User login
Children and COVID: Weekly cases top 200,000, vaccinations down
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.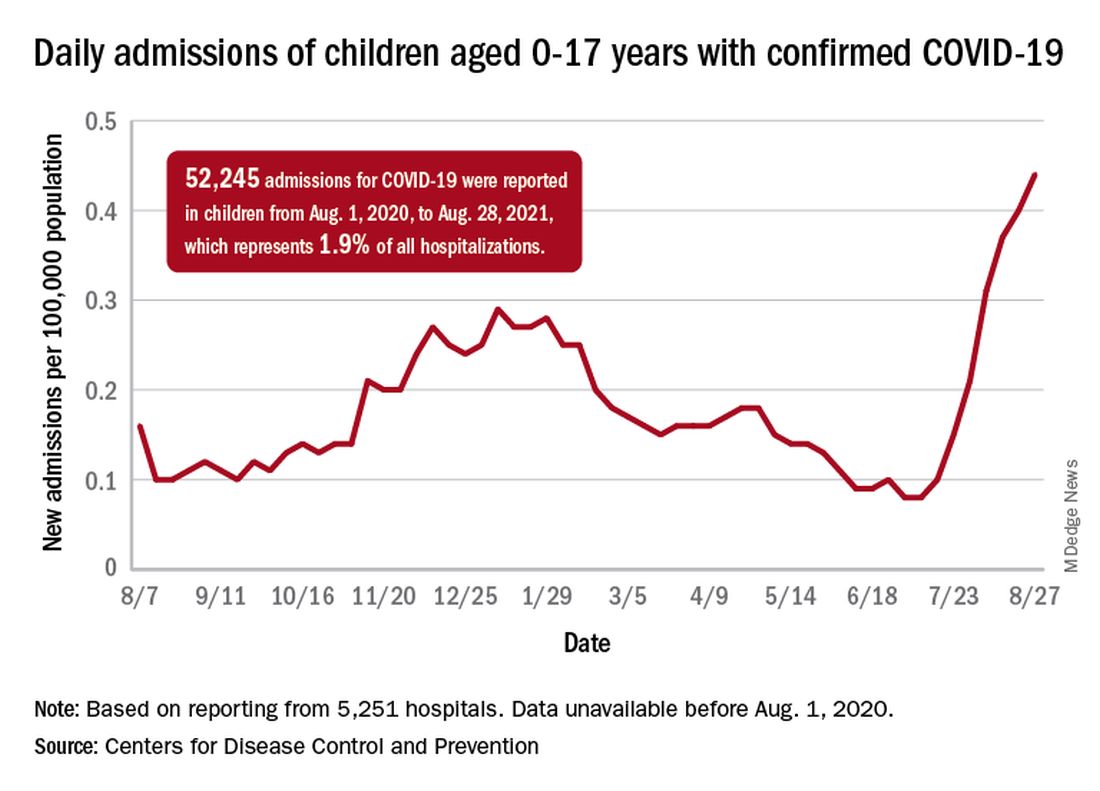
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
Neuropsychiatry affects pediatric OCD treatment
Treatment of pediatric obsessive-compulsive disorder (OCD) has evolved in recent years, with more attention given to some of the neuropsychiatric underpinnings of the condition and how they can affect treatment response.
At the Focus on Neuropsychiatry 2021 meeting, Jeffrey Strawn, MD, outlined some of the neuropsychiatry affecting disease and potential mechanisms to help control obsessions and behaviors, and how they may fit with some therapeutic regimens.
Dr. Strawn discussed the psychological construct of cognitive control, which can provide patients an “out” from the cycle of obsession/fear/worry and compulsion/avoidance. In the face of distress, compulsion and avoidance lead to relief, which reinforces the obsession/fear/worry; this in turn leads to more distress.
“We have an escape door for this circuit” in the form of cognitive control, said Dr. Strawn, who is an associate professor of pediatrics at Cincinnati Children’s Hospital Medical Center.
Cognitive control is linked to insight, which can in turn increase adaptive behaviors that help the patient resist the compulsion. Patients won’t eliminate distress, but they can be helped to make it more tolerable. Therapists can then help them move toward goal-directed thoughts and behaviors. Cognitive control is associated with several neural networks, but Dr. Strawn focused on two: the frontoparietal network, associated with top-down regulation; and the cingular-opercular network. Both of these are engaged during cognitive control processes, and play a role inhibitory control and error monitoring.
Dr. Strawn discussed a recent study that explored the neurofunctional basis of treatment. It compared the effects of a stress management therapy and cognitive-behavioral therapy (CBT) in children and adults with OCD at 6 and 12 weeks. The study found similar symptom reductions in both adults and adolescents in both intervention groups.
Before initiating treatment, the researchers conducted functional MRI scans of participants while conducting an incentive flanker task, which reveals brain activity in response to cognitive control and reward processing.
A larger therapeutic response was found in the CBT group among patients who had a larger pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control, as well as those with more activation within the medial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. On the other hand, within the stress management therapy group, treatment responses were better among those who had lower pretreatment activation among overlapping regions.
“There was a difference in terms of the neurofunctional predictors of treatment response. One of the key regions is the medial prefrontal cortex as well as the rostral anterior cingulate,” said Dr. Strawn, at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
On the neuropharmacology side, numerous medications have been approved for OCD. Dr. Strawn highlighted some studies to illustrate general OCD treatment concepts. That included the 2004 Pediatric OCD Treatment Study, which was one of the only trials to compare placebo with an SSRI, CBT, and the combination of SSRI and CBT. It showed the best results with combination therapy, and the difference appeared early in the treatment course.
That study had aggressive dosing, which led to some issues with sertraline tolerability. Dr. Strawn showed results of a study at his institution which showed that the drug levels of pediatric patients treated with sertraline depended on CYP2C19 metabolism, which affects overall exposure and peak dose concentration. In pediatric populations, some SSRIs clear more slowly and can have high peak concentrations. SSRIs have more side effects than serotonin and norepinephrine reuptake inhibitors in both anxiety disorders and OCD. A key difference between the two is that SSRI treatment is associated with greater frequency of activation, which is difficult to define, but includes restlessness and agitation and insomnia in the beginning stages of treatment.
SSRIs also lead to improvement early in the course of treatment, which was shown in a meta-analysis of nine trials. However, the same study showed that clomipramine is associated with a faster and greater magnitude of improvement, compared with SSRIs, even when the latter are dosed aggressively.
Clomipramine is a potent inhibitor of both serotonin and norepinephrine reuptake. It is recommended to monitor clomipramine levels in pediatric OCD patients, and Dr. Strawn suggested that monitoring should include both the parent drug and its primary metabolite, norclomipramine. At a given dose, there can be a great deal of variation in drug level. The clomipramine/norclomipramine ratio can provide information about the patient’s metabolic state, as well as drug adherence.
Dr. Strawn noted that peak levels occur around 1-3 hours after the dose, “and we really do want at least a 12-hour trough level.” EKGs should be performed at baseline and after any titration of clomipramine dose.
He also discussed pediatric OCD patients with OCD and tics. About one-third of Tourette syndrome patients experience OCD at some point. Tics often improve, whereas OCD more often persists. Tics that co-occur with OCD are associated with a lesser response to SSRI treatment, but not CBT treatment. Similarly, patients with hoarding tendencies are about one-third less likely to respond to SSRIs, CBT, or combination therapy.
Dr. Strawn discussed the concept of accommodation, in which family members cope with a patient’s behavior by altering routines to minimize distress and impairment. This may take the form of facilitating rituals, providing reassurance about a patient’s fears, acquiescing to demands, reducing the child’s day-to-day responsibilities, or helping the child complete tasks. Such actions are well intentioned, but they undermine cognitive control, negatively reinforce symptom engagement, and are associated with functional impairment. Reassurance is the most important behavior, occurring in more than half of patients, and it’s measurable. Parental involvement with rituals is also a concern. “This is associated with higher levels of child OCD severity, as well as parental psychopathology, and lower family cohesion. So
New developments in neurobiology and neuropsychology have changed the view of exposure. The old model emphasized the child’s fear rating as an index of corrective learning. The idea was that habituation would decrease anxiety and distress from future exposures. The new model revolves around inhibitory learning theory, which focuses on the variability of distress and aims to increase tolerance of distress. Another goal is to develop new, non-threat associations.
Finally, Dr. Strawn pointed out predictors of poor outcomes in pediatric OCD, including factors such as compulsion severity, oppositional behavior, frequent handwashing, functional impairment, lack of insight, externalizing symptoms, and possibly hoarding. Problematic family characteristics include higher levels of accommodation, parental anxiety, low family cohesion, and high levels of conflict. “The last three really represent a very concerning triad of family behaviors that may necessitate specific family work in order to facilitate the recovery of the pediatric patient,” Dr. Strawn said.
During the question-and-answer session after the talk, Dr. Strawn was asked whether there might be an inflammatory component to OCD, and whether pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) might be a prodromal condition. He noted that some studies have shown a relationship, but results have been mixed, with lots of heterogeneity within the studied populations. To be suspicious that a patient had OCD resulting from PANDAS would require a high threshold, including an acute onset of symptoms. “This is a situation also where I would tend to involve consultation with some other specialties, including neurology. And obviously there would be follow-up in terms of the general workup,” he said.
Dr. Strawn has received research funding from Allergan, Otsuka, and Myriad Genetics. He has consulted for Myriad Genetics, and is a speaker for CMEology and the Neuroscience Education Institute.
Treatment of pediatric obsessive-compulsive disorder (OCD) has evolved in recent years, with more attention given to some of the neuropsychiatric underpinnings of the condition and how they can affect treatment response.
At the Focus on Neuropsychiatry 2021 meeting, Jeffrey Strawn, MD, outlined some of the neuropsychiatry affecting disease and potential mechanisms to help control obsessions and behaviors, and how they may fit with some therapeutic regimens.
Dr. Strawn discussed the psychological construct of cognitive control, which can provide patients an “out” from the cycle of obsession/fear/worry and compulsion/avoidance. In the face of distress, compulsion and avoidance lead to relief, which reinforces the obsession/fear/worry; this in turn leads to more distress.
“We have an escape door for this circuit” in the form of cognitive control, said Dr. Strawn, who is an associate professor of pediatrics at Cincinnati Children’s Hospital Medical Center.
Cognitive control is linked to insight, which can in turn increase adaptive behaviors that help the patient resist the compulsion. Patients won’t eliminate distress, but they can be helped to make it more tolerable. Therapists can then help them move toward goal-directed thoughts and behaviors. Cognitive control is associated with several neural networks, but Dr. Strawn focused on two: the frontoparietal network, associated with top-down regulation; and the cingular-opercular network. Both of these are engaged during cognitive control processes, and play a role inhibitory control and error monitoring.
Dr. Strawn discussed a recent study that explored the neurofunctional basis of treatment. It compared the effects of a stress management therapy and cognitive-behavioral therapy (CBT) in children and adults with OCD at 6 and 12 weeks. The study found similar symptom reductions in both adults and adolescents in both intervention groups.
Before initiating treatment, the researchers conducted functional MRI scans of participants while conducting an incentive flanker task, which reveals brain activity in response to cognitive control and reward processing.
A larger therapeutic response was found in the CBT group among patients who had a larger pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control, as well as those with more activation within the medial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. On the other hand, within the stress management therapy group, treatment responses were better among those who had lower pretreatment activation among overlapping regions.
“There was a difference in terms of the neurofunctional predictors of treatment response. One of the key regions is the medial prefrontal cortex as well as the rostral anterior cingulate,” said Dr. Strawn, at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
On the neuropharmacology side, numerous medications have been approved for OCD. Dr. Strawn highlighted some studies to illustrate general OCD treatment concepts. That included the 2004 Pediatric OCD Treatment Study, which was one of the only trials to compare placebo with an SSRI, CBT, and the combination of SSRI and CBT. It showed the best results with combination therapy, and the difference appeared early in the treatment course.
That study had aggressive dosing, which led to some issues with sertraline tolerability. Dr. Strawn showed results of a study at his institution which showed that the drug levels of pediatric patients treated with sertraline depended on CYP2C19 metabolism, which affects overall exposure and peak dose concentration. In pediatric populations, some SSRIs clear more slowly and can have high peak concentrations. SSRIs have more side effects than serotonin and norepinephrine reuptake inhibitors in both anxiety disorders and OCD. A key difference between the two is that SSRI treatment is associated with greater frequency of activation, which is difficult to define, but includes restlessness and agitation and insomnia in the beginning stages of treatment.
SSRIs also lead to improvement early in the course of treatment, which was shown in a meta-analysis of nine trials. However, the same study showed that clomipramine is associated with a faster and greater magnitude of improvement, compared with SSRIs, even when the latter are dosed aggressively.
Clomipramine is a potent inhibitor of both serotonin and norepinephrine reuptake. It is recommended to monitor clomipramine levels in pediatric OCD patients, and Dr. Strawn suggested that monitoring should include both the parent drug and its primary metabolite, norclomipramine. At a given dose, there can be a great deal of variation in drug level. The clomipramine/norclomipramine ratio can provide information about the patient’s metabolic state, as well as drug adherence.
Dr. Strawn noted that peak levels occur around 1-3 hours after the dose, “and we really do want at least a 12-hour trough level.” EKGs should be performed at baseline and after any titration of clomipramine dose.
He also discussed pediatric OCD patients with OCD and tics. About one-third of Tourette syndrome patients experience OCD at some point. Tics often improve, whereas OCD more often persists. Tics that co-occur with OCD are associated with a lesser response to SSRI treatment, but not CBT treatment. Similarly, patients with hoarding tendencies are about one-third less likely to respond to SSRIs, CBT, or combination therapy.
Dr. Strawn discussed the concept of accommodation, in which family members cope with a patient’s behavior by altering routines to minimize distress and impairment. This may take the form of facilitating rituals, providing reassurance about a patient’s fears, acquiescing to demands, reducing the child’s day-to-day responsibilities, or helping the child complete tasks. Such actions are well intentioned, but they undermine cognitive control, negatively reinforce symptom engagement, and are associated with functional impairment. Reassurance is the most important behavior, occurring in more than half of patients, and it’s measurable. Parental involvement with rituals is also a concern. “This is associated with higher levels of child OCD severity, as well as parental psychopathology, and lower family cohesion. So
New developments in neurobiology and neuropsychology have changed the view of exposure. The old model emphasized the child’s fear rating as an index of corrective learning. The idea was that habituation would decrease anxiety and distress from future exposures. The new model revolves around inhibitory learning theory, which focuses on the variability of distress and aims to increase tolerance of distress. Another goal is to develop new, non-threat associations.
