User login
Ticagrelor/aspirin combo: Fewer repeat strokes and deaths, but more bleeds
, new data show. However, severe bleeding was more common in the ticagrelor/aspirin group than in the aspirin-only group.
“We found that ticagrelor plus aspirin reduced the risk of stroke or death, compared to aspirin alone in patients presenting acutely with stroke or TIA,” reported lead author S. Claiborne Johnston, MD, PhD, dean and vice president for medical affairs, Dell Medical School, the University of Texas, Austin.
Although the combination also increased the risk for major hemorrhage, that increase was small and would not overwhelm the benefit, he said.
The study was published online July 16 in The New England Journal of Medicine.
Attractive properties
“Lots of patients have stroke in the days to weeks after first presenting with a stroke or TIA,” said Dr. Johnston, who is also the Frank and Charmaine Denius Distinguished Dean’s Chair at Dell Medical School. “Aspirin has been the standard of care but is only partially effective. Clopidogrel plus aspirin is another option that has recently been proven, [but] ticagrelor has attractive properties as an antiplatelet agent and works synergistically with aspirin,” he added.
Ticagrelor is a direct-acting antiplatelet agent that does not depend on metabolic activation and that “reversibly binds” and inhibits the P2Y12 receptor on platelets. Previous research has evaluated clopidogrel and aspirin for the secondary prevention of ischemic stroke or TIA. In an earlier trial, ticagrelor was no better than aspirin in preventing these subsequent events. However, the investigators noted that the combination of the two drugs has not been well studied.
The randomized, placebo-controlled, double-blind trial involved 11,016 patients at 414 sites in 28 countries. Patients who had experienced mild to moderate acute noncardioembolic ischemic stroke (mean age, 65 years; 39% women; roughly 54% White) were randomly assigned to receive either ticagrelor plus aspirin (n = 5,523) or aspirin alone (n = 5,493) for 30 days. Of these patients, 91% had sustained a stroke, and 9% had sustained a TIA.
Thirty days was chosen as the treatment period because the risk for subsequent stroke tends to occur mainly in the first month after an acute ischemic stroke or TIA. The primary outcome was “a composite of stroke or death in a time-to-first-event analysis from randomization to 30 days of follow-up.” For the study, “stroke” encompassed ischemic, hemorrhagic, or stroke of undetermined type, and “death” included deaths of all causes. Secondary outcomes included first subsequent ischemic stroke and disability (defined as a score of >1 on the Rankin Scale).
Almost all patients (99.5%) were taking aspirin during the treatment period, and most were also taking an antihypertensive and a statin (74% and 83%, respectively).
Patients in the ticagrelor/aspirin group had fewer primary-outcome events in comparison with those in the aspirin-only group (303 patients [5.5%] vs. 362 patients [6.6%]; hazard ratio, 0.83; 95% confidence interval, 0.71-0.96; P = 0.02). Incidence of subsequent ischemic stroke were similarly lower in the ticagrelor/aspirin group in comparison with the aspirin-only group (276 patients [5.0%] vs. 345 patients [6.3%]; HR, 0.79; 95% CI, 0.68-0.93; P = .004).
On the other hand, there was no significant difference between the groups in the incidence of overall disability (23.8% of the patients in the ticagrelor/aspirin group and in 24.1% of the patients in the aspirin group; odds ratio, 0.98; 95% CI, 0.89-1.07; P = .61).
There were differences between the groups in severe bleeding, which occurred in 28 patients (0.5%) in the ticagrelor/aspirin group and in seven patients (0.15) in the ticagrelor group (HR, 3.99; 95% CI, 1.74-9.14; P = .001). Moreover, more patients in the ticagrelor/aspirin group experienced a composite of intracranial hemorrhage or fatal bleeding compared with the aspirin-only group (0.4% vs 0.1%). Fatal bleeding occurred in 0.2% of patients in the ticagrelor/aspirin group versus 0.1% of patients in the aspirin group. More patients in the ticagrelor-aspirin group permanently discontinued the treatment because of bleeding than in the aspirin-only group (2.8% vs. 0.6%).
“The benefit from treatment with ticagrelor/aspirin, as compared with aspirin alone, would be expected to result in a number needed to treat of 92 to prevent one primary outcome event, and a number needed to harm of 263 for severe bleeding,” the authors noted.
Risks versus benefits
Commenting on the study, Konark Malhotra, MD, a vascular neurologist at Allegheny Health Network, Pittsburgh, noted that ticagrelor is an antiplatelet medication “that adds to the armamentarium of stroke neurologists for the treatment of mild acute ischemic or high-risk TIA patients.” Dr. Malhotra, who was not involved with the study, added that the “combined use of ticagrelor and aspirin is effective in the reduction of ischemic events, however, at the expense of increased risk of bleeding events.”
In an accompanying editorial, Peter Rothwell, MD, PhD, of the Wolfson Center for Prevention of Stroke and Dementia, Nuffield Department of Clinical Neurosciences at the University of Oxford (England) who was not involved with the study, suggested that the “bleeding risk associated with ticagrelor and aspirin might exceed the benefit among lower-risk patients who make up the majority in practice, and so the results should not be overgeneralized.” Moreover, “regardless of which combination of antiplatelet therapy is favored for the high-risk minority, all patients should receive aspirin immediately after TIA, unless aspirin is contraindicated.”
He noted that “too many patients are sent home from emergency departments without this simple treatment that substantially reduces the risk and severity of early recurrent stroke.”
The study was supported by AstraZeneca. Dr. Johnston has received a grant from AstraZeneca and nonfinancial support from SANOFI. Dr. Rothwell has received personal fees from Bayer and BMS. Dr. Malhotra has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new data show. However, severe bleeding was more common in the ticagrelor/aspirin group than in the aspirin-only group.
“We found that ticagrelor plus aspirin reduced the risk of stroke or death, compared to aspirin alone in patients presenting acutely with stroke or TIA,” reported lead author S. Claiborne Johnston, MD, PhD, dean and vice president for medical affairs, Dell Medical School, the University of Texas, Austin.
Although the combination also increased the risk for major hemorrhage, that increase was small and would not overwhelm the benefit, he said.
The study was published online July 16 in The New England Journal of Medicine.
Attractive properties
“Lots of patients have stroke in the days to weeks after first presenting with a stroke or TIA,” said Dr. Johnston, who is also the Frank and Charmaine Denius Distinguished Dean’s Chair at Dell Medical School. “Aspirin has been the standard of care but is only partially effective. Clopidogrel plus aspirin is another option that has recently been proven, [but] ticagrelor has attractive properties as an antiplatelet agent and works synergistically with aspirin,” he added.
Ticagrelor is a direct-acting antiplatelet agent that does not depend on metabolic activation and that “reversibly binds” and inhibits the P2Y12 receptor on platelets. Previous research has evaluated clopidogrel and aspirin for the secondary prevention of ischemic stroke or TIA. In an earlier trial, ticagrelor was no better than aspirin in preventing these subsequent events. However, the investigators noted that the combination of the two drugs has not been well studied.
The randomized, placebo-controlled, double-blind trial involved 11,016 patients at 414 sites in 28 countries. Patients who had experienced mild to moderate acute noncardioembolic ischemic stroke (mean age, 65 years; 39% women; roughly 54% White) were randomly assigned to receive either ticagrelor plus aspirin (n = 5,523) or aspirin alone (n = 5,493) for 30 days. Of these patients, 91% had sustained a stroke, and 9% had sustained a TIA.
Thirty days was chosen as the treatment period because the risk for subsequent stroke tends to occur mainly in the first month after an acute ischemic stroke or TIA. The primary outcome was “a composite of stroke or death in a time-to-first-event analysis from randomization to 30 days of follow-up.” For the study, “stroke” encompassed ischemic, hemorrhagic, or stroke of undetermined type, and “death” included deaths of all causes. Secondary outcomes included first subsequent ischemic stroke and disability (defined as a score of >1 on the Rankin Scale).
Almost all patients (99.5%) were taking aspirin during the treatment period, and most were also taking an antihypertensive and a statin (74% and 83%, respectively).
Patients in the ticagrelor/aspirin group had fewer primary-outcome events in comparison with those in the aspirin-only group (303 patients [5.5%] vs. 362 patients [6.6%]; hazard ratio, 0.83; 95% confidence interval, 0.71-0.96; P = 0.02). Incidence of subsequent ischemic stroke were similarly lower in the ticagrelor/aspirin group in comparison with the aspirin-only group (276 patients [5.0%] vs. 345 patients [6.3%]; HR, 0.79; 95% CI, 0.68-0.93; P = .004).
On the other hand, there was no significant difference between the groups in the incidence of overall disability (23.8% of the patients in the ticagrelor/aspirin group and in 24.1% of the patients in the aspirin group; odds ratio, 0.98; 95% CI, 0.89-1.07; P = .61).
There were differences between the groups in severe bleeding, which occurred in 28 patients (0.5%) in the ticagrelor/aspirin group and in seven patients (0.15) in the ticagrelor group (HR, 3.99; 95% CI, 1.74-9.14; P = .001). Moreover, more patients in the ticagrelor/aspirin group experienced a composite of intracranial hemorrhage or fatal bleeding compared with the aspirin-only group (0.4% vs 0.1%). Fatal bleeding occurred in 0.2% of patients in the ticagrelor/aspirin group versus 0.1% of patients in the aspirin group. More patients in the ticagrelor-aspirin group permanently discontinued the treatment because of bleeding than in the aspirin-only group (2.8% vs. 0.6%).
“The benefit from treatment with ticagrelor/aspirin, as compared with aspirin alone, would be expected to result in a number needed to treat of 92 to prevent one primary outcome event, and a number needed to harm of 263 for severe bleeding,” the authors noted.
Risks versus benefits
Commenting on the study, Konark Malhotra, MD, a vascular neurologist at Allegheny Health Network, Pittsburgh, noted that ticagrelor is an antiplatelet medication “that adds to the armamentarium of stroke neurologists for the treatment of mild acute ischemic or high-risk TIA patients.” Dr. Malhotra, who was not involved with the study, added that the “combined use of ticagrelor and aspirin is effective in the reduction of ischemic events, however, at the expense of increased risk of bleeding events.”
In an accompanying editorial, Peter Rothwell, MD, PhD, of the Wolfson Center for Prevention of Stroke and Dementia, Nuffield Department of Clinical Neurosciences at the University of Oxford (England) who was not involved with the study, suggested that the “bleeding risk associated with ticagrelor and aspirin might exceed the benefit among lower-risk patients who make up the majority in practice, and so the results should not be overgeneralized.” Moreover, “regardless of which combination of antiplatelet therapy is favored for the high-risk minority, all patients should receive aspirin immediately after TIA, unless aspirin is contraindicated.”
He noted that “too many patients are sent home from emergency departments without this simple treatment that substantially reduces the risk and severity of early recurrent stroke.”
The study was supported by AstraZeneca. Dr. Johnston has received a grant from AstraZeneca and nonfinancial support from SANOFI. Dr. Rothwell has received personal fees from Bayer and BMS. Dr. Malhotra has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new data show. However, severe bleeding was more common in the ticagrelor/aspirin group than in the aspirin-only group.
“We found that ticagrelor plus aspirin reduced the risk of stroke or death, compared to aspirin alone in patients presenting acutely with stroke or TIA,” reported lead author S. Claiborne Johnston, MD, PhD, dean and vice president for medical affairs, Dell Medical School, the University of Texas, Austin.
Although the combination also increased the risk for major hemorrhage, that increase was small and would not overwhelm the benefit, he said.
The study was published online July 16 in The New England Journal of Medicine.
Attractive properties
“Lots of patients have stroke in the days to weeks after first presenting with a stroke or TIA,” said Dr. Johnston, who is also the Frank and Charmaine Denius Distinguished Dean’s Chair at Dell Medical School. “Aspirin has been the standard of care but is only partially effective. Clopidogrel plus aspirin is another option that has recently been proven, [but] ticagrelor has attractive properties as an antiplatelet agent and works synergistically with aspirin,” he added.
Ticagrelor is a direct-acting antiplatelet agent that does not depend on metabolic activation and that “reversibly binds” and inhibits the P2Y12 receptor on platelets. Previous research has evaluated clopidogrel and aspirin for the secondary prevention of ischemic stroke or TIA. In an earlier trial, ticagrelor was no better than aspirin in preventing these subsequent events. However, the investigators noted that the combination of the two drugs has not been well studied.
The randomized, placebo-controlled, double-blind trial involved 11,016 patients at 414 sites in 28 countries. Patients who had experienced mild to moderate acute noncardioembolic ischemic stroke (mean age, 65 years; 39% women; roughly 54% White) were randomly assigned to receive either ticagrelor plus aspirin (n = 5,523) or aspirin alone (n = 5,493) for 30 days. Of these patients, 91% had sustained a stroke, and 9% had sustained a TIA.
Thirty days was chosen as the treatment period because the risk for subsequent stroke tends to occur mainly in the first month after an acute ischemic stroke or TIA. The primary outcome was “a composite of stroke or death in a time-to-first-event analysis from randomization to 30 days of follow-up.” For the study, “stroke” encompassed ischemic, hemorrhagic, or stroke of undetermined type, and “death” included deaths of all causes. Secondary outcomes included first subsequent ischemic stroke and disability (defined as a score of >1 on the Rankin Scale).
