User login
What’s Eating You? The South African Fattail Scorpion Revisited
Identification
The South African fattail scorpion (Parabuthus transvaalicus)(Figure) is one of the most poisonous scorpions in southern Africa.1 A member of the Buthidae scorpion family, it can grow as long as 15 cm and is dark brown-black with lighter red-brown pincers. Similar to other fattail scorpions, it has slender pincers (pedipalps) and a thick square tail (the telson). Parabuthus transvaalicus inhabits hot dry deserts, scrublands, and semiarid regions.1,2 It also is popular in exotic pet collections, the most common source of stings in the United States.

Stings and Envenomation
Scorpions with thicker tails generally have more potent venom than those with slender tails and thick pincers. Venom is injected by a stinger at the tip of the telson1; P transvaalicus also can spray venom as far as 3 m.1,2 Venom is not known to cause toxicity through skin contact but could represent a hazard if sprayed in the eye.
Scorpion toxins are a group of complex neurotoxins that act on sodium channels, either retarding inactivation (α toxin) or enhancing activation (β toxin), causing massive depolarization of excitable cells.1,3 The toxin causes neurons to fire repetitively.4 Neurotransmitters—noradrenaline, adrenaline, and acetylcholine—cause the observed sympathetic, parasympathetic, and skeletal muscle effects.1
Incidence
Worldwide, more than 1.2 million individuals are stung by a scorpion annually, causing more than 3250 deaths a year.5 Adults are stung more often, but children experience more severe envenomation, are more likely to develop severe illness requiring intensive supportive care, and have a higher mortality.4
As many as one-third of patients stung by a Parabuthus scorpion develop neuromuscular toxicity, which can be life-threatening.6 In a study of 277 envenomations by P transvaalicus, 10% of patients developed severe symptoms and 5 died. Children younger than 10 years and adults older than 50 years are at greatest risk for
Clinical Presentation
The clinical presentation of scorpion envenomation varies with the species involved, the amount of venom injected, and the victim’s weight and baseline health.1 Scorpion envenomation is divided into 4 grades based on the severity of a sting:
• Grade I: pain and paresthesia at the envenomation site; usually, no local inflammation
• Grade II: local symptoms as well as more remote pain and paresthesia; pain can radiate up the affected limb
• Grade III: cranial nerve or somatic skeletal neuromuscular dysfunction; either presentation can have associated autonomic dysfunction
• Grade IV: both cranial nerve and somatic skeletal neuromuscular dysfunction, with associated auto-nomic dysfunction
The initial symptom of a scorpion sting is intense burning pain. The sting site might be unimpressive, with only a mild local reaction. Symptoms usually progress to maximum severity within 5 hours.1 Muscle pain, cramps, and weakness are prominent. The patient might have difficulty walking and swallowing, with increased salivation and drooling, and visual disturbance with abnormal eye movements. Pulse, blood pressure, and temperature often are elevated. The patient might be hyperreflexic with clonus.1,6
Symptoms of increased sympathetic activity are hypertension, tachycardia, cardiac dysrhythmia, perspiration, hyperglycemia, and restlessness.1,2 Parasympathetic effects are increased salivation, hypotension, bradycardia, and gastric distension. Skeletal muscle effects include tremors and involuntary muscle movement, which can be severe. Cranial nerve dysfunction may manifest as dysphagia, drooling, abnormal eye movements, blurred vision, slurred speech, and tongue fasciculations. Subsequent development of muscle weakness, bulbar paralysis, and difficulty breathing may be caused by depletion of neurotransmitters after prolonged excessive neuronal activity.1
Distinctive Signs in Younger Patients
A child who is stung by a scorpion might have symptoms similar to those seen in an adult victim but can also experience an extreme form of restlessness that indicates severe envenomation characterized by inability to lay still, violent muscle twitching, and uncontrollable flailing of extremities. The child might have facial grimacing, with lip-smacking and chewing motions. In addition, bulbar paralysis and respiratory distress are more likely in children who have been stung than in adults.1,2
Management
Treatment of a P transvaalicus sting is directed at “scorpionism,” envenomation that is associated with systemic symptoms that can be life-threatening. Treatment comprises support of vital functions, symptomatic measures, and injection of antivenin.8
Support of Vital Functions
In adults, systemic symptoms can be delayed as long as 8 hours after the sting. However, most severe cases usually are evident within 60 minutes; infants can reach grade IV as quickly as 15 to 30 minutes.9,10 Loss of pharyngeal reflexes and development of respiratory distress are ominous warning signs requiring immediate respiratory support. Respiratory failure is the most common cause of death.1 An asymptomatic child should be admitted to a hospital for observation for a minimum of 12 hours if the species of scorpion was not identified.2
Pain Relief
Most patients cannot tolerate an ice pack because of severe hyperesthesia. Infiltration of the local sting site with an anesthetic generally is safe and can provide some local pain relief. Intravenous fentanyl has been used in closely monitored patients because the drug is not associated with histamine release. Medications that cause release of histamine, such as morphine, can exacerbate or confuse the clinical picture.
Antivenin
Scorpion antivenin contains purified IgG fragments; allergic reactions are now rare. The sooner antivenin is administered, the greater the benefit. When administered early, it can prevent many of the most serious complications.7 In a randomized, double-blind study of critically ill children with clinically significant signs of scorpion envenomation, intravenous administration of scorpion-specific fragment antigen-binding 2 (F[(ab’]2) antivenin resulted in resolution of clinical symptoms within 4 hours.11
When managing grade III or IV scorpion envenomation, all patients should be admitted to a medical facility equipped to provide intensive supportive care; consider consultation with a regional poison control center. The World Health Organization maintains an international poison control center (at https://www.who.int/ipcs/poisons/centre/en/) with regional telephone numbers; alternatively, in the United States, call the nationwide telephone number of the Poison Control Center (800-222-1222).
The World Health Organization has identified declining production of antivenin as a crisis.12
Resolution
Symptoms of envenomation typically resolve 9 to 30 hours after a sting in a patient with grade III or IV envenomation not treated with antivenin.4 However, pain and paresthesia occasionally last as long as 2 weeks. In rare cases, more long-term sequelae of burning paresthesia persist for months.4
Conclusion
It is important for dermatologists to be aware of the potential for life-threatening envenomation by certain scorpion species native to southern Africa. In the United States, stings of these species most often are seen in patients with a pet collection, but late sequelae also can be seen in travelers returning from an endemic region. The site of a sting often appears unimpressive initially, but severe hyperesthesia is common. Patients with cardiac, neurologic, or respiratory symptoms require intensive supportive care. Proper care can be lifesaving.
- Müller GJ, Modler H, Wium CA, et al. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012;30:356-361.
- Müller GJ. Scorpionism in South Africa. a report of 42 serious scorpion envenomations. S Afr Med J. 1993;83:405-411.
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, et al. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328-342.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71-79.
- Bergman NJ. Clinical description of Parabuthus transvaalicus scorpionism in Zimbabwe. Toxicon. 1997;35:759-771.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165-173.
- Santos MS, Silva CG, Neto BS, et al. Clinical and epidemiological aspects of scorpionism in the world: a systematic review. Wilderness Environ Med. 2016;27:504-518.
- Amaral CF, Rezende NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon. 1997;35:997-998.
- Bergman NJ. Scorpion sting in Zimbabwe. S Afr Med J. 1997;87:163-167.
- Boyer LV, Theodorou AA, Berg RA, et al; Arizona Envenomation Investigators. antivenom for critically ill children with neurotoxicity from scorpion stings. N Engl J Med. 2009;360:2090-2098.
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541-557.
Identification
The South African fattail scorpion (Parabuthus transvaalicus)(Figure) is one of the most poisonous scorpions in southern Africa.1 A member of the Buthidae scorpion family, it can grow as long as 15 cm and is dark brown-black with lighter red-brown pincers. Similar to other fattail scorpions, it has slender pincers (pedipalps) and a thick square tail (the telson). Parabuthus transvaalicus inhabits hot dry deserts, scrublands, and semiarid regions.1,2 It also is popular in exotic pet collections, the most common source of stings in the United States.

Stings and Envenomation
Scorpions with thicker tails generally have more potent venom than those with slender tails and thick pincers. Venom is injected by a stinger at the tip of the telson1; P transvaalicus also can spray venom as far as 3 m.1,2 Venom is not known to cause toxicity through skin contact but could represent a hazard if sprayed in the eye.
Scorpion toxins are a group of complex neurotoxins that act on sodium channels, either retarding inactivation (α toxin) or enhancing activation (β toxin), causing massive depolarization of excitable cells.1,3 The toxin causes neurons to fire repetitively.4 Neurotransmitters—noradrenaline, adrenaline, and acetylcholine—cause the observed sympathetic, parasympathetic, and skeletal muscle effects.1
Incidence
Worldwide, more than 1.2 million individuals are stung by a scorpion annually, causing more than 3250 deaths a year.5 Adults are stung more often, but children experience more severe envenomation, are more likely to develop severe illness requiring intensive supportive care, and have a higher mortality.4
As many as one-third of patients stung by a Parabuthus scorpion develop neuromuscular toxicity, which can be life-threatening.6 In a study of 277 envenomations by P transvaalicus, 10% of patients developed severe symptoms and 5 died. Children younger than 10 years and adults older than 50 years are at greatest risk for
Clinical Presentation
The clinical presentation of scorpion envenomation varies with the species involved, the amount of venom injected, and the victim’s weight and baseline health.1 Scorpion envenomation is divided into 4 grades based on the severity of a sting:
• Grade I: pain and paresthesia at the envenomation site; usually, no local inflammation
• Grade II: local symptoms as well as more remote pain and paresthesia; pain can radiate up the affected limb
• Grade III: cranial nerve or somatic skeletal neuromuscular dysfunction; either presentation can have associated autonomic dysfunction
• Grade IV: both cranial nerve and somatic skeletal neuromuscular dysfunction, with associated auto-nomic dysfunction
The initial symptom of a scorpion sting is intense burning pain. The sting site might be unimpressive, with only a mild local reaction. Symptoms usually progress to maximum severity within 5 hours.1 Muscle pain, cramps, and weakness are prominent. The patient might have difficulty walking and swallowing, with increased salivation and drooling, and visual disturbance with abnormal eye movements. Pulse, blood pressure, and temperature often are elevated. The patient might be hyperreflexic with clonus.1,6
Symptoms of increased sympathetic activity are hypertension, tachycardia, cardiac dysrhythmia, perspiration, hyperglycemia, and restlessness.1,2 Parasympathetic effects are increased salivation, hypotension, bradycardia, and gastric distension. Skeletal muscle effects include tremors and involuntary muscle movement, which can be severe. Cranial nerve dysfunction may manifest as dysphagia, drooling, abnormal eye movements, blurred vision, slurred speech, and tongue fasciculations. Subsequent development of muscle weakness, bulbar paralysis, and difficulty breathing may be caused by depletion of neurotransmitters after prolonged excessive neuronal activity.1
Distinctive Signs in Younger Patients
A child who is stung by a scorpion might have symptoms similar to those seen in an adult victim but can also experience an extreme form of restlessness that indicates severe envenomation characterized by inability to lay still, violent muscle twitching, and uncontrollable flailing of extremities. The child might have facial grimacing, with lip-smacking and chewing motions. In addition, bulbar paralysis and respiratory distress are more likely in children who have been stung than in adults.1,2
Management
Treatment of a P transvaalicus sting is directed at “scorpionism,” envenomation that is associated with systemic symptoms that can be life-threatening. Treatment comprises support of vital functions, symptomatic measures, and injection of antivenin.8
Support of Vital Functions
In adults, systemic symptoms can be delayed as long as 8 hours after the sting. However, most severe cases usually are evident within 60 minutes; infants can reach grade IV as quickly as 15 to 30 minutes.9,10 Loss of pharyngeal reflexes and development of respiratory distress are ominous warning signs requiring immediate respiratory support. Respiratory failure is the most common cause of death.1 An asymptomatic child should be admitted to a hospital for observation for a minimum of 12 hours if the species of scorpion was not identified.2
Pain Relief
Most patients cannot tolerate an ice pack because of severe hyperesthesia. Infiltration of the local sting site with an anesthetic generally is safe and can provide some local pain relief. Intravenous fentanyl has been used in closely monitored patients because the drug is not associated with histamine release. Medications that cause release of histamine, such as morphine, can exacerbate or confuse the clinical picture.
Antivenin
Scorpion antivenin contains purified IgG fragments; allergic reactions are now rare. The sooner antivenin is administered, the greater the benefit. When administered early, it can prevent many of the most serious complications.7 In a randomized, double-blind study of critically ill children with clinically significant signs of scorpion envenomation, intravenous administration of scorpion-specific fragment antigen-binding 2 (F[(ab’]2) antivenin resulted in resolution of clinical symptoms within 4 hours.11
When managing grade III or IV scorpion envenomation, all patients should be admitted to a medical facility equipped to provide intensive supportive care; consider consultation with a regional poison control center. The World Health Organization maintains an international poison control center (at https://www.who.int/ipcs/poisons/centre/en/) with regional telephone numbers; alternatively, in the United States, call the nationwide telephone number of the Poison Control Center (800-222-1222).
