User login
The Mississippi solution
I agree wholeheartedly with Dr. William G. Wilkoff’s doubts that an increase in medical schools/students and/or foreign medical graduates is the answer to the physician shortage felt by many areas of the country (Letters From Maine, “Help Wanted,” Nov. 2019, page 19). All you have to do is look at the glut of physicians – and just about any other profession – in metropolitan areas versus rural America, and ask basic questions regarding why those doctors practice where they do. You will quickly discover that most are willing to trade the possibility of a higher salary in areas where their presence is more needed to achieve more school choices, jobs for a spouse, and likely a more favorable call schedule. Something more attractive than salary or the prospect of more “elbow room” is desired.
Here in Mississippi we may have found an answer to the problem. A few years ago our state legislature started the Mississippi Rural Health Scholarship Program that pays for recipients to attend a state-run medical school on scholarship in exchange for agreeing to practice at least 4 years in a rural area of the state (less than 20k population) following their primary care residency (family medicine, pediatrics, ob.gyn., med-peds, internal medicine, and, recently added, psychiatry). Although a recent increase in the number of pediatric residency slots at our state’s sole program will no doubt also have a positive effect to this end, such a scholarship program as the one implemented by Mississippi is the best way to compete with the various intangibles that lead people to choose bigger cities over rural areas of the state to practice their trade. Once there, many – like myself – will find that such a practice is not only a good business decision but often is a wonderful place to raise a family. Meanwhile, our own practice just added a fourth physician as a result of said Rural Health Scholarship Program, and we could not be more satisfied with the result.
I agree wholeheartedly with Dr. William G. Wilkoff’s doubts that an increase in medical schools/students and/or foreign medical graduates is the answer to the physician shortage felt by many areas of the country (Letters From Maine, “Help Wanted,” Nov. 2019, page 19). All you have to do is look at the glut of physicians – and just about any other profession – in metropolitan areas versus rural America, and ask basic questions regarding why those doctors practice where they do. You will quickly discover that most are willing to trade the possibility of a higher salary in areas where their presence is more needed to achieve more school choices, jobs for a spouse, and likely a more favorable call schedule. Something more attractive than salary or the prospect of more “elbow room” is desired.
Here in Mississippi we may have found an answer to the problem. A few years ago our state legislature started the Mississippi Rural Health Scholarship Program that pays for recipients to attend a state-run medical school on scholarship in exchange for agreeing to practice at least 4 years in a rural area of the state (less than 20k population) following their primary care residency (family medicine, pediatrics, ob.gyn., med-peds, internal medicine, and, recently added, psychiatry). Although a recent increase in the number of pediatric residency slots at our state’s sole program will no doubt also have a positive effect to this end, such a scholarship program as the one implemented by Mississippi is the best way to compete with the various intangibles that lead people to choose bigger cities over rural areas of the state to practice their trade. Once there, many – like myself – will find that such a practice is not only a good business decision but often is a wonderful place to raise a family. Meanwhile, our own practice just added a fourth physician as a result of said Rural Health Scholarship Program, and we could not be more satisfied with the result.
I agree wholeheartedly with Dr. William G. Wilkoff’s doubts that an increase in medical schools/students and/or foreign medical graduates is the answer to the physician shortage felt by many areas of the country (Letters From Maine, “Help Wanted,” Nov. 2019, page 19). All you have to do is look at the glut of physicians – and just about any other profession – in metropolitan areas versus rural America, and ask basic questions regarding why those doctors practice where they do. You will quickly discover that most are willing to trade the possibility of a higher salary in areas where their presence is more needed to achieve more school choices, jobs for a spouse, and likely a more favorable call schedule. Something more attractive than salary or the prospect of more “elbow room” is desired.
Here in Mississippi we may have found an answer to the problem. A few years ago our state legislature started the Mississippi Rural Health Scholarship Program that pays for recipients to attend a state-run medical school on scholarship in exchange for agreeing to practice at least 4 years in a rural area of the state (less than 20k population) following their primary care residency (family medicine, pediatrics, ob.gyn., med-peds, internal medicine, and, recently added, psychiatry). Although a recent increase in the number of pediatric residency slots at our state’s sole program will no doubt also have a positive effect to this end, such a scholarship program as the one implemented by Mississippi is the best way to compete with the various intangibles that lead people to choose bigger cities over rural areas of the state to practice their trade. Once there, many – like myself – will find that such a practice is not only a good business decision but often is a wonderful place to raise a family. Meanwhile, our own practice just added a fourth physician as a result of said Rural Health Scholarship Program, and we could not be more satisfied with the result.
Vaccinating most girls could eliminate cervical cancer within a century
Cervical cancer is the second most common cancer among women in lower- and middle-income countries, but universal human papillomavirus vaccination for girls would reduce new cervical cancer cases by about 90% over the next century, according to researchers.
Adding twice-lifetime cervical screening with human papillomavirus (HPV) testing would further reduce the incidence of cervical cancer, including in countries with the highest burden, the researchers reported in The Lancet.
Marc Brisson, PhD, of Laval University, Quebec City, and colleagues conducted this study using three models identified by the World Health Organization. The models were used to project reductions in cervical cancer incidence for women in 78 low- and middle-income countries based on the following HPV vaccination and screening scenarios:
- Universal girls-only vaccination at age 9 years, assuming 90% of girls vaccinated and a vaccine that is perfectly effective
- Girls-only vaccination plus cervical screening with HPV testing at age 35 years
- Girls-only vaccination plus screening at ages 35 and 45.
All three scenarios modeled would result in the elimination of cervical cancer, Dr. Brisson and colleagues found. Elimination was defined as four or fewer new cases per 100,000 women-years.
The simplest scenario, universal girls-only vaccination, was predicted to reduce age-standardized cervical cancer incidence from 19.8 cases per 100,000 women-years to 2.1 cases per 100,000 women-years (89.4% reduction) by 2120. That amounts to about 61 million potential cases avoided, with elimination targets reached in 60% of the countries studied.
HPV vaccination plus one-time screening was predicted to reduce the incidence of cervical cancer to 1.0 case per 100,000 women-years (95.0% reduction), and HPV vaccination plus twice-lifetime screening was predicted to reduce the incidence to 0.7 cases per 100,000 women-years (96.7% reduction).
Dr. Brisson and colleagues reported that, for the countries with the highest burden of cervical cancer (more than 25 cases per 100,000 women-years), adding screening would be necessary to achieve elimination.
To meet the same targets across all 78 countries, “our models predict that scale-up of both girls-only HPV vaccination and twice-lifetime screening is necessary, with 90% HPV vaccination coverage, 90% screening uptake, and long-term protection against HPV types 16, 18, 31, 33, 45, 52, and 58,” the researchers wrote.
Dr. Brisson and colleagues claimed that a strength of this study is the modeling approach, which compared three models “that have been extensively peer reviewed and validated with postvaccination surveillance data.”
The researchers acknowledged, however, that their modeling could not account for variations in sexual behavior from country to country, and the study was not designed to anticipate behavioral or technological changes that could affect cervical cancer incidence in the decades to come.
The study was funded by the WHO, the United Nations, and the Canadian and Australian governments. The WHO contributed to the study design, data analysis and interpretation, and writing of the manuscript. Two study authors reported receiving indirect industry funding for a cervical screening trial in Australia.
SOURCE: Brisson M et al. Lancet. 2020 Jan 30. doi: 10.1016/S0140-6736(20)30068-4.
Cervical cancer is the second most common cancer among women in lower- and middle-income countries, but universal human papillomavirus vaccination for girls would reduce new cervical cancer cases by about 90% over the next century, according to researchers.
Adding twice-lifetime cervical screening with human papillomavirus (HPV) testing would further reduce the incidence of cervical cancer, including in countries with the highest burden, the researchers reported in The Lancet.
Marc Brisson, PhD, of Laval University, Quebec City, and colleagues conducted this study using three models identified by the World Health Organization. The models were used to project reductions in cervical cancer incidence for women in 78 low- and middle-income countries based on the following HPV vaccination and screening scenarios:
- Universal girls-only vaccination at age 9 years, assuming 90% of girls vaccinated and a vaccine that is perfectly effective
- Girls-only vaccination plus cervical screening with HPV testing at age 35 years
- Girls-only vaccination plus screening at ages 35 and 45.
All three scenarios modeled would result in the elimination of cervical cancer, Dr. Brisson and colleagues found. Elimination was defined as four or fewer new cases per 100,000 women-years.
The simplest scenario, universal girls-only vaccination, was predicted to reduce age-standardized cervical cancer incidence from 19.8 cases per 100,000 women-years to 2.1 cases per 100,000 women-years (89.4% reduction) by 2120. That amounts to about 61 million potential cases avoided, with elimination targets reached in 60% of the countries studied.
HPV vaccination plus one-time screening was predicted to reduce the incidence of cervical cancer to 1.0 case per 100,000 women-years (95.0% reduction), and HPV vaccination plus twice-lifetime screening was predicted to reduce the incidence to 0.7 cases per 100,000 women-years (96.7% reduction).
Dr. Brisson and colleagues reported that, for the countries with the highest burden of cervical cancer (more than 25 cases per 100,000 women-years), adding screening would be necessary to achieve elimination.
To meet the same targets across all 78 countries, “our models predict that scale-up of both girls-only HPV vaccination and twice-lifetime screening is necessary, with 90% HPV vaccination coverage, 90% screening uptake, and long-term protection against HPV types 16, 18, 31, 33, 45, 52, and 58,” the researchers wrote.
Dr. Brisson and colleagues claimed that a strength of this study is the modeling approach, which compared three models “that have been extensively peer reviewed and validated with postvaccination surveillance data.”
The researchers acknowledged, however, that their modeling could not account for variations in sexual behavior from country to country, and the study was not designed to anticipate behavioral or technological changes that could affect cervical cancer incidence in the decades to come.
The study was funded by the WHO, the United Nations, and the Canadian and Australian governments. The WHO contributed to the study design, data analysis and interpretation, and writing of the manuscript. Two study authors reported receiving indirect industry funding for a cervical screening trial in Australia.
SOURCE: Brisson M et al. Lancet. 2020 Jan 30. doi: 10.1016/S0140-6736(20)30068-4.
Cervical cancer is the second most common cancer among women in lower- and middle-income countries, but universal human papillomavirus vaccination for girls would reduce new cervical cancer cases by about 90% over the next century, according to researchers.
Adding twice-lifetime cervical screening with human papillomavirus (HPV) testing would further reduce the incidence of cervical cancer, including in countries with the highest burden, the researchers reported in The Lancet.
Marc Brisson, PhD, of Laval University, Quebec City, and colleagues conducted this study using three models identified by the World Health Organization. The models were used to project reductions in cervical cancer incidence for women in 78 low- and middle-income countries based on the following HPV vaccination and screening scenarios:
- Universal girls-only vaccination at age 9 years, assuming 90% of girls vaccinated and a vaccine that is perfectly effective
- Girls-only vaccination plus cervical screening with HPV testing at age 35 years
- Girls-only vaccination plus screening at ages 35 and 45.
All three scenarios modeled would result in the elimination of cervical cancer, Dr. Brisson and colleagues found. Elimination was defined as four or fewer new cases per 100,000 women-years.
