User login
Chilblain-like lesions reported in children thought to have COVID-19
Two and elsewhere.
These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, from hospitals in Milan and Madrid, published in Pediatric Dermatology.
In the first study, Cristiana Colonna, MD, and colleagues at Hospital Maggiore Polyclinic in Milan described four cases of chilblain-like lesions in children ages 5-11 years with mild COVID-19 symptoms.
In the second, David Andina, MD, and colleagues in the ED and the departments of dermatology and pathology at the Child Jesus University Children’s Hospital in Madrid published a retrospective study of 22 cases in children and adolescents ages 6-17 years who reported to the hospital ED from April 6 to 17, the peak of the pandemic in Madrid.
In all four of the Milan cases, the skin lesions appeared several days after the onset of COVID-19 symptoms, although all four patients initially tested negative for COVID-19. However, Dr. Colonna and colleagues wrote that, “given the fact that the sensitivity and specificity of both nasopharyngeal swabs and antibody tests for COVID-19 (when available) are not 100% reliable, the question of the origin of these strange chilblain-like lesions is still elusive.” Until further studies are available, they emphasized that clinicians should be “alert to the presentation of chilblain-like findings” in children with mild symptoms “as a possible sign of COVID-19 infection.”
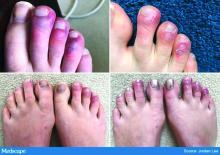
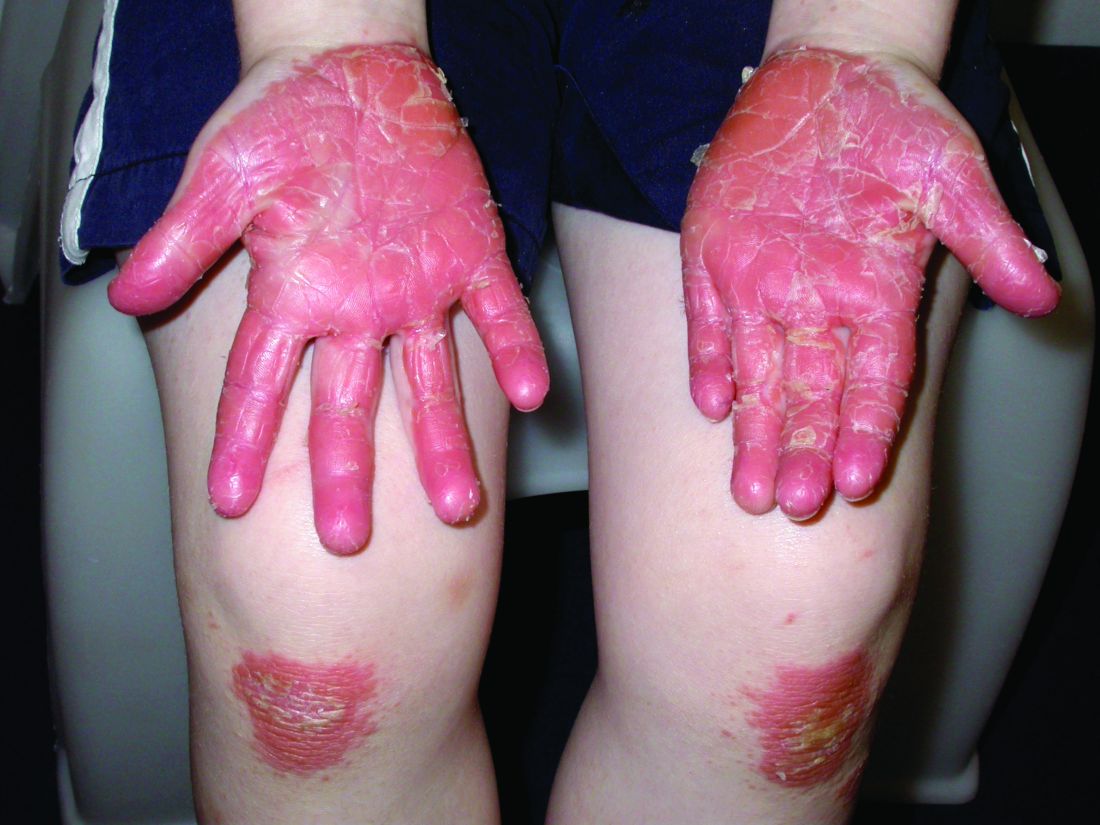
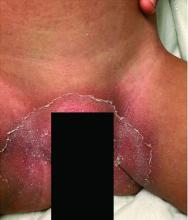
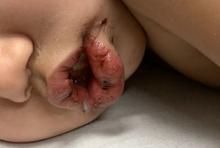
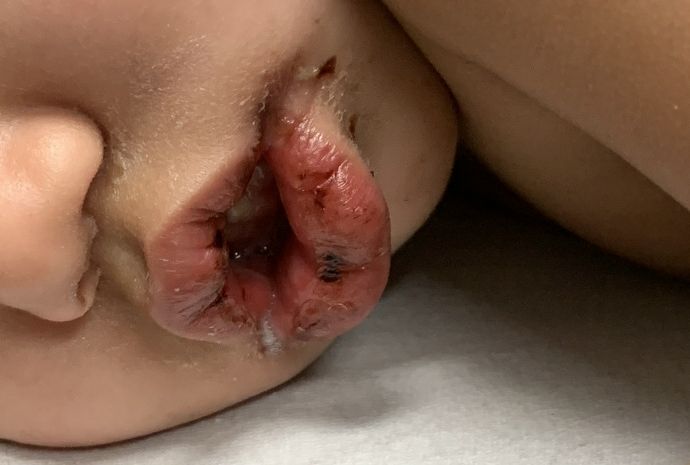
All the patients had lesions on their feet or toes, and a 5-year-old boy also had lesions on the right hand. One patient, an 11-year-old girl, had a biopsy that revealed dense lymphocytic perivascular cuffing and periadnexal infiltration.
“The finding of an elevated d-dimer in one of our patients, along with the clinical features suggestive of a vasoocclusive phenomenon, supports consideration of laboratory evaluation for coagulation defects in asymptomatic or mildly symptomatic children with acrovasculitis-like findings,” Dr. Colonna and colleagues wrote. None of the four cases in Milan required treatment, with three cases resolving within 5 days.
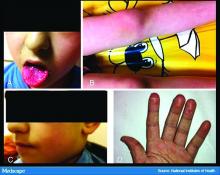
Like the Milan cases, all 22 patients in the Madrid series had foot or toe lesions and three had lesions on the fingers. This larger series also reported more detailed symptoms about the lesions: pruritus in nine patients (41%) and mild pain in seven (32%). A total of 10 patients had systemic symptoms of COVID-19, predominantly cough and rhinorrhea in 9 patients (41%), but 2 (9%) had abdominal pain and diarrhea. These symptoms, the authors said, appeared a median of 14 days (range, 1-28 days) before they developed chilblains.
A total of 19 patients were tested for COVID-19, but only 1 was positive.
This retrospective study also included contact information, with one patient having household contact with a single confirmed case of COVID-19; 12 patients recalled household contact who were considered probable cases of COVID-19, with respiratory symptoms.
Skin biopsies were obtained from the acral lesions in six patients, all showing similar results, although with varying degrees of intensity. All biopsies showed features of lymphocytic vasculopathy. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis described as “mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.”
The only treatments Dr. Andina and colleagues reported were oral analgesics for pain and oral antihistamines for pruritus when needed. One patient was given topical corticosteroids and another a short course of oral steroids, both for erythema multiforme.
Dr. Andina and colleagues wrote that the skin lesions in these patients “were unequivocally categorized as chilblains, both clinically and histopathologically,” and, after 7-10 days, began to fade. None of the patients had complications, and had an “excellent outcome,” they noted.
Dr. Colonna and colleagues had no conflicts of interest to declare. Dr. Andina and colleagues provided no disclosure statement.
SOURCES: Colonna C et al. Ped Derm. 2020 May 6. doi: 10.1111/pde.14210; Andina D et al. Ped Derm. 2020 May 9. doi: 10.1111/pde.14215.
Two and elsewhere.
These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, from hospitals in Milan and Madrid, published in Pediatric Dermatology.
In the first study, Cristiana Colonna, MD, and colleagues at Hospital Maggiore Polyclinic in Milan described four cases of chilblain-like lesions in children ages 5-11 years with mild COVID-19 symptoms.
In the second, David Andina, MD, and colleagues in the ED and the departments of dermatology and pathology at the Child Jesus University Children’s Hospital in Madrid published a retrospective study of 22 cases in children and adolescents ages 6-17 years who reported to the hospital ED from April 6 to 17, the peak of the pandemic in Madrid.
In all four of the Milan cases, the skin lesions appeared several days after the onset of COVID-19 symptoms, although all four patients initially tested negative for COVID-19. However, Dr. Colonna and colleagues wrote that, “given the fact that the sensitivity and specificity of both nasopharyngeal swabs and antibody tests for COVID-19 (when available) are not 100% reliable, the question of the origin of these strange chilblain-like lesions is still elusive.” Until further studies are available, they emphasized that clinicians should be “alert to the presentation of chilblain-like findings” in children with mild symptoms “as a possible sign of COVID-19 infection.”
All the patients had lesions on their feet or toes, and a 5-year-old boy also had lesions on the right hand. One patient, an 11-year-old girl, had a biopsy that revealed dense lymphocytic perivascular cuffing and periadnexal infiltration.
“The finding of an elevated d-dimer in one of our patients, along with the clinical features suggestive of a vasoocclusive phenomenon, supports consideration of laboratory evaluation for coagulation defects in asymptomatic or mildly symptomatic children with acrovasculitis-like findings,” Dr. Colonna and colleagues wrote. None of the four cases in Milan required treatment, with three cases resolving within 5 days.
Like the Milan cases, all 22 patients in the Madrid series had foot or toe lesions and three had lesions on the fingers. This larger series also reported more detailed symptoms about the lesions: pruritus in nine patients (41%) and mild pain in seven (32%). A total of 10 patients had systemic symptoms of COVID-19, predominantly cough and rhinorrhea in 9 patients (41%), but 2 (9%) had abdominal pain and diarrhea. These symptoms, the authors said, appeared a median of 14 days (range, 1-28 days) before they developed chilblains.
A total of 19 patients were tested for COVID-19, but only 1 was positive.
This retrospective study also included contact information, with one patient having household contact with a single confirmed case of COVID-19; 12 patients recalled household contact who were considered probable cases of COVID-19, with respiratory symptoms.
