User login
Disseminated Papules and Nodules on the Skin and Oral Mucosa in an Infant
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
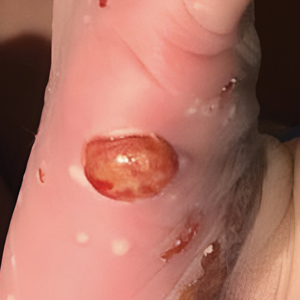
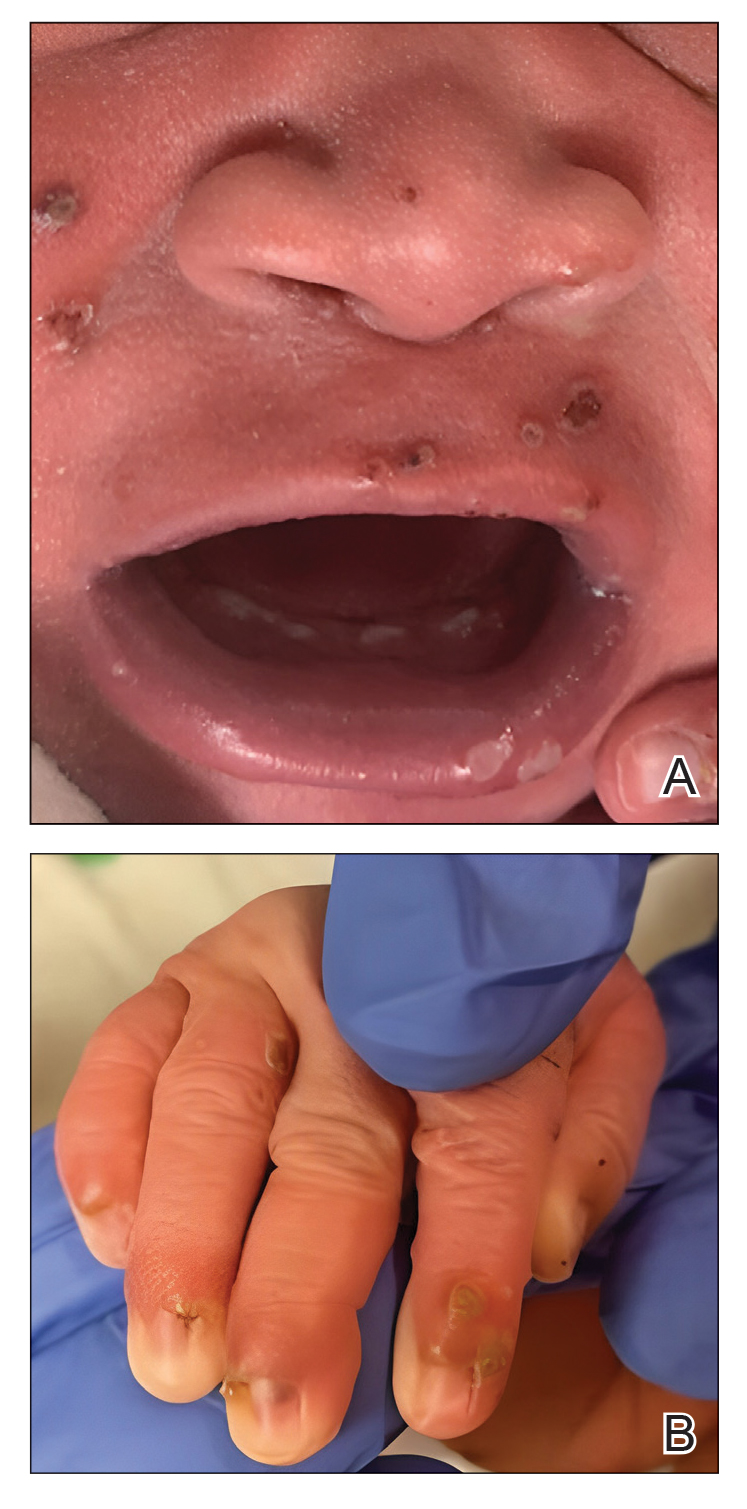
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.
Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.

Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.

Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
A 38-week-old infant boy presented at birth with disseminated, nonblanching, purple to dark red papules and nodules on the skin and oral mucosa. He was born spontaneously after an uncomplicated pregnancy. The mother experienced an episode of oral herpes simplex virus during pregnancy. The infant was otherwise healthy. Laboratory tests including a complete blood cell count and routine serum biochemical analyses were within reference range; however, an infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies. Ophthalmologic and auditory screenings were normal.
Young vapers and smokers beware: Eye problems abound
Adolescents and young adults who smoked and vaped were more likely to report ocular problems including dryness, redness, pain, blurry vision, light sensitivity, and headaches, according to an observational study published in JAMA Ophthalmology.
Eye symptoms were significantly worse among young people who reported using both cigarettes and e-cigarettes than for those who said they used only one of the products, according to researchers. Symptoms were particularly frequent and severe among those who had used both products in the prior week.
“In ophthalmology clinics, I’ve increasingly noticed patients, particularly adolescents and young adults, presenting with eye-related symptoms such as dryness, irritation, and even vision disturbances,” said Anne Xuan-Lan Nguyen, MDCM, an ophthalmology resident at the University of Toronto, who led the study.
Many of these patients said they did not use contact lenses or take medications associated with eye problems, but they did report a history of using e-cigarettes and cigarettes.
This “sparked my curiosity about the possible link between smoking or vaping and ocular symptoms,” Dr. Nguyen, who conducted the research as a medical student at McGill University in Montreal, told this news organization.
E-cigarettes are the most popular tobacco product among young people. Public health data show an increasing trend toward both vaping and smoking cigarettes, known as dual use. An estimated 40% of middle- and high school–aged tobacco users report using two or more tobacco products, according to the Centers for Disease Control and Prevention. Cigarette use has been linked to ocular damage, but the effects of e-cigarettes on eyesight and the combined effect with cigarettes are not as well known.
Dr. Nguyen and her colleagues surveyed more than 4,000 people aged 13-24 about their use of cigarettes or e-cigarettes in the last 30 days, the last 7 days, or ever. Half said they had never used any tobacco product and one quarter reported having used cigarettes, vapes, or both in the last month. More than 900 respondents said they had used one or both tobacco products in the last week.
Of the respondents who had ever vaped, 55.9% said they also used cigarettes. These dual users reported more severe and frequent eye symptoms compared with users of either product alone. Up to 4% of respondents who had ever been a dual user reported daily, severe, or very severe ocular symptoms – more than in the cigarette-only or e-cigarette-only groups.
More frequent tobacco use also was associated with more ocular symptoms. Young people who smoked or vaped in the previous week reported more symptoms than did the 30-day group, who reported more symptoms than the ever-user group (those who had taken at least a puff but not in the last month).
“All these conditions we know are worse as you get older,” said Laura B. Enyedi, MD, pediatric ophthalmologist at the Duke Eye Center in Durham, N.C., who was not associated with the study. “So if young people are having symptoms, it doesn’t bode well for them as they age.”
E-cigarette use alone did not appear to be linked to eye ailments, according to the findings. But to Dr. Nguyen’s surprise the survey results showed users of vaping products spent the most time worried about their eye health compared with all other participants. Users who smoked only cigarettes reported ocular symptoms, but not as severe or frequent as those of dual users.
The researchers hypothesized that ocular problems caused by vapes and cigarettes could be classified as oxidative damage. The combustion of the cigarette and the e-cigarette solvent (propylene glycol) potentially generates free radicals that can cause oxidative stress, damaging the ocular surface and film, Dr. Nguyen said.
Ophthalmologists are “always asking about contact lens use, lid hygiene, and screen time. Here’s another thing to consider when we get those common, nonspecific complaints of symptoms like dryness, redness, and burning,” Dr. Enyedi said.
Given the observational nature of the study, the researchers cannot confirm that dual use causes ocular symptoms. But given the public health challenge that tobacco use already presents for young people, the findings provide yet another reason to counsel against tobacco use and provide cessation options, Dr. Nguyen said.
“This study is just one of many, many studies showing a significant relationship among smoking, e-cigarette use, and health outcomes,” said Bonnie Halpern-Felsher, PhD, professor of pediatrics at Stanford (Calif.) University and a coauthor of the study. “We clearly need to help young people not use at all, or quit or cut back if using.”
This study was supported by the Taube Research Faculty Scholar Endowment; the National Heart, Lung, and Blood Institute; the Food and Drug Administration Center for Tobacco Products; the National Cancer Institute; the Stanford Maternal and Child Health Research Institute; and the Research to Prevent Blindness and National Eye Institute. Dr. Halpern-Felsher reported receiving personal fees as an expert scientist in litigation against some e-cigarette companies. The other study authors and Dr. Enyedi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adolescents and young adults who smoked and vaped were more likely to report ocular problems including dryness, redness, pain, blurry vision, light sensitivity, and headaches, according to an observational study published in JAMA Ophthalmology.
Eye symptoms were significantly worse among young people who reported using both cigarettes and e-cigarettes than for those who said they used only one of the products, according to researchers. Symptoms were particularly frequent and severe among those who had used both products in the prior week.
“In ophthalmology clinics, I’ve increasingly noticed patients, particularly adolescents and young adults, presenting with eye-related symptoms such as dryness, irritation, and even vision disturbances,” said Anne Xuan-Lan Nguyen, MDCM, an ophthalmology resident at the University of Toronto, who led the study.
Many of these patients said they did not use contact lenses or take medications associated with eye problems, but they did report a history of using e-cigarettes and cigarettes.
This “sparked my curiosity about the possible link between smoking or vaping and ocular symptoms,” Dr. Nguyen, who conducted the research as a medical student at McGill University in Montreal, told this news organization.
E-cigarettes are the most popular tobacco product among young people. Public health data show an increasing trend toward both vaping and smoking cigarettes, known as dual use. An estimated 40% of middle- and high school–aged tobacco users report using two or more tobacco products, according to the Centers for Disease Control and Prevention. Cigarette use has been linked to ocular damage, but the effects of e-cigarettes on eyesight and the combined effect with cigarettes are not as well known.
Dr. Nguyen and her colleagues surveyed more than 4,000 people aged 13-24 about their use of cigarettes or e-cigarettes in the last 30 days, the last 7 days, or ever. Half said they had never used any tobacco product and one quarter reported having used cigarettes, vapes, or both in the last month. More than 900 respondents said they had used one or both tobacco products in the last week.
Of the respondents who had ever vaped, 55.9% said they also used cigarettes. These dual users reported more severe and frequent eye symptoms compared with users of either product alone. Up to 4% of respondents who had ever been a dual user reported daily, severe, or very severe ocular symptoms – more than in the cigarette-only or e-cigarette-only groups.
More frequent tobacco use also was associated with more ocular symptoms. Young people who smoked or vaped in the previous week reported more symptoms than did the 30-day group, who reported more symptoms than the ever-user group (those who had taken at least a puff but not in the last month).
“All these conditions we know are worse as you get older,” said Laura B. Enyedi, MD, pediatric ophthalmologist at the Duke Eye Center in Durham, N.C., who was not associated with the study. “So if young people are having symptoms, it doesn’t bode well for them as they age.”
E-cigarette use alone did not appear to be linked to eye ailments, according to the findings. But to Dr. Nguyen’s surprise the survey results showed users of vaping products spent the most time worried about their eye health compared with all other participants. Users who smoked only cigarettes reported ocular symptoms, but not as severe or frequent as those of dual users.
The researchers hypothesized that ocular problems caused by vapes and cigarettes could be classified as oxidative damage. The combustion of the cigarette and the e-cigarette solvent (propylene glycol) potentially generates free radicals that can cause oxidative stress, damaging the ocular surface and film, Dr. Nguyen said.
Ophthalmologists are “always asking about contact lens use, lid hygiene, and screen time. Here’s another thing to consider when we get those common, nonspecific complaints of symptoms like dryness, redness, and burning,” Dr. Enyedi said.
Given the observational nature of the study, the researchers cannot confirm that dual use causes ocular symptoms. But given the public health challenge that tobacco use already presents for young people, the findings provide yet another reason to counsel against tobacco use and provide cessation options, Dr. Nguyen said.
“This study is just one of many, many studies showing a significant relationship among smoking, e-cigarette use, and health outcomes,” said Bonnie Halpern-Felsher, PhD, professor of pediatrics at Stanford (Calif.) University and a coauthor of the study. “We clearly need to help young people not use at all, or quit or cut back if using.”
This study was supported by the Taube Research Faculty Scholar Endowment; the National Heart, Lung, and Blood Institute; the Food and Drug Administration Center for Tobacco Products; the National Cancer Institute; the Stanford Maternal and Child Health Research Institute; and the Research to Prevent Blindness and National Eye Institute. Dr. Halpern-Felsher reported receiving personal fees as an expert scientist in litigation against some e-cigarette companies. The other study authors and Dr. Enyedi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adolescents and young adults who smoked and vaped were more likely to report ocular problems including dryness, redness, pain, blurry vision, light sensitivity, and headaches, according to an observational study published in JAMA Ophthalmology.
Eye symptoms were significantly worse among young people who reported using both cigarettes and e-cigarettes than for those who said they used only one of the products, according to researchers. Symptoms were particularly frequent and severe among those who had used both products in the prior week.
