User login
Functional MRI shows that empathetic remarks reduce pain
These are the results of a study, recently published in the Proceedings of the National Academy of Sciences, that was conducted by a team led by neuroscientist Dan-Mikael Ellingsen, PhD, from Oslo University Hospital.
The researchers used functional MRI to scan the brains of 20 patients with chronic pain to investigate how a physician’s demeanor may affect patients’ sensitivity to pain, including effects in the central nervous system. During the scans, which were conducted in two sessions, the patients’ legs were exposed to stimuli that ranged from painless to moderately painful. The patients recorded perceived pain intensity using a scale. The physicians also underwent fMRI.
Half of the patients were subjected to the pain stimuli while alone; the other half were subjected to pain while in the presence of a physician. The latter group of patients was divided into two subgroups. Half of the patients had spoken to the accompanying physician before the examination. They discussed the history of the patient’s condition to date, among other things. The other half underwent the brain scans without any prior interaction with a physician.
Worse when alone
Dr. Ellingsen and his colleagues found that patients who were alone during the examination reported greater pain than those who were in the presence of a physician, even though they were subjected to stimuli of the same intensity. In instances in which the physician and patient had already spoken before the brain scan, patients additionally felt that the physician was empathetic and understood their pain. Furthermore, the physicians were better able to estimate the pain that their patients experienced.
The patients who had a physician by their side consistently experienced pain that was milder than the pain experienced by those who were alone. For pairs that had spoken beforehand, the patients considered their physician to be better able to understand their pain, and the physicians estimated the perceived pain intensity of their patients more accurately.
Evidence of trust
There was greater activity in the dorsolateral and ventrolateral prefrontal cortex, as well as in the primary and secondary somatosensory areas, in patients in the subgroup that had spoken to a physician. For the physicians, compared with the comparison group, there was an increase in correspondence between activity in the dorsolateral prefrontal cortex and activity in the secondary somatosensory areas of patients, which is a brain region that is known to react to pain. The brain activity correlation increased in line with the self-reported mutual trust between the physician and patient.
“These results prove that empathy and support can decrease pain intensity,” the investigators write. The data shed light on the brain processes behind the social modulation of pain during the interaction between the physician and the patient. Concordances in the brain are increased by greater therapeutic alliance.
Beyond medication
Winfried Meissner, MD, head of the pain clinic at the department of anesthesiology and intensive care medicine at Jena University Hospital, Germany, and former president of the German Pain Society, said in an interview: “I view this as a vital study that impressively demonstrates that effective, intensive pain therapy is not just a case of administering the correct analgesic.”
“Instead, a focus should be placed on what common sense tells us, which is just how crucial an empathetic attitude from physicians and good communication with patients are when it comes to the success of any therapy,” Dr. Meissner added. Unfortunately, such an attitude and such communication often are not provided in clinical practice because of limitations on time.
“Now, with objectively collected data from patients and physicians, [Dr.] Ellingsen’s team has been able to demonstrate that human interaction has a decisive impact on the treatment of patients experiencing pain,” said Dr. Meissner. “The study should encourage practitioners to treat communication just as seriously as the pharmacology of analgesics.”
Perception and attitude
“The study shows remarkably well that empathetic conversation between the physician and patient represents a valuable therapeutic method and should be recognized as such,” emphasized Dr. Meissner. Of course, conversation cannot replace pharmacologic treatment, but it can supplement and reinforce it. Furthermore, a physician’s empathy presumably has an effect that is at least as great as a suitable analgesic.
“Pain is more than just sensory perception,” explained Dr. Meissner. “We all know that it has a strong affective component, and perception is greatly determined by context.” This can be seen, for example, in athletes, who often attribute less importance to their pain and can successfully perform competitively despite a painful injury.
Positive expectations
Dr. Meissner advised all physicians to treat patients with pain empathetically. He encourages them to ask patients about their pain, accompanying symptoms, possible fears, and other mental stress and to take these factors seriously.
Moreover, the findings accentuate the effect of prescribed analgesics. “Numerous studies have meanwhile shown that the more positive a patient’s expectations, the better the effect of a medication,” said Dr. Meissner. “We physicians must exploit this effect, too.”
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
These are the results of a study, recently published in the Proceedings of the National Academy of Sciences, that was conducted by a team led by neuroscientist Dan-Mikael Ellingsen, PhD, from Oslo University Hospital.
The researchers used functional MRI to scan the brains of 20 patients with chronic pain to investigate how a physician’s demeanor may affect patients’ sensitivity to pain, including effects in the central nervous system. During the scans, which were conducted in two sessions, the patients’ legs were exposed to stimuli that ranged from painless to moderately painful. The patients recorded perceived pain intensity using a scale. The physicians also underwent fMRI.
Half of the patients were subjected to the pain stimuli while alone; the other half were subjected to pain while in the presence of a physician. The latter group of patients was divided into two subgroups. Half of the patients had spoken to the accompanying physician before the examination. They discussed the history of the patient’s condition to date, among other things. The other half underwent the brain scans without any prior interaction with a physician.
Worse when alone
Dr. Ellingsen and his colleagues found that patients who were alone during the examination reported greater pain than those who were in the presence of a physician, even though they were subjected to stimuli of the same intensity. In instances in which the physician and patient had already spoken before the brain scan, patients additionally felt that the physician was empathetic and understood their pain. Furthermore, the physicians were better able to estimate the pain that their patients experienced.
The patients who had a physician by their side consistently experienced pain that was milder than the pain experienced by those who were alone. For pairs that had spoken beforehand, the patients considered their physician to be better able to understand their pain, and the physicians estimated the perceived pain intensity of their patients more accurately.
Evidence of trust
There was greater activity in the dorsolateral and ventrolateral prefrontal cortex, as well as in the primary and secondary somatosensory areas, in patients in the subgroup that had spoken to a physician. For the physicians, compared with the comparison group, there was an increase in correspondence between activity in the dorsolateral prefrontal cortex and activity in the secondary somatosensory areas of patients, which is a brain region that is known to react to pain. The brain activity correlation increased in line with the self-reported mutual trust between the physician and patient.
“These results prove that empathy and support can decrease pain intensity,” the investigators write. The data shed light on the brain processes behind the social modulation of pain during the interaction between the physician and the patient. Concordances in the brain are increased by greater therapeutic alliance.
Beyond medication
Winfried Meissner, MD, head of the pain clinic at the department of anesthesiology and intensive care medicine at Jena University Hospital, Germany, and former president of the German Pain Society, said in an interview: “I view this as a vital study that impressively demonstrates that effective, intensive pain therapy is not just a case of administering the correct analgesic.”
“Instead, a focus should be placed on what common sense tells us, which is just how crucial an empathetic attitude from physicians and good communication with patients are when it comes to the success of any therapy,” Dr. Meissner added. Unfortunately, such an attitude and such communication often are not provided in clinical practice because of limitations on time.
“Now, with objectively collected data from patients and physicians, [Dr.] Ellingsen’s team has been able to demonstrate that human interaction has a decisive impact on the treatment of patients experiencing pain,” said Dr. Meissner. “The study should encourage practitioners to treat communication just as seriously as the pharmacology of analgesics.”
Perception and attitude
“The study shows remarkably well that empathetic conversation between the physician and patient represents a valuable therapeutic method and should be recognized as such,” emphasized Dr. Meissner. Of course, conversation cannot replace pharmacologic treatment, but it can supplement and reinforce it. Furthermore, a physician’s empathy presumably has an effect that is at least as great as a suitable analgesic.
“Pain is more than just sensory perception,” explained Dr. Meissner. “We all know that it has a strong affective component, and perception is greatly determined by context.” This can be seen, for example, in athletes, who often attribute less importance to their pain and can successfully perform competitively despite a painful injury.
Positive expectations
Dr. Meissner advised all physicians to treat patients with pain empathetically. He encourages them to ask patients about their pain, accompanying symptoms, possible fears, and other mental stress and to take these factors seriously.
Moreover, the findings accentuate the effect of prescribed analgesics. “Numerous studies have meanwhile shown that the more positive a patient’s expectations, the better the effect of a medication,” said Dr. Meissner. “We physicians must exploit this effect, too.”
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
These are the results of a study, recently published in the Proceedings of the National Academy of Sciences, that was conducted by a team led by neuroscientist Dan-Mikael Ellingsen, PhD, from Oslo University Hospital.
The researchers used functional MRI to scan the brains of 20 patients with chronic pain to investigate how a physician’s demeanor may affect patients’ sensitivity to pain, including effects in the central nervous system. During the scans, which were conducted in two sessions, the patients’ legs were exposed to stimuli that ranged from painless to moderately painful. The patients recorded perceived pain intensity using a scale. The physicians also underwent fMRI.
Half of the patients were subjected to the pain stimuli while alone; the other half were subjected to pain while in the presence of a physician. The latter group of patients was divided into two subgroups. Half of the patients had spoken to the accompanying physician before the examination. They discussed the history of the patient’s condition to date, among other things. The other half underwent the brain scans without any prior interaction with a physician.
Worse when alone
Dr. Ellingsen and his colleagues found that patients who were alone during the examination reported greater pain than those who were in the presence of a physician, even though they were subjected to stimuli of the same intensity. In instances in which the physician and patient had already spoken before the brain scan, patients additionally felt that the physician was empathetic and understood their pain. Furthermore, the physicians were better able to estimate the pain that their patients experienced.
The patients who had a physician by their side consistently experienced pain that was milder than the pain experienced by those who were alone. For pairs that had spoken beforehand, the patients considered their physician to be better able to understand their pain, and the physicians estimated the perceived pain intensity of their patients more accurately.
Evidence of trust
There was greater activity in the dorsolateral and ventrolateral prefrontal cortex, as well as in the primary and secondary somatosensory areas, in patients in the subgroup that had spoken to a physician. For the physicians, compared with the comparison group, there was an increase in correspondence between activity in the dorsolateral prefrontal cortex and activity in the secondary somatosensory areas of patients, which is a brain region that is known to react to pain. The brain activity correlation increased in line with the self-reported mutual trust between the physician and patient.
“These results prove that empathy and support can decrease pain intensity,” the investigators write. The data shed light on the brain processes behind the social modulation of pain during the interaction between the physician and the patient. Concordances in the brain are increased by greater therapeutic alliance.
