User login
Study finds nadolol noninferior to propranolol for infantile hemangiomas
according to a study published in JAMA Pediatrics.
“In our experience, nadolol is preferable to propranolol given its observed efficacy and similar safety profile [and] its more predictable metabolism that does not involve the liver,” lead author Elena Pope, MD, told this news organization. “In addition, the fact that nadolol is less lipophilic than propranolol makes it less likely to cross the blood-brain barrier and potentially affect the central nervous system,” added Dr. Pope, who is head of the division of pediatric dermatology at the Hospital for Sick Children, Toronto, and professor of pediatric medicine at the University of Toronto.
The prospective double-blind, randomized noninferiority study was conducted between 2016 and 2020 at two tertiary academic pediatric dermatology clinics in Ontario, Canada. It included 71 infants with a corrected gestational age of 1-6 months whose hemangiomas were greater than 1.5 cm on the face or 3 cm or greater on another body part and had the potential to cause functional impairment or cosmetic disfigurement.
Patients were randomized to either nadolol (oral suspension, 10 mg/mL) or propranolol (oral suspension, 5 mg/mL) beginning at a dose of 0.5 mg/kg per day twice a day and titrated weekly by 0.5 mg/kg per day until the maximum dose of 2 mg/kg per day. The dose was then adjusted until week 24, based on patient weight and clinical response, after which parents could choose to continue the infant on the assigned medication or switch to the other one. Follow-up visits occurred every 2 months after that until week 52.
For the main study outcome, measured by visual analog scale (VAS) scores at week 24, the between-group differences of IH size and color from baseline were 8.8 and 17.1, respectively, in favor of the nadolol group, the researchers report, with similar results seen at week 52. Safety data were similar for both treatments, “demonstrating that nadolol was noninferior to propranolol,” they write.
Additionally, the mean size involution, compared with baseline was 97.9% in the nadolol group and 89.1% in the propranolol group, and the mean color fading was 94.5% in the nadolol group, compared with 80.5% in the propranolol group. During the study, nadolol was also “59% faster in achieving 75% shrinkage of IH, compared with propranolol (P = .02) and 105% faster in achieving 100% shrinkage (P = .07),” they add.
“A considerable portion of patients experienced at least one mild adverse event (77.1% vs. 94.4% at 0-24 weeks and 84.2% vs. 74.2% at 24-52 weeks in the nadolol group vs. the propranolol group, respectively), with a median of two in each intervention group,” they noted, adding that while these numbers are high, they are similar to those in previous clinical trials.
“The efficacy data coupled with a more predictable pharmacokinetic profile and lower chance of crossing the blood-brain barrier may make nadolol a favorable alternative intervention in patients with IHs,” the authors conclude. However, they add that “further studies are needed to prove superiority over propranolol.”
Asked to comment on the results, Ilona J. Frieden, MD, director of the Birthmarks & Vascular Anomalies Center at the University of California, San Francisco, said that while this is a “very interesting study and deserves further consideration,” the findings do not reach the level at which they would change guidelines. “The vast majority of patients being treated with a systemic medication for IH are in fact getting propranolol,” said Dr. Frieden, coauthor of the American Academy of Pediatrics Clinical Practice Guideline for the Management of Infantile Hemangiomas.
“Though this study – designed as a noninferiority study – does seem to show slightly better outcomes from nadolol versus propranolol … it is a relatively small study,” she told this news organization. “Infantile hemangiomas are a very heterogeneous group, and larger studies and longer-term outcome data would be needed to truly compare the two modalities of treatment.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics, which described the death of a 10-week-old girl 7 weeks after starting nadolol for IH. The infant was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “Although we debated the conclusion of that report in terms of death attribution to nadolol, one practical pearl is to instruct the parents to discontinue nadolol if the baby has no bowel movements for more than 3 days,” Dr. Pope advised.
The author of that case report, Eric McGillis, MD, program director of clinical pharmacology and toxicology and an emergency physician at Alberta Health Services, in Calgary, Alt., said the conclusion of his report has been taken out of context. “We acknowledge that our case report, like any case report, cannot prove causation,” he told this news organization. “We hypothesized that nadolol may have contributed to the death of the infant based on the limited pharmacokinetic data currently available for nadolol in infants. Nadolol is largely eliminated in the feces and infants may have infrequent stooling based on diet and other factors; therefore, nadolol may accumulate,” he noted.
The infant in the case report did not have a bowel movement for 10 days “and had an elevated postmortem cardiac nadolol concentration in the absence of another obvious cause of death. More pharmacokinetic studies on nadolol in this population are needed to substantiate our hypothesis. However, in the meantime, we agree that having parents monitor stool output for dose adjustments makes practical sense and can potentially reduce harm.”
Dr. Pope presented the results of the study earlier this year at the annual meeting of the Society for Pediatric Dermatology.
The study was supported by Physician Services, Ont. Dr. Pope has reported serving as an advisory board member for Boehringer Ingelheim, Novartis, Sanofi Genzyme, and Timber. Other authors have reported receiving personal fees from Pierre Fabre during the conduct of the study, as well as personal fees from Amgen, Ipsen, Novartis, Pfizer, and Sanofi Genzyme; grants from AbbVie, Clementia, Mayne Pharma, and Sanofi Genzyme; and grants and personal fees from Venthera. One author has a patent for a new topical treatment of IH. Dr. Frieden has reported being a consultant for Pfizer (data safety board), Novartis, and Venthera. Dr. McGillis has reported no relevant financial relationships.
Commentary by Lawrence W. Eichenfield, MD
The treatment of functionally significant and deforming hemangiomas has been revolutionized by propranolol, developed after the observation by Christine Léauté-Labrèze, MD, that a child who developed hypertension as a side effect of systemic steroids for a nasal hemangioma and was prescribed propranolol for the hypertension had rapid shrinkage of the hemangioma. The study by Pope and colleagues assesses nadolol as an alternative to propranolol, showing noninferiority and in some parameters improved outcomes and speed of response. The drug appeared to be fairly well tolerated in the study, though there is a prior published case report of a death from nadolol use for hemangioma treatment from a different Canadian center. Nadolol may be an important alternative to propranolol; however, propranolol remains the only FDA-approved medication for infantile hemangiomas and the generally recommended medication in the American Academy of Pediatrics guidelines for management of infantile hemangiomas.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
according to a study published in JAMA Pediatrics.
“In our experience, nadolol is preferable to propranolol given its observed efficacy and similar safety profile [and] its more predictable metabolism that does not involve the liver,” lead author Elena Pope, MD, told this news organization. “In addition, the fact that nadolol is less lipophilic than propranolol makes it less likely to cross the blood-brain barrier and potentially affect the central nervous system,” added Dr. Pope, who is head of the division of pediatric dermatology at the Hospital for Sick Children, Toronto, and professor of pediatric medicine at the University of Toronto.
The prospective double-blind, randomized noninferiority study was conducted between 2016 and 2020 at two tertiary academic pediatric dermatology clinics in Ontario, Canada. It included 71 infants with a corrected gestational age of 1-6 months whose hemangiomas were greater than 1.5 cm on the face or 3 cm or greater on another body part and had the potential to cause functional impairment or cosmetic disfigurement.
Patients were randomized to either nadolol (oral suspension, 10 mg/mL) or propranolol (oral suspension, 5 mg/mL) beginning at a dose of 0.5 mg/kg per day twice a day and titrated weekly by 0.5 mg/kg per day until the maximum dose of 2 mg/kg per day. The dose was then adjusted until week 24, based on patient weight and clinical response, after which parents could choose to continue the infant on the assigned medication or switch to the other one. Follow-up visits occurred every 2 months after that until week 52.
For the main study outcome, measured by visual analog scale (VAS) scores at week 24, the between-group differences of IH size and color from baseline were 8.8 and 17.1, respectively, in favor of the nadolol group, the researchers report, with similar results seen at week 52. Safety data were similar for both treatments, “demonstrating that nadolol was noninferior to propranolol,” they write.
Additionally, the mean size involution, compared with baseline was 97.9% in the nadolol group and 89.1% in the propranolol group, and the mean color fading was 94.5% in the nadolol group, compared with 80.5% in the propranolol group. During the study, nadolol was also “59% faster in achieving 75% shrinkage of IH, compared with propranolol (P = .02) and 105% faster in achieving 100% shrinkage (P = .07),” they add.
“A considerable portion of patients experienced at least one mild adverse event (77.1% vs. 94.4% at 0-24 weeks and 84.2% vs. 74.2% at 24-52 weeks in the nadolol group vs. the propranolol group, respectively), with a median of two in each intervention group,” they noted, adding that while these numbers are high, they are similar to those in previous clinical trials.
“The efficacy data coupled with a more predictable pharmacokinetic profile and lower chance of crossing the blood-brain barrier may make nadolol a favorable alternative intervention in patients with IHs,” the authors conclude. However, they add that “further studies are needed to prove superiority over propranolol.”
Asked to comment on the results, Ilona J. Frieden, MD, director of the Birthmarks & Vascular Anomalies Center at the University of California, San Francisco, said that while this is a “very interesting study and deserves further consideration,” the findings do not reach the level at which they would change guidelines. “The vast majority of patients being treated with a systemic medication for IH are in fact getting propranolol,” said Dr. Frieden, coauthor of the American Academy of Pediatrics Clinical Practice Guideline for the Management of Infantile Hemangiomas.
“Though this study – designed as a noninferiority study – does seem to show slightly better outcomes from nadolol versus propranolol … it is a relatively small study,” she told this news organization. “Infantile hemangiomas are a very heterogeneous group, and larger studies and longer-term outcome data would be needed to truly compare the two modalities of treatment.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics, which described the death of a 10-week-old girl 7 weeks after starting nadolol for IH. The infant was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “Although we debated the conclusion of that report in terms of death attribution to nadolol, one practical pearl is to instruct the parents to discontinue nadolol if the baby has no bowel movements for more than 3 days,” Dr. Pope advised.
The author of that case report, Eric McGillis, MD, program director of clinical pharmacology and toxicology and an emergency physician at Alberta Health Services, in Calgary, Alt., said the conclusion of his report has been taken out of context. “We acknowledge that our case report, like any case report, cannot prove causation,” he told this news organization. “We hypothesized that nadolol may have contributed to the death of the infant based on the limited pharmacokinetic data currently available for nadolol in infants. Nadolol is largely eliminated in the feces and infants may have infrequent stooling based on diet and other factors; therefore, nadolol may accumulate,” he noted.
The infant in the case report did not have a bowel movement for 10 days “and had an elevated postmortem cardiac nadolol concentration in the absence of another obvious cause of death. More pharmacokinetic studies on nadolol in this population are needed to substantiate our hypothesis. However, in the meantime, we agree that having parents monitor stool output for dose adjustments makes practical sense and can potentially reduce harm.”
Dr. Pope presented the results of the study earlier this year at the annual meeting of the Society for Pediatric Dermatology.
The study was supported by Physician Services, Ont. Dr. Pope has reported serving as an advisory board member for Boehringer Ingelheim, Novartis, Sanofi Genzyme, and Timber. Other authors have reported receiving personal fees from Pierre Fabre during the conduct of the study, as well as personal fees from Amgen, Ipsen, Novartis, Pfizer, and Sanofi Genzyme; grants from AbbVie, Clementia, Mayne Pharma, and Sanofi Genzyme; and grants and personal fees from Venthera. One author has a patent for a new topical treatment of IH. Dr. Frieden has reported being a consultant for Pfizer (data safety board), Novartis, and Venthera. Dr. McGillis has reported no relevant financial relationships.
Commentary by Lawrence W. Eichenfield, MD
The treatment of functionally significant and deforming hemangiomas has been revolutionized by propranolol, developed after the observation by Christine Léauté-Labrèze, MD, that a child who developed hypertension as a side effect of systemic steroids for a nasal hemangioma and was prescribed propranolol for the hypertension had rapid shrinkage of the hemangioma. The study by Pope and colleagues assesses nadolol as an alternative to propranolol, showing noninferiority and in some parameters improved outcomes and speed of response. The drug appeared to be fairly well tolerated in the study, though there is a prior published case report of a death from nadolol use for hemangioma treatment from a different Canadian center. Nadolol may be an important alternative to propranolol; however, propranolol remains the only FDA-approved medication for infantile hemangiomas and the generally recommended medication in the American Academy of Pediatrics guidelines for management of infantile hemangiomas.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
according to a study published in JAMA Pediatrics.
“In our experience, nadolol is preferable to propranolol given its observed efficacy and similar safety profile [and] its more predictable metabolism that does not involve the liver,” lead author Elena Pope, MD, told this news organization. “In addition, the fact that nadolol is less lipophilic than propranolol makes it less likely to cross the blood-brain barrier and potentially affect the central nervous system,” added Dr. Pope, who is head of the division of pediatric dermatology at the Hospital for Sick Children, Toronto, and professor of pediatric medicine at the University of Toronto.
The prospective double-blind, randomized noninferiority study was conducted between 2016 and 2020 at two tertiary academic pediatric dermatology clinics in Ontario, Canada. It included 71 infants with a corrected gestational age of 1-6 months whose hemangiomas were greater than 1.5 cm on the face or 3 cm or greater on another body part and had the potential to cause functional impairment or cosmetic disfigurement.
Patients were randomized to either nadolol (oral suspension, 10 mg/mL) or propranolol (oral suspension, 5 mg/mL) beginning at a dose of 0.5 mg/kg per day twice a day and titrated weekly by 0.5 mg/kg per day until the maximum dose of 2 mg/kg per day. The dose was then adjusted until week 24, based on patient weight and clinical response, after which parents could choose to continue the infant on the assigned medication or switch to the other one. Follow-up visits occurred every 2 months after that until week 52.
