User login
Delayed umbilical cord clamping improves outcomes in very preterm infants
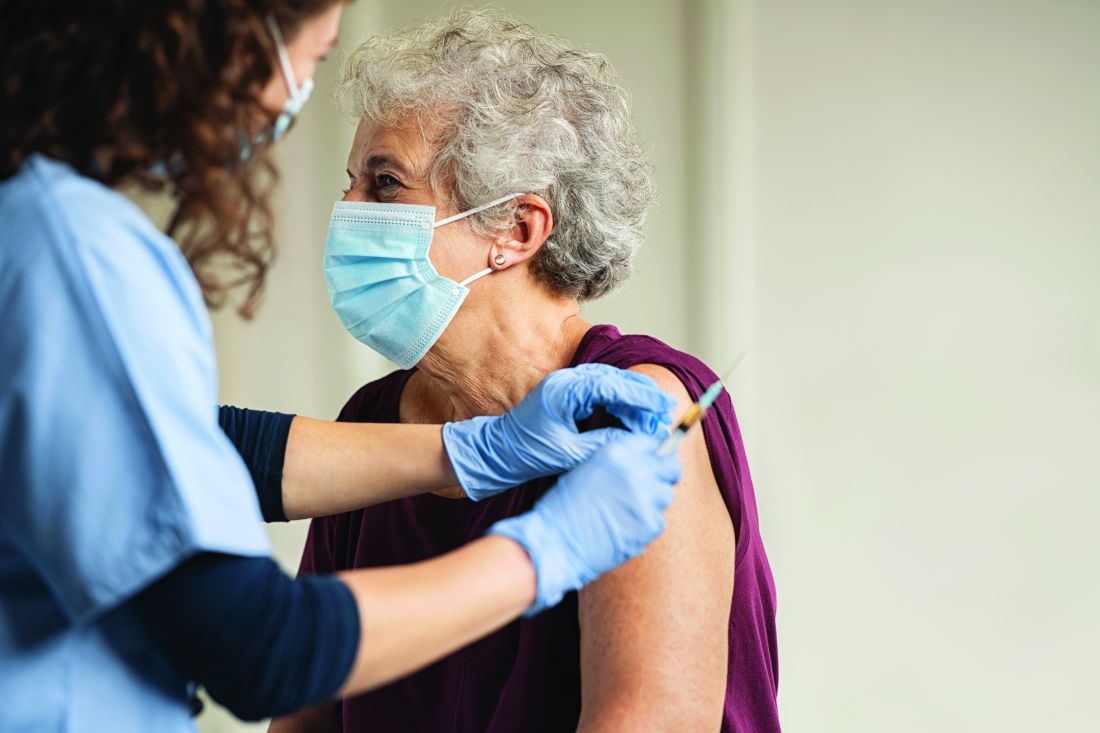
Delayed umbilical cord clamping for at least 60 seconds after birth significantly reduced death or disability in infants of less than 30 weeks’ gestation, according to data from nearly 1,500 infants.
The burden of disability and mortality for babies born before 30 weeks’ gestation remains high, especially in low- and middle-income countries, wrote Kristy P. Robledo, PhD, of the University of Sydney, Australia, and colleagues. Delayed clamping of the umbilical cord is a simple procedure that may improve mortality in this population, but more research is needed; recommended times to delayed clamping range from 30 seconds to 3 minutes, they noted.
In a study published in The Lancet Child & Adolescent Health, the researchers randomized 767 very preterm infants to delayed clamping at least 60 seconds after birth and 764 to immediate clamping. Of these, 384 were multiple births (who were individually randomized), 862 were male, and 505 were born before 27 weeks’ gestation. The primary outcome was death or disability at 2 years of age. Major disability was defined as cerebral palsy, severe visual loss, deafness requiring a hearing aid or cochlear implants, major language or speech problems, or cognitive delay at 2 years corrected age. The median time to clamping was 60 seconds in the delayed group and 5 seconds in the immediate group.
Primary outcome data were available for 1,419 infants. Death or major disability occurred in 29% of infants assigned to delayed clamping compared to 34% of those assigned to immediate clamping (relative risk 0.83, P = .010). The infants were part of the APTS Childhood Follow-Up Study, an open-label superiority trial conducted in Australia and New Zealand.
By age 2 years, 8% of infants in the delayed group and 11% of those in the immediate group had died; 23% and 26%, respectively, met criteria for major disability. The impact of delayed clamping translates to a 30% reduction in relative risk of mortality at 2 years of age, but no significant impact on major disability, the researchers wrote.
The findings were limited by several factors including the unblinded study design, lack of data on heart rate or time to first breath, and the clamping prior to 60 seconds in 26% of infants in the delayed group based on clinical concerns for these specific infants, the researchers noted.
However, the results were strengthened by the large size, low risk of bias, and specific primary outcome, they said. The data support findings from recent systematic reviews and highlight the need for further trials to evaluate delayed clamping at different time points, with larger populations, inclusion of time to first breath and heart rate, and improved measures of disability, the researchers added.
In clinical practice, “Given that aiming to delay cord clamping for 60 seconds or more improved 2-year outcomes and short-term hematological measures with no evidence of significant harm, it seems reasonable to conclude that delayed clamping is appropriate as standard care in very preterm infants,” they concluded.
Accepting simple intervention could have great impact
This study is important in light of the overwhelming burden of preterm birth on the health care system and society as a whole, Lisette D. Tanner, MD, of Emory University, Atlanta, said in an interview.
“Preterm birth is associated with billions in health care costs each year, and a large portion of that money is directed to the complications associated with preterm birth, such as early intervention services, educational support, and ongoing medical care,” Dr. Tanner said. “This study is particularly timely, as we are quickly approaching 2030, the deadline for achieving the United Nations Sustainable Development Goal of ending preventable deaths of newborns and children under 5 years of age,” she said. The goal involves “all countries aiming to reduce neonatal mortality to at least as low as 12 per 1,000 live births and under-5 mortality to at least as low as 25 per 1,000 live births. Effective treatments to reduce infant and child mortality would make strong inroads toward this goal,” she explained.
Dr. Tanner said she was not surprised by the findings because previous studies have shown similar results. “However, the large, multicenter nature of this study provides additional weight to recommendations to delay cord clamping as standard practice,” she said.
“The findings of this study support the recommendations of a number of large organizations,” said Dr. Tanner. “The World Health Organization recommends that the umbilical cord not be clamped earlier than 1 minute after birth in term or preterm infants who do not require positive pressure ventilation. The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics now recommend a delay in umbilical cord clamping in vigorous term and preterm infants for at least 30–60 seconds after birth,” she said. “The Royal College of Obstetricians and Gynaecologists also recommends deferring umbilical cord clamping for healthy term and preterm infants for at least 2 minutes after birth,” she added.
However, “the delay in adoption of this guidelines in practice appears to be related to some concerns regarding universal adoption of this approach,” Dr. Tanner noted. “Some clinicians have suggested that delayed cord clamping could delay vital neonatal resuscitative efforts, leading to worse neonatal outcomes, but this concern has not been borne out in the data, as all guidelines specifically state that this intervention is for vigorous newborns,” she said. “In fact, in preterm infants, delayed cord clamping is associated with improved transitional circulation, decreased need for blood transfusion, and lower incidence of necrotizing enterocolitis and intraventricular hemorrhage,” Dr. Tanner emphasized. “Additionally, concerns persist that delayed cord clamping could lead to excessive transfusion with resultant polycythemia. Again, no data have supported this claim to date,” she said.
“Finally, some clinicians are concerned that delayed clamping could lead to delay in addressing maternal complications of birth such as hemorrhage, but studies have shown the opposite; delayed umbilical cord clamping has not been associated with an increased risk of postpartum hemorrhage or increased blood loss at delivery, nor has it been with a difference in the need for blood transfusion,” said Dr. Tanner.
Ideally, practitioners will become more comfortable in delaying cord clamping as a routine practice as more data demonstrating the safety and benefit of this easy intervention are disseminated, she said.
Additional research delineating which gestational ages benefit most from delayed cord clamping would help direct education efforts to implement this intervention, Dr. Tanner noted.
The study was funded by the Australian National Health and Medical Research Council. The researchers and Dr. Tanner had no financial conflicts to disclose.
Delayed umbilical cord clamping for at least 60 seconds after birth significantly reduced death or disability in infants of less than 30 weeks’ gestation, according to data from nearly 1,500 infants.
The burden of disability and mortality for babies born before 30 weeks’ gestation remains high, especially in low- and middle-income countries, wrote Kristy P. Robledo, PhD, of the University of Sydney, Australia, and colleagues. Delayed clamping of the umbilical cord is a simple procedure that may improve mortality in this population, but more research is needed; recommended times to delayed clamping range from 30 seconds to 3 minutes, they noted.
