User login
Influenza vaccine efficacy called undiminished in MS
, Jackie Nguyen reported at the virtual annual meeting of the Consortium of Multiple Sclerosis Centers (CMSC). She presented a systematic review and meta-analysis of nine published cohort studies including 417 MS patients and more than 500 healthy controls, all of whom received inactivated seasonal influenza vaccine.
The impetus for this project was a recognition that the great majority of the research on the impact of influenza vaccine in patients with MS has focused on safety and MS relapse rates. In contrast, the nine studies included in the meta-analysis contained data on influenza vaccine efficacy as reflected in the ability to mount an adequate immune response. This was defined in standard fashion either by seroconversion, which required at least a fourfold increase in antibody titers following vaccination, or seroprotection, with a postvaccination antihemagglutination immunoglobulin G titer of at least 40. The analysis included patients with MS irrespective of disease duration or severity or treatment regimen, noted Ms. Nguyen, a third-year medical student at Nova Southeastern University College of Allopathic Medicine in Davie, Fla.
The researchers found that there was no significant difference between patients with MS and healthy controls in the rates of an adequate immune response for influenza H1N1, H3N2, or influenza B virus. “The vaccine should thus continue to be recommended for MS patients, as the data shows it to be efficacious,” she said.
Her conclusion is consistent with guidance provided in the American Academy of Neurology’s 2019 practice guideline update on immunization in MS, highlighted elsewhere at CMSC 2020 in a presentation by Marijean Buhse, PhD, of Stony Brook University in New York.
The guideline, updated for the first time in 17 years, states that all MS patients should be advised to receive influenza vaccine annually: “With known risks of exacerbation and other morbidity with influenza infection and no identified risks of exacerbation with influenza vaccines, benefits of influenza vaccination outweigh the risks in most scenarios. The exception involves the relatively few MS patients having a specific contraindication to the influenza vaccine, such as a previous severe reaction, noted Dr. Buhse, who wasn’t involved in developing the evidence-based guidelines.
The available evidence indicates that some but not all disease-modifying therapies for MS reduce the effectiveness of vaccination against influenza.
According to the guideline, “it is possible” that persons with MS being treated with glatiramer acetate have a reduced likelihood of seroprotection from influenza vaccine, a conclusion the guidelines committee drew with “low confidence in the evidence.” Further, the guideline states that “it is probable” MS patients on fingolimod have a lower likelihood of obtaining seroprotection from influenza vaccine than patients not on the drug, with moderate confidence in the evidence. Also, it is deemed probable that patients with MS who are taking mitoxantrone have a reduced likelihood of response to influenza vaccination, compared with healthy controls. But it is probable that patients with MS who are receiving interferon-beta have no diminution in the likelihood of seroprotection. According to the guideline, there is insufficient evidence to say whether patients with MS who are on natalizumab, teriflunomide, or methotrexate have a diminished response to influenza vaccination.
Dr. Buhse noted that rituximab is off-label therapy for MS, so there are no data available regarding the likelihood of seroprotection in response to influenza vaccination in that setting. However, rituximab profoundly decreases the immunogenicity of influenza and pneumococcal vaccines in rheumatoid arthritis patients. It is therefore recommended that inactivated influenza vaccine be given to patients with MS at least 2 weeks prior to starting rituximab or 6 months after the last dose in order to optimize the humoral results. Ms. Nguyen reported having no financial conflicts regarding her presentation. Dr. Buhse reported having received honoraria from Genzyme and Biogen.
, Jackie Nguyen reported at the virtual annual meeting of the Consortium of Multiple Sclerosis Centers (CMSC). She presented a systematic review and meta-analysis of nine published cohort studies including 417 MS patients and more than 500 healthy controls, all of whom received inactivated seasonal influenza vaccine.
The impetus for this project was a recognition that the great majority of the research on the impact of influenza vaccine in patients with MS has focused on safety and MS relapse rates. In contrast, the nine studies included in the meta-analysis contained data on influenza vaccine efficacy as reflected in the ability to mount an adequate immune response. This was defined in standard fashion either by seroconversion, which required at least a fourfold increase in antibody titers following vaccination, or seroprotection, with a postvaccination antihemagglutination immunoglobulin G titer of at least 40. The analysis included patients with MS irrespective of disease duration or severity or treatment regimen, noted Ms. Nguyen, a third-year medical student at Nova Southeastern University College of Allopathic Medicine in Davie, Fla.
The researchers found that there was no significant difference between patients with MS and healthy controls in the rates of an adequate immune response for influenza H1N1, H3N2, or influenza B virus. “The vaccine should thus continue to be recommended for MS patients, as the data shows it to be efficacious,” she said.
Her conclusion is consistent with guidance provided in the American Academy of Neurology’s 2019 practice guideline update on immunization in MS, highlighted elsewhere at CMSC 2020 in a presentation by Marijean Buhse, PhD, of Stony Brook University in New York.
The guideline, updated for the first time in 17 years, states that all MS patients should be advised to receive influenza vaccine annually: “With known risks of exacerbation and other morbidity with influenza infection and no identified risks of exacerbation with influenza vaccines, benefits of influenza vaccination outweigh the risks in most scenarios. The exception involves the relatively few MS patients having a specific contraindication to the influenza vaccine, such as a previous severe reaction, noted Dr. Buhse, who wasn’t involved in developing the evidence-based guidelines.
The available evidence indicates that some but not all disease-modifying therapies for MS reduce the effectiveness of vaccination against influenza.
According to the guideline, “it is possible” that persons with MS being treated with glatiramer acetate have a reduced likelihood of seroprotection from influenza vaccine, a conclusion the guidelines committee drew with “low confidence in the evidence.” Further, the guideline states that “it is probable” MS patients on fingolimod have a lower likelihood of obtaining seroprotection from influenza vaccine than patients not on the drug, with moderate confidence in the evidence. Also, it is deemed probable that patients with MS who are taking mitoxantrone have a reduced likelihood of response to influenza vaccination, compared with healthy controls. But it is probable that patients with MS who are receiving interferon-beta have no diminution in the likelihood of seroprotection. According to the guideline, there is insufficient evidence to say whether patients with MS who are on natalizumab, teriflunomide, or methotrexate have a diminished response to influenza vaccination.
Dr. Buhse noted that rituximab is off-label therapy for MS, so there are no data available regarding the likelihood of seroprotection in response to influenza vaccination in that setting. However, rituximab profoundly decreases the immunogenicity of influenza and pneumococcal vaccines in rheumatoid arthritis patients. It is therefore recommended that inactivated influenza vaccine be given to patients with MS at least 2 weeks prior to starting rituximab or 6 months after the last dose in order to optimize the humoral results. Ms. Nguyen reported having no financial conflicts regarding her presentation. Dr. Buhse reported having received honoraria from Genzyme and Biogen.
, Jackie Nguyen reported at the virtual annual meeting of the Consortium of Multiple Sclerosis Centers (CMSC). She presented a systematic review and meta-analysis of nine published cohort studies including 417 MS patients and more than 500 healthy controls, all of whom received inactivated seasonal influenza vaccine.
The impetus for this project was a recognition that the great majority of the research on the impact of influenza vaccine in patients with MS has focused on safety and MS relapse rates. In contrast, the nine studies included in the meta-analysis contained data on influenza vaccine efficacy as reflected in the ability to mount an adequate immune response. This was defined in standard fashion either by seroconversion, which required at least a fourfold increase in antibody titers following vaccination, or seroprotection, with a postvaccination antihemagglutination immunoglobulin G titer of at least 40. The analysis included patients with MS irrespective of disease duration or severity or treatment regimen, noted Ms. Nguyen, a third-year medical student at Nova Southeastern University College of Allopathic Medicine in Davie, Fla.
The researchers found that there was no significant difference between patients with MS and healthy controls in the rates of an adequate immune response for influenza H1N1, H3N2, or influenza B virus. “The vaccine should thus continue to be recommended for MS patients, as the data shows it to be efficacious,” she said.
Her conclusion is consistent with guidance provided in the American Academy of Neurology’s 2019 practice guideline update on immunization in MS, highlighted elsewhere at CMSC 2020 in a presentation by Marijean Buhse, PhD, of Stony Brook University in New York.
The guideline, updated for the first time in 17 years, states that all MS patients should be advised to receive influenza vaccine annually: “With known risks of exacerbation and other morbidity with influenza infection and no identified risks of exacerbation with influenza vaccines, benefits of influenza vaccination outweigh the risks in most scenarios. The exception involves the relatively few MS patients having a specific contraindication to the influenza vaccine, such as a previous severe reaction, noted Dr. Buhse, who wasn’t involved in developing the evidence-based guidelines.
The available evidence indicates that some but not all disease-modifying therapies for MS reduce the effectiveness of vaccination against influenza.
According to the guideline, “it is possible” that persons with MS being treated with glatiramer acetate have a reduced likelihood of seroprotection from influenza vaccine, a conclusion the guidelines committee drew with “low confidence in the evidence.” Further, the guideline states that “it is probable” MS patients on fingolimod have a lower likelihood of obtaining seroprotection from influenza vaccine than patients not on the drug, with moderate confidence in the evidence. Also, it is deemed probable that patients with MS who are taking mitoxantrone have a reduced likelihood of response to influenza vaccination, compared with healthy controls. But it is probable that patients with MS who are receiving interferon-beta have no diminution in the likelihood of seroprotection. According to the guideline, there is insufficient evidence to say whether patients with MS who are on natalizumab, teriflunomide, or methotrexate have a diminished response to influenza vaccination.
Dr. Buhse noted that rituximab is off-label therapy for MS, so there are no data available regarding the likelihood of seroprotection in response to influenza vaccination in that setting. However, rituximab profoundly decreases the immunogenicity of influenza and pneumococcal vaccines in rheumatoid arthritis patients. It is therefore recommended that inactivated influenza vaccine be given to patients with MS at least 2 weeks prior to starting rituximab or 6 months after the last dose in order to optimize the humoral results. Ms. Nguyen reported having no financial conflicts regarding her presentation. Dr. Buhse reported having received honoraria from Genzyme and Biogen.
REPORTING FROM CMSC 2020
Healthy Aging Project-Brain: A Psychoeducational and Motivational Group for Older Veterans
With a rapidly growing older adult population, increased attention has been given to cognitive changes that occur with age, with a focus on optimizing the cognitive health of aging individuals.1 Given the absence of pharmaceutical treatments to prevent cognitive decline, there is an increased need for health care systems to offer alternative or behavioral interventions that can mitigate the effects of cognitive decline in aging.
Notably, many individuals are able to maintain or even improve cognitive functioning throughout their lifespan, with some research implicating health behaviors as an important factor for promoting brain health with age. Specifically, sleep, exercise, eating habits, social engagement, and cognitive stimulation have been linked to improved cognitive functioning.2-8 In addition to the potential benefits for brain health, there is evidence that greater investment in attaining health goals is associated with subjective reports of higher well-being, fewer mental health symptoms, lower physical health stresses, decreased caregiver burden, and increased functional independence linked with longer independent living.9 The latter has a substantial financial impact, such that the positive consequence of increased independence is likely staving off the need for admission to assisted living and adult family homes, which can be costly.
Despite the role of health behaviors in brain aging and overall health and functioning, research indicates that only a small number of older adults (12.8%) follow recommended guidelines for healthy lifestyle factors.10 Education has been identified as one factor associated with the likelihood of engaging in positive health behaviors, prompting the delivery of health-education interventions. Most psychoeducational interventions have traditionally focused on one aspect of behavior change at a time (eg, sleep); however, Gross and colleaguesconducted a meta-analysis of cognitive interventions and in addition to the overall positive benefits (effect size 0.38), they also found suggestive evidence that interventions that combined multiple training strategies were associated with larger training gains (P = .04) after adjusting for multiple comparisons.11 For example, Miller and colleagues found a significant improvement on both subjective and objective measures of memory following a multicomponent approach that combined training in memory skills, stress reduction, nutrition, and physical activity.12
In addition to the potential positive impacts of health behaviors on brain health, findings suggest that targeted emphasis on health behavior change may have the potential to stave off mild cognitiveimpairment (MCI) or dementia even if for a short time. Given the increasing prevalence rates of MCI with age (6.7% in adults aged 60-64 years, reaching 25.2% in adults aged 80-84 years13) and dementia (prevalence of MCI converting to dementia is 18-40%14), as well as the corresponding emotional, financial, and family-oriented consequences (eg, impact on the well-being of family caregivers), the need for behavioral interventions that seek to optimize brain health is becoming increasingly apparent.
More than 9 million veterans are now aged ≥ 65 years.15 In addition to representing nearly half of all veterans and a sizable portion of aging adults in the US, older veterans are at increased risk of frailty, mortality, and high rates of chronic medical/mental health conditions that can lead to accelerated cognitive aging.6-17 Together, these conditions highlight the importance of developing comprehensive psychoeducational and behavioral interventions in this population. To address this need, we developed a novel psychoeducation and behavior change group called the Healthy Aging Project-Brain (HAP-B, pronounced “happy”). The HAP-B intervention was designed to promote healthy brain aging by using empirically supported health behavior change strategies, including education, personalized goal setting, and community support. The primary aim of this project was to develop and implement an intervention that was feasible and acceptable (eg, could be implemented in our setting, was appropriate for a veteran population) and to determine any positive outcomes/preliminary effects on overall health and well-being.
