User login
An Interdisciplinary Approach to Educating Medical Students About Dementia Assessment and Treatment Planning
The global burden of dementia is increasing at an alarming pace and is estimated to soon affect 81 million individuals worldwide.1 The World Health Organization and the Institute of Medicine have recommended greater dementia awareness and education.2,3 Despite this emphasis on dementia education, many general practitioners consider dementia care beyond their clinical domain and feel that specialists, such as geriatricians, geriatric psychiatrists, or neurologists should address dementia assessment and treatment. 4 Unfortunately, the geriatric health care workforce has been shrinking. The American Geriatrics Society estimates the need for 30,000 geriatricians by 2030, although there are only 7,300 board-certified geriatricians currently in the US.5 There is an urgent need for educating all medical trainees in dementia care regardless of their specialization interest. As the largest underwriter of graduate medical education in the US, the US Department of Veterans Affairs (VA) is well placed for rolling out focused dementia education. Training needs to be practical, brief, and responsive to knowledge gaps to reach the most trainees.
Despite growing emphasis on geriatric training, many medical students have limited experience with patients with dementia or their caregivers, lack exposure to interdisciplinary teams, have a poor attitude toward geriatric patients, and display specific knowledge gaps in dementia assessment and management. 6-9 Other knowledge gaps noted in medical students included assessing behavioral problems, function, safety, and caregiver burden. Medical students also had limited exposure to interdisciplinary team dementia assessment and management.
Our goal was to develop a multicomponent, experiential, brief curriculum using team-based learning to expose senior medical students to interdisciplinary assessment of dementia. The curriculum was developed with input from the interdisciplinary team to address dementia knowledge gaps while providing an opportunity to interact with caregivers. The curriculum targeted all medical students regardless of their interest in geriatrics. Particular emphasis was placed on systems-based learning and the importance of teamwork in managing complex conditions such as dementia. Students were taught that incorporating interdisciplinary input would be more effective during dementia care planning rather than developing specialized knowledge.
Methods
Our team developed a curriculum for fourthyear medical students who rotated through the VA Memory Disorders Clinic as a part of their geriatric medicine clerkship at the University of Arkansas for Medical Sciences in Little Rock. The Memory Disorders Clinic is a consultation practice at the Central Arkansas Veterans Healthcare System (CAVHS) where patients with memory problems are evaluated by a team consisting of a geriatric psychiatrist, a geriatrician, a social worker, and a neuropsychologist. Each specialist addresses specific areas of dementia assessment and management. The curriculum included didactics, clinical experience, and team-based learning.
Didactics
An hour-long didactic session lead by the team geriatrician provided a general overview of interdisciplinary assessment of dementia to groups of 2 to 3 students at a time. The geriatrician presented an overview of dementia types, comorbidities, medications that affect memory, details of the physical examination, and laboratory, cognitive, and behavioral assessments along with treatment plan development. Students also learned about the roles of the social worker, geriatrician, neuropsychologist, and geriatric psychiatrist in the clinic. Pictographs and pie charts highlighted the role of disciplines in assessing and managing aspects of dementia.
The social work evaluation included advance care planning, functional assessment, safety assessment (driving, guns, wandering behaviors, etc), home safety evaluation, support system, and financial evaluation. Each medical student received a binder with local resources to become familiar with the depth and breadth of agencies involved in dementia care. Each medical student learned how to administer the Zarit Burden Scale to assess caregiver burden.10 The details of the geriatrician assessment included reviewing medical comorbidities and medications contributing to dementia, a physical examination, including a focused neurologic examination, laboratory assessment, and judicious use of neuroimaging.
The neuropsychology assessment education included a battery of tests and assessments. The global screening instruments included the Modified Mini-Mental State examination (3MS), Montreal Cognitive Assessment (MoCA), and Saint Louis University Mental Status examination (SLUMS).11-13 Executive function is evaluated using the Trails Making Test A and Trails Making Test B, Controlled Oral Word Association Test, Semantic Fluency Test, and Repeatable Battery for the Assessment of Neuropsychological Status test. Cognitive tests were compared and age- , education-, and race-adjusted norms for rating scales were listed if available. Each student was expected to show proficiency in ≥ 2 cognitive screening instruments (3MS, MoCA, or SLUMS). The geriatric psychiatry assessment included clinical history, onset, and course of memory problems from patient and caregiver perspectives, the Neuropsychiatric Inventory for assessing behavioral problems, employing the clinical dementia rating scale, integrating the team data, summarizing assessment, and formulating a treatment plan.14
Clinical
Students had a single clinical exposure. Students followed 1 patient and his or her caregiver through the team assessment and observed each provider’s assessment to learn interview techniques to adapt to the patient’s sensory or cognitive impairment and become familiar with different tools and devices used in the dementia clinic, such as hearing amplifiers. Each specialist provided hands-on experience. This encounter helped the students connect with caregivers and appreciate their role in patient care.
Systems learning was an important component integrated throughout the clinical experience. Examples include using video teleconferences to communicate findings among team members and electronic health records to seamlessly obtain and integrate data. Students learned how to create worksheets to graph laboratory data such as B12, thyroid-stimulating hormone, and rapid plasma regain levels. Student gained experience in using applications to retrieve neuroimaging data, results of sleep studies, and other data. Many patients had not received the results of their sleep study, and students had the responsibility to share these reports, including the number of apneic episodes. Students used the VA Computerized Patient Record System for reviewing patient records. One particularly useful tool was Joint Legacy Viewer, a remote access tool used to retrieve data on veterans from anywhere within the US. Students were also trained on medication and consult order menus in the system.
Team-Based
Learning The objectives of the team-based learning section were to teach students basic concepts of integrating the interdisciplinary assessment and formulating a treatment plan, to provide an opportunity to present their case in a group format, to discuss the differential diagnosis, management and treatment plan with a geriatrician in the team-based learning format, and to answer questions from other students. The instructors developed a set of prepared take-home points (Table 1). The team-based learning sessions were structured so that all take-home points were covered.
Evaluations
Evaluations were performed before and immediately after the clinical experience. In preevaluation, students reported the frequency of their participation in an interdisciplinary team assessment of any condition and specifically for dementia. In pre- and postevaluation, students rated their perception of the role of interdisciplinary team members in assessing and managing dementia, their personal abilities to assess cognition, behavioral problems, caregiver burden, and their perception of the impact of behavioral problems on dementia care. A Likert scale (poor = 1; fair = 2; good = 3; very good = 4; and excellent = 5) was employed (eApendices 1 and 2 can be found at doi:10.12788/fp.0052). The only demographic information collected was the student’s gender. Semistructured interviews were conducted to assess students’ current knowledge, experience, and needs. These interviews lasted about 20 minutes and collected information regarding the students’ knowledge about cognitive and behavioral problems in general and those occurring in dementia, their experience with screening, and any problems they encountered.
Statistical Analysis
Student baseline characteristics were assessed. Pre- and postassessments were analyzed with the McNemar test for paired data, and associations with experience were evaluated using χ2 tests. Ratings were dichotomized as very good/excellent vs poor/fair/ good because our educational goal was “very good” to “excellent” experience in dementia care and to avoid expected small cell counts. Two-sided P < .05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide v5.1.
Results
One hundred fourth-year medical students participated, including 54 women. Thirtysix percent reported they had not previously attended an interdisciplinary team assessment for dementia, while 18% stated that they had attended only 1 interdisciplinary team assessment for dementia.
Before the education, students rated their dementia ability as poor. Only 2% (1 of 54), of those with 0 to 1 assessment experience rated their ability for assessing dementia with an interdisciplinary team format as very good/excellent compared with 20% (9/46) of those previously attending ≥ 2 assessments (P = .03); other ratings of ability were not associated with prior experience.
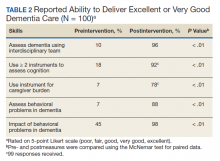
There was a significant change in the students’ self-efficacy ratings pre- to postassessment (P < .05) (Table 2). Only 10% rated their ability to assess for dementia as very good/excellent in before the intervention compared with 96% in postassessment (P < .01). Students’ perception of the impact of behavioral problems on dementia care improved significantly (45% to 98%, P < .01). Similarly, student’s perception of their ability to assess behavioral problems, caregiver burden, and cognition improved significantly from 7 to 88%; 7 to 78%, and 18 to 92%, respectively (P < .01). Students perception of the role of social worker, neuropsychologist, geriatrician, and geriatric psychiatrist also improved significantly for most measures from 81 to 98% (P = .02), 87 to 98% (P = .05), 94 to 99% (P = .06), and 88 to 100% (P = .01), respectively.
The semistructured interviews revealed that awareness of behavioral problems associated with dementia varied for different behavioral problems. Although many students showed familiarity with depression, agitation, and psychosis, they were not comfortable assessing them in a patient with dementia. These students were less aware of other behavioral problems such as disinhibition, apathy, and movement disorders. Deficits were noted in the skill of administering commonly used global cognitive screens, such as the Mini-Mental State Examination (MMSE).15
In semistructured interviews, only 7% of senior medical students were comfortable assessing behavioral problems associated with dementia. Most were not aware of any validated rating scale to assess neuropsychiatric symptoms. Similarly, only 7% of students were comfortable assessing caregiver burden, and most were not aware of any validated rating scale to assess caregiver burden. Only 1 in 5 students were comfortable using 2 cognitive screens to assess cognitive deficits. Many students stated that they were not routinely expected to perform common cognitive screens, such as the MMSE during their medical training except students who had expressed an interest in psychiatry and were expected to be proficient in the MMSE. Most students were making common mistakes, such as converting the 3-command task to 3 individual single commands, helping too much with serial 7s, and giving too much positive feedback throughout the test.
Discussion
Significant knowledge gaps regarding dementia were found in our study, which is in keeping with other studies in the area. Dementia knowledge deficits among medical trainees have been identified in the United Kingdom, Australia, and the US.6-9
In our study, a brief multicomponent experiential curriculum improved senior medical students’ perception and self-efficacy in diagnosing dementia. This is in keeping with other studies, such as the PAIRS Program.7 Findings from another study indicated that education for geriatric- oriented physicians should focus on experiential learning components through observation and interaction with older adults.16
A background of direct experience with older adults is associated with more positive attitudes toward older adults and increased interest in geriatric medicine.16 In our study, the exposure was brief; therefore, the results could not be compared with other long-term exposure studies. However, even with this brief intervention most students reported being comfortable with assessing caregiver burden (78%), behavioral problems of dementia (88%), and using ≥ 2 cognitive screens (92%). Comfortable in dementia assessment increased after the intervention from 10% to 96%. This finding is encouraging because brief multicomponent dementia education can be devised easily. This finding needs to be taken with caution because we did not conduct a formal skills evaluation.
A unique component of our experience was to learn medical students’ perception about the impact of neuropsychiatric symptoms on the trajectory, outcomes, and management of dementia. These symptoms included delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, and appetite and eating. Less than half the students thought that neuropsychiatric symptoms had a significant impact on dementia before the experience. Through didactics, systematic assessment of neuropsychiatric symptoms and interaction with caregivers, > 98% of students learned that these symptoms have a significant impact on dementia management.
This experience also emphasized the role of several disciplines in dementia assessment and management. Students’ experience positively influenced appreciation of the role of the memory clinic team. Our hope is that students will seek input from social workers, neuropsychologists, and other team members when working with patients with dementia or their caregivers. The common reason why primary care physicians focus on an exclusive medical model is the time commitment for communicating with an interdisciplinary team. Students experienced the feasibility of the interdisciplinary team involvement and how technology could be used for synchronous and asynchronous communication among team members. Medical students also were introduced to complex billing codes used when ≥ 3 disciplines assess/manage a geriatric patient.
Limitations
This study is limited by the lack of long-term follow-up evaluations, no metrics for practice changes clinical outcomes, and implementation in a single medical school. The postexperience evaluation in this study was performed immediately after the intervention. Long-term follow-up would inform whether the changes noted are durable. Because of the brief nature of our intervention, we do not believe that it would change practice in clinical care. It will be informative to follow this cohort of students to study whether their clinical approach to dementia care changes. The intervention needs to be replicated in other medical schools and in more heterogeneous groups to generalize the results of the study.
Conclusions
Senior medical students are not routinely exposed to interdisciplinary team assessments. Dementia knowledge gaps were prevalent in this cohort of senior medical students. Providing interdisciplinary geriatric educational experience improved their perception of their ability to assess for dementia and their recognition of the roles of interdisciplinary team members. Plans are in place to continue and expand the program to other complex geriatric syndromes.
Acknowledgments
Poster presented at the 2019 annual meeting of the American Geriatrics Society. Oral presentation at the same meeting as part of the select Geriatric Education Methods and Materials Swap workshop.
1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. doi:10.1016/S0140-6736(05)67889-0
2. Janca A, Aarli JA, Prilipko L, Dua T, Saxena S, Saraceno B. WHO/WFN survey of neurological services: a worldwide perspective. J Neurol Sci. 2006;247(1):29-34. doi:10.1016/j.jns.2006.03.003
3. Wilkins KM, Blazek MC, Brooks WB, Lehmann SW, Popeo D, Wagenaar D. Six things all medical students need to know about geriatric psychiatry (and how to teach them). Acad Psychiatry. 2017;41(5):693-700. doi:10.1007/s40596-017-0691-7
4. Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461- 467. doi:10.1093/ageing/afh140
5. Lester PE, Dharmarajan TS, Weinstein E. The looming geriatrician shortage: ramifications and solutions. J Aging Health. 2019:898264319879325. doi:10.1177/0898264319879325
6. Struck BD, Bernard MA, Teasdale TA; Oklahoma University Geriatric Education G. Effect of a mandatory geriatric medicine clerkship on third-year students. J Am Geriatr Soc. 2005;53(11):2007-2011. doi:10.1111/j.1532-5415.2005.00473.x
7. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer’s disease: the PAIRS Program. BMC Med Educ. 2012;12:80. doi:10.1186/1472-6920-12-80
8. Nagle BJ, Usita PM, Edland SD. United States medical students’ knowledge of Alzheimer disease. J Educ Eval Health Prof. 2013;10:4. doi:10.3352/jeehp.2013.10.4
9. Scott TL, Kugelman M, Tulloch K. How medical professional students view older people with dementia: Implications for education and practice. PLoS One. 2019;14(11):e0225329. doi:10.1371/journal.pone.0225329.
10. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655. doi:10.1093/geront/20.6.649
11. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7
12. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Ger iatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x
13. Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910. doi:10.1097/01.JGP.0000221510.33817.86
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi:10.1212/wnl.44.12.2308
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
16. Fitzgerald JT, Wray LA, Halter JB, Williams BC, Supiano MA. Relating medical students’ knowledge, attitudes, and experience to an interest in geriatric medicine. Gerontologist. 2003;43(6):849-855. doi:10.1093/geront/43.6.849
The global burden of dementia is increasing at an alarming pace and is estimated to soon affect 81 million individuals worldwide.1 The World Health Organization and the Institute of Medicine have recommended greater dementia awareness and education.2,3 Despite this emphasis on dementia education, many general practitioners consider dementia care beyond their clinical domain and feel that specialists, such as geriatricians, geriatric psychiatrists, or neurologists should address dementia assessment and treatment. 4 Unfortunately, the geriatric health care workforce has been shrinking. The American Geriatrics Society estimates the need for 30,000 geriatricians by 2030, although there are only 7,300 board-certified geriatricians currently in the US.5 There is an urgent need for educating all medical trainees in dementia care regardless of their specialization interest. As the largest underwriter of graduate medical education in the US, the US Department of Veterans Affairs (VA) is well placed for rolling out focused dementia education. Training needs to be practical, brief, and responsive to knowledge gaps to reach the most trainees.
Despite growing emphasis on geriatric training, many medical students have limited experience with patients with dementia or their caregivers, lack exposure to interdisciplinary teams, have a poor attitude toward geriatric patients, and display specific knowledge gaps in dementia assessment and management. 6-9 Other knowledge gaps noted in medical students included assessing behavioral problems, function, safety, and caregiver burden. Medical students also had limited exposure to interdisciplinary team dementia assessment and management.
Our goal was to develop a multicomponent, experiential, brief curriculum using team-based learning to expose senior medical students to interdisciplinary assessment of dementia. The curriculum was developed with input from the interdisciplinary team to address dementia knowledge gaps while providing an opportunity to interact with caregivers. The curriculum targeted all medical students regardless of their interest in geriatrics. Particular emphasis was placed on systems-based learning and the importance of teamwork in managing complex conditions such as dementia. Students were taught that incorporating interdisciplinary input would be more effective during dementia care planning rather than developing specialized knowledge.
Methods
Our team developed a curriculum for fourthyear medical students who rotated through the VA Memory Disorders Clinic as a part of their geriatric medicine clerkship at the University of Arkansas for Medical Sciences in Little Rock. The Memory Disorders Clinic is a consultation practice at the Central Arkansas Veterans Healthcare System (CAVHS) where patients with memory problems are evaluated by a team consisting of a geriatric psychiatrist, a geriatrician, a social worker, and a neuropsychologist. Each specialist addresses specific areas of dementia assessment and management. The curriculum included didactics, clinical experience, and team-based learning.
Didactics
An hour-long didactic session lead by the team geriatrician provided a general overview of interdisciplinary assessment of dementia to groups of 2 to 3 students at a time. The geriatrician presented an overview of dementia types, comorbidities, medications that affect memory, details of the physical examination, and laboratory, cognitive, and behavioral assessments along with treatment plan development. Students also learned about the roles of the social worker, geriatrician, neuropsychologist, and geriatric psychiatrist in the clinic. Pictographs and pie charts highlighted the role of disciplines in assessing and managing aspects of dementia.
The social work evaluation included advance care planning, functional assessment, safety assessment (driving, guns, wandering behaviors, etc), home safety evaluation, support system, and financial evaluation. Each medical student received a binder with local resources to become familiar with the depth and breadth of agencies involved in dementia care. Each medical student learned how to administer the Zarit Burden Scale to assess caregiver burden.10 The details of the geriatrician assessment included reviewing medical comorbidities and medications contributing to dementia, a physical examination, including a focused neurologic examination, laboratory assessment, and judicious use of neuroimaging.
