User login
Study spotlights the skin microbiome’s evolving nature
, while the skin microbiome of the mothers of the children whose microbiome was analyzed remained relatively constant over the same time period.
The findings come from what is believed to be the longest longitudinal study specific to the skin microbiome of infants and mothers.
“Even at 10 years, the skin microbiome does not look like an adult skin microbiome, based on composition of the ecosystem,” lead author Kimberly A. Capone, PhD, said in an interview during the virtual annual meeting of the American Academy of Dermatology. “The diversity of the microbiome in children’s skin is distinct to that of an adult’s skin. We all have the same bacteria present, but in children it is distributed differently because the bacteria set themselves up based on the nutrients and topography that they find on the skin. Since infant skin is unique to infants, so too is their microbiome when we compare it to adults. It’s been fascinating to observe these children grow and mature, and follow the skin microbiome along this same period.”
During five time points over a period of 10 years, Dr. Capone and her colleagues at the Skillman, N.J.–based Johnson & Johnson Consumer Experience Center, a research and development site, used 16s rRNA gene sequencing to evaluate the skin microbiome on the forearms and foreheads of 30 mothers and their 31 children. The study participants had Fitzpatrick skin types I-IV and the mean age of mothers was 37 years. “We used 16s rRNA gene sequencing for microbiome analysis at the beginning, as that was what was available 10 years ago,” said Dr. Capone, head of the microbiome platform for Johnson & Johnson Consumer Health. “Since then, we continue to use 16s for continuity, but also collected additional swabs for deeper analysis later.”
She and her colleagues often draw samples from the forearm in baby skin clinical studies, “as the arm is a good site to collect relevant data on the body overall,” she explained. “We chose the forearm and forehead specifically here so we can make same body site comparisons to adult sample data which we took from the mothers on the same areas of their body.” Time point 1 was 3 months to 1 year, time point 2 was 2-3 years, time point 3 was 5-6 years, time point 4 was 7-8 years, and time point 5 was 9-10 years.
The researchers found that the skin of infants during the first few weeks of life was similar at age 3 and 4 years. “From that second time point on, we see significant increases in richness and diversity, richness being presence or absence of various bacterial species, and diversity being the relative abundance of those species,” Dr. Capone said. “What you’re basically seeing is that there are new organisms, i.e., richness, coming into the microbiome. We start to detect new ones. Over time, the ecosystem is expanding. It’s evolving because it’s not yet set up.” The evolving skin microbiome on children was dominated by Streptococcus and Staphylococcus. In addition, children had higher levels of Streptococcus, Moraxella, Granulicatella, Gemella and Veillonella, compared with their adult mothers. Adult skin was colonized predominantly by Propionibacterium/Cutibacterium and Staphylococcus.
“The skin microbiome also increased in diversity over time on the forearm, but not face, of the mothers,” Dr. Capone said. “Previous studies have shown how stable the adult skin microbiome is, so it’s intriguing to see the changes that we saw on the mothers in this study.”
The increase in skin microbiome diversity observed in the children is likely due to a variety of factors, she continued, including inherent growth and development, dietary changes, as well as exposure to various environments and other people. “The fact remains that diversity is increasing over time, as the ecosystem evolves,” she said. “Eventually, the skin microbiome will become ‘adultlike’ in puberty, when lipid production increases. This drives increases in Cutibacterium acnes, particularly on the face.”
She acknowledged certain limitations of the study, including its relatively small size and the fact that some of the original subjects did not return for microbiome analysis at later time points. “We need larger cohort studies, additional deeper sequence data, metabolomics and transcriptomics to better understand the function of the skin microbiome over these various ages,” she said.
The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
, while the skin microbiome of the mothers of the children whose microbiome was analyzed remained relatively constant over the same time period.
The findings come from what is believed to be the longest longitudinal study specific to the skin microbiome of infants and mothers.
“Even at 10 years, the skin microbiome does not look like an adult skin microbiome, based on composition of the ecosystem,” lead author Kimberly A. Capone, PhD, said in an interview during the virtual annual meeting of the American Academy of Dermatology. “The diversity of the microbiome in children’s skin is distinct to that of an adult’s skin. We all have the same bacteria present, but in children it is distributed differently because the bacteria set themselves up based on the nutrients and topography that they find on the skin. Since infant skin is unique to infants, so too is their microbiome when we compare it to adults. It’s been fascinating to observe these children grow and mature, and follow the skin microbiome along this same period.”
During five time points over a period of 10 years, Dr. Capone and her colleagues at the Skillman, N.J.–based Johnson & Johnson Consumer Experience Center, a research and development site, used 16s rRNA gene sequencing to evaluate the skin microbiome on the forearms and foreheads of 30 mothers and their 31 children. The study participants had Fitzpatrick skin types I-IV and the mean age of mothers was 37 years. “We used 16s rRNA gene sequencing for microbiome analysis at the beginning, as that was what was available 10 years ago,” said Dr. Capone, head of the microbiome platform for Johnson & Johnson Consumer Health. “Since then, we continue to use 16s for continuity, but also collected additional swabs for deeper analysis later.”
She and her colleagues often draw samples from the forearm in baby skin clinical studies, “as the arm is a good site to collect relevant data on the body overall,” she explained. “We chose the forearm and forehead specifically here so we can make same body site comparisons to adult sample data which we took from the mothers on the same areas of their body.” Time point 1 was 3 months to 1 year, time point 2 was 2-3 years, time point 3 was 5-6 years, time point 4 was 7-8 years, and time point 5 was 9-10 years.