The World Health Organization has identified declining production of antivenin as a crisis.12
Resolution
Symptoms of envenomation typically resolve 9 to 30 hours after a sting in a patient with grade III or IV envenomation not treated with antivenin.4 However, pain and paresthesia occasionally last as long as 2 weeks. In rare cases, more long-term sequelae of burning paresthesia persist for months.4
Conclusion
It is important for dermatologists to be aware of the potential for life-threatening envenomation by certain scorpion species native to southern Africa. In the United States, stings of these species most often are seen in patients with a pet collection, but late sequelae also can be seen in travelers returning from an endemic region. The site of a sting often appears unimpressive initially, but severe hyperesthesia is common. Patients with cardiac, neurologic, or respiratory symptoms require intensive supportive care. Proper care can be lifesaving.
Identification
The South African fattail scorpion (Parabuthus transvaalicus)(Figure) is one of the most poisonous scorpions in southern Africa.1 A member of the Buthidae scorpion family, it can grow as long as 15 cm and is dark brown-black with lighter red-brown pincers. Similar to other fattail scorpions, it has slender pincers (pedipalps) and a thick square tail (the telson). Parabuthus transvaalicus inhabits hot dry deserts, scrublands, and semiarid regions.1,2 It also is popular in exotic pet collections, the most common source of stings in the United States.

Stings and Envenomation
Scorpions with thicker tails generally have more potent venom than those with slender tails and thick pincers. Venom is injected by a stinger at the tip of the telson1; P transvaalicus also can spray venom as far as 3 m.1,2 Venom is not known to cause toxicity through skin contact but could represent a hazard if sprayed in the eye.
Scorpion toxins are a group of complex neurotoxins that act on sodium channels, either retarding inactivation (α toxin) or enhancing activation (β toxin), causing massive depolarization of excitable cells.1,3 The toxin causes neurons to fire repetitively.4 Neurotransmitters—noradrenaline, adrenaline, and acetylcholine—cause the observed sympathetic, parasympathetic, and skeletal muscle effects.1
Incidence
Worldwide, more than 1.2 million individuals are stung by a scorpion annually, causing more than 3250 deaths a year.5 Adults are stung more often, but children experience more severe envenomation, are more likely to develop severe illness requiring intensive supportive care, and have a higher mortality.4
As many as one-third of patients stung by a Parabuthus scorpion develop neuromuscular toxicity, which can be life-threatening.6 In a study of 277 envenomations by P transvaalicus, 10% of patients developed severe symptoms and 5 died. Children younger than 10 years and adults older than 50 years are at greatest risk for
Clinical Presentation
The clinical presentation of scorpion envenomation varies with the species involved, the amount of venom injected, and the victim’s weight and baseline health.1 Scorpion envenomation is divided into 4 grades based on the severity of a sting:
• Grade I: pain and paresthesia at the envenomation site; usually, no local inflammation
• Grade II: local symptoms as well as more remote pain and paresthesia; pain can radiate up the affected limb
• Grade III: cranial nerve or somatic skeletal neuromuscular dysfunction; either presentation can have associated autonomic dysfunction
• Grade IV: both cranial nerve and somatic skeletal neuromuscular dysfunction, with associated auto-nomic dysfunction
The initial symptom of a scorpion sting is intense burning pain. The sting site might be unimpressive, with only a mild local reaction. Symptoms usually progress to maximum severity within 5 hours.1 Muscle pain, cramps, and weakness are prominent. The patient might have difficulty walking and swallowing, with increased salivation and drooling, and visual disturbance with abnormal eye movements. Pulse, blood pressure, and temperature often are elevated. The patient might be hyperreflexic with clonus.1,6
Symptoms of increased sympathetic activity are hypertension, tachycardia, cardiac dysrhythmia, perspiration, hyperglycemia, and restlessness.1,2 Parasympathetic effects are increased salivation, hypotension, bradycardia, and gastric distension. Skeletal muscle effects include tremors and involuntary muscle movement, which can be severe. Cranial nerve dysfunction may manifest as dysphagia, drooling, abnormal eye movements, blurred vision, slurred speech, and tongue fasciculations. Subsequent development of muscle weakness, bulbar paralysis, and difficulty breathing may be caused by depletion of neurotransmitters after prolonged excessive neuronal activity.1
Distinctive Signs in Younger Patients
A child who is stung by a scorpion might have symptoms similar to those seen in an adult victim but can also experience an extreme form of restlessness that indicates severe envenomation characterized by inability to lay still, violent muscle twitching, and uncontrollable flailing of extremities. The child might have facial grimacing, with lip-smacking and chewing motions. In addition, bulbar paralysis and respiratory distress are more likely in children who have been stung than in adults.1,2
Management
Treatment of a P transvaalicus sting is directed at “scorpionism,” envenomation that is associated with systemic symptoms that can be life-threatening. Treatment comprises support of vital functions, symptomatic measures, and injection of antivenin.8
Support of Vital Functions
In adults, systemic symptoms can be delayed as long as 8 hours after the sting. However, most severe cases usually are evident within 60 minutes; infants can reach grade IV as quickly as 15 to 30 minutes.9,10 Loss of pharyngeal reflexes and development of respiratory distress are ominous warning signs requiring immediate respiratory support. Respiratory failure is the most common cause of death.1 An asymptomatic child should be admitted to a hospital for observation for a minimum of 12 hours if the species of scorpion was not identified.2
Pain Relief
Most patients cannot tolerate an ice pack because of severe hyperesthesia. Infiltration of the local sting site with an anesthetic generally is safe and can provide some local pain relief. Intravenous fentanyl has been used in closely monitored patients because the drug is not associated with histamine release. Medications that cause release of histamine, such as morphine, can exacerbate or confuse the clinical picture.
Antivenin
Scorpion antivenin contains purified IgG fragments; allergic reactions are now rare. The sooner antivenin is administered, the greater the benefit. When administered early, it can prevent many of the most serious complications.7 In a randomized, double-blind study of critically ill children with clinically significant signs of scorpion envenomation, intravenous administration of scorpion-specific fragment antigen-binding 2 (F[(ab’]2) antivenin resulted in resolution of clinical symptoms within 4 hours.11
When managing grade III or IV scorpion envenomation, all patients should be admitted to a medical facility equipped to provide intensive supportive care; consider consultation with a regional poison control center. The World Health Organization maintains an international poison control center (at https://www.who.int/ipcs/poisons/centre/en/) with regional telephone numbers; alternatively, in the United States, call the nationwide telephone number of the Poison Control Center (800-222-1222).
The World Health Organization has identified declining production of antivenin as a crisis.12
Resolution
Symptoms of envenomation typically resolve 9 to 30 hours after a sting in a patient with grade III or IV envenomation not treated with antivenin.4 However, pain and paresthesia occasionally last as long as 2 weeks. In rare cases, more long-term sequelae of burning paresthesia persist for months.4
Conclusion
It is important for dermatologists to be aware of the potential for life-threatening envenomation by certain scorpion species native to southern Africa. In the United States, stings of these species most often are seen in patients with a pet collection, but late sequelae also can be seen in travelers returning from an endemic region. The site of a sting often appears unimpressive initially, but severe hyperesthesia is common. Patients with cardiac, neurologic, or respiratory symptoms require intensive supportive care. Proper care can be lifesaving.
- Müller GJ, Modler H, Wium CA, et al. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012;30:356-361.
- Müller GJ. Scorpionism in South Africa. a report of 42 serious scorpion envenomations. S Afr Med J. 1993;83:405-411.
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, et al. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328-342.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71-79.
- Bergman NJ. Clinical description of Parabuthus transvaalicus scorpionism in Zimbabwe. Toxicon. 1997;35:759-771.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165-173.
- Santos MS, Silva CG, Neto BS, et al. Clinical and epidemiological aspects of scorpionism in the world: a systematic review. Wilderness Environ Med. 2016;27:504-518.
- Amaral CF, Rezende NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon. 1997;35:997-998.
- Bergman NJ. Scorpion sting in Zimbabwe. S Afr Med J. 1997;87:163-167.
- Boyer LV, Theodorou AA, Berg RA, et al; Arizona Envenomation Investigators. antivenom for critically ill children with neurotoxicity from scorpion stings. N Engl J Med. 2009;360:2090-2098.
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541-557.
- Müller GJ, Modler H, Wium CA, et al. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012;30:356-361.
- Müller GJ. Scorpionism in South Africa. a report of 42 serious scorpion envenomations. S Afr Med J. 1993;83:405-411.
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, et al. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328-342.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71-79.
- Bergman NJ. Clinical description of Parabuthus transvaalicus scorpionism in Zimbabwe. Toxicon. 1997;35:759-771.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165-173.
- Santos MS, Silva CG, Neto BS, et al. Clinical and epidemiological aspects of scorpionism in the world: a systematic review. Wilderness Environ Med. 2016;27:504-518.
- Amaral CF, Rezende NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon. 1997;35:997-998.
- Bergman NJ. Scorpion sting in Zimbabwe. S Afr Med J. 1997;87:163-167.
- Boyer LV, Theodorou AA, Berg RA, et al; Arizona Envenomation Investigators. antivenom for critically ill children with neurotoxicity from scorpion stings. N Engl J Med. 2009;360:2090-2098.
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541-557.
Practice Points
- Exotic and dangerous pets are becoming more popular. Scorpion stings cause potentially life-threatening neurotoxicity, with children particularly susceptible.
- Fattail scorpions are particularly dangerous and physicians should be aware that their stings may be encountered worldwide.
- Symptoms present 1 to 8 hours after envenomation, with severe cases showing hyperreflexia, clonus, difficulty swallowing, and respiratory distress. The sting site may be unimpressive.
Liraglutide ‘option’ for treating pediatric type 2 diabetes
BARCELONA – The glucagon-like peptide-1 receptor agonist (GLP-1 RA) liraglutide added onto metformin with or without basal insulin effectively reduced hemoglobin A1c and fasting plasma glucose levels in children with type 2 diabetes in the 52-week ELLIPSE study.
The primary endpoint of the trial, which was the mean change in HbA1c from baseline to 26 weeks, was met, with a greater percentage point decrease with liraglutide (Victoza) than placebo (–0.64 vs. +0.42), with an estimated treatment difference of –1.06 percentage points (P less than .001). At the end of the study, the percentage point changes were –0.50 and +0.80, with a between-group difference of –1.30 in favor of liraglutide.
“Those of us working in pediatric practice are seeing an increasing demand for our clinical services in children with type 2 diabetes,” study investigator Timothy Barrett, PhD, MBBS, observed at the annual meeting of the European Association for the Study of Diabetes. This reflects the increasing prevalence of type 2 diabetes in this age group and is most likely linked to the rising rates of obesity and overweight that have been reported widely in young people in recent years, he added.
“Unfortunately, we look with envy upon our adult physician colleagues, and the range of treatments they have available to treat type 2 diabetes in adults.” In pediatrics, the only licensed treatments that have been available until recently were metformin and insulin, with the latter being an “illogical treatment to treat those with obesity-related diabetes.” The study’s findings, however, support liraglutide as another option to consider, said Dr. Barrett, a pediatric endocrinologist and professor of pediatrics and child health based at the University of Birmingham, England.
“Liraglutide at doses of up to 1.8 mg/day when added to metformin, and basal insulin if required, does seem to offer an additional treatment option for children and young people with type 2 diabetes who require improved glycemic control after they’ve reached a maximum dose of metformin,” he said.
ELLIPSE (Evaluation of Liraglutide in Pediatrics with Diabetes) was a multicenter, randomized, parallel group, placebo-controlled trial to assess the efficacy and safety of liraglutide as an add-on treatment to metformin, with or without basal insulin, in 134 overweight or obese children and adolescents (aged 10-17) with type 2 diabetes.
For inclusion, patients had to be able to complete the trial before their 18th birthday, and have an HbA1c of at least 7% if being treated with diet and exercise, or 6.5% or higher if already being treated with metformin, with or without insulin. Body mass index had to be above the 85the percentile for their age and sex.
Of 307 children and adolescents screened at 84 centers in 25 countries, 135 were randomized and 134 were treated between 2012 and 2018. Screening took place over a period of 2 weeks, after which time those eligible for the trial underwent a 3- to 4-week period where their dose of metformin was titrated if needed followed by an 8-week maintenance period. Only after that was randomization to liraglutide or placebo done, with the GLP-1R started at a subcutaneous dose of 0.6 mg and titrated up to 1.2 or 1.8 mg over 3 days to achieve a fasting plasma glucose (FPG) of less than 6.1 mmol/L (110 mg/dL). However, not all patients were escalated to the top dose, Dr. Barrett noted.
