User login
Study finds sharp drop in opioid scripts among most specialties
The volume of prescription opioids dispensed at retail pharmacies in the United States dropped by 21% in recent years amid efforts to reduce unnecessary use of the painkillers, but the rate of decline varied greatly among types of patients and by type of clinician, a study found.
In a brief report published by Annals of Internal Medicine, researchers from the nonprofit RAND Corp reported an analysis of opioid prescriptions from two periods, 2008-2009 and 2017-2018.
The researchers sought to assess total opioid use rather than simply track the number of pills dispensed. So they used days’ supply and total daily dose to calculate per capita morphine milligram equivalents (MME) for opioid prescriptions, write Bradley D. Stein, MD, PhD, MPH, the study’s lead author and a senior physician researcher at RAND Corp, and his coauthors in their paper.
For the study, the researchers used data from the consulting firm IQVIA, which they say covers about 90% of U.S. prescriptions. Total opioid volume per capita by prescriptions filled in retail pharmacies decreased from 951.4 MME in 2008-2009 to 749.3 MME in 2017-2018, Dr. Stein’s group found.
(In 2020, IQVIA separately said that prescription opioid use per adult in this country rose from an average of 16 pills, or 134 MMEs, in 1992 to a peak of about 55 pills a person, or 790 MMEs, in 2011. By 2019, opioid use per adult had declined to 29 pills and 366 MMEs per capita.)
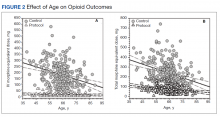
The RAND report found substantial variation in opioid volume by type of insurance, including a 41.5% decline (636.5 MME to 372.6 MME) among people covered by commercial health plans. That exceeded the 27.7% drop seen for people enrolled in Medicaid (646.8 MME to 467.7 MME). The decline was smaller (17.5%; 2,780.2 MME to 2,294.2 MME) for those on Medicare, who as a group used the most opioids.
‘Almost functions as a Rorschach test’
The causes of the decline are easy to guess, although definitive conclusions are impossible, Dr. Stein told this news organization.
Significant work has been done in recent years to change attitudes about opioid prescriptions by physicians, researchers, and lawmakers. Aggressive promotion of prescription painkillers, particularly Purdue Pharma’s OxyContin, in the 1990s, is widely cited as the triggering event for the national opioid crisis.
In response, states created databases known as prescription drug monitoring programs. The Centers for Disease Control and Prevention in 2016 issued guidelines intended to curb unnecessary use of opioids. The guidelines noted that other medicines could treat chronic pain without raising the risk of addiction. The Choosing Wisely campaign, run by a foundation of the American Board of Internal Medicine, also offered recommendations about limiting use of opioids. And insurers have restricted access to opioids through the prior authorization process. As a result, researchers will make their own guesses at the causes of the decline in opioid prescriptions, based on their own experiences and research interests, Dr. Stein said.
“It almost functions as a Rorschach test,” he said.
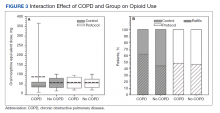
Dr. Stein’s group also looked at trends among medical specialties. They found the largest reduction between 2008-2009 and 2017-2018 among emergency physicians (70.5% drop from 99,254.5 MME to 29,234.3 MME), psychiatrists (67.2% drop from 50,464.3 MME to 16,533.0 MME) and oncologists (59.5% drop from 51,731.2 MME to 20,941.4).
Among surgeons, the RAND researchers found a drop of 49.3% from 220,764.6 to 111,904.4. Among dentists, they found a drop of 41.3% from 22,345.3 to 13,126.1.
Among pain specialists, they found a drop of 15.4% from 1,020,808.4 MME to 863,140.7 MME.
Among adult primary care clinicians, Dr. Stein and his colleagues found a drop of 40% from 651,489.4 MME in 2008-2009 to 390,841.0 MME in 2017-2018.
However, one of the groups tracked in the study increased the volume of opioid prescriptions written: advanced practice providers, among whom scripts for the drugs rose 22.7%, from 112,873.9 MME to 138,459.3 MME.
Dr. Stein said he suspects that this gain reflects a change in the nature of the practice of primary care, with nurse practitioners and physician assistants taking more active roles in treatment of patients. Some of the reduction seen among primary care clinicians who treat adults may reflect a shift in which medical personnel in a practice write the opioid prescriptions.
Still, the trends in general seen by Dr. Stein and coauthors are encouraging, even if further study of these patterns is needed, he said.
“This is one of those papers that I think potentially raises as many questions as it provides answers for,” he said.
What’s missing
Maya Hambright, MD, a family medicine physician in New York’s Hudson Valley, who has been working mainly in addiction in response to the opioid overdose crisis, observed that the drop in total prescribed volume of prescription painkillers does not necessarily translate into a reduction in use of opioids
“No one is taking fewer opioids,” Dr. Hambright told this news organization. “I can say that comfortably. They are just getting them from other sources.”
CDC data support Dr. Hambright’s view.
An estimated 100,306 people in the United States died of a drug overdose in the 12 months that ended in April 2021, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the CDC.
Dr. Hambright said more physicians need to be involved in prescribing medication-assisted treatment (MAT).
The federal government has in the past year loosened restrictions on a requirement, known as an X waiver. Certain clinicians have been exempted from training requirements, as explained in the frequently asked questions page on the Substance Abuse and Mental Health Services Administration website.
SAMHSA says legislation is required to eliminate the waiver. As of Dec. 30, 2021, more than half of the members of the U.S. House of Representatives were listed as sponsors of the Mainstreaming Addiction Treatment (MAT) Act (HR 1384), which would end the need for X waivers. The bill has the backing of 187 Democrats and 43 Republicans.
At this time, too many physicians shy away from offering MAT, Dr. Hambright said.
“People are still scared of it,” she said. “People don’t want to deal with addicts.”
But Dr. Hambright said it’s well worth the initial time invested in having the needed conversations with patients about MAT.
“Afterwards, it’s so straightforward. People feel better. They’re healthier. It’s amazing,” she said. “You’re changing lives.”
The research was supported by grants from the National Institutes of Health. Dr. Stein and coauthors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The volume of prescription opioids dispensed at retail pharmacies in the United States dropped by 21% in recent years amid efforts to reduce unnecessary use of the painkillers, but the rate of decline varied greatly among types of patients and by type of clinician, a study found.
In a brief report published by Annals of Internal Medicine, researchers from the nonprofit RAND Corp reported an analysis of opioid prescriptions from two periods, 2008-2009 and 2017-2018.
The researchers sought to assess total opioid use rather than simply track the number of pills dispensed. So they used days’ supply and total daily dose to calculate per capita morphine milligram equivalents (MME) for opioid prescriptions, write Bradley D. Stein, MD, PhD, MPH, the study’s lead author and a senior physician researcher at RAND Corp, and his coauthors in their paper.
For the study, the researchers used data from the consulting firm IQVIA, which they say covers about 90% of U.S. prescriptions. Total opioid volume per capita by prescriptions filled in retail pharmacies decreased from 951.4 MME in 2008-2009 to 749.3 MME in 2017-2018, Dr. Stein’s group found.
(In 2020, IQVIA separately said that prescription opioid use per adult in this country rose from an average of 16 pills, or 134 MMEs, in 1992 to a peak of about 55 pills a person, or 790 MMEs, in 2011. By 2019, opioid use per adult had declined to 29 pills and 366 MMEs per capita.)
The RAND report found substantial variation in opioid volume by type of insurance, including a 41.5% decline (636.5 MME to 372.6 MME) among people covered by commercial health plans. That exceeded the 27.7% drop seen for people enrolled in Medicaid (646.8 MME to 467.7 MME). The decline was smaller (17.5%; 2,780.2 MME to 2,294.2 MME) for those on Medicare, who as a group used the most opioids.
‘Almost functions as a Rorschach test’
The causes of the decline are easy to guess, although definitive conclusions are impossible, Dr. Stein told this news organization.
Significant work has been done in recent years to change attitudes about opioid prescriptions by physicians, researchers, and lawmakers. Aggressive promotion of prescription painkillers, particularly Purdue Pharma’s OxyContin, in the 1990s, is widely cited as the triggering event for the national opioid crisis.
In response, states created databases known as prescription drug monitoring programs. The Centers for Disease Control and Prevention in 2016 issued guidelines intended to curb unnecessary use of opioids. The guidelines noted that other medicines could treat chronic pain without raising the risk of addiction. The Choosing Wisely campaign, run by a foundation of the American Board of Internal Medicine, also offered recommendations about limiting use of opioids. And insurers have restricted access to opioids through the prior authorization process. As a result, researchers will make their own guesses at the causes of the decline in opioid prescriptions, based on their own experiences and research interests, Dr. Stein said.
“It almost functions as a Rorschach test,” he said.
Dr. Stein’s group also looked at trends among medical specialties. They found the largest reduction between 2008-2009 and 2017-2018 among emergency physicians (70.5% drop from 99,254.5 MME to 29,234.3 MME), psychiatrists (67.2% drop from 50,464.3 MME to 16,533.0 MME) and oncologists (59.5% drop from 51,731.2 MME to 20,941.4).
Among surgeons, the RAND researchers found a drop of 49.3% from 220,764.6 to 111,904.4. Among dentists, they found a drop of 41.3% from 22,345.3 to 13,126.1.
Among pain specialists, they found a drop of 15.4% from 1,020,808.4 MME to 863,140.7 MME.
Among adult primary care clinicians, Dr. Stein and his colleagues found a drop of 40% from 651,489.4 MME in 2008-2009 to 390,841.0 MME in 2017-2018.
However, one of the groups tracked in the study increased the volume of opioid prescriptions written: advanced practice providers, among whom scripts for the drugs rose 22.7%, from 112,873.9 MME to 138,459.3 MME.
Dr. Stein said he suspects that this gain reflects a change in the nature of the practice of primary care, with nurse practitioners and physician assistants taking more active roles in treatment of patients. Some of the reduction seen among primary care clinicians who treat adults may reflect a shift in which medical personnel in a practice write the opioid prescriptions.
Still, the trends in general seen by Dr. Stein and coauthors are encouraging, even if further study of these patterns is needed, he said.
“This is one of those papers that I think potentially raises as many questions as it provides answers for,” he said.
What’s missing
Maya Hambright, MD, a family medicine physician in New York’s Hudson Valley, who has been working mainly in addiction in response to the opioid overdose crisis, observed that the drop in total prescribed volume of prescription painkillers does not necessarily translate into a reduction in use of opioids
“No one is taking fewer opioids,” Dr. Hambright told this news organization. “I can say that comfortably. They are just getting them from other sources.”
CDC data support Dr. Hambright’s view.
An estimated 100,306 people in the United States died of a drug overdose in the 12 months that ended in April 2021, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the CDC.
Dr. Hambright said more physicians need to be involved in prescribing medication-assisted treatment (MAT).
The federal government has in the past year loosened restrictions on a requirement, known as an X waiver. Certain clinicians have been exempted from training requirements, as explained in the frequently asked questions page on the Substance Abuse and Mental Health Services Administration website.
SAMHSA says legislation is required to eliminate the waiver. As of Dec. 30, 2021, more than half of the members of the U.S. House of Representatives were listed as sponsors of the Mainstreaming Addiction Treatment (MAT) Act (HR 1384), which would end the need for X waivers. The bill has the backing of 187 Democrats and 43 Republicans.
At this time, too many physicians shy away from offering MAT, Dr. Hambright said.
“People are still scared of it,” she said. “People don’t want to deal with addicts.”
But Dr. Hambright said it’s well worth the initial time invested in having the needed conversations with patients about MAT.
“Afterwards, it’s so straightforward. People feel better. They’re healthier. It’s amazing,” she said. “You’re changing lives.”
The research was supported by grants from the National Institutes of Health. Dr. Stein and coauthors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The volume of prescription opioids dispensed at retail pharmacies in the United States dropped by 21% in recent years amid efforts to reduce unnecessary use of the painkillers, but the rate of decline varied greatly among types of patients and by type of clinician, a study found.
In a brief report published by Annals of Internal Medicine, researchers from the nonprofit RAND Corp reported an analysis of opioid prescriptions from two periods, 2008-2009 and 2017-2018.
The researchers sought to assess total opioid use rather than simply track the number of pills dispensed. So they used days’ supply and total daily dose to calculate per capita morphine milligram equivalents (MME) for opioid prescriptions, write Bradley D. Stein, MD, PhD, MPH, the study’s lead author and a senior physician researcher at RAND Corp, and his coauthors in their paper.
For the study, the researchers used data from the consulting firm IQVIA, which they say covers about 90% of U.S. prescriptions. Total opioid volume per capita by prescriptions filled in retail pharmacies decreased from 951.4 MME in 2008-2009 to 749.3 MME in 2017-2018, Dr. Stein’s group found.
(In 2020, IQVIA separately said that prescription opioid use per adult in this country rose from an average of 16 pills, or 134 MMEs, in 1992 to a peak of about 55 pills a person, or 790 MMEs, in 2011. By 2019, opioid use per adult had declined to 29 pills and 366 MMEs per capita.)
The RAND report found substantial variation in opioid volume by type of insurance, including a 41.5% decline (636.5 MME to 372.6 MME) among people covered by commercial health plans. That exceeded the 27.7% drop seen for people enrolled in Medicaid (646.8 MME to 467.7 MME). The decline was smaller (17.5%; 2,780.2 MME to 2,294.2 MME) for those on Medicare, who as a group used the most opioids.
‘Almost functions as a Rorschach test’
The causes of the decline are easy to guess, although definitive conclusions are impossible, Dr. Stein told this news organization.
Significant work has been done in recent years to change attitudes about opioid prescriptions by physicians, researchers, and lawmakers. Aggressive promotion of prescription painkillers, particularly Purdue Pharma’s OxyContin, in the 1990s, is widely cited as the triggering event for the national opioid crisis.
In response, states created databases known as prescription drug monitoring programs. The Centers for Disease Control and Prevention in 2016 issued guidelines intended to curb unnecessary use of opioids. The guidelines noted that other medicines could treat chronic pain without raising the risk of addiction. The Choosing Wisely campaign, run by a foundation of the American Board of Internal Medicine, also offered recommendations about limiting use of opioids. And insurers have restricted access to opioids through the prior authorization process. As a result, researchers will make their own guesses at the causes of the decline in opioid prescriptions, based on their own experiences and research interests, Dr. Stein said.
“It almost functions as a Rorschach test,” he said.
Dr. Stein’s group also looked at trends among medical specialties. They found the largest reduction between 2008-2009 and 2017-2018 among emergency physicians (70.5% drop from 99,254.5 MME to 29,234.3 MME), psychiatrists (67.2% drop from 50,464.3 MME to 16,533.0 MME) and oncologists (59.5% drop from 51,731.2 MME to 20,941.4).
Among surgeons, the RAND researchers found a drop of 49.3% from 220,764.6 to 111,904.4. Among dentists, they found a drop of 41.3% from 22,345.3 to 13,126.1.
Among pain specialists, they found a drop of 15.4% from 1,020,808.4 MME to 863,140.7 MME.
Among adult primary care clinicians, Dr. Stein and his colleagues found a drop of 40% from 651,489.4 MME in 2008-2009 to 390,841.0 MME in 2017-2018.
However, one of the groups tracked in the study increased the volume of opioid prescriptions written: advanced practice providers, among whom scripts for the drugs rose 22.7%, from 112,873.9 MME to 138,459.3 MME.
Dr. Stein said he suspects that this gain reflects a change in the nature of the practice of primary care, with nurse practitioners and physician assistants taking more active roles in treatment of patients. Some of the reduction seen among primary care clinicians who treat adults may reflect a shift in which medical personnel in a practice write the opioid prescriptions.
Still, the trends in general seen by Dr. Stein and coauthors are encouraging, even if further study of these patterns is needed, he said.
“This is one of those papers that I think potentially raises as many questions as it provides answers for,” he said.
What’s missing
Maya Hambright, MD, a family medicine physician in New York’s Hudson Valley, who has been working mainly in addiction in response to the opioid overdose crisis, observed that the drop in total prescribed volume of prescription painkillers does not necessarily translate into a reduction in use of opioids
“No one is taking fewer opioids,” Dr. Hambright told this news organization. “I can say that comfortably. They are just getting them from other sources.”
CDC data support Dr. Hambright’s view.
An estimated 100,306 people in the United States died of a drug overdose in the 12 months that ended in April 2021, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the CDC.
Dr. Hambright said more physicians need to be involved in prescribing medication-assisted treatment (MAT).
The federal government has in the past year loosened restrictions on a requirement, known as an X waiver. Certain clinicians have been exempted from training requirements, as explained in the frequently asked questions page on the Substance Abuse and Mental Health Services Administration website.
SAMHSA says legislation is required to eliminate the waiver. As of Dec. 30, 2021, more than half of the members of the U.S. House of Representatives were listed as sponsors of the Mainstreaming Addiction Treatment (MAT) Act (HR 1384), which would end the need for X waivers. The bill has the backing of 187 Democrats and 43 Republicans.
At this time, too many physicians shy away from offering MAT, Dr. Hambright said.
“People are still scared of it,” she said. “People don’t want to deal with addicts.”
But Dr. Hambright said it’s well worth the initial time invested in having the needed conversations with patients about MAT.
“Afterwards, it’s so straightforward. People feel better. They’re healthier. It’s amazing,” she said. “You’re changing lives.”
The research was supported by grants from the National Institutes of Health. Dr. Stein and coauthors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AAN updates treatment guidance on painful diabetic neuropathy
Painful diabetic neuropathy is very common and can greatly affect an individual’s quality of life, guideline author Brian Callaghan, MD, University of Michigan, Ann Arbor, noted in a news release.
“This guideline aims to help neurologists and other doctors provide the highest quality patient care based on the latest evidence,” Dr. Callaghan said.
The recommendations update the 2011 AAN guideline on the treatment of painful diabetic neuropathy. The new guidance was published online Dec. 27, 2021, in Neurology and has been endorsed by the American Association of Neuromuscular & Electrodiagnostic Medicine.
Multiple options
To update the guideline, an expert panel reviewed data from more than 100 randomized controlled trials published from January 2008 to April 2020.
The panel noted that more than 16% of individuals with diabetes experience painful diabetic neuropathy, but it often goes unrecognized and untreated. The guideline recommends clinicians assess patients with diabetes for peripheral neuropathic pain and its effect on their function and quality of life.