“In ophthalmology clinics, I’ve increasingly noticed patients, particularly adolescents and young adults, presenting with eye-related symptoms such as dryness, irritation, and even vision disturbances,” said Anne Xuan-Lan Nguyen, MDCM, an ophthalmology resident at the University of Toronto, who led the study.
Many of these patients said they did not use contact lenses or take medications associated with eye problems, but they did report a history of using e-cigarettes and cigarettes.
This “sparked my curiosity about the possible link between smoking or vaping and ocular symptoms,” Dr. Nguyen, who conducted the research as a medical student at McGill University in Montreal, told this news organization.
E-cigarettes are the most popular tobacco product among young people. Public health data show an increasing trend toward both vaping and smoking cigarettes, known as dual use. An estimated 40% of middle- and high school–aged tobacco users report using two or more tobacco products, according to the Centers for Disease Control and Prevention. Cigarette use has been linked to ocular damage, but the effects of e-cigarettes on eyesight and the combined effect with cigarettes are not as well known.
Dr. Nguyen and her colleagues surveyed more than 4,000 people aged 13-24 about their use of cigarettes or e-cigarettes in the last 30 days, the last 7 days, or ever. Half said they had never used any tobacco product and one quarter reported having used cigarettes, vapes, or both in the last month. More than 900 respondents said they had used one or both tobacco products in the last week.
Of the respondents who had ever vaped, 55.9% said they also used cigarettes. These dual users reported more severe and frequent eye symptoms compared with users of either product alone. Up to 4% of respondents who had ever been a dual user reported daily, severe, or very severe ocular symptoms – more than in the cigarette-only or e-cigarette-only groups.
More frequent tobacco use also was associated with more ocular symptoms. Young people who smoked or vaped in the previous week reported more symptoms than did the 30-day group, who reported more symptoms than the ever-user group (those who had taken at least a puff but not in the last month).
“All these conditions we know are worse as you get older,” said Laura B. Enyedi, MD, pediatric ophthalmologist at the Duke Eye Center in Durham, N.C., who was not associated with the study. “So if young people are having symptoms, it doesn’t bode well for them as they age.”
E-cigarette use alone did not appear to be linked to eye ailments, according to the findings. But to Dr. Nguyen’s surprise the survey results showed users of vaping products spent the most time worried about their eye health compared with all other participants. Users who smoked only cigarettes reported ocular symptoms, but not as severe or frequent as those of dual users.
The researchers hypothesized that ocular problems caused by vapes and cigarettes could be classified as oxidative damage. The combustion of the cigarette and the e-cigarette solvent (propylene glycol) potentially generates free radicals that can cause oxidative stress, damaging the ocular surface and film, Dr. Nguyen said.
Ophthalmologists are “always asking about contact lens use, lid hygiene, and screen time. Here’s another thing to consider when we get those common, nonspecific complaints of symptoms like dryness, redness, and burning,” Dr. Enyedi said.
Given the observational nature of the study, the researchers cannot confirm that dual use causes ocular symptoms. But given the public health challenge that tobacco use already presents for young people, the findings provide yet another reason to counsel against tobacco use and provide cessation options, Dr. Nguyen said.
“This study is just one of many, many studies showing a significant relationship among smoking, e-cigarette use, and health outcomes,” said Bonnie Halpern-Felsher, PhD, professor of pediatrics at Stanford (Calif.) University and a coauthor of the study. “We clearly need to help young people not use at all, or quit or cut back if using.”
This study was supported by the Taube Research Faculty Scholar Endowment; the National Heart, Lung, and Blood Institute; the Food and Drug Administration Center for Tobacco Products; the National Cancer Institute; the Stanford Maternal and Child Health Research Institute; and the Research to Prevent Blindness and National Eye Institute. Dr. Halpern-Felsher reported receiving personal fees as an expert scientist in litigation against some e-cigarette companies. The other study authors and Dr. Enyedi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA OPHTHALMOLOGY
New guideline for managing toothache in children
Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or both medications together can effectively manage a child’s toothache as a stopgap until definitive treatment is available, according to a new guideline.
The guideline, published in the September issue of the Journal of the American Dental Association, does not recommend opioids for a toothache or after tooth extraction in this population.
Opioid prescriptions for children entail risk for hospitalization and death. Yet, some dentists continued to prescribe contraindicated opioids to young children after a Food and Drug Administration warning in 2017 about the use of tramadol and codeine in this population, the guideline notes.
Opioid prescribing to children also continued after the American Academy of Pediatric Dentistry in 2018 recommended acetaminophen and NSAIDs as first-line medications for pain management and said that the use of opioids should be “rare.”
Although the new guidance, which also covers pain management after tooth extraction, is geared toward general dentists, it could help emergency clinicians and primary care providers manage children’s pain when definitive treatment is not immediately available, the authors noted.
Definitive treatment could include pulpectomy, nonsurgical root canal, incision for drainage of an abscess, or tooth extraction.
If definitive care in 2-3 days is not possible, parents should let the health care team know, the guideline says.
“These pharmacologic strategies will alleviate dental pain temporarily until a referral for definitive dental treatment is in place,” the authors wrote.
The American Dental Association (ADA) endorsed the new guideline, which was developed by researchers with the ADA Science & Research Institute, the University of Pittsburgh School of Dental Medicine, and the Center for Integrative Global Oral Health at the University of Pennsylvania School of Dental Medicine in Philadelphia.
The guideline recommends ibuprofen and, for children older than 2 years, naproxen as NSAID options. The use of naproxen in children younger than 12 years for this purpose is off label, they noted.
The guideline suggests doses of acetaminophen and NSAIDs on the basis of age and weight that may differ from those on medication packaging.
“When acetaminophen or NSAIDs are administered as directed, the risk of harm to children from either medication is low,” the guideline states.
“While prescribing opioids to children has become less frequent overall, this guideline ensures that both dentists and parents have evidence-based recommendations to determine the most appropriate treatment for dental pain,” senior guideline author Paul Moore, DMD, PhD, MPH, professor emeritus at the University of Pittsburgh’s School of Dental Medicine, said in a news release from the ADA. “Parents and caregivers can take comfort that widely available medications that have no abuse potential, such as acetaminophen or ibuprofen, are safe and effective for helping their children find relief from short-term dental pain.”
A 2018 review by Dr. Moore and coauthors found that NSAIDs, with or without acetaminophen, were effective and minimized adverse events, relative to opioids, for acute dental pain across ages.
The new recommendations for children will “allow for better treatment of this kind of pain” and “will help prevent unnecessary prescribing of medications with abuse potential, including opioids,” Patrizia Cavazzoni, MD, director of the FDA Center for Drug Evaluation and Research, said in the news release.
The report stems from a 3-year, $1.5 million grant awarded by the FDA in 2020 to the University of Pittsburgh and the ADA Science & Research Institute to develop a clinical practice guideline for the management of acute pain in dentistry in children, adolescents, and adults. The recommendations for adolescents and adults are still in development.
The report was supported by an FDA grant, and the guideline authors received technical and methodologic support from the agency. Some authors disclosed ties to pharmaceutical companies.
A version of this article appeared on Medscape.com.
Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or both medications together can effectively manage a child’s toothache as a stopgap until definitive treatment is available, according to a new guideline.
The guideline, published in the September issue of the Journal of the American Dental Association, does not recommend opioids for a toothache or after tooth extraction in this population.
Opioid prescriptions for children entail risk for hospitalization and death. Yet, some dentists continued to prescribe contraindicated opioids to young children after a Food and Drug Administration warning in 2017 about the use of tramadol and codeine in this population, the guideline notes.
Opioid prescribing to children also continued after the American Academy of Pediatric Dentistry in 2018 recommended acetaminophen and NSAIDs as first-line medications for pain management and said that the use of opioids should be “rare.”
Although the new guidance, which also covers pain management after tooth extraction, is geared toward general dentists, it could help emergency clinicians and primary care providers manage children’s pain when definitive treatment is not immediately available, the authors noted.
Definitive treatment could include pulpectomy, nonsurgical root canal, incision for drainage of an abscess, or tooth extraction.
If definitive care in 2-3 days is not possible, parents should let the health care team know, the guideline says.
“These pharmacologic strategies will alleviate dental pain temporarily until a referral for definitive dental treatment is in place,” the authors wrote.
The American Dental Association (ADA) endorsed the new guideline, which was developed by researchers with the ADA Science & Research Institute, the University of Pittsburgh School of Dental Medicine, and the Center for Integrative Global Oral Health at the University of Pennsylvania School of Dental Medicine in Philadelphia.
The guideline recommends ibuprofen and, for children older than 2 years, naproxen as NSAID options. The use of naproxen in children younger than 12 years for this purpose is off label, they noted.
The guideline suggests doses of acetaminophen and NSAIDs on the basis of age and weight that may differ from those on medication packaging.
“When acetaminophen or NSAIDs are administered as directed, the risk of harm to children from either medication is low,” the guideline states.
“While prescribing opioids to children has become less frequent overall, this guideline ensures that both dentists and parents have evidence-based recommendations to determine the most appropriate treatment for dental pain,” senior guideline author Paul Moore, DMD, PhD, MPH, professor emeritus at the University of Pittsburgh’s School of Dental Medicine, said in a news release from the ADA. “Parents and caregivers can take comfort that widely available medications that have no abuse potential, such as acetaminophen or ibuprofen, are safe and effective for helping their children find relief from short-term dental pain.”
A 2018 review by Dr. Moore and coauthors found that NSAIDs, with or without acetaminophen, were effective and minimized adverse events, relative to opioids, for acute dental pain across ages.
The new recommendations for children will “allow for better treatment of this kind of pain” and “will help prevent unnecessary prescribing of medications with abuse potential, including opioids,” Patrizia Cavazzoni, MD, director of the FDA Center for Drug Evaluation and Research, said in the news release.
The report stems from a 3-year, $1.5 million grant awarded by the FDA in 2020 to the University of Pittsburgh and the ADA Science & Research Institute to develop a clinical practice guideline for the management of acute pain in dentistry in children, adolescents, and adults. The recommendations for adolescents and adults are still in development.
The report was supported by an FDA grant, and the guideline authors received technical and methodologic support from the agency. Some authors disclosed ties to pharmaceutical companies.
A version of this article appeared on Medscape.com.
Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or both medications together can effectively manage a child’s toothache as a stopgap until definitive treatment is available, according to a new guideline.
The guideline, published in the September issue of the Journal of the American Dental Association, does not recommend opioids for a toothache or after tooth extraction in this population.
Opioid prescriptions for children entail risk for hospitalization and death. Yet, some dentists continued to prescribe contraindicated opioids to young children after a Food and Drug Administration warning in 2017 about the use of tramadol and codeine in this population, the guideline notes.
Opioid prescribing to children also continued after the American Academy of Pediatric Dentistry in 2018 recommended acetaminophen and NSAIDs as first-line medications for pain management and said that the use of opioids should be “rare.”
Although the new guidance, which also covers pain management after tooth extraction, is geared toward general dentists, it could help emergency clinicians and primary care providers manage children’s pain when definitive treatment is not immediately available, the authors noted.
Definitive treatment could include pulpectomy, nonsurgical root canal, incision for drainage of an abscess, or tooth extraction.
If definitive care in 2-3 days is not possible, parents should let the health care team know, the guideline says.
“These pharmacologic strategies will alleviate dental pain temporarily until a referral for definitive dental treatment is in place,” the authors wrote.
The American Dental Association (ADA) endorsed the new guideline, which was developed by researchers with the ADA Science & Research Institute, the University of Pittsburgh School of Dental Medicine, and the Center for Integrative Global Oral Health at the University of Pennsylvania School of Dental Medicine in Philadelphia.
The guideline recommends ibuprofen and, for children older than 2 years, naproxen as NSAID options. The use of naproxen in children younger than 12 years for this purpose is off label, they noted.
The guideline suggests doses of acetaminophen and NSAIDs on the basis of age and weight that may differ from those on medication packaging.
“When acetaminophen or NSAIDs are administered as directed, the risk of harm to children from either medication is low,” the guideline states.
“While prescribing opioids to children has become less frequent overall, this guideline ensures that both dentists and parents have evidence-based recommendations to determine the most appropriate treatment for dental pain,” senior guideline author Paul Moore, DMD, PhD, MPH, professor emeritus at the University of Pittsburgh’s School of Dental Medicine, said in a news release from the ADA. “Parents and caregivers can take comfort that widely available medications that have no abuse potential, such as acetaminophen or ibuprofen, are safe and effective for helping their children find relief from short-term dental pain.”
A 2018 review by Dr. Moore and coauthors found that NSAIDs, with or without acetaminophen, were effective and minimized adverse events, relative to opioids, for acute dental pain across ages.
The new recommendations for children will “allow for better treatment of this kind of pain” and “will help prevent unnecessary prescribing of medications with abuse potential, including opioids,” Patrizia Cavazzoni, MD, director of the FDA Center for Drug Evaluation and Research, said in the news release.
The report stems from a 3-year, $1.5 million grant awarded by the FDA in 2020 to the University of Pittsburgh and the ADA Science & Research Institute to develop a clinical practice guideline for the management of acute pain in dentistry in children, adolescents, and adults. The recommendations for adolescents and adults are still in development.
The report was supported by an FDA grant, and the guideline authors received technical and methodologic support from the agency. Some authors disclosed ties to pharmaceutical companies.
A version of this article appeared on Medscape.com.
Gender-affirming surgeries nearly tripled between 2016 and 2019: Study
a new study published in JAMA Network Open found.