For the main study outcome, measured by visual analog scale (VAS) scores at week 24, the between-group differences of IH size and color from baseline were 8.8 and 17.1, respectively, in favor of the nadolol group, the researchers report, with similar results seen at week 52. Safety data were similar for both treatments, “demonstrating that nadolol was noninferior to propranolol,” they write.
Additionally, the mean size involution, compared with baseline was 97.9% in the nadolol group and 89.1% in the propranolol group, and the mean color fading was 94.5% in the nadolol group, compared with 80.5% in the propranolol group. During the study, nadolol was also “59% faster in achieving 75% shrinkage of IH, compared with propranolol (P = .02) and 105% faster in achieving 100% shrinkage (P = .07),” they add.
“A considerable portion of patients experienced at least one mild adverse event (77.1% vs. 94.4% at 0-24 weeks and 84.2% vs. 74.2% at 24-52 weeks in the nadolol group vs. the propranolol group, respectively), with a median of two in each intervention group,” they noted, adding that while these numbers are high, they are similar to those in previous clinical trials.
“The efficacy data coupled with a more predictable pharmacokinetic profile and lower chance of crossing the blood-brain barrier may make nadolol a favorable alternative intervention in patients with IHs,” the authors conclude. However, they add that “further studies are needed to prove superiority over propranolol.”
Asked to comment on the results, Ilona J. Frieden, MD, director of the Birthmarks & Vascular Anomalies Center at the University of California, San Francisco, said that while this is a “very interesting study and deserves further consideration,” the findings do not reach the level at which they would change guidelines. “The vast majority of patients being treated with a systemic medication for IH are in fact getting propranolol,” said Dr. Frieden, coauthor of the American Academy of Pediatrics Clinical Practice Guideline for the Management of Infantile Hemangiomas.
“Though this study – designed as a noninferiority study – does seem to show slightly better outcomes from nadolol versus propranolol … it is a relatively small study,” she told this news organization. “Infantile hemangiomas are a very heterogeneous group, and larger studies and longer-term outcome data would be needed to truly compare the two modalities of treatment.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics, which described the death of a 10-week-old girl 7 weeks after starting nadolol for IH. The infant was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “Although we debated the conclusion of that report in terms of death attribution to nadolol, one practical pearl is to instruct the parents to discontinue nadolol if the baby has no bowel movements for more than 3 days,” Dr. Pope advised.
The author of that case report, Eric McGillis, MD, program director of clinical pharmacology and toxicology and an emergency physician at Alberta Health Services, in Calgary, Alt., said the conclusion of his report has been taken out of context. “We acknowledge that our case report, like any case report, cannot prove causation,” he told this news organization. “We hypothesized that nadolol may have contributed to the death of the infant based on the limited pharmacokinetic data currently available for nadolol in infants. Nadolol is largely eliminated in the feces and infants may have infrequent stooling based on diet and other factors; therefore, nadolol may accumulate,” he noted.
The infant in the case report did not have a bowel movement for 10 days “and had an elevated postmortem cardiac nadolol concentration in the absence of another obvious cause of death. More pharmacokinetic studies on nadolol in this population are needed to substantiate our hypothesis. However, in the meantime, we agree that having parents monitor stool output for dose adjustments makes practical sense and can potentially reduce harm.”
Dr. Pope presented the results of the study earlier this year at the annual meeting of the Society for Pediatric Dermatology.
The study was supported by Physician Services, Ont. Dr. Pope has reported serving as an advisory board member for Boehringer Ingelheim, Novartis, Sanofi Genzyme, and Timber. Other authors have reported receiving personal fees from Pierre Fabre during the conduct of the study, as well as personal fees from Amgen, Ipsen, Novartis, Pfizer, and Sanofi Genzyme; grants from AbbVie, Clementia, Mayne Pharma, and Sanofi Genzyme; and grants and personal fees from Venthera. One author has a patent for a new topical treatment of IH. Dr. Frieden has reported being a consultant for Pfizer (data safety board), Novartis, and Venthera. Dr. McGillis has reported no relevant financial relationships.
Commentary by Lawrence W. Eichenfield, MD
The treatment of functionally significant and deforming hemangiomas has been revolutionized by propranolol, developed after the observation by Christine Léauté-Labrèze, MD, that a child who developed hypertension as a side effect of systemic steroids for a nasal hemangioma and was prescribed propranolol for the hypertension had rapid shrinkage of the hemangioma. The study by Pope and colleagues assesses nadolol as an alternative to propranolol, showing noninferiority and in some parameters improved outcomes and speed of response. The drug appeared to be fairly well tolerated in the study, though there is a prior published case report of a death from nadolol use for hemangioma treatment from a different Canadian center. Nadolol may be an important alternative to propranolol; however, propranolol remains the only FDA-approved medication for infantile hemangiomas and the generally recommended medication in the American Academy of Pediatrics guidelines for management of infantile hemangiomas.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM JAMA PEDIATRICS
Pfizer COVID vaccine is 100% effective in adolescents: Study
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Surveillance for measles is a victim of the COVID pandemic
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
30% of docs say they don’t want own kids 5-11 to get COVID vaccine
A Medscape
Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did not want their kids to get the COVID-19 vaccine; 13% were unsure. Among pharmacists, 31% said they would not get them vaccinated and 9% were unsure.
Clinicians were more likely to want vaccinations for their kids 5-11 than were 510 consumers polled by WebMD at the same time. Overall, 49% of the consumers who had kids that age did not want them to get the COVID-19 vaccine.
On November 2, Centers for Disease Control and Prevention (CDC) Director Rochelle P. Walensky, MD, MPH, endorsed the CDC Advisory Committee on Immunization Practices’ recommendation that children 5-11 be vaccinated with the Pfizer-BioNTech pediatric vaccine. That decision expanded vaccine recommendations to about 28 million children in the United States.
The CDC states that, in clinical trials, the Pfizer vaccine had more than 90% efficacy in preventing laboratory-confirmed COVID-19 infection in children 5 to 15 years old, and that the immune response in children ages 5-15 equaled the immune response in people 16 to 25 years old.
The Medscape poll, fielded from November 3 to November 11, included 325 physicians, 793 nurses/APRNs, and 151 pharmacists.
How safe is the vaccine?
Clinicians were asked how confident they were that the vaccine is safe for that age group, and 66% of physicians, 52% of nurses/APRNs, and 66% of pharmacists said they were somewhat or very confident.
Among consumers overall in the WebMD poll, 56% said they were confident or somewhat confident that the vaccine is safe in that age group.
Among adolescents and young adults, rare cases of myocarditis and pericarditis in adolescents and young adults have been reported. According to the CDC, “[I]n one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12-17 years.”
Known and potential benefits of COVID-19 vaccination outweigh the risks, including the possible risk for myocarditis or pericarditis, the CDC states.
Across clinician types, women edged out their male counterparts on confidence in the vaccine’ s safety: 71% vs 65% among physicians, 55% vs 45% among nurses/APRNs, and 68% vs 60% among pharmacists.
Among both physicians and nurses, younger physicians (under 45) tended to have greater confidence in the vaccine’ s safety: 72% vs 64% (physicians), 54% vs 51% (nurses/APRNs), and 71% vs 59% (pharmacists).
The difference in confidence was clear between vaccinated and unvaccinated physicians. All of the unvaccinated physicians who responded to the poll said they had no confidence in the vaccine for kids. Among unvaccinated nurses/APRNs, 2% were somewhat confident in the vaccine for kids under 12.
Knowledge about smaller dosage
The clinicians were asked about whether they were aware, before reading the poll question, that the Pfizer vaccine for children and the proposed Moderna vaccine for children in this age group (5-11) would have a different dosage.
The dose for kids 5-11 is 10 micrograms rather than 30 micrograms for people at least 12 years old. Children 5-11 receive a second dose 21 days or more after their first shot. The formulation comes with an orange cap, and a smaller needle is used.
Knowledge on the lower dose was highest among pharmacists (91% said they knew), followed by physicians (84%) and nurses (79%).
The poll also asked whether the COVID-19 vaccine should be added to the list of childhood immunizations. Responses varied widely and uncertainty was evident.
Notably, female physicians were more likely to say it should be added to the list of immunizations than were their male counterparts: 46% vs 35% (physicians), 26% vs 22% (nurses/APRNs), and 33% vs 30% (pharmacists).
A version of this article first appeared on Medscape.com.
A Medscape
Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did not want their kids to get the COVID-19 vaccine; 13% were unsure. Among pharmacists, 31% said they would not get them vaccinated and 9% were unsure.
Clinicians were more likely to want vaccinations for their kids 5-11 than were 510 consumers polled by WebMD at the same time. Overall, 49% of the consumers who had kids that age did not want them to get the COVID-19 vaccine.
On November 2, Centers for Disease Control and Prevention (CDC) Director Rochelle P. Walensky, MD, MPH, endorsed the CDC Advisory Committee on Immunization Practices’ recommendation that children 5-11 be vaccinated with the Pfizer-BioNTech pediatric vaccine. That decision expanded vaccine recommendations to about 28 million children in the United States.
The CDC states that, in clinical trials, the Pfizer vaccine had more than 90% efficacy in preventing laboratory-confirmed COVID-19 infection in children 5 to 15 years old, and that the immune response in children ages 5-15 equaled the immune response in people 16 to 25 years old.
The Medscape poll, fielded from November 3 to November 11, included 325 physicians, 793 nurses/APRNs, and 151 pharmacists.
How safe is the vaccine?
Clinicians were asked how confident they were that the vaccine is safe for that age group, and 66% of physicians, 52% of nurses/APRNs, and 66% of pharmacists said they were somewhat or very confident.
Among consumers overall in the WebMD poll, 56% said they were confident or somewhat confident that the vaccine is safe in that age group.
Among adolescents and young adults, rare cases of myocarditis and pericarditis in adolescents and young adults have been reported. According to the CDC, “[I]n one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12-17 years.”
Known and potential benefits of COVID-19 vaccination outweigh the risks, including the possible risk for myocarditis or pericarditis, the CDC states.
Across clinician types, women edged out their male counterparts on confidence in the vaccine’ s safety: 71% vs 65% among physicians, 55% vs 45% among nurses/APRNs, and 68% vs 60% among pharmacists.
Among both physicians and nurses, younger physicians (under 45) tended to have greater confidence in the vaccine’ s safety: 72% vs 64% (physicians), 54% vs 51% (nurses/APRNs), and 71% vs 59% (pharmacists).
The difference in confidence was clear between vaccinated and unvaccinated physicians. All of the unvaccinated physicians who responded to the poll said they had no confidence in the vaccine for kids. Among unvaccinated nurses/APRNs, 2% were somewhat confident in the vaccine for kids under 12.
Knowledge about smaller dosage
The clinicians were asked about whether they were aware, before reading the poll question, that the Pfizer vaccine for children and the proposed Moderna vaccine for children in this age group (5-11) would have a different dosage.
The dose for kids 5-11 is 10 micrograms rather than 30 micrograms for people at least 12 years old. Children 5-11 receive a second dose 21 days or more after their first shot. The formulation comes with an orange cap, and a smaller needle is used.
Knowledge on the lower dose was highest among pharmacists (91% said they knew), followed by physicians (84%) and nurses (79%).
The poll also asked whether the COVID-19 vaccine should be added to the list of childhood immunizations. Responses varied widely and uncertainty was evident.
Notably, female physicians were more likely to say it should be added to the list of immunizations than were their male counterparts: 46% vs 35% (physicians), 26% vs 22% (nurses/APRNs), and 33% vs 30% (pharmacists).
A version of this article first appeared on Medscape.com.
A Medscape
Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did not want their kids to get the COVID-19 vaccine; 13% were unsure. Among pharmacists, 31% said they would not get them vaccinated and 9% were unsure.
Clinicians were more likely to want vaccinations for their kids 5-11 than were 510 consumers polled by WebMD at the same time. Overall, 49% of the consumers who had kids that age did not want them to get the COVID-19 vaccine.
On November 2, Centers for Disease Control and Prevention (CDC) Director Rochelle P. Walensky, MD, MPH, endorsed the CDC Advisory Committee on Immunization Practices’ recommendation that children 5-11 be vaccinated with the Pfizer-BioNTech pediatric vaccine. That decision expanded vaccine recommendations to about 28 million children in the United States.
The CDC states that, in clinical trials, the Pfizer vaccine had more than 90% efficacy in preventing laboratory-confirmed COVID-19 infection in children 5 to 15 years old, and that the immune response in children ages 5-15 equaled the immune response in people 16 to 25 years old.
The Medscape poll, fielded from November 3 to November 11, included 325 physicians, 793 nurses/APRNs, and 151 pharmacists.
How safe is the vaccine?
Clinicians were asked how confident they were that the vaccine is safe for that age group, and 66% of physicians, 52% of nurses/APRNs, and 66% of pharmacists said they were somewhat or very confident.
Among consumers overall in the WebMD poll, 56% said they were confident or somewhat confident that the vaccine is safe in that age group.
Among adolescents and young adults, rare cases of myocarditis and pericarditis in adolescents and young adults have been reported. According to the CDC, “[I]n one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12-17 years.”
Known and potential benefits of COVID-19 vaccination outweigh the risks, including the possible risk for myocarditis or pericarditis, the CDC states.
Across clinician types, women edged out their male counterparts on confidence in the vaccine’ s safety: 71% vs 65% among physicians, 55% vs 45% among nurses/APRNs, and 68% vs 60% among pharmacists.
Among both physicians and nurses, younger physicians (under 45) tended to have greater confidence in the vaccine’ s safety: 72% vs 64% (physicians), 54% vs 51% (nurses/APRNs), and 71% vs 59% (pharmacists).
The difference in confidence was clear between vaccinated and unvaccinated physicians. All of the unvaccinated physicians who responded to the poll said they had no confidence in the vaccine for kids. Among unvaccinated nurses/APRNs, 2% were somewhat confident in the vaccine for kids under 12.