Beyond medication
Winfried Meissner, MD, head of the pain clinic at the department of anesthesiology and intensive care medicine at Jena University Hospital, Germany, and former president of the German Pain Society, said in an interview: “I view this as a vital study that impressively demonstrates that effective, intensive pain therapy is not just a case of administering the correct analgesic.”
“Instead, a focus should be placed on what common sense tells us, which is just how crucial an empathetic attitude from physicians and good communication with patients are when it comes to the success of any therapy,” Dr. Meissner added. Unfortunately, such an attitude and such communication often are not provided in clinical practice because of limitations on time.
“Now, with objectively collected data from patients and physicians, [Dr.] Ellingsen’s team has been able to demonstrate that human interaction has a decisive impact on the treatment of patients experiencing pain,” said Dr. Meissner. “The study should encourage practitioners to treat communication just as seriously as the pharmacology of analgesics.”
Perception and attitude
“The study shows remarkably well that empathetic conversation between the physician and patient represents a valuable therapeutic method and should be recognized as such,” emphasized Dr. Meissner. Of course, conversation cannot replace pharmacologic treatment, but it can supplement and reinforce it. Furthermore, a physician’s empathy presumably has an effect that is at least as great as a suitable analgesic.
“Pain is more than just sensory perception,” explained Dr. Meissner. “We all know that it has a strong affective component, and perception is greatly determined by context.” This can be seen, for example, in athletes, who often attribute less importance to their pain and can successfully perform competitively despite a painful injury.
Positive expectations
Dr. Meissner advised all physicians to treat patients with pain empathetically. He encourages them to ask patients about their pain, accompanying symptoms, possible fears, and other mental stress and to take these factors seriously.
Moreover, the findings accentuate the effect of prescribed analgesics. “Numerous studies have meanwhile shown that the more positive a patient’s expectations, the better the effect of a medication,” said Dr. Meissner. “We physicians must exploit this effect, too.”
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
FROM THE PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Opioid initiation in dementia tied to an 11-fold increased risk of death
Opioid initiation for older adults with dementia is linked to a significantly increased risk of death, especially in the first 2 weeks, when the risk is elevated 11-fold, new research shows.
“We expected that opioids would be associated with an increased risk of death, but we are surprised by the magnitude,” study investigator Christina Jensen-Dahm, MD, PhD, with the Danish Dementia Research Centre, Copenhagen University Hospital, Rigshospitalet, Denmark, told this news organization.
“It’s important that physicians carefully evaluate the risk and benefits if considering initiating an opioid, and this is particularly important in elderly with dementia,” Dr. Jensen-Dahm added.
The findings were presented at the Alzheimer’s Association International Conference.
Risky business
Using Danish nationwide registries, the researchers analyzed data on all 75,471 adults in Denmark who were aged 65 and older and had been diagnosed with dementia between 2008 and 2018. A total of 31,619 individuals (42%) filled a prescription for an opioid. These “exposed” individuals were matched to 63,235 unexposed individuals.
Among the exposed group, 10,474 (33%) died within 180 days after starting opioid therapy, compared with 3,980 (6.4%) in the unexposed group.
After adjusting for potential differences between groups, new use of an opioid was associated with a greater than fourfold excess mortality risk (adjusted hazard ratio, 4.16; 95% confidence interval, 4.00-4.33).
New use of a strong opioid – defined as morphine, oxycodone, ketobemidone, hydromorphone, pethidine, buprenorphine, and fentanyl – was associated with a greater than sixfold increase in mortality risk (aHR, 6.42; 95% CI, 6.08-6.79).
Among those who used fentanyl patches as their first opioid, 65% died within the first 180 days, compared with 6.7% in the unexposed – an eightfold increased mortality risk (aHR, 8.04; 95% CI, 7.01-9.22).
For all opioids, the risk was greatest in the first 14 days, with a nearly 11-fold increased risk of mortality (aHR, 10.8; 95% CI, 9.74-11.99). However, there remained a twofold increase in risk after taking opioids for 90 days (aHR, 2.32; 95% CI, 2.17-2.48).
“Opioids are associated with severe and well-known side effects, such as sedation, confusion, respiratory depression, falls, and in the most severe cases, death. In the general population, opioids have been associated with an increased risk of death, and similar to ours, greatest in the first 14 days,” said Dr. Jensen-Dahm.
Need to weigh risks, benefits
Commenting on the study, Percy Griffin, PhD, director of scientific engagement at the Alzheimer’s Association, told this news organization that the use of strong opioids has “increased considerably over the past decade among older people with dementia. Opioid therapy should only be considered for pain if the benefits are anticipated to outweigh the risks in individuals who are living with dementia.”
“Opioids are very powerful drugs, and while we need to see additional research in more diverse populations, these initial findings indicate they may put older adults with dementia at much higher risk of death,” Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, added in a conference statement.
“Pain should not go undiagnosed or untreated, in particular in people living with dementia, who may not be able to effectively articulate the location and severity of the pain,” Dr. Purcell added.
These new findings further emphasize the need for discussion between patient, family, and physician. Decisions about prescribing pain medication should be thought through carefully, and if used, there needs to be careful monitoring of the patient, said Dr. Purcell.
The study was supported by a grant from the Capital Region of Denmark. Dr. Jensen-Dahm, Dr. Griffin, and Dr. Purcell have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioid initiation for older adults with dementia is linked to a significantly increased risk of death, especially in the first 2 weeks, when the risk is elevated 11-fold, new research shows.
“We expected that opioids would be associated with an increased risk of death, but we are surprised by the magnitude,” study investigator Christina Jensen-Dahm, MD, PhD, with the Danish Dementia Research Centre, Copenhagen University Hospital, Rigshospitalet, Denmark, told this news organization.
“It’s important that physicians carefully evaluate the risk and benefits if considering initiating an opioid, and this is particularly important in elderly with dementia,” Dr. Jensen-Dahm added.
The findings were presented at the Alzheimer’s Association International Conference.
Risky business
Using Danish nationwide registries, the researchers analyzed data on all 75,471 adults in Denmark who were aged 65 and older and had been diagnosed with dementia between 2008 and 2018. A total of 31,619 individuals (42%) filled a prescription for an opioid. These “exposed” individuals were matched to 63,235 unexposed individuals.
Among the exposed group, 10,474 (33%) died within 180 days after starting opioid therapy, compared with 3,980 (6.4%) in the unexposed group.
After adjusting for potential differences between groups, new use of an opioid was associated with a greater than fourfold excess mortality risk (adjusted hazard ratio, 4.16; 95% confidence interval, 4.00-4.33).
New use of a strong opioid – defined as morphine, oxycodone, ketobemidone, hydromorphone, pethidine, buprenorphine, and fentanyl – was associated with a greater than sixfold increase in mortality risk (aHR, 6.42; 95% CI, 6.08-6.79).
Among those who used fentanyl patches as their first opioid, 65% died within the first 180 days, compared with 6.7% in the unexposed – an eightfold increased mortality risk (aHR, 8.04; 95% CI, 7.01-9.22).
For all opioids, the risk was greatest in the first 14 days, with a nearly 11-fold increased risk of mortality (aHR, 10.8; 95% CI, 9.74-11.99). However, there remained a twofold increase in risk after taking opioids for 90 days (aHR, 2.32; 95% CI, 2.17-2.48).
“Opioids are associated with severe and well-known side effects, such as sedation, confusion, respiratory depression, falls, and in the most severe cases, death. In the general population, opioids have been associated with an increased risk of death, and similar to ours, greatest in the first 14 days,” said Dr. Jensen-Dahm.
Need to weigh risks, benefits
Commenting on the study, Percy Griffin, PhD, director of scientific engagement at the Alzheimer’s Association, told this news organization that the use of strong opioids has “increased considerably over the past decade among older people with dementia. Opioid therapy should only be considered for pain if the benefits are anticipated to outweigh the risks in individuals who are living with dementia.”
“Opioids are very powerful drugs, and while we need to see additional research in more diverse populations, these initial findings indicate they may put older adults with dementia at much higher risk of death,” Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, added in a conference statement.
“Pain should not go undiagnosed or untreated, in particular in people living with dementia, who may not be able to effectively articulate the location and severity of the pain,” Dr. Purcell added.
These new findings further emphasize the need for discussion between patient, family, and physician. Decisions about prescribing pain medication should be thought through carefully, and if used, there needs to be careful monitoring of the patient, said Dr. Purcell.
The study was supported by a grant from the Capital Region of Denmark. Dr. Jensen-Dahm, Dr. Griffin, and Dr. Purcell have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioid initiation for older adults with dementia is linked to a significantly increased risk of death, especially in the first 2 weeks, when the risk is elevated 11-fold, new research shows.
“We expected that opioids would be associated with an increased risk of death, but we are surprised by the magnitude,” study investigator Christina Jensen-Dahm, MD, PhD, with the Danish Dementia Research Centre, Copenhagen University Hospital, Rigshospitalet, Denmark, told this news organization.
“It’s important that physicians carefully evaluate the risk and benefits if considering initiating an opioid, and this is particularly important in elderly with dementia,” Dr. Jensen-Dahm added.
The findings were presented at the Alzheimer’s Association International Conference.
Risky business
Using Danish nationwide registries, the researchers analyzed data on all 75,471 adults in Denmark who were aged 65 and older and had been diagnosed with dementia between 2008 and 2018. A total of 31,619 individuals (42%) filled a prescription for an opioid. These “exposed” individuals were matched to 63,235 unexposed individuals.
Among the exposed group, 10,474 (33%) died within 180 days after starting opioid therapy, compared with 3,980 (6.4%) in the unexposed group.
After adjusting for potential differences between groups, new use of an opioid was associated with a greater than fourfold excess mortality risk (adjusted hazard ratio, 4.16; 95% confidence interval, 4.00-4.33).
New use of a strong opioid – defined as morphine, oxycodone, ketobemidone, hydromorphone, pethidine, buprenorphine, and fentanyl – was associated with a greater than sixfold increase in mortality risk (aHR, 6.42; 95% CI, 6.08-6.79).
Among those who used fentanyl patches as their first opioid, 65% died within the first 180 days, compared with 6.7% in the unexposed – an eightfold increased mortality risk (aHR, 8.04; 95% CI, 7.01-9.22).
For all opioids, the risk was greatest in the first 14 days, with a nearly 11-fold increased risk of mortality (aHR, 10.8; 95% CI, 9.74-11.99). However, there remained a twofold increase in risk after taking opioids for 90 days (aHR, 2.32; 95% CI, 2.17-2.48).
