User login
A large proportion of migraine patients are not offered preventive treatment
, new research suggests. Investigators found that among patients with migraine who are eligible for preventive therapy, more than a third were not offered this option. In addition, fewer than 10% were currently taking preventive medication, and an additional 10% had discontinued preventive therapy.
“We confirmed that as of 2012 to 2013 – the years these data were collected from a large, comprehensive survey – gaps in care remained,” said study investigator Stephanie J. Nahas, MD, director of the headache medicine fellowship program, Thomas Jefferson University, Philadelphia. “In this preventive-eligible population, 35% reported never even being offered preventive medication.”
Furthermore, only 28% of patients taking preventive medication experienced a reduction in headache frequency to less than 4 days per month, which is a primary goal of treatment, said Dr. Nahas. Disease burden, as measured with scales of disability and affective comorbidities, remained substantial.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Lack of efficacy?
In 2019, the American Headache Society published a position statement recommending that preventive treatment be considered for patients who have migraine and four or more monthly headache days (MHDs), regardless of their level of associated disability. However, previous data suggest few patients who are eligible for preventive treatment receive it. In addition, many who have used preventive medications do not adhere to their regimens because of problems with tolerability, efficacy, or both.
To identify treatment gaps and characterize self-reported use of preventive medications for migraine, the investigators examined data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study, a web-based survey conducted in a representative U.S. sample from September 2012 through November 2013.
The survey identified and characterized patients who met modified criteria for migraine consistent with those in ICHD-3. The researchers classified respondents who had migraine and four or more MHDs as potentially eligible for migraine preventive treatment.
The investigators assessed the study population’s use of oral preventive medications, migraine-related disability and burden, willingness to take preventive treatment, and reasons for discontinuation.
Assessments included the Migraine Disability Assessment Questionnaire, the Patient Health Questionnaire–9 for depression, the Generalized Anxiety Disorder 7-Item Scale, the Migraine Specific Quality of Life questionnaire, and the Migraine Symptom Severity Scale.
In all, 16,789 respondents met criteria for migraine, and 6,579 (39.2%) reported having at least four MHDs. The median age of this subgroup that was eligible for preventive treatment was 40.3 years, and approximately 79% were women.
Only 9.8% of respondents who were eligible for preventive medications were currently using an oral preventive medication. Among those who had ever tried an oral preventive medication, 53.6% discontinued it. Efficacy for patients who used medications appeared to be inadequate. Among all current users of preventive treatment, 68.4% continued to have at least four MHDs.
The researchers assessed treatment eligibility among patients not taking preventive medication. Among respondents who had never used a preventive treatment, 35.7% were eligible to receive it. Among all users who had discontinued preventive medication, 61.0% were still eligible to receive it.
Attitudes toward injectables
Among respondents who had never used a preventive treatment, 64.3% had zero to three MHDs. The remaining 35.7% had 4-7, 8-14, or 15 or more MHDs. Among current users of preventive treatments, 68.4% had four or more MHDs. Among those who had discontinued preventive treatment, 61.0% had four or more MHDs.
Patients who have never used preventive medication “have substantial management gaps,” said Dr. Nahas. High proportions of these patients have moderate or severe disability (64.7%), depression (43%), and anxiety (39%). The rates of these outcomes are higher in users who discontinued treatment, likely because of confounding by indication, she added.
The prevalence of anxiety was similar between those who currently used, formerly used, or never used preventive medications. However, there were differences between never-users and current or former users with respect to moderate to severe depression (never-users, 43%; current users, 49.4%; discontinued users, 46.5%) and moderate to severe disability (never-users, 64.7%; current users, 80.4%; discontinued users, 78.9%).
In all, 44.6% of those who discontinued preventive therapy reported safety and tolerability problems as reasons for stopping treatment. In addition, 39.7% reported that these medications did not prevent enough headaches. Some patients reported partial or temporary efficacy as a reason for discontinuation. Other reasons were related to health care costs and access and personal preferences. Only 9.2% of patients who discontinued treatment said that their headaches improved enough to stop medication.
The investigators also analyzed respondents’ interest in preventive therapies. Among respondents who had never used preventive therapies, 61.8% of those who were eligible to use them were somewhat or very interested in trying an oral prescription medication for migraine prevention. However, 59.1% of never-users who were eligible for preventive medications were not at all interested, not sure, or needed more information about trying an injectable preventive medication. About 40% were not at all interested in injectables. In general, current users and those who had discontinued medication were more interested in preventive medication, including injectables.
‘Disheartening’ discontinuation rates
There are likely multiple reasons for the low rate of migraine prevention treatment, said Dr. Nahas. Many people with migraine never consult a clinician, owing to factors such as stigma, cost, lack of access, and lack of awareness. In addition, patients with migraine are frequently misdiagnosed, she added.
“Other data suggest that only about a quarter of people with episodic migraine and under 5% of people with chronic migraine consult a clinician, receive an accurate diagnosis, and are prescribed appropriate therapy,” said Dr. Nahas.
When the data in this analysis were gathered, public awareness of migraine was much lower than it is today, and injectable migraine therapies had not gained broad acceptance, she noted. Dr. Nahas added it is possible that attitudes toward injectable preventive medications have changed.
“Would people still prefer daily oral medications? We can’t know for sure until we start asking,” she said. In addition, scientific advances and educational outreach have increased clinicians’ awareness, interest, and skill regarding injectable medications, she said.
“I would certainly hope to see that a much greater proportion of preventive-eligible persons with migraine were at least offered, if not currently taking, preventive medication,” said Dr. Nahas. “But there’s no pleasing everyone, so I think we would still see somewhat disheartening discontinuation rates. The reasons for discontinuation, however, might be less typified by concerns about safety and tolerability.”
Still relevant
Commenting on the study, Mia Tova Minen, MD, chief of headache research and associate professor of neurology and population health at NYU Langone Health, New York, noted that although CaMEO is an older study, its results are still highly relevant.
“Unfortunately, primary care providers are still uncomfortable prescribing migraine preventive medications, and this accounts for the large percentage [of patients] with migraine who, while eligible for migraine preventive therapy, are not offered it,” she said.
Although the public and primary care physicians are now more aware of preventive treatments for migraine, “the number of people offered migraine preventive medication still needs to increase dramatically,” said Dr. Minen.
The American Academy of Neurology’s guidelines for migraine prevention were published in 2012 and are currently being updated. The updated guidelines may include new evidence for candesartan and emerging treatments, such as melatonin and aerobic exercise.
“It is my hope that primary care providers will become more comfortable prescribing migraine preventive medications sooner,” said Dr. Minen.
The current findings suggest a need for additional ways of educating patients with migraine who are eligible for preventive therapies so that they can advocate for themselves, she added. They also suggest the idea of demanding more insurance coverage of behavioral therapies for migraine, because data indicate that these treatments have long-term efficacy and good safety profiles, said Dr. Minen.
An ‘invisible’ disorder
Also commenting on the study, Barbara L. Nye, MD, director of the headache fellowship and codirector of the headache clinic at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said the CaMEO cohort likely is representative of the general population of patients with migraine.
She noted that a significant weakness of the current study is that it examined data collected before the Food and Drug Administration approved monoclonal antibodies and therefore does not reflect patients’ current experience with medications.
“I believe that the attitudes and fears surrounding the use of injectable medication are now likely far less than previously reported, given the positive track record the new generation of once-a-month injectable medications has,” said Dr. Nye.
The findings reinforce the idea that either patients are not talking to their primary care physicians about their headaches and disability or that clinicians are not asking about them, she added. “Both issues are likely linked to the stigma that this disease state has surrounding it. This is an invisible neurological disorder to most,” Dr. Nye said.
The study was sponsored by Allergan before it was acquired by AbbVie. Dr. Nahas has served as a consultant, advisory board member, or speaker for AbbVie/Allergan, Alder/Lundbeck, Amgen/Novartis, Biohaven, Eli Lilly, Impel, Nesos Corp, Supernus, Teva, Theranica, and Zosano. She has not received and will not receive monetary compensation for this research. Dr. Minen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that among patients with migraine who are eligible for preventive therapy, more than a third were not offered this option. In addition, fewer than 10% were currently taking preventive medication, and an additional 10% had discontinued preventive therapy.
“We confirmed that as of 2012 to 2013 – the years these data were collected from a large, comprehensive survey – gaps in care remained,” said study investigator Stephanie J. Nahas, MD, director of the headache medicine fellowship program, Thomas Jefferson University, Philadelphia. “In this preventive-eligible population, 35% reported never even being offered preventive medication.”
Furthermore, only 28% of patients taking preventive medication experienced a reduction in headache frequency to less than 4 days per month, which is a primary goal of treatment, said Dr. Nahas. Disease burden, as measured with scales of disability and affective comorbidities, remained substantial.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Lack of efficacy?
In 2019, the American Headache Society published a position statement recommending that preventive treatment be considered for patients who have migraine and four or more monthly headache days (MHDs), regardless of their level of associated disability. However, previous data suggest few patients who are eligible for preventive treatment receive it. In addition, many who have used preventive medications do not adhere to their regimens because of problems with tolerability, efficacy, or both.
To identify treatment gaps and characterize self-reported use of preventive medications for migraine, the investigators examined data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study, a web-based survey conducted in a representative U.S. sample from September 2012 through November 2013.
The survey identified and characterized patients who met modified criteria for migraine consistent with those in ICHD-3. The researchers classified respondents who had migraine and four or more MHDs as potentially eligible for migraine preventive treatment.
The investigators assessed the study population’s use of oral preventive medications, migraine-related disability and burden, willingness to take preventive treatment, and reasons for discontinuation.
Assessments included the Migraine Disability Assessment Questionnaire, the Patient Health Questionnaire–9 for depression, the Generalized Anxiety Disorder 7-Item Scale, the Migraine Specific Quality of Life questionnaire, and the Migraine Symptom Severity Scale.
In all, 16,789 respondents met criteria for migraine, and 6,579 (39.2%) reported having at least four MHDs. The median age of this subgroup that was eligible for preventive treatment was 40.3 years, and approximately 79% were women.
Only 9.8% of respondents who were eligible for preventive medications were currently using an oral preventive medication. Among those who had ever tried an oral preventive medication, 53.6% discontinued it. Efficacy for patients who used medications appeared to be inadequate. Among all current users of preventive treatment, 68.4% continued to have at least four MHDs.
The researchers assessed treatment eligibility among patients not taking preventive medication. Among respondents who had never used a preventive treatment, 35.7% were eligible to receive it. Among all users who had discontinued preventive medication, 61.0% were still eligible to receive it.
Attitudes toward injectables
Among respondents who had never used a preventive treatment, 64.3% had zero to three MHDs. The remaining 35.7% had 4-7, 8-14, or 15 or more MHDs. Among current users of preventive treatments, 68.4% had four or more MHDs. Among those who had discontinued preventive treatment, 61.0% had four or more MHDs.
Patients who have never used preventive medication “have substantial management gaps,” said Dr. Nahas. High proportions of these patients have moderate or severe disability (64.7%), depression (43%), and anxiety (39%). The rates of these outcomes are higher in users who discontinued treatment, likely because of confounding by indication, she added.
The prevalence of anxiety was similar between those who currently used, formerly used, or never used preventive medications. However, there were differences between never-users and current or former users with respect to moderate to severe depression (never-users, 43%; current users, 49.4%; discontinued users, 46.5%) and moderate to severe disability (never-users, 64.7%; current users, 80.4%; discontinued users, 78.9%).
In all, 44.6% of those who discontinued preventive therapy reported safety and tolerability problems as reasons for stopping treatment. In addition, 39.7% reported that these medications did not prevent enough headaches. Some patients reported partial or temporary efficacy as a reason for discontinuation. Other reasons were related to health care costs and access and personal preferences. Only 9.2% of patients who discontinued treatment said that their headaches improved enough to stop medication.
The investigators also analyzed respondents’ interest in preventive therapies. Among respondents who had never used preventive therapies, 61.8% of those who were eligible to use them were somewhat or very interested in trying an oral prescription medication for migraine prevention. However, 59.1% of never-users who were eligible for preventive medications were not at all interested, not sure, or needed more information about trying an injectable preventive medication. About 40% were not at all interested in injectables. In general, current users and those who had discontinued medication were more interested in preventive medication, including injectables.
‘Disheartening’ discontinuation rates
There are likely multiple reasons for the low rate of migraine prevention treatment, said Dr. Nahas. Many people with migraine never consult a clinician, owing to factors such as stigma, cost, lack of access, and lack of awareness. In addition, patients with migraine are frequently misdiagnosed, she added.
“Other data suggest that only about a quarter of people with episodic migraine and under 5% of people with chronic migraine consult a clinician, receive an accurate diagnosis, and are prescribed appropriate therapy,” said Dr. Nahas.
When the data in this analysis were gathered, public awareness of migraine was much lower than it is today, and injectable migraine therapies had not gained broad acceptance, she noted. Dr. Nahas added it is possible that attitudes toward injectable preventive medications have changed.
“Would people still prefer daily oral medications? We can’t know for sure until we start asking,” she said. In addition, scientific advances and educational outreach have increased clinicians’ awareness, interest, and skill regarding injectable medications, she said.
“I would certainly hope to see that a much greater proportion of preventive-eligible persons with migraine were at least offered, if not currently taking, preventive medication,” said Dr. Nahas. “But there’s no pleasing everyone, so I think we would still see somewhat disheartening discontinuation rates. The reasons for discontinuation, however, might be less typified by concerns about safety and tolerability.”
Still relevant
Commenting on the study, Mia Tova Minen, MD, chief of headache research and associate professor of neurology and population health at NYU Langone Health, New York, noted that although CaMEO is an older study, its results are still highly relevant.
“Unfortunately, primary care providers are still uncomfortable prescribing migraine preventive medications, and this accounts for the large percentage [of patients] with migraine who, while eligible for migraine preventive therapy, are not offered it,” she said.
Although the public and primary care physicians are now more aware of preventive treatments for migraine, “the number of people offered migraine preventive medication still needs to increase dramatically,” said Dr. Minen.
The American Academy of Neurology’s guidelines for migraine prevention were published in 2012 and are currently being updated. The updated guidelines may include new evidence for candesartan and emerging treatments, such as melatonin and aerobic exercise.
“It is my hope that primary care providers will become more comfortable prescribing migraine preventive medications sooner,” said Dr. Minen.
The current findings suggest a need for additional ways of educating patients with migraine who are eligible for preventive therapies so that they can advocate for themselves, she added. They also suggest the idea of demanding more insurance coverage of behavioral therapies for migraine, because data indicate that these treatments have long-term efficacy and good safety profiles, said Dr. Minen.
An ‘invisible’ disorder
Also commenting on the study, Barbara L. Nye, MD, director of the headache fellowship and codirector of the headache clinic at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said the CaMEO cohort likely is representative of the general population of patients with migraine.
She noted that a significant weakness of the current study is that it examined data collected before the Food and Drug Administration approved monoclonal antibodies and therefore does not reflect patients’ current experience with medications.
“I believe that the attitudes and fears surrounding the use of injectable medication are now likely far less than previously reported, given the positive track record the new generation of once-a-month injectable medications has,” said Dr. Nye.
The findings reinforce the idea that either patients are not talking to their primary care physicians about their headaches and disability or that clinicians are not asking about them, she added. “Both issues are likely linked to the stigma that this disease state has surrounding it. This is an invisible neurological disorder to most,” Dr. Nye said.
The study was sponsored by Allergan before it was acquired by AbbVie. Dr. Nahas has served as a consultant, advisory board member, or speaker for AbbVie/Allergan, Alder/Lundbeck, Amgen/Novartis, Biohaven, Eli Lilly, Impel, Nesos Corp, Supernus, Teva, Theranica, and Zosano. She has not received and will not receive monetary compensation for this research. Dr. Minen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that among patients with migraine who are eligible for preventive therapy, more than a third were not offered this option. In addition, fewer than 10% were currently taking preventive medication, and an additional 10% had discontinued preventive therapy.
“We confirmed that as of 2012 to 2013 – the years these data were collected from a large, comprehensive survey – gaps in care remained,” said study investigator Stephanie J. Nahas, MD, director of the headache medicine fellowship program, Thomas Jefferson University, Philadelphia. “In this preventive-eligible population, 35% reported never even being offered preventive medication.”
Furthermore, only 28% of patients taking preventive medication experienced a reduction in headache frequency to less than 4 days per month, which is a primary goal of treatment, said Dr. Nahas. Disease burden, as measured with scales of disability and affective comorbidities, remained substantial.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Lack of efficacy?
In 2019, the American Headache Society published a position statement recommending that preventive treatment be considered for patients who have migraine and four or more monthly headache days (MHDs), regardless of their level of associated disability. However, previous data suggest few patients who are eligible for preventive treatment receive it. In addition, many who have used preventive medications do not adhere to their regimens because of problems with tolerability, efficacy, or both.
To identify treatment gaps and characterize self-reported use of preventive medications for migraine, the investigators examined data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study, a web-based survey conducted in a representative U.S. sample from September 2012 through November 2013.
The survey identified and characterized patients who met modified criteria for migraine consistent with those in ICHD-3. The researchers classified respondents who had migraine and four or more MHDs as potentially eligible for migraine preventive treatment.
The investigators assessed the study population’s use of oral preventive medications, migraine-related disability and burden, willingness to take preventive treatment, and reasons for discontinuation.
Assessments included the Migraine Disability Assessment Questionnaire, the Patient Health Questionnaire–9 for depression, the Generalized Anxiety Disorder 7-Item Scale, the Migraine Specific Quality of Life questionnaire, and the Migraine Symptom Severity Scale.
In all, 16,789 respondents met criteria for migraine, and 6,579 (39.2%) reported having at least four MHDs. The median age of this subgroup that was eligible for preventive treatment was 40.3 years, and approximately 79% were women.
Only 9.8% of respondents who were eligible for preventive medications were currently using an oral preventive medication. Among those who had ever tried an oral preventive medication, 53.6% discontinued it. Efficacy for patients who used medications appeared to be inadequate. Among all current users of preventive treatment, 68.4% continued to have at least four MHDs.
The researchers assessed treatment eligibility among patients not taking preventive medication. Among respondents who had never used a preventive treatment, 35.7% were eligible to receive it. Among all users who had discontinued preventive medication, 61.0% were still eligible to receive it.
Attitudes toward injectables
Among respondents who had never used a preventive treatment, 64.3% had zero to three MHDs. The remaining 35.7% had 4-7, 8-14, or 15 or more MHDs. Among current users of preventive treatments, 68.4% had four or more MHDs. Among those who had discontinued preventive treatment, 61.0% had four or more MHDs.
Patients who have never used preventive medication “have substantial management gaps,” said Dr. Nahas. High proportions of these patients have moderate or severe disability (64.7%), depression (43%), and anxiety (39%). The rates of these outcomes are higher in users who discontinued treatment, likely because of confounding by indication, she added.
The prevalence of anxiety was similar between those who currently used, formerly used, or never used preventive medications. However, there were differences between never-users and current or former users with respect to moderate to severe depression (never-users, 43%; current users, 49.4%; discontinued users, 46.5%) and moderate to severe disability (never-users, 64.7%; current users, 80.4%; discontinued users, 78.9%).
In all, 44.6% of those who discontinued preventive therapy reported safety and tolerability problems as reasons for stopping treatment. In addition, 39.7% reported that these medications did not prevent enough headaches. Some patients reported partial or temporary efficacy as a reason for discontinuation. Other reasons were related to health care costs and access and personal preferences. Only 9.2% of patients who discontinued treatment said that their headaches improved enough to stop medication.
The investigators also analyzed respondents’ interest in preventive therapies. Among respondents who had never used preventive therapies, 61.8% of those who were eligible to use them were somewhat or very interested in trying an oral prescription medication for migraine prevention. However, 59.1% of never-users who were eligible for preventive medications were not at all interested, not sure, or needed more information about trying an injectable preventive medication. About 40% were not at all interested in injectables. In general, current users and those who had discontinued medication were more interested in preventive medication, including injectables.
‘Disheartening’ discontinuation rates
There are likely multiple reasons for the low rate of migraine prevention treatment, said Dr. Nahas. Many people with migraine never consult a clinician, owing to factors such as stigma, cost, lack of access, and lack of awareness. In addition, patients with migraine are frequently misdiagnosed, she added.
“Other data suggest that only about a quarter of people with episodic migraine and under 5% of people with chronic migraine consult a clinician, receive an accurate diagnosis, and are prescribed appropriate therapy,” said Dr. Nahas.
When the data in this analysis were gathered, public awareness of migraine was much lower than it is today, and injectable migraine therapies had not gained broad acceptance, she noted. Dr. Nahas added it is possible that attitudes toward injectable preventive medications have changed.
“Would people still prefer daily oral medications? We can’t know for sure until we start asking,” she said. In addition, scientific advances and educational outreach have increased clinicians’ awareness, interest, and skill regarding injectable medications, she said.
“I would certainly hope to see that a much greater proportion of preventive-eligible persons with migraine were at least offered, if not currently taking, preventive medication,” said Dr. Nahas. “But there’s no pleasing everyone, so I think we would still see somewhat disheartening discontinuation rates. The reasons for discontinuation, however, might be less typified by concerns about safety and tolerability.”
Still relevant
Commenting on the study, Mia Tova Minen, MD, chief of headache research and associate professor of neurology and population health at NYU Langone Health, New York, noted that although CaMEO is an older study, its results are still highly relevant.
“Unfortunately, primary care providers are still uncomfortable prescribing migraine preventive medications, and this accounts for the large percentage [of patients] with migraine who, while eligible for migraine preventive therapy, are not offered it,” she said.
Although the public and primary care physicians are now more aware of preventive treatments for migraine, “the number of people offered migraine preventive medication still needs to increase dramatically,” said Dr. Minen.
The American Academy of Neurology’s guidelines for migraine prevention were published in 2012 and are currently being updated. The updated guidelines may include new evidence for candesartan and emerging treatments, such as melatonin and aerobic exercise.
“It is my hope that primary care providers will become more comfortable prescribing migraine preventive medications sooner,” said Dr. Minen.
The current findings suggest a need for additional ways of educating patients with migraine who are eligible for preventive therapies so that they can advocate for themselves, she added. They also suggest the idea of demanding more insurance coverage of behavioral therapies for migraine, because data indicate that these treatments have long-term efficacy and good safety profiles, said Dr. Minen.
An ‘invisible’ disorder
Also commenting on the study, Barbara L. Nye, MD, director of the headache fellowship and codirector of the headache clinic at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said the CaMEO cohort likely is representative of the general population of patients with migraine.
She noted that a significant weakness of the current study is that it examined data collected before the Food and Drug Administration approved monoclonal antibodies and therefore does not reflect patients’ current experience with medications.
“I believe that the attitudes and fears surrounding the use of injectable medication are now likely far less than previously reported, given the positive track record the new generation of once-a-month injectable medications has,” said Dr. Nye.
The findings reinforce the idea that either patients are not talking to their primary care physicians about their headaches and disability or that clinicians are not asking about them, she added. “Both issues are likely linked to the stigma that this disease state has surrounding it. This is an invisible neurological disorder to most,” Dr. Nye said.
The study was sponsored by Allergan before it was acquired by AbbVie. Dr. Nahas has served as a consultant, advisory board member, or speaker for AbbVie/Allergan, Alder/Lundbeck, Amgen/Novartis, Biohaven, Eli Lilly, Impel, Nesos Corp, Supernus, Teva, Theranica, and Zosano. She has not received and will not receive monetary compensation for this research. Dr. Minen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHS 2021
Physician convicted in buprenorphine scheme faces up to 20 years in prison
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
Combination therapy may benefit patients with migraine
, according to a large retrospective analysis. The results lend hope that the combination may be synergistic, according to Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad. Dr. Blumenfeld presented at the American Headache Society’s 2021 annual meeting. The study was published online April 21 in Pain Therapy.
The retrospective analysis showed a 4-day reduction in headache days per month. In contrast, in the pivotal study for erenumab, the most commonly used anti-CGRP antibody among subjects in the study, showed a 2-day benefit in a subanalysis of patients who had failed at least two oral preventives.
There is mechanistic evidence to suggest the two therapies could be synergistic. OnabotulinumtoxinA is believed to inhibit the release of CGRP, and antibodies reduce CGRP levels. OnabotulinumtoxinA prevents activation of C-fibers in the trigeminal sensory afferents, but does not affect A-delta fibers.
On the other hand, most data indicate that the anti-CGRP antibody fremanezumab prevents activation of A-delta but not C-fibers, and a recent review argues that CGRP antibody nonresponders may have migraines driven by C-fibers or other pathways. “Thus, concomitant use of medications blocking the activation of meningeal C-fibers may provide a synergistic effect on the trigeminal nociceptive pathway,” the authors wrote.
Study finding match clinical practice
The results of the new study strengthen the case that the combination is effective, though proof would require prospective, randomized trials. “I think that it really gives credibility to what we are seeing in practice, which is that combined therapy often is much better than therapy with onabotulinumtoxinA alone, said Deborah Friedman, MD, MPH, who was asked to comment on the findings. Dr. Friedman is professor of neurology and ophthalmology at the University of Texas, Dallas.
The extra 4 migraine-free days per month is a significant benefit, said Stewart Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “It’s an extra month and a half of no disability per year, and that’s on top of what onabotulinumtoxinA does. So it’s really a very important clinical finding,” Dr. Tepper said in an interview.
Many insurance companies refuse to pay for the combination therapy, despite the fact that relatively few migraine patients would likely seek it out, according to Dr. Friedman. “It’s just kind of a shame,” she said.
Insurance companies often object that the combination therapy is experimental, despite the widespread use of combination therapies in migraine. “It’s no more experimental in my opinion than any other combination of medications that we use. For people that have severe migraine, we use combination therapy all the time,” said Dr. Friedman.
Improvements with combination therapy
The study was a chart review of 257 patients who started on onabotulinumtoxinA and later initiated anti-CGRP antibody therapy. A total of 104 completed four visits after initiation of anti-CGRP antibody therapy (completers). Before starting any therapy, patients reported an average of 21 headache days per month in the overall group, and 22 among completers. That frequency dropped to 12 in both groups after onabotulinumtoxinA therapy (overall group difference, –9 days; 95% confidence interval, –8 to –11 days; completers group difference, –10; 95% CI, –7 to –12 days).
