User login
Sleep time ‘sweet spot’ to slow cognitive decline identified?
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis, said in an interview.
The study, published online Oct. 20, 2021, in the journal Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, AD, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of AD, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid-beta, a hallmark sign of AD.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University.
Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta 42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR greater than 0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of AD pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale–Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale–Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both).
The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta 42 ratio, apo E four-allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of AD could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis, said in an interview.
The study, published online Oct. 20, 2021, in the journal Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, AD, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of AD, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid-beta, a hallmark sign of AD.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University.
Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta 42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR greater than 0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of AD pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale–Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale–Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both).
The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta 42 ratio, apo E four-allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of AD could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis, said in an interview.
The study, published online Oct. 20, 2021, in the journal Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, AD, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of AD, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid-beta, a hallmark sign of AD.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University.
Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta 42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR greater than 0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of AD pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale–Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale–Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both).
The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta 42 ratio, apo E four-allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of AD could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MS and COVID: Docs switched DMTs but maybe didn’t need to
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
FROM CMSC 2021
Autism prevalence in children as high as 10% in some New Jersey communities
Investigators found that up to 10% of children in some of the state’s school districts have an ASD diagnosis vs. the national average of just under 2%.
School districts with higher ASD prevalence in the study have expansive health and educational programs in place to diagnose and support children with ASD, which likely contributed to the higher caseloads, senior investigator Walter Zahorodny, PhD, associate professor of pediatrics at New Jersey Medical School in Newark, said in an interview.
“When you have those players on the ground, it’s likely we’re going to be finding more, if not almost all, of the children with autism in a given district,” said Dr. Zahorodny, director of the New Jersey Autism Study for the Centers for Disease Control and Prevention.
The study was published online Oct. 21 in the journal Autism Research.
Local analysis
Researchers used the Autism and Developmental Disabilities Monitoring (ADDM) Network, a surveillance method developed by the CDC that includes data collected from health and special education records of children living in 11 communities across the United States. New Jersey is one of the ADDM participating sites.
National data are important, but Dr. Zahorodny and colleagues wanted to examine ASD prevalence at a more granular level, comparing prevalence district by district.
They examined data from 5,453 children who were 8 years old in 2016 and attended public school in Essex, Hudson, Ocean, and Union counties.
The prevalence of ASD was 36 children per 1,000 overall. Hudson County reported the lowest rate, at 31 cases per 1,000 children, and Ocean County reported the highest, at 54 cases per 1,000 children.
Across the region, ASD prevalence was four times higher in boys vs. girls, mirroring national statistics.
High ASD prevalence was more likely in mid-socioeconomic status districts (prevalence ratio [PR], 1.2; P = .01) and in larger school districts (PR, 1.3; P = .004).
Hispanic children had significantly lower ASD prevalence overall compared with White children (PR, 0.6; P < .001). In fact, prevalence was 30%-60% lower among Hispanic children in three of four counties compared with White children.
Another study is underway to better understand why autism rates were lower in specific districts and Hispanic children overall, but Dr. Zahorodny said one possibility is a lack of resources in those districts.
Will new methodology miss cases?
The study’s methodology was used by the CDC from 2000 to 2016 and includes assessment of children who have an ASD diagnosis, and children who haven’t received a diagnosis but have documented behaviors consistent with ASD.
In 2018, it was replaced with a less comprehensive approach that relies only on children with an ASD diagnosis. Data using this new methodology have not yet been reported.
In the new study from New Jersey, 767 children with autism were diagnosed by a pediatrician, neurologist, or other community provider. The remaining 175 children with autism, 18.6% of the total cohort, did not have an ASD diagnosis but met the ADDM case definition.
Under the new methodology, those children would not be counted.
“Something could be lost in the new methodology in terms of usefulness of the information because when the estimates are incomplete or low, that might lead people to make the wrong judgments when they make decisions about resources,” Dr. Zahorodny said.
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. The study authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that up to 10% of children in some of the state’s school districts have an ASD diagnosis vs. the national average of just under 2%.
School districts with higher ASD prevalence in the study have expansive health and educational programs in place to diagnose and support children with ASD, which likely contributed to the higher caseloads, senior investigator Walter Zahorodny, PhD, associate professor of pediatrics at New Jersey Medical School in Newark, said in an interview.
“When you have those players on the ground, it’s likely we’re going to be finding more, if not almost all, of the children with autism in a given district,” said Dr. Zahorodny, director of the New Jersey Autism Study for the Centers for Disease Control and Prevention.
The study was published online Oct. 21 in the journal Autism Research.
Local analysis
Researchers used the Autism and Developmental Disabilities Monitoring (ADDM) Network, a surveillance method developed by the CDC that includes data collected from health and special education records of children living in 11 communities across the United States. New Jersey is one of the ADDM participating sites.
National data are important, but Dr. Zahorodny and colleagues wanted to examine ASD prevalence at a more granular level, comparing prevalence district by district.
They examined data from 5,453 children who were 8 years old in 2016 and attended public school in Essex, Hudson, Ocean, and Union counties.
The prevalence of ASD was 36 children per 1,000 overall. Hudson County reported the lowest rate, at 31 cases per 1,000 children, and Ocean County reported the highest, at 54 cases per 1,000 children.
Across the region, ASD prevalence was four times higher in boys vs. girls, mirroring national statistics.
High ASD prevalence was more likely in mid-socioeconomic status districts (prevalence ratio [PR], 1.2; P = .01) and in larger school districts (PR, 1.3; P = .004).
Hispanic children had significantly lower ASD prevalence overall compared with White children (PR, 0.6; P < .001). In fact, prevalence was 30%-60% lower among Hispanic children in three of four counties compared with White children.
Another study is underway to better understand why autism rates were lower in specific districts and Hispanic children overall, but Dr. Zahorodny said one possibility is a lack of resources in those districts.
Will new methodology miss cases?
The study’s methodology was used by the CDC from 2000 to 2016 and includes assessment of children who have an ASD diagnosis, and children who haven’t received a diagnosis but have documented behaviors consistent with ASD.
In 2018, it was replaced with a less comprehensive approach that relies only on children with an ASD diagnosis. Data using this new methodology have not yet been reported.
In the new study from New Jersey, 767 children with autism were diagnosed by a pediatrician, neurologist, or other community provider. The remaining 175 children with autism, 18.6% of the total cohort, did not have an ASD diagnosis but met the ADDM case definition.
Under the new methodology, those children would not be counted.
“Something could be lost in the new methodology in terms of usefulness of the information because when the estimates are incomplete or low, that might lead people to make the wrong judgments when they make decisions about resources,” Dr. Zahorodny said.
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. The study authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that up to 10% of children in some of the state’s school districts have an ASD diagnosis vs. the national average of just under 2%.
School districts with higher ASD prevalence in the study have expansive health and educational programs in place to diagnose and support children with ASD, which likely contributed to the higher caseloads, senior investigator Walter Zahorodny, PhD, associate professor of pediatrics at New Jersey Medical School in Newark, said in an interview.
“When you have those players on the ground, it’s likely we’re going to be finding more, if not almost all, of the children with autism in a given district,” said Dr. Zahorodny, director of the New Jersey Autism Study for the Centers for Disease Control and Prevention.
The study was published online Oct. 21 in the journal Autism Research.
Local analysis
Researchers used the Autism and Developmental Disabilities Monitoring (ADDM) Network, a surveillance method developed by the CDC that includes data collected from health and special education records of children living in 11 communities across the United States. New Jersey is one of the ADDM participating sites.
National data are important, but Dr. Zahorodny and colleagues wanted to examine ASD prevalence at a more granular level, comparing prevalence district by district.
They examined data from 5,453 children who were 8 years old in 2016 and attended public school in Essex, Hudson, Ocean, and Union counties.
The prevalence of ASD was 36 children per 1,000 overall. Hudson County reported the lowest rate, at 31 cases per 1,000 children, and Ocean County reported the highest, at 54 cases per 1,000 children.
Across the region, ASD prevalence was four times higher in boys vs. girls, mirroring national statistics.
High ASD prevalence was more likely in mid-socioeconomic status districts (prevalence ratio [PR], 1.2; P = .01) and in larger school districts (PR, 1.3; P = .004).
Hispanic children had significantly lower ASD prevalence overall compared with White children (PR, 0.6; P < .001). In fact, prevalence was 30%-60% lower among Hispanic children in three of four counties compared with White children.
