User login
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
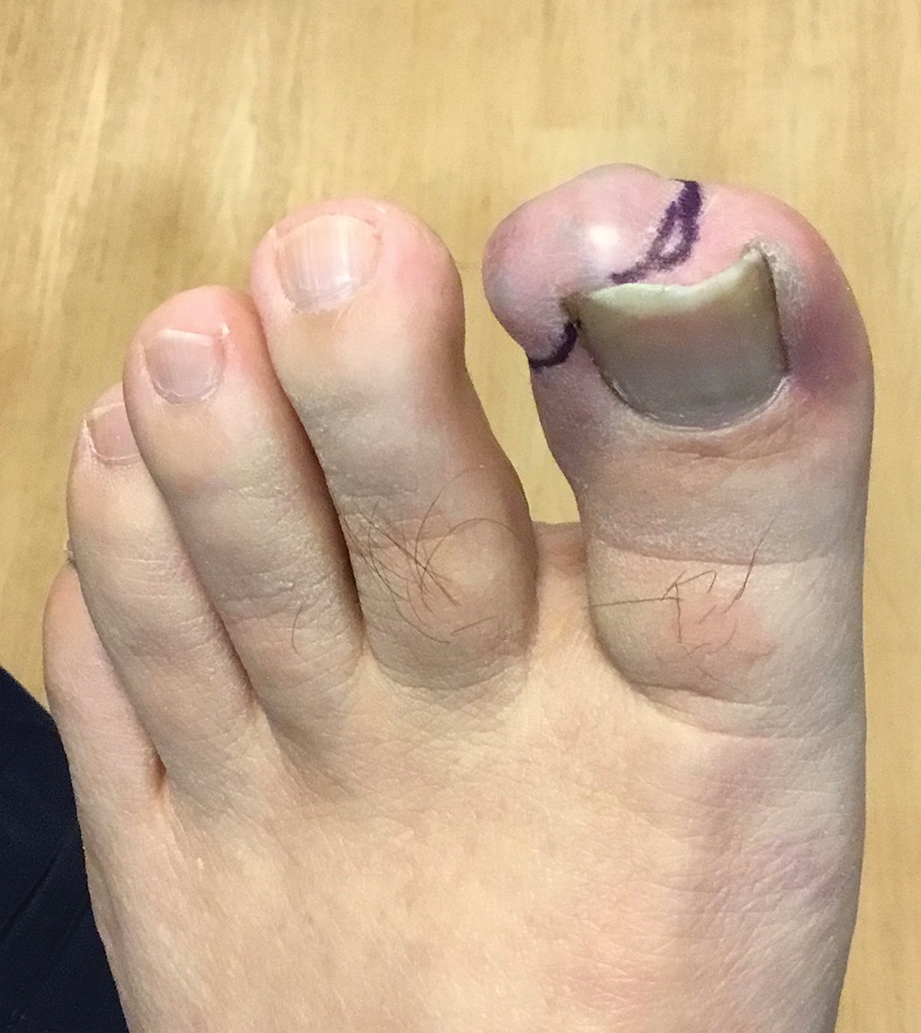
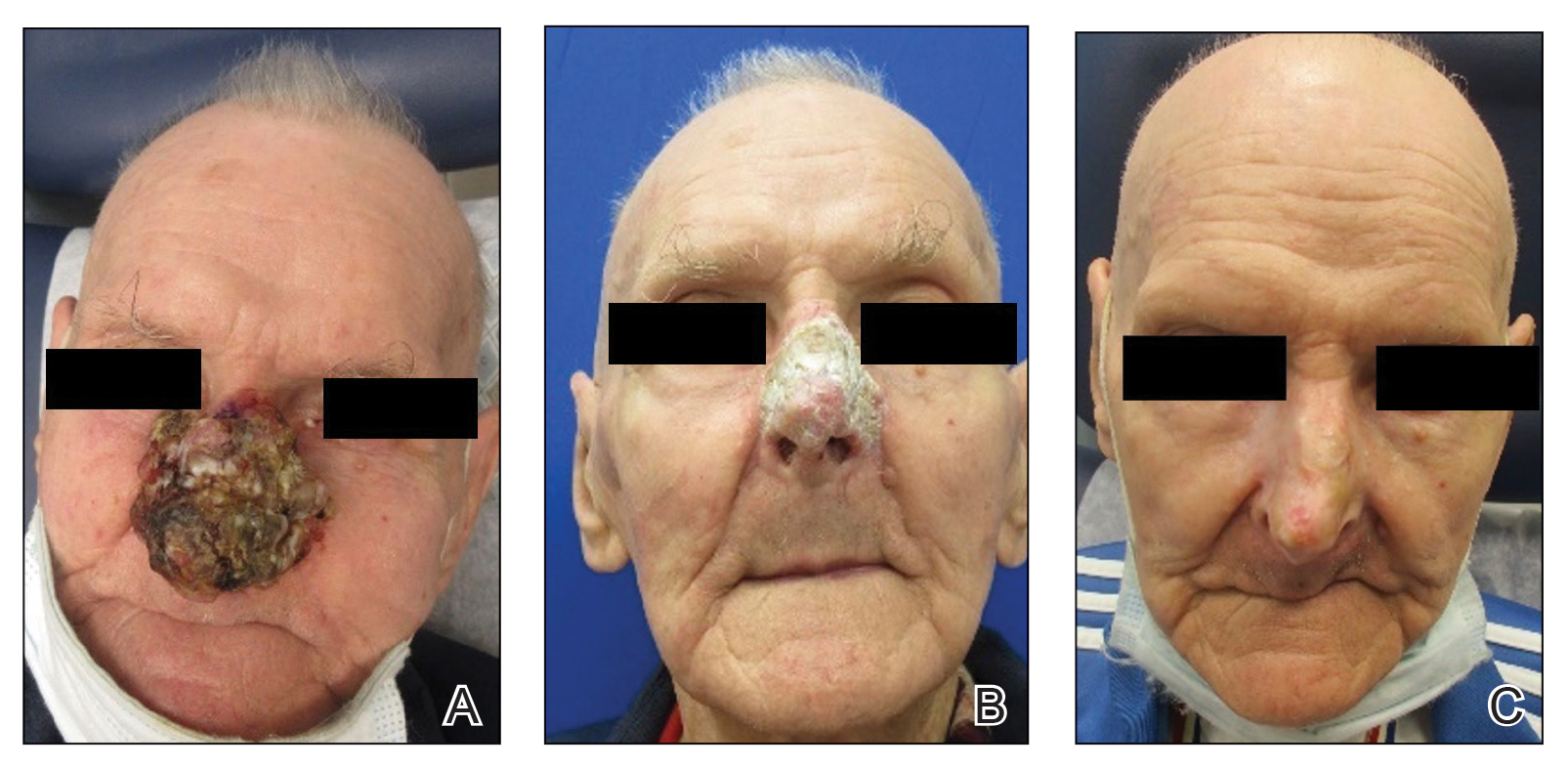
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.
Comment
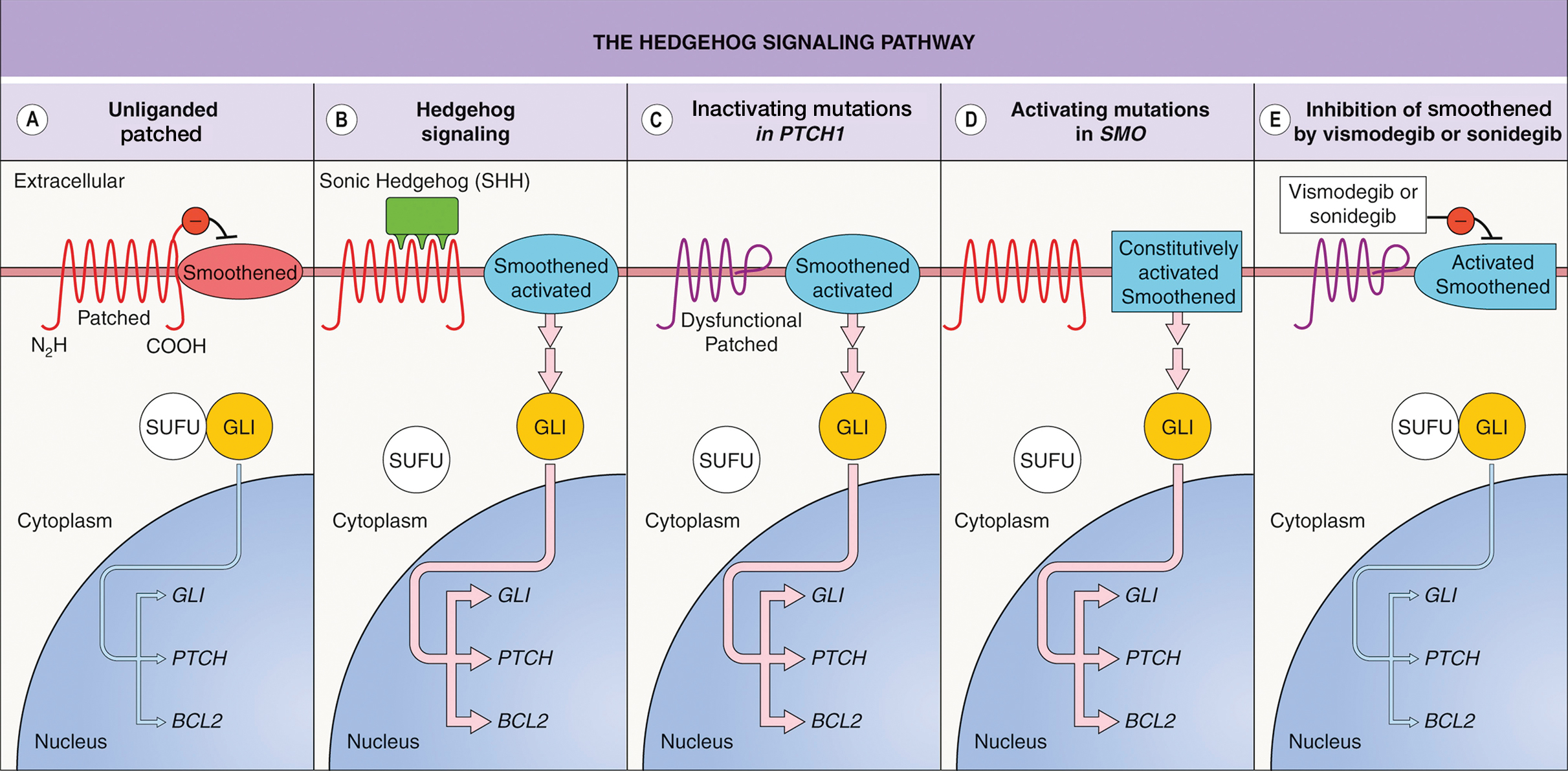
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4
Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
PRACTICE POINTS
- Dermatologists should consider using vismodegib for treatment of unresectable basal cell carcinoma.
- Vismodegib dosing regimens can vary; drug holidays can be used to mitigate adverse effects while maintaining desirable treatment outcomes.
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4
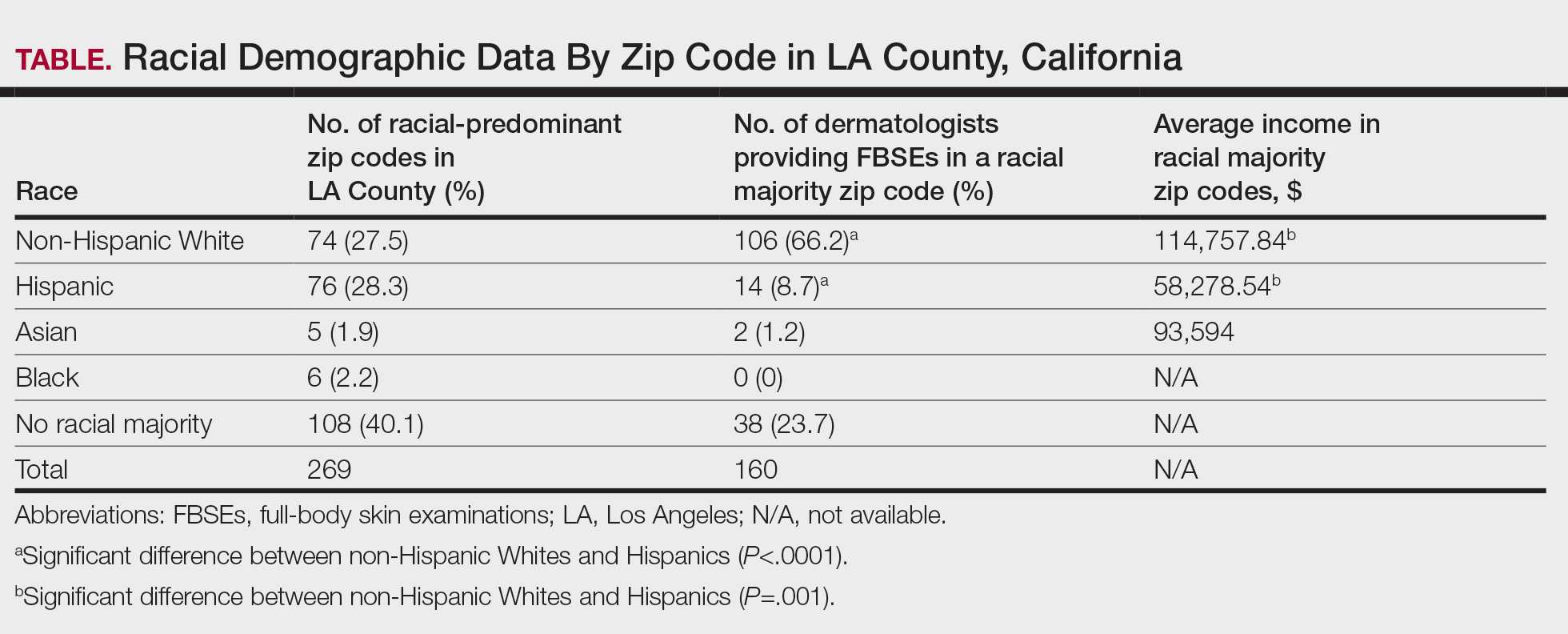
In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4

In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4

In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
PRACTICE POINTS
- Socioeconomic and racial disparities impact access to full-body skin examinations (FBSEs) in Los Angeles County.