A total of 77.8% of subjects in the overall cohort took erenumab, 16.3% took galcanezumab, and 5.8% took fremanezumab. In the completers cohort, the percentages were 84.5%, 10.7%, and 4.9%, respectively.
Compared with baseline, both completers and noncompleters had clinically significant improvements in disability, as measured by at least a 5-point improvement in Migraine Disability Assessment (MIDAS) score at the 3-month visit (–5.8 for completers and –6.3 for the overall cohort group), the 6-month visit (–6.6 and –11.1), the 9-month visit (–8.3 and –6.1), and 1 year (–12.7 and –8.4).
At the first visit, 33.0% of completers had at least a 5-point reduction in MIDAS, as did 36.0% of the overall cohort group, and the trend continued at 6 months (39.8% and 45.1%), 9 months (43.7% and 43.7%), and at 1 year (45.3% and 44.8%).
The study was funded by Allergan. Dr. Blumenfeld has served on advisory boards for Aeon, AbbVie, Amgen, Alder, Biohaven, Teva, Supernus, Promius, Eaglet, and Lilly, and has received funding for speaking from AbbVie, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, Eli Lilly, Lundbeck, Novartis, and Theranica. Dr. Tepper has consulted for Teva. Dr. Friedman has been on the advisory board for Allergan, Amgen, Lundbeck, Eli Lilly, and Teva Pharmaceuticals, and has received grant support from Allergan and Eli Lilly.
, according to a large retrospective analysis. The results lend hope that the combination may be synergistic, according to Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad. Dr. Blumenfeld presented at the American Headache Society’s 2021 annual meeting. The study was published online April 21 in Pain Therapy.
The retrospective analysis showed a 4-day reduction in headache days per month. In contrast, in the pivotal study for erenumab, the most commonly used anti-CGRP antibody among subjects in the study, showed a 2-day benefit in a subanalysis of patients who had failed at least two oral preventives.
There is mechanistic evidence to suggest the two therapies could be synergistic. OnabotulinumtoxinA is believed to inhibit the release of CGRP, and antibodies reduce CGRP levels. OnabotulinumtoxinA prevents activation of C-fibers in the trigeminal sensory afferents, but does not affect A-delta fibers.
On the other hand, most data indicate that the anti-CGRP antibody fremanezumab prevents activation of A-delta but not C-fibers, and a recent review argues that CGRP antibody nonresponders may have migraines driven by C-fibers or other pathways. “Thus, concomitant use of medications blocking the activation of meningeal C-fibers may provide a synergistic effect on the trigeminal nociceptive pathway,” the authors wrote.
Study finding match clinical practice
The results of the new study strengthen the case that the combination is effective, though proof would require prospective, randomized trials. “I think that it really gives credibility to what we are seeing in practice, which is that combined therapy often is much better than therapy with onabotulinumtoxinA alone, said Deborah Friedman, MD, MPH, who was asked to comment on the findings. Dr. Friedman is professor of neurology and ophthalmology at the University of Texas, Dallas.
The extra 4 migraine-free days per month is a significant benefit, said Stewart Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “It’s an extra month and a half of no disability per year, and that’s on top of what onabotulinumtoxinA does. So it’s really a very important clinical finding,” Dr. Tepper said in an interview.
Many insurance companies refuse to pay for the combination therapy, despite the fact that relatively few migraine patients would likely seek it out, according to Dr. Friedman. “It’s just kind of a shame,” she said.
Insurance companies often object that the combination therapy is experimental, despite the widespread use of combination therapies in migraine. “It’s no more experimental in my opinion than any other combination of medications that we use. For people that have severe migraine, we use combination therapy all the time,” said Dr. Friedman.
Improvements with combination therapy
The study was a chart review of 257 patients who started on onabotulinumtoxinA and later initiated anti-CGRP antibody therapy. A total of 104 completed four visits after initiation of anti-CGRP antibody therapy (completers). Before starting any therapy, patients reported an average of 21 headache days per month in the overall group, and 22 among completers. That frequency dropped to 12 in both groups after onabotulinumtoxinA therapy (overall group difference, –9 days; 95% confidence interval, –8 to –11 days; completers group difference, –10; 95% CI, –7 to –12 days).
A total of 77.8% of subjects in the overall cohort took erenumab, 16.3% took galcanezumab, and 5.8% took fremanezumab. In the completers cohort, the percentages were 84.5%, 10.7%, and 4.9%, respectively.
Compared with baseline, both completers and noncompleters had clinically significant improvements in disability, as measured by at least a 5-point improvement in Migraine Disability Assessment (MIDAS) score at the 3-month visit (–5.8 for completers and –6.3 for the overall cohort group), the 6-month visit (–6.6 and –11.1), the 9-month visit (–8.3 and –6.1), and 1 year (–12.7 and –8.4).
At the first visit, 33.0% of completers had at least a 5-point reduction in MIDAS, as did 36.0% of the overall cohort group, and the trend continued at 6 months (39.8% and 45.1%), 9 months (43.7% and 43.7%), and at 1 year (45.3% and 44.8%).
The study was funded by Allergan. Dr. Blumenfeld has served on advisory boards for Aeon, AbbVie, Amgen, Alder, Biohaven, Teva, Supernus, Promius, Eaglet, and Lilly, and has received funding for speaking from AbbVie, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, Eli Lilly, Lundbeck, Novartis, and Theranica. Dr. Tepper has consulted for Teva. Dr. Friedman has been on the advisory board for Allergan, Amgen, Lundbeck, Eli Lilly, and Teva Pharmaceuticals, and has received grant support from Allergan and Eli Lilly.
, according to a large retrospective analysis. The results lend hope that the combination may be synergistic, according to Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad. Dr. Blumenfeld presented at the American Headache Society’s 2021 annual meeting. The study was published online April 21 in Pain Therapy.
The retrospective analysis showed a 4-day reduction in headache days per month. In contrast, in the pivotal study for erenumab, the most commonly used anti-CGRP antibody among subjects in the study, showed a 2-day benefit in a subanalysis of patients who had failed at least two oral preventives.
There is mechanistic evidence to suggest the two therapies could be synergistic. OnabotulinumtoxinA is believed to inhibit the release of CGRP, and antibodies reduce CGRP levels. OnabotulinumtoxinA prevents activation of C-fibers in the trigeminal sensory afferents, but does not affect A-delta fibers.
On the other hand, most data indicate that the anti-CGRP antibody fremanezumab prevents activation of A-delta but not C-fibers, and a recent review argues that CGRP antibody nonresponders may have migraines driven by C-fibers or other pathways. “Thus, concomitant use of medications blocking the activation of meningeal C-fibers may provide a synergistic effect on the trigeminal nociceptive pathway,” the authors wrote.
Study finding match clinical practice
The results of the new study strengthen the case that the combination is effective, though proof would require prospective, randomized trials. “I think that it really gives credibility to what we are seeing in practice, which is that combined therapy often is much better than therapy with onabotulinumtoxinA alone, said Deborah Friedman, MD, MPH, who was asked to comment on the findings. Dr. Friedman is professor of neurology and ophthalmology at the University of Texas, Dallas.
The extra 4 migraine-free days per month is a significant benefit, said Stewart Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “It’s an extra month and a half of no disability per year, and that’s on top of what onabotulinumtoxinA does. So it’s really a very important clinical finding,” Dr. Tepper said in an interview.
Many insurance companies refuse to pay for the combination therapy, despite the fact that relatively few migraine patients would likely seek it out, according to Dr. Friedman. “It’s just kind of a shame,” she said.
Insurance companies often object that the combination therapy is experimental, despite the widespread use of combination therapies in migraine. “It’s no more experimental in my opinion than any other combination of medications that we use. For people that have severe migraine, we use combination therapy all the time,” said Dr. Friedman.
Improvements with combination therapy
The study was a chart review of 257 patients who started on onabotulinumtoxinA and later initiated anti-CGRP antibody therapy. A total of 104 completed four visits after initiation of anti-CGRP antibody therapy (completers). Before starting any therapy, patients reported an average of 21 headache days per month in the overall group, and 22 among completers. That frequency dropped to 12 in both groups after onabotulinumtoxinA therapy (overall group difference, –9 days; 95% confidence interval, –8 to –11 days; completers group difference, –10; 95% CI, –7 to –12 days).
A total of 77.8% of subjects in the overall cohort took erenumab, 16.3% took galcanezumab, and 5.8% took fremanezumab. In the completers cohort, the percentages were 84.5%, 10.7%, and 4.9%, respectively.
Compared with baseline, both completers and noncompleters had clinically significant improvements in disability, as measured by at least a 5-point improvement in Migraine Disability Assessment (MIDAS) score at the 3-month visit (–5.8 for completers and –6.3 for the overall cohort group), the 6-month visit (–6.6 and –11.1), the 9-month visit (–8.3 and –6.1), and 1 year (–12.7 and –8.4).
At the first visit, 33.0% of completers had at least a 5-point reduction in MIDAS, as did 36.0% of the overall cohort group, and the trend continued at 6 months (39.8% and 45.1%), 9 months (43.7% and 43.7%), and at 1 year (45.3% and 44.8%).
The study was funded by Allergan. Dr. Blumenfeld has served on advisory boards for Aeon, AbbVie, Amgen, Alder, Biohaven, Teva, Supernus, Promius, Eaglet, and Lilly, and has received funding for speaking from AbbVie, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, Eli Lilly, Lundbeck, Novartis, and Theranica. Dr. Tepper has consulted for Teva. Dr. Friedman has been on the advisory board for Allergan, Amgen, Lundbeck, Eli Lilly, and Teva Pharmaceuticals, and has received grant support from Allergan and Eli Lilly.
FROM AHS 2021
Not your ordinary neuropathy
She has had a diagnosis of type 2 diabetes for the past 4 years. She initially presented with polyuria/polydipsia and a hemoglobin A1c level of 9.5. She has previously not tolerated metformin, and did not want to take any subsequent medications. She was seen 4 months ago and at that time had an A1c level of 12.5. She decided she wanted to really treat her diabetes as well as she could. She started consuming a low carbohydrate diet, restarted metformin and began using a continuous glucose monitor. She also started taking nighttime glargine insulin, and mealtime insulin apart. She reports she lost 20 pounds over the past 4 months, her blood sugars now run between 100-120 fasting, and up to 180 before meals. She has had a severe, sharp pain in both of her feet over the past month that is interfering with sleep and makes walking painful for her. An exam reveals hyperesthesia of both feet, and her A1c level is 7.5. What is the most likely cause of her neuropathic symptoms?
A. Vitamin B12 deficiency
B. Diabetic neuropathy
C. Insulin neuritis
D. Charcot-Marie-Tooth disease
The most likely cause
In this case, certainly considering vitamin B12 deficiency is reasonable. It is highly unlikely though, given the rapidity of onset of symptoms, and that the patient has been on metformin for a very short period of time. Chronic metformin use is associated with low B12 levels, and the American Diabetes Association has advised that regular monitoring of vitamin B12 levels should be done on patients who are on long-term metformin.1
Diabetic neuropathy is also unlikely, given the rapidity of symptoms in this patient. What is most likely in this patient is treatment-induced neuropathy (TIN), first described with the name “insulin neuritis”.
Research on TIN
Gibbons and colleagues evaluated 16 patients with diabetes with recent marked, rapid improvement in glycemic control who developed a sudden, painful neuropathy.2 All developed symptoms within 8 weeks of intensive glucose control, with 69% having autonomic dysfunction as well, and all developing worsening retinopathy.
Gibbons and Freeman did a retrospective study of patients referred to a diabetic neuropathy clinic over a 5-year period to try to understand how prevalent TIN is.3
A total of 954 patients were evaluated for diabetic neuropathy. Treatment induced neuropathy was defined as a painful neuropathy and/or autonomic dysfunction occurring within 8 weeks of intensified treatment and a drop of the A1c level greater than 2 over a 3-month period.
A total of 104 patients (10.9%) met the criteria for treatment induced neuropathy. Patients who had a decrease in A1c had a much greater chance of developing a painful or autonomic neuropathy than patients who had no change in A1c (P < .0001). The same patients had a much higher risk of developing retinopathy (P < .001). The greater the reduction in A1c, the greater the risk. Patients whose A1c decreased by 2%-3% over 3 months had an absolute risk of 20%, whereas those with a A1c decease of greater than 4% had an 80% absolute risk.
Siddique and colleagues reported on three cases with very different clinical presentations of TIN.4 One patient had an acute third nerve palsy, another patient had a lumbosacral radiculoplexus neuropathy, and the third patient presented with a diffuse painful sensory neuropathy and postural hypotension.
Most patients improve over time from their neuropathic symptoms, with better recovery in patients with type 1 diabetes.2
Pearl
Strongly consider treatment induced neuropathy in your patients with diabetes who present with acute painful neuropathy and/or autonomic dysfunction in the setting of rapid improvement of glucose control.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. American Diabetes Association. Diabetes Care. 2019 Jan;42(Suppl 1):S90-102.
2. Gibbons CH and Freeman R. Ann Neurol 2010; 67:534–41.
3. Gibbons CH and Freeman R. Brain. 2015;138:43-52.
4. Siddique N et al. Endocrinol Diabetes Metab Case Rep. 2020 Feb 26;2020:19-0140.
She has had a diagnosis of type 2 diabetes for the past 4 years. She initially presented with polyuria/polydipsia and a hemoglobin A1c level of 9.5. She has previously not tolerated metformin, and did not want to take any subsequent medications. She was seen 4 months ago and at that time had an A1c level of 12.5. She decided she wanted to really treat her diabetes as well as she could. She started consuming a low carbohydrate diet, restarted metformin and began using a continuous glucose monitor. She also started taking nighttime glargine insulin, and mealtime insulin apart. She reports she lost 20 pounds over the past 4 months, her blood sugars now run between 100-120 fasting, and up to 180 before meals. She has had a severe, sharp pain in both of her feet over the past month that is interfering with sleep and makes walking painful for her. An exam reveals hyperesthesia of both feet, and her A1c level is 7.5. What is the most likely cause of her neuropathic symptoms?
A. Vitamin B12 deficiency
B. Diabetic neuropathy
C. Insulin neuritis
D. Charcot-Marie-Tooth disease
The most likely cause
In this case, certainly considering vitamin B12 deficiency is reasonable. It is highly unlikely though, given the rapidity of onset of symptoms, and that the patient has been on metformin for a very short period of time. Chronic metformin use is associated with low B12 levels, and the American Diabetes Association has advised that regular monitoring of vitamin B12 levels should be done on patients who are on long-term metformin.1
Diabetic neuropathy is also unlikely, given the rapidity of symptoms in this patient. What is most likely in this patient is treatment-induced neuropathy (TIN), first described with the name “insulin neuritis”.
Research on TIN
Gibbons and colleagues evaluated 16 patients with diabetes with recent marked, rapid improvement in glycemic control who developed a sudden, painful neuropathy.2 All developed symptoms within 8 weeks of intensive glucose control, with 69% having autonomic dysfunction as well, and all developing worsening retinopathy.
Gibbons and Freeman did a retrospective study of patients referred to a diabetic neuropathy clinic over a 5-year period to try to understand how prevalent TIN is.3
A total of 954 patients were evaluated for diabetic neuropathy. Treatment induced neuropathy was defined as a painful neuropathy and/or autonomic dysfunction occurring within 8 weeks of intensified treatment and a drop of the A1c level greater than 2 over a 3-month period.
A total of 104 patients (10.9%) met the criteria for treatment induced neuropathy. Patients who had a decrease in A1c had a much greater chance of developing a painful or autonomic neuropathy than patients who had no change in A1c (P < .0001). The same patients had a much higher risk of developing retinopathy (P < .001). The greater the reduction in A1c, the greater the risk. Patients whose A1c decreased by 2%-3% over 3 months had an absolute risk of 20%, whereas those with a A1c decease of greater than 4% had an 80% absolute risk.
Siddique and colleagues reported on three cases with very different clinical presentations of TIN.4 One patient had an acute third nerve palsy, another patient had a lumbosacral radiculoplexus neuropathy, and the third patient presented with a diffuse painful sensory neuropathy and postural hypotension.
Most patients improve over time from their neuropathic symptoms, with better recovery in patients with type 1 diabetes.2
Pearl
Strongly consider treatment induced neuropathy in your patients with diabetes who present with acute painful neuropathy and/or autonomic dysfunction in the setting of rapid improvement of glucose control.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. American Diabetes Association. Diabetes Care. 2019 Jan;42(Suppl 1):S90-102.
2. Gibbons CH and Freeman R. Ann Neurol 2010; 67:534–41.
3. Gibbons CH and Freeman R. Brain. 2015;138:43-52.
4. Siddique N et al. Endocrinol Diabetes Metab Case Rep. 2020 Feb 26;2020:19-0140.
She has had a diagnosis of type 2 diabetes for the past 4 years. She initially presented with polyuria/polydipsia and a hemoglobin A1c level of 9.5. She has previously not tolerated metformin, and did not want to take any subsequent medications. She was seen 4 months ago and at that time had an A1c level of 12.5. She decided she wanted to really treat her diabetes as well as she could. She started consuming a low carbohydrate diet, restarted metformin and began using a continuous glucose monitor. She also started taking nighttime glargine insulin, and mealtime insulin apart. She reports she lost 20 pounds over the past 4 months, her blood sugars now run between 100-120 fasting, and up to 180 before meals. She has had a severe, sharp pain in both of her feet over the past month that is interfering with sleep and makes walking painful for her. An exam reveals hyperesthesia of both feet, and her A1c level is 7.5. What is the most likely cause of her neuropathic symptoms?
A. Vitamin B12 deficiency
B. Diabetic neuropathy
C. Insulin neuritis
D. Charcot-Marie-Tooth disease
The most likely cause
In this case, certainly considering vitamin B12 deficiency is reasonable. It is highly unlikely though, given the rapidity of onset of symptoms, and that the patient has been on metformin for a very short period of time. Chronic metformin use is associated with low B12 levels, and the American Diabetes Association has advised that regular monitoring of vitamin B12 levels should be done on patients who are on long-term metformin.1
Diabetic neuropathy is also unlikely, given the rapidity of symptoms in this patient. What is most likely in this patient is treatment-induced neuropathy (TIN), first described with the name “insulin neuritis”.
Research on TIN
Gibbons and colleagues evaluated 16 patients with diabetes with recent marked, rapid improvement in glycemic control who developed a sudden, painful neuropathy.2 All developed symptoms within 8 weeks of intensive glucose control, with 69% having autonomic dysfunction as well, and all developing worsening retinopathy.
Gibbons and Freeman did a retrospective study of patients referred to a diabetic neuropathy clinic over a 5-year period to try to understand how prevalent TIN is.3
A total of 954 patients were evaluated for diabetic neuropathy. Treatment induced neuropathy was defined as a painful neuropathy and/or autonomic dysfunction occurring within 8 weeks of intensified treatment and a drop of the A1c level greater than 2 over a 3-month period.
A total of 104 patients (10.9%) met the criteria for treatment induced neuropathy. Patients who had a decrease in A1c had a much greater chance of developing a painful or autonomic neuropathy than patients who had no change in A1c (P < .0001). The same patients had a much higher risk of developing retinopathy (P < .001). The greater the reduction in A1c, the greater the risk. Patients whose A1c decreased by 2%-3% over 3 months had an absolute risk of 20%, whereas those with a A1c decease of greater than 4% had an 80% absolute risk.
Siddique and colleagues reported on three cases with very different clinical presentations of TIN.4 One patient had an acute third nerve palsy, another patient had a lumbosacral radiculoplexus neuropathy, and the third patient presented with a diffuse painful sensory neuropathy and postural hypotension.
Most patients improve over time from their neuropathic symptoms, with better recovery in patients with type 1 diabetes.2
Pearl
Strongly consider treatment induced neuropathy in your patients with diabetes who present with acute painful neuropathy and/or autonomic dysfunction in the setting of rapid improvement of glucose control.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. American Diabetes Association. Diabetes Care. 2019 Jan;42(Suppl 1):S90-102.
2. Gibbons CH and Freeman R. Ann Neurol 2010; 67:534–41.
3. Gibbons CH and Freeman R. Brain. 2015;138:43-52.
4. Siddique N et al. Endocrinol Diabetes Metab Case Rep. 2020 Feb 26;2020:19-0140.
Language barrier may contribute to ob.gyn. pain management disparities
Obstetric patients whose first language is not English received fewer pain assessments and fewer doses of NSAIDs and oxycodone therapeutic equivalents (OTEs) following cesarean deliveries, according to a retrospective cohort study poster presented at the 2021 annual meeting of the American College of Obstetricians and Gynecologists.
The findings “may indicate language as a barrier for equitable pain management in the postpartum period,” concluded Alison Wiles, MD, a resident at Mount Sinai South Nassau in Oceanside, N.Y., and colleagues. They recommended “scheduled pain assessment and around the clock nonopioid medication administration” as potential ways to reduce the disparities.
“Racial and ethnic disparities in pain management have been well documented in both inpatient and outpatient settings, [and] similar disparities exist within postpartum pain management,” the researchers note in their background material. They also note that non-Hispanic White communities tend to have a higher incidence of opioid misuse.
The researchers conducted a retrospective study of 327 women who had cesarean deliveries from January to June 2018 at Mount Sinai South Nassau Hospital. They excluded women who underwent cesarean hysterectomies, received general anesthesia or patient-controlled analgesia, had a history of drug use, or had allergies to opiates. They did not note incidence of uterine fibroids, endometriosis, or other gynecologic conditions aside from delivery that could cause pain.
The population included a similar number of non-Hispanic White women (n = 111) and Hispanic women (n = 125). The remaining study participants included 32 non-Hispanic Black women and 59 women who were Asian or had another race/ethnicity. The women’s average age was 31, which was statistically similar across all four race/ethnicity groups. Average body mass index of participants was also similar, ranging from 32 to 34.6 kg/m2, across all four demographic groups.
About half of all the women (52%) had a previous cesarean delivery, but rates were significantly different between groups: 31% of non-Hispanic Black women and 58% of Hispanic women had a prior cesarean, compared to 50% of non-Hispanic White, Asian, and other women (P < .05).
Half the women in the study overall (50.5%) had public insurance, but the proportion of those with public insurance differed significantly by racial/ethnic demographics. Less than a quarter of Asian/other women (23%) had public insurance, compared with 78% of Hispanic women, 74% of non-Hispanic White women, and 59% of non-Hispanic Black women (P < .0001).
Most of the women (76%) spoke English as their primary language, which included nearly all the women in each demographic group except Hispanic, in which 58% of the women’s primary language was Spanish or another language (P < .0001).
Hispanic patients received an average of 10 pain assessments after their cesarean, compared with an average of 11 in each of the other demographic groups (P = .02). Similarly, English speakers received an average 11 pain assessments, but those who primarily spoke Spanish or another language received 10 (P = .01).
The differences between English and non-English speakers were reflected in who received pain medication even though pain scores were the same between the two groups. English speakers received an average two doses of NSAIDs in the first 24 hours post partum, compared with one dose for those who spoke a primary language other than English (P = .03). At 24-48 hours post partum, those who spoke English received an average three NSAID doses, compared with two among those whose primary language was Spanish or another language (P = .03).
There was no difference between language groups in doses of OTEs in the first 24 hours post partum, but differences did occur on the second day. Women who primarily spoke English received an average four OTE doses in the 24-48 hours post partum, compared with two doses given to women who spoke a non-English primary language (P = .03).
Differences were less consistent or not significant when looking solely at race/ethnicity. All four groups received an average of two NSAID doses in the first 24 hours post partum, but second-day rates varied. Non-Hispanic White women and Asian/other women received an average three doses from 24 to 48 hours post partum while non-Hispanic Black women received one and Hispanic women received two (P = .0009).
No statistically significant differences in OTE doses occurred across the groups in the first 24 hours, but from 24 to 48 hours, the average two doses received by Hispanic women and 3 doses received by Asian women differed significantly from the average four doses received by non-Hispanic White women and the average five doses received by non-Hispanic Black women (P =.01).
“Non-Hispanic Black patients had higher OTE doses and fewer NSAID doses in the 24- to 48-hour postpartum period despite no differences in severe pain scores,” the authors also reported.
“These findings are surprising given the standardized protocols in place designed to assess and treat pain post partum,” Etoi A. Garrison, MD, PhD, an associate professor of maternal-fetal medicine at Vanderbilt University Medical Center, Memphis, Tenn., said in an interview. ” Protocols should minimize bias and promote equitable delivery of care.”
Dr. Garrison said it’s important to find out why these discrepancies exist even when ready access to interpretation services exist in the hospital.
“An important component of health care disparity research is to hear directly from patients themselves about their experiences,” Dr. Garrison said. “Often the patient voice is an overlooked and underappreciated resource. I hope that future iterations of this work include patient perceptions about the adequacy of postpartum care and provide more information about how health care delivery can be tailored to the unique needs of this vulnerable population.”
The authors reported no disclosures. Dr Garrison reported receiving a grant from the State of Tennessee Maternal Mortality Review Committee to Create an Unconscious Bias Faculty Train-the-Trainer Program.
Obstetric patients whose first language is not English received fewer pain assessments and fewer doses of NSAIDs and oxycodone therapeutic equivalents (OTEs) following cesarean deliveries, according to a retrospective cohort study poster presented at the 2021 annual meeting of the American College of Obstetricians and Gynecologists.
The findings “may indicate language as a barrier for equitable pain management in the postpartum period,” concluded Alison Wiles, MD, a resident at Mount Sinai South Nassau in Oceanside, N.Y., and colleagues. They recommended “scheduled pain assessment and around the clock nonopioid medication administration” as potential ways to reduce the disparities.
“Racial and ethnic disparities in pain management have been well documented in both inpatient and outpatient settings, [and] similar disparities exist within postpartum pain management,” the researchers note in their background material. They also note that non-Hispanic White communities tend to have a higher incidence of opioid misuse.
The researchers conducted a retrospective study of 327 women who had cesarean deliveries from January to June 2018 at Mount Sinai South Nassau Hospital. They excluded women who underwent cesarean hysterectomies, received general anesthesia or patient-controlled analgesia, had a history of drug use, or had allergies to opiates. They did not note incidence of uterine fibroids, endometriosis, or other gynecologic conditions aside from delivery that could cause pain.