In a study published in The Lancet Child & Adolescent Health, the researchers randomized 767 very preterm infants to delayed clamping at least 60 seconds after birth and 764 to immediate clamping. Of these, 384 were multiple births (who were individually randomized), 862 were male, and 505 were born before 27 weeks’ gestation. The primary outcome was death or disability at 2 years of age. Major disability was defined as cerebral palsy, severe visual loss, deafness requiring a hearing aid or cochlear implants, major language or speech problems, or cognitive delay at 2 years corrected age. The median time to clamping was 60 seconds in the delayed group and 5 seconds in the immediate group.
Primary outcome data were available for 1,419 infants. Death or major disability occurred in 29% of infants assigned to delayed clamping compared to 34% of those assigned to immediate clamping (relative risk 0.83, P = .010). The infants were part of the APTS Childhood Follow-Up Study, an open-label superiority trial conducted in Australia and New Zealand.
By age 2 years, 8% of infants in the delayed group and 11% of those in the immediate group had died; 23% and 26%, respectively, met criteria for major disability. The impact of delayed clamping translates to a 30% reduction in relative risk of mortality at 2 years of age, but no significant impact on major disability, the researchers wrote.
The findings were limited by several factors including the unblinded study design, lack of data on heart rate or time to first breath, and the clamping prior to 60 seconds in 26% of infants in the delayed group based on clinical concerns for these specific infants, the researchers noted.
However, the results were strengthened by the large size, low risk of bias, and specific primary outcome, they said. The data support findings from recent systematic reviews and highlight the need for further trials to evaluate delayed clamping at different time points, with larger populations, inclusion of time to first breath and heart rate, and improved measures of disability, the researchers added.
In clinical practice, “Given that aiming to delay cord clamping for 60 seconds or more improved 2-year outcomes and short-term hematological measures with no evidence of significant harm, it seems reasonable to conclude that delayed clamping is appropriate as standard care in very preterm infants,” they concluded.
Accepting simple intervention could have great impact
This study is important in light of the overwhelming burden of preterm birth on the health care system and society as a whole, Lisette D. Tanner, MD, of Emory University, Atlanta, said in an interview.
“Preterm birth is associated with billions in health care costs each year, and a large portion of that money is directed to the complications associated with preterm birth, such as early intervention services, educational support, and ongoing medical care,” Dr. Tanner said. “This study is particularly timely, as we are quickly approaching 2030, the deadline for achieving the United Nations Sustainable Development Goal of ending preventable deaths of newborns and children under 5 years of age,” she said. The goal involves “all countries aiming to reduce neonatal mortality to at least as low as 12 per 1,000 live births and under-5 mortality to at least as low as 25 per 1,000 live births. Effective treatments to reduce infant and child mortality would make strong inroads toward this goal,” she explained.
Dr. Tanner said she was not surprised by the findings because previous studies have shown similar results. “However, the large, multicenter nature of this study provides additional weight to recommendations to delay cord clamping as standard practice,” she said.
“The findings of this study support the recommendations of a number of large organizations,” said Dr. Tanner. “The World Health Organization recommends that the umbilical cord not be clamped earlier than 1 minute after birth in term or preterm infants who do not require positive pressure ventilation. The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics now recommend a delay in umbilical cord clamping in vigorous term and preterm infants for at least 30–60 seconds after birth,” she said. “The Royal College of Obstetricians and Gynaecologists also recommends deferring umbilical cord clamping for healthy term and preterm infants for at least 2 minutes after birth,” she added.
However, “the delay in adoption of this guidelines in practice appears to be related to some concerns regarding universal adoption of this approach,” Dr. Tanner noted. “Some clinicians have suggested that delayed cord clamping could delay vital neonatal resuscitative efforts, leading to worse neonatal outcomes, but this concern has not been borne out in the data, as all guidelines specifically state that this intervention is for vigorous newborns,” she said. “In fact, in preterm infants, delayed cord clamping is associated with improved transitional circulation, decreased need for blood transfusion, and lower incidence of necrotizing enterocolitis and intraventricular hemorrhage,” Dr. Tanner emphasized. “Additionally, concerns persist that delayed cord clamping could lead to excessive transfusion with resultant polycythemia. Again, no data have supported this claim to date,” she said.
“Finally, some clinicians are concerned that delayed clamping could lead to delay in addressing maternal complications of birth such as hemorrhage, but studies have shown the opposite; delayed umbilical cord clamping has not been associated with an increased risk of postpartum hemorrhage or increased blood loss at delivery, nor has it been with a difference in the need for blood transfusion,” said Dr. Tanner.
Ideally, practitioners will become more comfortable in delaying cord clamping as a routine practice as more data demonstrating the safety and benefit of this easy intervention are disseminated, she said.
Additional research delineating which gestational ages benefit most from delayed cord clamping would help direct education efforts to implement this intervention, Dr. Tanner noted.
The study was funded by the Australian National Health and Medical Research Council. The researchers and Dr. Tanner had no financial conflicts to disclose.
Delayed umbilical cord clamping for at least 60 seconds after birth significantly reduced death or disability in infants of less than 30 weeks’ gestation, according to data from nearly 1,500 infants.
The burden of disability and mortality for babies born before 30 weeks’ gestation remains high, especially in low- and middle-income countries, wrote Kristy P. Robledo, PhD, of the University of Sydney, Australia, and colleagues. Delayed clamping of the umbilical cord is a simple procedure that may improve mortality in this population, but more research is needed; recommended times to delayed clamping range from 30 seconds to 3 minutes, they noted.
In a study published in The Lancet Child & Adolescent Health, the researchers randomized 767 very preterm infants to delayed clamping at least 60 seconds after birth and 764 to immediate clamping. Of these, 384 were multiple births (who were individually randomized), 862 were male, and 505 were born before 27 weeks’ gestation. The primary outcome was death or disability at 2 years of age. Major disability was defined as cerebral palsy, severe visual loss, deafness requiring a hearing aid or cochlear implants, major language or speech problems, or cognitive delay at 2 years corrected age. The median time to clamping was 60 seconds in the delayed group and 5 seconds in the immediate group.
Primary outcome data were available for 1,419 infants. Death or major disability occurred in 29% of infants assigned to delayed clamping compared to 34% of those assigned to immediate clamping (relative risk 0.83, P = .010). The infants were part of the APTS Childhood Follow-Up Study, an open-label superiority trial conducted in Australia and New Zealand.
By age 2 years, 8% of infants in the delayed group and 11% of those in the immediate group had died; 23% and 26%, respectively, met criteria for major disability. The impact of delayed clamping translates to a 30% reduction in relative risk of mortality at 2 years of age, but no significant impact on major disability, the researchers wrote.
The findings were limited by several factors including the unblinded study design, lack of data on heart rate or time to first breath, and the clamping prior to 60 seconds in 26% of infants in the delayed group based on clinical concerns for these specific infants, the researchers noted.
However, the results were strengthened by the large size, low risk of bias, and specific primary outcome, they said. The data support findings from recent systematic reviews and highlight the need for further trials to evaluate delayed clamping at different time points, with larger populations, inclusion of time to first breath and heart rate, and improved measures of disability, the researchers added.
In clinical practice, “Given that aiming to delay cord clamping for 60 seconds or more improved 2-year outcomes and short-term hematological measures with no evidence of significant harm, it seems reasonable to conclude that delayed clamping is appropriate as standard care in very preterm infants,” they concluded.
Accepting simple intervention could have great impact
This study is important in light of the overwhelming burden of preterm birth on the health care system and society as a whole, Lisette D. Tanner, MD, of Emory University, Atlanta, said in an interview.
“Preterm birth is associated with billions in health care costs each year, and a large portion of that money is directed to the complications associated with preterm birth, such as early intervention services, educational support, and ongoing medical care,” Dr. Tanner said. “This study is particularly timely, as we are quickly approaching 2030, the deadline for achieving the United Nations Sustainable Development Goal of ending preventable deaths of newborns and children under 5 years of age,” she said. The goal involves “all countries aiming to reduce neonatal mortality to at least as low as 12 per 1,000 live births and under-5 mortality to at least as low as 25 per 1,000 live births. Effective treatments to reduce infant and child mortality would make strong inroads toward this goal,” she explained.
Dr. Tanner said she was not surprised by the findings because previous studies have shown similar results. “However, the large, multicenter nature of this study provides additional weight to recommendations to delay cord clamping as standard practice,” she said.
“The findings of this study support the recommendations of a number of large organizations,” said Dr. Tanner. “The World Health Organization recommends that the umbilical cord not be clamped earlier than 1 minute after birth in term or preterm infants who do not require positive pressure ventilation. The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics now recommend a delay in umbilical cord clamping in vigorous term and preterm infants for at least 30–60 seconds after birth,” she said. “The Royal College of Obstetricians and Gynaecologists also recommends deferring umbilical cord clamping for healthy term and preterm infants for at least 2 minutes after birth,” she added.
However, “the delay in adoption of this guidelines in practice appears to be related to some concerns regarding universal adoption of this approach,” Dr. Tanner noted. “Some clinicians have suggested that delayed cord clamping could delay vital neonatal resuscitative efforts, leading to worse neonatal outcomes, but this concern has not been borne out in the data, as all guidelines specifically state that this intervention is for vigorous newborns,” she said. “In fact, in preterm infants, delayed cord clamping is associated with improved transitional circulation, decreased need for blood transfusion, and lower incidence of necrotizing enterocolitis and intraventricular hemorrhage,” Dr. Tanner emphasized. “Additionally, concerns persist that delayed cord clamping could lead to excessive transfusion with resultant polycythemia. Again, no data have supported this claim to date,” she said.