Finally, Dr. Strawn pointed out predictors of poor outcomes in pediatric OCD, including factors such as compulsion severity, oppositional behavior, frequent handwashing, functional impairment, lack of insight, externalizing symptoms, and possibly hoarding. Problematic family characteristics include higher levels of accommodation, parental anxiety, low family cohesion, and high levels of conflict. “The last three really represent a very concerning triad of family behaviors that may necessitate specific family work in order to facilitate the recovery of the pediatric patient,” Dr. Strawn said.
During the question-and-answer session after the talk, Dr. Strawn was asked whether there might be an inflammatory component to OCD, and whether pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) might be a prodromal condition. He noted that some studies have shown a relationship, but results have been mixed, with lots of heterogeneity within the studied populations. To be suspicious that a patient had OCD resulting from PANDAS would require a high threshold, including an acute onset of symptoms. “This is a situation also where I would tend to involve consultation with some other specialties, including neurology. And obviously there would be follow-up in terms of the general workup,” he said.
Dr. Strawn has received research funding from Allergan, Otsuka, and Myriad Genetics. He has consulted for Myriad Genetics, and is a speaker for CMEology and the Neuroscience Education Institute.
Treatment of pediatric obsessive-compulsive disorder (OCD) has evolved in recent years, with more attention given to some of the neuropsychiatric underpinnings of the condition and how they can affect treatment response.
At the Focus on Neuropsychiatry 2021 meeting, Jeffrey Strawn, MD, outlined some of the neuropsychiatry affecting disease and potential mechanisms to help control obsessions and behaviors, and how they may fit with some therapeutic regimens.
Dr. Strawn discussed the psychological construct of cognitive control, which can provide patients an “out” from the cycle of obsession/fear/worry and compulsion/avoidance. In the face of distress, compulsion and avoidance lead to relief, which reinforces the obsession/fear/worry; this in turn leads to more distress.
“We have an escape door for this circuit” in the form of cognitive control, said Dr. Strawn, who is an associate professor of pediatrics at Cincinnati Children’s Hospital Medical Center.
Cognitive control is linked to insight, which can in turn increase adaptive behaviors that help the patient resist the compulsion. Patients won’t eliminate distress, but they can be helped to make it more tolerable. Therapists can then help them move toward goal-directed thoughts and behaviors. Cognitive control is associated with several neural networks, but Dr. Strawn focused on two: the frontoparietal network, associated with top-down regulation; and the cingular-opercular network. Both of these are engaged during cognitive control processes, and play a role inhibitory control and error monitoring.
Dr. Strawn discussed a recent study that explored the neurofunctional basis of treatment. It compared the effects of a stress management therapy and cognitive-behavioral therapy (CBT) in children and adults with OCD at 6 and 12 weeks. The study found similar symptom reductions in both adults and adolescents in both intervention groups.
Before initiating treatment, the researchers conducted functional MRI scans of participants while conducting an incentive flanker task, which reveals brain activity in response to cognitive control and reward processing.
A larger therapeutic response was found in the CBT group among patients who had a larger pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control, as well as those with more activation within the medial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. On the other hand, within the stress management therapy group, treatment responses were better among those who had lower pretreatment activation among overlapping regions.
“There was a difference in terms of the neurofunctional predictors of treatment response. One of the key regions is the medial prefrontal cortex as well as the rostral anterior cingulate,” said Dr. Strawn, at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
On the neuropharmacology side, numerous medications have been approved for OCD. Dr. Strawn highlighted some studies to illustrate general OCD treatment concepts. That included the 2004 Pediatric OCD Treatment Study, which was one of the only trials to compare placebo with an SSRI, CBT, and the combination of SSRI and CBT. It showed the best results with combination therapy, and the difference appeared early in the treatment course.
That study had aggressive dosing, which led to some issues with sertraline tolerability. Dr. Strawn showed results of a study at his institution which showed that the drug levels of pediatric patients treated with sertraline depended on CYP2C19 metabolism, which affects overall exposure and peak dose concentration. In pediatric populations, some SSRIs clear more slowly and can have high peak concentrations. SSRIs have more side effects than serotonin and norepinephrine reuptake inhibitors in both anxiety disorders and OCD. A key difference between the two is that SSRI treatment is associated with greater frequency of activation, which is difficult to define, but includes restlessness and agitation and insomnia in the beginning stages of treatment.
SSRIs also lead to improvement early in the course of treatment, which was shown in a meta-analysis of nine trials. However, the same study showed that clomipramine is associated with a faster and greater magnitude of improvement, compared with SSRIs, even when the latter are dosed aggressively.
Clomipramine is a potent inhibitor of both serotonin and norepinephrine reuptake. It is recommended to monitor clomipramine levels in pediatric OCD patients, and Dr. Strawn suggested that monitoring should include both the parent drug and its primary metabolite, norclomipramine. At a given dose, there can be a great deal of variation in drug level. The clomipramine/norclomipramine ratio can provide information about the patient’s metabolic state, as well as drug adherence.
Dr. Strawn noted that peak levels occur around 1-3 hours after the dose, “and we really do want at least a 12-hour trough level.” EKGs should be performed at baseline and after any titration of clomipramine dose.
He also discussed pediatric OCD patients with OCD and tics. About one-third of Tourette syndrome patients experience OCD at some point. Tics often improve, whereas OCD more often persists. Tics that co-occur with OCD are associated with a lesser response to SSRI treatment, but not CBT treatment. Similarly, patients with hoarding tendencies are about one-third less likely to respond to SSRIs, CBT, or combination therapy.
Dr. Strawn discussed the concept of accommodation, in which family members cope with a patient’s behavior by altering routines to minimize distress and impairment. This may take the form of facilitating rituals, providing reassurance about a patient’s fears, acquiescing to demands, reducing the child’s day-to-day responsibilities, or helping the child complete tasks. Such actions are well intentioned, but they undermine cognitive control, negatively reinforce symptom engagement, and are associated with functional impairment. Reassurance is the most important behavior, occurring in more than half of patients, and it’s measurable. Parental involvement with rituals is also a concern. “This is associated with higher levels of child OCD severity, as well as parental psychopathology, and lower family cohesion. So
New developments in neurobiology and neuropsychology have changed the view of exposure. The old model emphasized the child’s fear rating as an index of corrective learning. The idea was that habituation would decrease anxiety and distress from future exposures. The new model revolves around inhibitory learning theory, which focuses on the variability of distress and aims to increase tolerance of distress. Another goal is to develop new, non-threat associations.
Finally, Dr. Strawn pointed out predictors of poor outcomes in pediatric OCD, including factors such as compulsion severity, oppositional behavior, frequent handwashing, functional impairment, lack of insight, externalizing symptoms, and possibly hoarding. Problematic family characteristics include higher levels of accommodation, parental anxiety, low family cohesion, and high levels of conflict. “The last three really represent a very concerning triad of family behaviors that may necessitate specific family work in order to facilitate the recovery of the pediatric patient,” Dr. Strawn said.
During the question-and-answer session after the talk, Dr. Strawn was asked whether there might be an inflammatory component to OCD, and whether pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) might be a prodromal condition. He noted that some studies have shown a relationship, but results have been mixed, with lots of heterogeneity within the studied populations. To be suspicious that a patient had OCD resulting from PANDAS would require a high threshold, including an acute onset of symptoms. “This is a situation also where I would tend to involve consultation with some other specialties, including neurology. And obviously there would be follow-up in terms of the general workup,” he said.
Dr. Strawn has received research funding from Allergan, Otsuka, and Myriad Genetics. He has consulted for Myriad Genetics, and is a speaker for CMEology and the Neuroscience Education Institute.
FROM FOCUS ON NEUROPSYCHIATRY 2021
Reassuring data on long-term outcomes among kids with MIS-C
Most children who develop multisystemic inflammatory syndrome (MIS-C) after infection with SARS-CoV-2 recover relatively quickly and without significant sequelae, according to a research letter published online in JAMA Pediatrics.
“The results of this research letter offer some reassurance as has been the case with other longitudinal reports, that children with MIS-C largely recover from the illness with minimal sequelae,” said Kanwal M. Farooqi, MD, a pediatric cardiologist from Columbia University Irving Medical Center, New York.
“This is despite the severity of the initial clinical presentation, which can be quite significant with signs of systemic inflammation, hypotension, and need for ICU-level care,” continued Dr. Farooqi, who was not involved in the study.
Given that little is known about the medium- and long-term effects of MIS-C following infection with COVID-19, Patrick Davies, MRCPCH, Nottingham (England) University Hospitals NHS Trust, and colleagues reviewed data from one of the earliest multicenter national cohorts of children in the United Kingdom. The cohort included children admitted to the hospital prior to May 10, 2020, and the analysis was based on data from 68 of 76 (89%) patients of the initial surviving cohort. Information regarding critical care readmissions and outpatient follow-up visits up to April 1, 2021 (1-year post admission), was included in the analysis.
Overall laboratory results appeared normal for most children at 50 days post admission, including neutrophils, platelets, ferritin, creatinine, and alanine transaminase. Just 3% (2/65 test results) of children showed elevated levels of C-reactive protein, 3% (2/59 test results) for D-dimer, and 2% (1/60 test results) for troponin.
Based on echocardiographic data, 14 of the 19 patients who presented with aneurysms had resolution. Nine of 10 patients who presented with “bright” coronary arteries had resolution and only one progressed to having unresolved coronary artery aneurysms with the latest follow-up at 86 days post admission. All of the 38 patients who presented with impaired function without aneurysm had recovered by day 74.
Of the six patients with ongoing echocardiographic abnormalities, all had aneurysmal changes noted on echocardiograms performed between 86 and 336 days post admission. The authors were surprised to find that troponin levels in this group were lower when compared with others in the cohort (0.06 ng/mL [interquartile range, 0.02-0.418 ng/mL] vs. 0.157 ng/mL [0.033-0.81 ng/mL]; P = .02).
These six patients ranged in age from 0 to 13 years (median age, 8.75 years); five were Afro Caribbean boys and one was a White girl.
The researchers acknowledged that, despite coming from a nationwide data set, the interpretation of this data is limited given the small size of the cohort and the lack of standardized follow-up protocol available at the time.
When asked how this data might inform follow-up guidance for children post COVID infection, Dr. Farooqi said, “although it appears from the data that we have seen in the last few months that the patients recover relatively quickly from MIS-C, I believe it is reasonable to evaluate them at 6-month intervals for the second year until we have more information regarding longer-term outcomes.”
The study authors and Dr. Farooqi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most children who develop multisystemic inflammatory syndrome (MIS-C) after infection with SARS-CoV-2 recover relatively quickly and without significant sequelae, according to a research letter published online in JAMA Pediatrics.
“The results of this research letter offer some reassurance as has been the case with other longitudinal reports, that children with MIS-C largely recover from the illness with minimal sequelae,” said Kanwal M. Farooqi, MD, a pediatric cardiologist from Columbia University Irving Medical Center, New York.
“This is despite the severity of the initial clinical presentation, which can be quite significant with signs of systemic inflammation, hypotension, and need for ICU-level care,” continued Dr. Farooqi, who was not involved in the study.
Given that little is known about the medium- and long-term effects of MIS-C following infection with COVID-19, Patrick Davies, MRCPCH, Nottingham (England) University Hospitals NHS Trust, and colleagues reviewed data from one of the earliest multicenter national cohorts of children in the United Kingdom. The cohort included children admitted to the hospital prior to May 10, 2020, and the analysis was based on data from 68 of 76 (89%) patients of the initial surviving cohort. Information regarding critical care readmissions and outpatient follow-up visits up to April 1, 2021 (1-year post admission), was included in the analysis.
Overall laboratory results appeared normal for most children at 50 days post admission, including neutrophils, platelets, ferritin, creatinine, and alanine transaminase. Just 3% (2/65 test results) of children showed elevated levels of C-reactive protein, 3% (2/59 test results) for D-dimer, and 2% (1/60 test results) for troponin.
Based on echocardiographic data, 14 of the 19 patients who presented with aneurysms had resolution. Nine of 10 patients who presented with “bright” coronary arteries had resolution and only one progressed to having unresolved coronary artery aneurysms with the latest follow-up at 86 days post admission. All of the 38 patients who presented with impaired function without aneurysm had recovered by day 74.
Of the six patients with ongoing echocardiographic abnormalities, all had aneurysmal changes noted on echocardiograms performed between 86 and 336 days post admission. The authors were surprised to find that troponin levels in this group were lower when compared with others in the cohort (0.06 ng/mL [interquartile range, 0.02-0.418 ng/mL] vs. 0.157 ng/mL [0.033-0.81 ng/mL]; P = .02).
These six patients ranged in age from 0 to 13 years (median age, 8.75 years); five were Afro Caribbean boys and one was a White girl.
The researchers acknowledged that, despite coming from a nationwide data set, the interpretation of this data is limited given the small size of the cohort and the lack of standardized follow-up protocol available at the time.
When asked how this data might inform follow-up guidance for children post COVID infection, Dr. Farooqi said, “although it appears from the data that we have seen in the last few months that the patients recover relatively quickly from MIS-C, I believe it is reasonable to evaluate them at 6-month intervals for the second year until we have more information regarding longer-term outcomes.”
The study authors and Dr. Farooqi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most children who develop multisystemic inflammatory syndrome (MIS-C) after infection with SARS-CoV-2 recover relatively quickly and without significant sequelae, according to a research letter published online in JAMA Pediatrics.
“The results of this research letter offer some reassurance as has been the case with other longitudinal reports, that children with MIS-C largely recover from the illness with minimal sequelae,” said Kanwal M. Farooqi, MD, a pediatric cardiologist from Columbia University Irving Medical Center, New York.
“This is despite the severity of the initial clinical presentation, which can be quite significant with signs of systemic inflammation, hypotension, and need for ICU-level care,” continued Dr. Farooqi, who was not involved in the study.
Given that little is known about the medium- and long-term effects of MIS-C following infection with COVID-19, Patrick Davies, MRCPCH, Nottingham (England) University Hospitals NHS Trust, and colleagues reviewed data from one of the earliest multicenter national cohorts of children in the United Kingdom. The cohort included children admitted to the hospital prior to May 10, 2020, and the analysis was based on data from 68 of 76 (89%) patients of the initial surviving cohort. Information regarding critical care readmissions and outpatient follow-up visits up to April 1, 2021 (1-year post admission), was included in the analysis.