The population included a similar number of non-Hispanic White women (n = 111) and Hispanic women (n = 125). The remaining study participants included 32 non-Hispanic Black women and 59 women who were Asian or had another race/ethnicity. The women’s average age was 31, which was statistically similar across all four race/ethnicity groups. Average body mass index of participants was also similar, ranging from 32 to 34.6 kg/m2, across all four demographic groups.
About half of all the women (52%) had a previous cesarean delivery, but rates were significantly different between groups: 31% of non-Hispanic Black women and 58% of Hispanic women had a prior cesarean, compared to 50% of non-Hispanic White, Asian, and other women (P < .05).
Half the women in the study overall (50.5%) had public insurance, but the proportion of those with public insurance differed significantly by racial/ethnic demographics. Less than a quarter of Asian/other women (23%) had public insurance, compared with 78% of Hispanic women, 74% of non-Hispanic White women, and 59% of non-Hispanic Black women (P < .0001).
Most of the women (76%) spoke English as their primary language, which included nearly all the women in each demographic group except Hispanic, in which 58% of the women’s primary language was Spanish or another language (P < .0001).
Hispanic patients received an average of 10 pain assessments after their cesarean, compared with an average of 11 in each of the other demographic groups (P = .02). Similarly, English speakers received an average 11 pain assessments, but those who primarily spoke Spanish or another language received 10 (P = .01).
The differences between English and non-English speakers were reflected in who received pain medication even though pain scores were the same between the two groups. English speakers received an average two doses of NSAIDs in the first 24 hours post partum, compared with one dose for those who spoke a primary language other than English (P = .03). At 24-48 hours post partum, those who spoke English received an average three NSAID doses, compared with two among those whose primary language was Spanish or another language (P = .03).
There was no difference between language groups in doses of OTEs in the first 24 hours post partum, but differences did occur on the second day. Women who primarily spoke English received an average four OTE doses in the 24-48 hours post partum, compared with two doses given to women who spoke a non-English primary language (P = .03).
Differences were less consistent or not significant when looking solely at race/ethnicity. All four groups received an average of two NSAID doses in the first 24 hours post partum, but second-day rates varied. Non-Hispanic White women and Asian/other women received an average three doses from 24 to 48 hours post partum while non-Hispanic Black women received one and Hispanic women received two (P = .0009).
No statistically significant differences in OTE doses occurred across the groups in the first 24 hours, but from 24 to 48 hours, the average two doses received by Hispanic women and 3 doses received by Asian women differed significantly from the average four doses received by non-Hispanic White women and the average five doses received by non-Hispanic Black women (P =.01).
“Non-Hispanic Black patients had higher OTE doses and fewer NSAID doses in the 24- to 48-hour postpartum period despite no differences in severe pain scores,” the authors also reported.
“These findings are surprising given the standardized protocols in place designed to assess and treat pain post partum,” Etoi A. Garrison, MD, PhD, an associate professor of maternal-fetal medicine at Vanderbilt University Medical Center, Memphis, Tenn., said in an interview. ” Protocols should minimize bias and promote equitable delivery of care.”
Dr. Garrison said it’s important to find out why these discrepancies exist even when ready access to interpretation services exist in the hospital.
“An important component of health care disparity research is to hear directly from patients themselves about their experiences,” Dr. Garrison said. “Often the patient voice is an overlooked and underappreciated resource. I hope that future iterations of this work include patient perceptions about the adequacy of postpartum care and provide more information about how health care delivery can be tailored to the unique needs of this vulnerable population.”
The authors reported no disclosures. Dr Garrison reported receiving a grant from the State of Tennessee Maternal Mortality Review Committee to Create an Unconscious Bias Faculty Train-the-Trainer Program.
Obstetric patients whose first language is not English received fewer pain assessments and fewer doses of NSAIDs and oxycodone therapeutic equivalents (OTEs) following cesarean deliveries, according to a retrospective cohort study poster presented at the 2021 annual meeting of the American College of Obstetricians and Gynecologists.
The findings “may indicate language as a barrier for equitable pain management in the postpartum period,” concluded Alison Wiles, MD, a resident at Mount Sinai South Nassau in Oceanside, N.Y., and colleagues. They recommended “scheduled pain assessment and around the clock nonopioid medication administration” as potential ways to reduce the disparities.
“Racial and ethnic disparities in pain management have been well documented in both inpatient and outpatient settings, [and] similar disparities exist within postpartum pain management,” the researchers note in their background material. They also note that non-Hispanic White communities tend to have a higher incidence of opioid misuse.
The researchers conducted a retrospective study of 327 women who had cesarean deliveries from January to June 2018 at Mount Sinai South Nassau Hospital. They excluded women who underwent cesarean hysterectomies, received general anesthesia or patient-controlled analgesia, had a history of drug use, or had allergies to opiates. They did not note incidence of uterine fibroids, endometriosis, or other gynecologic conditions aside from delivery that could cause pain.
The population included a similar number of non-Hispanic White women (n = 111) and Hispanic women (n = 125). The remaining study participants included 32 non-Hispanic Black women and 59 women who were Asian or had another race/ethnicity. The women’s average age was 31, which was statistically similar across all four race/ethnicity groups. Average body mass index of participants was also similar, ranging from 32 to 34.6 kg/m2, across all four demographic groups.
About half of all the women (52%) had a previous cesarean delivery, but rates were significantly different between groups: 31% of non-Hispanic Black women and 58% of Hispanic women had a prior cesarean, compared to 50% of non-Hispanic White, Asian, and other women (P < .05).
Half the women in the study overall (50.5%) had public insurance, but the proportion of those with public insurance differed significantly by racial/ethnic demographics. Less than a quarter of Asian/other women (23%) had public insurance, compared with 78% of Hispanic women, 74% of non-Hispanic White women, and 59% of non-Hispanic Black women (P < .0001).
Most of the women (76%) spoke English as their primary language, which included nearly all the women in each demographic group except Hispanic, in which 58% of the women’s primary language was Spanish or another language (P < .0001).
Hispanic patients received an average of 10 pain assessments after their cesarean, compared with an average of 11 in each of the other demographic groups (P = .02). Similarly, English speakers received an average 11 pain assessments, but those who primarily spoke Spanish or another language received 10 (P = .01).
The differences between English and non-English speakers were reflected in who received pain medication even though pain scores were the same between the two groups. English speakers received an average two doses of NSAIDs in the first 24 hours post partum, compared with one dose for those who spoke a primary language other than English (P = .03). At 24-48 hours post partum, those who spoke English received an average three NSAID doses, compared with two among those whose primary language was Spanish or another language (P = .03).
There was no difference between language groups in doses of OTEs in the first 24 hours post partum, but differences did occur on the second day. Women who primarily spoke English received an average four OTE doses in the 24-48 hours post partum, compared with two doses given to women who spoke a non-English primary language (P = .03).
Differences were less consistent or not significant when looking solely at race/ethnicity. All four groups received an average of two NSAID doses in the first 24 hours post partum, but second-day rates varied. Non-Hispanic White women and Asian/other women received an average three doses from 24 to 48 hours post partum while non-Hispanic Black women received one and Hispanic women received two (P = .0009).
No statistically significant differences in OTE doses occurred across the groups in the first 24 hours, but from 24 to 48 hours, the average two doses received by Hispanic women and 3 doses received by Asian women differed significantly from the average four doses received by non-Hispanic White women and the average five doses received by non-Hispanic Black women (P =.01).
“Non-Hispanic Black patients had higher OTE doses and fewer NSAID doses in the 24- to 48-hour postpartum period despite no differences in severe pain scores,” the authors also reported.
“These findings are surprising given the standardized protocols in place designed to assess and treat pain post partum,” Etoi A. Garrison, MD, PhD, an associate professor of maternal-fetal medicine at Vanderbilt University Medical Center, Memphis, Tenn., said in an interview. ” Protocols should minimize bias and promote equitable delivery of care.”
Dr. Garrison said it’s important to find out why these discrepancies exist even when ready access to interpretation services exist in the hospital.
“An important component of health care disparity research is to hear directly from patients themselves about their experiences,” Dr. Garrison said. “Often the patient voice is an overlooked and underappreciated resource. I hope that future iterations of this work include patient perceptions about the adequacy of postpartum care and provide more information about how health care delivery can be tailored to the unique needs of this vulnerable population.”
The authors reported no disclosures. Dr Garrison reported receiving a grant from the State of Tennessee Maternal Mortality Review Committee to Create an Unconscious Bias Faculty Train-the-Trainer Program.
FROM ACOG 2021
Is Person-Centered Physical Activity–Promoting Intervention for Individuals With CWP More Effective With Digital Support or Telephone Support?
Study Overview
Objective. To determine the effectiveness of a person-centered intervention (comprising personalized and cocreated treatment plans to promote physical activity) for individuals with chronic widespread pain when delivered with digital eHealth support compared with standard telephone follow-up.
Design. Single-blinded multicenter randomized controlled trial.
Settings and participants. Participants with chronic widespread pain (CWP) who had participated in a pain management program from 2010–16 at 5 primary health care rehabilitation centers in 5 cities or towns in the western part of Sweden were invited to join the study between March 2018 and April 2019 via letter providing information about the intervention. The letter was followed by a phone call 1-2 weeks later to screen for inclusion and exclusion criteria and interest in participating. Additional participants were invited to participate via a newspaper advertisement in 1 of the 5 cities.
Inclusion criteria were Swedish-speaking persons aged 20–65 years with CWP (defined as having pain in both sides of the body, pain above and below the waist, and axial pain for at least 3 months). Exclusion criteria included having other severe somatic or psychiatric disorders, dominating causes of pain other than CWP, or other severe disease interfering with the ability to be physically active, pregnancy, not having access to a smartphone or a computer, inability to speak or understand Swedish, ongoing physiotherapy treatment, and already exercising regularly. Of 716 people initially assessed for eligibility, 425 completed telephone screening, and 139 were randomized (using block randomization) to either the intervention arm (n = 69) or the active control arm (n = 70). Due to the nature of the intervention, it was not possible to blind the participants or the physiotherapist to group allocation. All participants provided written informed consent.
The 2 groups underwent the same first individual meeting with a physiotherapist to cocreate a health plan with physical activities, and, if needed, stress management, based on each participant’s individual preferences, obstacles, goals, and resources. The difference between the groups was the type of follow-up support. Participants in the intervention group had 1 follow-up meeting with the physiotherapist a week after the initial meeting (to review and adjust the health plan as needed) and thereafter were supported through a digital e-health platform (accessed via the participant’s smartphone or computer) during the 6-month follow-up period. Participants were encouraged to access the platform once a week to answer questions regarding their health, and the extent to which they had been able to manage their health plan during the previous week. In addition, the participant and physiotherapist could communicate via the platform as needed. Participants in the active control group had 1 follow-up phone call with the physiotherapist 1 month after the initial meeting (similarly to review and adjust the health plan as needed), and no further contact or support from the physiotherapist during the 6-month follow-up period.
Measures and analysis. The primary outcome measure was pain intensity during the previous week assessed with a 0–100 subscale from the Fibromyalgia Impact Questionnaire (FIQ-pain). Secondary outcome measures included overall health status (via FIQ-total with 10 subscales), global fatigue (via FIQ-fatigue subscale), multidimensional fatigue (via Multidimensional Fatigue Inventory, a 20-item questionnaire rated on a 1-5 Likert scale), clinical manifestations of stress (via Stress and Crisis Inventory, a 35-item questionnaire rated on a 0-4 Likert scale), self-efficacy (via General Self-Efficacy Scale, a 10-item questionnaire rated on a 1-4 Likert scale), health-related quality of life (via Short Form 36, specifically the Physical Component Summary composite score), leisure-time physical activity (via Leisure Time Physical Activity Instrument), and physical function (via 1-min chair-stand test). Additional demographic data on age, pain localization, pharmacological treatment, tobacco use, country of birth, level of education, family status, economic status, work status, sick-leave, and disability pension were collected via a questionnaire.
Between-group differences for changes in outcomes from baseline to 6-month follow-up were calculated using the Mann–Whitney U test for continuous data, and Pearson’s χ2 or Fisher’s exact test for categorical data. Significance level was set at 5% with no adjustment for multiple comparisons. All analyses were made according to intention-to-treat by originally assigned group; missing cases were not included in the analysis.
Main results. Participants consisted of primarily middle-age, middle income, educated (> 12 years of education) females, with > 60% of participants working at least part-time (between-group differences in baseline data and demographic data not detailed in the article). A total of 29 participants were lost to follow-up. In the intervention group, lost-to-follow up participants were older, performed fewer hours of physical activity, and had lower mental fatigue at baseline, compared with those who were lost to follow-up in the active control group.
In between-group analyses, there were no significant differences in the primary outcome (pain intensity) from baseline to 6-month follow-up. The only significant difference in secondary outcomes was seen in global fatigue – the active control group improved significantly compared with the intervention group (P = .004).
In the intervention group, 87% of participants used the digital platform. Among these users, 35% contacted the physiotherapist (75% of these communications were health- or study-related issues, 25% were issues with the digital platform), 33% were contacted by the physiotherapist (96% of these communications were about the health plan and physical activity), and 32% never had any contact with the physiotherapist. There was a significant difference in the primary outcome (pain intensity) from baseline to 6-month follow-up between platform users and non-users (P = .03, mean change [SD] 3.8 [19.66] mm vs –20.5 [6.36] mm, respectively).
Conclusion. No significant differences were found between the groups after 6 months (except for a significant decrease in global fatigue in the active control group compared with the intervention group). Further development of interventions to support persons with CWP to maintain regular physical activity is needed.
Commentary
Chronic widespread pain is a disorder characterized by diffuse body pain persisting for at least 3 months.1-2 It has been associated with lost work productivity, mental ill health, and reduced quality of life. The development of clinically effective and cost-effective pain management strategies for CWP is challenging given the syndrome complexity and heterogenous symptomology. Thus, multimodal, multidisciplinary management is widely advocated, often a combination of education and self-management, with integration of physical, non-pharmacological and pharmacological treatments.1-3 Of note, physical exercise and cognitive behavioral therapy are 2 non-pharmacological treatments that hold some promise based on available evidence.
The pervasiveness of technology in nearly all aspects of daily life has corresponded with the development of implementation of a wide range of technology-based interventions for health purposes.4 Examples of electronic health or eHealth modalities include internet-based, telephone supported, interactive voice-response, videoconferencing, mobile apps, and virtual reality. While the use of technology in chronic pain management interventions has increased in recent years, the literature is still limited, heterogenous, and provides limited evidence on the efficacy of eHealth/digital interventions, let alone which specific modalities are most effective.4-9
This study adds to the literature as a randomized controlled trial evaluating the effectiveness of a person-centered intervention for individuals with CWP delivered with digital eHealth support compared with standard telephone follow-up. Results showed no significant difference in the primary outcome of pain intensity and nearly all secondary outcomes between the intervention group (supported by the digital platform) and the active control group (supported by a follow-up phone call). Further, intervention participants who did not use the platform improved significantly more in pain intensity than those who used the platform.
While these results imply that digital support does not contribute to improvements in the outcomes measured, it is important these findings are interpreted with caution given the limitations of the study design as well as limitations with the intervention itself. Importantly, while this study was designed as a randomized controlled trial, the authors indicated that it was not possible to blind the participants or the physiotherapist to group allocation, which may have impacted their behaviors while in the study. In addition, as the authors note, an intervention aimed at increasing physical activity should ideally include an objective measure of activity and this was lacking in this study. The use of an actigraphy device for example would have provided objective, continuous data on movement and could have helped assess an important outcome measure – whether participants reached their physical activity goals or had increased their overall physical activity. In the analysis, there was no adjustment for multiple comparisons or use of imputation methods to handle missing values. Further, it was unclear whether differences in baseline data were evaluated and taken into consideration in between-group analyses. Lastly, results are only attributable to the eHealth mode used in this study (digital web-based with limited mechanisms of behavior change and engagement built-in) and thus should not be generalized to all digital/eHealth interventions persons with CWP.
Applications for Clinical Practice
While the results of this study failed to demonstrate significant differences between a physical activity-promoting intervention for persons with CWP with digital follow-up vs telephone follow-up, it remains important to consider person-centered principles when offering CWP management support. In this spirit, clinicians should consider a management approach that takes into account the individual’s knowledge, resources, and barriers, and also actively involves the patient in treatment planning to enhance the patient’s self-efficacy to manage their health. In addition, individual preference for a specific (or combination of) eHealth/digital modality should be considered and used to guide a comprehensive management plan, as well as used as a complementary modality to face-to-face care/support.
1. Bee, P, McBeth, J, MacFarlane, GJ, Lovell K. Managing chronic widespread pain in primary care: a qualitative study of patient perspectives and implications for treatment delivery. BMC Musculoskelet Disord. 2016;17(1):354.
2. Whibley D, Dean LE, Basu N. Management of Widespread Pain and Fibromyalgia. Curr Treatm Opt Rheumatol. 2016;2(4):312-320.
3. Takai Y, Yamamoto-Mitani N, Abe Y, Suzuki M. Literature review of pain management for people with chronic pain. Jpn J Nurs Sci. 2015;12(3):167-183.
4. Slattery BW, Haugh S, O’Connor L, et al. An Evaluation of the Effectiveness of the Modalities Used to Deliver Electronic Health Interventions for Chronic Pain: Systematic Review With Network Meta-Analysis. J Med Internet Res. 2019;21(7):e11086.
5. Heapy AA, Higgins DM, Cervone D, et al. A Systematic Review of Technology-assisted Self-Management Interventions for Chronic Pain. Clin J Pain. 2015;31(6):470-492.
6. Martin CL, Bakker CJ, Breth MS, et al. The efficacy of mobile health interventions used to manage acute or chronic pain: A systematic review. Res Nurs Health. 2021 Feb;44(1):111-128.
7. Bhattarai P, Phillips JL. The role of digital health technologies in management of pain in older people: An integrative review. Arch Gerontol and Geriatr. 2017;68:14-24.
8. Bhatia A, Kara J, Janmohamed T, et al. User Engagement and Clinical Impact of the Manage My Pain App in Patients With Chronic Pain: A Real-World, Multi-site Trial. JMIR Mhealth Uhealth. 2021;9(3):e26528.
9. Nevedal DC, Wang C, Oberleitner L, et al. Effects of an individually tailored Web-based chronic pain management program on pain severity, psychological health, and functioning. J Med Internet Res. 2013;15(9):e201.
Study Overview
Objective. To determine the effectiveness of a person-centered intervention (comprising personalized and cocreated treatment plans to promote physical activity) for individuals with chronic widespread pain when delivered with digital eHealth support compared with standard telephone follow-up.
Design. Single-blinded multicenter randomized controlled trial.
Settings and participants. Participants with chronic widespread pain (CWP) who had participated in a pain management program from 2010–16 at 5 primary health care rehabilitation centers in 5 cities or towns in the western part of Sweden were invited to join the study between March 2018 and April 2019 via letter providing information about the intervention. The letter was followed by a phone call 1-2 weeks later to screen for inclusion and exclusion criteria and interest in participating. Additional participants were invited to participate via a newspaper advertisement in 1 of the 5 cities.
Inclusion criteria were Swedish-speaking persons aged 20–65 years with CWP (defined as having pain in both sides of the body, pain above and below the waist, and axial pain for at least 3 months). Exclusion criteria included having other severe somatic or psychiatric disorders, dominating causes of pain other than CWP, or other severe disease interfering with the ability to be physically active, pregnancy, not having access to a smartphone or a computer, inability to speak or understand Swedish, ongoing physiotherapy treatment, and already exercising regularly. Of 716 people initially assessed for eligibility, 425 completed telephone screening, and 139 were randomized (using block randomization) to either the intervention arm (n = 69) or the active control arm (n = 70). Due to the nature of the intervention, it was not possible to blind the participants or the physiotherapist to group allocation. All participants provided written informed consent.
The 2 groups underwent the same first individual meeting with a physiotherapist to cocreate a health plan with physical activities, and, if needed, stress management, based on each participant’s individual preferences, obstacles, goals, and resources. The difference between the groups was the type of follow-up support. Participants in the intervention group had 1 follow-up meeting with the physiotherapist a week after the initial meeting (to review and adjust the health plan as needed) and thereafter were supported through a digital e-health platform (accessed via the participant’s smartphone or computer) during the 6-month follow-up period. Participants were encouraged to access the platform once a week to answer questions regarding their health, and the extent to which they had been able to manage their health plan during the previous week. In addition, the participant and physiotherapist could communicate via the platform as needed. Participants in the active control group had 1 follow-up phone call with the physiotherapist 1 month after the initial meeting (similarly to review and adjust the health plan as needed), and no further contact or support from the physiotherapist during the 6-month follow-up period.
Measures and analysis. The primary outcome measure was pain intensity during the previous week assessed with a 0–100 subscale from the Fibromyalgia Impact Questionnaire (FIQ-pain). Secondary outcome measures included overall health status (via FIQ-total with 10 subscales), global fatigue (via FIQ-fatigue subscale), multidimensional fatigue (via Multidimensional Fatigue Inventory, a 20-item questionnaire rated on a 1-5 Likert scale), clinical manifestations of stress (via Stress and Crisis Inventory, a 35-item questionnaire rated on a 0-4 Likert scale), self-efficacy (via General Self-Efficacy Scale, a 10-item questionnaire rated on a 1-4 Likert scale), health-related quality of life (via Short Form 36, specifically the Physical Component Summary composite score), leisure-time physical activity (via Leisure Time Physical Activity Instrument), and physical function (via 1-min chair-stand test). Additional demographic data on age, pain localization, pharmacological treatment, tobacco use, country of birth, level of education, family status, economic status, work status, sick-leave, and disability pension were collected via a questionnaire.
Between-group differences for changes in outcomes from baseline to 6-month follow-up were calculated using the Mann–Whitney U test for continuous data, and Pearson’s χ2 or Fisher’s exact test for categorical data. Significance level was set at 5% with no adjustment for multiple comparisons. All analyses were made according to intention-to-treat by originally assigned group; missing cases were not included in the analysis.
Main results. Participants consisted of primarily middle-age, middle income, educated (> 12 years of education) females, with > 60% of participants working at least part-time (between-group differences in baseline data and demographic data not detailed in the article). A total of 29 participants were lost to follow-up. In the intervention group, lost-to-follow up participants were older, performed fewer hours of physical activity, and had lower mental fatigue at baseline, compared with those who were lost to follow-up in the active control group.
In between-group analyses, there were no significant differences in the primary outcome (pain intensity) from baseline to 6-month follow-up. The only significant difference in secondary outcomes was seen in global fatigue – the active control group improved significantly compared with the intervention group (P = .004).
In the intervention group, 87% of participants used the digital platform. Among these users, 35% contacted the physiotherapist (75% of these communications were health- or study-related issues, 25% were issues with the digital platform), 33% were contacted by the physiotherapist (96% of these communications were about the health plan and physical activity), and 32% never had any contact with the physiotherapist. There was a significant difference in the primary outcome (pain intensity) from baseline to 6-month follow-up between platform users and non-users (P = .03, mean change [SD] 3.8 [19.66] mm vs –20.5 [6.36] mm, respectively).
Conclusion. No significant differences were found between the groups after 6 months (except for a significant decrease in global fatigue in the active control group compared with the intervention group). Further development of interventions to support persons with CWP to maintain regular physical activity is needed.
Commentary
Chronic widespread pain is a disorder characterized by diffuse body pain persisting for at least 3 months.1-2 It has been associated with lost work productivity, mental ill health, and reduced quality of life. The development of clinically effective and cost-effective pain management strategies for CWP is challenging given the syndrome complexity and heterogenous symptomology. Thus, multimodal, multidisciplinary management is widely advocated, often a combination of education and self-management, with integration of physical, non-pharmacological and pharmacological treatments.1-3 Of note, physical exercise and cognitive behavioral therapy are 2 non-pharmacological treatments that hold some promise based on available evidence.
The pervasiveness of technology in nearly all aspects of daily life has corresponded with the development of implementation of a wide range of technology-based interventions for health purposes.4 Examples of electronic health or eHealth modalities include internet-based, telephone supported, interactive voice-response, videoconferencing, mobile apps, and virtual reality. While the use of technology in chronic pain management interventions has increased in recent years, the literature is still limited, heterogenous, and provides limited evidence on the efficacy of eHealth/digital interventions, let alone which specific modalities are most effective.4-9
This study adds to the literature as a randomized controlled trial evaluating the effectiveness of a person-centered intervention for individuals with CWP delivered with digital eHealth support compared with standard telephone follow-up. Results showed no significant difference in the primary outcome of pain intensity and nearly all secondary outcomes between the intervention group (supported by the digital platform) and the active control group (supported by a follow-up phone call). Further, intervention participants who did not use the platform improved significantly more in pain intensity than those who used the platform.
While these results imply that digital support does not contribute to improvements in the outcomes measured, it is important these findings are interpreted with caution given the limitations of the study design as well as limitations with the intervention itself. Importantly, while this study was designed as a randomized controlled trial, the authors indicated that it was not possible to blind the participants or the physiotherapist to group allocation, which may have impacted their behaviors while in the study. In addition, as the authors note, an intervention aimed at increasing physical activity should ideally include an objective measure of activity and this was lacking in this study. The use of an actigraphy device for example would have provided objective, continuous data on movement and could have helped assess an important outcome measure – whether participants reached their physical activity goals or had increased their overall physical activity. In the analysis, there was no adjustment for multiple comparisons or use of imputation methods to handle missing values. Further, it was unclear whether differences in baseline data were evaluated and taken into consideration in between-group analyses. Lastly, results are only attributable to the eHealth mode used in this study (digital web-based with limited mechanisms of behavior change and engagement built-in) and thus should not be generalized to all digital/eHealth interventions persons with CWP.
Applications for Clinical Practice
While the results of this study failed to demonstrate significant differences between a physical activity-promoting intervention for persons with CWP with digital follow-up vs telephone follow-up, it remains important to consider person-centered principles when offering CWP management support. In this spirit, clinicians should consider a management approach that takes into account the individual’s knowledge, resources, and barriers, and also actively involves the patient in treatment planning to enhance the patient’s self-efficacy to manage their health. In addition, individual preference for a specific (or combination of) eHealth/digital modality should be considered and used to guide a comprehensive management plan, as well as used as a complementary modality to face-to-face care/support.