The researchers found that the skin of infants during the first few weeks of life was similar at age 3 and 4 years. “From that second time point on, we see significant increases in richness and diversity, richness being presence or absence of various bacterial species, and diversity being the relative abundance of those species,” Dr. Capone said. “What you’re basically seeing is that there are new organisms, i.e., richness, coming into the microbiome. We start to detect new ones. Over time, the ecosystem is expanding. It’s evolving because it’s not yet set up.” The evolving skin microbiome on children was dominated by Streptococcus and Staphylococcus. In addition, children had higher levels of Streptococcus, Moraxella, Granulicatella, Gemella and Veillonella, compared with their adult mothers. Adult skin was colonized predominantly by Propionibacterium/Cutibacterium and Staphylococcus.
“The skin microbiome also increased in diversity over time on the forearm, but not face, of the mothers,” Dr. Capone said. “Previous studies have shown how stable the adult skin microbiome is, so it’s intriguing to see the changes that we saw on the mothers in this study.”
The increase in skin microbiome diversity observed in the children is likely due to a variety of factors, she continued, including inherent growth and development, dietary changes, as well as exposure to various environments and other people. “The fact remains that diversity is increasing over time, as the ecosystem evolves,” she said. “Eventually, the skin microbiome will become ‘adultlike’ in puberty, when lipid production increases. This drives increases in Cutibacterium acnes, particularly on the face.”
She acknowledged certain limitations of the study, including its relatively small size and the fact that some of the original subjects did not return for microbiome analysis at later time points. “We need larger cohort studies, additional deeper sequence data, metabolomics and transcriptomics to better understand the function of the skin microbiome over these various ages,” she said.
The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
, while the skin microbiome of the mothers of the children whose microbiome was analyzed remained relatively constant over the same time period.
The findings come from what is believed to be the longest longitudinal study specific to the skin microbiome of infants and mothers.
“Even at 10 years, the skin microbiome does not look like an adult skin microbiome, based on composition of the ecosystem,” lead author Kimberly A. Capone, PhD, said in an interview during the virtual annual meeting of the American Academy of Dermatology. “The diversity of the microbiome in children’s skin is distinct to that of an adult’s skin. We all have the same bacteria present, but in children it is distributed differently because the bacteria set themselves up based on the nutrients and topography that they find on the skin. Since infant skin is unique to infants, so too is their microbiome when we compare it to adults. It’s been fascinating to observe these children grow and mature, and follow the skin microbiome along this same period.”
During five time points over a period of 10 years, Dr. Capone and her colleagues at the Skillman, N.J.–based Johnson & Johnson Consumer Experience Center, a research and development site, used 16s rRNA gene sequencing to evaluate the skin microbiome on the forearms and foreheads of 30 mothers and their 31 children. The study participants had Fitzpatrick skin types I-IV and the mean age of mothers was 37 years. “We used 16s rRNA gene sequencing for microbiome analysis at the beginning, as that was what was available 10 years ago,” said Dr. Capone, head of the microbiome platform for Johnson & Johnson Consumer Health. “Since then, we continue to use 16s for continuity, but also collected additional swabs for deeper analysis later.”
She and her colleagues often draw samples from the forearm in baby skin clinical studies, “as the arm is a good site to collect relevant data on the body overall,” she explained. “We chose the forearm and forehead specifically here so we can make same body site comparisons to adult sample data which we took from the mothers on the same areas of their body.” Time point 1 was 3 months to 1 year, time point 2 was 2-3 years, time point 3 was 5-6 years, time point 4 was 7-8 years, and time point 5 was 9-10 years.
The researchers found that the skin of infants during the first few weeks of life was similar at age 3 and 4 years. “From that second time point on, we see significant increases in richness and diversity, richness being presence or absence of various bacterial species, and diversity being the relative abundance of those species,” Dr. Capone said. “What you’re basically seeing is that there are new organisms, i.e., richness, coming into the microbiome. We start to detect new ones. Over time, the ecosystem is expanding. It’s evolving because it’s not yet set up.” The evolving skin microbiome on children was dominated by Streptococcus and Staphylococcus. In addition, children had higher levels of Streptococcus, Moraxella, Granulicatella, Gemella and Veillonella, compared with their adult mothers. Adult skin was colonized predominantly by Propionibacterium/Cutibacterium and Staphylococcus.
“The skin microbiome also increased in diversity over time on the forearm, but not face, of the mothers,” Dr. Capone said. “Previous studies have shown how stable the adult skin microbiome is, so it’s intriguing to see the changes that we saw on the mothers in this study.”
The increase in skin microbiome diversity observed in the children is likely due to a variety of factors, she continued, including inherent growth and development, dietary changes, as well as exposure to various environments and other people. “The fact remains that diversity is increasing over time, as the ecosystem evolves,” she said. “Eventually, the skin microbiome will become ‘adultlike’ in puberty, when lipid production increases. This drives increases in Cutibacterium acnes, particularly on the face.”
She acknowledged certain limitations of the study, including its relatively small size and the fact that some of the original subjects did not return for microbiome analysis at later time points. “We need larger cohort studies, additional deeper sequence data, metabolomics and transcriptomics to better understand the function of the skin microbiome over these various ages,” she said.
The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
FROM AAD 20
Key clinical point: The skin’s microbial diversity changes with increasing age in children while remaining stable in adult mothers.
Major finding: The skin microbiome in children becomes more diverse between the ages of 3-4 to age 10.
