User login
Optimal Cosmetic Outcomes for Basal Cell Carcinoma: A Retrospective Study of Nonablative Laser Management
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
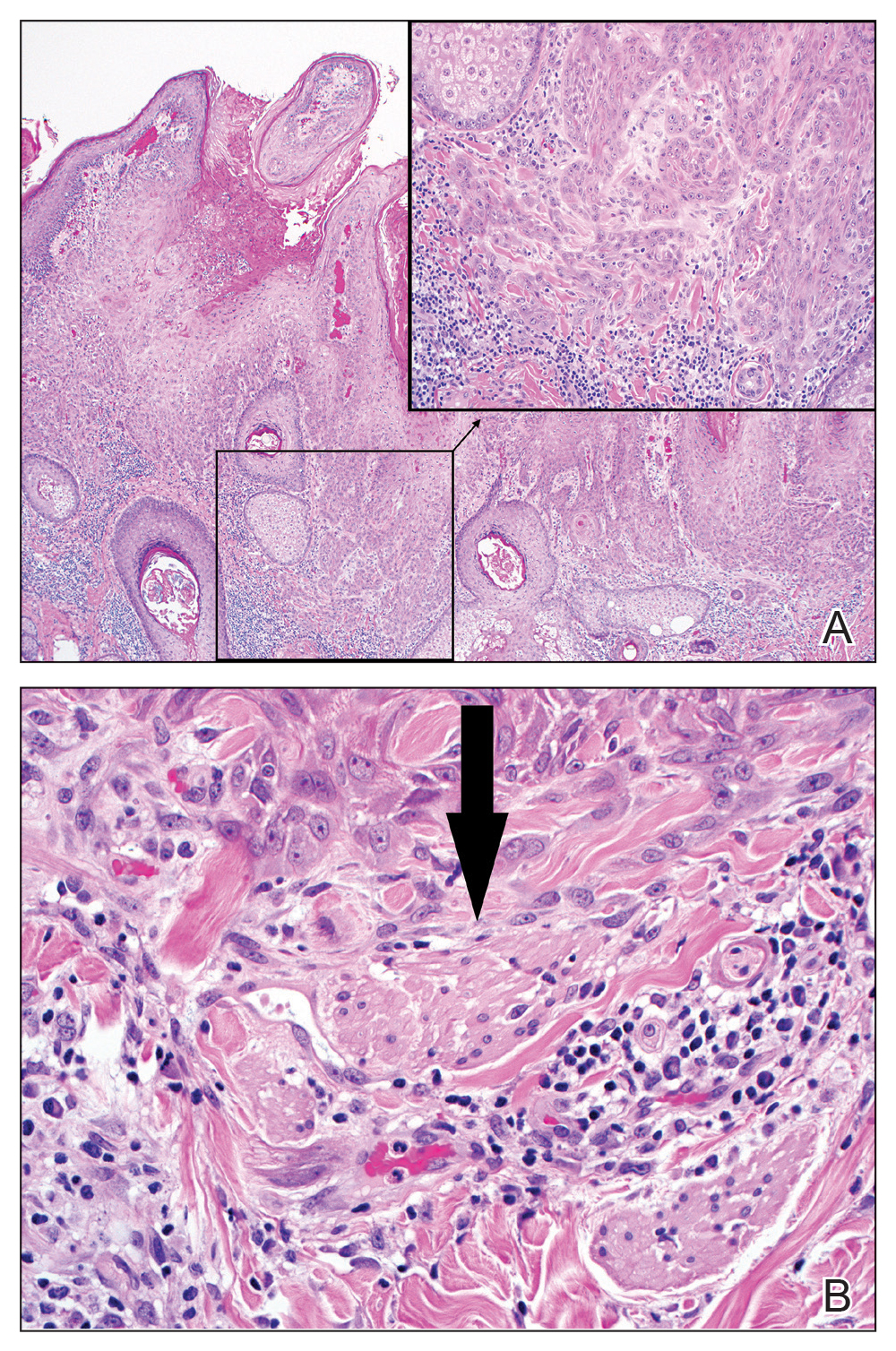
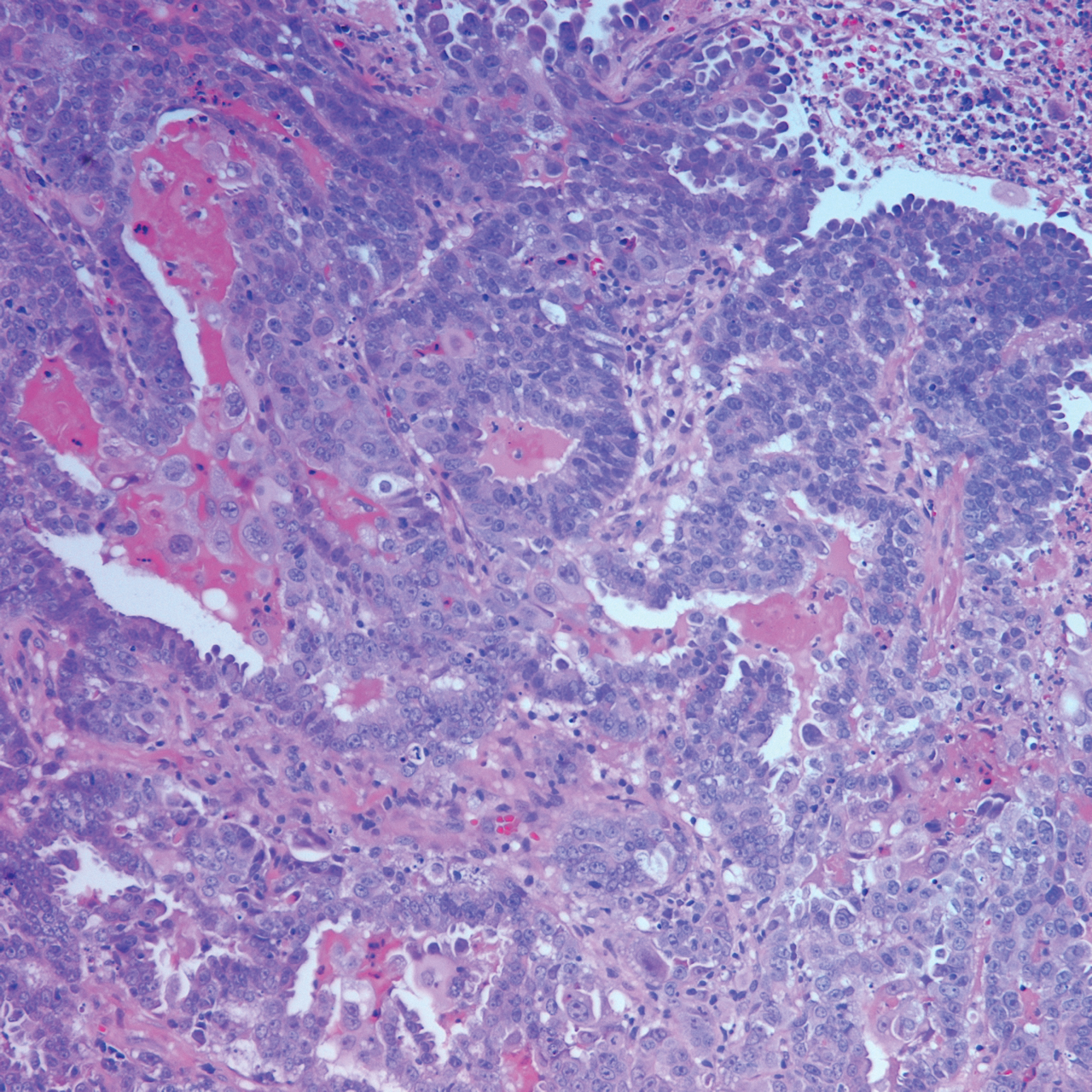
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
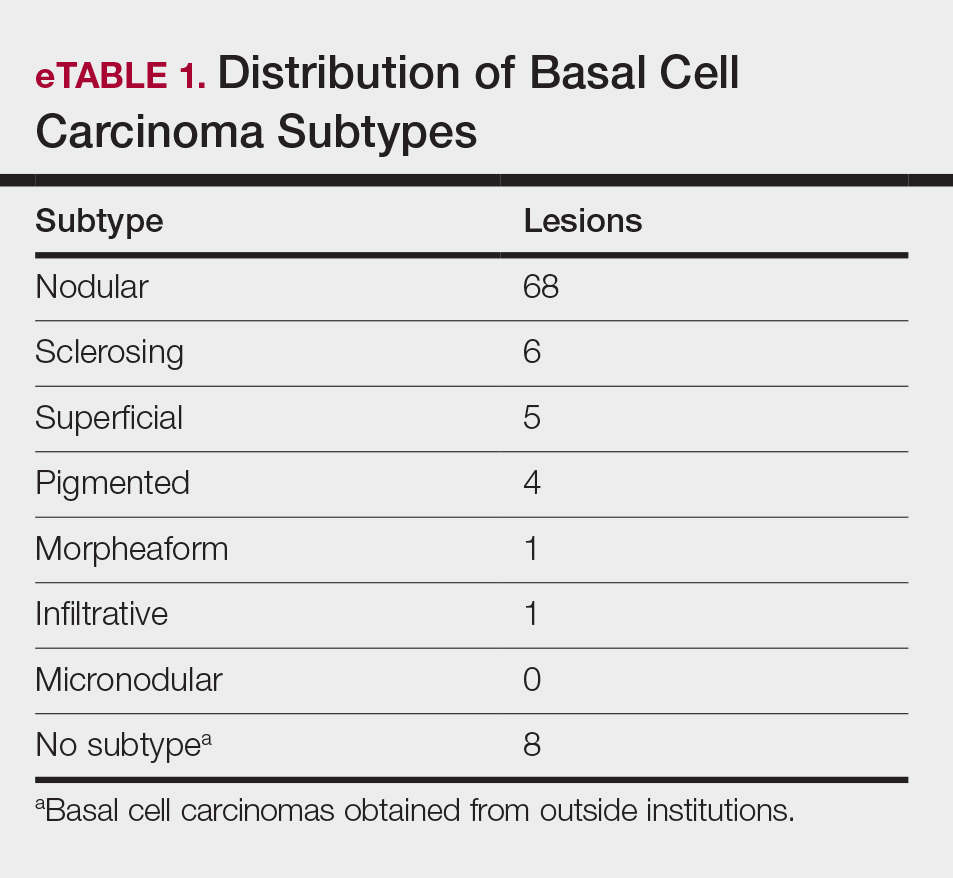
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.
Tumor Clearance
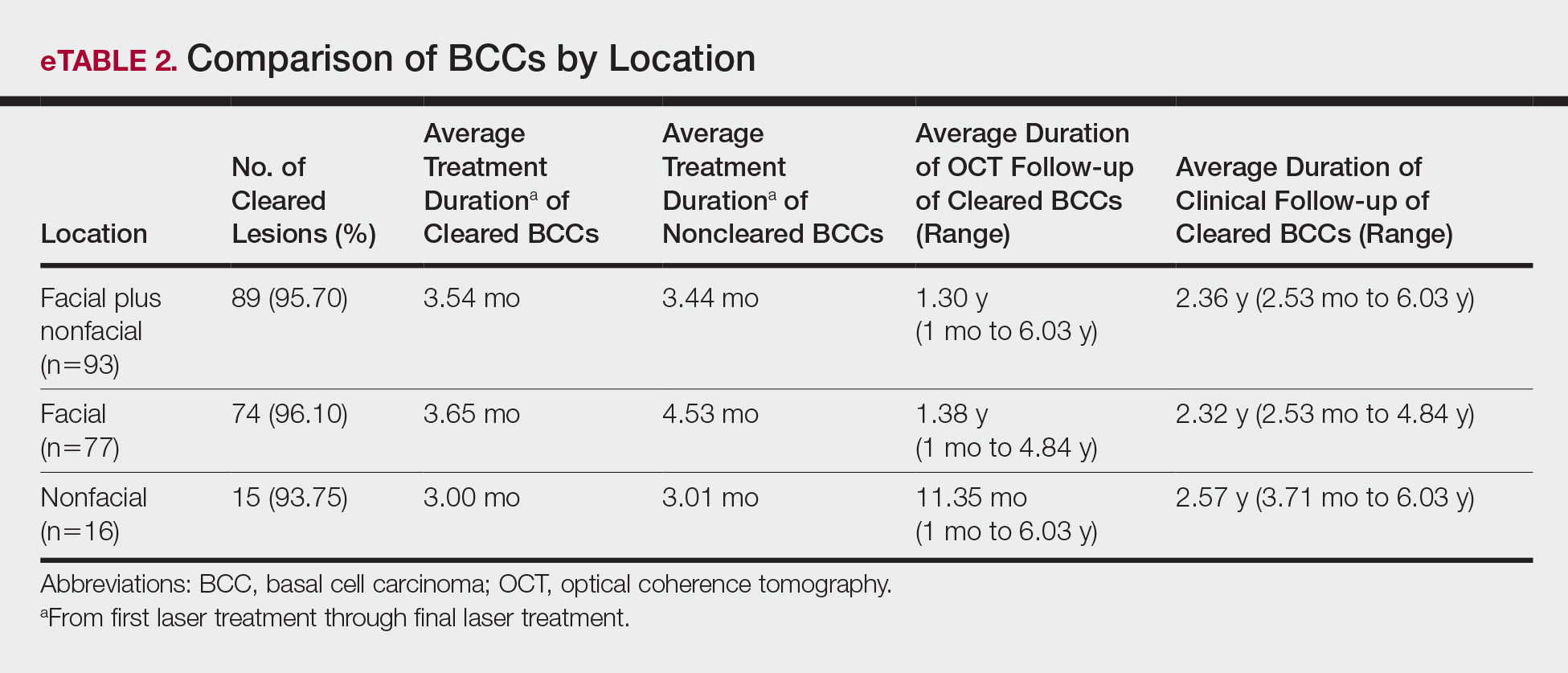
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.
Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
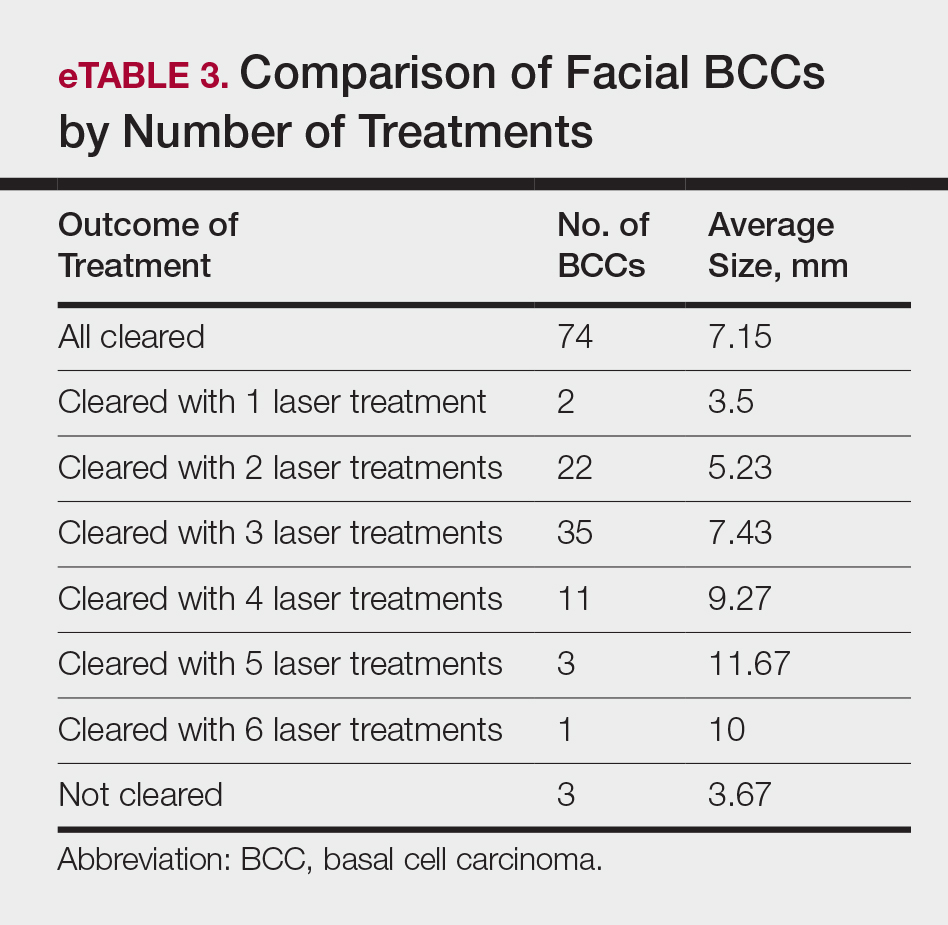
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).
Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.
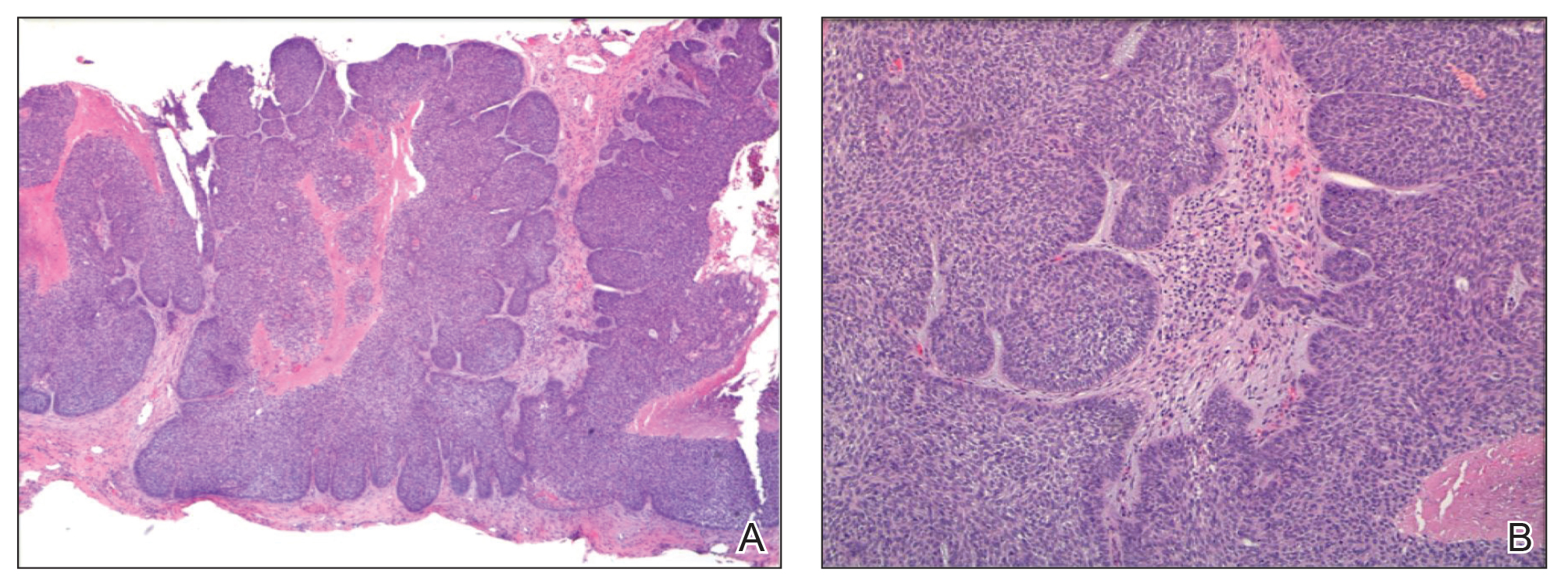
Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
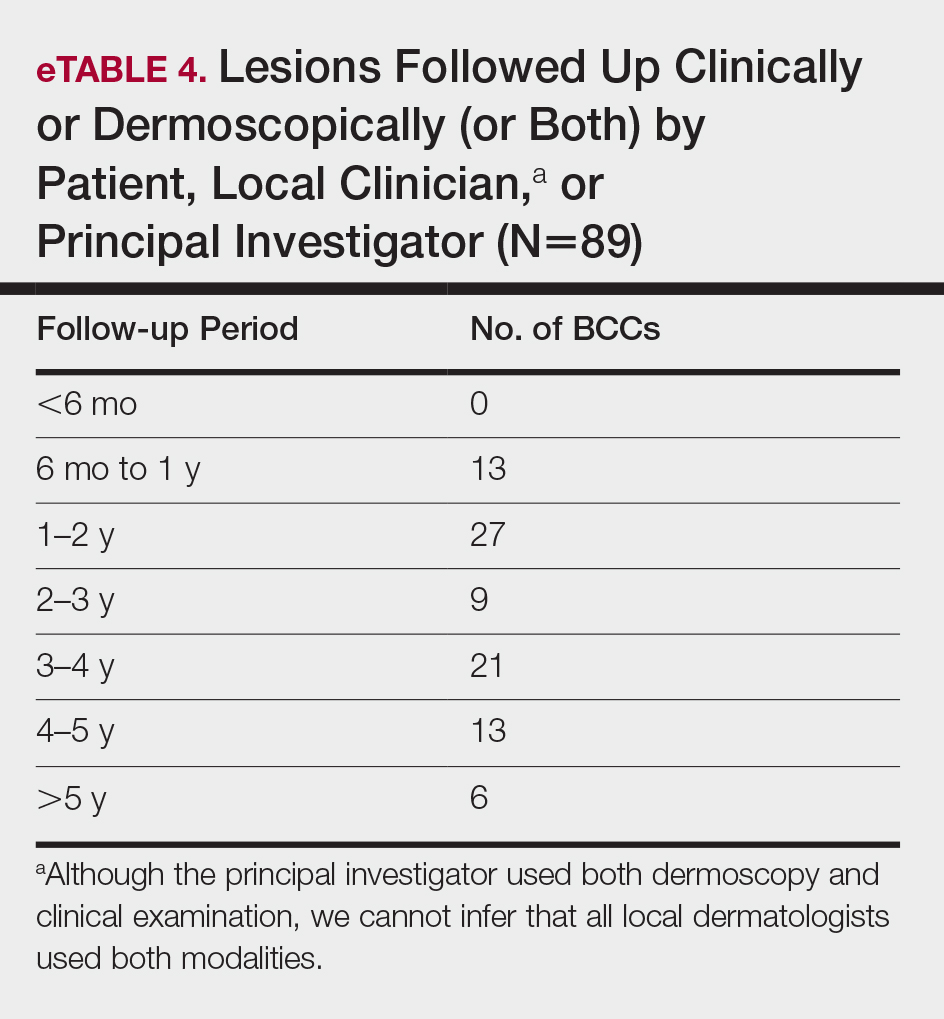
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.
Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
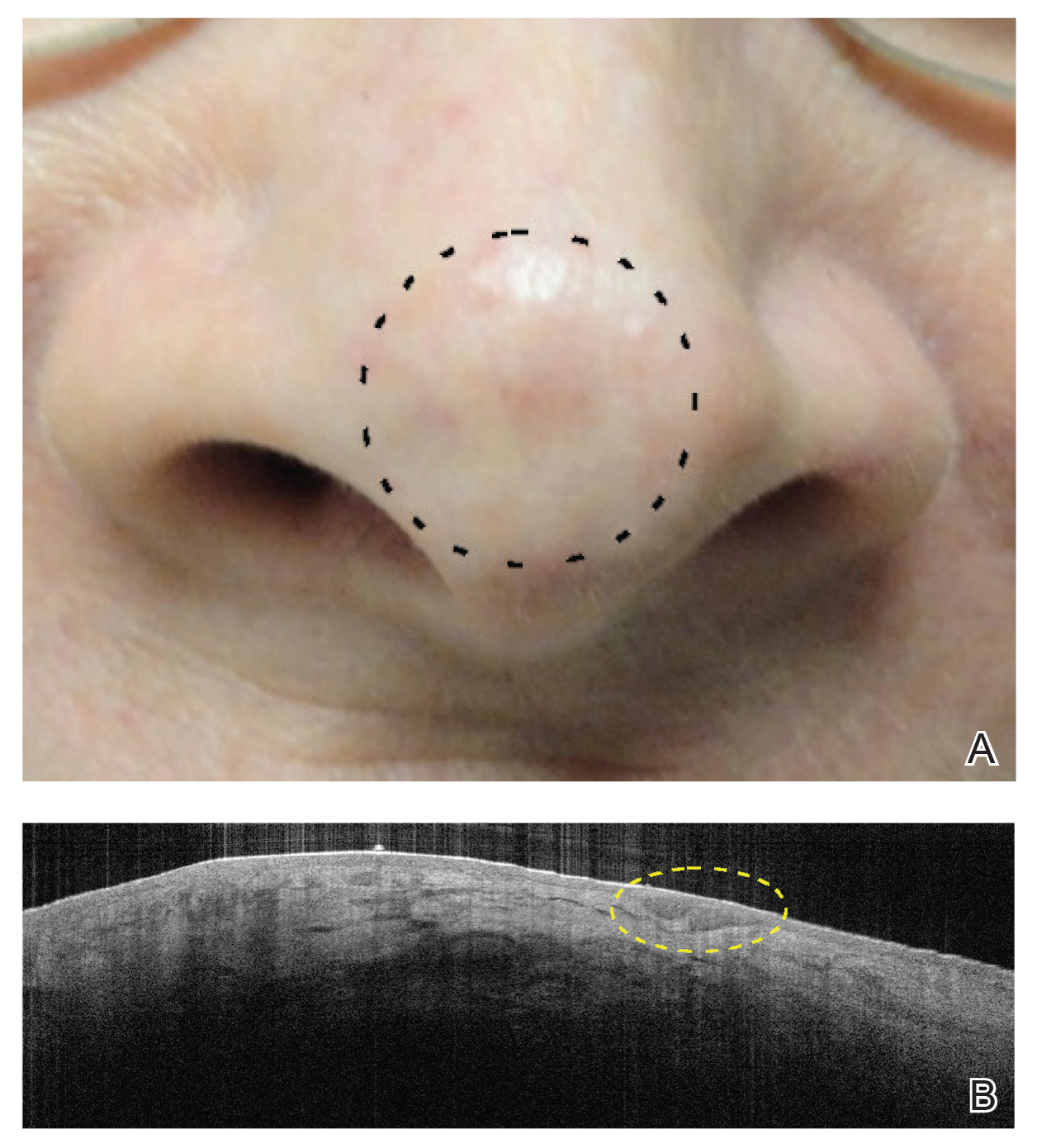
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29
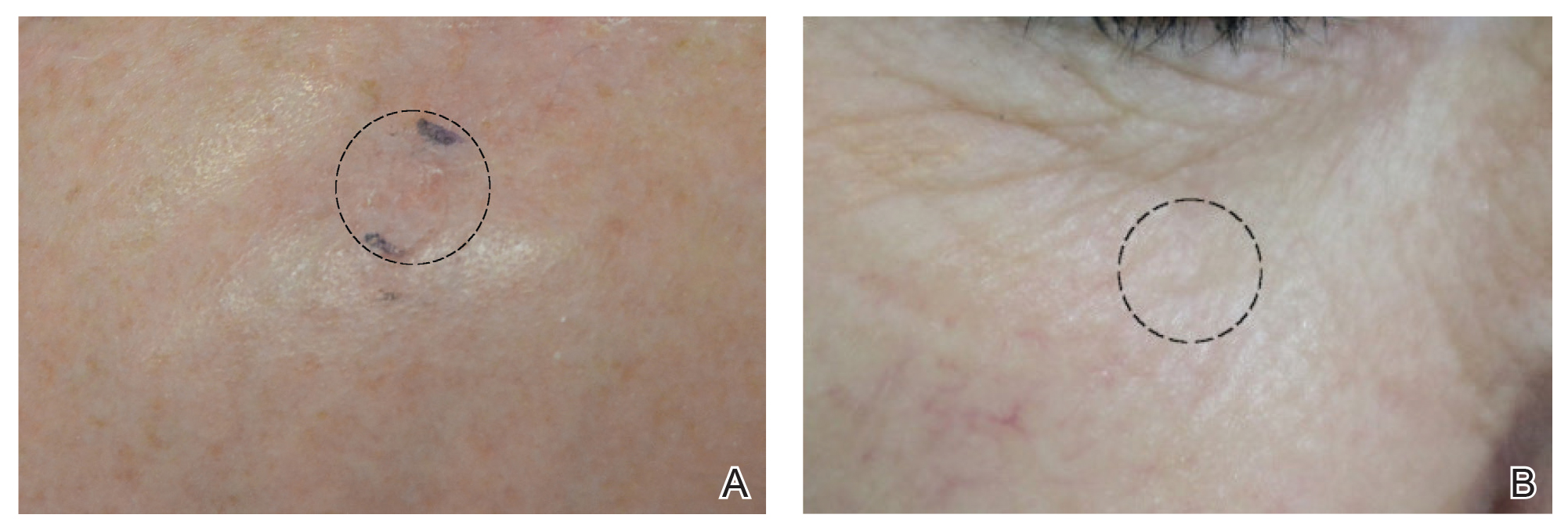
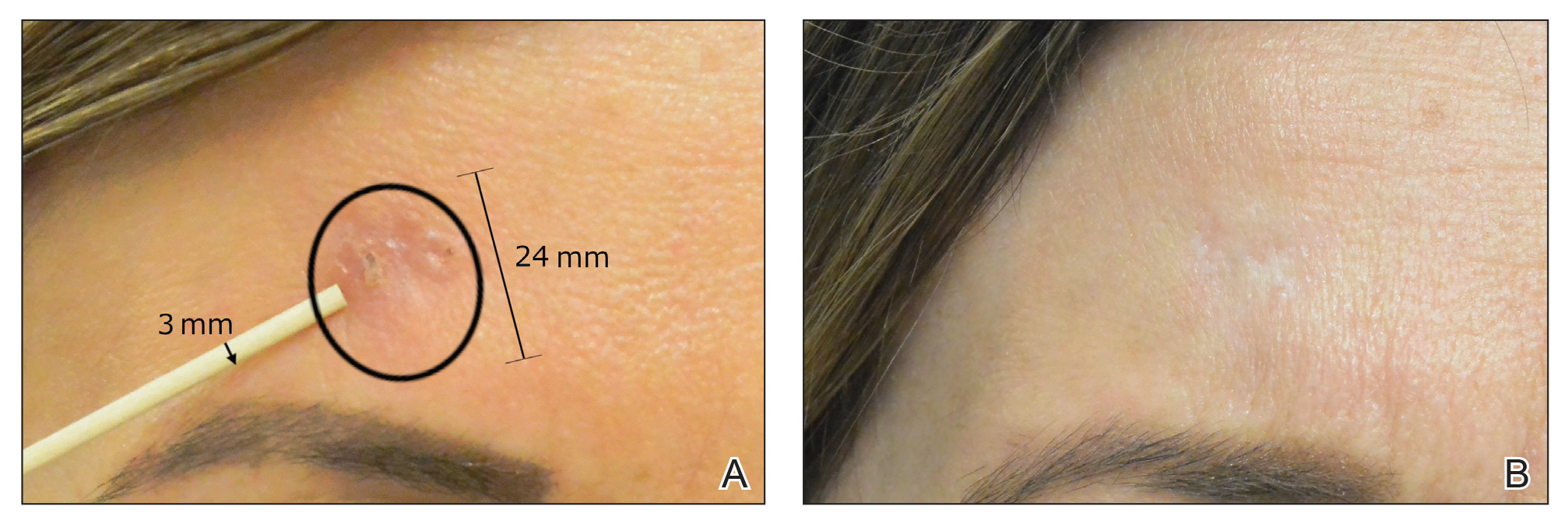
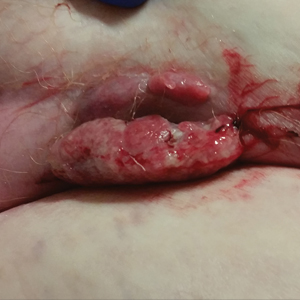
After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.
there was no clinical evidence of residual BCC.