Before prescribing treatment, health providers should determine if the patient also has mood or sleep problems as both can influence pain perception.
The guideline recommends offering one of four classes of oral medications found to be effective for neuropathic pain: tricyclic antidepressants such as amitriptyline, nortriptyline, or imipramine; serotonin norepinephrine reuptake inhibitors such as duloxetine, venlafaxine, or desvenlafaxine; gabapentinoids such as gabapentin or pregabalin; and/or sodium channel blockers such as carbamazepine, oxcarbazepine, lamotrigine, or lacosamide.
All four classes of medications have “comparable effect sizes just above or just below our cutoff for a medium effect size” (standardized median difference, 0.5), the panel noted.
In addition, “new studies on sodium channel blockers published since the last guideline have resulted in these drugs now being recommended and considered as effective at providing pain relief as the other drug classes recommended in this guideline,” said Dr. Callaghan.
When an initial medication fails to provide meaningful improvement in pain, or produces significant side effects, a trial of another medication from a different class is recommended.
Pain reduction, not elimination
Opioids are not recommended for painful diabetic neuropathy. Not only do they come with risks, there is also no strong evidence they are effective for painful diabetic neuropathy in the long term, the panel wrote. Tramadol and tapentadol are also not recommended for the treatment of painful diabetic neuropathy.
“Current evidence suggests that the risks of the use of opioids for painful diabetic neuropathy therapy outweigh the benefits, so they should not be prescribed,” Dr. Callaghan said.
For patients interested in trying topical, nontraditional, or nondrug interventions to reduce pain, the guideline recommends a number of options including capsaicin, glyceryl trinitrate spray, and Citrullus colocynthis. Ginkgo biloba, exercise, mindfulness, cognitive-behavioral therapy, and tai chi are also suggested.
“It is important to note that the recommended drugs and topical treatments in this guideline may not eliminate pain, but they have been shown to reduce pain,” Dr. Callaghan said. “The good news is there are many treatment options for painful diabetic neuropathy, so a treatment plan can be tailored specifically to each person living with this condition.”
Along with the updated guideline, the AAN has also published a new Polyneuropathy Quality Measurement Set to assist neurologists and other health care providers in treating patients with painful diabetic neuropathy.
The updated guideline was developed with financial support from the AAN.
A version of this article first appeared on Medscape.com.
Painful diabetic neuropathy is very common and can greatly affect an individual’s quality of life, guideline author Brian Callaghan, MD, University of Michigan, Ann Arbor, noted in a news release.
“This guideline aims to help neurologists and other doctors provide the highest quality patient care based on the latest evidence,” Dr. Callaghan said.
The recommendations update the 2011 AAN guideline on the treatment of painful diabetic neuropathy. The new guidance was published online Dec. 27, 2021, in Neurology and has been endorsed by the American Association of Neuromuscular & Electrodiagnostic Medicine.
Multiple options
To update the guideline, an expert panel reviewed data from more than 100 randomized controlled trials published from January 2008 to April 2020.
The panel noted that more than 16% of individuals with diabetes experience painful diabetic neuropathy, but it often goes unrecognized and untreated. The guideline recommends clinicians assess patients with diabetes for peripheral neuropathic pain and its effect on their function and quality of life.
Before prescribing treatment, health providers should determine if the patient also has mood or sleep problems as both can influence pain perception.
The guideline recommends offering one of four classes of oral medications found to be effective for neuropathic pain: tricyclic antidepressants such as amitriptyline, nortriptyline, or imipramine; serotonin norepinephrine reuptake inhibitors such as duloxetine, venlafaxine, or desvenlafaxine; gabapentinoids such as gabapentin or pregabalin; and/or sodium channel blockers such as carbamazepine, oxcarbazepine, lamotrigine, or lacosamide.
All four classes of medications have “comparable effect sizes just above or just below our cutoff for a medium effect size” (standardized median difference, 0.5), the panel noted.
In addition, “new studies on sodium channel blockers published since the last guideline have resulted in these drugs now being recommended and considered as effective at providing pain relief as the other drug classes recommended in this guideline,” said Dr. Callaghan.
When an initial medication fails to provide meaningful improvement in pain, or produces significant side effects, a trial of another medication from a different class is recommended.
Pain reduction, not elimination
Opioids are not recommended for painful diabetic neuropathy. Not only do they come with risks, there is also no strong evidence they are effective for painful diabetic neuropathy in the long term, the panel wrote. Tramadol and tapentadol are also not recommended for the treatment of painful diabetic neuropathy.
“Current evidence suggests that the risks of the use of opioids for painful diabetic neuropathy therapy outweigh the benefits, so they should not be prescribed,” Dr. Callaghan said.
For patients interested in trying topical, nontraditional, or nondrug interventions to reduce pain, the guideline recommends a number of options including capsaicin, glyceryl trinitrate spray, and Citrullus colocynthis. Ginkgo biloba, exercise, mindfulness, cognitive-behavioral therapy, and tai chi are also suggested.
“It is important to note that the recommended drugs and topical treatments in this guideline may not eliminate pain, but they have been shown to reduce pain,” Dr. Callaghan said. “The good news is there are many treatment options for painful diabetic neuropathy, so a treatment plan can be tailored specifically to each person living with this condition.”
Along with the updated guideline, the AAN has also published a new Polyneuropathy Quality Measurement Set to assist neurologists and other health care providers in treating patients with painful diabetic neuropathy.
The updated guideline was developed with financial support from the AAN.
A version of this article first appeared on Medscape.com.
Painful diabetic neuropathy is very common and can greatly affect an individual’s quality of life, guideline author Brian Callaghan, MD, University of Michigan, Ann Arbor, noted in a news release.
“This guideline aims to help neurologists and other doctors provide the highest quality patient care based on the latest evidence,” Dr. Callaghan said.
The recommendations update the 2011 AAN guideline on the treatment of painful diabetic neuropathy. The new guidance was published online Dec. 27, 2021, in Neurology and has been endorsed by the American Association of Neuromuscular & Electrodiagnostic Medicine.
Multiple options
To update the guideline, an expert panel reviewed data from more than 100 randomized controlled trials published from January 2008 to April 2020.
The panel noted that more than 16% of individuals with diabetes experience painful diabetic neuropathy, but it often goes unrecognized and untreated. The guideline recommends clinicians assess patients with diabetes for peripheral neuropathic pain and its effect on their function and quality of life.
Before prescribing treatment, health providers should determine if the patient also has mood or sleep problems as both can influence pain perception.
The guideline recommends offering one of four classes of oral medications found to be effective for neuropathic pain: tricyclic antidepressants such as amitriptyline, nortriptyline, or imipramine; serotonin norepinephrine reuptake inhibitors such as duloxetine, venlafaxine, or desvenlafaxine; gabapentinoids such as gabapentin or pregabalin; and/or sodium channel blockers such as carbamazepine, oxcarbazepine, lamotrigine, or lacosamide.
All four classes of medications have “comparable effect sizes just above or just below our cutoff for a medium effect size” (standardized median difference, 0.5), the panel noted.
In addition, “new studies on sodium channel blockers published since the last guideline have resulted in these drugs now being recommended and considered as effective at providing pain relief as the other drug classes recommended in this guideline,” said Dr. Callaghan.
When an initial medication fails to provide meaningful improvement in pain, or produces significant side effects, a trial of another medication from a different class is recommended.
Pain reduction, not elimination
Opioids are not recommended for painful diabetic neuropathy. Not only do they come with risks, there is also no strong evidence they are effective for painful diabetic neuropathy in the long term, the panel wrote. Tramadol and tapentadol are also not recommended for the treatment of painful diabetic neuropathy.
“Current evidence suggests that the risks of the use of opioids for painful diabetic neuropathy therapy outweigh the benefits, so they should not be prescribed,” Dr. Callaghan said.
For patients interested in trying topical, nontraditional, or nondrug interventions to reduce pain, the guideline recommends a number of options including capsaicin, glyceryl trinitrate spray, and Citrullus colocynthis. Ginkgo biloba, exercise, mindfulness, cognitive-behavioral therapy, and tai chi are also suggested.
“It is important to note that the recommended drugs and topical treatments in this guideline may not eliminate pain, but they have been shown to reduce pain,” Dr. Callaghan said. “The good news is there are many treatment options for painful diabetic neuropathy, so a treatment plan can be tailored specifically to each person living with this condition.”
Along with the updated guideline, the AAN has also published a new Polyneuropathy Quality Measurement Set to assist neurologists and other health care providers in treating patients with painful diabetic neuropathy.
The updated guideline was developed with financial support from the AAN.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
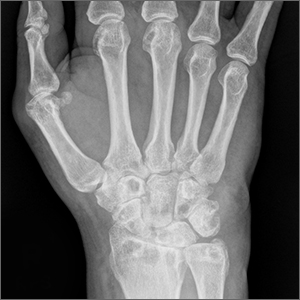
Wrist pain and swelling
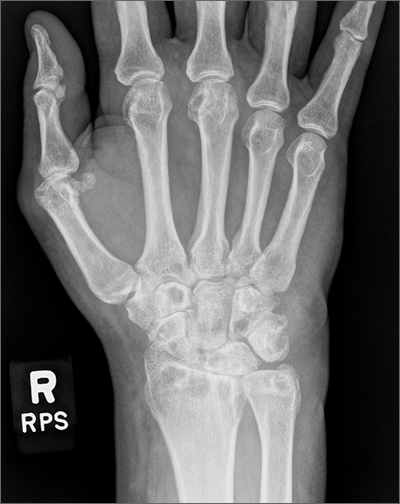
Bilateral wrist pain with associated swelling consistent with synovitis pointed to an inflammatory arthritis confirmed by x-ray imaging. An elevated erythrocyte sedimentation rate (109 mm/hr), rheumatoid factor (314 IU/mL), and cyclic citrullinated peptide (34.5 EU/mL) confirmed the diagnosis of rheumatoid arthritis (RA). Hepatitis and tuberculosis screens were negative and uric acid was normal.
The patient’s radiographic imaging of the wrists revealed mild-to-moderate narrowing of the radiocarpal and midcarpal joints, multiple scattered cyst-like and erosive changes throughout, and mild-to-moderate soft tissue edema. These findings were consistent with a diagnosis of RA. Additionally, the radiographs showed cortical irregularity of the proximal ulnar aspect of the lunate, consistent with ulnar abutment syndrome, a degenerative condition in which the ulnar head abuts the triangular fibrocartilage complex and ulnar-sided carpal bones.
Some of the first changes that can be observed radiographically in RA include soft tissue swelling and periarticular osteopenia.1 As the disease progresses, bony erosions, especially in the metacarpophalangeal and proximal interphalangeal joints, can be observed. Additional findings with active disease include joint space narrowing and deformities, such as joint subluxation. Erosions of cartilage and bone can also occur in some other forms of inflammatory and gouty arthropathies, so it is important to consider differential diagnoses in the case of ambiguous laboratory findings.
The primary disease-modifying antirheumatic drug (DMARD) used for treatment of RA is methotrexate.2 DMARDs take weeks to months before there is noticeable improvement and should be used in combination with anti-inflammatory agents such as nonsteroidal anti-inflammatory drugs or glucocorticoids. Response rates to DMARDs decrease over time. In the case of drug resistance, combination therapy (eg, methotrexate plus sulfasalazine and hydroxychloroquine, or methotrexate plus a tumor necrosis factor inhibitor) can be used. For acute flares, patients can undergo intra-articular glucocorticoid injections if a limited number of joints are affected. Widespread flares can be treated with oral glucocorticoids. Severe flares can be treated with pulse intravenous methylprednisolone.
Our patient was referred to Rheumatology for prompt treatment. He was started on DMARD therapy (methotrexate 12.5 mg weekly) with daily folic acid and a plan to increase the methotrexate to 25 mg after the third week of therapy. He was also prescribed oral prednisone to have on hand for flares, and azithromycin to treat possible future infections. Additionally, the patient underwent bilateral steroid wrist injections at the clinic.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Rachel Ruckman, BS, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26-34. doi: 10.1002/art.1780350105
2. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903-911. doi: 10.1016/S0140-6736(01)06075-5

Bilateral wrist pain with associated swelling consistent with synovitis pointed to an inflammatory arthritis confirmed by x-ray imaging. An elevated erythrocyte sedimentation rate (109 mm/hr), rheumatoid factor (314 IU/mL), and cyclic citrullinated peptide (34.5 EU/mL) confirmed the diagnosis of rheumatoid arthritis (RA). Hepatitis and tuberculosis screens were negative and uric acid was normal.
The patient’s radiographic imaging of the wrists revealed mild-to-moderate narrowing of the radiocarpal and midcarpal joints, multiple scattered cyst-like and erosive changes throughout, and mild-to-moderate soft tissue edema. These findings were consistent with a diagnosis of RA. Additionally, the radiographs showed cortical irregularity of the proximal ulnar aspect of the lunate, consistent with ulnar abutment syndrome, a degenerative condition in which the ulnar head abuts the triangular fibrocartilage complex and ulnar-sided carpal bones.
Some of the first changes that can be observed radiographically in RA include soft tissue swelling and periarticular osteopenia.1 As the disease progresses, bony erosions, especially in the metacarpophalangeal and proximal interphalangeal joints, can be observed. Additional findings with active disease include joint space narrowing and deformities, such as joint subluxation. Erosions of cartilage and bone can also occur in some other forms of inflammatory and gouty arthropathies, so it is important to consider differential diagnoses in the case of ambiguous laboratory findings.
The primary disease-modifying antirheumatic drug (DMARD) used for treatment of RA is methotrexate.2 DMARDs take weeks to months before there is noticeable improvement and should be used in combination with anti-inflammatory agents such as nonsteroidal anti-inflammatory drugs or glucocorticoids. Response rates to DMARDs decrease over time. In the case of drug resistance, combination therapy (eg, methotrexate plus sulfasalazine and hydroxychloroquine, or methotrexate plus a tumor necrosis factor inhibitor) can be used. For acute flares, patients can undergo intra-articular glucocorticoid injections if a limited number of joints are affected. Widespread flares can be treated with oral glucocorticoids. Severe flares can be treated with pulse intravenous methylprednisolone.
Our patient was referred to Rheumatology for prompt treatment. He was started on DMARD therapy (methotrexate 12.5 mg weekly) with daily folic acid and a plan to increase the methotrexate to 25 mg after the third week of therapy. He was also prescribed oral prednisone to have on hand for flares, and azithromycin to treat possible future infections. Additionally, the patient underwent bilateral steroid wrist injections at the clinic.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Rachel Ruckman, BS, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Bilateral wrist pain with associated swelling consistent with synovitis pointed to an inflammatory arthritis confirmed by x-ray imaging. An elevated erythrocyte sedimentation rate (109 mm/hr), rheumatoid factor (314 IU/mL), and cyclic citrullinated peptide (34.5 EU/mL) confirmed the diagnosis of rheumatoid arthritis (RA). Hepatitis and tuberculosis screens were negative and uric acid was normal.
The patient’s radiographic imaging of the wrists revealed mild-to-moderate narrowing of the radiocarpal and midcarpal joints, multiple scattered cyst-like and erosive changes throughout, and mild-to-moderate soft tissue edema. These findings were consistent with a diagnosis of RA. Additionally, the radiographs showed cortical irregularity of the proximal ulnar aspect of the lunate, consistent with ulnar abutment syndrome, a degenerative condition in which the ulnar head abuts the triangular fibrocartilage complex and ulnar-sided carpal bones.
Some of the first changes that can be observed radiographically in RA include soft tissue swelling and periarticular osteopenia.1 As the disease progresses, bony erosions, especially in the metacarpophalangeal and proximal interphalangeal joints, can be observed. Additional findings with active disease include joint space narrowing and deformities, such as joint subluxation. Erosions of cartilage and bone can also occur in some other forms of inflammatory and gouty arthropathies, so it is important to consider differential diagnoses in the case of ambiguous laboratory findings.
The primary disease-modifying antirheumatic drug (DMARD) used for treatment of RA is methotrexate.2 DMARDs take weeks to months before there is noticeable improvement and should be used in combination with anti-inflammatory agents such as nonsteroidal anti-inflammatory drugs or glucocorticoids. Response rates to DMARDs decrease over time. In the case of drug resistance, combination therapy (eg, methotrexate plus sulfasalazine and hydroxychloroquine, or methotrexate plus a tumor necrosis factor inhibitor) can be used. For acute flares, patients can undergo intra-articular glucocorticoid injections if a limited number of joints are affected. Widespread flares can be treated with oral glucocorticoids. Severe flares can be treated with pulse intravenous methylprednisolone.
Our patient was referred to Rheumatology for prompt treatment. He was started on DMARD therapy (methotrexate 12.5 mg weekly) with daily folic acid and a plan to increase the methotrexate to 25 mg after the third week of therapy. He was also prescribed oral prednisone to have on hand for flares, and azithromycin to treat possible future infections. Additionally, the patient underwent bilateral steroid wrist injections at the clinic.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Rachel Ruckman, BS, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26-34. doi: 10.1002/art.1780350105
2. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903-911. doi: 10.1016/S0140-6736(01)06075-5
1. van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26-34. doi: 10.1002/art.1780350105
2. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903-911. doi: 10.1016/S0140-6736(01)06075-5
EMA panel backs linzagolix for uterine fibroid symptoms
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) on December 17 recommended approval of linzagolix (Yselty, ObsEva), an oral gonadotropin-releasing hormone (GnRH) antagonist, for the management of moderate to severe symptoms of uterine fibroids (UF) in adult women of reproductive age.
If approved, linzagolix – which is taken once per day – would become the first GnRH receptor antagonist with a nonhormonal option to reach the market. The U.S. Food and Drug Administration in November accepted ObsEva’s new drug application for the medication, with a decision expected by September 2022.
“The positive CHMP opinion is an important milestone for millions of women in the EU living with UF to address the diverse medical needs of the women who suffer from this condition,” said Brian O’Callaghan, CEO of ObsEva, in a statement. “We will continue our productive, ongoing dialogue with [the] EMA toward potential marketing authorization in the EU and, in parallel, continue to work with the FDA to advance linzagolix through the U.S. regulatory process.”