Almost all patients (99.5%) were taking aspirin during the treatment period, and most were also taking an antihypertensive and a statin (74% and 83%, respectively).
Patients in the ticagrelor/aspirin group had fewer primary-outcome events in comparison with those in the aspirin-only group (303 patients [5.5%] vs. 362 patients [6.6%]; hazard ratio, 0.83; 95% confidence interval, 0.71-0.96; P = 0.02). Incidence of subsequent ischemic stroke were similarly lower in the ticagrelor/aspirin group in comparison with the aspirin-only group (276 patients [5.0%] vs. 345 patients [6.3%]; HR, 0.79; 95% CI, 0.68-0.93; P = .004).
On the other hand, there was no significant difference between the groups in the incidence of overall disability (23.8% of the patients in the ticagrelor/aspirin group and in 24.1% of the patients in the aspirin group; odds ratio, 0.98; 95% CI, 0.89-1.07; P = .61).
There were differences between the groups in severe bleeding, which occurred in 28 patients (0.5%) in the ticagrelor/aspirin group and in seven patients (0.15) in the ticagrelor group (HR, 3.99; 95% CI, 1.74-9.14; P = .001). Moreover, more patients in the ticagrelor/aspirin group experienced a composite of intracranial hemorrhage or fatal bleeding compared with the aspirin-only group (0.4% vs 0.1%). Fatal bleeding occurred in 0.2% of patients in the ticagrelor/aspirin group versus 0.1% of patients in the aspirin group. More patients in the ticagrelor-aspirin group permanently discontinued the treatment because of bleeding than in the aspirin-only group (2.8% vs. 0.6%).
“The benefit from treatment with ticagrelor/aspirin, as compared with aspirin alone, would be expected to result in a number needed to treat of 92 to prevent one primary outcome event, and a number needed to harm of 263 for severe bleeding,” the authors noted.
Risks versus benefits
Commenting on the study, Konark Malhotra, MD, a vascular neurologist at Allegheny Health Network, Pittsburgh, noted that ticagrelor is an antiplatelet medication “that adds to the armamentarium of stroke neurologists for the treatment of mild acute ischemic or high-risk TIA patients.” Dr. Malhotra, who was not involved with the study, added that the “combined use of ticagrelor and aspirin is effective in the reduction of ischemic events, however, at the expense of increased risk of bleeding events.”
In an accompanying editorial, Peter Rothwell, MD, PhD, of the Wolfson Center for Prevention of Stroke and Dementia, Nuffield Department of Clinical Neurosciences at the University of Oxford (England) who was not involved with the study, suggested that the “bleeding risk associated with ticagrelor and aspirin might exceed the benefit among lower-risk patients who make up the majority in practice, and so the results should not be overgeneralized.” Moreover, “regardless of which combination of antiplatelet therapy is favored for the high-risk minority, all patients should receive aspirin immediately after TIA, unless aspirin is contraindicated.”
He noted that “too many patients are sent home from emergency departments without this simple treatment that substantially reduces the risk and severity of early recurrent stroke.”
The study was supported by AstraZeneca. Dr. Johnston has received a grant from AstraZeneca and nonfinancial support from SANOFI. Dr. Rothwell has received personal fees from Bayer and BMS. Dr. Malhotra has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From New England Journal of Medicine
Managing amidst COVID-19 (and everything else that ails us)
This year, medical media has been dominated by reporting on the devastating COVID-19 pandemic. Many studies and analyses have shown that staying at home, social distancing, quarantining of close contacts, and wearing face masks and face shields are effective ways of preventing spread.
Although initially there were no known effective treatments for severe COVID-19 infection (other than oxygen and ventilator support), we now know that dexamethasone,1 remdesivir,2 and convalescent plasma3 are effective in lessening the severity of illness and perhaps preventing death. That said, we will continue to struggle with COVID-19 for the foreseeable future.
But other medical illnesses actually predominate in terms of morbidity and mortality, even during this pandemic. For example, although there has been an average of roughly 5600 COVID-19-related deaths per week for the past 4 months,4 there are, on average, more than 54,000 deaths per week in the United States from other causes.5 This means that we must continue to tend to the other health care needs of our patients even as we deal with COVID-19.
In that light, JFP continues to publish practical, evidence-based clinical reviews designed to keep family physicians and other primary health care clinicians up to date on a variety of topics. For instance, in this issue of JFP, we have articles on:
- Opioid prescribing. Although opioids have risks, they remain potent medications for relief from acute pain, as well as cancer-related pain and chronic pain not sufficiently treated with other medications. Mahvan et al provide expert advice on maximizing benefit and minimizing the risks of opioid prescribing.
- Secondary ischemic stroke prevention. For patients who have suffered a transient ischemic attack or minor stroke, a mainstay of prevention is antiplatelet therapy. Aspirin alone used to be the treatment of choice, but research has demonstrated the value of adding another antiplatelet agent. Helmer et al’s thorough review reminds us that the antiplatelet drug of choice, in addition to aspirin, is clopidogrel, which should be used only for the first 30 days after the event because of an increased bleeding risk.
- Combatting Clostridioides difficile infection. CDI has been a difficult condition to treat, especially in high-risk patients. Zukauckas et al provide a comprehensive review of diagnosis and management. Vancomycin is now the drug of choice, and fecal transplant is highly effective in preventing recurrent CDI.
This diverse range of timely, practical, evidence-based guidance—in addition to coverage of COVID-19 and other rapidly emerging medical news stories—can all be found on our Web site at www.mdedge.com/familymedicine. We remain committed to supplying you with all of the information you need to provide your patients with the very best care—no matter what brings them in to see you.
1. Low-cost dexamethasone reduces death by up to one third in hospitalized patients with severe respiratory complications of COVID-19. Recovery: Randomised Evaluation of COVID-19 Therapy Web site. June 16, 2020. www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. Accessed July 1, 2020.
2. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—preliminary report [published online ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2007764.
3. Li L, Zhang W, Hu Y, et. al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial [published online ahead of print]. JAMA. doi:10.1001/jama.2020.10044.
4. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765.
5. Xu J, Murphy SL, Kochanek KD, et al. Mortality in the United States, 2018. NCHS Data Brief. 2020;1-8.
This year, medical media has been dominated by reporting on the devastating COVID-19 pandemic. Many studies and analyses have shown that staying at home, social distancing, quarantining of close contacts, and wearing face masks and face shields are effective ways of preventing spread.
Although initially there were no known effective treatments for severe COVID-19 infection (other than oxygen and ventilator support), we now know that dexamethasone,1 remdesivir,2 and convalescent plasma3 are effective in lessening the severity of illness and perhaps preventing death. That said, we will continue to struggle with COVID-19 for the foreseeable future.
But other medical illnesses actually predominate in terms of morbidity and mortality, even during this pandemic. For example, although there has been an average of roughly 5600 COVID-19-related deaths per week for the past 4 months,4 there are, on average, more than 54,000 deaths per week in the United States from other causes.5 This means that we must continue to tend to the other health care needs of our patients even as we deal with COVID-19.
In that light, JFP continues to publish practical, evidence-based clinical reviews designed to keep family physicians and other primary health care clinicians up to date on a variety of topics. For instance, in this issue of JFP, we have articles on:
- Opioid prescribing. Although opioids have risks, they remain potent medications for relief from acute pain, as well as cancer-related pain and chronic pain not sufficiently treated with other medications. Mahvan et al provide expert advice on maximizing benefit and minimizing the risks of opioid prescribing.
- Secondary ischemic stroke prevention. For patients who have suffered a transient ischemic attack or minor stroke, a mainstay of prevention is antiplatelet therapy. Aspirin alone used to be the treatment of choice, but research has demonstrated the value of adding another antiplatelet agent. Helmer et al’s thorough review reminds us that the antiplatelet drug of choice, in addition to aspirin, is clopidogrel, which should be used only for the first 30 days after the event because of an increased bleeding risk.
- Combatting Clostridioides difficile infection. CDI has been a difficult condition to treat, especially in high-risk patients. Zukauckas et al provide a comprehensive review of diagnosis and management. Vancomycin is now the drug of choice, and fecal transplant is highly effective in preventing recurrent CDI.
This diverse range of timely, practical, evidence-based guidance—in addition to coverage of COVID-19 and other rapidly emerging medical news stories—can all be found on our Web site at www.mdedge.com/familymedicine. We remain committed to supplying you with all of the information you need to provide your patients with the very best care—no matter what brings them in to see you.
This year, medical media has been dominated by reporting on the devastating COVID-19 pandemic. Many studies and analyses have shown that staying at home, social distancing, quarantining of close contacts, and wearing face masks and face shields are effective ways of preventing spread.
Although initially there were no known effective treatments for severe COVID-19 infection (other than oxygen and ventilator support), we now know that dexamethasone,1 remdesivir,2 and convalescent plasma3 are effective in lessening the severity of illness and perhaps preventing death. That said, we will continue to struggle with COVID-19 for the foreseeable future.
But other medical illnesses actually predominate in terms of morbidity and mortality, even during this pandemic. For example, although there has been an average of roughly 5600 COVID-19-related deaths per week for the past 4 months,4 there are, on average, more than 54,000 deaths per week in the United States from other causes.5 This means that we must continue to tend to the other health care needs of our patients even as we deal with COVID-19.
In that light, JFP continues to publish practical, evidence-based clinical reviews designed to keep family physicians and other primary health care clinicians up to date on a variety of topics. For instance, in this issue of JFP, we have articles on:
- Opioid prescribing. Although opioids have risks, they remain potent medications for relief from acute pain, as well as cancer-related pain and chronic pain not sufficiently treated with other medications. Mahvan et al provide expert advice on maximizing benefit and minimizing the risks of opioid prescribing.
- Secondary ischemic stroke prevention. For patients who have suffered a transient ischemic attack or minor stroke, a mainstay of prevention is antiplatelet therapy. Aspirin alone used to be the treatment of choice, but research has demonstrated the value of adding another antiplatelet agent. Helmer et al’s thorough review reminds us that the antiplatelet drug of choice, in addition to aspirin, is clopidogrel, which should be used only for the first 30 days after the event because of an increased bleeding risk.
- Combatting Clostridioides difficile infection. CDI has been a difficult condition to treat, especially in high-risk patients. Zukauckas et al provide a comprehensive review of diagnosis and management. Vancomycin is now the drug of choice, and fecal transplant is highly effective in preventing recurrent CDI.
This diverse range of timely, practical, evidence-based guidance—in addition to coverage of COVID-19 and other rapidly emerging medical news stories—can all be found on our Web site at www.mdedge.com/familymedicine. We remain committed to supplying you with all of the information you need to provide your patients with the very best care—no matter what brings them in to see you.
1. Low-cost dexamethasone reduces death by up to one third in hospitalized patients with severe respiratory complications of COVID-19. Recovery: Randomised Evaluation of COVID-19 Therapy Web site. June 16, 2020. www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. Accessed July 1, 2020.
2. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—preliminary report [published online ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2007764.
3. Li L, Zhang W, Hu Y, et. al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial [published online ahead of print]. JAMA. doi:10.1001/jama.2020.10044.
4. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765.
5. Xu J, Murphy SL, Kochanek KD, et al. Mortality in the United States, 2018. NCHS Data Brief. 2020;1-8.
1. Low-cost dexamethasone reduces death by up to one third in hospitalized patients with severe respiratory complications of COVID-19. Recovery: Randomised Evaluation of COVID-19 Therapy Web site. June 16, 2020. www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. Accessed July 1, 2020.
2. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—preliminary report [published online ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2007764.
3. Li L, Zhang W, Hu Y, et. al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial [published online ahead of print]. JAMA. doi:10.1001/jama.2020.10044.
4. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765.
5. Xu J, Murphy SL, Kochanek KD, et al. Mortality in the United States, 2018. NCHS Data Brief. 2020;1-8.
Dual antiplatelet Tx for stroke prevention: Worth the risk?