Another study is underway to better understand why autism rates were lower in specific districts and Hispanic children overall, but Dr. Zahorodny said one possibility is a lack of resources in those districts.
Will new methodology miss cases?
The study’s methodology was used by the CDC from 2000 to 2016 and includes assessment of children who have an ASD diagnosis, and children who haven’t received a diagnosis but have documented behaviors consistent with ASD.
In 2018, it was replaced with a less comprehensive approach that relies only on children with an ASD diagnosis. Data using this new methodology have not yet been reported.
In the new study from New Jersey, 767 children with autism were diagnosed by a pediatrician, neurologist, or other community provider. The remaining 175 children with autism, 18.6% of the total cohort, did not have an ASD diagnosis but met the ADDM case definition.
Under the new methodology, those children would not be counted.
“Something could be lost in the new methodology in terms of usefulness of the information because when the estimates are incomplete or low, that might lead people to make the wrong judgments when they make decisions about resources,” Dr. Zahorodny said.
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. The study authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ohio records more deaths than births for first time
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
Sleep time ‘sweet spot’ to slow cognitive decline identified?
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
From Brain
Opioid-induced adrenal insufficiency for the hospitalist
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
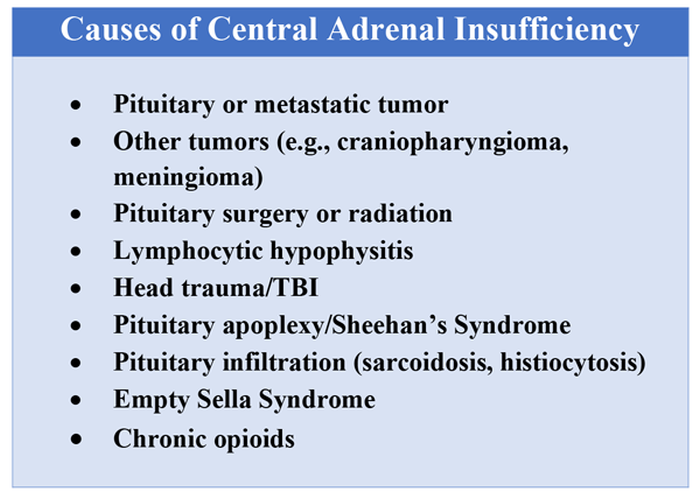
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4
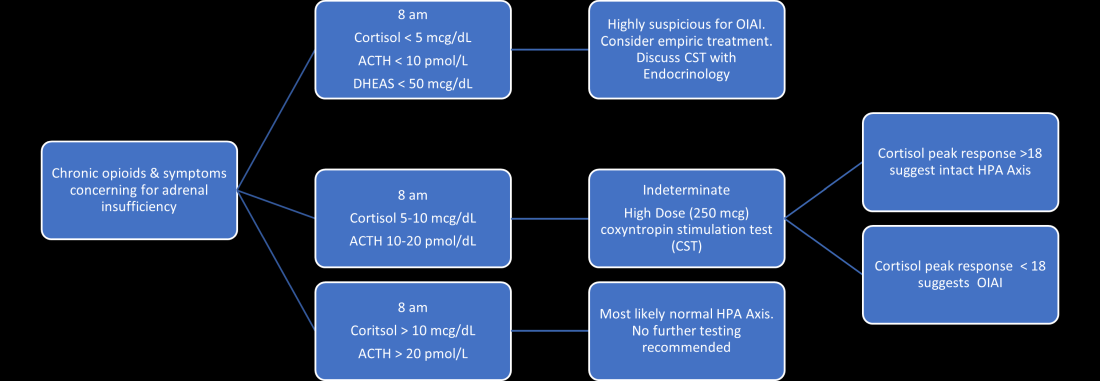
The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.
To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.
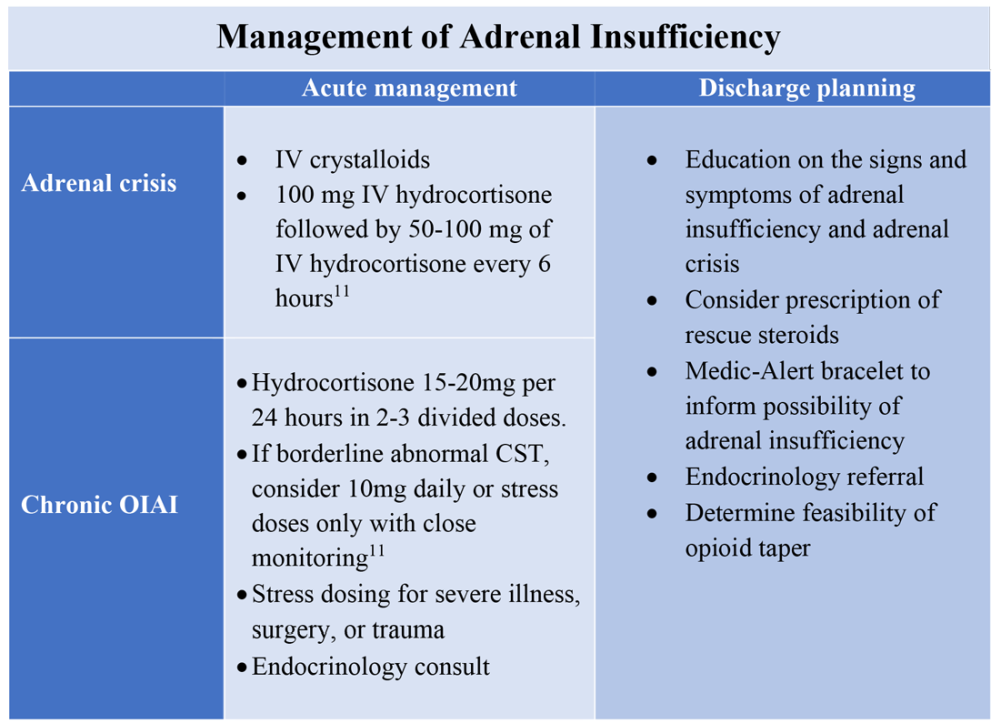
All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Consider OIAI, even among patients with common infections
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Steroid-induced psychosis in MS? Quetiapine may help
a new case review says.
“Our case-report study observed that quetiapine was effective at decreasing irritability, reducing psychological distress, and improving sleep in patients with MS who experienced psychosis symptoms compared with patients who received no treatment. This has changed our practice as we now counsel all patients about the potential side effect of steroid-induced psychosis and discuss treatment options,” said Olinka Hrebicek, MD, medical director of Vancouver Island Multiple Sclerosis Clinic in Victoria, B.C., who was scheduled to present the study findings at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
According to Dr. Hrebicek, who spoke in an interview, nursing staff and neurologists at the Canadian clinic had typically attributed symptoms such as irritability, anger, insomnia, and psychological distress to the stress of experiencing a relapse. The treatment often was a prescription for a benzodiazepine or zopiclone.
In fact, she and colleagues wrote in their report, psychosis following treatment with high-dose corticosteroids for MS may be underreported.
“The purpose of the study was to determine whether quetiapine was effective for treating symptoms of steroid-induced psychosis in patients with MS,” study coauthor and clinic research assistant Niall Murphy said in an interview. “We also wanted to highlight the importance of looking for symptoms of steroid-induced psychosis as this is likely not the primary concern when treating patients for a relapse. In addition, nurses and neurologists may have less experience with the spectrum of clinical symptoms of psychosis than psychiatrists.”
For the case review, researchers examined 10 reports (8 female) of patients who had signs of psychiatric distress after treatment with steroids. Eight of the patients were treated with quetiapine (six female, two male).
All those who took quetiapine experienced benefits, while the two others didn’t improve.
Commenting on the study, E. Sherwood Brown, MD, PhD, MBA, professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas, said in an interview that psychosis may not appear as expected in patients who develop it as a result of corticosteroid use. “Typically, psychosis refers to delusions, hallucinations, or disorganized thought processes. However, with corticosteroids severe mood and cognitive changes [for example, delirium] are also often included in the definition. Mild mood and memory changes appear to be fairly common with prescription corticosteroids. More severe symptoms are less common.”
Higher doses of corticosteroids – like those used in MS – boost the risk of psychosis, said Dr. Brown, who was not involved in the study.