Breast and chest surgeries were the most common procedures performed, and the number of surgical procedures carried out increased with age. The researchers said that, in addition to legal shifts, the established safety of the surgeries and resulting increase in quality of life may also help explain the increase.
“The point of this is to raise awareness and to really document the patterns of care in the United States,” said Jason Wright, MD, an associate professor at Columbia University, New York. “We hope that people understand that these procedures are being performed more commonly and they’re out there.”
A study published in 2022 in JAMA Pediatrics found that the number of chest reconstruction surgeries among U.S. adolescents rose fourfold between 2016 and 2019.
The new study included data from 2016 to 2020 in the Nationwide Ambulatory Surgery Sample and the National Inpatient Sample. More than 48,000 patients with diagnosis codes for gender identity disorder, transsexualism, or a personal history of sex reassignment were identified. Age ranges were grouped as 12-18 (7.7%), 19-30 (52.3%), and 31-40 (21.8%).
The number of gender-affirming procedures rose from 4,552 in 2016 to a peak of 13,011 in 2019. (A slight decline to 12,818 procedures in 2020 was attributed to the COVID-19 pandemic.) The surgeries were grouped into three categories: breast and chest procedures, which occurred in 56.6% of patients; genital reconstructive surgeries (35.1%), and other facial cosmetic procedures (13.9%).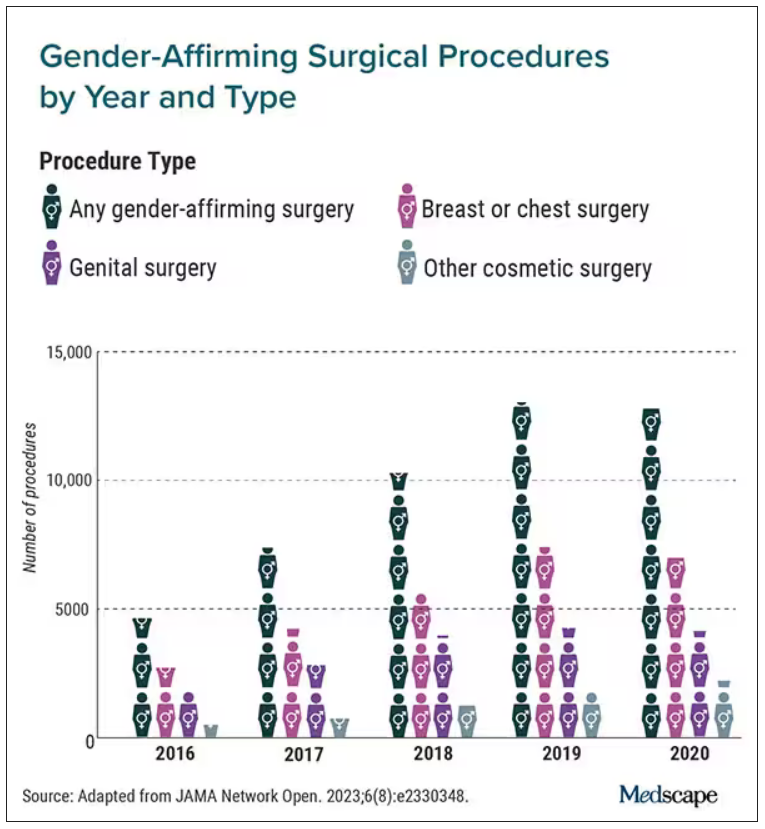
“We really wanted to try to make this as representative as we could,” Dr. Wright said. “I think this is really the best estimates that are available to date.”
Chest and breast procedures made up a higher percentage of surgeries in younger patients, while genital surgical procedures made up a higher percentage in older patients. For example, patients aged 19-30 made up 59.1% of breast or chest surgeries and 44.2% of genital surgeries. However, those aged 31-40 accounted for 26.2% of genital surgeries and 18.1% of breast or chest surgeries. For ages 41-50, the spread was more than double, accounting for 12.8% of genital surgeries and only 6.1% of breast or chest surgeries, according to the researchers.
Undocumented uptick
In addition to more inclusive health insurance, Dr. Wright said the increase in these procedures can also be attributed to studies showing their safety and the long-term association with high patient satisfaction.
Kevin Wang, MD, medical director of Providence–Swedish Health Services’ LGBTQIA+ program in Seattle, agreed that changes in health insurance coverage for gender-affirming surgery likely account in part for their increase. But he added that more clinicians are performing these procedures.
He said gender-affirming surgeries improve quality of life for the people who undergo them. The American Academy of Pediatrics has said it would be conducting a thorough review of the effects of transgender care on youth. A 2018 policy statement from the group said transgender youth should “have access to comprehensive, gender-affirming, and developmentally appropriate health care that is provided in a safe and inclusive clinical space.”
Dr. Wright cited several limitations to his group’s study that may result in the undercapture of transgender individuals and gender-affirming surgery; in particular, while the study captured inpatient and ambulatory surgical procedures in large, nationwide datasets, a small number of the procedures could have been performed in other settings.
Guiding a patient through gender-affirming care and surgical procedures can be an arduous process, including understanding their goals, using hormone therapy, and making referrals to specialists. Dr. Wang said he works to maximize his patients’ physical, mental, and emotional health, and helps them understand the risks.
He cited the double standard of a cisgender woman wanting breast augmentation without justification, but someone who identifies as transgender has many more boxes to check – for example, seeing a behavior health specialist to demonstrate they understand the risks and securing a letter of support from their primary care physician to undergo a similar procedure.
“It’s just interesting how the transgender community has to jump through so many more barriers and hoops for affirming, lifesaving procedures where you have other people who are doing it for aesthetic purposes and do not require any type of authorization,” Dr. Wang said.
Dr. Wright said he hopes the findings call attention to the need for more professionals working in the gender-affirming care field.
“I think for the medical community, it’s important to raise the idea that these procedures are becoming more common,” Dr. Wright said. “We are going to need specialists who have expertise in transgender care and surgeons who have the ability to perform these operations. Hopefully, this sheds light on the resources that are going to be required to care for these patients going forward.”
Dr. Wright reported receiving grants from Merck and personal fees from UpToDate outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
a new study published in JAMA Network Open found.
Breast and chest surgeries were the most common procedures performed, and the number of surgical procedures carried out increased with age. The researchers said that, in addition to legal shifts, the established safety of the surgeries and resulting increase in quality of life may also help explain the increase.
“The point of this is to raise awareness and to really document the patterns of care in the United States,” said Jason Wright, MD, an associate professor at Columbia University, New York. “We hope that people understand that these procedures are being performed more commonly and they’re out there.”
A study published in 2022 in JAMA Pediatrics found that the number of chest reconstruction surgeries among U.S. adolescents rose fourfold between 2016 and 2019.
The new study included data from 2016 to 2020 in the Nationwide Ambulatory Surgery Sample and the National Inpatient Sample. More than 48,000 patients with diagnosis codes for gender identity disorder, transsexualism, or a personal history of sex reassignment were identified. Age ranges were grouped as 12-18 (7.7%), 19-30 (52.3%), and 31-40 (21.8%).
The number of gender-affirming procedures rose from 4,552 in 2016 to a peak of 13,011 in 2019. (A slight decline to 12,818 procedures in 2020 was attributed to the COVID-19 pandemic.) The surgeries were grouped into three categories: breast and chest procedures, which occurred in 56.6% of patients; genital reconstructive surgeries (35.1%), and other facial cosmetic procedures (13.9%).
“We really wanted to try to make this as representative as we could,” Dr. Wright said. “I think this is really the best estimates that are available to date.”
Chest and breast procedures made up a higher percentage of surgeries in younger patients, while genital surgical procedures made up a higher percentage in older patients. For example, patients aged 19-30 made up 59.1% of breast or chest surgeries and 44.2% of genital surgeries. However, those aged 31-40 accounted for 26.2% of genital surgeries and 18.1% of breast or chest surgeries. For ages 41-50, the spread was more than double, accounting for 12.8% of genital surgeries and only 6.1% of breast or chest surgeries, according to the researchers.
Undocumented uptick
In addition to more inclusive health insurance, Dr. Wright said the increase in these procedures can also be attributed to studies showing their safety and the long-term association with high patient satisfaction.
Kevin Wang, MD, medical director of Providence–Swedish Health Services’ LGBTQIA+ program in Seattle, agreed that changes in health insurance coverage for gender-affirming surgery likely account in part for their increase. But he added that more clinicians are performing these procedures.
He said gender-affirming surgeries improve quality of life for the people who undergo them. The American Academy of Pediatrics has said it would be conducting a thorough review of the effects of transgender care on youth. A 2018 policy statement from the group said transgender youth should “have access to comprehensive, gender-affirming, and developmentally appropriate health care that is provided in a safe and inclusive clinical space.”
Dr. Wright cited several limitations to his group’s study that may result in the undercapture of transgender individuals and gender-affirming surgery; in particular, while the study captured inpatient and ambulatory surgical procedures in large, nationwide datasets, a small number of the procedures could have been performed in other settings.
Guiding a patient through gender-affirming care and surgical procedures can be an arduous process, including understanding their goals, using hormone therapy, and making referrals to specialists. Dr. Wang said he works to maximize his patients’ physical, mental, and emotional health, and helps them understand the risks.
He cited the double standard of a cisgender woman wanting breast augmentation without justification, but someone who identifies as transgender has many more boxes to check – for example, seeing a behavior health specialist to demonstrate they understand the risks and securing a letter of support from their primary care physician to undergo a similar procedure.
“It’s just interesting how the transgender community has to jump through so many more barriers and hoops for affirming, lifesaving procedures where you have other people who are doing it for aesthetic purposes and do not require any type of authorization,” Dr. Wang said.
Dr. Wright said he hopes the findings call attention to the need for more professionals working in the gender-affirming care field.
“I think for the medical community, it’s important to raise the idea that these procedures are becoming more common,” Dr. Wright said. “We are going to need specialists who have expertise in transgender care and surgeons who have the ability to perform these operations. Hopefully, this sheds light on the resources that are going to be required to care for these patients going forward.”
Dr. Wright reported receiving grants from Merck and personal fees from UpToDate outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
a new study published in JAMA Network Open found.
Breast and chest surgeries were the most common procedures performed, and the number of surgical procedures carried out increased with age. The researchers said that, in addition to legal shifts, the established safety of the surgeries and resulting increase in quality of life may also help explain the increase.
“The point of this is to raise awareness and to really document the patterns of care in the United States,” said Jason Wright, MD, an associate professor at Columbia University, New York. “We hope that people understand that these procedures are being performed more commonly and they’re out there.”
A study published in 2022 in JAMA Pediatrics found that the number of chest reconstruction surgeries among U.S. adolescents rose fourfold between 2016 and 2019.
The new study included data from 2016 to 2020 in the Nationwide Ambulatory Surgery Sample and the National Inpatient Sample. More than 48,000 patients with diagnosis codes for gender identity disorder, transsexualism, or a personal history of sex reassignment were identified. Age ranges were grouped as 12-18 (7.7%), 19-30 (52.3%), and 31-40 (21.8%).
The number of gender-affirming procedures rose from 4,552 in 2016 to a peak of 13,011 in 2019. (A slight decline to 12,818 procedures in 2020 was attributed to the COVID-19 pandemic.) The surgeries were grouped into three categories: breast and chest procedures, which occurred in 56.6% of patients; genital reconstructive surgeries (35.1%), and other facial cosmetic procedures (13.9%).
“We really wanted to try to make this as representative as we could,” Dr. Wright said. “I think this is really the best estimates that are available to date.”
Chest and breast procedures made up a higher percentage of surgeries in younger patients, while genital surgical procedures made up a higher percentage in older patients. For example, patients aged 19-30 made up 59.1% of breast or chest surgeries and 44.2% of genital surgeries. However, those aged 31-40 accounted for 26.2% of genital surgeries and 18.1% of breast or chest surgeries. For ages 41-50, the spread was more than double, accounting for 12.8% of genital surgeries and only 6.1% of breast or chest surgeries, according to the researchers.
Undocumented uptick
In addition to more inclusive health insurance, Dr. Wright said the increase in these procedures can also be attributed to studies showing their safety and the long-term association with high patient satisfaction.
Kevin Wang, MD, medical director of Providence–Swedish Health Services’ LGBTQIA+ program in Seattle, agreed that changes in health insurance coverage for gender-affirming surgery likely account in part for their increase. But he added that more clinicians are performing these procedures.
He said gender-affirming surgeries improve quality of life for the people who undergo them. The American Academy of Pediatrics has said it would be conducting a thorough review of the effects of transgender care on youth. A 2018 policy statement from the group said transgender youth should “have access to comprehensive, gender-affirming, and developmentally appropriate health care that is provided in a safe and inclusive clinical space.”
Dr. Wright cited several limitations to his group’s study that may result in the undercapture of transgender individuals and gender-affirming surgery; in particular, while the study captured inpatient and ambulatory surgical procedures in large, nationwide datasets, a small number of the procedures could have been performed in other settings.
Guiding a patient through gender-affirming care and surgical procedures can be an arduous process, including understanding their goals, using hormone therapy, and making referrals to specialists. Dr. Wang said he works to maximize his patients’ physical, mental, and emotional health, and helps them understand the risks.
He cited the double standard of a cisgender woman wanting breast augmentation without justification, but someone who identifies as transgender has many more boxes to check – for example, seeing a behavior health specialist to demonstrate they understand the risks and securing a letter of support from their primary care physician to undergo a similar procedure.
