User login
Maternal COVID-19 vaccine curbs infant infection
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
FROM THE BMJ
IVF-conceived children show strong developmental performance
In vitro fertilization has been around long enough that researchers can now compare developmental and academic achievements between these children and peers at school age.
Amber Kennedy, MBBS, and colleagues did just that. They found little difference in these milestones between a total of 11,059 IVF-conceived children and 401,654 spontaneously conceived children in a new study.
“Parents considering IVF and health care professionals can be reassured that the school age developmental and educational outcomes of IVF-conceived children are equivalent to their peers,” said Dr. Kennedy, lead author and obstetrician and gynecologist at Mercy Hospital for Women at the University of Melbourne.
The findings were published online in PLOS Medicine.
“Overall, we know that children born through IVF are doing fine in terms of health, but also emotionally and cognitively. So I wasn’t surprised. I live in this world,” said Ariadna Cymet Lanski, PsyD, chair of the American Society for Reproductive Medicine Mental Health Professional Group, who was not affiliated with the study.
Some previous researchers linked conception via IVF to an increased risk of congenital abnormalities, autism spectrum disorder, developmental delay, and intellectual disability.
Asked why the current study did not find increased risks, Dr. Kennedy said, “Our population included a relatively recent birth cohort, which may explain some differences from previous studies as IVF practices have evolved over time.”
An estimated 8 million people worldwide have been conceived through IVF since the first birth in 1978, the researchers said. In Australia, this has grown from 2% of births in the year 2000 to now nearly 5% or 1 in 20 live births, Dr. Kennedy noted. “Consequently, it is important to understand the longer-term outcomes for this population of children.”
Along with senior author Anthea Lindquist, MBBS, Dr. Kennedy and colleagues studied 585,659 single births in Victoria, Australia, between 2005 and 2014. They did not include multiple births such as twins or triplets.
The investigators compared 4,697 children conceived via IVF and 168,503 others conceived spontaneously using a standard developmental measure, the Australian Early Developmental Census (AEDC). They also assessed 8,976 children in the IVF group and 333,335 other children on a standard educational measure, the National Assessment Program–Literacy and Numeracy (NAPLAN).
For example, the developmental census measures developmental vulnerability. Dr. Kennedy and colleagues found a 0.3% difference in favor of IVF-conceived children, which statistically was no different than zero.
Similarly, the researchers reported that IVF conception had essentially no effect on overall the literacy score, with an adjusted average difference of 0.03.
Dr. Lanski said the results should be reassuring for people considering IVF. “I can see the value of the study.” The findings “probably brings a lot of comfort ... if you want to build a family, and medically this is what’s recommended.”
Not all IVF techniques are the same, and the researchers want to take a deeper dive to evaluate any distinctions among them. For example, Dr. Kennedy said, “We plan to investigate the same school-aged outcomes after specific IVF-associated techniques.”
A version of this article first appeared on WebMD.com.
In vitro fertilization has been around long enough that researchers can now compare developmental and academic achievements between these children and peers at school age.
Amber Kennedy, MBBS, and colleagues did just that. They found little difference in these milestones between a total of 11,059 IVF-conceived children and 401,654 spontaneously conceived children in a new study.
“Parents considering IVF and health care professionals can be reassured that the school age developmental and educational outcomes of IVF-conceived children are equivalent to their peers,” said Dr. Kennedy, lead author and obstetrician and gynecologist at Mercy Hospital for Women at the University of Melbourne.
The findings were published online in PLOS Medicine.
“Overall, we know that children born through IVF are doing fine in terms of health, but also emotionally and cognitively. So I wasn’t surprised. I live in this world,” said Ariadna Cymet Lanski, PsyD, chair of the American Society for Reproductive Medicine Mental Health Professional Group, who was not affiliated with the study.
Some previous researchers linked conception via IVF to an increased risk of congenital abnormalities, autism spectrum disorder, developmental delay, and intellectual disability.
Asked why the current study did not find increased risks, Dr. Kennedy said, “Our population included a relatively recent birth cohort, which may explain some differences from previous studies as IVF practices have evolved over time.”
An estimated 8 million people worldwide have been conceived through IVF since the first birth in 1978, the researchers said. In Australia, this has grown from 2% of births in the year 2000 to now nearly 5% or 1 in 20 live births, Dr. Kennedy noted. “Consequently, it is important to understand the longer-term outcomes for this population of children.”
Along with senior author Anthea Lindquist, MBBS, Dr. Kennedy and colleagues studied 585,659 single births in Victoria, Australia, between 2005 and 2014. They did not include multiple births such as twins or triplets.
The investigators compared 4,697 children conceived via IVF and 168,503 others conceived spontaneously using a standard developmental measure, the Australian Early Developmental Census (AEDC). They also assessed 8,976 children in the IVF group and 333,335 other children on a standard educational measure, the National Assessment Program–Literacy and Numeracy (NAPLAN).
For example, the developmental census measures developmental vulnerability. Dr. Kennedy and colleagues found a 0.3% difference in favor of IVF-conceived children, which statistically was no different than zero.
Similarly, the researchers reported that IVF conception had essentially no effect on overall the literacy score, with an adjusted average difference of 0.03.
Dr. Lanski said the results should be reassuring for people considering IVF. “I can see the value of the study.” The findings “probably brings a lot of comfort ... if you want to build a family, and medically this is what’s recommended.”
Not all IVF techniques are the same, and the researchers want to take a deeper dive to evaluate any distinctions among them. For example, Dr. Kennedy said, “We plan to investigate the same school-aged outcomes after specific IVF-associated techniques.”
A version of this article first appeared on WebMD.com.
In vitro fertilization has been around long enough that researchers can now compare developmental and academic achievements between these children and peers at school age.
Amber Kennedy, MBBS, and colleagues did just that. They found little difference in these milestones between a total of 11,059 IVF-conceived children and 401,654 spontaneously conceived children in a new study.
“Parents considering IVF and health care professionals can be reassured that the school age developmental and educational outcomes of IVF-conceived children are equivalent to their peers,” said Dr. Kennedy, lead author and obstetrician and gynecologist at Mercy Hospital for Women at the University of Melbourne.
