User login
Report describes intoxication with new psychoactive substance
When evaluated at local emergency departments, lethargy and slurred speech were the most common clinical findings.
One student had mild respiratory depression with a respiratory rate of 10 breaths per minute.
“All patients had sufficient clinical improvement within 6 hours such that they could be discharged from the hospital,” according to a description of the cases that was published online in Pediatrics.
The report is the first to detail clinical toxicity from flualprazolam, and “it is likely that physicians will again encounter patients” with intoxication from this new psychoactive drug, said Adam Blumenberg, MD, of Oregon Health & Science University in Portland and colleagues.
Internet purchasing has increased rates of exposure to new psychoactive substances since the early 2000s, and law enforcement agents have seized tons of these drugs. “In the United States, the incidence of exposures to designer benzodiazepines in particular has been rising since 2014,” the authors said.
According to an addiction researcher, the COVID-19 pandemic may exacerbate abuse of designer benzodiazepines.
“This is an important paper describing what medical examiners, pathologists, and emergency rooms have been seeing recently – an increase in designer benzodiazepines,” commented Mark S. Gold, MD, adjunct professor of psychiatry at Washington University in St. Louis. “Recent increases in these drugs have started to be seen in many locations as the traditional drugs of abuse, grown and distributed in bulk, have been disrupted” by the pandemic, he said in an interview. Although it may be too early for such cases to appear in Centers for Disease Control and Prevention reports, they can be described in studies like this one and, “I suspect, sadly, in medical examiner case reports.”
Flualprazolam, known colloquially as Hulk, is structurally related to the Food and Drug Administration–approved drugs alprazolam and triazolam. During 1 week in June 2019, the patients in Oregon received the drug as a free sample from another student from their Oregon high school. They believed it was commercial Xanax (alprazolam). “The flualprazolam tablets were identical in appearance and labeling to 2-mg tablets of alprazolam,” according to the report. “This indicates an intentionally counterfeit product entering the drug supply chain.”
Five of the six patients were boys, and they ranged in age from 14 to 16 years. The patient with mild respiratory depression received 0.4-mg naloxone, which physicians gave empirically because of the unknown identity of the drug, but did not respond. Two of the six patients initially felt drowsy but were asymptomatic during the clinical evaluation.
A urine immunoassay was performed in five of the patients, and all tested positive for benzodiazepines. One patient also tested positive for cannabinoids. Analysis of a tablet fragment revealed that it contained flualprazolam.
“Although flualprazolam intoxication cannot be clinically differentiated from that of other benzodiazepines without advanced testing, patient management should be the same,” Dr. Blumenberg and coauthors said. “For mild to moderate intoxication, patients should be treated with close monitoring and supportive care until symptom resolution. The benzodiazepine antidote flumazenil may be considered a safe and effective antidote in pediatric patients with significant CNS or respiratory depression. In patients for whom there is a concern of benzodiazepine dependence and flumazenil-induced seizures, airway protection and mechanical ventilation may be considered.”
Although patients rarely die from isolated benzodiazepine toxicity, death from respiratory depression or aspiration is more common when benzodiazepine toxicity occurs “in combination with alcohol, opioids, or other sedatives,” the authors noted. In addition, counterfeit alprazolam tablets have contained adulterants such as fentanyl and the opioid U-47700, which can be deadly.
The authors had no relevant financial disclosures, and there was no external funding for the study.
SOURCE: Blumenberg A et al. Pediatrics. 2020 Jun 24. doi: 10.1542/peds.2019-2953.
When evaluated at local emergency departments, lethargy and slurred speech were the most common clinical findings.
One student had mild respiratory depression with a respiratory rate of 10 breaths per minute.
“All patients had sufficient clinical improvement within 6 hours such that they could be discharged from the hospital,” according to a description of the cases that was published online in Pediatrics.
The report is the first to detail clinical toxicity from flualprazolam, and “it is likely that physicians will again encounter patients” with intoxication from this new psychoactive drug, said Adam Blumenberg, MD, of Oregon Health & Science University in Portland and colleagues.
Internet purchasing has increased rates of exposure to new psychoactive substances since the early 2000s, and law enforcement agents have seized tons of these drugs. “In the United States, the incidence of exposures to designer benzodiazepines in particular has been rising since 2014,” the authors said.
According to an addiction researcher, the COVID-19 pandemic may exacerbate abuse of designer benzodiazepines.
“This is an important paper describing what medical examiners, pathologists, and emergency rooms have been seeing recently – an increase in designer benzodiazepines,” commented Mark S. Gold, MD, adjunct professor of psychiatry at Washington University in St. Louis. “Recent increases in these drugs have started to be seen in many locations as the traditional drugs of abuse, grown and distributed in bulk, have been disrupted” by the pandemic, he said in an interview. Although it may be too early for such cases to appear in Centers for Disease Control and Prevention reports, they can be described in studies like this one and, “I suspect, sadly, in medical examiner case reports.”
Flualprazolam, known colloquially as Hulk, is structurally related to the Food and Drug Administration–approved drugs alprazolam and triazolam. During 1 week in June 2019, the patients in Oregon received the drug as a free sample from another student from their Oregon high school. They believed it was commercial Xanax (alprazolam). “The flualprazolam tablets were identical in appearance and labeling to 2-mg tablets of alprazolam,” according to the report. “This indicates an intentionally counterfeit product entering the drug supply chain.”
Five of the six patients were boys, and they ranged in age from 14 to 16 years. The patient with mild respiratory depression received 0.4-mg naloxone, which physicians gave empirically because of the unknown identity of the drug, but did not respond. Two of the six patients initially felt drowsy but were asymptomatic during the clinical evaluation.
A urine immunoassay was performed in five of the patients, and all tested positive for benzodiazepines. One patient also tested positive for cannabinoids. Analysis of a tablet fragment revealed that it contained flualprazolam.
“Although flualprazolam intoxication cannot be clinically differentiated from that of other benzodiazepines without advanced testing, patient management should be the same,” Dr. Blumenberg and coauthors said. “For mild to moderate intoxication, patients should be treated with close monitoring and supportive care until symptom resolution. The benzodiazepine antidote flumazenil may be considered a safe and effective antidote in pediatric patients with significant CNS or respiratory depression. In patients for whom there is a concern of benzodiazepine dependence and flumazenil-induced seizures, airway protection and mechanical ventilation may be considered.”
Although patients rarely die from isolated benzodiazepine toxicity, death from respiratory depression or aspiration is more common when benzodiazepine toxicity occurs “in combination with alcohol, opioids, or other sedatives,” the authors noted. In addition, counterfeit alprazolam tablets have contained adulterants such as fentanyl and the opioid U-47700, which can be deadly.
The authors had no relevant financial disclosures, and there was no external funding for the study.
SOURCE: Blumenberg A et al. Pediatrics. 2020 Jun 24. doi: 10.1542/peds.2019-2953.
When evaluated at local emergency departments, lethargy and slurred speech were the most common clinical findings.
One student had mild respiratory depression with a respiratory rate of 10 breaths per minute.
“All patients had sufficient clinical improvement within 6 hours such that they could be discharged from the hospital,” according to a description of the cases that was published online in Pediatrics.
The report is the first to detail clinical toxicity from flualprazolam, and “it is likely that physicians will again encounter patients” with intoxication from this new psychoactive drug, said Adam Blumenberg, MD, of Oregon Health & Science University in Portland and colleagues.
Internet purchasing has increased rates of exposure to new psychoactive substances since the early 2000s, and law enforcement agents have seized tons of these drugs. “In the United States, the incidence of exposures to designer benzodiazepines in particular has been rising since 2014,” the authors said.
According to an addiction researcher, the COVID-19 pandemic may exacerbate abuse of designer benzodiazepines.
“This is an important paper describing what medical examiners, pathologists, and emergency rooms have been seeing recently – an increase in designer benzodiazepines,” commented Mark S. Gold, MD, adjunct professor of psychiatry at Washington University in St. Louis. “Recent increases in these drugs have started to be seen in many locations as the traditional drugs of abuse, grown and distributed in bulk, have been disrupted” by the pandemic, he said in an interview. Although it may be too early for such cases to appear in Centers for Disease Control and Prevention reports, they can be described in studies like this one and, “I suspect, sadly, in medical examiner case reports.”
Flualprazolam, known colloquially as Hulk, is structurally related to the Food and Drug Administration–approved drugs alprazolam and triazolam. During 1 week in June 2019, the patients in Oregon received the drug as a free sample from another student from their Oregon high school. They believed it was commercial Xanax (alprazolam). “The flualprazolam tablets were identical in appearance and labeling to 2-mg tablets of alprazolam,” according to the report. “This indicates an intentionally counterfeit product entering the drug supply chain.”
Five of the six patients were boys, and they ranged in age from 14 to 16 years. The patient with mild respiratory depression received 0.4-mg naloxone, which physicians gave empirically because of the unknown identity of the drug, but did not respond. Two of the six patients initially felt drowsy but were asymptomatic during the clinical evaluation.
A urine immunoassay was performed in five of the patients, and all tested positive for benzodiazepines. One patient also tested positive for cannabinoids. Analysis of a tablet fragment revealed that it contained flualprazolam.
“Although flualprazolam intoxication cannot be clinically differentiated from that of other benzodiazepines without advanced testing, patient management should be the same,” Dr. Blumenberg and coauthors said. “For mild to moderate intoxication, patients should be treated with close monitoring and supportive care until symptom resolution. The benzodiazepine antidote flumazenil may be considered a safe and effective antidote in pediatric patients with significant CNS or respiratory depression. In patients for whom there is a concern of benzodiazepine dependence and flumazenil-induced seizures, airway protection and mechanical ventilation may be considered.”
Although patients rarely die from isolated benzodiazepine toxicity, death from respiratory depression or aspiration is more common when benzodiazepine toxicity occurs “in combination with alcohol, opioids, or other sedatives,” the authors noted. In addition, counterfeit alprazolam tablets have contained adulterants such as fentanyl and the opioid U-47700, which can be deadly.
The authors had no relevant financial disclosures, and there was no external funding for the study.
SOURCE: Blumenberg A et al. Pediatrics. 2020 Jun 24. doi: 10.1542/peds.2019-2953.
FROM PEDIATRICS
Encourage parents to follow pediatric plans for vaccination
Outpatient medical care has been severely disrupted during the COVID-19 pandemic with a reduction of nearly 70% in outpatient visits since March before starting to rebound, Melinda Wharton, MD, said at the virtual meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Pediatrics was among the hardest hit specialties, with a 62% reduction in outpatient visits by April 5, said Dr. Wharton, director of the immunization services division at the CDC’s National Center for Immunization and Respiratory Diseases. However, visits for all pediatric age groups increased in May, compared with April, and the CDC emphasized the need to educate families about the importance of routine vaccination and well-child visits, Dr. Wharton said.
The CDC strategies to support routine childhood vaccination include monitoring vaccination service delivery to inform targeted interventions, said Dr. Wharton. In addition, the CDC will continue to support providers by identifying gaps in the Vaccines For Children (VFC) program network, increasing VFC funding, developing guidance materials, and identifying policy interventions.
Many small practices have struggled during the pandemic, and financial support is available through the Provider Relief Fund, which is now available to all Medicaid and Children’s Health Insurance Program (CHIP) providers, said Dr. Wharton.
because more families may now qualify for the program because of changes in job status and income, and parents may not be aware that their children may be eligible, she said.
“Vaccination is an essential medical service for all children and adolescents, ideally in the medical home,” Dr. Wharton said. The CDC’s interim guidance for immunization during the COVID-19 pandemic calls for administering all current or overdue vaccines according to the routine immunization schedule during the same visit, and implementing strategies to get patients caught up, prioritizing newborns, infants, and children up to age 24 months. The guidance includes details on safe delivery of vaccines, including physical distance and the use of personal protective equipment.
In addition, encourage parents to return for well-child visits, and use reminder systems to help keep patients current on visits and vaccines. “Discuss the safety protocols that have been put in place,” Dr. Wharton emphasized. The CDC also offers resources for providers to help communicate with parents about routine vaccination.
Looking ahead, back-to-school vaccination requirements “provide a critical checkpoint for children’s vaccination status,” Dr. Wharton said. Catch-up vaccination during the summer will help clinical capacity manage back-to-school and influenza vaccination in the fall, she emphasized. “Influenza vaccination will be an important strategy to decrease stress on our health care system.”
Flu vaccination strategies should focus on adults at higher risk for COVID-19 infections, such as health care providers. In addition, identifying and reducing disparities will be important for future COVID-19 vaccines, as well as for the flu this season, she noted.
View the complete guidance online.
Dr. Wharton had no relevant financial disclosures.
Outpatient medical care has been severely disrupted during the COVID-19 pandemic with a reduction of nearly 70% in outpatient visits since March before starting to rebound, Melinda Wharton, MD, said at the virtual meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Pediatrics was among the hardest hit specialties, with a 62% reduction in outpatient visits by April 5, said Dr. Wharton, director of the immunization services division at the CDC’s National Center for Immunization and Respiratory Diseases. However, visits for all pediatric age groups increased in May, compared with April, and the CDC emphasized the need to educate families about the importance of routine vaccination and well-child visits, Dr. Wharton said.
The CDC strategies to support routine childhood vaccination include monitoring vaccination service delivery to inform targeted interventions, said Dr. Wharton. In addition, the CDC will continue to support providers by identifying gaps in the Vaccines For Children (VFC) program network, increasing VFC funding, developing guidance materials, and identifying policy interventions.
Many small practices have struggled during the pandemic, and financial support is available through the Provider Relief Fund, which is now available to all Medicaid and Children’s Health Insurance Program (CHIP) providers, said Dr. Wharton.
because more families may now qualify for the program because of changes in job status and income, and parents may not be aware that their children may be eligible, she said.
“Vaccination is an essential medical service for all children and adolescents, ideally in the medical home,” Dr. Wharton said. The CDC’s interim guidance for immunization during the COVID-19 pandemic calls for administering all current or overdue vaccines according to the routine immunization schedule during the same visit, and implementing strategies to get patients caught up, prioritizing newborns, infants, and children up to age 24 months. The guidance includes details on safe delivery of vaccines, including physical distance and the use of personal protective equipment.
In addition, encourage parents to return for well-child visits, and use reminder systems to help keep patients current on visits and vaccines. “Discuss the safety protocols that have been put in place,” Dr. Wharton emphasized. The CDC also offers resources for providers to help communicate with parents about routine vaccination.
Looking ahead, back-to-school vaccination requirements “provide a critical checkpoint for children’s vaccination status,” Dr. Wharton said. Catch-up vaccination during the summer will help clinical capacity manage back-to-school and influenza vaccination in the fall, she emphasized. “Influenza vaccination will be an important strategy to decrease stress on our health care system.”
Flu vaccination strategies should focus on adults at higher risk for COVID-19 infections, such as health care providers. In addition, identifying and reducing disparities will be important for future COVID-19 vaccines, as well as for the flu this season, she noted.