“It’s just interesting how the transgender community has to jump through so many more barriers and hoops for affirming, lifesaving procedures where you have other people who are doing it for aesthetic purposes and do not require any type of authorization,” Dr. Wang said.
Dr. Wright said he hopes the findings call attention to the need for more professionals working in the gender-affirming care field.
“I think for the medical community, it’s important to raise the idea that these procedures are becoming more common,” Dr. Wright said. “We are going to need specialists who have expertise in transgender care and surgeons who have the ability to perform these operations. Hopefully, this sheds light on the resources that are going to be required to care for these patients going forward.”
Dr. Wright reported receiving grants from Merck and personal fees from UpToDate outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
CTE common among young athletes in largest brain donor study
Analysis of brain tissue from athletes who were exposed to RHIs and died before the age of 30 revealed neuropathological evidence of shrinkage of the brain and microscopic changes that indicate a breach of the blood-brain barrier. The case series also identified the first known American female athlete with CTE.
Nearly all of those with CTE had a mild form of the disease and 71% played only at the amateur level in youth, high school, or college sports.
“A lot of people think CTE is a result of high-level, professional play such as football, ice hockey, and boxing, but it can affect amateur athletes and can affect people at a young age,” lead author Ann McKee, MD, professor of neurology and pathology and director of the Chronic Traumatic Encephalopathy Center at Boston University, said in an interview.
The findings were published online in JAMA Neurology.
A rare look
Brain donation at younger ages is rare, so most of what is known about CTE comes from studies in older athletes.
“We’ve always known that young people could develop this disease early after just amateur high school, youth, and college exposure, but this is the largest study of donor brains at this age,” Dr. McKee said.
The case series included 152 brains of athletes who played contact sports, experienced RHIs, and died before age 30. The tissues are part of the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank and were donated between February 2008 and September 2022.
Researchers reviewed the donors’ medical records and conducted retrospective interviews with the donors’ next of kin to assess cognitive symptoms, mood disturbances, and neurobehavioral issues.
Donors died between the ages of 13 and 29 years, 92.8% were male and 73% were White. In 57.2% of the cases, suicide was the cause of death, with no difference between those with or without CTE.
CTE was neuropathologically diagnosed in 41.4% of athletes, using diagnostic criteria developed by the National Institute of Neurological Disorders and Stroke.
More than 95% had mild CTE. Diagnosis was associated with older age (mean difference, 3.92 years; P < .001) and significantly more years of exposure to contact sports (11.6 vs. 8.8 years).
Among those with CTE, 71.4% played amateur sports, including football (60.9%), soccer (17.2%), hockey (7.8%), and wrestling (7%).
The cohort includes the first known American female athlete with CTE. Recruiting female brain donors has always been a challenge, Dr. McKee said. In this study, females comprised about 7% of the entire cohort and tended to be younger and play fewer years of a sport, compared with their male counterparts. All of that could lower their risk for CTE, Dr. McKee said.
“We don’t have enough brain donations to make any comments about differences between the genders, but we’ve always known that women can develop CTE,” she said. “It’s been reported after domestic violence and in an autistic woman who was a headbanger, so it was just a matter of time before we found our first case.”
Early stage of CTE?
Neuropathological analysis revealed neuronal p-tau aggregates in all CTE cases, a hallmark of the disease.
Young athletes with CTE had significantly more ventricular dilatation, suggesting atrophy or shrinkage of the brain, and more cavum septum pellucidum.
“I was surprised that even at this very young age group we could see structural changes to the gross pathology,” Dr. McKee said.
Investigators also found evidence of perivascular macrophages in the deep white matter, a microscopic change that correlated with CTE and years of play and indicates a breach of the blood-brain barrier that could allow pro-inflammatory molecules to enter the brain, setting up a neuroinflammatory response.
“Neuroinflammation is a very early change after repetitive head impacts, as well as in CTE,” Dr. McKee said. “This may be one of the mechanisms by which the inflammation starts, meaning microvascular injury might be an integral part of the pathogenesis of CTE.”
A message for clinicians
All athletes had symptoms of mood and neurobehavioral dysfunction common in people with RHIs. There were no significant differences in those clinical symptoms based on CTE diagnosis, which is likely related to the retrospective nature of the clinical evaluations, Dr. McKee said.
While the study leaves many questions about CTE in younger athletes unanswered, there is a message for clinicians and for patients in the findings, she said.
For clinicians, it’s important to note that “this young population of amateur athletes can be very symptomatic, and in all likelihood, a lot of these symptoms are reversible with proper care and management,” Dr. McKee said.
“For individual athletes, it’s important to note that 58% of this cohort did not have CTE, so just because you have these symptoms is not an indication that you have a neurodegenerative disease,” she added.
The study was funded by Andlinger Foundation, the National Football League, Mac Parkman Foundation, National Operating Committee on Standards for Athletic Equipment, and the Nick and Lynn Buoniconti Foundation, World Wrestling Entertainment, Alzheimer’s Association, National Institutes of Health, Concussion Legacy Foundation, U.S. Department of Defense and the U.S. Department of Veterans Affairs. Dr. McKee is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the NIH and Department of Veteran Affairs and other funding from the Buoniconti Foundation and Mac Parkman Foundation during the conduct of the study.
A version of this article appeared on Medscape.com.
Analysis of brain tissue from athletes who were exposed to RHIs and died before the age of 30 revealed neuropathological evidence of shrinkage of the brain and microscopic changes that indicate a breach of the blood-brain barrier. The case series also identified the first known American female athlete with CTE.
Nearly all of those with CTE had a mild form of the disease and 71% played only at the amateur level in youth, high school, or college sports.
“A lot of people think CTE is a result of high-level, professional play such as football, ice hockey, and boxing, but it can affect amateur athletes and can affect people at a young age,” lead author Ann McKee, MD, professor of neurology and pathology and director of the Chronic Traumatic Encephalopathy Center at Boston University, said in an interview.
The findings were published online in JAMA Neurology.
A rare look
Brain donation at younger ages is rare, so most of what is known about CTE comes from studies in older athletes.
“We’ve always known that young people could develop this disease early after just amateur high school, youth, and college exposure, but this is the largest study of donor brains at this age,” Dr. McKee said.
The case series included 152 brains of athletes who played contact sports, experienced RHIs, and died before age 30. The tissues are part of the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank and were donated between February 2008 and September 2022.
Researchers reviewed the donors’ medical records and conducted retrospective interviews with the donors’ next of kin to assess cognitive symptoms, mood disturbances, and neurobehavioral issues.
Donors died between the ages of 13 and 29 years, 92.8% were male and 73% were White. In 57.2% of the cases, suicide was the cause of death, with no difference between those with or without CTE.
CTE was neuropathologically diagnosed in 41.4% of athletes, using diagnostic criteria developed by the National Institute of Neurological Disorders and Stroke.
More than 95% had mild CTE. Diagnosis was associated with older age (mean difference, 3.92 years; P < .001) and significantly more years of exposure to contact sports (11.6 vs. 8.8 years).
Among those with CTE, 71.4% played amateur sports, including football (60.9%), soccer (17.2%), hockey (7.8%), and wrestling (7%).
The cohort includes the first known American female athlete with CTE. Recruiting female brain donors has always been a challenge, Dr. McKee said. In this study, females comprised about 7% of the entire cohort and tended to be younger and play fewer years of a sport, compared with their male counterparts. All of that could lower their risk for CTE, Dr. McKee said.
“We don’t have enough brain donations to make any comments about differences between the genders, but we’ve always known that women can develop CTE,” she said. “It’s been reported after domestic violence and in an autistic woman who was a headbanger, so it was just a matter of time before we found our first case.”
Early stage of CTE?
Neuropathological analysis revealed neuronal p-tau aggregates in all CTE cases, a hallmark of the disease.
Young athletes with CTE had significantly more ventricular dilatation, suggesting atrophy or shrinkage of the brain, and more cavum septum pellucidum.
“I was surprised that even at this very young age group we could see structural changes to the gross pathology,” Dr. McKee said.
Investigators also found evidence of perivascular macrophages in the deep white matter, a microscopic change that correlated with CTE and years of play and indicates a breach of the blood-brain barrier that could allow pro-inflammatory molecules to enter the brain, setting up a neuroinflammatory response.
“Neuroinflammation is a very early change after repetitive head impacts, as well as in CTE,” Dr. McKee said. “This may be one of the mechanisms by which the inflammation starts, meaning microvascular injury might be an integral part of the pathogenesis of CTE.”
A message for clinicians
All athletes had symptoms of mood and neurobehavioral dysfunction common in people with RHIs. There were no significant differences in those clinical symptoms based on CTE diagnosis, which is likely related to the retrospective nature of the clinical evaluations, Dr. McKee said.
While the study leaves many questions about CTE in younger athletes unanswered, there is a message for clinicians and for patients in the findings, she said.
For clinicians, it’s important to note that “this young population of amateur athletes can be very symptomatic, and in all likelihood, a lot of these symptoms are reversible with proper care and management,” Dr. McKee said.
“For individual athletes, it’s important to note that 58% of this cohort did not have CTE, so just because you have these symptoms is not an indication that you have a neurodegenerative disease,” she added.
The study was funded by Andlinger Foundation, the National Football League, Mac Parkman Foundation, National Operating Committee on Standards for Athletic Equipment, and the Nick and Lynn Buoniconti Foundation, World Wrestling Entertainment, Alzheimer’s Association, National Institutes of Health, Concussion Legacy Foundation, U.S. Department of Defense and the U.S. Department of Veterans Affairs. Dr. McKee is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the NIH and Department of Veteran Affairs and other funding from the Buoniconti Foundation and Mac Parkman Foundation during the conduct of the study.
A version of this article appeared on Medscape.com.
Analysis of brain tissue from athletes who were exposed to RHIs and died before the age of 30 revealed neuropathological evidence of shrinkage of the brain and microscopic changes that indicate a breach of the blood-brain barrier. The case series also identified the first known American female athlete with CTE.
Nearly all of those with CTE had a mild form of the disease and 71% played only at the amateur level in youth, high school, or college sports.
“A lot of people think CTE is a result of high-level, professional play such as football, ice hockey, and boxing, but it can affect amateur athletes and can affect people at a young age,” lead author Ann McKee, MD, professor of neurology and pathology and director of the Chronic Traumatic Encephalopathy Center at Boston University, said in an interview.
The findings were published online in JAMA Neurology.
A rare look
Brain donation at younger ages is rare, so most of what is known about CTE comes from studies in older athletes.
“We’ve always known that young people could develop this disease early after just amateur high school, youth, and college exposure, but this is the largest study of donor brains at this age,” Dr. McKee said.
The case series included 152 brains of athletes who played contact sports, experienced RHIs, and died before age 30. The tissues are part of the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank and were donated between February 2008 and September 2022.
Researchers reviewed the donors’ medical records and conducted retrospective interviews with the donors’ next of kin to assess cognitive symptoms, mood disturbances, and neurobehavioral issues.
Donors died between the ages of 13 and 29 years, 92.8% were male and 73% were White. In 57.2% of the cases, suicide was the cause of death, with no difference between those with or without CTE.
CTE was neuropathologically diagnosed in 41.4% of athletes, using diagnostic criteria developed by the National Institute of Neurological Disorders and Stroke.
More than 95% had mild CTE. Diagnosis was associated with older age (mean difference, 3.92 years; P < .001) and significantly more years of exposure to contact sports (11.6 vs. 8.8 years).
Among those with CTE, 71.4% played amateur sports, including football (60.9%), soccer (17.2%), hockey (7.8%), and wrestling (7%).
The cohort includes the first known American female athlete with CTE. Recruiting female brain donors has always been a challenge, Dr. McKee said. In this study, females comprised about 7% of the entire cohort and tended to be younger and play fewer years of a sport, compared with their male counterparts. All of that could lower their risk for CTE, Dr. McKee said.
“We don’t have enough brain donations to make any comments about differences between the genders, but we’ve always known that women can develop CTE,” she said. “It’s been reported after domestic violence and in an autistic woman who was a headbanger, so it was just a matter of time before we found our first case.”
Early stage of CTE?
Neuropathological analysis revealed neuronal p-tau aggregates in all CTE cases, a hallmark of the disease.
Young athletes with CTE had significantly more ventricular dilatation, suggesting atrophy or shrinkage of the brain, and more cavum septum pellucidum.
“I was surprised that even at this very young age group we could see structural changes to the gross pathology,” Dr. McKee said.
Investigators also found evidence of perivascular macrophages in the deep white matter, a microscopic change that correlated with CTE and years of play and indicates a breach of the blood-brain barrier that could allow pro-inflammatory molecules to enter the brain, setting up a neuroinflammatory response.
“Neuroinflammation is a very early change after repetitive head impacts, as well as in CTE,” Dr. McKee said. “This may be one of the mechanisms by which the inflammation starts, meaning microvascular injury might be an integral part of the pathogenesis of CTE.”
A message for clinicians
All athletes had symptoms of mood and neurobehavioral dysfunction common in people with RHIs. There were no significant differences in those clinical symptoms based on CTE diagnosis, which is likely related to the retrospective nature of the clinical evaluations, Dr. McKee said.
While the study leaves many questions about CTE in younger athletes unanswered, there is a message for clinicians and for patients in the findings, she said.
For clinicians, it’s important to note that “this young population of amateur athletes can be very symptomatic, and in all likelihood, a lot of these symptoms are reversible with proper care and management,” Dr. McKee said.
