User login
Patch testing in children: An evolving science
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
FROM SPD 2020
Atretic Cephalocele With Hypertrichosis
To the Editor:
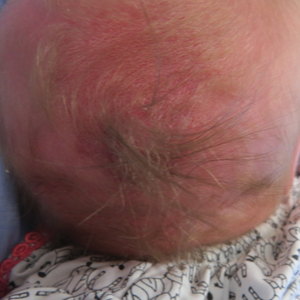
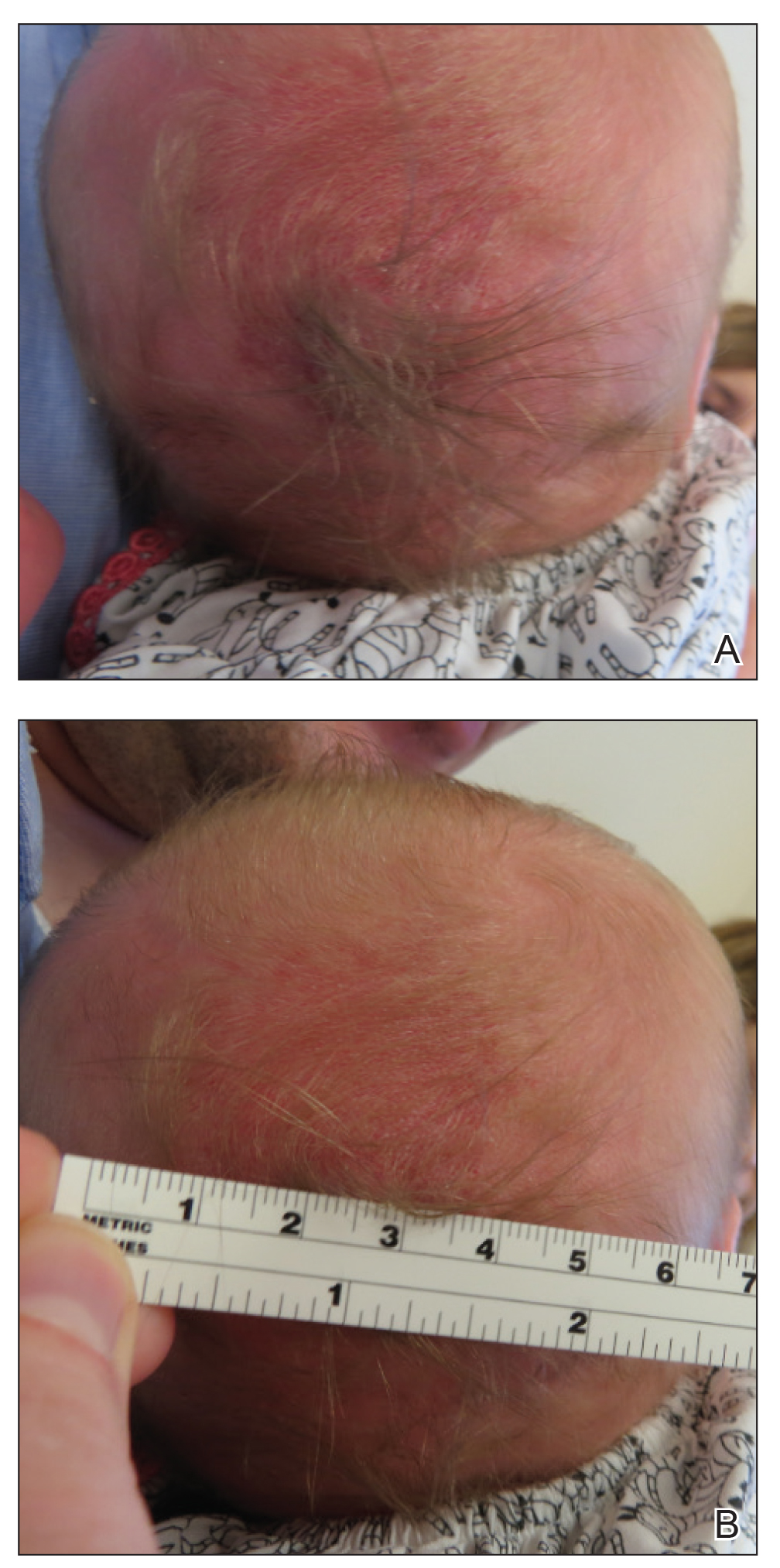
A 2-week-old female infant presented to our dermatology clinic for evaluation of a 4.0×4.5-cm pink-red patch with a 1-cm central nodule and an overlying tuft of hair on the midline occipital region (Figure). The patient was born at 39 weeks’ gestation to nonconsanguineous parents via a normal spontaneous vaginal delivery and had an unremarkable prenatal course with no complications since birth. The red patch and tuft of hair were noted at birth, and the parents reported that the redness varied somewhat in size throughout the day and from day to day. An initial neurologic workup revealed no gross neurologic abnormalities. A head ultrasound revealed a soft-tissue hypervascular nodule that appeared separate from bony structures but showed evidence of a necklike extension from the nodule to the underlying soft tissues. The ultrasound could not definitively rule out intracranial extension; gross brain structures appeared normal. The initial differential diagnosis consisted of a congenital hemangioma (either a rapidly involuting or noninvoluting subtype), meningioma, or cephalocele.
Consultation with the pediatric neurosurgery service was sought, and magnetic resonance imaging of the head was performed, which demonstrated a cystic lesion within the subcutaneous soft tissue in the midline posterior scalp approximately 2 cm above the torcula. There also was a thin stalk extending from the cyst and going through an osseous defect within the occipital bone and attaching to the falx cerebri. There was no evidence of any venous communication with the cerebral sinus tracts or intraparenchymal extension. No intracranial abnormalities were noted. Given the radiographic evidence, a presumptive diagnosis of an atretic cephalocele was made with the plan for surgical repair.
The patient was re-evaluated at 3 and 4 months of age; there were no changes in the size or appearance of the lesion, and she continued to meet all developmental milestones. At 9 months of age the patient underwent uncomplicated neurosurgery to repair the cephalocele. Histopathologic examination of the resected lesion was consistent with an atretic cephalocele and showed positive staining for epithelial membrane antigen, which further confirmed a meningothelial origin; no glial elements were identified. The postoperative course was uncomplicated, and the patient was healing well at a follow-up examination 2 weeks after the procedure.
This case highlights the importance of an extensive workup when a patient presents with a midline lesion and hypertrichosis. The patient’s red patch, excluding the hair tuft, was reminiscent of a vascular malformation or hemangioma precursor lesion given the hypervascularity, the history of the lesion being present since birth, the lack of neurologic symptomatology, and the history of meeting all developmental milestones. The differential diagnosis for this patient was extensive, as many neurologic conditions present with cutaneous findings. Having central nervous system (CNS) and cutaneous comorbidities coincide underscores their common neuroectodermal origin during embryogenesis.1,2
Atretic cephalocele is a rare diagnosis, with the prevalence of cephaloceles estimated to be 0.8 to 3.0 per 10,000 births.3 It typically occurs in either the parietal or occipital scalp as a skin nodule with a hair tuft or alopecic lesion with or without a hair collar. A cephalocele is defined as a skin-covered protrusion of intracranial contents through a bony defect. Central nervous system tissue, meninges, or cerebrospinal fluid can protrude outside the skull with this condition. An atretic cephalocele refers to a cephalocele that arrested in development and represents approximately 40% to 50% of all cephaloceles.4 Various hypotheses have explained the development of atretic cephaloceles: it represents a neural crest remnant, regression of a meningocele in utero, injury of multipotential mesenchymal cells, and either failure of the neural tube to close or reopening of the neural tube after closure.4-6 There is evidence of developmental defects in skin appendages including sweat and sebaceous glands, arrector pili muscles, and hair follicles in and around the skin overlying the cephalocele, suggesting that there is a developmental abnormality of not only the CNS but also the cutaneous tissue.5 Typical radiographic findings include a cystic lesion with underlying defect in the skull. A vertical positioning of the straight sinus also has been demonstrated to be a consistent finding that can aid in diagnosis.4
Imaging is of utmost importance when a patient presents with a tuft of hair on the scalp to rule out intracranial extension and associated abnormalities such as gray matter heterotopia, hypogenesis of the corpus callosum, hydrocephalus, and Dandy-Walker and Walker-Warburg syndromes, which have all been associated with atretic cephaloceles.4,7 The impact of location of the intracranial abnormality on prognosis has been contested, with some reporting a better prognosis with occipital cephalocele vs parietal cephalocele while others have found the opposite to be true.6,7
Cutaneous abnormalities presenting with hypertrichosis (ie, hair tuft, hair collar) and/or capillary malformations increase the likelihood of a cranial dysraphism, especially when these findings present together and occur in and around the midline. Clinical examination cannot rule out an underlying connection to the CNS; these findings require appropriate radiographic imaging assessment prior to any procedural intervention.
- Drolet BA, Clowry L, McTigue K, et al. The hair collar sign: marker for cranial dysraphism. Pediatrics. 1995;96(2, pt 1):309-313.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Carvalho DR, Giuliani LR, Simão GN, et al. Autosomal dominant atretic cephalocele with phenotype variability: report of a Brazilian family with six affected in four generation. Am J Med Genet A. 2006;140:1458-1462.
- Bick DS, Brockland JJ, Scott AR. A scalp lesion with intracranial extension. atretic cephalocele. JAMA Otolaryngol Head Neck Surg. 2015;141:289-290.
- Fukuyama M, Tanese K, Yasuda F, et al. Two cases of atretic cephalocele, and histological evaluation of skin appendages in the surrounding skin. Clin Exp Dermatol. 2016;41:48-52.
- Martinez-Lage JF, Sola J, Casas C, et al. Atretic cephalocele: the tip of the iceberg. J Neurosurg. 1992;77:230-235.
- Yakota A, Kajiwara H, Kohchi M, et al. Parietal cephalocele: clinical importance of its atretic form and associated malformation. J Neurosurg. 1988;69:545-551.
To the Editor:
A 2-week-old female infant presented to our dermatology clinic for evaluation of a 4.0×4.5-cm pink-red patch with a 1-cm central nodule and an overlying tuft of hair on the midline occipital region (Figure). The patient was born at 39 weeks’ gestation to nonconsanguineous parents via a normal spontaneous vaginal delivery and had an unremarkable prenatal course with no complications since birth. The red patch and tuft of hair were noted at birth, and the parents reported that the redness varied somewhat in size throughout the day and from day to day. An initial neurologic workup revealed no gross neurologic abnormalities. A head ultrasound revealed a soft-tissue hypervascular nodule that appeared separate from bony structures but showed evidence of a necklike extension from the nodule to the underlying soft tissues. The ultrasound could not definitively rule out intracranial extension; gross brain structures appeared normal. The initial differential diagnosis consisted of a congenital hemangioma (either a rapidly involuting or noninvoluting subtype), meningioma, or cephalocele.

Consultation with the pediatric neurosurgery service was sought, and magnetic resonance imaging of the head was performed, which demonstrated a cystic lesion within the subcutaneous soft tissue in the midline posterior scalp approximately 2 cm above the torcula. There also was a thin stalk extending from the cyst and going through an osseous defect within the occipital bone and attaching to the falx cerebri. There was no evidence of any venous communication with the cerebral sinus tracts or intraparenchymal extension. No intracranial abnormalities were noted. Given the radiographic evidence, a presumptive diagnosis of an atretic cephalocele was made with the plan for surgical repair.
The patient was re-evaluated at 3 and 4 months of age; there were no changes in the size or appearance of the lesion, and she continued to meet all developmental milestones. At 9 months of age the patient underwent uncomplicated neurosurgery to repair the cephalocele. Histopathologic examination of the resected lesion was consistent with an atretic cephalocele and showed positive staining for epithelial membrane antigen, which further confirmed a meningothelial origin; no glial elements were identified. The postoperative course was uncomplicated, and the patient was healing well at a follow-up examination 2 weeks after the procedure.