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
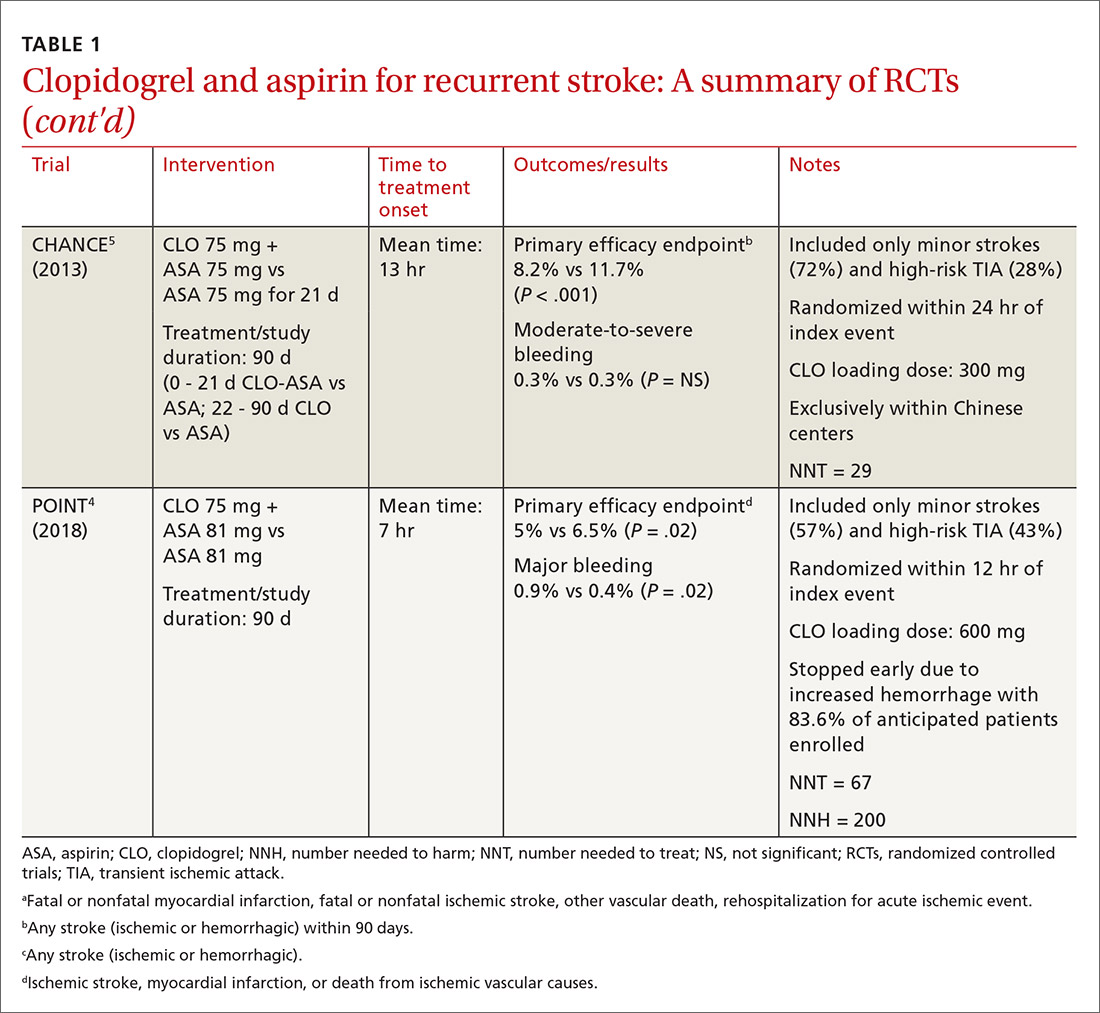
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
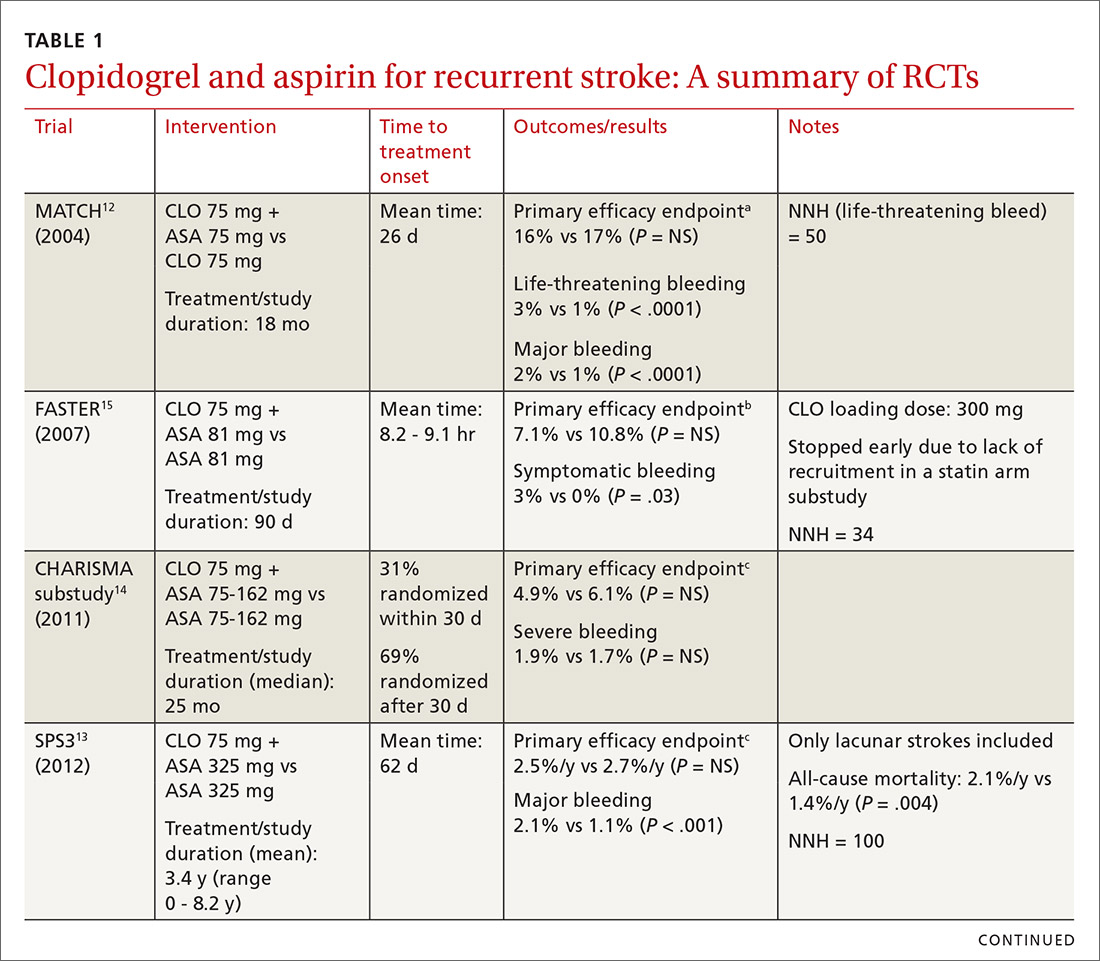
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
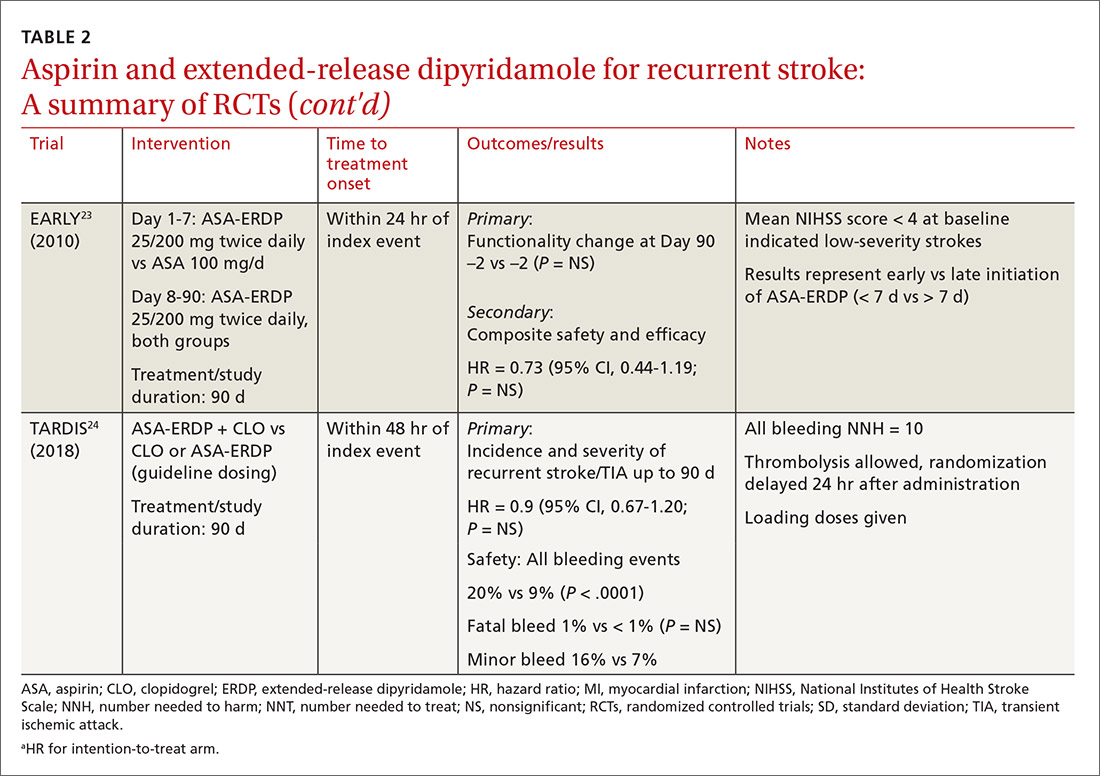
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
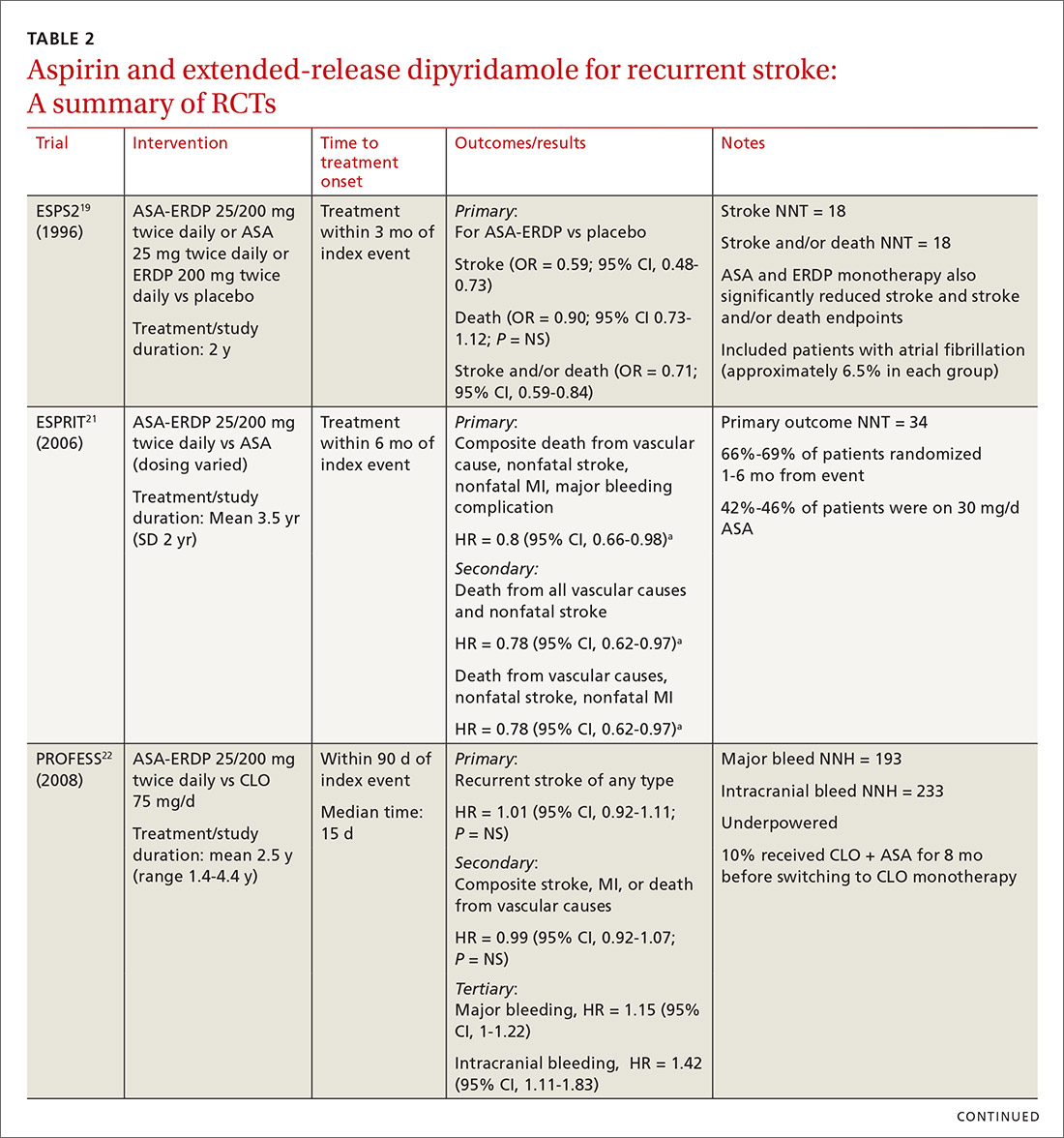
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; [email protected].
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15

Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14

Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.

The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.

CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; [email protected].
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15

Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14

Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.

The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.

CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; [email protected].
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
PRACTICE RECOMMENDATIONS
› Initiate combined clopidogrel plus aspirin within 24 hours of a minor stroke or TIA and continue for no longer than 1 month; then switch patients to aspirin or clopidogrel monotherapy. A
› Do not use combined clopidogrel plus aspirin for long-term secondary stroke prevention. A
› Limit use of aspirin plus extended-release dipyridamole as a first choice for secondary stroke prevention because of limitations in efficacy and poor tolerability. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Cardiovascular risk factors tied to midlife cognitive decline
new research shows. The findings suggest that the relationship between CVRFs and cognition becomes evident much earlier than previously realized. Investigators found that individuals who smoked were 65% more likely to have accelerated cognitive decline, those with hypertension were 87% more likely, and individuals with diabetes had nearly a 200% increased risk.
“What is new here is that almost no one has looked at cardiovascular risk factors in such a young age [mean, 50 years] and cognitive change in middle age from 50 to 55 or so. Almost all other studies have looked at mid- or late-life cardiovascular risk factors and late-life cognition or dementia,” said study investigator Kristine Yaffe, MD.
