User login
Baby’s Rash Causes Family Feud
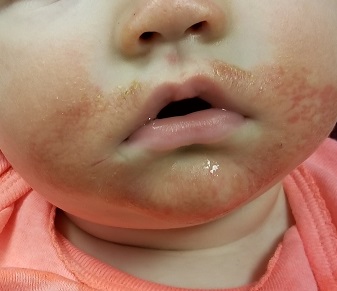
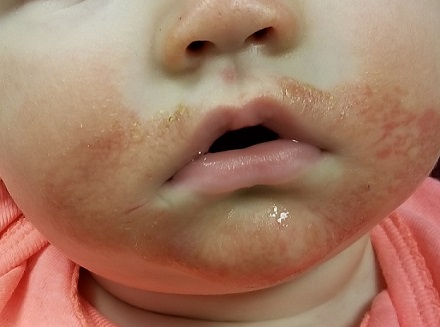
Since birth, this 5-month-old boy has had a facial rash that comes and goes. At times severe, it is the source of much familial disagreement about its cause: Some say the problem is related to food, while others are sure it represents infection.
Several medications, including triple-antibiotic ointment and nystatin cream, have been tried. None have had much effect.
The child is well in all other respects—gaining weight as expected and experiencing normal growth and development. The rash does not appear to bother him as much as it bothers his family to see.
Further questioning reveals a strong family history of seasonal allergies, eczema, and asthma. Notably, all affected individuals have long since outgrown those problems.
EXAMINATION
The child is in no apparent distress but is noted to have nasal congestion, with continual mouth breathing. Overall, his skin is quite dry and fair.
The rash itself is rather florid, affecting the perioral area and spreading onto the cheeks in a symmetrical configuration. The skin in these areas is focally erythematous, though not swollen. It is also quite scaly in places, giving the appearance of, as his parents note, “chapped” skin. Examination of the diaper area reveals a similar look focally.
What’s the diagnosis?
DISCUSSION
This case is typical of those seen multiple times daily in primary care and dermatology offices—hardly surprising, since atopic dermatitis (AD) affects about 20% of all newborns in this and other developed countries. In very young children, AD primarily affects the face and diaper area, as well as the trunk. About 50% of affected patients will have cradle cap—as did this child in his first month of life, we subsequently learned.
The tendency to develop AD is inherited. It is not related to food, although children with AD could develop a food allergy. However, it would more likely manifest with gastrointestinal symptoms. Another myth embedded in Western culture is that AD is caused by exposure to a particular laundry detergent.
Rather, this child and others like him have inherited dry, thin, overreactive skin that is bathed early on with nasal secretions, bacteria, and saliva. Later, their eczema will migrate to areas that stay moist, such as the antecubital and popliteal folds, or to the area around the neck, where the itching can be intense—which of course causes the child to scratch, in turn worsening the problem.
Patient/parent education is the key to dealing with AD and can be bolstered by handouts or direction to reliable websites. Besides objectifying the problem, these resources detail its nature and outline the need for daily bathing with mild cleansers, generous application of heavy moisturizers, careful use of topical corticosteroid creams or ointments (eg, 2.5% hydrocortisone), and avoidance of woolen clothing or bedding.
Another important component of patient/parent education is the reassurance that eczema will not scar the patient. It does occasionally become severe enough to require a short course of oral antibiotics (eg, cephalexin) or even the use of oral prednisolone. Since AD is not a histamine-driven process, antihistamines are ineffective for eczema.
TAKE-HOME LEARNING POINTS
- Atopic dermatitis (AD) is extremely common, affecting 20% of all newborns in developed countries.
- Infantile eczema typically centers on the face, particularly the perioral area.
- Later, it begins to involve the antecubital and popliteal areas, which stay moist a good part of the time.
- Most children outgrow the worst of the problem—and go on to have children who develop it.
Since birth, this 5-month-old boy has had a facial rash that comes and goes. At times severe, it is the source of much familial disagreement about its cause: Some say the problem is related to food, while others are sure it represents infection.
Several medications, including triple-antibiotic ointment and nystatin cream, have been tried. None have had much effect.
The child is well in all other respects—gaining weight as expected and experiencing normal growth and development. The rash does not appear to bother him as much as it bothers his family to see.
Further questioning reveals a strong family history of seasonal allergies, eczema, and asthma. Notably, all affected individuals have long since outgrown those problems.

EXAMINATION
The child is in no apparent distress but is noted to have nasal congestion, with continual mouth breathing. Overall, his skin is quite dry and fair.
The rash itself is rather florid, affecting the perioral area and spreading onto the cheeks in a symmetrical configuration. The skin in these areas is focally erythematous, though not swollen. It is also quite scaly in places, giving the appearance of, as his parents note, “chapped” skin. Examination of the diaper area reveals a similar look focally.
What’s the diagnosis?
DISCUSSION
This case is typical of those seen multiple times daily in primary care and dermatology offices—hardly surprising, since atopic dermatitis (AD) affects about 20% of all newborns in this and other developed countries. In very young children, AD primarily affects the face and diaper area, as well as the trunk. About 50% of affected patients will have cradle cap—as did this child in his first month of life, we subsequently learned.
The tendency to develop AD is inherited. It is not related to food, although children with AD could develop a food allergy. However, it would more likely manifest with gastrointestinal symptoms. Another myth embedded in Western culture is that AD is caused by exposure to a particular laundry detergent.
Rather, this child and others like him have inherited dry, thin, overreactive skin that is bathed early on with nasal secretions, bacteria, and saliva. Later, their eczema will migrate to areas that stay moist, such as the antecubital and popliteal folds, or to the area around the neck, where the itching can be intense—which of course causes the child to scratch, in turn worsening the problem.
Patient/parent education is the key to dealing with AD and can be bolstered by handouts or direction to reliable websites. Besides objectifying the problem, these resources detail its nature and outline the need for daily bathing with mild cleansers, generous application of heavy moisturizers, careful use of topical corticosteroid creams or ointments (eg, 2.5% hydrocortisone), and avoidance of woolen clothing or bedding.
Another important component of patient/parent education is the reassurance that eczema will not scar the patient. It does occasionally become severe enough to require a short course of oral antibiotics (eg, cephalexin) or even the use of oral prednisolone. Since AD is not a histamine-driven process, antihistamines are ineffective for eczema.
TAKE-HOME LEARNING POINTS
- Atopic dermatitis (AD) is extremely common, affecting 20% of all newborns in developed countries.
- Infantile eczema typically centers on the face, particularly the perioral area.
- Later, it begins to involve the antecubital and popliteal areas, which stay moist a good part of the time.
- Most children outgrow the worst of the problem—and go on to have children who develop it.
Since birth, this 5-month-old boy has had a facial rash that comes and goes. At times severe, it is the source of much familial disagreement about its cause: Some say the problem is related to food, while others are sure it represents infection.
Several medications, including triple-antibiotic ointment and nystatin cream, have been tried. None have had much effect.
The child is well in all other respects—gaining weight as expected and experiencing normal growth and development. The rash does not appear to bother him as much as it bothers his family to see.
Further questioning reveals a strong family history of seasonal allergies, eczema, and asthma. Notably, all affected individuals have long since outgrown those problems.

EXAMINATION
The child is in no apparent distress but is noted to have nasal congestion, with continual mouth breathing. Overall, his skin is quite dry and fair.
The rash itself is rather florid, affecting the perioral area and spreading onto the cheeks in a symmetrical configuration. The skin in these areas is focally erythematous, though not swollen. It is also quite scaly in places, giving the appearance of, as his parents note, “chapped” skin. Examination of the diaper area reveals a similar look focally.
What’s the diagnosis?
DISCUSSION
This case is typical of those seen multiple times daily in primary care and dermatology offices—hardly surprising, since atopic dermatitis (AD) affects about 20% of all newborns in this and other developed countries. In very young children, AD primarily affects the face and diaper area, as well as the trunk. About 50% of affected patients will have cradle cap—as did this child in his first month of life, we subsequently learned.
The tendency to develop AD is inherited. It is not related to food, although children with AD could develop a food allergy. However, it would more likely manifest with gastrointestinal symptoms. Another myth embedded in Western culture is that AD is caused by exposure to a particular laundry detergent.
Rather, this child and others like him have inherited dry, thin, overreactive skin that is bathed early on with nasal secretions, bacteria, and saliva. Later, their eczema will migrate to areas that stay moist, such as the antecubital and popliteal folds, or to the area around the neck, where the itching can be intense—which of course causes the child to scratch, in turn worsening the problem.
Patient/parent education is the key to dealing with AD and can be bolstered by handouts or direction to reliable websites. Besides objectifying the problem, these resources detail its nature and outline the need for daily bathing with mild cleansers, generous application of heavy moisturizers, careful use of topical corticosteroid creams or ointments (eg, 2.5% hydrocortisone), and avoidance of woolen clothing or bedding.