This case highlights the importance of an extensive workup when a patient presents with a midline lesion and hypertrichosis. The patient’s red patch, excluding the hair tuft, was reminiscent of a vascular malformation or hemangioma precursor lesion given the hypervascularity, the history of the lesion being present since birth, the lack of neurologic symptomatology, and the history of meeting all developmental milestones. The differential diagnosis for this patient was extensive, as many neurologic conditions present with cutaneous findings. Having central nervous system (CNS) and cutaneous comorbidities coincide underscores their common neuroectodermal origin during embryogenesis.1,2
Atretic cephalocele is a rare diagnosis, with the prevalence of cephaloceles estimated to be 0.8 to 3.0 per 10,000 births.3 It typically occurs in either the parietal or occipital scalp as a skin nodule with a hair tuft or alopecic lesion with or without a hair collar. A cephalocele is defined as a skin-covered protrusion of intracranial contents through a bony defect. Central nervous system tissue, meninges, or cerebrospinal fluid can protrude outside the skull with this condition. An atretic cephalocele refers to a cephalocele that arrested in development and represents approximately 40% to 50% of all cephaloceles.4 Various hypotheses have explained the development of atretic cephaloceles: it represents a neural crest remnant, regression of a meningocele in utero, injury of multipotential mesenchymal cells, and either failure of the neural tube to close or reopening of the neural tube after closure.4-6 There is evidence of developmental defects in skin appendages including sweat and sebaceous glands, arrector pili muscles, and hair follicles in and around the skin overlying the cephalocele, suggesting that there is a developmental abnormality of not only the CNS but also the cutaneous tissue.5 Typical radiographic findings include a cystic lesion with underlying defect in the skull. A vertical positioning of the straight sinus also has been demonstrated to be a consistent finding that can aid in diagnosis.4
Imaging is of utmost importance when a patient presents with a tuft of hair on the scalp to rule out intracranial extension and associated abnormalities such as gray matter heterotopia, hypogenesis of the corpus callosum, hydrocephalus, and Dandy-Walker and Walker-Warburg syndromes, which have all been associated with atretic cephaloceles.4,7 The impact of location of the intracranial abnormality on prognosis has been contested, with some reporting a better prognosis with occipital cephalocele vs parietal cephalocele while others have found the opposite to be true.6,7
Cutaneous abnormalities presenting with hypertrichosis (ie, hair tuft, hair collar) and/or capillary malformations increase the likelihood of a cranial dysraphism, especially when these findings present together and occur in and around the midline. Clinical examination cannot rule out an underlying connection to the CNS; these findings require appropriate radiographic imaging assessment prior to any procedural intervention.
To the Editor:
A 2-week-old female infant presented to our dermatology clinic for evaluation of a 4.0×4.5-cm pink-red patch with a 1-cm central nodule and an overlying tuft of hair on the midline occipital region (Figure). The patient was born at 39 weeks’ gestation to nonconsanguineous parents via a normal spontaneous vaginal delivery and had an unremarkable prenatal course with no complications since birth. The red patch and tuft of hair were noted at birth, and the parents reported that the redness varied somewhat in size throughout the day and from day to day. An initial neurologic workup revealed no gross neurologic abnormalities. A head ultrasound revealed a soft-tissue hypervascular nodule that appeared separate from bony structures but showed evidence of a necklike extension from the nodule to the underlying soft tissues. The ultrasound could not definitively rule out intracranial extension; gross brain structures appeared normal. The initial differential diagnosis consisted of a congenital hemangioma (either a rapidly involuting or noninvoluting subtype), meningioma, or cephalocele.

Consultation with the pediatric neurosurgery service was sought, and magnetic resonance imaging of the head was performed, which demonstrated a cystic lesion within the subcutaneous soft tissue in the midline posterior scalp approximately 2 cm above the torcula. There also was a thin stalk extending from the cyst and going through an osseous defect within the occipital bone and attaching to the falx cerebri. There was no evidence of any venous communication with the cerebral sinus tracts or intraparenchymal extension. No intracranial abnormalities were noted. Given the radiographic evidence, a presumptive diagnosis of an atretic cephalocele was made with the plan for surgical repair.
The patient was re-evaluated at 3 and 4 months of age; there were no changes in the size or appearance of the lesion, and she continued to meet all developmental milestones. At 9 months of age the patient underwent uncomplicated neurosurgery to repair the cephalocele. Histopathologic examination of the resected lesion was consistent with an atretic cephalocele and showed positive staining for epithelial membrane antigen, which further confirmed a meningothelial origin; no glial elements were identified. The postoperative course was uncomplicated, and the patient was healing well at a follow-up examination 2 weeks after the procedure.
This case highlights the importance of an extensive workup when a patient presents with a midline lesion and hypertrichosis. The patient’s red patch, excluding the hair tuft, was reminiscent of a vascular malformation or hemangioma precursor lesion given the hypervascularity, the history of the lesion being present since birth, the lack of neurologic symptomatology, and the history of meeting all developmental milestones. The differential diagnosis for this patient was extensive, as many neurologic conditions present with cutaneous findings. Having central nervous system (CNS) and cutaneous comorbidities coincide underscores their common neuroectodermal origin during embryogenesis.1,2
Atretic cephalocele is a rare diagnosis, with the prevalence of cephaloceles estimated to be 0.8 to 3.0 per 10,000 births.3 It typically occurs in either the parietal or occipital scalp as a skin nodule with a hair tuft or alopecic lesion with or without a hair collar. A cephalocele is defined as a skin-covered protrusion of intracranial contents through a bony defect. Central nervous system tissue, meninges, or cerebrospinal fluid can protrude outside the skull with this condition. An atretic cephalocele refers to a cephalocele that arrested in development and represents approximately 40% to 50% of all cephaloceles.4 Various hypotheses have explained the development of atretic cephaloceles: it represents a neural crest remnant, regression of a meningocele in utero, injury of multipotential mesenchymal cells, and either failure of the neural tube to close or reopening of the neural tube after closure.4-6 There is evidence of developmental defects in skin appendages including sweat and sebaceous glands, arrector pili muscles, and hair follicles in and around the skin overlying the cephalocele, suggesting that there is a developmental abnormality of not only the CNS but also the cutaneous tissue.5 Typical radiographic findings include a cystic lesion with underlying defect in the skull. A vertical positioning of the straight sinus also has been demonstrated to be a consistent finding that can aid in diagnosis.4
Imaging is of utmost importance when a patient presents with a tuft of hair on the scalp to rule out intracranial extension and associated abnormalities such as gray matter heterotopia, hypogenesis of the corpus callosum, hydrocephalus, and Dandy-Walker and Walker-Warburg syndromes, which have all been associated with atretic cephaloceles.4,7 The impact of location of the intracranial abnormality on prognosis has been contested, with some reporting a better prognosis with occipital cephalocele vs parietal cephalocele while others have found the opposite to be true.6,7
Cutaneous abnormalities presenting with hypertrichosis (ie, hair tuft, hair collar) and/or capillary malformations increase the likelihood of a cranial dysraphism, especially when these findings present together and occur in and around the midline. Clinical examination cannot rule out an underlying connection to the CNS; these findings require appropriate radiographic imaging assessment prior to any procedural intervention.
- Drolet BA, Clowry L, McTigue K, et al. The hair collar sign: marker for cranial dysraphism. Pediatrics. 1995;96(2, pt 1):309-313.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Carvalho DR, Giuliani LR, Simão GN, et al. Autosomal dominant atretic cephalocele with phenotype variability: report of a Brazilian family with six affected in four generation. Am J Med Genet A. 2006;140:1458-1462.
- Bick DS, Brockland JJ, Scott AR. A scalp lesion with intracranial extension. atretic cephalocele. JAMA Otolaryngol Head Neck Surg. 2015;141:289-290.
- Fukuyama M, Tanese K, Yasuda F, et al. Two cases of atretic cephalocele, and histological evaluation of skin appendages in the surrounding skin. Clin Exp Dermatol. 2016;41:48-52.
- Martinez-Lage JF, Sola J, Casas C, et al. Atretic cephalocele: the tip of the iceberg. J Neurosurg. 1992;77:230-235.
- Yakota A, Kajiwara H, Kohchi M, et al. Parietal cephalocele: clinical importance of its atretic form and associated malformation. J Neurosurg. 1988;69:545-551.
- Drolet BA, Clowry L, McTigue K, et al. The hair collar sign: marker for cranial dysraphism. Pediatrics. 1995;96(2, pt 1):309-313.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Carvalho DR, Giuliani LR, Simão GN, et al. Autosomal dominant atretic cephalocele with phenotype variability: report of a Brazilian family with six affected in four generation. Am J Med Genet A. 2006;140:1458-1462.
- Bick DS, Brockland JJ, Scott AR. A scalp lesion with intracranial extension. atretic cephalocele. JAMA Otolaryngol Head Neck Surg. 2015;141:289-290.
- Fukuyama M, Tanese K, Yasuda F, et al. Two cases of atretic cephalocele, and histological evaluation of skin appendages in the surrounding skin. Clin Exp Dermatol. 2016;41:48-52.
- Martinez-Lage JF, Sola J, Casas C, et al. Atretic cephalocele: the tip of the iceberg. J Neurosurg. 1992;77:230-235.
- Yakota A, Kajiwara H, Kohchi M, et al. Parietal cephalocele: clinical importance of its atretic form and associated malformation. J Neurosurg. 1988;69:545-551.
Practice Points
- Atretic cephalocele is a rare diagnosis occurring on the scalp as a nodule with an overlying hair tuft or alopecia with or without a hair collar.
- Imaging is of utmost importance when presented with a tuft of hair on the midline to rule out intracranial extension and associated abnormalities.
Stress, COVID-19 contribute to mental health concerns in college students
Socioeconomic, technological, cultural, and historical conditions are contributing to a mental health crisis among college students in the United States, according to Anthony L. Rostain, MD, MA, in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
A recent National College Health Assessment published in fall of 2018 by the American College Health Association found that one in four college students had some kind of diagnosable mental illness, and 44% had symptoms of depression within the past year.
The assessment also found that college students felt overwhelmed (86%), felt sad (68%), felt very lonely (63%), had overwhelming anxiety (62%), experienced feelings of hopelessness (53%), or were depressed to the point where functioning was difficult (41%), all of which was higher than in previous years. Students also were more likely than in previous years to engage in interpersonal violence (17%), seriously consider suicide (11%), intentionally hurt themselves (7.4%), and attempt suicide (1.9%). According to the organization Active Minds, suicide is a leading cause of death in college students.
This shift in mental health for individuals in Generation Z, those born between the mid-1990s and early 2010s, can be attributed to historical events since the turn of the century, Dr. Rostain said at the meeting, presented by Global Academy for Medical Education. The Sept. 11, 2001, attacks, wars in Iraq and Afghanistan, the financial crisis of 2007-2008, school shootings, globalization leading to economic uncertainty, the 24-hour news cycle and continuous media exposure, and the influence of the Internet have all influenced Gen Z’s identity.
“Growing up immersed in the Internet certainly has its advantages, but also maybe created some vulnerabilities in our young people,” he said.
Concerns about climate change, the burden of higher education and student debt, and the COVID-19 pandemic also have contributed to anxiety in this group. In a spring survey of students published by Active Minds about COVID-19 and its impact on mental health, 91% of students reported having stress or anxiety, 81% were disappointed or sad, 80% said they felt lonely or isolated, 56% had relocated as a result of the pandemic, and 48% reported financial setbacks tied to COVID-19.
“Anxiety seems to have become a feature of modern life,” said Dr. Rostain, who is director of education at the department of psychiatry and professor of psychiatry at the Hospital of the University of Pennsylvania in Philadelphia.