The mean age of patients in the trial was 14.5 years; about 60% of patients were female. The duration of diabetes was about 1.9 years and the average body weight and BMI a respective 91 kg and 33 kg/m2.
Over the course of the study, FPG fell by 1.06 mmol/L at week 26 and 1.03 mmol/L at week 52 in the liraglutide group but rose in the placebo group by 0.80 and 0.78 mmol/L, respectively. The estimated treatment difference was –1.88 (P = .002) and –1.81 at 26 an 52 weeks, respectively.
What was “a really gratifying to see,” said Dr. Barrett, was that the proportion of children and young people achieving a glycemic target of an HbA1c of less than 7% by the end of the double-blind treatment period was significantly higher in the liraglutide than placebo group, at 63.7% and 36.5%, respectively.
Most of the adverse effects seen in the study were gastrointestinal symptoms, including nausea, vomiting, and diarrhea, in about 20% of liraglutide-treated patients, compared with roughly 10% of placebo-treated patients. “This is really reflected in the adult studies as well, and many of these were thankfully transient.”
As for hypoglycemia, Dr. Barrett reported that there was a higher rate in liraglutide- than placebo-treated patients (45.5% vs. 25% for any event), although there were no severe episodes in the liraglutide group and one in the placebo group. Almost a third (31%) of hypoglycemic episodes were asymptomatic, versus 17.6% for the placebo group.
“This is the first successfully completed phase 3 trial showing efficacy of a noninsulin agent, in this case, for children who do not get managed solely on metformin monotherapy,” Dr. Barrett said.
The Food and Drug Administration has approved liraglutide for use in pediatric patients 10 years or older with type 2 diabetes, based in part on results of the ELLIPSE results, Novo Nordisk announced in June. The trial results were published prior to the EASD meeting (Tamborlane WV et al. N Engl J Med. 2019 Aug 15;381:637-46).
Novo Nordisk initiated and funded the trial, and most of the investigators reported receiving funds from the company outside the submitted work. Dr Barrett disclosed being a consultant to and/or receiving honoraria from AstraZeneca, Novo Nordisk and Servier.
SOURCE: Barrett T et al. EASD 2019. Abstract 84.
BARCELONA – The glucagon-like peptide-1 receptor agonist (GLP-1 RA) liraglutide added onto metformin with or without basal insulin effectively reduced hemoglobin A1c and fasting plasma glucose levels in children with type 2 diabetes in the 52-week ELLIPSE study.
The primary endpoint of the trial, which was the mean change in HbA1c from baseline to 26 weeks, was met, with a greater percentage point decrease with liraglutide (Victoza) than placebo (–0.64 vs. +0.42), with an estimated treatment difference of –1.06 percentage points (P less than .001). At the end of the study, the percentage point changes were –0.50 and +0.80, with a between-group difference of –1.30 in favor of liraglutide.
“Those of us working in pediatric practice are seeing an increasing demand for our clinical services in children with type 2 diabetes,” study investigator Timothy Barrett, PhD, MBBS, observed at the annual meeting of the European Association for the Study of Diabetes. This reflects the increasing prevalence of type 2 diabetes in this age group and is most likely linked to the rising rates of obesity and overweight that have been reported widely in young people in recent years, he added.
“Unfortunately, we look with envy upon our adult physician colleagues, and the range of treatments they have available to treat type 2 diabetes in adults.” In pediatrics, the only licensed treatments that have been available until recently were metformin and insulin, with the latter being an “illogical treatment to treat those with obesity-related diabetes.” The study’s findings, however, support liraglutide as another option to consider, said Dr. Barrett, a pediatric endocrinologist and professor of pediatrics and child health based at the University of Birmingham, England.
“Liraglutide at doses of up to 1.8 mg/day when added to metformin, and basal insulin if required, does seem to offer an additional treatment option for children and young people with type 2 diabetes who require improved glycemic control after they’ve reached a maximum dose of metformin,” he said.
ELLIPSE (Evaluation of Liraglutide in Pediatrics with Diabetes) was a multicenter, randomized, parallel group, placebo-controlled trial to assess the efficacy and safety of liraglutide as an add-on treatment to metformin, with or without basal insulin, in 134 overweight or obese children and adolescents (aged 10-17) with type 2 diabetes.
For inclusion, patients had to be able to complete the trial before their 18th birthday, and have an HbA1c of at least 7% if being treated with diet and exercise, or 6.5% or higher if already being treated with metformin, with or without insulin. Body mass index had to be above the 85the percentile for their age and sex.
Of 307 children and adolescents screened at 84 centers in 25 countries, 135 were randomized and 134 were treated between 2012 and 2018. Screening took place over a period of 2 weeks, after which time those eligible for the trial underwent a 3- to 4-week period where their dose of metformin was titrated if needed followed by an 8-week maintenance period. Only after that was randomization to liraglutide or placebo done, with the GLP-1R started at a subcutaneous dose of 0.6 mg and titrated up to 1.2 or 1.8 mg over 3 days to achieve a fasting plasma glucose (FPG) of less than 6.1 mmol/L (110 mg/dL). However, not all patients were escalated to the top dose, Dr. Barrett noted.
The mean age of patients in the trial was 14.5 years; about 60% of patients were female. The duration of diabetes was about 1.9 years and the average body weight and BMI a respective 91 kg and 33 kg/m2.
Over the course of the study, FPG fell by 1.06 mmol/L at week 26 and 1.03 mmol/L at week 52 in the liraglutide group but rose in the placebo group by 0.80 and 0.78 mmol/L, respectively. The estimated treatment difference was –1.88 (P = .002) and –1.81 at 26 an 52 weeks, respectively.
What was “a really gratifying to see,” said Dr. Barrett, was that the proportion of children and young people achieving a glycemic target of an HbA1c of less than 7% by the end of the double-blind treatment period was significantly higher in the liraglutide than placebo group, at 63.7% and 36.5%, respectively.
Most of the adverse effects seen in the study were gastrointestinal symptoms, including nausea, vomiting, and diarrhea, in about 20% of liraglutide-treated patients, compared with roughly 10% of placebo-treated patients. “This is really reflected in the adult studies as well, and many of these were thankfully transient.”
As for hypoglycemia, Dr. Barrett reported that there was a higher rate in liraglutide- than placebo-treated patients (45.5% vs. 25% for any event), although there were no severe episodes in the liraglutide group and one in the placebo group. Almost a third (31%) of hypoglycemic episodes were asymptomatic, versus 17.6% for the placebo group.
“This is the first successfully completed phase 3 trial showing efficacy of a noninsulin agent, in this case, for children who do not get managed solely on metformin monotherapy,” Dr. Barrett said.
The Food and Drug Administration has approved liraglutide for use in pediatric patients 10 years or older with type 2 diabetes, based in part on results of the ELLIPSE results, Novo Nordisk announced in June. The trial results were published prior to the EASD meeting (Tamborlane WV et al. N Engl J Med. 2019 Aug 15;381:637-46).
Novo Nordisk initiated and funded the trial, and most of the investigators reported receiving funds from the company outside the submitted work. Dr Barrett disclosed being a consultant to and/or receiving honoraria from AstraZeneca, Novo Nordisk and Servier.
SOURCE: Barrett T et al. EASD 2019. Abstract 84.
BARCELONA – The glucagon-like peptide-1 receptor agonist (GLP-1 RA) liraglutide added onto metformin with or without basal insulin effectively reduced hemoglobin A1c and fasting plasma glucose levels in children with type 2 diabetes in the 52-week ELLIPSE study.
The primary endpoint of the trial, which was the mean change in HbA1c from baseline to 26 weeks, was met, with a greater percentage point decrease with liraglutide (Victoza) than placebo (–0.64 vs. +0.42), with an estimated treatment difference of –1.06 percentage points (P less than .001). At the end of the study, the percentage point changes were –0.50 and +0.80, with a between-group difference of –1.30 in favor of liraglutide.
“Those of us working in pediatric practice are seeing an increasing demand for our clinical services in children with type 2 diabetes,” study investigator Timothy Barrett, PhD, MBBS, observed at the annual meeting of the European Association for the Study of Diabetes. This reflects the increasing prevalence of type 2 diabetes in this age group and is most likely linked to the rising rates of obesity and overweight that have been reported widely in young people in recent years, he added.
“Unfortunately, we look with envy upon our adult physician colleagues, and the range of treatments they have available to treat type 2 diabetes in adults.” In pediatrics, the only licensed treatments that have been available until recently were metformin and insulin, with the latter being an “illogical treatment to treat those with obesity-related diabetes.” The study’s findings, however, support liraglutide as another option to consider, said Dr. Barrett, a pediatric endocrinologist and professor of pediatrics and child health based at the University of Birmingham, England.
“Liraglutide at doses of up to 1.8 mg/day when added to metformin, and basal insulin if required, does seem to offer an additional treatment option for children and young people with type 2 diabetes who require improved glycemic control after they’ve reached a maximum dose of metformin,” he said.
ELLIPSE (Evaluation of Liraglutide in Pediatrics with Diabetes) was a multicenter, randomized, parallel group, placebo-controlled trial to assess the efficacy and safety of liraglutide as an add-on treatment to metformin, with or without basal insulin, in 134 overweight or obese children and adolescents (aged 10-17) with type 2 diabetes.
For inclusion, patients had to be able to complete the trial before their 18th birthday, and have an HbA1c of at least 7% if being treated with diet and exercise, or 6.5% or higher if already being treated with metformin, with or without insulin. Body mass index had to be above the 85the percentile for their age and sex.
Of 307 children and adolescents screened at 84 centers in 25 countries, 135 were randomized and 134 were treated between 2012 and 2018. Screening took place over a period of 2 weeks, after which time those eligible for the trial underwent a 3- to 4-week period where their dose of metformin was titrated if needed followed by an 8-week maintenance period. Only after that was randomization to liraglutide or placebo done, with the GLP-1R started at a subcutaneous dose of 0.6 mg and titrated up to 1.2 or 1.8 mg over 3 days to achieve a fasting plasma glucose (FPG) of less than 6.1 mmol/L (110 mg/dL). However, not all patients were escalated to the top dose, Dr. Barrett noted.
The mean age of patients in the trial was 14.5 years; about 60% of patients were female. The duration of diabetes was about 1.9 years and the average body weight and BMI a respective 91 kg and 33 kg/m2.
Over the course of the study, FPG fell by 1.06 mmol/L at week 26 and 1.03 mmol/L at week 52 in the liraglutide group but rose in the placebo group by 0.80 and 0.78 mmol/L, respectively. The estimated treatment difference was –1.88 (P = .002) and –1.81 at 26 an 52 weeks, respectively.
What was “a really gratifying to see,” said Dr. Barrett, was that the proportion of children and young people achieving a glycemic target of an HbA1c of less than 7% by the end of the double-blind treatment period was significantly higher in the liraglutide than placebo group, at 63.7% and 36.5%, respectively.
Most of the adverse effects seen in the study were gastrointestinal symptoms, including nausea, vomiting, and diarrhea, in about 20% of liraglutide-treated patients, compared with roughly 10% of placebo-treated patients. “This is really reflected in the adult studies as well, and many of these were thankfully transient.”
As for hypoglycemia, Dr. Barrett reported that there was a higher rate in liraglutide- than placebo-treated patients (45.5% vs. 25% for any event), although there were no severe episodes in the liraglutide group and one in the placebo group. Almost a third (31%) of hypoglycemic episodes were asymptomatic, versus 17.6% for the placebo group.
“This is the first successfully completed phase 3 trial showing efficacy of a noninsulin agent, in this case, for children who do not get managed solely on metformin monotherapy,” Dr. Barrett said.
The Food and Drug Administration has approved liraglutide for use in pediatric patients 10 years or older with type 2 diabetes, based in part on results of the ELLIPSE results, Novo Nordisk announced in June. The trial results were published prior to the EASD meeting (Tamborlane WV et al. N Engl J Med. 2019 Aug 15;381:637-46).
Novo Nordisk initiated and funded the trial, and most of the investigators reported receiving funds from the company outside the submitted work. Dr Barrett disclosed being a consultant to and/or receiving honoraria from AstraZeneca, Novo Nordisk and Servier.
SOURCE: Barrett T et al. EASD 2019. Abstract 84.
REPORTING FROM EASD 2019
High maternal lead levels linked to children’s obesity
Children born to mothers with high blood levels of lead have an increased risk of being overweight or obese, particularly if their mothers are also overweight, according to new research.
Adequate maternal plasma levels of folate, however, mitigated this risk.
“When considered simultaneously, maternal lead exposure, rather than early childhood lead exposure, contributed to overweight/obesity risk in a dose-response fashion across multiple developmental stages (preschool age, school age and early adolescence) and amplified intergenerational overweight/obesity risk (additively with maternal overweight/obesity),” Guoying Wang, MD, PhD, of Johns Hopkins Bloomberg School of Public Health, Baltimore, and associates, reported in JAMA Network Open.
