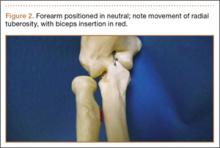
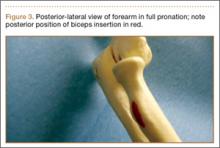
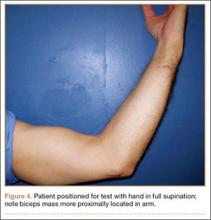
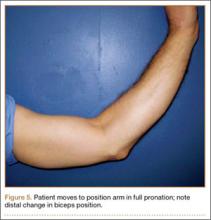
User login
Isolated Avulsion of Extensor Carpi Radialis Longus and Brachioradialis Origins: A Case Report and Surgical Repair Technique
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
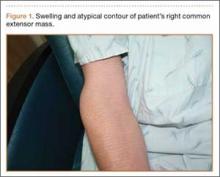
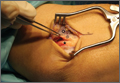
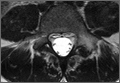
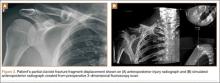
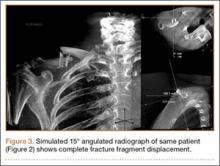
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
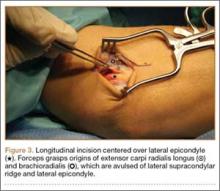
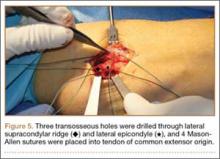
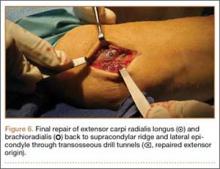
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
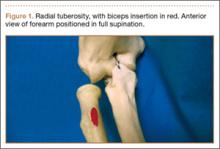
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
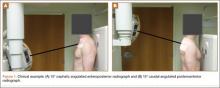
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
Avascular Necrosis of Trochlea After Supracondylar Humerus Fractures in Children
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
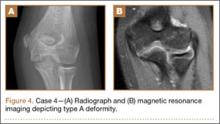
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
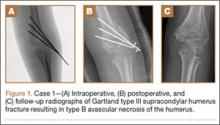
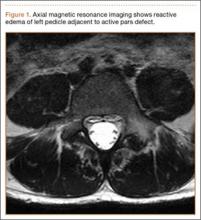
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
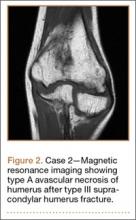
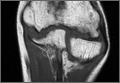
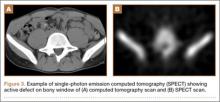
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
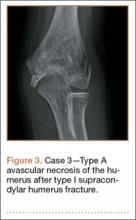
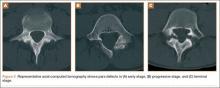
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
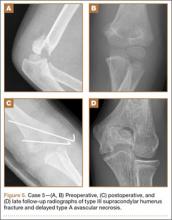
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
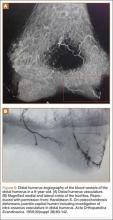
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
Invasive Compartment Pressure Testing for Chronic Exertional Compartment Syndrome: A Survey of Clinical Practice Among Military Orthopedic Surgeons
Chronic exertional compartment syndrome (CECS) is a common cause of leg pain during exertion in athletic and active-duty populations.1 It is caused by an increase in intramuscular pressure to a point that the tissues within the involved compartment become ischemic because of a decrease in arteriolar blood flow.2 This relative ischemia causes pain and may also be associated with neurologic symptoms. By definition, the pain associated with CECS resolves with rest. Patients typically describe a feeling of fullness or tightness, which eventually evolves into pain as they continue exercising. Pain onset is usually predictable and reproducible after a finite amount of time and/or intensity of exercise.
The differential diagnosis of leg pain during exercise includes CECS, medial tibial stress syndrome, popliteal entrapment syndrome, myopathy, peripheral nerve entrapment syndromes, stress fracture, and effort-induced rhabdomyolysis.3 CECS can be differentiated from other causes of leg pain with measurement of compartment pressures (the standard recommendation).4 Compartment pressure measurement, however, is invasive, time-consuming, and painful and may be associated with bleeding risk, infection, and nerve injury. Noninvasive means of testing for CECS (eg, magnetic resonance imaging [MRI], near-infrared spectroscopy [NIRS], thallium stress testing) remain experimental and expensive and are not easily accessible at all institutions.5-8 While invasive compartment pressure (ICP) testing remains an important tool in the diagnosis of CECS, its criteria and execution vary considerably. Aweid and colleagues4 performed a meta-analysis of use of ICP testing in the diagnosis of CECS and concluded that, though elevated ICP measurements are accepted as the gold standard for diagnosing CECS, the criteria outlined for a positive test lack high-level supporting evidence. In addition, how the test is performed has been inconsistent across studies—further clouding the literature.4
The review by Aweid and colleagues4 highlights the deficiencies in diagnosing CECS by ICP testing. In clinical practice, ICP testing is challenging for both the patient and physician. As other validated, less-invasive tests are lacking, emphasis should remain on the history and the physical examination. Although all athletic populations are at risk for CECS, the active-duty military population is at particularly high risk because of the physical requirements and demands of military service.1,9
We surveyed military orthopedic surgeons to investigate the clinical practice of performing ICP testing in patients with suspected CECS. We hypothesized that the rate of ICP testing among military orthopedic surgeons would not be 100% for patients with the typical signs and symptoms of CECS.
Materials and Methods
This study was approved by the institutional review board at Wright-Patterson Medical Center at Wright-Patterson Air Force Base in Ohio. A link to an online survey was distributed by email to members of the Society of Military Orthopaedic Surgeons. The anonymous survey polled the surgeons regarding basic demographic data and clinical practice as it pertains to the evaluation and treatment of CECS. No patient-protected health information was obtained. Survey results were compiled in a Microsoft Excel file for analysis.
Results
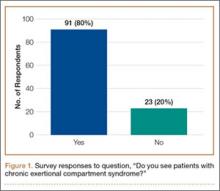
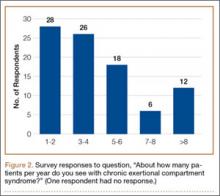
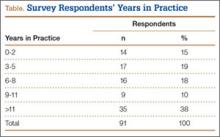
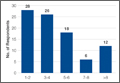
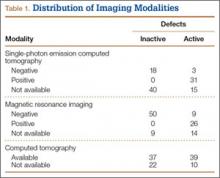
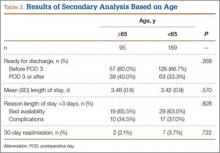
The survey was distributed to 606 email accounts; the response rate was 19% (114/606). Ninety-one surgeons (80%) indicated they have patients with CECS in their practice (Figure 1). Surgeons were asked how many CECS patients they see per year (responses are summarized in Figure 2) and how many years they have been in practice (Table).
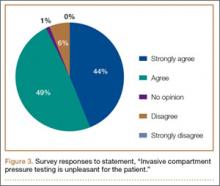
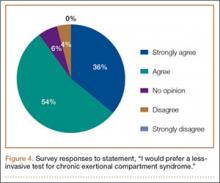
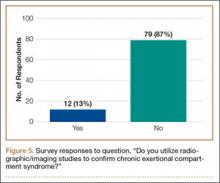
Ninety-three percent of the respondents agreed or strongly agreed that ICP testing is unpleasant for the patient (Figure 3), and 90% would prefer a less-invasive test for confirmatory testing for CECS (Figure 4). Only 13% of respondents indicated they actually use noninvasive modalities (eg, MRI, NIRS) to confirm the diagnosis of CECS (Figure 5).
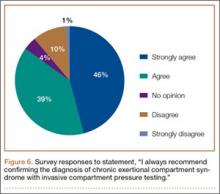
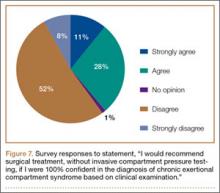
Respondents were asked about the practice of using ICP testing in the diagnosis of CECS (responses are summarized in Figures 6, 7). Although 85% of respondents agreed or strongly agreed with always confirming the diagnosis of CECS with ICP testing, 39% stated they would recommend surgical treatment without ICP testing if they were confident about the diagnosis based on clinical examination findings.
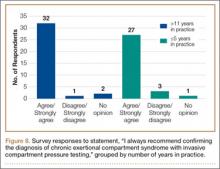
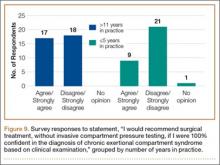
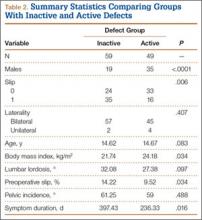
To better understand the apparent discrepancy between the percentage of surgeons who agreed or strongly agreed with always recommending ICP testing (85%) and the percentage who would recommend treatment without testing (39%), responses were stratified by clinical experience. Surgeons in practice more than 11 years (n = 35) were compared with those in practice 5 years or less (n = 31) (Table). Although the vast majority (85%) of respondents from both groups agreed or strongly agreed with always recommending ICP testing, 49% of those in practice more than 11 years and 29% in practice 5 years or less indicated they would recommend surgical treatment for CECS based solely on clinical examination findings (Figures 8, 9).
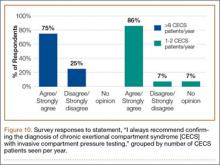
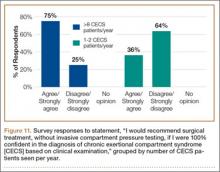
Responses were also stratified by number of CECS patients seen by each surgeon per year. Twenty-eight respondents saw 1 or 2 patients per year, and 12 saw more than 8 patients per year—31% and 13% of the total number of respondents, respectively. Of the respondents who saw 1 or 2 patients, 86% (24/28) agreed or strongly agreed with always recommending ICP testing—comparable to the 75% (9/12) who saw more than 8 patients (Figure 10). However, of the respondents who saw 1 or 2 patients, 36% (10/28) indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% (9/12) who saw more than 8 patients (Figure 11).
Discussion
CECS is a common cause of leg pain and a significant cause of disability among the active-duty military population. This was illustrated in 2 recent studies by Waterman and colleagues.1,9 The first1 investigated failure rates and disability after surgery for CECS among those on active duty. The authors showed that CECS is a substantial contributor to lower extremity disability in the military population and that there is a substantial risk for persistent symptoms despite surgical treatment. Nearly 1 in 5 patients experienced surgical failure after elective fasciotomy, and about 28% of patients were unable to return to the full activity required in the military. The second, more recent study9 was an epidemiologic study of risk factors associated with CECS in a physically active military population. The authors identified 4100 cases diagnosed between 2006 and 2011—representing an overall annual incidence of 0.49 per 1000 at-risk person-years, or about 683 cases per year; the authors also showed the incidence increased during the time period studied.
The diagnosis of CECS remains imperfect. A clinical history of exercise-induced lower leg pain that is relieved with rest suggests the diagnosis, but a confirmatory test is needed to distinguish CECS from other causes of exercise-induced leg pain. Although direct measurement of compartment pressures is the test used most often, it is invasive and time-consuming, can be uncomfortable for the patient, and may be associated with bleeding risk, infection, and nerve injury. Pedowitz and colleagues10 described the ICP testing criteria now generally used in the diagnosis of CECS. Unfortunately, there is little objective evidence supporting these criteria.4 Although less invasive tests (eg, MRI, NIRS) have been described,5-8 they may not be readily available across institutions, and further study is needed to validate their use in diagnosing CECS.
While an objective, validated test or measurement for confirming the diagnosis of CECS remains elusive, the outcomes after surgical treatment of CECS also remain imperfect. Surgery consists of both open and endoscopically assisted fasciotomy of the involved compartments.2,11-17 Reports of improvement after treatment range from 81% to 100%3; however, symptom relief does not come for all patients, particularly those in the military. Waterman and colleagues1 found a failure rate of about 20% among an active-duty military population. Packer and colleagues18 examined civilians with CECS, treated both operatively and nonoperatively. Patients in this series were diagnosed with CECS based on clinical symptoms as well as compartment pressure measurements according to the Pedowitz criteria. Although overall outcomes were better for operatively treated patients than for nonoperatively treated patients, only 47% of patients were completely pain-free, and 21% were unable to return to full activity.
More recent studies have explored use of other nonoperative treatment modalities. Diebal and colleagues19,20 used a running retraining program to treat patients with CECS. They based this treatment on the hypothesis that a heel-strike running pattern is associated with increased anterior compartment pressures.21 CECS patients who underwent a 6-week systematic treatment program focused on forefoot running, stride shortening, and hamstring activation during push-off experienced a decrease in clinical symptoms and posttreatment intracompartmental pressures.20 The improvements in clinical scores were maintained at 1-year follow-up. Another nonoperative intervention, recently described by Isner-Horobeti and colleagues,22 involves injecting botulinum toxin A (BoNT-A) into the anterior and lateral compartments of the leg. Sixteen patients with CECS received BoNT-A injections. On average, intracompartmental pressures were lower after injection than they were before injection. In addition, exertional pain was eliminated in 15 patients at an average follow-up of 4.4 months.
This survey-based study examined the practice patterns of military orthopedic surgeons who performed ICP testing for cases of suspected CECS. Our hypothesis was that, though ICP testing is the most commonly accepted method for confirming the diagnosis of CECS, the ICP testing rate would not be 100% among those surveyed.
The results of our study uncover an apparent inconsistency in survey responses among physicians who evaluate and treat patients with CECS. About 85% of respondents stated they would always recommend confirming the diagnosis of CECS with ICP testing. However, about 40% stated they would recommend surgical treatment without confirmatory testing if they were confident about the diagnosis based on clinical findings. In other words, only 60% of the respondents disagreed or strongly disagreed with pursuing surgical treatment without testing. One would expect a closer correlation between respondents who would always recommend ICP testing and those who disagreed with recommending surgical treatment without ICP testing. This raises the question of what actually occurs when CECS is suspected in clinical practice.
To better understand the apparent discrepancy between respondents who agreed or strongly agreed with always recommending ICP testing and respondents who would recommend treatment without testing, we grouped responses by clinical experience. Although 85% of respondents (no matter the number of years in practice) agreed or strongly agreed with the statement, “I always recommend confirming the diagnosis of CECS with ICP measurements,” 49% of those in practice more than 11 years (vs. 29% of those in practice 5 years or less) agreed or strongly agreed with recommending surgery without testing if they were 100% confident about the diagnosis of CECS based solely on clinical findings. This may suggest that, though most agreed that the gold standard for confirming the diagnosis of CECS remains ICP testing, those with more clinical experience were more comfortable forgoing this diagnostic measure and recommending treatment without testing.
Another measure of clinical experience used in this survey was based on number of CECS patients seen per year. Responses of surgeons who saw 1 or 2 patients with CECS per year were compared with responses of surgeons who saw more than 8 patients with CECS per year. Of the respondents who saw 1 or 2 patients, 86% agreed or strongly agreed with always recommending ICP testing to confirm CECS—comparable to the 75% who saw more than 8 patients. However, of the respondents who saw 1 or 2 patients, 36% indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% who saw more than 8 patients.
Responses regarding the absolute of always recommending ICP testing and the absolute of being 100% confident about the clinical diagnosis of CECS highlight differences between the surgeons with more experience (>11 years in practice, >8 CECS patients per year) and those with less experience (≤5 years in practice, 1 or 2 CECS patients per year). Surgeons in practice longer, and surgeons who saw more patients with suspected CECS per year, were more likely to recommend surgical treatment based solely on clinical findings.
Conclusion
CECS can cause debilitating activity-related leg pain in both civilian and military populations. Treatment with fasciotomy is often curative, but a significant number of patients may continue to have pain and disability. As the incidence of treatment failures may be higher in the military than in civilians, proper evaluation of patients with suspected CECS is particularly important for military orthopedic surgeons. The diagnosis of CECS can be challenging to both the clinician and patient, and diagnostic modalities remain imperfect. The results of this study highlight this, revealing less than 100% agreement regarding use of ICP testing (the gold standard) for diagnosis of CECS.
This study also highlights the need for an improved method of diagnosing CECS since 93% of respondents agreed or strongly agreed that ICP testing is unpleasant for the patient, and 90% would prefer a less-invasive test. In addition, the ICP testing criteria for establishing the diagnosis of CECS remain inconsistent. If a reliable, consistent, and less-invasive test were available, perhaps there would be less variability in practitioners’ evaluations of patients with CECS.
This study shows an inconsistency among military orthopedic surgeons evaluating and treating patients with CECS. As testing modalities for CECS remain imperfect, clinical acumen and experience assume an important role in the assessment of patients with suspected CECS.
