User login
Novel score spots high-risk febrile children in ED
LJUBLJANA, SLOVENIA – A new age-adjusted quick Sequential Organ Failure Assessment (qSOFA) score designed for use in children presenting to the ED with fever showed good predictive value for admission to critical care within the next 48 hours, Aakash Khanijau, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In the needle-in-a-haystack scenario that’s seen in pediatric emergency departments, our novel, age-adjusted qSOFA score could potentially improve the rapid identification and treatment of children with suspected sepsis presenting to the ED,” said Dr. Khanijau of the University of Liverpool (England).
He presented an exceptionally large retrospective validation study of the score’s performance in 12,393 children (median age, 2.5 years) who presented to EDs with fever, of whom 1,521 were admitted for suspected sepsis. Of the hospitalized children, 145 were admitted to critical care within the first 48 hours.
The pediatric qSOFA score had 72% sensitivity and 85% specificity for critical care admission within 48 hours, with a positive predictive value of 5.4% and, more importantly, a whopping negative predictive value of 99.6%.
“That very high negative predictive value underlines the powerful discriminatory nature of our tool in the emergency department setting,” Dr. Khanijau observed, adding that the score’s area under the receiver operating characteristic curve was 0.81, which is considered a good predictive value.
The impetus for developing an age-adjusted pediatric qSOFA score stems from the fact that the original qSOFA score was designed for rapid assessment of adults with suspected sepsis and isn’t applicable in children. Other existing scores, including SIRS (the Systemic Inflammatory Response Syndrome criteria), the full SOFA, and PELOD-2 (the Pediatric Logistic Organ Dysfunction score), take longer to determine than the adapted qSOFA in a setting where speed is of the essence, he explained.
The original qSOFA components are altered mentation, systolic blood pressure, and respiratory rate. The novel score developed by Dr. Khanijau and coworkers swaps out systolic BP in favor of capillary refill time and age-adjusted heart rate using the thresholds previously established in a landmark study from the Children’s Hospital of Philadelphia (Pediatrics. 2013 Apr;131[4]:e1150-7.)
“Our reasoning here is that arterial hypertension is known to be a much later sign of circulatory compromise in children and may provide less discriminatory value than signs such as delayed capillary refill time and tachycardia early in presentation in the emergency department,” according to Dr. Khanijau.
The novel scoring system features four criteria. One point each is given for a capillary refill time of 3 seconds or longer; anything less than “Alert” on the Alert, Responds to Voice, Respond to Pain, and Unresponsive scale; a heart rate above the 99th percentile on the age-adjusted curves; and a respiratory rate above the age-adjusted 99th percentile. Thus, scores can range from 0 to 4. In the validation study, a score of 2 or more spelled a 890% increased likelihood of being admitted to a critical care setting within 48 hours. It was also associated with a 100-fold increased likelihood of death during the hospitalization, which occurred in 10 children.
Asked how the new predictive score could change clinical management, Dr. Khanijau replied, “I think the key thing it does here is it identifies the children at risk of requiring critical care and should therefore motivate us in the children achieving that threshold to promptly investigate thoroughly for suspected sepsis using the more comprehensive tools, like the full SOFA.”
He reported having no financial conflicts of interest regarding his study.
SOURCE: Khanijau A et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – A new age-adjusted quick Sequential Organ Failure Assessment (qSOFA) score designed for use in children presenting to the ED with fever showed good predictive value for admission to critical care within the next 48 hours, Aakash Khanijau, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In the needle-in-a-haystack scenario that’s seen in pediatric emergency departments, our novel, age-adjusted qSOFA score could potentially improve the rapid identification and treatment of children with suspected sepsis presenting to the ED,” said Dr. Khanijau of the University of Liverpool (England).
He presented an exceptionally large retrospective validation study of the score’s performance in 12,393 children (median age, 2.5 years) who presented to EDs with fever, of whom 1,521 were admitted for suspected sepsis. Of the hospitalized children, 145 were admitted to critical care within the first 48 hours.
The pediatric qSOFA score had 72% sensitivity and 85% specificity for critical care admission within 48 hours, with a positive predictive value of 5.4% and, more importantly, a whopping negative predictive value of 99.6%.
“That very high negative predictive value underlines the powerful discriminatory nature of our tool in the emergency department setting,” Dr. Khanijau observed, adding that the score’s area under the receiver operating characteristic curve was 0.81, which is considered a good predictive value.
The impetus for developing an age-adjusted pediatric qSOFA score stems from the fact that the original qSOFA score was designed for rapid assessment of adults with suspected sepsis and isn’t applicable in children. Other existing scores, including SIRS (the Systemic Inflammatory Response Syndrome criteria), the full SOFA, and PELOD-2 (the Pediatric Logistic Organ Dysfunction score), take longer to determine than the adapted qSOFA in a setting where speed is of the essence, he explained.
The original qSOFA components are altered mentation, systolic blood pressure, and respiratory rate. The novel score developed by Dr. Khanijau and coworkers swaps out systolic BP in favor of capillary refill time and age-adjusted heart rate using the thresholds previously established in a landmark study from the Children’s Hospital of Philadelphia (Pediatrics. 2013 Apr;131[4]:e1150-7.)
“Our reasoning here is that arterial hypertension is known to be a much later sign of circulatory compromise in children and may provide less discriminatory value than signs such as delayed capillary refill time and tachycardia early in presentation in the emergency department,” according to Dr. Khanijau.
The novel scoring system features four criteria. One point each is given for a capillary refill time of 3 seconds or longer; anything less than “Alert” on the Alert, Responds to Voice, Respond to Pain, and Unresponsive scale; a heart rate above the 99th percentile on the age-adjusted curves; and a respiratory rate above the age-adjusted 99th percentile. Thus, scores can range from 0 to 4. In the validation study, a score of 2 or more spelled a 890% increased likelihood of being admitted to a critical care setting within 48 hours. It was also associated with a 100-fold increased likelihood of death during the hospitalization, which occurred in 10 children.
Asked how the new predictive score could change clinical management, Dr. Khanijau replied, “I think the key thing it does here is it identifies the children at risk of requiring critical care and should therefore motivate us in the children achieving that threshold to promptly investigate thoroughly for suspected sepsis using the more comprehensive tools, like the full SOFA.”
He reported having no financial conflicts of interest regarding his study.
SOURCE: Khanijau A et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – A new age-adjusted quick Sequential Organ Failure Assessment (qSOFA) score designed for use in children presenting to the ED with fever showed good predictive value for admission to critical care within the next 48 hours, Aakash Khanijau, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In the needle-in-a-haystack scenario that’s seen in pediatric emergency departments, our novel, age-adjusted qSOFA score could potentially improve the rapid identification and treatment of children with suspected sepsis presenting to the ED,” said Dr. Khanijau of the University of Liverpool (England).
He presented an exceptionally large retrospective validation study of the score’s performance in 12,393 children (median age, 2.5 years) who presented to EDs with fever, of whom 1,521 were admitted for suspected sepsis. Of the hospitalized children, 145 were admitted to critical care within the first 48 hours.
The pediatric qSOFA score had 72% sensitivity and 85% specificity for critical care admission within 48 hours, with a positive predictive value of 5.4% and, more importantly, a whopping negative predictive value of 99.6%.
“That very high negative predictive value underlines the powerful discriminatory nature of our tool in the emergency department setting,” Dr. Khanijau observed, adding that the score’s area under the receiver operating characteristic curve was 0.81, which is considered a good predictive value.
The impetus for developing an age-adjusted pediatric qSOFA score stems from the fact that the original qSOFA score was designed for rapid assessment of adults with suspected sepsis and isn’t applicable in children. Other existing scores, including SIRS (the Systemic Inflammatory Response Syndrome criteria), the full SOFA, and PELOD-2 (the Pediatric Logistic Organ Dysfunction score), take longer to determine than the adapted qSOFA in a setting where speed is of the essence, he explained.
The original qSOFA components are altered mentation, systolic blood pressure, and respiratory rate. The novel score developed by Dr. Khanijau and coworkers swaps out systolic BP in favor of capillary refill time and age-adjusted heart rate using the thresholds previously established in a landmark study from the Children’s Hospital of Philadelphia (Pediatrics. 2013 Apr;131[4]:e1150-7.)
“Our reasoning here is that arterial hypertension is known to be a much later sign of circulatory compromise in children and may provide less discriminatory value than signs such as delayed capillary refill time and tachycardia early in presentation in the emergency department,” according to Dr. Khanijau.
The novel scoring system features four criteria. One point each is given for a capillary refill time of 3 seconds or longer; anything less than “Alert” on the Alert, Responds to Voice, Respond to Pain, and Unresponsive scale; a heart rate above the 99th percentile on the age-adjusted curves; and a respiratory rate above the age-adjusted 99th percentile. Thus, scores can range from 0 to 4. In the validation study, a score of 2 or more spelled a 890% increased likelihood of being admitted to a critical care setting within 48 hours. It was also associated with a 100-fold increased likelihood of death during the hospitalization, which occurred in 10 children.
Asked how the new predictive score could change clinical management, Dr. Khanijau replied, “I think the key thing it does here is it identifies the children at risk of requiring critical care and should therefore motivate us in the children achieving that threshold to promptly investigate thoroughly for suspected sepsis using the more comprehensive tools, like the full SOFA.”
He reported having no financial conflicts of interest regarding his study.
SOURCE: Khanijau A et al. ESPID 2019, Abstract.
REPORTING FROM ESPID 2019
Procalcitonin advocated to help rule out bacterial infections
SEATTLE – Procalcitonin, a marker of bacterial infection, rises and peaks sooner than C-reactive protein (CRP), and is especially useful to help rule out invasive bacterial infections in young infants and pediatric community acquired pneumonia due to typical bacteria, according to a presentation at the 2019 Pediatric Hospital Medicine Conference.
It’s “excellent for identifying low risk patients” and has the potential to decrease lumbar punctures and antibiotic exposure, but “the specificity isn’t great,” so there’s the potential for false positives, said Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
There was great interest in procalcitonin at the meeting; the presentation room was packed, with a line out the door. It’s used mostly in Europe at this point. Testing is available in many U.S. hospitals, but a large majority of audience members, when polled, said they don’t currently use it in clinical practice, and that it’s not a part of diagnostic algorithms at their institutions.
Levels of procalcitonin, a calcitonin precursor normally produced by the thyroid, are low or undetectable in healthy people, but inflammation, be it from infectious or noninfectious causes, triggers production by parenchymal cells throughout the body.
Levels began to rise as early as 2.5 hours after healthy subjects in one study were injected with bacterial endotoxins, and peaked as early as 6 hours; CRP, in contrast, started to rise after 12 hours, and peaked at 30 hours. Procalcitonin levels also seem to correlate with bacterial load and severity of infection, said Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University (J Pediatr Intensive Care. 2016 Dec;5[4]:162-71).