Overall laboratory results appeared normal for most children at 50 days post admission, including neutrophils, platelets, ferritin, creatinine, and alanine transaminase. Just 3% (2/65 test results) of children showed elevated levels of C-reactive protein, 3% (2/59 test results) for D-dimer, and 2% (1/60 test results) for troponin.
Based on echocardiographic data, 14 of the 19 patients who presented with aneurysms had resolution. Nine of 10 patients who presented with “bright” coronary arteries had resolution and only one progressed to having unresolved coronary artery aneurysms with the latest follow-up at 86 days post admission. All of the 38 patients who presented with impaired function without aneurysm had recovered by day 74.
Of the six patients with ongoing echocardiographic abnormalities, all had aneurysmal changes noted on echocardiograms performed between 86 and 336 days post admission. The authors were surprised to find that troponin levels in this group were lower when compared with others in the cohort (0.06 ng/mL [interquartile range, 0.02-0.418 ng/mL] vs. 0.157 ng/mL [0.033-0.81 ng/mL]; P = .02).
These six patients ranged in age from 0 to 13 years (median age, 8.75 years); five were Afro Caribbean boys and one was a White girl.
The researchers acknowledged that, despite coming from a nationwide data set, the interpretation of this data is limited given the small size of the cohort and the lack of standardized follow-up protocol available at the time.
When asked how this data might inform follow-up guidance for children post COVID infection, Dr. Farooqi said, “although it appears from the data that we have seen in the last few months that the patients recover relatively quickly from MIS-C, I believe it is reasonable to evaluate them at 6-month intervals for the second year until we have more information regarding longer-term outcomes.”
The study authors and Dr. Farooqi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA OKs IV Briviact for seizures in kids as young as 1 month
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
Alcohol use by young adolescents drops during pandemic
The restrictions resulting from the COVID-19 pandemic altered patterns of substance use by early adolescents to less alcohol use and greater use and misuse of nicotine and prescription drugs, based on data from more than 7,000 youth aged 10-14 years.
Substance use in early adolescence is a function of many environmental factors including substance availability, parent and peer use, and family function, as well as macroeconomic factors, William E. Pelham III, PhD, of the University of California, San Diego, and colleagues wrote. “Thus, it is critical to evaluate how substance use during early adolescence has been impacted by the coronavirus disease 2019 (COVID-19) pandemic, a source of large and sustained disruptions to adolescents’ daily lives in terms of education, contact with family/friends, and health behaviors.”
In a prospective, community-based cohort study, published in the Journal of Adolescent Health, the researchers conducted a three-wave assessment of substance use between May 2020 and August 2020, and reviewed prepandemic assessments from 2018 to 2019. The participants included 7,842 adolescents with an average age of 12 years who were initially enrolled in the Adolescent Brain Cognitive Development (ABCD) study at age 9-10 years. At the start of the study, 48% of the participants were female, 20% were Hispanic, 15% were Black, and 2% were Asian. Participants completed three online surveys between May 2020 and August 2020.
Each survey included the number of days in the past 30 days in which the adolescents drank alcohol; smoked cigarettes; used electronic nicotine delivery systems; smoked a cigar, hookah, or pipe; used smokeless tobacco products; used a cannabis product; abused prescription drugs; used inhalants; or used any other drugs. The response scale was 0 days to 10-plus days.
The overall prevalence of substance use among young adolescents was similar between prepandemic and pandemic periods; however fewer respondents reported using alcohol, but more reported using nicotine or misusing prescription medications.
Across all three survey periods, 7.4% of youth reported any substance use, 3.4% reported ever using alcohol, and 3.2% reported ever using nicotine. Of those who reported substance use, 79% reported 1-2 days of use in the past month, and 87% reported using a single substance.
In comparing prepandemic and pandemic substance use, the prevalence of alcohol use in the past 30 days decreased significantly, from 2.1% to 0.8%. However, use of nicotine increased significantly from 0% to 1.3%, and misuse of prescription drugs increased significantly from 0% to 0.6%. “Changes in the rates of use of any substance, cannabis, or inhalants were not statistically significant,” the researchers wrote.
Sex and ethnicity were not associated with substance use during the pandemic, but rates of substance use were higher among youth whose parents were unmarried or had lower levels of education, and among those with preexisting externalizing and internalizing behaviors. Youth who reported higher levels of uncertainty related to COVID-19 were significantly more likely to report substance use; additionally, stress, anxiety, and depressive symptoms were positively association with any substance use during the pandemic survey periods. Youth whose parents experienced hardship or whose parents used alcohol or drugs also were more likely to report substance use.
“Stability in the overall rate of substance use in this cohort is reassuring given that the pandemic has brought increases in teens’ unoccupied time, stress, and loneliness, reduced access to support services, and disruptions to routines and family/parenting practices, all of which might be expected to have increased youth substance use,” the researchers noted. The findings do not explain the decreased alcohol use, but the researchers cited possible reasons for reduced alcohol use including lack of contact with friends and social activities, and greater supervision by parents.
The study findings were limited by several factors including the comparison of prepandemic and pandemic substance use in younger adolescents, which may not reflect changes in substance use in older adolescents. The study also could not establish causality, and did not account for the intensity of substance use, such as number of drinks, the researchers wrote. However, the results were strengthened by the longitudinal design and large, diverse study population, and the use of prepandemic assessments that allowed evaluation of changes over time.
Overall, the results highlight the importance of preexisting and acute risk protective factors in mitigating substance use in young adolescents, and suggest the potential of economic support for families and emotional support for youth as ways to reduce risk, the researchers concluded.
Predicting use and identifying risk factors
“It was important to conduct research at this time so we know how trends have changed during the pandemic,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview. The research helps clinicians “so we can better predict which substances our patients may be using, especially those with preexisting psychological conditions and those at socioeconomic disadvantage.
“I was surprised by the increased prescription drug use, but it make sense, as adolescents are at home more and may be illicitly using their parents medications,” Dr. Kinsella noted. “I think as they go back to school, trends will shift back to where they were as they will be spending more time with friends.” The take-home message to clinicians is the increased use of nicotine and prescription drugs during the pandemic, and future research should focus on substance use trends in 14- to 20-year-olds.
The ABCD study was supported by the National Institutes of Health, and the current study also received support from the National Science Foundation and Children and Screens: Institute of Digital Media and Child Development. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The restrictions resulting from the COVID-19 pandemic altered patterns of substance use by early adolescents to less alcohol use and greater use and misuse of nicotine and prescription drugs, based on data from more than 7,000 youth aged 10-14 years.
Substance use in early adolescence is a function of many environmental factors including substance availability, parent and peer use, and family function, as well as macroeconomic factors, William E. Pelham III, PhD, of the University of California, San Diego, and colleagues wrote. “Thus, it is critical to evaluate how substance use during early adolescence has been impacted by the coronavirus disease 2019 (COVID-19) pandemic, a source of large and sustained disruptions to adolescents’ daily lives in terms of education, contact with family/friends, and health behaviors.”
In a prospective, community-based cohort study, published in the Journal of Adolescent Health, the researchers conducted a three-wave assessment of substance use between May 2020 and August 2020, and reviewed prepandemic assessments from 2018 to 2019. The participants included 7,842 adolescents with an average age of 12 years who were initially enrolled in the Adolescent Brain Cognitive Development (ABCD) study at age 9-10 years. At the start of the study, 48% of the participants were female, 20% were Hispanic, 15% were Black, and 2% were Asian. Participants completed three online surveys between May 2020 and August 2020.
Each survey included the number of days in the past 30 days in which the adolescents drank alcohol; smoked cigarettes; used electronic nicotine delivery systems; smoked a cigar, hookah, or pipe; used smokeless tobacco products; used a cannabis product; abused prescription drugs; used inhalants; or used any other drugs. The response scale was 0 days to 10-plus days.
The overall prevalence of substance use among young adolescents was similar between prepandemic and pandemic periods; however fewer respondents reported using alcohol, but more reported using nicotine or misusing prescription medications.
Across all three survey periods, 7.4% of youth reported any substance use, 3.4% reported ever using alcohol, and 3.2% reported ever using nicotine. Of those who reported substance use, 79% reported 1-2 days of use in the past month, and 87% reported using a single substance.
In comparing prepandemic and pandemic substance use, the prevalence of alcohol use in the past 30 days decreased significantly, from 2.1% to 0.8%. However, use of nicotine increased significantly from 0% to 1.3%, and misuse of prescription drugs increased significantly from 0% to 0.6%. “Changes in the rates of use of any substance, cannabis, or inhalants were not statistically significant,” the researchers wrote.
Sex and ethnicity were not associated with substance use during the pandemic, but rates of substance use were higher among youth whose parents were unmarried or had lower levels of education, and among those with preexisting externalizing and internalizing behaviors. Youth who reported higher levels of uncertainty related to COVID-19 were significantly more likely to report substance use; additionally, stress, anxiety, and depressive symptoms were positively association with any substance use during the pandemic survey periods. Youth whose parents experienced hardship or whose parents used alcohol or drugs also were more likely to report substance use.
“Stability in the overall rate of substance use in this cohort is reassuring given that the pandemic has brought increases in teens’ unoccupied time, stress, and loneliness, reduced access to support services, and disruptions to routines and family/parenting practices, all of which might be expected to have increased youth substance use,” the researchers noted. The findings do not explain the decreased alcohol use, but the researchers cited possible reasons for reduced alcohol use including lack of contact with friends and social activities, and greater supervision by parents.
The study findings were limited by several factors including the comparison of prepandemic and pandemic substance use in younger adolescents, which may not reflect changes in substance use in older adolescents. The study also could not establish causality, and did not account for the intensity of substance use, such as number of drinks, the researchers wrote. However, the results were strengthened by the longitudinal design and large, diverse study population, and the use of prepandemic assessments that allowed evaluation of changes over time.
Overall, the results highlight the importance of preexisting and acute risk protective factors in mitigating substance use in young adolescents, and suggest the potential of economic support for families and emotional support for youth as ways to reduce risk, the researchers concluded.
Predicting use and identifying risk factors
“It was important to conduct research at this time so we know how trends have changed during the pandemic,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview. The research helps clinicians “so we can better predict which substances our patients may be using, especially those with preexisting psychological conditions and those at socioeconomic disadvantage.
“I was surprised by the increased prescription drug use, but it make sense, as adolescents are at home more and may be illicitly using their parents medications,” Dr. Kinsella noted. “I think as they go back to school, trends will shift back to where they were as they will be spending more time with friends.” The take-home message to clinicians is the increased use of nicotine and prescription drugs during the pandemic, and future research should focus on substance use trends in 14- to 20-year-olds.
The ABCD study was supported by the National Institutes of Health, and the current study also received support from the National Science Foundation and Children and Screens: Institute of Digital Media and Child Development. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The restrictions resulting from the COVID-19 pandemic altered patterns of substance use by early adolescents to less alcohol use and greater use and misuse of nicotine and prescription drugs, based on data from more than 7,000 youth aged 10-14 years.
Substance use in early adolescence is a function of many environmental factors including substance availability, parent and peer use, and family function, as well as macroeconomic factors, William E. Pelham III, PhD, of the University of California, San Diego, and colleagues wrote. “Thus, it is critical to evaluate how substance use during early adolescence has been impacted by the coronavirus disease 2019 (COVID-19) pandemic, a source of large and sustained disruptions to adolescents’ daily lives in terms of education, contact with family/friends, and health behaviors.”
In a prospective, community-based cohort study, published in the Journal of Adolescent Health, the researchers conducted a three-wave assessment of substance use between May 2020 and August 2020, and reviewed prepandemic assessments from 2018 to 2019. The participants included 7,842 adolescents with an average age of 12 years who were initially enrolled in the Adolescent Brain Cognitive Development (ABCD) study at age 9-10 years. At the start of the study, 48% of the participants were female, 20% were Hispanic, 15% were Black, and 2% were Asian. Participants completed three online surveys between May 2020 and August 2020.
Each survey included the number of days in the past 30 days in which the adolescents drank alcohol; smoked cigarettes; used electronic nicotine delivery systems; smoked a cigar, hookah, or pipe; used smokeless tobacco products; used a cannabis product; abused prescription drugs; used inhalants; or used any other drugs. The response scale was 0 days to 10-plus days.
The overall prevalence of substance use among young adolescents was similar between prepandemic and pandemic periods; however fewer respondents reported using alcohol, but more reported using nicotine or misusing prescription medications.
Across all three survey periods, 7.4% of youth reported any substance use, 3.4% reported ever using alcohol, and 3.2% reported ever using nicotine. Of those who reported substance use, 79% reported 1-2 days of use in the past month, and 87% reported using a single substance.
In comparing prepandemic and pandemic substance use, the prevalence of alcohol use in the past 30 days decreased significantly, from 2.1% to 0.8%. However, use of nicotine increased significantly from 0% to 1.3%, and misuse of prescription drugs increased significantly from 0% to 0.6%. “Changes in the rates of use of any substance, cannabis, or inhalants were not statistically significant,” the researchers wrote.
Sex and ethnicity were not associated with substance use during the pandemic, but rates of substance use were higher among youth whose parents were unmarried or had lower levels of education, and among those with preexisting externalizing and internalizing behaviors. Youth who reported higher levels of uncertainty related to COVID-19 were significantly more likely to report substance use; additionally, stress, anxiety, and depressive symptoms were positively association with any substance use during the pandemic survey periods. Youth whose parents experienced hardship or whose parents used alcohol or drugs also were more likely to report substance use.
“Stability in the overall rate of substance use in this cohort is reassuring given that the pandemic has brought increases in teens’ unoccupied time, stress, and loneliness, reduced access to support services, and disruptions to routines and family/parenting practices, all of which might be expected to have increased youth substance use,” the researchers noted. The findings do not explain the decreased alcohol use, but the researchers cited possible reasons for reduced alcohol use including lack of contact with friends and social activities, and greater supervision by parents.
The study findings were limited by several factors including the comparison of prepandemic and pandemic substance use in younger adolescents, which may not reflect changes in substance use in older adolescents. The study also could not establish causality, and did not account for the intensity of substance use, such as number of drinks, the researchers wrote. However, the results were strengthened by the longitudinal design and large, diverse study population, and the use of prepandemic assessments that allowed evaluation of changes over time.
Overall, the results highlight the importance of preexisting and acute risk protective factors in mitigating substance use in young adolescents, and suggest the potential of economic support for families and emotional support for youth as ways to reduce risk, the researchers concluded.
Predicting use and identifying risk factors
“It was important to conduct research at this time so we know how trends have changed during the pandemic,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview. The research helps clinicians “so we can better predict which substances our patients may be using, especially those with preexisting psychological conditions and those at socioeconomic disadvantage.