The simplest scenario, universal girls-only vaccination, was predicted to reduce age-standardized cervical cancer incidence from 19.8 cases per 100,000 women-years to 2.1 cases per 100,000 women-years (89.4% reduction) by 2120. That amounts to about 61 million potential cases avoided, with elimination targets reached in 60% of the countries studied.
HPV vaccination plus one-time screening was predicted to reduce the incidence of cervical cancer to 1.0 case per 100,000 women-years (95.0% reduction), and HPV vaccination plus twice-lifetime screening was predicted to reduce the incidence to 0.7 cases per 100,000 women-years (96.7% reduction).
Dr. Brisson and colleagues reported that, for the countries with the highest burden of cervical cancer (more than 25 cases per 100,000 women-years), adding screening would be necessary to achieve elimination.
To meet the same targets across all 78 countries, “our models predict that scale-up of both girls-only HPV vaccination and twice-lifetime screening is necessary, with 90% HPV vaccination coverage, 90% screening uptake, and long-term protection against HPV types 16, 18, 31, 33, 45, 52, and 58,” the researchers wrote.
Dr. Brisson and colleagues claimed that a strength of this study is the modeling approach, which compared three models “that have been extensively peer reviewed and validated with postvaccination surveillance data.”
The researchers acknowledged, however, that their modeling could not account for variations in sexual behavior from country to country, and the study was not designed to anticipate behavioral or technological changes that could affect cervical cancer incidence in the decades to come.
The study was funded by the WHO, the United Nations, and the Canadian and Australian governments. The WHO contributed to the study design, data analysis and interpretation, and writing of the manuscript. Two study authors reported receiving indirect industry funding for a cervical screening trial in Australia.
SOURCE: Brisson M et al. Lancet. 2020 Jan 30. doi: 10.1016/S0140-6736(20)30068-4.
FROM THE LANCET
What to do when stimulants fail for ADHD
NEW ORLEANS – A variety of reasons can contribute to the failure of stimulants to treat ADHD in children, such as comorbidities, missed diagnoses, inadequate medication dosage, side effects, major life changes, and other factors in the home or school environments, said Alison Schonwald, MD, of Harvard Medical School, Boston.
Stimulant medications indicated for ADHD usually work in 70%-75% of school-age children, but that leaves one in four children whose condition can be more challenging to treat, she said.
“Look around you,” Dr. Schonwald told a packed room at the annual meeting of the American Academy of Pediatrics. “You’re not the only one struggling with this topic.” She sprinkled her presentation with case studies of patients with ADHD for whom stimulants weren’t working, examples that the audience clearly found familiar.
The three steps you already know to do with treatment-resistant children sound simple: assess the child for factors linked to their poor response; develop a new treatment plan; and use Food and Drug Administration-approved nonstimulant medications, including off-label options, in a new plan.
But in the office, the process can be anything but simple when you must consider school and family environments, comorbidities, and other factors potentially complicating the child’s ability to function well.
Comorbidities
To start, Dr. Schonwald provided a chart of common coexisting problems in children with ADHD that included the recommended assessment and intervention:
- Mood and self-esteem issues call for the depression section of the patient health questionnaire (PHQ9) and Moods and Feelings questionnaire (MFQ), followed by interventions such as individual and peer group therapy and exercise.
- Anxiety can be assessed with the Screen for Child Anxiety Related Disorders (SCARED) and Spence Children’s Anxiety Scale, then treated similarly to mood and self-esteem issues.
- Bullying or trauma require taking a history during an interview, and treatment with individual and peer group therapy.
- Substance abuse should be assessed with the CRAFFT screening tool (Car, Relax, Alone, Forget, Friends, Trouble) and Screening to Brief Intervention (S2BI) Tool, then treated according to best practices.
- Executive function, low cognitive abilities, and poor adaptive skills require a review of the child’s Individualized Education Program (IEP) testing, followed by personalized school and home interventions.
- Poor social skills, assessed in an interview, also require personalized interventions at home and in school.
Doctors also may need to consider other common comorbidities in children with ADHD, such as bipolar disorder, depression, learning disabilities, oppositional defiant disorder, and tic disorders.
Tic disorders typically have an onset around 7 years old and peak in midadolescence, declining in late teen years. An estimated 35%-90% of children with Tourette syndrome have ADHD, Dr. Schonwald said (Dev Med Child Neurol. 2006 Jul;48[7]:616-21).
Managing treatment with stimulants
A common dosage amount for stimulants is 2.5-5 mg, but that dose may be too low for children, Dr. Schonwald said. She recommended increasing it until an effect is seen and stopping at the effective dose level the child can tolerate. The maximum recommended by the FDA is 60 mg/day for short-acting stimulants and 72 mg/day for extended-release ones, but some research has shown dosage can go even higher without causing toxic effects (J Child Adolesc Psychopharmacol. 2010 Feb;20[1]:49-54).
Dr. Schonwald also suggested trying both methylphenidate and amphetamine medication, while recognizing the latter tends to have more stimulant-related side effects.
Adherence is another consideration because multiple studies show high rates of noncompliance or discontinuation, such as up to 19% discontinuation for long-acting and 38% for short-acting stimulants (J Clin Psychiatry. 2015 Nov;76(11):e1459-68; Postgrad Med. 2012 May;124(3):139-48). A study of a school cohort in Philadelphia found only about one in five children were adherent (J Am Acad Child Adolesc Psychiatry. 2011 May;50[5]:480-9).
One potential solution to adherence challenges are pill reminder smartphone apps, such as Medisafe Medication Management, Pill Reminder-All in One, MyTherapy: Medication Reminder, and CareZone.
Dr. Schonwald noted several factors that can influence children’s response to stimulants. Among children with comorbid intellectual disability, for example, the response rate is lower than the average 75% of children without the disability, hovering around 40%-50% (Res Dev Disabil. 2018 Dec;83:217-32). Those who get more sleep tend to have improved attention, compared with children with less sleep (Atten Defic Hyperact Disord. 2017 Mar;9[1]:31-38).
She also offered strategies to manage problematic adverse effects from stimulants. Those experiencing weight loss can take their stimulant after breakfast, drink whole milk, and consider taking drug holidays.
To reduce stomachaches, children should take their medication with food, and you should look at whether the child is taking the lowest effective dose they can and whether anxiety may be involved. Similarly, children with headaches should take stimulants with food, and you should look at the dosage and ask whether the patient is getting adequate sleep.
Strategies to address difficulty falling asleep can include taking the stimulant earlier in the day or switching to a shorter-acting form, dexmethylphenidate, or another stimulant. If they’re having trouble staying asleep, inquire about sleep hygiene, and look for associations with other factors that might explain why the child is experiencing new problems with staying asleep. If these strategies are unsuccessful, you can consider prescribing melatonin or clonidine.
Alternatives to stimulants
Several medications besides stimulants are available to prescribe to children with ADHD if they aren’t responding adequately to stimulants, Dr. Schonwald said.
Atomoxetine performed better than placebo in treatment studies, with similar weight loss effects, albeit the lowest mean effect size in clinician ratings (Lancet Psychiatry. 2018 Sep;5[9]:727-38). Dr. Schonwald recommended starting atomoxetine in children under 40 kg at 0.5 mg/kg for 4 days, then increasing to 1.2 mg/kg/day. For children over 40 kg, the dose can start at 40 mg. Maximum dose can range from 1.4 to 1.8 mg/kg or 100 mg/day.
About 7% of white children and 2% of African American children are poor metabolizers of atomoxetine, and the drug has interactions with dextromethorphan, fluoxetine, and paroxetine, she noted. Side effects can include abdominal pain, dry mouth, fatigue, mood swings, nausea, and vomiting.
Two alpha-adrenergics that you can consider are clonidine and guanfacine. Clonidine, a hypotensive drug given at a dose of 0.05-0.2 mg up to three times a day, is helpful for hyperactivity and impulsivity rather than attention difficulties. Side effects can include depression, headache, rebound hypertension, and sedation, and it’s only FDA approved for ages 12 years and older.
An extended release version of clonidine (Kapvay) is approved for monotherapy or adjunctive therapy for ADHD; it led to improvements in ADHD–Rating Scale-IV scores as soon as the second week in an 8-week randomized controlled trial. Mild to moderate somnolence was the most common adverse event, and changes on electrocardiograms were minor (J Am Acad Child Adolesc Psychiatry. 2011 Feb;50[2]:171-9).
Guanfacine, also a hypotensive drug, given at a dose of 0.5-2 mg up to three times a day, has fewer data about its use for ADHD but appears to treat attention problems more effectively than hyperactivity. Also approved only for ages 12 years and older, guanfacine is less sedating, and its side effects can include agitation, headache , and insomnia. An extended-release version of guanfacine (brand name Intuniv) showed statistically significant reductions in ADHD Rating Scale-IV scores in a 9-week, double-blind, randomized, controlled trial. Side effects including fatigue, sedation, and somnolence occurred in the first 2 weeks but generally resolved, and participants returned to baseline during dose maintenance and tapering (J Am Acad Child Adolesc Psychiatry. 2009 Feb;48[2]:155-65).
Intuniv doses should start at 1 mg/day and increase no more than 1 mg/week, Dr. Schonwald said, until reaching a maintenance dose of 1-4 mg once daily, depending on the patient’s clinical response and tolerability. Children also must be able to swallow the pill whole.
Treating preschoolers
Preschool children are particularly difficult to diagnose given their normal range of temperament and development, Dr. Schonwald said. Their symptoms could be resulting from another diagnosis or from circumstances in the environment.
You should consider potential comorbidities and whether the child’s symptoms are situational or pervasive. About 55% of preschoolers have at least one comorbidity, she said (Infants & Young Children. 2006 Apr-Jun;19[2]:109-122.)
That said, stimulants usually are effective in very young children whose primary concern is ADHD. In a randomized controlled trial of 303 preschoolers, significantly more children experienced reduced ADHD symptoms with methylphenidate than with placebo. The trial’s “data suggest that preschoolers with ADHD need to start with low methylphenidate doses. Treatment may best begin using methylphenidate–immediate release at 2.5 mg twice daily, and then be increased to 7.5 mg three times a day during the course of 1 week. The mean optimal total daily [methylphenidate] dose for preschoolers was 14.2 plus or minus 8.1 mg/day” (J Am Acad Child Adolesc Psychiatry. 2006 Nov;45[11]:1284-93).
In treating preschoolers, if the patient’s symptoms appear to get worse after starting a stimulant, you can consider a medication change. If symptoms are much worse, consider a lower dose or a different stimulant class, or whether the diagnosis is appropriate.
Five common components of poor behavior in preschoolers with ADHD include agitation, anxiety, explosively, hyperactivity, and impulsivity. If these issues are occurring throughout the day, consider reducing the dose or switching drug classes.
If it’s only occurring in the morning, Dr. Schonwald said, optimize the morning structure and consider giving the medication earlier in the morning or adding a short-acting booster. If it’s occurring in late afternoon, consider a booster and reducing high-demand activities for the child.
If a preschooler experiences some benefit from the stimulant but still has problems, adjunctive atomoxetine or an alpha adrenergic may help. Those medications also are recommended if the child has no benefit with the stimulant or cannot tolerate the lowest therapeutic dose.