Due to time, the presenters focused their talk on community acquired pneumonia (CAP) and invasive bacterial infections (IBI) in young infants, meaning essentially bacteremia and meningitis.
Different studies use different cutoffs, but a procalcitonin below, for instance, 0.5 ng/mL is “certainly more sensitive [for IBI] than any single biomarker we currently use,” including CRP, white blood cells, and absolute neutrophil count (ANC). “If it’s negative, you’re really confident it’s negative,” but “a positive test does not necessarily indicate the presence of IBI,” Dr. McCulloh said (Pediatrics. 2012 Nov;130[5]:815-22).
“Procalcitonin works really well as part of a validated step-wise rule” that includes, for instance, CRP and ANC; “I think that’s where its utility is. On its own, it is not a substitute for you examining the patient and doing your basic risk stratification, but it may enhance your decision making incrementally above what we currently have,” he said.
Meanwhile, in a study of 532 children a median age of 2.4 years with radiographically confirmed CAP, procalcitonin levels were a median of 6.1 ng/mL in children whose pneumonia was caused by Streptococcus pneumoniae or other typical bacteria, and no child infected with typical bacteria had a level under 0.1 ng/mL. Below that level, “you can be very sure you do not have typical bacteria pneumonia,” said Marie Wang, MD, also a pediatric infectious disease specialist at Stanford (J Pediatric Infect Dis Soc. 2018 Feb 19;7[1]:46-53).
As procalcitonin levels went up, the likelihood of having bacterial pneumonia increased; at 2 ng/mL, 26% of subjects were infected with typical bacteria, “but even in that group, 58% still had viral infection, so you are still detecting a lot of viral” disease, she said.
Prolcalcitonin-guided therapy – antibiotics until patients fall below a level of 0.25 ng/ml, for instance – has also been associated with decreased antibiotic exposure (Respir Med. 2011 Dec;105[12]:1939-45).
The speakers had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Procalcitonin, a marker of bacterial infection, rises and peaks sooner than C-reactive protein (CRP), and is especially useful to help rule out invasive bacterial infections in young infants and pediatric community acquired pneumonia due to typical bacteria, according to a presentation at the 2019 Pediatric Hospital Medicine Conference.
It’s “excellent for identifying low risk patients” and has the potential to decrease lumbar punctures and antibiotic exposure, but “the specificity isn’t great,” so there’s the potential for false positives, said Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
There was great interest in procalcitonin at the meeting; the presentation room was packed, with a line out the door. It’s used mostly in Europe at this point. Testing is available in many U.S. hospitals, but a large majority of audience members, when polled, said they don’t currently use it in clinical practice, and that it’s not a part of diagnostic algorithms at their institutions.
Levels of procalcitonin, a calcitonin precursor normally produced by the thyroid, are low or undetectable in healthy people, but inflammation, be it from infectious or noninfectious causes, triggers production by parenchymal cells throughout the body.
Levels began to rise as early as 2.5 hours after healthy subjects in one study were injected with bacterial endotoxins, and peaked as early as 6 hours; CRP, in contrast, started to rise after 12 hours, and peaked at 30 hours. Procalcitonin levels also seem to correlate with bacterial load and severity of infection, said Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University (J Pediatr Intensive Care. 2016 Dec;5[4]:162-71).
Due to time, the presenters focused their talk on community acquired pneumonia (CAP) and invasive bacterial infections (IBI) in young infants, meaning essentially bacteremia and meningitis.
Different studies use different cutoffs, but a procalcitonin below, for instance, 0.5 ng/mL is “certainly more sensitive [for IBI] than any single biomarker we currently use,” including CRP, white blood cells, and absolute neutrophil count (ANC). “If it’s negative, you’re really confident it’s negative,” but “a positive test does not necessarily indicate the presence of IBI,” Dr. McCulloh said (Pediatrics. 2012 Nov;130[5]:815-22).
“Procalcitonin works really well as part of a validated step-wise rule” that includes, for instance, CRP and ANC; “I think that’s where its utility is. On its own, it is not a substitute for you examining the patient and doing your basic risk stratification, but it may enhance your decision making incrementally above what we currently have,” he said.
Meanwhile, in a study of 532 children a median age of 2.4 years with radiographically confirmed CAP, procalcitonin levels were a median of 6.1 ng/mL in children whose pneumonia was caused by Streptococcus pneumoniae or other typical bacteria, and no child infected with typical bacteria had a level under 0.1 ng/mL. Below that level, “you can be very sure you do not have typical bacteria pneumonia,” said Marie Wang, MD, also a pediatric infectious disease specialist at Stanford (J Pediatric Infect Dis Soc. 2018 Feb 19;7[1]:46-53).
As procalcitonin levels went up, the likelihood of having bacterial pneumonia increased; at 2 ng/mL, 26% of subjects were infected with typical bacteria, “but even in that group, 58% still had viral infection, so you are still detecting a lot of viral” disease, she said.
Prolcalcitonin-guided therapy – antibiotics until patients fall below a level of 0.25 ng/ml, for instance – has also been associated with decreased antibiotic exposure (Respir Med. 2011 Dec;105[12]:1939-45).
The speakers had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Procalcitonin, a marker of bacterial infection, rises and peaks sooner than C-reactive protein (CRP), and is especially useful to help rule out invasive bacterial infections in young infants and pediatric community acquired pneumonia due to typical bacteria, according to a presentation at the 2019 Pediatric Hospital Medicine Conference.
It’s “excellent for identifying low risk patients” and has the potential to decrease lumbar punctures and antibiotic exposure, but “the specificity isn’t great,” so there’s the potential for false positives, said Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
There was great interest in procalcitonin at the meeting; the presentation room was packed, with a line out the door. It’s used mostly in Europe at this point. Testing is available in many U.S. hospitals, but a large majority of audience members, when polled, said they don’t currently use it in clinical practice, and that it’s not a part of diagnostic algorithms at their institutions.
Levels of procalcitonin, a calcitonin precursor normally produced by the thyroid, are low or undetectable in healthy people, but inflammation, be it from infectious or noninfectious causes, triggers production by parenchymal cells throughout the body.
Levels began to rise as early as 2.5 hours after healthy subjects in one study were injected with bacterial endotoxins, and peaked as early as 6 hours; CRP, in contrast, started to rise after 12 hours, and peaked at 30 hours. Procalcitonin levels also seem to correlate with bacterial load and severity of infection, said Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University (J Pediatr Intensive Care. 2016 Dec;5[4]:162-71).
Due to time, the presenters focused their talk on community acquired pneumonia (CAP) and invasive bacterial infections (IBI) in young infants, meaning essentially bacteremia and meningitis.
Different studies use different cutoffs, but a procalcitonin below, for instance, 0.5 ng/mL is “certainly more sensitive [for IBI] than any single biomarker we currently use,” including CRP, white blood cells, and absolute neutrophil count (ANC). “If it’s negative, you’re really confident it’s negative,” but “a positive test does not necessarily indicate the presence of IBI,” Dr. McCulloh said (Pediatrics. 2012 Nov;130[5]:815-22).
“Procalcitonin works really well as part of a validated step-wise rule” that includes, for instance, CRP and ANC; “I think that’s where its utility is. On its own, it is not a substitute for you examining the patient and doing your basic risk stratification, but it may enhance your decision making incrementally above what we currently have,” he said.
Meanwhile, in a study of 532 children a median age of 2.4 years with radiographically confirmed CAP, procalcitonin levels were a median of 6.1 ng/mL in children whose pneumonia was caused by Streptococcus pneumoniae or other typical bacteria, and no child infected with typical bacteria had a level under 0.1 ng/mL. Below that level, “you can be very sure you do not have typical bacteria pneumonia,” said Marie Wang, MD, also a pediatric infectious disease specialist at Stanford (J Pediatric Infect Dis Soc. 2018 Feb 19;7[1]:46-53).
As procalcitonin levels went up, the likelihood of having bacterial pneumonia increased; at 2 ng/mL, 26% of subjects were infected with typical bacteria, “but even in that group, 58% still had viral infection, so you are still detecting a lot of viral” disease, she said.
Prolcalcitonin-guided therapy – antibiotics until patients fall below a level of 0.25 ng/ml, for instance – has also been associated with decreased antibiotic exposure (Respir Med. 2011 Dec;105[12]:1939-45).
The speakers had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
EXPERT ANALYSIS FROM PHM 2019
Mortality is high in pediatric superrefractory status epilepticus
BANGKOK – presented by Maggie Lo Yee Yau, MD, at the International Epilepsy Congress.
“Death in these children usually occurred within the first few days after admission to the pediatric ICU,” she said at the congress sponsored by the International League Against Epilepsy.
The study included 15 consecutive patients aged between 1 month and 17 years treated for superrefractory status epilepticus (SRSE) during 2011-2017 at the Chinese University of Hong Kong, where Dr. Yau practices. Seven children died during their index hospital admission, with a median time to death of 8 days. Two more died within several years post discharge.
Morbidity was substantial: At follow-up 1 year after the index episode of SRSE, two patients had a Glasgow Outcome Scale (GOS) score of 3, indicative of severe disability; three patients had moderate disability, with a GOS of 4; and two patients were in a vegetative state, with a GOS of 2, both of whom subsequently died of aspiration pneumonia. Only 1 of the 15 patients had a good recovery. Through 8 years of follow-up, all six survivors had epilepsy. Common nonneurologic deficits included a predisposition to a variety of infections.
By way of background, Dr. Yau noted that convulsive status epilepticus is the most common neurologic emergency in children, with an incidence of about 20 episodes per 100,000. Of affected children, 10%-40% develop refractory status, with reported mortality rates of 16%-43%. SRSE is a term reserved for persistent or recurrent seizures 24 hours or more after onset of general anesthesia for management of refractory status.
The impetus for Dr. Yau’s study was the dearth of data on SRSE in children. The literature consists of a few case series totaling well under 100 patients.
The Hong Kong case series included 15 patients with SRSE who had a median age of 7.9 years, only 1 of whom had preexisting epilepsy, a case of epileptic encephalopathy with severe developmental delay. Of the 15, 12 were boys. The patients were placed on a median of four antiepileptic drugs. Those who survived to discharge spent a median of 17.8 days under general anesthesia and 42.5 days in the pediatric ICU.
The SRSE etiologies included febrile infection–related epilepsy syndrome in two cases, four serious infections, four cases of autoimmune etiology, two cases of epileptic encephalopathy, one patient with hypoxia caused by severe croup, and two of unknown origin despite intensive work-up.
The four in-hospital deaths caused by acute cerebral edema occurred a median 6.5 days after admission. There were also two deaths because of uncontrolled sepsis and one because of intraventricular bleeding secondary to thrombotic thrombocytopenic purpura thought to have occurred as a complication of interactions between the numerous prescribed medications. All six children with an infectious or unknown etiology died in hospital, whereas none of those with an autoimmune etiology, epileptic encephalopathy, or hypoxia did. Duration of anesthesia did not predict mortality.