Skin biopsies were obtained from the acral lesions in six patients, all showing similar results, although with varying degrees of intensity. All biopsies showed features of lymphocytic vasculopathy. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis described as “mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.”
The only treatments Dr. Andina and colleagues reported were oral analgesics for pain and oral antihistamines for pruritus when needed. One patient was given topical corticosteroids and another a short course of oral steroids, both for erythema multiforme.
Dr. Andina and colleagues wrote that the skin lesions in these patients “were unequivocally categorized as chilblains, both clinically and histopathologically,” and, after 7-10 days, began to fade. None of the patients had complications, and had an “excellent outcome,” they noted.
Dr. Colonna and colleagues had no conflicts of interest to declare. Dr. Andina and colleagues provided no disclosure statement.
SOURCES: Colonna C et al. Ped Derm. 2020 May 6. doi: 10.1111/pde.14210; Andina D et al. Ped Derm. 2020 May 9. doi: 10.1111/pde.14215.
Two and elsewhere.
These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, from hospitals in Milan and Madrid, published in Pediatric Dermatology.
In the first study, Cristiana Colonna, MD, and colleagues at Hospital Maggiore Polyclinic in Milan described four cases of chilblain-like lesions in children ages 5-11 years with mild COVID-19 symptoms.
In the second, David Andina, MD, and colleagues in the ED and the departments of dermatology and pathology at the Child Jesus University Children’s Hospital in Madrid published a retrospective study of 22 cases in children and adolescents ages 6-17 years who reported to the hospital ED from April 6 to 17, the peak of the pandemic in Madrid.
In all four of the Milan cases, the skin lesions appeared several days after the onset of COVID-19 symptoms, although all four patients initially tested negative for COVID-19. However, Dr. Colonna and colleagues wrote that, “given the fact that the sensitivity and specificity of both nasopharyngeal swabs and antibody tests for COVID-19 (when available) are not 100% reliable, the question of the origin of these strange chilblain-like lesions is still elusive.” Until further studies are available, they emphasized that clinicians should be “alert to the presentation of chilblain-like findings” in children with mild symptoms “as a possible sign of COVID-19 infection.”
All the patients had lesions on their feet or toes, and a 5-year-old boy also had lesions on the right hand. One patient, an 11-year-old girl, had a biopsy that revealed dense lymphocytic perivascular cuffing and periadnexal infiltration.
“The finding of an elevated d-dimer in one of our patients, along with the clinical features suggestive of a vasoocclusive phenomenon, supports consideration of laboratory evaluation for coagulation defects in asymptomatic or mildly symptomatic children with acrovasculitis-like findings,” Dr. Colonna and colleagues wrote. None of the four cases in Milan required treatment, with three cases resolving within 5 days.
Like the Milan cases, all 22 patients in the Madrid series had foot or toe lesions and three had lesions on the fingers. This larger series also reported more detailed symptoms about the lesions: pruritus in nine patients (41%) and mild pain in seven (32%). A total of 10 patients had systemic symptoms of COVID-19, predominantly cough and rhinorrhea in 9 patients (41%), but 2 (9%) had abdominal pain and diarrhea. These symptoms, the authors said, appeared a median of 14 days (range, 1-28 days) before they developed chilblains.
A total of 19 patients were tested for COVID-19, but only 1 was positive.
This retrospective study also included contact information, with one patient having household contact with a single confirmed case of COVID-19; 12 patients recalled household contact who were considered probable cases of COVID-19, with respiratory symptoms.
Skin biopsies were obtained from the acral lesions in six patients, all showing similar results, although with varying degrees of intensity. All biopsies showed features of lymphocytic vasculopathy. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis described as “mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.”
The only treatments Dr. Andina and colleagues reported were oral analgesics for pain and oral antihistamines for pruritus when needed. One patient was given topical corticosteroids and another a short course of oral steroids, both for erythema multiforme.
Dr. Andina and colleagues wrote that the skin lesions in these patients “were unequivocally categorized as chilblains, both clinically and histopathologically,” and, after 7-10 days, began to fade. None of the patients had complications, and had an “excellent outcome,” they noted.
Dr. Colonna and colleagues had no conflicts of interest to declare. Dr. Andina and colleagues provided no disclosure statement.
SOURCES: Colonna C et al. Ped Derm. 2020 May 6. doi: 10.1111/pde.14210; Andina D et al. Ped Derm. 2020 May 9. doi: 10.1111/pde.14215.
FROM PEDIATRIC DERMATOLOGY
School intervention on mental illness stigma boosts treatment-seeking
A school curriculum–based intervention aimed at reducing the stigma of mental illness was associated with a nearly fourfold increase in the likelihood of youth with significant symptoms seeking treatment.
Writing in Pediatrics, researchers reported the outcome of a 2-year, longitudinal, cluster-randomized trial involving 416 students in sixth-grade classes in 14 schools across Texas.
The intervention was a school-based curriculum program called Eliminating the Stigma of Differences (ESD); a 3-hour, three-module curriculum program delivered over 1 week, which contained a mix of teaching, group discussion, and homework exercises.
One module explored the idea of difference; the definition, causes and consequences of stigma; ways to end stigma; and the description, causes and treatments of mental illness as well as the barriers to seeking help. The other two modules explored specific mental illnesses in more detail but with content designed to stimulate empathy.
The study compared this with two other interventions – in-class presentations and discussions led by two young adults with a history of mental illness; or exposure to anti-stigma printed materials – and a no-intervention control.
The study found that involvement with the curriculum program was associated with a significant and sustained increase in knowledge of and attitudes to mental illness compared with the control and other interventions, and with significant decreases in social distance, which measures the extent to which children are unwilling to interact with someone who is identified as having a mental illness. This association was seen even after the researchers controlled for other factors such as a participants’ knowledge of or attitudes toward mental illness before the intervention, their age, sex, race or ethnicity, or their parents’ educational level.
“Our study, in combination with other studies, suggests strongly that youth can be positively influenced at a relatively young age, fostering changes in mental health attitudes and behaviors that last, as our study has shown, for at least 2 years,” wrote Bruce G. Link, PhD, of the School of Public Policy at the University of California, Riverside, and coauthors.
The study also found that among youth who were experiencing a high level of symptoms of mental illness, the curriculum-based intervention was associated with nearly fourfold higher odds of seeking treatment (odd ratio 3.9, P < .05), after adjustment for similar covariates.
The authors looked separately at whether this self-reported treatment-seeking was the first time that students had sought treatment, a continuation of treatment seeking, or a return to it. All three showed similar odds ratios but small sample sizes meant they did not reach statistical significance.
“We do know that negative attitudes toward mental illnesses and the exceptionally large percentage of people who experience but do not receive treatment for such illnesses are problems that have been with us for a long time,” Dr. Link and associates said. “Interventions such as ESD represent a partial but positive response to this public mental health challenge.”
The intervention didn’t lead to a significant increase in treatment-seeking behavior among students with low levels of mental illness symptoms.
There were no significant differences in the effectiveness of the intervention across race or ethnicity, sex, education level of caregivers, or the baseline attitudes toward mental illness. The only exception was seen with Latino youth, where the intervention was not associated with a decrease in social distancing.
Contact intervention, in which two young people with a history of mental illness came to talk to classes and participate in discussions, was not associated with any significant changes in attitudes.
“A potential explanation is that contact is not as effective in youth, a possibility that is supported by a meta-analysis showing diminished effects of contact compared with educational interventions in adolescents,” Dr. Link and associates said.
In an accompanying editorial, Nathaniel Beers, MD, of Children’s National Hospital in Washington, and Dr. Shashank V. Joshi, MD, of Stanford (Calif.) University, wrote that more than one-fifth of children and youth in the United States are diagnosed with behavioral health needs before they reach the age of 18, but the perception of stigma can make families reluctant to access treatment.
“Previous research has highlighted the importance of stigma reduction in school-based settings as a crucial component in changing the social norms about seeking help among diverse youth populations,” they said. Reducing stigma also can reduce detrimental outcomes from social isolation and bullying.
Dr. Beers and Dr. Joshi noted that school-based interventions can have a substantial and lasting effect, with the benefit of influencing parents and staff in addition to students.
“Combined with screening and improved access to school-based mental health services, this curriculum could add a critical component to addressing the mental health needs of children and youth in the United States,” they concluded.
The study was supported by the National Institute of Mental Health and National Institutes of Health. The authors said they had no relevant financial disclosures. Dr. Beers and Dr. Joshi received no external funding, and they said they had no relevant financial disclosures.
SOURCES: Link B. et al. Pediatrics 2020, May 20. doi: 10.1542/peds.2019-0780; Beers N and Joshi SV. Pediatrics. 2020, May 20. doi: 10.1542/peds.2020-0127.
A school curriculum–based intervention aimed at reducing the stigma of mental illness was associated with a nearly fourfold increase in the likelihood of youth with significant symptoms seeking treatment.
Writing in Pediatrics, researchers reported the outcome of a 2-year, longitudinal, cluster-randomized trial involving 416 students in sixth-grade classes in 14 schools across Texas.
The intervention was a school-based curriculum program called Eliminating the Stigma of Differences (ESD); a 3-hour, three-module curriculum program delivered over 1 week, which contained a mix of teaching, group discussion, and homework exercises.
One module explored the idea of difference; the definition, causes and consequences of stigma; ways to end stigma; and the description, causes and treatments of mental illness as well as the barriers to seeking help. The other two modules explored specific mental illnesses in more detail but with content designed to stimulate empathy.
The study compared this with two other interventions – in-class presentations and discussions led by two young adults with a history of mental illness; or exposure to anti-stigma printed materials – and a no-intervention control.
The study found that involvement with the curriculum program was associated with a significant and sustained increase in knowledge of and attitudes to mental illness compared with the control and other interventions, and with significant decreases in social distance, which measures the extent to which children are unwilling to interact with someone who is identified as having a mental illness. This association was seen even after the researchers controlled for other factors such as a participants’ knowledge of or attitudes toward mental illness before the intervention, their age, sex, race or ethnicity, or their parents’ educational level.
“Our study, in combination with other studies, suggests strongly that youth can be positively influenced at a relatively young age, fostering changes in mental health attitudes and behaviors that last, as our study has shown, for at least 2 years,” wrote Bruce G. Link, PhD, of the School of Public Policy at the University of California, Riverside, and coauthors.
The study also found that among youth who were experiencing a high level of symptoms of mental illness, the curriculum-based intervention was associated with nearly fourfold higher odds of seeking treatment (odd ratio 3.9, P < .05), after adjustment for similar covariates.
The authors looked separately at whether this self-reported treatment-seeking was the first time that students had sought treatment, a continuation of treatment seeking, or a return to it. All three showed similar odds ratios but small sample sizes meant they did not reach statistical significance.