Study details: A longitudinal analysis of 30 mothers and their 31 children.
Disclosures: The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
Source: Capone K et al. AAD 20, Abstract F053.
Perfect storm of SARS-CoV-2 during flu season
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at [email protected].
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at [email protected].
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at [email protected].
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
Pediatric Dermatology: A Supplement to Pediatric News & Dermatology News
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
New long-term data for antipsychotic in pediatric bipolar depression
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.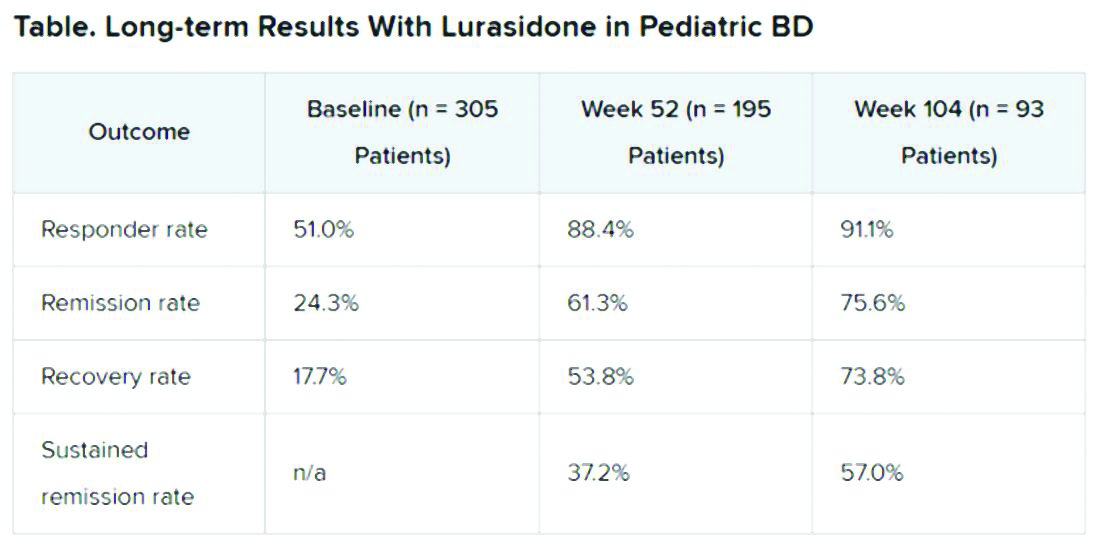
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
FROM ASCP 2020
Liposomal bupivacaine excreted in breast milk, but levels appear safe
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
FROM OBSTETRICS & GYNECOLOGY
EULAR’s COVID-19 recommendations offer no surprises
As might be expected, the “EULAR [European League Against Rheumatism] provisional recommendations for the management of rheumatic and musculoskeletal diseases [RMDs] in the context of SARS-CoV-2” concur with much of the guidance already released on how best to manage patients during the current pandemic.
Highlights of the five overarching principles are that, contrary to earlier expectations, “there is no indication that patients with RMDs have an additional, or have a higher, risk of contracting the virus, or that they fare a worse course” than the general population, said the task force convener Robert Landewé, MD, PhD, professor of rheumatology at the University of Amsterdam.
“The second pertinent highlight is that, when it comes to managerial discussions, whether or not to stop or to start treatment for RMDs, rheumatologists should definitely be involved,” Dr. Landewé said during a live session at the annual European Congress of Rheumatology, held online this year due to COVID-19. “In practice, something that happens very often is that immunosuppressive drugs are stopped by medical specialists involved in the care of COVID but without any expertise in treating patients with rheumatic diseases. We should try to avoid that situation.”
The third highlight, something many rheumatologists may already be well aware of, is that rheumatology drugs are being used to treat COVID-19 patients without RMDs and a shortage of disease-modifying antirheumatic drugs (DMARDs) agents is a real possibility. As such, the fifth overarching highlight states that the availability of both synthetic and biologic DMARDs is “a delicate societal responsibility” and that “the off-label use of DMARDs in COVID-19 outside the context of clinical trials should be discouraged.”
The EULAR recommendation are now published online in Annals of the Rheumatic Diseases and they are “what you could call an unprecedented set of recommendations,” Dr. Landewé said. “We have never done this before,” he added, referring to the speed and way in which they had to be put together, remotely, and with little scientific evidence currently available. “Three months ago we hadn’t even heard about the virus.”
From the first patient being identified in the Hubei province of China in November 2019, to the first U.S. patient in the state of Washington on Jan. 20, 2020, and to the first European patient identified a little over 10 days later, the COVID-19 pandemic has taken the world by storm. It was only declared a pandemic on March 11, 2020, however, and Dr. Landewé noted that the response to the pandemic had been very variable – some countries locking down their borders early, while others took their time to make an appropriate response, if at all.
The rheumatology community was particularly concerned, Dr. Landewé said, because people with autoimmune diseases who were taking immunosuppressant drugs might be at higher risk for becoming infected with SARS-CoV-2, and may be at higher risk than others for a worse disease course. Thankfully, that seems not to be the case according to data that are emerging from new registries that have been set up, including EULAR’s own COVID-19 registry.
There are 13 recommendations that cover 4 themes: general measures and prevention of SARS-CoV-2 infection; the management of RMD patients during the pandemic; the management of RMD patients who have COVID-19; and the prevention of other pulmonary infections in RMD patients.
