User login
Erenumab beats topiramate for migraine in first head-to-head trial
, according to data from almost 800 patients in the first head-to-head trial of its kind.
The findings suggest that erenumab may help overcome longstanding issues with migraine medication adherence, and additional supportive data may alter treatment sequencing, reported lead author Uwe Reuter, MD, professor at Charité Universitätsmedizin Berlin, and colleagues.
“So far, no study has been done in order to compare the efficacy of a monoclonal antibody targeting the CGRP pathway to that of a standard of care oral preventive drug,” the investigators wrote in Cephalalgia.
The phase 4 HER-MES trial aimed to address this knowledge gap by enrolling 777 adult patients with a history of migraine. All patients reported migraine with or without aura for at least 1 year prior to screening. At baseline, most patients (65%) reported 8-14 migraine days per months, followed by 4-7 days (24.0%), and at least 15 days (11.0%). No patients had previously received topiramate or a CGRP-targeting agent.
“HER-MES includes a broad migraine population with two-thirds of the patients in the high-frequency migraine spectrum,” the investigators noted. “Despite a mean disease duration of about 20 years, almost 60% of the patients had not received previous prophylactic treatment, which underlines the long-standing problem of undertreatment in migraine.”
The trial had a double-dummy design; patients were randomized in a 1:1 ratio to receive either subcutaneous erenumab (70 or 140 mg/month) plus oral placebo, or oral topiramate (50-100 mg/day) plus subcutaneous placebo. The topiramate dose was uptitrated over the first 6 weeks. Treatments were given for a total of 24 weeks or until discontinuation due to adverse events, which was the primary endpoint. The secondary endpoint was efficacy over months 4-6, defined as at least 50% reduction in monthly migraine days, compared with baseline. Other patient-reported outcomes were also evaluated.
After 24 weeks, 95.1% of patients were still enrolled in the trial. Discontinuations due to adverse events were almost four times as common in the topiramate group than the erenumab group (38.9% vs. 10.6%; odds ratio [OR], 0.19; confidence interval, 0.13-0.27; P less than .001). Efficacy findings followed suit, with 55.4% of patients in the erenumab group reporting at least 50% reduction in monthly migraine days, compared with 31.2% of patients in the topiramate group (OR, 2.76; 95% CI, 2.06-3.71; P less than.001).
Erenumab significantly improved monthly migraine days, headache impact test (HIT-6) scores, and short form health survey version (SF-35v2) scores, including physical and mental components (P less than .001 for all).
Safety profiles aligned with previous findings.
“Compared to topiramate, treatment with erenumab has a superior tolerability profile and a significantly higher efficacy,” the investigators concluded. “HER-MES supports the potential of erenumab in overcoming issues of low adherence in clinical practice observed with topiramate, lessening migraine burden, and improving quality of life in a broad migraine population.”
Superior tolerability
Commenting on the study, Alan Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews, said this is “a very important, very well conducted trial that documents what many of us already suspected; erenumab clearly has better tolerability than topiramate as well as better efficacy.”
Dr. Rapoport, a past president of the International Headache Society, said the study highlights an area of unmet need in neurology practice.
“Despite most patients in the trial having chronic headaches for 20 years, 60% of them had never received preventive treatment,” he said, noting that this reflects current practice in the United States.
Dr. Rapoport said primary care providers in the United States prescribe preventive migraine medications to 10%-15% of eligible patients. Prescribing rates for general neurologists are slightly higher, he said, ranging from 35% to 40%, while headache specialists prescribe 70%-90% of the time.
“How can we improve this situation?” Dr. Rapoport asked. “For years we have tried to improve it with education, but we need to do a better job. We need to educate our primary care physicians in more practical ways. We have to teach them how to make a diagnosis of high frequency migraine and chronic migraine and strongly suggest that those patients be put on appropriate preventive medications.”
Barriers to care may be systemic, according to Dr. Rapoport.
“One issue in the U.S. is that patients with commercial insurance are almost always required to fail two or three categories of older oral preventive migraine medications before they can get a monoclonal antibody or gepants for prevention,” he said. “It would be good if we could change that system so that patients that absolutely need the better tolerated, more effective preventive medications could get them sooner rather than later. This will help them feel and function better, with less pain, and eventually bring down the cost of migraine therapy.”
While Dr. Reuter and colleagues concluded that revised treatment sequencing may be warranted after more trials show similar results, Dr. Rapoport suggested that “this was such a large, well-performed, 6-month study with few dropouts, that further trials to confirm these findings are unnecessary, in my opinion.”
The HER-MES trial was funded by Novartis. Dr. Reuter and colleagues disclosed additional relationships with Eli Lilly, Teva Pharmaceutical, Allergan, and others. Dr. Rapoport was involved in early topiramate trials for prevention and migraine, and is a speaker for Amgen.
, according to data from almost 800 patients in the first head-to-head trial of its kind.
The findings suggest that erenumab may help overcome longstanding issues with migraine medication adherence, and additional supportive data may alter treatment sequencing, reported lead author Uwe Reuter, MD, professor at Charité Universitätsmedizin Berlin, and colleagues.
“So far, no study has been done in order to compare the efficacy of a monoclonal antibody targeting the CGRP pathway to that of a standard of care oral preventive drug,” the investigators wrote in Cephalalgia.
The phase 4 HER-MES trial aimed to address this knowledge gap by enrolling 777 adult patients with a history of migraine. All patients reported migraine with or without aura for at least 1 year prior to screening. At baseline, most patients (65%) reported 8-14 migraine days per months, followed by 4-7 days (24.0%), and at least 15 days (11.0%). No patients had previously received topiramate or a CGRP-targeting agent.
“HER-MES includes a broad migraine population with two-thirds of the patients in the high-frequency migraine spectrum,” the investigators noted. “Despite a mean disease duration of about 20 years, almost 60% of the patients had not received previous prophylactic treatment, which underlines the long-standing problem of undertreatment in migraine.”
The trial had a double-dummy design; patients were randomized in a 1:1 ratio to receive either subcutaneous erenumab (70 or 140 mg/month) plus oral placebo, or oral topiramate (50-100 mg/day) plus subcutaneous placebo. The topiramate dose was uptitrated over the first 6 weeks. Treatments were given for a total of 24 weeks or until discontinuation due to adverse events, which was the primary endpoint. The secondary endpoint was efficacy over months 4-6, defined as at least 50% reduction in monthly migraine days, compared with baseline. Other patient-reported outcomes were also evaluated.
After 24 weeks, 95.1% of patients were still enrolled in the trial. Discontinuations due to adverse events were almost four times as common in the topiramate group than the erenumab group (38.9% vs. 10.6%; odds ratio [OR], 0.19; confidence interval, 0.13-0.27; P less than .001). Efficacy findings followed suit, with 55.4% of patients in the erenumab group reporting at least 50% reduction in monthly migraine days, compared with 31.2% of patients in the topiramate group (OR, 2.76; 95% CI, 2.06-3.71; P less than.001).
Erenumab significantly improved monthly migraine days, headache impact test (HIT-6) scores, and short form health survey version (SF-35v2) scores, including physical and mental components (P less than .001 for all).
Safety profiles aligned with previous findings.
“Compared to topiramate, treatment with erenumab has a superior tolerability profile and a significantly higher efficacy,” the investigators concluded. “HER-MES supports the potential of erenumab in overcoming issues of low adherence in clinical practice observed with topiramate, lessening migraine burden, and improving quality of life in a broad migraine population.”
Superior tolerability
Commenting on the study, Alan Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews, said this is “a very important, very well conducted trial that documents what many of us already suspected; erenumab clearly has better tolerability than topiramate as well as better efficacy.”
Dr. Rapoport, a past president of the International Headache Society, said the study highlights an area of unmet need in neurology practice.
“Despite most patients in the trial having chronic headaches for 20 years, 60% of them had never received preventive treatment,” he said, noting that this reflects current practice in the United States.
Dr. Rapoport said primary care providers in the United States prescribe preventive migraine medications to 10%-15% of eligible patients. Prescribing rates for general neurologists are slightly higher, he said, ranging from 35% to 40%, while headache specialists prescribe 70%-90% of the time.
“How can we improve this situation?” Dr. Rapoport asked. “For years we have tried to improve it with education, but we need to do a better job. We need to educate our primary care physicians in more practical ways. We have to teach them how to make a diagnosis of high frequency migraine and chronic migraine and strongly suggest that those patients be put on appropriate preventive medications.”
Barriers to care may be systemic, according to Dr. Rapoport.
“One issue in the U.S. is that patients with commercial insurance are almost always required to fail two or three categories of older oral preventive migraine medications before they can get a monoclonal antibody or gepants for prevention,” he said. “It would be good if we could change that system so that patients that absolutely need the better tolerated, more effective preventive medications could get them sooner rather than later. This will help them feel and function better, with less pain, and eventually bring down the cost of migraine therapy.”