- Most dermatologists included in this study were accepting new patients for a FBSE.
- There are significantly more dermatologists in predominantly non-Hispanic White zip codes than in predominantly Hispanic zip codes in Los Angeles County.
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
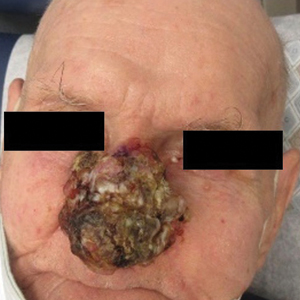
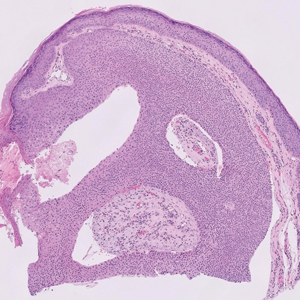
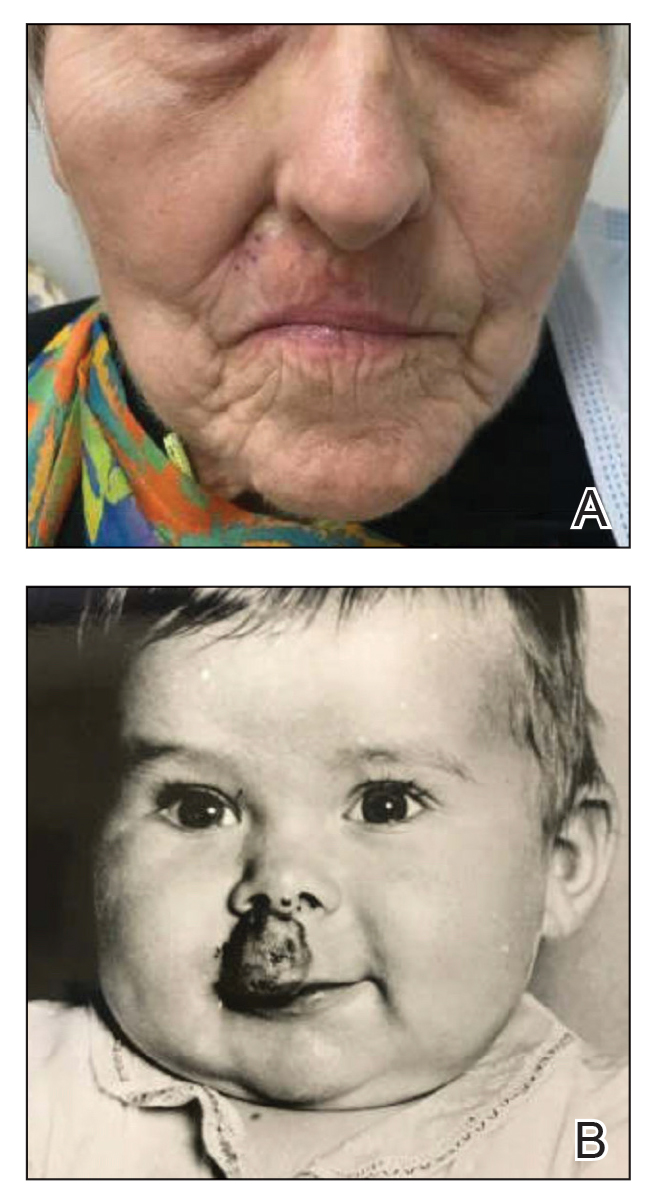
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.
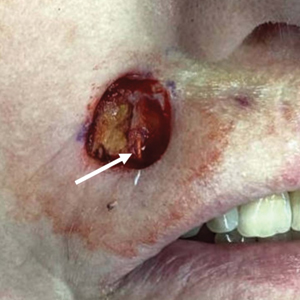
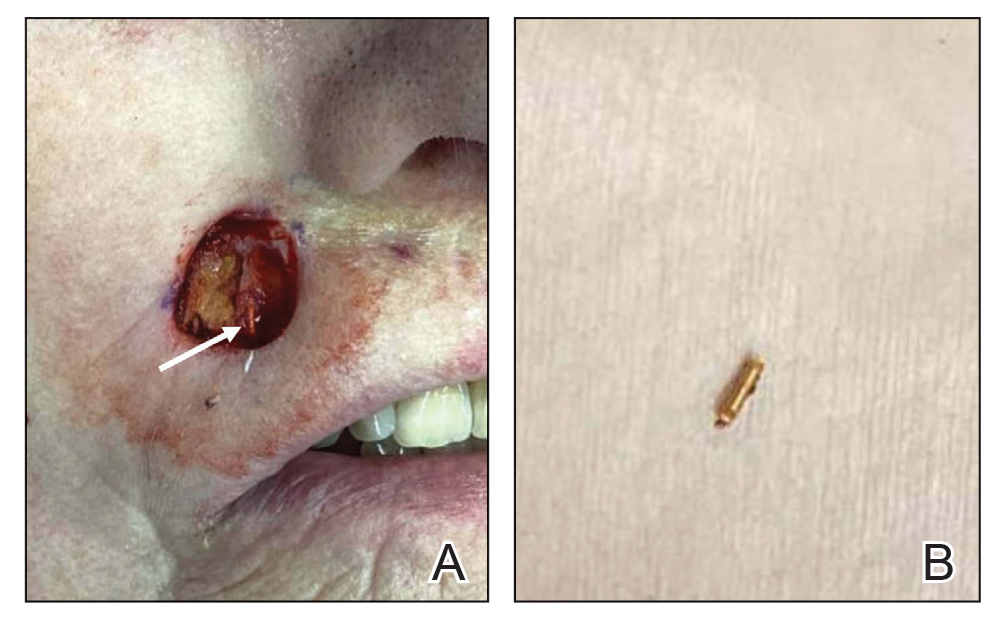
Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.
Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.

Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.

Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.

Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.

Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
PRACTICE POINTS
- Historical use of ionizing radiation to treat skin disease is a risk factor for basal cell carcinoma (BCC).
- Mohs micrographic surgery is the treatment of choice for BCC in high-risk areas such as the nose, eyelids, and lips, where tissue conservation and complete margin control are essential.
- Elderly patients should be screened for less common sources of radiation exposure for better risk stratification and to determine appropriate intervals for follow-up with a dermatologist.
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
To the Editor:
The incidence of nonmelanoma skin cancer (NMSC) is rapidly increasing worldwide. Due to its highly curable nature when treated early, accurate diagnosis is the cornerstone to good patient outcomes.1 Accurate diagnosis of skin cancer and subsequent treatment decisions rely heavily on the congruence between clinical observations and histopathologic assessments. Clinical misdiagnosis of a malignant lesion can lead to delayed and suboptimal treatment, which may contribute to serious complications such as metastasis or even mortality. In this study, data from clinically diagnosed basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) were compared to their identified histopathologic subtype classifications. The accuracy of the clinical diagnosis of these NMSCs was assessed by determining the rate of misdiagnosis and the respective positive predictive value (PPV).
A retrospective review of medical records from a private dermatology practice in Lubbock, Texas, was conducted to identify patients diagnosed with NMSC from January 1, 2017, through December 31, 2021. A total of 11,229 NMSCs were diagnosed and treated in 5877 patients. Of the NMSCs diagnosed, 11,145 were identified as keratinocyte carcinomas and were classified as BCCs or SCCs. The accuracy of the clinical diagnoses was determined by comparison to the histologic subtype identified via biopsy of the lesion. Although the use of a dermatoscope during the clinical encounter was not formally recorded, reports from the examining dermatologists indicated it was not used in the majority of cases.
If a lesion was clinically diagnosed as a BCC but was identified as a subtype of SCC on histology (or vice versa), the lesion was considered to be mismatched. The number of mismatched lesions and the mismatch rate for each lesion type/subtype is recorded in the Table. Of the total 11,145 keratinocyte carcinomas included in our study, there was an overall 10.63% mismatch rate, with 1185 of the malignancies having a differing clinical diagnosis (eg, BCC vs SCC) from the histologic findings. The clinical mismatch rate was notably higher for SCC compared to BCC (15.83% vs 7.03%, respectively).
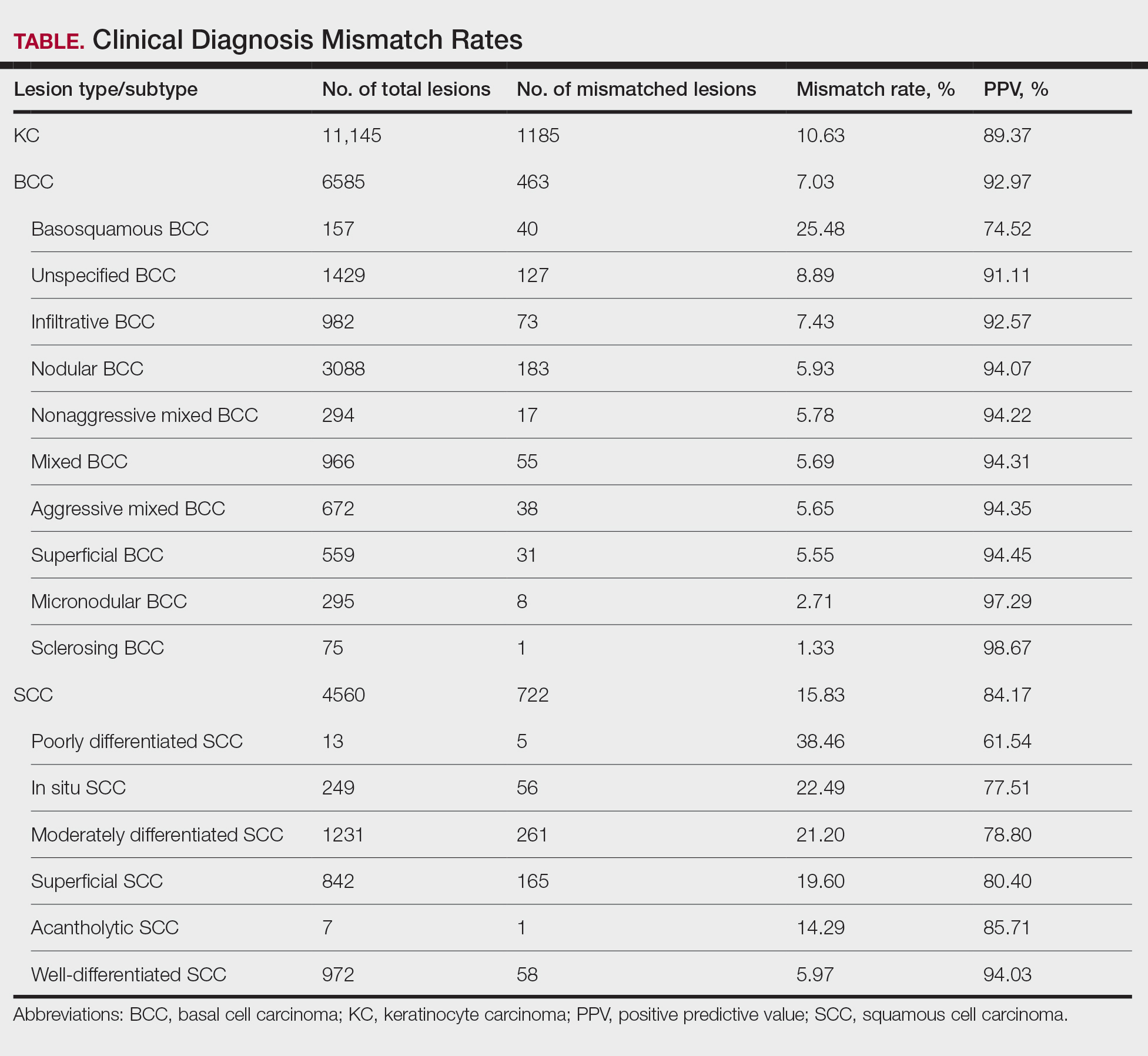
The Table provides a breakdown of the BCC subtypes identified by histology with their computed mismatch rate and PPV. It is worth clarifying that lesions classified as more than one BCC subtype per the histologic findings were diagnosed as mixed BCC; these were further classified as mixed-aggressive BCC (if at least one aggressive BCC subtype was present) and mixed nonaggressive BCC (if no aggressive BCC subtype was present). Overall, BCCs were less likely to be misdiagnosed, with an average PPV of 92.97% compared to 84.17% for SCCs. Basosquamous BCC was the BCC subtype with the highest mismatch rate (25.48%), while sclerosing BCC has the lowest overall mismatch rate (1.33%). The most common malignancy was BCC, with nodular BCC being the most common subtype.
The Table also breaks down the SCC subtypes, reporting the most commonly misdiagnosed of any BCC or SCC subtype to be poorly differentiated SCC (mismatch rate, 38.46%). The lowest mismatch rate of the SCC subtypes was 5.97% for well-differentiated SCC.
There was an overall PPV of 89.37% in clinically evaluated malignancies and their respective histologic subtypes. Basal cell carcinoma had a lower overall mismatch rate of 7.03% compared to 15.83% in SCC. The most common misdiagnosis was attributed to poorly differentiated SCC (mismatch rate, 38.46%), while the least common misdiagnosed malignancy was sclerosing BCC (1.33%). The high mismatch rate of poorly differentiated SCC may be due to its diverging presentation from a typical SCC as a flat lesion with the absence of scaling, keratin, or bleeding, leading to the misdiagnosis of BCC.2
Accurate clinical diagnosis of NMSCs is the basis for further evaluation and treatment that should ensue in a timely manner; however, accurately identifying BCCs vs SCCs solely based on clinical examination can be challenging due to variable manifestations and overlapping features. Basal cell carcinoma commonly presents as a shiny pink/flesh-colored nodule, macule, or patch with surface telangiectasia, sometimes appearing with ulceration or crusting.3 Alternatively, SCC typically appears as a firm, sharply demarcated, red nodule with a thick overlying scale.4 Definitive diagnoses can be difficult upon clinical examination since these features can be shared between the 2 subtypes. To aid in these uncertainties, a growing number of clinicians are implementing the use of dermoscopy in their everyday practice.