Other investigators have reported that younger age is associated with higher mortality, but that was not true in the Hong Kong experience. Neither of the two children aged less than 3 years died during their index hospitalization. All 7 deaths occurred in the 13 children age 3 years or older.
When asked whether she thought SRSE or the underlying disorder was the bigger contributor to mortality, Dr. Yau replied that she believes the prolonged refractory seizures may have worsened cerebral edema in some patients and thereby have been the cause of death.
She reported having no financial conflicts regarding her study.
BANGKOK – presented by Maggie Lo Yee Yau, MD, at the International Epilepsy Congress.
“Death in these children usually occurred within the first few days after admission to the pediatric ICU,” she said at the congress sponsored by the International League Against Epilepsy.
The study included 15 consecutive patients aged between 1 month and 17 years treated for superrefractory status epilepticus (SRSE) during 2011-2017 at the Chinese University of Hong Kong, where Dr. Yau practices. Seven children died during their index hospital admission, with a median time to death of 8 days. Two more died within several years post discharge.
Morbidity was substantial: At follow-up 1 year after the index episode of SRSE, two patients had a Glasgow Outcome Scale (GOS) score of 3, indicative of severe disability; three patients had moderate disability, with a GOS of 4; and two patients were in a vegetative state, with a GOS of 2, both of whom subsequently died of aspiration pneumonia. Only 1 of the 15 patients had a good recovery. Through 8 years of follow-up, all six survivors had epilepsy. Common nonneurologic deficits included a predisposition to a variety of infections.
By way of background, Dr. Yau noted that convulsive status epilepticus is the most common neurologic emergency in children, with an incidence of about 20 episodes per 100,000. Of affected children, 10%-40% develop refractory status, with reported mortality rates of 16%-43%. SRSE is a term reserved for persistent or recurrent seizures 24 hours or more after onset of general anesthesia for management of refractory status.
The impetus for Dr. Yau’s study was the dearth of data on SRSE in children. The literature consists of a few case series totaling well under 100 patients.
The Hong Kong case series included 15 patients with SRSE who had a median age of 7.9 years, only 1 of whom had preexisting epilepsy, a case of epileptic encephalopathy with severe developmental delay. Of the 15, 12 were boys. The patients were placed on a median of four antiepileptic drugs. Those who survived to discharge spent a median of 17.8 days under general anesthesia and 42.5 days in the pediatric ICU.
The SRSE etiologies included febrile infection–related epilepsy syndrome in two cases, four serious infections, four cases of autoimmune etiology, two cases of epileptic encephalopathy, one patient with hypoxia caused by severe croup, and two of unknown origin despite intensive work-up.
The four in-hospital deaths caused by acute cerebral edema occurred a median 6.5 days after admission. There were also two deaths because of uncontrolled sepsis and one because of intraventricular bleeding secondary to thrombotic thrombocytopenic purpura thought to have occurred as a complication of interactions between the numerous prescribed medications. All six children with an infectious or unknown etiology died in hospital, whereas none of those with an autoimmune etiology, epileptic encephalopathy, or hypoxia did. Duration of anesthesia did not predict mortality.
Other investigators have reported that younger age is associated with higher mortality, but that was not true in the Hong Kong experience. Neither of the two children aged less than 3 years died during their index hospitalization. All 7 deaths occurred in the 13 children age 3 years or older.
When asked whether she thought SRSE or the underlying disorder was the bigger contributor to mortality, Dr. Yau replied that she believes the prolonged refractory seizures may have worsened cerebral edema in some patients and thereby have been the cause of death.
She reported having no financial conflicts regarding her study.
BANGKOK – presented by Maggie Lo Yee Yau, MD, at the International Epilepsy Congress.
“Death in these children usually occurred within the first few days after admission to the pediatric ICU,” she said at the congress sponsored by the International League Against Epilepsy.
The study included 15 consecutive patients aged between 1 month and 17 years treated for superrefractory status epilepticus (SRSE) during 2011-2017 at the Chinese University of Hong Kong, where Dr. Yau practices. Seven children died during their index hospital admission, with a median time to death of 8 days. Two more died within several years post discharge.
Morbidity was substantial: At follow-up 1 year after the index episode of SRSE, two patients had a Glasgow Outcome Scale (GOS) score of 3, indicative of severe disability; three patients had moderate disability, with a GOS of 4; and two patients were in a vegetative state, with a GOS of 2, both of whom subsequently died of aspiration pneumonia. Only 1 of the 15 patients had a good recovery. Through 8 years of follow-up, all six survivors had epilepsy. Common nonneurologic deficits included a predisposition to a variety of infections.
By way of background, Dr. Yau noted that convulsive status epilepticus is the most common neurologic emergency in children, with an incidence of about 20 episodes per 100,000. Of affected children, 10%-40% develop refractory status, with reported mortality rates of 16%-43%. SRSE is a term reserved for persistent or recurrent seizures 24 hours or more after onset of general anesthesia for management of refractory status.
The impetus for Dr. Yau’s study was the dearth of data on SRSE in children. The literature consists of a few case series totaling well under 100 patients.
The Hong Kong case series included 15 patients with SRSE who had a median age of 7.9 years, only 1 of whom had preexisting epilepsy, a case of epileptic encephalopathy with severe developmental delay. Of the 15, 12 were boys. The patients were placed on a median of four antiepileptic drugs. Those who survived to discharge spent a median of 17.8 days under general anesthesia and 42.5 days in the pediatric ICU.
The SRSE etiologies included febrile infection–related epilepsy syndrome in two cases, four serious infections, four cases of autoimmune etiology, two cases of epileptic encephalopathy, one patient with hypoxia caused by severe croup, and two of unknown origin despite intensive work-up.
The four in-hospital deaths caused by acute cerebral edema occurred a median 6.5 days after admission. There were also two deaths because of uncontrolled sepsis and one because of intraventricular bleeding secondary to thrombotic thrombocytopenic purpura thought to have occurred as a complication of interactions between the numerous prescribed medications. All six children with an infectious or unknown etiology died in hospital, whereas none of those with an autoimmune etiology, epileptic encephalopathy, or hypoxia did. Duration of anesthesia did not predict mortality.
Other investigators have reported that younger age is associated with higher mortality, but that was not true in the Hong Kong experience. Neither of the two children aged less than 3 years died during their index hospitalization. All 7 deaths occurred in the 13 children age 3 years or older.
When asked whether she thought SRSE or the underlying disorder was the bigger contributor to mortality, Dr. Yau replied that she believes the prolonged refractory seizures may have worsened cerebral edema in some patients and thereby have been the cause of death.
She reported having no financial conflicts regarding her study.
REPORTING FROM IEC 2019
Dupilumab found effective for adolescents with moderate to severe AD
AUSTIN, TEX. – according to results of a phase 3 study.
“Dupilumab works as effectively in adolescents as in adults,” Randy Prescilla, MD, one of the study authors, said in an interview at the annual meeting of the Society for Pediatric Dermatology. “It gives us promise that we could go into other age groups with the same optimism. We are enrolling patients in even younger age groups.”
The double-blind, placebo-controlled study analyzed the efficacy and safety of dupilumab monotherapy in patients between the ages of 12 and 17 years with moderate to severe atopic dermatitis (AD) inadequately controlled with topical therapies. In the United States, dupilumab is approved for those aged 12 years and older with moderate to severe disease inadequately controlled by topical prescription treatments or when those therapies are not advisable.
For the 16-week study, Dr. Prescilla, global medical affairs director of pediatric dermatology for Sanofi Genzyme, and colleagues randomized 251 patients to one of three groups: dupilumab every 2 weeks (200 mg if baseline weight was less than 60 kg; 300 mg if that weight was 60 kg or more); 300 mg dupilumab every 4 weeks; or placebo every 2 weeks.
At week 16, a significantly higher proportion of patients in the two drug treatment groups had Investigator’s Global Assessment scores of 0/1, compared with those in the placebo group (24.4%, 17.9%, and 2.4%) as well as a significantly higher percentage of patients who achieved at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) score (41.5%, 38.1%, and 8.2%).
In addition, patients in the two drug treatment groups experienced improved percent change in least square-means on the EASI from baseline to week 16, compared with those in the placebo group (–65.9%, –64.8%, and –23.6%), the Peak Pruritus Numerical Rating Scale (–47.9%, –45.5%, and –19.0%), body surface area affected by AD (–30.1%, –33.4%, and –11.7%), and in the SCORing AD clinical tool (P less than .001 for all comparisons).
Between baseline and week 16, scores on the Children’s Dermatology Life Quality Index and Patient-Oriented Eczema Measure improved significantly in the two dupilumab groups, compared with the placebo group. The rate of skin infection was higher in the placebo group (20%), compared with 11% in the group that received dupilumab every 2 weeks and 13.3% in the group receiving the drug every 4 weeks.
Conjunctivitis occurred more frequently with dupilumab treatment (9.8% in the every-2-weeks dupilumab group, 10.8% in the every-4-weeks dupilumab group, and 4.7% in the placebo group) as did injection site reactions (8.5%, 6.0%, and 3.5%). Two adverse events, one of which was serious, occurred in the placebo group.
Dr. Prescilla acknowledged certain limitations of the study, including its small sample size and the fact that it was limited to 16 weeks. “However, smaller sample size and duration are typical for this type of study and in line with the study design of the SOLO 1 and SOLO 2 studies in adults,” he said.
On Aug. 6, the European Commission extended the marketing authorization for dupilumab in the European Union to include adolescents 12-17 years of age with moderate to severe atopic dermatitis who are candidates for systemic therapy. On the same day, Sanofi Genzyme and Regeneron announced positive topline results in a phase 3 trial in children aged 6-11 years with severe AD.
The study’s principal investigator was Amy S. Paller, MD. The study was funded by Sanofi Genzyme and Regeneron. Dr. Prescilla is an employee of Sanofi Genzyme.
AUSTIN, TEX. – according to results of a phase 3 study.
“Dupilumab works as effectively in adolescents as in adults,” Randy Prescilla, MD, one of the study authors, said in an interview at the annual meeting of the Society for Pediatric Dermatology. “It gives us promise that we could go into other age groups with the same optimism. We are enrolling patients in even younger age groups.”
The double-blind, placebo-controlled study analyzed the efficacy and safety of dupilumab monotherapy in patients between the ages of 12 and 17 years with moderate to severe atopic dermatitis (AD) inadequately controlled with topical therapies. In the United States, dupilumab is approved for those aged 12 years and older with moderate to severe disease inadequately controlled by topical prescription treatments or when those therapies are not advisable.