“For individual athletes, it’s important to note that 58% of this cohort did not have CTE, so just because you have these symptoms is not an indication that you have a neurodegenerative disease,” she added.
The study was funded by Andlinger Foundation, the National Football League, Mac Parkman Foundation, National Operating Committee on Standards for Athletic Equipment, and the Nick and Lynn Buoniconti Foundation, World Wrestling Entertainment, Alzheimer’s Association, National Institutes of Health, Concussion Legacy Foundation, U.S. Department of Defense and the U.S. Department of Veterans Affairs. Dr. McKee is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the NIH and Department of Veteran Affairs and other funding from the Buoniconti Foundation and Mac Parkman Foundation during the conduct of the study.
A version of this article appeared on Medscape.com.
AAP’s hearing test clinical update is the first since 2009
The update is the first since 2009.
The AAP’s clinical report was published online in Pediatrics.
Charles Bower, MD, with the department of otolaryngology at Arkansas Children’s Hospital in Little Rock, led the research team representing AAP’s Committee on Practice and Ambulatory Medicine, section on otolaryngology and head and neck surgery.
The report details how primary care clinicians can detect changes in hearing status by age.
Eliminating terms such as ‘failed’ or ‘impairment’
A key change in this report is that it no longer uses terms such as “loss,” “failed,” or “impairment,” “to reflect that children who are deaf or hard of hearing (D/HH) are equal, healthy, and whole,” the authors wrote.
The report’s recommendations are based on the literature and engagement with deaf and hard of hearing professionals and partner organizations, such as the National Association of the Deaf, working with the AAP Early Hearing Detection and Intervention program.
Birth to 5 a critical time
The authors noted that early medical support for hearing is especially important between birth and 5 years of age. That span is a critical time for brain and language development.
Parents and caregivers are often the first to notice a child’s inattention or erratic responses to sound, they wrote, and it’s important to address these concerns with a pediatrician even if the child has passed a newborn hearing test after birth.
Among recommendations in the update:
- All children should have an objective, evidence-based risk assessment for changes in hearing.
- Children at all ages should have prompt screening if there is clinical or caregiver concern about hearing.
- A child who screens positive for atypical hearing in one or both ears should be referred to an audiologist for diagnostic consultation and testing.
- Because standard testing for children with developmental or behavioral health conditions may be impossible or inaccurate, referral may be more appropriate to audiology for electrophysiological hearing testing using auditory brainstem response (ABR) with sedation.
- To prevent false negatives and to avoid delays in identification, access to language, and support, screening tests should not be repeated more than once before referral to audiology.
Additional recommendations
The report authors pointed out that genetic causes may affect hearing and may show up beyond the newborn period.
They wrote that congenital cytomegalovirus (cCMV) infection is the most common infectious cause of childhood sensorineural hearing change and accounts for 25% of deaf and hard of hearing children at age 4.
Meningitis and otitis media also are leading causes of a change in hearing.
Judith E.C. Lieu, MD, MSPH, professor, program director and vice-chair for education in the department of otolaryngology and head and neck surgery at Washington University in St. Louis, who was not part of the research team, said screening recommendations have not changed much in the update, but she highlighted some points.
She noted that tympanometry is not listed as a method of hearing screening in primary care.
“I agree that tympanogram is not a hearing screening. It is an adjunct to look at middle ear function, but that doesn’t necessarily mean it looks for hearing,” she said.
Dr. Lieu says she does take issue with the stated length of one of the tests in the paper. She said she is concerned that the pure-tone audiometry test for ages 4 through adolescence is listed as taking 30 minutes in a primary care setting. She said she worries that pediatricians will be put off by reading that it is a 30-minute test.
“Honestly, in my experience, it doesn’t take 30 minutes. Maybe 10 minutes,” she said. “I don’t know any pediatrician who could devote 30 minutes to one screening test.”
Development milestones have been adjusted
Also different in these recommendations are the developmental and speech milestones updated according to the most recent AAP information, Dr. Lieu said. Though the new milestones don’t change by much, they are important to note, she said, such as updated guidance on when to be concerned about speech delay.
She said she wished the guidance included more about hearing loss in older children.
The report authors stated that about 1 to 3 per 1,000 children have atypical hearing at birth and similar numbers become deaf or hard of hearing later in childhood.
But Dr. Lieu says that statistic may give the wrong impression about frequency of atypical hearing.
“Hearing loss increases during childhood,” she pointed out. “By the time they hit about age 18, about 15% of kids have some kind of hearing loss.”
“I don’t think it’s made clear to pediatricians that this is not 1 or 2 in a thousand children – this happens much more frequently,” she said.
The report authors and Dr. Lieu report no relevant financial relationships.
The update is the first since 2009.
The AAP’s clinical report was published online in Pediatrics.
Charles Bower, MD, with the department of otolaryngology at Arkansas Children’s Hospital in Little Rock, led the research team representing AAP’s Committee on Practice and Ambulatory Medicine, section on otolaryngology and head and neck surgery.
The report details how primary care clinicians can detect changes in hearing status by age.
Eliminating terms such as ‘failed’ or ‘impairment’
A key change in this report is that it no longer uses terms such as “loss,” “failed,” or “impairment,” “to reflect that children who are deaf or hard of hearing (D/HH) are equal, healthy, and whole,” the authors wrote.
The report’s recommendations are based on the literature and engagement with deaf and hard of hearing professionals and partner organizations, such as the National Association of the Deaf, working with the AAP Early Hearing Detection and Intervention program.
Birth to 5 a critical time
The authors noted that early medical support for hearing is especially important between birth and 5 years of age. That span is a critical time for brain and language development.
Parents and caregivers are often the first to notice a child’s inattention or erratic responses to sound, they wrote, and it’s important to address these concerns with a pediatrician even if the child has passed a newborn hearing test after birth.
Among recommendations in the update:
- All children should have an objective, evidence-based risk assessment for changes in hearing.
- Children at all ages should have prompt screening if there is clinical or caregiver concern about hearing.
- A child who screens positive for atypical hearing in one or both ears should be referred to an audiologist for diagnostic consultation and testing.
- Because standard testing for children with developmental or behavioral health conditions may be impossible or inaccurate, referral may be more appropriate to audiology for electrophysiological hearing testing using auditory brainstem response (ABR) with sedation.
- To prevent false negatives and to avoid delays in identification, access to language, and support, screening tests should not be repeated more than once before referral to audiology.
Additional recommendations
The report authors pointed out that genetic causes may affect hearing and may show up beyond the newborn period.
They wrote that congenital cytomegalovirus (cCMV) infection is the most common infectious cause of childhood sensorineural hearing change and accounts for 25% of deaf and hard of hearing children at age 4.
Meningitis and otitis media also are leading causes of a change in hearing.
Judith E.C. Lieu, MD, MSPH, professor, program director and vice-chair for education in the department of otolaryngology and head and neck surgery at Washington University in St. Louis, who was not part of the research team, said screening recommendations have not changed much in the update, but she highlighted some points.
She noted that tympanometry is not listed as a method of hearing screening in primary care.
“I agree that tympanogram is not a hearing screening. It is an adjunct to look at middle ear function, but that doesn’t necessarily mean it looks for hearing,” she said.
Dr. Lieu says she does take issue with the stated length of one of the tests in the paper. She said she is concerned that the pure-tone audiometry test for ages 4 through adolescence is listed as taking 30 minutes in a primary care setting. She said she worries that pediatricians will be put off by reading that it is a 30-minute test.
“Honestly, in my experience, it doesn’t take 30 minutes. Maybe 10 minutes,” she said. “I don’t know any pediatrician who could devote 30 minutes to one screening test.”
Development milestones have been adjusted
Also different in these recommendations are the developmental and speech milestones updated according to the most recent AAP information, Dr. Lieu said. Though the new milestones don’t change by much, they are important to note, she said, such as updated guidance on when to be concerned about speech delay.
She said she wished the guidance included more about hearing loss in older children.
The report authors stated that about 1 to 3 per 1,000 children have atypical hearing at birth and similar numbers become deaf or hard of hearing later in childhood.
But Dr. Lieu says that statistic may give the wrong impression about frequency of atypical hearing.
“Hearing loss increases during childhood,” she pointed out. “By the time they hit about age 18, about 15% of kids have some kind of hearing loss.”
“I don’t think it’s made clear to pediatricians that this is not 1 or 2 in a thousand children – this happens much more frequently,” she said.
The report authors and Dr. Lieu report no relevant financial relationships.
The update is the first since 2009.
The AAP’s clinical report was published online in Pediatrics.
Charles Bower, MD, with the department of otolaryngology at Arkansas Children’s Hospital in Little Rock, led the research team representing AAP’s Committee on Practice and Ambulatory Medicine, section on otolaryngology and head and neck surgery.
The report details how primary care clinicians can detect changes in hearing status by age.
Eliminating terms such as ‘failed’ or ‘impairment’
A key change in this report is that it no longer uses terms such as “loss,” “failed,” or “impairment,” “to reflect that children who are deaf or hard of hearing (D/HH) are equal, healthy, and whole,” the authors wrote.
The report’s recommendations are based on the literature and engagement with deaf and hard of hearing professionals and partner organizations, such as the National Association of the Deaf, working with the AAP Early Hearing Detection and Intervention program.
Birth to 5 a critical time
The authors noted that early medical support for hearing is especially important between birth and 5 years of age. That span is a critical time for brain and language development.
Parents and caregivers are often the first to notice a child’s inattention or erratic responses to sound, they wrote, and it’s important to address these concerns with a pediatrician even if the child has passed a newborn hearing test after birth.
Among recommendations in the update:
- All children should have an objective, evidence-based risk assessment for changes in hearing.
- Children at all ages should have prompt screening if there is clinical or caregiver concern about hearing.
- A child who screens positive for atypical hearing in one or both ears should be referred to an audiologist for diagnostic consultation and testing.
- Because standard testing for children with developmental or behavioral health conditions may be impossible or inaccurate, referral may be more appropriate to audiology for electrophysiological hearing testing using auditory brainstem response (ABR) with sedation.
- To prevent false negatives and to avoid delays in identification, access to language, and support, screening tests should not be repeated more than once before referral to audiology.
Additional recommendations
The report authors pointed out that genetic causes may affect hearing and may show up beyond the newborn period.
They wrote that congenital cytomegalovirus (cCMV) infection is the most common infectious cause of childhood sensorineural hearing change and accounts for 25% of deaf and hard of hearing children at age 4.
Meningitis and otitis media also are leading causes of a change in hearing.
Judith E.C. Lieu, MD, MSPH, professor, program director and vice-chair for education in the department of otolaryngology and head and neck surgery at Washington University in St. Louis, who was not part of the research team, said screening recommendations have not changed much in the update, but she highlighted some points.
She noted that tympanometry is not listed as a method of hearing screening in primary care.
“I agree that tympanogram is not a hearing screening. It is an adjunct to look at middle ear function, but that doesn’t necessarily mean it looks for hearing,” she said.
Dr. Lieu says she does take issue with the stated length of one of the tests in the paper. She said she is concerned that the pure-tone audiometry test for ages 4 through adolescence is listed as taking 30 minutes in a primary care setting. She said she worries that pediatricians will be put off by reading that it is a 30-minute test.
“Honestly, in my experience, it doesn’t take 30 minutes. Maybe 10 minutes,” she said. “I don’t know any pediatrician who could devote 30 minutes to one screening test.”
Development milestones have been adjusted
Also different in these recommendations are the developmental and speech milestones updated according to the most recent AAP information, Dr. Lieu said. Though the new milestones don’t change by much, they are important to note, she said, such as updated guidance on when to be concerned about speech delay.
She said she wished the guidance included more about hearing loss in older children.
The report authors stated that about 1 to 3 per 1,000 children have atypical hearing at birth and similar numbers become deaf or hard of hearing later in childhood.
But Dr. Lieu says that statistic may give the wrong impression about frequency of atypical hearing.
“Hearing loss increases during childhood,” she pointed out. “By the time they hit about age 18, about 15% of kids have some kind of hearing loss.”
“I don’t think it’s made clear to pediatricians that this is not 1 or 2 in a thousand children – this happens much more frequently,” she said.
The report authors and Dr. Lieu report no relevant financial relationships.
FROM PEDIATRICS
First guidelines developed for childhood eosinophilic GI disorders beyond eosinophilic esophagitis
The limited scope and depth of existing literature on childhood eosinophilic gastrointestinal disorders (EGIDs) beyond eosinophilic esophagitis (EoE) spurred an international group of researchers and clinicians to develop the first clinical practice guidelines for diagnosing and treating these rare conditions.
They were developed jointly by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition.
Non-EoE EGIDs are rare chronic inflammatory disorders of the gastrointestinal tract, estimated at less than 200,000 cases annually in the United States, with unknown long-term consequences, Glenn Furuta, MD, professor of pediatrics at the University of Colorado at Denver and section head of gastroenterology at Children’s Hospital Colorado, both in Aurora, said in an interview
“There are many unmet needs. Research has been limited and has not progressed at the pace we want it to,” added Dr. Furuta, who is corresponding author of the guidelines.
The guidelines were published online in the Journal of Pediatric Gastroenterology & Nutrition, by lead author Alexandra Papadopoulou, MD, division of gastroenterology and hepatology, first department of pediatrics, University of Athens, and Children’s Hospital Agia Sofia, also in Athens, and colleagues.