“I was surprised by the increased prescription drug use, but it make sense, as adolescents are at home more and may be illicitly using their parents medications,” Dr. Kinsella noted. “I think as they go back to school, trends will shift back to where they were as they will be spending more time with friends.” The take-home message to clinicians is the increased use of nicotine and prescription drugs during the pandemic, and future research should focus on substance use trends in 14- to 20-year-olds.
The ABCD study was supported by the National Institutes of Health, and the current study also received support from the National Science Foundation and Children and Screens: Institute of Digital Media and Child Development. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
FROM THE JOURNAL OF ADOLESCENT HEALTH
Children’s upper airways primed to combat SARS-CoV-2 infection
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study evaluates OTC treatments for molluscum contagiosum
“It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them,” senior author Elaine Siegfried, MD, said in an interview following the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session.
In the text of their abstract, Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and coauthors Isaac Hoft, of Open Mind Holistics in Ft. Collins, Colo., and Samantha K. Ong, BA, a student at SLU, noted that MC primarily infects children, with an annual incidence of 8%. “Although the disease is self-limited, associated symptoms, contagion and an average 1-year duration prompt concern and frequent medical visits,” they wrote.
The optimal treatment for MC has not been defined and there is currently no approved medication approved for the condition, although three products are in development: VP-102 (cantharidin) by Verrica Pharmaceuticals; SB206, a topical antiviral by Novan; and 10%-15% KOH formulation by the Gurina Foundation.
But many OTC products have been marketed to treat the condition. To identify the OTC products and to assess accompanying information related to safety, efficacy, and cost, the researchers performed an internet search using the terms “molluscum” plus “treatment,” “treatment at home,” “relief,” and “medication.” Eight products were identified for analysis: Conzerol (Elroselabs), Molleave (Innovative Med), Mollenol (Jeva Laboratories), MolluscumBLAST (Revitalize Life Organics), Molluscum Away Patches (Molluscum Away), Naturasil (Nature’s Innovation), Terrasil (Advanced Skincare % Topical Solutions), and Zymaderm (Naturopathix). Package sizes ranged from 0.78 to 1.5 ounces, and prices ranged from about $19 to almost $55.
Dr. Siegfried and colleagues found that all products provided instructions on application and use but most package labels did not include sufficient information about their plant-based ingredients or appropriate dosing. Six of the eight products contained Thuja occidentalis (Arbor vitae), a coniferous cedar whose essential oil has been used in homeopathic products for its anti-inflammatory and antiviral properties. Lemon extract, tea tree oil, and other botanicals were present in no more than three products each. Only two of the products provided information about the number of lesions that could be treated per package.
“The lack of national oversight as well as robust methods for high-level data analysis make safety and efficacy unclear for a Thuja extract marketed to treat MC,” the researchers wrote. “Numerous adverse drug events and positive intradermal skin tests related to Thuja have been reported.”
Dr. Siegfried added that many OTC products offer a money-back guarantee, “so when seeing a patient who failed to respond to one of these products, encourage them, at least, to request a refund, but to also submit a comment about lack of efficacy, in order to provide more balanced Internet information.”
Dr. Siegfried disclosed that she has served as an investigator and consultant for Verrica Pharmaceuticals, and as a consultant and Data Safety Monitoring board member for Novan, two of the companies currently developing drugs to treat molluscum. Her coauthors had no conflicts of interest to disclose.
“It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them,” senior author Elaine Siegfried, MD, said in an interview following the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session.
In the text of their abstract, Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and coauthors Isaac Hoft, of Open Mind Holistics in Ft. Collins, Colo., and Samantha K. Ong, BA, a student at SLU, noted that MC primarily infects children, with an annual incidence of 8%. “Although the disease is self-limited, associated symptoms, contagion and an average 1-year duration prompt concern and frequent medical visits,” they wrote.
The optimal treatment for MC has not been defined and there is currently no approved medication approved for the condition, although three products are in development: VP-102 (cantharidin) by Verrica Pharmaceuticals; SB206, a topical antiviral by Novan; and 10%-15% KOH formulation by the Gurina Foundation.
But many OTC products have been marketed to treat the condition. To identify the OTC products and to assess accompanying information related to safety, efficacy, and cost, the researchers performed an internet search using the terms “molluscum” plus “treatment,” “treatment at home,” “relief,” and “medication.” Eight products were identified for analysis: Conzerol (Elroselabs), Molleave (Innovative Med), Mollenol (Jeva Laboratories), MolluscumBLAST (Revitalize Life Organics), Molluscum Away Patches (Molluscum Away), Naturasil (Nature’s Innovation), Terrasil (Advanced Skincare % Topical Solutions), and Zymaderm (Naturopathix). Package sizes ranged from 0.78 to 1.5 ounces, and prices ranged from about $19 to almost $55.
Dr. Siegfried and colleagues found that all products provided instructions on application and use but most package labels did not include sufficient information about their plant-based ingredients or appropriate dosing. Six of the eight products contained Thuja occidentalis (Arbor vitae), a coniferous cedar whose essential oil has been used in homeopathic products for its anti-inflammatory and antiviral properties. Lemon extract, tea tree oil, and other botanicals were present in no more than three products each. Only two of the products provided information about the number of lesions that could be treated per package.
“The lack of national oversight as well as robust methods for high-level data analysis make safety and efficacy unclear for a Thuja extract marketed to treat MC,” the researchers wrote. “Numerous adverse drug events and positive intradermal skin tests related to Thuja have been reported.”
Dr. Siegfried added that many OTC products offer a money-back guarantee, “so when seeing a patient who failed to respond to one of these products, encourage them, at least, to request a refund, but to also submit a comment about lack of efficacy, in order to provide more balanced Internet information.”
Dr. Siegfried disclosed that she has served as an investigator and consultant for Verrica Pharmaceuticals, and as a consultant and Data Safety Monitoring board member for Novan, two of the companies currently developing drugs to treat molluscum. Her coauthors had no conflicts of interest to disclose.
“It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them,” senior author Elaine Siegfried, MD, said in an interview following the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session.
In the text of their abstract, Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and coauthors Isaac Hoft, of Open Mind Holistics in Ft. Collins, Colo., and Samantha K. Ong, BA, a student at SLU, noted that MC primarily infects children, with an annual incidence of 8%. “Although the disease is self-limited, associated symptoms, contagion and an average 1-year duration prompt concern and frequent medical visits,” they wrote.
The optimal treatment for MC has not been defined and there is currently no approved medication approved for the condition, although three products are in development: VP-102 (cantharidin) by Verrica Pharmaceuticals; SB206, a topical antiviral by Novan; and 10%-15% KOH formulation by the Gurina Foundation.
But many OTC products have been marketed to treat the condition. To identify the OTC products and to assess accompanying information related to safety, efficacy, and cost, the researchers performed an internet search using the terms “molluscum” plus “treatment,” “treatment at home,” “relief,” and “medication.” Eight products were identified for analysis: Conzerol (Elroselabs), Molleave (Innovative Med), Mollenol (Jeva Laboratories), MolluscumBLAST (Revitalize Life Organics), Molluscum Away Patches (Molluscum Away), Naturasil (Nature’s Innovation), Terrasil (Advanced Skincare % Topical Solutions), and Zymaderm (Naturopathix). Package sizes ranged from 0.78 to 1.5 ounces, and prices ranged from about $19 to almost $55.
Dr. Siegfried and colleagues found that all products provided instructions on application and use but most package labels did not include sufficient information about their plant-based ingredients or appropriate dosing. Six of the eight products contained Thuja occidentalis (Arbor vitae), a coniferous cedar whose essential oil has been used in homeopathic products for its anti-inflammatory and antiviral properties. Lemon extract, tea tree oil, and other botanicals were present in no more than three products each. Only two of the products provided information about the number of lesions that could be treated per package.
“The lack of national oversight as well as robust methods for high-level data analysis make safety and efficacy unclear for a Thuja extract marketed to treat MC,” the researchers wrote. “Numerous adverse drug events and positive intradermal skin tests related to Thuja have been reported.”
Dr. Siegfried added that many OTC products offer a money-back guarantee, “so when seeing a patient who failed to respond to one of these products, encourage them, at least, to request a refund, but to also submit a comment about lack of efficacy, in order to provide more balanced Internet information.”
Dr. Siegfried disclosed that she has served as an investigator and consultant for Verrica Pharmaceuticals, and as a consultant and Data Safety Monitoring board member for Novan, two of the companies currently developing drugs to treat molluscum. Her coauthors had no conflicts of interest to disclose.
FROM SPD 2021
EAACI review urges reduction in antibiotic overuse with allergy
Urgent recommendations from a European Academy of Allergy and Clinical Immunology (EAACI) task force are aimed at reducing antibiotic overuse with allergic disease.
Top recommendations include limiting antibiotic therapy in pregnancy and early childhood to help reduce the allergy epidemic in children, and restricting antibiotic therapy in exacerbations and chronic treatment of allergic diseases, especially asthma and atopic dermatitis.
The review, by lead author Gerdien Tramper-Stranders, MD, PhD, department of pediatrics, Franciscus Gasthuis & Vlietland Hospital, Rotterdam, the Netherlands, and colleagues, was published online Aug. 13 in the journal Allergy.
Several studies have shown that use of antibiotics in childhood and during pregnancy is associated with disturbing the intestinal and respiratory microbiome, which in turn leads to dysbiosis and an increased risk of acquiring allergic diseases, the authors noted.
In addition, patients with allergic diseases such as asthma have a higher risk of being prescribed antibiotics for infections compared with the general population, despite lack of clear clinical benefit.
“In fact, there are no clear data supporting antibiotic prescriptions for acute exacerbations; and clinical and/or laboratory criteria are lacking,” the authors wrote.
Despite that lack of data, antibiotics are often prescribed for exacerbations along with oral corticosteroids, Dr. Tramper-Stranders said in an interview. Some patients may benefit from antibiotics in a flare-up, she said, but more research is needed to determine which ones.
Dr. Tramper-Stranders said Franciscus has begun a large study that includes patients with asthma exacerbations to find biomarkers that might predict the type or origin of exacerbation to personalize treatment.
Recommendations have global relevance
She said although the recommendations are coming from the EAACI group, they apply worldwide.
“Especially in countries outside Northern Europe, antibiotic use is tremendous, leading to high rates of antibiotic resistance; but also increasing the risk for developing allergic diseases when prescribed in infancy,” she said.
She pointed out that in the United States, as many as one in six children receive unnecessary antibiotics for an asthma exacerbation. Overtreatment in adults with flare-ups is also prevalent, at rates from 40%-50%.
Millie Kwan, MD, PhD, an allergy specialist at University of North Carolina in Chapel Hill, said in an interview that in the U.S. there’s been a culture change in the direction of antibiotic restraint – but there are still problems.
“It’s a lot easier for us to whip out our prescription pads and prescribe antibiotics for an asthma patient who’s having a flare-up or a patient who has atopic dermatitis before addressing the underlying mechanism directly,” Dr. Kwan said. She agreed that antibiotic overuse is prevalent in pregnancies in the U.S., and she said that starts with the high prevalence of cesarean births. Nearly one-third of all births in the U.S. are by C-section, twice the rate recommended by the World Health Organization.
“Just bypassing the birth canal actually changes what kind of microflora the infant is being exposed to,” Dr. Kwan said. “That’s the first huge problem.”
The second problem, she said, is the potential for overuse of antibiotics with the surgical procedure.
The researchers wrote that pre-, pro- or postbiotics might alter the course of allergic disease, but clear evidence is lacking.
Until now, Dr. Tramper-Stranders said, pre- or probiotic treatment in infancy, irrespective of previous antibiotic use, has not proved effective in preventing allergies.
Data describing the effect of pre- or probiotics after an antibiotic course are scarce, are limited to older children and adults, and are focused on short-term effects, such as diarrhea prevention, she explained.
Dr. Kwan says she agrees that current data are not strong enough to recommend one over another.
“We don’t even know what the normal amount of bacteria should be to constitute an environment where the immune system develops ‘normally,’ “ she said.
Antibiotics should be prescribed cautiously and by following current recommendations to use the narrowest spectrum available, the authors wrote. Future research in antibiotic stewardship should incorporate biomarker-guided therapy to determine which patients might benefit most from antibiotic therapy.
“Practicing antibiotic stewardship needs recurrent attention and we hope that with this initiative, we specifically reach allergy doctors who will rethink their next [antibiotic] prescription. Within our EAACI task force, we will next work on a guideline for rational antibiotic use in asthma,” Dr. Tramper-Stranders said.
The review’s authors and Dr. Kwan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Urgent recommendations from a European Academy of Allergy and Clinical Immunology (EAACI) task force are aimed at reducing antibiotic overuse with allergic disease.
Top recommendations include limiting antibiotic therapy in pregnancy and early childhood to help reduce the allergy epidemic in children, and restricting antibiotic therapy in exacerbations and chronic treatment of allergic diseases, especially asthma and atopic dermatitis.
The review, by lead author Gerdien Tramper-Stranders, MD, PhD, department of pediatrics, Franciscus Gasthuis & Vlietland Hospital, Rotterdam, the Netherlands, and colleagues, was published online Aug. 13 in the journal Allergy.
Several studies have shown that use of antibiotics in childhood and during pregnancy is associated with disturbing the intestinal and respiratory microbiome, which in turn leads to dysbiosis and an increased risk of acquiring allergic diseases, the authors noted.
In addition, patients with allergic diseases such as asthma have a higher risk of being prescribed antibiotics for infections compared with the general population, despite lack of clear clinical benefit.
“In fact, there are no clear data supporting antibiotic prescriptions for acute exacerbations; and clinical and/or laboratory criteria are lacking,” the authors wrote.
Despite that lack of data, antibiotics are often prescribed for exacerbations along with oral corticosteroids, Dr. Tramper-Stranders said in an interview. Some patients may benefit from antibiotics in a flare-up, she said, but more research is needed to determine which ones.
Dr. Tramper-Stranders said Franciscus has begun a large study that includes patients with asthma exacerbations to find biomarkers that might predict the type or origin of exacerbation to personalize treatment.
Recommendations have global relevance
She said although the recommendations are coming from the EAACI group, they apply worldwide.
“Especially in countries outside Northern Europe, antibiotic use is tremendous, leading to high rates of antibiotic resistance; but also increasing the risk for developing allergic diseases when prescribed in infancy,” she said.