Dermoscopy is an extremely useful tool in improving the diagnostic accuracy of skin cancers compared to examination with the naked eye, as it provides detailed visualization of specific structures and patterns in skin cancer lesions.5 The dermoscopic appearance of BCC is characterized by pearly blue-gray or translucent globules with arborizing vessels, spoke-wheel structures, and leaflike areas.5,6 Conversely, dermoscopic features of SCC may include a milky-red globule with a scaly, sharply demarcated, crusted lesion with polymorphous vasculature, sometimes resembling a persistent sore or nonhealing wound.4,5 Though the use of dermoscopy can aid in diagnosis upon initial examination, certain factors such as trauma, ulceration, and previous treatments that distorted the lesion’s architecture may lead to misdiagnosis. Furthermore, the distinct vascular patterns found in BCC and SCC may be mistaken for each other and therefore lead to misdiagnosis upon examination.7 Other variables that may complicate diagnosis include the location of the lesion, its size, and the presence of other skin conditions or nearby lesions.
The primary limitation of the current study was the limited scope of the data, as they were derived from patients seen at one private dermatology practice, preventing the generalizability of our findings. However, our results show trends similar to those observed in other studies analyzing the clinical accuracy of skin cancer diagnoses, with higher PPVs for BCC compared to SCC. A study by Ahnlide and Bjellerup8 was based in a hospital dermatology department and demonstrated a PPV of 85.5% for BCC compared to 92.97% in our study; for SCC, the PPV was 67.3% compared to 84.17% in our study. In another study by Heal et al,9 data were collected from an Australian registry that included records of all histologically confirmed skin cancers from December 1996 to October 1999 from 202 general practitioners and 42 specialists, including 1 dermatologist. The PPVs for BCC and SCC were 72.7% and 49.4%, respectively. Although our results indicated higher PPVs compared to these 2 studies, some of the discrepancies can be accounted for by the differences in clinical setting as well as the lack of expertise of nondermatologist physicians in identifying skin malignancies in the study by Heal et al.9
The current study was further limited by the lack of data quantifying the number of lesions clinically suspected to be malignant but found to be histologically benign. It is typical for clinicians to have a low threshold to biopsy a suspicious lesion with atypical features (eg, rapid evolution and growth, bleeding, crusting). Furthermore, the identification of risk factors in the patient’s medical and family history (eg, exposure to radiation, personal or family history of skin cancers) can heavily influence a clinician’s decision to biopsy a lesion with an atypical appearance.10 Many benign lesions are biopsied to avoid missing a diagnosis of malignancy. Consequently, our results suggest a high degree of clinical misdiagnosis of BCCs and SCCs. Obtaining data on the number of lesions suspected to be BCC or SCC that were found to be histologically benign would be a valuable addition to our study, as it would provide a measurable insight into the sensitivity of clinicians’ decision-making to identify a lesion as suspicious and warranting biopsy.
While clinical diagnosis plays a vital role in identifying suspected NMSCs such as BCC and SCC, its accuracy can be limited even with the use of dermoscopy. Overall, our data have shown a high rate of diagnostic accuracy upon suspicion of malignancy, but the different variables that affect clinical presentation promote histologic diagnosis to prevail as the gold standard.
- Seyed Ahadi M, Firooz A, Rahimi H, et al. Clinical diagnosis has a high negative predictive value in evaluation of malignant skin lesions. Dermatol Res Pract. 2021;2021:6618990. doi:10.1155/2021/6618990
- Lallas A, Pyne J, Kyrgidis A, et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308- 1315. doi:10.1111/bjd.13510
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. September 19, 2022. In: StatPearls. StatPearls Publishing; 2023.
- Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J Am Acad Dermatol. 2015;73:968-975. doi:10.1016/j.jaad.2015.08.041
- Wolner ZJ, Yélamos O, Liopyris K, et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35:417-437. doi:10.1016/j.det.2017.06.003
- Reiter O, Mimouni I, Dusza S, et al. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2021;85:653-664. doi:10.1016/j.jaad.2019.11.008
- Pruneda C, Ramesh M, Hope L, et al. Nonmelanoma skin cancers: diagnostic accuracy of midlevel providers versus dermatologists. The Dermatologist. March 2023. Accessed March 18, 2025. https://www.hmpgloballearningnetwork.com/site/thederm/feature-story/nonmelanoma-skin-cancers-diagnostic-accuracy-midlevel-providers-vs
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Derm Venereol. 2013;93:305-308. doi:10.2340/00015555-1560
- Heal CF, Raasch BA, Buettner PG, et al. Accuracy of clinical diagnosis of skin lesions. Br J Dermatol. 2008;159:661-668.
- Fu S, Kim S, Wasko C. Dermatological guide for primary care physicians: full body skin checks, skin cancer detection, and patient education on self-skin checks and sun protection. Proc (Bayl Univ Med Cent). 2024;37:647-654. doi:10.1080/08998280.2024.2351751
To the Editor:
The incidence of nonmelanoma skin cancer (NMSC) is rapidly increasing worldwide. Due to its highly curable nature when treated early, accurate diagnosis is the cornerstone to good patient outcomes.1 Accurate diagnosis of skin cancer and subsequent treatment decisions rely heavily on the congruence between clinical observations and histopathologic assessments. Clinical misdiagnosis of a malignant lesion can lead to delayed and suboptimal treatment, which may contribute to serious complications such as metastasis or even mortality. In this study, data from clinically diagnosed basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) were compared to their identified histopathologic subtype classifications. The accuracy of the clinical diagnosis of these NMSCs was assessed by determining the rate of misdiagnosis and the respective positive predictive value (PPV).
A retrospective review of medical records from a private dermatology practice in Lubbock, Texas, was conducted to identify patients diagnosed with NMSC from January 1, 2017, through December 31, 2021. A total of 11,229 NMSCs were diagnosed and treated in 5877 patients. Of the NMSCs diagnosed, 11,145 were identified as keratinocyte carcinomas and were classified as BCCs or SCCs. The accuracy of the clinical diagnoses was determined by comparison to the histologic subtype identified via biopsy of the lesion. Although the use of a dermatoscope during the clinical encounter was not formally recorded, reports from the examining dermatologists indicated it was not used in the majority of cases.
If a lesion was clinically diagnosed as a BCC but was identified as a subtype of SCC on histology (or vice versa), the lesion was considered to be mismatched. The number of mismatched lesions and the mismatch rate for each lesion type/subtype is recorded in the Table. Of the total 11,145 keratinocyte carcinomas included in our study, there was an overall 10.63% mismatch rate, with 1185 of the malignancies having a differing clinical diagnosis (eg, BCC vs SCC) from the histologic findings. The clinical mismatch rate was notably higher for SCC compared to BCC (15.83% vs 7.03%, respectively).

The Table provides a breakdown of the BCC subtypes identified by histology with their computed mismatch rate and PPV. It is worth clarifying that lesions classified as more than one BCC subtype per the histologic findings were diagnosed as mixed BCC; these were further classified as mixed-aggressive BCC (if at least one aggressive BCC subtype was present) and mixed nonaggressive BCC (if no aggressive BCC subtype was present). Overall, BCCs were less likely to be misdiagnosed, with an average PPV of 92.97% compared to 84.17% for SCCs. Basosquamous BCC was the BCC subtype with the highest mismatch rate (25.48%), while sclerosing BCC has the lowest overall mismatch rate (1.33%). The most common malignancy was BCC, with nodular BCC being the most common subtype.
The Table also breaks down the SCC subtypes, reporting the most commonly misdiagnosed of any BCC or SCC subtype to be poorly differentiated SCC (mismatch rate, 38.46%). The lowest mismatch rate of the SCC subtypes was 5.97% for well-differentiated SCC.
There was an overall PPV of 89.37% in clinically evaluated malignancies and their respective histologic subtypes. Basal cell carcinoma had a lower overall mismatch rate of 7.03% compared to 15.83% in SCC. The most common misdiagnosis was attributed to poorly differentiated SCC (mismatch rate, 38.46%), while the least common misdiagnosed malignancy was sclerosing BCC (1.33%). The high mismatch rate of poorly differentiated SCC may be due to its diverging presentation from a typical SCC as a flat lesion with the absence of scaling, keratin, or bleeding, leading to the misdiagnosis of BCC.2
Accurate clinical diagnosis of NMSCs is the basis for further evaluation and treatment that should ensue in a timely manner; however, accurately identifying BCCs vs SCCs solely based on clinical examination can be challenging due to variable manifestations and overlapping features. Basal cell carcinoma commonly presents as a shiny pink/flesh-colored nodule, macule, or patch with surface telangiectasia, sometimes appearing with ulceration or crusting.3 Alternatively, SCC typically appears as a firm, sharply demarcated, red nodule with a thick overlying scale.4 Definitive diagnoses can be difficult upon clinical examination since these features can be shared between the 2 subtypes. To aid in these uncertainties, a growing number of clinicians are implementing the use of dermoscopy in their everyday practice.
Dermoscopy is an extremely useful tool in improving the diagnostic accuracy of skin cancers compared to examination with the naked eye, as it provides detailed visualization of specific structures and patterns in skin cancer lesions.5 The dermoscopic appearance of BCC is characterized by pearly blue-gray or translucent globules with arborizing vessels, spoke-wheel structures, and leaflike areas.5,6 Conversely, dermoscopic features of SCC may include a milky-red globule with a scaly, sharply demarcated, crusted lesion with polymorphous vasculature, sometimes resembling a persistent sore or nonhealing wound.4,5 Though the use of dermoscopy can aid in diagnosis upon initial examination, certain factors such as trauma, ulceration, and previous treatments that distorted the lesion’s architecture may lead to misdiagnosis. Furthermore, the distinct vascular patterns found in BCC and SCC may be mistaken for each other and therefore lead to misdiagnosis upon examination.7 Other variables that may complicate diagnosis include the location of the lesion, its size, and the presence of other skin conditions or nearby lesions.
The primary limitation of the current study was the limited scope of the data, as they were derived from patients seen at one private dermatology practice, preventing the generalizability of our findings. However, our results show trends similar to those observed in other studies analyzing the clinical accuracy of skin cancer diagnoses, with higher PPVs for BCC compared to SCC. A study by Ahnlide and Bjellerup8 was based in a hospital dermatology department and demonstrated a PPV of 85.5% for BCC compared to 92.97% in our study; for SCC, the PPV was 67.3% compared to 84.17% in our study. In another study by Heal et al,9 data were collected from an Australian registry that included records of all histologically confirmed skin cancers from December 1996 to October 1999 from 202 general practitioners and 42 specialists, including 1 dermatologist. The PPVs for BCC and SCC were 72.7% and 49.4%, respectively. Although our results indicated higher PPVs compared to these 2 studies, some of the discrepancies can be accounted for by the differences in clinical setting as well as the lack of expertise of nondermatologist physicians in identifying skin malignancies in the study by Heal et al.9
The current study was further limited by the lack of data quantifying the number of lesions clinically suspected to be malignant but found to be histologically benign. It is typical for clinicians to have a low threshold to biopsy a suspicious lesion with atypical features (eg, rapid evolution and growth, bleeding, crusting). Furthermore, the identification of risk factors in the patient’s medical and family history (eg, exposure to radiation, personal or family history of skin cancers) can heavily influence a clinician’s decision to biopsy a lesion with an atypical appearance.10 Many benign lesions are biopsied to avoid missing a diagnosis of malignancy. Consequently, our results suggest a high degree of clinical misdiagnosis of BCCs and SCCs. Obtaining data on the number of lesions suspected to be BCC or SCC that were found to be histologically benign would be a valuable addition to our study, as it would provide a measurable insight into the sensitivity of clinicians’ decision-making to identify a lesion as suspicious and warranting biopsy.
While clinical diagnosis plays a vital role in identifying suspected NMSCs such as BCC and SCC, its accuracy can be limited even with the use of dermoscopy. Overall, our data have shown a high rate of diagnostic accuracy upon suspicion of malignancy, but the different variables that affect clinical presentation promote histologic diagnosis to prevail as the gold standard.
To the Editor:
The incidence of nonmelanoma skin cancer (NMSC) is rapidly increasing worldwide. Due to its highly curable nature when treated early, accurate diagnosis is the cornerstone to good patient outcomes.1 Accurate diagnosis of skin cancer and subsequent treatment decisions rely heavily on the congruence between clinical observations and histopathologic assessments. Clinical misdiagnosis of a malignant lesion can lead to delayed and suboptimal treatment, which may contribute to serious complications such as metastasis or even mortality. In this study, data from clinically diagnosed basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) were compared to their identified histopathologic subtype classifications. The accuracy of the clinical diagnosis of these NMSCs was assessed by determining the rate of misdiagnosis and the respective positive predictive value (PPV).
A retrospective review of medical records from a private dermatology practice in Lubbock, Texas, was conducted to identify patients diagnosed with NMSC from January 1, 2017, through December 31, 2021. A total of 11,229 NMSCs were diagnosed and treated in 5877 patients. Of the NMSCs diagnosed, 11,145 were identified as keratinocyte carcinomas and were classified as BCCs or SCCs. The accuracy of the clinical diagnoses was determined by comparison to the histologic subtype identified via biopsy of the lesion. Although the use of a dermatoscope during the clinical encounter was not formally recorded, reports from the examining dermatologists indicated it was not used in the majority of cases.
If a lesion was clinically diagnosed as a BCC but was identified as a subtype of SCC on histology (or vice versa), the lesion was considered to be mismatched. The number of mismatched lesions and the mismatch rate for each lesion type/subtype is recorded in the Table. Of the total 11,145 keratinocyte carcinomas included in our study, there was an overall 10.63% mismatch rate, with 1185 of the malignancies having a differing clinical diagnosis (eg, BCC vs SCC) from the histologic findings. The clinical mismatch rate was notably higher for SCC compared to BCC (15.83% vs 7.03%, respectively).

The Table provides a breakdown of the BCC subtypes identified by histology with their computed mismatch rate and PPV. It is worth clarifying that lesions classified as more than one BCC subtype per the histologic findings were diagnosed as mixed BCC; these were further classified as mixed-aggressive BCC (if at least one aggressive BCC subtype was present) and mixed nonaggressive BCC (if no aggressive BCC subtype was present). Overall, BCCs were less likely to be misdiagnosed, with an average PPV of 92.97% compared to 84.17% for SCCs. Basosquamous BCC was the BCC subtype with the highest mismatch rate (25.48%), while sclerosing BCC has the lowest overall mismatch rate (1.33%). The most common malignancy was BCC, with nodular BCC being the most common subtype.