Highlighting the first three general recommendations, Dr. Landewé said: “Follow the regular guidelines in your country; if a patient with RMD does not have symptoms of COVID-19, simply continue RMD treatments,” albeit with a couple of exceptions.
The next four recommendation highlights are to avoid visits to the hospital or to the office; use remote monitoring via the telephone, for example; and if visits cannot be avoided, then take appropriate precautions. Finally, if you suspect a patient has COVID-19, do a test.
If patients test positive, then the next four recommendations cover what to do, such as continuing use of RMD treatments, but in the case of glucocorticoids this should be the lowest possible dose necessary. There is no consensus on what to do in cases of mild symptoms; the recommendation is to “decide on a case-by-case basis,” said Dr. Landewé. If a patient’s symptoms worsen, then “seek expert advice immediately and follow local treatment recommendations. The rheumatologist is not the expert to treat COVID-19,” he added. That responsibility lies with the pulmonologist, infectious disease specialist, or maybe the intensive care specialist, depending on local situations.
On the whole, the EULAR recommendations are pretty similar to those already released by the American College of Rheumatology, said Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha. The ACR recommendations are “slightly more prescriptive”, he suggested, with 25 final guidance statements. For example, general statements focused not only on the use of glucocorticoids, but also other medicines, such as antihypertensives.
“There’s really not a [lot of], I would say, major differences in the two efforts and that’s ... somewhat reassuring that we’re approaching the unknown from very different parts of the world, and driving in a very similar place,” commented Dr. Mikuls, who is a member of the ACR COVID-19 recommendations task force.
“I think one of the very important similarities that I would highlight is that, in the absence of known exposure, in the absence of COVID-19 infection, our panel felt very strongly about the importance of continuing rheumatic disease treatments,” Dr. Mikuls observed. The ACR guidelines also touch upon societal perspectives, including “some statements that were made very specific to lupus, and the use of antimalarials, given supply chain issues that we have encountered.”
Dr. Mikuls also said that the American recommendations emphasized that “you really have to manage active inflammatory rheumatic disease. Even in the context of the COVID-19 pandemic, given what we saw as the potential risk of unchecked inflammation and unchecked rheumatic disease.”
One notable difference, however, is that the European recommendations advise on immunizations and pneumonia prophylaxis, saying that all patients without COVID-19 symptoms should make sure they are up to date with any recommended vaccinations, “with a particular focus on pneumococcal and influenza vaccinations,” Dr. Landewé said.
Another difference is that the ACR recommendations are a living document and could potentially be updated monthly if the evidence arrives to allow that. In that sense, the American guidance is more agile, with EULAR expecting to update its recommendations every 3 months.
“The current evidence is extremely sparse and fragmented,” Dr. Landewé said. “We, as a task force are essentially flying blindly. We also have to cover many jurisdictions within Europe, with many conflicting opinions. So the last word to say is that updates are truly necessary, but we have to wait a while.”
SOURCE: Landewé RB et al. Ann Rheum Dis. 2020 Jun 5. doi: 10.1136/annrheumdis-2020-217877.
As might be expected, the “EULAR [European League Against Rheumatism] provisional recommendations for the management of rheumatic and musculoskeletal diseases [RMDs] in the context of SARS-CoV-2” concur with much of the guidance already released on how best to manage patients during the current pandemic.
Highlights of the five overarching principles are that, contrary to earlier expectations, “there is no indication that patients with RMDs have an additional, or have a higher, risk of contracting the virus, or that they fare a worse course” than the general population, said the task force convener Robert Landewé, MD, PhD, professor of rheumatology at the University of Amsterdam.
“The second pertinent highlight is that, when it comes to managerial discussions, whether or not to stop or to start treatment for RMDs, rheumatologists should definitely be involved,” Dr. Landewé said during a live session at the annual European Congress of Rheumatology, held online this year due to COVID-19. “In practice, something that happens very often is that immunosuppressive drugs are stopped by medical specialists involved in the care of COVID but without any expertise in treating patients with rheumatic diseases. We should try to avoid that situation.”
The third highlight, something many rheumatologists may already be well aware of, is that rheumatology drugs are being used to treat COVID-19 patients without RMDs and a shortage of disease-modifying antirheumatic drugs (DMARDs) agents is a real possibility. As such, the fifth overarching highlight states that the availability of both synthetic and biologic DMARDs is “a delicate societal responsibility” and that “the off-label use of DMARDs in COVID-19 outside the context of clinical trials should be discouraged.”
The EULAR recommendation are now published online in Annals of the Rheumatic Diseases and they are “what you could call an unprecedented set of recommendations,” Dr. Landewé said. “We have never done this before,” he added, referring to the speed and way in which they had to be put together, remotely, and with little scientific evidence currently available. “Three months ago we hadn’t even heard about the virus.”
From the first patient being identified in the Hubei province of China in November 2019, to the first U.S. patient in the state of Washington on Jan. 20, 2020, and to the first European patient identified a little over 10 days later, the COVID-19 pandemic has taken the world by storm. It was only declared a pandemic on March 11, 2020, however, and Dr. Landewé noted that the response to the pandemic had been very variable – some countries locking down their borders early, while others took their time to make an appropriate response, if at all.
The rheumatology community was particularly concerned, Dr. Landewé said, because people with autoimmune diseases who were taking immunosuppressant drugs might be at higher risk for becoming infected with SARS-CoV-2, and may be at higher risk than others for a worse disease course. Thankfully, that seems not to be the case according to data that are emerging from new registries that have been set up, including EULAR’s own COVID-19 registry.