Methods
We recruited veterans aged ≥ 50 years through primary care clinics and self-referrals via flyers in the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS), Seattle Division hospital. We targeted the “worried well” and welcomed veterans with MCI and mental health diagnoses. Notably, if there were significant mental health and/or substance use concerns, we encouraged veterans to seek focused care and stabilization prior to or concurrent with group participation. Exclusion criteria included presence of suicidality/homicidality, untreated or unstable substance use disorder, or a diagnosis of dementia. Exclusion criteria were assessed by the referring health care providers (HCPs), when appropriate, and through a health record review. Group facilitators used their clinical judgment to monitor participants if they began experiencing more severe cognitive impairment or acute mental health concerns. Although we did not encounter any of these instances, facilitators were prepared to discuss any concerns with the veteran and their referring HCP. Participants sampled were from 1 of 5 groups offered between January 2018 and March 2019. A waiver from the institutional review board was obtained after meeting criteria for quality improvement/quality assurance (QI/QA) for this study.
Procedures
At the initial stages of development, our team conducted a needs assessment to identify health-related areas where HCPs felt veterans would benefit from additional education and support. The needs assessment was conducted across primary care, geriatric extended care, and the Geriatric Research, Education, and Clinical Center (GRECC) at VAPSHCS. Combining the needs assessment results with the available research base, we identified sleep, physical activity, social engagement, and cognitive stimulation as areas for focus. Notably, although nutrition has been identified as an important factor in cognitive aging, a diet and nutrition class was already available to older veterans at the Seattle VA; hence, we chose to limit overlap by not covering this topic in our group.
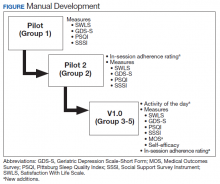
The group was offered on a quarterly basis as six 90-minute psychoeducational classes to allow time for didactics, discussion, and practice without overloading participants with information. Each group consisted of 4 to 9 veterans led by 2 cofacilitators. Group structure allowed for feedback and ideas from group members as well as accountability for engaging in behavior change. Cognitive functioning was not formally evaluated. Attendees were asked but not required to complete questionnaires before the classes began and again at completion. In addition at the completion of each group, feedback was collected from veterans and used to modify group content (Figure).
Two pilot groups were implemented in early and mid-2018 with iterative changes after each group. Then we revised the assessment battery and implemented the current version (v1.0), which was first offered in the fall of 2018 and was used with the final 3 groups. Noteworthy changes included weekly check-ins to assess use of health behavior logs and progress toward individual goals, additional pre-and postgroup measures, and in vivo skills practice relevant to the topic being discussed that day.
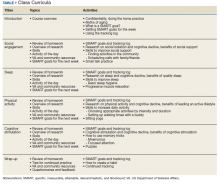
Each session began with a check-in, which included a review of daily logs and SMART (specific, measurable, attainable, relevant/realistic, and timebound) goals from the previous week.18 This allowed for praise/reinforcement of health behaviors as well as discussion of potential barriers. Second, an overview of research focusing on the relationship between aging, brain health, and the topic of the day was presented. As an example, in the discussion of social engagement, research was presented about the link between social isolation and cognitive decline; the indirect benefits of social support (eg, social support is linked to improved physical and mental health, which, in turn, is associated with less cognitive decline); and the direct benefits of social support (eg, high levels of emotional support are associated with better cognitive function) (Table 1).6
Next, facilitators reviewed skills and strategies to improve functioning in the topic of discussion. During the social engagement group, for example, facilitators discussed tips to improve social skills (eg, asking open-ended questions) and how to build social support into a daily routine (eg, scheduling weekly phone calls with family and friends). Following this discussion of skills, an activity was practiced, reinforcing learned material. During the social engagement group, veterans were invited to use small talk strategies with fellow group members. Finally, group sessions ended with each participant identifying a SMART goal for the coming week and troubleshooting potential barriers to success. SMART goals were kept broad, so veterans could choose a goal related to the topic discussed at the group that day (eg, scheduling a phone call with a friend twice in the coming week during the social engagement-focused group) or choose any other goal to focus on (eg, a sleep-related goal). Similarly, goals could change week to week, or could remain the same throughout the 6-week classes.
Measures
The questionnaires used for QI/QA analyses included the Satisfaction with Life Scale (SWLS); Geriatric Depression Scale-Short Form (GDS-S); Social Support Survey Instrument (SSSI); Pittsburg Sleep Quality Index (PSQI); Medical Outcomes Survey-Short Form (MOS-36 SF); and a self-efficacy scale (adapted from Huckans and colleagues for traumatic brain injury).19-24 Written feedback was collected at the end of the last group to assess perception of progress, self-perceived behavior change, what was helpful or unhelpful, and how likely the participants were to recommend the group to other veterans (0 to 3, very unlikely to very likely).
To promote consistency with other health and behavior change interventions at the VA, HAP-B used resources from the Whole Health model SMART goals. Research supports the use of self-monitoring techniques like SMART goals for behavior change.25
To facilitate skills practice and self-monitoring between classes, veterans were asked to complete 2 homework assignments. First, at the end of each group, each veteran identified a specific SMART goal to focus on and track in the coming week. Goals were unique to each veteran and allowed to change from week to week. Group discussion around SMART goals involved plans for how to address potential barriers; progress toward goals was discussed at the beginning of the following group. Second, veterans were asked to complete a worksheet used to track progress toward the weekly SMART goal and the specific health behaviors related to the 4 domains targeted by HAP-B. For example, when tracking sleep behaviors, veterans noted bedtime, waketime, number of times they woke up during the night, and length of daytime naps if applicable. Tracking logs were provided at the end of each class for personal purposes only. We asked veterans to rate themselves each week on whether they used the tracking sheet to monitor health behaviors; and how successful they were at accomplishing their previously identified SMART goal. We recorded responses on a 0 to 2 scale (0, not good; 1, fair; 2, good). This rating system was developed and implemented in later groups to promote self-monitoring, accountability, and discussion of potential barriers. However, due to the small sample that completed these ratings and the absence of objective corroborating data, these ratings were not included in the current analyses.
Every participant received a manual in binder format, which provided the didactic information for each group session, skills and strategies discussed in each session, and relevant resources in both the VA and community. For example, social engagement resources included information about volunteer opportunities, VA groups that focus on developing interpersonal skills, and recommendations from past group members on social events (eg, dance lessons at a senior center). We also developed a facilitator version of the manual in which we added comments and guidance on topics for discussion. Materials were developed with the goal of optimizing the ease of dissemination to other sites.
Results
Across the 5 groups, 31 veterans enrolled as participants and completed the initial intake measures, with an average of 6 participants per group (range 4-9). The majority (80%) attended at least 5 of the 6 classes. The mean
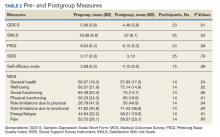
At the start of the class, the mean (SD) reports of participants were mild depressive symptoms 5.96 (3.8) on the GDS scale, moderate levels of self-efficacy 3.69 (0.5) on the self-efficacy scale, and moderate levels of satisfaction with life 18.08 (6.8) on the SWLS scale (Table 2). Data from 25 of 31 veterans who completed both pregroup and postgroup surveys were analyzed and paired samples t tests without corrections indicated a reduction in depressive symptoms (P = .01), improved self-efficacy (P = .08), and improved satisfaction with life (P = .03). There were no significant differences in self-reported sleep quality or perceived social support from pregroup to postgroup evaluations. Because the sample size was smaller for the MOS-36, which was not used until group 3, and the subscales are composed of few items each, we conducted exploratory analyses of the 8 MOS-36 subscales and found that well-being, physical functioning, role limitations due to physical and emotional functioning, and energy/fatigue significantly improved over time (Ps < .04).
Twenty-eight veterans provided written feedback following the final session. Qualitative feedback received at the completion of the group focused on participants’ desire for increased number of classes, longer sessions (eg, 2 participants recommended lengthening the group to 2 hours), and integrating mindfulness-based activities into each class. Participants rated themselves somewhat likely to very likely to recommend this group to other veterans (mean, 2.9 [SD, 0.4]).
Discussion
The ability and need to promote brain health with age is an emerging priority as our aging population grows. A growing body of evidence supports the role of health behaviors in healthy brain aging. Education and skills training in a group setting provides a supportive, cost-effective approach for increasing overall health in aging adults. Yet older adults are statistically less likely to engage in these behaviors on a regular basis. The current investigation provides preliminary support for a model of care that uses a comprehensive, experiential psychoeducational approach to facilitate behavior change in older adults. Our aim was to develop and implement an intervention that was feasible and acceptable to our older veterans and to determine any positive outcomes/preliminary effects on overall health and well-being.
Participants indicated that they enjoyed the group, learned new skills (per participant feedback and facilitator observation), and experienced improvements in mood, self-efficacy, and life satisfaction. Given the participants’ positive response to the group and its content, as well as continued referrals by HCPs to this group and low difficulty with ongoing recruitment, this program was deemed both feasible and acceptable in our veteran health care setting. Questions remain about the extent to which participants modified their health behaviors given that we did not collect objective measurements of behaviors (eg, time spent exercising), the duration of behavior change (ie, how long during and after the group were behaviors maintained), and the role of premorbid or concurrent characteristics that may moderate the effect of the intervention on health-related outcomes (eg, sleep quality, perceived social support, overall functioning, concurrent interventions, medications).
Strengths and Limitations
This study had a limited sample size and no control group. However, evidence of significant improvements in depressive symptoms, self-efficacy, and life satisfaction in the development groups without a control group is encouraging. This is particularly noteworthy given that older veterans as a group have higher rates of frailty and mortality than do other similarly aged counterparts.17An additional weakness is the absence of a brief cognitive assessment or other formal assessment as part of the inclusion/exclusion criteria. However, this program development project provides data from a realistic condition (recruited broadly and with few exclusions, offered in similar format as other VA classes), thus adding strength to the interpretation and possibly the generalizability of these findings.
Conclusions
Future directions include disseminating HAP-B materials and procedures across a variety of sites, both VA and non-VA. In line with this goal, we hope to increase sample size and sample diversity while optimizing protocol integrity during the exportation phase. With a greater sample size and power, we aim to examine the role of self-efficacy and other premorbid factors (eg, cognitive functioning at baseline) as mediators for observed changes in pre-/postmeasures and outcomes. We also hope to incorporate objective measures of behavior change, such as fitness trackers, heart rate/pulse monitors, and actigraphy for monitoring sleep. Finally, we are interested in conducting follow-up with past and future participants to detect changes that may occur with learning new skills following the completion of the group (eg, changes in sleep behavior that take time to take effect) and the extent to which participants continue to use the health behavior skills and strategies to maintain or enhance progress in behavioral goals. Finally, although this intervention was initially designed for use with older veterans receiving health care through the VA, we believe the concepts and work products described here can be used with older adults across a wide range of health care settings. Providers interested in trialing HAP-B at their local site are encouraged to contact the authors.
1. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-20.
2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):85-592. doi:10.1093/sleep/33.5.585
3. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12-31. doi:10.1016/j.arr.2014.05.002
4. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251-1257. doi:10.1001/archinternmed.2011.277
5. World Health Organization. Interventions on diet and physical activity: what works. https://www.who.int/dietphysicalactivity/whatworks/en/. Published 2009. Accessed June 19, 2020.
6. Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243-255. doi:10.1037//0278-6133.20.4.243
7. La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation and cognitive exercises. Clin Geriatr Med. 2010;26(1):99-111. doi:10.1016/j.cger.2009.11.003
8. Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychol Aging. 2002;17(4):548-557. doi:10.1037//0882-7974.17.4.548
9. Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: the importance of health engagement control strategies. Health Psychol. 2002;21(4):340-348. doi:10.1037//0278-6133.21.4.340
10. Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27(suppl 2):25-33. doi:10.1016/j.amepre.2004.04.022
11. Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health. 2012;16(6):722-734. doi:10.1080/13607863.2012.667783
12. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J Geriat Psychiatry. 2012;20(6):514-523. doi:10.1097/JGP.0b013e318227f821
13. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
14. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi:10.1016/S0140-6736(06)68542-5
15. US Department of Veteran Affairs, National Center for Veteran Analysis and Statistics.Veteran population. 2020. https://www.va.gov/vetdata/Veteran_Population.asp. Updated May 21, 2020 . Accessed June 17, 2020.
16. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique healthcare needs of the patient population served by the Department of Veterans Affairs. RAND Health Q. 2016;5(4):13.
17. Orkaby AR, Nussbaum L, Ho Y, et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
18. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70(11):35-36.
19. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi:10.1207/s15327752jpa4901-13
20. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165-173. doi:10.1300/J018v05n01_09
21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi:10.1016/0277-9536(91)90150-b
22. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4
23. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
24. Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43-60. doi:10.1682/jrrd.2009.02.0019
25. Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. doi:10.1016/j.pec.2011.07.018
With a rapidly growing older adult population, increased attention has been given to cognitive changes that occur with age, with a focus on optimizing the cognitive health of aging individuals.1 Given the absence of pharmaceutical treatments to prevent cognitive decline, there is an increased need for health care systems to offer alternative or behavioral interventions that can mitigate the effects of cognitive decline in aging.
