Exercises to regain range of motion, such as stretching and doing ankle “ABCs,” should begin within 48 to 72 hours of the initial injury (TABLE 1).9-11
Continue to: Phase 2
Phase 2: Once the patient has achieved full range of motion and pain is controlled, begin the process of regaining strength. The 4-way ankle exercise program (with elastic tubing) is an easy at-home exercise that has been shown to improve strength in plantar flexion, dorsiflexion, eversion, and inversion (TABLE 1).9-11
Phase 3: Once your patient is able to bear full weight with little to no pain, begin a balance program (TABLE 19-11). This is the most frequently neglected component of rehabilitation and the most common reason patients return with chronic ankle pain or repeat ankle injuries. Deficits in postural stability and balance have been reported in unstable ankles following acute ankle sprains,10,12-15 and studies have shown that individuals with poor stability are at a greater risk of injury.13-16

For most lateral ankle sprains, patients can expect time to recovery to range from 2 to 8 weeks. Longer recoveries are associated with more severe injuries or those that involve the syndesmosis.
Plantar fasciitis
Plantar fasciitis (PF) of the foot can be frustrating for a patient due to its chronic nature. Most patients will present with pain in the heel that is aggravated by weight-bearing activities. A conservative management program that focuses on reducing pain and inflammation, reducing tissue stress, and restoring strength and flexibility has been shown to be effective for this type of injury.17,18

Step 1: Reduce pain and inflammation. Deep-tissue massage and cryotherapy are easy ways to help with pain and inflammation. Deep-tissue massage can be accomplished by rolling the bottom of the foot on a golf or lacrosse ball. A favorite recommendation of ours to reduce inflammation is to use the ice-cup massage, mentioned earlier, for 5 minutes. Or rolling the bottom of the foot on a frozen water bottle will accomplish both tasks at once (TABLE 217,18).
Step 2: Reduce tissue stress. Management tools commonly used to reduce tissue stress are OTC orthotics and night splints. The night splint has been shown to improve symptoms,but patients often stop using it due to discomfort.19 Many kinds of night splints are available, but we have found that the sock variety with a strap to keep the foot in dorsiflexion is best tolerated, and it should be covered by most care plans.
Continue to: Step 3
Step 3: Restore muscle strength and flexibility. Restoring flexibility of the gastrocnemius and soleus is most frequently recommended for treating PF. Strengthening exercises that involve intrinsic and extrinsic muscles of the foot and ankle are also essential.17,18 Helpful exercises include those listed in TABLE 1.9-11 Additionally, an eccentric heel stretch can help to alleviate PF symptoms (TABLE 217,18).
A reasonable timeline for follow-up on newly diagnosed PF is 4 to 6 weeks. While many patients will not have recovered in that time, the goal is to document progress in recovery. If no progress is made, consider other treatment modalities.

Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS) is one of the most common orthopedic complaints, estimated to comprise 7.3% of all orthopedic visits.20 Commonly called “runner’s knee,” PFPS is the leading cause of anterior knee pain in active individuals. Studies suggest a gender bias, with PFPS being diagnosed more frequently in females than in males, particularly between the ages of 10 and 19.20 Often, there is vague anterior knee pain, or pain that worsens with activities such as climbing hills or stairs, or with long sitting or when fatigued.
In general, unbalanced patellar tracking within the trochlear groove likely leads to this pain. Multiple contributory factors have been described; however, evidence increasingly has shown that deficiencies in hip strength may contribute significantly to maltracking of the patella with resultant pain. Specifically, weakness in hip external rotators and abductors is associated with abnormal lower extremity mechanics.21 One randomized controlled trial by Ferber et al found that therapy protocols directed at hip and core strength showed earlier resolution of pain and greater strength when compared with knee protocols alone.22
We routinely talk to patients about how the knee is the “victim” caught between weak hips and/or flat feet. It is prudent to look for both in the office visit. This can be done with one simple maneuver: Ask your patient to do a squat followed by 3 or 4 single-leg squats on each side. This will often reveal dysfunction at the foot/ankle or weakness in the hips/core as demonstrated by pronated feet (along with valgus tracking of the knees inward) or loss of balance upon squatting.
There is general consensus that a nonsurgical approach is the mainstay of treatment for PFPS.23 Pelvic stabilization and hip strengthening are standard components along with treatment protocols of exercises tailored to one’s individual weaknesses.
Numerous types of exercises do not require specialized equipment and can be taught in the office (TABLE 324). Explain to patients that the recovery process may take several months. Monthly follow-up to document progress is essential and helps to ensure compliance with one’s home program.


Continue to : Neck pain
Neck pain
The annual prevalence of nonspecific neck pain ranges from 27% to 48%, with 70% of individuals being afflicted at some time in their lives.25 First rule out any neurologic factors that might suggest cervical disc disease or spinal stenosis. If a patient describes weakness or sensory changes along one or both upper extremities, obtain imaging and consider more formalized therapy with a physical therapist.
In patients without any red flags, investigate possible biomechanical causes. It is essential to review the patient’s work and home habits, particularly in light of COVID-19, to determine if adjustments may be needed. Factors to consider are desk and computer setups at work or home, reading or laptop use in bed, sleep habits, and frequency of cellular phone calls/texting.26 A formal ergonomic assessment of the patient’s workplace may be helpful.