The committee’s positive opinion was based on 52-week results from PRIMROSE 1 and PRIMROSE 2 phase 3 trials, involving more than 1,000 patients in the United States and Europe, as well as results from 76-week follow-up studies of patients in those trials. The two phase 3 trials assessed a 200-mg and 100-mg dose of linzagolix, with and without hormone add-back therapy (ABT; 1 mg estradiol and 0.5 mg norethisterone acetate).
According to ObsEVA, both trials met their primary endpoints, with all doses showing statistically significant and clinically relevant reductions in heavy menstrual bleeding (HMB) compared to placebo. The trials also achieved several secondary endpoints, including reduction in pain, rates of amenorrhea, time to reduced HMB, and amenorrhea and for the high dose without ABT, reductions in uterine and fibroid volume, the company said.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) on December 17 recommended approval of linzagolix (Yselty, ObsEva), an oral gonadotropin-releasing hormone (GnRH) antagonist, for the management of moderate to severe symptoms of uterine fibroids (UF) in adult women of reproductive age.
If approved, linzagolix – which is taken once per day – would become the first GnRH receptor antagonist with a nonhormonal option to reach the market. The U.S. Food and Drug Administration in November accepted ObsEva’s new drug application for the medication, with a decision expected by September 2022.
“The positive CHMP opinion is an important milestone for millions of women in the EU living with UF to address the diverse medical needs of the women who suffer from this condition,” said Brian O’Callaghan, CEO of ObsEva, in a statement. “We will continue our productive, ongoing dialogue with [the] EMA toward potential marketing authorization in the EU and, in parallel, continue to work with the FDA to advance linzagolix through the U.S. regulatory process.”
The committee’s positive opinion was based on 52-week results from PRIMROSE 1 and PRIMROSE 2 phase 3 trials, involving more than 1,000 patients in the United States and Europe, as well as results from 76-week follow-up studies of patients in those trials. The two phase 3 trials assessed a 200-mg and 100-mg dose of linzagolix, with and without hormone add-back therapy (ABT; 1 mg estradiol and 0.5 mg norethisterone acetate).
According to ObsEVA, both trials met their primary endpoints, with all doses showing statistically significant and clinically relevant reductions in heavy menstrual bleeding (HMB) compared to placebo. The trials also achieved several secondary endpoints, including reduction in pain, rates of amenorrhea, time to reduced HMB, and amenorrhea and for the high dose without ABT, reductions in uterine and fibroid volume, the company said.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) on December 17 recommended approval of linzagolix (Yselty, ObsEva), an oral gonadotropin-releasing hormone (GnRH) antagonist, for the management of moderate to severe symptoms of uterine fibroids (UF) in adult women of reproductive age.
If approved, linzagolix – which is taken once per day – would become the first GnRH receptor antagonist with a nonhormonal option to reach the market. The U.S. Food and Drug Administration in November accepted ObsEva’s new drug application for the medication, with a decision expected by September 2022.
“The positive CHMP opinion is an important milestone for millions of women in the EU living with UF to address the diverse medical needs of the women who suffer from this condition,” said Brian O’Callaghan, CEO of ObsEva, in a statement. “We will continue our productive, ongoing dialogue with [the] EMA toward potential marketing authorization in the EU and, in parallel, continue to work with the FDA to advance linzagolix through the U.S. regulatory process.”
The committee’s positive opinion was based on 52-week results from PRIMROSE 1 and PRIMROSE 2 phase 3 trials, involving more than 1,000 patients in the United States and Europe, as well as results from 76-week follow-up studies of patients in those trials. The two phase 3 trials assessed a 200-mg and 100-mg dose of linzagolix, with and without hormone add-back therapy (ABT; 1 mg estradiol and 0.5 mg norethisterone acetate).
According to ObsEVA, both trials met their primary endpoints, with all doses showing statistically significant and clinically relevant reductions in heavy menstrual bleeding (HMB) compared to placebo. The trials also achieved several secondary endpoints, including reduction in pain, rates of amenorrhea, time to reduced HMB, and amenorrhea and for the high dose without ABT, reductions in uterine and fibroid volume, the company said.
A version of this article first appeared on Medscape.com.
Califf plans work on opioids, accelerated approvals on return to FDA
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Ginger for migraine: A new review
in patients who do not want to use or don’t have access to prescription medications, new data suggest.
Conducted by investigators at the National Institute of Mental Health and Neurosciences, Bangalore, India, the review showed ginger root can relieve migraine-related pain, nausea, and vomiting. However, the evidence does not support ginger’s use as a first-line therapy for acute migraine or for migraine prevention.
Study author Chittaranjan Andrade, MD, professor of clinical psychopharmacology and neurotoxicology at the institute, said in an interview that the evidence base is still “too small” to support formal clinical recommendations. However, he added, ginger can be considered as a viable “home-remedy option” for acute migraine.
The review was published online Dec. 2 in The Journal of Clinical Psychiatry.
Potential uses
Used for centuries in traditional medicine, much of the preclinical and clinical research has examined the potential of raw ginger, ginger extracts, and ginger constituents to prevent and treat a wide range of medical conditions. These include nausea and vomiting associated with pregnancy, chemotherapy, postoperative states, motion sickness, and other diseases and disorders, said Dr. Andrade.
Ginger has “long been recommended as an effective home remedy for the acute treatment of migraine, relieving both headache and the associated nausea,” Dr. Andrade noted.
One recommended recipe is stirring half a teaspoon of ground ginger into a glass of water and drinking the “ginger juice,” while another is to drink hot tea made from a teaspoon of freshly ground ginger.
“Patients with a number of common ailments, including migraine, are sometimes caught without medicines; or they may have poor access to medicines,” Dr. Andrade said. “I came across a reference to the use of ginger for migraine in a book on home remedies and I thought that if the research literature supports the use of ginger for migraine episodes, such patients could benefit.”
Large treatment gap
The review and meta-analysis included three randomized controlled trials with 227 patients looking at ginger versus placebo for the treatment.
One of the studies investigated the therapeutic efficacy of a specific proprietary formulation of ginger, combined with feverfew, while two trials were independent of industry.
Of these two, one examined the benefit of add-on dry ginger extract (400 mg; 5% active gingerols) in 50 patients who were also taking ketoprofen to treat migraine episodes, while the other examined the 3-month efficacy of daily dry ginger extract for migraine prophylaxis in 107 patients.
The two studies that examined the therapeutic efficacy of ginger versus placebo showed ginger reduced mean pain scores at 2 hours (mean difference, –1.27 [95% confidence interval, –1.46 to 1,07]) and also increased the proportion of patients who were pain free at 2 hours (RR, 1.79 [1.04 to 3.09]). In addition, compared to placebo, ginger halved the risk of migraine-related nausea and vomiting in all of the studies and was not associated with an increased risk of adverse events.
One RCT investigated prophylactic efficacy and found it to be more effective than placebo in bringing a ≥ 50% reduction in the frequency of monthly migraine episodes (in 42% versus 39% of patients, respectively), but the difference was not deemed statistically significant. In addition, there were no significant differences between the groups in days of pain, severe pain, days requiring use of analgesics, number of migraine episodes, and maximum duration of migraine episodes.
Dr. Andrade noted that ginger has many chemical constituents, including phenolic compounds, terpenes, polysaccharides, lipids, and organic acids of which 6-shogaol, 6-gingerol, and 10-dehydrogingerdione “may be important.”
It also has antioxidant and anti-inflammatory effects, lowering prostaglandins, and reducing several serum lipid and glycemic measures. Additionally, it has “putative” vasculoprotective effects, he added.
“Ginger has a large number of chemical constituents and we do not know which of these, separately or in combination, will help relieve migraine,” he said. “We won’t know the answer unless clinical trials are conducted with the individual constituents rather than with ginger extract.” He compared this to the study of omega-3 fatty acids rather than fish and nuts for various neuropsychiatric or cardiovascular indications.
Nevertheless, given the high global prevalence of migraine and the “large treatment gap [of migraine] in primary care,” it could be common for many affected patients to experience episodes of migraine headache “without recourse to recommended pharmacologic relief,” he noted. “In such cases, the availability of a simple home remedy, such as ginger, could be helpful.”
‘Good additional tool’
Commenting on the study for this news organization, Jessica Ailani, MD, director, MedStar Georgetown Headache Center and professor of clinical neurology, MedStar Georgetown University Hospital, Washington, said that for “people with migraine who are seeking treatment with minimal side effects that they can obtain without counsel of a health care provider, ginger is a good additional tool to have.”
Dr. Ailani, vice cochair of strategic planning in the MedStar department of neurology, who was not involved with the study, said that clinicians can “consider suggesting ginger to patients with migraine that have associated nausea who are interested in nonpharmacologic ways to treat symptoms.”
Since there are “many other effective ways to treat migraine,” she advises “conversing with the patient about speed of onset of efficacy, along with tolerability, and return of migraine symptoms as important factors to evaluate when choosing and staying with a treatment.”
Also commenting on the study for this news organization, Nada Hindiyeh, MD, clinical associate professor, department of neurology, Stanford (Calif.) University, called it a “nice summary of the objective research available for the use of ginger in acute and preventive treatment of migraine.”
Although there is insufficient literature evaluating ginger alone in migraine treatment, so “no definitive conclusions can be drawn,” since it appears to be safe and “somewhat helpful for migraine-associated nausea and vomiting and possibly in frequency of migraine reduction, it remains a considerable alternative for those seeking nonprescription options,” said Dr. Hindiyeh, who was not involved with the study.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made to charities. He has received payments for developing educational materials for scientific initiatives and programs. Dr. Ailani reports honoraria for independent consulting from various pharmaceutical companies and clinical trial grants to her institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly, Satsuma, and Zosano. Dr. Hindiyeh discloses no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in patients who do not want to use or don’t have access to prescription medications, new data suggest.
Conducted by investigators at the National Institute of Mental Health and Neurosciences, Bangalore, India, the review showed ginger root can relieve migraine-related pain, nausea, and vomiting. However, the evidence does not support ginger’s use as a first-line therapy for acute migraine or for migraine prevention.
Study author Chittaranjan Andrade, MD, professor of clinical psychopharmacology and neurotoxicology at the institute, said in an interview that the evidence base is still “too small” to support formal clinical recommendations. However, he added, ginger can be considered as a viable “home-remedy option” for acute migraine.
The review was published online Dec. 2 in The Journal of Clinical Psychiatry.
Potential uses
Used for centuries in traditional medicine, much of the preclinical and clinical research has examined the potential of raw ginger, ginger extracts, and ginger constituents to prevent and treat a wide range of medical conditions. These include nausea and vomiting associated with pregnancy, chemotherapy, postoperative states, motion sickness, and other diseases and disorders, said Dr. Andrade.
Ginger has “long been recommended as an effective home remedy for the acute treatment of migraine, relieving both headache and the associated nausea,” Dr. Andrade noted.
One recommended recipe is stirring half a teaspoon of ground ginger into a glass of water and drinking the “ginger juice,” while another is to drink hot tea made from a teaspoon of freshly ground ginger.
“Patients with a number of common ailments, including migraine, are sometimes caught without medicines; or they may have poor access to medicines,” Dr. Andrade said. “I came across a reference to the use of ginger for migraine in a book on home remedies and I thought that if the research literature supports the use of ginger for migraine episodes, such patients could benefit.”
Large treatment gap
The review and meta-analysis included three randomized controlled trials with 227 patients looking at ginger versus placebo for the treatment.
One of the studies investigated the therapeutic efficacy of a specific proprietary formulation of ginger, combined with feverfew, while two trials were independent of industry.
Of these two, one examined the benefit of add-on dry ginger extract (400 mg; 5% active gingerols) in 50 patients who were also taking ketoprofen to treat migraine episodes, while the other examined the 3-month efficacy of daily dry ginger extract for migraine prophylaxis in 107 patients.
The two studies that examined the therapeutic efficacy of ginger versus placebo showed ginger reduced mean pain scores at 2 hours (mean difference, –1.27 [95% confidence interval, –1.46 to 1,07]) and also increased the proportion of patients who were pain free at 2 hours (RR, 1.79 [1.04 to 3.09]). In addition, compared to placebo, ginger halved the risk of migraine-related nausea and vomiting in all of the studies and was not associated with an increased risk of adverse events.
One RCT investigated prophylactic efficacy and found it to be more effective than placebo in bringing a ≥ 50% reduction in the frequency of monthly migraine episodes (in 42% versus 39% of patients, respectively), but the difference was not deemed statistically significant. In addition, there were no significant differences between the groups in days of pain, severe pain, days requiring use of analgesics, number of migraine episodes, and maximum duration of migraine episodes.
Dr. Andrade noted that ginger has many chemical constituents, including phenolic compounds, terpenes, polysaccharides, lipids, and organic acids of which 6-shogaol, 6-gingerol, and 10-dehydrogingerdione “may be important.”
It also has antioxidant and anti-inflammatory effects, lowering prostaglandins, and reducing several serum lipid and glycemic measures. Additionally, it has “putative” vasculoprotective effects, he added.
“Ginger has a large number of chemical constituents and we do not know which of these, separately or in combination, will help relieve migraine,” he said. “We won’t know the answer unless clinical trials are conducted with the individual constituents rather than with ginger extract.” He compared this to the study of omega-3 fatty acids rather than fish and nuts for various neuropsychiatric or cardiovascular indications.
Nevertheless, given the high global prevalence of migraine and the “large treatment gap [of migraine] in primary care,” it could be common for many affected patients to experience episodes of migraine headache “without recourse to recommended pharmacologic relief,” he noted. “In such cases, the availability of a simple home remedy, such as ginger, could be helpful.”
‘Good additional tool’
Commenting on the study for this news organization, Jessica Ailani, MD, director, MedStar Georgetown Headache Center and professor of clinical neurology, MedStar Georgetown University Hospital, Washington, said that for “people with migraine who are seeking treatment with minimal side effects that they can obtain without counsel of a health care provider, ginger is a good additional tool to have.”
Dr. Ailani, vice cochair of strategic planning in the MedStar department of neurology, who was not involved with the study, said that clinicians can “consider suggesting ginger to patients with migraine that have associated nausea who are interested in nonpharmacologic ways to treat symptoms.”
Since there are “many other effective ways to treat migraine,” she advises “conversing with the patient about speed of onset of efficacy, along with tolerability, and return of migraine symptoms as important factors to evaluate when choosing and staying with a treatment.”
Also commenting on the study for this news organization, Nada Hindiyeh, MD, clinical associate professor, department of neurology, Stanford (Calif.) University, called it a “nice summary of the objective research available for the use of ginger in acute and preventive treatment of migraine.”
Although there is insufficient literature evaluating ginger alone in migraine treatment, so “no definitive conclusions can be drawn,” since it appears to be safe and “somewhat helpful for migraine-associated nausea and vomiting and possibly in frequency of migraine reduction, it remains a considerable alternative for those seeking nonprescription options,” said Dr. Hindiyeh, who was not involved with the study.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made to charities. He has received payments for developing educational materials for scientific initiatives and programs. Dr. Ailani reports honoraria for independent consulting from various pharmaceutical companies and clinical trial grants to her institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly, Satsuma, and Zosano. Dr. Hindiyeh discloses no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in patients who do not want to use or don’t have access to prescription medications, new data suggest.
Conducted by investigators at the National Institute of Mental Health and Neurosciences, Bangalore, India, the review showed ginger root can relieve migraine-related pain, nausea, and vomiting. However, the evidence does not support ginger’s use as a first-line therapy for acute migraine or for migraine prevention.
Study author Chittaranjan Andrade, MD, professor of clinical psychopharmacology and neurotoxicology at the institute, said in an interview that the evidence base is still “too small” to support formal clinical recommendations. However, he added, ginger can be considered as a viable “home-remedy option” for acute migraine.
The review was published online Dec. 2 in The Journal of Clinical Psychiatry.
Potential uses
Used for centuries in traditional medicine, much of the preclinical and clinical research has examined the potential of raw ginger, ginger extracts, and ginger constituents to prevent and treat a wide range of medical conditions. These include nausea and vomiting associated with pregnancy, chemotherapy, postoperative states, motion sickness, and other diseases and disorders, said Dr. Andrade.
Ginger has “long been recommended as an effective home remedy for the acute treatment of migraine, relieving both headache and the associated nausea,” Dr. Andrade noted.
One recommended recipe is stirring half a teaspoon of ground ginger into a glass of water and drinking the “ginger juice,” while another is to drink hot tea made from a teaspoon of freshly ground ginger.
“Patients with a number of common ailments, including migraine, are sometimes caught without medicines; or they may have poor access to medicines,” Dr. Andrade said. “I came across a reference to the use of ginger for migraine in a book on home remedies and I thought that if the research literature supports the use of ginger for migraine episodes, such patients could benefit.”
Large treatment gap
The review and meta-analysis included three randomized controlled trials with 227 patients looking at ginger versus placebo for the treatment.
One of the studies investigated the therapeutic efficacy of a specific proprietary formulation of ginger, combined with feverfew, while two trials were independent of industry.
Of these two, one examined the benefit of add-on dry ginger extract (400 mg; 5% active gingerols) in 50 patients who were also taking ketoprofen to treat migraine episodes, while the other examined the 3-month efficacy of daily dry ginger extract for migraine prophylaxis in 107 patients.
The two studies that examined the therapeutic efficacy of ginger versus placebo showed ginger reduced mean pain scores at 2 hours (mean difference, –1.27 [95% confidence interval, –1.46 to 1,07]) and also increased the proportion of patients who were pain free at 2 hours (RR, 1.79 [1.04 to 3.09]). In addition, compared to placebo, ginger halved the risk of migraine-related nausea and vomiting in all of the studies and was not associated with an increased risk of adverse events.
One RCT investigated prophylactic efficacy and found it to be more effective than placebo in bringing a ≥ 50% reduction in the frequency of monthly migraine episodes (in 42% versus 39% of patients, respectively), but the difference was not deemed statistically significant. In addition, there were no significant differences between the groups in days of pain, severe pain, days requiring use of analgesics, number of migraine episodes, and maximum duration of migraine episodes.
Dr. Andrade noted that ginger has many chemical constituents, including phenolic compounds, terpenes, polysaccharides, lipids, and organic acids of which 6-shogaol, 6-gingerol, and 10-dehydrogingerdione “may be important.”
It also has antioxidant and anti-inflammatory effects, lowering prostaglandins, and reducing several serum lipid and glycemic measures. Additionally, it has “putative” vasculoprotective effects, he added.