For the 16-week study, Dr. Prescilla, global medical affairs director of pediatric dermatology for Sanofi Genzyme, and colleagues randomized 251 patients to one of three groups: dupilumab every 2 weeks (200 mg if baseline weight was less than 60 kg; 300 mg if that weight was 60 kg or more); 300 mg dupilumab every 4 weeks; or placebo every 2 weeks.
At week 16, a significantly higher proportion of patients in the two drug treatment groups had Investigator’s Global Assessment scores of 0/1, compared with those in the placebo group (24.4%, 17.9%, and 2.4%) as well as a significantly higher percentage of patients who achieved at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) score (41.5%, 38.1%, and 8.2%).
In addition, patients in the two drug treatment groups experienced improved percent change in least square-means on the EASI from baseline to week 16, compared with those in the placebo group (–65.9%, –64.8%, and –23.6%), the Peak Pruritus Numerical Rating Scale (–47.9%, –45.5%, and –19.0%), body surface area affected by AD (–30.1%, –33.4%, and –11.7%), and in the SCORing AD clinical tool (P less than .001 for all comparisons).
Between baseline and week 16, scores on the Children’s Dermatology Life Quality Index and Patient-Oriented Eczema Measure improved significantly in the two dupilumab groups, compared with the placebo group. The rate of skin infection was higher in the placebo group (20%), compared with 11% in the group that received dupilumab every 2 weeks and 13.3% in the group receiving the drug every 4 weeks.
Conjunctivitis occurred more frequently with dupilumab treatment (9.8% in the every-2-weeks dupilumab group, 10.8% in the every-4-weeks dupilumab group, and 4.7% in the placebo group) as did injection site reactions (8.5%, 6.0%, and 3.5%). Two adverse events, one of which was serious, occurred in the placebo group.
Dr. Prescilla acknowledged certain limitations of the study, including its small sample size and the fact that it was limited to 16 weeks. “However, smaller sample size and duration are typical for this type of study and in line with the study design of the SOLO 1 and SOLO 2 studies in adults,” he said.
On Aug. 6, the European Commission extended the marketing authorization for dupilumab in the European Union to include adolescents 12-17 years of age with moderate to severe atopic dermatitis who are candidates for systemic therapy. On the same day, Sanofi Genzyme and Regeneron announced positive topline results in a phase 3 trial in children aged 6-11 years with severe AD.
The study’s principal investigator was Amy S. Paller, MD. The study was funded by Sanofi Genzyme and Regeneron. Dr. Prescilla is an employee of Sanofi Genzyme.
AUSTIN, TEX. – according to results of a phase 3 study.
“Dupilumab works as effectively in adolescents as in adults,” Randy Prescilla, MD, one of the study authors, said in an interview at the annual meeting of the Society for Pediatric Dermatology. “It gives us promise that we could go into other age groups with the same optimism. We are enrolling patients in even younger age groups.”
The double-blind, placebo-controlled study analyzed the efficacy and safety of dupilumab monotherapy in patients between the ages of 12 and 17 years with moderate to severe atopic dermatitis (AD) inadequately controlled with topical therapies. In the United States, dupilumab is approved for those aged 12 years and older with moderate to severe disease inadequately controlled by topical prescription treatments or when those therapies are not advisable.
For the 16-week study, Dr. Prescilla, global medical affairs director of pediatric dermatology for Sanofi Genzyme, and colleagues randomized 251 patients to one of three groups: dupilumab every 2 weeks (200 mg if baseline weight was less than 60 kg; 300 mg if that weight was 60 kg or more); 300 mg dupilumab every 4 weeks; or placebo every 2 weeks.
At week 16, a significantly higher proportion of patients in the two drug treatment groups had Investigator’s Global Assessment scores of 0/1, compared with those in the placebo group (24.4%, 17.9%, and 2.4%) as well as a significantly higher percentage of patients who achieved at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) score (41.5%, 38.1%, and 8.2%).
In addition, patients in the two drug treatment groups experienced improved percent change in least square-means on the EASI from baseline to week 16, compared with those in the placebo group (–65.9%, –64.8%, and –23.6%), the Peak Pruritus Numerical Rating Scale (–47.9%, –45.5%, and –19.0%), body surface area affected by AD (–30.1%, –33.4%, and –11.7%), and in the SCORing AD clinical tool (P less than .001 for all comparisons).
Between baseline and week 16, scores on the Children’s Dermatology Life Quality Index and Patient-Oriented Eczema Measure improved significantly in the two dupilumab groups, compared with the placebo group. The rate of skin infection was higher in the placebo group (20%), compared with 11% in the group that received dupilumab every 2 weeks and 13.3% in the group receiving the drug every 4 weeks.
Conjunctivitis occurred more frequently with dupilumab treatment (9.8% in the every-2-weeks dupilumab group, 10.8% in the every-4-weeks dupilumab group, and 4.7% in the placebo group) as did injection site reactions (8.5%, 6.0%, and 3.5%). Two adverse events, one of which was serious, occurred in the placebo group.
Dr. Prescilla acknowledged certain limitations of the study, including its small sample size and the fact that it was limited to 16 weeks. “However, smaller sample size and duration are typical for this type of study and in line with the study design of the SOLO 1 and SOLO 2 studies in adults,” he said.
On Aug. 6, the European Commission extended the marketing authorization for dupilumab in the European Union to include adolescents 12-17 years of age with moderate to severe atopic dermatitis who are candidates for systemic therapy. On the same day, Sanofi Genzyme and Regeneron announced positive topline results in a phase 3 trial in children aged 6-11 years with severe AD.
The study’s principal investigator was Amy S. Paller, MD. The study was funded by Sanofi Genzyme and Regeneron. Dr. Prescilla is an employee of Sanofi Genzyme.
REPORTING FROM SPD 2019
Neonatal epileptic syndromes are surprisingly common
BANGKOK – Katherine B. Howell, MD, reported at the International Epilepsy Congress.
“This is an important finding. It’s a considerably larger number than might have been expected and likely has two contributing factors. Neonatal seizures were previously not considered epilepsy, so many previous studies excluded neonates and the conditions were underrecognized. And our large number of ictal EEGs allowed identification of ictal activation, which is a feature of EIMFS [epilepsy of infancy with migrating focal seizures]. Without those ictal recordings, the diagnosis of EIMFS may not have been made,” according to Dr. Howell, a neurologist at the Royal Children’s Hospital and University of Melbourne.
She presented a population-based study of all infants born with severe epilepsies of infancy (SEI) during a 2-year period in the Australian state of Victoria, which is considered an ideal environment for epidemiologic studies because government-funded health care is available to all. SEI was defined as seizures beginning before age 18 months, occurring at a rate of at least one per day for 1 week or weekly for 1 month, refractory to adequate trials of at least two antiepileptic drugs, and accompanied by an epileptiform EEG abnormality. Her focus was on the electroclinical phenotypes of the affected children because of the high clinical utility of this information.
“Assigning an epileptic syndrome is highly useful for clinician-to-clinician communication of an infant’s phenotype. It guides investigation of etiology and possibly selection of optimal therapy, such as steroids in West syndrome. And it can inform prognosis,” Dr. Howell said at the congress sponsored by the International League Against Epilepsy.
She and her coinvestigators analyzed the detailed records of all 114 infants with SEI born during the study period. The incidence was 1 in 2,000 live births.
“Among infants with epilepsy, this patient group with SEI is most critical to better understand. Effective treatment is often not available, the seizure and developmental outcomes are frequently devastating, and the health burden massive,” the neurologist observed.
The full spectrum of SEI
With the help of ictal EEGs, home seizure recordings, MRI scans, and genomic testing, the investigators were able to classify more than 85% of the infants. About 64% had a prototypic syndrome at onset, such as West syndrome, which accounted for 33% of all SEI, or Dravet syndrome, which was diagnosed in 3%.
The prevalence of the prototypic neonatal and early infantile epileptic syndromes was notably higher than previously reported by others: EIMFS accounted for 9% of total SEI, early infantile epileptic encephalopathy (EIEE) for 7%, and early myoclonic encephalopathy (EME) for 2%. This translated to an incidence of 1 in 28,000 live births for EIEE, 1 in 111,000 for EME, and 1 in 22,500 for EIMFS.
“While neither EIEE nor EIMFS are common, these incidences are actually not that much lower than the reported incidence of Dravet syndrome,” the neurologist pointed out.
About 36% of SEI didn’t fit into any of the prototypic syndromes. However, more than half of this subgroup, or 19% of total SEI, were prototypic syndrome like, a designation Dr. Howell and her coworkers used for cases that possessed most but not all of the well-recognized features of a particular prototypic syndrome; for example, West syndrome–like seizures but without hypsarrhythmia. Whether these prototypic syndrome-like SEI have etiologies and outcomes similar to or distinct from the prototypic syndromes remains a topic for further study.
SEI etiologies
A total of 14 patients had SEI because of an acquired syndrome attributed to brain injury, 31 were because of brain malformation, 21 involved single gene disorders, 9 were of chromosomal etiology, and 7 had a metabolic cause.
The key finding with regard to etiology was the glaring difference between children with West syndrome, its variants, or unifocal epilepsies as compared with the rest of the SEI patients. Those with West syndrome, a West syndrome–like designation, or unifocal epilepsies most commonly had a structural etiology for their SEI. Indeed, of the 52 children with West syndrome or a variant, 10 had an acquired brain injury as their etiology and 17 had a brain malformation. And of the 12 patients with unifocal SEI, 1 had a brain injury and 9 had brain malformations.
In contrast, children with neonatal or early infantile epileptic syndromes had predominantly genetic rather than structural etiologies. Of the 20 children with EIEE, EIMFS, or EME, none had brain injury as the etiology, only 1 had a brain malformation, but 9 had a single gene or chromosomal etiology.
Outcomes
“The outcome data highlight the extreme severity of SEI and the imperative for novel treatments: 16% mortality overall, so one in six was deceased by age 2 years. The infants who died after the neonatal period all had profound delays, and almost all had ongoing seizures until their death. Most survivors also had developmental delay, with severity ranging from mild to moderate in 49% to severe/profound in 41%. Just 10 of 114 children had normal development,” Dr. Howell reported.
However, there was a notable difference in outcomes between the various syndromes, and this information is highly relevant prognostically. Of the 20 children with neonatal and early infantile epileptic syndromes, 11 died and the other 9 had profound developmental delay. In contrast, the outlook was far better for children with West syndrome, West syndrome–like variants, or focal epilepsies: Among 64 affected patients, there were just 2 deaths, normal development in 9 patients, mild to moderate developmental delay in 34, and severe/profound delay in 19.
Dr. Howell reported having no financial conflicts regarding this study, which was supported by governmental research grants.
SOURCE: Howell KB et al. IEC 2019, Abstract P053.
BANGKOK – Katherine B. Howell, MD, reported at the International Epilepsy Congress.