1. Waterman BR, Laughlin M, Kilcoyne K, Cameron KL, Owens BD. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95(7):592-596.
2. Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36-40.
3. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268-276.
4. Aweid O, Del Buono A, Malliaras P, et al. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22(4):356-370.
5. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42(3):385-392.
6. van den Brand JG, Verleisdonk EJ, van der Werken C. Near infrared spectroscopy in the diagnosis of chronic exertional compartment syndrome. Am J Sports Med. 2004;32(2):452-456.
7. van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699-704.
8. Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321-325.
9. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
10. Pedowitz RA, Hargens AR, Mubarek SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35-40.1. Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65(9):1245-1251.
12. Rorabeck CH, Fowler PJ, Nott L. The results of fasciotomy in the management of chronic exertional compartment syndrome. Am J Sports Med. 1988;16(3):224-227.
13. Detmer DE, Sharpe K, Sufit RL, Girdley FM. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13(3):162-170.
14. Stein DA, Sennett BJ. One-portal endoscopically assisted fasciotomy for exertional compartment syndrome. Arthroscopy. 2005;21(1):108-112.
15. Fronek J, Mubarak SJ, Hargens AR, et al. Management of chronic exertional anterior compartment syndrome of the lower extremity. Clin Orthop Relat Res. 1989;(220):217-227.
16. Leversedge FJ, Casey PJ, Seiler JG 3rd, Xerogeanes JW. Endoscopically assisted fasciotomy: description of technique and in vitro assessment of lower-leg compartment decompression. Am J Sports Med. 2002;30(2):272-278.
17. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21(6):811-817.
18. Packer JD, Day MS, Nguyen JT, Hobart SJ, Hannafin JA, Metzl JD. Functional outcomes and patient satisfaction after fasciotomy for chronic exertional compartment syndrome. Am J Sports Med. 2013;41(2):430-436.
19. Diebal AR, Gregory R, Alitz C, Gerber JP. Effects of forefoot running on chronic exertional compartment syndrome: a case series. Int J Sports Phys Ther. 2011;6(4):312-321.
20. Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060-1067.
21. Kirby RL, McDermott AG. Anterior tibial compartment pressures during running with rearfoot and forefoot landing styles. Arch Phys Med Rehabil. 1983;64(7):296-299.
22. Isner-Horobeti ME, Dufour SP, Blaes C, Lecocq J. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41(11):2558-2566.
Chronic exertional compartment syndrome (CECS) is a common cause of leg pain during exertion in athletic and active-duty populations.1 It is caused by an increase in intramuscular pressure to a point that the tissues within the involved compartment become ischemic because of a decrease in arteriolar blood flow.2 This relative ischemia causes pain and may also be associated with neurologic symptoms. By definition, the pain associated with CECS resolves with rest. Patients typically describe a feeling of fullness or tightness, which eventually evolves into pain as they continue exercising. Pain onset is usually predictable and reproducible after a finite amount of time and/or intensity of exercise.
The differential diagnosis of leg pain during exercise includes CECS, medial tibial stress syndrome, popliteal entrapment syndrome, myopathy, peripheral nerve entrapment syndromes, stress fracture, and effort-induced rhabdomyolysis.3 CECS can be differentiated from other causes of leg pain with measurement of compartment pressures (the standard recommendation).4 Compartment pressure measurement, however, is invasive, time-consuming, and painful and may be associated with bleeding risk, infection, and nerve injury. Noninvasive means of testing for CECS (eg, magnetic resonance imaging [MRI], near-infrared spectroscopy [NIRS], thallium stress testing) remain experimental and expensive and are not easily accessible at all institutions.5-8 While invasive compartment pressure (ICP) testing remains an important tool in the diagnosis of CECS, its criteria and execution vary considerably. Aweid and colleagues4 performed a meta-analysis of use of ICP testing in the diagnosis of CECS and concluded that, though elevated ICP measurements are accepted as the gold standard for diagnosing CECS, the criteria outlined for a positive test lack high-level supporting evidence. In addition, how the test is performed has been inconsistent across studies—further clouding the literature.4
The review by Aweid and colleagues4 highlights the deficiencies in diagnosing CECS by ICP testing. In clinical practice, ICP testing is challenging for both the patient and physician. As other validated, less-invasive tests are lacking, emphasis should remain on the history and the physical examination. Although all athletic populations are at risk for CECS, the active-duty military population is at particularly high risk because of the physical requirements and demands of military service.1,9
We surveyed military orthopedic surgeons to investigate the clinical practice of performing ICP testing in patients with suspected CECS. We hypothesized that the rate of ICP testing among military orthopedic surgeons would not be 100% for patients with the typical signs and symptoms of CECS.
Materials and Methods
This study was approved by the institutional review board at Wright-Patterson Medical Center at Wright-Patterson Air Force Base in Ohio. A link to an online survey was distributed by email to members of the Society of Military Orthopaedic Surgeons. The anonymous survey polled the surgeons regarding basic demographic data and clinical practice as it pertains to the evaluation and treatment of CECS. No patient-protected health information was obtained. Survey results were compiled in a Microsoft Excel file for analysis.
Results
The survey was distributed to 606 email accounts; the response rate was 19% (114/606). Ninety-one surgeons (80%) indicated they have patients with CECS in their practice (Figure 1). Surgeons were asked how many CECS patients they see per year (responses are summarized in Figure 2) and how many years they have been in practice (Table).
Ninety-three percent of the respondents agreed or strongly agreed that ICP testing is unpleasant for the patient (Figure 3), and 90% would prefer a less-invasive test for confirmatory testing for CECS (Figure 4). Only 13% of respondents indicated they actually use noninvasive modalities (eg, MRI, NIRS) to confirm the diagnosis of CECS (Figure 5).
Respondents were asked about the practice of using ICP testing in the diagnosis of CECS (responses are summarized in Figures 6, 7). Although 85% of respondents agreed or strongly agreed with always confirming the diagnosis of CECS with ICP testing, 39% stated they would recommend surgical treatment without ICP testing if they were confident about the diagnosis based on clinical examination findings.
To better understand the apparent discrepancy between the percentage of surgeons who agreed or strongly agreed with always recommending ICP testing (85%) and the percentage who would recommend treatment without testing (39%), responses were stratified by clinical experience. Surgeons in practice more than 11 years (n = 35) were compared with those in practice 5 years or less (n = 31) (Table). Although the vast majority (85%) of respondents from both groups agreed or strongly agreed with always recommending ICP testing, 49% of those in practice more than 11 years and 29% in practice 5 years or less indicated they would recommend surgical treatment for CECS based solely on clinical examination findings (Figures 8, 9).
Responses were also stratified by number of CECS patients seen by each surgeon per year. Twenty-eight respondents saw 1 or 2 patients per year, and 12 saw more than 8 patients per year—31% and 13% of the total number of respondents, respectively. Of the respondents who saw 1 or 2 patients, 86% (24/28) agreed or strongly agreed with always recommending ICP testing—comparable to the 75% (9/12) who saw more than 8 patients (Figure 10). However, of the respondents who saw 1 or 2 patients, 36% (10/28) indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% (9/12) who saw more than 8 patients (Figure 11).
Discussion
CECS is a common cause of leg pain and a significant cause of disability among the active-duty military population. This was illustrated in 2 recent studies by Waterman and colleagues.1,9 The first1 investigated failure rates and disability after surgery for CECS among those on active duty. The authors showed that CECS is a substantial contributor to lower extremity disability in the military population and that there is a substantial risk for persistent symptoms despite surgical treatment. Nearly 1 in 5 patients experienced surgical failure after elective fasciotomy, and about 28% of patients were unable to return to the full activity required in the military. The second, more recent study9 was an epidemiologic study of risk factors associated with CECS in a physically active military population. The authors identified 4100 cases diagnosed between 2006 and 2011—representing an overall annual incidence of 0.49 per 1000 at-risk person-years, or about 683 cases per year; the authors also showed the incidence increased during the time period studied.
The diagnosis of CECS remains imperfect. A clinical history of exercise-induced lower leg pain that is relieved with rest suggests the diagnosis, but a confirmatory test is needed to distinguish CECS from other causes of exercise-induced leg pain. Although direct measurement of compartment pressures is the test used most often, it is invasive and time-consuming, can be uncomfortable for the patient, and may be associated with bleeding risk, infection, and nerve injury. Pedowitz and colleagues10 described the ICP testing criteria now generally used in the diagnosis of CECS. Unfortunately, there is little objective evidence supporting these criteria.4 Although less invasive tests (eg, MRI, NIRS) have been described,5-8 they may not be readily available across institutions, and further study is needed to validate their use in diagnosing CECS.
While an objective, validated test or measurement for confirming the diagnosis of CECS remains elusive, the outcomes after surgical treatment of CECS also remain imperfect. Surgery consists of both open and endoscopically assisted fasciotomy of the involved compartments.2,11-17 Reports of improvement after treatment range from 81% to 100%3; however, symptom relief does not come for all patients, particularly those in the military. Waterman and colleagues1 found a failure rate of about 20% among an active-duty military population. Packer and colleagues18 examined civilians with CECS, treated both operatively and nonoperatively. Patients in this series were diagnosed with CECS based on clinical symptoms as well as compartment pressure measurements according to the Pedowitz criteria. Although overall outcomes were better for operatively treated patients than for nonoperatively treated patients, only 47% of patients were completely pain-free, and 21% were unable to return to full activity.
More recent studies have explored use of other nonoperative treatment modalities. Diebal and colleagues19,20 used a running retraining program to treat patients with CECS. They based this treatment on the hypothesis that a heel-strike running pattern is associated with increased anterior compartment pressures.21 CECS patients who underwent a 6-week systematic treatment program focused on forefoot running, stride shortening, and hamstring activation during push-off experienced a decrease in clinical symptoms and posttreatment intracompartmental pressures.20 The improvements in clinical scores were maintained at 1-year follow-up. Another nonoperative intervention, recently described by Isner-Horobeti and colleagues,22 involves injecting botulinum toxin A (BoNT-A) into the anterior and lateral compartments of the leg. Sixteen patients with CECS received BoNT-A injections. On average, intracompartmental pressures were lower after injection than they were before injection. In addition, exertional pain was eliminated in 15 patients at an average follow-up of 4.4 months.
This survey-based study examined the practice patterns of military orthopedic surgeons who performed ICP testing for cases of suspected CECS. Our hypothesis was that, though ICP testing is the most commonly accepted method for confirming the diagnosis of CECS, the ICP testing rate would not be 100% among those surveyed.
The results of our study uncover an apparent inconsistency in survey responses among physicians who evaluate and treat patients with CECS. About 85% of respondents stated they would always recommend confirming the diagnosis of CECS with ICP testing. However, about 40% stated they would recommend surgical treatment without confirmatory testing if they were confident about the diagnosis based on clinical findings. In other words, only 60% of the respondents disagreed or strongly disagreed with pursuing surgical treatment without testing. One would expect a closer correlation between respondents who would always recommend ICP testing and those who disagreed with recommending surgical treatment without ICP testing. This raises the question of what actually occurs when CECS is suspected in clinical practice.
To better understand the apparent discrepancy between respondents who agreed or strongly agreed with always recommending ICP testing and respondents who would recommend treatment without testing, we grouped responses by clinical experience. Although 85% of respondents (no matter the number of years in practice) agreed or strongly agreed with the statement, “I always recommend confirming the diagnosis of CECS with ICP measurements,” 49% of those in practice more than 11 years (vs. 29% of those in practice 5 years or less) agreed or strongly agreed with recommending surgery without testing if they were 100% confident about the diagnosis of CECS based solely on clinical findings. This may suggest that, though most agreed that the gold standard for confirming the diagnosis of CECS remains ICP testing, those with more clinical experience were more comfortable forgoing this diagnostic measure and recommending treatment without testing.
Another measure of clinical experience used in this survey was based on number of CECS patients seen per year. Responses of surgeons who saw 1 or 2 patients with CECS per year were compared with responses of surgeons who saw more than 8 patients with CECS per year. Of the respondents who saw 1 or 2 patients, 86% agreed or strongly agreed with always recommending ICP testing to confirm CECS—comparable to the 75% who saw more than 8 patients. However, of the respondents who saw 1 or 2 patients, 36% indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% who saw more than 8 patients.
Responses regarding the absolute of always recommending ICP testing and the absolute of being 100% confident about the clinical diagnosis of CECS highlight differences between the surgeons with more experience (>11 years in practice, >8 CECS patients per year) and those with less experience (≤5 years in practice, 1 or 2 CECS patients per year). Surgeons in practice longer, and surgeons who saw more patients with suspected CECS per year, were more likely to recommend surgical treatment based solely on clinical findings.
Conclusion
CECS can cause debilitating activity-related leg pain in both civilian and military populations. Treatment with fasciotomy is often curative, but a significant number of patients may continue to have pain and disability. As the incidence of treatment failures may be higher in the military than in civilians, proper evaluation of patients with suspected CECS is particularly important for military orthopedic surgeons. The diagnosis of CECS can be challenging to both the clinician and patient, and diagnostic modalities remain imperfect. The results of this study highlight this, revealing less than 100% agreement regarding use of ICP testing (the gold standard) for diagnosis of CECS.
This study also highlights the need for an improved method of diagnosing CECS since 93% of respondents agreed or strongly agreed that ICP testing is unpleasant for the patient, and 90% would prefer a less-invasive test. In addition, the ICP testing criteria for establishing the diagnosis of CECS remain inconsistent. If a reliable, consistent, and less-invasive test were available, perhaps there would be less variability in practitioners’ evaluations of patients with CECS.
This study shows an inconsistency among military orthopedic surgeons evaluating and treating patients with CECS. As testing modalities for CECS remain imperfect, clinical acumen and experience assume an important role in the assessment of patients with suspected CECS.
Chronic exertional compartment syndrome (CECS) is a common cause of leg pain during exertion in athletic and active-duty populations.1 It is caused by an increase in intramuscular pressure to a point that the tissues within the involved compartment become ischemic because of a decrease in arteriolar blood flow.2 This relative ischemia causes pain and may also be associated with neurologic symptoms. By definition, the pain associated with CECS resolves with rest. Patients typically describe a feeling of fullness or tightness, which eventually evolves into pain as they continue exercising. Pain onset is usually predictable and reproducible after a finite amount of time and/or intensity of exercise.
The differential diagnosis of leg pain during exercise includes CECS, medial tibial stress syndrome, popliteal entrapment syndrome, myopathy, peripheral nerve entrapment syndromes, stress fracture, and effort-induced rhabdomyolysis.3 CECS can be differentiated from other causes of leg pain with measurement of compartment pressures (the standard recommendation).4 Compartment pressure measurement, however, is invasive, time-consuming, and painful and may be associated with bleeding risk, infection, and nerve injury. Noninvasive means of testing for CECS (eg, magnetic resonance imaging [MRI], near-infrared spectroscopy [NIRS], thallium stress testing) remain experimental and expensive and are not easily accessible at all institutions.5-8 While invasive compartment pressure (ICP) testing remains an important tool in the diagnosis of CECS, its criteria and execution vary considerably. Aweid and colleagues4 performed a meta-analysis of use of ICP testing in the diagnosis of CECS and concluded that, though elevated ICP measurements are accepted as the gold standard for diagnosing CECS, the criteria outlined for a positive test lack high-level supporting evidence. In addition, how the test is performed has been inconsistent across studies—further clouding the literature.4
The review by Aweid and colleagues4 highlights the deficiencies in diagnosing CECS by ICP testing. In clinical practice, ICP testing is challenging for both the patient and physician. As other validated, less-invasive tests are lacking, emphasis should remain on the history and the physical examination. Although all athletic populations are at risk for CECS, the active-duty military population is at particularly high risk because of the physical requirements and demands of military service.1,9
We surveyed military orthopedic surgeons to investigate the clinical practice of performing ICP testing in patients with suspected CECS. We hypothesized that the rate of ICP testing among military orthopedic surgeons would not be 100% for patients with the typical signs and symptoms of CECS.
Materials and Methods
This study was approved by the institutional review board at Wright-Patterson Medical Center at Wright-Patterson Air Force Base in Ohio. A link to an online survey was distributed by email to members of the Society of Military Orthopaedic Surgeons. The anonymous survey polled the surgeons regarding basic demographic data and clinical practice as it pertains to the evaluation and treatment of CECS. No patient-protected health information was obtained. Survey results were compiled in a Microsoft Excel file for analysis.
Results
The survey was distributed to 606 email accounts; the response rate was 19% (114/606). Ninety-one surgeons (80%) indicated they have patients with CECS in their practice (Figure 1). Surgeons were asked how many CECS patients they see per year (responses are summarized in Figure 2) and how many years they have been in practice (Table).