Notably, many individuals are able to maintain or even improve cognitive functioning throughout their lifespan, with some research implicating health behaviors as an important factor for promoting brain health with age. Specifically, sleep, exercise, eating habits, social engagement, and cognitive stimulation have been linked to improved cognitive functioning.2-8 In addition to the potential benefits for brain health, there is evidence that greater investment in attaining health goals is associated with subjective reports of higher well-being, fewer mental health symptoms, lower physical health stresses, decreased caregiver burden, and increased functional independence linked with longer independent living.9 The latter has a substantial financial impact, such that the positive consequence of increased independence is likely staving off the need for admission to assisted living and adult family homes, which can be costly.
Despite the role of health behaviors in brain aging and overall health and functioning, research indicates that only a small number of older adults (12.8%) follow recommended guidelines for healthy lifestyle factors.10 Education has been identified as one factor associated with the likelihood of engaging in positive health behaviors, prompting the delivery of health-education interventions. Most psychoeducational interventions have traditionally focused on one aspect of behavior change at a time (eg, sleep); however, Gross and colleaguesconducted a meta-analysis of cognitive interventions and in addition to the overall positive benefits (effect size 0.38), they also found suggestive evidence that interventions that combined multiple training strategies were associated with larger training gains (P = .04) after adjusting for multiple comparisons.11 For example, Miller and colleagues found a significant improvement on both subjective and objective measures of memory following a multicomponent approach that combined training in memory skills, stress reduction, nutrition, and physical activity.12
In addition to the potential positive impacts of health behaviors on brain health, findings suggest that targeted emphasis on health behavior change may have the potential to stave off mild cognitiveimpairment (MCI) or dementia even if for a short time. Given the increasing prevalence rates of MCI with age (6.7% in adults aged 60-64 years, reaching 25.2% in adults aged 80-84 years13) and dementia (prevalence of MCI converting to dementia is 18-40%14), as well as the corresponding emotional, financial, and family-oriented consequences (eg, impact on the well-being of family caregivers), the need for behavioral interventions that seek to optimize brain health is becoming increasingly apparent.
More than 9 million veterans are now aged ≥ 65 years.15 In addition to representing nearly half of all veterans and a sizable portion of aging adults in the US, older veterans are at increased risk of frailty, mortality, and high rates of chronic medical/mental health conditions that can lead to accelerated cognitive aging.6-17 Together, these conditions highlight the importance of developing comprehensive psychoeducational and behavioral interventions in this population. To address this need, we developed a novel psychoeducation and behavior change group called the Healthy Aging Project-Brain (HAP-B, pronounced “happy”). The HAP-B intervention was designed to promote healthy brain aging by using empirically supported health behavior change strategies, including education, personalized goal setting, and community support. The primary aim of this project was to develop and implement an intervention that was feasible and acceptable (eg, could be implemented in our setting, was appropriate for a veteran population) and to determine any positive outcomes/preliminary effects on overall health and well-being.
Methods
We recruited veterans aged ≥ 50 years through primary care clinics and self-referrals via flyers in the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS), Seattle Division hospital. We targeted the “worried well” and welcomed veterans with MCI and mental health diagnoses. Notably, if there were significant mental health and/or substance use concerns, we encouraged veterans to seek focused care and stabilization prior to or concurrent with group participation. Exclusion criteria included presence of suicidality/homicidality, untreated or unstable substance use disorder, or a diagnosis of dementia. Exclusion criteria were assessed by the referring health care providers (HCPs), when appropriate, and through a health record review. Group facilitators used their clinical judgment to monitor participants if they began experiencing more severe cognitive impairment or acute mental health concerns. Although we did not encounter any of these instances, facilitators were prepared to discuss any concerns with the veteran and their referring HCP. Participants sampled were from 1 of 5 groups offered between January 2018 and March 2019. A waiver from the institutional review board was obtained after meeting criteria for quality improvement/quality assurance (QI/QA) for this study.
Procedures
At the initial stages of development, our team conducted a needs assessment to identify health-related areas where HCPs felt veterans would benefit from additional education and support. The needs assessment was conducted across primary care, geriatric extended care, and the Geriatric Research, Education, and Clinical Center (GRECC) at VAPSHCS. Combining the needs assessment results with the available research base, we identified sleep, physical activity, social engagement, and cognitive stimulation as areas for focus. Notably, although nutrition has been identified as an important factor in cognitive aging, a diet and nutrition class was already available to older veterans at the Seattle VA; hence, we chose to limit overlap by not covering this topic in our group.
The group was offered on a quarterly basis as six 90-minute psychoeducational classes to allow time for didactics, discussion, and practice without overloading participants with information. Each group consisted of 4 to 9 veterans led by 2 cofacilitators. Group structure allowed for feedback and ideas from group members as well as accountability for engaging in behavior change. Cognitive functioning was not formally evaluated. Attendees were asked but not required to complete questionnaires before the classes began and again at completion. In addition at the completion of each group, feedback was collected from veterans and used to modify group content (Figure).
Two pilot groups were implemented in early and mid-2018 with iterative changes after each group. Then we revised the assessment battery and implemented the current version (v1.0), which was first offered in the fall of 2018 and was used with the final 3 groups. Noteworthy changes included weekly check-ins to assess use of health behavior logs and progress toward individual goals, additional pre-and postgroup measures, and in vivo skills practice relevant to the topic being discussed that day.
Each session began with a check-in, which included a review of daily logs and SMART (specific, measurable, attainable, relevant/realistic, and timebound) goals from the previous week.18 This allowed for praise/reinforcement of health behaviors as well as discussion of potential barriers. Second, an overview of research focusing on the relationship between aging, brain health, and the topic of the day was presented. As an example, in the discussion of social engagement, research was presented about the link between social isolation and cognitive decline; the indirect benefits of social support (eg, social support is linked to improved physical and mental health, which, in turn, is associated with less cognitive decline); and the direct benefits of social support (eg, high levels of emotional support are associated with better cognitive function) (Table 1).6
Next, facilitators reviewed skills and strategies to improve functioning in the topic of discussion. During the social engagement group, for example, facilitators discussed tips to improve social skills (eg, asking open-ended questions) and how to build social support into a daily routine (eg, scheduling weekly phone calls with family and friends). Following this discussion of skills, an activity was practiced, reinforcing learned material. During the social engagement group, veterans were invited to use small talk strategies with fellow group members. Finally, group sessions ended with each participant identifying a SMART goal for the coming week and troubleshooting potential barriers to success. SMART goals were kept broad, so veterans could choose a goal related to the topic discussed at the group that day (eg, scheduling a phone call with a friend twice in the coming week during the social engagement-focused group) or choose any other goal to focus on (eg, a sleep-related goal). Similarly, goals could change week to week, or could remain the same throughout the 6-week classes.
Measures
The questionnaires used for QI/QA analyses included the Satisfaction with Life Scale (SWLS); Geriatric Depression Scale-Short Form (GDS-S); Social Support Survey Instrument (SSSI); Pittsburg Sleep Quality Index (PSQI); Medical Outcomes Survey-Short Form (MOS-36 SF); and a self-efficacy scale (adapted from Huckans and colleagues for traumatic brain injury).19-24 Written feedback was collected at the end of the last group to assess perception of progress, self-perceived behavior change, what was helpful or unhelpful, and how likely the participants were to recommend the group to other veterans (0 to 3, very unlikely to very likely).
To promote consistency with other health and behavior change interventions at the VA, HAP-B used resources from the Whole Health model SMART goals. Research supports the use of self-monitoring techniques like SMART goals for behavior change.25
To facilitate skills practice and self-monitoring between classes, veterans were asked to complete 2 homework assignments. First, at the end of each group, each veteran identified a specific SMART goal to focus on and track in the coming week. Goals were unique to each veteran and allowed to change from week to week. Group discussion around SMART goals involved plans for how to address potential barriers; progress toward goals was discussed at the beginning of the following group. Second, veterans were asked to complete a worksheet used to track progress toward the weekly SMART goal and the specific health behaviors related to the 4 domains targeted by HAP-B. For example, when tracking sleep behaviors, veterans noted bedtime, waketime, number of times they woke up during the night, and length of daytime naps if applicable. Tracking logs were provided at the end of each class for personal purposes only. We asked veterans to rate themselves each week on whether they used the tracking sheet to monitor health behaviors; and how successful they were at accomplishing their previously identified SMART goal. We recorded responses on a 0 to 2 scale (0, not good; 1, fair; 2, good). This rating system was developed and implemented in later groups to promote self-monitoring, accountability, and discussion of potential barriers. However, due to the small sample that completed these ratings and the absence of objective corroborating data, these ratings were not included in the current analyses.
Every participant received a manual in binder format, which provided the didactic information for each group session, skills and strategies discussed in each session, and relevant resources in both the VA and community. For example, social engagement resources included information about volunteer opportunities, VA groups that focus on developing interpersonal skills, and recommendations from past group members on social events (eg, dance lessons at a senior center). We also developed a facilitator version of the manual in which we added comments and guidance on topics for discussion. Materials were developed with the goal of optimizing the ease of dissemination to other sites.
Results
Across the 5 groups, 31 veterans enrolled as participants and completed the initial intake measures, with an average of 6 participants per group (range 4-9). The majority (80%) attended at least 5 of the 6 classes. The mean
At the start of the class, the mean (SD) reports of participants were mild depressive symptoms 5.96 (3.8) on the GDS scale, moderate levels of self-efficacy 3.69 (0.5) on the self-efficacy scale, and moderate levels of satisfaction with life 18.08 (6.8) on the SWLS scale (Table 2). Data from 25 of 31 veterans who completed both pregroup and postgroup surveys were analyzed and paired samples t tests without corrections indicated a reduction in depressive symptoms (P = .01), improved self-efficacy (P = .08), and improved satisfaction with life (P = .03). There were no significant differences in self-reported sleep quality or perceived social support from pregroup to postgroup evaluations. Because the sample size was smaller for the MOS-36, which was not used until group 3, and the subscales are composed of few items each, we conducted exploratory analyses of the 8 MOS-36 subscales and found that well-being, physical functioning, role limitations due to physical and emotional functioning, and energy/fatigue significantly improved over time (Ps < .04).
Twenty-eight veterans provided written feedback following the final session. Qualitative feedback received at the completion of the group focused on participants’ desire for increased number of classes, longer sessions (eg, 2 participants recommended lengthening the group to 2 hours), and integrating mindfulness-based activities into each class. Participants rated themselves somewhat likely to very likely to recommend this group to other veterans (mean, 2.9 [SD, 0.4]).
Discussion
The ability and need to promote brain health with age is an emerging priority as our aging population grows. A growing body of evidence supports the role of health behaviors in healthy brain aging. Education and skills training in a group setting provides a supportive, cost-effective approach for increasing overall health in aging adults. Yet older adults are statistically less likely to engage in these behaviors on a regular basis. The current investigation provides preliminary support for a model of care that uses a comprehensive, experiential psychoeducational approach to facilitate behavior change in older adults. Our aim was to develop and implement an intervention that was feasible and acceptable to our older veterans and to determine any positive outcomes/preliminary effects on overall health and well-being.
Participants indicated that they enjoyed the group, learned new skills (per participant feedback and facilitator observation), and experienced improvements in mood, self-efficacy, and life satisfaction. Given the participants’ positive response to the group and its content, as well as continued referrals by HCPs to this group and low difficulty with ongoing recruitment, this program was deemed both feasible and acceptable in our veteran health care setting. Questions remain about the extent to which participants modified their health behaviors given that we did not collect objective measurements of behaviors (eg, time spent exercising), the duration of behavior change (ie, how long during and after the group were behaviors maintained), and the role of premorbid or concurrent characteristics that may moderate the effect of the intervention on health-related outcomes (eg, sleep quality, perceived social support, overall functioning, concurrent interventions, medications).
Strengths and Limitations
This study had a limited sample size and no control group. However, evidence of significant improvements in depressive symptoms, self-efficacy, and life satisfaction in the development groups without a control group is encouraging. This is particularly noteworthy given that older veterans as a group have higher rates of frailty and mortality than do other similarly aged counterparts.17An additional weakness is the absence of a brief cognitive assessment or other formal assessment as part of the inclusion/exclusion criteria. However, this program development project provides data from a realistic condition (recruited broadly and with few exclusions, offered in similar format as other VA classes), thus adding strength to the interpretation and possibly the generalizability of these findings.
Conclusions
Future directions include disseminating HAP-B materials and procedures across a variety of sites, both VA and non-VA. In line with this goal, we hope to increase sample size and sample diversity while optimizing protocol integrity during the exportation phase. With a greater sample size and power, we aim to examine the role of self-efficacy and other premorbid factors (eg, cognitive functioning at baseline) as mediators for observed changes in pre-/postmeasures and outcomes. We also hope to incorporate objective measures of behavior change, such as fitness trackers, heart rate/pulse monitors, and actigraphy for monitoring sleep. Finally, we are interested in conducting follow-up with past and future participants to detect changes that may occur with learning new skills following the completion of the group (eg, changes in sleep behavior that take time to take effect) and the extent to which participants continue to use the health behavior skills and strategies to maintain or enhance progress in behavioral goals. Finally, although this intervention was initially designed for use with older veterans receiving health care through the VA, we believe the concepts and work products described here can be used with older adults across a wide range of health care settings. Providers interested in trialing HAP-B at their local site are encouraged to contact the authors.