“Opioids are associated with severe and well-known side effects, such as sedation, confusion, respiratory depression, falls, and in the most severe cases, death. In the general population, opioids have been associated with an increased risk of death, and similar to ours, greatest in the first 14 days,” said Dr. Jensen-Dahm.
Need to weigh risks, benefits
Commenting on the study, Percy Griffin, PhD, director of scientific engagement at the Alzheimer’s Association, told this news organization that the use of strong opioids has “increased considerably over the past decade among older people with dementia. Opioid therapy should only be considered for pain if the benefits are anticipated to outweigh the risks in individuals who are living with dementia.”
“Opioids are very powerful drugs, and while we need to see additional research in more diverse populations, these initial findings indicate they may put older adults with dementia at much higher risk of death,” Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, added in a conference statement.
“Pain should not go undiagnosed or untreated, in particular in people living with dementia, who may not be able to effectively articulate the location and severity of the pain,” Dr. Purcell added.
These new findings further emphasize the need for discussion between patient, family, and physician. Decisions about prescribing pain medication should be thought through carefully, and if used, there needs to be careful monitoring of the patient, said Dr. Purcell.
The study was supported by a grant from the Capital Region of Denmark. Dr. Jensen-Dahm, Dr. Griffin, and Dr. Purcell have no relevant disclosures.
A version of this article first appeared on Medscape.com.
From AAIC 2023
Fibromyalgia linked to higher mortality risk
People who experience chronic pain and tiredness from fibromyalgia have an increased risk for all-cause mortality, a new analysis of evidence says.
The condition can lead people to be vulnerable to accidents, infections, and even suicide, according to the report published in RMD Open.
The researchers suggest that care providers monitor physical and mental health to lower the dangers.
People with fibromyalgia often have other health issues, including rheumatic, gut, neurological, and mental health disorders, according to The BMJ. More and more people are being diagnosed with fibromyalgia. The cause of the illness remains unclear.
The researchers looked at eight studies published between 1999 and 2020 and pooled results from six of them. The studies involved a total of 188,000 adults.
The analysis of the data revealed that fibromyalgia was linked to a 27% greater risk of death from all causes.
Those with fibromyalgia were at a 44% greater risk of infections, including pneumonia. Their suicide risk was more than three times higher.
The greater risk of all-cause death could result from fatigue, poor sleep, and concentration problems, The BMJ said.
The patients had a 12% lower risk of dying from cancer, the analysis found. This could be because they tend to make more visits to health care professionals, the authors suggest.
“Fibromyalgia is often called an ‘imaginary condition,’ with ongoing debates on the legitimacy and clinical usefulness of this diagnosis. Our review provides further proof that fibromyalgia patients should be taken seriously, with particular focus on screening for suicidal ideation, prevention of accidents, and prevention and treatment of infections,” the researchers say.
A version of this article appeared on WebMD.com.
People who experience chronic pain and tiredness from fibromyalgia have an increased risk for all-cause mortality, a new analysis of evidence says.
The condition can lead people to be vulnerable to accidents, infections, and even suicide, according to the report published in RMD Open.
The researchers suggest that care providers monitor physical and mental health to lower the dangers.
People with fibromyalgia often have other health issues, including rheumatic, gut, neurological, and mental health disorders, according to The BMJ. More and more people are being diagnosed with fibromyalgia. The cause of the illness remains unclear.
The researchers looked at eight studies published between 1999 and 2020 and pooled results from six of them. The studies involved a total of 188,000 adults.
The analysis of the data revealed that fibromyalgia was linked to a 27% greater risk of death from all causes.
Those with fibromyalgia were at a 44% greater risk of infections, including pneumonia. Their suicide risk was more than three times higher.
The greater risk of all-cause death could result from fatigue, poor sleep, and concentration problems, The BMJ said.
The patients had a 12% lower risk of dying from cancer, the analysis found. This could be because they tend to make more visits to health care professionals, the authors suggest.
“Fibromyalgia is often called an ‘imaginary condition,’ with ongoing debates on the legitimacy and clinical usefulness of this diagnosis. Our review provides further proof that fibromyalgia patients should be taken seriously, with particular focus on screening for suicidal ideation, prevention of accidents, and prevention and treatment of infections,” the researchers say.
A version of this article appeared on WebMD.com.
People who experience chronic pain and tiredness from fibromyalgia have an increased risk for all-cause mortality, a new analysis of evidence says.
The condition can lead people to be vulnerable to accidents, infections, and even suicide, according to the report published in RMD Open.
The researchers suggest that care providers monitor physical and mental health to lower the dangers.
People with fibromyalgia often have other health issues, including rheumatic, gut, neurological, and mental health disorders, according to The BMJ. More and more people are being diagnosed with fibromyalgia. The cause of the illness remains unclear.
The researchers looked at eight studies published between 1999 and 2020 and pooled results from six of them. The studies involved a total of 188,000 adults.
The analysis of the data revealed that fibromyalgia was linked to a 27% greater risk of death from all causes.
Those with fibromyalgia were at a 44% greater risk of infections, including pneumonia. Their suicide risk was more than three times higher.
The greater risk of all-cause death could result from fatigue, poor sleep, and concentration problems, The BMJ said.
The patients had a 12% lower risk of dying from cancer, the analysis found. This could be because they tend to make more visits to health care professionals, the authors suggest.
“Fibromyalgia is often called an ‘imaginary condition,’ with ongoing debates on the legitimacy and clinical usefulness of this diagnosis. Our review provides further proof that fibromyalgia patients should be taken seriously, with particular focus on screening for suicidal ideation, prevention of accidents, and prevention and treatment of infections,” the researchers say.
A version of this article appeared on WebMD.com.
Medical cannabis does not reduce use of prescription meds
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
‘Landmark’ trial shows opioids for back, neck pain no better than placebo
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
New international guidelines on opioid deprescribing
An expert panel of pain management clinicians has released what they say are the first international guidelines for general practitioners on opioid analgesic deprescribing in adults.
The recommendations describe best practices for stopping opioid therapy and emphasize slow tapering and individualized deprescribing plans tailored to each patient.
Developed by general practitioners, pain specialists, addiction specialists, pharmacists, registered nurses, consumers, and physiotherapists, the guidelines note that deprescribing may not be appropriate for every patient and that stopping abruptly can be associated with an increased risk of overdose.
“Internationally, we were seeing significant harms from opioids but also significant harms from unsolicited and abrupt opioid cessation,” said lead author Aili Langford, PhD, who conducted the study as a doctoral student at the University of Sydney. “It was clear that recommendations to support safe and person-centered opioid deprescribing were required.”
The findings were published online in the Medical Journal of Australia.
Deprescribing plan
The consensus guidelines include 11 recommendations for deprescribing in adult patients who take at least one opioid for any type of pain.
Recommendations include implementing a deprescribing plan when opioids are first prescribed and gradual and individualized deprescribing, with regular monitoring and review.
Clinicians should consider opioid deprescribing in patients who experience no clinically meaningful improvement in function, quality of life, or pain at high risk with opioid therapy, they note. Patients who are at high risk for opioid-related harm are also good candidates for deprescribing.
Stopping opioid therapy is not recommended for patients with severe opioid use disorder (OUD). In those patients, medication-assisted OUD treatment and other evidence-based interventions are recommended.
“Opioids can be effective in pain management,” co-author Carl Schneider, PhD, an associate professor of pharmacy at the University of Sydney, said in a press release. “However, over the longer term, the risk of harms may outweigh the benefits.”
A ‘global problem’
Commenting on the guidelines, Orman Trent Hall, DO, assistant professor of addiction medicine, department of psychiatry and behavioral health at the Ohio State University Wexner Medical Center, Columbus, said they are similar to recommendations published by the U.S. Centers for Disease Control and Prevention in 2016 and 2022 but offer additional information that could be helpful.
“This new guideline provides more explicit advice about tapering and withdrawal management, which may be useful to practitioners. The opioid crisis is a global problem, and while individual countries may require local solutions, the new international guideline may offer a framework for approaching this issue,” he said.
The guideline’s emphasis on the potential risks of deprescribing in some patients is also key, Dr. Hall added. Patients who are tapering off opioid therapy may have worsening pain and loss of function that can affect their quality of life.
“Patients may also experience psychological harm and increased risk of opioid use disorder and death by suicide following opioid deprescribing,” Dr. Hall said. “Therefore, it is important for providers to carefully weigh the risks of prescribing and deprescribing and engage patients with person-centered communication and shared decision-making.”
The work was funded by grants from the University of Sydney and the National Health and Medical Research Council. Full disclosures are available in the original article. Dr. Hall has provided expert opinion to the health care consultancy firm Lumanity and Emergent BioSolutions regarding the overdose crisis.
A version of this article originally appeared on Medscape.com.
An expert panel of pain management clinicians has released what they say are the first international guidelines for general practitioners on opioid analgesic deprescribing in adults.
The recommendations describe best practices for stopping opioid therapy and emphasize slow tapering and individualized deprescribing plans tailored to each patient.
Developed by general practitioners, pain specialists, addiction specialists, pharmacists, registered nurses, consumers, and physiotherapists, the guidelines note that deprescribing may not be appropriate for every patient and that stopping abruptly can be associated with an increased risk of overdose.
“Internationally, we were seeing significant harms from opioids but also significant harms from unsolicited and abrupt opioid cessation,” said lead author Aili Langford, PhD, who conducted the study as a doctoral student at the University of Sydney. “It was clear that recommendations to support safe and person-centered opioid deprescribing were required.”
The findings were published online in the Medical Journal of Australia.
Deprescribing plan
The consensus guidelines include 11 recommendations for deprescribing in adult patients who take at least one opioid for any type of pain.
Recommendations include implementing a deprescribing plan when opioids are first prescribed and gradual and individualized deprescribing, with regular monitoring and review.
Clinicians should consider opioid deprescribing in patients who experience no clinically meaningful improvement in function, quality of life, or pain at high risk with opioid therapy, they note. Patients who are at high risk for opioid-related harm are also good candidates for deprescribing.
Stopping opioid therapy is not recommended for patients with severe opioid use disorder (OUD). In those patients, medication-assisted OUD treatment and other evidence-based interventions are recommended.
“Opioids can be effective in pain management,” co-author Carl Schneider, PhD, an associate professor of pharmacy at the University of Sydney, said in a press release. “However, over the longer term, the risk of harms may outweigh the benefits.”