Dr. Schonwald said she had no relevant financial disclosures.
NEW ORLEANS – A variety of reasons can contribute to the failure of stimulants to treat ADHD in children, such as comorbidities, missed diagnoses, inadequate medication dosage, side effects, major life changes, and other factors in the home or school environments, said Alison Schonwald, MD, of Harvard Medical School, Boston.
Stimulant medications indicated for ADHD usually work in 70%-75% of school-age children, but that leaves one in four children whose condition can be more challenging to treat, she said.
“Look around you,” Dr. Schonwald told a packed room at the annual meeting of the American Academy of Pediatrics. “You’re not the only one struggling with this topic.” She sprinkled her presentation with case studies of patients with ADHD for whom stimulants weren’t working, examples that the audience clearly found familiar.
The three steps you already know to do with treatment-resistant children sound simple: assess the child for factors linked to their poor response; develop a new treatment plan; and use Food and Drug Administration-approved nonstimulant medications, including off-label options, in a new plan.
But in the office, the process can be anything but simple when you must consider school and family environments, comorbidities, and other factors potentially complicating the child’s ability to function well.
Comorbidities
To start, Dr. Schonwald provided a chart of common coexisting problems in children with ADHD that included the recommended assessment and intervention:
- Mood and self-esteem issues call for the depression section of the patient health questionnaire (PHQ9) and Moods and Feelings questionnaire (MFQ), followed by interventions such as individual and peer group therapy and exercise.
- Anxiety can be assessed with the Screen for Child Anxiety Related Disorders (SCARED) and Spence Children’s Anxiety Scale, then treated similarly to mood and self-esteem issues.
- Bullying or trauma require taking a history during an interview, and treatment with individual and peer group therapy.
- Substance abuse should be assessed with the CRAFFT screening tool (Car, Relax, Alone, Forget, Friends, Trouble) and Screening to Brief Intervention (S2BI) Tool, then treated according to best practices.
- Executive function, low cognitive abilities, and poor adaptive skills require a review of the child’s Individualized Education Program (IEP) testing, followed by personalized school and home interventions.
- Poor social skills, assessed in an interview, also require personalized interventions at home and in school.
Doctors also may need to consider other common comorbidities in children with ADHD, such as bipolar disorder, depression, learning disabilities, oppositional defiant disorder, and tic disorders.
Tic disorders typically have an onset around 7 years old and peak in midadolescence, declining in late teen years. An estimated 35%-90% of children with Tourette syndrome have ADHD, Dr. Schonwald said (Dev Med Child Neurol. 2006 Jul;48[7]:616-21).
Managing treatment with stimulants
A common dosage amount for stimulants is 2.5-5 mg, but that dose may be too low for children, Dr. Schonwald said. She recommended increasing it until an effect is seen and stopping at the effective dose level the child can tolerate. The maximum recommended by the FDA is 60 mg/day for short-acting stimulants and 72 mg/day for extended-release ones, but some research has shown dosage can go even higher without causing toxic effects (J Child Adolesc Psychopharmacol. 2010 Feb;20[1]:49-54).
Dr. Schonwald also suggested trying both methylphenidate and amphetamine medication, while recognizing the latter tends to have more stimulant-related side effects.
Adherence is another consideration because multiple studies show high rates of noncompliance or discontinuation, such as up to 19% discontinuation for long-acting and 38% for short-acting stimulants (J Clin Psychiatry. 2015 Nov;76(11):e1459-68; Postgrad Med. 2012 May;124(3):139-48). A study of a school cohort in Philadelphia found only about one in five children were adherent (J Am Acad Child Adolesc Psychiatry. 2011 May;50[5]:480-9).
One potential solution to adherence challenges are pill reminder smartphone apps, such as Medisafe Medication Management, Pill Reminder-All in One, MyTherapy: Medication Reminder, and CareZone.
Dr. Schonwald noted several factors that can influence children’s response to stimulants. Among children with comorbid intellectual disability, for example, the response rate is lower than the average 75% of children without the disability, hovering around 40%-50% (Res Dev Disabil. 2018 Dec;83:217-32). Those who get more sleep tend to have improved attention, compared with children with less sleep (Atten Defic Hyperact Disord. 2017 Mar;9[1]:31-38).
She also offered strategies to manage problematic adverse effects from stimulants. Those experiencing weight loss can take their stimulant after breakfast, drink whole milk, and consider taking drug holidays.
To reduce stomachaches, children should take their medication with food, and you should look at whether the child is taking the lowest effective dose they can and whether anxiety may be involved. Similarly, children with headaches should take stimulants with food, and you should look at the dosage and ask whether the patient is getting adequate sleep.
Strategies to address difficulty falling asleep can include taking the stimulant earlier in the day or switching to a shorter-acting form, dexmethylphenidate, or another stimulant. If they’re having trouble staying asleep, inquire about sleep hygiene, and look for associations with other factors that might explain why the child is experiencing new problems with staying asleep. If these strategies are unsuccessful, you can consider prescribing melatonin or clonidine.
Alternatives to stimulants
Several medications besides stimulants are available to prescribe to children with ADHD if they aren’t responding adequately to stimulants, Dr. Schonwald said.
Atomoxetine performed better than placebo in treatment studies, with similar weight loss effects, albeit the lowest mean effect size in clinician ratings (Lancet Psychiatry. 2018 Sep;5[9]:727-38). Dr. Schonwald recommended starting atomoxetine in children under 40 kg at 0.5 mg/kg for 4 days, then increasing to 1.2 mg/kg/day. For children over 40 kg, the dose can start at 40 mg. Maximum dose can range from 1.4 to 1.8 mg/kg or 100 mg/day.
About 7% of white children and 2% of African American children are poor metabolizers of atomoxetine, and the drug has interactions with dextromethorphan, fluoxetine, and paroxetine, she noted. Side effects can include abdominal pain, dry mouth, fatigue, mood swings, nausea, and vomiting.
Two alpha-adrenergics that you can consider are clonidine and guanfacine. Clonidine, a hypotensive drug given at a dose of 0.05-0.2 mg up to three times a day, is helpful for hyperactivity and impulsivity rather than attention difficulties. Side effects can include depression, headache, rebound hypertension, and sedation, and it’s only FDA approved for ages 12 years and older.
An extended release version of clonidine (Kapvay) is approved for monotherapy or adjunctive therapy for ADHD; it led to improvements in ADHD–Rating Scale-IV scores as soon as the second week in an 8-week randomized controlled trial. Mild to moderate somnolence was the most common adverse event, and changes on electrocardiograms were minor (J Am Acad Child Adolesc Psychiatry. 2011 Feb;50[2]:171-9).
Guanfacine, also a hypotensive drug, given at a dose of 0.5-2 mg up to three times a day, has fewer data about its use for ADHD but appears to treat attention problems more effectively than hyperactivity. Also approved only for ages 12 years and older, guanfacine is less sedating, and its side effects can include agitation, headache , and insomnia. An extended-release version of guanfacine (brand name Intuniv) showed statistically significant reductions in ADHD Rating Scale-IV scores in a 9-week, double-blind, randomized, controlled trial. Side effects including fatigue, sedation, and somnolence occurred in the first 2 weeks but generally resolved, and participants returned to baseline during dose maintenance and tapering (J Am Acad Child Adolesc Psychiatry. 2009 Feb;48[2]:155-65).
Intuniv doses should start at 1 mg/day and increase no more than 1 mg/week, Dr. Schonwald said, until reaching a maintenance dose of 1-4 mg once daily, depending on the patient’s clinical response and tolerability. Children also must be able to swallow the pill whole.
Treating preschoolers
Preschool children are particularly difficult to diagnose given their normal range of temperament and development, Dr. Schonwald said. Their symptoms could be resulting from another diagnosis or from circumstances in the environment.
You should consider potential comorbidities and whether the child’s symptoms are situational or pervasive. About 55% of preschoolers have at least one comorbidity, she said (Infants & Young Children. 2006 Apr-Jun;19[2]:109-122.)
That said, stimulants usually are effective in very young children whose primary concern is ADHD. In a randomized controlled trial of 303 preschoolers, significantly more children experienced reduced ADHD symptoms with methylphenidate than with placebo. The trial’s “data suggest that preschoolers with ADHD need to start with low methylphenidate doses. Treatment may best begin using methylphenidate–immediate release at 2.5 mg twice daily, and then be increased to 7.5 mg three times a day during the course of 1 week. The mean optimal total daily [methylphenidate] dose for preschoolers was 14.2 plus or minus 8.1 mg/day” (J Am Acad Child Adolesc Psychiatry. 2006 Nov;45[11]:1284-93).
In treating preschoolers, if the patient’s symptoms appear to get worse after starting a stimulant, you can consider a medication change. If symptoms are much worse, consider a lower dose or a different stimulant class, or whether the diagnosis is appropriate.
Five common components of poor behavior in preschoolers with ADHD include agitation, anxiety, explosively, hyperactivity, and impulsivity. If these issues are occurring throughout the day, consider reducing the dose or switching drug classes.
If it’s only occurring in the morning, Dr. Schonwald said, optimize the morning structure and consider giving the medication earlier in the morning or adding a short-acting booster. If it’s occurring in late afternoon, consider a booster and reducing high-demand activities for the child.
If a preschooler experiences some benefit from the stimulant but still has problems, adjunctive atomoxetine or an alpha adrenergic may help. Those medications also are recommended if the child has no benefit with the stimulant or cannot tolerate the lowest therapeutic dose.
Dr. Schonwald said she had no relevant financial disclosures.
NEW ORLEANS – A variety of reasons can contribute to the failure of stimulants to treat ADHD in children, such as comorbidities, missed diagnoses, inadequate medication dosage, side effects, major life changes, and other factors in the home or school environments, said Alison Schonwald, MD, of Harvard Medical School, Boston.
Stimulant medications indicated for ADHD usually work in 70%-75% of school-age children, but that leaves one in four children whose condition can be more challenging to treat, she said.
“Look around you,” Dr. Schonwald told a packed room at the annual meeting of the American Academy of Pediatrics. “You’re not the only one struggling with this topic.” She sprinkled her presentation with case studies of patients with ADHD for whom stimulants weren’t working, examples that the audience clearly found familiar.
The three steps you already know to do with treatment-resistant children sound simple: assess the child for factors linked to their poor response; develop a new treatment plan; and use Food and Drug Administration-approved nonstimulant medications, including off-label options, in a new plan.
But in the office, the process can be anything but simple when you must consider school and family environments, comorbidities, and other factors potentially complicating the child’s ability to function well.
Comorbidities
To start, Dr. Schonwald provided a chart of common coexisting problems in children with ADHD that included the recommended assessment and intervention:
- Mood and self-esteem issues call for the depression section of the patient health questionnaire (PHQ9) and Moods and Feelings questionnaire (MFQ), followed by interventions such as individual and peer group therapy and exercise.
- Anxiety can be assessed with the Screen for Child Anxiety Related Disorders (SCARED) and Spence Children’s Anxiety Scale, then treated similarly to mood and self-esteem issues.
- Bullying or trauma require taking a history during an interview, and treatment with individual and peer group therapy.