Another important component of patient/parent education is the reassurance that eczema will not scar the patient. It does occasionally become severe enough to require a short course of oral antibiotics (eg, cephalexin) or even the use of oral prednisolone. Since AD is not a histamine-driven process, antihistamines are ineffective for eczema.
TAKE-HOME LEARNING POINTS
- Atopic dermatitis (AD) is extremely common, affecting 20% of all newborns in developed countries.
- Infantile eczema typically centers on the face, particularly the perioral area.
- Later, it begins to involve the antecubital and popliteal areas, which stay moist a good part of the time.
- Most children outgrow the worst of the problem—and go on to have children who develop it.
New scale could measure vaccine hesitancy in developing countries
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
New analysis challenges fluid resuscitation guidelines for patients in shock
Although guideline recommended, according to a detailed analysis of available data, including a randomized trial.
Several sets of guidelines for resuscitation of patients in shock have advocated volume expansion with bolus intravenous fluid, but that recommendation was based on expected physiologic benefits not a randomized trial. The only randomized trial associated this approach showed increased mortality, and a new analysis of these and other data appears to explain why.
According to the findings of a study lead by Michael Levin, MD, of the department of medicine at Imperial College London and colleagues, “volume resuscitation is associated with deterioration of respiratory function and neurological function in some patients.” Their study was published in Lancet Respiratory Medicine. The authors stated that saline-induced hyperchloremic acidosis appears to have been “a major contributor” to the observed increase in adverse outcomes.
The key take home message is that “normal saline and other unbuffered crystalloid solutions should be avoided in resuscitating seriously ill patients,” according to the authors, who believe the findings might be relevant to adults as well as children.
The controversy about the role of volume expansion for management of shock was ignited by a 2011 trial called FEAST (N Engl J Med. 2011;364:2483-95). That trial, which randomized African children with severe febrile illness to a bolus of 20-40 mg of 5% albumin solution, a bolus of 0.9% saline solution, or no bolus, was halted early when 48-hour mortality data showed a lower death rate in the no bolus group (7.3%) than either the albumin (10.6%) or saline (10.5%) bolus groups.
The FEAST result was unexpected and so contrary to accepted thinking that it prompted widespread debate, including whether findings in the resource-poor area of the world where the FEAST trial was conducted could be extrapolated to centers elsewhere in the world. Arguing for benefit, fluid resuscitation is known to increase pulse pressure and urinary output. Arguing against benefit, pulmonary edema is a known complication of bolus fluid replacement.
In an attempt to address and potentially resolve this controversy, data collected in the FEAST trial along with four other sets of data involving volume expansion in critically ill children were evaluated with a focus on changes in cardiovascular, neurological, and respiratory function. Analysis of blood biochemistry and blood oxygen transport were also conducted.
The cardiovascular, respiratory, and neurologic functions were scored on the basis of objective measurements, such as heart rate, respiratory rate, and blood pressure. These measures were evaluated prior to fluid administration and at 1 hour, 4 hours, 8 hours, 24 hours, and 48 hours after fluid administration. Odds ratio (OR) of an adverse outcome were evaluated in the context of each 10-unit change in these scores.
Relative to baseline, there was worsening respiratory and neurological function after fluid administration. Although cardiovascular function improved, hemoglobin concentrations were lower in those who received fluid than in those who did not. Fluid resuscitation was also associated with lower bicarbonate and increased base deficit and chloride at 24 hours.
Regression modeling with physiological variables suggests “that the increased mortality in FEAST can be explained by bolus-induced worsening in respiratory and neurological function, hemodilution, and hyperchloremic acidosis,” according to the authors.
Analyses of the four other sets of data, which included children treated for meningococcal sepsis in the United Kingdom, acutely ill with malaria treated in Malawi, and cohorts of children in South Africa and a London hospital for acute illnesses, provided supportive data.
Although this analysis does not address the value of administering buffered solutions in low volumes, the authors concluded that the data from the FEAST trial are generalizable. They challenge the routine use of bolus infusions of saline or albumin in the initial management of shock, which has been guideline recommended. The risks of fluid resuscitation might be particularly high among children who already have compromised respiratory or neurologic function.
SOURCE: Levin M et al. Lancet Respir Med. 2019;7:581-93.
Although guideline recommended, according to a detailed analysis of available data, including a randomized trial.
Several sets of guidelines for resuscitation of patients in shock have advocated volume expansion with bolus intravenous fluid, but that recommendation was based on expected physiologic benefits not a randomized trial. The only randomized trial associated this approach showed increased mortality, and a new analysis of these and other data appears to explain why.
According to the findings of a study lead by Michael Levin, MD, of the department of medicine at Imperial College London and colleagues, “volume resuscitation is associated with deterioration of respiratory function and neurological function in some patients.” Their study was published in Lancet Respiratory Medicine. The authors stated that saline-induced hyperchloremic acidosis appears to have been “a major contributor” to the observed increase in adverse outcomes.
The key take home message is that “normal saline and other unbuffered crystalloid solutions should be avoided in resuscitating seriously ill patients,” according to the authors, who believe the findings might be relevant to adults as well as children.
The controversy about the role of volume expansion for management of shock was ignited by a 2011 trial called FEAST (N Engl J Med. 2011;364:2483-95). That trial, which randomized African children with severe febrile illness to a bolus of 20-40 mg of 5% albumin solution, a bolus of 0.9% saline solution, or no bolus, was halted early when 48-hour mortality data showed a lower death rate in the no bolus group (7.3%) than either the albumin (10.6%) or saline (10.5%) bolus groups.
The FEAST result was unexpected and so contrary to accepted thinking that it prompted widespread debate, including whether findings in the resource-poor area of the world where the FEAST trial was conducted could be extrapolated to centers elsewhere in the world. Arguing for benefit, fluid resuscitation is known to increase pulse pressure and urinary output. Arguing against benefit, pulmonary edema is a known complication of bolus fluid replacement.
In an attempt to address and potentially resolve this controversy, data collected in the FEAST trial along with four other sets of data involving volume expansion in critically ill children were evaluated with a focus on changes in cardiovascular, neurological, and respiratory function. Analysis of blood biochemistry and blood oxygen transport were also conducted.
The cardiovascular, respiratory, and neurologic functions were scored on the basis of objective measurements, such as heart rate, respiratory rate, and blood pressure. These measures were evaluated prior to fluid administration and at 1 hour, 4 hours, 8 hours, 24 hours, and 48 hours after fluid administration. Odds ratio (OR) of an adverse outcome were evaluated in the context of each 10-unit change in these scores.
Relative to baseline, there was worsening respiratory and neurological function after fluid administration. Although cardiovascular function improved, hemoglobin concentrations were lower in those who received fluid than in those who did not. Fluid resuscitation was also associated with lower bicarbonate and increased base deficit and chloride at 24 hours.
Regression modeling with physiological variables suggests “that the increased mortality in FEAST can be explained by bolus-induced worsening in respiratory and neurological function, hemodilution, and hyperchloremic acidosis,” according to the authors.
Analyses of the four other sets of data, which included children treated for meningococcal sepsis in the United Kingdom, acutely ill with malaria treated in Malawi, and cohorts of children in South Africa and a London hospital for acute illnesses, provided supportive data.
Although this analysis does not address the value of administering buffered solutions in low volumes, the authors concluded that the data from the FEAST trial are generalizable. They challenge the routine use of bolus infusions of saline or albumin in the initial management of shock, which has been guideline recommended. The risks of fluid resuscitation might be particularly high among children who already have compromised respiratory or neurologic function.
SOURCE: Levin M et al. Lancet Respir Med. 2019;7:581-93.
Although guideline recommended, according to a detailed analysis of available data, including a randomized trial.
Several sets of guidelines for resuscitation of patients in shock have advocated volume expansion with bolus intravenous fluid, but that recommendation was based on expected physiologic benefits not a randomized trial. The only randomized trial associated this approach showed increased mortality, and a new analysis of these and other data appears to explain why.
According to the findings of a study lead by Michael Levin, MD, of the department of medicine at Imperial College London and colleagues, “volume resuscitation is associated with deterioration of respiratory function and neurological function in some patients.” Their study was published in Lancet Respiratory Medicine. The authors stated that saline-induced hyperchloremic acidosis appears to have been “a major contributor” to the observed increase in adverse outcomes.
The key take home message is that “normal saline and other unbuffered crystalloid solutions should be avoided in resuscitating seriously ill patients,” according to the authors, who believe the findings might be relevant to adults as well as children.
The controversy about the role of volume expansion for management of shock was ignited by a 2011 trial called FEAST (N Engl J Med. 2011;364:2483-95). That trial, which randomized African children with severe febrile illness to a bolus of 20-40 mg of 5% albumin solution, a bolus of 0.9% saline solution, or no bolus, was halted early when 48-hour mortality data showed a lower death rate in the no bolus group (7.3%) than either the albumin (10.6%) or saline (10.5%) bolus groups.