A ‘global problem’
Commenting on the guidelines, Orman Trent Hall, DO, assistant professor of addiction medicine, department of psychiatry and behavioral health at the Ohio State University Wexner Medical Center, Columbus, said they are similar to recommendations published by the U.S. Centers for Disease Control and Prevention in 2016 and 2022 but offer additional information that could be helpful.
“This new guideline provides more explicit advice about tapering and withdrawal management, which may be useful to practitioners. The opioid crisis is a global problem, and while individual countries may require local solutions, the new international guideline may offer a framework for approaching this issue,” he said.
The guideline’s emphasis on the potential risks of deprescribing in some patients is also key, Dr. Hall added. Patients who are tapering off opioid therapy may have worsening pain and loss of function that can affect their quality of life.
“Patients may also experience psychological harm and increased risk of opioid use disorder and death by suicide following opioid deprescribing,” Dr. Hall said. “Therefore, it is important for providers to carefully weigh the risks of prescribing and deprescribing and engage patients with person-centered communication and shared decision-making.”
The work was funded by grants from the University of Sydney and the National Health and Medical Research Council. Full disclosures are available in the original article. Dr. Hall has provided expert opinion to the health care consultancy firm Lumanity and Emergent BioSolutions regarding the overdose crisis.
A version of this article originally appeared on Medscape.com.
An expert panel of pain management clinicians has released what they say are the first international guidelines for general practitioners on opioid analgesic deprescribing in adults.
The recommendations describe best practices for stopping opioid therapy and emphasize slow tapering and individualized deprescribing plans tailored to each patient.
Developed by general practitioners, pain specialists, addiction specialists, pharmacists, registered nurses, consumers, and physiotherapists, the guidelines note that deprescribing may not be appropriate for every patient and that stopping abruptly can be associated with an increased risk of overdose.
“Internationally, we were seeing significant harms from opioids but also significant harms from unsolicited and abrupt opioid cessation,” said lead author Aili Langford, PhD, who conducted the study as a doctoral student at the University of Sydney. “It was clear that recommendations to support safe and person-centered opioid deprescribing were required.”
The findings were published online in the Medical Journal of Australia.
Deprescribing plan
The consensus guidelines include 11 recommendations for deprescribing in adult patients who take at least one opioid for any type of pain.
Recommendations include implementing a deprescribing plan when opioids are first prescribed and gradual and individualized deprescribing, with regular monitoring and review.
Clinicians should consider opioid deprescribing in patients who experience no clinically meaningful improvement in function, quality of life, or pain at high risk with opioid therapy, they note. Patients who are at high risk for opioid-related harm are also good candidates for deprescribing.
Stopping opioid therapy is not recommended for patients with severe opioid use disorder (OUD). In those patients, medication-assisted OUD treatment and other evidence-based interventions are recommended.
“Opioids can be effective in pain management,” co-author Carl Schneider, PhD, an associate professor of pharmacy at the University of Sydney, said in a press release. “However, over the longer term, the risk of harms may outweigh the benefits.”
A ‘global problem’
Commenting on the guidelines, Orman Trent Hall, DO, assistant professor of addiction medicine, department of psychiatry and behavioral health at the Ohio State University Wexner Medical Center, Columbus, said they are similar to recommendations published by the U.S. Centers for Disease Control and Prevention in 2016 and 2022 but offer additional information that could be helpful.
“This new guideline provides more explicit advice about tapering and withdrawal management, which may be useful to practitioners. The opioid crisis is a global problem, and while individual countries may require local solutions, the new international guideline may offer a framework for approaching this issue,” he said.
The guideline’s emphasis on the potential risks of deprescribing in some patients is also key, Dr. Hall added. Patients who are tapering off opioid therapy may have worsening pain and loss of function that can affect their quality of life.
“Patients may also experience psychological harm and increased risk of opioid use disorder and death by suicide following opioid deprescribing,” Dr. Hall said. “Therefore, it is important for providers to carefully weigh the risks of prescribing and deprescribing and engage patients with person-centered communication and shared decision-making.”
The work was funded by grants from the University of Sydney and the National Health and Medical Research Council. Full disclosures are available in the original article. Dr. Hall has provided expert opinion to the health care consultancy firm Lumanity and Emergent BioSolutions regarding the overdose crisis.
A version of this article originally appeared on Medscape.com.
New DEA CME mandate affects 2 million prescribers
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.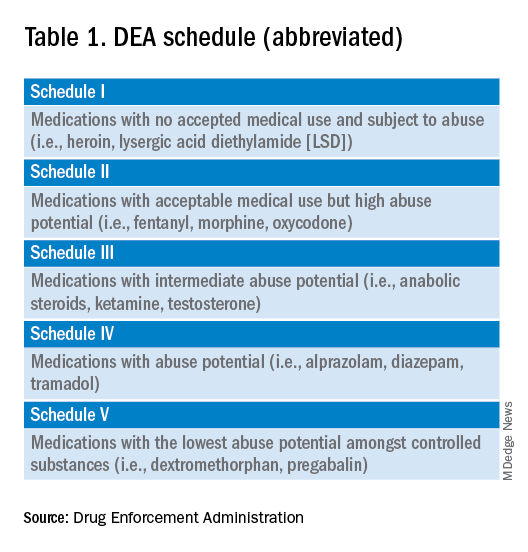
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 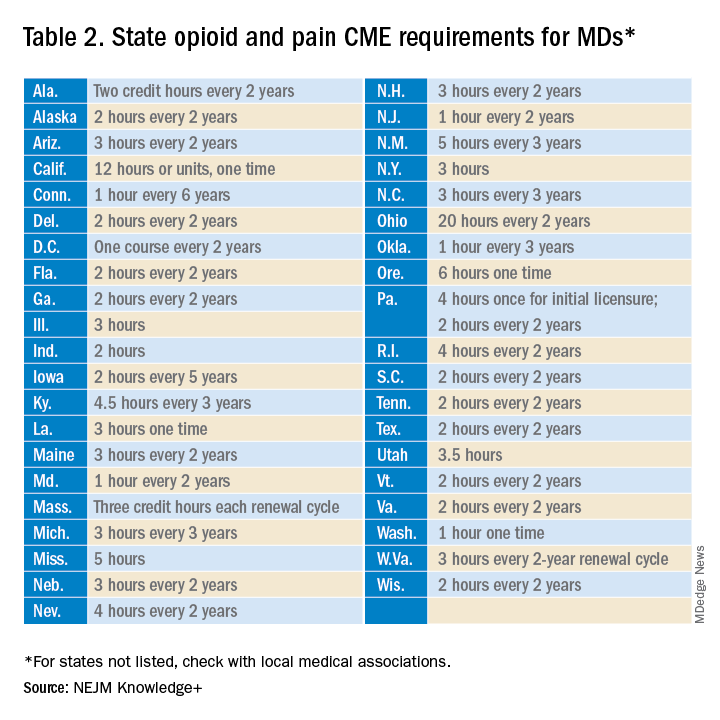
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
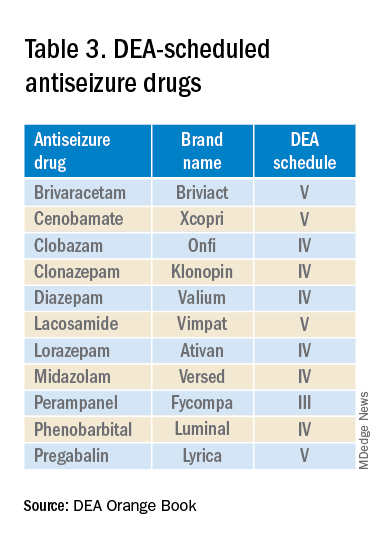
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).
Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
Migraine device expands treatment possibilities
AUSTIN, TEX – Migraine treatment and prevention is challenging in any population, but some present even more difficulties. Pregnant women and pediatric patients are two such groups where physicians and patients may be hesitant to use drugs.
Neuromodulation devices are proven alternatives to medical interventions, and the remote electrical neuromodulation device Nerivio (Theranica) was cleared by the Food and Drug Administration for acute treatment of migraine patients aged 12 and over in 2021. In March 2023, the agency expanded the clearance to include prevention of migration in adolescents aged 12 and over as well as adults.
Two studies presented at the annual meeting of the American Headache Society showed The latter study yielded similar findings to adults and was used by FDA in its decision to expand the device’s indication in adolescents in 2023, according to Teshamae Monteith, MD, who presented the study at a poster session.
The device, worn on the arm, allows the user to modulate the intensity of the stimulation so that it activates nociceptive pain receptors, but not in a painful way. “Each [patient] raises the intensity until it feels strong, yet comfortable, and when that happens, they activate the nociceptive receptors and the arm sends a signal all the way back up to the brainstem, where the pain control area is. Activating it causes the release of neurotransmitters that inhibit pain. That inhibition is a global pain inhibition mechanism, which causes inhibition of the migraine pain, and also the symptoms associated with migraine like photophobia and vomiting,” said Alit Stark-Inbar, PhD, who presented the study of treatment of pregnant women during a poster session.
Declining treatment days over time in adolescents
Dr. Monteith’s team studied high-frequency remote electrical neuromodulation device use in adolescents who had migraine on 10 days or more per month. They also required at least three treatment days in months 2 and 3 to control for the possibility that patients might stop using the device because they couldn’t afford it or for some reason other than efficacy or because their migraines went away.
The study included 83 adolescents aged 12-17 (mean, 15.9 years, 89% female). In the first month of use, the mean number of migraine treatment days was 12.6, which dropped to 9.0 in month 2 (P < .001), and 7.4 in month 3 (P < .001 from month 2). At 2 hours after treatment, 61.9% had pain relief, 24.5% had freedom from pain, 67.4% had functional disability relief, and 41.3% had functional disability freedom.
“It parallels the findings of the randomized, sham-controlled study in adults. The safety profile was excellent with just one person complaining of minor discomfort of the arm that resolved after treatment. The combination of the exceedingly safe profile and the likelihood of efficacy based on using monthly migraine treatment days as a proxy, the FDA decided to clear this for an adolescent indication,” said Dr. Monteith, associate professor of clinical neurology and chief of the headache division at the University of Miami.