With these, we provide guidance for clinicians to better understand the conditions and also how to diagnose and initiate care for patients with these rare diseases, said Dr. Furuta.
Difficult-to-diagnose conditions
Guideline development involved a working group of 26 pediatric gastroenterologists, adult gastroenterologists, allergists/immunologists, and pathologists from 16 countries across five continents. The consensus document includes 34 statements based on available evidence and 41 recommendations based on expert opinion and best clinical practices. In cases where the supporting evidence was weak but agreement was strong, the authors issued conditional recommendations.
The guidelines subdivide the non-EoE EGIDs according to inflammation location: eosinophilic gastritis, eosinophilic duodenitis (EoD), eosinophilic colitis, and eosinophilic enteritis. The latter can be further subdivided into EoD, eosinophilic jejunitis, and eosinophilic ileitis.
Non-EoE EGIDs are hard to diagnose because symptoms are relatively nonspecific and may include abdominal pain, vomiting, diarrhea, and bloody stools, all of which could have any number of underlying causes, Dr. Furuta said.
If you are treating a patient who is not getting better with such symptoms as persisting infections, acid-related problems, significant bleeding leading to anemia, intestinal perforation or obstruction, or low serum protein leading to swelling, then you should think that something else is going on that requires more of an evaluation, Dr. Furuta noted.
Patients with personal or family histories of eosinophilic or allergic disease should raise greater suspicion, Dr. Furuta said. “The next step requires an endoscopy and biopsy.”
Awareness of non-EoE EGIDs has been higher among pediatric gastroenterologists than among those treating adult disease because pediatric gastroenterologists have always obtained biopsies of the intestinal tract, Dr. Furuta noted.
The guidelines recommend that diagnosis of non-EoE EGIDs in children and adolescents must include signs or symptoms of gastrointestinal dysfunction, dense eosinophilic infiltrates found in mucosal or full-thickness biopsies above organ-specific threshold values included in the document, and absence of other diseases associated with GI mucosal eosinophilic inflammation.
Individualized treatment
The authors noted that the strength of recommendations varies with the often-modest availability of randomized controlled trial data on treatment efficacy.
For example, they recommended that systemic steroids be considered to induce remission but only conditionally recommend topical steroids. They conditionally recommend consideration of empiric elimination diets and conditionally recommend against using food allergy testing to guide diet.
The choice of treatment should be individualized on the basis of the affected GI segment, severity of the disease, patient characteristics, and family resources and capabilities, the authors wrote.
“We’ve provided guidance on how to care for patients based on the consensus of experts who have the necessary experience and knowledge base,” Dr. Furuta said. “Our ability to say: ‘Here are the established treatments,’ is lacking, though. We need research studies to verify that our recommended approaches are indeed correct.”
The authors conditionally recommended that treatment goals include achieving symptom resolution, improving gross endoscopic and histologic abnormalities, promoting normal childhood growth and development, and preventing disease complications.
No pediatric study has determined the natural history of non-EoE EGIDs, and no study of maintenance therapy has been conducted, the authors noted.
For this reason, they conditionally recommended that the clinical decision to continue therapy should be discussed with patients and their parents/caregivers, and those discussions include the benefits and risk of long-term treatment, its cost, and its impact on health-related quality of life.
A starting point for patient management
In a comment, Vincent Mukkada, MD, professor of pediatrics at the University of Cincinnati and an attending physician in gastroenterology, hepatology, and nutrition at Cincinnati Children’s Hospital and Medical Center, observed that, though improved awareness among pediatric gastroenterologists may account for some of the increase in GI eosinophil disease, the incidence is also likely growing.
“We’re looking for them much more,” said Dr. Mukkada.
“But I also think they’re increasing, just like all other atopic diseases. We’re not sure why,” he added.
“The hope is that these guidelines will allow nonsubspecialized gastroenterologists and allergists feel comfortable to at least start on the journey of managing these patients. And, for pediatricians who learn that their patient has received a non-EoE EGID diagnosis, they can go to the summary figures in this one document and very quickly get an overview of the disease and its course,” Dr. Mukkada said.
Guideline development was funded by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. The authors and Dr. Mukkada reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The limited scope and depth of existing literature on childhood eosinophilic gastrointestinal disorders (EGIDs) beyond eosinophilic esophagitis (EoE) spurred an international group of researchers and clinicians to develop the first clinical practice guidelines for diagnosing and treating these rare conditions.
They were developed jointly by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition.
Non-EoE EGIDs are rare chronic inflammatory disorders of the gastrointestinal tract, estimated at less than 200,000 cases annually in the United States, with unknown long-term consequences, Glenn Furuta, MD, professor of pediatrics at the University of Colorado at Denver and section head of gastroenterology at Children’s Hospital Colorado, both in Aurora, said in an interview
“There are many unmet needs. Research has been limited and has not progressed at the pace we want it to,” added Dr. Furuta, who is corresponding author of the guidelines.
The guidelines were published online in the Journal of Pediatric Gastroenterology & Nutrition, by lead author Alexandra Papadopoulou, MD, division of gastroenterology and hepatology, first department of pediatrics, University of Athens, and Children’s Hospital Agia Sofia, also in Athens, and colleagues.
With these, we provide guidance for clinicians to better understand the conditions and also how to diagnose and initiate care for patients with these rare diseases, said Dr. Furuta.
Difficult-to-diagnose conditions
Guideline development involved a working group of 26 pediatric gastroenterologists, adult gastroenterologists, allergists/immunologists, and pathologists from 16 countries across five continents. The consensus document includes 34 statements based on available evidence and 41 recommendations based on expert opinion and best clinical practices. In cases where the supporting evidence was weak but agreement was strong, the authors issued conditional recommendations.
The guidelines subdivide the non-EoE EGIDs according to inflammation location: eosinophilic gastritis, eosinophilic duodenitis (EoD), eosinophilic colitis, and eosinophilic enteritis. The latter can be further subdivided into EoD, eosinophilic jejunitis, and eosinophilic ileitis.
Non-EoE EGIDs are hard to diagnose because symptoms are relatively nonspecific and may include abdominal pain, vomiting, diarrhea, and bloody stools, all of which could have any number of underlying causes, Dr. Furuta said.
If you are treating a patient who is not getting better with such symptoms as persisting infections, acid-related problems, significant bleeding leading to anemia, intestinal perforation or obstruction, or low serum protein leading to swelling, then you should think that something else is going on that requires more of an evaluation, Dr. Furuta noted.
Patients with personal or family histories of eosinophilic or allergic disease should raise greater suspicion, Dr. Furuta said. “The next step requires an endoscopy and biopsy.”
Awareness of non-EoE EGIDs has been higher among pediatric gastroenterologists than among those treating adult disease because pediatric gastroenterologists have always obtained biopsies of the intestinal tract, Dr. Furuta noted.
The guidelines recommend that diagnosis of non-EoE EGIDs in children and adolescents must include signs or symptoms of gastrointestinal dysfunction, dense eosinophilic infiltrates found in mucosal or full-thickness biopsies above organ-specific threshold values included in the document, and absence of other diseases associated with GI mucosal eosinophilic inflammation.
Individualized treatment
The authors noted that the strength of recommendations varies with the often-modest availability of randomized controlled trial data on treatment efficacy.
For example, they recommended that systemic steroids be considered to induce remission but only conditionally recommend topical steroids. They conditionally recommend consideration of empiric elimination diets and conditionally recommend against using food allergy testing to guide diet.
The choice of treatment should be individualized on the basis of the affected GI segment, severity of the disease, patient characteristics, and family resources and capabilities, the authors wrote.
“We’ve provided guidance on how to care for patients based on the consensus of experts who have the necessary experience and knowledge base,” Dr. Furuta said. “Our ability to say: ‘Here are the established treatments,’ is lacking, though. We need research studies to verify that our recommended approaches are indeed correct.”
The authors conditionally recommended that treatment goals include achieving symptom resolution, improving gross endoscopic and histologic abnormalities, promoting normal childhood growth and development, and preventing disease complications.
No pediatric study has determined the natural history of non-EoE EGIDs, and no study of maintenance therapy has been conducted, the authors noted.
For this reason, they conditionally recommended that the clinical decision to continue therapy should be discussed with patients and their parents/caregivers, and those discussions include the benefits and risk of long-term treatment, its cost, and its impact on health-related quality of life.
A starting point for patient management
In a comment, Vincent Mukkada, MD, professor of pediatrics at the University of Cincinnati and an attending physician in gastroenterology, hepatology, and nutrition at Cincinnati Children’s Hospital and Medical Center, observed that, though improved awareness among pediatric gastroenterologists may account for some of the increase in GI eosinophil disease, the incidence is also likely growing.
“We’re looking for them much more,” said Dr. Mukkada.
“But I also think they’re increasing, just like all other atopic diseases. We’re not sure why,” he added.
“The hope is that these guidelines will allow nonsubspecialized gastroenterologists and allergists feel comfortable to at least start on the journey of managing these patients. And, for pediatricians who learn that their patient has received a non-EoE EGID diagnosis, they can go to the summary figures in this one document and very quickly get an overview of the disease and its course,” Dr. Mukkada said.
Guideline development was funded by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. The authors and Dr. Mukkada reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The limited scope and depth of existing literature on childhood eosinophilic gastrointestinal disorders (EGIDs) beyond eosinophilic esophagitis (EoE) spurred an international group of researchers and clinicians to develop the first clinical practice guidelines for diagnosing and treating these rare conditions.
They were developed jointly by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition.
Non-EoE EGIDs are rare chronic inflammatory disorders of the gastrointestinal tract, estimated at less than 200,000 cases annually in the United States, with unknown long-term consequences, Glenn Furuta, MD, professor of pediatrics at the University of Colorado at Denver and section head of gastroenterology at Children’s Hospital Colorado, both in Aurora, said in an interview
“There are many unmet needs. Research has been limited and has not progressed at the pace we want it to,” added Dr. Furuta, who is corresponding author of the guidelines.
The guidelines were published online in the Journal of Pediatric Gastroenterology & Nutrition, by lead author Alexandra Papadopoulou, MD, division of gastroenterology and hepatology, first department of pediatrics, University of Athens, and Children’s Hospital Agia Sofia, also in Athens, and colleagues.
With these, we provide guidance for clinicians to better understand the conditions and also how to diagnose and initiate care for patients with these rare diseases, said Dr. Furuta.
Difficult-to-diagnose conditions
Guideline development involved a working group of 26 pediatric gastroenterologists, adult gastroenterologists, allergists/immunologists, and pathologists from 16 countries across five continents. The consensus document includes 34 statements based on available evidence and 41 recommendations based on expert opinion and best clinical practices. In cases where the supporting evidence was weak but agreement was strong, the authors issued conditional recommendations.
The guidelines subdivide the non-EoE EGIDs according to inflammation location: eosinophilic gastritis, eosinophilic duodenitis (EoD), eosinophilic colitis, and eosinophilic enteritis. The latter can be further subdivided into EoD, eosinophilic jejunitis, and eosinophilic ileitis.
Non-EoE EGIDs are hard to diagnose because symptoms are relatively nonspecific and may include abdominal pain, vomiting, diarrhea, and bloody stools, all of which could have any number of underlying causes, Dr. Furuta said.
If you are treating a patient who is not getting better with such symptoms as persisting infections, acid-related problems, significant bleeding leading to anemia, intestinal perforation or obstruction, or low serum protein leading to swelling, then you should think that something else is going on that requires more of an evaluation, Dr. Furuta noted.
Patients with personal or family histories of eosinophilic or allergic disease should raise greater suspicion, Dr. Furuta said. “The next step requires an endoscopy and biopsy.”
Awareness of non-EoE EGIDs has been higher among pediatric gastroenterologists than among those treating adult disease because pediatric gastroenterologists have always obtained biopsies of the intestinal tract, Dr. Furuta noted.
The guidelines recommend that diagnosis of non-EoE EGIDs in children and adolescents must include signs or symptoms of gastrointestinal dysfunction, dense eosinophilic infiltrates found in mucosal or full-thickness biopsies above organ-specific threshold values included in the document, and absence of other diseases associated with GI mucosal eosinophilic inflammation.
Individualized treatment
The authors noted that the strength of recommendations varies with the often-modest availability of randomized controlled trial data on treatment efficacy.
For example, they recommended that systemic steroids be considered to induce remission but only conditionally recommend topical steroids. They conditionally recommend consideration of empiric elimination diets and conditionally recommend against using food allergy testing to guide diet.
The choice of treatment should be individualized on the basis of the affected GI segment, severity of the disease, patient characteristics, and family resources and capabilities, the authors wrote.
“We’ve provided guidance on how to care for patients based on the consensus of experts who have the necessary experience and knowledge base,” Dr. Furuta said. “Our ability to say: ‘Here are the established treatments,’ is lacking, though. We need research studies to verify that our recommended approaches are indeed correct.”
The authors conditionally recommended that treatment goals include achieving symptom resolution, improving gross endoscopic and histologic abnormalities, promoting normal childhood growth and development, and preventing disease complications.
No pediatric study has determined the natural history of non-EoE EGIDs, and no study of maintenance therapy has been conducted, the authors noted.
For this reason, they conditionally recommended that the clinical decision to continue therapy should be discussed with patients and their parents/caregivers, and those discussions include the benefits and risk of long-term treatment, its cost, and its impact on health-related quality of life.
A starting point for patient management
In a comment, Vincent Mukkada, MD, professor of pediatrics at the University of Cincinnati and an attending physician in gastroenterology, hepatology, and nutrition at Cincinnati Children’s Hospital and Medical Center, observed that, though improved awareness among pediatric gastroenterologists may account for some of the increase in GI eosinophil disease, the incidence is also likely growing.
“We’re looking for them much more,” said Dr. Mukkada.
“But I also think they’re increasing, just like all other atopic diseases. We’re not sure why,” he added.
“The hope is that these guidelines will allow nonsubspecialized gastroenterologists and allergists feel comfortable to at least start on the journey of managing these patients. And, for pediatricians who learn that their patient has received a non-EoE EGID diagnosis, they can go to the summary figures in this one document and very quickly get an overview of the disease and its course,” Dr. Mukkada said.
Guideline development was funded by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. The authors and Dr. Mukkada reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PEDIATRIC GASTROENTEROLOGY & NUTRITION
Consider housing insecurity, other issues when managing challenging skin diseases in children, expert says
ASHEVILLE, N.C. – , according to a pediatric dermatologist who addressed the annual meeting of the Society for Pediatric Dermatology.
As a general principle for treating chronic skin conditions in children who are not doing well, it is reasonable to draw out information about a patient’s access to adequate housing, nutrition, and other basic needs, George Hightower, MD, PhD, of the division of pediatric and adolescent dermatology, University of California, San Diego, said at the meeting.
“We need conversations about where patients play, learn, and rest their heads at night,” said Dr. Hightower, who conducts research in this area. Fundamental components of well-being, such as stable housing and secure access to nutrition “are inseparable” from a child’s health, he noted.
“What are the stakes?” he asked. For many children, these factors might mean the difference between effective and poor control of the diseases for which the patient is seeking care.
To illustrate the point, Dr. Hightower used hidradenitis suppurativa (HS), a disease that appears to be on the rise among adolescents, as an example of why patient circumstances matter and should be considered. A complex disorder that is more prevalent in resource-poor communities, HS is difficult to control, often requiring extended periods of treatment with medications that can involve complex dosing or regular infusions.
“There is a need for medical providers to help the patient plan for this chronic illness,” said Dr. Hightower, referring to the importance of close follow-up. In adolescents, HS can be sufficiently disruptive from both the physical and psychological perspective that poor control can “derail future aspirations” by complicating educational endeavors and social interactions.
Dr. Hightower acknowledged that simply documenting housing insecurity or other issues does not solve these problems, but he does believe that developing a sensitivity to these obstacles to health care is a first step. It is a process that should permeate into medical training, health care research, and strategies to improve outcomes.
“The connections between fair housing and clinical practice may appear tenuous and inconsequential to the care provided by medical specialists,” Dr. Hightower said, but he emphasized that there are clear consequences when these factors contribute to inadequate control of such diseases as HS. As a source of missed appointments and disjointed care, an unstable home life can be an important barrier to disease control – and because of scarring nodules, fistulae, pain, school absences, and social isolation, complications can be dire.
Solutions to insecure housing are not typically available to an individual clinician, but the awareness that this can be a factor can help both physicians and patients begin to think about the role this plays in impairing recovery and what solutions might be found to modify the impact. Awareness not just among individual clinicians but a broader consortium of those working to improve health care outcomes is needed to “challenge the way we are doing medicine,” he said.
While conversations about the social determinants of health, including access to resources within patients’ neighborhoods, schools, and environment, can demonstrate concern about how to address obstacles, it can also be part of a reorientation to think beyond treatment for the underlying pathology alone. Eliciting trust and emphasizing the importance of environmental barriers to adequate care can be positive steps on the path to solutions.
Participatory action research
Relevant to this orientation, Dr. Hightower spoke about participatory action research (PAR), which provides a framework for patients to participate in the planning of clinical studies to effect change, not just serve as subjects in these studies.
The assumption of PAR is that “all people have valuable knowledge about their lives and experiences,” Dr. Hightower said. From this assumption, individuals who have been historically marginalized by race, income, or other factors can help define the problems from the patient’s perspective and, from there, create studies to seek solutions.
PAR is consistent with a patient-centered approach to medical care, which Dr. Hightower called “the future of medicine.” It involves a big-picture approach to look beyond disease pathology and symptoms to factors that might be creating susceptibility to disease and undermining health care.
Organized medicine alone cannot solve the cause of social inequities leading to disparate risks for disease and risks of inadequate health care, but Dr. Hightower argued that these inequities should not be ignored. He believes medical trainees should learn how to elicit information about the barriers to adequate health care and be aware of solutions, such as fair housing policies.
While he believes that PAR is an example of a pathway to problem solving, he suggested that a comprehensive approach requires an effective method of communication between providers and patients that would lead to a collaborative and mutually reinforcing approach.
“How do we ensure that individuals from communities most impacted by health disparities are treated fairly and empowered to address these disparities?” Dr. Hightower asked. He said that this is the direction of his own research and the issues that inhibit adequate treatment of many dermatologic diseases, as well as other types of disease, in childhood.
Craig Burkhart, MD, director of a private pediatric and adolescent dermatology practice in Cary, N.C., said that Dr. Hightower’s message is relevant. The value of considering and addressing the psychological well-being of patients of any age is not a new concept, but he acknowledged that he, for one, has not routinely inquired about obstacles to follow-up care if there is a signal that this might be an issue.
“As dermatologists, we focus on the acute complaints. We want to make the patient better,” said Dr. Burkhart, who moderated the session in which Dr. Hightower spoke. He agreed with Dr. Hightower that environmental factors make a difference on the road to recovery for a patient, and his presentation was a good reminder, he said, to consider the patient’s circumstances when response to treatment is inadequate, particularly in chronic diseases like HS, for which comprehensive care and close follow-up are needed.
Dr. Hightower and Dr. Burkhart report no potential conflicts of interest.
ASHEVILLE, N.C. – , according to a pediatric dermatologist who addressed the annual meeting of the Society for Pediatric Dermatology.
As a general principle for treating chronic skin conditions in children who are not doing well, it is reasonable to draw out information about a patient’s access to adequate housing, nutrition, and other basic needs, George Hightower, MD, PhD, of the division of pediatric and adolescent dermatology, University of California, San Diego, said at the meeting.
“We need conversations about where patients play, learn, and rest their heads at night,” said Dr. Hightower, who conducts research in this area. Fundamental components of well-being, such as stable housing and secure access to nutrition “are inseparable” from a child’s health, he noted.
“What are the stakes?” he asked. For many children, these factors might mean the difference between effective and poor control of the diseases for which the patient is seeking care.
To illustrate the point, Dr. Hightower used hidradenitis suppurativa (HS), a disease that appears to be on the rise among adolescents, as an example of why patient circumstances matter and should be considered. A complex disorder that is more prevalent in resource-poor communities, HS is difficult to control, often requiring extended periods of treatment with medications that can involve complex dosing or regular infusions.
“There is a need for medical providers to help the patient plan for this chronic illness,” said Dr. Hightower, referring to the importance of close follow-up. In adolescents, HS can be sufficiently disruptive from both the physical and psychological perspective that poor control can “derail future aspirations” by complicating educational endeavors and social interactions.
Dr. Hightower acknowledged that simply documenting housing insecurity or other issues does not solve these problems, but he does believe that developing a sensitivity to these obstacles to health care is a first step. It is a process that should permeate into medical training, health care research, and strategies to improve outcomes.
“The connections between fair housing and clinical practice may appear tenuous and inconsequential to the care provided by medical specialists,” Dr. Hightower said, but he emphasized that there are clear consequences when these factors contribute to inadequate control of such diseases as HS. As a source of missed appointments and disjointed care, an unstable home life can be an important barrier to disease control – and because of scarring nodules, fistulae, pain, school absences, and social isolation, complications can be dire.
Solutions to insecure housing are not typically available to an individual clinician, but the awareness that this can be a factor can help both physicians and patients begin to think about the role this plays in impairing recovery and what solutions might be found to modify the impact. Awareness not just among individual clinicians but a broader consortium of those working to improve health care outcomes is needed to “challenge the way we are doing medicine,” he said.
While conversations about the social determinants of health, including access to resources within patients’ neighborhoods, schools, and environment, can demonstrate concern about how to address obstacles, it can also be part of a reorientation to think beyond treatment for the underlying pathology alone. Eliciting trust and emphasizing the importance of environmental barriers to adequate care can be positive steps on the path to solutions.
Participatory action research
Relevant to this orientation, Dr. Hightower spoke about participatory action research (PAR), which provides a framework for patients to participate in the planning of clinical studies to effect change, not just serve as subjects in these studies.
The assumption of PAR is that “all people have valuable knowledge about their lives and experiences,” Dr. Hightower said. From this assumption, individuals who have been historically marginalized by race, income, or other factors can help define the problems from the patient’s perspective and, from there, create studies to seek solutions.
PAR is consistent with a patient-centered approach to medical care, which Dr. Hightower called “the future of medicine.” It involves a big-picture approach to look beyond disease pathology and symptoms to factors that might be creating susceptibility to disease and undermining health care.
Organized medicine alone cannot solve the cause of social inequities leading to disparate risks for disease and risks of inadequate health care, but Dr. Hightower argued that these inequities should not be ignored. He believes medical trainees should learn how to elicit information about the barriers to adequate health care and be aware of solutions, such as fair housing policies.
While he believes that PAR is an example of a pathway to problem solving, he suggested that a comprehensive approach requires an effective method of communication between providers and patients that would lead to a collaborative and mutually reinforcing approach.
“How do we ensure that individuals from communities most impacted by health disparities are treated fairly and empowered to address these disparities?” Dr. Hightower asked. He said that this is the direction of his own research and the issues that inhibit adequate treatment of many dermatologic diseases, as well as other types of disease, in childhood.
Craig Burkhart, MD, director of a private pediatric and adolescent dermatology practice in Cary, N.C., said that Dr. Hightower’s message is relevant. The value of considering and addressing the psychological well-being of patients of any age is not a new concept, but he acknowledged that he, for one, has not routinely inquired about obstacles to follow-up care if there is a signal that this might be an issue.
“As dermatologists, we focus on the acute complaints. We want to make the patient better,” said Dr. Burkhart, who moderated the session in which Dr. Hightower spoke. He agreed with Dr. Hightower that environmental factors make a difference on the road to recovery for a patient, and his presentation was a good reminder, he said, to consider the patient’s circumstances when response to treatment is inadequate, particularly in chronic diseases like HS, for which comprehensive care and close follow-up are needed.
Dr. Hightower and Dr. Burkhart report no potential conflicts of interest.
ASHEVILLE, N.C. – , according to a pediatric dermatologist who addressed the annual meeting of the Society for Pediatric Dermatology.
As a general principle for treating chronic skin conditions in children who are not doing well, it is reasonable to draw out information about a patient’s access to adequate housing, nutrition, and other basic needs, George Hightower, MD, PhD, of the division of pediatric and adolescent dermatology, University of California, San Diego, said at the meeting.
“We need conversations about where patients play, learn, and rest their heads at night,” said Dr. Hightower, who conducts research in this area. Fundamental components of well-being, such as stable housing and secure access to nutrition “are inseparable” from a child’s health, he noted.
“What are the stakes?” he asked. For many children, these factors might mean the difference between effective and poor control of the diseases for which the patient is seeking care.
To illustrate the point, Dr. Hightower used hidradenitis suppurativa (HS), a disease that appears to be on the rise among adolescents, as an example of why patient circumstances matter and should be considered. A complex disorder that is more prevalent in resource-poor communities, HS is difficult to control, often requiring extended periods of treatment with medications that can involve complex dosing or regular infusions.
“There is a need for medical providers to help the patient plan for this chronic illness,” said Dr. Hightower, referring to the importance of close follow-up. In adolescents, HS can be sufficiently disruptive from both the physical and psychological perspective that poor control can “derail future aspirations” by complicating educational endeavors and social interactions.
Dr. Hightower acknowledged that simply documenting housing insecurity or other issues does not solve these problems, but he does believe that developing a sensitivity to these obstacles to health care is a first step. It is a process that should permeate into medical training, health care research, and strategies to improve outcomes.
“The connections between fair housing and clinical practice may appear tenuous and inconsequential to the care provided by medical specialists,” Dr. Hightower said, but he emphasized that there are clear consequences when these factors contribute to inadequate control of such diseases as HS. As a source of missed appointments and disjointed care, an unstable home life can be an important barrier to disease control – and because of scarring nodules, fistulae, pain, school absences, and social isolation, complications can be dire.
Solutions to insecure housing are not typically available to an individual clinician, but the awareness that this can be a factor can help both physicians and patients begin to think about the role this plays in impairing recovery and what solutions might be found to modify the impact. Awareness not just among individual clinicians but a broader consortium of those working to improve health care outcomes is needed to “challenge the way we are doing medicine,” he said.
While conversations about the social determinants of health, including access to resources within patients’ neighborhoods, schools, and environment, can demonstrate concern about how to address obstacles, it can also be part of a reorientation to think beyond treatment for the underlying pathology alone. Eliciting trust and emphasizing the importance of environmental barriers to adequate care can be positive steps on the path to solutions.
Participatory action research
Relevant to this orientation, Dr. Hightower spoke about participatory action research (PAR), which provides a framework for patients to participate in the planning of clinical studies to effect change, not just serve as subjects in these studies.