“Our culture, which often has a prominent emotional tone of fear, tends to promote cognitive distortions in which everyone is perceiving danger at every turn.” in this group, he noted. While people should be washing their hands and staying safe through social distancing during the pandemic, “we don’t want people to stop functioning, planning the future, and really in college students’ case, studying and getting ready for their careers,” he said. Parents can hinder those goals through intensively parenting or “overparenting” their children, which can result in destructive perfectionism, anxiety and depression, abject fear of failure and risk avoidance, and a focus on the external aspects of life rather than internal feelings.
Heavier alcohol use and amphetamine use also is on the rise in college students, Dr. Rostain said. Increased stimulant use in young adults is attributed to greater access to prescription drugs prior to college, greater prevalence of attention-deficit/hyperactivity disorder (ADHD), peer pressure, and influence from marketing and media messaging, he said. Another important change is the rise of smartphones and the Internet, which might drive the need to be constantly connected and compete for attention.
“This is the first generation who had constant access to the Internet. Smartphones in particular are everywhere, and we think this is another important factor in considering what might be happening to young people,” Dr. Rostain said.
Developing problem-solving and conflict resolution skills, developing coping mechanisms, being able to regulate emotions, finding optimism toward the future, having access to mental health services, and having cultural or religious beliefs with a negative view of suicide are all protective factors that promote resiliency in young people, Dr. Rostain said. Other protective factors include the development of socio-emotional readiness skills, such as conscientiousness, self-management, interpersonal skills, self-control, task persistence, risk management, self-acceptance, and having an open mindset or seeking help when needed. However, he noted, family is one area that can be both a help or a risk to mental health.
“Family attachments and supportive relationships in the family are really critical in predicting good outcomes. By the same token, families that are conflicted, where there’s a lot of stress or there’s a lot of turmoil and/or where resources are not available, that may be a risk factor to coping in young adulthood,” he said.
Individual resilience can be developed through learning from mistakes and overcoming mindset barriers, such as feelings of not belonging, concerns about disappointing one’s parents, worries about not making it, or fears of being different.
On campus, best practices and emerging trends include wellness and resiliency programs, reducing stigma, engagement from students, training of faculty and staff, crisis management plans, telehealth counseling, substance abuse programs, postvention support after suicide, collaboration with mental health providers, and support for diverse populations.
“The best schools are the ones that promote communication and that invite families to be involved early on because parents and families can be and need to be educated about what to do to prevent adverse outcomes of young people who are really at risk,” Dr. Rostain said. “It takes a village to raise a child, and it takes the same village to bring someone from adolescence to young adulthood.”
Family-based intervention has also shown promise, he said, but clinicians should watch for signs that a family is not willing to undergo therapy, is scapegoating a college student, or there are signs of boundary violations, violence, or sexual abuse in the family – or attempts to undermine treatment.
Specific to COVID-19, campus mental health services should focus on routine, self-care, physical activity, and connections with other people while also space for grieving lost experiences, facing uncertainty, developing resilience, and finding meaning. In the family, challenges around COVID-19 can include issues of physical distancing and quarantine, anxiety about becoming infected with the virus, economic insecurity, managing conflicts, setting and enforcing boundaries in addition to providing mutual support, and finding new meaning during the pandemic.
“I think these are the challenges, but we think this whole process of people living together and handling life in a way they’ve never expected to may hold some silver linings,” Dr. Rostain said. “It may be a way of addressing many issues that were never addressed before the young person went off to college.”
Global Academy and this news organization are owned by the same parent company. Dr. Rostain reported receiving royalties from Routledge/Taylor Francis Group and St. Martin’s Press, scientific advisory board honoraria from Arbor and Shire/Takeda, consulting fees from the National Football League and Tris Pharmaceuticals, and has presented CME sessions for American Psychiatric Publishing, Global Medical Education, Shire/Takeda, and the U.S. Psychiatric Congress.
Socioeconomic, technological, cultural, and historical conditions are contributing to a mental health crisis among college students in the United States, according to Anthony L. Rostain, MD, MA, in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
A recent National College Health Assessment published in fall of 2018 by the American College Health Association found that one in four college students had some kind of diagnosable mental illness, and 44% had symptoms of depression within the past year.
The assessment also found that college students felt overwhelmed (86%), felt sad (68%), felt very lonely (63%), had overwhelming anxiety (62%), experienced feelings of hopelessness (53%), or were depressed to the point where functioning was difficult (41%), all of which was higher than in previous years. Students also were more likely than in previous years to engage in interpersonal violence (17%), seriously consider suicide (11%), intentionally hurt themselves (7.4%), and attempt suicide (1.9%). According to the organization Active Minds, suicide is a leading cause of death in college students.
This shift in mental health for individuals in Generation Z, those born between the mid-1990s and early 2010s, can be attributed to historical events since the turn of the century, Dr. Rostain said at the meeting, presented by Global Academy for Medical Education. The Sept. 11, 2001, attacks, wars in Iraq and Afghanistan, the financial crisis of 2007-2008, school shootings, globalization leading to economic uncertainty, the 24-hour news cycle and continuous media exposure, and the influence of the Internet have all influenced Gen Z’s identity.
“Growing up immersed in the Internet certainly has its advantages, but also maybe created some vulnerabilities in our young people,” he said.
Concerns about climate change, the burden of higher education and student debt, and the COVID-19 pandemic also have contributed to anxiety in this group. In a spring survey of students published by Active Minds about COVID-19 and its impact on mental health, 91% of students reported having stress or anxiety, 81% were disappointed or sad, 80% said they felt lonely or isolated, 56% had relocated as a result of the pandemic, and 48% reported financial setbacks tied to COVID-19.
“Anxiety seems to have become a feature of modern life,” said Dr. Rostain, who is director of education at the department of psychiatry and professor of psychiatry at the Hospital of the University of Pennsylvania in Philadelphia.
“Our culture, which often has a prominent emotional tone of fear, tends to promote cognitive distortions in which everyone is perceiving danger at every turn.” in this group, he noted. While people should be washing their hands and staying safe through social distancing during the pandemic, “we don’t want people to stop functioning, planning the future, and really in college students’ case, studying and getting ready for their careers,” he said. Parents can hinder those goals through intensively parenting or “overparenting” their children, which can result in destructive perfectionism, anxiety and depression, abject fear of failure and risk avoidance, and a focus on the external aspects of life rather than internal feelings.
Heavier alcohol use and amphetamine use also is on the rise in college students, Dr. Rostain said. Increased stimulant use in young adults is attributed to greater access to prescription drugs prior to college, greater prevalence of attention-deficit/hyperactivity disorder (ADHD), peer pressure, and influence from marketing and media messaging, he said. Another important change is the rise of smartphones and the Internet, which might drive the need to be constantly connected and compete for attention.
“This is the first generation who had constant access to the Internet. Smartphones in particular are everywhere, and we think this is another important factor in considering what might be happening to young people,” Dr. Rostain said.
Developing problem-solving and conflict resolution skills, developing coping mechanisms, being able to regulate emotions, finding optimism toward the future, having access to mental health services, and having cultural or religious beliefs with a negative view of suicide are all protective factors that promote resiliency in young people, Dr. Rostain said. Other protective factors include the development of socio-emotional readiness skills, such as conscientiousness, self-management, interpersonal skills, self-control, task persistence, risk management, self-acceptance, and having an open mindset or seeking help when needed. However, he noted, family is one area that can be both a help or a risk to mental health.
“Family attachments and supportive relationships in the family are really critical in predicting good outcomes. By the same token, families that are conflicted, where there’s a lot of stress or there’s a lot of turmoil and/or where resources are not available, that may be a risk factor to coping in young adulthood,” he said.
Individual resilience can be developed through learning from mistakes and overcoming mindset barriers, such as feelings of not belonging, concerns about disappointing one’s parents, worries about not making it, or fears of being different.
On campus, best practices and emerging trends include wellness and resiliency programs, reducing stigma, engagement from students, training of faculty and staff, crisis management plans, telehealth counseling, substance abuse programs, postvention support after suicide, collaboration with mental health providers, and support for diverse populations.
“The best schools are the ones that promote communication and that invite families to be involved early on because parents and families can be and need to be educated about what to do to prevent adverse outcomes of young people who are really at risk,” Dr. Rostain said. “It takes a village to raise a child, and it takes the same village to bring someone from adolescence to young adulthood.”
Family-based intervention has also shown promise, he said, but clinicians should watch for signs that a family is not willing to undergo therapy, is scapegoating a college student, or there are signs of boundary violations, violence, or sexual abuse in the family – or attempts to undermine treatment.
Specific to COVID-19, campus mental health services should focus on routine, self-care, physical activity, and connections with other people while also space for grieving lost experiences, facing uncertainty, developing resilience, and finding meaning. In the family, challenges around COVID-19 can include issues of physical distancing and quarantine, anxiety about becoming infected with the virus, economic insecurity, managing conflicts, setting and enforcing boundaries in addition to providing mutual support, and finding new meaning during the pandemic.
“I think these are the challenges, but we think this whole process of people living together and handling life in a way they’ve never expected to may hold some silver linings,” Dr. Rostain said. “It may be a way of addressing many issues that were never addressed before the young person went off to college.”
Global Academy and this news organization are owned by the same parent company. Dr. Rostain reported receiving royalties from Routledge/Taylor Francis Group and St. Martin’s Press, scientific advisory board honoraria from Arbor and Shire/Takeda, consulting fees from the National Football League and Tris Pharmaceuticals, and has presented CME sessions for American Psychiatric Publishing, Global Medical Education, Shire/Takeda, and the U.S. Psychiatric Congress.
Socioeconomic, technological, cultural, and historical conditions are contributing to a mental health crisis among college students in the United States, according to Anthony L. Rostain, MD, MA, in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
A recent National College Health Assessment published in fall of 2018 by the American College Health Association found that one in four college students had some kind of diagnosable mental illness, and 44% had symptoms of depression within the past year.
The assessment also found that college students felt overwhelmed (86%), felt sad (68%), felt very lonely (63%), had overwhelming anxiety (62%), experienced feelings of hopelessness (53%), or were depressed to the point where functioning was difficult (41%), all of which was higher than in previous years. Students also were more likely than in previous years to engage in interpersonal violence (17%), seriously consider suicide (11%), intentionally hurt themselves (7.4%), and attempt suicide (1.9%). According to the organization Active Minds, suicide is a leading cause of death in college students.
This shift in mental health for individuals in Generation Z, those born between the mid-1990s and early 2010s, can be attributed to historical events since the turn of the century, Dr. Rostain said at the meeting, presented by Global Academy for Medical Education. The Sept. 11, 2001, attacks, wars in Iraq and Afghanistan, the financial crisis of 2007-2008, school shootings, globalization leading to economic uncertainty, the 24-hour news cycle and continuous media exposure, and the influence of the Internet have all influenced Gen Z’s identity.
“Growing up immersed in the Internet certainly has its advantages, but also maybe created some vulnerabilities in our young people,” he said.
Concerns about climate change, the burden of higher education and student debt, and the COVID-19 pandemic also have contributed to anxiety in this group. In a spring survey of students published by Active Minds about COVID-19 and its impact on mental health, 91% of students reported having stress or anxiety, 81% were disappointed or sad, 80% said they felt lonely or isolated, 56% had relocated as a result of the pandemic, and 48% reported financial setbacks tied to COVID-19.
“Anxiety seems to have become a feature of modern life,” said Dr. Rostain, who is director of education at the department of psychiatry and professor of psychiatry at the Hospital of the University of Pennsylvania in Philadelphia.
“Our culture, which often has a prominent emotional tone of fear, tends to promote cognitive distortions in which everyone is perceiving danger at every turn.” in this group, he noted. While people should be washing their hands and staying safe through social distancing during the pandemic, “we don’t want people to stop functioning, planning the future, and really in college students’ case, studying and getting ready for their careers,” he said. Parents can hinder those goals through intensively parenting or “overparenting” their children, which can result in destructive perfectionism, anxiety and depression, abject fear of failure and risk avoidance, and a focus on the external aspects of life rather than internal feelings.
