User login
Sexual function in transfeminine patients following gender-affirming vaginoplasty
For many patients, sexual function is an important component of a healthy quality of life.1 However, to many transgender individuals, their sexual organs are often a source of gender dysphoria, which can significantly inhibit sexual activity with their partners. Patients who seek gender-affirming surgery not only hope to have these feelings of dysphoria alleviated but also desire improvement in sexual function after surgery. While the medical and psychiatric criteria for patients seeking vaginoplasty procedures are well established by the World Professional Association for Transgender Health,2 there is little guidance surrounding the discourse surgeons should have regarding sexual function pre- and postsurgery.
Setting realistic expectations is one of the major challenges surgeons and patients alike face in preoperative and postoperative encounters. Patients not only are tasked with recovering from a major surgical procedure, but must also now learn their new anatomy, which includes learning how to urinate, maintain proper neovaginal hygiene, and experience sexual pleasure.
Given the permanence of these procedures and the possibility of loss of sexual function, the surgeon must ensure that patients truly comprehend the nature of the procedure and its complications. During the preoperative consultation, the surgeon must inquire about any desire for future fertility, discuss any history of pelvic radiation, epispadias, hypospadias, current erectile dysfunction, libido, comorbid medical conditions (such as diabetes or smoking), current sexual practices, and overall patient goals regarding their surgical outcome.
The vast majority of patients state they will experience a significant decrease in gender dysphoria with the removal of their current natal male genitalia.1 However, some patients have very specific preferences regarding the cosmetic appearance of vulvar structures. Others have more functional concerns about neovaginal depth and the ability to have receptive penetrative intercourse. It is important to note that not all transgender women have male partners. Furthermore, whether patients have male or female partners, some patients do not desire the ability to have penetrative intercourse and/or do not want to undergo the potential complications of a full-depth vaginoplasty. In these patients, offering a “shallow depth” vaginoplasty may be acceptable.
It is useful in the consultation to discuss a patient’s sexual partners and sexual practices in order to best determine the type of procedure that may be appropriate for a patient. In my practice, I emphasize that full-depth vaginoplasties require a lifelong commitment of dilation to maintain patency. Unlike cisgender women, patients must also douche to ensure appropriate vaginal hygiene. Regarding cosmetic preferences patients may have, it is essential to educate patients on the significant variation in the appearance of vulvar structures among both cisgender and transgender women.
During the surgical consultation, I review which structures from their natal genitalia are removed and which structures are utilized to create the neo–vulvar-vaginal anatomy. The testicles and spermatic cord are excised. The dorsal neurovascular bundle of the penile shaft and portion of the dorsal aspect of the glans penis are used to create the neoclitoris. A combination of penile shaft skin and scrotal skin is used to line the neovaginal canal. The erectile tissue of the penile shaft is also resected and the natal urethra is shortened and spatulated to create the urethral plate and urethral meatus. I also remind patients that the prostate remains intact during vaginoplasty procedures. Unless patients undergo the colonic interposition vaginoplasty and in some cases the peritoneal vaginoplasty, the neovaginal canal is not self-lubricating, nor will patients experience ejaculation after surgery. In the presurgical period, I often remind patients that the location of erogenous sensation after surgery will be altered and the method by which they self-stimulate will also be different. It is also essential to document whether patients can achieve satisfactory orgasms presurgically in order to determine adequate sexual function in the postoperative period.
It cannot be emphasized enough that the best predictor of unsatisfactory sexual function after genital gender-affirming surgery is poor sexual function prior to surgery.1,3
Retention of sexual function after gender-affirming genital surgery is common, with studies citing a range of 70%-90% of patients reporting their ability to regularly achieve an orgasm after surgery.1,4 In some cases, patients will report issues with sexual function after surgery despite having no prior history of sexual dysfunction. If patients present with complaints of postsurgical anorgasmia, the provider should rule out insufficient time for wound healing and resolution of surgery-site pain, and determine if there was an intraoperative injury to the neurovascular bundle or significant clitoral necrosis. A thorough genital exam should include a sensory examination of the neoclitoris and the introitus and neovaginal canal for signs of scarring, stenosis, loss of vaginal depth, or high-tone pelvic-floor dysfunction.
Unfortunately, if the neurovascular bundle is injured or if a patient experienced clitoral necrosis, the likelihood of a patient regaining sensation is decreased, although there are currently no studies examining the exact rates. It is also important to reassure patients that wound healing after surgery and relearning sexual function is not linear. I encourage patients to initially self-stimulate without a partner as they learn their new anatomy in order to remove any potential performance anxiety a partner could cause immediately after surgery. Similar to the approach to sexual dysfunction in cisgender patients, referral to a specialist in sexual health and/or pelvic floor physical therapy are useful adjuncts, depending on the findings from the physical exam and patient symptoms.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Garcia MM. Clin Plastic Surg. 2018;45:437-46.
2. Eli Coleman WB et al. “Standards of care for the health of transsexual, transgender, and gender non-conforming people” 7th version. World Professional Association for Transgender Health: 2012.
3. Garcia MM et al. Transl Androl Urol. 2014;3:156.
4. Ferrando CA, Bowers ML. “Genital gender confirmation surgery for patients assigned male at birth” In: Ferrando CA, ed. “Comprehensive care for the transgender patient” Philadelphia: Elsevier, 2020:82-92.
For many patients, sexual function is an important component of a healthy quality of life.1 However, to many transgender individuals, their sexual organs are often a source of gender dysphoria, which can significantly inhibit sexual activity with their partners. Patients who seek gender-affirming surgery not only hope to have these feelings of dysphoria alleviated but also desire improvement in sexual function after surgery. While the medical and psychiatric criteria for patients seeking vaginoplasty procedures are well established by the World Professional Association for Transgender Health,2 there is little guidance surrounding the discourse surgeons should have regarding sexual function pre- and postsurgery.
Setting realistic expectations is one of the major challenges surgeons and patients alike face in preoperative and postoperative encounters. Patients not only are tasked with recovering from a major surgical procedure, but must also now learn their new anatomy, which includes learning how to urinate, maintain proper neovaginal hygiene, and experience sexual pleasure.
Given the permanence of these procedures and the possibility of loss of sexual function, the surgeon must ensure that patients truly comprehend the nature of the procedure and its complications. During the preoperative consultation, the surgeon must inquire about any desire for future fertility, discuss any history of pelvic radiation, epispadias, hypospadias, current erectile dysfunction, libido, comorbid medical conditions (such as diabetes or smoking), current sexual practices, and overall patient goals regarding their surgical outcome.
The vast majority of patients state they will experience a significant decrease in gender dysphoria with the removal of their current natal male genitalia.1 However, some patients have very specific preferences regarding the cosmetic appearance of vulvar structures. Others have more functional concerns about neovaginal depth and the ability to have receptive penetrative intercourse. It is important to note that not all transgender women have male partners. Furthermore, whether patients have male or female partners, some patients do not desire the ability to have penetrative intercourse and/or do not want to undergo the potential complications of a full-depth vaginoplasty. In these patients, offering a “shallow depth” vaginoplasty may be acceptable.
It is useful in the consultation to discuss a patient’s sexual partners and sexual practices in order to best determine the type of procedure that may be appropriate for a patient. In my practice, I emphasize that full-depth vaginoplasties require a lifelong commitment of dilation to maintain patency. Unlike cisgender women, patients must also douche to ensure appropriate vaginal hygiene. Regarding cosmetic preferences patients may have, it is essential to educate patients on the significant variation in the appearance of vulvar structures among both cisgender and transgender women.
During the surgical consultation, I review which structures from their natal genitalia are removed and which structures are utilized to create the neo–vulvar-vaginal anatomy. The testicles and spermatic cord are excised. The dorsal neurovascular bundle of the penile shaft and portion of the dorsal aspect of the glans penis are used to create the neoclitoris. A combination of penile shaft skin and scrotal skin is used to line the neovaginal canal. The erectile tissue of the penile shaft is also resected and the natal urethra is shortened and spatulated to create the urethral plate and urethral meatus. I also remind patients that the prostate remains intact during vaginoplasty procedures. Unless patients undergo the colonic interposition vaginoplasty and in some cases the peritoneal vaginoplasty, the neovaginal canal is not self-lubricating, nor will patients experience ejaculation after surgery. In the presurgical period, I often remind patients that the location of erogenous sensation after surgery will be altered and the method by which they self-stimulate will also be different. It is also essential to document whether patients can achieve satisfactory orgasms presurgically in order to determine adequate sexual function in the postoperative period.
It cannot be emphasized enough that the best predictor of unsatisfactory sexual function after genital gender-affirming surgery is poor sexual function prior to surgery.1,3
Retention of sexual function after gender-affirming genital surgery is common, with studies citing a range of 70%-90% of patients reporting their ability to regularly achieve an orgasm after surgery.1,4 In some cases, patients will report issues with sexual function after surgery despite having no prior history of sexual dysfunction. If patients present with complaints of postsurgical anorgasmia, the provider should rule out insufficient time for wound healing and resolution of surgery-site pain, and determine if there was an intraoperative injury to the neurovascular bundle or significant clitoral necrosis. A thorough genital exam should include a sensory examination of the neoclitoris and the introitus and neovaginal canal for signs of scarring, stenosis, loss of vaginal depth, or high-tone pelvic-floor dysfunction.
Unfortunately, if the neurovascular bundle is injured or if a patient experienced clitoral necrosis, the likelihood of a patient regaining sensation is decreased, although there are currently no studies examining the exact rates. It is also important to reassure patients that wound healing after surgery and relearning sexual function is not linear. I encourage patients to initially self-stimulate without a partner as they learn their new anatomy in order to remove any potential performance anxiety a partner could cause immediately after surgery. Similar to the approach to sexual dysfunction in cisgender patients, referral to a specialist in sexual health and/or pelvic floor physical therapy are useful adjuncts, depending on the findings from the physical exam and patient symptoms.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Garcia MM. Clin Plastic Surg. 2018;45:437-46.