“Ginger has a large number of chemical constituents and we do not know which of these, separately or in combination, will help relieve migraine,” he said. “We won’t know the answer unless clinical trials are conducted with the individual constituents rather than with ginger extract.” He compared this to the study of omega-3 fatty acids rather than fish and nuts for various neuropsychiatric or cardiovascular indications.
Nevertheless, given the high global prevalence of migraine and the “large treatment gap [of migraine] in primary care,” it could be common for many affected patients to experience episodes of migraine headache “without recourse to recommended pharmacologic relief,” he noted. “In such cases, the availability of a simple home remedy, such as ginger, could be helpful.”
‘Good additional tool’
Commenting on the study for this news organization, Jessica Ailani, MD, director, MedStar Georgetown Headache Center and professor of clinical neurology, MedStar Georgetown University Hospital, Washington, said that for “people with migraine who are seeking treatment with minimal side effects that they can obtain without counsel of a health care provider, ginger is a good additional tool to have.”
Dr. Ailani, vice cochair of strategic planning in the MedStar department of neurology, who was not involved with the study, said that clinicians can “consider suggesting ginger to patients with migraine that have associated nausea who are interested in nonpharmacologic ways to treat symptoms.”
Since there are “many other effective ways to treat migraine,” she advises “conversing with the patient about speed of onset of efficacy, along with tolerability, and return of migraine symptoms as important factors to evaluate when choosing and staying with a treatment.”
Also commenting on the study for this news organization, Nada Hindiyeh, MD, clinical associate professor, department of neurology, Stanford (Calif.) University, called it a “nice summary of the objective research available for the use of ginger in acute and preventive treatment of migraine.”
Although there is insufficient literature evaluating ginger alone in migraine treatment, so “no definitive conclusions can be drawn,” since it appears to be safe and “somewhat helpful for migraine-associated nausea and vomiting and possibly in frequency of migraine reduction, it remains a considerable alternative for those seeking nonprescription options,” said Dr. Hindiyeh, who was not involved with the study.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made to charities. He has received payments for developing educational materials for scientific initiatives and programs. Dr. Ailani reports honoraria for independent consulting from various pharmaceutical companies and clinical trial grants to her institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly, Satsuma, and Zosano. Dr. Hindiyeh discloses no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Are newer migraine therapies better? It depends
The findings, published in JAMA Network Open, “may imply that triptans will remain the current mainstay of specific acute migraine treatment,” suggested senior author Shuu-Jiun Wang, MD, from the National Yang Ming Chiao Tung University, and the Taipei Veterans General Hospital, both in Taipei, Taiwan, and his coauthors. However, lasmiditan (a 5-hydroxytryptamine1F receptor agonist) and rimegepant and ubrogepant (both calcitonin gene-related peptide [CGRP] antagonists) might still have unique advantages, since triptans are contraindicated for patients with cardiovascular risks, they said.
The systemic review and meta-analysis showed that, for the outcome of pain freedom and pain relief at 2 hours after the dose, the three newer agents worked better than placebo, but were inferior to most triptans. However, ubrogepant and rimegepant, which received U.S. Food and Drug Administration approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively, might be associated with fewer risks of adverse events (AEs), compared with triptans. “These new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction,” they wrote.
The meta-analysis included 64 randomized, controlled trials involving 46,442 participants (74%-87% female across studies; age range, 36-43 years). All studies examined clinically relevant outcomes in patients with International Headache Society criteria for migraine, and compared currently available migraine-specific acute treatments with each other or placebo. The drugs were examined at doses with widespread clinical use and included: ergotamine, dihydroergotamine, sumatriptan, zolmitriptan, naratriptan, rizatriptan, almotriptan, eletriptan, frovatriptan, lasmiditan, rimegepant, and ubrogepant.
The findings showed that all drug treatments were associated with a higher odds ratio for pain freedom, compared with placebo, except for sumatriptan, 10-mg nasal spray. The most effective drug was eletriptan 40 mg (OR, 5.59), and the least effective was lasmiditan 50 mg (OR, 1.65). Most triptans were associated with higher ORs for both pain freedom and pain relief at 2 hours, compared with lasmiditan, rimegepant, or ubrogepant, while comparisons between lasmiditan, rimegepant, and ubrogepant for these outcomes showed no statistically significant difference, they reported.
Lasmiditan was associated with the highest risk of any AEs, “however, the AEs were tolerable and were not considered serious. … Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan,” they wrote. Certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs, compared with the CGRP antagonists. “Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%).”
Finally, the authors noted that their observations of successful treatment with 5-hydroxytriptamine1F receptor agonists and CGRP antagonists “reveals that vasoconstriction is not essential for antimigraine therapy.” which could have implications for future pharmaceutical development.
Older and newer medications each have advantages
“Triptans will be around for a long time, but the newer medications are here to stay,” said Alan M. Rapoport, MD, in reaction to the study. “Before this publication, we knew that the 2-hour efficacy results of the newer medications were not quite as good as the faster-acting triptans; and after this network meta-analysis we are more sure of that,” said Dr. Rapoport, of the department of neurology at University of California, Los Angeles. “But the fact that the three newer medications do not constrict blood vessels and can easily be given even to patients with contraindications to triptans, or patients that simply are at greater risk due to obesity, smoking history, family history, diabetes, lack of exercise, or higher lipid levels, puts them into a desirable category.”
Calling it a “very carefully done” systematic review, Dr. Rapoport had a few caveats about the strength of the research. The trials that were included were not identically designed and were performed in different areas, by different investigators, on different patients, he noted. They were also not head-to-head trials “which ensures that the resultant data are more pure.” The studies also looked only at rapid results at 2 hours after dosing. “In my experience, patients are often satisfied with the response times from these newer agents; and doctors and patients both are happy that they are not vasoconstrictive,” he said. “The researchers also omitted studies looking at zolmitriptan nasal spray, which I have found to be rapid in onset and efficacious with few adverse events.”
Finally, Dr. Rapoport noted that one condition not examined in the review was medication overuse headache (MOH), which is “a major problem with patients that have high-frequency episodic migraine and chronic migraine. To our knowledge thus far, the two gepants (ubrogepant and rimegepant) do not appear to cause MOH when taken frequently, and these agents may end up being a treatment for this condition.”
Dr Wang reported receiving personal fees from Eli Lilly, Daiichi-Sankyo, Norvatis Taiwan, Biogen, Pfizer, and Bayer; and grants from AbbVie, Norvatis, Eli Lilly, Taiwan Ministry of Technology and Science, Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. No other disclosures were reported. Dr. Rapoport serves as an advisor for AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano; he is on the Speakers Bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries. He is Editor-in-Chief of Neurology Reviews.
The study was funded by the Ministry of Science and Technology, Taiwan, Ministry of Education, Taiwan, and the Brain Research Center, National Yang Ming Chiao Tung University.
The findings, published in JAMA Network Open, “may imply that triptans will remain the current mainstay of specific acute migraine treatment,” suggested senior author Shuu-Jiun Wang, MD, from the National Yang Ming Chiao Tung University, and the Taipei Veterans General Hospital, both in Taipei, Taiwan, and his coauthors. However, lasmiditan (a 5-hydroxytryptamine1F receptor agonist) and rimegepant and ubrogepant (both calcitonin gene-related peptide [CGRP] antagonists) might still have unique advantages, since triptans are contraindicated for patients with cardiovascular risks, they said.
The systemic review and meta-analysis showed that, for the outcome of pain freedom and pain relief at 2 hours after the dose, the three newer agents worked better than placebo, but were inferior to most triptans. However, ubrogepant and rimegepant, which received U.S. Food and Drug Administration approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively, might be associated with fewer risks of adverse events (AEs), compared with triptans. “These new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction,” they wrote.
The meta-analysis included 64 randomized, controlled trials involving 46,442 participants (74%-87% female across studies; age range, 36-43 years). All studies examined clinically relevant outcomes in patients with International Headache Society criteria for migraine, and compared currently available migraine-specific acute treatments with each other or placebo. The drugs were examined at doses with widespread clinical use and included: ergotamine, dihydroergotamine, sumatriptan, zolmitriptan, naratriptan, rizatriptan, almotriptan, eletriptan, frovatriptan, lasmiditan, rimegepant, and ubrogepant.
The findings showed that all drug treatments were associated with a higher odds ratio for pain freedom, compared with placebo, except for sumatriptan, 10-mg nasal spray. The most effective drug was eletriptan 40 mg (OR, 5.59), and the least effective was lasmiditan 50 mg (OR, 1.65). Most triptans were associated with higher ORs for both pain freedom and pain relief at 2 hours, compared with lasmiditan, rimegepant, or ubrogepant, while comparisons between lasmiditan, rimegepant, and ubrogepant for these outcomes showed no statistically significant difference, they reported.
Lasmiditan was associated with the highest risk of any AEs, “however, the AEs were tolerable and were not considered serious. … Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan,” they wrote. Certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs, compared with the CGRP antagonists. “Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%).”
Finally, the authors noted that their observations of successful treatment with 5-hydroxytriptamine1F receptor agonists and CGRP antagonists “reveals that vasoconstriction is not essential for antimigraine therapy.” which could have implications for future pharmaceutical development.
Older and newer medications each have advantages
“Triptans will be around for a long time, but the newer medications are here to stay,” said Alan M. Rapoport, MD, in reaction to the study. “Before this publication, we knew that the 2-hour efficacy results of the newer medications were not quite as good as the faster-acting triptans; and after this network meta-analysis we are more sure of that,” said Dr. Rapoport, of the department of neurology at University of California, Los Angeles. “But the fact that the three newer medications do not constrict blood vessels and can easily be given even to patients with contraindications to triptans, or patients that simply are at greater risk due to obesity, smoking history, family history, diabetes, lack of exercise, or higher lipid levels, puts them into a desirable category.”
Calling it a “very carefully done” systematic review, Dr. Rapoport had a few caveats about the strength of the research. The trials that were included were not identically designed and were performed in different areas, by different investigators, on different patients, he noted. They were also not head-to-head trials “which ensures that the resultant data are more pure.” The studies also looked only at rapid results at 2 hours after dosing. “In my experience, patients are often satisfied with the response times from these newer agents; and doctors and patients both are happy that they are not vasoconstrictive,” he said. “The researchers also omitted studies looking at zolmitriptan nasal spray, which I have found to be rapid in onset and efficacious with few adverse events.”
Finally, Dr. Rapoport noted that one condition not examined in the review was medication overuse headache (MOH), which is “a major problem with patients that have high-frequency episodic migraine and chronic migraine. To our knowledge thus far, the two gepants (ubrogepant and rimegepant) do not appear to cause MOH when taken frequently, and these agents may end up being a treatment for this condition.”
Dr Wang reported receiving personal fees from Eli Lilly, Daiichi-Sankyo, Norvatis Taiwan, Biogen, Pfizer, and Bayer; and grants from AbbVie, Norvatis, Eli Lilly, Taiwan Ministry of Technology and Science, Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. No other disclosures were reported. Dr. Rapoport serves as an advisor for AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano; he is on the Speakers Bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries. He is Editor-in-Chief of Neurology Reviews.
The study was funded by the Ministry of Science and Technology, Taiwan, Ministry of Education, Taiwan, and the Brain Research Center, National Yang Ming Chiao Tung University.
The findings, published in JAMA Network Open, “may imply that triptans will remain the current mainstay of specific acute migraine treatment,” suggested senior author Shuu-Jiun Wang, MD, from the National Yang Ming Chiao Tung University, and the Taipei Veterans General Hospital, both in Taipei, Taiwan, and his coauthors. However, lasmiditan (a 5-hydroxytryptamine1F receptor agonist) and rimegepant and ubrogepant (both calcitonin gene-related peptide [CGRP] antagonists) might still have unique advantages, since triptans are contraindicated for patients with cardiovascular risks, they said.
The systemic review and meta-analysis showed that, for the outcome of pain freedom and pain relief at 2 hours after the dose, the three newer agents worked better than placebo, but were inferior to most triptans. However, ubrogepant and rimegepant, which received U.S. Food and Drug Administration approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively, might be associated with fewer risks of adverse events (AEs), compared with triptans. “These new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction,” they wrote.
The meta-analysis included 64 randomized, controlled trials involving 46,442 participants (74%-87% female across studies; age range, 36-43 years). All studies examined clinically relevant outcomes in patients with International Headache Society criteria for migraine, and compared currently available migraine-specific acute treatments with each other or placebo. The drugs were examined at doses with widespread clinical use and included: ergotamine, dihydroergotamine, sumatriptan, zolmitriptan, naratriptan, rizatriptan, almotriptan, eletriptan, frovatriptan, lasmiditan, rimegepant, and ubrogepant.
The findings showed that all drug treatments were associated with a higher odds ratio for pain freedom, compared with placebo, except for sumatriptan, 10-mg nasal spray. The most effective drug was eletriptan 40 mg (OR, 5.59), and the least effective was lasmiditan 50 mg (OR, 1.65). Most triptans were associated with higher ORs for both pain freedom and pain relief at 2 hours, compared with lasmiditan, rimegepant, or ubrogepant, while comparisons between lasmiditan, rimegepant, and ubrogepant for these outcomes showed no statistically significant difference, they reported.
Lasmiditan was associated with the highest risk of any AEs, “however, the AEs were tolerable and were not considered serious. … Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan,” they wrote. Certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs, compared with the CGRP antagonists. “Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%).”
Finally, the authors noted that their observations of successful treatment with 5-hydroxytriptamine1F receptor agonists and CGRP antagonists “reveals that vasoconstriction is not essential for antimigraine therapy.” which could have implications for future pharmaceutical development.
Older and newer medications each have advantages
“Triptans will be around for a long time, but the newer medications are here to stay,” said Alan M. Rapoport, MD, in reaction to the study. “Before this publication, we knew that the 2-hour efficacy results of the newer medications were not quite as good as the faster-acting triptans; and after this network meta-analysis we are more sure of that,” said Dr. Rapoport, of the department of neurology at University of California, Los Angeles. “But the fact that the three newer medications do not constrict blood vessels and can easily be given even to patients with contraindications to triptans, or patients that simply are at greater risk due to obesity, smoking history, family history, diabetes, lack of exercise, or higher lipid levels, puts them into a desirable category.”
Calling it a “very carefully done” systematic review, Dr. Rapoport had a few caveats about the strength of the research. The trials that were included were not identically designed and were performed in different areas, by different investigators, on different patients, he noted. They were also not head-to-head trials “which ensures that the resultant data are more pure.” The studies also looked only at rapid results at 2 hours after dosing. “In my experience, patients are often satisfied with the response times from these newer agents; and doctors and patients both are happy that they are not vasoconstrictive,” he said. “The researchers also omitted studies looking at zolmitriptan nasal spray, which I have found to be rapid in onset and efficacious with few adverse events.”
Finally, Dr. Rapoport noted that one condition not examined in the review was medication overuse headache (MOH), which is “a major problem with patients that have high-frequency episodic migraine and chronic migraine. To our knowledge thus far, the two gepants (ubrogepant and rimegepant) do not appear to cause MOH when taken frequently, and these agents may end up being a treatment for this condition.”
Dr Wang reported receiving personal fees from Eli Lilly, Daiichi-Sankyo, Norvatis Taiwan, Biogen, Pfizer, and Bayer; and grants from AbbVie, Norvatis, Eli Lilly, Taiwan Ministry of Technology and Science, Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. No other disclosures were reported. Dr. Rapoport serves as an advisor for AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano; he is on the Speakers Bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries. He is Editor-in-Chief of Neurology Reviews.
The study was funded by the Ministry of Science and Technology, Taiwan, Ministry of Education, Taiwan, and the Brain Research Center, National Yang Ming Chiao Tung University.
FROM JAMA NETWORK OPEN
Multimodal Pain Management With Adductor Canal Block Decreases Opioid Consumption Following Total Knee Arthroplasty
Ease of access to opioids in the perioperative period is a risk factor for opioid misuse and has been identified as a strong risk factor for heroin use.1,2 Three-quarters of today’s heroin users were introduced to opioids through prescription medications.2 The United States accounts for about 80% of the global opioid supply consumption, and deaths from opioid overdose are increasing: 70,630 deaths in 2019 alone.3,4
The Centers for Disease Control and Prevention (CDC) has called for changes in opioid prescribing. The American Academy of Orthopaedic Surgeons (AAOS) also has published an information statement with strategies to decrease opioid misuse and abuse.5,6 Arthroplasty surgeons have recently focused on decreasing use of opioids in total knee arthroplasty (TKA), a procedure traditionally associated with high levels of opioid consumption and historical reliance on opioid monotherapy for postoperative analgesia.7,8 From a clinical perspective, prolonged postoperative opioid use contributes to poorer surgical outcomes due to increased risk of complications, including stiffness, infection, and revision TKA.9
Multimodal pain regimens are increasingly being used to control postoperative pain as data supports their efficacy.10,11 Previous studies have found that simultaneous modulation of multiple pain pathways decreases narcotics consumption and improves patient outcomes.12,13 Along with other adjuvant therapies, peripheral nerve blocks, such as adductor canal block (ACB) and femoral nerve block (FNB), have been used to decrease postoperative pain.14 Studies have shown that ACB has fewer complications and shorter functional recovery times compared with FNB.15,16 The distribution of the ACB excludes the femoral nerve, thus preserving greater quadriceps strength while providing equivalent levels of analgesia compared with FNB.15,17,18 The ACB has shown decreased near-fall events and improved balance scores in the immediate postoperative period.19
Our study analyzed opioid consumption patterns of TKA patients from a US Department of Veterans Affairs (VA) medical center before and after the institution of a multimodal analgesic protocol using ACB. The primary purpose of this study was to determine whether a protocol that included intraoperative spinal anesthesia with a postoperative multimodal analgesic regimen and ACB was associated with a decreased postoperative opioid requirement when compared with patients who received intraoperative general anesthesia and a traditional opioid regimen. Secondary outcomes included the effect of opioid consumption on range of motion on postoperative day (POD) 1 and number of opioid prescriptions written at the first postoperative clinic visit.
Methods
Approval for the study was obtained from the institutional review board at the Dayton Veterans Affairs Medical Center (DVAMC) in Ohio. A retrospective chart review was performed to collect data from all patients undergoing TKA at DVAMC from June 1, 2011, through December 31, 2015. Exclusion criteria included multiple surgeries in the study time frame, documented chronic pain, allergy to local anesthetics, daily preoperative use of opioids, and incomplete data in the health record.