The device design is convenient, according to Dr. Monteith. “The arm is just an easy place to stimulate. It’s a wearable device, and it’s 45 minutes [of treatment] and it’s app controlled. You know adolescents like their technology. They can track their symptoms here, and there’s some biobehavioral power to this because they can do biobehavioral exercises in addition to receiving the simulation,” she said.
The fact that the device is discrete is also an advantage for adolescents in school. “You have to go to the nurse to get your medication versus a device, you can just put it on, it’s easy, no one sees it, and no one’s making fun of you,” said Dr. Monteith.
Advantages for adolescents
The device offers a useful alternative to medication, according to Alan M. Rapoport, MD, who was asked for comment on the adolescent study. “I’d rather not give medication and certainly not preventive medication to an adolescent,” he said. He noted that over-the-counter acute care migraine medications such as aspirin or acetaminophen and combination medications with caffeine, as well as prescription medications such as triptans, “all have possible side effects, and when used to an increased extent can even cause medication overuse headache, increasing the severity and frequency of headache and migraine days per month,” Dr. Rapoport said. Using an effective device with almost no side effects is preferable to any of these acute care medications, especially if there are several headaches a month,” he said. Some newer medications that block calcitonin gene-related peptide might be quite effective when they are approved for adolescents, and should have few adverse events, he added.
In the past, Dr. Rapoport has favored biofeedback training for acute and especially preventive treatment of migraine in adolescents. “[Remote electrical neuromodulation] seems to do just as well, children enjoy it, and it’s easier for a patient to do at home,” said Dr. Rapoport.
Biofeedback training is usually taught to patients by a PhD psychologist. Once the patients have been on the biofeedback equipment and learn the techniques, they can practice on their own at home without equipment. “This new device treatment using Nerivio for acute care and prevention of migraine in adults and children 12 and older, where they can easily apply the device in almost any situation, whether they are at home or possibly even in school or out and about, looks very promising,” said Dr. Rapoport. It is quite effective and has almost no adverse events, which is what you really want, especially for adolescents,” he said.
Also asked to comment on the study of remote electrical neuromodulation use in adolescents, Abraham Avi Ashkenazi, MD, director of the Headache Clinic at Shaare Zedek Medical Center in Jerusalem, who attended the session, was enthusiastic, and said he has begun using it in his own practice. “It shows that remote electrical neuromodulation can not only be effective for the acute migraine attack, but also has a potential preventive effect on future migraine attacks. [This] actually makes sense, because we know that the more migraine attacks a person has, the more likely they are to progress to a more chronic form of the disease,” he said in an interview.
Asked what distinguishes REN from other neuromodulation therapies such as vagus nerve stimulation or transcranial magnetic stimulation (TMS), Dr. Ashkenazi said: “It’s just a different way of modulating the brain system via a different mechanism. In both ways, though, the advantage is that there are literally no adverse effects, as opposed to drug treatment.”
An alternative during pregnancy
Adolescents aren’t the only population where there is reluctance to use medication. Physicians have been prescribing the device for pregnant women, who are reluctant to take medication due to concerns effects on the fetus. However, pregnant women were not included in the pivotal studies. “They expect it to be safe. This study was done in order to validate that assumption. We reached out to women who either used the device during pregnancy or women from the same database who started it using afterwards, but did not use it during the pregnancy,” said Dr. Stark-Inbar, vice president of medical information at Theranica.
The study included 140 women, 59 in the remote electrical neuromodulation device group and 81 controls. The primary endpoint was gestational age, which was 38 weeks and 5 days in the remote electrical neuromodulation device group and 39 weeks among controls (P = .150). There were no significant between-group differences with respect to newborn birth weight, miscarriage rate, preterm birth rate, birth defect rate, developmental milestone rate, or emergency department visit rate.
Dr. Monteith and Dr. Ashkenazi have no relevant financial disclosures. Dr. Rapoport advises AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica. He is on the speakers bureau of AbbVie, Dr. Reddy’s, Impel, Pfizer and Teva Pharmaceutical Industries. Dr. Rapoport is the editor-in-chief of Neurology Reviews and on the editorial board of CNS Drugs.
AUSTIN, TEX – Migraine treatment and prevention is challenging in any population, but some present even more difficulties. Pregnant women and pediatric patients are two such groups where physicians and patients may be hesitant to use drugs.
Neuromodulation devices are proven alternatives to medical interventions, and the remote electrical neuromodulation device Nerivio (Theranica) was cleared by the Food and Drug Administration for acute treatment of migraine patients aged 12 and over in 2021. In March 2023, the agency expanded the clearance to include prevention of migration in adolescents aged 12 and over as well as adults.
Two studies presented at the annual meeting of the American Headache Society showed The latter study yielded similar findings to adults and was used by FDA in its decision to expand the device’s indication in adolescents in 2023, according to Teshamae Monteith, MD, who presented the study at a poster session.
The device, worn on the arm, allows the user to modulate the intensity of the stimulation so that it activates nociceptive pain receptors, but not in a painful way. “Each [patient] raises the intensity until it feels strong, yet comfortable, and when that happens, they activate the nociceptive receptors and the arm sends a signal all the way back up to the brainstem, where the pain control area is. Activating it causes the release of neurotransmitters that inhibit pain. That inhibition is a global pain inhibition mechanism, which causes inhibition of the migraine pain, and also the symptoms associated with migraine like photophobia and vomiting,” said Alit Stark-Inbar, PhD, who presented the study of treatment of pregnant women during a poster session.
Declining treatment days over time in adolescents
Dr. Monteith’s team studied high-frequency remote electrical neuromodulation device use in adolescents who had migraine on 10 days or more per month. They also required at least three treatment days in months 2 and 3 to control for the possibility that patients might stop using the device because they couldn’t afford it or for some reason other than efficacy or because their migraines went away.
The study included 83 adolescents aged 12-17 (mean, 15.9 years, 89% female). In the first month of use, the mean number of migraine treatment days was 12.6, which dropped to 9.0 in month 2 (P < .001), and 7.4 in month 3 (P < .001 from month 2). At 2 hours after treatment, 61.9% had pain relief, 24.5% had freedom from pain, 67.4% had functional disability relief, and 41.3% had functional disability freedom.
“It parallels the findings of the randomized, sham-controlled study in adults. The safety profile was excellent with just one person complaining of minor discomfort of the arm that resolved after treatment. The combination of the exceedingly safe profile and the likelihood of efficacy based on using monthly migraine treatment days as a proxy, the FDA decided to clear this for an adolescent indication,” said Dr. Monteith, associate professor of clinical neurology and chief of the headache division at the University of Miami.
The device design is convenient, according to Dr. Monteith. “The arm is just an easy place to stimulate. It’s a wearable device, and it’s 45 minutes [of treatment] and it’s app controlled. You know adolescents like their technology. They can track their symptoms here, and there’s some biobehavioral power to this because they can do biobehavioral exercises in addition to receiving the simulation,” she said.
The fact that the device is discrete is also an advantage for adolescents in school. “You have to go to the nurse to get your medication versus a device, you can just put it on, it’s easy, no one sees it, and no one’s making fun of you,” said Dr. Monteith.
Advantages for adolescents
The device offers a useful alternative to medication, according to Alan M. Rapoport, MD, who was asked for comment on the adolescent study. “I’d rather not give medication and certainly not preventive medication to an adolescent,” he said. He noted that over-the-counter acute care migraine medications such as aspirin or acetaminophen and combination medications with caffeine, as well as prescription medications such as triptans, “all have possible side effects, and when used to an increased extent can even cause medication overuse headache, increasing the severity and frequency of headache and migraine days per month,” Dr. Rapoport said. Using an effective device with almost no side effects is preferable to any of these acute care medications, especially if there are several headaches a month,” he said. Some newer medications that block calcitonin gene-related peptide might be quite effective when they are approved for adolescents, and should have few adverse events, he added.
In the past, Dr. Rapoport has favored biofeedback training for acute and especially preventive treatment of migraine in adolescents. “[Remote electrical neuromodulation] seems to do just as well, children enjoy it, and it’s easier for a patient to do at home,” said Dr. Rapoport.
Biofeedback training is usually taught to patients by a PhD psychologist. Once the patients have been on the biofeedback equipment and learn the techniques, they can practice on their own at home without equipment. “This new device treatment using Nerivio for acute care and prevention of migraine in adults and children 12 and older, where they can easily apply the device in almost any situation, whether they are at home or possibly even in school or out and about, looks very promising,” said Dr. Rapoport. It is quite effective and has almost no adverse events, which is what you really want, especially for adolescents,” he said.
Also asked to comment on the study of remote electrical neuromodulation use in adolescents, Abraham Avi Ashkenazi, MD, director of the Headache Clinic at Shaare Zedek Medical Center in Jerusalem, who attended the session, was enthusiastic, and said he has begun using it in his own practice. “It shows that remote electrical neuromodulation can not only be effective for the acute migraine attack, but also has a potential preventive effect on future migraine attacks. [This] actually makes sense, because we know that the more migraine attacks a person has, the more likely they are to progress to a more chronic form of the disease,” he said in an interview.
Asked what distinguishes REN from other neuromodulation therapies such as vagus nerve stimulation or transcranial magnetic stimulation (TMS), Dr. Ashkenazi said: “It’s just a different way of modulating the brain system via a different mechanism. In both ways, though, the advantage is that there are literally no adverse effects, as opposed to drug treatment.”
An alternative during pregnancy
Adolescents aren’t the only population where there is reluctance to use medication. Physicians have been prescribing the device for pregnant women, who are reluctant to take medication due to concerns effects on the fetus. However, pregnant women were not included in the pivotal studies. “They expect it to be safe. This study was done in order to validate that assumption. We reached out to women who either used the device during pregnancy or women from the same database who started it using afterwards, but did not use it during the pregnancy,” said Dr. Stark-Inbar, vice president of medical information at Theranica.
The study included 140 women, 59 in the remote electrical neuromodulation device group and 81 controls. The primary endpoint was gestational age, which was 38 weeks and 5 days in the remote electrical neuromodulation device group and 39 weeks among controls (P = .150). There were no significant between-group differences with respect to newborn birth weight, miscarriage rate, preterm birth rate, birth defect rate, developmental milestone rate, or emergency department visit rate.
Dr. Monteith and Dr. Ashkenazi have no relevant financial disclosures. Dr. Rapoport advises AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica. He is on the speakers bureau of AbbVie, Dr. Reddy’s, Impel, Pfizer and Teva Pharmaceutical Industries. Dr. Rapoport is the editor-in-chief of Neurology Reviews and on the editorial board of CNS Drugs.