The Table also breaks down the SCC subtypes, reporting the most commonly misdiagnosed of any BCC or SCC subtype to be poorly differentiated SCC (mismatch rate, 38.46%). The lowest mismatch rate of the SCC subtypes was 5.97% for well-differentiated SCC.
There was an overall PPV of 89.37% in clinically evaluated malignancies and their respective histologic subtypes. Basal cell carcinoma had a lower overall mismatch rate of 7.03% compared to 15.83% in SCC. The most common misdiagnosis was attributed to poorly differentiated SCC (mismatch rate, 38.46%), while the least common misdiagnosed malignancy was sclerosing BCC (1.33%). The high mismatch rate of poorly differentiated SCC may be due to its diverging presentation from a typical SCC as a flat lesion with the absence of scaling, keratin, or bleeding, leading to the misdiagnosis of BCC.2
Accurate clinical diagnosis of NMSCs is the basis for further evaluation and treatment that should ensue in a timely manner; however, accurately identifying BCCs vs SCCs solely based on clinical examination can be challenging due to variable manifestations and overlapping features. Basal cell carcinoma commonly presents as a shiny pink/flesh-colored nodule, macule, or patch with surface telangiectasia, sometimes appearing with ulceration or crusting.3 Alternatively, SCC typically appears as a firm, sharply demarcated, red nodule with a thick overlying scale.4 Definitive diagnoses can be difficult upon clinical examination since these features can be shared between the 2 subtypes. To aid in these uncertainties, a growing number of clinicians are implementing the use of dermoscopy in their everyday practice.
Dermoscopy is an extremely useful tool in improving the diagnostic accuracy of skin cancers compared to examination with the naked eye, as it provides detailed visualization of specific structures and patterns in skin cancer lesions.5 The dermoscopic appearance of BCC is characterized by pearly blue-gray or translucent globules with arborizing vessels, spoke-wheel structures, and leaflike areas.5,6 Conversely, dermoscopic features of SCC may include a milky-red globule with a scaly, sharply demarcated, crusted lesion with polymorphous vasculature, sometimes resembling a persistent sore or nonhealing wound.4,5 Though the use of dermoscopy can aid in diagnosis upon initial examination, certain factors such as trauma, ulceration, and previous treatments that distorted the lesion’s architecture may lead to misdiagnosis. Furthermore, the distinct vascular patterns found in BCC and SCC may be mistaken for each other and therefore lead to misdiagnosis upon examination.7 Other variables that may complicate diagnosis include the location of the lesion, its size, and the presence of other skin conditions or nearby lesions.
The primary limitation of the current study was the limited scope of the data, as they were derived from patients seen at one private dermatology practice, preventing the generalizability of our findings. However, our results show trends similar to those observed in other studies analyzing the clinical accuracy of skin cancer diagnoses, with higher PPVs for BCC compared to SCC. A study by Ahnlide and Bjellerup8 was based in a hospital dermatology department and demonstrated a PPV of 85.5% for BCC compared to 92.97% in our study; for SCC, the PPV was 67.3% compared to 84.17% in our study. In another study by Heal et al,9 data were collected from an Australian registry that included records of all histologically confirmed skin cancers from December 1996 to October 1999 from 202 general practitioners and 42 specialists, including 1 dermatologist. The PPVs for BCC and SCC were 72.7% and 49.4%, respectively. Although our results indicated higher PPVs compared to these 2 studies, some of the discrepancies can be accounted for by the differences in clinical setting as well as the lack of expertise of nondermatologist physicians in identifying skin malignancies in the study by Heal et al.9
The current study was further limited by the lack of data quantifying the number of lesions clinically suspected to be malignant but found to be histologically benign. It is typical for clinicians to have a low threshold to biopsy a suspicious lesion with atypical features (eg, rapid evolution and growth, bleeding, crusting). Furthermore, the identification of risk factors in the patient’s medical and family history (eg, exposure to radiation, personal or family history of skin cancers) can heavily influence a clinician’s decision to biopsy a lesion with an atypical appearance.10 Many benign lesions are biopsied to avoid missing a diagnosis of malignancy. Consequently, our results suggest a high degree of clinical misdiagnosis of BCCs and SCCs. Obtaining data on the number of lesions suspected to be BCC or SCC that were found to be histologically benign would be a valuable addition to our study, as it would provide a measurable insight into the sensitivity of clinicians’ decision-making to identify a lesion as suspicious and warranting biopsy.
While clinical diagnosis plays a vital role in identifying suspected NMSCs such as BCC and SCC, its accuracy can be limited even with the use of dermoscopy. Overall, our data have shown a high rate of diagnostic accuracy upon suspicion of malignancy, but the different variables that affect clinical presentation promote histologic diagnosis to prevail as the gold standard.
- Seyed Ahadi M, Firooz A, Rahimi H, et al. Clinical diagnosis has a high negative predictive value in evaluation of malignant skin lesions. Dermatol Res Pract. 2021;2021:6618990. doi:10.1155/2021/6618990
- Lallas A, Pyne J, Kyrgidis A, et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308- 1315. doi:10.1111/bjd.13510
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. September 19, 2022. In: StatPearls. StatPearls Publishing; 2023.
- Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J Am Acad Dermatol. 2015;73:968-975. doi:10.1016/j.jaad.2015.08.041
- Wolner ZJ, Yélamos O, Liopyris K, et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35:417-437. doi:10.1016/j.det.2017.06.003
- Reiter O, Mimouni I, Dusza S, et al. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2021;85:653-664. doi:10.1016/j.jaad.2019.11.008
- Pruneda C, Ramesh M, Hope L, et al. Nonmelanoma skin cancers: diagnostic accuracy of midlevel providers versus dermatologists. The Dermatologist. March 2023. Accessed March 18, 2025. https://www.hmpgloballearningnetwork.com/site/thederm/feature-story/nonmelanoma-skin-cancers-diagnostic-accuracy-midlevel-providers-vs
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Derm Venereol. 2013;93:305-308. doi:10.2340/00015555-1560
- Heal CF, Raasch BA, Buettner PG, et al. Accuracy of clinical diagnosis of skin lesions. Br J Dermatol. 2008;159:661-668.
- Fu S, Kim S, Wasko C. Dermatological guide for primary care physicians: full body skin checks, skin cancer detection, and patient education on self-skin checks and sun protection. Proc (Bayl Univ Med Cent). 2024;37:647-654. doi:10.1080/08998280.2024.2351751
- Seyed Ahadi M, Firooz A, Rahimi H, et al. Clinical diagnosis has a high negative predictive value in evaluation of malignant skin lesions. Dermatol Res Pract. 2021;2021:6618990. doi:10.1155/2021/6618990
- Lallas A, Pyne J, Kyrgidis A, et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308- 1315. doi:10.1111/bjd.13510
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. September 19, 2022. In: StatPearls. StatPearls Publishing; 2023.
- Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J Am Acad Dermatol. 2015;73:968-975. doi:10.1016/j.jaad.2015.08.041
- Wolner ZJ, Yélamos O, Liopyris K, et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35:417-437. doi:10.1016/j.det.2017.06.003
- Reiter O, Mimouni I, Dusza S, et al. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2021;85:653-664. doi:10.1016/j.jaad.2019.11.008
- Pruneda C, Ramesh M, Hope L, et al. Nonmelanoma skin cancers: diagnostic accuracy of midlevel providers versus dermatologists. The Dermatologist. March 2023. Accessed March 18, 2025. https://www.hmpgloballearningnetwork.com/site/thederm/feature-story/nonmelanoma-skin-cancers-diagnostic-accuracy-midlevel-providers-vs
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Derm Venereol. 2013;93:305-308. doi:10.2340/00015555-1560
- Heal CF, Raasch BA, Buettner PG, et al. Accuracy of clinical diagnosis of skin lesions. Br J Dermatol. 2008;159:661-668.
- Fu S, Kim S, Wasko C. Dermatological guide for primary care physicians: full body skin checks, skin cancer detection, and patient education on self-skin checks and sun protection. Proc (Bayl Univ Med Cent). 2024;37:647-654. doi:10.1080/08998280.2024.2351751
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
PRACTICE POINTS
- Malignant lesions may be misdiagnosed when assessments are guided by clinical features that align with typical presentations of other lesion types, potentially leading to diagnostic errors among experienced clinicians.
- Although dermoscopy is a beneficial tool in examining potential skin cancers, clinical observations should not bypass the gold standard of histopathologic examination.
Vascular Nodule on the Upper Chest
Vascular Nodule on the Upper Chest
THE DIAGNOSIS: Metastatic Renal Cell Carcinoma
The shave biopsy revealed large cells with prominent nucleoli, clear cytoplasm, and thin cell borders in a nestlike arrangement (Figure 1). Immunohistochemical examination was negative for cytokeratin 5/6 and positive for PAX8 (Figure 2), which finalized the diagnosis of metastatic renal cell carcinoma (RCC). Later, our patient had a core biopsy-proven metastasis to the C6 spinous process, with concern for additional metastasis to the liver and lungs on positron emission tomography. Our patient’s treatment plan included pembrolizumab and axitinib to manage further cutaneous metastasis and radiation therapy for the C6 spinous process metastasis.
Renal cell carcinoma denotes cancer originating from the renal epithelium and is the most common kidney tumor in adults.1 Renal cell carcinoma accounts for more than 90% of kidney malignancies in the United States and has 3 main subtypes: clear cell RCC, papillary RCC, and chromophobe RCC.2 About 25% of cases metastasize, commonly to the lungs, liver, bones, lymph nodes, contralateral kidney, and adrenal glands.3
Cutaneous metastasis of RCC is rare, with an incidence of approximately 3.3%.4 Notably, 80% to 90% of patients with metastatic skin lesions had a prior diagnosis of RCC.2 Skin metastases associated with RCC predominantly are found on the face and scalp, appearing as nodular, swiftly expanding, circular, or oval-shaped growths. The robust vascular element of these lesions can lead to confusion with regard to the proper diagnosis, as they often resemble hemangiomas, pyogenic granulomas, or Kaposi sarcomas.4
Many cutaneous metastases linked to RCC exhibit a histomorphologic pattern consistent with clear cell adenocarcinoma.2 The malignant cells are large and possess transparent cytoplasm, round to oval nuclei, and prominent nucleoli. The cells can form glandular, acinar, or papillary arrangements; extravasated red blood cells frequently are found within the surrounding fibrovascular tissue.5 The presence of cytoplasmic glycogen can be revealed through periodic acid–Schiff staining. Other immunohistochemical markers commonly used to identify skin metastasis of RCC include epithelioid membrane antigen, carcinoembryonic antigen, and CD-10.1
Various mechanisms are involved in the cutaneous metastases of RCC. The most common pathway involves infiltration of the skin directly overlying the malignant renal mass; additional potential mechanisms include the introduction of abnormal cells into the skin during surgical or diagnostic interventions and their dissemination through the lymphatic system or bloodstream.1 Among urogenital malignancies other than RCC, skin metastases predominantly manifest in the abdominal region.2 Conversely, the head and neck region are more frequently impacted in RCC. The vascular composition of these tumors plays a role in facilitating the extension of cancer cells through the bloodstream, fostering the emergence of distant metastases.6
The development of cutaneous metastasis in RCC is associated with a poor prognosis, as most patients die within 6 months of detection.3 Treatment options thus are limited and palliative. Although local excision is an alternative treatment for localized cutaneous metastasis, it often provides little benefit in the presence of extensive metastasis; radiotherapy also has been shown to have a limited effect on primary RCC, though its devascularization of the lesion may be effective in metastatic cases.5 Immune checkpoint inhibitors such as nivolumab and ipilimumab have improved progression-free survival in patients with metastatic RCC, though uncertainty remains regarding their efficacy in attenuating cutaneous metastasis.5,6
- Kanwal R. Metastasis in renal cell carcinoma: biology and treatment. Adv Cancer Biol Metastasis. 2023;7:100094. doi:10.1016 /j.adcanc.2023.100094
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. doi:10.1093/annonc/mdr362
- Lorenzo-Rios D, Cruzval-O’Reilly E, Rabelo-Cartagena J. Facial cutaneous metastasis in renal cell carcinoma. Cureus. 2020;12:E12093. doi:10.7759/cureus.12093
- Iliescu CA, Beiu C, Racovit·a¢ A, et al. Atypical presentation of rapidly progressive cutaneous metastases of clear cell renal carcinoma: a case report. Medicina. 2024;60:1797. doi:10.3390/medicina60111797
- Joyce MJ. Management of skeletal metastases in renal cell carcinoma patients. In: Bukowski RM, Novick AC, eds. Clinical Management of Renal Tumors. Springer; 2008: 421-459.
THE DIAGNOSIS: Metastatic Renal Cell Carcinoma
The shave biopsy revealed large cells with prominent nucleoli, clear cytoplasm, and thin cell borders in a nestlike arrangement (Figure 1). Immunohistochemical examination was negative for cytokeratin 5/6 and positive for PAX8 (Figure 2), which finalized the diagnosis of metastatic renal cell carcinoma (RCC). Later, our patient had a core biopsy-proven metastasis to the C6 spinous process, with concern for additional metastasis to the liver and lungs on positron emission tomography. Our patient’s treatment plan included pembrolizumab and axitinib to manage further cutaneous metastasis and radiation therapy for the C6 spinous process metastasis.