- Substance abuse should be assessed with the CRAFFT screening tool (Car, Relax, Alone, Forget, Friends, Trouble) and Screening to Brief Intervention (S2BI) Tool, then treated according to best practices.
- Executive function, low cognitive abilities, and poor adaptive skills require a review of the child’s Individualized Education Program (IEP) testing, followed by personalized school and home interventions.
- Poor social skills, assessed in an interview, also require personalized interventions at home and in school.
Doctors also may need to consider other common comorbidities in children with ADHD, such as bipolar disorder, depression, learning disabilities, oppositional defiant disorder, and tic disorders.
Tic disorders typically have an onset around 7 years old and peak in midadolescence, declining in late teen years. An estimated 35%-90% of children with Tourette syndrome have ADHD, Dr. Schonwald said (Dev Med Child Neurol. 2006 Jul;48[7]:616-21).
Managing treatment with stimulants
A common dosage amount for stimulants is 2.5-5 mg, but that dose may be too low for children, Dr. Schonwald said. She recommended increasing it until an effect is seen and stopping at the effective dose level the child can tolerate. The maximum recommended by the FDA is 60 mg/day for short-acting stimulants and 72 mg/day for extended-release ones, but some research has shown dosage can go even higher without causing toxic effects (J Child Adolesc Psychopharmacol. 2010 Feb;20[1]:49-54).
Dr. Schonwald also suggested trying both methylphenidate and amphetamine medication, while recognizing the latter tends to have more stimulant-related side effects.
Adherence is another consideration because multiple studies show high rates of noncompliance or discontinuation, such as up to 19% discontinuation for long-acting and 38% for short-acting stimulants (J Clin Psychiatry. 2015 Nov;76(11):e1459-68; Postgrad Med. 2012 May;124(3):139-48). A study of a school cohort in Philadelphia found only about one in five children were adherent (J Am Acad Child Adolesc Psychiatry. 2011 May;50[5]:480-9).
One potential solution to adherence challenges are pill reminder smartphone apps, such as Medisafe Medication Management, Pill Reminder-All in One, MyTherapy: Medication Reminder, and CareZone.
Dr. Schonwald noted several factors that can influence children’s response to stimulants. Among children with comorbid intellectual disability, for example, the response rate is lower than the average 75% of children without the disability, hovering around 40%-50% (Res Dev Disabil. 2018 Dec;83:217-32). Those who get more sleep tend to have improved attention, compared with children with less sleep (Atten Defic Hyperact Disord. 2017 Mar;9[1]:31-38).
She also offered strategies to manage problematic adverse effects from stimulants. Those experiencing weight loss can take their stimulant after breakfast, drink whole milk, and consider taking drug holidays.
To reduce stomachaches, children should take their medication with food, and you should look at whether the child is taking the lowest effective dose they can and whether anxiety may be involved. Similarly, children with headaches should take stimulants with food, and you should look at the dosage and ask whether the patient is getting adequate sleep.
Strategies to address difficulty falling asleep can include taking the stimulant earlier in the day or switching to a shorter-acting form, dexmethylphenidate, or another stimulant. If they’re having trouble staying asleep, inquire about sleep hygiene, and look for associations with other factors that might explain why the child is experiencing new problems with staying asleep. If these strategies are unsuccessful, you can consider prescribing melatonin or clonidine.
Alternatives to stimulants
Several medications besides stimulants are available to prescribe to children with ADHD if they aren’t responding adequately to stimulants, Dr. Schonwald said.
Atomoxetine performed better than placebo in treatment studies, with similar weight loss effects, albeit the lowest mean effect size in clinician ratings (Lancet Psychiatry. 2018 Sep;5[9]:727-38). Dr. Schonwald recommended starting atomoxetine in children under 40 kg at 0.5 mg/kg for 4 days, then increasing to 1.2 mg/kg/day. For children over 40 kg, the dose can start at 40 mg. Maximum dose can range from 1.4 to 1.8 mg/kg or 100 mg/day.
About 7% of white children and 2% of African American children are poor metabolizers of atomoxetine, and the drug has interactions with dextromethorphan, fluoxetine, and paroxetine, she noted. Side effects can include abdominal pain, dry mouth, fatigue, mood swings, nausea, and vomiting.
Two alpha-adrenergics that you can consider are clonidine and guanfacine. Clonidine, a hypotensive drug given at a dose of 0.05-0.2 mg up to three times a day, is helpful for hyperactivity and impulsivity rather than attention difficulties. Side effects can include depression, headache, rebound hypertension, and sedation, and it’s only FDA approved for ages 12 years and older.
An extended release version of clonidine (Kapvay) is approved for monotherapy or adjunctive therapy for ADHD; it led to improvements in ADHD–Rating Scale-IV scores as soon as the second week in an 8-week randomized controlled trial. Mild to moderate somnolence was the most common adverse event, and changes on electrocardiograms were minor (J Am Acad Child Adolesc Psychiatry. 2011 Feb;50[2]:171-9).
Guanfacine, also a hypotensive drug, given at a dose of 0.5-2 mg up to three times a day, has fewer data about its use for ADHD but appears to treat attention problems more effectively than hyperactivity. Also approved only for ages 12 years and older, guanfacine is less sedating, and its side effects can include agitation, headache , and insomnia. An extended-release version of guanfacine (brand name Intuniv) showed statistically significant reductions in ADHD Rating Scale-IV scores in a 9-week, double-blind, randomized, controlled trial. Side effects including fatigue, sedation, and somnolence occurred in the first 2 weeks but generally resolved, and participants returned to baseline during dose maintenance and tapering (J Am Acad Child Adolesc Psychiatry. 2009 Feb;48[2]:155-65).
Intuniv doses should start at 1 mg/day and increase no more than 1 mg/week, Dr. Schonwald said, until reaching a maintenance dose of 1-4 mg once daily, depending on the patient’s clinical response and tolerability. Children also must be able to swallow the pill whole.
Treating preschoolers
Preschool children are particularly difficult to diagnose given their normal range of temperament and development, Dr. Schonwald said. Their symptoms could be resulting from another diagnosis or from circumstances in the environment.
You should consider potential comorbidities and whether the child’s symptoms are situational or pervasive. About 55% of preschoolers have at least one comorbidity, she said (Infants & Young Children. 2006 Apr-Jun;19[2]:109-122.)
That said, stimulants usually are effective in very young children whose primary concern is ADHD. In a randomized controlled trial of 303 preschoolers, significantly more children experienced reduced ADHD symptoms with methylphenidate than with placebo. The trial’s “data suggest that preschoolers with ADHD need to start with low methylphenidate doses. Treatment may best begin using methylphenidate–immediate release at 2.5 mg twice daily, and then be increased to 7.5 mg three times a day during the course of 1 week. The mean optimal total daily [methylphenidate] dose for preschoolers was 14.2 plus or minus 8.1 mg/day” (J Am Acad Child Adolesc Psychiatry. 2006 Nov;45[11]:1284-93).
In treating preschoolers, if the patient’s symptoms appear to get worse after starting a stimulant, you can consider a medication change. If symptoms are much worse, consider a lower dose or a different stimulant class, or whether the diagnosis is appropriate.
Five common components of poor behavior in preschoolers with ADHD include agitation, anxiety, explosively, hyperactivity, and impulsivity. If these issues are occurring throughout the day, consider reducing the dose or switching drug classes.
If it’s only occurring in the morning, Dr. Schonwald said, optimize the morning structure and consider giving the medication earlier in the morning or adding a short-acting booster. If it’s occurring in late afternoon, consider a booster and reducing high-demand activities for the child.
If a preschooler experiences some benefit from the stimulant but still has problems, adjunctive atomoxetine or an alpha adrenergic may help. Those medications also are recommended if the child has no benefit with the stimulant or cannot tolerate the lowest therapeutic dose.
Dr. Schonwald said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 2019
Delaying flu vaccine didn’t drop fever rate for childhood immunizations
according to a randomized trial.
An increased risk for febrile seizures had been seen when the three vaccines were administered together, wrote Emmanuel B. Walter, MD, MPH, and coauthors, so they constructed a trial that compared a simultaneous administration strategy that delayed inactivated influenza vaccine (IIV) administration by about 2 weeks.
In all, 221 children aged 12-16 months were enrolled in the randomized study. A total of 110 children received quadrivalent IIV (IIV4), DTaP, and 13-valent pneumococcal conjugate vaccine (PCV13) simultaneously and returned for a dental health education visit 2 weeks later. For 111 children, DTaP and PCV13 were administered at study visit 1, and IIV4 was given along with dental health education 2 weeks later. Most children in both groups also received at least one nonstudy vaccine at the first study visit. Eleven children in the simultaneous group and four in the sequential group didn’t complete the study.
There was no difference between study groups in the combined rates of fever on the first 2 days after study visits 1 and 2 taken together: 8% of children in the simultaneous group and 9% of those in the sequential group had fever of 38° C or higher (adjusted relative risk, 0.87; 95% confidence interval, 0.36-2.10).
However, children in the simultaneous group were more likely to receive antipyretic medication in the first 2 days after visit 1 (37% versus 22%; P = .020), reported Dr. Walter, professor of pediatrics at Duke University, Durham, N.C., and coauthors. Because it’s rare for febrile seizures to occur after immunization, the authors didn’t make the occurrence of febrile seizure a primary or secondary endpoint of the study; no seizures occurred in study participants. They did hypothesize that the total proportion of children having fever would be higher in the simultaneous than in the sequential group – a hypothesis not supported by the study findings.
Children were excluded, or their study vaccinations were delayed, if they had received antipyretic medication within the 72 hours preceding the visit or at the study visit, or if they had a temperature of 38° C or more.
Parents monitored participants’ temperatures for 8 days after visits by using a study-provided temporal thermometer once daily at about the same time, and also by checking the temperature if their child felt feverish. Parents also recorded any antipyretic use, medical care, other symptoms, and febrile seizures.
The study was stopped earlier than anticipated because unexpectedly high levels of influenza activity made it unethical to delay influenza immunization, explained Dr. Walter and coauthors.
Participants were a median 15 months old; most were non-Hispanic white and had private insurance. Most participants didn’t attend day care.
“Nearly all fever episodes and days of fever on days 1-2 after the study visits occurred after visit 1,” reported Dr. Walter and coinvestigators. They saw no difference between groups in the proportion of children who had a fever of 38.6° C on days 1-2 after either study visit.
The mean peak temperature – about 38.5° C – on combined study visits 1 and 2 didn’t differ between groups. Similarly, for those participants who had a fever, the mean postvisit fever duration of 1.3 days was identical between groups.
Parents also were asked about their perceptions of the vaccination schedule their children received. Over half of parents overall (56%) reported that they disliked having to bring their child in for two separate clinic visits, with more parents in the sequential group than the simultaneous group reporting this (65% versus 48%).
Generalizability of the findings and comparison with previous studies are limited, noted Dr. Walter and coinvestigators, because the composition of influenza vaccine varies from year to year. No signal for seizures was seen in the Vaccine Safety Datalink after IIV during the 2017-2018 influenza season, wrote the investigators. The 2010-2011 influenza season’s IIV formulation was associated with increased febrile seizure risk, indicating that the IIV formulation for that year may have been more pyrogenic than the 2017-2018 formulation.