“This is an important finding. It’s a considerably larger number than might have been expected and likely has two contributing factors. Neonatal seizures were previously not considered epilepsy, so many previous studies excluded neonates and the conditions were underrecognized. And our large number of ictal EEGs allowed identification of ictal activation, which is a feature of EIMFS [epilepsy of infancy with migrating focal seizures]. Without those ictal recordings, the diagnosis of EIMFS may not have been made,” according to Dr. Howell, a neurologist at the Royal Children’s Hospital and University of Melbourne.
She presented a population-based study of all infants born with severe epilepsies of infancy (SEI) during a 2-year period in the Australian state of Victoria, which is considered an ideal environment for epidemiologic studies because government-funded health care is available to all. SEI was defined as seizures beginning before age 18 months, occurring at a rate of at least one per day for 1 week or weekly for 1 month, refractory to adequate trials of at least two antiepileptic drugs, and accompanied by an epileptiform EEG abnormality. Her focus was on the electroclinical phenotypes of the affected children because of the high clinical utility of this information.
“Assigning an epileptic syndrome is highly useful for clinician-to-clinician communication of an infant’s phenotype. It guides investigation of etiology and possibly selection of optimal therapy, such as steroids in West syndrome. And it can inform prognosis,” Dr. Howell said at the congress sponsored by the International League Against Epilepsy.
She and her coinvestigators analyzed the detailed records of all 114 infants with SEI born during the study period. The incidence was 1 in 2,000 live births.
“Among infants with epilepsy, this patient group with SEI is most critical to better understand. Effective treatment is often not available, the seizure and developmental outcomes are frequently devastating, and the health burden massive,” the neurologist observed.
The full spectrum of SEI
With the help of ictal EEGs, home seizure recordings, MRI scans, and genomic testing, the investigators were able to classify more than 85% of the infants. About 64% had a prototypic syndrome at onset, such as West syndrome, which accounted for 33% of all SEI, or Dravet syndrome, which was diagnosed in 3%.
The prevalence of the prototypic neonatal and early infantile epileptic syndromes was notably higher than previously reported by others: EIMFS accounted for 9% of total SEI, early infantile epileptic encephalopathy (EIEE) for 7%, and early myoclonic encephalopathy (EME) for 2%. This translated to an incidence of 1 in 28,000 live births for EIEE, 1 in 111,000 for EME, and 1 in 22,500 for EIMFS.
“While neither EIEE nor EIMFS are common, these incidences are actually not that much lower than the reported incidence of Dravet syndrome,” the neurologist pointed out.
About 36% of SEI didn’t fit into any of the prototypic syndromes. However, more than half of this subgroup, or 19% of total SEI, were prototypic syndrome like, a designation Dr. Howell and her coworkers used for cases that possessed most but not all of the well-recognized features of a particular prototypic syndrome; for example, West syndrome–like seizures but without hypsarrhythmia. Whether these prototypic syndrome-like SEI have etiologies and outcomes similar to or distinct from the prototypic syndromes remains a topic for further study.
SEI etiologies
A total of 14 patients had SEI because of an acquired syndrome attributed to brain injury, 31 were because of brain malformation, 21 involved single gene disorders, 9 were of chromosomal etiology, and 7 had a metabolic cause.
The key finding with regard to etiology was the glaring difference between children with West syndrome, its variants, or unifocal epilepsies as compared with the rest of the SEI patients. Those with West syndrome, a West syndrome–like designation, or unifocal epilepsies most commonly had a structural etiology for their SEI. Indeed, of the 52 children with West syndrome or a variant, 10 had an acquired brain injury as their etiology and 17 had a brain malformation. And of the 12 patients with unifocal SEI, 1 had a brain injury and 9 had brain malformations.
In contrast, children with neonatal or early infantile epileptic syndromes had predominantly genetic rather than structural etiologies. Of the 20 children with EIEE, EIMFS, or EME, none had brain injury as the etiology, only 1 had a brain malformation, but 9 had a single gene or chromosomal etiology.
Outcomes
“The outcome data highlight the extreme severity of SEI and the imperative for novel treatments: 16% mortality overall, so one in six was deceased by age 2 years. The infants who died after the neonatal period all had profound delays, and almost all had ongoing seizures until their death. Most survivors also had developmental delay, with severity ranging from mild to moderate in 49% to severe/profound in 41%. Just 10 of 114 children had normal development,” Dr. Howell reported.
However, there was a notable difference in outcomes between the various syndromes, and this information is highly relevant prognostically. Of the 20 children with neonatal and early infantile epileptic syndromes, 11 died and the other 9 had profound developmental delay. In contrast, the outlook was far better for children with West syndrome, West syndrome–like variants, or focal epilepsies: Among 64 affected patients, there were just 2 deaths, normal development in 9 patients, mild to moderate developmental delay in 34, and severe/profound delay in 19.
Dr. Howell reported having no financial conflicts regarding this study, which was supported by governmental research grants.
SOURCE: Howell KB et al. IEC 2019, Abstract P053.
BANGKOK – Katherine B. Howell, MD, reported at the International Epilepsy Congress.
“This is an important finding. It’s a considerably larger number than might have been expected and likely has two contributing factors. Neonatal seizures were previously not considered epilepsy, so many previous studies excluded neonates and the conditions were underrecognized. And our large number of ictal EEGs allowed identification of ictal activation, which is a feature of EIMFS [epilepsy of infancy with migrating focal seizures]. Without those ictal recordings, the diagnosis of EIMFS may not have been made,” according to Dr. Howell, a neurologist at the Royal Children’s Hospital and University of Melbourne.
She presented a population-based study of all infants born with severe epilepsies of infancy (SEI) during a 2-year period in the Australian state of Victoria, which is considered an ideal environment for epidemiologic studies because government-funded health care is available to all. SEI was defined as seizures beginning before age 18 months, occurring at a rate of at least one per day for 1 week or weekly for 1 month, refractory to adequate trials of at least two antiepileptic drugs, and accompanied by an epileptiform EEG abnormality. Her focus was on the electroclinical phenotypes of the affected children because of the high clinical utility of this information.
“Assigning an epileptic syndrome is highly useful for clinician-to-clinician communication of an infant’s phenotype. It guides investigation of etiology and possibly selection of optimal therapy, such as steroids in West syndrome. And it can inform prognosis,” Dr. Howell said at the congress sponsored by the International League Against Epilepsy.
She and her coinvestigators analyzed the detailed records of all 114 infants with SEI born during the study period. The incidence was 1 in 2,000 live births.
“Among infants with epilepsy, this patient group with SEI is most critical to better understand. Effective treatment is often not available, the seizure and developmental outcomes are frequently devastating, and the health burden massive,” the neurologist observed.
The full spectrum of SEI
With the help of ictal EEGs, home seizure recordings, MRI scans, and genomic testing, the investigators were able to classify more than 85% of the infants. About 64% had a prototypic syndrome at onset, such as West syndrome, which accounted for 33% of all SEI, or Dravet syndrome, which was diagnosed in 3%.
The prevalence of the prototypic neonatal and early infantile epileptic syndromes was notably higher than previously reported by others: EIMFS accounted for 9% of total SEI, early infantile epileptic encephalopathy (EIEE) for 7%, and early myoclonic encephalopathy (EME) for 2%. This translated to an incidence of 1 in 28,000 live births for EIEE, 1 in 111,000 for EME, and 1 in 22,500 for EIMFS.
“While neither EIEE nor EIMFS are common, these incidences are actually not that much lower than the reported incidence of Dravet syndrome,” the neurologist pointed out.
About 36% of SEI didn’t fit into any of the prototypic syndromes. However, more than half of this subgroup, or 19% of total SEI, were prototypic syndrome like, a designation Dr. Howell and her coworkers used for cases that possessed most but not all of the well-recognized features of a particular prototypic syndrome; for example, West syndrome–like seizures but without hypsarrhythmia. Whether these prototypic syndrome-like SEI have etiologies and outcomes similar to or distinct from the prototypic syndromes remains a topic for further study.
SEI etiologies
A total of 14 patients had SEI because of an acquired syndrome attributed to brain injury, 31 were because of brain malformation, 21 involved single gene disorders, 9 were of chromosomal etiology, and 7 had a metabolic cause.
The key finding with regard to etiology was the glaring difference between children with West syndrome, its variants, or unifocal epilepsies as compared with the rest of the SEI patients. Those with West syndrome, a West syndrome–like designation, or unifocal epilepsies most commonly had a structural etiology for their SEI. Indeed, of the 52 children with West syndrome or a variant, 10 had an acquired brain injury as their etiology and 17 had a brain malformation. And of the 12 patients with unifocal SEI, 1 had a brain injury and 9 had brain malformations.
In contrast, children with neonatal or early infantile epileptic syndromes had predominantly genetic rather than structural etiologies. Of the 20 children with EIEE, EIMFS, or EME, none had brain injury as the etiology, only 1 had a brain malformation, but 9 had a single gene or chromosomal etiology.
Outcomes
“The outcome data highlight the extreme severity of SEI and the imperative for novel treatments: 16% mortality overall, so one in six was deceased by age 2 years. The infants who died after the neonatal period all had profound delays, and almost all had ongoing seizures until their death. Most survivors also had developmental delay, with severity ranging from mild to moderate in 49% to severe/profound in 41%. Just 10 of 114 children had normal development,” Dr. Howell reported.
However, there was a notable difference in outcomes between the various syndromes, and this information is highly relevant prognostically. Of the 20 children with neonatal and early infantile epileptic syndromes, 11 died and the other 9 had profound developmental delay. In contrast, the outlook was far better for children with West syndrome, West syndrome–like variants, or focal epilepsies: Among 64 affected patients, there were just 2 deaths, normal development in 9 patients, mild to moderate developmental delay in 34, and severe/profound delay in 19.
Dr. Howell reported having no financial conflicts regarding this study, which was supported by governmental research grants.
SOURCE: Howell KB et al. IEC 2019, Abstract P053.
REPORTING FROM IEC 2019
PROMIS tools provide useful data for managing rheumatology patients
LAKE BUENA VISTA, FLA. –
The PROMIS tools – which like most patient-reported outcome (PRO) measurement tools are designed to evaluate and monitor physical, mental, and social health – can be used both for the general population and for individuals living with chronic conditions, Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham (UAB), said at the annual meeting of the Florida Society of Rheumatology.
The tools take a deeper dive into various symptoms and their effects; for instance, with respect to physical health, they measure fatigue, physical function, sleep disturbance, pain intensity, and pain interference – the extent to which pain “messes your patient’s life up,” explained Dr. Curtis, who also is codirector of the UAB Pharmacoepidemiology and Pharmacoeconomics Unit.
Additional physical health domains that PROs measure include dyspnea, gastrointestinal symptoms, pain behavior, pain quality, sexual function, and sleep-related impairment.