Ninety-three percent of the respondents agreed or strongly agreed that ICP testing is unpleasant for the patient (Figure 3), and 90% would prefer a less-invasive test for confirmatory testing for CECS (Figure 4). Only 13% of respondents indicated they actually use noninvasive modalities (eg, MRI, NIRS) to confirm the diagnosis of CECS (Figure 5).
Respondents were asked about the practice of using ICP testing in the diagnosis of CECS (responses are summarized in Figures 6, 7). Although 85% of respondents agreed or strongly agreed with always confirming the diagnosis of CECS with ICP testing, 39% stated they would recommend surgical treatment without ICP testing if they were confident about the diagnosis based on clinical examination findings.
To better understand the apparent discrepancy between the percentage of surgeons who agreed or strongly agreed with always recommending ICP testing (85%) and the percentage who would recommend treatment without testing (39%), responses were stratified by clinical experience. Surgeons in practice more than 11 years (n = 35) were compared with those in practice 5 years or less (n = 31) (Table). Although the vast majority (85%) of respondents from both groups agreed or strongly agreed with always recommending ICP testing, 49% of those in practice more than 11 years and 29% in practice 5 years or less indicated they would recommend surgical treatment for CECS based solely on clinical examination findings (Figures 8, 9).
Responses were also stratified by number of CECS patients seen by each surgeon per year. Twenty-eight respondents saw 1 or 2 patients per year, and 12 saw more than 8 patients per year—31% and 13% of the total number of respondents, respectively. Of the respondents who saw 1 or 2 patients, 86% (24/28) agreed or strongly agreed with always recommending ICP testing—comparable to the 75% (9/12) who saw more than 8 patients (Figure 10). However, of the respondents who saw 1 or 2 patients, 36% (10/28) indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% (9/12) who saw more than 8 patients (Figure 11).
Discussion
CECS is a common cause of leg pain and a significant cause of disability among the active-duty military population. This was illustrated in 2 recent studies by Waterman and colleagues.1,9 The first1 investigated failure rates and disability after surgery for CECS among those on active duty. The authors showed that CECS is a substantial contributor to lower extremity disability in the military population and that there is a substantial risk for persistent symptoms despite surgical treatment. Nearly 1 in 5 patients experienced surgical failure after elective fasciotomy, and about 28% of patients were unable to return to the full activity required in the military. The second, more recent study9 was an epidemiologic study of risk factors associated with CECS in a physically active military population. The authors identified 4100 cases diagnosed between 2006 and 2011—representing an overall annual incidence of 0.49 per 1000 at-risk person-years, or about 683 cases per year; the authors also showed the incidence increased during the time period studied.
The diagnosis of CECS remains imperfect. A clinical history of exercise-induced lower leg pain that is relieved with rest suggests the diagnosis, but a confirmatory test is needed to distinguish CECS from other causes of exercise-induced leg pain. Although direct measurement of compartment pressures is the test used most often, it is invasive and time-consuming, can be uncomfortable for the patient, and may be associated with bleeding risk, infection, and nerve injury. Pedowitz and colleagues10 described the ICP testing criteria now generally used in the diagnosis of CECS. Unfortunately, there is little objective evidence supporting these criteria.4 Although less invasive tests (eg, MRI, NIRS) have been described,5-8 they may not be readily available across institutions, and further study is needed to validate their use in diagnosing CECS.
While an objective, validated test or measurement for confirming the diagnosis of CECS remains elusive, the outcomes after surgical treatment of CECS also remain imperfect. Surgery consists of both open and endoscopically assisted fasciotomy of the involved compartments.2,11-17 Reports of improvement after treatment range from 81% to 100%3; however, symptom relief does not come for all patients, particularly those in the military. Waterman and colleagues1 found a failure rate of about 20% among an active-duty military population. Packer and colleagues18 examined civilians with CECS, treated both operatively and nonoperatively. Patients in this series were diagnosed with CECS based on clinical symptoms as well as compartment pressure measurements according to the Pedowitz criteria. Although overall outcomes were better for operatively treated patients than for nonoperatively treated patients, only 47% of patients were completely pain-free, and 21% were unable to return to full activity.
More recent studies have explored use of other nonoperative treatment modalities. Diebal and colleagues19,20 used a running retraining program to treat patients with CECS. They based this treatment on the hypothesis that a heel-strike running pattern is associated with increased anterior compartment pressures.21 CECS patients who underwent a 6-week systematic treatment program focused on forefoot running, stride shortening, and hamstring activation during push-off experienced a decrease in clinical symptoms and posttreatment intracompartmental pressures.20 The improvements in clinical scores were maintained at 1-year follow-up. Another nonoperative intervention, recently described by Isner-Horobeti and colleagues,22 involves injecting botulinum toxin A (BoNT-A) into the anterior and lateral compartments of the leg. Sixteen patients with CECS received BoNT-A injections. On average, intracompartmental pressures were lower after injection than they were before injection. In addition, exertional pain was eliminated in 15 patients at an average follow-up of 4.4 months.
This survey-based study examined the practice patterns of military orthopedic surgeons who performed ICP testing for cases of suspected CECS. Our hypothesis was that, though ICP testing is the most commonly accepted method for confirming the diagnosis of CECS, the ICP testing rate would not be 100% among those surveyed.
The results of our study uncover an apparent inconsistency in survey responses among physicians who evaluate and treat patients with CECS. About 85% of respondents stated they would always recommend confirming the diagnosis of CECS with ICP testing. However, about 40% stated they would recommend surgical treatment without confirmatory testing if they were confident about the diagnosis based on clinical findings. In other words, only 60% of the respondents disagreed or strongly disagreed with pursuing surgical treatment without testing. One would expect a closer correlation between respondents who would always recommend ICP testing and those who disagreed with recommending surgical treatment without ICP testing. This raises the question of what actually occurs when CECS is suspected in clinical practice.
To better understand the apparent discrepancy between respondents who agreed or strongly agreed with always recommending ICP testing and respondents who would recommend treatment without testing, we grouped responses by clinical experience. Although 85% of respondents (no matter the number of years in practice) agreed or strongly agreed with the statement, “I always recommend confirming the diagnosis of CECS with ICP measurements,” 49% of those in practice more than 11 years (vs. 29% of those in practice 5 years or less) agreed or strongly agreed with recommending surgery without testing if they were 100% confident about the diagnosis of CECS based solely on clinical findings. This may suggest that, though most agreed that the gold standard for confirming the diagnosis of CECS remains ICP testing, those with more clinical experience were more comfortable forgoing this diagnostic measure and recommending treatment without testing.
Another measure of clinical experience used in this survey was based on number of CECS patients seen per year. Responses of surgeons who saw 1 or 2 patients with CECS per year were compared with responses of surgeons who saw more than 8 patients with CECS per year. Of the respondents who saw 1 or 2 patients, 86% agreed or strongly agreed with always recommending ICP testing to confirm CECS—comparable to the 75% who saw more than 8 patients. However, of the respondents who saw 1 or 2 patients, 36% indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% who saw more than 8 patients.
Responses regarding the absolute of always recommending ICP testing and the absolute of being 100% confident about the clinical diagnosis of CECS highlight differences between the surgeons with more experience (>11 years in practice, >8 CECS patients per year) and those with less experience (≤5 years in practice, 1 or 2 CECS patients per year). Surgeons in practice longer, and surgeons who saw more patients with suspected CECS per year, were more likely to recommend surgical treatment based solely on clinical findings.
Conclusion
CECS can cause debilitating activity-related leg pain in both civilian and military populations. Treatment with fasciotomy is often curative, but a significant number of patients may continue to have pain and disability. As the incidence of treatment failures may be higher in the military than in civilians, proper evaluation of patients with suspected CECS is particularly important for military orthopedic surgeons. The diagnosis of CECS can be challenging to both the clinician and patient, and diagnostic modalities remain imperfect. The results of this study highlight this, revealing less than 100% agreement regarding use of ICP testing (the gold standard) for diagnosis of CECS.
This study also highlights the need for an improved method of diagnosing CECS since 93% of respondents agreed or strongly agreed that ICP testing is unpleasant for the patient, and 90% would prefer a less-invasive test. In addition, the ICP testing criteria for establishing the diagnosis of CECS remain inconsistent. If a reliable, consistent, and less-invasive test were available, perhaps there would be less variability in practitioners’ evaluations of patients with CECS.
This study shows an inconsistency among military orthopedic surgeons evaluating and treating patients with CECS. As testing modalities for CECS remain imperfect, clinical acumen and experience assume an important role in the assessment of patients with suspected CECS.
1. Waterman BR, Laughlin M, Kilcoyne K, Cameron KL, Owens BD. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95(7):592-596.
2. Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36-40.
3. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268-276.
4. Aweid O, Del Buono A, Malliaras P, et al. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22(4):356-370.
5. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42(3):385-392.
6. van den Brand JG, Verleisdonk EJ, van der Werken C. Near infrared spectroscopy in the diagnosis of chronic exertional compartment syndrome. Am J Sports Med. 2004;32(2):452-456.
7. van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699-704.
8. Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321-325.
9. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
10. Pedowitz RA, Hargens AR, Mubarek SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35-40.1. Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65(9):1245-1251.
12. Rorabeck CH, Fowler PJ, Nott L. The results of fasciotomy in the management of chronic exertional compartment syndrome. Am J Sports Med. 1988;16(3):224-227.
13. Detmer DE, Sharpe K, Sufit RL, Girdley FM. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13(3):162-170.
14. Stein DA, Sennett BJ. One-portal endoscopically assisted fasciotomy for exertional compartment syndrome. Arthroscopy. 2005;21(1):108-112.
15. Fronek J, Mubarak SJ, Hargens AR, et al. Management of chronic exertional anterior compartment syndrome of the lower extremity. Clin Orthop Relat Res. 1989;(220):217-227.
16. Leversedge FJ, Casey PJ, Seiler JG 3rd, Xerogeanes JW. Endoscopically assisted fasciotomy: description of technique and in vitro assessment of lower-leg compartment decompression. Am J Sports Med. 2002;30(2):272-278.
17. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21(6):811-817.
18. Packer JD, Day MS, Nguyen JT, Hobart SJ, Hannafin JA, Metzl JD. Functional outcomes and patient satisfaction after fasciotomy for chronic exertional compartment syndrome. Am J Sports Med. 2013;41(2):430-436.
19. Diebal AR, Gregory R, Alitz C, Gerber JP. Effects of forefoot running on chronic exertional compartment syndrome: a case series. Int J Sports Phys Ther. 2011;6(4):312-321.
20. Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060-1067.
21. Kirby RL, McDermott AG. Anterior tibial compartment pressures during running with rearfoot and forefoot landing styles. Arch Phys Med Rehabil. 1983;64(7):296-299.
22. Isner-Horobeti ME, Dufour SP, Blaes C, Lecocq J. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41(11):2558-2566.
1. Waterman BR, Laughlin M, Kilcoyne K, Cameron KL, Owens BD. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95(7):592-596.
2. Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36-40.
3. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268-276.
4. Aweid O, Del Buono A, Malliaras P, et al. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22(4):356-370.
5. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42(3):385-392.
6. van den Brand JG, Verleisdonk EJ, van der Werken C. Near infrared spectroscopy in the diagnosis of chronic exertional compartment syndrome. Am J Sports Med. 2004;32(2):452-456.
7. van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699-704.
8. Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321-325.
9. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
10. Pedowitz RA, Hargens AR, Mubarek SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35-40.1. Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65(9):1245-1251.
12. Rorabeck CH, Fowler PJ, Nott L. The results of fasciotomy in the management of chronic exertional compartment syndrome. Am J Sports Med. 1988;16(3):224-227.
13. Detmer DE, Sharpe K, Sufit RL, Girdley FM. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13(3):162-170.
14. Stein DA, Sennett BJ. One-portal endoscopically assisted fasciotomy for exertional compartment syndrome. Arthroscopy. 2005;21(1):108-112.
15. Fronek J, Mubarak SJ, Hargens AR, et al. Management of chronic exertional anterior compartment syndrome of the lower extremity. Clin Orthop Relat Res. 1989;(220):217-227.
16. Leversedge FJ, Casey PJ, Seiler JG 3rd, Xerogeanes JW. Endoscopically assisted fasciotomy: description of technique and in vitro assessment of lower-leg compartment decompression. Am J Sports Med. 2002;30(2):272-278.
17. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21(6):811-817.
18. Packer JD, Day MS, Nguyen JT, Hobart SJ, Hannafin JA, Metzl JD. Functional outcomes and patient satisfaction after fasciotomy for chronic exertional compartment syndrome. Am J Sports Med. 2013;41(2):430-436.
19. Diebal AR, Gregory R, Alitz C, Gerber JP. Effects of forefoot running on chronic exertional compartment syndrome: a case series. Int J Sports Phys Ther. 2011;6(4):312-321.
20. Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060-1067.
21. Kirby RL, McDermott AG. Anterior tibial compartment pressures during running with rearfoot and forefoot landing styles. Arch Phys Med Rehabil. 1983;64(7):296-299.
22. Isner-Horobeti ME, Dufour SP, Blaes C, Lecocq J. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41(11):2558-2566.
Characteristics Associated With Active Defects in Juvenile Spondylolysis
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Business and Practice Management Knowledge Deficiencies in Graduating Orthopedic Residents
With the increasing complexity of health care policy, significant changes in reimbursement and payer sources, and constant push to improve the cost-efficiency of care delivery, there has been a growing focus on the importance of business knowledge and practice management (PM) skills among physicians. Family medicine was the first specialty to require PM training during residency; other specialities have begun implementing business training into their residency curriculum.1 In 1999, the Accreditation Council for Graduate Medical Education (ACGME) identified 6 core competencies that should be included in resident training. One of these core competencies involves training in health care systems and PM.2,3
Residency program directors have also recognized the need for business training among residents. One study that surveyed general surgery program directors found that more than 87% agreed that residents should be trained in business and PM.4 Although these directors recognized the need for training, they also acknowledged the current deficiency: more than 70% thought their current trainees were inadequately trained in business and PM. Similarly, residents and physicians in multiple specialties have reported significant deficiencies in their training and knowledge of PM and business principles.5-11 For example, in a recent survey of ophthalmologists who had been in practice less than 5 years, 70% reported being not very well or not at all trained in overall PM skills during residency.5 Yet, most respondents thought training in this area was the responsibility of the training program.
The call for more business and PM training during residency has been tempered by increasing demands on medical and surgical skills training and time limitations such as duty-hour restrictions. These limitations reinforce the need to find efficient and effective means of teaching necessary business knowledge and PM skills. Paramount to doing this is recognizing the difference between general knowledge and functional knowledge—essentially, what is specifically needed to function effectively in practice.
We conducted a study not only to determine the general level of knowledge that physicians have in different business and PM topics when they complete their residency, but also to evaluate the level of knowledge that graduating physicians need in different business and PM topics in order to function effectively in a medical practice. Toward this end, we developed a novel model that could help determine the level of the functional knowledge deficiency (FKD) of particular business topics. We thought this model would allow us to quantify how much knowledge physicians needed to acquire in a given topic in order to function effectively in practice. We hypothesized that graduating residents would report overall low levels of business knowledge and high FKDs.
Materials and Methods
To minimize variability in the specific type and amount of business training received, we focused this study on a single institution that had maintained a uniform business management curriculum over an extended period. The business training program provided to residents in the orthopedic surgery residency at this institution included 6 hours of didactic lectures on various business topics annually. This program has been in place for more than 15 years and has not undergone any significant changes during that time.
Using the program’s alumni directory, we emailed a cover letter and an 11-question survey to all 332 residents and fellows who had completed their residency or fellowship training at our institution between 1970 and 2008. Anyone who did not reply to the email was mailed a copy of the cover letter and the survey.
The first 4 survey questions involved the demographics of the surgeon and the surgeon’s practice. Subsequent questions focused on the surgeon’s understanding of 9 different general business and PM topics and their importance in the practice. The topics were marketing, business operations, human resources, contract negotiations, malpractice issues, coding/billing, medical records management, accounting, and economic analytical tools. The surgeon was asked to use a 10-point scale ranging from 1 (“knew nothing at all”) to 10 (“complete understanding”) to rate his or her understanding of each topic at the completion of residency. The surgeon was also asked to rate how important it was to understand that topic in the surgeon’s current practice. Again, a 10-point scale was used: 1 (“not important at all”) to 10 (“absolutely vital”) (Figure).