A mainstay in treating mechanical neck pain is alleviating trapezial tightness or spasm. Manipulative therapies such as osteopathic manipulation, massage, and chiropractic care can provide pain relief in the acute setting as well as help with control of chronic symptoms.27 A simple self-care tool is using a tennis ball to massage the trapezial muscles. This can be accomplished by having the patient position the tennis ball along the upper trapezial muscles, holding it in place by leaning against a wall, and initiating self-massage. Another method of self-massage is to put 2 tennis balls in an athletic tube sock and tie off the end, place the sock on the floor, and lie on it in the supine position.
There is also evidence that exercise of any kind can help control neck pain.28,29 The easiest exercises one can offer a patient with neck stiffness, or even mild cervical strains, is self-directed stretching through gentle pressure applied in all 4 directions on the neck. This technique can be repeated hourly both at work and at home (TABLE 4).

Reminders that can help ensure success
You can use the approaches described here for numerous other MSK conditions in helping patients on the road to recovery.
After the acute phase, advise patients to
• apply heat to the affected area before exercising. This can help bring blood flow to the region and promote ease of movement.
• continue icing the area following rehabilitation exercises in order to control exercise-induced inflammation.
• report any changing symptoms such as worsening pain, numbness, or weakness.
These techniques are one step in the recovery process. A home program can benefit the patient either alone or in combination with more advanced techniques that are best accomplished under the watchful eye of a physical or occupational therapist.
CORRESPONDENCE
Carrie A. Jaworski, MD, FAAFP, FACSM, 2180 Pfingsten Road, Suite 3100, Glenview, IL 60026; [email protected]
The mainstay of treatment for many musculoskeletal (MSK) complaints is physical or occupational therapy. But often an individual’s underlying biomechanical issue is one that can be easily addressed with a home exercise plan, and, in light of the COVID-19 pandemic, patients may wish to avoid in-person physical therapy. This article describes the rationale for, and methods of providing, home exercises for several MSK conditions commonly seen in the primary care setting.
General rehabilitation principles: First things first
With basic MSK complaints, focus on controlling pain and swelling before undertaking restoration of function. Tailor pharmacologic and nonpharmacologic options to the patient’s needs, using first-line modalities such as ice and compression to reduce inflammation, and prescribing scheduled doses of an anti-inflammatory medication to help with both pain and inflammation.
Once pain is sufficiently controlled, have patients begin basic rehabilitation with simple range-of-motion exercises that move the injured region through normal patterns, as tolerated. Later, the patient can progress through more specific exercises to return the injured region to full functional capacity.
Explain to patients that it takes about 7 to 10 days of consistent care to decrease inflammation, but that they should begin prescribed exercises once they are able to tolerate them. Plan a follow-up visit in 2 to 3 weeks to check on the patient’s response to prescribed care.
Which is better, ice or heat?
Ice and heat are both commonly used to treat MSK injuries and pain, although scrutiny of the use of either intervention has increased. Despite the widespread use of these modalities, there is little evidence to support their effect on patient outcomes. The historical consensus has been that ice decreases pain, inflammation, and edema,while heat can facilitate movement in rehabilitation by improving blood flow and decreasing stiffness.1-3 In our practice, we encourage use of both topical modalities as a way to start exercise therapy when pain from the acute injury limits participation. Patients often ask which modality they should use. Ice is generally applied in the acute injury phase (48-72 hours after injury), while heat has been thought to be more beneficial in the chronic stages.
Ccontinue to: When and how to apply ice
When and how to apply ice. Applying an ice pack or a bag of frozen vegetables directly to the affected area will help control pain and swelling. Ice should be applied for 15 to 20 minutes at a time, once an hour. If a patient has sensitivity to cold or if the ice pack is a gel-type, have the patient place a layer (eg, towel) between the ice and skin to avoid injury to the skin. Additional caution should be exercised in patients with peripheral vascular disease, cryoglobulinemia, Raynaud disease, or a history of frostbite at the site.4
An alternative method we sometimes recommend is ice-cup massage. The patient can fill a small paper cup with water and freeze it. The cup is then used to massage the injured area, providing a more active method of icing whereby the cold can penetrate more quickly. Ice-cup massage should be done for 5 to 10 minutes, 3 to 4 times a day.
When and how to apply heat. Heat will help relax and loosen muscles and is a preferred treatment for older injuries, chronic pain, muscle tension, and spasms.5 Because heat can increase blood flow and, likely, inflammation, it should not be used in the acute injury phase. A heating pad or a warm, wet towel can be applied for up to 20 minutes at a time to help relieve pain and tension. Heat is also beneficial before participating in rehab activities as a method of “warming up” a recently injured area.6 However, ice should still be used following activity to prevent any new inflammation.
Anti-inflammatory medications
For an acute injury, nonsteroidal anti-inflammatory drugs (NSAIDs) not only can decrease inflammation and aid in healing but can alleviate pain. We typically start with over-the-counter (OTC) NSAIDs taken on a schedule. A good suggestion is to have the patient take the scheduled NSAID with food for 7 to 10 days or until symptoms subside.
Topical analgesics
Because oral medications can occasionally cause adverse effects or be contraindicated in some patients, topical analgesics can be a good substitute due to their minimal adverse effects. Acceptable topical medications include NSAIDs, lidocaine, menthol, and arnica. Other than prescribed topical NSAIDs, these products can be applied directly to the painful area on an as-needed basis. Often, a topical patch is a nice option to recommend for use during work or school, and a topical cream or ointment can be used at bedtime.