These are “things that, honestly, we don’t talk about much as a field, but absolutely affect patients with autoimmune diseases,” he said. “You know, sexual function – that doesn’t come up in my practice spontaneously very often, but there are ways you can quantify that, and for many patients that’s actually a big deal.”
The domains measured by PROMIS tools for mental health look at anxiety and depression, but also delve into alcohol use, anger, cognitive function, life satisfaction, self-efficacy for managing chronic conditions, substance use, and more. The domains for social health address ability to participate in social roles and activities, as well as companionship, satisfaction with social roles and activity, social isolation, and social support.
“You can’t go on a hike with friends [and] be far from a bathroom, because you have bad arthritis and you have Crohn’s disease. Well, that’s kind of an important thing that may or may not come up in your discussions about inflammatory arthritis associated with [inflammatory bowel disease],” he said.
Another example is a patient who is embarrassed attending social functions or wearing a swimsuit because of really bad psoriasis.
“These are the kinds of things that I’m suggesting you and I probably want to measure if we’re providing holistic care to rheumatology patients,” Dr. Curtis said.
The PROMIS tools provide a simple, user-friendly means for doing so in English, Spanish, and many other languages, he noted.
All the scales use the same 1-100 scoring range, which simplifies measurements. They are available for free by download and can be printed or used electronically for use in the office, at home, on the web, and via smartphone.
The NIH developed the PROMIS tools several years ago and validated them for multiple chronic disease populations, Dr. Curtis said, adding that the tools include multiple individual domains and overall “profiles” of varying lengths.
Most are fixed-length scales that are between 4 and 10 questions and can be completed within 30-60 seconds per scale, so several scales can be completed within 5-10 minutes.
However, some scales are longer and provide greater detail.
“The nice thing is that if you ask a few more questions you can get more precise information – there’s more of a floor and ceiling. You can detect people who do really well. You can distinguish between the marathon runners and the 5K-ers and the people who can walk 2 miles but aren’t going to run a race,” he explained.
Further, the PROMIS tools, like the 36-item Short Form Health Survey (SF-36), are benchmarked against the U.S. adult population, allowing for assessment of how a specific drug or treatment “impacts your arthritis patient on a scale that would also be relevant for somebody who doesn’t have arthritis, they have diabetes.”
The metrics and scales are the same, and that can be helpful when trying to get a payer to pay for a particular drug, he said.
“None of these are rheumatology specific; this puts PROs into a language that can help rheumatology contend for the value of the care that we provide on a scale that would be relevant for any other chronic illness, even for nonrheumatology patients,” he explained.
In addition, minimally important differences (group mean change of about 2-3 units) and minimally clinical important differences for individuals (5 units) have been established.
“So we know what the numbers mean, and this is true for all of the scales,” he said.
PROMIS tools also include computer-adaptive testing (CAT) versions, which helps to personalize the scales to provide more precise information for a given patient and eliminate irrelevant information.
Of note, PROMIS health measures are among the data that can be tracked on a smartphone using Arthritis Power, an arthritis research registry developed with the help of a recent infrastructure grant awarded to the Center for Education and Research and Therapeutics of Musculoskeletal Disorders at UAB, Dr. Curtis said.
The measures were also shown in the AWARE study to track closely with other measures, including the Clinical Disease Activity Index (CDAI), and with patient improvement on therapy.
“So these PROMIS scores are tracking with things that you and I are familiar with ... and it looks like these scores are faithfully tracking, over time, patients getting better on therapies that we would expect them to,” he said. “I think this is additional validation – not just from the National Institutes of Health and a decade of research by lots of different groups, but in our own field – that these actually correlate with disease activity ... and that when you start an effective therapy like a [tumor necrosis factor inhibitor] they’re going to improve as you would anticipate.”
Dr. Curtis reported funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Patient-Centered Outcomes Research Institute. He has also consulted for or received research grants from Amgen, AbbVie, Bristol-Myers Squibb, CORRONA, Lilly, Janssen, Myriad, Novartis, Roche, Pfizer, and Sanofi/Regeneron.
LAKE BUENA VISTA, FLA. –
The PROMIS tools – which like most patient-reported outcome (PRO) measurement tools are designed to evaluate and monitor physical, mental, and social health – can be used both for the general population and for individuals living with chronic conditions, Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham (UAB), said at the annual meeting of the Florida Society of Rheumatology.
The tools take a deeper dive into various symptoms and their effects; for instance, with respect to physical health, they measure fatigue, physical function, sleep disturbance, pain intensity, and pain interference – the extent to which pain “messes your patient’s life up,” explained Dr. Curtis, who also is codirector of the UAB Pharmacoepidemiology and Pharmacoeconomics Unit.
Additional physical health domains that PROs measure include dyspnea, gastrointestinal symptoms, pain behavior, pain quality, sexual function, and sleep-related impairment.
These are “things that, honestly, we don’t talk about much as a field, but absolutely affect patients with autoimmune diseases,” he said. “You know, sexual function – that doesn’t come up in my practice spontaneously very often, but there are ways you can quantify that, and for many patients that’s actually a big deal.”
The domains measured by PROMIS tools for mental health look at anxiety and depression, but also delve into alcohol use, anger, cognitive function, life satisfaction, self-efficacy for managing chronic conditions, substance use, and more. The domains for social health address ability to participate in social roles and activities, as well as companionship, satisfaction with social roles and activity, social isolation, and social support.
“You can’t go on a hike with friends [and] be far from a bathroom, because you have bad arthritis and you have Crohn’s disease. Well, that’s kind of an important thing that may or may not come up in your discussions about inflammatory arthritis associated with [inflammatory bowel disease],” he said.
Another example is a patient who is embarrassed attending social functions or wearing a swimsuit because of really bad psoriasis.
“These are the kinds of things that I’m suggesting you and I probably want to measure if we’re providing holistic care to rheumatology patients,” Dr. Curtis said.
The PROMIS tools provide a simple, user-friendly means for doing so in English, Spanish, and many other languages, he noted.
All the scales use the same 1-100 scoring range, which simplifies measurements. They are available for free by download and can be printed or used electronically for use in the office, at home, on the web, and via smartphone.
The NIH developed the PROMIS tools several years ago and validated them for multiple chronic disease populations, Dr. Curtis said, adding that the tools include multiple individual domains and overall “profiles” of varying lengths.
Most are fixed-length scales that are between 4 and 10 questions and can be completed within 30-60 seconds per scale, so several scales can be completed within 5-10 minutes.
However, some scales are longer and provide greater detail.
“The nice thing is that if you ask a few more questions you can get more precise information – there’s more of a floor and ceiling. You can detect people who do really well. You can distinguish between the marathon runners and the 5K-ers and the people who can walk 2 miles but aren’t going to run a race,” he explained.
Further, the PROMIS tools, like the 36-item Short Form Health Survey (SF-36), are benchmarked against the U.S. adult population, allowing for assessment of how a specific drug or treatment “impacts your arthritis patient on a scale that would also be relevant for somebody who doesn’t have arthritis, they have diabetes.”
The metrics and scales are the same, and that can be helpful when trying to get a payer to pay for a particular drug, he said.
“None of these are rheumatology specific; this puts PROs into a language that can help rheumatology contend for the value of the care that we provide on a scale that would be relevant for any other chronic illness, even for nonrheumatology patients,” he explained.
In addition, minimally important differences (group mean change of about 2-3 units) and minimally clinical important differences for individuals (5 units) have been established.
“So we know what the numbers mean, and this is true for all of the scales,” he said.
PROMIS tools also include computer-adaptive testing (CAT) versions, which helps to personalize the scales to provide more precise information for a given patient and eliminate irrelevant information.
Of note, PROMIS health measures are among the data that can be tracked on a smartphone using Arthritis Power, an arthritis research registry developed with the help of a recent infrastructure grant awarded to the Center for Education and Research and Therapeutics of Musculoskeletal Disorders at UAB, Dr. Curtis said.
The measures were also shown in the AWARE study to track closely with other measures, including the Clinical Disease Activity Index (CDAI), and with patient improvement on therapy.
“So these PROMIS scores are tracking with things that you and I are familiar with ... and it looks like these scores are faithfully tracking, over time, patients getting better on therapies that we would expect them to,” he said. “I think this is additional validation – not just from the National Institutes of Health and a decade of research by lots of different groups, but in our own field – that these actually correlate with disease activity ... and that when you start an effective therapy like a [tumor necrosis factor inhibitor] they’re going to improve as you would anticipate.”
Dr. Curtis reported funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Patient-Centered Outcomes Research Institute. He has also consulted for or received research grants from Amgen, AbbVie, Bristol-Myers Squibb, CORRONA, Lilly, Janssen, Myriad, Novartis, Roche, Pfizer, and Sanofi/Regeneron.
LAKE BUENA VISTA, FLA. –
The PROMIS tools – which like most patient-reported outcome (PRO) measurement tools are designed to evaluate and monitor physical, mental, and social health – can be used both for the general population and for individuals living with chronic conditions, Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham (UAB), said at the annual meeting of the Florida Society of Rheumatology.
The tools take a deeper dive into various symptoms and their effects; for instance, with respect to physical health, they measure fatigue, physical function, sleep disturbance, pain intensity, and pain interference – the extent to which pain “messes your patient’s life up,” explained Dr. Curtis, who also is codirector of the UAB Pharmacoepidemiology and Pharmacoeconomics Unit.
Additional physical health domains that PROs measure include dyspnea, gastrointestinal symptoms, pain behavior, pain quality, sexual function, and sleep-related impairment.
These are “things that, honestly, we don’t talk about much as a field, but absolutely affect patients with autoimmune diseases,” he said. “You know, sexual function – that doesn’t come up in my practice spontaneously very often, but there are ways you can quantify that, and for many patients that’s actually a big deal.”
The domains measured by PROMIS tools for mental health look at anxiety and depression, but also delve into alcohol use, anger, cognitive function, life satisfaction, self-efficacy for managing chronic conditions, substance use, and more. The domains for social health address ability to participate in social roles and activities, as well as companionship, satisfaction with social roles and activity, social isolation, and social support.
“You can’t go on a hike with friends [and] be far from a bathroom, because you have bad arthritis and you have Crohn’s disease. Well, that’s kind of an important thing that may or may not come up in your discussions about inflammatory arthritis associated with [inflammatory bowel disease],” he said.
Another example is a patient who is embarrassed attending social functions or wearing a swimsuit because of really bad psoriasis.
“These are the kinds of things that I’m suggesting you and I probably want to measure if we’re providing holistic care to rheumatology patients,” Dr. Curtis said.
The PROMIS tools provide a simple, user-friendly means for doing so in English, Spanish, and many other languages, he noted.
All the scales use the same 1-100 scoring range, which simplifies measurements. They are available for free by download and can be printed or used electronically for use in the office, at home, on the web, and via smartphone.