2. Eli Coleman WB et al. “Standards of care for the health of transsexual, transgender, and gender non-conforming people” 7th version. World Professional Association for Transgender Health: 2012.
3. Garcia MM et al. Transl Androl Urol. 2014;3:156.
4. Ferrando CA, Bowers ML. “Genital gender confirmation surgery for patients assigned male at birth” In: Ferrando CA, ed. “Comprehensive care for the transgender patient” Philadelphia: Elsevier, 2020:82-92.
For many patients, sexual function is an important component of a healthy quality of life.1 However, to many transgender individuals, their sexual organs are often a source of gender dysphoria, which can significantly inhibit sexual activity with their partners. Patients who seek gender-affirming surgery not only hope to have these feelings of dysphoria alleviated but also desire improvement in sexual function after surgery. While the medical and psychiatric criteria for patients seeking vaginoplasty procedures are well established by the World Professional Association for Transgender Health,2 there is little guidance surrounding the discourse surgeons should have regarding sexual function pre- and postsurgery.
Setting realistic expectations is one of the major challenges surgeons and patients alike face in preoperative and postoperative encounters. Patients not only are tasked with recovering from a major surgical procedure, but must also now learn their new anatomy, which includes learning how to urinate, maintain proper neovaginal hygiene, and experience sexual pleasure.
Given the permanence of these procedures and the possibility of loss of sexual function, the surgeon must ensure that patients truly comprehend the nature of the procedure and its complications. During the preoperative consultation, the surgeon must inquire about any desire for future fertility, discuss any history of pelvic radiation, epispadias, hypospadias, current erectile dysfunction, libido, comorbid medical conditions (such as diabetes or smoking), current sexual practices, and overall patient goals regarding their surgical outcome.
The vast majority of patients state they will experience a significant decrease in gender dysphoria with the removal of their current natal male genitalia.1 However, some patients have very specific preferences regarding the cosmetic appearance of vulvar structures. Others have more functional concerns about neovaginal depth and the ability to have receptive penetrative intercourse. It is important to note that not all transgender women have male partners. Furthermore, whether patients have male or female partners, some patients do not desire the ability to have penetrative intercourse and/or do not want to undergo the potential complications of a full-depth vaginoplasty. In these patients, offering a “shallow depth” vaginoplasty may be acceptable.
It is useful in the consultation to discuss a patient’s sexual partners and sexual practices in order to best determine the type of procedure that may be appropriate for a patient. In my practice, I emphasize that full-depth vaginoplasties require a lifelong commitment of dilation to maintain patency. Unlike cisgender women, patients must also douche to ensure appropriate vaginal hygiene. Regarding cosmetic preferences patients may have, it is essential to educate patients on the significant variation in the appearance of vulvar structures among both cisgender and transgender women.
During the surgical consultation, I review which structures from their natal genitalia are removed and which structures are utilized to create the neo–vulvar-vaginal anatomy. The testicles and spermatic cord are excised. The dorsal neurovascular bundle of the penile shaft and portion of the dorsal aspect of the glans penis are used to create the neoclitoris. A combination of penile shaft skin and scrotal skin is used to line the neovaginal canal. The erectile tissue of the penile shaft is also resected and the natal urethra is shortened and spatulated to create the urethral plate and urethral meatus. I also remind patients that the prostate remains intact during vaginoplasty procedures. Unless patients undergo the colonic interposition vaginoplasty and in some cases the peritoneal vaginoplasty, the neovaginal canal is not self-lubricating, nor will patients experience ejaculation after surgery. In the presurgical period, I often remind patients that the location of erogenous sensation after surgery will be altered and the method by which they self-stimulate will also be different. It is also essential to document whether patients can achieve satisfactory orgasms presurgically in order to determine adequate sexual function in the postoperative period.
It cannot be emphasized enough that the best predictor of unsatisfactory sexual function after genital gender-affirming surgery is poor sexual function prior to surgery.1,3
Retention of sexual function after gender-affirming genital surgery is common, with studies citing a range of 70%-90% of patients reporting their ability to regularly achieve an orgasm after surgery.1,4 In some cases, patients will report issues with sexual function after surgery despite having no prior history of sexual dysfunction. If patients present with complaints of postsurgical anorgasmia, the provider should rule out insufficient time for wound healing and resolution of surgery-site pain, and determine if there was an intraoperative injury to the neurovascular bundle or significant clitoral necrosis. A thorough genital exam should include a sensory examination of the neoclitoris and the introitus and neovaginal canal for signs of scarring, stenosis, loss of vaginal depth, or high-tone pelvic-floor dysfunction.
Unfortunately, if the neurovascular bundle is injured or if a patient experienced clitoral necrosis, the likelihood of a patient regaining sensation is decreased, although there are currently no studies examining the exact rates. It is also important to reassure patients that wound healing after surgery and relearning sexual function is not linear. I encourage patients to initially self-stimulate without a partner as they learn their new anatomy in order to remove any potential performance anxiety a partner could cause immediately after surgery. Similar to the approach to sexual dysfunction in cisgender patients, referral to a specialist in sexual health and/or pelvic floor physical therapy are useful adjuncts, depending on the findings from the physical exam and patient symptoms.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Garcia MM. Clin Plastic Surg. 2018;45:437-46.
2. Eli Coleman WB et al. “Standards of care for the health of transsexual, transgender, and gender non-conforming people” 7th version. World Professional Association for Transgender Health: 2012.
3. Garcia MM et al. Transl Androl Urol. 2014;3:156.
4. Ferrando CA, Bowers ML. “Genital gender confirmation surgery for patients assigned male at birth” In: Ferrando CA, ed. “Comprehensive care for the transgender patient” Philadelphia: Elsevier, 2020:82-92.
LGBTQ students would get new protections under Biden plan
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
Combo of excision, cryosurgery found to benefit keloid scar outcomes
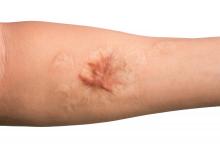
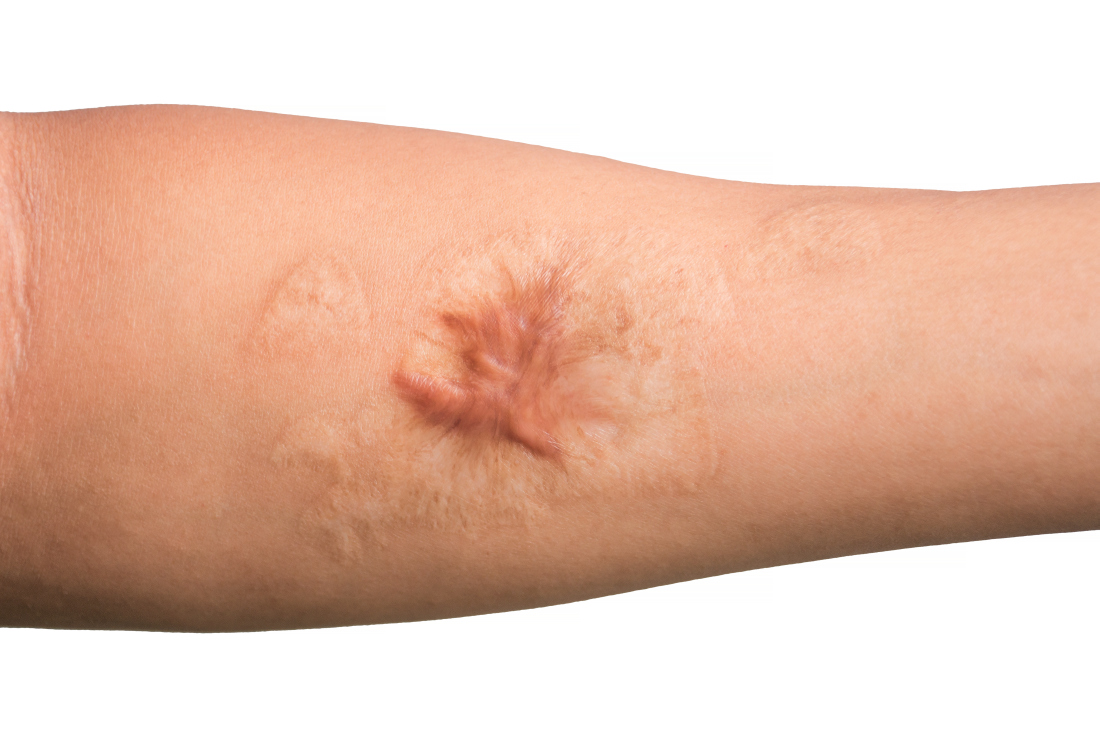
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
FROM JAMA DERMATOLOGY
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Breast anatomy and augmentation in transfeminine individuals
Augmentation mammaplasty, otherwise known as a breast augmentation, is one of the most common cosmetic procedures performed in cisgender females. Gynecologists routinely perform annual breast examinations and order screening mammography in cisgender women with breast implants. Similarly, there is an increasing number of transgender women seeking breast augmentation – with approximately 60%-70% of patients having desired or undergone the procedure.1 Consequently, these patients are instructed by their surgeons to follow up with gynecologists for annual examinations and screening. While there are many similarities in technique and procedure, there are nuances in patient demographics, anatomy, and surgical technique that obstetricians/gynecologists should be aware of when examining these patients or prior to referring them to a surgeon for augmentation.2
Many patients who are dissatisfied with breast size from hormone therapy alone will seek out augmentation mammaplasty. In patients taking estrogen for hormone therapy, breast growth will commence around 2-3 months and peak over 1-2 years.3 Unlike chest surgery for transmasculine individuals, it is recommended that transfeminine patients seeking breast augmentation wait a minimum of 12 months before to surgery to allow for maximum breast enlargement. As with breast growth in cisgender females, the extent of breast development is multifactorial and varies from individual to individual. Current literature does not suggest that estrogen type or dose affects the ultimate breast size; however, younger age, tissue sensitivity, and body weight may affect breast volume.3 Referral to a genetic counselor and preoperative imaging may be necessary if a patient has a history concerning for a genetic or familial predisposition to breast cancer.