Knowledge about smaller dosage
The clinicians were asked about whether they were aware, before reading the poll question, that the Pfizer vaccine for children and the proposed Moderna vaccine for children in this age group (5-11) would have a different dosage.
The dose for kids 5-11 is 10 micrograms rather than 30 micrograms for people at least 12 years old. Children 5-11 receive a second dose 21 days or more after their first shot. The formulation comes with an orange cap, and a smaller needle is used.
Knowledge on the lower dose was highest among pharmacists (91% said they knew), followed by physicians (84%) and nurses (79%).
The poll also asked whether the COVID-19 vaccine should be added to the list of childhood immunizations. Responses varied widely and uncertainty was evident.
Notably, female physicians were more likely to say it should be added to the list of immunizations than were their male counterparts: 46% vs 35% (physicians), 26% vs 22% (nurses/APRNs), and 33% vs 30% (pharmacists).
A version of this article first appeared on Medscape.com.
Children and COVID: New cases increase for third straight week
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.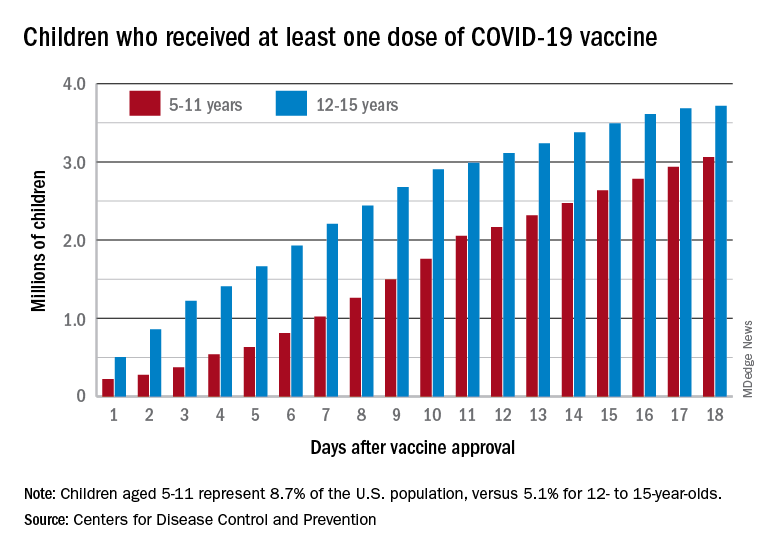
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
Penicillin slows latent rheumatic heart disease progression
In a randomized controlled trial of close to 1,000 Ugandan children and youth with latent rheumatic heart disease (RHD), those who received monthly injections of penicillin G benzathine for 2 years had less disease progression than those who did not.
RHD, a valvular heart disease caused by rheumatic fever that develops after untreated Streptococcus pyogenes infection, is the most common acquired cardiovascular disease among children and young adults.
“It is clear that secondary antibiotic prophylaxis can improve outcomes for children with echo-detected rheumatic RHD,” co–lead author of the study, Andrea Z. Beaton, MD, said in an interview.
“There is huge potential here, but we are not quite ready to advocate for this strategy as a broad public health approach,” said Dr. Beaton, a pediatric cardiologist at Cincinnati Children’s Hospital Medical Center.
“We need to understand more the practical translation of this strategy to a low-resourced public health system at scale, improve [penicillin G benzathine] supply, and improve community and health care worker knowledge of this disease.”
Dr. Beaton presented the findings at the American Heart Association scientific sessions, and the study was simultaneously published in the New England Journal of Medicine on Nov. 13, 2021.
The GOAL trial – or the Gwoko Adunu pa Lutino trial, meaning “protect the heart of a child” – screened 102,200 children and adolescents aged 5-17. Of these kids and teenagers, 926 (0.9%) were diagnosed with latent RHD based on a confirmatory electrocardiogram.
“For now, I would say, if you are screening, then kids found to have latent RHD should be put on prophylaxis,” Dr. Beaton said.
“I think this is also a powerful call for more research [severely lacking in RHD],” to improve risk stratification, determine how to implement screening and prophylaxis programs, and develop new and better approaches for RHD prevention and care.
“This essential trial partially addresses the clinical equipoise that has developed regarding penicillin administration in latent RHD,” said Gabriele Rossi, MD, MPH, who was not involved with this research.
It showed that, out of the final 818 participants included in the modified intention-to-treat analysis, a total of 3 (0.8%) in the prophylaxis group had echocardiographic progression at 2 years, compared with 33 participants (8.2%) in the control group (risk difference, −7.5 percentage points; 95% confidence interval, −10.2 to −4.7; P < .001).
“This is a significant difference,” Dr. Rossi, from Médecins Sans Frontières (Doctors Without Borders), Brussels, said in an interview, noting that, however, it is not known what happens after 2 years.
The authors estimated that 13 children or adolescents with latent rheumatic heart disease would need to be treated to prevent disease progression in one person at 2 years, which is “acceptable,” he continued.
However, “screening, diagnosis, clinical follow-up, treatment, and program management [would] require substantial strengthening of health systems and the workforce, which is still far from being realizable in many African and low-income country settings,” Dr. Rossi noted.
Related study in Italy
Previously, Dr. Rossi and colleagues conducted a trial, published in 2019, that showed it was feasible to screen for asymptomatic RHD among refugee/migrant children and youths in Rome.
From February 2016 to January 2018, they screened more than 650 refugee/migrant children and adolescents who were younger than 18. They came largely from Egypt (65%) but also from 22 other countries and were often unaccompanied or with just one parent.
The number needed to screen was 5 to identify a child/youth with borderline RHD and around 40 to identify a child/youth with definite RHD.
Dr. Rossi noted that local resurgences of RHD have also been also documented in high-income countries such as Europe, Australia, New Zealand, Canada, and the United States, often among disadvantaged indigenous people, as described in a 2018 Letter to the Editor in the New England Journal of Medicine.
Dr. Beaton noted that a review of 10-year data (2008-2018) from 22 U.S. pediatric institutions showed that in the United States the prevalence of RHD “is higher in immigrant children from RHD endemic areas, but because of total numbers, more RHD cases than not are domestic.” Children living in more deprived communities are at risk for more severe disease, and the burden in U.S. territories is also quite high.
Screening and secondary prophylaxis
The aim of the current GOAL study was to evaluate if screening and treatment with penicillin G benzathine could detect and prevent progression of latent rheumatic heart disease in 5- to 17-year-olds living in Gulu, Uganda. The trial was conducted from July 2018 to October 2020.
“School education and community sensitization was done prior to the trial,” through radio shows or school-based education, Dr. Beaton explained. About 99% of the children/adolescents/families agreed to be screened.
The group has been conducting echo screening research in Uganda for 10 years, she noted. They have developed peer group and case manager strategies to aid participant retention, as they describe in an article about the study protocol.
The screening echocardiograms were interpreted by about 30 providers and four cardiologists reviewed confirmatory echocardiograms.
Two participants in the prophylaxis group had serious adverse events that were attributable to receipt of prophylaxis, including one episode of a mild anaphylactic reaction (representing <0.1% of all administered doses of prophylaxis).
Once children and adolescents have moderate/severe RHD, there is not much that can be done in lower- and middle-income countries, where surgery for this is uncommon, Dr. Beaton explained. Around 30% of children and adolescents with this condition who come to clinical attention in Uganda die within 9 months.
Further research
Dr. Beaton and colleagues have just started a trial to investigate the burden of RHD among Native American youth, which has not been studied since the 1970s.
They also have an ongoing study looking at the efficacy of a pragmatic, community-based sore throat program to prevent RHD.
“Unfortunately, this strategy has not worked well in low-to-middle income countries, for a variety of reasons so far,” Dr. Beaton noted, and the cost-effectiveness of this preventive strategy is questionable.
The trial was supported by the Thrasher Research Fund, Gift of Life International, Children’s National Hospital Foundation (Zachary Blumenfeld Fund and Race for Every Child [Team Jocelyn]), the Elias-Ginsburg Family, Wiley Rein, Philips Foundation, AT&T Foundation, Heart Healers International, the Karp Family Foundation, Huron Philanthropies, and the Cincinnati Children’s Hospital Heart Institute Research Core. Dr. Beaton and Dr. Rossi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled trial of close to 1,000 Ugandan children and youth with latent rheumatic heart disease (RHD), those who received monthly injections of penicillin G benzathine for 2 years had less disease progression than those who did not.
RHD, a valvular heart disease caused by rheumatic fever that develops after untreated Streptococcus pyogenes infection, is the most common acquired cardiovascular disease among children and young adults.
“It is clear that secondary antibiotic prophylaxis can improve outcomes for children with echo-detected rheumatic RHD,” co–lead author of the study, Andrea Z. Beaton, MD, said in an interview.
“There is huge potential here, but we are not quite ready to advocate for this strategy as a broad public health approach,” said Dr. Beaton, a pediatric cardiologist at Cincinnati Children’s Hospital Medical Center.
“We need to understand more the practical translation of this strategy to a low-resourced public health system at scale, improve [penicillin G benzathine] supply, and improve community and health care worker knowledge of this disease.”
Dr. Beaton presented the findings at the American Heart Association scientific sessions, and the study was simultaneously published in the New England Journal of Medicine on Nov. 13, 2021.
The GOAL trial – or the Gwoko Adunu pa Lutino trial, meaning “protect the heart of a child” – screened 102,200 children and adolescents aged 5-17. Of these kids and teenagers, 926 (0.9%) were diagnosed with latent RHD based on a confirmatory electrocardiogram.
“For now, I would say, if you are screening, then kids found to have latent RHD should be put on prophylaxis,” Dr. Beaton said.
“I think this is also a powerful call for more research [severely lacking in RHD],” to improve risk stratification, determine how to implement screening and prophylaxis programs, and develop new and better approaches for RHD prevention and care.
“This essential trial partially addresses the clinical equipoise that has developed regarding penicillin administration in latent RHD,” said Gabriele Rossi, MD, MPH, who was not involved with this research.
It showed that, out of the final 818 participants included in the modified intention-to-treat analysis, a total of 3 (0.8%) in the prophylaxis group had echocardiographic progression at 2 years, compared with 33 participants (8.2%) in the control group (risk difference, −7.5 percentage points; 95% confidence interval, −10.2 to −4.7; P < .001).
“This is a significant difference,” Dr. Rossi, from Médecins Sans Frontières (Doctors Without Borders), Brussels, said in an interview, noting that, however, it is not known what happens after 2 years.
The authors estimated that 13 children or adolescents with latent rheumatic heart disease would need to be treated to prevent disease progression in one person at 2 years, which is “acceptable,” he continued.
However, “screening, diagnosis, clinical follow-up, treatment, and program management [would] require substantial strengthening of health systems and the workforce, which is still far from being realizable in many African and low-income country settings,” Dr. Rossi noted.
Related study in Italy
Previously, Dr. Rossi and colleagues conducted a trial, published in 2019, that showed it was feasible to screen for asymptomatic RHD among refugee/migrant children and youths in Rome.
From February 2016 to January 2018, they screened more than 650 refugee/migrant children and adolescents who were younger than 18. They came largely from Egypt (65%) but also from 22 other countries and were often unaccompanied or with just one parent.
The number needed to screen was 5 to identify a child/youth with borderline RHD and around 40 to identify a child/youth with definite RHD.
Dr. Rossi noted that local resurgences of RHD have also been also documented in high-income countries such as Europe, Australia, New Zealand, Canada, and the United States, often among disadvantaged indigenous people, as described in a 2018 Letter to the Editor in the New England Journal of Medicine.
Dr. Beaton noted that a review of 10-year data (2008-2018) from 22 U.S. pediatric institutions showed that in the United States the prevalence of RHD “is higher in immigrant children from RHD endemic areas, but because of total numbers, more RHD cases than not are domestic.” Children living in more deprived communities are at risk for more severe disease, and the burden in U.S. territories is also quite high.
Screening and secondary prophylaxis
The aim of the current GOAL study was to evaluate if screening and treatment with penicillin G benzathine could detect and prevent progression of latent rheumatic heart disease in 5- to 17-year-olds living in Gulu, Uganda. The trial was conducted from July 2018 to October 2020.
“School education and community sensitization was done prior to the trial,” through radio shows or school-based education, Dr. Beaton explained. About 99% of the children/adolescents/families agreed to be screened.
The group has been conducting echo screening research in Uganda for 10 years, she noted. They have developed peer group and case manager strategies to aid participant retention, as they describe in an article about the study protocol.
The screening echocardiograms were interpreted by about 30 providers and four cardiologists reviewed confirmatory echocardiograms.
Two participants in the prophylaxis group had serious adverse events that were attributable to receipt of prophylaxis, including one episode of a mild anaphylactic reaction (representing <0.1% of all administered doses of prophylaxis).
Once children and adolescents have moderate/severe RHD, there is not much that can be done in lower- and middle-income countries, where surgery for this is uncommon, Dr. Beaton explained. Around 30% of children and adolescents with this condition who come to clinical attention in Uganda die within 9 months.
Further research
Dr. Beaton and colleagues have just started a trial to investigate the burden of RHD among Native American youth, which has not been studied since the 1970s.
They also have an ongoing study looking at the efficacy of a pragmatic, community-based sore throat program to prevent RHD.
“Unfortunately, this strategy has not worked well in low-to-middle income countries, for a variety of reasons so far,” Dr. Beaton noted, and the cost-effectiveness of this preventive strategy is questionable.