AUSTIN, TEX – Migraine treatment and prevention is challenging in any population, but some present even more difficulties. Pregnant women and pediatric patients are two such groups where physicians and patients may be hesitant to use drugs.
Neuromodulation devices are proven alternatives to medical interventions, and the remote electrical neuromodulation device Nerivio (Theranica) was cleared by the Food and Drug Administration for acute treatment of migraine patients aged 12 and over in 2021. In March 2023, the agency expanded the clearance to include prevention of migration in adolescents aged 12 and over as well as adults.
Two studies presented at the annual meeting of the American Headache Society showed The latter study yielded similar findings to adults and was used by FDA in its decision to expand the device’s indication in adolescents in 2023, according to Teshamae Monteith, MD, who presented the study at a poster session.
The device, worn on the arm, allows the user to modulate the intensity of the stimulation so that it activates nociceptive pain receptors, but not in a painful way. “Each [patient] raises the intensity until it feels strong, yet comfortable, and when that happens, they activate the nociceptive receptors and the arm sends a signal all the way back up to the brainstem, where the pain control area is. Activating it causes the release of neurotransmitters that inhibit pain. That inhibition is a global pain inhibition mechanism, which causes inhibition of the migraine pain, and also the symptoms associated with migraine like photophobia and vomiting,” said Alit Stark-Inbar, PhD, who presented the study of treatment of pregnant women during a poster session.
Declining treatment days over time in adolescents
Dr. Monteith’s team studied high-frequency remote electrical neuromodulation device use in adolescents who had migraine on 10 days or more per month. They also required at least three treatment days in months 2 and 3 to control for the possibility that patients might stop using the device because they couldn’t afford it or for some reason other than efficacy or because their migraines went away.
The study included 83 adolescents aged 12-17 (mean, 15.9 years, 89% female). In the first month of use, the mean number of migraine treatment days was 12.6, which dropped to 9.0 in month 2 (P < .001), and 7.4 in month 3 (P < .001 from month 2). At 2 hours after treatment, 61.9% had pain relief, 24.5% had freedom from pain, 67.4% had functional disability relief, and 41.3% had functional disability freedom.
“It parallels the findings of the randomized, sham-controlled study in adults. The safety profile was excellent with just one person complaining of minor discomfort of the arm that resolved after treatment. The combination of the exceedingly safe profile and the likelihood of efficacy based on using monthly migraine treatment days as a proxy, the FDA decided to clear this for an adolescent indication,” said Dr. Monteith, associate professor of clinical neurology and chief of the headache division at the University of Miami.
The device design is convenient, according to Dr. Monteith. “The arm is just an easy place to stimulate. It’s a wearable device, and it’s 45 minutes [of treatment] and it’s app controlled. You know adolescents like their technology. They can track their symptoms here, and there’s some biobehavioral power to this because they can do biobehavioral exercises in addition to receiving the simulation,” she said.
The fact that the device is discrete is also an advantage for adolescents in school. “You have to go to the nurse to get your medication versus a device, you can just put it on, it’s easy, no one sees it, and no one’s making fun of you,” said Dr. Monteith.
Advantages for adolescents
The device offers a useful alternative to medication, according to Alan M. Rapoport, MD, who was asked for comment on the adolescent study. “I’d rather not give medication and certainly not preventive medication to an adolescent,” he said. He noted that over-the-counter acute care migraine medications such as aspirin or acetaminophen and combination medications with caffeine, as well as prescription medications such as triptans, “all have possible side effects, and when used to an increased extent can even cause medication overuse headache, increasing the severity and frequency of headache and migraine days per month,” Dr. Rapoport said. Using an effective device with almost no side effects is preferable to any of these acute care medications, especially if there are several headaches a month,” he said. Some newer medications that block calcitonin gene-related peptide might be quite effective when they are approved for adolescents, and should have few adverse events, he added.
In the past, Dr. Rapoport has favored biofeedback training for acute and especially preventive treatment of migraine in adolescents. “[Remote electrical neuromodulation] seems to do just as well, children enjoy it, and it’s easier for a patient to do at home,” said Dr. Rapoport.
Biofeedback training is usually taught to patients by a PhD psychologist. Once the patients have been on the biofeedback equipment and learn the techniques, they can practice on their own at home without equipment. “This new device treatment using Nerivio for acute care and prevention of migraine in adults and children 12 and older, where they can easily apply the device in almost any situation, whether they are at home or possibly even in school or out and about, looks very promising,” said Dr. Rapoport. It is quite effective and has almost no adverse events, which is what you really want, especially for adolescents,” he said.
Also asked to comment on the study of remote electrical neuromodulation use in adolescents, Abraham Avi Ashkenazi, MD, director of the Headache Clinic at Shaare Zedek Medical Center in Jerusalem, who attended the session, was enthusiastic, and said he has begun using it in his own practice. “It shows that remote electrical neuromodulation can not only be effective for the acute migraine attack, but also has a potential preventive effect on future migraine attacks. [This] actually makes sense, because we know that the more migraine attacks a person has, the more likely they are to progress to a more chronic form of the disease,” he said in an interview.
Asked what distinguishes REN from other neuromodulation therapies such as vagus nerve stimulation or transcranial magnetic stimulation (TMS), Dr. Ashkenazi said: “It’s just a different way of modulating the brain system via a different mechanism. In both ways, though, the advantage is that there are literally no adverse effects, as opposed to drug treatment.”
An alternative during pregnancy
Adolescents aren’t the only population where there is reluctance to use medication. Physicians have been prescribing the device for pregnant women, who are reluctant to take medication due to concerns effects on the fetus. However, pregnant women were not included in the pivotal studies. “They expect it to be safe. This study was done in order to validate that assumption. We reached out to women who either used the device during pregnancy or women from the same database who started it using afterwards, but did not use it during the pregnancy,” said Dr. Stark-Inbar, vice president of medical information at Theranica.
The study included 140 women, 59 in the remote electrical neuromodulation device group and 81 controls. The primary endpoint was gestational age, which was 38 weeks and 5 days in the remote electrical neuromodulation device group and 39 weeks among controls (P = .150). There were no significant between-group differences with respect to newborn birth weight, miscarriage rate, preterm birth rate, birth defect rate, developmental milestone rate, or emergency department visit rate.
Dr. Monteith and Dr. Ashkenazi have no relevant financial disclosures. Dr. Rapoport advises AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica. He is on the speakers bureau of AbbVie, Dr. Reddy’s, Impel, Pfizer and Teva Pharmaceutical Industries. Dr. Rapoport is the editor-in-chief of Neurology Reviews and on the editorial board of CNS Drugs.
AT AHS 2023
Consider mental health and social factors in management of sickle cell disease
Complications from sickle cell disease (SCD) can affect education and life opportunities, and these complications have been associated with social determinants of health such as socioeconomic status, depression, health literacy, and level of education, according to Kelly M. Harris, PhD, of Washington University in St. Louis, and colleagues.
Pain is a hallmark of SCD, and “the current climate around pain management and opioid use has specific implications for individuals with [SCD], especially youth,” Dr. Harris said in an interview.
In a study published in JAMA Network Open, the researchers analyzed 2,264 participants (average age, 27.9 years; 56.2% were female) in the Sickle Cell Disease Implementation Consortium a study that includes patient assessment, treatment, and creation of a longitudinal registry.
The participants completed the Adult Sickle Cell Quality of Life Measurement Information System to provide data on the frequency and severity of pain episodes related to SCD over the past 12 months. Multivariable regression analysis was used to examine the associations of education, employment, and mental health with pain frequency and severity.
Overall, 79.8% of participants reported severe pain, and 47.8% reported more than four episodes of pain in the past year.
Notably, 20% of the participants were diagnosed with depression, and increased pain frequency was significantly associated with depression, although no significant association appeared between pain severity and depression, the researchers said.
A total of 47% of the participants reported using pain medication and 49% reported using hydroxyurea. In addition, 628 participants (28.0%) underwent regular blood transfusions.
Neither education level nor income was associated with increased pain frequency or severity. Age younger than 18 years was significantly associated with both pain frequency and severity, as was daily used of pain medication. Unemployment and female sex also were associated with increased pain frequency.
The findings were limited by several factors including the cross-sectional design that prevents conclusions of causality, and by the reliance on patient reports of depression, which likely led to underreporting, the researchers noted.
However, the results are consistent with previous studies suggesting that pain and negative feelings were associated with reduced quality of life in SCD patients, especially younger patients, and support the need to screen SCD patients for depression, especially those who report more severe and/or more frequent pain, they said.
Take a comprehensive approach to a complex condition
“When treating pain, we cannot just rely on medication,” Dr. Harris said. “It is important that providers consider the full experiences of patients and pursue holistic and comprehensive treatment approaches to reducing pain. Screening for depression should be a regular practice, particularly for patients experiencing frequent and/or severe pain.
“Racial discrimination, stigma, and bias impact pain diagnosis and treatment for individuals with SCD,” said Dr. Harris. “Increasing awareness of the associations between depression and pain frequency and severity ... may help address these barriers.”
Data highlight treatment gaps
Alexander A. Boucher, MD, a member of the division of pediatric hematology and oncology at the University of Minnesota, Minneapolis, noted the researchers included patients as young as midadolescence, with a majority being under 35 years old. “The 18- to 30-year-old range is an especially high-risk age window for increased acute health care utilization, even compared with other chronic adolescent/young adult conditions. “The demographics in the study group also reasonably approximate those for young adults with SCD in urban centers. By taking a multicenter approach across a several-state region, I believe the findings offer better generalizability, since health care access and mental health access can vary state-by-state,” and the current results show a more standard experience.
“It was a bit surprising that female [sex] maintained such an association with pain across the different components of the study,” and that the pain peak was in the 25- to 34-year-old age range, said Dr. Boucher. However, anecdotally, the late teens and early 20s “can be laden with mental health concerns due to the life transitions that accompany most people at that time. The note that hydroxyurea use was associated with more pain and depression symptoms was interesting, and serves as a reminder that what is happening to the red blood cells and in the blood vessels, such as red blood cell breakdown, sickling, and vaso-occlusion are only a part of what causes pain, and hydroxyurea is not likely to play a role in mitigating mental health aspects of pain.”