She pointed out that in the United States, as many as one in six children receive unnecessary antibiotics for an asthma exacerbation. Overtreatment in adults with flare-ups is also prevalent, at rates from 40%-50%.
Millie Kwan, MD, PhD, an allergy specialist at University of North Carolina in Chapel Hill, said in an interview that in the U.S. there’s been a culture change in the direction of antibiotic restraint – but there are still problems.
“It’s a lot easier for us to whip out our prescription pads and prescribe antibiotics for an asthma patient who’s having a flare-up or a patient who has atopic dermatitis before addressing the underlying mechanism directly,” Dr. Kwan said. She agreed that antibiotic overuse is prevalent in pregnancies in the U.S., and she said that starts with the high prevalence of cesarean births. Nearly one-third of all births in the U.S. are by C-section, twice the rate recommended by the World Health Organization.
“Just bypassing the birth canal actually changes what kind of microflora the infant is being exposed to,” Dr. Kwan said. “That’s the first huge problem.”
The second problem, she said, is the potential for overuse of antibiotics with the surgical procedure.
The researchers wrote that pre-, pro- or postbiotics might alter the course of allergic disease, but clear evidence is lacking.
Until now, Dr. Tramper-Stranders said, pre- or probiotic treatment in infancy, irrespective of previous antibiotic use, has not proved effective in preventing allergies.
Data describing the effect of pre- or probiotics after an antibiotic course are scarce, are limited to older children and adults, and are focused on short-term effects, such as diarrhea prevention, she explained.
Dr. Kwan says she agrees that current data are not strong enough to recommend one over another.
“We don’t even know what the normal amount of bacteria should be to constitute an environment where the immune system develops ‘normally,’ “ she said.
Antibiotics should be prescribed cautiously and by following current recommendations to use the narrowest spectrum available, the authors wrote. Future research in antibiotic stewardship should incorporate biomarker-guided therapy to determine which patients might benefit most from antibiotic therapy.
“Practicing antibiotic stewardship needs recurrent attention and we hope that with this initiative, we specifically reach allergy doctors who will rethink their next [antibiotic] prescription. Within our EAACI task force, we will next work on a guideline for rational antibiotic use in asthma,” Dr. Tramper-Stranders said.
The review’s authors and Dr. Kwan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Urgent recommendations from a European Academy of Allergy and Clinical Immunology (EAACI) task force are aimed at reducing antibiotic overuse with allergic disease.
Top recommendations include limiting antibiotic therapy in pregnancy and early childhood to help reduce the allergy epidemic in children, and restricting antibiotic therapy in exacerbations and chronic treatment of allergic diseases, especially asthma and atopic dermatitis.
The review, by lead author Gerdien Tramper-Stranders, MD, PhD, department of pediatrics, Franciscus Gasthuis & Vlietland Hospital, Rotterdam, the Netherlands, and colleagues, was published online Aug. 13 in the journal Allergy.
Several studies have shown that use of antibiotics in childhood and during pregnancy is associated with disturbing the intestinal and respiratory microbiome, which in turn leads to dysbiosis and an increased risk of acquiring allergic diseases, the authors noted.
In addition, patients with allergic diseases such as asthma have a higher risk of being prescribed antibiotics for infections compared with the general population, despite lack of clear clinical benefit.
“In fact, there are no clear data supporting antibiotic prescriptions for acute exacerbations; and clinical and/or laboratory criteria are lacking,” the authors wrote.
Despite that lack of data, antibiotics are often prescribed for exacerbations along with oral corticosteroids, Dr. Tramper-Stranders said in an interview. Some patients may benefit from antibiotics in a flare-up, she said, but more research is needed to determine which ones.
Dr. Tramper-Stranders said Franciscus has begun a large study that includes patients with asthma exacerbations to find biomarkers that might predict the type or origin of exacerbation to personalize treatment.
Recommendations have global relevance
She said although the recommendations are coming from the EAACI group, they apply worldwide.
“Especially in countries outside Northern Europe, antibiotic use is tremendous, leading to high rates of antibiotic resistance; but also increasing the risk for developing allergic diseases when prescribed in infancy,” she said.
She pointed out that in the United States, as many as one in six children receive unnecessary antibiotics for an asthma exacerbation. Overtreatment in adults with flare-ups is also prevalent, at rates from 40%-50%.
Millie Kwan, MD, PhD, an allergy specialist at University of North Carolina in Chapel Hill, said in an interview that in the U.S. there’s been a culture change in the direction of antibiotic restraint – but there are still problems.
“It’s a lot easier for us to whip out our prescription pads and prescribe antibiotics for an asthma patient who’s having a flare-up or a patient who has atopic dermatitis before addressing the underlying mechanism directly,” Dr. Kwan said. She agreed that antibiotic overuse is prevalent in pregnancies in the U.S., and she said that starts with the high prevalence of cesarean births. Nearly one-third of all births in the U.S. are by C-section, twice the rate recommended by the World Health Organization.
“Just bypassing the birth canal actually changes what kind of microflora the infant is being exposed to,” Dr. Kwan said. “That’s the first huge problem.”
The second problem, she said, is the potential for overuse of antibiotics with the surgical procedure.
The researchers wrote that pre-, pro- or postbiotics might alter the course of allergic disease, but clear evidence is lacking.
Until now, Dr. Tramper-Stranders said, pre- or probiotic treatment in infancy, irrespective of previous antibiotic use, has not proved effective in preventing allergies.
Data describing the effect of pre- or probiotics after an antibiotic course are scarce, are limited to older children and adults, and are focused on short-term effects, such as diarrhea prevention, she explained.
Dr. Kwan says she agrees that current data are not strong enough to recommend one over another.
“We don’t even know what the normal amount of bacteria should be to constitute an environment where the immune system develops ‘normally,’ “ she said.
Antibiotics should be prescribed cautiously and by following current recommendations to use the narrowest spectrum available, the authors wrote. Future research in antibiotic stewardship should incorporate biomarker-guided therapy to determine which patients might benefit most from antibiotic therapy.
“Practicing antibiotic stewardship needs recurrent attention and we hope that with this initiative, we specifically reach allergy doctors who will rethink their next [antibiotic] prescription. Within our EAACI task force, we will next work on a guideline for rational antibiotic use in asthma,” Dr. Tramper-Stranders said.
The review’s authors and Dr. Kwan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pandemic unveils growing suicide crisis for communities of color
This story is a collaboration between KHN and “Science Friday.”
Rafiah Maxie has been a licensed clinical social worker in the Chicago area for a decade. Throughout that time, she’d viewed suicide as a problem most prevalent among middle-aged white men.
Until May 27, 2020.
That day, Maxie’s 19-year-old son, Jamal Clay – who loved playing the trumpet and participating in theater, who would help her unload groceries from the car and raise funds for the March of the Dimes – killed himself in their garage.
“Now I cannot blink without seeing my son hanging,” said Maxie, who is Black.
Clay’s death, along with the suicides of more than 100 other Black residents in Illinois last year, has led locals to call for new prevention efforts focused on Black communities. In 2020, during the pandemic’s first year, suicides among White residents decreased compared with previous years, while they increased among Black residents, according to state data.
But this is not a local problem. Nor is it limited to the pandemic.
Interviews with a dozen suicide researchers, data collected from states across the country, and a review of decades of research revealed that suicide is a growing crisis for communities of color – one that plagued them well before the pandemic and has only been exacerbated since.
Overall suicide rates in the U.S. decreased in 2019 and 2020. National and local studies attribute the trend to a drop among White Americans, who make up the majority of suicide deaths. Meanwhile, rates for Black, Hispanic, and Asian Americans – though lower than those of their white peers – continued to climb in many states. (Suicide rates have been consistently high for Native Americans.)
“COVID created more transparency regarding what we already knew was happening,” said Sonyia Richardson, a licensed clinical social worker who focuses on serving people of color, and assistant professor at the University of North Carolina–Charlotte, where she researches suicide. When you put the suicide rates of all communities in one bucket, “that bucket says it’s getting better and what we’re doing is working,” she said. “But that’s not the case for communities of color.”
Losing generations
Although the suicide rate is highest among middle-aged White men, young people of color are emerging as particularly at risk.
Research shows Black kids younger than 13 die by suicide at nearly twice the rate of White kids and, over time, their suicide rates have grown even as rates have decreased for White children. Among teenagers and young adults, suicide deaths have increased more than 45% for Black Americans and about 40% for Asian Americans in the 7 years ending in 2019. Other concerning trends in suicide attempts date to the ’90s.
“We have to pay attention now because if you’re out of the first decade of life and think life is not worth pursuing, that’s a signal to say something is going really wrong.”
These statistics also refute traditional ideas that suicide doesn’t happen in certain ethnic or minority populations because they’re “protected” and “resilient” or the “model minority,” said Kiara Alvarez, a researcher and psychologist at Massachusetts General Hospital who focuses on suicide among Hispanic and immigrant populations.
Although these groups may have had low suicide rates historically, that’s changing, she said.
Paul Chin lost his 17-year-old brother, Chris, to suicide in 2009. A poem Chris wrote in high school about his heritage has left Chin, 8 years his senior, wondering if his brother struggled to feel accepted in the U.S., despite being born and raised in New York.
Growing up, Asian Americans weren’t represented in lessons at school or in pop culture, said Chin, now 37. Even in clinical research on suicide as well as other health topics, kids like Chris are underrepresented, with less than 1% of federal research funding focused on Asian Americans.
It wasn’t until the pandemic, and the concurrent rise in hate crimes against Asian Americans, that Chin saw national attention on the community’s mental health. He hopes the interest is not short-lived.
Suicide is the leading cause of death for Asian Americans ages 15 to 24, yet “that doesn’t get enough attention,” Chin said. “It’s important to continue to share these stories.”
Kathy Williams, who is Black, has been on a similar mission since her 15-year-old son, Torian Graves, died by suicide in 1996. People didn’t talk about suicide in the Black community then, she said. So she started raising the topic at her church in Durham, N.C., and in local schools. She wanted Black families to know the warning signs and society at large to recognize the seriousness of the problem.
The pandemic may have highlighted this, Williams said, but “it has always happened. Always.”
Pandemic sheds light on the triggers
Pinpointing the root causes of rising suicide within communities of color has proved difficult. How much stems from mental illness? How much from socioeconomic changes like job losses or social isolation? Now, COVID-19 may offer some clues.
Recent decades have been marked by growing economic instability, a widening racial wealth gap, and more public attention on police killings of unarmed Black and Brown people, said Michael Lindsey, executive director of the New York University McSilver Institute for Poverty Policy and Research.
With social media, youths face racism on more fronts than their parents did, said Leslie Adams, assistant professor in the department of mental health at Johns Hopkins Bloomberg School of Public Health.
Each of these factors has been shown to affect suicide risk. For example, experiencing racism and sexism together is linked to a threefold increase in suicidal thoughts for Asian American women, said Brian Keum, assistant professor at UCLA, based on preliminary research findings.
COVID-19 intensified these hardships among communities of color, with disproportionate numbers of lost loved ones, lost jobs, and lost housing. The murder of George Floyd prompted widespread racial unrest, and Asian Americans saw an increase in hate crimes.
At the same time, studies in Connecticut and Maryland found that suicide rates rose within these populations and dropped for their White counterparts.
“It’s not just a problem within the person, but societal issues that need to be addressed,” said Shari Jager-Hyman, assistant professor of psychiatry at the University of Pennsylvania.
Lessons from Texas
In Texas, COVID-19 hit Hispanics especially hard. As of July 2021, they accounted for 45% of all COVID-19 deaths and disproportionately lost jobs. Individuals living in the U.S. without authorization were generally not eligible for unemployment benefits or federal stimulus checks.
During this time, suicide deaths among Hispanic Texans climbed from 847 deaths in 2019 to 962 deaths in 2020, according to preliminary state data. Suicide deaths rose for Black Texans and residents classified as “other” races or ethnicities, but decreased for White Texans.
The numbers didn’t surprise Marc Mendiola. The 20-year-old grew up in a majority-Hispanic community on the south side of San Antonio. Even before the pandemic, he often heard classmates say they were suicidal. Many faced dire finances at home, sometimes living without electricity, food, or water. Those who sought mental health treatment often found services prohibitively expensive or inaccessible because they weren’t offered in Spanish.
“These are conditions the community has always been in,” Mendiola said. “But with the pandemic, it’s even worse.”
Four years ago, Mendiola and his classmates at South San High School began advocating for mental health services. In late 2019, just months before COVID-19 struck, their vision became reality. Six community agencies partnered to offer free services to students and their families across three school districts.
Richard Davidson, chief operating officer of Family Service, one of the groups in the collaborative, said the number of students discussing economic stressors has been on the rise since April 2020. More than 90% of the students who received services in the first half of 2021 were Hispanic, and nearly 10% reported thoughts of suicide or self-harm, program data show. None died by suicide.
Many students are so worried about what’s for dinner the next day that they’re not able to see a future beyond that, Davidson said. That’s when suicide can feel like a viable option.
“One of the things we do is help them see … that despite this situation now, you can create a vision for your future,” Davidson said.
A good future
Researchers say the promise of a good future is often overlooked in suicide prevention, perhaps because achieving it is so challenging. It requires economic and social growth and breaking systemic barriers.
Tevis Simon works to address all those fronts. As a child in West Baltimore, Simon, who is Black, faced poverty and trauma. As an adult, she attempted suicide three times. But now she shares her story with youths across the city to inspire them to overcome challenges. She also talks to politicians, law enforcement agencies, and public policy officials about their responsibilities.
“We can’t not talk about race,” said Simon, 43. “We can’t not talk about systematic oppression. We cannot not talk about these conditions that affect our mental well-being and our feeling and desire to live.”
For Jamal Clay in Illinois, the systemic barriers started early. Before his suicide last year, he had tried to harm himself when he was 12 and the victim of bullies. At that time, he was hospitalized for a few days and told to follow up with outpatient therapy, said his mother, Maxie.
But it was difficult to find therapists who accepted Medicaid, she said. When Maxie finally found one, there was a 60-day wait. Other therapists canceled appointments, she said.
“So we worked on our own,” Maxie said, relying on church and community. Her son seemed to improve. “We thought we closed that chapter in our lives.”
But when the pandemic hit, everything got worse, she said. Clay came home from college and worked at an Amazon warehouse. On drives to and from work, he was frequently pulled over by police. He stopped wearing hats so officers would consider him less intimidating, Maxie said.
“He felt uncomfortable being out in the street,” she said.