“These findings support the hypothesis that the obesity epidemic could be related to environmental chemical exposures in utero and raise the possibility that optimal maternal folate supplementation may help counteract the adverse effects of environmental lead exposure,” the authors wrote.
The prospective urban, low-income cohort study, which ran from 2002 to 2013, involved 1,442 mother-child pairs who joined the study when the children were born and attended follow-up visits at Boston Medical Center. The mean age of the mothers was 29 years, and the children were, on average, 8 years old at follow-up. Half the children were male; 67% of mothers were black, and 20% were Latina.
The researchers collected maternal blood samples within 24-72 hours after birth to measure red blood cell lead levels and plasma folate levels. Children’s whole-blood lead levels were measured during the first lead screening of their well child visits, at a median 10 months of age. Researchers tracked children’s body mass index Z-score and defined overweight/obesity as exceeding the 85th national percentile for their age and sex.
Detectable lead was present in all the mothers’ blood samples. The median maternal red blood cell lead level was 2.5 mcg/dL, although black mothers tended to have higher lead exposure than that of other racial groups. Median maternal plasma folate level was 32 nmol/L. Children’s blood lead levels were a median 1.4 mcg/dL, and their median BMI Z-score was 0.78.
Children whose mothers had red blood cell lead levels of 5.0 mcg/dL or greater (16%) had 65% greater odds of being overweight or obese compared with children whose mothers’ lead level was less than 2 mcg/dL, after adjustment for maternal education, race/ethnicity, smoking status, parity, diabetes, hypertensive disorder, preterm birth, fetal growth, and breastfeeding status (odds ratio [OR], 1.65; 95% confidence internal [CI], 1.18-2.32). Only 5.2% of children had whole-blood lead levels of 5 mcg/dL or greater.
“Mothers with the highest red blood cell lead levels were older and multiparous, were more likely to be black and nonsmokers, had lower plasma folate levels and were more likely to have prepregnancy overweight/obesity and diabetes,” the authors reported.
The dose-response association did not lose significance when the researchers adjusted for children’s blood lead levels, maternal age, cesarean delivery, term births only, and black race. Nor did it change in a subset of children when the researchers adjusted for children’s physical activity.
The strength of the association increased when mothers also had a BMI greater than the average/healthy range. Children were more than four times more likely to be overweight or obese if their mothers were overweight or obese and had lead levels greater than 5.0 mcg/dL, compared with nonoverweight mothers with levels below 2 mcg/dL (OR, 4.24; 95% CI, 2.64-6.82).
Among children whose mothers were overweight/obese and had high blood lead levels, however, high folate levels appeared protective against obesity. These children had a 41% lower risk of being overweight or obese, compared with others in their group, if their mothers had plasma folate levels of at least 20 nmol/L (OR, 0.59 CI, 0.36-0.95; P = .03).
According to an invited commentary, “approximately 140,000 new chemicals and pesticides have appeared since 1950,” with “universal human exposure to approximately 5,000 of those,” wrote Marco Sanchez-Guerra, PhD, of the National Institute of Perinatology in Mexico City, and coauthors Andres Cardenas, PhD, of the University of California, Berkeley, and Citlalli Osorio-Yáñez, PhD, of the National Autonomous University of Mexico in Mexico City. Yet fewer than half of those chemicals have been tested for safety or toxic effect, the editorialists wrote, and scientists know little of their potential reproductive harm.
Dr. Sanchez-Guerra, Dr. Cardenas, and Dr. Osorio-Yáñez agreed with the study authors that elevated lead exposures, especially from gasoline before lead was removed in the United States in 1975, may partly explain the current epidemic of obesity.
“Identifying preventable prenatal causes of obesity is a cornerstone in the fight against the obesity epidemic,” the editorialists said. While most recommendations center on changes to diet and physical activity, environmental factors during pregnancy could be involved in childhood obesity as well.
“The study by Wang et al. opens the door to new questions about whether adequate folate intake might modify the adverse effects of other chemical exposures,” they continued, noting other research suggesting a protective effect from folate against health effects of air pollution exposure. “These efforts could yield substantial public health benefits and represent novel tools in fighting the obesity epidemic,” they concluded.
The research was funded by the National Institutes of Health and the U.S. Department of Health and Human Services. Neither the study authors nor the editorialists had industry financial disclosures.
SOURCES: Wang G et al. JAMA Netw Open. 2019;2(10):e1912343. doi: 10.1001/jamanetworkopen.2019.12343; Sanchez-Guerra M et al. JAMA Netw Open. 2019;2(10):e1912334. doi: 10.1001/jamanetworkopen.2019.12334.
Children born to mothers with high blood levels of lead have an increased risk of being overweight or obese, particularly if their mothers are also overweight, according to new research.
Adequate maternal plasma levels of folate, however, mitigated this risk.
“When considered simultaneously, maternal lead exposure, rather than early childhood lead exposure, contributed to overweight/obesity risk in a dose-response fashion across multiple developmental stages (preschool age, school age and early adolescence) and amplified intergenerational overweight/obesity risk (additively with maternal overweight/obesity),” Guoying Wang, MD, PhD, of Johns Hopkins Bloomberg School of Public Health, Baltimore, and associates, reported in JAMA Network Open.
“These findings support the hypothesis that the obesity epidemic could be related to environmental chemical exposures in utero and raise the possibility that optimal maternal folate supplementation may help counteract the adverse effects of environmental lead exposure,” the authors wrote.
The prospective urban, low-income cohort study, which ran from 2002 to 2013, involved 1,442 mother-child pairs who joined the study when the children were born and attended follow-up visits at Boston Medical Center. The mean age of the mothers was 29 years, and the children were, on average, 8 years old at follow-up. Half the children were male; 67% of mothers were black, and 20% were Latina.
The researchers collected maternal blood samples within 24-72 hours after birth to measure red blood cell lead levels and plasma folate levels. Children’s whole-blood lead levels were measured during the first lead screening of their well child visits, at a median 10 months of age. Researchers tracked children’s body mass index Z-score and defined overweight/obesity as exceeding the 85th national percentile for their age and sex.
Detectable lead was present in all the mothers’ blood samples. The median maternal red blood cell lead level was 2.5 mcg/dL, although black mothers tended to have higher lead exposure than that of other racial groups. Median maternal plasma folate level was 32 nmol/L. Children’s blood lead levels were a median 1.4 mcg/dL, and their median BMI Z-score was 0.78.
Children whose mothers had red blood cell lead levels of 5.0 mcg/dL or greater (16%) had 65% greater odds of being overweight or obese compared with children whose mothers’ lead level was less than 2 mcg/dL, after adjustment for maternal education, race/ethnicity, smoking status, parity, diabetes, hypertensive disorder, preterm birth, fetal growth, and breastfeeding status (odds ratio [OR], 1.65; 95% confidence internal [CI], 1.18-2.32). Only 5.2% of children had whole-blood lead levels of 5 mcg/dL or greater.
“Mothers with the highest red blood cell lead levels were older and multiparous, were more likely to be black and nonsmokers, had lower plasma folate levels and were more likely to have prepregnancy overweight/obesity and diabetes,” the authors reported.
The dose-response association did not lose significance when the researchers adjusted for children’s blood lead levels, maternal age, cesarean delivery, term births only, and black race. Nor did it change in a subset of children when the researchers adjusted for children’s physical activity.
The strength of the association increased when mothers also had a BMI greater than the average/healthy range. Children were more than four times more likely to be overweight or obese if their mothers were overweight or obese and had lead levels greater than 5.0 mcg/dL, compared with nonoverweight mothers with levels below 2 mcg/dL (OR, 4.24; 95% CI, 2.64-6.82).
Among children whose mothers were overweight/obese and had high blood lead levels, however, high folate levels appeared protective against obesity. These children had a 41% lower risk of being overweight or obese, compared with others in their group, if their mothers had plasma folate levels of at least 20 nmol/L (OR, 0.59 CI, 0.36-0.95; P = .03).
According to an invited commentary, “approximately 140,000 new chemicals and pesticides have appeared since 1950,” with “universal human exposure to approximately 5,000 of those,” wrote Marco Sanchez-Guerra, PhD, of the National Institute of Perinatology in Mexico City, and coauthors Andres Cardenas, PhD, of the University of California, Berkeley, and Citlalli Osorio-Yáñez, PhD, of the National Autonomous University of Mexico in Mexico City. Yet fewer than half of those chemicals have been tested for safety or toxic effect, the editorialists wrote, and scientists know little of their potential reproductive harm.
Dr. Sanchez-Guerra, Dr. Cardenas, and Dr. Osorio-Yáñez agreed with the study authors that elevated lead exposures, especially from gasoline before lead was removed in the United States in 1975, may partly explain the current epidemic of obesity.
“Identifying preventable prenatal causes of obesity is a cornerstone in the fight against the obesity epidemic,” the editorialists said. While most recommendations center on changes to diet and physical activity, environmental factors during pregnancy could be involved in childhood obesity as well.
“The study by Wang et al. opens the door to new questions about whether adequate folate intake might modify the adverse effects of other chemical exposures,” they continued, noting other research suggesting a protective effect from folate against health effects of air pollution exposure. “These efforts could yield substantial public health benefits and represent novel tools in fighting the obesity epidemic,” they concluded.
The research was funded by the National Institutes of Health and the U.S. Department of Health and Human Services. Neither the study authors nor the editorialists had industry financial disclosures.
SOURCES: Wang G et al. JAMA Netw Open. 2019;2(10):e1912343. doi: 10.1001/jamanetworkopen.2019.12343; Sanchez-Guerra M et al. JAMA Netw Open. 2019;2(10):e1912334. doi: 10.1001/jamanetworkopen.2019.12334.
Children born to mothers with high blood levels of lead have an increased risk of being overweight or obese, particularly if their mothers are also overweight, according to new research.
Adequate maternal plasma levels of folate, however, mitigated this risk.
“When considered simultaneously, maternal lead exposure, rather than early childhood lead exposure, contributed to overweight/obesity risk in a dose-response fashion across multiple developmental stages (preschool age, school age and early adolescence) and amplified intergenerational overweight/obesity risk (additively with maternal overweight/obesity),” Guoying Wang, MD, PhD, of Johns Hopkins Bloomberg School of Public Health, Baltimore, and associates, reported in JAMA Network Open.
“These findings support the hypothesis that the obesity epidemic could be related to environmental chemical exposures in utero and raise the possibility that optimal maternal folate supplementation may help counteract the adverse effects of environmental lead exposure,” the authors wrote.
The prospective urban, low-income cohort study, which ran from 2002 to 2013, involved 1,442 mother-child pairs who joined the study when the children were born and attended follow-up visits at Boston Medical Center. The mean age of the mothers was 29 years, and the children were, on average, 8 years old at follow-up. Half the children were male; 67% of mothers were black, and 20% were Latina.
The researchers collected maternal blood samples within 24-72 hours after birth to measure red blood cell lead levels and plasma folate levels. Children’s whole-blood lead levels were measured during the first lead screening of their well child visits, at a median 10 months of age. Researchers tracked children’s body mass index Z-score and defined overweight/obesity as exceeding the 85th national percentile for their age and sex.
Detectable lead was present in all the mothers’ blood samples. The median maternal red blood cell lead level was 2.5 mcg/dL, although black mothers tended to have higher lead exposure than that of other racial groups. Median maternal plasma folate level was 32 nmol/L. Children’s blood lead levels were a median 1.4 mcg/dL, and their median BMI Z-score was 0.78.
Children whose mothers had red blood cell lead levels of 5.0 mcg/dL or greater (16%) had 65% greater odds of being overweight or obese compared with children whose mothers’ lead level was less than 2 mcg/dL, after adjustment for maternal education, race/ethnicity, smoking status, parity, diabetes, hypertensive disorder, preterm birth, fetal growth, and breastfeeding status (odds ratio [OR], 1.65; 95% confidence internal [CI], 1.18-2.32). Only 5.2% of children had whole-blood lead levels of 5 mcg/dL or greater.
“Mothers with the highest red blood cell lead levels were older and multiparous, were more likely to be black and nonsmokers, had lower plasma folate levels and were more likely to have prepregnancy overweight/obesity and diabetes,” the authors reported.
The dose-response association did not lose significance when the researchers adjusted for children’s blood lead levels, maternal age, cesarean delivery, term births only, and black race. Nor did it change in a subset of children when the researchers adjusted for children’s physical activity.
The strength of the association increased when mothers also had a BMI greater than the average/healthy range. Children were more than four times more likely to be overweight or obese if their mothers were overweight or obese and had lead levels greater than 5.0 mcg/dL, compared with nonoverweight mothers with levels below 2 mcg/dL (OR, 4.24; 95% CI, 2.64-6.82).