As for quetiapine, Dr. Brown said it could be a good treatment option. “The use of quetiapine, a drug approved for schizophrenia and mania, is consistent with the idea suggested in the literature that the symptoms with corticosteroids tend to be similar to those of bipolar disorder and that they respond to medications for bipolar disorder,” he said. “A potential concern is that both corticosteroids and quetiapine can cause weight gain. However, this may not be a major problem with a brief course of the corticosteroids. It would be great to see a randomized, controlled trial.”
In British Columbia, the Victoria clinic has changed policy as a result of the analysis, Dr. Hrebicek said. “Nurses and physicians now ask more specific questions to decide if patients are experiencing symptoms of steroid-induced psychosis and whether they should be treated with an antipsychotic medication.”
And now, report coauthor Mr. Murphy said, “our clinic proactively offers patients a prescription for quetiapine that they can fill if they are experiencing symptoms of steroid psychosis.”
Dr. Brown supported the new policy of alerting patients to the psychosis risk. “Counseling patients about common side effects is a good idea,” he said. “I have seen data suggesting that patients may be hesitant to report psychiatric symptoms with corticosteroids to their physicians. Letting them know about the potential for these kinds of side effects might make them more forthcoming in reporting this side effect.”
No study funding is reported. The study authors reported no disclosures. Dr. Brown has a National Institutes of Health grant for studying the effect of corticosteroids on the brain.
a new case review says.
“Our case-report study observed that quetiapine was effective at decreasing irritability, reducing psychological distress, and improving sleep in patients with MS who experienced psychosis symptoms compared with patients who received no treatment. This has changed our practice as we now counsel all patients about the potential side effect of steroid-induced psychosis and discuss treatment options,” said Olinka Hrebicek, MD, medical director of Vancouver Island Multiple Sclerosis Clinic in Victoria, B.C., who was scheduled to present the study findings at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
According to Dr. Hrebicek, who spoke in an interview, nursing staff and neurologists at the Canadian clinic had typically attributed symptoms such as irritability, anger, insomnia, and psychological distress to the stress of experiencing a relapse. The treatment often was a prescription for a benzodiazepine or zopiclone.
In fact, she and colleagues wrote in their report, psychosis following treatment with high-dose corticosteroids for MS may be underreported.
“The purpose of the study was to determine whether quetiapine was effective for treating symptoms of steroid-induced psychosis in patients with MS,” study coauthor and clinic research assistant Niall Murphy said in an interview. “We also wanted to highlight the importance of looking for symptoms of steroid-induced psychosis as this is likely not the primary concern when treating patients for a relapse. In addition, nurses and neurologists may have less experience with the spectrum of clinical symptoms of psychosis than psychiatrists.”
For the case review, researchers examined 10 reports (8 female) of patients who had signs of psychiatric distress after treatment with steroids. Eight of the patients were treated with quetiapine (six female, two male).
All those who took quetiapine experienced benefits, while the two others didn’t improve.
Commenting on the study, E. Sherwood Brown, MD, PhD, MBA, professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas, said in an interview that psychosis may not appear as expected in patients who develop it as a result of corticosteroid use. “Typically, psychosis refers to delusions, hallucinations, or disorganized thought processes. However, with corticosteroids severe mood and cognitive changes [for example, delirium] are also often included in the definition. Mild mood and memory changes appear to be fairly common with prescription corticosteroids. More severe symptoms are less common.”
Higher doses of corticosteroids – like those used in MS – boost the risk of psychosis, said Dr. Brown, who was not involved in the study.
As for quetiapine, Dr. Brown said it could be a good treatment option. “The use of quetiapine, a drug approved for schizophrenia and mania, is consistent with the idea suggested in the literature that the symptoms with corticosteroids tend to be similar to those of bipolar disorder and that they respond to medications for bipolar disorder,” he said. “A potential concern is that both corticosteroids and quetiapine can cause weight gain. However, this may not be a major problem with a brief course of the corticosteroids. It would be great to see a randomized, controlled trial.”
In British Columbia, the Victoria clinic has changed policy as a result of the analysis, Dr. Hrebicek said. “Nurses and physicians now ask more specific questions to decide if patients are experiencing symptoms of steroid-induced psychosis and whether they should be treated with an antipsychotic medication.”
And now, report coauthor Mr. Murphy said, “our clinic proactively offers patients a prescription for quetiapine that they can fill if they are experiencing symptoms of steroid psychosis.”
Dr. Brown supported the new policy of alerting patients to the psychosis risk. “Counseling patients about common side effects is a good idea,” he said. “I have seen data suggesting that patients may be hesitant to report psychiatric symptoms with corticosteroids to their physicians. Letting them know about the potential for these kinds of side effects might make them more forthcoming in reporting this side effect.”
No study funding is reported. The study authors reported no disclosures. Dr. Brown has a National Institutes of Health grant for studying the effect of corticosteroids on the brain.
a new case review says.
“Our case-report study observed that quetiapine was effective at decreasing irritability, reducing psychological distress, and improving sleep in patients with MS who experienced psychosis symptoms compared with patients who received no treatment. This has changed our practice as we now counsel all patients about the potential side effect of steroid-induced psychosis and discuss treatment options,” said Olinka Hrebicek, MD, medical director of Vancouver Island Multiple Sclerosis Clinic in Victoria, B.C., who was scheduled to present the study findings at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
According to Dr. Hrebicek, who spoke in an interview, nursing staff and neurologists at the Canadian clinic had typically attributed symptoms such as irritability, anger, insomnia, and psychological distress to the stress of experiencing a relapse. The treatment often was a prescription for a benzodiazepine or zopiclone.
In fact, she and colleagues wrote in their report, psychosis following treatment with high-dose corticosteroids for MS may be underreported.
“The purpose of the study was to determine whether quetiapine was effective for treating symptoms of steroid-induced psychosis in patients with MS,” study coauthor and clinic research assistant Niall Murphy said in an interview. “We also wanted to highlight the importance of looking for symptoms of steroid-induced psychosis as this is likely not the primary concern when treating patients for a relapse. In addition, nurses and neurologists may have less experience with the spectrum of clinical symptoms of psychosis than psychiatrists.”
For the case review, researchers examined 10 reports (8 female) of patients who had signs of psychiatric distress after treatment with steroids. Eight of the patients were treated with quetiapine (six female, two male).
All those who took quetiapine experienced benefits, while the two others didn’t improve.
Commenting on the study, E. Sherwood Brown, MD, PhD, MBA, professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas, said in an interview that psychosis may not appear as expected in patients who develop it as a result of corticosteroid use. “Typically, psychosis refers to delusions, hallucinations, or disorganized thought processes. However, with corticosteroids severe mood and cognitive changes [for example, delirium] are also often included in the definition. Mild mood and memory changes appear to be fairly common with prescription corticosteroids. More severe symptoms are less common.”
Higher doses of corticosteroids – like those used in MS – boost the risk of psychosis, said Dr. Brown, who was not involved in the study.
As for quetiapine, Dr. Brown said it could be a good treatment option. “The use of quetiapine, a drug approved for schizophrenia and mania, is consistent with the idea suggested in the literature that the symptoms with corticosteroids tend to be similar to those of bipolar disorder and that they respond to medications for bipolar disorder,” he said. “A potential concern is that both corticosteroids and quetiapine can cause weight gain. However, this may not be a major problem with a brief course of the corticosteroids. It would be great to see a randomized, controlled trial.”
In British Columbia, the Victoria clinic has changed policy as a result of the analysis, Dr. Hrebicek said. “Nurses and physicians now ask more specific questions to decide if patients are experiencing symptoms of steroid-induced psychosis and whether they should be treated with an antipsychotic medication.”
And now, report coauthor Mr. Murphy said, “our clinic proactively offers patients a prescription for quetiapine that they can fill if they are experiencing symptoms of steroid psychosis.”
Dr. Brown supported the new policy of alerting patients to the psychosis risk. “Counseling patients about common side effects is a good idea,” he said. “I have seen data suggesting that patients may be hesitant to report psychiatric symptoms with corticosteroids to their physicians. Letting them know about the potential for these kinds of side effects might make them more forthcoming in reporting this side effect.”
No study funding is reported. The study authors reported no disclosures. Dr. Brown has a National Institutes of Health grant for studying the effect of corticosteroids on the brain.
FROM CMSC 2021
Antiepileptic medications linked to increased priapism risk
Several antiepileptic drugs (AEDs) are associated with an increased risk for priapism, new research suggests.