The FEAST result was unexpected and so contrary to accepted thinking that it prompted widespread debate, including whether findings in the resource-poor area of the world where the FEAST trial was conducted could be extrapolated to centers elsewhere in the world. Arguing for benefit, fluid resuscitation is known to increase pulse pressure and urinary output. Arguing against benefit, pulmonary edema is a known complication of bolus fluid replacement.
In an attempt to address and potentially resolve this controversy, data collected in the FEAST trial along with four other sets of data involving volume expansion in critically ill children were evaluated with a focus on changes in cardiovascular, neurological, and respiratory function. Analysis of blood biochemistry and blood oxygen transport were also conducted.
The cardiovascular, respiratory, and neurologic functions were scored on the basis of objective measurements, such as heart rate, respiratory rate, and blood pressure. These measures were evaluated prior to fluid administration and at 1 hour, 4 hours, 8 hours, 24 hours, and 48 hours after fluid administration. Odds ratio (OR) of an adverse outcome were evaluated in the context of each 10-unit change in these scores.
Relative to baseline, there was worsening respiratory and neurological function after fluid administration. Although cardiovascular function improved, hemoglobin concentrations were lower in those who received fluid than in those who did not. Fluid resuscitation was also associated with lower bicarbonate and increased base deficit and chloride at 24 hours.
Regression modeling with physiological variables suggests “that the increased mortality in FEAST can be explained by bolus-induced worsening in respiratory and neurological function, hemodilution, and hyperchloremic acidosis,” according to the authors.
Analyses of the four other sets of data, which included children treated for meningococcal sepsis in the United Kingdom, acutely ill with malaria treated in Malawi, and cohorts of children in South Africa and a London hospital for acute illnesses, provided supportive data.
Although this analysis does not address the value of administering buffered solutions in low volumes, the authors concluded that the data from the FEAST trial are generalizable. They challenge the routine use of bolus infusions of saline or albumin in the initial management of shock, which has been guideline recommended. The risks of fluid resuscitation might be particularly high among children who already have compromised respiratory or neurologic function.
SOURCE: Levin M et al. Lancet Respir Med. 2019;7:581-93.
FROM THE LANCET RESPIRATORY MEDICINE
Little association found between in utero H1N1 vaccine and 5-year health outcomes
according to Laura K. Walsh of the University of Ottawa and associates.
The investigators conducted a population-based retrospective cohort study from November 2009 to October 2010 of all live births within the province of Ontario. Of the 104,249 eligible live births reported to the Ontario birth registry, 31,295 were exposed to the H1N1 vaccine in utero. After adjustment, there were no significant differences in the women who did and did not receive vaccines during pregnancy, according to the study, published in the BMJ.
After a median follow-up of 5 years, 14% of children received an asthma diagnosis, with a median age at diagnosis of 1.8 years. Children were more likely to receive an asthma diagnosis if their mothers had a preexisting condition or if they were born preterm. At follow-up, 34% of children had at least one upper respiratory tract infection. Sensory disorder, neoplasm, and pediatric complex chronic condition were rare, each occurring in less than 1% of the study cohort (BMJ. 2019 Jul 10. doi: 10.1136/bmj.l4151).
No significant association was found between prenatal exposure to the H1N1 vaccine and upper or lower respiratory infections, otitis media, all infections, neoplasms, sensory disorders, rates of urgent and inpatient health services use, pediatric complex chronic conditions, or mortality. A weak but significant association was observed for asthma (adjusted hazard ratio, 1.05; 95% confidence interval, 1.02-1.09), and a weak inverse association was found for gastrointestinal infections (adjusted incidence rate ratio, 0.94; 95% CI, 0.91-0.98).
“Although we observed a small, but statistically significant, increase in pediatric asthma and a reduction in gastrointestinal infections, we are not aware of any biologic mechanisms to explain these findings. Future studies in different settings and with different influenza vaccine formulations will be important for developing the evidence base on longer-term pediatric outcomes following influenza vaccination during pregnancy,” the investigators concluded.
The study was funded by grants from the Canadian Institutes of Health Research and the Institute for Clinical Evaluative Sciences.
according to Laura K. Walsh of the University of Ottawa and associates.
The investigators conducted a population-based retrospective cohort study from November 2009 to October 2010 of all live births within the province of Ontario. Of the 104,249 eligible live births reported to the Ontario birth registry, 31,295 were exposed to the H1N1 vaccine in utero. After adjustment, there were no significant differences in the women who did and did not receive vaccines during pregnancy, according to the study, published in the BMJ.
After a median follow-up of 5 years, 14% of children received an asthma diagnosis, with a median age at diagnosis of 1.8 years. Children were more likely to receive an asthma diagnosis if their mothers had a preexisting condition or if they were born preterm. At follow-up, 34% of children had at least one upper respiratory tract infection. Sensory disorder, neoplasm, and pediatric complex chronic condition were rare, each occurring in less than 1% of the study cohort (BMJ. 2019 Jul 10. doi: 10.1136/bmj.l4151).
No significant association was found between prenatal exposure to the H1N1 vaccine and upper or lower respiratory infections, otitis media, all infections, neoplasms, sensory disorders, rates of urgent and inpatient health services use, pediatric complex chronic conditions, or mortality. A weak but significant association was observed for asthma (adjusted hazard ratio, 1.05; 95% confidence interval, 1.02-1.09), and a weak inverse association was found for gastrointestinal infections (adjusted incidence rate ratio, 0.94; 95% CI, 0.91-0.98).
“Although we observed a small, but statistically significant, increase in pediatric asthma and a reduction in gastrointestinal infections, we are not aware of any biologic mechanisms to explain these findings. Future studies in different settings and with different influenza vaccine formulations will be important for developing the evidence base on longer-term pediatric outcomes following influenza vaccination during pregnancy,” the investigators concluded.
The study was funded by grants from the Canadian Institutes of Health Research and the Institute for Clinical Evaluative Sciences.
according to Laura K. Walsh of the University of Ottawa and associates.
The investigators conducted a population-based retrospective cohort study from November 2009 to October 2010 of all live births within the province of Ontario. Of the 104,249 eligible live births reported to the Ontario birth registry, 31,295 were exposed to the H1N1 vaccine in utero. After adjustment, there were no significant differences in the women who did and did not receive vaccines during pregnancy, according to the study, published in the BMJ.
After a median follow-up of 5 years, 14% of children received an asthma diagnosis, with a median age at diagnosis of 1.8 years. Children were more likely to receive an asthma diagnosis if their mothers had a preexisting condition or if they were born preterm. At follow-up, 34% of children had at least one upper respiratory tract infection. Sensory disorder, neoplasm, and pediatric complex chronic condition were rare, each occurring in less than 1% of the study cohort (BMJ. 2019 Jul 10. doi: 10.1136/bmj.l4151).
No significant association was found between prenatal exposure to the H1N1 vaccine and upper or lower respiratory infections, otitis media, all infections, neoplasms, sensory disorders, rates of urgent and inpatient health services use, pediatric complex chronic conditions, or mortality. A weak but significant association was observed for asthma (adjusted hazard ratio, 1.05; 95% confidence interval, 1.02-1.09), and a weak inverse association was found for gastrointestinal infections (adjusted incidence rate ratio, 0.94; 95% CI, 0.91-0.98).
“Although we observed a small, but statistically significant, increase in pediatric asthma and a reduction in gastrointestinal infections, we are not aware of any biologic mechanisms to explain these findings. Future studies in different settings and with different influenza vaccine formulations will be important for developing the evidence base on longer-term pediatric outcomes following influenza vaccination during pregnancy,” the investigators concluded.
The study was funded by grants from the Canadian Institutes of Health Research and the Institute for Clinical Evaluative Sciences.
FROM THE BMJ
Universal adolescent education on healthy relationships needed
Sexually active adolescent girls face reproductive coercion (RC) and adolescent relationship abuse (ARA), but there seems to be no statistically significant demographic factors, so education should be universally provided, wrote Amber L. Hill, MSPH, and colleagues in Obstetrics & Gynecology.
Ms. Hill of the University of Pittsburgh and colleagues conducted a secondary analysis of data from a cross-sectional baseline survey that had been used in a cluster-randomized trial. The SHARP (School Health Center Healthy Adolescent Relationship Program) trial, investigated an educational intervention regarding healthy relationships. Their analysis included survey data for 550 sexually active girls aged 14-19 years who’d received services from any of eight student health centers across Northern California during the 2012-2013 school year.
The investigators explained that ARA includes physical, sexual, and emotional abuse among adolescents in a romantic relationship; they further described RC as a form of ARA that increases risks of unintended pregnancy, such as contraceptive sabotage, condom manipulation, and pregnancy coercion. RC was defined as a positive response on a 10-item validated measure, and ARA was defined by positive response to at least one of three items that had been derived from Conflict Tactics Scale 2 and the Sexual Experiences Survey.
Among all females in the analysis, 12% reported reproductive coercion, and 17% reported relationship abuse . Black and Hispanic girls were the most likely to report RC, each at 15%; white girls were the most likely to report ARA at 22%. However, none of the demographic differences evaluated in this analysis, including these, were statistically significant, the authors cautioned.