Study Overview
Objective. To determine the effectiveness of a person-centered intervention (comprising personalized and cocreated treatment plans to promote physical activity) for individuals with chronic widespread pain when delivered with digital eHealth support compared with standard telephone follow-up.
Design. Single-blinded multicenter randomized controlled trial.
Settings and participants. Participants with chronic widespread pain (CWP) who had participated in a pain management program from 2010–16 at 5 primary health care rehabilitation centers in 5 cities or towns in the western part of Sweden were invited to join the study between March 2018 and April 2019 via letter providing information about the intervention. The letter was followed by a phone call 1-2 weeks later to screen for inclusion and exclusion criteria and interest in participating. Additional participants were invited to participate via a newspaper advertisement in 1 of the 5 cities.
Inclusion criteria were Swedish-speaking persons aged 20–65 years with CWP (defined as having pain in both sides of the body, pain above and below the waist, and axial pain for at least 3 months). Exclusion criteria included having other severe somatic or psychiatric disorders, dominating causes of pain other than CWP, or other severe disease interfering with the ability to be physically active, pregnancy, not having access to a smartphone or a computer, inability to speak or understand Swedish, ongoing physiotherapy treatment, and already exercising regularly. Of 716 people initially assessed for eligibility, 425 completed telephone screening, and 139 were randomized (using block randomization) to either the intervention arm (n = 69) or the active control arm (n = 70). Due to the nature of the intervention, it was not possible to blind the participants or the physiotherapist to group allocation. All participants provided written informed consent.
The 2 groups underwent the same first individual meeting with a physiotherapist to cocreate a health plan with physical activities, and, if needed, stress management, based on each participant’s individual preferences, obstacles, goals, and resources. The difference between the groups was the type of follow-up support. Participants in the intervention group had 1 follow-up meeting with the physiotherapist a week after the initial meeting (to review and adjust the health plan as needed) and thereafter were supported through a digital e-health platform (accessed via the participant’s smartphone or computer) during the 6-month follow-up period. Participants were encouraged to access the platform once a week to answer questions regarding their health, and the extent to which they had been able to manage their health plan during the previous week. In addition, the participant and physiotherapist could communicate via the platform as needed. Participants in the active control group had 1 follow-up phone call with the physiotherapist 1 month after the initial meeting (similarly to review and adjust the health plan as needed), and no further contact or support from the physiotherapist during the 6-month follow-up period.
Measures and analysis. The primary outcome measure was pain intensity during the previous week assessed with a 0–100 subscale from the Fibromyalgia Impact Questionnaire (FIQ-pain). Secondary outcome measures included overall health status (via FIQ-total with 10 subscales), global fatigue (via FIQ-fatigue subscale), multidimensional fatigue (via Multidimensional Fatigue Inventory, a 20-item questionnaire rated on a 1-5 Likert scale), clinical manifestations of stress (via Stress and Crisis Inventory, a 35-item questionnaire rated on a 0-4 Likert scale), self-efficacy (via General Self-Efficacy Scale, a 10-item questionnaire rated on a 1-4 Likert scale), health-related quality of life (via Short Form 36, specifically the Physical Component Summary composite score), leisure-time physical activity (via Leisure Time Physical Activity Instrument), and physical function (via 1-min chair-stand test). Additional demographic data on age, pain localization, pharmacological treatment, tobacco use, country of birth, level of education, family status, economic status, work status, sick-leave, and disability pension were collected via a questionnaire.
Between-group differences for changes in outcomes from baseline to 6-month follow-up were calculated using the Mann–Whitney U test for continuous data, and Pearson’s χ2 or Fisher’s exact test for categorical data. Significance level was set at 5% with no adjustment for multiple comparisons. All analyses were made according to intention-to-treat by originally assigned group; missing cases were not included in the analysis.
Main results. Participants consisted of primarily middle-age, middle income, educated (> 12 years of education) females, with > 60% of participants working at least part-time (between-group differences in baseline data and demographic data not detailed in the article). A total of 29 participants were lost to follow-up. In the intervention group, lost-to-follow up participants were older, performed fewer hours of physical activity, and had lower mental fatigue at baseline, compared with those who were lost to follow-up in the active control group.
In between-group analyses, there were no significant differences in the primary outcome (pain intensity) from baseline to 6-month follow-up. The only significant difference in secondary outcomes was seen in global fatigue – the active control group improved significantly compared with the intervention group (P = .004).
In the intervention group, 87% of participants used the digital platform. Among these users, 35% contacted the physiotherapist (75% of these communications were health- or study-related issues, 25% were issues with the digital platform), 33% were contacted by the physiotherapist (96% of these communications were about the health plan and physical activity), and 32% never had any contact with the physiotherapist. There was a significant difference in the primary outcome (pain intensity) from baseline to 6-month follow-up between platform users and non-users (P = .03, mean change [SD] 3.8 [19.66] mm vs –20.5 [6.36] mm, respectively).
Conclusion. No significant differences were found between the groups after 6 months (except for a significant decrease in global fatigue in the active control group compared with the intervention group). Further development of interventions to support persons with CWP to maintain regular physical activity is needed.
Commentary
Chronic widespread pain is a disorder characterized by diffuse body pain persisting for at least 3 months.1-2 It has been associated with lost work productivity, mental ill health, and reduced quality of life. The development of clinically effective and cost-effective pain management strategies for CWP is challenging given the syndrome complexity and heterogenous symptomology. Thus, multimodal, multidisciplinary management is widely advocated, often a combination of education and self-management, with integration of physical, non-pharmacological and pharmacological treatments.1-3 Of note, physical exercise and cognitive behavioral therapy are 2 non-pharmacological treatments that hold some promise based on available evidence.
The pervasiveness of technology in nearly all aspects of daily life has corresponded with the development of implementation of a wide range of technology-based interventions for health purposes.4 Examples of electronic health or eHealth modalities include internet-based, telephone supported, interactive voice-response, videoconferencing, mobile apps, and virtual reality. While the use of technology in chronic pain management interventions has increased in recent years, the literature is still limited, heterogenous, and provides limited evidence on the efficacy of eHealth/digital interventions, let alone which specific modalities are most effective.4-9
This study adds to the literature as a randomized controlled trial evaluating the effectiveness of a person-centered intervention for individuals with CWP delivered with digital eHealth support compared with standard telephone follow-up. Results showed no significant difference in the primary outcome of pain intensity and nearly all secondary outcomes between the intervention group (supported by the digital platform) and the active control group (supported by a follow-up phone call). Further, intervention participants who did not use the platform improved significantly more in pain intensity than those who used the platform.
While these results imply that digital support does not contribute to improvements in the outcomes measured, it is important these findings are interpreted with caution given the limitations of the study design as well as limitations with the intervention itself. Importantly, while this study was designed as a randomized controlled trial, the authors indicated that it was not possible to blind the participants or the physiotherapist to group allocation, which may have impacted their behaviors while in the study. In addition, as the authors note, an intervention aimed at increasing physical activity should ideally include an objective measure of activity and this was lacking in this study. The use of an actigraphy device for example would have provided objective, continuous data on movement and could have helped assess an important outcome measure – whether participants reached their physical activity goals or had increased their overall physical activity. In the analysis, there was no adjustment for multiple comparisons or use of imputation methods to handle missing values. Further, it was unclear whether differences in baseline data were evaluated and taken into consideration in between-group analyses. Lastly, results are only attributable to the eHealth mode used in this study (digital web-based with limited mechanisms of behavior change and engagement built-in) and thus should not be generalized to all digital/eHealth interventions persons with CWP.
Applications for Clinical Practice
While the results of this study failed to demonstrate significant differences between a physical activity-promoting intervention for persons with CWP with digital follow-up vs telephone follow-up, it remains important to consider person-centered principles when offering CWP management support. In this spirit, clinicians should consider a management approach that takes into account the individual’s knowledge, resources, and barriers, and also actively involves the patient in treatment planning to enhance the patient’s self-efficacy to manage their health. In addition, individual preference for a specific (or combination of) eHealth/digital modality should be considered and used to guide a comprehensive management plan, as well as used as a complementary modality to face-to-face care/support.
1. Bee, P, McBeth, J, MacFarlane, GJ, Lovell K. Managing chronic widespread pain in primary care: a qualitative study of patient perspectives and implications for treatment delivery. BMC Musculoskelet Disord. 2016;17(1):354.
2. Whibley D, Dean LE, Basu N. Management of Widespread Pain and Fibromyalgia. Curr Treatm Opt Rheumatol. 2016;2(4):312-320.
3. Takai Y, Yamamoto-Mitani N, Abe Y, Suzuki M. Literature review of pain management for people with chronic pain. Jpn J Nurs Sci. 2015;12(3):167-183.
4. Slattery BW, Haugh S, O’Connor L, et al. An Evaluation of the Effectiveness of the Modalities Used to Deliver Electronic Health Interventions for Chronic Pain: Systematic Review With Network Meta-Analysis. J Med Internet Res. 2019;21(7):e11086.
5. Heapy AA, Higgins DM, Cervone D, et al. A Systematic Review of Technology-assisted Self-Management Interventions for Chronic Pain. Clin J Pain. 2015;31(6):470-492.
6. Martin CL, Bakker CJ, Breth MS, et al. The efficacy of mobile health interventions used to manage acute or chronic pain: A systematic review. Res Nurs Health. 2021 Feb;44(1):111-128.
7. Bhattarai P, Phillips JL. The role of digital health technologies in management of pain in older people: An integrative review. Arch Gerontol and Geriatr. 2017;68:14-24.
8. Bhatia A, Kara J, Janmohamed T, et al. User Engagement and Clinical Impact of the Manage My Pain App in Patients With Chronic Pain: A Real-World, Multi-site Trial. JMIR Mhealth Uhealth. 2021;9(3):e26528.
9. Nevedal DC, Wang C, Oberleitner L, et al. Effects of an individually tailored Web-based chronic pain management program on pain severity, psychological health, and functioning. J Med Internet Res. 2013;15(9):e201.
1. Bee, P, McBeth, J, MacFarlane, GJ, Lovell K. Managing chronic widespread pain in primary care: a qualitative study of patient perspectives and implications for treatment delivery. BMC Musculoskelet Disord. 2016;17(1):354.
2. Whibley D, Dean LE, Basu N. Management of Widespread Pain and Fibromyalgia. Curr Treatm Opt Rheumatol. 2016;2(4):312-320.
3. Takai Y, Yamamoto-Mitani N, Abe Y, Suzuki M. Literature review of pain management for people with chronic pain. Jpn J Nurs Sci. 2015;12(3):167-183.
4. Slattery BW, Haugh S, O’Connor L, et al. An Evaluation of the Effectiveness of the Modalities Used to Deliver Electronic Health Interventions for Chronic Pain: Systematic Review With Network Meta-Analysis. J Med Internet Res. 2019;21(7):e11086.
5. Heapy AA, Higgins DM, Cervone D, et al. A Systematic Review of Technology-assisted Self-Management Interventions for Chronic Pain. Clin J Pain. 2015;31(6):470-492.
6. Martin CL, Bakker CJ, Breth MS, et al. The efficacy of mobile health interventions used to manage acute or chronic pain: A systematic review. Res Nurs Health. 2021 Feb;44(1):111-128.
7. Bhattarai P, Phillips JL. The role of digital health technologies in management of pain in older people: An integrative review. Arch Gerontol and Geriatr. 2017;68:14-24.
8. Bhatia A, Kara J, Janmohamed T, et al. User Engagement and Clinical Impact of the Manage My Pain App in Patients With Chronic Pain: A Real-World, Multi-site Trial. JMIR Mhealth Uhealth. 2021;9(3):e26528.
9. Nevedal DC, Wang C, Oberleitner L, et al. Effects of an individually tailored Web-based chronic pain management program on pain severity, psychological health, and functioning. J Med Internet Res. 2013;15(9):e201.
Lesions in pelvis may be ‘tip of the iceberg’ in endometriosis
Recognizing the systemic effects of endometriosis may help doctors better understand the experiences of patients with the disease and guide the approach to diagnosis and treatment, according to the president of the American Society for Reproductive Medicine (ASRM).
, Hugh S. Taylor, MD, said at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Its systemic manifestations may explain why women with endometriosis tend to have a lower body mass index, compared with women without the disease, Dr. Taylor said.
“Stem cells, microRNAs, and generalized inflammation are some of the mechanisms that mediate these long-range effects on distant organ systems,” he said.
Studies have indicated that lesions in the pelvis do not fully explain the disease, and investigators continue to elucidate how “endometriosis that we see in the pelvis is really just the tip of the iceberg,” said Dr. Taylor, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn.
Pain, including dysmenorrhea, pelvic pain, and dyspareunia, “can be just as bad with ... stage 1 disease as it can be with stage 4 disease,” he said.
Some patients may not have pain, but have infertility. Other women are asymptomatic, and doctors find endometriosis incidentally.
One common definition of endometriosis – ectopic endometrial glands and stroma predominantly caused by retrograde menstruation – “probably overly simplifies this complex disease,” said Dr. Taylor, who reviewed the current understanding of endometriosis in an article in The Lancet. “The lesions in the pelvis are important. We see them. We treat them. But endometriosis has ... effects throughout the body.”
Dr. Taylor’s research group has shown that stem cells are a potential source of endometriosis. “There are cells from the endometriosis that can be found traveling in the circulation,” but their effects are unclear, he said.
Levels of several microRNAs may be increased or decreased in women with endometriosis, and these altered levels may induce the production of inflammatory cytokines. They also may serve as the basis of a blood test for endometriosis that could be ready for clinical use soon, Dr. Taylor said.
In a mouse model of endometriosis, the disease changes the electrophysiology of the brain and behavior. “We see changes in anxiety induced by endometriosis. We see changes in pain sensitivity induced by endometriosis. And we also see an increase in depression induced by endometriosis in this animal model,” Dr. Taylor said.
Although surgical therapy treats local disease, medical therapy may be needed to treat the systemic manifestations.
During a question-and-answer period after the presentation, Marcelle I. Cedars, MD, asked whether analgesic and hormonal management may be sufficient when a woman has suspected or laparoscopically diagnosed endometriosis and pain is the primary complaint.
“Given the understanding of endometriosis, how would you suggest approaching treatment?” asked Dr. Cedars, president elect of the ASRM and director of the division of reproductive endocrinology and infertility at the University of California, San Francisco.
Analgesic and hormonal therapies remain “the best treatments we have,” Dr. Taylor said. He starts treatment with an oral contraceptive and a nonsteroidal anti-inflammatory medication – “not only for pain relief but to tamp some of the inflammation associated with endometriosis,” he said. If an oral contraceptive does not work, a gonadotropin-releasing hormone antagonist typically is the next step.
Dr. Taylor has disclosed ties to Dot Lab and AbbVie. Dr. Cedars had no disclosures.
Recognizing the systemic effects of endometriosis may help doctors better understand the experiences of patients with the disease and guide the approach to diagnosis and treatment, according to the president of the American Society for Reproductive Medicine (ASRM).
, Hugh S. Taylor, MD, said at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Its systemic manifestations may explain why women with endometriosis tend to have a lower body mass index, compared with women without the disease, Dr. Taylor said.
“Stem cells, microRNAs, and generalized inflammation are some of the mechanisms that mediate these long-range effects on distant organ systems,” he said.
Studies have indicated that lesions in the pelvis do not fully explain the disease, and investigators continue to elucidate how “endometriosis that we see in the pelvis is really just the tip of the iceberg,” said Dr. Taylor, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn.
Pain, including dysmenorrhea, pelvic pain, and dyspareunia, “can be just as bad with ... stage 1 disease as it can be with stage 4 disease,” he said.
Some patients may not have pain, but have infertility. Other women are asymptomatic, and doctors find endometriosis incidentally.
One common definition of endometriosis – ectopic endometrial glands and stroma predominantly caused by retrograde menstruation – “probably overly simplifies this complex disease,” said Dr. Taylor, who reviewed the current understanding of endometriosis in an article in The Lancet. “The lesions in the pelvis are important. We see them. We treat them. But endometriosis has ... effects throughout the body.”
Dr. Taylor’s research group has shown that stem cells are a potential source of endometriosis. “There are cells from the endometriosis that can be found traveling in the circulation,” but their effects are unclear, he said.
Levels of several microRNAs may be increased or decreased in women with endometriosis, and these altered levels may induce the production of inflammatory cytokines. They also may serve as the basis of a blood test for endometriosis that could be ready for clinical use soon, Dr. Taylor said.
In a mouse model of endometriosis, the disease changes the electrophysiology of the brain and behavior. “We see changes in anxiety induced by endometriosis. We see changes in pain sensitivity induced by endometriosis. And we also see an increase in depression induced by endometriosis in this animal model,” Dr. Taylor said.
Although surgical therapy treats local disease, medical therapy may be needed to treat the systemic manifestations.
During a question-and-answer period after the presentation, Marcelle I. Cedars, MD, asked whether analgesic and hormonal management may be sufficient when a woman has suspected or laparoscopically diagnosed endometriosis and pain is the primary complaint.
“Given the understanding of endometriosis, how would you suggest approaching treatment?” asked Dr. Cedars, president elect of the ASRM and director of the division of reproductive endocrinology and infertility at the University of California, San Francisco.
Analgesic and hormonal therapies remain “the best treatments we have,” Dr. Taylor said. He starts treatment with an oral contraceptive and a nonsteroidal anti-inflammatory medication – “not only for pain relief but to tamp some of the inflammation associated with endometriosis,” he said. If an oral contraceptive does not work, a gonadotropin-releasing hormone antagonist typically is the next step.
Dr. Taylor has disclosed ties to Dot Lab and AbbVie. Dr. Cedars had no disclosures.
Recognizing the systemic effects of endometriosis may help doctors better understand the experiences of patients with the disease and guide the approach to diagnosis and treatment, according to the president of the American Society for Reproductive Medicine (ASRM).
, Hugh S. Taylor, MD, said at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Its systemic manifestations may explain why women with endometriosis tend to have a lower body mass index, compared with women without the disease, Dr. Taylor said.
“Stem cells, microRNAs, and generalized inflammation are some of the mechanisms that mediate these long-range effects on distant organ systems,” he said.
Studies have indicated that lesions in the pelvis do not fully explain the disease, and investigators continue to elucidate how “endometriosis that we see in the pelvis is really just the tip of the iceberg,” said Dr. Taylor, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn.
Pain, including dysmenorrhea, pelvic pain, and dyspareunia, “can be just as bad with ... stage 1 disease as it can be with stage 4 disease,” he said.
Some patients may not have pain, but have infertility. Other women are asymptomatic, and doctors find endometriosis incidentally.
One common definition of endometriosis – ectopic endometrial glands and stroma predominantly caused by retrograde menstruation – “probably overly simplifies this complex disease,” said Dr. Taylor, who reviewed the current understanding of endometriosis in an article in The Lancet. “The lesions in the pelvis are important. We see them. We treat them. But endometriosis has ... effects throughout the body.”
Dr. Taylor’s research group has shown that stem cells are a potential source of endometriosis. “There are cells from the endometriosis that can be found traveling in the circulation,” but their effects are unclear, he said.
Levels of several microRNAs may be increased or decreased in women with endometriosis, and these altered levels may induce the production of inflammatory cytokines. They also may serve as the basis of a blood test for endometriosis that could be ready for clinical use soon, Dr. Taylor said.
In a mouse model of endometriosis, the disease changes the electrophysiology of the brain and behavior. “We see changes in anxiety induced by endometriosis. We see changes in pain sensitivity induced by endometriosis. And we also see an increase in depression induced by endometriosis in this animal model,” Dr. Taylor said.
Although surgical therapy treats local disease, medical therapy may be needed to treat the systemic manifestations.
During a question-and-answer period after the presentation, Marcelle I. Cedars, MD, asked whether analgesic and hormonal management may be sufficient when a woman has suspected or laparoscopically diagnosed endometriosis and pain is the primary complaint.
“Given the understanding of endometriosis, how would you suggest approaching treatment?” asked Dr. Cedars, president elect of the ASRM and director of the division of reproductive endocrinology and infertility at the University of California, San Francisco.
Analgesic and hormonal therapies remain “the best treatments we have,” Dr. Taylor said. He starts treatment with an oral contraceptive and a nonsteroidal anti-inflammatory medication – “not only for pain relief but to tamp some of the inflammation associated with endometriosis,” he said. If an oral contraceptive does not work, a gonadotropin-releasing hormone antagonist typically is the next step.
Dr. Taylor has disclosed ties to Dot Lab and AbbVie. Dr. Cedars had no disclosures.
FROM ACOG 2021
Which comes first in osteoarthritis: The damage or the pain?
Is innervation of cartilage the driving force behind development of osteoarthritis and subsequent pain, or is the degeneration of joints in osteoarthritis affecting nerves and creating pain?
This was the question underpinning a fascinating debate at the OARSI 2021 World Congress, featuring two giants of the OA research community: Anne-Marie Malfait, MD, PhD, professor of medicine in the division of rheumatology at Rush Medical College, Chicago, and Stefan Lohmander, MD, PhD, professor emeritus of orthopedics at Lund (Sweden) University in Sweden.
At stake in the discussion is a greater understanding of the physiological processes that underpin both the development of OA in joints and the experience of pain in patients with OA.
Dr. Lohmander started by pointing out that, while pain is the primary symptoms of OA, it does not always overlap with the physiological processes of the disease, as measured by techniques such as MRI, x-ray, biomarkers, and gait analysis.
“This lack of complete overlap is often a problem when doing our clinical trials,” Dr. Lohmander told the conference, sponsored by Osteoarthritis Research Society International. “When talking about osteoarthritis, we also need to remind ourselves every so often that we are speaking of either the symptoms or the disease and maybe not always the both of them.”
While a healthy joint has pain receptors everywhere but the cartilage, studies have found that the osteoarthritic joint brings blood vessels, sensory nerves, and cells expressing nerve growth factor from the subchondral bone into even noncalcified articular cartilage, he said.
These nociceptor neurons are mechanosensitive, so mechanical injury to the joint triggers inflammation, and the inflammatory proteins themselves act on the nociceptors to generate pain signals in the brain, “so clearly, it is the joint that signals the brain,” Dr. Lohmander said.
However, Dr. Malfait pointed out that there is a body of evidence from animal studies showing that the absence of sensory nerves in joints – either from disease or removal – is associated with the onset or worsening of OA.
“Healthy nerves are really important to ensure healthy joints,” Dr. Malfait said. She said age-related loss of sensory nerves always preceded age-related OA, and was also associated with age-related loss of proprioception and vibratory perception.
Interestingly, animal studies suggest that removing intra-articular nociceptors can actually have a protective effect on the osteoarthritic joint, Dr. Malfait said. Studies in humans who have experienced neurologic lesions also suggests improvement in conditions such as rheumatoid arthritis.
She raised the idea of neurogenic inflammation: that peripheral neurons are releasing vasoactive mediators that contribute to inflammation in tissues. “These nerves and nerve products are talking to all the different cells in the joints,” she said.
Defending his argument that joint pathology is the cause of pain, not the pain causing the joint pathology, Dr. Lohmander gave the example of studies that looked at radiographic abnormalities between two knees of the same patient who also had discordant pain measures for each knee. This research “showed strong association between radiographic osteoarthritis and knee pain, supporting the argument that structural abnormalities cause knee pain,” he said.
Martin van der Esch, PhD, of the Amsterdam University of Applied Sciences, said the debate was one of the highlights of the conference because it addressed such an important and longstanding question in OA.
“Is osteoarthritis leading to a generalized pain, so involvement of the nervous system, but the source – the causality – is in the joint?” he said in an interview. “Or is it the other way around, so that means is there first a problem inside the nervous system – including also the vascular system – and which is presented in the joint?”
It is more than an academic discussion because the conclusions of that could mean different treatment approaches are needed for different groups of patients, and raises the different ways of thinking about OA, he said.
None of the sources for this story declared having any relevant conflicts of interest.
Is innervation of cartilage the driving force behind development of osteoarthritis and subsequent pain, or is the degeneration of joints in osteoarthritis affecting nerves and creating pain?
This was the question underpinning a fascinating debate at the OARSI 2021 World Congress, featuring two giants of the OA research community: Anne-Marie Malfait, MD, PhD, professor of medicine in the division of rheumatology at Rush Medical College, Chicago, and Stefan Lohmander, MD, PhD, professor emeritus of orthopedics at Lund (Sweden) University in Sweden.
At stake in the discussion is a greater understanding of the physiological processes that underpin both the development of OA in joints and the experience of pain in patients with OA.
Dr. Lohmander started by pointing out that, while pain is the primary symptoms of OA, it does not always overlap with the physiological processes of the disease, as measured by techniques such as MRI, x-ray, biomarkers, and gait analysis.
“This lack of complete overlap is often a problem when doing our clinical trials,” Dr. Lohmander told the conference, sponsored by Osteoarthritis Research Society International. “When talking about osteoarthritis, we also need to remind ourselves every so often that we are speaking of either the symptoms or the disease and maybe not always the both of them.”
While a healthy joint has pain receptors everywhere but the cartilage, studies have found that the osteoarthritic joint brings blood vessels, sensory nerves, and cells expressing nerve growth factor from the subchondral bone into even noncalcified articular cartilage, he said.
These nociceptor neurons are mechanosensitive, so mechanical injury to the joint triggers inflammation, and the inflammatory proteins themselves act on the nociceptors to generate pain signals in the brain, “so clearly, it is the joint that signals the brain,” Dr. Lohmander said.
However, Dr. Malfait pointed out that there is a body of evidence from animal studies showing that the absence of sensory nerves in joints – either from disease or removal – is associated with the onset or worsening of OA.
“Healthy nerves are really important to ensure healthy joints,” Dr. Malfait said. She said age-related loss of sensory nerves always preceded age-related OA, and was also associated with age-related loss of proprioception and vibratory perception.
Interestingly, animal studies suggest that removing intra-articular nociceptors can actually have a protective effect on the osteoarthritic joint, Dr. Malfait said. Studies in humans who have experienced neurologic lesions also suggests improvement in conditions such as rheumatoid arthritis.