The NIH developed the PROMIS tools several years ago and validated them for multiple chronic disease populations, Dr. Curtis said, adding that the tools include multiple individual domains and overall “profiles” of varying lengths.
Most are fixed-length scales that are between 4 and 10 questions and can be completed within 30-60 seconds per scale, so several scales can be completed within 5-10 minutes.
However, some scales are longer and provide greater detail.
“The nice thing is that if you ask a few more questions you can get more precise information – there’s more of a floor and ceiling. You can detect people who do really well. You can distinguish between the marathon runners and the 5K-ers and the people who can walk 2 miles but aren’t going to run a race,” he explained.
Further, the PROMIS tools, like the 36-item Short Form Health Survey (SF-36), are benchmarked against the U.S. adult population, allowing for assessment of how a specific drug or treatment “impacts your arthritis patient on a scale that would also be relevant for somebody who doesn’t have arthritis, they have diabetes.”
The metrics and scales are the same, and that can be helpful when trying to get a payer to pay for a particular drug, he said.
“None of these are rheumatology specific; this puts PROs into a language that can help rheumatology contend for the value of the care that we provide on a scale that would be relevant for any other chronic illness, even for nonrheumatology patients,” he explained.
In addition, minimally important differences (group mean change of about 2-3 units) and minimally clinical important differences for individuals (5 units) have been established.
“So we know what the numbers mean, and this is true for all of the scales,” he said.
PROMIS tools also include computer-adaptive testing (CAT) versions, which helps to personalize the scales to provide more precise information for a given patient and eliminate irrelevant information.
Of note, PROMIS health measures are among the data that can be tracked on a smartphone using Arthritis Power, an arthritis research registry developed with the help of a recent infrastructure grant awarded to the Center for Education and Research and Therapeutics of Musculoskeletal Disorders at UAB, Dr. Curtis said.
The measures were also shown in the AWARE study to track closely with other measures, including the Clinical Disease Activity Index (CDAI), and with patient improvement on therapy.
“So these PROMIS scores are tracking with things that you and I are familiar with ... and it looks like these scores are faithfully tracking, over time, patients getting better on therapies that we would expect them to,” he said. “I think this is additional validation – not just from the National Institutes of Health and a decade of research by lots of different groups, but in our own field – that these actually correlate with disease activity ... and that when you start an effective therapy like a [tumor necrosis factor inhibitor] they’re going to improve as you would anticipate.”
Dr. Curtis reported funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Patient-Centered Outcomes Research Institute. He has also consulted for or received research grants from Amgen, AbbVie, Bristol-Myers Squibb, CORRONA, Lilly, Janssen, Myriad, Novartis, Roche, Pfizer, and Sanofi/Regeneron.
EXPERT ANALYSIS FROM FSR 2019
In newborns, concentrated urine helps rule out UTI
SEATTLE – according to investigators at the University of Texas Health Science Center, Houston.
The researchers found that urine testing negative for nitrites with a specific gravity above 1.015 in children up to 2 months old had a sensitivity of 53% for ruling out UTIs, but that urine with a specific gravity below that mark had a sensitivity of just 14%. The finding “should be taken into account when interpreting nitrite results ... in this high-risk population,” they concluded.
Bacteria in the bladder convert nitrates to nitrites, so positive results are pretty much pathognomonic for UTIs, with a specificity of nearly 100%, according to the researchers.
Negative results, however, don’t reliably rule out infection, and are even less reliable in infants because they urinate frequently, which means they usually flush out bacteria before they have enough time to make the conversion, which takes several hours, they said.
The lead investigator Raymond Parlar-Chun, MD, an assistant professor of pediatrics at the University of Texas McGovern Medical School in Houston, said he had a hunch that negative results might be more reliable when newborns urinate less frequently and have more concentrated urine.
He and his team reviewed data collected on 413 infants up to 2 months old who were admitted for fever workup and treated for UTIs both in the hospital and after discharge. Nitrite results were stratified by urine concentration. A specific gravity of 1.015 was used as the cutoff between concentrated and dilute urine, which was “midway between the parameters reported” in every urinalysis, Dr. Parlar-Chun said.
Although the sensitivity of concentrated urine was only 53%, “it’s a stark difference from” the 14% in dilute urine, he said.“You should take a look at specific gravity to interpret nitrites. If urine is concentrated, you have [more confidence] that you don’t have a UTI if you’re negative. It’s better than taking [nitrites] at face value.”
The subjects were 31 days old, on average, and 62% were boys; 112 had a specific gravity above 1.015, and 301 below.
There was no external funding, and Dr. Parlar-Chun didn’t have any disclosures.
SEATTLE – according to investigators at the University of Texas Health Science Center, Houston.
The researchers found that urine testing negative for nitrites with a specific gravity above 1.015 in children up to 2 months old had a sensitivity of 53% for ruling out UTIs, but that urine with a specific gravity below that mark had a sensitivity of just 14%. The finding “should be taken into account when interpreting nitrite results ... in this high-risk population,” they concluded.
Bacteria in the bladder convert nitrates to nitrites, so positive results are pretty much pathognomonic for UTIs, with a specificity of nearly 100%, according to the researchers.
Negative results, however, don’t reliably rule out infection, and are even less reliable in infants because they urinate frequently, which means they usually flush out bacteria before they have enough time to make the conversion, which takes several hours, they said.
The lead investigator Raymond Parlar-Chun, MD, an assistant professor of pediatrics at the University of Texas McGovern Medical School in Houston, said he had a hunch that negative results might be more reliable when newborns urinate less frequently and have more concentrated urine.
He and his team reviewed data collected on 413 infants up to 2 months old who were admitted for fever workup and treated for UTIs both in the hospital and after discharge. Nitrite results were stratified by urine concentration. A specific gravity of 1.015 was used as the cutoff between concentrated and dilute urine, which was “midway between the parameters reported” in every urinalysis, Dr. Parlar-Chun said.
Although the sensitivity of concentrated urine was only 53%, “it’s a stark difference from” the 14% in dilute urine, he said.“You should take a look at specific gravity to interpret nitrites. If urine is concentrated, you have [more confidence] that you don’t have a UTI if you’re negative. It’s better than taking [nitrites] at face value.”
The subjects were 31 days old, on average, and 62% were boys; 112 had a specific gravity above 1.015, and 301 below.
There was no external funding, and Dr. Parlar-Chun didn’t have any disclosures.
SEATTLE – according to investigators at the University of Texas Health Science Center, Houston.
The researchers found that urine testing negative for nitrites with a specific gravity above 1.015 in children up to 2 months old had a sensitivity of 53% for ruling out UTIs, but that urine with a specific gravity below that mark had a sensitivity of just 14%. The finding “should be taken into account when interpreting nitrite results ... in this high-risk population,” they concluded.
Bacteria in the bladder convert nitrates to nitrites, so positive results are pretty much pathognomonic for UTIs, with a specificity of nearly 100%, according to the researchers.
Negative results, however, don’t reliably rule out infection, and are even less reliable in infants because they urinate frequently, which means they usually flush out bacteria before they have enough time to make the conversion, which takes several hours, they said.
The lead investigator Raymond Parlar-Chun, MD, an assistant professor of pediatrics at the University of Texas McGovern Medical School in Houston, said he had a hunch that negative results might be more reliable when newborns urinate less frequently and have more concentrated urine.
He and his team reviewed data collected on 413 infants up to 2 months old who were admitted for fever workup and treated for UTIs both in the hospital and after discharge. Nitrite results were stratified by urine concentration. A specific gravity of 1.015 was used as the cutoff between concentrated and dilute urine, which was “midway between the parameters reported” in every urinalysis, Dr. Parlar-Chun said.
Although the sensitivity of concentrated urine was only 53%, “it’s a stark difference from” the 14% in dilute urine, he said.“You should take a look at specific gravity to interpret nitrites. If urine is concentrated, you have [more confidence] that you don’t have a UTI if you’re negative. It’s better than taking [nitrites] at face value.”
The subjects were 31 days old, on average, and 62% were boys; 112 had a specific gravity above 1.015, and 301 below.
There was no external funding, and Dr. Parlar-Chun didn’t have any disclosures.
REPORTING FROM PHM 2019
New RSV vaccine immunogenicity improved with protein engineering
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
FROM SCIENCE
Key clinical point:
Major finding: Epitope-neutralizing activity is 5-10 times greater 12 weeks after baseline with a 50 mcg or 150 mcg with and without alum adjuvant.
Study details: The findings are based on a prespecified interim analysis of 90 healthy adult participants in a phase 1, randomized, trial of DS-Cav1.
Disclosures: The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several authors are inventors on patents for stabilizing the RSV F protein.
Source: Crank MC et al. Science. 2019;365(6452):505-9.
Hospital slashes S. aureus vancomycin resistance
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
REPORTING FROM ESPID 2019
Key clinical point: Staphylococcus aureus vancomycin MIC creep is reversible through dedicated antimicrobial stewardship.
Major finding: The prevalence of hGISA in MRSA and MSSA specimens improved from 25% during 2002-2009 to 6% during 2010-2017 at one German tertiary children’s hospital.
Study details: This was a retrospective single-center analysis of vancomycin resistance trends over time in 540 S. aureus specimens gathered in 2002-2017.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.
Rubbery Nodule on the Face of an Infant
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 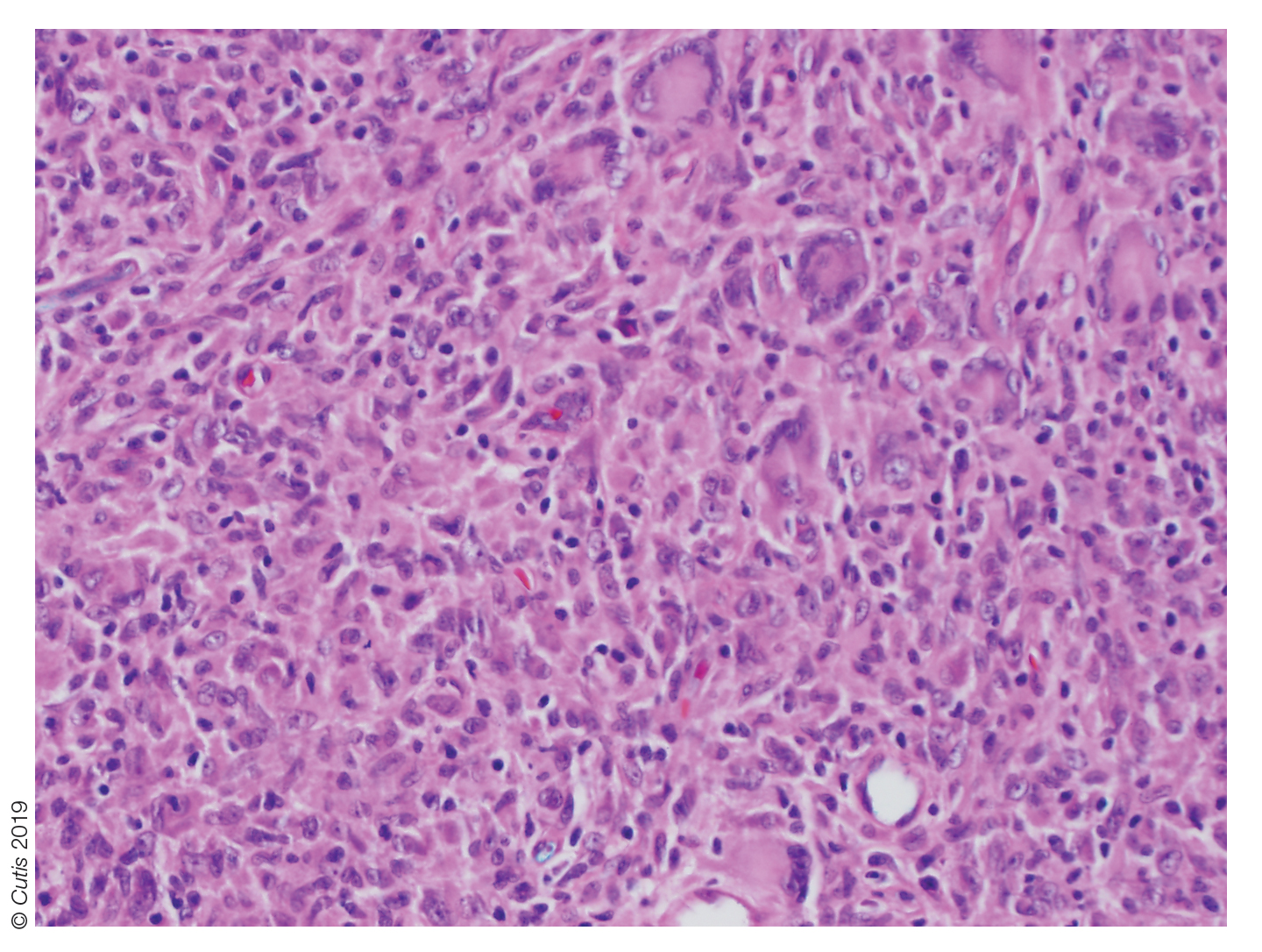
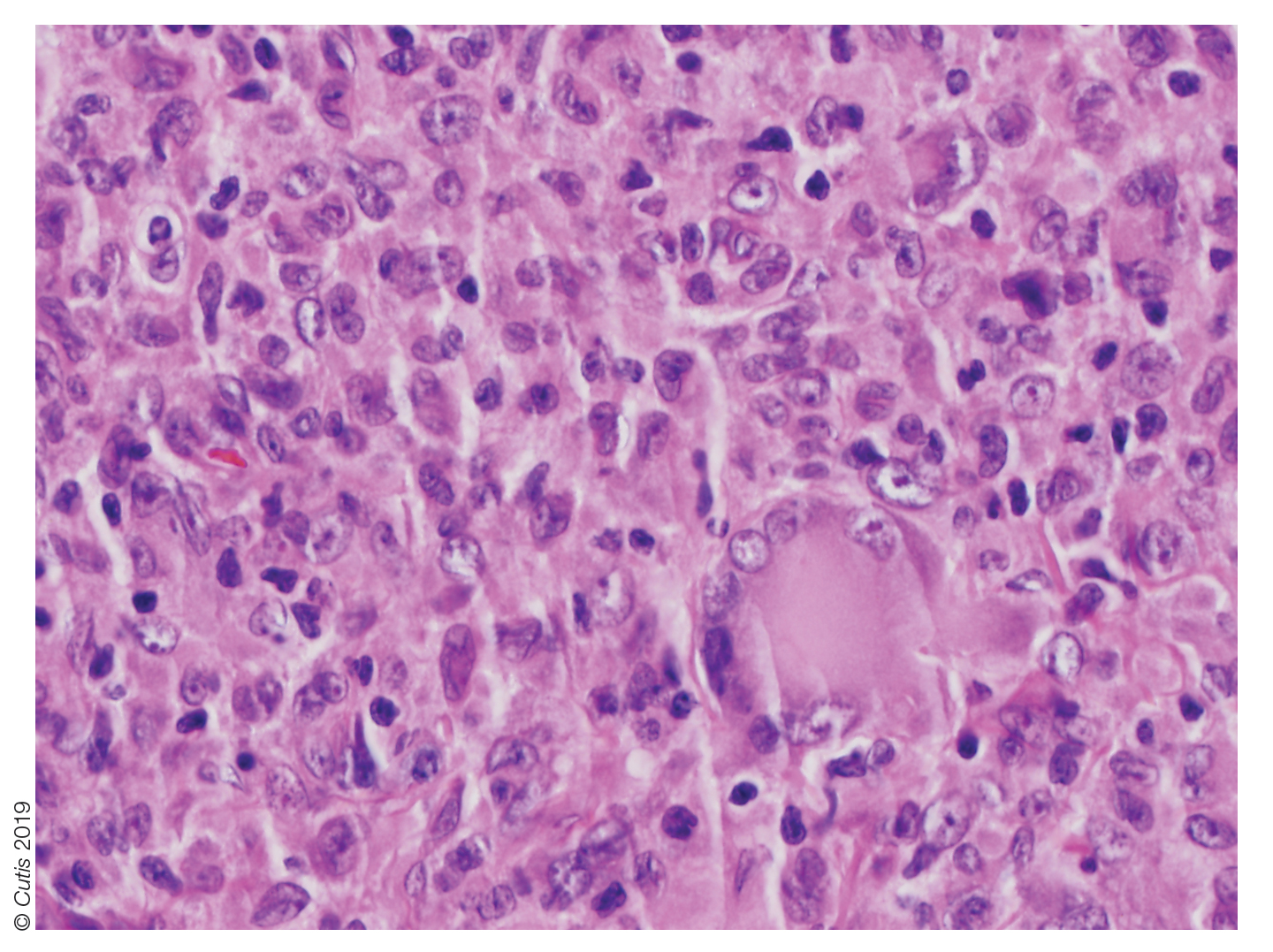
Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13
Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 

Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13

Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 

Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13

Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
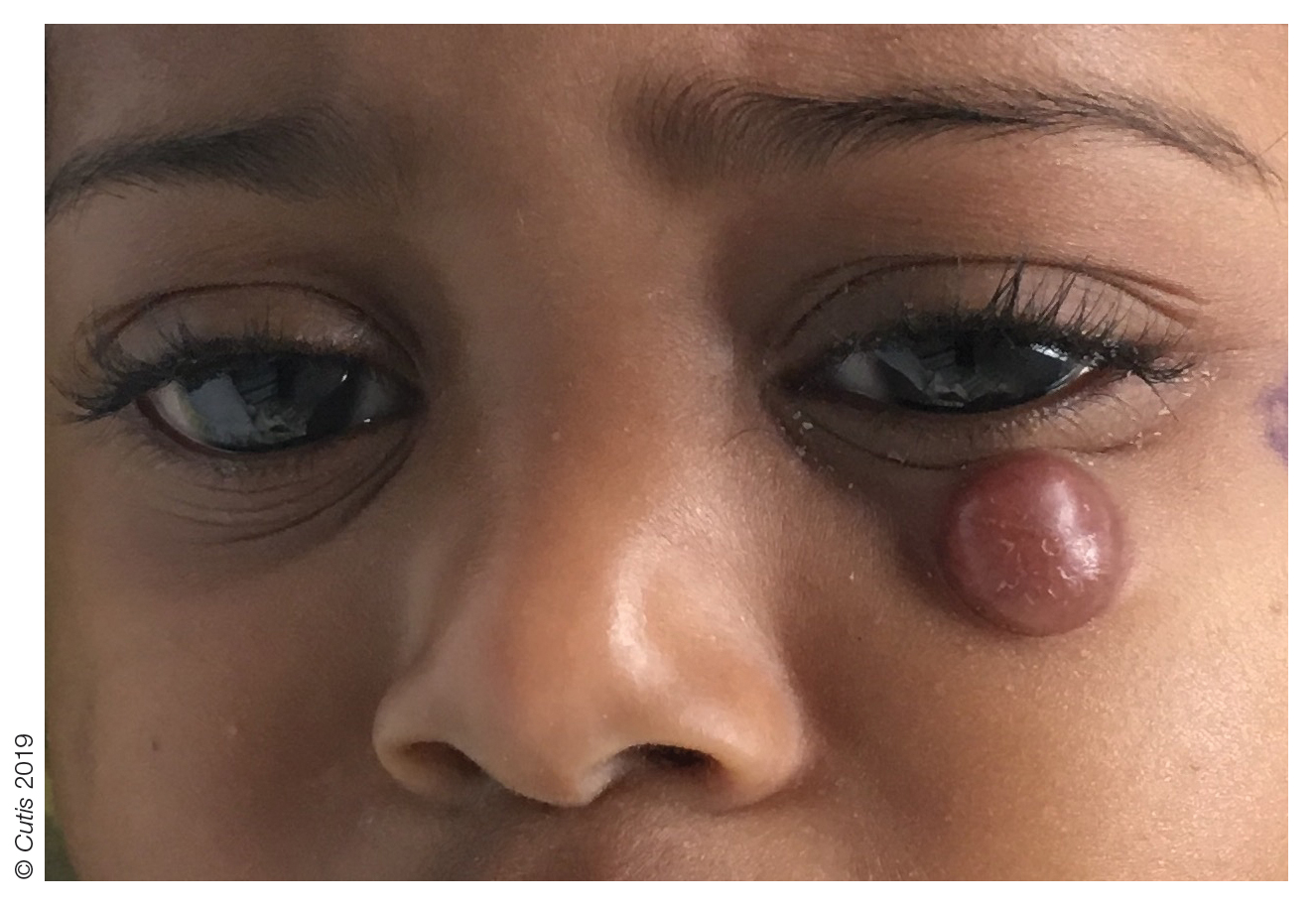
A 10-month-old girl presented with a facial nodule of 7 months’ duration that started as a small lesion. On physical examination, a single 10×10-mm, nontender, well-circumscribed, firm, freely mobile nodule was observed in the left infraorbital area. The patient was born full term at 37 weeks’ gestation via spontaneous vaginal delivery and had no other notable findings on physical examination. Excision was performed by an oculoplastic surgeon. Pathology revealed a relatively well-circumscribed, diffuse, dermal infiltrate of cells arranged in short fascicles and a storiform pattern. The cells had abundant clear to amphophilic cytoplasm, ovoid to reniform nuclei with vesicular chromatin and focal grooves, and diffuse CD68+ immunoreactivity, as well as scattered S-100–positive cells. The patient did well with the excision and no new lesions have developed.