User login
Lupus mutation may unlock targeted drugs for patient subset
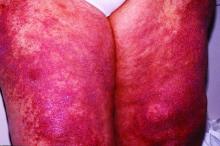
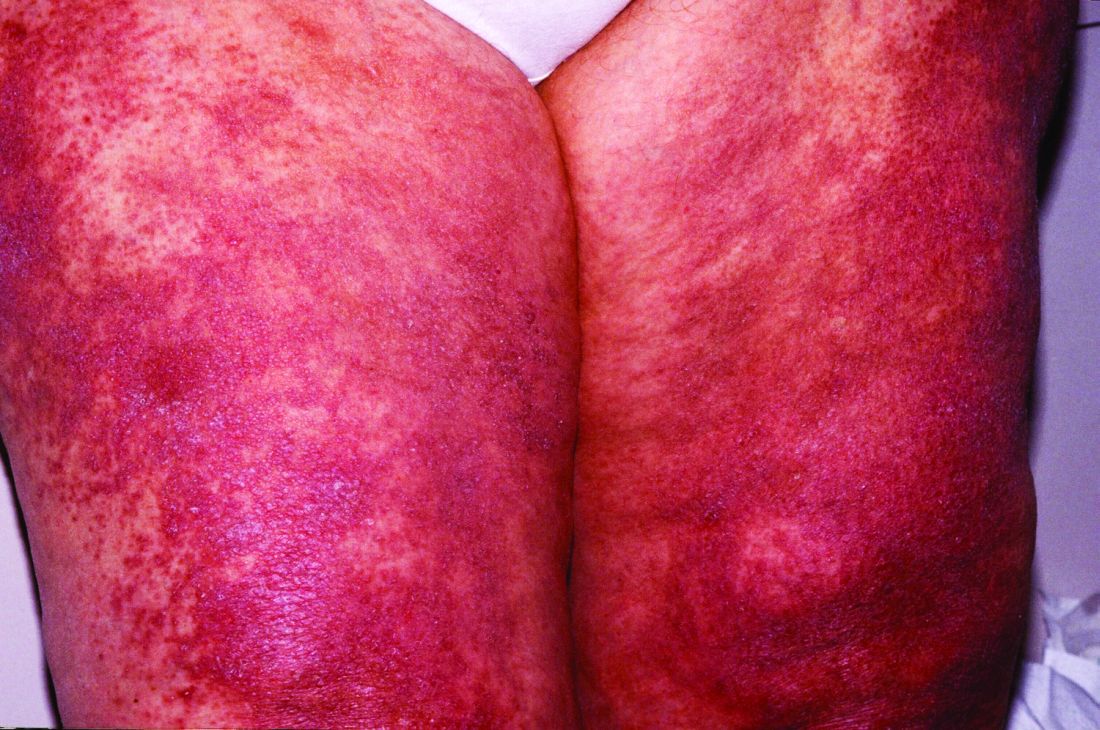
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.

“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.

“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.

“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE
High maternal, fetal morbidity rates in SLE pregnancies
COPENHAGEN – Pregnant women with systemic lupus erythematosus (SLE) are at significantly higher risk of requiring transfusion, developing a cerebrovascular disorder, or developing acute renal failure than pregnant women without SLE, a review of data from an American national sample indicates.
Pregnant women with SLE also have a twofold-higher risk for premature delivery, and a threefold risk of having a fetus with intrauterine growth restriction than their pregnant counterparts without SLE, reported Bella Mehta, MBBS, MS, MD, a rheumatologist at the Hospital for Special Surgery in New York.
“Severe maternal morbidity and fetal morbidity still remain high, but this work can help inform physicians and counsel patients for pregnancy planning and management,” she said at the annual European Congress of Rheumatology.
Although in-hospital maternal and fetal mortality rates for women with SLE have declined over the past 2 decades, the same cannot be said for morbidities, prompting the investigators to conduct a study to determine the proportion of fetal and maternal morbidity in SLE deliveries, compared with non-SLE deliveries over a decade.
Inpatient Sample
Dr. Mehta and colleagues studied retrospective data on 40 million delivery-related admissions from the National Inpatient Sample database. Of these patients, 51,161 had a diagnosis of SLE.
They identified all delivery-related hospital admissions for patients with and without SLE from 2008 through 2017 using diagnostic codes.
The researchers looked at fetal morbidity indicators, including preterm delivery and intrauterine growth restriction, and used the Centers for Disease Control and Prevention standard definition of severe maternal morbidity as “unexpected outcomes of labor and delivery that result in significant short- or long- term consequences to a woman’s health.”
They identified 21 severe maternal morbidity outcomes, including blood transfusion requirements, acute renal failure, eclampsia and disseminated intravascular coagulation, cardiovascular and peripheral vascular disorders, and general medical issues (hysterectomy, shock, sepsis, adult respiratory distress syndrome, severe anesthesia complications, temporary tracheostomy, and ventilation).
Study results
Women with SLE were slightly older at the time of delivery (mean age, 30.05 vs. 29.19 years) and had more comorbidities, according to the Elixhauser Comorbidity Scale, with 97.84% of women in this group having one to four comorbidities, compared with 19.4% of women without SLE.