There are 13 recommendations that cover 4 themes: general measures and prevention of SARS-CoV-2 infection; the management of RMD patients during the pandemic; the management of RMD patients who have COVID-19; and the prevention of other pulmonary infections in RMD patients.
Highlighting the first three general recommendations, Dr. Landewé said: “Follow the regular guidelines in your country; if a patient with RMD does not have symptoms of COVID-19, simply continue RMD treatments,” albeit with a couple of exceptions.
The next four recommendation highlights are to avoid visits to the hospital or to the office; use remote monitoring via the telephone, for example; and if visits cannot be avoided, then take appropriate precautions. Finally, if you suspect a patient has COVID-19, do a test.
If patients test positive, then the next four recommendations cover what to do, such as continuing use of RMD treatments, but in the case of glucocorticoids this should be the lowest possible dose necessary. There is no consensus on what to do in cases of mild symptoms; the recommendation is to “decide on a case-by-case basis,” said Dr. Landewé. If a patient’s symptoms worsen, then “seek expert advice immediately and follow local treatment recommendations. The rheumatologist is not the expert to treat COVID-19,” he added. That responsibility lies with the pulmonologist, infectious disease specialist, or maybe the intensive care specialist, depending on local situations.
On the whole, the EULAR recommendations are pretty similar to those already released by the American College of Rheumatology, said Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha. The ACR recommendations are “slightly more prescriptive”, he suggested, with 25 final guidance statements. For example, general statements focused not only on the use of glucocorticoids, but also other medicines, such as antihypertensives.
“There’s really not a [lot of], I would say, major differences in the two efforts and that’s ... somewhat reassuring that we’re approaching the unknown from very different parts of the world, and driving in a very similar place,” commented Dr. Mikuls, who is a member of the ACR COVID-19 recommendations task force.
“I think one of the very important similarities that I would highlight is that, in the absence of known exposure, in the absence of COVID-19 infection, our panel felt very strongly about the importance of continuing rheumatic disease treatments,” Dr. Mikuls observed. The ACR guidelines also touch upon societal perspectives, including “some statements that were made very specific to lupus, and the use of antimalarials, given supply chain issues that we have encountered.”
Dr. Mikuls also said that the American recommendations emphasized that “you really have to manage active inflammatory rheumatic disease. Even in the context of the COVID-19 pandemic, given what we saw as the potential risk of unchecked inflammation and unchecked rheumatic disease.”
One notable difference, however, is that the European recommendations advise on immunizations and pneumonia prophylaxis, saying that all patients without COVID-19 symptoms should make sure they are up to date with any recommended vaccinations, “with a particular focus on pneumococcal and influenza vaccinations,” Dr. Landewé said.
Another difference is that the ACR recommendations are a living document and could potentially be updated monthly if the evidence arrives to allow that. In that sense, the American guidance is more agile, with EULAR expecting to update its recommendations every 3 months.
“The current evidence is extremely sparse and fragmented,” Dr. Landewé said. “We, as a task force are essentially flying blindly. We also have to cover many jurisdictions within Europe, with many conflicting opinions. So the last word to say is that updates are truly necessary, but we have to wait a while.”
SOURCE: Landewé RB et al. Ann Rheum Dis. 2020 Jun 5. doi: 10.1136/annrheumdis-2020-217877.
As might be expected, the “EULAR [European League Against Rheumatism] provisional recommendations for the management of rheumatic and musculoskeletal diseases [RMDs] in the context of SARS-CoV-2” concur with much of the guidance already released on how best to manage patients during the current pandemic.
Highlights of the five overarching principles are that, contrary to earlier expectations, “there is no indication that patients with RMDs have an additional, or have a higher, risk of contracting the virus, or that they fare a worse course” than the general population, said the task force convener Robert Landewé, MD, PhD, professor of rheumatology at the University of Amsterdam.
“The second pertinent highlight is that, when it comes to managerial discussions, whether or not to stop or to start treatment for RMDs, rheumatologists should definitely be involved,” Dr. Landewé said during a live session at the annual European Congress of Rheumatology, held online this year due to COVID-19. “In practice, something that happens very often is that immunosuppressive drugs are stopped by medical specialists involved in the care of COVID but without any expertise in treating patients with rheumatic diseases. We should try to avoid that situation.”
The third highlight, something many rheumatologists may already be well aware of, is that rheumatology drugs are being used to treat COVID-19 patients without RMDs and a shortage of disease-modifying antirheumatic drugs (DMARDs) agents is a real possibility. As such, the fifth overarching highlight states that the availability of both synthetic and biologic DMARDs is “a delicate societal responsibility” and that “the off-label use of DMARDs in COVID-19 outside the context of clinical trials should be discouraged.”
The EULAR recommendation are now published online in Annals of the Rheumatic Diseases and they are “what you could call an unprecedented set of recommendations,” Dr. Landewé said. “We have never done this before,” he added, referring to the speed and way in which they had to be put together, remotely, and with little scientific evidence currently available. “Three months ago we hadn’t even heard about the virus.”
From the first patient being identified in the Hubei province of China in November 2019, to the first U.S. patient in the state of Washington on Jan. 20, 2020, and to the first European patient identified a little over 10 days later, the COVID-19 pandemic has taken the world by storm. It was only declared a pandemic on March 11, 2020, however, and Dr. Landewé noted that the response to the pandemic had been very variable – some countries locking down their borders early, while others took their time to make an appropriate response, if at all.