She raised the idea of neurogenic inflammation: that peripheral neurons are releasing vasoactive mediators that contribute to inflammation in tissues. “These nerves and nerve products are talking to all the different cells in the joints,” she said.
Defending his argument that joint pathology is the cause of pain, not the pain causing the joint pathology, Dr. Lohmander gave the example of studies that looked at radiographic abnormalities between two knees of the same patient who also had discordant pain measures for each knee. This research “showed strong association between radiographic osteoarthritis and knee pain, supporting the argument that structural abnormalities cause knee pain,” he said.
Martin van der Esch, PhD, of the Amsterdam University of Applied Sciences, said the debate was one of the highlights of the conference because it addressed such an important and longstanding question in OA.
“Is osteoarthritis leading to a generalized pain, so involvement of the nervous system, but the source – the causality – is in the joint?” he said in an interview. “Or is it the other way around, so that means is there first a problem inside the nervous system – including also the vascular system – and which is presented in the joint?”
It is more than an academic discussion because the conclusions of that could mean different treatment approaches are needed for different groups of patients, and raises the different ways of thinking about OA, he said.
None of the sources for this story declared having any relevant conflicts of interest.
Is innervation of cartilage the driving force behind development of osteoarthritis and subsequent pain, or is the degeneration of joints in osteoarthritis affecting nerves and creating pain?
This was the question underpinning a fascinating debate at the OARSI 2021 World Congress, featuring two giants of the OA research community: Anne-Marie Malfait, MD, PhD, professor of medicine in the division of rheumatology at Rush Medical College, Chicago, and Stefan Lohmander, MD, PhD, professor emeritus of orthopedics at Lund (Sweden) University in Sweden.
At stake in the discussion is a greater understanding of the physiological processes that underpin both the development of OA in joints and the experience of pain in patients with OA.
Dr. Lohmander started by pointing out that, while pain is the primary symptoms of OA, it does not always overlap with the physiological processes of the disease, as measured by techniques such as MRI, x-ray, biomarkers, and gait analysis.
“This lack of complete overlap is often a problem when doing our clinical trials,” Dr. Lohmander told the conference, sponsored by Osteoarthritis Research Society International. “When talking about osteoarthritis, we also need to remind ourselves every so often that we are speaking of either the symptoms or the disease and maybe not always the both of them.”
While a healthy joint has pain receptors everywhere but the cartilage, studies have found that the osteoarthritic joint brings blood vessels, sensory nerves, and cells expressing nerve growth factor from the subchondral bone into even noncalcified articular cartilage, he said.
These nociceptor neurons are mechanosensitive, so mechanical injury to the joint triggers inflammation, and the inflammatory proteins themselves act on the nociceptors to generate pain signals in the brain, “so clearly, it is the joint that signals the brain,” Dr. Lohmander said.
However, Dr. Malfait pointed out that there is a body of evidence from animal studies showing that the absence of sensory nerves in joints – either from disease or removal – is associated with the onset or worsening of OA.
“Healthy nerves are really important to ensure healthy joints,” Dr. Malfait said. She said age-related loss of sensory nerves always preceded age-related OA, and was also associated with age-related loss of proprioception and vibratory perception.
Interestingly, animal studies suggest that removing intra-articular nociceptors can actually have a protective effect on the osteoarthritic joint, Dr. Malfait said. Studies in humans who have experienced neurologic lesions also suggests improvement in conditions such as rheumatoid arthritis.
She raised the idea of neurogenic inflammation: that peripheral neurons are releasing vasoactive mediators that contribute to inflammation in tissues. “These nerves and nerve products are talking to all the different cells in the joints,” she said.
Defending his argument that joint pathology is the cause of pain, not the pain causing the joint pathology, Dr. Lohmander gave the example of studies that looked at radiographic abnormalities between two knees of the same patient who also had discordant pain measures for each knee. This research “showed strong association between radiographic osteoarthritis and knee pain, supporting the argument that structural abnormalities cause knee pain,” he said.
Martin van der Esch, PhD, of the Amsterdam University of Applied Sciences, said the debate was one of the highlights of the conference because it addressed such an important and longstanding question in OA.
“Is osteoarthritis leading to a generalized pain, so involvement of the nervous system, but the source – the causality – is in the joint?” he said in an interview. “Or is it the other way around, so that means is there first a problem inside the nervous system – including also the vascular system – and which is presented in the joint?”
It is more than an academic discussion because the conclusions of that could mean different treatment approaches are needed for different groups of patients, and raises the different ways of thinking about OA, he said.
None of the sources for this story declared having any relevant conflicts of interest.
FROM OARSI 2021
How to help runners steer clear of injury
Approximately 60 million people in the United States run for exercise at least once a calendar year, with approximately 11 million of them running > 100 days a year.1,2 Running is an affordable, convenient, and efficient form of exercise, whose benefits include a decrease in the risk of all-cause early mortality, cancer, and diabetes; an improved lipid profile; and better mental health.3
However, running is also the cause of a significant percentage of exercise-associated injuries: More than 60% of runners report overuse injury annually.4 Given the high incidence of running-related injury, an important component of primary care is accurately diagnosing and managing such injuries and counseling patients about how to prevent them.

This article reviews risk factors for running-related injury and summarizes evidence-based recommendations for prevention.
CASE
During a health maintenance examination, Clara K, a 47-year-old woman who is obese (body mass index [BMI], 34) and has bilateral knee osteoarthritis (OA), inquires about establishing a weight-loss strategy. Ms. K is interested in starting an exercise regimen involving running but is worried about provoking a flare of OA pain.
Risk factors for running injuries
Several risk factors—some modifiable, others nonmodifiable—are associated with running-related injury (TABLE 14-16). In addition, research suggests that other variables once thought to be risk factors, such as running surface and the Q-angle (described later), are not associated with running-related injury.
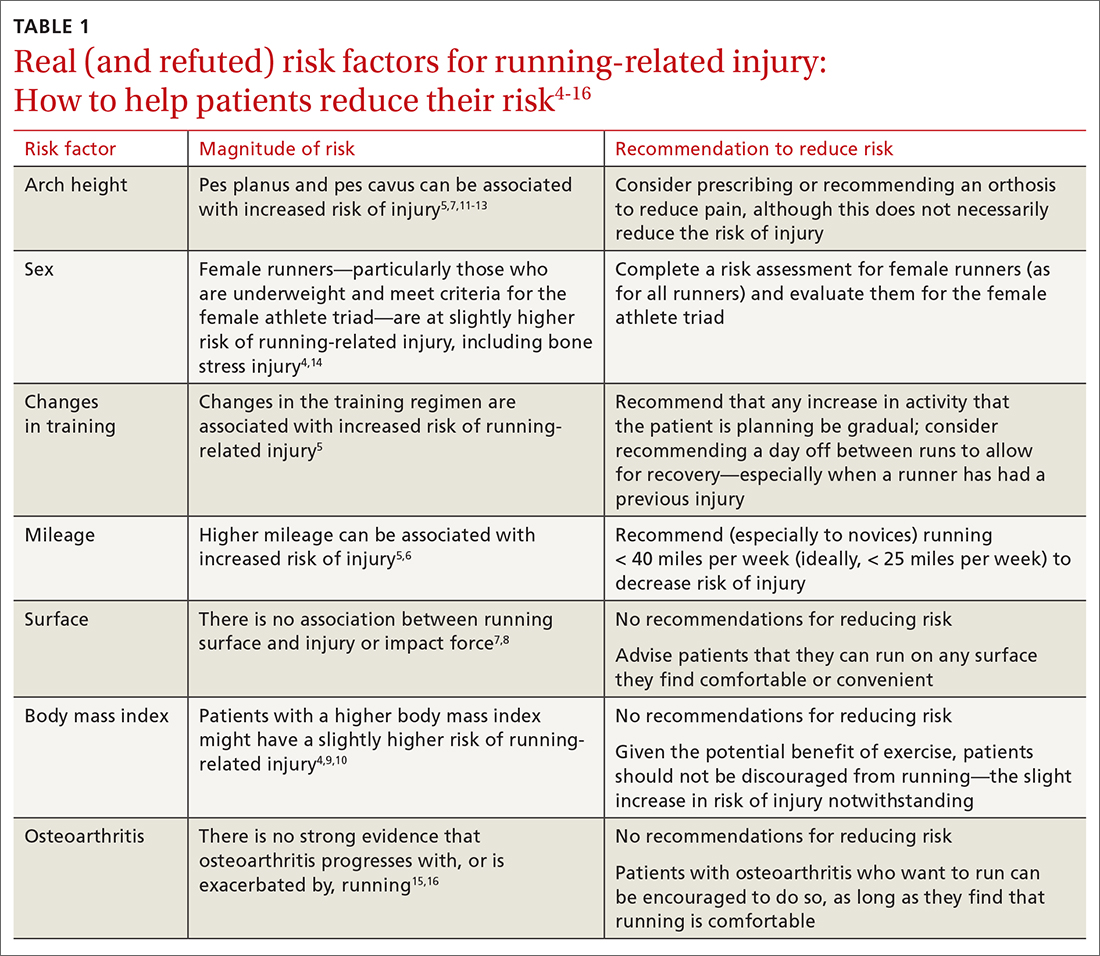
Modifiable risk factors
Changes in a training regimen or type of training. Many runners escalate training regimens as their fitness improves. Increasing mileage and changing the type of training (such as introducing hills or interval training) are independent risk factors for sustaining injury.5
The traditional recommendation has been for a runner to slowly increase or modify training with a 10% weekly increase in mileage or intensity.17 However, a randomized controlled trial failed to show a lower incidence of injury among amateur runners who adopted a graded exercise program.18 Regardless: It is still prudent to recommend a gradual increase in activity, such as taking ≥ 1 day off between running workouts or starting with a walking or jogging program, especially when there is a history of injury.19
Continue to: Excessive mileage
Excessive mileage. Many runners aspire to complete high-mileage runs. There is low-quality evidence demonstrating that high-mileage running, especially > 40 miles per week, is associated with increased risk of running-related injury.5 Injuries that occur with higher mileage are more often those of the hip and hamstring.5 A study noted that running ≤ 25 miles a week was protective against calf injury.6
Overall, there is little evidence to show that high-mileage running is associated with increased risk of running-related injury. However, this is still a risk factor that you should address with patients who have a running program—especially novices and those who ramp up mileage quickly.
Type of surface. Access to running surfaces—concrete, pavement, trails, treadmills, and athletic tracks—varies by time of day and season. Softer surfaces include treadmill, tracks, and trails; harder surfaces include asphalt and concrete.
There are limited data linking running surface with risk of injury.7 A study did not find an association between peak impact force based on running surface8; the authors hypothesized that runners compensate for a harder surface by making kinematic adjustments to minimize impact. With no strong evidence to link running-related injury to a particular running surface, patients should not be restricted to a softer running surface unless they notice a difference in comfort, because it is likely that they can compensate for a harder surface by adapting their gait.
Patients can therefore be counseled to run locally on sidewalks and neighborhood streets—if safe to do so—instead of obtaining a gym membership or driving to run on a trail. Such reassurance can increase a patient’s access to running and reduce barriers to exercise.
Continue to: BMI
BMI. Elevated BMI increases joint contact forces, which might increase risk of pain and injury.20 Results of studies investigating the link between BMI and running injury are mixed; some report that, in regard to bone stress injury, overweight BMI (> 25) is a risk factor for male runners and underweight BMI (< 18.5) is a risk factor for female runners.4,6 An observational study concluded that, among half-marathon and marathon runners, there was no significant increase in race-related injury, based on BMI.9 However, another study showed a higher rate of running-related injury in novice runners who had a higher BMI.10 A prospective cohort study found that runners with a higher BMI reported increased knee stiffness, which can place a runner at higher risk of overuse injury.4
Although these results conflict, there is consistency in the finding that obese novice runners are likely at increased risk of running-related injury; it is reasonable, therefore, for you to discuss strategies to reduce the risk of other modifiable factors, especially among obese novice runners. Patients with a higher BMI should not be discouraged from running, because exercise in combination with healthy eating habits is essential to decrease the myriad adverse health outcomes associated with obesity.
Female runners with a lower BMI, especially in the presence of other components of the female athlete triad (inadequate nutrition, amenorrhea, and low bone density), should be counseled about their increased risk of bone stress injury.21 Notably, a study of female US Navy recruits randomized to receive a trial of dietary supplementation of vitamin D plus calcium, or placebo, showed a 21% lower incidence of bone stress injury in the active-treatment group.22 To mitigate risk of injury associated with low BMI and the female athlete triad, therefore, a multidisciplinary approach of nutrition intervention, dietary optimization of vitamin D and calcium, and, possibly, activity modification should be implemented when appropriate.
Running gait. A study using 2-dimensional gait analysis to visualize biomechanical running patterns in injured and noninjured runners found that, in regard to mechanical variables, running-related injury was most strongly associated with contralateral pelvic drop.23 Gait retraining can be employed to help decrease contralateral pelvic drop.24 In addition, pelvic drop is often a result of weak gluteal muscles, and can be improved by doing strengthening exercises at home or with physical therapy.
Longer stride is also associated with running-related injury.25 A study showed improvement in patellofemoral pain by having runners increase stride rate by 10%, which reduces stride length to a significant degree.25,26 These improvements were maintained at 1-month and 3-month follow-up, and required only 1 gait retraining session.
Continue to: Get analysis is not feasible...
Gait analysis is not feasible in most primary care clinics. Instead, patients who run and (1) in whom pain persists despite more traditional treatments and (2) who have had recurring injury should be referred to a gait lab for analysis, usually by a physical therapist.
Nonmodifiable risk factors
Arch height. A high arch (pes cavus) is associated with increased risk of running-related injury, including bone stress injury, Achilles tendinopathy, plantar fasciitis, and patellofemoral pain syndrome.5 The mechanism of injury is thought to be increased forefoot loading forces.1
A review article showed that patients with pes cavus have reduced pain when using an orthosis, although there is no associated decrease in the risk of injury.5 To the contrary, a prospective study concluded that arch height was unrelated to increased risk of running-related injury.7
Evidence regarding flat feet (pes planus) and risk of injury is also mixed. Some studies show that pes planus is not associated with increased risk of injury in athletes.12 A cross-sectional study in older patients showed those with pes planus morphology had a higher rate of knee pain and wearing away of medial compartment cartilage.13 Because this study comprised only older adults, it is not generalizable to runners—nor can conclusions be drawn about causation, given the cross-sectional nature of the study.
Although a foot orthosis can correct mechanical differences caused by pes planus morphology, there is not enough evidence to conclude that correction results in a lower rate of injury. In sum, data are mixed with regard to arch height as a risk factor for running-related injury.
Continue to: Patients with...
Patients with pes planus or pes cavus should not be discouraged from running, however. If they experience pain with running, they might benefit from a trial of arch support inserts; or consider referral to an orthotist for evaluation for a custom orthosis.
Sex. Based on a prospective cohort study, female runners have a slightly higher rate of running injury than male counterparts.4 Similarly, a study showed that female military members generally had a higher incidence of stress fractures than male military members—specifically, femoral shaft and neck stress fractures.14 Runners who fall in the spectrum of the female athlete triad, as described earlier, are particularly vulnerable to bone stress injury. It is reasonable, therefore, to review risk factors for injury with female runners (as it is with all runners), especially those who have sustained a prior running-related injury.
Increased Q-angle (an obsolete risk factor). The Q-angle is approximated by drawing a line from the anterior superior iliac spine to the patella and a second line from the patella to the tibial tubercle. In males, a normal Q-angle is 14°; in females, 17° (SD = 4.5°). The Q-angle can be obtained by goniometric or radiographic measurement.
An increased Q-angle had been considered an intrinsic risk factor for running injury but has not been shown to be associated with increased risk of running-related injury or patellofemoral pain syndrome.27,28 Because the Q-angle is not a clinically relevant tool in assessing risk of injury, do not routinely measure it or include it in risk-factor counseling.
OA. Based on a systematic review of observational studies, data are inconclusive with regard to whether running contributes to, or is protective against, knee OA.15 In a large cohort study, running (1) was protective against development of hip OA and (2) decreased the risk of requiring hip replacement.29 This finding was supported by animal-model research that concluded that it is inactivity that results in thinning of articular cartilage.29 In addition, a systematic review of randomized controlled trials concluded that knee joint-loading exercises are not harmful to articular cartilage (this is low-quality evidence, however).16
Continue to: Given that there...
Given that there are no high-quality studies suggesting that running contributes to or exacerbates OA, patients with OA can be counseled to start or continue running as tolerated because the health benefit of running likely outweighs risk. Patients with pre-existing moderate-to-severe OA might report knee and hip pain that is already exacerbated by certain activities; if a high-impact activity, such as running, makes that pain worse, exercise counseling that you provide can be tailored to include lower-impact alternatives, such as swimming, cycling, or an elliptical workout.
CASE
In response to Ms. K’s interest in beginning an exercise regimen that includes running, you perform a complete routine pre-participation evaluation and appropriate cardiac screening. You discuss risk factors for running injury, focusing on modifiable risk factors.
Ms. K is perimenopausal but reports a history of regular menstrual cycles. She eats a relatively well-balanced diet. You advise that her BMI should not restrict her from incorporating running into her fitness regimen. Also, you reassure her that she should not restrict running based on a diagnosis of OA; instead, you advise her to monitor her symptoms and reconsider her program if running makes her knee pain worse.
At this point, Ms. K is ready to run. She tells you that, based on your guidance, she feels more comfortable and safe starting a running program.
Preventing injury
After reviewing risk factors for running-related injury with patients, encourage other evidence-based methods of reducing that risk.
Continue to: Shoes
Shoes
The running shoe industry offers a variety of running shoes, from minimalist shoes to cushioned stability shoes that vary based on the amount of cushioning, level of motion control, and amount of heel-to-toe drop. With so many options, new runners might wonder which shoes can reduce their risk of injury and how they should select a pair.
Stability. A characteristic of running shoes promoted by the industry is their stability: ie, their motion control. Stability shoes are marketed to runners who overpronate and therefore limit motion to prevent overpronation. The benefit of stability shoes, or stability insoles, is unclear.30 A randomized controlled trial showed that, in runners who overpronate, motion-control shoes reduced their risk of injury.31 However, another study assessed whether shoes that had been “prescribed” based on foot morphology and stride reduced the risk of injury (compared to neutral, cushioned shoes) and found no change in the incidence of soft-tissue injury.32 Given no strong evidence to suggest otherwise, runners can be advised to buy shoes based on comfort rather than on foot morphology or running stride.
Heel-to-toe drop. Another component of shoe variability is heel-to-toe drop (the height difference between heel and forefoot). A study suggests that moderate-to-high (8-12 mm) heel-to-toe drop is associated with a reduced risk of running injury.33 Barefoot running shoes, which, typically, have no heel-to-toe drop, are associated with increased risk of injury—specifically, foot stress fracture (especially in runners who are even moderately overweight).34,35
Shoe age and shoe wear can be modified to reduce injury. There is evidence that running shoes lose approximately 50% of cushioning after 300 to 500 miles of use.36 Another study found that rotating running shoes—ideally, different types or brands—can lead to fewer running-related injuries.37
In general, patients can be counseled to use shoes that feel comfortable, as long as they replace them regularly (TABLE 2). Runners can also consider alternating pairs of different running shoes between runs. Overweight runners should avoid minimally cushioned and low heel-to-toe drop running shoes.

Continue to: Cross-training
Cross-training
Cross-training exercises for runners include cycling, an elliptical workout, swimming, and weightlifting. Incorporating cross-training can be protective against running injury because cross-training requires different movement patterns, prevents overuse, and equalizes muscle imbalances that occur with running.7 In addition, replacing running with a cross-training activity can decrease weekly running time and mileage, which can further reduce risk of running-related injury.7 Runners—especially higher-mileage runners—should be encouraged to incorporate cross-training into their workout regimen to decrease their risk of injury.
Stretching. The authors of a Cochrane review concluded that there is no significant reduction in injury associated with hamstring or gastrocnemius stretching.32 A small randomized, controlled, crossover study concluded that participants subjectively felt their performance was better when warm-ups included stretching.38 This perceived improvement in performance was similar between groups who completed dynamic or static stretching. However, no difference was noted in flexibility or objective performance between groups who stretched or did not stretch before activity.
Although there is no supporting evidence that stretching reduces the risk of injury, stretching is a low-risk intervention. Because stretching might provide subjective benefit to runners, you need not discourage patients from including this activity in their running program.
CORRESPONDENCE
Kartik Sidhar, MD, 15370 Huff Way, Brookfield, WI, 53005; [email protected]
1. Brown CR Jr. Common injuries from running. In: Imboden JB, Hellerman, DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. McGraw-Hill; 2013.
2. Lange D. Running & jogging - statistic and facts. Statista Web site. November 16, 2020. Accessed March 28, 2021. www.statista.com/topics/1743/running-and-jogging/
3. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541-556. doi:10.1097/HCO.0000000000000437
4. Messier SP, Martin DF, Mihalko SL, et al. A 2-year prospective cohort study of overuse running injuries: The Runners and Injury Longitudinal Study (TRAILS). Am J Sports Med. 2018;46:2211-2221. doi:10.1177/0363546518773755
5. Fields KB, Sykes JC, Walker KM, et al. Prevention of running injuries. Curr Sports Med Rep. 2010;9:176-182. doi:10.1249/JSR.0b013e3181de7ec5
6. van der Worp MP, ten Haaf DSM, van Cingel R. Injuries in runners; a systematic review on risk factors and sex differences. PLoS One. 2015;10:1-18. doi:10.1371/journal.pone.0114937
7. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244. doi:10.1136/bjsm.37.3.239
8. Dixon SJ, Collop AC, Batt ME. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919-1926. doi:10.1097/00005768-200011000-00016
9. Vadeboncoeur TF, Silvers SM, Taylor WC, et al. Impact of a high body mass index on lower extremity injury in marathon/half-marathon participants. J Phys Act Health. 2012;9:96-103. doi:10.1123/jpah.9.1.96
10. Buist I, Bredeweg SW. Higher risk of injury in overweight novice runners. Br J Sports Med. 2011;45:338. http://dx.doi.org/10.1136/bjsm.2011.084038.79
11. Cowan DN, Jones BH, Robinson JR. Foot morphologic characteristics and risk of Exercise-related injury. Arch Fam Med. 1993;2:773-777. doi:10.1001/archfami.2.7.773
12. Michelson JD, Durant DM, McFarland E. The injury risk associated with pes planus in athletes. Foot Ankle Int. 2002;23:629-633. doi: 10.1177/107110070202300708
13. Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken). 2011;63:937-944. doi:10.1002/acr.20431
14. Waterman BR, Gun B, Bader JO, et al. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181:1308-1313. doi:10.7205/MILMED-D-15-00571
15. Timmins KA, Leech RD, Batt ME, et al. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447-1457. doi:10.1177/0363546516657531
16. Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2019;53:940-947. doi:10.1136/bjsports-2017-098661
17. Johnston CAM, Taunton JE, Lloyd-Smith DR, et al. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101-1109.
18. Buist I, Bredeweg SW, van Mechelen W, et al. No effect of a graded training program on the number of running-related injuries in novice runners: a randomized controlled trial. Am J Sports Med. 2008;36:33-39. doi:10.1177/0363546507307505
19. Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44:749-765. doi:10.2519/jospt.2014.5334
20. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209-214. doi:10.1016/j.gaitpost.2019.07.307
21. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139-149. doi:10.1016/j.pmr.2015.08.008
22. Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. doi:10.1359/jbmr.080102
23. Bramah C, Preece SJ, Gill N, et al. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46:3023-3031. doi:10.1177/0363546518793657
24. Willy RW, Scholz PT, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol Avon). 2012;27:1045-1051. doi:10.1016/j.clinbiomech.2012.07.011
25. Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014;6:210-217. doi:10.1177/1941738113508544
26. Bramah C, Preece SJ, Gill N et al. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. Am J Sports Med. 2019;47:3406-3413. doi: 10.1177/0363546519879693
27. Ramskov D, Jensen ML, Obling K, et al. No association between q-angle and foot posture with running-related injuries: a 10 week prospective follow-up study. Int J Sports Phys Ther. 2013;8:407-415.
28. Almeida GPL, Silva AP, França FJR, et al. Q-angle in patellofemoral pain: relationship with dynamic knee valgus, hip abductor torque, pain and function. Rev Bras Ortop. 2016;51:181-186. doi:10.1016/j.rboe.2016.01.010
29. Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292-1297. doi:10.1249/MSS.0b013e3182885f26
30. Nigg BM, Baltich J, Hoerzer S, et al. Running shoes and running injuries: mythbusting and a proposal for two new paradigms: ‘Preferred movement path’ and ‘comfort filter.’ Br J Sports Med. 2015;49:1290-1294. doi:10.1136/bjsports-2015-095054
31. Malisoux L, Chambon N, Delattre N, et al. Injury risk in runners using standard or motion control shoes: a randomised controlled trial with participant and assessor blinding. Br J Sports Med. 2016;50:481-487. doi:10.1136/bjsports-2015-095031
32. Yeung SS, Yeung EW, Gillespie LD. Interventions for preventing lower limb soft-tissue running injuries. Cochrane Database Syst Rev. 2011(7):CD001256. doi:10.1002/14651858.cd001256.pub2
33. Malisoux L, Chambon N, Urhausen A, et al. Influence of the heel-to-toe drop of standard cushioned running shoes on injury risk in leisure-time runners: a randomized controlled trial with 6-month follow-up. Am J Sports Med. 2016;44:2933-2940. doi:10.1177/0363546516654690
34. Ryan M, Elashi M, Newsham-West R, et al. Examining injury risk and pain perception in runners using minimalist footwear. Br J Sports Med. 2014;48:1257-1262. doi:10.1136/bjsports-2012-092061
35. Fuller JT, Thewlis D, Buckley JD, et al.. Body mass and weekly training distance influence the pain and injuries experienced by runners using minimalist shoes: a randomized controlled trial. Am J Sports Med. 2017;45:1162-1170. doi:10.1177/0363546516682497
36. Cook SD, Kester MA, Brunet ME. Shock absorption characteristics of running shoes. Am J Sports Med. 1985;13:248-253. doi.org/10.1177/036354658501300406
37. Malisoux L, Ramesh J, Mann R, et al. Can parallel use of different running shoes decrease running-related injury risk? Scand J Med Sci Sport. 2015;25:110-115. doi:10.1111/sms.12154
38. Blazevich AJ, Gill ND, Kvorning T, et al. No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Med Sci Sports Exerc. 2018;50:1258-1266. doi:10.1249/MSS.0000000000001539
Approximately 60 million people in the United States run for exercise at least once a calendar year, with approximately 11 million of them running > 100 days a year.1,2 Running is an affordable, convenient, and efficient form of exercise, whose benefits include a decrease in the risk of all-cause early mortality, cancer, and diabetes; an improved lipid profile; and better mental health.3
However, running is also the cause of a significant percentage of exercise-associated injuries: More than 60% of runners report overuse injury annually.4 Given the high incidence of running-related injury, an important component of primary care is accurately diagnosing and managing such injuries and counseling patients about how to prevent them.