View the complete guidance online.
Dr. Wharton had no relevant financial disclosures.
Outpatient medical care has been severely disrupted during the COVID-19 pandemic with a reduction of nearly 70% in outpatient visits since March before starting to rebound, Melinda Wharton, MD, said at the virtual meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Pediatrics was among the hardest hit specialties, with a 62% reduction in outpatient visits by April 5, said Dr. Wharton, director of the immunization services division at the CDC’s National Center for Immunization and Respiratory Diseases. However, visits for all pediatric age groups increased in May, compared with April, and the CDC emphasized the need to educate families about the importance of routine vaccination and well-child visits, Dr. Wharton said.
The CDC strategies to support routine childhood vaccination include monitoring vaccination service delivery to inform targeted interventions, said Dr. Wharton. In addition, the CDC will continue to support providers by identifying gaps in the Vaccines For Children (VFC) program network, increasing VFC funding, developing guidance materials, and identifying policy interventions.
Many small practices have struggled during the pandemic, and financial support is available through the Provider Relief Fund, which is now available to all Medicaid and Children’s Health Insurance Program (CHIP) providers, said Dr. Wharton.
because more families may now qualify for the program because of changes in job status and income, and parents may not be aware that their children may be eligible, she said.
“Vaccination is an essential medical service for all children and adolescents, ideally in the medical home,” Dr. Wharton said. The CDC’s interim guidance for immunization during the COVID-19 pandemic calls for administering all current or overdue vaccines according to the routine immunization schedule during the same visit, and implementing strategies to get patients caught up, prioritizing newborns, infants, and children up to age 24 months. The guidance includes details on safe delivery of vaccines, including physical distance and the use of personal protective equipment.
In addition, encourage parents to return for well-child visits, and use reminder systems to help keep patients current on visits and vaccines. “Discuss the safety protocols that have been put in place,” Dr. Wharton emphasized. The CDC also offers resources for providers to help communicate with parents about routine vaccination.
Looking ahead, back-to-school vaccination requirements “provide a critical checkpoint for children’s vaccination status,” Dr. Wharton said. Catch-up vaccination during the summer will help clinical capacity manage back-to-school and influenza vaccination in the fall, she emphasized. “Influenza vaccination will be an important strategy to decrease stress on our health care system.”
Flu vaccination strategies should focus on adults at higher risk for COVID-19 infections, such as health care providers. In addition, identifying and reducing disparities will be important for future COVID-19 vaccines, as well as for the flu this season, she noted.
View the complete guidance online.
Dr. Wharton had no relevant financial disclosures.
Tumor markers are ‘valuable’ for relapse detection in rare CNS tumors
according to a pooled analysis of cooperative group trials.
The findings suggest a role for the routine use of tumor markers for surveillance in CNS-NGGCT patients, said Adriana Fonseca, MD, a pediatric neuro-oncology fellow at the Hospital for Sick Children in Toronto.
She presented these findings as part of the American Society of Clinical Oncology virtual scientific program.
This pooled analysis represents the largest prospective cohort to date of relapsed intracranial germ cell tumors, Dr. Fonseca said. The analysis included 483 patients enrolled in five prospective CNS-NGGCT trials between 1989 and 2016. There were 106 patients who relapsed after the end of therapy; the relapsed patients had a median age of 13 years (range, 1-30 years) at diagnosis and 82% were male.
Tumor marker utility
There were 86 patients with tumor marker assessments at diagnosis, and 83 had tumor marker elevations in serum, cerebrospinal fluid (CSF), or both.
The three patients without tumor marker elevations at diagnosis had mixed GCT, choriocarcinoma, and yolk sac tumor, which are usually associated with tumor marker elevation, so this will be investigated further, Dr. Fonseca said.
The sensitivity of tumor markers at diagnosis was 94% for serum, 83% for CSF, and 97% for either serum or CSF.
The median time to relapse was 15.5 months. Relapses were local in 45 patients (44%), distant in 32 (33%), and combined in 22 (21%). Three intracranial relapses were located outside of the radiation field and were classified as distant.
Only two patients presented with isolated tumor marker elevations as the sole evidence of relapse, and the elevations usually preceded the presence of macroscopic disease, Dr. Fonseca said.
At the time of relapse, 88% of patients (n = 73) had tumor marker elevations. The sensitivity of tumor markers was 82% in serum, 85% in CSF, and 88% in either.
To better understand if tumor markers can be used for surveillance, the researchers analyzed data from patients who had either serum or CSF tumor marker levels available at both diagnosis and relapse.
Of the 74 evaluable patients who had elevated tumor markers at diagnosis, 68 had elevated tumor markers at relapse as well. This means 92% of relapsed patients were detectable by tumor markers, Dr. Fonseca said.
“Only six patients had tumor marker–negative relapses, and interestingly, one patient who was tumor marker negative at diagnosis relapsed with tumor markers positive,” she added.
Rationale and next steps
CNS-NGGCTs are rare and heterogeneous tumors that respond best when treated using multimodal approaches, including surgical resection, chemotherapy, and radiation, according to Dr. Fonseca. The 5-year event-free and overall survival rates range from 72%-84% and 82%-93% respectively.
“GCTs are unique as they express tumor markers, such as AFP and beta-HCG, which we know are sensitive and specific and used for diagnostic and monitoring purposes,” Dr. Fonseca said.
Current surveillance strategies use a combination of brain and spine MRI and serum tumor markers with declining frequency over time.
“CSF tumor markers are not performed during follow-up, and they are usually obtained only at the time of relapse,” Dr. Fonseca said. “But what is the best surveillance strategy? We have to remember that some of our patients require sedation to undergo MRI, and recurrent sedations in children have been recently associated with potential detrimental neurocognitive effects.”
Similarly, the administration of gadolinium used for MRI has been associated with an increased risk of renal fibrosis and negative neurological outcomes.
“Additionally, nonspecific areas of enhancement are commonly encountered and can lead to unnecessary further investigations,” Dr. Fonseca said, adding that this can contribute to patients’ and parents’ anxiety and to increased overall health care costs and resource utilization.
Recent Children’s Oncology Group data showed that 98% of patients with extracranial germ cell tumors who relapsed were detectable by tumor markers alone, and this led to a change in surveillance guidelines for those patients. This raised the question as to whether a similar approach could be used in CNS-NCCGTs, Dr. Fonseca explained.
“We hypothesized that tumor markers alone may be sufficient for relapse detection in children and adolescents treated for CNS-NGGCT, and hence, the frequency and associated risk with serial MRIs could be safely avoided,” she said.
Though this study was limited by missing data in some cases, the inclusion of trials from different eras, and the use of different detection techniques across trials, the findings confirm the sensitivity of tumor markers in this setting.
“Tumor markers represent a valuable surveillance strategy with the potential to reduce MRI frequency in these patients,” Dr. Fonseca said. “Additionally, the higher proportion of tumor marker–negative relapses, compared to extracranial GCTs, suggests a different biological behavior. Further studies to investigate the biology of the primary versus relapsed samples in GCTs are currently needed.”
Dr. Fonseca and colleagues are “currently undertaking some correlative outcomes analyses to try to understand if the elevation or nonelevation to tumor markers is correlated with survival. We also would like to elucidate the optimal MRI frequency required for surveillance,” she said.
Dr. Fonseca reported having no disclosures, and the researchers disclosed no funding for the study.
[email protected]
SOURCE: Fonseca A et al. ASCO 2020, Abstract 2503.
according to a pooled analysis of cooperative group trials.
The findings suggest a role for the routine use of tumor markers for surveillance in CNS-NGGCT patients, said Adriana Fonseca, MD, a pediatric neuro-oncology fellow at the Hospital for Sick Children in Toronto.
She presented these findings as part of the American Society of Clinical Oncology virtual scientific program.
This pooled analysis represents the largest prospective cohort to date of relapsed intracranial germ cell tumors, Dr. Fonseca said. The analysis included 483 patients enrolled in five prospective CNS-NGGCT trials between 1989 and 2016. There were 106 patients who relapsed after the end of therapy; the relapsed patients had a median age of 13 years (range, 1-30 years) at diagnosis and 82% were male.
Tumor marker utility
There were 86 patients with tumor marker assessments at diagnosis, and 83 had tumor marker elevations in serum, cerebrospinal fluid (CSF), or both.
The three patients without tumor marker elevations at diagnosis had mixed GCT, choriocarcinoma, and yolk sac tumor, which are usually associated with tumor marker elevation, so this will be investigated further, Dr. Fonseca said.
The sensitivity of tumor markers at diagnosis was 94% for serum, 83% for CSF, and 97% for either serum or CSF.
The median time to relapse was 15.5 months. Relapses were local in 45 patients (44%), distant in 32 (33%), and combined in 22 (21%). Three intracranial relapses were located outside of the radiation field and were classified as distant.
Only two patients presented with isolated tumor marker elevations as the sole evidence of relapse, and the elevations usually preceded the presence of macroscopic disease, Dr. Fonseca said.
At the time of relapse, 88% of patients (n = 73) had tumor marker elevations. The sensitivity of tumor markers was 82% in serum, 85% in CSF, and 88% in either.
To better understand if tumor markers can be used for surveillance, the researchers analyzed data from patients who had either serum or CSF tumor marker levels available at both diagnosis and relapse.
Of the 74 evaluable patients who had elevated tumor markers at diagnosis, 68 had elevated tumor markers at relapse as well. This means 92% of relapsed patients were detectable by tumor markers, Dr. Fonseca said.
“Only six patients had tumor marker–negative relapses, and interestingly, one patient who was tumor marker negative at diagnosis relapsed with tumor markers positive,” she added.
Rationale and next steps
CNS-NGGCTs are rare and heterogeneous tumors that respond best when treated using multimodal approaches, including surgical resection, chemotherapy, and radiation, according to Dr. Fonseca. The 5-year event-free and overall survival rates range from 72%-84% and 82%-93% respectively.
“GCTs are unique as they express tumor markers, such as AFP and beta-HCG, which we know are sensitive and specific and used for diagnostic and monitoring purposes,” Dr. Fonseca said.
Current surveillance strategies use a combination of brain and spine MRI and serum tumor markers with declining frequency over time.
“CSF tumor markers are not performed during follow-up, and they are usually obtained only at the time of relapse,” Dr. Fonseca said. “But what is the best surveillance strategy? We have to remember that some of our patients require sedation to undergo MRI, and recurrent sedations in children have been recently associated with potential detrimental neurocognitive effects.”
Similarly, the administration of gadolinium used for MRI has been associated with an increased risk of renal fibrosis and negative neurological outcomes.
“Additionally, nonspecific areas of enhancement are commonly encountered and can lead to unnecessary further investigations,” Dr. Fonseca said, adding that this can contribute to patients’ and parents’ anxiety and to increased overall health care costs and resource utilization.
Recent Children’s Oncology Group data showed that 98% of patients with extracranial germ cell tumors who relapsed were detectable by tumor markers alone, and this led to a change in surveillance guidelines for those patients. This raised the question as to whether a similar approach could be used in CNS-NCCGTs, Dr. Fonseca explained.
“We hypothesized that tumor markers alone may be sufficient for relapse detection in children and adolescents treated for CNS-NGGCT, and hence, the frequency and associated risk with serial MRIs could be safely avoided,” she said.
Though this study was limited by missing data in some cases, the inclusion of trials from different eras, and the use of different detection techniques across trials, the findings confirm the sensitivity of tumor markers in this setting.
“Tumor markers represent a valuable surveillance strategy with the potential to reduce MRI frequency in these patients,” Dr. Fonseca said. “Additionally, the higher proportion of tumor marker–negative relapses, compared to extracranial GCTs, suggests a different biological behavior. Further studies to investigate the biology of the primary versus relapsed samples in GCTs are currently needed.”
Dr. Fonseca and colleagues are “currently undertaking some correlative outcomes analyses to try to understand if the elevation or nonelevation to tumor markers is correlated with survival. We also would like to elucidate the optimal MRI frequency required for surveillance,” she said.
Dr. Fonseca reported having no disclosures, and the researchers disclosed no funding for the study.
[email protected]
SOURCE: Fonseca A et al. ASCO 2020, Abstract 2503.
according to a pooled analysis of cooperative group trials.
The findings suggest a role for the routine use of tumor markers for surveillance in CNS-NGGCT patients, said Adriana Fonseca, MD, a pediatric neuro-oncology fellow at the Hospital for Sick Children in Toronto.
She presented these findings as part of the American Society of Clinical Oncology virtual scientific program.
This pooled analysis represents the largest prospective cohort to date of relapsed intracranial germ cell tumors, Dr. Fonseca said. The analysis included 483 patients enrolled in five prospective CNS-NGGCT trials between 1989 and 2016. There were 106 patients who relapsed after the end of therapy; the relapsed patients had a median age of 13 years (range, 1-30 years) at diagnosis and 82% were male.
Tumor marker utility
There were 86 patients with tumor marker assessments at diagnosis, and 83 had tumor marker elevations in serum, cerebrospinal fluid (CSF), or both.
The three patients without tumor marker elevations at diagnosis had mixed GCT, choriocarcinoma, and yolk sac tumor, which are usually associated with tumor marker elevation, so this will be investigated further, Dr. Fonseca said.
The sensitivity of tumor markers at diagnosis was 94% for serum, 83% for CSF, and 97% for either serum or CSF.
The median time to relapse was 15.5 months. Relapses were local in 45 patients (44%), distant in 32 (33%), and combined in 22 (21%). Three intracranial relapses were located outside of the radiation field and were classified as distant.
Only two patients presented with isolated tumor marker elevations as the sole evidence of relapse, and the elevations usually preceded the presence of macroscopic disease, Dr. Fonseca said.
At the time of relapse, 88% of patients (n = 73) had tumor marker elevations. The sensitivity of tumor markers was 82% in serum, 85% in CSF, and 88% in either.
To better understand if tumor markers can be used for surveillance, the researchers analyzed data from patients who had either serum or CSF tumor marker levels available at both diagnosis and relapse.
Of the 74 evaluable patients who had elevated tumor markers at diagnosis, 68 had elevated tumor markers at relapse as well. This means 92% of relapsed patients were detectable by tumor markers, Dr. Fonseca said.
“Only six patients had tumor marker–negative relapses, and interestingly, one patient who was tumor marker negative at diagnosis relapsed with tumor markers positive,” she added.
Rationale and next steps
CNS-NGGCTs are rare and heterogeneous tumors that respond best when treated using multimodal approaches, including surgical resection, chemotherapy, and radiation, according to Dr. Fonseca. The 5-year event-free and overall survival rates range from 72%-84% and 82%-93% respectively.
“GCTs are unique as they express tumor markers, such as AFP and beta-HCG, which we know are sensitive and specific and used for diagnostic and monitoring purposes,” Dr. Fonseca said.
Current surveillance strategies use a combination of brain and spine MRI and serum tumor markers with declining frequency over time.