Cosmetic Outcome
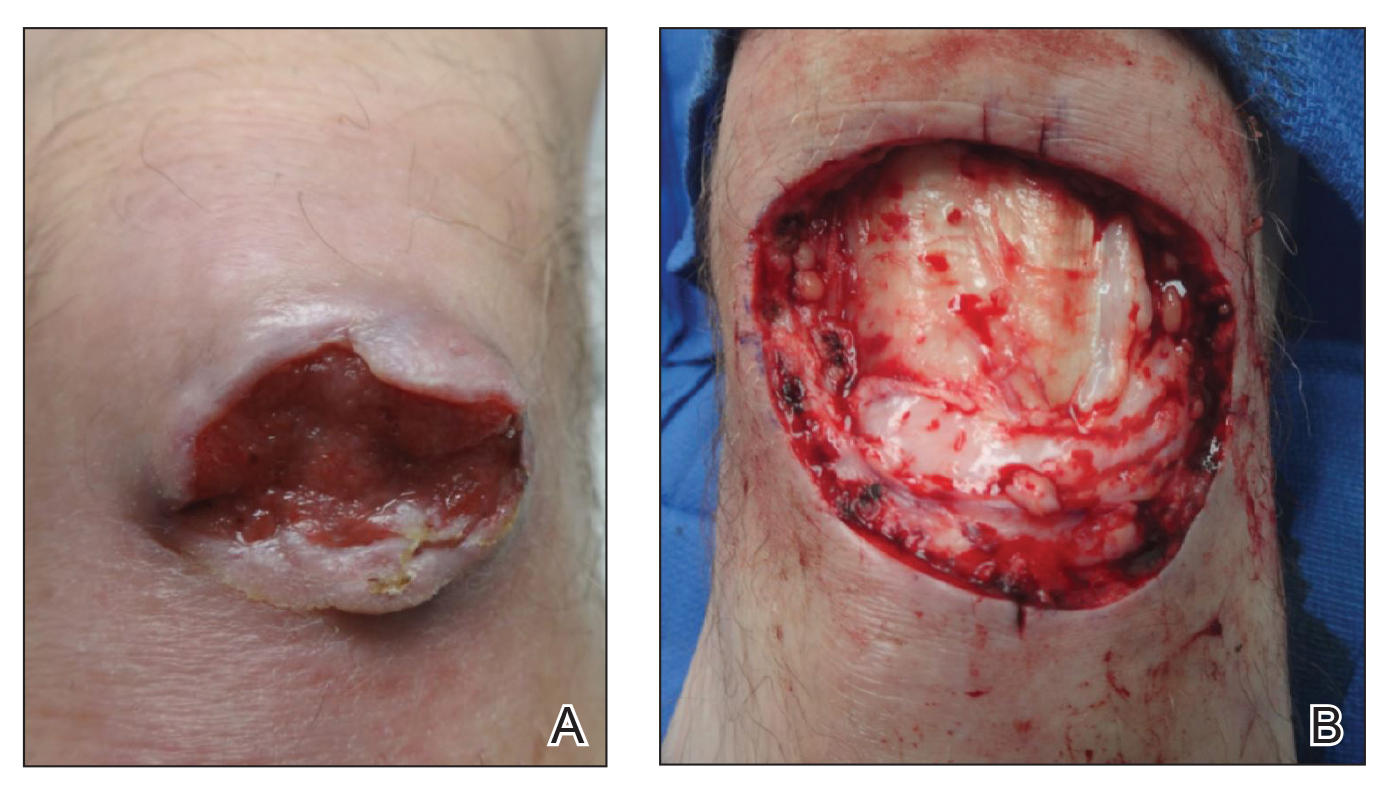
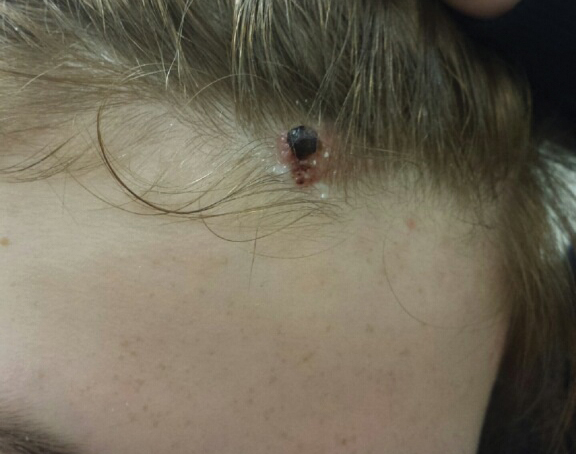
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.
Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.
Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).
Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.
Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.
Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29
After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.
there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.
Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.
Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).
Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.
Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.
Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29
After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.
there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
Practice Points
- A major benefit of nonablative laser therapy over more invasive options in the management of basal cell carcinoma (BCC) is minimal scarring.
- When patients are managed with nonablative laser therapy, follow-up with clinical, dermoscopic, and/or noninvasive imaging is more efficient during treatment as well as when assessing for recurrences.
- Optical coherence tomography in combination with nonablative laser therapy allows for detection of residual skin cancers that would not be evident on clinical and/or dermoscopic examination.
- Utilizing early detection techniques, such as a color wheel dermoscopic approach, along with other noninvasive imaging modalities facilitates the use of less invasive treatment options for primary and/or recurrent BCCs.
Basal Cell Carcinoma Masquerading as a Dermoid Cyst and Bursitis of the Knee
Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer in the United States. It develops most often on sun-exposed skin, including the face and neck. Although BCCs are slow-growing tumors that rarely metastasize, they can cause notable local destruction with disfigurement if neglected or inadequately treated. Basal cell carcinoma arising on the legs is relatively uncommon.1,2 We present an interesting case of delayed diagnosis of BCC on the left knee due to earlier misdiagnoses of a dermoid cyst and bursitis.
Case Report
A 67-year-old man with no history of skin cancer presented with a painful growing tumor on the left knee of approximately 2 years’ duration. The patient’s primary care physician as well as a general surgeon initially diagnosed it as a dermoid cyst and bursitis. The nodule failed to respond to conservative therapy with nonsteroidal anti-inflammatory drugs and continued to grow until it began to ulcerate. Concerned about the possibility of septic arthritis, the patient’s primary care physician referred him to the emergency department. He was subsequently sent to the dermatology clinic.
On examination by dermatology, a 6.3×4.4-cm, tender, mobile, ulcerated nodule was noted on the left knee (Figure 1A). No popliteal or inguinal lymph nodes were palpable. Basal cell carcinoma, squamous cell carcinoma, or atypical infection (eg, Leishmania, deep fungal, mycobacterial) was suspected clinically. The patient underwent a diagnostic skin biopsy; hematoxylin and eosin–stained sections revealed lobular proliferation of basaloid cells with peripheral palisading and central tumoral necrosis, consistent with primary BCC (Figure 2).
Given the size of the tumor, the patient was referred for Mohs micrographic surgery and eventual reconstruction by a plastic surgeon. The tumor was cleared after 2 stages of Mohs surgery, with a final wound size of 7.7×5.4 cm (Figure 1B). Plastic surgery later performed a gastrocnemius muscle flap with a split-thickness skin graft (175 cm2) to repair the wound.
Comment
Exposure to UV radiation is the primary causative agent of most BCCs, accounting for the preferential distribution of these tumors on sun-exposed areas of the body. Approximately 80% of BCCs are located on the head and neck, 10% occur on the trunk, and only 8% are found on the lower extremities.1
Giant BCC, the finding in this case, is defined by the American Joint Committee on Cancer as a tumor larger than 5 cm in diameter. Fewer than 1% of all BCCs achieve this size; they appear more commonly on the back where they can go unnoticed.2 Neglect and inadequate treatment of the primary tumor are the most important contributing factors to the size of giant BCCs. Giant BCCs also have more aggressive biologic behavior, with an increased risk for local invasion and metastasis.3 In this case, the lesion was larger than 5 cm in diameter and occurred on the lower extremity rather than on the trunk.
This case is unusual because delayed diagnosis of BCC was the result of misdiagnoses of a dermoid cyst and bursitis, with a diagnostic skin biopsy demonstrating BCC almost 2 years later. It should be emphasized that early diagnosis and treatment could prevent tumor expansion. Physicians should have a high degree of suspicion for BCC, especially when a dermoid cyst and knee bursitis fail to respond to conservative management.
- Pearson G, King LE, Boyd AS. Basal cell carcinoma of the lower extremities. Int J Dermatol. 1999;38:852-854.
- Arnaiz J, Gallardo E, Piedra T, et al. Giant basal cell carcinoma on the lower leg: MRI findings. J Plast Reconstr Aesthet Surg. 2007;60:1167-1168.
- Randle HW. Giant basal cell carcinoma [letter]. Int J Dermatol. 1996;35:222-223.
Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer in the United States. It develops most often on sun-exposed skin, including the face and neck. Although BCCs are slow-growing tumors that rarely metastasize, they can cause notable local destruction with disfigurement if neglected or inadequately treated. Basal cell carcinoma arising on the legs is relatively uncommon.1,2 We present an interesting case of delayed diagnosis of BCC on the left knee due to earlier misdiagnoses of a dermoid cyst and bursitis.
Case Report
A 67-year-old man with no history of skin cancer presented with a painful growing tumor on the left knee of approximately 2 years’ duration. The patient’s primary care physician as well as a general surgeon initially diagnosed it as a dermoid cyst and bursitis. The nodule failed to respond to conservative therapy with nonsteroidal anti-inflammatory drugs and continued to grow until it began to ulcerate. Concerned about the possibility of septic arthritis, the patient’s primary care physician referred him to the emergency department. He was subsequently sent to the dermatology clinic.
On examination by dermatology, a 6.3×4.4-cm, tender, mobile, ulcerated nodule was noted on the left knee (Figure 1A). No popliteal or inguinal lymph nodes were palpable. Basal cell carcinoma, squamous cell carcinoma, or atypical infection (eg, Leishmania, deep fungal, mycobacterial) was suspected clinically. The patient underwent a diagnostic skin biopsy; hematoxylin and eosin–stained sections revealed lobular proliferation of basaloid cells with peripheral palisading and central tumoral necrosis, consistent with primary BCC (Figure 2).
Given the size of the tumor, the patient was referred for Mohs micrographic surgery and eventual reconstruction by a plastic surgeon. The tumor was cleared after 2 stages of Mohs surgery, with a final wound size of 7.7×5.4 cm (Figure 1B). Plastic surgery later performed a gastrocnemius muscle flap with a split-thickness skin graft (175 cm2) to repair the wound.
Comment
Exposure to UV radiation is the primary causative agent of most BCCs, accounting for the preferential distribution of these tumors on sun-exposed areas of the body. Approximately 80% of BCCs are located on the head and neck, 10% occur on the trunk, and only 8% are found on the lower extremities.1
Giant BCC, the finding in this case, is defined by the American Joint Committee on Cancer as a tumor larger than 5 cm in diameter. Fewer than 1% of all BCCs achieve this size; they appear more commonly on the back where they can go unnoticed.2 Neglect and inadequate treatment of the primary tumor are the most important contributing factors to the size of giant BCCs. Giant BCCs also have more aggressive biologic behavior, with an increased risk for local invasion and metastasis.3 In this case, the lesion was larger than 5 cm in diameter and occurred on the lower extremity rather than on the trunk.
This case is unusual because delayed diagnosis of BCC was the result of misdiagnoses of a dermoid cyst and bursitis, with a diagnostic skin biopsy demonstrating BCC almost 2 years later. It should be emphasized that early diagnosis and treatment could prevent tumor expansion. Physicians should have a high degree of suspicion for BCC, especially when a dermoid cyst and knee bursitis fail to respond to conservative management.
Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer in the United States. It develops most often on sun-exposed skin, including the face and neck. Although BCCs are slow-growing tumors that rarely metastasize, they can cause notable local destruction with disfigurement if neglected or inadequately treated. Basal cell carcinoma arising on the legs is relatively uncommon.1,2 We present an interesting case of delayed diagnosis of BCC on the left knee due to earlier misdiagnoses of a dermoid cyst and bursitis.
Case Report
A 67-year-old man with no history of skin cancer presented with a painful growing tumor on the left knee of approximately 2 years’ duration. The patient’s primary care physician as well as a general surgeon initially diagnosed it as a dermoid cyst and bursitis. The nodule failed to respond to conservative therapy with nonsteroidal anti-inflammatory drugs and continued to grow until it began to ulcerate. Concerned about the possibility of septic arthritis, the patient’s primary care physician referred him to the emergency department. He was subsequently sent to the dermatology clinic.
On examination by dermatology, a 6.3×4.4-cm, tender, mobile, ulcerated nodule was noted on the left knee (Figure 1A). No popliteal or inguinal lymph nodes were palpable. Basal cell carcinoma, squamous cell carcinoma, or atypical infection (eg, Leishmania, deep fungal, mycobacterial) was suspected clinically. The patient underwent a diagnostic skin biopsy; hematoxylin and eosin–stained sections revealed lobular proliferation of basaloid cells with peripheral palisading and central tumoral necrosis, consistent with primary BCC (Figure 2).
Given the size of the tumor, the patient was referred for Mohs micrographic surgery and eventual reconstruction by a plastic surgeon. The tumor was cleared after 2 stages of Mohs surgery, with a final wound size of 7.7×5.4 cm (Figure 1B). Plastic surgery later performed a gastrocnemius muscle flap with a split-thickness skin graft (175 cm2) to repair the wound.
Comment
Exposure to UV radiation is the primary causative agent of most BCCs, accounting for the preferential distribution of these tumors on sun-exposed areas of the body. Approximately 80% of BCCs are located on the head and neck, 10% occur on the trunk, and only 8% are found on the lower extremities.1
Giant BCC, the finding in this case, is defined by the American Joint Committee on Cancer as a tumor larger than 5 cm in diameter. Fewer than 1% of all BCCs achieve this size; they appear more commonly on the back where they can go unnoticed.2 Neglect and inadequate treatment of the primary tumor are the most important contributing factors to the size of giant BCCs. Giant BCCs also have more aggressive biologic behavior, with an increased risk for local invasion and metastasis.3 In this case, the lesion was larger than 5 cm in diameter and occurred on the lower extremity rather than on the trunk.
This case is unusual because delayed diagnosis of BCC was the result of misdiagnoses of a dermoid cyst and bursitis, with a diagnostic skin biopsy demonstrating BCC almost 2 years later. It should be emphasized that early diagnosis and treatment could prevent tumor expansion. Physicians should have a high degree of suspicion for BCC, especially when a dermoid cyst and knee bursitis fail to respond to conservative management.
- Pearson G, King LE, Boyd AS. Basal cell carcinoma of the lower extremities. Int J Dermatol. 1999;38:852-854.
- Arnaiz J, Gallardo E, Piedra T, et al. Giant basal cell carcinoma on the lower leg: MRI findings. J Plast Reconstr Aesthet Surg. 2007;60:1167-1168.