The research was published online July 15 in Neurology.
New insight
Previous research has shown a strong association between CVRFs and a greater risk for cognitive decline and dementia in late life, but the investigators note that data about the influence of CVRFs on cognition in midlife are “sparse.” Longitudinal studies have also shown that several cognitive domains – particularly processing speed and executive function – may start to decline in midlife, but whether CVRFs, many of which also emerge in midlife, contribute to these changes is unclear.
To assess the effect of CVRFs on cognitive changes in midlife, the investigators analyzed data from the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a large cohort of Black and White men and women.
The analysis was based on data from 2,675 participants who underwent CVRF assessment and cognitive testing at baseline and 5 years later. At baseline, participants’ mean age was 50.2 years. Approximately 57% of participants were women, 55% were White, and the mean number of years of education was 15. At study outset, 43% (n = 1,133) of participants were considered obese, 31% (n = 826) had hypertension, 15% (n = 701) were current smokers, 11% (n = 290) had diabetes, and 9% (n = 248) had high cholesterol.
Cognition was assessed using the Digit Symbol Substitution Test, which measures processing speed and executive function; the Stroop Test, which measures executive function; and the Rey Auditory Verbal Learning Test, which measures verbal memory.
Dose-dependent effect
Overall results showed that, for 5% of participants, cognitive decline was accelerated at 5 years. In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs. 4.3%; odds ratio, 1.79, 95% confidence interval, 1.27-2.52), diabetes (10.3% vs. 4.7%; OR, 2.33; 95% CI, 1.53-3.56), and smoking (7.7% current smokers vs. 4.3% never smokers; OR, 1.87; 95% CI, 1.21-2.90). After adjusting for age, sex, and race, the associations remained significant.
The researchers found no significant effect of high cholesterol (6.9% vs. 5.2%; OR, 1.35; 95% CI, 0.80-2.28) or obesity (6.1% vs. 4.8%; OR, 1.29; 95% CI, 0.92-1.82) on accelerated cognitive decline.
Compared with participants with no CVRFs, the likelihood of accelerated cognitive decline was higher for individuals with one or two risk factors (OR, 1.94; 95% CI, 1.16-3.25) and was higher still for those with three or more risk factors (OR, 3.51; 95% CI, 2.05-6.00).
The fact that there was no association between midlife cognitive decline and obesity or high cholesterol did not come as a surprise, said Dr. Yaffe. “Most studies have not shown a consistent finding with high cholesterol and later-life cognition, so it is not surprising we did not see one in midlife, when there is not as much cognitive change.”
The study’s results, said Dr. Yaffe, provide physicians with another good reason to help patients address CVRFs and to work with them to lower blood pressure, stop smoking, reduce diabetes incidence, or control diabetes.
Dr. Yaffe said she and her colleagues plan further research into CVRFs and accelerated cognitive decline. “We want to know if this earlier cognitive decline [in midlife] is connected to greater decline later in life. We also want to know if improving these risk factors in midlife might prevent or slow dementia later.”
More to explore
Commenting on the findings, Michelle M. Mielke, PhD, professor of epidemiology and neurology at Mayo Clinic, Rochester, Minn., said one of the study’s main implications “is that the prevention and treatment of midlife hypertension and diabetes and smoking cessation directly impacts shorter-term changes in cognition.”
She added that the study also provides a foundation for answering further questions about the effects of CVRFs on cognition in midlife. For example, questions about sex differences remain unanswered. Men develop CVRFs earlier than women, but the investigators did not provide the prevalence of cardiovascular risk factors by sex.
“It was also not reported whether a specific midlife cardiovascular risk factor was more strongly associated with accelerated cognitive decline for women or for men,” she said. In addition, the mean age of the population at baseline is the approximate age of the onset of menopause, after which cardiovascular risk factors increase among women.
“Additional research is needed to understand the emergence of cardiovascular risk factors pre- versus post menopause on subsequent cognition and also consider the use of menopausal hormone therapy,” said Dr. Mielke.
“Another future research avenue is to further understand the impact of antihypertensive and diabetes medications,” she continued. “For example, in the current study, it was not clear how many [participants] with hypertension were treated versus untreated and whether this impacted subsequent cognition. Similarly, it is not known whether specific antihypertensives are more beneficial for cognition in midlife.”
CARDIA is supported by the National Heart, Lung, and Blood Institute; the University of Alabama at Birmingham; Northwestern University, Chicago; the University of Minnesota; and the Kaiser Foundation Research Institute. Dr. Yaffe serves on data safety monitoring boards for Eli Lilly and studies sponsored by the National Institute on Aging. She is a board member of Alector and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Dr. Mielke has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. The findings suggest that the relationship between CVRFs and cognition becomes evident much earlier than previously realized. Investigators found that individuals who smoked were 65% more likely to have accelerated cognitive decline, those with hypertension were 87% more likely, and individuals with diabetes had nearly a 200% increased risk.
“What is new here is that almost no one has looked at cardiovascular risk factors in such a young age [mean, 50 years] and cognitive change in middle age from 50 to 55 or so. Almost all other studies have looked at mid- or late-life cardiovascular risk factors and late-life cognition or dementia,” said study investigator Kristine Yaffe, MD.
The research was published online July 15 in Neurology.
New insight
Previous research has shown a strong association between CVRFs and a greater risk for cognitive decline and dementia in late life, but the investigators note that data about the influence of CVRFs on cognition in midlife are “sparse.” Longitudinal studies have also shown that several cognitive domains – particularly processing speed and executive function – may start to decline in midlife, but whether CVRFs, many of which also emerge in midlife, contribute to these changes is unclear.
To assess the effect of CVRFs on cognitive changes in midlife, the investigators analyzed data from the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a large cohort of Black and White men and women.
The analysis was based on data from 2,675 participants who underwent CVRF assessment and cognitive testing at baseline and 5 years later. At baseline, participants’ mean age was 50.2 years. Approximately 57% of participants were women, 55% were White, and the mean number of years of education was 15. At study outset, 43% (n = 1,133) of participants were considered obese, 31% (n = 826) had hypertension, 15% (n = 701) were current smokers, 11% (n = 290) had diabetes, and 9% (n = 248) had high cholesterol.
Cognition was assessed using the Digit Symbol Substitution Test, which measures processing speed and executive function; the Stroop Test, which measures executive function; and the Rey Auditory Verbal Learning Test, which measures verbal memory.
Dose-dependent effect
Overall results showed that, for 5% of participants, cognitive decline was accelerated at 5 years. In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs. 4.3%; odds ratio, 1.79, 95% confidence interval, 1.27-2.52), diabetes (10.3% vs. 4.7%; OR, 2.33; 95% CI, 1.53-3.56), and smoking (7.7% current smokers vs. 4.3% never smokers; OR, 1.87; 95% CI, 1.21-2.90). After adjusting for age, sex, and race, the associations remained significant.
The researchers found no significant effect of high cholesterol (6.9% vs. 5.2%; OR, 1.35; 95% CI, 0.80-2.28) or obesity (6.1% vs. 4.8%; OR, 1.29; 95% CI, 0.92-1.82) on accelerated cognitive decline.
Compared with participants with no CVRFs, the likelihood of accelerated cognitive decline was higher for individuals with one or two risk factors (OR, 1.94; 95% CI, 1.16-3.25) and was higher still for those with three or more risk factors (OR, 3.51; 95% CI, 2.05-6.00).
The fact that there was no association between midlife cognitive decline and obesity or high cholesterol did not come as a surprise, said Dr. Yaffe. “Most studies have not shown a consistent finding with high cholesterol and later-life cognition, so it is not surprising we did not see one in midlife, when there is not as much cognitive change.”
The study’s results, said Dr. Yaffe, provide physicians with another good reason to help patients address CVRFs and to work with them to lower blood pressure, stop smoking, reduce diabetes incidence, or control diabetes.
Dr. Yaffe said she and her colleagues plan further research into CVRFs and accelerated cognitive decline. “We want to know if this earlier cognitive decline [in midlife] is connected to greater decline later in life. We also want to know if improving these risk factors in midlife might prevent or slow dementia later.”
More to explore
Commenting on the findings, Michelle M. Mielke, PhD, professor of epidemiology and neurology at Mayo Clinic, Rochester, Minn., said one of the study’s main implications “is that the prevention and treatment of midlife hypertension and diabetes and smoking cessation directly impacts shorter-term changes in cognition.”
She added that the study also provides a foundation for answering further questions about the effects of CVRFs on cognition in midlife. For example, questions about sex differences remain unanswered. Men develop CVRFs earlier than women, but the investigators did not provide the prevalence of cardiovascular risk factors by sex.
“It was also not reported whether a specific midlife cardiovascular risk factor was more strongly associated with accelerated cognitive decline for women or for men,” she said. In addition, the mean age of the population at baseline is the approximate age of the onset of menopause, after which cardiovascular risk factors increase among women.
“Additional research is needed to understand the emergence of cardiovascular risk factors pre- versus post menopause on subsequent cognition and also consider the use of menopausal hormone therapy,” said Dr. Mielke.
“Another future research avenue is to further understand the impact of antihypertensive and diabetes medications,” she continued. “For example, in the current study, it was not clear how many [participants] with hypertension were treated versus untreated and whether this impacted subsequent cognition. Similarly, it is not known whether specific antihypertensives are more beneficial for cognition in midlife.”
CARDIA is supported by the National Heart, Lung, and Blood Institute; the University of Alabama at Birmingham; Northwestern University, Chicago; the University of Minnesota; and the Kaiser Foundation Research Institute. Dr. Yaffe serves on data safety monitoring boards for Eli Lilly and studies sponsored by the National Institute on Aging. She is a board member of Alector and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Dr. Mielke has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. The findings suggest that the relationship between CVRFs and cognition becomes evident much earlier than previously realized. Investigators found that individuals who smoked were 65% more likely to have accelerated cognitive decline, those with hypertension were 87% more likely, and individuals with diabetes had nearly a 200% increased risk.
“What is new here is that almost no one has looked at cardiovascular risk factors in such a young age [mean, 50 years] and cognitive change in middle age from 50 to 55 or so. Almost all other studies have looked at mid- or late-life cardiovascular risk factors and late-life cognition or dementia,” said study investigator Kristine Yaffe, MD.
The research was published online July 15 in Neurology.
New insight
Previous research has shown a strong association between CVRFs and a greater risk for cognitive decline and dementia in late life, but the investigators note that data about the influence of CVRFs on cognition in midlife are “sparse.” Longitudinal studies have also shown that several cognitive domains – particularly processing speed and executive function – may start to decline in midlife, but whether CVRFs, many of which also emerge in midlife, contribute to these changes is unclear.
To assess the effect of CVRFs on cognitive changes in midlife, the investigators analyzed data from the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a large cohort of Black and White men and women.
The analysis was based on data from 2,675 participants who underwent CVRF assessment and cognitive testing at baseline and 5 years later. At baseline, participants’ mean age was 50.2 years. Approximately 57% of participants were women, 55% were White, and the mean number of years of education was 15. At study outset, 43% (n = 1,133) of participants were considered obese, 31% (n = 826) had hypertension, 15% (n = 701) were current smokers, 11% (n = 290) had diabetes, and 9% (n = 248) had high cholesterol.
Cognition was assessed using the Digit Symbol Substitution Test, which measures processing speed and executive function; the Stroop Test, which measures executive function; and the Rey Auditory Verbal Learning Test, which measures verbal memory.
Dose-dependent effect
Overall results showed that, for 5% of participants, cognitive decline was accelerated at 5 years. In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs. 4.3%; odds ratio, 1.79, 95% confidence interval, 1.27-2.52), diabetes (10.3% vs. 4.7%; OR, 2.33; 95% CI, 1.53-3.56), and smoking (7.7% current smokers vs. 4.3% never smokers; OR, 1.87; 95% CI, 1.21-2.90). After adjusting for age, sex, and race, the associations remained significant.
The researchers found no significant effect of high cholesterol (6.9% vs. 5.2%; OR, 1.35; 95% CI, 0.80-2.28) or obesity (6.1% vs. 4.8%; OR, 1.29; 95% CI, 0.92-1.82) on accelerated cognitive decline.
Compared with participants with no CVRFs, the likelihood of accelerated cognitive decline was higher for individuals with one or two risk factors (OR, 1.94; 95% CI, 1.16-3.25) and was higher still for those with three or more risk factors (OR, 3.51; 95% CI, 2.05-6.00).
The fact that there was no association between midlife cognitive decline and obesity or high cholesterol did not come as a surprise, said Dr. Yaffe. “Most studies have not shown a consistent finding with high cholesterol and later-life cognition, so it is not surprising we did not see one in midlife, when there is not as much cognitive change.”
The study’s results, said Dr. Yaffe, provide physicians with another good reason to help patients address CVRFs and to work with them to lower blood pressure, stop smoking, reduce diabetes incidence, or control diabetes.
Dr. Yaffe said she and her colleagues plan further research into CVRFs and accelerated cognitive decline. “We want to know if this earlier cognitive decline [in midlife] is connected to greater decline later in life. We also want to know if improving these risk factors in midlife might prevent or slow dementia later.”