Renal cell carcinoma denotes cancer originating from the renal epithelium and is the most common kidney tumor in adults.1 Renal cell carcinoma accounts for more than 90% of kidney malignancies in the United States and has 3 main subtypes: clear cell RCC, papillary RCC, and chromophobe RCC.2 About 25% of cases metastasize, commonly to the lungs, liver, bones, lymph nodes, contralateral kidney, and adrenal glands.3
Cutaneous metastasis of RCC is rare, with an incidence of approximately 3.3%.4 Notably, 80% to 90% of patients with metastatic skin lesions had a prior diagnosis of RCC.2 Skin metastases associated with RCC predominantly are found on the face and scalp, appearing as nodular, swiftly expanding, circular, or oval-shaped growths. The robust vascular element of these lesions can lead to confusion with regard to the proper diagnosis, as they often resemble hemangiomas, pyogenic granulomas, or Kaposi sarcomas.4
Many cutaneous metastases linked to RCC exhibit a histomorphologic pattern consistent with clear cell adenocarcinoma.2 The malignant cells are large and possess transparent cytoplasm, round to oval nuclei, and prominent nucleoli. The cells can form glandular, acinar, or papillary arrangements; extravasated red blood cells frequently are found within the surrounding fibrovascular tissue.5 The presence of cytoplasmic glycogen can be revealed through periodic acid–Schiff staining. Other immunohistochemical markers commonly used to identify skin metastasis of RCC include epithelioid membrane antigen, carcinoembryonic antigen, and CD-10.1
Various mechanisms are involved in the cutaneous metastases of RCC. The most common pathway involves infiltration of the skin directly overlying the malignant renal mass; additional potential mechanisms include the introduction of abnormal cells into the skin during surgical or diagnostic interventions and their dissemination through the lymphatic system or bloodstream.1 Among urogenital malignancies other than RCC, skin metastases predominantly manifest in the abdominal region.2 Conversely, the head and neck region are more frequently impacted in RCC. The vascular composition of these tumors plays a role in facilitating the extension of cancer cells through the bloodstream, fostering the emergence of distant metastases.6
The development of cutaneous metastasis in RCC is associated with a poor prognosis, as most patients die within 6 months of detection.3 Treatment options thus are limited and palliative. Although local excision is an alternative treatment for localized cutaneous metastasis, it often provides little benefit in the presence of extensive metastasis; radiotherapy also has been shown to have a limited effect on primary RCC, though its devascularization of the lesion may be effective in metastatic cases.5 Immune checkpoint inhibitors such as nivolumab and ipilimumab have improved progression-free survival in patients with metastatic RCC, though uncertainty remains regarding their efficacy in attenuating cutaneous metastasis.5,6
THE DIAGNOSIS: Metastatic Renal Cell Carcinoma
The shave biopsy revealed large cells with prominent nucleoli, clear cytoplasm, and thin cell borders in a nestlike arrangement (Figure 1). Immunohistochemical examination was negative for cytokeratin 5/6 and positive for PAX8 (Figure 2), which finalized the diagnosis of metastatic renal cell carcinoma (RCC). Later, our patient had a core biopsy-proven metastasis to the C6 spinous process, with concern for additional metastasis to the liver and lungs on positron emission tomography. Our patient’s treatment plan included pembrolizumab and axitinib to manage further cutaneous metastasis and radiation therapy for the C6 spinous process metastasis.


Renal cell carcinoma denotes cancer originating from the renal epithelium and is the most common kidney tumor in adults.1 Renal cell carcinoma accounts for more than 90% of kidney malignancies in the United States and has 3 main subtypes: clear cell RCC, papillary RCC, and chromophobe RCC.2 About 25% of cases metastasize, commonly to the lungs, liver, bones, lymph nodes, contralateral kidney, and adrenal glands.3
Cutaneous metastasis of RCC is rare, with an incidence of approximately 3.3%.4 Notably, 80% to 90% of patients with metastatic skin lesions had a prior diagnosis of RCC.2 Skin metastases associated with RCC predominantly are found on the face and scalp, appearing as nodular, swiftly expanding, circular, or oval-shaped growths. The robust vascular element of these lesions can lead to confusion with regard to the proper diagnosis, as they often resemble hemangiomas, pyogenic granulomas, or Kaposi sarcomas.4
Many cutaneous metastases linked to RCC exhibit a histomorphologic pattern consistent with clear cell adenocarcinoma.2 The malignant cells are large and possess transparent cytoplasm, round to oval nuclei, and prominent nucleoli. The cells can form glandular, acinar, or papillary arrangements; extravasated red blood cells frequently are found within the surrounding fibrovascular tissue.5 The presence of cytoplasmic glycogen can be revealed through periodic acid–Schiff staining. Other immunohistochemical markers commonly used to identify skin metastasis of RCC include epithelioid membrane antigen, carcinoembryonic antigen, and CD-10.1
Various mechanisms are involved in the cutaneous metastases of RCC. The most common pathway involves infiltration of the skin directly overlying the malignant renal mass; additional potential mechanisms include the introduction of abnormal cells into the skin during surgical or diagnostic interventions and their dissemination through the lymphatic system or bloodstream.1 Among urogenital malignancies other than RCC, skin metastases predominantly manifest in the abdominal region.2 Conversely, the head and neck region are more frequently impacted in RCC. The vascular composition of these tumors plays a role in facilitating the extension of cancer cells through the bloodstream, fostering the emergence of distant metastases.6
The development of cutaneous metastasis in RCC is associated with a poor prognosis, as most patients die within 6 months of detection.3 Treatment options thus are limited and palliative. Although local excision is an alternative treatment for localized cutaneous metastasis, it often provides little benefit in the presence of extensive metastasis; radiotherapy also has been shown to have a limited effect on primary RCC, though its devascularization of the lesion may be effective in metastatic cases.5 Immune checkpoint inhibitors such as nivolumab and ipilimumab have improved progression-free survival in patients with metastatic RCC, though uncertainty remains regarding their efficacy in attenuating cutaneous metastasis.5,6
- Kanwal R. Metastasis in renal cell carcinoma: biology and treatment. Adv Cancer Biol Metastasis. 2023;7:100094. doi:10.1016 /j.adcanc.2023.100094
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. doi:10.1093/annonc/mdr362
- Lorenzo-Rios D, Cruzval-O’Reilly E, Rabelo-Cartagena J. Facial cutaneous metastasis in renal cell carcinoma. Cureus. 2020;12:E12093. doi:10.7759/cureus.12093
- Iliescu CA, Beiu C, Racovit·a¢ A, et al. Atypical presentation of rapidly progressive cutaneous metastases of clear cell renal carcinoma: a case report. Medicina. 2024;60:1797. doi:10.3390/medicina60111797
- Joyce MJ. Management of skeletal metastases in renal cell carcinoma patients. In: Bukowski RM, Novick AC, eds. Clinical Management of Renal Tumors. Springer; 2008: 421-459.
- Kanwal R. Metastasis in renal cell carcinoma: biology and treatment. Adv Cancer Biol Metastasis. 2023;7:100094. doi:10.1016 /j.adcanc.2023.100094
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. doi:10.1093/annonc/mdr362
- Lorenzo-Rios D, Cruzval-O’Reilly E, Rabelo-Cartagena J. Facial cutaneous metastasis in renal cell carcinoma. Cureus. 2020;12:E12093. doi:10.7759/cureus.12093
- Iliescu CA, Beiu C, Racovit·a¢ A, et al. Atypical presentation of rapidly progressive cutaneous metastases of clear cell renal carcinoma: a case report. Medicina. 2024;60:1797. doi:10.3390/medicina60111797
- Joyce MJ. Management of skeletal metastases in renal cell carcinoma patients. In: Bukowski RM, Novick AC, eds. Clinical Management of Renal Tumors. Springer; 2008: 421-459.
Vascular Nodule on the Upper Chest
Vascular Nodule on the Upper Chest
A 45-year-old man presented to the dermatology clinic with a bleeding nodule on the upper chest of 2 months’ duration. He had a history of a low-grade mucoepidermoid carcinoma of the left parotid gland that was diagnosed 14 years prior and was treated via parotidectomy with 1 positive lymph node removed. Two months prior to the current presentation, the patient presented to the emergency department with unintentional weight loss and fatigue and subsequently was diagnosed with clear cell renal cell carcinoma that was treated via radical nephrectomy.
At the current presentation, the patient denied any recent fatigue, fever, weight loss, shortness of breath, or abdominal pain but reported neck stiffness. Physical examination revealed a solitary, smooth, vascular, 1.5×1.5 cm nodule on the left upper chest with no overlying skin changes. The remainder of the skin examination was unremarkable. A shave biopsy of the nodule was performed.
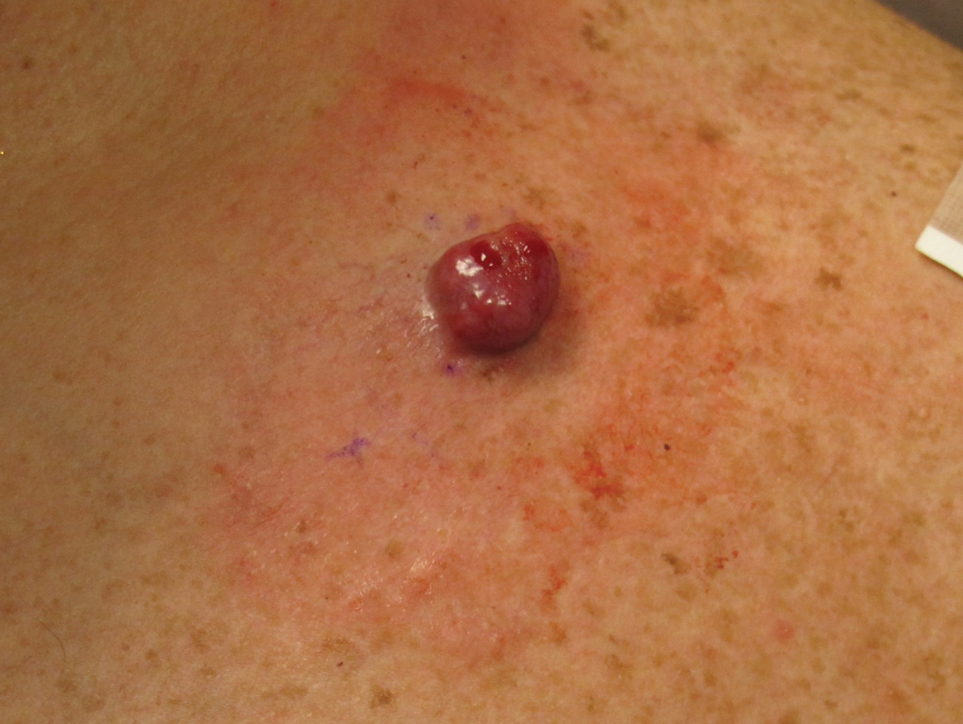
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11
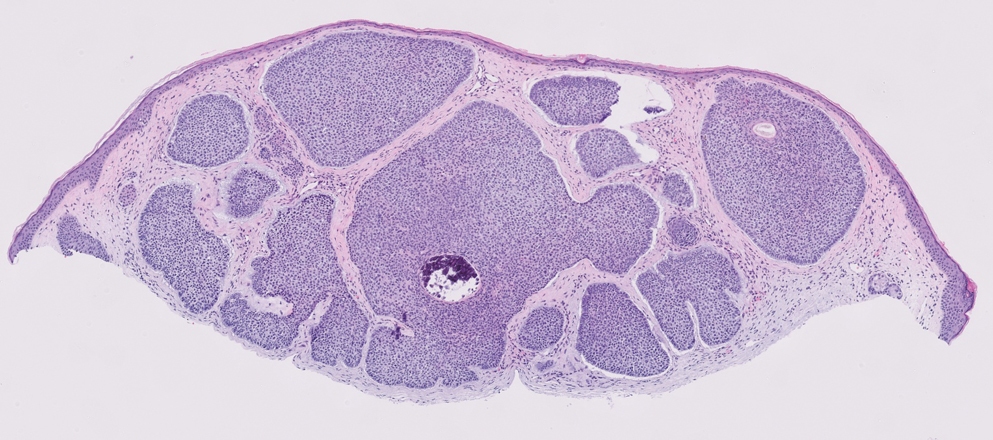
Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5
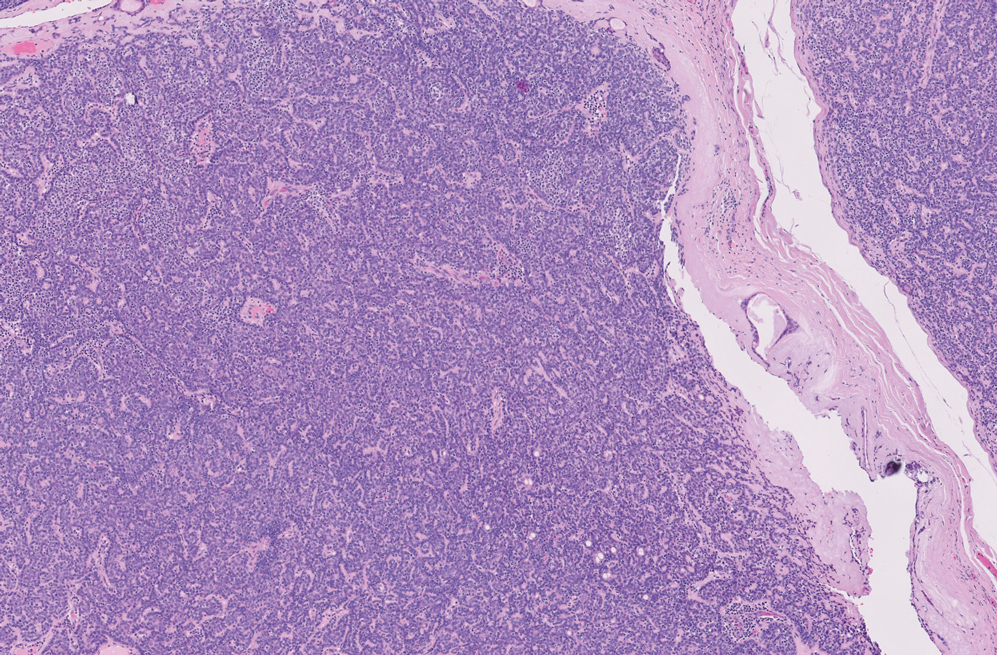
Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13
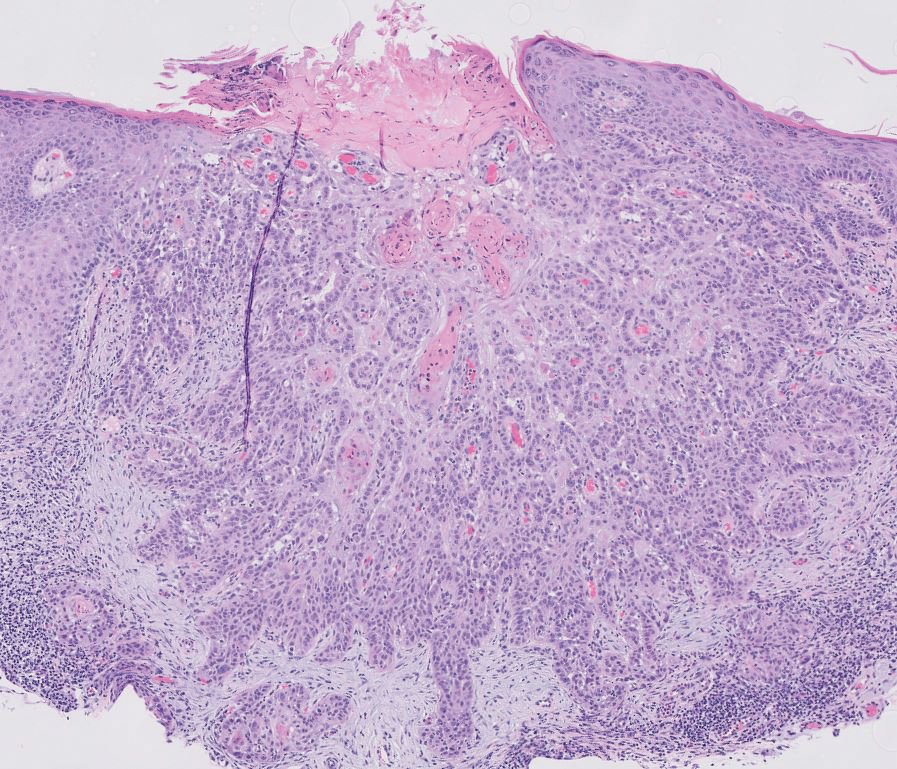
Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4
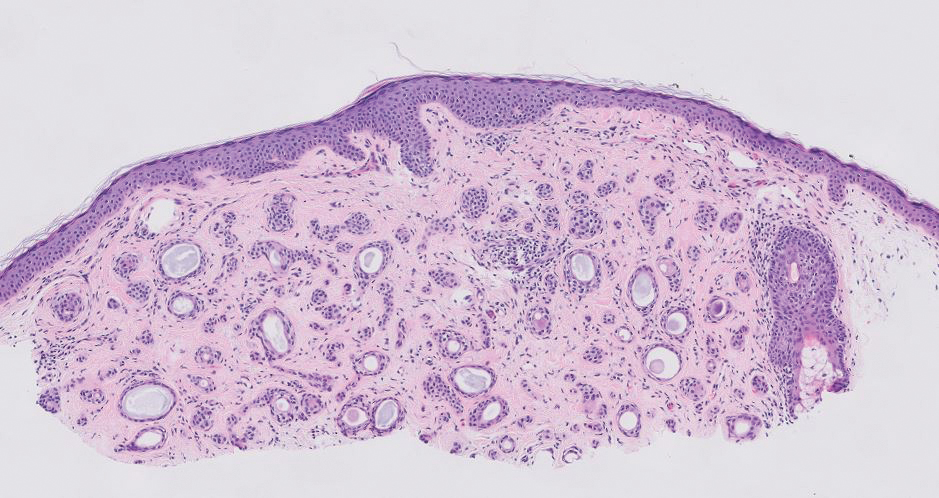
Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
A 57-year-old man with no notable medical history presented to the dermatology clinic for evaluation of an asymptomatic papule on the left lower eyelid. The patient reported that the lesion seemed to wax and wane in size over time. Physical examination revealed a small, pink, verrucous papule on the left lower eyelid. A shave biopsy of the lesion revealed a well-circumscribed collection of small, monomorphic, cuboidal cells with basophilic round nuclei, inconspicuous nucleoli, and compact eosinophilic cytoplasm (top) with focal areas of duct formation (bottom) that was sharply demarcated from normal keratinocytes.