One of the limitations of this study is that its sample was limited to school health centers in Northern California so it may not be generalizable. Furthermore, its cross-sectional design limits causal inference.
“By highlighting the relevance of reproductive coercion in adolescence, this study substantiates the urgent need for developmentally appropriate interventions,” Ms. Hill and associates concluded.
The authors did not report any potential conflicts of interest. Grants from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Center for Advancing Translational Sciences of the National Institutes of Health supported the study.
SOURCE: Hill AL et al. Obstet Gynecol. 2019;134(2):351-9.
Sexually active adolescent girls face reproductive coercion (RC) and adolescent relationship abuse (ARA), but there seems to be no statistically significant demographic factors, so education should be universally provided, wrote Amber L. Hill, MSPH, and colleagues in Obstetrics & Gynecology.
Ms. Hill of the University of Pittsburgh and colleagues conducted a secondary analysis of data from a cross-sectional baseline survey that had been used in a cluster-randomized trial. The SHARP (School Health Center Healthy Adolescent Relationship Program) trial, investigated an educational intervention regarding healthy relationships. Their analysis included survey data for 550 sexually active girls aged 14-19 years who’d received services from any of eight student health centers across Northern California during the 2012-2013 school year.
The investigators explained that ARA includes physical, sexual, and emotional abuse among adolescents in a romantic relationship; they further described RC as a form of ARA that increases risks of unintended pregnancy, such as contraceptive sabotage, condom manipulation, and pregnancy coercion. RC was defined as a positive response on a 10-item validated measure, and ARA was defined by positive response to at least one of three items that had been derived from Conflict Tactics Scale 2 and the Sexual Experiences Survey.
Among all females in the analysis, 12% reported reproductive coercion, and 17% reported relationship abuse . Black and Hispanic girls were the most likely to report RC, each at 15%; white girls were the most likely to report ARA at 22%. However, none of the demographic differences evaluated in this analysis, including these, were statistically significant, the authors cautioned.
One of the limitations of this study is that its sample was limited to school health centers in Northern California so it may not be generalizable. Furthermore, its cross-sectional design limits causal inference.
“By highlighting the relevance of reproductive coercion in adolescence, this study substantiates the urgent need for developmentally appropriate interventions,” Ms. Hill and associates concluded.
The authors did not report any potential conflicts of interest. Grants from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Center for Advancing Translational Sciences of the National Institutes of Health supported the study.
SOURCE: Hill AL et al. Obstet Gynecol. 2019;134(2):351-9.
Sexually active adolescent girls face reproductive coercion (RC) and adolescent relationship abuse (ARA), but there seems to be no statistically significant demographic factors, so education should be universally provided, wrote Amber L. Hill, MSPH, and colleagues in Obstetrics & Gynecology.
Ms. Hill of the University of Pittsburgh and colleagues conducted a secondary analysis of data from a cross-sectional baseline survey that had been used in a cluster-randomized trial. The SHARP (School Health Center Healthy Adolescent Relationship Program) trial, investigated an educational intervention regarding healthy relationships. Their analysis included survey data for 550 sexually active girls aged 14-19 years who’d received services from any of eight student health centers across Northern California during the 2012-2013 school year.
The investigators explained that ARA includes physical, sexual, and emotional abuse among adolescents in a romantic relationship; they further described RC as a form of ARA that increases risks of unintended pregnancy, such as contraceptive sabotage, condom manipulation, and pregnancy coercion. RC was defined as a positive response on a 10-item validated measure, and ARA was defined by positive response to at least one of three items that had been derived from Conflict Tactics Scale 2 and the Sexual Experiences Survey.
Among all females in the analysis, 12% reported reproductive coercion, and 17% reported relationship abuse . Black and Hispanic girls were the most likely to report RC, each at 15%; white girls were the most likely to report ARA at 22%. However, none of the demographic differences evaluated in this analysis, including these, were statistically significant, the authors cautioned.
One of the limitations of this study is that its sample was limited to school health centers in Northern California so it may not be generalizable. Furthermore, its cross-sectional design limits causal inference.
“By highlighting the relevance of reproductive coercion in adolescence, this study substantiates the urgent need for developmentally appropriate interventions,” Ms. Hill and associates concluded.
The authors did not report any potential conflicts of interest. Grants from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Center for Advancing Translational Sciences of the National Institutes of Health supported the study.
SOURCE: Hill AL et al. Obstet Gynecol. 2019;134(2):351-9.
FROM OBSTETRICS & GYNECOLOGY
Acne before puberty: When to treat, when to worry
NEWPORT BEACH, CALIF. – according to Sheila Fallon Friedlander, MD.
“This is something you are going to see in your practice,” said Dr. Friedlander, a pediatric dermatologists at Rady Children’s Hospital–San Diego. It’s important to know when it’s time to be concerned and when another condition may be masquerading as acne, she said at the at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Dr. Friedlander, who is professor of dermatology and pediatrics at the University of California, San Diego, talked about treating acne in the following prepubertal age groups:
Neonatal acne (ages birth to 4 weeks)
Acne appears in this population up to 20% of the time, according to research, and it is much more common in males than in females, at a ratio of five to one.
The cause is “most likely the relationship between placental androgens and the baby’s adrenal glands,” Dr. Friedlander said. However, something more serious could be going on. “Look at the child and see if he’s sick. If he looks sick, then we need to worry.”
Hormonal abnormalities also could be a cause, she said. Refer a baby to a specialist if there are other signs of hyperandrogenism. However, “the likelihood is very low,” and she’s never needed to refer a neonate with acne for evaluation.
As for treatment, she said, “Mainly, I’m using tincture of time.” However, “many of my mothers have told me that topical yogurt application will work.” Why yogurt? It’s possible that its bacteria could play a role in combating acne, she said.
Masquerader alert! Beware of neonatal cephalic pustulosis, Dr. Friedlander cautioned, which may be an inflammatory response to yeast. Ketoconazole cream may be helpful.
Infantile acne (ages 0-12 months)
This form of acne is more common in males and may hint at the future development of severe adolescent acne. It does resolve but it may take months or years, Dr. Friedlander said.
In general, this acne isn’t a sign of something more serious. “You do not need to go crazy with the work-up,” she said. “With mild to moderate disease, with nothing else suspicious, I don’t do a big work-up.”
However, do consider whether the child is undergoing precocious puberty, Dr. Friedlander said. Signs include axillary hair, pubic hair, and body odor.
As for treatment of infantile acne, “start out topically” and consider options such as Bactrim (sulfamethoxazole/trimethoprim) and erythromycin.
Masquerader alert! Idiopathic facial aseptic granuloma can be mistaken for acne and abscess, and ultrasound is helpful to confirm it. “It’s not so easy to treat,” she said. “Ivermectin may be helpful. Sometimes you do cultures and make sure something else isn’t going on.”
Midchildhood (ages 1-7 years)
“It’s not as common to have acne develop in this age group, but when it develops you need to be concerned,” Dr. Friedlander said. “This is the age period when there is more often something really wrong.”
Be on the lookout for a family history of hormonal abnormalities, and check if the child is on medication. “You need to look carefully,” she said, adding that it’s important to check for signs of premature puberty such as giant spikes in growth, abnormally large hands and feet, genital changes, and body odor. Check blood pressure if you’re worried about an adrenal tumor.
It’s possible for children to develop precocious puberty – with acne – because of exposure to testosterone gel used by a father. Dehydroepiandrosterone (DHEA) creams also may cause the condition. “The more creams out there with androgenic effects, the more we may see it,” Dr. Friedlander said. “This is something to ask about because families may not be forthcoming.”
Masquerader alert! Perioral dermatitis may look like acne, and it may be linked to inhaled or topical steroids, she said.
Other masqueraders include demodex folliculitis, angiofibromas (think tuberous sclerosis), and keratosis pilaris (the most common type of bump on a children aged 1-7 years). The latter condition “is not the end of the world,” said Dr. Friedlander, who added that “I’ve never cured anyone of it.”
Prepubertal acne (ages 7 years to puberty)
Acne in this group is generally not worrisome, Dr. Friedlander said, but investigate further if there’s significant inflammation and signs of early sexual development or virilization.
Benzoyl peroxide wash may be enough to help the condition initially, and consider topical clindamycin or a combination product. “Start out slow,” she said. Twice a week to start might be appropriate. Moisturizers can be helpful, as can topical adapalene.
Also, keep in mind that even mild acne can be emotionally devastating to a child in this age group and worthy of treatment. “Your assessment may be very different than hers,” she said. It’s possible that “she has a few lesions, but she feels like an outcast.”
Dr. Friedlander reported no relevant financial disclosures. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – according to Sheila Fallon Friedlander, MD.