“CSF tumor markers are not performed during follow-up, and they are usually obtained only at the time of relapse,” Dr. Fonseca said. “But what is the best surveillance strategy? We have to remember that some of our patients require sedation to undergo MRI, and recurrent sedations in children have been recently associated with potential detrimental neurocognitive effects.”
Similarly, the administration of gadolinium used for MRI has been associated with an increased risk of renal fibrosis and negative neurological outcomes.
“Additionally, nonspecific areas of enhancement are commonly encountered and can lead to unnecessary further investigations,” Dr. Fonseca said, adding that this can contribute to patients’ and parents’ anxiety and to increased overall health care costs and resource utilization.
Recent Children’s Oncology Group data showed that 98% of patients with extracranial germ cell tumors who relapsed were detectable by tumor markers alone, and this led to a change in surveillance guidelines for those patients. This raised the question as to whether a similar approach could be used in CNS-NCCGTs, Dr. Fonseca explained.
“We hypothesized that tumor markers alone may be sufficient for relapse detection in children and adolescents treated for CNS-NGGCT, and hence, the frequency and associated risk with serial MRIs could be safely avoided,” she said.
Though this study was limited by missing data in some cases, the inclusion of trials from different eras, and the use of different detection techniques across trials, the findings confirm the sensitivity of tumor markers in this setting.
“Tumor markers represent a valuable surveillance strategy with the potential to reduce MRI frequency in these patients,” Dr. Fonseca said. “Additionally, the higher proportion of tumor marker–negative relapses, compared to extracranial GCTs, suggests a different biological behavior. Further studies to investigate the biology of the primary versus relapsed samples in GCTs are currently needed.”
Dr. Fonseca and colleagues are “currently undertaking some correlative outcomes analyses to try to understand if the elevation or nonelevation to tumor markers is correlated with survival. We also would like to elucidate the optimal MRI frequency required for surveillance,” she said.
Dr. Fonseca reported having no disclosures, and the researchers disclosed no funding for the study.
[email protected]
SOURCE: Fonseca A et al. ASCO 2020, Abstract 2503.
FROM ASCO 2020
Suicide thoughts, attempts in adolescence correlate with mental health symptoms
About one in five adolescents has thought about suicide, about 10% have experienced serious suicidal ideation, and 7% have attempted suicide by age 20 years, according to a longitudinal study of Canadian adolescents published online in Pediatrics.
In multivariable analyses, depression and anxiety were independently associated with passive and serious suicidal ideation at some ages, but none of the externalizing problems were significantly associated with passive or serious suicidal ideation. However, “both depressive and conduct symptoms [were] independently associated with suicidal risk,” the researchers found. Most adolescents with suicidal ideation or suicide attempt met criteria for at least one mental health problem.
“These findings suggest that suicide risk should be systematically assessed in adolescents who present with mental health symptoms and not solely in adolescents with clinically diagnosed mental disorders,” said Massimiliano Orri, PhD, and colleagues. Dr. Orri is affiliated with the McGill Group for Suicide Studies, Douglas Mental Health University Institute, Montreal, and the University of Bordeaux (France).
To document the prevalence of passive or serious suicidal ideation and suicide attempt from ages 13-20 years and examine correlations with mental health symptoms, Dr. Orri and colleagues analyzed data from 1,618 participants in the Quebec Longitudinal Study of Child Development. The population-based study follows individuals born in 1997 and 1998 in Quebec. Participants answered questions about suicidal ideation or suicide attempt in the past year at ages 13, 15, 17, or 20 years (“Did you ever think about suicide?” “Did you ever seriously think of attempting suicide?” and “How many times did you attempt suicide?”). The researchers assessed symptoms of mental health problems using self-report questionnaires.
Lifetime prevalence of suicide-related outcomes was higher for female participants than for male participants. The prevalence of passive suicidal ideation was 28% in females versus 15% in males. The prevalence of serious suicidal ideation was 12% in females versus 8% in males. The prevalence of suicide attempt was 9% in females versus 4% in males. “Sex differences in suicidal ideation and suicide attempt might be attributed to various factors, such as mental health (e.g., higher prevalence of depression in female participants) or social stigma (e.g., greater stigma around suicide in male than in female participants),” the authors wrote.
In the entire cohort, the prevalence of passive suicidal ideation increased from 12% at 13 years to 18% at 17 years. The prevalence of serious suicidal ideation increased from 3% at 13 years to 10% at 20 years. The prevalence of suicide attempt was approximately 4% at each age.
“Although having a major depressive episode is a well-known risk factor of suicidal ideation and suicide attempt, our study adds to the general body of knowledge by showing associations with suicide-related outcomes across the full spectrum of depressive symptoms,” Dr. Orri and colleagues wrote. “This suggests that youth who present with depressive symptoms (and not solely those who are clinically depressed) may be more likely to experience suicidal ideation or attempt suicide.”
The estimated rates of serious suicidal ideation and attempted suicide by age 20 years are consistent with previous U.S. and Canadian surveys. Sample attrition, the use of different questionnaires in early and late adolescence, and the lack of information about substance use and psychotic symptoms are among the study’s limitations.
Six of the authors were supported by grants from a variety of Canadian and European agencies and the American Foundation for Suicide Prevention. All of the authors said they had no relevant financial disclosures.
SOURCE: Orri M et al. Pediatrics. 2020 Jun 8. doi: 10.1542/peds.2019-3823.
Interestingly, this study by Orri et al. found that there was not a peak in suicide attempts in mid-adolescence; instead, rates of attempts were stable throughout adolescence and serious suicidal ideation actually increased with age. This was an unexpected finding for me, and something I will be more mindful about in my clinical practice when seeing older teens and young adults. Additionally, all mental health problems – not just depression – evaluated in univariate analyses in the study were associated with suicidal thoughts and attempts. On multivariable analysis that accounted for the impact of the effect of comorbid mental health symptoms, depressive symptoms had the highest and most consistent correlation to suicidal thoughts, and conduct symptoms were associated with an increase in suicide attempts. The authors conclude that youth with mental health symptoms – not just those who meet diagnostic criteria – should be assessed for suicide risk.
In my subspecialty practice, we screen every new patient for suicide regardless of the reason for their visit and more often for those with mental health symptoms. I know this may seem onerous, but screening and counseling typically take under 5 minutes – and in many instances around 1-2 minutes. Having ready-to-go resources including mental health professionals to refer to, screening algorithms (such as protocols published in MedEdPORTAL or Family Practice Management), and suicide prevention resources for patients and family for those who screen positive can help expedite this process. I think these recommendations can be adapted with relative ease into any visit for a teen or young adult who is presenting with a mental health complaint.
Kelly A. Curran, MD, is an assistant professor of pediatrics at the University of Oklahoma in Oklahoma City. She is a member of the Pediatric News editorial advisory board. Dr. Curran said she had no relevant financial disclosures.
Interestingly, this study by Orri et al. found that there was not a peak in suicide attempts in mid-adolescence; instead, rates of attempts were stable throughout adolescence and serious suicidal ideation actually increased with age. This was an unexpected finding for me, and something I will be more mindful about in my clinical practice when seeing older teens and young adults. Additionally, all mental health problems – not just depression – evaluated in univariate analyses in the study were associated with suicidal thoughts and attempts. On multivariable analysis that accounted for the impact of the effect of comorbid mental health symptoms, depressive symptoms had the highest and most consistent correlation to suicidal thoughts, and conduct symptoms were associated with an increase in suicide attempts. The authors conclude that youth with mental health symptoms – not just those who meet diagnostic criteria – should be assessed for suicide risk.
In my subspecialty practice, we screen every new patient for suicide regardless of the reason for their visit and more often for those with mental health symptoms. I know this may seem onerous, but screening and counseling typically take under 5 minutes – and in many instances around 1-2 minutes. Having ready-to-go resources including mental health professionals to refer to, screening algorithms (such as protocols published in MedEdPORTAL or Family Practice Management), and suicide prevention resources for patients and family for those who screen positive can help expedite this process. I think these recommendations can be adapted with relative ease into any visit for a teen or young adult who is presenting with a mental health complaint.
Kelly A. Curran, MD, is an assistant professor of pediatrics at the University of Oklahoma in Oklahoma City. She is a member of the Pediatric News editorial advisory board. Dr. Curran said she had no relevant financial disclosures.
Interestingly, this study by Orri et al. found that there was not a peak in suicide attempts in mid-adolescence; instead, rates of attempts were stable throughout adolescence and serious suicidal ideation actually increased with age. This was an unexpected finding for me, and something I will be more mindful about in my clinical practice when seeing older teens and young adults. Additionally, all mental health problems – not just depression – evaluated in univariate analyses in the study were associated with suicidal thoughts and attempts. On multivariable analysis that accounted for the impact of the effect of comorbid mental health symptoms, depressive symptoms had the highest and most consistent correlation to suicidal thoughts, and conduct symptoms were associated with an increase in suicide attempts. The authors conclude that youth with mental health symptoms – not just those who meet diagnostic criteria – should be assessed for suicide risk.
In my subspecialty practice, we screen every new patient for suicide regardless of the reason for their visit and more often for those with mental health symptoms. I know this may seem onerous, but screening and counseling typically take under 5 minutes – and in many instances around 1-2 minutes. Having ready-to-go resources including mental health professionals to refer to, screening algorithms (such as protocols published in MedEdPORTAL or Family Practice Management), and suicide prevention resources for patients and family for those who screen positive can help expedite this process. I think these recommendations can be adapted with relative ease into any visit for a teen or young adult who is presenting with a mental health complaint.
Kelly A. Curran, MD, is an assistant professor of pediatrics at the University of Oklahoma in Oklahoma City. She is a member of the Pediatric News editorial advisory board. Dr. Curran said she had no relevant financial disclosures.
About one in five adolescents has thought about suicide, about 10% have experienced serious suicidal ideation, and 7% have attempted suicide by age 20 years, according to a longitudinal study of Canadian adolescents published online in Pediatrics.
In multivariable analyses, depression and anxiety were independently associated with passive and serious suicidal ideation at some ages, but none of the externalizing problems were significantly associated with passive or serious suicidal ideation. However, “both depressive and conduct symptoms [were] independently associated with suicidal risk,” the researchers found. Most adolescents with suicidal ideation or suicide attempt met criteria for at least one mental health problem.
“These findings suggest that suicide risk should be systematically assessed in adolescents who present with mental health symptoms and not solely in adolescents with clinically diagnosed mental disorders,” said Massimiliano Orri, PhD, and colleagues. Dr. Orri is affiliated with the McGill Group for Suicide Studies, Douglas Mental Health University Institute, Montreal, and the University of Bordeaux (France).
To document the prevalence of passive or serious suicidal ideation and suicide attempt from ages 13-20 years and examine correlations with mental health symptoms, Dr. Orri and colleagues analyzed data from 1,618 participants in the Quebec Longitudinal Study of Child Development. The population-based study follows individuals born in 1997 and 1998 in Quebec. Participants answered questions about suicidal ideation or suicide attempt in the past year at ages 13, 15, 17, or 20 years (“Did you ever think about suicide?” “Did you ever seriously think of attempting suicide?” and “How many times did you attempt suicide?”). The researchers assessed symptoms of mental health problems using self-report questionnaires.
Lifetime prevalence of suicide-related outcomes was higher for female participants than for male participants. The prevalence of passive suicidal ideation was 28% in females versus 15% in males. The prevalence of serious suicidal ideation was 12% in females versus 8% in males. The prevalence of suicide attempt was 9% in females versus 4% in males. “Sex differences in suicidal ideation and suicide attempt might be attributed to various factors, such as mental health (e.g., higher prevalence of depression in female participants) or social stigma (e.g., greater stigma around suicide in male than in female participants),” the authors wrote.
In the entire cohort, the prevalence of passive suicidal ideation increased from 12% at 13 years to 18% at 17 years. The prevalence of serious suicidal ideation increased from 3% at 13 years to 10% at 20 years. The prevalence of suicide attempt was approximately 4% at each age.
“Although having a major depressive episode is a well-known risk factor of suicidal ideation and suicide attempt, our study adds to the general body of knowledge by showing associations with suicide-related outcomes across the full spectrum of depressive symptoms,” Dr. Orri and colleagues wrote. “This suggests that youth who present with depressive symptoms (and not solely those who are clinically depressed) may be more likely to experience suicidal ideation or attempt suicide.”
The estimated rates of serious suicidal ideation and attempted suicide by age 20 years are consistent with previous U.S. and Canadian surveys. Sample attrition, the use of different questionnaires in early and late adolescence, and the lack of information about substance use and psychotic symptoms are among the study’s limitations.
Six of the authors were supported by grants from a variety of Canadian and European agencies and the American Foundation for Suicide Prevention. All of the authors said they had no relevant financial disclosures.
SOURCE: Orri M et al. Pediatrics. 2020 Jun 8. doi: 10.1542/peds.2019-3823.
About one in five adolescents has thought about suicide, about 10% have experienced serious suicidal ideation, and 7% have attempted suicide by age 20 years, according to a longitudinal study of Canadian adolescents published online in Pediatrics.
In multivariable analyses, depression and anxiety were independently associated with passive and serious suicidal ideation at some ages, but none of the externalizing problems were significantly associated with passive or serious suicidal ideation. However, “both depressive and conduct symptoms [were] independently associated with suicidal risk,” the researchers found. Most adolescents with suicidal ideation or suicide attempt met criteria for at least one mental health problem.
“These findings suggest that suicide risk should be systematically assessed in adolescents who present with mental health symptoms and not solely in adolescents with clinically diagnosed mental disorders,” said Massimiliano Orri, PhD, and colleagues. Dr. Orri is affiliated with the McGill Group for Suicide Studies, Douglas Mental Health University Institute, Montreal, and the University of Bordeaux (France).
To document the prevalence of passive or serious suicidal ideation and suicide attempt from ages 13-20 years and examine correlations with mental health symptoms, Dr. Orri and colleagues analyzed data from 1,618 participants in the Quebec Longitudinal Study of Child Development. The population-based study follows individuals born in 1997 and 1998 in Quebec. Participants answered questions about suicidal ideation or suicide attempt in the past year at ages 13, 15, 17, or 20 years (“Did you ever think about suicide?” “Did you ever seriously think of attempting suicide?” and “How many times did you attempt suicide?”). The researchers assessed symptoms of mental health problems using self-report questionnaires.
Lifetime prevalence of suicide-related outcomes was higher for female participants than for male participants. The prevalence of passive suicidal ideation was 28% in females versus 15% in males. The prevalence of serious suicidal ideation was 12% in females versus 8% in males. The prevalence of suicide attempt was 9% in females versus 4% in males. “Sex differences in suicidal ideation and suicide attempt might be attributed to various factors, such as mental health (e.g., higher prevalence of depression in female participants) or social stigma (e.g., greater stigma around suicide in male than in female participants),” the authors wrote.