“We do know that negative attitudes toward mental illnesses and the exceptionally large percentage of people who experience but do not receive treatment for such illnesses are problems that have been with us for a long time,” Dr. Link and associates said. “Interventions such as ESD represent a partial but positive response to this public mental health challenge.”
The intervention didn’t lead to a significant increase in treatment-seeking behavior among students with low levels of mental illness symptoms.
There were no significant differences in the effectiveness of the intervention across race or ethnicity, sex, education level of caregivers, or the baseline attitudes toward mental illness. The only exception was seen with Latino youth, where the intervention was not associated with a decrease in social distancing.
Contact intervention, in which two young people with a history of mental illness came to talk to classes and participate in discussions, was not associated with any significant changes in attitudes.
“A potential explanation is that contact is not as effective in youth, a possibility that is supported by a meta-analysis showing diminished effects of contact compared with educational interventions in adolescents,” Dr. Link and associates said.
In an accompanying editorial, Nathaniel Beers, MD, of Children’s National Hospital in Washington, and Dr. Shashank V. Joshi, MD, of Stanford (Calif.) University, wrote that more than one-fifth of children and youth in the United States are diagnosed with behavioral health needs before they reach the age of 18, but the perception of stigma can make families reluctant to access treatment.
“Previous research has highlighted the importance of stigma reduction in school-based settings as a crucial component in changing the social norms about seeking help among diverse youth populations,” they said. Reducing stigma also can reduce detrimental outcomes from social isolation and bullying.
Dr. Beers and Dr. Joshi noted that school-based interventions can have a substantial and lasting effect, with the benefit of influencing parents and staff in addition to students.
“Combined with screening and improved access to school-based mental health services, this curriculum could add a critical component to addressing the mental health needs of children and youth in the United States,” they concluded.
The study was supported by the National Institute of Mental Health and National Institutes of Health. The authors said they had no relevant financial disclosures. Dr. Beers and Dr. Joshi received no external funding, and they said they had no relevant financial disclosures.
SOURCES: Link B. et al. Pediatrics 2020, May 20. doi: 10.1542/peds.2019-0780; Beers N and Joshi SV. Pediatrics. 2020, May 20. doi: 10.1542/peds.2020-0127.
A school curriculum–based intervention aimed at reducing the stigma of mental illness was associated with a nearly fourfold increase in the likelihood of youth with significant symptoms seeking treatment.
Writing in Pediatrics, researchers reported the outcome of a 2-year, longitudinal, cluster-randomized trial involving 416 students in sixth-grade classes in 14 schools across Texas.
The intervention was a school-based curriculum program called Eliminating the Stigma of Differences (ESD); a 3-hour, three-module curriculum program delivered over 1 week, which contained a mix of teaching, group discussion, and homework exercises.
One module explored the idea of difference; the definition, causes and consequences of stigma; ways to end stigma; and the description, causes and treatments of mental illness as well as the barriers to seeking help. The other two modules explored specific mental illnesses in more detail but with content designed to stimulate empathy.
The study compared this with two other interventions – in-class presentations and discussions led by two young adults with a history of mental illness; or exposure to anti-stigma printed materials – and a no-intervention control.
The study found that involvement with the curriculum program was associated with a significant and sustained increase in knowledge of and attitudes to mental illness compared with the control and other interventions, and with significant decreases in social distance, which measures the extent to which children are unwilling to interact with someone who is identified as having a mental illness. This association was seen even after the researchers controlled for other factors such as a participants’ knowledge of or attitudes toward mental illness before the intervention, their age, sex, race or ethnicity, or their parents’ educational level.
“Our study, in combination with other studies, suggests strongly that youth can be positively influenced at a relatively young age, fostering changes in mental health attitudes and behaviors that last, as our study has shown, for at least 2 years,” wrote Bruce G. Link, PhD, of the School of Public Policy at the University of California, Riverside, and coauthors.
The study also found that among youth who were experiencing a high level of symptoms of mental illness, the curriculum-based intervention was associated with nearly fourfold higher odds of seeking treatment (odd ratio 3.9, P < .05), after adjustment for similar covariates.
The authors looked separately at whether this self-reported treatment-seeking was the first time that students had sought treatment, a continuation of treatment seeking, or a return to it. All three showed similar odds ratios but small sample sizes meant they did not reach statistical significance.
“We do know that negative attitudes toward mental illnesses and the exceptionally large percentage of people who experience but do not receive treatment for such illnesses are problems that have been with us for a long time,” Dr. Link and associates said. “Interventions such as ESD represent a partial but positive response to this public mental health challenge.”
The intervention didn’t lead to a significant increase in treatment-seeking behavior among students with low levels of mental illness symptoms.
There were no significant differences in the effectiveness of the intervention across race or ethnicity, sex, education level of caregivers, or the baseline attitudes toward mental illness. The only exception was seen with Latino youth, where the intervention was not associated with a decrease in social distancing.
Contact intervention, in which two young people with a history of mental illness came to talk to classes and participate in discussions, was not associated with any significant changes in attitudes.
“A potential explanation is that contact is not as effective in youth, a possibility that is supported by a meta-analysis showing diminished effects of contact compared with educational interventions in adolescents,” Dr. Link and associates said.
In an accompanying editorial, Nathaniel Beers, MD, of Children’s National Hospital in Washington, and Dr. Shashank V. Joshi, MD, of Stanford (Calif.) University, wrote that more than one-fifth of children and youth in the United States are diagnosed with behavioral health needs before they reach the age of 18, but the perception of stigma can make families reluctant to access treatment.
“Previous research has highlighted the importance of stigma reduction in school-based settings as a crucial component in changing the social norms about seeking help among diverse youth populations,” they said. Reducing stigma also can reduce detrimental outcomes from social isolation and bullying.
Dr. Beers and Dr. Joshi noted that school-based interventions can have a substantial and lasting effect, with the benefit of influencing parents and staff in addition to students.
“Combined with screening and improved access to school-based mental health services, this curriculum could add a critical component to addressing the mental health needs of children and youth in the United States,” they concluded.
The study was supported by the National Institute of Mental Health and National Institutes of Health. The authors said they had no relevant financial disclosures. Dr. Beers and Dr. Joshi received no external funding, and they said they had no relevant financial disclosures.
SOURCES: Link B. et al. Pediatrics 2020, May 20. doi: 10.1542/peds.2019-0780; Beers N and Joshi SV. Pediatrics. 2020, May 20. doi: 10.1542/peds.2020-0127.
FROM PEDIATRICS
Key clinical point: A curriculum-based intervention addressing stigma and mental illness had a significant impact on attitudes and treatment-seeking.
Major finding:
Study details: A longitudinal, cluster-randomized trial involving 416 students in sixth-grade classes across 14 schools.
Disclosures: The study was supported by the National Institute of Mental Health and National Institutes of Health. The authors said they had no relevant financial disclosures.
Source: Link B. et al. Pediatrics 2020 May 20. doi: 10.1542/peds.2019-0780.
Radiation-associated childhood cancer quantified in congenital heart disease
Children with congenital heart disease exposed to low-dose ionizing radiation from cardiac procedures had a cancer risk more than triple that of pediatric congenital heart disease (CHD) patients without such exposures, according to a large Canadian nested case-control study presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This cancer risk was dose dependent. It rose stepwise with the number of cardiac procedures involving exposure to low-dose ionizing radiation (LDIR) and the total radiation dose. Moreover, roughly 80% of the cancers were of types known to be associated with radiation exposure in children, reported Elie Ganni, a medical student at McGill University, Montreal, working with MAUDE, the McGill Adult Unit for Congenital Heart Disease.
The MAUDE group previously published the first large, population-based study analyzing the association between LDIR from cardiac procedures and incident cancer in adults with CHD. The study, which included nearly 25,000 adult CHD patients aged 18-64 years with more than 250,000 person-years of follow-up, concluded that individuals with LDIR exposure from six or more cardiac procedures had a 140% greater cancer incidence than those with no or one exposure (Circulation. 2018 Mar 27;137[13]:1334-45).
Because children are considered to be more sensitive to the carcinogenic effects of LDIR than adults, the MAUDE group next did a similar study in a pediatric CHD population included in the Quebec Congenital Heart Disease Database. This nested case-control study included 232 children with CHD who were first diagnosed with cancer at a median age of 3.9 years and 8,160 pediatric CHD controls matched for gender and birth year. About 76% of cancers were diagnosed before age 7, 20% at ages 7-12 years, and the remaining 4% at ages 13-18. Hematologic malignancies accounted for 61% of the pediatric cancers, CNS cancers for another 12.5%, and thyroid cancers 6.6%; all three types of cancer are associated with radiation exposure.
After excluding all cardiac procedures involving LDIR performed within 6 months prior to cancer diagnosis, the risk of developing a pediatric cancer was 230% greater in children with LDIR exposure from cardiac procedures than in CHD patients without such exposure. For every 4 mSv in estimated LDIR exposure from cardiac procedures, the risk of cancer rose by 15.5%. In contrast, in the earlier study in adults with CHD, cancer risk climbed by 10% per 10 mSv. Patients with six or more LDIR cardiac procedures – not at all unusual in contemporary practice – were 2.4 times more likely to have cancer than those with no or one such radiation exposure.
Current ACC guidelines on radiation exposure from cardiac procedures recommend calculating an individual’s lifetime attributable cancer incidence and mortality risks, as well as adhering to the time-honored principle of ensuring that radiation exposure is as low as reasonably achievable without sacrificing quality of care.
“Our findings strongly support these ACC recommendations and moreover suggest that radiation surveillance for patients with congenital heart disease should be considered using radiation badges. Also, cancer surveillance guidelines should be considered for CHD patients exposed to LDIR,” Mr. Ganni said.
These suggestions for creation of patient radiation passports and cancer surveillance guidelines take on greater weight in light of two trends: the increasing life expectancy of children with CHD during the past 3 decades as a result of procedural advances that entail LDIR exposure, mostly for imaging, and the growing number of such procedures performed per patient earlier and earlier in life.
He and the MAUDE group plan to confirm their latest findings in other, larger data sets and hope to identify threshold effects for LDIR for specific cancers, with hematologic malignancies as the top priority.
Mr. Ganni reported having no financial conflicts regarding his study, funded by the Heart and Stroke Foundation of Canada, the Quebec Foundation for Health Research, and the Canadian Institutes for Health Research.