Implant selection and placement is determined by a variety of factors. While the overall principles of augmentation mammaplasty are essentially the same, there are anatomic differences in transfeminine patients that surgeons must take into consideration at the time of the consultation and during the surgery itself. For example, the pectoralis major muscle is more defined, there is a longer sternal notch-to-nipple distance, the chest wall is broader and more barrel-shaped, and there is a shorter distance between the nipple and the inframammary crease.2-4 As a result of the broader chest wall, it is extremely difficult to achieve central cleavage even with larger implant selection. The surgeon must also ensure that the nipple and areola overlie the implant centrally. Medial placement of the implant will result in lateral displacement of the nipples, which can have an unsatisfactory cosmetic appearance.
Incision location can be axillary, inframammary, or even transareolar, although the latter is less common due to the smaller areolar size and larger implant choice.3 If the inframammary incision is used, it should be placed lower than the natural inframammary fold because the distance between the inferior areolar margin and inframammary fold is shorter and will expand after the implant is placed.4 While both saline and silicone implants are available, many surgeons (myself included), favor more form-stable silicone implants. Given the association between anaplastic large-cell lymphoma and textured implants, many surgeons also use nontextured, or smooth, cohesive gel silicone implants.5
Pocket selection of the implant itself can be subglandular – directly under the breast mound – or subpectoral – behind the pectoralis muscle. For patients with a pinch test of greater than 1.5 cm (outside of the area of the breast bud), good skin softening, and marked pectoralis hypertrophy, subglandular placement is reasonable.6 In thin patients with minimal breast development, subglandular placement can result in a “double-mound” appearance and can lead to visible implant edges on the periphery.6 Use of the subpectoral plane is more common and is associated with less implant visibility due to an increased amount of soft-tissue coverage and has lower rates of capsular contracture.4 However, due to the more robust pectoralis muscle in transfeminine patients, implant displacement can occur more frequently compared to subglandular placement. The surgeon and patient must have a thorough discussion about the location of the incision, implant material, and pocket placement along with the benefits and complications of the surgical plan.
Complications of augmentation mammaplasty are rare. However, when they occur it can include capsular contracture, breast asymmetry, hematoma formation, loss of nipple sensation, implant malposition, implant displacement below the inframammary crease, implant rupture, and need for revisional surgery.7 If an obstetrician/gynecologist observes any of the aforementioned findings in a postoperative patient, consultation and referral to a plastic surgeon is imperative.
Postoperative assessment and screening are mandatory in all patients who undergo breast augmentation. It is important for the gynecologist to note the incision placement, know the type of implant used (saline or silicone), and delineate where the implant was placed. If silicone implants are used, breast MRI is more sensitive in detecting implant rupture compared to mammography alone. Given the relatively poor epidemiologic data on breast cancer in transgender women, the Endocrine Society recommends that these patients follow the same screening guidelines as cisgender women.4,6
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Wierckx K et al. J Sex Med. 2014;11(5):1240-7.
2. Mehra G et al. Plast Reconstr Surg Glob Open 2021 Jan 21;9(1):e3362. doi: 10.1097/GOX.0000000000003362.
3. Schecter LS, Schechter RB. Breast and chest surgery for transgender patients. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier, 2020:73-81.
4. Colebunders B et al. Top surgery. In: Salgado CJ et al. ed. Gender Affirmation: Medical and Surgical Perspectives. New York, NY: Thieme, 2017:51-66.
5. De Boer M et al. Aesthet Surg J. 2017;37:NP83-NP87.
6. Coon D et al. Plast Reconstr Surg. 2020 Jun;145(6):1343-53.
7. Kanhai RC et al. Br J Plast Surg. 2000;53:209-11.
Augmentation mammaplasty, otherwise known as a breast augmentation, is one of the most common cosmetic procedures performed in cisgender females. Gynecologists routinely perform annual breast examinations and order screening mammography in cisgender women with breast implants. Similarly, there is an increasing number of transgender women seeking breast augmentation – with approximately 60%-70% of patients having desired or undergone the procedure.1 Consequently, these patients are instructed by their surgeons to follow up with gynecologists for annual examinations and screening. While there are many similarities in technique and procedure, there are nuances in patient demographics, anatomy, and surgical technique that obstetricians/gynecologists should be aware of when examining these patients or prior to referring them to a surgeon for augmentation.2
Many patients who are dissatisfied with breast size from hormone therapy alone will seek out augmentation mammaplasty. In patients taking estrogen for hormone therapy, breast growth will commence around 2-3 months and peak over 1-2 years.3 Unlike chest surgery for transmasculine individuals, it is recommended that transfeminine patients seeking breast augmentation wait a minimum of 12 months before to surgery to allow for maximum breast enlargement. As with breast growth in cisgender females, the extent of breast development is multifactorial and varies from individual to individual. Current literature does not suggest that estrogen type or dose affects the ultimate breast size; however, younger age, tissue sensitivity, and body weight may affect breast volume.3 Referral to a genetic counselor and preoperative imaging may be necessary if a patient has a history concerning for a genetic or familial predisposition to breast cancer.
Implant selection and placement is determined by a variety of factors. While the overall principles of augmentation mammaplasty are essentially the same, there are anatomic differences in transfeminine patients that surgeons must take into consideration at the time of the consultation and during the surgery itself. For example, the pectoralis major muscle is more defined, there is a longer sternal notch-to-nipple distance, the chest wall is broader and more barrel-shaped, and there is a shorter distance between the nipple and the inframammary crease.2-4 As a result of the broader chest wall, it is extremely difficult to achieve central cleavage even with larger implant selection. The surgeon must also ensure that the nipple and areola overlie the implant centrally. Medial placement of the implant will result in lateral displacement of the nipples, which can have an unsatisfactory cosmetic appearance.
Incision location can be axillary, inframammary, or even transareolar, although the latter is less common due to the smaller areolar size and larger implant choice.3 If the inframammary incision is used, it should be placed lower than the natural inframammary fold because the distance between the inferior areolar margin and inframammary fold is shorter and will expand after the implant is placed.4 While both saline and silicone implants are available, many surgeons (myself included), favor more form-stable silicone implants. Given the association between anaplastic large-cell lymphoma and textured implants, many surgeons also use nontextured, or smooth, cohesive gel silicone implants.5
Pocket selection of the implant itself can be subglandular – directly under the breast mound – or subpectoral – behind the pectoralis muscle. For patients with a pinch test of greater than 1.5 cm (outside of the area of the breast bud), good skin softening, and marked pectoralis hypertrophy, subglandular placement is reasonable.6 In thin patients with minimal breast development, subglandular placement can result in a “double-mound” appearance and can lead to visible implant edges on the periphery.6 Use of the subpectoral plane is more common and is associated with less implant visibility due to an increased amount of soft-tissue coverage and has lower rates of capsular contracture.4 However, due to the more robust pectoralis muscle in transfeminine patients, implant displacement can occur more frequently compared to subglandular placement. The surgeon and patient must have a thorough discussion about the location of the incision, implant material, and pocket placement along with the benefits and complications of the surgical plan.
Complications of augmentation mammaplasty are rare. However, when they occur it can include capsular contracture, breast asymmetry, hematoma formation, loss of nipple sensation, implant malposition, implant displacement below the inframammary crease, implant rupture, and need for revisional surgery.7 If an obstetrician/gynecologist observes any of the aforementioned findings in a postoperative patient, consultation and referral to a plastic surgeon is imperative.
Postoperative assessment and screening are mandatory in all patients who undergo breast augmentation. It is important for the gynecologist to note the incision placement, know the type of implant used (saline or silicone), and delineate where the implant was placed. If silicone implants are used, breast MRI is more sensitive in detecting implant rupture compared to mammography alone. Given the relatively poor epidemiologic data on breast cancer in transgender women, the Endocrine Society recommends that these patients follow the same screening guidelines as cisgender women.4,6
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Wierckx K et al. J Sex Med. 2014;11(5):1240-7.
2. Mehra G et al. Plast Reconstr Surg Glob Open 2021 Jan 21;9(1):e3362. doi: 10.1097/GOX.0000000000003362.
3. Schecter LS, Schechter RB. Breast and chest surgery for transgender patients. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier, 2020:73-81.
4. Colebunders B et al. Top surgery. In: Salgado CJ et al. ed. Gender Affirmation: Medical and Surgical Perspectives. New York, NY: Thieme, 2017:51-66.
5. De Boer M et al. Aesthet Surg J. 2017;37:NP83-NP87.
6. Coon D et al. Plast Reconstr Surg. 2020 Jun;145(6):1343-53.
7. Kanhai RC et al. Br J Plast Surg. 2000;53:209-11.
Augmentation mammaplasty, otherwise known as a breast augmentation, is one of the most common cosmetic procedures performed in cisgender females. Gynecologists routinely perform annual breast examinations and order screening mammography in cisgender women with breast implants. Similarly, there is an increasing number of transgender women seeking breast augmentation – with approximately 60%-70% of patients having desired or undergone the procedure.1 Consequently, these patients are instructed by their surgeons to follow up with gynecologists for annual examinations and screening. While there are many similarities in technique and procedure, there are nuances in patient demographics, anatomy, and surgical technique that obstetricians/gynecologists should be aware of when examining these patients or prior to referring them to a surgeon for augmentation.2
Many patients who are dissatisfied with breast size from hormone therapy alone will seek out augmentation mammaplasty. In patients taking estrogen for hormone therapy, breast growth will commence around 2-3 months and peak over 1-2 years.3 Unlike chest surgery for transmasculine individuals, it is recommended that transfeminine patients seeking breast augmentation wait a minimum of 12 months before to surgery to allow for maximum breast enlargement. As with breast growth in cisgender females, the extent of breast development is multifactorial and varies from individual to individual. Current literature does not suggest that estrogen type or dose affects the ultimate breast size; however, younger age, tissue sensitivity, and body weight may affect breast volume.3 Referral to a genetic counselor and preoperative imaging may be necessary if a patient has a history concerning for a genetic or familial predisposition to breast cancer.