All surgeries were performed by 2 staff arthroplasty surgeons at a single VAMC. All patients attended a preoperative visit where a history, physical, and anesthesia evaluation were performed, and watched an educational video detailing surgical indications and postoperative rehabilitation. All surgeries were performed with tourniquets and a periarticular injection was performed at the conclusion of each case. Surgeon 1 treatment of choice was 10 mL 0.5% bupivacaine, whereas surgeon 2 performed a posterior capsular injection of 30 mL 0.25% bupivacaine and a periarticular injection of 30 mg ketorolac in 10 mL 0.25% bupivacaine with epinephrine.
Prior to August 2014, general endotracheal anesthesia was used intraoperatively. A patient-controlled analgesia (PCA) pump of morphine or hydromorphone and additional oral oxycodone or hydrocodone was used for postoperative pain. PCA pumps were patient dependent. In the control group, 245 patients received the morphine PCA while 61 received the hydromorphone PCA. Morphine PCA dosing consisted of 1-mg doses every 10 minutes with potential baseline infusion rates of 0.5 to 1.0 mg/h and a 4-hour limit of 20 mg. Hydromorphone PCA dosing consisted of 0.2 to 0.4-mg doses with a potential continuous dose of 0.2 to 0.4 mg/h and a 4-hour limit of 4 mg.
In August 2014, a new analgesic protocol was adopted for TKA consisting of intraoperative spinal anesthesia (0.75% bupivacaine) with IV sedation (propofol), a postoperative multimodal analgesic regimen, an ACB performed in the postanesthesia care unit (PACU), and opioids as needed (protocol group). The ACB catheter was a 0.5% ropivo caine hydrochloride injection. It was attached to a local anesthetic fixed flow rate pump that administers 0.5% ropivacaine without epinephrine at 8 mL/h and was removed on POD 5 by the patient. The multimodal medication regimen included IV ketorolac 15 mg every 6 hours for 3 doses, gabapentin 300 mg every 8 hours, acetaminophen 975 mg every 8 hours, meloxicam 7.5 mg daily, tramadol 50 mg every 6 hours, oxycodone 5 mg 1 to 2 tabs every 4 hours as needed, and IV hydromorphone 0.5 mg every 4 hours as needed for breakthrough pain.
Preoperative demographic characteristics were collected (Table 1). Data on all IV and oral opioid requirements were collected for both groups, converted to morphine milligram equivalents (MME), and a total morphine equivalent dose (MED) was calculated.20,21
In April 2015, a separate protocol change occurred at the DVAMC with the goal of discharge on POD 1. To standardize outcomes before and after this change, data collection regarding opioid requirements was concluded at midnight on POD 1. If a patient was discharged before midnight on POD 1, opioid requirement through the time of discharge was collected. All surgeries were performed in the morning to early afternoon; however, specific surgical times were not collected. Patients were also evaluated by a physical therapist on POD 0, and maximal knee flexion and extension were measured on POD 1. Patients were discharged with prescriptions for oxycodone/acetaminophen and tramadol and were seen 3 weeks later for their first postoperative visit. Opioid refills at the first postoperative visit were recorded. All statistical analyses were performed in SAS 9.4 with significance set to α = 0.05. Between-groups differences in preoperative and perioperative characteristics as well as postoperative outcomes were analyzed using independent samples t tests for continuous variables and Fisher exact tests for dichotomous discrete variables. Where groups differed for a pre- or perioperative variable, linear mixed models analysis was used to determine whether IV, oral, and total MEDs were significantly affected by the interaction between the pre- or perioperative variable with analgesia group. For refills at the postoperative visit, the effects of pre- or perioperative differences were tested using χ2 tests. Effect sizes for outcome variables were estimated using Cohen d and probability of superiority (Δ) for continuous variables, and relative risk (RR) in the case of discrete variables.22
Results
During the study period from June 1, 2011, through December 31, 2015, 533 eligible TKAs were performed, 306 in the control group and 227 in the protocol group. The groups had similar sex distribution; body mass index; knee range of motion; diagnoses of diabetes mellitus, coronary artery disease, and chronic kidney disease; and history of deep vein thrombosis (DVT) or pulmonary embolism (P ≥ .05). The protocol group was significantly older (P = .04) and had a significantly higher rate of chronic obstructive pulmonary disease (COPD) (P = .002). There were no significant differences between number of procedures performed by surgeon (P = .48) or total tourniquet time (P = .13) (Table 2). Mean (SD) length of stay was significantly greater in the control group compared with the protocol group (2.5 [1.3] vs 1.4 [0.7] days, P < .001).
Figure 1 shows the distributions of each type of opioid used. Compared with the control group, the protocol group had a significantly lower mean (SD) IV opioid use: 178.2 (98.0) MED vs 12.0 (24.6) MED (P < .001; d = 2.19; Δ = 0.94) and mean (SD) total opioid use: 241.7 (120.1) MED vs 74.8 (42.7) MED (P < .001; d = 1.76; Δ = 0.89). Mean (SD) oral opioid use did not differ between groups (control, 63.6 [45.4] MED; protocol, 62.9 [31.4] MED; P = .85; d = 0.02; Δ = 0.51). A significantly lower percentage of patients in the protocol group received additional opioids at the 3-week follow-up when compared to the control group: 46.7% vs 61.3%, respectively (P < .001; RR, 0.76; 95% CI, 0.65-0.90).
There were no significant differences in postoperative mean (SD) maximum knee flexion (control, 67.2 [15.7]°; protocol, 67.8 [19.2]°; P = .72; d = 0.03; Δ = 0.51) or mean (SD) total flexion/extension arc (control, 66.2 [15.9]°; protocol, 67.9 [19.4]°; P = .32; d = 0.10; Δ = 0.53). Mean (SD) postoperative maximum knee extension was significantly higher in the protocol group compared with the control group (-0.1 [2.1]° vs 1.0 [3.7]°; P < .001; d = 0.35; Δ = 0.60). More patients in the protocol group (92.5%) were discharged to home compared with the control group (86.6%) (P = .02; RR, 1.07; 95% CI, 1.01-1.13).
Because age and rates of COPD differed between groups, sensitivity analyses were conducted to determine whether these variables influenced postoperative opioid use. The relationship between age and group was significant for IV (P < .001) and total opioid use (P < .001). Younger patients received higher MED doses than older patients within the control group, while dosages were fairly consistent regardless of age in the protocol group (Figure 2). There was no significance in age interaction effect with regard to oral opioids (P = .83) nor opioid refills at 3-week follow-up (P = .24).
The sensitivity analysis for COPD found that a diagnosis of COPD did not significantly influence utilization of IV opioids (P = .10), or total opioids (P = .68). There was a significant interaction effect for oral opioids (Figure 3). Patients in the control group with COPD required significantly higher mean (SD) oral opioids than patients without COPD (91.5 [123.9] MED and 62.0 [36.0] MED, respectively; P = .03). In the control group, the χ2 test was significant regarding opioid prescription refills at the 3-week visit (P = .004) with 62.4% of patients with COPD requiring refills vs 44.4% without COPD (P = .004). There was no difference in refills in the protocol group (46.4% vs 48.4%).
Finally, 2-sided independent samples t test evaluated total MED use between the 2 surgeons. There was no difference in total MED per patient for the surgeons. In the control group, mean (SD) total MED for surgeon 1 was 232.9 (118.7) MED vs 252.8 (121.5) MED for surgeon 2 (P = .18). In the protocol group, the mean (SD) total MED was 72.5 (43.2) and 77.4 (42.1) for surgeon 1 and surgeon 2, respectively (P = .39).
Discussion
Coordinated efforts with major medical organizations are being made to decrease opioid prescriptions and exposure.5,6 To our knowledge, no study has quantified a decrease in opioid requirement in a VA population after implementation of a protocol that includes intraoperative spinal anesthesia and a postoperative multimodal analgesic regimen including ACB after TKA. The analgesic protocol described in this study aligns with recommendations from both the CDC and the AAOS to decrease opioid use and misuse by maximizing nonopioid medications and limiting the size and number of opioid prescriptions. However, public and medical opinion of opioids as well as prescribing practices have changed over time with a trend toward lower opioid use. The interventions, as part of the described protocol, are a result of these changes and attempt to minimize opioid use while maximizing postoperative analgesia.
Our data showed a significant decrease in total opioid use through POD 1, IV opioid use, and opioid prescriptions provided at the first postoperative visit. The protocol group used only 6.7% of the IV opioids and 30.9% of the total opioids that were used by the control group. The substantial difference in IV opioid requirement, 166.2 MED, is equivalent to 8 mg of IV hydromorphone or 55 mg of IV morphine. The difference in total opioid requirement was similar at 166.9 MED, equivalent to 111 mg of oral oxycodone.
Decreasing opioid use has the additional benefit of improving outcomes, as higher doses of opioids have been associated with increased length of stay, greater rates of DVT, and postoperative infection.23 These complications occurred in a stepwise manner, suggesting a dose-response gradient that makes the sizable decrease noted in our data of greater relevance.23 While the adverse effects (AEs) of opioids are well known, there are limited data on opioid dosing and its effect on perioperative outcomes.23
A significant decrease in the percentage of patients receiving an opioid prescription at the first postoperative visit suggests a decrease in the number of patients on prolonged opioids after TKA with implementation of modern analgesic modalities. The duration of postoperative opioid use has been found to be the strongest predictor of misuse, and each postoperative refill increases the probability of misuse by 44%.24 In addition, opioid use for > 3 months after TKA is associated with increased risk of periprosthetic infection, increased overall revision rate, and stiffness at 1 year postoperatively.9 While not entirely under the control of the surgeon, measures to decrease the number of postoperative opioid refills may lead to a decrease in opioid misuse.
In the control group, older patients tended to receive less opioids. This is likely due to physiologic changes in opioid metabolism associated with aging, including decreased renal and hepatic opioid metabolism and alterations in overall body composition that increase relative potency and duration of action of opioids in a geriatric population.25,26 No difference in opioid use by age was found for the protocol group.
Patients in the protocol group demonstrated significantly greater maximal knee extension on POD 1 compared with the control group. No difference in maximal flexion was found. This difference in extension may partially be explained by the use of an ACB. One benefit of ACB is greater quadriceps strength and fewer near-fall events when compared with FNB.15,19
Our results corroborate the findings of similar studies. A randomized controlled trial comparing a multimodal analgesic regimen with a periarticular injection without a postoperative ACB to a hydromorphone PCA revealed a significant decrease in opioid use in the multimodal analgesic group.27 Along with lower opioid requirements, the multimodal analgesic group had lower visual analog scale pain scores, fewer AEs, faster progression to physical therapy milestones, and higher satisfaction.27 Recent guidelines from the French Society of Anaesthesia and Intensive Care Medicine recommend against the use of gabapentin as a method of postoperative pain control. However, this specifically refers to the preoperative administration of gabapentin. This same set of guidelines later cites a high level of evidence suggesting patients undergoing arthroplasty benefit more from gabapentinoids.28 Multiple analgesic protocols that include gabapentin as a part of a multimodal approach have been shown to have positive results.13,29
In our study, patients receiving the multimodal analgesic regimen were significantly more likely to be discharged home rather than to postacute care facilities, which have been associated with increased rates of major complications, 30-day readmission, and 30-day reoperation.30,31 In addition, discharge to an inpatient rehabilitation or skilled nursing facility has not been found to result in higher functional outcomes, despite $3.2 billion spent yearly on rehabilitation services after primary TKA.32,33
A component of our described analgesic protocol included spinal anesthesia intraoperatively. The differences between groups regarding anesthesia type can be attributed to this protocol change. A significantly greater percentage of patients in the protocol group received spinal anesthesia, while more patients in the control group received general anesthesia. While patients who received spinal anesthesia may have enhanced analgesia in the immediate postoperative period, no differences in opioid outcomes were seen based on anesthesia type. Known benefits of intraoperative spinal anesthesia include decreased perioperative blood loss and a smaller decrease in hemoglobin postoperatively, as well as lower rates of in-hospital complications, including pulmonary embolism, pneumonia, cerebrovascular events, and acute renal failure.34
Limitations
A number of limitations of this study should be noted. One was a protocol change regarding length of stay, which occurred during the study period and resulted in a significantly shorter length of stay in the protocol group. As a result, opioid use data were analyzed only through midnight at the end of POD 1. Patients who were discharged on POD 1 did not have opioid use data available for the full duration of the first POD, which may exaggerate the decrease in opioid requirements, as opioids used after discharge but prior to midnight on POD 1 were not recorded. However, opioids taken at home are oral with a low MME compared with IV opioids received by hospitalized patients in the control group. In addition, if taken as prescribed, patients at home would only have enough time to take a few doses of opioids prior to the midnight cutoff. We do not believe this difference in time of opioid use meaningfully affected the data. An additional limitation includes the variability between periarticular injections between surgeons. While the percentage of patients that received injections from surgeon 1 vs surgeon 2 were similar, it cannot be ruled out as a potential confounding factor. Other limitations include a lack of pain scores to compare subjective pain ratings, the retrospective nature of the study, and a largely homogenous male VA population.
Conclusions
Ease of access to opioids is a risk factor for opioid abuse, which itself is a risk factor for subsequent heroin use.1,2 The CDC and AAOS have thus published recommendations regarding opioid prescribing practices to decrease opioid use and abuse.5,6 Our described protocol, which aligns with these recommendations, resulted in a significant decrease in IV opioid requirement, total opioid requirement, and lower rates of opioid prescriptions provided at the first postoperative visit. These promising findings demonstrate a lower percentage of patients on long-term opioids after TKA and a significantly decreased cumulative opioid exposure.
1. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44. doi:10.1016/j.drugpo.2011.05.014
2. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi:10.1016/j.drugalcdep.2013.01.007
3. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(suppl 2):S63-S88.
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349-358. Published 2018 Mar 30. doi:10.15585/mmwr.mm6712a1
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. American Academy of Orthopaedic Surgeons. Information statement: opioid use, misuse, and abuse in orthopaedic practice. Published October 2015. Accessed November 12, 2021. https://aaos.org/globalassets/about /bylaws-library/information-statements/1045-opioid-use -misuse-and-abuse-in-practice.pdf
7. Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty. 2017;32(8):2395-2398. doi:10.1016/j.arth.2017.02.060
8. Gerner P, Poeran J, Cozowicz C, Mörwald EE, Zubizarreta N, Mazumdar M, Memtsoudis SG, Multimodal pain management in total hip and knee arthroplasty: trends over the last 10 years. Abstract presented at: American Society of Anesthesiologists Annual Meeting; October 21, 2017; Boston, MA.
9. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
10. Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
11. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.doi:10.1001/jamasurg.2017.0898
12. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthoplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
13. Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty. 2017;32(9S):S69-S73. doi:10.1016/j.arth.2017.05.002
14. Ardon AE, Clendenen SR, Porter SB, Robards CB, Greengrass RA. Opioid consumption in total knee arthroplasty patients: a retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J Clin Anesth. 2016;31:19-26. doi:10.1016/j.jclinane.2015.12.014
15. Li D, Ma GG. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2614-2619. doi:10.1007/s00167-015-3874-3
16. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925-933. doi:10.1007/s00264-015-2998-x
17. Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221-226.
18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540-550. doi:10.1097/ALN.0000000000000119
19. Thacher RR, Hickernell TR, Grosso MJ, et al. Decreased risk of knee buckling with adductor canal block versus femoral nerve block in total knee arthroplasty: a retrospective cohort study. Arthroplasty Today. 2017;3(4):281-285. Published 2017 Apr 15. doi:10.1016/j.artd.2017.02.008
20. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain. 2008;24(6):521-527. doi:10.1097/AJP.0b013e318169d03b
21. Kishner S. Opioid equivalents and conversions: overview. Published January 29, 2018. Accessed November 12, 2021. https://emedicine.medscape.com/article/2138678 -overview#a1
22. Ruscio J, Mullen T. Confidence intervals for the probability of superiority effect size measure and the area under a receiver operating characteristic curve. Multivariate Behav Res. 2012;47(2):201-223. doi:10.1080/00273171.2012.658329
23. Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic orthopedic surgery. Pain. 2017;158(12):2422-2430. doi:10.1097/j.pain.0000000000001047
24. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
25. Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37(1):17- 40. doi:10.2165/00003088-199937010-00002
26. Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396-1430. doi:10.1592/phco.2005.25.10.1396
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329- 334. doi:10.1016/j.arth.2013.06.005
28. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi:10.1016/j.accpm.2019.02.011
29. Han C, Li XD, Jiang HQ, Ma JX, Ma XL. The use of gabapentin in the management of postoperative pain after total knee arthroplasty: A PRISMA-compliant metaanalysis of randomized controlled trials [published correction appears in Medicine (Baltimore). 2016 Jul 18;95(28):e0916]. Medicine (Baltimore). 2016;95(23):e3883. doi:10.1097/MD.0000000000003883
30. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day postdischarge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32(9S):S113-S118. doi:10.1016/j.arth.2017.01.039
31. Pelt CE, Gililland JM, Erickson JA, Trimble DE, Anderson MB, Peters CL. Improving value in total joint arthroplasty: a comprehensive patient education and management program decreases discharge to post-acute care facilities and post-operative complications. J Arthroplasty. 2018;33(1):14-18. doi:10.1016/j.arth.2017.08.003
32. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33(6):1663-1667. doi:10.1016/j.arth.2017.12.033
33. Lavernia CJ, D’Apuzzo MR, Hernandez VH, Lee DJ, Rossi MD. Postdischarge costs in arthroplasty surgery. J Arthroplasty. 2006;21(6 Suppl 2):144-150. doi:10.1016/j.arth.2006.05.003
Ease of access to opioids in the perioperative period is a risk factor for opioid misuse and has been identified as a strong risk factor for heroin use.1,2 Three-quarters of today’s heroin users were introduced to opioids through prescription medications.2 The United States accounts for about 80% of the global opioid supply consumption, and deaths from opioid overdose are increasing: 70,630 deaths in 2019 alone.3,4
The Centers for Disease Control and Prevention (CDC) has called for changes in opioid prescribing. The American Academy of Orthopaedic Surgeons (AAOS) also has published an information statement with strategies to decrease opioid misuse and abuse.5,6 Arthroplasty surgeons have recently focused on decreasing use of opioids in total knee arthroplasty (TKA), a procedure traditionally associated with high levels of opioid consumption and historical reliance on opioid monotherapy for postoperative analgesia.7,8 From a clinical perspective, prolonged postoperative opioid use contributes to poorer surgical outcomes due to increased risk of complications, including stiffness, infection, and revision TKA.9
Multimodal pain regimens are increasingly being used to control postoperative pain as data supports their efficacy.10,11 Previous studies have found that simultaneous modulation of multiple pain pathways decreases narcotics consumption and improves patient outcomes.12,13 Along with other adjuvant therapies, peripheral nerve blocks, such as adductor canal block (ACB) and femoral nerve block (FNB), have been used to decrease postoperative pain.14 Studies have shown that ACB has fewer complications and shorter functional recovery times compared with FNB.15,16 The distribution of the ACB excludes the femoral nerve, thus preserving greater quadriceps strength while providing equivalent levels of analgesia compared with FNB.15,17,18 The ACB has shown decreased near-fall events and improved balance scores in the immediate postoperative period.19
Our study analyzed opioid consumption patterns of TKA patients from a US Department of Veterans Affairs (VA) medical center before and after the institution of a multimodal analgesic protocol using ACB. The primary purpose of this study was to determine whether a protocol that included intraoperative spinal anesthesia with a postoperative multimodal analgesic regimen and ACB was associated with a decreased postoperative opioid requirement when compared with patients who received intraoperative general anesthesia and a traditional opioid regimen. Secondary outcomes included the effect of opioid consumption on range of motion on postoperative day (POD) 1 and number of opioid prescriptions written at the first postoperative clinic visit.