After analyzing U.S. adverse event reporting data, investigators found that among nearly 200 cases of priapism, a persistent, often painful erection unrelated to sexual interest or stimulation that lasts more than 4 hours, eight AEDs were associated with a positive “safety signal” for priapism.
These included valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine. Of these, valpromide had the largest association.
“Based on our results, we would recommend to clinicians to be cautious about the possibility of encountering priapism” in patients receiving the eight AEDs identified, lead researcher Ana Pejcic, PhD, department of pharmacology and toxicology, University of Kragujevac, Serbia, told meeting attendees.
If clinicians encounter such cases, they should be “reported to the regulatory authorities,” Dr. Pejcic added.
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
Noteworthy limitations
Dr. Pejcic told this news organization that the safety signal with AEDs “does not directly mean that a medicine has caused the reported adverse event” because an illness or other drug taken by the patient could be responsible instead.
She also noted that the U.S. Food and Drug Administration’s Adverse Event Reporting System relies on “spontaneous reports of adverse events,” which have multiple limitations.
These limitations include that the FDA “does not require that a causal relationship between a drug and event be proven, and reports do not always have enough information to properly evaluate an event.”
Nevertheless, Dr. Pejcic added that if a causal relationship was to be shown, the underlying mechanism could be linked to the pharmacological properties of the individual antiepileptic, such as altered alpha-1 adrenergic receptor expression or increased dopamine release.
Still, that would require “further evaluation in larger pharmacoepidemiological studies, with adjustment for potential confounding variables,” she said.
Replication needed
Priapism has recently been observed in case reports in association with the use of some AEDs. In addition, use of the drugs has been associated with hypo- and hypersexuality, as well as erectile and ejaculatory dysfunction.
Because the relationship between priapism and AED use “has not been well characterized,” the researchers mined data from the FDA’s Adverse Event Reporting System.
They examined entries from the first quarter of 2004 and the third quarter of 2020, focusing on 47 AEDs from the N03A subgroup of the Anatomical Therapeutic Chemical Classification System.
The researchers identified 8,122,037 cases for data analysis, of which 1,936 involved priapism as an adverse event. In total, 16 antiepileptic medications had at least one case of an adverse event involving priapism.
A positive safety signal was defined as a Proportional Reporting Ratio (PRR) of at least two, a chi-squared of at least four, or three or more cases. The signal was detected for valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine.
The largest association with priapism was with valpromide, at a PRR of 61.79. That was followed by PRR of 9.61 for brivaracetam, 7.28 for valproic acid, and 3.23 for topiramate.
“Considering that the proportionality analysis we applied in our study is used for hypothesis generation, our results will need to confirm in large cohorts and case-control studies,” said Dr. Pejcic.
New and important hypothesis?
Commenting on the study, Daniel Goldenholz, MD, PhD, instructor in the Division of Epilepsy, Beth Israel Deaconess Medical Center, Boston, said priapism is not something that practicing epileptologists are instructed “to look for.”
He noted that “the idea of looking for a hidden signal in a massive database like this is very appealing” because it could reveal patterns that were previously undetected.
However, the event rate in the study suggests priapism, which “in the right context would be considered a medical emergency, [is] relatively uncommon,” said Dr. Goldenholz, who was not involved with the research.
He noted that medications that could cause priapism, “such as antidepressants, blood pressure meds, and anticoagulants,” are commonly used by many people – including those with epilepsy.
It is consequently possible that “the finding from this study can be explained by comorbid medical problems,” Dr. Goldenholz said. This is particularly likely because many of the AEDs in question “have been on the market for decades,” he added.
“If a seemingly dangerous symptom would be happening as a result of one of these medications, ,” he said.
Still, Dr. Goldenholz noted that it is “possible that these authors have a new and important hypothesis which must now be tested: Does priapism occur in patients with antiseizure medications when other causes are already ruled out?”
The investigators and Dr. Goldenholz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several antiepileptic drugs (AEDs) are associated with an increased risk for priapism, new research suggests.
After analyzing U.S. adverse event reporting data, investigators found that among nearly 200 cases of priapism, a persistent, often painful erection unrelated to sexual interest or stimulation that lasts more than 4 hours, eight AEDs were associated with a positive “safety signal” for priapism.
These included valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine. Of these, valpromide had the largest association.
“Based on our results, we would recommend to clinicians to be cautious about the possibility of encountering priapism” in patients receiving the eight AEDs identified, lead researcher Ana Pejcic, PhD, department of pharmacology and toxicology, University of Kragujevac, Serbia, told meeting attendees.
If clinicians encounter such cases, they should be “reported to the regulatory authorities,” Dr. Pejcic added.
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
Noteworthy limitations
Dr. Pejcic told this news organization that the safety signal with AEDs “does not directly mean that a medicine has caused the reported adverse event” because an illness or other drug taken by the patient could be responsible instead.
She also noted that the U.S. Food and Drug Administration’s Adverse Event Reporting System relies on “spontaneous reports of adverse events,” which have multiple limitations.
These limitations include that the FDA “does not require that a causal relationship between a drug and event be proven, and reports do not always have enough information to properly evaluate an event.”
Nevertheless, Dr. Pejcic added that if a causal relationship was to be shown, the underlying mechanism could be linked to the pharmacological properties of the individual antiepileptic, such as altered alpha-1 adrenergic receptor expression or increased dopamine release.
Still, that would require “further evaluation in larger pharmacoepidemiological studies, with adjustment for potential confounding variables,” she said.
Replication needed
Priapism has recently been observed in case reports in association with the use of some AEDs. In addition, use of the drugs has been associated with hypo- and hypersexuality, as well as erectile and ejaculatory dysfunction.
Because the relationship between priapism and AED use “has not been well characterized,” the researchers mined data from the FDA’s Adverse Event Reporting System.
They examined entries from the first quarter of 2004 and the third quarter of 2020, focusing on 47 AEDs from the N03A subgroup of the Anatomical Therapeutic Chemical Classification System.
The researchers identified 8,122,037 cases for data analysis, of which 1,936 involved priapism as an adverse event. In total, 16 antiepileptic medications had at least one case of an adverse event involving priapism.
A positive safety signal was defined as a Proportional Reporting Ratio (PRR) of at least two, a chi-squared of at least four, or three or more cases. The signal was detected for valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine.
The largest association with priapism was with valpromide, at a PRR of 61.79. That was followed by PRR of 9.61 for brivaracetam, 7.28 for valproic acid, and 3.23 for topiramate.
“Considering that the proportionality analysis we applied in our study is used for hypothesis generation, our results will need to confirm in large cohorts and case-control studies,” said Dr. Pejcic.
New and important hypothesis?
Commenting on the study, Daniel Goldenholz, MD, PhD, instructor in the Division of Epilepsy, Beth Israel Deaconess Medical Center, Boston, said priapism is not something that practicing epileptologists are instructed “to look for.”
He noted that “the idea of looking for a hidden signal in a massive database like this is very appealing” because it could reveal patterns that were previously undetected.
However, the event rate in the study suggests priapism, which “in the right context would be considered a medical emergency, [is] relatively uncommon,” said Dr. Goldenholz, who was not involved with the research.
He noted that medications that could cause priapism, “such as antidepressants, blood pressure meds, and anticoagulants,” are commonly used by many people – including those with epilepsy.
It is consequently possible that “the finding from this study can be explained by comorbid medical problems,” Dr. Goldenholz said. This is particularly likely because many of the AEDs in question “have been on the market for decades,” he added.
“If a seemingly dangerous symptom would be happening as a result of one of these medications, ,” he said.
Still, Dr. Goldenholz noted that it is “possible that these authors have a new and important hypothesis which must now be tested: Does priapism occur in patients with antiseizure medications when other causes are already ruled out?”
The investigators and Dr. Goldenholz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several antiepileptic drugs (AEDs) are associated with an increased risk for priapism, new research suggests.
After analyzing U.S. adverse event reporting data, investigators found that among nearly 200 cases of priapism, a persistent, often painful erection unrelated to sexual interest or stimulation that lasts more than 4 hours, eight AEDs were associated with a positive “safety signal” for priapism.
These included valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine. Of these, valpromide had the largest association.
“Based on our results, we would recommend to clinicians to be cautious about the possibility of encountering priapism” in patients receiving the eight AEDs identified, lead researcher Ana Pejcic, PhD, department of pharmacology and toxicology, University of Kragujevac, Serbia, told meeting attendees.