The findings were published online in PLOS Medicine.
“Overall, we know that children born through IVF are doing fine in terms of health, but also emotionally and cognitively. So I wasn’t surprised. I live in this world,” said Ariadna Cymet Lanski, PsyD, chair of the American Society for Reproductive Medicine Mental Health Professional Group, who was not affiliated with the study.
Some previous researchers linked conception via IVF to an increased risk of congenital abnormalities, autism spectrum disorder, developmental delay, and intellectual disability.
Asked why the current study did not find increased risks, Dr. Kennedy said, “Our population included a relatively recent birth cohort, which may explain some differences from previous studies as IVF practices have evolved over time.”
An estimated 8 million people worldwide have been conceived through IVF since the first birth in 1978, the researchers said. In Australia, this has grown from 2% of births in the year 2000 to now nearly 5% or 1 in 20 live births, Dr. Kennedy noted. “Consequently, it is important to understand the longer-term outcomes for this population of children.”
Along with senior author Anthea Lindquist, MBBS, Dr. Kennedy and colleagues studied 585,659 single births in Victoria, Australia, between 2005 and 2014. They did not include multiple births such as twins or triplets.
The investigators compared 4,697 children conceived via IVF and 168,503 others conceived spontaneously using a standard developmental measure, the Australian Early Developmental Census (AEDC). They also assessed 8,976 children in the IVF group and 333,335 other children on a standard educational measure, the National Assessment Program–Literacy and Numeracy (NAPLAN).
For example, the developmental census measures developmental vulnerability. Dr. Kennedy and colleagues found a 0.3% difference in favor of IVF-conceived children, which statistically was no different than zero.
Similarly, the researchers reported that IVF conception had essentially no effect on overall the literacy score, with an adjusted average difference of 0.03.
Dr. Lanski said the results should be reassuring for people considering IVF. “I can see the value of the study.” The findings “probably brings a lot of comfort ... if you want to build a family, and medically this is what’s recommended.”
Not all IVF techniques are the same, and the researchers want to take a deeper dive to evaluate any distinctions among them. For example, Dr. Kennedy said, “We plan to investigate the same school-aged outcomes after specific IVF-associated techniques.”
A version of this article first appeared on WebMD.com.
FROM PLOS MEDICINE
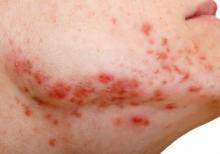
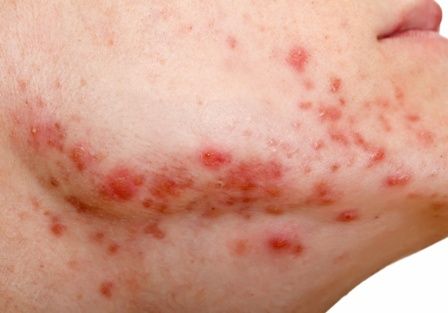
Study documents link between preadolescent acne and elevated BMI
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
FROM PEDIATRIC DERMATOLOGY
Can pediatricians’ offices be urgent care centers again?
If you live in a suburban or semirural community you have seen at least one urgent care center open up in the last decade. They now number nearly 12,000 nationwide and are growing in number at a 7% rate. Urgent care center patient volume surged during the pandemic and an industry trade group reports it has risen 60% since 2019 (Meyerson N. Why urgent care centers are popping up everywhere. CNN Business. 2023 Jan 28).
According to a report on the CNN Business website, this growth is the result of “convenience, gaps in primary care, high costs of emergency room visits, and increased investment by health systems and equity groups.” Initially, these centers were generally staffed by physicians (70% in 2009) but as of 2022 this number has fallen to 16%. While there are conflicting data to support the claim that urgent care centers are overprescribing, it is pretty clear that their presence in a community encourages fragmented care and weakens established provider-patient relationships. One study has shown that although urgent care centers can prevent a costly emergency room visit ($1,649/visit) this advantage is offset by urgent care cost of more than $6,000.
In the same CNN report, Susan Kressly MD, chair of the AAP’s Private Payer Advocacy Advisory Committee, said: “There’s a need to keep up with society’s demand for quick turnaround, on-demand services that can’t be supported by underfunded primary care.”
Her observation suggests that there is an accelerating demand for timely primary care services. From my perch here in semirural Maine, I don’t see an increasing or unreasonable demand for timeliness by patients and families. Two decades ago, the practice I was in offered evening and weekend morning office hours and call-in times when patientsor parents could speak directly to a physician. These avenues of accessibility have disappeared community wide.
Back in the 1990s “the medical home” was all the buzz. We were encouraged to be the first and primary place to go for a broad range of preventive and responsive care. One-stop shopping at its best. Now it’s “knock, knock ... is anybody home?” Not if it’s getting dark, or it’s the weekend, or you have a minor injury. “Please call the urgent care center.”
I will admit that our dedicated call-in times were unusual and probably not sustainable for most practices. But, most practices back then would see children with acute illness and minor scrapes and trauma on a same-day basis. We dressed burns, splinted joints, and closed minor lacerations. What has changed to create the void that urgent care centers see as an opportunity to make money?
One explanation is the difficulty in finding folks (both providers and support people) who are willing to work a schedule that includes evenings and weekends. One study predicts that there will be a shortfall of 55,000 primary care physicians in the next decade, regardless of their work-life balance preferences. Sometimes it is a lack of creativity and foresight in creating flexible booking schedules that include ample time for patient- and parent-friendly same-day appointments. Minor injuries and skin problems can usually be managed quickly and effectively by an experienced clinician. Unquestionably, one of the big changes has been the shift in the patient mix leaning more toward time-consuming mental health complaints, which make it more difficult to leave open same-day slots. Restoring pediatricians’ offices to their former role as urgent care centers will require training not just more primary care physicians but also mental health consultants and providers.
First, we must decide that we want to become a real medical home that answers to a knock with a receptive response at almost any hour. By failing to accept the challenge of seeing our patients in a timely manner for their minor problems we will continue to fragment their care and threaten to make our relationship with them increasingly irrelevant.