When the surveys were returned, their data were compiled and analyzed to determine the overall knowledge levels for each topic and the levels based on years in practice, type of practice, and level of involvement in PM. We also wanted to determine the amount of business knowledge that they needed in order to function effectively in practice (and that they lacked at time of graduation). We defined this as the FKD at graduation and calculated it as the difference between the surgeon’s reported importance of a topic in his or her current practice and his or her level of understanding of that topic at graduation. A larger FKD score represented greater deficiency, with a maximal possible FKD score of 9. A score of 0 would reflect an appropriate amount of knowledge to function effectively, and a negative score would reflect a knowledge surplus. Using the demographic information from the survey, we were then able to further analyze the levels of overall knowledge as well as the FKD for each topic with respect to length of time in practice, type of practice, and the surgeon’s involvement in PM.
We evaluated the reported levels of knowledge based on both type of practice (academic, hospital-employed, private practice) and who managed the practice (physician, nonphysician). Academic practices were defined as those associated with an academic medical center; hospital-employed practices were those in which the physician was an employee of a health system not associated with an academic medical center; and private practices were defined as physician-owned orthopedic practices not associated with an academic medical center. Regarding management, practices in which physicians were primarily responsible for the daily operations of the practice were considered physician-managed; conversely, practices in which operations were controlled by either employed or institutionally assigned administration were defined as nonphysician-managed.
Statistical analysis of the results for different practice types and levels of involvement in management was performed for both general knowledge and FKD. Means, medians, and standard deviations were calculated. One-way analysis of variance or t tests were then used to examine mean differences overall and within each business topic. When a difference was found, a post hoc Tukey multiple range test was performed to identify it. Differences at P < .05 were considered significant.
Results
One hundred eighty-two surgeons answered the survey, yielding a response rate of 55%. All had completed their training at our institution. Seven respondents were removed from the study because they had retired from practice (5) or had returned incomplete surveys (2).
The overall self-rated level of business knowledge of all responding surgeons at the conclusion of their training was 2.4 on the 10-point scale (Table 1). Specifically, physicians reported the lowest levels of business understanding in economic analytical tools (1.5), human resources (1.7), and contract negotiations (1.9), suggesting minimal knowledge of these topics generally. They reported the highest levels of knowledge in medical records management (3.8) and malpractice issues (3.3). Even these topics, however, still reflected overall low levels of knowledge.
There was no statistically significant difference between private practice and academic physicians. In addition, surgeons in physician-managed practices reported significantly (P = .045) higher levels of understanding of economic analytical tools than surgeons in nonphysician-managed practices (Table 1). There were no other statistically significant differences among groups.
The overall calculated FKD for all surgeons was 5.6. FKDs were calculated for all 9 business topics. The worst FKDs were in business operations (6.4) and coding/billing (6.3). The topic with the least deficiency (lowest FKD) was in medical records management (4.2) (Table 2).
Surgeons’ FKDs based on practice type (academic, hospital-employed, private practice) were compared to identify potentially significant differences. Hospital-employed physicians had the lowest overall FKD (4.0), followed by physicians in academic practices (5.1) and private practices (5.9). Hospital-employed physicians reported statistically significantly better (lower) FKDs in comparison with physicians in private practice in multiple topics, including human resources, contract negotiations, malpractice issues, coding/billing, and accounting (Table 2). Similarly, physicians in academic practices also had statistically significantly better FKDs than physicians in private practice in the topics of business operations, contract negotiations, and billing/coding. Compared with hospital-employed physicians, physicians in academic practices had significantly more knowledge about marketing, business operations, and accounting. Physicians in private practice did not have significantly better FKDs in any topic in comparison with hospital-employed or academic physicians. There was no significant difference in FKDs for medical records management or economic analytical tools based on practice type.
Comparisons based on PM involvement showed that physicians in practices with nonphysician management had only a slightly better FKD (5.6) at graduation than those in practices with physician involvement (5.7). None of the 9 topics was statistically significant different based on physician involvement in PM.
Discussion
Building a successful medical practice has become more difficult for graduating orthopedic surgery residents because of an increasingly complex health care system, shrinking reimbursement rates, and looming regulatory changes. These challenges have reinforced the importance of teaching residents the necessary PM knowledge and skills to function effectively in a medical practice. Multiple studies from different specialties surveying or testing graduating residents and young practicing physicians on their business management knowledge or specific business topics have shown severe deficiencies.5-11 Unfortunately, graduating orthopedic surgery residents also appear inadequately prepared in PM. In a study of resident coding/billing knowledge, Gill and Schutt6 surveyed 2006 graduating orthopedic residents and found that only 13% felt confident in their coding ability. Our study results add to our understanding of multiple PM topics and demonstrate graduating orthopedic residents’ deficiencies throughout these topics.
Increased efforts to develop business management training programs and curricula have helped improve both overall PM and business knowledge in other specialties.12-15 ACGME now requires 100 hours of PM training among family medicine residency programs.16 A curriculum instituted in a general surgery residency focused on improving coding found that accuracy improved from 36% to 88% over 12 months.13 A family practice residency instituted a “simulated practice” model for its residents to improve practical PM learning and found statistically significant improvement over their prior didactic lectures.15 However, there continues to be significant variability in the topics and methods covered in business management curricula as programs struggle to determine how to most effectively use their limited time to prepare graduating residents.
In this study, we introduced the concept of FKD. With limited time available for teaching business knowledge and PM skills in residency, it has become imperative that training be efficient and effective. The FKD model can improve training efficiency by directing training to the topics that will produce the highest yield in preparing physicians for practice. As our results demonstrate, topics with the lowest levels of knowledge among surgeons often are not the same as the topics that are most needed to function effectively in practice (Table 3). The FKD model identifies deficiencies in practical, applicable knowledge rather than focusing on a general knowledge level. We suspect that focusing on topics with a high FKD would provide a higher yield in preparing physicians for practice. As such, our results suggest that training in business operations and coding/billing would likely provide the highest practical value, despite the fact that these were not the areas of least general knowledge.
Another finding of this study was the FKD difference based on type of practice. Compared with private practice physicians, hospital-employed or academic physicians had substantially lower overall FKDs and significantly lower FKDs in several specific topics. However, these FKD differences exist despite minimal differences in overall levels of knowledge. This would suggest that less business knowledge was needed by physicians to enter these types of practices compared with traditional private practice. We speculate that this may be one factor influencing the recent trend by graduating orthopedic residents to take hospital-employed positions, as these positions may appear less demanding in terms of learning the management aspects of the new practice.
Our results also showed slightly higher reported average business knowledge and lower FKD reported by those who had recently completed training (within 2-5 years) versus those in practice much longer. This is particularly interesting, as our institution has maintained the same lecture-based program for many years without significant changes. Although these differences may not be statistically significant, they may reflect an increased interest in and attention to learning PM skills while in training. However, we acknowledge this is only one of many possible explanations for these findings.
This study had several limitations. First, all respondents were graduates of a single institution. We were trying to limit the variability in business training, but this also limits the scope of the results. Second, self-ratings on surveys provide subjective measures of business knowledge and functional knowledge. Scores may vary based on individuals’ understanding of given topics, or they may inaccurately represent their level of understanding. This is especially true of respondents who graduated from residency, for example, 20 years earlier—their survey responses may reflect erroneous recollection of business training at time of graduation compared with respondents who graduated more recently. Conversely, more recent graduates may not have a fully formed or accurate picture of how much business knowledge is required to function in practice. Nevertheless, we found no significant differences in measured parameters based on graduation date, so we chose not to exclude older respondents, which also may have weakened our data pool. Further, FKDs are relative values used to compare subjective deficiencies rather than absolute scores of specific general knowledge. As such, subjectivity, including recollection of business training, is inherent in the model used in this study.
Conclusion
Graduating orthopedic surgeons currently appear inadequately prepared to effectively manage business issues in their practices, as evidenced by their low overall knowledge levels and high FKDs. The novel FKD model described in this study helps define FKD levels and identify topics that may provide the highest yield in improving effectiveness in practice. Residency curricula focused on improving business and PM knowledge, particularly in the topics with the highest FKDs (eg, business operations, coding/billing), may improve training efficiency in these areas. Further studies with larger numbers of physicians across multiple institutions are needed to confirm these findings and to validate the FKD concept.
1. Rose EA, Neale AV, Rathur WA. Teaching practice management during residency. Fam Med. 1999;31(2):107-113.
2. Accreditation Council for Graduate Medical Education. ACGME common program requirements. http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Updated June 9, 2013. Accessed August 25, 2015.
3. Itani K. A positive approach to core competencies and benchmarks for graduate medical education. Am J Surg. 2002;184(3):196-203.
4. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
5. McDonnell PJ, Kirwan TJ, Brinton GS, et al. Perceptions of recent ophthalmology residency graduates regarding preparation for practice. Ophthalmology. 2007;114(2):387-391.
6. Gill JB, Schutt RC Jr. Practice management education in orthopaedic surgical residencies. J Bone Joint Surg Am. 2007;89(1):216-219.
7. Satiani B. Business knowledge in surgeons. Am J Surg. 2004;188(1):13-16.
8. Cantor JC, Baker LC, Hughes RG. Preparedness for practice. Young physicians’ views of their professional education. JAMA. 1993;270(9):1035-1040.
9. Fakhry SM, Robinson L, Hendershot K, Reines HD. Surgical residents’ knowledge of documentation and coding for professional services: an opportunity for a focused educational offering. Am J Surg. 2007;194(2):263-267.
10. Williford LE, Ling FW, Summitt RL Jr, Stovall TG. Practice management in obstetrics and gynecology residency curriculum. Obstet Gynecol. 1999;94(3):476-479.
11. Andreae MC, Dunham K, Freed GL. Inadequate training in billing and coding as perceived by recent pediatric graduates. Clin Pediatr. 2009;48(9):939-944.
12. Kolva DE, Barzee KA, Morley CP. Practice management residency curricula: a systematic literature review. Fam Med. 2009;41(6):411-419.
13. Jones K, Lebron RA, Mangram A, Dunn E. Practice management education during surgical residency. Am J Surg. 2008;196(6):878-881.
14. Kerfoot BP, Conlin PR, Travison T, McMahon GT. Web-based education in systems-based practice: a randomized trial. Arch Intern Med. 2007;167(4):361-366.
15. LoPresti L, Ginn P, Treat R. Using a simulated practice to improve practice management learning. Fam Med. 2009;41(9):640-645.
16. Accreditation Council for Graduate Medical Education. Family medicine program requirements. https://www.acgme.org/acgmeweb/tabid/132/ProgramandInstitutionalAccreditation/MedicalSpecialties/FamilyMedicine.aspx. Accessed September 23, 2015.
With the increasing complexity of health care policy, significant changes in reimbursement and payer sources, and constant push to improve the cost-efficiency of care delivery, there has been a growing focus on the importance of business knowledge and practice management (PM) skills among physicians. Family medicine was the first specialty to require PM training during residency; other specialities have begun implementing business training into their residency curriculum.1 In 1999, the Accreditation Council for Graduate Medical Education (ACGME) identified 6 core competencies that should be included in resident training. One of these core competencies involves training in health care systems and PM.2,3
Residency program directors have also recognized the need for business training among residents. One study that surveyed general surgery program directors found that more than 87% agreed that residents should be trained in business and PM.4 Although these directors recognized the need for training, they also acknowledged the current deficiency: more than 70% thought their current trainees were inadequately trained in business and PM. Similarly, residents and physicians in multiple specialties have reported significant deficiencies in their training and knowledge of PM and business principles.5-11 For example, in a recent survey of ophthalmologists who had been in practice less than 5 years, 70% reported being not very well or not at all trained in overall PM skills during residency.5 Yet, most respondents thought training in this area was the responsibility of the training program.
The call for more business and PM training during residency has been tempered by increasing demands on medical and surgical skills training and time limitations such as duty-hour restrictions. These limitations reinforce the need to find efficient and effective means of teaching necessary business knowledge and PM skills. Paramount to doing this is recognizing the difference between general knowledge and functional knowledge—essentially, what is specifically needed to function effectively in practice.
We conducted a study not only to determine the general level of knowledge that physicians have in different business and PM topics when they complete their residency, but also to evaluate the level of knowledge that graduating physicians need in different business and PM topics in order to function effectively in a medical practice. Toward this end, we developed a novel model that could help determine the level of the functional knowledge deficiency (FKD) of particular business topics. We thought this model would allow us to quantify how much knowledge physicians needed to acquire in a given topic in order to function effectively in practice. We hypothesized that graduating residents would report overall low levels of business knowledge and high FKDs.
Materials and Methods
To minimize variability in the specific type and amount of business training received, we focused this study on a single institution that had maintained a uniform business management curriculum over an extended period. The business training program provided to residents in the orthopedic surgery residency at this institution included 6 hours of didactic lectures on various business topics annually. This program has been in place for more than 15 years and has not undergone any significant changes during that time.
Using the program’s alumni directory, we emailed a cover letter and an 11-question survey to all 332 residents and fellows who had completed their residency or fellowship training at our institution between 1970 and 2008. Anyone who did not reply to the email was mailed a copy of the cover letter and the survey.
The first 4 survey questions involved the demographics of the surgeon and the surgeon’s practice. Subsequent questions focused on the surgeon’s understanding of 9 different general business and PM topics and their importance in the practice. The topics were marketing, business operations, human resources, contract negotiations, malpractice issues, coding/billing, medical records management, accounting, and economic analytical tools. The surgeon was asked to use a 10-point scale ranging from 1 (“knew nothing at all”) to 10 (“complete understanding”) to rate his or her understanding of each topic at the completion of residency. The surgeon was also asked to rate how important it was to understand that topic in the surgeon’s current practice. Again, a 10-point scale was used: 1 (“not important at all”) to 10 (“absolutely vital”) (Figure).
When the surveys were returned, their data were compiled and analyzed to determine the overall knowledge levels for each topic and the levels based on years in practice, type of practice, and level of involvement in PM. We also wanted to determine the amount of business knowledge that they needed in order to function effectively in practice (and that they lacked at time of graduation). We defined this as the FKD at graduation and calculated it as the difference between the surgeon’s reported importance of a topic in his or her current practice and his or her level of understanding of that topic at graduation. A larger FKD score represented greater deficiency, with a maximal possible FKD score of 9. A score of 0 would reflect an appropriate amount of knowledge to function effectively, and a negative score would reflect a knowledge surplus. Using the demographic information from the survey, we were then able to further analyze the levels of overall knowledge as well as the FKD for each topic with respect to length of time in practice, type of practice, and the surgeon’s involvement in PM.
We evaluated the reported levels of knowledge based on both type of practice (academic, hospital-employed, private practice) and who managed the practice (physician, nonphysician). Academic practices were defined as those associated with an academic medical center; hospital-employed practices were those in which the physician was an employee of a health system not associated with an academic medical center; and private practices were defined as physician-owned orthopedic practices not associated with an academic medical center. Regarding management, practices in which physicians were primarily responsible for the daily operations of the practice were considered physician-managed; conversely, practices in which operations were controlled by either employed or institutionally assigned administration were defined as nonphysician-managed.
Statistical analysis of the results for different practice types and levels of involvement in management was performed for both general knowledge and FKD. Means, medians, and standard deviations were calculated. One-way analysis of variance or t tests were then used to examine mean differences overall and within each business topic. When a difference was found, a post hoc Tukey multiple range test was performed to identify it. Differences at P < .05 were considered significant.
Results
One hundred eighty-two surgeons answered the survey, yielding a response rate of 55%. All had completed their training at our institution. Seven respondents were removed from the study because they had retired from practice (5) or had returned incomplete surveys (2).
The overall self-rated level of business knowledge of all responding surgeons at the conclusion of their training was 2.4 on the 10-point scale (Table 1). Specifically, physicians reported the lowest levels of business understanding in economic analytical tools (1.5), human resources (1.7), and contract negotiations (1.9), suggesting minimal knowledge of these topics generally. They reported the highest levels of knowledge in medical records management (3.8) and malpractice issues (3.3). Even these topics, however, still reflected overall low levels of knowledge.
There was no statistically significant difference between private practice and academic physicians. In addition, surgeons in physician-managed practices reported significantly (P = .045) higher levels of understanding of economic analytical tools than surgeons in nonphysician-managed practices (Table 1). There were no other statistically significant differences among groups.
The overall calculated FKD for all surgeons was 5.6. FKDs were calculated for all 9 business topics. The worst FKDs were in business operations (6.4) and coding/billing (6.3). The topic with the least deficiency (lowest FKD) was in medical records management (4.2) (Table 2).