The rheumatology community was particularly concerned, Dr. Landewé said, because people with autoimmune diseases who were taking immunosuppressant drugs might be at higher risk for becoming infected with SARS-CoV-2, and may be at higher risk than others for a worse disease course. Thankfully, that seems not to be the case according to data that are emerging from new registries that have been set up, including EULAR’s own COVID-19 registry.
There are 13 recommendations that cover 4 themes: general measures and prevention of SARS-CoV-2 infection; the management of RMD patients during the pandemic; the management of RMD patients who have COVID-19; and the prevention of other pulmonary infections in RMD patients.
Highlighting the first three general recommendations, Dr. Landewé said: “Follow the regular guidelines in your country; if a patient with RMD does not have symptoms of COVID-19, simply continue RMD treatments,” albeit with a couple of exceptions.
The next four recommendation highlights are to avoid visits to the hospital or to the office; use remote monitoring via the telephone, for example; and if visits cannot be avoided, then take appropriate precautions. Finally, if you suspect a patient has COVID-19, do a test.
If patients test positive, then the next four recommendations cover what to do, such as continuing use of RMD treatments, but in the case of glucocorticoids this should be the lowest possible dose necessary. There is no consensus on what to do in cases of mild symptoms; the recommendation is to “decide on a case-by-case basis,” said Dr. Landewé. If a patient’s symptoms worsen, then “seek expert advice immediately and follow local treatment recommendations. The rheumatologist is not the expert to treat COVID-19,” he added. That responsibility lies with the pulmonologist, infectious disease specialist, or maybe the intensive care specialist, depending on local situations.
On the whole, the EULAR recommendations are pretty similar to those already released by the American College of Rheumatology, said Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha. The ACR recommendations are “slightly more prescriptive”, he suggested, with 25 final guidance statements. For example, general statements focused not only on the use of glucocorticoids, but also other medicines, such as antihypertensives.
“There’s really not a [lot of], I would say, major differences in the two efforts and that’s ... somewhat reassuring that we’re approaching the unknown from very different parts of the world, and driving in a very similar place,” commented Dr. Mikuls, who is a member of the ACR COVID-19 recommendations task force.
“I think one of the very important similarities that I would highlight is that, in the absence of known exposure, in the absence of COVID-19 infection, our panel felt very strongly about the importance of continuing rheumatic disease treatments,” Dr. Mikuls observed. The ACR guidelines also touch upon societal perspectives, including “some statements that were made very specific to lupus, and the use of antimalarials, given supply chain issues that we have encountered.”
Dr. Mikuls also said that the American recommendations emphasized that “you really have to manage active inflammatory rheumatic disease. Even in the context of the COVID-19 pandemic, given what we saw as the potential risk of unchecked inflammation and unchecked rheumatic disease.”
One notable difference, however, is that the European recommendations advise on immunizations and pneumonia prophylaxis, saying that all patients without COVID-19 symptoms should make sure they are up to date with any recommended vaccinations, “with a particular focus on pneumococcal and influenza vaccinations,” Dr. Landewé said.
Another difference is that the ACR recommendations are a living document and could potentially be updated monthly if the evidence arrives to allow that. In that sense, the American guidance is more agile, with EULAR expecting to update its recommendations every 3 months.
“The current evidence is extremely sparse and fragmented,” Dr. Landewé said. “We, as a task force are essentially flying blindly. We also have to cover many jurisdictions within Europe, with many conflicting opinions. So the last word to say is that updates are truly necessary, but we have to wait a while.”
SOURCE: Landewé RB et al. Ann Rheum Dis. 2020 Jun 5. doi: 10.1136/annrheumdis-2020-217877.
FROM THE EULAR 2020 E-CONGRESS
Baloxavir effective, well tolerated for influenza treatment in children
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Does cultural tailoring of sexual health programs lead to safer behavior?
including abstinence, condom use, and number of sex partners, a meta-analysis of 12 studies suggests.
Furthermore, cultural tailoring may contribute to a program’s success, the data indicate.
“It is important that culturally tailored sexual health programs be available to Hispanic communities across the United States,” the study authors stated in Pediatrics.
To examine the effects of sexual health interventions on behavioral outcomes among Hispanic adolescents and factors that may influence the success of an intervention, Reina Evans, a doctoral student in the department of psychology at North Carolina State University in Raleigh, and colleagues systemically reviewed published studies that included Hispanic adolescents in the United States. Included studies evaluated a sexual health intervention using an experimental or quasiexperimental design and assessed a behavioral outcome.
The researchers synthesized effect sizes from 12 studies that included 4,673 Hispanic adolescents. “As the indicator of effect size, the standardized mean difference, Cohen’s d, was used,” they said. Effect size was interpreted as small at 0.20, medium at 0.50, and large at 0.80.
Sexual health interventions improved abstinence (d = 0.15), condom use (d = 0.44), number of sex partners (d = –0.19), and sexual health knowledge (d = 0.40), compared with control conditions.
Eight of the 12 interventions incorporated Hispanic-specific practices and values such as familialism into the intervention materials. Culturally tailored interventions produced greater change in condom use, compared with interventions that were not culturally tailored. One intervention with a large effect on condom use was developed by researchers in collaboration with community members, the authors said. Another program with a large effect on condom use was designed for Hispanic families.
Ten of the 12 studies included males and females, and two included only females. Intervention dose ranged from less than 10 hours of program content to more than 20 hours of content.