With a rapidly growing older adult population, increased attention has been given to cognitive changes that occur with age, with a focus on optimizing the cognitive health of aging individuals.1 Given the absence of pharmaceutical treatments to prevent cognitive decline, there is an increased need for health care systems to offer alternative or behavioral interventions that can mitigate the effects of cognitive decline in aging.
Notably, many individuals are able to maintain or even improve cognitive functioning throughout their lifespan, with some research implicating health behaviors as an important factor for promoting brain health with age. Specifically, sleep, exercise, eating habits, social engagement, and cognitive stimulation have been linked to improved cognitive functioning.2-8 In addition to the potential benefits for brain health, there is evidence that greater investment in attaining health goals is associated with subjective reports of higher well-being, fewer mental health symptoms, lower physical health stresses, decreased caregiver burden, and increased functional independence linked with longer independent living.9 The latter has a substantial financial impact, such that the positive consequence of increased independence is likely staving off the need for admission to assisted living and adult family homes, which can be costly.
Despite the role of health behaviors in brain aging and overall health and functioning, research indicates that only a small number of older adults (12.8%) follow recommended guidelines for healthy lifestyle factors.10 Education has been identified as one factor associated with the likelihood of engaging in positive health behaviors, prompting the delivery of health-education interventions. Most psychoeducational interventions have traditionally focused on one aspect of behavior change at a time (eg, sleep); however, Gross and colleaguesconducted a meta-analysis of cognitive interventions and in addition to the overall positive benefits (effect size 0.38), they also found suggestive evidence that interventions that combined multiple training strategies were associated with larger training gains (P = .04) after adjusting for multiple comparisons.11 For example, Miller and colleagues found a significant improvement on both subjective and objective measures of memory following a multicomponent approach that combined training in memory skills, stress reduction, nutrition, and physical activity.12
In addition to the potential positive impacts of health behaviors on brain health, findings suggest that targeted emphasis on health behavior change may have the potential to stave off mild cognitiveimpairment (MCI) or dementia even if for a short time. Given the increasing prevalence rates of MCI with age (6.7% in adults aged 60-64 years, reaching 25.2% in adults aged 80-84 years13) and dementia (prevalence of MCI converting to dementia is 18-40%14), as well as the corresponding emotional, financial, and family-oriented consequences (eg, impact on the well-being of family caregivers), the need for behavioral interventions that seek to optimize brain health is becoming increasingly apparent.
More than 9 million veterans are now aged ≥ 65 years.15 In addition to representing nearly half of all veterans and a sizable portion of aging adults in the US, older veterans are at increased risk of frailty, mortality, and high rates of chronic medical/mental health conditions that can lead to accelerated cognitive aging.6-17 Together, these conditions highlight the importance of developing comprehensive psychoeducational and behavioral interventions in this population. To address this need, we developed a novel psychoeducation and behavior change group called the Healthy Aging Project-Brain (HAP-B, pronounced “happy”). The HAP-B intervention was designed to promote healthy brain aging by using empirically supported health behavior change strategies, including education, personalized goal setting, and community support. The primary aim of this project was to develop and implement an intervention that was feasible and acceptable (eg, could be implemented in our setting, was appropriate for a veteran population) and to determine any positive outcomes/preliminary effects on overall health and well-being.
Methods
We recruited veterans aged ≥ 50 years through primary care clinics and self-referrals via flyers in the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS), Seattle Division hospital. We targeted the “worried well” and welcomed veterans with MCI and mental health diagnoses. Notably, if there were significant mental health and/or substance use concerns, we encouraged veterans to seek focused care and stabilization prior to or concurrent with group participation. Exclusion criteria included presence of suicidality/homicidality, untreated or unstable substance use disorder, or a diagnosis of dementia. Exclusion criteria were assessed by the referring health care providers (HCPs), when appropriate, and through a health record review. Group facilitators used their clinical judgment to monitor participants if they began experiencing more severe cognitive impairment or acute mental health concerns. Although we did not encounter any of these instances, facilitators were prepared to discuss any concerns with the veteran and their referring HCP. Participants sampled were from 1 of 5 groups offered between January 2018 and March 2019. A waiver from the institutional review board was obtained after meeting criteria for quality improvement/quality assurance (QI/QA) for this study.
Procedures
At the initial stages of development, our team conducted a needs assessment to identify health-related areas where HCPs felt veterans would benefit from additional education and support. The needs assessment was conducted across primary care, geriatric extended care, and the Geriatric Research, Education, and Clinical Center (GRECC) at VAPSHCS. Combining the needs assessment results with the available research base, we identified sleep, physical activity, social engagement, and cognitive stimulation as areas for focus. Notably, although nutrition has been identified as an important factor in cognitive aging, a diet and nutrition class was already available to older veterans at the Seattle VA; hence, we chose to limit overlap by not covering this topic in our group.
The group was offered on a quarterly basis as six 90-minute psychoeducational classes to allow time for didactics, discussion, and practice without overloading participants with information. Each group consisted of 4 to 9 veterans led by 2 cofacilitators. Group structure allowed for feedback and ideas from group members as well as accountability for engaging in behavior change. Cognitive functioning was not formally evaluated. Attendees were asked but not required to complete questionnaires before the classes began and again at completion. In addition at the completion of each group, feedback was collected from veterans and used to modify group content (Figure).
Two pilot groups were implemented in early and mid-2018 with iterative changes after each group. Then we revised the assessment battery and implemented the current version (v1.0), which was first offered in the fall of 2018 and was used with the final 3 groups. Noteworthy changes included weekly check-ins to assess use of health behavior logs and progress toward individual goals, additional pre-and postgroup measures, and in vivo skills practice relevant to the topic being discussed that day.
Each session began with a check-in, which included a review of daily logs and SMART (specific, measurable, attainable, relevant/realistic, and timebound) goals from the previous week.18 This allowed for praise/reinforcement of health behaviors as well as discussion of potential barriers. Second, an overview of research focusing on the relationship between aging, brain health, and the topic of the day was presented. As an example, in the discussion of social engagement, research was presented about the link between social isolation and cognitive decline; the indirect benefits of social support (eg, social support is linked to improved physical and mental health, which, in turn, is associated with less cognitive decline); and the direct benefits of social support (eg, high levels of emotional support are associated with better cognitive function) (Table 1).6
Next, facilitators reviewed skills and strategies to improve functioning in the topic of discussion. During the social engagement group, for example, facilitators discussed tips to improve social skills (eg, asking open-ended questions) and how to build social support into a daily routine (eg, scheduling weekly phone calls with family and friends). Following this discussion of skills, an activity was practiced, reinforcing learned material. During the social engagement group, veterans were invited to use small talk strategies with fellow group members. Finally, group sessions ended with each participant identifying a SMART goal for the coming week and troubleshooting potential barriers to success. SMART goals were kept broad, so veterans could choose a goal related to the topic discussed at the group that day (eg, scheduling a phone call with a friend twice in the coming week during the social engagement-focused group) or choose any other goal to focus on (eg, a sleep-related goal). Similarly, goals could change week to week, or could remain the same throughout the 6-week classes.
Measures
The questionnaires used for QI/QA analyses included the Satisfaction with Life Scale (SWLS); Geriatric Depression Scale-Short Form (GDS-S); Social Support Survey Instrument (SSSI); Pittsburg Sleep Quality Index (PSQI); Medical Outcomes Survey-Short Form (MOS-36 SF); and a self-efficacy scale (adapted from Huckans and colleagues for traumatic brain injury).19-24 Written feedback was collected at the end of the last group to assess perception of progress, self-perceived behavior change, what was helpful or unhelpful, and how likely the participants were to recommend the group to other veterans (0 to 3, very unlikely to very likely).
To promote consistency with other health and behavior change interventions at the VA, HAP-B used resources from the Whole Health model SMART goals. Research supports the use of self-monitoring techniques like SMART goals for behavior change.25
To facilitate skills practice and self-monitoring between classes, veterans were asked to complete 2 homework assignments. First, at the end of each group, each veteran identified a specific SMART goal to focus on and track in the coming week. Goals were unique to each veteran and allowed to change from week to week. Group discussion around SMART goals involved plans for how to address potential barriers; progress toward goals was discussed at the beginning of the following group. Second, veterans were asked to complete a worksheet used to track progress toward the weekly SMART goal and the specific health behaviors related to the 4 domains targeted by HAP-B. For example, when tracking sleep behaviors, veterans noted bedtime, waketime, number of times they woke up during the night, and length of daytime naps if applicable. Tracking logs were provided at the end of each class for personal purposes only. We asked veterans to rate themselves each week on whether they used the tracking sheet to monitor health behaviors; and how successful they were at accomplishing their previously identified SMART goal. We recorded responses on a 0 to 2 scale (0, not good; 1, fair; 2, good). This rating system was developed and implemented in later groups to promote self-monitoring, accountability, and discussion of potential barriers. However, due to the small sample that completed these ratings and the absence of objective corroborating data, these ratings were not included in the current analyses.
Every participant received a manual in binder format, which provided the didactic information for each group session, skills and strategies discussed in each session, and relevant resources in both the VA and community. For example, social engagement resources included information about volunteer opportunities, VA groups that focus on developing interpersonal skills, and recommendations from past group members on social events (eg, dance lessons at a senior center). We also developed a facilitator version of the manual in which we added comments and guidance on topics for discussion. Materials were developed with the goal of optimizing the ease of dissemination to other sites.
Results
Across the 5 groups, 31 veterans enrolled as participants and completed the initial intake measures, with an average of 6 participants per group (range 4-9). The majority (80%) attended at least 5 of the 6 classes. The mean
At the start of the class, the mean (SD) reports of participants were mild depressive symptoms 5.96 (3.8) on the GDS scale, moderate levels of self-efficacy 3.69 (0.5) on the self-efficacy scale, and moderate levels of satisfaction with life 18.08 (6.8) on the SWLS scale (Table 2). Data from 25 of 31 veterans who completed both pregroup and postgroup surveys were analyzed and paired samples t tests without corrections indicated a reduction in depressive symptoms (P = .01), improved self-efficacy (P = .08), and improved satisfaction with life (P = .03). There were no significant differences in self-reported sleep quality or perceived social support from pregroup to postgroup evaluations. Because the sample size was smaller for the MOS-36, which was not used until group 3, and the subscales are composed of few items each, we conducted exploratory analyses of the 8 MOS-36 subscales and found that well-being, physical functioning, role limitations due to physical and emotional functioning, and energy/fatigue significantly improved over time (Ps < .04).
Twenty-eight veterans provided written feedback following the final session. Qualitative feedback received at the completion of the group focused on participants’ desire for increased number of classes, longer sessions (eg, 2 participants recommended lengthening the group to 2 hours), and integrating mindfulness-based activities into each class. Participants rated themselves somewhat likely to very likely to recommend this group to other veterans (mean, 2.9 [SD, 0.4]).
Discussion
The ability and need to promote brain health with age is an emerging priority as our aging population grows. A growing body of evidence supports the role of health behaviors in healthy brain aging. Education and skills training in a group setting provides a supportive, cost-effective approach for increasing overall health in aging adults. Yet older adults are statistically less likely to engage in these behaviors on a regular basis. The current investigation provides preliminary support for a model of care that uses a comprehensive, experiential psychoeducational approach to facilitate behavior change in older adults. Our aim was to develop and implement an intervention that was feasible and acceptable to our older veterans and to determine any positive outcomes/preliminary effects on overall health and well-being.
Participants indicated that they enjoyed the group, learned new skills (per participant feedback and facilitator observation), and experienced improvements in mood, self-efficacy, and life satisfaction. Given the participants’ positive response to the group and its content, as well as continued referrals by HCPs to this group and low difficulty with ongoing recruitment, this program was deemed both feasible and acceptable in our veteran health care setting. Questions remain about the extent to which participants modified their health behaviors given that we did not collect objective measurements of behaviors (eg, time spent exercising), the duration of behavior change (ie, how long during and after the group were behaviors maintained), and the role of premorbid or concurrent characteristics that may moderate the effect of the intervention on health-related outcomes (eg, sleep quality, perceived social support, overall functioning, concurrent interventions, medications).
Strengths and Limitations
This study had a limited sample size and no control group. However, evidence of significant improvements in depressive symptoms, self-efficacy, and life satisfaction in the development groups without a control group is encouraging. This is particularly noteworthy given that older veterans as a group have higher rates of frailty and mortality than do other similarly aged counterparts.17An additional weakness is the absence of a brief cognitive assessment or other formal assessment as part of the inclusion/exclusion criteria. However, this program development project provides data from a realistic condition (recruited broadly and with few exclusions, offered in similar format as other VA classes), thus adding strength to the interpretation and possibly the generalizability of these findings.