Heavier alcohol use and amphetamine use also is on the rise in college students, Dr. Rostain said. Increased stimulant use in young adults is attributed to greater access to prescription drugs prior to college, greater prevalence of attention-deficit/hyperactivity disorder (ADHD), peer pressure, and influence from marketing and media messaging, he said. Another important change is the rise of smartphones and the Internet, which might drive the need to be constantly connected and compete for attention.
“This is the first generation who had constant access to the Internet. Smartphones in particular are everywhere, and we think this is another important factor in considering what might be happening to young people,” Dr. Rostain said.
Developing problem-solving and conflict resolution skills, developing coping mechanisms, being able to regulate emotions, finding optimism toward the future, having access to mental health services, and having cultural or religious beliefs with a negative view of suicide are all protective factors that promote resiliency in young people, Dr. Rostain said. Other protective factors include the development of socio-emotional readiness skills, such as conscientiousness, self-management, interpersonal skills, self-control, task persistence, risk management, self-acceptance, and having an open mindset or seeking help when needed. However, he noted, family is one area that can be both a help or a risk to mental health.
“Family attachments and supportive relationships in the family are really critical in predicting good outcomes. By the same token, families that are conflicted, where there’s a lot of stress or there’s a lot of turmoil and/or where resources are not available, that may be a risk factor to coping in young adulthood,” he said.
Individual resilience can be developed through learning from mistakes and overcoming mindset barriers, such as feelings of not belonging, concerns about disappointing one’s parents, worries about not making it, or fears of being different.
On campus, best practices and emerging trends include wellness and resiliency programs, reducing stigma, engagement from students, training of faculty and staff, crisis management plans, telehealth counseling, substance abuse programs, postvention support after suicide, collaboration with mental health providers, and support for diverse populations.
“The best schools are the ones that promote communication and that invite families to be involved early on because parents and families can be and need to be educated about what to do to prevent adverse outcomes of young people who are really at risk,” Dr. Rostain said. “It takes a village to raise a child, and it takes the same village to bring someone from adolescence to young adulthood.”
Family-based intervention has also shown promise, he said, but clinicians should watch for signs that a family is not willing to undergo therapy, is scapegoating a college student, or there are signs of boundary violations, violence, or sexual abuse in the family – or attempts to undermine treatment.
Specific to COVID-19, campus mental health services should focus on routine, self-care, physical activity, and connections with other people while also space for grieving lost experiences, facing uncertainty, developing resilience, and finding meaning. In the family, challenges around COVID-19 can include issues of physical distancing and quarantine, anxiety about becoming infected with the virus, economic insecurity, managing conflicts, setting and enforcing boundaries in addition to providing mutual support, and finding new meaning during the pandemic.
“I think these are the challenges, but we think this whole process of people living together and handling life in a way they’ve never expected to may hold some silver linings,” Dr. Rostain said. “It may be a way of addressing many issues that were never addressed before the young person went off to college.”
Global Academy and this news organization are owned by the same parent company. Dr. Rostain reported receiving royalties from Routledge/Taylor Francis Group and St. Martin’s Press, scientific advisory board honoraria from Arbor and Shire/Takeda, consulting fees from the National Football League and Tris Pharmaceuticals, and has presented CME sessions for American Psychiatric Publishing, Global Medical Education, Shire/Takeda, and the U.S. Psychiatric Congress.
EXPERT ANALYSIS FROM CP/AACP 2020 PSYCHIATRY UPDATE
Acute EVALI remains a diagnosis of exclusion
according to a synthesis of current information presented at the virtual Pediatric Hospital Medicine.
Respiratory symptoms, including cough, chest pain, and shortness of breath are common but so are constitutive symptoms, including fever, sore throat, muscle aches, nausea and vomiting, said Yamini Kuchipudi, MD, a staff physician at Cincinnati Children’s Hospital, during the session at the virtual meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
If EVALI is not considered across this broad array of symptoms, of which respiratory complaints might not be the most prominent at the time of presentation, the diagnosis might be delayed, Dr. Kuchipudi warned during the virtual meeting.
Teenagers and young adults are the most common users of e-cigarettes and vaping devices. In these patients or in any individual suspected of having EVALI, Dr. Kuchipudi recommended posing questions about vaping relatively early in the work-up “in a confidential and nonjudgmental way.”
Eliciting a truthful history will be particularly important, because the risk of EVALI appears to be largely related to vaping with tetrahydrocannabinol (THC)-containing products rather than with nicotine alone. Although the exact cause of EVALI is not yet completely clear, this condition is now strongly associated with additives to the THC, according to Issa Hanna, MD, of the department of pediatrics at the University of Florida, Jacksonville.
“E-liquid contains products like hydrocarbons, vitamin E acetate, and heavy metals that appear to damage the alveolar epithelium by direct cellular inflammation,” Dr. Hanna explained.
These products are not only found in THC processed for vaping but also for dabbing, a related but different form of inhalation that involves vaporization of highly concentrated THC waxes or resins. Dr. Hanna suggested that the decline in reported cases of EVALI, which has followed the peak incidence in September 2019, is likely to be related to a decline in THC additives as well as greater caution among users.
E-cigarettes were introduced in 2007, according to Dr. Hanna, but EVALI was not widely recognized until cases began accruing early in 2019. By June 2019, the growing number of case reports had attracted the attention of the media as well as public health officials, intensifying the effort to isolate the risks and causes.
Consistent with greater use of e-cigarettes and vaping among younger individuals, nearly 80% of the 2,807 patients hospitalized for EVALI in the United States by February of this year occurred in individuals aged less than 35 years, according to data released by the Centers for Disease Control and Prevention. The median age was less than 25 years. Of these hospitalizations, 68 deaths (2.5%) in 29 states and Washington, D.C., were attributed to EVALI.
Because of the nonspecific symptoms and lack of a definitive diagnostic test, EVALI is considered a diagnosis of exclusion, according to Abigail Musial, MD, who is completing a fellowship in hospital medicine at Cincinnati Children’s. She presented a case in which a patient suspected of EVALI went home after symptoms abated on steroids.
“Less than 24 hours later, she returned to the ED with tachypnea and hypoxemia,” Dr. Musial recounted. Although a chest x-ray at the initial evaluation showed lung opacities, a repeat chest x-ray when she returned to the ED showed bilateral worsening of these opacities and persistent elevation of inflammatory markers.
“She was started on steroids and also on antibiotics,” Dr. Musial said. “She was weaned quickly from oxygen once the steroids were started and was discharged on hospital day 3.”
For patients suspected of EVALI, COVID-19 testing should be part of the work-up, according to Dr. Kuchipudi. She also recommended an x-ray or CT scan of the lung as well as an evaluation of inflammatory markers.
Dr. Kuchipudi said that more invasive studies than lung function tests, such as bronchoalveolar lavage or lung biopsy, might be considered when severe symptoms make aggressive diagnostic studies attractive.
Steroids and antibiotics typically lead to control of acute symptoms, but patients should be clinically stable for 24-48 hours prior to hospital discharge, according to Dr. Kuchipudi. Follow-up after discharge should include lung function tests and imaging 2-4 weeks later to confirm resolution of abnormalities.
Dr. Kuchipudi stressed the opportunity that an episode of EVALI provides to induce patients to give up nicotine and vaping entirely. Such strategies, such as a nicotine patch, deserve consideration, but she also cautioned that e-cigarettes for smoking cessation should not be recommended to EVALI patients.
The speakers reported no potential conflicts of interest relevant to this study.
according to a synthesis of current information presented at the virtual Pediatric Hospital Medicine.
Respiratory symptoms, including cough, chest pain, and shortness of breath are common but so are constitutive symptoms, including fever, sore throat, muscle aches, nausea and vomiting, said Yamini Kuchipudi, MD, a staff physician at Cincinnati Children’s Hospital, during the session at the virtual meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
If EVALI is not considered across this broad array of symptoms, of which respiratory complaints might not be the most prominent at the time of presentation, the diagnosis might be delayed, Dr. Kuchipudi warned during the virtual meeting.
Teenagers and young adults are the most common users of e-cigarettes and vaping devices. In these patients or in any individual suspected of having EVALI, Dr. Kuchipudi recommended posing questions about vaping relatively early in the work-up “in a confidential and nonjudgmental way.”
Eliciting a truthful history will be particularly important, because the risk of EVALI appears to be largely related to vaping with tetrahydrocannabinol (THC)-containing products rather than with nicotine alone. Although the exact cause of EVALI is not yet completely clear, this condition is now strongly associated with additives to the THC, according to Issa Hanna, MD, of the department of pediatrics at the University of Florida, Jacksonville.
“E-liquid contains products like hydrocarbons, vitamin E acetate, and heavy metals that appear to damage the alveolar epithelium by direct cellular inflammation,” Dr. Hanna explained.
These products are not only found in THC processed for vaping but also for dabbing, a related but different form of inhalation that involves vaporization of highly concentrated THC waxes or resins. Dr. Hanna suggested that the decline in reported cases of EVALI, which has followed the peak incidence in September 2019, is likely to be related to a decline in THC additives as well as greater caution among users.
E-cigarettes were introduced in 2007, according to Dr. Hanna, but EVALI was not widely recognized until cases began accruing early in 2019. By June 2019, the growing number of case reports had attracted the attention of the media as well as public health officials, intensifying the effort to isolate the risks and causes.
Consistent with greater use of e-cigarettes and vaping among younger individuals, nearly 80% of the 2,807 patients hospitalized for EVALI in the United States by February of this year occurred in individuals aged less than 35 years, according to data released by the Centers for Disease Control and Prevention. The median age was less than 25 years. Of these hospitalizations, 68 deaths (2.5%) in 29 states and Washington, D.C., were attributed to EVALI.
Because of the nonspecific symptoms and lack of a definitive diagnostic test, EVALI is considered a diagnosis of exclusion, according to Abigail Musial, MD, who is completing a fellowship in hospital medicine at Cincinnati Children’s. She presented a case in which a patient suspected of EVALI went home after symptoms abated on steroids.
“Less than 24 hours later, she returned to the ED with tachypnea and hypoxemia,” Dr. Musial recounted. Although a chest x-ray at the initial evaluation showed lung opacities, a repeat chest x-ray when she returned to the ED showed bilateral worsening of these opacities and persistent elevation of inflammatory markers.
“She was started on steroids and also on antibiotics,” Dr. Musial said. “She was weaned quickly from oxygen once the steroids were started and was discharged on hospital day 3.”
For patients suspected of EVALI, COVID-19 testing should be part of the work-up, according to Dr. Kuchipudi. She also recommended an x-ray or CT scan of the lung as well as an evaluation of inflammatory markers.
Dr. Kuchipudi said that more invasive studies than lung function tests, such as bronchoalveolar lavage or lung biopsy, might be considered when severe symptoms make aggressive diagnostic studies attractive.
Steroids and antibiotics typically lead to control of acute symptoms, but patients should be clinically stable for 24-48 hours prior to hospital discharge, according to Dr. Kuchipudi. Follow-up after discharge should include lung function tests and imaging 2-4 weeks later to confirm resolution of abnormalities.
Dr. Kuchipudi stressed the opportunity that an episode of EVALI provides to induce patients to give up nicotine and vaping entirely. Such strategies, such as a nicotine patch, deserve consideration, but she also cautioned that e-cigarettes for smoking cessation should not be recommended to EVALI patients.
The speakers reported no potential conflicts of interest relevant to this study.
according to a synthesis of current information presented at the virtual Pediatric Hospital Medicine.
Respiratory symptoms, including cough, chest pain, and shortness of breath are common but so are constitutive symptoms, including fever, sore throat, muscle aches, nausea and vomiting, said Yamini Kuchipudi, MD, a staff physician at Cincinnati Children’s Hospital, during the session at the virtual meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
If EVALI is not considered across this broad array of symptoms, of which respiratory complaints might not be the most prominent at the time of presentation, the diagnosis might be delayed, Dr. Kuchipudi warned during the virtual meeting.