Surgeons’ FKDs based on practice type (academic, hospital-employed, private practice) were compared to identify potentially significant differences. Hospital-employed physicians had the lowest overall FKD (4.0), followed by physicians in academic practices (5.1) and private practices (5.9). Hospital-employed physicians reported statistically significantly better (lower) FKDs in comparison with physicians in private practice in multiple topics, including human resources, contract negotiations, malpractice issues, coding/billing, and accounting (Table 2). Similarly, physicians in academic practices also had statistically significantly better FKDs than physicians in private practice in the topics of business operations, contract negotiations, and billing/coding. Compared with hospital-employed physicians, physicians in academic practices had significantly more knowledge about marketing, business operations, and accounting. Physicians in private practice did not have significantly better FKDs in any topic in comparison with hospital-employed or academic physicians. There was no significant difference in FKDs for medical records management or economic analytical tools based on practice type.
Comparisons based on PM involvement showed that physicians in practices with nonphysician management had only a slightly better FKD (5.6) at graduation than those in practices with physician involvement (5.7). None of the 9 topics was statistically significant different based on physician involvement in PM.
Discussion
Building a successful medical practice has become more difficult for graduating orthopedic surgery residents because of an increasingly complex health care system, shrinking reimbursement rates, and looming regulatory changes. These challenges have reinforced the importance of teaching residents the necessary PM knowledge and skills to function effectively in a medical practice. Multiple studies from different specialties surveying or testing graduating residents and young practicing physicians on their business management knowledge or specific business topics have shown severe deficiencies.5-11 Unfortunately, graduating orthopedic surgery residents also appear inadequately prepared in PM. In a study of resident coding/billing knowledge, Gill and Schutt6 surveyed 2006 graduating orthopedic residents and found that only 13% felt confident in their coding ability. Our study results add to our understanding of multiple PM topics and demonstrate graduating orthopedic residents’ deficiencies throughout these topics.
Increased efforts to develop business management training programs and curricula have helped improve both overall PM and business knowledge in other specialties.12-15 ACGME now requires 100 hours of PM training among family medicine residency programs.16 A curriculum instituted in a general surgery residency focused on improving coding found that accuracy improved from 36% to 88% over 12 months.13 A family practice residency instituted a “simulated practice” model for its residents to improve practical PM learning and found statistically significant improvement over their prior didactic lectures.15 However, there continues to be significant variability in the topics and methods covered in business management curricula as programs struggle to determine how to most effectively use their limited time to prepare graduating residents.
In this study, we introduced the concept of FKD. With limited time available for teaching business knowledge and PM skills in residency, it has become imperative that training be efficient and effective. The FKD model can improve training efficiency by directing training to the topics that will produce the highest yield in preparing physicians for practice. As our results demonstrate, topics with the lowest levels of knowledge among surgeons often are not the same as the topics that are most needed to function effectively in practice (Table 3). The FKD model identifies deficiencies in practical, applicable knowledge rather than focusing on a general knowledge level. We suspect that focusing on topics with a high FKD would provide a higher yield in preparing physicians for practice. As such, our results suggest that training in business operations and coding/billing would likely provide the highest practical value, despite the fact that these were not the areas of least general knowledge.
Another finding of this study was the FKD difference based on type of practice. Compared with private practice physicians, hospital-employed or academic physicians had substantially lower overall FKDs and significantly lower FKDs in several specific topics. However, these FKD differences exist despite minimal differences in overall levels of knowledge. This would suggest that less business knowledge was needed by physicians to enter these types of practices compared with traditional private practice. We speculate that this may be one factor influencing the recent trend by graduating orthopedic residents to take hospital-employed positions, as these positions may appear less demanding in terms of learning the management aspects of the new practice.
Our results also showed slightly higher reported average business knowledge and lower FKD reported by those who had recently completed training (within 2-5 years) versus those in practice much longer. This is particularly interesting, as our institution has maintained the same lecture-based program for many years without significant changes. Although these differences may not be statistically significant, they may reflect an increased interest in and attention to learning PM skills while in training. However, we acknowledge this is only one of many possible explanations for these findings.
This study had several limitations. First, all respondents were graduates of a single institution. We were trying to limit the variability in business training, but this also limits the scope of the results. Second, self-ratings on surveys provide subjective measures of business knowledge and functional knowledge. Scores may vary based on individuals’ understanding of given topics, or they may inaccurately represent their level of understanding. This is especially true of respondents who graduated from residency, for example, 20 years earlier—their survey responses may reflect erroneous recollection of business training at time of graduation compared with respondents who graduated more recently. Conversely, more recent graduates may not have a fully formed or accurate picture of how much business knowledge is required to function in practice. Nevertheless, we found no significant differences in measured parameters based on graduation date, so we chose not to exclude older respondents, which also may have weakened our data pool. Further, FKDs are relative values used to compare subjective deficiencies rather than absolute scores of specific general knowledge. As such, subjectivity, including recollection of business training, is inherent in the model used in this study.
Conclusion
Graduating orthopedic surgeons currently appear inadequately prepared to effectively manage business issues in their practices, as evidenced by their low overall knowledge levels and high FKDs. The novel FKD model described in this study helps define FKD levels and identify topics that may provide the highest yield in improving effectiveness in practice. Residency curricula focused on improving business and PM knowledge, particularly in the topics with the highest FKDs (eg, business operations, coding/billing), may improve training efficiency in these areas. Further studies with larger numbers of physicians across multiple institutions are needed to confirm these findings and to validate the FKD concept.
With the increasing complexity of health care policy, significant changes in reimbursement and payer sources, and constant push to improve the cost-efficiency of care delivery, there has been a growing focus on the importance of business knowledge and practice management (PM) skills among physicians. Family medicine was the first specialty to require PM training during residency; other specialities have begun implementing business training into their residency curriculum.1 In 1999, the Accreditation Council for Graduate Medical Education (ACGME) identified 6 core competencies that should be included in resident training. One of these core competencies involves training in health care systems and PM.2,3
Residency program directors have also recognized the need for business training among residents. One study that surveyed general surgery program directors found that more than 87% agreed that residents should be trained in business and PM.4 Although these directors recognized the need for training, they also acknowledged the current deficiency: more than 70% thought their current trainees were inadequately trained in business and PM. Similarly, residents and physicians in multiple specialties have reported significant deficiencies in their training and knowledge of PM and business principles.5-11 For example, in a recent survey of ophthalmologists who had been in practice less than 5 years, 70% reported being not very well or not at all trained in overall PM skills during residency.5 Yet, most respondents thought training in this area was the responsibility of the training program.
The call for more business and PM training during residency has been tempered by increasing demands on medical and surgical skills training and time limitations such as duty-hour restrictions. These limitations reinforce the need to find efficient and effective means of teaching necessary business knowledge and PM skills. Paramount to doing this is recognizing the difference between general knowledge and functional knowledge—essentially, what is specifically needed to function effectively in practice.
We conducted a study not only to determine the general level of knowledge that physicians have in different business and PM topics when they complete their residency, but also to evaluate the level of knowledge that graduating physicians need in different business and PM topics in order to function effectively in a medical practice. Toward this end, we developed a novel model that could help determine the level of the functional knowledge deficiency (FKD) of particular business topics. We thought this model would allow us to quantify how much knowledge physicians needed to acquire in a given topic in order to function effectively in practice. We hypothesized that graduating residents would report overall low levels of business knowledge and high FKDs.
Materials and Methods
To minimize variability in the specific type and amount of business training received, we focused this study on a single institution that had maintained a uniform business management curriculum over an extended period. The business training program provided to residents in the orthopedic surgery residency at this institution included 6 hours of didactic lectures on various business topics annually. This program has been in place for more than 15 years and has not undergone any significant changes during that time.
Using the program’s alumni directory, we emailed a cover letter and an 11-question survey to all 332 residents and fellows who had completed their residency or fellowship training at our institution between 1970 and 2008. Anyone who did not reply to the email was mailed a copy of the cover letter and the survey.
The first 4 survey questions involved the demographics of the surgeon and the surgeon’s practice. Subsequent questions focused on the surgeon’s understanding of 9 different general business and PM topics and their importance in the practice. The topics were marketing, business operations, human resources, contract negotiations, malpractice issues, coding/billing, medical records management, accounting, and economic analytical tools. The surgeon was asked to use a 10-point scale ranging from 1 (“knew nothing at all”) to 10 (“complete understanding”) to rate his or her understanding of each topic at the completion of residency. The surgeon was also asked to rate how important it was to understand that topic in the surgeon’s current practice. Again, a 10-point scale was used: 1 (“not important at all”) to 10 (“absolutely vital”) (Figure).
When the surveys were returned, their data were compiled and analyzed to determine the overall knowledge levels for each topic and the levels based on years in practice, type of practice, and level of involvement in PM. We also wanted to determine the amount of business knowledge that they needed in order to function effectively in practice (and that they lacked at time of graduation). We defined this as the FKD at graduation and calculated it as the difference between the surgeon’s reported importance of a topic in his or her current practice and his or her level of understanding of that topic at graduation. A larger FKD score represented greater deficiency, with a maximal possible FKD score of 9. A score of 0 would reflect an appropriate amount of knowledge to function effectively, and a negative score would reflect a knowledge surplus. Using the demographic information from the survey, we were then able to further analyze the levels of overall knowledge as well as the FKD for each topic with respect to length of time in practice, type of practice, and the surgeon’s involvement in PM.
We evaluated the reported levels of knowledge based on both type of practice (academic, hospital-employed, private practice) and who managed the practice (physician, nonphysician). Academic practices were defined as those associated with an academic medical center; hospital-employed practices were those in which the physician was an employee of a health system not associated with an academic medical center; and private practices were defined as physician-owned orthopedic practices not associated with an academic medical center. Regarding management, practices in which physicians were primarily responsible for the daily operations of the practice were considered physician-managed; conversely, practices in which operations were controlled by either employed or institutionally assigned administration were defined as nonphysician-managed.
Statistical analysis of the results for different practice types and levels of involvement in management was performed for both general knowledge and FKD. Means, medians, and standard deviations were calculated. One-way analysis of variance or t tests were then used to examine mean differences overall and within each business topic. When a difference was found, a post hoc Tukey multiple range test was performed to identify it. Differences at P < .05 were considered significant.
Results
One hundred eighty-two surgeons answered the survey, yielding a response rate of 55%. All had completed their training at our institution. Seven respondents were removed from the study because they had retired from practice (5) or had returned incomplete surveys (2).
The overall self-rated level of business knowledge of all responding surgeons at the conclusion of their training was 2.4 on the 10-point scale (Table 1). Specifically, physicians reported the lowest levels of business understanding in economic analytical tools (1.5), human resources (1.7), and contract negotiations (1.9), suggesting minimal knowledge of these topics generally. They reported the highest levels of knowledge in medical records management (3.8) and malpractice issues (3.3). Even these topics, however, still reflected overall low levels of knowledge.
There was no statistically significant difference between private practice and academic physicians. In addition, surgeons in physician-managed practices reported significantly (P = .045) higher levels of understanding of economic analytical tools than surgeons in nonphysician-managed practices (Table 1). There were no other statistically significant differences among groups.
The overall calculated FKD for all surgeons was 5.6. FKDs were calculated for all 9 business topics. The worst FKDs were in business operations (6.4) and coding/billing (6.3). The topic with the least deficiency (lowest FKD) was in medical records management (4.2) (Table 2).
Surgeons’ FKDs based on practice type (academic, hospital-employed, private practice) were compared to identify potentially significant differences. Hospital-employed physicians had the lowest overall FKD (4.0), followed by physicians in academic practices (5.1) and private practices (5.9). Hospital-employed physicians reported statistically significantly better (lower) FKDs in comparison with physicians in private practice in multiple topics, including human resources, contract negotiations, malpractice issues, coding/billing, and accounting (Table 2). Similarly, physicians in academic practices also had statistically significantly better FKDs than physicians in private practice in the topics of business operations, contract negotiations, and billing/coding. Compared with hospital-employed physicians, physicians in academic practices had significantly more knowledge about marketing, business operations, and accounting. Physicians in private practice did not have significantly better FKDs in any topic in comparison with hospital-employed or academic physicians. There was no significant difference in FKDs for medical records management or economic analytical tools based on practice type.
Comparisons based on PM involvement showed that physicians in practices with nonphysician management had only a slightly better FKD (5.6) at graduation than those in practices with physician involvement (5.7). None of the 9 topics was statistically significant different based on physician involvement in PM.
Discussion
Building a successful medical practice has become more difficult for graduating orthopedic surgery residents because of an increasingly complex health care system, shrinking reimbursement rates, and looming regulatory changes. These challenges have reinforced the importance of teaching residents the necessary PM knowledge and skills to function effectively in a medical practice. Multiple studies from different specialties surveying or testing graduating residents and young practicing physicians on their business management knowledge or specific business topics have shown severe deficiencies.5-11 Unfortunately, graduating orthopedic surgery residents also appear inadequately prepared in PM. In a study of resident coding/billing knowledge, Gill and Schutt6 surveyed 2006 graduating orthopedic residents and found that only 13% felt confident in their coding ability. Our study results add to our understanding of multiple PM topics and demonstrate graduating orthopedic residents’ deficiencies throughout these topics.
Increased efforts to develop business management training programs and curricula have helped improve both overall PM and business knowledge in other specialties.12-15 ACGME now requires 100 hours of PM training among family medicine residency programs.16 A curriculum instituted in a general surgery residency focused on improving coding found that accuracy improved from 36% to 88% over 12 months.13 A family practice residency instituted a “simulated practice” model for its residents to improve practical PM learning and found statistically significant improvement over their prior didactic lectures.15 However, there continues to be significant variability in the topics and methods covered in business management curricula as programs struggle to determine how to most effectively use their limited time to prepare graduating residents.
In this study, we introduced the concept of FKD. With limited time available for teaching business knowledge and PM skills in residency, it has become imperative that training be efficient and effective. The FKD model can improve training efficiency by directing training to the topics that will produce the highest yield in preparing physicians for practice. As our results demonstrate, topics with the lowest levels of knowledge among surgeons often are not the same as the topics that are most needed to function effectively in practice (Table 3). The FKD model identifies deficiencies in practical, applicable knowledge rather than focusing on a general knowledge level. We suspect that focusing on topics with a high FKD would provide a higher yield in preparing physicians for practice. As such, our results suggest that training in business operations and coding/billing would likely provide the highest practical value, despite the fact that these were not the areas of least general knowledge.
Another finding of this study was the FKD difference based on type of practice. Compared with private practice physicians, hospital-employed or academic physicians had substantially lower overall FKDs and significantly lower FKDs in several specific topics. However, these FKD differences exist despite minimal differences in overall levels of knowledge. This would suggest that less business knowledge was needed by physicians to enter these types of practices compared with traditional private practice. We speculate that this may be one factor influencing the recent trend by graduating orthopedic residents to take hospital-employed positions, as these positions may appear less demanding in terms of learning the management aspects of the new practice.
Our results also showed slightly higher reported average business knowledge and lower FKD reported by those who had recently completed training (within 2-5 years) versus those in practice much longer. This is particularly interesting, as our institution has maintained the same lecture-based program for many years without significant changes. Although these differences may not be statistically significant, they may reflect an increased interest in and attention to learning PM skills while in training. However, we acknowledge this is only one of many possible explanations for these findings.
This study had several limitations. First, all respondents were graduates of a single institution. We were trying to limit the variability in business training, but this also limits the scope of the results. Second, self-ratings on surveys provide subjective measures of business knowledge and functional knowledge. Scores may vary based on individuals’ understanding of given topics, or they may inaccurately represent their level of understanding. This is especially true of respondents who graduated from residency, for example, 20 years earlier—their survey responses may reflect erroneous recollection of business training at time of graduation compared with respondents who graduated more recently. Conversely, more recent graduates may not have a fully formed or accurate picture of how much business knowledge is required to function in practice. Nevertheless, we found no significant differences in measured parameters based on graduation date, so we chose not to exclude older respondents, which also may have weakened our data pool. Further, FKDs are relative values used to compare subjective deficiencies rather than absolute scores of specific general knowledge. As such, subjectivity, including recollection of business training, is inherent in the model used in this study.
Conclusion
Graduating orthopedic surgeons currently appear inadequately prepared to effectively manage business issues in their practices, as evidenced by their low overall knowledge levels and high FKDs. The novel FKD model described in this study helps define FKD levels and identify topics that may provide the highest yield in improving effectiveness in practice. Residency curricula focused on improving business and PM knowledge, particularly in the topics with the highest FKDs (eg, business operations, coding/billing), may improve training efficiency in these areas. Further studies with larger numbers of physicians across multiple institutions are needed to confirm these findings and to validate the FKD concept.