While Dr. Reuter and colleagues concluded that revised treatment sequencing may be warranted after more trials show similar results, Dr. Rapoport suggested that “this was such a large, well-performed, 6-month study with few dropouts, that further trials to confirm these findings are unnecessary, in my opinion.”
The HER-MES trial was funded by Novartis. Dr. Reuter and colleagues disclosed additional relationships with Eli Lilly, Teva Pharmaceutical, Allergan, and others. Dr. Rapoport was involved in early topiramate trials for prevention and migraine, and is a speaker for Amgen.
, according to data from almost 800 patients in the first head-to-head trial of its kind.
The findings suggest that erenumab may help overcome longstanding issues with migraine medication adherence, and additional supportive data may alter treatment sequencing, reported lead author Uwe Reuter, MD, professor at Charité Universitätsmedizin Berlin, and colleagues.
“So far, no study has been done in order to compare the efficacy of a monoclonal antibody targeting the CGRP pathway to that of a standard of care oral preventive drug,” the investigators wrote in Cephalalgia.
The phase 4 HER-MES trial aimed to address this knowledge gap by enrolling 777 adult patients with a history of migraine. All patients reported migraine with or without aura for at least 1 year prior to screening. At baseline, most patients (65%) reported 8-14 migraine days per months, followed by 4-7 days (24.0%), and at least 15 days (11.0%). No patients had previously received topiramate or a CGRP-targeting agent.
“HER-MES includes a broad migraine population with two-thirds of the patients in the high-frequency migraine spectrum,” the investigators noted. “Despite a mean disease duration of about 20 years, almost 60% of the patients had not received previous prophylactic treatment, which underlines the long-standing problem of undertreatment in migraine.”
The trial had a double-dummy design; patients were randomized in a 1:1 ratio to receive either subcutaneous erenumab (70 or 140 mg/month) plus oral placebo, or oral topiramate (50-100 mg/day) plus subcutaneous placebo. The topiramate dose was uptitrated over the first 6 weeks. Treatments were given for a total of 24 weeks or until discontinuation due to adverse events, which was the primary endpoint. The secondary endpoint was efficacy over months 4-6, defined as at least 50% reduction in monthly migraine days, compared with baseline. Other patient-reported outcomes were also evaluated.
After 24 weeks, 95.1% of patients were still enrolled in the trial. Discontinuations due to adverse events were almost four times as common in the topiramate group than the erenumab group (38.9% vs. 10.6%; odds ratio [OR], 0.19; confidence interval, 0.13-0.27; P less than .001). Efficacy findings followed suit, with 55.4% of patients in the erenumab group reporting at least 50% reduction in monthly migraine days, compared with 31.2% of patients in the topiramate group (OR, 2.76; 95% CI, 2.06-3.71; P less than.001).
Erenumab significantly improved monthly migraine days, headache impact test (HIT-6) scores, and short form health survey version (SF-35v2) scores, including physical and mental components (P less than .001 for all).
Safety profiles aligned with previous findings.
“Compared to topiramate, treatment with erenumab has a superior tolerability profile and a significantly higher efficacy,” the investigators concluded. “HER-MES supports the potential of erenumab in overcoming issues of low adherence in clinical practice observed with topiramate, lessening migraine burden, and improving quality of life in a broad migraine population.”
Superior tolerability
Commenting on the study, Alan Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews, said this is “a very important, very well conducted trial that documents what many of us already suspected; erenumab clearly has better tolerability than topiramate as well as better efficacy.”
Dr. Rapoport, a past president of the International Headache Society, said the study highlights an area of unmet need in neurology practice.
“Despite most patients in the trial having chronic headaches for 20 years, 60% of them had never received preventive treatment,” he said, noting that this reflects current practice in the United States.
Dr. Rapoport said primary care providers in the United States prescribe preventive migraine medications to 10%-15% of eligible patients. Prescribing rates for general neurologists are slightly higher, he said, ranging from 35% to 40%, while headache specialists prescribe 70%-90% of the time.
“How can we improve this situation?” Dr. Rapoport asked. “For years we have tried to improve it with education, but we need to do a better job. We need to educate our primary care physicians in more practical ways. We have to teach them how to make a diagnosis of high frequency migraine and chronic migraine and strongly suggest that those patients be put on appropriate preventive medications.”
Barriers to care may be systemic, according to Dr. Rapoport.
“One issue in the U.S. is that patients with commercial insurance are almost always required to fail two or three categories of older oral preventive migraine medications before they can get a monoclonal antibody or gepants for prevention,” he said. “It would be good if we could change that system so that patients that absolutely need the better tolerated, more effective preventive medications could get them sooner rather than later. This will help them feel and function better, with less pain, and eventually bring down the cost of migraine therapy.”
While Dr. Reuter and colleagues concluded that revised treatment sequencing may be warranted after more trials show similar results, Dr. Rapoport suggested that “this was such a large, well-performed, 6-month study with few dropouts, that further trials to confirm these findings are unnecessary, in my opinion.”
The HER-MES trial was funded by Novartis. Dr. Reuter and colleagues disclosed additional relationships with Eli Lilly, Teva Pharmaceutical, Allergan, and others. Dr. Rapoport was involved in early topiramate trials for prevention and migraine, and is a speaker for Amgen.
FROM CEPHALALGIA
U.S. overdose deaths hit an all-time high
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
Which injections are effective for lateral epicondylitis?
EVIDENCE SUMMARY
Neither corticosteroids nor platelet-rich plasma are superior to placebo
A 2014 systematic review of RCTs of nonsurgical treatments for lateral epicondylitis identified 4 studies comparing corticosteroid injections to saline or anesthetic injections.1 In the first study, investigators followed 64 patients for 6 months. Both groups significantly improved from baseline, but there were no differences in pain or function at 1 or 6 months. Skin discoloration occurred in 2 patients who received lidocaine injection and 1 who received dexamethasone.2
In a second RCT of patients with symptoms for > 4 weeks, 39 participants were randomized to either betamethasone/bupivacaine or bupivacaine-only injections. In-person follow-up occurred at 4 and 8 weeks and telephone follow-up at 6 months. Both groups statistically improved from baseline to 6 months. No differences were seen between groups in pain or functional improvement at 4, 8, or 26 weeks, but the betamethasone group showed statistically greater improvement on the Visual Analog Scale (VAS) from 8 weeks to the final 6-month telephone follow-up. No functional assessments were reported at 6 months.3
The third RCT of 165 patients with lateral epicondylitis for > 6 weeks evaluated 4 intervention groups: corticosteroid injection with/without physiotherapy and placebo (small-volume saline) injection with/without physiotherapy. At the end of 1 year, the corticosteroid injection groups had less complete recovery (83% vs 96%; relative risk [RR] = 0.86; 99% CI, 0.75-0.99) and more recurrences (54% vs 12%; RR = 0.23; 99% CI, 0.10-0.51) than the placebo groups.4
The fourth RCT randomized 120 patients to either 2 mL lidocaine or 1 mL lidocaine plus 1 mL of triamcinolone. At 1-year follow-up, 57 of 60 lidocaine-injected patients had an excellent recovery and 56 of 60 triamcinolone plus lidocaine patients had an excellent recovery.5
Platelet-rich plasma. A meta-analysis6 of RCTs of PRP vs saline injections included 5 trials and 276 patients with a mean age of 48 years; duration of follow-up was 2 to 12 months. No significant differences were found between the groups for pain score—measured by VAS or the Patient-Rated Tennis Elbow Evaluation (PRTEE)—(standardized mean difference [SMD] = –0.51; 95% CI, –1.32 to –0.30) nor for functional score (SMD = 0.07; 95% CI, –0.46 to 0.33). Two of the trials reported adverse reactions of pain around the injection site: 16% to 20% in the PRP group vs 8% to 15% in the saline group.
Corticosteroids and PRP. A 2013 3-armed RCT7 (n = 60) compared 1-time injections of PRP, corticosteroid, and saline for treatment of lateral epicondylitis. Pain was evaluated at 1 and 3 months using the PRTEE. Compared to saline, corticosteroid showed a statistically significant, but not a minimum clinically important, reduction (8% greater improvement) at 1 month but not at 3 months. PRP pain reduction at both 1 and 3 months was not significantly different from placebo. Importantly, a small sample size combined with a high dropout rate (> 70%) limit validity of this study.
Botulinum toxin shows modest pain improvement, but …
A 2017 meta-analysis8 of 4 RCTs (n = 278) compared the effectiveness of botulinum toxin vs saline injection and other nonsurgical treatments for lateral epicondylitis. The studies compared the mean differences in pain relief and hand grip strength in adult patients with lateral epicondylitis symptoms for at least 3 months. Compared with saline injection, botulinum toxin injection significantly reduced pain to a small or medium SMD, at 2 to 4 weeks post injection (SMD = –0.73; 95% CI, –1.29 to –0.17); 8 to 12 weeks post injection (SMD = –0.45; 95% CI, –0.74 to –0.15); and 16+ weeks post injection (SMD = –0.54; 95% CI, –0.98 to –0.11). Harm from botulinum toxin was greater than from saline or corticosteroid, with a significant reduction in grip strength at 2 to 4 weeks (SMD = –0.33; 95% CI, –0.59 to –0.08).