This article reviews risk factors for running-related injury and summarizes evidence-based recommendations for prevention.
CASE
During a health maintenance examination, Clara K, a 47-year-old woman who is obese (body mass index [BMI], 34) and has bilateral knee osteoarthritis (OA), inquires about establishing a weight-loss strategy. Ms. K is interested in starting an exercise regimen involving running but is worried about provoking a flare of OA pain.
Risk factors for running injuries
Several risk factors—some modifiable, others nonmodifiable—are associated with running-related injury (TABLE 14-16). In addition, research suggests that other variables once thought to be risk factors, such as running surface and the Q-angle (described later), are not associated with running-related injury.

Modifiable risk factors
Changes in a training regimen or type of training. Many runners escalate training regimens as their fitness improves. Increasing mileage and changing the type of training (such as introducing hills or interval training) are independent risk factors for sustaining injury.5
The traditional recommendation has been for a runner to slowly increase or modify training with a 10% weekly increase in mileage or intensity.17 However, a randomized controlled trial failed to show a lower incidence of injury among amateur runners who adopted a graded exercise program.18 Regardless: It is still prudent to recommend a gradual increase in activity, such as taking ≥ 1 day off between running workouts or starting with a walking or jogging program, especially when there is a history of injury.19
Continue to: Excessive mileage
Excessive mileage. Many runners aspire to complete high-mileage runs. There is low-quality evidence demonstrating that high-mileage running, especially > 40 miles per week, is associated with increased risk of running-related injury.5 Injuries that occur with higher mileage are more often those of the hip and hamstring.5 A study noted that running ≤ 25 miles a week was protective against calf injury.6
Overall, there is little evidence to show that high-mileage running is associated with increased risk of running-related injury. However, this is still a risk factor that you should address with patients who have a running program—especially novices and those who ramp up mileage quickly.
Type of surface. Access to running surfaces—concrete, pavement, trails, treadmills, and athletic tracks—varies by time of day and season. Softer surfaces include treadmill, tracks, and trails; harder surfaces include asphalt and concrete.
There are limited data linking running surface with risk of injury.7 A study did not find an association between peak impact force based on running surface8; the authors hypothesized that runners compensate for a harder surface by making kinematic adjustments to minimize impact. With no strong evidence to link running-related injury to a particular running surface, patients should not be restricted to a softer running surface unless they notice a difference in comfort, because it is likely that they can compensate for a harder surface by adapting their gait.
Patients can therefore be counseled to run locally on sidewalks and neighborhood streets—if safe to do so—instead of obtaining a gym membership or driving to run on a trail. Such reassurance can increase a patient’s access to running and reduce barriers to exercise.
Continue to: BMI
BMI. Elevated BMI increases joint contact forces, which might increase risk of pain and injury.20 Results of studies investigating the link between BMI and running injury are mixed; some report that, in regard to bone stress injury, overweight BMI (> 25) is a risk factor for male runners and underweight BMI (< 18.5) is a risk factor for female runners.4,6 An observational study concluded that, among half-marathon and marathon runners, there was no significant increase in race-related injury, based on BMI.9 However, another study showed a higher rate of running-related injury in novice runners who had a higher BMI.10 A prospective cohort study found that runners with a higher BMI reported increased knee stiffness, which can place a runner at higher risk of overuse injury.4
Although these results conflict, there is consistency in the finding that obese novice runners are likely at increased risk of running-related injury; it is reasonable, therefore, for you to discuss strategies to reduce the risk of other modifiable factors, especially among obese novice runners. Patients with a higher BMI should not be discouraged from running, because exercise in combination with healthy eating habits is essential to decrease the myriad adverse health outcomes associated with obesity.
Female runners with a lower BMI, especially in the presence of other components of the female athlete triad (inadequate nutrition, amenorrhea, and low bone density), should be counseled about their increased risk of bone stress injury.21 Notably, a study of female US Navy recruits randomized to receive a trial of dietary supplementation of vitamin D plus calcium, or placebo, showed a 21% lower incidence of bone stress injury in the active-treatment group.22 To mitigate risk of injury associated with low BMI and the female athlete triad, therefore, a multidisciplinary approach of nutrition intervention, dietary optimization of vitamin D and calcium, and, possibly, activity modification should be implemented when appropriate.
Running gait. A study using 2-dimensional gait analysis to visualize biomechanical running patterns in injured and noninjured runners found that, in regard to mechanical variables, running-related injury was most strongly associated with contralateral pelvic drop.23 Gait retraining can be employed to help decrease contralateral pelvic drop.24 In addition, pelvic drop is often a result of weak gluteal muscles, and can be improved by doing strengthening exercises at home or with physical therapy.
Longer stride is also associated with running-related injury.25 A study showed improvement in patellofemoral pain by having runners increase stride rate by 10%, which reduces stride length to a significant degree.25,26 These improvements were maintained at 1-month and 3-month follow-up, and required only 1 gait retraining session.
Continue to: Get analysis is not feasible...
Gait analysis is not feasible in most primary care clinics. Instead, patients who run and (1) in whom pain persists despite more traditional treatments and (2) who have had recurring injury should be referred to a gait lab for analysis, usually by a physical therapist.
Nonmodifiable risk factors
Arch height. A high arch (pes cavus) is associated with increased risk of running-related injury, including bone stress injury, Achilles tendinopathy, plantar fasciitis, and patellofemoral pain syndrome.5 The mechanism of injury is thought to be increased forefoot loading forces.1
A review article showed that patients with pes cavus have reduced pain when using an orthosis, although there is no associated decrease in the risk of injury.5 To the contrary, a prospective study concluded that arch height was unrelated to increased risk of running-related injury.7
Evidence regarding flat feet (pes planus) and risk of injury is also mixed. Some studies show that pes planus is not associated with increased risk of injury in athletes.12 A cross-sectional study in older patients showed those with pes planus morphology had a higher rate of knee pain and wearing away of medial compartment cartilage.13 Because this study comprised only older adults, it is not generalizable to runners—nor can conclusions be drawn about causation, given the cross-sectional nature of the study.
Although a foot orthosis can correct mechanical differences caused by pes planus morphology, there is not enough evidence to conclude that correction results in a lower rate of injury. In sum, data are mixed with regard to arch height as a risk factor for running-related injury.
Continue to: Patients with...
Patients with pes planus or pes cavus should not be discouraged from running, however. If they experience pain with running, they might benefit from a trial of arch support inserts; or consider referral to an orthotist for evaluation for a custom orthosis.
Sex. Based on a prospective cohort study, female runners have a slightly higher rate of running injury than male counterparts.4 Similarly, a study showed that female military members generally had a higher incidence of stress fractures than male military members—specifically, femoral shaft and neck stress fractures.14 Runners who fall in the spectrum of the female athlete triad, as described earlier, are particularly vulnerable to bone stress injury. It is reasonable, therefore, to review risk factors for injury with female runners (as it is with all runners), especially those who have sustained a prior running-related injury.
Increased Q-angle (an obsolete risk factor). The Q-angle is approximated by drawing a line from the anterior superior iliac spine to the patella and a second line from the patella to the tibial tubercle. In males, a normal Q-angle is 14°; in females, 17° (SD = 4.5°). The Q-angle can be obtained by goniometric or radiographic measurement.
An increased Q-angle had been considered an intrinsic risk factor for running injury but has not been shown to be associated with increased risk of running-related injury or patellofemoral pain syndrome.27,28 Because the Q-angle is not a clinically relevant tool in assessing risk of injury, do not routinely measure it or include it in risk-factor counseling.
OA. Based on a systematic review of observational studies, data are inconclusive with regard to whether running contributes to, or is protective against, knee OA.15 In a large cohort study, running (1) was protective against development of hip OA and (2) decreased the risk of requiring hip replacement.29 This finding was supported by animal-model research that concluded that it is inactivity that results in thinning of articular cartilage.29 In addition, a systematic review of randomized controlled trials concluded that knee joint-loading exercises are not harmful to articular cartilage (this is low-quality evidence, however).16
Continue to: Given that there...
Given that there are no high-quality studies suggesting that running contributes to or exacerbates OA, patients with OA can be counseled to start or continue running as tolerated because the health benefit of running likely outweighs risk. Patients with pre-existing moderate-to-severe OA might report knee and hip pain that is already exacerbated by certain activities; if a high-impact activity, such as running, makes that pain worse, exercise counseling that you provide can be tailored to include lower-impact alternatives, such as swimming, cycling, or an elliptical workout.
CASE
In response to Ms. K’s interest in beginning an exercise regimen that includes running, you perform a complete routine pre-participation evaluation and appropriate cardiac screening. You discuss risk factors for running injury, focusing on modifiable risk factors.
Ms. K is perimenopausal but reports a history of regular menstrual cycles. She eats a relatively well-balanced diet. You advise that her BMI should not restrict her from incorporating running into her fitness regimen. Also, you reassure her that she should not restrict running based on a diagnosis of OA; instead, you advise her to monitor her symptoms and reconsider her program if running makes her knee pain worse.
At this point, Ms. K is ready to run. She tells you that, based on your guidance, she feels more comfortable and safe starting a running program.
Preventing injury
After reviewing risk factors for running-related injury with patients, encourage other evidence-based methods of reducing that risk.
Continue to: Shoes
Shoes
The running shoe industry offers a variety of running shoes, from minimalist shoes to cushioned stability shoes that vary based on the amount of cushioning, level of motion control, and amount of heel-to-toe drop. With so many options, new runners might wonder which shoes can reduce their risk of injury and how they should select a pair.
Stability. A characteristic of running shoes promoted by the industry is their stability: ie, their motion control. Stability shoes are marketed to runners who overpronate and therefore limit motion to prevent overpronation. The benefit of stability shoes, or stability insoles, is unclear.30 A randomized controlled trial showed that, in runners who overpronate, motion-control shoes reduced their risk of injury.31 However, another study assessed whether shoes that had been “prescribed” based on foot morphology and stride reduced the risk of injury (compared to neutral, cushioned shoes) and found no change in the incidence of soft-tissue injury.32 Given no strong evidence to suggest otherwise, runners can be advised to buy shoes based on comfort rather than on foot morphology or running stride.
Heel-to-toe drop. Another component of shoe variability is heel-to-toe drop (the height difference between heel and forefoot). A study suggests that moderate-to-high (8-12 mm) heel-to-toe drop is associated with a reduced risk of running injury.33 Barefoot running shoes, which, typically, have no heel-to-toe drop, are associated with increased risk of injury—specifically, foot stress fracture (especially in runners who are even moderately overweight).34,35
Shoe age and shoe wear can be modified to reduce injury. There is evidence that running shoes lose approximately 50% of cushioning after 300 to 500 miles of use.36 Another study found that rotating running shoes—ideally, different types or brands—can lead to fewer running-related injuries.37
In general, patients can be counseled to use shoes that feel comfortable, as long as they replace them regularly (TABLE 2). Runners can also consider alternating pairs of different running shoes between runs. Overweight runners should avoid minimally cushioned and low heel-to-toe drop running shoes.

Continue to: Cross-training
Cross-training
Cross-training exercises for runners include cycling, an elliptical workout, swimming, and weightlifting. Incorporating cross-training can be protective against running injury because cross-training requires different movement patterns, prevents overuse, and equalizes muscle imbalances that occur with running.7 In addition, replacing running with a cross-training activity can decrease weekly running time and mileage, which can further reduce risk of running-related injury.7 Runners—especially higher-mileage runners—should be encouraged to incorporate cross-training into their workout regimen to decrease their risk of injury.
Stretching. The authors of a Cochrane review concluded that there is no significant reduction in injury associated with hamstring or gastrocnemius stretching.32 A small randomized, controlled, crossover study concluded that participants subjectively felt their performance was better when warm-ups included stretching.38 This perceived improvement in performance was similar between groups who completed dynamic or static stretching. However, no difference was noted in flexibility or objective performance between groups who stretched or did not stretch before activity.
Although there is no supporting evidence that stretching reduces the risk of injury, stretching is a low-risk intervention. Because stretching might provide subjective benefit to runners, you need not discourage patients from including this activity in their running program.
CORRESPONDENCE
Kartik Sidhar, MD, 15370 Huff Way, Brookfield, WI, 53005; [email protected]
Approximately 60 million people in the United States run for exercise at least once a calendar year, with approximately 11 million of them running > 100 days a year.1,2 Running is an affordable, convenient, and efficient form of exercise, whose benefits include a decrease in the risk of all-cause early mortality, cancer, and diabetes; an improved lipid profile; and better mental health.3
However, running is also the cause of a significant percentage of exercise-associated injuries: More than 60% of runners report overuse injury annually.4 Given the high incidence of running-related injury, an important component of primary care is accurately diagnosing and managing such injuries and counseling patients about how to prevent them.

This article reviews risk factors for running-related injury and summarizes evidence-based recommendations for prevention.
CASE
During a health maintenance examination, Clara K, a 47-year-old woman who is obese (body mass index [BMI], 34) and has bilateral knee osteoarthritis (OA), inquires about establishing a weight-loss strategy. Ms. K is interested in starting an exercise regimen involving running but is worried about provoking a flare of OA pain.
Risk factors for running injuries
Several risk factors—some modifiable, others nonmodifiable—are associated with running-related injury (TABLE 14-16). In addition, research suggests that other variables once thought to be risk factors, such as running surface and the Q-angle (described later), are not associated with running-related injury.

Modifiable risk factors
Changes in a training regimen or type of training. Many runners escalate training regimens as their fitness improves. Increasing mileage and changing the type of training (such as introducing hills or interval training) are independent risk factors for sustaining injury.5
The traditional recommendation has been for a runner to slowly increase or modify training with a 10% weekly increase in mileage or intensity.17 However, a randomized controlled trial failed to show a lower incidence of injury among amateur runners who adopted a graded exercise program.18 Regardless: It is still prudent to recommend a gradual increase in activity, such as taking ≥ 1 day off between running workouts or starting with a walking or jogging program, especially when there is a history of injury.19
Continue to: Excessive mileage
Excessive mileage. Many runners aspire to complete high-mileage runs. There is low-quality evidence demonstrating that high-mileage running, especially > 40 miles per week, is associated with increased risk of running-related injury.5 Injuries that occur with higher mileage are more often those of the hip and hamstring.5 A study noted that running ≤ 25 miles a week was protective against calf injury.6
Overall, there is little evidence to show that high-mileage running is associated with increased risk of running-related injury. However, this is still a risk factor that you should address with patients who have a running program—especially novices and those who ramp up mileage quickly.
Type of surface. Access to running surfaces—concrete, pavement, trails, treadmills, and athletic tracks—varies by time of day and season. Softer surfaces include treadmill, tracks, and trails; harder surfaces include asphalt and concrete.
There are limited data linking running surface with risk of injury.7 A study did not find an association between peak impact force based on running surface8; the authors hypothesized that runners compensate for a harder surface by making kinematic adjustments to minimize impact. With no strong evidence to link running-related injury to a particular running surface, patients should not be restricted to a softer running surface unless they notice a difference in comfort, because it is likely that they can compensate for a harder surface by adapting their gait.
Patients can therefore be counseled to run locally on sidewalks and neighborhood streets—if safe to do so—instead of obtaining a gym membership or driving to run on a trail. Such reassurance can increase a patient’s access to running and reduce barriers to exercise.
Continue to: BMI
BMI. Elevated BMI increases joint contact forces, which might increase risk of pain and injury.20 Results of studies investigating the link between BMI and running injury are mixed; some report that, in regard to bone stress injury, overweight BMI (> 25) is a risk factor for male runners and underweight BMI (< 18.5) is a risk factor for female runners.4,6 An observational study concluded that, among half-marathon and marathon runners, there was no significant increase in race-related injury, based on BMI.9 However, another study showed a higher rate of running-related injury in novice runners who had a higher BMI.10 A prospective cohort study found that runners with a higher BMI reported increased knee stiffness, which can place a runner at higher risk of overuse injury.4
Although these results conflict, there is consistency in the finding that obese novice runners are likely at increased risk of running-related injury; it is reasonable, therefore, for you to discuss strategies to reduce the risk of other modifiable factors, especially among obese novice runners. Patients with a higher BMI should not be discouraged from running, because exercise in combination with healthy eating habits is essential to decrease the myriad adverse health outcomes associated with obesity.
Female runners with a lower BMI, especially in the presence of other components of the female athlete triad (inadequate nutrition, amenorrhea, and low bone density), should be counseled about their increased risk of bone stress injury.21 Notably, a study of female US Navy recruits randomized to receive a trial of dietary supplementation of vitamin D plus calcium, or placebo, showed a 21% lower incidence of bone stress injury in the active-treatment group.22 To mitigate risk of injury associated with low BMI and the female athlete triad, therefore, a multidisciplinary approach of nutrition intervention, dietary optimization of vitamin D and calcium, and, possibly, activity modification should be implemented when appropriate.
Running gait. A study using 2-dimensional gait analysis to visualize biomechanical running patterns in injured and noninjured runners found that, in regard to mechanical variables, running-related injury was most strongly associated with contralateral pelvic drop.23 Gait retraining can be employed to help decrease contralateral pelvic drop.24 In addition, pelvic drop is often a result of weak gluteal muscles, and can be improved by doing strengthening exercises at home or with physical therapy.
Longer stride is also associated with running-related injury.25 A study showed improvement in patellofemoral pain by having runners increase stride rate by 10%, which reduces stride length to a significant degree.25,26 These improvements were maintained at 1-month and 3-month follow-up, and required only 1 gait retraining session.
Continue to: Get analysis is not feasible...
Gait analysis is not feasible in most primary care clinics. Instead, patients who run and (1) in whom pain persists despite more traditional treatments and (2) who have had recurring injury should be referred to a gait lab for analysis, usually by a physical therapist.
Nonmodifiable risk factors
Arch height. A high arch (pes cavus) is associated with increased risk of running-related injury, including bone stress injury, Achilles tendinopathy, plantar fasciitis, and patellofemoral pain syndrome.5 The mechanism of injury is thought to be increased forefoot loading forces.1
A review article showed that patients with pes cavus have reduced pain when using an orthosis, although there is no associated decrease in the risk of injury.5 To the contrary, a prospective study concluded that arch height was unrelated to increased risk of running-related injury.7
Evidence regarding flat feet (pes planus) and risk of injury is also mixed. Some studies show that pes planus is not associated with increased risk of injury in athletes.12 A cross-sectional study in older patients showed those with pes planus morphology had a higher rate of knee pain and wearing away of medial compartment cartilage.13 Because this study comprised only older adults, it is not generalizable to runners—nor can conclusions be drawn about causation, given the cross-sectional nature of the study.
Although a foot orthosis can correct mechanical differences caused by pes planus morphology, there is not enough evidence to conclude that correction results in a lower rate of injury. In sum, data are mixed with regard to arch height as a risk factor for running-related injury.
Continue to: Patients with...
Patients with pes planus or pes cavus should not be discouraged from running, however. If they experience pain with running, they might benefit from a trial of arch support inserts; or consider referral to an orthotist for evaluation for a custom orthosis.
Sex. Based on a prospective cohort study, female runners have a slightly higher rate of running injury than male counterparts.4 Similarly, a study showed that female military members generally had a higher incidence of stress fractures than male military members—specifically, femoral shaft and neck stress fractures.14 Runners who fall in the spectrum of the female athlete triad, as described earlier, are particularly vulnerable to bone stress injury. It is reasonable, therefore, to review risk factors for injury with female runners (as it is with all runners), especially those who have sustained a prior running-related injury.
Increased Q-angle (an obsolete risk factor). The Q-angle is approximated by drawing a line from the anterior superior iliac spine to the patella and a second line from the patella to the tibial tubercle. In males, a normal Q-angle is 14°; in females, 17° (SD = 4.5°). The Q-angle can be obtained by goniometric or radiographic measurement.
An increased Q-angle had been considered an intrinsic risk factor for running injury but has not been shown to be associated with increased risk of running-related injury or patellofemoral pain syndrome.27,28 Because the Q-angle is not a clinically relevant tool in assessing risk of injury, do not routinely measure it or include it in risk-factor counseling.
OA. Based on a systematic review of observational studies, data are inconclusive with regard to whether running contributes to, or is protective against, knee OA.15 In a large cohort study, running (1) was protective against development of hip OA and (2) decreased the risk of requiring hip replacement.29 This finding was supported by animal-model research that concluded that it is inactivity that results in thinning of articular cartilage.29 In addition, a systematic review of randomized controlled trials concluded that knee joint-loading exercises are not harmful to articular cartilage (this is low-quality evidence, however).16
Continue to: Given that there...
Given that there are no high-quality studies suggesting that running contributes to or exacerbates OA, patients with OA can be counseled to start or continue running as tolerated because the health benefit of running likely outweighs risk. Patients with pre-existing moderate-to-severe OA might report knee and hip pain that is already exacerbated by certain activities; if a high-impact activity, such as running, makes that pain worse, exercise counseling that you provide can be tailored to include lower-impact alternatives, such as swimming, cycling, or an elliptical workout.
CASE
In response to Ms. K’s interest in beginning an exercise regimen that includes running, you perform a complete routine pre-participation evaluation and appropriate cardiac screening. You discuss risk factors for running injury, focusing on modifiable risk factors.
Ms. K is perimenopausal but reports a history of regular menstrual cycles. She eats a relatively well-balanced diet. You advise that her BMI should not restrict her from incorporating running into her fitness regimen. Also, you reassure her that she should not restrict running based on a diagnosis of OA; instead, you advise her to monitor her symptoms and reconsider her program if running makes her knee pain worse.
At this point, Ms. K is ready to run. She tells you that, based on your guidance, she feels more comfortable and safe starting a running program.
Preventing injury
After reviewing risk factors for running-related injury with patients, encourage other evidence-based methods of reducing that risk.
Continue to: Shoes
Shoes
The running shoe industry offers a variety of running shoes, from minimalist shoes to cushioned stability shoes that vary based on the amount of cushioning, level of motion control, and amount of heel-to-toe drop. With so many options, new runners might wonder which shoes can reduce their risk of injury and how they should select a pair.
Stability. A characteristic of running shoes promoted by the industry is their stability: ie, their motion control. Stability shoes are marketed to runners who overpronate and therefore limit motion to prevent overpronation. The benefit of stability shoes, or stability insoles, is unclear.30 A randomized controlled trial showed that, in runners who overpronate, motion-control shoes reduced their risk of injury.31 However, another study assessed whether shoes that had been “prescribed” based on foot morphology and stride reduced the risk of injury (compared to neutral, cushioned shoes) and found no change in the incidence of soft-tissue injury.32 Given no strong evidence to suggest otherwise, runners can be advised to buy shoes based on comfort rather than on foot morphology or running stride.
Heel-to-toe drop. Another component of shoe variability is heel-to-toe drop (the height difference between heel and forefoot). A study suggests that moderate-to-high (8-12 mm) heel-to-toe drop is associated with a reduced risk of running injury.33 Barefoot running shoes, which, typically, have no heel-to-toe drop, are associated with increased risk of injury—specifically, foot stress fracture (especially in runners who are even moderately overweight).34,35
Shoe age and shoe wear can be modified to reduce injury. There is evidence that running shoes lose approximately 50% of cushioning after 300 to 500 miles of use.36 Another study found that rotating running shoes—ideally, different types or brands—can lead to fewer running-related injuries.37
In general, patients can be counseled to use shoes that feel comfortable, as long as they replace them regularly (TABLE 2). Runners can also consider alternating pairs of different running shoes between runs. Overweight runners should avoid minimally cushioned and low heel-to-toe drop running shoes.

Continue to: Cross-training
Cross-training
Cross-training exercises for runners include cycling, an elliptical workout, swimming, and weightlifting. Incorporating cross-training can be protective against running injury because cross-training requires different movement patterns, prevents overuse, and equalizes muscle imbalances that occur with running.7 In addition, replacing running with a cross-training activity can decrease weekly running time and mileage, which can further reduce risk of running-related injury.7 Runners—especially higher-mileage runners—should be encouraged to incorporate cross-training into their workout regimen to decrease their risk of injury.
Stretching. The authors of a Cochrane review concluded that there is no significant reduction in injury associated with hamstring or gastrocnemius stretching.32 A small randomized, controlled, crossover study concluded that participants subjectively felt their performance was better when warm-ups included stretching.38 This perceived improvement in performance was similar between groups who completed dynamic or static stretching. However, no difference was noted in flexibility or objective performance between groups who stretched or did not stretch before activity.
Although there is no supporting evidence that stretching reduces the risk of injury, stretching is a low-risk intervention. Because stretching might provide subjective benefit to runners, you need not discourage patients from including this activity in their running program.
CORRESPONDENCE
Kartik Sidhar, MD, 15370 Huff Way, Brookfield, WI, 53005; [email protected]
1. Brown CR Jr. Common injuries from running. In: Imboden JB, Hellerman, DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. McGraw-Hill; 2013.