1. Rose EA, Neale AV, Rathur WA. Teaching practice management during residency. Fam Med. 1999;31(2):107-113.
2. Accreditation Council for Graduate Medical Education. ACGME common program requirements. http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Updated June 9, 2013. Accessed August 25, 2015.
3. Itani K. A positive approach to core competencies and benchmarks for graduate medical education. Am J Surg. 2002;184(3):196-203.
4. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
5. McDonnell PJ, Kirwan TJ, Brinton GS, et al. Perceptions of recent ophthalmology residency graduates regarding preparation for practice. Ophthalmology. 2007;114(2):387-391.
6. Gill JB, Schutt RC Jr. Practice management education in orthopaedic surgical residencies. J Bone Joint Surg Am. 2007;89(1):216-219.
7. Satiani B. Business knowledge in surgeons. Am J Surg. 2004;188(1):13-16.
8. Cantor JC, Baker LC, Hughes RG. Preparedness for practice. Young physicians’ views of their professional education. JAMA. 1993;270(9):1035-1040.
9. Fakhry SM, Robinson L, Hendershot K, Reines HD. Surgical residents’ knowledge of documentation and coding for professional services: an opportunity for a focused educational offering. Am J Surg. 2007;194(2):263-267.
10. Williford LE, Ling FW, Summitt RL Jr, Stovall TG. Practice management in obstetrics and gynecology residency curriculum. Obstet Gynecol. 1999;94(3):476-479.
11. Andreae MC, Dunham K, Freed GL. Inadequate training in billing and coding as perceived by recent pediatric graduates. Clin Pediatr. 2009;48(9):939-944.
12. Kolva DE, Barzee KA, Morley CP. Practice management residency curricula: a systematic literature review. Fam Med. 2009;41(6):411-419.
13. Jones K, Lebron RA, Mangram A, Dunn E. Practice management education during surgical residency. Am J Surg. 2008;196(6):878-881.
14. Kerfoot BP, Conlin PR, Travison T, McMahon GT. Web-based education in systems-based practice: a randomized trial. Arch Intern Med. 2007;167(4):361-366.
15. LoPresti L, Ginn P, Treat R. Using a simulated practice to improve practice management learning. Fam Med. 2009;41(9):640-645.
16. Accreditation Council for Graduate Medical Education. Family medicine program requirements. https://www.acgme.org/acgmeweb/tabid/132/ProgramandInstitutionalAccreditation/MedicalSpecialties/FamilyMedicine.aspx. Accessed September 23, 2015.
1. Rose EA, Neale AV, Rathur WA. Teaching practice management during residency. Fam Med. 1999;31(2):107-113.
2. Accreditation Council for Graduate Medical Education. ACGME common program requirements. http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Updated June 9, 2013. Accessed August 25, 2015.
3. Itani K. A positive approach to core competencies and benchmarks for graduate medical education. Am J Surg. 2002;184(3):196-203.
4. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
5. McDonnell PJ, Kirwan TJ, Brinton GS, et al. Perceptions of recent ophthalmology residency graduates regarding preparation for practice. Ophthalmology. 2007;114(2):387-391.
6. Gill JB, Schutt RC Jr. Practice management education in orthopaedic surgical residencies. J Bone Joint Surg Am. 2007;89(1):216-219.
7. Satiani B. Business knowledge in surgeons. Am J Surg. 2004;188(1):13-16.
8. Cantor JC, Baker LC, Hughes RG. Preparedness for practice. Young physicians’ views of their professional education. JAMA. 1993;270(9):1035-1040.
9. Fakhry SM, Robinson L, Hendershot K, Reines HD. Surgical residents’ knowledge of documentation and coding for professional services: an opportunity for a focused educational offering. Am J Surg. 2007;194(2):263-267.
10. Williford LE, Ling FW, Summitt RL Jr, Stovall TG. Practice management in obstetrics and gynecology residency curriculum. Obstet Gynecol. 1999;94(3):476-479.
11. Andreae MC, Dunham K, Freed GL. Inadequate training in billing and coding as perceived by recent pediatric graduates. Clin Pediatr. 2009;48(9):939-944.
12. Kolva DE, Barzee KA, Morley CP. Practice management residency curricula: a systematic literature review. Fam Med. 2009;41(6):411-419.
13. Jones K, Lebron RA, Mangram A, Dunn E. Practice management education during surgical residency. Am J Surg. 2008;196(6):878-881.
14. Kerfoot BP, Conlin PR, Travison T, McMahon GT. Web-based education in systems-based practice: a randomized trial. Arch Intern Med. 2007;167(4):361-366.
15. LoPresti L, Ginn P, Treat R. Using a simulated practice to improve practice management learning. Fam Med. 2009;41(9):640-645.
16. Accreditation Council for Graduate Medical Education. Family medicine program requirements. https://www.acgme.org/acgmeweb/tabid/132/ProgramandInstitutionalAccreditation/MedicalSpecialties/FamilyMedicine.aspx. Accessed September 23, 2015.
Current Evidence Does Not Support Medicare’s 3-Day Rule in Primary Total Joint Arthroplasty
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
Using 3-Dimensional Fluoroscopy to Assess Acute Clavicle Fracture Displacement: A Radiographic Study
Clavicle fractures are common injuries, accounting for 2.6% to 5% of all adult fractures.1,2 Most clavicle fractures (69%-82%) occur in the middle third or midshaft.3,4 Midshaft clavicle fractures are often treated successfully with nonoperative means consisting of shoulder immobilization with either a sling or a figure-of-8 brace. Operative indications historically have been limited to open or impending open injuries and to patients with underlying neurovascular compromise. However, recent clinical studies have found that fractures with particular characteristics may benefit from surgical fixation. Important relative indications for open reduction and internal fixation of midshaft clavicle fractures are complete fracture fragment displacement with no cortical contact, and fractures with axial shortening of more than 20 mm.5,6
Accurately determining the extent of displacement and shortening can therefore be important in guiding treatment recommendations. The standard radiographic view for a clavicle fracture is upright or supine anteroposterior (AP). Typically, an AP radiograph with cephalic tilt of about 20° is obtained as well. On occasion, other supplemental radiographs, such as a 45° angulated view, as originally described by Quesada,7 are obtained. To our knowledge, the literature includes only 2 reports of studies that have compared different radiographic views and their accuracy in measuring fracture shortening8,9; no study has determined the best radiographic view for evaluating fracture displacement.
We conducted a study to determine which radiographic view best captures the most fracture fragment displacement. Acute midshaft clavicle fractures were assessed with simulated angled radiographs created from preoperative upright 3-dimensional (3-D) fluoroscopy scans. Our hypothesis was that a radiographic view with 20° of cephalic tilt would most often detect the most fracture displacement. In addition, we retrospectively reviewed our study patients’ initial AP injury radiographs to determine if obtaining a different view at maximum displacement would have helped identify a larger number of completely displaced midshaft clavicle fractures.
Patients and Methods
Institutional review board approval was obtained. Using our institution’s trauma registry database, we retrospectively identified 10 cases of patients who had undergone preoperative 3-D fluoroscopy for midshaft clavicle fractures. Study inclusion criteria were age 18 years or older, acute midshaft clavicle fracture, and preoperative 3-D fluoroscopy scan of clavicle available. Pediatric patients, nonacute injuries, and clavicle fractures of the lateral or medial third were excluded.
Three-dimensional fluoroscopy was used when the treating surgeon deemed it necessary for preoperative planning. All imaging was performed with a Philips MultiDiagnost Eleva 3-D fluoroscopy imager with patients in the upright standing position. (Informed patient consent was obtained.) Software bundled with the imager was used to create representative radiographs of differing angulation.
The common practice at most institutions is to obtain 2 radiographic views as part of a standard clavicle series. The additional AP angulated radiograph typically is obtained with 20° to 45° cephalic tilt from the horizontal axis. Therefore, simulated radiographs ranging from 15° to 50° of angulation in 5° increments were created, and the amount of superior displacement of the medial fragment was measured. As the simulated views were constructed from a 3-D composite image, there was none of the magnification error that occurs with AP or posteroanterior (PA) views. The stated degree of angulation mimics a radiograph’s AP cephalic tilt or PA caudal tilt (Figures 1A, 1B). For all radiographic images, displacement between fracture fragments was determined by measuring the distance between the superior cortices at the fracture site of the medial and lateral fragments. Each simulated radiograph was measured by 2 readers using standard computerized radiographic measurement tools. Final displacement was taken as the mean of the 2 measurements.
After determining which radiographic angulation demonstrated the largest number of maximally displaced fractures, we compared the simulated radiographs at that angulation with the injury AP images for all patients. Total number of patients with a completely displaced midshaft clavicle fracture and no cortical contact was recorded for the 2 radiographic views.
The Orthopaedic Trauma Association classification system8 was used to classify the clavicle fractures. Statistical analysis was performed with the Fisher exact test and a regression model, using SPSS Version 19.0 (IBM SPSS Statistics).
Results
Ten patients met the study inclusion criteria. Mean age was 32.9 years (range, 18-65 years). Seven of the 10 patients were male. Six patients had right-side clavicle fractures. Of the 10 patients, 5 had the comminuted wedge fracture pattern (15-B2.3), 2 had the simple spiral pattern (15-B1.1), 2 had the spiral wedge pattern (15-B2.1), and 1 had the oblique pattern (15-B1.2).
Table 1 summarizes the fracture displacement measurements obtained with the different radiographic views. Of the 10 cases, 5 showed the most displacement with the 15° tilted view (P = .004), and the other 5 showed maximum displacement with different radiographic angulations. In addition, 6 patients showed the least displacement with the 50° angulated view (P < .001). Results of the regression analysis are summarized in Tables 2 and 3.
Initial horizontal AP imaging showed completely displaced midshaft clavicle fractures in 9 of the 10 patients, and 15° simulated radiographs showed completely displaced fractures in all 10 patients (P = .50).
Discussion
Our study results demonstrated that an upright 15° radiographic tilt (AP cephalad or PA caudal) identified the most fracture displacement in the most patients with acute midshaft clavicle fractures. To our knowledge, this is the first study to identify the radiographic angulation that best shows the most clavicle fracture fragment displacement.
Other investigators have studied the accuracy of different radiographic views in the assessment of midshaft clavicle fractures, but they concentrated on fracture shortening. Smekal and colleagues9 used computed tomography (CT) and 3 different radiographic views to evaluate malunited midshaft clavicle fractures. Comparing the horizontal clavicular length measurements obtained with radiographs and CT scans, they determined that PA thoracic radiographs were in highest agreement with the CT scans. The results, however, were not statistically significant. In their study, supine CT was successful because the fractures were healed, and the displacement and shortening amounts were not affected by patient position. Sharr and Mohammed10 studied the accuracy of different views in the assessment of clavicle length in an articulated cadaver specimen. They obtained multiple AP and PA radiographs of different horizontal (medial, lateral) and vertical (cephalad, caudal) angulations. Actual clavicle length was then directly measured and compared with the length measured on the different views. The authors concluded that a PA 15° caudal radiograph was most accurate in assessing clavicular length. Both Smekal and colleagues9 and Sharr and Mohammed10 recommended the PA radiograph because it decreases the degree of magnification on AP radiographs by minimizing the film-to-object distance.
Our findings are important because more accurate determination of fracture displacement in patients with midshaft clavicle fractures may change clinical management. Nowak and colleagues11 investigated various patient and clavicle fracture characteristics that were predictive of a higher rate of long-term sequelae. They found that complete fracture displacement was the strongest radiographic predictor of patients’ beliefs that they were fully recovered from injury at final follow-up. The authors concluded that fractures with no bony contact should receive more “active” management. Robinson and colleagues12 studied a cohort of patients with nonoperatively managed midshaft clavicle fractures and concluded that complete fracture displacement significantly increased risk for nonunion (this risk was 2.3 times higher in patients with displaced fractures than in patients with nondisplaced fractures). Last, McKee and colleagues13 found that shoulder strength and endurance were significantly decreased in nonoperatively treated displaced midshaft clavicle fractures than in the same patients’ uninjured shoulders.
Extending the results of these studies, recent prospective randomized control trials and a meta-analysis have compared the clinical outcomes of nonoperatively and operatively managed displaced midshaft clavicle fractures.14-18 With few exceptions, these studies found improved clinical results with operative fixation. In one such study, the Canadian Orthopaedic Trauma Society14 randomized patients with displaced midshaft clavicle fractures to either operative plate fixation or sling immobilization. The operative group was found to have improved Disability of the Arm, Shoulder, and Hand scores, improved Constant shoulder scores, increased patient satisfaction, faster mean time to bony fracture union, higher satisfaction with shoulder appearance, and lower rates of nonunion and malunion. Given the results of these studies, accurate identification of a displaced midshaft clavicle fracture with no cortical contact is fundamental in deciding whether to recommend operative fixation.
Retrospective review of our cohort’s initial radiographs revealed 1 case in which the patient’s completely displaced midshaft clavicle fracture would not have been diagnosed solely with an AP horizontal image. Cortical contact was seen on a standard AP clavicle radiograph (Figures 2A, 2B), and a 15° tilt radiograph created from 3-D fluoroscopy scan showed complete fracture fragment displacement (Figure 3). A change in fracture classification from partially displaced to fully displaced could alter the type of management used by a treating surgeon.
There were obvious weaknesses to this study. First, its sample size was small (10 patients). Nevertheless, we had sufficient numbers to find a statistically significant angulation. Second, a wider range of radiographic angles could have been studied. Our intent, however, was to investigate the accuracy of the 2 most common supplementary clavicle views (20° and 45° cephalic tilt). Therefore, we selected a range of simulated radiographs that began 5° outside these angulations. Third, we measured only the degree of fracture displacement; we were unable to accurately access fracture shortening, as the 3-D fluoroscopic images were limited to the injured clavicles. A potential solution to this problem is to widen the exposure field in order to include the entire chest and allow clavicular length comparison against the uninjured side. Doing this would have been possible, but at the expense of increasing the patient’s radiation exposure.
This innovative study used 3-D fluoroscopy to capture clavicle fracture images with patients in an upright position. Unlike standard CT, in which patients are supine, this 3-D imaging technology better emulates the patient positioning used for upright radiographs, thereby avoiding potential fracture fragment alignment changes caused by shifts in body position. In addition, 3-D fluoroscopy allows us to create multiple precise simulated radiographic angulations without the magnification error of AP radiographs and, to a lesser extent, PA radiographs. Having a standing PA 15° caudal tilt radiograph obviates the need for CT with 3-D reconstruction. More fine detail may be revealed by CT with 3-D reconstruction than by a standing PA 15° caudal tilt radiograph, but the patient faces less radiation risk and cost with the radiograph.
There is no consensus as to what constitutes the standard radiographic series for clavicle fractures. Radiographic technique can vary with respect to supplemental view angulation, supine or upright patient positioning, and AP or PA radiographic views. Although our study did not address the effect of supine versus upright patient positioning on acute midshaft clavicle fracture displacement, we think that, for all clinical and research purposes, upright 15° caudal PA radiographs should be obtained for patients with acute midshaft clavicle fractures.
Conclusion
Our retrospective study of 10 patients with acute midshaft clavicle fractures and preoperative upright 3-D fluoroscopy scans found that a 15° angulated radiograph most often demonstrated the most fracture fragment displacement. Given these findings, we recommend obtaining an additional PA 15° caudal radiograph in the upright position for patients with midshaft clavicle fractures to best assess the extent of fracture displacement. Accurately identifying the degree of fracture displacement is important, as operative management of completely displaced fractures has been shown to improve clinical outcomes.
1. Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452-456.
2. Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clin Orthop Relat Res. 1994;(300):127-132.
3. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
4. Rowe CR. An atlas of anatomy and treatment of midclavicular fractures. Clin Orthop Relat Res. 1968;(58):29-42.
5. Jeray KJ. Acute midshaft clavicular fracture. J Am Acad Orthop Surg. 2007;15(4):239-248.
6. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
7. Quesada F. Technique for the roentgen diagnosis of fractures of the clavicle. Surg Gynecol Obstet. 1926;42:424-428.
8. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
9. Smekal V, Deml C, Irenberger A, et al. Length determination in midshaft clavicle fractures: validation of measurement. J Orthop Trauma. 2008;22(7):458-462.
10. Sharr JR, Mohammed KD. Optimizing the radiographic technique in clavicular fractures. J Shoulder Elbow Surg. 2003;12(2):170-172.
11. Nowak J, Holgersson M, Larsson S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J Shoulder Elbow Surg. 2004;13(5):479-486.
12. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365.