Implant selection and placement is determined by a variety of factors. While the overall principles of augmentation mammaplasty are essentially the same, there are anatomic differences in transfeminine patients that surgeons must take into consideration at the time of the consultation and during the surgery itself. For example, the pectoralis major muscle is more defined, there is a longer sternal notch-to-nipple distance, the chest wall is broader and more barrel-shaped, and there is a shorter distance between the nipple and the inframammary crease.2-4 As a result of the broader chest wall, it is extremely difficult to achieve central cleavage even with larger implant selection. The surgeon must also ensure that the nipple and areola overlie the implant centrally. Medial placement of the implant will result in lateral displacement of the nipples, which can have an unsatisfactory cosmetic appearance.
Incision location can be axillary, inframammary, or even transareolar, although the latter is less common due to the smaller areolar size and larger implant choice.3 If the inframammary incision is used, it should be placed lower than the natural inframammary fold because the distance between the inferior areolar margin and inframammary fold is shorter and will expand after the implant is placed.4 While both saline and silicone implants are available, many surgeons (myself included), favor more form-stable silicone implants. Given the association between anaplastic large-cell lymphoma and textured implants, many surgeons also use nontextured, or smooth, cohesive gel silicone implants.5
Pocket selection of the implant itself can be subglandular – directly under the breast mound – or subpectoral – behind the pectoralis muscle. For patients with a pinch test of greater than 1.5 cm (outside of the area of the breast bud), good skin softening, and marked pectoralis hypertrophy, subglandular placement is reasonable.6 In thin patients with minimal breast development, subglandular placement can result in a “double-mound” appearance and can lead to visible implant edges on the periphery.6 Use of the subpectoral plane is more common and is associated with less implant visibility due to an increased amount of soft-tissue coverage and has lower rates of capsular contracture.4 However, due to the more robust pectoralis muscle in transfeminine patients, implant displacement can occur more frequently compared to subglandular placement. The surgeon and patient must have a thorough discussion about the location of the incision, implant material, and pocket placement along with the benefits and complications of the surgical plan.
Complications of augmentation mammaplasty are rare. However, when they occur it can include capsular contracture, breast asymmetry, hematoma formation, loss of nipple sensation, implant malposition, implant displacement below the inframammary crease, implant rupture, and need for revisional surgery.7 If an obstetrician/gynecologist observes any of the aforementioned findings in a postoperative patient, consultation and referral to a plastic surgeon is imperative.
Postoperative assessment and screening are mandatory in all patients who undergo breast augmentation. It is important for the gynecologist to note the incision placement, know the type of implant used (saline or silicone), and delineate where the implant was placed. If silicone implants are used, breast MRI is more sensitive in detecting implant rupture compared to mammography alone. Given the relatively poor epidemiologic data on breast cancer in transgender women, the Endocrine Society recommends that these patients follow the same screening guidelines as cisgender women.4,6
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Wierckx K et al. J Sex Med. 2014;11(5):1240-7.
2. Mehra G et al. Plast Reconstr Surg Glob Open 2021 Jan 21;9(1):e3362. doi: 10.1097/GOX.0000000000003362.
3. Schecter LS, Schechter RB. Breast and chest surgery for transgender patients. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier, 2020:73-81.
4. Colebunders B et al. Top surgery. In: Salgado CJ et al. ed. Gender Affirmation: Medical and Surgical Perspectives. New York, NY: Thieme, 2017:51-66.
5. De Boer M et al. Aesthet Surg J. 2017;37:NP83-NP87.
6. Coon D et al. Plast Reconstr Surg. 2020 Jun;145(6):1343-53.
7. Kanhai RC et al. Br J Plast Surg. 2000;53:209-11.
Surgeons successfully reattach testis after wrong-site surgery
NEW YORK (Reuters) – After doctors removed the wrong testis from a young man, the search was on for a surgeon who might be willing to try to replant it.
A new case report details the experience of a 25-year-old patient who had developed testicular pain and a palpable mass in his right testis; he went to a local hospital for a radical orchiectomy only to have the surgical team remove the left – wrong – testis.
Once the team recognized their error, they began searching for a center with microsurgical capacity to replant the testis.
“The take-home message is that microsurgery can be used to reattach an organ, in the case of a wrong-site surgery,” lead author Dr. Fatma Tuncer, a microsurgery fellow at the Cleveland Clinic, in Ohio, at the time of the surgery, told Reuters Health by email. She is now an assistant professor of plastic surgery at the University of Utah.
“The vast majority of surgeries, including urologic procedures will never have such an event, but there are helpful groups of physicians that are available to reduce the morbidity of such an event,” said coauthor Dr. Brian Gastman, a professor of surgery at the Case Western School of Medicine and a surgeon at the Cleveland Clinic.
“We were, I believe, the third one contacted, each one causing a greater time of ischemia,” Dr. Gastman told Reuters Health by email. “I accepted the patient and in doing so had the buy-in of my urology and anesthesia colleagues.”
Once Dr. Gastman and his team agreed to take on the task, the patient, and his testis, were flown to Cleveland. Once the patient arrived, he was counseled on the risks and benefits of the surgery. After agreeing to the surgery, the patient was taken to the OR immediately by the plastic surgery and urology teams.
Prior to initiating anesthesia, the testicle was examined and the urology team performed testicular sperm extraction as the patient did not have any biological children. The sperm were transported to a CLIA-certified andrology lab and were cryopreserved.
Next, the team examined the testis and spermatic cord under the microscope. The team identified the testicular artery, veins and vas deferens and marked them with prolene sutures. They next placed the testis in a moist gauze over ice until the recipient vessels were prepared.
After the team reconnected vessels, they observed strong arterial and venous Doppler flow on both testicular vessels and the testis itself. Five days after the replantation surgery, the team performed a radical orchiectomy on the correct side.
Dr. Gastman isn’t sure how well the testis will perform over time. “I cannot speak too much on this as it is ongoing,” he said. “But he will likely need some level of hormonal supplementation. I can state that the testis is alive and palpable.”
This is a “very interesting paper,” said Dr. Miroslav Djordjevic, a professor of urology at the Icahn School of Medicine at Mount Sinai, New York. “Congratulations to colleagues for a great idea for solving this wrong-site surgery with very precise microsurgical technique and new insight in the fight to save the organs.”
Still, Dr. Djordjevic told Reuters Health by email, “postoperatively, the authors confirmed there was not complete testicular function based on testosterone levels and hypotrophy of the reimplanted testis. The main reason is the time between removal and reimplanation. Based on experiences with testicular torsion, four to six hours is the maximum that will offer restoration of volume and function. Here, a longer period (10 hours) resulted in poor outcomes.”
“Our experience with testicular implantation in monozygotic twins showed great success (Belgrade University, Serbia, December 2019, personal report) because the cold ischemia was only one hour,” Dr. Djordjevic said.
Reuters Health Information © 2022
NEW YORK (Reuters) – After doctors removed the wrong testis from a young man, the search was on for a surgeon who might be willing to try to replant it.
A new case report details the experience of a 25-year-old patient who had developed testicular pain and a palpable mass in his right testis; he went to a local hospital for a radical orchiectomy only to have the surgical team remove the left – wrong – testis.
Once the team recognized their error, they began searching for a center with microsurgical capacity to replant the testis.
“The take-home message is that microsurgery can be used to reattach an organ, in the case of a wrong-site surgery,” lead author Dr. Fatma Tuncer, a microsurgery fellow at the Cleveland Clinic, in Ohio, at the time of the surgery, told Reuters Health by email. She is now an assistant professor of plastic surgery at the University of Utah.
“The vast majority of surgeries, including urologic procedures will never have such an event, but there are helpful groups of physicians that are available to reduce the morbidity of such an event,” said coauthor Dr. Brian Gastman, a professor of surgery at the Case Western School of Medicine and a surgeon at the Cleveland Clinic.
“We were, I believe, the third one contacted, each one causing a greater time of ischemia,” Dr. Gastman told Reuters Health by email. “I accepted the patient and in doing so had the buy-in of my urology and anesthesia colleagues.”
Once Dr. Gastman and his team agreed to take on the task, the patient, and his testis, were flown to Cleveland. Once the patient arrived, he was counseled on the risks and benefits of the surgery. After agreeing to the surgery, the patient was taken to the OR immediately by the plastic surgery and urology teams.
Prior to initiating anesthesia, the testicle was examined and the urology team performed testicular sperm extraction as the patient did not have any biological children. The sperm were transported to a CLIA-certified andrology lab and were cryopreserved.
Next, the team examined the testis and spermatic cord under the microscope. The team identified the testicular artery, veins and vas deferens and marked them with prolene sutures. They next placed the testis in a moist gauze over ice until the recipient vessels were prepared.
After the team reconnected vessels, they observed strong arterial and venous Doppler flow on both testicular vessels and the testis itself. Five days after the replantation surgery, the team performed a radical orchiectomy on the correct side.
Dr. Gastman isn’t sure how well the testis will perform over time. “I cannot speak too much on this as it is ongoing,” he said. “But he will likely need some level of hormonal supplementation. I can state that the testis is alive and palpable.”
This is a “very interesting paper,” said Dr. Miroslav Djordjevic, a professor of urology at the Icahn School of Medicine at Mount Sinai, New York. “Congratulations to colleagues for a great idea for solving this wrong-site surgery with very precise microsurgical technique and new insight in the fight to save the organs.”
Still, Dr. Djordjevic told Reuters Health by email, “postoperatively, the authors confirmed there was not complete testicular function based on testosterone levels and hypotrophy of the reimplanted testis. The main reason is the time between removal and reimplanation. Based on experiences with testicular torsion, four to six hours is the maximum that will offer restoration of volume and function. Here, a longer period (10 hours) resulted in poor outcomes.”