In the entire cohort, the prevalence of passive suicidal ideation increased from 12% at 13 years to 18% at 17 years. The prevalence of serious suicidal ideation increased from 3% at 13 years to 10% at 20 years. The prevalence of suicide attempt was approximately 4% at each age.
“Although having a major depressive episode is a well-known risk factor of suicidal ideation and suicide attempt, our study adds to the general body of knowledge by showing associations with suicide-related outcomes across the full spectrum of depressive symptoms,” Dr. Orri and colleagues wrote. “This suggests that youth who present with depressive symptoms (and not solely those who are clinically depressed) may be more likely to experience suicidal ideation or attempt suicide.”
The estimated rates of serious suicidal ideation and attempted suicide by age 20 years are consistent with previous U.S. and Canadian surveys. Sample attrition, the use of different questionnaires in early and late adolescence, and the lack of information about substance use and psychotic symptoms are among the study’s limitations.
Six of the authors were supported by grants from a variety of Canadian and European agencies and the American Foundation for Suicide Prevention. All of the authors said they had no relevant financial disclosures.
SOURCE: Orri M et al. Pediatrics. 2020 Jun 8. doi: 10.1542/peds.2019-3823.
FROM PEDIATRICS
ACIP approves flu vaccine recommendations for 2020-2021
– Fluzone high-dose quadrivalent, which replaces the trivalent Fluzone high-dose and Fluad quadrivalent (Seqirus), according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
At a virtual meeting on June 24, the committee voted unanimously to approve the vaccine recommendations for annual influenza immunization of all individuals aged 6 months and older. They also voted to accept some guidance and language changes to the recommendations.
The past flu season was unique in its overlap with the emergence of the COVID-19 coronavirus, which likely contributed to a third peak in reported cases of influenza-like illness at approximately week 14 of last season, said Lisa Grohskopf, MD, of the CDC’s influenza division, who presented data on last year’s activity and the updates for next season.
The CDC estimates that 39,000,000-56,000,000 flu illnesses occurred in the United States from Oct. 1, 2019, to April 4, 2020, said Dr. Grohskopf. Estimates also suggest as many as 740,000 hospitalizations and 62,000 deaths related to the seasonal flu.
Preliminary results of vaccine effectiveness showed 39% overall for the 2019-2020 season, with more substantial protection against influenza B and lower protection against A/H1N1pmd09.
Vaccine safety data from the Vaccine Adverse Event Reporting System and Vaccine Safety Datalink showed no new safety concerns for any flu vaccine types used last year, Dr. Grohskopf noted.
Based on this information, three components (A/H1N1pdm09, A/H3N2, and B/Victoria) have been updated for the 2020-2021 vaccines, said Dr. Grohskopf. The egg-based influenza vaccines will include hemagglutinin derived from an A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09–like virus, an A/Hong Kong/2671/2019(H3N2)–like virus and a B/Washington/02/2019 (Victoria lineage)–like virus, and (for quadrivalent vaccines) a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
Nonegg vaccines will contain hemagglutinin derived from an A/Hawaii/70/2019 (H1N1)pdm09–like virus, an A/Hong Kong/45/2019 (H3N2)–like virus, a B/Washington/02/2019 (Victoria lineage)–like virus, and a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
New guidance for next year’s flu season includes a change to the language in the contraindications and precautions table to simply read “Contraindications,” with more details in the text explaining package insert contraindications and ACIP recommendations, Dr. Grohskopf said. In addition, updated guidance clarifies that live-attenuated influenza vaccine quadravalents (LAIV4) should not be used in patients with cochlear implants, active cerebrospinal fluid leaks, and anatomical or functional asplenia, based on ACIP’s review of the latest evidence and the availability of alternative vaccines.
ACIP also updated guidance on the use of antivirals and LAIV4. Based on half-lives, language was added indicating that clinicians should assume interference if antivirals are given within certain intervals of LAIV4, Dr. Grohskopf explained. “Newer antivirals peramivir and baloxavir have longer half-lives than oseltamivir and zanamivir, and insufficient data are available on the use of LAIV4 in the setting of antiviral use.”
The ACIP members had no financial conflicts to disclose.
– Fluzone high-dose quadrivalent, which replaces the trivalent Fluzone high-dose and Fluad quadrivalent (Seqirus), according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
At a virtual meeting on June 24, the committee voted unanimously to approve the vaccine recommendations for annual influenza immunization of all individuals aged 6 months and older. They also voted to accept some guidance and language changes to the recommendations.
The past flu season was unique in its overlap with the emergence of the COVID-19 coronavirus, which likely contributed to a third peak in reported cases of influenza-like illness at approximately week 14 of last season, said Lisa Grohskopf, MD, of the CDC’s influenza division, who presented data on last year’s activity and the updates for next season.
The CDC estimates that 39,000,000-56,000,000 flu illnesses occurred in the United States from Oct. 1, 2019, to April 4, 2020, said Dr. Grohskopf. Estimates also suggest as many as 740,000 hospitalizations and 62,000 deaths related to the seasonal flu.
Preliminary results of vaccine effectiveness showed 39% overall for the 2019-2020 season, with more substantial protection against influenza B and lower protection against A/H1N1pmd09.
Vaccine safety data from the Vaccine Adverse Event Reporting System and Vaccine Safety Datalink showed no new safety concerns for any flu vaccine types used last year, Dr. Grohskopf noted.
Based on this information, three components (A/H1N1pdm09, A/H3N2, and B/Victoria) have been updated for the 2020-2021 vaccines, said Dr. Grohskopf. The egg-based influenza vaccines will include hemagglutinin derived from an A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09–like virus, an A/Hong Kong/2671/2019(H3N2)–like virus and a B/Washington/02/2019 (Victoria lineage)–like virus, and (for quadrivalent vaccines) a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
Nonegg vaccines will contain hemagglutinin derived from an A/Hawaii/70/2019 (H1N1)pdm09–like virus, an A/Hong Kong/45/2019 (H3N2)–like virus, a B/Washington/02/2019 (Victoria lineage)–like virus, and a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
New guidance for next year’s flu season includes a change to the language in the contraindications and precautions table to simply read “Contraindications,” with more details in the text explaining package insert contraindications and ACIP recommendations, Dr. Grohskopf said. In addition, updated guidance clarifies that live-attenuated influenza vaccine quadravalents (LAIV4) should not be used in patients with cochlear implants, active cerebrospinal fluid leaks, and anatomical or functional asplenia, based on ACIP’s review of the latest evidence and the availability of alternative vaccines.
ACIP also updated guidance on the use of antivirals and LAIV4. Based on half-lives, language was added indicating that clinicians should assume interference if antivirals are given within certain intervals of LAIV4, Dr. Grohskopf explained. “Newer antivirals peramivir and baloxavir have longer half-lives than oseltamivir and zanamivir, and insufficient data are available on the use of LAIV4 in the setting of antiviral use.”
The ACIP members had no financial conflicts to disclose.
– Fluzone high-dose quadrivalent, which replaces the trivalent Fluzone high-dose and Fluad quadrivalent (Seqirus), according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
At a virtual meeting on June 24, the committee voted unanimously to approve the vaccine recommendations for annual influenza immunization of all individuals aged 6 months and older. They also voted to accept some guidance and language changes to the recommendations.
The past flu season was unique in its overlap with the emergence of the COVID-19 coronavirus, which likely contributed to a third peak in reported cases of influenza-like illness at approximately week 14 of last season, said Lisa Grohskopf, MD, of the CDC’s influenza division, who presented data on last year’s activity and the updates for next season.
The CDC estimates that 39,000,000-56,000,000 flu illnesses occurred in the United States from Oct. 1, 2019, to April 4, 2020, said Dr. Grohskopf. Estimates also suggest as many as 740,000 hospitalizations and 62,000 deaths related to the seasonal flu.
Preliminary results of vaccine effectiveness showed 39% overall for the 2019-2020 season, with more substantial protection against influenza B and lower protection against A/H1N1pmd09.
Vaccine safety data from the Vaccine Adverse Event Reporting System and Vaccine Safety Datalink showed no new safety concerns for any flu vaccine types used last year, Dr. Grohskopf noted.
Based on this information, three components (A/H1N1pdm09, A/H3N2, and B/Victoria) have been updated for the 2020-2021 vaccines, said Dr. Grohskopf. The egg-based influenza vaccines will include hemagglutinin derived from an A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09–like virus, an A/Hong Kong/2671/2019(H3N2)–like virus and a B/Washington/02/2019 (Victoria lineage)–like virus, and (for quadrivalent vaccines) a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
Nonegg vaccines will contain hemagglutinin derived from an A/Hawaii/70/2019 (H1N1)pdm09–like virus, an A/Hong Kong/45/2019 (H3N2)–like virus, a B/Washington/02/2019 (Victoria lineage)–like virus, and a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
New guidance for next year’s flu season includes a change to the language in the contraindications and precautions table to simply read “Contraindications,” with more details in the text explaining package insert contraindications and ACIP recommendations, Dr. Grohskopf said. In addition, updated guidance clarifies that live-attenuated influenza vaccine quadravalents (LAIV4) should not be used in patients with cochlear implants, active cerebrospinal fluid leaks, and anatomical or functional asplenia, based on ACIP’s review of the latest evidence and the availability of alternative vaccines.
ACIP also updated guidance on the use of antivirals and LAIV4. Based on half-lives, language was added indicating that clinicians should assume interference if antivirals are given within certain intervals of LAIV4, Dr. Grohskopf explained. “Newer antivirals peramivir and baloxavir have longer half-lives than oseltamivir and zanamivir, and insufficient data are available on the use of LAIV4 in the setting of antiviral use.”
The ACIP members had no financial conflicts to disclose.
Newborn ear lesion
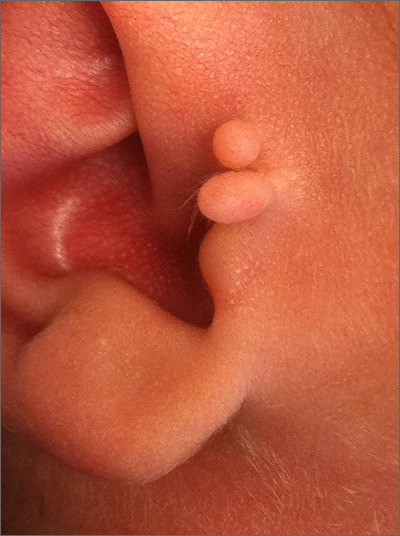
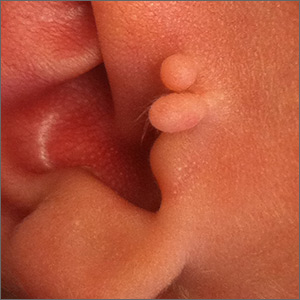
This lesion was diagnosed as an accessory tragus. Although sometimes called a preauricular skin tag, it is more aptly named accessory tragus since it arises embryonically along with the tragus from the first branchial arch. It usually is slightly anterior to the ear and can be seen unilaterally or bilaterally. The lesions usually feel firm to palpation due to an underlying cartilaginous component.
While this finding can be seen in congenital syndromes—most commonly oculoauriculovertebral syndrome (also known as Goldenhar syndrome)—it usually occurs in isolation. If there are no other abnormalities on exam to suggest a congenital syndrome, no additional work-up is necessary.
Concerns have been raised about a possible association with decreased hearing. Routine hearing screens are performed in the United States and this child’s screen was normal, so no further investigation for inner ear abnormalities was warranted.
These lesions usually are asymptomatic (unless traumatized). If removal is desired, it is important to remove the cartilaginous component, which can be deep and require additional dissection rather than simple transection at the base. Incomplete removal of the cartilage can cause chondritis, impede skin healing, and lead to infection.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Bahrani B, Khachemoune A. Review of accessory tragus with highlights of its associated syndromes. Int J Dermatol. 2014;53:1442-1446.

This lesion was diagnosed as an accessory tragus. Although sometimes called a preauricular skin tag, it is more aptly named accessory tragus since it arises embryonically along with the tragus from the first branchial arch. It usually is slightly anterior to the ear and can be seen unilaterally or bilaterally. The lesions usually feel firm to palpation due to an underlying cartilaginous component.
While this finding can be seen in congenital syndromes—most commonly oculoauriculovertebral syndrome (also known as Goldenhar syndrome)—it usually occurs in isolation. If there are no other abnormalities on exam to suggest a congenital syndrome, no additional work-up is necessary.
Concerns have been raised about a possible association with decreased hearing. Routine hearing screens are performed in the United States and this child’s screen was normal, so no further investigation for inner ear abnormalities was warranted.
These lesions usually are asymptomatic (unless traumatized). If removal is desired, it is important to remove the cartilaginous component, which can be deep and require additional dissection rather than simple transection at the base. Incomplete removal of the cartilage can cause chondritis, impede skin healing, and lead to infection.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This lesion was diagnosed as an accessory tragus. Although sometimes called a preauricular skin tag, it is more aptly named accessory tragus since it arises embryonically along with the tragus from the first branchial arch. It usually is slightly anterior to the ear and can be seen unilaterally or bilaterally. The lesions usually feel firm to palpation due to an underlying cartilaginous component.
While this finding can be seen in congenital syndromes—most commonly oculoauriculovertebral syndrome (also known as Goldenhar syndrome)—it usually occurs in isolation. If there are no other abnormalities on exam to suggest a congenital syndrome, no additional work-up is necessary.
Concerns have been raised about a possible association with decreased hearing. Routine hearing screens are performed in the United States and this child’s screen was normal, so no further investigation for inner ear abnormalities was warranted.
These lesions usually are asymptomatic (unless traumatized). If removal is desired, it is important to remove the cartilaginous component, which can be deep and require additional dissection rather than simple transection at the base. Incomplete removal of the cartilage can cause chondritis, impede skin healing, and lead to infection.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Bahrani B, Khachemoune A. Review of accessory tragus with highlights of its associated syndromes. Int J Dermatol. 2014;53:1442-1446.
Bahrani B, Khachemoune A. Review of accessory tragus with highlights of its associated syndromes. Int J Dermatol. 2014;53:1442-1446.
New quadrivalent meningococcal vaccine joins VFC arsenal
No changes to the current meningococcal vaccination recommendations were made. The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted 14-0 to include MenACWY-TT as an option for vaccination against meningococcal serogroups A, C, W, and Y in the VFC program. The vote took place in a virtual meeting held on June 24.
The currently available MenACWY vaccines in the United States are MenACWY-D (Menactra), MenACWY-CRW (Menveo), and MenACWY-TT (MedQuadfi), with MenACWY-TT approved by the Food and Drug Administration in April 2020.
Meningococcal vaccination is currently recommended for adolescents, with one dose at age 11 or 12 years and a booster at age 16 years, as well as individuals aged 2 months and older at increased risk for meningococcal disease, according to Lucy McNamara, PhD, of the CDC’s National Center for Immunization and Respiratory Diseases.
Dr. McNamara presented considerations from the Meningococcal Work Group, which determined that the inclusion of MenACWY-TT “is of public health importance given recent vaccine licensure and to support security of vaccine supply.”
The Work Group reviewed 10 studies (phase 2 or 3) of MenACWY-TT that included data on short-term immune response, persistence of immune response, immune interference because of coadministration with other routine adolescent vaccines, and incidence of serious adverse events. Overall, the data showed noninferiority of MenACWY-TT, compared with other available products, in terms of response rates, as well as higher levels of immune response in some studies. Serious adverse events were similar, and none determined to be associated with the vaccines.
ACIP member Paul Hunter, MD, of the University of Milwaukee, Wisc., expressed some concerns about pain or side effects for the new vaccine and Tdap when given together. However, a study of coadministration of MedACWY-TT and Tdap, compared with Tdap alone, showed no impact on geometric mean titer ratios.
Overall, the Work Group concluded that “desirable effects outweigh undesirable effects” and that the data favor the inclusion of MenACWY-TT as an option for meningococcal vaccination.
The committee members and Dr. McNamara had no relevant financial conflicts to disclose.
No changes to the current meningococcal vaccination recommendations were made. The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted 14-0 to include MenACWY-TT as an option for vaccination against meningococcal serogroups A, C, W, and Y in the VFC program. The vote took place in a virtual meeting held on June 24.
The currently available MenACWY vaccines in the United States are MenACWY-D (Menactra), MenACWY-CRW (Menveo), and MenACWY-TT (MedQuadfi), with MenACWY-TT approved by the Food and Drug Administration in April 2020.
Meningococcal vaccination is currently recommended for adolescents, with one dose at age 11 or 12 years and a booster at age 16 years, as well as individuals aged 2 months and older at increased risk for meningococcal disease, according to Lucy McNamara, PhD, of the CDC’s National Center for Immunization and Respiratory Diseases.
Dr. McNamara presented considerations from the Meningococcal Work Group, which determined that the inclusion of MenACWY-TT “is of public health importance given recent vaccine licensure and to support security of vaccine supply.”
The Work Group reviewed 10 studies (phase 2 or 3) of MenACWY-TT that included data on short-term immune response, persistence of immune response, immune interference because of coadministration with other routine adolescent vaccines, and incidence of serious adverse events. Overall, the data showed noninferiority of MenACWY-TT, compared with other available products, in terms of response rates, as well as higher levels of immune response in some studies. Serious adverse events were similar, and none determined to be associated with the vaccines.
ACIP member Paul Hunter, MD, of the University of Milwaukee, Wisc., expressed some concerns about pain or side effects for the new vaccine and Tdap when given together. However, a study of coadministration of MedACWY-TT and Tdap, compared with Tdap alone, showed no impact on geometric mean titer ratios.
Overall, the Work Group concluded that “desirable effects outweigh undesirable effects” and that the data favor the inclusion of MenACWY-TT as an option for meningococcal vaccination.
The committee members and Dr. McNamara had no relevant financial conflicts to disclose.
No changes to the current meningococcal vaccination recommendations were made. The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted 14-0 to include MenACWY-TT as an option for vaccination against meningococcal serogroups A, C, W, and Y in the VFC program. The vote took place in a virtual meeting held on June 24.
The currently available MenACWY vaccines in the United States are MenACWY-D (Menactra), MenACWY-CRW (Menveo), and MenACWY-TT (MedQuadfi), with MenACWY-TT approved by the Food and Drug Administration in April 2020.
Meningococcal vaccination is currently recommended for adolescents, with one dose at age 11 or 12 years and a booster at age 16 years, as well as individuals aged 2 months and older at increased risk for meningococcal disease, according to Lucy McNamara, PhD, of the CDC’s National Center for Immunization and Respiratory Diseases.
Dr. McNamara presented considerations from the Meningococcal Work Group, which determined that the inclusion of MenACWY-TT “is of public health importance given recent vaccine licensure and to support security of vaccine supply.”
The Work Group reviewed 10 studies (phase 2 or 3) of MenACWY-TT that included data on short-term immune response, persistence of immune response, immune interference because of coadministration with other routine adolescent vaccines, and incidence of serious adverse events. Overall, the data showed noninferiority of MenACWY-TT, compared with other available products, in terms of response rates, as well as higher levels of immune response in some studies. Serious adverse events were similar, and none determined to be associated with the vaccines.
ACIP member Paul Hunter, MD, of the University of Milwaukee, Wisc., expressed some concerns about pain or side effects for the new vaccine and Tdap when given together. However, a study of coadministration of MedACWY-TT and Tdap, compared with Tdap alone, showed no impact on geometric mean titer ratios.
Overall, the Work Group concluded that “desirable effects outweigh undesirable effects” and that the data favor the inclusion of MenACWY-TT as an option for meningococcal vaccination.
The committee members and Dr. McNamara had no relevant financial conflicts to disclose.
ACR issues guidances for MIS-C and pediatric rheumatic disease during pandemic
Two new clinical guidance documents from the American College of Rheumatology provide evidence-based recommendations for managing pediatric rheumatic disease during the COVID-19 pandemic as well as diagnostic and treatment recommendations for multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 infection.
Although several children’s hospitals have published their treatment protocols for MIS-C since the condition’s initial discovery, the ACR appears to be the first medical organization to review all the most current evidence to issue interim guidance with the expectations that it will change as more data become available.
“It is challenging having to make recommendations not having a lot of scientific evidence, but we still felt we had to use whatever’s out there to the best of our ability and use our experience to put together these recommendations,” Dawn M. Wahezi, MD, chief of pediatric rheumatology at Children’s Hospital at Montefiore and an associate professor of pediatrics at Albert Einstein College of Medicine, New York, said in an interview.
“We wanted to be mindful of the fact that there are things we know and things we don’t know, and we have to be careful about what we’re recommending,” said Dr. Wahezi, a member of the ACR working group that assembled the recommendations for pediatric rheumatic disease management during the pandemic. “We’re recommending the best we can at this moment, but if there are new studies that come out and suggest otherwise, we will definitely have to go back and amend the document.”
The foremost priority of the pediatric rheumatic disease guidance focuses on maintaining control of the disease and avoiding flares that may put children at greater risk of infection. Dr. Wahezi said the ACR has received many calls from patients and clinicians asking whether patients should continue their immunosuppressant medications. Fear of the coronavirus infection, medication shortages, difficulty getting to the pharmacy, uneasiness about going to the clinic or hospital for infusions, and other barriers may have led to gaps in medication.
“We didn’t want people to be too quick to hold patients’ medications just because they were scared of COVID,” Dr. Wahezi said. “If they did have medication stopped for one reason or another and their disease flared, having active disease, regardless of which disease it is, actually puts you at higher risk for infection. By controlling their disease, that would be the way to protect them the most.”
A key takeaway in the guidance on MIS-C, meanwhile, is an emphasis on its rarity lest physicians be too quick to diagnose it and miss another serious condition with overlapping symptoms, explained Lauren Henderson, MD, an attending rheumatologist at Boston Children’s Hospital and assistant professor of pediatrics at Harvard Medical School, Boston. Dr. Henderson participated in the ACR group that wrote the MIS-C guidance.
“The first thing we want to be thoughtful about clinically is to recognize that children in general with the acute infectious phase of SARS-CoV-2 have mild symptoms and generally do well,” Dr. Henderson said. “From what we can tell from all the data, MIS-C is rare. That really needs to be considered when clinicians on the ground are doing the diagnostic evaluation” because of concerns that clinicians “could rush to diagnose and treat patients with MIS-C and miss important diagnoses like malignancies and infections.”
Management of pediatric rheumatic disease during the pandemic
The COVID-19 clinical guidance for managing pediatric rheumatic disease grew from the work of the North American Pediatric Rheumatology Clinical Guidance Task Force, which included seven pediatric rheumatologists, two pediatric infectious disease physicians, one adult rheumatologist, and one pediatric nurse practitioner. The general guidance covers usual preventive measures for reducing risk for COVID-19 infection, the recommendation that children continue to receive recommended vaccines unless contraindicated by medication, and routine in-person visits for ophthalmologic surveillance of those with a history of uveitis or at high risk for chronic uveitis. The guidance also notes the risk of mental health concerns, such as depression and anxiety, related to quarantine and the pandemic.
The top recommendation is initiation or continuation of all medications necessary to control underlying disease, including NSAIDs, hydroxychloroquine, ACE inhibitors/angiotensin II receptor blockers, colchicine, conventional disease-modifying antirheumatic drugs (cDMARDs), biologic DMARDs, and targeted synthetic DMARDs. Even patients who may have had exposure to COVID-19 or who have an asymptomatic COVID-19 infection should continue to take these medications with the exception of ACEi/ARBs.
In those with pediatric rheumatic disease who have a symptomatic COVID-19 infection, “NSAIDs, HCQ, and colchicine may be continued, if necessary, to control underlying disease,” as can interleukin (IL)-1 and IL-6 inhibitors, but “cDMARDs, bDMARDs [except IL-1 and IL-6 inhibitors] and tsDMARDs should be temporarily delayed or withheld,” according to the guidance. Glucocorticoids can be continued at the lowest possible dose to control disease.
“There’s nothing in the literature that suggests people who have rheumatic disease, especially children, and people who are on these medications, really are at increased risk for COVID-19,” Dr. Wahezi said. “That’s why we didn’t want people to be overcautious in stopping medications when the main priority is to control their disease.”
She noted some experts’ speculations that these medications may actually benefit patients with rheumatic disease who develop a COVID-19 infection because the medications keep the immune response in check. “If you allow them to have this dysregulated immune response and have active disease, you’re potentially putting them at greater risk,” Dr. Wahezi said, although she stressed that inadequate evidence exists to support these speculations right now.
Lack of evidence has been the biggest challenge all around with developing this guidance, she said.
“Because this is such an unprecedented situation and because people are so desperate to find treatments both for the illness and to protect those at risk for it, there are lots of people trying to put evidence out there, but it may not be the best-quality evidence,” Dr. Wahezi said.
Insufficient evidence also drove the group’s determination that “SARS-CoV-2 antibody testing is not useful in informing on the history of infection or risk of reinfection,” as the guidance states. Too much variability in the assays exist, Dr. Wahezi said, and, further, it’s unclear what the clinical significance of a positive test would be.
“We didn’t want anyone to feel they had to make clinical decisions based on the results of that antibody testing,” she said. “Even if the test is accurate, we don’t know how to interpret it because it’s so new.”
The guidance also notes that patients with stable disease and previously stable lab markers on stable doses of their medication may be able to extend the interval for medication toxicity lab testing a few months if there is concern about exposure to COVID-19 to get the blood work.
“If you’re just starting a medicine or there’s someone who’s had abnormalities with the medicine in the past or you’re making medication adjustments, you wouldn’t do it in those scenarios, but if there’s someone who’s been on the drug for a long time and are nervous to get [blood] drawn, it’s probably okay to delay it,” Dr. Wahezi said. Lab work for disease activity measures, on the other hand, remain particularly important, especially since telemedicine visits may require clinicians to rely on lab results more than previously.
Management of MIS-C associated with COVID-19
The task force that developed guidance for the new inflammatory condition recently linked to SARS-CoV-2 infections in children included nine pediatric rheumatologists, two adult rheumatologists, two pediatric cardiologists, two pediatric infectious disease specialists, and one pediatric critical care physician.
The guidance includes a figure for the diagnostic pathway in evaluating children suspected of having MIS-C and extensive detail on diagnostic work-up, but the task force intentionally avoided providing a case definition for the condition. Existing case definitions from the Centers for Disease Control and Prevention, World Health Organization, and the United Kingdom’s Royal College of Paediatrics and Child Health differ from one another and are based on unclear evidence, Dr. Henderson noted. “We really don’t have enough data to know the sensitivity and specificity of each parameter, and until that’s available, we didn’t want to add to the confusion,” she said.
The guidance also stresses that MIS-C is a rare complication, so patients suspected of having the condition who do not have “life-threatening manifestations should undergo diagnostic evaluation for MIS-C as well as other possible infectious and noninfectious etiologies before immunomodulatory treatment is initiated,” the guidance states.
Unless a child is in shock or otherwise requires urgent care, physicians should take the time to complete the diagnostic work-up while monitoring the child, Dr. Henderson said. If the child does have MIS-C, the guidance currently recommends intravenous immunoglobulin (IVIG) and/or glucocorticoids to prevent coronary artery aneurysms, the same treatment other institutions have been recommending.
“We don’t have rigorous comparative studies looking at different types of treatments,” Dr. Henderson said, noting that the vast majority of children in the literature received IVIG and/or glucocorticoid treatment. “Often children really responded quite forcefully to those treatments, but we don’t have high-quality data yet to know that this treatment is better than supportive care or another medication.”
Dr. Henderson also stressed the importance of children receiving care at a facility with the necessary expertise to manage MIS-C and receiving long-term follow-up care from a multidisciplinary clinical team that includes a rheumatologist, an infectious disease doctor, a cardiologist, and possibly a hematologist.
“Making sure children are admitted to a hospital that has the resources and are followed by physicians with expertise or understanding of the intricacies of MIS-C is really important,” she said, particularly for children with cardiac involvement. “We don’t know if all the kids presenting with left ventricular dysfunction and shock are at risk for having myocardial fibrosis down the line,” she noted. “There is so much we do not understand and very little data to guide us on what to do, so these children really need to be under the care of a cardiologist and rheumatologist to make sure that their care is tailored to them.”
Although MIS-C shares overlapping symptoms with Kawasaki disease, it’s still unclear how similar or different the two conditions are, Dr. Henderson said.
“We can definitely say that when we look at MIS-C and compare it to historical groups of Kawasaki disease before the pandemic, there are definitely different features in the MIS-C group,” she said. Kawasaki disease generally only affects children under age 5, whereas MIS-C patients run the gamut from age 1-17. Racial demographics are also different, with a higher proportion of black children affected by MIS-C.
It’s possible that the pathophysiology of both conditions will turn out to be similar, particularly given the hypothesis that Kawasaki disease is triggered by infections in genetically predisposed people. However, the severity of symptoms and risk of aneurysms appear greater with MIS-C so far.
“The degree to which these patients are presenting with left ventricular dysfunction and shock is much higher than what we’ve seen previously,” Dr. Henderson said. “Children can have aneurysms even if they don’t meet all the Kawasaki disease features, which makes it feel that this is somehow clinically different from what we’ve seen before. It’s not just the kids who have the rash and the conjunctivitis and the extremity changes and oral changes who have the aneurysms.”
The reason for including both IVIG and glucocorticoids as possible first-line drugs to prevent aneurysms is that some evidence suggests children with MIS-C may have higher levels of IVIG resistance, she said.
Like Dr. Wahezi, Dr. Henderson emphasized the necessarily transient nature of these recommendations.
“These recommendations will almost certainly change based on evolving understanding of MIS-C and the data,” Dr. Henderson said, adding that this new, unique condition highlights the importance of including children in allocating funding for research and in clinical trials.
“Children are not always identical to adults, and it’s really important that we have high-quality data to inform our decisions about how to care for them,” she said.
Dr. Wahezi had no disclosures. Dr. Henderson has consulted for Sobi and Adaptive Technologies. The guidelines did not note other disclosures for members of the ACR groups.
SOURCES: COVID-19 Clinical Guidance for Pediatric Patients with Rheumatic Disease and Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19
Two new clinical guidance documents from the American College of Rheumatology provide evidence-based recommendations for managing pediatric rheumatic disease during the COVID-19 pandemic as well as diagnostic and treatment recommendations for multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 infection.
Although several children’s hospitals have published their treatment protocols for MIS-C since the condition’s initial discovery, the ACR appears to be the first medical organization to review all the most current evidence to issue interim guidance with the expectations that it will change as more data become available.
“It is challenging having to make recommendations not having a lot of scientific evidence, but we still felt we had to use whatever’s out there to the best of our ability and use our experience to put together these recommendations,” Dawn M. Wahezi, MD, chief of pediatric rheumatology at Children’s Hospital at Montefiore and an associate professor of pediatrics at Albert Einstein College of Medicine, New York, said in an interview.
“We wanted to be mindful of the fact that there are things we know and things we don’t know, and we have to be careful about what we’re recommending,” said Dr. Wahezi, a member of the ACR working group that assembled the recommendations for pediatric rheumatic disease management during the pandemic. “We’re recommending the best we can at this moment, but if there are new studies that come out and suggest otherwise, we will definitely have to go back and amend the document.”
The foremost priority of the pediatric rheumatic disease guidance focuses on maintaining control of the disease and avoiding flares that may put children at greater risk of infection. Dr. Wahezi said the ACR has received many calls from patients and clinicians asking whether patients should continue their immunosuppressant medications. Fear of the coronavirus infection, medication shortages, difficulty getting to the pharmacy, uneasiness about going to the clinic or hospital for infusions, and other barriers may have led to gaps in medication.
“We didn’t want people to be too quick to hold patients’ medications just because they were scared of COVID,” Dr. Wahezi said. “If they did have medication stopped for one reason or another and their disease flared, having active disease, regardless of which disease it is, actually puts you at higher risk for infection. By controlling their disease, that would be the way to protect them the most.”
A key takeaway in the guidance on MIS-C, meanwhile, is an emphasis on its rarity lest physicians be too quick to diagnose it and miss another serious condition with overlapping symptoms, explained Lauren Henderson, MD, an attending rheumatologist at Boston Children’s Hospital and assistant professor of pediatrics at Harvard Medical School, Boston. Dr. Henderson participated in the ACR group that wrote the MIS-C guidance.
“The first thing we want to be thoughtful about clinically is to recognize that children in general with the acute infectious phase of SARS-CoV-2 have mild symptoms and generally do well,” Dr. Henderson said. “From what we can tell from all the data, MIS-C is rare. That really needs to be considered when clinicians on the ground are doing the diagnostic evaluation” because of concerns that clinicians “could rush to diagnose and treat patients with MIS-C and miss important diagnoses like malignancies and infections.”
Management of pediatric rheumatic disease during the pandemic
The COVID-19 clinical guidance for managing pediatric rheumatic disease grew from the work of the North American Pediatric Rheumatology Clinical Guidance Task Force, which included seven pediatric rheumatologists, two pediatric infectious disease physicians, one adult rheumatologist, and one pediatric nurse practitioner. The general guidance covers usual preventive measures for reducing risk for COVID-19 infection, the recommendation that children continue to receive recommended vaccines unless contraindicated by medication, and routine in-person visits for ophthalmologic surveillance of those with a history of uveitis or at high risk for chronic uveitis. The guidance also notes the risk of mental health concerns, such as depression and anxiety, related to quarantine and the pandemic.
The top recommendation is initiation or continuation of all medications necessary to control underlying disease, including NSAIDs, hydroxychloroquine, ACE inhibitors/angiotensin II receptor blockers, colchicine, conventional disease-modifying antirheumatic drugs (cDMARDs), biologic DMARDs, and targeted synthetic DMARDs. Even patients who may have had exposure to COVID-19 or who have an asymptomatic COVID-19 infection should continue to take these medications with the exception of ACEi/ARBs.
In those with pediatric rheumatic disease who have a symptomatic COVID-19 infection, “NSAIDs, HCQ, and colchicine may be continued, if necessary, to control underlying disease,” as can interleukin (IL)-1 and IL-6 inhibitors, but “cDMARDs, bDMARDs [except IL-1 and IL-6 inhibitors] and tsDMARDs should be temporarily delayed or withheld,” according to the guidance. Glucocorticoids can be continued at the lowest possible dose to control disease.
“There’s nothing in the literature that suggests people who have rheumatic disease, especially children, and people who are on these medications, really are at increased risk for COVID-19,” Dr. Wahezi said. “That’s why we didn’t want people to be overcautious in stopping medications when the main priority is to control their disease.”
She noted some experts’ speculations that these medications may actually benefit patients with rheumatic disease who develop a COVID-19 infection because the medications keep the immune response in check. “If you allow them to have this dysregulated immune response and have active disease, you’re potentially putting them at greater risk,” Dr. Wahezi said, although she stressed that inadequate evidence exists to support these speculations right now.
Lack of evidence has been the biggest challenge all around with developing this guidance, she said.
“Because this is such an unprecedented situation and because people are so desperate to find treatments both for the illness and to protect those at risk for it, there are lots of people trying to put evidence out there, but it may not be the best-quality evidence,” Dr. Wahezi said.
Insufficient evidence also drove the group’s determination that “SARS-CoV-2 antibody testing is not useful in informing on the history of infection or risk of reinfection,” as the guidance states. Too much variability in the assays exist, Dr. Wahezi said, and, further, it’s unclear what the clinical significance of a positive test would be.
“We didn’t want anyone to feel they had to make clinical decisions based on the results of that antibody testing,” she said. “Even if the test is accurate, we don’t know how to interpret it because it’s so new.”
The guidance also notes that patients with stable disease and previously stable lab markers on stable doses of their medication may be able to extend the interval for medication toxicity lab testing a few months if there is concern about exposure to COVID-19 to get the blood work.
“If you’re just starting a medicine or there’s someone who’s had abnormalities with the medicine in the past or you’re making medication adjustments, you wouldn’t do it in those scenarios, but if there’s someone who’s been on the drug for a long time and are nervous to get [blood] drawn, it’s probably okay to delay it,” Dr. Wahezi said. Lab work for disease activity measures, on the other hand, remain particularly important, especially since telemedicine visits may require clinicians to rely on lab results more than previously.
Management of MIS-C associated with COVID-19
The task force that developed guidance for the new inflammatory condition recently linked to SARS-CoV-2 infections in children included nine pediatric rheumatologists, two adult rheumatologists, two pediatric cardiologists, two pediatric infectious disease specialists, and one pediatric critical care physician.
The guidance includes a figure for the diagnostic pathway in evaluating children suspected of having MIS-C and extensive detail on diagnostic work-up, but the task force intentionally avoided providing a case definition for the condition. Existing case definitions from the Centers for Disease Control and Prevention, World Health Organization, and the United Kingdom’s Royal College of Paediatrics and Child Health differ from one another and are based on unclear evidence, Dr. Henderson noted. “We really don’t have enough data to know the sensitivity and specificity of each parameter, and until that’s available, we didn’t want to add to the confusion,” she said.
The guidance also stresses that MIS-C is a rare complication, so patients suspected of having the condition who do not have “life-threatening manifestations should undergo diagnostic evaluation for MIS-C as well as other possible infectious and noninfectious etiologies before immunomodulatory treatment is initiated,” the guidance states.
Unless a child is in shock or otherwise requires urgent care, physicians should take the time to complete the diagnostic work-up while monitoring the child, Dr. Henderson said. If the child does have MIS-C, the guidance currently recommends intravenous immunoglobulin (IVIG) and/or glucocorticoids to prevent coronary artery aneurysms, the same treatment other institutions have been recommending.
“We don’t have rigorous comparative studies looking at different types of treatments,” Dr. Henderson said, noting that the vast majority of children in the literature received IVIG and/or glucocorticoid treatment. “Often children really responded quite forcefully to those treatments, but we don’t have high-quality data yet to know that this treatment is better than supportive care or another medication.”
Dr. Henderson also stressed the importance of children receiving care at a facility with the necessary expertise to manage MIS-C and receiving long-term follow-up care from a multidisciplinary clinical team that includes a rheumatologist, an infectious disease doctor, a cardiologist, and possibly a hematologist.
“Making sure children are admitted to a hospital that has the resources and are followed by physicians with expertise or understanding of the intricacies of MIS-C is really important,” she said, particularly for children with cardiac involvement. “We don’t know if all the kids presenting with left ventricular dysfunction and shock are at risk for having myocardial fibrosis down the line,” she noted. “There is so much we do not understand and very little data to guide us on what to do, so these children really need to be under the care of a cardiologist and rheumatologist to make sure that their care is tailored to them.”
Although MIS-C shares overlapping symptoms with Kawasaki disease, it’s still unclear how similar or different the two conditions are, Dr. Henderson said.
“We can definitely say that when we look at MIS-C and compare it to historical groups of Kawasaki disease before the pandemic, there are definitely different features in the MIS-C group,” she said. Kawasaki disease generally only affects children under age 5, whereas MIS-C patients run the gamut from age 1-17. Racial demographics are also different, with a higher proportion of black children affected by MIS-C.
It’s possible that the pathophysiology of both conditions will turn out to be similar, particularly given the hypothesis that Kawasaki disease is triggered by infections in genetically predisposed people. However, the severity of symptoms and risk of aneurysms appear greater with MIS-C so far.
“The degree to which these patients are presenting with left ventricular dysfunction and shock is much higher than what we’ve seen previously,” Dr. Henderson said. “Children can have aneurysms even if they don’t meet all the Kawasaki disease features, which makes it feel that this is somehow clinically different from what we’ve seen before. It’s not just the kids who have the rash and the conjunctivitis and the extremity changes and oral changes who have the aneurysms.”
The reason for including both IVIG and glucocorticoids as possible first-line drugs to prevent aneurysms is that some evidence suggests children with MIS-C may have higher levels of IVIG resistance, she said.
Like Dr. Wahezi, Dr. Henderson emphasized the necessarily transient nature of these recommendations.
“These recommendations will almost certainly change based on evolving understanding of MIS-C and the data,” Dr. Henderson said, adding that this new, unique condition highlights the importance of including children in allocating funding for research and in clinical trials.
“Children are not always identical to adults, and it’s really important that we have high-quality data to inform our decisions about how to care for them,” she said.
Dr. Wahezi had no disclosures. Dr. Henderson has consulted for Sobi and Adaptive Technologies. The guidelines did not note other disclosures for members of the ACR groups.
SOURCES: COVID-19 Clinical Guidance for Pediatric Patients with Rheumatic Disease and Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19
Two new clinical guidance documents from the American College of Rheumatology provide evidence-based recommendations for managing pediatric rheumatic disease during the COVID-19 pandemic as well as diagnostic and treatment recommendations for multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 infection.
Although several children’s hospitals have published their treatment protocols for MIS-C since the condition’s initial discovery, the ACR appears to be the first medical organization to review all the most current evidence to issue interim guidance with the expectations that it will change as more data become available.
“It is challenging having to make recommendations not having a lot of scientific evidence, but we still felt we had to use whatever’s out there to the best of our ability and use our experience to put together these recommendations,” Dawn M. Wahezi, MD, chief of pediatric rheumatology at Children’s Hospital at Montefiore and an associate professor of pediatrics at Albert Einstein College of Medicine, New York, said in an interview.
“We wanted to be mindful of the fact that there are things we know and things we don’t know, and we have to be careful about what we’re recommending,” said Dr. Wahezi, a member of the ACR working group that assembled the recommendations for pediatric rheumatic disease management during the pandemic. “We’re recommending the best we can at this moment, but if there are new studies that come out and suggest otherwise, we will definitely have to go back and amend the document.”
The foremost priority of the pediatric rheumatic disease guidance focuses on maintaining control of the disease and avoiding flares that may put children at greater risk of infection. Dr. Wahezi said the ACR has received many calls from patients and clinicians asking whether patients should continue their immunosuppressant medications. Fear of the coronavirus infection, medication shortages, difficulty getting to the pharmacy, uneasiness about going to the clinic or hospital for infusions, and other barriers may have led to gaps in medication.
“We didn’t want people to be too quick to hold patients’ medications just because they were scared of COVID,” Dr. Wahezi said. “If they did have medication stopped for one reason or another and their disease flared, having active disease, regardless of which disease it is, actually puts you at higher risk for infection. By controlling their disease, that would be the way to protect them the most.”
A key takeaway in the guidance on MIS-C, meanwhile, is an emphasis on its rarity lest physicians be too quick to diagnose it and miss another serious condition with overlapping symptoms, explained Lauren Henderson, MD, an attending rheumatologist at Boston Children’s Hospital and assistant professor of pediatrics at Harvard Medical School, Boston. Dr. Henderson participated in the ACR group that wrote the MIS-C guidance.
“The first thing we want to be thoughtful about clinically is to recognize that children in general with the acute infectious phase of SARS-CoV-2 have mild symptoms and generally do well,” Dr. Henderson said. “From what we can tell from all the data, MIS-C is rare. That really needs to be considered when clinicians on the ground are doing the diagnostic evaluation” because of concerns that clinicians “could rush to diagnose and treat patients with MIS-C and miss important diagnoses like malignancies and infections.”
Management of pediatric rheumatic disease during the pandemic
The COVID-19 clinical guidance for managing pediatric rheumatic disease grew from the work of the North American Pediatric Rheumatology Clinical Guidance Task Force, which included seven pediatric rheumatologists, two pediatric infectious disease physicians, one adult rheumatologist, and one pediatric nurse practitioner. The general guidance covers usual preventive measures for reducing risk for COVID-19 infection, the recommendation that children continue to receive recommended vaccines unless contraindicated by medication, and routine in-person visits for ophthalmologic surveillance of those with a history of uveitis or at high risk for chronic uveitis. The guidance also notes the risk of mental health concerns, such as depression and anxiety, related to quarantine and the pandemic.
The top recommendation is initiation or continuation of all medications necessary to control underlying disease, including NSAIDs, hydroxychloroquine, ACE inhibitors/angiotensin II receptor blockers, colchicine, conventional disease-modifying antirheumatic drugs (cDMARDs), biologic DMARDs, and targeted synthetic DMARDs. Even patients who may have had exposure to COVID-19 or who have an asymptomatic COVID-19 infection should continue to take these medications with the exception of ACEi/ARBs.
In those with pediatric rheumatic disease who have a symptomatic COVID-19 infection, “NSAIDs, HCQ, and colchicine may be continued, if necessary, to control underlying disease,” as can interleukin (IL)-1 and IL-6 inhibitors, but “cDMARDs, bDMARDs [except IL-1 and IL-6 inhibitors] and tsDMARDs should be temporarily delayed or withheld,” according to the guidance. Glucocorticoids can be continued at the lowest possible dose to control disease.
“There’s nothing in the literature that suggests people who have rheumatic disease, especially children, and people who are on these medications, really are at increased risk for COVID-19,” Dr. Wahezi said. “That’s why we didn’t want people to be overcautious in stopping medications when the main priority is to control their disease.”
She noted some experts’ speculations that these medications may actually benefit patients with rheumatic disease who develop a COVID-19 infection because the medications keep the immune response in check. “If you allow them to have this dysregulated immune response and have active disease, you’re potentially putting them at greater risk,” Dr. Wahezi said, although she stressed that inadequate evidence exists to support these speculations right now.
Lack of evidence has been the biggest challenge all around with developing this guidance, she said.
“Because this is such an unprecedented situation and because people are so desperate to find treatments both for the illness and to protect those at risk for it, there are lots of people trying to put evidence out there, but it may not be the best-quality evidence,” Dr. Wahezi said.
Insufficient evidence also drove the group’s determination that “SARS-CoV-2 antibody testing is not useful in informing on the history of infection or risk of reinfection,” as the guidance states. Too much variability in the assays exist, Dr. Wahezi said, and, further, it’s unclear what the clinical significance of a positive test would be.
“We didn’t want anyone to feel they had to make clinical decisions based on the results of that antibody testing,” she said. “Even if the test is accurate, we don’t know how to interpret it because it’s so new.”
The guidance also notes that patients with stable disease and previously stable lab markers on stable doses of their medication may be able to extend the interval for medication toxicity lab testing a few months if there is concern about exposure to COVID-19 to get the blood work.
“If you’re just starting a medicine or there’s someone who’s had abnormalities with the medicine in the past or you’re making medication adjustments, you wouldn’t do it in those scenarios, but if there’s someone who’s been on the drug for a long time and are nervous to get [blood] drawn, it’s probably okay to delay it,” Dr. Wahezi said. Lab work for disease activity measures, on the other hand, remain particularly important, especially since telemedicine visits may require clinicians to rely on lab results more than previously.
Management of MIS-C associated with COVID-19
The task force that developed guidance for the new inflammatory condition recently linked to SARS-CoV-2 infections in children included nine pediatric rheumatologists, two adult rheumatologists, two pediatric cardiologists, two pediatric infectious disease specialists, and one pediatric critical care physician.
The guidance includes a figure for the diagnostic pathway in evaluating children suspected of having MIS-C and extensive detail on diagnostic work-up, but the task force intentionally avoided providing a case definition for the condition. Existing case definitions from the Centers for Disease Control and Prevention, World Health Organization, and the United Kingdom’s Royal College of Paediatrics and Child Health differ from one another and are based on unclear evidence, Dr. Henderson noted. “We really don’t have enough data to know the sensitivity and specificity of each parameter, and until that’s available, we didn’t want to add to the confusion,” she said.
The guidance also stresses that MIS-C is a rare complication, so patients suspected of having the condition who do not have “life-threatening manifestations should undergo diagnostic evaluation for MIS-C as well as other possible infectious and noninfectious etiologies before immunomodulatory treatment is initiated,” the guidance states.
Unless a child is in shock or otherwise requires urgent care, physicians should take the time to complete the diagnostic work-up while monitoring the child, Dr. Henderson said. If the child does have MIS-C, the guidance currently recommends intravenous immunoglobulin (IVIG) and/or glucocorticoids to prevent coronary artery aneurysms, the same treatment other institutions have been recommending.
“We don’t have rigorous comparative studies looking at different types of treatments,” Dr. Henderson said, noting that the vast majority of children in the literature received IVIG and/or glucocorticoid treatment. “Often children really responded quite forcefully to those treatments, but we don’t have high-quality data yet to know that this treatment is better than supportive care or another medication.”
Dr. Henderson also stressed the importance of children receiving care at a facility with the necessary expertise to manage MIS-C and receiving long-term follow-up care from a multidisciplinary clinical team that includes a rheumatologist, an infectious disease doctor, a cardiologist, and possibly a hematologist.
“Making sure children are admitted to a hospital that has the resources and are followed by physicians with expertise or understanding of the intricacies of MIS-C is really important,” she said, particularly for children with cardiac involvement. “We don’t know if all the kids presenting with left ventricular dysfunction and shock are at risk for having myocardial fibrosis down the line,” she noted. “There is so much we do not understand and very little data to guide us on what to do, so these children really need to be under the care of a cardiologist and rheumatologist to make sure that their care is tailored to them.”
Although MIS-C shares overlapping symptoms with Kawasaki disease, it’s still unclear how similar or different the two conditions are, Dr. Henderson said.
“We can definitely say that when we look at MIS-C and compare it to historical groups of Kawasaki disease before the pandemic, there are definitely different features in the MIS-C group,” she said. Kawasaki disease generally only affects children under age 5, whereas MIS-C patients run the gamut from age 1-17. Racial demographics are also different, with a higher proportion of black children affected by MIS-C.
It’s possible that the pathophysiology of both conditions will turn out to be similar, particularly given the hypothesis that Kawasaki disease is triggered by infections in genetically predisposed people. However, the severity of symptoms and risk of aneurysms appear greater with MIS-C so far.
“The degree to which these patients are presenting with left ventricular dysfunction and shock is much higher than what we’ve seen previously,” Dr. Henderson said. “Children can have aneurysms even if they don’t meet all the Kawasaki disease features, which makes it feel that this is somehow clinically different from what we’ve seen before. It’s not just the kids who have the rash and the conjunctivitis and the extremity changes and oral changes who have the aneurysms.”
The reason for including both IVIG and glucocorticoids as possible first-line drugs to prevent aneurysms is that some evidence suggests children with MIS-C may have higher levels of IVIG resistance, she said.
Like Dr. Wahezi, Dr. Henderson emphasized the necessarily transient nature of these recommendations.
“These recommendations will almost certainly change based on evolving understanding of MIS-C and the data,” Dr. Henderson said, adding that this new, unique condition highlights the importance of including children in allocating funding for research and in clinical trials.
“Children are not always identical to adults, and it’s really important that we have high-quality data to inform our decisions about how to care for them,” she said.
Dr. Wahezi had no disclosures. Dr. Henderson has consulted for Sobi and Adaptive Technologies. The guidelines did not note other disclosures for members of the ACR groups.
SOURCES: COVID-19 Clinical Guidance for Pediatric Patients with Rheumatic Disease and Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19
Experts publish imaging recommendations for pediatric COVID-19
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has devised recommendations for ordering imaging studies in suspected cases of the infection.
The review also included useful radiographic findings to help in the differential diagnosis of COVID-19 pneumonia from other respiratory infections. Alexandra M. Foust, DO, of Boston Children’s Hospital, and colleagues reported the summary of findings and recommendations in Pediatric Pulmonology.
“Pediatricians face numerous challenges created by increasing reports of severe COVID-19 related findings in affected children,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. “[The current review] represents a multinational collaboration to provide up to date information and key imaging findings to guide chest physicians caring for children with pneumonia symptoms during the COVID-19 pandemic.”
Clinical presentation in children
In general, pediatric patients infected with the virus show milder symptoms compared with adults, and based on the limited evidence reported to date, the most common clinical symptoms of COVID-19 in children are rhinorrhea and/or nasal congestion, fever and cough with sore throat, fatigue or dyspnea, and diarrhea.
As with other viral pneumonias in children, the laboratory parameters are usually nonspecific; however, while the complete blood count (CBC) is often normal, lymphopenia, thrombocytopenia, and neutropenia have been reported in some cases of pediatric COVID-19, the authors noted.
The current Centers for Disease Control and Prevention (CDC) recommendation for initial diagnosis of SARS-CoV-2 is obtaining a nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) testing, they explained.
Role of imaging in diagnosis
The researchers reported that current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as a upfront test to diagnose pediatric COVID-19, but they may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course.
The potential benefits of utilizing radiologic evaluation, such as establishing a baseline for monitoring disease progression, must be balanced with potential drawbacks, which include radiation exposure, and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Recommendations for ordering imaging studies
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia.
The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia.
“The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Dr. Cataletto. “By synthesizing the available imaging case series and guidelines, this primer provides a useful tool for the practicing pulmonologist,” she explained.
Key recommendations: CXR
“For pediatric patients with suspected or known COVID-19 infection with moderate to severe clinical symptoms requiring hospitalization (i.e., hypoxia, moderate or severe dyspnea, signs of sepsis, shock, cardiovascular compromise, altered mentation), CXR is usually indicated to establish an imaging baseline and to assess for an alternative diagnosis,” they recommended.
“Sequential CXRs may be helpful to assess pediatric patients with COVID-19 who demonstrate worsening clinical symptoms or to assess response to supportive therapy,” they wrote.
Key recommendations: CT
“Due to the increased radiation sensitivity of pediatric patients, chest CT is not recommended as an initial diagnostic test for pediatric patients with known or suspected COVID-19 pneumonia,” they explained.
The guide also included several considerations around the differential diagnosis of COVID-19 pneumonia from other pediatric lung disorders, including immune-related conditions, infectious etiologies, hematological dyscrasias, and inhalation-related lung injury.
As best practice recommendations for COVID-19 continue to evolve, the availability of practical clinical decision-making tools becomes essential to ensure optimal patient care.
No funding sources or financial disclosures were reported in the manuscript.
SOURCE: Foust AM et al. Pediatr Pulmonol. 2020 May 28. doi: 10.1002/ppul.24870.
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has devised recommendations for ordering imaging studies in suspected cases of the infection.
The review also included useful radiographic findings to help in the differential diagnosis of COVID-19 pneumonia from other respiratory infections. Alexandra M. Foust, DO, of Boston Children’s Hospital, and colleagues reported the summary of findings and recommendations in Pediatric Pulmonology.
“Pediatricians face numerous challenges created by increasing reports of severe COVID-19 related findings in affected children,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. “[The current review] represents a multinational collaboration to provide up to date information and key imaging findings to guide chest physicians caring for children with pneumonia symptoms during the COVID-19 pandemic.”
Clinical presentation in children
In general, pediatric patients infected with the virus show milder symptoms compared with adults, and based on the limited evidence reported to date, the most common clinical symptoms of COVID-19 in children are rhinorrhea and/or nasal congestion, fever and cough with sore throat, fatigue or dyspnea, and diarrhea.
As with other viral pneumonias in children, the laboratory parameters are usually nonspecific; however, while the complete blood count (CBC) is often normal, lymphopenia, thrombocytopenia, and neutropenia have been reported in some cases of pediatric COVID-19, the authors noted.
The current Centers for Disease Control and Prevention (CDC) recommendation for initial diagnosis of SARS-CoV-2 is obtaining a nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) testing, they explained.
Role of imaging in diagnosis
The researchers reported that current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as a upfront test to diagnose pediatric COVID-19, but they may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course.
The potential benefits of utilizing radiologic evaluation, such as establishing a baseline for monitoring disease progression, must be balanced with potential drawbacks, which include radiation exposure, and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Recommendations for ordering imaging studies
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia.
The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia.
“The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Dr. Cataletto. “By synthesizing the available imaging case series and guidelines, this primer provides a useful tool for the practicing pulmonologist,” she explained.
Key recommendations: CXR
“For pediatric patients with suspected or known COVID-19 infection with moderate to severe clinical symptoms requiring hospitalization (i.e., hypoxia, moderate or severe dyspnea, signs of sepsis, shock, cardiovascular compromise, altered mentation), CXR is usually indicated to establish an imaging baseline and to assess for an alternative diagnosis,” they recommended.
“Sequential CXRs may be helpful to assess pediatric patients with COVID-19 who demonstrate worsening clinical symptoms or to assess response to supportive therapy,” they wrote.
Key recommendations: CT
“Due to the increased radiation sensitivity of pediatric patients, chest CT is not recommended as an initial diagnostic test for pediatric patients with known or suspected COVID-19 pneumonia,” they explained.
The guide also included several considerations around the differential diagnosis of COVID-19 pneumonia from other pediatric lung disorders, including immune-related conditions, infectious etiologies, hematological dyscrasias, and inhalation-related lung injury.
As best practice recommendations for COVID-19 continue to evolve, the availability of practical clinical decision-making tools becomes essential to ensure optimal patient care.
No funding sources or financial disclosures were reported in the manuscript.
SOURCE: Foust AM et al. Pediatr Pulmonol. 2020 May 28. doi: 10.1002/ppul.24870.
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has devised recommendations for ordering imaging studies in suspected cases of the infection.
The review also included useful radiographic findings to help in the differential diagnosis of COVID-19 pneumonia from other respiratory infections. Alexandra M. Foust, DO, of Boston Children’s Hospital, and colleagues reported the summary of findings and recommendations in Pediatric Pulmonology.
“Pediatricians face numerous challenges created by increasing reports of severe COVID-19 related findings in affected children,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. “[The current review] represents a multinational collaboration to provide up to date information and key imaging findings to guide chest physicians caring for children with pneumonia symptoms during the COVID-19 pandemic.”
Clinical presentation in children
In general, pediatric patients infected with the virus show milder symptoms compared with adults, and based on the limited evidence reported to date, the most common clinical symptoms of COVID-19 in children are rhinorrhea and/or nasal congestion, fever and cough with sore throat, fatigue or dyspnea, and diarrhea.
As with other viral pneumonias in children, the laboratory parameters are usually nonspecific; however, while the complete blood count (CBC) is often normal, lymphopenia, thrombocytopenia, and neutropenia have been reported in some cases of pediatric COVID-19, the authors noted.
The current Centers for Disease Control and Prevention (CDC) recommendation for initial diagnosis of SARS-CoV-2 is obtaining a nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) testing, they explained.
Role of imaging in diagnosis
The researchers reported that current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as a upfront test to diagnose pediatric COVID-19, but they may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course.
The potential benefits of utilizing radiologic evaluation, such as establishing a baseline for monitoring disease progression, must be balanced with potential drawbacks, which include radiation exposure, and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Recommendations for ordering imaging studies
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia.
The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia.
“The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Dr. Cataletto. “By synthesizing the available imaging case series and guidelines, this primer provides a useful tool for the practicing pulmonologist,” she explained.
Key recommendations: CXR
“For pediatric patients with suspected or known COVID-19 infection with moderate to severe clinical symptoms requiring hospitalization (i.e., hypoxia, moderate or severe dyspnea, signs of sepsis, shock, cardiovascular compromise, altered mentation), CXR is usually indicated to establish an imaging baseline and to assess for an alternative diagnosis,” they recommended.
“Sequential CXRs may be helpful to assess pediatric patients with COVID-19 who demonstrate worsening clinical symptoms or to assess response to supportive therapy,” they wrote.
Key recommendations: CT
“Due to the increased radiation sensitivity of pediatric patients, chest CT is not recommended as an initial diagnostic test for pediatric patients with known or suspected COVID-19 pneumonia,” they explained.
The guide also included several considerations around the differential diagnosis of COVID-19 pneumonia from other pediatric lung disorders, including immune-related conditions, infectious etiologies, hematological dyscrasias, and inhalation-related lung injury.
As best practice recommendations for COVID-19 continue to evolve, the availability of practical clinical decision-making tools becomes essential to ensure optimal patient care.
No funding sources or financial disclosures were reported in the manuscript.
SOURCE: Foust AM et al. Pediatr Pulmonol. 2020 May 28. doi: 10.1002/ppul.24870.
FROM PEDIATRIC PULMONOLOGY
What’s pushing cannabis use in first-episode psychosis?
The desire to feel better is a major driver for patients with first-episode psychosis (FEP) to turn to cannabis, new research shows.
An analysis of more than 1,300 individuals from six European countries showed patients with FEP were four times more likely than their healthy peers to start smoking cannabis in order to make themselves feel better.
The results also revealed that initiating cannabis use to feel better was associated with a more than tripled risk of being a daily user.
as well as offer an opportunity for psychoeducation – particularly as the reasons for starting cannabis appear to influence frequency of use, study investigator Edoardo Spinazzola, MD, Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, said in an interview.
Patients who start smoking cannabis because their friends or family partakes may benefit from therapies that encourage more “assertiveness” and being “socially comfortable without the substance,” Dr. Spinazzola said, noting that it might also be beneficial to identify the specific cause of the psychological discomfort driving cannabis use, such as depression, and specifically treat that issue.
The results were scheduled to be presented at the Congress of the Schizophrenia International Research Society 2020, but the meeting was canceled because of the coronavirus pandemic.
Answering the skeptics
Previous studies suggest that cannabis use can increase risk for psychosis up to 290%, with both frequency of use and potency playing a role, the researchers noted.
However, they added that “skeptics” argue the association could be caused by individuals with psychosis using cannabis as a form of self-medication, the comorbid effect of other psychogenic drugs, or a common genetic vulnerability between cannabis use and psychosis.
The reasons for starting cannabis use remain “largely unexplored,” so the researchers examined records from the European network of national schizophrenia networks studying Gene-Environment Interactions (EU-GEI) database, which includes patients with FEP and healthy individuals acting as controls from France, Italy, the Netherlands, Spain, United Kingdom, and Brazil.
The analysis included 1,347 individuals, of whom 446 had a diagnosis of nonaffective psychosis, 89 had bipolar disorder, and 58 had psychotic depression.
Reasons to start smoking cannabis and patterns of use were determined using the modified version of the Cannabis Experiences Questionnaire.
Results showed that participants who started cannabis to feel better were significantly more likely to be younger, have fewer years of education, to be black or of mixed ethnicity, to be single, or to not be living independently than those who started it because their friends or family were using it (P < .001 for all comparisons).
In addition, 68% of the patients with FEP and 85% of the healthy controls started using cannabis because friends or family were using it. In contrast, 18% of those with FEP versus 5% of controls starting using cannabis to feel better; 13% versus 10%, respectively, started using for “other reasons.”
After taking into account gender, age, ethnicity, and study site, the patients with FEP were significantly more likely than their healthy peers to have started using cannabis to feel better (relative risk ratio, 4.67; P < .001).
Starting to smoke cannabis to feel better versus any other reason was associated with an increased frequency of use in both those with and without FEP, with an RRR of 2.9 for using the drug more than once a week (P = .001) and an RRR of 3.13 for daily use (P < .001). However, the association was stronger in the healthy controls than in those with FEP, with an RRR for daily use of 4.45 versus 3.11, respectively.
The investigators also examined whether there was a link between reasons to start smoking and an individual’s polygenic risk score (PRS) for developing schizophrenia.
Multinomial regression indicated that PRS was not associated with starting cannabis to feel better or because friends were using it. However, there was an association between PRS score and starting the drug because family members were using it (RRR, 0.68; P < .05).
Complex association
Gabriella Gobbi, MD, PhD, professor in the neurobiological psychiatry unit, department of psychiatry, at McGill University, Montreal, said the data confirm “what we already know about cannabis.”
She noted that one of the “major causes” of young people starting cannabis is the social environment, while the desire to use the drug to feel better is linked to “the fact that cannabis, in a lot of cases, is used as a self-medication” in order to be calmer and as a relief from anxiety.
There is a “very complex” association between using cannabis to feel better and the self-medication seen with cigarette smoking and alcohol in patients with schizophrenia, said Dr. Gobbi, who was not involved with the research.
“When we talk about [patients using] cannabis, alcohol, and cigarettes, actually we’re talking about the same group of people,” she said.
Although “it is true they say that people look to cigarettes, tobacco, and alcohol to feel happier because they are depressed, the risk of psychosis is only for cannabis,” she added. “It is very low for alcohol and tobacco.”
As a result, Dr. Gobbi said she and her colleagues are “very worried” about the consequences for mental health of the legalization of cannabis consumption in Canada in October 2018 with the passing of the Cannabis Act.
Although there are no firm statistics yet, she has observed that since the law was passed, cannabis use has stabilized at a lower level among adolescents. “But now we have another population of people aged 34 and older that consume cannabis,” she said.
Particularly when considering the impact of higher strength cannabis on psychosis risk, Dr. Gobbi believes the increase in consumption in this age group will result in a “more elevated” risk for mental health issues.
Dr. Spinazzola and Dr. Gobbi have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The desire to feel better is a major driver for patients with first-episode psychosis (FEP) to turn to cannabis, new research shows.
An analysis of more than 1,300 individuals from six European countries showed patients with FEP were four times more likely than their healthy peers to start smoking cannabis in order to make themselves feel better.
The results also revealed that initiating cannabis use to feel better was associated with a more than tripled risk of being a daily user.
as well as offer an opportunity for psychoeducation – particularly as the reasons for starting cannabis appear to influence frequency of use, study investigator Edoardo Spinazzola, MD, Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, said in an interview.
Patients who start smoking cannabis because their friends or family partakes may benefit from therapies that encourage more “assertiveness” and being “socially comfortable without the substance,” Dr. Spinazzola said, noting that it might also be beneficial to identify the specific cause of the psychological discomfort driving cannabis use, such as depression, and specifically treat that issue.
The results were scheduled to be presented at the Congress of the Schizophrenia International Research Society 2020, but the meeting was canceled because of the coronavirus pandemic.
Answering the skeptics
Previous studies suggest that cannabis use can increase risk for psychosis up to 290%, with both frequency of use and potency playing a role, the researchers noted.
However, they added that “skeptics” argue the association could be caused by individuals with psychosis using cannabis as a form of self-medication, the comorbid effect of other psychogenic drugs, or a common genetic vulnerability between cannabis use and psychosis.
The reasons for starting cannabis use remain “largely unexplored,” so the researchers examined records from the European network of national schizophrenia networks studying Gene-Environment Interactions (EU-GEI) database, which includes patients with FEP and healthy individuals acting as controls from France, Italy, the Netherlands, Spain, United Kingdom, and Brazil.
The analysis included 1,347 individuals, of whom 446 had a diagnosis of nonaffective psychosis, 89 had bipolar disorder, and 58 had psychotic depression.
Reasons to start smoking cannabis and patterns of use were determined using the modified version of the Cannabis Experiences Questionnaire.
Results showed that participants who started cannabis to feel better were significantly more likely to be younger, have fewer years of education, to be black or of mixed ethnicity, to be single, or to not be living independently than those who started it because their friends or family were using it (P < .001 for all comparisons).
In addition, 68% of the patients with FEP and 85% of the healthy controls started using cannabis because friends or family were using it. In contrast, 18% of those with FEP versus 5% of controls starting using cannabis to feel better; 13% versus 10%, respectively, started using for “other reasons.”
After taking into account gender, age, ethnicity, and study site, the patients with FEP were significantly more likely than their healthy peers to have started using cannabis to feel better (relative risk ratio, 4.67; P < .001).
Starting to smoke cannabis to feel better versus any other reason was associated with an increased frequency of use in both those with and without FEP, with an RRR of 2.9 for using the drug more than once a week (P = .001) and an RRR of 3.13 for daily use (P < .001). However, the association was stronger in the healthy controls than in those with FEP, with an RRR for daily use of 4.45 versus 3.11, respectively.
The investigators also examined whether there was a link between reasons to start smoking and an individual’s polygenic risk score (PRS) for developing schizophrenia.
Multinomial regression indicated that PRS was not associated with starting cannabis to feel better or because friends were using it. However, there was an association between PRS score and starting the drug because family members were using it (RRR, 0.68; P < .05).
Complex association
Gabriella Gobbi, MD, PhD, professor in the neurobiological psychiatry unit, department of psychiatry, at McGill University, Montreal, said the data confirm “what we already know about cannabis.”
She noted that one of the “major causes” of young people starting cannabis is the social environment, while the desire to use the drug to feel better is linked to “the fact that cannabis, in a lot of cases, is used as a self-medication” in order to be calmer and as a relief from anxiety.
There is a “very complex” association between using cannabis to feel better and the self-medication seen with cigarette smoking and alcohol in patients with schizophrenia, said Dr. Gobbi, who was not involved with the research.
“When we talk about [patients using] cannabis, alcohol, and cigarettes, actually we’re talking about the same group of people,” she said.
Although “it is true they say that people look to cigarettes, tobacco, and alcohol to feel happier because they are depressed, the risk of psychosis is only for cannabis,” she added. “It is very low for alcohol and tobacco.”
As a result, Dr. Gobbi said she and her colleagues are “very worried” about the consequences for mental health of the legalization of cannabis consumption in Canada in October 2018 with the passing of the Cannabis Act.
Although there are no firm statistics yet, she has observed that since the law was passed, cannabis use has stabilized at a lower level among adolescents. “But now we have another population of people aged 34 and older that consume cannabis,” she said.
Particularly when considering the impact of higher strength cannabis on psychosis risk, Dr. Gobbi believes the increase in consumption in this age group will result in a “more elevated” risk for mental health issues.
Dr. Spinazzola and Dr. Gobbi have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The desire to feel better is a major driver for patients with first-episode psychosis (FEP) to turn to cannabis, new research shows.
An analysis of more than 1,300 individuals from six European countries showed patients with FEP were four times more likely than their healthy peers to start smoking cannabis in order to make themselves feel better.
The results also revealed that initiating cannabis use to feel better was associated with a more than tripled risk of being a daily user.
as well as offer an opportunity for psychoeducation – particularly as the reasons for starting cannabis appear to influence frequency of use, study investigator Edoardo Spinazzola, MD, Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, said in an interview.
Patients who start smoking cannabis because their friends or family partakes may benefit from therapies that encourage more “assertiveness” and being “socially comfortable without the substance,” Dr. Spinazzola said, noting that it might also be beneficial to identify the specific cause of the psychological discomfort driving cannabis use, such as depression, and specifically treat that issue.
The results were scheduled to be presented at the Congress of the Schizophrenia International Research Society 2020, but the meeting was canceled because of the coronavirus pandemic.
Answering the skeptics
Previous studies suggest that cannabis use can increase risk for psychosis up to 290%, with both frequency of use and potency playing a role, the researchers noted.
However, they added that “skeptics” argue the association could be caused by individuals with psychosis using cannabis as a form of self-medication, the comorbid effect of other psychogenic drugs, or a common genetic vulnerability between cannabis use and psychosis.
The reasons for starting cannabis use remain “largely unexplored,” so the researchers examined records from the European network of national schizophrenia networks studying Gene-Environment Interactions (EU-GEI) database, which includes patients with FEP and healthy individuals acting as controls from France, Italy, the Netherlands, Spain, United Kingdom, and Brazil.
The analysis included 1,347 individuals, of whom 446 had a diagnosis of nonaffective psychosis, 89 had bipolar disorder, and 58 had psychotic depression.
Reasons to start smoking cannabis and patterns of use were determined using the modified version of the Cannabis Experiences Questionnaire.
Results showed that participants who started cannabis to feel better were significantly more likely to be younger, have fewer years of education, to be black or of mixed ethnicity, to be single, or to not be living independently than those who started it because their friends or family were using it (P < .001 for all comparisons).
In addition, 68% of the patients with FEP and 85% of the healthy controls started using cannabis because friends or family were using it. In contrast, 18% of those with FEP versus 5% of controls starting using cannabis to feel better; 13% versus 10%, respectively, started using for “other reasons.”
After taking into account gender, age, ethnicity, and study site, the patients with FEP were significantly more likely than their healthy peers to have started using cannabis to feel better (relative risk ratio, 4.67; P < .001).
Starting to smoke cannabis to feel better versus any other reason was associated with an increased frequency of use in both those with and without FEP, with an RRR of 2.9 for using the drug more than once a week (P = .001) and an RRR of 3.13 for daily use (P < .001). However, the association was stronger in the healthy controls than in those with FEP, with an RRR for daily use of 4.45 versus 3.11, respectively.
The investigators also examined whether there was a link between reasons to start smoking and an individual’s polygenic risk score (PRS) for developing schizophrenia.
Multinomial regression indicated that PRS was not associated with starting cannabis to feel better or because friends were using it. However, there was an association between PRS score and starting the drug because family members were using it (RRR, 0.68; P < .05).
Complex association
Gabriella Gobbi, MD, PhD, professor in the neurobiological psychiatry unit, department of psychiatry, at McGill University, Montreal, said the data confirm “what we already know about cannabis.”
She noted that one of the “major causes” of young people starting cannabis is the social environment, while the desire to use the drug to feel better is linked to “the fact that cannabis, in a lot of cases, is used as a self-medication” in order to be calmer and as a relief from anxiety.
There is a “very complex” association between using cannabis to feel better and the self-medication seen with cigarette smoking and alcohol in patients with schizophrenia, said Dr. Gobbi, who was not involved with the research.
“When we talk about [patients using] cannabis, alcohol, and cigarettes, actually we’re talking about the same group of people,” she said.
Although “it is true they say that people look to cigarettes, tobacco, and alcohol to feel happier because they are depressed, the risk of psychosis is only for cannabis,” she added. “It is very low for alcohol and tobacco.”
As a result, Dr. Gobbi said she and her colleagues are “very worried” about the consequences for mental health of the legalization of cannabis consumption in Canada in October 2018 with the passing of the Cannabis Act.
Although there are no firm statistics yet, she has observed that since the law was passed, cannabis use has stabilized at a lower level among adolescents. “But now we have another population of people aged 34 and older that consume cannabis,” she said.
Particularly when considering the impact of higher strength cannabis on psychosis risk, Dr. Gobbi believes the increase in consumption in this age group will result in a “more elevated” risk for mental health issues.
Dr. Spinazzola and Dr. Gobbi have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SIRS 2020