Among children whose mothers were overweight/obese and had high blood lead levels, however, high folate levels appeared protective against obesity. These children had a 41% lower risk of being overweight or obese, compared with others in their group, if their mothers had plasma folate levels of at least 20 nmol/L (OR, 0.59 CI, 0.36-0.95; P = .03).
According to an invited commentary, “approximately 140,000 new chemicals and pesticides have appeared since 1950,” with “universal human exposure to approximately 5,000 of those,” wrote Marco Sanchez-Guerra, PhD, of the National Institute of Perinatology in Mexico City, and coauthors Andres Cardenas, PhD, of the University of California, Berkeley, and Citlalli Osorio-Yáñez, PhD, of the National Autonomous University of Mexico in Mexico City. Yet fewer than half of those chemicals have been tested for safety or toxic effect, the editorialists wrote, and scientists know little of their potential reproductive harm.
Dr. Sanchez-Guerra, Dr. Cardenas, and Dr. Osorio-Yáñez agreed with the study authors that elevated lead exposures, especially from gasoline before lead was removed in the United States in 1975, may partly explain the current epidemic of obesity.
“Identifying preventable prenatal causes of obesity is a cornerstone in the fight against the obesity epidemic,” the editorialists said. While most recommendations center on changes to diet and physical activity, environmental factors during pregnancy could be involved in childhood obesity as well.
“The study by Wang et al. opens the door to new questions about whether adequate folate intake might modify the adverse effects of other chemical exposures,” they continued, noting other research suggesting a protective effect from folate against health effects of air pollution exposure. “These efforts could yield substantial public health benefits and represent novel tools in fighting the obesity epidemic,” they concluded.
The research was funded by the National Institutes of Health and the U.S. Department of Health and Human Services. Neither the study authors nor the editorialists had industry financial disclosures.
SOURCES: Wang G et al. JAMA Netw Open. 2019;2(10):e1912343. doi: 10.1001/jamanetworkopen.2019.12343; Sanchez-Guerra M et al. JAMA Netw Open. 2019;2(10):e1912334. doi: 10.1001/jamanetworkopen.2019.12334.
FROM JAMA NETWORK OPEN
Higher teen pregnancy risk in girls with ADHD
Teenage girls with ADHD may be at greater risk of pregnancy than their unaffected peers, which suggests they may benefit from targeted interventions to prevent teen pregnancy.
A Swedish nationwide cohort study published in JAMA Network Open examined data from 384,103 nulliparous women and girls who gave birth between 2007-2014, of whom, 6,410 (1.7%) had received treatment for ADHD.
While the overall rate of teenage births was 3%, the rate among women and girls with ADHD was 15.3%, which represents a greater than sixfold higher odds of giving birth below the age of 20 years (odds ratio, 6.23; 95% confidence interval, 5.80-6.68).
“Becoming a mother at such early age is associated with long-term adverse outcomes for both women and their children,” wrote Charlotte Skoglund, PhD, of the department of clinical neuroscience at the Karolinska Institute in Stockholm and coauthors. “Consequently, our findings argue for an improvement in the standard of care for women and girls with ADHD, including active efforts to prevent teenage pregnancies and address comorbid medical and psychiatric conditions.”
The study also found women and girls with ADHD were significantly more likely to be underweight (OR, 1.29; 95% CI, 1.12-1.49) or have a body mass index greater than 40 kg/m2 (OR, 2.01; 95% CI, 1.60-2.52) when compared with those without ADHD.
They were also six times more likely to smoke, were nearly seven times more likely to continue smoking into their third trimester of pregnancy, and had a 20-fold higher odds of alcohol and substance use disorder. Among individuals who had been diagnosed with ADHD, 7.6% continued to use stimulant and nonstimulant ADHD medication during pregnancy, and 16.4% used antidepressants during pregnancy.
Psychiatric comorbidities were also significantly more common among individuals with ADHD in the year preceding pregnancy, compared with those without ADHD. The authors saw a 17-fold higher odds of receiving a diagnosis of bipolar disorder, nearly 8-fold higher odds of a diagnosis of schizophrenia or other psychotic disorder, and 22-fold higher odds of being diagnosed with emotionally unstable personality disorder among women and girls with ADHD versus those without.
The authors commented that antenatal care should focus on trying to reduce such obstetric risk factors in these women, but also pointed out that ADHD in women and girls was still underdiagnosed and undertreated.
Commenting on the association between ADHD and teenage pregnancy, the authors noted that women and girls with ADHD may be less likely to receive adequate contraceptive counseling and less likely to access, respond to, and act on counseling. They may also experience more adverse effects from hormonal contraceptives.
While Swedish youth clinics enable easier and low-cost access to counseling and contraception, the authors called for greater collaboration between psychiatric care clinics and specialized youth clinics to provide adequate care for women and girls with ADHD.
Three authors declared advisory board positions, grants, personal fees, and speakers’ fees from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Skoglund C et al. JAMA Netw Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.12463
Teenage girls with ADHD may be at greater risk of pregnancy than their unaffected peers, which suggests they may benefit from targeted interventions to prevent teen pregnancy.
A Swedish nationwide cohort study published in JAMA Network Open examined data from 384,103 nulliparous women and girls who gave birth between 2007-2014, of whom, 6,410 (1.7%) had received treatment for ADHD.
While the overall rate of teenage births was 3%, the rate among women and girls with ADHD was 15.3%, which represents a greater than sixfold higher odds of giving birth below the age of 20 years (odds ratio, 6.23; 95% confidence interval, 5.80-6.68).
“Becoming a mother at such early age is associated with long-term adverse outcomes for both women and their children,” wrote Charlotte Skoglund, PhD, of the department of clinical neuroscience at the Karolinska Institute in Stockholm and coauthors. “Consequently, our findings argue for an improvement in the standard of care for women and girls with ADHD, including active efforts to prevent teenage pregnancies and address comorbid medical and psychiatric conditions.”
The study also found women and girls with ADHD were significantly more likely to be underweight (OR, 1.29; 95% CI, 1.12-1.49) or have a body mass index greater than 40 kg/m2 (OR, 2.01; 95% CI, 1.60-2.52) when compared with those without ADHD.
They were also six times more likely to smoke, were nearly seven times more likely to continue smoking into their third trimester of pregnancy, and had a 20-fold higher odds of alcohol and substance use disorder. Among individuals who had been diagnosed with ADHD, 7.6% continued to use stimulant and nonstimulant ADHD medication during pregnancy, and 16.4% used antidepressants during pregnancy.
Psychiatric comorbidities were also significantly more common among individuals with ADHD in the year preceding pregnancy, compared with those without ADHD. The authors saw a 17-fold higher odds of receiving a diagnosis of bipolar disorder, nearly 8-fold higher odds of a diagnosis of schizophrenia or other psychotic disorder, and 22-fold higher odds of being diagnosed with emotionally unstable personality disorder among women and girls with ADHD versus those without.
The authors commented that antenatal care should focus on trying to reduce such obstetric risk factors in these women, but also pointed out that ADHD in women and girls was still underdiagnosed and undertreated.
Commenting on the association between ADHD and teenage pregnancy, the authors noted that women and girls with ADHD may be less likely to receive adequate contraceptive counseling and less likely to access, respond to, and act on counseling. They may also experience more adverse effects from hormonal contraceptives.
While Swedish youth clinics enable easier and low-cost access to counseling and contraception, the authors called for greater collaboration between psychiatric care clinics and specialized youth clinics to provide adequate care for women and girls with ADHD.
Three authors declared advisory board positions, grants, personal fees, and speakers’ fees from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Skoglund C et al. JAMA Netw Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.12463
Teenage girls with ADHD may be at greater risk of pregnancy than their unaffected peers, which suggests they may benefit from targeted interventions to prevent teen pregnancy.
A Swedish nationwide cohort study published in JAMA Network Open examined data from 384,103 nulliparous women and girls who gave birth between 2007-2014, of whom, 6,410 (1.7%) had received treatment for ADHD.
While the overall rate of teenage births was 3%, the rate among women and girls with ADHD was 15.3%, which represents a greater than sixfold higher odds of giving birth below the age of 20 years (odds ratio, 6.23; 95% confidence interval, 5.80-6.68).
“Becoming a mother at such early age is associated with long-term adverse outcomes for both women and their children,” wrote Charlotte Skoglund, PhD, of the department of clinical neuroscience at the Karolinska Institute in Stockholm and coauthors. “Consequently, our findings argue for an improvement in the standard of care for women and girls with ADHD, including active efforts to prevent teenage pregnancies and address comorbid medical and psychiatric conditions.”
The study also found women and girls with ADHD were significantly more likely to be underweight (OR, 1.29; 95% CI, 1.12-1.49) or have a body mass index greater than 40 kg/m2 (OR, 2.01; 95% CI, 1.60-2.52) when compared with those without ADHD.
They were also six times more likely to smoke, were nearly seven times more likely to continue smoking into their third trimester of pregnancy, and had a 20-fold higher odds of alcohol and substance use disorder. Among individuals who had been diagnosed with ADHD, 7.6% continued to use stimulant and nonstimulant ADHD medication during pregnancy, and 16.4% used antidepressants during pregnancy.
Psychiatric comorbidities were also significantly more common among individuals with ADHD in the year preceding pregnancy, compared with those without ADHD. The authors saw a 17-fold higher odds of receiving a diagnosis of bipolar disorder, nearly 8-fold higher odds of a diagnosis of schizophrenia or other psychotic disorder, and 22-fold higher odds of being diagnosed with emotionally unstable personality disorder among women and girls with ADHD versus those without.
The authors commented that antenatal care should focus on trying to reduce such obstetric risk factors in these women, but also pointed out that ADHD in women and girls was still underdiagnosed and undertreated.
Commenting on the association between ADHD and teenage pregnancy, the authors noted that women and girls with ADHD may be less likely to receive adequate contraceptive counseling and less likely to access, respond to, and act on counseling. They may also experience more adverse effects from hormonal contraceptives.
While Swedish youth clinics enable easier and low-cost access to counseling and contraception, the authors called for greater collaboration between psychiatric care clinics and specialized youth clinics to provide adequate care for women and girls with ADHD.
Three authors declared advisory board positions, grants, personal fees, and speakers’ fees from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Skoglund C et al. JAMA Netw Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.12463
FROM JAMA NETWORK OPEN
NICUs admitting more normal-weight newborns
Almost half of the newborns admitted to U.S. neonatal intensive care units in 2017 were of normal birth weight, according to a new report from the Dartmouth Institute for Health Policy & Clinical Practice.
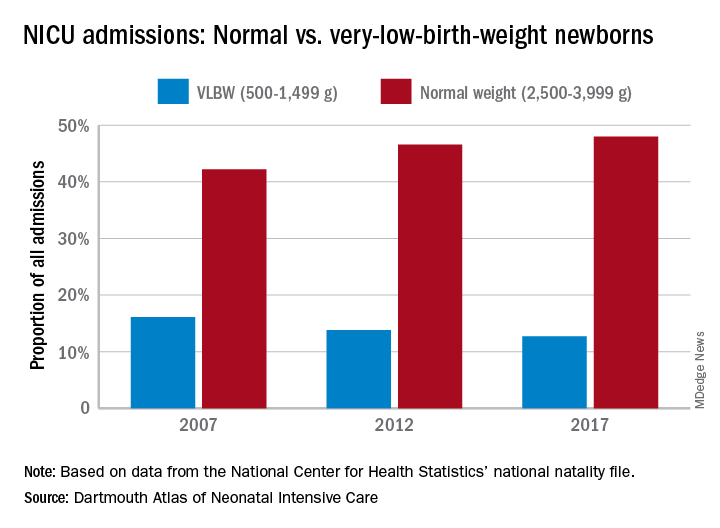
The proportion of NICU admissions involving normal-weight (2,500-3,999 g) newborns increased from 42% in 2007 to 48% in 2017, investigators said in the Dartmouth Atlas of Neonatal Intensive Care. Over that same period, admissions of very-low-birth-weight (500-1,499 g) babies dropped from 16% to 13% of the total.
Those changes were part of a larger, longer-term trend. “The expansion of NICUs and beds in recent decades has been associated with changes in the newborn population receiving NICU care,” the investigators said in the report.
The number of NICU beds increased by 65% from 1995 to 2013, and the number of neonatologists rose by 75% from 1996 to 2013. “At the same time, ” they said in a written statement.
The increases in NICU and neonatologist supply, however, did not always follow the need for such care. Areas of the country with high rates of newborn prematurity, or of risk factors such as low maternal education levels or high cesarean section rates, do not have higher supplies of NICU beds or neonatologists, the researchers noted.
“We should not spare a dollar in providing the best care for newborns. But spending more doesn’t help infants if they could receive the care they need in a maternity unit or home with their mothers. It is very troubling that such a valuable and expensive health care resource is not distributed where it is needed,” said principal author David C. Goodman, MD, of the Dartmouth Institute for Health Policy & Clinical Practice in Lebanon, N.H.