Children with congenital heart disease exposed to low-dose ionizing radiation from cardiac procedures had a cancer risk more than triple that of pediatric congenital heart disease (CHD) patients without such exposures, according to a large Canadian nested case-control study presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This cancer risk was dose dependent. It rose stepwise with the number of cardiac procedures involving exposure to low-dose ionizing radiation (LDIR) and the total radiation dose. Moreover, roughly 80% of the cancers were of types known to be associated with radiation exposure in children, reported Elie Ganni, a medical student at McGill University, Montreal, working with MAUDE, the McGill Adult Unit for Congenital Heart Disease.
The MAUDE group previously published the first large, population-based study analyzing the association between LDIR from cardiac procedures and incident cancer in adults with CHD. The study, which included nearly 25,000 adult CHD patients aged 18-64 years with more than 250,000 person-years of follow-up, concluded that individuals with LDIR exposure from six or more cardiac procedures had a 140% greater cancer incidence than those with no or one exposure (Circulation. 2018 Mar 27;137[13]:1334-45).
Because children are considered to be more sensitive to the carcinogenic effects of LDIR than adults, the MAUDE group next did a similar study in a pediatric CHD population included in the Quebec Congenital Heart Disease Database. This nested case-control study included 232 children with CHD who were first diagnosed with cancer at a median age of 3.9 years and 8,160 pediatric CHD controls matched for gender and birth year. About 76% of cancers were diagnosed before age 7, 20% at ages 7-12 years, and the remaining 4% at ages 13-18. Hematologic malignancies accounted for 61% of the pediatric cancers, CNS cancers for another 12.5%, and thyroid cancers 6.6%; all three types of cancer are associated with radiation exposure.
After excluding all cardiac procedures involving LDIR performed within 6 months prior to cancer diagnosis, the risk of developing a pediatric cancer was 230% greater in children with LDIR exposure from cardiac procedures than in CHD patients without such exposure. For every 4 mSv in estimated LDIR exposure from cardiac procedures, the risk of cancer rose by 15.5%. In contrast, in the earlier study in adults with CHD, cancer risk climbed by 10% per 10 mSv. Patients with six or more LDIR cardiac procedures – not at all unusual in contemporary practice – were 2.4 times more likely to have cancer than those with no or one such radiation exposure.
Current ACC guidelines on radiation exposure from cardiac procedures recommend calculating an individual’s lifetime attributable cancer incidence and mortality risks, as well as adhering to the time-honored principle of ensuring that radiation exposure is as low as reasonably achievable without sacrificing quality of care.
“Our findings strongly support these ACC recommendations and moreover suggest that radiation surveillance for patients with congenital heart disease should be considered using radiation badges. Also, cancer surveillance guidelines should be considered for CHD patients exposed to LDIR,” Mr. Ganni said.
These suggestions for creation of patient radiation passports and cancer surveillance guidelines take on greater weight in light of two trends: the increasing life expectancy of children with CHD during the past 3 decades as a result of procedural advances that entail LDIR exposure, mostly for imaging, and the growing number of such procedures performed per patient earlier and earlier in life.
He and the MAUDE group plan to confirm their latest findings in other, larger data sets and hope to identify threshold effects for LDIR for specific cancers, with hematologic malignancies as the top priority.
Mr. Ganni reported having no financial conflicts regarding his study, funded by the Heart and Stroke Foundation of Canada, the Quebec Foundation for Health Research, and the Canadian Institutes for Health Research.
Children with congenital heart disease exposed to low-dose ionizing radiation from cardiac procedures had a cancer risk more than triple that of pediatric congenital heart disease (CHD) patients without such exposures, according to a large Canadian nested case-control study presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This cancer risk was dose dependent. It rose stepwise with the number of cardiac procedures involving exposure to low-dose ionizing radiation (LDIR) and the total radiation dose. Moreover, roughly 80% of the cancers were of types known to be associated with radiation exposure in children, reported Elie Ganni, a medical student at McGill University, Montreal, working with MAUDE, the McGill Adult Unit for Congenital Heart Disease.
The MAUDE group previously published the first large, population-based study analyzing the association between LDIR from cardiac procedures and incident cancer in adults with CHD. The study, which included nearly 25,000 adult CHD patients aged 18-64 years with more than 250,000 person-years of follow-up, concluded that individuals with LDIR exposure from six or more cardiac procedures had a 140% greater cancer incidence than those with no or one exposure (Circulation. 2018 Mar 27;137[13]:1334-45).
Because children are considered to be more sensitive to the carcinogenic effects of LDIR than adults, the MAUDE group next did a similar study in a pediatric CHD population included in the Quebec Congenital Heart Disease Database. This nested case-control study included 232 children with CHD who were first diagnosed with cancer at a median age of 3.9 years and 8,160 pediatric CHD controls matched for gender and birth year. About 76% of cancers were diagnosed before age 7, 20% at ages 7-12 years, and the remaining 4% at ages 13-18. Hematologic malignancies accounted for 61% of the pediatric cancers, CNS cancers for another 12.5%, and thyroid cancers 6.6%; all three types of cancer are associated with radiation exposure.
After excluding all cardiac procedures involving LDIR performed within 6 months prior to cancer diagnosis, the risk of developing a pediatric cancer was 230% greater in children with LDIR exposure from cardiac procedures than in CHD patients without such exposure. For every 4 mSv in estimated LDIR exposure from cardiac procedures, the risk of cancer rose by 15.5%. In contrast, in the earlier study in adults with CHD, cancer risk climbed by 10% per 10 mSv. Patients with six or more LDIR cardiac procedures – not at all unusual in contemporary practice – were 2.4 times more likely to have cancer than those with no or one such radiation exposure.
Current ACC guidelines on radiation exposure from cardiac procedures recommend calculating an individual’s lifetime attributable cancer incidence and mortality risks, as well as adhering to the time-honored principle of ensuring that radiation exposure is as low as reasonably achievable without sacrificing quality of care.
“Our findings strongly support these ACC recommendations and moreover suggest that radiation surveillance for patients with congenital heart disease should be considered using radiation badges. Also, cancer surveillance guidelines should be considered for CHD patients exposed to LDIR,” Mr. Ganni said.
These suggestions for creation of patient radiation passports and cancer surveillance guidelines take on greater weight in light of two trends: the increasing life expectancy of children with CHD during the past 3 decades as a result of procedural advances that entail LDIR exposure, mostly for imaging, and the growing number of such procedures performed per patient earlier and earlier in life.
He and the MAUDE group plan to confirm their latest findings in other, larger data sets and hope to identify threshold effects for LDIR for specific cancers, with hematologic malignancies as the top priority.
Mr. Ganni reported having no financial conflicts regarding his study, funded by the Heart and Stroke Foundation of Canada, the Quebec Foundation for Health Research, and the Canadian Institutes for Health Research.
FROM ACC 2020
Vitamin D intake among U.S. infants has not improved, despite guidance
with breastfed infants less likely to have adequate levels than formula-fed infants, according to results of a study.
The American Association of Pediatrics has recommended since 2008 that breastfeeding babies under 1 year of age receive 400 IU of vitamin D supplementation daily, usually in the form of drops, to prevent rickets. For formula-fed infants, the AAP recommends that infants be fed one liter of formula daily, as formulas must contain 400 IU of vitamin D per liter.
A study looking at caregiver-reported dietary data through 2012 suggested that the guideline was having little impact, with only 27% of U.S. infants considered to be getting adequate vitamin D. The same researchers have now updated those findings with data through 2016 to report virtually no improvement over time. For their research, published in Pediatrics, Alan E. Simon, MD, of the National Institutes of Health in Rockville, Md., and Katherine A. Ahrens, PhD, of the University of Southern Maine in Portland, analyzed data for 1,435 infants aged 0-11 months. All data were recorded during 2009-2016 as part of the ongoing National Health and Nutrition Examination Survey (NHANES).
Overall, 27% of infants in the study were considered likely to meet the guidelines. Among nonbreastfeeding infants, 31% were deemed to have adequate levels, compared with 21% of breastfeeding infants (P less than .01).
Parents’ income and education affected infants’ likelihood of meeting guidelines. Breastfeeding infants in families with incomes above 400% of the federal poverty level were twice as likely to meet guidelines (31% vs. 14%-16% for lower income brackets, P less than .05). Babies from families whose head of household had a college degree had a 26% likelihood of having enough vitamin D, compared with less than 11% of those in whose parents had less than a high school education (P less than .05). Babies from families with private insurance also had a better chance of meeting guidelines, compared with those with public insurance (24% vs. 13%; P less than .05).
Ethnicity was seen as affecting vitamin D intake only insofar as some groups had more formula use than breastfeeding. The only ethnic or racial subgroup in the study that saw more than 40% of infants likely to meet guidelines was nonbreastfeeding infants of Asian, American Indian, Native Hawaiian or Pacific Islander, or multiracial parentage, with 46% considered to have adequate vitamin D levels. This group makes up 6% of the infant population in the United States.
“Reasons for low rates of meeting guidelines in the United States and little improvement over time are not fully known,” Dr. Simon and Dr. Ahrens wrote in their analysis. “One factor may be that the impact of low vitamin D in infancy is not highly visible to physicians because rickets is an uncommon diagnosis in the United States.” They noted that recent studies from Canada, where public health officials have done more to promote supplementation, have shown rates of adequate vitamin D in breastfeeding babies to be as high as 90%.
The researchers listed among limitations of their study the fact that the data source, NHANES, captured nutrition information only for the previous 24 hours; that it relied on parental report, and did not confirm serum levels of vitamin D; and that it was possible that cow’s milk – which is not recommended before age 1 but frequently given to older infants anyway – could be a hidden source of vitamin D that was not taken into consideration.
In an editorial comment, Jaspreet Loyal, MD, and Annette Cameron, MD, of Yale University in New Haven, Conn., faulted “a combination of inconsistent prescribing by clinicians and poor adherence to the use of a supplement by parents of infants … further complicated by a lack of awareness of the consequences of vitamin D deficiency in infants among the public” for the low adherence to guidelines in the United States, compared with other countries.
Also, the editorialists noted, the dropper used to administer liquid supplements has been associated with “inconsistent precision” and concerns about infants gagging on the liquid. More research is needed to better understand “prescribing patterns, barriers to adherence by parents of infants, and alternate strategies for vitamin D supplementation to inform novel public health programs in the United States,” they wrote.
The National Institutes of Health funded the study, and Dr. Ahrens is supported by a faculty development grant from the Maine Economic Improvement Fund. The researchers declared no conflicts of interest. Dr. Loyal and Dr. Cameron disclosed no funding and no relevant financial disclosures.