- Randle HW. Giant basal cell carcinoma [letter]. Int J Dermatol. 1996;35:222-223.
- Pearson G, King LE, Boyd AS. Basal cell carcinoma of the lower extremities. Int J Dermatol. 1999;38:852-854.
- Arnaiz J, Gallardo E, Piedra T, et al. Giant basal cell carcinoma on the lower leg: MRI findings. J Plast Reconstr Aesthet Surg. 2007;60:1167-1168.
- Randle HW. Giant basal cell carcinoma [letter]. Int J Dermatol. 1996;35:222-223.
Practice Points
- This case highlights an unusual presentation of basal cell carcinoma masquerading as bursitis.
- Clinicians should be aware of confirmation bias, especially when multiple physicians and specialists are involved in a case.
- When the initial clinical impression is not corroborated by objective data or the condition is not responding to conventional therapy, it is important for clinicians to revisit the possibility of an inaccurate diagnosis.
Quantity and Characteristics of Flap or Graft Repairs for Skin Cancer on the Nose or Ears: A Comparison Between Mohs Micrographic Surgery and Plastic Surgery
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.
Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.
Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.
Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
Practice Points
- Patients and nondermatologist physicians may be unaware of how frequently Mohs surgeons perform complex surgical repairs compared to other specialists.
- Compared to plastic surgeons, Mohs surgeons performed a larger number of complex skin cancer repairs on the nose or ears with similar-sized defects.
- Primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer through awareness of this type of data.
The Dayanara Effect: Increasing Skin Cancer Awareness in the Hispanic Community
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Squamous Cell Carcinoma With Perineural Involvement in Nevus Sebaceus
First reported in 1895, nevus sebaceus (NS) is a con genital papillomatous hamartoma most commonly found on the scalp and face. 1 Lesions typically are yellow-orange plaques and often are hairless. Nevus sebaceus is most prominent in the few first months after birth and again at puberty during development of the sebaceous glands. Development of epithelial hyperplasia, cysts, verrucas, and benign or malignant tumors has been reported. 1 The most common benign tumors are syringocystadenoma papilliferum and trichoblastoma. Cases of malignancy are rare, and basal cell carcinoma is the predominant form (approximately 2% of cases). Squamous cell carcinoma (SCC) and adnexal carcinoma are reported at even lower rates. 1 Malignant transformation occurring during childhood is extremely uncommon. According to a PubMed search of articles indexed for MEDLINE using the terms nevus sebaceous, malignancy, and squamous cell carcinoma and narrowing the results to children, there have been only 4 prior reports of SCC developing within an NS in a child. 2-5 We report a case of SCC arising in an NS in a 13-year-old adolescent girl with perineural invasion.
Case Report
A 13-year-old fair-skinned adolescent girl presented with a hairless 2×2.5-cm yellow plaque at the hairline on the anterior central scalp. The plaque had been present since birth and had progressively developed a superiorly located 3×5-mm erythematous verrucous nodule (Figure 1) with an approximate height of 6 mm over the last year. The nodule was subjected to regular trauma and bled with minimal insult. The patient appeared otherwise healthy, with no history of skin cancer or other chronic medical conditions. There was no evidence of lymphadenopathy on examination, and no other skin abnormalities were noted. There was no reported family history of skin cancer or chronic skin conditions suggestive of increased risk for cancer or other pathologic dermatoses. Differential diagnoses for the plaque and nodule complex included verruca, Spitz nevus, or secondary neoplasm within NS.
Excision was conducted under local anesthesia without complication. An elliptical section of skin measuring 0.8×2.5 cm was excised to a depth of 3 mm. The resulting wound was closed using a complex linear repair. The section was placed in formalin specimen transport medium and sent to Walter Reed National Military Medical Center (Bethesda, Maryland). Microscopic examination of the specimen revealed features typical for NS, including mild verrucous epidermal hyperplasia, sebaceous gland hyperplasia, presence of apocrine glands, and hamartomatous follicular proliferations (Figure 2). An even more papillomatous epidermal proliferation that was comprised of atypical squamous cells was present within the lesion. Similar atypical squamous cells infiltrated the superficial dermis in nests, cords, and single cells (Figure 3A). One focus showed perineural invasion with a small superficial nerve fiber surrounded by SCC (Figure 3B). The tumor was completely excised, with negative surgical margins extending approximately 2 mm. Adjuvant radiation therapy and further specialized Mohs micrographic excision were not performed because of the clear histologic appearance of the carcinoma and strong evidence of complete excision.
At 2-week follow-up, the surgical scar on the anterior central forehead was well healed without evidence of SCC recurrence. On physical examination there was neither lymphadenopathy nor signs of neurologic deficit, except for superficial cutaneous hypoesthesia in the immediate area surrounding the healed site. Following discussion with the patient and her parents, it was decided that the patient would obtain baseline laboratory tests, chest radiography, and abdominal ultrasonography, and she would undergo serial follow-up examinations every 3 months for the next 2 years. Annual follow-up was recommended after 2 years, with the caveat to return sooner if recurrence or symptoms were to arise.
Comment
Historically, there has been variability in the histopathologic interpretation of SCC in NS in the literature. Retrospective analysis of the histologic evidence of SCC in the 2 earliest possible cases of pediatric SCC in NS have been questioned due to the lack of clinical data presented and the possibility that the diagnosis of SCC was inaccurate.6 Our case was histopathologically interpreted as superficially invasive, well-differentiated SCC arising within an NS; therefore, we classified this case as SCC and took every precaution to ensure the lesion was completely excised, given the potentially invasive nature of SCC.
Our case is unique because it represents SCC in NS with histologic evidence of perineural involvement. Perineural invasion is a major route of tumor spread in SCC and may result in increased occurrence of regional lymph node spread and distant metastases, with path of least resistance or neural cell adhesion as possible spreading methods.7-9 However, there is a notable amount of prognostic variability based on tumor type, the nerve involved, and degree of involvement.9 It is common for cutaneous SCC to occur with invasion of small intradermal nerves, but a poor outcome is less likely in asymptomatic patients who have perineural involvement that was incidentally discovered on histologic examination.10
In our patient, the entire tumor was completely removed with local excision. Recurrence of the SCC or future symptoms of deep neural invasion were not anticipated given the postoperative evidence of clear margins in the excised skin and subdermal structures as well as the lack of preoperative and postoperative symptoms. Close clinical follow-up was warranted to monitor for early signs of recurrence or neural involvement. We have confidence that the planned follow-up regimen in our patient will reveal any early signs of new occurrence or recurrence.
In the case of recurrence, Mohs micrographic surgery would likely be indicated. We elected not to treat with adjuvant radiotherapy because its benefit in cutaneous SCC with perineural invasion is debatable based on the lack of randomized controlled clinical evidence.10,11 The patient obtained postoperative baseline complete blood cell count with differential, posterior/anterior and lateral chest radiographs, as well as abdominal ultrasonography. Each returned negative findings of hematologic or distant organ metastases, with subsequent follow-up visits also negative for any new concerning findings.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Aguayo R, Pallares J, Cassanova JM, et al. Squamous cell carcinoma developing in Jadassohn’s sebaceous nevus: case report and review of the literature. Dermatol Surg. 2010;36:1763-1768.
- Taher M, Feibleman C, Bennet R. Squamous cell carcinoma arising in a nevus sebaceous of Jadassohn in a 9-year-old girl: treatment using Mohs micrographic surgery with literature review. Dermatol Surg. 2010;36:1203-1208.
- Hidvegi NC, Kangesu L, Wolfe KQ. Squamous cell carcinoma complicating naevus sebaceous of Jadassohn in a child. Br J Plast Surg. 2003;56:50-52.
- Belhadjali H, Moussa A, Yahia S, et al. Simultaneous occurrence of squamous cell carcinomas within a nevus sebaceous of Jadassohn in an 11-year-old girl. Pediatr Dermatol. 2009;26:236-237.
- Wilson-Jones EW, Heyl T. Naevus sebaceus: a report of 140 cases with special regard to the development of secondary malignant tumors. Br J Dermatol. 1970;82:99-117.
- Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through perineural peripheral nerves. Am J Surg. 1963;106:651-667.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
- Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
- Cottel WI. Perineural invasion by squamous cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
- Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301-308.
First reported in 1895, nevus sebaceus (NS) is a con genital papillomatous hamartoma most commonly found on the scalp and face. 1 Lesions typically are yellow-orange plaques and often are hairless. Nevus sebaceus is most prominent in the few first months after birth and again at puberty during development of the sebaceous glands. Development of epithelial hyperplasia, cysts, verrucas, and benign or malignant tumors has been reported. 1 The most common benign tumors are syringocystadenoma papilliferum and trichoblastoma. Cases of malignancy are rare, and basal cell carcinoma is the predominant form (approximately 2% of cases). Squamous cell carcinoma (SCC) and adnexal carcinoma are reported at even lower rates. 1 Malignant transformation occurring during childhood is extremely uncommon. According to a PubMed search of articles indexed for MEDLINE using the terms nevus sebaceous, malignancy, and squamous cell carcinoma and narrowing the results to children, there have been only 4 prior reports of SCC developing within an NS in a child. 2-5 We report a case of SCC arising in an NS in a 13-year-old adolescent girl with perineural invasion.
Case Report
A 13-year-old fair-skinned adolescent girl presented with a hairless 2×2.5-cm yellow plaque at the hairline on the anterior central scalp. The plaque had been present since birth and had progressively developed a superiorly located 3×5-mm erythematous verrucous nodule (Figure 1) with an approximate height of 6 mm over the last year. The nodule was subjected to regular trauma and bled with minimal insult. The patient appeared otherwise healthy, with no history of skin cancer or other chronic medical conditions. There was no evidence of lymphadenopathy on examination, and no other skin abnormalities were noted. There was no reported family history of skin cancer or chronic skin conditions suggestive of increased risk for cancer or other pathologic dermatoses. Differential diagnoses for the plaque and nodule complex included verruca, Spitz nevus, or secondary neoplasm within NS.
Excision was conducted under local anesthesia without complication. An elliptical section of skin measuring 0.8×2.5 cm was excised to a depth of 3 mm. The resulting wound was closed using a complex linear repair. The section was placed in formalin specimen transport medium and sent to Walter Reed National Military Medical Center (Bethesda, Maryland). Microscopic examination of the specimen revealed features typical for NS, including mild verrucous epidermal hyperplasia, sebaceous gland hyperplasia, presence of apocrine glands, and hamartomatous follicular proliferations (Figure 2). An even more papillomatous epidermal proliferation that was comprised of atypical squamous cells was present within the lesion. Similar atypical squamous cells infiltrated the superficial dermis in nests, cords, and single cells (Figure 3A). One focus showed perineural invasion with a small superficial nerve fiber surrounded by SCC (Figure 3B). The tumor was completely excised, with negative surgical margins extending approximately 2 mm. Adjuvant radiation therapy and further specialized Mohs micrographic excision were not performed because of the clear histologic appearance of the carcinoma and strong evidence of complete excision.
At 2-week follow-up, the surgical scar on the anterior central forehead was well healed without evidence of SCC recurrence. On physical examination there was neither lymphadenopathy nor signs of neurologic deficit, except for superficial cutaneous hypoesthesia in the immediate area surrounding the healed site. Following discussion with the patient and her parents, it was decided that the patient would obtain baseline laboratory tests, chest radiography, and abdominal ultrasonography, and she would undergo serial follow-up examinations every 3 months for the next 2 years. Annual follow-up was recommended after 2 years, with the caveat to return sooner if recurrence or symptoms were to arise.
Comment
Historically, there has been variability in the histopathologic interpretation of SCC in NS in the literature. Retrospective analysis of the histologic evidence of SCC in the 2 earliest possible cases of pediatric SCC in NS have been questioned due to the lack of clinical data presented and the possibility that the diagnosis of SCC was inaccurate.6 Our case was histopathologically interpreted as superficially invasive, well-differentiated SCC arising within an NS; therefore, we classified this case as SCC and took every precaution to ensure the lesion was completely excised, given the potentially invasive nature of SCC.
Our case is unique because it represents SCC in NS with histologic evidence of perineural involvement. Perineural invasion is a major route of tumor spread in SCC and may result in increased occurrence of regional lymph node spread and distant metastases, with path of least resistance or neural cell adhesion as possible spreading methods.7-9 However, there is a notable amount of prognostic variability based on tumor type, the nerve involved, and degree of involvement.9 It is common for cutaneous SCC to occur with invasion of small intradermal nerves, but a poor outcome is less likely in asymptomatic patients who have perineural involvement that was incidentally discovered on histologic examination.10
In our patient, the entire tumor was completely removed with local excision. Recurrence of the SCC or future symptoms of deep neural invasion were not anticipated given the postoperative evidence of clear margins in the excised skin and subdermal structures as well as the lack of preoperative and postoperative symptoms. Close clinical follow-up was warranted to monitor for early signs of recurrence or neural involvement. We have confidence that the planned follow-up regimen in our patient will reveal any early signs of new occurrence or recurrence.
In the case of recurrence, Mohs micrographic surgery would likely be indicated. We elected not to treat with adjuvant radiotherapy because its benefit in cutaneous SCC with perineural invasion is debatable based on the lack of randomized controlled clinical evidence.10,11 The patient obtained postoperative baseline complete blood cell count with differential, posterior/anterior and lateral chest radiographs, as well as abdominal ultrasonography. Each returned negative findings of hematologic or distant organ metastases, with subsequent follow-up visits also negative for any new concerning findings.
First reported in 1895, nevus sebaceus (NS) is a con genital papillomatous hamartoma most commonly found on the scalp and face. 1 Lesions typically are yellow-orange plaques and often are hairless. Nevus sebaceus is most prominent in the few first months after birth and again at puberty during development of the sebaceous glands. Development of epithelial hyperplasia, cysts, verrucas, and benign or malignant tumors has been reported. 1 The most common benign tumors are syringocystadenoma papilliferum and trichoblastoma. Cases of malignancy are rare, and basal cell carcinoma is the predominant form (approximately 2% of cases). Squamous cell carcinoma (SCC) and adnexal carcinoma are reported at even lower rates. 1 Malignant transformation occurring during childhood is extremely uncommon. According to a PubMed search of articles indexed for MEDLINE using the terms nevus sebaceous, malignancy, and squamous cell carcinoma and narrowing the results to children, there have been only 4 prior reports of SCC developing within an NS in a child. 2-5 We report a case of SCC arising in an NS in a 13-year-old adolescent girl with perineural invasion.
Case Report
A 13-year-old fair-skinned adolescent girl presented with a hairless 2×2.5-cm yellow plaque at the hairline on the anterior central scalp. The plaque had been present since birth and had progressively developed a superiorly located 3×5-mm erythematous verrucous nodule (Figure 1) with an approximate height of 6 mm over the last year. The nodule was subjected to regular trauma and bled with minimal insult. The patient appeared otherwise healthy, with no history of skin cancer or other chronic medical conditions. There was no evidence of lymphadenopathy on examination, and no other skin abnormalities were noted. There was no reported family history of skin cancer or chronic skin conditions suggestive of increased risk for cancer or other pathologic dermatoses. Differential diagnoses for the plaque and nodule complex included verruca, Spitz nevus, or secondary neoplasm within NS.