More to explore
Commenting on the findings, Michelle M. Mielke, PhD, professor of epidemiology and neurology at Mayo Clinic, Rochester, Minn., said one of the study’s main implications “is that the prevention and treatment of midlife hypertension and diabetes and smoking cessation directly impacts shorter-term changes in cognition.”
She added that the study also provides a foundation for answering further questions about the effects of CVRFs on cognition in midlife. For example, questions about sex differences remain unanswered. Men develop CVRFs earlier than women, but the investigators did not provide the prevalence of cardiovascular risk factors by sex.
“It was also not reported whether a specific midlife cardiovascular risk factor was more strongly associated with accelerated cognitive decline for women or for men,” she said. In addition, the mean age of the population at baseline is the approximate age of the onset of menopause, after which cardiovascular risk factors increase among women.
“Additional research is needed to understand the emergence of cardiovascular risk factors pre- versus post menopause on subsequent cognition and also consider the use of menopausal hormone therapy,” said Dr. Mielke.
“Another future research avenue is to further understand the impact of antihypertensive and diabetes medications,” she continued. “For example, in the current study, it was not clear how many [participants] with hypertension were treated versus untreated and whether this impacted subsequent cognition. Similarly, it is not known whether specific antihypertensives are more beneficial for cognition in midlife.”
CARDIA is supported by the National Heart, Lung, and Blood Institute; the University of Alabama at Birmingham; Northwestern University, Chicago; the University of Minnesota; and the Kaiser Foundation Research Institute. Dr. Yaffe serves on data safety monitoring boards for Eli Lilly and studies sponsored by the National Institute on Aging. She is a board member of Alector and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Dr. Mielke has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Still no clear answer on intranasal insulin for MCI and Alzheimer’s disease
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY
Repetitive hits to the head tied to depression, poor cognition in later life
A history of repetitive hits to the head (RHI), even without noticeable symptoms, is linked to a significantly increased risk of depression and poorer cognition later in life, new research shows.
“We found that a history of exposure to [repetitive hits to the head] from contact sports, military service, or physical abuse, as well as a history of TBI (traumatic brain injury), corresponded to more symptoms of later life depression and worse cognitive function,” lead author Michael Alosco, PhD, associate professor of neurology and codirector of the Boston University Alzheimer’s Disease Center Clinical Core, told Medscape Medical News.
He added that the findings underscore the importance of assessing repetitive head impacts (RHI).
The study was published online June 26 in Neurology.
Largest study to date
It is well known that sustaining a TBI is associated with worse later life cognition or mood problems, said Alosco. However, in the current research the investigators hypothesized that RHI may be a key driver of some of these outcomes, Alosco said.
Previous studies have been small or have only examined male former football players.
“What’s unique about our study is that we focused on a history of RHIs, and it is the largest study of its kind, incorporating over 30,000 males and females with different types of exposure to these RHIs.”
The researchers used data from the Brain Health Registry, an internet-based registry that longitudinally monitors cognition and functioning of participants (age 40 years and older).
Participants completed the Ohio State University TBI Identification Method (OSU TBI-ID) and answered a yes/no question: “Have you ever had a period of time in which you experienced multiple, repeated impacts to your head (eg, history of abuse, contact sports, military duty)?”
Participants also completed the Geriatric Depression Scale (GDS-15), the CogState Battery (CBB), and the Lumos Labs NeuroCognitive Performance Tests (NCPT). Demographic information included age, sex, race/ethnicity, and level of education.
Negative synergistic effect
Of the total sample (N = 13,323, mean age 62 years, 72.5% female, 88.6% White) 725 participants (5%) reported exposure to RHI, with contact sports as the most common cause, followed by physical abuse and then military duty; about 55% (7277 participants) reported TBI.
The researchers noted that 44.4% of those exposed to RHI and 70.3% of those who reported TBI were female. However, those with a history of contact sports were predominantly male and those reporting a history of abuse were predominantly women.
Among study participants who completed the GDS-15, 16.4% reported symptoms of depression, similar to rates reported among community-dwelling older adults.
Compared to the unexposed group, participants who reported TBI with loss of consciousness (LOC) and participants who reported TBI without LOC both had higher scores on the GDS-15 (beta = 0.75 [95% CI, 0.59-0.91] and beta = 0.43 [95% CI, 0.31-0.54], respectively).
A history of RHI was associated with an even higher depression score (beta = 1.24 [95% CI, 0.36-2.12).
Depression increased in tandem with increased exposure, with the lowest GDS-15 scores found in the unexposed group and subsequent increases in scores as exposure to RHI was introduced and TBI severity increased. The GDS scores were highest in those who had RHI plus TBI with LOC.
Participants with a history of RHI and/or TBI also had worse scores on tests of memory, learning, processing speed, and reaction time, compared with unexposed participants.
In particular, TBI with LOC had the most neuropsychological associations.
TBI without LOC had a negative effect on CogState tests measuring Identification and processing speed (beta = 0.004 [95% CI, 0-0.01] and beta = 0.004 [95% CI, 0.0002-0.01], respectively), whereas RHI predicted a worse processing speed score (beta = .02 [95% CI, 0.01-0.05]).
The presence of both RHI and TBI (with or without LOC) had a “synergistic negative effect” on neuropsychological performance, with a “consistent statistically significant finding” for worse neuropsychological test performance for those who had RHI and TBI with LOC, compared with those who had not sustained RHI.
Alosco said the findings highlight the need for clinicians to educate and inform parents/guardians of kids playing (or considering playing) contact sports about the research and potential risks associated with these activities.
If we want to prevent long-term problems, one way is not to expose [people] to these hits. Everyone takes risks in life with everything, but the more we can understand and mitigate the risks, the better,” Alosco said.
“A significant contribution”
Commenting on the findings for Medscape Medical News, Temitayo Oyegbile-Chidi, MD, PhD, a pediatric neurologist with Health Peak Inc, McLean, Virginia, and a member of the American Academy of Neurology, said the study “makes a significant contribution to the literature, as neurologists who specialized in TBI have long yearned to understand the long-term effects of repeated head impact on the brain and cognition.”
Clinicians should “inquire about a history of prior head impacts on all our patients, regardless of age, especially if they are experiencing or showing signs of unexpected cognitive dysfunction or mental health concerns,” said Oyegbile-Chidi, who was not involved with the study.
For those who have sustained single or repeated head impacts with or without associated LOC in the past, “it is important … to keep in mind that depression and cognitive dysfunction may persist or present even many years after the impact was sustained,” she added.
The study was supported by a grant from the National Institutes of Health. Alosco has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Oyegbile-Chidi has disclosed no relevant financial relationships.
A history of repetitive hits to the head (RHI), even without noticeable symptoms, is linked to a significantly increased risk of depression and poorer cognition later in life, new research shows.
“We found that a history of exposure to [repetitive hits to the head] from contact sports, military service, or physical abuse, as well as a history of TBI (traumatic brain injury), corresponded to more symptoms of later life depression and worse cognitive function,” lead author Michael Alosco, PhD, associate professor of neurology and codirector of the Boston University Alzheimer’s Disease Center Clinical Core, told Medscape Medical News.
He added that the findings underscore the importance of assessing repetitive head impacts (RHI).
The study was published online June 26 in Neurology.
Largest study to date
It is well known that sustaining a TBI is associated with worse later life cognition or mood problems, said Alosco. However, in the current research the investigators hypothesized that RHI may be a key driver of some of these outcomes, Alosco said.
Previous studies have been small or have only examined male former football players.
“What’s unique about our study is that we focused on a history of RHIs, and it is the largest study of its kind, incorporating over 30,000 males and females with different types of exposure to these RHIs.”
The researchers used data from the Brain Health Registry, an internet-based registry that longitudinally monitors cognition and functioning of participants (age 40 years and older).
Participants completed the Ohio State University TBI Identification Method (OSU TBI-ID) and answered a yes/no question: “Have you ever had a period of time in which you experienced multiple, repeated impacts to your head (eg, history of abuse, contact sports, military duty)?”
Participants also completed the Geriatric Depression Scale (GDS-15), the CogState Battery (CBB), and the Lumos Labs NeuroCognitive Performance Tests (NCPT). Demographic information included age, sex, race/ethnicity, and level of education.
Negative synergistic effect
Of the total sample (N = 13,323, mean age 62 years, 72.5% female, 88.6% White) 725 participants (5%) reported exposure to RHI, with contact sports as the most common cause, followed by physical abuse and then military duty; about 55% (7277 participants) reported TBI.
The researchers noted that 44.4% of those exposed to RHI and 70.3% of those who reported TBI were female. However, those with a history of contact sports were predominantly male and those reporting a history of abuse were predominantly women.
Among study participants who completed the GDS-15, 16.4% reported symptoms of depression, similar to rates reported among community-dwelling older adults.
Compared to the unexposed group, participants who reported TBI with loss of consciousness (LOC) and participants who reported TBI without LOC both had higher scores on the GDS-15 (beta = 0.75 [95% CI, 0.59-0.91] and beta = 0.43 [95% CI, 0.31-0.54], respectively).
A history of RHI was associated with an even higher depression score (beta = 1.24 [95% CI, 0.36-2.12).
Depression increased in tandem with increased exposure, with the lowest GDS-15 scores found in the unexposed group and subsequent increases in scores as exposure to RHI was introduced and TBI severity increased. The GDS scores were highest in those who had RHI plus TBI with LOC.
Participants with a history of RHI and/or TBI also had worse scores on tests of memory, learning, processing speed, and reaction time, compared with unexposed participants.
In particular, TBI with LOC had the most neuropsychological associations.
TBI without LOC had a negative effect on CogState tests measuring Identification and processing speed (beta = 0.004 [95% CI, 0-0.01] and beta = 0.004 [95% CI, 0.0002-0.01], respectively), whereas RHI predicted a worse processing speed score (beta = .02 [95% CI, 0.01-0.05]).
The presence of both RHI and TBI (with or without LOC) had a “synergistic negative effect” on neuropsychological performance, with a “consistent statistically significant finding” for worse neuropsychological test performance for those who had RHI and TBI with LOC, compared with those who had not sustained RHI.
Alosco said the findings highlight the need for clinicians to educate and inform parents/guardians of kids playing (or considering playing) contact sports about the research and potential risks associated with these activities.
If we want to prevent long-term problems, one way is not to expose [people] to these hits. Everyone takes risks in life with everything, but the more we can understand and mitigate the risks, the better,” Alosco said.
“A significant contribution”
Commenting on the findings for Medscape Medical News, Temitayo Oyegbile-Chidi, MD, PhD, a pediatric neurologist with Health Peak Inc, McLean, Virginia, and a member of the American Academy of Neurology, said the study “makes a significant contribution to the literature, as neurologists who specialized in TBI have long yearned to understand the long-term effects of repeated head impact on the brain and cognition.”
Clinicians should “inquire about a history of prior head impacts on all our patients, regardless of age, especially if they are experiencing or showing signs of unexpected cognitive dysfunction or mental health concerns,” said Oyegbile-Chidi, who was not involved with the study.
For those who have sustained single or repeated head impacts with or without associated LOC in the past, “it is important … to keep in mind that depression and cognitive dysfunction may persist or present even many years after the impact was sustained,” she added.
The study was supported by a grant from the National Institutes of Health. Alosco has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Oyegbile-Chidi has disclosed no relevant financial relationships.
A history of repetitive hits to the head (RHI), even without noticeable symptoms, is linked to a significantly increased risk of depression and poorer cognition later in life, new research shows.
“We found that a history of exposure to [repetitive hits to the head] from contact sports, military service, or physical abuse, as well as a history of TBI (traumatic brain injury), corresponded to more symptoms of later life depression and worse cognitive function,” lead author Michael Alosco, PhD, associate professor of neurology and codirector of the Boston University Alzheimer’s Disease Center Clinical Core, told Medscape Medical News.
He added that the findings underscore the importance of assessing repetitive head impacts (RHI).
The study was published online June 26 in Neurology.
Largest study to date
It is well known that sustaining a TBI is associated with worse later life cognition or mood problems, said Alosco. However, in the current research the investigators hypothesized that RHI may be a key driver of some of these outcomes, Alosco said.
Previous studies have been small or have only examined male former football players.
“What’s unique about our study is that we focused on a history of RHIs, and it is the largest study of its kind, incorporating over 30,000 males and females with different types of exposure to these RHIs.”
The researchers used data from the Brain Health Registry, an internet-based registry that longitudinally monitors cognition and functioning of participants (age 40 years and older).
Participants completed the Ohio State University TBI Identification Method (OSU TBI-ID) and answered a yes/no question: “Have you ever had a period of time in which you experienced multiple, repeated impacts to your head (eg, history of abuse, contact sports, military duty)?”
Participants also completed the Geriatric Depression Scale (GDS-15), the CogState Battery (CBB), and the Lumos Labs NeuroCognitive Performance Tests (NCPT). Demographic information included age, sex, race/ethnicity, and level of education.
Negative synergistic effect
Of the total sample (N = 13,323, mean age 62 years, 72.5% female, 88.6% White) 725 participants (5%) reported exposure to RHI, with contact sports as the most common cause, followed by physical abuse and then military duty; about 55% (7277 participants) reported TBI.
The researchers noted that 44.4% of those exposed to RHI and 70.3% of those who reported TBI were female. However, those with a history of contact sports were predominantly male and those reporting a history of abuse were predominantly women.
Among study participants who completed the GDS-15, 16.4% reported symptoms of depression, similar to rates reported among community-dwelling older adults.