SOURCE: Simon AE and Ahrens KA. Pediatrics 2020 May. doi: 10.1542/peds.2019-3574; Loyal J and Cameron A. Pediatrics. 2020 May. doi: 10.1542/peds.2020-0504.
with breastfed infants less likely to have adequate levels than formula-fed infants, according to results of a study.
The American Association of Pediatrics has recommended since 2008 that breastfeeding babies under 1 year of age receive 400 IU of vitamin D supplementation daily, usually in the form of drops, to prevent rickets. For formula-fed infants, the AAP recommends that infants be fed one liter of formula daily, as formulas must contain 400 IU of vitamin D per liter.
A study looking at caregiver-reported dietary data through 2012 suggested that the guideline was having little impact, with only 27% of U.S. infants considered to be getting adequate vitamin D. The same researchers have now updated those findings with data through 2016 to report virtually no improvement over time. For their research, published in Pediatrics, Alan E. Simon, MD, of the National Institutes of Health in Rockville, Md., and Katherine A. Ahrens, PhD, of the University of Southern Maine in Portland, analyzed data for 1,435 infants aged 0-11 months. All data were recorded during 2009-2016 as part of the ongoing National Health and Nutrition Examination Survey (NHANES).
Overall, 27% of infants in the study were considered likely to meet the guidelines. Among nonbreastfeeding infants, 31% were deemed to have adequate levels, compared with 21% of breastfeeding infants (P less than .01).
Parents’ income and education affected infants’ likelihood of meeting guidelines. Breastfeeding infants in families with incomes above 400% of the federal poverty level were twice as likely to meet guidelines (31% vs. 14%-16% for lower income brackets, P less than .05). Babies from families whose head of household had a college degree had a 26% likelihood of having enough vitamin D, compared with less than 11% of those in whose parents had less than a high school education (P less than .05). Babies from families with private insurance also had a better chance of meeting guidelines, compared with those with public insurance (24% vs. 13%; P less than .05).
Ethnicity was seen as affecting vitamin D intake only insofar as some groups had more formula use than breastfeeding. The only ethnic or racial subgroup in the study that saw more than 40% of infants likely to meet guidelines was nonbreastfeeding infants of Asian, American Indian, Native Hawaiian or Pacific Islander, or multiracial parentage, with 46% considered to have adequate vitamin D levels. This group makes up 6% of the infant population in the United States.
“Reasons for low rates of meeting guidelines in the United States and little improvement over time are not fully known,” Dr. Simon and Dr. Ahrens wrote in their analysis. “One factor may be that the impact of low vitamin D in infancy is not highly visible to physicians because rickets is an uncommon diagnosis in the United States.” They noted that recent studies from Canada, where public health officials have done more to promote supplementation, have shown rates of adequate vitamin D in breastfeeding babies to be as high as 90%.
The researchers listed among limitations of their study the fact that the data source, NHANES, captured nutrition information only for the previous 24 hours; that it relied on parental report, and did not confirm serum levels of vitamin D; and that it was possible that cow’s milk – which is not recommended before age 1 but frequently given to older infants anyway – could be a hidden source of vitamin D that was not taken into consideration.
In an editorial comment, Jaspreet Loyal, MD, and Annette Cameron, MD, of Yale University in New Haven, Conn., faulted “a combination of inconsistent prescribing by clinicians and poor adherence to the use of a supplement by parents of infants … further complicated by a lack of awareness of the consequences of vitamin D deficiency in infants among the public” for the low adherence to guidelines in the United States, compared with other countries.
Also, the editorialists noted, the dropper used to administer liquid supplements has been associated with “inconsistent precision” and concerns about infants gagging on the liquid. More research is needed to better understand “prescribing patterns, barriers to adherence by parents of infants, and alternate strategies for vitamin D supplementation to inform novel public health programs in the United States,” they wrote.
The National Institutes of Health funded the study, and Dr. Ahrens is supported by a faculty development grant from the Maine Economic Improvement Fund. The researchers declared no conflicts of interest. Dr. Loyal and Dr. Cameron disclosed no funding and no relevant financial disclosures.
SOURCE: Simon AE and Ahrens KA. Pediatrics 2020 May. doi: 10.1542/peds.2019-3574; Loyal J and Cameron A. Pediatrics. 2020 May. doi: 10.1542/peds.2020-0504.
with breastfed infants less likely to have adequate levels than formula-fed infants, according to results of a study.
The American Association of Pediatrics has recommended since 2008 that breastfeeding babies under 1 year of age receive 400 IU of vitamin D supplementation daily, usually in the form of drops, to prevent rickets. For formula-fed infants, the AAP recommends that infants be fed one liter of formula daily, as formulas must contain 400 IU of vitamin D per liter.
A study looking at caregiver-reported dietary data through 2012 suggested that the guideline was having little impact, with only 27% of U.S. infants considered to be getting adequate vitamin D. The same researchers have now updated those findings with data through 2016 to report virtually no improvement over time. For their research, published in Pediatrics, Alan E. Simon, MD, of the National Institutes of Health in Rockville, Md., and Katherine A. Ahrens, PhD, of the University of Southern Maine in Portland, analyzed data for 1,435 infants aged 0-11 months. All data were recorded during 2009-2016 as part of the ongoing National Health and Nutrition Examination Survey (NHANES).
Overall, 27% of infants in the study were considered likely to meet the guidelines. Among nonbreastfeeding infants, 31% were deemed to have adequate levels, compared with 21% of breastfeeding infants (P less than .01).
Parents’ income and education affected infants’ likelihood of meeting guidelines. Breastfeeding infants in families with incomes above 400% of the federal poverty level were twice as likely to meet guidelines (31% vs. 14%-16% for lower income brackets, P less than .05). Babies from families whose head of household had a college degree had a 26% likelihood of having enough vitamin D, compared with less than 11% of those in whose parents had less than a high school education (P less than .05). Babies from families with private insurance also had a better chance of meeting guidelines, compared with those with public insurance (24% vs. 13%; P less than .05).
Ethnicity was seen as affecting vitamin D intake only insofar as some groups had more formula use than breastfeeding. The only ethnic or racial subgroup in the study that saw more than 40% of infants likely to meet guidelines was nonbreastfeeding infants of Asian, American Indian, Native Hawaiian or Pacific Islander, or multiracial parentage, with 46% considered to have adequate vitamin D levels. This group makes up 6% of the infant population in the United States.
“Reasons for low rates of meeting guidelines in the United States and little improvement over time are not fully known,” Dr. Simon and Dr. Ahrens wrote in their analysis. “One factor may be that the impact of low vitamin D in infancy is not highly visible to physicians because rickets is an uncommon diagnosis in the United States.” They noted that recent studies from Canada, where public health officials have done more to promote supplementation, have shown rates of adequate vitamin D in breastfeeding babies to be as high as 90%.
The researchers listed among limitations of their study the fact that the data source, NHANES, captured nutrition information only for the previous 24 hours; that it relied on parental report, and did not confirm serum levels of vitamin D; and that it was possible that cow’s milk – which is not recommended before age 1 but frequently given to older infants anyway – could be a hidden source of vitamin D that was not taken into consideration.
In an editorial comment, Jaspreet Loyal, MD, and Annette Cameron, MD, of Yale University in New Haven, Conn., faulted “a combination of inconsistent prescribing by clinicians and poor adherence to the use of a supplement by parents of infants … further complicated by a lack of awareness of the consequences of vitamin D deficiency in infants among the public” for the low adherence to guidelines in the United States, compared with other countries.
Also, the editorialists noted, the dropper used to administer liquid supplements has been associated with “inconsistent precision” and concerns about infants gagging on the liquid. More research is needed to better understand “prescribing patterns, barriers to adherence by parents of infants, and alternate strategies for vitamin D supplementation to inform novel public health programs in the United States,” they wrote.
The National Institutes of Health funded the study, and Dr. Ahrens is supported by a faculty development grant from the Maine Economic Improvement Fund. The researchers declared no conflicts of interest. Dr. Loyal and Dr. Cameron disclosed no funding and no relevant financial disclosures.
SOURCE: Simon AE and Ahrens KA. Pediatrics 2020 May. doi: 10.1542/peds.2019-3574; Loyal J and Cameron A. Pediatrics. 2020 May. doi: 10.1542/peds.2020-0504.
FROM PEDIATRICS
COVID-19 in kids: Severe illness most common in infants, teens
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
FROM THE JOURNAL OF PEDIATRICS
New research confirms the efficacy and safety of onasemnogene abeparvovec for SMA
The research was presented online as part of the 2020 AAN Science Highlights.
SMA results from a mutation in SMN1, which encodes the SMN protein necessary for motor function. Deficiency of this protein causes motor neurons to die, resulting in severe muscle weakness. At 2 years of age, untreated patients with SMA type 1 generally die or require permanent ventilation.
The Food and Drug Administration approved onasemnogene abeparvovec-xioi under the brand name Zolgensma in May 2019. The gene-replacement therapy, which is administered once intravenously, delivers a fully functional copy of human SMN1 into the target motor neuron cells. It is indicated as treatment for SMA in infants younger than 2 years of age.
Preliminary STR1VE data
Preliminary data from the phase 3 STR1VE study were scheduled to be presented at the meeting. The open-label, single-arm, single-dose study enrolled symptomatic patients with SMA type 1 (SMA1) at multiple US sites. Enrollment was completed in May 2019.
The study included 10 male patients and 12 female patients. Participants’ mean age at dosing was 3.7 months. Of 19 patients who could have reached age 13.6 months at data cutoff, 17 (89.5%) were surviving without permanent ventilation, compared with a 25% survival rate among untreated patients. One of the 19 patients died, and the event was judged to be unrelated to treatment. Another of the 19 reached a respiratory endpoint or withdrew consent.
The population’s mean baseline Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) score was 32. This score increased by 6.9, 11.7, and 14.3 points at months 1, 3, and 5, respectively. Half of the 22 infants sat independently for 30 or more seconds, and this milestone was achieved at a mean of 8.2 months after treatment. Five of six (83%) patients age 18 months or older sat independently for 30 or more seconds, which was one of the study’s primary endpoints. As of March 8, 2019, treatment-emergent adverse events of special interest were transient and not associated with any sequelae.
The STR1VE study was sponsored by AveXis, the maker of onasemnogene abeparvovec-xioi. Several of the investigators are employees of AveXis, and others received funding from the company.
Long-term follow-up in START
Long-term follow-up data for participants in the phase 1/2a START study also were scheduled to be presented. Patients who completed START were eligible to participate, and the trial’s primary aim was to evaluate the long-term safety of onasemnogene abeparvovec-xioi. Patients are intended to have five annual visits, followed by 10 annual phone calls, and the investigators request local physicians or neurologists to transfer patient records. Safety assessments include medical history and record review, physical examination, clinical laboratory evaluation, and pulmonary assessments. Efficacy assessments include evaluation of the maintenance of developmental milestones.