“This is something you are going to see in your practice,” said Dr. Friedlander, a pediatric dermatologists at Rady Children’s Hospital–San Diego. It’s important to know when it’s time to be concerned and when another condition may be masquerading as acne, she said at the at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Dr. Friedlander, who is professor of dermatology and pediatrics at the University of California, San Diego, talked about treating acne in the following prepubertal age groups:
Neonatal acne (ages birth to 4 weeks)
Acne appears in this population up to 20% of the time, according to research, and it is much more common in males than in females, at a ratio of five to one.
The cause is “most likely the relationship between placental androgens and the baby’s adrenal glands,” Dr. Friedlander said. However, something more serious could be going on. “Look at the child and see if he’s sick. If he looks sick, then we need to worry.”
Hormonal abnormalities also could be a cause, she said. Refer a baby to a specialist if there are other signs of hyperandrogenism. However, “the likelihood is very low,” and she’s never needed to refer a neonate with acne for evaluation.
As for treatment, she said, “Mainly, I’m using tincture of time.” However, “many of my mothers have told me that topical yogurt application will work.” Why yogurt? It’s possible that its bacteria could play a role in combating acne, she said.
Masquerader alert! Beware of neonatal cephalic pustulosis, Dr. Friedlander cautioned, which may be an inflammatory response to yeast. Ketoconazole cream may be helpful.
Infantile acne (ages 0-12 months)
This form of acne is more common in males and may hint at the future development of severe adolescent acne. It does resolve but it may take months or years, Dr. Friedlander said.
In general, this acne isn’t a sign of something more serious. “You do not need to go crazy with the work-up,” she said. “With mild to moderate disease, with nothing else suspicious, I don’t do a big work-up.”
However, do consider whether the child is undergoing precocious puberty, Dr. Friedlander said. Signs include axillary hair, pubic hair, and body odor.
As for treatment of infantile acne, “start out topically” and consider options such as Bactrim (sulfamethoxazole/trimethoprim) and erythromycin.
Masquerader alert! Idiopathic facial aseptic granuloma can be mistaken for acne and abscess, and ultrasound is helpful to confirm it. “It’s not so easy to treat,” she said. “Ivermectin may be helpful. Sometimes you do cultures and make sure something else isn’t going on.”
Midchildhood (ages 1-7 years)
“It’s not as common to have acne develop in this age group, but when it develops you need to be concerned,” Dr. Friedlander said. “This is the age period when there is more often something really wrong.”
Be on the lookout for a family history of hormonal abnormalities, and check if the child is on medication. “You need to look carefully,” she said, adding that it’s important to check for signs of premature puberty such as giant spikes in growth, abnormally large hands and feet, genital changes, and body odor. Check blood pressure if you’re worried about an adrenal tumor.
It’s possible for children to develop precocious puberty – with acne – because of exposure to testosterone gel used by a father. Dehydroepiandrosterone (DHEA) creams also may cause the condition. “The more creams out there with androgenic effects, the more we may see it,” Dr. Friedlander said. “This is something to ask about because families may not be forthcoming.”
Masquerader alert! Perioral dermatitis may look like acne, and it may be linked to inhaled or topical steroids, she said.
Other masqueraders include demodex folliculitis, angiofibromas (think tuberous sclerosis), and keratosis pilaris (the most common type of bump on a children aged 1-7 years). The latter condition “is not the end of the world,” said Dr. Friedlander, who added that “I’ve never cured anyone of it.”
Prepubertal acne (ages 7 years to puberty)
Acne in this group is generally not worrisome, Dr. Friedlander said, but investigate further if there’s significant inflammation and signs of early sexual development or virilization.
Benzoyl peroxide wash may be enough to help the condition initially, and consider topical clindamycin or a combination product. “Start out slow,” she said. Twice a week to start might be appropriate. Moisturizers can be helpful, as can topical adapalene.
Also, keep in mind that even mild acne can be emotionally devastating to a child in this age group and worthy of treatment. “Your assessment may be very different than hers,” she said. It’s possible that “she has a few lesions, but she feels like an outcast.”
Dr. Friedlander reported no relevant financial disclosures. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – according to Sheila Fallon Friedlander, MD.
“This is something you are going to see in your practice,” said Dr. Friedlander, a pediatric dermatologists at Rady Children’s Hospital–San Diego. It’s important to know when it’s time to be concerned and when another condition may be masquerading as acne, she said at the at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Dr. Friedlander, who is professor of dermatology and pediatrics at the University of California, San Diego, talked about treating acne in the following prepubertal age groups:
Neonatal acne (ages birth to 4 weeks)
Acne appears in this population up to 20% of the time, according to research, and it is much more common in males than in females, at a ratio of five to one.
The cause is “most likely the relationship between placental androgens and the baby’s adrenal glands,” Dr. Friedlander said. However, something more serious could be going on. “Look at the child and see if he’s sick. If he looks sick, then we need to worry.”
Hormonal abnormalities also could be a cause, she said. Refer a baby to a specialist if there are other signs of hyperandrogenism. However, “the likelihood is very low,” and she’s never needed to refer a neonate with acne for evaluation.
As for treatment, she said, “Mainly, I’m using tincture of time.” However, “many of my mothers have told me that topical yogurt application will work.” Why yogurt? It’s possible that its bacteria could play a role in combating acne, she said.
Masquerader alert! Beware of neonatal cephalic pustulosis, Dr. Friedlander cautioned, which may be an inflammatory response to yeast. Ketoconazole cream may be helpful.
Infantile acne (ages 0-12 months)
This form of acne is more common in males and may hint at the future development of severe adolescent acne. It does resolve but it may take months or years, Dr. Friedlander said.
In general, this acne isn’t a sign of something more serious. “You do not need to go crazy with the work-up,” she said. “With mild to moderate disease, with nothing else suspicious, I don’t do a big work-up.”
However, do consider whether the child is undergoing precocious puberty, Dr. Friedlander said. Signs include axillary hair, pubic hair, and body odor.
As for treatment of infantile acne, “start out topically” and consider options such as Bactrim (sulfamethoxazole/trimethoprim) and erythromycin.
Masquerader alert! Idiopathic facial aseptic granuloma can be mistaken for acne and abscess, and ultrasound is helpful to confirm it. “It’s not so easy to treat,” she said. “Ivermectin may be helpful. Sometimes you do cultures and make sure something else isn’t going on.”
Midchildhood (ages 1-7 years)
“It’s not as common to have acne develop in this age group, but when it develops you need to be concerned,” Dr. Friedlander said. “This is the age period when there is more often something really wrong.”
Be on the lookout for a family history of hormonal abnormalities, and check if the child is on medication. “You need to look carefully,” she said, adding that it’s important to check for signs of premature puberty such as giant spikes in growth, abnormally large hands and feet, genital changes, and body odor. Check blood pressure if you’re worried about an adrenal tumor.
It’s possible for children to develop precocious puberty – with acne – because of exposure to testosterone gel used by a father. Dehydroepiandrosterone (DHEA) creams also may cause the condition. “The more creams out there with androgenic effects, the more we may see it,” Dr. Friedlander said. “This is something to ask about because families may not be forthcoming.”
Masquerader alert! Perioral dermatitis may look like acne, and it may be linked to inhaled or topical steroids, she said.
Other masqueraders include demodex folliculitis, angiofibromas (think tuberous sclerosis), and keratosis pilaris (the most common type of bump on a children aged 1-7 years). The latter condition “is not the end of the world,” said Dr. Friedlander, who added that “I’ve never cured anyone of it.”
Prepubertal acne (ages 7 years to puberty)
Acne in this group is generally not worrisome, Dr. Friedlander said, but investigate further if there’s significant inflammation and signs of early sexual development or virilization.
Benzoyl peroxide wash may be enough to help the condition initially, and consider topical clindamycin or a combination product. “Start out slow,” she said. Twice a week to start might be appropriate. Moisturizers can be helpful, as can topical adapalene.
Also, keep in mind that even mild acne can be emotionally devastating to a child in this age group and worthy of treatment. “Your assessment may be very different than hers,” she said. It’s possible that “she has a few lesions, but she feels like an outcast.”
Dr. Friedlander reported no relevant financial disclosures. SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Measles cases have slowed but not stopped
The United States continues to slowly add new cases of measles to 2019’s postelimination-record total, but California was officially removed from the outbreak list this week, according to the Centers for Disease Control and Prevention.
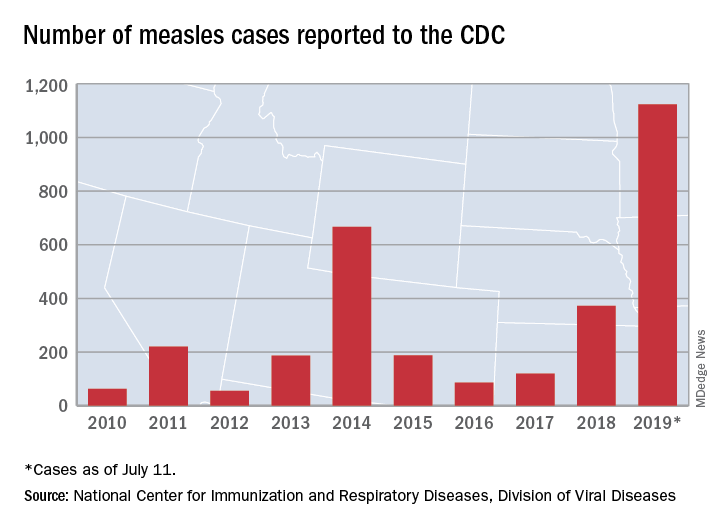
That is the highest number of cases reported since measles was declared eliminated in 2000 and the most in a single year since 1992.