Compared to the unexposed group, participants who reported TBI with loss of consciousness (LOC) and participants who reported TBI without LOC both had higher scores on the GDS-15 (beta = 0.75 [95% CI, 0.59-0.91] and beta = 0.43 [95% CI, 0.31-0.54], respectively).
A history of RHI was associated with an even higher depression score (beta = 1.24 [95% CI, 0.36-2.12).
Depression increased in tandem with increased exposure, with the lowest GDS-15 scores found in the unexposed group and subsequent increases in scores as exposure to RHI was introduced and TBI severity increased. The GDS scores were highest in those who had RHI plus TBI with LOC.
Participants with a history of RHI and/or TBI also had worse scores on tests of memory, learning, processing speed, and reaction time, compared with unexposed participants.
In particular, TBI with LOC had the most neuropsychological associations.
TBI without LOC had a negative effect on CogState tests measuring Identification and processing speed (beta = 0.004 [95% CI, 0-0.01] and beta = 0.004 [95% CI, 0.0002-0.01], respectively), whereas RHI predicted a worse processing speed score (beta = .02 [95% CI, 0.01-0.05]).
The presence of both RHI and TBI (with or without LOC) had a “synergistic negative effect” on neuropsychological performance, with a “consistent statistically significant finding” for worse neuropsychological test performance for those who had RHI and TBI with LOC, compared with those who had not sustained RHI.
Alosco said the findings highlight the need for clinicians to educate and inform parents/guardians of kids playing (or considering playing) contact sports about the research and potential risks associated with these activities.
If we want to prevent long-term problems, one way is not to expose [people] to these hits. Everyone takes risks in life with everything, but the more we can understand and mitigate the risks, the better,” Alosco said.
“A significant contribution”
Commenting on the findings for Medscape Medical News, Temitayo Oyegbile-Chidi, MD, PhD, a pediatric neurologist with Health Peak Inc, McLean, Virginia, and a member of the American Academy of Neurology, said the study “makes a significant contribution to the literature, as neurologists who specialized in TBI have long yearned to understand the long-term effects of repeated head impact on the brain and cognition.”
Clinicians should “inquire about a history of prior head impacts on all our patients, regardless of age, especially if they are experiencing or showing signs of unexpected cognitive dysfunction or mental health concerns,” said Oyegbile-Chidi, who was not involved with the study.
For those who have sustained single or repeated head impacts with or without associated LOC in the past, “it is important … to keep in mind that depression and cognitive dysfunction may persist or present even many years after the impact was sustained,” she added.
The study was supported by a grant from the National Institutes of Health. Alosco has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Oyegbile-Chidi has disclosed no relevant financial relationships.
Move over supplements, here come medical foods
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
Blood biomarker detects concussion, shows severity, predicts recovery
(TBI), new research indicates.
“Blood NfL may be used to aid in the diagnosis of patients with concussion or mild TBI [and] to identify individuals at increased risk of developing persistent postconcussive symptoms following TBI,” said lead author Pashtun Shahim, MD, PhD, National Institutes of Health Clinical Center, Bethesda, Md.
“This study is the first to do a detailed assessment of serum NfL chain and advanced brain imaging in multiple cohorts, brain injury severities, and time points after injury. The cohorts included professional athletes and nonathletes, and over time up to 5 years after TBI,” Dr. Shahim added.
The study was published online July 8 in Neurology.
Rapid indicator of neuronal damage
The researchers studied two cohorts of patients with head injuries. In the first, they determined serum and CSF NfL chain levels in professional Swedish ice hockey players (median age, 27 years), including 45 with acute concussion, 31 with repetitive concussions and persistent post-concussive symptoms (PCS), 28 who contributed samples during preseason with no recent concussion, and 14 healthy nonathletes.
CSF and serum NfL concentrations were closely correlated (r = 0.71; P < .0001). Serum NfL distinguished players with persistent PCS due to repetitive concussions from preseason concussion-free players, with an area under the receiver operating characteristic curve of 0.97. Higher CSF and serum NfL levels were associated with a higher number of concussions and severity of PCS after 1 year.
The second cohort involved 230 clinic-based adults (mean age, 43 years), including 162 with TBI and 68 healthy controls. In this cohort, patients with TBI had increased serum NfL concentrations compared with controls for up to 5 years, and these concentrations were able to distinguish between mild, moderate, and severe TBI. Serum NfL also correlated with measures of functional outcome, MRI brain atrophy, and diffusion tensor imaging estimates of traumatic axonal injury.
“Our findings suggest that NfL concentrations in serum offer rapid and accessible means of assessing and predicting neuronal damage in patients with TBI,” the investigators wrote.
What’s needed going forward, said Dr. Shahim, is “validation in larger cohorts for determining what levels of NfL in blood may be associated with a specific type of TBI, and what the levels are in healthy individuals of different ages.”
Not ready for prime time
In an accompanying editorial, Christopher Filley, MD, University of Colorado at Denver, Aurora, noted that NfL “may prove useful in identifying TBI patients at risk for prolonged symptoms and in enabling more focused treatment for these individuals.”
“These reports are richly laden with acute and longitudinal data that not only support the use of NfL as a convenient diagnostic test for TBI, but plausibly correlate with the neuropathology of TBI that is thought to play a major role in immediate and lasting cognitive disability,” he wrote.
Although the origin of TBI-induced cognitive decline is not entirely explained by traumatic axonal injury, “NfL appears to have much promise as a blood test that relates directly to the ubiquitous white matter damage of TBI, revealing a great deal about not only whether a TBI occurred, but also the extent of injury sustained, and how this injury may affect patient outcome for years thereafter,” Dr. Filley wrote.
However, he cautioned more research is needed before the blood test can be routinely applied to TBI diagnosis in clinical practice. “Among the hurdles still ahead are the standardization of measurement techniques across analytical platforms, and the determination of precise cutoffs between normal and abnormal values in different ages groups and at varying levels of TBI severity,” Dr. Filley noted.
The research was supported by the National Institutes of Health, the Department of Defense, the Center for Neuroscience and Regenerative Medicine at the Uniformed Services University, and the Swedish Research Council. Dr. Shahim and Dr. Filley have reported no relevant financial relationships.
This article first appeared on Medscape.com.
(TBI), new research indicates.
“Blood NfL may be used to aid in the diagnosis of patients with concussion or mild TBI [and] to identify individuals at increased risk of developing persistent postconcussive symptoms following TBI,” said lead author Pashtun Shahim, MD, PhD, National Institutes of Health Clinical Center, Bethesda, Md.
“This study is the first to do a detailed assessment of serum NfL chain and advanced brain imaging in multiple cohorts, brain injury severities, and time points after injury. The cohorts included professional athletes and nonathletes, and over time up to 5 years after TBI,” Dr. Shahim added.
The study was published online July 8 in Neurology.
Rapid indicator of neuronal damage
The researchers studied two cohorts of patients with head injuries. In the first, they determined serum and CSF NfL chain levels in professional Swedish ice hockey players (median age, 27 years), including 45 with acute concussion, 31 with repetitive concussions and persistent post-concussive symptoms (PCS), 28 who contributed samples during preseason with no recent concussion, and 14 healthy nonathletes.
CSF and serum NfL concentrations were closely correlated (r = 0.71; P < .0001). Serum NfL distinguished players with persistent PCS due to repetitive concussions from preseason concussion-free players, with an area under the receiver operating characteristic curve of 0.97. Higher CSF and serum NfL levels were associated with a higher number of concussions and severity of PCS after 1 year.
The second cohort involved 230 clinic-based adults (mean age, 43 years), including 162 with TBI and 68 healthy controls. In this cohort, patients with TBI had increased serum NfL concentrations compared with controls for up to 5 years, and these concentrations were able to distinguish between mild, moderate, and severe TBI. Serum NfL also correlated with measures of functional outcome, MRI brain atrophy, and diffusion tensor imaging estimates of traumatic axonal injury.
“Our findings suggest that NfL concentrations in serum offer rapid and accessible means of assessing and predicting neuronal damage in patients with TBI,” the investigators wrote.
What’s needed going forward, said Dr. Shahim, is “validation in larger cohorts for determining what levels of NfL in blood may be associated with a specific type of TBI, and what the levels are in healthy individuals of different ages.”
Not ready for prime time
In an accompanying editorial, Christopher Filley, MD, University of Colorado at Denver, Aurora, noted that NfL “may prove useful in identifying TBI patients at risk for prolonged symptoms and in enabling more focused treatment for these individuals.”
“These reports are richly laden with acute and longitudinal data that not only support the use of NfL as a convenient diagnostic test for TBI, but plausibly correlate with the neuropathology of TBI that is thought to play a major role in immediate and lasting cognitive disability,” he wrote.
Although the origin of TBI-induced cognitive decline is not entirely explained by traumatic axonal injury, “NfL appears to have much promise as a blood test that relates directly to the ubiquitous white matter damage of TBI, revealing a great deal about not only whether a TBI occurred, but also the extent of injury sustained, and how this injury may affect patient outcome for years thereafter,” Dr. Filley wrote.
However, he cautioned more research is needed before the blood test can be routinely applied to TBI diagnosis in clinical practice. “Among the hurdles still ahead are the standardization of measurement techniques across analytical platforms, and the determination of precise cutoffs between normal and abnormal values in different ages groups and at varying levels of TBI severity,” Dr. Filley noted.
The research was supported by the National Institutes of Health, the Department of Defense, the Center for Neuroscience and Regenerative Medicine at the Uniformed Services University, and the Swedish Research Council. Dr. Shahim and Dr. Filley have reported no relevant financial relationships.
This article first appeared on Medscape.com.
(TBI), new research indicates.
“Blood NfL may be used to aid in the diagnosis of patients with concussion or mild TBI [and] to identify individuals at increased risk of developing persistent postconcussive symptoms following TBI,” said lead author Pashtun Shahim, MD, PhD, National Institutes of Health Clinical Center, Bethesda, Md.
“This study is the first to do a detailed assessment of serum NfL chain and advanced brain imaging in multiple cohorts, brain injury severities, and time points after injury. The cohorts included professional athletes and nonathletes, and over time up to 5 years after TBI,” Dr. Shahim added.
The study was published online July 8 in Neurology.
Rapid indicator of neuronal damage
The researchers studied two cohorts of patients with head injuries. In the first, they determined serum and CSF NfL chain levels in professional Swedish ice hockey players (median age, 27 years), including 45 with acute concussion, 31 with repetitive concussions and persistent post-concussive symptoms (PCS), 28 who contributed samples during preseason with no recent concussion, and 14 healthy nonathletes.
CSF and serum NfL concentrations were closely correlated (r = 0.71; P < .0001). Serum NfL distinguished players with persistent PCS due to repetitive concussions from preseason concussion-free players, with an area under the receiver operating characteristic curve of 0.97. Higher CSF and serum NfL levels were associated with a higher number of concussions and severity of PCS after 1 year.
The second cohort involved 230 clinic-based adults (mean age, 43 years), including 162 with TBI and 68 healthy controls. In this cohort, patients with TBI had increased serum NfL concentrations compared with controls for up to 5 years, and these concentrations were able to distinguish between mild, moderate, and severe TBI. Serum NfL also correlated with measures of functional outcome, MRI brain atrophy, and diffusion tensor imaging estimates of traumatic axonal injury.
“Our findings suggest that NfL concentrations in serum offer rapid and accessible means of assessing and predicting neuronal damage in patients with TBI,” the investigators wrote.
What’s needed going forward, said Dr. Shahim, is “validation in larger cohorts for determining what levels of NfL in blood may be associated with a specific type of TBI, and what the levels are in healthy individuals of different ages.”
Not ready for prime time
In an accompanying editorial, Christopher Filley, MD, University of Colorado at Denver, Aurora, noted that NfL “may prove useful in identifying TBI patients at risk for prolonged symptoms and in enabling more focused treatment for these individuals.”
“These reports are richly laden with acute and longitudinal data that not only support the use of NfL as a convenient diagnostic test for TBI, but plausibly correlate with the neuropathology of TBI that is thought to play a major role in immediate and lasting cognitive disability,” he wrote.
Although the origin of TBI-induced cognitive decline is not entirely explained by traumatic axonal injury, “NfL appears to have much promise as a blood test that relates directly to the ubiquitous white matter damage of TBI, revealing a great deal about not only whether a TBI occurred, but also the extent of injury sustained, and how this injury may affect patient outcome for years thereafter,” Dr. Filley wrote.
However, he cautioned more research is needed before the blood test can be routinely applied to TBI diagnosis in clinical practice. “Among the hurdles still ahead are the standardization of measurement techniques across analytical platforms, and the determination of precise cutoffs between normal and abnormal values in different ages groups and at varying levels of TBI severity,” Dr. Filley noted.
The research was supported by the National Institutes of Health, the Department of Defense, the Center for Neuroscience and Regenerative Medicine at the Uniformed Services University, and the Swedish Research Council. Dr. Shahim and Dr. Filley have reported no relevant financial relationships.
This article first appeared on Medscape.com.
New insight into neurobehavioral effects of legalized cannabis
Researchers have published one of the first studies to characterize the association between the consumption of legal cannabis and subsequent pharmacologic and neurobehavioral outcomes, with somewhat surprising results.
The study showed that, although cannabis consumption did not affect most short-term neurobehavioral measures, it delayed recall memory and impaired balance.