“Finally, some clinicians are concerned that delayed clamping could lead to delay in addressing maternal complications of birth such as hemorrhage, but studies have shown the opposite; delayed umbilical cord clamping has not been associated with an increased risk of postpartum hemorrhage or increased blood loss at delivery, nor has it been with a difference in the need for blood transfusion,” said Dr. Tanner.
Ideally, practitioners will become more comfortable in delaying cord clamping as a routine practice as more data demonstrating the safety and benefit of this easy intervention are disseminated, she said.
Additional research delineating which gestational ages benefit most from delayed cord clamping would help direct education efforts to implement this intervention, Dr. Tanner noted.
The study was funded by the Australian National Health and Medical Research Council. The researchers and Dr. Tanner had no financial conflicts to disclose.
FROM THE LANCET CHILD & ADOLESCENT HEALTH
Sputum biomarkers may predict COPD exacerbations
Examining sputum from patients with chronic obstructive pulmonary disease may help predict the course of the disease.
A mass spectrometric panel of biomarkers related to mucus hydration and inflammation examined in sputa showed elevated levels of metabolites from multiple pathways in patients with COPD. These correlated with sputum neutrophil counts and COPD exacerbations. In particular, sialic acid and hypoxanthine concentrations were strongly associated with disease severity, according to a study reported in the journal CHEST® authored by Charles R. Esther Jr. MD, PhD, and colleagues.
Given that an improved understanding of the pathways associated with airway pathophysiology in COPD will identify new predictive biomarkers and novel therapeutic targets, Dr. Esther and colleagues posed the question: Which physiologic pathways are altered and predict exacerbations in the airways of subjects with COPD?
They noted that in persons with COPD – characterized by dominant small airway obstruction associated with airway inflammation – multiple inflammatory pathways, as well as indices of oxidative stress (including oxidized glutathione and 8-isoprostane), are elevated in sputum. Because inflammation is a challenging therapeutic target, identification of other biologic pathways involved in COPD pathogenesis could point to novel biomarkers and therapeutic targets.
Using this approach in cystic fibrosis (CF), the authors have previously identified small molecule metabolites correlated with airway inflammation. Findings from that research supported development of a mass spectrometric biomarker panel for simultaneous measurement of inflammatory markers coupled to biomarkers of mucus hydration. The researchers applied this technology to sputum supernatants collected through the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), which included subjects with COPD, as well as relevant smoking and nonsmoking controls.
Addressing inflammation
“Inhaled steroids are really more effective for allergic inflammation as in asthma and less so for the neutrophilic inflammation that dominates in COPD. The challenge is that neutrophilic inflammation is also a key response to infection, and it’s really hard to find an anti-inflammatory that suppresses neutrophilic inflammation well enough to get clinical benefit but not so much that the patient becomes vulnerable to infection. Lots of clinical trials of anti-inflammatories in cystic fibrosis or COPD have been stopped because treated subjects had more trouble with infection,” Dr. Esther stated in an interview,
The investigators analyzed cell-free sputum supernatants from 980 subjects, including samples from 77 healthy nonsmokers (NS), 341 ever-smokers with preserved spirometry (SPS), and 562 subjects with COPD (178 GOLD [Global Initiative for Chronic Obstructive Lung Disease]1, 303 GOLD 2, and 81 GOLD 3). Among the subjects with COPD, elevated biomarkers from multiple pathways correlated with sputum neutrophil counts.
The most significant analytes (at FDR [False Discovery Rate] 0.1) were sialic acid (a mucin marker), hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione, with sialic acid and hypoxanthine strongly associated with measures of disease severity. Elevation of sialic acid and hypoxanthine were associated with shorter time to exacerbation and improved prediction models of future exacerbations.
Study results
Sialic acid was elevated in all GOLD groups relative to NS healthy controls, with a 2.8-fold (0.44 log) increase in GOLD 2 and 3.7 fold (0.56 log) increase in GOLD 3 relative to NS. Sialic acid was also elevated in the most severe disease cohorts (GOLD 2 and GOLD 3) relative to smokers with preserved spirometry (SPS) and those with less severe disease (GOLD 1).
Because mucin secretion and inflammation are also related to the pathophysiology of pulmonary exacerbations, Dr. Esther and colleagues had hypothesized that sputum biomarkers would be predictive of future exacerbations. Within the full cohort, both sialic acid and hypoxanthine were significantly elevated in those who had multiple (two or more) pulmonary exacerbations relative to those who had none (P = .001). Similar, though less significant findings were observed for xanthine (P = .01), methylthioadenosine (P = .01), adenine (P = .01), and glutathione (P = .01).
Sputum tests needed
While tests still need to be developed, Dr. Esther noted in an interview that they would be based on well-established technologies commonly utilized in clinical laboratories. “Sputum biomarkers of mucus hydration and adenosine metabolism could help clinicians predict which patients with COPD are likely to experience multiple pulmonary exacerbations. Tests would be applied to patients with COPD at higher risk for exacerbations; for example, those who have low lung function or a history of prior exacerbations.”
Dr. Esther noted that these biomarkers could be helpful in developing novel therapies. “Using sialic acid to assess mucus concentrations is much easier than other methods, so it could help in developing mucolytic treatments. Also, adenosine metabolism represents a novel therapeutic target in COPD. Drugs that modify adenosine metabolism that have been approved for other diseases such as gout could be tested in COPD. As with mucus hydration, the biomarkers we identified (particularly hypoxanthine) could be utilized to make sure that novel therapies are having the intended impact on airway adenosine metabolism.”
The research was supported by SPIROMICS (funded by NIH and the COPD Foundation). Dr. Esther reported having no relevant disclosures.
Examining sputum from patients with chronic obstructive pulmonary disease may help predict the course of the disease.
A mass spectrometric panel of biomarkers related to mucus hydration and inflammation examined in sputa showed elevated levels of metabolites from multiple pathways in patients with COPD. These correlated with sputum neutrophil counts and COPD exacerbations. In particular, sialic acid and hypoxanthine concentrations were strongly associated with disease severity, according to a study reported in the journal CHEST® authored by Charles R. Esther Jr. MD, PhD, and colleagues.
Given that an improved understanding of the pathways associated with airway pathophysiology in COPD will identify new predictive biomarkers and novel therapeutic targets, Dr. Esther and colleagues posed the question: Which physiologic pathways are altered and predict exacerbations in the airways of subjects with COPD?
They noted that in persons with COPD – characterized by dominant small airway obstruction associated with airway inflammation – multiple inflammatory pathways, as well as indices of oxidative stress (including oxidized glutathione and 8-isoprostane), are elevated in sputum. Because inflammation is a challenging therapeutic target, identification of other biologic pathways involved in COPD pathogenesis could point to novel biomarkers and therapeutic targets.
Using this approach in cystic fibrosis (CF), the authors have previously identified small molecule metabolites correlated with airway inflammation. Findings from that research supported development of a mass spectrometric biomarker panel for simultaneous measurement of inflammatory markers coupled to biomarkers of mucus hydration. The researchers applied this technology to sputum supernatants collected through the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), which included subjects with COPD, as well as relevant smoking and nonsmoking controls.
Addressing inflammation
“Inhaled steroids are really more effective for allergic inflammation as in asthma and less so for the neutrophilic inflammation that dominates in COPD. The challenge is that neutrophilic inflammation is also a key response to infection, and it’s really hard to find an anti-inflammatory that suppresses neutrophilic inflammation well enough to get clinical benefit but not so much that the patient becomes vulnerable to infection. Lots of clinical trials of anti-inflammatories in cystic fibrosis or COPD have been stopped because treated subjects had more trouble with infection,” Dr. Esther stated in an interview,
The investigators analyzed cell-free sputum supernatants from 980 subjects, including samples from 77 healthy nonsmokers (NS), 341 ever-smokers with preserved spirometry (SPS), and 562 subjects with COPD (178 GOLD [Global Initiative for Chronic Obstructive Lung Disease]1, 303 GOLD 2, and 81 GOLD 3). Among the subjects with COPD, elevated biomarkers from multiple pathways correlated with sputum neutrophil counts.
The most significant analytes (at FDR [False Discovery Rate] 0.1) were sialic acid (a mucin marker), hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione, with sialic acid and hypoxanthine strongly associated with measures of disease severity. Elevation of sialic acid and hypoxanthine were associated with shorter time to exacerbation and improved prediction models of future exacerbations.
Study results
Sialic acid was elevated in all GOLD groups relative to NS healthy controls, with a 2.8-fold (0.44 log) increase in GOLD 2 and 3.7 fold (0.56 log) increase in GOLD 3 relative to NS. Sialic acid was also elevated in the most severe disease cohorts (GOLD 2 and GOLD 3) relative to smokers with preserved spirometry (SPS) and those with less severe disease (GOLD 1).