Continue to: Prolotherapy needs further study
Prolotherapy needs further study
A 2008 RCT9 of 20 adults with at least 6 months of lateral epicondylitis received either prolotherapy (1 part 5% sodium morrhuate, 1.5 parts 50% dextrose, 0.5 parts 4% lidocaine, 0.5 parts 0.5% bupivacaine HCl, and 3.5 parts normal saline) injections or 0.9% saline injections at baseline, 4 weeks, and 8 weeks. On a 10-point Likert scale, the prolotherapy group had a lower mean pain score at 16 weeks than the saline injection group (0.5 vs 3.5), but not at 8 weeks (3.3 vs 3.6). This pilot study’s results are limited by its small sample size.
Hyaluronic acid improves pain, but not enough
A 2010 double-blind RCT10 (n = 331) compared hyaluronic acid injection vs saline injection in treatment of lateral epicondylitis in adults with > 3 months of symptoms. Two injections were performed 1 week apart, with follow-up at 30 days and at 1 year after the first injection. VAS score in the hyaluronic acid group, at rest and after grip testing, was significantly different (statistically) than in the placebo group but did not meet criteria for minimum clinically important improvement. Review of the literature showed limited follow-up studies on hyaluronic acid for lateral epicondylitis to confirm this RCT.
Autologous blood has no advantage over placebo
The only RCT of autologous blood compared to saline injections11 included patients with lateral epicondylitis for < 6 months: 10 saline injections vs 9 autologous blood injections. Patient scores on the Disabilities of the Arm, Shoulder, and Hand scale (which measures symptoms from 0 to 100; lower is better) showed no difference but favored the saline injections at 2-month (28 vs 20) and 6-month (20 vs 10) follow-up.
Editor’s takeaway
Limiting the evidence review to studies with a placebo comparator clarifies the lack of effectiveness of lateral epicondylitis injections. Neither corticosteroid, platelet-rich plasma, botulinum toxin, prolotherapy, hyaluronic acid, or autologous blood injections have proven superior to saline or anesthetic injections. However, all injections that contained “placebo” significantly improved lateralepicondylitis.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Lindenhovius A, Henket M, Gilligan BP, et al. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg Am. 2008;33:909-919. doi: 10.1016/j.jhsa.2008.02.004
3. Newcomer KL, Laskowski ER, Idank DM, et al. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11:214-222. doi: 10.1097/00042752-200110000-00002
4. Coombes BK, Bisset L, Brooks P, et al. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309:461-469. doi: 10.1001/jama.2013.129
5. Altay T, Gunal I, Ozturk H. Local injection treatment for lateral epicondylitis. Clin Orthop Relat Res. 2002;398:127-130.
6. Simental-Mendía M, Vilchez-Cavazos F, Álvarez-Villalobos N, et al. Clinical efficacy of platelet-rich plasma in the treatment of lateral epicondylitis: a systematic review and meta-analysis of randomized placebo-controlled clinical trials. Clin Rheumatol. 2020;39:2255-2265. doi: 10.1007/s10067-020-05000-y
7. Krogh T, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich-plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635. doi:10.1177/0363546512472975
8. Lin Y, Wu W, Hsu Y, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2017;32:131-145. doi:10.1177/0269215517702517
9. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sports Med. 2008;18:248-254. doi: 10.1097/JSM.0b013e318170fc87
10. Petrella R, Cogliano A, Decaria J, et al. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:4. doi: 10.1186/1758-2555-2-4
11. Wolf JM, Ozer K, Scott F, et al. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am. 2011;36:1269-1272. doi: 10.1016/j.jhsa.2011.05.014
EVIDENCE SUMMARY
Neither corticosteroids nor platelet-rich plasma are superior to placebo
A 2014 systematic review of RCTs of nonsurgical treatments for lateral epicondylitis identified 4 studies comparing corticosteroid injections to saline or anesthetic injections.1 In the first study, investigators followed 64 patients for 6 months. Both groups significantly improved from baseline, but there were no differences in pain or function at 1 or 6 months. Skin discoloration occurred in 2 patients who received lidocaine injection and 1 who received dexamethasone.2
In a second RCT of patients with symptoms for > 4 weeks, 39 participants were randomized to either betamethasone/bupivacaine or bupivacaine-only injections. In-person follow-up occurred at 4 and 8 weeks and telephone follow-up at 6 months. Both groups statistically improved from baseline to 6 months. No differences were seen between groups in pain or functional improvement at 4, 8, or 26 weeks, but the betamethasone group showed statistically greater improvement on the Visual Analog Scale (VAS) from 8 weeks to the final 6-month telephone follow-up. No functional assessments were reported at 6 months.3
The third RCT of 165 patients with lateral epicondylitis for > 6 weeks evaluated 4 intervention groups: corticosteroid injection with/without physiotherapy and placebo (small-volume saline) injection with/without physiotherapy. At the end of 1 year, the corticosteroid injection groups had less complete recovery (83% vs 96%; relative risk [RR] = 0.86; 99% CI, 0.75-0.99) and more recurrences (54% vs 12%; RR = 0.23; 99% CI, 0.10-0.51) than the placebo groups.4
The fourth RCT randomized 120 patients to either 2 mL lidocaine or 1 mL lidocaine plus 1 mL of triamcinolone. At 1-year follow-up, 57 of 60 lidocaine-injected patients had an excellent recovery and 56 of 60 triamcinolone plus lidocaine patients had an excellent recovery.5
Platelet-rich plasma. A meta-analysis6 of RCTs of PRP vs saline injections included 5 trials and 276 patients with a mean age of 48 years; duration of follow-up was 2 to 12 months. No significant differences were found between the groups for pain score—measured by VAS or the Patient-Rated Tennis Elbow Evaluation (PRTEE)—(standardized mean difference [SMD] = –0.51; 95% CI, –1.32 to –0.30) nor for functional score (SMD = 0.07; 95% CI, –0.46 to 0.33). Two of the trials reported adverse reactions of pain around the injection site: 16% to 20% in the PRP group vs 8% to 15% in the saline group.
Corticosteroids and PRP. A 2013 3-armed RCT7 (n = 60) compared 1-time injections of PRP, corticosteroid, and saline for treatment of lateral epicondylitis. Pain was evaluated at 1 and 3 months using the PRTEE. Compared to saline, corticosteroid showed a statistically significant, but not a minimum clinically important, reduction (8% greater improvement) at 1 month but not at 3 months. PRP pain reduction at both 1 and 3 months was not significantly different from placebo. Importantly, a small sample size combined with a high dropout rate (> 70%) limit validity of this study.
Botulinum toxin shows modest pain improvement, but …
A 2017 meta-analysis8 of 4 RCTs (n = 278) compared the effectiveness of botulinum toxin vs saline injection and other nonsurgical treatments for lateral epicondylitis. The studies compared the mean differences in pain relief and hand grip strength in adult patients with lateral epicondylitis symptoms for at least 3 months. Compared with saline injection, botulinum toxin injection significantly reduced pain to a small or medium SMD, at 2 to 4 weeks post injection (SMD = –0.73; 95% CI, –1.29 to –0.17); 8 to 12 weeks post injection (SMD = –0.45; 95% CI, –0.74 to –0.15); and 16+ weeks post injection (SMD = –0.54; 95% CI, –0.98 to –0.11). Harm from botulinum toxin was greater than from saline or corticosteroid, with a significant reduction in grip strength at 2 to 4 weeks (SMD = –0.33; 95% CI, –0.59 to –0.08).
Continue to: Prolotherapy needs further study
Prolotherapy needs further study
A 2008 RCT9 of 20 adults with at least 6 months of lateral epicondylitis received either prolotherapy (1 part 5% sodium morrhuate, 1.5 parts 50% dextrose, 0.5 parts 4% lidocaine, 0.5 parts 0.5% bupivacaine HCl, and 3.5 parts normal saline) injections or 0.9% saline injections at baseline, 4 weeks, and 8 weeks. On a 10-point Likert scale, the prolotherapy group had a lower mean pain score at 16 weeks than the saline injection group (0.5 vs 3.5), but not at 8 weeks (3.3 vs 3.6). This pilot study’s results are limited by its small sample size.
Hyaluronic acid improves pain, but not enough
A 2010 double-blind RCT10 (n = 331) compared hyaluronic acid injection vs saline injection in treatment of lateral epicondylitis in adults with > 3 months of symptoms. Two injections were performed 1 week apart, with follow-up at 30 days and at 1 year after the first injection. VAS score in the hyaluronic acid group, at rest and after grip testing, was significantly different (statistically) than in the placebo group but did not meet criteria for minimum clinically important improvement. Review of the literature showed limited follow-up studies on hyaluronic acid for lateral epicondylitis to confirm this RCT.
Autologous blood has no advantage over placebo
The only RCT of autologous blood compared to saline injections11 included patients with lateral epicondylitis for < 6 months: 10 saline injections vs 9 autologous blood injections. Patient scores on the Disabilities of the Arm, Shoulder, and Hand scale (which measures symptoms from 0 to 100; lower is better) showed no difference but favored the saline injections at 2-month (28 vs 20) and 6-month (20 vs 10) follow-up.
Editor’s takeaway
Limiting the evidence review to studies with a placebo comparator clarifies the lack of effectiveness of lateral epicondylitis injections. Neither corticosteroid, platelet-rich plasma, botulinum toxin, prolotherapy, hyaluronic acid, or autologous blood injections have proven superior to saline or anesthetic injections. However, all injections that contained “placebo” significantly improved lateralepicondylitis.
EVIDENCE SUMMARY
Neither corticosteroids nor platelet-rich plasma are superior to placebo
A 2014 systematic review of RCTs of nonsurgical treatments for lateral epicondylitis identified 4 studies comparing corticosteroid injections to saline or anesthetic injections.1 In the first study, investigators followed 64 patients for 6 months. Both groups significantly improved from baseline, but there were no differences in pain or function at 1 or 6 months. Skin discoloration occurred in 2 patients who received lidocaine injection and 1 who received dexamethasone.2
In a second RCT of patients with symptoms for > 4 weeks, 39 participants were randomized to either betamethasone/bupivacaine or bupivacaine-only injections. In-person follow-up occurred at 4 and 8 weeks and telephone follow-up at 6 months. Both groups statistically improved from baseline to 6 months. No differences were seen between groups in pain or functional improvement at 4, 8, or 26 weeks, but the betamethasone group showed statistically greater improvement on the Visual Analog Scale (VAS) from 8 weeks to the final 6-month telephone follow-up. No functional assessments were reported at 6 months.3
The third RCT of 165 patients with lateral epicondylitis for > 6 weeks evaluated 4 intervention groups: corticosteroid injection with/without physiotherapy and placebo (small-volume saline) injection with/without physiotherapy. At the end of 1 year, the corticosteroid injection groups had less complete recovery (83% vs 96%; relative risk [RR] = 0.86; 99% CI, 0.75-0.99) and more recurrences (54% vs 12%; RR = 0.23; 99% CI, 0.10-0.51) than the placebo groups.4
The fourth RCT randomized 120 patients to either 2 mL lidocaine or 1 mL lidocaine plus 1 mL of triamcinolone. At 1-year follow-up, 57 of 60 lidocaine-injected patients had an excellent recovery and 56 of 60 triamcinolone plus lidocaine patients had an excellent recovery.5
Platelet-rich plasma. A meta-analysis6 of RCTs of PRP vs saline injections included 5 trials and 276 patients with a mean age of 48 years; duration of follow-up was 2 to 12 months. No significant differences were found between the groups for pain score—measured by VAS or the Patient-Rated Tennis Elbow Evaluation (PRTEE)—(standardized mean difference [SMD] = –0.51; 95% CI, –1.32 to –0.30) nor for functional score (SMD = 0.07; 95% CI, –0.46 to 0.33). Two of the trials reported adverse reactions of pain around the injection site: 16% to 20% in the PRP group vs 8% to 15% in the saline group.
Corticosteroids and PRP. A 2013 3-armed RCT7 (n = 60) compared 1-time injections of PRP, corticosteroid, and saline for treatment of lateral epicondylitis. Pain was evaluated at 1 and 3 months using the PRTEE. Compared to saline, corticosteroid showed a statistically significant, but not a minimum clinically important, reduction (8% greater improvement) at 1 month but not at 3 months. PRP pain reduction at both 1 and 3 months was not significantly different from placebo. Importantly, a small sample size combined with a high dropout rate (> 70%) limit validity of this study.
Botulinum toxin shows modest pain improvement, but …
A 2017 meta-analysis8 of 4 RCTs (n = 278) compared the effectiveness of botulinum toxin vs saline injection and other nonsurgical treatments for lateral epicondylitis. The studies compared the mean differences in pain relief and hand grip strength in adult patients with lateral epicondylitis symptoms for at least 3 months. Compared with saline injection, botulinum toxin injection significantly reduced pain to a small or medium SMD, at 2 to 4 weeks post injection (SMD = –0.73; 95% CI, –1.29 to –0.17); 8 to 12 weeks post injection (SMD = –0.45; 95% CI, –0.74 to –0.15); and 16+ weeks post injection (SMD = –0.54; 95% CI, –0.98 to –0.11). Harm from botulinum toxin was greater than from saline or corticosteroid, with a significant reduction in grip strength at 2 to 4 weeks (SMD = –0.33; 95% CI, –0.59 to –0.08).
Continue to: Prolotherapy needs further study
Prolotherapy needs further study
A 2008 RCT9 of 20 adults with at least 6 months of lateral epicondylitis received either prolotherapy (1 part 5% sodium morrhuate, 1.5 parts 50% dextrose, 0.5 parts 4% lidocaine, 0.5 parts 0.5% bupivacaine HCl, and 3.5 parts normal saline) injections or 0.9% saline injections at baseline, 4 weeks, and 8 weeks. On a 10-point Likert scale, the prolotherapy group had a lower mean pain score at 16 weeks than the saline injection group (0.5 vs 3.5), but not at 8 weeks (3.3 vs 3.6). This pilot study’s results are limited by its small sample size.
Hyaluronic acid improves pain, but not enough
A 2010 double-blind RCT10 (n = 331) compared hyaluronic acid injection vs saline injection in treatment of lateral epicondylitis in adults with > 3 months of symptoms. Two injections were performed 1 week apart, with follow-up at 30 days and at 1 year after the first injection. VAS score in the hyaluronic acid group, at rest and after grip testing, was significantly different (statistically) than in the placebo group but did not meet criteria for minimum clinically important improvement. Review of the literature showed limited follow-up studies on hyaluronic acid for lateral epicondylitis to confirm this RCT.
Autologous blood has no advantage over placebo
The only RCT of autologous blood compared to saline injections11 included patients with lateral epicondylitis for < 6 months: 10 saline injections vs 9 autologous blood injections. Patient scores on the Disabilities of the Arm, Shoulder, and Hand scale (which measures symptoms from 0 to 100; lower is better) showed no difference but favored the saline injections at 2-month (28 vs 20) and 6-month (20 vs 10) follow-up.
Editor’s takeaway
Limiting the evidence review to studies with a placebo comparator clarifies the lack of effectiveness of lateral epicondylitis injections. Neither corticosteroid, platelet-rich plasma, botulinum toxin, prolotherapy, hyaluronic acid, or autologous blood injections have proven superior to saline or anesthetic injections. However, all injections that contained “placebo” significantly improved lateralepicondylitis.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Lindenhovius A, Henket M, Gilligan BP, et al. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg Am. 2008;33:909-919. doi: 10.1016/j.jhsa.2008.02.004
3. Newcomer KL, Laskowski ER, Idank DM, et al. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11:214-222. doi: 10.1097/00042752-200110000-00002
4. Coombes BK, Bisset L, Brooks P, et al. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309:461-469. doi: 10.1001/jama.2013.129
5. Altay T, Gunal I, Ozturk H. Local injection treatment for lateral epicondylitis. Clin Orthop Relat Res. 2002;398:127-130.
6. Simental-Mendía M, Vilchez-Cavazos F, Álvarez-Villalobos N, et al. Clinical efficacy of platelet-rich plasma in the treatment of lateral epicondylitis: a systematic review and meta-analysis of randomized placebo-controlled clinical trials. Clin Rheumatol. 2020;39:2255-2265. doi: 10.1007/s10067-020-05000-y
7. Krogh T, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich-plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635. doi:10.1177/0363546512472975
8. Lin Y, Wu W, Hsu Y, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2017;32:131-145. doi:10.1177/0269215517702517
9. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sports Med. 2008;18:248-254. doi: 10.1097/JSM.0b013e318170fc87
10. Petrella R, Cogliano A, Decaria J, et al. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:4. doi: 10.1186/1758-2555-2-4
11. Wolf JM, Ozer K, Scott F, et al. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am. 2011;36:1269-1272. doi: 10.1016/j.jhsa.2011.05.014
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Lindenhovius A, Henket M, Gilligan BP, et al. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg Am. 2008;33:909-919. doi: 10.1016/j.jhsa.2008.02.004
3. Newcomer KL, Laskowski ER, Idank DM, et al. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11:214-222. doi: 10.1097/00042752-200110000-00002
4. Coombes BK, Bisset L, Brooks P, et al. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309:461-469. doi: 10.1001/jama.2013.129
5. Altay T, Gunal I, Ozturk H. Local injection treatment for lateral epicondylitis. Clin Orthop Relat Res. 2002;398:127-130.
6. Simental-Mendía M, Vilchez-Cavazos F, Álvarez-Villalobos N, et al. Clinical efficacy of platelet-rich plasma in the treatment of lateral epicondylitis: a systematic review and meta-analysis of randomized placebo-controlled clinical trials. Clin Rheumatol. 2020;39:2255-2265. doi: 10.1007/s10067-020-05000-y
7. Krogh T, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich-plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635. doi:10.1177/0363546512472975
8. Lin Y, Wu W, Hsu Y, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2017;32:131-145. doi:10.1177/0269215517702517
9. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sports Med. 2008;18:248-254. doi: 10.1097/JSM.0b013e318170fc87
10. Petrella R, Cogliano A, Decaria J, et al. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:4. doi: 10.1186/1758-2555-2-4
11. Wolf JM, Ozer K, Scott F, et al. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am. 2011;36:1269-1272. doi: 10.1016/j.jhsa.2011.05.014
EVIDENCE-BASED ANSWER:
Placebo injections actually improve lateral epicondylitis at high rates. No other injections convincingly improve it better than placebo.
Corticosteroid injection is not superior to saline or anesthetic injection (strength of recommendation [SOR] A, systematic review of randomized controlled trials [RCTs]). Platelet-rich plasma (PRP) injection is not superior to saline injection (SOR A, meta-analysis of RCTs).
Botulinum toxin injection, compared to saline injection, modestly improved pain in lateral epicondylitis, but with short-term grip-strength weakness (SOR A, meta-analysis of RCTs). Prolotherapy injection, compared to saline injection, improved pain at 16-week, but not at 8-week, follow-up (SOR B, one small pilot RCT).
Hyaluronic acid injection, compared to saline injection, resulted in a statistically significant pain reduction (6%) but did not achieve the minimum clinically important difference (SOR B, single RCT). Autologous blood injection, compared to saline injection, did not improve disability ratings (SOR B, one small RCT).
When the evidence suggests that placebo is best
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
Botulinum toxin for chronic pain: What's on the horizon?
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.
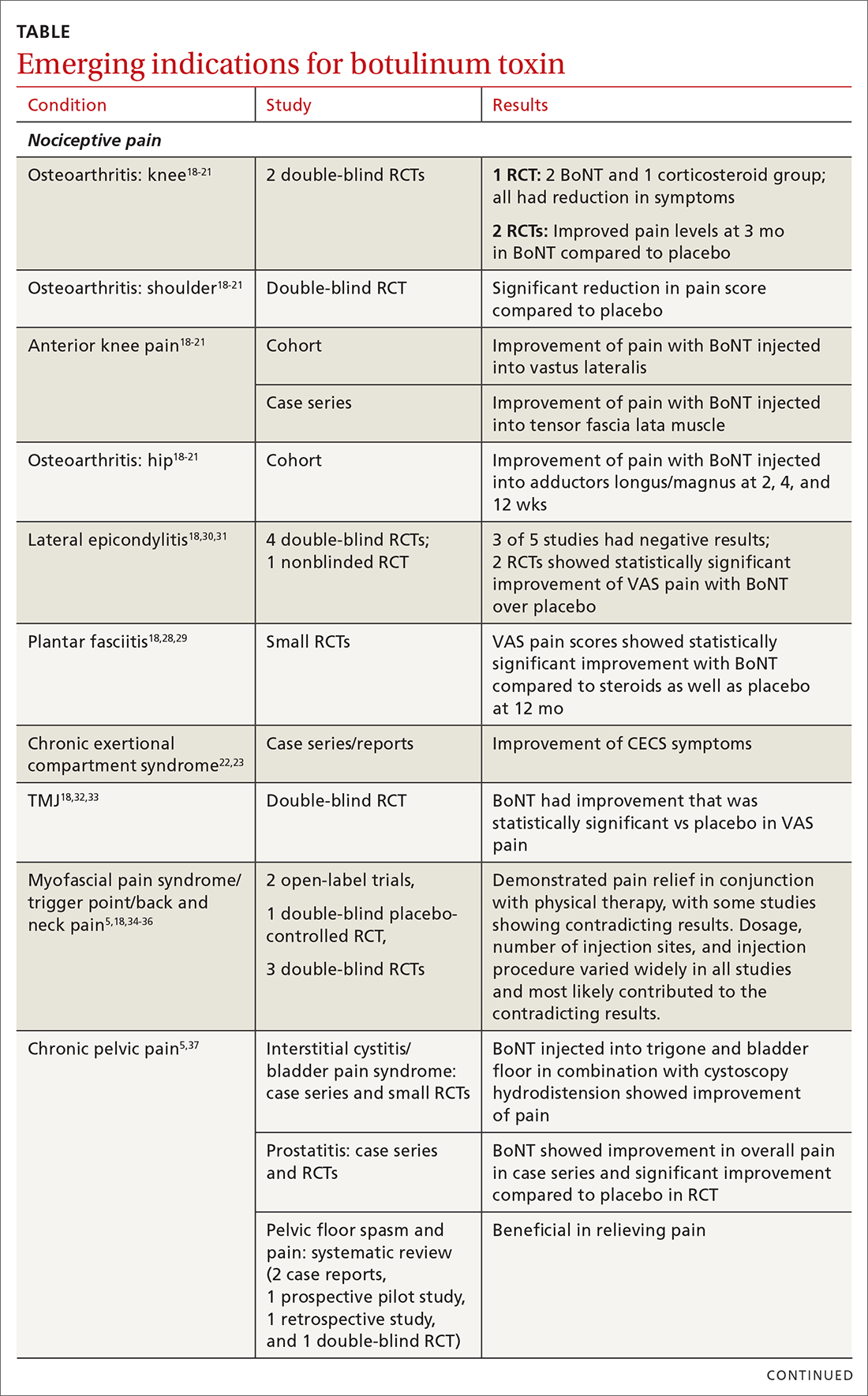
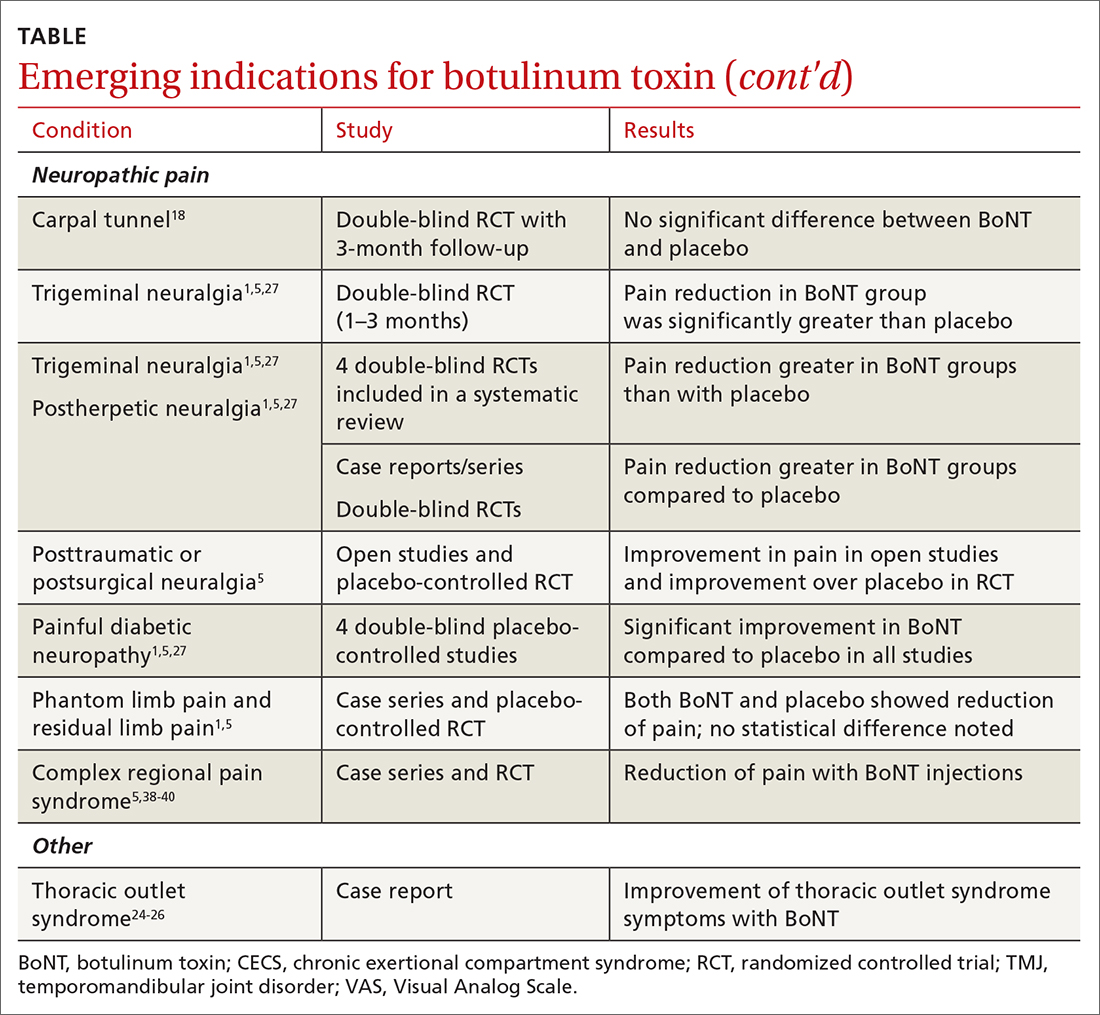
The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.
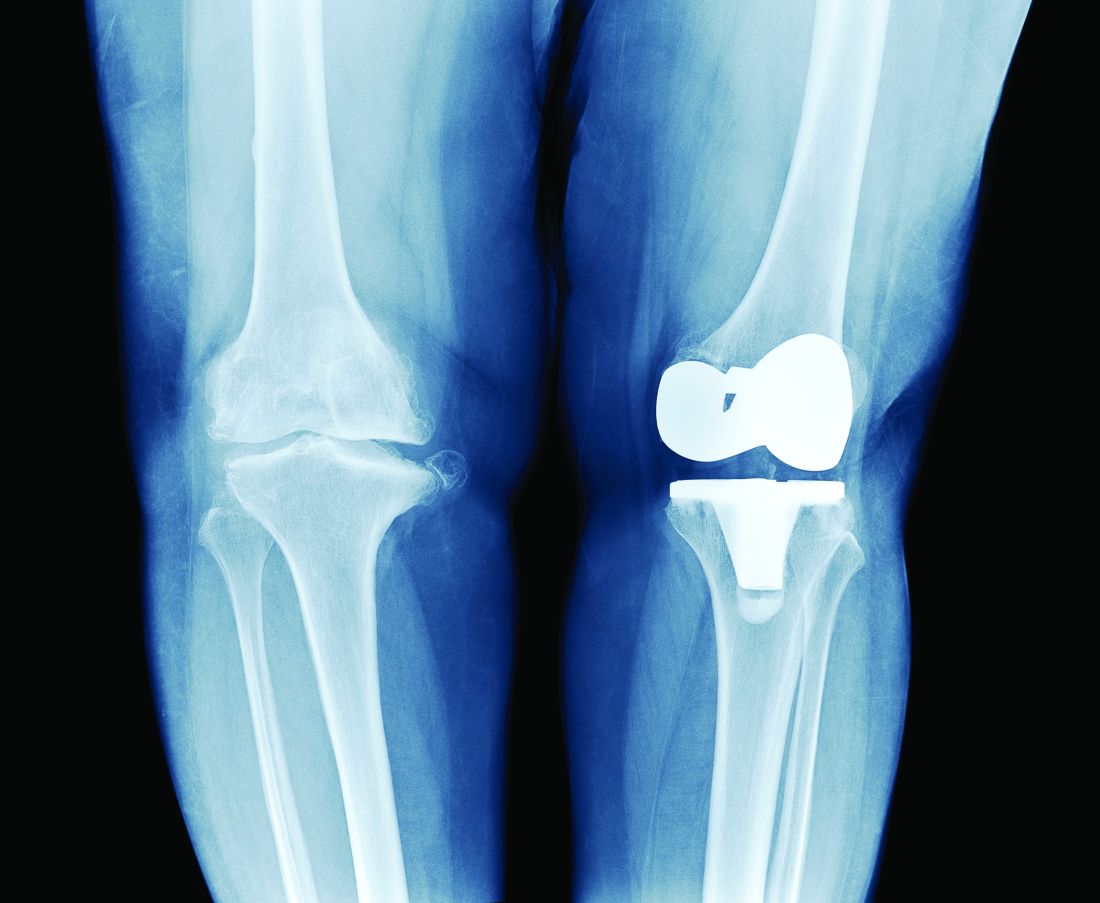
Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
PRACTICE RECOMMENDATIONS
› Consider botulinum toxin (BoNT) for patients with headache, spasticity, or cervical dystonia, as the FDA has approved BoNT for pain relief in these conditions. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Tips and tools to help refine your approach to chest pain
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3

First challenge: The scale of the differential Dx
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).
History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.
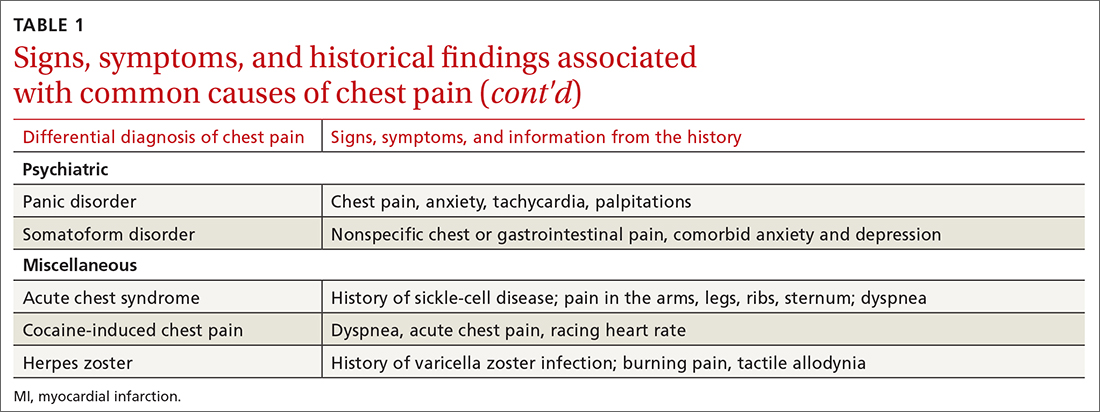
History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.
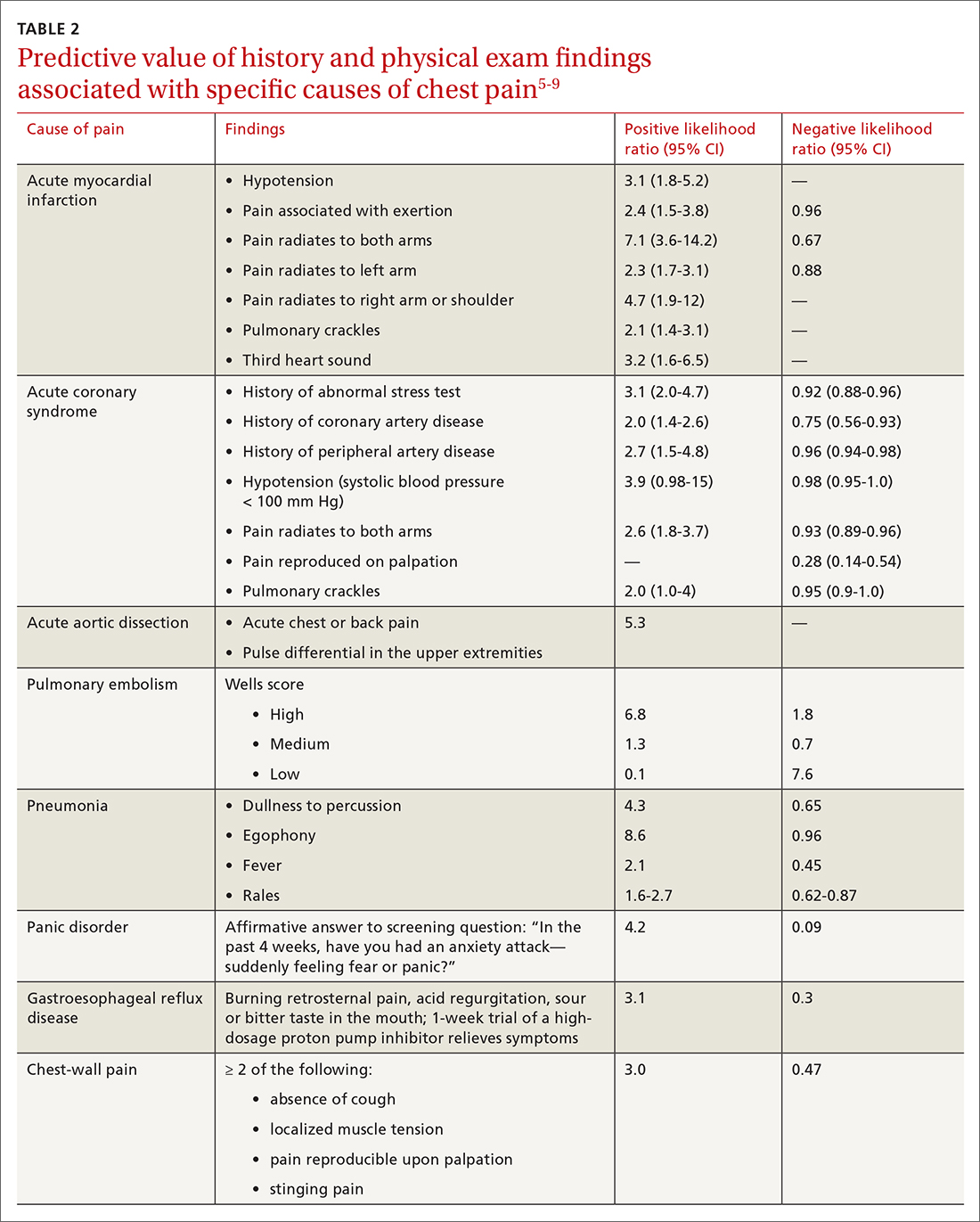
The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4
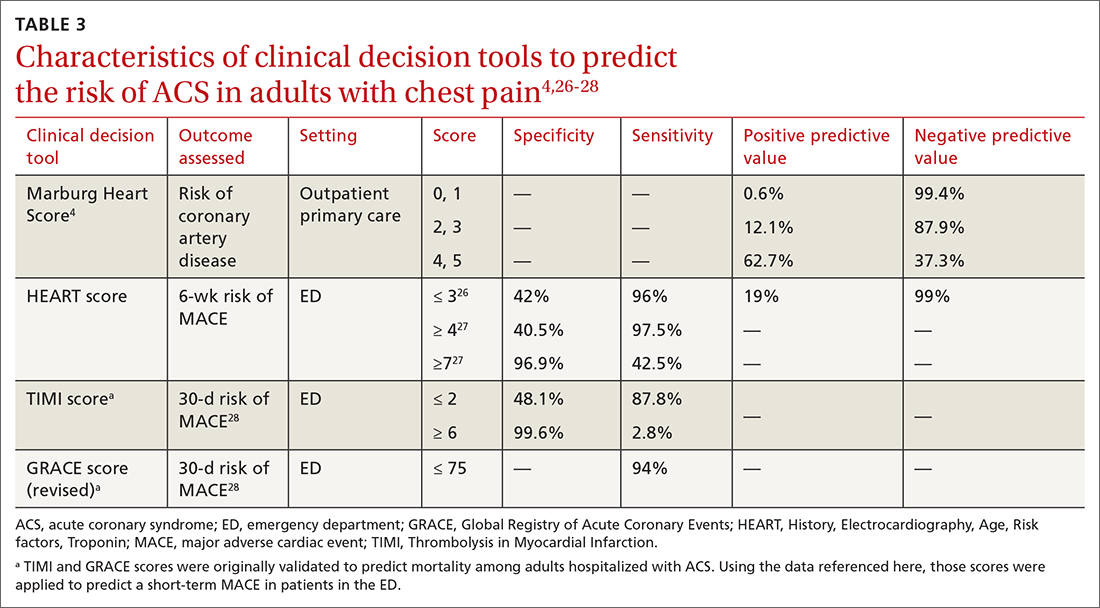
This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35
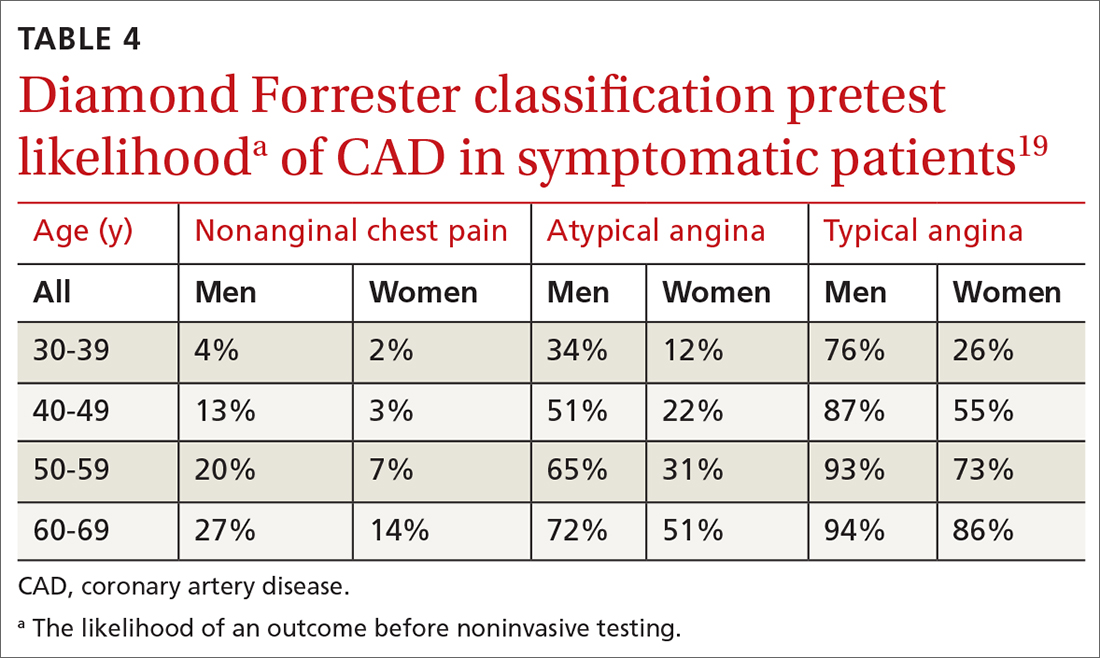
Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).
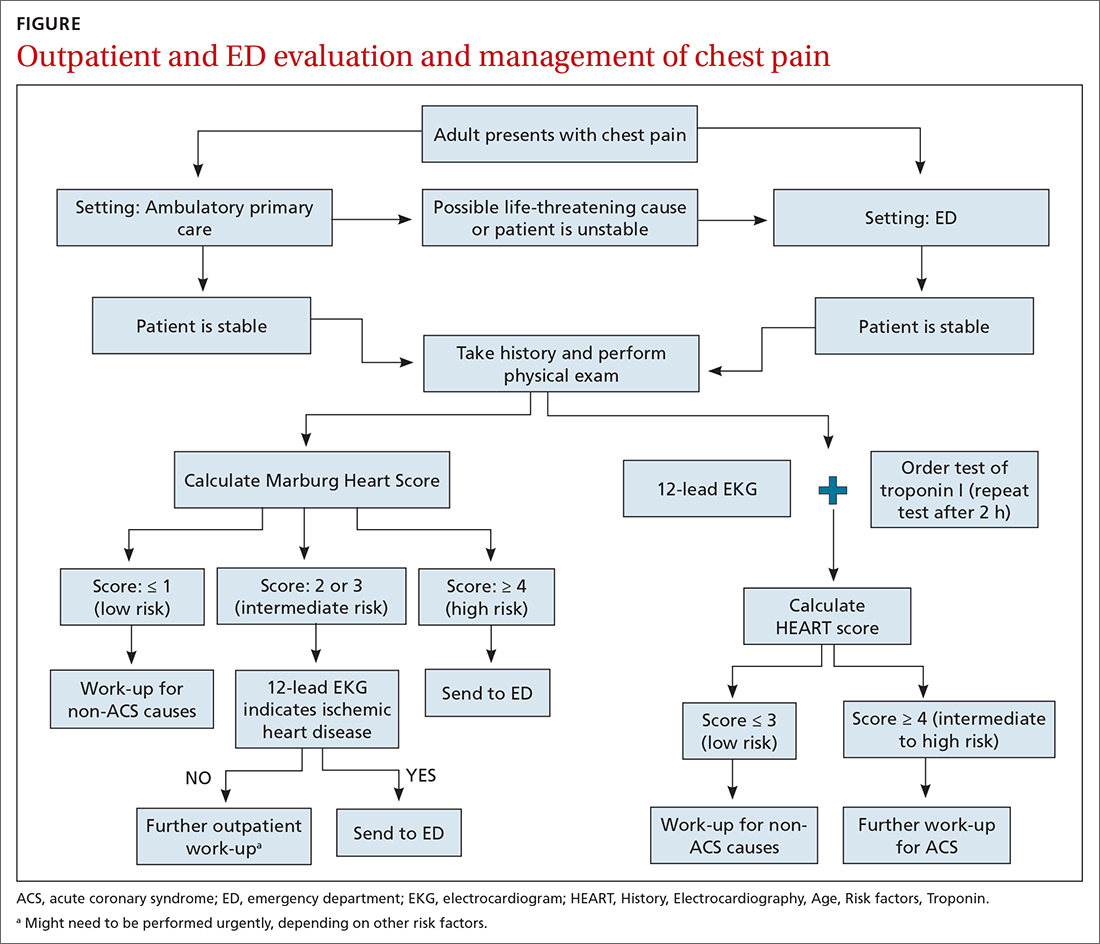
When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3

First challenge: The scale of the differential Dx
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).

History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.

History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.

The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4

This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35

Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).

When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3

First challenge: The scale of the differential Dx
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).

History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.

History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.

The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4

This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35

Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).

When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
PRACTICE RECOMMENDATIONS
› Use the highly sensitive Marburg Heart Score to rule out coronary artery disease as a cause of chest pain in the ambulatory care setting. B
› Consider a prior normal stress test result nonpredictive of outcome in a patient presenting with chest pain. Patients with such a history of testing have a risk of a 30-day adverse cardiac event that is similar to the risk seen in patients who have never had a stress test. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
a Risk factors include hypertension, hypercholesterolemia, diabetes, obesity (body mass index > 30), smoking (current, or smoking cessation for ≤ 3 mo), and family history of CAD (ie, parent or sibling affected before 65 years of age). Atherosclerotic disease includes history of AMI, percutaneous coronary intervention or coronary artery bypass grafting, stroke, or peripheral artery disease.
Small fiber neuropathy is rising in the U.S., but why is a mystery
The exact reason for the increase in isolated SFN “remains unclear,” said Christopher J. Klein, MD, of the Mayo Clinic in Rochester, Minn. However, “we noted during the study period the population has had increased BMI, which appears to be a risk factor for this disorder, with many (50%) developing either glucose impairment or frank diabetes during the study period even if not present at first small fiber neuropathy presentation, also with associated higher triglyceride levels,” he explained.
The study was published online October 27 in Neurology.
Significant upward trend
Investigators reviewed the records of all 94 adults diagnosed with pure SFN (no large fiber involvement) between 1998 and 2017 in Olmsted and adjacent counties in Minnesota – and compared them with 282 adults of similar age and gender who did not have neuropathy.
The incidence of SFN over the entire study period was 1.3 per 100,000 per year and the prevalence was 13.3 per 100,000.
There was a “significant upward trend” in SFN incidence over the study period that could not be attributed to the availability of intraepidermal nerve fiber density testing, the authors reported.
The median age of onset of SFN was 54 years and two-thirds were women (67%).
Diabetes, obesity, and hypertriglyceridemia were significantly more common in patients with SFN compared with matched controls. These metabolic risk factors are also associated with peripheral neuropathy regardless of fiber type.
Autonomic symptoms were common and generally mild, affecting 85% of patients with SFN, and included male erectile dysfunction, constipation, light-headedness and palpitations, urinary symptoms, diarrhea, dry eyes and mouth, sweat abnormalities, and gastroparesis.
Insomnia and use of opioid pain medication were more common in those with SFN than matched controls.
More than one-third (36%) of patients with SFN developed large fiber neuropathy an average of 5.3 years after developing SFN.
During an average follow-up of 6.1 years, adults with SFN were significantly more likely to suffer myocardial infarction (46% vs. 27%; odds ratio, 2.0; 95% CI, 1.8-4.9), congestive heart failure (27% vs. 12%; OR, 2.6; 95% CI, 1.4-4.8), peripheral vascular disease (22% vs. 6%; OR, 4.0; 95% CI, 1.9-8.1), stroke (24% vs. 10%; OR, 2.8; 95% CI, 1.5-5.3), diabetes (51% vs. 22%; OR, 4.6; 95% CI, 2.8-7.6) and rheumatologic disease (30% vs. 7%; OR, 5.3; 95% CI, 2.8-10.4).
For 70% of patients, no cause for SFN could be determined. Diabetes (15%) was the most common cause identified. Other less common causes included Sjögren syndrome, lupus, amyloidosis, and Fabry disease.
“It is important to quantitatively diagnose patients with SFN as many non-neurological musculoskeletal causes can mimic the disorder,” said Dr. Klein.
“If rates of progression are rapid, sinister causes such as out-of-control diabetes, hereditary [transthyretin] TTR amyloidosis, and Fabry disease can be responsible. For other patients, rates of progression are slow and generally do not lead to significant neurologic impairments,” he added.
“However,” he said, “internal medicine follow-up is important for all as this disorder associates with development with higher risk of cardiovascular disease, including commonly heart attacks.”
Of note, although mean age at death was not significantly different in patients with SFN than controls (70 vs. 73 years), there was a significantly higher number of deaths in patients with SFN (n = 18; 19%) than in matched controls (n = 35; 12%) from the time of symptom onset, the researchers reported.
Important research
This “important” study sheds light on the comorbidities and longitudinal consequences of SFN, wrote Brian Callaghan, MD, with the University of Michigan, Ann Arbor, and J. Robinson Singleton, MD, with the University of Utah, Salt Lake City, in an accompanying editorial in Neurology.
The study demonstrates clearly that SFN has “metabolic risk factors similar to those seen for sensory predominant peripheral neuropathies affecting a broader range of fiber types. As a result, therapies that address metabolic risk factors are likely to help prevent or treat both conditions,” they wrote.
Dr. Callaghan and Dr. Singleton added that a key strength of the study is the detailed follow-up that examines SFN progression over time. “The authors found that patients with SFN do not report high disability and that progression tends to be slow. Therefore, patients with SFN can be counseled that progression and disability are likely to be modest in most cases. However, when patients do progress quickly, uncommon etiologies should be sought,” the editorialists wrote.
The study was supported by the Mayo Clinic Foundation, Mayo Clinic Center for Individualized Medicine, and Mayo Clinic Center of MS and Autoimmune Neurology. Dr. Klein has received teaching honorarium from Ackea pharmaceuticals for lectures on hereditary transthyretin amyloidosis and Fabry disease, consulted for Pfizer regarding tafamidis (all compensation for consulting activities is paid directly to Mayo Clinic), and participated in the clinical trials for inotersen and patisiran but received no personal compensation for his participation. Dr. Callaghan consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Singleton has consulted for Regenacy.
A version of this article first appeared on Medscape.com.
The exact reason for the increase in isolated SFN “remains unclear,” said Christopher J. Klein, MD, of the Mayo Clinic in Rochester, Minn. However, “we noted during the study period the population has had increased BMI, which appears to be a risk factor for this disorder, with many (50%) developing either glucose impairment or frank diabetes during the study period even if not present at first small fiber neuropathy presentation, also with associated higher triglyceride levels,” he explained.
The study was published online October 27 in Neurology.
Significant upward trend
Investigators reviewed the records of all 94 adults diagnosed with pure SFN (no large fiber involvement) between 1998 and 2017 in Olmsted and adjacent counties in Minnesota – and compared them with 282 adults of similar age and gender who did not have neuropathy.
The incidence of SFN over the entire study period was 1.3 per 100,000 per year and the prevalence was 13.3 per 100,000.
There was a “significant upward trend” in SFN incidence over the study period that could not be attributed to the availability of intraepidermal nerve fiber density testing, the authors reported.
The median age of onset of SFN was 54 years and two-thirds were women (67%).
Diabetes, obesity, and hypertriglyceridemia were significantly more common in patients with SFN compared with matched controls. These metabolic risk factors are also associated with peripheral neuropathy regardless of fiber type.
Autonomic symptoms were common and generally mild, affecting 85% of patients with SFN, and included male erectile dysfunction, constipation, light-headedness and palpitations, urinary symptoms, diarrhea, dry eyes and mouth, sweat abnormalities, and gastroparesis.
Insomnia and use of opioid pain medication were more common in those with SFN than matched controls.
More than one-third (36%) of patients with SFN developed large fiber neuropathy an average of 5.3 years after developing SFN.
During an average follow-up of 6.1 years, adults with SFN were significantly more likely to suffer myocardial infarction (46% vs. 27%; odds ratio, 2.0; 95% CI, 1.8-4.9), congestive heart failure (27% vs. 12%; OR, 2.6; 95% CI, 1.4-4.8), peripheral vascular disease (22% vs. 6%; OR, 4.0; 95% CI, 1.9-8.1), stroke (24% vs. 10%; OR, 2.8; 95% CI, 1.5-5.3), diabetes (51% vs. 22%; OR, 4.6; 95% CI, 2.8-7.6) and rheumatologic disease (30% vs. 7%; OR, 5.3; 95% CI, 2.8-10.4).
For 70% of patients, no cause for SFN could be determined. Diabetes (15%) was the most common cause identified. Other less common causes included Sjögren syndrome, lupus, amyloidosis, and Fabry disease.
“It is important to quantitatively diagnose patients with SFN as many non-neurological musculoskeletal causes can mimic the disorder,” said Dr. Klein.
“If rates of progression are rapid, sinister causes such as out-of-control diabetes, hereditary [transthyretin] TTR amyloidosis, and Fabry disease can be responsible. For other patients, rates of progression are slow and generally do not lead to significant neurologic impairments,” he added.
“However,” he said, “internal medicine follow-up is important for all as this disorder associates with development with higher risk of cardiovascular disease, including commonly heart attacks.”
Of note, although mean age at death was not significantly different in patients with SFN than controls (70 vs. 73 years), there was a significantly higher number of deaths in patients with SFN (n = 18; 19%) than in matched controls (n = 35; 12%) from the time of symptom onset, the researchers reported.
Important research
This “important” study sheds light on the comorbidities and longitudinal consequences of SFN, wrote Brian Callaghan, MD, with the University of Michigan, Ann Arbor, and J. Robinson Singleton, MD, with the University of Utah, Salt Lake City, in an accompanying editorial in Neurology.
The study demonstrates clearly that SFN has “metabolic risk factors similar to those seen for sensory predominant peripheral neuropathies affecting a broader range of fiber types. As a result, therapies that address metabolic risk factors are likely to help prevent or treat both conditions,” they wrote.
Dr. Callaghan and Dr. Singleton added that a key strength of the study is the detailed follow-up that examines SFN progression over time. “The authors found that patients with SFN do not report high disability and that progression tends to be slow. Therefore, patients with SFN can be counseled that progression and disability are likely to be modest in most cases. However, when patients do progress quickly, uncommon etiologies should be sought,” the editorialists wrote.
The study was supported by the Mayo Clinic Foundation, Mayo Clinic Center for Individualized Medicine, and Mayo Clinic Center of MS and Autoimmune Neurology. Dr. Klein has received teaching honorarium from Ackea pharmaceuticals for lectures on hereditary transthyretin amyloidosis and Fabry disease, consulted for Pfizer regarding tafamidis (all compensation for consulting activities is paid directly to Mayo Clinic), and participated in the clinical trials for inotersen and patisiran but received no personal compensation for his participation. Dr. Callaghan consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Singleton has consulted for Regenacy.
A version of this article first appeared on Medscape.com.
The exact reason for the increase in isolated SFN “remains unclear,” said Christopher J. Klein, MD, of the Mayo Clinic in Rochester, Minn. However, “we noted during the study period the population has had increased BMI, which appears to be a risk factor for this disorder, with many (50%) developing either glucose impairment or frank diabetes during the study period even if not present at first small fiber neuropathy presentation, also with associated higher triglyceride levels,” he explained.
The study was published online October 27 in Neurology.
Significant upward trend
Investigators reviewed the records of all 94 adults diagnosed with pure SFN (no large fiber involvement) between 1998 and 2017 in Olmsted and adjacent counties in Minnesota – and compared them with 282 adults of similar age and gender who did not have neuropathy.
The incidence of SFN over the entire study period was 1.3 per 100,000 per year and the prevalence was 13.3 per 100,000.
There was a “significant upward trend” in SFN incidence over the study period that could not be attributed to the availability of intraepidermal nerve fiber density testing, the authors reported.
The median age of onset of SFN was 54 years and two-thirds were women (67%).
Diabetes, obesity, and hypertriglyceridemia were significantly more common in patients with SFN compared with matched controls. These metabolic risk factors are also associated with peripheral neuropathy regardless of fiber type.
Autonomic symptoms were common and generally mild, affecting 85% of patients with SFN, and included male erectile dysfunction, constipation, light-headedness and palpitations, urinary symptoms, diarrhea, dry eyes and mouth, sweat abnormalities, and gastroparesis.
Insomnia and use of opioid pain medication were more common in those with SFN than matched controls.
More than one-third (36%) of patients with SFN developed large fiber neuropathy an average of 5.3 years after developing SFN.
During an average follow-up of 6.1 years, adults with SFN were significantly more likely to suffer myocardial infarction (46% vs. 27%; odds ratio, 2.0; 95% CI, 1.8-4.9), congestive heart failure (27% vs. 12%; OR, 2.6; 95% CI, 1.4-4.8), peripheral vascular disease (22% vs. 6%; OR, 4.0; 95% CI, 1.9-8.1), stroke (24% vs. 10%; OR, 2.8; 95% CI, 1.5-5.3), diabetes (51% vs. 22%; OR, 4.6; 95% CI, 2.8-7.6) and rheumatologic disease (30% vs. 7%; OR, 5.3; 95% CI, 2.8-10.4).
For 70% of patients, no cause for SFN could be determined. Diabetes (15%) was the most common cause identified. Other less common causes included Sjögren syndrome, lupus, amyloidosis, and Fabry disease.
“It is important to quantitatively diagnose patients with SFN as many non-neurological musculoskeletal causes can mimic the disorder,” said Dr. Klein.
“If rates of progression are rapid, sinister causes such as out-of-control diabetes, hereditary [transthyretin] TTR amyloidosis, and Fabry disease can be responsible. For other patients, rates of progression are slow and generally do not lead to significant neurologic impairments,” he added.
“However,” he said, “internal medicine follow-up is important for all as this disorder associates with development with higher risk of cardiovascular disease, including commonly heart attacks.”
Of note, although mean age at death was not significantly different in patients with SFN than controls (70 vs. 73 years), there was a significantly higher number of deaths in patients with SFN (n = 18; 19%) than in matched controls (n = 35; 12%) from the time of symptom onset, the researchers reported.
Important research
This “important” study sheds light on the comorbidities and longitudinal consequences of SFN, wrote Brian Callaghan, MD, with the University of Michigan, Ann Arbor, and J. Robinson Singleton, MD, with the University of Utah, Salt Lake City, in an accompanying editorial in Neurology.
The study demonstrates clearly that SFN has “metabolic risk factors similar to those seen for sensory predominant peripheral neuropathies affecting a broader range of fiber types. As a result, therapies that address metabolic risk factors are likely to help prevent or treat both conditions,” they wrote.
Dr. Callaghan and Dr. Singleton added that a key strength of the study is the detailed follow-up that examines SFN progression over time. “The authors found that patients with SFN do not report high disability and that progression tends to be slow. Therefore, patients with SFN can be counseled that progression and disability are likely to be modest in most cases. However, when patients do progress quickly, uncommon etiologies should be sought,” the editorialists wrote.
The study was supported by the Mayo Clinic Foundation, Mayo Clinic Center for Individualized Medicine, and Mayo Clinic Center of MS and Autoimmune Neurology. Dr. Klein has received teaching honorarium from Ackea pharmaceuticals for lectures on hereditary transthyretin amyloidosis and Fabry disease, consulted for Pfizer regarding tafamidis (all compensation for consulting activities is paid directly to Mayo Clinic), and participated in the clinical trials for inotersen and patisiran but received no personal compensation for his participation. Dr. Callaghan consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Singleton has consulted for Regenacy.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Patients given NSAIDs over antiemetics for headaches spend less time in the ED
based on data from approximately 7,000 patients.
Headache is the fourth-most common chief complaint in the ED, accounting for approximately 3% of all ED visits, said Philip Wang, a medical student at the Cleveland Clinic, in a presentation at the annual meeting of the American College of Emergency Physicians.
A variety of pharmacotherapies are used to manage headache, which leads to a range of resource use, he said.
To understand the association between route of drug administration and length of ED stay, Mr. Wang and colleagues reviewed data from 7,233 visits by 6,715 patients at any of the 21 Cleveland Clinic Health System EDs in 2018 with headache as the primary discharge diagnosis. Patients admitted to the hospital were excluded; those treated with opioids, antiemetics, and/or NSAIDs were included. The average age of the study population was 31 years, 57% were White, and approximately half were Medicaid or Medicare patients.
Approximately 68% of patients received antiemetics, 66.8% received NSAIDs, and 9.8% received opioids. Approximately 42% of patients received parenteral-only treatment and 42% received oral-only treatment; 15% received mixed treatment. The average length of ED stay was 202 minutes.
In a multivariate analysis adjusted for sex, age, income, race, insurance status, ED type, and arrival time, treatment with oral drugs only was associated with an 11% reduction of length of stay, compared with treatment with parenteral medication only (P < .001). However, the length of stay for patients treated with mixed route of administration was 10% longer, compared with parenteral only (P < .001).
In terms of drug class (a secondary outcome), patients treated with opioids had a 10% increase in length of stay (P < .01) and those treated with antiemetics had a 14% increase in length of stay; however, patients treated with NSAIDs had a 7% decrease in length of stay.
The study findings were limited in part by the challenge of isolating patients presenting with a primary headache diagnosis, Mr. Wang noted in the presentation.
The challenge of controlling for all the potential factors impacting length of stay, which is “provider, resource, and situation dependent,” is an additional limitation, he said.
However, the results show that route of administration has a significant impact on length of ED stay in patients presenting with headache, he concluded.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
based on data from approximately 7,000 patients.
Headache is the fourth-most common chief complaint in the ED, accounting for approximately 3% of all ED visits, said Philip Wang, a medical student at the Cleveland Clinic, in a presentation at the annual meeting of the American College of Emergency Physicians.
A variety of pharmacotherapies are used to manage headache, which leads to a range of resource use, he said.
To understand the association between route of drug administration and length of ED stay, Mr. Wang and colleagues reviewed data from 7,233 visits by 6,715 patients at any of the 21 Cleveland Clinic Health System EDs in 2018 with headache as the primary discharge diagnosis. Patients admitted to the hospital were excluded; those treated with opioids, antiemetics, and/or NSAIDs were included. The average age of the study population was 31 years, 57% were White, and approximately half were Medicaid or Medicare patients.
Approximately 68% of patients received antiemetics, 66.8% received NSAIDs, and 9.8% received opioids. Approximately 42% of patients received parenteral-only treatment and 42% received oral-only treatment; 15% received mixed treatment. The average length of ED stay was 202 minutes.
In a multivariate analysis adjusted for sex, age, income, race, insurance status, ED type, and arrival time, treatment with oral drugs only was associated with an 11% reduction of length of stay, compared with treatment with parenteral medication only (P < .001). However, the length of stay for patients treated with mixed route of administration was 10% longer, compared with parenteral only (P < .001).
In terms of drug class (a secondary outcome), patients treated with opioids had a 10% increase in length of stay (P < .01) and those treated with antiemetics had a 14% increase in length of stay; however, patients treated with NSAIDs had a 7% decrease in length of stay.
The study findings were limited in part by the challenge of isolating patients presenting with a primary headache diagnosis, Mr. Wang noted in the presentation.
The challenge of controlling for all the potential factors impacting length of stay, which is “provider, resource, and situation dependent,” is an additional limitation, he said.
However, the results show that route of administration has a significant impact on length of ED stay in patients presenting with headache, he concluded.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
based on data from approximately 7,000 patients.
Headache is the fourth-most common chief complaint in the ED, accounting for approximately 3% of all ED visits, said Philip Wang, a medical student at the Cleveland Clinic, in a presentation at the annual meeting of the American College of Emergency Physicians.
A variety of pharmacotherapies are used to manage headache, which leads to a range of resource use, he said.
To understand the association between route of drug administration and length of ED stay, Mr. Wang and colleagues reviewed data from 7,233 visits by 6,715 patients at any of the 21 Cleveland Clinic Health System EDs in 2018 with headache as the primary discharge diagnosis. Patients admitted to the hospital were excluded; those treated with opioids, antiemetics, and/or NSAIDs were included. The average age of the study population was 31 years, 57% were White, and approximately half were Medicaid or Medicare patients.
Approximately 68% of patients received antiemetics, 66.8% received NSAIDs, and 9.8% received opioids. Approximately 42% of patients received parenteral-only treatment and 42% received oral-only treatment; 15% received mixed treatment. The average length of ED stay was 202 minutes.
In a multivariate analysis adjusted for sex, age, income, race, insurance status, ED type, and arrival time, treatment with oral drugs only was associated with an 11% reduction of length of stay, compared with treatment with parenteral medication only (P < .001). However, the length of stay for patients treated with mixed route of administration was 10% longer, compared with parenteral only (P < .001).
In terms of drug class (a secondary outcome), patients treated with opioids had a 10% increase in length of stay (P < .01) and those treated with antiemetics had a 14% increase in length of stay; however, patients treated with NSAIDs had a 7% decrease in length of stay.
The study findings were limited in part by the challenge of isolating patients presenting with a primary headache diagnosis, Mr. Wang noted in the presentation.
The challenge of controlling for all the potential factors impacting length of stay, which is “provider, resource, and situation dependent,” is an additional limitation, he said.
However, the results show that route of administration has a significant impact on length of ED stay in patients presenting with headache, he concluded.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PT may lower risk of long-term opioid use after knee replacement
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
Warn patients about illicit drugs doctored with fentanyl
Fentanyl is now threatening overdoses in patients exposed to essentially any of the full array of recreational drugs – not just opioids – that are being sold illicitly, according to an overview of the problem presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Fentanyl can now be found in cocaine and methamphetamine. At this point, there is really no way to predict what is in a [street] drug,” Edwin A. Salsitz, MD, said at the meeting, sponsored by Medscape Live. He is associate clinical professor of medicine who works in the division of chemical dependency at Mount Sinai Beth Israel Medical Center in New York.
As proof of the frequency with which fentanyl is now being used as an additive, most patients with a drug use disorder, regardless of their drug of choice, are testing positive for fentanyl at Dr. Salsitz’s center. Many of those with positive fentanyl tests are unaware that their drugs had been doctored with this agent.
Relative to drugs sold as an opioid, such as heroin or oxycodone, the fentanyl dose in nonopioid drugs is typically more modest, but Dr. Salsitz pointed out that those expecting cocaine or methamphetamine often “have no heroin tolerance, so they are more vulnerable” to the adverse effects of fentanyl, including an overdose.
Although opioid tolerance might improve the chances for surviving a fentanyl overdose, the toxicology of fentanyl is not the same as other opioids. Death from heroin is typically a result of respiratory depression, but the onset is relatively slow, providing a greater opportunity to administer a reversal agent, such as naloxone.
Fentanyl not only produces respiratory depression but skeletal muscle rigidity. The rapid onset of “wooden chest syndrome” can occur within minutes, making the opportunity for intervention much smaller, Dr. Salsitz said.
To illustrate the phenomenon, Dr. Salsitz recounted a case.
After an argument with his mother, a 26-year-old male with a long history of intravenous drug use went to his bedroom. His mother, responding to the sound of a loud thud, rushed to the bedroom to find her son on the floor with a needle still in his arm. Resuscitation efforts by the mother and by the emergency responders, who arrived quickly, failed.
“The speed of his death made it clear that it was fentanyl related, and the postmortem toxicology confirmed that the exposure involved both heroin and fentanyl,” Dr. Salsitz said.
After the first wave of deaths in the opioid epidemic, which was attributed to inappropriate use of prescription opioids, the second wave was driven by heroin. In that wave, patients who became addicted to prescription opioids but were having more difficulty gaining access to them, turned to far cheaper and readily available street heroin. The third wave, driven by fentanyl, began several years ago when sellers of heroin began adding this synthetic opioid, which is relatively cheap, to intensify the high.
It is not expected to end quickly. The fentanyl added to heroin was never a prescription version. Rather, Dr. Salsitz said, it is synthesized in laboratories in China, Mexico, and the United States. It is relatively easy to produce and compact, which makes it easy to transport.
Exacerbating the risks that fentanyl poses when added to street drugs, even more potent versions, such as carfentanil, are also being added to cocaine, methamphetamines, and other nonopioid illicit drugs. When compared on a per-milligram basis, fentanyl is about 100 times more potent than heroin, but carfentanil is about 100 times more potent than fentanyl, according to Dr. Salsitz.
When the third wave of deaths in the opioid epidemic began around 2013, prescriptions of fentanyl, like many other opioid-type therapies were declining. The “perfect storm” that initiated the opioid epidemic was a product of intense focus on pain control and a misperception that prescription opioids posed a low risk of abuse potential, Dr. Salsitz said. By the time fentanyl was driving opioid deaths, the risks of opioids were widely appreciated and their use for prescription analgesia was declining.
Citing several cases, Dr. Salsitz noted that only 20 years after clinicians were being successfully sued for not offering enough analgesia, they were now going to jail for prescribing these drugs too liberally.
According to Dr. Salsitz, While psychiatrists might not have a role in this issue, Dr. Salsitz did see a role for these specialists in protecting patients from the adverse consequences of using illicit drugs doctored with fentanyl.
Noting that individuals with psychiatric disorders are more likely than the general population to self-medicate with drugs purchased illegally, Dr. Salsitz encouraged psychiatrists “to get involved” in asking about drug use and counseling patients on the risks of fentanyl substitution or additives.
“The message is that no one knows what are in these drugs, anymore,” he said.
In addition to making patients aware that many street drugs are now contaminated with fentanyl, Dr. Salsitz provided some safety tips. He suggested instructing patients to take a low dose of any newly acquired drug to gauge its effect, to avoid taking drugs alone, and to avoid mixing drugs. He also recommended using rapid fentanyl test strips in order to detect fentanyl contamination.
Even for the many psychiatrists who do not feel comfortable managing addiction, Dr. Salsitz recommended a proactive approach to address the current threat.
Test strips as an intervention
The seriousness of fentanyl contamination of illicit drugs, including cocaine and methamphetamine, was corroborated by two investigators at the School of Public Health and the Albert Einstein Medical School of Brown University, Providence, R.I. Brandon D.L. Marshall, PhD, associate professor of epidemiology in the School of Public Health, called fentanyl-contaminated cannabis “extremely rare,” but he said that it is being found in counterfeit prescription pills as well as in crystal methamphetamine and in both crack and powder cocaine.
He also advocated the use of fentanyl test strips.
“Test strips are an efficient, inexpensive, and effective way to determine whether fentanyl or related analogs are present in illicit drugs,” he said, noting that he is involved in a trial designed to determine whether fentanyl test strips can reduce the risk of fatal and nonfatal overdoses.
In a pilot study conducted in Baltimore, 69% of the 103 participants engaged in harm reduction behavior after using a fentanyl test strip and receiving a positive result (Addict Behav. 2020;110:106529). It is notable that 86% of the participants had a least one positive result when using the strips. More than half were surprised by the result.
One of the findings from this study was “that the lasting benefit of fentanyl test strip distribution is the opportunity to engage in discussions around safety and relationship building with historically underserved communities,” said the lead author, Ju Nyeong Park, PhD, assistant professor of medicine and epidemiology at Brown University. She moved to Brown after performing this work at Johns Hopkins University, Baltimore.
Dr. Park noted that “many patients in the community already know that they are using drugs containing fentanyl,” but for those who are concerned and wish to avoid contaminated drugs, fentanyl test strips “are a quick screening tool.” However, while the strips are helpful, she cautioned that they cannot be considered a definitive tool for detecting harm in illicit drugs.
“There may also be other chemicals present in tested drugs that confer risk,” she said.
Medscape Live and this news organization are owned by the same parent company. Dr. Salsitz, Dr. Marshall, and Dr. Park reported no potential conflicts of interest.
Fentanyl is now threatening overdoses in patients exposed to essentially any of the full array of recreational drugs – not just opioids – that are being sold illicitly, according to an overview of the problem presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Fentanyl can now be found in cocaine and methamphetamine. At this point, there is really no way to predict what is in a [street] drug,” Edwin A. Salsitz, MD, said at the meeting, sponsored by Medscape Live. He is associate clinical professor of medicine who works in the division of chemical dependency at Mount Sinai Beth Israel Medical Center in New York.
As proof of the frequency with which fentanyl is now being used as an additive, most patients with a drug use disorder, regardless of their drug of choice, are testing positive for fentanyl at Dr. Salsitz’s center. Many of those with positive fentanyl tests are unaware that their drugs had been doctored with this agent.
Relative to drugs sold as an opioid, such as heroin or oxycodone, the fentanyl dose in nonopioid drugs is typically more modest, but Dr. Salsitz pointed out that those expecting cocaine or methamphetamine often “have no heroin tolerance, so they are more vulnerable” to the adverse effects of fentanyl, including an overdose.
Although opioid tolerance might improve the chances for surviving a fentanyl overdose, the toxicology of fentanyl is not the same as other opioids. Death from heroin is typically a result of respiratory depression, but the onset is relatively slow, providing a greater opportunity to administer a reversal agent, such as naloxone.
Fentanyl not only produces respiratory depression but skeletal muscle rigidity. The rapid onset of “wooden chest syndrome” can occur within minutes, making the opportunity for intervention much smaller, Dr. Salsitz said.
To illustrate the phenomenon, Dr. Salsitz recounted a case.
After an argument with his mother, a 26-year-old male with a long history of intravenous drug use went to his bedroom. His mother, responding to the sound of a loud thud, rushed to the bedroom to find her son on the floor with a needle still in his arm. Resuscitation efforts by the mother and by the emergency responders, who arrived quickly, failed.
“The speed of his death made it clear that it was fentanyl related, and the postmortem toxicology confirmed that the exposure involved both heroin and fentanyl,” Dr. Salsitz said.
After the first wave of deaths in the opioid epidemic, which was attributed to inappropriate use of prescription opioids, the second wave was driven by heroin. In that wave, patients who became addicted to prescription opioids but were having more difficulty gaining access to them, turned to far cheaper and readily available street heroin. The third wave, driven by fentanyl, began several years ago when sellers of heroin began adding this synthetic opioid, which is relatively cheap, to intensify the high.
It is not expected to end quickly. The fentanyl added to heroin was never a prescription version. Rather, Dr. Salsitz said, it is synthesized in laboratories in China, Mexico, and the United States. It is relatively easy to produce and compact, which makes it easy to transport.
Exacerbating the risks that fentanyl poses when added to street drugs, even more potent versions, such as carfentanil, are also being added to cocaine, methamphetamines, and other nonopioid illicit drugs. When compared on a per-milligram basis, fentanyl is about 100 times more potent than heroin, but carfentanil is about 100 times more potent than fentanyl, according to Dr. Salsitz.
When the third wave of deaths in the opioid epidemic began around 2013, prescriptions of fentanyl, like many other opioid-type therapies were declining. The “perfect storm” that initiated the opioid epidemic was a product of intense focus on pain control and a misperception that prescription opioids posed a low risk of abuse potential, Dr. Salsitz said. By the time fentanyl was driving opioid deaths, the risks of opioids were widely appreciated and their use for prescription analgesia was declining.
Citing several cases, Dr. Salsitz noted that only 20 years after clinicians were being successfully sued for not offering enough analgesia, they were now going to jail for prescribing these drugs too liberally.
According to Dr. Salsitz, While psychiatrists might not have a role in this issue, Dr. Salsitz did see a role for these specialists in protecting patients from the adverse consequences of using illicit drugs doctored with fentanyl.
Noting that individuals with psychiatric disorders are more likely than the general population to self-medicate with drugs purchased illegally, Dr. Salsitz encouraged psychiatrists “to get involved” in asking about drug use and counseling patients on the risks of fentanyl substitution or additives.
“The message is that no one knows what are in these drugs, anymore,” he said.
In addition to making patients aware that many street drugs are now contaminated with fentanyl, Dr. Salsitz provided some safety tips. He suggested instructing patients to take a low dose of any newly acquired drug to gauge its effect, to avoid taking drugs alone, and to avoid mixing drugs. He also recommended using rapid fentanyl test strips in order to detect fentanyl contamination.
Even for the many psychiatrists who do not feel comfortable managing addiction, Dr. Salsitz recommended a proactive approach to address the current threat.
Test strips as an intervention
The seriousness of fentanyl contamination of illicit drugs, including cocaine and methamphetamine, was corroborated by two investigators at the School of Public Health and the Albert Einstein Medical School of Brown University, Providence, R.I. Brandon D.L. Marshall, PhD, associate professor of epidemiology in the School of Public Health, called fentanyl-contaminated cannabis “extremely rare,” but he said that it is being found in counterfeit prescription pills as well as in crystal methamphetamine and in both crack and powder cocaine.
He also advocated the use of fentanyl test strips.
“Test strips are an efficient, inexpensive, and effective way to determine whether fentanyl or related analogs are present in illicit drugs,” he said, noting that he is involved in a trial designed to determine whether fentanyl test strips can reduce the risk of fatal and nonfatal overdoses.
In a pilot study conducted in Baltimore, 69% of the 103 participants engaged in harm reduction behavior after using a fentanyl test strip and receiving a positive result (Addict Behav. 2020;110:106529). It is notable that 86% of the participants had a least one positive result when using the strips. More than half were surprised by the result.
One of the findings from this study was “that the lasting benefit of fentanyl test strip distribution is the opportunity to engage in discussions around safety and relationship building with historically underserved communities,” said the lead author, Ju Nyeong Park, PhD, assistant professor of medicine and epidemiology at Brown University. She moved to Brown after performing this work at Johns Hopkins University, Baltimore.
Dr. Park noted that “many patients in the community already know that they are using drugs containing fentanyl,” but for those who are concerned and wish to avoid contaminated drugs, fentanyl test strips “are a quick screening tool.” However, while the strips are helpful, she cautioned that they cannot be considered a definitive tool for detecting harm in illicit drugs.
“There may also be other chemicals present in tested drugs that confer risk,” she said.
Medscape Live and this news organization are owned by the same parent company. Dr. Salsitz, Dr. Marshall, and Dr. Park reported no potential conflicts of interest.
Fentanyl is now threatening overdoses in patients exposed to essentially any of the full array of recreational drugs – not just opioids – that are being sold illicitly, according to an overview of the problem presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Fentanyl can now be found in cocaine and methamphetamine. At this point, there is really no way to predict what is in a [street] drug,” Edwin A. Salsitz, MD, said at the meeting, sponsored by Medscape Live. He is associate clinical professor of medicine who works in the division of chemical dependency at Mount Sinai Beth Israel Medical Center in New York.
As proof of the frequency with which fentanyl is now being used as an additive, most patients with a drug use disorder, regardless of their drug of choice, are testing positive for fentanyl at Dr. Salsitz’s center. Many of those with positive fentanyl tests are unaware that their drugs had been doctored with this agent.
Relative to drugs sold as an opioid, such as heroin or oxycodone, the fentanyl dose in nonopioid drugs is typically more modest, but Dr. Salsitz pointed out that those expecting cocaine or methamphetamine often “have no heroin tolerance, so they are more vulnerable” to the adverse effects of fentanyl, including an overdose.
Although opioid tolerance might improve the chances for surviving a fentanyl overdose, the toxicology of fentanyl is not the same as other opioids. Death from heroin is typically a result of respiratory depression, but the onset is relatively slow, providing a greater opportunity to administer a reversal agent, such as naloxone.
Fentanyl not only produces respiratory depression but skeletal muscle rigidity. The rapid onset of “wooden chest syndrome” can occur within minutes, making the opportunity for intervention much smaller, Dr. Salsitz said.
To illustrate the phenomenon, Dr. Salsitz recounted a case.
After an argument with his mother, a 26-year-old male with a long history of intravenous drug use went to his bedroom. His mother, responding to the sound of a loud thud, rushed to the bedroom to find her son on the floor with a needle still in his arm. Resuscitation efforts by the mother and by the emergency responders, who arrived quickly, failed.
“The speed of his death made it clear that it was fentanyl related, and the postmortem toxicology confirmed that the exposure involved both heroin and fentanyl,” Dr. Salsitz said.
After the first wave of deaths in the opioid epidemic, which was attributed to inappropriate use of prescription opioids, the second wave was driven by heroin. In that wave, patients who became addicted to prescription opioids but were having more difficulty gaining access to them, turned to far cheaper and readily available street heroin. The third wave, driven by fentanyl, began several years ago when sellers of heroin began adding this synthetic opioid, which is relatively cheap, to intensify the high.
It is not expected to end quickly. The fentanyl added to heroin was never a prescription version. Rather, Dr. Salsitz said, it is synthesized in laboratories in China, Mexico, and the United States. It is relatively easy to produce and compact, which makes it easy to transport.
Exacerbating the risks that fentanyl poses when added to street drugs, even more potent versions, such as carfentanil, are also being added to cocaine, methamphetamines, and other nonopioid illicit drugs. When compared on a per-milligram basis, fentanyl is about 100 times more potent than heroin, but carfentanil is about 100 times more potent than fentanyl, according to Dr. Salsitz.
When the third wave of deaths in the opioid epidemic began around 2013, prescriptions of fentanyl, like many other opioid-type therapies were declining. The “perfect storm” that initiated the opioid epidemic was a product of intense focus on pain control and a misperception that prescription opioids posed a low risk of abuse potential, Dr. Salsitz said. By the time fentanyl was driving opioid deaths, the risks of opioids were widely appreciated and their use for prescription analgesia was declining.
Citing several cases, Dr. Salsitz noted that only 20 years after clinicians were being successfully sued for not offering enough analgesia, they were now going to jail for prescribing these drugs too liberally.
According to Dr. Salsitz, While psychiatrists might not have a role in this issue, Dr. Salsitz did see a role for these specialists in protecting patients from the adverse consequences of using illicit drugs doctored with fentanyl.
Noting that individuals with psychiatric disorders are more likely than the general population to self-medicate with drugs purchased illegally, Dr. Salsitz encouraged psychiatrists “to get involved” in asking about drug use and counseling patients on the risks of fentanyl substitution or additives.
“The message is that no one knows what are in these drugs, anymore,” he said.
In addition to making patients aware that many street drugs are now contaminated with fentanyl, Dr. Salsitz provided some safety tips. He suggested instructing patients to take a low dose of any newly acquired drug to gauge its effect, to avoid taking drugs alone, and to avoid mixing drugs. He also recommended using rapid fentanyl test strips in order to detect fentanyl contamination.
Even for the many psychiatrists who do not feel comfortable managing addiction, Dr. Salsitz recommended a proactive approach to address the current threat.
Test strips as an intervention
The seriousness of fentanyl contamination of illicit drugs, including cocaine and methamphetamine, was corroborated by two investigators at the School of Public Health and the Albert Einstein Medical School of Brown University, Providence, R.I. Brandon D.L. Marshall, PhD, associate professor of epidemiology in the School of Public Health, called fentanyl-contaminated cannabis “extremely rare,” but he said that it is being found in counterfeit prescription pills as well as in crystal methamphetamine and in both crack and powder cocaine.
He also advocated the use of fentanyl test strips.
“Test strips are an efficient, inexpensive, and effective way to determine whether fentanyl or related analogs are present in illicit drugs,” he said, noting that he is involved in a trial designed to determine whether fentanyl test strips can reduce the risk of fatal and nonfatal overdoses.
In a pilot study conducted in Baltimore, 69% of the 103 participants engaged in harm reduction behavior after using a fentanyl test strip and receiving a positive result (Addict Behav. 2020;110:106529). It is notable that 86% of the participants had a least one positive result when using the strips. More than half were surprised by the result.
One of the findings from this study was “that the lasting benefit of fentanyl test strip distribution is the opportunity to engage in discussions around safety and relationship building with historically underserved communities,” said the lead author, Ju Nyeong Park, PhD, assistant professor of medicine and epidemiology at Brown University. She moved to Brown after performing this work at Johns Hopkins University, Baltimore.
Dr. Park noted that “many patients in the community already know that they are using drugs containing fentanyl,” but for those who are concerned and wish to avoid contaminated drugs, fentanyl test strips “are a quick screening tool.” However, while the strips are helpful, she cautioned that they cannot be considered a definitive tool for detecting harm in illicit drugs.
“There may also be other chemicals present in tested drugs that confer risk,” she said.
Medscape Live and this news organization are owned by the same parent company. Dr. Salsitz, Dr. Marshall, and Dr. Park reported no potential conflicts of interest.
FROM PSYCHOPHARMACOLOGY UPDATE