Teenagers and young adults are the most common users of e-cigarettes and vaping devices. In these patients or in any individual suspected of having EVALI, Dr. Kuchipudi recommended posing questions about vaping relatively early in the work-up “in a confidential and nonjudgmental way.”
Eliciting a truthful history will be particularly important, because the risk of EVALI appears to be largely related to vaping with tetrahydrocannabinol (THC)-containing products rather than with nicotine alone. Although the exact cause of EVALI is not yet completely clear, this condition is now strongly associated with additives to the THC, according to Issa Hanna, MD, of the department of pediatrics at the University of Florida, Jacksonville.
“E-liquid contains products like hydrocarbons, vitamin E acetate, and heavy metals that appear to damage the alveolar epithelium by direct cellular inflammation,” Dr. Hanna explained.
These products are not only found in THC processed for vaping but also for dabbing, a related but different form of inhalation that involves vaporization of highly concentrated THC waxes or resins. Dr. Hanna suggested that the decline in reported cases of EVALI, which has followed the peak incidence in September 2019, is likely to be related to a decline in THC additives as well as greater caution among users.
E-cigarettes were introduced in 2007, according to Dr. Hanna, but EVALI was not widely recognized until cases began accruing early in 2019. By June 2019, the growing number of case reports had attracted the attention of the media as well as public health officials, intensifying the effort to isolate the risks and causes.
Consistent with greater use of e-cigarettes and vaping among younger individuals, nearly 80% of the 2,807 patients hospitalized for EVALI in the United States by February of this year occurred in individuals aged less than 35 years, according to data released by the Centers for Disease Control and Prevention. The median age was less than 25 years. Of these hospitalizations, 68 deaths (2.5%) in 29 states and Washington, D.C., were attributed to EVALI.
Because of the nonspecific symptoms and lack of a definitive diagnostic test, EVALI is considered a diagnosis of exclusion, according to Abigail Musial, MD, who is completing a fellowship in hospital medicine at Cincinnati Children’s. She presented a case in which a patient suspected of EVALI went home after symptoms abated on steroids.
“Less than 24 hours later, she returned to the ED with tachypnea and hypoxemia,” Dr. Musial recounted. Although a chest x-ray at the initial evaluation showed lung opacities, a repeat chest x-ray when she returned to the ED showed bilateral worsening of these opacities and persistent elevation of inflammatory markers.
“She was started on steroids and also on antibiotics,” Dr. Musial said. “She was weaned quickly from oxygen once the steroids were started and was discharged on hospital day 3.”
For patients suspected of EVALI, COVID-19 testing should be part of the work-up, according to Dr. Kuchipudi. She also recommended an x-ray or CT scan of the lung as well as an evaluation of inflammatory markers.
Dr. Kuchipudi said that more invasive studies than lung function tests, such as bronchoalveolar lavage or lung biopsy, might be considered when severe symptoms make aggressive diagnostic studies attractive.
Steroids and antibiotics typically lead to control of acute symptoms, but patients should be clinically stable for 24-48 hours prior to hospital discharge, according to Dr. Kuchipudi. Follow-up after discharge should include lung function tests and imaging 2-4 weeks later to confirm resolution of abnormalities.
Dr. Kuchipudi stressed the opportunity that an episode of EVALI provides to induce patients to give up nicotine and vaping entirely. Such strategies, such as a nicotine patch, deserve consideration, but she also cautioned that e-cigarettes for smoking cessation should not be recommended to EVALI patients.
The speakers reported no potential conflicts of interest relevant to this study.
FROM PHM20
Behind the mask
Bicycling has always been part of who I am because it offered me the freedom to explore as a preteen. As an adult I have always been a bicycle commuter and a very visible part of the community as I pedal around town to do my errands. But, I didn’t always wear a helmet ... because well, I just didn’t. I saw the helmet as a nuisance with very little benefit to myself. Eventually, when bike races required helmets I bought one just for the competitions. Until one day about 30 years ago when the mother of a child I was seeing in the office said, “Dr. Wilkoff, you know as an influential member of this community, particularly its children, you should be wearing a helmet.” My wife had been badgering me for years but this woman’s courage to speak up embarrassed me into changing my ways.
For some, maybe many, people, wearing a mask during the COVID-19 pandemic is a nuisance and an assault on their independence just as I viewed a bicycle helmet. Initially there was some information being circulated that any mask less robust than a N-95 had very little if any effect, either as protection or as way to decrease spread. I certainly had my doubts about the value of mask other than as a statement of solidarity. However, we are now learning that masks can serve an important role along with social distancing in a comprehensive community effort to minimize contagion.
In light of this new information, why are there are still people who won’t wear a mask? It may be that they are receiving their news filtered through a lens that discredits science. But, it is more likely the result of the same mindset that permeates the anti-vaccine faction that the common good is less important than personal freedom to follow their beliefs.
Do we have any tools at our disposal to increase the number of folks wearing masks? Based on our experience with attempts to convince those who are anti-vaccine, education will be ineffective in shifting the focus from personal freedom to a commitment to the welfare of the community at large. Shaming might be effective, but it runs the risk of igniting conflicts and further widening the gaps in our society. Some establishments have been effective in simply saying “no mask, no entry,” but this runs the same risk of creating friction depending on the community and the situation.
The ship may have already sailed on our best opportunity to achieve community compliance when the leaders of our national government have chosen to ignore their obligation to set an example by refusing to wear masks. I fear that the wedge has already been set and the widening of the gap between those who see their responsibility to the community at large and those who do not will continue to grow.
I am fortunate to live in a town whose residents look out for each other and have relied on local leaders to set an example in the absence of leadership on a national level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Bicycling has always been part of who I am because it offered me the freedom to explore as a preteen. As an adult I have always been a bicycle commuter and a very visible part of the community as I pedal around town to do my errands. But, I didn’t always wear a helmet ... because well, I just didn’t. I saw the helmet as a nuisance with very little benefit to myself. Eventually, when bike races required helmets I bought one just for the competitions. Until one day about 30 years ago when the mother of a child I was seeing in the office said, “Dr. Wilkoff, you know as an influential member of this community, particularly its children, you should be wearing a helmet.” My wife had been badgering me for years but this woman’s courage to speak up embarrassed me into changing my ways.
For some, maybe many, people, wearing a mask during the COVID-19 pandemic is a nuisance and an assault on their independence just as I viewed a bicycle helmet. Initially there was some information being circulated that any mask less robust than a N-95 had very little if any effect, either as protection or as way to decrease spread. I certainly had my doubts about the value of mask other than as a statement of solidarity. However, we are now learning that masks can serve an important role along with social distancing in a comprehensive community effort to minimize contagion.
In light of this new information, why are there are still people who won’t wear a mask? It may be that they are receiving their news filtered through a lens that discredits science. But, it is more likely the result of the same mindset that permeates the anti-vaccine faction that the common good is less important than personal freedom to follow their beliefs.
Do we have any tools at our disposal to increase the number of folks wearing masks? Based on our experience with attempts to convince those who are anti-vaccine, education will be ineffective in shifting the focus from personal freedom to a commitment to the welfare of the community at large. Shaming might be effective, but it runs the risk of igniting conflicts and further widening the gaps in our society. Some establishments have been effective in simply saying “no mask, no entry,” but this runs the same risk of creating friction depending on the community and the situation.
The ship may have already sailed on our best opportunity to achieve community compliance when the leaders of our national government have chosen to ignore their obligation to set an example by refusing to wear masks. I fear that the wedge has already been set and the widening of the gap between those who see their responsibility to the community at large and those who do not will continue to grow.
I am fortunate to live in a town whose residents look out for each other and have relied on local leaders to set an example in the absence of leadership on a national level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Bicycling has always been part of who I am because it offered me the freedom to explore as a preteen. As an adult I have always been a bicycle commuter and a very visible part of the community as I pedal around town to do my errands. But, I didn’t always wear a helmet ... because well, I just didn’t. I saw the helmet as a nuisance with very little benefit to myself. Eventually, when bike races required helmets I bought one just for the competitions. Until one day about 30 years ago when the mother of a child I was seeing in the office said, “Dr. Wilkoff, you know as an influential member of this community, particularly its children, you should be wearing a helmet.” My wife had been badgering me for years but this woman’s courage to speak up embarrassed me into changing my ways.
For some, maybe many, people, wearing a mask during the COVID-19 pandemic is a nuisance and an assault on their independence just as I viewed a bicycle helmet. Initially there was some information being circulated that any mask less robust than a N-95 had very little if any effect, either as protection or as way to decrease spread. I certainly had my doubts about the value of mask other than as a statement of solidarity. However, we are now learning that masks can serve an important role along with social distancing in a comprehensive community effort to minimize contagion.
In light of this new information, why are there are still people who won’t wear a mask? It may be that they are receiving their news filtered through a lens that discredits science. But, it is more likely the result of the same mindset that permeates the anti-vaccine faction that the common good is less important than personal freedom to follow their beliefs.
Do we have any tools at our disposal to increase the number of folks wearing masks? Based on our experience with attempts to convince those who are anti-vaccine, education will be ineffective in shifting the focus from personal freedom to a commitment to the welfare of the community at large. Shaming might be effective, but it runs the risk of igniting conflicts and further widening the gaps in our society. Some establishments have been effective in simply saying “no mask, no entry,” but this runs the same risk of creating friction depending on the community and the situation.
The ship may have already sailed on our best opportunity to achieve community compliance when the leaders of our national government have chosen to ignore their obligation to set an example by refusing to wear masks. I fear that the wedge has already been set and the widening of the gap between those who see their responsibility to the community at large and those who do not will continue to grow.
I am fortunate to live in a town whose residents look out for each other and have relied on local leaders to set an example in the absence of leadership on a national level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Early screening may halve breast cancer mortality in childhood cancer survivors
Two strategies – annual mammography with MRI and annual MRI alone – at least halved breast cancer mortality when started at the ages of 25 or 30 years.
Jennifer M. Yeh, PhD, of Harvard Medical School in Boston and colleagues reported these results in the Annals of Internal Medicine.
When cost was also considered, 30 years emerged as the preferred starting age, dropping the incremental cost-effectiveness ratio (ICER) below the generally accepted threshold of $100,000 per quality-adjusted life-year gained.
“Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with these patients,” Dr. Yeh and colleagues wrote.
“Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors,” the authors recommended. “Our findings also highlight the importance of ensuring that survivors have access to health insurance coverage for MRI screening.”
Implications for awareness, coverage
“My hope is that, by showing the significantly decreased risk of death associated with early breast cancer screening, with harm-benefit ratios considerably lower than benchmarks for average-risk women, this study will help health insurance companies see the benefit in covering early screening for at-risk survivors,” commented Karen E. Effinger, MD, of Emory University, Atlanta, and the Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta.
“In many survivors, the cost of current screening [as recommended by] guidelines is prohibitive,” added Dr. Effinger, who was not involved in the current study.
The main concern regarding the study’s findings is generalizability to the contemporary era, given the use of a cohort diagnosed and treated decades ago and changes in radiation techniques and dosing since then, she noted in an interview. This limitation was addressed in a sensitivity analysis that halved the women’s base-case lifetime risk of breast cancer and still netted similar results.
“However, it will take many years to determine the true risk reduction of our current treatment strategies,” Dr. Effinger acknowledged.
“It is crucial that we improve our education of both survivors and our colleagues who care for these survivors, especially in regard to risk of subsequent malignancies and the benefits of screening,” Dr. Effinger maintained. “While many people are aware of the risk of breast cancer associated with BRCA mutations, the increased risk in survivors of childhood cancer is not as recognized by nononcologists. This study reinforces that increasing this awareness can save lives.”