The end of outbreak-related activity in California leaves three locations still dealing with ongoing cases: Rockland County, N.Y.; New York City; and King, Pierce, and Snohomish Counties in Washington, the CDC said.
Those three jurisdictions currently report the following:
- reported four new cases from July 3 to July 11 and is up to 175 cases for the year.
- had one new case from July 1 to July 8 and is now at 564 for the year.
- reported two cases from July 1 to July 10 and is now at 10 for the year (the other two counties have a total of three cases). Clark County in Washington reported 71 cases in an earlier, unrelated outbreak.
The United States continues to slowly add new cases of measles to 2019’s postelimination-record total, but California was officially removed from the outbreak list this week, according to the Centers for Disease Control and Prevention.

That is the highest number of cases reported since measles was declared eliminated in 2000 and the most in a single year since 1992.
The end of outbreak-related activity in California leaves three locations still dealing with ongoing cases: Rockland County, N.Y.; New York City; and King, Pierce, and Snohomish Counties in Washington, the CDC said.
Those three jurisdictions currently report the following:
- reported four new cases from July 3 to July 11 and is up to 175 cases for the year.
- had one new case from July 1 to July 8 and is now at 564 for the year.
- reported two cases from July 1 to July 10 and is now at 10 for the year (the other two counties have a total of three cases). Clark County in Washington reported 71 cases in an earlier, unrelated outbreak.
The United States continues to slowly add new cases of measles to 2019’s postelimination-record total, but California was officially removed from the outbreak list this week, according to the Centers for Disease Control and Prevention.

That is the highest number of cases reported since measles was declared eliminated in 2000 and the most in a single year since 1992.
The end of outbreak-related activity in California leaves three locations still dealing with ongoing cases: Rockland County, N.Y.; New York City; and King, Pierce, and Snohomish Counties in Washington, the CDC said.
Those three jurisdictions currently report the following:
- reported four new cases from July 3 to July 11 and is up to 175 cases for the year.
- had one new case from July 1 to July 8 and is now at 564 for the year.
- reported two cases from July 1 to July 10 and is now at 10 for the year (the other two counties have a total of three cases). Clark County in Washington reported 71 cases in an earlier, unrelated outbreak.
Obesity tied to relapse in young patients with multiple sclerosis
, results of a recent large, single-center study show. The rate of switching to second-line disease-modifying therapy was consequently about 50% higher among the obese children in the study, which included a total of 453 pediatric patients.
The link between obesity and treatment response suggests that the management of these younger patients with MS could be improved through weight loss or body mass index (BMI)-adjusted dosing, according to Peter Huppke, MD, of Georg August University in Göttingen, Germany, and co-investigators.
“The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response,” Dr. Huppke and co-authors said in a report on their study, which was published online ahead of print July 15 in JAMA Neurology.
This is believed to be the first-ever study to find an association between BMI and treatment response in pediatric patients with MS, according to the authors, who said they also confirmed a link between obesity and MS.
Specifically, obesity increased MS susceptibility by two-fold as compared with healthy controls, a finding that they said adds to a small but growing body of evidence that high BMI is associated with increased risk of the disease in these younger individuals.
This retrospective study included 453 pediatric patients with MS treated at the Center for MS in Childhood and Adolescence in Göttingen, Germany between 1990 and 2016. About two-thirds were female and the mean age at MS diagnosis was about 14 years.
Of those patients, 126 (27.8%) were classified as obese based on a BMI greater than the 90th percentile, according to the report.
Dr. Huppke and co-investigators found that high BMI was linked to a significantly increased odds of pediatric MS, with odds ratios of 2.19 (95% CI, 1.5-3.1; P < 0.001) in girls and 2.14 (95% CI, 1.3-3.5; P = 0.003) in boys.
A total of 277 of these pediatric patients received a first-line disease-modifying therapy for 6 months or longer, including 249 treated with interferon beta and 51 treated with glatiramer.
Relapses were more common in obese patients, according to the report. with an annualized relapse rate of 1.29, compared to just 0.72 for those who were not overweight (P < 0.001).
Consequently, likelihood of receiving a second-line treatment was about 1.5 times higher in the obese or extremely obese patients, investigators said.
“A healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs,” they concluded in the report, adding that BMI-adjusted dosing may “increase the value” of first-line disease-modifying therapies.
Dr. Huppke reported disclosures related to Bayer Health Care, Merck Serono, and Novartis not associated with the current study.
SOURCE: Huppke B, et al. JAMA Neurol. 2019 Jul 15. doi: 10.1001/jamaneurol.2019.1997
, results of a recent large, single-center study show. The rate of switching to second-line disease-modifying therapy was consequently about 50% higher among the obese children in the study, which included a total of 453 pediatric patients.
The link between obesity and treatment response suggests that the management of these younger patients with MS could be improved through weight loss or body mass index (BMI)-adjusted dosing, according to Peter Huppke, MD, of Georg August University in Göttingen, Germany, and co-investigators.
“The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response,” Dr. Huppke and co-authors said in a report on their study, which was published online ahead of print July 15 in JAMA Neurology.
This is believed to be the first-ever study to find an association between BMI and treatment response in pediatric patients with MS, according to the authors, who said they also confirmed a link between obesity and MS.
Specifically, obesity increased MS susceptibility by two-fold as compared with healthy controls, a finding that they said adds to a small but growing body of evidence that high BMI is associated with increased risk of the disease in these younger individuals.
This retrospective study included 453 pediatric patients with MS treated at the Center for MS in Childhood and Adolescence in Göttingen, Germany between 1990 and 2016. About two-thirds were female and the mean age at MS diagnosis was about 14 years.
Of those patients, 126 (27.8%) were classified as obese based on a BMI greater than the 90th percentile, according to the report.
Dr. Huppke and co-investigators found that high BMI was linked to a significantly increased odds of pediatric MS, with odds ratios of 2.19 (95% CI, 1.5-3.1; P < 0.001) in girls and 2.14 (95% CI, 1.3-3.5; P = 0.003) in boys.
A total of 277 of these pediatric patients received a first-line disease-modifying therapy for 6 months or longer, including 249 treated with interferon beta and 51 treated with glatiramer.
Relapses were more common in obese patients, according to the report. with an annualized relapse rate of 1.29, compared to just 0.72 for those who were not overweight (P < 0.001).
Consequently, likelihood of receiving a second-line treatment was about 1.5 times higher in the obese or extremely obese patients, investigators said.
“A healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs,” they concluded in the report, adding that BMI-adjusted dosing may “increase the value” of first-line disease-modifying therapies.
Dr. Huppke reported disclosures related to Bayer Health Care, Merck Serono, and Novartis not associated with the current study.
SOURCE: Huppke B, et al. JAMA Neurol. 2019 Jul 15. doi: 10.1001/jamaneurol.2019.1997
, results of a recent large, single-center study show. The rate of switching to second-line disease-modifying therapy was consequently about 50% higher among the obese children in the study, which included a total of 453 pediatric patients.
The link between obesity and treatment response suggests that the management of these younger patients with MS could be improved through weight loss or body mass index (BMI)-adjusted dosing, according to Peter Huppke, MD, of Georg August University in Göttingen, Germany, and co-investigators.
“The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response,” Dr. Huppke and co-authors said in a report on their study, which was published online ahead of print July 15 in JAMA Neurology.
This is believed to be the first-ever study to find an association between BMI and treatment response in pediatric patients with MS, according to the authors, who said they also confirmed a link between obesity and MS.
Specifically, obesity increased MS susceptibility by two-fold as compared with healthy controls, a finding that they said adds to a small but growing body of evidence that high BMI is associated with increased risk of the disease in these younger individuals.
This retrospective study included 453 pediatric patients with MS treated at the Center for MS in Childhood and Adolescence in Göttingen, Germany between 1990 and 2016. About two-thirds were female and the mean age at MS diagnosis was about 14 years.
Of those patients, 126 (27.8%) were classified as obese based on a BMI greater than the 90th percentile, according to the report.
Dr. Huppke and co-investigators found that high BMI was linked to a significantly increased odds of pediatric MS, with odds ratios of 2.19 (95% CI, 1.5-3.1; P < 0.001) in girls and 2.14 (95% CI, 1.3-3.5; P = 0.003) in boys.
A total of 277 of these pediatric patients received a first-line disease-modifying therapy for 6 months or longer, including 249 treated with interferon beta and 51 treated with glatiramer.