Almost half of the newborns admitted to U.S. neonatal intensive care units in 2017 were of normal birth weight, according to a new report from the Dartmouth Institute for Health Policy & Clinical Practice.

The proportion of NICU admissions involving normal-weight (2,500-3,999 g) newborns increased from 42% in 2007 to 48% in 2017, investigators said in the Dartmouth Atlas of Neonatal Intensive Care. Over that same period, admissions of very-low-birth-weight (500-1,499 g) babies dropped from 16% to 13% of the total.
Those changes were part of a larger, longer-term trend. “The expansion of NICUs and beds in recent decades has been associated with changes in the newborn population receiving NICU care,” the investigators said in the report.
The number of NICU beds increased by 65% from 1995 to 2013, and the number of neonatologists rose by 75% from 1996 to 2013. “At the same time, ” they said in a written statement.
The increases in NICU and neonatologist supply, however, did not always follow the need for such care. Areas of the country with high rates of newborn prematurity, or of risk factors such as low maternal education levels or high cesarean section rates, do not have higher supplies of NICU beds or neonatologists, the researchers noted.
“We should not spare a dollar in providing the best care for newborns. But spending more doesn’t help infants if they could receive the care they need in a maternity unit or home with their mothers. It is very troubling that such a valuable and expensive health care resource is not distributed where it is needed,” said principal author David C. Goodman, MD, of the Dartmouth Institute for Health Policy & Clinical Practice in Lebanon, N.H.
Almost half of the newborns admitted to U.S. neonatal intensive care units in 2017 were of normal birth weight, according to a new report from the Dartmouth Institute for Health Policy & Clinical Practice.

The proportion of NICU admissions involving normal-weight (2,500-3,999 g) newborns increased from 42% in 2007 to 48% in 2017, investigators said in the Dartmouth Atlas of Neonatal Intensive Care. Over that same period, admissions of very-low-birth-weight (500-1,499 g) babies dropped from 16% to 13% of the total.
Those changes were part of a larger, longer-term trend. “The expansion of NICUs and beds in recent decades has been associated with changes in the newborn population receiving NICU care,” the investigators said in the report.
The number of NICU beds increased by 65% from 1995 to 2013, and the number of neonatologists rose by 75% from 1996 to 2013. “At the same time, ” they said in a written statement.
The increases in NICU and neonatologist supply, however, did not always follow the need for such care. Areas of the country with high rates of newborn prematurity, or of risk factors such as low maternal education levels or high cesarean section rates, do not have higher supplies of NICU beds or neonatologists, the researchers noted.
“We should not spare a dollar in providing the best care for newborns. But spending more doesn’t help infants if they could receive the care they need in a maternity unit or home with their mothers. It is very troubling that such a valuable and expensive health care resource is not distributed where it is needed,” said principal author David C. Goodman, MD, of the Dartmouth Institute for Health Policy & Clinical Practice in Lebanon, N.H.
Preemptive pacifier promotion
I recently encountered an article aimed at parents who were struggling with what to do about their child’s persistent attachment to his pacifier (“How to Ditch the Pacifier,” by Anna Nowogrodski, New York Times, 2019 Sept. 16). For the most part, the author presented a sampling of sound advice from pediatricians and other health experts.
Most children will abandon their pacifiers at a time that is consistent with their developmental stage. Pacifiers seldom do any permanent damage, although they aren’t terribly appealing to look at when hanging out of a toddling toddler’s mouth. Parents were urged to be patient and consistent and were told that allowing the gooey thing to self-destruct often works, as does accelerating the process with a razor blade. Enlisting the aid of the Pacifier Fairy was suggested, but I’m not so sure that would work terribly well.
As I finished perusing the article, I couldn’t help think of how this vexing issue of pacifier removal can be avoided if parents follow a simple rule when they first introduced a pacifier to their child. If experienced parents think back to when they first resorted to using the pacifier, it wasn’t because the plastic and rubber gadget was a family heirloom that had been passed down from generation to generation like an engraved silver spoon. It wasn’t because the dentist told them that children who use pacifiers are less likely to need braces on their teeth. Nor was it a rumor filtered down from speech therapists that pacifiers improve articulation.
Parents reach for a pacifier in hopes that it will help their child will fall asleep. I think most parents of older children agree that at the beginning the pacifier was first and foremost a sleep aid. But here is where the critical oversight occurs: If you give your children pacifiers when you want them to go to sleep, why not simply add the stipulation of where you would like them to go to sleep as well?
Most parents prefer that their children sleep in their own space. We can argue of whether that should be in a side sleeper or their own crib, but most parents don’t want their 3-year-olds sleeping in their bed. Nor do they want their children sleeping on the couch in the living room with them while they watch a movie at 10:30 at night. And as pediatricians, we prefer that children not sleep with their necks flexed in a car seat or baby rocker, particularly if they’re a preemie.
Augmenting the primary association between sleep and the pacifier by adding a place has several important advantages. It gives parents more control of where their children will sleep or, more importantly, where they won’t be sleeping. It helps transitions to nonhome sleeping places like day care and long trips to grandma’s house go more smoothly.
Even more importantly, the crib/pacifier association helps parents who have had trouble reading their children’s cues. If they want a pacifier, it means they are tired and want to go to where the pacifier lives: bed
Finally, maintaining the link between sleeping and the pacifier promotes a more natural weaning process than going cold turkey or hiring the Pacifier Fairy. As naps disappear, the pacifier gradually become a less obvious accessory in the child’s life. However, it may linger in the background as a reminder of when the child needs some restorative sleep.
Of course, helping parents to think clearly enough to create and enforce a simple rule long enough to forge a healthy association when they are sleep deprived themselves is just another one of those challenges we must accept as concerned primary care pediatricians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired? The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
I recently encountered an article aimed at parents who were struggling with what to do about their child’s persistent attachment to his pacifier (“How to Ditch the Pacifier,” by Anna Nowogrodski, New York Times, 2019 Sept. 16). For the most part, the author presented a sampling of sound advice from pediatricians and other health experts.
Most children will abandon their pacifiers at a time that is consistent with their developmental stage. Pacifiers seldom do any permanent damage, although they aren’t terribly appealing to look at when hanging out of a toddling toddler’s mouth. Parents were urged to be patient and consistent and were told that allowing the gooey thing to self-destruct often works, as does accelerating the process with a razor blade. Enlisting the aid of the Pacifier Fairy was suggested, but I’m not so sure that would work terribly well.
As I finished perusing the article, I couldn’t help think of how this vexing issue of pacifier removal can be avoided if parents follow a simple rule when they first introduced a pacifier to their child. If experienced parents think back to when they first resorted to using the pacifier, it wasn’t because the plastic and rubber gadget was a family heirloom that had been passed down from generation to generation like an engraved silver spoon. It wasn’t because the dentist told them that children who use pacifiers are less likely to need braces on their teeth. Nor was it a rumor filtered down from speech therapists that pacifiers improve articulation.
Parents reach for a pacifier in hopes that it will help their child will fall asleep. I think most parents of older children agree that at the beginning the pacifier was first and foremost a sleep aid. But here is where the critical oversight occurs: If you give your children pacifiers when you want them to go to sleep, why not simply add the stipulation of where you would like them to go to sleep as well?
Most parents prefer that their children sleep in their own space. We can argue of whether that should be in a side sleeper or their own crib, but most parents don’t want their 3-year-olds sleeping in their bed. Nor do they want their children sleeping on the couch in the living room with them while they watch a movie at 10:30 at night. And as pediatricians, we prefer that children not sleep with their necks flexed in a car seat or baby rocker, particularly if they’re a preemie.
Augmenting the primary association between sleep and the pacifier by adding a place has several important advantages. It gives parents more control of where their children will sleep or, more importantly, where they won’t be sleeping. It helps transitions to nonhome sleeping places like day care and long trips to grandma’s house go more smoothly.
Even more importantly, the crib/pacifier association helps parents who have had trouble reading their children’s cues. If they want a pacifier, it means they are tired and want to go to where the pacifier lives: bed
Finally, maintaining the link between sleeping and the pacifier promotes a more natural weaning process than going cold turkey or hiring the Pacifier Fairy. As naps disappear, the pacifier gradually become a less obvious accessory in the child’s life. However, it may linger in the background as a reminder of when the child needs some restorative sleep.
Of course, helping parents to think clearly enough to create and enforce a simple rule long enough to forge a healthy association when they are sleep deprived themselves is just another one of those challenges we must accept as concerned primary care pediatricians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired? The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
I recently encountered an article aimed at parents who were struggling with what to do about their child’s persistent attachment to his pacifier (“How to Ditch the Pacifier,” by Anna Nowogrodski, New York Times, 2019 Sept. 16). For the most part, the author presented a sampling of sound advice from pediatricians and other health experts.
Most children will abandon their pacifiers at a time that is consistent with their developmental stage. Pacifiers seldom do any permanent damage, although they aren’t terribly appealing to look at when hanging out of a toddling toddler’s mouth. Parents were urged to be patient and consistent and were told that allowing the gooey thing to self-destruct often works, as does accelerating the process with a razor blade. Enlisting the aid of the Pacifier Fairy was suggested, but I’m not so sure that would work terribly well.
As I finished perusing the article, I couldn’t help think of how this vexing issue of pacifier removal can be avoided if parents follow a simple rule when they first introduced a pacifier to their child. If experienced parents think back to when they first resorted to using the pacifier, it wasn’t because the plastic and rubber gadget was a family heirloom that had been passed down from generation to generation like an engraved silver spoon. It wasn’t because the dentist told them that children who use pacifiers are less likely to need braces on their teeth. Nor was it a rumor filtered down from speech therapists that pacifiers improve articulation.
Parents reach for a pacifier in hopes that it will help their child will fall asleep. I think most parents of older children agree that at the beginning the pacifier was first and foremost a sleep aid. But here is where the critical oversight occurs: If you give your children pacifiers when you want them to go to sleep, why not simply add the stipulation of where you would like them to go to sleep as well?
Most parents prefer that their children sleep in their own space. We can argue of whether that should be in a side sleeper or their own crib, but most parents don’t want their 3-year-olds sleeping in their bed. Nor do they want their children sleeping on the couch in the living room with them while they watch a movie at 10:30 at night. And as pediatricians, we prefer that children not sleep with their necks flexed in a car seat or baby rocker, particularly if they’re a preemie.
Augmenting the primary association between sleep and the pacifier by adding a place has several important advantages. It gives parents more control of where their children will sleep or, more importantly, where they won’t be sleeping. It helps transitions to nonhome sleeping places like day care and long trips to grandma’s house go more smoothly.
Even more importantly, the crib/pacifier association helps parents who have had trouble reading their children’s cues. If they want a pacifier, it means they are tired and want to go to where the pacifier lives: bed
Finally, maintaining the link between sleeping and the pacifier promotes a more natural weaning process than going cold turkey or hiring the Pacifier Fairy. As naps disappear, the pacifier gradually become a less obvious accessory in the child’s life. However, it may linger in the background as a reminder of when the child needs some restorative sleep.
Of course, helping parents to think clearly enough to create and enforce a simple rule long enough to forge a healthy association when they are sleep deprived themselves is just another one of those challenges we must accept as concerned primary care pediatricians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired? The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
FDA approves rituximab to treat children with rare vasculitis
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
Apple cider vinegar soaks fall short in atopic dermatitis
, in a pilot split-arm study.
The aim of the study was to evaluate the effects of diluted apple cider vinegar application on transepidermal water loss (TEWL) and pH on skin affected by AD and on healthy skin, according to Lydia A. Luu of the department of dermatology at University of Virginia, Charlottesville, and colleagues. “Acetic acid, particularly apple cider vinegar, is prominent among emerging natural remedies used in AD. Therefore, determining the safety of this commonly used product is crucial,” they wrote in the study, published in Pediatric Dermatology.
In total, 11 patients with AD and 11 healthy controls were included; most of those with AD were considered mild (36.4%) or moderate (45.5%). Participants had not used systemic or topical antimicrobial treatments in the month preceding the study, and they were aged 12 years and older (mean ages were 20.6 years in the AD group and 28.8 years among controls). Those with AD had significantly elevated TEWL at baseline, compared with controls.
For 14 days, study participants soaked one forearm in dilute apple cider vinegar (0.5% acetic acid) and the other in tap water for 10 minutes daily. Changes in pH and TEWL before and after application were measured.
The researchers found that TEWL significantly increased immediately post treatment (at 0 and 15 minutes) in both groups, dropping to baseline at 30 minutes among those with AD and at 60 minutes among controls.
Skin pH was similar in both groups at baseline (4.86-4.88). After the cider vinegar soak, there was a transient reduction in skin pH among AD patients that lasted for 15 minutes among those with AD and 60 minutes in controls. This finding “suggests temporary acidification of the skin that has theoretical benefit of correcting disrupted skin pH in AD,” the authors wrote, noting that increased TEWL and alkaline skin pH is common among people with AD because of skin barrier dysfunction.