The neuropsychology assessment education included a battery of tests and assessments. The global screening instruments included the Modified Mini-Mental State examination (3MS), Montreal Cognitive Assessment (MoCA), and Saint Louis University Mental Status examination (SLUMS).11-13 Executive function is evaluated using the Trails Making Test A and Trails Making Test B, Controlled Oral Word Association Test, Semantic Fluency Test, and Repeatable Battery for the Assessment of Neuropsychological Status test. Cognitive tests were compared and age- , education-, and race-adjusted norms for rating scales were listed if available. Each student was expected to show proficiency in ≥ 2 cognitive screening instruments (3MS, MoCA, or SLUMS). The geriatric psychiatry assessment included clinical history, onset, and course of memory problems from patient and caregiver perspectives, the Neuropsychiatric Inventory for assessing behavioral problems, employing the clinical dementia rating scale, integrating the team data, summarizing assessment, and formulating a treatment plan.14
Clinical
Students had a single clinical exposure. Students followed 1 patient and his or her caregiver through the team assessment and observed each provider’s assessment to learn interview techniques to adapt to the patient’s sensory or cognitive impairment and become familiar with different tools and devices used in the dementia clinic, such as hearing amplifiers. Each specialist provided hands-on experience. This encounter helped the students connect with caregivers and appreciate their role in patient care.
Systems learning was an important component integrated throughout the clinical experience. Examples include using video teleconferences to communicate findings among team members and electronic health records to seamlessly obtain and integrate data. Students learned how to create worksheets to graph laboratory data such as B12, thyroid-stimulating hormone, and rapid plasma regain levels. Student gained experience in using applications to retrieve neuroimaging data, results of sleep studies, and other data. Many patients had not received the results of their sleep study, and students had the responsibility to share these reports, including the number of apneic episodes. Students used the VA Computerized Patient Record System for reviewing patient records. One particularly useful tool was Joint Legacy Viewer, a remote access tool used to retrieve data on veterans from anywhere within the US. Students were also trained on medication and consult order menus in the system.
Team-Based
Learning The objectives of the team-based learning section were to teach students basic concepts of integrating the interdisciplinary assessment and formulating a treatment plan, to provide an opportunity to present their case in a group format, to discuss the differential diagnosis, management and treatment plan with a geriatrician in the team-based learning format, and to answer questions from other students. The instructors developed a set of prepared take-home points (Table 1). The team-based learning sessions were structured so that all take-home points were covered.
Evaluations
Evaluations were performed before and immediately after the clinical experience. In preevaluation, students reported the frequency of their participation in an interdisciplinary team assessment of any condition and specifically for dementia. In pre- and postevaluation, students rated their perception of the role of interdisciplinary team members in assessing and managing dementia, their personal abilities to assess cognition, behavioral problems, caregiver burden, and their perception of the impact of behavioral problems on dementia care. A Likert scale (poor = 1; fair = 2; good = 3; very good = 4; and excellent = 5) was employed (eApendices 1 and 2 can be found at doi:10.12788/fp.0052). The only demographic information collected was the student’s gender. Semistructured interviews were conducted to assess students’ current knowledge, experience, and needs. These interviews lasted about 20 minutes and collected information regarding the students’ knowledge about cognitive and behavioral problems in general and those occurring in dementia, their experience with screening, and any problems they encountered.
Statistical Analysis
Student baseline characteristics were assessed. Pre- and postassessments were analyzed with the McNemar test for paired data, and associations with experience were evaluated using χ2 tests. Ratings were dichotomized as very good/excellent vs poor/fair/ good because our educational goal was “very good” to “excellent” experience in dementia care and to avoid expected small cell counts. Two-sided P < .05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide v5.1.
Results
One hundred fourth-year medical students participated, including 54 women. Thirtysix percent reported they had not previously attended an interdisciplinary team assessment for dementia, while 18% stated that they had attended only 1 interdisciplinary team assessment for dementia.
Before the education, students rated their dementia ability as poor. Only 2% (1 of 54), of those with 0 to 1 assessment experience rated their ability for assessing dementia with an interdisciplinary team format as very good/excellent compared with 20% (9/46) of those previously attending ≥ 2 assessments (P = .03); other ratings of ability were not associated with prior experience.
There was a significant change in the students’ self-efficacy ratings pre- to postassessment (P < .05) (Table 2). Only 10% rated their ability to assess for dementia as very good/excellent in before the intervention compared with 96% in postassessment (P < .01). Students’ perception of the impact of behavioral problems on dementia care improved significantly (45% to 98%, P < .01). Similarly, student’s perception of their ability to assess behavioral problems, caregiver burden, and cognition improved significantly from 7 to 88%; 7 to 78%, and 18 to 92%, respectively (P < .01). Students perception of the role of social worker, neuropsychologist, geriatrician, and geriatric psychiatrist also improved significantly for most measures from 81 to 98% (P = .02), 87 to 98% (P = .05), 94 to 99% (P = .06), and 88 to 100% (P = .01), respectively.
The semistructured interviews revealed that awareness of behavioral problems associated with dementia varied for different behavioral problems. Although many students showed familiarity with depression, agitation, and psychosis, they were not comfortable assessing them in a patient with dementia. These students were less aware of other behavioral problems such as disinhibition, apathy, and movement disorders. Deficits were noted in the skill of administering commonly used global cognitive screens, such as the Mini-Mental State Examination (MMSE).15
In semistructured interviews, only 7% of senior medical students were comfortable assessing behavioral problems associated with dementia. Most were not aware of any validated rating scale to assess neuropsychiatric symptoms. Similarly, only 7% of students were comfortable assessing caregiver burden, and most were not aware of any validated rating scale to assess caregiver burden. Only 1 in 5 students were comfortable using 2 cognitive screens to assess cognitive deficits. Many students stated that they were not routinely expected to perform common cognitive screens, such as the MMSE during their medical training except students who had expressed an interest in psychiatry and were expected to be proficient in the MMSE. Most students were making common mistakes, such as converting the 3-command task to 3 individual single commands, helping too much with serial 7s, and giving too much positive feedback throughout the test.
Discussion
Significant knowledge gaps regarding dementia were found in our study, which is in keeping with other studies in the area. Dementia knowledge deficits among medical trainees have been identified in the United Kingdom, Australia, and the US.6-9
In our study, a brief multicomponent experiential curriculum improved senior medical students’ perception and self-efficacy in diagnosing dementia. This is in keeping with other studies, such as the PAIRS Program.7 Findings from another study indicated that education for geriatric- oriented physicians should focus on experiential learning components through observation and interaction with older adults.16
A background of direct experience with older adults is associated with more positive attitudes toward older adults and increased interest in geriatric medicine.16 In our study, the exposure was brief; therefore, the results could not be compared with other long-term exposure studies. However, even with this brief intervention most students reported being comfortable with assessing caregiver burden (78%), behavioral problems of dementia (88%), and using ≥ 2 cognitive screens (92%). Comfortable in dementia assessment increased after the intervention from 10% to 96%. This finding is encouraging because brief multicomponent dementia education can be devised easily. This finding needs to be taken with caution because we did not conduct a formal skills evaluation.
A unique component of our experience was to learn medical students’ perception about the impact of neuropsychiatric symptoms on the trajectory, outcomes, and management of dementia. These symptoms included delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, and appetite and eating. Less than half the students thought that neuropsychiatric symptoms had a significant impact on dementia before the experience. Through didactics, systematic assessment of neuropsychiatric symptoms and interaction with caregivers, > 98% of students learned that these symptoms have a significant impact on dementia management.
This experience also emphasized the role of several disciplines in dementia assessment and management. Students’ experience positively influenced appreciation of the role of the memory clinic team. Our hope is that students will seek input from social workers, neuropsychologists, and other team members when working with patients with dementia or their caregivers. The common reason why primary care physicians focus on an exclusive medical model is the time commitment for communicating with an interdisciplinary team. Students experienced the feasibility of the interdisciplinary team involvement and how technology could be used for synchronous and asynchronous communication among team members. Medical students also were introduced to complex billing codes used when ≥ 3 disciplines assess/manage a geriatric patient.
Limitations
This study is limited by the lack of long-term follow-up evaluations, no metrics for practice changes clinical outcomes, and implementation in a single medical school. The postexperience evaluation in this study was performed immediately after the intervention. Long-term follow-up would inform whether the changes noted are durable. Because of the brief nature of our intervention, we do not believe that it would change practice in clinical care. It will be informative to follow this cohort of students to study whether their clinical approach to dementia care changes. The intervention needs to be replicated in other medical schools and in more heterogeneous groups to generalize the results of the study.
Conclusions
Senior medical students are not routinely exposed to interdisciplinary team assessments. Dementia knowledge gaps were prevalent in this cohort of senior medical students. Providing interdisciplinary geriatric educational experience improved their perception of their ability to assess for dementia and their recognition of the roles of interdisciplinary team members. Plans are in place to continue and expand the program to other complex geriatric syndromes.
Acknowledgments
Poster presented at the 2019 annual meeting of the American Geriatrics Society. Oral presentation at the same meeting as part of the select Geriatric Education Methods and Materials Swap workshop.
The global burden of dementia is increasing at an alarming pace and is estimated to soon affect 81 million individuals worldwide.1 The World Health Organization and the Institute of Medicine have recommended greater dementia awareness and education.2,3 Despite this emphasis on dementia education, many general practitioners consider dementia care beyond their clinical domain and feel that specialists, such as geriatricians, geriatric psychiatrists, or neurologists should address dementia assessment and treatment. 4 Unfortunately, the geriatric health care workforce has been shrinking. The American Geriatrics Society estimates the need for 30,000 geriatricians by 2030, although there are only 7,300 board-certified geriatricians currently in the US.5 There is an urgent need for educating all medical trainees in dementia care regardless of their specialization interest. As the largest underwriter of graduate medical education in the US, the US Department of Veterans Affairs (VA) is well placed for rolling out focused dementia education. Training needs to be practical, brief, and responsive to knowledge gaps to reach the most trainees.
Despite growing emphasis on geriatric training, many medical students have limited experience with patients with dementia or their caregivers, lack exposure to interdisciplinary teams, have a poor attitude toward geriatric patients, and display specific knowledge gaps in dementia assessment and management. 6-9 Other knowledge gaps noted in medical students included assessing behavioral problems, function, safety, and caregiver burden. Medical students also had limited exposure to interdisciplinary team dementia assessment and management.
Our goal was to develop a multicomponent, experiential, brief curriculum using team-based learning to expose senior medical students to interdisciplinary assessment of dementia. The curriculum was developed with input from the interdisciplinary team to address dementia knowledge gaps while providing an opportunity to interact with caregivers. The curriculum targeted all medical students regardless of their interest in geriatrics. Particular emphasis was placed on systems-based learning and the importance of teamwork in managing complex conditions such as dementia. Students were taught that incorporating interdisciplinary input would be more effective during dementia care planning rather than developing specialized knowledge.
Methods
Our team developed a curriculum for fourthyear medical students who rotated through the VA Memory Disorders Clinic as a part of their geriatric medicine clerkship at the University of Arkansas for Medical Sciences in Little Rock. The Memory Disorders Clinic is a consultation practice at the Central Arkansas Veterans Healthcare System (CAVHS) where patients with memory problems are evaluated by a team consisting of a geriatric psychiatrist, a geriatrician, a social worker, and a neuropsychologist. Each specialist addresses specific areas of dementia assessment and management. The curriculum included didactics, clinical experience, and team-based learning.
Didactics
An hour-long didactic session lead by the team geriatrician provided a general overview of interdisciplinary assessment of dementia to groups of 2 to 3 students at a time. The geriatrician presented an overview of dementia types, comorbidities, medications that affect memory, details of the physical examination, and laboratory, cognitive, and behavioral assessments along with treatment plan development. Students also learned about the roles of the social worker, geriatrician, neuropsychologist, and geriatric psychiatrist in the clinic. Pictographs and pie charts highlighted the role of disciplines in assessing and managing aspects of dementia.
The social work evaluation included advance care planning, functional assessment, safety assessment (driving, guns, wandering behaviors, etc), home safety evaluation, support system, and financial evaluation. Each medical student received a binder with local resources to become familiar with the depth and breadth of agencies involved in dementia care. Each medical student learned how to administer the Zarit Burden Scale to assess caregiver burden.10 The details of the geriatrician assessment included reviewing medical comorbidities and medications contributing to dementia, a physical examination, including a focused neurologic examination, laboratory assessment, and judicious use of neuroimaging.
The neuropsychology assessment education included a battery of tests and assessments. The global screening instruments included the Modified Mini-Mental State examination (3MS), Montreal Cognitive Assessment (MoCA), and Saint Louis University Mental Status examination (SLUMS).11-13 Executive function is evaluated using the Trails Making Test A and Trails Making Test B, Controlled Oral Word Association Test, Semantic Fluency Test, and Repeatable Battery for the Assessment of Neuropsychological Status test. Cognitive tests were compared and age- , education-, and race-adjusted norms for rating scales were listed if available. Each student was expected to show proficiency in ≥ 2 cognitive screening instruments (3MS, MoCA, or SLUMS). The geriatric psychiatry assessment included clinical history, onset, and course of memory problems from patient and caregiver perspectives, the Neuropsychiatric Inventory for assessing behavioral problems, employing the clinical dementia rating scale, integrating the team data, summarizing assessment, and formulating a treatment plan.14
Clinical
Students had a single clinical exposure. Students followed 1 patient and his or her caregiver through the team assessment and observed each provider’s assessment to learn interview techniques to adapt to the patient’s sensory or cognitive impairment and become familiar with different tools and devices used in the dementia clinic, such as hearing amplifiers. Each specialist provided hands-on experience. This encounter helped the students connect with caregivers and appreciate their role in patient care.
Systems learning was an important component integrated throughout the clinical experience. Examples include using video teleconferences to communicate findings among team members and electronic health records to seamlessly obtain and integrate data. Students learned how to create worksheets to graph laboratory data such as B12, thyroid-stimulating hormone, and rapid plasma regain levels. Student gained experience in using applications to retrieve neuroimaging data, results of sleep studies, and other data. Many patients had not received the results of their sleep study, and students had the responsibility to share these reports, including the number of apneic episodes. Students used the VA Computerized Patient Record System for reviewing patient records. One particularly useful tool was Joint Legacy Viewer, a remote access tool used to retrieve data on veterans from anywhere within the US. Students were also trained on medication and consult order menus in the system.
Team-Based
Learning The objectives of the team-based learning section were to teach students basic concepts of integrating the interdisciplinary assessment and formulating a treatment plan, to provide an opportunity to present their case in a group format, to discuss the differential diagnosis, management and treatment plan with a geriatrician in the team-based learning format, and to answer questions from other students. The instructors developed a set of prepared take-home points (Table 1). The team-based learning sessions were structured so that all take-home points were covered.
Evaluations
Evaluations were performed before and immediately after the clinical experience. In preevaluation, students reported the frequency of their participation in an interdisciplinary team assessment of any condition and specifically for dementia. In pre- and postevaluation, students rated their perception of the role of interdisciplinary team members in assessing and managing dementia, their personal abilities to assess cognition, behavioral problems, caregiver burden, and their perception of the impact of behavioral problems on dementia care. A Likert scale (poor = 1; fair = 2; good = 3; very good = 4; and excellent = 5) was employed (eApendices 1 and 2 can be found at doi:10.12788/fp.0052). The only demographic information collected was the student’s gender. Semistructured interviews were conducted to assess students’ current knowledge, experience, and needs. These interviews lasted about 20 minutes and collected information regarding the students’ knowledge about cognitive and behavioral problems in general and those occurring in dementia, their experience with screening, and any problems they encountered.
Statistical Analysis
Student baseline characteristics were assessed. Pre- and postassessments were analyzed with the McNemar test for paired data, and associations with experience were evaluated using χ2 tests. Ratings were dichotomized as very good/excellent vs poor/fair/ good because our educational goal was “very good” to “excellent” experience in dementia care and to avoid expected small cell counts. Two-sided P < .05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide v5.1.
Results
One hundred fourth-year medical students participated, including 54 women. Thirtysix percent reported they had not previously attended an interdisciplinary team assessment for dementia, while 18% stated that they had attended only 1 interdisciplinary team assessment for dementia.
Before the education, students rated their dementia ability as poor. Only 2% (1 of 54), of those with 0 to 1 assessment experience rated their ability for assessing dementia with an interdisciplinary team format as very good/excellent compared with 20% (9/46) of those previously attending ≥ 2 assessments (P = .03); other ratings of ability were not associated with prior experience.
There was a significant change in the students’ self-efficacy ratings pre- to postassessment (P < .05) (Table 2). Only 10% rated their ability to assess for dementia as very good/excellent in before the intervention compared with 96% in postassessment (P < .01). Students’ perception of the impact of behavioral problems on dementia care improved significantly (45% to 98%, P < .01). Similarly, student’s perception of their ability to assess behavioral problems, caregiver burden, and cognition improved significantly from 7 to 88%; 7 to 78%, and 18 to 92%, respectively (P < .01). Students perception of the role of social worker, neuropsychologist, geriatrician, and geriatric psychiatrist also improved significantly for most measures from 81 to 98% (P = .02), 87 to 98% (P = .05), 94 to 99% (P = .06), and 88 to 100% (P = .01), respectively.
The semistructured interviews revealed that awareness of behavioral problems associated with dementia varied for different behavioral problems. Although many students showed familiarity with depression, agitation, and psychosis, they were not comfortable assessing them in a patient with dementia. These students were less aware of other behavioral problems such as disinhibition, apathy, and movement disorders. Deficits were noted in the skill of administering commonly used global cognitive screens, such as the Mini-Mental State Examination (MMSE).15
In semistructured interviews, only 7% of senior medical students were comfortable assessing behavioral problems associated with dementia. Most were not aware of any validated rating scale to assess neuropsychiatric symptoms. Similarly, only 7% of students were comfortable assessing caregiver burden, and most were not aware of any validated rating scale to assess caregiver burden. Only 1 in 5 students were comfortable using 2 cognitive screens to assess cognitive deficits. Many students stated that they were not routinely expected to perform common cognitive screens, such as the MMSE during their medical training except students who had expressed an interest in psychiatry and were expected to be proficient in the MMSE. Most students were making common mistakes, such as converting the 3-command task to 3 individual single commands, helping too much with serial 7s, and giving too much positive feedback throughout the test.
Discussion
Significant knowledge gaps regarding dementia were found in our study, which is in keeping with other studies in the area. Dementia knowledge deficits among medical trainees have been identified in the United Kingdom, Australia, and the US.6-9
In our study, a brief multicomponent experiential curriculum improved senior medical students’ perception and self-efficacy in diagnosing dementia. This is in keeping with other studies, such as the PAIRS Program.7 Findings from another study indicated that education for geriatric- oriented physicians should focus on experiential learning components through observation and interaction with older adults.16
A background of direct experience with older adults is associated with more positive attitudes toward older adults and increased interest in geriatric medicine.16 In our study, the exposure was brief; therefore, the results could not be compared with other long-term exposure studies. However, even with this brief intervention most students reported being comfortable with assessing caregiver burden (78%), behavioral problems of dementia (88%), and using ≥ 2 cognitive screens (92%). Comfortable in dementia assessment increased after the intervention from 10% to 96%. This finding is encouraging because brief multicomponent dementia education can be devised easily. This finding needs to be taken with caution because we did not conduct a formal skills evaluation.