In educating their patients about preventive care, health care providers must strike “a fine balance between discussing the risks and benefits of screening without provoking significant anxiety,” she concluded. “It is important for survivors to establish care with a primary care provider in order to develop trust and receive the guidance they need to decrease the risk of early mortality.”
Study details
Dr. Yeh and colleagues developed models to compare outcomes with various screening strategies among women aged 20 years who had received chest radiotherapy for childhood cancer during 1970-1986. The women had been diagnosed with Hodgkin lymphoma (55%), Wilms tumor (12%), non-Hodgkin lymphoma (8%), and other cancers.
The investigators conducted their analysis using data from the Childhood Cancer Survivor Study and other published sources, a lifetime time horizon, and a payer perspective.
The team assessed three strategies: no screening; digital mammography with MRI screening starting at 25 years of age (the current Children’s Oncology Group recommendation), 30 years, or 35 years and continuing to 74 years of age; and MRI only starting at age 25, 30, or 35 years and continuing to age 74 years.
The main study results showed that, without screening, women who had received chest radiation for childhood cancer had a 10%-11% lifetime risk of breast cancer mortality across models.
Relative to no screening, starting at age 25 years, the largest share of deaths was averted with the strategy of annual mammography with MRI – 56.3%-71.2% – or with the strategy of annual MRI alone – 55.7%-62.0%.
These two strategies also yielded the most screening tests, as well as the most false-positive test results and benign biopsy results.
For women who started screening at age 25, there were 4,188-4,879 false-positive test results per 1,000 women for mammography plus MRI and 3,283-3,764 false-positive results per 1,000 women for MRI alone.
For women who started screening at age 25, there were 1,340-1,561 benign biopsy results per 1,000 women for mammography plus MRI and 1,248-1,430 benign results per 1,000 women for MRI alone.
After cost was factored in, beginning screening at age 30 emerged as the preferred strategy to achieve an ICER threshold of less than $100,000 per quality-adjusted life-year gained.
When started at 30 years of age, annual mammography with MRI averted 54.7%-68.8% of breast cancer deaths, with an ICER of $25,400-$113,200 per quality-adjusted life-year gained. Annual MRI alone averted 54.0%-60.0% of breast cancer deaths, with an ICER of $21,800-$50,580 per quality-adjusted life-year gained.
This research was supported by grants from the National Cancer Institute, American Cancer Society, and American Lebanese Syrian Associated Charities. The authors disclosed relationships with GE Healthcare and Biovector. Dr. Effinger disclosed no relevant conflicts of interest.
SOURCE: Yeh JM et al. Ann Intern Med. 2020 Jul 7. doi: 10.7326/M19-3481.
Two strategies – annual mammography with MRI and annual MRI alone – at least halved breast cancer mortality when started at the ages of 25 or 30 years.
Jennifer M. Yeh, PhD, of Harvard Medical School in Boston and colleagues reported these results in the Annals of Internal Medicine.
When cost was also considered, 30 years emerged as the preferred starting age, dropping the incremental cost-effectiveness ratio (ICER) below the generally accepted threshold of $100,000 per quality-adjusted life-year gained.
“Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with these patients,” Dr. Yeh and colleagues wrote.
“Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors,” the authors recommended. “Our findings also highlight the importance of ensuring that survivors have access to health insurance coverage for MRI screening.”
Implications for awareness, coverage
“My hope is that, by showing the significantly decreased risk of death associated with early breast cancer screening, with harm-benefit ratios considerably lower than benchmarks for average-risk women, this study will help health insurance companies see the benefit in covering early screening for at-risk survivors,” commented Karen E. Effinger, MD, of Emory University, Atlanta, and the Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta.
“In many survivors, the cost of current screening [as recommended by] guidelines is prohibitive,” added Dr. Effinger, who was not involved in the current study.
The main concern regarding the study’s findings is generalizability to the contemporary era, given the use of a cohort diagnosed and treated decades ago and changes in radiation techniques and dosing since then, she noted in an interview. This limitation was addressed in a sensitivity analysis that halved the women’s base-case lifetime risk of breast cancer and still netted similar results.
“However, it will take many years to determine the true risk reduction of our current treatment strategies,” Dr. Effinger acknowledged.
“It is crucial that we improve our education of both survivors and our colleagues who care for these survivors, especially in regard to risk of subsequent malignancies and the benefits of screening,” Dr. Effinger maintained. “While many people are aware of the risk of breast cancer associated with BRCA mutations, the increased risk in survivors of childhood cancer is not as recognized by nononcologists. This study reinforces that increasing this awareness can save lives.”
In educating their patients about preventive care, health care providers must strike “a fine balance between discussing the risks and benefits of screening without provoking significant anxiety,” she concluded. “It is important for survivors to establish care with a primary care provider in order to develop trust and receive the guidance they need to decrease the risk of early mortality.”
Study details
Dr. Yeh and colleagues developed models to compare outcomes with various screening strategies among women aged 20 years who had received chest radiotherapy for childhood cancer during 1970-1986. The women had been diagnosed with Hodgkin lymphoma (55%), Wilms tumor (12%), non-Hodgkin lymphoma (8%), and other cancers.
The investigators conducted their analysis using data from the Childhood Cancer Survivor Study and other published sources, a lifetime time horizon, and a payer perspective.
The team assessed three strategies: no screening; digital mammography with MRI screening starting at 25 years of age (the current Children’s Oncology Group recommendation), 30 years, or 35 years and continuing to 74 years of age; and MRI only starting at age 25, 30, or 35 years and continuing to age 74 years.
The main study results showed that, without screening, women who had received chest radiation for childhood cancer had a 10%-11% lifetime risk of breast cancer mortality across models.
Relative to no screening, starting at age 25 years, the largest share of deaths was averted with the strategy of annual mammography with MRI – 56.3%-71.2% – or with the strategy of annual MRI alone – 55.7%-62.0%.
These two strategies also yielded the most screening tests, as well as the most false-positive test results and benign biopsy results.
For women who started screening at age 25, there were 4,188-4,879 false-positive test results per 1,000 women for mammography plus MRI and 3,283-3,764 false-positive results per 1,000 women for MRI alone.
For women who started screening at age 25, there were 1,340-1,561 benign biopsy results per 1,000 women for mammography plus MRI and 1,248-1,430 benign results per 1,000 women for MRI alone.
After cost was factored in, beginning screening at age 30 emerged as the preferred strategy to achieve an ICER threshold of less than $100,000 per quality-adjusted life-year gained.
When started at 30 years of age, annual mammography with MRI averted 54.7%-68.8% of breast cancer deaths, with an ICER of $25,400-$113,200 per quality-adjusted life-year gained. Annual MRI alone averted 54.0%-60.0% of breast cancer deaths, with an ICER of $21,800-$50,580 per quality-adjusted life-year gained.
This research was supported by grants from the National Cancer Institute, American Cancer Society, and American Lebanese Syrian Associated Charities. The authors disclosed relationships with GE Healthcare and Biovector. Dr. Effinger disclosed no relevant conflicts of interest.
SOURCE: Yeh JM et al. Ann Intern Med. 2020 Jul 7. doi: 10.7326/M19-3481.
Two strategies – annual mammography with MRI and annual MRI alone – at least halved breast cancer mortality when started at the ages of 25 or 30 years.
Jennifer M. Yeh, PhD, of Harvard Medical School in Boston and colleagues reported these results in the Annals of Internal Medicine.
When cost was also considered, 30 years emerged as the preferred starting age, dropping the incremental cost-effectiveness ratio (ICER) below the generally accepted threshold of $100,000 per quality-adjusted life-year gained.
“Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with these patients,” Dr. Yeh and colleagues wrote.
“Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors,” the authors recommended. “Our findings also highlight the importance of ensuring that survivors have access to health insurance coverage for MRI screening.”
Implications for awareness, coverage
“My hope is that, by showing the significantly decreased risk of death associated with early breast cancer screening, with harm-benefit ratios considerably lower than benchmarks for average-risk women, this study will help health insurance companies see the benefit in covering early screening for at-risk survivors,” commented Karen E. Effinger, MD, of Emory University, Atlanta, and the Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta.
“In many survivors, the cost of current screening [as recommended by] guidelines is prohibitive,” added Dr. Effinger, who was not involved in the current study.
The main concern regarding the study’s findings is generalizability to the contemporary era, given the use of a cohort diagnosed and treated decades ago and changes in radiation techniques and dosing since then, she noted in an interview. This limitation was addressed in a sensitivity analysis that halved the women’s base-case lifetime risk of breast cancer and still netted similar results.
“However, it will take many years to determine the true risk reduction of our current treatment strategies,” Dr. Effinger acknowledged.
“It is crucial that we improve our education of both survivors and our colleagues who care for these survivors, especially in regard to risk of subsequent malignancies and the benefits of screening,” Dr. Effinger maintained. “While many people are aware of the risk of breast cancer associated with BRCA mutations, the increased risk in survivors of childhood cancer is not as recognized by nononcologists. This study reinforces that increasing this awareness can save lives.”
In educating their patients about preventive care, health care providers must strike “a fine balance between discussing the risks and benefits of screening without provoking significant anxiety,” she concluded. “It is important for survivors to establish care with a primary care provider in order to develop trust and receive the guidance they need to decrease the risk of early mortality.”
Study details
Dr. Yeh and colleagues developed models to compare outcomes with various screening strategies among women aged 20 years who had received chest radiotherapy for childhood cancer during 1970-1986. The women had been diagnosed with Hodgkin lymphoma (55%), Wilms tumor (12%), non-Hodgkin lymphoma (8%), and other cancers.
The investigators conducted their analysis using data from the Childhood Cancer Survivor Study and other published sources, a lifetime time horizon, and a payer perspective.
The team assessed three strategies: no screening; digital mammography with MRI screening starting at 25 years of age (the current Children’s Oncology Group recommendation), 30 years, or 35 years and continuing to 74 years of age; and MRI only starting at age 25, 30, or 35 years and continuing to age 74 years.
The main study results showed that, without screening, women who had received chest radiation for childhood cancer had a 10%-11% lifetime risk of breast cancer mortality across models.
Relative to no screening, starting at age 25 years, the largest share of deaths was averted with the strategy of annual mammography with MRI – 56.3%-71.2% – or with the strategy of annual MRI alone – 55.7%-62.0%.
These two strategies also yielded the most screening tests, as well as the most false-positive test results and benign biopsy results.
For women who started screening at age 25, there were 4,188-4,879 false-positive test results per 1,000 women for mammography plus MRI and 3,283-3,764 false-positive results per 1,000 women for MRI alone.
For women who started screening at age 25, there were 1,340-1,561 benign biopsy results per 1,000 women for mammography plus MRI and 1,248-1,430 benign results per 1,000 women for MRI alone.
After cost was factored in, beginning screening at age 30 emerged as the preferred strategy to achieve an ICER threshold of less than $100,000 per quality-adjusted life-year gained.
When started at 30 years of age, annual mammography with MRI averted 54.7%-68.8% of breast cancer deaths, with an ICER of $25,400-$113,200 per quality-adjusted life-year gained. Annual MRI alone averted 54.0%-60.0% of breast cancer deaths, with an ICER of $21,800-$50,580 per quality-adjusted life-year gained.
This research was supported by grants from the National Cancer Institute, American Cancer Society, and American Lebanese Syrian Associated Charities. The authors disclosed relationships with GE Healthcare and Biovector. Dr. Effinger disclosed no relevant conflicts of interest.
SOURCE: Yeh JM et al. Ann Intern Med. 2020 Jul 7. doi: 10.7326/M19-3481.
FROM ANNALS OF INTERNAL MEDICINE
AAP releases new policy statement on barrier protection for teens
For adolescent patients, routinely take a sexual history, discuss the use of barrier methods, and perform relevant examinations, screenings, and vaccinations, according to a new policy statement on barrier protection use from the American Academy of Pediatrics’ Committee on Adolescence.