The assumption of PAR is that “all people have valuable knowledge about their lives and experiences,” Dr. Hightower said. From this assumption, individuals who have been historically marginalized by race, income, or other factors can help define the problems from the patient’s perspective and, from there, create studies to seek solutions.
PAR is consistent with a patient-centered approach to medical care, which Dr. Hightower called “the future of medicine.” It involves a big-picture approach to look beyond disease pathology and symptoms to factors that might be creating susceptibility to disease and undermining health care.
Organized medicine alone cannot solve the cause of social inequities leading to disparate risks for disease and risks of inadequate health care, but Dr. Hightower argued that these inequities should not be ignored. He believes medical trainees should learn how to elicit information about the barriers to adequate health care and be aware of solutions, such as fair housing policies.
While he believes that PAR is an example of a pathway to problem solving, he suggested that a comprehensive approach requires an effective method of communication between providers and patients that would lead to a collaborative and mutually reinforcing approach.
“How do we ensure that individuals from communities most impacted by health disparities are treated fairly and empowered to address these disparities?” Dr. Hightower asked. He said that this is the direction of his own research and the issues that inhibit adequate treatment of many dermatologic diseases, as well as other types of disease, in childhood.
Craig Burkhart, MD, director of a private pediatric and adolescent dermatology practice in Cary, N.C., said that Dr. Hightower’s message is relevant. The value of considering and addressing the psychological well-being of patients of any age is not a new concept, but he acknowledged that he, for one, has not routinely inquired about obstacles to follow-up care if there is a signal that this might be an issue.
“As dermatologists, we focus on the acute complaints. We want to make the patient better,” said Dr. Burkhart, who moderated the session in which Dr. Hightower spoke. He agreed with Dr. Hightower that environmental factors make a difference on the road to recovery for a patient, and his presentation was a good reminder, he said, to consider the patient’s circumstances when response to treatment is inadequate, particularly in chronic diseases like HS, for which comprehensive care and close follow-up are needed.
Dr. Hightower and Dr. Burkhart report no potential conflicts of interest.
AT SPD 2023
New RSV shot is a monoclonal antibody, not a vaccine
For the first time in the fall of 2023, families will be offered season-long protection for infants and some children against respiratory syncytial virus (RSV).
The Food and Drug Administration in July approved a prevention called nirsevimab (Beyfortus, AstraZeneca/Sanofi) and it is expected to be widely rolled out in the coming weeks as the RSV season begins.
And monoclonal antibodies are often used for treatment rather than prevention.
Adding to potential confusion is the fact the Centers for Disease Control and Prevention has included nirsevimab in the Vaccines for Children program, which covers the costs for uninsured kids and makes it more accessible.
Nirsevimab is approved for infants (up to 8 months old) born during or entering their first RSV season, and in children up to 2 years of age who are still vulnerable to severe RSV through their second season.
It’s recommended that all infants get one injection in their first 8 months for prevention instead of the previous monthly shots used to help prevent kids at high risk from getting severe RSV.
If monoclonal antibodies can be used for preventing disease in infants, could they become a viable vaccine alternative for adults?
Specialists say no.
That’s partly because of the difference in body size. Although an injection is an option for a newborn, pediatricians suggest, it would take far too much of the treatment to work as a shot for adults.
Ruth Karron, MD, an expert in pediatric infectious diseases at Johns Hopkins Medicine, Baltimore, said that, while vaccines come in small amounts and activate immune cells, monoclonal antibodies are more like a drug, with the dose based on weight.
“You’d have to give it intravenously,” for larger doses, she explained, which has never been studied before and would also be very expensive. “It really couldn’t be an option for adults.”
What’s the difference between vaccines and antibodies?
Monoclonal antibodies are proteins made in a lab to mimic the immune system’s ability to fight pathogens such as viruses.
Dr. Karron explained that a wide variety of monoclonal antibodies have long been used to treat diseases such as cancers and autoimmune disease. In recent years, the antibodies have been used to treat COVID.
Monoclonal antibodies have also been used to treat RSV in children, but the effects don’t last long – they confer passive immunity and “when it’s gone, it’s gone,” Dr. Karron said.
That means kids at high risk for severe RSV have had to get monthly injections.
But with nirsevimab, the mutated antibodies stay in circulation longer so they can last 5 or 6 months, enough to cover the RSV season, Dr. Karron explained. “It’s highly, highly effective.”
Vaccines train the body
“The idea with vaccines is that you engage the individual’s immune system. You teach it to make antibodies,” Dr. Karron said. Conversely, “you give an antibody and it’s good for as long as the antibody lasts. It’s not teaching your body anything.”
Frank Esper, MD, a pediatric infectious disease specialist at Cleveland Clinic Children’s Hospital, said monoclonal antibody protection for RSV is particularly welcome. “We’ve been trying to make an RSV vaccine since the 1960s and have done nothing but fail miserably.”
“The best thing is always a vaccine,” Dr. Esper said, explaining that vaccines teach the body to make its own antibodies and confer long-term protection and are “probably more efficacious than anything that’s ever manmade.
“But since we’ve really not done very well for pediatric RSV vaccines, nirsevimab is certainly something I’m looking forward to,” he said.
Fast-acting monoclonal antibodies
An advantage for monoclonal antibodies is that they start working almost immediately.
Children can get sick with RSV in the first few months of life so the speed of the monoclonal antibodies to begin protection is important, Dr. Esper said, adding that RSV “is the worst during the first year of life.”
The peak age for babies getting infected enough to require hospitalization is about 2 months, he said.
By 14 months, he said, kids’ immune systems and airways have matured enough “that it’s not nearly as bad.”
To get protection from a vaccine, he added, “usually takes 2-4 weeks from the time you get your shot to the time you see some benefit. With an antibody, you’re bypassing the processing that the body has to do, and it goes straight to ‘protection’ mode,” Dr. Esper said. “You get protected pretty much as soon as you get the antibody.”
A version of this article first appeared on Medscape.com.
For the first time in the fall of 2023, families will be offered season-long protection for infants and some children against respiratory syncytial virus (RSV).
The Food and Drug Administration in July approved a prevention called nirsevimab (Beyfortus, AstraZeneca/Sanofi) and it is expected to be widely rolled out in the coming weeks as the RSV season begins.
And monoclonal antibodies are often used for treatment rather than prevention.
Adding to potential confusion is the fact the Centers for Disease Control and Prevention has included nirsevimab in the Vaccines for Children program, which covers the costs for uninsured kids and makes it more accessible.
Nirsevimab is approved for infants (up to 8 months old) born during or entering their first RSV season, and in children up to 2 years of age who are still vulnerable to severe RSV through their second season.
It’s recommended that all infants get one injection in their first 8 months for prevention instead of the previous monthly shots used to help prevent kids at high risk from getting severe RSV.
If monoclonal antibodies can be used for preventing disease in infants, could they become a viable vaccine alternative for adults?
Specialists say no.
That’s partly because of the difference in body size. Although an injection is an option for a newborn, pediatricians suggest, it would take far too much of the treatment to work as a shot for adults.
Ruth Karron, MD, an expert in pediatric infectious diseases at Johns Hopkins Medicine, Baltimore, said that, while vaccines come in small amounts and activate immune cells, monoclonal antibodies are more like a drug, with the dose based on weight.
“You’d have to give it intravenously,” for larger doses, she explained, which has never been studied before and would also be very expensive. “It really couldn’t be an option for adults.”
What’s the difference between vaccines and antibodies?
Monoclonal antibodies are proteins made in a lab to mimic the immune system’s ability to fight pathogens such as viruses.
Dr. Karron explained that a wide variety of monoclonal antibodies have long been used to treat diseases such as cancers and autoimmune disease. In recent years, the antibodies have been used to treat COVID.
Monoclonal antibodies have also been used to treat RSV in children, but the effects don’t last long – they confer passive immunity and “when it’s gone, it’s gone,” Dr. Karron said.
That means kids at high risk for severe RSV have had to get monthly injections.
But with nirsevimab, the mutated antibodies stay in circulation longer so they can last 5 or 6 months, enough to cover the RSV season, Dr. Karron explained. “It’s highly, highly effective.”
Vaccines train the body
“The idea with vaccines is that you engage the individual’s immune system. You teach it to make antibodies,” Dr. Karron said. Conversely, “you give an antibody and it’s good for as long as the antibody lasts. It’s not teaching your body anything.”
Frank Esper, MD, a pediatric infectious disease specialist at Cleveland Clinic Children’s Hospital, said monoclonal antibody protection for RSV is particularly welcome. “We’ve been trying to make an RSV vaccine since the 1960s and have done nothing but fail miserably.”
“The best thing is always a vaccine,” Dr. Esper said, explaining that vaccines teach the body to make its own antibodies and confer long-term protection and are “probably more efficacious than anything that’s ever manmade.
“But since we’ve really not done very well for pediatric RSV vaccines, nirsevimab is certainly something I’m looking forward to,” he said.
Fast-acting monoclonal antibodies
An advantage for monoclonal antibodies is that they start working almost immediately.
Children can get sick with RSV in the first few months of life so the speed of the monoclonal antibodies to begin protection is important, Dr. Esper said, adding that RSV “is the worst during the first year of life.”
The peak age for babies getting infected enough to require hospitalization is about 2 months, he said.
By 14 months, he said, kids’ immune systems and airways have matured enough “that it’s not nearly as bad.”
To get protection from a vaccine, he added, “usually takes 2-4 weeks from the time you get your shot to the time you see some benefit. With an antibody, you’re bypassing the processing that the body has to do, and it goes straight to ‘protection’ mode,” Dr. Esper said. “You get protected pretty much as soon as you get the antibody.”
A version of this article first appeared on Medscape.com.
For the first time in the fall of 2023, families will be offered season-long protection for infants and some children against respiratory syncytial virus (RSV).
The Food and Drug Administration in July approved a prevention called nirsevimab (Beyfortus, AstraZeneca/Sanofi) and it is expected to be widely rolled out in the coming weeks as the RSV season begins.
And monoclonal antibodies are often used for treatment rather than prevention.
Adding to potential confusion is the fact the Centers for Disease Control and Prevention has included nirsevimab in the Vaccines for Children program, which covers the costs for uninsured kids and makes it more accessible.
Nirsevimab is approved for infants (up to 8 months old) born during or entering their first RSV season, and in children up to 2 years of age who are still vulnerable to severe RSV through their second season.
It’s recommended that all infants get one injection in their first 8 months for prevention instead of the previous monthly shots used to help prevent kids at high risk from getting severe RSV.
If monoclonal antibodies can be used for preventing disease in infants, could they become a viable vaccine alternative for adults?
Specialists say no.
That’s partly because of the difference in body size. Although an injection is an option for a newborn, pediatricians suggest, it would take far too much of the treatment to work as a shot for adults.
Ruth Karron, MD, an expert in pediatric infectious diseases at Johns Hopkins Medicine, Baltimore, said that, while vaccines come in small amounts and activate immune cells, monoclonal antibodies are more like a drug, with the dose based on weight.
“You’d have to give it intravenously,” for larger doses, she explained, which has never been studied before and would also be very expensive. “It really couldn’t be an option for adults.”
What’s the difference between vaccines and antibodies?
Monoclonal antibodies are proteins made in a lab to mimic the immune system’s ability to fight pathogens such as viruses.
Dr. Karron explained that a wide variety of monoclonal antibodies have long been used to treat diseases such as cancers and autoimmune disease. In recent years, the antibodies have been used to treat COVID.
Monoclonal antibodies have also been used to treat RSV in children, but the effects don’t last long – they confer passive immunity and “when it’s gone, it’s gone,” Dr. Karron said.
That means kids at high risk for severe RSV have had to get monthly injections.
But with nirsevimab, the mutated antibodies stay in circulation longer so they can last 5 or 6 months, enough to cover the RSV season, Dr. Karron explained. “It’s highly, highly effective.”
Vaccines train the body
“The idea with vaccines is that you engage the individual’s immune system. You teach it to make antibodies,” Dr. Karron said. Conversely, “you give an antibody and it’s good for as long as the antibody lasts. It’s not teaching your body anything.”
Frank Esper, MD, a pediatric infectious disease specialist at Cleveland Clinic Children’s Hospital, said monoclonal antibody protection for RSV is particularly welcome. “We’ve been trying to make an RSV vaccine since the 1960s and have done nothing but fail miserably.”
“The best thing is always a vaccine,” Dr. Esper said, explaining that vaccines teach the body to make its own antibodies and confer long-term protection and are “probably more efficacious than anything that’s ever manmade.
“But since we’ve really not done very well for pediatric RSV vaccines, nirsevimab is certainly something I’m looking forward to,” he said.
Fast-acting monoclonal antibodies
An advantage for monoclonal antibodies is that they start working almost immediately.
Children can get sick with RSV in the first few months of life so the speed of the monoclonal antibodies to begin protection is important, Dr. Esper said, adding that RSV “is the worst during the first year of life.”
The peak age for babies getting infected enough to require hospitalization is about 2 months, he said.
By 14 months, he said, kids’ immune systems and airways have matured enough “that it’s not nearly as bad.”
To get protection from a vaccine, he added, “usually takes 2-4 weeks from the time you get your shot to the time you see some benefit. With an antibody, you’re bypassing the processing that the body has to do, and it goes straight to ‘protection’ mode,” Dr. Esper said. “You get protected pretty much as soon as you get the antibody.”
A version of this article first appeared on Medscape.com.
Dupilumab gains off-label uses as clinicians turn to drug for more indications
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.