A unique component of our experience was to learn medical students’ perception about the impact of neuropsychiatric symptoms on the trajectory, outcomes, and management of dementia. These symptoms included delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, and appetite and eating. Less than half the students thought that neuropsychiatric symptoms had a significant impact on dementia before the experience. Through didactics, systematic assessment of neuropsychiatric symptoms and interaction with caregivers, > 98% of students learned that these symptoms have a significant impact on dementia management.
This experience also emphasized the role of several disciplines in dementia assessment and management. Students’ experience positively influenced appreciation of the role of the memory clinic team. Our hope is that students will seek input from social workers, neuropsychologists, and other team members when working with patients with dementia or their caregivers. The common reason why primary care physicians focus on an exclusive medical model is the time commitment for communicating with an interdisciplinary team. Students experienced the feasibility of the interdisciplinary team involvement and how technology could be used for synchronous and asynchronous communication among team members. Medical students also were introduced to complex billing codes used when ≥ 3 disciplines assess/manage a geriatric patient.
Limitations
This study is limited by the lack of long-term follow-up evaluations, no metrics for practice changes clinical outcomes, and implementation in a single medical school. The postexperience evaluation in this study was performed immediately after the intervention. Long-term follow-up would inform whether the changes noted are durable. Because of the brief nature of our intervention, we do not believe that it would change practice in clinical care. It will be informative to follow this cohort of students to study whether their clinical approach to dementia care changes. The intervention needs to be replicated in other medical schools and in more heterogeneous groups to generalize the results of the study.
Conclusions
Senior medical students are not routinely exposed to interdisciplinary team assessments. Dementia knowledge gaps were prevalent in this cohort of senior medical students. Providing interdisciplinary geriatric educational experience improved their perception of their ability to assess for dementia and their recognition of the roles of interdisciplinary team members. Plans are in place to continue and expand the program to other complex geriatric syndromes.
Acknowledgments
Poster presented at the 2019 annual meeting of the American Geriatrics Society. Oral presentation at the same meeting as part of the select Geriatric Education Methods and Materials Swap workshop.
1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. doi:10.1016/S0140-6736(05)67889-0
2. Janca A, Aarli JA, Prilipko L, Dua T, Saxena S, Saraceno B. WHO/WFN survey of neurological services: a worldwide perspective. J Neurol Sci. 2006;247(1):29-34. doi:10.1016/j.jns.2006.03.003
3. Wilkins KM, Blazek MC, Brooks WB, Lehmann SW, Popeo D, Wagenaar D. Six things all medical students need to know about geriatric psychiatry (and how to teach them). Acad Psychiatry. 2017;41(5):693-700. doi:10.1007/s40596-017-0691-7
4. Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461- 467. doi:10.1093/ageing/afh140
5. Lester PE, Dharmarajan TS, Weinstein E. The looming geriatrician shortage: ramifications and solutions. J Aging Health. 2019:898264319879325. doi:10.1177/0898264319879325
6. Struck BD, Bernard MA, Teasdale TA; Oklahoma University Geriatric Education G. Effect of a mandatory geriatric medicine clerkship on third-year students. J Am Geriatr Soc. 2005;53(11):2007-2011. doi:10.1111/j.1532-5415.2005.00473.x
7. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer’s disease: the PAIRS Program. BMC Med Educ. 2012;12:80. doi:10.1186/1472-6920-12-80
8. Nagle BJ, Usita PM, Edland SD. United States medical students’ knowledge of Alzheimer disease. J Educ Eval Health Prof. 2013;10:4. doi:10.3352/jeehp.2013.10.4
9. Scott TL, Kugelman M, Tulloch K. How medical professional students view older people with dementia: Implications for education and practice. PLoS One. 2019;14(11):e0225329. doi:10.1371/journal.pone.0225329.
10. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655. doi:10.1093/geront/20.6.649
11. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7
12. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Ger iatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x
13. Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910. doi:10.1097/01.JGP.0000221510.33817.86
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi:10.1212/wnl.44.12.2308
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
16. Fitzgerald JT, Wray LA, Halter JB, Williams BC, Supiano MA. Relating medical students’ knowledge, attitudes, and experience to an interest in geriatric medicine. Gerontologist. 2003;43(6):849-855. doi:10.1093/geront/43.6.849
1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. doi:10.1016/S0140-6736(05)67889-0
2. Janca A, Aarli JA, Prilipko L, Dua T, Saxena S, Saraceno B. WHO/WFN survey of neurological services: a worldwide perspective. J Neurol Sci. 2006;247(1):29-34. doi:10.1016/j.jns.2006.03.003
3. Wilkins KM, Blazek MC, Brooks WB, Lehmann SW, Popeo D, Wagenaar D. Six things all medical students need to know about geriatric psychiatry (and how to teach them). Acad Psychiatry. 2017;41(5):693-700. doi:10.1007/s40596-017-0691-7
4. Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461- 467. doi:10.1093/ageing/afh140
5. Lester PE, Dharmarajan TS, Weinstein E. The looming geriatrician shortage: ramifications and solutions. J Aging Health. 2019:898264319879325. doi:10.1177/0898264319879325
6. Struck BD, Bernard MA, Teasdale TA; Oklahoma University Geriatric Education G. Effect of a mandatory geriatric medicine clerkship on third-year students. J Am Geriatr Soc. 2005;53(11):2007-2011. doi:10.1111/j.1532-5415.2005.00473.x
7. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer’s disease: the PAIRS Program. BMC Med Educ. 2012;12:80. doi:10.1186/1472-6920-12-80
8. Nagle BJ, Usita PM, Edland SD. United States medical students’ knowledge of Alzheimer disease. J Educ Eval Health Prof. 2013;10:4. doi:10.3352/jeehp.2013.10.4
9. Scott TL, Kugelman M, Tulloch K. How medical professional students view older people with dementia: Implications for education and practice. PLoS One. 2019;14(11):e0225329. doi:10.1371/journal.pone.0225329.
10. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655. doi:10.1093/geront/20.6.649
11. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7
12. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Ger iatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x
13. Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910. doi:10.1097/01.JGP.0000221510.33817.86
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi:10.1212/wnl.44.12.2308
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
16. Fitzgerald JT, Wray LA, Halter JB, Williams BC, Supiano MA. Relating medical students’ knowledge, attitudes, and experience to an interest in geriatric medicine. Gerontologist. 2003;43(6):849-855. doi:10.1093/geront/43.6.849
28-year-old woman • weakness • anxiety • altered mental status • Dx?
THE CASE
A 28-year-old woman with an extensive psychiatric history—including generalized anxiety disorder, panic disorder, and recent postpartum depression—presented with a chief complaint of right leg weakness. She stated this weakness had begun 4 days earlier. It occurred episodically and was preceded by tingling and cramping sensations. Each episode lasted a couple of minutes and spontaneously resolved. Associated with it, she experienced slurred speech and altered mentation. There was no loss of consciousness and no pain. A panic attack usually followed, consisting of feelings of impending doom, rapid breathing, palpitations, and nausea.
She had 3 prior diagnostic evaluations for this same chief complaint, twice in an emergency department (ED) and once with her primary care physician. These evaluations included lab work and extensive head imaging, which demonstrated no acute intracranial pathology. At each previous presentation, the diagnosis was an exacerbation of her anxiety disorder, and she was treated with lorazepam.
At the current presentation, her vital signs were stable. Examination revealed a notably anxious patient. She repeatedly expressed concern that she might have a brain tumor or some other deadly disease, as she had a family history of brain cancer. Her physical exam was entirely normal, including normal strength, sensation, and reflexes in all extremities.
Further head imaging (computed tomography, CT angiography, and magnetic resonance imaging of the brain) failed to reveal an etiology of her symptoms. With no clear organic cause, her medical providers again suspected an anxiety or panic episode. She was given reassurance, and an outpatient neurology consult was arranged.
THE DIAGNOSIS
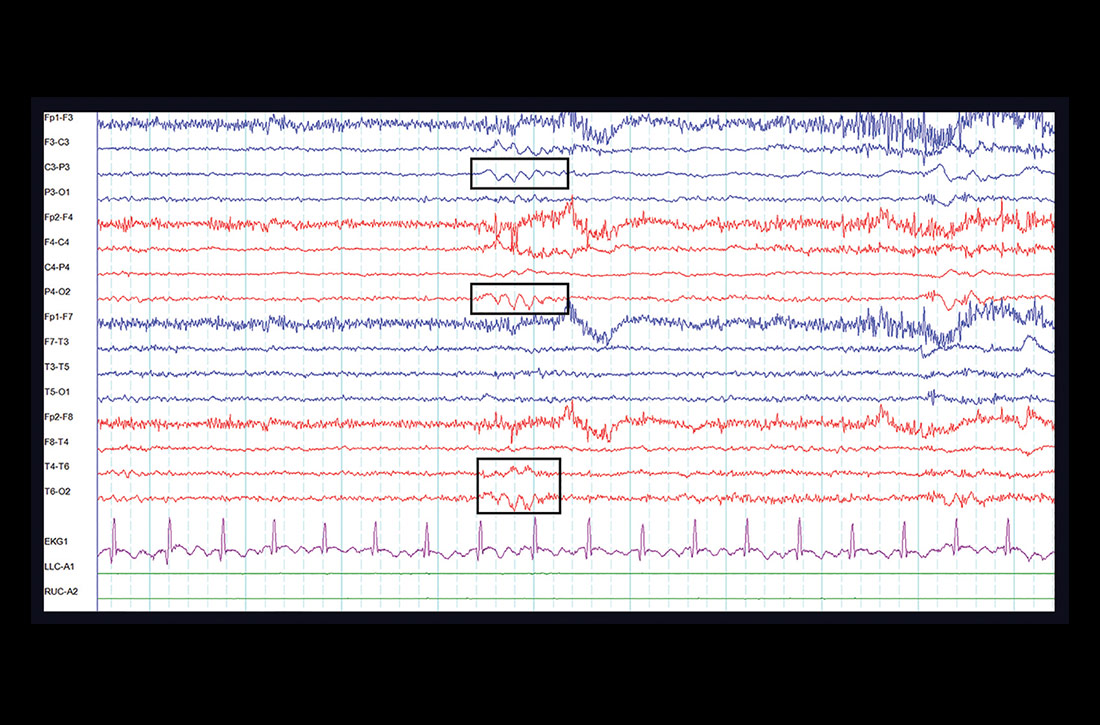
One week later, at her outpatient neurology appointment, an electroencephalogram (EEG) was performed. Following photic stimulation, the EEG showed multiple right- and left-hemisphere foci of cortical hyperexcitability including a subtle sharp component (see FIGURE). Immediately following the longest of these episodes, the patient expressed a sense of anxiety and an altered sensorium similar to her prior presentations.

The EEG findings, in addition to the postictal anxiety symptoms and clinical history, were all important components that led the treating neurologist to the diagnosis of localization-related (focal) epilepsy.1 The patient was started on oxcarbazepine, a first-line anti-epileptic medication used in the treatment of focal epilepsy.2 She is being followed by a neurologist regularly and after optimizing her anti-epileptic medication, is no longer having seizures.
DISCUSSION
The difficulty of this case stems from the atypical presentation of the patient’s seizures. The key step to the correct diagnosis was a neurological consultation and an ensuing EEG. However, the patient received a vast spectrum of care, including multiple work-ups, prior to a conclusive diagnosis—which highlights an important issue health care providers must address.
Continue to: The role of bias
The role of bias. From the patient’s initial visits to the ED to her hospital admission, there was a prominent affixation, known as the anchoring bias,3 by the clinicians providing her care: All were focused heavily on her psychiatric features. Conversely, the evaluation for patients with suspected psychiatric diagnoses should focus on successfully ruling out major organic etiology with a broad differential diagnosis. It is crucial for providers to take a step back and make a conscious attempt to avoid fixation on a particular diagnosis, especially when it is psychiatric in nature. This allows the provider to actively consider alternative explanations for a patient presentation and work through a more encompassing differential.
The distinguishing symptoms. There is a common association between comorbid mood disorders (eg, depression, anxiety) and epilepsy.4 Another clue is ictal anxiety or nervousness, which is commonly observed in patients with partial seizures (and occurred with our patient).
These ictal episodes can be difficult to identify within the context of an isolated psychiatric diagnosis.5 The distinction can be clarified by the presence of associated somatic symptoms, which in this case included unilateral cramping, paresthesia, and weakness. These symptoms should clue in a practitioner to the possibility of underlying neurologic pathology, which should prompt the ordering of either an EEG or, at minimum, a neurological consultation.
THE TAKEAWAY
This case report shows how anchoring bias can lead to a delay in diagnosis and treatment. Avoidance of this type of bias requires heightened cognitive awareness by medical providers. A more system-based approach is to have structured diagnostic assessments,6 such as conducting a thorough neurological exam for patients with somatic symptoms and exacerbating comorbid psychiatric conditions.
It may also help to review cases like this with colleagues from diverse disciplinary backgrounds, highlighting thought processes and sharing uncertainty.3 These processes may shed light on confounding diagnoses that might be playing a role in a patient’s presentation and ultimately aid in the decision-making process.
CORRESPONDENCE
Paimon Ameli, DO, Naval Medical Center San Diego, 34800 Bob Wilson Drive, San Diego, CA 92134; [email protected]
1. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522-530.
2. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000-1015.
3. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
4. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
5. López-Gómez M, Espinola M, Ramirez-Bermudez J, et al. Clinical presentation of anxiety among patients with epilepsy. Neuropsychiatr Dis Treat. 2008;4:1235-1239.
6. Etchells E. Anchoring bias with critical implications. Published June 2015. Patient Safety Network. https://psnet.ahrq.gov/web-mm/anchoring-bias-critical-implications. Accessed September 29, 2020.
THE CASE
A 28-year-old woman with an extensive psychiatric history—including generalized anxiety disorder, panic disorder, and recent postpartum depression—presented with a chief complaint of right leg weakness. She stated this weakness had begun 4 days earlier. It occurred episodically and was preceded by tingling and cramping sensations. Each episode lasted a couple of minutes and spontaneously resolved. Associated with it, she experienced slurred speech and altered mentation. There was no loss of consciousness and no pain. A panic attack usually followed, consisting of feelings of impending doom, rapid breathing, palpitations, and nausea.
She had 3 prior diagnostic evaluations for this same chief complaint, twice in an emergency department (ED) and once with her primary care physician. These evaluations included lab work and extensive head imaging, which demonstrated no acute intracranial pathology. At each previous presentation, the diagnosis was an exacerbation of her anxiety disorder, and she was treated with lorazepam.
At the current presentation, her vital signs were stable. Examination revealed a notably anxious patient. She repeatedly expressed concern that she might have a brain tumor or some other deadly disease, as she had a family history of brain cancer. Her physical exam was entirely normal, including normal strength, sensation, and reflexes in all extremities.
Further head imaging (computed tomography, CT angiography, and magnetic resonance imaging of the brain) failed to reveal an etiology of her symptoms. With no clear organic cause, her medical providers again suspected an anxiety or panic episode. She was given reassurance, and an outpatient neurology consult was arranged.
THE DIAGNOSIS
One week later, at her outpatient neurology appointment, an electroencephalogram (EEG) was performed. Following photic stimulation, the EEG showed multiple right- and left-hemisphere foci of cortical hyperexcitability including a subtle sharp component (see FIGURE). Immediately following the longest of these episodes, the patient expressed a sense of anxiety and an altered sensorium similar to her prior presentations.

The EEG findings, in addition to the postictal anxiety symptoms and clinical history, were all important components that led the treating neurologist to the diagnosis of localization-related (focal) epilepsy.1 The patient was started on oxcarbazepine, a first-line anti-epileptic medication used in the treatment of focal epilepsy.2 She is being followed by a neurologist regularly and after optimizing her anti-epileptic medication, is no longer having seizures.
DISCUSSION
The difficulty of this case stems from the atypical presentation of the patient’s seizures. The key step to the correct diagnosis was a neurological consultation and an ensuing EEG. However, the patient received a vast spectrum of care, including multiple work-ups, prior to a conclusive diagnosis—which highlights an important issue health care providers must address.
Continue to: The role of bias
The role of bias. From the patient’s initial visits to the ED to her hospital admission, there was a prominent affixation, known as the anchoring bias,3 by the clinicians providing her care: All were focused heavily on her psychiatric features. Conversely, the evaluation for patients with suspected psychiatric diagnoses should focus on successfully ruling out major organic etiology with a broad differential diagnosis. It is crucial for providers to take a step back and make a conscious attempt to avoid fixation on a particular diagnosis, especially when it is psychiatric in nature. This allows the provider to actively consider alternative explanations for a patient presentation and work through a more encompassing differential.
The distinguishing symptoms. There is a common association between comorbid mood disorders (eg, depression, anxiety) and epilepsy.4 Another clue is ictal anxiety or nervousness, which is commonly observed in patients with partial seizures (and occurred with our patient).
These ictal episodes can be difficult to identify within the context of an isolated psychiatric diagnosis.5 The distinction can be clarified by the presence of associated somatic symptoms, which in this case included unilateral cramping, paresthesia, and weakness. These symptoms should clue in a practitioner to the possibility of underlying neurologic pathology, which should prompt the ordering of either an EEG or, at minimum, a neurological consultation.
THE TAKEAWAY
This case report shows how anchoring bias can lead to a delay in diagnosis and treatment. Avoidance of this type of bias requires heightened cognitive awareness by medical providers. A more system-based approach is to have structured diagnostic assessments,6 such as conducting a thorough neurological exam for patients with somatic symptoms and exacerbating comorbid psychiatric conditions.
It may also help to review cases like this with colleagues from diverse disciplinary backgrounds, highlighting thought processes and sharing uncertainty.3 These processes may shed light on confounding diagnoses that might be playing a role in a patient’s presentation and ultimately aid in the decision-making process.
CORRESPONDENCE
Paimon Ameli, DO, Naval Medical Center San Diego, 34800 Bob Wilson Drive, San Diego, CA 92134; [email protected]
THE CASE
A 28-year-old woman with an extensive psychiatric history—including generalized anxiety disorder, panic disorder, and recent postpartum depression—presented with a chief complaint of right leg weakness. She stated this weakness had begun 4 days earlier. It occurred episodically and was preceded by tingling and cramping sensations. Each episode lasted a couple of minutes and spontaneously resolved. Associated with it, she experienced slurred speech and altered mentation. There was no loss of consciousness and no pain. A panic attack usually followed, consisting of feelings of impending doom, rapid breathing, palpitations, and nausea.
She had 3 prior diagnostic evaluations for this same chief complaint, twice in an emergency department (ED) and once with her primary care physician. These evaluations included lab work and extensive head imaging, which demonstrated no acute intracranial pathology. At each previous presentation, the diagnosis was an exacerbation of her anxiety disorder, and she was treated with lorazepam.
At the current presentation, her vital signs were stable. Examination revealed a notably anxious patient. She repeatedly expressed concern that she might have a brain tumor or some other deadly disease, as she had a family history of brain cancer. Her physical exam was entirely normal, including normal strength, sensation, and reflexes in all extremities.
Further head imaging (computed tomography, CT angiography, and magnetic resonance imaging of the brain) failed to reveal an etiology of her symptoms. With no clear organic cause, her medical providers again suspected an anxiety or panic episode. She was given reassurance, and an outpatient neurology consult was arranged.
THE DIAGNOSIS
One week later, at her outpatient neurology appointment, an electroencephalogram (EEG) was performed. Following photic stimulation, the EEG showed multiple right- and left-hemisphere foci of cortical hyperexcitability including a subtle sharp component (see FIGURE). Immediately following the longest of these episodes, the patient expressed a sense of anxiety and an altered sensorium similar to her prior presentations.

The EEG findings, in addition to the postictal anxiety symptoms and clinical history, were all important components that led the treating neurologist to the diagnosis of localization-related (focal) epilepsy.1 The patient was started on oxcarbazepine, a first-line anti-epileptic medication used in the treatment of focal epilepsy.2 She is being followed by a neurologist regularly and after optimizing her anti-epileptic medication, is no longer having seizures.
DISCUSSION
The difficulty of this case stems from the atypical presentation of the patient’s seizures. The key step to the correct diagnosis was a neurological consultation and an ensuing EEG. However, the patient received a vast spectrum of care, including multiple work-ups, prior to a conclusive diagnosis—which highlights an important issue health care providers must address.
Continue to: The role of bias
The role of bias. From the patient’s initial visits to the ED to her hospital admission, there was a prominent affixation, known as the anchoring bias,3 by the clinicians providing her care: All were focused heavily on her psychiatric features. Conversely, the evaluation for patients with suspected psychiatric diagnoses should focus on successfully ruling out major organic etiology with a broad differential diagnosis. It is crucial for providers to take a step back and make a conscious attempt to avoid fixation on a particular diagnosis, especially when it is psychiatric in nature. This allows the provider to actively consider alternative explanations for a patient presentation and work through a more encompassing differential.
The distinguishing symptoms. There is a common association between comorbid mood disorders (eg, depression, anxiety) and epilepsy.4 Another clue is ictal anxiety or nervousness, which is commonly observed in patients with partial seizures (and occurred with our patient).
These ictal episodes can be difficult to identify within the context of an isolated psychiatric diagnosis.5 The distinction can be clarified by the presence of associated somatic symptoms, which in this case included unilateral cramping, paresthesia, and weakness. These symptoms should clue in a practitioner to the possibility of underlying neurologic pathology, which should prompt the ordering of either an EEG or, at minimum, a neurological consultation.
THE TAKEAWAY
This case report shows how anchoring bias can lead to a delay in diagnosis and treatment. Avoidance of this type of bias requires heightened cognitive awareness by medical providers. A more system-based approach is to have structured diagnostic assessments,6 such as conducting a thorough neurological exam for patients with somatic symptoms and exacerbating comorbid psychiatric conditions.
It may also help to review cases like this with colleagues from diverse disciplinary backgrounds, highlighting thought processes and sharing uncertainty.3 These processes may shed light on confounding diagnoses that might be playing a role in a patient’s presentation and ultimately aid in the decision-making process.
CORRESPONDENCE
Paimon Ameli, DO, Naval Medical Center San Diego, 34800 Bob Wilson Drive, San Diego, CA 92134; [email protected]
1. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522-530.
2. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000-1015.
3. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
4. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
5. López-Gómez M, Espinola M, Ramirez-Bermudez J, et al. Clinical presentation of anxiety among patients with epilepsy. Neuropsychiatr Dis Treat. 2008;4:1235-1239.
6. Etchells E. Anchoring bias with critical implications. Published June 2015. Patient Safety Network. https://psnet.ahrq.gov/web-mm/anchoring-bias-critical-implications. Accessed September 29, 2020.
1. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522-530.
2. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000-1015.
3. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
4. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
5. López-Gómez M, Espinola M, Ramirez-Bermudez J, et al. Clinical presentation of anxiety among patients with epilepsy. Neuropsychiatr Dis Treat. 2008;4:1235-1239.
6. Etchells E. Anchoring bias with critical implications. Published June 2015. Patient Safety Network. https://psnet.ahrq.gov/web-mm/anchoring-bias-critical-implications. Accessed September 29, 2020.
Telemedicine feasible and reliable in Parkinson’s trial
, a 1-year, phase 3 clinical trial has shown. The trial was an add-on study involving a subset of subjects from the STEADY-PD III trial of isradipine in early Parkinson’s disease.
Although the trial was conducted before SARS-CoV-2 arrived on the scene, the findings have particular relevance for being able to conduct a variety of clinical trials in the face of COVID-19 and the need to limit in-person interactions.
The 40 participants used tablets to complete three remote, video-based assessments during 1 year, with each remote visit planned to be completed within 4 weeks of an in-person visit. It was easy to enroll patients, and they completed about 95% of planned visits, said neurologist Christopher Tarolli, MD, of the University of Rochester (N.Y.).
He presented the study findings at the Movement Disorder Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
“The visits were clearly feasible, and we were able to do them [84%] within that 4-week time frame around the in-person visit,” he said. “The visits were also reasonably reliable, particularly so for what we call the nonmotor outcomes and the patient-reported outcomes.”
In-person versus remote assessment
For the remote visits, participants completed primarily the same battery of tests as the in-person visits. Responses on the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) subscales demonstrated “that there was excellent correlation between patient-reported and nonmotor outcome measures and moderate correlation between in-person and remote-performed motor assessments,” Dr. Tarolli said.
He explained that the study used modified motor assessments (MDS-UPDRS Part III) that excluded testing of rigidity and postural instability, which require hands-on testing by a trained examiner and thus are impossible to do remotely.
Additionally, the somewhat lower correlation on this subscale was probably the result of different investigators conducting in-person versus remote assessments, with a subset of in-person investigators who tended to rate participants more severely driving down the correlation. “I think if these methods were applied in future trials, the in-person and remote investigators would optimally be the same person,” Dr. Tarolli suggested.
Room for error?
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that “the reliability of UPDRS [part] III is where I would want to have, for sure, a little bit more of a deep dive. … possibly the same patient be rated by the same person.”
She also noted that doing remote and in-person assessments within 4 weeks of each other leaves a lot of room for variability. “You could see the same patient in the morning and then do UPDRS in the afternoon, and it can be totally different depending on when you meet the person,” she said.
Only so much testing can be done remotely. Nonetheless, she questioned whether it is really a valid UPDRS if rigidity and postural stability measures are eliminated. “[Is] this now a new modified UPDRS that we’re going to use that is as good as the old UPDRS moving forward, a home version of UPDRS or whatever we’re going to call it?”
Dr. Subramanian mentioned that patients have told her that UPDRS part III does not really measure what is most important to them, such as making pastries for their grandchildren rather than rapidly tapping their fingers.
“That speaks a little bit to the fact that we should have more patient-centered outcomes and things that patients can report. … things that are not going to require necessarily an in-person exam as maybe measures that really can be used moving forward in studies,” she suggested.
Patient satisfaction with remote visits
Greater than 90% of the patients were satisfied or very satisfied overall with the remote visits, including the convenience, comfort, and connection (using the devices and Internet connection), with “patients describing enjoying being able to do these visits from the comfort of their own home, not having to travel,” Dr. Tarolli said. Not having to drive in an ‘off’ state “was actually something that some participants identified as a safety benefit from this as well.”
There was also a time benefit to the patients and investigators. The average length of the remote visits was 54.3 minutes each versus 74 minutes of interaction for in-person visits, mainly a result of more efficient hand-offs between the neurologist and the study coordinator during the remote visits, plus being able to pause the remote visit to give a medication dose time to take effect.
For the patient, there was a large amount of time saved when travel time was considered – a total of 190.2 minutes on average for travel and testing for the in-person visits.
About three-quarters (76%) of the study patients said that remote visits would increase their likelihood of participating in future trials. However, that result may be skewed by the fact that these were already people willing to participate in a remote trial, so the generalizability of the result may be affected. Nonetheless, Dr. Tarolli said he thinks that, as technology gets better and older people become more comfortable with it, remote visits within Parkinson’s research studies may become more common.
One caveat he mentioned is that, with remote visits, the neurologist misses a chance to observe a patient’s whole body and construct a global impression of how he or she is moving. On the other hand, remote video gives the investigator the chance to see the living environment of the patient and suggest changes for safety, such as to reduce the risk of falling for a person with unsteadiness of gait living in a crowded house.
“It really allows us to make a more holistic assessment of how our patient is functioning outside the clinic, which I think we’ve traditionally had really no way of doing,” Dr. Tarolli said.
His final suggestion for anyone contemplating conducting studies with remote visits is to develop a team that is comfortable troubleshooting the technological aspects of those visits.
UCLA’s Dr. Subramanian lauded the University of Rochester team for their efforts in moving remote visits forward. “They’re at the cutting edge of these sorts of things,” she said. “So I’m assuming that they’ll come out with more things [for visits] to become better that are going to move this forward, which is exciting.”
Dr. Tarolli has disclosed no relevant financial relationships. Dr. Subramanian has given talks for Acorda Pharmaceuticals and Acadia Pharmaceuticals in the past. The study had only university, government, foundation, and other nonprofit support.
A version of this article originally appeared on Medscape.com.
, a 1-year, phase 3 clinical trial has shown. The trial was an add-on study involving a subset of subjects from the STEADY-PD III trial of isradipine in early Parkinson’s disease.
Although the trial was conducted before SARS-CoV-2 arrived on the scene, the findings have particular relevance for being able to conduct a variety of clinical trials in the face of COVID-19 and the need to limit in-person interactions.
The 40 participants used tablets to complete three remote, video-based assessments during 1 year, with each remote visit planned to be completed within 4 weeks of an in-person visit. It was easy to enroll patients, and they completed about 95% of planned visits, said neurologist Christopher Tarolli, MD, of the University of Rochester (N.Y.).
He presented the study findings at the Movement Disorder Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
“The visits were clearly feasible, and we were able to do them [84%] within that 4-week time frame around the in-person visit,” he said. “The visits were also reasonably reliable, particularly so for what we call the nonmotor outcomes and the patient-reported outcomes.”
In-person versus remote assessment
For the remote visits, participants completed primarily the same battery of tests as the in-person visits. Responses on the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) subscales demonstrated “that there was excellent correlation between patient-reported and nonmotor outcome measures and moderate correlation between in-person and remote-performed motor assessments,” Dr. Tarolli said.
He explained that the study used modified motor assessments (MDS-UPDRS Part III) that excluded testing of rigidity and postural instability, which require hands-on testing by a trained examiner and thus are impossible to do remotely.
Additionally, the somewhat lower correlation on this subscale was probably the result of different investigators conducting in-person versus remote assessments, with a subset of in-person investigators who tended to rate participants more severely driving down the correlation. “I think if these methods were applied in future trials, the in-person and remote investigators would optimally be the same person,” Dr. Tarolli suggested.
Room for error?
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that “the reliability of UPDRS [part] III is where I would want to have, for sure, a little bit more of a deep dive. … possibly the same patient be rated by the same person.”
She also noted that doing remote and in-person assessments within 4 weeks of each other leaves a lot of room for variability. “You could see the same patient in the morning and then do UPDRS in the afternoon, and it can be totally different depending on when you meet the person,” she said.
Only so much testing can be done remotely. Nonetheless, she questioned whether it is really a valid UPDRS if rigidity and postural stability measures are eliminated. “[Is] this now a new modified UPDRS that we’re going to use that is as good as the old UPDRS moving forward, a home version of UPDRS or whatever we’re going to call it?”
Dr. Subramanian mentioned that patients have told her that UPDRS part III does not really measure what is most important to them, such as making pastries for their grandchildren rather than rapidly tapping their fingers.
“That speaks a little bit to the fact that we should have more patient-centered outcomes and things that patients can report. … things that are not going to require necessarily an in-person exam as maybe measures that really can be used moving forward in studies,” she suggested.
Patient satisfaction with remote visits
Greater than 90% of the patients were satisfied or very satisfied overall with the remote visits, including the convenience, comfort, and connection (using the devices and Internet connection), with “patients describing enjoying being able to do these visits from the comfort of their own home, not having to travel,” Dr. Tarolli said. Not having to drive in an ‘off’ state “was actually something that some participants identified as a safety benefit from this as well.”
There was also a time benefit to the patients and investigators. The average length of the remote visits was 54.3 minutes each versus 74 minutes of interaction for in-person visits, mainly a result of more efficient hand-offs between the neurologist and the study coordinator during the remote visits, plus being able to pause the remote visit to give a medication dose time to take effect.
For the patient, there was a large amount of time saved when travel time was considered – a total of 190.2 minutes on average for travel and testing for the in-person visits.
About three-quarters (76%) of the study patients said that remote visits would increase their likelihood of participating in future trials. However, that result may be skewed by the fact that these were already people willing to participate in a remote trial, so the generalizability of the result may be affected. Nonetheless, Dr. Tarolli said he thinks that, as technology gets better and older people become more comfortable with it, remote visits within Parkinson’s research studies may become more common.
One caveat he mentioned is that, with remote visits, the neurologist misses a chance to observe a patient’s whole body and construct a global impression of how he or she is moving. On the other hand, remote video gives the investigator the chance to see the living environment of the patient and suggest changes for safety, such as to reduce the risk of falling for a person with unsteadiness of gait living in a crowded house.
“It really allows us to make a more holistic assessment of how our patient is functioning outside the clinic, which I think we’ve traditionally had really no way of doing,” Dr. Tarolli said.
His final suggestion for anyone contemplating conducting studies with remote visits is to develop a team that is comfortable troubleshooting the technological aspects of those visits.
UCLA’s Dr. Subramanian lauded the University of Rochester team for their efforts in moving remote visits forward. “They’re at the cutting edge of these sorts of things,” she said. “So I’m assuming that they’ll come out with more things [for visits] to become better that are going to move this forward, which is exciting.”
Dr. Tarolli has disclosed no relevant financial relationships. Dr. Subramanian has given talks for Acorda Pharmaceuticals and Acadia Pharmaceuticals in the past. The study had only university, government, foundation, and other nonprofit support.
A version of this article originally appeared on Medscape.com.
, a 1-year, phase 3 clinical trial has shown. The trial was an add-on study involving a subset of subjects from the STEADY-PD III trial of isradipine in early Parkinson’s disease.
Although the trial was conducted before SARS-CoV-2 arrived on the scene, the findings have particular relevance for being able to conduct a variety of clinical trials in the face of COVID-19 and the need to limit in-person interactions.
The 40 participants used tablets to complete three remote, video-based assessments during 1 year, with each remote visit planned to be completed within 4 weeks of an in-person visit. It was easy to enroll patients, and they completed about 95% of planned visits, said neurologist Christopher Tarolli, MD, of the University of Rochester (N.Y.).
He presented the study findings at the Movement Disorder Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
“The visits were clearly feasible, and we were able to do them [84%] within that 4-week time frame around the in-person visit,” he said. “The visits were also reasonably reliable, particularly so for what we call the nonmotor outcomes and the patient-reported outcomes.”
In-person versus remote assessment
For the remote visits, participants completed primarily the same battery of tests as the in-person visits. Responses on the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) subscales demonstrated “that there was excellent correlation between patient-reported and nonmotor outcome measures and moderate correlation between in-person and remote-performed motor assessments,” Dr. Tarolli said.
He explained that the study used modified motor assessments (MDS-UPDRS Part III) that excluded testing of rigidity and postural instability, which require hands-on testing by a trained examiner and thus are impossible to do remotely.
Additionally, the somewhat lower correlation on this subscale was probably the result of different investigators conducting in-person versus remote assessments, with a subset of in-person investigators who tended to rate participants more severely driving down the correlation. “I think if these methods were applied in future trials, the in-person and remote investigators would optimally be the same person,” Dr. Tarolli suggested.
Room for error?
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that “the reliability of UPDRS [part] III is where I would want to have, for sure, a little bit more of a deep dive. … possibly the same patient be rated by the same person.”
She also noted that doing remote and in-person assessments within 4 weeks of each other leaves a lot of room for variability. “You could see the same patient in the morning and then do UPDRS in the afternoon, and it can be totally different depending on when you meet the person,” she said.
Only so much testing can be done remotely. Nonetheless, she questioned whether it is really a valid UPDRS if rigidity and postural stability measures are eliminated. “[Is] this now a new modified UPDRS that we’re going to use that is as good as the old UPDRS moving forward, a home version of UPDRS or whatever we’re going to call it?”
Dr. Subramanian mentioned that patients have told her that UPDRS part III does not really measure what is most important to them, such as making pastries for their grandchildren rather than rapidly tapping their fingers.
“That speaks a little bit to the fact that we should have more patient-centered outcomes and things that patients can report. … things that are not going to require necessarily an in-person exam as maybe measures that really can be used moving forward in studies,” she suggested.
Patient satisfaction with remote visits
Greater than 90% of the patients were satisfied or very satisfied overall with the remote visits, including the convenience, comfort, and connection (using the devices and Internet connection), with “patients describing enjoying being able to do these visits from the comfort of their own home, not having to travel,” Dr. Tarolli said. Not having to drive in an ‘off’ state “was actually something that some participants identified as a safety benefit from this as well.”
There was also a time benefit to the patients and investigators. The average length of the remote visits was 54.3 minutes each versus 74 minutes of interaction for in-person visits, mainly a result of more efficient hand-offs between the neurologist and the study coordinator during the remote visits, plus being able to pause the remote visit to give a medication dose time to take effect.
For the patient, there was a large amount of time saved when travel time was considered – a total of 190.2 minutes on average for travel and testing for the in-person visits.
About three-quarters (76%) of the study patients said that remote visits would increase their likelihood of participating in future trials. However, that result may be skewed by the fact that these were already people willing to participate in a remote trial, so the generalizability of the result may be affected. Nonetheless, Dr. Tarolli said he thinks that, as technology gets better and older people become more comfortable with it, remote visits within Parkinson’s research studies may become more common.
One caveat he mentioned is that, with remote visits, the neurologist misses a chance to observe a patient’s whole body and construct a global impression of how he or she is moving. On the other hand, remote video gives the investigator the chance to see the living environment of the patient and suggest changes for safety, such as to reduce the risk of falling for a person with unsteadiness of gait living in a crowded house.
“It really allows us to make a more holistic assessment of how our patient is functioning outside the clinic, which I think we’ve traditionally had really no way of doing,” Dr. Tarolli said.
His final suggestion for anyone contemplating conducting studies with remote visits is to develop a team that is comfortable troubleshooting the technological aspects of those visits.
UCLA’s Dr. Subramanian lauded the University of Rochester team for their efforts in moving remote visits forward. “They’re at the cutting edge of these sorts of things,” she said. “So I’m assuming that they’ll come out with more things [for visits] to become better that are going to move this forward, which is exciting.”
Dr. Tarolli has disclosed no relevant financial relationships. Dr. Subramanian has given talks for Acorda Pharmaceuticals and Acadia Pharmaceuticals in the past. The study had only university, government, foundation, and other nonprofit support.
A version of this article originally appeared on Medscape.com.
Bacteria may be associated with risk of MS relapse
No broad differences in gut bacterial composition, however, are associated with risk of relapse, according to the investigators. The findings were presented at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Previous research has found an association between Blautia stercoris and disease activity in other immune-mediated diseases such as systemic lupus. Although the current study is the largest in patients with MS that includes data about the microbiome and relapses, its findings require replication, said Mary Horton, a doctoral candidate in epidemiology at the University of California, Berkeley.
Gut microbes digest food, produce vitamins (for example, B12 and K), create a barrier against pathogens, and regulate the immune system, among other tasks. Most current knowledge about the gut microbiome in MS comes from studies of patients with adult-onset MS. In 2016, Tremlett et al. found an increase in Desulfovibrionaceae and a decrease in Lachnospiraceae and Ruminococcaceae in patients with pediatric-onset MS. They also found that a decrease in Fusobacteria was associated with risk of relapse in this population.
Advanced analytical methods
Using a larger sample size and newer analytical methods than in the study by Tremlett and colleagues, Ms. Horton’s group sought to determine whether features of the gut microbiome are associated with relapse. From 2014 to 2018, the investigators recruited 53 patients with pediatric-onset MS from the University of California, San Francisco, and six centers in the U.S. Network of Pediatric MS Centers. At baseline, they collected stool samples, blood samples, information about past relapses, medication records, demographics, and environmental factors. At each relapse, the investigators collected information about the patient’s current and past medication use and about relapses that the patient had had since the previous visit.
Ms. Horton and colleagues analyzed the stool samples using 16S rRNA sequencing of the V4 region. They identified amplicon sequence variants (ASVs), which are used to define species of bacteria, with the Divisive Amplicon Denoising Algorithm-2 (DADA2). Taxonomies were assigned using the naive Bayesian classifier method, and the read count was normalized using multiple rarefaction.
The investigators identified ASV clusters using weighted genetic correlation network analysis (WGCNA). To evaluate whether individual ASVs were associated with relapse, they used a Prentice, Williams, and Peterson (PWP) recurrent event model, an extension of the Cox proportional hazards model.
The role of methanogenesis
Ms. Horton and colleagues included 53 patients (72% girls) in their study. The population’s mean age was 14.3 years at disease onset and 15.5 years at stool sample collection. About 70% of patients were White, and about 36% were Hispanic. Mean disease duration was 1.3 years, and median Expanded Disability Status Scale score was 1.0.
Approximately 45% of participants had one relapse, and 30% had more than one relapse during the subsequent mean follow-up of 2.5 years. About 91% of patients used a disease-modifying therapy during follow-up.
Gut bacterial abundance was broadly similar between patients who relapsed during the study period and those who did not. Of 270 ASVs included in the analyses, 20 were nominally associated with risk of relapse. Blautia stercoris had the most significant association with relapse risk (hazard ratio, 2.50). Blautia massiliensis also was among the 20 ASVs associated with risk of relapse.
WGCNA identified six ASV clusters. Higher values of one cluster’s eigengene were significantly associated with higher relapse risk (HR, 1.23). The following four ASVs nominally associated with higher relapse risk were in this cluster: Blautia massiliensis, Dorea longicatena, Coprococcus comes, and an unknown species in genus Subdoligranulum.
When Ms. Horton and colleagues examined the pathways from these bacterial species, they found 10 that were significantly associated with the risk of relapse. Four of these 10 pathways are involved in methane production, which suggests the involvement of methanogenesis pathways in relapse.
Although the investigators used advanced techniques for genetic and statistical analysis, the study’s sample size is small, Ms. Horton acknowledged. In addition, the conclusions that can be drawn from observational data are limited.
These suggest several avenues for future research. “There is a big question about how the different treatments that people are on when they are experiencing relapses might impact the microbiome,” said Ms. Horton. “Is the microbiome impacting your treatment response, or is it the reverse?” Investigators also could examine why the methane production pathway is overrepresented among people with MS who have relapses. “Which specific archaea might be leading to that increase in methane is a ripe future study question. Just what that means for health is really unknown.”
The National MS Society and the National Institute of Neurological Disorders and Stroke provided funding for the study. Ms. Horton had no disclosures.
SOURCE: Horton M et al. MSVirtual2020, Abstract LB01.05.
No broad differences in gut bacterial composition, however, are associated with risk of relapse, according to the investigators. The findings were presented at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Previous research has found an association between Blautia stercoris and disease activity in other immune-mediated diseases such as systemic lupus. Although the current study is the largest in patients with MS that includes data about the microbiome and relapses, its findings require replication, said Mary Horton, a doctoral candidate in epidemiology at the University of California, Berkeley.
Gut microbes digest food, produce vitamins (for example, B12 and K), create a barrier against pathogens, and regulate the immune system, among other tasks. Most current knowledge about the gut microbiome in MS comes from studies of patients with adult-onset MS. In 2016, Tremlett et al. found an increase in Desulfovibrionaceae and a decrease in Lachnospiraceae and Ruminococcaceae in patients with pediatric-onset MS. They also found that a decrease in Fusobacteria was associated with risk of relapse in this population.
Advanced analytical methods
Using a larger sample size and newer analytical methods than in the study by Tremlett and colleagues, Ms. Horton’s group sought to determine whether features of the gut microbiome are associated with relapse. From 2014 to 2018, the investigators recruited 53 patients with pediatric-onset MS from the University of California, San Francisco, and six centers in the U.S. Network of Pediatric MS Centers. At baseline, they collected stool samples, blood samples, information about past relapses, medication records, demographics, and environmental factors. At each relapse, the investigators collected information about the patient’s current and past medication use and about relapses that the patient had had since the previous visit.
Ms. Horton and colleagues analyzed the stool samples using 16S rRNA sequencing of the V4 region. They identified amplicon sequence variants (ASVs), which are used to define species of bacteria, with the Divisive Amplicon Denoising Algorithm-2 (DADA2). Taxonomies were assigned using the naive Bayesian classifier method, and the read count was normalized using multiple rarefaction.
The investigators identified ASV clusters using weighted genetic correlation network analysis (WGCNA). To evaluate whether individual ASVs were associated with relapse, they used a Prentice, Williams, and Peterson (PWP) recurrent event model, an extension of the Cox proportional hazards model.
The role of methanogenesis
Ms. Horton and colleagues included 53 patients (72% girls) in their study. The population’s mean age was 14.3 years at disease onset and 15.5 years at stool sample collection. About 70% of patients were White, and about 36% were Hispanic. Mean disease duration was 1.3 years, and median Expanded Disability Status Scale score was 1.0.
Approximately 45% of participants had one relapse, and 30% had more than one relapse during the subsequent mean follow-up of 2.5 years. About 91% of patients used a disease-modifying therapy during follow-up.
Gut bacterial abundance was broadly similar between patients who relapsed during the study period and those who did not. Of 270 ASVs included in the analyses, 20 were nominally associated with risk of relapse. Blautia stercoris had the most significant association with relapse risk (hazard ratio, 2.50). Blautia massiliensis also was among the 20 ASVs associated with risk of relapse.
WGCNA identified six ASV clusters. Higher values of one cluster’s eigengene were significantly associated with higher relapse risk (HR, 1.23). The following four ASVs nominally associated with higher relapse risk were in this cluster: Blautia massiliensis, Dorea longicatena, Coprococcus comes, and an unknown species in genus Subdoligranulum.
When Ms. Horton and colleagues examined the pathways from these bacterial species, they found 10 that were significantly associated with the risk of relapse. Four of these 10 pathways are involved in methane production, which suggests the involvement of methanogenesis pathways in relapse.
Although the investigators used advanced techniques for genetic and statistical analysis, the study’s sample size is small, Ms. Horton acknowledged. In addition, the conclusions that can be drawn from observational data are limited.
These suggest several avenues for future research. “There is a big question about how the different treatments that people are on when they are experiencing relapses might impact the microbiome,” said Ms. Horton. “Is the microbiome impacting your treatment response, or is it the reverse?” Investigators also could examine why the methane production pathway is overrepresented among people with MS who have relapses. “Which specific archaea might be leading to that increase in methane is a ripe future study question. Just what that means for health is really unknown.”
The National MS Society and the National Institute of Neurological Disorders and Stroke provided funding for the study. Ms. Horton had no disclosures.
SOURCE: Horton M et al. MSVirtual2020, Abstract LB01.05.
No broad differences in gut bacterial composition, however, are associated with risk of relapse, according to the investigators. The findings were presented at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Previous research has found an association between Blautia stercoris and disease activity in other immune-mediated diseases such as systemic lupus. Although the current study is the largest in patients with MS that includes data about the microbiome and relapses, its findings require replication, said Mary Horton, a doctoral candidate in epidemiology at the University of California, Berkeley.
Gut microbes digest food, produce vitamins (for example, B12 and K), create a barrier against pathogens, and regulate the immune system, among other tasks. Most current knowledge about the gut microbiome in MS comes from studies of patients with adult-onset MS. In 2016, Tremlett et al. found an increase in Desulfovibrionaceae and a decrease in Lachnospiraceae and Ruminococcaceae in patients with pediatric-onset MS. They also found that a decrease in Fusobacteria was associated with risk of relapse in this population.
Advanced analytical methods
Using a larger sample size and newer analytical methods than in the study by Tremlett and colleagues, Ms. Horton’s group sought to determine whether features of the gut microbiome are associated with relapse. From 2014 to 2018, the investigators recruited 53 patients with pediatric-onset MS from the University of California, San Francisco, and six centers in the U.S. Network of Pediatric MS Centers. At baseline, they collected stool samples, blood samples, information about past relapses, medication records, demographics, and environmental factors. At each relapse, the investigators collected information about the patient’s current and past medication use and about relapses that the patient had had since the previous visit.
Ms. Horton and colleagues analyzed the stool samples using 16S rRNA sequencing of the V4 region. They identified amplicon sequence variants (ASVs), which are used to define species of bacteria, with the Divisive Amplicon Denoising Algorithm-2 (DADA2). Taxonomies were assigned using the naive Bayesian classifier method, and the read count was normalized using multiple rarefaction.
The investigators identified ASV clusters using weighted genetic correlation network analysis (WGCNA). To evaluate whether individual ASVs were associated with relapse, they used a Prentice, Williams, and Peterson (PWP) recurrent event model, an extension of the Cox proportional hazards model.
The role of methanogenesis
Ms. Horton and colleagues included 53 patients (72% girls) in their study. The population’s mean age was 14.3 years at disease onset and 15.5 years at stool sample collection. About 70% of patients were White, and about 36% were Hispanic. Mean disease duration was 1.3 years, and median Expanded Disability Status Scale score was 1.0.
Approximately 45% of participants had one relapse, and 30% had more than one relapse during the subsequent mean follow-up of 2.5 years. About 91% of patients used a disease-modifying therapy during follow-up.
Gut bacterial abundance was broadly similar between patients who relapsed during the study period and those who did not. Of 270 ASVs included in the analyses, 20 were nominally associated with risk of relapse. Blautia stercoris had the most significant association with relapse risk (hazard ratio, 2.50). Blautia massiliensis also was among the 20 ASVs associated with risk of relapse.
WGCNA identified six ASV clusters. Higher values of one cluster’s eigengene were significantly associated with higher relapse risk (HR, 1.23). The following four ASVs nominally associated with higher relapse risk were in this cluster: Blautia massiliensis, Dorea longicatena, Coprococcus comes, and an unknown species in genus Subdoligranulum.
When Ms. Horton and colleagues examined the pathways from these bacterial species, they found 10 that were significantly associated with the risk of relapse. Four of these 10 pathways are involved in methane production, which suggests the involvement of methanogenesis pathways in relapse.
Although the investigators used advanced techniques for genetic and statistical analysis, the study’s sample size is small, Ms. Horton acknowledged. In addition, the conclusions that can be drawn from observational data are limited.
These suggest several avenues for future research. “There is a big question about how the different treatments that people are on when they are experiencing relapses might impact the microbiome,” said Ms. Horton. “Is the microbiome impacting your treatment response, or is it the reverse?” Investigators also could examine why the methane production pathway is overrepresented among people with MS who have relapses. “Which specific archaea might be leading to that increase in methane is a ripe future study question. Just what that means for health is really unknown.”
The National MS Society and the National Institute of Neurological Disorders and Stroke provided funding for the study. Ms. Horton had no disclosures.
SOURCE: Horton M et al. MSVirtual2020, Abstract LB01.05.
From MSVirtual2020
COVID-19 risks are no higher in patients with multiple sclerosis
new U.S. data suggest. A separate study from the United Kingdom also found similar trends of rates of COVID-19 infection in patients with MS and the general population.
Both studies were presented Sept. 26 at a special session on multiple sclerosis and COVID-19 at a final “Encore” event as part of the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
The U.S. data appear consistent with studies from several other countries, in that worse COVID-19 outcomes increase with age and higher disability levels, both of which would be expected from findings in the general population.
The U.S. data also show a clear effect of race in MS, with higher rates of adverse COVID-19 outcomes in Black patients, again in line with what is seen in the general population.
“I would say the results from our study and in general do not suggest that MS itself is associated with higher risks of severe COVID-19 outcomes, compared with the general population,” said Amber Salter, PhD.
Dr. Salter, who is assistant professor of biostatistics at Washington University, St. Louis, presented data from the COViMS North American registry, set up for health care providers to report persons with MS who are infected with COVID-19.
The COViMS registry so far has information on 858 patients with MS who have COVID-19 (80% verified by a positive test), as reported from 150 different health care providers in the United States and Canada. The average age was 48 years, with average disease duration of 13.6 years. MS clinical course was reported as relapsing remitting in 78%, secondary progressive in 15%, and primary progressive in 5%. Most patients (72%) were fully ambulatory, 16% could walk with assistance, and 12% were nonambulatory.
Severe COVID-19 outcomes were classified as mortality (which occurred in 5.7% of the cohort), mortality/ICU admission (13.6%) and mortality/ICU admission/hospitalization (30.2%).
Results were adjusted for many different covariates, including sex, age, smoking, MS clinical course (relapsing, progressive), disease duration, ambulation, individual comorbidities (cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic lung disease, diabetes, hypertension, morbid obesity), and disease-modifying therapy use.
In multivariable logistic regression analyses, older age, having chronic renal disease, and being nonambulatory were consistently associated with increased odds of poorer outcomes. Chronic kidney disease had the strongest association with mortality (odds ratio, 28.6; P < .001). Other factors associated with mortality included cardiovascular disease (OR, 4.35; P = .009); age (OR per 10 years, 1.91; P = .012), and male sex (OR, 2.60; P = .041).
Patients who were nonambulatory had a higher risk of mortality/ICU admission/hospitalization (OR, 3.32; P = .003). This endpoint was also increased in patients on anti-CD20 drugs, compared with other disease-modifying treatment (OR, 2.31; P = .002), consistent with results from at least two other studies.
Disease-modifying therapy in general was not associated with an increased risk of worse outcomes. “There was some concern at the outset about the effect of disease-modifying therapies on COVID-19 outcomes, but most studies have not found an increased risk of worse outcomes in patients on such drug treatments, with the possible exception of anti-CD20 drugs,” Dr. Salter said.
“Some disease-modifying therapies may actually be protective (particularly interferon) and studies are investigating whether they may have a role in the treatment of COVID-19,” she added.
“The factors in MS patients that we and others have found to be associated with worse COVID-19 outcomes may not be specific to MS. Older age is known to be a primary risk factor for worse COVID-19 outcomes in the general population, and increasing disability presumably tracks with worse general heath,” Dr. Salter commented.
“I would say the overall data are fairly reassuring for MS patents,” she concluded.
Black patients have higher risk
One worrying finding in the North American data, however, was the effect of race. “We found an independent effect of race for worse COVID-19 outcomes in MS patients,” Dr. Slater said.
Of the 858 patients in the COViMS registry, 65.7% were White and 26.1% were Black. Black individuals were more likely to be younger, never smokers, have shorter MS duration, a relapsing MS course, and have comorbidities, compared with White patients. A higher proportion of Black patients had hypertension (40.2% vs 19.5%) and morbid obesity (17% vs. 9.5%).
Results showed that mortality rates were not statistically different between White and Black patients, but Black race was associated with increased risk of mortality and/or ICU admission, compared with White patients (16.9% vs. 12.8%), and multivariate logistic regression analysis showed Black race was independently associated with mortality/ICU admission after adjustments for covariates (OR, 3.7; P = .002).
Black race was also associated with increased risk of mortality/ICU admission/hospital admission (35.8% vs. 30.2%), and after adjustment for covariates this was found to be an independent predictor (OR, 1.7; P = .04).
“This higher COVID-19 risk in Black individuals is also seen in the general population, so these results are not that surprising and it doesn’t appear to be an effect specific to MS patients,” Dr. Salter commented.
U.K. data on risk of contracting COVID-19
A U.K. study also suggested race to be an independent predictor in the risk of contracting COVID-19 in patients with MS.
The study of more than 5,000 patients with MS showed that those from a Black, Asian, and Minority Ethnic group were twice as likely to report having COVID-19 than those who were White.
The study, which was conducted during the U.K. lockdown, also found that the trend of COVID-19 infection in patients with MS is comparable with that of the U.K. general population.
Presenting the data, Afagh Garjani, MD, concluded: “During a period with strict physical distancing measures, patients with MS are not at an increased risk of contracting COVID-19.”
Dr. Garjani, a neurology clinical research fellow at the University of Nottingham, (England), explained that the COVID-19 pandemic has introduced uncertainties into the MS community, and the focus so far has been the severity of infection among people with MS who have COVID-19.
“This approach has left questions about the risk of contracting disease in people with MS unanswered, which has implications as society gradually returns to normal,” she said.
Dr. Garjani presented data from the United Kingdom MS Register (UKMSR), which has been collecting demographic and MS-related data since 2011 from patients with MS throughout the United Kingdom.
On March 17 – just before the lockdown in United Kingdom – existing participants of the UKMSR were asked to join the COVID-19 study. The study was also advertised through social media. In this ongoing study, people with MS answered a COVID-19–related survey at participation and a different follow-up survey every 2 weeks depending on whether they contracted COVID-19.
The COVID-19 study included 5,309 patients with MS. The mean age of the study population was 52.4 years, 76.1% were female, and 95.7% were White. Of the 5,309 patients, 535 (10%) reported a self-diagnosis of COVID-19. Because of limited availability of tests in the United Kingdom at the time, only 75 patents had a positive polymerase chain reaction result.
“To our knowledge, this is the largest community-based study of COVID-19 in patients with MS worldwide,” Dr. Garjani said. She presented results from the period March 23 to June 24, when the United Kingdom was in a period of lockdown with vulnerable groups encouraged to self-isolate completely.
In this MS cohort, 47% reported self-isolating at some point. Those at older age and higher Expanded Disability Status Scale (EDSS) score were more likely to have self-isolated.
The researchers did not find that patients with progressive MS or those on disease-modifying therapies in general isolated more, but patients on monoclonal antibody drugs and fingolimod were more likely to self-isolate versus those on other therapies. “This may be because there are concerns about infection with these drugs and patients on these therapies may be more concerned about contracting COVID-19,” Dr. Garjani suggested.
In terms of contracting COVID, the researchers found a reduced risk of COVID-19 (self-diagnosed) in patients with older age and higher EDSS. “This is not really surprising that these patients were more likely to self-isolate,” Dr. Garjani commented.
No association was seen between type of MS, disease duration, disease-modifying therapy in general, and risk of COVID-19. No individual drug treatment increased risk versus no therapy or versus self-injectables. But there was an increased risk of contracting the virus in patients whose race was Black, Asian, or Minority Ethnic (OR, 2.2), which is in line with findings from the general population.
“This study is unique – the denominator is all people with MS. We are looking primarily at the risk of contracting COVID-19. Other studies are focusing more on people with MS who have COVID and assessing risk of a severe COVID outcome. Our results are not contradicting the findings from those studies,” Dr. Garjani said.
The results were similar only when patients with a confirmed COVID-19 test were considered.
In terms of outcomes in those who reported COVID-19 infection, preliminary results have not shown any MS factors – such as EDSS, age, type of MS, drug therapy in general – to be associated with outcome.
“Since the COVID-19 outbreak started there has been concern among MS patients, especially among those on disease-modifying therapies, about whether they are at increased risk of infection and severe disease,” Dr. Garjani said.
“We found similar trends of rates of infection in MS patients and the general population, and no signal of increased risks in those with higher EDSS or progressive MS. The caveat is that this study was conducted in a period of lockdown, but we adjusted for self-isolating behavior in the multivariable regression analysis,” she noted.
Dr. Salter is a statistical editor for the American Heart Association journal Circulation: Cardiovascular Imaging. Dr. Garjani has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new U.S. data suggest. A separate study from the United Kingdom also found similar trends of rates of COVID-19 infection in patients with MS and the general population.
Both studies were presented Sept. 26 at a special session on multiple sclerosis and COVID-19 at a final “Encore” event as part of the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
The U.S. data appear consistent with studies from several other countries, in that worse COVID-19 outcomes increase with age and higher disability levels, both of which would be expected from findings in the general population.
The U.S. data also show a clear effect of race in MS, with higher rates of adverse COVID-19 outcomes in Black patients, again in line with what is seen in the general population.
“I would say the results from our study and in general do not suggest that MS itself is associated with higher risks of severe COVID-19 outcomes, compared with the general population,” said Amber Salter, PhD.
Dr. Salter, who is assistant professor of biostatistics at Washington University, St. Louis, presented data from the COViMS North American registry, set up for health care providers to report persons with MS who are infected with COVID-19.
The COViMS registry so far has information on 858 patients with MS who have COVID-19 (80% verified by a positive test), as reported from 150 different health care providers in the United States and Canada. The average age was 48 years, with average disease duration of 13.6 years. MS clinical course was reported as relapsing remitting in 78%, secondary progressive in 15%, and primary progressive in 5%. Most patients (72%) were fully ambulatory, 16% could walk with assistance, and 12% were nonambulatory.
Severe COVID-19 outcomes were classified as mortality (which occurred in 5.7% of the cohort), mortality/ICU admission (13.6%) and mortality/ICU admission/hospitalization (30.2%).
Results were adjusted for many different covariates, including sex, age, smoking, MS clinical course (relapsing, progressive), disease duration, ambulation, individual comorbidities (cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic lung disease, diabetes, hypertension, morbid obesity), and disease-modifying therapy use.
In multivariable logistic regression analyses, older age, having chronic renal disease, and being nonambulatory were consistently associated with increased odds of poorer outcomes. Chronic kidney disease had the strongest association with mortality (odds ratio, 28.6; P < .001). Other factors associated with mortality included cardiovascular disease (OR, 4.35; P = .009); age (OR per 10 years, 1.91; P = .012), and male sex (OR, 2.60; P = .041).
Patients who were nonambulatory had a higher risk of mortality/ICU admission/hospitalization (OR, 3.32; P = .003). This endpoint was also increased in patients on anti-CD20 drugs, compared with other disease-modifying treatment (OR, 2.31; P = .002), consistent with results from at least two other studies.
Disease-modifying therapy in general was not associated with an increased risk of worse outcomes. “There was some concern at the outset about the effect of disease-modifying therapies on COVID-19 outcomes, but most studies have not found an increased risk of worse outcomes in patients on such drug treatments, with the possible exception of anti-CD20 drugs,” Dr. Salter said.
“Some disease-modifying therapies may actually be protective (particularly interferon) and studies are investigating whether they may have a role in the treatment of COVID-19,” she added.
“The factors in MS patients that we and others have found to be associated with worse COVID-19 outcomes may not be specific to MS. Older age is known to be a primary risk factor for worse COVID-19 outcomes in the general population, and increasing disability presumably tracks with worse general heath,” Dr. Salter commented.
“I would say the overall data are fairly reassuring for MS patents,” she concluded.
Black patients have higher risk
One worrying finding in the North American data, however, was the effect of race. “We found an independent effect of race for worse COVID-19 outcomes in MS patients,” Dr. Slater said.
Of the 858 patients in the COViMS registry, 65.7% were White and 26.1% were Black. Black individuals were more likely to be younger, never smokers, have shorter MS duration, a relapsing MS course, and have comorbidities, compared with White patients. A higher proportion of Black patients had hypertension (40.2% vs 19.5%) and morbid obesity (17% vs. 9.5%).
Results showed that mortality rates were not statistically different between White and Black patients, but Black race was associated with increased risk of mortality and/or ICU admission, compared with White patients (16.9% vs. 12.8%), and multivariate logistic regression analysis showed Black race was independently associated with mortality/ICU admission after adjustments for covariates (OR, 3.7; P = .002).
Black race was also associated with increased risk of mortality/ICU admission/hospital admission (35.8% vs. 30.2%), and after adjustment for covariates this was found to be an independent predictor (OR, 1.7; P = .04).
“This higher COVID-19 risk in Black individuals is also seen in the general population, so these results are not that surprising and it doesn’t appear to be an effect specific to MS patients,” Dr. Salter commented.
U.K. data on risk of contracting COVID-19
A U.K. study also suggested race to be an independent predictor in the risk of contracting COVID-19 in patients with MS.
The study of more than 5,000 patients with MS showed that those from a Black, Asian, and Minority Ethnic group were twice as likely to report having COVID-19 than those who were White.
The study, which was conducted during the U.K. lockdown, also found that the trend of COVID-19 infection in patients with MS is comparable with that of the U.K. general population.
Presenting the data, Afagh Garjani, MD, concluded: “During a period with strict physical distancing measures, patients with MS are not at an increased risk of contracting COVID-19.”
Dr. Garjani, a neurology clinical research fellow at the University of Nottingham, (England), explained that the COVID-19 pandemic has introduced uncertainties into the MS community, and the focus so far has been the severity of infection among people with MS who have COVID-19.
“This approach has left questions about the risk of contracting disease in people with MS unanswered, which has implications as society gradually returns to normal,” she said.
Dr. Garjani presented data from the United Kingdom MS Register (UKMSR), which has been collecting demographic and MS-related data since 2011 from patients with MS throughout the United Kingdom.
On March 17 – just before the lockdown in United Kingdom – existing participants of the UKMSR were asked to join the COVID-19 study. The study was also advertised through social media. In this ongoing study, people with MS answered a COVID-19–related survey at participation and a different follow-up survey every 2 weeks depending on whether they contracted COVID-19.
The COVID-19 study included 5,309 patients with MS. The mean age of the study population was 52.4 years, 76.1% were female, and 95.7% were White. Of the 5,309 patients, 535 (10%) reported a self-diagnosis of COVID-19. Because of limited availability of tests in the United Kingdom at the time, only 75 patents had a positive polymerase chain reaction result.
“To our knowledge, this is the largest community-based study of COVID-19 in patients with MS worldwide,” Dr. Garjani said. She presented results from the period March 23 to June 24, when the United Kingdom was in a period of lockdown with vulnerable groups encouraged to self-isolate completely.
In this MS cohort, 47% reported self-isolating at some point. Those at older age and higher Expanded Disability Status Scale (EDSS) score were more likely to have self-isolated.
The researchers did not find that patients with progressive MS or those on disease-modifying therapies in general isolated more, but patients on monoclonal antibody drugs and fingolimod were more likely to self-isolate versus those on other therapies. “This may be because there are concerns about infection with these drugs and patients on these therapies may be more concerned about contracting COVID-19,” Dr. Garjani suggested.
In terms of contracting COVID, the researchers found a reduced risk of COVID-19 (self-diagnosed) in patients with older age and higher EDSS. “This is not really surprising that these patients were more likely to self-isolate,” Dr. Garjani commented.
No association was seen between type of MS, disease duration, disease-modifying therapy in general, and risk of COVID-19. No individual drug treatment increased risk versus no therapy or versus self-injectables. But there was an increased risk of contracting the virus in patients whose race was Black, Asian, or Minority Ethnic (OR, 2.2), which is in line with findings from the general population.
“This study is unique – the denominator is all people with MS. We are looking primarily at the risk of contracting COVID-19. Other studies are focusing more on people with MS who have COVID and assessing risk of a severe COVID outcome. Our results are not contradicting the findings from those studies,” Dr. Garjani said.
The results were similar only when patients with a confirmed COVID-19 test were considered.
In terms of outcomes in those who reported COVID-19 infection, preliminary results have not shown any MS factors – such as EDSS, age, type of MS, drug therapy in general – to be associated with outcome.
“Since the COVID-19 outbreak started there has been concern among MS patients, especially among those on disease-modifying therapies, about whether they are at increased risk of infection and severe disease,” Dr. Garjani said.
“We found similar trends of rates of infection in MS patients and the general population, and no signal of increased risks in those with higher EDSS or progressive MS. The caveat is that this study was conducted in a period of lockdown, but we adjusted for self-isolating behavior in the multivariable regression analysis,” she noted.
Dr. Salter is a statistical editor for the American Heart Association journal Circulation: Cardiovascular Imaging. Dr. Garjani has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new U.S. data suggest. A separate study from the United Kingdom also found similar trends of rates of COVID-19 infection in patients with MS and the general population.
Both studies were presented Sept. 26 at a special session on multiple sclerosis and COVID-19 at a final “Encore” event as part of the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
The U.S. data appear consistent with studies from several other countries, in that worse COVID-19 outcomes increase with age and higher disability levels, both of which would be expected from findings in the general population.
The U.S. data also show a clear effect of race in MS, with higher rates of adverse COVID-19 outcomes in Black patients, again in line with what is seen in the general population.
“I would say the results from our study and in general do not suggest that MS itself is associated with higher risks of severe COVID-19 outcomes, compared with the general population,” said Amber Salter, PhD.
Dr. Salter, who is assistant professor of biostatistics at Washington University, St. Louis, presented data from the COViMS North American registry, set up for health care providers to report persons with MS who are infected with COVID-19.
The COViMS registry so far has information on 858 patients with MS who have COVID-19 (80% verified by a positive test), as reported from 150 different health care providers in the United States and Canada. The average age was 48 years, with average disease duration of 13.6 years. MS clinical course was reported as relapsing remitting in 78%, secondary progressive in 15%, and primary progressive in 5%. Most patients (72%) were fully ambulatory, 16% could walk with assistance, and 12% were nonambulatory.
Severe COVID-19 outcomes were classified as mortality (which occurred in 5.7% of the cohort), mortality/ICU admission (13.6%) and mortality/ICU admission/hospitalization (30.2%).
Results were adjusted for many different covariates, including sex, age, smoking, MS clinical course (relapsing, progressive), disease duration, ambulation, individual comorbidities (cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic lung disease, diabetes, hypertension, morbid obesity), and disease-modifying therapy use.
In multivariable logistic regression analyses, older age, having chronic renal disease, and being nonambulatory were consistently associated with increased odds of poorer outcomes. Chronic kidney disease had the strongest association with mortality (odds ratio, 28.6; P < .001). Other factors associated with mortality included cardiovascular disease (OR, 4.35; P = .009); age (OR per 10 years, 1.91; P = .012), and male sex (OR, 2.60; P = .041).
Patients who were nonambulatory had a higher risk of mortality/ICU admission/hospitalization (OR, 3.32; P = .003). This endpoint was also increased in patients on anti-CD20 drugs, compared with other disease-modifying treatment (OR, 2.31; P = .002), consistent with results from at least two other studies.
Disease-modifying therapy in general was not associated with an increased risk of worse outcomes. “There was some concern at the outset about the effect of disease-modifying therapies on COVID-19 outcomes, but most studies have not found an increased risk of worse outcomes in patients on such drug treatments, with the possible exception of anti-CD20 drugs,” Dr. Salter said.
“Some disease-modifying therapies may actually be protective (particularly interferon) and studies are investigating whether they may have a role in the treatment of COVID-19,” she added.
“The factors in MS patients that we and others have found to be associated with worse COVID-19 outcomes may not be specific to MS. Older age is known to be a primary risk factor for worse COVID-19 outcomes in the general population, and increasing disability presumably tracks with worse general heath,” Dr. Salter commented.
“I would say the overall data are fairly reassuring for MS patents,” she concluded.
Black patients have higher risk
One worrying finding in the North American data, however, was the effect of race. “We found an independent effect of race for worse COVID-19 outcomes in MS patients,” Dr. Slater said.
Of the 858 patients in the COViMS registry, 65.7% were White and 26.1% were Black. Black individuals were more likely to be younger, never smokers, have shorter MS duration, a relapsing MS course, and have comorbidities, compared with White patients. A higher proportion of Black patients had hypertension (40.2% vs 19.5%) and morbid obesity (17% vs. 9.5%).
Results showed that mortality rates were not statistically different between White and Black patients, but Black race was associated with increased risk of mortality and/or ICU admission, compared with White patients (16.9% vs. 12.8%), and multivariate logistic regression analysis showed Black race was independently associated with mortality/ICU admission after adjustments for covariates (OR, 3.7; P = .002).
Black race was also associated with increased risk of mortality/ICU admission/hospital admission (35.8% vs. 30.2%), and after adjustment for covariates this was found to be an independent predictor (OR, 1.7; P = .04).
“This higher COVID-19 risk in Black individuals is also seen in the general population, so these results are not that surprising and it doesn’t appear to be an effect specific to MS patients,” Dr. Salter commented.
U.K. data on risk of contracting COVID-19
A U.K. study also suggested race to be an independent predictor in the risk of contracting COVID-19 in patients with MS.
The study of more than 5,000 patients with MS showed that those from a Black, Asian, and Minority Ethnic group were twice as likely to report having COVID-19 than those who were White.
The study, which was conducted during the U.K. lockdown, also found that the trend of COVID-19 infection in patients with MS is comparable with that of the U.K. general population.
Presenting the data, Afagh Garjani, MD, concluded: “During a period with strict physical distancing measures, patients with MS are not at an increased risk of contracting COVID-19.”
Dr. Garjani, a neurology clinical research fellow at the University of Nottingham, (England), explained that the COVID-19 pandemic has introduced uncertainties into the MS community, and the focus so far has been the severity of infection among people with MS who have COVID-19.
“This approach has left questions about the risk of contracting disease in people with MS unanswered, which has implications as society gradually returns to normal,” she said.
Dr. Garjani presented data from the United Kingdom MS Register (UKMSR), which has been collecting demographic and MS-related data since 2011 from patients with MS throughout the United Kingdom.
On March 17 – just before the lockdown in United Kingdom – existing participants of the UKMSR were asked to join the COVID-19 study. The study was also advertised through social media. In this ongoing study, people with MS answered a COVID-19–related survey at participation and a different follow-up survey every 2 weeks depending on whether they contracted COVID-19.
The COVID-19 study included 5,309 patients with MS. The mean age of the study population was 52.4 years, 76.1% were female, and 95.7% were White. Of the 5,309 patients, 535 (10%) reported a self-diagnosis of COVID-19. Because of limited availability of tests in the United Kingdom at the time, only 75 patents had a positive polymerase chain reaction result.
“To our knowledge, this is the largest community-based study of COVID-19 in patients with MS worldwide,” Dr. Garjani said. She presented results from the period March 23 to June 24, when the United Kingdom was in a period of lockdown with vulnerable groups encouraged to self-isolate completely.
In this MS cohort, 47% reported self-isolating at some point. Those at older age and higher Expanded Disability Status Scale (EDSS) score were more likely to have self-isolated.
The researchers did not find that patients with progressive MS or those on disease-modifying therapies in general isolated more, but patients on monoclonal antibody drugs and fingolimod were more likely to self-isolate versus those on other therapies. “This may be because there are concerns about infection with these drugs and patients on these therapies may be more concerned about contracting COVID-19,” Dr. Garjani suggested.
In terms of contracting COVID, the researchers found a reduced risk of COVID-19 (self-diagnosed) in patients with older age and higher EDSS. “This is not really surprising that these patients were more likely to self-isolate,” Dr. Garjani commented.
No association was seen between type of MS, disease duration, disease-modifying therapy in general, and risk of COVID-19. No individual drug treatment increased risk versus no therapy or versus self-injectables. But there was an increased risk of contracting the virus in patients whose race was Black, Asian, or Minority Ethnic (OR, 2.2), which is in line with findings from the general population.
“This study is unique – the denominator is all people with MS. We are looking primarily at the risk of contracting COVID-19. Other studies are focusing more on people with MS who have COVID and assessing risk of a severe COVID outcome. Our results are not contradicting the findings from those studies,” Dr. Garjani said.
The results were similar only when patients with a confirmed COVID-19 test were considered.
In terms of outcomes in those who reported COVID-19 infection, preliminary results have not shown any MS factors – such as EDSS, age, type of MS, drug therapy in general – to be associated with outcome.
“Since the COVID-19 outbreak started there has been concern among MS patients, especially among those on disease-modifying therapies, about whether they are at increased risk of infection and severe disease,” Dr. Garjani said.
“We found similar trends of rates of infection in MS patients and the general population, and no signal of increased risks in those with higher EDSS or progressive MS. The caveat is that this study was conducted in a period of lockdown, but we adjusted for self-isolating behavior in the multivariable regression analysis,” she noted.
Dr. Salter is a statistical editor for the American Heart Association journal Circulation: Cardiovascular Imaging. Dr. Garjani has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM MSVIRTUAL2020
Stroke may be the first symptom of COVID-19 in younger patients
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Neurology
Music’s charms may soothe heart failure’s effects
Music listening and singing each showed early, promising evidence for producing cardiovascular benefits, part of a burgeoning area of research that is exploring and documenting ways to effectively use music to improve health.
A study run at four centers in Italy randomized 159 patients with heart failure, primarily New York Heart Association class I or II disease, to either a daily regimen of at least 30 minutes spent listening to music daily or to a control group that received usual care with no music prescription. After 3 months, the 82 patients in the daily music-listening group had a statistically significant improvement in their Minnesota Living with Heart Failure Questionnaire scores, compared with 77 controls for the study’s primary outcome measure. The results also showed significant benefits, compared with placebo, for other, secondary efficacy measures including improvements in anxiety, depression, sleep quality, and cognition.
Although the results are considered preliminary, they drew significant attention when published in July 2020 (J Card Fail. 2020 Jul 1;26[7]:541-9), where it was accompanied by two editorials in the same issue as well as an editor’s statement. All these commentators as well as other experts interested in music as medicine gathered to further discuss the topic during a panel session at the virtual annual meeting of the Heart Failure Society of America.
Music as a calming influence
The source of the primary benefits seen in this Italian study likely involved “emotional, psychological, and relaxation,” suggested Jerome L. Fleg, MD, program officer for clinical cardiovascular disease at the National Heart, Lung, and Blood Institute in Bethesda, Md. Researchers had used calming potential as a major criterion when selecting the 80 classical pieces that the heart failure patients in the intervention arm of the study could shuffle on their play lists.
“The tempo/rhythm was set up in a range between 60 and 80 beats per minute, because this range mirrors the human heart rate and facilitates relaxation,” the investigators said in their published report. Unfortunately, noted Dr. Fleg, the study lacked physiologic and biomarker measurements that could have provided objective evidence of effects from music. And the study failed to include a control arm of patients instructed to spend 30 minutes a day resting and relaxing without instruction to listen to music, he noted.
Dr. Fleg had authored one of the July editorials, where he said “It is hoped that findings from these studies and others can expand the scientific evidence for music-based interventions and bring these therapies into clinical practice. The current study from Burrai et al. is a positive step in this direction for patients with heart failure.” (J Card Fail. 2020 Jul 1;26[7]: 550-1). What’s needed now, he added during the virtual session, are “more objective data” to better and more comprehensively document the benefits from a music-based intervention in patients with heart failure.
An add-on to standard care
The findings in heart failure patients follows a growing literature that’s shown music can generate a restful state by doing things like activating autonomic parasympathetic outflow while dampening sympathetic outflow. This produces moderation in mood and emotion as well as depressed heart rate, lowered blood pressure, and slowed respiration, commented Emmeline Edwards, PhD director of the division of extramural research of the National Center for Complementary and Integrative Health in Bethesda, Md. Music also seems able to stimulate higher-order brain regions that can result in reduced psychological stress, anxiety, and depression.
“It’s a promising protective intervention to add to standard care for cardiac patients,” Dr. Edwards said during the virtual session. “Music is part of the toolbox for managing symptoms and improving health and well-being.”
“Music is not a substitute for standard therapy, but could add to it,” declared Dr. Fleg.
The already-established intervention known as music therapy has identified music’s ability to modulate breathing as an important mediator of music’s effect.
“Breathing is one of the few physiological processes that can be voluntarily controlled making it a viable target for intervention,” noted opera soprano Renée Fleming and Sheri L. Robb, PhD, in the second editorial that accompanied the Italian heart failure report (J Card Fail. 2020 Jul 1;26[7]:552-4). The music-listening intervention “may have had more effect if they had used compositional features [of the music] to teach patients how to structure their breathing,” said Dr. Robb, a music therapist at Indiana University–Purdue University Indianapolis, during the virtual session.
Another variable to consider is the type of music. “What is the emotional response to the music, and how does that affect heart rate,” wondered Dr. Robb, a professor at the Indiana University School of Nursing in Indianapolis.
Music as exercise
The division that Dr. Edwards directs recently funded a pilot study that assessed the feasibility of using music to stimulate activity and improve breathing another way, by repurposing singing as a novel form of rehabilitative exercise.
The pilot study enrolled patients with coronary disease into a randomized study that tested whether a 14-minute session of supervised singing could produce acute improvement in vascular function, “a biomarker for the risk of future cardiovascular disease events,” explained Jacqueline P. Kulinski, MD, a preventive cardiologist at the Medical College of Wisconsin in Milwaukee. Dr. Kulinski did not report details of her yet-unpublished study, but said that her initial findings held promise for developing musical activities such as singing as a novel way to stimulate therapeutic physical activity in patients with heart disease.
“It’s exciting to see this signal” of benefit. “I envision music therapy as a part of cardiac rehabilitation, or an alternative for patients who can’t participate in traditional rehab,” Dr. Kulinski said during the virtual session. “I think of singing as a physical activity, as exercise, and using this exercise as medicine.”
Harmonizing with the NIH
“Singing is like swimming: You need to hold your breath,” agreed Ms. Fleming, who participated on the virtual panel and has spearheaded a collaboration between the National Institutes of Health and the Kennedy Center for the Performing Arts, the Sound Health Initiative, that’s coordinating research into the connections between music and health. Ms. Fleming helped launch the Sound Health Initiative in 2017 by coauthoring a JAMA article with the NIH director that spelled out the rationale and goals of the project (JAMA. 2017 Jun 27;317[24]:2470-1), and by launching a lecture tour on the topic in a presentation she calls Music and the Mind.
Ms. Fleming has given her talk in more than 30 locations worldwide, and she’s found that “audiences love” the combination of neuroscience and music that her talks cover, she said. Her lectures highlight that, in addition to cardiovascular disease, the potential for music therapy and related interventions has been shown in patients with disorders that include autism, psychosis, pain, Parkinson’s disease, Alzheimer’s disease, and epilepsy.
The research highlighted in the session “opens new doors to prevention and treatment strategies using music for patients with heart failure and cardiovascular disease,” summed up Biykem Bozkurt, MD, professor of medicine at the Baylor College of Medicine in Houston and president of the Heart Failure Society of America, who helped organize the virtual session.
Dr. Fleg, Dr. Edwards, Dr. Robb, Dr Kulinski, Ms. Fleming, and Dr. Bozkurt had no relevant financial disclosures.
Music listening and singing each showed early, promising evidence for producing cardiovascular benefits, part of a burgeoning area of research that is exploring and documenting ways to effectively use music to improve health.
A study run at four centers in Italy randomized 159 patients with heart failure, primarily New York Heart Association class I or II disease, to either a daily regimen of at least 30 minutes spent listening to music daily or to a control group that received usual care with no music prescription. After 3 months, the 82 patients in the daily music-listening group had a statistically significant improvement in their Minnesota Living with Heart Failure Questionnaire scores, compared with 77 controls for the study’s primary outcome measure. The results also showed significant benefits, compared with placebo, for other, secondary efficacy measures including improvements in anxiety, depression, sleep quality, and cognition.
Although the results are considered preliminary, they drew significant attention when published in July 2020 (J Card Fail. 2020 Jul 1;26[7]:541-9), where it was accompanied by two editorials in the same issue as well as an editor’s statement. All these commentators as well as other experts interested in music as medicine gathered to further discuss the topic during a panel session at the virtual annual meeting of the Heart Failure Society of America.
Music as a calming influence
The source of the primary benefits seen in this Italian study likely involved “emotional, psychological, and relaxation,” suggested Jerome L. Fleg, MD, program officer for clinical cardiovascular disease at the National Heart, Lung, and Blood Institute in Bethesda, Md. Researchers had used calming potential as a major criterion when selecting the 80 classical pieces that the heart failure patients in the intervention arm of the study could shuffle on their play lists.
“The tempo/rhythm was set up in a range between 60 and 80 beats per minute, because this range mirrors the human heart rate and facilitates relaxation,” the investigators said in their published report. Unfortunately, noted Dr. Fleg, the study lacked physiologic and biomarker measurements that could have provided objective evidence of effects from music. And the study failed to include a control arm of patients instructed to spend 30 minutes a day resting and relaxing without instruction to listen to music, he noted.
Dr. Fleg had authored one of the July editorials, where he said “It is hoped that findings from these studies and others can expand the scientific evidence for music-based interventions and bring these therapies into clinical practice. The current study from Burrai et al. is a positive step in this direction for patients with heart failure.” (J Card Fail. 2020 Jul 1;26[7]: 550-1). What’s needed now, he added during the virtual session, are “more objective data” to better and more comprehensively document the benefits from a music-based intervention in patients with heart failure.
An add-on to standard care
The findings in heart failure patients follows a growing literature that’s shown music can generate a restful state by doing things like activating autonomic parasympathetic outflow while dampening sympathetic outflow. This produces moderation in mood and emotion as well as depressed heart rate, lowered blood pressure, and slowed respiration, commented Emmeline Edwards, PhD director of the division of extramural research of the National Center for Complementary and Integrative Health in Bethesda, Md. Music also seems able to stimulate higher-order brain regions that can result in reduced psychological stress, anxiety, and depression.
“It’s a promising protective intervention to add to standard care for cardiac patients,” Dr. Edwards said during the virtual session. “Music is part of the toolbox for managing symptoms and improving health and well-being.”
“Music is not a substitute for standard therapy, but could add to it,” declared Dr. Fleg.
The already-established intervention known as music therapy has identified music’s ability to modulate breathing as an important mediator of music’s effect.
“Breathing is one of the few physiological processes that can be voluntarily controlled making it a viable target for intervention,” noted opera soprano Renée Fleming and Sheri L. Robb, PhD, in the second editorial that accompanied the Italian heart failure report (J Card Fail. 2020 Jul 1;26[7]:552-4). The music-listening intervention “may have had more effect if they had used compositional features [of the music] to teach patients how to structure their breathing,” said Dr. Robb, a music therapist at Indiana University–Purdue University Indianapolis, during the virtual session.
Another variable to consider is the type of music. “What is the emotional response to the music, and how does that affect heart rate,” wondered Dr. Robb, a professor at the Indiana University School of Nursing in Indianapolis.
Music as exercise
The division that Dr. Edwards directs recently funded a pilot study that assessed the feasibility of using music to stimulate activity and improve breathing another way, by repurposing singing as a novel form of rehabilitative exercise.
The pilot study enrolled patients with coronary disease into a randomized study that tested whether a 14-minute session of supervised singing could produce acute improvement in vascular function, “a biomarker for the risk of future cardiovascular disease events,” explained Jacqueline P. Kulinski, MD, a preventive cardiologist at the Medical College of Wisconsin in Milwaukee. Dr. Kulinski did not report details of her yet-unpublished study, but said that her initial findings held promise for developing musical activities such as singing as a novel way to stimulate therapeutic physical activity in patients with heart disease.
“It’s exciting to see this signal” of benefit. “I envision music therapy as a part of cardiac rehabilitation, or an alternative for patients who can’t participate in traditional rehab,” Dr. Kulinski said during the virtual session. “I think of singing as a physical activity, as exercise, and using this exercise as medicine.”
Harmonizing with the NIH
“Singing is like swimming: You need to hold your breath,” agreed Ms. Fleming, who participated on the virtual panel and has spearheaded a collaboration between the National Institutes of Health and the Kennedy Center for the Performing Arts, the Sound Health Initiative, that’s coordinating research into the connections between music and health. Ms. Fleming helped launch the Sound Health Initiative in 2017 by coauthoring a JAMA article with the NIH director that spelled out the rationale and goals of the project (JAMA. 2017 Jun 27;317[24]:2470-1), and by launching a lecture tour on the topic in a presentation she calls Music and the Mind.
Ms. Fleming has given her talk in more than 30 locations worldwide, and she’s found that “audiences love” the combination of neuroscience and music that her talks cover, she said. Her lectures highlight that, in addition to cardiovascular disease, the potential for music therapy and related interventions has been shown in patients with disorders that include autism, psychosis, pain, Parkinson’s disease, Alzheimer’s disease, and epilepsy.
The research highlighted in the session “opens new doors to prevention and treatment strategies using music for patients with heart failure and cardiovascular disease,” summed up Biykem Bozkurt, MD, professor of medicine at the Baylor College of Medicine in Houston and president of the Heart Failure Society of America, who helped organize the virtual session.
Dr. Fleg, Dr. Edwards, Dr. Robb, Dr Kulinski, Ms. Fleming, and Dr. Bozkurt had no relevant financial disclosures.
Music listening and singing each showed early, promising evidence for producing cardiovascular benefits, part of a burgeoning area of research that is exploring and documenting ways to effectively use music to improve health.
A study run at four centers in Italy randomized 159 patients with heart failure, primarily New York Heart Association class I or II disease, to either a daily regimen of at least 30 minutes spent listening to music daily or to a control group that received usual care with no music prescription. After 3 months, the 82 patients in the daily music-listening group had a statistically significant improvement in their Minnesota Living with Heart Failure Questionnaire scores, compared with 77 controls for the study’s primary outcome measure. The results also showed significant benefits, compared with placebo, for other, secondary efficacy measures including improvements in anxiety, depression, sleep quality, and cognition.
Although the results are considered preliminary, they drew significant attention when published in July 2020 (J Card Fail. 2020 Jul 1;26[7]:541-9), where it was accompanied by two editorials in the same issue as well as an editor’s statement. All these commentators as well as other experts interested in music as medicine gathered to further discuss the topic during a panel session at the virtual annual meeting of the Heart Failure Society of America.
Music as a calming influence
The source of the primary benefits seen in this Italian study likely involved “emotional, psychological, and relaxation,” suggested Jerome L. Fleg, MD, program officer for clinical cardiovascular disease at the National Heart, Lung, and Blood Institute in Bethesda, Md. Researchers had used calming potential as a major criterion when selecting the 80 classical pieces that the heart failure patients in the intervention arm of the study could shuffle on their play lists.
“The tempo/rhythm was set up in a range between 60 and 80 beats per minute, because this range mirrors the human heart rate and facilitates relaxation,” the investigators said in their published report. Unfortunately, noted Dr. Fleg, the study lacked physiologic and biomarker measurements that could have provided objective evidence of effects from music. And the study failed to include a control arm of patients instructed to spend 30 minutes a day resting and relaxing without instruction to listen to music, he noted.
Dr. Fleg had authored one of the July editorials, where he said “It is hoped that findings from these studies and others can expand the scientific evidence for music-based interventions and bring these therapies into clinical practice. The current study from Burrai et al. is a positive step in this direction for patients with heart failure.” (J Card Fail. 2020 Jul 1;26[7]: 550-1). What’s needed now, he added during the virtual session, are “more objective data” to better and more comprehensively document the benefits from a music-based intervention in patients with heart failure.
An add-on to standard care
The findings in heart failure patients follows a growing literature that’s shown music can generate a restful state by doing things like activating autonomic parasympathetic outflow while dampening sympathetic outflow. This produces moderation in mood and emotion as well as depressed heart rate, lowered blood pressure, and slowed respiration, commented Emmeline Edwards, PhD director of the division of extramural research of the National Center for Complementary and Integrative Health in Bethesda, Md. Music also seems able to stimulate higher-order brain regions that can result in reduced psychological stress, anxiety, and depression.
“It’s a promising protective intervention to add to standard care for cardiac patients,” Dr. Edwards said during the virtual session. “Music is part of the toolbox for managing symptoms and improving health and well-being.”
“Music is not a substitute for standard therapy, but could add to it,” declared Dr. Fleg.
The already-established intervention known as music therapy has identified music’s ability to modulate breathing as an important mediator of music’s effect.
“Breathing is one of the few physiological processes that can be voluntarily controlled making it a viable target for intervention,” noted opera soprano Renée Fleming and Sheri L. Robb, PhD, in the second editorial that accompanied the Italian heart failure report (J Card Fail. 2020 Jul 1;26[7]:552-4). The music-listening intervention “may have had more effect if they had used compositional features [of the music] to teach patients how to structure their breathing,” said Dr. Robb, a music therapist at Indiana University–Purdue University Indianapolis, during the virtual session.
Another variable to consider is the type of music. “What is the emotional response to the music, and how does that affect heart rate,” wondered Dr. Robb, a professor at the Indiana University School of Nursing in Indianapolis.
Music as exercise
The division that Dr. Edwards directs recently funded a pilot study that assessed the feasibility of using music to stimulate activity and improve breathing another way, by repurposing singing as a novel form of rehabilitative exercise.
The pilot study enrolled patients with coronary disease into a randomized study that tested whether a 14-minute session of supervised singing could produce acute improvement in vascular function, “a biomarker for the risk of future cardiovascular disease events,” explained Jacqueline P. Kulinski, MD, a preventive cardiologist at the Medical College of Wisconsin in Milwaukee. Dr. Kulinski did not report details of her yet-unpublished study, but said that her initial findings held promise for developing musical activities such as singing as a novel way to stimulate therapeutic physical activity in patients with heart disease.
“It’s exciting to see this signal” of benefit. “I envision music therapy as a part of cardiac rehabilitation, or an alternative for patients who can’t participate in traditional rehab,” Dr. Kulinski said during the virtual session. “I think of singing as a physical activity, as exercise, and using this exercise as medicine.”
Harmonizing with the NIH
“Singing is like swimming: You need to hold your breath,” agreed Ms. Fleming, who participated on the virtual panel and has spearheaded a collaboration between the National Institutes of Health and the Kennedy Center for the Performing Arts, the Sound Health Initiative, that’s coordinating research into the connections between music and health. Ms. Fleming helped launch the Sound Health Initiative in 2017 by coauthoring a JAMA article with the NIH director that spelled out the rationale and goals of the project (JAMA. 2017 Jun 27;317[24]:2470-1), and by launching a lecture tour on the topic in a presentation she calls Music and the Mind.
Ms. Fleming has given her talk in more than 30 locations worldwide, and she’s found that “audiences love” the combination of neuroscience and music that her talks cover, she said. Her lectures highlight that, in addition to cardiovascular disease, the potential for music therapy and related interventions has been shown in patients with disorders that include autism, psychosis, pain, Parkinson’s disease, Alzheimer’s disease, and epilepsy.
The research highlighted in the session “opens new doors to prevention and treatment strategies using music for patients with heart failure and cardiovascular disease,” summed up Biykem Bozkurt, MD, professor of medicine at the Baylor College of Medicine in Houston and president of the Heart Failure Society of America, who helped organize the virtual session.
Dr. Fleg, Dr. Edwards, Dr. Robb, Dr Kulinski, Ms. Fleming, and Dr. Bozkurt had no relevant financial disclosures.
Nerve damage linked to prone positioning in COVID-19
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF ANAESTHESIOLOGY
Geriatric patients: My three rules for them
I have been in practice for 31 years, so many of my patients are now in their 80s and 90s. Practices age with us, and I have been seeing many of these patients for 25-30 years.
Absolutely, positively make sure you move!
Our older patients often have many reasons not to move, including pain from arthritis, deconditioning, muscle weakness, fatigue, and depression. “Keeping moving” is probably the most important thing a patient can do for their health.
Holme and Anderssen studied a large cohort of men for cardiovascular risk in 1972 and again in 2000. The surviving men were followed over an additional 12 years.1 They found that 30 minutes of physical activity 6 days a week was associated with a 40% reduction in mortality. Sedentary men had a reduced life expectancy of about 5 years, compared with men who were moderately to vigorously physically active.
Stewart etal. studied the benefit of physical activity in people with stable coronary disease.2 They concluded that, in patients with stable coronary heart disease, more physical activity was associated with lower mortality, and the largest benefit occurred in the sedentary patient groups and the highest cardiac risk groups.
Saint-Maurice et al. studied the effects of total daily step count and step intensity on mortality risk.3 They found that the risk of all-cause mortality decreases as the total number of daily steps increases, but that the speed of those steps did not make a difference. This is very encouraging data for our elderly patients. Moving is the secret, even if it may not be moving at a fast pace!
Never, ever get on a ladder!
This one should be part of every geriatric’s assessment and every Medicare wellness exam. I first experienced the horror of what can happen when elderly people climb when a 96-year-old healthy patient of mine fell off his roof and died. I never thought to tell him climbing on the roof was an awful idea.
Akland et al. looked at the epidemiology and outcomes of ladder-related falls that required ICU admission.4 Hospital mortality was 26%, and almost all of the mortalities occurred in older males in domestic falls, who died as a result of traumatic brain injury. Fewer than half of the survivors were living independently 1 year after the fall.
Valmuur et al. studied ladder related falls in Australia.5 They found that rates of ladder related falls requiring hospitalization rose from about 20/100,000 for men ages 15-29 years to 78/100,000 for men aged over 60 years. Of those who died from fall-related injury, 82% were over the age of 60, with more than 70% dying from head injuries.
Schaffarczyk et al. looked at the impact of nonoccupational falls from ladders in men aged over 50 years.6 The mean age of the patients in the study was 64 years (range, 50-85), with 27% suffering severe trauma. There was a striking impact on long-term function occurring in over half the study patients. The authors did interviews with patients in follow-up long after the falls and found that most never thought of themselves at risk for a fall, and after the experience of a bad fall, would never consider going on a ladder again. I think it is important for health care professionals to discuss the dangers of ladder use with our older patients, pointing out the higher risk of falling and the potential for the fall to be a life-changing or life-ending event.
Let them eat!
Many patients have a reduced appetite as they age. We work hard with our patients to choose a healthy diet throughout their lives, to help ward off obesity, treat hypertension, prevent or control diabetes, or provide heart health. Many patients just stop being interested in food, reduce intake, and may lose weight and muscle mass. When my patients pass the age of 85, I change my focus to encouraging them to eat for calories, socialization, and joy. I think the marginal benefits of more restrictive diets are small, compared with the benefits of helping your patients enjoy eating again. I ask patients what their very favorite foods are and encourage them to have them.
Pearl
Keep your patients eating and moving, except not onto a ladder!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Holme I, Anderssen SA. Increases in physical activity is as important as smoking cessation for reduction in total mortality in elderly men: 12 years of follow-up of the Oslo II study. Br J Sports Med. 2015; 49:743-8.
2. Stewart RAH et al. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol. 2017 Oct 3;70(14):1689-1700..
3. Saint-Maurice PF et al. Association of daily step count and step intensity with mortality among U.S. adults. JAMA 2020;323:1151-60.
4. Ackland HM et al. Danger at every rung: Epidemiology and outcomes of ICU-admitted ladder-related trauma. Injury. 2016;47:1109-117.
5. Vallmuur K et al. Falls from ladders in Australia: comparing occupational and nonoccupational injuries across age groups. Aust N Z J Public Health. 2016 Dec;40(6):559-63.
6. Schaffarczyk K et al. Nonoccupational falls from ladders in men 50 years and over: Contributing factors and impact. Injury. 2020 Aug;51(8):1798-1804.
I have been in practice for 31 years, so many of my patients are now in their 80s and 90s. Practices age with us, and I have been seeing many of these patients for 25-30 years.
Absolutely, positively make sure you move!
Our older patients often have many reasons not to move, including pain from arthritis, deconditioning, muscle weakness, fatigue, and depression. “Keeping moving” is probably the most important thing a patient can do for their health.
Holme and Anderssen studied a large cohort of men for cardiovascular risk in 1972 and again in 2000. The surviving men were followed over an additional 12 years.1 They found that 30 minutes of physical activity 6 days a week was associated with a 40% reduction in mortality. Sedentary men had a reduced life expectancy of about 5 years, compared with men who were moderately to vigorously physically active.
Stewart etal. studied the benefit of physical activity in people with stable coronary disease.2 They concluded that, in patients with stable coronary heart disease, more physical activity was associated with lower mortality, and the largest benefit occurred in the sedentary patient groups and the highest cardiac risk groups.
Saint-Maurice et al. studied the effects of total daily step count and step intensity on mortality risk.3 They found that the risk of all-cause mortality decreases as the total number of daily steps increases, but that the speed of those steps did not make a difference. This is very encouraging data for our elderly patients. Moving is the secret, even if it may not be moving at a fast pace!
Never, ever get on a ladder!
This one should be part of every geriatric’s assessment and every Medicare wellness exam. I first experienced the horror of what can happen when elderly people climb when a 96-year-old healthy patient of mine fell off his roof and died. I never thought to tell him climbing on the roof was an awful idea.
Akland et al. looked at the epidemiology and outcomes of ladder-related falls that required ICU admission.4 Hospital mortality was 26%, and almost all of the mortalities occurred in older males in domestic falls, who died as a result of traumatic brain injury. Fewer than half of the survivors were living independently 1 year after the fall.
Valmuur et al. studied ladder related falls in Australia.5 They found that rates of ladder related falls requiring hospitalization rose from about 20/100,000 for men ages 15-29 years to 78/100,000 for men aged over 60 years. Of those who died from fall-related injury, 82% were over the age of 60, with more than 70% dying from head injuries.
Schaffarczyk et al. looked at the impact of nonoccupational falls from ladders in men aged over 50 years.6 The mean age of the patients in the study was 64 years (range, 50-85), with 27% suffering severe trauma. There was a striking impact on long-term function occurring in over half the study patients. The authors did interviews with patients in follow-up long after the falls and found that most never thought of themselves at risk for a fall, and after the experience of a bad fall, would never consider going on a ladder again. I think it is important for health care professionals to discuss the dangers of ladder use with our older patients, pointing out the higher risk of falling and the potential for the fall to be a life-changing or life-ending event.
Let them eat!
Many patients have a reduced appetite as they age. We work hard with our patients to choose a healthy diet throughout their lives, to help ward off obesity, treat hypertension, prevent or control diabetes, or provide heart health. Many patients just stop being interested in food, reduce intake, and may lose weight and muscle mass. When my patients pass the age of 85, I change my focus to encouraging them to eat for calories, socialization, and joy. I think the marginal benefits of more restrictive diets are small, compared with the benefits of helping your patients enjoy eating again. I ask patients what their very favorite foods are and encourage them to have them.
Pearl
Keep your patients eating and moving, except not onto a ladder!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Holme I, Anderssen SA. Increases in physical activity is as important as smoking cessation for reduction in total mortality in elderly men: 12 years of follow-up of the Oslo II study. Br J Sports Med. 2015; 49:743-8.
2. Stewart RAH et al. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol. 2017 Oct 3;70(14):1689-1700..
3. Saint-Maurice PF et al. Association of daily step count and step intensity with mortality among U.S. adults. JAMA 2020;323:1151-60.
4. Ackland HM et al. Danger at every rung: Epidemiology and outcomes of ICU-admitted ladder-related trauma. Injury. 2016;47:1109-117.
5. Vallmuur K et al. Falls from ladders in Australia: comparing occupational and nonoccupational injuries across age groups. Aust N Z J Public Health. 2016 Dec;40(6):559-63.
6. Schaffarczyk K et al. Nonoccupational falls from ladders in men 50 years and over: Contributing factors and impact. Injury. 2020 Aug;51(8):1798-1804.
I have been in practice for 31 years, so many of my patients are now in their 80s and 90s. Practices age with us, and I have been seeing many of these patients for 25-30 years.
Absolutely, positively make sure you move!
Our older patients often have many reasons not to move, including pain from arthritis, deconditioning, muscle weakness, fatigue, and depression. “Keeping moving” is probably the most important thing a patient can do for their health.
Holme and Anderssen studied a large cohort of men for cardiovascular risk in 1972 and again in 2000. The surviving men were followed over an additional 12 years.1 They found that 30 minutes of physical activity 6 days a week was associated with a 40% reduction in mortality. Sedentary men had a reduced life expectancy of about 5 years, compared with men who were moderately to vigorously physically active.
Stewart etal. studied the benefit of physical activity in people with stable coronary disease.2 They concluded that, in patients with stable coronary heart disease, more physical activity was associated with lower mortality, and the largest benefit occurred in the sedentary patient groups and the highest cardiac risk groups.
Saint-Maurice et al. studied the effects of total daily step count and step intensity on mortality risk.3 They found that the risk of all-cause mortality decreases as the total number of daily steps increases, but that the speed of those steps did not make a difference. This is very encouraging data for our elderly patients. Moving is the secret, even if it may not be moving at a fast pace!
Never, ever get on a ladder!
This one should be part of every geriatric’s assessment and every Medicare wellness exam. I first experienced the horror of what can happen when elderly people climb when a 96-year-old healthy patient of mine fell off his roof and died. I never thought to tell him climbing on the roof was an awful idea.
Akland et al. looked at the epidemiology and outcomes of ladder-related falls that required ICU admission.4 Hospital mortality was 26%, and almost all of the mortalities occurred in older males in domestic falls, who died as a result of traumatic brain injury. Fewer than half of the survivors were living independently 1 year after the fall.
Valmuur et al. studied ladder related falls in Australia.5 They found that rates of ladder related falls requiring hospitalization rose from about 20/100,000 for men ages 15-29 years to 78/100,000 for men aged over 60 years. Of those who died from fall-related injury, 82% were over the age of 60, with more than 70% dying from head injuries.
Schaffarczyk et al. looked at the impact of nonoccupational falls from ladders in men aged over 50 years.6 The mean age of the patients in the study was 64 years (range, 50-85), with 27% suffering severe trauma. There was a striking impact on long-term function occurring in over half the study patients. The authors did interviews with patients in follow-up long after the falls and found that most never thought of themselves at risk for a fall, and after the experience of a bad fall, would never consider going on a ladder again. I think it is important for health care professionals to discuss the dangers of ladder use with our older patients, pointing out the higher risk of falling and the potential for the fall to be a life-changing or life-ending event.
Let them eat!
Many patients have a reduced appetite as they age. We work hard with our patients to choose a healthy diet throughout their lives, to help ward off obesity, treat hypertension, prevent or control diabetes, or provide heart health. Many patients just stop being interested in food, reduce intake, and may lose weight and muscle mass. When my patients pass the age of 85, I change my focus to encouraging them to eat for calories, socialization, and joy. I think the marginal benefits of more restrictive diets are small, compared with the benefits of helping your patients enjoy eating again. I ask patients what their very favorite foods are and encourage them to have them.
Pearl
Keep your patients eating and moving, except not onto a ladder!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Holme I, Anderssen SA. Increases in physical activity is as important as smoking cessation for reduction in total mortality in elderly men: 12 years of follow-up of the Oslo II study. Br J Sports Med. 2015; 49:743-8.
2. Stewart RAH et al. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol. 2017 Oct 3;70(14):1689-1700..
3. Saint-Maurice PF et al. Association of daily step count and step intensity with mortality among U.S. adults. JAMA 2020;323:1151-60.
4. Ackland HM et al. Danger at every rung: Epidemiology and outcomes of ICU-admitted ladder-related trauma. Injury. 2016;47:1109-117.
5. Vallmuur K et al. Falls from ladders in Australia: comparing occupational and nonoccupational injuries across age groups. Aust N Z J Public Health. 2016 Dec;40(6):559-63.
6. Schaffarczyk K et al. Nonoccupational falls from ladders in men 50 years and over: Contributing factors and impact. Injury. 2020 Aug;51(8):1798-1804.
Listening to Mozart helps tame epilepsy
Listening to Mozart’s piano music improves epilepsy, according to a meta-analysis presented at the virtual congress of the European College of Neuropsychopharmacology.
The results of the meta-analysis of 12 published studies of the so-called Mozart Effect that met rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines demonstrate that listening to Mozart results in significant reductions in both epileptic seizure frequency and interictal epileptiform discharges (IED), compared with baseline.
The benefits were apparent during and after even a single listening session, although the effect was greater with regular daily listening sessions, according to Gianluca Sesso, MD, a resident in child and adolescent psychiatry at the University of Pisa (Italy.)
“Obviously other music may have similar effects, but it may be that Mozart’s sonatas have distinctive rhythmic structures which are particularly suited to working on epilepsy,” he speculated, adding that the mechanism involved in the Mozart Effect on brain systems remains unclear.
“The highly consistent results of our meta-analysis strongly suggest that music-based neurostimulation may improve the clinical outcome in epilepsy by reducing seizures and IED, and thus deserves to be included in the set of nonpharmacologic complementary approaches for treating epilepsy,” Dr. Sesso added.
Four studies examined the effects of listening to Mozart’s Sonata for Two Pianos in D, K.448, the most-studied piece of music as a treatment for epilepsy. The data documented a 31% reduction in seizure frequency and 28% decrease in IED during a single listen, and a 79% reduction in IED after long-term Mozart music therapy. Similarly, studies demonstrated that listening to a set of Mozart’s compositions resulted in a 36% reduction in IED during and 38% decrease after a single listen, while regular listening in a prolonged treatment period resulted in a 66% reduction in seizure frequency from baseline.
Several studies compared the benefits of listening to K. 488 with those accrued through listening to Piano Sonata No. 16 in C major, K. 545. There was no significant difference between the two, according to Dr. Sesso.
He reported having no financial conflicts regarding his meta-analysis, carried out free of commercial support.
The full details of the meta-analysis were recently published in Clinical Neurophysiology.
Listening to Mozart’s piano music improves epilepsy, according to a meta-analysis presented at the virtual congress of the European College of Neuropsychopharmacology.
The results of the meta-analysis of 12 published studies of the so-called Mozart Effect that met rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines demonstrate that listening to Mozart results in significant reductions in both epileptic seizure frequency and interictal epileptiform discharges (IED), compared with baseline.
The benefits were apparent during and after even a single listening session, although the effect was greater with regular daily listening sessions, according to Gianluca Sesso, MD, a resident in child and adolescent psychiatry at the University of Pisa (Italy.)
“Obviously other music may have similar effects, but it may be that Mozart’s sonatas have distinctive rhythmic structures which are particularly suited to working on epilepsy,” he speculated, adding that the mechanism involved in the Mozart Effect on brain systems remains unclear.
“The highly consistent results of our meta-analysis strongly suggest that music-based neurostimulation may improve the clinical outcome in epilepsy by reducing seizures and IED, and thus deserves to be included in the set of nonpharmacologic complementary approaches for treating epilepsy,” Dr. Sesso added.
Four studies examined the effects of listening to Mozart’s Sonata for Two Pianos in D, K.448, the most-studied piece of music as a treatment for epilepsy. The data documented a 31% reduction in seizure frequency and 28% decrease in IED during a single listen, and a 79% reduction in IED after long-term Mozart music therapy. Similarly, studies demonstrated that listening to a set of Mozart’s compositions resulted in a 36% reduction in IED during and 38% decrease after a single listen, while regular listening in a prolonged treatment period resulted in a 66% reduction in seizure frequency from baseline.
Several studies compared the benefits of listening to K. 488 with those accrued through listening to Piano Sonata No. 16 in C major, K. 545. There was no significant difference between the two, according to Dr. Sesso.
He reported having no financial conflicts regarding his meta-analysis, carried out free of commercial support.
The full details of the meta-analysis were recently published in Clinical Neurophysiology.
Listening to Mozart’s piano music improves epilepsy, according to a meta-analysis presented at the virtual congress of the European College of Neuropsychopharmacology.
The results of the meta-analysis of 12 published studies of the so-called Mozart Effect that met rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines demonstrate that listening to Mozart results in significant reductions in both epileptic seizure frequency and interictal epileptiform discharges (IED), compared with baseline.
The benefits were apparent during and after even a single listening session, although the effect was greater with regular daily listening sessions, according to Gianluca Sesso, MD, a resident in child and adolescent psychiatry at the University of Pisa (Italy.)
“Obviously other music may have similar effects, but it may be that Mozart’s sonatas have distinctive rhythmic structures which are particularly suited to working on epilepsy,” he speculated, adding that the mechanism involved in the Mozart Effect on brain systems remains unclear.
“The highly consistent results of our meta-analysis strongly suggest that music-based neurostimulation may improve the clinical outcome in epilepsy by reducing seizures and IED, and thus deserves to be included in the set of nonpharmacologic complementary approaches for treating epilepsy,” Dr. Sesso added.
Four studies examined the effects of listening to Mozart’s Sonata for Two Pianos in D, K.448, the most-studied piece of music as a treatment for epilepsy. The data documented a 31% reduction in seizure frequency and 28% decrease in IED during a single listen, and a 79% reduction in IED after long-term Mozart music therapy. Similarly, studies demonstrated that listening to a set of Mozart’s compositions resulted in a 36% reduction in IED during and 38% decrease after a single listen, while regular listening in a prolonged treatment period resulted in a 66% reduction in seizure frequency from baseline.
Several studies compared the benefits of listening to K. 488 with those accrued through listening to Piano Sonata No. 16 in C major, K. 545. There was no significant difference between the two, according to Dr. Sesso.
He reported having no financial conflicts regarding his meta-analysis, carried out free of commercial support.
The full details of the meta-analysis were recently published in Clinical Neurophysiology.
FROM ECNP 2020