As of May 31, 2019, 13 patients in two cohorts had been enrolled and had had a baseline visit. For patients in Cohort 2, the mean age and time since dosing were 4.2 years and 3.9 years, respectively. All patients in Cohort 2 were alive and did not require permanent ventilation. Participants did not lose any developmental milestones that they had achieved at the end of START. Two patients were able to walk, and two could stand with assistance during long-term follow-up. This result suggests the durability of the treatment’s effect. No new treatment-related serious adverse events or adverse events of special interest had occurred as of March 8, 2019.
“We know from accumulating experience that treating infants by gene therapy is safe,” said Jerry R. Mendell, MD, the principal investigator and an attending neurologist at Nationwide Children’s Hospital in Columbus, Ohio. “Of the 15 patients we had in our first trial, only four adverse events related to the gene delivery were encountered, and only two of these were considered serious adverse events [i.e., liver enzymes that were 10 times greater than normal laboratory levels]. These laboratory tests occurred without accompanying clinical symptoms or signs. All were suppressed by corticosteroids and related to the liver inflammation. This pattern of safety has been seen in our very large gene therapy experience. No long-term surprises were encountered.”
The START study was sponsored by AveXis. Several of the investigators are employees of AveXis, and others received funding from the company.
Update on the SPR1NT study
Interim safety and efficacy data from the ongoing SPR1NT study, which includes presymptomatic patients, also were scheduled to be presented. The trial “was built on the basic premise that spinal motor neuron degeneration associated with SMN protein deficiency begins in utero, continues to progress rapidly during the first months of life, and is irreversible,” said Kevin Strauss, MD, medical director of the Clinic for Special Children in Strasburg, Pennsylvania. “SPR1NT leveraged the advantages conferred by carrier testing and newborn screening programs for SMA, which allowed the first 22 children enrolled to have a confirmed molecular diagnosis between 1 and 26 days of postnatal life, before the onset of dysphagia, respiratory compromise, or overt weakness.”
In this multicenter, open-label, phase 3 trial, presymptomatic patients age 6 weeks or younger who are expected to develop SMA receive onasemnogene abeparvovec-xioi once and are evaluated during 18 or 24 months. The primary outcomes are sitting for 30 or more seconds for infants with two copies of SMN2 and standing unassisted for infants with three copies of SMN2.
As of December 31, 2019, 29 infants had been treated in the efficacy group at a mean age of 20.6 days among infants with two copies of SMN2 and 28.7 days among infants with three copies of SMN2. All patients are alive, and no patient in SPR1NT required ventilation support at last visit. Among 14 patients with two copies of SMN2, all achieved CHOP INTEND scores of 50 or greater, which exceeds the maximal score observed in untreated patients. Eight have achieved sitting, seven of whom achieved it within the World Health Organization sitting age range of 3.8-9.2 months. The other six patients have not yet passed the WHO developmental window. Among 15 patients with three copies of SMN2, four stood independently and three walked independently, all within the WHO developmental windows of 6.9-16.9 months and 8.2-17.6 months, respectively. The other patients have not yet passed the WHO developmental window. No patient in either cohort required a feeding tube, and most remained within the normal weight range. Treatment-emergent adverse events of special interest were reported in 16 patients. The study is ongoing, and patients continue to meet primary endpoints.
“Comparing functional and motor indices between these two groups [i.e., patients with two copies of SMN2 and those with three copies] should contribute to our understanding of how motor neuron loss during fetal development may impact long-term neurological outcomes over the arc of life and could even form a basis for considering antenatal gene therapy for severe forms of SMA,” said Dr. Strauss.
SPR1NT was funded by AveXis. Several of the investigators are employees of AveXis, and others received funding from the company.
Combination therapy may be a possibility
A benefit of onasemnogene abeparvovec-xioi is that the adeno-associated virus that delivers it does not integrate itself into the genome, said Darryl C. De Vivo, MD, Sidney Carter professor of neurology and professor of pediatrics at Columbia University in New York. “The bad news is that every time the cell divides, the gene therapy goes to one of the two daughter cells, but not to both. ... That means the effectiveness, in theory, would be reduced by 50% with each cell division, possibly affecting the durability of treatment.” The fact that brain and spinal cord neurons are presumed to be fully populated around the time of birth partly mitigates this concern, he added. “There isn’t too much additional cell division going on in neurons after birth at a time when the gene therapy would be administered.”
Furthermore, the cellular distribution of the gene therapy within the nervous system, which is unclear, might affect the therapy’s effect. “These are largely unanswered questions,” said Dr. De Vivo. “The answers to these questions only will come with continued observation of patients who have been treated.”
Considering that nusinersen, the antisense oligonucleotide also approved for SMA, targets SMN2, and the gene therapy replaces SMN1, “there may be some wisdom in thinking about combination therapy,” said Dr. De Vivo. “There’s no doubt that these therapeutic agents are effective,” and continued follow-up will clarify their comparative efficacy, he concluded.
SOURCES: Day JW, et al. AAN 2020. Abstract S27.001. Mendell JR, et al. AAN 2020. Abstract S27.002. Strauss KA, et al. AAN 2020. Abstract S27.003.
The research was presented online as part of the 2020 AAN Science Highlights.
SMA results from a mutation in SMN1, which encodes the SMN protein necessary for motor function. Deficiency of this protein causes motor neurons to die, resulting in severe muscle weakness. At 2 years of age, untreated patients with SMA type 1 generally die or require permanent ventilation.
The Food and Drug Administration approved onasemnogene abeparvovec-xioi under the brand name Zolgensma in May 2019. The gene-replacement therapy, which is administered once intravenously, delivers a fully functional copy of human SMN1 into the target motor neuron cells. It is indicated as treatment for SMA in infants younger than 2 years of age.
Preliminary STR1VE data
Preliminary data from the phase 3 STR1VE study were scheduled to be presented at the meeting. The open-label, single-arm, single-dose study enrolled symptomatic patients with SMA type 1 (SMA1) at multiple US sites. Enrollment was completed in May 2019.
The study included 10 male patients and 12 female patients. Participants’ mean age at dosing was 3.7 months. Of 19 patients who could have reached age 13.6 months at data cutoff, 17 (89.5%) were surviving without permanent ventilation, compared with a 25% survival rate among untreated patients. One of the 19 patients died, and the event was judged to be unrelated to treatment. Another of the 19 reached a respiratory endpoint or withdrew consent.
The population’s mean baseline Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) score was 32. This score increased by 6.9, 11.7, and 14.3 points at months 1, 3, and 5, respectively. Half of the 22 infants sat independently for 30 or more seconds, and this milestone was achieved at a mean of 8.2 months after treatment. Five of six (83%) patients age 18 months or older sat independently for 30 or more seconds, which was one of the study’s primary endpoints. As of March 8, 2019, treatment-emergent adverse events of special interest were transient and not associated with any sequelae.
The STR1VE study was sponsored by AveXis, the maker of onasemnogene abeparvovec-xioi. Several of the investigators are employees of AveXis, and others received funding from the company.
Long-term follow-up in START
Long-term follow-up data for participants in the phase 1/2a START study also were scheduled to be presented. Patients who completed START were eligible to participate, and the trial’s primary aim was to evaluate the long-term safety of onasemnogene abeparvovec-xioi. Patients are intended to have five annual visits, followed by 10 annual phone calls, and the investigators request local physicians or neurologists to transfer patient records. Safety assessments include medical history and record review, physical examination, clinical laboratory evaluation, and pulmonary assessments. Efficacy assessments include evaluation of the maintenance of developmental milestones.
As of May 31, 2019, 13 patients in two cohorts had been enrolled and had had a baseline visit. For patients in Cohort 2, the mean age and time since dosing were 4.2 years and 3.9 years, respectively. All patients in Cohort 2 were alive and did not require permanent ventilation. Participants did not lose any developmental milestones that they had achieved at the end of START. Two patients were able to walk, and two could stand with assistance during long-term follow-up. This result suggests the durability of the treatment’s effect. No new treatment-related serious adverse events or adverse events of special interest had occurred as of March 8, 2019.
“We know from accumulating experience that treating infants by gene therapy is safe,” said Jerry R. Mendell, MD, the principal investigator and an attending neurologist at Nationwide Children’s Hospital in Columbus, Ohio. “Of the 15 patients we had in our first trial, only four adverse events related to the gene delivery were encountered, and only two of these were considered serious adverse events [i.e., liver enzymes that were 10 times greater than normal laboratory levels]. These laboratory tests occurred without accompanying clinical symptoms or signs. All were suppressed by corticosteroids and related to the liver inflammation. This pattern of safety has been seen in our very large gene therapy experience. No long-term surprises were encountered.”
The START study was sponsored by AveXis. Several of the investigators are employees of AveXis, and others received funding from the company.
Update on the SPR1NT study
Interim safety and efficacy data from the ongoing SPR1NT study, which includes presymptomatic patients, also were scheduled to be presented. The trial “was built on the basic premise that spinal motor neuron degeneration associated with SMN protein deficiency begins in utero, continues to progress rapidly during the first months of life, and is irreversible,” said Kevin Strauss, MD, medical director of the Clinic for Special Children in Strasburg, Pennsylvania. “SPR1NT leveraged the advantages conferred by carrier testing and newborn screening programs for SMA, which allowed the first 22 children enrolled to have a confirmed molecular diagnosis between 1 and 26 days of postnatal life, before the onset of dysphagia, respiratory compromise, or overt weakness.”
In this multicenter, open-label, phase 3 trial, presymptomatic patients age 6 weeks or younger who are expected to develop SMA receive onasemnogene abeparvovec-xioi once and are evaluated during 18 or 24 months. The primary outcomes are sitting for 30 or more seconds for infants with two copies of SMN2 and standing unassisted for infants with three copies of SMN2.
As of December 31, 2019, 29 infants had been treated in the efficacy group at a mean age of 20.6 days among infants with two copies of SMN2 and 28.7 days among infants with three copies of SMN2. All patients are alive, and no patient in SPR1NT required ventilation support at last visit. Among 14 patients with two copies of SMN2, all achieved CHOP INTEND scores of 50 or greater, which exceeds the maximal score observed in untreated patients. Eight have achieved sitting, seven of whom achieved it within the World Health Organization sitting age range of 3.8-9.2 months. The other six patients have not yet passed the WHO developmental window. Among 15 patients with three copies of SMN2, four stood independently and three walked independently, all within the WHO developmental windows of 6.9-16.9 months and 8.2-17.6 months, respectively. The other patients have not yet passed the WHO developmental window. No patient in either cohort required a feeding tube, and most remained within the normal weight range. Treatment-emergent adverse events of special interest were reported in 16 patients. The study is ongoing, and patients continue to meet primary endpoints.