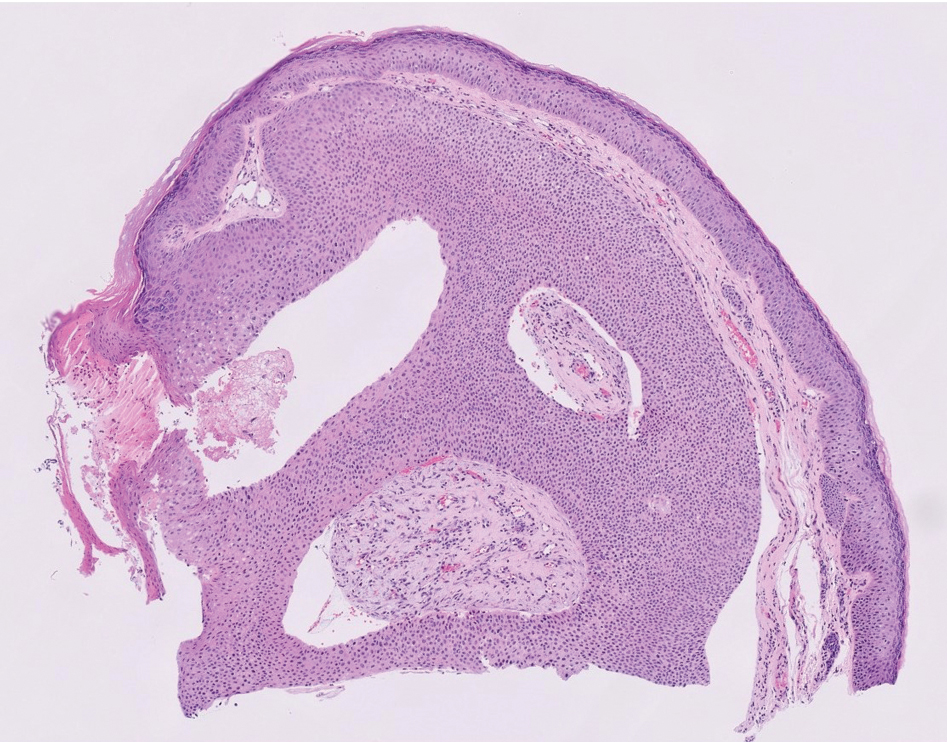
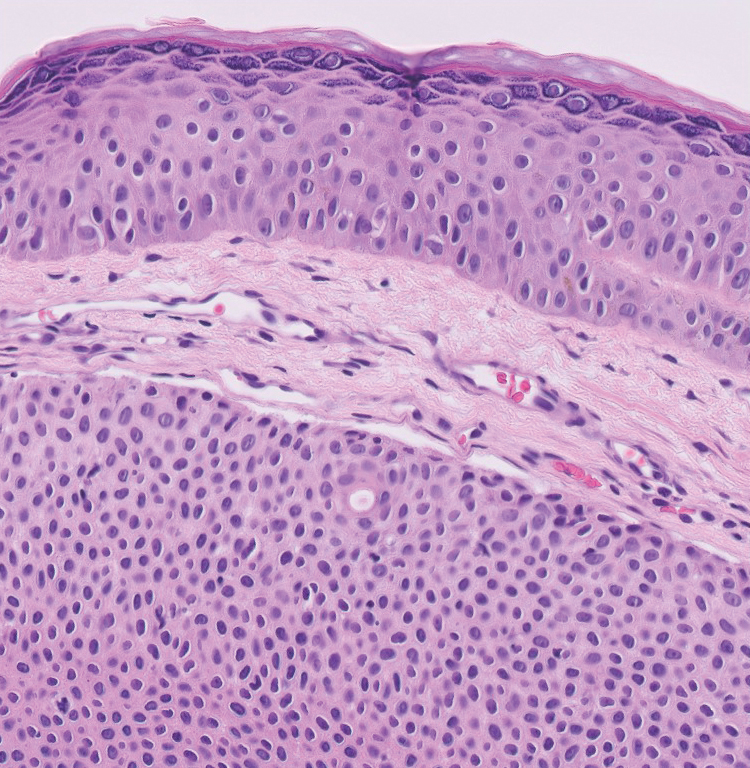
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
To the Editor:
Cutaneous metastasis of prostate cancer is rare and portends a bleak prognosis. Diagnosis of the primary cancer can be challenging, as skin metastasis can mimic a variety of conditions. We report a case of metastatic prostatic adenocarcinoma confirmed via biopsy of a new skin lesion.
A 97-year-old man presented to the dermatology clinic for routine follow-up of psoriasis. During the visit, a family member mentioned a new bleeding lesion on the left shoulder. It was not known how long the lesion had been present. Four months prior, the patient had a prostate-specific antigen (PSA) level of 582 ng/mL (reference range, 0-6.5 ng/mL), and computed tomography of the chest had shown innumerable pulmonary nodules in addition to lymphadenopathy of the left axilla, clavicle, and mediastinum. The imaging was ordered by the patient’s urologist as part of routine workup, as he had a history of obstructive renal failure and was being monitored for an indwelling catheter. Two months later, a bone scan ordered by the urologist due to high PSA levels showed extensive osteoblastic metastatic disease throughout the axial and proximal appendicular skeleton. The elevated PSA levels and findings of pulmonary and osteoblastic metastasis suggested a diagnosis of metastatic prostatic adenocarcinoma, but no confirmatory biopsy was performed following the imaging because the patient’s family declined additional workup or intervention.
Physical examination at the current presentation revealed an 8-mm brown papule with an overlying blue-white veil (Figure 1). There were no other skin findings. Primary differential diagnoses included metastatic prostate cancer, nodular melanoma, and traumatized seborrheic keratosis. A shave biopsy of the lesion showed multiple glandular structures infiltrating the dermis lined by monomorphic epithelial cells with prominent eosinophilic nucleoli (Figures 2 and 3). Focal cribriform architecture of the glands was present as well as dermal hemorrhage and a lymphohistiocytic infiltrate (Figure 2A). Interestingly, in-transit vascular metastases were confirmed with the support of ERG, CD34, and CD31 immunohistochemical staining of the vessels.
Immunohistochemical staining was positive for PSA (Figure 2B), NKX 3.1, and ERG in the invasive glandular structures, which also displayed patchy weak staining with AMACR. Staining was negative for prostein, cytokeratin (CK) 7, CK20, CK5/6, p63, p40, CDX2, and thyroid transcription factor 1. These findings were consistent with a diagnosis of cutaneous metastatic prostatic adenocarcinoma. Next-generation sequencing showed trans-membrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) fusion compatible with the positive ERG immunohistochemical staining. The patient and family declined any treatment due to his age, comorbidities, and rapid decline. He died 2 months after diagnosis of the skin metastasis.
Aside from nonmelanoma skin cancer, prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in the United States.1 It most commonly metastasizes to the bones, nonregional lymph nodes, liver, and thorax.2 Metastasis to the skin is very rare, with only a 0.36% incidence.3 When prostate cancer does metastasize to the skin, the prognosis is poor, with an estimated mean survival of 7 months after diagnosis of cutaneous metastasis.4 Our patient’s survival time was even shorter—only 2 months after diagnosis of cutaneous metastasis, likely the result of his late diagnosis.
Clinically, cutaneous metastasis of prostate cancer can manifest as a wide variety of lesions; in one report of 78 cases, 56 (72%) were hard nodules, 11 (14%) were single nodules, 5 (7%) were edema or lymphedema, and 5 (7%) were an unspecific rash.4 Diagnosis of cutaneous metastasis of prostate cancer can be challenging, as it often is mistaken for other skin conditions including herpes zoster, basal cell carcinoma, angiosarcoma, cellulitis, mammary Paget disease, telangiectasia, pyoderma, morphea, and trichoepithelioma.5 In our patient, the clinical appearance of the lesion resembled a nodular melanoma. Thus, in patients with a history of prostate cancer, it is important to keep cutaneous metastasis in the differential when examining the skin because of the prognostic implications. Cutaneous metastasis of prostate cancer often indicates a poor prognosis.
In a report of 78 patients, the most common sites of skin metastasis for prostate cancer were the inguinal area and penis (28% [22/78]), abdomen (23% [18/78]), head and neck (16% [12/78]), and chest (14% [11/78]); the extremities and back were less frequently involved (10% [8/78] and 9% [7/78], respectively).4 Generally, cutaneous metastasis of internal malignancies involves the deep dermis and the subcutaneous tissue. It is common for cutaneous metastases to show histologic features of the primary tumor, as we saw in our patient. In a case series with 45 histologic diagnoses of cutaneous metastases from internal malignancies, 75.5% (34/45) of cases showed morphologic features of the primary tumor.6 However, this is not always the case, and the histologic appearance may vary. Metastatic prostate cancer may manifest as sheets, nests, or cords and often may have nuclear pleomorphism with prominent nucleoli.7
Immunohistochemical staining can help make a definitive diagnosis and differentiate the source of the tumor. Prostate cancer metastases often will stain positive for NKX3.1, PSA, AMACR, ERG, PSMA, and prosaposin, with PSA being the most specific marker.7,8 In our patient, no prostate biopsy had been performed, thus the skin biopsy was the diagnostic tissue for the prostatic adenocarcinoma.
Next-generation sequencing showed a TMPRSS2- ERG fusion, which commonly is seen in prostate cancer.9 A search of Google Scholar using the terms next-generation sequencing, cutaneous metastasis, and prostate adenocarcinoma yielded 3 additional cases of cutaneous metastasis of prostate cancer in which next-generation sequencing was performed.10-12 One case showed mutations of the tumor protein 53 (TP53) and phosphatase and tensin homolog (PTEN) genes; one showed just a TP53 mutation; and one showed inactivation of the breast cancer predisposition gene 2 (BRCA2) and amplification of MYC proto-oncogene, BHLH transcription factor (MYC) and fibroblast growth factor receptor 1 (FGFR1).10,11,12 While limited by a small number of reported cases, there does not appear to be a repeating mutation to suggest a genetic mechanism of skin metastasis.
The route of cutaneous metastasis of prostate cancer still is unclear, but hypothesized mechanisms include hematogenous or lymphatic spread, direct infiltration, or implantation from a surgical scar.11 When cutaneous involvement occurs in an area far from the primary tumor, it is thought to be the result of hematogenous spread, which would be consistent with our patient’s findings.13 Given the role of Batson venous plexus as a conduit from the prostate to the vertebral column for metastatic spread and considering the location of the lesion on our patient’s back, we hypothesized that the mechanism of metastasis to the skin was from vascular extension of the metastatic foci involving the vertebrae.