It will mean rethinking how we schedule ourselves and our offices. It may require taking a hard look at how we spend our professional time. For example are annual checkups a must for every child at every age? Are all follow-up visits equally important? Would a phone call be just as effective? Most of all it will require adopting a mindset that we want to be complete physicians for our patients.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
If you live in a suburban or semirural community you have seen at least one urgent care center open up in the last decade. They now number nearly 12,000 nationwide and are growing in number at a 7% rate. Urgent care center patient volume surged during the pandemic and an industry trade group reports it has risen 60% since 2019 (Meyerson N. Why urgent care centers are popping up everywhere. CNN Business. 2023 Jan 28).
According to a report on the CNN Business website, this growth is the result of “convenience, gaps in primary care, high costs of emergency room visits, and increased investment by health systems and equity groups.” Initially, these centers were generally staffed by physicians (70% in 2009) but as of 2022 this number has fallen to 16%. While there are conflicting data to support the claim that urgent care centers are overprescribing, it is pretty clear that their presence in a community encourages fragmented care and weakens established provider-patient relationships. One study has shown that although urgent care centers can prevent a costly emergency room visit ($1,649/visit) this advantage is offset by urgent care cost of more than $6,000.
In the same CNN report, Susan Kressly MD, chair of the AAP’s Private Payer Advocacy Advisory Committee, said: “There’s a need to keep up with society’s demand for quick turnaround, on-demand services that can’t be supported by underfunded primary care.”
Her observation suggests that there is an accelerating demand for timely primary care services. From my perch here in semirural Maine, I don’t see an increasing or unreasonable demand for timeliness by patients and families. Two decades ago, the practice I was in offered evening and weekend morning office hours and call-in times when patientsor parents could speak directly to a physician. These avenues of accessibility have disappeared community wide.
Back in the 1990s “the medical home” was all the buzz. We were encouraged to be the first and primary place to go for a broad range of preventive and responsive care. One-stop shopping at its best. Now it’s “knock, knock ... is anybody home?” Not if it’s getting dark, or it’s the weekend, or you have a minor injury. “Please call the urgent care center.”
I will admit that our dedicated call-in times were unusual and probably not sustainable for most practices. But, most practices back then would see children with acute illness and minor scrapes and trauma on a same-day basis. We dressed burns, splinted joints, and closed minor lacerations. What has changed to create the void that urgent care centers see as an opportunity to make money?
One explanation is the difficulty in finding folks (both providers and support people) who are willing to work a schedule that includes evenings and weekends. One study predicts that there will be a shortfall of 55,000 primary care physicians in the next decade, regardless of their work-life balance preferences. Sometimes it is a lack of creativity and foresight in creating flexible booking schedules that include ample time for patient- and parent-friendly same-day appointments. Minor injuries and skin problems can usually be managed quickly and effectively by an experienced clinician. Unquestionably, one of the big changes has been the shift in the patient mix leaning more toward time-consuming mental health complaints, which make it more difficult to leave open same-day slots. Restoring pediatricians’ offices to their former role as urgent care centers will require training not just more primary care physicians but also mental health consultants and providers.
First, we must decide that we want to become a real medical home that answers to a knock with a receptive response at almost any hour. By failing to accept the challenge of seeing our patients in a timely manner for their minor problems we will continue to fragment their care and threaten to make our relationship with them increasingly irrelevant.
It will mean rethinking how we schedule ourselves and our offices. It may require taking a hard look at how we spend our professional time. For example are annual checkups a must for every child at every age? Are all follow-up visits equally important? Would a phone call be just as effective? Most of all it will require adopting a mindset that we want to be complete physicians for our patients.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
If you live in a suburban or semirural community you have seen at least one urgent care center open up in the last decade. They now number nearly 12,000 nationwide and are growing in number at a 7% rate. Urgent care center patient volume surged during the pandemic and an industry trade group reports it has risen 60% since 2019 (Meyerson N. Why urgent care centers are popping up everywhere. CNN Business. 2023 Jan 28).
According to a report on the CNN Business website, this growth is the result of “convenience, gaps in primary care, high costs of emergency room visits, and increased investment by health systems and equity groups.” Initially, these centers were generally staffed by physicians (70% in 2009) but as of 2022 this number has fallen to 16%. While there are conflicting data to support the claim that urgent care centers are overprescribing, it is pretty clear that their presence in a community encourages fragmented care and weakens established provider-patient relationships. One study has shown that although urgent care centers can prevent a costly emergency room visit ($1,649/visit) this advantage is offset by urgent care cost of more than $6,000.
In the same CNN report, Susan Kressly MD, chair of the AAP’s Private Payer Advocacy Advisory Committee, said: “There’s a need to keep up with society’s demand for quick turnaround, on-demand services that can’t be supported by underfunded primary care.”
Her observation suggests that there is an accelerating demand for timely primary care services. From my perch here in semirural Maine, I don’t see an increasing or unreasonable demand for timeliness by patients and families. Two decades ago, the practice I was in offered evening and weekend morning office hours and call-in times when patientsor parents could speak directly to a physician. These avenues of accessibility have disappeared community wide.
Back in the 1990s “the medical home” was all the buzz. We were encouraged to be the first and primary place to go for a broad range of preventive and responsive care. One-stop shopping at its best. Now it’s “knock, knock ... is anybody home?” Not if it’s getting dark, or it’s the weekend, or you have a minor injury. “Please call the urgent care center.”
I will admit that our dedicated call-in times were unusual and probably not sustainable for most practices. But, most practices back then would see children with acute illness and minor scrapes and trauma on a same-day basis. We dressed burns, splinted joints, and closed minor lacerations. What has changed to create the void that urgent care centers see as an opportunity to make money?
One explanation is the difficulty in finding folks (both providers and support people) who are willing to work a schedule that includes evenings and weekends. One study predicts that there will be a shortfall of 55,000 primary care physicians in the next decade, regardless of their work-life balance preferences. Sometimes it is a lack of creativity and foresight in creating flexible booking schedules that include ample time for patient- and parent-friendly same-day appointments. Minor injuries and skin problems can usually be managed quickly and effectively by an experienced clinician. Unquestionably, one of the big changes has been the shift in the patient mix leaning more toward time-consuming mental health complaints, which make it more difficult to leave open same-day slots. Restoring pediatricians’ offices to their former role as urgent care centers will require training not just more primary care physicians but also mental health consultants and providers.