If clinicians encounter such cases, they should be “reported to the regulatory authorities,” Dr. Pejcic added.
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
Noteworthy limitations
Dr. Pejcic told this news organization that the safety signal with AEDs “does not directly mean that a medicine has caused the reported adverse event” because an illness or other drug taken by the patient could be responsible instead.
She also noted that the U.S. Food and Drug Administration’s Adverse Event Reporting System relies on “spontaneous reports of adverse events,” which have multiple limitations.
These limitations include that the FDA “does not require that a causal relationship between a drug and event be proven, and reports do not always have enough information to properly evaluate an event.”
Nevertheless, Dr. Pejcic added that if a causal relationship was to be shown, the underlying mechanism could be linked to the pharmacological properties of the individual antiepileptic, such as altered alpha-1 adrenergic receptor expression or increased dopamine release.
Still, that would require “further evaluation in larger pharmacoepidemiological studies, with adjustment for potential confounding variables,” she said.
Replication needed
Priapism has recently been observed in case reports in association with the use of some AEDs. In addition, use of the drugs has been associated with hypo- and hypersexuality, as well as erectile and ejaculatory dysfunction.
Because the relationship between priapism and AED use “has not been well characterized,” the researchers mined data from the FDA’s Adverse Event Reporting System.
They examined entries from the first quarter of 2004 and the third quarter of 2020, focusing on 47 AEDs from the N03A subgroup of the Anatomical Therapeutic Chemical Classification System.
The researchers identified 8,122,037 cases for data analysis, of which 1,936 involved priapism as an adverse event. In total, 16 antiepileptic medications had at least one case of an adverse event involving priapism.
A positive safety signal was defined as a Proportional Reporting Ratio (PRR) of at least two, a chi-squared of at least four, or three or more cases. The signal was detected for valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine.
The largest association with priapism was with valpromide, at a PRR of 61.79. That was followed by PRR of 9.61 for brivaracetam, 7.28 for valproic acid, and 3.23 for topiramate.
“Considering that the proportionality analysis we applied in our study is used for hypothesis generation, our results will need to confirm in large cohorts and case-control studies,” said Dr. Pejcic.
New and important hypothesis?
Commenting on the study, Daniel Goldenholz, MD, PhD, instructor in the Division of Epilepsy, Beth Israel Deaconess Medical Center, Boston, said priapism is not something that practicing epileptologists are instructed “to look for.”
He noted that “the idea of looking for a hidden signal in a massive database like this is very appealing” because it could reveal patterns that were previously undetected.
However, the event rate in the study suggests priapism, which “in the right context would be considered a medical emergency, [is] relatively uncommon,” said Dr. Goldenholz, who was not involved with the research.
He noted that medications that could cause priapism, “such as antidepressants, blood pressure meds, and anticoagulants,” are commonly used by many people – including those with epilepsy.
It is consequently possible that “the finding from this study can be explained by comorbid medical problems,” Dr. Goldenholz said. This is particularly likely because many of the AEDs in question “have been on the market for decades,” he added.
“If a seemingly dangerous symptom would be happening as a result of one of these medications, ,” he said.
Still, Dr. Goldenholz noted that it is “possible that these authors have a new and important hypothesis which must now be tested: Does priapism occur in patients with antiseizure medications when other causes are already ruled out?”
The investigators and Dr. Goldenholz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECNP 2021
Stem cell transplant benefits in secondary progressive MS
, a new Italian study suggests.
In the study, stem cell transplant was associated with a slowing of disability progression and a higher likelihood of disability improvement in patients with secondary progressive MS compared with other disease-modifying therapies.
“Our study shows that, although limited, sustained disability improvement is still possible during early active secondary progressive MS and seems to be more likely with stem cell transplant than other disease-modifying treatments,” said lead author Giacomo Boffa, MD, University of Genoa, Italy.
“Brain penetrating–intent immune suppression in long-term immunological reconstitution within the central nervous system could be responsible for this clinical efficacy,” he added.
Dr. Boffa presented the research at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
He explained that compartmentalized inflation in the brain parenchyma, left meninges, and the cerebral spinal fluid (CSF) is a key driver of disability worsening in secondary progressive MS.
Although initial studies did not reveal an effect of disease-modifying therapies in secondary progressive MS, recent randomized trials have established some benefit of siponimod in reducing the risk of disability worsening, and consistent with this finding, observational studies have suggested that other available immunotherapies may also be beneficial for active secondary progressive MS, Dr. Boffa noted.
“Autologous hematopoietic stem cell transplantation has been widely investigated for the treatment of refractory MS, and although the ideal candidate for this procedure is a young patient with relapsing-remitting MS, there is some evidence to suggest that the procedure could also slow down neurological disability in patients with secondary progressive MS,” Dr. Boffa said.
“Indeed, all the drugs used in the transplant technology share the ability to cross the blood-brain barrier and exert a strong immunosuppressant effect within the brain parenchyma and CSF,” he added.
Comparing treatment regimens
The aim of the current study was to compare the effect of autologous hematopoietic stem cell transplantation with other immunotherapies on disability worsening in patients with active secondary progressive MS.
Study endpoints included the Expanded Disability Status Scale (EDSS) score, 6-month worsening and improvement in disability, and sustained disability improvement over time.
The researchers studied patients with secondary progressive MS who had received autologous hematopoietic stem cell transplantation and were included in the Italian Bone Marrow Transplant Group. They were compared with a control group of patients in the Italian MS registry who had started a nontransplant disease-modifying therapy after the diagnosis of secondary progressive MS.
To control for many different variables, two separate analyses were preformed. One analyses was a propensity-score approach (patients were matched based on their propensity to receive bone marrow transplant or one of the other disease-modifying therapies). The other analysis used an overlap weighting approach (each patient was given a weight proportional to the probability of them belonging to the other treatment group).
The final cohort consisted of 79 bone marrow transplant recipients and 1,975 patients who had received other disease-modifying therapies.
Before matching, patients in the control group were older, had a longer disease duration, and had a lower annualized relapse rate than transplanted patients.
After propensity-score matching, there were 69 transplanted patients and 217 control patients who were well balanced in terms of clinical and demographic characteristics. After overlap weighting, the entire cohort was also well balanced for these variables.
In terms of the primary endpoint, stem cell transplant stabilized the EDSS score over time, while patients treated with other disease-modifying therapies had continuous progression of the EDSS score over time.
In the propensity-matched analysis, the EDSS score improved by 0.013 points per year in the stem cell transplant group compared with a worsening of 0.157 points per year in the control group. Similar results were seen in the overlap-weighting analysis.
The effect of stem cell transplant on EDSS score translated into a significantly delayed time to confirmed disability progression in the stem cell transplant group compared with the control group (HR, 0.5; P = .005), Dr. Boffa reported.
Five years after the procedure, 62% of the transplant group were free of disability progression, compared with around 20% of the control group.
Patients in the transplanted group were also more likely to show disability improvement over time. Five years after the procedure, almost 20% of the stem cell transplant group still maintained a disability improvement compared with only 4% of patients treated with other disease-modifying therapies.
“Our study population was composed of relatively young patients (average age 38 years) with clinical activity during secondary progressive MS, and the results of this study would not be applicable to patients with secondary progressive MS patients without signs of inflammatory activity,” Dr. Boffa commented.
“But on the other hand, our results reinforce the notion that ongoing inflammation during progressive MS requires adequate immunotherapy,” he added.
A version of this article first appeared on Medscape.com.
, a new Italian study suggests.
In the study, stem cell transplant was associated with a slowing of disability progression and a higher likelihood of disability improvement in patients with secondary progressive MS compared with other disease-modifying therapies.
“Our study shows that, although limited, sustained disability improvement is still possible during early active secondary progressive MS and seems to be more likely with stem cell transplant than other disease-modifying treatments,” said lead author Giacomo Boffa, MD, University of Genoa, Italy.
“Brain penetrating–intent immune suppression in long-term immunological reconstitution within the central nervous system could be responsible for this clinical efficacy,” he added.
Dr. Boffa presented the research at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
He explained that compartmentalized inflation in the brain parenchyma, left meninges, and the cerebral spinal fluid (CSF) is a key driver of disability worsening in secondary progressive MS.