Excision was conducted under local anesthesia without complication. An elliptical section of skin measuring 0.8×2.5 cm was excised to a depth of 3 mm. The resulting wound was closed using a complex linear repair. The section was placed in formalin specimen transport medium and sent to Walter Reed National Military Medical Center (Bethesda, Maryland). Microscopic examination of the specimen revealed features typical for NS, including mild verrucous epidermal hyperplasia, sebaceous gland hyperplasia, presence of apocrine glands, and hamartomatous follicular proliferations (Figure 2). An even more papillomatous epidermal proliferation that was comprised of atypical squamous cells was present within the lesion. Similar atypical squamous cells infiltrated the superficial dermis in nests, cords, and single cells (Figure 3A). One focus showed perineural invasion with a small superficial nerve fiber surrounded by SCC (Figure 3B). The tumor was completely excised, with negative surgical margins extending approximately 2 mm. Adjuvant radiation therapy and further specialized Mohs micrographic excision were not performed because of the clear histologic appearance of the carcinoma and strong evidence of complete excision.
At 2-week follow-up, the surgical scar on the anterior central forehead was well healed without evidence of SCC recurrence. On physical examination there was neither lymphadenopathy nor signs of neurologic deficit, except for superficial cutaneous hypoesthesia in the immediate area surrounding the healed site. Following discussion with the patient and her parents, it was decided that the patient would obtain baseline laboratory tests, chest radiography, and abdominal ultrasonography, and she would undergo serial follow-up examinations every 3 months for the next 2 years. Annual follow-up was recommended after 2 years, with the caveat to return sooner if recurrence or symptoms were to arise.
Comment
Historically, there has been variability in the histopathologic interpretation of SCC in NS in the literature. Retrospective analysis of the histologic evidence of SCC in the 2 earliest possible cases of pediatric SCC in NS have been questioned due to the lack of clinical data presented and the possibility that the diagnosis of SCC was inaccurate.6 Our case was histopathologically interpreted as superficially invasive, well-differentiated SCC arising within an NS; therefore, we classified this case as SCC and took every precaution to ensure the lesion was completely excised, given the potentially invasive nature of SCC.
Our case is unique because it represents SCC in NS with histologic evidence of perineural involvement. Perineural invasion is a major route of tumor spread in SCC and may result in increased occurrence of regional lymph node spread and distant metastases, with path of least resistance or neural cell adhesion as possible spreading methods.7-9 However, there is a notable amount of prognostic variability based on tumor type, the nerve involved, and degree of involvement.9 It is common for cutaneous SCC to occur with invasion of small intradermal nerves, but a poor outcome is less likely in asymptomatic patients who have perineural involvement that was incidentally discovered on histologic examination.10
In our patient, the entire tumor was completely removed with local excision. Recurrence of the SCC or future symptoms of deep neural invasion were not anticipated given the postoperative evidence of clear margins in the excised skin and subdermal structures as well as the lack of preoperative and postoperative symptoms. Close clinical follow-up was warranted to monitor for early signs of recurrence or neural involvement. We have confidence that the planned follow-up regimen in our patient will reveal any early signs of new occurrence or recurrence.
In the case of recurrence, Mohs micrographic surgery would likely be indicated. We elected not to treat with adjuvant radiotherapy because its benefit in cutaneous SCC with perineural invasion is debatable based on the lack of randomized controlled clinical evidence.10,11 The patient obtained postoperative baseline complete blood cell count with differential, posterior/anterior and lateral chest radiographs, as well as abdominal ultrasonography. Each returned negative findings of hematologic or distant organ metastases, with subsequent follow-up visits also negative for any new concerning findings.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Aguayo R, Pallares J, Cassanova JM, et al. Squamous cell carcinoma developing in Jadassohn’s sebaceous nevus: case report and review of the literature. Dermatol Surg. 2010;36:1763-1768.
- Taher M, Feibleman C, Bennet R. Squamous cell carcinoma arising in a nevus sebaceous of Jadassohn in a 9-year-old girl: treatment using Mohs micrographic surgery with literature review. Dermatol Surg. 2010;36:1203-1208.
- Hidvegi NC, Kangesu L, Wolfe KQ. Squamous cell carcinoma complicating naevus sebaceous of Jadassohn in a child. Br J Plast Surg. 2003;56:50-52.
- Belhadjali H, Moussa A, Yahia S, et al. Simultaneous occurrence of squamous cell carcinomas within a nevus sebaceous of Jadassohn in an 11-year-old girl. Pediatr Dermatol. 2009;26:236-237.
- Wilson-Jones EW, Heyl T. Naevus sebaceus: a report of 140 cases with special regard to the development of secondary malignant tumors. Br J Dermatol. 1970;82:99-117.
- Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through perineural peripheral nerves. Am J Surg. 1963;106:651-667.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
- Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
- Cottel WI. Perineural invasion by squamous cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
- Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301-308.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Aguayo R, Pallares J, Cassanova JM, et al. Squamous cell carcinoma developing in Jadassohn’s sebaceous nevus: case report and review of the literature. Dermatol Surg. 2010;36:1763-1768.
- Taher M, Feibleman C, Bennet R. Squamous cell carcinoma arising in a nevus sebaceous of Jadassohn in a 9-year-old girl: treatment using Mohs micrographic surgery with literature review. Dermatol Surg. 2010;36:1203-1208.
- Hidvegi NC, Kangesu L, Wolfe KQ. Squamous cell carcinoma complicating naevus sebaceous of Jadassohn in a child. Br J Plast Surg. 2003;56:50-52.
- Belhadjali H, Moussa A, Yahia S, et al. Simultaneous occurrence of squamous cell carcinomas within a nevus sebaceous of Jadassohn in an 11-year-old girl. Pediatr Dermatol. 2009;26:236-237.
- Wilson-Jones EW, Heyl T. Naevus sebaceus: a report of 140 cases with special regard to the development of secondary malignant tumors. Br J Dermatol. 1970;82:99-117.
- Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through perineural peripheral nerves. Am J Surg. 1963;106:651-667.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
- Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
- Cottel WI. Perineural invasion by squamous cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
- Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301-308.
Practice Points
- Nevus sebaceus (NS) is frequently found on the scalp and may increase in size during puberty.
- Commonly found additional neoplasms within NS include trichoblastoma and syringocystadenoma papilliferum. Malignancies are possible but rare.
Dr. Douglas Paauw gives updates on antihypertensives, statins, SGLT2 inhibitors
PHILADELPHIA – in a video interview at the annual meeting of the American College of Physicians.
Dr. Paauw, professor of medicine at the University of Washington, Seattle, began by discussing some of the issues that occurred with antihypertensive drugs in the past year. These included the link between hydrochlorothiazide use and the increased risk of nonmelanoma skin cancers, the recalls of many drug lots of angiotensin II receptor blockers, and a study that found an increased risk of lung cancer in people who were taking ACE inhibitors.
He then described the findings of studies that examined the links between statins and muscle pain and other new research on these drugs.
He also warned physicians to be particularity cautious about prescribing sodium-glucose cotransporter 2 inhibitors to certain kinds of patients.
Dr. Paauw concluded by explaining why clarithromycin is his most hated drug.
Dr. Paauw is also the Rathmann Family Foundation Endowed Chair for Patient-Centered Clinical Education and the medicine clerkship director at the University of Washington.
PHILADELPHIA – in a video interview at the annual meeting of the American College of Physicians.
Dr. Paauw, professor of medicine at the University of Washington, Seattle, began by discussing some of the issues that occurred with antihypertensive drugs in the past year. These included the link between hydrochlorothiazide use and the increased risk of nonmelanoma skin cancers, the recalls of many drug lots of angiotensin II receptor blockers, and a study that found an increased risk of lung cancer in people who were taking ACE inhibitors.
He then described the findings of studies that examined the links between statins and muscle pain and other new research on these drugs.
He also warned physicians to be particularity cautious about prescribing sodium-glucose cotransporter 2 inhibitors to certain kinds of patients.
Dr. Paauw concluded by explaining why clarithromycin is his most hated drug.
Dr. Paauw is also the Rathmann Family Foundation Endowed Chair for Patient-Centered Clinical Education and the medicine clerkship director at the University of Washington.
PHILADELPHIA – in a video interview at the annual meeting of the American College of Physicians.
Dr. Paauw, professor of medicine at the University of Washington, Seattle, began by discussing some of the issues that occurred with antihypertensive drugs in the past year. These included the link between hydrochlorothiazide use and the increased risk of nonmelanoma skin cancers, the recalls of many drug lots of angiotensin II receptor blockers, and a study that found an increased risk of lung cancer in people who were taking ACE inhibitors.
He then described the findings of studies that examined the links between statins and muscle pain and other new research on these drugs.
He also warned physicians to be particularity cautious about prescribing sodium-glucose cotransporter 2 inhibitors to certain kinds of patients.
Dr. Paauw concluded by explaining why clarithromycin is his most hated drug.
Dr. Paauw is also the Rathmann Family Foundation Endowed Chair for Patient-Centered Clinical Education and the medicine clerkship director at the University of Washington.
REPORTING FROM INTERNAL MEDICINE 2019
The Evolution of the Micrographic Surgery and Dermatologic Oncology Fellowship
Originating in 1968, the dermatologic surgery fellowship is as young as many dermatologists in practice today. Not surprisingly, the blossoming fellowship has undergone its fair share of both growth and growing pains over the last 5 decades.
A Brief History
The first dermatologic surgery fellowship was born in 1968 when Dr. Perry Robins established a program at the New York University Medical Center for training in chemosurgery.1 The fellowship quickly underwent notable change with the rising popularity of the fresh tissue technique, which was first performed by Dr. Fred Mohs in 1953 and made popular following publication of a series of landmark articles on the technique by Drs. Sam Stegman and Theodore Tromovitch in the late 1960s and early 1970s. The fellowship correspondingly saw a rise in fresh tissue technique training, accompanied by a decline in chemosurgery training. In 1974, Dr. Daniel Jones coined the term micrographic surgery to describe the favored technique, and at the 1985 annual meeting of the American College of Chemosurgery, the name of the technique was changed to Mohs micrographic surgery.1
By 1995, the fellowship was officially named Procedural Dermatology, and programs were exclusively accredited by the American College of Mohs Surgery (ACMS). A 1-year Procedural Dermatology fellowship gained accreditation by the Accreditation Council for Graduate Medical Education (ACGME) in 2003.2 Beginning in July 2013, all fellowship programs in the United States fell under the governance of the ACGME; however, the ACMS has remained the sponsor of the matching process.3 In 2014, the ACGME changed the name of the fellowship to Micrographic Surgery and Dermatologic Oncology (MSDO).2 Fellowship training today is centered on the core elements of cutaneous oncologic surgery, cutaneous reconstructive surgery, and dermatologic oncology; however, the scope of training in technologies and techniques offered has continued to broaden.4 Many programs now offer additional training in cosmetic and other procedural dermatology. To date, there are 76 accredited MSDO fellowship training programs in the United States and more than 1500 fellowship-trained micrographic surgeons.2,4
Trends in Program and Match Statistics
As the role of dermatologic surgery within the field of dermatology continues to expand, the MSDO fellowship has become increasingly popular over the last decade. From 2005 to 2018, applicants participating in the fellowship match increased by 34%.3 Despite the fellowship’s growing popularity, programs participating in the match have remained largely stable from 2005 to 2018, with 50 positions offered in 2005 and 58 in 2018. The match rate has correspondingly decreased from 66.2% in 2005 to 61.1% in 2018.3
Changes in the Match Process
The fellowship match is processed by the SF Match and sponsored by the ACMS. Over the last decade, programs have increasingly opted for exemptions from participation in the SF Match. In 2005, there were 8 match exemptions. In 2018, there were 20.4 Despite the increasing popularity of match exemptions, in October 2018 the ACMS Board of Directors approved a new policy that eliminated match exemptions, with the exception of applicants on active military duty and international (non-Canadian) applicants. All other applicants applying for a fellowship position for the 2020-2021 academic year must participate in the match.4 This new policy attempts to ensure a fair match process, especially for applicants who have trained at a program without an affiliated MSDO fellowship.
The Road to Board Certification
Further growth during the fellowship’s mid-adult years centered on the long-contested debate on subspecialty board certification. In 2009, an American Society for Dermatologic Surgery membership survey demonstrated an overwhelming majority in opposition. In 2014, the debate resurfaced. At the 2016 American Society for Dermatologic Surgery annual meeting, former American Academy of Dermatology presidents Brett Coldiron, MD, and Darrell S. Rigel, MD, MS, conveyed opposing positions, after which an audience survey demonstrated a 69% opposition rate. Proponents continued to argue that board certification would decrease divisiveness in the specialty, create a better brand, help to obtain a Medicare specialty designation that could help prevent exclusion of Mohs surgeons from insurance networks, give allopathic dermatologists the same opportunity for certification as osteopathic counterparts, and demonstrate competence to the public. Those in opposition argued that the term dermatologic oncology erroneously suggests general dermatologists are not experts in the treatment of skin cancers, practices may be restricted by carriers misusing the new credential, and subspecialty certification would actually create division among practicing dermatologists.5
Following years of debate, the American Board of Dermatology’s proposal to offer subspecialty certification in Micrographic Dermatologic Surgery was submitted to the American Board of Medical Specialties and approved on October 26, 2018. The name of the new subspecialty (Micrographic Dermatologic Surgery) is different than that of the fellowship (Micrographic Surgery and Dermatologic Oncology), a decision reached in response to diplomats indicating discomfort with the term oncology potentially misleading the public that general dermatologists do not treat skin cancer. Per the American Board of Dermatology official website, the first certification examination likely will take place in about 2 years. A maintenance of certification examination for the subspecialty will be required every 10 years.6
Final Thoughts
During its short history, the MSDO fellowship has undergone a notable evolution in recognition, popularity among residents, match process, and board certification, which attests to its adaptability over time and growing prominence.
- Robins P, Ebede TL, Hale EK. The evolution of Mohs micrographic surgery. Skin Cancer Foundation website. https://www.skincancer.org/skin-cancer-information/mohs-surgery/evolution-of-mohs. Updated July 13, 2016. Accessed April 17, 2019.
- Micrographic surgery and dermatologic oncology. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-surgery-and-dermatologic-oncology.aspx. Accessed April 9, 2019.
- Micrographic Surgery and Dermatologic Oncology Fellowship. San Francisco Match website. https://sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology. Accessed April 9, 2019.
- ACMS fellowship training. American College of Mohs Surgery website. https://www.mohscollege.org/fellowship-training. Accessed April 9, 2019.
- Should the ABD offer a Mohs surgery sub-certification? Dermatology World. April 26, 2017. https://www.aad.org/dw/dw-weekly/should-the-abd-offer-a-mohs-surgery-sub-certification. Accessed April 9, 2019.
- ABD Micrographic Dermatologic Surgery (MDS) subspecialty certification. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed April 9, 2019.
Originating in 1968, the dermatologic surgery fellowship is as young as many dermatologists in practice today. Not surprisingly, the blossoming fellowship has undergone its fair share of both growth and growing pains over the last 5 decades.