Also, children deemed at higher risk of febrile seizure were excluded from the study, so findings may have limited applicability to these children. The lack of parental blinding also may have influenced antipyretic administration or other symptom reporting, although objective temperature measurement should not have been affected by the lack of blinding, wrote Dr. Walker and collaborators.
The study was funded by the Centers for Disease Control and Prevention. One coauthor reported potential conflicts of interest from financial support received from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Protein Science, Dynavax, and Medimmune. The remaining authors have no relevant financial disclosures.
SOURCE: Walter EB et al. Pediatrics. 2020;145(3):e20191909.
according to a randomized trial.
An increased risk for febrile seizures had been seen when the three vaccines were administered together, wrote Emmanuel B. Walter, MD, MPH, and coauthors, so they constructed a trial that compared a simultaneous administration strategy that delayed inactivated influenza vaccine (IIV) administration by about 2 weeks.
In all, 221 children aged 12-16 months were enrolled in the randomized study. A total of 110 children received quadrivalent IIV (IIV4), DTaP, and 13-valent pneumococcal conjugate vaccine (PCV13) simultaneously and returned for a dental health education visit 2 weeks later. For 111 children, DTaP and PCV13 were administered at study visit 1, and IIV4 was given along with dental health education 2 weeks later. Most children in both groups also received at least one nonstudy vaccine at the first study visit. Eleven children in the simultaneous group and four in the sequential group didn’t complete the study.
There was no difference between study groups in the combined rates of fever on the first 2 days after study visits 1 and 2 taken together: 8% of children in the simultaneous group and 9% of those in the sequential group had fever of 38° C or higher (adjusted relative risk, 0.87; 95% confidence interval, 0.36-2.10).
However, children in the simultaneous group were more likely to receive antipyretic medication in the first 2 days after visit 1 (37% versus 22%; P = .020), reported Dr. Walter, professor of pediatrics at Duke University, Durham, N.C., and coauthors. Because it’s rare for febrile seizures to occur after immunization, the authors didn’t make the occurrence of febrile seizure a primary or secondary endpoint of the study; no seizures occurred in study participants. They did hypothesize that the total proportion of children having fever would be higher in the simultaneous than in the sequential group – a hypothesis not supported by the study findings.
Children were excluded, or their study vaccinations were delayed, if they had received antipyretic medication within the 72 hours preceding the visit or at the study visit, or if they had a temperature of 38° C or more.
Parents monitored participants’ temperatures for 8 days after visits by using a study-provided temporal thermometer once daily at about the same time, and also by checking the temperature if their child felt feverish. Parents also recorded any antipyretic use, medical care, other symptoms, and febrile seizures.
The study was stopped earlier than anticipated because unexpectedly high levels of influenza activity made it unethical to delay influenza immunization, explained Dr. Walter and coauthors.
Participants were a median 15 months old; most were non-Hispanic white and had private insurance. Most participants didn’t attend day care.
“Nearly all fever episodes and days of fever on days 1-2 after the study visits occurred after visit 1,” reported Dr. Walter and coinvestigators. They saw no difference between groups in the proportion of children who had a fever of 38.6° C on days 1-2 after either study visit.
The mean peak temperature – about 38.5° C – on combined study visits 1 and 2 didn’t differ between groups. Similarly, for those participants who had a fever, the mean postvisit fever duration of 1.3 days was identical between groups.
Parents also were asked about their perceptions of the vaccination schedule their children received. Over half of parents overall (56%) reported that they disliked having to bring their child in for two separate clinic visits, with more parents in the sequential group than the simultaneous group reporting this (65% versus 48%).
Generalizability of the findings and comparison with previous studies are limited, noted Dr. Walter and coinvestigators, because the composition of influenza vaccine varies from year to year. No signal for seizures was seen in the Vaccine Safety Datalink after IIV during the 2017-2018 influenza season, wrote the investigators. The 2010-2011 influenza season’s IIV formulation was associated with increased febrile seizure risk, indicating that the IIV formulation for that year may have been more pyrogenic than the 2017-2018 formulation.
Also, children deemed at higher risk of febrile seizure were excluded from the study, so findings may have limited applicability to these children. The lack of parental blinding also may have influenced antipyretic administration or other symptom reporting, although objective temperature measurement should not have been affected by the lack of blinding, wrote Dr. Walker and collaborators.
The study was funded by the Centers for Disease Control and Prevention. One coauthor reported potential conflicts of interest from financial support received from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Protein Science, Dynavax, and Medimmune. The remaining authors have no relevant financial disclosures.
SOURCE: Walter EB et al. Pediatrics. 2020;145(3):e20191909.
according to a randomized trial.
An increased risk for febrile seizures had been seen when the three vaccines were administered together, wrote Emmanuel B. Walter, MD, MPH, and coauthors, so they constructed a trial that compared a simultaneous administration strategy that delayed inactivated influenza vaccine (IIV) administration by about 2 weeks.
In all, 221 children aged 12-16 months were enrolled in the randomized study. A total of 110 children received quadrivalent IIV (IIV4), DTaP, and 13-valent pneumococcal conjugate vaccine (PCV13) simultaneously and returned for a dental health education visit 2 weeks later. For 111 children, DTaP and PCV13 were administered at study visit 1, and IIV4 was given along with dental health education 2 weeks later. Most children in both groups also received at least one nonstudy vaccine at the first study visit. Eleven children in the simultaneous group and four in the sequential group didn’t complete the study.
There was no difference between study groups in the combined rates of fever on the first 2 days after study visits 1 and 2 taken together: 8% of children in the simultaneous group and 9% of those in the sequential group had fever of 38° C or higher (adjusted relative risk, 0.87; 95% confidence interval, 0.36-2.10).
However, children in the simultaneous group were more likely to receive antipyretic medication in the first 2 days after visit 1 (37% versus 22%; P = .020), reported Dr. Walter, professor of pediatrics at Duke University, Durham, N.C., and coauthors. Because it’s rare for febrile seizures to occur after immunization, the authors didn’t make the occurrence of febrile seizure a primary or secondary endpoint of the study; no seizures occurred in study participants. They did hypothesize that the total proportion of children having fever would be higher in the simultaneous than in the sequential group – a hypothesis not supported by the study findings.
Children were excluded, or their study vaccinations were delayed, if they had received antipyretic medication within the 72 hours preceding the visit or at the study visit, or if they had a temperature of 38° C or more.
Parents monitored participants’ temperatures for 8 days after visits by using a study-provided temporal thermometer once daily at about the same time, and also by checking the temperature if their child felt feverish. Parents also recorded any antipyretic use, medical care, other symptoms, and febrile seizures.
The study was stopped earlier than anticipated because unexpectedly high levels of influenza activity made it unethical to delay influenza immunization, explained Dr. Walter and coauthors.
Participants were a median 15 months old; most were non-Hispanic white and had private insurance. Most participants didn’t attend day care.
“Nearly all fever episodes and days of fever on days 1-2 after the study visits occurred after visit 1,” reported Dr. Walter and coinvestigators. They saw no difference between groups in the proportion of children who had a fever of 38.6° C on days 1-2 after either study visit.
The mean peak temperature – about 38.5° C – on combined study visits 1 and 2 didn’t differ between groups. Similarly, for those participants who had a fever, the mean postvisit fever duration of 1.3 days was identical between groups.
Parents also were asked about their perceptions of the vaccination schedule their children received. Over half of parents overall (56%) reported that they disliked having to bring their child in for two separate clinic visits, with more parents in the sequential group than the simultaneous group reporting this (65% versus 48%).
Generalizability of the findings and comparison with previous studies are limited, noted Dr. Walter and coinvestigators, because the composition of influenza vaccine varies from year to year. No signal for seizures was seen in the Vaccine Safety Datalink after IIV during the 2017-2018 influenza season, wrote the investigators. The 2010-2011 influenza season’s IIV formulation was associated with increased febrile seizure risk, indicating that the IIV formulation for that year may have been more pyrogenic than the 2017-2018 formulation.
Also, children deemed at higher risk of febrile seizure were excluded from the study, so findings may have limited applicability to these children. The lack of parental blinding also may have influenced antipyretic administration or other symptom reporting, although objective temperature measurement should not have been affected by the lack of blinding, wrote Dr. Walker and collaborators.
The study was funded by the Centers for Disease Control and Prevention. One coauthor reported potential conflicts of interest from financial support received from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Protein Science, Dynavax, and Medimmune. The remaining authors have no relevant financial disclosures.
SOURCE: Walter EB et al. Pediatrics. 2020;145(3):e20191909.
FROM PEDIATRICS
Key clinical point: Fevers were no less common when influenza vaccine was delayed for children receiving DTaP and pneumococcal vaccinations.
Major finding: There was no difference between study groups in the combined rates of fever on the first 2 days after study visits 1 and 2 taken together: 8% of children in the simultaneous group and 9% of those in the sequential group had fever of 38° C or higher (adjusted relative risk, 0.87).
Study details: Randomized, nonblinded trial of 221 children aged 12-16 months receiving scheduled vaccinations.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. One coauthor reported financial support received from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Protein Science, Dynavax, and Medimmune.
Source: Walter EB et al. Pediatrics. 2020;145(3):e20191909.
Losartan showing promise in pediatric epidermolysis bullosa trial
LONDON – Treatment with the in an early clinical study.
In the ongoing phase 1/2 REFLECT (Recessive dystrophic EB: Mechanisms of fibrosis and its prevention with Losartan in vivo) trial, involving 29 children, no severe complications have been noted so far, according to one of the study investigators, Dimitra Kiritsi, MD, of the University of Freiburg, Germany. At the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), she presented interim data on 18 patients in the trial, emphasizing that the primary aim of the trial was to evaluate the safety of this treatment approach.
Over the 2 years the trial has been underway, 65 adverse events have been reported, of which 4 have been severe. Two of these were bacterial infections that required hospital treatment and the other two were a reduction in the general health condition of the child.
Losartan is an angiotensin-II receptor blocker (ARB) that has been in clinical use for more than 25 years in adults and 15 years in children over the age of 6 years.
The drug may be used for treating recessive dystrophic EB (RDEB) in the future, Dr. Kiritsi said, because it attenuates tumor necrosis factor–beta (TGF-beta) signaling, which is thought to be involved in the fibrotic process. So while it may not target the genetic defect, it could help ameliorate the effects of the disease.
The precursor to REFLECT was a study performed in a mouse disease model of EB (EMBO Mol Med. 2015;7:1211-28) where a reduction in fibrotic scarring was seen with losartan with “remarkable effects” on “mitten” deformity, Dr. Kiritsi said. The results of that study suggested that the earlier treatment with losartan was started in the course of the disease, the better the effect, she added. (Mitten deformity is the result of fused skin between the fingers or toes, and the subsequent buildup of fibrotic tissue causes the hand or foot to contract.)
REFLECT is an investigator-initiated trial that started in 2017 and is being funded by DEBRA International. It is a dual-center, nonrandomized, single-arm study in which children aged 3-16 years with RDEB are treated with losartan for 10 months, with follow-up at 3 months.