The trial was supported by the Thrasher Research Fund, Gift of Life International, Children’s National Hospital Foundation (Zachary Blumenfeld Fund and Race for Every Child [Team Jocelyn]), the Elias-Ginsburg Family, Wiley Rein, Philips Foundation, AT&T Foundation, Heart Healers International, the Karp Family Foundation, Huron Philanthropies, and the Cincinnati Children’s Hospital Heart Institute Research Core. Dr. Beaton and Dr. Rossi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled trial of close to 1,000 Ugandan children and youth with latent rheumatic heart disease (RHD), those who received monthly injections of penicillin G benzathine for 2 years had less disease progression than those who did not.
RHD, a valvular heart disease caused by rheumatic fever that develops after untreated Streptococcus pyogenes infection, is the most common acquired cardiovascular disease among children and young adults.
“It is clear that secondary antibiotic prophylaxis can improve outcomes for children with echo-detected rheumatic RHD,” co–lead author of the study, Andrea Z. Beaton, MD, said in an interview.
“There is huge potential here, but we are not quite ready to advocate for this strategy as a broad public health approach,” said Dr. Beaton, a pediatric cardiologist at Cincinnati Children’s Hospital Medical Center.
“We need to understand more the practical translation of this strategy to a low-resourced public health system at scale, improve [penicillin G benzathine] supply, and improve community and health care worker knowledge of this disease.”
Dr. Beaton presented the findings at the American Heart Association scientific sessions, and the study was simultaneously published in the New England Journal of Medicine on Nov. 13, 2021.
The GOAL trial – or the Gwoko Adunu pa Lutino trial, meaning “protect the heart of a child” – screened 102,200 children and adolescents aged 5-17. Of these kids and teenagers, 926 (0.9%) were diagnosed with latent RHD based on a confirmatory electrocardiogram.
“For now, I would say, if you are screening, then kids found to have latent RHD should be put on prophylaxis,” Dr. Beaton said.
“I think this is also a powerful call for more research [severely lacking in RHD],” to improve risk stratification, determine how to implement screening and prophylaxis programs, and develop new and better approaches for RHD prevention and care.
“This essential trial partially addresses the clinical equipoise that has developed regarding penicillin administration in latent RHD,” said Gabriele Rossi, MD, MPH, who was not involved with this research.
It showed that, out of the final 818 participants included in the modified intention-to-treat analysis, a total of 3 (0.8%) in the prophylaxis group had echocardiographic progression at 2 years, compared with 33 participants (8.2%) in the control group (risk difference, −7.5 percentage points; 95% confidence interval, −10.2 to −4.7; P < .001).
“This is a significant difference,” Dr. Rossi, from Médecins Sans Frontières (Doctors Without Borders), Brussels, said in an interview, noting that, however, it is not known what happens after 2 years.
The authors estimated that 13 children or adolescents with latent rheumatic heart disease would need to be treated to prevent disease progression in one person at 2 years, which is “acceptable,” he continued.
However, “screening, diagnosis, clinical follow-up, treatment, and program management [would] require substantial strengthening of health systems and the workforce, which is still far from being realizable in many African and low-income country settings,” Dr. Rossi noted.
Related study in Italy
Previously, Dr. Rossi and colleagues conducted a trial, published in 2019, that showed it was feasible to screen for asymptomatic RHD among refugee/migrant children and youths in Rome.
From February 2016 to January 2018, they screened more than 650 refugee/migrant children and adolescents who were younger than 18. They came largely from Egypt (65%) but also from 22 other countries and were often unaccompanied or with just one parent.
The number needed to screen was 5 to identify a child/youth with borderline RHD and around 40 to identify a child/youth with definite RHD.
Dr. Rossi noted that local resurgences of RHD have also been also documented in high-income countries such as Europe, Australia, New Zealand, Canada, and the United States, often among disadvantaged indigenous people, as described in a 2018 Letter to the Editor in the New England Journal of Medicine.
Dr. Beaton noted that a review of 10-year data (2008-2018) from 22 U.S. pediatric institutions showed that in the United States the prevalence of RHD “is higher in immigrant children from RHD endemic areas, but because of total numbers, more RHD cases than not are domestic.” Children living in more deprived communities are at risk for more severe disease, and the burden in U.S. territories is also quite high.
Screening and secondary prophylaxis
The aim of the current GOAL study was to evaluate if screening and treatment with penicillin G benzathine could detect and prevent progression of latent rheumatic heart disease in 5- to 17-year-olds living in Gulu, Uganda. The trial was conducted from July 2018 to October 2020.
“School education and community sensitization was done prior to the trial,” through radio shows or school-based education, Dr. Beaton explained. About 99% of the children/adolescents/families agreed to be screened.
The group has been conducting echo screening research in Uganda for 10 years, she noted. They have developed peer group and case manager strategies to aid participant retention, as they describe in an article about the study protocol.
The screening echocardiograms were interpreted by about 30 providers and four cardiologists reviewed confirmatory echocardiograms.
Two participants in the prophylaxis group had serious adverse events that were attributable to receipt of prophylaxis, including one episode of a mild anaphylactic reaction (representing <0.1% of all administered doses of prophylaxis).
Once children and adolescents have moderate/severe RHD, there is not much that can be done in lower- and middle-income countries, where surgery for this is uncommon, Dr. Beaton explained. Around 30% of children and adolescents with this condition who come to clinical attention in Uganda die within 9 months.
Further research
Dr. Beaton and colleagues have just started a trial to investigate the burden of RHD among Native American youth, which has not been studied since the 1970s.
They also have an ongoing study looking at the efficacy of a pragmatic, community-based sore throat program to prevent RHD.
“Unfortunately, this strategy has not worked well in low-to-middle income countries, for a variety of reasons so far,” Dr. Beaton noted, and the cost-effectiveness of this preventive strategy is questionable.
The trial was supported by the Thrasher Research Fund, Gift of Life International, Children’s National Hospital Foundation (Zachary Blumenfeld Fund and Race for Every Child [Team Jocelyn]), the Elias-Ginsburg Family, Wiley Rein, Philips Foundation, AT&T Foundation, Heart Healers International, the Karp Family Foundation, Huron Philanthropies, and the Cincinnati Children’s Hospital Heart Institute Research Core. Dr. Beaton and Dr. Rossi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
HCV screening in pregnancy: Reducing the risk for casualties in the quest for elimination
Because hepatitis C virus (HCV) infection is typically asymptomatic, its presence can easily be overlooked without appropriate screening efforts. For those screening efforts to be effective, they must keep pace with the changing demographic face of this increasingly prevalent but treatable disease.
Perhaps the most dramatic shift in HCV demographics in recent years has been the increase of infections among those born after 1965, a trend primarily driven by the opioid epidemic. In addition, data from the National Notifiable Diseases Surveillance System show that cases of diagnosed HCV doubled among women of childbearing age from 2006 to 2014, with new infections in younger women surpassing those in older age groups.
With such trends in mind, the Centers for Disease Control and Prevention broadened their recommendations regarding HCV in 2020 to include one-time testing in all adults aged 18 years and older and screening of all pregnant women during each pregnancy, except where the prevalence of infection is less than 0.1%, a threshold that no state has yet achieved.
The US Preventive Services Task Force (USPSTF) subsequently followed suit in their own recommendations.
The American Association for the Study of Liver Diseases/Infectious Diseases Society of America have long advocated for extensive expansion in their screening recommendations for HCV, including pregnancy.
Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine did not immediately adopt these recommendations, they have since endorsed them in May 2021 and June 2021, respectively.
The hepatologist perspective
As a practicing hepatologist, this seems like an uncontroversial recommendation. Obstetricians already screen for hepatitis B virus in each pregnancy. It should be easy to add HCV testing to the same lab testing.
Risk-based screening has repeatedly been demonstrated to be ineffective. It should be easier to test all women than to ask prying questions about high-risk behaviors.
Given the increase of injection drug use and resultant HCV infections in women of childbearing age, this seems like a perfect opportunity to identify chronically infected women and counsel them on transmission and cure. And pregnancy is also unique in that it is a time of near-universal health coverage.
Let’s address some of the operational issues.
The diagnostic cascade for HCV can be made very easy. HCV antibody testing is our standard screening test and, when positive, can automatically reflex to HCV polymerase chain reaction (PCR), the diagnostic test. Thus, with one blood sample, you can both screen for and diagnose infection.
Current guidelines do not recommend treating HCV during pregnancy, although therapy can be considered on an individual basis. Linkage to a knowledgeable provider who can discuss transmission and treatment, as well as assess the stage of liver injury, should decrease the burden on the ob.gyn.
The impact on pregnancy is marginal. HCV should not change either the mode of delivery or the decision to breastfeed. The AASLD/IDSA guidance outlines only four recommendations for monitoring during pregnancy:
- Obtain HCV RNA to see whether the infection is active and assess liver function at initiation of prenatal care.
- Prenatal care should be tailored to the pregnancy. There is no modification recommended to decrease mother-to-child transmission (MTCT).
- Be aware that intrahepatic is more common with HCV.
- Women with have a higher rate of adverse outcomes and should be linked to a high-risk obstetrics specialist.
But of course, what seems easy to one specialist may not be true of another. With that in mind, let’s hear the ob.gyn. perspective on these updated screening recommendations.
The ob.gyn. perspective
Recent guidelines from the CDC, ACOG, and SMFM recommend universal screening for HCV in all pregnant women. The increased availability of highly effective antiviral regimens makes universal screening a logical strategy, especially to identify candidates for this curative treatment. What is questionable, however, is the recommended timing by which this screening should take place.
HCV screening during pregnancy, as currently recommended, provides no immediate benefit for the pregnant woman or the fetus/neonate, given that antiviral treatments have not been approved during gestation, and there are no known measures that decrease MTCT or change routine perinatal care.
We also must not forget that a significant proportion of women in the United States, particularly those with limited resources, do not receive prenatal care at all. Most of them, however, will present to a hospital for delivery. Consequently, compliance with screening might be higher if performed at the time of delivery rather than antepartum.
Deferring screening until the intrapartum or immediate postpartum period, at least until antiviral treatment during pregnancy becomes a reality, was discussed. The rationale was that this approach might obviate the need to deal with the unintended consequences and burden of testing for HCV during pregnancy. Ultimately, ACOG and SMFM fell in line with the CDC recommendations.
Despite the lack of robust evidence regarding the risk for MTCT associated with commonly performed obstetric procedures (for example, genetic amniocentesis, artificial rupture of the membranes during labor, placement of an intrauterine pressure catheter), clinicians may be reluctant to perform them in HCV-infected women, resulting in potential deviations from the obstetric standard of care.
Similarly, it is likely that patients may choose to have a cesarean delivery for the sole purpose of decreasing MTCT, despite the lack of evidence for this. Such ill-advised patient-driven decisions are increasingly likely in the current environment, where social media can rapidly disseminate misinformation.
Implications for pediatric patients
One cannot isolate HCV screening in pregnancy from the consequences that may potentially occur as part of the infant’s transition to the care of a pediatrician.
Even though MTCT is estimated to occur in just 5%-15% of cases, all children born to HCV viremic mothers should be screened for HCV.
Traditionally, screening for HCV antibodies occurred after 18 months of age. In those who test positive, HCV PCR testing is recommended at 3 years. However, this algorithm is being called into question because only approximately one-third of infants are successfully screened.
HCV RNA testing in the first year after birth has been suggested. However, even proponents of this approach concur that all management decisions should be deferred until after the age of 3 years, when medications are approved for pediatric use.
In addition, HCV testing would be required again before considering therapy because children have higher rates of spontaneous clearance.
Seeking consensus beyond the controversy
Controversy remains surrounding the most recent update to the HCV screening guidelines. The current recommendation to screen during pregnancy cannot modify the risk for MTCT, has no impact on decisions regarding mode of delivery or breastfeeding, and could potentially cause harm by making obstetricians defer necessary invasive procedures even though there are no data linking them to an increase in MTCT.
Yet after extensive debate, the CDC, USPSTF, AASLD/IDSA, ACOG, and SMFM all developed their current recommendations to initiate HCV screening during pregnancy. To make this successful, screening algorithms need to be simple and consistent across all society recommendations.
HCV antibody testing should always reflex to the diagnostic test (HCV PCR) to allow confirmation in those who test positive without requiring an additional blood test. Viremic mothers (those who are HCV positive on PCR) should be linked to a provider who can discuss prognosis, transmission, and treatment. The importance of screening the infant also must be communicated to the parents and pediatrician alike.
Dr. Reau has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Gilead, Arbutus, Intercept, and Salix; received research grants from AbbVie and Gilead; and received income from AASLD. Dr. Pacheco disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Because hepatitis C virus (HCV) infection is typically asymptomatic, its presence can easily be overlooked without appropriate screening efforts. For those screening efforts to be effective, they must keep pace with the changing demographic face of this increasingly prevalent but treatable disease.
Perhaps the most dramatic shift in HCV demographics in recent years has been the increase of infections among those born after 1965, a trend primarily driven by the opioid epidemic. In addition, data from the National Notifiable Diseases Surveillance System show that cases of diagnosed HCV doubled among women of childbearing age from 2006 to 2014, with new infections in younger women surpassing those in older age groups.
With such trends in mind, the Centers for Disease Control and Prevention broadened their recommendations regarding HCV in 2020 to include one-time testing in all adults aged 18 years and older and screening of all pregnant women during each pregnancy, except where the prevalence of infection is less than 0.1%, a threshold that no state has yet achieved.
The US Preventive Services Task Force (USPSTF) subsequently followed suit in their own recommendations.
The American Association for the Study of Liver Diseases/Infectious Diseases Society of America have long advocated for extensive expansion in their screening recommendations for HCV, including pregnancy.
Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine did not immediately adopt these recommendations, they have since endorsed them in May 2021 and June 2021, respectively.
The hepatologist perspective
As a practicing hepatologist, this seems like an uncontroversial recommendation. Obstetricians already screen for hepatitis B virus in each pregnancy. It should be easy to add HCV testing to the same lab testing.