Definitions of abstinence and time frames for reporting recent condom use varied across studies, the researchers noted. Data about patient characteristics, such as the percentage of participants born in the United States, and pregnancy outcomes were limited. These domains could be areas of future research.
“Latinx adolescents are disproportionately burdened with unplanned pregnancy and STIs [sexually transmitted infections]. In this meta-analysis, it is shown that sexual health interventions can play a role in combating these health disparities,” Ms. Evans and associates said.
Among Hispanic adolescents, persistent disparities in sexual and reproductive health “remain a national public health priority,” and “strengthening the effects of future ... interventions for Hispanic adolescents is needed,” said Vincent Guilamo-Ramos, PhD, MPH, and colleagues in an accompanying editorial. Dr. Guilamo-Ramos is a professor of social work and director and founder of the Center for Latino Adolescent and Family Health at New York University.
“Evans et al. highlighted that reporting on the foreign-born participant proportions was incomplete across studies, thereby excluding this clinical heterogeneity domain from formal moderation analyses,” said Dr. Guilamo-Ramos and colleagues. People who develop Hispanic sexual and reproductive health interventions may consider whether this domain or other domains moderate intervention effectiveness.
Although sensitivity analyses focused on several potential sources of bias, “other domains of potential methodologic heterogeneity, such as refusal bias, differential attrition, or information bias, remained unaccounted for,” they said.
“Attention to clinical, methodologic, and statistical heterogeneity across studies can yield insights into factors associated with bolstering intervention effectiveness. Cultural tailoring to increase the effectiveness of condom interventions for Hispanic adolescents is one such intervention effect modifier,” Dr. Guilamo-Ramos and associates concluded.
The study authors had no relevant financial disclosures. The research was supported by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS and Sexually Transmitted Disease Prevention, Indiana University School of Public Health–Bloomington, and the Center for Family and Community Engagement, North Carolina State University. The editorialists are supported by the William T. Grant Foundation and the National Institutes of Health. In addition, Dr. Guilamo-Ramos has received grants and personal fees from ViiV Healthcare outside the submitted work and serves as a member of the U.S. Presidential Advisory Council on HIV/AIDS and as the vice chair of the board of directors of the Latino Commission on AIDS. His coauthors had no relevant financial disclosures.
SOURCES: Evans R et al. Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2019-3572; Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2020-1406.
including abstinence, condom use, and number of sex partners, a meta-analysis of 12 studies suggests.
Furthermore, cultural tailoring may contribute to a program’s success, the data indicate.
“It is important that culturally tailored sexual health programs be available to Hispanic communities across the United States,” the study authors stated in Pediatrics.
To examine the effects of sexual health interventions on behavioral outcomes among Hispanic adolescents and factors that may influence the success of an intervention, Reina Evans, a doctoral student in the department of psychology at North Carolina State University in Raleigh, and colleagues systemically reviewed published studies that included Hispanic adolescents in the United States. Included studies evaluated a sexual health intervention using an experimental or quasiexperimental design and assessed a behavioral outcome.
The researchers synthesized effect sizes from 12 studies that included 4,673 Hispanic adolescents. “As the indicator of effect size, the standardized mean difference, Cohen’s d, was used,” they said. Effect size was interpreted as small at 0.20, medium at 0.50, and large at 0.80.
Sexual health interventions improved abstinence (d = 0.15), condom use (d = 0.44), number of sex partners (d = –0.19), and sexual health knowledge (d = 0.40), compared with control conditions.
Eight of the 12 interventions incorporated Hispanic-specific practices and values such as familialism into the intervention materials. Culturally tailored interventions produced greater change in condom use, compared with interventions that were not culturally tailored. One intervention with a large effect on condom use was developed by researchers in collaboration with community members, the authors said. Another program with a large effect on condom use was designed for Hispanic families.
Ten of the 12 studies included males and females, and two included only females. Intervention dose ranged from less than 10 hours of program content to more than 20 hours of content.
Definitions of abstinence and time frames for reporting recent condom use varied across studies, the researchers noted. Data about patient characteristics, such as the percentage of participants born in the United States, and pregnancy outcomes were limited. These domains could be areas of future research.
“Latinx adolescents are disproportionately burdened with unplanned pregnancy and STIs [sexually transmitted infections]. In this meta-analysis, it is shown that sexual health interventions can play a role in combating these health disparities,” Ms. Evans and associates said.
Among Hispanic adolescents, persistent disparities in sexual and reproductive health “remain a national public health priority,” and “strengthening the effects of future ... interventions for Hispanic adolescents is needed,” said Vincent Guilamo-Ramos, PhD, MPH, and colleagues in an accompanying editorial. Dr. Guilamo-Ramos is a professor of social work and director and founder of the Center for Latino Adolescent and Family Health at New York University.
“Evans et al. highlighted that reporting on the foreign-born participant proportions was incomplete across studies, thereby excluding this clinical heterogeneity domain from formal moderation analyses,” said Dr. Guilamo-Ramos and colleagues. People who develop Hispanic sexual and reproductive health interventions may consider whether this domain or other domains moderate intervention effectiveness.
Although sensitivity analyses focused on several potential sources of bias, “other domains of potential methodologic heterogeneity, such as refusal bias, differential attrition, or information bias, remained unaccounted for,” they said.
“Attention to clinical, methodologic, and statistical heterogeneity across studies can yield insights into factors associated with bolstering intervention effectiveness. Cultural tailoring to increase the effectiveness of condom interventions for Hispanic adolescents is one such intervention effect modifier,” Dr. Guilamo-Ramos and associates concluded.