Although initial studies did not reveal an effect of disease-modifying therapies in secondary progressive MS, recent randomized trials have established some benefit of siponimod in reducing the risk of disability worsening, and consistent with this finding, observational studies have suggested that other available immunotherapies may also be beneficial for active secondary progressive MS, Dr. Boffa noted.
“Autologous hematopoietic stem cell transplantation has been widely investigated for the treatment of refractory MS, and although the ideal candidate for this procedure is a young patient with relapsing-remitting MS, there is some evidence to suggest that the procedure could also slow down neurological disability in patients with secondary progressive MS,” Dr. Boffa said.
“Indeed, all the drugs used in the transplant technology share the ability to cross the blood-brain barrier and exert a strong immunosuppressant effect within the brain parenchyma and CSF,” he added.
Comparing treatment regimens
The aim of the current study was to compare the effect of autologous hematopoietic stem cell transplantation with other immunotherapies on disability worsening in patients with active secondary progressive MS.
Study endpoints included the Expanded Disability Status Scale (EDSS) score, 6-month worsening and improvement in disability, and sustained disability improvement over time.
The researchers studied patients with secondary progressive MS who had received autologous hematopoietic stem cell transplantation and were included in the Italian Bone Marrow Transplant Group. They were compared with a control group of patients in the Italian MS registry who had started a nontransplant disease-modifying therapy after the diagnosis of secondary progressive MS.
To control for many different variables, two separate analyses were preformed. One analyses was a propensity-score approach (patients were matched based on their propensity to receive bone marrow transplant or one of the other disease-modifying therapies). The other analysis used an overlap weighting approach (each patient was given a weight proportional to the probability of them belonging to the other treatment group).
The final cohort consisted of 79 bone marrow transplant recipients and 1,975 patients who had received other disease-modifying therapies.
Before matching, patients in the control group were older, had a longer disease duration, and had a lower annualized relapse rate than transplanted patients.
After propensity-score matching, there were 69 transplanted patients and 217 control patients who were well balanced in terms of clinical and demographic characteristics. After overlap weighting, the entire cohort was also well balanced for these variables.
In terms of the primary endpoint, stem cell transplant stabilized the EDSS score over time, while patients treated with other disease-modifying therapies had continuous progression of the EDSS score over time.
In the propensity-matched analysis, the EDSS score improved by 0.013 points per year in the stem cell transplant group compared with a worsening of 0.157 points per year in the control group. Similar results were seen in the overlap-weighting analysis.
The effect of stem cell transplant on EDSS score translated into a significantly delayed time to confirmed disability progression in the stem cell transplant group compared with the control group (HR, 0.5; P = .005), Dr. Boffa reported.
Five years after the procedure, 62% of the transplant group were free of disability progression, compared with around 20% of the control group.
Patients in the transplanted group were also more likely to show disability improvement over time. Five years after the procedure, almost 20% of the stem cell transplant group still maintained a disability improvement compared with only 4% of patients treated with other disease-modifying therapies.
“Our study population was composed of relatively young patients (average age 38 years) with clinical activity during secondary progressive MS, and the results of this study would not be applicable to patients with secondary progressive MS patients without signs of inflammatory activity,” Dr. Boffa commented.
“But on the other hand, our results reinforce the notion that ongoing inflammation during progressive MS requires adequate immunotherapy,” he added.
A version of this article first appeared on Medscape.com.
, a new Italian study suggests.
In the study, stem cell transplant was associated with a slowing of disability progression and a higher likelihood of disability improvement in patients with secondary progressive MS compared with other disease-modifying therapies.
“Our study shows that, although limited, sustained disability improvement is still possible during early active secondary progressive MS and seems to be more likely with stem cell transplant than other disease-modifying treatments,” said lead author Giacomo Boffa, MD, University of Genoa, Italy.
“Brain penetrating–intent immune suppression in long-term immunological reconstitution within the central nervous system could be responsible for this clinical efficacy,” he added.
Dr. Boffa presented the research at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
He explained that compartmentalized inflation in the brain parenchyma, left meninges, and the cerebral spinal fluid (CSF) is a key driver of disability worsening in secondary progressive MS.
Although initial studies did not reveal an effect of disease-modifying therapies in secondary progressive MS, recent randomized trials have established some benefit of siponimod in reducing the risk of disability worsening, and consistent with this finding, observational studies have suggested that other available immunotherapies may also be beneficial for active secondary progressive MS, Dr. Boffa noted.
“Autologous hematopoietic stem cell transplantation has been widely investigated for the treatment of refractory MS, and although the ideal candidate for this procedure is a young patient with relapsing-remitting MS, there is some evidence to suggest that the procedure could also slow down neurological disability in patients with secondary progressive MS,” Dr. Boffa said.
“Indeed, all the drugs used in the transplant technology share the ability to cross the blood-brain barrier and exert a strong immunosuppressant effect within the brain parenchyma and CSF,” he added.
Comparing treatment regimens
The aim of the current study was to compare the effect of autologous hematopoietic stem cell transplantation with other immunotherapies on disability worsening in patients with active secondary progressive MS.
Study endpoints included the Expanded Disability Status Scale (EDSS) score, 6-month worsening and improvement in disability, and sustained disability improvement over time.
The researchers studied patients with secondary progressive MS who had received autologous hematopoietic stem cell transplantation and were included in the Italian Bone Marrow Transplant Group. They were compared with a control group of patients in the Italian MS registry who had started a nontransplant disease-modifying therapy after the diagnosis of secondary progressive MS.
To control for many different variables, two separate analyses were preformed. One analyses was a propensity-score approach (patients were matched based on their propensity to receive bone marrow transplant or one of the other disease-modifying therapies). The other analysis used an overlap weighting approach (each patient was given a weight proportional to the probability of them belonging to the other treatment group).
The final cohort consisted of 79 bone marrow transplant recipients and 1,975 patients who had received other disease-modifying therapies.
Before matching, patients in the control group were older, had a longer disease duration, and had a lower annualized relapse rate than transplanted patients.
After propensity-score matching, there were 69 transplanted patients and 217 control patients who were well balanced in terms of clinical and demographic characteristics. After overlap weighting, the entire cohort was also well balanced for these variables.
In terms of the primary endpoint, stem cell transplant stabilized the EDSS score over time, while patients treated with other disease-modifying therapies had continuous progression of the EDSS score over time.
In the propensity-matched analysis, the EDSS score improved by 0.013 points per year in the stem cell transplant group compared with a worsening of 0.157 points per year in the control group. Similar results were seen in the overlap-weighting analysis.
The effect of stem cell transplant on EDSS score translated into a significantly delayed time to confirmed disability progression in the stem cell transplant group compared with the control group (HR, 0.5; P = .005), Dr. Boffa reported.
Five years after the procedure, 62% of the transplant group were free of disability progression, compared with around 20% of the control group.
Patients in the transplanted group were also more likely to show disability improvement over time. Five years after the procedure, almost 20% of the stem cell transplant group still maintained a disability improvement compared with only 4% of patients treated with other disease-modifying therapies.
“Our study population was composed of relatively young patients (average age 38 years) with clinical activity during secondary progressive MS, and the results of this study would not be applicable to patients with secondary progressive MS patients without signs of inflammatory activity,” Dr. Boffa commented.
“But on the other hand, our results reinforce the notion that ongoing inflammation during progressive MS requires adequate immunotherapy,” he added.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2021
Updated MS guidelines advocate earlier, more aggressive treatment
and include a recommendation for siponimod (Mayzent) in progressive MS, as well as a general emphasis toward earlier and more aggressive treatment.
The updated guidelines were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and are the result of a collaboration between ECTRIMS and the European Academy of Neurology (EAN).
Maria Pia Amato, MD, ECTRIMS president and co-chair of the guidelines steering committee, noted that the European MS treatment guidelines were last published in 2018. “Since then more trials have been published, and we felt this was a good time to incorporate the new evidence into updated guidelines,” she said.
“As before, the updated guidelines contain a number of core questions that address the efficacy of disease-modifying therapies, early treatment decisions, disease/treatment response monitoring and treatment modifications, treatment suspension and disease reactivation, and pregnancy and breastfeeding,” Dr. Amato said.
New recommendations
New features of the updated guidelines include a recommendation for siponimod for secondary progressive MS with evidence of disease inflammatory activity; in addition, there is more emphasis on starting treatment early, with greater consideration of higher efficacy drugs, depending on the characteristics of the disease and the patient, Dr. Amato commented.