Methods
Approval for the study was obtained from the institutional review board at the Dayton Veterans Affairs Medical Center (DVAMC) in Ohio. A retrospective chart review was performed to collect data from all patients undergoing TKA at DVAMC from June 1, 2011, through December 31, 2015. Exclusion criteria included multiple surgeries in the study time frame, documented chronic pain, allergy to local anesthetics, daily preoperative use of opioids, and incomplete data in the health record.
All surgeries were performed by 2 staff arthroplasty surgeons at a single VAMC. All patients attended a preoperative visit where a history, physical, and anesthesia evaluation were performed, and watched an educational video detailing surgical indications and postoperative rehabilitation. All surgeries were performed with tourniquets and a periarticular injection was performed at the conclusion of each case. Surgeon 1 treatment of choice was 10 mL 0.5% bupivacaine, whereas surgeon 2 performed a posterior capsular injection of 30 mL 0.25% bupivacaine and a periarticular injection of 30 mg ketorolac in 10 mL 0.25% bupivacaine with epinephrine.
Prior to August 2014, general endotracheal anesthesia was used intraoperatively. A patient-controlled analgesia (PCA) pump of morphine or hydromorphone and additional oral oxycodone or hydrocodone was used for postoperative pain. PCA pumps were patient dependent. In the control group, 245 patients received the morphine PCA while 61 received the hydromorphone PCA. Morphine PCA dosing consisted of 1-mg doses every 10 minutes with potential baseline infusion rates of 0.5 to 1.0 mg/h and a 4-hour limit of 20 mg. Hydromorphone PCA dosing consisted of 0.2 to 0.4-mg doses with a potential continuous dose of 0.2 to 0.4 mg/h and a 4-hour limit of 4 mg.
In August 2014, a new analgesic protocol was adopted for TKA consisting of intraoperative spinal anesthesia (0.75% bupivacaine) with IV sedation (propofol), a postoperative multimodal analgesic regimen, an ACB performed in the postanesthesia care unit (PACU), and opioids as needed (protocol group). The ACB catheter was a 0.5% ropivo caine hydrochloride injection. It was attached to a local anesthetic fixed flow rate pump that administers 0.5% ropivacaine without epinephrine at 8 mL/h and was removed on POD 5 by the patient. The multimodal medication regimen included IV ketorolac 15 mg every 6 hours for 3 doses, gabapentin 300 mg every 8 hours, acetaminophen 975 mg every 8 hours, meloxicam 7.5 mg daily, tramadol 50 mg every 6 hours, oxycodone 5 mg 1 to 2 tabs every 4 hours as needed, and IV hydromorphone 0.5 mg every 4 hours as needed for breakthrough pain.
Preoperative demographic characteristics were collected (Table 1). Data on all IV and oral opioid requirements were collected for both groups, converted to morphine milligram equivalents (MME), and a total morphine equivalent dose (MED) was calculated.20,21
In April 2015, a separate protocol change occurred at the DVAMC with the goal of discharge on POD 1. To standardize outcomes before and after this change, data collection regarding opioid requirements was concluded at midnight on POD 1. If a patient was discharged before midnight on POD 1, opioid requirement through the time of discharge was collected. All surgeries were performed in the morning to early afternoon; however, specific surgical times were not collected. Patients were also evaluated by a physical therapist on POD 0, and maximal knee flexion and extension were measured on POD 1. Patients were discharged with prescriptions for oxycodone/acetaminophen and tramadol and were seen 3 weeks later for their first postoperative visit. Opioid refills at the first postoperative visit were recorded. All statistical analyses were performed in SAS 9.4 with significance set to α = 0.05. Between-groups differences in preoperative and perioperative characteristics as well as postoperative outcomes were analyzed using independent samples t tests for continuous variables and Fisher exact tests for dichotomous discrete variables. Where groups differed for a pre- or perioperative variable, linear mixed models analysis was used to determine whether IV, oral, and total MEDs were significantly affected by the interaction between the pre- or perioperative variable with analgesia group. For refills at the postoperative visit, the effects of pre- or perioperative differences were tested using χ2 tests. Effect sizes for outcome variables were estimated using Cohen d and probability of superiority (Δ) for continuous variables, and relative risk (RR) in the case of discrete variables.22
Results
During the study period from June 1, 2011, through December 31, 2015, 533 eligible TKAs were performed, 306 in the control group and 227 in the protocol group. The groups had similar sex distribution; body mass index; knee range of motion; diagnoses of diabetes mellitus, coronary artery disease, and chronic kidney disease; and history of deep vein thrombosis (DVT) or pulmonary embolism (P ≥ .05). The protocol group was significantly older (P = .04) and had a significantly higher rate of chronic obstructive pulmonary disease (COPD) (P = .002). There were no significant differences between number of procedures performed by surgeon (P = .48) or total tourniquet time (P = .13) (Table 2). Mean (SD) length of stay was significantly greater in the control group compared with the protocol group (2.5 [1.3] vs 1.4 [0.7] days, P < .001).
Figure 1 shows the distributions of each type of opioid used. Compared with the control group, the protocol group had a significantly lower mean (SD) IV opioid use: 178.2 (98.0) MED vs 12.0 (24.6) MED (P < .001; d = 2.19; Δ = 0.94) and mean (SD) total opioid use: 241.7 (120.1) MED vs 74.8 (42.7) MED (P < .001; d = 1.76; Δ = 0.89). Mean (SD) oral opioid use did not differ between groups (control, 63.6 [45.4] MED; protocol, 62.9 [31.4] MED; P = .85; d = 0.02; Δ = 0.51). A significantly lower percentage of patients in the protocol group received additional opioids at the 3-week follow-up when compared to the control group: 46.7% vs 61.3%, respectively (P < .001; RR, 0.76; 95% CI, 0.65-0.90).
There were no significant differences in postoperative mean (SD) maximum knee flexion (control, 67.2 [15.7]°; protocol, 67.8 [19.2]°; P = .72; d = 0.03; Δ = 0.51) or mean (SD) total flexion/extension arc (control, 66.2 [15.9]°; protocol, 67.9 [19.4]°; P = .32; d = 0.10; Δ = 0.53). Mean (SD) postoperative maximum knee extension was significantly higher in the protocol group compared with the control group (-0.1 [2.1]° vs 1.0 [3.7]°; P < .001; d = 0.35; Δ = 0.60). More patients in the protocol group (92.5%) were discharged to home compared with the control group (86.6%) (P = .02; RR, 1.07; 95% CI, 1.01-1.13).
Because age and rates of COPD differed between groups, sensitivity analyses were conducted to determine whether these variables influenced postoperative opioid use. The relationship between age and group was significant for IV (P < .001) and total opioid use (P < .001). Younger patients received higher MED doses than older patients within the control group, while dosages were fairly consistent regardless of age in the protocol group (Figure 2). There was no significance in age interaction effect with regard to oral opioids (P = .83) nor opioid refills at 3-week follow-up (P = .24).
The sensitivity analysis for COPD found that a diagnosis of COPD did not significantly influence utilization of IV opioids (P = .10), or total opioids (P = .68). There was a significant interaction effect for oral opioids (Figure 3). Patients in the control group with COPD required significantly higher mean (SD) oral opioids than patients without COPD (91.5 [123.9] MED and 62.0 [36.0] MED, respectively; P = .03). In the control group, the χ2 test was significant regarding opioid prescription refills at the 3-week visit (P = .004) with 62.4% of patients with COPD requiring refills vs 44.4% without COPD (P = .004). There was no difference in refills in the protocol group (46.4% vs 48.4%).
Finally, 2-sided independent samples t test evaluated total MED use between the 2 surgeons. There was no difference in total MED per patient for the surgeons. In the control group, mean (SD) total MED for surgeon 1 was 232.9 (118.7) MED vs 252.8 (121.5) MED for surgeon 2 (P = .18). In the protocol group, the mean (SD) total MED was 72.5 (43.2) and 77.4 (42.1) for surgeon 1 and surgeon 2, respectively (P = .39).
Discussion
Coordinated efforts with major medical organizations are being made to decrease opioid prescriptions and exposure.5,6 To our knowledge, no study has quantified a decrease in opioid requirement in a VA population after implementation of a protocol that includes intraoperative spinal anesthesia and a postoperative multimodal analgesic regimen including ACB after TKA. The analgesic protocol described in this study aligns with recommendations from both the CDC and the AAOS to decrease opioid use and misuse by maximizing nonopioid medications and limiting the size and number of opioid prescriptions. However, public and medical opinion of opioids as well as prescribing practices have changed over time with a trend toward lower opioid use. The interventions, as part of the described protocol, are a result of these changes and attempt to minimize opioid use while maximizing postoperative analgesia.
Our data showed a significant decrease in total opioid use through POD 1, IV opioid use, and opioid prescriptions provided at the first postoperative visit. The protocol group used only 6.7% of the IV opioids and 30.9% of the total opioids that were used by the control group. The substantial difference in IV opioid requirement, 166.2 MED, is equivalent to 8 mg of IV hydromorphone or 55 mg of IV morphine. The difference in total opioid requirement was similar at 166.9 MED, equivalent to 111 mg of oral oxycodone.
Decreasing opioid use has the additional benefit of improving outcomes, as higher doses of opioids have been associated with increased length of stay, greater rates of DVT, and postoperative infection.23 These complications occurred in a stepwise manner, suggesting a dose-response gradient that makes the sizable decrease noted in our data of greater relevance.23 While the adverse effects (AEs) of opioids are well known, there are limited data on opioid dosing and its effect on perioperative outcomes.23
A significant decrease in the percentage of patients receiving an opioid prescription at the first postoperative visit suggests a decrease in the number of patients on prolonged opioids after TKA with implementation of modern analgesic modalities. The duration of postoperative opioid use has been found to be the strongest predictor of misuse, and each postoperative refill increases the probability of misuse by 44%.24 In addition, opioid use for > 3 months after TKA is associated with increased risk of periprosthetic infection, increased overall revision rate, and stiffness at 1 year postoperatively.9 While not entirely under the control of the surgeon, measures to decrease the number of postoperative opioid refills may lead to a decrease in opioid misuse.
In the control group, older patients tended to receive less opioids. This is likely due to physiologic changes in opioid metabolism associated with aging, including decreased renal and hepatic opioid metabolism and alterations in overall body composition that increase relative potency and duration of action of opioids in a geriatric population.25,26 No difference in opioid use by age was found for the protocol group.
Patients in the protocol group demonstrated significantly greater maximal knee extension on POD 1 compared with the control group. No difference in maximal flexion was found. This difference in extension may partially be explained by the use of an ACB. One benefit of ACB is greater quadriceps strength and fewer near-fall events when compared with FNB.15,19
Our results corroborate the findings of similar studies. A randomized controlled trial comparing a multimodal analgesic regimen with a periarticular injection without a postoperative ACB to a hydromorphone PCA revealed a significant decrease in opioid use in the multimodal analgesic group.27 Along with lower opioid requirements, the multimodal analgesic group had lower visual analog scale pain scores, fewer AEs, faster progression to physical therapy milestones, and higher satisfaction.27 Recent guidelines from the French Society of Anaesthesia and Intensive Care Medicine recommend against the use of gabapentin as a method of postoperative pain control. However, this specifically refers to the preoperative administration of gabapentin. This same set of guidelines later cites a high level of evidence suggesting patients undergoing arthroplasty benefit more from gabapentinoids.28 Multiple analgesic protocols that include gabapentin as a part of a multimodal approach have been shown to have positive results.13,29
In our study, patients receiving the multimodal analgesic regimen were significantly more likely to be discharged home rather than to postacute care facilities, which have been associated with increased rates of major complications, 30-day readmission, and 30-day reoperation.30,31 In addition, discharge to an inpatient rehabilitation or skilled nursing facility has not been found to result in higher functional outcomes, despite $3.2 billion spent yearly on rehabilitation services after primary TKA.32,33
A component of our described analgesic protocol included spinal anesthesia intraoperatively. The differences between groups regarding anesthesia type can be attributed to this protocol change. A significantly greater percentage of patients in the protocol group received spinal anesthesia, while more patients in the control group received general anesthesia. While patients who received spinal anesthesia may have enhanced analgesia in the immediate postoperative period, no differences in opioid outcomes were seen based on anesthesia type. Known benefits of intraoperative spinal anesthesia include decreased perioperative blood loss and a smaller decrease in hemoglobin postoperatively, as well as lower rates of in-hospital complications, including pulmonary embolism, pneumonia, cerebrovascular events, and acute renal failure.34
Limitations
A number of limitations of this study should be noted. One was a protocol change regarding length of stay, which occurred during the study period and resulted in a significantly shorter length of stay in the protocol group. As a result, opioid use data were analyzed only through midnight at the end of POD 1. Patients who were discharged on POD 1 did not have opioid use data available for the full duration of the first POD, which may exaggerate the decrease in opioid requirements, as opioids used after discharge but prior to midnight on POD 1 were not recorded. However, opioids taken at home are oral with a low MME compared with IV opioids received by hospitalized patients in the control group. In addition, if taken as prescribed, patients at home would only have enough time to take a few doses of opioids prior to the midnight cutoff. We do not believe this difference in time of opioid use meaningfully affected the data. An additional limitation includes the variability between periarticular injections between surgeons. While the percentage of patients that received injections from surgeon 1 vs surgeon 2 were similar, it cannot be ruled out as a potential confounding factor. Other limitations include a lack of pain scores to compare subjective pain ratings, the retrospective nature of the study, and a largely homogenous male VA population.
Conclusions
Ease of access to opioids is a risk factor for opioid abuse, which itself is a risk factor for subsequent heroin use.1,2 The CDC and AAOS have thus published recommendations regarding opioid prescribing practices to decrease opioid use and abuse.5,6 Our described protocol, which aligns with these recommendations, resulted in a significant decrease in IV opioid requirement, total opioid requirement, and lower rates of opioid prescriptions provided at the first postoperative visit. These promising findings demonstrate a lower percentage of patients on long-term opioids after TKA and a significantly decreased cumulative opioid exposure.
Ease of access to opioids in the perioperative period is a risk factor for opioid misuse and has been identified as a strong risk factor for heroin use.1,2 Three-quarters of today’s heroin users were introduced to opioids through prescription medications.2 The United States accounts for about 80% of the global opioid supply consumption, and deaths from opioid overdose are increasing: 70,630 deaths in 2019 alone.3,4
The Centers for Disease Control and Prevention (CDC) has called for changes in opioid prescribing. The American Academy of Orthopaedic Surgeons (AAOS) also has published an information statement with strategies to decrease opioid misuse and abuse.5,6 Arthroplasty surgeons have recently focused on decreasing use of opioids in total knee arthroplasty (TKA), a procedure traditionally associated with high levels of opioid consumption and historical reliance on opioid monotherapy for postoperative analgesia.7,8 From a clinical perspective, prolonged postoperative opioid use contributes to poorer surgical outcomes due to increased risk of complications, including stiffness, infection, and revision TKA.9
Multimodal pain regimens are increasingly being used to control postoperative pain as data supports their efficacy.10,11 Previous studies have found that simultaneous modulation of multiple pain pathways decreases narcotics consumption and improves patient outcomes.12,13 Along with other adjuvant therapies, peripheral nerve blocks, such as adductor canal block (ACB) and femoral nerve block (FNB), have been used to decrease postoperative pain.14 Studies have shown that ACB has fewer complications and shorter functional recovery times compared with FNB.15,16 The distribution of the ACB excludes the femoral nerve, thus preserving greater quadriceps strength while providing equivalent levels of analgesia compared with FNB.15,17,18 The ACB has shown decreased near-fall events and improved balance scores in the immediate postoperative period.19
Our study analyzed opioid consumption patterns of TKA patients from a US Department of Veterans Affairs (VA) medical center before and after the institution of a multimodal analgesic protocol using ACB. The primary purpose of this study was to determine whether a protocol that included intraoperative spinal anesthesia with a postoperative multimodal analgesic regimen and ACB was associated with a decreased postoperative opioid requirement when compared with patients who received intraoperative general anesthesia and a traditional opioid regimen. Secondary outcomes included the effect of opioid consumption on range of motion on postoperative day (POD) 1 and number of opioid prescriptions written at the first postoperative clinic visit.
Methods
Approval for the study was obtained from the institutional review board at the Dayton Veterans Affairs Medical Center (DVAMC) in Ohio. A retrospective chart review was performed to collect data from all patients undergoing TKA at DVAMC from June 1, 2011, through December 31, 2015. Exclusion criteria included multiple surgeries in the study time frame, documented chronic pain, allergy to local anesthetics, daily preoperative use of opioids, and incomplete data in the health record.