First, we must decide that we want to become a real medical home that answers to a knock with a receptive response at almost any hour. By failing to accept the challenge of seeing our patients in a timely manner for their minor problems we will continue to fragment their care and threaten to make our relationship with them increasingly irrelevant.
It will mean rethinking how we schedule ourselves and our offices. It may require taking a hard look at how we spend our professional time. For example are annual checkups a must for every child at every age? Are all follow-up visits equally important? Would a phone call be just as effective? Most of all it will require adopting a mindset that we want to be complete physicians for our patients.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Parents driving the ‘talk’ supports healthy sexual behaviors in GBQ teens and young adults
When it comes to sexual health education in the United States, one thing is abundantly clear: It’s a messy patchwork of programs, topics, and criteria. Only 29 states and the District of Columbia currently mandate sexual health education. Sixteen states have an abstinence-only curriculum, whereas 13 do not require that instruction be age-appropriate, inclusive, medically accurate, or evidence-based/informed. And this is just the tip of the iceberg, according to a 2022 report issued by the Sex Ed for Social Change organization.
Parents should take an inclusive approach to sex communication and create a safe space for discussing sex and sexual orientation, said almost all (96.7%) of male young adults who participated in a qualitative study. This would help reinforce acceptance and parents could possibly serve as a proxy for children who’ve not yet disclosed their sexual orientation. Yet, few parents are equipped or prepared to have these meaningful conversations with gay, bisexual, queer, or gender-diverse children, despite the fact that they are especially vulnerable to poor sexual health outcomes, bullying, abuse, and mental health challenges, as well as high-risk sexual behaviors.
“Parents are sexual socialization agents,” Dalmacio Dennis Flores, PhD, ACRN, assistant professor of nursing at the University of Pennsylvania, Philadelphia, told this news organization. “It’s through the information that they convey, the way that they normalize rituals and expectations, that they inform young people of all of societal expectations or roles they’ll be fulfilling in the future.”
Dr. Flores is lead author of a study published in the Journal of Adolescent Health. He and his colleagues collected perspectives on comprehensive, inclusive, and age-appropriate parent-child sex communications from 30 GBQ adolescent males aged 15-20 years who were already “out” to their parents. Participants were asked to sort through 28 preprinted note cards containing broad sexual health topics (for instance, human anatomy, dating, sexually transmitted infections) as well as topics theoretically specific to GBQ individuals (for example, anal sex), and were asked to add additional topics that they felt were missing. They were then directed to recommend topics along with ideal timing (that is, elementary, middle, or high school) for these conversations.
Study findings also underscored the importance of initiating comprehensive sexuality talks as early as elementary school age – namely to start preparing GBQ children for inevitable adversities that they were likely to encounter later in life, as well as to form building blocks for more mature, in-depth discussions during high school.
Importantly, these recommendations generally align with those aimed at heterosexual youth.
“When we refer to topics for elementary school, they are general parameters of what kids might be interested in or want to hear more about; it’s not planting a seed,” explained Dr. Flores.
Eva Goldfarb, PhD, LHD, MA, professor of public health at Montclair (N.J.) State University, agreed. “We always talk about (in sex education) to follow young people’s lead. If your child is asking you a question, they deserve a response,” said Dr. Goldfarb, who wasn’t involved in the study. “It doesn’t mean you have to give a detailed- level explanation but if they’re asking about it, it means that they are thinking about it. But it’s really important for all young people to know all of this information.”
Along those lines, participants deemed that fundamental issues about bodies (for example, human anatomy, reproduction), different sexual orientations, and an introduction to foundational issues (like privacy, peer or social pressure, sexual abuse) would help elementary-aged children to normalize discussions about sex, anatomy, and sexual orientation.
Middle school conversations were ideally more in-depth to reflect the time when young people are beginning to explore and accept their social and sexual identities. Topics of discussion might include types of sexual intercourse (anal, oral, and vaginal), health promotion strategies (abstinence, condoms, and contraception), possible adverse outcomes of condomless intercourse (HIV, STIs), considerations about engaging in sexual intercourse (including readiness, negotiating boundaries, virginity), and interpersonal safety (for instance, sexting, alcohol/drugs/chemsex, sexual coercion, and partner abuse/violence).
Finally, high school age recommendations focused on socio-relational topics (such as hook-up culture, technology/online dating, and multiple or concurrent sex partners), which are most relevant during a time when adolescents are most prone to experimentation and risk-taking.
Acknowledging that the study approach was novel, Dr. Flores noted that hearing about these topics from the youth perspective allowed parents to prepare. “Communication is better when it’s anticipated vs. reactive,” he said.
Last but not least, clinicians also have an important role in supporting these conversations.
“We’ve always looked at sex communication as a dyadic process, as a parent bestowing wisdom on a child who doesn’t have that knowledge yet. But it can be a triadic model,” said Dr. Flores. “Providers can encourage parents to ask if a child is dating or is familiar with ways to protect themselves or provide consent, and act as a resource exclusively to troubleshoot emergent issues.”
This study was funded by the National Institutes of Health. The study also received supplementary funding from the Surgeon General C. Everett Koop HIV/AIDS Research Award. Dr. Flores and Dr. Goldfarb report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
When it comes to sexual health education in the United States, one thing is abundantly clear: It’s a messy patchwork of programs, topics, and criteria. Only 29 states and the District of Columbia currently mandate sexual health education. Sixteen states have an abstinence-only curriculum, whereas 13 do not require that instruction be age-appropriate, inclusive, medically accurate, or evidence-based/informed. And this is just the tip of the iceberg, according to a 2022 report issued by the Sex Ed for Social Change organization.
Parents should take an inclusive approach to sex communication and create a safe space for discussing sex and sexual orientation, said almost all (96.7%) of male young adults who participated in a qualitative study. This would help reinforce acceptance and parents could possibly serve as a proxy for children who’ve not yet disclosed their sexual orientation. Yet, few parents are equipped or prepared to have these meaningful conversations with gay, bisexual, queer, or gender-diverse children, despite the fact that they are especially vulnerable to poor sexual health outcomes, bullying, abuse, and mental health challenges, as well as high-risk sexual behaviors.