The study authors had no relevant financial disclosures. The research was supported by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS and Sexually Transmitted Disease Prevention, Indiana University School of Public Health–Bloomington, and the Center for Family and Community Engagement, North Carolina State University. The editorialists are supported by the William T. Grant Foundation and the National Institutes of Health. In addition, Dr. Guilamo-Ramos has received grants and personal fees from ViiV Healthcare outside the submitted work and serves as a member of the U.S. Presidential Advisory Council on HIV/AIDS and as the vice chair of the board of directors of the Latino Commission on AIDS. His coauthors had no relevant financial disclosures.
SOURCES: Evans R et al. Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2019-3572; Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2020-1406.
including abstinence, condom use, and number of sex partners, a meta-analysis of 12 studies suggests.
Furthermore, cultural tailoring may contribute to a program’s success, the data indicate.
“It is important that culturally tailored sexual health programs be available to Hispanic communities across the United States,” the study authors stated in Pediatrics.
To examine the effects of sexual health interventions on behavioral outcomes among Hispanic adolescents and factors that may influence the success of an intervention, Reina Evans, a doctoral student in the department of psychology at North Carolina State University in Raleigh, and colleagues systemically reviewed published studies that included Hispanic adolescents in the United States. Included studies evaluated a sexual health intervention using an experimental or quasiexperimental design and assessed a behavioral outcome.
The researchers synthesized effect sizes from 12 studies that included 4,673 Hispanic adolescents. “As the indicator of effect size, the standardized mean difference, Cohen’s d, was used,” they said. Effect size was interpreted as small at 0.20, medium at 0.50, and large at 0.80.
Sexual health interventions improved abstinence (d = 0.15), condom use (d = 0.44), number of sex partners (d = –0.19), and sexual health knowledge (d = 0.40), compared with control conditions.
Eight of the 12 interventions incorporated Hispanic-specific practices and values such as familialism into the intervention materials. Culturally tailored interventions produced greater change in condom use, compared with interventions that were not culturally tailored. One intervention with a large effect on condom use was developed by researchers in collaboration with community members, the authors said. Another program with a large effect on condom use was designed for Hispanic families.
Ten of the 12 studies included males and females, and two included only females. Intervention dose ranged from less than 10 hours of program content to more than 20 hours of content.
Definitions of abstinence and time frames for reporting recent condom use varied across studies, the researchers noted. Data about patient characteristics, such as the percentage of participants born in the United States, and pregnancy outcomes were limited. These domains could be areas of future research.
“Latinx adolescents are disproportionately burdened with unplanned pregnancy and STIs [sexually transmitted infections]. In this meta-analysis, it is shown that sexual health interventions can play a role in combating these health disparities,” Ms. Evans and associates said.
Among Hispanic adolescents, persistent disparities in sexual and reproductive health “remain a national public health priority,” and “strengthening the effects of future ... interventions for Hispanic adolescents is needed,” said Vincent Guilamo-Ramos, PhD, MPH, and colleagues in an accompanying editorial. Dr. Guilamo-Ramos is a professor of social work and director and founder of the Center for Latino Adolescent and Family Health at New York University.
“Evans et al. highlighted that reporting on the foreign-born participant proportions was incomplete across studies, thereby excluding this clinical heterogeneity domain from formal moderation analyses,” said Dr. Guilamo-Ramos and colleagues. People who develop Hispanic sexual and reproductive health interventions may consider whether this domain or other domains moderate intervention effectiveness.
Although sensitivity analyses focused on several potential sources of bias, “other domains of potential methodologic heterogeneity, such as refusal bias, differential attrition, or information bias, remained unaccounted for,” they said.
“Attention to clinical, methodologic, and statistical heterogeneity across studies can yield insights into factors associated with bolstering intervention effectiveness. Cultural tailoring to increase the effectiveness of condom interventions for Hispanic adolescents is one such intervention effect modifier,” Dr. Guilamo-Ramos and associates concluded.
The study authors had no relevant financial disclosures. The research was supported by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS and Sexually Transmitted Disease Prevention, Indiana University School of Public Health–Bloomington, and the Center for Family and Community Engagement, North Carolina State University. The editorialists are supported by the William T. Grant Foundation and the National Institutes of Health. In addition, Dr. Guilamo-Ramos has received grants and personal fees from ViiV Healthcare outside the submitted work and serves as a member of the U.S. Presidential Advisory Council on HIV/AIDS and as the vice chair of the board of directors of the Latino Commission on AIDS. His coauthors had no relevant financial disclosures.
SOURCES: Evans R et al. Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2019-3572; Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2020-1406.
FROM PEDIATRICS
Key clinical point: Among Hispanic adolescents, sexual health interventions have a small but significant effect on improving safe sexual behavior. Cultural tailoring may contribute to a program’s success.
Major finding: Sexual health interventions improved abstinence (d = 0.15), condom use (d = 0.44), number of sex partners (d = –0.19), and sexual health knowledge (d = 0.40), compared with control conditions.
Study details: A meta-analysis of 12 studies with 4,673 participants.
Disclosures: The study authors had no relevant financial disclosures. The research was supported by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS and Sexually Transmitted Disease Prevention, Indiana University School of Public Health–Bloomington, and the Center for Family and Community Engagement, North Carolina State University.
Source: Evans R et al. Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2019-3572.
Kids with food allergies the newest victims of COVID-19?
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
In-hospital formula feeding more than doubles odds of early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
FROM PEDIATRICS