“Our experience with testicular implantation in monozygotic twins showed great success (Belgrade University, Serbia, December 2019, personal report) because the cold ischemia was only one hour,” Dr. Djordjevic said.
Reuters Health Information © 2022
NEW YORK (Reuters) – After doctors removed the wrong testis from a young man, the search was on for a surgeon who might be willing to try to replant it.
A new case report details the experience of a 25-year-old patient who had developed testicular pain and a palpable mass in his right testis; he went to a local hospital for a radical orchiectomy only to have the surgical team remove the left – wrong – testis.
Once the team recognized their error, they began searching for a center with microsurgical capacity to replant the testis.
“The take-home message is that microsurgery can be used to reattach an organ, in the case of a wrong-site surgery,” lead author Dr. Fatma Tuncer, a microsurgery fellow at the Cleveland Clinic, in Ohio, at the time of the surgery, told Reuters Health by email. She is now an assistant professor of plastic surgery at the University of Utah.
“The vast majority of surgeries, including urologic procedures will never have such an event, but there are helpful groups of physicians that are available to reduce the morbidity of such an event,” said coauthor Dr. Brian Gastman, a professor of surgery at the Case Western School of Medicine and a surgeon at the Cleveland Clinic.
“We were, I believe, the third one contacted, each one causing a greater time of ischemia,” Dr. Gastman told Reuters Health by email. “I accepted the patient and in doing so had the buy-in of my urology and anesthesia colleagues.”
Once Dr. Gastman and his team agreed to take on the task, the patient, and his testis, were flown to Cleveland. Once the patient arrived, he was counseled on the risks and benefits of the surgery. After agreeing to the surgery, the patient was taken to the OR immediately by the plastic surgery and urology teams.
Prior to initiating anesthesia, the testicle was examined and the urology team performed testicular sperm extraction as the patient did not have any biological children. The sperm were transported to a CLIA-certified andrology lab and were cryopreserved.
Next, the team examined the testis and spermatic cord under the microscope. The team identified the testicular artery, veins and vas deferens and marked them with prolene sutures. They next placed the testis in a moist gauze over ice until the recipient vessels were prepared.
After the team reconnected vessels, they observed strong arterial and venous Doppler flow on both testicular vessels and the testis itself. Five days after the replantation surgery, the team performed a radical orchiectomy on the correct side.
Dr. Gastman isn’t sure how well the testis will perform over time. “I cannot speak too much on this as it is ongoing,” he said. “But he will likely need some level of hormonal supplementation. I can state that the testis is alive and palpable.”
This is a “very interesting paper,” said Dr. Miroslav Djordjevic, a professor of urology at the Icahn School of Medicine at Mount Sinai, New York. “Congratulations to colleagues for a great idea for solving this wrong-site surgery with very precise microsurgical technique and new insight in the fight to save the organs.”
Still, Dr. Djordjevic told Reuters Health by email, “postoperatively, the authors confirmed there was not complete testicular function based on testosterone levels and hypotrophy of the reimplanted testis. The main reason is the time between removal and reimplanation. Based on experiences with testicular torsion, four to six hours is the maximum that will offer restoration of volume and function. Here, a longer period (10 hours) resulted in poor outcomes.”
“Our experience with testicular implantation in monozygotic twins showed great success (Belgrade University, Serbia, December 2019, personal report) because the cold ischemia was only one hour,” Dr. Djordjevic said.
Reuters Health Information © 2022
Detransition, baby: Examining factors leading to ‘detransitioning’ and regret in the transgender community
Over the holiday season I had the pleasure of finally reading the national bestseller, Detransition, Baby. On the surface, the story depicts the complex relationships between Reese, a transgender woman who strongly desires a family, her ex-wife, Ames – a transgender woman who detransitioned to live as a cisgender man – and Ames’ cisgender female partner, who is unexpectedly pregnant with his child. The story delves much deeper than the relationships between these characters, as it exceptionally articulates many of the emotional intricacies of the transgender experience and addresses one of the most taboo topics in the transgender community – detransitioning and regret.
The terms “transition” and “detransition” have fallen out of favor in the vernacular of the transgender population as they incorrectly imply that gender identity is contingent upon gender-affirmation processes.1,2 More importantly, the terms “detransition” and regret are not synonymous. Conflating these terms has undermined the intrinsic nature of gender identity, which has resulted in political and legal consequences seeking to limit or outright ban care for transgender patients.
As a gender-affirming surgeon, one of the most common questions I get asked is the rate of regret patients have after their surgeries. While I have no issue answering the question when it is presented, I do not hesitate to point out some of the problematic subtext inherent in such inquiries. Within the line of questioning, many often comment, “It’s so permanent,” “I can’t believe people can do this to their bodies,” or “How sure are patients before undergoing these surgeries?” While these comments and queries can be downright offensive, they seem to stem from the difficulty people have comprehending gender dysphoria and the painstaking steps people take to affirm their identity. The implication of these comments also reveals a more deep-seated issue – general distrust of individual bodily autonomy, personal identity, and choice.
For the obstetrician-gynecologist, understanding the concept of autonomous, patient-centered decision-making should be second nature, as we face a similar line of interrogation when discussing abortion, contraception, and pregnancy. No other field faces this level of scrutiny when it comes to defending a patient’s bodily autonomy. For example, given the history of reproductive injustice with tubal ligation procedures, the American College of Obstetricians and Gynecologists has issued clear guidelines regarding counseling of women while acknowledging the tenuous history of these procedures with minority subgroups. According to their committee opinion, ethical counseling for such a permanent procedure involves understanding the content of information presented to the patient, how that information is conveyed, and self-reflection on the part of the provider.3 The approach to counseling and understanding gender-affirming care is no different.
I want to be clear that regret after gender-affirming surgery is rare, occurring in 0%-3.8% of patients.4-6 In a separate study, 91% of patients expressed significant improvement in quality of life after surgery.7 However, what is disheartening about patients who experience surgical regret is that it originates from continued difficulty from the transition process itself and ongoing discrimination – even though the patient’s physical characteristics match their gender identity.4-6 Similarly, in another survey which examined 17,151 participants who had pursued gender affirmation (broadly defined), approximately 2,242 (13.1%) reported a history of detransition.2 Among these adults, the vast majority (82.5%), cited external factors such as school harassment, sexual violence, family pressure, and social stigma as reasons for detransitioning.2 Other associated factors included male sex assigned at birth, nonbinary gender identity, bisexual orientation, and having an unsupportive family.2
When Ames is explaining his “detransition” to his cisfemale partner, he states: “I got sick of living as trans …[sic]… I am trans, but I don’t need to do trans.”8 While there is still more research needed to further understand detransitioning and surgical regret, these few studies demonstrate a heart-breaking reality – in many aspects of our society it is still extremely difficult to live as a transgender person.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She did not report any disclosures.
References
1. National LGBTQIA+ Health Education Center, A program of the Fenway Institute: LGBTQIA+ glossary of terms for health care teams. 2020. Available at www.lgbtqiahealtheducation.org/wp-content/uploads/2020/10/Glossary-2020.08.30.pdf. Accessed Dec. 30, 2021.
2. Turban JL et al. LGBT Health 2021;8(4):273-80.
3. Sterilization of women: Ethical issues and considerations. Committee Opinion No. 695. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;129:e109-16.
4. Ruppin U, Pfafflin F. Arch Sex Behav. 2015;44:1321-9.
5. Lawrence AA. Arch Sex Behav. 2003;32:299-315.
6. Landen M et al. Acta Psychiatr Scand. 1998;97:284-9.
7. Papdopulos NA et al. J Sex Med. 2017;14(5):721-30.
8. Peters T. Detransition, Baby. New York: Penguin Random House, 2021.
Over the holiday season I had the pleasure of finally reading the national bestseller, Detransition, Baby. On the surface, the story depicts the complex relationships between Reese, a transgender woman who strongly desires a family, her ex-wife, Ames – a transgender woman who detransitioned to live as a cisgender man – and Ames’ cisgender female partner, who is unexpectedly pregnant with his child. The story delves much deeper than the relationships between these characters, as it exceptionally articulates many of the emotional intricacies of the transgender experience and addresses one of the most taboo topics in the transgender community – detransitioning and regret.
The terms “transition” and “detransition” have fallen out of favor in the vernacular of the transgender population as they incorrectly imply that gender identity is contingent upon gender-affirmation processes.1,2 More importantly, the terms “detransition” and regret are not synonymous. Conflating these terms has undermined the intrinsic nature of gender identity, which has resulted in political and legal consequences seeking to limit or outright ban care for transgender patients.
As a gender-affirming surgeon, one of the most common questions I get asked is the rate of regret patients have after their surgeries. While I have no issue answering the question when it is presented, I do not hesitate to point out some of the problematic subtext inherent in such inquiries. Within the line of questioning, many often comment, “It’s so permanent,” “I can’t believe people can do this to their bodies,” or “How sure are patients before undergoing these surgeries?” While these comments and queries can be downright offensive, they seem to stem from the difficulty people have comprehending gender dysphoria and the painstaking steps people take to affirm their identity. The implication of these comments also reveals a more deep-seated issue – general distrust of individual bodily autonomy, personal identity, and choice.
For the obstetrician-gynecologist, understanding the concept of autonomous, patient-centered decision-making should be second nature, as we face a similar line of interrogation when discussing abortion, contraception, and pregnancy. No other field faces this level of scrutiny when it comes to defending a patient’s bodily autonomy. For example, given the history of reproductive injustice with tubal ligation procedures, the American College of Obstetricians and Gynecologists has issued clear guidelines regarding counseling of women while acknowledging the tenuous history of these procedures with minority subgroups. According to their committee opinion, ethical counseling for such a permanent procedure involves understanding the content of information presented to the patient, how that information is conveyed, and self-reflection on the part of the provider.3 The approach to counseling and understanding gender-affirming care is no different.
I want to be clear that regret after gender-affirming surgery is rare, occurring in 0%-3.8% of patients.4-6 In a separate study, 91% of patients expressed significant improvement in quality of life after surgery.7 However, what is disheartening about patients who experience surgical regret is that it originates from continued difficulty from the transition process itself and ongoing discrimination – even though the patient’s physical characteristics match their gender identity.4-6 Similarly, in another survey which examined 17,151 participants who had pursued gender affirmation (broadly defined), approximately 2,242 (13.1%) reported a history of detransition.2 Among these adults, the vast majority (82.5%), cited external factors such as school harassment, sexual violence, family pressure, and social stigma as reasons for detransitioning.2 Other associated factors included male sex assigned at birth, nonbinary gender identity, bisexual orientation, and having an unsupportive family.2
When Ames is explaining his “detransition” to his cisfemale partner, he states: “I got sick of living as trans …[sic]… I am trans, but I don’t need to do trans.”8 While there is still more research needed to further understand detransitioning and surgical regret, these few studies demonstrate a heart-breaking reality – in many aspects of our society it is still extremely difficult to live as a transgender person.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She did not report any disclosures.
References
1. National LGBTQIA+ Health Education Center, A program of the Fenway Institute: LGBTQIA+ glossary of terms for health care teams. 2020. Available at www.lgbtqiahealtheducation.org/wp-content/uploads/2020/10/Glossary-2020.08.30.pdf. Accessed Dec. 30, 2021.
2. Turban JL et al. LGBT Health 2021;8(4):273-80.
3. Sterilization of women: Ethical issues and considerations. Committee Opinion No. 695. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;129:e109-16.
4. Ruppin U, Pfafflin F. Arch Sex Behav. 2015;44:1321-9.
5. Lawrence AA. Arch Sex Behav. 2003;32:299-315.
6. Landen M et al. Acta Psychiatr Scand. 1998;97:284-9.
7. Papdopulos NA et al. J Sex Med. 2017;14(5):721-30.
8. Peters T. Detransition, Baby. New York: Penguin Random House, 2021.
Over the holiday season I had the pleasure of finally reading the national bestseller, Detransition, Baby. On the surface, the story depicts the complex relationships between Reese, a transgender woman who strongly desires a family, her ex-wife, Ames – a transgender woman who detransitioned to live as a cisgender man – and Ames’ cisgender female partner, who is unexpectedly pregnant with his child. The story delves much deeper than the relationships between these characters, as it exceptionally articulates many of the emotional intricacies of the transgender experience and addresses one of the most taboo topics in the transgender community – detransitioning and regret.
The terms “transition” and “detransition” have fallen out of favor in the vernacular of the transgender population as they incorrectly imply that gender identity is contingent upon gender-affirmation processes.1,2 More importantly, the terms “detransition” and regret are not synonymous. Conflating these terms has undermined the intrinsic nature of gender identity, which has resulted in political and legal consequences seeking to limit or outright ban care for transgender patients.
As a gender-affirming surgeon, one of the most common questions I get asked is the rate of regret patients have after their surgeries. While I have no issue answering the question when it is presented, I do not hesitate to point out some of the problematic subtext inherent in such inquiries. Within the line of questioning, many often comment, “It’s so permanent,” “I can’t believe people can do this to their bodies,” or “How sure are patients before undergoing these surgeries?” While these comments and queries can be downright offensive, they seem to stem from the difficulty people have comprehending gender dysphoria and the painstaking steps people take to affirm their identity. The implication of these comments also reveals a more deep-seated issue – general distrust of individual bodily autonomy, personal identity, and choice.
For the obstetrician-gynecologist, understanding the concept of autonomous, patient-centered decision-making should be second nature, as we face a similar line of interrogation when discussing abortion, contraception, and pregnancy. No other field faces this level of scrutiny when it comes to defending a patient’s bodily autonomy. For example, given the history of reproductive injustice with tubal ligation procedures, the American College of Obstetricians and Gynecologists has issued clear guidelines regarding counseling of women while acknowledging the tenuous history of these procedures with minority subgroups. According to their committee opinion, ethical counseling for such a permanent procedure involves understanding the content of information presented to the patient, how that information is conveyed, and self-reflection on the part of the provider.3 The approach to counseling and understanding gender-affirming care is no different.
I want to be clear that regret after gender-affirming surgery is rare, occurring in 0%-3.8% of patients.4-6 In a separate study, 91% of patients expressed significant improvement in quality of life after surgery.7 However, what is disheartening about patients who experience surgical regret is that it originates from continued difficulty from the transition process itself and ongoing discrimination – even though the patient’s physical characteristics match their gender identity.4-6 Similarly, in another survey which examined 17,151 participants who had pursued gender affirmation (broadly defined), approximately 2,242 (13.1%) reported a history of detransition.2 Among these adults, the vast majority (82.5%), cited external factors such as school harassment, sexual violence, family pressure, and social stigma as reasons for detransitioning.2 Other associated factors included male sex assigned at birth, nonbinary gender identity, bisexual orientation, and having an unsupportive family.2
When Ames is explaining his “detransition” to his cisfemale partner, he states: “I got sick of living as trans …[sic]… I am trans, but I don’t need to do trans.”8 While there is still more research needed to further understand detransitioning and surgical regret, these few studies demonstrate a heart-breaking reality – in many aspects of our society it is still extremely difficult to live as a transgender person.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She did not report any disclosures.
References
1. National LGBTQIA+ Health Education Center, A program of the Fenway Institute: LGBTQIA+ glossary of terms for health care teams. 2020. Available at www.lgbtqiahealtheducation.org/wp-content/uploads/2020/10/Glossary-2020.08.30.pdf. Accessed Dec. 30, 2021.
2. Turban JL et al. LGBT Health 2021;8(4):273-80.
3. Sterilization of women: Ethical issues and considerations. Committee Opinion No. 695. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;129:e109-16.
4. Ruppin U, Pfafflin F. Arch Sex Behav. 2015;44:1321-9.
5. Lawrence AA. Arch Sex Behav. 2003;32:299-315.
6. Landen M et al. Acta Psychiatr Scand. 1998;97:284-9.
7. Papdopulos NA et al. J Sex Med. 2017;14(5):721-30.
8. Peters T. Detransition, Baby. New York: Penguin Random House, 2021.
Dermatologists take to TikTok to share their own ‘hacks’
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.

The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.

The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.

The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
Filler complications involving vascular necrosis, vision changes on the rise
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM ASDS 2021
ED docs are cleaning up the messes of medical tourism
It was a typical, busy evening shift in the emergency department (ED) when Steve Carroll, DO, an emergency medicine physician in the Philadelphia area, noticed an odd listing on the tracking board. In the waiting room, there was someone whose chief complaint was that she needed to have surgical drains pulled.
According to the woman’s chart, she’d undergone liposuction in Miami a week before. The surgeon had effectively relinquished all follow-up care to the woman’s local ED.
Dr. Carroll searched the name of her surgeon and found that his site “specifically advertised medical tourism,” Dr. Carroll said. The site lured patients with the idea of recovering by the beach and that a local nurse would come to their room every day.
But when Dr. Carroll told the patient that her surgeon should be the one who removes the drains, she became concerned. She didn’t know that her surgeon wasn’t providing the standard of care, he said. Somewhat appalled that a board-certified plastic surgeon would place the burden of follow-up care on an ED doctor hundreds of miles away, Dr. Carroll posted the case to Twitter and several Facebook groups.
“Yes I could refuse to take [the drains] out but that’s not patient-centered care,” Dr. Carroll wrote in a Twitter thread. “It’s unfairly shifting routine outpatient surgical followup (and liability) onto me and extra cost to [the patient].” Comments from ED physicians and sympathetic surgeons across the country flowed in. Dr. Carroll quickly realized his situation was part of a much larger problem than he’d thought.
Dr. Carroll’s patient told him that the Miami surgery cost less than undergoing the surgery locally; that’s why she’d made the trip. She’s not alone. Traveling to get the lowest price for a plastic surgery procedure has been a rising phenomenon since the early 2000s, according to the American Society of Plastic Surgeons (ASPS). Many countries are actively fostering their medical tourism industries, as are states such as Florida.
People have long traveled to get the best medical care. But “medical tourism is completely different,” said Alan Matarasso, MD, FACS, a Manhattan-based plastic surgeon and member of the ASPS Executive Committee. “People [are] traveling to get a simultaneous vacation or lower cost,” he said.
Choosing facilities on the basis of these criteria comes with myriad problems, and the quality of medical care may be lower. It’s difficult to verify the credentials of the surgeons, anesthesiologists, and facilities involved. Medical records can be in a different language, and traveling immediately after surgery increases the risk for pulmonary embolism and death, not to mention the added complications of traveling and being a surgical patient during the COVID-19 pandemic, he said.
Typically, surgeons are protective of their patients. But Murtaza Akhter, MD, an emergency medicine physician based in Miami, says it’s the opposite with the medical tourism surgeons whose patients regularly end up in his ED. “There’s almost no ownership,” he said. “Every time, [the patients] say, ‘My doctor isn’t responding,’ or they said go to the ER.” And that’s before they’ve even made it out of Miami.
The most common cosmetic surgery complications Dr. Akhter sees occur in patients who’ve undergone so-called Brazilian butt lifts. They show up in his ED face down, suffering from severe blood loss. He has them undergo a transfusion and maybe some imaging, but if they need a higher degree of care, they have to be transferred. “There’s a reason it’s cheaper,” he said.
Medical tourism mishaps are such a regular occurrence in Miami that no one flinches when the patients show up in the ED, Dr. Akhter said. He had begun to think he was overreacting to the problem until he saw Dr. Carroll’s Twitter thread.
“Since it’s daily, I just thought maybe I had gone crazy and that it’s considered normal for plastic surgeons to do this. Thanks for making me feel sane again,” Dr. Akhter tweeted in a reply to Dr. Carroll.
There are no reliable data as to of how often or where such surgeries are occurring or of patients’ outcomes. But Nicholas Genes, MD, an ED physician in Manhattan, says he sees far more postsurgical patients who traveled for their procedures than ones who underwent surgery locally. He can’t say for certain whether that’s because procedures performed by doctors in New York City have fewer complications or the physicians just handle postprocedure problems themselves.
In a 2021 systematic review of aesthetic breast surgeries performed through medical tourism, researchers found that of 171 patients who traveled for surgery, 88 (51%) had a total of 106 complications that required returning to the operating room and undergoing general anesthesia. They also found that 39% of breast augmentation implant surgeries required either a unilateral or bilateral explantation procedure after patients returned home.
The rate of complications was higher than the study authors had expected. “These are totally elective procedures,” Dr. Matarasso said. “They should be optimized.” And high rates of complications come with hefty price tags.
The cost of managing these complications, which falls to the home healthcare system or the patient themselves, can range from $5,500 (determined on the basis of data from a 2019 study in the United Kingdom) to as much as $123,000, researchers in New York City calculated, if the patient develops a complicated mycobacterium infection.
“In your effort to get a good deal or around the system, you could still end up with a lot of extensive medical bills if something goes wrong,” Dr. Genes said.
The liability dilemma
Many of the ED physicians Dr. Carroll heard from said that they wouldn’t have treated the woman who needed to have drains removed. Unlike the Brazilian-butt-lifts-gone-wrong in Miami or the complications Dr. Genes sees in New York City, Dr. Carroll’s patient wasn’t in a state of emergency. Most ED physicians said they would have sent her on her way to find a surgeon.
“In general, we shouldn’t be doing things we aren’t trained to do. It’s sort of a slippery slope,” Dr. Genes said. He’s comfortable with removing stitches, but for surgical drains and plastic apparatuses, “I don’t feel particularly well trained. I’d have to consult a colleague in general surgery,” he said. When he does get one of these patients, he works the phones to find a plastic surgeon who will see the patient, something he says their original plastic surgeon should have done.
“Sitting there with the patient, I felt a little bad for her,” Dr. Carroll said. “I knew if I didn’t do it, it would be weeks while she bounced around to urgent care, primary care, and finally found a surgeon.” But by removing the drains, he did shift some of the liability to himself. “If she developed a wound infection, then I’m on the hook for [that],” he said. “If I send her away, I have less liability but didn’t quite do the right thing for the patient.”
In replies to Dr. Carroll’s thread, some doctors debated whether these types of cases, particularly those in which surgeons forgo follow-up care, could be considered medical abandonment. Legal experts say that’s not exactly the case, at least it would not be the case with Dr. Carroll’s patient.
“I don’t think they’ve abandoned the patient; I think they’ve abandoned care,” said Michael Flynn, JD, professor of personal injury law at Nova Southeastern University, in Fort Lauderdale–Davie, Fla. “And that abandonment of follow-up care, if it falls below the standard of what medical professionals should do, then it’s malpractice.”
“The doctor didn’t just walk away and become unreachable,” said Bernard Black, JD, a medical malpractice attorney and law professor at Northwestern University, in Evanston, Ill. Technically, the surgeon referred the patient to the ED. Mr. Black agreed that it sounds more like a question of malpractice, “but without real damages, there’s no claim.”
Even if not illegal, sending these patients to the ED is still highly unethical, Dr. Carroll said. The authors of a 2014 article in Aesthetic Plastic Surgery concur: “It is the duty and ethical responsibility of plastic surgeons to prevent unnecessary complications following tourism medicine by adequately counseling patients, defining perioperative treatment protocols, and reporting complications to regional and specialty-specific governing bodies,” they write.
Sometimes patients need to travel, Dr. Matarasso said. Recently, three out-of-state patients came to him for procedures. Two stayed in Manhattan until their follow-up care was finished; he arranged care elsewhere for the third. It’s the operating surgeon’s job to connect patients with someone who can provide follow-up care when they go home, Dr. Matarasso said. If a surgeon doesn’t have a connection in a patient’s home city, the ASPS has a referral service to help, he said.
“My frustration was never with the patient,” Dr. Carroll said. “No one should feel bad about coming to an ED for literally anything, and I mean that. My frustration is with the surgeon who didn’t go the one extra step to arrange her follow-up.”
A version of this article first appeared on Medscape.com.
It was a typical, busy evening shift in the emergency department (ED) when Steve Carroll, DO, an emergency medicine physician in the Philadelphia area, noticed an odd listing on the tracking board. In the waiting room, there was someone whose chief complaint was that she needed to have surgical drains pulled.
According to the woman’s chart, she’d undergone liposuction in Miami a week before. The surgeon had effectively relinquished all follow-up care to the woman’s local ED.
Dr. Carroll searched the name of her surgeon and found that his site “specifically advertised medical tourism,” Dr. Carroll said. The site lured patients with the idea of recovering by the beach and that a local nurse would come to their room every day.
But when Dr. Carroll told the patient that her surgeon should be the one who removes the drains, she became concerned. She didn’t know that her surgeon wasn’t providing the standard of care, he said. Somewhat appalled that a board-certified plastic surgeon would place the burden of follow-up care on an ED doctor hundreds of miles away, Dr. Carroll posted the case to Twitter and several Facebook groups.
“Yes I could refuse to take [the drains] out but that’s not patient-centered care,” Dr. Carroll wrote in a Twitter thread. “It’s unfairly shifting routine outpatient surgical followup (and liability) onto me and extra cost to [the patient].” Comments from ED physicians and sympathetic surgeons across the country flowed in. Dr. Carroll quickly realized his situation was part of a much larger problem than he’d thought.
Dr. Carroll’s patient told him that the Miami surgery cost less than undergoing the surgery locally; that’s why she’d made the trip. She’s not alone. Traveling to get the lowest price for a plastic surgery procedure has been a rising phenomenon since the early 2000s, according to the American Society of Plastic Surgeons (ASPS). Many countries are actively fostering their medical tourism industries, as are states such as Florida.
People have long traveled to get the best medical care. But “medical tourism is completely different,” said Alan Matarasso, MD, FACS, a Manhattan-based plastic surgeon and member of the ASPS Executive Committee. “People [are] traveling to get a simultaneous vacation or lower cost,” he said.
Choosing facilities on the basis of these criteria comes with myriad problems, and the quality of medical care may be lower. It’s difficult to verify the credentials of the surgeons, anesthesiologists, and facilities involved. Medical records can be in a different language, and traveling immediately after surgery increases the risk for pulmonary embolism and death, not to mention the added complications of traveling and being a surgical patient during the COVID-19 pandemic, he said.
Typically, surgeons are protective of their patients. But Murtaza Akhter, MD, an emergency medicine physician based in Miami, says it’s the opposite with the medical tourism surgeons whose patients regularly end up in his ED. “There’s almost no ownership,” he said. “Every time, [the patients] say, ‘My doctor isn’t responding,’ or they said go to the ER.” And that’s before they’ve even made it out of Miami.
The most common cosmetic surgery complications Dr. Akhter sees occur in patients who’ve undergone so-called Brazilian butt lifts. They show up in his ED face down, suffering from severe blood loss. He has them undergo a transfusion and maybe some imaging, but if they need a higher degree of care, they have to be transferred. “There’s a reason it’s cheaper,” he said.
Medical tourism mishaps are such a regular occurrence in Miami that no one flinches when the patients show up in the ED, Dr. Akhter said. He had begun to think he was overreacting to the problem until he saw Dr. Carroll’s Twitter thread.
“Since it’s daily, I just thought maybe I had gone crazy and that it’s considered normal for plastic surgeons to do this. Thanks for making me feel sane again,” Dr. Akhter tweeted in a reply to Dr. Carroll.
There are no reliable data as to of how often or where such surgeries are occurring or of patients’ outcomes. But Nicholas Genes, MD, an ED physician in Manhattan, says he sees far more postsurgical patients who traveled for their procedures than ones who underwent surgery locally. He can’t say for certain whether that’s because procedures performed by doctors in New York City have fewer complications or the physicians just handle postprocedure problems themselves.
In a 2021 systematic review of aesthetic breast surgeries performed through medical tourism, researchers found that of 171 patients who traveled for surgery, 88 (51%) had a total of 106 complications that required returning to the operating room and undergoing general anesthesia. They also found that 39% of breast augmentation implant surgeries required either a unilateral or bilateral explantation procedure after patients returned home.
The rate of complications was higher than the study authors had expected. “These are totally elective procedures,” Dr. Matarasso said. “They should be optimized.” And high rates of complications come with hefty price tags.
The cost of managing these complications, which falls to the home healthcare system or the patient themselves, can range from $5,500 (determined on the basis of data from a 2019 study in the United Kingdom) to as much as $123,000, researchers in New York City calculated, if the patient develops a complicated mycobacterium infection.
“In your effort to get a good deal or around the system, you could still end up with a lot of extensive medical bills if something goes wrong,” Dr. Genes said.
The liability dilemma
Many of the ED physicians Dr. Carroll heard from said that they wouldn’t have treated the woman who needed to have drains removed. Unlike the Brazilian-butt-lifts-gone-wrong in Miami or the complications Dr. Genes sees in New York City, Dr. Carroll’s patient wasn’t in a state of emergency. Most ED physicians said they would have sent her on her way to find a surgeon.
“In general, we shouldn’t be doing things we aren’t trained to do. It’s sort of a slippery slope,” Dr. Genes said. He’s comfortable with removing stitches, but for surgical drains and plastic apparatuses, “I don’t feel particularly well trained. I’d have to consult a colleague in general surgery,” he said. When he does get one of these patients, he works the phones to find a plastic surgeon who will see the patient, something he says their original plastic surgeon should have done.
“Sitting there with the patient, I felt a little bad for her,” Dr. Carroll said. “I knew if I didn’t do it, it would be weeks while she bounced around to urgent care, primary care, and finally found a surgeon.” But by removing the drains, he did shift some of the liability to himself. “If she developed a wound infection, then I’m on the hook for [that],” he said. “If I send her away, I have less liability but didn’t quite do the right thing for the patient.”
In replies to Dr. Carroll’s thread, some doctors debated whether these types of cases, particularly those in which surgeons forgo follow-up care, could be considered medical abandonment. Legal experts say that’s not exactly the case, at least it would not be the case with Dr. Carroll’s patient.
“I don’t think they’ve abandoned the patient; I think they’ve abandoned care,” said Michael Flynn, JD, professor of personal injury law at Nova Southeastern University, in Fort Lauderdale–Davie, Fla. “And that abandonment of follow-up care, if it falls below the standard of what medical professionals should do, then it’s malpractice.”
“The doctor didn’t just walk away and become unreachable,” said Bernard Black, JD, a medical malpractice attorney and law professor at Northwestern University, in Evanston, Ill. Technically, the surgeon referred the patient to the ED. Mr. Black agreed that it sounds more like a question of malpractice, “but without real damages, there’s no claim.”
Even if not illegal, sending these patients to the ED is still highly unethical, Dr. Carroll said. The authors of a 2014 article in Aesthetic Plastic Surgery concur: “It is the duty and ethical responsibility of plastic surgeons to prevent unnecessary complications following tourism medicine by adequately counseling patients, defining perioperative treatment protocols, and reporting complications to regional and specialty-specific governing bodies,” they write.
Sometimes patients need to travel, Dr. Matarasso said. Recently, three out-of-state patients came to him for procedures. Two stayed in Manhattan until their follow-up care was finished; he arranged care elsewhere for the third. It’s the operating surgeon’s job to connect patients with someone who can provide follow-up care when they go home, Dr. Matarasso said. If a surgeon doesn’t have a connection in a patient’s home city, the ASPS has a referral service to help, he said.
“My frustration was never with the patient,” Dr. Carroll said. “No one should feel bad about coming to an ED for literally anything, and I mean that. My frustration is with the surgeon who didn’t go the one extra step to arrange her follow-up.”
A version of this article first appeared on Medscape.com.
It was a typical, busy evening shift in the emergency department (ED) when Steve Carroll, DO, an emergency medicine physician in the Philadelphia area, noticed an odd listing on the tracking board. In the waiting room, there was someone whose chief complaint was that she needed to have surgical drains pulled.
According to the woman’s chart, she’d undergone liposuction in Miami a week before. The surgeon had effectively relinquished all follow-up care to the woman’s local ED.
Dr. Carroll searched the name of her surgeon and found that his site “specifically advertised medical tourism,” Dr. Carroll said. The site lured patients with the idea of recovering by the beach and that a local nurse would come to their room every day.
But when Dr. Carroll told the patient that her surgeon should be the one who removes the drains, she became concerned. She didn’t know that her surgeon wasn’t providing the standard of care, he said. Somewhat appalled that a board-certified plastic surgeon would place the burden of follow-up care on an ED doctor hundreds of miles away, Dr. Carroll posted the case to Twitter and several Facebook groups.
“Yes I could refuse to take [the drains] out but that’s not patient-centered care,” Dr. Carroll wrote in a Twitter thread. “It’s unfairly shifting routine outpatient surgical followup (and liability) onto me and extra cost to [the patient].” Comments from ED physicians and sympathetic surgeons across the country flowed in. Dr. Carroll quickly realized his situation was part of a much larger problem than he’d thought.
Dr. Carroll’s patient told him that the Miami surgery cost less than undergoing the surgery locally; that’s why she’d made the trip. She’s not alone. Traveling to get the lowest price for a plastic surgery procedure has been a rising phenomenon since the early 2000s, according to the American Society of Plastic Surgeons (ASPS). Many countries are actively fostering their medical tourism industries, as are states such as Florida.
People have long traveled to get the best medical care. But “medical tourism is completely different,” said Alan Matarasso, MD, FACS, a Manhattan-based plastic surgeon and member of the ASPS Executive Committee. “People [are] traveling to get a simultaneous vacation or lower cost,” he said.
Choosing facilities on the basis of these criteria comes with myriad problems, and the quality of medical care may be lower. It’s difficult to verify the credentials of the surgeons, anesthesiologists, and facilities involved. Medical records can be in a different language, and traveling immediately after surgery increases the risk for pulmonary embolism and death, not to mention the added complications of traveling and being a surgical patient during the COVID-19 pandemic, he said.
Typically, surgeons are protective of their patients. But Murtaza Akhter, MD, an emergency medicine physician based in Miami, says it’s the opposite with the medical tourism surgeons whose patients regularly end up in his ED. “There’s almost no ownership,” he said. “Every time, [the patients] say, ‘My doctor isn’t responding,’ or they said go to the ER.” And that’s before they’ve even made it out of Miami.
The most common cosmetic surgery complications Dr. Akhter sees occur in patients who’ve undergone so-called Brazilian butt lifts. They show up in his ED face down, suffering from severe blood loss. He has them undergo a transfusion and maybe some imaging, but if they need a higher degree of care, they have to be transferred. “There’s a reason it’s cheaper,” he said.
Medical tourism mishaps are such a regular occurrence in Miami that no one flinches when the patients show up in the ED, Dr. Akhter said. He had begun to think he was overreacting to the problem until he saw Dr. Carroll’s Twitter thread.
“Since it’s daily, I just thought maybe I had gone crazy and that it’s considered normal for plastic surgeons to do this. Thanks for making me feel sane again,” Dr. Akhter tweeted in a reply to Dr. Carroll.
There are no reliable data as to of how often or where such surgeries are occurring or of patients’ outcomes. But Nicholas Genes, MD, an ED physician in Manhattan, says he sees far more postsurgical patients who traveled for their procedures than ones who underwent surgery locally. He can’t say for certain whether that’s because procedures performed by doctors in New York City have fewer complications or the physicians just handle postprocedure problems themselves.
In a 2021 systematic review of aesthetic breast surgeries performed through medical tourism, researchers found that of 171 patients who traveled for surgery, 88 (51%) had a total of 106 complications that required returning to the operating room and undergoing general anesthesia. They also found that 39% of breast augmentation implant surgeries required either a unilateral or bilateral explantation procedure after patients returned home.
The rate of complications was higher than the study authors had expected. “These are totally elective procedures,” Dr. Matarasso said. “They should be optimized.” And high rates of complications come with hefty price tags.
The cost of managing these complications, which falls to the home healthcare system or the patient themselves, can range from $5,500 (determined on the basis of data from a 2019 study in the United Kingdom) to as much as $123,000, researchers in New York City calculated, if the patient develops a complicated mycobacterium infection.
“In your effort to get a good deal or around the system, you could still end up with a lot of extensive medical bills if something goes wrong,” Dr. Genes said.
The liability dilemma
Many of the ED physicians Dr. Carroll heard from said that they wouldn’t have treated the woman who needed to have drains removed. Unlike the Brazilian-butt-lifts-gone-wrong in Miami or the complications Dr. Genes sees in New York City, Dr. Carroll’s patient wasn’t in a state of emergency. Most ED physicians said they would have sent her on her way to find a surgeon.
“In general, we shouldn’t be doing things we aren’t trained to do. It’s sort of a slippery slope,” Dr. Genes said. He’s comfortable with removing stitches, but for surgical drains and plastic apparatuses, “I don’t feel particularly well trained. I’d have to consult a colleague in general surgery,” he said. When he does get one of these patients, he works the phones to find a plastic surgeon who will see the patient, something he says their original plastic surgeon should have done.
“Sitting there with the patient, I felt a little bad for her,” Dr. Carroll said. “I knew if I didn’t do it, it would be weeks while she bounced around to urgent care, primary care, and finally found a surgeon.” But by removing the drains, he did shift some of the liability to himself. “If she developed a wound infection, then I’m on the hook for [that],” he said. “If I send her away, I have less liability but didn’t quite do the right thing for the patient.”
In replies to Dr. Carroll’s thread, some doctors debated whether these types of cases, particularly those in which surgeons forgo follow-up care, could be considered medical abandonment. Legal experts say that’s not exactly the case, at least it would not be the case with Dr. Carroll’s patient.
“I don’t think they’ve abandoned the patient; I think they’ve abandoned care,” said Michael Flynn, JD, professor of personal injury law at Nova Southeastern University, in Fort Lauderdale–Davie, Fla. “And that abandonment of follow-up care, if it falls below the standard of what medical professionals should do, then it’s malpractice.”
“The doctor didn’t just walk away and become unreachable,” said Bernard Black, JD, a medical malpractice attorney and law professor at Northwestern University, in Evanston, Ill. Technically, the surgeon referred the patient to the ED. Mr. Black agreed that it sounds more like a question of malpractice, “but without real damages, there’s no claim.”
Even if not illegal, sending these patients to the ED is still highly unethical, Dr. Carroll said. The authors of a 2014 article in Aesthetic Plastic Surgery concur: “It is the duty and ethical responsibility of plastic surgeons to prevent unnecessary complications following tourism medicine by adequately counseling patients, defining perioperative treatment protocols, and reporting complications to regional and specialty-specific governing bodies,” they write.
Sometimes patients need to travel, Dr. Matarasso said. Recently, three out-of-state patients came to him for procedures. Two stayed in Manhattan until their follow-up care was finished; he arranged care elsewhere for the third. It’s the operating surgeon’s job to connect patients with someone who can provide follow-up care when they go home, Dr. Matarasso said. If a surgeon doesn’t have a connection in a patient’s home city, the ASPS has a referral service to help, he said.
“My frustration was never with the patient,” Dr. Carroll said. “No one should feel bad about coming to an ED for literally anything, and I mean that. My frustration is with the surgeon who didn’t go the one extra step to arrange her follow-up.”
A version of this article first appeared on Medscape.com.