13. McKee MD, Pedersen EM, Jones C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006;88(1):35-40.
14. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
15. Judd DB, Pallis MP, Smith E, Bottoni CR. Acute operative stabilization versus nonoperative management of clavicle fractures. Am J Orthop. 2009;38(7):341-345.
16. Smekal V, Irenberger A, Struve P, Wambacher M, Krappinger D, Kralinger FS. Elastic stable intramedullary nailing versus nonoperative treatment of displaced midshaft clavicular fractures—a randomized, controlled, clinical trial. J Orthop Trauma. 2009;23(2):106-112.
17. Witzel K. Intramedullary osteosynthesis in fractures of the mid-third of the clavicle in sports traumatology [in German]. Z Orthop Unfall. 2007;145(5):639-642.
18. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
Clavicle fractures are common injuries, accounting for 2.6% to 5% of all adult fractures.1,2 Most clavicle fractures (69%-82%) occur in the middle third or midshaft.3,4 Midshaft clavicle fractures are often treated successfully with nonoperative means consisting of shoulder immobilization with either a sling or a figure-of-8 brace. Operative indications historically have been limited to open or impending open injuries and to patients with underlying neurovascular compromise. However, recent clinical studies have found that fractures with particular characteristics may benefit from surgical fixation. Important relative indications for open reduction and internal fixation of midshaft clavicle fractures are complete fracture fragment displacement with no cortical contact, and fractures with axial shortening of more than 20 mm.5,6
Accurately determining the extent of displacement and shortening can therefore be important in guiding treatment recommendations. The standard radiographic view for a clavicle fracture is upright or supine anteroposterior (AP). Typically, an AP radiograph with cephalic tilt of about 20° is obtained as well. On occasion, other supplemental radiographs, such as a 45° angulated view, as originally described by Quesada,7 are obtained. To our knowledge, the literature includes only 2 reports of studies that have compared different radiographic views and their accuracy in measuring fracture shortening8,9; no study has determined the best radiographic view for evaluating fracture displacement.
We conducted a study to determine which radiographic view best captures the most fracture fragment displacement. Acute midshaft clavicle fractures were assessed with simulated angled radiographs created from preoperative upright 3-dimensional (3-D) fluoroscopy scans. Our hypothesis was that a radiographic view with 20° of cephalic tilt would most often detect the most fracture displacement. In addition, we retrospectively reviewed our study patients’ initial AP injury radiographs to determine if obtaining a different view at maximum displacement would have helped identify a larger number of completely displaced midshaft clavicle fractures.
Patients and Methods
Institutional review board approval was obtained. Using our institution’s trauma registry database, we retrospectively identified 10 cases of patients who had undergone preoperative 3-D fluoroscopy for midshaft clavicle fractures. Study inclusion criteria were age 18 years or older, acute midshaft clavicle fracture, and preoperative 3-D fluoroscopy scan of clavicle available. Pediatric patients, nonacute injuries, and clavicle fractures of the lateral or medial third were excluded.
Three-dimensional fluoroscopy was used when the treating surgeon deemed it necessary for preoperative planning. All imaging was performed with a Philips MultiDiagnost Eleva 3-D fluoroscopy imager with patients in the upright standing position. (Informed patient consent was obtained.) Software bundled with the imager was used to create representative radiographs of differing angulation.
The common practice at most institutions is to obtain 2 radiographic views as part of a standard clavicle series. The additional AP angulated radiograph typically is obtained with 20° to 45° cephalic tilt from the horizontal axis. Therefore, simulated radiographs ranging from 15° to 50° of angulation in 5° increments were created, and the amount of superior displacement of the medial fragment was measured. As the simulated views were constructed from a 3-D composite image, there was none of the magnification error that occurs with AP or posteroanterior (PA) views. The stated degree of angulation mimics a radiograph’s AP cephalic tilt or PA caudal tilt (Figures 1A, 1B). For all radiographic images, displacement between fracture fragments was determined by measuring the distance between the superior cortices at the fracture site of the medial and lateral fragments. Each simulated radiograph was measured by 2 readers using standard computerized radiographic measurement tools. Final displacement was taken as the mean of the 2 measurements.
After determining which radiographic angulation demonstrated the largest number of maximally displaced fractures, we compared the simulated radiographs at that angulation with the injury AP images for all patients. Total number of patients with a completely displaced midshaft clavicle fracture and no cortical contact was recorded for the 2 radiographic views.
The Orthopaedic Trauma Association classification system8 was used to classify the clavicle fractures. Statistical analysis was performed with the Fisher exact test and a regression model, using SPSS Version 19.0 (IBM SPSS Statistics).
Results
Ten patients met the study inclusion criteria. Mean age was 32.9 years (range, 18-65 years). Seven of the 10 patients were male. Six patients had right-side clavicle fractures. Of the 10 patients, 5 had the comminuted wedge fracture pattern (15-B2.3), 2 had the simple spiral pattern (15-B1.1), 2 had the spiral wedge pattern (15-B2.1), and 1 had the oblique pattern (15-B1.2).
Table 1 summarizes the fracture displacement measurements obtained with the different radiographic views. Of the 10 cases, 5 showed the most displacement with the 15° tilted view (P = .004), and the other 5 showed maximum displacement with different radiographic angulations. In addition, 6 patients showed the least displacement with the 50° angulated view (P < .001). Results of the regression analysis are summarized in Tables 2 and 3.
Initial horizontal AP imaging showed completely displaced midshaft clavicle fractures in 9 of the 10 patients, and 15° simulated radiographs showed completely displaced fractures in all 10 patients (P = .50).
Discussion
Our study results demonstrated that an upright 15° radiographic tilt (AP cephalad or PA caudal) identified the most fracture displacement in the most patients with acute midshaft clavicle fractures. To our knowledge, this is the first study to identify the radiographic angulation that best shows the most clavicle fracture fragment displacement.
Other investigators have studied the accuracy of different radiographic views in the assessment of midshaft clavicle fractures, but they concentrated on fracture shortening. Smekal and colleagues9 used computed tomography (CT) and 3 different radiographic views to evaluate malunited midshaft clavicle fractures. Comparing the horizontal clavicular length measurements obtained with radiographs and CT scans, they determined that PA thoracic radiographs were in highest agreement with the CT scans. The results, however, were not statistically significant. In their study, supine CT was successful because the fractures were healed, and the displacement and shortening amounts were not affected by patient position. Sharr and Mohammed10 studied the accuracy of different views in the assessment of clavicle length in an articulated cadaver specimen. They obtained multiple AP and PA radiographs of different horizontal (medial, lateral) and vertical (cephalad, caudal) angulations. Actual clavicle length was then directly measured and compared with the length measured on the different views. The authors concluded that a PA 15° caudal radiograph was most accurate in assessing clavicular length. Both Smekal and colleagues9 and Sharr and Mohammed10 recommended the PA radiograph because it decreases the degree of magnification on AP radiographs by minimizing the film-to-object distance.
Our findings are important because more accurate determination of fracture displacement in patients with midshaft clavicle fractures may change clinical management. Nowak and colleagues11 investigated various patient and clavicle fracture characteristics that were predictive of a higher rate of long-term sequelae. They found that complete fracture displacement was the strongest radiographic predictor of patients’ beliefs that they were fully recovered from injury at final follow-up. The authors concluded that fractures with no bony contact should receive more “active” management. Robinson and colleagues12 studied a cohort of patients with nonoperatively managed midshaft clavicle fractures and concluded that complete fracture displacement significantly increased risk for nonunion (this risk was 2.3 times higher in patients with displaced fractures than in patients with nondisplaced fractures). Last, McKee and colleagues13 found that shoulder strength and endurance were significantly decreased in nonoperatively treated displaced midshaft clavicle fractures than in the same patients’ uninjured shoulders.
Extending the results of these studies, recent prospective randomized control trials and a meta-analysis have compared the clinical outcomes of nonoperatively and operatively managed displaced midshaft clavicle fractures.14-18 With few exceptions, these studies found improved clinical results with operative fixation. In one such study, the Canadian Orthopaedic Trauma Society14 randomized patients with displaced midshaft clavicle fractures to either operative plate fixation or sling immobilization. The operative group was found to have improved Disability of the Arm, Shoulder, and Hand scores, improved Constant shoulder scores, increased patient satisfaction, faster mean time to bony fracture union, higher satisfaction with shoulder appearance, and lower rates of nonunion and malunion. Given the results of these studies, accurate identification of a displaced midshaft clavicle fracture with no cortical contact is fundamental in deciding whether to recommend operative fixation.
Retrospective review of our cohort’s initial radiographs revealed 1 case in which the patient’s completely displaced midshaft clavicle fracture would not have been diagnosed solely with an AP horizontal image. Cortical contact was seen on a standard AP clavicle radiograph (Figures 2A, 2B), and a 15° tilt radiograph created from 3-D fluoroscopy scan showed complete fracture fragment displacement (Figure 3). A change in fracture classification from partially displaced to fully displaced could alter the type of management used by a treating surgeon.
There were obvious weaknesses to this study. First, its sample size was small (10 patients). Nevertheless, we had sufficient numbers to find a statistically significant angulation. Second, a wider range of radiographic angles could have been studied. Our intent, however, was to investigate the accuracy of the 2 most common supplementary clavicle views (20° and 45° cephalic tilt). Therefore, we selected a range of simulated radiographs that began 5° outside these angulations. Third, we measured only the degree of fracture displacement; we were unable to accurately access fracture shortening, as the 3-D fluoroscopic images were limited to the injured clavicles. A potential solution to this problem is to widen the exposure field in order to include the entire chest and allow clavicular length comparison against the uninjured side. Doing this would have been possible, but at the expense of increasing the patient’s radiation exposure.
This innovative study used 3-D fluoroscopy to capture clavicle fracture images with patients in an upright position. Unlike standard CT, in which patients are supine, this 3-D imaging technology better emulates the patient positioning used for upright radiographs, thereby avoiding potential fracture fragment alignment changes caused by shifts in body position. In addition, 3-D fluoroscopy allows us to create multiple precise simulated radiographic angulations without the magnification error of AP radiographs and, to a lesser extent, PA radiographs. Having a standing PA 15° caudal tilt radiograph obviates the need for CT with 3-D reconstruction. More fine detail may be revealed by CT with 3-D reconstruction than by a standing PA 15° caudal tilt radiograph, but the patient faces less radiation risk and cost with the radiograph.
There is no consensus as to what constitutes the standard radiographic series for clavicle fractures. Radiographic technique can vary with respect to supplemental view angulation, supine or upright patient positioning, and AP or PA radiographic views. Although our study did not address the effect of supine versus upright patient positioning on acute midshaft clavicle fracture displacement, we think that, for all clinical and research purposes, upright 15° caudal PA radiographs should be obtained for patients with acute midshaft clavicle fractures.
Conclusion
Our retrospective study of 10 patients with acute midshaft clavicle fractures and preoperative upright 3-D fluoroscopy scans found that a 15° angulated radiograph most often demonstrated the most fracture fragment displacement. Given these findings, we recommend obtaining an additional PA 15° caudal radiograph in the upright position for patients with midshaft clavicle fractures to best assess the extent of fracture displacement. Accurately identifying the degree of fracture displacement is important, as operative management of completely displaced fractures has been shown to improve clinical outcomes.
Clavicle fractures are common injuries, accounting for 2.6% to 5% of all adult fractures.1,2 Most clavicle fractures (69%-82%) occur in the middle third or midshaft.3,4 Midshaft clavicle fractures are often treated successfully with nonoperative means consisting of shoulder immobilization with either a sling or a figure-of-8 brace. Operative indications historically have been limited to open or impending open injuries and to patients with underlying neurovascular compromise. However, recent clinical studies have found that fractures with particular characteristics may benefit from surgical fixation. Important relative indications for open reduction and internal fixation of midshaft clavicle fractures are complete fracture fragment displacement with no cortical contact, and fractures with axial shortening of more than 20 mm.5,6
Accurately determining the extent of displacement and shortening can therefore be important in guiding treatment recommendations. The standard radiographic view for a clavicle fracture is upright or supine anteroposterior (AP). Typically, an AP radiograph with cephalic tilt of about 20° is obtained as well. On occasion, other supplemental radiographs, such as a 45° angulated view, as originally described by Quesada,7 are obtained. To our knowledge, the literature includes only 2 reports of studies that have compared different radiographic views and their accuracy in measuring fracture shortening8,9; no study has determined the best radiographic view for evaluating fracture displacement.
We conducted a study to determine which radiographic view best captures the most fracture fragment displacement. Acute midshaft clavicle fractures were assessed with simulated angled radiographs created from preoperative upright 3-dimensional (3-D) fluoroscopy scans. Our hypothesis was that a radiographic view with 20° of cephalic tilt would most often detect the most fracture displacement. In addition, we retrospectively reviewed our study patients’ initial AP injury radiographs to determine if obtaining a different view at maximum displacement would have helped identify a larger number of completely displaced midshaft clavicle fractures.
Patients and Methods
Institutional review board approval was obtained. Using our institution’s trauma registry database, we retrospectively identified 10 cases of patients who had undergone preoperative 3-D fluoroscopy for midshaft clavicle fractures. Study inclusion criteria were age 18 years or older, acute midshaft clavicle fracture, and preoperative 3-D fluoroscopy scan of clavicle available. Pediatric patients, nonacute injuries, and clavicle fractures of the lateral or medial third were excluded.
Three-dimensional fluoroscopy was used when the treating surgeon deemed it necessary for preoperative planning. All imaging was performed with a Philips MultiDiagnost Eleva 3-D fluoroscopy imager with patients in the upright standing position. (Informed patient consent was obtained.) Software bundled with the imager was used to create representative radiographs of differing angulation.
The common practice at most institutions is to obtain 2 radiographic views as part of a standard clavicle series. The additional AP angulated radiograph typically is obtained with 20° to 45° cephalic tilt from the horizontal axis. Therefore, simulated radiographs ranging from 15° to 50° of angulation in 5° increments were created, and the amount of superior displacement of the medial fragment was measured. As the simulated views were constructed from a 3-D composite image, there was none of the magnification error that occurs with AP or posteroanterior (PA) views. The stated degree of angulation mimics a radiograph’s AP cephalic tilt or PA caudal tilt (Figures 1A, 1B). For all radiographic images, displacement between fracture fragments was determined by measuring the distance between the superior cortices at the fracture site of the medial and lateral fragments. Each simulated radiograph was measured by 2 readers using standard computerized radiographic measurement tools. Final displacement was taken as the mean of the 2 measurements.
After determining which radiographic angulation demonstrated the largest number of maximally displaced fractures, we compared the simulated radiographs at that angulation with the injury AP images for all patients. Total number of patients with a completely displaced midshaft clavicle fracture and no cortical contact was recorded for the 2 radiographic views.
The Orthopaedic Trauma Association classification system8 was used to classify the clavicle fractures. Statistical analysis was performed with the Fisher exact test and a regression model, using SPSS Version 19.0 (IBM SPSS Statistics).
Results
Ten patients met the study inclusion criteria. Mean age was 32.9 years (range, 18-65 years). Seven of the 10 patients were male. Six patients had right-side clavicle fractures. Of the 10 patients, 5 had the comminuted wedge fracture pattern (15-B2.3), 2 had the simple spiral pattern (15-B1.1), 2 had the spiral wedge pattern (15-B2.1), and 1 had the oblique pattern (15-B1.2).
Table 1 summarizes the fracture displacement measurements obtained with the different radiographic views. Of the 10 cases, 5 showed the most displacement with the 15° tilted view (P = .004), and the other 5 showed maximum displacement with different radiographic angulations. In addition, 6 patients showed the least displacement with the 50° angulated view (P < .001). Results of the regression analysis are summarized in Tables 2 and 3.
Initial horizontal AP imaging showed completely displaced midshaft clavicle fractures in 9 of the 10 patients, and 15° simulated radiographs showed completely displaced fractures in all 10 patients (P = .50).
Discussion
Our study results demonstrated that an upright 15° radiographic tilt (AP cephalad or PA caudal) identified the most fracture displacement in the most patients with acute midshaft clavicle fractures. To our knowledge, this is the first study to identify the radiographic angulation that best shows the most clavicle fracture fragment displacement.