“Parents are sexual socialization agents,” Dalmacio Dennis Flores, PhD, ACRN, assistant professor of nursing at the University of Pennsylvania, Philadelphia, told this news organization. “It’s through the information that they convey, the way that they normalize rituals and expectations, that they inform young people of all of societal expectations or roles they’ll be fulfilling in the future.”
Dr. Flores is lead author of a study published in the Journal of Adolescent Health. He and his colleagues collected perspectives on comprehensive, inclusive, and age-appropriate parent-child sex communications from 30 GBQ adolescent males aged 15-20 years who were already “out” to their parents. Participants were asked to sort through 28 preprinted note cards containing broad sexual health topics (for instance, human anatomy, dating, sexually transmitted infections) as well as topics theoretically specific to GBQ individuals (for example, anal sex), and were asked to add additional topics that they felt were missing. They were then directed to recommend topics along with ideal timing (that is, elementary, middle, or high school) for these conversations.
Study findings also underscored the importance of initiating comprehensive sexuality talks as early as elementary school age – namely to start preparing GBQ children for inevitable adversities that they were likely to encounter later in life, as well as to form building blocks for more mature, in-depth discussions during high school.
Importantly, these recommendations generally align with those aimed at heterosexual youth.
“When we refer to topics for elementary school, they are general parameters of what kids might be interested in or want to hear more about; it’s not planting a seed,” explained Dr. Flores.
Eva Goldfarb, PhD, LHD, MA, professor of public health at Montclair (N.J.) State University, agreed. “We always talk about (in sex education) to follow young people’s lead. If your child is asking you a question, they deserve a response,” said Dr. Goldfarb, who wasn’t involved in the study. “It doesn’t mean you have to give a detailed- level explanation but if they’re asking about it, it means that they are thinking about it. But it’s really important for all young people to know all of this information.”
Along those lines, participants deemed that fundamental issues about bodies (for example, human anatomy, reproduction), different sexual orientations, and an introduction to foundational issues (like privacy, peer or social pressure, sexual abuse) would help elementary-aged children to normalize discussions about sex, anatomy, and sexual orientation.
Middle school conversations were ideally more in-depth to reflect the time when young people are beginning to explore and accept their social and sexual identities. Topics of discussion might include types of sexual intercourse (anal, oral, and vaginal), health promotion strategies (abstinence, condoms, and contraception), possible adverse outcomes of condomless intercourse (HIV, STIs), considerations about engaging in sexual intercourse (including readiness, negotiating boundaries, virginity), and interpersonal safety (for instance, sexting, alcohol/drugs/chemsex, sexual coercion, and partner abuse/violence).
Finally, high school age recommendations focused on socio-relational topics (such as hook-up culture, technology/online dating, and multiple or concurrent sex partners), which are most relevant during a time when adolescents are most prone to experimentation and risk-taking.
Acknowledging that the study approach was novel, Dr. Flores noted that hearing about these topics from the youth perspective allowed parents to prepare. “Communication is better when it’s anticipated vs. reactive,” he said.
Last but not least, clinicians also have an important role in supporting these conversations.
“We’ve always looked at sex communication as a dyadic process, as a parent bestowing wisdom on a child who doesn’t have that knowledge yet. But it can be a triadic model,” said Dr. Flores. “Providers can encourage parents to ask if a child is dating or is familiar with ways to protect themselves or provide consent, and act as a resource exclusively to troubleshoot emergent issues.”
This study was funded by the National Institutes of Health. The study also received supplementary funding from the Surgeon General C. Everett Koop HIV/AIDS Research Award. Dr. Flores and Dr. Goldfarb report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
When it comes to sexual health education in the United States, one thing is abundantly clear: It’s a messy patchwork of programs, topics, and criteria. Only 29 states and the District of Columbia currently mandate sexual health education. Sixteen states have an abstinence-only curriculum, whereas 13 do not require that instruction be age-appropriate, inclusive, medically accurate, or evidence-based/informed. And this is just the tip of the iceberg, according to a 2022 report issued by the Sex Ed for Social Change organization.
Parents should take an inclusive approach to sex communication and create a safe space for discussing sex and sexual orientation, said almost all (96.7%) of male young adults who participated in a qualitative study. This would help reinforce acceptance and parents could possibly serve as a proxy for children who’ve not yet disclosed their sexual orientation. Yet, few parents are equipped or prepared to have these meaningful conversations with gay, bisexual, queer, or gender-diverse children, despite the fact that they are especially vulnerable to poor sexual health outcomes, bullying, abuse, and mental health challenges, as well as high-risk sexual behaviors.
“Parents are sexual socialization agents,” Dalmacio Dennis Flores, PhD, ACRN, assistant professor of nursing at the University of Pennsylvania, Philadelphia, told this news organization. “It’s through the information that they convey, the way that they normalize rituals and expectations, that they inform young people of all of societal expectations or roles they’ll be fulfilling in the future.”
Dr. Flores is lead author of a study published in the Journal of Adolescent Health. He and his colleagues collected perspectives on comprehensive, inclusive, and age-appropriate parent-child sex communications from 30 GBQ adolescent males aged 15-20 years who were already “out” to their parents. Participants were asked to sort through 28 preprinted note cards containing broad sexual health topics (for instance, human anatomy, dating, sexually transmitted infections) as well as topics theoretically specific to GBQ individuals (for example, anal sex), and were asked to add additional topics that they felt were missing. They were then directed to recommend topics along with ideal timing (that is, elementary, middle, or high school) for these conversations.
Study findings also underscored the importance of initiating comprehensive sexuality talks as early as elementary school age – namely to start preparing GBQ children for inevitable adversities that they were likely to encounter later in life, as well as to form building blocks for more mature, in-depth discussions during high school.
Importantly, these recommendations generally align with those aimed at heterosexual youth.