Various secondary endpoints were included to look for the first signs of any efficacy: the Physician’s Global Assessment (PGA), the Birmingham Epidermolysis Bullosa Severity Score (BEBS), the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), the Itch Assessment Scale for the Pediatric Burn Patients, and two quality of life indices: the Quality of Life in EB (QOLEB) questionnaire and the Children’s Dermatology Life Quality Index (CDLQI).
Dr. Kiritsi highlighted a few of the secondary endpoint findings, saying that reduced BEBS scores showed there was “amelioration of the patients’ phenotype” and that EBDASI scores also decreased, with “nearly 60% of the patients having significant improvement of their skin disease.” Importantly, itch improved in most of the patients, she said. Reductions in CDLQI were observed, “meaning that quality of life was significantly better at the end of the trial.” There were also decreases in inflammatory markers, such as C-reactive protein, interleukin-6, and TNF-alpha.
Although there is no validated tool available to assess hand function, Dr. Kiritsi and her team used their own morphometric scoring instrument to measure how far the hand could stretch; their evaluations suggested that this measure improved – or at least did not worsen – with losartan treatment, she noted.
A larger, randomized trial is needed to confirm if there is any benefit of losartan, but first, a new, easy-to-swallow losartan formulation needs to be developed specifically for EB in the pediatric population, Dr. Kiritsi said. Although a pediatric suspension of losartan was previously available, it is no longer on the market, so the next step is to develop a formulation that could be used in a pivotal clinical trial, she noted.
“Losartan faces fewer technical hurdles compared to other novel treatments as it is an established medicine,” Dr. Kiritsi and associates observed in a poster presentation. There are still economic hurdles, however, since “with losartan patents expired, companies cannot expect to recoup an investment into clinical studies” and alternative funding sources are needed.
In 2019, losartan was granted an orphan drug designation for the treatment of EB from both the Food and Drug Administration and the European Medicines Agency, but its use remains off label in children. “We decided to treat children,” Dr. Kiritsi said, “because we wanted to start as early as possible. If you already have mitten deformities, these cannot be reversed.”
DEBRA International funded the study. Dr. Kiritsi received research support from Rheacell GmbH and honoraria or consultation fees from Amryt Pharma and Rheacell GmbH. She has received other support from DEBRA International, EB Research Partnership, Fritz Thyssen Stiftung, German Research Foundation (funding of research projects), and 3R Pharma Consulting and Midas Pharma GmbH (consultation for losartan new drug formulation).
SOURCE: Kiritsi D et al. EB 2020. Poster 47.
LONDON – Treatment with the in an early clinical study.
In the ongoing phase 1/2 REFLECT (Recessive dystrophic EB: Mechanisms of fibrosis and its prevention with Losartan in vivo) trial, involving 29 children, no severe complications have been noted so far, according to one of the study investigators, Dimitra Kiritsi, MD, of the University of Freiburg, Germany. At the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), she presented interim data on 18 patients in the trial, emphasizing that the primary aim of the trial was to evaluate the safety of this treatment approach.
Over the 2 years the trial has been underway, 65 adverse events have been reported, of which 4 have been severe. Two of these were bacterial infections that required hospital treatment and the other two were a reduction in the general health condition of the child.
Losartan is an angiotensin-II receptor blocker (ARB) that has been in clinical use for more than 25 years in adults and 15 years in children over the age of 6 years.
The drug may be used for treating recessive dystrophic EB (RDEB) in the future, Dr. Kiritsi said, because it attenuates tumor necrosis factor–beta (TGF-beta) signaling, which is thought to be involved in the fibrotic process. So while it may not target the genetic defect, it could help ameliorate the effects of the disease.
The precursor to REFLECT was a study performed in a mouse disease model of EB (EMBO Mol Med. 2015;7:1211-28) where a reduction in fibrotic scarring was seen with losartan with “remarkable effects” on “mitten” deformity, Dr. Kiritsi said. The results of that study suggested that the earlier treatment with losartan was started in the course of the disease, the better the effect, she added. (Mitten deformity is the result of fused skin between the fingers or toes, and the subsequent buildup of fibrotic tissue causes the hand or foot to contract.)
REFLECT is an investigator-initiated trial that started in 2017 and is being funded by DEBRA International. It is a dual-center, nonrandomized, single-arm study in which children aged 3-16 years with RDEB are treated with losartan for 10 months, with follow-up at 3 months.
Various secondary endpoints were included to look for the first signs of any efficacy: the Physician’s Global Assessment (PGA), the Birmingham Epidermolysis Bullosa Severity Score (BEBS), the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), the Itch Assessment Scale for the Pediatric Burn Patients, and two quality of life indices: the Quality of Life in EB (QOLEB) questionnaire and the Children’s Dermatology Life Quality Index (CDLQI).
Dr. Kiritsi highlighted a few of the secondary endpoint findings, saying that reduced BEBS scores showed there was “amelioration of the patients’ phenotype” and that EBDASI scores also decreased, with “nearly 60% of the patients having significant improvement of their skin disease.” Importantly, itch improved in most of the patients, she said. Reductions in CDLQI were observed, “meaning that quality of life was significantly better at the end of the trial.” There were also decreases in inflammatory markers, such as C-reactive protein, interleukin-6, and TNF-alpha.
Although there is no validated tool available to assess hand function, Dr. Kiritsi and her team used their own morphometric scoring instrument to measure how far the hand could stretch; their evaluations suggested that this measure improved – or at least did not worsen – with losartan treatment, she noted.
A larger, randomized trial is needed to confirm if there is any benefit of losartan, but first, a new, easy-to-swallow losartan formulation needs to be developed specifically for EB in the pediatric population, Dr. Kiritsi said. Although a pediatric suspension of losartan was previously available, it is no longer on the market, so the next step is to develop a formulation that could be used in a pivotal clinical trial, she noted.
“Losartan faces fewer technical hurdles compared to other novel treatments as it is an established medicine,” Dr. Kiritsi and associates observed in a poster presentation. There are still economic hurdles, however, since “with losartan patents expired, companies cannot expect to recoup an investment into clinical studies” and alternative funding sources are needed.
In 2019, losartan was granted an orphan drug designation for the treatment of EB from both the Food and Drug Administration and the European Medicines Agency, but its use remains off label in children. “We decided to treat children,” Dr. Kiritsi said, “because we wanted to start as early as possible. If you already have mitten deformities, these cannot be reversed.”
DEBRA International funded the study. Dr. Kiritsi received research support from Rheacell GmbH and honoraria or consultation fees from Amryt Pharma and Rheacell GmbH. She has received other support from DEBRA International, EB Research Partnership, Fritz Thyssen Stiftung, German Research Foundation (funding of research projects), and 3R Pharma Consulting and Midas Pharma GmbH (consultation for losartan new drug formulation).
SOURCE: Kiritsi D et al. EB 2020. Poster 47.
LONDON – Treatment with the in an early clinical study.
In the ongoing phase 1/2 REFLECT (Recessive dystrophic EB: Mechanisms of fibrosis and its prevention with Losartan in vivo) trial, involving 29 children, no severe complications have been noted so far, according to one of the study investigators, Dimitra Kiritsi, MD, of the University of Freiburg, Germany. At the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), she presented interim data on 18 patients in the trial, emphasizing that the primary aim of the trial was to evaluate the safety of this treatment approach.
Over the 2 years the trial has been underway, 65 adverse events have been reported, of which 4 have been severe. Two of these were bacterial infections that required hospital treatment and the other two were a reduction in the general health condition of the child.
Losartan is an angiotensin-II receptor blocker (ARB) that has been in clinical use for more than 25 years in adults and 15 years in children over the age of 6 years.
The drug may be used for treating recessive dystrophic EB (RDEB) in the future, Dr. Kiritsi said, because it attenuates tumor necrosis factor–beta (TGF-beta) signaling, which is thought to be involved in the fibrotic process. So while it may not target the genetic defect, it could help ameliorate the effects of the disease.
The precursor to REFLECT was a study performed in a mouse disease model of EB (EMBO Mol Med. 2015;7:1211-28) where a reduction in fibrotic scarring was seen with losartan with “remarkable effects” on “mitten” deformity, Dr. Kiritsi said. The results of that study suggested that the earlier treatment with losartan was started in the course of the disease, the better the effect, she added. (Mitten deformity is the result of fused skin between the fingers or toes, and the subsequent buildup of fibrotic tissue causes the hand or foot to contract.)
REFLECT is an investigator-initiated trial that started in 2017 and is being funded by DEBRA International. It is a dual-center, nonrandomized, single-arm study in which children aged 3-16 years with RDEB are treated with losartan for 10 months, with follow-up at 3 months.
Various secondary endpoints were included to look for the first signs of any efficacy: the Physician’s Global Assessment (PGA), the Birmingham Epidermolysis Bullosa Severity Score (BEBS), the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), the Itch Assessment Scale for the Pediatric Burn Patients, and two quality of life indices: the Quality of Life in EB (QOLEB) questionnaire and the Children’s Dermatology Life Quality Index (CDLQI).
Dr. Kiritsi highlighted a few of the secondary endpoint findings, saying that reduced BEBS scores showed there was “amelioration of the patients’ phenotype” and that EBDASI scores also decreased, with “nearly 60% of the patients having significant improvement of their skin disease.” Importantly, itch improved in most of the patients, she said. Reductions in CDLQI were observed, “meaning that quality of life was significantly better at the end of the trial.” There were also decreases in inflammatory markers, such as C-reactive protein, interleukin-6, and TNF-alpha.
Although there is no validated tool available to assess hand function, Dr. Kiritsi and her team used their own morphometric scoring instrument to measure how far the hand could stretch; their evaluations suggested that this measure improved – or at least did not worsen – with losartan treatment, she noted.
A larger, randomized trial is needed to confirm if there is any benefit of losartan, but first, a new, easy-to-swallow losartan formulation needs to be developed specifically for EB in the pediatric population, Dr. Kiritsi said. Although a pediatric suspension of losartan was previously available, it is no longer on the market, so the next step is to develop a formulation that could be used in a pivotal clinical trial, she noted.
“Losartan faces fewer technical hurdles compared to other novel treatments as it is an established medicine,” Dr. Kiritsi and associates observed in a poster presentation. There are still economic hurdles, however, since “with losartan patents expired, companies cannot expect to recoup an investment into clinical studies” and alternative funding sources are needed.
In 2019, losartan was granted an orphan drug designation for the treatment of EB from both the Food and Drug Administration and the European Medicines Agency, but its use remains off label in children. “We decided to treat children,” Dr. Kiritsi said, “because we wanted to start as early as possible. If you already have mitten deformities, these cannot be reversed.”
DEBRA International funded the study. Dr. Kiritsi received research support from Rheacell GmbH and honoraria or consultation fees from Amryt Pharma and Rheacell GmbH. She has received other support from DEBRA International, EB Research Partnership, Fritz Thyssen Stiftung, German Research Foundation (funding of research projects), and 3R Pharma Consulting and Midas Pharma GmbH (consultation for losartan new drug formulation).
SOURCE: Kiritsi D et al. EB 2020. Poster 47.
REPORTING FROM EB 2020
New Barbie lineup includes a doll with vitiligo
A new line of Barbie dolls unveiled by Mattel earlier this month includes one with vitiligo, much to the delight of clinicians who treat children and adolescents with the condition.