2. Lange D. Running & jogging - statistic and facts. Statista Web site. November 16, 2020. Accessed March 28, 2021. www.statista.com/topics/1743/running-and-jogging/
3. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541-556. doi:10.1097/HCO.0000000000000437
4. Messier SP, Martin DF, Mihalko SL, et al. A 2-year prospective cohort study of overuse running injuries: The Runners and Injury Longitudinal Study (TRAILS). Am J Sports Med. 2018;46:2211-2221. doi:10.1177/0363546518773755
5. Fields KB, Sykes JC, Walker KM, et al. Prevention of running injuries. Curr Sports Med Rep. 2010;9:176-182. doi:10.1249/JSR.0b013e3181de7ec5
6. van der Worp MP, ten Haaf DSM, van Cingel R. Injuries in runners; a systematic review on risk factors and sex differences. PLoS One. 2015;10:1-18. doi:10.1371/journal.pone.0114937
7. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244. doi:10.1136/bjsm.37.3.239
8. Dixon SJ, Collop AC, Batt ME. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919-1926. doi:10.1097/00005768-200011000-00016
9. Vadeboncoeur TF, Silvers SM, Taylor WC, et al. Impact of a high body mass index on lower extremity injury in marathon/half-marathon participants. J Phys Act Health. 2012;9:96-103. doi:10.1123/jpah.9.1.96
10. Buist I, Bredeweg SW. Higher risk of injury in overweight novice runners. Br J Sports Med. 2011;45:338. http://dx.doi.org/10.1136/bjsm.2011.084038.79
11. Cowan DN, Jones BH, Robinson JR. Foot morphologic characteristics and risk of Exercise-related injury. Arch Fam Med. 1993;2:773-777. doi:10.1001/archfami.2.7.773
12. Michelson JD, Durant DM, McFarland E. The injury risk associated with pes planus in athletes. Foot Ankle Int. 2002;23:629-633. doi: 10.1177/107110070202300708
13. Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken). 2011;63:937-944. doi:10.1002/acr.20431
14. Waterman BR, Gun B, Bader JO, et al. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181:1308-1313. doi:10.7205/MILMED-D-15-00571
15. Timmins KA, Leech RD, Batt ME, et al. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447-1457. doi:10.1177/0363546516657531
16. Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2019;53:940-947. doi:10.1136/bjsports-2017-098661
17. Johnston CAM, Taunton JE, Lloyd-Smith DR, et al. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101-1109.
18. Buist I, Bredeweg SW, van Mechelen W, et al. No effect of a graded training program on the number of running-related injuries in novice runners: a randomized controlled trial. Am J Sports Med. 2008;36:33-39. doi:10.1177/0363546507307505
19. Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44:749-765. doi:10.2519/jospt.2014.5334
20. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209-214. doi:10.1016/j.gaitpost.2019.07.307
21. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139-149. doi:10.1016/j.pmr.2015.08.008
22. Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. doi:10.1359/jbmr.080102
23. Bramah C, Preece SJ, Gill N, et al. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46:3023-3031. doi:10.1177/0363546518793657
24. Willy RW, Scholz PT, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol Avon). 2012;27:1045-1051. doi:10.1016/j.clinbiomech.2012.07.011
25. Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014;6:210-217. doi:10.1177/1941738113508544
26. Bramah C, Preece SJ, Gill N et al. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. Am J Sports Med. 2019;47:3406-3413. doi: 10.1177/0363546519879693
27. Ramskov D, Jensen ML, Obling K, et al. No association between q-angle and foot posture with running-related injuries: a 10 week prospective follow-up study. Int J Sports Phys Ther. 2013;8:407-415.
28. Almeida GPL, Silva AP, França FJR, et al. Q-angle in patellofemoral pain: relationship with dynamic knee valgus, hip abductor torque, pain and function. Rev Bras Ortop. 2016;51:181-186. doi:10.1016/j.rboe.2016.01.010
29. Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292-1297. doi:10.1249/MSS.0b013e3182885f26
30. Nigg BM, Baltich J, Hoerzer S, et al. Running shoes and running injuries: mythbusting and a proposal for two new paradigms: ‘Preferred movement path’ and ‘comfort filter.’ Br J Sports Med. 2015;49:1290-1294. doi:10.1136/bjsports-2015-095054
31. Malisoux L, Chambon N, Delattre N, et al. Injury risk in runners using standard or motion control shoes: a randomised controlled trial with participant and assessor blinding. Br J Sports Med. 2016;50:481-487. doi:10.1136/bjsports-2015-095031
32. Yeung SS, Yeung EW, Gillespie LD. Interventions for preventing lower limb soft-tissue running injuries. Cochrane Database Syst Rev. 2011(7):CD001256. doi:10.1002/14651858.cd001256.pub2
33. Malisoux L, Chambon N, Urhausen A, et al. Influence of the heel-to-toe drop of standard cushioned running shoes on injury risk in leisure-time runners: a randomized controlled trial with 6-month follow-up. Am J Sports Med. 2016;44:2933-2940. doi:10.1177/0363546516654690
34. Ryan M, Elashi M, Newsham-West R, et al. Examining injury risk and pain perception in runners using minimalist footwear. Br J Sports Med. 2014;48:1257-1262. doi:10.1136/bjsports-2012-092061
35. Fuller JT, Thewlis D, Buckley JD, et al.. Body mass and weekly training distance influence the pain and injuries experienced by runners using minimalist shoes: a randomized controlled trial. Am J Sports Med. 2017;45:1162-1170. doi:10.1177/0363546516682497
36. Cook SD, Kester MA, Brunet ME. Shock absorption characteristics of running shoes. Am J Sports Med. 1985;13:248-253. doi.org/10.1177/036354658501300406
37. Malisoux L, Ramesh J, Mann R, et al. Can parallel use of different running shoes decrease running-related injury risk? Scand J Med Sci Sport. 2015;25:110-115. doi:10.1111/sms.12154
38. Blazevich AJ, Gill ND, Kvorning T, et al. No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Med Sci Sports Exerc. 2018;50:1258-1266. doi:10.1249/MSS.0000000000001539
1. Brown CR Jr. Common injuries from running. In: Imboden JB, Hellerman, DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. McGraw-Hill; 2013.
2. Lange D. Running & jogging - statistic and facts. Statista Web site. November 16, 2020. Accessed March 28, 2021. www.statista.com/topics/1743/running-and-jogging/
3. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541-556. doi:10.1097/HCO.0000000000000437
4. Messier SP, Martin DF, Mihalko SL, et al. A 2-year prospective cohort study of overuse running injuries: The Runners and Injury Longitudinal Study (TRAILS). Am J Sports Med. 2018;46:2211-2221. doi:10.1177/0363546518773755
5. Fields KB, Sykes JC, Walker KM, et al. Prevention of running injuries. Curr Sports Med Rep. 2010;9:176-182. doi:10.1249/JSR.0b013e3181de7ec5
6. van der Worp MP, ten Haaf DSM, van Cingel R. Injuries in runners; a systematic review on risk factors and sex differences. PLoS One. 2015;10:1-18. doi:10.1371/journal.pone.0114937
7. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244. doi:10.1136/bjsm.37.3.239
8. Dixon SJ, Collop AC, Batt ME. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919-1926. doi:10.1097/00005768-200011000-00016
9. Vadeboncoeur TF, Silvers SM, Taylor WC, et al. Impact of a high body mass index on lower extremity injury in marathon/half-marathon participants. J Phys Act Health. 2012;9:96-103. doi:10.1123/jpah.9.1.96
10. Buist I, Bredeweg SW. Higher risk of injury in overweight novice runners. Br J Sports Med. 2011;45:338. http://dx.doi.org/10.1136/bjsm.2011.084038.79
11. Cowan DN, Jones BH, Robinson JR. Foot morphologic characteristics and risk of Exercise-related injury. Arch Fam Med. 1993;2:773-777. doi:10.1001/archfami.2.7.773
12. Michelson JD, Durant DM, McFarland E. The injury risk associated with pes planus in athletes. Foot Ankle Int. 2002;23:629-633. doi: 10.1177/107110070202300708
13. Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken). 2011;63:937-944. doi:10.1002/acr.20431
14. Waterman BR, Gun B, Bader JO, et al. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181:1308-1313. doi:10.7205/MILMED-D-15-00571
15. Timmins KA, Leech RD, Batt ME, et al. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447-1457. doi:10.1177/0363546516657531
16. Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2019;53:940-947. doi:10.1136/bjsports-2017-098661
17. Johnston CAM, Taunton JE, Lloyd-Smith DR, et al. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101-1109.
18. Buist I, Bredeweg SW, van Mechelen W, et al. No effect of a graded training program on the number of running-related injuries in novice runners: a randomized controlled trial. Am J Sports Med. 2008;36:33-39. doi:10.1177/0363546507307505
19. Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44:749-765. doi:10.2519/jospt.2014.5334
20. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209-214. doi:10.1016/j.gaitpost.2019.07.307
21. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139-149. doi:10.1016/j.pmr.2015.08.008
22. Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. doi:10.1359/jbmr.080102
23. Bramah C, Preece SJ, Gill N, et al. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46:3023-3031. doi:10.1177/0363546518793657
24. Willy RW, Scholz PT, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol Avon). 2012;27:1045-1051. doi:10.1016/j.clinbiomech.2012.07.011
25. Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014;6:210-217. doi:10.1177/1941738113508544
26. Bramah C, Preece SJ, Gill N et al. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. Am J Sports Med. 2019;47:3406-3413. doi: 10.1177/0363546519879693
27. Ramskov D, Jensen ML, Obling K, et al. No association between q-angle and foot posture with running-related injuries: a 10 week prospective follow-up study. Int J Sports Phys Ther. 2013;8:407-415.
28. Almeida GPL, Silva AP, França FJR, et al. Q-angle in patellofemoral pain: relationship with dynamic knee valgus, hip abductor torque, pain and function. Rev Bras Ortop. 2016;51:181-186. doi:10.1016/j.rboe.2016.01.010
29. Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292-1297. doi:10.1249/MSS.0b013e3182885f26
30. Nigg BM, Baltich J, Hoerzer S, et al. Running shoes and running injuries: mythbusting and a proposal for two new paradigms: ‘Preferred movement path’ and ‘comfort filter.’ Br J Sports Med. 2015;49:1290-1294. doi:10.1136/bjsports-2015-095054
31. Malisoux L, Chambon N, Delattre N, et al. Injury risk in runners using standard or motion control shoes: a randomised controlled trial with participant and assessor blinding. Br J Sports Med. 2016;50:481-487. doi:10.1136/bjsports-2015-095031
32. Yeung SS, Yeung EW, Gillespie LD. Interventions for preventing lower limb soft-tissue running injuries. Cochrane Database Syst Rev. 2011(7):CD001256. doi:10.1002/14651858.cd001256.pub2
33. Malisoux L, Chambon N, Urhausen A, et al. Influence of the heel-to-toe drop of standard cushioned running shoes on injury risk in leisure-time runners: a randomized controlled trial with 6-month follow-up. Am J Sports Med. 2016;44:2933-2940. doi:10.1177/0363546516654690
34. Ryan M, Elashi M, Newsham-West R, et al. Examining injury risk and pain perception in runners using minimalist footwear. Br J Sports Med. 2014;48:1257-1262. doi:10.1136/bjsports-2012-092061
35. Fuller JT, Thewlis D, Buckley JD, et al.. Body mass and weekly training distance influence the pain and injuries experienced by runners using minimalist shoes: a randomized controlled trial. Am J Sports Med. 2017;45:1162-1170. doi:10.1177/0363546516682497
36. Cook SD, Kester MA, Brunet ME. Shock absorption characteristics of running shoes. Am J Sports Med. 1985;13:248-253. doi.org/10.1177/036354658501300406
37. Malisoux L, Ramesh J, Mann R, et al. Can parallel use of different running shoes decrease running-related injury risk? Scand J Med Sci Sport. 2015;25:110-115. doi:10.1111/sms.12154
38. Blazevich AJ, Gill ND, Kvorning T, et al. No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Med Sci Sports Exerc. 2018;50:1258-1266. doi:10.1249/MSS.0000000000001539
PRACTICE RECOMMENDATIONS
› Counsel runners to cross-train, replace shoes regularly, and use shoes with moderate-to-high (8-12 mm) heel-to-toe drop. C
› Don’t discourage running for exercise, as long as it is tolerated, in patients who have osteoarthritis. C
› Encourage moderation in running distance and intensity, especially in novice runners. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Veteran and Provider Perspectives on Telehealth for Vocational Rehabilitation Services
Vocational rehabilitation (VR) interventions are offered through Compensated Work Therapy (CWT) as part of clinical care in the Veterans Health Administration (VHA) to improve employment and quality of life outcomes for veterans with life-altering disabilities.1–5 CWT vocational services range from assessment, vocational counseling, and treatment plan development to job placement, coaching, and follow-along support.1 However, many veterans receive care in community-based clinics that are not staffed with a VR specialist (VRS) to provide these services.6–8 Telehealth may increase patient access to VR, especially for rural veterans and those with travel barriers, but it is not known whether veterans and VRS would find this to be a satisfactory service delivery method.8,9 This paper examines veteran and VRS provider perspectives on VR provided by telehealth (VRtele) as part of a VHA clinical demonstration project. To our knowledge, this is the first report of using real-time, clinic-based VRtele.
Methods
The Rural Veterans Supported Employment Telerehabilitation Initiative (RVSETI) was conducted as a field-initiated demonstration project at 2 US Department of Veterans Affairs (VA) medical centers (VAMCs) in Florida between 2014 and 2016: James A. Haley Veterans’ Hospital & Clinics (Tampa) and Malcom Randall VAMC (Gainesville). This retrospective evaluation of its first year did not require institutional review board approval as it was determined to be a quality improvement project by the local research service.
The patient population for the project was veterans with disabilities who were referred by clinical consults to the CWT service, a recovery-oriented vocational program. During the project years, veterans were offered the option of receiving VR services, such as supported employment, community-based employment services, or vocational assistance, through VRtele rather than traditional face-to-face meetings. The specific interventions delivered included patient orientation, interview assessment, treatment plan development, referral activities, vocational counseling, assessment of workplace for accommodation needs, vocational case management, and other employment supports. VR staff participating in the project included 2 VR supervisors, 1 supported employment mentor trainer, and 5 VRSs.
Each clinic was set up for VRtele, and codes were added to the electronic health record (EHR) to ensure proper documentation. Participating VRSs completed teleconferencing training, including a skills assessment using the equipment for real-time, high-quality video streaming over an encrypted network to provide services in a patient’s home or other remote locations. VRS staff provided veterans with instructions on using a VA-provided tablet or their own device and assisted them with establishing connectivity with the network. Video equipment included speakers, camera, and headphones connected to the desktop computer or laptop of the VRS. A patient’s first VRtele
Demographic data, primary diagnosis, VR usage data, and zip codes of participating veterans were extracted from the EHR. Veterans completed a 2-part satisfaction survey administered 90 days after enrollment and at discharge. Part 1 was composed of 15 items, most with a 5-point Likert scale (higher ratings indicated greater satisfaction), on various aspects of the VRtele experience, such as audio and video quality and wait times.10 Part 2 addressed VR services and the VRS and consisted of 8 Likert scale items with the option to add a comment for each and 2 open-ended items that asked the participant to list what they liked best and least about VRtele.
Semistructured, in-person 30- to 60-minute interviews were conducted with VRSs at the initiation of VRtele
After ≥ 2 months of VRtele use
Analyses
Descriptive statistics were used for EHR data and satisfaction surveys. For qualitative analysis, each transcript was read in full by 2 researchers to get an overview of the data, and a rapid analysis approach was used to identify central themes focused on how technology was used and the experiences of the participants.11,12 Relevant text for each topic was tabulated, and a summary table was created that highlighted overlapping ideas discussed by the interviewees as well as differences.
Results
Of the 22 veterans who participated in the project, 11 completed satisfaction surveys and 4 participated in qualitative interviews. The rural and nonrural groups did not differ demographically or by diagnosis, which was predominantly mental health related. Only 1 veteran in each group owned a tablet; the majority of both groups required VA-issued devices: 80% (n = 8) rural and 91.7% (n = 11) nonrural. The number of VRtele sessions for the groups also was similar, 53 for rural and 60 for nonrural, as was the mean (SD) number of sessions per veteran: 5.3 (SD, 3.2) rural and 5.0 (SD, 2.5) urban. Overall, 63 miles per session were saved, mostly for rural veterans, and the number of mean (SD) miles saved per veteran was greater for rural than nonrural veterans: 379.2 (243.0) and 256.1 (275.9), respectively. One veteran who moved to a different state during the program continued VRtele at the new location. In a qualitative sampling of 5 VRtele sessions, all the VRSs used office desktop computers.
Level of satisfaction with aspects of VRtele related to the technology rated was consistently > 4 on the Likert scale. The lowest mean (SD) ratings were 4.2 (1.0) for audio quality and 4.4 (0.5) for video quality, and the highest rating was given for equipment operation explanation and privacy was respected, 4.9 (0.3) for both. All questions related to satisfaction with services were also rated high: The mean (SD) lowest ratings were 4.3 (1.0) given to both vocational needs 4.3 (1.0) and tasks effectively helped achieve goals 4.3 (0.7). The highest mean (SD) ratings were 4.6 (0.5) given to VR program service explained and 4.7 (0.5) for appointment timeliness.
Qualitative Results
At first, some VRSs thought the teleconferencing system might be difficult or awkward to use, but they found it easier to set up than expected and seamless to use. VRS staff reported being surprised at how well it worked despite some issues that occurred with loading the software. Once loaded, however, the connection worked well, one VRS noting that following step-by-step instructions solved the problem. Some VRSs indicated they did not invite all the veterans on their caseload to participate in VRtele due to concerns with the patient’s familiarity with technology, but one VRS stated, “I haven’t had anybody that failed to do a [session] that I couldn’t get them up and running within a few minutes.”
When working in the community, VRSs reported using laptops for VRtele but found that these devices were unreliable due to lack of internet access and were slow to start; several VRSs thought tablets would have been more helpful. Some veterans reported technical glitches, lack of comfort with technology, or a problem with sound due to a tablet’s protective case blocking the speakers. To solve the sound issue, a veteran used headphones. This veteran also explained that the log-on process required a new password every time, so he would keep a pen and paper ready to write it down. Because signing in and setting up takes a little time, this veteran and his VRS agreed to start connecting 5 minutes before their meeting time to allow for that set- up time.
Initially, some VRSs expressed concern that transitioning to VRtele would affect the quality of interactions with the veterans. However, VRSs also identified strengths of VRtele, including flexibility, saved time, and increased interaction. One VRS discussed a veteran’s adaptation by saying, “I think he feels even more involved in his plan [and] enjoys the increased interaction.” Veterans reported enjoying using tablets and identified the main strength of VRtele as being able to talk face-to-face with the VRS. Echoing the VRSs, veterans reported teleconferencing saved time by avoiding travel and enabled spontaneous meetings. One of the veterans summed up the benefits of using VRtele: “I’d rather just connect. It’s going to take us 40 to 50 minutes [to meet in person] when we can just connect right here and it takes 15 to 20. We don’t have to go through the driving.… So this right here, doing it ahead of time and having the appointment, it’s a lot easier.”
In their interviews, VRSs talked about enjoying VRtele. A VRS explained: “It makes it a lot easier. It makes me feel less guilty. This way [veterans] don’t have to use their gas money, use their time. I know [the veteran] had something else he needed to do today.” Thus, both veterans and VRSs were satisfied with their VRtele experiences.
Discussion
This first report on the perspective of providers and veterans using VRtele suggests that it is a viable option for service delivery and that is highly satisfactory for serving veterans with disabilities, many of whom live in rural areas or have travel barriers. These findings are consistent with data on telerehabilitation for veterans with cognitive, physical, and mental disabilities.13-22 Further, the data support the notion of using VRtele to facilitate long-term VR follow-up for persons with disabilities, as illustrated by successful continuation of vocational services after a veteran moved out of state.23
Similar to other reports, our experience highlighted 2 factors that affect successful VRtele: (1) Troubleshooting technology barriers for both VR providers and clients; and (2) supportive leadership to facilitate implementation
Changes to technology and increased usage of VA Video Connect may indicate that the barriers identified from the earlier process described here have been diminished or eliminated. More evaluation is needed to assess whether system upgrades have increased ease of use and access for veterans with disabilities.
Conclusions
Encouragingly, this clinical demonstration project showed that both providers and clients recognize the benefits of VRtele. Patient satisfaction and decreased travel costs were clear advantages to using VRtele for this small group of veterans who had barriers to care due to travel or disability barriers. As this program evaluation was limited by a small sample, absence of a comparison group, and lack of outcome data (eg, employment rates, hours, wages, retention), future research is needed on implementation and outcomes of VRtele
Acknowledgments
The authors thank Lynn Dirk, MAMC, for substantial editorial assistance. This material was based on work supported by Rural Veterans Supported Employment TeleRehabilitation Initiative (RVSETI), funded by the VA Office of Rural Health (Project # N08-FY14Q3-S2-P01222) and by support of the VA Health Services Research and Development Service. This work was presented in part at the 114th Annual Meeting of the American Anthropological Association at Denver, Colorado, November 21, 2015; a field-based Health Services Research and Development Service meeting, US Department of Veterans Affairs at Washington, DC, September 12, 2016; and the 2016 Annual Conference of the American Congress for Rehabilitation Medicine at Chicago, Illinois, October-November 2016.
1. Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA therapeutic and supported employment services programs. Psychiatr Serv. 2017;68(9):938-946. doi:10.1176/appi.ps.201600412
2. Davis LL, Kyriakides TC, Suris AM, et al. Effect of evidence-based supported employment vs transitional work on achieving steady work among veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2018;75(4):316. doi:10.1001/jamapsychiatry.2017.4472
3. Ottomanelli L, Goetz LL, Suris A, et al. Effectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747. doi:10.1016/j.apmr.2012.01.002
4. Ottomanelli L, Goetz LL, Barnett SD, et al. Individual placement and support in spinal cord injury: a longitudinal observational study of employment outcomes. Arch Phys Med Rehabil. 2017;98(8):1567-1575. doi:10.1016/j.apmr.2016.12.010
5. Cotner BA, Ottomanelli L, O’Connor DR, Njoh EN, Barnett SD, Miech EJ. Quality of life outcomes for veterans with spinal cord injury receiving individual placement and support (IPS). Top Spinal Cord Inj Rehabil. 2018;24(4):325-335. doi:10.1310/sci17-00046
6. Metzel DS, Giordano A. Locations of employment services and people with disabilities: a geographical analysis of accessibility. J Disabil Policy Stud. 2007;18(2):88-97. doi:10.1177/10442073070180020501
7. Landon T, Connor A, McKnight-Lizotte M, Peña J. Rehabilitation counseling in rural settings: a phenomenological study on barriers and supports. J Rehabil. 2019;85(2):47-57.
8. Riemer-Reiss M. Vocational rehabilitation counseling at a distance: Challenges, strategies and ethics to consider. J Rehabil. 2000;66(1):11-17.
9. Schmeler MR, Schein RM, McCue M, Betz K. Telerehabilitation clinical and vocational applications for assistive technology: research, opportunities, and challenges. Int J Telerehabilitation. 2009;1(1):59-72.
10. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370. doi:10.1682/JRRD.2014.10.0239
11. McMullen CK, Ash JS, Sittig DF, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299-307. doi:10.3414/ME10-01-0042
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
13. Egede LE, Acierno R, Knapp RG, et al. Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry. 2015;2(8):693-701. doi:10.1016/S2215-0366(15)00122-4
14. Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086-1093. doi:10.1007/s11606-007-0201-9
15. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58. doi:10.1001/jamapsychiatry.2014.1575
16. Grubbs KM, Fortney JC, Dean T, Williams JS, Godleski L. A comparison of mental health diagnoses treated via interactive video and face to face in the Veterans Healthcare Administration. Telemed E-Health. 2015;21(7):564-566. doi:10.1089/tmj.2014.0152
17. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213. doi:10.1177/1357633X15572201
18. Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed E-Health. 2010;16(4):417-423. doi:10.1089/tmj.2009.0118
19. Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147-154. doi:10.1089/tmj.2004.10.147
20. Dallolio L, Menarini M, China S, et al. Functional and clinical outcomes of telemedicine in patients with spinal cord injury. Arch Phys Med Rehabil. 2008;89(12):2332-2341. doi:10.1016/j.apmr.2008.06.012
21. Houlihan BV, Jette A, Friedman RH, et al. A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715-720.doi:10.1038/sc.2013.45
22. Smith MW, Hill ML, Hopkins KL, Kiratli BJ, Cronkite RC. A modeled analysis of telehealth methods for treating pressure ulcers after spinal cord injury. Int J Telemed Appl. 2012;2012:1-10. doi:10.1155/2012/729492
23. Balcazar FE, Keys CB, Davis M, Lardon C, Jones C. Strengths and challenges of intervention research in vocational rehabilitation: an illustration of agency-university collaboration. J Rehabil. 2005;71(2):40-48.
24. Martinez RN, Hogan TP, Balbale S, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E Health. 2017;23(7):567-576. doi:10.1089/tmj.2016.0200
25. Martinez RN, Hogan TP, Lones K, et al. Evaluation and treatment of mild traumatic brain injury through the implementation of clinical video telehealth: provider perspectives from the Veterans Health Administration. PM R. 2017;9(3):231-240. doi:10.1016/j.pmrj.2016.07.002
26. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309-313. doi:10.1177/1357633X20916567
27. Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019; 36(3):122-128.
28. US Department of Veterans Affairs. Veterans VA Video Connect. Published May 22, 2020. Accessed May 29, 2020. https://mobile.va.gov/app/va-video-connect#AppDescription.
29. US Department of Veterans Affairs. VA telehealth at home. Accessed May 29, 2020. https://telehealth.va.gov/type/home
Vocational rehabilitation (VR) interventions are offered through Compensated Work Therapy (CWT) as part of clinical care in the Veterans Health Administration (VHA) to improve employment and quality of life outcomes for veterans with life-altering disabilities.1–5 CWT vocational services range from assessment, vocational counseling, and treatment plan development to job placement, coaching, and follow-along support.1 However, many veterans receive care in community-based clinics that are not staffed with a VR specialist (VRS) to provide these services.6–8 Telehealth may increase patient access to VR, especially for rural veterans and those with travel barriers, but it is not known whether veterans and VRS would find this to be a satisfactory service delivery method.8,9 This paper examines veteran and VRS provider perspectives on VR provided by telehealth (VRtele) as part of a VHA clinical demonstration project. To our knowledge, this is the first report of using real-time, clinic-based VRtele.