“When we refer to topics for elementary school, they are general parameters of what kids might be interested in or want to hear more about; it’s not planting a seed,” explained Dr. Flores.
Eva Goldfarb, PhD, LHD, MA, professor of public health at Montclair (N.J.) State University, agreed. “We always talk about (in sex education) to follow young people’s lead. If your child is asking you a question, they deserve a response,” said Dr. Goldfarb, who wasn’t involved in the study. “It doesn’t mean you have to give a detailed- level explanation but if they’re asking about it, it means that they are thinking about it. But it’s really important for all young people to know all of this information.”
Along those lines, participants deemed that fundamental issues about bodies (for example, human anatomy, reproduction), different sexual orientations, and an introduction to foundational issues (like privacy, peer or social pressure, sexual abuse) would help elementary-aged children to normalize discussions about sex, anatomy, and sexual orientation.
Middle school conversations were ideally more in-depth to reflect the time when young people are beginning to explore and accept their social and sexual identities. Topics of discussion might include types of sexual intercourse (anal, oral, and vaginal), health promotion strategies (abstinence, condoms, and contraception), possible adverse outcomes of condomless intercourse (HIV, STIs), considerations about engaging in sexual intercourse (including readiness, negotiating boundaries, virginity), and interpersonal safety (for instance, sexting, alcohol/drugs/chemsex, sexual coercion, and partner abuse/violence).
Finally, high school age recommendations focused on socio-relational topics (such as hook-up culture, technology/online dating, and multiple or concurrent sex partners), which are most relevant during a time when adolescents are most prone to experimentation and risk-taking.
Acknowledging that the study approach was novel, Dr. Flores noted that hearing about these topics from the youth perspective allowed parents to prepare. “Communication is better when it’s anticipated vs. reactive,” he said.
Last but not least, clinicians also have an important role in supporting these conversations.
“We’ve always looked at sex communication as a dyadic process, as a parent bestowing wisdom on a child who doesn’t have that knowledge yet. But it can be a triadic model,” said Dr. Flores. “Providers can encourage parents to ask if a child is dating or is familiar with ways to protect themselves or provide consent, and act as a resource exclusively to troubleshoot emergent issues.”
This study was funded by the National Institutes of Health. The study also received supplementary funding from the Surgeon General C. Everett Koop HIV/AIDS Research Award. Dr. Flores and Dr. Goldfarb report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A technicality could keep RSV shots from kids in need
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Large cohort study finds isotretinoin not associated with IBD
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Advice on antibiotics for kids during shortages
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Genetic testing in the PICU prompts meaningful changes in care
according to a new study presented at the Society of Critical Care Medicine’s 2023 Critical Care Congress.
“We have had a lot of success using genome sequencing to help not only with diagnosis, but also changes in management,” lead author Katherine Rodriguez, MD, a pediatric critical care fellow physician at Rady Children’s Hospital, San Diego, told this news organization.
However, data on the use of rapid whole genome sequencing (rWGS) in the pediatric intensive care unit (PICU) are limited, and data from multiple institutions are lacking, Dr. Rodriguez said. In the current study, data from multiple hospitals allowed the researchers to examine differences in management across institutions, she said.
Dr. Rodriguez, with principal investigator Nicole Coufal, MD, also of Rady Children’s, and colleagues conducted the study at three children’s hospitals from March 2019 to July 2022. The study population included 80 children whose origin of illness was uncertain. The patients underwent rWGS testing in the PICU or cardiac ICU setting. The patients ranged in age from 0 to 17 years; 64% were younger than 1 year, (mean age, 2.8 years); 56% were male, and 59% were White.
After rWGS testing, 65% of the children were positive for a genetic variant. The data prompted changes to care for 42% of these patients; 38% of the changes occurred during the patient’s PICU stay, including medication changes and procedures that were either avoided or completed.
The remaining 62% of the changes were subacute and affected management for the remainder of the child’s hospitalization and after discharge, Dr. Rodriguez explained in her presentation.
The average turnaround time for the testing was 10 days, which is important to an intensivist, who may have been hesitant to order tests because of the time involved, Dr. Rodriguez said. The current study shows that “we can get test results in a reasonable time to make meaningful changes in care,” she told this news organization.
Choosing which patients to test can be a challenge for clinicians, Dr. Rodriguez acknowledged. “We have gotten a sense of which patients are likely to have diagnostic or not diagnostic genomes, but it is also a gut feeling,” she said.
“If this child is your patient and you are concerned, if they seem sicker than expected, or have a concerning family history, then send the test,” she said. “It is becoming more affordable, and can come back quickly enough to guide treatment while the patient is still in the ICU.”
In the current study, the greatest diagnostic utility appeared in patients with cardiac symptoms, such as congenital heart disease, sudden cardiac arrest, or suspected channelopathy, Dr. Rodriguez said in her presentation.
Patients with suspected neurological disease had a 50% rate of molecular diagnosis. “Interestingly, 74% of patients with respiratory disease where an underlying genetic etiology was suspected received a molecular diagnosis,” although rWGS was not applied to general populations with RSV or other respiratory illnesses, she said.
In her presentation, Dr. Rodriguez shared examples of how genetic testing had a dramatic impact on patient survival. In one case, a 14-year-old girl presented in cardiac arrest and was found to have new-onset dilated cardiomyopathy. Whether the etiology was acquired or infectious and possibly reversible or genetic was unclear, she said.
“A diagnostic genome result within 48 hours indicated a genetic etiology,” she said. The patient was listed for heart transplant despite the incomplete infectious workup, and received a successful heart transplant 1 week after admission, Dr. Rodriguez said.
Guidelines for which PICU patients should undergo genetic testing do not yet exist, Dr. Rodriguez told this news organization. “We are trying to find some more meaningful parameters where we can say that a patient has a high pretest possibility of a genetic condition,” she said.
“Increasing availability of rWGS can significantly impact patient care and assist families in making difficult decisions during times of critical illness,” she said.
Insurance coverage and testing access are improving, said Dr. Rodriguez. Medicaid policies exist for neonates/infants in the ICU in several states, including Oregon, California, Michigan, Maryland, and Louisiana, she said. In some areas, hospitals may pay for testing for these children if insurance will not, she added.