Maxie is still trying to make sense of what happened the day Clay died. But she’s found meaning in starting a nonprofit called Soul Survivors of Chicago. Through the organization, she provides education, scholarships and shoes – including Jamal’s old ones – to those impacted by violence, suicide, and trauma.
“My son won’t be able to have a first interview in [those] shoes. He won’t be able to have a nice jump shot or go to church or even meet his wife,” Maxie said.
But she hopes his shoes will carry someone else to a good future.
[Editor’s note: For the purposes of this story, “people of color” or “communities of color” refers to any racial or ethnic populations whose members do not identify as White, including those who are multiracial. Hispanics can be of any race or combination of races.]
KHN senior correspondent JoNel Aleccia contributed to this report. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
This story is a collaboration between KHN and “Science Friday.”
Rafiah Maxie has been a licensed clinical social worker in the Chicago area for a decade. Throughout that time, she’d viewed suicide as a problem most prevalent among middle-aged white men.
Until May 27, 2020.
That day, Maxie’s 19-year-old son, Jamal Clay – who loved playing the trumpet and participating in theater, who would help her unload groceries from the car and raise funds for the March of the Dimes – killed himself in their garage.
“Now I cannot blink without seeing my son hanging,” said Maxie, who is Black.
Clay’s death, along with the suicides of more than 100 other Black residents in Illinois last year, has led locals to call for new prevention efforts focused on Black communities. In 2020, during the pandemic’s first year, suicides among White residents decreased compared with previous years, while they increased among Black residents, according to state data.
But this is not a local problem. Nor is it limited to the pandemic.
Interviews with a dozen suicide researchers, data collected from states across the country, and a review of decades of research revealed that suicide is a growing crisis for communities of color – one that plagued them well before the pandemic and has only been exacerbated since.
Overall suicide rates in the U.S. decreased in 2019 and 2020. National and local studies attribute the trend to a drop among White Americans, who make up the majority of suicide deaths. Meanwhile, rates for Black, Hispanic, and Asian Americans – though lower than those of their white peers – continued to climb in many states. (Suicide rates have been consistently high for Native Americans.)
“COVID created more transparency regarding what we already knew was happening,” said Sonyia Richardson, a licensed clinical social worker who focuses on serving people of color, and assistant professor at the University of North Carolina–Charlotte, where she researches suicide. When you put the suicide rates of all communities in one bucket, “that bucket says it’s getting better and what we’re doing is working,” she said. “But that’s not the case for communities of color.”
Losing generations
Although the suicide rate is highest among middle-aged White men, young people of color are emerging as particularly at risk.
Research shows Black kids younger than 13 die by suicide at nearly twice the rate of White kids and, over time, their suicide rates have grown even as rates have decreased for White children. Among teenagers and young adults, suicide deaths have increased more than 45% for Black Americans and about 40% for Asian Americans in the 7 years ending in 2019. Other concerning trends in suicide attempts date to the ’90s.
“We have to pay attention now because if you’re out of the first decade of life and think life is not worth pursuing, that’s a signal to say something is going really wrong.”
These statistics also refute traditional ideas that suicide doesn’t happen in certain ethnic or minority populations because they’re “protected” and “resilient” or the “model minority,” said Kiara Alvarez, a researcher and psychologist at Massachusetts General Hospital who focuses on suicide among Hispanic and immigrant populations.
Although these groups may have had low suicide rates historically, that’s changing, she said.
Paul Chin lost his 17-year-old brother, Chris, to suicide in 2009. A poem Chris wrote in high school about his heritage has left Chin, 8 years his senior, wondering if his brother struggled to feel accepted in the U.S., despite being born and raised in New York.
Growing up, Asian Americans weren’t represented in lessons at school or in pop culture, said Chin, now 37. Even in clinical research on suicide as well as other health topics, kids like Chris are underrepresented, with less than 1% of federal research funding focused on Asian Americans.
It wasn’t until the pandemic, and the concurrent rise in hate crimes against Asian Americans, that Chin saw national attention on the community’s mental health. He hopes the interest is not short-lived.
Suicide is the leading cause of death for Asian Americans ages 15 to 24, yet “that doesn’t get enough attention,” Chin said. “It’s important to continue to share these stories.”
Kathy Williams, who is Black, has been on a similar mission since her 15-year-old son, Torian Graves, died by suicide in 1996. People didn’t talk about suicide in the Black community then, she said. So she started raising the topic at her church in Durham, N.C., and in local schools. She wanted Black families to know the warning signs and society at large to recognize the seriousness of the problem.
The pandemic may have highlighted this, Williams said, but “it has always happened. Always.”
Pandemic sheds light on the triggers
Pinpointing the root causes of rising suicide within communities of color has proved difficult. How much stems from mental illness? How much from socioeconomic changes like job losses or social isolation? Now, COVID-19 may offer some clues.
Recent decades have been marked by growing economic instability, a widening racial wealth gap, and more public attention on police killings of unarmed Black and Brown people, said Michael Lindsey, executive director of the New York University McSilver Institute for Poverty Policy and Research.
With social media, youths face racism on more fronts than their parents did, said Leslie Adams, assistant professor in the department of mental health at Johns Hopkins Bloomberg School of Public Health.
Each of these factors has been shown to affect suicide risk. For example, experiencing racism and sexism together is linked to a threefold increase in suicidal thoughts for Asian American women, said Brian Keum, assistant professor at UCLA, based on preliminary research findings.
COVID-19 intensified these hardships among communities of color, with disproportionate numbers of lost loved ones, lost jobs, and lost housing. The murder of George Floyd prompted widespread racial unrest, and Asian Americans saw an increase in hate crimes.
At the same time, studies in Connecticut and Maryland found that suicide rates rose within these populations and dropped for their White counterparts.
“It’s not just a problem within the person, but societal issues that need to be addressed,” said Shari Jager-Hyman, assistant professor of psychiatry at the University of Pennsylvania.
Lessons from Texas
In Texas, COVID-19 hit Hispanics especially hard. As of July 2021, they accounted for 45% of all COVID-19 deaths and disproportionately lost jobs. Individuals living in the U.S. without authorization were generally not eligible for unemployment benefits or federal stimulus checks.
During this time, suicide deaths among Hispanic Texans climbed from 847 deaths in 2019 to 962 deaths in 2020, according to preliminary state data. Suicide deaths rose for Black Texans and residents classified as “other” races or ethnicities, but decreased for White Texans.
The numbers didn’t surprise Marc Mendiola. The 20-year-old grew up in a majority-Hispanic community on the south side of San Antonio. Even before the pandemic, he often heard classmates say they were suicidal. Many faced dire finances at home, sometimes living without electricity, food, or water. Those who sought mental health treatment often found services prohibitively expensive or inaccessible because they weren’t offered in Spanish.
“These are conditions the community has always been in,” Mendiola said. “But with the pandemic, it’s even worse.”
Four years ago, Mendiola and his classmates at South San High School began advocating for mental health services. In late 2019, just months before COVID-19 struck, their vision became reality. Six community agencies partnered to offer free services to students and their families across three school districts.
Richard Davidson, chief operating officer of Family Service, one of the groups in the collaborative, said the number of students discussing economic stressors has been on the rise since April 2020. More than 90% of the students who received services in the first half of 2021 were Hispanic, and nearly 10% reported thoughts of suicide or self-harm, program data show. None died by suicide.
Many students are so worried about what’s for dinner the next day that they’re not able to see a future beyond that, Davidson said. That’s when suicide can feel like a viable option.
“One of the things we do is help them see … that despite this situation now, you can create a vision for your future,” Davidson said.
A good future
Researchers say the promise of a good future is often overlooked in suicide prevention, perhaps because achieving it is so challenging. It requires economic and social growth and breaking systemic barriers.
Tevis Simon works to address all those fronts. As a child in West Baltimore, Simon, who is Black, faced poverty and trauma. As an adult, she attempted suicide three times. But now she shares her story with youths across the city to inspire them to overcome challenges. She also talks to politicians, law enforcement agencies, and public policy officials about their responsibilities.
“We can’t not talk about race,” said Simon, 43. “We can’t not talk about systematic oppression. We cannot not talk about these conditions that affect our mental well-being and our feeling and desire to live.”
For Jamal Clay in Illinois, the systemic barriers started early. Before his suicide last year, he had tried to harm himself when he was 12 and the victim of bullies. At that time, he was hospitalized for a few days and told to follow up with outpatient therapy, said his mother, Maxie.
But it was difficult to find therapists who accepted Medicaid, she said. When Maxie finally found one, there was a 60-day wait. Other therapists canceled appointments, she said.
“So we worked on our own,” Maxie said, relying on church and community. Her son seemed to improve. “We thought we closed that chapter in our lives.”
But when the pandemic hit, everything got worse, she said. Clay came home from college and worked at an Amazon warehouse. On drives to and from work, he was frequently pulled over by police. He stopped wearing hats so officers would consider him less intimidating, Maxie said.
“He felt uncomfortable being out in the street,” she said.
Maxie is still trying to make sense of what happened the day Clay died. But she’s found meaning in starting a nonprofit called Soul Survivors of Chicago. Through the organization, she provides education, scholarships and shoes – including Jamal’s old ones – to those impacted by violence, suicide, and trauma.
“My son won’t be able to have a first interview in [those] shoes. He won’t be able to have a nice jump shot or go to church or even meet his wife,” Maxie said.
But she hopes his shoes will carry someone else to a good future.
[Editor’s note: For the purposes of this story, “people of color” or “communities of color” refers to any racial or ethnic populations whose members do not identify as White, including those who are multiracial. Hispanics can be of any race or combination of races.]
KHN senior correspondent JoNel Aleccia contributed to this report. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
This story is a collaboration between KHN and “Science Friday.”
Rafiah Maxie has been a licensed clinical social worker in the Chicago area for a decade. Throughout that time, she’d viewed suicide as a problem most prevalent among middle-aged white men.
Until May 27, 2020.
That day, Maxie’s 19-year-old son, Jamal Clay – who loved playing the trumpet and participating in theater, who would help her unload groceries from the car and raise funds for the March of the Dimes – killed himself in their garage.
“Now I cannot blink without seeing my son hanging,” said Maxie, who is Black.
Clay’s death, along with the suicides of more than 100 other Black residents in Illinois last year, has led locals to call for new prevention efforts focused on Black communities. In 2020, during the pandemic’s first year, suicides among White residents decreased compared with previous years, while they increased among Black residents, according to state data.
But this is not a local problem. Nor is it limited to the pandemic.
Interviews with a dozen suicide researchers, data collected from states across the country, and a review of decades of research revealed that suicide is a growing crisis for communities of color – one that plagued them well before the pandemic and has only been exacerbated since.
Overall suicide rates in the U.S. decreased in 2019 and 2020. National and local studies attribute the trend to a drop among White Americans, who make up the majority of suicide deaths. Meanwhile, rates for Black, Hispanic, and Asian Americans – though lower than those of their white peers – continued to climb in many states. (Suicide rates have been consistently high for Native Americans.)
“COVID created more transparency regarding what we already knew was happening,” said Sonyia Richardson, a licensed clinical social worker who focuses on serving people of color, and assistant professor at the University of North Carolina–Charlotte, where she researches suicide. When you put the suicide rates of all communities in one bucket, “that bucket says it’s getting better and what we’re doing is working,” she said. “But that’s not the case for communities of color.”
Losing generations
Although the suicide rate is highest among middle-aged White men, young people of color are emerging as particularly at risk.
Research shows Black kids younger than 13 die by suicide at nearly twice the rate of White kids and, over time, their suicide rates have grown even as rates have decreased for White children. Among teenagers and young adults, suicide deaths have increased more than 45% for Black Americans and about 40% for Asian Americans in the 7 years ending in 2019. Other concerning trends in suicide attempts date to the ’90s.
“We have to pay attention now because if you’re out of the first decade of life and think life is not worth pursuing, that’s a signal to say something is going really wrong.”
These statistics also refute traditional ideas that suicide doesn’t happen in certain ethnic or minority populations because they’re “protected” and “resilient” or the “model minority,” said Kiara Alvarez, a researcher and psychologist at Massachusetts General Hospital who focuses on suicide among Hispanic and immigrant populations.
Although these groups may have had low suicide rates historically, that’s changing, she said.
Paul Chin lost his 17-year-old brother, Chris, to suicide in 2009. A poem Chris wrote in high school about his heritage has left Chin, 8 years his senior, wondering if his brother struggled to feel accepted in the U.S., despite being born and raised in New York.
Growing up, Asian Americans weren’t represented in lessons at school or in pop culture, said Chin, now 37. Even in clinical research on suicide as well as other health topics, kids like Chris are underrepresented, with less than 1% of federal research funding focused on Asian Americans.
It wasn’t until the pandemic, and the concurrent rise in hate crimes against Asian Americans, that Chin saw national attention on the community’s mental health. He hopes the interest is not short-lived.
Suicide is the leading cause of death for Asian Americans ages 15 to 24, yet “that doesn’t get enough attention,” Chin said. “It’s important to continue to share these stories.”
Kathy Williams, who is Black, has been on a similar mission since her 15-year-old son, Torian Graves, died by suicide in 1996. People didn’t talk about suicide in the Black community then, she said. So she started raising the topic at her church in Durham, N.C., and in local schools. She wanted Black families to know the warning signs and society at large to recognize the seriousness of the problem.
The pandemic may have highlighted this, Williams said, but “it has always happened. Always.”
Pandemic sheds light on the triggers
Pinpointing the root causes of rising suicide within communities of color has proved difficult. How much stems from mental illness? How much from socioeconomic changes like job losses or social isolation? Now, COVID-19 may offer some clues.
Recent decades have been marked by growing economic instability, a widening racial wealth gap, and more public attention on police killings of unarmed Black and Brown people, said Michael Lindsey, executive director of the New York University McSilver Institute for Poverty Policy and Research.
With social media, youths face racism on more fronts than their parents did, said Leslie Adams, assistant professor in the department of mental health at Johns Hopkins Bloomberg School of Public Health.
Each of these factors has been shown to affect suicide risk. For example, experiencing racism and sexism together is linked to a threefold increase in suicidal thoughts for Asian American women, said Brian Keum, assistant professor at UCLA, based on preliminary research findings.
COVID-19 intensified these hardships among communities of color, with disproportionate numbers of lost loved ones, lost jobs, and lost housing. The murder of George Floyd prompted widespread racial unrest, and Asian Americans saw an increase in hate crimes.
At the same time, studies in Connecticut and Maryland found that suicide rates rose within these populations and dropped for their White counterparts.