The investigation also showed that users of much more potent cannabis concentrates actually demonstrated similar or lower levels of subjective drug intoxication and short-term impairment than did their counterparts who used lower-potency forms of the cannabis flower.
“It does not appear that the potency being used matters that much,” senior investigator Kent E. Hutchison, PhD, said in an interview. “People seem to be titrating to a certain level of intoxication or a certain level of feeling high. And for some people that requires a lot of drug, and for other people not as much.”
added Dr. Hutchison, a professor of psychology and neuroscience at the University of Colorado Boulder.
The study was published online June 10 in JAMA Psychiatry.
Widespread availability, little research
Recreational cannabis is now legal in 11 states and the District of Columbia, while medical cannabis is legal in 33. However, despite growing popularity of cannabis, there is little research on its potential health and biobehavioral risks, largely because of federal restrictions on cannabis research.
Cannabis users typically consume various forms of the cannabis flower, which can boast concentrations of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC) of up to 30%. However, use of concentrated forms of cannabis – which are made by extracting plant cannabinoids into a different form – is increasing.
Such formulations can boast THC concentrations as high as 90%. Nevertheless, data regarding the relative risks of these higher-strength products are limited.
Previous research has shown a variety of negative short-term and long-term neurobehavioral effects associated with cannabis use, including harmful cognitive and motor effects. Extended exposure to THC may also negatively affect brain regions that are associated with the control of coordinated movement, and create brain-activation deficits in motor control regions that persist well beyond the effects of short-term intoxication.
Despite such findings, Dr. Hutchison said the existing literature on the subject does not yield a real-world view of current cannabis use because it tends to focus on low-THC products that are increasingly less common in today’s legal market.
Given such shortcomings, the investigators wanted to address persistent questions surrounding the neurobehavioral effects of legal cannabis flower products (16% or 24% THC) and cannabis concentrate products (70% or 90% THC). In doing so, they examined three primary topics:
- The association between short-term use of these products and THC plasma levels; subjective intoxication; and mood, cognitive performance, and balance
- Differences in such associations between users of cannabis flower and concentrate products
- Potential variations in these associations by THC potency
High- versus low-potency varieties
The study included 133 individuals (aged 21-70 years), who were designated as either cannabis flower users or cannabis concentrate users. Participants had all used cannabis at least four times in the previous month with no adverse reaction and were not receiving treatment for a psychotic disorder or bipolar disorder.
Participants were randomly assigned to consume either higher-potency or lower-potency products that had been purchased from a local dispensary. Flower users were randomized to purchase 3 g of either a 16% THC or 24% THC product, while concentrate users were randomized to purchase 1g of either 70% THC or 90% THC.
Participants completed a series of four assessments, one at baseline and three others at a mobile laboratory. The mobile laboratory assessments occurred before, immediately after, and one hour after participants had all consumed their cannabis ad libitum.
Of the original cohort of 133 participants, 55 flower cannabis users (mean age, 28.8 years; 46% women) and 66 concentrate cannabis users (mean age, 28.3 years; 45% women) complied with the study’s instructions and had complete data.
The study’s primary outcome measures included plasma cannabinoids, subjective drug intoxication and mood, and neurobehavioral outcomes such as attention, memory, inhibitory control, and balance.
Mixed results
With respect to cannabis concentrations, results showed that users of concentrate exhibited higher levels of both plasma THC and the active metabolite of THC (11-hydroxy-delta9-THC) across all points than did their counterparts who used cannabis flower products.
Specifically, mean plasma THC levels were 1,016 ± 1,380 mcg/ml in concentrate users and 455±503 mcg/mL in flower users after ad libitum cannabis consumption. Nevertheless, self-reported levels of intoxication were no different between users of cannabis flower or concentrate products.
Although results also showed that most neurobehavioral measures were not altered by short-term cannabis consumption, there were some notable exceptions. There was a negative linear effect with delayed verbal recall errors, suggesting poorer performance after cannabis use (F1, 203 = 32.31; P < .001).
On the other hand, investigators found a positive linear effect with inhibitory control and working memory, which actually suggests better performance after cannabis use. This finding, the researchers note, may be the result of a practice effect. Cannabis flower users performed better across all inhibitory control assessments.
The researchers also tested participants’ balance with their eyes open and closed. In the eyes-open condition, they found a trend toward impaired balance after cannabis use, though this normalized within an hour. When subjects closed their eyes, however, researchers observed a significant short-term increase in sway after cannabis use, which fell back to pre-use levels one hour after use (F1, 203 = 18.88; P < .001).
Of note, outcomes did not differ between groups according to the type of cannabis product consumed or its relative potency.
The study yielded several surprising findings, beginning with self-reported intoxication levels, which were not statistically significant between different cannabis flower and concentrate users, despite significantly different plasma THC levels between the two groups.
Dr. Hutchison explained that this may be the result of greater THC tolerance among concentrate users, THC saturation of cannabinoid receptors, or interindividual differences among users with respect to cannabis metabolism or sensitivity.
“I thought for sure that high-potency users would be much more compromised,” he said. “I guess it just goes to show we have a lot to learn about how these things work.”
Additionally, there were virtually no significant changes in acute performance after cannabis use, with the exception of delayed verbal recall. In fact, the most marked change observed in the study was the effect of cannabis on balance immediately after drug use, though these changes seemed to abate within an hour.
Nevertheless, the study highlights several potential public health implications of cannabis consumption, Dr. Hutchison added. “What happens when people with high blood concentrations decide to quit?” he asked. “Do they have trouble quitting? Do they have withdrawal symptoms?”
The long-term effects of cannabis use is another important question that still needs to be answered, he added.
Finally, Dr. Hutchison noted that, although the study showed little difference between users of cannabis flower and concentrates, study participants were all experienced users.
“There is certainly the potential for harm when a naive person uses cannabis concentrate,” he said. “Suddenly they have way more THC than they thought they were going to get, and that’s where a lot of people get into trouble with cannabis.”
Pitfalls and hurdles
In an accompanying editorial, Margaret Haney, PhD, of Columbia University Irving Medical Center, New York, explained that cannabis’ awkward position as simultaneously legal and illegal, medical and recreational, has hampered researchers’ ability to study its effects as comprehensively as they would otherwise like.
“With a federally illegal drug legalized in individual states, scientists constrained, and federal agencies somewhat silent, clinicians have none of the data that guide their decisions for other medications (eg, which indication, product, cannabinoid ratio, dose, or route of administration; what risks for individual patients [eg, pregnant, adolescent, psychiatric?]),” Dr. Haney wrote.
These pitfalls are compounded by the significant regulatory hurdles.
“The FDA is appropriately cautious about what it allows scientists to test in patients, and none of the products available in dispensaries or online have undergone the safety and manufacturing procedures needed for FDA approval,” she continued. “How then to conduct the studies so needed?”
Yet as Haney noted, giving cannabinoid researchers a Schedule I exemption may help address many of the barriers facing these scientists. Such a move, she said, would increase the number of randomized controlled trials being performed, “and thereby begin to breach the divide between the use of these products and empirical evidence.”
Dr. Hutchison has disclosed no relevant financial relationships. Dr. Haney disclosed funding from the US National Institute on Drug Abuse and from the Thompson Family Foundation Initiative. The study was funded by the NIH and Colorado Department of Public Health and Environment.
A version of this article originally appeared on Medscape.com.
Researchers have published one of the first studies to characterize the association between the consumption of legal cannabis and subsequent pharmacologic and neurobehavioral outcomes, with somewhat surprising results.
The study showed that, although cannabis consumption did not affect most short-term neurobehavioral measures, it delayed recall memory and impaired balance.
The investigation also showed that users of much more potent cannabis concentrates actually demonstrated similar or lower levels of subjective drug intoxication and short-term impairment than did their counterparts who used lower-potency forms of the cannabis flower.
“It does not appear that the potency being used matters that much,” senior investigator Kent E. Hutchison, PhD, said in an interview. “People seem to be titrating to a certain level of intoxication or a certain level of feeling high. And for some people that requires a lot of drug, and for other people not as much.”
added Dr. Hutchison, a professor of psychology and neuroscience at the University of Colorado Boulder.
The study was published online June 10 in JAMA Psychiatry.
Widespread availability, little research
Recreational cannabis is now legal in 11 states and the District of Columbia, while medical cannabis is legal in 33. However, despite growing popularity of cannabis, there is little research on its potential health and biobehavioral risks, largely because of federal restrictions on cannabis research.
Cannabis users typically consume various forms of the cannabis flower, which can boast concentrations of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC) of up to 30%. However, use of concentrated forms of cannabis – which are made by extracting plant cannabinoids into a different form – is increasing.
Such formulations can boast THC concentrations as high as 90%. Nevertheless, data regarding the relative risks of these higher-strength products are limited.
Previous research has shown a variety of negative short-term and long-term neurobehavioral effects associated with cannabis use, including harmful cognitive and motor effects. Extended exposure to THC may also negatively affect brain regions that are associated with the control of coordinated movement, and create brain-activation deficits in motor control regions that persist well beyond the effects of short-term intoxication.
Despite such findings, Dr. Hutchison said the existing literature on the subject does not yield a real-world view of current cannabis use because it tends to focus on low-THC products that are increasingly less common in today’s legal market.
Given such shortcomings, the investigators wanted to address persistent questions surrounding the neurobehavioral effects of legal cannabis flower products (16% or 24% THC) and cannabis concentrate products (70% or 90% THC). In doing so, they examined three primary topics:
- The association between short-term use of these products and THC plasma levels; subjective intoxication; and mood, cognitive performance, and balance
- Differences in such associations between users of cannabis flower and concentrate products
- Potential variations in these associations by THC potency
High- versus low-potency varieties
The study included 133 individuals (aged 21-70 years), who were designated as either cannabis flower users or cannabis concentrate users. Participants had all used cannabis at least four times in the previous month with no adverse reaction and were not receiving treatment for a psychotic disorder or bipolar disorder.
Participants were randomly assigned to consume either higher-potency or lower-potency products that had been purchased from a local dispensary. Flower users were randomized to purchase 3 g of either a 16% THC or 24% THC product, while concentrate users were randomized to purchase 1g of either 70% THC or 90% THC.
Participants completed a series of four assessments, one at baseline and three others at a mobile laboratory. The mobile laboratory assessments occurred before, immediately after, and one hour after participants had all consumed their cannabis ad libitum.
Of the original cohort of 133 participants, 55 flower cannabis users (mean age, 28.8 years; 46% women) and 66 concentrate cannabis users (mean age, 28.3 years; 45% women) complied with the study’s instructions and had complete data.
The study’s primary outcome measures included plasma cannabinoids, subjective drug intoxication and mood, and neurobehavioral outcomes such as attention, memory, inhibitory control, and balance.
Mixed results
With respect to cannabis concentrations, results showed that users of concentrate exhibited higher levels of both plasma THC and the active metabolite of THC (11-hydroxy-delta9-THC) across all points than did their counterparts who used cannabis flower products.
Specifically, mean plasma THC levels were 1,016 ± 1,380 mcg/ml in concentrate users and 455±503 mcg/mL in flower users after ad libitum cannabis consumption. Nevertheless, self-reported levels of intoxication were no different between users of cannabis flower or concentrate products.
Although results also showed that most neurobehavioral measures were not altered by short-term cannabis consumption, there were some notable exceptions. There was a negative linear effect with delayed verbal recall errors, suggesting poorer performance after cannabis use (F1, 203 = 32.31; P < .001).
On the other hand, investigators found a positive linear effect with inhibitory control and working memory, which actually suggests better performance after cannabis use. This finding, the researchers note, may be the result of a practice effect. Cannabis flower users performed better across all inhibitory control assessments.
The researchers also tested participants’ balance with their eyes open and closed. In the eyes-open condition, they found a trend toward impaired balance after cannabis use, though this normalized within an hour. When subjects closed their eyes, however, researchers observed a significant short-term increase in sway after cannabis use, which fell back to pre-use levels one hour after use (F1, 203 = 18.88; P < .001).
Of note, outcomes did not differ between groups according to the type of cannabis product consumed or its relative potency.
The study yielded several surprising findings, beginning with self-reported intoxication levels, which were not statistically significant between different cannabis flower and concentrate users, despite significantly different plasma THC levels between the two groups.
Dr. Hutchison explained that this may be the result of greater THC tolerance among concentrate users, THC saturation of cannabinoid receptors, or interindividual differences among users with respect to cannabis metabolism or sensitivity.
“I thought for sure that high-potency users would be much more compromised,” he said. “I guess it just goes to show we have a lot to learn about how these things work.”
Additionally, there were virtually no significant changes in acute performance after cannabis use, with the exception of delayed verbal recall. In fact, the most marked change observed in the study was the effect of cannabis on balance immediately after drug use, though these changes seemed to abate within an hour.
Nevertheless, the study highlights several potential public health implications of cannabis consumption, Dr. Hutchison added. “What happens when people with high blood concentrations decide to quit?” he asked. “Do they have trouble quitting? Do they have withdrawal symptoms?”
The long-term effects of cannabis use is another important question that still needs to be answered, he added.
Finally, Dr. Hutchison noted that, although the study showed little difference between users of cannabis flower and concentrates, study participants were all experienced users.
“There is certainly the potential for harm when a naive person uses cannabis concentrate,” he said. “Suddenly they have way more THC than they thought they were going to get, and that’s where a lot of people get into trouble with cannabis.”