Dr. Rodriguez and colleagues are continuing to enroll patients in a prospective study of the impact of rWGS, with the addition of a fourth study site and inclusion of family surveys. “We also will be looking at a secondary analysis of cost savings and benefits,” she said.
Ultimately, the current study should be empowering to physicians, “especially if they don’t have good access to geneticists,” Dr. Rodriguez said in an interview.
The study received no outside funding. Dr. Rodriguez reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study presented at the Society of Critical Care Medicine’s 2023 Critical Care Congress.
“We have had a lot of success using genome sequencing to help not only with diagnosis, but also changes in management,” lead author Katherine Rodriguez, MD, a pediatric critical care fellow physician at Rady Children’s Hospital, San Diego, told this news organization.
However, data on the use of rapid whole genome sequencing (rWGS) in the pediatric intensive care unit (PICU) are limited, and data from multiple institutions are lacking, Dr. Rodriguez said. In the current study, data from multiple hospitals allowed the researchers to examine differences in management across institutions, she said.
Dr. Rodriguez, with principal investigator Nicole Coufal, MD, also of Rady Children’s, and colleagues conducted the study at three children’s hospitals from March 2019 to July 2022. The study population included 80 children whose origin of illness was uncertain. The patients underwent rWGS testing in the PICU or cardiac ICU setting. The patients ranged in age from 0 to 17 years; 64% were younger than 1 year, (mean age, 2.8 years); 56% were male, and 59% were White.
After rWGS testing, 65% of the children were positive for a genetic variant. The data prompted changes to care for 42% of these patients; 38% of the changes occurred during the patient’s PICU stay, including medication changes and procedures that were either avoided or completed.
The remaining 62% of the changes were subacute and affected management for the remainder of the child’s hospitalization and after discharge, Dr. Rodriguez explained in her presentation.
The average turnaround time for the testing was 10 days, which is important to an intensivist, who may have been hesitant to order tests because of the time involved, Dr. Rodriguez said. The current study shows that “we can get test results in a reasonable time to make meaningful changes in care,” she told this news organization.
Choosing which patients to test can be a challenge for clinicians, Dr. Rodriguez acknowledged. “We have gotten a sense of which patients are likely to have diagnostic or not diagnostic genomes, but it is also a gut feeling,” she said.
“If this child is your patient and you are concerned, if they seem sicker than expected, or have a concerning family history, then send the test,” she said. “It is becoming more affordable, and can come back quickly enough to guide treatment while the patient is still in the ICU.”
In the current study, the greatest diagnostic utility appeared in patients with cardiac symptoms, such as congenital heart disease, sudden cardiac arrest, or suspected channelopathy, Dr. Rodriguez said in her presentation.
Patients with suspected neurological disease had a 50% rate of molecular diagnosis. “Interestingly, 74% of patients with respiratory disease where an underlying genetic etiology was suspected received a molecular diagnosis,” although rWGS was not applied to general populations with RSV or other respiratory illnesses, she said.
In her presentation, Dr. Rodriguez shared examples of how genetic testing had a dramatic impact on patient survival. In one case, a 14-year-old girl presented in cardiac arrest and was found to have new-onset dilated cardiomyopathy. Whether the etiology was acquired or infectious and possibly reversible or genetic was unclear, she said.
“A diagnostic genome result within 48 hours indicated a genetic etiology,” she said. The patient was listed for heart transplant despite the incomplete infectious workup, and received a successful heart transplant 1 week after admission, Dr. Rodriguez said.
Guidelines for which PICU patients should undergo genetic testing do not yet exist, Dr. Rodriguez told this news organization. “We are trying to find some more meaningful parameters where we can say that a patient has a high pretest possibility of a genetic condition,” she said.
“Increasing availability of rWGS can significantly impact patient care and assist families in making difficult decisions during times of critical illness,” she said.
Insurance coverage and testing access are improving, said Dr. Rodriguez. Medicaid policies exist for neonates/infants in the ICU in several states, including Oregon, California, Michigan, Maryland, and Louisiana, she said. In some areas, hospitals may pay for testing for these children if insurance will not, she added.
Dr. Rodriguez and colleagues are continuing to enroll patients in a prospective study of the impact of rWGS, with the addition of a fourth study site and inclusion of family surveys. “We also will be looking at a secondary analysis of cost savings and benefits,” she said.
Ultimately, the current study should be empowering to physicians, “especially if they don’t have good access to geneticists,” Dr. Rodriguez said in an interview.
The study received no outside funding. Dr. Rodriguez reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study presented at the Society of Critical Care Medicine’s 2023 Critical Care Congress.
“We have had a lot of success using genome sequencing to help not only with diagnosis, but also changes in management,” lead author Katherine Rodriguez, MD, a pediatric critical care fellow physician at Rady Children’s Hospital, San Diego, told this news organization.
However, data on the use of rapid whole genome sequencing (rWGS) in the pediatric intensive care unit (PICU) are limited, and data from multiple institutions are lacking, Dr. Rodriguez said. In the current study, data from multiple hospitals allowed the researchers to examine differences in management across institutions, she said.
Dr. Rodriguez, with principal investigator Nicole Coufal, MD, also of Rady Children’s, and colleagues conducted the study at three children’s hospitals from March 2019 to July 2022. The study population included 80 children whose origin of illness was uncertain. The patients underwent rWGS testing in the PICU or cardiac ICU setting. The patients ranged in age from 0 to 17 years; 64% were younger than 1 year, (mean age, 2.8 years); 56% were male, and 59% were White.
After rWGS testing, 65% of the children were positive for a genetic variant. The data prompted changes to care for 42% of these patients; 38% of the changes occurred during the patient’s PICU stay, including medication changes and procedures that were either avoided or completed.
The remaining 62% of the changes were subacute and affected management for the remainder of the child’s hospitalization and after discharge, Dr. Rodriguez explained in her presentation.
The average turnaround time for the testing was 10 days, which is important to an intensivist, who may have been hesitant to order tests because of the time involved, Dr. Rodriguez said. The current study shows that “we can get test results in a reasonable time to make meaningful changes in care,” she told this news organization.