“It’s not just a problem within the person, but societal issues that need to be addressed,” said Shari Jager-Hyman, assistant professor of psychiatry at the University of Pennsylvania.
Lessons from Texas
In Texas, COVID-19 hit Hispanics especially hard. As of July 2021, they accounted for 45% of all COVID-19 deaths and disproportionately lost jobs. Individuals living in the U.S. without authorization were generally not eligible for unemployment benefits or federal stimulus checks.
During this time, suicide deaths among Hispanic Texans climbed from 847 deaths in 2019 to 962 deaths in 2020, according to preliminary state data. Suicide deaths rose for Black Texans and residents classified as “other” races or ethnicities, but decreased for White Texans.
The numbers didn’t surprise Marc Mendiola. The 20-year-old grew up in a majority-Hispanic community on the south side of San Antonio. Even before the pandemic, he often heard classmates say they were suicidal. Many faced dire finances at home, sometimes living without electricity, food, or water. Those who sought mental health treatment often found services prohibitively expensive or inaccessible because they weren’t offered in Spanish.
“These are conditions the community has always been in,” Mendiola said. “But with the pandemic, it’s even worse.”
Four years ago, Mendiola and his classmates at South San High School began advocating for mental health services. In late 2019, just months before COVID-19 struck, their vision became reality. Six community agencies partnered to offer free services to students and their families across three school districts.
Richard Davidson, chief operating officer of Family Service, one of the groups in the collaborative, said the number of students discussing economic stressors has been on the rise since April 2020. More than 90% of the students who received services in the first half of 2021 were Hispanic, and nearly 10% reported thoughts of suicide or self-harm, program data show. None died by suicide.
Many students are so worried about what’s for dinner the next day that they’re not able to see a future beyond that, Davidson said. That’s when suicide can feel like a viable option.
“One of the things we do is help them see … that despite this situation now, you can create a vision for your future,” Davidson said.
A good future
Researchers say the promise of a good future is often overlooked in suicide prevention, perhaps because achieving it is so challenging. It requires economic and social growth and breaking systemic barriers.
Tevis Simon works to address all those fronts. As a child in West Baltimore, Simon, who is Black, faced poverty and trauma. As an adult, she attempted suicide three times. But now she shares her story with youths across the city to inspire them to overcome challenges. She also talks to politicians, law enforcement agencies, and public policy officials about their responsibilities.
“We can’t not talk about race,” said Simon, 43. “We can’t not talk about systematic oppression. We cannot not talk about these conditions that affect our mental well-being and our feeling and desire to live.”
For Jamal Clay in Illinois, the systemic barriers started early. Before his suicide last year, he had tried to harm himself when he was 12 and the victim of bullies. At that time, he was hospitalized for a few days and told to follow up with outpatient therapy, said his mother, Maxie.
But it was difficult to find therapists who accepted Medicaid, she said. When Maxie finally found one, there was a 60-day wait. Other therapists canceled appointments, she said.
“So we worked on our own,” Maxie said, relying on church and community. Her son seemed to improve. “We thought we closed that chapter in our lives.”
But when the pandemic hit, everything got worse, she said. Clay came home from college and worked at an Amazon warehouse. On drives to and from work, he was frequently pulled over by police. He stopped wearing hats so officers would consider him less intimidating, Maxie said.
“He felt uncomfortable being out in the street,” she said.
Maxie is still trying to make sense of what happened the day Clay died. But she’s found meaning in starting a nonprofit called Soul Survivors of Chicago. Through the organization, she provides education, scholarships and shoes – including Jamal’s old ones – to those impacted by violence, suicide, and trauma.
“My son won’t be able to have a first interview in [those] shoes. He won’t be able to have a nice jump shot or go to church or even meet his wife,” Maxie said.
But she hopes his shoes will carry someone else to a good future.
[Editor’s note: For the purposes of this story, “people of color” or “communities of color” refers to any racial or ethnic populations whose members do not identify as White, including those who are multiracial. Hispanics can be of any race or combination of races.]
KHN senior correspondent JoNel Aleccia contributed to this report. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Peanut allergy patients reap continuing benefits past first year, Palforzia study shows
A recent analysis of 142 peanut-allergic children treated for 1.5 to 2 years with a licensed oral immunotherapy (OIT) product confirms what various smaller studies have shown: Maintaining treatment for longer periods improves protection and reduces adverse effects. The findings offer some reassurance regarding the controversial approach, which has become available at a small number of clinics yet faces an uncertain future.
The new study, published July 28 in Allergy, included a subset of patients who chose to complete an extension of the phase 3 PALISADE trial of Palforzia, a proprietary set of premeasured peanut flour capsules developed by Aimmune Therapeutics.
Palforzia was approved last year for children aged 4 to 17 years with peanut allergy – one of the most common food allergies, affecting around 2% of children in the United States and Europe. The treatment is not a cure – patients must still watch what they eat and carry epinephrine for emergency reactions – but it helps build protection through daily ingestion of gradually increasing amounts of the allergen over a period of months.
In the 1-year PALISADE trial, which enrolled 496 peanut-allergic children at 66 sites in North America and Europe, participants received daily doses of study drug or placebo. The dose of the drug was escalated from 3 mg to 300 mg over 6 months; the 300-mg dose was then maintained for another 6 months. By the end of the study, about two-thirds of the children who underwent treatment could safely consume at least 600 mg of peanut protein, about the equivalent of two peanuts.
Could protection be increased with further treatment, and what would be required to sustain it? To address these questions, PALISADE patients who successfully reached the 600-mg threshold, along with those from the placebo group, were invited to participate in Aimmune’s open-label follow-on study. The extension study also explored whether protection could be maintained with less frequent dosing.
Among the 358 eligible participants who opted into the 1-year extension study, 256 came from the PALISADE treatment arm. These children were assigned to five cohorts to continue for 6 months or 12 months with daily or less frequent doses. Within the 6-month group, all started with the 300-mg daily dose. A subset received two doses a week. Within the 12-month group, some patients maintained daily dosing throughout; others received doses every other day, twice weekly, or once every 2 weeks.
The children who continued daily maintenance dosing the longest gained the most protection. Those in less-frequent dosing groups experienced more adverse events than those who received doses every day, the company reported last December in The Journal of Allergy and Clinical Immunology: In Practice.
More than a quarter (97 of 358, or 27.1%) of participants failed to complete the extension. Families could withdraw any time for any reason. Participating in an OIT trial is demanding – it requires office visits for dosing adjustments and blood tests, rest periods, keeping symptom logs in which daily doses are recorded, and possible allergic reactions from the treatment itself. “A common reason for ‘withdrawal of consent’ in clinical studies is the inconvenience of remaining in a long-term study,” Mohamed Yassine, MD, Aimmune’s senior vice present of medical affairs, said via email.
Attrition was concentrated within certain subgroups. Most participants in (88.7%; 102 of 115) PALISADE who received placebo elected to enter the open-label extension; nearly half did not finish. Dropout rates were also high (29.2%) for non-daily dosing participants who had come from the PALISADE treatment arm.
The authors did not report on those high-dropout groups. Instead, they focused their analysis on the 142 treated PALISADE participants who continued daily dosing through the extension – 110 patients for a total of about 1.5 years and 32 patients for about 2 years. In a subgroup analysis, 48.1% of children in the 1.5-year group upped their tolerance to 2,000 mg peanut protein, and even more (80.8%) in the 2-year group reached that threshold – all while taking a 300-mg maintenance dose.
Those who remained on treatment longer also had fewer adverse events. At the exit food challenge, 24% of the 1.5-year participants had reactions that required epinephrine, but among 2-year participants, only 3.8% needed the rescue medication.
Continuing therapy past the first year seemed to have additional benefits, Sandra Hong, MD, director of the Cleveland Clinic Food Allergy Center of Excellence, said in an interview. Dr. Hong was not involved in the new research and has no financial ties with Aimmune or other food allergy companies. “Not only can you ingest more, but your reaction when you do react is going to be less,” she says.
Palforzia is only available through a risk evaluation and mitigation strategy (REMS) program, which educates patients, health care professionals, and pharmacies about immunotherapy risks and precautionary measures. As of last summer, before Aimmune was acquired by Nestlé Health Science, about 100 allergists in the United States had enrolled patients in the REMS program. Families can find allergists who are certified to prescribe Palforzia using the website’s Certified Participant Locator.
Although the field at large remains apprehensive about OIT and other forms of immunotherapy, an estimated 200 or more U.S. clinics are administering home-grown OIT using commercial food products, says Richard Wasserman, an OIT pioneer whose clinic in Dallas has treated allergies to about 20 foods since the practice started offering the therapy in 2008. OIT practitioners have treated more than 15,000 food allergy patients nationwide, Dr. Wasserman said via email, yet they make up just a tiny fraction of the more than 6,000 board-certified allergists in the United States.
Whether using Palforzia or nonproprietary food products, oral immunotherapy requires a lot of time and effort – not just for patients but also practitioners. “You need more space. You need more staffing. Patients doing oral challenges stay in your office for 4 to 5 hours, and we have one-to-one nursing care for them,” said Dr. Hong. “So it’s a lot of resources.”
Her team has treated about 20 children with Palforzia since the Cleveland Clinic began offering the therapy last summer. Dr. Hong and coworkers have administered OIT using commercial peanut flour and peanut butter to some 80 peanut-allergic toddlers younger than 4 years who are too young to receive for the U.S. Food and Drug Administration–approved treatment. Their early data, which were presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology in February, suggest that toddlers get complete OIT more quickly with fewer side effects than older children, Dr. Hong says. A recent study of preschoolers in Canada also found that nonproprietary OIT is very safe and effective in this younger set and could be cost-saving in the long run.
By comparison, Palforzia, which has a list price of $890 per month, was judged to be less cost-effective in analyses by academic allergists and by the Institute for Clinical and Economic Review. But through a copay savings program, depending on their insurance coverage, some eligible families can pay as little as $20 per month for the FDA-approved treatment.
Because the therapy is time consuming for families and is resource intensive for practices, questions remain as to how long and how frequently patients need to remain on treatment to sustain protection. Do they need to keep taking Palforzia, or “can we switch them to an equivalent amount of food and not bother with the study drug?” said Edwin Kim, director of the UNC Food Allergy Initiative, Chapel Hill, North Carolina, and study investigator for several Palforzia trials, in an interview.
The Food Allergy Support Team, a nonprofit group started by Dr. Wasserman and colleagues, publishes best practices and meets annually to discuss research and protocols. However, the best maintenance dose, the best dosing frequency, and the duration of daily dosing that yields the best outcomes are not known, Dr. Wasserman says.
“We think the best way to answer that question is with a regulated, pharmaceutical-grade form of peanut protein,” Dr. Yassine said.
The field’s experience with Palforzia raises a dilemma: Does its approval legitimize oral immunotherapy in general, or will rigorous, multi-million dollar trials be needed to approve products for each food or combination of foods? About 32 million people in the United States have food allergies – about 1 in 10 adults and 1 in 13 children.
“I think the field has always grappled with that, honestly,” said Stacie Jones, MD, professor of pediatrics and chief of allergy and immunology at the University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, in an interview. Home-grown OIT is “easier to do when you have high control of your small patient volumes or you’re in a clinical trial,” said Dr. Jones, who has served as an investigator on Palforzia trials and last year received more than $30,000 in consulting fees from Aimmune. “It becomes a very different situation when it becomes a national or an international recommended therapy.”
The Canadian Society of Allergy and Clinical Immunology has published clinical practice guidelines and provides practical information on its website on how to implement OIT – including protocols for dozens of foods and diary sheets for patients to log doses and symptoms.
However, U.S. professional societies still consider OIT investigational and suggest that it will not be approved by the FDA. “As a field, are we willing to wait 4 to 5 more years for an egg product? Should we? Are we willing?” said Dr. Kim. “These are tough questions.”
Stacie M. Jones reports advisory board fees, Aimmune Therapeutics, FARE; personal fees, DBV Technologies; clinical trials grants, Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi, Regeneron, FARE, Genentech, and NIH-NIAID. Edwin Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; grant support from the NIH’s National Institute of Allergy and Infectious Diseases, National Center for Complementary and Integrative Health and Immune Tolerance Network; Food Allergy Research and Education, and the Wallace Research Foundation. Richard Wasserman receives consulting fees from Aimmune Therapeutics and DBV Technologies. Mohamed Yassine is employed by Aimmune Therapeutics. Sandra Hong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent analysis of 142 peanut-allergic children treated for 1.5 to 2 years with a licensed oral immunotherapy (OIT) product confirms what various smaller studies have shown: Maintaining treatment for longer periods improves protection and reduces adverse effects. The findings offer some reassurance regarding the controversial approach, which has become available at a small number of clinics yet faces an uncertain future.
The new study, published July 28 in Allergy, included a subset of patients who chose to complete an extension of the phase 3 PALISADE trial of Palforzia, a proprietary set of premeasured peanut flour capsules developed by Aimmune Therapeutics.
Palforzia was approved last year for children aged 4 to 17 years with peanut allergy – one of the most common food allergies, affecting around 2% of children in the United States and Europe. The treatment is not a cure – patients must still watch what they eat and carry epinephrine for emergency reactions – but it helps build protection through daily ingestion of gradually increasing amounts of the allergen over a period of months.
In the 1-year PALISADE trial, which enrolled 496 peanut-allergic children at 66 sites in North America and Europe, participants received daily doses of study drug or placebo. The dose of the drug was escalated from 3 mg to 300 mg over 6 months; the 300-mg dose was then maintained for another 6 months. By the end of the study, about two-thirds of the children who underwent treatment could safely consume at least 600 mg of peanut protein, about the equivalent of two peanuts.
Could protection be increased with further treatment, and what would be required to sustain it? To address these questions, PALISADE patients who successfully reached the 600-mg threshold, along with those from the placebo group, were invited to participate in Aimmune’s open-label follow-on study. The extension study also explored whether protection could be maintained with less frequent dosing.
Among the 358 eligible participants who opted into the 1-year extension study, 256 came from the PALISADE treatment arm. These children were assigned to five cohorts to continue for 6 months or 12 months with daily or less frequent doses. Within the 6-month group, all started with the 300-mg daily dose. A subset received two doses a week. Within the 12-month group, some patients maintained daily dosing throughout; others received doses every other day, twice weekly, or once every 2 weeks.
The children who continued daily maintenance dosing the longest gained the most protection. Those in less-frequent dosing groups experienced more adverse events than those who received doses every day, the company reported last December in The Journal of Allergy and Clinical Immunology: In Practice.
More than a quarter (97 of 358, or 27.1%) of participants failed to complete the extension. Families could withdraw any time for any reason. Participating in an OIT trial is demanding – it requires office visits for dosing adjustments and blood tests, rest periods, keeping symptom logs in which daily doses are recorded, and possible allergic reactions from the treatment itself. “A common reason for ‘withdrawal of consent’ in clinical studies is the inconvenience of remaining in a long-term study,” Mohamed Yassine, MD, Aimmune’s senior vice present of medical affairs, said via email.
Attrition was concentrated within certain subgroups. Most participants in (88.7%; 102 of 115) PALISADE who received placebo elected to enter the open-label extension; nearly half did not finish. Dropout rates were also high (29.2%) for non-daily dosing participants who had come from the PALISADE treatment arm.
The authors did not report on those high-dropout groups. Instead, they focused their analysis on the 142 treated PALISADE participants who continued daily dosing through the extension – 110 patients for a total of about 1.5 years and 32 patients for about 2 years. In a subgroup analysis, 48.1% of children in the 1.5-year group upped their tolerance to 2,000 mg peanut protein, and even more (80.8%) in the 2-year group reached that threshold – all while taking a 300-mg maintenance dose.
Those who remained on treatment longer also had fewer adverse events. At the exit food challenge, 24% of the 1.5-year participants had reactions that required epinephrine, but among 2-year participants, only 3.8% needed the rescue medication.
Continuing therapy past the first year seemed to have additional benefits, Sandra Hong, MD, director of the Cleveland Clinic Food Allergy Center of Excellence, said in an interview. Dr. Hong was not involved in the new research and has no financial ties with Aimmune or other food allergy companies. “Not only can you ingest more, but your reaction when you do react is going to be less,” she says.
Palforzia is only available through a risk evaluation and mitigation strategy (REMS) program, which educates patients, health care professionals, and pharmacies about immunotherapy risks and precautionary measures. As of last summer, before Aimmune was acquired by Nestlé Health Science, about 100 allergists in the United States had enrolled patients in the REMS program. Families can find allergists who are certified to prescribe Palforzia using the website’s Certified Participant Locator.
Although the field at large remains apprehensive about OIT and other forms of immunotherapy, an estimated 200 or more U.S. clinics are administering home-grown OIT using commercial food products, says Richard Wasserman, an OIT pioneer whose clinic in Dallas has treated allergies to about 20 foods since the practice started offering the therapy in 2008. OIT practitioners have treated more than 15,000 food allergy patients nationwide, Dr. Wasserman said via email, yet they make up just a tiny fraction of the more than 6,000 board-certified allergists in the United States.
Whether using Palforzia or nonproprietary food products, oral immunotherapy requires a lot of time and effort – not just for patients but also practitioners. “You need more space. You need more staffing. Patients doing oral challenges stay in your office for 4 to 5 hours, and we have one-to-one nursing care for them,” said Dr. Hong. “So it’s a lot of resources.”
Her team has treated about 20 children with Palforzia since the Cleveland Clinic began offering the therapy last summer. Dr. Hong and coworkers have administered OIT using commercial peanut flour and peanut butter to some 80 peanut-allergic toddlers younger than 4 years who are too young to receive for the U.S. Food and Drug Administration–approved treatment. Their early data, which were presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology in February, suggest that toddlers get complete OIT more quickly with fewer side effects than older children, Dr. Hong says. A recent study of preschoolers in Canada also found that nonproprietary OIT is very safe and effective in this younger set and could be cost-saving in the long run.
By comparison, Palforzia, which has a list price of $890 per month, was judged to be less cost-effective in analyses by academic allergists and by the Institute for Clinical and Economic Review. But through a copay savings program, depending on their insurance coverage, some eligible families can pay as little as $20 per month for the FDA-approved treatment.
Because the therapy is time consuming for families and is resource intensive for practices, questions remain as to how long and how frequently patients need to remain on treatment to sustain protection. Do they need to keep taking Palforzia, or “can we switch them to an equivalent amount of food and not bother with the study drug?” said Edwin Kim, director of the UNC Food Allergy Initiative, Chapel Hill, North Carolina, and study investigator for several Palforzia trials, in an interview.
The Food Allergy Support Team, a nonprofit group started by Dr. Wasserman and colleagues, publishes best practices and meets annually to discuss research and protocols. However, the best maintenance dose, the best dosing frequency, and the duration of daily dosing that yields the best outcomes are not known, Dr. Wasserman says.
“We think the best way to answer that question is with a regulated, pharmaceutical-grade form of peanut protein,” Dr. Yassine said.
The field’s experience with Palforzia raises a dilemma: Does its approval legitimize oral immunotherapy in general, or will rigorous, multi-million dollar trials be needed to approve products for each food or combination of foods? About 32 million people in the United States have food allergies – about 1 in 10 adults and 1 in 13 children.
“I think the field has always grappled with that, honestly,” said Stacie Jones, MD, professor of pediatrics and chief of allergy and immunology at the University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, in an interview. Home-grown OIT is “easier to do when you have high control of your small patient volumes or you’re in a clinical trial,” said Dr. Jones, who has served as an investigator on Palforzia trials and last year received more than $30,000 in consulting fees from Aimmune. “It becomes a very different situation when it becomes a national or an international recommended therapy.”
The Canadian Society of Allergy and Clinical Immunology has published clinical practice guidelines and provides practical information on its website on how to implement OIT – including protocols for dozens of foods and diary sheets for patients to log doses and symptoms.
However, U.S. professional societies still consider OIT investigational and suggest that it will not be approved by the FDA. “As a field, are we willing to wait 4 to 5 more years for an egg product? Should we? Are we willing?” said Dr. Kim. “These are tough questions.”
Stacie M. Jones reports advisory board fees, Aimmune Therapeutics, FARE; personal fees, DBV Technologies; clinical trials grants, Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi, Regeneron, FARE, Genentech, and NIH-NIAID. Edwin Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; grant support from the NIH’s National Institute of Allergy and Infectious Diseases, National Center for Complementary and Integrative Health and Immune Tolerance Network; Food Allergy Research and Education, and the Wallace Research Foundation. Richard Wasserman receives consulting fees from Aimmune Therapeutics and DBV Technologies. Mohamed Yassine is employed by Aimmune Therapeutics. Sandra Hong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent analysis of 142 peanut-allergic children treated for 1.5 to 2 years with a licensed oral immunotherapy (OIT) product confirms what various smaller studies have shown: Maintaining treatment for longer periods improves protection and reduces adverse effects. The findings offer some reassurance regarding the controversial approach, which has become available at a small number of clinics yet faces an uncertain future.
The new study, published July 28 in Allergy, included a subset of patients who chose to complete an extension of the phase 3 PALISADE trial of Palforzia, a proprietary set of premeasured peanut flour capsules developed by Aimmune Therapeutics.
Palforzia was approved last year for children aged 4 to 17 years with peanut allergy – one of the most common food allergies, affecting around 2% of children in the United States and Europe. The treatment is not a cure – patients must still watch what they eat and carry epinephrine for emergency reactions – but it helps build protection through daily ingestion of gradually increasing amounts of the allergen over a period of months.
In the 1-year PALISADE trial, which enrolled 496 peanut-allergic children at 66 sites in North America and Europe, participants received daily doses of study drug or placebo. The dose of the drug was escalated from 3 mg to 300 mg over 6 months; the 300-mg dose was then maintained for another 6 months. By the end of the study, about two-thirds of the children who underwent treatment could safely consume at least 600 mg of peanut protein, about the equivalent of two peanuts.
Could protection be increased with further treatment, and what would be required to sustain it? To address these questions, PALISADE patients who successfully reached the 600-mg threshold, along with those from the placebo group, were invited to participate in Aimmune’s open-label follow-on study. The extension study also explored whether protection could be maintained with less frequent dosing.
Among the 358 eligible participants who opted into the 1-year extension study, 256 came from the PALISADE treatment arm. These children were assigned to five cohorts to continue for 6 months or 12 months with daily or less frequent doses. Within the 6-month group, all started with the 300-mg daily dose. A subset received two doses a week. Within the 12-month group, some patients maintained daily dosing throughout; others received doses every other day, twice weekly, or once every 2 weeks.
The children who continued daily maintenance dosing the longest gained the most protection. Those in less-frequent dosing groups experienced more adverse events than those who received doses every day, the company reported last December in The Journal of Allergy and Clinical Immunology: In Practice.
More than a quarter (97 of 358, or 27.1%) of participants failed to complete the extension. Families could withdraw any time for any reason. Participating in an OIT trial is demanding – it requires office visits for dosing adjustments and blood tests, rest periods, keeping symptom logs in which daily doses are recorded, and possible allergic reactions from the treatment itself. “A common reason for ‘withdrawal of consent’ in clinical studies is the inconvenience of remaining in a long-term study,” Mohamed Yassine, MD, Aimmune’s senior vice present of medical affairs, said via email.
Attrition was concentrated within certain subgroups. Most participants in (88.7%; 102 of 115) PALISADE who received placebo elected to enter the open-label extension; nearly half did not finish. Dropout rates were also high (29.2%) for non-daily dosing participants who had come from the PALISADE treatment arm.
The authors did not report on those high-dropout groups. Instead, they focused their analysis on the 142 treated PALISADE participants who continued daily dosing through the extension – 110 patients for a total of about 1.5 years and 32 patients for about 2 years. In a subgroup analysis, 48.1% of children in the 1.5-year group upped their tolerance to 2,000 mg peanut protein, and even more (80.8%) in the 2-year group reached that threshold – all while taking a 300-mg maintenance dose.
Those who remained on treatment longer also had fewer adverse events. At the exit food challenge, 24% of the 1.5-year participants had reactions that required epinephrine, but among 2-year participants, only 3.8% needed the rescue medication.
Continuing therapy past the first year seemed to have additional benefits, Sandra Hong, MD, director of the Cleveland Clinic Food Allergy Center of Excellence, said in an interview. Dr. Hong was not involved in the new research and has no financial ties with Aimmune or other food allergy companies. “Not only can you ingest more, but your reaction when you do react is going to be less,” she says.
Palforzia is only available through a risk evaluation and mitigation strategy (REMS) program, which educates patients, health care professionals, and pharmacies about immunotherapy risks and precautionary measures. As of last summer, before Aimmune was acquired by Nestlé Health Science, about 100 allergists in the United States had enrolled patients in the REMS program. Families can find allergists who are certified to prescribe Palforzia using the website’s Certified Participant Locator.
Although the field at large remains apprehensive about OIT and other forms of immunotherapy, an estimated 200 or more U.S. clinics are administering home-grown OIT using commercial food products, says Richard Wasserman, an OIT pioneer whose clinic in Dallas has treated allergies to about 20 foods since the practice started offering the therapy in 2008. OIT practitioners have treated more than 15,000 food allergy patients nationwide, Dr. Wasserman said via email, yet they make up just a tiny fraction of the more than 6,000 board-certified allergists in the United States.
Whether using Palforzia or nonproprietary food products, oral immunotherapy requires a lot of time and effort – not just for patients but also practitioners. “You need more space. You need more staffing. Patients doing oral challenges stay in your office for 4 to 5 hours, and we have one-to-one nursing care for them,” said Dr. Hong. “So it’s a lot of resources.”
Her team has treated about 20 children with Palforzia since the Cleveland Clinic began offering the therapy last summer. Dr. Hong and coworkers have administered OIT using commercial peanut flour and peanut butter to some 80 peanut-allergic toddlers younger than 4 years who are too young to receive for the U.S. Food and Drug Administration–approved treatment. Their early data, which were presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology in February, suggest that toddlers get complete OIT more quickly with fewer side effects than older children, Dr. Hong says. A recent study of preschoolers in Canada also found that nonproprietary OIT is very safe and effective in this younger set and could be cost-saving in the long run.
By comparison, Palforzia, which has a list price of $890 per month, was judged to be less cost-effective in analyses by academic allergists and by the Institute for Clinical and Economic Review. But through a copay savings program, depending on their insurance coverage, some eligible families can pay as little as $20 per month for the FDA-approved treatment.
Because the therapy is time consuming for families and is resource intensive for practices, questions remain as to how long and how frequently patients need to remain on treatment to sustain protection. Do they need to keep taking Palforzia, or “can we switch them to an equivalent amount of food and not bother with the study drug?” said Edwin Kim, director of the UNC Food Allergy Initiative, Chapel Hill, North Carolina, and study investigator for several Palforzia trials, in an interview.
The Food Allergy Support Team, a nonprofit group started by Dr. Wasserman and colleagues, publishes best practices and meets annually to discuss research and protocols. However, the best maintenance dose, the best dosing frequency, and the duration of daily dosing that yields the best outcomes are not known, Dr. Wasserman says.
“We think the best way to answer that question is with a regulated, pharmaceutical-grade form of peanut protein,” Dr. Yassine said.
The field’s experience with Palforzia raises a dilemma: Does its approval legitimize oral immunotherapy in general, or will rigorous, multi-million dollar trials be needed to approve products for each food or combination of foods? About 32 million people in the United States have food allergies – about 1 in 10 adults and 1 in 13 children.
“I think the field has always grappled with that, honestly,” said Stacie Jones, MD, professor of pediatrics and chief of allergy and immunology at the University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, in an interview. Home-grown OIT is “easier to do when you have high control of your small patient volumes or you’re in a clinical trial,” said Dr. Jones, who has served as an investigator on Palforzia trials and last year received more than $30,000 in consulting fees from Aimmune. “It becomes a very different situation when it becomes a national or an international recommended therapy.”
The Canadian Society of Allergy and Clinical Immunology has published clinical practice guidelines and provides practical information on its website on how to implement OIT – including protocols for dozens of foods and diary sheets for patients to log doses and symptoms.
However, U.S. professional societies still consider OIT investigational and suggest that it will not be approved by the FDA. “As a field, are we willing to wait 4 to 5 more years for an egg product? Should we? Are we willing?” said Dr. Kim. “These are tough questions.”
Stacie M. Jones reports advisory board fees, Aimmune Therapeutics, FARE; personal fees, DBV Technologies; clinical trials grants, Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi, Regeneron, FARE, Genentech, and NIH-NIAID. Edwin Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; grant support from the NIH’s National Institute of Allergy and Infectious Diseases, National Center for Complementary and Integrative Health and Immune Tolerance Network; Food Allergy Research and Education, and the Wallace Research Foundation. Richard Wasserman receives consulting fees from Aimmune Therapeutics and DBV Technologies. Mohamed Yassine is employed by Aimmune Therapeutics. Sandra Hong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.