Because mucin secretion and inflammation are also related to the pathophysiology of pulmonary exacerbations, Dr. Esther and colleagues had hypothesized that sputum biomarkers would be predictive of future exacerbations. Within the full cohort, both sialic acid and hypoxanthine were significantly elevated in those who had multiple (two or more) pulmonary exacerbations relative to those who had none (P = .001). Similar, though less significant findings were observed for xanthine (P = .01), methylthioadenosine (P = .01), adenine (P = .01), and glutathione (P = .01).
Sputum tests needed
While tests still need to be developed, Dr. Esther noted in an interview that they would be based on well-established technologies commonly utilized in clinical laboratories. “Sputum biomarkers of mucus hydration and adenosine metabolism could help clinicians predict which patients with COPD are likely to experience multiple pulmonary exacerbations. Tests would be applied to patients with COPD at higher risk for exacerbations; for example, those who have low lung function or a history of prior exacerbations.”
Dr. Esther noted that these biomarkers could be helpful in developing novel therapies. “Using sialic acid to assess mucus concentrations is much easier than other methods, so it could help in developing mucolytic treatments. Also, adenosine metabolism represents a novel therapeutic target in COPD. Drugs that modify adenosine metabolism that have been approved for other diseases such as gout could be tested in COPD. As with mucus hydration, the biomarkers we identified (particularly hypoxanthine) could be utilized to make sure that novel therapies are having the intended impact on airway adenosine metabolism.”
The research was supported by SPIROMICS (funded by NIH and the COPD Foundation). Dr. Esther reported having no relevant disclosures.
Examining sputum from patients with chronic obstructive pulmonary disease may help predict the course of the disease.
A mass spectrometric panel of biomarkers related to mucus hydration and inflammation examined in sputa showed elevated levels of metabolites from multiple pathways in patients with COPD. These correlated with sputum neutrophil counts and COPD exacerbations. In particular, sialic acid and hypoxanthine concentrations were strongly associated with disease severity, according to a study reported in the journal CHEST® authored by Charles R. Esther Jr. MD, PhD, and colleagues.
Given that an improved understanding of the pathways associated with airway pathophysiology in COPD will identify new predictive biomarkers and novel therapeutic targets, Dr. Esther and colleagues posed the question: Which physiologic pathways are altered and predict exacerbations in the airways of subjects with COPD?
They noted that in persons with COPD – characterized by dominant small airway obstruction associated with airway inflammation – multiple inflammatory pathways, as well as indices of oxidative stress (including oxidized glutathione and 8-isoprostane), are elevated in sputum. Because inflammation is a challenging therapeutic target, identification of other biologic pathways involved in COPD pathogenesis could point to novel biomarkers and therapeutic targets.
Using this approach in cystic fibrosis (CF), the authors have previously identified small molecule metabolites correlated with airway inflammation. Findings from that research supported development of a mass spectrometric biomarker panel for simultaneous measurement of inflammatory markers coupled to biomarkers of mucus hydration. The researchers applied this technology to sputum supernatants collected through the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), which included subjects with COPD, as well as relevant smoking and nonsmoking controls.
Addressing inflammation
“Inhaled steroids are really more effective for allergic inflammation as in asthma and less so for the neutrophilic inflammation that dominates in COPD. The challenge is that neutrophilic inflammation is also a key response to infection, and it’s really hard to find an anti-inflammatory that suppresses neutrophilic inflammation well enough to get clinical benefit but not so much that the patient becomes vulnerable to infection. Lots of clinical trials of anti-inflammatories in cystic fibrosis or COPD have been stopped because treated subjects had more trouble with infection,” Dr. Esther stated in an interview,
The investigators analyzed cell-free sputum supernatants from 980 subjects, including samples from 77 healthy nonsmokers (NS), 341 ever-smokers with preserved spirometry (SPS), and 562 subjects with COPD (178 GOLD [Global Initiative for Chronic Obstructive Lung Disease]1, 303 GOLD 2, and 81 GOLD 3). Among the subjects with COPD, elevated biomarkers from multiple pathways correlated with sputum neutrophil counts.
The most significant analytes (at FDR [False Discovery Rate] 0.1) were sialic acid (a mucin marker), hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione, with sialic acid and hypoxanthine strongly associated with measures of disease severity. Elevation of sialic acid and hypoxanthine were associated with shorter time to exacerbation and improved prediction models of future exacerbations.
Study results
Sialic acid was elevated in all GOLD groups relative to NS healthy controls, with a 2.8-fold (0.44 log) increase in GOLD 2 and 3.7 fold (0.56 log) increase in GOLD 3 relative to NS. Sialic acid was also elevated in the most severe disease cohorts (GOLD 2 and GOLD 3) relative to smokers with preserved spirometry (SPS) and those with less severe disease (GOLD 1).
Because mucin secretion and inflammation are also related to the pathophysiology of pulmonary exacerbations, Dr. Esther and colleagues had hypothesized that sputum biomarkers would be predictive of future exacerbations. Within the full cohort, both sialic acid and hypoxanthine were significantly elevated in those who had multiple (two or more) pulmonary exacerbations relative to those who had none (P = .001). Similar, though less significant findings were observed for xanthine (P = .01), methylthioadenosine (P = .01), adenine (P = .01), and glutathione (P = .01).
Sputum tests needed
While tests still need to be developed, Dr. Esther noted in an interview that they would be based on well-established technologies commonly utilized in clinical laboratories. “Sputum biomarkers of mucus hydration and adenosine metabolism could help clinicians predict which patients with COPD are likely to experience multiple pulmonary exacerbations. Tests would be applied to patients with COPD at higher risk for exacerbations; for example, those who have low lung function or a history of prior exacerbations.”
Dr. Esther noted that these biomarkers could be helpful in developing novel therapies. “Using sialic acid to assess mucus concentrations is much easier than other methods, so it could help in developing mucolytic treatments. Also, adenosine metabolism represents a novel therapeutic target in COPD. Drugs that modify adenosine metabolism that have been approved for other diseases such as gout could be tested in COPD. As with mucus hydration, the biomarkers we identified (particularly hypoxanthine) could be utilized to make sure that novel therapies are having the intended impact on airway adenosine metabolism.”
The research was supported by SPIROMICS (funded by NIH and the COPD Foundation). Dr. Esther reported having no relevant disclosures.
FROM THE JOURNAL CHEST®
D-dimer thresholds rule out PE in meta-analysis
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
FROM THE ANNALS OF INTERNAL MEDICINE
Booster recommendations for pregnant women, teens, and other groups explained
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
Idiopathic pulmonary fibrosis – a mortality predictor found?
A low lymphocyte-to-monocyte ratio (LMR) is associated with worse survival in newly-diagnosed patients with idiopathic pulmonary fibrosis (IPF), according to a new retrospective, single-center analysis. Patients with both IPF and lung cancer also had a lower LMR than patients with IPF alone.
The study, published online in Respiratory Medicine, was conducted among 77 newly diagnosed patients, 40 end-stage IPF patients, and 17 patients with IPF and lung cancer. All received at least 1 year of antifibrotic therapy (pirfenidone or nintedanib). The researchers collected demographic and clinical data between December 2014 and December 2020.
The disease course of IPF is difficult to predict, with some patients progressing slowly, and others suffer a rapid decline to respiratory failure. Previous studies found that higher levels of monocytes are associated with higher mortality in IPF and other fibrotic lung disease, and both neutrophil-to-lymphocyte ratio (NLR) and LMR have been shown to predict mortality in lung cancer.
A previous study found that IPF patients had a higher NLR and a lower LMR than controls, but that research did not consider the impact of antifibrotic treatment, which may improve outcomes.
There has been accumulating cellular and molecular evidence that leukocyte population abnormalities are associated with IPF outcomes, but that work was more discovery based and relied on tests that aren’t readily available clinically, said Erica L. Herzog, MD, PhD, who was asked to comment on the study. “It’s provided a lot of insight into potential new mechanisms and potential biomarkers, but their clinical utility for patients is limited. So the use of lymphocyte-to-monocyte ratios that can be obtained from a complete blood cell count, which is a test that can be done in any hospital, would really be a game-changer in terms of predictive algorithms for patients with IPF,” said Dr. Herzog, who is a professor of medicine and pathology at Yale University, New Haven, Conn., and director of the Yale ILD Center of Excellence.
In humans, abnormalities in circulating monocytes and lymphocytes have individually been linked worse IPF outcomes. Animal studies have implicated monocyte-derived cells in lung fibrosis, but because animal studies have shown that lymphocyte populations are not required for fibrosis, more work is needed.
“I think what we’re finding is that lymphocytes probably have a regulatory role, and there’s probably a protective population and potentially a pathogenic population. Something about the balance between adaptive immunity, which is reflected by your lymphocytes, and innate immunity, which is reflected by your monocytes. Something about that balance is important for tissue homeostasis, and then when it’s disrupted or perturbed, fibrosis ensues,” said Dr. Herzog.