The findings that overall pain frequency and related pain medication use were associated with higher depression rates “may in part reflect a blind spot for physicians and medical teams, who often resort back to physical pain-based heuristics.” Physicians may misunderstand chronic pain and its management and look for quick fixes for pain out of uncertainty or urgency, said Dr. Boucher. “This serves to diminish the perspectives of patients as people first (not embodiments of a disease) and can lead to missed opportunities to tackle mental health challenges.”
Barriers and limitations
There are barriers to mental health screening in hematology care,” Dr. Boucher said. First, most hematologists are not experts in mental health and while they may have some from their medical training in these disorders, it can be difficult to maintain the level of health literacy needed to stay up to date on treatments. Second, depression screening may not be part of regular patient intake and the Patient Health Questionnaire–2 or PHQ-9 offer only short-term (2-week) snapshots of depression.
“Perhaps most critically, even if we do successfully screen, the access to mental health specialists is severely limited, just as it is across the medical landscape, so intervention opportunities may be suboptimal,” said Dr. Boucher. The problem is magnified if, as the current study suggests, the rates of depression in SCD are approximately three times greater than the population overall.
In the current study, “the fact that only half of those who self-reported depression symptoms actually had depression documented as a diagnosis in their medical records suggests that we are missing a lot of patients affected by mental health disturbances.”
This study is limited in measurement of the contribution of social determinants of health, he said, as they were primarily focused on employment status and income. The study does not describe other factors like support systems, housing, and transportation.
“I would like to see studies that not only identify associated drivers of pain, but also offer evidence for successful interventions,” Dr. Boucher said, and these studies should include patient-centered interventions versus disease-centered interventions.
Undertreatment persists
Other concerns with sickle cell anemia include the underuse of hydroxyurea to reduce complications associated with the disease such as pain, stroke, and even early death. Another recent study in JAMA Network Open suggested that use of hydroxyurea remained low in children and youth despite the issuing of guidelines, and that underserved populations were especially affected. In that study, the researchers found that the patients’ annual days’ supply of hydroxyurea in New York state did not change significantly after the guideline update.
SCD also has been associated with increased risk of other poor outcomes, such as stillbirth and increased risk of poor COVID-19–related outcomes and COVID-19–related deaths.
The study by Dr. Harris and colleagues was supported by the National Institutes of Health through the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Dr. Harris had no financial conflicts to disclose. The hydroxyurea study was supported by the Agency for Healthcare Research and Quality and the NHLBI. The researchers had no financial conflicts to disclose. Dr. Boucher disclosed conducting research with SCL Behring, but had no relevant financial conflicts.
Complications from sickle cell disease (SCD) can affect education and life opportunities, and these complications have been associated with social determinants of health such as socioeconomic status, depression, health literacy, and level of education, according to Kelly M. Harris, PhD, of Washington University in St. Louis, and colleagues.
Pain is a hallmark of SCD, and “the current climate around pain management and opioid use has specific implications for individuals with [SCD], especially youth,” Dr. Harris said in an interview.
In a study published in JAMA Network Open, the researchers analyzed 2,264 participants (average age, 27.9 years; 56.2% were female) in the Sickle Cell Disease Implementation Consortium a study that includes patient assessment, treatment, and creation of a longitudinal registry.
The participants completed the Adult Sickle Cell Quality of Life Measurement Information System to provide data on the frequency and severity of pain episodes related to SCD over the past 12 months. Multivariable regression analysis was used to examine the associations of education, employment, and mental health with pain frequency and severity.
Overall, 79.8% of participants reported severe pain, and 47.8% reported more than four episodes of pain in the past year.
Notably, 20% of the participants were diagnosed with depression, and increased pain frequency was significantly associated with depression, although no significant association appeared between pain severity and depression, the researchers said.
A total of 47% of the participants reported using pain medication and 49% reported using hydroxyurea. In addition, 628 participants (28.0%) underwent regular blood transfusions.
Neither education level nor income was associated with increased pain frequency or severity. Age younger than 18 years was significantly associated with both pain frequency and severity, as was daily used of pain medication. Unemployment and female sex also were associated with increased pain frequency.
The findings were limited by several factors including the cross-sectional design that prevents conclusions of causality, and by the reliance on patient reports of depression, which likely led to underreporting, the researchers noted.
However, the results are consistent with previous studies suggesting that pain and negative feelings were associated with reduced quality of life in SCD patients, especially younger patients, and support the need to screen SCD patients for depression, especially those who report more severe and/or more frequent pain, they said.
Take a comprehensive approach to a complex condition
“When treating pain, we cannot just rely on medication,” Dr. Harris said. “It is important that providers consider the full experiences of patients and pursue holistic and comprehensive treatment approaches to reducing pain. Screening for depression should be a regular practice, particularly for patients experiencing frequent and/or severe pain.
“Racial discrimination, stigma, and bias impact pain diagnosis and treatment for individuals with SCD,” said Dr. Harris. “Increasing awareness of the associations between depression and pain frequency and severity ... may help address these barriers.”
Data highlight treatment gaps
Alexander A. Boucher, MD, a member of the division of pediatric hematology and oncology at the University of Minnesota, Minneapolis, noted the researchers included patients as young as midadolescence, with a majority being under 35 years old. “The 18- to 30-year-old range is an especially high-risk age window for increased acute health care utilization, even compared with other chronic adolescent/young adult conditions. “The demographics in the study group also reasonably approximate those for young adults with SCD in urban centers. By taking a multicenter approach across a several-state region, I believe the findings offer better generalizability, since health care access and mental health access can vary state-by-state,” and the current results show a more standard experience.
“It was a bit surprising that female [sex] maintained such an association with pain across the different components of the study,” and that the pain peak was in the 25- to 34-year-old age range, said Dr. Boucher. However, anecdotally, the late teens and early 20s “can be laden with mental health concerns due to the life transitions that accompany most people at that time. The note that hydroxyurea use was associated with more pain and depression symptoms was interesting, and serves as a reminder that what is happening to the red blood cells and in the blood vessels, such as red blood cell breakdown, sickling, and vaso-occlusion are only a part of what causes pain, and hydroxyurea is not likely to play a role in mitigating mental health aspects of pain.”
The findings that overall pain frequency and related pain medication use were associated with higher depression rates “may in part reflect a blind spot for physicians and medical teams, who often resort back to physical pain-based heuristics.” Physicians may misunderstand chronic pain and its management and look for quick fixes for pain out of uncertainty or urgency, said Dr. Boucher. “This serves to diminish the perspectives of patients as people first (not embodiments of a disease) and can lead to missed opportunities to tackle mental health challenges.”
Barriers and limitations
There are barriers to mental health screening in hematology care,” Dr. Boucher said. First, most hematologists are not experts in mental health and while they may have some from their medical training in these disorders, it can be difficult to maintain the level of health literacy needed to stay up to date on treatments. Second, depression screening may not be part of regular patient intake and the Patient Health Questionnaire–2 or PHQ-9 offer only short-term (2-week) snapshots of depression.
“Perhaps most critically, even if we do successfully screen, the access to mental health specialists is severely limited, just as it is across the medical landscape, so intervention opportunities may be suboptimal,” said Dr. Boucher. The problem is magnified if, as the current study suggests, the rates of depression in SCD are approximately three times greater than the population overall.
In the current study, “the fact that only half of those who self-reported depression symptoms actually had depression documented as a diagnosis in their medical records suggests that we are missing a lot of patients affected by mental health disturbances.”
This study is limited in measurement of the contribution of social determinants of health, he said, as they were primarily focused on employment status and income. The study does not describe other factors like support systems, housing, and transportation.
“I would like to see studies that not only identify associated drivers of pain, but also offer evidence for successful interventions,” Dr. Boucher said, and these studies should include patient-centered interventions versus disease-centered interventions.
Undertreatment persists
Other concerns with sickle cell anemia include the underuse of hydroxyurea to reduce complications associated with the disease such as pain, stroke, and even early death. Another recent study in JAMA Network Open suggested that use of hydroxyurea remained low in children and youth despite the issuing of guidelines, and that underserved populations were especially affected. In that study, the researchers found that the patients’ annual days’ supply of hydroxyurea in New York state did not change significantly after the guideline update.
SCD also has been associated with increased risk of other poor outcomes, such as stillbirth and increased risk of poor COVID-19–related outcomes and COVID-19–related deaths.
The study by Dr. Harris and colleagues was supported by the National Institutes of Health through the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Dr. Harris had no financial conflicts to disclose. The hydroxyurea study was supported by the Agency for Healthcare Research and Quality and the NHLBI. The researchers had no financial conflicts to disclose. Dr. Boucher disclosed conducting research with SCL Behring, but had no relevant financial conflicts.
Complications from sickle cell disease (SCD) can affect education and life opportunities, and these complications have been associated with social determinants of health such as socioeconomic status, depression, health literacy, and level of education, according to Kelly M. Harris, PhD, of Washington University in St. Louis, and colleagues.
Pain is a hallmark of SCD, and “the current climate around pain management and opioid use has specific implications for individuals with [SCD], especially youth,” Dr. Harris said in an interview.
In a study published in JAMA Network Open, the researchers analyzed 2,264 participants (average age, 27.9 years; 56.2% were female) in the Sickle Cell Disease Implementation Consortium a study that includes patient assessment, treatment, and creation of a longitudinal registry.
The participants completed the Adult Sickle Cell Quality of Life Measurement Information System to provide data on the frequency and severity of pain episodes related to SCD over the past 12 months. Multivariable regression analysis was used to examine the associations of education, employment, and mental health with pain frequency and severity.
Overall, 79.8% of participants reported severe pain, and 47.8% reported more than four episodes of pain in the past year.
Notably, 20% of the participants were diagnosed with depression, and increased pain frequency was significantly associated with depression, although no significant association appeared between pain severity and depression, the researchers said.
A total of 47% of the participants reported using pain medication and 49% reported using hydroxyurea. In addition, 628 participants (28.0%) underwent regular blood transfusions.
Neither education level nor income was associated with increased pain frequency or severity. Age younger than 18 years was significantly associated with both pain frequency and severity, as was daily used of pain medication. Unemployment and female sex also were associated with increased pain frequency.
The findings were limited by several factors including the cross-sectional design that prevents conclusions of causality, and by the reliance on patient reports of depression, which likely led to underreporting, the researchers noted.
However, the results are consistent with previous studies suggesting that pain and negative feelings were associated with reduced quality of life in SCD patients, especially younger patients, and support the need to screen SCD patients for depression, especially those who report more severe and/or more frequent pain, they said.