Risk-based screening has repeatedly been demonstrated to be ineffective. It should be easier to test all women than to ask prying questions about high-risk behaviors.
Given the increase of injection drug use and resultant HCV infections in women of childbearing age, this seems like a perfect opportunity to identify chronically infected women and counsel them on transmission and cure. And pregnancy is also unique in that it is a time of near-universal health coverage.
Let’s address some of the operational issues.
The diagnostic cascade for HCV can be made very easy. HCV antibody testing is our standard screening test and, when positive, can automatically reflex to HCV polymerase chain reaction (PCR), the diagnostic test. Thus, with one blood sample, you can both screen for and diagnose infection.
Current guidelines do not recommend treating HCV during pregnancy, although therapy can be considered on an individual basis. Linkage to a knowledgeable provider who can discuss transmission and treatment, as well as assess the stage of liver injury, should decrease the burden on the ob.gyn.
The impact on pregnancy is marginal. HCV should not change either the mode of delivery or the decision to breastfeed. The AASLD/IDSA guidance outlines only four recommendations for monitoring during pregnancy:
- Obtain HCV RNA to see whether the infection is active and assess liver function at initiation of prenatal care.
- Prenatal care should be tailored to the pregnancy. There is no modification recommended to decrease mother-to-child transmission (MTCT).
- Be aware that intrahepatic is more common with HCV.
- Women with have a higher rate of adverse outcomes and should be linked to a high-risk obstetrics specialist.
But of course, what seems easy to one specialist may not be true of another. With that in mind, let’s hear the ob.gyn. perspective on these updated screening recommendations.
The ob.gyn. perspective
Recent guidelines from the CDC, ACOG, and SMFM recommend universal screening for HCV in all pregnant women. The increased availability of highly effective antiviral regimens makes universal screening a logical strategy, especially to identify candidates for this curative treatment. What is questionable, however, is the recommended timing by which this screening should take place.
HCV screening during pregnancy, as currently recommended, provides no immediate benefit for the pregnant woman or the fetus/neonate, given that antiviral treatments have not been approved during gestation, and there are no known measures that decrease MTCT or change routine perinatal care.
We also must not forget that a significant proportion of women in the United States, particularly those with limited resources, do not receive prenatal care at all. Most of them, however, will present to a hospital for delivery. Consequently, compliance with screening might be higher if performed at the time of delivery rather than antepartum.
Deferring screening until the intrapartum or immediate postpartum period, at least until antiviral treatment during pregnancy becomes a reality, was discussed. The rationale was that this approach might obviate the need to deal with the unintended consequences and burden of testing for HCV during pregnancy. Ultimately, ACOG and SMFM fell in line with the CDC recommendations.
Despite the lack of robust evidence regarding the risk for MTCT associated with commonly performed obstetric procedures (for example, genetic amniocentesis, artificial rupture of the membranes during labor, placement of an intrauterine pressure catheter), clinicians may be reluctant to perform them in HCV-infected women, resulting in potential deviations from the obstetric standard of care.
Similarly, it is likely that patients may choose to have a cesarean delivery for the sole purpose of decreasing MTCT, despite the lack of evidence for this. Such ill-advised patient-driven decisions are increasingly likely in the current environment, where social media can rapidly disseminate misinformation.
Implications for pediatric patients
One cannot isolate HCV screening in pregnancy from the consequences that may potentially occur as part of the infant’s transition to the care of a pediatrician.
Even though MTCT is estimated to occur in just 5%-15% of cases, all children born to HCV viremic mothers should be screened for HCV.
Traditionally, screening for HCV antibodies occurred after 18 months of age. In those who test positive, HCV PCR testing is recommended at 3 years. However, this algorithm is being called into question because only approximately one-third of infants are successfully screened.
HCV RNA testing in the first year after birth has been suggested. However, even proponents of this approach concur that all management decisions should be deferred until after the age of 3 years, when medications are approved for pediatric use.
In addition, HCV testing would be required again before considering therapy because children have higher rates of spontaneous clearance.
Seeking consensus beyond the controversy
Controversy remains surrounding the most recent update to the HCV screening guidelines. The current recommendation to screen during pregnancy cannot modify the risk for MTCT, has no impact on decisions regarding mode of delivery or breastfeeding, and could potentially cause harm by making obstetricians defer necessary invasive procedures even though there are no data linking them to an increase in MTCT.
Yet after extensive debate, the CDC, USPSTF, AASLD/IDSA, ACOG, and SMFM all developed their current recommendations to initiate HCV screening during pregnancy. To make this successful, screening algorithms need to be simple and consistent across all society recommendations.
HCV antibody testing should always reflex to the diagnostic test (HCV PCR) to allow confirmation in those who test positive without requiring an additional blood test. Viremic mothers (those who are HCV positive on PCR) should be linked to a provider who can discuss prognosis, transmission, and treatment. The importance of screening the infant also must be communicated to the parents and pediatrician alike.
Dr. Reau has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Gilead, Arbutus, Intercept, and Salix; received research grants from AbbVie and Gilead; and received income from AASLD. Dr. Pacheco disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Because hepatitis C virus (HCV) infection is typically asymptomatic, its presence can easily be overlooked without appropriate screening efforts. For those screening efforts to be effective, they must keep pace with the changing demographic face of this increasingly prevalent but treatable disease.
Perhaps the most dramatic shift in HCV demographics in recent years has been the increase of infections among those born after 1965, a trend primarily driven by the opioid epidemic. In addition, data from the National Notifiable Diseases Surveillance System show that cases of diagnosed HCV doubled among women of childbearing age from 2006 to 2014, with new infections in younger women surpassing those in older age groups.
With such trends in mind, the Centers for Disease Control and Prevention broadened their recommendations regarding HCV in 2020 to include one-time testing in all adults aged 18 years and older and screening of all pregnant women during each pregnancy, except where the prevalence of infection is less than 0.1%, a threshold that no state has yet achieved.
The US Preventive Services Task Force (USPSTF) subsequently followed suit in their own recommendations.
The American Association for the Study of Liver Diseases/Infectious Diseases Society of America have long advocated for extensive expansion in their screening recommendations for HCV, including pregnancy.
Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine did not immediately adopt these recommendations, they have since endorsed them in May 2021 and June 2021, respectively.
The hepatologist perspective
As a practicing hepatologist, this seems like an uncontroversial recommendation. Obstetricians already screen for hepatitis B virus in each pregnancy. It should be easy to add HCV testing to the same lab testing.
Risk-based screening has repeatedly been demonstrated to be ineffective. It should be easier to test all women than to ask prying questions about high-risk behaviors.
Given the increase of injection drug use and resultant HCV infections in women of childbearing age, this seems like a perfect opportunity to identify chronically infected women and counsel them on transmission and cure. And pregnancy is also unique in that it is a time of near-universal health coverage.
Let’s address some of the operational issues.
The diagnostic cascade for HCV can be made very easy. HCV antibody testing is our standard screening test and, when positive, can automatically reflex to HCV polymerase chain reaction (PCR), the diagnostic test. Thus, with one blood sample, you can both screen for and diagnose infection.
Current guidelines do not recommend treating HCV during pregnancy, although therapy can be considered on an individual basis. Linkage to a knowledgeable provider who can discuss transmission and treatment, as well as assess the stage of liver injury, should decrease the burden on the ob.gyn.
The impact on pregnancy is marginal. HCV should not change either the mode of delivery or the decision to breastfeed. The AASLD/IDSA guidance outlines only four recommendations for monitoring during pregnancy:
- Obtain HCV RNA to see whether the infection is active and assess liver function at initiation of prenatal care.
- Prenatal care should be tailored to the pregnancy. There is no modification recommended to decrease mother-to-child transmission (MTCT).
- Be aware that intrahepatic is more common with HCV.
- Women with have a higher rate of adverse outcomes and should be linked to a high-risk obstetrics specialist.
But of course, what seems easy to one specialist may not be true of another. With that in mind, let’s hear the ob.gyn. perspective on these updated screening recommendations.
The ob.gyn. perspective
Recent guidelines from the CDC, ACOG, and SMFM recommend universal screening for HCV in all pregnant women. The increased availability of highly effective antiviral regimens makes universal screening a logical strategy, especially to identify candidates for this curative treatment. What is questionable, however, is the recommended timing by which this screening should take place.
HCV screening during pregnancy, as currently recommended, provides no immediate benefit for the pregnant woman or the fetus/neonate, given that antiviral treatments have not been approved during gestation, and there are no known measures that decrease MTCT or change routine perinatal care.
We also must not forget that a significant proportion of women in the United States, particularly those with limited resources, do not receive prenatal care at all. Most of them, however, will present to a hospital for delivery. Consequently, compliance with screening might be higher if performed at the time of delivery rather than antepartum.
Deferring screening until the intrapartum or immediate postpartum period, at least until antiviral treatment during pregnancy becomes a reality, was discussed. The rationale was that this approach might obviate the need to deal with the unintended consequences and burden of testing for HCV during pregnancy. Ultimately, ACOG and SMFM fell in line with the CDC recommendations.
Despite the lack of robust evidence regarding the risk for MTCT associated with commonly performed obstetric procedures (for example, genetic amniocentesis, artificial rupture of the membranes during labor, placement of an intrauterine pressure catheter), clinicians may be reluctant to perform them in HCV-infected women, resulting in potential deviations from the obstetric standard of care.
Similarly, it is likely that patients may choose to have a cesarean delivery for the sole purpose of decreasing MTCT, despite the lack of evidence for this. Such ill-advised patient-driven decisions are increasingly likely in the current environment, where social media can rapidly disseminate misinformation.
Implications for pediatric patients
One cannot isolate HCV screening in pregnancy from the consequences that may potentially occur as part of the infant’s transition to the care of a pediatrician.
Even though MTCT is estimated to occur in just 5%-15% of cases, all children born to HCV viremic mothers should be screened for HCV.
Traditionally, screening for HCV antibodies occurred after 18 months of age. In those who test positive, HCV PCR testing is recommended at 3 years. However, this algorithm is being called into question because only approximately one-third of infants are successfully screened.
HCV RNA testing in the first year after birth has been suggested. However, even proponents of this approach concur that all management decisions should be deferred until after the age of 3 years, when medications are approved for pediatric use.
In addition, HCV testing would be required again before considering therapy because children have higher rates of spontaneous clearance.
Seeking consensus beyond the controversy
Controversy remains surrounding the most recent update to the HCV screening guidelines. The current recommendation to screen during pregnancy cannot modify the risk for MTCT, has no impact on decisions regarding mode of delivery or breastfeeding, and could potentially cause harm by making obstetricians defer necessary invasive procedures even though there are no data linking them to an increase in MTCT.
Yet after extensive debate, the CDC, USPSTF, AASLD/IDSA, ACOG, and SMFM all developed their current recommendations to initiate HCV screening during pregnancy. To make this successful, screening algorithms need to be simple and consistent across all society recommendations.
HCV antibody testing should always reflex to the diagnostic test (HCV PCR) to allow confirmation in those who test positive without requiring an additional blood test. Viremic mothers (those who are HCV positive on PCR) should be linked to a provider who can discuss prognosis, transmission, and treatment. The importance of screening the infant also must be communicated to the parents and pediatrician alike.
Dr. Reau has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Gilead, Arbutus, Intercept, and Salix; received research grants from AbbVie and Gilead; and received income from AASLD. Dr. Pacheco disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Schools, pediatricians look to make up lost ground on non–COVID-19 vaccinations
WESTMINSTER, COLO. – Melissa Blatzer was determined to get her three children caught up on their routine immunizations on a recent Saturday morning at a walk-in clinic in this Denver suburb. It had been about a year since the kids’ last shots, a delay Ms. Blatzer chalked up to the pandemic.
Two-year-old Lincoln Blatzer, in his fleece dinosaur pajamas, waited anxiously in line for his hepatitis A vaccine. His siblings, 14-year-old Nyla Kusumah and 11-year-old Nevan Kusumah, were there for their TDAP, HPV and meningococcal vaccines, plus a COVID-19 shot for Nyla.
“You don’t have to make an appointment and you can take all three at once,” said Ms. Blatzer, who lives several miles away in Commerce City. That convenience outweighed the difficulty of getting everyone up early on a weekend.
Child health experts hope community clinics like this – along with the return to in-person classes, more well-child visits, and the rollout of COVID shots for younger children – can help boost routine childhood immunizations, which dropped during the pandemic. Despite a rebound, immunization rates are still lower than in 2019, and disparities in rates between racial and economic groups, particularly for Black children, have been exacerbated.
“We’re still not back to where we need to be,” said Sean O’Leary, MD, a pediatric infectious disease doctor at Children’s Hospital Colorado and a professor of pediatrics at the University of Colorado at Denver, Aurora.
Routine immunizations protect children against 16 infectious diseases, including measles, diphtheria and chickenpox, and inhibit transmission to the community.
The rollout of COVID shots for younger kids is an opportunity to catch up on routine vaccinations, said Dr. O’Leary, adding that children can receive these vaccines together. Primary care practices, where many children are likely to receive the COVID shots, usually have other childhood vaccines on hand.
“It’s really important that parents and health care providers work together so that all children are up to date on these recommended vaccines,” said Malini DeSilva, MD, an internist and pediatrician at HealthPartners in the Minneapolis–St. Paul area. “Not only for the child’s health but for our community’s health.”
People were reluctant to come out for routine immunizations at the height of the pandemic, said Karen Miller, an immunization nurse manager for the Denver area’s Tri-County Health Department, which ran the Westminster clinic. National and global data confirm what Ms. Miller saw on the ground.
Global vaccine coverage in children fell from 2019 to 2020, according to a recent study by scientists at the Centers for Disease Control and Prevention, the World Health Organization, and UNICEF. Reasons included reduced access, lack of transportation, worries about COVID exposure and supply chain interruptions, the study said.