“Comparing functional and motor indices between these two groups [i.e., patients with two copies of SMN2 and those with three copies] should contribute to our understanding of how motor neuron loss during fetal development may impact long-term neurological outcomes over the arc of life and could even form a basis for considering antenatal gene therapy for severe forms of SMA,” said Dr. Strauss.
SPR1NT was funded by AveXis. Several of the investigators are employees of AveXis, and others received funding from the company.
Combination therapy may be a possibility
A benefit of onasemnogene abeparvovec-xioi is that the adeno-associated virus that delivers it does not integrate itself into the genome, said Darryl C. De Vivo, MD, Sidney Carter professor of neurology and professor of pediatrics at Columbia University in New York. “The bad news is that every time the cell divides, the gene therapy goes to one of the two daughter cells, but not to both. ... That means the effectiveness, in theory, would be reduced by 50% with each cell division, possibly affecting the durability of treatment.” The fact that brain and spinal cord neurons are presumed to be fully populated around the time of birth partly mitigates this concern, he added. “There isn’t too much additional cell division going on in neurons after birth at a time when the gene therapy would be administered.”
Furthermore, the cellular distribution of the gene therapy within the nervous system, which is unclear, might affect the therapy’s effect. “These are largely unanswered questions,” said Dr. De Vivo. “The answers to these questions only will come with continued observation of patients who have been treated.”
Considering that nusinersen, the antisense oligonucleotide also approved for SMA, targets SMN2, and the gene therapy replaces SMN1, “there may be some wisdom in thinking about combination therapy,” said Dr. De Vivo. “There’s no doubt that these therapeutic agents are effective,” and continued follow-up will clarify their comparative efficacy, he concluded.
SOURCES: Day JW, et al. AAN 2020. Abstract S27.001. Mendell JR, et al. AAN 2020. Abstract S27.002. Strauss KA, et al. AAN 2020. Abstract S27.003.
The research was presented online as part of the 2020 AAN Science Highlights.
SMA results from a mutation in SMN1, which encodes the SMN protein necessary for motor function. Deficiency of this protein causes motor neurons to die, resulting in severe muscle weakness. At 2 years of age, untreated patients with SMA type 1 generally die or require permanent ventilation.
The Food and Drug Administration approved onasemnogene abeparvovec-xioi under the brand name Zolgensma in May 2019. The gene-replacement therapy, which is administered once intravenously, delivers a fully functional copy of human SMN1 into the target motor neuron cells. It is indicated as treatment for SMA in infants younger than 2 years of age.
Preliminary STR1VE data
Preliminary data from the phase 3 STR1VE study were scheduled to be presented at the meeting. The open-label, single-arm, single-dose study enrolled symptomatic patients with SMA type 1 (SMA1) at multiple US sites. Enrollment was completed in May 2019.
The study included 10 male patients and 12 female patients. Participants’ mean age at dosing was 3.7 months. Of 19 patients who could have reached age 13.6 months at data cutoff, 17 (89.5%) were surviving without permanent ventilation, compared with a 25% survival rate among untreated patients. One of the 19 patients died, and the event was judged to be unrelated to treatment. Another of the 19 reached a respiratory endpoint or withdrew consent.
The population’s mean baseline Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) score was 32. This score increased by 6.9, 11.7, and 14.3 points at months 1, 3, and 5, respectively. Half of the 22 infants sat independently for 30 or more seconds, and this milestone was achieved at a mean of 8.2 months after treatment. Five of six (83%) patients age 18 months or older sat independently for 30 or more seconds, which was one of the study’s primary endpoints. As of March 8, 2019, treatment-emergent adverse events of special interest were transient and not associated with any sequelae.
The STR1VE study was sponsored by AveXis, the maker of onasemnogene abeparvovec-xioi. Several of the investigators are employees of AveXis, and others received funding from the company.
Long-term follow-up in START
Long-term follow-up data for participants in the phase 1/2a START study also were scheduled to be presented. Patients who completed START were eligible to participate, and the trial’s primary aim was to evaluate the long-term safety of onasemnogene abeparvovec-xioi. Patients are intended to have five annual visits, followed by 10 annual phone calls, and the investigators request local physicians or neurologists to transfer patient records. Safety assessments include medical history and record review, physical examination, clinical laboratory evaluation, and pulmonary assessments. Efficacy assessments include evaluation of the maintenance of developmental milestones.
As of May 31, 2019, 13 patients in two cohorts had been enrolled and had had a baseline visit. For patients in Cohort 2, the mean age and time since dosing were 4.2 years and 3.9 years, respectively. All patients in Cohort 2 were alive and did not require permanent ventilation. Participants did not lose any developmental milestones that they had achieved at the end of START. Two patients were able to walk, and two could stand with assistance during long-term follow-up. This result suggests the durability of the treatment’s effect. No new treatment-related serious adverse events or adverse events of special interest had occurred as of March 8, 2019.
“We know from accumulating experience that treating infants by gene therapy is safe,” said Jerry R. Mendell, MD, the principal investigator and an attending neurologist at Nationwide Children’s Hospital in Columbus, Ohio. “Of the 15 patients we had in our first trial, only four adverse events related to the gene delivery were encountered, and only two of these were considered serious adverse events [i.e., liver enzymes that were 10 times greater than normal laboratory levels]. These laboratory tests occurred without accompanying clinical symptoms or signs. All were suppressed by corticosteroids and related to the liver inflammation. This pattern of safety has been seen in our very large gene therapy experience. No long-term surprises were encountered.”
The START study was sponsored by AveXis. Several of the investigators are employees of AveXis, and others received funding from the company.
Update on the SPR1NT study
Interim safety and efficacy data from the ongoing SPR1NT study, which includes presymptomatic patients, also were scheduled to be presented. The trial “was built on the basic premise that spinal motor neuron degeneration associated with SMN protein deficiency begins in utero, continues to progress rapidly during the first months of life, and is irreversible,” said Kevin Strauss, MD, medical director of the Clinic for Special Children in Strasburg, Pennsylvania. “SPR1NT leveraged the advantages conferred by carrier testing and newborn screening programs for SMA, which allowed the first 22 children enrolled to have a confirmed molecular diagnosis between 1 and 26 days of postnatal life, before the onset of dysphagia, respiratory compromise, or overt weakness.”
In this multicenter, open-label, phase 3 trial, presymptomatic patients age 6 weeks or younger who are expected to develop SMA receive onasemnogene abeparvovec-xioi once and are evaluated during 18 or 24 months. The primary outcomes are sitting for 30 or more seconds for infants with two copies of SMN2 and standing unassisted for infants with three copies of SMN2.
As of December 31, 2019, 29 infants had been treated in the efficacy group at a mean age of 20.6 days among infants with two copies of SMN2 and 28.7 days among infants with three copies of SMN2. All patients are alive, and no patient in SPR1NT required ventilation support at last visit. Among 14 patients with two copies of SMN2, all achieved CHOP INTEND scores of 50 or greater, which exceeds the maximal score observed in untreated patients. Eight have achieved sitting, seven of whom achieved it within the World Health Organization sitting age range of 3.8-9.2 months. The other six patients have not yet passed the WHO developmental window. Among 15 patients with three copies of SMN2, four stood independently and three walked independently, all within the WHO developmental windows of 6.9-16.9 months and 8.2-17.6 months, respectively. The other patients have not yet passed the WHO developmental window. No patient in either cohort required a feeding tube, and most remained within the normal weight range. Treatment-emergent adverse events of special interest were reported in 16 patients. The study is ongoing, and patients continue to meet primary endpoints.
“Comparing functional and motor indices between these two groups [i.e., patients with two copies of SMN2 and those with three copies] should contribute to our understanding of how motor neuron loss during fetal development may impact long-term neurological outcomes over the arc of life and could even form a basis for considering antenatal gene therapy for severe forms of SMA,” said Dr. Strauss.
SPR1NT was funded by AveXis. Several of the investigators are employees of AveXis, and others received funding from the company.
Combination therapy may be a possibility
A benefit of onasemnogene abeparvovec-xioi is that the adeno-associated virus that delivers it does not integrate itself into the genome, said Darryl C. De Vivo, MD, Sidney Carter professor of neurology and professor of pediatrics at Columbia University in New York. “The bad news is that every time the cell divides, the gene therapy goes to one of the two daughter cells, but not to both. ... That means the effectiveness, in theory, would be reduced by 50% with each cell division, possibly affecting the durability of treatment.” The fact that brain and spinal cord neurons are presumed to be fully populated around the time of birth partly mitigates this concern, he added. “There isn’t too much additional cell division going on in neurons after birth at a time when the gene therapy would be administered.”
Furthermore, the cellular distribution of the gene therapy within the nervous system, which is unclear, might affect the therapy’s effect. “These are largely unanswered questions,” said Dr. De Vivo. “The answers to these questions only will come with continued observation of patients who have been treated.”
Considering that nusinersen, the antisense oligonucleotide also approved for SMA, targets SMN2, and the gene therapy replaces SMN1, “there may be some wisdom in thinking about combination therapy,” said Dr. De Vivo. “There’s no doubt that these therapeutic agents are effective,” and continued follow-up will clarify their comparative efficacy, he concluded.
SOURCES: Day JW, et al. AAN 2020. Abstract S27.001. Mendell JR, et al. AAN 2020. Abstract S27.002. Strauss KA, et al. AAN 2020. Abstract S27.003.
FROM AAN 2020
Dermatologic changes with COVID-19: What we know and don’t know
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
Distracted driving laws reduce teen driver deaths
While car crashes are still the leading cause of death among adolescents in the United States, the expansion of state laws restricting cell phone use or texting while driving has pushed down death rates for teen drivers, a study has found.
However, the researchers wrote that the type of law and the manner of enforcement bear on how much teen road deaths are reduced.
In an article published in Pediatrics, Michael R. Flaherty, DO, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues used data from the Fatality Analysis Reporting System, a national database of motor vehicle deaths in the United States, to identify 38,215 fatal crashes nationwide involving teen drivers from 2007 to 2017.
During that same time period, in which a majority of states began to adopt some form of “distracted driving” legislation prohibiting texting or all handheld cell phone use, fatal crashes involving 16- to 19-year-old drivers decreased from 30 in 100,000 persons to 19 in 100,000.
Under primarily enforced laws – those that make texting an offense for which police can stop and cite a driver – 16- to 19-year-old drivers saw a 29% lower driver fatality rate, compared with those living in states with no texting laws (adjusted incidence rate ratio, 0.71; 95% confidence interval, 0.67-0.76).
Under secondarily enforced bans, deaths of drivers aged 16-19 were reduced 15%, compared with no restrictions (aIRR, 0.85; 95% CI, 0.77-0.95).
Importantly, state laws limiting texting and cell phone use had to apply to drivers of all ages to be protective, the investigators found. Laws banning cell phone use only among novice drivers, which have been adopted in many states, were not seen lowering teen driver fatality rates. At the time of this study in 2017, “40 states had primary enforcement texting bans, 6 states had secondary enforcement texting bans, 34 states banned all cellphone use for novice drivers, and 12 banned handheld cellphones for all drivers, they reported.
Dr. Flaherty and colleagues noted that their study was the first to look in detail at the effects of anti–distracted driving laws on teen drivers specifically. They noted among the study’s limitations that the database used did not capture nonfatal accidents, and that the findings could not be adjusted for social or technological changes such as alcohol use trends among teens or safety improvements to cars.
In an accompanying editorial, Catherine C. McDonald, PhD, RN, and M. Kit Delgado, MD, of the University of Pennsylvania, Philadelphia, along with Mark R. Zonfrillo, MD, of Brown University, Providence, R.I., wrote that the findings show “reducing adolescent [crash] fatalities is not just about targeting laws to the adolescent drivers who are at elevated crash risk but also the other drivers who share the road with them.”
“The basic concepts related to eyes on the road, hands on the wheel, and mind on the task of driving are fundamental to driver safety. There is no one cause to pinpoint for adolescent motor vehicle crashes because there are multiple contributing factors, including inexperience, maturational development, and risk-taking.” they wrote.
Noting that nearly half of high school–aged drivers acknowledge texting while driving, the editorialists argued that most states still had room to “refine existing laws or implement new laws” to help reduce fatalities associated with adolescent drivers. “In the meantime, other technological and behavioral approaches may be needed to encourage adolescent drivers to act in their own and society’s best interests and comply with the law.”
Dr. Flaherty and colleagues declared no external funding for their study or financial conflicts of interest. Dr. McDonald, Dr. Delgado, and Dr. Zonfrillo declared funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development related to their editorial and no relevant financial disclosures.
SOURCE: Flaherty M et al. Pediatrics. 2020;145(6):e20193621; McDonald CC et al. Pediatrics. 2020;145(6):e20200419.
While car crashes are still the leading cause of death among adolescents in the United States, the expansion of state laws restricting cell phone use or texting while driving has pushed down death rates for teen drivers, a study has found.
However, the researchers wrote that the type of law and the manner of enforcement bear on how much teen road deaths are reduced.
In an article published in Pediatrics, Michael R. Flaherty, DO, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues used data from the Fatality Analysis Reporting System, a national database of motor vehicle deaths in the United States, to identify 38,215 fatal crashes nationwide involving teen drivers from 2007 to 2017.
During that same time period, in which a majority of states began to adopt some form of “distracted driving” legislation prohibiting texting or all handheld cell phone use, fatal crashes involving 16- to 19-year-old drivers decreased from 30 in 100,000 persons to 19 in 100,000.
Under primarily enforced laws – those that make texting an offense for which police can stop and cite a driver – 16- to 19-year-old drivers saw a 29% lower driver fatality rate, compared with those living in states with no texting laws (adjusted incidence rate ratio, 0.71; 95% confidence interval, 0.67-0.76).
Under secondarily enforced bans, deaths of drivers aged 16-19 were reduced 15%, compared with no restrictions (aIRR, 0.85; 95% CI, 0.77-0.95).
Importantly, state laws limiting texting and cell phone use had to apply to drivers of all ages to be protective, the investigators found. Laws banning cell phone use only among novice drivers, which have been adopted in many states, were not seen lowering teen driver fatality rates. At the time of this study in 2017, “40 states had primary enforcement texting bans, 6 states had secondary enforcement texting bans, 34 states banned all cellphone use for novice drivers, and 12 banned handheld cellphones for all drivers, they reported.
Dr. Flaherty and colleagues noted that their study was the first to look in detail at the effects of anti–distracted driving laws on teen drivers specifically. They noted among the study’s limitations that the database used did not capture nonfatal accidents, and that the findings could not be adjusted for social or technological changes such as alcohol use trends among teens or safety improvements to cars.
In an accompanying editorial, Catherine C. McDonald, PhD, RN, and M. Kit Delgado, MD, of the University of Pennsylvania, Philadelphia, along with Mark R. Zonfrillo, MD, of Brown University, Providence, R.I., wrote that the findings show “reducing adolescent [crash] fatalities is not just about targeting laws to the adolescent drivers who are at elevated crash risk but also the other drivers who share the road with them.”
“The basic concepts related to eyes on the road, hands on the wheel, and mind on the task of driving are fundamental to driver safety. There is no one cause to pinpoint for adolescent motor vehicle crashes because there are multiple contributing factors, including inexperience, maturational development, and risk-taking.” they wrote.
Noting that nearly half of high school–aged drivers acknowledge texting while driving, the editorialists argued that most states still had room to “refine existing laws or implement new laws” to help reduce fatalities associated with adolescent drivers. “In the meantime, other technological and behavioral approaches may be needed to encourage adolescent drivers to act in their own and society’s best interests and comply with the law.”
Dr. Flaherty and colleagues declared no external funding for their study or financial conflicts of interest. Dr. McDonald, Dr. Delgado, and Dr. Zonfrillo declared funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development related to their editorial and no relevant financial disclosures.
SOURCE: Flaherty M et al. Pediatrics. 2020;145(6):e20193621; McDonald CC et al. Pediatrics. 2020;145(6):e20200419.
While car crashes are still the leading cause of death among adolescents in the United States, the expansion of state laws restricting cell phone use or texting while driving has pushed down death rates for teen drivers, a study has found.
However, the researchers wrote that the type of law and the manner of enforcement bear on how much teen road deaths are reduced.
In an article published in Pediatrics, Michael R. Flaherty, DO, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues used data from the Fatality Analysis Reporting System, a national database of motor vehicle deaths in the United States, to identify 38,215 fatal crashes nationwide involving teen drivers from 2007 to 2017.
During that same time period, in which a majority of states began to adopt some form of “distracted driving” legislation prohibiting texting or all handheld cell phone use, fatal crashes involving 16- to 19-year-old drivers decreased from 30 in 100,000 persons to 19 in 100,000.
Under primarily enforced laws – those that make texting an offense for which police can stop and cite a driver – 16- to 19-year-old drivers saw a 29% lower driver fatality rate, compared with those living in states with no texting laws (adjusted incidence rate ratio, 0.71; 95% confidence interval, 0.67-0.76).
Under secondarily enforced bans, deaths of drivers aged 16-19 were reduced 15%, compared with no restrictions (aIRR, 0.85; 95% CI, 0.77-0.95).
Importantly, state laws limiting texting and cell phone use had to apply to drivers of all ages to be protective, the investigators found. Laws banning cell phone use only among novice drivers, which have been adopted in many states, were not seen lowering teen driver fatality rates. At the time of this study in 2017, “40 states had primary enforcement texting bans, 6 states had secondary enforcement texting bans, 34 states banned all cellphone use for novice drivers, and 12 banned handheld cellphones for all drivers, they reported.
Dr. Flaherty and colleagues noted that their study was the first to look in detail at the effects of anti–distracted driving laws on teen drivers specifically. They noted among the study’s limitations that the database used did not capture nonfatal accidents, and that the findings could not be adjusted for social or technological changes such as alcohol use trends among teens or safety improvements to cars.
In an accompanying editorial, Catherine C. McDonald, PhD, RN, and M. Kit Delgado, MD, of the University of Pennsylvania, Philadelphia, along with Mark R. Zonfrillo, MD, of Brown University, Providence, R.I., wrote that the findings show “reducing adolescent [crash] fatalities is not just about targeting laws to the adolescent drivers who are at elevated crash risk but also the other drivers who share the road with them.”
“The basic concepts related to eyes on the road, hands on the wheel, and mind on the task of driving are fundamental to driver safety. There is no one cause to pinpoint for adolescent motor vehicle crashes because there are multiple contributing factors, including inexperience, maturational development, and risk-taking.” they wrote.
Noting that nearly half of high school–aged drivers acknowledge texting while driving, the editorialists argued that most states still had room to “refine existing laws or implement new laws” to help reduce fatalities associated with adolescent drivers. “In the meantime, other technological and behavioral approaches may be needed to encourage adolescent drivers to act in their own and society’s best interests and comply with the law.”
Dr. Flaherty and colleagues declared no external funding for their study or financial conflicts of interest. Dr. McDonald, Dr. Delgado, and Dr. Zonfrillo declared funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development related to their editorial and no relevant financial disclosures.
SOURCE: Flaherty M et al. Pediatrics. 2020;145(6):e20193621; McDonald CC et al. Pediatrics. 2020;145(6):e20200419.
FROM PEDIATRICS
What's your diagnosis?
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
A 10-year-old, otherwise healthy female with no prior significant medical history is brought into clinic for evaluation of orange-red scaly papules and plaques that first started on the face, neck, and fingers and began spreading to the trunk, arms, and knees. The mother of the patient also had noticed thickening of the skin on her palms and soles. The rash has been present for 2 months. Patient does not appear to be itchy, and otherwise is in normal state without pain, fever, drainage from sites, or known exposures. She was initially treated with topical triamcinolone with minimal improvement.
On physical exam, she is noted to have reddish-orange hyperkeratotic scaling papules coalescing into large plaques with follicular prominence diffusely on the face, neck, trunk, and upper extremities with smaller islands of skin that are normal-appearing. There is diffuse fine scale throughout the scalp and thickening of the skin on the palms and soles with a yellowish waxy appearance.
A toddler with a fever and desquamating perineal rash
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
An otherwise healthy 18-month-old female presented to the emergency department with 5 days of fever, erythema, fissuring of the lips, conjunctival injection, and a desquamating perineal rash. In addition, she had nasal congestion and cough for which she was started on amoxicillin 2 days prior to presentation given concern for pneumonia.
On exam, she was also noted to have several palpable cervical lymph nodes and edematous hands with overlying erythema. Laboratory evaluation was notable for respiratory syncytial virus positivity by polymerase chain reaction assay, leukocytosis, and elevated inflammatory markers (erythrocyte sedimentation rate and C-reactive protein).