Take a comprehensive approach to a complex condition
“When treating pain, we cannot just rely on medication,” Dr. Harris said. “It is important that providers consider the full experiences of patients and pursue holistic and comprehensive treatment approaches to reducing pain. Screening for depression should be a regular practice, particularly for patients experiencing frequent and/or severe pain.
“Racial discrimination, stigma, and bias impact pain diagnosis and treatment for individuals with SCD,” said Dr. Harris. “Increasing awareness of the associations between depression and pain frequency and severity ... may help address these barriers.”
Data highlight treatment gaps
Alexander A. Boucher, MD, a member of the division of pediatric hematology and oncology at the University of Minnesota, Minneapolis, noted the researchers included patients as young as midadolescence, with a majority being under 35 years old. “The 18- to 30-year-old range is an especially high-risk age window for increased acute health care utilization, even compared with other chronic adolescent/young adult conditions. “The demographics in the study group also reasonably approximate those for young adults with SCD in urban centers. By taking a multicenter approach across a several-state region, I believe the findings offer better generalizability, since health care access and mental health access can vary state-by-state,” and the current results show a more standard experience.
“It was a bit surprising that female [sex] maintained such an association with pain across the different components of the study,” and that the pain peak was in the 25- to 34-year-old age range, said Dr. Boucher. However, anecdotally, the late teens and early 20s “can be laden with mental health concerns due to the life transitions that accompany most people at that time. The note that hydroxyurea use was associated with more pain and depression symptoms was interesting, and serves as a reminder that what is happening to the red blood cells and in the blood vessels, such as red blood cell breakdown, sickling, and vaso-occlusion are only a part of what causes pain, and hydroxyurea is not likely to play a role in mitigating mental health aspects of pain.”
The findings that overall pain frequency and related pain medication use were associated with higher depression rates “may in part reflect a blind spot for physicians and medical teams, who often resort back to physical pain-based heuristics.” Physicians may misunderstand chronic pain and its management and look for quick fixes for pain out of uncertainty or urgency, said Dr. Boucher. “This serves to diminish the perspectives of patients as people first (not embodiments of a disease) and can lead to missed opportunities to tackle mental health challenges.”
Barriers and limitations
There are barriers to mental health screening in hematology care,” Dr. Boucher said. First, most hematologists are not experts in mental health and while they may have some from their medical training in these disorders, it can be difficult to maintain the level of health literacy needed to stay up to date on treatments. Second, depression screening may not be part of regular patient intake and the Patient Health Questionnaire–2 or PHQ-9 offer only short-term (2-week) snapshots of depression.
“Perhaps most critically, even if we do successfully screen, the access to mental health specialists is severely limited, just as it is across the medical landscape, so intervention opportunities may be suboptimal,” said Dr. Boucher. The problem is magnified if, as the current study suggests, the rates of depression in SCD are approximately three times greater than the population overall.
In the current study, “the fact that only half of those who self-reported depression symptoms actually had depression documented as a diagnosis in their medical records suggests that we are missing a lot of patients affected by mental health disturbances.”
This study is limited in measurement of the contribution of social determinants of health, he said, as they were primarily focused on employment status and income. The study does not describe other factors like support systems, housing, and transportation.
“I would like to see studies that not only identify associated drivers of pain, but also offer evidence for successful interventions,” Dr. Boucher said, and these studies should include patient-centered interventions versus disease-centered interventions.
Undertreatment persists
Other concerns with sickle cell anemia include the underuse of hydroxyurea to reduce complications associated with the disease such as pain, stroke, and even early death. Another recent study in JAMA Network Open suggested that use of hydroxyurea remained low in children and youth despite the issuing of guidelines, and that underserved populations were especially affected. In that study, the researchers found that the patients’ annual days’ supply of hydroxyurea in New York state did not change significantly after the guideline update.
SCD also has been associated with increased risk of other poor outcomes, such as stillbirth and increased risk of poor COVID-19–related outcomes and COVID-19–related deaths.
The study by Dr. Harris and colleagues was supported by the National Institutes of Health through the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Dr. Harris had no financial conflicts to disclose. The hydroxyurea study was supported by the Agency for Healthcare Research and Quality and the NHLBI. The researchers had no financial conflicts to disclose. Dr. Boucher disclosed conducting research with SCL Behring, but had no relevant financial conflicts.
FROM JAMA NETWORK OPEN
Are you a physician ... or a vending machine?
When we address this problem with patients, some become immediately defensive, making it difficult to modify treatment regimens. It’s almost as if people believe that they have a “right” to their medications and nobody should dare take them away. Even when I think the interaction goes relatively smoothly, the outcome usually shows otherwise.
I will decrease gabapentin from 3,200 mg per day and they will come back with cyclobenzaprine from the urgent care center down the block.
I try to stop an abused amphetamine and dextroamphetamine, and not only do the drugs show up in the urine toxicology test a month later (from the brother’s girlfriend’s sister) but the screening will be positive for cocaine (from the sister’s boyfriend’s brother) and probably alprazolam, too.
People want what they want, and I believe what they want is the overwhelming need not to feel, and especially to not feel our natural and uncomfortable states of pain, sadness, anxiety, fatigue, and discomfort (sometimes all at once). They will use anything orally or intravenously or nasally to make those feelings go away.
I am an addiction specialist so I write this commentary out of care and concern and recognition of how much, pain both physical and psychic, people suffer.
Perhaps we as physicians are conditioned to believe that we must prescribe “something” to the patient who is uncomfortable and sitting in front of us. In general we are sympathetic to the needs of those who come to us in distress, and we try our best to help reduce their symptoms.
I know that we cannot simply “fire” people, because these patients are ours to take care of; they are our responsibility, though this is our overused response to “difficult” patients.
And I know that we have insufficient replacements for these medications. We stopped prescribing oxycodone and now people are on gabapentin in the highest doses, diversion is up, and so is its abuse.
Many of us regularly teach about breathing and mindfulness. I discuss trauma and talk therapy. I order physical therapy and walking regimens and podcasts. But our relationship is transactional, and in prescribing a medication, I have shown them that I am hearing them. I hate this feeling of being trapped.
I spend much of my day negotiating and drive home at night feeling like nothing more than a vending machine.
Dr. Hambright is with the department of addiction medicine at Samaritan Daytop Village, Ellenville, N.Y., and Samadhi Recovery Community Outreach Center, Kingston, N.Y. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When we address this problem with patients, some become immediately defensive, making it difficult to modify treatment regimens. It’s almost as if people believe that they have a “right” to their medications and nobody should dare take them away. Even when I think the interaction goes relatively smoothly, the outcome usually shows otherwise.
I will decrease gabapentin from 3,200 mg per day and they will come back with cyclobenzaprine from the urgent care center down the block.
I try to stop an abused amphetamine and dextroamphetamine, and not only do the drugs show up in the urine toxicology test a month later (from the brother’s girlfriend’s sister) but the screening will be positive for cocaine (from the sister’s boyfriend’s brother) and probably alprazolam, too.
People want what they want, and I believe what they want is the overwhelming need not to feel, and especially to not feel our natural and uncomfortable states of pain, sadness, anxiety, fatigue, and discomfort (sometimes all at once). They will use anything orally or intravenously or nasally to make those feelings go away.
I am an addiction specialist so I write this commentary out of care and concern and recognition of how much, pain both physical and psychic, people suffer.
Perhaps we as physicians are conditioned to believe that we must prescribe “something” to the patient who is uncomfortable and sitting in front of us. In general we are sympathetic to the needs of those who come to us in distress, and we try our best to help reduce their symptoms.
I know that we cannot simply “fire” people, because these patients are ours to take care of; they are our responsibility, though this is our overused response to “difficult” patients.
And I know that we have insufficient replacements for these medications. We stopped prescribing oxycodone and now people are on gabapentin in the highest doses, diversion is up, and so is its abuse.
Many of us regularly teach about breathing and mindfulness. I discuss trauma and talk therapy. I order physical therapy and walking regimens and podcasts. But our relationship is transactional, and in prescribing a medication, I have shown them that I am hearing them. I hate this feeling of being trapped.
I spend much of my day negotiating and drive home at night feeling like nothing more than a vending machine.
Dr. Hambright is with the department of addiction medicine at Samaritan Daytop Village, Ellenville, N.Y., and Samadhi Recovery Community Outreach Center, Kingston, N.Y. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When we address this problem with patients, some become immediately defensive, making it difficult to modify treatment regimens. It’s almost as if people believe that they have a “right” to their medications and nobody should dare take them away. Even when I think the interaction goes relatively smoothly, the outcome usually shows otherwise.
I will decrease gabapentin from 3,200 mg per day and they will come back with cyclobenzaprine from the urgent care center down the block.
I try to stop an abused amphetamine and dextroamphetamine, and not only do the drugs show up in the urine toxicology test a month later (from the brother’s girlfriend’s sister) but the screening will be positive for cocaine (from the sister’s boyfriend’s brother) and probably alprazolam, too.
People want what they want, and I believe what they want is the overwhelming need not to feel, and especially to not feel our natural and uncomfortable states of pain, sadness, anxiety, fatigue, and discomfort (sometimes all at once). They will use anything orally or intravenously or nasally to make those feelings go away.
I am an addiction specialist so I write this commentary out of care and concern and recognition of how much, pain both physical and psychic, people suffer.
Perhaps we as physicians are conditioned to believe that we must prescribe “something” to the patient who is uncomfortable and sitting in front of us. In general we are sympathetic to the needs of those who come to us in distress, and we try our best to help reduce their symptoms.
I know that we cannot simply “fire” people, because these patients are ours to take care of; they are our responsibility, though this is our overused response to “difficult” patients.
And I know that we have insufficient replacements for these medications. We stopped prescribing oxycodone and now people are on gabapentin in the highest doses, diversion is up, and so is its abuse.
Many of us regularly teach about breathing and mindfulness. I discuss trauma and talk therapy. I order physical therapy and walking regimens and podcasts. But our relationship is transactional, and in prescribing a medication, I have shown them that I am hearing them. I hate this feeling of being trapped.
I spend much of my day negotiating and drive home at night feeling like nothing more than a vending machine.
Dr. Hambright is with the department of addiction medicine at Samaritan Daytop Village, Ellenville, N.Y., and Samadhi Recovery Community Outreach Center, Kingston, N.Y. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.