Relapses were more common in obese patients, according to the report. with an annualized relapse rate of 1.29, compared to just 0.72 for those who were not overweight (P < 0.001).
Consequently, likelihood of receiving a second-line treatment was about 1.5 times higher in the obese or extremely obese patients, investigators said.
“A healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs,” they concluded in the report, adding that BMI-adjusted dosing may “increase the value” of first-line disease-modifying therapies.
Dr. Huppke reported disclosures related to Bayer Health Care, Merck Serono, and Novartis not associated with the current study.
SOURCE: Huppke B, et al. JAMA Neurol. 2019 Jul 15. doi: 10.1001/jamaneurol.2019.1997
FROM JAMA NEUROLOGY
Key clinical point: Obese children and adolescents with MS had about twice as many relapses on first-line treatment as compared with their non-obese counterparts.
Major finding: The annualized relapse rate was 1.29 for obese pediatric patients, compared to 0.72 for those who were not overweight (P < 0.001).
Study details: Retrospective study including 453 patients with pediatric MS treated at a center in Göttingen, Germany between 1990 and 2016.
Disclosures: The senior author reported disclosures related to Bayer Health Care, Merck Serono, and Novartis unrelated to the this study.
Source: Huppke B, et al. JAMA Neurol. 2019 Jul 15.
Topical calcineurin inhibitors are an effective treatment option for pediatric periorificial dermatitis
AUSTIN, TEX. – , results from a retrospective cohort study showed.
The mainstays of treatment for POD include topical and oral antibiotics. In an interview prior to the annual meeting of the Society for Pediatric Dermatology, Ayelet Ollech, MD, said that the most common systemic agents used include erythromycin, azithromycin, and, in patients older than 8-10 years of age, minocycline or doxycycline. Topical agents, which are often used as monotherapy in mild disease, include metronidazole, clindamycin, erythromycin, sodium sulfacetamide, and, less often, azelaic acid, topical retinoids, and ivermectin. “TCIs (pimecrolimus 1% cream and tacrolimus 0.03% or 0.1% ointment) are a good steroid sparing option for POD,” said Dr. Ollech, a pediatric dermatology fellow at Ann & Robert H. Lurie Children’s Hospital of Chicago. “In the adult population, two randomized controlled studies of pimecrolimus 1% cream showed good results. In the pediatric population, there are only a few case series and case reports of TCIs for the treatment of POD.”
In what is believed to be the largest study of its kind, Dr. Ollech, Anthony J. Mancini, MD, and colleagues assessed the clinical utility of TCI in 132 pediatric patients with POD who were treated in the division of dermatology at Children’s Hospital of Chicago between 2008 and 2018. The researchers made note of epidemiologic variables, personal and family medical histories, possible triggers, duration of illness, previous treatments, distribution (periocular, perinasal, perioral, extra facial regions), severity of POD, treatment(s) prescribed, duration of therapy, clinical response, recurrences, and side effects. In an effort to capture missing data, the researchers performed follow-up via telephone for all patients who lacked appropriate follow-up documentation in the medical record.
Of the 132 patients, the female: male ratio was 1.2:1 and the median age at diagnosis was 4.2 years. About one-third of patients (33%) had involvement of one region, 38% had involvement of two regions, 26% had involvement of three regions, and 3% patients had involvement of all regions. The most common disorders on medical history were atopic dermatitis and asthma (in 29% and 17% of patients, respectively).
Dr. Ollech reported that 72 of the 132 patients (55%) had evaluable follow up data via either medical record documentation or the phone questionnaire. Of these, 67% were treated with TCI alone, 19% were treated with a combination of TCI and topical metronidazole, and 10% were treated with a combination of TCI and a systemic antibiotic. The median duration of treatment was 60 days. The researchers observed complete response in 65% of patients treated with TCI alone, in 64% of those treated with TCI and metronidazole, and in 70% of those treated with TCI and a systemic antibiotic. Adverse events attributed to TCI were rare and mild in severity.
“We were surprised that there were almost no reported side effects from the usage of TCIs as it is known that these agents can cause a burning or stinging sensation,” Dr. Ollech said. “Only one case described this side effect. We found 30% of the patients to have associated atopic dermatitis (AD) as well as a few patients with irritant dermatitis. We were also surprised how convenient the TCI treatment was for a patient who had POD and concomitant facial AD or even irritant dermatitis as an agent that can treat both. This can be very helpful for the parents that apply the medication to have a single solution to more than one rash.”
The researchers noted recurrence of POD in 14% of patients overall, including 6% of patients treated with TCI alone, 29% of patients treated with TCI and metronidazole, and 30% of patients treated with TCI and a systemic antibiotic.
Dr. Ollech acknowledged certain limitations of the study, including its retrospective design and lack of a control group. She and her colleagues reported having no financial disclosures.
SOURCE: Ollech A et al. SPD 2019, poster 23.
AUSTIN, TEX. – , results from a retrospective cohort study showed.
The mainstays of treatment for POD include topical and oral antibiotics. In an interview prior to the annual meeting of the Society for Pediatric Dermatology, Ayelet Ollech, MD, said that the most common systemic agents used include erythromycin, azithromycin, and, in patients older than 8-10 years of age, minocycline or doxycycline. Topical agents, which are often used as monotherapy in mild disease, include metronidazole, clindamycin, erythromycin, sodium sulfacetamide, and, less often, azelaic acid, topical retinoids, and ivermectin. “TCIs (pimecrolimus 1% cream and tacrolimus 0.03% or 0.1% ointment) are a good steroid sparing option for POD,” said Dr. Ollech, a pediatric dermatology fellow at Ann & Robert H. Lurie Children’s Hospital of Chicago. “In the adult population, two randomized controlled studies of pimecrolimus 1% cream showed good results. In the pediatric population, there are only a few case series and case reports of TCIs for the treatment of POD.”
In what is believed to be the largest study of its kind, Dr. Ollech, Anthony J. Mancini, MD, and colleagues assessed the clinical utility of TCI in 132 pediatric patients with POD who were treated in the division of dermatology at Children’s Hospital of Chicago between 2008 and 2018. The researchers made note of epidemiologic variables, personal and family medical histories, possible triggers, duration of illness, previous treatments, distribution (periocular, perinasal, perioral, extra facial regions), severity of POD, treatment(s) prescribed, duration of therapy, clinical response, recurrences, and side effects. In an effort to capture missing data, the researchers performed follow-up via telephone for all patients who lacked appropriate follow-up documentation in the medical record.
Of the 132 patients, the female: male ratio was 1.2:1 and the median age at diagnosis was 4.2 years. About one-third of patients (33%) had involvement of one region, 38% had involvement of two regions, 26% had involvement of three regions, and 3% patients had involvement of all regions. The most common disorders on medical history were atopic dermatitis and asthma (in 29% and 17% of patients, respectively).
Dr. Ollech reported that 72 of the 132 patients (55%) had evaluable follow up data via either medical record documentation or the phone questionnaire. Of these, 67% were treated with TCI alone, 19% were treated with a combination of TCI and topical metronidazole, and 10% were treated with a combination of TCI and a systemic antibiotic. The median duration of treatment was 60 days. The researchers observed complete response in 65% of patients treated with TCI alone, in 64% of those treated with TCI and metronidazole, and in 70% of those treated with TCI and a systemic antibiotic. Adverse events attributed to TCI were rare and mild in severity.
“We were surprised that there were almost no reported side effects from the usage of TCIs as it is known that these agents can cause a burning or stinging sensation,” Dr. Ollech said. “Only one case described this side effect. We found 30% of the patients to have associated atopic dermatitis (AD) as well as a few patients with irritant dermatitis. We were also surprised how convenient the TCI treatment was for a patient who had POD and concomitant facial AD or even irritant dermatitis as an agent that can treat both. This can be very helpful for the parents that apply the medication to have a single solution to more than one rash.”
The researchers noted recurrence of POD in 14% of patients overall, including 6% of patients treated with TCI alone, 29% of patients treated with TCI and metronidazole, and 30% of patients treated with TCI and a systemic antibiotic.
Dr. Ollech acknowledged certain limitations of the study, including its retrospective design and lack of a control group. She and her colleagues reported having no financial disclosures.
SOURCE: Ollech A et al. SPD 2019, poster 23.
AUSTIN, TEX. – , results from a retrospective cohort study showed.
The mainstays of treatment for POD include topical and oral antibiotics. In an interview prior to the annual meeting of the Society for Pediatric Dermatology, Ayelet Ollech, MD, said that the most common systemic agents used include erythromycin, azithromycin, and, in patients older than 8-10 years of age, minocycline or doxycycline. Topical agents, which are often used as monotherapy in mild disease, include metronidazole, clindamycin, erythromycin, sodium sulfacetamide, and, less often, azelaic acid, topical retinoids, and ivermectin. “TCIs (pimecrolimus 1% cream and tacrolimus 0.03% or 0.1% ointment) are a good steroid sparing option for POD,” said Dr. Ollech, a pediatric dermatology fellow at Ann & Robert H. Lurie Children’s Hospital of Chicago. “In the adult population, two randomized controlled studies of pimecrolimus 1% cream showed good results. In the pediatric population, there are only a few case series and case reports of TCIs for the treatment of POD.”