A Brief History
The first dermatologic surgery fellowship was born in 1968 when Dr. Perry Robins established a program at the New York University Medical Center for training in chemosurgery.1 The fellowship quickly underwent notable change with the rising popularity of the fresh tissue technique, which was first performed by Dr. Fred Mohs in 1953 and made popular following publication of a series of landmark articles on the technique by Drs. Sam Stegman and Theodore Tromovitch in the late 1960s and early 1970s. The fellowship correspondingly saw a rise in fresh tissue technique training, accompanied by a decline in chemosurgery training. In 1974, Dr. Daniel Jones coined the term micrographic surgery to describe the favored technique, and at the 1985 annual meeting of the American College of Chemosurgery, the name of the technique was changed to Mohs micrographic surgery.1
By 1995, the fellowship was officially named Procedural Dermatology, and programs were exclusively accredited by the American College of Mohs Surgery (ACMS). A 1-year Procedural Dermatology fellowship gained accreditation by the Accreditation Council for Graduate Medical Education (ACGME) in 2003.2 Beginning in July 2013, all fellowship programs in the United States fell under the governance of the ACGME; however, the ACMS has remained the sponsor of the matching process.3 In 2014, the ACGME changed the name of the fellowship to Micrographic Surgery and Dermatologic Oncology (MSDO).2 Fellowship training today is centered on the core elements of cutaneous oncologic surgery, cutaneous reconstructive surgery, and dermatologic oncology; however, the scope of training in technologies and techniques offered has continued to broaden.4 Many programs now offer additional training in cosmetic and other procedural dermatology. To date, there are 76 accredited MSDO fellowship training programs in the United States and more than 1500 fellowship-trained micrographic surgeons.2,4
Trends in Program and Match Statistics
As the role of dermatologic surgery within the field of dermatology continues to expand, the MSDO fellowship has become increasingly popular over the last decade. From 2005 to 2018, applicants participating in the fellowship match increased by 34%.3 Despite the fellowship’s growing popularity, programs participating in the match have remained largely stable from 2005 to 2018, with 50 positions offered in 2005 and 58 in 2018. The match rate has correspondingly decreased from 66.2% in 2005 to 61.1% in 2018.3
Changes in the Match Process
The fellowship match is processed by the SF Match and sponsored by the ACMS. Over the last decade, programs have increasingly opted for exemptions from participation in the SF Match. In 2005, there were 8 match exemptions. In 2018, there were 20.4 Despite the increasing popularity of match exemptions, in October 2018 the ACMS Board of Directors approved a new policy that eliminated match exemptions, with the exception of applicants on active military duty and international (non-Canadian) applicants. All other applicants applying for a fellowship position for the 2020-2021 academic year must participate in the match.4 This new policy attempts to ensure a fair match process, especially for applicants who have trained at a program without an affiliated MSDO fellowship.
The Road to Board Certification
Further growth during the fellowship’s mid-adult years centered on the long-contested debate on subspecialty board certification. In 2009, an American Society for Dermatologic Surgery membership survey demonstrated an overwhelming majority in opposition. In 2014, the debate resurfaced. At the 2016 American Society for Dermatologic Surgery annual meeting, former American Academy of Dermatology presidents Brett Coldiron, MD, and Darrell S. Rigel, MD, MS, conveyed opposing positions, after which an audience survey demonstrated a 69% opposition rate. Proponents continued to argue that board certification would decrease divisiveness in the specialty, create a better brand, help to obtain a Medicare specialty designation that could help prevent exclusion of Mohs surgeons from insurance networks, give allopathic dermatologists the same opportunity for certification as osteopathic counterparts, and demonstrate competence to the public. Those in opposition argued that the term dermatologic oncology erroneously suggests general dermatologists are not experts in the treatment of skin cancers, practices may be restricted by carriers misusing the new credential, and subspecialty certification would actually create division among practicing dermatologists.5
Following years of debate, the American Board of Dermatology’s proposal to offer subspecialty certification in Micrographic Dermatologic Surgery was submitted to the American Board of Medical Specialties and approved on October 26, 2018. The name of the new subspecialty (Micrographic Dermatologic Surgery) is different than that of the fellowship (Micrographic Surgery and Dermatologic Oncology), a decision reached in response to diplomats indicating discomfort with the term oncology potentially misleading the public that general dermatologists do not treat skin cancer. Per the American Board of Dermatology official website, the first certification examination likely will take place in about 2 years. A maintenance of certification examination for the subspecialty will be required every 10 years.6
Final Thoughts
During its short history, the MSDO fellowship has undergone a notable evolution in recognition, popularity among residents, match process, and board certification, which attests to its adaptability over time and growing prominence.
Originating in 1968, the dermatologic surgery fellowship is as young as many dermatologists in practice today. Not surprisingly, the blossoming fellowship has undergone its fair share of both growth and growing pains over the last 5 decades.
A Brief History
The first dermatologic surgery fellowship was born in 1968 when Dr. Perry Robins established a program at the New York University Medical Center for training in chemosurgery.1 The fellowship quickly underwent notable change with the rising popularity of the fresh tissue technique, which was first performed by Dr. Fred Mohs in 1953 and made popular following publication of a series of landmark articles on the technique by Drs. Sam Stegman and Theodore Tromovitch in the late 1960s and early 1970s. The fellowship correspondingly saw a rise in fresh tissue technique training, accompanied by a decline in chemosurgery training. In 1974, Dr. Daniel Jones coined the term micrographic surgery to describe the favored technique, and at the 1985 annual meeting of the American College of Chemosurgery, the name of the technique was changed to Mohs micrographic surgery.1
By 1995, the fellowship was officially named Procedural Dermatology, and programs were exclusively accredited by the American College of Mohs Surgery (ACMS). A 1-year Procedural Dermatology fellowship gained accreditation by the Accreditation Council for Graduate Medical Education (ACGME) in 2003.2 Beginning in July 2013, all fellowship programs in the United States fell under the governance of the ACGME; however, the ACMS has remained the sponsor of the matching process.3 In 2014, the ACGME changed the name of the fellowship to Micrographic Surgery and Dermatologic Oncology (MSDO).2 Fellowship training today is centered on the core elements of cutaneous oncologic surgery, cutaneous reconstructive surgery, and dermatologic oncology; however, the scope of training in technologies and techniques offered has continued to broaden.4 Many programs now offer additional training in cosmetic and other procedural dermatology. To date, there are 76 accredited MSDO fellowship training programs in the United States and more than 1500 fellowship-trained micrographic surgeons.2,4
Trends in Program and Match Statistics
As the role of dermatologic surgery within the field of dermatology continues to expand, the MSDO fellowship has become increasingly popular over the last decade. From 2005 to 2018, applicants participating in the fellowship match increased by 34%.3 Despite the fellowship’s growing popularity, programs participating in the match have remained largely stable from 2005 to 2018, with 50 positions offered in 2005 and 58 in 2018. The match rate has correspondingly decreased from 66.2% in 2005 to 61.1% in 2018.3
Changes in the Match Process
The fellowship match is processed by the SF Match and sponsored by the ACMS. Over the last decade, programs have increasingly opted for exemptions from participation in the SF Match. In 2005, there were 8 match exemptions. In 2018, there were 20.4 Despite the increasing popularity of match exemptions, in October 2018 the ACMS Board of Directors approved a new policy that eliminated match exemptions, with the exception of applicants on active military duty and international (non-Canadian) applicants. All other applicants applying for a fellowship position for the 2020-2021 academic year must participate in the match.4 This new policy attempts to ensure a fair match process, especially for applicants who have trained at a program without an affiliated MSDO fellowship.
The Road to Board Certification
Further growth during the fellowship’s mid-adult years centered on the long-contested debate on subspecialty board certification. In 2009, an American Society for Dermatologic Surgery membership survey demonstrated an overwhelming majority in opposition. In 2014, the debate resurfaced. At the 2016 American Society for Dermatologic Surgery annual meeting, former American Academy of Dermatology presidents Brett Coldiron, MD, and Darrell S. Rigel, MD, MS, conveyed opposing positions, after which an audience survey demonstrated a 69% opposition rate. Proponents continued to argue that board certification would decrease divisiveness in the specialty, create a better brand, help to obtain a Medicare specialty designation that could help prevent exclusion of Mohs surgeons from insurance networks, give allopathic dermatologists the same opportunity for certification as osteopathic counterparts, and demonstrate competence to the public. Those in opposition argued that the term dermatologic oncology erroneously suggests general dermatologists are not experts in the treatment of skin cancers, practices may be restricted by carriers misusing the new credential, and subspecialty certification would actually create division among practicing dermatologists.5
Following years of debate, the American Board of Dermatology’s proposal to offer subspecialty certification in Micrographic Dermatologic Surgery was submitted to the American Board of Medical Specialties and approved on October 26, 2018. The name of the new subspecialty (Micrographic Dermatologic Surgery) is different than that of the fellowship (Micrographic Surgery and Dermatologic Oncology), a decision reached in response to diplomats indicating discomfort with the term oncology potentially misleading the public that general dermatologists do not treat skin cancer. Per the American Board of Dermatology official website, the first certification examination likely will take place in about 2 years. A maintenance of certification examination for the subspecialty will be required every 10 years.6
Final Thoughts
During its short history, the MSDO fellowship has undergone a notable evolution in recognition, popularity among residents, match process, and board certification, which attests to its adaptability over time and growing prominence.
- Robins P, Ebede TL, Hale EK. The evolution of Mohs micrographic surgery. Skin Cancer Foundation website. https://www.skincancer.org/skin-cancer-information/mohs-surgery/evolution-of-mohs. Updated July 13, 2016. Accessed April 17, 2019.
- Micrographic surgery and dermatologic oncology. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-surgery-and-dermatologic-oncology.aspx. Accessed April 9, 2019.
- Micrographic Surgery and Dermatologic Oncology Fellowship. San Francisco Match website. https://sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology. Accessed April 9, 2019.
- ACMS fellowship training. American College of Mohs Surgery website. https://www.mohscollege.org/fellowship-training. Accessed April 9, 2019.
- Should the ABD offer a Mohs surgery sub-certification? Dermatology World. April 26, 2017. https://www.aad.org/dw/dw-weekly/should-the-abd-offer-a-mohs-surgery-sub-certification. Accessed April 9, 2019.
- ABD Micrographic Dermatologic Surgery (MDS) subspecialty certification. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed April 9, 2019.
- Robins P, Ebede TL, Hale EK. The evolution of Mohs micrographic surgery. Skin Cancer Foundation website. https://www.skincancer.org/skin-cancer-information/mohs-surgery/evolution-of-mohs. Updated July 13, 2016. Accessed April 17, 2019.
- Micrographic surgery and dermatologic oncology. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-surgery-and-dermatologic-oncology.aspx. Accessed April 9, 2019.
- Micrographic Surgery and Dermatologic Oncology Fellowship. San Francisco Match website. https://sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology. Accessed April 9, 2019.
- ACMS fellowship training. American College of Mohs Surgery website. https://www.mohscollege.org/fellowship-training. Accessed April 9, 2019.
- Should the ABD offer a Mohs surgery sub-certification? Dermatology World. April 26, 2017. https://www.aad.org/dw/dw-weekly/should-the-abd-offer-a-mohs-surgery-sub-certification. Accessed April 9, 2019.
- ABD Micrographic Dermatologic Surgery (MDS) subspecialty certification. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed April 9, 2019.
Resident Pearl
- Residents should be aware of recent changes to the Micrographic Surgery and Dermatologic Oncology fellowship: the elimination of fellowship match exemptions for most applicants for the upcoming 2019-2020 academic year, the American Board of Medical Specialties approval of subspecialty certification in Micrographic Dermatologic Surgery, and the likelihood of the first subspecialty certification examination in the next 2 years.
Cutaneous Metastasis of Endometrial Carcinoma: An Unusual and Dramatic Presentation
Case Report
A 62-year-old woman presented with multiple large friable tumors of the abdominal panniculus. The patient also reported an unintentional 75-lb weight loss over the last 9 months as well as vaginal bleeding and fecal discharge from the vagina of 2 weeks’ duration. The patient had a surgical and medical history of a robotic-assisted hysterectomy and bilateral salpingo-oophorectomy performed 4 years prior to presentation. Final surgical pathology showed complex atypical endometrial hyperplasia with no adenocarcinoma identified.
Physical examination revealed multiple large, friable, exophytic tumors of the left side of the lower abdominal panniculus within close vicinity of the patient’s abdominal hysterectomy scars (Figure 1). The largest lesion measured approximately 6 cm in length. Laboratory values were elevated for carcinoembryonic antigen (5.9 ng/mL [reference range, <3.0 ng/mL]) and cancer antigen 125 (202 U/mL [reference range, <35 U/mL]). Computed tomography of the abdomen and pelvis revealed diffuse metastatic disease.
Comment
Incidence and Pathogenesis
Endometrial carcinoma is the most common gynecologic malignancy in the United States, but it rarely progresses to disseminated disease because of routine gynecologic examinations and the low threshold for surgical intervention. Cutaneous metastases represent one of the rarest presentations of disseminated disease, occurring in only 0.8% of those diagnosed with endometrial carcinoma.1 Cutaneous metastases occur almost exclusively in women older than 50 years and typically appear several months to years after hysterectomy. Although the exact pathogenesis is unknown, it is theorized that small foci of malignant cells may be seeded during surgery, leading to visceral and cutaneous involvement.
Clinical Presentation
Lesions vary morphologically, most commonly presenting as nonspecific, painless, hemorrhagic nodules. Lesions typically present in areas of direct local extension; prior radiotherapy; or areas of initial surgery, as was the case with our patient.2 Approximately 20 cases of umbilical involvement (Sister Mary Joseph nodule) have been reported in the literature. These cases are thought to occur from direct local spread of disease from the peritoneum.3 Hematogenous and lymphatic spread to distant sites such as the scalp and mandible also have been reported. More than 50% of patients will have underlying visceral metastatic disease at the time of diagnosis.3
Histopathologic Findings
Histopathology varies with the morphology of the underlying primary tumor, with endometrioid adenocarcinoma being the most common form associated with cutaneous metastasis, as was the case with our patient.4 Histology is characterized by dermal proliferation of atypical glandular epithelium with diffuse hemorrhage. Staining typically is positive for CK7 and negative for CK20 and CDX2.5 Histopathology and immunohistochemical staining are not specific for diagnosis and must be correlated with clinical history.
Management and Prognosis
Similar to cutaneous metastasis in other internal malignancies, prognosis is poor, as widespread dissemination of the underlying malignancy typically is present. Mean life expectancy is 4 to 12 months.6 Treatment is primarily palliative, as chemotherapy and radiotherapy are largely ineffective.
Conclusion
Our patient represents a dramatic form of cutaneous extension of a common disease. Dermatologists often are consulted because of the nonspecific nature of the lesions and must be conscious of this entity. As with other cutaneous metastases, a thorough medical and surgical history in conjunction with histopathology are necessary for an accurate diagnosis.
- Atallah D, el Kassis N, Lutfallah F, et al. Cutaneous metastasis in endometrial cancer: once in a blue moon—case report. World J Surg Oncol. 2014;12:86.
- Temkin SM, Hellman M, Lee YC, et al. Surgical resection of vulvar metastases of endometrial cancer: a presentation of two cases. J Low Genit Tract Dis. 2007;11:118-121.
- Kushner DM, Lurain JR, Fu TS, et al. Endometrial adenocarcinoma metastatic to the scalp: case report and literature review. Gynecol Oncol. 1997;65:530-533.
- El M’rabet FZ, Hottinger A, George AC. Cutaneous metastasis of endometrial carcinoma: a case report and literature review. J Clin Gynecol Obstet. 2012;1:19-23.
- Stonard CM, Manek S. Cutaneous metastasis from an endometrial carcinoma: a case history and review of the literature. Histopathology. 2003;43:201-203
- Damewood MD, Rosenshein NB, Grumbine FC, et al. Cutaneous metastasis of endometrial carcinoma. Cancer. 1980;46:1471-1477.
Case Report
A 62-year-old woman presented with multiple large friable tumors of the abdominal panniculus. The patient also reported an unintentional 75-lb weight loss over the last 9 months as well as vaginal bleeding and fecal discharge from the vagina of 2 weeks’ duration. The patient had a surgical and medical history of a robotic-assisted hysterectomy and bilateral salpingo-oophorectomy performed 4 years prior to presentation. Final surgical pathology showed complex atypical endometrial hyperplasia with no adenocarcinoma identified.
Physical examination revealed multiple large, friable, exophytic tumors of the left side of the lower abdominal panniculus within close vicinity of the patient’s abdominal hysterectomy scars (Figure 1). The largest lesion measured approximately 6 cm in length. Laboratory values were elevated for carcinoembryonic antigen (5.9 ng/mL [reference range, <3.0 ng/mL]) and cancer antigen 125 (202 U/mL [reference range, <35 U/mL]). Computed tomography of the abdomen and pelvis revealed diffuse metastatic disease.
Comment
Incidence and Pathogenesis
Endometrial carcinoma is the most common gynecologic malignancy in the United States, but it rarely progresses to disseminated disease because of routine gynecologic examinations and the low threshold for surgical intervention. Cutaneous metastases represent one of the rarest presentations of disseminated disease, occurring in only 0.8% of those diagnosed with endometrial carcinoma.1 Cutaneous metastases occur almost exclusively in women older than 50 years and typically appear several months to years after hysterectomy. Although the exact pathogenesis is unknown, it is theorized that small foci of malignant cells may be seeded during surgery, leading to visceral and cutaneous involvement.
Clinical Presentation
Lesions vary morphologically, most commonly presenting as nonspecific, painless, hemorrhagic nodules. Lesions typically present in areas of direct local extension; prior radiotherapy; or areas of initial surgery, as was the case with our patient.2 Approximately 20 cases of umbilical involvement (Sister Mary Joseph nodule) have been reported in the literature. These cases are thought to occur from direct local spread of disease from the peritoneum.3 Hematogenous and lymphatic spread to distant sites such as the scalp and mandible also have been reported. More than 50% of patients will have underlying visceral metastatic disease at the time of diagnosis.3
Histopathologic Findings
Histopathology varies with the morphology of the underlying primary tumor, with endometrioid adenocarcinoma being the most common form associated with cutaneous metastasis, as was the case with our patient.4 Histology is characterized by dermal proliferation of atypical glandular epithelium with diffuse hemorrhage. Staining typically is positive for CK7 and negative for CK20 and CDX2.5 Histopathology and immunohistochemical staining are not specific for diagnosis and must be correlated with clinical history.
Management and Prognosis
Similar to cutaneous metastasis in other internal malignancies, prognosis is poor, as widespread dissemination of the underlying malignancy typically is present. Mean life expectancy is 4 to 12 months.6 Treatment is primarily palliative, as chemotherapy and radiotherapy are largely ineffective.
Conclusion
Our patient represents a dramatic form of cutaneous extension of a common disease. Dermatologists often are consulted because of the nonspecific nature of the lesions and must be conscious of this entity. As with other cutaneous metastases, a thorough medical and surgical history in conjunction with histopathology are necessary for an accurate diagnosis.
Case Report
A 62-year-old woman presented with multiple large friable tumors of the abdominal panniculus. The patient also reported an unintentional 75-lb weight loss over the last 9 months as well as vaginal bleeding and fecal discharge from the vagina of 2 weeks’ duration. The patient had a surgical and medical history of a robotic-assisted hysterectomy and bilateral salpingo-oophorectomy performed 4 years prior to presentation. Final surgical pathology showed complex atypical endometrial hyperplasia with no adenocarcinoma identified.
Physical examination revealed multiple large, friable, exophytic tumors of the left side of the lower abdominal panniculus within close vicinity of the patient’s abdominal hysterectomy scars (Figure 1). The largest lesion measured approximately 6 cm in length. Laboratory values were elevated for carcinoembryonic antigen (5.9 ng/mL [reference range, <3.0 ng/mL]) and cancer antigen 125 (202 U/mL [reference range, <35 U/mL]). Computed tomography of the abdomen and pelvis revealed diffuse metastatic disease.
Comment
Incidence and Pathogenesis
Endometrial carcinoma is the most common gynecologic malignancy in the United States, but it rarely progresses to disseminated disease because of routine gynecologic examinations and the low threshold for surgical intervention. Cutaneous metastases represent one of the rarest presentations of disseminated disease, occurring in only 0.8% of those diagnosed with endometrial carcinoma.1 Cutaneous metastases occur almost exclusively in women older than 50 years and typically appear several months to years after hysterectomy. Although the exact pathogenesis is unknown, it is theorized that small foci of malignant cells may be seeded during surgery, leading to visceral and cutaneous involvement.
Clinical Presentation
Lesions vary morphologically, most commonly presenting as nonspecific, painless, hemorrhagic nodules. Lesions typically present in areas of direct local extension; prior radiotherapy; or areas of initial surgery, as was the case with our patient.2 Approximately 20 cases of umbilical involvement (Sister Mary Joseph nodule) have been reported in the literature. These cases are thought to occur from direct local spread of disease from the peritoneum.3 Hematogenous and lymphatic spread to distant sites such as the scalp and mandible also have been reported. More than 50% of patients will have underlying visceral metastatic disease at the time of diagnosis.3
Histopathologic Findings
Histopathology varies with the morphology of the underlying primary tumor, with endometrioid adenocarcinoma being the most common form associated with cutaneous metastasis, as was the case with our patient.4 Histology is characterized by dermal proliferation of atypical glandular epithelium with diffuse hemorrhage. Staining typically is positive for CK7 and negative for CK20 and CDX2.5 Histopathology and immunohistochemical staining are not specific for diagnosis and must be correlated with clinical history.
Management and Prognosis
Similar to cutaneous metastasis in other internal malignancies, prognosis is poor, as widespread dissemination of the underlying malignancy typically is present. Mean life expectancy is 4 to 12 months.6 Treatment is primarily palliative, as chemotherapy and radiotherapy are largely ineffective.
Conclusion
Our patient represents a dramatic form of cutaneous extension of a common disease. Dermatologists often are consulted because of the nonspecific nature of the lesions and must be conscious of this entity. As with other cutaneous metastases, a thorough medical and surgical history in conjunction with histopathology are necessary for an accurate diagnosis.
- Atallah D, el Kassis N, Lutfallah F, et al. Cutaneous metastasis in endometrial cancer: once in a blue moon—case report. World J Surg Oncol. 2014;12:86.
- Temkin SM, Hellman M, Lee YC, et al. Surgical resection of vulvar metastases of endometrial cancer: a presentation of two cases. J Low Genit Tract Dis. 2007;11:118-121.
- Kushner DM, Lurain JR, Fu TS, et al. Endometrial adenocarcinoma metastatic to the scalp: case report and literature review. Gynecol Oncol. 1997;65:530-533.
- El M’rabet FZ, Hottinger A, George AC. Cutaneous metastasis of endometrial carcinoma: a case report and literature review. J Clin Gynecol Obstet. 2012;1:19-23.
- Stonard CM, Manek S. Cutaneous metastasis from an endometrial carcinoma: a case history and review of the literature. Histopathology. 2003;43:201-203
- Damewood MD, Rosenshein NB, Grumbine FC, et al. Cutaneous metastasis of endometrial carcinoma. Cancer. 1980;46:1471-1477.
- Atallah D, el Kassis N, Lutfallah F, et al. Cutaneous metastasis in endometrial cancer: once in a blue moon—case report. World J Surg Oncol. 2014;12:86.
- Temkin SM, Hellman M, Lee YC, et al. Surgical resection of vulvar metastases of endometrial cancer: a presentation of two cases. J Low Genit Tract Dis. 2007;11:118-121.
- Kushner DM, Lurain JR, Fu TS, et al. Endometrial adenocarcinoma metastatic to the scalp: case report and literature review. Gynecol Oncol. 1997;65:530-533.
- El M’rabet FZ, Hottinger A, George AC. Cutaneous metastasis of endometrial carcinoma: a case report and literature review. J Clin Gynecol Obstet. 2012;1:19-23.
- Stonard CM, Manek S. Cutaneous metastasis from an endometrial carcinoma: a case history and review of the literature. Histopathology. 2003;43:201-203
- Damewood MD, Rosenshein NB, Grumbine FC, et al. Cutaneous metastasis of endometrial carcinoma. Cancer. 1980;46:1471-1477.
Practice Points
- Cutaneous metastases of endometrial carcinoma are extremely rare and typically present in areas of direct local spread.
- As with other cutaneous metastases, lesions often are nonspecific, making history and histopathology essential for diagnosis.
Leukemia Cutis–Associated Leonine Facies and Eyebrow Loss
To the Editor:
I read with interest the informative Cutis case report by Krooks and Weatherall1 in which the authors not only described the case of a 66-year-old man whose diagnosis of bone marrow biopsy–confirmed acute myeloid leukemia (AML) presented concurrently with skin biopsy–confirmed leukemia cutis but also discussed the poor prognosis of individuals with acute myelogenous leukemia cutis. Their patient died within 5 weeks of establishing the diagnosis. In addition, lateral and frontal photographs of the patient’s face demonstrated diffuse infiltrative plaques of leukemia cutis; he had swollen eyelids and lips with distortion of the nose secondary to dermal infiltration of leukemic myeloid cells.1 Although not emphasized by the authors, the patient appeared to have a leonine facies and at least partial loss of the lateral eyebrows.
Malignancy-associated leonine facies resulting from infiltration of the skin by neoplastic cells has been reported in a patient with metastatic breast carcinoma.2,3 However, it predominantly occurs in patients with hematologic dyscrasias such as leukemia cutis, lymphoma (ie, cutaneous B cell, cutaneous T cell, Hodgkin), plasmacytoma, and systemic mastocytosis.3,4 The report by Krooks and Weatherall1 adds AML-associated leukemia cutis to the previously observed types of leukemia cutis–related leonine facies in patients with acute lymphocytic leukemia, acute myelomonocytic leukemia, and chronic lymphocytic leukemia.3,4
Partial or complete loss of eyebrows in the setting of leonine facies has a limited differential diagnosis.3,5 In addition to cancer, the associated disorders include adnexal mucin deposition (alopecia mucinosis), granulomatous conditions (sarcoidosis), infectious diseases (leprosy), inherited syndromes (Setleis syndrome), photoallergic dermatoses (actinic reticuloid), and viral conditions (viral-associated trichodysplasia).3-9 Neoplasms associated with leonine facies and eyebrow loss include lymphomas (mycosis fungoides and unspecified cutaneous T-cell lymphoma), systemic mastocytosis and leukemia cutis secondary to acute lymphocytic leukemia, acute myelomonocytic leukemia, and now AML.1,3-5
The eyebrow loss associated with leonine facies often is not reversible once the causative cell of the associated condition (eg, granulomas of mycobacteria-infected histiocytes in leprosy, neoplastic lymphocytes in cutaneous T-cell lymphoma) has infiltrated the area of the eyebrows and abolished the preexisting hair follicles; however, follow-up descriptions of patients after treatment of other conditions that cause eyebrow loss usually are not reported. Indeed, there was partial reappearance of the eyebrows in a woman with systemic mastocytosis–associated loss of the eyebrows after malignancy-related treatment was reinitiated and the infiltrative facial plaques that had created her leonine facies had decreased in size.5 It is reasonable to speculate that the eyebrows may have reappeared in the patient reported by Krooks and Weatherall1 and his leonine facies–associated facial plaques may have resolved if he had underwent and responded to treatment with antineoplastic chemotherapy.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Jin CC, Martinelli PT, Cohen PR. What are these erythematous skin lesions? leukemia cutis. The Dermatologist. 2012;20:46-50.
- Chodkiewicz HM, Cohen PR. Systemic mastocytosis-associated leonine facies and eyebrow loss. South Med J. 2011;104:236-238.
- Cohen PR, Rapini RP, Beran M. Infiltrated blue-gray plaques in a patient with leukemia. Chloroma (granulocytic sarcoma). Arch Dermatol. 1987;123:251, 254.
- Cohen PR. Leonine facies associated with eyebrow loss. Int J Dermatol. 2014;53:e148-e149.
- Ravic-Nikolic A, Milicic V, Ristic G, et al. Actinic reticuloid presented as facies leonine. Int J Dermatol. 2012;51:234-236.
- Jacob Raja SA, Raja JJ, Vijayashree R, et al. Evaluation of oral and periodontal status of leprosy patients in Dindigul district. J Pharm Bioallied Sci. 2016;8(suppl 1):S119-S121.
- McGaughran J, Aftimos S. Setleis syndrome: three new cases and a review of the literature. Am J Med Genet. 2002;111:376-380.
- Benoit T, Bacelieri R, Morrell DS, et al. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871-874.
To the Editor:
I read with interest the informative Cutis case report by Krooks and Weatherall1 in which the authors not only described the case of a 66-year-old man whose diagnosis of bone marrow biopsy–confirmed acute myeloid leukemia (AML) presented concurrently with skin biopsy–confirmed leukemia cutis but also discussed the poor prognosis of individuals with acute myelogenous leukemia cutis. Their patient died within 5 weeks of establishing the diagnosis. In addition, lateral and frontal photographs of the patient’s face demonstrated diffuse infiltrative plaques of leukemia cutis; he had swollen eyelids and lips with distortion of the nose secondary to dermal infiltration of leukemic myeloid cells.1 Although not emphasized by the authors, the patient appeared to have a leonine facies and at least partial loss of the lateral eyebrows.
Malignancy-associated leonine facies resulting from infiltration of the skin by neoplastic cells has been reported in a patient with metastatic breast carcinoma.2,3 However, it predominantly occurs in patients with hematologic dyscrasias such as leukemia cutis, lymphoma (ie, cutaneous B cell, cutaneous T cell, Hodgkin), plasmacytoma, and systemic mastocytosis.3,4 The report by Krooks and Weatherall1 adds AML-associated leukemia cutis to the previously observed types of leukemia cutis–related leonine facies in patients with acute lymphocytic leukemia, acute myelomonocytic leukemia, and chronic lymphocytic leukemia.3,4
Partial or complete loss of eyebrows in the setting of leonine facies has a limited differential diagnosis.3,5 In addition to cancer, the associated disorders include adnexal mucin deposition (alopecia mucinosis), granulomatous conditions (sarcoidosis), infectious diseases (leprosy), inherited syndromes (Setleis syndrome), photoallergic dermatoses (actinic reticuloid), and viral conditions (viral-associated trichodysplasia).3-9 Neoplasms associated with leonine facies and eyebrow loss include lymphomas (mycosis fungoides and unspecified cutaneous T-cell lymphoma), systemic mastocytosis and leukemia cutis secondary to acute lymphocytic leukemia, acute myelomonocytic leukemia, and now AML.1,3-5
The eyebrow loss associated with leonine facies often is not reversible once the causative cell of the associated condition (eg, granulomas of mycobacteria-infected histiocytes in leprosy, neoplastic lymphocytes in cutaneous T-cell lymphoma) has infiltrated the area of the eyebrows and abolished the preexisting hair follicles; however, follow-up descriptions of patients after treatment of other conditions that cause eyebrow loss usually are not reported. Indeed, there was partial reappearance of the eyebrows in a woman with systemic mastocytosis–associated loss of the eyebrows after malignancy-related treatment was reinitiated and the infiltrative facial plaques that had created her leonine facies had decreased in size.5 It is reasonable to speculate that the eyebrows may have reappeared in the patient reported by Krooks and Weatherall1 and his leonine facies–associated facial plaques may have resolved if he had underwent and responded to treatment with antineoplastic chemotherapy.
To the Editor:
I read with interest the informative Cutis case report by Krooks and Weatherall1 in which the authors not only described the case of a 66-year-old man whose diagnosis of bone marrow biopsy–confirmed acute myeloid leukemia (AML) presented concurrently with skin biopsy–confirmed leukemia cutis but also discussed the poor prognosis of individuals with acute myelogenous leukemia cutis. Their patient died within 5 weeks of establishing the diagnosis. In addition, lateral and frontal photographs of the patient’s face demonstrated diffuse infiltrative plaques of leukemia cutis; he had swollen eyelids and lips with distortion of the nose secondary to dermal infiltration of leukemic myeloid cells.1 Although not emphasized by the authors, the patient appeared to have a leonine facies and at least partial loss of the lateral eyebrows.
Malignancy-associated leonine facies resulting from infiltration of the skin by neoplastic cells has been reported in a patient with metastatic breast carcinoma.2,3 However, it predominantly occurs in patients with hematologic dyscrasias such as leukemia cutis, lymphoma (ie, cutaneous B cell, cutaneous T cell, Hodgkin), plasmacytoma, and systemic mastocytosis.3,4 The report by Krooks and Weatherall1 adds AML-associated leukemia cutis to the previously observed types of leukemia cutis–related leonine facies in patients with acute lymphocytic leukemia, acute myelomonocytic leukemia, and chronic lymphocytic leukemia.3,4
Partial or complete loss of eyebrows in the setting of leonine facies has a limited differential diagnosis.3,5 In addition to cancer, the associated disorders include adnexal mucin deposition (alopecia mucinosis), granulomatous conditions (sarcoidosis), infectious diseases (leprosy), inherited syndromes (Setleis syndrome), photoallergic dermatoses (actinic reticuloid), and viral conditions (viral-associated trichodysplasia).3-9 Neoplasms associated with leonine facies and eyebrow loss include lymphomas (mycosis fungoides and unspecified cutaneous T-cell lymphoma), systemic mastocytosis and leukemia cutis secondary to acute lymphocytic leukemia, acute myelomonocytic leukemia, and now AML.1,3-5
The eyebrow loss associated with leonine facies often is not reversible once the causative cell of the associated condition (eg, granulomas of mycobacteria-infected histiocytes in leprosy, neoplastic lymphocytes in cutaneous T-cell lymphoma) has infiltrated the area of the eyebrows and abolished the preexisting hair follicles; however, follow-up descriptions of patients after treatment of other conditions that cause eyebrow loss usually are not reported. Indeed, there was partial reappearance of the eyebrows in a woman with systemic mastocytosis–associated loss of the eyebrows after malignancy-related treatment was reinitiated and the infiltrative facial plaques that had created her leonine facies had decreased in size.5 It is reasonable to speculate that the eyebrows may have reappeared in the patient reported by Krooks and Weatherall1 and his leonine facies–associated facial plaques may have resolved if he had underwent and responded to treatment with antineoplastic chemotherapy.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Jin CC, Martinelli PT, Cohen PR. What are these erythematous skin lesions? leukemia cutis. The Dermatologist. 2012;20:46-50.
- Chodkiewicz HM, Cohen PR. Systemic mastocytosis-associated leonine facies and eyebrow loss. South Med J. 2011;104:236-238.
- Cohen PR, Rapini RP, Beran M. Infiltrated blue-gray plaques in a patient with leukemia. Chloroma (granulocytic sarcoma). Arch Dermatol. 1987;123:251, 254.
- Cohen PR. Leonine facies associated with eyebrow loss. Int J Dermatol. 2014;53:e148-e149.
- Ravic-Nikolic A, Milicic V, Ristic G, et al. Actinic reticuloid presented as facies leonine. Int J Dermatol. 2012;51:234-236.
- Jacob Raja SA, Raja JJ, Vijayashree R, et al. Evaluation of oral and periodontal status of leprosy patients in Dindigul district. J Pharm Bioallied Sci. 2016;8(suppl 1):S119-S121.
- McGaughran J, Aftimos S. Setleis syndrome: three new cases and a review of the literature. Am J Med Genet. 2002;111:376-380.
- Benoit T, Bacelieri R, Morrell DS, et al. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871-874.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Jin CC, Martinelli PT, Cohen PR. What are these erythematous skin lesions? leukemia cutis. The Dermatologist. 2012;20:46-50.
- Chodkiewicz HM, Cohen PR. Systemic mastocytosis-associated leonine facies and eyebrow loss. South Med J. 2011;104:236-238.
- Cohen PR, Rapini RP, Beran M. Infiltrated blue-gray plaques in a patient with leukemia. Chloroma (granulocytic sarcoma). Arch Dermatol. 1987;123:251, 254.
- Cohen PR. Leonine facies associated with eyebrow loss. Int J Dermatol. 2014;53:e148-e149.
- Ravic-Nikolic A, Milicic V, Ristic G, et al. Actinic reticuloid presented as facies leonine. Int J Dermatol. 2012;51:234-236.
- Jacob Raja SA, Raja JJ, Vijayashree R, et al. Evaluation of oral and periodontal status of leprosy patients in Dindigul district. J Pharm Bioallied Sci. 2016;8(suppl 1):S119-S121.
- McGaughran J, Aftimos S. Setleis syndrome: three new cases and a review of the literature. Am J Med Genet. 2002;111:376-380.
- Benoit T, Bacelieri R, Morrell DS, et al. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871-874.
TNF inhibitor–induced psoriasis in IBD patients a consideration
WASHINGTON – (IBD), Sophia Delano, MD, said during a session on the cutaneous effects of IBD at the annual meeting of the American Academy of Dermatology.
This is a paradoxical reaction, which can happen “weeks to years after starting a TNF blocker,” with about 70% of cases occurring during the first year of therapy, said Dr. Delano, an attending physician in the dermatology program at Boston Children’s Hospital.
Those receiving infliximab are more likely to develop TNF inhibitor–induced psoriasis, compared with those on adalimumab or etanercept. TNF inhibitor–induced psoriasis may not track with gastrointestinal activity, and some patients whose gastrointestinal disease is responding to treatment can begin to develop psoriasis, she noted.
The clinical presentation of TNF inhibitor–induced psoriasis can also vary. In one study of 216 cases, 26.9% of patients had a mixed morphology, with the most common presentations including plaque psoriasis (44.8%) and palmoplantar pustular psoriasis (36.3%). Other presentations were psoriasiform dermatitis (19.9%), scalp involvement with alopecia (7.5%), and generalized pustular psoriasis (10.9%). Locations affected were the soles of the feet (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%), Dr. Delano said.
TNF inhibitor–induced psoriasis is likely a class effect, she said, noting that, in the same review, symptoms resolved in 47.7% of patients who discontinued TNF inhibitors, in 36.7% of patients who switched to another TNF inhibitor, and in 32.9% of patients who continued their original therapy (J Am Acad Dermatol. 2017 Feb;76[2]:334-41). In the study, Crohn’s disease and RA were the most common diseases, in 40.7% and 37.0% of the patients, respectively.
There have been case reports of TNF antagonist–induced lupus-like syndrome (TAILS), which is more common in patients with RA and ulcerative colitis. TAILS occurs more often in women than in men; can present similarly to systemic lupus erythematosus, subacute cutaneous lupus erythematosus, and chronic cutaneous lupus; and resolves by stopping TNF inhibitor treatment, Dr. Delano said.
Skin cancer risk, infections, and injection site reactions
Both adult and pediatric patients treated with TNF inhibitors for IBD may be at increased risk for lymphoma, visceral tumors, melanoma, and nonmelanoma skin cancers. Dr. Delano referred to a study published in 2014, which identified 972 reports of melanoma in the Food and Drug Administration’s Adverse Event Reporting System database associated with TNF inhibitor use; of these, 69 cases involved patients using more than one TNF inhibitor. Infliximab, golimumab, etanercept, and adalimumab were associated with a safety signal for melanoma, but not certolizumab (Br J Dermatol. 2014 May;170[5]:1170-2).
Dr. Delano observed that thiopurines such as azathioprine are also associated with an increased cancer risk, as noted in one retrospective study that found that the risk of nonmelanoma skin cancer was 2.1 times higher in a mostly white male cohort with ulcerative colitis during treatment with thiopurines, compared with patients not treated with thiopurines (Am J Gastroenterol. 2014 Nov;109[11]:1781-93). A greater duration of treatment (more than 6 months) and higher doses were associated with higher risks.
Adalimumab, golimumab, and certolizumab can also cause injection site reactions, typically within 1- 2 days of injection, said Dr. Delano. In these cases, symptoms of erythema, warmth, burning, or pruritus are worse at the beginning of treatment and can be relieved by rotating the injection site as well as providing cool compresses, topical steroids, antihistamines, and supportive care.
“If you have a patient with a worsening reaction, consider it may represent the type 1 IgE-related hypersensitivity requiring desensitization to continue that systemic,” she noted.
Cutaneous bacterial, fungal, and viral infections such as molluscum contagiosum, verruca vulgaris, herpes simplex, and varicella zoster can occur as a result of TNF inhibition as well, and can be difficult to clear because of immunosuppression, she added.
Dr. Delano reported no relevant conflicts of interest.
WASHINGTON – (IBD), Sophia Delano, MD, said during a session on the cutaneous effects of IBD at the annual meeting of the American Academy of Dermatology.
This is a paradoxical reaction, which can happen “weeks to years after starting a TNF blocker,” with about 70% of cases occurring during the first year of therapy, said Dr. Delano, an attending physician in the dermatology program at Boston Children’s Hospital.
Those receiving infliximab are more likely to develop TNF inhibitor–induced psoriasis, compared with those on adalimumab or etanercept. TNF inhibitor–induced psoriasis may not track with gastrointestinal activity, and some patients whose gastrointestinal disease is responding to treatment can begin to develop psoriasis, she noted.
The clinical presentation of TNF inhibitor–induced psoriasis can also vary. In one study of 216 cases, 26.9% of patients had a mixed morphology, with the most common presentations including plaque psoriasis (44.8%) and palmoplantar pustular psoriasis (36.3%). Other presentations were psoriasiform dermatitis (19.9%), scalp involvement with alopecia (7.5%), and generalized pustular psoriasis (10.9%). Locations affected were the soles of the feet (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%), Dr. Delano said.
TNF inhibitor–induced psoriasis is likely a class effect, she said, noting that, in the same review, symptoms resolved in 47.7% of patients who discontinued TNF inhibitors, in 36.7% of patients who switched to another TNF inhibitor, and in 32.9% of patients who continued their original therapy (J Am Acad Dermatol. 2017 Feb;76[2]:334-41). In the study, Crohn’s disease and RA were the most common diseases, in 40.7% and 37.0% of the patients, respectively.
There have been case reports of TNF antagonist–induced lupus-like syndrome (TAILS), which is more common in patients with RA and ulcerative colitis. TAILS occurs more often in women than in men; can present similarly to systemic lupus erythematosus, subacute cutaneous lupus erythematosus, and chronic cutaneous lupus; and resolves by stopping TNF inhibitor treatment, Dr. Delano said.
Skin cancer risk, infections, and injection site reactions
Both adult and pediatric patients treated with TNF inhibitors for IBD may be at increased risk for lymphoma, visceral tumors, melanoma, and nonmelanoma skin cancers. Dr. Delano referred to a study published in 2014, which identified 972 reports of melanoma in the Food and Drug Administration’s Adverse Event Reporting System database associated with TNF inhibitor use; of these, 69 cases involved patients using more than one TNF inhibitor. Infliximab, golimumab, etanercept, and adalimumab were associated with a safety signal for melanoma, but not certolizumab (Br J Dermatol. 2014 May;170[5]:1170-2).
Dr. Delano observed that thiopurines such as azathioprine are also associated with an increased cancer risk, as noted in one retrospective study that found that the risk of nonmelanoma skin cancer was 2.1 times higher in a mostly white male cohort with ulcerative colitis during treatment with thiopurines, compared with patients not treated with thiopurines (Am J Gastroenterol. 2014 Nov;109[11]:1781-93). A greater duration of treatment (more than 6 months) and higher doses were associated with higher risks.
Adalimumab, golimumab, and certolizumab can also cause injection site reactions, typically within 1- 2 days of injection, said Dr. Delano. In these cases, symptoms of erythema, warmth, burning, or pruritus are worse at the beginning of treatment and can be relieved by rotating the injection site as well as providing cool compresses, topical steroids, antihistamines, and supportive care.
“If you have a patient with a worsening reaction, consider it may represent the type 1 IgE-related hypersensitivity requiring desensitization to continue that systemic,” she noted.
Cutaneous bacterial, fungal, and viral infections such as molluscum contagiosum, verruca vulgaris, herpes simplex, and varicella zoster can occur as a result of TNF inhibition as well, and can be difficult to clear because of immunosuppression, she added.
Dr. Delano reported no relevant conflicts of interest.
WASHINGTON – (IBD), Sophia Delano, MD, said during a session on the cutaneous effects of IBD at the annual meeting of the American Academy of Dermatology.
This is a paradoxical reaction, which can happen “weeks to years after starting a TNF blocker,” with about 70% of cases occurring during the first year of therapy, said Dr. Delano, an attending physician in the dermatology program at Boston Children’s Hospital.
Those receiving infliximab are more likely to develop TNF inhibitor–induced psoriasis, compared with those on adalimumab or etanercept. TNF inhibitor–induced psoriasis may not track with gastrointestinal activity, and some patients whose gastrointestinal disease is responding to treatment can begin to develop psoriasis, she noted.
The clinical presentation of TNF inhibitor–induced psoriasis can also vary. In one study of 216 cases, 26.9% of patients had a mixed morphology, with the most common presentations including plaque psoriasis (44.8%) and palmoplantar pustular psoriasis (36.3%). Other presentations were psoriasiform dermatitis (19.9%), scalp involvement with alopecia (7.5%), and generalized pustular psoriasis (10.9%). Locations affected were the soles of the feet (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%), Dr. Delano said.
TNF inhibitor–induced psoriasis is likely a class effect, she said, noting that, in the same review, symptoms resolved in 47.7% of patients who discontinued TNF inhibitors, in 36.7% of patients who switched to another TNF inhibitor, and in 32.9% of patients who continued their original therapy (J Am Acad Dermatol. 2017 Feb;76[2]:334-41). In the study, Crohn’s disease and RA were the most common diseases, in 40.7% and 37.0% of the patients, respectively.
There have been case reports of TNF antagonist–induced lupus-like syndrome (TAILS), which is more common in patients with RA and ulcerative colitis. TAILS occurs more often in women than in men; can present similarly to systemic lupus erythematosus, subacute cutaneous lupus erythematosus, and chronic cutaneous lupus; and resolves by stopping TNF inhibitor treatment, Dr. Delano said.
Skin cancer risk, infections, and injection site reactions
Both adult and pediatric patients treated with TNF inhibitors for IBD may be at increased risk for lymphoma, visceral tumors, melanoma, and nonmelanoma skin cancers. Dr. Delano referred to a study published in 2014, which identified 972 reports of melanoma in the Food and Drug Administration’s Adverse Event Reporting System database associated with TNF inhibitor use; of these, 69 cases involved patients using more than one TNF inhibitor. Infliximab, golimumab, etanercept, and adalimumab were associated with a safety signal for melanoma, but not certolizumab (Br J Dermatol. 2014 May;170[5]:1170-2).
Dr. Delano observed that thiopurines such as azathioprine are also associated with an increased cancer risk, as noted in one retrospective study that found that the risk of nonmelanoma skin cancer was 2.1 times higher in a mostly white male cohort with ulcerative colitis during treatment with thiopurines, compared with patients not treated with thiopurines (Am J Gastroenterol. 2014 Nov;109[11]:1781-93). A greater duration of treatment (more than 6 months) and higher doses were associated with higher risks.
Adalimumab, golimumab, and certolizumab can also cause injection site reactions, typically within 1- 2 days of injection, said Dr. Delano. In these cases, symptoms of erythema, warmth, burning, or pruritus are worse at the beginning of treatment and can be relieved by rotating the injection site as well as providing cool compresses, topical steroids, antihistamines, and supportive care.
“If you have a patient with a worsening reaction, consider it may represent the type 1 IgE-related hypersensitivity requiring desensitization to continue that systemic,” she noted.
Cutaneous bacterial, fungal, and viral infections such as molluscum contagiosum, verruca vulgaris, herpes simplex, and varicella zoster can occur as a result of TNF inhibition as well, and can be difficult to clear because of immunosuppression, she added.
Dr. Delano reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAD 2019