“When I see young children and adolescents with vitiligo, it is very common for me to feel their emotional suffering from their skin condition,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center in Dallas said in an interview. “Kids can be cruel. Name calling, social ostracizing, [and] effects on self-esteem are all things I have seen amongst my patients and their families in their own struggles with vitiligo.”
According to a brand communications representative from toymaker Mattel, which began manufacturing Barbie dolls in 1959, the company worked with a board-certified dermatologist to include a doll with vitiligo in its 2020 “Fashionistas” line. “As we continue to redefine what it means to be a ‘Barbie’ or look like Barbie, offering a doll with vitiligo in our main doll line allows kids to play out even more stories they see in the world around them,” the representative wrote in an email message. Other dolls debuting as part of the lineup include one with no hair, one with a darker skin tone that uses a gold prosthetic limb, and a Ken doll with long rooted hair (think Jeff Spicoli in “Fast Times at Ridgemont High,” but about six inches longer).
Such efforts to celebrate diversity and inclusiveness go far in helping children and young adults to embrace their skin and their own identities, said Dr. Desai, the immediate past president of the Skin of Color Society and a member of the American Academy of Dermatology board of directors. “One nuance, perhaps even more important, is that the Barbie can help to break down barriers, create awareness, and potentially even reduce bullying, stigma, and lack of knowledge about vitiligo amongst the general public who don’t understand vitiligo,” he said. “I hope the public and social media will embrace this new Barbie. Who knows? Pretty soon, vitiligo may no longer be a ‘thing’ that causes ‘stares’ and ‘glares.’ ”
Referring to the Barbie with no hair in the new line of dolls, the Mattel statement said, “ if a girl is experiencing hair loss for any reason, she can see herself reflected in the line.”
In 2019, Mattel introduced a lineup of Barbie dolls reflecting permanent disabilities, including one with a prosthetic limb. For that effort, the company collaborated with then-12-year-old Jordan Reeves, the “Born Just Right” coauthor “who is on a mission to build creative solutions that help kids with disabilities, to create a play experience that is as representative as possible,” the Mattel representative wrote.
A new line of Barbie dolls unveiled by Mattel earlier this month includes one with vitiligo, much to the delight of clinicians who treat children and adolescents with the condition.
“When I see young children and adolescents with vitiligo, it is very common for me to feel their emotional suffering from their skin condition,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center in Dallas said in an interview. “Kids can be cruel. Name calling, social ostracizing, [and] effects on self-esteem are all things I have seen amongst my patients and their families in their own struggles with vitiligo.”
According to a brand communications representative from toymaker Mattel, which began manufacturing Barbie dolls in 1959, the company worked with a board-certified dermatologist to include a doll with vitiligo in its 2020 “Fashionistas” line. “As we continue to redefine what it means to be a ‘Barbie’ or look like Barbie, offering a doll with vitiligo in our main doll line allows kids to play out even more stories they see in the world around them,” the representative wrote in an email message. Other dolls debuting as part of the lineup include one with no hair, one with a darker skin tone that uses a gold prosthetic limb, and a Ken doll with long rooted hair (think Jeff Spicoli in “Fast Times at Ridgemont High,” but about six inches longer).
Such efforts to celebrate diversity and inclusiveness go far in helping children and young adults to embrace their skin and their own identities, said Dr. Desai, the immediate past president of the Skin of Color Society and a member of the American Academy of Dermatology board of directors. “One nuance, perhaps even more important, is that the Barbie can help to break down barriers, create awareness, and potentially even reduce bullying, stigma, and lack of knowledge about vitiligo amongst the general public who don’t understand vitiligo,” he said. “I hope the public and social media will embrace this new Barbie. Who knows? Pretty soon, vitiligo may no longer be a ‘thing’ that causes ‘stares’ and ‘glares.’ ”
Referring to the Barbie with no hair in the new line of dolls, the Mattel statement said, “ if a girl is experiencing hair loss for any reason, she can see herself reflected in the line.”
In 2019, Mattel introduced a lineup of Barbie dolls reflecting permanent disabilities, including one with a prosthetic limb. For that effort, the company collaborated with then-12-year-old Jordan Reeves, the “Born Just Right” coauthor “who is on a mission to build creative solutions that help kids with disabilities, to create a play experience that is as representative as possible,” the Mattel representative wrote.
A new line of Barbie dolls unveiled by Mattel earlier this month includes one with vitiligo, much to the delight of clinicians who treat children and adolescents with the condition.
“When I see young children and adolescents with vitiligo, it is very common for me to feel their emotional suffering from their skin condition,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center in Dallas said in an interview. “Kids can be cruel. Name calling, social ostracizing, [and] effects on self-esteem are all things I have seen amongst my patients and their families in their own struggles with vitiligo.”
According to a brand communications representative from toymaker Mattel, which began manufacturing Barbie dolls in 1959, the company worked with a board-certified dermatologist to include a doll with vitiligo in its 2020 “Fashionistas” line. “As we continue to redefine what it means to be a ‘Barbie’ or look like Barbie, offering a doll with vitiligo in our main doll line allows kids to play out even more stories they see in the world around them,” the representative wrote in an email message. Other dolls debuting as part of the lineup include one with no hair, one with a darker skin tone that uses a gold prosthetic limb, and a Ken doll with long rooted hair (think Jeff Spicoli in “Fast Times at Ridgemont High,” but about six inches longer).
Such efforts to celebrate diversity and inclusiveness go far in helping children and young adults to embrace their skin and their own identities, said Dr. Desai, the immediate past president of the Skin of Color Society and a member of the American Academy of Dermatology board of directors. “One nuance, perhaps even more important, is that the Barbie can help to break down barriers, create awareness, and potentially even reduce bullying, stigma, and lack of knowledge about vitiligo amongst the general public who don’t understand vitiligo,” he said. “I hope the public and social media will embrace this new Barbie. Who knows? Pretty soon, vitiligo may no longer be a ‘thing’ that causes ‘stares’ and ‘glares.’ ”
Referring to the Barbie with no hair in the new line of dolls, the Mattel statement said, “ if a girl is experiencing hair loss for any reason, she can see herself reflected in the line.”
In 2019, Mattel introduced a lineup of Barbie dolls reflecting permanent disabilities, including one with a prosthetic limb. For that effort, the company collaborated with then-12-year-old Jordan Reeves, the “Born Just Right” coauthor “who is on a mission to build creative solutions that help kids with disabilities, to create a play experience that is as representative as possible,” the Mattel representative wrote.
Don’t forget about the flu: 2019-2010 season is not over
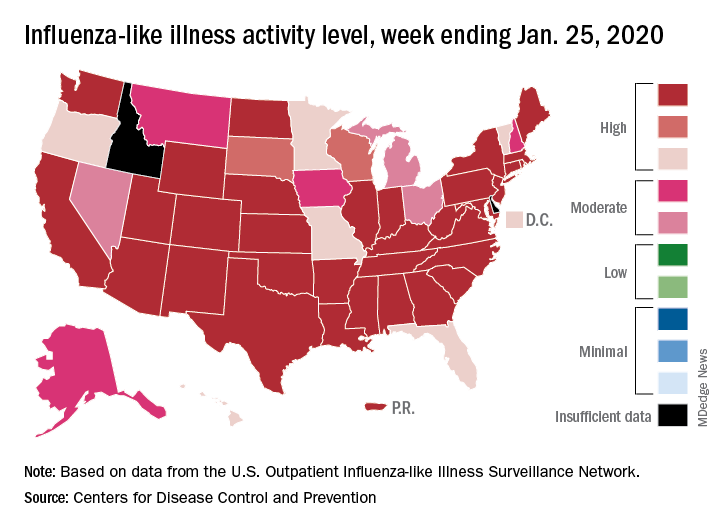
Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.

Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.

Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.
High cost of wound dressings for epidermolysis bullosa highlighted
LONDON – More than £2.8 million (RDEB), according to a report at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Results from the Prospective Epidermolysis Bullosa Longitudinal Evaluation Study (PEBLES), which is looking at the natural history of RDEB, showed that wound dressing and bandage costs were highest for study participants with the generalized severe (RDEB-GS) subtype, at just over £85,156 (about $112,450) per patient annually. Respective yearly costs for the generalized intermediate (RDEB-GI) and inversa (RDEB-INV) subtypes were £10,112 (about $13,350) and £1,699 (about $2,240) per patient.
Looking at the costs associated with EB is important, said one of the lead investigators for PEBLES, Jemima Mellerio, MD, FRCP, consultant dermatologist at St John’s Institute of Dermatology, at Guy’s & St. Thomas’ NHS Foundation Trust, London.
“If we are going to justify the kind of expenditure [associated with new treatments], we need to know that what we are treating is already a significant burden on our health care systems,” Dr. Mellerio said.
PEBLES is an ongoing London-based registry study that is enrolling patients with all subtypes of RDEB. Data are collected via a tablet device and include demographic data, information on clinical features, results of skin biopsies and genetic tests, and laboratory findings, as well as objective disease severity and subjective patient-orientated outcome scores.
So far, 60 patients – 49 adults and 11 children – have been enrolled in PEBLES since November 2014: 26 with RDEB-GS, 23 with RDEB-GI, 9 with RDEB-INV, and 2 with the pruriginosa RDEB subtype (RDEB-PR).
Most of the participants (71%) changed all their wound dressings at one time, patching up when required. Fourteen of 49 participants had paid people to help them change their dressings and when the total cost of combined wound dressings and paid care was taken into consideration, the mean annual cost per patient was around £2,500 (about $3,300) for RDEB-INV, £10,375 (about $13,700) per patient for RDEB-GS, and a staggering £98,000 (about $129,000) per patient for RDEB-GS. The total annual cost of dressings and associated care was an estimated £3,184,229 (about $4.2 million).
In addition to data on the cost of wound dressings, data on itch and pain and quality of life were presented at the EB World Congress and discussed by Dr. Mellerio.
A total of 42 participants older than 8 years of age had itch measured via the Leuven Itch Scale, she reported, noting that itch was a consistent symptom across all subtypes of RDEB. Itch is important as it not only causes problems with skin lesions and healing, but also significantly affects sleep and has a negative impact on patients’ mood, she emphasized.
Despite experiencing itch, more than half (58%) of participants were not using any kind of treatment for itch. This “likely reflects the lack of effectiveness of current medication for this debilitating symptom,” Dr. Mellerio and associates noted in one of their poster presentations of PEBLES data.
When treatment was used for itch, it consisted mainly of antihistamines (19% of patients), emollients (19%), or a combination of both (4%). However, treatment was generally “not very good,” with a satisfaction score of just 5 on a scale of 10, Dr. Mellerio pointed out. Participants “reported frustration with the lack of effective treatment for itch,” she said.
Itch was associated with disturbed sleep 1-3 nights per week in 20%-40% of participants, and every night in 20%-30%.
Pain was found to be a significant problem, with a median level of background pain scored as 4 on a 10-cm visual analog scale and a higher level (6) when associated with dressing changes.
Data on how RDEB affected quality of life were reported for 39 adults completing the 17-item Quality of Life in EB Questionnaire (QOLEB) and eight children who were able to complete the Pediatric Quality of Life Inventory (PedsQL) with the aid of their parents.