Conclusions
Future directions include disseminating HAP-B materials and procedures across a variety of sites, both VA and non-VA. In line with this goal, we hope to increase sample size and sample diversity while optimizing protocol integrity during the exportation phase. With a greater sample size and power, we aim to examine the role of self-efficacy and other premorbid factors (eg, cognitive functioning at baseline) as mediators for observed changes in pre-/postmeasures and outcomes. We also hope to incorporate objective measures of behavior change, such as fitness trackers, heart rate/pulse monitors, and actigraphy for monitoring sleep. Finally, we are interested in conducting follow-up with past and future participants to detect changes that may occur with learning new skills following the completion of the group (eg, changes in sleep behavior that take time to take effect) and the extent to which participants continue to use the health behavior skills and strategies to maintain or enhance progress in behavioral goals. Finally, although this intervention was initially designed for use with older veterans receiving health care through the VA, we believe the concepts and work products described here can be used with older adults across a wide range of health care settings. Providers interested in trialing HAP-B at their local site are encouraged to contact the authors.
1. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-20.
2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):85-592. doi:10.1093/sleep/33.5.585
3. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12-31. doi:10.1016/j.arr.2014.05.002
4. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251-1257. doi:10.1001/archinternmed.2011.277
5. World Health Organization. Interventions on diet and physical activity: what works. https://www.who.int/dietphysicalactivity/whatworks/en/. Published 2009. Accessed June 19, 2020.
6. Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243-255. doi:10.1037//0278-6133.20.4.243
7. La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation and cognitive exercises. Clin Geriatr Med. 2010;26(1):99-111. doi:10.1016/j.cger.2009.11.003
8. Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychol Aging. 2002;17(4):548-557. doi:10.1037//0882-7974.17.4.548
9. Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: the importance of health engagement control strategies. Health Psychol. 2002;21(4):340-348. doi:10.1037//0278-6133.21.4.340
10. Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27(suppl 2):25-33. doi:10.1016/j.amepre.2004.04.022
11. Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health. 2012;16(6):722-734. doi:10.1080/13607863.2012.667783
12. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J Geriat Psychiatry. 2012;20(6):514-523. doi:10.1097/JGP.0b013e318227f821
13. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
14. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi:10.1016/S0140-6736(06)68542-5
15. US Department of Veteran Affairs, National Center for Veteran Analysis and Statistics.Veteran population. 2020. https://www.va.gov/vetdata/Veteran_Population.asp. Updated May 21, 2020 . Accessed June 17, 2020.
16. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique healthcare needs of the patient population served by the Department of Veterans Affairs. RAND Health Q. 2016;5(4):13.
17. Orkaby AR, Nussbaum L, Ho Y, et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
18. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70(11):35-36.
19. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi:10.1207/s15327752jpa4901-13
20. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165-173. doi:10.1300/J018v05n01_09
21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi:10.1016/0277-9536(91)90150-b
22. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4
23. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
24. Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43-60. doi:10.1682/jrrd.2009.02.0019
25. Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. doi:10.1016/j.pec.2011.07.018
1. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-20.
2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):85-592. doi:10.1093/sleep/33.5.585
3. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12-31. doi:10.1016/j.arr.2014.05.002
4. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251-1257. doi:10.1001/archinternmed.2011.277
5. World Health Organization. Interventions on diet and physical activity: what works. https://www.who.int/dietphysicalactivity/whatworks/en/. Published 2009. Accessed June 19, 2020.
6. Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243-255. doi:10.1037//0278-6133.20.4.243
7. La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation and cognitive exercises. Clin Geriatr Med. 2010;26(1):99-111. doi:10.1016/j.cger.2009.11.003
8. Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychol Aging. 2002;17(4):548-557. doi:10.1037//0882-7974.17.4.548
9. Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: the importance of health engagement control strategies. Health Psychol. 2002;21(4):340-348. doi:10.1037//0278-6133.21.4.340
10. Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27(suppl 2):25-33. doi:10.1016/j.amepre.2004.04.022
11. Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health. 2012;16(6):722-734. doi:10.1080/13607863.2012.667783
12. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J Geriat Psychiatry. 2012;20(6):514-523. doi:10.1097/JGP.0b013e318227f821
13. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
14. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi:10.1016/S0140-6736(06)68542-5
15. US Department of Veteran Affairs, National Center for Veteran Analysis and Statistics.Veteran population. 2020. https://www.va.gov/vetdata/Veteran_Population.asp. Updated May 21, 2020 . Accessed June 17, 2020.
16. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique healthcare needs of the patient population served by the Department of Veterans Affairs. RAND Health Q. 2016;5(4):13.
17. Orkaby AR, Nussbaum L, Ho Y, et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
18. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70(11):35-36.
19. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi:10.1207/s15327752jpa4901-13
20. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165-173. doi:10.1300/J018v05n01_09
21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi:10.1016/0277-9536(91)90150-b
22. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4
23. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
24. Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43-60. doi:10.1682/jrrd.2009.02.0019
25. Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. doi:10.1016/j.pec.2011.07.018
Does moderate drinking slow cognitive decline?
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
Epilepsy after TBI linked to worse 12-month outcomes
findings from an analysis of a large, prospective database suggest. “We found that patients essentially have a 10-times greater risk of developing posttraumatic epilepsy and seizures at 12 months [post injury] if the presenting Glasgow Coma Scale GCS) is less than 8,” said lead author John F. Burke, MD, PhD, University of California, San Francisco, in presenting the findings as part of the virtual annual meeting of the American Association of Neurological Surgeons.
Assessing risk factors
While posttraumatic epilepsy represents an estimated 20% of all cases of symptomatic epilepsy, many questions remain on those most at risk and on the long-term effects of posttraumatic epilepsy on TBI outcomes. To probe those issues, Dr. Burke and colleagues turned to the multicenter TRACK-TBI database, which has prospective, longitudinal data on more than 2,700 patients with traumatic brain injuries and is considered the largest source of prospective data on posttraumatic epilepsy.
Using the criteria of no previous epilepsy and having 12 months of follow-up, the team identified 1,493 patients with TBI. In addition, investigators identified 182 orthopedic controls (included and prospectively followed because they have injuries but not specifically head trauma) and 210 controls who are friends of the patients and who do not have injuries but allow researchers to control for socioeconomic and environmental factors.
Of the 1,493 patients with TBI, 41 (2.7%) were determined to have posttraumatic epilepsy, assessed according to a National Institute of Neurological Disorders and Stroke epilepsy screening questionnaire, which is designed to identify patients with posttraumatic epilepsy symptoms. There were no reports of epilepsy symptoms using the screening tool among the controls. Dr. Burke noted that the 2.7% was in agreement with historical reports.
In comparing patients with TBI who did and did not have posttraumatic epilepsy, no differences were observed in the groups in terms of gender, although there was a trend toward younger age among those with PTE (mean age, 35.4 years with posttraumatic injury vs. 41.5 without; P = .05).
A major risk factor for the development of posttraumatic epilepsy was presenting GCS scores. Among those with scores of less than 8, indicative of severe injury, the rate of posttraumatic epilepsy was 6% at 6 months and 12.5% at 12 months. In contrast, those with TBI presenting with GCS scores between 13 and 15, indicative of minor injury, had an incidence of posttraumatic epilepsy of 0.9% at 6 months and 1.4% at 12 months.
Imaging findings in the two groups showed that hemorrhage detected on CT imaging was associated with a significantly higher risk for posttraumatic epilepsy (P < .001).
“The main takeaway is that any hemorrhage in the brain is a major risk factor for developing seizures,” Dr. Burke said. “Whether it is subdural, epidural blood, subarachnoid or contusion, any blood confers a very [high] risk for developing seizures.”
Posttraumatic epilepsy was linked to poorer longer-term outcomes even for patients with lesser injury: Among those with TBI and GCS of 13-15, the mean Glasgow Outcome Scale Extended (GOSE) score at 12 months among those without posttraumatic epilepsy was 7, indicative of a good recovery with minor defects, whereas the mean GOSE score for those with PTE was 4.6, indicative of moderate to severe disability (P < .001).
“It was surprising to us that PTE-positive patients had a very significant decrease in GOSE, compared to PTE-negative patients,” Dr. Burke said. “There was a nearly 2-point drop in the GOSE and that was extremely significant.”
A multivariate analysis showed there was still a significant independent risk for a poor GOSE score with posttraumatic epilepsy after controlling for GCS score, head CT findings, and age (P < .001).
The authors also looked at mood outcomes using the Brief Symptom Inventory–18, which showed significant worse effect in those with posttraumatic epilepsy after multivariate adjustment (P = .01). Additionally, a highly significant worse effect in cognitive outcomes on the Rivermead cognitive metric was observed with posttraumatic epilepsy (P = .001).
“On all metrics tested, posttraumatic epilepsy worsened outcomes,” Dr. Burke said.
He noted that the study has some key limitations, including the 12-month follow-up. A previous study showed a linear increase in posttraumatic follow-up up to 30 years. “The fact that we found 41 patients at 12 months indicates there are probably more that are out there who are going to develop seizures, but because we don’t have the follow-up we can’t look at that.”
Although the screening questionnaires are effective, “the issue is these people are not being seen by an epileptologist or having scalp EEG done, and we need a more accurate way to do this,” he said. A new study, TRACK-TBI EPI, will address those limitations and a host of other issues with a 5-year follow-up.
Capturing the nuances of brain injury
Commenting on the study as a discussant, neurosurgeon Uzma Samadani, MD, PhD, of the Minneapolis Veterans Affairs Medical Center and CentraCare in Minneapolis, suggested that the future work should focus on issues including the wide-ranging mechanisms that could explain the seizure activity.
“For example, it’s known that posttraumatic epilepsy or seizures can be triggered by abnormal conductivity due to multiple different mechanisms associated with brain injury, such as endocrine dysfunction, cortical-spreading depression, and many others,” said Dr. Samadani, who has been a researcher on the TRACK-TBI study.
Factors ranging from genetic differences to comorbid conditions such as alcoholism can play a role in brain injury susceptibility, Dr. Samadani added. Furthermore, outcome measures currently available simply may not capture the unknown nuances of brain injury.
“We have to ask, are these an all-or-none phenomena, or is aberrant electrical activity after brain injury a continuum of dysfunction?” Dr. Samadani speculated.
“I would caution that we are likely underestimating the non–easily measurable consequences of brain injury,” she said. “And the better we can quantitate susceptibility, classify the nature of injury and target acute management, the less posttraumatic epilepsy/aberrant electrical activity our patients will have.”
Dr. Burke and Dr. Samadani disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
findings from an analysis of a large, prospective database suggest. “We found that patients essentially have a 10-times greater risk of developing posttraumatic epilepsy and seizures at 12 months [post injury] if the presenting Glasgow Coma Scale GCS) is less than 8,” said lead author John F. Burke, MD, PhD, University of California, San Francisco, in presenting the findings as part of the virtual annual meeting of the American Association of Neurological Surgeons.
Assessing risk factors
While posttraumatic epilepsy represents an estimated 20% of all cases of symptomatic epilepsy, many questions remain on those most at risk and on the long-term effects of posttraumatic epilepsy on TBI outcomes. To probe those issues, Dr. Burke and colleagues turned to the multicenter TRACK-TBI database, which has prospective, longitudinal data on more than 2,700 patients with traumatic brain injuries and is considered the largest source of prospective data on posttraumatic epilepsy.
Using the criteria of no previous epilepsy and having 12 months of follow-up, the team identified 1,493 patients with TBI. In addition, investigators identified 182 orthopedic controls (included and prospectively followed because they have injuries but not specifically head trauma) and 210 controls who are friends of the patients and who do not have injuries but allow researchers to control for socioeconomic and environmental factors.
Of the 1,493 patients with TBI, 41 (2.7%) were determined to have posttraumatic epilepsy, assessed according to a National Institute of Neurological Disorders and Stroke epilepsy screening questionnaire, which is designed to identify patients with posttraumatic epilepsy symptoms. There were no reports of epilepsy symptoms using the screening tool among the controls. Dr. Burke noted that the 2.7% was in agreement with historical reports.
In comparing patients with TBI who did and did not have posttraumatic epilepsy, no differences were observed in the groups in terms of gender, although there was a trend toward younger age among those with PTE (mean age, 35.4 years with posttraumatic injury vs. 41.5 without; P = .05).
A major risk factor for the development of posttraumatic epilepsy was presenting GCS scores. Among those with scores of less than 8, indicative of severe injury, the rate of posttraumatic epilepsy was 6% at 6 months and 12.5% at 12 months. In contrast, those with TBI presenting with GCS scores between 13 and 15, indicative of minor injury, had an incidence of posttraumatic epilepsy of 0.9% at 6 months and 1.4% at 12 months.
Imaging findings in the two groups showed that hemorrhage detected on CT imaging was associated with a significantly higher risk for posttraumatic epilepsy (P < .001).
“The main takeaway is that any hemorrhage in the brain is a major risk factor for developing seizures,” Dr. Burke said. “Whether it is subdural, epidural blood, subarachnoid or contusion, any blood confers a very [high] risk for developing seizures.”
Posttraumatic epilepsy was linked to poorer longer-term outcomes even for patients with lesser injury: Among those with TBI and GCS of 13-15, the mean Glasgow Outcome Scale Extended (GOSE) score at 12 months among those without posttraumatic epilepsy was 7, indicative of a good recovery with minor defects, whereas the mean GOSE score for those with PTE was 4.6, indicative of moderate to severe disability (P < .001).
“It was surprising to us that PTE-positive patients had a very significant decrease in GOSE, compared to PTE-negative patients,” Dr. Burke said. “There was a nearly 2-point drop in the GOSE and that was extremely significant.”
A multivariate analysis showed there was still a significant independent risk for a poor GOSE score with posttraumatic epilepsy after controlling for GCS score, head CT findings, and age (P < .001).
The authors also looked at mood outcomes using the Brief Symptom Inventory–18, which showed significant worse effect in those with posttraumatic epilepsy after multivariate adjustment (P = .01). Additionally, a highly significant worse effect in cognitive outcomes on the Rivermead cognitive metric was observed with posttraumatic epilepsy (P = .001).
“On all metrics tested, posttraumatic epilepsy worsened outcomes,” Dr. Burke said.
He noted that the study has some key limitations, including the 12-month follow-up. A previous study showed a linear increase in posttraumatic follow-up up to 30 years. “The fact that we found 41 patients at 12 months indicates there are probably more that are out there who are going to develop seizures, but because we don’t have the follow-up we can’t look at that.”
Although the screening questionnaires are effective, “the issue is these people are not being seen by an epileptologist or having scalp EEG done, and we need a more accurate way to do this,” he said. A new study, TRACK-TBI EPI, will address those limitations and a host of other issues with a 5-year follow-up.
Capturing the nuances of brain injury
Commenting on the study as a discussant, neurosurgeon Uzma Samadani, MD, PhD, of the Minneapolis Veterans Affairs Medical Center and CentraCare in Minneapolis, suggested that the future work should focus on issues including the wide-ranging mechanisms that could explain the seizure activity.
“For example, it’s known that posttraumatic epilepsy or seizures can be triggered by abnormal conductivity due to multiple different mechanisms associated with brain injury, such as endocrine dysfunction, cortical-spreading depression, and many others,” said Dr. Samadani, who has been a researcher on the TRACK-TBI study.
Factors ranging from genetic differences to comorbid conditions such as alcoholism can play a role in brain injury susceptibility, Dr. Samadani added. Furthermore, outcome measures currently available simply may not capture the unknown nuances of brain injury.
“We have to ask, are these an all-or-none phenomena, or is aberrant electrical activity after brain injury a continuum of dysfunction?” Dr. Samadani speculated.
“I would caution that we are likely underestimating the non–easily measurable consequences of brain injury,” she said. “And the better we can quantitate susceptibility, classify the nature of injury and target acute management, the less posttraumatic epilepsy/aberrant electrical activity our patients will have.”
Dr. Burke and Dr. Samadani disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
findings from an analysis of a large, prospective database suggest. “We found that patients essentially have a 10-times greater risk of developing posttraumatic epilepsy and seizures at 12 months [post injury] if the presenting Glasgow Coma Scale GCS) is less than 8,” said lead author John F. Burke, MD, PhD, University of California, San Francisco, in presenting the findings as part of the virtual annual meeting of the American Association of Neurological Surgeons.
Assessing risk factors
While posttraumatic epilepsy represents an estimated 20% of all cases of symptomatic epilepsy, many questions remain on those most at risk and on the long-term effects of posttraumatic epilepsy on TBI outcomes. To probe those issues, Dr. Burke and colleagues turned to the multicenter TRACK-TBI database, which has prospective, longitudinal data on more than 2,700 patients with traumatic brain injuries and is considered the largest source of prospective data on posttraumatic epilepsy.
Using the criteria of no previous epilepsy and having 12 months of follow-up, the team identified 1,493 patients with TBI. In addition, investigators identified 182 orthopedic controls (included and prospectively followed because they have injuries but not specifically head trauma) and 210 controls who are friends of the patients and who do not have injuries but allow researchers to control for socioeconomic and environmental factors.
Of the 1,493 patients with TBI, 41 (2.7%) were determined to have posttraumatic epilepsy, assessed according to a National Institute of Neurological Disorders and Stroke epilepsy screening questionnaire, which is designed to identify patients with posttraumatic epilepsy symptoms. There were no reports of epilepsy symptoms using the screening tool among the controls. Dr. Burke noted that the 2.7% was in agreement with historical reports.
In comparing patients with TBI who did and did not have posttraumatic epilepsy, no differences were observed in the groups in terms of gender, although there was a trend toward younger age among those with PTE (mean age, 35.4 years with posttraumatic injury vs. 41.5 without; P = .05).
A major risk factor for the development of posttraumatic epilepsy was presenting GCS scores. Among those with scores of less than 8, indicative of severe injury, the rate of posttraumatic epilepsy was 6% at 6 months and 12.5% at 12 months. In contrast, those with TBI presenting with GCS scores between 13 and 15, indicative of minor injury, had an incidence of posttraumatic epilepsy of 0.9% at 6 months and 1.4% at 12 months.
Imaging findings in the two groups showed that hemorrhage detected on CT imaging was associated with a significantly higher risk for posttraumatic epilepsy (P < .001).
“The main takeaway is that any hemorrhage in the brain is a major risk factor for developing seizures,” Dr. Burke said. “Whether it is subdural, epidural blood, subarachnoid or contusion, any blood confers a very [high] risk for developing seizures.”
Posttraumatic epilepsy was linked to poorer longer-term outcomes even for patients with lesser injury: Among those with TBI and GCS of 13-15, the mean Glasgow Outcome Scale Extended (GOSE) score at 12 months among those without posttraumatic epilepsy was 7, indicative of a good recovery with minor defects, whereas the mean GOSE score for those with PTE was 4.6, indicative of moderate to severe disability (P < .001).
“It was surprising to us that PTE-positive patients had a very significant decrease in GOSE, compared to PTE-negative patients,” Dr. Burke said. “There was a nearly 2-point drop in the GOSE and that was extremely significant.”
A multivariate analysis showed there was still a significant independent risk for a poor GOSE score with posttraumatic epilepsy after controlling for GCS score, head CT findings, and age (P < .001).
The authors also looked at mood outcomes using the Brief Symptom Inventory–18, which showed significant worse effect in those with posttraumatic epilepsy after multivariate adjustment (P = .01). Additionally, a highly significant worse effect in cognitive outcomes on the Rivermead cognitive metric was observed with posttraumatic epilepsy (P = .001).
“On all metrics tested, posttraumatic epilepsy worsened outcomes,” Dr. Burke said.
He noted that the study has some key limitations, including the 12-month follow-up. A previous study showed a linear increase in posttraumatic follow-up up to 30 years. “The fact that we found 41 patients at 12 months indicates there are probably more that are out there who are going to develop seizures, but because we don’t have the follow-up we can’t look at that.”
Although the screening questionnaires are effective, “the issue is these people are not being seen by an epileptologist or having scalp EEG done, and we need a more accurate way to do this,” he said. A new study, TRACK-TBI EPI, will address those limitations and a host of other issues with a 5-year follow-up.
Capturing the nuances of brain injury
Commenting on the study as a discussant, neurosurgeon Uzma Samadani, MD, PhD, of the Minneapolis Veterans Affairs Medical Center and CentraCare in Minneapolis, suggested that the future work should focus on issues including the wide-ranging mechanisms that could explain the seizure activity.
“For example, it’s known that posttraumatic epilepsy or seizures can be triggered by abnormal conductivity due to multiple different mechanisms associated with brain injury, such as endocrine dysfunction, cortical-spreading depression, and many others,” said Dr. Samadani, who has been a researcher on the TRACK-TBI study.
Factors ranging from genetic differences to comorbid conditions such as alcoholism can play a role in brain injury susceptibility, Dr. Samadani added. Furthermore, outcome measures currently available simply may not capture the unknown nuances of brain injury.
“We have to ask, are these an all-or-none phenomena, or is aberrant electrical activity after brain injury a continuum of dysfunction?” Dr. Samadani speculated.
“I would caution that we are likely underestimating the non–easily measurable consequences of brain injury,” she said. “And the better we can quantitate susceptibility, classify the nature of injury and target acute management, the less posttraumatic epilepsy/aberrant electrical activity our patients will have.”
Dr. Burke and Dr. Samadani disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AANS 2020
Use of nonopioid pain meds is on the rise
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Higher stroke rates seen among patients with COVID-19 compared with influenza
, according to a retrospective cohort study conducted at New York–Presbyterian Hospital and Weill Cornell Medicine, New York. “These findings suggest that clinicians should be vigilant for symptoms and signs of acute ischemic stroke in patients with COVID-19 so that time-sensitive interventions, such as thrombolysis and thrombectomy, can be instituted if possible to reduce the burden of long-term disability,” wrote Alexander E. Merkler and colleagues. Their report is in JAMA Neurology.
While several recent publications have “raised the possibility” of this link, none have had an appropriate control group, noted Dr. Merkler of the department of neurology, Weill Cornell Medicine. “Further elucidation of thrombotic mechanisms in patients with COVID-19 may yield better strategies to prevent disabling thrombotic complications like ischemic stroke,” he added.
An increased risk of stroke
The study included 1,916 adults with confirmed COVID-19 (median age 64 years) who were either hospitalized or visited an emergency department between March 4 and May 2, 2020. These cases were compared with a historical cohort of 1,486 patients (median age 62 years) who were hospitalized with laboratory-confirmed influenza A or B between January 1, 2016, and May 31, 2018.
Among the patients with COVID-19, a diagnosis of cerebrovascular disease during hospitalization, a brain computed tomography (CT), or brain magnetic resonance imaging (MRI) was an indication of possible ischemic stroke. These records were then independently reviewed by two board-certified attending neurologists (with a third resolving any disagreement) to adjudicate a final stroke diagnosis. In the influenza cohort, the Cornell Acute Stroke Academic Registry (CAESAR) was used to ascertain ischemic strokes.
The study identified 31 patients with stroke among the COVID-19 cohort (1.6%; 95% confidence interval, 1.1%-2.3%) and 3 in the influenza cohort (0.2%; 95% CI, 0.0%-0.6%). After adjustment for age, sex, and race, stroke risk was almost 8 times higher in the COVID-19 cohort (OR, 7.6; 95% CI, 2.3-25.2).
This association “persisted across multiple sensitivity analyses, with the magnitude of relative associations ranging from 4.0 to 9,” wrote the authors. “This included a sensitivity analysis that adjusted for the number of vascular risk factors and ICU admissions (OR, 4.6; 95% CI, 1.4-15.7).”
The median age of patients with COVID-19 and stroke was 69 years, and the median duration of COVID-19 symptom onset to stroke diagnosis was 16 days. Stroke symptoms were the presenting complaint in only 26% of the patients, while the remainder developing stroke while hospitalized, and more than a third (35%) of all strokes occurred in patients who were mechanically ventilated with severe COVID-19. Inpatient mortality was considerably higher among patients with COVID-19 with stroke versus without (32% vs. 14%; P = .003).
In patients with COVID-19 “most ischemic strokes occurred in older age groups, those with traditional stroke risk factors, and people of color,” wrote the authors. “We also noted that initial plasma D-dimer levels were nearly 3-fold higher in those who received a diagnosis of ischemic stroke than in those who did not” (1.930 mcg/mL vs. 0.682 mcg/mL).
The authors suggested several possible explanations for the elevated risk of stroke in COVID-19. Acute viral illnesses are known to trigger inflammation, and COVID-19 in particular is associated with “a vigorous inflammatory response accompanied by coagulopathy, with elevated D-dimer levels and the frequent presence of antiphospholipid antibodies,” they wrote. The infection is also associated with more severe respiratory syndrome compared with influenza, as well as a heightened risk for complications such as atrial arrhythmias, myocardial infarction, heart failure, myocarditis, and venous thromboses, all of which likely contribute to the risk of ischemic stroke.”
COVID or conventional risk factors?
Asked to comment on the study, Benedict Michael, MBChB (Hons), MRCP (Neurol), PhD, from the United Kingdom’s Coronerve Studies Group, a collaborative initiative to study the neurological features of COVID-19, said in an interview that “this study suggests many cases of stroke are occurring in older patients with multiple existing conventional and well recognized risks for stroke, and may simply represent decompensation during sepsis.”
Dr. Michael, a senior clinician scientist fellow at the University of Liverpool and an honorary consultant neurologist at the Walton Centre, was the senior author on a recently published UK-wide surveillance study on the neurological and neuropsychiatric complications of COVID-19 (Lancet Psychiatry. 2020 Jun 25. doi: 10.1016/S2215-0366[20]30287-X).
He said among patients in the New York study, “those with COVID and a stroke appeared to have many conventional risk factors for stroke (and often at higher percentages than COVID patients without a stroke), e.g. hypertension, overweight, diabetes, hyperlipidemia, existing vascular disease affecting the coronary arteries and atrial fibrillation. To establish evidence-based treatment pathways, clearly further studies are needed to determine the biological mechanisms underlying the seemingly higher rate of stroke with COVID-19 than influenza; but this must especially focus on those younger patients without conventional risk factors for stroke (which are largely not included in this study).”
SOURCE: Merkler AE et al. JAMA Neurol. doi: 10.1001/jamaneurol.2020.2730.
, according to a retrospective cohort study conducted at New York–Presbyterian Hospital and Weill Cornell Medicine, New York. “These findings suggest that clinicians should be vigilant for symptoms and signs of acute ischemic stroke in patients with COVID-19 so that time-sensitive interventions, such as thrombolysis and thrombectomy, can be instituted if possible to reduce the burden of long-term disability,” wrote Alexander E. Merkler and colleagues. Their report is in JAMA Neurology.
While several recent publications have “raised the possibility” of this link, none have had an appropriate control group, noted Dr. Merkler of the department of neurology, Weill Cornell Medicine. “Further elucidation of thrombotic mechanisms in patients with COVID-19 may yield better strategies to prevent disabling thrombotic complications like ischemic stroke,” he added.
An increased risk of stroke
The study included 1,916 adults with confirmed COVID-19 (median age 64 years) who were either hospitalized or visited an emergency department between March 4 and May 2, 2020. These cases were compared with a historical cohort of 1,486 patients (median age 62 years) who were hospitalized with laboratory-confirmed influenza A or B between January 1, 2016, and May 31, 2018.
Among the patients with COVID-19, a diagnosis of cerebrovascular disease during hospitalization, a brain computed tomography (CT), or brain magnetic resonance imaging (MRI) was an indication of possible ischemic stroke. These records were then independently reviewed by two board-certified attending neurologists (with a third resolving any disagreement) to adjudicate a final stroke diagnosis. In the influenza cohort, the Cornell Acute Stroke Academic Registry (CAESAR) was used to ascertain ischemic strokes.
The study identified 31 patients with stroke among the COVID-19 cohort (1.6%; 95% confidence interval, 1.1%-2.3%) and 3 in the influenza cohort (0.2%; 95% CI, 0.0%-0.6%). After adjustment for age, sex, and race, stroke risk was almost 8 times higher in the COVID-19 cohort (OR, 7.6; 95% CI, 2.3-25.2).
This association “persisted across multiple sensitivity analyses, with the magnitude of relative associations ranging from 4.0 to 9,” wrote the authors. “This included a sensitivity analysis that adjusted for the number of vascular risk factors and ICU admissions (OR, 4.6; 95% CI, 1.4-15.7).”
The median age of patients with COVID-19 and stroke was 69 years, and the median duration of COVID-19 symptom onset to stroke diagnosis was 16 days. Stroke symptoms were the presenting complaint in only 26% of the patients, while the remainder developing stroke while hospitalized, and more than a third (35%) of all strokes occurred in patients who were mechanically ventilated with severe COVID-19. Inpatient mortality was considerably higher among patients with COVID-19 with stroke versus without (32% vs. 14%; P = .003).
In patients with COVID-19 “most ischemic strokes occurred in older age groups, those with traditional stroke risk factors, and people of color,” wrote the authors. “We also noted that initial plasma D-dimer levels were nearly 3-fold higher in those who received a diagnosis of ischemic stroke than in those who did not” (1.930 mcg/mL vs. 0.682 mcg/mL).
The authors suggested several possible explanations for the elevated risk of stroke in COVID-19. Acute viral illnesses are known to trigger inflammation, and COVID-19 in particular is associated with “a vigorous inflammatory response accompanied by coagulopathy, with elevated D-dimer levels and the frequent presence of antiphospholipid antibodies,” they wrote. The infection is also associated with more severe respiratory syndrome compared with influenza, as well as a heightened risk for complications such as atrial arrhythmias, myocardial infarction, heart failure, myocarditis, and venous thromboses, all of which likely contribute to the risk of ischemic stroke.”
COVID or conventional risk factors?
Asked to comment on the study, Benedict Michael, MBChB (Hons), MRCP (Neurol), PhD, from the United Kingdom’s Coronerve Studies Group, a collaborative initiative to study the neurological features of COVID-19, said in an interview that “this study suggests many cases of stroke are occurring in older patients with multiple existing conventional and well recognized risks for stroke, and may simply represent decompensation during sepsis.”
Dr. Michael, a senior clinician scientist fellow at the University of Liverpool and an honorary consultant neurologist at the Walton Centre, was the senior author on a recently published UK-wide surveillance study on the neurological and neuropsychiatric complications of COVID-19 (Lancet Psychiatry. 2020 Jun 25. doi: 10.1016/S2215-0366[20]30287-X).
He said among patients in the New York study, “those with COVID and a stroke appeared to have many conventional risk factors for stroke (and often at higher percentages than COVID patients without a stroke), e.g. hypertension, overweight, diabetes, hyperlipidemia, existing vascular disease affecting the coronary arteries and atrial fibrillation. To establish evidence-based treatment pathways, clearly further studies are needed to determine the biological mechanisms underlying the seemingly higher rate of stroke with COVID-19 than influenza; but this must especially focus on those younger patients without conventional risk factors for stroke (which are largely not included in this study).”
SOURCE: Merkler AE et al. JAMA Neurol. doi: 10.1001/jamaneurol.2020.2730.
, according to a retrospective cohort study conducted at New York–Presbyterian Hospital and Weill Cornell Medicine, New York. “These findings suggest that clinicians should be vigilant for symptoms and signs of acute ischemic stroke in patients with COVID-19 so that time-sensitive interventions, such as thrombolysis and thrombectomy, can be instituted if possible to reduce the burden of long-term disability,” wrote Alexander E. Merkler and colleagues. Their report is in JAMA Neurology.
While several recent publications have “raised the possibility” of this link, none have had an appropriate control group, noted Dr. Merkler of the department of neurology, Weill Cornell Medicine. “Further elucidation of thrombotic mechanisms in patients with COVID-19 may yield better strategies to prevent disabling thrombotic complications like ischemic stroke,” he added.
An increased risk of stroke
The study included 1,916 adults with confirmed COVID-19 (median age 64 years) who were either hospitalized or visited an emergency department between March 4 and May 2, 2020. These cases were compared with a historical cohort of 1,486 patients (median age 62 years) who were hospitalized with laboratory-confirmed influenza A or B between January 1, 2016, and May 31, 2018.
Among the patients with COVID-19, a diagnosis of cerebrovascular disease during hospitalization, a brain computed tomography (CT), or brain magnetic resonance imaging (MRI) was an indication of possible ischemic stroke. These records were then independently reviewed by two board-certified attending neurologists (with a third resolving any disagreement) to adjudicate a final stroke diagnosis. In the influenza cohort, the Cornell Acute Stroke Academic Registry (CAESAR) was used to ascertain ischemic strokes.
The study identified 31 patients with stroke among the COVID-19 cohort (1.6%; 95% confidence interval, 1.1%-2.3%) and 3 in the influenza cohort (0.2%; 95% CI, 0.0%-0.6%). After adjustment for age, sex, and race, stroke risk was almost 8 times higher in the COVID-19 cohort (OR, 7.6; 95% CI, 2.3-25.2).
This association “persisted across multiple sensitivity analyses, with the magnitude of relative associations ranging from 4.0 to 9,” wrote the authors. “This included a sensitivity analysis that adjusted for the number of vascular risk factors and ICU admissions (OR, 4.6; 95% CI, 1.4-15.7).”
The median age of patients with COVID-19 and stroke was 69 years, and the median duration of COVID-19 symptom onset to stroke diagnosis was 16 days. Stroke symptoms were the presenting complaint in only 26% of the patients, while the remainder developing stroke while hospitalized, and more than a third (35%) of all strokes occurred in patients who were mechanically ventilated with severe COVID-19. Inpatient mortality was considerably higher among patients with COVID-19 with stroke versus without (32% vs. 14%; P = .003).
In patients with COVID-19 “most ischemic strokes occurred in older age groups, those with traditional stroke risk factors, and people of color,” wrote the authors. “We also noted that initial plasma D-dimer levels were nearly 3-fold higher in those who received a diagnosis of ischemic stroke than in those who did not” (1.930 mcg/mL vs. 0.682 mcg/mL).
The authors suggested several possible explanations for the elevated risk of stroke in COVID-19. Acute viral illnesses are known to trigger inflammation, and COVID-19 in particular is associated with “a vigorous inflammatory response accompanied by coagulopathy, with elevated D-dimer levels and the frequent presence of antiphospholipid antibodies,” they wrote. The infection is also associated with more severe respiratory syndrome compared with influenza, as well as a heightened risk for complications such as atrial arrhythmias, myocardial infarction, heart failure, myocarditis, and venous thromboses, all of which likely contribute to the risk of ischemic stroke.”
COVID or conventional risk factors?
Asked to comment on the study, Benedict Michael, MBChB (Hons), MRCP (Neurol), PhD, from the United Kingdom’s Coronerve Studies Group, a collaborative initiative to study the neurological features of COVID-19, said in an interview that “this study suggests many cases of stroke are occurring in older patients with multiple existing conventional and well recognized risks for stroke, and may simply represent decompensation during sepsis.”
Dr. Michael, a senior clinician scientist fellow at the University of Liverpool and an honorary consultant neurologist at the Walton Centre, was the senior author on a recently published UK-wide surveillance study on the neurological and neuropsychiatric complications of COVID-19 (Lancet Psychiatry. 2020 Jun 25. doi: 10.1016/S2215-0366[20]30287-X).
He said among patients in the New York study, “those with COVID and a stroke appeared to have many conventional risk factors for stroke (and often at higher percentages than COVID patients without a stroke), e.g. hypertension, overweight, diabetes, hyperlipidemia, existing vascular disease affecting the coronary arteries and atrial fibrillation. To establish evidence-based treatment pathways, clearly further studies are needed to determine the biological mechanisms underlying the seemingly higher rate of stroke with COVID-19 than influenza; but this must especially focus on those younger patients without conventional risk factors for stroke (which are largely not included in this study).”
SOURCE: Merkler AE et al. JAMA Neurol. doi: 10.1001/jamaneurol.2020.2730.
FROM JAMA NEUROLOGY
Migraine is often a deciding factor in pregnancy planning
new research shows. Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
Although women with migraine who avoided pregnancy believed their migraines would worsen during pregnancy or make their pregnancy difficult, previous observational research indicates that migraine often improves during pregnancy.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix, Arizona.
The findings were presented at the virtual annual meeting of the American Headache Society.
Plans for the future
There is a paucity of research on the effects of migraine on pregnancy planning, the researchers noted. The few studies that have investigated this issue have focused on women’s previous family planning decisions and experience rather than on plans for the future, the researchers noted.
To evaluate how migraine in women influences pregnancy planning, the investigators analyzed data from the American Registry for Migraine Research (ARMR). The registry, which was established by the American Migraine Foundation, collects clinical data about individuals with migraine and other headache disorders from multiple centers.
Participants eligible for the current analysis were women who had been diagnosed with migraine on the basis of the International Classification of Headache Disorders–3 criteria. All completed the ARMR questionnaire between February 2016 and September 2019. The investigators excluded patients with trigeminal autonomic cephalalgia, secondary headache, painful cranial neuropathies, other facial pain, and other headaches.
They identified 895 eligible women with migraine. Of these, 607 completed the pregnancy question. Among those participants, 121 women (19.9%) reported that migraine was a factor in their decision to not become pregnant. Of this group, 70 (11.5%) reported that migraine was a “significant” factor in deciding to not have children, and 8.4% said it was “somewhat” of a factor. The remainder of the cohort (479) reported that migraine had no influence on their pregnancy plans.
There were no between-group differences by race, marital status, employment, or income. This finding suggests that sociodemographic differences “have less impact on pregnancy planning than migraine-specific characteristics like headache frequency and experience with having migraine attacks triggered by menstruation,” Dr. Ishii said.
“Substantial burden”
Not surprisingly, women who avoided pregnancy had fewer children than the rest of the sample. About 60% of those who made the decision to not become pregnant had no children, and 72% had not been pregnant since they began experiencing migraine.
Compared with women who reported that migraine had no influence on their pregnancy plans, those who avoided pregnancy were more likely to have chronic migraine at 81.8% versus 70.2%. They were also more likely to have menstrual migraine at 4.1% versus 1%. In addition, women who decided to not have children because of migraine were significantly younger at an average age of 37.5 versus 47.2 years.
The number of days with headache per 3-month interval was 53.9 among women who avoided pregnancy versus 42.5 among the other women. The Migraine Disability Assessment score was also higher for women who avoided pregnancy (132.5) than for it was the other women (91.7), indicating more severe disability.
In addition, more of the women who avoided pregnancy had a history of depression (48.8%) compared with the other women (37.7%). The average score on the Patient Health Questionnaire–4 was higher among women who avoided pregnancy (4.0) than among other women (3.1), which indicates greater anxiety or depression. Among women who avoided pregnancy, 72.5% believed their migraine would worsen during pregnancy, and 68.3% believed that migraine would make pregnancy very difficult.
“Clinicians need to recognize that migraine often has a substantial burden on multiple aspects of life, including one’s plans for having children,” Dr. Ishii said.
“Clinicians should educate their patients who are considering pregnancy about the most likely course of migraine during pregnancy, migraine treatment during pregnancy, and the potential impacts of migraine and its treatment on pregnancy outcomes,” he added.
More education needed
Commenting on the study, Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center, Irvine, California, said that not knowing how pregnancy is going to affect patients’ migraines can be “very scary” for women. In addition, patients often wonder what migraine treatments they can safely take once they do become pregnant, said Dr. Hutchinson, who was not involved in the research.
She noted that advantages of the ARMR data are that they are derived from a multicenter study and that migraine diagnoses were made by a headache specialist. A potential limitation of the study is that the population may not reflect outcomes of the millions of women who have migraine and become pregnant but never see a specialist.
“These findings show that more education is needed,” Dr. Hutchinson said.
Most women, especially those who have migraine without aura, note improvement with migraine during pregnancy, primarily because of the high, steady levels of estradiol, especially in the second and third trimesters, she said. In light of this, neurologists should reassure women that migraine is not a contraindication to pregnancy, she added.
There is also a need for additional research to assess how past experience with migraine and pregnancy influences a woman’s comfort level with additional pregnancies. Studies as to which treatments are safest for acute and preventive treatment of migraine during prepregnancy, pregnancy, and lactation are also needed, Dr. Hutchinson noted.
“If women knew they had treatment options that were evidence-based, they might be much more comfortable contemplating a pregnancy,” she said.
Dr. Ishii and Dr. Hutchinson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
Although women with migraine who avoided pregnancy believed their migraines would worsen during pregnancy or make their pregnancy difficult, previous observational research indicates that migraine often improves during pregnancy.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix, Arizona.
The findings were presented at the virtual annual meeting of the American Headache Society.
Plans for the future
There is a paucity of research on the effects of migraine on pregnancy planning, the researchers noted. The few studies that have investigated this issue have focused on women’s previous family planning decisions and experience rather than on plans for the future, the researchers noted.
To evaluate how migraine in women influences pregnancy planning, the investigators analyzed data from the American Registry for Migraine Research (ARMR). The registry, which was established by the American Migraine Foundation, collects clinical data about individuals with migraine and other headache disorders from multiple centers.
Participants eligible for the current analysis were women who had been diagnosed with migraine on the basis of the International Classification of Headache Disorders–3 criteria. All completed the ARMR questionnaire between February 2016 and September 2019. The investigators excluded patients with trigeminal autonomic cephalalgia, secondary headache, painful cranial neuropathies, other facial pain, and other headaches.
They identified 895 eligible women with migraine. Of these, 607 completed the pregnancy question. Among those participants, 121 women (19.9%) reported that migraine was a factor in their decision to not become pregnant. Of this group, 70 (11.5%) reported that migraine was a “significant” factor in deciding to not have children, and 8.4% said it was “somewhat” of a factor. The remainder of the cohort (479) reported that migraine had no influence on their pregnancy plans.
There were no between-group differences by race, marital status, employment, or income. This finding suggests that sociodemographic differences “have less impact on pregnancy planning than migraine-specific characteristics like headache frequency and experience with having migraine attacks triggered by menstruation,” Dr. Ishii said.
“Substantial burden”
Not surprisingly, women who avoided pregnancy had fewer children than the rest of the sample. About 60% of those who made the decision to not become pregnant had no children, and 72% had not been pregnant since they began experiencing migraine.
Compared with women who reported that migraine had no influence on their pregnancy plans, those who avoided pregnancy were more likely to have chronic migraine at 81.8% versus 70.2%. They were also more likely to have menstrual migraine at 4.1% versus 1%. In addition, women who decided to not have children because of migraine were significantly younger at an average age of 37.5 versus 47.2 years.
The number of days with headache per 3-month interval was 53.9 among women who avoided pregnancy versus 42.5 among the other women. The Migraine Disability Assessment score was also higher for women who avoided pregnancy (132.5) than for it was the other women (91.7), indicating more severe disability.
In addition, more of the women who avoided pregnancy had a history of depression (48.8%) compared with the other women (37.7%). The average score on the Patient Health Questionnaire–4 was higher among women who avoided pregnancy (4.0) than among other women (3.1), which indicates greater anxiety or depression. Among women who avoided pregnancy, 72.5% believed their migraine would worsen during pregnancy, and 68.3% believed that migraine would make pregnancy very difficult.
“Clinicians need to recognize that migraine often has a substantial burden on multiple aspects of life, including one’s plans for having children,” Dr. Ishii said.
“Clinicians should educate their patients who are considering pregnancy about the most likely course of migraine during pregnancy, migraine treatment during pregnancy, and the potential impacts of migraine and its treatment on pregnancy outcomes,” he added.
More education needed
Commenting on the study, Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center, Irvine, California, said that not knowing how pregnancy is going to affect patients’ migraines can be “very scary” for women. In addition, patients often wonder what migraine treatments they can safely take once they do become pregnant, said Dr. Hutchinson, who was not involved in the research.
She noted that advantages of the ARMR data are that they are derived from a multicenter study and that migraine diagnoses were made by a headache specialist. A potential limitation of the study is that the population may not reflect outcomes of the millions of women who have migraine and become pregnant but never see a specialist.
“These findings show that more education is needed,” Dr. Hutchinson said.
Most women, especially those who have migraine without aura, note improvement with migraine during pregnancy, primarily because of the high, steady levels of estradiol, especially in the second and third trimesters, she said. In light of this, neurologists should reassure women that migraine is not a contraindication to pregnancy, she added.
There is also a need for additional research to assess how past experience with migraine and pregnancy influences a woman’s comfort level with additional pregnancies. Studies as to which treatments are safest for acute and preventive treatment of migraine during prepregnancy, pregnancy, and lactation are also needed, Dr. Hutchinson noted.
“If women knew they had treatment options that were evidence-based, they might be much more comfortable contemplating a pregnancy,” she said.
Dr. Ishii and Dr. Hutchinson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
Although women with migraine who avoided pregnancy believed their migraines would worsen during pregnancy or make their pregnancy difficult, previous observational research indicates that migraine often improves during pregnancy.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix, Arizona.
The findings were presented at the virtual annual meeting of the American Headache Society.
Plans for the future
There is a paucity of research on the effects of migraine on pregnancy planning, the researchers noted. The few studies that have investigated this issue have focused on women’s previous family planning decisions and experience rather than on plans for the future, the researchers noted.
To evaluate how migraine in women influences pregnancy planning, the investigators analyzed data from the American Registry for Migraine Research (ARMR). The registry, which was established by the American Migraine Foundation, collects clinical data about individuals with migraine and other headache disorders from multiple centers.
Participants eligible for the current analysis were women who had been diagnosed with migraine on the basis of the International Classification of Headache Disorders–3 criteria. All completed the ARMR questionnaire between February 2016 and September 2019. The investigators excluded patients with trigeminal autonomic cephalalgia, secondary headache, painful cranial neuropathies, other facial pain, and other headaches.
They identified 895 eligible women with migraine. Of these, 607 completed the pregnancy question. Among those participants, 121 women (19.9%) reported that migraine was a factor in their decision to not become pregnant. Of this group, 70 (11.5%) reported that migraine was a “significant” factor in deciding to not have children, and 8.4% said it was “somewhat” of a factor. The remainder of the cohort (479) reported that migraine had no influence on their pregnancy plans.
There were no between-group differences by race, marital status, employment, or income. This finding suggests that sociodemographic differences “have less impact on pregnancy planning than migraine-specific characteristics like headache frequency and experience with having migraine attacks triggered by menstruation,” Dr. Ishii said.
“Substantial burden”
Not surprisingly, women who avoided pregnancy had fewer children than the rest of the sample. About 60% of those who made the decision to not become pregnant had no children, and 72% had not been pregnant since they began experiencing migraine.
Compared with women who reported that migraine had no influence on their pregnancy plans, those who avoided pregnancy were more likely to have chronic migraine at 81.8% versus 70.2%. They were also more likely to have menstrual migraine at 4.1% versus 1%. In addition, women who decided to not have children because of migraine were significantly younger at an average age of 37.5 versus 47.2 years.
The number of days with headache per 3-month interval was 53.9 among women who avoided pregnancy versus 42.5 among the other women. The Migraine Disability Assessment score was also higher for women who avoided pregnancy (132.5) than for it was the other women (91.7), indicating more severe disability.
In addition, more of the women who avoided pregnancy had a history of depression (48.8%) compared with the other women (37.7%). The average score on the Patient Health Questionnaire–4 was higher among women who avoided pregnancy (4.0) than among other women (3.1), which indicates greater anxiety or depression. Among women who avoided pregnancy, 72.5% believed their migraine would worsen during pregnancy, and 68.3% believed that migraine would make pregnancy very difficult.
“Clinicians need to recognize that migraine often has a substantial burden on multiple aspects of life, including one’s plans for having children,” Dr. Ishii said.
“Clinicians should educate their patients who are considering pregnancy about the most likely course of migraine during pregnancy, migraine treatment during pregnancy, and the potential impacts of migraine and its treatment on pregnancy outcomes,” he added.
More education needed
Commenting on the study, Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center, Irvine, California, said that not knowing how pregnancy is going to affect patients’ migraines can be “very scary” for women. In addition, patients often wonder what migraine treatments they can safely take once they do become pregnant, said Dr. Hutchinson, who was not involved in the research.
She noted that advantages of the ARMR data are that they are derived from a multicenter study and that migraine diagnoses were made by a headache specialist. A potential limitation of the study is that the population may not reflect outcomes of the millions of women who have migraine and become pregnant but never see a specialist.
“These findings show that more education is needed,” Dr. Hutchinson said.
Most women, especially those who have migraine without aura, note improvement with migraine during pregnancy, primarily because of the high, steady levels of estradiol, especially in the second and third trimesters, she said. In light of this, neurologists should reassure women that migraine is not a contraindication to pregnancy, she added.
There is also a need for additional research to assess how past experience with migraine and pregnancy influences a woman’s comfort level with additional pregnancies. Studies as to which treatments are safest for acute and preventive treatment of migraine during prepregnancy, pregnancy, and lactation are also needed, Dr. Hutchinson noted.
“If women knew they had treatment options that were evidence-based, they might be much more comfortable contemplating a pregnancy,” she said.
Dr. Ishii and Dr. Hutchinson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AHS 2020
Daily Recap: Hospitalized COVID patients need MRIs; Americans vote for face masks
Here are the stories our MDedge editors across specialties think you need to know about today:
Three stages to COVID-19 brain damage, new review suggests
A new review outlined a three-stage classification of the impact of COVID-19 on the central nervous system and recommended all hospitalized patients with the virus undergo MRI to flag potential neurologic damage and inform postdischarge monitoring.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” said lead author Majid Fotuhi, MD, PhD. The review was published online in the Journal of Alzheimer’s Disease. Read more.
Topline results for novel intranasal med to treat opioid overdose
Topline results show positive results for the experimental intranasal nalmefene product OX125 for opioid overdose reversal, Orexo, the drug’s manufacturer, announced.
A crossover, comparative bioavailability study was conducted in healthy volunteers to assess nalmefene absorption of three development formulations of OX125. Preliminary results showed “extensive and rapid absorption” across all three formulations versus an intramuscular injection of nalmefene, Orexo reported.
“As the U.S. heroin crisis has developed to a fentanyl crisis, the medical need for novel and more powerful opioid rescue medications is vast,” Nikolaj Sørensen, president and CEO of Orexo, said in a press release. Read more.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
Regarding regular testing, 66% of Republicans and those leaning Republican said that such testing was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Read more.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders.
Of gastric band patients, “almost 20% did not think they made the right decision,” he said. As for RYGP patients, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Three stages to COVID-19 brain damage, new review suggests
A new review outlined a three-stage classification of the impact of COVID-19 on the central nervous system and recommended all hospitalized patients with the virus undergo MRI to flag potential neurologic damage and inform postdischarge monitoring.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” said lead author Majid Fotuhi, MD, PhD. The review was published online in the Journal of Alzheimer’s Disease. Read more.
Topline results for novel intranasal med to treat opioid overdose
Topline results show positive results for the experimental intranasal nalmefene product OX125 for opioid overdose reversal, Orexo, the drug’s manufacturer, announced.
A crossover, comparative bioavailability study was conducted in healthy volunteers to assess nalmefene absorption of three development formulations of OX125. Preliminary results showed “extensive and rapid absorption” across all three formulations versus an intramuscular injection of nalmefene, Orexo reported.
“As the U.S. heroin crisis has developed to a fentanyl crisis, the medical need for novel and more powerful opioid rescue medications is vast,” Nikolaj Sørensen, president and CEO of Orexo, said in a press release. Read more.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
Regarding regular testing, 66% of Republicans and those leaning Republican said that such testing was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Read more.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders.
Of gastric band patients, “almost 20% did not think they made the right decision,” he said. As for RYGP patients, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Three stages to COVID-19 brain damage, new review suggests
A new review outlined a three-stage classification of the impact of COVID-19 on the central nervous system and recommended all hospitalized patients with the virus undergo MRI to flag potential neurologic damage and inform postdischarge monitoring.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” said lead author Majid Fotuhi, MD, PhD. The review was published online in the Journal of Alzheimer’s Disease. Read more.
Topline results for novel intranasal med to treat opioid overdose
Topline results show positive results for the experimental intranasal nalmefene product OX125 for opioid overdose reversal, Orexo, the drug’s manufacturer, announced.
A crossover, comparative bioavailability study was conducted in healthy volunteers to assess nalmefene absorption of three development formulations of OX125. Preliminary results showed “extensive and rapid absorption” across all three formulations versus an intramuscular injection of nalmefene, Orexo reported.
“As the U.S. heroin crisis has developed to a fentanyl crisis, the medical need for novel and more powerful opioid rescue medications is vast,” Nikolaj Sørensen, president and CEO of Orexo, said in a press release. Read more.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
Regarding regular testing, 66% of Republicans and those leaning Republican said that such testing was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Read more.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders.
Of gastric band patients, “almost 20% did not think they made the right decision,” he said. As for RYGP patients, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Three stages to COVID-19 brain damage, new review suggests
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Psychiatric manifestations of sport-related concussion
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.
Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.

Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.

Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.