Our case highlights the importance of considering cutaneous involvement of prostatic adenocarcinoma in patients with new skin lesions, particularly in the setting of a known or suspected prostate malignancy. Skin metastasis can have a range of manifestations and provides prognostic information that can help determine the course of treatment.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2023. Accessed November 11, 2024. https://www.cdc.gov/cancer/dataviz
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210-216. doi:10.1002/pros.22742
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026. doi:10.1016/j.urology.2004.01.014
- Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684. doi:10.1111/j.1600-0560.2007.00873.x
- Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600. doi:10.1111/j.1365-2133.2006.07696.x
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614. doi:10.1684/ejd.2017.3142
- Onalaja-Underwood AA, Sokumbi O. Eruptive papules as a cutaneous manifestation of metastatic prostate adenocarcinoma. Am J Dermatopathol. 2023;45:828-830. doi:10.1097/DAD.0000000000002559
- Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907-923. doi:10.1016/s0022-5347(17)38491-4
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450-5458. doi:10.3892/mmr.2017.7281
- Sharma H, Franklin M, Braunberger R, et al. Cutaneous metastasis from prostate cancer: a case report with literature review. Curr Probl Cancer Case Rep. 2022;7:100175. doi:10.1016/j.cpccr.2022.100175
- Dills A, Obi O, Bustos K, et al. Cutaneous manifestation of prostate adenocarcinoma: a rare presentation of a common disease. J Investig Med High Impact Case Rep. 2021;9:2324709621990769. doi:10.1177/2324709621990769
- Fadel CA, Kallab AM. Cutaneous scrotal metastasis secondary to primary prostate adenocarcinoma responding to immunotherapy. Ann Intern Med: Clinical Cases. 2022;1. doi:10.7326/aimcc.2022.0682
- Powell FC, Venencie PY, Winkelmann RK. Metastatic prostate carcinoma manifesting as penile nodules. Arch Dermatol. 1984;120:1604- 1606. doi:10.1001/archderm.1984.01650480066022
To the Editor:
Cutaneous metastasis of prostate cancer is rare and portends a bleak prognosis. Diagnosis of the primary cancer can be challenging, as skin metastasis can mimic a variety of conditions. We report a case of metastatic prostatic adenocarcinoma confirmed via biopsy of a new skin lesion.
A 97-year-old man presented to the dermatology clinic for routine follow-up of psoriasis. During the visit, a family member mentioned a new bleeding lesion on the left shoulder. It was not known how long the lesion had been present. Four months prior, the patient had a prostate-specific antigen (PSA) level of 582 ng/mL (reference range, 0-6.5 ng/mL), and computed tomography of the chest had shown innumerable pulmonary nodules in addition to lymphadenopathy of the left axilla, clavicle, and mediastinum. The imaging was ordered by the patient’s urologist as part of routine workup, as he had a history of obstructive renal failure and was being monitored for an indwelling catheter. Two months later, a bone scan ordered by the urologist due to high PSA levels showed extensive osteoblastic metastatic disease throughout the axial and proximal appendicular skeleton. The elevated PSA levels and findings of pulmonary and osteoblastic metastasis suggested a diagnosis of metastatic prostatic adenocarcinoma, but no confirmatory biopsy was performed following the imaging because the patient’s family declined additional workup or intervention.
Physical examination at the current presentation revealed an 8-mm brown papule with an overlying blue-white veil (Figure 1). There were no other skin findings. Primary differential diagnoses included metastatic prostate cancer, nodular melanoma, and traumatized seborrheic keratosis. A shave biopsy of the lesion showed multiple glandular structures infiltrating the dermis lined by monomorphic epithelial cells with prominent eosinophilic nucleoli (Figures 2 and 3). Focal cribriform architecture of the glands was present as well as dermal hemorrhage and a lymphohistiocytic infiltrate (Figure 2A). Interestingly, in-transit vascular metastases were confirmed with the support of ERG, CD34, and CD31 immunohistochemical staining of the vessels.



Immunohistochemical staining was positive for PSA (Figure 2B), NKX 3.1, and ERG in the invasive glandular structures, which also displayed patchy weak staining with AMACR. Staining was negative for prostein, cytokeratin (CK) 7, CK20, CK5/6, p63, p40, CDX2, and thyroid transcription factor 1. These findings were consistent with a diagnosis of cutaneous metastatic prostatic adenocarcinoma. Next-generation sequencing showed trans-membrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) fusion compatible with the positive ERG immunohistochemical staining. The patient and family declined any treatment due to his age, comorbidities, and rapid decline. He died 2 months after diagnosis of the skin metastasis.
Aside from nonmelanoma skin cancer, prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in the United States.1 It most commonly metastasizes to the bones, nonregional lymph nodes, liver, and thorax.2 Metastasis to the skin is very rare, with only a 0.36% incidence.3 When prostate cancer does metastasize to the skin, the prognosis is poor, with an estimated mean survival of 7 months after diagnosis of cutaneous metastasis.4 Our patient’s survival time was even shorter—only 2 months after diagnosis of cutaneous metastasis, likely the result of his late diagnosis.
Clinically, cutaneous metastasis of prostate cancer can manifest as a wide variety of lesions; in one report of 78 cases, 56 (72%) were hard nodules, 11 (14%) were single nodules, 5 (7%) were edema or lymphedema, and 5 (7%) were an unspecific rash.4 Diagnosis of cutaneous metastasis of prostate cancer can be challenging, as it often is mistaken for other skin conditions including herpes zoster, basal cell carcinoma, angiosarcoma, cellulitis, mammary Paget disease, telangiectasia, pyoderma, morphea, and trichoepithelioma.5 In our patient, the clinical appearance of the lesion resembled a nodular melanoma. Thus, in patients with a history of prostate cancer, it is important to keep cutaneous metastasis in the differential when examining the skin because of the prognostic implications. Cutaneous metastasis of prostate cancer often indicates a poor prognosis.
In a report of 78 patients, the most common sites of skin metastasis for prostate cancer were the inguinal area and penis (28% [22/78]), abdomen (23% [18/78]), head and neck (16% [12/78]), and chest (14% [11/78]); the extremities and back were less frequently involved (10% [8/78] and 9% [7/78], respectively).4 Generally, cutaneous metastasis of internal malignancies involves the deep dermis and the subcutaneous tissue. It is common for cutaneous metastases to show histologic features of the primary tumor, as we saw in our patient. In a case series with 45 histologic diagnoses of cutaneous metastases from internal malignancies, 75.5% (34/45) of cases showed morphologic features of the primary tumor.6 However, this is not always the case, and the histologic appearance may vary. Metastatic prostate cancer may manifest as sheets, nests, or cords and often may have nuclear pleomorphism with prominent nucleoli.7
Immunohistochemical staining can help make a definitive diagnosis and differentiate the source of the tumor. Prostate cancer metastases often will stain positive for NKX3.1, PSA, AMACR, ERG, PSMA, and prosaposin, with PSA being the most specific marker.7,8 In our patient, no prostate biopsy had been performed, thus the skin biopsy was the diagnostic tissue for the prostatic adenocarcinoma.
Next-generation sequencing showed a TMPRSS2- ERG fusion, which commonly is seen in prostate cancer.9 A search of Google Scholar using the terms next-generation sequencing, cutaneous metastasis, and prostate adenocarcinoma yielded 3 additional cases of cutaneous metastasis of prostate cancer in which next-generation sequencing was performed.10-12 One case showed mutations of the tumor protein 53 (TP53) and phosphatase and tensin homolog (PTEN) genes; one showed just a TP53 mutation; and one showed inactivation of the breast cancer predisposition gene 2 (BRCA2) and amplification of MYC proto-oncogene, BHLH transcription factor (MYC) and fibroblast growth factor receptor 1 (FGFR1).10,11,12 While limited by a small number of reported cases, there does not appear to be a repeating mutation to suggest a genetic mechanism of skin metastasis.
The route of cutaneous metastasis of prostate cancer still is unclear, but hypothesized mechanisms include hematogenous or lymphatic spread, direct infiltration, or implantation from a surgical scar.11 When cutaneous involvement occurs in an area far from the primary tumor, it is thought to be the result of hematogenous spread, which would be consistent with our patient’s findings.13 Given the role of Batson venous plexus as a conduit from the prostate to the vertebral column for metastatic spread and considering the location of the lesion on our patient’s back, we hypothesized that the mechanism of metastasis to the skin was from vascular extension of the metastatic foci involving the vertebrae.
Our case highlights the importance of considering cutaneous involvement of prostatic adenocarcinoma in patients with new skin lesions, particularly in the setting of a known or suspected prostate malignancy. Skin metastasis can have a range of manifestations and provides prognostic information that can help determine the course of treatment.
To the Editor:
Cutaneous metastasis of prostate cancer is rare and portends a bleak prognosis. Diagnosis of the primary cancer can be challenging, as skin metastasis can mimic a variety of conditions. We report a case of metastatic prostatic adenocarcinoma confirmed via biopsy of a new skin lesion.
A 97-year-old man presented to the dermatology clinic for routine follow-up of psoriasis. During the visit, a family member mentioned a new bleeding lesion on the left shoulder. It was not known how long the lesion had been present. Four months prior, the patient had a prostate-specific antigen (PSA) level of 582 ng/mL (reference range, 0-6.5 ng/mL), and computed tomography of the chest had shown innumerable pulmonary nodules in addition to lymphadenopathy of the left axilla, clavicle, and mediastinum. The imaging was ordered by the patient’s urologist as part of routine workup, as he had a history of obstructive renal failure and was being monitored for an indwelling catheter. Two months later, a bone scan ordered by the urologist due to high PSA levels showed extensive osteoblastic metastatic disease throughout the axial and proximal appendicular skeleton. The elevated PSA levels and findings of pulmonary and osteoblastic metastasis suggested a diagnosis of metastatic prostatic adenocarcinoma, but no confirmatory biopsy was performed following the imaging because the patient’s family declined additional workup or intervention.
Physical examination at the current presentation revealed an 8-mm brown papule with an overlying blue-white veil (Figure 1). There were no other skin findings. Primary differential diagnoses included metastatic prostate cancer, nodular melanoma, and traumatized seborrheic keratosis. A shave biopsy of the lesion showed multiple glandular structures infiltrating the dermis lined by monomorphic epithelial cells with prominent eosinophilic nucleoli (Figures 2 and 3). Focal cribriform architecture of the glands was present as well as dermal hemorrhage and a lymphohistiocytic infiltrate (Figure 2A). Interestingly, in-transit vascular metastases were confirmed with the support of ERG, CD34, and CD31 immunohistochemical staining of the vessels.



Immunohistochemical staining was positive for PSA (Figure 2B), NKX 3.1, and ERG in the invasive glandular structures, which also displayed patchy weak staining with AMACR. Staining was negative for prostein, cytokeratin (CK) 7, CK20, CK5/6, p63, p40, CDX2, and thyroid transcription factor 1. These findings were consistent with a diagnosis of cutaneous metastatic prostatic adenocarcinoma. Next-generation sequencing showed trans-membrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) fusion compatible with the positive ERG immunohistochemical staining. The patient and family declined any treatment due to his age, comorbidities, and rapid decline. He died 2 months after diagnosis of the skin metastasis.
Aside from nonmelanoma skin cancer, prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in the United States.1 It most commonly metastasizes to the bones, nonregional lymph nodes, liver, and thorax.2 Metastasis to the skin is very rare, with only a 0.36% incidence.3 When prostate cancer does metastasize to the skin, the prognosis is poor, with an estimated mean survival of 7 months after diagnosis of cutaneous metastasis.4 Our patient’s survival time was even shorter—only 2 months after diagnosis of cutaneous metastasis, likely the result of his late diagnosis.
Clinically, cutaneous metastasis of prostate cancer can manifest as a wide variety of lesions; in one report of 78 cases, 56 (72%) were hard nodules, 11 (14%) were single nodules, 5 (7%) were edema or lymphedema, and 5 (7%) were an unspecific rash.4 Diagnosis of cutaneous metastasis of prostate cancer can be challenging, as it often is mistaken for other skin conditions including herpes zoster, basal cell carcinoma, angiosarcoma, cellulitis, mammary Paget disease, telangiectasia, pyoderma, morphea, and trichoepithelioma.5 In our patient, the clinical appearance of the lesion resembled a nodular melanoma. Thus, in patients with a history of prostate cancer, it is important to keep cutaneous metastasis in the differential when examining the skin because of the prognostic implications. Cutaneous metastasis of prostate cancer often indicates a poor prognosis.
In a report of 78 patients, the most common sites of skin metastasis for prostate cancer were the inguinal area and penis (28% [22/78]), abdomen (23% [18/78]), head and neck (16% [12/78]), and chest (14% [11/78]); the extremities and back were less frequently involved (10% [8/78] and 9% [7/78], respectively).4 Generally, cutaneous metastasis of internal malignancies involves the deep dermis and the subcutaneous tissue. It is common for cutaneous metastases to show histologic features of the primary tumor, as we saw in our patient. In a case series with 45 histologic diagnoses of cutaneous metastases from internal malignancies, 75.5% (34/45) of cases showed morphologic features of the primary tumor.6 However, this is not always the case, and the histologic appearance may vary. Metastatic prostate cancer may manifest as sheets, nests, or cords and often may have nuclear pleomorphism with prominent nucleoli.7
Immunohistochemical staining can help make a definitive diagnosis and differentiate the source of the tumor. Prostate cancer metastases often will stain positive for NKX3.1, PSA, AMACR, ERG, PSMA, and prosaposin, with PSA being the most specific marker.7,8 In our patient, no prostate biopsy had been performed, thus the skin biopsy was the diagnostic tissue for the prostatic adenocarcinoma.