Choosing which patients to test can be a challenge for clinicians, Dr. Rodriguez acknowledged. “We have gotten a sense of which patients are likely to have diagnostic or not diagnostic genomes, but it is also a gut feeling,” she said.
“If this child is your patient and you are concerned, if they seem sicker than expected, or have a concerning family history, then send the test,” she said. “It is becoming more affordable, and can come back quickly enough to guide treatment while the patient is still in the ICU.”
In the current study, the greatest diagnostic utility appeared in patients with cardiac symptoms, such as congenital heart disease, sudden cardiac arrest, or suspected channelopathy, Dr. Rodriguez said in her presentation.
Patients with suspected neurological disease had a 50% rate of molecular diagnosis. “Interestingly, 74% of patients with respiratory disease where an underlying genetic etiology was suspected received a molecular diagnosis,” although rWGS was not applied to general populations with RSV or other respiratory illnesses, she said.
In her presentation, Dr. Rodriguez shared examples of how genetic testing had a dramatic impact on patient survival. In one case, a 14-year-old girl presented in cardiac arrest and was found to have new-onset dilated cardiomyopathy. Whether the etiology was acquired or infectious and possibly reversible or genetic was unclear, she said.
“A diagnostic genome result within 48 hours indicated a genetic etiology,” she said. The patient was listed for heart transplant despite the incomplete infectious workup, and received a successful heart transplant 1 week after admission, Dr. Rodriguez said.
Guidelines for which PICU patients should undergo genetic testing do not yet exist, Dr. Rodriguez told this news organization. “We are trying to find some more meaningful parameters where we can say that a patient has a high pretest possibility of a genetic condition,” she said.
“Increasing availability of rWGS can significantly impact patient care and assist families in making difficult decisions during times of critical illness,” she said.
Insurance coverage and testing access are improving, said Dr. Rodriguez. Medicaid policies exist for neonates/infants in the ICU in several states, including Oregon, California, Michigan, Maryland, and Louisiana, she said. In some areas, hospitals may pay for testing for these children if insurance will not, she added.
Dr. Rodriguez and colleagues are continuing to enroll patients in a prospective study of the impact of rWGS, with the addition of a fourth study site and inclusion of family surveys. “We also will be looking at a secondary analysis of cost savings and benefits,” she said.
Ultimately, the current study should be empowering to physicians, “especially if they don’t have good access to geneticists,” Dr. Rodriguez said in an interview.
The study received no outside funding. Dr. Rodriguez reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCCM 2023
Brain scans show effect of poverty, stress on Black children
Childhood stress can change the brain negatively, according to a new study that says Black children are affected more because they experience more poverty and adversity.
“The researchers analyzed MRI scans to identify small differences in the volume of certain brain structures, and said these could accumulate as children age and play a role in the later development of mental health problems,” STAT News reported. “The finding, part of an emerging research field looking at how racism and other social factors may affect the physical architecture of the brain, may help explain longstanding racial disparities in the prevalence of psychiatric disorders such as PTSD.”
The study was published in The American Journal of Psychiatry.
Brain development is affected by “disparities faced by certain groups of people,” even among children as young as 9 years old, said Nathaniel Harnett, an assistant professor of psychiatry at Harvard Medical School, Boston, and the study’s senior author. “If we’re going to treat the world as colorblind, we’re not going to create mental health solutions that are effective for all people.”
The study used evidence from the Adolescent Brain Cognitive Development Study, which the National Institutes of Health established in 2015 to study the brains and experiences of thousands of American children through early adulthood.
Brain scans revealed that Black children had less gray matter in 11 of 14 brain areas that were examined. Disparities in 8 of the 14 brain areas were affected by childhood adversity, particularly poverty.
A version of this article first appeared on WebMD.com.
Childhood stress can change the brain negatively, according to a new study that says Black children are affected more because they experience more poverty and adversity.
“The researchers analyzed MRI scans to identify small differences in the volume of certain brain structures, and said these could accumulate as children age and play a role in the later development of mental health problems,” STAT News reported. “The finding, part of an emerging research field looking at how racism and other social factors may affect the physical architecture of the brain, may help explain longstanding racial disparities in the prevalence of psychiatric disorders such as PTSD.”
The study was published in The American Journal of Psychiatry.
Brain development is affected by “disparities faced by certain groups of people,” even among children as young as 9 years old, said Nathaniel Harnett, an assistant professor of psychiatry at Harvard Medical School, Boston, and the study’s senior author. “If we’re going to treat the world as colorblind, we’re not going to create mental health solutions that are effective for all people.”
The study used evidence from the Adolescent Brain Cognitive Development Study, which the National Institutes of Health established in 2015 to study the brains and experiences of thousands of American children through early adulthood.
Brain scans revealed that Black children had less gray matter in 11 of 14 brain areas that were examined. Disparities in 8 of the 14 brain areas were affected by childhood adversity, particularly poverty.
A version of this article first appeared on WebMD.com.
Childhood stress can change the brain negatively, according to a new study that says Black children are affected more because they experience more poverty and adversity.
“The researchers analyzed MRI scans to identify small differences in the volume of certain brain structures, and said these could accumulate as children age and play a role in the later development of mental health problems,” STAT News reported. “The finding, part of an emerging research field looking at how racism and other social factors may affect the physical architecture of the brain, may help explain longstanding racial disparities in the prevalence of psychiatric disorders such as PTSD.”
The study was published in The American Journal of Psychiatry.
Brain development is affected by “disparities faced by certain groups of people,” even among children as young as 9 years old, said Nathaniel Harnett, an assistant professor of psychiatry at Harvard Medical School, Boston, and the study’s senior author. “If we’re going to treat the world as colorblind, we’re not going to create mental health solutions that are effective for all people.”
The study used evidence from the Adolescent Brain Cognitive Development Study, which the National Institutes of Health established in 2015 to study the brains and experiences of thousands of American children through early adulthood.
Brain scans revealed that Black children had less gray matter in 11 of 14 brain areas that were examined. Disparities in 8 of the 14 brain areas were affected by childhood adversity, particularly poverty.
A version of this article first appeared on WebMD.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY