User login
Lesions on the Thigh After an Organ Transplant
The Diagnosis: Microcystic Lymphatic Malformation
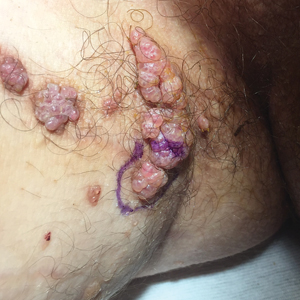
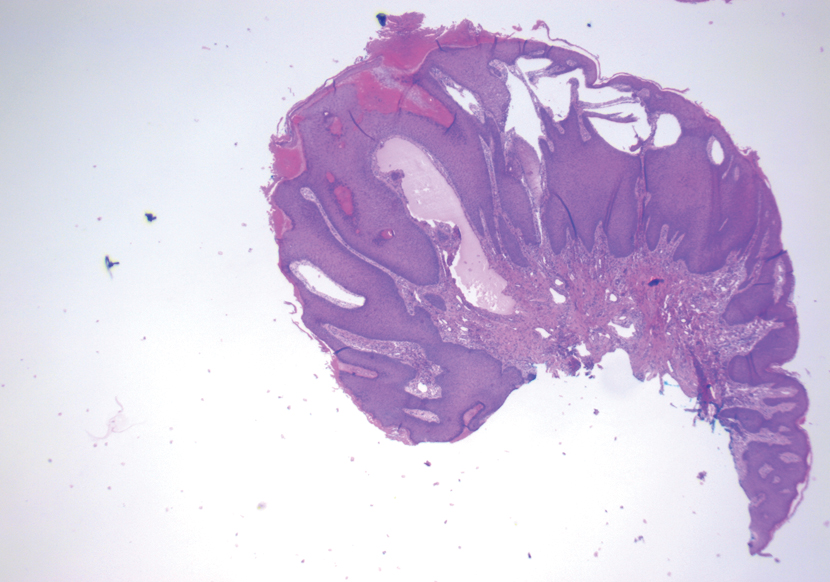
The shave biopsy demonstrated numerous thin-walled vascular spaces filled with lymphatic fluid within the dermis (Figure), consistent with a diagnosis of microcystic lymphatic malformation (LM). Lymphatic malformations represent a class of benign vascular lesions consisting of anomalous or dilated lymphatic vessels, which can be broadly categorized as macrocystic (formerly cavernous lymphangioma or cystic hygroma), microcystic (formerly lymphangioma circumscriptum), or mixed.1 Patients often will present with pruritus, crusting, secondary infection, edema, or oozing.2 The superficial blebs of microcystic LMs resemble frog spawn and range in color from clear to pink, brawny, or deep maroon.3 Although the lymphatic vessels involved in microcystic LMs appear disconnected from the major lymphatic circulation,3 systemic fluid overload could plausibly promote lesional swelling and tenderness; we attributed our patient's worsening symptoms to the cumulative 7.8 L of intravenous fluid he received intraoperatively during his cardiac transplant. The excess fluid allowed communication between lymphatic cisterns and thin-walled vesicles on the skin surface through dilated channels. Overall, LMs represent roughly 26% of pediatric benign vascular tumors and approximately 4% of all vascular tumors.4
Although microcystic LMs may appear especially vascular or verrucous, the differential diagnosis for our patient's LM included condyloma acuminatum,5,6 condyloma lata,7 epidermal nevus, and lymphangiosarcoma. Epidermal nevi are congenital lesions, varying in appearance from velvety to verrucous patches and plaques that often evolve during puberty and become thicker, more verrucous, and hyperpigmented. Keratinocytic epidermal nevus syndromes and other entities such as nevus sebaceous have been associated with somatic mutations affecting proteins in the fibroblast growth factor receptor signaling pathway (eg, FGFR3, HRAS).8 Although the clinical appearance alone may be similar, lymphangiosarcoma can be distinguished from LM via biopsy.
There are several methods to diagnose LM. Duplex sonography is possibly the best noninvasive method to identify the flow between venous valves. Magnetic resonance imaging can detect larger occurrences of LM, and lymphangiography can be utilized to confirm a normal or abnormal lymphatic network.4 Treatment options are broad, including surgical excision, laser ablation, and topical sirolimus. Hypertonic saline sclerotherapy can be injected into the afflicted lymphatic channels to decrease inflammation, erythema, and hyperpigmentation without further treatment or major side effects.4
However, the benefits of sclerotherapy alone in the treatment of LM often come gradually, and radiofrequency ablation may need to be utilized to achieve more immediate results.2 Overall, outcomes are highly variable, but favorable outcomes often can be difficult to obtain due to a high recurrence rate.2,8 Our patient's symptoms improved during his postoperative recovery, and he declined further intervention.
- Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178-185. doi:10.1053/j.sempedsurg.2014.07.002
- Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010;36:1711-1717. doi:10.1111/j.1524-4725.2010.01723.x
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111 /j.1365-4632.2009.04226.x
- Bikowski JB, Dumont AM. Lymphangioma circumscriptum: treatment with hypertonic saline sclerotherapy. J Am Acad Dermatol. 2005;53:442-444. doi:10.1016/j.jaad.2005.04.086
- Costa-Silva M, Fernandes I, Rodrigues AG, et al. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92:675-681. doi:10.1590 /abd1806-4841.201756411
- Darmstadt GL. Perianal lymphangioma circumscriptum mistaken for genital warts. Pediatrics 1996;98;461.
- Bruins FG, van Deudekom FJA, de Vries HJC. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259. doi:10.1136 /bmj.h1259
- Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi:10.1111 /pde.13273
The Diagnosis: Microcystic Lymphatic Malformation
The shave biopsy demonstrated numerous thin-walled vascular spaces filled with lymphatic fluid within the dermis (Figure), consistent with a diagnosis of microcystic lymphatic malformation (LM). Lymphatic malformations represent a class of benign vascular lesions consisting of anomalous or dilated lymphatic vessels, which can be broadly categorized as macrocystic (formerly cavernous lymphangioma or cystic hygroma), microcystic (formerly lymphangioma circumscriptum), or mixed.1 Patients often will present with pruritus, crusting, secondary infection, edema, or oozing.2 The superficial blebs of microcystic LMs resemble frog spawn and range in color from clear to pink, brawny, or deep maroon.3 Although the lymphatic vessels involved in microcystic LMs appear disconnected from the major lymphatic circulation,3 systemic fluid overload could plausibly promote lesional swelling and tenderness; we attributed our patient's worsening symptoms to the cumulative 7.8 L of intravenous fluid he received intraoperatively during his cardiac transplant. The excess fluid allowed communication between lymphatic cisterns and thin-walled vesicles on the skin surface through dilated channels. Overall, LMs represent roughly 26% of pediatric benign vascular tumors and approximately 4% of all vascular tumors.4

Although microcystic LMs may appear especially vascular or verrucous, the differential diagnosis for our patient's LM included condyloma acuminatum,5,6 condyloma lata,7 epidermal nevus, and lymphangiosarcoma. Epidermal nevi are congenital lesions, varying in appearance from velvety to verrucous patches and plaques that often evolve during puberty and become thicker, more verrucous, and hyperpigmented. Keratinocytic epidermal nevus syndromes and other entities such as nevus sebaceous have been associated with somatic mutations affecting proteins in the fibroblast growth factor receptor signaling pathway (eg, FGFR3, HRAS).8 Although the clinical appearance alone may be similar, lymphangiosarcoma can be distinguished from LM via biopsy.
There are several methods to diagnose LM. Duplex sonography is possibly the best noninvasive method to identify the flow between venous valves. Magnetic resonance imaging can detect larger occurrences of LM, and lymphangiography can be utilized to confirm a normal or abnormal lymphatic network.4 Treatment options are broad, including surgical excision, laser ablation, and topical sirolimus. Hypertonic saline sclerotherapy can be injected into the afflicted lymphatic channels to decrease inflammation, erythema, and hyperpigmentation without further treatment or major side effects.4
However, the benefits of sclerotherapy alone in the treatment of LM often come gradually, and radiofrequency ablation may need to be utilized to achieve more immediate results.2 Overall, outcomes are highly variable, but favorable outcomes often can be difficult to obtain due to a high recurrence rate.2,8 Our patient's symptoms improved during his postoperative recovery, and he declined further intervention.
The Diagnosis: Microcystic Lymphatic Malformation
The shave biopsy demonstrated numerous thin-walled vascular spaces filled with lymphatic fluid within the dermis (Figure), consistent with a diagnosis of microcystic lymphatic malformation (LM). Lymphatic malformations represent a class of benign vascular lesions consisting of anomalous or dilated lymphatic vessels, which can be broadly categorized as macrocystic (formerly cavernous lymphangioma or cystic hygroma), microcystic (formerly lymphangioma circumscriptum), or mixed.1 Patients often will present with pruritus, crusting, secondary infection, edema, or oozing.2 The superficial blebs of microcystic LMs resemble frog spawn and range in color from clear to pink, brawny, or deep maroon.3 Although the lymphatic vessels involved in microcystic LMs appear disconnected from the major lymphatic circulation,3 systemic fluid overload could plausibly promote lesional swelling and tenderness; we attributed our patient's worsening symptoms to the cumulative 7.8 L of intravenous fluid he received intraoperatively during his cardiac transplant. The excess fluid allowed communication between lymphatic cisterns and thin-walled vesicles on the skin surface through dilated channels. Overall, LMs represent roughly 26% of pediatric benign vascular tumors and approximately 4% of all vascular tumors.4

Although microcystic LMs may appear especially vascular or verrucous, the differential diagnosis for our patient's LM included condyloma acuminatum,5,6 condyloma lata,7 epidermal nevus, and lymphangiosarcoma. Epidermal nevi are congenital lesions, varying in appearance from velvety to verrucous patches and plaques that often evolve during puberty and become thicker, more verrucous, and hyperpigmented. Keratinocytic epidermal nevus syndromes and other entities such as nevus sebaceous have been associated with somatic mutations affecting proteins in the fibroblast growth factor receptor signaling pathway (eg, FGFR3, HRAS).8 Although the clinical appearance alone may be similar, lymphangiosarcoma can be distinguished from LM via biopsy.
There are several methods to diagnose LM. Duplex sonography is possibly the best noninvasive method to identify the flow between venous valves. Magnetic resonance imaging can detect larger occurrences of LM, and lymphangiography can be utilized to confirm a normal or abnormal lymphatic network.4 Treatment options are broad, including surgical excision, laser ablation, and topical sirolimus. Hypertonic saline sclerotherapy can be injected into the afflicted lymphatic channels to decrease inflammation, erythema, and hyperpigmentation without further treatment or major side effects.4
However, the benefits of sclerotherapy alone in the treatment of LM often come gradually, and radiofrequency ablation may need to be utilized to achieve more immediate results.2 Overall, outcomes are highly variable, but favorable outcomes often can be difficult to obtain due to a high recurrence rate.2,8 Our patient's symptoms improved during his postoperative recovery, and he declined further intervention.
- Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178-185. doi:10.1053/j.sempedsurg.2014.07.002
- Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010;36:1711-1717. doi:10.1111/j.1524-4725.2010.01723.x
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111 /j.1365-4632.2009.04226.x
- Bikowski JB, Dumont AM. Lymphangioma circumscriptum: treatment with hypertonic saline sclerotherapy. J Am Acad Dermatol. 2005;53:442-444. doi:10.1016/j.jaad.2005.04.086
- Costa-Silva M, Fernandes I, Rodrigues AG, et al. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92:675-681. doi:10.1590 /abd1806-4841.201756411
- Darmstadt GL. Perianal lymphangioma circumscriptum mistaken for genital warts. Pediatrics 1996;98;461.
- Bruins FG, van Deudekom FJA, de Vries HJC. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259. doi:10.1136 /bmj.h1259
- Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi:10.1111 /pde.13273
- Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178-185. doi:10.1053/j.sempedsurg.2014.07.002
- Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010;36:1711-1717. doi:10.1111/j.1524-4725.2010.01723.x
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111 /j.1365-4632.2009.04226.x
- Bikowski JB, Dumont AM. Lymphangioma circumscriptum: treatment with hypertonic saline sclerotherapy. J Am Acad Dermatol. 2005;53:442-444. doi:10.1016/j.jaad.2005.04.086
- Costa-Silva M, Fernandes I, Rodrigues AG, et al. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92:675-681. doi:10.1590 /abd1806-4841.201756411
- Darmstadt GL. Perianal lymphangioma circumscriptum mistaken for genital warts. Pediatrics 1996;98;461.
- Bruins FG, van Deudekom FJA, de Vries HJC. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259. doi:10.1136 /bmj.h1259
- Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi:10.1111 /pde.13273
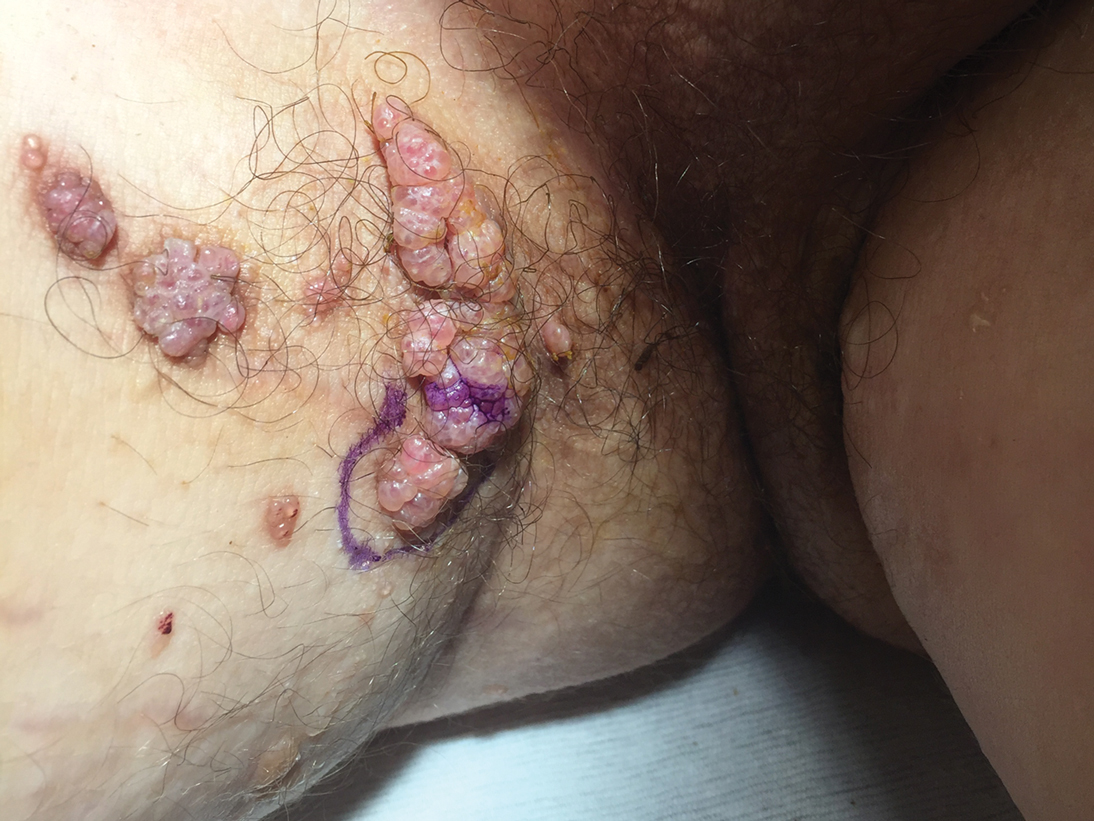
A 17-year-old adolescent boy presented with increasingly painful genital warts on the right thigh, groin, and scrotum that had been present since birth. The patient had a medical history of cardiac transplantation in the months prior to presentation and was on immunosuppressive therapy. The lesions had become more swollen and bothersome in the weeks following the transplantation and now prevented him from ambulating due to discomfort. He denied any history of sexual contact or oral lesions. Physical examination revealed numerous translucent and hemorrhagic vesicles clustered and linearly distributed on the right medial thigh. A shave biopsy of a vesicle was performed.
Targeted therapies for vascular anomalies continue to be refined
“The medicines we had were believed to be antiangiogenic and they were used not only for tumors but for all sorts of malformations,” Dr. Adams, a pediatric hematologist-oncologist at Children’s Hospital of Philadelphia, recalled during the annual meeting of the Society for Pediatric Dermatology. “I didn’t understand how so many different phenotypes could respond to the same medicine. Not all of them did, but some did have some response.”
She also grew frustrated by the lack of clinical trials and collaborative research groups involving patients with vascular anomalies. “I called this the chicken soup of medical management,” she said. “As we got more involved in vascular anomalies, the power of one patient or that power of a few patients led us in a direction for improved medical management. Or knowledge was gained by one patient who failed all noted medical management and led us into a direction repurposing a drug that actually wound up working.”
Propranolol, for example, became a key medicine for the treatment of vascular anomalies when it was found to improve hemangiomas in children who were given the drug for other reasons. “From this observation a key prospective study was performed and this beta-blocker became FDA approved for the treatment of complicated hemangiomas,” said Dr. Adams, who directs the hospital’s Comprehensive Vascular Anomalies Program. “That was how a bedside observation let to bench intervention, and how presently we are investigating bench interventions related to the mechanism of propranolol therapy.”
Then there is the story of the mammalian target of rapamycin (mTOR) inhibitor sirolimus. In her previous role as medical director of the Hemangioma and Vascular Malformation Center at Cincinnati Children’s Hospital, Dr. Adams and colleagues cared for an infant who presented with a Kaposiform hemangioendothelioma (KHE). “At that time, she was given our standard of practice for the treatment, but our standard of practice was not good enough,” she said.
While other options were being discussed for this patient, “we had been doing some collaborative work with pathology and nephrology on the PIKC3A pathway, because we knew that germline mutations of TEK were involved in this pathway, and we knew that 50% of patients with PTEN mutations had vascular anomalies. So, we hypothesized that this pathway was involved in vascular anomalies.”
They also had earlier success using mTOR inhibition for tuberous sclerosis patients with angiomyolipomas and patients with neurofibromatosis. “We needed a medicine that could be given orally because we did not think this patient was going to do well, so we started her on sirolimus,” Dr. Adams said. “She had a great response. This was followed by a phase 2 study, which proved efficacy and led to discovery of biomarkers.” This is where the angiopoietin-2 story started, she said, noting that this biomarker is now used “to differentiate KLA [Kaposiform lymphangiomatosis] from KHE and KLAs and KHE from other disorders.”
This bedside work helped researchers to better understand the mechanism of action in other disorders, such as observing somatic mutations in PIK3CA in patients with CLOVES syndrome. “This meant that we could now correlate the phenotype to the genotype, and it opened up targeted therapy with developmental therapeutics that were already in use for oncology,” Dr. Adams said. “We know we had mTOR inhibition with sirolimus and everolimus. We now have an AKT inhibitor, a PIK3CA inhibitor, and we now have another side of the pathway which deals with RASopathies, and some other medicines that we can use.”
Miransertib, a potent PAN-AKT inhibitor initially used for breast cancer, is currently being evaluated in open-label, phase 1 and 2 trials in patients with PIK3CA-related overgrowth spectrum (PROS) and Proteus syndrome. The dose used in a pilot study is about one-sixth of the dose used for oncology patients, Dr. Adams said.
She and her colleagues used miransertib to treat a 3-year-old with CLOVES syndrome who had lipomatous infiltration of the abdomen and retroperitoneum with failure to thrive. “He was not eating and was G-tube dependent,” she recalled. “After a month of therapy, he started eating and had improvement in his quality of life,” although despite this improvement volumetric MRI remained unchanged.
Advances in bench to bedside approaches are also under way. Hakon Hakonarson, MD, PhD, the founding director of the Center for Applied Genomics at CHOP, has discovered several genes with in vitro testing and zebra fish modeling, which has been followed by testing medicines on patients.
One such patient, according to Dr. Adams, had a severe central conducting lymphatic anomaly, with a pericardial effusion and significant dysfunction of the central conducting system. The patient was found to have an ARAF mutation, which induces ERK activation. “ERK is downstream of MEK, so the question was whether a MEK inhibitor, trametinib, could be used to treat this patient,” she said. “Trametinib was first used in tissue culture, then used in a zebra fish model and it showed some positive results. Then it was taken to the patient, who had improvement of pulmonary function, remodeling of the lymphatic system, and decrease in the size of his legs.”
Other antiangiogenic agents being used for the treatment of vascular anomalies include bevacizumab, which is being used in hereditary hemorrhagic telangiectasia, and thalidomide for HHT and arteriovenous malformations. For more information, Dr. Adams recommended a comprehensive review of vascular anomalies, related genes, and treatments that was published in Circulation Research.
The goal of future drug therapies is to support normal growth, “so we don’t need a maximum tolerated dose,” Dr. Adams said. “We need to be very careful of short-term and long-term side effects.”
Going forward, she said that she would like to see more natural history studies of vascular anomalies, improved outcome measures for clinical trials, adaptive study design, preclinical testing, animal model studies, universal availability of genomic testing, improvement of NIH funding, research collaboration nationally and internationally, and industry support.
Dr. Adams disclosed that she is a consultant to Venthera and Novartis.
“The medicines we had were believed to be antiangiogenic and they were used not only for tumors but for all sorts of malformations,” Dr. Adams, a pediatric hematologist-oncologist at Children’s Hospital of Philadelphia, recalled during the annual meeting of the Society for Pediatric Dermatology. “I didn’t understand how so many different phenotypes could respond to the same medicine. Not all of them did, but some did have some response.”
She also grew frustrated by the lack of clinical trials and collaborative research groups involving patients with vascular anomalies. “I called this the chicken soup of medical management,” she said. “As we got more involved in vascular anomalies, the power of one patient or that power of a few patients led us in a direction for improved medical management. Or knowledge was gained by one patient who failed all noted medical management and led us into a direction repurposing a drug that actually wound up working.”
Propranolol, for example, became a key medicine for the treatment of vascular anomalies when it was found to improve hemangiomas in children who were given the drug for other reasons. “From this observation a key prospective study was performed and this beta-blocker became FDA approved for the treatment of complicated hemangiomas,” said Dr. Adams, who directs the hospital’s Comprehensive Vascular Anomalies Program. “That was how a bedside observation let to bench intervention, and how presently we are investigating bench interventions related to the mechanism of propranolol therapy.”
Then there is the story of the mammalian target of rapamycin (mTOR) inhibitor sirolimus. In her previous role as medical director of the Hemangioma and Vascular Malformation Center at Cincinnati Children’s Hospital, Dr. Adams and colleagues cared for an infant who presented with a Kaposiform hemangioendothelioma (KHE). “At that time, she was given our standard of practice for the treatment, but our standard of practice was not good enough,” she said.
While other options were being discussed for this patient, “we had been doing some collaborative work with pathology and nephrology on the PIKC3A pathway, because we knew that germline mutations of TEK were involved in this pathway, and we knew that 50% of patients with PTEN mutations had vascular anomalies. So, we hypothesized that this pathway was involved in vascular anomalies.”
They also had earlier success using mTOR inhibition for tuberous sclerosis patients with angiomyolipomas and patients with neurofibromatosis. “We needed a medicine that could be given orally because we did not think this patient was going to do well, so we started her on sirolimus,” Dr. Adams said. “She had a great response. This was followed by a phase 2 study, which proved efficacy and led to discovery of biomarkers.” This is where the angiopoietin-2 story started, she said, noting that this biomarker is now used “to differentiate KLA [Kaposiform lymphangiomatosis] from KHE and KLAs and KHE from other disorders.”
This bedside work helped researchers to better understand the mechanism of action in other disorders, such as observing somatic mutations in PIK3CA in patients with CLOVES syndrome. “This meant that we could now correlate the phenotype to the genotype, and it opened up targeted therapy with developmental therapeutics that were already in use for oncology,” Dr. Adams said. “We know we had mTOR inhibition with sirolimus and everolimus. We now have an AKT inhibitor, a PIK3CA inhibitor, and we now have another side of the pathway which deals with RASopathies, and some other medicines that we can use.”
Miransertib, a potent PAN-AKT inhibitor initially used for breast cancer, is currently being evaluated in open-label, phase 1 and 2 trials in patients with PIK3CA-related overgrowth spectrum (PROS) and Proteus syndrome. The dose used in a pilot study is about one-sixth of the dose used for oncology patients, Dr. Adams said.
She and her colleagues used miransertib to treat a 3-year-old with CLOVES syndrome who had lipomatous infiltration of the abdomen and retroperitoneum with failure to thrive. “He was not eating and was G-tube dependent,” she recalled. “After a month of therapy, he started eating and had improvement in his quality of life,” although despite this improvement volumetric MRI remained unchanged.
Advances in bench to bedside approaches are also under way. Hakon Hakonarson, MD, PhD, the founding director of the Center for Applied Genomics at CHOP, has discovered several genes with in vitro testing and zebra fish modeling, which has been followed by testing medicines on patients.
One such patient, according to Dr. Adams, had a severe central conducting lymphatic anomaly, with a pericardial effusion and significant dysfunction of the central conducting system. The patient was found to have an ARAF mutation, which induces ERK activation. “ERK is downstream of MEK, so the question was whether a MEK inhibitor, trametinib, could be used to treat this patient,” she said. “Trametinib was first used in tissue culture, then used in a zebra fish model and it showed some positive results. Then it was taken to the patient, who had improvement of pulmonary function, remodeling of the lymphatic system, and decrease in the size of his legs.”
Other antiangiogenic agents being used for the treatment of vascular anomalies include bevacizumab, which is being used in hereditary hemorrhagic telangiectasia, and thalidomide for HHT and arteriovenous malformations. For more information, Dr. Adams recommended a comprehensive review of vascular anomalies, related genes, and treatments that was published in Circulation Research.
The goal of future drug therapies is to support normal growth, “so we don’t need a maximum tolerated dose,” Dr. Adams said. “We need to be very careful of short-term and long-term side effects.”
Going forward, she said that she would like to see more natural history studies of vascular anomalies, improved outcome measures for clinical trials, adaptive study design, preclinical testing, animal model studies, universal availability of genomic testing, improvement of NIH funding, research collaboration nationally and internationally, and industry support.
Dr. Adams disclosed that she is a consultant to Venthera and Novartis.
“The medicines we had were believed to be antiangiogenic and they were used not only for tumors but for all sorts of malformations,” Dr. Adams, a pediatric hematologist-oncologist at Children’s Hospital of Philadelphia, recalled during the annual meeting of the Society for Pediatric Dermatology. “I didn’t understand how so many different phenotypes could respond to the same medicine. Not all of them did, but some did have some response.”
She also grew frustrated by the lack of clinical trials and collaborative research groups involving patients with vascular anomalies. “I called this the chicken soup of medical management,” she said. “As we got more involved in vascular anomalies, the power of one patient or that power of a few patients led us in a direction for improved medical management. Or knowledge was gained by one patient who failed all noted medical management and led us into a direction repurposing a drug that actually wound up working.”
Propranolol, for example, became a key medicine for the treatment of vascular anomalies when it was found to improve hemangiomas in children who were given the drug for other reasons. “From this observation a key prospective study was performed and this beta-blocker became FDA approved for the treatment of complicated hemangiomas,” said Dr. Adams, who directs the hospital’s Comprehensive Vascular Anomalies Program. “That was how a bedside observation let to bench intervention, and how presently we are investigating bench interventions related to the mechanism of propranolol therapy.”
Then there is the story of the mammalian target of rapamycin (mTOR) inhibitor sirolimus. In her previous role as medical director of the Hemangioma and Vascular Malformation Center at Cincinnati Children’s Hospital, Dr. Adams and colleagues cared for an infant who presented with a Kaposiform hemangioendothelioma (KHE). “At that time, she was given our standard of practice for the treatment, but our standard of practice was not good enough,” she said.
While other options were being discussed for this patient, “we had been doing some collaborative work with pathology and nephrology on the PIKC3A pathway, because we knew that germline mutations of TEK were involved in this pathway, and we knew that 50% of patients with PTEN mutations had vascular anomalies. So, we hypothesized that this pathway was involved in vascular anomalies.”
They also had earlier success using mTOR inhibition for tuberous sclerosis patients with angiomyolipomas and patients with neurofibromatosis. “We needed a medicine that could be given orally because we did not think this patient was going to do well, so we started her on sirolimus,” Dr. Adams said. “She had a great response. This was followed by a phase 2 study, which proved efficacy and led to discovery of biomarkers.” This is where the angiopoietin-2 story started, she said, noting that this biomarker is now used “to differentiate KLA [Kaposiform lymphangiomatosis] from KHE and KLAs and KHE from other disorders.”
This bedside work helped researchers to better understand the mechanism of action in other disorders, such as observing somatic mutations in PIK3CA in patients with CLOVES syndrome. “This meant that we could now correlate the phenotype to the genotype, and it opened up targeted therapy with developmental therapeutics that were already in use for oncology,” Dr. Adams said. “We know we had mTOR inhibition with sirolimus and everolimus. We now have an AKT inhibitor, a PIK3CA inhibitor, and we now have another side of the pathway which deals with RASopathies, and some other medicines that we can use.”
Miransertib, a potent PAN-AKT inhibitor initially used for breast cancer, is currently being evaluated in open-label, phase 1 and 2 trials in patients with PIK3CA-related overgrowth spectrum (PROS) and Proteus syndrome. The dose used in a pilot study is about one-sixth of the dose used for oncology patients, Dr. Adams said.
She and her colleagues used miransertib to treat a 3-year-old with CLOVES syndrome who had lipomatous infiltration of the abdomen and retroperitoneum with failure to thrive. “He was not eating and was G-tube dependent,” she recalled. “After a month of therapy, he started eating and had improvement in his quality of life,” although despite this improvement volumetric MRI remained unchanged.
Advances in bench to bedside approaches are also under way. Hakon Hakonarson, MD, PhD, the founding director of the Center for Applied Genomics at CHOP, has discovered several genes with in vitro testing and zebra fish modeling, which has been followed by testing medicines on patients.
One such patient, according to Dr. Adams, had a severe central conducting lymphatic anomaly, with a pericardial effusion and significant dysfunction of the central conducting system. The patient was found to have an ARAF mutation, which induces ERK activation. “ERK is downstream of MEK, so the question was whether a MEK inhibitor, trametinib, could be used to treat this patient,” she said. “Trametinib was first used in tissue culture, then used in a zebra fish model and it showed some positive results. Then it was taken to the patient, who had improvement of pulmonary function, remodeling of the lymphatic system, and decrease in the size of his legs.”
Other antiangiogenic agents being used for the treatment of vascular anomalies include bevacizumab, which is being used in hereditary hemorrhagic telangiectasia, and thalidomide for HHT and arteriovenous malformations. For more information, Dr. Adams recommended a comprehensive review of vascular anomalies, related genes, and treatments that was published in Circulation Research.
The goal of future drug therapies is to support normal growth, “so we don’t need a maximum tolerated dose,” Dr. Adams said. “We need to be very careful of short-term and long-term side effects.”
Going forward, she said that she would like to see more natural history studies of vascular anomalies, improved outcome measures for clinical trials, adaptive study design, preclinical testing, animal model studies, universal availability of genomic testing, improvement of NIH funding, research collaboration nationally and internationally, and industry support.
Dr. Adams disclosed that she is a consultant to Venthera and Novartis.
FROM SPD 2021
FDA approves first once-weekly growth hormone for children
The U.S. Food and Drug Administration has approved lonapegsomatropin (Skytrofa, Ascendis Pharma), the first weekly subcutaneous injectable growth hormone for children with growth hormone deficiency (GHD).
The approval was based on the findings of the 52-week, phase 3 heiGHt trial in 161 treatment-naive pediatric patients with GHD, which was recently published in the Journal of Clinical Endocrinology & Metabolism.
Since 1987, the standard treatment for pediatric GHD, in which the pituitary gland does not produce enough growth hormone, has been a daily injection of somatropin (recombinant DNA human growth hormone).
“I am excited to be able to reduce the number of shots for some children requiring growth hormone therapy” with this new dosing option, Bradley S. Miller, MD, PhD, who was not involved with the research, said in an email.
“I am hopeful that a once-weekly growth hormone option will improve adherence to growth hormone therapy, leading to improved growth and metabolic outcomes,” added Dr. Miller, professor and division director, pediatric endocrinology, at the University of Minnesota Masonic Children’s Hospital, Minneapolis.
Lonapegsomatropin is approved for the treatment of pediatric patients age 1 year and older who weigh at least 11.5 kg (25.4 pounds) and have short stature due to inadequate secretion of endogenous growth hormone, according to the prescribing information.
The drug molecule consists of a prodrug of somatropin that is inactive when it is bound to a proprietary TransCon (transient conjugation) inert carrier using a TransCon linker. The three-part molecule breaks apart after injection, exposing the active somatropin that is slowly released.
The heiGHt trial demonstrated noninferiority of lonapegsomatropin to somatropin daily injections. Children who received weekly lonapegsomatropin grew 11.2 cm (4.4 inches) per year, whereas those who received an equivalent total dose of somatropin daily injections grew 10.3 cm (4.1 inches) per year.
Safety outcomes – the ratio of bone age to chronologic age, adverse events, tolerability, and immunogenicity – were similar in both groups.
Anticipated uptake, other drugs on horizon
Lonapegsomatropin is expected to be available shortly in the United States along with a suite of patient support programs, according to a company press release.
“The impact of the approval of lonapegsomatropin on clinical practice will depend upon its availability, coverage by insurance providers, and patient/provider comfort with using a new product,” Dr. Miller said.
For most pediatric endocrinologists, daily growth hormone has been available their entire careers, so he expects it will take some time for the pediatric endocrinology community to be comfortable prescribing long-acting growth hormone (LAGH), the name given to the once-weekly products.
In the meantime, an FDA decision on another once-weekly growth hormone, somatrogon (OPKO Health/Pfizer) for children with GHD is expected very soon, in October 2021.
And a weekly injectable somapacitan (Sogroya, Novo Nordisk), approved by the FDA in September last year for adults with GHD, is also being studied in children, with estimated study completion in 2024.
“Approval of more LAGH molecules, approval of LAGH for more indications, real-world evidence of safety, efficacy, and improved adherence, and personal experience with LAGH will all likely lead to increased LAGH use over time,” Dr. Miller speculated.
“Over the long-term, I expect insurance providers will cover LAGH products,” he surmised, “but that the price will be similar to or slightly higher than daily growth hormone.”
However, if improved adherence with LAGH is demonstrated and associated with better treatment outcomes, the price of LAGH will likely increase and use of daily growth hormone will decrease, he predicts.
Paul Saenger, MD, who was not involved with the research, believes “all three long-acting growth hormone drugs will eventually be approved for GHD in children.”
“The price will be the same or may be at most 10% more than daily growth hormone replacement,” Dr. Saenger, a pediatric endocrinologist and clinical assistant professor at NYU Long Island School of Medicine, New York, said in an email.
However, daily subcutaneous injections will still be warranted for certain children with GHD, Dr. Miller noted.
“Daily growth hormone may be better than LAGH for a small number of children who have severe GHD associated with hypoglycemia,” he said. “The low levels of growth hormone at the end of the weekly interval of LAGH may allow hypoglycemia to occur in this population.”
Phase 3 trial in 161 treatment-naive children with GHD
The heiGHt trial randomized treatment-naive prepubertal children with GHD 2:1 to weekly lonapegsomatropin or daily somatropin (Genotropin, Pfizer) at 73 sites in 15 countries.
The children were a mean age of 8.5 years (range, 3.2-13.1 years), 82% were boys, and 94% were White.
There were no reported serious adverse events or discontinuations related to lonapegsomatropin.
The most common adverse reactions in ≥5% of these pediatric patients were viral infection (15%), pyrexia (15%), cough (11%), nausea and vomiting (11%), hemorrhage (7%), diarrhea (6%), abdominal pain (6%), and arthralgia and arthritis (6%).
Both study groups reported low incidences of transient, non-neutralizing anti-hGH binding antibodies and no cases of persistent antibodies.
Trial limitations include the fact the study was not blinded (as patients received a weekly or daily injection) and drug doses were fixed at 0.24 mg human growth hormone/kg/week, although in real-world clinical practice, doses may be titrated.
Lonapegsomatropin has been studied in more than 300 children with GHD in the phase 3 program in the heiGHt trial (treatment-naive patients), fliGHt trial (treatment-experienced patients), and enliGHten trial (an ongoing long-term extension trial that includes some patients who have been taking lonapegsomatropin for more than 4 years).
The study was sponsored by Ascendis Pharma. Some of the phase 3 study authors are company employees.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved lonapegsomatropin (Skytrofa, Ascendis Pharma), the first weekly subcutaneous injectable growth hormone for children with growth hormone deficiency (GHD).
The approval was based on the findings of the 52-week, phase 3 heiGHt trial in 161 treatment-naive pediatric patients with GHD, which was recently published in the Journal of Clinical Endocrinology & Metabolism.
Since 1987, the standard treatment for pediatric GHD, in which the pituitary gland does not produce enough growth hormone, has been a daily injection of somatropin (recombinant DNA human growth hormone).
“I am excited to be able to reduce the number of shots for some children requiring growth hormone therapy” with this new dosing option, Bradley S. Miller, MD, PhD, who was not involved with the research, said in an email.
“I am hopeful that a once-weekly growth hormone option will improve adherence to growth hormone therapy, leading to improved growth and metabolic outcomes,” added Dr. Miller, professor and division director, pediatric endocrinology, at the University of Minnesota Masonic Children’s Hospital, Minneapolis.
Lonapegsomatropin is approved for the treatment of pediatric patients age 1 year and older who weigh at least 11.5 kg (25.4 pounds) and have short stature due to inadequate secretion of endogenous growth hormone, according to the prescribing information.
The drug molecule consists of a prodrug of somatropin that is inactive when it is bound to a proprietary TransCon (transient conjugation) inert carrier using a TransCon linker. The three-part molecule breaks apart after injection, exposing the active somatropin that is slowly released.
The heiGHt trial demonstrated noninferiority of lonapegsomatropin to somatropin daily injections. Children who received weekly lonapegsomatropin grew 11.2 cm (4.4 inches) per year, whereas those who received an equivalent total dose of somatropin daily injections grew 10.3 cm (4.1 inches) per year.
Safety outcomes – the ratio of bone age to chronologic age, adverse events, tolerability, and immunogenicity – were similar in both groups.
Anticipated uptake, other drugs on horizon
Lonapegsomatropin is expected to be available shortly in the United States along with a suite of patient support programs, according to a company press release.
“The impact of the approval of lonapegsomatropin on clinical practice will depend upon its availability, coverage by insurance providers, and patient/provider comfort with using a new product,” Dr. Miller said.
For most pediatric endocrinologists, daily growth hormone has been available their entire careers, so he expects it will take some time for the pediatric endocrinology community to be comfortable prescribing long-acting growth hormone (LAGH), the name given to the once-weekly products.
In the meantime, an FDA decision on another once-weekly growth hormone, somatrogon (OPKO Health/Pfizer) for children with GHD is expected very soon, in October 2021.
And a weekly injectable somapacitan (Sogroya, Novo Nordisk), approved by the FDA in September last year for adults with GHD, is also being studied in children, with estimated study completion in 2024.
“Approval of more LAGH molecules, approval of LAGH for more indications, real-world evidence of safety, efficacy, and improved adherence, and personal experience with LAGH will all likely lead to increased LAGH use over time,” Dr. Miller speculated.
“Over the long-term, I expect insurance providers will cover LAGH products,” he surmised, “but that the price will be similar to or slightly higher than daily growth hormone.”
However, if improved adherence with LAGH is demonstrated and associated with better treatment outcomes, the price of LAGH will likely increase and use of daily growth hormone will decrease, he predicts.
Paul Saenger, MD, who was not involved with the research, believes “all three long-acting growth hormone drugs will eventually be approved for GHD in children.”
“The price will be the same or may be at most 10% more than daily growth hormone replacement,” Dr. Saenger, a pediatric endocrinologist and clinical assistant professor at NYU Long Island School of Medicine, New York, said in an email.
However, daily subcutaneous injections will still be warranted for certain children with GHD, Dr. Miller noted.
“Daily growth hormone may be better than LAGH for a small number of children who have severe GHD associated with hypoglycemia,” he said. “The low levels of growth hormone at the end of the weekly interval of LAGH may allow hypoglycemia to occur in this population.”
Phase 3 trial in 161 treatment-naive children with GHD
The heiGHt trial randomized treatment-naive prepubertal children with GHD 2:1 to weekly lonapegsomatropin or daily somatropin (Genotropin, Pfizer) at 73 sites in 15 countries.
The children were a mean age of 8.5 years (range, 3.2-13.1 years), 82% were boys, and 94% were White.
There were no reported serious adverse events or discontinuations related to lonapegsomatropin.
The most common adverse reactions in ≥5% of these pediatric patients were viral infection (15%), pyrexia (15%), cough (11%), nausea and vomiting (11%), hemorrhage (7%), diarrhea (6%), abdominal pain (6%), and arthralgia and arthritis (6%).
Both study groups reported low incidences of transient, non-neutralizing anti-hGH binding antibodies and no cases of persistent antibodies.
Trial limitations include the fact the study was not blinded (as patients received a weekly or daily injection) and drug doses were fixed at 0.24 mg human growth hormone/kg/week, although in real-world clinical practice, doses may be titrated.
Lonapegsomatropin has been studied in more than 300 children with GHD in the phase 3 program in the heiGHt trial (treatment-naive patients), fliGHt trial (treatment-experienced patients), and enliGHten trial (an ongoing long-term extension trial that includes some patients who have been taking lonapegsomatropin for more than 4 years).
The study was sponsored by Ascendis Pharma. Some of the phase 3 study authors are company employees.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved lonapegsomatropin (Skytrofa, Ascendis Pharma), the first weekly subcutaneous injectable growth hormone for children with growth hormone deficiency (GHD).
The approval was based on the findings of the 52-week, phase 3 heiGHt trial in 161 treatment-naive pediatric patients with GHD, which was recently published in the Journal of Clinical Endocrinology & Metabolism.
Since 1987, the standard treatment for pediatric GHD, in which the pituitary gland does not produce enough growth hormone, has been a daily injection of somatropin (recombinant DNA human growth hormone).
“I am excited to be able to reduce the number of shots for some children requiring growth hormone therapy” with this new dosing option, Bradley S. Miller, MD, PhD, who was not involved with the research, said in an email.
“I am hopeful that a once-weekly growth hormone option will improve adherence to growth hormone therapy, leading to improved growth and metabolic outcomes,” added Dr. Miller, professor and division director, pediatric endocrinology, at the University of Minnesota Masonic Children’s Hospital, Minneapolis.
Lonapegsomatropin is approved for the treatment of pediatric patients age 1 year and older who weigh at least 11.5 kg (25.4 pounds) and have short stature due to inadequate secretion of endogenous growth hormone, according to the prescribing information.
The drug molecule consists of a prodrug of somatropin that is inactive when it is bound to a proprietary TransCon (transient conjugation) inert carrier using a TransCon linker. The three-part molecule breaks apart after injection, exposing the active somatropin that is slowly released.
The heiGHt trial demonstrated noninferiority of lonapegsomatropin to somatropin daily injections. Children who received weekly lonapegsomatropin grew 11.2 cm (4.4 inches) per year, whereas those who received an equivalent total dose of somatropin daily injections grew 10.3 cm (4.1 inches) per year.
Safety outcomes – the ratio of bone age to chronologic age, adverse events, tolerability, and immunogenicity – were similar in both groups.
Anticipated uptake, other drugs on horizon
Lonapegsomatropin is expected to be available shortly in the United States along with a suite of patient support programs, according to a company press release.
“The impact of the approval of lonapegsomatropin on clinical practice will depend upon its availability, coverage by insurance providers, and patient/provider comfort with using a new product,” Dr. Miller said.
For most pediatric endocrinologists, daily growth hormone has been available their entire careers, so he expects it will take some time for the pediatric endocrinology community to be comfortable prescribing long-acting growth hormone (LAGH), the name given to the once-weekly products.
In the meantime, an FDA decision on another once-weekly growth hormone, somatrogon (OPKO Health/Pfizer) for children with GHD is expected very soon, in October 2021.
And a weekly injectable somapacitan (Sogroya, Novo Nordisk), approved by the FDA in September last year for adults with GHD, is also being studied in children, with estimated study completion in 2024.
“Approval of more LAGH molecules, approval of LAGH for more indications, real-world evidence of safety, efficacy, and improved adherence, and personal experience with LAGH will all likely lead to increased LAGH use over time,” Dr. Miller speculated.
“Over the long-term, I expect insurance providers will cover LAGH products,” he surmised, “but that the price will be similar to or slightly higher than daily growth hormone.”
However, if improved adherence with LAGH is demonstrated and associated with better treatment outcomes, the price of LAGH will likely increase and use of daily growth hormone will decrease, he predicts.
Paul Saenger, MD, who was not involved with the research, believes “all three long-acting growth hormone drugs will eventually be approved for GHD in children.”
“The price will be the same or may be at most 10% more than daily growth hormone replacement,” Dr. Saenger, a pediatric endocrinologist and clinical assistant professor at NYU Long Island School of Medicine, New York, said in an email.
However, daily subcutaneous injections will still be warranted for certain children with GHD, Dr. Miller noted.
“Daily growth hormone may be better than LAGH for a small number of children who have severe GHD associated with hypoglycemia,” he said. “The low levels of growth hormone at the end of the weekly interval of LAGH may allow hypoglycemia to occur in this population.”
Phase 3 trial in 161 treatment-naive children with GHD
The heiGHt trial randomized treatment-naive prepubertal children with GHD 2:1 to weekly lonapegsomatropin or daily somatropin (Genotropin, Pfizer) at 73 sites in 15 countries.
The children were a mean age of 8.5 years (range, 3.2-13.1 years), 82% were boys, and 94% were White.
There were no reported serious adverse events or discontinuations related to lonapegsomatropin.
The most common adverse reactions in ≥5% of these pediatric patients were viral infection (15%), pyrexia (15%), cough (11%), nausea and vomiting (11%), hemorrhage (7%), diarrhea (6%), abdominal pain (6%), and arthralgia and arthritis (6%).
Both study groups reported low incidences of transient, non-neutralizing anti-hGH binding antibodies and no cases of persistent antibodies.
Trial limitations include the fact the study was not blinded (as patients received a weekly or daily injection) and drug doses were fixed at 0.24 mg human growth hormone/kg/week, although in real-world clinical practice, doses may be titrated.
Lonapegsomatropin has been studied in more than 300 children with GHD in the phase 3 program in the heiGHt trial (treatment-naive patients), fliGHt trial (treatment-experienced patients), and enliGHten trial (an ongoing long-term extension trial that includes some patients who have been taking lonapegsomatropin for more than 4 years).
The study was sponsored by Ascendis Pharma. Some of the phase 3 study authors are company employees.
A version of this article first appeared on Medscape.com.
A long look at long haulers
With the number of pediatric infections with SARS-CoV-2 rising it is not surprising that children with persistent symptoms are beginning to accumulate. Who are these pediatric “long haulers” and do they differ from their adult counterparts? The answer is far from clear because the terms “long COVID” and “long hauler” are not well defined. But, I suspect we will find that they will be similar in most respects.
In a recent Guest Essay in the New York Times, two medical school professors attempt to inject some common sense into the long hauler phenomenon. (“The Truth About Long Covid is Complicated. Better Treatment Isn’t,” Adam Gaffney and Zackary Berger, The New York Times, Aug. 18, 2021).
The authors divide the patients with long COVID into three categories. The first includes those who are complaining of persistent cough and fatigue for up to 3 months, a not unexpected course for patients recovering from a significant respiratory illness like pneumonia.
The second group comprises patients who developed acute respiratory distress syndrome during the course of their SARS-CoV-2 infection. These unfortunate individuals likely incurred lung damage that may have triggered renal damage and delirium and may never regain full function.
The third group of patients reports a wide variety of less specific symptoms including, but not limited to, severe fatigue, brain fog, shortness of breath, gastrointestinal symptoms, chronic pain, and palpitations.
The authors of the essay refer to several studies in which there was little if any correlation between these patients’ complaints and their antibody levels. In fact, one study of adolescents found that in a group with similar symptoms many of the individuals had no serologic evidence of SARS-CoV-2 infection.
Unfortunately, the lay public, the media, and some physicians make no distinction between these three groups and lump them all under the same long COVID umbrella. The resulting confusion seeds unwarranted anxiety among the first and third groups and may prevent some individuals from receiving the appropriate attention they deserve.
I suspect that like me, many of you see some similarities between this third group of long COVID patients and adolescents whose persistent symptoms don’t quite fit with their primary illness. Patients labeled as having post-concussion syndrome or “chronic Lyme disease” come immediately to mind. In both conditions, many of the patients had little if any evidence of severe insult from the initial event but continue to complain about a variety of symptoms including severe fatigue and brain fog.
We have done a very poor job of properly managing these patients. And there are a lot of them. A large part of the problem is labeling. In the old days one might have said these patients were having “psychosomatic” symptoms. But, while it may be an accurate description, like the term “retardation” it has been permanently tarnished. Fortunately, most of us are smart enough to avoid telling these patients that it is all in their heads.
However, convincing an individual that many of his symptoms may be the result of the psychological insult from the original disease compounded by other stresses and lifestyle factors can be a difficult sell. The task is made particularly difficult when there continue to be physicians who will miss or ignore the obvious and embark on therapeutic endeavors that are not only ineffective but can serve as a distraction from the real work of listening to and engaging these patients whose suffering may be just as real as that of those long haulers with structural damage.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
With the number of pediatric infections with SARS-CoV-2 rising it is not surprising that children with persistent symptoms are beginning to accumulate. Who are these pediatric “long haulers” and do they differ from their adult counterparts? The answer is far from clear because the terms “long COVID” and “long hauler” are not well defined. But, I suspect we will find that they will be similar in most respects.
In a recent Guest Essay in the New York Times, two medical school professors attempt to inject some common sense into the long hauler phenomenon. (“The Truth About Long Covid is Complicated. Better Treatment Isn’t,” Adam Gaffney and Zackary Berger, The New York Times, Aug. 18, 2021).
The authors divide the patients with long COVID into three categories. The first includes those who are complaining of persistent cough and fatigue for up to 3 months, a not unexpected course for patients recovering from a significant respiratory illness like pneumonia.
The second group comprises patients who developed acute respiratory distress syndrome during the course of their SARS-CoV-2 infection. These unfortunate individuals likely incurred lung damage that may have triggered renal damage and delirium and may never regain full function.
The third group of patients reports a wide variety of less specific symptoms including, but not limited to, severe fatigue, brain fog, shortness of breath, gastrointestinal symptoms, chronic pain, and palpitations.
The authors of the essay refer to several studies in which there was little if any correlation between these patients’ complaints and their antibody levels. In fact, one study of adolescents found that in a group with similar symptoms many of the individuals had no serologic evidence of SARS-CoV-2 infection.
Unfortunately, the lay public, the media, and some physicians make no distinction between these three groups and lump them all under the same long COVID umbrella. The resulting confusion seeds unwarranted anxiety among the first and third groups and may prevent some individuals from receiving the appropriate attention they deserve.
I suspect that like me, many of you see some similarities between this third group of long COVID patients and adolescents whose persistent symptoms don’t quite fit with their primary illness. Patients labeled as having post-concussion syndrome or “chronic Lyme disease” come immediately to mind. In both conditions, many of the patients had little if any evidence of severe insult from the initial event but continue to complain about a variety of symptoms including severe fatigue and brain fog.
We have done a very poor job of properly managing these patients. And there are a lot of them. A large part of the problem is labeling. In the old days one might have said these patients were having “psychosomatic” symptoms. But, while it may be an accurate description, like the term “retardation” it has been permanently tarnished. Fortunately, most of us are smart enough to avoid telling these patients that it is all in their heads.
However, convincing an individual that many of his symptoms may be the result of the psychological insult from the original disease compounded by other stresses and lifestyle factors can be a difficult sell. The task is made particularly difficult when there continue to be physicians who will miss or ignore the obvious and embark on therapeutic endeavors that are not only ineffective but can serve as a distraction from the real work of listening to and engaging these patients whose suffering may be just as real as that of those long haulers with structural damage.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
With the number of pediatric infections with SARS-CoV-2 rising it is not surprising that children with persistent symptoms are beginning to accumulate. Who are these pediatric “long haulers” and do they differ from their adult counterparts? The answer is far from clear because the terms “long COVID” and “long hauler” are not well defined. But, I suspect we will find that they will be similar in most respects.
In a recent Guest Essay in the New York Times, two medical school professors attempt to inject some common sense into the long hauler phenomenon. (“The Truth About Long Covid is Complicated. Better Treatment Isn’t,” Adam Gaffney and Zackary Berger, The New York Times, Aug. 18, 2021).
The authors divide the patients with long COVID into three categories. The first includes those who are complaining of persistent cough and fatigue for up to 3 months, a not unexpected course for patients recovering from a significant respiratory illness like pneumonia.
The second group comprises patients who developed acute respiratory distress syndrome during the course of their SARS-CoV-2 infection. These unfortunate individuals likely incurred lung damage that may have triggered renal damage and delirium and may never regain full function.
The third group of patients reports a wide variety of less specific symptoms including, but not limited to, severe fatigue, brain fog, shortness of breath, gastrointestinal symptoms, chronic pain, and palpitations.
The authors of the essay refer to several studies in which there was little if any correlation between these patients’ complaints and their antibody levels. In fact, one study of adolescents found that in a group with similar symptoms many of the individuals had no serologic evidence of SARS-CoV-2 infection.
Unfortunately, the lay public, the media, and some physicians make no distinction between these three groups and lump them all under the same long COVID umbrella. The resulting confusion seeds unwarranted anxiety among the first and third groups and may prevent some individuals from receiving the appropriate attention they deserve.
I suspect that like me, many of you see some similarities between this third group of long COVID patients and adolescents whose persistent symptoms don’t quite fit with their primary illness. Patients labeled as having post-concussion syndrome or “chronic Lyme disease” come immediately to mind. In both conditions, many of the patients had little if any evidence of severe insult from the initial event but continue to complain about a variety of symptoms including severe fatigue and brain fog.
We have done a very poor job of properly managing these patients. And there are a lot of them. A large part of the problem is labeling. In the old days one might have said these patients were having “psychosomatic” symptoms. But, while it may be an accurate description, like the term “retardation” it has been permanently tarnished. Fortunately, most of us are smart enough to avoid telling these patients that it is all in their heads.
However, convincing an individual that many of his symptoms may be the result of the psychological insult from the original disease compounded by other stresses and lifestyle factors can be a difficult sell. The task is made particularly difficult when there continue to be physicians who will miss or ignore the obvious and embark on therapeutic endeavors that are not only ineffective but can serve as a distraction from the real work of listening to and engaging these patients whose suffering may be just as real as that of those long haulers with structural damage.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Ask about itch and joint pain in pediatric psoriasis patients, expert advises
During the annual meeting of the Society for Pediatric Dermatology, Amy S. Paller, MD, MS, marveled on the remarkable advances in the treatment of inflammatory skin disorders during the past 2 decades.
“We’ve come a long way, from disease features being red, thick, and scaly and being treated with nonspecific therapy like topical steroids, keratolytics, and tar, to understanding disease pathogenesis and finding new targeted therapies for inflammatory skin disorders in children,” said Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago. “There are now studies moving forward with gene correction, gene replacement, the gene product replaced, or pathway inhibition to prevent the effects of genetic change.”
Technology is leading the way in generating new therapeutic advances, she continued, beyond traditional “omics” to lipidomics, metabolomics, glycomics, and kinomics. “This has enabled us to find new genetic disorders and their causes, to look at changes in gene expression patterns, and to look at changes in protein expression patterns that give us clues as to how to move forward with better therapy,” she said. “When we’re talking about new insights into pathogenesis-based therapy, we’re talking largely about understanding the pathways that lead to either inflammation or promoting cell proliferation and abnormal differentiation.”
Treating pediatric psoriasis
. “First of all, ask about itch and pain with these patients,” she advised. “Interviews have shown that 61% of children experience some itch, 39% have pain or stinging, and in the ixekizumab trials, 72% had what’s considered meaningful itch, with at least 4 out of 10 (mean intensity 5.3) on the itch numeric rating scale. Little is known about the itch associated with psoriasis and its underlying cause – unrelated to the IL-4/IL-13 pathway activation of atopic dermatitis – but it’s worth asking about. I find that itch of the scalp is especially a problem in psoriasis.”
Physicians should also ask pediatric psoriasis patients about joint pain, because about 1% of them have psoriatic arthritis, which is much less common than in adults, “but important to find and manage,” she added. Dr. Paller recommends the new R-JET rapid joint exam technique, which is accompanied by a three-question survey and body diagram that facilitates identification of true arthritis, “so you can know how quickly to refer”.
Several studies have described an increased risk of metabolic syndrome in adolescents with pediatric psoriasis and now in prepubertal children with the disease. In a recent study of 60 consecutive prepubertal children with psoriasis, 70% of whom had mild disease, 40% were overweight or obese, 53% had central obesity, 27% had high levels of the HOMA-IR (homeostasis model assessment of insulin resistance) despite generally normal levels of fasting glucose, and 30% met criteria for metabolic syndrome.
“This really struck me because our AAD [American Academy of Dermatology] guidelines did not recommend screening for type 2 diabetes in prepubertal children, even if overweight, because the risk is so small,” Dr. Paller said. “This report suggests that we may need to reconsider this recommendation in prepubertal children with psoriasis.”
Meanwhile, the number of medications approved by the Food and Drug Administration and the European Medicines Agency for children with psoriasis who are 6 years of age and above continues to expand, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-23 inhibitors, and IL-17 inhibitors. Most children can now achieve a PASI 90 within 12 weeks with the IL-23 inhibitor ustekinumab and the IL-17 inhibitors ixekizumab and secukinumab, Dr. Paller said.
In the ixekizumab trial, there are head-to-head comparison data in a European arm that involved the use of etanercept, she said. “What’s most noticeable is the significant difference in those who were able to achieve PASI 90 or above with this IL-17 inhibitor, versus etanercept,” which she added, raises the question of whether aiming for a PASI 75 is adequate, "or should we strive for PASI 90?” A pediatric psoriasis study published in 2020 found that the greatest improvement in quality of life was associated with a PASI 90 and use of systemic treatments.
Looking forward, phase 3 clinical trials are underway in pediatric patients with moderate to severe psoriasis for guselkumab, tildrakizumab, risankizumab, certolizumab, bimekizumab, and brodalumab. “The cost of all of these biologics is high, however. I remind everyone that we still have methotrexate,” she said. “The risk of side effects with our low-dose methotrexate treatment for psoriasis remains low, but methotrexate doesn’t hit these [high] PASI numbers and it’s much slower in its onset than biologics.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi.
Commentary by Robert Sidbury, MD, MPH
Dr. Paller reminds us of some essential features of pediatric psoriasis:
• It can hurt. Ask your patients if it does.
• It can itch. Look for excoriations, especially in the scalp.
• It is often associated with metabolic syndrome, so check relevant biometrics and labs, and consider coincident insulin resistance.
• Our traditional clinical trial target of PASI75, or a 75% reduction in body surface area involvement, is just not good enough. Studies have shown that the most meaningful quality-of-life gains come at PASI90 or above.
• With our newer biologics, such as IL-12/23 blockers (for instance, ustekinumab) and IL-17 blockers (for example, ixekizumab and secukinumab), PASI90 and better is a reasonable expectation, not a pipe dream.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
During the annual meeting of the Society for Pediatric Dermatology, Amy S. Paller, MD, MS, marveled on the remarkable advances in the treatment of inflammatory skin disorders during the past 2 decades.
“We’ve come a long way, from disease features being red, thick, and scaly and being treated with nonspecific therapy like topical steroids, keratolytics, and tar, to understanding disease pathogenesis and finding new targeted therapies for inflammatory skin disorders in children,” said Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago. “There are now studies moving forward with gene correction, gene replacement, the gene product replaced, or pathway inhibition to prevent the effects of genetic change.”
Technology is leading the way in generating new therapeutic advances, she continued, beyond traditional “omics” to lipidomics, metabolomics, glycomics, and kinomics. “This has enabled us to find new genetic disorders and their causes, to look at changes in gene expression patterns, and to look at changes in protein expression patterns that give us clues as to how to move forward with better therapy,” she said. “When we’re talking about new insights into pathogenesis-based therapy, we’re talking largely about understanding the pathways that lead to either inflammation or promoting cell proliferation and abnormal differentiation.”
Treating pediatric psoriasis
. “First of all, ask about itch and pain with these patients,” she advised. “Interviews have shown that 61% of children experience some itch, 39% have pain or stinging, and in the ixekizumab trials, 72% had what’s considered meaningful itch, with at least 4 out of 10 (mean intensity 5.3) on the itch numeric rating scale. Little is known about the itch associated with psoriasis and its underlying cause – unrelated to the IL-4/IL-13 pathway activation of atopic dermatitis – but it’s worth asking about. I find that itch of the scalp is especially a problem in psoriasis.”
Physicians should also ask pediatric psoriasis patients about joint pain, because about 1% of them have psoriatic arthritis, which is much less common than in adults, “but important to find and manage,” she added. Dr. Paller recommends the new R-JET rapid joint exam technique, which is accompanied by a three-question survey and body diagram that facilitates identification of true arthritis, “so you can know how quickly to refer”.
Several studies have described an increased risk of metabolic syndrome in adolescents with pediatric psoriasis and now in prepubertal children with the disease. In a recent study of 60 consecutive prepubertal children with psoriasis, 70% of whom had mild disease, 40% were overweight or obese, 53% had central obesity, 27% had high levels of the HOMA-IR (homeostasis model assessment of insulin resistance) despite generally normal levels of fasting glucose, and 30% met criteria for metabolic syndrome.
“This really struck me because our AAD [American Academy of Dermatology] guidelines did not recommend screening for type 2 diabetes in prepubertal children, even if overweight, because the risk is so small,” Dr. Paller said. “This report suggests that we may need to reconsider this recommendation in prepubertal children with psoriasis.”
Meanwhile, the number of medications approved by the Food and Drug Administration and the European Medicines Agency for children with psoriasis who are 6 years of age and above continues to expand, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-23 inhibitors, and IL-17 inhibitors. Most children can now achieve a PASI 90 within 12 weeks with the IL-23 inhibitor ustekinumab and the IL-17 inhibitors ixekizumab and secukinumab, Dr. Paller said.
In the ixekizumab trial, there are head-to-head comparison data in a European arm that involved the use of etanercept, she said. “What’s most noticeable is the significant difference in those who were able to achieve PASI 90 or above with this IL-17 inhibitor, versus etanercept,” which she added, raises the question of whether aiming for a PASI 75 is adequate, "or should we strive for PASI 90?” A pediatric psoriasis study published in 2020 found that the greatest improvement in quality of life was associated with a PASI 90 and use of systemic treatments.
Looking forward, phase 3 clinical trials are underway in pediatric patients with moderate to severe psoriasis for guselkumab, tildrakizumab, risankizumab, certolizumab, bimekizumab, and brodalumab. “The cost of all of these biologics is high, however. I remind everyone that we still have methotrexate,” she said. “The risk of side effects with our low-dose methotrexate treatment for psoriasis remains low, but methotrexate doesn’t hit these [high] PASI numbers and it’s much slower in its onset than biologics.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi.
Commentary by Robert Sidbury, MD, MPH
Dr. Paller reminds us of some essential features of pediatric psoriasis:
• It can hurt. Ask your patients if it does.
• It can itch. Look for excoriations, especially in the scalp.
• It is often associated with metabolic syndrome, so check relevant biometrics and labs, and consider coincident insulin resistance.
• Our traditional clinical trial target of PASI75, or a 75% reduction in body surface area involvement, is just not good enough. Studies have shown that the most meaningful quality-of-life gains come at PASI90 or above.
• With our newer biologics, such as IL-12/23 blockers (for instance, ustekinumab) and IL-17 blockers (for example, ixekizumab and secukinumab), PASI90 and better is a reasonable expectation, not a pipe dream.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
During the annual meeting of the Society for Pediatric Dermatology, Amy S. Paller, MD, MS, marveled on the remarkable advances in the treatment of inflammatory skin disorders during the past 2 decades.
“We’ve come a long way, from disease features being red, thick, and scaly and being treated with nonspecific therapy like topical steroids, keratolytics, and tar, to understanding disease pathogenesis and finding new targeted therapies for inflammatory skin disorders in children,” said Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago. “There are now studies moving forward with gene correction, gene replacement, the gene product replaced, or pathway inhibition to prevent the effects of genetic change.”
Technology is leading the way in generating new therapeutic advances, she continued, beyond traditional “omics” to lipidomics, metabolomics, glycomics, and kinomics. “This has enabled us to find new genetic disorders and their causes, to look at changes in gene expression patterns, and to look at changes in protein expression patterns that give us clues as to how to move forward with better therapy,” she said. “When we’re talking about new insights into pathogenesis-based therapy, we’re talking largely about understanding the pathways that lead to either inflammation or promoting cell proliferation and abnormal differentiation.”
Treating pediatric psoriasis
. “First of all, ask about itch and pain with these patients,” she advised. “Interviews have shown that 61% of children experience some itch, 39% have pain or stinging, and in the ixekizumab trials, 72% had what’s considered meaningful itch, with at least 4 out of 10 (mean intensity 5.3) on the itch numeric rating scale. Little is known about the itch associated with psoriasis and its underlying cause – unrelated to the IL-4/IL-13 pathway activation of atopic dermatitis – but it’s worth asking about. I find that itch of the scalp is especially a problem in psoriasis.”
Physicians should also ask pediatric psoriasis patients about joint pain, because about 1% of them have psoriatic arthritis, which is much less common than in adults, “but important to find and manage,” she added. Dr. Paller recommends the new R-JET rapid joint exam technique, which is accompanied by a three-question survey and body diagram that facilitates identification of true arthritis, “so you can know how quickly to refer”.
Several studies have described an increased risk of metabolic syndrome in adolescents with pediatric psoriasis and now in prepubertal children with the disease. In a recent study of 60 consecutive prepubertal children with psoriasis, 70% of whom had mild disease, 40% were overweight or obese, 53% had central obesity, 27% had high levels of the HOMA-IR (homeostasis model assessment of insulin resistance) despite generally normal levels of fasting glucose, and 30% met criteria for metabolic syndrome.
“This really struck me because our AAD [American Academy of Dermatology] guidelines did not recommend screening for type 2 diabetes in prepubertal children, even if overweight, because the risk is so small,” Dr. Paller said. “This report suggests that we may need to reconsider this recommendation in prepubertal children with psoriasis.”
Meanwhile, the number of medications approved by the Food and Drug Administration and the European Medicines Agency for children with psoriasis who are 6 years of age and above continues to expand, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-23 inhibitors, and IL-17 inhibitors. Most children can now achieve a PASI 90 within 12 weeks with the IL-23 inhibitor ustekinumab and the IL-17 inhibitors ixekizumab and secukinumab, Dr. Paller said.
In the ixekizumab trial, there are head-to-head comparison data in a European arm that involved the use of etanercept, she said. “What’s most noticeable is the significant difference in those who were able to achieve PASI 90 or above with this IL-17 inhibitor, versus etanercept,” which she added, raises the question of whether aiming for a PASI 75 is adequate, "or should we strive for PASI 90?” A pediatric psoriasis study published in 2020 found that the greatest improvement in quality of life was associated with a PASI 90 and use of systemic treatments.
Looking forward, phase 3 clinical trials are underway in pediatric patients with moderate to severe psoriasis for guselkumab, tildrakizumab, risankizumab, certolizumab, bimekizumab, and brodalumab. “The cost of all of these biologics is high, however. I remind everyone that we still have methotrexate,” she said. “The risk of side effects with our low-dose methotrexate treatment for psoriasis remains low, but methotrexate doesn’t hit these [high] PASI numbers and it’s much slower in its onset than biologics.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi.
Commentary by Robert Sidbury, MD, MPH
Dr. Paller reminds us of some essential features of pediatric psoriasis:
• It can hurt. Ask your patients if it does.
• It can itch. Look for excoriations, especially in the scalp.
• It is often associated with metabolic syndrome, so check relevant biometrics and labs, and consider coincident insulin resistance.
• Our traditional clinical trial target of PASI75, or a 75% reduction in body surface area involvement, is just not good enough. Studies have shown that the most meaningful quality-of-life gains come at PASI90 or above.
• With our newer biologics, such as IL-12/23 blockers (for instance, ustekinumab) and IL-17 blockers (for example, ixekizumab and secukinumab), PASI90 and better is a reasonable expectation, not a pipe dream.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
FROM SPD 2021
COVID-19 linked to baby bust in high-income countries
In an assessment of the pandemic’s early effects, Arnstein Aassve, PhD, and colleagues found a significant COVID-19–related decline in crude birth rates (CBRs) in 7 of 22 high-income countries, particularly in Southwestern Europe.
Dr. Aassve, an economist at the Carlo F. Dondena Center for Research on Social Dynamics and Public Policy at the Università Commerciale Luigi Bocconi, Milan, and colleagues report the results in an article published online August 30 in the Proceedings of the National Academy of Sciences.
Defining the start of the COVID-19 pandemic as February 2020, the study identifies strong declines in Italy (-9.1%), Hungary (-8.5%), Spain (-8.4%), and Portugal (-6.6%) beyond those predicted by past trends. In the United States, CBRs fell by 7.1% relative to 2019 for births occurring in Nov. and Dec. 2020 following conceptions in February and March of that year.
Significant declines in CBR also occurred in Belgium, Austria, and Singapore.
A year-to-year comparison of the mean for monthly CBRs per 1,000 population before and during the pandemic suggests a negative difference for all countries studied except for Denmark, Finland, Germany, and the Netherlands, Dr. Aassve and colleagues write. These findings may have policy implications for childcare, housing, and the labor market.
The Milan researchers compared monthly vital statistics data on live births from the international Human Fertility Database for the period of Jan. 2016 to March 2021. These figures reflect conceptions carried to term between April 2015 and June 2020. The 22 countries in the analysis represent 37% of the total reported COVID-19 cases and 34% of deaths worldwide.
The study findings align with surveys on “fertility intentions” collected early in the first COVID-19 wave in Germany, France, Spain, and the United Kingdom. These surveys indicated that 73% of people who were planning pregnancies in 2020 either decided to delay the pregnancy or they abandoned their plans.
“The popular media speculated that the lockdown would lead to a baby boom, as couples spent more time together,” Dr. Aassve told this news organization. “There’s very little evidence of this when you look to previous disasters and shocks, and the first data suggest more of an immediate collapse than a boom. But as you also see from the paper, the collapse is not seen everywhere.” Other current studies suggest the fertility drop is immediate but temporary, says Dr. Aassve, who is also a professor of demography.
Interestingly, Dr. Aassve and colleagues found that CBRs were relatively stable in Northern Europe. The authors point to supportive social and family policies in that region that might have reduced the effect of the pandemic on births. “These factors are likely to affect CBRs in the subsequent pandemic waves,” they write. They call for future studies to assess the full population implications of the pandemic, the moderating impact of policy interventions, and the nexus between short- and long-run effects in relation to the various waves of the COVID-19 pandemic.
Rebounds
Some regions have already reported a rebound from the COVID-19 fertility trough. Molly J. Stout, MD, director of maternal fetal medicine at the University of Michigan, Ann Arbor, and colleagues used electronic medical records to predict a surge in births after the initial decline.
“The surge we’ve seen at the end of this summer is exceeding the usual annual birth rate, as predicted,” she said in an interview. “But I think there’ll be a return to normal after this transient escalation. I don’t think birth rates will stay elevated above the normal because the birth surge is a temporary response to an event, although there will likely be regional differences.”
Looking ahead, Dr. Stout, who was not involved in Dr. Aassve’s analysis, is not certain how a fourth pandemic wave might ultimately modify a couple’s overall family size. But the toll the health crisis has taken on working women who have been forced to withdraw from the economy because of a lack of childcare points to a societal need that should be addressed.
According to Philip N. Cohen, PhD, a professor of sociology at the University of Maryland, College Park, who’s been tracking fertility trends since the onset of the COVID-19 emergency, the pandemic has combined a health crisis with an economic crisis, along with “the additional factor of social distancing and isolation, which all contributed to the decline in birth rates. Some people changed their plans to hold off on having children, while others didn’t get pregnant because they weren’t socializing and meeting people as much.”
Dr. Cohen, who was not involved in the study by Dr. Aassve and associates, said his provisional data show that although in many places, birth rates have rebounded more or less to prepandemic levels after a nadir around Jan. 2021, some areas of the United States still show substantially lower rates, including California, Hawaii, and Oregon.
As to the duration of the pandemic effect, Dr. Aassve cautions that his group’s estimates refer to the first wave only. “We then have the second, third, and currently the fourth wave. We can’t be sure about the impact of these waves on fertility since the data are not there yet, but I’d be surprised if they didn’t continue to have an impact on fertility rates,” he said.
Dr. Cohen agreed: “Some people who delayed childbearing will make up the delay. However, whenever there’s a delay, there’s inevitably some portion of the decline that’s not recouped.”
As for the wider effect across the world, Dr. Aassve said his team’s figures derive from high-income countries where data are readily available. For middle- and low-income countries, fewer data exist, and the quality of those data is not as good.
The lessons from this and other upheavals teach us that unforeseen shocks almost always have a negative impact on fertility, says Dr. Aassve. “[B]ut these effects may be separate from existing declining trends. The issue here is that those overall declining trends may be driven by other factors. In contrast, the shock of the pandemic is short-lived, and we may return to normal rather quickly. But if the pandemic also impacts other societal structures, such as the occupational and industrial sectors, then the pandemic might exacerbate the negative trend.”
The study was supported by funding from the European Research Council for funding under the European Union’s Horizon 2020 Research and Innovation Programme. The study authors, Dr. Stout, and Dr. Cohen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an assessment of the pandemic’s early effects, Arnstein Aassve, PhD, and colleagues found a significant COVID-19–related decline in crude birth rates (CBRs) in 7 of 22 high-income countries, particularly in Southwestern Europe.
Dr. Aassve, an economist at the Carlo F. Dondena Center for Research on Social Dynamics and Public Policy at the Università Commerciale Luigi Bocconi, Milan, and colleagues report the results in an article published online August 30 in the Proceedings of the National Academy of Sciences.
Defining the start of the COVID-19 pandemic as February 2020, the study identifies strong declines in Italy (-9.1%), Hungary (-8.5%), Spain (-8.4%), and Portugal (-6.6%) beyond those predicted by past trends. In the United States, CBRs fell by 7.1% relative to 2019 for births occurring in Nov. and Dec. 2020 following conceptions in February and March of that year.
Significant declines in CBR also occurred in Belgium, Austria, and Singapore.
A year-to-year comparison of the mean for monthly CBRs per 1,000 population before and during the pandemic suggests a negative difference for all countries studied except for Denmark, Finland, Germany, and the Netherlands, Dr. Aassve and colleagues write. These findings may have policy implications for childcare, housing, and the labor market.
The Milan researchers compared monthly vital statistics data on live births from the international Human Fertility Database for the period of Jan. 2016 to March 2021. These figures reflect conceptions carried to term between April 2015 and June 2020. The 22 countries in the analysis represent 37% of the total reported COVID-19 cases and 34% of deaths worldwide.
The study findings align with surveys on “fertility intentions” collected early in the first COVID-19 wave in Germany, France, Spain, and the United Kingdom. These surveys indicated that 73% of people who were planning pregnancies in 2020 either decided to delay the pregnancy or they abandoned their plans.
“The popular media speculated that the lockdown would lead to a baby boom, as couples spent more time together,” Dr. Aassve told this news organization. “There’s very little evidence of this when you look to previous disasters and shocks, and the first data suggest more of an immediate collapse than a boom. But as you also see from the paper, the collapse is not seen everywhere.” Other current studies suggest the fertility drop is immediate but temporary, says Dr. Aassve, who is also a professor of demography.
Interestingly, Dr. Aassve and colleagues found that CBRs were relatively stable in Northern Europe. The authors point to supportive social and family policies in that region that might have reduced the effect of the pandemic on births. “These factors are likely to affect CBRs in the subsequent pandemic waves,” they write. They call for future studies to assess the full population implications of the pandemic, the moderating impact of policy interventions, and the nexus between short- and long-run effects in relation to the various waves of the COVID-19 pandemic.
Rebounds
Some regions have already reported a rebound from the COVID-19 fertility trough. Molly J. Stout, MD, director of maternal fetal medicine at the University of Michigan, Ann Arbor, and colleagues used electronic medical records to predict a surge in births after the initial decline.
“The surge we’ve seen at the end of this summer is exceeding the usual annual birth rate, as predicted,” she said in an interview. “But I think there’ll be a return to normal after this transient escalation. I don’t think birth rates will stay elevated above the normal because the birth surge is a temporary response to an event, although there will likely be regional differences.”
Looking ahead, Dr. Stout, who was not involved in Dr. Aassve’s analysis, is not certain how a fourth pandemic wave might ultimately modify a couple’s overall family size. But the toll the health crisis has taken on working women who have been forced to withdraw from the economy because of a lack of childcare points to a societal need that should be addressed.
According to Philip N. Cohen, PhD, a professor of sociology at the University of Maryland, College Park, who’s been tracking fertility trends since the onset of the COVID-19 emergency, the pandemic has combined a health crisis with an economic crisis, along with “the additional factor of social distancing and isolation, which all contributed to the decline in birth rates. Some people changed their plans to hold off on having children, while others didn’t get pregnant because they weren’t socializing and meeting people as much.”
Dr. Cohen, who was not involved in the study by Dr. Aassve and associates, said his provisional data show that although in many places, birth rates have rebounded more or less to prepandemic levels after a nadir around Jan. 2021, some areas of the United States still show substantially lower rates, including California, Hawaii, and Oregon.
As to the duration of the pandemic effect, Dr. Aassve cautions that his group’s estimates refer to the first wave only. “We then have the second, third, and currently the fourth wave. We can’t be sure about the impact of these waves on fertility since the data are not there yet, but I’d be surprised if they didn’t continue to have an impact on fertility rates,” he said.
Dr. Cohen agreed: “Some people who delayed childbearing will make up the delay. However, whenever there’s a delay, there’s inevitably some portion of the decline that’s not recouped.”
As for the wider effect across the world, Dr. Aassve said his team’s figures derive from high-income countries where data are readily available. For middle- and low-income countries, fewer data exist, and the quality of those data is not as good.
The lessons from this and other upheavals teach us that unforeseen shocks almost always have a negative impact on fertility, says Dr. Aassve. “[B]ut these effects may be separate from existing declining trends. The issue here is that those overall declining trends may be driven by other factors. In contrast, the shock of the pandemic is short-lived, and we may return to normal rather quickly. But if the pandemic also impacts other societal structures, such as the occupational and industrial sectors, then the pandemic might exacerbate the negative trend.”
The study was supported by funding from the European Research Council for funding under the European Union’s Horizon 2020 Research and Innovation Programme. The study authors, Dr. Stout, and Dr. Cohen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an assessment of the pandemic’s early effects, Arnstein Aassve, PhD, and colleagues found a significant COVID-19–related decline in crude birth rates (CBRs) in 7 of 22 high-income countries, particularly in Southwestern Europe.
Dr. Aassve, an economist at the Carlo F. Dondena Center for Research on Social Dynamics and Public Policy at the Università Commerciale Luigi Bocconi, Milan, and colleagues report the results in an article published online August 30 in the Proceedings of the National Academy of Sciences.
Defining the start of the COVID-19 pandemic as February 2020, the study identifies strong declines in Italy (-9.1%), Hungary (-8.5%), Spain (-8.4%), and Portugal (-6.6%) beyond those predicted by past trends. In the United States, CBRs fell by 7.1% relative to 2019 for births occurring in Nov. and Dec. 2020 following conceptions in February and March of that year.
Significant declines in CBR also occurred in Belgium, Austria, and Singapore.
A year-to-year comparison of the mean for monthly CBRs per 1,000 population before and during the pandemic suggests a negative difference for all countries studied except for Denmark, Finland, Germany, and the Netherlands, Dr. Aassve and colleagues write. These findings may have policy implications for childcare, housing, and the labor market.
The Milan researchers compared monthly vital statistics data on live births from the international Human Fertility Database for the period of Jan. 2016 to March 2021. These figures reflect conceptions carried to term between April 2015 and June 2020. The 22 countries in the analysis represent 37% of the total reported COVID-19 cases and 34% of deaths worldwide.
The study findings align with surveys on “fertility intentions” collected early in the first COVID-19 wave in Germany, France, Spain, and the United Kingdom. These surveys indicated that 73% of people who were planning pregnancies in 2020 either decided to delay the pregnancy or they abandoned their plans.
“The popular media speculated that the lockdown would lead to a baby boom, as couples spent more time together,” Dr. Aassve told this news organization. “There’s very little evidence of this when you look to previous disasters and shocks, and the first data suggest more of an immediate collapse than a boom. But as you also see from the paper, the collapse is not seen everywhere.” Other current studies suggest the fertility drop is immediate but temporary, says Dr. Aassve, who is also a professor of demography.
Interestingly, Dr. Aassve and colleagues found that CBRs were relatively stable in Northern Europe. The authors point to supportive social and family policies in that region that might have reduced the effect of the pandemic on births. “These factors are likely to affect CBRs in the subsequent pandemic waves,” they write. They call for future studies to assess the full population implications of the pandemic, the moderating impact of policy interventions, and the nexus between short- and long-run effects in relation to the various waves of the COVID-19 pandemic.
Rebounds
Some regions have already reported a rebound from the COVID-19 fertility trough. Molly J. Stout, MD, director of maternal fetal medicine at the University of Michigan, Ann Arbor, and colleagues used electronic medical records to predict a surge in births after the initial decline.
“The surge we’ve seen at the end of this summer is exceeding the usual annual birth rate, as predicted,” she said in an interview. “But I think there’ll be a return to normal after this transient escalation. I don’t think birth rates will stay elevated above the normal because the birth surge is a temporary response to an event, although there will likely be regional differences.”
Looking ahead, Dr. Stout, who was not involved in Dr. Aassve’s analysis, is not certain how a fourth pandemic wave might ultimately modify a couple’s overall family size. But the toll the health crisis has taken on working women who have been forced to withdraw from the economy because of a lack of childcare points to a societal need that should be addressed.
According to Philip N. Cohen, PhD, a professor of sociology at the University of Maryland, College Park, who’s been tracking fertility trends since the onset of the COVID-19 emergency, the pandemic has combined a health crisis with an economic crisis, along with “the additional factor of social distancing and isolation, which all contributed to the decline in birth rates. Some people changed their plans to hold off on having children, while others didn’t get pregnant because they weren’t socializing and meeting people as much.”
Dr. Cohen, who was not involved in the study by Dr. Aassve and associates, said his provisional data show that although in many places, birth rates have rebounded more or less to prepandemic levels after a nadir around Jan. 2021, some areas of the United States still show substantially lower rates, including California, Hawaii, and Oregon.
As to the duration of the pandemic effect, Dr. Aassve cautions that his group’s estimates refer to the first wave only. “We then have the second, third, and currently the fourth wave. We can’t be sure about the impact of these waves on fertility since the data are not there yet, but I’d be surprised if they didn’t continue to have an impact on fertility rates,” he said.
Dr. Cohen agreed: “Some people who delayed childbearing will make up the delay. However, whenever there’s a delay, there’s inevitably some portion of the decline that’s not recouped.”
As for the wider effect across the world, Dr. Aassve said his team’s figures derive from high-income countries where data are readily available. For middle- and low-income countries, fewer data exist, and the quality of those data is not as good.
The lessons from this and other upheavals teach us that unforeseen shocks almost always have a negative impact on fertility, says Dr. Aassve. “[B]ut these effects may be separate from existing declining trends. The issue here is that those overall declining trends may be driven by other factors. In contrast, the shock of the pandemic is short-lived, and we may return to normal rather quickly. But if the pandemic also impacts other societal structures, such as the occupational and industrial sectors, then the pandemic might exacerbate the negative trend.”
The study was supported by funding from the European Research Council for funding under the European Union’s Horizon 2020 Research and Innovation Programme. The study authors, Dr. Stout, and Dr. Cohen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three JAK inhibitors get boxed warnings, modified indications
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
Bystander rescue breathing CPR in kids tied to better survival
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Office-based pediatricians unprepared for emergencies
Emergency preparedness in U.S. pediatric offices is variable and less than ideal, especially in smaller independent practices, a 15-month multicenter study has found.
Researchers led by Kamal Abulebda, MD, associate professor of clinical pediatrics in the division of pediatric critical care medicine at Indiana University and Riley Hospital for Children in Indianapolis, report that adherence to the 2007 policy statement of the American Academy of Pediatrics on emergency preparedness in pediatric primary care offices was suboptimal across 42 offices in 9 states. They suggest that academic and community partnerships use in-situ simulation exercises to address preparedness gaps and implement standard procedures for contacting emergency medical services.
The group’s findings were published online in Pediatrics. “These data can be used to guide the development of interventions to improve emergency preparedness and care delivery in pediatric offices, Dr. Abulebda and coauthors wrote, noting that theirs is the first multicenter study to directly measure preparedness and quality of care in pediatric offices.
According to the authors, the incidence of a child’s requiring emergent stabilization in an individual office ranges from weekly to monthly, with seizures and respiratory distress being the most common events.
The study was conducted from 2018 to 2020 by 48 national teams participating in in-situ simulated sessions in the ambulatory setting. Office teams, recruited from practices by members of regional academic medical centers, included two patients – a child with respiratory distress and a child with a seizure. Almost 40% were from Indiana.
The scenarios and checklists for the mock exercises were created by content experts in pediatric emergency medicine and critical care using evidence-based guidelines and best practices.
Previous research has relied on self-reported surveys rather than direct measurement to assess adherence to the AAP guidelines, the authors say. In-person surveys assessed adherence to AAP recommendations for emergency preparedness. In-person surveys were, however, used to gauge adherence to AAP recommendations for emergency preparedness.
Findings
The overall mean emergency preparedness score was 74.7% (standard deviation [SD] 12.9), with an unweighted percentage of adherence to checklists calculated for each case. By emergency type, the median asthma case performance score was 63.6% (interquartile range [IQR] 43.2-81.2), and the median seizure case score was 69.2% (IQR 46.2-80.8).
On the measure of essential equipment and supplies, the mean subscore (relating to availability of such items as oxygen sources, suction devices, and epinephrine, for example) was 82.2% (SD 15.1).
As for recommended policies and protocols (e.g., regular assessment of the office, maintenance of emergency equipment and medications) the mean subscore fell to 57.1% (SD 25.6).
In multivariable analyses, offices with a standardized procedure for contacting EMS had a higher rate of activating that service during the simulations.
Independent practices and smaller total staff size were associated with lower preparedness compared with larger groups: beta = –11.89, 95% confidence interval [CI], 19.33-4.45).
Higher annual patient volume and larger total staff size were slightly associated with higher scores (beta = .001, 95% CI, .00-001, P = .017; and beta = .51, 95% CI, .19-.83, P = .002, respectively).
Affiliation with an academic medical center and the presence of learners were not associated with higher scores. And in multivariable regression, a higher annual patient volume lost its significant association with greater preparedness.
So why the lag in preparedness despite the long-standing AAP recommendations? “It’s most likely due to the rare occurrence of these emergencies in the office setting, in addition to most offices’ dependence on EMS when they encounter pediatric emergencies in their setting,” Dr. Abulebda said in an interview. “A 2018 study published by Yuknis and associates demonstrated that the average time from EMS notification to arrival on scene was just 6 minutes.”
In other study findings, 82% of offices did not have an infant bag valve mask and would therefore need to wait for EMS to administer lifesaving ventilation. “This highlights the need to have this equipment available and maintain the skills necessary to care for patients in respiratory distress, the most common emergency encountered in the office setting,” Dr. Abulebda and associates wrote.
A cardiac arrest board is another example of a potentially lifesaving piece of equipment that was not available in the majority of offices, likely because of the rarity of this event in the office setting, but lack of this item may result in poor cardiopulmonary resuscitation quality before the arrival of EMS.
In an accompanying editorial, Jesse Hackell, MD, a pediatrician at Boston Children’s Health Physicians and New York Medical College in Pomona, N.Y., noted that data from 2 decades ago suggested that many pediatric offices saw multiple children requiring emergency intervention each week. More recent figures, however, indicate the situation has evolved, with fewer than 1% of current pediatric EMS transports originating from the office setting.
Dr. Hackell agrees that implementation of AAP recommendations has been far from universal and cites the cost of equipment and supplies as well as a lack of access to training and evaluation as significant barriers to implementation. “In addition, the infrequent occurrence of these emergencies makes maintenance of resuscitation skills even more difficult without frequent practice,” he wrote.
Further complicating the issue, preparedness needs vary with practice location, the response time of local EMS, and proximity to an emergency department. “Pediatric offices in more rural areas, which are farther from these services, will require more equipment and more skills to provide optimal emergency care to children living in these underresourced areas,” he wrote.
He called for equitable distribution of preparedness training, equipment, and staffing, with guidance designed to meet patient needs and ensure optimal outcomes. “In discussion of recommendations, one should consider the likely conditions requiring this response, availability of resources beyond the pediatric office, and ongoing training and support needed to maintain provider skills at the level needed for a successful response to any pediatric emergency,” Dr. Hackell wrote.
This study was supported by grants from Indiana University Health Values and the RBaby Foundation. One study coauthor is a board observer of a medical device company. No other authors disclosed financial relationships relevant to this work. Dr. Hackell has disclosed having no competing interests.
Emergency preparedness in U.S. pediatric offices is variable and less than ideal, especially in smaller independent practices, a 15-month multicenter study has found.
Researchers led by Kamal Abulebda, MD, associate professor of clinical pediatrics in the division of pediatric critical care medicine at Indiana University and Riley Hospital for Children in Indianapolis, report that adherence to the 2007 policy statement of the American Academy of Pediatrics on emergency preparedness in pediatric primary care offices was suboptimal across 42 offices in 9 states. They suggest that academic and community partnerships use in-situ simulation exercises to address preparedness gaps and implement standard procedures for contacting emergency medical services.
The group’s findings were published online in Pediatrics. “These data can be used to guide the development of interventions to improve emergency preparedness and care delivery in pediatric offices, Dr. Abulebda and coauthors wrote, noting that theirs is the first multicenter study to directly measure preparedness and quality of care in pediatric offices.
According to the authors, the incidence of a child’s requiring emergent stabilization in an individual office ranges from weekly to monthly, with seizures and respiratory distress being the most common events.
The study was conducted from 2018 to 2020 by 48 national teams participating in in-situ simulated sessions in the ambulatory setting. Office teams, recruited from practices by members of regional academic medical centers, included two patients – a child with respiratory distress and a child with a seizure. Almost 40% were from Indiana.
The scenarios and checklists for the mock exercises were created by content experts in pediatric emergency medicine and critical care using evidence-based guidelines and best practices.
Previous research has relied on self-reported surveys rather than direct measurement to assess adherence to the AAP guidelines, the authors say. In-person surveys assessed adherence to AAP recommendations for emergency preparedness. In-person surveys were, however, used to gauge adherence to AAP recommendations for emergency preparedness.
Findings
The overall mean emergency preparedness score was 74.7% (standard deviation [SD] 12.9), with an unweighted percentage of adherence to checklists calculated for each case. By emergency type, the median asthma case performance score was 63.6% (interquartile range [IQR] 43.2-81.2), and the median seizure case score was 69.2% (IQR 46.2-80.8).
On the measure of essential equipment and supplies, the mean subscore (relating to availability of such items as oxygen sources, suction devices, and epinephrine, for example) was 82.2% (SD 15.1).
As for recommended policies and protocols (e.g., regular assessment of the office, maintenance of emergency equipment and medications) the mean subscore fell to 57.1% (SD 25.6).
In multivariable analyses, offices with a standardized procedure for contacting EMS had a higher rate of activating that service during the simulations.
Independent practices and smaller total staff size were associated with lower preparedness compared with larger groups: beta = –11.89, 95% confidence interval [CI], 19.33-4.45).
Higher annual patient volume and larger total staff size were slightly associated with higher scores (beta = .001, 95% CI, .00-001, P = .017; and beta = .51, 95% CI, .19-.83, P = .002, respectively).
Affiliation with an academic medical center and the presence of learners were not associated with higher scores. And in multivariable regression, a higher annual patient volume lost its significant association with greater preparedness.
So why the lag in preparedness despite the long-standing AAP recommendations? “It’s most likely due to the rare occurrence of these emergencies in the office setting, in addition to most offices’ dependence on EMS when they encounter pediatric emergencies in their setting,” Dr. Abulebda said in an interview. “A 2018 study published by Yuknis and associates demonstrated that the average time from EMS notification to arrival on scene was just 6 minutes.”
In other study findings, 82% of offices did not have an infant bag valve mask and would therefore need to wait for EMS to administer lifesaving ventilation. “This highlights the need to have this equipment available and maintain the skills necessary to care for patients in respiratory distress, the most common emergency encountered in the office setting,” Dr. Abulebda and associates wrote.
A cardiac arrest board is another example of a potentially lifesaving piece of equipment that was not available in the majority of offices, likely because of the rarity of this event in the office setting, but lack of this item may result in poor cardiopulmonary resuscitation quality before the arrival of EMS.
In an accompanying editorial, Jesse Hackell, MD, a pediatrician at Boston Children’s Health Physicians and New York Medical College in Pomona, N.Y., noted that data from 2 decades ago suggested that many pediatric offices saw multiple children requiring emergency intervention each week. More recent figures, however, indicate the situation has evolved, with fewer than 1% of current pediatric EMS transports originating from the office setting.
Dr. Hackell agrees that implementation of AAP recommendations has been far from universal and cites the cost of equipment and supplies as well as a lack of access to training and evaluation as significant barriers to implementation. “In addition, the infrequent occurrence of these emergencies makes maintenance of resuscitation skills even more difficult without frequent practice,” he wrote.
Further complicating the issue, preparedness needs vary with practice location, the response time of local EMS, and proximity to an emergency department. “Pediatric offices in more rural areas, which are farther from these services, will require more equipment and more skills to provide optimal emergency care to children living in these underresourced areas,” he wrote.
He called for equitable distribution of preparedness training, equipment, and staffing, with guidance designed to meet patient needs and ensure optimal outcomes. “In discussion of recommendations, one should consider the likely conditions requiring this response, availability of resources beyond the pediatric office, and ongoing training and support needed to maintain provider skills at the level needed for a successful response to any pediatric emergency,” Dr. Hackell wrote.
This study was supported by grants from Indiana University Health Values and the RBaby Foundation. One study coauthor is a board observer of a medical device company. No other authors disclosed financial relationships relevant to this work. Dr. Hackell has disclosed having no competing interests.
Emergency preparedness in U.S. pediatric offices is variable and less than ideal, especially in smaller independent practices, a 15-month multicenter study has found.
Researchers led by Kamal Abulebda, MD, associate professor of clinical pediatrics in the division of pediatric critical care medicine at Indiana University and Riley Hospital for Children in Indianapolis, report that adherence to the 2007 policy statement of the American Academy of Pediatrics on emergency preparedness in pediatric primary care offices was suboptimal across 42 offices in 9 states. They suggest that academic and community partnerships use in-situ simulation exercises to address preparedness gaps and implement standard procedures for contacting emergency medical services.
The group’s findings were published online in Pediatrics. “These data can be used to guide the development of interventions to improve emergency preparedness and care delivery in pediatric offices, Dr. Abulebda and coauthors wrote, noting that theirs is the first multicenter study to directly measure preparedness and quality of care in pediatric offices.
According to the authors, the incidence of a child’s requiring emergent stabilization in an individual office ranges from weekly to monthly, with seizures and respiratory distress being the most common events.
The study was conducted from 2018 to 2020 by 48 national teams participating in in-situ simulated sessions in the ambulatory setting. Office teams, recruited from practices by members of regional academic medical centers, included two patients – a child with respiratory distress and a child with a seizure. Almost 40% were from Indiana.
The scenarios and checklists for the mock exercises were created by content experts in pediatric emergency medicine and critical care using evidence-based guidelines and best practices.
Previous research has relied on self-reported surveys rather than direct measurement to assess adherence to the AAP guidelines, the authors say. In-person surveys assessed adherence to AAP recommendations for emergency preparedness. In-person surveys were, however, used to gauge adherence to AAP recommendations for emergency preparedness.
Findings
The overall mean emergency preparedness score was 74.7% (standard deviation [SD] 12.9), with an unweighted percentage of adherence to checklists calculated for each case. By emergency type, the median asthma case performance score was 63.6% (interquartile range [IQR] 43.2-81.2), and the median seizure case score was 69.2% (IQR 46.2-80.8).
On the measure of essential equipment and supplies, the mean subscore (relating to availability of such items as oxygen sources, suction devices, and epinephrine, for example) was 82.2% (SD 15.1).
As for recommended policies and protocols (e.g., regular assessment of the office, maintenance of emergency equipment and medications) the mean subscore fell to 57.1% (SD 25.6).
In multivariable analyses, offices with a standardized procedure for contacting EMS had a higher rate of activating that service during the simulations.
Independent practices and smaller total staff size were associated with lower preparedness compared with larger groups: beta = –11.89, 95% confidence interval [CI], 19.33-4.45).
Higher annual patient volume and larger total staff size were slightly associated with higher scores (beta = .001, 95% CI, .00-001, P = .017; and beta = .51, 95% CI, .19-.83, P = .002, respectively).
Affiliation with an academic medical center and the presence of learners were not associated with higher scores. And in multivariable regression, a higher annual patient volume lost its significant association with greater preparedness.
So why the lag in preparedness despite the long-standing AAP recommendations? “It’s most likely due to the rare occurrence of these emergencies in the office setting, in addition to most offices’ dependence on EMS when they encounter pediatric emergencies in their setting,” Dr. Abulebda said in an interview. “A 2018 study published by Yuknis and associates demonstrated that the average time from EMS notification to arrival on scene was just 6 minutes.”
In other study findings, 82% of offices did not have an infant bag valve mask and would therefore need to wait for EMS to administer lifesaving ventilation. “This highlights the need to have this equipment available and maintain the skills necessary to care for patients in respiratory distress, the most common emergency encountered in the office setting,” Dr. Abulebda and associates wrote.
A cardiac arrest board is another example of a potentially lifesaving piece of equipment that was not available in the majority of offices, likely because of the rarity of this event in the office setting, but lack of this item may result in poor cardiopulmonary resuscitation quality before the arrival of EMS.
In an accompanying editorial, Jesse Hackell, MD, a pediatrician at Boston Children’s Health Physicians and New York Medical College in Pomona, N.Y., noted that data from 2 decades ago suggested that many pediatric offices saw multiple children requiring emergency intervention each week. More recent figures, however, indicate the situation has evolved, with fewer than 1% of current pediatric EMS transports originating from the office setting.
Dr. Hackell agrees that implementation of AAP recommendations has been far from universal and cites the cost of equipment and supplies as well as a lack of access to training and evaluation as significant barriers to implementation. “In addition, the infrequent occurrence of these emergencies makes maintenance of resuscitation skills even more difficult without frequent practice,” he wrote.
Further complicating the issue, preparedness needs vary with practice location, the response time of local EMS, and proximity to an emergency department. “Pediatric offices in more rural areas, which are farther from these services, will require more equipment and more skills to provide optimal emergency care to children living in these underresourced areas,” he wrote.
He called for equitable distribution of preparedness training, equipment, and staffing, with guidance designed to meet patient needs and ensure optimal outcomes. “In discussion of recommendations, one should consider the likely conditions requiring this response, availability of resources beyond the pediatric office, and ongoing training and support needed to maintain provider skills at the level needed for a successful response to any pediatric emergency,” Dr. Hackell wrote.
This study was supported by grants from Indiana University Health Values and the RBaby Foundation. One study coauthor is a board observer of a medical device company. No other authors disclosed financial relationships relevant to this work. Dr. Hackell has disclosed having no competing interests.
FROM PEDIATRICS
Pediatric-Onset Refractory Lupus Erythematosus Panniculitis Treated With Rituximab
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.
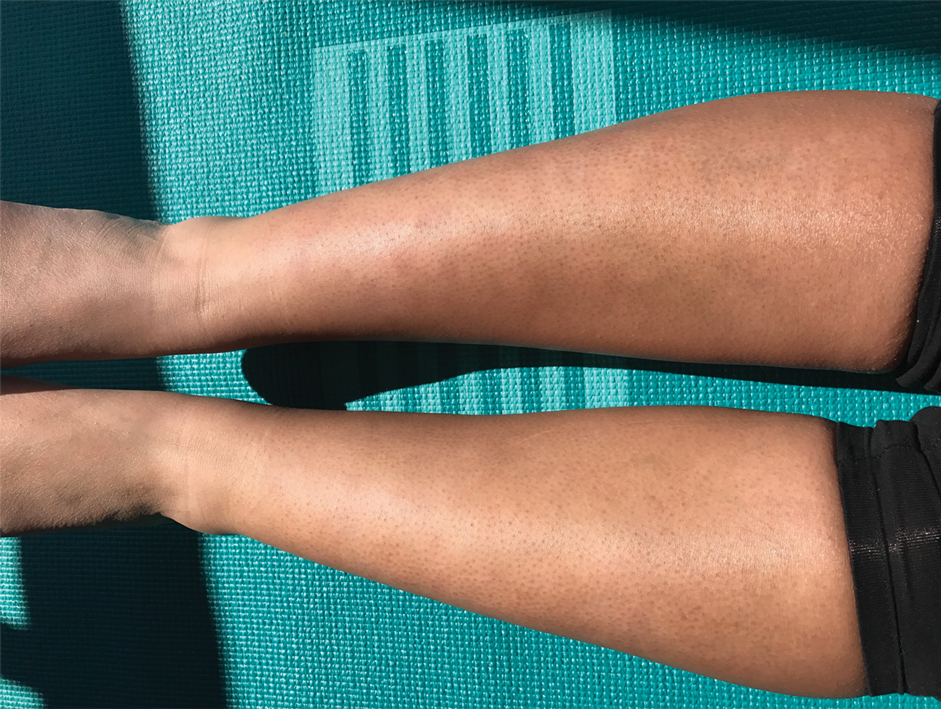
Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.
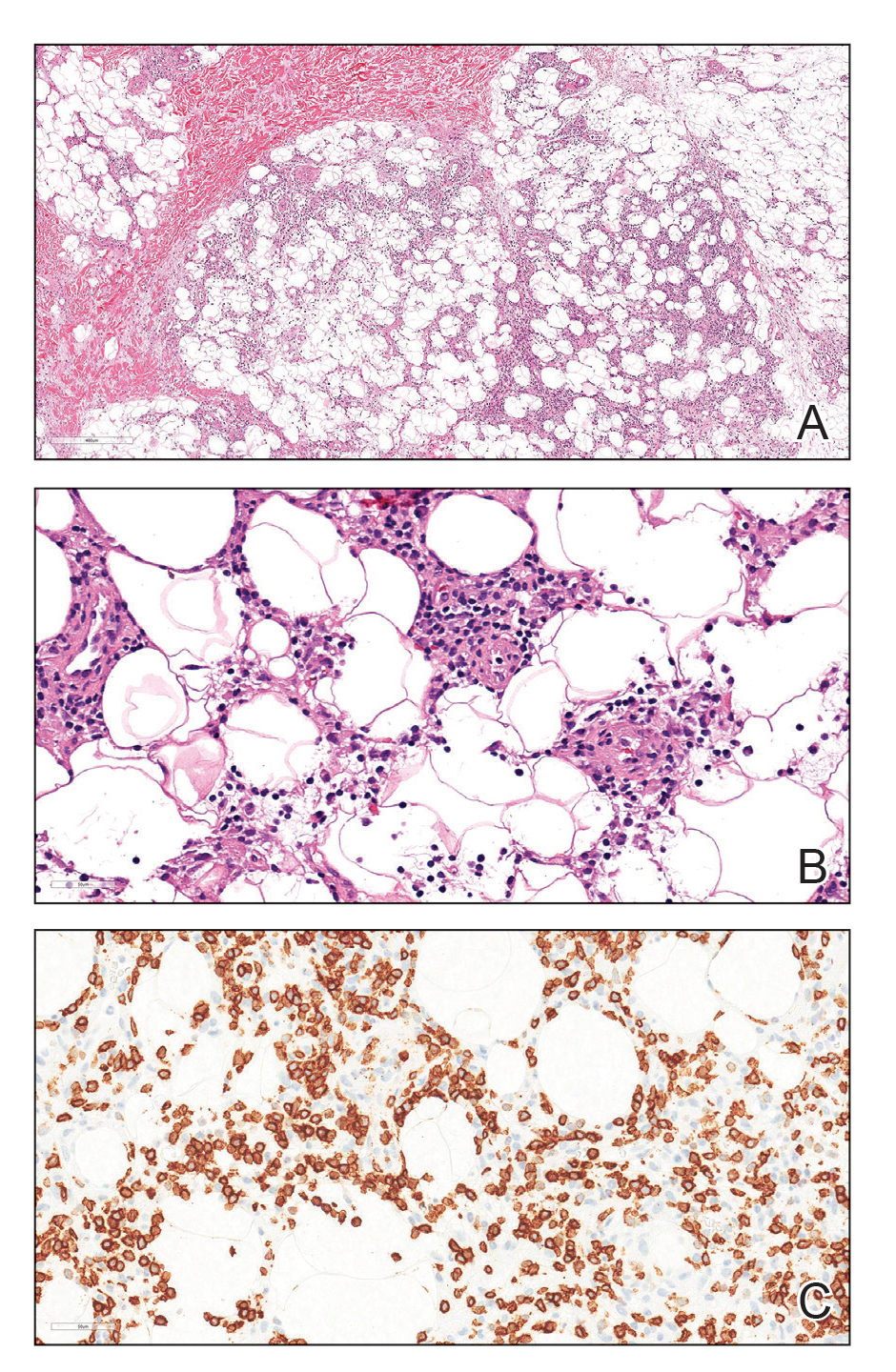

Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.

Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.


Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.

Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.


Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
Practice Points
- Lupus erythematosus panniculitis (LEP) is rare in the pediatric population and often is difficult to treat.
- Rituximab can be an effective treatment option for refractory LEP.
- Before the initiation of rituximab, a biopsy is warranted to rule out subcutaneous T-cell lymphoma, which can mimic LEP and hemophagocytic lymphohistiocytosis–associated panniculitis.