“We also provided more detailed information on disease-modifying therapy use in pregnancy and breastfeeding and also for women with high disease activity who desire to become pregnant,” she added.
Other new features include the introduction of clinical questions dealing with treatment safety and monitoring (for example, for natalizumab) and also considering the current COVID-19 pandemic scenario; switching strategies with more detailed practical indications on timing; and long lasting effects of drugs such as alemtuzumab and cladribine, Dr. Amato said.
The updated guidelines include the following recommendations:
- The entire spectrum of disease-modifying drugs should be prescribed by a neurologist with expertise in MS and ready access to adequate infrastructure to provide proper monitoring of patents, comprehensive assessment, early detection of side effects, and the capacity to address those side effects promptly.
- Offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) highly suggestive of MS and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS.
- For patients with relapsing-remitting MS, the choice between a wide range of available drugs (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, fingolimod, ozanimod, ponesimod, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab), from modestly to highly effective, will depend on factors including: underlying disability progression, disease severity/clinical or radiological activity, patient characteristics and morbidity, drug safety profile, family planning, and patient preferences.
Progressive MS
- For patients with secondary progressive MS with evidence of inflammatory activity (relapses and/or MRI activity), offer treatment with siponimod. Treatment with other therapies used for relapsing remitting MS may also be considered.
- For secondary progressive MS without evidence of inflammatory activity, particularly in young patients and those in whom progression has started recently, consider treatment with siponimod or anti-CD20 monoclonal antibodies, taking into account that there is scarce evidence to support their use in this setting.
- For patients with active secondary progressive MS when there is no other therapy available, consider treatment with mitoxantrone, taking into account the safety concerns and tolerability issues of this agent.
- Consider ocrelizumab for patients with primary progressive MS, particularly early and active (clinically and/or radiologically) disease.
Emphasis toward higher-efficacy drugs
- Consider choosing a higher-efficacy disease-modifying drug early on, according to disease activity (either clinically or on MRI).
- Offer a more efficacious drug to patients who show evidence of disease activity with their current treatment.
- When treatment with a high-efficacy drug is stopped, whether because of inefficacy or risk of adverse effects, consider starting another high-efficacy drug, taking into account clinical and MRI disease activity before and during treatment, pharmacokinetics and biological activity of the previous drug, and the potential for resumed disease activity or even rebound syndrome (particularly with natalizumab and S1P modulators).
- In the stable patient (clinically and on MRI) who shows no safety or tolerability issues, consider continuing treatment with disease-modifying therapy, taking into account patient characteristics and comorbidities, drug safety profile, family planning, and patient preferences.
Recommendations for pregnancy and breastfeeding
Recommendations for pregnant women and mothers who choose to breastfeed include:
- Advise women who wish to become pregnant to plan their pregnancy beforehand.
- Advise women of childbearing potential that MS disease-modifying therapies are not licensed during pregnancy, with the exception of interferons and glatiramer acetate.
- For women planning a pregnancy, offer interferons and glatiramer acetate and consider continuing these agents during pregnancy after assessment of risk and benefits. Consider using dimethyl fumarate until pregnancy is confirmed and stopping during pregnancy after assessment of the risks and benefits.
- For women with highly active disease who wish to become pregnant, there are a number of therapeutic options:
1) treatment with long lasting effects such as alemtuzumab or cladribine provided that at least 4 or 6 months respectively have elapsed between the last dose and conception2) treatment with anti-CD20 drugs before pregnancy with advice to wait for 2-6 months after the last infusion before becoming pregnant and to avoid further infusions during pregnancy, or3) for patients treated with natalizumab, consider continuing treatment during pregnancy using a 6-week extended dosage regimen until the end of the second trimester or up until week 34 and resuming after delivery (in newborns exposed to natalizumab, check for hematological abnormalities and liver function)
- Only interferons and ofatumumab are currently approved during breastfeeding.
Treatment safety/monitoring
- When treating patients with natalizumab and after a period of stability, consider switching to a 6-week interval regimen in order to minimize the risk of progressive multifocal leukoencephalopathy (PML).
- Consider treatment with high-efficacy drugs including natalizumab in patients with high disease activity, in whom a quick therapeutic effect is required, taking into account the risk of PML in John Cunningham virus (JCV)-positive patients, as well as the therapeutic lag of the different disease-modifying drugs.
- Ideally, prioritize vaccination against COVID-19 before starting immunosuppressive disease-modifying treatments to achieve the highest protection rate possible.
Long-lasting treatments
- When using long-lasting treatments (alemtuzumab or cladribine) in patients who experience disease activity before the treatment is completed (between the first and second cycles), consider waiting until completion of the therapeutic regimen before switching to other drugs.
- Consider offering additional courses of alemtuzumab after the first two cycles at least 1 year apart from each other when disease activity has not remitted completely or reappears after a period of stability, taking into account the balance between the potential benefits and side effects.
A version of this article first appeared on Medscape.com.
and include a recommendation for siponimod (Mayzent) in progressive MS, as well as a general emphasis toward earlier and more aggressive treatment.
The updated guidelines were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and are the result of a collaboration between ECTRIMS and the European Academy of Neurology (EAN).
Maria Pia Amato, MD, ECTRIMS president and co-chair of the guidelines steering committee, noted that the European MS treatment guidelines were last published in 2018. “Since then more trials have been published, and we felt this was a good time to incorporate the new evidence into updated guidelines,” she said.
“As before, the updated guidelines contain a number of core questions that address the efficacy of disease-modifying therapies, early treatment decisions, disease/treatment response monitoring and treatment modifications, treatment suspension and disease reactivation, and pregnancy and breastfeeding,” Dr. Amato said.
New recommendations
New features of the updated guidelines include a recommendation for siponimod for secondary progressive MS with evidence of disease inflammatory activity; in addition, there is more emphasis on starting treatment early, with greater consideration of higher efficacy drugs, depending on the characteristics of the disease and the patient, Dr. Amato commented.
“We also provided more detailed information on disease-modifying therapy use in pregnancy and breastfeeding and also for women with high disease activity who desire to become pregnant,” she added.
Other new features include the introduction of clinical questions dealing with treatment safety and monitoring (for example, for natalizumab) and also considering the current COVID-19 pandemic scenario; switching strategies with more detailed practical indications on timing; and long lasting effects of drugs such as alemtuzumab and cladribine, Dr. Amato said.
The updated guidelines include the following recommendations:
- The entire spectrum of disease-modifying drugs should be prescribed by a neurologist with expertise in MS and ready access to adequate infrastructure to provide proper monitoring of patents, comprehensive assessment, early detection of side effects, and the capacity to address those side effects promptly.
- Offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) highly suggestive of MS and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS.
- For patients with relapsing-remitting MS, the choice between a wide range of available drugs (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, fingolimod, ozanimod, ponesimod, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab), from modestly to highly effective, will depend on factors including: underlying disability progression, disease severity/clinical or radiological activity, patient characteristics and morbidity, drug safety profile, family planning, and patient preferences.
Progressive MS
- For patients with secondary progressive MS with evidence of inflammatory activity (relapses and/or MRI activity), offer treatment with siponimod. Treatment with other therapies used for relapsing remitting MS may also be considered.
- For secondary progressive MS without evidence of inflammatory activity, particularly in young patients and those in whom progression has started recently, consider treatment with siponimod or anti-CD20 monoclonal antibodies, taking into account that there is scarce evidence to support their use in this setting.
- For patients with active secondary progressive MS when there is no other therapy available, consider treatment with mitoxantrone, taking into account the safety concerns and tolerability issues of this agent.
- Consider ocrelizumab for patients with primary progressive MS, particularly early and active (clinically and/or radiologically) disease.
Emphasis toward higher-efficacy drugs
- Consider choosing a higher-efficacy disease-modifying drug early on, according to disease activity (either clinically or on MRI).
- Offer a more efficacious drug to patients who show evidence of disease activity with their current treatment.
- When treatment with a high-efficacy drug is stopped, whether because of inefficacy or risk of adverse effects, consider starting another high-efficacy drug, taking into account clinical and MRI disease activity before and during treatment, pharmacokinetics and biological activity of the previous drug, and the potential for resumed disease activity or even rebound syndrome (particularly with natalizumab and S1P modulators).