Next-generation sequencing showed a TMPRSS2- ERG fusion, which commonly is seen in prostate cancer.9 A search of Google Scholar using the terms next-generation sequencing, cutaneous metastasis, and prostate adenocarcinoma yielded 3 additional cases of cutaneous metastasis of prostate cancer in which next-generation sequencing was performed.10-12 One case showed mutations of the tumor protein 53 (TP53) and phosphatase and tensin homolog (PTEN) genes; one showed just a TP53 mutation; and one showed inactivation of the breast cancer predisposition gene 2 (BRCA2) and amplification of MYC proto-oncogene, BHLH transcription factor (MYC) and fibroblast growth factor receptor 1 (FGFR1).10,11,12 While limited by a small number of reported cases, there does not appear to be a repeating mutation to suggest a genetic mechanism of skin metastasis.
The route of cutaneous metastasis of prostate cancer still is unclear, but hypothesized mechanisms include hematogenous or lymphatic spread, direct infiltration, or implantation from a surgical scar.11 When cutaneous involvement occurs in an area far from the primary tumor, it is thought to be the result of hematogenous spread, which would be consistent with our patient’s findings.13 Given the role of Batson venous plexus as a conduit from the prostate to the vertebral column for metastatic spread and considering the location of the lesion on our patient’s back, we hypothesized that the mechanism of metastasis to the skin was from vascular extension of the metastatic foci involving the vertebrae.
Our case highlights the importance of considering cutaneous involvement of prostatic adenocarcinoma in patients with new skin lesions, particularly in the setting of a known or suspected prostate malignancy. Skin metastasis can have a range of manifestations and provides prognostic information that can help determine the course of treatment.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2023. Accessed November 11, 2024. https://www.cdc.gov/cancer/dataviz
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210-216. doi:10.1002/pros.22742
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026. doi:10.1016/j.urology.2004.01.014
- Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684. doi:10.1111/j.1600-0560.2007.00873.x
- Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600. doi:10.1111/j.1365-2133.2006.07696.x
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614. doi:10.1684/ejd.2017.3142
- Onalaja-Underwood AA, Sokumbi O. Eruptive papules as a cutaneous manifestation of metastatic prostate adenocarcinoma. Am J Dermatopathol. 2023;45:828-830. doi:10.1097/DAD.0000000000002559
- Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907-923. doi:10.1016/s0022-5347(17)38491-4
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450-5458. doi:10.3892/mmr.2017.7281
- Sharma H, Franklin M, Braunberger R, et al. Cutaneous metastasis from prostate cancer: a case report with literature review. Curr Probl Cancer Case Rep. 2022;7:100175. doi:10.1016/j.cpccr.2022.100175
- Dills A, Obi O, Bustos K, et al. Cutaneous manifestation of prostate adenocarcinoma: a rare presentation of a common disease. J Investig Med High Impact Case Rep. 2021;9:2324709621990769. doi:10.1177/2324709621990769
- Fadel CA, Kallab AM. Cutaneous scrotal metastasis secondary to primary prostate adenocarcinoma responding to immunotherapy. Ann Intern Med: Clinical Cases. 2022;1. doi:10.7326/aimcc.2022.0682
- Powell FC, Venencie PY, Winkelmann RK. Metastatic prostate carcinoma manifesting as penile nodules. Arch Dermatol. 1984;120:1604- 1606. doi:10.1001/archderm.1984.01650480066022
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2023. Accessed November 11, 2024. https://www.cdc.gov/cancer/dataviz
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210-216. doi:10.1002/pros.22742
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026. doi:10.1016/j.urology.2004.01.014
- Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684. doi:10.1111/j.1600-0560.2007.00873.x
- Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600. doi:10.1111/j.1365-2133.2006.07696.x
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614. doi:10.1684/ejd.2017.3142
- Onalaja-Underwood AA, Sokumbi O. Eruptive papules as a cutaneous manifestation of metastatic prostate adenocarcinoma. Am J Dermatopathol. 2023;45:828-830. doi:10.1097/DAD.0000000000002559
- Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907-923. doi:10.1016/s0022-5347(17)38491-4
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450-5458. doi:10.3892/mmr.2017.7281
- Sharma H, Franklin M, Braunberger R, et al. Cutaneous metastasis from prostate cancer: a case report with literature review. Curr Probl Cancer Case Rep. 2022;7:100175. doi:10.1016/j.cpccr.2022.100175
- Dills A, Obi O, Bustos K, et al. Cutaneous manifestation of prostate adenocarcinoma: a rare presentation of a common disease. J Investig Med High Impact Case Rep. 2021;9:2324709621990769. doi:10.1177/2324709621990769
- Fadel CA, Kallab AM. Cutaneous scrotal metastasis secondary to primary prostate adenocarcinoma responding to immunotherapy. Ann Intern Med: Clinical Cases. 2022;1. doi:10.7326/aimcc.2022.0682
- Powell FC, Venencie PY, Winkelmann RK. Metastatic prostate carcinoma manifesting as penile nodules. Arch Dermatol. 1984;120:1604- 1606. doi:10.1001/archderm.1984.01650480066022
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
PRACTICE POINTS
- Cutaneous metastasis of prostate cancer can have various manifestations and portends a poor prognosis.
- New skin lesions that develop in patients with a high clinical suspicion for prostate cancer warrant consideration of cutaneous metastasis.
Merkel Cell Carcinoma Less Common, With higher Mortality Than Melanoma
TOPLINE:
that also reported that male gender, older age, and exposure to ultraviolet radiation (UVR) are significant risk factors.
METHODOLOGY:
- Researchers identified 19,444 MCC cases and 646,619 melanoma cases diagnosed between 2000 and 2021 using data from the Surveillance, Epidemiology, and End Results (SEER) Program.
- Ambient UVR exposure data were obtained from the National Aeronautics and Space Administration’s total ozone mapping spectrometer database.
- Risk factors and cancer-specific mortality rates were evaluated for both cancers.
TAKEAWAY:
- Incidence rates per 100,000 person-years of MCC and melanoma were 0.8 and 27.3, respectively.
- Men (adjusted incidence rate ratio [IRR], 1.72 for MCC and 1.23 for melanoma), older age groups (IRR: 2.69 for MCC and 1.62 for melanoma among those 70-79 years; and 5.68 for MCC and 2.26 for melanoma among those 80 years or older) showed higher incidences of MCC and melanoma. Non-Hispanic White individuals were at higher risk for MCC and melanoma than other racial/ethnic groups.
- Exposure to UVR was associated with higher incidences of melanoma (IRR, 1.24-1.49) and MCC (IRR, 1.15-1.20) in non-Hispanic White individuals, particularly on the head and neck. These associations were unclear among racial/ethnic groups.
- Individuals with MCC had a higher risk for cancer-specific mortality than those with melanoma (adjusted hazard ratio [HR], 2.33; 95% CI, 2.26-2.42). Cancer-specific survival for both cancers improved for cases diagnosed during 2012-2021 vs 2004-2011 (MCC: HR, 0.83; 95% CI, 0.78-0.89; melanoma: HR, 0.75; 95% CI, 0.74-0.76).
IN PRACTICE:
“MCC and melanoma are aggressive skin cancers with similar risk factors including male sex, older age, and UV radiation exposure. Clinicians should be alert to diagnosis of these cancers to allow for prompt treatment,” the authors wrote, adding: “It is encouraging that survival for both cancers has increased in recent years, with the largest gains in survival seen in distant stage melanoma, coinciding with the approval of BRAF and PD-1 inhibitors used for distant stage disease,” although mortality for advanced stage tumors “continues to be very high.”
SOURCE:
The study was led by Jacob T. Tribble, BA, National Cancer Institute, Rockville, Maryland. It was published online on January 5 in the Journal of Investigative Dermatology.
LIMITATIONS:
The study relied on SEER’s general staging system rather than the American Joint Committee on Cancer standard, and UVR exposure estimates did not account for individual sun protection behaviors or prior residential history. Race and ethnicity served as a proxy for UVR sensitivity, which may introduce misclassification bias.
DISCLOSURES:
The research was supported by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the American Association for Dental Research, and the Colgate-Palmolive Company. The authors reported no conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also reported that male gender, older age, and exposure to ultraviolet radiation (UVR) are significant risk factors.
METHODOLOGY:
- Researchers identified 19,444 MCC cases and 646,619 melanoma cases diagnosed between 2000 and 2021 using data from the Surveillance, Epidemiology, and End Results (SEER) Program.
- Ambient UVR exposure data were obtained from the National Aeronautics and Space Administration’s total ozone mapping spectrometer database.
- Risk factors and cancer-specific mortality rates were evaluated for both cancers.
TAKEAWAY:
- Incidence rates per 100,000 person-years of MCC and melanoma were 0.8 and 27.3, respectively.
- Men (adjusted incidence rate ratio [IRR], 1.72 for MCC and 1.23 for melanoma), older age groups (IRR: 2.69 for MCC and 1.62 for melanoma among those 70-79 years; and 5.68 for MCC and 2.26 for melanoma among those 80 years or older) showed higher incidences of MCC and melanoma. Non-Hispanic White individuals were at higher risk for MCC and melanoma than other racial/ethnic groups.
- Exposure to UVR was associated with higher incidences of melanoma (IRR, 1.24-1.49) and MCC (IRR, 1.15-1.20) in non-Hispanic White individuals, particularly on the head and neck. These associations were unclear among racial/ethnic groups.
- Individuals with MCC had a higher risk for cancer-specific mortality than those with melanoma (adjusted hazard ratio [HR], 2.33; 95% CI, 2.26-2.42). Cancer-specific survival for both cancers improved for cases diagnosed during 2012-2021 vs 2004-2011 (MCC: HR, 0.83; 95% CI, 0.78-0.89; melanoma: HR, 0.75; 95% CI, 0.74-0.76).
IN PRACTICE:
“MCC and melanoma are aggressive skin cancers with similar risk factors including male sex, older age, and UV radiation exposure. Clinicians should be alert to diagnosis of these cancers to allow for prompt treatment,” the authors wrote, adding: “It is encouraging that survival for both cancers has increased in recent years, with the largest gains in survival seen in distant stage melanoma, coinciding with the approval of BRAF and PD-1 inhibitors used for distant stage disease,” although mortality for advanced stage tumors “continues to be very high.”
SOURCE:
The study was led by Jacob T. Tribble, BA, National Cancer Institute, Rockville, Maryland. It was published online on January 5 in the Journal of Investigative Dermatology.
LIMITATIONS:
The study relied on SEER’s general staging system rather than the American Joint Committee on Cancer standard, and UVR exposure estimates did not account for individual sun protection behaviors or prior residential history. Race and ethnicity served as a proxy for UVR sensitivity, which may introduce misclassification bias.
DISCLOSURES:
The research was supported by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the American Association for Dental Research, and the Colgate-Palmolive Company. The authors reported no conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also reported that male gender, older age, and exposure to ultraviolet radiation (UVR) are significant risk factors.
METHODOLOGY:
- Researchers identified 19,444 MCC cases and 646,619 melanoma cases diagnosed between 2000 and 2021 using data from the Surveillance, Epidemiology, and End Results (SEER) Program.
- Ambient UVR exposure data were obtained from the National Aeronautics and Space Administration’s total ozone mapping spectrometer database.
- Risk factors and cancer-specific mortality rates were evaluated for both cancers.
TAKEAWAY:
- Incidence rates per 100,000 person-years of MCC and melanoma were 0.8 and 27.3, respectively.
- Men (adjusted incidence rate ratio [IRR], 1.72 for MCC and 1.23 for melanoma), older age groups (IRR: 2.69 for MCC and 1.62 for melanoma among those 70-79 years; and 5.68 for MCC and 2.26 for melanoma among those 80 years or older) showed higher incidences of MCC and melanoma. Non-Hispanic White individuals were at higher risk for MCC and melanoma than other racial/ethnic groups.
- Exposure to UVR was associated with higher incidences of melanoma (IRR, 1.24-1.49) and MCC (IRR, 1.15-1.20) in non-Hispanic White individuals, particularly on the head and neck. These associations were unclear among racial/ethnic groups.
- Individuals with MCC had a higher risk for cancer-specific mortality than those with melanoma (adjusted hazard ratio [HR], 2.33; 95% CI, 2.26-2.42). Cancer-specific survival for both cancers improved for cases diagnosed during 2012-2021 vs 2004-2011 (MCC: HR, 0.83; 95% CI, 0.78-0.89; melanoma: HR, 0.75; 95% CI, 0.74-0.76).
IN PRACTICE:
“MCC and melanoma are aggressive skin cancers with similar risk factors including male sex, older age, and UV radiation exposure. Clinicians should be alert to diagnosis of these cancers to allow for prompt treatment,” the authors wrote, adding: “It is encouraging that survival for both cancers has increased in recent years, with the largest gains in survival seen in distant stage melanoma, coinciding with the approval of BRAF and PD-1 inhibitors used for distant stage disease,” although mortality for advanced stage tumors “continues to be very high.”
SOURCE:
The study was led by Jacob T. Tribble, BA, National Cancer Institute, Rockville, Maryland. It was published online on January 5 in the Journal of Investigative Dermatology.
LIMITATIONS:
The study relied on SEER’s general staging system rather than the American Joint Committee on Cancer standard, and UVR exposure estimates did not account for individual sun protection behaviors or prior residential history. Race and ethnicity served as a proxy for UVR sensitivity, which may introduce misclassification bias.
DISCLOSURES:
The research was supported by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the American Association for Dental Research, and the Colgate-Palmolive Company. The authors reported no conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
MRI-Invisible Prostate Lesions: Are They Dangerous?
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
Recurrent Nodule on the First Toe
Recurrent Nodule on the First Toe
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.
Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.

Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.

Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
Recurrent Nodule on the First Toe
Recurrent Nodule on the First Toe
A 56-year-old man was referred to the dermatology clinic for treatment of a recurrent nodule on the left first toe. The lesion first appeared 12 years prior and was resected by an outside dermatologist, who diagnosed the lesion as benign based on biopsy results. Approximately 10 years later, the lesion began to grow back with a similar appearance to the original nodule; it again was diagnosed as benign based on another biopsy and excised by the outside dermatologist. Two years later, the patient had a second recurrence of the lesion, which was excised by his dermatologist. The biopsy report at that time identified the lesion as a low-grade adnexal neoplasm. The patient had a rapid recurrence of the tumor after 6 months and was referred to our clinic for Mohs micrographic surgery. Physical examination revealed a tender, 2.5×1.8-cm, firm, exophytic, subcutaneous nodule on the left first toe with no associated lymphadenopathy.