Third doses of the DTP vaccine and of the polio vaccine decreased from 86% of all eligible children in 2019 to 83% in 2020, according to the study. Worldwide, 22.7 million children had not had their third dose of DTP in 2020, compared with 19 million in 2019. Three doses are far more effective than one or two at protecting children and communities.
In the United States, researchers who studied 2019 and 2020 data on routine vaccinations in California, Colorado, Minnesota, Oregon, Washington, and Wisconsin found substantial disruptions in vaccination rates during the pandemic that continued into September 2020. For example, the percentage of 7-month-old babies who were up to date on vaccinations decreased from 81% in September 2019 to 74% a year later.
The proportion of Black children up to date on immunizations in almost all age groups was lower than that of children in other racial and ethnic groups. This was most pronounced in those turning 18 months old: Only 41% of Black children that age were caught up on vaccinations in September 2020, compared with 57% of all children at 18 months, said Dr. DeSilva, who led that study.
A CDC study of data from the National Immunization Surveys found that race and ethnicity, poverty, and lack of insurance created the greatest disparities in vaccination rates, and the authors noted that extra efforts are needed to counter the pandemic’s disruptions.
In addition to the problems caused by COVID, Ms. Miller said, competing life priorities like work and school impede families from keeping up with shots. Weekend vaccination clinics can help working parents get their children caught up on routine immunizations while they get a flu or COVID shot. Ms. Miller and O’Leary also said reminders via phone, text or email can boost immunizations.
“Vaccines are so effective that I think it’s easy for families to put immunizations on the back burner because we don’t often hear about these diseases,” she said.
It’s a long and nasty list that includes hepatitis A and B, measles, mumps, whooping cough, polio, rubella, rotavirus, pneumococcus, tetanus, diphtheria, human papillomavirus, and meningococcal disease, among others. Even small drops in vaccination coverage can lead to outbreaks. And measles is the perfect example that worries experts, particularly as international travel opens up.
“Measles is among the most contagious diseases known to humankind, meaning that we have to keep very high vaccination coverage to keep it from spreading,” said Dr. O’Leary.
In 2019, 22 measles outbreaks occurred in 17 states in mostly unvaccinated children and adults. Dr. O’Leary said outbreaks in New York City were contained because surrounding areas had high vaccination coverage. But an outbreak in an undervaccinated community still could spread beyond its borders.
In some states a significant number of parents were opposed to routine childhood vaccines even before the pandemic for religious or personal reasons, posing another challenge for health professionals. For example, 87% of Colorado kindergartners were vaccinated against measles, mumps, and rubella during the 2018-19 school year, one of the nation’s lowest rates.
Those rates bumped up to 91% in 2019-20 but are still below the CDC’s target of 95%.
Dr. O’Leary said he does not see the same level of hesitancy for routine immunizations as for COVID. “There has always been vaccine hesitancy and vaccine refusers. But we’ve maintained vaccination rates north of 90% for all routine childhood vaccines for a long time now,” he said.
Dr. DeSilva said the “ripple effects” of missed vaccinations earlier in the pandemic continued into 2021. As children returned to in-person learning this fall, schools may have been the first place families heard about missed vaccinations. Individual states set vaccination requirements, and allowable exemptions, for entry at schools and child care facilities. In 2020, Colorado passed a school entry immunization law that tightened allowable exemptions.
“Schools, where vaccination requirements are generally enforced, are stretched thin for a variety of reasons, including COVID,” said Dr. O’Leary, adding that managing vaccine requirements may be more difficult for some, but not all, schools.
Anayeli Dominguez, 13, was at the Westminster clinic for a Tdap vaccine because her middle school had noticed she was not up to date.
“School nurses play an important role in helping identify students in need of immunizations, and also by connecting families to resources both within the district and in the larger community,” said Denver Public Schools spokesperson Will Jones.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
WESTMINSTER, COLO. – Melissa Blatzer was determined to get her three children caught up on their routine immunizations on a recent Saturday morning at a walk-in clinic in this Denver suburb. It had been about a year since the kids’ last shots, a delay Ms. Blatzer chalked up to the pandemic.
Two-year-old Lincoln Blatzer, in his fleece dinosaur pajamas, waited anxiously in line for his hepatitis A vaccine. His siblings, 14-year-old Nyla Kusumah and 11-year-old Nevan Kusumah, were there for their TDAP, HPV and meningococcal vaccines, plus a COVID-19 shot for Nyla.
“You don’t have to make an appointment and you can take all three at once,” said Ms. Blatzer, who lives several miles away in Commerce City. That convenience outweighed the difficulty of getting everyone up early on a weekend.
Child health experts hope community clinics like this – along with the return to in-person classes, more well-child visits, and the rollout of COVID shots for younger children – can help boost routine childhood immunizations, which dropped during the pandemic. Despite a rebound, immunization rates are still lower than in 2019, and disparities in rates between racial and economic groups, particularly for Black children, have been exacerbated.
“We’re still not back to where we need to be,” said Sean O’Leary, MD, a pediatric infectious disease doctor at Children’s Hospital Colorado and a professor of pediatrics at the University of Colorado at Denver, Aurora.
Routine immunizations protect children against 16 infectious diseases, including measles, diphtheria and chickenpox, and inhibit transmission to the community.
The rollout of COVID shots for younger kids is an opportunity to catch up on routine vaccinations, said Dr. O’Leary, adding that children can receive these vaccines together. Primary care practices, where many children are likely to receive the COVID shots, usually have other childhood vaccines on hand.
“It’s really important that parents and health care providers work together so that all children are up to date on these recommended vaccines,” said Malini DeSilva, MD, an internist and pediatrician at HealthPartners in the Minneapolis–St. Paul area. “Not only for the child’s health but for our community’s health.”
People were reluctant to come out for routine immunizations at the height of the pandemic, said Karen Miller, an immunization nurse manager for the Denver area’s Tri-County Health Department, which ran the Westminster clinic. National and global data confirm what Ms. Miller saw on the ground.
Global vaccine coverage in children fell from 2019 to 2020, according to a recent study by scientists at the Centers for Disease Control and Prevention, the World Health Organization, and UNICEF. Reasons included reduced access, lack of transportation, worries about COVID exposure and supply chain interruptions, the study said.
Third doses of the DTP vaccine and of the polio vaccine decreased from 86% of all eligible children in 2019 to 83% in 2020, according to the study. Worldwide, 22.7 million children had not had their third dose of DTP in 2020, compared with 19 million in 2019. Three doses are far more effective than one or two at protecting children and communities.
In the United States, researchers who studied 2019 and 2020 data on routine vaccinations in California, Colorado, Minnesota, Oregon, Washington, and Wisconsin found substantial disruptions in vaccination rates during the pandemic that continued into September 2020. For example, the percentage of 7-month-old babies who were up to date on vaccinations decreased from 81% in September 2019 to 74% a year later.
The proportion of Black children up to date on immunizations in almost all age groups was lower than that of children in other racial and ethnic groups. This was most pronounced in those turning 18 months old: Only 41% of Black children that age were caught up on vaccinations in September 2020, compared with 57% of all children at 18 months, said Dr. DeSilva, who led that study.
A CDC study of data from the National Immunization Surveys found that race and ethnicity, poverty, and lack of insurance created the greatest disparities in vaccination rates, and the authors noted that extra efforts are needed to counter the pandemic’s disruptions.
In addition to the problems caused by COVID, Ms. Miller said, competing life priorities like work and school impede families from keeping up with shots. Weekend vaccination clinics can help working parents get their children caught up on routine immunizations while they get a flu or COVID shot. Ms. Miller and O’Leary also said reminders via phone, text or email can boost immunizations.
“Vaccines are so effective that I think it’s easy for families to put immunizations on the back burner because we don’t often hear about these diseases,” she said.
It’s a long and nasty list that includes hepatitis A and B, measles, mumps, whooping cough, polio, rubella, rotavirus, pneumococcus, tetanus, diphtheria, human papillomavirus, and meningococcal disease, among others. Even small drops in vaccination coverage can lead to outbreaks. And measles is the perfect example that worries experts, particularly as international travel opens up.
“Measles is among the most contagious diseases known to humankind, meaning that we have to keep very high vaccination coverage to keep it from spreading,” said Dr. O’Leary.
In 2019, 22 measles outbreaks occurred in 17 states in mostly unvaccinated children and adults. Dr. O’Leary said outbreaks in New York City were contained because surrounding areas had high vaccination coverage. But an outbreak in an undervaccinated community still could spread beyond its borders.
In some states a significant number of parents were opposed to routine childhood vaccines even before the pandemic for religious or personal reasons, posing another challenge for health professionals. For example, 87% of Colorado kindergartners were vaccinated against measles, mumps, and rubella during the 2018-19 school year, one of the nation’s lowest rates.
Those rates bumped up to 91% in 2019-20 but are still below the CDC’s target of 95%.
Dr. O’Leary said he does not see the same level of hesitancy for routine immunizations as for COVID. “There has always been vaccine hesitancy and vaccine refusers. But we’ve maintained vaccination rates north of 90% for all routine childhood vaccines for a long time now,” he said.
Dr. DeSilva said the “ripple effects” of missed vaccinations earlier in the pandemic continued into 2021. As children returned to in-person learning this fall, schools may have been the first place families heard about missed vaccinations. Individual states set vaccination requirements, and allowable exemptions, for entry at schools and child care facilities. In 2020, Colorado passed a school entry immunization law that tightened allowable exemptions.
“Schools, where vaccination requirements are generally enforced, are stretched thin for a variety of reasons, including COVID,” said Dr. O’Leary, adding that managing vaccine requirements may be more difficult for some, but not all, schools.
Anayeli Dominguez, 13, was at the Westminster clinic for a Tdap vaccine because her middle school had noticed she was not up to date.
“School nurses play an important role in helping identify students in need of immunizations, and also by connecting families to resources both within the district and in the larger community,” said Denver Public Schools spokesperson Will Jones.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
WESTMINSTER, COLO. – Melissa Blatzer was determined to get her three children caught up on their routine immunizations on a recent Saturday morning at a walk-in clinic in this Denver suburb. It had been about a year since the kids’ last shots, a delay Ms. Blatzer chalked up to the pandemic.
Two-year-old Lincoln Blatzer, in his fleece dinosaur pajamas, waited anxiously in line for his hepatitis A vaccine. His siblings, 14-year-old Nyla Kusumah and 11-year-old Nevan Kusumah, were there for their TDAP, HPV and meningococcal vaccines, plus a COVID-19 shot for Nyla.
“You don’t have to make an appointment and you can take all three at once,” said Ms. Blatzer, who lives several miles away in Commerce City. That convenience outweighed the difficulty of getting everyone up early on a weekend.
Child health experts hope community clinics like this – along with the return to in-person classes, more well-child visits, and the rollout of COVID shots for younger children – can help boost routine childhood immunizations, which dropped during the pandemic. Despite a rebound, immunization rates are still lower than in 2019, and disparities in rates between racial and economic groups, particularly for Black children, have been exacerbated.
“We’re still not back to where we need to be,” said Sean O’Leary, MD, a pediatric infectious disease doctor at Children’s Hospital Colorado and a professor of pediatrics at the University of Colorado at Denver, Aurora.
Routine immunizations protect children against 16 infectious diseases, including measles, diphtheria and chickenpox, and inhibit transmission to the community.
The rollout of COVID shots for younger kids is an opportunity to catch up on routine vaccinations, said Dr. O’Leary, adding that children can receive these vaccines together. Primary care practices, where many children are likely to receive the COVID shots, usually have other childhood vaccines on hand.
“It’s really important that parents and health care providers work together so that all children are up to date on these recommended vaccines,” said Malini DeSilva, MD, an internist and pediatrician at HealthPartners in the Minneapolis–St. Paul area. “Not only for the child’s health but for our community’s health.”
People were reluctant to come out for routine immunizations at the height of the pandemic, said Karen Miller, an immunization nurse manager for the Denver area’s Tri-County Health Department, which ran the Westminster clinic. National and global data confirm what Ms. Miller saw on the ground.
Global vaccine coverage in children fell from 2019 to 2020, according to a recent study by scientists at the Centers for Disease Control and Prevention, the World Health Organization, and UNICEF. Reasons included reduced access, lack of transportation, worries about COVID exposure and supply chain interruptions, the study said.
Third doses of the DTP vaccine and of the polio vaccine decreased from 86% of all eligible children in 2019 to 83% in 2020, according to the study. Worldwide, 22.7 million children had not had their third dose of DTP in 2020, compared with 19 million in 2019. Three doses are far more effective than one or two at protecting children and communities.
In the United States, researchers who studied 2019 and 2020 data on routine vaccinations in California, Colorado, Minnesota, Oregon, Washington, and Wisconsin found substantial disruptions in vaccination rates during the pandemic that continued into September 2020. For example, the percentage of 7-month-old babies who were up to date on vaccinations decreased from 81% in September 2019 to 74% a year later.
The proportion of Black children up to date on immunizations in almost all age groups was lower than that of children in other racial and ethnic groups. This was most pronounced in those turning 18 months old: Only 41% of Black children that age were caught up on vaccinations in September 2020, compared with 57% of all children at 18 months, said Dr. DeSilva, who led that study.
A CDC study of data from the National Immunization Surveys found that race and ethnicity, poverty, and lack of insurance created the greatest disparities in vaccination rates, and the authors noted that extra efforts are needed to counter the pandemic’s disruptions.
In addition to the problems caused by COVID, Ms. Miller said, competing life priorities like work and school impede families from keeping up with shots. Weekend vaccination clinics can help working parents get their children caught up on routine immunizations while they get a flu or COVID shot. Ms. Miller and O’Leary also said reminders via phone, text or email can boost immunizations.