Continue to: Graduated rehabilitation
Graduated rehabilitation
The following 4 common MSK injuries are ones that can benefit from a graduated approach to rehabilitation at home.
Lateral ankle sprain
Lateral ankle sprain, usually resulting from an inversion mechanism, is the most common type of acute ankle sprain seen in primary care and sports medicine settings.7-9 The injury causes lateral ankle pain and swelling, decreased range of motion and strength, and pain with weight-bearing activities.
Treatment and rehabilitation after this type of injury are critical to restoring normal function and increasing the likelihood of returning to pre-injury levels of activity.9,10 Goals for an acute ankle sprain include controlling swelling, regaining full range of motion, increasing muscle strength and power, and improving balance.
Phase 1: Immediately following injury, have the patient protect the injured area with rest, ice, compression, and elevation (RICE). This will help to decrease swelling and pain. Exercises to regain range of motion, such as stretching and doing ankle “ABCs,” should begin within 48 to 72 hours of the initial injury (TABLE 1).9-11
Continue to: Phase 2
Phase 2: Once the patient has achieved full range of motion and pain is controlled, begin the process of regaining strength. The 4-way ankle exercise program (with elastic tubing) is an easy at-home exercise that has been shown to improve strength in plantar flexion, dorsiflexion, eversion, and inversion (TABLE 1).9-11
Phase 3: Once your patient is able to bear full weight with little to no pain, begin a balance program (TABLE 19-11). This is the most frequently neglected component of rehabilitation and the most common reason patients return with chronic ankle pain or repeat ankle injuries. Deficits in postural stability and balance have been reported in unstable ankles following acute ankle sprains,10,12-15 and studies have shown that individuals with poor stability are at a greater risk of injury.13-16

For most lateral ankle sprains, patients can expect time to recovery to range from 2 to 8 weeks. Longer recoveries are associated with more severe injuries or those that involve the syndesmosis.
Plantar fasciitis
Plantar fasciitis (PF) of the foot can be frustrating for a patient due to its chronic nature. Most patients will present with pain in the heel that is aggravated by weight-bearing activities. A conservative management program that focuses on reducing pain and inflammation, reducing tissue stress, and restoring strength and flexibility has been shown to be effective for this type of injury.17,18

Step 1: Reduce pain and inflammation. Deep-tissue massage and cryotherapy are easy ways to help with pain and inflammation. Deep-tissue massage can be accomplished by rolling the bottom of the foot on a golf or lacrosse ball. A favorite recommendation of ours to reduce inflammation is to use the ice-cup massage, mentioned earlier, for 5 minutes. Or rolling the bottom of the foot on a frozen water bottle will accomplish both tasks at once (TABLE 217,18).
Step 2: Reduce tissue stress. Management tools commonly used to reduce tissue stress are OTC orthotics and night splints. The night splint has been shown to improve symptoms,but patients often stop using it due to discomfort.19 Many kinds of night splints are available, but we have found that the sock variety with a strap to keep the foot in dorsiflexion is best tolerated, and it should be covered by most care plans.
Continue to: Step 3
Step 3: Restore muscle strength and flexibility. Restoring flexibility of the gastrocnemius and soleus is most frequently recommended for treating PF. Strengthening exercises that involve intrinsic and extrinsic muscles of the foot and ankle are also essential.17,18 Helpful exercises include those listed in TABLE 1.9-11 Additionally, an eccentric heel stretch can help to alleviate PF symptoms (TABLE 217,18).
A reasonable timeline for follow-up on newly diagnosed PF is 4 to 6 weeks. While many patients will not have recovered in that time, the goal is to document progress in recovery. If no progress is made, consider other treatment modalities.

Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS) is one of the most common orthopedic complaints, estimated to comprise 7.3% of all orthopedic visits.20 Commonly called “runner’s knee,” PFPS is the leading cause of anterior knee pain in active individuals. Studies suggest a gender bias, with PFPS being diagnosed more frequently in females than in males, particularly between the ages of 10 and 19.20 Often, there is vague anterior knee pain, or pain that worsens with activities such as climbing hills or stairs, or with long sitting or when fatigued.
In general, unbalanced patellar tracking within the trochlear groove likely leads to this pain. Multiple contributory factors have been described; however, evidence increasingly has shown that deficiencies in hip strength may contribute significantly to maltracking of the patella with resultant pain. Specifically, weakness in hip external rotators and abductors is associated with abnormal lower extremity mechanics.21 One randomized controlled trial by Ferber et al found that therapy protocols directed at hip and core strength showed earlier resolution of pain and greater strength when compared with knee protocols alone.22
We routinely talk to patients about how the knee is the “victim” caught between weak hips and/or flat feet. It is prudent to look for both in the office visit. This can be done with one simple maneuver: Ask your patient to do a squat followed by 3 or 4 single-leg squats on each side. This will often reveal dysfunction at the foot/ankle or weakness in the hips/core as demonstrated by pronated feet (along with valgus tracking of the knees inward) or loss of balance upon squatting.
There is general consensus that a nonsurgical approach is the mainstay of treatment for PFPS.23 Pelvic stabilization and hip strengthening are standard components along with treatment protocols of exercises tailored to one’s individual weaknesses.
Numerous types of exercises do not require specialized equipment and can be taught in the office (TABLE 324). Explain to patients that the recovery process may take several months. Monthly follow-up to document progress is essential and helps to ensure compliance with one’s home program.


Continue to : Neck pain
Neck pain
The annual prevalence of nonspecific neck pain ranges from 27% to 48%, with 70% of individuals being afflicted at some time in their lives.25 First rule out any neurologic factors that might suggest cervical disc disease or spinal stenosis. If a patient describes weakness or sensory changes along one or both upper extremities, obtain imaging and consider more formalized therapy with a physical therapist.
In patients without any red flags, investigate possible biomechanical causes. It is essential to review the patient’s work and home habits, particularly in light of COVID-19, to determine if adjustments may be needed. Factors to consider are desk and computer setups at work or home, reading or laptop use in bed, sleep habits, and frequency of cellular phone calls/texting.26 A formal ergonomic assessment of the patient’s workplace may be helpful.
A mainstay in treating mechanical neck pain is alleviating trapezial tightness or spasm. Manipulative therapies such as osteopathic manipulation, massage, and chiropractic care can provide pain relief in the acute setting as well as help with control of chronic symptoms.27 A simple self-care tool is using a tennis ball to massage the trapezial muscles. This can be accomplished by having the patient position the tennis ball along the upper trapezial muscles, holding it in place by leaning against a wall, and initiating self-massage. Another method of self-massage is to put 2 tennis balls in an athletic tube sock and tie off the end, place the sock on the floor, and lie on it in the supine position.
There is also evidence that exercise of any kind can help control neck pain.28,29 The easiest exercises one can offer a patient with neck stiffness, or even mild cervical strains, is self-directed stretching through gentle pressure applied in all 4 directions on the neck. This technique can be repeated hourly both at work and at home (TABLE 4).

Reminders that can help ensure success
You can use the approaches described here for numerous other MSK conditions in helping patients on the road to recovery.
After the acute phase, advise patients to
• apply heat to the affected area before exercising. This can help bring blood flow to the region and promote ease of movement.
• continue icing the area following rehabilitation exercises in order to control exercise-induced inflammation.
• report any changing symptoms such as worsening pain, numbness, or weakness.
These techniques are one step in the recovery process. A home program can benefit the patient either alone or in combination with more advanced techniques that are best accomplished under the watchful eye of a physical or occupational therapist.
CORRESPONDENCE
Carrie A. Jaworski, MD, FAAFP, FACSM, 2180 Pfingsten Road, Suite 3100, Glenview, IL 60026; [email protected]
1. Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39:88-94.
2. Ho SS, Coel MN, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537-540.
3. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57-65.
4. Bleakley CM, O’Connor S, Tully MA, et al. The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain. BMC Musculoskelet Disord. 2007;8:125.
5. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395-403.
6. Cetin N, Aytar A, Atalay A, et al. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87:443-451.
7. Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284.
8. Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94.
9. Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002(3):CD003762.
10. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Ath Train. 2002;37:413-429.
11. Hü bscher M, Zech A, Pfeifer K, et al. Neuromuscular training for sports injury prevention: a systematic review. Med Sci Sports Exerc. 2010;42:413-421.
12. Emery CA, Meeuwisse WH. The effectiveness of a neuromuscular prevention strategy to reduce injuries in youth soccer: a cluster-randomised controlled trial. Br J Sports Med. 2010;44:555-562.
13. Tiemstra JD. Update on acute ankle sprains. Am Fam Physician. 2012;85:1170-1176.
14. Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Ath Train. 2002;37:376-380.
15. Schiftan GS, Ross LA, Hahne AJ. The effectiveness of proprioceptive training in preventing ankle sprains in sporting populations: a systematic review and meta-analysis. J Sci Med Sport. 2015;18:238–244.
16. Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684
17. Thompson JV, Saini SS, Reb CW, et al. Diagnosis and management of plantar fasciitis. J Am Osteopath Assoc. 2014;114:900-906.
18. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88:1775-1781.

19. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
20. Glaviano NR, Key M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. J Sports Phys Ther. 2015;10: 281-290.
21. Louden JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11: 820-830.
22. Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Ath Train. 2015;50: 366-377.
23. Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2013;41:31-49.
24. Bolgla LA. Hip strength and kinematics in patellofemoral syndrome. In: Brotzman SB, Manske RC eds. Clinical Orthopaedic Rehabilitation. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011:273-274.
25. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl 4):S39-S51.
26. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol. 2007; 21:447-463.
27. Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502.
28. Bronfort G, Evans R, Anderson A, et al. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1-10.
29. Evans R, Bronfort G, Bittell S, et al. A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients. J Manipulative Physiol Ther. 2003;26:403-411.
1. Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39:88-94.
2. Ho SS, Coel MN, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537-540.
3. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57-65.
4. Bleakley CM, O’Connor S, Tully MA, et al. The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain. BMC Musculoskelet Disord. 2007;8:125.
5. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395-403.
6. Cetin N, Aytar A, Atalay A, et al. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87:443-451.
7. Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284.
8. Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94.
9. Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002(3):CD003762.
10. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Ath Train. 2002;37:413-429.
11. Hü bscher M, Zech A, Pfeifer K, et al. Neuromuscular training for sports injury prevention: a systematic review. Med Sci Sports Exerc. 2010;42:413-421.
12. Emery CA, Meeuwisse WH. The effectiveness of a neuromuscular prevention strategy to reduce injuries in youth soccer: a cluster-randomised controlled trial. Br J Sports Med. 2010;44:555-562.
13. Tiemstra JD. Update on acute ankle sprains. Am Fam Physician. 2012;85:1170-1176.
14. Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Ath Train. 2002;37:376-380.
15. Schiftan GS, Ross LA, Hahne AJ. The effectiveness of proprioceptive training in preventing ankle sprains in sporting populations: a systematic review and meta-analysis. J Sci Med Sport. 2015;18:238–244.
16. Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684
17. Thompson JV, Saini SS, Reb CW, et al. Diagnosis and management of plantar fasciitis. J Am Osteopath Assoc. 2014;114:900-906.
18. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88:1775-1781.

19. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
20. Glaviano NR, Key M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. J Sports Phys Ther. 2015;10: 281-290.
21. Louden JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11: 820-830.
22. Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Ath Train. 2015;50: 366-377.
23. Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2013;41:31-49.
24. Bolgla LA. Hip strength and kinematics in patellofemoral syndrome. In: Brotzman SB, Manske RC eds. Clinical Orthopaedic Rehabilitation. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011:273-274.
25. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl 4):S39-S51.
26. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol. 2007; 21:447-463.
27. Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502.
28. Bronfort G, Evans R, Anderson A, et al. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1-10.
29. Evans R, Bronfort G, Bittell S, et al. A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients. J Manipulative Physiol Ther. 2003;26:403-411.
PRACTICE RECOMMENDATIONS
❯ Have patients apply ice to an acute injury for 15 to 20 minutes at a time to help control inflammation, and prescribe an anti-inflammatory medication, if indicated. A
❯ Reserve heat application for use following the acute phase of injury to decrease stiffness. A
❯ Instruct patients who have an acute lateral ankle sprain to begin “ankle ABCs” and other range-of-motion exercises once acute pain subsides. C
❯ Consider recommending an eccentric heel stretch to help alleviate plantar fasciitis symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Cognitive Behavioral Therapy Plus Placebo Is Inferior to NSAID Therapy for Arthritis Pain
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
JIA guideline calls for earlier use of targeted therapies
A draft guideline for the management of patients with juvenile idiopathic arthritis reflects changes in therapy away from reliance on NSAIDs and glucocorticoids and toward earlier introduction of biologic disease-modifying antirheumatic drugs (DMARDs).
The guideline, described in an oral session during the virtual annual meeting of the American College of Rheumatology, contains weighted recommendations for the treatment of JIA, including therapeutic approaches for oligoarthritis, tempromandibular joint (TMJ) arthritis, and systemic JIA (sJIA). The recommendations were the result of expert consensus and literature review using GRADE methodology, with input from clinicians, as well as patients and parents.
“Although evidence remains very low and many recommendations are conditional, the inclusion of parents and patients in the decision-making process strengthens their validity,” said project principal investigator Karen Onel, MD, of the Hospital for Special Surgery and Weill Cornell Medicine, both in New York.
She added that “it’s important to remember that these guidelines are meant to be guidelines; clinical care remains in the hands of the provider and the patient, and we endorse the importance of shared decision-making in coming to these agreements.”
Dr. Onel outlined key recommendations for patients for whom a diagnosis of JIA has already been made and who have no contraindications to recommended therapies. The strength of the recommendations (strong or conditional) and evidence levels (high, moderate, low, very low) were also reported.
Oligoarthritis with fewer than five involved joints
For these patients, intra-articular glucocorticoids (IAGC) are recommended as a part of initial therapy (strong, very low evidence).
Triamcinolone acetonide is the preferred agent in this situation (strong, low evidence).
The guideline also has a conditional recommendation (very low evidence) for a trial of consistent NSAIDS as part of initial therapy and a conditional recommendation against oral glucocorticoids for initial therapy (very low evidence).
Patients with no or incomplete responses or intolerance to NSAIDS and/or IAGC may be tried on a nonbiologic DMARD (strong, very low evidence), with methotrexate as the preferred agent (conditional, low evidence).
If the patient has no response or an inadequate response to at least one nonbiologic DMARD, biologic DMARDs are recommended (strong, very low evidence), with no preferred agent.
The guideline also conditionally recommends (all with very low evidence) using risk factors and validated disease activity measures to guide treatment decisions, as well as imaging guidance of joints that are difficult to access or to localize the distribution of inflammation.
TMJ arthritis
For patients with temporomandibular joint arthritis, isolated or not, IAGCs are conditionally recommended as part of initial therapy (very low evidence) with no preferred agents. The guideline also conditionally recommends in favor of a trial of consistent NSAIDs, and against oral glucocorticoids in initial therapy (evidence for both very low).
Recommendations for patients with TMJ with no or an incomplete response to the initial therapy are the same as for patients with oligoarthritis, with no preferred agent.
sJIA without macrophage activation syndrome
For patients with sJIA without macrophage activation syndrome (MAS), NSAIDS are conditionally recommended as initial monotherapy (very low evidence). Biologic DMARDS (including interleukin-1 and IL-6 inhibitors) are also recommended, conditionally, as initial monotherapy, with no preferred agent.
If the patient has an inadequate response or intolerance to NSAIDS and at least one nonbiologic DMARD, a single biologic DMARD is recommended over a combination of nonbiologic therapies (strong, very low evidence).
“However, there have been reports of emergent, highly severe lung disease associated with the use of biologics in children with systemic JIA, especially in those who are young, with chronic macrophage activation syndrome, and those with trisomy 21. More information is needed to clarify the safety of these agents,” Dr. Onel said.
There is a conditional recommendation against oral glucocorticoids as initial monotherapy, and strong recommendation against nonbiologic DMARDs as initial monotherapy (both very low evidence).
sJIA with MAS
“Macrophage activation syndrome is a major cause of morbidity and mortality for children with sJIA. Cytokine storm and secondary hemophagocytic syndrome can be seen with any rheumatic disease, but are most commonly seen with sJIA,” she said.
The features of MAS include fever, high ferritin levels, cytopenias, elevated liver-function test results, and high triglyceride levels.
For these patients, glucocorticoids are recommended as initial monotherapy (conditional, very low evidence). Biologic DMARDs (IL-1 and IL-6 inhibitors) are recommended over calcineurin inhibitors for achieving inactive disease and resolution of MAS (conditional, very low evidence). There is no preferred agent.
For patients with residual arthritis and an incomplete response to IL-1 or IL-6 inhibitors, biologic and nonbiologic DMARDs are recommended over chronic glucocorticoids (strong, very low evidence). There is no preferred agent.
After an MAS inactive disease state has been attained, the guideline recommends tapering and discontinuing glucocorticoids (strong, very low evidence) and the same for biologic DMARDs (conditional, very low evidence).
All children with JIA
In addition to the recommendations on specific clinical situations, the guideline includes recommendations for all children with JIA on medication monitoring, laboratory testing, and infection screening, as well as immunization and nonpharmacologic management.
A rheumatologist who was not involved in development of the guidelines commented on the importance of optimal management of JIA.
“Children are not immune from devastating rheumatic diseases, and the largest group is juvenile idiopathic arthritis. In my clinic, I have patients in their 30s, 40s, and 50s who have adult persistence of their arthritis from JIA who have permanent joint damage and even ongoing hard-to-control disease, and it has to do with the lack of therapies in the 1990s,” said Donald Thomas, MD, from Arthritis and Pain Associates of Prince George’s County (Md.).
“Today when we get a young adult transitioned from the pediatric clinic they’re usually in remission or have low disease activity because these treatments have paralleled those of our adult RA patients. Yet they do [provide clinicians with] unique challenges, with stunting of growth, macrophage activiation syndrome, and having to work with family members of the patient,” he said at a press briefing he moderated following the presentation of RA and JIA guidelines.
Eyal Muscal, MD, associate professor of pediatrics and rheumatology at Baylor College of Medicine, Houston, said in an interview that the guidelines clarify recommendations about earlier use of targeted therapies, primarily biologics.
“This will not change care, but hopefully remind all to adopt such strategies. Yet earlier utilization of often expensive biologic agents is delayed by administrative and insurance hurdles in the U.S. and access to these medications globally. I hope the guidelines will enhance advocacy on a state, national, and global stage,” he said when asked for comment.
The guideline development process is supported by ACR. Dr. Onel, Dr. Thomas, and Dr. Muscal reported no relevant conflicts of interest.
SOURCE: Onel K et al. ACR 2020, Presented November 8.
A draft guideline for the management of patients with juvenile idiopathic arthritis reflects changes in therapy away from reliance on NSAIDs and glucocorticoids and toward earlier introduction of biologic disease-modifying antirheumatic drugs (DMARDs).
The guideline, described in an oral session during the virtual annual meeting of the American College of Rheumatology, contains weighted recommendations for the treatment of JIA, including therapeutic approaches for oligoarthritis, tempromandibular joint (TMJ) arthritis, and systemic JIA (sJIA). The recommendations were the result of expert consensus and literature review using GRADE methodology, with input from clinicians, as well as patients and parents.
“Although evidence remains very low and many recommendations are conditional, the inclusion of parents and patients in the decision-making process strengthens their validity,” said project principal investigator Karen Onel, MD, of the Hospital for Special Surgery and Weill Cornell Medicine, both in New York.
She added that “it’s important to remember that these guidelines are meant to be guidelines; clinical care remains in the hands of the provider and the patient, and we endorse the importance of shared decision-making in coming to these agreements.”
Dr. Onel outlined key recommendations for patients for whom a diagnosis of JIA has already been made and who have no contraindications to recommended therapies. The strength of the recommendations (strong or conditional) and evidence levels (high, moderate, low, very low) were also reported.
Oligoarthritis with fewer than five involved joints
For these patients, intra-articular glucocorticoids (IAGC) are recommended as a part of initial therapy (strong, very low evidence).
Triamcinolone acetonide is the preferred agent in this situation (strong, low evidence).
The guideline also has a conditional recommendation (very low evidence) for a trial of consistent NSAIDS as part of initial therapy and a conditional recommendation against oral glucocorticoids for initial therapy (very low evidence).
Patients with no or incomplete responses or intolerance to NSAIDS and/or IAGC may be tried on a nonbiologic DMARD (strong, very low evidence), with methotrexate as the preferred agent (conditional, low evidence).
If the patient has no response or an inadequate response to at least one nonbiologic DMARD, biologic DMARDs are recommended (strong, very low evidence), with no preferred agent.
The guideline also conditionally recommends (all with very low evidence) using risk factors and validated disease activity measures to guide treatment decisions, as well as imaging guidance of joints that are difficult to access or to localize the distribution of inflammation.
TMJ arthritis
For patients with temporomandibular joint arthritis, isolated or not, IAGCs are conditionally recommended as part of initial therapy (very low evidence) with no preferred agents. The guideline also conditionally recommends in favor of a trial of consistent NSAIDs, and against oral glucocorticoids in initial therapy (evidence for both very low).
Recommendations for patients with TMJ with no or an incomplete response to the initial therapy are the same as for patients with oligoarthritis, with no preferred agent.
sJIA without macrophage activation syndrome
For patients with sJIA without macrophage activation syndrome (MAS), NSAIDS are conditionally recommended as initial monotherapy (very low evidence). Biologic DMARDS (including interleukin-1 and IL-6 inhibitors) are also recommended, conditionally, as initial monotherapy, with no preferred agent.
If the patient has an inadequate response or intolerance to NSAIDS and at least one nonbiologic DMARD, a single biologic DMARD is recommended over a combination of nonbiologic therapies (strong, very low evidence).
“However, there have been reports of emergent, highly severe lung disease associated with the use of biologics in children with systemic JIA, especially in those who are young, with chronic macrophage activation syndrome, and those with trisomy 21. More information is needed to clarify the safety of these agents,” Dr. Onel said.
There is a conditional recommendation against oral glucocorticoids as initial monotherapy, and strong recommendation against nonbiologic DMARDs as initial monotherapy (both very low evidence).
sJIA with MAS
“Macrophage activation syndrome is a major cause of morbidity and mortality for children with sJIA. Cytokine storm and secondary hemophagocytic syndrome can be seen with any rheumatic disease, but are most commonly seen with sJIA,” she said.
The features of MAS include fever, high ferritin levels, cytopenias, elevated liver-function test results, and high triglyceride levels.
For these patients, glucocorticoids are recommended as initial monotherapy (conditional, very low evidence). Biologic DMARDs (IL-1 and IL-6 inhibitors) are recommended over calcineurin inhibitors for achieving inactive disease and resolution of MAS (conditional, very low evidence). There is no preferred agent.
For patients with residual arthritis and an incomplete response to IL-1 or IL-6 inhibitors, biologic and nonbiologic DMARDs are recommended over chronic glucocorticoids (strong, very low evidence). There is no preferred agent.
After an MAS inactive disease state has been attained, the guideline recommends tapering and discontinuing glucocorticoids (strong, very low evidence) and the same for biologic DMARDs (conditional, very low evidence).
All children with JIA
In addition to the recommendations on specific clinical situations, the guideline includes recommendations for all children with JIA on medication monitoring, laboratory testing, and infection screening, as well as immunization and nonpharmacologic management.
A rheumatologist who was not involved in development of the guidelines commented on the importance of optimal management of JIA.
“Children are not immune from devastating rheumatic diseases, and the largest group is juvenile idiopathic arthritis. In my clinic, I have patients in their 30s, 40s, and 50s who have adult persistence of their arthritis from JIA who have permanent joint damage and even ongoing hard-to-control disease, and it has to do with the lack of therapies in the 1990s,” said Donald Thomas, MD, from Arthritis and Pain Associates of Prince George’s County (Md.).
“Today when we get a young adult transitioned from the pediatric clinic they’re usually in remission or have low disease activity because these treatments have paralleled those of our adult RA patients. Yet they do [provide clinicians with] unique challenges, with stunting of growth, macrophage activiation syndrome, and having to work with family members of the patient,” he said at a press briefing he moderated following the presentation of RA and JIA guidelines.
Eyal Muscal, MD, associate professor of pediatrics and rheumatology at Baylor College of Medicine, Houston, said in an interview that the guidelines clarify recommendations about earlier use of targeted therapies, primarily biologics.
“This will not change care, but hopefully remind all to adopt such strategies. Yet earlier utilization of often expensive biologic agents is delayed by administrative and insurance hurdles in the U.S. and access to these medications globally. I hope the guidelines will enhance advocacy on a state, national, and global stage,” he said when asked for comment.
The guideline development process is supported by ACR. Dr. Onel, Dr. Thomas, and Dr. Muscal reported no relevant conflicts of interest.
SOURCE: Onel K et al. ACR 2020, Presented November 8.
A draft guideline for the management of patients with juvenile idiopathic arthritis reflects changes in therapy away from reliance on NSAIDs and glucocorticoids and toward earlier introduction of biologic disease-modifying antirheumatic drugs (DMARDs).
The guideline, described in an oral session during the virtual annual meeting of the American College of Rheumatology, contains weighted recommendations for the treatment of JIA, including therapeutic approaches for oligoarthritis, tempromandibular joint (TMJ) arthritis, and systemic JIA (sJIA). The recommendations were the result of expert consensus and literature review using GRADE methodology, with input from clinicians, as well as patients and parents.
“Although evidence remains very low and many recommendations are conditional, the inclusion of parents and patients in the decision-making process strengthens their validity,” said project principal investigator Karen Onel, MD, of the Hospital for Special Surgery and Weill Cornell Medicine, both in New York.
She added that “it’s important to remember that these guidelines are meant to be guidelines; clinical care remains in the hands of the provider and the patient, and we endorse the importance of shared decision-making in coming to these agreements.”
Dr. Onel outlined key recommendations for patients for whom a diagnosis of JIA has already been made and who have no contraindications to recommended therapies. The strength of the recommendations (strong or conditional) and evidence levels (high, moderate, low, very low) were also reported.
Oligoarthritis with fewer than five involved joints
For these patients, intra-articular glucocorticoids (IAGC) are recommended as a part of initial therapy (strong, very low evidence).
Triamcinolone acetonide is the preferred agent in this situation (strong, low evidence).
The guideline also has a conditional recommendation (very low evidence) for a trial of consistent NSAIDS as part of initial therapy and a conditional recommendation against oral glucocorticoids for initial therapy (very low evidence).
Patients with no or incomplete responses or intolerance to NSAIDS and/or IAGC may be tried on a nonbiologic DMARD (strong, very low evidence), with methotrexate as the preferred agent (conditional, low evidence).
If the patient has no response or an inadequate response to at least one nonbiologic DMARD, biologic DMARDs are recommended (strong, very low evidence), with no preferred agent.
The guideline also conditionally recommends (all with very low evidence) using risk factors and validated disease activity measures to guide treatment decisions, as well as imaging guidance of joints that are difficult to access or to localize the distribution of inflammation.
TMJ arthritis
For patients with temporomandibular joint arthritis, isolated or not, IAGCs are conditionally recommended as part of initial therapy (very low evidence) with no preferred agents. The guideline also conditionally recommends in favor of a trial of consistent NSAIDs, and against oral glucocorticoids in initial therapy (evidence for both very low).
Recommendations for patients with TMJ with no or an incomplete response to the initial therapy are the same as for patients with oligoarthritis, with no preferred agent.
sJIA without macrophage activation syndrome
For patients with sJIA without macrophage activation syndrome (MAS), NSAIDS are conditionally recommended as initial monotherapy (very low evidence). Biologic DMARDS (including interleukin-1 and IL-6 inhibitors) are also recommended, conditionally, as initial monotherapy, with no preferred agent.
If the patient has an inadequate response or intolerance to NSAIDS and at least one nonbiologic DMARD, a single biologic DMARD is recommended over a combination of nonbiologic therapies (strong, very low evidence).
“However, there have been reports of emergent, highly severe lung disease associated with the use of biologics in children with systemic JIA, especially in those who are young, with chronic macrophage activation syndrome, and those with trisomy 21. More information is needed to clarify the safety of these agents,” Dr. Onel said.
There is a conditional recommendation against oral glucocorticoids as initial monotherapy, and strong recommendation against nonbiologic DMARDs as initial monotherapy (both very low evidence).
sJIA with MAS
“Macrophage activation syndrome is a major cause of morbidity and mortality for children with sJIA. Cytokine storm and secondary hemophagocytic syndrome can be seen with any rheumatic disease, but are most commonly seen with sJIA,” she said.
The features of MAS include fever, high ferritin levels, cytopenias, elevated liver-function test results, and high triglyceride levels.
For these patients, glucocorticoids are recommended as initial monotherapy (conditional, very low evidence). Biologic DMARDs (IL-1 and IL-6 inhibitors) are recommended over calcineurin inhibitors for achieving inactive disease and resolution of MAS (conditional, very low evidence). There is no preferred agent.
For patients with residual arthritis and an incomplete response to IL-1 or IL-6 inhibitors, biologic and nonbiologic DMARDs are recommended over chronic glucocorticoids (strong, very low evidence). There is no preferred agent.
After an MAS inactive disease state has been attained, the guideline recommends tapering and discontinuing glucocorticoids (strong, very low evidence) and the same for biologic DMARDs (conditional, very low evidence).
All children with JIA
In addition to the recommendations on specific clinical situations, the guideline includes recommendations for all children with JIA on medication monitoring, laboratory testing, and infection screening, as well as immunization and nonpharmacologic management.
A rheumatologist who was not involved in development of the guidelines commented on the importance of optimal management of JIA.
“Children are not immune from devastating rheumatic diseases, and the largest group is juvenile idiopathic arthritis. In my clinic, I have patients in their 30s, 40s, and 50s who have adult persistence of their arthritis from JIA who have permanent joint damage and even ongoing hard-to-control disease, and it has to do with the lack of therapies in the 1990s,” said Donald Thomas, MD, from Arthritis and Pain Associates of Prince George’s County (Md.).
“Today when we get a young adult transitioned from the pediatric clinic they’re usually in remission or have low disease activity because these treatments have paralleled those of our adult RA patients. Yet they do [provide clinicians with] unique challenges, with stunting of growth, macrophage activiation syndrome, and having to work with family members of the patient,” he said at a press briefing he moderated following the presentation of RA and JIA guidelines.
Eyal Muscal, MD, associate professor of pediatrics and rheumatology at Baylor College of Medicine, Houston, said in an interview that the guidelines clarify recommendations about earlier use of targeted therapies, primarily biologics.
“This will not change care, but hopefully remind all to adopt such strategies. Yet earlier utilization of often expensive biologic agents is delayed by administrative and insurance hurdles in the U.S. and access to these medications globally. I hope the guidelines will enhance advocacy on a state, national, and global stage,” he said when asked for comment.
The guideline development process is supported by ACR. Dr. Onel, Dr. Thomas, and Dr. Muscal reported no relevant conflicts of interest.
SOURCE: Onel K et al. ACR 2020, Presented November 8.
FROM ACR 2020