All surgeries were performed by 2 staff arthroplasty surgeons at a single VAMC. All patients attended a preoperative visit where a history, physical, and anesthesia evaluation were performed, and watched an educational video detailing surgical indications and postoperative rehabilitation. All surgeries were performed with tourniquets and a periarticular injection was performed at the conclusion of each case. Surgeon 1 treatment of choice was 10 mL 0.5% bupivacaine, whereas surgeon 2 performed a posterior capsular injection of 30 mL 0.25% bupivacaine and a periarticular injection of 30 mg ketorolac in 10 mL 0.25% bupivacaine with epinephrine.
Prior to August 2014, general endotracheal anesthesia was used intraoperatively. A patient-controlled analgesia (PCA) pump of morphine or hydromorphone and additional oral oxycodone or hydrocodone was used for postoperative pain. PCA pumps were patient dependent. In the control group, 245 patients received the morphine PCA while 61 received the hydromorphone PCA. Morphine PCA dosing consisted of 1-mg doses every 10 minutes with potential baseline infusion rates of 0.5 to 1.0 mg/h and a 4-hour limit of 20 mg. Hydromorphone PCA dosing consisted of 0.2 to 0.4-mg doses with a potential continuous dose of 0.2 to 0.4 mg/h and a 4-hour limit of 4 mg.
In August 2014, a new analgesic protocol was adopted for TKA consisting of intraoperative spinal anesthesia (0.75% bupivacaine) with IV sedation (propofol), a postoperative multimodal analgesic regimen, an ACB performed in the postanesthesia care unit (PACU), and opioids as needed (protocol group). The ACB catheter was a 0.5% ropivo caine hydrochloride injection. It was attached to a local anesthetic fixed flow rate pump that administers 0.5% ropivacaine without epinephrine at 8 mL/h and was removed on POD 5 by the patient. The multimodal medication regimen included IV ketorolac 15 mg every 6 hours for 3 doses, gabapentin 300 mg every 8 hours, acetaminophen 975 mg every 8 hours, meloxicam 7.5 mg daily, tramadol 50 mg every 6 hours, oxycodone 5 mg 1 to 2 tabs every 4 hours as needed, and IV hydromorphone 0.5 mg every 4 hours as needed for breakthrough pain.
Preoperative demographic characteristics were collected (Table 1). Data on all IV and oral opioid requirements were collected for both groups, converted to morphine milligram equivalents (MME), and a total morphine equivalent dose (MED) was calculated.20,21
In April 2015, a separate protocol change occurred at the DVAMC with the goal of discharge on POD 1. To standardize outcomes before and after this change, data collection regarding opioid requirements was concluded at midnight on POD 1. If a patient was discharged before midnight on POD 1, opioid requirement through the time of discharge was collected. All surgeries were performed in the morning to early afternoon; however, specific surgical times were not collected. Patients were also evaluated by a physical therapist on POD 0, and maximal knee flexion and extension were measured on POD 1. Patients were discharged with prescriptions for oxycodone/acetaminophen and tramadol and were seen 3 weeks later for their first postoperative visit. Opioid refills at the first postoperative visit were recorded. All statistical analyses were performed in SAS 9.4 with significance set to α = 0.05. Between-groups differences in preoperative and perioperative characteristics as well as postoperative outcomes were analyzed using independent samples t tests for continuous variables and Fisher exact tests for dichotomous discrete variables. Where groups differed for a pre- or perioperative variable, linear mixed models analysis was used to determine whether IV, oral, and total MEDs were significantly affected by the interaction between the pre- or perioperative variable with analgesia group. For refills at the postoperative visit, the effects of pre- or perioperative differences were tested using χ2 tests. Effect sizes for outcome variables were estimated using Cohen d and probability of superiority (Δ) for continuous variables, and relative risk (RR) in the case of discrete variables.22
Results
During the study period from June 1, 2011, through December 31, 2015, 533 eligible TKAs were performed, 306 in the control group and 227 in the protocol group. The groups had similar sex distribution; body mass index; knee range of motion; diagnoses of diabetes mellitus, coronary artery disease, and chronic kidney disease; and history of deep vein thrombosis (DVT) or pulmonary embolism (P ≥ .05). The protocol group was significantly older (P = .04) and had a significantly higher rate of chronic obstructive pulmonary disease (COPD) (P = .002). There were no significant differences between number of procedures performed by surgeon (P = .48) or total tourniquet time (P = .13) (Table 2). Mean (SD) length of stay was significantly greater in the control group compared with the protocol group (2.5 [1.3] vs 1.4 [0.7] days, P < .001).
Figure 1 shows the distributions of each type of opioid used. Compared with the control group, the protocol group had a significantly lower mean (SD) IV opioid use: 178.2 (98.0) MED vs 12.0 (24.6) MED (P < .001; d = 2.19; Δ = 0.94) and mean (SD) total opioid use: 241.7 (120.1) MED vs 74.8 (42.7) MED (P < .001; d = 1.76; Δ = 0.89). Mean (SD) oral opioid use did not differ between groups (control, 63.6 [45.4] MED; protocol, 62.9 [31.4] MED; P = .85; d = 0.02; Δ = 0.51). A significantly lower percentage of patients in the protocol group received additional opioids at the 3-week follow-up when compared to the control group: 46.7% vs 61.3%, respectively (P < .001; RR, 0.76; 95% CI, 0.65-0.90).
There were no significant differences in postoperative mean (SD) maximum knee flexion (control, 67.2 [15.7]°; protocol, 67.8 [19.2]°; P = .72; d = 0.03; Δ = 0.51) or mean (SD) total flexion/extension arc (control, 66.2 [15.9]°; protocol, 67.9 [19.4]°; P = .32; d = 0.10; Δ = 0.53). Mean (SD) postoperative maximum knee extension was significantly higher in the protocol group compared with the control group (-0.1 [2.1]° vs 1.0 [3.7]°; P < .001; d = 0.35; Δ = 0.60). More patients in the protocol group (92.5%) were discharged to home compared with the control group (86.6%) (P = .02; RR, 1.07; 95% CI, 1.01-1.13).
Because age and rates of COPD differed between groups, sensitivity analyses were conducted to determine whether these variables influenced postoperative opioid use. The relationship between age and group was significant for IV (P < .001) and total opioid use (P < .001). Younger patients received higher MED doses than older patients within the control group, while dosages were fairly consistent regardless of age in the protocol group (Figure 2). There was no significance in age interaction effect with regard to oral opioids (P = .83) nor opioid refills at 3-week follow-up (P = .24).
The sensitivity analysis for COPD found that a diagnosis of COPD did not significantly influence utilization of IV opioids (P = .10), or total opioids (P = .68). There was a significant interaction effect for oral opioids (Figure 3). Patients in the control group with COPD required significantly higher mean (SD) oral opioids than patients without COPD (91.5 [123.9] MED and 62.0 [36.0] MED, respectively; P = .03). In the control group, the χ2 test was significant regarding opioid prescription refills at the 3-week visit (P = .004) with 62.4% of patients with COPD requiring refills vs 44.4% without COPD (P = .004). There was no difference in refills in the protocol group (46.4% vs 48.4%).
Finally, 2-sided independent samples t test evaluated total MED use between the 2 surgeons. There was no difference in total MED per patient for the surgeons. In the control group, mean (SD) total MED for surgeon 1 was 232.9 (118.7) MED vs 252.8 (121.5) MED for surgeon 2 (P = .18). In the protocol group, the mean (SD) total MED was 72.5 (43.2) and 77.4 (42.1) for surgeon 1 and surgeon 2, respectively (P = .39).
Discussion
Coordinated efforts with major medical organizations are being made to decrease opioid prescriptions and exposure.5,6 To our knowledge, no study has quantified a decrease in opioid requirement in a VA population after implementation of a protocol that includes intraoperative spinal anesthesia and a postoperative multimodal analgesic regimen including ACB after TKA. The analgesic protocol described in this study aligns with recommendations from both the CDC and the AAOS to decrease opioid use and misuse by maximizing nonopioid medications and limiting the size and number of opioid prescriptions. However, public and medical opinion of opioids as well as prescribing practices have changed over time with a trend toward lower opioid use. The interventions, as part of the described protocol, are a result of these changes and attempt to minimize opioid use while maximizing postoperative analgesia.
Our data showed a significant decrease in total opioid use through POD 1, IV opioid use, and opioid prescriptions provided at the first postoperative visit. The protocol group used only 6.7% of the IV opioids and 30.9% of the total opioids that were used by the control group. The substantial difference in IV opioid requirement, 166.2 MED, is equivalent to 8 mg of IV hydromorphone or 55 mg of IV morphine. The difference in total opioid requirement was similar at 166.9 MED, equivalent to 111 mg of oral oxycodone.
Decreasing opioid use has the additional benefit of improving outcomes, as higher doses of opioids have been associated with increased length of stay, greater rates of DVT, and postoperative infection.23 These complications occurred in a stepwise manner, suggesting a dose-response gradient that makes the sizable decrease noted in our data of greater relevance.23 While the adverse effects (AEs) of opioids are well known, there are limited data on opioid dosing and its effect on perioperative outcomes.23
A significant decrease in the percentage of patients receiving an opioid prescription at the first postoperative visit suggests a decrease in the number of patients on prolonged opioids after TKA with implementation of modern analgesic modalities. The duration of postoperative opioid use has been found to be the strongest predictor of misuse, and each postoperative refill increases the probability of misuse by 44%.24 In addition, opioid use for > 3 months after TKA is associated with increased risk of periprosthetic infection, increased overall revision rate, and stiffness at 1 year postoperatively.9 While not entirely under the control of the surgeon, measures to decrease the number of postoperative opioid refills may lead to a decrease in opioid misuse.
In the control group, older patients tended to receive less opioids. This is likely due to physiologic changes in opioid metabolism associated with aging, including decreased renal and hepatic opioid metabolism and alterations in overall body composition that increase relative potency and duration of action of opioids in a geriatric population.25,26 No difference in opioid use by age was found for the protocol group.
Patients in the protocol group demonstrated significantly greater maximal knee extension on POD 1 compared with the control group. No difference in maximal flexion was found. This difference in extension may partially be explained by the use of an ACB. One benefit of ACB is greater quadriceps strength and fewer near-fall events when compared with FNB.15,19
Our results corroborate the findings of similar studies. A randomized controlled trial comparing a multimodal analgesic regimen with a periarticular injection without a postoperative ACB to a hydromorphone PCA revealed a significant decrease in opioid use in the multimodal analgesic group.27 Along with lower opioid requirements, the multimodal analgesic group had lower visual analog scale pain scores, fewer AEs, faster progression to physical therapy milestones, and higher satisfaction.27 Recent guidelines from the French Society of Anaesthesia and Intensive Care Medicine recommend against the use of gabapentin as a method of postoperative pain control. However, this specifically refers to the preoperative administration of gabapentin. This same set of guidelines later cites a high level of evidence suggesting patients undergoing arthroplasty benefit more from gabapentinoids.28 Multiple analgesic protocols that include gabapentin as a part of a multimodal approach have been shown to have positive results.13,29
In our study, patients receiving the multimodal analgesic regimen were significantly more likely to be discharged home rather than to postacute care facilities, which have been associated with increased rates of major complications, 30-day readmission, and 30-day reoperation.30,31 In addition, discharge to an inpatient rehabilitation or skilled nursing facility has not been found to result in higher functional outcomes, despite $3.2 billion spent yearly on rehabilitation services after primary TKA.32,33
A component of our described analgesic protocol included spinal anesthesia intraoperatively. The differences between groups regarding anesthesia type can be attributed to this protocol change. A significantly greater percentage of patients in the protocol group received spinal anesthesia, while more patients in the control group received general anesthesia. While patients who received spinal anesthesia may have enhanced analgesia in the immediate postoperative period, no differences in opioid outcomes were seen based on anesthesia type. Known benefits of intraoperative spinal anesthesia include decreased perioperative blood loss and a smaller decrease in hemoglobin postoperatively, as well as lower rates of in-hospital complications, including pulmonary embolism, pneumonia, cerebrovascular events, and acute renal failure.34
Limitations
A number of limitations of this study should be noted. One was a protocol change regarding length of stay, which occurred during the study period and resulted in a significantly shorter length of stay in the protocol group. As a result, opioid use data were analyzed only through midnight at the end of POD 1. Patients who were discharged on POD 1 did not have opioid use data available for the full duration of the first POD, which may exaggerate the decrease in opioid requirements, as opioids used after discharge but prior to midnight on POD 1 were not recorded. However, opioids taken at home are oral with a low MME compared with IV opioids received by hospitalized patients in the control group. In addition, if taken as prescribed, patients at home would only have enough time to take a few doses of opioids prior to the midnight cutoff. We do not believe this difference in time of opioid use meaningfully affected the data. An additional limitation includes the variability between periarticular injections between surgeons. While the percentage of patients that received injections from surgeon 1 vs surgeon 2 were similar, it cannot be ruled out as a potential confounding factor. Other limitations include a lack of pain scores to compare subjective pain ratings, the retrospective nature of the study, and a largely homogenous male VA population.
Conclusions
Ease of access to opioids is a risk factor for opioid abuse, which itself is a risk factor for subsequent heroin use.1,2 The CDC and AAOS have thus published recommendations regarding opioid prescribing practices to decrease opioid use and abuse.5,6 Our described protocol, which aligns with these recommendations, resulted in a significant decrease in IV opioid requirement, total opioid requirement, and lower rates of opioid prescriptions provided at the first postoperative visit. These promising findings demonstrate a lower percentage of patients on long-term opioids after TKA and a significantly decreased cumulative opioid exposure.
1. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44. doi:10.1016/j.drugpo.2011.05.014
2. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi:10.1016/j.drugalcdep.2013.01.007
3. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(suppl 2):S63-S88.
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349-358. Published 2018 Mar 30. doi:10.15585/mmwr.mm6712a1
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. American Academy of Orthopaedic Surgeons. Information statement: opioid use, misuse, and abuse in orthopaedic practice. Published October 2015. Accessed November 12, 2021. https://aaos.org/globalassets/about /bylaws-library/information-statements/1045-opioid-use -misuse-and-abuse-in-practice.pdf
7. Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty. 2017;32(8):2395-2398. doi:10.1016/j.arth.2017.02.060
8. Gerner P, Poeran J, Cozowicz C, Mörwald EE, Zubizarreta N, Mazumdar M, Memtsoudis SG, Multimodal pain management in total hip and knee arthroplasty: trends over the last 10 years. Abstract presented at: American Society of Anesthesiologists Annual Meeting; October 21, 2017; Boston, MA.
9. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
10. Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
11. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.doi:10.1001/jamasurg.2017.0898
12. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthoplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
13. Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty. 2017;32(9S):S69-S73. doi:10.1016/j.arth.2017.05.002
14. Ardon AE, Clendenen SR, Porter SB, Robards CB, Greengrass RA. Opioid consumption in total knee arthroplasty patients: a retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J Clin Anesth. 2016;31:19-26. doi:10.1016/j.jclinane.2015.12.014
15. Li D, Ma GG. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2614-2619. doi:10.1007/s00167-015-3874-3
16. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925-933. doi:10.1007/s00264-015-2998-x
17. Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221-226.
18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540-550. doi:10.1097/ALN.0000000000000119
19. Thacher RR, Hickernell TR, Grosso MJ, et al. Decreased risk of knee buckling with adductor canal block versus femoral nerve block in total knee arthroplasty: a retrospective cohort study. Arthroplasty Today. 2017;3(4):281-285. Published 2017 Apr 15. doi:10.1016/j.artd.2017.02.008
20. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain. 2008;24(6):521-527. doi:10.1097/AJP.0b013e318169d03b
21. Kishner S. Opioid equivalents and conversions: overview. Published January 29, 2018. Accessed November 12, 2021. https://emedicine.medscape.com/article/2138678 -overview#a1
22. Ruscio J, Mullen T. Confidence intervals for the probability of superiority effect size measure and the area under a receiver operating characteristic curve. Multivariate Behav Res. 2012;47(2):201-223. doi:10.1080/00273171.2012.658329
23. Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic orthopedic surgery. Pain. 2017;158(12):2422-2430. doi:10.1097/j.pain.0000000000001047
24. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
25. Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37(1):17- 40. doi:10.2165/00003088-199937010-00002
26. Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396-1430. doi:10.1592/phco.2005.25.10.1396
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329- 334. doi:10.1016/j.arth.2013.06.005
28. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi:10.1016/j.accpm.2019.02.011
29. Han C, Li XD, Jiang HQ, Ma JX, Ma XL. The use of gabapentin in the management of postoperative pain after total knee arthroplasty: A PRISMA-compliant metaanalysis of randomized controlled trials [published correction appears in Medicine (Baltimore). 2016 Jul 18;95(28):e0916]. Medicine (Baltimore). 2016;95(23):e3883. doi:10.1097/MD.0000000000003883
30. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day postdischarge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32(9S):S113-S118. doi:10.1016/j.arth.2017.01.039
31. Pelt CE, Gililland JM, Erickson JA, Trimble DE, Anderson MB, Peters CL. Improving value in total joint arthroplasty: a comprehensive patient education and management program decreases discharge to post-acute care facilities and post-operative complications. J Arthroplasty. 2018;33(1):14-18. doi:10.1016/j.arth.2017.08.003
32. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33(6):1663-1667. doi:10.1016/j.arth.2017.12.033
33. Lavernia CJ, D’Apuzzo MR, Hernandez VH, Lee DJ, Rossi MD. Postdischarge costs in arthroplasty surgery. J Arthroplasty. 2006;21(6 Suppl 2):144-150. doi:10.1016/j.arth.2006.05.003
1. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44. doi:10.1016/j.drugpo.2011.05.014
2. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi:10.1016/j.drugalcdep.2013.01.007
3. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(suppl 2):S63-S88.
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349-358. Published 2018 Mar 30. doi:10.15585/mmwr.mm6712a1
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. American Academy of Orthopaedic Surgeons. Information statement: opioid use, misuse, and abuse in orthopaedic practice. Published October 2015. Accessed November 12, 2021. https://aaos.org/globalassets/about /bylaws-library/information-statements/1045-opioid-use -misuse-and-abuse-in-practice.pdf
7. Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty. 2017;32(8):2395-2398. doi:10.1016/j.arth.2017.02.060
8. Gerner P, Poeran J, Cozowicz C, Mörwald EE, Zubizarreta N, Mazumdar M, Memtsoudis SG, Multimodal pain management in total hip and knee arthroplasty: trends over the last 10 years. Abstract presented at: American Society of Anesthesiologists Annual Meeting; October 21, 2017; Boston, MA.
9. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
10. Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
11. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.doi:10.1001/jamasurg.2017.0898
12. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthoplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
13. Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty. 2017;32(9S):S69-S73. doi:10.1016/j.arth.2017.05.002
14. Ardon AE, Clendenen SR, Porter SB, Robards CB, Greengrass RA. Opioid consumption in total knee arthroplasty patients: a retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J Clin Anesth. 2016;31:19-26. doi:10.1016/j.jclinane.2015.12.014
15. Li D, Ma GG. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2614-2619. doi:10.1007/s00167-015-3874-3
16. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925-933. doi:10.1007/s00264-015-2998-x
17. Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221-226.
18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540-550. doi:10.1097/ALN.0000000000000119
19. Thacher RR, Hickernell TR, Grosso MJ, et al. Decreased risk of knee buckling with adductor canal block versus femoral nerve block in total knee arthroplasty: a retrospective cohort study. Arthroplasty Today. 2017;3(4):281-285. Published 2017 Apr 15. doi:10.1016/j.artd.2017.02.008
20. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain. 2008;24(6):521-527. doi:10.1097/AJP.0b013e318169d03b
21. Kishner S. Opioid equivalents and conversions: overview. Published January 29, 2018. Accessed November 12, 2021. https://emedicine.medscape.com/article/2138678 -overview#a1
22. Ruscio J, Mullen T. Confidence intervals for the probability of superiority effect size measure and the area under a receiver operating characteristic curve. Multivariate Behav Res. 2012;47(2):201-223. doi:10.1080/00273171.2012.658329
23. Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic orthopedic surgery. Pain. 2017;158(12):2422-2430. doi:10.1097/j.pain.0000000000001047
24. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
25. Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37(1):17- 40. doi:10.2165/00003088-199937010-00002
26. Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396-1430. doi:10.1592/phco.2005.25.10.1396
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329- 334. doi:10.1016/j.arth.2013.06.005
28. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi:10.1016/j.accpm.2019.02.011
29. Han C, Li XD, Jiang HQ, Ma JX, Ma XL. The use of gabapentin in the management of postoperative pain after total knee arthroplasty: A PRISMA-compliant metaanalysis of randomized controlled trials [published correction appears in Medicine (Baltimore). 2016 Jul 18;95(28):e0916]. Medicine (Baltimore). 2016;95(23):e3883. doi:10.1097/MD.0000000000003883
30. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day postdischarge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32(9S):S113-S118. doi:10.1016/j.arth.2017.01.039
31. Pelt CE, Gililland JM, Erickson JA, Trimble DE, Anderson MB, Peters CL. Improving value in total joint arthroplasty: a comprehensive patient education and management program decreases discharge to post-acute care facilities and post-operative complications. J Arthroplasty. 2018;33(1):14-18. doi:10.1016/j.arth.2017.08.003
32. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33(6):1663-1667. doi:10.1016/j.arth.2017.12.033
33. Lavernia CJ, D’Apuzzo MR, Hernandez VH, Lee DJ, Rossi MD. Postdischarge costs in arthroplasty surgery. J Arthroplasty. 2006;21(6 Suppl 2):144-150. doi:10.1016/j.arth.2006.05.003
Short-acting opioids needed for withdrawal in U.S. hospitals, say experts
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Neurologist guilty of overprescribing thousands of doses of painkillers
Ohio doctor convicted of prescribing unnecessary controlled substances, fraud
A federal jury found William R. Bauer, 84, of Port Clinton, Ohio, guilty of prescribing powerful controlled substances, including opioids, to patients without medical necessity and outside the usual course of medical practice.
Dr. Bauer, a neurologist with over 50 years of experience, was convicted of 76 counts of distribution of controlled substances and 25 counts of healthcare fraud. According to television station WTOL, a federal indictment from 2019 listed 270 charges against the physician.
Federal officials claim that through his practice in Bellevue, Ohio, Dr. Bauer repeatedly prescribed controlled substances, including oxycodone, fentanyl, morphine, and tramadol, outside the usual course of professional practice and without legitimate medical purpose. The charges focused on 14 of his patients, to whom he prescribed high doses of opioids and other controlled substances without medical necessity. He also prescribed dangerous drug combinations. He ignored patients’ signs of addiction and abuse, such as early requests for refills, claims that medications had been lost, and claims that family members were stealing pills.
Dr. Bauer was also convicted of healthcare fraud for regularly administering epidural injections and trigger-point injections without medical necessity. Because these injections failed to meet the procedural requirements, they were rendered ineffective and were fraudulently billed to insurers. Dr. Bauer’s illegal prescriptions resulted in insurers paying for these medically unnecessary controlled substances.
Evidence at trial indicated that between January 2007 and August 16, 2019, Dr. Bauer prescribed controlled substances outside the usual course of medical practice and for illegitimate medical purposes. Insurers paid for these medically unnecessary controlled substances as well.
He will be sentenced at a later date.
Lab pays $1.2 million to resolve allegations of false claims for drug testing
Bluewater Toxicology, LLC, a clinical laboratory in Mount Washington, Ky., has agreed to pay $1.2 million to resolve civil allegations that it violated the False Claims Act.
The U.S. Department of Justice alleged three issues relating to claims for urine drug testing services that Bluewater submitted to Medicare, Kentucky Medicaid, Indiana Medicaid, TRICARE, and CHAMPVA. First, Bluewater submitted claims in which it misrepresented the number of drug classes it tested. Bluewater claimed it conducted definitive urine drug tests of 22 or more drug classes. In truth, Bluewater tested for fewer than 22 drug classes and secured reimbursement for drug tests that it did not conduct.
Second, Bluewater submitted certain claims without sufficient documentation to support the physician’s intent to order the test that was billed. In this way Bluewater obtained further unwarranted reimbursements.
Finally, Bluewater billed Medicare for specimen validity testing, a quality control process used to analyze a urine specimen to ensure that it has not been diluted or adulterated. Since January 2014, Medicare’s guidance has stated that specimen validity testing should not be separately billed to Medicare, but Bluewater did so anyway.
Home care company owner pays $1 million in Medicare fraud restitution
Richard Wennerberg, 72, of Grantham, N.H., pleaded guilty and was sentenced to two counts of class B felony Medicaid fraud, according to the New Hampshire Department of Justice.
Mr. Wennerberg is the owner of Alternative Care @ Home, LLC, a company licensed to provide in-home personal care services to Medicaid beneficiaries. He also pleaded guilty to a third charge of Medicaid fraud, through which Alternative Care @ Home, LLC, will be excluded from future participation in federal healthcare programs.
According to New Hampshire officials, Mr. Wennerberg submitted claims for reimbursement for in-home, personal care services that were never provided. Wennerberg billed Medicaid up to the maximum hours allowed under certain clients’ service authorizations, knowing that his employees did not provide care for all of those hours. He would use the difference to reimburse some caregivers for mileage.
Mr. Wennerberg will serve 1 year in state prison and will pay $1 million in restitution.
North Carolina wins two “Operation Root Canal” settlements
North Carolina Attorney General Josh Stein announced two separate civil settlements with ProHealth Dental Inc and Henry W. Davis, Jr, DDS, as part of Operation Root Canal, an ongoing effort to find and stop healthcare fraud among dental practitioners. The settlements, totaling $75,000, resolve allegations of the submission of false claims to the North Carolina Medicaid program.
In Operation Root Canal, the state Medicaid investigations department reviews billing practices for a wide variety of dental services, including dental cleanings, use of nitrous oxide, repetitive restorations on the same tooth, palliative care, and upcoding of patient examinations. In total, the operation has netted more than $7 million for the state.
The recent settlement relates to a prior criminal plea the attorney general’s Medicaid Investigations Division obtained involving Mr. Christian Ekberg, of Maryland, who was sentenced to 18 months in prison for healthcare fraud and was ordered to pay $173,870.12 to the North Carolina Medicaid Fund in restitution. Ekberg was an officer and minority shareholder of ProHealth Dental, a company that entered into a practice management agreement with Henry W. Davis, Jr, DDS., a North Carolina dentist and Medicaid practitioner who provided dental services to patients living in skilled nursing facilities throughout North Carolina. ProHealth Dental would provide professional management services to Dr. Davis, including submitting Medicaid claims. The company submitted claims for dental services that Dr. Davis did not perform on Medicaid recipients.
A version of this article first appeared on Medscape.com.
Ohio doctor convicted of prescribing unnecessary controlled substances, fraud
Ohio doctor convicted of prescribing unnecessary controlled substances, fraud
A federal jury found William R. Bauer, 84, of Port Clinton, Ohio, guilty of prescribing powerful controlled substances, including opioids, to patients without medical necessity and outside the usual course of medical practice.
Dr. Bauer, a neurologist with over 50 years of experience, was convicted of 76 counts of distribution of controlled substances and 25 counts of healthcare fraud. According to television station WTOL, a federal indictment from 2019 listed 270 charges against the physician.
Federal officials claim that through his practice in Bellevue, Ohio, Dr. Bauer repeatedly prescribed controlled substances, including oxycodone, fentanyl, morphine, and tramadol, outside the usual course of professional practice and without legitimate medical purpose. The charges focused on 14 of his patients, to whom he prescribed high doses of opioids and other controlled substances without medical necessity. He also prescribed dangerous drug combinations. He ignored patients’ signs of addiction and abuse, such as early requests for refills, claims that medications had been lost, and claims that family members were stealing pills.
Dr. Bauer was also convicted of healthcare fraud for regularly administering epidural injections and trigger-point injections without medical necessity. Because these injections failed to meet the procedural requirements, they were rendered ineffective and were fraudulently billed to insurers. Dr. Bauer’s illegal prescriptions resulted in insurers paying for these medically unnecessary controlled substances.
Evidence at trial indicated that between January 2007 and August 16, 2019, Dr. Bauer prescribed controlled substances outside the usual course of medical practice and for illegitimate medical purposes. Insurers paid for these medically unnecessary controlled substances as well.
He will be sentenced at a later date.
Lab pays $1.2 million to resolve allegations of false claims for drug testing
Bluewater Toxicology, LLC, a clinical laboratory in Mount Washington, Ky., has agreed to pay $1.2 million to resolve civil allegations that it violated the False Claims Act.
The U.S. Department of Justice alleged three issues relating to claims for urine drug testing services that Bluewater submitted to Medicare, Kentucky Medicaid, Indiana Medicaid, TRICARE, and CHAMPVA. First, Bluewater submitted claims in which it misrepresented the number of drug classes it tested. Bluewater claimed it conducted definitive urine drug tests of 22 or more drug classes. In truth, Bluewater tested for fewer than 22 drug classes and secured reimbursement for drug tests that it did not conduct.
Second, Bluewater submitted certain claims without sufficient documentation to support the physician’s intent to order the test that was billed. In this way Bluewater obtained further unwarranted reimbursements.
Finally, Bluewater billed Medicare for specimen validity testing, a quality control process used to analyze a urine specimen to ensure that it has not been diluted or adulterated. Since January 2014, Medicare’s guidance has stated that specimen validity testing should not be separately billed to Medicare, but Bluewater did so anyway.
Home care company owner pays $1 million in Medicare fraud restitution
Richard Wennerberg, 72, of Grantham, N.H., pleaded guilty and was sentenced to two counts of class B felony Medicaid fraud, according to the New Hampshire Department of Justice.
Mr. Wennerberg is the owner of Alternative Care @ Home, LLC, a company licensed to provide in-home personal care services to Medicaid beneficiaries. He also pleaded guilty to a third charge of Medicaid fraud, through which Alternative Care @ Home, LLC, will be excluded from future participation in federal healthcare programs.
According to New Hampshire officials, Mr. Wennerberg submitted claims for reimbursement for in-home, personal care services that were never provided. Wennerberg billed Medicaid up to the maximum hours allowed under certain clients’ service authorizations, knowing that his employees did not provide care for all of those hours. He would use the difference to reimburse some caregivers for mileage.
Mr. Wennerberg will serve 1 year in state prison and will pay $1 million in restitution.
North Carolina wins two “Operation Root Canal” settlements
North Carolina Attorney General Josh Stein announced two separate civil settlements with ProHealth Dental Inc and Henry W. Davis, Jr, DDS, as part of Operation Root Canal, an ongoing effort to find and stop healthcare fraud among dental practitioners. The settlements, totaling $75,000, resolve allegations of the submission of false claims to the North Carolina Medicaid program.
In Operation Root Canal, the state Medicaid investigations department reviews billing practices for a wide variety of dental services, including dental cleanings, use of nitrous oxide, repetitive restorations on the same tooth, palliative care, and upcoding of patient examinations. In total, the operation has netted more than $7 million for the state.
The recent settlement relates to a prior criminal plea the attorney general’s Medicaid Investigations Division obtained involving Mr. Christian Ekberg, of Maryland, who was sentenced to 18 months in prison for healthcare fraud and was ordered to pay $173,870.12 to the North Carolina Medicaid Fund in restitution. Ekberg was an officer and minority shareholder of ProHealth Dental, a company that entered into a practice management agreement with Henry W. Davis, Jr, DDS., a North Carolina dentist and Medicaid practitioner who provided dental services to patients living in skilled nursing facilities throughout North Carolina. ProHealth Dental would provide professional management services to Dr. Davis, including submitting Medicaid claims. The company submitted claims for dental services that Dr. Davis did not perform on Medicaid recipients.
A version of this article first appeared on Medscape.com.
A federal jury found William R. Bauer, 84, of Port Clinton, Ohio, guilty of prescribing powerful controlled substances, including opioids, to patients without medical necessity and outside the usual course of medical practice.
Dr. Bauer, a neurologist with over 50 years of experience, was convicted of 76 counts of distribution of controlled substances and 25 counts of healthcare fraud. According to television station WTOL, a federal indictment from 2019 listed 270 charges against the physician.
Federal officials claim that through his practice in Bellevue, Ohio, Dr. Bauer repeatedly prescribed controlled substances, including oxycodone, fentanyl, morphine, and tramadol, outside the usual course of professional practice and without legitimate medical purpose. The charges focused on 14 of his patients, to whom he prescribed high doses of opioids and other controlled substances without medical necessity. He also prescribed dangerous drug combinations. He ignored patients’ signs of addiction and abuse, such as early requests for refills, claims that medications had been lost, and claims that family members were stealing pills.
Dr. Bauer was also convicted of healthcare fraud for regularly administering epidural injections and trigger-point injections without medical necessity. Because these injections failed to meet the procedural requirements, they were rendered ineffective and were fraudulently billed to insurers. Dr. Bauer’s illegal prescriptions resulted in insurers paying for these medically unnecessary controlled substances.
Evidence at trial indicated that between January 2007 and August 16, 2019, Dr. Bauer prescribed controlled substances outside the usual course of medical practice and for illegitimate medical purposes. Insurers paid for these medically unnecessary controlled substances as well.
He will be sentenced at a later date.
Lab pays $1.2 million to resolve allegations of false claims for drug testing
Bluewater Toxicology, LLC, a clinical laboratory in Mount Washington, Ky., has agreed to pay $1.2 million to resolve civil allegations that it violated the False Claims Act.
The U.S. Department of Justice alleged three issues relating to claims for urine drug testing services that Bluewater submitted to Medicare, Kentucky Medicaid, Indiana Medicaid, TRICARE, and CHAMPVA. First, Bluewater submitted claims in which it misrepresented the number of drug classes it tested. Bluewater claimed it conducted definitive urine drug tests of 22 or more drug classes. In truth, Bluewater tested for fewer than 22 drug classes and secured reimbursement for drug tests that it did not conduct.
Second, Bluewater submitted certain claims without sufficient documentation to support the physician’s intent to order the test that was billed. In this way Bluewater obtained further unwarranted reimbursements.
Finally, Bluewater billed Medicare for specimen validity testing, a quality control process used to analyze a urine specimen to ensure that it has not been diluted or adulterated. Since January 2014, Medicare’s guidance has stated that specimen validity testing should not be separately billed to Medicare, but Bluewater did so anyway.
Home care company owner pays $1 million in Medicare fraud restitution
Richard Wennerberg, 72, of Grantham, N.H., pleaded guilty and was sentenced to two counts of class B felony Medicaid fraud, according to the New Hampshire Department of Justice.
Mr. Wennerberg is the owner of Alternative Care @ Home, LLC, a company licensed to provide in-home personal care services to Medicaid beneficiaries. He also pleaded guilty to a third charge of Medicaid fraud, through which Alternative Care @ Home, LLC, will be excluded from future participation in federal healthcare programs.
According to New Hampshire officials, Mr. Wennerberg submitted claims for reimbursement for in-home, personal care services that were never provided. Wennerberg billed Medicaid up to the maximum hours allowed under certain clients’ service authorizations, knowing that his employees did not provide care for all of those hours. He would use the difference to reimburse some caregivers for mileage.
Mr. Wennerberg will serve 1 year in state prison and will pay $1 million in restitution.
North Carolina wins two “Operation Root Canal” settlements
North Carolina Attorney General Josh Stein announced two separate civil settlements with ProHealth Dental Inc and Henry W. Davis, Jr, DDS, as part of Operation Root Canal, an ongoing effort to find and stop healthcare fraud among dental practitioners. The settlements, totaling $75,000, resolve allegations of the submission of false claims to the North Carolina Medicaid program.
In Operation Root Canal, the state Medicaid investigations department reviews billing practices for a wide variety of dental services, including dental cleanings, use of nitrous oxide, repetitive restorations on the same tooth, palliative care, and upcoding of patient examinations. In total, the operation has netted more than $7 million for the state.
The recent settlement relates to a prior criminal plea the attorney general’s Medicaid Investigations Division obtained involving Mr. Christian Ekberg, of Maryland, who was sentenced to 18 months in prison for healthcare fraud and was ordered to pay $173,870.12 to the North Carolina Medicaid Fund in restitution. Ekberg was an officer and minority shareholder of ProHealth Dental, a company that entered into a practice management agreement with Henry W. Davis, Jr, DDS., a North Carolina dentist and Medicaid practitioner who provided dental services to patients living in skilled nursing facilities throughout North Carolina. ProHealth Dental would provide professional management services to Dr. Davis, including submitting Medicaid claims. The company submitted claims for dental services that Dr. Davis did not perform on Medicaid recipients.
A version of this article first appeared on Medscape.com.