“Vaccines are so effective that I think it’s easy for families to put immunizations on the back burner because we don’t often hear about these diseases,” she said.
It’s a long and nasty list that includes hepatitis A and B, measles, mumps, whooping cough, polio, rubella, rotavirus, pneumococcus, tetanus, diphtheria, human papillomavirus, and meningococcal disease, among others. Even small drops in vaccination coverage can lead to outbreaks. And measles is the perfect example that worries experts, particularly as international travel opens up.
“Measles is among the most contagious diseases known to humankind, meaning that we have to keep very high vaccination coverage to keep it from spreading,” said Dr. O’Leary.
In 2019, 22 measles outbreaks occurred in 17 states in mostly unvaccinated children and adults. Dr. O’Leary said outbreaks in New York City were contained because surrounding areas had high vaccination coverage. But an outbreak in an undervaccinated community still could spread beyond its borders.
In some states a significant number of parents were opposed to routine childhood vaccines even before the pandemic for religious or personal reasons, posing another challenge for health professionals. For example, 87% of Colorado kindergartners were vaccinated against measles, mumps, and rubella during the 2018-19 school year, one of the nation’s lowest rates.
Those rates bumped up to 91% in 2019-20 but are still below the CDC’s target of 95%.
Dr. O’Leary said he does not see the same level of hesitancy for routine immunizations as for COVID. “There has always been vaccine hesitancy and vaccine refusers. But we’ve maintained vaccination rates north of 90% for all routine childhood vaccines for a long time now,” he said.
Dr. DeSilva said the “ripple effects” of missed vaccinations earlier in the pandemic continued into 2021. As children returned to in-person learning this fall, schools may have been the first place families heard about missed vaccinations. Individual states set vaccination requirements, and allowable exemptions, for entry at schools and child care facilities. In 2020, Colorado passed a school entry immunization law that tightened allowable exemptions.
“Schools, where vaccination requirements are generally enforced, are stretched thin for a variety of reasons, including COVID,” said Dr. O’Leary, adding that managing vaccine requirements may be more difficult for some, but not all, schools.
Anayeli Dominguez, 13, was at the Westminster clinic for a Tdap vaccine because her middle school had noticed she was not up to date.
“School nurses play an important role in helping identify students in need of immunizations, and also by connecting families to resources both within the district and in the larger community,” said Denver Public Schools spokesperson Will Jones.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Topical options for acne patients continue to expand
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Intranasal oxytocin for autism promising – then came the data
When parents of children with autism spectrum disorder (ASD) participating in the largest clinical trial of intranasal oxytocin to date came in for follow-up visits with investigators, they reported marked improvement in the children’s social functioning.
Kids who rarely communicated with their families began to interact more. Those who usually preferred to isolate themselves started joining their parents for meals. It all seemed so promising – until the data came in.
“Those sounded like real improvements to me, and it seemed like they increased over the period of the study,” lead investigator Linmarie Sikich, MD, an associate clinical professor of psychiatry with Duke University School of Medicine and the Duke Center for Autism and Brain Development, Durham, N.C., told this news organization. “Turns out it wasn’t oxytocin that was making that difference.”
Researchers found that after 24 weeks of daily treatment with intranasal oxytocin, there were no significant differences in social functioning between children who received active treatment and those in the placebo group.
The much-anticipated results were published online in The New England Journal of Medicine. To say that they are disappointing, Dr. Sikich said, is an understatement.
Increase in off-label use
Most studies in mouse models of ASD and small trials in children produced conflicting results, although there were modest improvements in social functioning associated with the use of intranasal oxytocin. Some clinicians were already prescribing it off label.
On the basis of this research and early feedback from parents of children, Dr. Sikich and colleagues were hopeful.
However, results from a rigorous, 5-year, $11.4 million randomized trial were negative. Yet, parents were convinced their child improved during the study, and there was a significant increase in off-label prescribing of a treatment her research says doesn’t work. What’s next for oxytocin?
Known as the “love hormone,” oxytocin is a neurotransmitter that is primarily synthesized in the hypothalamus. It plays a role in childbirth and lactation and is also involved in the regulation of social functioning and emotions. Research suggests low oxytocin levels are associated with diminished social functioning, regardless of ASD status.
Its potential as an autism therapy for children has been under study for a decade. Some findings link its use to improvements in core deficits associated with ASD, including repetitive behaviors, fixated or restricted interest, and social communication. A study published in 2020 showed that the treatment improved symptoms in high-functioning adults with ASD.
These were mostly small studies and were underpowered to reliably detect an effect of the therapy on social functioning. They often involved only a single dose of oxytocin. Some studies showed improvements, but others did not.
Still, interest in the treatment grew. Physicians began prescribing it for children with ASD, and parents began buying products containing oxytocin on the internet. Researchers feared this off-label use was becoming widespread, despite inconclusive evidence of efficacy.
High hopes
With support from a National Institutes of Health grant, Dr. Sikich and her team designed a phase 2, multicenter, randomized, double-blind, placebo-controlled study to determine whether the use of oxytocin in children with ASD works and is safe.
The challenges began before they even enrolled a single child. A number of behavioral assessment tools are used to measure social function in ASD, but there is no consensus on which one is best.
A simple blood test could determine how much oxytocin from the nasal spray was absorbed in the blood, but identifying how much made it to the brain would require fMRI, which is expensive and is challenging to use in this study population. Then there was the acquisition of the drug itself.
The Food and Drug Administration has approved intravenous oxytocin for inducing labor. Intranasal oxytocin is not approved for any indication and isn’t available commercially in the United States. Patients or researchers must secure the drug from a manufacturer in a country where it is approved or order it from a U.S. pharmacy that is capable of compounding IV oxytocin into an intranasal formulation.
The pharmacy in Switzerland Dr. Sikich planned to use couldn’t make enough for the study. Contracting with a compounding pharmacy in the United States was significantly more expensive and time consuming, but it was the researchers’ only option.
“If it hadn’t been something we expected to have a major benefit, I think we would have given up the project at multiple points along the line due to all of these challenges,” said Dr. Sikich.
In August 2014, with all the pieces finally in place, researchers began enrolling children aged 3-17 years. The final cohort included 290 participants with ASD, 146 in the oxytocin group and 144 in the placebo group. Of these, 48% had minimal verbal fluency, and 52% had fluent verbal speech.
Participants received daily synthetic oxytocin or placebo via a nasal spray for 24 weeks. The daily oxytocin dose was 48 IU for the first 7 weeks. After that, the dosage could be titrated to a maximum of 80 IU/d. The mean maximal total daily dose of oxytocin throughout the study was 67.6 ± 16.9 IU.
‘It just didn’t work’
Both study groups showed improvement in social withdrawal beginning at 4 weeks and continuing throughout the trial, as determined on the basis of caretakers’ responses on the Aberrant Behavior Checklist Modified Social Withdrawal Subscale, the study’s primary outcome measure.
Sociability and social motivation also improved in both groups, as measured by the Pervasive Developmental Disorders Behavior Inventory and the Social Responsiveness Scale.
But by the end of the trial, the difference between the groups in improvement of social function wasn’t significant (difference, -0.2 points; P = .61) after adjusting for age, verbal fluency, and baseline oxytocin level.
“We were so convinced that it would work,” Dr. Sikich said, “but it just didn’t.”
From observation, parents were also convinced the therapy was working. At the trial’s conclusion, fewer than half of caregivers correctly guessed whether their child was in the treatment group or the placebo group.
A lot of development changes can happen in a child over 6 months. It’s possible the improvements would have occurred regardless of the trial, Dr. Sikich said. Parents’ perceptions could also be a placebo effect. Their child was in a clinical trial of a drug they believed could improve social functioning, so in their mind, it did.
Caregivers received training in how to identify certain behavioral changes, which may have helped them spot an existing positive change they had previously overlooked. Or they may have worked with their child more intently as a result of their participation in the trial.
“People may start doing more things or doing them more intensively or purposefully, consciously or subconsciously, to try to help their child improve the skills or behaviors targeted by the active therapy in the study,” Dr. Sikich said. “These are things that might really help the child move forward which are completely separate from the medication being studied.”
The safety analysis offered more hopeful results. Only one serious adverse event from the treatment was reported: A 17-year-old participant taking a daily dose of 48 IU experienced a sedating effect while driving and had an accident.
Too soon to walk away?
Perhaps the most important take-away from the study is that even if it’s safe, intranasal oxytocin as it is currently used doesn’t work and clinicians shouldn’t prescribe it, said Daniel Geschwind, MD, PhD, director of the University of California, Los Angeles (UCLA) Center for Autism Research, who penned a commentary on the study and discussed the findings with this news organization.
“This study shows that using oxytocin the way it’s used in the community right now is not helping anybody, so why put a child through that?” added Dr. Geschwind, who also is a professor of genetics, neurology, and psychiatry at UCLA.
The trial highlights areas that need to be addressed in order to improve research in the field, he said. Establishing a consensus process to measure social functioning and figuring out a better way to access intranasal oxytocin would lead to studies that are more conclusive, comparable, and less expensive. Dr. Sikich agrees.
Despite the findings, Dr. Geschwind and other autism researchers say it’s too soon to walk away from oxytocin altogether, although it may be time to change the approach to autism research.
“We have to take a page from the playbook of modern medicine in other areas and begin to recognize that these syndromes are incredibly heterogeneous,” Dr. Geschwind says. “We can surmise, although we don’t know, that there might be different biological forms of autism that have different pathways involved that are going to respond differently to different medications.”
Calling the researchers’ efforts “heroic,” Karen Parker, PhD, an associate professor and associate chair of psychiatry and behavioral sciences at Stanford (Calif.) University, says efficacy trials such as this one are critical. However, Dr. Parker said in an interview, there are a number of questions that the study didn’t address.
The majority of medication dispensed in a standard intranasal device is sprayed into the back of the throat. Regular blood tests confirmed that oxytocin was getting into participants’ system, but, given how quickly oxytocin degrades in the blood, Dr. Parker said it’s hard to know just how much reached the brain.
It’s also unclear whether the results would have been different had the treatment been paired with behavioral therapy, an approach Dr. Parker suggests might benefit a subset of children with ASD.
A 2017 study from Dr. Parker’s lab found that children with ASD whose use of oxytocin at baseline was low derived greater benefit from synthetic oxytocin, something the new study failed to find. Still, Dr. Parker said, it’s possible oxytocin might increase social motivation and increase a child’s receptiveness to behavioral therapy.
“When you see a negative trial like this, it decreases enthusiasm for the therapy for autism in this context,” Dr. Parker said. “I hope people who are studying these syndromes will continue to explore oxytocin as a therapy.”
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the Autism Centers of Excellence Program and the Department of Psychiatry and Behavioral Sciences at Duke University. Full disclosures of the authors’ possible conflicts of interest are available online.
A version of this article first appeared on Medscape.com.
When parents of children with autism spectrum disorder (ASD) participating in the largest clinical trial of intranasal oxytocin to date came in for follow-up visits with investigators, they reported marked improvement in the children’s social functioning.
Kids who rarely communicated with their families began to interact more. Those who usually preferred to isolate themselves started joining their parents for meals. It all seemed so promising – until the data came in.
“Those sounded like real improvements to me, and it seemed like they increased over the period of the study,” lead investigator Linmarie Sikich, MD, an associate clinical professor of psychiatry with Duke University School of Medicine and the Duke Center for Autism and Brain Development, Durham, N.C., told this news organization. “Turns out it wasn’t oxytocin that was making that difference.”
Researchers found that after 24 weeks of daily treatment with intranasal oxytocin, there were no significant differences in social functioning between children who received active treatment and those in the placebo group.
The much-anticipated results were published online in The New England Journal of Medicine. To say that they are disappointing, Dr. Sikich said, is an understatement.
Increase in off-label use
Most studies in mouse models of ASD and small trials in children produced conflicting results, although there were modest improvements in social functioning associated with the use of intranasal oxytocin. Some clinicians were already prescribing it off label.
On the basis of this research and early feedback from parents of children, Dr. Sikich and colleagues were hopeful.
However, results from a rigorous, 5-year, $11.4 million randomized trial were negative. Yet, parents were convinced their child improved during the study, and there was a significant increase in off-label prescribing of a treatment her research says doesn’t work. What’s next for oxytocin?
Known as the “love hormone,” oxytocin is a neurotransmitter that is primarily synthesized in the hypothalamus. It plays a role in childbirth and lactation and is also involved in the regulation of social functioning and emotions. Research suggests low oxytocin levels are associated with diminished social functioning, regardless of ASD status.
Its potential as an autism therapy for children has been under study for a decade. Some findings link its use to improvements in core deficits associated with ASD, including repetitive behaviors, fixated or restricted interest, and social communication. A study published in 2020 showed that the treatment improved symptoms in high-functioning adults with ASD.
These were mostly small studies and were underpowered to reliably detect an effect of the therapy on social functioning. They often involved only a single dose of oxytocin. Some studies showed improvements, but others did not.
Still, interest in the treatment grew. Physicians began prescribing it for children with ASD, and parents began buying products containing oxytocin on the internet. Researchers feared this off-label use was becoming widespread, despite inconclusive evidence of efficacy.
High hopes
With support from a National Institutes of Health grant, Dr. Sikich and her team designed a phase 2, multicenter, randomized, double-blind, placebo-controlled study to determine whether the use of oxytocin in children with ASD works and is safe.
The challenges began before they even enrolled a single child. A number of behavioral assessment tools are used to measure social function in ASD, but there is no consensus on which one is best.
A simple blood test could determine how much oxytocin from the nasal spray was absorbed in the blood, but identifying how much made it to the brain would require fMRI, which is expensive and is challenging to use in this study population. Then there was the acquisition of the drug itself.
The Food and Drug Administration has approved intravenous oxytocin for inducing labor. Intranasal oxytocin is not approved for any indication and isn’t available commercially in the United States. Patients or researchers must secure the drug from a manufacturer in a country where it is approved or order it from a U.S. pharmacy that is capable of compounding IV oxytocin into an intranasal formulation.
The pharmacy in Switzerland Dr. Sikich planned to use couldn’t make enough for the study. Contracting with a compounding pharmacy in the United States was significantly more expensive and time consuming, but it was the researchers’ only option.
“If it hadn’t been something we expected to have a major benefit, I think we would have given up the project at multiple points along the line due to all of these challenges,” said Dr. Sikich.
In August 2014, with all the pieces finally in place, researchers began enrolling children aged 3-17 years. The final cohort included 290 participants with ASD, 146 in the oxytocin group and 144 in the placebo group. Of these, 48% had minimal verbal fluency, and 52% had fluent verbal speech.
Participants received daily synthetic oxytocin or placebo via a nasal spray for 24 weeks. The daily oxytocin dose was 48 IU for the first 7 weeks. After that, the dosage could be titrated to a maximum of 80 IU/d. The mean maximal total daily dose of oxytocin throughout the study was 67.6 ± 16.9 IU.
‘It just didn’t work’
Both study groups showed improvement in social withdrawal beginning at 4 weeks and continuing throughout the trial, as determined on the basis of caretakers’ responses on the Aberrant Behavior Checklist Modified Social Withdrawal Subscale, the study’s primary outcome measure.
Sociability and social motivation also improved in both groups, as measured by the Pervasive Developmental Disorders Behavior Inventory and the Social Responsiveness Scale.
But by the end of the trial, the difference between the groups in improvement of social function wasn’t significant (difference, -0.2 points; P = .61) after adjusting for age, verbal fluency, and baseline oxytocin level.
“We were so convinced that it would work,” Dr. Sikich said, “but it just didn’t.”
From observation, parents were also convinced the therapy was working. At the trial’s conclusion, fewer than half of caregivers correctly guessed whether their child was in the treatment group or the placebo group.
A lot of development changes can happen in a child over 6 months. It’s possible the improvements would have occurred regardless of the trial, Dr. Sikich said. Parents’ perceptions could also be a placebo effect. Their child was in a clinical trial of a drug they believed could improve social functioning, so in their mind, it did.
Caregivers received training in how to identify certain behavioral changes, which may have helped them spot an existing positive change they had previously overlooked. Or they may have worked with their child more intently as a result of their participation in the trial.
“People may start doing more things or doing them more intensively or purposefully, consciously or subconsciously, to try to help their child improve the skills or behaviors targeted by the active therapy in the study,” Dr. Sikich said. “These are things that might really help the child move forward which are completely separate from the medication being studied.”
The safety analysis offered more hopeful results. Only one serious adverse event from the treatment was reported: A 17-year-old participant taking a daily dose of 48 IU experienced a sedating effect while driving and had an accident.
Too soon to walk away?
Perhaps the most important take-away from the study is that even if it’s safe, intranasal oxytocin as it is currently used doesn’t work and clinicians shouldn’t prescribe it, said Daniel Geschwind, MD, PhD, director of the University of California, Los Angeles (UCLA) Center for Autism Research, who penned a commentary on the study and discussed the findings with this news organization.
“This study shows that using oxytocin the way it’s used in the community right now is not helping anybody, so why put a child through that?” added Dr. Geschwind, who also is a professor of genetics, neurology, and psychiatry at UCLA.
The trial highlights areas that need to be addressed in order to improve research in the field, he said. Establishing a consensus process to measure social functioning and figuring out a better way to access intranasal oxytocin would lead to studies that are more conclusive, comparable, and less expensive. Dr. Sikich agrees.
Despite the findings, Dr. Geschwind and other autism researchers say it’s too soon to walk away from oxytocin altogether, although it may be time to change the approach to autism research.
“We have to take a page from the playbook of modern medicine in other areas and begin to recognize that these syndromes are incredibly heterogeneous,” Dr. Geschwind says. “We can surmise, although we don’t know, that there might be different biological forms of autism that have different pathways involved that are going to respond differently to different medications.”
Calling the researchers’ efforts “heroic,” Karen Parker, PhD, an associate professor and associate chair of psychiatry and behavioral sciences at Stanford (Calif.) University, says efficacy trials such as this one are critical. However, Dr. Parker said in an interview, there are a number of questions that the study didn’t address.
The majority of medication dispensed in a standard intranasal device is sprayed into the back of the throat. Regular blood tests confirmed that oxytocin was getting into participants’ system, but, given how quickly oxytocin degrades in the blood, Dr. Parker said it’s hard to know just how much reached the brain.
It’s also unclear whether the results would have been different had the treatment been paired with behavioral therapy, an approach Dr. Parker suggests might benefit a subset of children with ASD.
A 2017 study from Dr. Parker’s lab found that children with ASD whose use of oxytocin at baseline was low derived greater benefit from synthetic oxytocin, something the new study failed to find. Still, Dr. Parker said, it’s possible oxytocin might increase social motivation and increase a child’s receptiveness to behavioral therapy.
“When you see a negative trial like this, it decreases enthusiasm for the therapy for autism in this context,” Dr. Parker said. “I hope people who are studying these syndromes will continue to explore oxytocin as a therapy.”
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the Autism Centers of Excellence Program and the Department of Psychiatry and Behavioral Sciences at Duke University. Full disclosures of the authors’ possible conflicts of interest are available online.
A version of this article first appeared on Medscape.com.
When parents of children with autism spectrum disorder (ASD) participating in the largest clinical trial of intranasal oxytocin to date came in for follow-up visits with investigators, they reported marked improvement in the children’s social functioning.
Kids who rarely communicated with their families began to interact more. Those who usually preferred to isolate themselves started joining their parents for meals. It all seemed so promising – until the data came in.
“Those sounded like real improvements to me, and it seemed like they increased over the period of the study,” lead investigator Linmarie Sikich, MD, an associate clinical professor of psychiatry with Duke University School of Medicine and the Duke Center for Autism and Brain Development, Durham, N.C., told this news organization. “Turns out it wasn’t oxytocin that was making that difference.”
Researchers found that after 24 weeks of daily treatment with intranasal oxytocin, there were no significant differences in social functioning between children who received active treatment and those in the placebo group.
The much-anticipated results were published online in The New England Journal of Medicine. To say that they are disappointing, Dr. Sikich said, is an understatement.
Increase in off-label use
Most studies in mouse models of ASD and small trials in children produced conflicting results, although there were modest improvements in social functioning associated with the use of intranasal oxytocin. Some clinicians were already prescribing it off label.
On the basis of this research and early feedback from parents of children, Dr. Sikich and colleagues were hopeful.
However, results from a rigorous, 5-year, $11.4 million randomized trial were negative. Yet, parents were convinced their child improved during the study, and there was a significant increase in off-label prescribing of a treatment her research says doesn’t work. What’s next for oxytocin?
Known as the “love hormone,” oxytocin is a neurotransmitter that is primarily synthesized in the hypothalamus. It plays a role in childbirth and lactation and is also involved in the regulation of social functioning and emotions. Research suggests low oxytocin levels are associated with diminished social functioning, regardless of ASD status.
Its potential as an autism therapy for children has been under study for a decade. Some findings link its use to improvements in core deficits associated with ASD, including repetitive behaviors, fixated or restricted interest, and social communication. A study published in 2020 showed that the treatment improved symptoms in high-functioning adults with ASD.
These were mostly small studies and were underpowered to reliably detect an effect of the therapy on social functioning. They often involved only a single dose of oxytocin. Some studies showed improvements, but others did not.
Still, interest in the treatment grew. Physicians began prescribing it for children with ASD, and parents began buying products containing oxytocin on the internet. Researchers feared this off-label use was becoming widespread, despite inconclusive evidence of efficacy.
High hopes
With support from a National Institutes of Health grant, Dr. Sikich and her team designed a phase 2, multicenter, randomized, double-blind, placebo-controlled study to determine whether the use of oxytocin in children with ASD works and is safe.
The challenges began before they even enrolled a single child. A number of behavioral assessment tools are used to measure social function in ASD, but there is no consensus on which one is best.
A simple blood test could determine how much oxytocin from the nasal spray was absorbed in the blood, but identifying how much made it to the brain would require fMRI, which is expensive and is challenging to use in this study population. Then there was the acquisition of the drug itself.
The Food and Drug Administration has approved intravenous oxytocin for inducing labor. Intranasal oxytocin is not approved for any indication and isn’t available commercially in the United States. Patients or researchers must secure the drug from a manufacturer in a country where it is approved or order it from a U.S. pharmacy that is capable of compounding IV oxytocin into an intranasal formulation.
The pharmacy in Switzerland Dr. Sikich planned to use couldn’t make enough for the study. Contracting with a compounding pharmacy in the United States was significantly more expensive and time consuming, but it was the researchers’ only option.
“If it hadn’t been something we expected to have a major benefit, I think we would have given up the project at multiple points along the line due to all of these challenges,” said Dr. Sikich.
In August 2014, with all the pieces finally in place, researchers began enrolling children aged 3-17 years. The final cohort included 290 participants with ASD, 146 in the oxytocin group and 144 in the placebo group. Of these, 48% had minimal verbal fluency, and 52% had fluent verbal speech.
Participants received daily synthetic oxytocin or placebo via a nasal spray for 24 weeks. The daily oxytocin dose was 48 IU for the first 7 weeks. After that, the dosage could be titrated to a maximum of 80 IU/d. The mean maximal total daily dose of oxytocin throughout the study was 67.6 ± 16.9 IU.
‘It just didn’t work’
Both study groups showed improvement in social withdrawal beginning at 4 weeks and continuing throughout the trial, as determined on the basis of caretakers’ responses on the Aberrant Behavior Checklist Modified Social Withdrawal Subscale, the study’s primary outcome measure.
Sociability and social motivation also improved in both groups, as measured by the Pervasive Developmental Disorders Behavior Inventory and the Social Responsiveness Scale.
But by the end of the trial, the difference between the groups in improvement of social function wasn’t significant (difference, -0.2 points; P = .61) after adjusting for age, verbal fluency, and baseline oxytocin level.
“We were so convinced that it would work,” Dr. Sikich said, “but it just didn’t.”
From observation, parents were also convinced the therapy was working. At the trial’s conclusion, fewer than half of caregivers correctly guessed whether their child was in the treatment group or the placebo group.
A lot of development changes can happen in a child over 6 months. It’s possible the improvements would have occurred regardless of the trial, Dr. Sikich said. Parents’ perceptions could also be a placebo effect. Their child was in a clinical trial of a drug they believed could improve social functioning, so in their mind, it did.
Caregivers received training in how to identify certain behavioral changes, which may have helped them spot an existing positive change they had previously overlooked. Or they may have worked with their child more intently as a result of their participation in the trial.
“People may start doing more things or doing them more intensively or purposefully, consciously or subconsciously, to try to help their child improve the skills or behaviors targeted by the active therapy in the study,” Dr. Sikich said. “These are things that might really help the child move forward which are completely separate from the medication being studied.”
The safety analysis offered more hopeful results. Only one serious adverse event from the treatment was reported: A 17-year-old participant taking a daily dose of 48 IU experienced a sedating effect while driving and had an accident.
Too soon to walk away?
Perhaps the most important take-away from the study is that even if it’s safe, intranasal oxytocin as it is currently used doesn’t work and clinicians shouldn’t prescribe it, said Daniel Geschwind, MD, PhD, director of the University of California, Los Angeles (UCLA) Center for Autism Research, who penned a commentary on the study and discussed the findings with this news organization.
“This study shows that using oxytocin the way it’s used in the community right now is not helping anybody, so why put a child through that?” added Dr. Geschwind, who also is a professor of genetics, neurology, and psychiatry at UCLA.
The trial highlights areas that need to be addressed in order to improve research in the field, he said. Establishing a consensus process to measure social functioning and figuring out a better way to access intranasal oxytocin would lead to studies that are more conclusive, comparable, and less expensive. Dr. Sikich agrees.
Despite the findings, Dr. Geschwind and other autism researchers say it’s too soon to walk away from oxytocin altogether, although it may be time to change the approach to autism research.
“We have to take a page from the playbook of modern medicine in other areas and begin to recognize that these syndromes are incredibly heterogeneous,” Dr. Geschwind says. “We can surmise, although we don’t know, that there might be different biological forms of autism that have different pathways involved that are going to respond differently to different medications.”
Calling the researchers’ efforts “heroic,” Karen Parker, PhD, an associate professor and associate chair of psychiatry and behavioral sciences at Stanford (Calif.) University, says efficacy trials such as this one are critical. However, Dr. Parker said in an interview, there are a number of questions that the study didn’t address.
The majority of medication dispensed in a standard intranasal device is sprayed into the back of the throat. Regular blood tests confirmed that oxytocin was getting into participants’ system, but, given how quickly oxytocin degrades in the blood, Dr. Parker said it’s hard to know just how much reached the brain.
It’s also unclear whether the results would have been different had the treatment been paired with behavioral therapy, an approach Dr. Parker suggests might benefit a subset of children with ASD.
A 2017 study from Dr. Parker’s lab found that children with ASD whose use of oxytocin at baseline was low derived greater benefit from synthetic oxytocin, something the new study failed to find. Still, Dr. Parker said, it’s possible oxytocin might increase social motivation and increase a child’s receptiveness to behavioral therapy.
“When you see a negative trial like this, it decreases enthusiasm for the therapy for autism in this context,” Dr. Parker said. “I hope people who are studying these syndromes will continue to explore oxytocin as a therapy.”
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the Autism Centers of Excellence Program and the Department of Psychiatry and Behavioral Sciences at Duke University. Full disclosures of the authors’ possible conflicts of interest are available online.
A version of this article first appeared on Medscape.com.