Dr. Mellerio reported that adults with RDEB-GS had an overall QOLEB score of 24 out of 50, an indication that their condition had a severe impact on their quality of life. The effect on quality of life was greater in terms of their physical functioning than emotional well-being, with respective scores of 19 out of 36, and 5 out of a possible 15. Less impact on quality of life was reported by participants with other RDEB subtypes.
PedsQL scores for the children indicated there might be a lesser effect of physical functioning on quality of life but a greater effect of emotional well-being on quality of life, but the numbers were small. “Interestingly, parents tended to rate their children’s impact on quality of life much higher than the children themselves,” Dr. Mellerio said.
The point of PEBLES is to start to understand the natural history of RDEB and to identify endpoints that might help in clinical trials of potential new treatments. Discussing the next steps for PEBLES, Dr. Mellerio said the aim was to recruit more pediatric patients and look at other data sets, such as bone health. The PEBLES team also hopes to extend recruitment to include other United Kingdom, and ultimately international, EB centers and, perhaps eventually to start to include other types of EB, such as EB simplex.
PEBLES is funded by DEBRA UK. Dr. Mellerio is a PEBLES investigator but had no conflicts of interest to disclose.
SOURCE: Mellerio JE et al. EB 2020. Pillay EI et al. Poster 77; Jeffs E et al. Poster 74; Jeffs et al. Poster 75. https://ebworldcongress.org/.
LONDON – More than £2.8 million (RDEB), according to a report at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Results from the Prospective Epidermolysis Bullosa Longitudinal Evaluation Study (PEBLES), which is looking at the natural history of RDEB, showed that wound dressing and bandage costs were highest for study participants with the generalized severe (RDEB-GS) subtype, at just over £85,156 (about $112,450) per patient annually. Respective yearly costs for the generalized intermediate (RDEB-GI) and inversa (RDEB-INV) subtypes were £10,112 (about $13,350) and £1,699 (about $2,240) per patient.
Looking at the costs associated with EB is important, said one of the lead investigators for PEBLES, Jemima Mellerio, MD, FRCP, consultant dermatologist at St John’s Institute of Dermatology, at Guy’s & St. Thomas’ NHS Foundation Trust, London.
“If we are going to justify the kind of expenditure [associated with new treatments], we need to know that what we are treating is already a significant burden on our health care systems,” Dr. Mellerio said.
PEBLES is an ongoing London-based registry study that is enrolling patients with all subtypes of RDEB. Data are collected via a tablet device and include demographic data, information on clinical features, results of skin biopsies and genetic tests, and laboratory findings, as well as objective disease severity and subjective patient-orientated outcome scores.
So far, 60 patients – 49 adults and 11 children – have been enrolled in PEBLES since November 2014: 26 with RDEB-GS, 23 with RDEB-GI, 9 with RDEB-INV, and 2 with the pruriginosa RDEB subtype (RDEB-PR).
Most of the participants (71%) changed all their wound dressings at one time, patching up when required. Fourteen of 49 participants had paid people to help them change their dressings and when the total cost of combined wound dressings and paid care was taken into consideration, the mean annual cost per patient was around £2,500 (about $3,300) for RDEB-INV, £10,375 (about $13,700) per patient for RDEB-GS, and a staggering £98,000 (about $129,000) per patient for RDEB-GS. The total annual cost of dressings and associated care was an estimated £3,184,229 (about $4.2 million).
In addition to data on the cost of wound dressings, data on itch and pain and quality of life were presented at the EB World Congress and discussed by Dr. Mellerio.
A total of 42 participants older than 8 years of age had itch measured via the Leuven Itch Scale, she reported, noting that itch was a consistent symptom across all subtypes of RDEB. Itch is important as it not only causes problems with skin lesions and healing, but also significantly affects sleep and has a negative impact on patients’ mood, she emphasized.
Despite experiencing itch, more than half (58%) of participants were not using any kind of treatment for itch. This “likely reflects the lack of effectiveness of current medication for this debilitating symptom,” Dr. Mellerio and associates noted in one of their poster presentations of PEBLES data.
When treatment was used for itch, it consisted mainly of antihistamines (19% of patients), emollients (19%), or a combination of both (4%). However, treatment was generally “not very good,” with a satisfaction score of just 5 on a scale of 10, Dr. Mellerio pointed out. Participants “reported frustration with the lack of effective treatment for itch,” she said.
Itch was associated with disturbed sleep 1-3 nights per week in 20%-40% of participants, and every night in 20%-30%.
Pain was found to be a significant problem, with a median level of background pain scored as 4 on a 10-cm visual analog scale and a higher level (6) when associated with dressing changes.
Data on how RDEB affected quality of life were reported for 39 adults completing the 17-item Quality of Life in EB Questionnaire (QOLEB) and eight children who were able to complete the Pediatric Quality of Life Inventory (PedsQL) with the aid of their parents.
Dr. Mellerio reported that adults with RDEB-GS had an overall QOLEB score of 24 out of 50, an indication that their condition had a severe impact on their quality of life. The effect on quality of life was greater in terms of their physical functioning than emotional well-being, with respective scores of 19 out of 36, and 5 out of a possible 15. Less impact on quality of life was reported by participants with other RDEB subtypes.
PedsQL scores for the children indicated there might be a lesser effect of physical functioning on quality of life but a greater effect of emotional well-being on quality of life, but the numbers were small. “Interestingly, parents tended to rate their children’s impact on quality of life much higher than the children themselves,” Dr. Mellerio said.
The point of PEBLES is to start to understand the natural history of RDEB and to identify endpoints that might help in clinical trials of potential new treatments. Discussing the next steps for PEBLES, Dr. Mellerio said the aim was to recruit more pediatric patients and look at other data sets, such as bone health. The PEBLES team also hopes to extend recruitment to include other United Kingdom, and ultimately international, EB centers and, perhaps eventually to start to include other types of EB, such as EB simplex.
PEBLES is funded by DEBRA UK. Dr. Mellerio is a PEBLES investigator but had no conflicts of interest to disclose.
SOURCE: Mellerio JE et al. EB 2020. Pillay EI et al. Poster 77; Jeffs E et al. Poster 74; Jeffs et al. Poster 75. https://ebworldcongress.org/.
LONDON – More than £2.8 million (RDEB), according to a report at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Results from the Prospective Epidermolysis Bullosa Longitudinal Evaluation Study (PEBLES), which is looking at the natural history of RDEB, showed that wound dressing and bandage costs were highest for study participants with the generalized severe (RDEB-GS) subtype, at just over £85,156 (about $112,450) per patient annually. Respective yearly costs for the generalized intermediate (RDEB-GI) and inversa (RDEB-INV) subtypes were £10,112 (about $13,350) and £1,699 (about $2,240) per patient.
Looking at the costs associated with EB is important, said one of the lead investigators for PEBLES, Jemima Mellerio, MD, FRCP, consultant dermatologist at St John’s Institute of Dermatology, at Guy’s & St. Thomas’ NHS Foundation Trust, London.
“If we are going to justify the kind of expenditure [associated with new treatments], we need to know that what we are treating is already a significant burden on our health care systems,” Dr. Mellerio said.
PEBLES is an ongoing London-based registry study that is enrolling patients with all subtypes of RDEB. Data are collected via a tablet device and include demographic data, information on clinical features, results of skin biopsies and genetic tests, and laboratory findings, as well as objective disease severity and subjective patient-orientated outcome scores.
So far, 60 patients – 49 adults and 11 children – have been enrolled in PEBLES since November 2014: 26 with RDEB-GS, 23 with RDEB-GI, 9 with RDEB-INV, and 2 with the pruriginosa RDEB subtype (RDEB-PR).
Most of the participants (71%) changed all their wound dressings at one time, patching up when required. Fourteen of 49 participants had paid people to help them change their dressings and when the total cost of combined wound dressings and paid care was taken into consideration, the mean annual cost per patient was around £2,500 (about $3,300) for RDEB-INV, £10,375 (about $13,700) per patient for RDEB-GS, and a staggering £98,000 (about $129,000) per patient for RDEB-GS. The total annual cost of dressings and associated care was an estimated £3,184,229 (about $4.2 million).
In addition to data on the cost of wound dressings, data on itch and pain and quality of life were presented at the EB World Congress and discussed by Dr. Mellerio.
A total of 42 participants older than 8 years of age had itch measured via the Leuven Itch Scale, she reported, noting that itch was a consistent symptom across all subtypes of RDEB. Itch is important as it not only causes problems with skin lesions and healing, but also significantly affects sleep and has a negative impact on patients’ mood, she emphasized.
Despite experiencing itch, more than half (58%) of participants were not using any kind of treatment for itch. This “likely reflects the lack of effectiveness of current medication for this debilitating symptom,” Dr. Mellerio and associates noted in one of their poster presentations of PEBLES data.
When treatment was used for itch, it consisted mainly of antihistamines (19% of patients), emollients (19%), or a combination of both (4%). However, treatment was generally “not very good,” with a satisfaction score of just 5 on a scale of 10, Dr. Mellerio pointed out. Participants “reported frustration with the lack of effective treatment for itch,” she said.
Itch was associated with disturbed sleep 1-3 nights per week in 20%-40% of participants, and every night in 20%-30%.
Pain was found to be a significant problem, with a median level of background pain scored as 4 on a 10-cm visual analog scale and a higher level (6) when associated with dressing changes.
Data on how RDEB affected quality of life were reported for 39 adults completing the 17-item Quality of Life in EB Questionnaire (QOLEB) and eight children who were able to complete the Pediatric Quality of Life Inventory (PedsQL) with the aid of their parents.
Dr. Mellerio reported that adults with RDEB-GS had an overall QOLEB score of 24 out of 50, an indication that their condition had a severe impact on their quality of life. The effect on quality of life was greater in terms of their physical functioning than emotional well-being, with respective scores of 19 out of 36, and 5 out of a possible 15. Less impact on quality of life was reported by participants with other RDEB subtypes.
PedsQL scores for the children indicated there might be a lesser effect of physical functioning on quality of life but a greater effect of emotional well-being on quality of life, but the numbers were small. “Interestingly, parents tended to rate their children’s impact on quality of life much higher than the children themselves,” Dr. Mellerio said.
The point of PEBLES is to start to understand the natural history of RDEB and to identify endpoints that might help in clinical trials of potential new treatments. Discussing the next steps for PEBLES, Dr. Mellerio said the aim was to recruit more pediatric patients and look at other data sets, such as bone health. The PEBLES team also hopes to extend recruitment to include other United Kingdom, and ultimately international, EB centers and, perhaps eventually to start to include other types of EB, such as EB simplex.
PEBLES is funded by DEBRA UK. Dr. Mellerio is a PEBLES investigator but had no conflicts of interest to disclose.
SOURCE: Mellerio JE et al. EB 2020. Pillay EI et al. Poster 77; Jeffs E et al. Poster 74; Jeffs et al. Poster 75. https://ebworldcongress.org/.
REPORTING FROM EB 2020
Social media may negatively influence acne treatment
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
FROM PEDIATRIC DERMATOLOGY
Opioid use disorder in adolescents: An overview
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.

NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.

NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.

NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.