Methods
The Rural Veterans Supported Employment Telerehabilitation Initiative (RVSETI) was conducted as a field-initiated demonstration project at 2 US Department of Veterans Affairs (VA) medical centers (VAMCs) in Florida between 2014 and 2016: James A. Haley Veterans’ Hospital & Clinics (Tampa) and Malcom Randall VAMC (Gainesville). This retrospective evaluation of its first year did not require institutional review board approval as it was determined to be a quality improvement project by the local research service.
The patient population for the project was veterans with disabilities who were referred by clinical consults to the CWT service, a recovery-oriented vocational program. During the project years, veterans were offered the option of receiving VR services, such as supported employment, community-based employment services, or vocational assistance, through VRtele rather than traditional face-to-face meetings. The specific interventions delivered included patient orientation, interview assessment, treatment plan development, referral activities, vocational counseling, assessment of workplace for accommodation needs, vocational case management, and other employment supports. VR staff participating in the project included 2 VR supervisors, 1 supported employment mentor trainer, and 5 VRSs.
Each clinic was set up for VRtele, and codes were added to the electronic health record (EHR) to ensure proper documentation. Participating VRSs completed teleconferencing training, including a skills assessment using the equipment for real-time, high-quality video streaming over an encrypted network to provide services in a patient’s home or other remote locations. VRS staff provided veterans with instructions on using a VA-provided tablet or their own device and assisted them with establishing connectivity with the network. Video equipment included speakers, camera, and headphones connected to the desktop computer or laptop of the VRS. A patient’s first VRtele
Demographic data, primary diagnosis, VR usage data, and zip codes of participating veterans were extracted from the EHR. Veterans completed a 2-part satisfaction survey administered 90 days after enrollment and at discharge. Part 1 was composed of 15 items, most with a 5-point Likert scale (higher ratings indicated greater satisfaction), on various aspects of the VRtele experience, such as audio and video quality and wait times.10 Part 2 addressed VR services and the VRS and consisted of 8 Likert scale items with the option to add a comment for each and 2 open-ended items that asked the participant to list what they liked best and least about VRtele.
Semistructured, in-person 30- to 60-minute interviews were conducted with VRSs at the initiation of VRtele
After ≥ 2 months of VRtele use
Analyses
Descriptive statistics were used for EHR data and satisfaction surveys. For qualitative analysis, each transcript was read in full by 2 researchers to get an overview of the data, and a rapid analysis approach was used to identify central themes focused on how technology was used and the experiences of the participants.11,12 Relevant text for each topic was tabulated, and a summary table was created that highlighted overlapping ideas discussed by the interviewees as well as differences.
Results
Of the 22 veterans who participated in the project, 11 completed satisfaction surveys and 4 participated in qualitative interviews. The rural and nonrural groups did not differ demographically or by diagnosis, which was predominantly mental health related. Only 1 veteran in each group owned a tablet; the majority of both groups required VA-issued devices: 80% (n = 8) rural and 91.7% (n = 11) nonrural. The number of VRtele sessions for the groups also was similar, 53 for rural and 60 for nonrural, as was the mean (SD) number of sessions per veteran: 5.3 (SD, 3.2) rural and 5.0 (SD, 2.5) urban. Overall, 63 miles per session were saved, mostly for rural veterans, and the number of mean (SD) miles saved per veteran was greater for rural than nonrural veterans: 379.2 (243.0) and 256.1 (275.9), respectively. One veteran who moved to a different state during the program continued VRtele at the new location. In a qualitative sampling of 5 VRtele sessions, all the VRSs used office desktop computers.
Level of satisfaction with aspects of VRtele related to the technology rated was consistently > 4 on the Likert scale. The lowest mean (SD) ratings were 4.2 (1.0) for audio quality and 4.4 (0.5) for video quality, and the highest rating was given for equipment operation explanation and privacy was respected, 4.9 (0.3) for both. All questions related to satisfaction with services were also rated high: The mean (SD) lowest ratings were 4.3 (1.0) given to both vocational needs 4.3 (1.0) and tasks effectively helped achieve goals 4.3 (0.7). The highest mean (SD) ratings were 4.6 (0.5) given to VR program service explained and 4.7 (0.5) for appointment timeliness.
Qualitative Results
At first, some VRSs thought the teleconferencing system might be difficult or awkward to use, but they found it easier to set up than expected and seamless to use. VRS staff reported being surprised at how well it worked despite some issues that occurred with loading the software. Once loaded, however, the connection worked well, one VRS noting that following step-by-step instructions solved the problem. Some VRSs indicated they did not invite all the veterans on their caseload to participate in VRtele due to concerns with the patient’s familiarity with technology, but one VRS stated, “I haven’t had anybody that failed to do a [session] that I couldn’t get them up and running within a few minutes.”
When working in the community, VRSs reported using laptops for VRtele but found that these devices were unreliable due to lack of internet access and were slow to start; several VRSs thought tablets would have been more helpful. Some veterans reported technical glitches, lack of comfort with technology, or a problem with sound due to a tablet’s protective case blocking the speakers. To solve the sound issue, a veteran used headphones. This veteran also explained that the log-on process required a new password every time, so he would keep a pen and paper ready to write it down. Because signing in and setting up takes a little time, this veteran and his VRS agreed to start connecting 5 minutes before their meeting time to allow for that set- up time.
Initially, some VRSs expressed concern that transitioning to VRtele would affect the quality of interactions with the veterans. However, VRSs also identified strengths of VRtele, including flexibility, saved time, and increased interaction. One VRS discussed a veteran’s adaptation by saying, “I think he feels even more involved in his plan [and] enjoys the increased interaction.” Veterans reported enjoying using tablets and identified the main strength of VRtele as being able to talk face-to-face with the VRS. Echoing the VRSs, veterans reported teleconferencing saved time by avoiding travel and enabled spontaneous meetings. One of the veterans summed up the benefits of using VRtele: “I’d rather just connect. It’s going to take us 40 to 50 minutes [to meet in person] when we can just connect right here and it takes 15 to 20. We don’t have to go through the driving.… So this right here, doing it ahead of time and having the appointment, it’s a lot easier.”
In their interviews, VRSs talked about enjoying VRtele. A VRS explained: “It makes it a lot easier. It makes me feel less guilty. This way [veterans] don’t have to use their gas money, use their time. I know [the veteran] had something else he needed to do today.” Thus, both veterans and VRSs were satisfied with their VRtele experiences.
Discussion
This first report on the perspective of providers and veterans using VRtele suggests that it is a viable option for service delivery and that is highly satisfactory for serving veterans with disabilities, many of whom live in rural areas or have travel barriers. These findings are consistent with data on telerehabilitation for veterans with cognitive, physical, and mental disabilities.13-22 Further, the data support the notion of using VRtele to facilitate long-term VR follow-up for persons with disabilities, as illustrated by successful continuation of vocational services after a veteran moved out of state.23
Similar to other reports, our experience highlighted 2 factors that affect successful VRtele: (1) Troubleshooting technology barriers for both VR providers and clients; and (2) supportive leadership to facilitate implementation
Changes to technology and increased usage of VA Video Connect may indicate that the barriers identified from the earlier process described here have been diminished or eliminated. More evaluation is needed to assess whether system upgrades have increased ease of use and access for veterans with disabilities.
Conclusions
Encouragingly, this clinical demonstration project showed that both providers and clients recognize the benefits of VRtele. Patient satisfaction and decreased travel costs were clear advantages to using VRtele for this small group of veterans who had barriers to care due to travel or disability barriers. As this program evaluation was limited by a small sample, absence of a comparison group, and lack of outcome data (eg, employment rates, hours, wages, retention), future research is needed on implementation and outcomes of VRtele
Acknowledgments
The authors thank Lynn Dirk, MAMC, for substantial editorial assistance. This material was based on work supported by Rural Veterans Supported Employment TeleRehabilitation Initiative (RVSETI), funded by the VA Office of Rural Health (Project # N08-FY14Q3-S2-P01222) and by support of the VA Health Services Research and Development Service. This work was presented in part at the 114th Annual Meeting of the American Anthropological Association at Denver, Colorado, November 21, 2015; a field-based Health Services Research and Development Service meeting, US Department of Veterans Affairs at Washington, DC, September 12, 2016; and the 2016 Annual Conference of the American Congress for Rehabilitation Medicine at Chicago, Illinois, October-November 2016.
Vocational rehabilitation (VR) interventions are offered through Compensated Work Therapy (CWT) as part of clinical care in the Veterans Health Administration (VHA) to improve employment and quality of life outcomes for veterans with life-altering disabilities.1–5 CWT vocational services range from assessment, vocational counseling, and treatment plan development to job placement, coaching, and follow-along support.1 However, many veterans receive care in community-based clinics that are not staffed with a VR specialist (VRS) to provide these services.6–8 Telehealth may increase patient access to VR, especially for rural veterans and those with travel barriers, but it is not known whether veterans and VRS would find this to be a satisfactory service delivery method.8,9 This paper examines veteran and VRS provider perspectives on VR provided by telehealth (VRtele) as part of a VHA clinical demonstration project. To our knowledge, this is the first report of using real-time, clinic-based VRtele.
Methods
The Rural Veterans Supported Employment Telerehabilitation Initiative (RVSETI) was conducted as a field-initiated demonstration project at 2 US Department of Veterans Affairs (VA) medical centers (VAMCs) in Florida between 2014 and 2016: James A. Haley Veterans’ Hospital & Clinics (Tampa) and Malcom Randall VAMC (Gainesville). This retrospective evaluation of its first year did not require institutional review board approval as it was determined to be a quality improvement project by the local research service.
The patient population for the project was veterans with disabilities who were referred by clinical consults to the CWT service, a recovery-oriented vocational program. During the project years, veterans were offered the option of receiving VR services, such as supported employment, community-based employment services, or vocational assistance, through VRtele rather than traditional face-to-face meetings. The specific interventions delivered included patient orientation, interview assessment, treatment plan development, referral activities, vocational counseling, assessment of workplace for accommodation needs, vocational case management, and other employment supports. VR staff participating in the project included 2 VR supervisors, 1 supported employment mentor trainer, and 5 VRSs.
Each clinic was set up for VRtele, and codes were added to the electronic health record (EHR) to ensure proper documentation. Participating VRSs completed teleconferencing training, including a skills assessment using the equipment for real-time, high-quality video streaming over an encrypted network to provide services in a patient’s home or other remote locations. VRS staff provided veterans with instructions on using a VA-provided tablet or their own device and assisted them with establishing connectivity with the network. Video equipment included speakers, camera, and headphones connected to the desktop computer or laptop of the VRS. A patient’s first VRtele
Demographic data, primary diagnosis, VR usage data, and zip codes of participating veterans were extracted from the EHR. Veterans completed a 2-part satisfaction survey administered 90 days after enrollment and at discharge. Part 1 was composed of 15 items, most with a 5-point Likert scale (higher ratings indicated greater satisfaction), on various aspects of the VRtele experience, such as audio and video quality and wait times.10 Part 2 addressed VR services and the VRS and consisted of 8 Likert scale items with the option to add a comment for each and 2 open-ended items that asked the participant to list what they liked best and least about VRtele.
Semistructured, in-person 30- to 60-minute interviews were conducted with VRSs at the initiation of VRtele
After ≥ 2 months of VRtele use
Analyses
Descriptive statistics were used for EHR data and satisfaction surveys. For qualitative analysis, each transcript was read in full by 2 researchers to get an overview of the data, and a rapid analysis approach was used to identify central themes focused on how technology was used and the experiences of the participants.11,12 Relevant text for each topic was tabulated, and a summary table was created that highlighted overlapping ideas discussed by the interviewees as well as differences.
Results
Of the 22 veterans who participated in the project, 11 completed satisfaction surveys and 4 participated in qualitative interviews. The rural and nonrural groups did not differ demographically or by diagnosis, which was predominantly mental health related. Only 1 veteran in each group owned a tablet; the majority of both groups required VA-issued devices: 80% (n = 8) rural and 91.7% (n = 11) nonrural. The number of VRtele sessions for the groups also was similar, 53 for rural and 60 for nonrural, as was the mean (SD) number of sessions per veteran: 5.3 (SD, 3.2) rural and 5.0 (SD, 2.5) urban. Overall, 63 miles per session were saved, mostly for rural veterans, and the number of mean (SD) miles saved per veteran was greater for rural than nonrural veterans: 379.2 (243.0) and 256.1 (275.9), respectively. One veteran who moved to a different state during the program continued VRtele at the new location. In a qualitative sampling of 5 VRtele sessions, all the VRSs used office desktop computers.
Level of satisfaction with aspects of VRtele related to the technology rated was consistently > 4 on the Likert scale. The lowest mean (SD) ratings were 4.2 (1.0) for audio quality and 4.4 (0.5) for video quality, and the highest rating was given for equipment operation explanation and privacy was respected, 4.9 (0.3) for both. All questions related to satisfaction with services were also rated high: The mean (SD) lowest ratings were 4.3 (1.0) given to both vocational needs 4.3 (1.0) and tasks effectively helped achieve goals 4.3 (0.7). The highest mean (SD) ratings were 4.6 (0.5) given to VR program service explained and 4.7 (0.5) for appointment timeliness.
Qualitative Results
At first, some VRSs thought the teleconferencing system might be difficult or awkward to use, but they found it easier to set up than expected and seamless to use. VRS staff reported being surprised at how well it worked despite some issues that occurred with loading the software. Once loaded, however, the connection worked well, one VRS noting that following step-by-step instructions solved the problem. Some VRSs indicated they did not invite all the veterans on their caseload to participate in VRtele due to concerns with the patient’s familiarity with technology, but one VRS stated, “I haven’t had anybody that failed to do a [session] that I couldn’t get them up and running within a few minutes.”
When working in the community, VRSs reported using laptops for VRtele but found that these devices were unreliable due to lack of internet access and were slow to start; several VRSs thought tablets would have been more helpful. Some veterans reported technical glitches, lack of comfort with technology, or a problem with sound due to a tablet’s protective case blocking the speakers. To solve the sound issue, a veteran used headphones. This veteran also explained that the log-on process required a new password every time, so he would keep a pen and paper ready to write it down. Because signing in and setting up takes a little time, this veteran and his VRS agreed to start connecting 5 minutes before their meeting time to allow for that set- up time.
Initially, some VRSs expressed concern that transitioning to VRtele would affect the quality of interactions with the veterans. However, VRSs also identified strengths of VRtele, including flexibility, saved time, and increased interaction. One VRS discussed a veteran’s adaptation by saying, “I think he feels even more involved in his plan [and] enjoys the increased interaction.” Veterans reported enjoying using tablets and identified the main strength of VRtele as being able to talk face-to-face with the VRS. Echoing the VRSs, veterans reported teleconferencing saved time by avoiding travel and enabled spontaneous meetings. One of the veterans summed up the benefits of using VRtele: “I’d rather just connect. It’s going to take us 40 to 50 minutes [to meet in person] when we can just connect right here and it takes 15 to 20. We don’t have to go through the driving.… So this right here, doing it ahead of time and having the appointment, it’s a lot easier.”
In their interviews, VRSs talked about enjoying VRtele. A VRS explained: “It makes it a lot easier. It makes me feel less guilty. This way [veterans] don’t have to use their gas money, use their time. I know [the veteran] had something else he needed to do today.” Thus, both veterans and VRSs were satisfied with their VRtele experiences.
Discussion
This first report on the perspective of providers and veterans using VRtele suggests that it is a viable option for service delivery and that is highly satisfactory for serving veterans with disabilities, many of whom live in rural areas or have travel barriers. These findings are consistent with data on telerehabilitation for veterans with cognitive, physical, and mental disabilities.13-22 Further, the data support the notion of using VRtele to facilitate long-term VR follow-up for persons with disabilities, as illustrated by successful continuation of vocational services after a veteran moved out of state.23
Similar to other reports, our experience highlighted 2 factors that affect successful VRtele: (1) Troubleshooting technology barriers for both VR providers and clients; and (2) supportive leadership to facilitate implementation
Changes to technology and increased usage of VA Video Connect may indicate that the barriers identified from the earlier process described here have been diminished or eliminated. More evaluation is needed to assess whether system upgrades have increased ease of use and access for veterans with disabilities.
Conclusions
Encouragingly, this clinical demonstration project showed that both providers and clients recognize the benefits of VRtele. Patient satisfaction and decreased travel costs were clear advantages to using VRtele for this small group of veterans who had barriers to care due to travel or disability barriers. As this program evaluation was limited by a small sample, absence of a comparison group, and lack of outcome data (eg, employment rates, hours, wages, retention), future research is needed on implementation and outcomes of VRtele
Acknowledgments
The authors thank Lynn Dirk, MAMC, for substantial editorial assistance. This material was based on work supported by Rural Veterans Supported Employment TeleRehabilitation Initiative (RVSETI), funded by the VA Office of Rural Health (Project # N08-FY14Q3-S2-P01222) and by support of the VA Health Services Research and Development Service. This work was presented in part at the 114th Annual Meeting of the American Anthropological Association at Denver, Colorado, November 21, 2015; a field-based Health Services Research and Development Service meeting, US Department of Veterans Affairs at Washington, DC, September 12, 2016; and the 2016 Annual Conference of the American Congress for Rehabilitation Medicine at Chicago, Illinois, October-November 2016.
1. Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA therapeutic and supported employment services programs. Psychiatr Serv. 2017;68(9):938-946. doi:10.1176/appi.ps.201600412
2. Davis LL, Kyriakides TC, Suris AM, et al. Effect of evidence-based supported employment vs transitional work on achieving steady work among veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2018;75(4):316. doi:10.1001/jamapsychiatry.2017.4472
3. Ottomanelli L, Goetz LL, Suris A, et al. Effectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747. doi:10.1016/j.apmr.2012.01.002
4. Ottomanelli L, Goetz LL, Barnett SD, et al. Individual placement and support in spinal cord injury: a longitudinal observational study of employment outcomes. Arch Phys Med Rehabil. 2017;98(8):1567-1575. doi:10.1016/j.apmr.2016.12.010
5. Cotner BA, Ottomanelli L, O’Connor DR, Njoh EN, Barnett SD, Miech EJ. Quality of life outcomes for veterans with spinal cord injury receiving individual placement and support (IPS). Top Spinal Cord Inj Rehabil. 2018;24(4):325-335. doi:10.1310/sci17-00046
6. Metzel DS, Giordano A. Locations of employment services and people with disabilities: a geographical analysis of accessibility. J Disabil Policy Stud. 2007;18(2):88-97. doi:10.1177/10442073070180020501
7. Landon T, Connor A, McKnight-Lizotte M, Peña J. Rehabilitation counseling in rural settings: a phenomenological study on barriers and supports. J Rehabil. 2019;85(2):47-57.
8. Riemer-Reiss M. Vocational rehabilitation counseling at a distance: Challenges, strategies and ethics to consider. J Rehabil. 2000;66(1):11-17.
9. Schmeler MR, Schein RM, McCue M, Betz K. Telerehabilitation clinical and vocational applications for assistive technology: research, opportunities, and challenges. Int J Telerehabilitation. 2009;1(1):59-72.
10. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370. doi:10.1682/JRRD.2014.10.0239
11. McMullen CK, Ash JS, Sittig DF, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299-307. doi:10.3414/ME10-01-0042
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
13. Egede LE, Acierno R, Knapp RG, et al. Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry. 2015;2(8):693-701. doi:10.1016/S2215-0366(15)00122-4
14. Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086-1093. doi:10.1007/s11606-007-0201-9
15. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58. doi:10.1001/jamapsychiatry.2014.1575
16. Grubbs KM, Fortney JC, Dean T, Williams JS, Godleski L. A comparison of mental health diagnoses treated via interactive video and face to face in the Veterans Healthcare Administration. Telemed E-Health. 2015;21(7):564-566. doi:10.1089/tmj.2014.0152
17. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213. doi:10.1177/1357633X15572201
18. Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed E-Health. 2010;16(4):417-423. doi:10.1089/tmj.2009.0118
19. Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147-154. doi:10.1089/tmj.2004.10.147
20. Dallolio L, Menarini M, China S, et al. Functional and clinical outcomes of telemedicine in patients with spinal cord injury. Arch Phys Med Rehabil. 2008;89(12):2332-2341. doi:10.1016/j.apmr.2008.06.012
21. Houlihan BV, Jette A, Friedman RH, et al. A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715-720.doi:10.1038/sc.2013.45
22. Smith MW, Hill ML, Hopkins KL, Kiratli BJ, Cronkite RC. A modeled analysis of telehealth methods for treating pressure ulcers after spinal cord injury. Int J Telemed Appl. 2012;2012:1-10. doi:10.1155/2012/729492
23. Balcazar FE, Keys CB, Davis M, Lardon C, Jones C. Strengths and challenges of intervention research in vocational rehabilitation: an illustration of agency-university collaboration. J Rehabil. 2005;71(2):40-48.
24. Martinez RN, Hogan TP, Balbale S, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E Health. 2017;23(7):567-576. doi:10.1089/tmj.2016.0200
25. Martinez RN, Hogan TP, Lones K, et al. Evaluation and treatment of mild traumatic brain injury through the implementation of clinical video telehealth: provider perspectives from the Veterans Health Administration. PM R. 2017;9(3):231-240. doi:10.1016/j.pmrj.2016.07.002
26. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309-313. doi:10.1177/1357633X20916567
27. Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019; 36(3):122-128.
28. US Department of Veterans Affairs. Veterans VA Video Connect. Published May 22, 2020. Accessed May 29, 2020. https://mobile.va.gov/app/va-video-connect#AppDescription.
29. US Department of Veterans Affairs. VA telehealth at home. Accessed May 29, 2020. https://telehealth.va.gov/type/home
1. Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA therapeutic and supported employment services programs. Psychiatr Serv. 2017;68(9):938-946. doi:10.1176/appi.ps.201600412
2. Davis LL, Kyriakides TC, Suris AM, et al. Effect of evidence-based supported employment vs transitional work on achieving steady work among veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2018;75(4):316. doi:10.1001/jamapsychiatry.2017.4472
3. Ottomanelli L, Goetz LL, Suris A, et al. Effectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747. doi:10.1016/j.apmr.2012.01.002
4. Ottomanelli L, Goetz LL, Barnett SD, et al. Individual placement and support in spinal cord injury: a longitudinal observational study of employment outcomes. Arch Phys Med Rehabil. 2017;98(8):1567-1575. doi:10.1016/j.apmr.2016.12.010
5. Cotner BA, Ottomanelli L, O’Connor DR, Njoh EN, Barnett SD, Miech EJ. Quality of life outcomes for veterans with spinal cord injury receiving individual placement and support (IPS). Top Spinal Cord Inj Rehabil. 2018;24(4):325-335. doi:10.1310/sci17-00046
6. Metzel DS, Giordano A. Locations of employment services and people with disabilities: a geographical analysis of accessibility. J Disabil Policy Stud. 2007;18(2):88-97. doi:10.1177/10442073070180020501
7. Landon T, Connor A, McKnight-Lizotte M, Peña J. Rehabilitation counseling in rural settings: a phenomenological study on barriers and supports. J Rehabil. 2019;85(2):47-57.
8. Riemer-Reiss M. Vocational rehabilitation counseling at a distance: Challenges, strategies and ethics to consider. J Rehabil. 2000;66(1):11-17.
9. Schmeler MR, Schein RM, McCue M, Betz K. Telerehabilitation clinical and vocational applications for assistive technology: research, opportunities, and challenges. Int J Telerehabilitation. 2009;1(1):59-72.
10. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370. doi:10.1682/JRRD.2014.10.0239
11. McMullen CK, Ash JS, Sittig DF, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299-307. doi:10.3414/ME10-01-0042
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
13. Egede LE, Acierno R, Knapp RG, et al. Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry. 2015;2(8):693-701. doi:10.1016/S2215-0366(15)00122-4
14. Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086-1093. doi:10.1007/s11606-007-0201-9
15. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58. doi:10.1001/jamapsychiatry.2014.1575
16. Grubbs KM, Fortney JC, Dean T, Williams JS, Godleski L. A comparison of mental health diagnoses treated via interactive video and face to face in the Veterans Healthcare Administration. Telemed E-Health. 2015;21(7):564-566. doi:10.1089/tmj.2014.0152
17. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213. doi:10.1177/1357633X15572201
18. Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed E-Health. 2010;16(4):417-423. doi:10.1089/tmj.2009.0118
19. Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147-154. doi:10.1089/tmj.2004.10.147
20. Dallolio L, Menarini M, China S, et al. Functional and clinical outcomes of telemedicine in patients with spinal cord injury. Arch Phys Med Rehabil. 2008;89(12):2332-2341. doi:10.1016/j.apmr.2008.06.012
21. Houlihan BV, Jette A, Friedman RH, et al. A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715-720.doi:10.1038/sc.2013.45
22. Smith MW, Hill ML, Hopkins KL, Kiratli BJ, Cronkite RC. A modeled analysis of telehealth methods for treating pressure ulcers after spinal cord injury. Int J Telemed Appl. 2012;2012:1-10. doi:10.1155/2012/729492
23. Balcazar FE, Keys CB, Davis M, Lardon C, Jones C. Strengths and challenges of intervention research in vocational rehabilitation: an illustration of agency-university collaboration. J Rehabil. 2005;71(2):40-48.
24. Martinez RN, Hogan TP, Balbale S, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E Health. 2017;23(7):567-576. doi:10.1089/tmj.2016.0200
25. Martinez RN, Hogan TP, Lones K, et al. Evaluation and treatment of mild traumatic brain injury through the implementation of clinical video telehealth: provider perspectives from the Veterans Health Administration. PM R. 2017;9(3):231-240. doi:10.1016/j.pmrj.2016.07.002
26. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309-313. doi:10.1177/1357633X20916567
27. Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019; 36(3):122-128.
28. US Department of Veterans Affairs. Veterans VA Video Connect. Published May 22, 2020. Accessed May 29, 2020. https://mobile.va.gov/app/va-video-connect#AppDescription.
29. US Department of Veterans Affairs. VA telehealth at home. Accessed May 29, 2020. https://telehealth.va.gov/type/home