In what is believed to be the largest study of its kind, Dr. Ollech, Anthony J. Mancini, MD, and colleagues assessed the clinical utility of TCI in 132 pediatric patients with POD who were treated in the division of dermatology at Children’s Hospital of Chicago between 2008 and 2018. The researchers made note of epidemiologic variables, personal and family medical histories, possible triggers, duration of illness, previous treatments, distribution (periocular, perinasal, perioral, extra facial regions), severity of POD, treatment(s) prescribed, duration of therapy, clinical response, recurrences, and side effects. In an effort to capture missing data, the researchers performed follow-up via telephone for all patients who lacked appropriate follow-up documentation in the medical record.
Of the 132 patients, the female: male ratio was 1.2:1 and the median age at diagnosis was 4.2 years. About one-third of patients (33%) had involvement of one region, 38% had involvement of two regions, 26% had involvement of three regions, and 3% patients had involvement of all regions. The most common disorders on medical history were atopic dermatitis and asthma (in 29% and 17% of patients, respectively).
Dr. Ollech reported that 72 of the 132 patients (55%) had evaluable follow up data via either medical record documentation or the phone questionnaire. Of these, 67% were treated with TCI alone, 19% were treated with a combination of TCI and topical metronidazole, and 10% were treated with a combination of TCI and a systemic antibiotic. The median duration of treatment was 60 days. The researchers observed complete response in 65% of patients treated with TCI alone, in 64% of those treated with TCI and metronidazole, and in 70% of those treated with TCI and a systemic antibiotic. Adverse events attributed to TCI were rare and mild in severity.
“We were surprised that there were almost no reported side effects from the usage of TCIs as it is known that these agents can cause a burning or stinging sensation,” Dr. Ollech said. “Only one case described this side effect. We found 30% of the patients to have associated atopic dermatitis (AD) as well as a few patients with irritant dermatitis. We were also surprised how convenient the TCI treatment was for a patient who had POD and concomitant facial AD or even irritant dermatitis as an agent that can treat both. This can be very helpful for the parents that apply the medication to have a single solution to more than one rash.”
The researchers noted recurrence of POD in 14% of patients overall, including 6% of patients treated with TCI alone, 29% of patients treated with TCI and metronidazole, and 30% of patients treated with TCI and a systemic antibiotic.
Dr. Ollech acknowledged certain limitations of the study, including its retrospective design and lack of a control group. She and her colleagues reported having no financial disclosures.
SOURCE: Ollech A et al. SPD 2019, poster 23.
REPORTING FROM SPD 2019
Tocilizumab linked to refractory uveitis improvements in some JIA patients
MADRID – Tocilizumab was associated with improved vision in about half of 22 enrolled children with juvenile idiopathic arthritis (JIA) and uveitis refractory to tumor necrosis factor inhibitor (TNFi) therapy, based on results from an investigator-initiated phase 2 trial presented at the European Congress of Rheumatology.
At 12 weeks of treatment, 11 children in the study had some improvement in uveitis and 7 of them had achieved the primary study outcome, which was a two-step decrease in the level of inflammation as measured by the Standard of the Uveitis Nomenclature (SUN) criteria, Athimalaipet V. Ramanan, MBBS, of the department of paediatric rheumatology at the University Hospitals Bristol (England) NHS Foundation Trust, said. Ten of the 21 evaluable patients had no apparent response to tocilizumab. One study participant could not be included in the final analysis because of a violation in study protocol that involved use of disallowed concomitant medications.
“Although this study did not meet the prespecified criterion for efficacy (for most of those treated) ... almost half of those enrolled achieved some benefit,” reported Dr. Ramanan.
The multicenter investigator-initiated phase 2 trial conducted in the United Kingdom enrolled children with JIA and uveitis that was refractory to methotrexate and TNFi therapy. The study participants received methotrexate as well as 162 mg of tocilizumab administered subcutaneously every 2 weeks (those weighing less than 30 kg received tocilizumab treatment every 3 weeks).
Seven of 21 evaluable patients achieved the two-step reduction in inflammation that was the prespecified criterion for a response. For the study population overall, this fell short of statistical significance (P = .11).
However, Dr. Ramanan emphasized that another three patients achieved a one-step improvement, which he believes merits consideration as a sign of efficacy in a “very refractory group.” Moreover, three of four patients with macular edema at baseline had total resolution of this complication.
Other outcomes of interest monitored during the study included the safety and tolerability of tocilizumab and change in use of topical corticosteroids. There were no serious adverse events associated with tocilizumab in this study, according to Dr. Ramanan.
SOURCE: Ann Rheum Dis. Jun 2019;78(Suppl2)265, Abstract LB0011.
MADRID – Tocilizumab was associated with improved vision in about half of 22 enrolled children with juvenile idiopathic arthritis (JIA) and uveitis refractory to tumor necrosis factor inhibitor (TNFi) therapy, based on results from an investigator-initiated phase 2 trial presented at the European Congress of Rheumatology.
At 12 weeks of treatment, 11 children in the study had some improvement in uveitis and 7 of them had achieved the primary study outcome, which was a two-step decrease in the level of inflammation as measured by the Standard of the Uveitis Nomenclature (SUN) criteria, Athimalaipet V. Ramanan, MBBS, of the department of paediatric rheumatology at the University Hospitals Bristol (England) NHS Foundation Trust, said. Ten of the 21 evaluable patients had no apparent response to tocilizumab. One study participant could not be included in the final analysis because of a violation in study protocol that involved use of disallowed concomitant medications.
“Although this study did not meet the prespecified criterion for efficacy (for most of those treated) ... almost half of those enrolled achieved some benefit,” reported Dr. Ramanan.
The multicenter investigator-initiated phase 2 trial conducted in the United Kingdom enrolled children with JIA and uveitis that was refractory to methotrexate and TNFi therapy. The study participants received methotrexate as well as 162 mg of tocilizumab administered subcutaneously every 2 weeks (those weighing less than 30 kg received tocilizumab treatment every 3 weeks).
Seven of 21 evaluable patients achieved the two-step reduction in inflammation that was the prespecified criterion for a response. For the study population overall, this fell short of statistical significance (P = .11).
However, Dr. Ramanan emphasized that another three patients achieved a one-step improvement, which he believes merits consideration as a sign of efficacy in a “very refractory group.” Moreover, three of four patients with macular edema at baseline had total resolution of this complication.
Other outcomes of interest monitored during the study included the safety and tolerability of tocilizumab and change in use of topical corticosteroids. There were no serious adverse events associated with tocilizumab in this study, according to Dr. Ramanan.
SOURCE: Ann Rheum Dis. Jun 2019;78(Suppl2)265, Abstract LB0011.
MADRID – Tocilizumab was associated with improved vision in about half of 22 enrolled children with juvenile idiopathic arthritis (JIA) and uveitis refractory to tumor necrosis factor inhibitor (TNFi) therapy, based on results from an investigator-initiated phase 2 trial presented at the European Congress of Rheumatology.
At 12 weeks of treatment, 11 children in the study had some improvement in uveitis and 7 of them had achieved the primary study outcome, which was a two-step decrease in the level of inflammation as measured by the Standard of the Uveitis Nomenclature (SUN) criteria, Athimalaipet V. Ramanan, MBBS, of the department of paediatric rheumatology at the University Hospitals Bristol (England) NHS Foundation Trust, said. Ten of the 21 evaluable patients had no apparent response to tocilizumab. One study participant could not be included in the final analysis because of a violation in study protocol that involved use of disallowed concomitant medications.
“Although this study did not meet the prespecified criterion for efficacy (for most of those treated) ... almost half of those enrolled achieved some benefit,” reported Dr. Ramanan.
The multicenter investigator-initiated phase 2 trial conducted in the United Kingdom enrolled children with JIA and uveitis that was refractory to methotrexate and TNFi therapy. The study participants received methotrexate as well as 162 mg of tocilizumab administered subcutaneously every 2 weeks (those weighing less than 30 kg received tocilizumab treatment every 3 weeks).
Seven of 21 evaluable patients achieved the two-step reduction in inflammation that was the prespecified criterion for a response. For the study population overall, this fell short of statistical significance (P = .11).
However, Dr. Ramanan emphasized that another three patients achieved a one-step improvement, which he believes merits consideration as a sign of efficacy in a “very refractory group.” Moreover, three of four patients with macular edema at baseline had total resolution of this complication.
Other outcomes of interest monitored during the study included the safety and tolerability of tocilizumab and change in use of topical corticosteroids. There were no serious adverse events associated with tocilizumab in this study, according to Dr. Ramanan.
SOURCE: Ann Rheum Dis. Jun 2019;78(Suppl2)265, Abstract LB0011.
REPORTING FROM EULAR 2019 Congress