With respect to safety, 72.7% (16) of the participants experienced skin discomfort, mostly described as mild, limited to the vinegar-treated arm. After discontinuation, the majority of skin irritation resolved quickly, with no additional therapy.
The authors acknowledged two key limitations of the study were the homogeneous patient population and small sample size. “Although epidermal acidification would theoretically be beneficial in treating AD, our study shows that acidification by way of topical bathing in a 0.5% [apple cider vinegar] solution as performed in this study is not useful in AD treatment,” they wrote. “Further studies in a more diverse population will be necessary to fully characterize the risk/benefit profile of topical dilute apple cider vinegar treatments.”
The study was funded by the University of Virginia. The authors did not provide information on financial disclosures.
SOURCE: Luu LA et al. Pediatr Dermatol. 2019 Jul 22. doi: 10.1111/pde.13888.
, in a pilot split-arm study.
The aim of the study was to evaluate the effects of diluted apple cider vinegar application on transepidermal water loss (TEWL) and pH on skin affected by AD and on healthy skin, according to Lydia A. Luu of the department of dermatology at University of Virginia, Charlottesville, and colleagues. “Acetic acid, particularly apple cider vinegar, is prominent among emerging natural remedies used in AD. Therefore, determining the safety of this commonly used product is crucial,” they wrote in the study, published in Pediatric Dermatology.
In total, 11 patients with AD and 11 healthy controls were included; most of those with AD were considered mild (36.4%) or moderate (45.5%). Participants had not used systemic or topical antimicrobial treatments in the month preceding the study, and they were aged 12 years and older (mean ages were 20.6 years in the AD group and 28.8 years among controls). Those with AD had significantly elevated TEWL at baseline, compared with controls.
For 14 days, study participants soaked one forearm in dilute apple cider vinegar (0.5% acetic acid) and the other in tap water for 10 minutes daily. Changes in pH and TEWL before and after application were measured.
The researchers found that TEWL significantly increased immediately post treatment (at 0 and 15 minutes) in both groups, dropping to baseline at 30 minutes among those with AD and at 60 minutes among controls.
Skin pH was similar in both groups at baseline (4.86-4.88). After the cider vinegar soak, there was a transient reduction in skin pH among AD patients that lasted for 15 minutes among those with AD and 60 minutes in controls. This finding “suggests temporary acidification of the skin that has theoretical benefit of correcting disrupted skin pH in AD,” the authors wrote, noting that increased TEWL and alkaline skin pH is common among people with AD because of skin barrier dysfunction.
With respect to safety, 72.7% (16) of the participants experienced skin discomfort, mostly described as mild, limited to the vinegar-treated arm. After discontinuation, the majority of skin irritation resolved quickly, with no additional therapy.
The authors acknowledged two key limitations of the study were the homogeneous patient population and small sample size. “Although epidermal acidification would theoretically be beneficial in treating AD, our study shows that acidification by way of topical bathing in a 0.5% [apple cider vinegar] solution as performed in this study is not useful in AD treatment,” they wrote. “Further studies in a more diverse population will be necessary to fully characterize the risk/benefit profile of topical dilute apple cider vinegar treatments.”
The study was funded by the University of Virginia. The authors did not provide information on financial disclosures.
SOURCE: Luu LA et al. Pediatr Dermatol. 2019 Jul 22. doi: 10.1111/pde.13888.
, in a pilot split-arm study.
The aim of the study was to evaluate the effects of diluted apple cider vinegar application on transepidermal water loss (TEWL) and pH on skin affected by AD and on healthy skin, according to Lydia A. Luu of the department of dermatology at University of Virginia, Charlottesville, and colleagues. “Acetic acid, particularly apple cider vinegar, is prominent among emerging natural remedies used in AD. Therefore, determining the safety of this commonly used product is crucial,” they wrote in the study, published in Pediatric Dermatology.
In total, 11 patients with AD and 11 healthy controls were included; most of those with AD were considered mild (36.4%) or moderate (45.5%). Participants had not used systemic or topical antimicrobial treatments in the month preceding the study, and they were aged 12 years and older (mean ages were 20.6 years in the AD group and 28.8 years among controls). Those with AD had significantly elevated TEWL at baseline, compared with controls.
For 14 days, study participants soaked one forearm in dilute apple cider vinegar (0.5% acetic acid) and the other in tap water for 10 minutes daily. Changes in pH and TEWL before and after application were measured.
The researchers found that TEWL significantly increased immediately post treatment (at 0 and 15 minutes) in both groups, dropping to baseline at 30 minutes among those with AD and at 60 minutes among controls.
Skin pH was similar in both groups at baseline (4.86-4.88). After the cider vinegar soak, there was a transient reduction in skin pH among AD patients that lasted for 15 minutes among those with AD and 60 minutes in controls. This finding “suggests temporary acidification of the skin that has theoretical benefit of correcting disrupted skin pH in AD,” the authors wrote, noting that increased TEWL and alkaline skin pH is common among people with AD because of skin barrier dysfunction.
With respect to safety, 72.7% (16) of the participants experienced skin discomfort, mostly described as mild, limited to the vinegar-treated arm. After discontinuation, the majority of skin irritation resolved quickly, with no additional therapy.
The authors acknowledged two key limitations of the study were the homogeneous patient population and small sample size. “Although epidermal acidification would theoretically be beneficial in treating AD, our study shows that acidification by way of topical bathing in a 0.5% [apple cider vinegar] solution as performed in this study is not useful in AD treatment,” they wrote. “Further studies in a more diverse population will be necessary to fully characterize the risk/benefit profile of topical dilute apple cider vinegar treatments.”
The study was funded by the University of Virginia. The authors did not provide information on financial disclosures.
SOURCE: Luu LA et al. Pediatr Dermatol. 2019 Jul 22. doi: 10.1111/pde.13888.
FROM PEDIATRIC DERMATOLOGY
FDA expands Dysport’s upper-limb spasticity indication to children
The Food and Drug Administration has expanded the indication of abobotulinumtoxinA (Dysport) for upper-limb spasticity to include patients aged 2 years and older, according to a release from Ipsen. This botulinum toxin product received approval for this indication in adults in 2015 and approval for lower-limb spasticity in patients aged 2 years and older in 2016. Notably, Orphan Drug Exclusivity prevents it from being indicated for patients with cerebral palsy because another botulinum toxin product, onabotulinumtoxinA (Botox), already was approved for the indication in June 2019.
Spasticity affects the muscles and joints of extremities, especially in growing children, and is usually caused by nerve damage, such as head trauma or spinal cord injury. The degree of spasticity can vary from mild muscle stiffness to severe, painful, and uncontrollable muscle spasms.
AbobotulinumtoxinA was evaluated for upper-limb spasticity in a phase 3, randomized, double-blind, low-dose controlled, multicenter study; the study enrolled 210 children aged 2-17 years with the condition and a Modified Ashworth Scale grade 2 or greater for elbow and wrist flexors. The children were randomized 1:1:1 to injections of either 8 units/kg, 16 units/kg, or 2 units/kg into the elbow flexors and wrist flexors. At 6 weeks, there were statistically significant improvements in Modified Ashworth Scale grade, the primary endpoint, with least-square mean changes from baseline of –2.0, –2.3, and –1.6, respectively.
AbobotulinumtoxinA and all other botulinum toxin products carry a boxed warning, the most serious warning the FDA issues. This warning refers to risk of botulism-like symptoms caused by the botulinum toxin spreading away from the injection area; these symptoms can included sometimes life-threatening difficulty swallowing or breathing. AbobotulinumtoxinA is contraindicated in patients with known hypersensitivity to any botulinum toxin or any of the components, those with presence of infection at proposed injection site(s), and those with known allergy to cow’s milk protein. It is also important to note that botulinum toxin preparations are not interchangeable; the potency units of one are not the same as those of another. Full prescribing information can be found on the Ipsen website.
The Food and Drug Administration has expanded the indication of abobotulinumtoxinA (Dysport) for upper-limb spasticity to include patients aged 2 years and older, according to a release from Ipsen. This botulinum toxin product received approval for this indication in adults in 2015 and approval for lower-limb spasticity in patients aged 2 years and older in 2016. Notably, Orphan Drug Exclusivity prevents it from being indicated for patients with cerebral palsy because another botulinum toxin product, onabotulinumtoxinA (Botox), already was approved for the indication in June 2019.
Spasticity affects the muscles and joints of extremities, especially in growing children, and is usually caused by nerve damage, such as head trauma or spinal cord injury. The degree of spasticity can vary from mild muscle stiffness to severe, painful, and uncontrollable muscle spasms.
AbobotulinumtoxinA was evaluated for upper-limb spasticity in a phase 3, randomized, double-blind, low-dose controlled, multicenter study; the study enrolled 210 children aged 2-17 years with the condition and a Modified Ashworth Scale grade 2 or greater for elbow and wrist flexors. The children were randomized 1:1:1 to injections of either 8 units/kg, 16 units/kg, or 2 units/kg into the elbow flexors and wrist flexors. At 6 weeks, there were statistically significant improvements in Modified Ashworth Scale grade, the primary endpoint, with least-square mean changes from baseline of –2.0, –2.3, and –1.6, respectively.
AbobotulinumtoxinA and all other botulinum toxin products carry a boxed warning, the most serious warning the FDA issues. This warning refers to risk of botulism-like symptoms caused by the botulinum toxin spreading away from the injection area; these symptoms can included sometimes life-threatening difficulty swallowing or breathing. AbobotulinumtoxinA is contraindicated in patients with known hypersensitivity to any botulinum toxin or any of the components, those with presence of infection at proposed injection site(s), and those with known allergy to cow’s milk protein. It is also important to note that botulinum toxin preparations are not interchangeable; the potency units of one are not the same as those of another. Full prescribing information can be found on the Ipsen website.
The Food and Drug Administration has expanded the indication of abobotulinumtoxinA (Dysport) for upper-limb spasticity to include patients aged 2 years and older, according to a release from Ipsen. This botulinum toxin product received approval for this indication in adults in 2015 and approval for lower-limb spasticity in patients aged 2 years and older in 2016. Notably, Orphan Drug Exclusivity prevents it from being indicated for patients with cerebral palsy because another botulinum toxin product, onabotulinumtoxinA (Botox), already was approved for the indication in June 2019.
Spasticity affects the muscles and joints of extremities, especially in growing children, and is usually caused by nerve damage, such as head trauma or spinal cord injury. The degree of spasticity can vary from mild muscle stiffness to severe, painful, and uncontrollable muscle spasms.
AbobotulinumtoxinA was evaluated for upper-limb spasticity in a phase 3, randomized, double-blind, low-dose controlled, multicenter study; the study enrolled 210 children aged 2-17 years with the condition and a Modified Ashworth Scale grade 2 or greater for elbow and wrist flexors. The children were randomized 1:1:1 to injections of either 8 units/kg, 16 units/kg, or 2 units/kg into the elbow flexors and wrist flexors. At 6 weeks, there were statistically significant improvements in Modified Ashworth Scale grade, the primary endpoint, with least-square mean changes from baseline of –2.0, –2.3, and –1.6, respectively.
AbobotulinumtoxinA and all other botulinum toxin products carry a boxed warning, the most serious warning the FDA issues. This warning refers to risk of botulism-like symptoms caused by the botulinum toxin spreading away from the injection area; these symptoms can included sometimes life-threatening difficulty swallowing or breathing. AbobotulinumtoxinA is contraindicated in patients with known hypersensitivity to any botulinum toxin or any of the components, those with presence of infection at proposed injection site(s), and those with known allergy to cow’s milk protein. It is also important to note that botulinum toxin preparations are not interchangeable; the potency units of one are not the same as those of another. Full prescribing information can be found on the Ipsen website.
Universal ASD screening is feasible, but M-CHAT/F underperforms
, especially in children of color and in those from lower-income households, new research found.
Guidelines for universal autism spectrum disorder (ASD) screening currently conflict, Whitney Guthrie, PhD, of the Children’s Hospital of Philadelphia, and associates wrote in Pediatrics. The American Academy of Pediatrics recommends universal screening in children aged 18 months and 24 months to expedite earlier identification and diagnosis. However, the U.S. Preventive Services Task Force has concluded that “there is insufficient evidence to recommend universal screening, in part because of limited data on outcomes for children who screen negative and from diverse samples.”
Dr. Guthrie and associates conducted a study of 25,999 children aged 16-26 months who had a well-child visit between January 2011 and July 2015 at a Children’s Hospital of Philadelphia site that had implemented universal electronic screening. Of this group, 43% were white, 37% were black, and the remainder were Asian or of other/multiple races; 92% were non-Hispanic. The median parental income was $59,597, 54% had private insurance and 45.3% had public insurance/Medicaid, and 42% came from an urban primary care site while the rest came from suburban primary care.
Screening rates were good over the study period, with 91% of children undergoing at least one screen with the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F). While 50% were screened more than once, only 48% of children were screened at 18 and 24 months, as per the AAP guideline. Children who were screened multiple times were more likely to be white and non-Hispanic, to be from a suburban site, and to have higher incomes and private insurance.
After the first M-CHAT/F screen, 9.5% of children were positive, a rate comparable with that seen in other large-scale, U.S.-based studies. Of the 2,256 children who tested positive, 41% received a second screen; 782 (95%) of these children tested negative.
After the study period, most children (n = 20,437; 87%) continued receiving care with the Children’s Hospital of Philadelphia system and had diagnostic data available past the age of 4 years. ASD prevalence was 2%, giving the M-CHAT/F a sensitivity of 39%, a specificity of 95%, a positive predictive value of 15%, and a negative predictive value of 99%.
M-CHAT/F sensitivity was higher in older children (49% at 21-26 months vs. 35% at 16-20 months) and with repeated screenings (40% vs. 32%), and positive predictive value was lower in girls (8% vs. 20%). Specificity and positive predictive value were higher in white children (98% and 24%, respectively), compared with black children (92% and 12%, respectively), Asian children (90% and 11%, respectively), and those from other/multiple racial groups (94% and 13%, respectively). Higher-income families also saw increased specificity (97% vs. 92%) and positive predictive value (20% vs. 12%), compared with lower-income families.
While Dr. Guthrie and associates wrote of new methods of screening, such as parental reporting tools supported by picture or video and “direct data-gathering methods that leverage technological advances in computing and machine learning,” Lonnie Zwaigenbaum, MD, MSc, and Jonathon Maguire, MD, MSc, argued in an editorial, also published in Pediatrics, that the M-CHAT/F “remains a strong candidate” for universal ASD screening, despite the notable weaknesses.
“Ultimately, the potential added value of ASD screening must be considered relative to what would occur in its absence,” wrote Dr. Zwaigenbaum of the Women’s and Children’s Health Research Institute at the University of Alberta, Edmonton, and Dr. Maguire of St Michael’s Hospital, Toronto, and the University of Toronto. “Although it is difficult to object to the guidance from the U.S. Preventive Services Task Force to listen carefully to parents’ concerns, we must acknowledge the false dichotomy between screening and surveillance in this context. ... Why not use the best available measurement tools to identify developmental concerns with the highest possible accuracy?”
The study was funded by the Allerton Foundation, Eagles Charitable Foundation, and the National Institute of Mental Health; the study investigators reported that they had no conflicts of interest. Dr. Zwaigenbaum is a member of an independent data monitoring committee for a Roche-funded medication trial and Dr. Maguire reported receiving nonfinancial support from Ddrops for an investigator-initiated study on vitamin D and respiratory tract infections. Dr Zwaigenbaum is supported by the Stollery Children’s Hospital Foundation Chair in Autism Research. Dr Maguire is supported by St. Michael’s Hospital and the Hospital for Sick Children.
SOURCEs: Guthrie W et al. Pediatrics. 2019 Sep 27. doi: 10.1542/peds.2018-3963; Zwaigenbaum L, Maguire J. Pediatrics. 2019 Sep 27. doi: 10.1542/peds.2019-0925.
, especially in children of color and in those from lower-income households, new research found.
Guidelines for universal autism spectrum disorder (ASD) screening currently conflict, Whitney Guthrie, PhD, of the Children’s Hospital of Philadelphia, and associates wrote in Pediatrics. The American Academy of Pediatrics recommends universal screening in children aged 18 months and 24 months to expedite earlier identification and diagnosis. However, the U.S. Preventive Services Task Force has concluded that “there is insufficient evidence to recommend universal screening, in part because of limited data on outcomes for children who screen negative and from diverse samples.”
Dr. Guthrie and associates conducted a study of 25,999 children aged 16-26 months who had a well-child visit between January 2011 and July 2015 at a Children’s Hospital of Philadelphia site that had implemented universal electronic screening. Of this group, 43% were white, 37% were black, and the remainder were Asian or of other/multiple races; 92% were non-Hispanic. The median parental income was $59,597, 54% had private insurance and 45.3% had public insurance/Medicaid, and 42% came from an urban primary care site while the rest came from suburban primary care.
Screening rates were good over the study period, with 91% of children undergoing at least one screen with the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F). While 50% were screened more than once, only 48% of children were screened at 18 and 24 months, as per the AAP guideline. Children who were screened multiple times were more likely to be white and non-Hispanic, to be from a suburban site, and to have higher incomes and private insurance.
After the first M-CHAT/F screen, 9.5% of children were positive, a rate comparable with that seen in other large-scale, U.S.-based studies. Of the 2,256 children who tested positive, 41% received a second screen; 782 (95%) of these children tested negative.
After the study period, most children (n = 20,437; 87%) continued receiving care with the Children’s Hospital of Philadelphia system and had diagnostic data available past the age of 4 years. ASD prevalence was 2%, giving the M-CHAT/F a sensitivity of 39%, a specificity of 95%, a positive predictive value of 15%, and a negative predictive value of 99%.
M-CHAT/F sensitivity was higher in older children (49% at 21-26 months vs. 35% at 16-20 months) and with repeated screenings (40% vs. 32%), and positive predictive value was lower in girls (8% vs. 20%). Specificity and positive predictive value were higher in white children (98% and 24%, respectively), compared with black children (92% and 12%, respectively), Asian children (90% and 11%, respectively), and those from other/multiple racial groups (94% and 13%, respectively). Higher-income families also saw increased specificity (97% vs. 92%) and positive predictive value (20% vs. 12%), compared with lower-income families.
While Dr. Guthrie and associates wrote of new methods of screening, such as parental reporting tools supported by picture or video and “direct data-gathering methods that leverage technological advances in computing and machine learning,” Lonnie Zwaigenbaum, MD, MSc, and Jonathon Maguire, MD, MSc, argued in an editorial, also published in Pediatrics, that the M-CHAT/F “remains a strong candidate” for universal ASD screening, despite the notable weaknesses.
“Ultimately, the potential added value of ASD screening must be considered relative to what would occur in its absence,” wrote Dr. Zwaigenbaum of the Women’s and Children’s Health Research Institute at the University of Alberta, Edmonton, and Dr. Maguire of St Michael’s Hospital, Toronto, and the University of Toronto. “Although it is difficult to object to the guidance from the U.S. Preventive Services Task Force to listen carefully to parents’ concerns, we must acknowledge the false dichotomy between screening and surveillance in this context. ... Why not use the best available measurement tools to identify developmental concerns with the highest possible accuracy?”
The study was funded by the Allerton Foundation, Eagles Charitable Foundation, and the National Institute of Mental Health; the study investigators reported that they had no conflicts of interest. Dr. Zwaigenbaum is a member of an independent data monitoring committee for a Roche-funded medication trial and Dr. Maguire reported receiving nonfinancial support from Ddrops for an investigator-initiated study on vitamin D and respiratory tract infections. Dr Zwaigenbaum is supported by the Stollery Children’s Hospital Foundation Chair in Autism Research. Dr Maguire is supported by St. Michael’s Hospital and the Hospital for Sick Children.
SOURCEs: Guthrie W et al. Pediatrics. 2019 Sep 27. doi: 10.1542/peds.2018-3963; Zwaigenbaum L, Maguire J. Pediatrics. 2019 Sep 27. doi: 10.1542/peds.2019-0925.
, especially in children of color and in those from lower-income households, new research found.
Guidelines for universal autism spectrum disorder (ASD) screening currently conflict, Whitney Guthrie, PhD, of the Children’s Hospital of Philadelphia, and associates wrote in Pediatrics. The American Academy of Pediatrics recommends universal screening in children aged 18 months and 24 months to expedite earlier identification and diagnosis. However, the U.S. Preventive Services Task Force has concluded that “there is insufficient evidence to recommend universal screening, in part because of limited data on outcomes for children who screen negative and from diverse samples.”
Dr. Guthrie and associates conducted a study of 25,999 children aged 16-26 months who had a well-child visit between January 2011 and July 2015 at a Children’s Hospital of Philadelphia site that had implemented universal electronic screening. Of this group, 43% were white, 37% were black, and the remainder were Asian or of other/multiple races; 92% were non-Hispanic. The median parental income was $59,597, 54% had private insurance and 45.3% had public insurance/Medicaid, and 42% came from an urban primary care site while the rest came from suburban primary care.
Screening rates were good over the study period, with 91% of children undergoing at least one screen with the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F). While 50% were screened more than once, only 48% of children were screened at 18 and 24 months, as per the AAP guideline. Children who were screened multiple times were more likely to be white and non-Hispanic, to be from a suburban site, and to have higher incomes and private insurance.
After the first M-CHAT/F screen, 9.5% of children were positive, a rate comparable with that seen in other large-scale, U.S.-based studies. Of the 2,256 children who tested positive, 41% received a second screen; 782 (95%) of these children tested negative.
After the study period, most children (n = 20,437; 87%) continued receiving care with the Children’s Hospital of Philadelphia system and had diagnostic data available past the age of 4 years. ASD prevalence was 2%, giving the M-CHAT/F a sensitivity of 39%, a specificity of 95%, a positive predictive value of 15%, and a negative predictive value of 99%.
M-CHAT/F sensitivity was higher in older children (49% at 21-26 months vs. 35% at 16-20 months) and with repeated screenings (40% vs. 32%), and positive predictive value was lower in girls (8% vs. 20%). Specificity and positive predictive value were higher in white children (98% and 24%, respectively), compared with black children (92% and 12%, respectively), Asian children (90% and 11%, respectively), and those from other/multiple racial groups (94% and 13%, respectively). Higher-income families also saw increased specificity (97% vs. 92%) and positive predictive value (20% vs. 12%), compared with lower-income families.
While Dr. Guthrie and associates wrote of new methods of screening, such as parental reporting tools supported by picture or video and “direct data-gathering methods that leverage technological advances in computing and machine learning,” Lonnie Zwaigenbaum, MD, MSc, and Jonathon Maguire, MD, MSc, argued in an editorial, also published in Pediatrics, that the M-CHAT/F “remains a strong candidate” for universal ASD screening, despite the notable weaknesses.
“Ultimately, the potential added value of ASD screening must be considered relative to what would occur in its absence,” wrote Dr. Zwaigenbaum of the Women’s and Children’s Health Research Institute at the University of Alberta, Edmonton, and Dr. Maguire of St Michael’s Hospital, Toronto, and the University of Toronto. “Although it is difficult to object to the guidance from the U.S. Preventive Services Task Force to listen carefully to parents’ concerns, we must acknowledge the false dichotomy between screening and surveillance in this context. ... Why not use the best available measurement tools to identify developmental concerns with the highest possible accuracy?”
The study was funded by the Allerton Foundation, Eagles Charitable Foundation, and the National Institute of Mental Health; the study investigators reported that they had no conflicts of interest. Dr. Zwaigenbaum is a member of an independent data monitoring committee for a Roche-funded medication trial and Dr. Maguire reported receiving nonfinancial support from Ddrops for an investigator-initiated study on vitamin D and respiratory tract infections. Dr Zwaigenbaum is supported by the Stollery Children’s Hospital Foundation Chair in Autism Research. Dr Maguire is supported by St. Michael’s Hospital and the Hospital for Sick Children.
SOURCEs: Guthrie W et al. Pediatrics. 2019 Sep 27. doi: 10.1542/peds.2018-3963; Zwaigenbaum L, Maguire J. Pediatrics. 2019 Sep 27. doi: 10.1542/peds.2019-0925.
FROM PEDIATRICS
Key clinical point: Universal screening for autism in a primary care setting is possible, but accuracy of the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) tool was low, especially for children of color and those from lower-income households.
Major finding: Just over 90% of children received screening, with an autism spectrum disorder prevalence of 2%. The M-CHAT/F screen had a sensitivity of 39% and a positive predictive value of 15%
Study details: A total of 25,999 children aged 16-26 months who had a well-child visit between January 2011 and July 2015 at a Children’s Hospital of Philadelphia site that had implemented universal electronic screening.
Disclosures: The study was funded by the Allerton Foundation, Eagles Charitable Foundation, the National Institute of Mental Health, and the National Institutes of Health. The study investigators reported they had no conflicts of interest. Dr. Zwaigenbaum is a member of an independent data monitoring committee for a Roche-funded medication trial and Dr. Maguire reported receiving nonfinancial support from Ddrops for an investigator-initiated study on vitamin D and respiratory tract infections. Dr. Zwaigenbaum and Dr. Maguire receive hospital-supported funding.
Sources: Guthrie W et al. Pediatrics. 2019 Sep 27. doi: 10.1542/peds.2018-3963; Zwaigenbaum L, Maguire J. Pediatrics. 2019 Sep 27. doi: 10.1542/peds.2019-0925.