Other investigators have studied the accuracy of different radiographic views in the assessment of midshaft clavicle fractures, but they concentrated on fracture shortening. Smekal and colleagues9 used computed tomography (CT) and 3 different radiographic views to evaluate malunited midshaft clavicle fractures. Comparing the horizontal clavicular length measurements obtained with radiographs and CT scans, they determined that PA thoracic radiographs were in highest agreement with the CT scans. The results, however, were not statistically significant. In their study, supine CT was successful because the fractures were healed, and the displacement and shortening amounts were not affected by patient position. Sharr and Mohammed10 studied the accuracy of different views in the assessment of clavicle length in an articulated cadaver specimen. They obtained multiple AP and PA radiographs of different horizontal (medial, lateral) and vertical (cephalad, caudal) angulations. Actual clavicle length was then directly measured and compared with the length measured on the different views. The authors concluded that a PA 15° caudal radiograph was most accurate in assessing clavicular length. Both Smekal and colleagues9 and Sharr and Mohammed10 recommended the PA radiograph because it decreases the degree of magnification on AP radiographs by minimizing the film-to-object distance.
Our findings are important because more accurate determination of fracture displacement in patients with midshaft clavicle fractures may change clinical management. Nowak and colleagues11 investigated various patient and clavicle fracture characteristics that were predictive of a higher rate of long-term sequelae. They found that complete fracture displacement was the strongest radiographic predictor of patients’ beliefs that they were fully recovered from injury at final follow-up. The authors concluded that fractures with no bony contact should receive more “active” management. Robinson and colleagues12 studied a cohort of patients with nonoperatively managed midshaft clavicle fractures and concluded that complete fracture displacement significantly increased risk for nonunion (this risk was 2.3 times higher in patients with displaced fractures than in patients with nondisplaced fractures). Last, McKee and colleagues13 found that shoulder strength and endurance were significantly decreased in nonoperatively treated displaced midshaft clavicle fractures than in the same patients’ uninjured shoulders.
Extending the results of these studies, recent prospective randomized control trials and a meta-analysis have compared the clinical outcomes of nonoperatively and operatively managed displaced midshaft clavicle fractures.14-18 With few exceptions, these studies found improved clinical results with operative fixation. In one such study, the Canadian Orthopaedic Trauma Society14 randomized patients with displaced midshaft clavicle fractures to either operative plate fixation or sling immobilization. The operative group was found to have improved Disability of the Arm, Shoulder, and Hand scores, improved Constant shoulder scores, increased patient satisfaction, faster mean time to bony fracture union, higher satisfaction with shoulder appearance, and lower rates of nonunion and malunion. Given the results of these studies, accurate identification of a displaced midshaft clavicle fracture with no cortical contact is fundamental in deciding whether to recommend operative fixation.
Retrospective review of our cohort’s initial radiographs revealed 1 case in which the patient’s completely displaced midshaft clavicle fracture would not have been diagnosed solely with an AP horizontal image. Cortical contact was seen on a standard AP clavicle radiograph (Figures 2A, 2B), and a 15° tilt radiograph created from 3-D fluoroscopy scan showed complete fracture fragment displacement (Figure 3). A change in fracture classification from partially displaced to fully displaced could alter the type of management used by a treating surgeon.
There were obvious weaknesses to this study. First, its sample size was small (10 patients). Nevertheless, we had sufficient numbers to find a statistically significant angulation. Second, a wider range of radiographic angles could have been studied. Our intent, however, was to investigate the accuracy of the 2 most common supplementary clavicle views (20° and 45° cephalic tilt). Therefore, we selected a range of simulated radiographs that began 5° outside these angulations. Third, we measured only the degree of fracture displacement; we were unable to accurately access fracture shortening, as the 3-D fluoroscopic images were limited to the injured clavicles. A potential solution to this problem is to widen the exposure field in order to include the entire chest and allow clavicular length comparison against the uninjured side. Doing this would have been possible, but at the expense of increasing the patient’s radiation exposure.
This innovative study used 3-D fluoroscopy to capture clavicle fracture images with patients in an upright position. Unlike standard CT, in which patients are supine, this 3-D imaging technology better emulates the patient positioning used for upright radiographs, thereby avoiding potential fracture fragment alignment changes caused by shifts in body position. In addition, 3-D fluoroscopy allows us to create multiple precise simulated radiographic angulations without the magnification error of AP radiographs and, to a lesser extent, PA radiographs. Having a standing PA 15° caudal tilt radiograph obviates the need for CT with 3-D reconstruction. More fine detail may be revealed by CT with 3-D reconstruction than by a standing PA 15° caudal tilt radiograph, but the patient faces less radiation risk and cost with the radiograph.
There is no consensus as to what constitutes the standard radiographic series for clavicle fractures. Radiographic technique can vary with respect to supplemental view angulation, supine or upright patient positioning, and AP or PA radiographic views. Although our study did not address the effect of supine versus upright patient positioning on acute midshaft clavicle fracture displacement, we think that, for all clinical and research purposes, upright 15° caudal PA radiographs should be obtained for patients with acute midshaft clavicle fractures.
Conclusion
Our retrospective study of 10 patients with acute midshaft clavicle fractures and preoperative upright 3-D fluoroscopy scans found that a 15° angulated radiograph most often demonstrated the most fracture fragment displacement. Given these findings, we recommend obtaining an additional PA 15° caudal radiograph in the upright position for patients with midshaft clavicle fractures to best assess the extent of fracture displacement. Accurately identifying the degree of fracture displacement is important, as operative management of completely displaced fractures has been shown to improve clinical outcomes.
1. Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452-456.
2. Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clin Orthop Relat Res. 1994;(300):127-132.
3. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
4. Rowe CR. An atlas of anatomy and treatment of midclavicular fractures. Clin Orthop Relat Res. 1968;(58):29-42.
5. Jeray KJ. Acute midshaft clavicular fracture. J Am Acad Orthop Surg. 2007;15(4):239-248.
6. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
7. Quesada F. Technique for the roentgen diagnosis of fractures of the clavicle. Surg Gynecol Obstet. 1926;42:424-428.
8. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
9. Smekal V, Deml C, Irenberger A, et al. Length determination in midshaft clavicle fractures: validation of measurement. J Orthop Trauma. 2008;22(7):458-462.
10. Sharr JR, Mohammed KD. Optimizing the radiographic technique in clavicular fractures. J Shoulder Elbow Surg. 2003;12(2):170-172.
11. Nowak J, Holgersson M, Larsson S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J Shoulder Elbow Surg. 2004;13(5):479-486.
12. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365.
13. McKee MD, Pedersen EM, Jones C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006;88(1):35-40.
14. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
15. Judd DB, Pallis MP, Smith E, Bottoni CR. Acute operative stabilization versus nonoperative management of clavicle fractures. Am J Orthop. 2009;38(7):341-345.
16. Smekal V, Irenberger A, Struve P, Wambacher M, Krappinger D, Kralinger FS. Elastic stable intramedullary nailing versus nonoperative treatment of displaced midshaft clavicular fractures—a randomized, controlled, clinical trial. J Orthop Trauma. 2009;23(2):106-112.
17. Witzel K. Intramedullary osteosynthesis in fractures of the mid-third of the clavicle in sports traumatology [in German]. Z Orthop Unfall. 2007;145(5):639-642.
18. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
1. Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452-456.
2. Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clin Orthop Relat Res. 1994;(300):127-132.
3. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
4. Rowe CR. An atlas of anatomy and treatment of midclavicular fractures. Clin Orthop Relat Res. 1968;(58):29-42.
5. Jeray KJ. Acute midshaft clavicular fracture. J Am Acad Orthop Surg. 2007;15(4):239-248.
6. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
7. Quesada F. Technique for the roentgen diagnosis of fractures of the clavicle. Surg Gynecol Obstet. 1926;42:424-428.
8. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
9. Smekal V, Deml C, Irenberger A, et al. Length determination in midshaft clavicle fractures: validation of measurement. J Orthop Trauma. 2008;22(7):458-462.
10. Sharr JR, Mohammed KD. Optimizing the radiographic technique in clavicular fractures. J Shoulder Elbow Surg. 2003;12(2):170-172.
11. Nowak J, Holgersson M, Larsson S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J Shoulder Elbow Surg. 2004;13(5):479-486.
12. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365.
13. McKee MD, Pedersen EM, Jones C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006;88(1):35-40.
14. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
15. Judd DB, Pallis MP, Smith E, Bottoni CR. Acute operative stabilization versus nonoperative management of clavicle fractures. Am J Orthop. 2009;38(7):341-345.
16. Smekal V, Irenberger A, Struve P, Wambacher M, Krappinger D, Kralinger FS. Elastic stable intramedullary nailing versus nonoperative treatment of displaced midshaft clavicular fractures—a randomized, controlled, clinical trial. J Orthop Trauma. 2009;23(2):106-112.
17. Witzel K. Intramedullary osteosynthesis in fractures of the mid-third of the clavicle in sports traumatology [in German]. Z Orthop Unfall. 2007;145(5):639-642.
18. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
The Supination-Pronation Test for Distal Biceps Tendon Rupture
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
S. lugdunensis osteoarticular infection often linked to orthopedic devices
SAN DIEGO – Bone and joint infections caused by Staphylococcus lugdunensis are an underestimated hospital-acquired infection often associated with orthopedic devices, according to a multicenter study.
“Consider potential relapse even after 1 year of the end of antibiotic treatment and follow patients with bone and joint infections caused by S. lugdunensis for a minimum 2 years after the end of treatment,” lead study author Dr. Piseth Seng said in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
S. lugdunensis is a virulent coagulase-negative staphylococcus which behaves like S. aureus. Prior to the current study, only 47 cases are believed to be published in the medical literature, according to Dr. Seng of the department of internal medicine at Assistance Publique des Hôpitaux de Marseille (France). The purpose of the current study was to report a series of 138 cases of S. lugdunensis osteoarticular infection managed in nine hospital centers and three private clinics in France from January 1995 to December 2014.
The mean age of patients was 61 years, and 68% were male. Of the 138 cases, 113 (82%) were associated with an orthopedic device, including 2 cases of infection after anterior cruciate ligament reconstruction, 66 cases of prosthetic joint infection, and 3 cases of vertebral orthopedic device infection. The majority of orthopedic device infections (88%) occurred more than 1 month after implantation, while the remaining 12% occurred within the first month of implantation.
The researchers identified 30 cases (22%) of bone and joint infection that occurred in the absence of an orthopedic device, including 7 cases of arthritis, 21 cases of osteitis, and 2 cases of vertebral osteomyelitis.
The majority of patients (91%) received a combination of antibiotic and surgical treatment, including amputation (6%), orthopedic prosthesis removal (14%), internal orthopedic device removal (23%), and surgical debridement and retention of the orthopedic device (41%). The proportion of S. lugdunensis strains with reduced susceptibility to antistaphylococcal agents was low. Resistant strains included five to oxacillin, four to fosfomycin, two to fusidic acid, two to co-trimoxazole, one to rifampicin, and one to clindamycin.
To date, relapses have occurred in 19% of the 123 patients in whom researchers have complete follow-up data. The readmission rate among these patients was 76%, and four (3%) died of their infection. “These relapses were not associated with risk factor or comorbidity or polymicrobial infection,” noted Dr. Seng, who characterized the incidence of bone and joint infections caused by S. lugdunensis as being under reported. “S. lugdunensis is known as an organism forming biofilms, but treatment options (surgical debridement or prosthesis removal) did not influence clinical outcomes.”
The mean time to relapse was 305 days and no risk factor or comorbidity was associated with relapse.
Dr. Seng acknowledged that the study was limited by its retrospective design. He and his associates reported having no financial disclosures.
SAN DIEGO – Bone and joint infections caused by Staphylococcus lugdunensis are an underestimated hospital-acquired infection often associated with orthopedic devices, according to a multicenter study.
“Consider potential relapse even after 1 year of the end of antibiotic treatment and follow patients with bone and joint infections caused by S. lugdunensis for a minimum 2 years after the end of treatment,” lead study author Dr. Piseth Seng said in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
S. lugdunensis is a virulent coagulase-negative staphylococcus which behaves like S. aureus. Prior to the current study, only 47 cases are believed to be published in the medical literature, according to Dr. Seng of the department of internal medicine at Assistance Publique des Hôpitaux de Marseille (France). The purpose of the current study was to report a series of 138 cases of S. lugdunensis osteoarticular infection managed in nine hospital centers and three private clinics in France from January 1995 to December 2014.
The mean age of patients was 61 years, and 68% were male. Of the 138 cases, 113 (82%) were associated with an orthopedic device, including 2 cases of infection after anterior cruciate ligament reconstruction, 66 cases of prosthetic joint infection, and 3 cases of vertebral orthopedic device infection. The majority of orthopedic device infections (88%) occurred more than 1 month after implantation, while the remaining 12% occurred within the first month of implantation.
The researchers identified 30 cases (22%) of bone and joint infection that occurred in the absence of an orthopedic device, including 7 cases of arthritis, 21 cases of osteitis, and 2 cases of vertebral osteomyelitis.
The majority of patients (91%) received a combination of antibiotic and surgical treatment, including amputation (6%), orthopedic prosthesis removal (14%), internal orthopedic device removal (23%), and surgical debridement and retention of the orthopedic device (41%). The proportion of S. lugdunensis strains with reduced susceptibility to antistaphylococcal agents was low. Resistant strains included five to oxacillin, four to fosfomycin, two to fusidic acid, two to co-trimoxazole, one to rifampicin, and one to clindamycin.
To date, relapses have occurred in 19% of the 123 patients in whom researchers have complete follow-up data. The readmission rate among these patients was 76%, and four (3%) died of their infection. “These relapses were not associated with risk factor or comorbidity or polymicrobial infection,” noted Dr. Seng, who characterized the incidence of bone and joint infections caused by S. lugdunensis as being under reported. “S. lugdunensis is known as an organism forming biofilms, but treatment options (surgical debridement or prosthesis removal) did not influence clinical outcomes.”
The mean time to relapse was 305 days and no risk factor or comorbidity was associated with relapse.
Dr. Seng acknowledged that the study was limited by its retrospective design. He and his associates reported having no financial disclosures.
SAN DIEGO – Bone and joint infections caused by Staphylococcus lugdunensis are an underestimated hospital-acquired infection often associated with orthopedic devices, according to a multicenter study.
“Consider potential relapse even after 1 year of the end of antibiotic treatment and follow patients with bone and joint infections caused by S. lugdunensis for a minimum 2 years after the end of treatment,” lead study author Dr. Piseth Seng said in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
S. lugdunensis is a virulent coagulase-negative staphylococcus which behaves like S. aureus. Prior to the current study, only 47 cases are believed to be published in the medical literature, according to Dr. Seng of the department of internal medicine at Assistance Publique des Hôpitaux de Marseille (France). The purpose of the current study was to report a series of 138 cases of S. lugdunensis osteoarticular infection managed in nine hospital centers and three private clinics in France from January 1995 to December 2014.
The mean age of patients was 61 years, and 68% were male. Of the 138 cases, 113 (82%) were associated with an orthopedic device, including 2 cases of infection after anterior cruciate ligament reconstruction, 66 cases of prosthetic joint infection, and 3 cases of vertebral orthopedic device infection. The majority of orthopedic device infections (88%) occurred more than 1 month after implantation, while the remaining 12% occurred within the first month of implantation.
The researchers identified 30 cases (22%) of bone and joint infection that occurred in the absence of an orthopedic device, including 7 cases of arthritis, 21 cases of osteitis, and 2 cases of vertebral osteomyelitis.
The majority of patients (91%) received a combination of antibiotic and surgical treatment, including amputation (6%), orthopedic prosthesis removal (14%), internal orthopedic device removal (23%), and surgical debridement and retention of the orthopedic device (41%). The proportion of S. lugdunensis strains with reduced susceptibility to antistaphylococcal agents was low. Resistant strains included five to oxacillin, four to fosfomycin, two to fusidic acid, two to co-trimoxazole, one to rifampicin, and one to clindamycin.
To date, relapses have occurred in 19% of the 123 patients in whom researchers have complete follow-up data. The readmission rate among these patients was 76%, and four (3%) died of their infection. “These relapses were not associated with risk factor or comorbidity or polymicrobial infection,” noted Dr. Seng, who characterized the incidence of bone and joint infections caused by S. lugdunensis as being under reported. “S. lugdunensis is known as an organism forming biofilms, but treatment options (surgical debridement or prosthesis removal) did not influence clinical outcomes.”
The mean time to relapse was 305 days and no risk factor or comorbidity was associated with relapse.
Dr. Seng acknowledged that the study was limited by its retrospective design. He and his associates reported having no financial disclosures.
AT ICAAC 2015
Key clinical point: S. lugdunensis infections are often associated with orthopedic devices.
Major finding: Of 138 cases of S. lugdunensis osteoarticular infection, 113 (82%) were associated with an orthopedic device.
Data source: A retrospective study of 138 cases of S. lugdunensis osteoarticular infection managed in nine hospitals and three private clinics in France.
Disclosures: The researchers reported having no financial disclosures.
Is There a Link Between Diabetes and Bone Health?
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].