Pitfalls and hurdles
In an accompanying editorial, Margaret Haney, PhD, of Columbia University Irving Medical Center, New York, explained that cannabis’ awkward position as simultaneously legal and illegal, medical and recreational, has hampered researchers’ ability to study its effects as comprehensively as they would otherwise like.
“With a federally illegal drug legalized in individual states, scientists constrained, and federal agencies somewhat silent, clinicians have none of the data that guide their decisions for other medications (eg, which indication, product, cannabinoid ratio, dose, or route of administration; what risks for individual patients [eg, pregnant, adolescent, psychiatric?]),” Dr. Haney wrote.
These pitfalls are compounded by the significant regulatory hurdles.
“The FDA is appropriately cautious about what it allows scientists to test in patients, and none of the products available in dispensaries or online have undergone the safety and manufacturing procedures needed for FDA approval,” she continued. “How then to conduct the studies so needed?”
Yet as Haney noted, giving cannabinoid researchers a Schedule I exemption may help address many of the barriers facing these scientists. Such a move, she said, would increase the number of randomized controlled trials being performed, “and thereby begin to breach the divide between the use of these products and empirical evidence.”
Dr. Hutchison has disclosed no relevant financial relationships. Dr. Haney disclosed funding from the US National Institute on Drug Abuse and from the Thompson Family Foundation Initiative. The study was funded by the NIH and Colorado Department of Public Health and Environment.
A version of this article originally appeared on Medscape.com.
Researchers have published one of the first studies to characterize the association between the consumption of legal cannabis and subsequent pharmacologic and neurobehavioral outcomes, with somewhat surprising results.
The study showed that, although cannabis consumption did not affect most short-term neurobehavioral measures, it delayed recall memory and impaired balance.
The investigation also showed that users of much more potent cannabis concentrates actually demonstrated similar or lower levels of subjective drug intoxication and short-term impairment than did their counterparts who used lower-potency forms of the cannabis flower.
“It does not appear that the potency being used matters that much,” senior investigator Kent E. Hutchison, PhD, said in an interview. “People seem to be titrating to a certain level of intoxication or a certain level of feeling high. And for some people that requires a lot of drug, and for other people not as much.”
added Dr. Hutchison, a professor of psychology and neuroscience at the University of Colorado Boulder.
The study was published online June 10 in JAMA Psychiatry.
Widespread availability, little research
Recreational cannabis is now legal in 11 states and the District of Columbia, while medical cannabis is legal in 33. However, despite growing popularity of cannabis, there is little research on its potential health and biobehavioral risks, largely because of federal restrictions on cannabis research.
Cannabis users typically consume various forms of the cannabis flower, which can boast concentrations of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC) of up to 30%. However, use of concentrated forms of cannabis – which are made by extracting plant cannabinoids into a different form – is increasing.
Such formulations can boast THC concentrations as high as 90%. Nevertheless, data regarding the relative risks of these higher-strength products are limited.
Previous research has shown a variety of negative short-term and long-term neurobehavioral effects associated with cannabis use, including harmful cognitive and motor effects. Extended exposure to THC may also negatively affect brain regions that are associated with the control of coordinated movement, and create brain-activation deficits in motor control regions that persist well beyond the effects of short-term intoxication.
Despite such findings, Dr. Hutchison said the existing literature on the subject does not yield a real-world view of current cannabis use because it tends to focus on low-THC products that are increasingly less common in today’s legal market.
Given such shortcomings, the investigators wanted to address persistent questions surrounding the neurobehavioral effects of legal cannabis flower products (16% or 24% THC) and cannabis concentrate products (70% or 90% THC). In doing so, they examined three primary topics:
- The association between short-term use of these products and THC plasma levels; subjective intoxication; and mood, cognitive performance, and balance
- Differences in such associations between users of cannabis flower and concentrate products
- Potential variations in these associations by THC potency
High- versus low-potency varieties
The study included 133 individuals (aged 21-70 years), who were designated as either cannabis flower users or cannabis concentrate users. Participants had all used cannabis at least four times in the previous month with no adverse reaction and were not receiving treatment for a psychotic disorder or bipolar disorder.
Participants were randomly assigned to consume either higher-potency or lower-potency products that had been purchased from a local dispensary. Flower users were randomized to purchase 3 g of either a 16% THC or 24% THC product, while concentrate users were randomized to purchase 1g of either 70% THC or 90% THC.
Participants completed a series of four assessments, one at baseline and three others at a mobile laboratory. The mobile laboratory assessments occurred before, immediately after, and one hour after participants had all consumed their cannabis ad libitum.
Of the original cohort of 133 participants, 55 flower cannabis users (mean age, 28.8 years; 46% women) and 66 concentrate cannabis users (mean age, 28.3 years; 45% women) complied with the study’s instructions and had complete data.
The study’s primary outcome measures included plasma cannabinoids, subjective drug intoxication and mood, and neurobehavioral outcomes such as attention, memory, inhibitory control, and balance.
Mixed results
With respect to cannabis concentrations, results showed that users of concentrate exhibited higher levels of both plasma THC and the active metabolite of THC (11-hydroxy-delta9-THC) across all points than did their counterparts who used cannabis flower products.
Specifically, mean plasma THC levels were 1,016 ± 1,380 mcg/ml in concentrate users and 455±503 mcg/mL in flower users after ad libitum cannabis consumption. Nevertheless, self-reported levels of intoxication were no different between users of cannabis flower or concentrate products.
Although results also showed that most neurobehavioral measures were not altered by short-term cannabis consumption, there were some notable exceptions. There was a negative linear effect with delayed verbal recall errors, suggesting poorer performance after cannabis use (F1, 203 = 32.31; P < .001).
On the other hand, investigators found a positive linear effect with inhibitory control and working memory, which actually suggests better performance after cannabis use. This finding, the researchers note, may be the result of a practice effect. Cannabis flower users performed better across all inhibitory control assessments.
The researchers also tested participants’ balance with their eyes open and closed. In the eyes-open condition, they found a trend toward impaired balance after cannabis use, though this normalized within an hour. When subjects closed their eyes, however, researchers observed a significant short-term increase in sway after cannabis use, which fell back to pre-use levels one hour after use (F1, 203 = 18.88; P < .001).
Of note, outcomes did not differ between groups according to the type of cannabis product consumed or its relative potency.
The study yielded several surprising findings, beginning with self-reported intoxication levels, which were not statistically significant between different cannabis flower and concentrate users, despite significantly different plasma THC levels between the two groups.
Dr. Hutchison explained that this may be the result of greater THC tolerance among concentrate users, THC saturation of cannabinoid receptors, or interindividual differences among users with respect to cannabis metabolism or sensitivity.
“I thought for sure that high-potency users would be much more compromised,” he said. “I guess it just goes to show we have a lot to learn about how these things work.”
Additionally, there were virtually no significant changes in acute performance after cannabis use, with the exception of delayed verbal recall. In fact, the most marked change observed in the study was the effect of cannabis on balance immediately after drug use, though these changes seemed to abate within an hour.
Nevertheless, the study highlights several potential public health implications of cannabis consumption, Dr. Hutchison added. “What happens when people with high blood concentrations decide to quit?” he asked. “Do they have trouble quitting? Do they have withdrawal symptoms?”
The long-term effects of cannabis use is another important question that still needs to be answered, he added.
Finally, Dr. Hutchison noted that, although the study showed little difference between users of cannabis flower and concentrates, study participants were all experienced users.
“There is certainly the potential for harm when a naive person uses cannabis concentrate,” he said. “Suddenly they have way more THC than they thought they were going to get, and that’s where a lot of people get into trouble with cannabis.”
Pitfalls and hurdles
In an accompanying editorial, Margaret Haney, PhD, of Columbia University Irving Medical Center, New York, explained that cannabis’ awkward position as simultaneously legal and illegal, medical and recreational, has hampered researchers’ ability to study its effects as comprehensively as they would otherwise like.
“With a federally illegal drug legalized in individual states, scientists constrained, and federal agencies somewhat silent, clinicians have none of the data that guide their decisions for other medications (eg, which indication, product, cannabinoid ratio, dose, or route of administration; what risks for individual patients [eg, pregnant, adolescent, psychiatric?]),” Dr. Haney wrote.
These pitfalls are compounded by the significant regulatory hurdles.
“The FDA is appropriately cautious about what it allows scientists to test in patients, and none of the products available in dispensaries or online have undergone the safety and manufacturing procedures needed for FDA approval,” she continued. “How then to conduct the studies so needed?”
Yet as Haney noted, giving cannabinoid researchers a Schedule I exemption may help address many of the barriers facing these scientists. Such a move, she said, would increase the number of randomized controlled trials being performed, “and thereby begin to breach the divide between the use of these products and empirical evidence.”
Dr. Hutchison has disclosed no relevant financial relationships. Dr. Haney disclosed funding from the US National Institute on Drug Abuse and from the Thompson Family Foundation Initiative. The study was funded by the NIH and Colorado Department of Public Health and Environment.
A version of this article originally appeared on Medscape.com.
FDA expands Dysport use for cerebral palsy–related spasticity
– for patients as young as 2 years and older, according to manufacturer Ipsen Biopharmaceuticals.
When Dysport (abobotulinumtoxinA) initially was approved for treating pediatric lower limb spasticity by the FDA in 2016, Ipsen was granted Orphan Drug exclusivity for children whose lower-limb spasticity was caused by cerebral palsy. In 2019, Dysport was approved by the FDA for treating of upper-limb spasticity in children 2 years older. But if that spasticity was caused by cerebral palsy, Dysport could be used to treat it only through Orphan Drug exclusivity granted to another manufacturer, according to an Ipsen press release.
“The proactive step to resolve the uncertainty created by the previous CP [cerebral palsy] carve out enables us as physicians to prescribe consistent therapy for pediatric patients experiencing both upper- and lower-limb spasticity,” Sarah Helen Evans, MD, division chief of rehabilitation medicine in the department of pediatrics at the Children’s Hospital of Philadelphia, said in the press release.
The most common adverse effects among children with lower-limb spasticity treated with Dysport were nasopharyngitis, cough, and pyrexia. Among children with upper-limb spasticity, the most common effects associated with Dysport treatment were upper respiratory tract infection and pharyngitis.
The press release also included a warning of the distant spread of the botulinum toxin from the area of injection hours to weeks afterward, causing symptoms including blurred vision, generalized muscle weakness, and swallowing and breathing difficulties that can be life threatening; there have been reports of death.
Suspected adverse effects can be reported to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
– for patients as young as 2 years and older, according to manufacturer Ipsen Biopharmaceuticals.
When Dysport (abobotulinumtoxinA) initially was approved for treating pediatric lower limb spasticity by the FDA in 2016, Ipsen was granted Orphan Drug exclusivity for children whose lower-limb spasticity was caused by cerebral palsy. In 2019, Dysport was approved by the FDA for treating of upper-limb spasticity in children 2 years older. But if that spasticity was caused by cerebral palsy, Dysport could be used to treat it only through Orphan Drug exclusivity granted to another manufacturer, according to an Ipsen press release.
“The proactive step to resolve the uncertainty created by the previous CP [cerebral palsy] carve out enables us as physicians to prescribe consistent therapy for pediatric patients experiencing both upper- and lower-limb spasticity,” Sarah Helen Evans, MD, division chief of rehabilitation medicine in the department of pediatrics at the Children’s Hospital of Philadelphia, said in the press release.
The most common adverse effects among children with lower-limb spasticity treated with Dysport were nasopharyngitis, cough, and pyrexia. Among children with upper-limb spasticity, the most common effects associated with Dysport treatment were upper respiratory tract infection and pharyngitis.
The press release also included a warning of the distant spread of the botulinum toxin from the area of injection hours to weeks afterward, causing symptoms including blurred vision, generalized muscle weakness, and swallowing and breathing difficulties that can be life threatening; there have been reports of death.
Suspected adverse effects can be reported to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
– for patients as young as 2 years and older, according to manufacturer Ipsen Biopharmaceuticals.
When Dysport (abobotulinumtoxinA) initially was approved for treating pediatric lower limb spasticity by the FDA in 2016, Ipsen was granted Orphan Drug exclusivity for children whose lower-limb spasticity was caused by cerebral palsy. In 2019, Dysport was approved by the FDA for treating of upper-limb spasticity in children 2 years older. But if that spasticity was caused by cerebral palsy, Dysport could be used to treat it only through Orphan Drug exclusivity granted to another manufacturer, according to an Ipsen press release.
“The proactive step to resolve the uncertainty created by the previous CP [cerebral palsy] carve out enables us as physicians to prescribe consistent therapy for pediatric patients experiencing both upper- and lower-limb spasticity,” Sarah Helen Evans, MD, division chief of rehabilitation medicine in the department of pediatrics at the Children’s Hospital of Philadelphia, said in the press release.
The most common adverse effects among children with lower-limb spasticity treated with Dysport were nasopharyngitis, cough, and pyrexia. Among children with upper-limb spasticity, the most common effects associated with Dysport treatment were upper respiratory tract infection and pharyngitis.
The press release also included a warning of the distant spread of the botulinum toxin from the area of injection hours to weeks afterward, causing symptoms including blurred vision, generalized muscle weakness, and swallowing and breathing difficulties that can be life threatening; there have been reports of death.
Suspected adverse effects can be reported to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.