- In the stable patient (clinically and on MRI) who shows no safety or tolerability issues, consider continuing treatment with disease-modifying therapy, taking into account patient characteristics and comorbidities, drug safety profile, family planning, and patient preferences.
Recommendations for pregnancy and breastfeeding
Recommendations for pregnant women and mothers who choose to breastfeed include:
- Advise women who wish to become pregnant to plan their pregnancy beforehand.
- Advise women of childbearing potential that MS disease-modifying therapies are not licensed during pregnancy, with the exception of interferons and glatiramer acetate.
- For women planning a pregnancy, offer interferons and glatiramer acetate and consider continuing these agents during pregnancy after assessment of risk and benefits. Consider using dimethyl fumarate until pregnancy is confirmed and stopping during pregnancy after assessment of the risks and benefits.
- For women with highly active disease who wish to become pregnant, there are a number of therapeutic options:
1) treatment with long lasting effects such as alemtuzumab or cladribine provided that at least 4 or 6 months respectively have elapsed between the last dose and conception2) treatment with anti-CD20 drugs before pregnancy with advice to wait for 2-6 months after the last infusion before becoming pregnant and to avoid further infusions during pregnancy, or3) for patients treated with natalizumab, consider continuing treatment during pregnancy using a 6-week extended dosage regimen until the end of the second trimester or up until week 34 and resuming after delivery (in newborns exposed to natalizumab, check for hematological abnormalities and liver function)
- Only interferons and ofatumumab are currently approved during breastfeeding.
Treatment safety/monitoring
- When treating patients with natalizumab and after a period of stability, consider switching to a 6-week interval regimen in order to minimize the risk of progressive multifocal leukoencephalopathy (PML).
- Consider treatment with high-efficacy drugs including natalizumab in patients with high disease activity, in whom a quick therapeutic effect is required, taking into account the risk of PML in John Cunningham virus (JCV)-positive patients, as well as the therapeutic lag of the different disease-modifying drugs.
- Ideally, prioritize vaccination against COVID-19 before starting immunosuppressive disease-modifying treatments to achieve the highest protection rate possible.
Long-lasting treatments
- When using long-lasting treatments (alemtuzumab or cladribine) in patients who experience disease activity before the treatment is completed (between the first and second cycles), consider waiting until completion of the therapeutic regimen before switching to other drugs.
- Consider offering additional courses of alemtuzumab after the first two cycles at least 1 year apart from each other when disease activity has not remitted completely or reappears after a period of stability, taking into account the balance between the potential benefits and side effects.
A version of this article first appeared on Medscape.com.
and include a recommendation for siponimod (Mayzent) in progressive MS, as well as a general emphasis toward earlier and more aggressive treatment.
The updated guidelines were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and are the result of a collaboration between ECTRIMS and the European Academy of Neurology (EAN).
Maria Pia Amato, MD, ECTRIMS president and co-chair of the guidelines steering committee, noted that the European MS treatment guidelines were last published in 2018. “Since then more trials have been published, and we felt this was a good time to incorporate the new evidence into updated guidelines,” she said.
“As before, the updated guidelines contain a number of core questions that address the efficacy of disease-modifying therapies, early treatment decisions, disease/treatment response monitoring and treatment modifications, treatment suspension and disease reactivation, and pregnancy and breastfeeding,” Dr. Amato said.
New recommendations
New features of the updated guidelines include a recommendation for siponimod for secondary progressive MS with evidence of disease inflammatory activity; in addition, there is more emphasis on starting treatment early, with greater consideration of higher efficacy drugs, depending on the characteristics of the disease and the patient, Dr. Amato commented.
“We also provided more detailed information on disease-modifying therapy use in pregnancy and breastfeeding and also for women with high disease activity who desire to become pregnant,” she added.
Other new features include the introduction of clinical questions dealing with treatment safety and monitoring (for example, for natalizumab) and also considering the current COVID-19 pandemic scenario; switching strategies with more detailed practical indications on timing; and long lasting effects of drugs such as alemtuzumab and cladribine, Dr. Amato said.
The updated guidelines include the following recommendations:
- The entire spectrum of disease-modifying drugs should be prescribed by a neurologist with expertise in MS and ready access to adequate infrastructure to provide proper monitoring of patents, comprehensive assessment, early detection of side effects, and the capacity to address those side effects promptly.
- Offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) highly suggestive of MS and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS.
- For patients with relapsing-remitting MS, the choice between a wide range of available drugs (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, fingolimod, ozanimod, ponesimod, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab), from modestly to highly effective, will depend on factors including: underlying disability progression, disease severity/clinical or radiological activity, patient characteristics and morbidity, drug safety profile, family planning, and patient preferences.
Progressive MS
- For patients with secondary progressive MS with evidence of inflammatory activity (relapses and/or MRI activity), offer treatment with siponimod. Treatment with other therapies used for relapsing remitting MS may also be considered.
- For secondary progressive MS without evidence of inflammatory activity, particularly in young patients and those in whom progression has started recently, consider treatment with siponimod or anti-CD20 monoclonal antibodies, taking into account that there is scarce evidence to support their use in this setting.
- For patients with active secondary progressive MS when there is no other therapy available, consider treatment with mitoxantrone, taking into account the safety concerns and tolerability issues of this agent.
- Consider ocrelizumab for patients with primary progressive MS, particularly early and active (clinically and/or radiologically) disease.
Emphasis toward higher-efficacy drugs
- Consider choosing a higher-efficacy disease-modifying drug early on, according to disease activity (either clinically or on MRI).
- Offer a more efficacious drug to patients who show evidence of disease activity with their current treatment.
- When treatment with a high-efficacy drug is stopped, whether because of inefficacy or risk of adverse effects, consider starting another high-efficacy drug, taking into account clinical and MRI disease activity before and during treatment, pharmacokinetics and biological activity of the previous drug, and the potential for resumed disease activity or even rebound syndrome (particularly with natalizumab and S1P modulators).
- In the stable patient (clinically and on MRI) who shows no safety or tolerability issues, consider continuing treatment with disease-modifying therapy, taking into account patient characteristics and comorbidities, drug safety profile, family planning, and patient preferences.
Recommendations for pregnancy and breastfeeding
Recommendations for pregnant women and mothers who choose to breastfeed include:
- Advise women who wish to become pregnant to plan their pregnancy beforehand.
- Advise women of childbearing potential that MS disease-modifying therapies are not licensed during pregnancy, with the exception of interferons and glatiramer acetate.
- For women planning a pregnancy, offer interferons and glatiramer acetate and consider continuing these agents during pregnancy after assessment of risk and benefits. Consider using dimethyl fumarate until pregnancy is confirmed and stopping during pregnancy after assessment of the risks and benefits.
- For women with highly active disease who wish to become pregnant, there are a number of therapeutic options:
1) treatment with long lasting effects such as alemtuzumab or cladribine provided that at least 4 or 6 months respectively have elapsed between the last dose and conception2) treatment with anti-CD20 drugs before pregnancy with advice to wait for 2-6 months after the last infusion before becoming pregnant and to avoid further infusions during pregnancy, or3) for patients treated with natalizumab, consider continuing treatment during pregnancy using a 6-week extended dosage regimen until the end of the second trimester or up until week 34 and resuming after delivery (in newborns exposed to natalizumab, check for hematological abnormalities and liver function)
- Only interferons and ofatumumab are currently approved during breastfeeding.
Treatment safety/monitoring
- When treating patients with natalizumab and after a period of stability, consider switching to a 6-week interval regimen in order to minimize the risk of progressive multifocal leukoencephalopathy (PML).
- Consider treatment with high-efficacy drugs including natalizumab in patients with high disease activity, in whom a quick therapeutic effect is required, taking into account the risk of PML in John Cunningham virus (JCV)-positive patients, as well as the therapeutic lag of the different disease-modifying drugs.
- Ideally, prioritize vaccination against COVID-19 before starting immunosuppressive disease-modifying treatments to achieve the highest protection rate possible.
Long-lasting treatments
- When using long-lasting treatments (alemtuzumab or cladribine) in patients who experience disease activity before the treatment is completed (between the first and second cycles), consider waiting until completion of the therapeutic regimen before switching to other drugs.
- Consider offering additional courses of alemtuzumab after the first two cycles at least 1 year apart from each other when disease activity has not remitted completely or reappears after a period of stability, taking into account the balance between the potential benefits and side effects.
A version of this article first appeared on Medscape.com.
From ECTRIMS 2021