Study details
The newly diagnosed patients were older (mean age, 70 years) than the end-stage IPF patients (mean age, 60 years) and patients with IPF and lung cancer (mean age, 64 years; P < .0001).
Among newly diagnosed IPF patients, a receiving operating characteristic analysis before antifibrotic treatment determined a cutoff LMR value of less than 4.18, with an area under the curve of 0.67 (P = .025). Values below 4.18 were associated with shorter survival (hazard ratio, 6.88; P = .027).
Patients with LMR less than 4.18 were more likely to be men (89% vs. 67%; P = .036), had a lower percent predicted forced vital capacity (76% vs. 87%; P = .023), and were more likely to die or undergo lung transplant (34% vs. 5%; P = .009).
Mortality results
A Kaplan-Meier curve illustrated a stark difference between patients with LMR of 4.18 or higher, nearly all of whom remained alive out to almost 100 months of follow-up. Around 30% of those with LMR less than 4.18 remained alive while close to 100% of the patients with LMR below this value showed worsened outcomes. “You don’t normally see curves like that,” said Dr. Herzog.
There was no significant difference in blood cell counts and ratios at the time of IPF diagnosis and after 1 year of antifibrotic treatment, which suggests that the risk profile is independent of treatment, according to the study authors.
Among patient subgroups, those with IPF and lung cancer had the lowest mean LMR (2.2), followed by newly diagnosed patients (3.5), and those with end-stage disease (3.6; P < .0001).
The study authors reported financial relationships with various pharmaceutical companies. Dr. Herzog had no relevant financial disclosures.
A low lymphocyte-to-monocyte ratio (LMR) is associated with worse survival in newly-diagnosed patients with idiopathic pulmonary fibrosis (IPF), according to a new retrospective, single-center analysis. Patients with both IPF and lung cancer also had a lower LMR than patients with IPF alone.
The study, published online in Respiratory Medicine, was conducted among 77 newly diagnosed patients, 40 end-stage IPF patients, and 17 patients with IPF and lung cancer. All received at least 1 year of antifibrotic therapy (pirfenidone or nintedanib). The researchers collected demographic and clinical data between December 2014 and December 2020.
The disease course of IPF is difficult to predict, with some patients progressing slowly, and others suffer a rapid decline to respiratory failure. Previous studies found that higher levels of monocytes are associated with higher mortality in IPF and other fibrotic lung disease, and both neutrophil-to-lymphocyte ratio (NLR) and LMR have been shown to predict mortality in lung cancer.
A previous study found that IPF patients had a higher NLR and a lower LMR than controls, but that research did not consider the impact of antifibrotic treatment, which may improve outcomes.
There has been accumulating cellular and molecular evidence that leukocyte population abnormalities are associated with IPF outcomes, but that work was more discovery based and relied on tests that aren’t readily available clinically, said Erica L. Herzog, MD, PhD, who was asked to comment on the study. “It’s provided a lot of insight into potential new mechanisms and potential biomarkers, but their clinical utility for patients is limited. So the use of lymphocyte-to-monocyte ratios that can be obtained from a complete blood cell count, which is a test that can be done in any hospital, would really be a game-changer in terms of predictive algorithms for patients with IPF,” said Dr. Herzog, who is a professor of medicine and pathology at Yale University, New Haven, Conn., and director of the Yale ILD Center of Excellence.
In humans, abnormalities in circulating monocytes and lymphocytes have individually been linked worse IPF outcomes. Animal studies have implicated monocyte-derived cells in lung fibrosis, but because animal studies have shown that lymphocyte populations are not required for fibrosis, more work is needed.
“I think what we’re finding is that lymphocytes probably have a regulatory role, and there’s probably a protective population and potentially a pathogenic population. Something about the balance between adaptive immunity, which is reflected by your lymphocytes, and innate immunity, which is reflected by your monocytes. Something about that balance is important for tissue homeostasis, and then when it’s disrupted or perturbed, fibrosis ensues,” said Dr. Herzog.
Study details
The newly diagnosed patients were older (mean age, 70 years) than the end-stage IPF patients (mean age, 60 years) and patients with IPF and lung cancer (mean age, 64 years; P < .0001).
Among newly diagnosed IPF patients, a receiving operating characteristic analysis before antifibrotic treatment determined a cutoff LMR value of less than 4.18, with an area under the curve of 0.67 (P = .025). Values below 4.18 were associated with shorter survival (hazard ratio, 6.88; P = .027).
Patients with LMR less than 4.18 were more likely to be men (89% vs. 67%; P = .036), had a lower percent predicted forced vital capacity (76% vs. 87%; P = .023), and were more likely to die or undergo lung transplant (34% vs. 5%; P = .009).
Mortality results
A Kaplan-Meier curve illustrated a stark difference between patients with LMR of 4.18 or higher, nearly all of whom remained alive out to almost 100 months of follow-up. Around 30% of those with LMR less than 4.18 remained alive while close to 100% of the patients with LMR below this value showed worsened outcomes. “You don’t normally see curves like that,” said Dr. Herzog.
There was no significant difference in blood cell counts and ratios at the time of IPF diagnosis and after 1 year of antifibrotic treatment, which suggests that the risk profile is independent of treatment, according to the study authors.
Among patient subgroups, those with IPF and lung cancer had the lowest mean LMR (2.2), followed by newly diagnosed patients (3.5), and those with end-stage disease (3.6; P < .0001).
The study authors reported financial relationships with various pharmaceutical companies. Dr. Herzog had no relevant financial disclosures.
A low lymphocyte-to-monocyte ratio (LMR) is associated with worse survival in newly-diagnosed patients with idiopathic pulmonary fibrosis (IPF), according to a new retrospective, single-center analysis. Patients with both IPF and lung cancer also had a lower LMR than patients with IPF alone.
The study, published online in Respiratory Medicine, was conducted among 77 newly diagnosed patients, 40 end-stage IPF patients, and 17 patients with IPF and lung cancer. All received at least 1 year of antifibrotic therapy (pirfenidone or nintedanib). The researchers collected demographic and clinical data between December 2014 and December 2020.
The disease course of IPF is difficult to predict, with some patients progressing slowly, and others suffer a rapid decline to respiratory failure. Previous studies found that higher levels of monocytes are associated with higher mortality in IPF and other fibrotic lung disease, and both neutrophil-to-lymphocyte ratio (NLR) and LMR have been shown to predict mortality in lung cancer.
A previous study found that IPF patients had a higher NLR and a lower LMR than controls, but that research did not consider the impact of antifibrotic treatment, which may improve outcomes.
There has been accumulating cellular and molecular evidence that leukocyte population abnormalities are associated with IPF outcomes, but that work was more discovery based and relied on tests that aren’t readily available clinically, said Erica L. Herzog, MD, PhD, who was asked to comment on the study. “It’s provided a lot of insight into potential new mechanisms and potential biomarkers, but their clinical utility for patients is limited. So the use of lymphocyte-to-monocyte ratios that can be obtained from a complete blood cell count, which is a test that can be done in any hospital, would really be a game-changer in terms of predictive algorithms for patients with IPF,” said Dr. Herzog, who is a professor of medicine and pathology at Yale University, New Haven, Conn., and director of the Yale ILD Center of Excellence.
In humans, abnormalities in circulating monocytes and lymphocytes have individually been linked worse IPF outcomes. Animal studies have implicated monocyte-derived cells in lung fibrosis, but because animal studies have shown that lymphocyte populations are not required for fibrosis, more work is needed.
“I think what we’re finding is that lymphocytes probably have a regulatory role, and there’s probably a protective population and potentially a pathogenic population. Something about the balance between adaptive immunity, which is reflected by your lymphocytes, and innate immunity, which is reflected by your monocytes. Something about that balance is important for tissue homeostasis, and then when it’s disrupted or perturbed, fibrosis ensues,” said Dr. Herzog.
Study details
The newly diagnosed patients were older (mean age, 70 years) than the end-stage IPF patients (mean age, 60 years) and patients with IPF and lung cancer (mean age, 64 years; P < .0001).
Among newly diagnosed IPF patients, a receiving operating characteristic analysis before antifibrotic treatment determined a cutoff LMR value of less than 4.18, with an area under the curve of 0.67 (P = .025). Values below 4.18 were associated with shorter survival (hazard ratio, 6.88; P = .027).
Patients with LMR less than 4.18 were more likely to be men (89% vs. 67%; P = .036), had a lower percent predicted forced vital capacity (76% vs. 87%; P = .023), and were more likely to die or undergo lung transplant (34% vs. 5%; P = .009).
Mortality results
A Kaplan-Meier curve illustrated a stark difference between patients with LMR of 4.18 or higher, nearly all of whom remained alive out to almost 100 months of follow-up. Around 30% of those with LMR less than 4.18 remained alive while close to 100% of the patients with LMR below this value showed worsened outcomes. “You don’t normally see curves like that,” said Dr. Herzog.
There was no significant difference in blood cell counts and ratios at the time of IPF diagnosis and after 1 year of antifibrotic treatment, which suggests that the risk profile is independent of treatment, according to the study authors.
Among patient subgroups, those with IPF and lung cancer had the lowest mean LMR (2.2), followed by newly diagnosed patients (3.5), and those with end-stage disease (3.6; P < .0001).
The study authors reported financial relationships with various pharmaceutical companies. Dr. Herzog had no relevant financial disclosures.
FROM RESPIRATORY MEDICINE
Transitioning from fellow to attending
It’s day 1 of “attendingship,” and I’m back to wearing my white coat after years of being confident enough in myself to think I didn’t need it to look like “the doctor.” Is it okay to park here? Does my clinic have a staff bathroom? Will my log in work? Oh my gosh, how do I place orders?! I remember this feeling – it’s intern year all over again, except there’s no senior resident to rescue me now – here we go!
Starting off
As a new attending, the amount of responsibility can be intimidating and overwhelming. It is important to remember that you are not alone, you have a whole team supporting you whether you are in clinic or the ICU. Be sure to introduce yourself to those who you will be working with, get to know them, their roles, and figure out the best way that you can help each other with the ultimate goal of helping patients. In addition to meeting your own team, it is important to introduce yourself to your new colleagues – especially if you are new to the institution. Drs. Fielder and Sihag suggest putting together an introductory email to those who may be referring to you that includes an overview of what you do and how you can help, as well as your contact information. They also suggest maintaining an open line of communication and keeping the referring provider updated on your mutual patient (Fiedler AG, Sihag S. J Thorac Cardiovasc Surg. 2020 Mar;159[3]:1156-60). While this may sound antiquated, in my experience thus far, my colleagues have greatly appreciated this gesture.
Finding support
Even though you will be surrounded by a plethora of new colleagues, the transition to attending can be lonely – especially if you are moving to a new institution. Be sure to keep in touch with your co-residents, co-fellows, mentors, and, of course, your friends and family. Studies have shown that support mitigates stress and reduces job strain, which can lead to better health outcomes in the long term (Fiedler AG, Sihag S. [above]). Another great source of support for me is my CHEST colleagues. If you have not already, I highly suggest joining the CHEST Network(s) that aligns with your career interests. This is a great way to not only network with those who share the same niche as you but also to explore academic opportunities outside of your institution. Through the CHEST Home Mechanical Ventilation and Sleep NetWorks, I have gained mentors, made friends, and have become more involved in CHEST’s annual meeting, chairing my first session this year.
Staying organized
Adjusting to your new schedule can be just as hard as adjusting to a new role or new institution. After years of moving through the well-oiled, regimented machine that is medical training, there are suddenly no more rotations, no more research blocks, and no more protected time for learning. Dr. Okereke suggests creating a weekly calendar, which blocks time for not only your clinical duties but for studying (as you will be taking boards during your first year), academic endeavors (teaching and/or research), and, most importantly – for fun (Okereke I. J Thorac Cardiovasc Surg. 2020 Mar;159[3]:1161-2). Being cognizant about maintaining work-life balance is key once you become an attending. It is finally time to learn how to take time off, away from all things work, and to not feel guilty about it.
Saying no
This brings me to saying “no.” We are taught to say “yes” to every opportunity throughout our careers and, while that can certainly help us get far, it can also lead to burnout. Once you’re an attending, you’re in it for the long haul, so best to say yes to the things you are most interested in and “spark joy,” as Marie Kondo says, and say no to the things that do not make you happy and are not congruent with your overall goals. Fielder and Sihag (above) note that your division director or chief typically has a vision in mind for you within the department. It is important to communicate with leadership so that everyone is on the same page and the administrative and academic opportunities afforded to you are in alignment with your career goals going forward.
Teaching trainees
To prepare for teaching as an attending, Dr. Greco recommends starting during your own training. She suggests cataloging your study materials and notes for later reference, curating talks throughout your training, and exploring different rounding styles prior to graduation (Greco, A. CHEST Thought Leader Blog. 2021 June). To get more experience in formal speaking, Dr. Shen and colleagues encourage getting involved in resident noon conferences (Shen JZ, Memon AA, Lin C. Stroke. 2019 Sep;50[9]:e250-e252). A benefit of being a critical care attending is that you can gain experience teaching not only with the internal medicine residents but with emergency medicine, anesthesiology, and critical care advanced practice provider residents, as well.
While lecturing is one thing, teaching on service is a whole different ball game. No matter how young, fun, and relatable you think you are, you’re the boss now. You’re the giver of grades and the writer of evaluations. It is important to be self-aware of your influence and be deliberate with the environment you create on rounds and in clinic. Set expectations on day 1 so that everyone understands. Be open with what you are working on. For example, I make daily goals for myself that I share with the team before rounds. Drs. Fielder and Sihag (above) suggest sharing anecdotes from your own time in training that can help both you and your trainees remember that you were just in their shoes. Allowing yourself to be vulnerable creates a safe space in which your learners feel more comfortable doing the same.
Lastly, delegation is key. While many of us have done this since residency, Dr. Shen et al (above) suggest deliberately practicing this during fellowship. If you were the fellow who was able to handle a lot on your own, trust that your own fellows will be able to do that. Delegating to your trainees helps you improve personal and team efficiency, provides fellows with needed autonomy, and allows you to further grow into the role of attending physician.
Conclusion
While you may be nervous starting out, trust that you have been well trained and have the clinical knowledge and skills you need to do your job – you are ready. Get to know the staff you will be working with, your colleagues, and keep in touch with your co-trainees and mentors who have helped you along the way. Make daily goals for yourself, and make time to read and reflect so that you can continue to learn and grow. Most of all, make time for yourself, your friends, and your family, because after years of supporting you through all of your hard work, you’ve finally made it – congrats!
Dr. Greer is Assistant Professor of Medicine, Emory University Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Atlanta, GA.
It’s day 1 of “attendingship,” and I’m back to wearing my white coat after years of being confident enough in myself to think I didn’t need it to look like “the doctor.” Is it okay to park here? Does my clinic have a staff bathroom? Will my log in work? Oh my gosh, how do I place orders?! I remember this feeling – it’s intern year all over again, except there’s no senior resident to rescue me now – here we go!
Starting off
As a new attending, the amount of responsibility can be intimidating and overwhelming. It is important to remember that you are not alone, you have a whole team supporting you whether you are in clinic or the ICU. Be sure to introduce yourself to those who you will be working with, get to know them, their roles, and figure out the best way that you can help each other with the ultimate goal of helping patients. In addition to meeting your own team, it is important to introduce yourself to your new colleagues – especially if you are new to the institution. Drs. Fielder and Sihag suggest putting together an introductory email to those who may be referring to you that includes an overview of what you do and how you can help, as well as your contact information. They also suggest maintaining an open line of communication and keeping the referring provider updated on your mutual patient (Fiedler AG, Sihag S. J Thorac Cardiovasc Surg. 2020 Mar;159[3]:1156-60). While this may sound antiquated, in my experience thus far, my colleagues have greatly appreciated this gesture.
Finding support
Even though you will be surrounded by a plethora of new colleagues, the transition to attending can be lonely – especially if you are moving to a new institution. Be sure to keep in touch with your co-residents, co-fellows, mentors, and, of course, your friends and family. Studies have shown that support mitigates stress and reduces job strain, which can lead to better health outcomes in the long term (Fiedler AG, Sihag S. [above]). Another great source of support for me is my CHEST colleagues. If you have not already, I highly suggest joining the CHEST Network(s) that aligns with your career interests. This is a great way to not only network with those who share the same niche as you but also to explore academic opportunities outside of your institution. Through the CHEST Home Mechanical Ventilation and Sleep NetWorks, I have gained mentors, made friends, and have become more involved in CHEST’s annual meeting, chairing my first session this year.
Staying organized
Adjusting to your new schedule can be just as hard as adjusting to a new role or new institution. After years of moving through the well-oiled, regimented machine that is medical training, there are suddenly no more rotations, no more research blocks, and no more protected time for learning. Dr. Okereke suggests creating a weekly calendar, which blocks time for not only your clinical duties but for studying (as you will be taking boards during your first year), academic endeavors (teaching and/or research), and, most importantly – for fun (Okereke I. J Thorac Cardiovasc Surg. 2020 Mar;159[3]:1161-2). Being cognizant about maintaining work-life balance is key once you become an attending. It is finally time to learn how to take time off, away from all things work, and to not feel guilty about it.
Saying no
This brings me to saying “no.” We are taught to say “yes” to every opportunity throughout our careers and, while that can certainly help us get far, it can also lead to burnout. Once you’re an attending, you’re in it for the long haul, so best to say yes to the things you are most interested in and “spark joy,” as Marie Kondo says, and say no to the things that do not make you happy and are not congruent with your overall goals. Fielder and Sihag (above) note that your division director or chief typically has a vision in mind for you within the department. It is important to communicate with leadership so that everyone is on the same page and the administrative and academic opportunities afforded to you are in alignment with your career goals going forward.
Teaching trainees
To prepare for teaching as an attending, Dr. Greco recommends starting during your own training. She suggests cataloging your study materials and notes for later reference, curating talks throughout your training, and exploring different rounding styles prior to graduation (Greco, A. CHEST Thought Leader Blog. 2021 June). To get more experience in formal speaking, Dr. Shen and colleagues encourage getting involved in resident noon conferences (Shen JZ, Memon AA, Lin C. Stroke. 2019 Sep;50[9]:e250-e252). A benefit of being a critical care attending is that you can gain experience teaching not only with the internal medicine residents but with emergency medicine, anesthesiology, and critical care advanced practice provider residents, as well.
While lecturing is one thing, teaching on service is a whole different ball game. No matter how young, fun, and relatable you think you are, you’re the boss now. You’re the giver of grades and the writer of evaluations. It is important to be self-aware of your influence and be deliberate with the environment you create on rounds and in clinic. Set expectations on day 1 so that everyone understands. Be open with what you are working on. For example, I make daily goals for myself that I share with the team before rounds. Drs. Fielder and Sihag (above) suggest sharing anecdotes from your own time in training that can help both you and your trainees remember that you were just in their shoes. Allowing yourself to be vulnerable creates a safe space in which your learners feel more comfortable doing the same.
Lastly, delegation is key. While many of us have done this since residency, Dr. Shen et al (above) suggest deliberately practicing this during fellowship. If you were the fellow who was able to handle a lot on your own, trust that your own fellows will be able to do that. Delegating to your trainees helps you improve personal and team efficiency, provides fellows with needed autonomy, and allows you to further grow into the role of attending physician.
Conclusion
While you may be nervous starting out, trust that you have been well trained and have the clinical knowledge and skills you need to do your job – you are ready. Get to know the staff you will be working with, your colleagues, and keep in touch with your co-trainees and mentors who have helped you along the way. Make daily goals for yourself, and make time to read and reflect so that you can continue to learn and grow. Most of all, make time for yourself, your friends, and your family, because after years of supporting you through all of your hard work, you’ve finally made it – congrats!
Dr. Greer is Assistant Professor of Medicine, Emory University Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Atlanta, GA.
It’s day 1 of “attendingship,” and I’m back to wearing my white coat after years of being confident enough in myself to think I didn’t need it to look like “the doctor.” Is it okay to park here? Does my clinic have a staff bathroom? Will my log in work? Oh my gosh, how do I place orders?! I remember this feeling – it’s intern year all over again, except there’s no senior resident to rescue me now – here we go!
Starting off
As a new attending, the amount of responsibility can be intimidating and overwhelming. It is important to remember that you are not alone, you have a whole team supporting you whether you are in clinic or the ICU. Be sure to introduce yourself to those who you will be working with, get to know them, their roles, and figure out the best way that you can help each other with the ultimate goal of helping patients. In addition to meeting your own team, it is important to introduce yourself to your new colleagues – especially if you are new to the institution. Drs. Fielder and Sihag suggest putting together an introductory email to those who may be referring to you that includes an overview of what you do and how you can help, as well as your contact information. They also suggest maintaining an open line of communication and keeping the referring provider updated on your mutual patient (Fiedler AG, Sihag S. J Thorac Cardiovasc Surg. 2020 Mar;159[3]:1156-60). While this may sound antiquated, in my experience thus far, my colleagues have greatly appreciated this gesture.
Finding support
Even though you will be surrounded by a plethora of new colleagues, the transition to attending can be lonely – especially if you are moving to a new institution. Be sure to keep in touch with your co-residents, co-fellows, mentors, and, of course, your friends and family. Studies have shown that support mitigates stress and reduces job strain, which can lead to better health outcomes in the long term (Fiedler AG, Sihag S. [above]). Another great source of support for me is my CHEST colleagues. If you have not already, I highly suggest joining the CHEST Network(s) that aligns with your career interests. This is a great way to not only network with those who share the same niche as you but also to explore academic opportunities outside of your institution. Through the CHEST Home Mechanical Ventilation and Sleep NetWorks, I have gained mentors, made friends, and have become more involved in CHEST’s annual meeting, chairing my first session this year.
Staying organized
Adjusting to your new schedule can be just as hard as adjusting to a new role or new institution. After years of moving through the well-oiled, regimented machine that is medical training, there are suddenly no more rotations, no more research blocks, and no more protected time for learning. Dr. Okereke suggests creating a weekly calendar, which blocks time for not only your clinical duties but for studying (as you will be taking boards during your first year), academic endeavors (teaching and/or research), and, most importantly – for fun (Okereke I. J Thorac Cardiovasc Surg. 2020 Mar;159[3]:1161-2). Being cognizant about maintaining work-life balance is key once you become an attending. It is finally time to learn how to take time off, away from all things work, and to not feel guilty about it.
Saying no
This brings me to saying “no.” We are taught to say “yes” to every opportunity throughout our careers and, while that can certainly help us get far, it can also lead to burnout. Once you’re an attending, you’re in it for the long haul, so best to say yes to the things you are most interested in and “spark joy,” as Marie Kondo says, and say no to the things that do not make you happy and are not congruent with your overall goals. Fielder and Sihag (above) note that your division director or chief typically has a vision in mind for you within the department. It is important to communicate with leadership so that everyone is on the same page and the administrative and academic opportunities afforded to you are in alignment with your career goals going forward.
Teaching trainees
To prepare for teaching as an attending, Dr. Greco recommends starting during your own training. She suggests cataloging your study materials and notes for later reference, curating talks throughout your training, and exploring different rounding styles prior to graduation (Greco, A. CHEST Thought Leader Blog. 2021 June). To get more experience in formal speaking, Dr. Shen and colleagues encourage getting involved in resident noon conferences (Shen JZ, Memon AA, Lin C. Stroke. 2019 Sep;50[9]:e250-e252). A benefit of being a critical care attending is that you can gain experience teaching not only with the internal medicine residents but with emergency medicine, anesthesiology, and critical care advanced practice provider residents, as well.
While lecturing is one thing, teaching on service is a whole different ball game. No matter how young, fun, and relatable you think you are, you’re the boss now. You’re the giver of grades and the writer of evaluations. It is important to be self-aware of your influence and be deliberate with the environment you create on rounds and in clinic. Set expectations on day 1 so that everyone understands. Be open with what you are working on. For example, I make daily goals for myself that I share with the team before rounds. Drs. Fielder and Sihag (above) suggest sharing anecdotes from your own time in training that can help both you and your trainees remember that you were just in their shoes. Allowing yourself to be vulnerable creates a safe space in which your learners feel more comfortable doing the same.
Lastly, delegation is key. While many of us have done this since residency, Dr. Shen et al (above) suggest deliberately practicing this during fellowship. If you were the fellow who was able to handle a lot on your own, trust that your own fellows will be able to do that. Delegating to your trainees helps you improve personal and team efficiency, provides fellows with needed autonomy, and allows you to further grow into the role of attending physician.
Conclusion
While you may be nervous starting out, trust that you have been well trained and have the clinical knowledge and skills you need to do your job – you are ready. Get to know the staff you will be working with, your colleagues, and keep in touch with your co-trainees and mentors who have helped you along the way. Make daily goals for yourself, and make time to read and reflect so that you can continue to learn and grow. Most of all, make time for yourself, your friends, and your family, because after years of supporting you through all of your hard work, you’ve finally made it – congrats!
Dr. Greer is Assistant Professor of Medicine, Emory University Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Atlanta, GA.
Risk for severe COVID-19 and death plummets with Pfizer booster
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Intent to vaccinate kids against COVID higher among vaccinated parents
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
FROM JAMA PEDIATRICS
Apixaban outmatches rivaroxaban for VTE in study
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
No serious CV risks for elderly after Pfizer COVID-19 vaccine
A French population-based study provides further evidence that the BNT162b2 Pfizer-BioNTech mRNA COVID-19 vaccine does not increase the short-term risk for serious cardiovascular adverse events in older people.
The study showed no increased risk of myocardial infarction (MI), stroke, or pulmonary embolism (PE) following vaccination in adults aged 75 years or older in the 14 days following vaccination.
“These findings regarding the BNT162b2 vaccine’s short-term cardiovascular safety profile in older people are reassuring. They should be taken into account by doctors when considering implementing a third dose of the vaccine in older people,” Marie Joelle Jabagi, PharmD, PhD, with the French National Agency for Medicines and Health Products Safety, Saint-Denis, France, said in an interview.
The study was published as a research letter online Nov. 22 in JAMA.
The Pfizer-BioNTech mRNA vaccine was the first SARS-CoV-2 vaccine authorized in France and has been widely used in older people. The phase 3 trials of the vaccine showed no increase in cardiovascular events, but older people were underrepresented in the trials.
As of April 30, 2021, nearly 3.9 million French adults aged 75 or older had received at least one dose of the Pfizer COVID-19 vaccine and 3.2 million had received two doses.
Using the French National Health Data System linked to the national COVID-19 vaccination database, Dr. Jabagi and her colleagues identified all unvaccinated or vaccinated adults aged 75 and older who were hospitalized between Dec. 15, 2020, and April 30, 2021, for acute MI, hemorrhagic or ischemic stroke, or PE.
During the 4.5-month study period, 11,113 elderly were hospitalized for acute MI, 17,014 for ischemic stroke, 4,804 for hemorrhagic stroke, and 7,221 for PE. Of these, 58.6%, 54.0%, 42.7%, and 55.3%, respectively, had received at least one dose of vaccine.
In the 14 days following receipt of either dose, no significant increased risk was found for any outcome, the investigators report.
The relative incidence (RI) for MI after the first and second dose was 0.97 (95% CI, 0.88-1.06) and 1.04 (95% CI, 0.93-1.16), respectively.
For ischemic stroke, the RI was 0.90 after the first dose (95% CI, 0.84-0.98) and 0.92 (95% CI, 0.84-1.02) after the second; for hemorrhagic stroke, the RI was 0.90 (95% CI, 0.78-1.04) and 0.97 (95% CI, 0.81-1.15), respectively.
For PE, the RI was 0.85 (95% CI, 0.75-0.96) after the first dose and 1.10 (95% CI, 0.95-1.26) after the second dose.
There was also no significant increase for any of the cardiovascular events when the exposure risk window was subdivided into 1 to 7 days and 8 to 14 days.
“Evaluating the short-term risk of hospitalization for severe cardiovascular events after the BNT162b2 mRNA vaccine in older people was a priority, especially after signals for hypertension and cardiovascular, thromboembolic, and hemorrhagic events have been issued from spontaneous notification data,” Dr. Jabagi said in an interview.
“The results of this nationwide study provide further solid evidence regarding the lack of increase of serious cardiovascular adverse events in older people in the 14 days following both doses of the vaccine,” Dr. Jabagi said.
The French study supports a recent U.S. study of more than 6 million people demonstrating that serious health risks were no more common in the first 3 weeks after Pfizer/BioNTech or Moderna COVID-19 vaccination compared with 22 to 42 days later.
As previously reported by this news organization, mRNA vaccination was not associated with greater risks for Guillain-Barré syndrome, myocarditis/pericarditis, stroke, or 20 other serious outcomes.
The current study had no specific funding. Dr. Jabagi and colleagues have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A French population-based study provides further evidence that the BNT162b2 Pfizer-BioNTech mRNA COVID-19 vaccine does not increase the short-term risk for serious cardiovascular adverse events in older people.
The study showed no increased risk of myocardial infarction (MI), stroke, or pulmonary embolism (PE) following vaccination in adults aged 75 years or older in the 14 days following vaccination.
“These findings regarding the BNT162b2 vaccine’s short-term cardiovascular safety profile in older people are reassuring. They should be taken into account by doctors when considering implementing a third dose of the vaccine in older people,” Marie Joelle Jabagi, PharmD, PhD, with the French National Agency for Medicines and Health Products Safety, Saint-Denis, France, said in an interview.
The study was published as a research letter online Nov. 22 in JAMA.
The Pfizer-BioNTech mRNA vaccine was the first SARS-CoV-2 vaccine authorized in France and has been widely used in older people. The phase 3 trials of the vaccine showed no increase in cardiovascular events, but older people were underrepresented in the trials.
As of April 30, 2021, nearly 3.9 million French adults aged 75 or older had received at least one dose of the Pfizer COVID-19 vaccine and 3.2 million had received two doses.
Using the French National Health Data System linked to the national COVID-19 vaccination database, Dr. Jabagi and her colleagues identified all unvaccinated or vaccinated adults aged 75 and older who were hospitalized between Dec. 15, 2020, and April 30, 2021, for acute MI, hemorrhagic or ischemic stroke, or PE.
During the 4.5-month study period, 11,113 elderly were hospitalized for acute MI, 17,014 for ischemic stroke, 4,804 for hemorrhagic stroke, and 7,221 for PE. Of these, 58.6%, 54.0%, 42.7%, and 55.3%, respectively, had received at least one dose of vaccine.
In the 14 days following receipt of either dose, no significant increased risk was found for any outcome, the investigators report.
The relative incidence (RI) for MI after the first and second dose was 0.97 (95% CI, 0.88-1.06) and 1.04 (95% CI, 0.93-1.16), respectively.
For ischemic stroke, the RI was 0.90 after the first dose (95% CI, 0.84-0.98) and 0.92 (95% CI, 0.84-1.02) after the second; for hemorrhagic stroke, the RI was 0.90 (95% CI, 0.78-1.04) and 0.97 (95% CI, 0.81-1.15), respectively.
For PE, the RI was 0.85 (95% CI, 0.75-0.96) after the first dose and 1.10 (95% CI, 0.95-1.26) after the second dose.
There was also no significant increase for any of the cardiovascular events when the exposure risk window was subdivided into 1 to 7 days and 8 to 14 days.
“Evaluating the short-term risk of hospitalization for severe cardiovascular events after the BNT162b2 mRNA vaccine in older people was a priority, especially after signals for hypertension and cardiovascular, thromboembolic, and hemorrhagic events have been issued from spontaneous notification data,” Dr. Jabagi said in an interview.
“The results of this nationwide study provide further solid evidence regarding the lack of increase of serious cardiovascular adverse events in older people in the 14 days following both doses of the vaccine,” Dr. Jabagi said.
The French study supports a recent U.S. study of more than 6 million people demonstrating that serious health risks were no more common in the first 3 weeks after Pfizer/BioNTech or Moderna COVID-19 vaccination compared with 22 to 42 days later.
As previously reported by this news organization, mRNA vaccination was not associated with greater risks for Guillain-Barré syndrome, myocarditis/pericarditis, stroke, or 20 other serious outcomes.
The current study had no specific funding. Dr. Jabagi and colleagues have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A French population-based study provides further evidence that the BNT162b2 Pfizer-BioNTech mRNA COVID-19 vaccine does not increase the short-term risk for serious cardiovascular adverse events in older people.
The study showed no increased risk of myocardial infarction (MI), stroke, or pulmonary embolism (PE) following vaccination in adults aged 75 years or older in the 14 days following vaccination.
“These findings regarding the BNT162b2 vaccine’s short-term cardiovascular safety profile in older people are reassuring. They should be taken into account by doctors when considering implementing a third dose of the vaccine in older people,” Marie Joelle Jabagi, PharmD, PhD, with the French National Agency for Medicines and Health Products Safety, Saint-Denis, France, said in an interview.
The study was published as a research letter online Nov. 22 in JAMA.
The Pfizer-BioNTech mRNA vaccine was the first SARS-CoV-2 vaccine authorized in France and has been widely used in older people. The phase 3 trials of the vaccine showed no increase in cardiovascular events, but older people were underrepresented in the trials.
As of April 30, 2021, nearly 3.9 million French adults aged 75 or older had received at least one dose of the Pfizer COVID-19 vaccine and 3.2 million had received two doses.
Using the French National Health Data System linked to the national COVID-19 vaccination database, Dr. Jabagi and her colleagues identified all unvaccinated or vaccinated adults aged 75 and older who were hospitalized between Dec. 15, 2020, and April 30, 2021, for acute MI, hemorrhagic or ischemic stroke, or PE.
During the 4.5-month study period, 11,113 elderly were hospitalized for acute MI, 17,014 for ischemic stroke, 4,804 for hemorrhagic stroke, and 7,221 for PE. Of these, 58.6%, 54.0%, 42.7%, and 55.3%, respectively, had received at least one dose of vaccine.
In the 14 days following receipt of either dose, no significant increased risk was found for any outcome, the investigators report.
The relative incidence (RI) for MI after the first and second dose was 0.97 (95% CI, 0.88-1.06) and 1.04 (95% CI, 0.93-1.16), respectively.
For ischemic stroke, the RI was 0.90 after the first dose (95% CI, 0.84-0.98) and 0.92 (95% CI, 0.84-1.02) after the second; for hemorrhagic stroke, the RI was 0.90 (95% CI, 0.78-1.04) and 0.97 (95% CI, 0.81-1.15), respectively.
For PE, the RI was 0.85 (95% CI, 0.75-0.96) after the first dose and 1.10 (95% CI, 0.95-1.26) after the second dose.
There was also no significant increase for any of the cardiovascular events when the exposure risk window was subdivided into 1 to 7 days and 8 to 14 days.
“Evaluating the short-term risk of hospitalization for severe cardiovascular events after the BNT162b2 mRNA vaccine in older people was a priority, especially after signals for hypertension and cardiovascular, thromboembolic, and hemorrhagic events have been issued from spontaneous notification data,” Dr. Jabagi said in an interview.
“The results of this nationwide study provide further solid evidence regarding the lack of increase of serious cardiovascular adverse events in older people in the 14 days following both doses of the vaccine,” Dr. Jabagi said.
The French study supports a recent U.S. study of more than 6 million people demonstrating that serious health risks were no more common in the first 3 weeks after Pfizer/BioNTech or Moderna COVID-19 vaccination compared with 22 to 42 days later.
As previously reported by this news organization, mRNA vaccination was not associated with greater risks for Guillain-Barré syndrome, myocarditis/pericarditis, stroke, or 20 other serious outcomes.
The current study had no specific funding. Dr. Jabagi and colleagues have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.