The policy statement has been expanded to cover multiple types of sexual activity and methods of barrier protection. These include not only traditional condoms, but also internal condoms (available in the United States only by prescription) and dental dams (for use during oral sex) or a latex sheet. “Pediatricians and other clinicians are encouraged to provide barrier methods within their offices and support availability within their communities,” said Laura K. Grubb, MD, MPH, of Tufts Medical Center in Boston, who authored both the policy statement and the technical report.
Counsel adolescents that abstaining from sexual intercourse is the best way to prevent genital sexually transmitted infections (STIs), HIV infection, and unplanned pregnancy. Also encourage and support consistent, correct barrier method use – in addition to other reliable contraception, if patients are sexually active or are thinking about becoming sexually active – the policy statement notes. Emphasize that all partners share responsibility to prevent STIs and unplanned pregnancies. “Adolescents with intellectual and physical disabilities are an overlooked group when it comes to sexual behavior, but they have similar rates of sexual behaviors when compared with their peers without disabilities,” Dr. Grubb and colleagues emphasized in the policy statement.
This is key because Centers for Disease Control and Prevention 2017 data showed that in the United States, “456,000 adolescent and young women younger than 20 years became pregnant; 448,000 of those pregnancies were among 15- to 19-year-olds, and 7,400 were among those 14 years of age and younger,” according to the technical report accompanying the policy statement. Also, “new cases of STIs increased 31% in the United States from 2013 to 2017, with half of the 2.3 million new STIs reported each year among young people 15 to 24 years of age.”
Parents may need support and encouragement to talk with their children about sex, sexuality, and the use of barrier methods to prevent STIs. Dr. Grubb and colleagues recommend via the policy statement: “Actively communicate to parents and communities that making barrier methods available to adolescents does not increase the onset or frequency of adolescent sexual activity, and that use of barrier methods can help decrease rates of unintended pregnancy and acquisition of STIs.”
Use Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents, Fourth Edition, for guidance on supporting parents and adolescents in promoting healthy sexual development and sexuality, including discussions of barrier methods.
Some groups of adolescents may use barrier methods less consistently because they perceive themselves to be lower risk. These include adolescents who use preexposure prophylaxis or nonbarrier contraception, who identify as bisexual or lesbian, or who are in established relationships. Monitor these patients to assess their risk and need for additional counseling. In the technical report, studies are cited finding that barrier methods are used less consistently during oral sex and that condom use is lower among cisgender and transgender females, and among adolescents who self-identify as gay, lesbian, or bisexual, compared with other groups.
In the policy statement, Dr. Grubb and colleagues call on pediatricians to advocate for more research and better access to barrier methods, especially for higher-risk adolescents and those living in underserved areas. In particular, school education programs on barrier methods can reach large adolescent groups and provide a “comprehensive array of educational and health care resources.”
Katie Brigham, MD, a pediatrician at MassGeneral Hospital for Children in Boston, affirmed the recommendations in the new policy statement (which she did not help write or research). “Even though the pregnancy rate is dropping in the United States, STI rates are increasing, so it is vital that pediatricians and other providers of adolescents and young adults counsel all their patients, regardless of gender and sexual orientation, of the importance of barrier methods when having oral, vaginal, or anal sex,” she said in an interview.
Dr. Brigham praised the technical report, adding that she found no major weaknesses in its methodology. “For future research, it would be interesting to see if there are different rates of pregnancy and STIs in pediatric practices that provide condoms and other barrier methods free to their patients, compared to those that do not.”
No external funding sources were reported. Dr. Grubb and Dr. Brigham reported having no relevant financial disclosures.
SOURCE: Grubb LK et al. Pediatrics. 2020 Jul 20. doi: 10.1542/peds.2020-007237.
For adolescent patients, routinely take a sexual history, discuss the use of barrier methods, and perform relevant examinations, screenings, and vaccinations, according to a new policy statement on barrier protection use from the American Academy of Pediatrics’ Committee on Adolescence.
The policy statement has been expanded to cover multiple types of sexual activity and methods of barrier protection. These include not only traditional condoms, but also internal condoms (available in the United States only by prescription) and dental dams (for use during oral sex) or a latex sheet. “Pediatricians and other clinicians are encouraged to provide barrier methods within their offices and support availability within their communities,” said Laura K. Grubb, MD, MPH, of Tufts Medical Center in Boston, who authored both the policy statement and the technical report.
Counsel adolescents that abstaining from sexual intercourse is the best way to prevent genital sexually transmitted infections (STIs), HIV infection, and unplanned pregnancy. Also encourage and support consistent, correct barrier method use – in addition to other reliable contraception, if patients are sexually active or are thinking about becoming sexually active – the policy statement notes. Emphasize that all partners share responsibility to prevent STIs and unplanned pregnancies. “Adolescents with intellectual and physical disabilities are an overlooked group when it comes to sexual behavior, but they have similar rates of sexual behaviors when compared with their peers without disabilities,” Dr. Grubb and colleagues emphasized in the policy statement.
This is key because Centers for Disease Control and Prevention 2017 data showed that in the United States, “456,000 adolescent and young women younger than 20 years became pregnant; 448,000 of those pregnancies were among 15- to 19-year-olds, and 7,400 were among those 14 years of age and younger,” according to the technical report accompanying the policy statement. Also, “new cases of STIs increased 31% in the United States from 2013 to 2017, with half of the 2.3 million new STIs reported each year among young people 15 to 24 years of age.”
Parents may need support and encouragement to talk with their children about sex, sexuality, and the use of barrier methods to prevent STIs. Dr. Grubb and colleagues recommend via the policy statement: “Actively communicate to parents and communities that making barrier methods available to adolescents does not increase the onset or frequency of adolescent sexual activity, and that use of barrier methods can help decrease rates of unintended pregnancy and acquisition of STIs.”
Use Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents, Fourth Edition, for guidance on supporting parents and adolescents in promoting healthy sexual development and sexuality, including discussions of barrier methods.
Some groups of adolescents may use barrier methods less consistently because they perceive themselves to be lower risk. These include adolescents who use preexposure prophylaxis or nonbarrier contraception, who identify as bisexual or lesbian, or who are in established relationships. Monitor these patients to assess their risk and need for additional counseling. In the technical report, studies are cited finding that barrier methods are used less consistently during oral sex and that condom use is lower among cisgender and transgender females, and among adolescents who self-identify as gay, lesbian, or bisexual, compared with other groups.
In the policy statement, Dr. Grubb and colleagues call on pediatricians to advocate for more research and better access to barrier methods, especially for higher-risk adolescents and those living in underserved areas. In particular, school education programs on barrier methods can reach large adolescent groups and provide a “comprehensive array of educational and health care resources.”
Katie Brigham, MD, a pediatrician at MassGeneral Hospital for Children in Boston, affirmed the recommendations in the new policy statement (which she did not help write or research). “Even though the pregnancy rate is dropping in the United States, STI rates are increasing, so it is vital that pediatricians and other providers of adolescents and young adults counsel all their patients, regardless of gender and sexual orientation, of the importance of barrier methods when having oral, vaginal, or anal sex,” she said in an interview.
Dr. Brigham praised the technical report, adding that she found no major weaknesses in its methodology. “For future research, it would be interesting to see if there are different rates of pregnancy and STIs in pediatric practices that provide condoms and other barrier methods free to their patients, compared to those that do not.”
No external funding sources were reported. Dr. Grubb and Dr. Brigham reported having no relevant financial disclosures.
SOURCE: Grubb LK et al. Pediatrics. 2020 Jul 20. doi: 10.1542/peds.2020-007237.
For adolescent patients, routinely take a sexual history, discuss the use of barrier methods, and perform relevant examinations, screenings, and vaccinations, according to a new policy statement on barrier protection use from the American Academy of Pediatrics’ Committee on Adolescence.
The policy statement has been expanded to cover multiple types of sexual activity and methods of barrier protection. These include not only traditional condoms, but also internal condoms (available in the United States only by prescription) and dental dams (for use during oral sex) or a latex sheet. “Pediatricians and other clinicians are encouraged to provide barrier methods within their offices and support availability within their communities,” said Laura K. Grubb, MD, MPH, of Tufts Medical Center in Boston, who authored both the policy statement and the technical report.
Counsel adolescents that abstaining from sexual intercourse is the best way to prevent genital sexually transmitted infections (STIs), HIV infection, and unplanned pregnancy. Also encourage and support consistent, correct barrier method use – in addition to other reliable contraception, if patients are sexually active or are thinking about becoming sexually active – the policy statement notes. Emphasize that all partners share responsibility to prevent STIs and unplanned pregnancies. “Adolescents with intellectual and physical disabilities are an overlooked group when it comes to sexual behavior, but they have similar rates of sexual behaviors when compared with their peers without disabilities,” Dr. Grubb and colleagues emphasized in the policy statement.
This is key because Centers for Disease Control and Prevention 2017 data showed that in the United States, “456,000 adolescent and young women younger than 20 years became pregnant; 448,000 of those pregnancies were among 15- to 19-year-olds, and 7,400 were among those 14 years of age and younger,” according to the technical report accompanying the policy statement. Also, “new cases of STIs increased 31% in the United States from 2013 to 2017, with half of the 2.3 million new STIs reported each year among young people 15 to 24 years of age.”
Parents may need support and encouragement to talk with their children about sex, sexuality, and the use of barrier methods to prevent STIs. Dr. Grubb and colleagues recommend via the policy statement: “Actively communicate to parents and communities that making barrier methods available to adolescents does not increase the onset or frequency of adolescent sexual activity, and that use of barrier methods can help decrease rates of unintended pregnancy and acquisition of STIs.”
Use Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents, Fourth Edition, for guidance on supporting parents and adolescents in promoting healthy sexual development and sexuality, including discussions of barrier methods.
Some groups of adolescents may use barrier methods less consistently because they perceive themselves to be lower risk. These include adolescents who use preexposure prophylaxis or nonbarrier contraception, who identify as bisexual or lesbian, or who are in established relationships. Monitor these patients to assess their risk and need for additional counseling. In the technical report, studies are cited finding that barrier methods are used less consistently during oral sex and that condom use is lower among cisgender and transgender females, and among adolescents who self-identify as gay, lesbian, or bisexual, compared with other groups.
In the policy statement, Dr. Grubb and colleagues call on pediatricians to advocate for more research and better access to barrier methods, especially for higher-risk adolescents and those living in underserved areas. In particular, school education programs on barrier methods can reach large adolescent groups and provide a “comprehensive array of educational and health care resources.”
Katie Brigham, MD, a pediatrician at MassGeneral Hospital for Children in Boston, affirmed the recommendations in the new policy statement (which she did not help write or research). “Even though the pregnancy rate is dropping in the United States, STI rates are increasing, so it is vital that pediatricians and other providers of adolescents and young adults counsel all their patients, regardless of gender and sexual orientation, of the importance of barrier methods when having oral, vaginal, or anal sex,” she said in an interview.
Dr. Brigham praised the technical report, adding that she found no major weaknesses in its methodology. “For future research, it would be interesting to see if there are different rates of pregnancy and STIs in pediatric practices that provide condoms and other barrier methods free to their patients, compared to those that do not.”
No external funding sources were reported. Dr. Grubb and Dr. Brigham reported having no relevant financial disclosures.
SOURCE: Grubb LK et al. Pediatrics. 2020 Jul 20. doi: 10.1542/peds.2020-007237.
FROM PEDIATRICS
Consider adverse childhood experiences during the pandemic
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
No link between topical steroids and fracture risk found in children with atopic dermatitis
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
FROM SPD 2020
Schools can reopen safely with precautions, experts say
The absence of in-person school has harmed children in ways beyond loss of academic learning, according to Josh Sharfstein, MD, vice dean for public health practice and community engagement at the Johns Hopkins Bloomberg School of Public Health, Baltimore. In addition to learning, school is a place where many children receive breakfast and lunch every day, as well as support services and the benefits of being in a safe and secure environment, Dr. Sharfstein said in a press briefing sponsored by Johns Hopkins University.
However, although it is an important priority for children to return to school, “we are in the midst of a pandemic that poses real risk,” he said.
In the press briefing, several experts shared ideas and considerations for safely reopening K-12 schools in the fall of 2020.
Data from other countries where schools have reopened, notably Austria and Denmark, have been reassuring about the lack of transmission of SARS-CoV-2 among children in a school setting, said Jennifer Nuzzo, DrPH, an epidemiologist at the Johns Hopkins Center for Health Security. However, other countries where schools have reopened successfully have reported low levels of viral transmission locally, and a responsible strategy for school reopening in the United States should follow a similar plan, she said. In areas where transmission and infection rates are increasing “it may not be safe to reopen,” but in areas where rates are declining or stable, schools could potentially reopen if they follow safety measures.
Dr. Nuzzo suggested that Considerations include protocols for handwashing and sanitation, and maintaining physical distance by creative use of outdoor classrooms (weather permitting) or other spaces within school buildings. Transportation to and from school also will be an issue to address, she noted.
None of the strategies being considered will completely eliminate risk of SARS-CoV-2 infection in school settings, so allowing parents and students to opt out and choose distance learning will be important as well, said Dr. Nuzzo. In addition, schools may need to consider alternative roles for teachers and staff who don’t feel comfortable being in contact with students and fellow staff members. “All of these things are going to be hard,” Dr. Nuzzo acknowledged. “Hard should not be a deterrent,” to reopening schools, but “we acknowledge the resources that schools will need in order to do this.”
At present, all 50 states and the District of Columbia have released some type of plan for reopening schools, said Megan Collins, MD, MPH, codirector the Johns Hopkins Consortium for School-Based Health Solutions.
Dr. Collins and colleagues have developed a school reopening tracker, which is “a national snapshot of current reopening plans that have been released,” she said. The tracker is being updated continuously as plans evolve. The eSchool+ K-12 School Reopening Tracker identifies 12 reopening categories that states could potentially address in the plans. These categories are divided into Operational and Ethics/Equity. The operational categories include:
- Core academics
- SARS-CoV-2 protection
- Before and after school programs
- School access and transportation
- Student health services
- Food and nutrition.
Ethics/equity categories include the following:
- Parent choice
- Teacher and staff choice
- Children of poverty and systemic disadvantage
- Children with special needs/English as second language/gifted and twice exceptional
- Privacy
- Engagement and transparency.
As of July 15, 2020, 16 states (Arizona, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, North Carolina, North Dakota, Ohio, Rhode Island, Tennessee, Texas, Virginia, Washington, and Wisconsin) had addressed all 12 categories in their reopening plans, Dr. Collins said.
School reopening plans must take equity issues into account, said Annette Anderson, PhD, of the Johns Hopkins University School of Education.
Specifically, developing learning plans for special education students and others at the most risk for learning loss will be essential. “The digital divide has become a digital canyon” in some areas, Dr. Anderson noted, and schools need to rethink eligibility and work to provide access to devices for online learning for all students.
In addition, schools need to convince parents that schools are safe. She recommended that schools consider inviting parents and families to visit buildings in advance of reopening so they can see the safety measures, such as space between desks, cleaning stations, and other protective strategies.
The message to pediatricians and health care professionals when counseling families about returning individual children to school is to consider the risk to the child and the family directly in the context of the local plans, Dr. Sharfstein said during a question and answer session. “One school system’s plan is one school system’s plan,” he said, and added that families who are concerned about the risk should have an online option. However, “if you see a thoughtful approach” to reopening, with safety steps taken and parents informed, with protocols such as keeping small groups of children together to reduce transmission, “it is a pretty good trade-off,” and that is why the American Academy of Pediatrics currently favors children returning to school, he said.
The briefing participants had no relevant financial conflicts to disclose.
The absence of in-person school has harmed children in ways beyond loss of academic learning, according to Josh Sharfstein, MD, vice dean for public health practice and community engagement at the Johns Hopkins Bloomberg School of Public Health, Baltimore. In addition to learning, school is a place where many children receive breakfast and lunch every day, as well as support services and the benefits of being in a safe and secure environment, Dr. Sharfstein said in a press briefing sponsored by Johns Hopkins University.
However, although it is an important priority for children to return to school, “we are in the midst of a pandemic that poses real risk,” he said.
In the press briefing, several experts shared ideas and considerations for safely reopening K-12 schools in the fall of 2020.
Data from other countries where schools have reopened, notably Austria and Denmark, have been reassuring about the lack of transmission of SARS-CoV-2 among children in a school setting, said Jennifer Nuzzo, DrPH, an epidemiologist at the Johns Hopkins Center for Health Security. However, other countries where schools have reopened successfully have reported low levels of viral transmission locally, and a responsible strategy for school reopening in the United States should follow a similar plan, she said. In areas where transmission and infection rates are increasing “it may not be safe to reopen,” but in areas where rates are declining or stable, schools could potentially reopen if they follow safety measures.
Dr. Nuzzo suggested that Considerations include protocols for handwashing and sanitation, and maintaining physical distance by creative use of outdoor classrooms (weather permitting) or other spaces within school buildings. Transportation to and from school also will be an issue to address, she noted.
None of the strategies being considered will completely eliminate risk of SARS-CoV-2 infection in school settings, so allowing parents and students to opt out and choose distance learning will be important as well, said Dr. Nuzzo. In addition, schools may need to consider alternative roles for teachers and staff who don’t feel comfortable being in contact with students and fellow staff members. “All of these things are going to be hard,” Dr. Nuzzo acknowledged. “Hard should not be a deterrent,” to reopening schools, but “we acknowledge the resources that schools will need in order to do this.”
At present, all 50 states and the District of Columbia have released some type of plan for reopening schools, said Megan Collins, MD, MPH, codirector the Johns Hopkins Consortium for School-Based Health Solutions.
Dr. Collins and colleagues have developed a school reopening tracker, which is “a national snapshot of current reopening plans that have been released,” she said. The tracker is being updated continuously as plans evolve. The eSchool+ K-12 School Reopening Tracker identifies 12 reopening categories that states could potentially address in the plans. These categories are divided into Operational and Ethics/Equity. The operational categories include:
- Core academics
- SARS-CoV-2 protection
- Before and after school programs
- School access and transportation
- Student health services
- Food and nutrition.
Ethics/equity categories include the following:
- Parent choice
- Teacher and staff choice
- Children of poverty and systemic disadvantage
- Children with special needs/English as second language/gifted and twice exceptional
- Privacy
- Engagement and transparency.
As of July 15, 2020, 16 states (Arizona, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, North Carolina, North Dakota, Ohio, Rhode Island, Tennessee, Texas, Virginia, Washington, and Wisconsin) had addressed all 12 categories in their reopening plans, Dr. Collins said.
School reopening plans must take equity issues into account, said Annette Anderson, PhD, of the Johns Hopkins University School of Education.
Specifically, developing learning plans for special education students and others at the most risk for learning loss will be essential. “The digital divide has become a digital canyon” in some areas, Dr. Anderson noted, and schools need to rethink eligibility and work to provide access to devices for online learning for all students.
In addition, schools need to convince parents that schools are safe. She recommended that schools consider inviting parents and families to visit buildings in advance of reopening so they can see the safety measures, such as space between desks, cleaning stations, and other protective strategies.
The message to pediatricians and health care professionals when counseling families about returning individual children to school is to consider the risk to the child and the family directly in the context of the local plans, Dr. Sharfstein said during a question and answer session. “One school system’s plan is one school system’s plan,” he said, and added that families who are concerned about the risk should have an online option. However, “if you see a thoughtful approach” to reopening, with safety steps taken and parents informed, with protocols such as keeping small groups of children together to reduce transmission, “it is a pretty good trade-off,” and that is why the American Academy of Pediatrics currently favors children returning to school, he said.
The briefing participants had no relevant financial conflicts to disclose.
The absence of in-person school has harmed children in ways beyond loss of academic learning, according to Josh Sharfstein, MD, vice dean for public health practice and community engagement at the Johns Hopkins Bloomberg School of Public Health, Baltimore. In addition to learning, school is a place where many children receive breakfast and lunch every day, as well as support services and the benefits of being in a safe and secure environment, Dr. Sharfstein said in a press briefing sponsored by Johns Hopkins University.
However, although it is an important priority for children to return to school, “we are in the midst of a pandemic that poses real risk,” he said.
In the press briefing, several experts shared ideas and considerations for safely reopening K-12 schools in the fall of 2020.
Data from other countries where schools have reopened, notably Austria and Denmark, have been reassuring about the lack of transmission of SARS-CoV-2 among children in a school setting, said Jennifer Nuzzo, DrPH, an epidemiologist at the Johns Hopkins Center for Health Security. However, other countries where schools have reopened successfully have reported low levels of viral transmission locally, and a responsible strategy for school reopening in the United States should follow a similar plan, she said. In areas where transmission and infection rates are increasing “it may not be safe to reopen,” but in areas where rates are declining or stable, schools could potentially reopen if they follow safety measures.
Dr. Nuzzo suggested that Considerations include protocols for handwashing and sanitation, and maintaining physical distance by creative use of outdoor classrooms (weather permitting) or other spaces within school buildings. Transportation to and from school also will be an issue to address, she noted.
None of the strategies being considered will completely eliminate risk of SARS-CoV-2 infection in school settings, so allowing parents and students to opt out and choose distance learning will be important as well, said Dr. Nuzzo. In addition, schools may need to consider alternative roles for teachers and staff who don’t feel comfortable being in contact with students and fellow staff members. “All of these things are going to be hard,” Dr. Nuzzo acknowledged. “Hard should not be a deterrent,” to reopening schools, but “we acknowledge the resources that schools will need in order to do this.”
At present, all 50 states and the District of Columbia have released some type of plan for reopening schools, said Megan Collins, MD, MPH, codirector the Johns Hopkins Consortium for School-Based Health Solutions.
Dr. Collins and colleagues have developed a school reopening tracker, which is “a national snapshot of current reopening plans that have been released,” she said. The tracker is being updated continuously as plans evolve. The eSchool+ K-12 School Reopening Tracker identifies 12 reopening categories that states could potentially address in the plans. These categories are divided into Operational and Ethics/Equity. The operational categories include:
- Core academics
- SARS-CoV-2 protection
- Before and after school programs
- School access and transportation
- Student health services
- Food and nutrition.
Ethics/equity categories include the following:
- Parent choice
- Teacher and staff choice
- Children of poverty and systemic disadvantage
- Children with special needs/English as second language/gifted and twice exceptional
- Privacy
- Engagement and transparency.
As of July 15, 2020, 16 states (Arizona, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, North Carolina, North Dakota, Ohio, Rhode Island, Tennessee, Texas, Virginia, Washington, and Wisconsin) had addressed all 12 categories in their reopening plans, Dr. Collins said.
School reopening plans must take equity issues into account, said Annette Anderson, PhD, of the Johns Hopkins University School of Education.
Specifically, developing learning plans for special education students and others at the most risk for learning loss will be essential. “The digital divide has become a digital canyon” in some areas, Dr. Anderson noted, and schools need to rethink eligibility and work to provide access to devices for online learning for all students.
In addition, schools need to convince parents that schools are safe. She recommended that schools consider inviting parents and families to visit buildings in advance of reopening so they can see the safety measures, such as space between desks, cleaning stations, and other protective strategies.
The message to pediatricians and health care professionals when counseling families about returning individual children to school is to consider the risk to the child and the family directly in the context of the local plans, Dr. Sharfstein said during a question and answer session. “One school system’s plan is one school system’s plan,” he said, and added that families who are concerned about the risk should have an online option. However, “if you see a thoughtful approach” to reopening, with safety steps taken and parents informed, with protocols such as keeping small groups of children together to reduce transmission, “it is a pretty good trade-off,” and that is why the American Academy of Pediatrics currently favors children returning to school, he said.
The briefing participants had no relevant financial conflicts to disclose.