Dr. Mehta acknowledged that the study was limited by the inability to capture outpatient deliveries, although she noted that only about 1.3% of deliveries in the United States occur outside the inpatient setting.
In addition, she noted that the database does not include information on lupus disease activity, Apgar scores, SLE flares, the presence of nephritis, antiphospholipid or anti-Ro/SSA antibodies, or medication use.
A rheumatologist who was not involved in the study said in an interview that the data from this study are in line with those in other recently published studies.
“The problem is that these data were not corrected for further disease activity or drugs,” said Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented.
She said prospective studies that adjusted for factors such as SLE disease activity and medication use will be required to give clinicians a better understanding of how to manage pregnancies in women with SLE.
The study was supported by an award from Weill Cornell Medicine. Dr. Mehta and Dr. Förger reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Pregnant women with systemic lupus erythematosus (SLE) are at significantly higher risk of requiring transfusion, developing a cerebrovascular disorder, or developing acute renal failure than pregnant women without SLE, a review of data from an American national sample indicates.
Pregnant women with SLE also have a twofold-higher risk for premature delivery, and a threefold risk of having a fetus with intrauterine growth restriction than their pregnant counterparts without SLE, reported Bella Mehta, MBBS, MS, MD, a rheumatologist at the Hospital for Special Surgery in New York.
“Severe maternal morbidity and fetal morbidity still remain high, but this work can help inform physicians and counsel patients for pregnancy planning and management,” she said at the annual European Congress of Rheumatology.
Although in-hospital maternal and fetal mortality rates for women with SLE have declined over the past 2 decades, the same cannot be said for morbidities, prompting the investigators to conduct a study to determine the proportion of fetal and maternal morbidity in SLE deliveries, compared with non-SLE deliveries over a decade.
Inpatient Sample
Dr. Mehta and colleagues studied retrospective data on 40 million delivery-related admissions from the National Inpatient Sample database. Of these patients, 51,161 had a diagnosis of SLE.
They identified all delivery-related hospital admissions for patients with and without SLE from 2008 through 2017 using diagnostic codes.
The researchers looked at fetal morbidity indicators, including preterm delivery and intrauterine growth restriction, and used the Centers for Disease Control and Prevention standard definition of severe maternal morbidity as “unexpected outcomes of labor and delivery that result in significant short- or long- term consequences to a woman’s health.”
They identified 21 severe maternal morbidity outcomes, including blood transfusion requirements, acute renal failure, eclampsia and disseminated intravascular coagulation, cardiovascular and peripheral vascular disorders, and general medical issues (hysterectomy, shock, sepsis, adult respiratory distress syndrome, severe anesthesia complications, temporary tracheostomy, and ventilation).
Study results
Women with SLE were slightly older at the time of delivery (mean age, 30.05 vs. 29.19 years) and had more comorbidities, according to the Elixhauser Comorbidity Scale, with 97.84% of women in this group having one to four comorbidities, compared with 19.4% of women without SLE.

Dr. Mehta acknowledged that the study was limited by the inability to capture outpatient deliveries, although she noted that only about 1.3% of deliveries in the United States occur outside the inpatient setting.
In addition, she noted that the database does not include information on lupus disease activity, Apgar scores, SLE flares, the presence of nephritis, antiphospholipid or anti-Ro/SSA antibodies, or medication use.
A rheumatologist who was not involved in the study said in an interview that the data from this study are in line with those in other recently published studies.
“The problem is that these data were not corrected for further disease activity or drugs,” said Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented.
She said prospective studies that adjusted for factors such as SLE disease activity and medication use will be required to give clinicians a better understanding of how to manage pregnancies in women with SLE.
The study was supported by an award from Weill Cornell Medicine. Dr. Mehta and Dr. Förger reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Pregnant women with systemic lupus erythematosus (SLE) are at significantly higher risk of requiring transfusion, developing a cerebrovascular disorder, or developing acute renal failure than pregnant women without SLE, a review of data from an American national sample indicates.
Pregnant women with SLE also have a twofold-higher risk for premature delivery, and a threefold risk of having a fetus with intrauterine growth restriction than their pregnant counterparts without SLE, reported Bella Mehta, MBBS, MS, MD, a rheumatologist at the Hospital for Special Surgery in New York.
“Severe maternal morbidity and fetal morbidity still remain high, but this work can help inform physicians and counsel patients for pregnancy planning and management,” she said at the annual European Congress of Rheumatology.
Although in-hospital maternal and fetal mortality rates for women with SLE have declined over the past 2 decades, the same cannot be said for morbidities, prompting the investigators to conduct a study to determine the proportion of fetal and maternal morbidity in SLE deliveries, compared with non-SLE deliveries over a decade.
Inpatient Sample
Dr. Mehta and colleagues studied retrospective data on 40 million delivery-related admissions from the National Inpatient Sample database. Of these patients, 51,161 had a diagnosis of SLE.
They identified all delivery-related hospital admissions for patients with and without SLE from 2008 through 2017 using diagnostic codes.
The researchers looked at fetal morbidity indicators, including preterm delivery and intrauterine growth restriction, and used the Centers for Disease Control and Prevention standard definition of severe maternal morbidity as “unexpected outcomes of labor and delivery that result in significant short- or long- term consequences to a woman’s health.”
They identified 21 severe maternal morbidity outcomes, including blood transfusion requirements, acute renal failure, eclampsia and disseminated intravascular coagulation, cardiovascular and peripheral vascular disorders, and general medical issues (hysterectomy, shock, sepsis, adult respiratory distress syndrome, severe anesthesia complications, temporary tracheostomy, and ventilation).
Study results
Women with SLE were slightly older at the time of delivery (mean age, 30.05 vs. 29.19 years) and had more comorbidities, according to the Elixhauser Comorbidity Scale, with 97.84% of women in this group having one to four comorbidities, compared with 19.4% of women without SLE.

Dr. Mehta acknowledged that the study was limited by the inability to capture outpatient deliveries, although she noted that only about 1.3% of deliveries in the United States occur outside the inpatient setting.
In addition, she noted that the database does not include information on lupus disease activity, Apgar scores, SLE flares, the presence of nephritis, antiphospholipid or anti-Ro/SSA antibodies, or medication use.
A rheumatologist who was not involved in the study said in an interview that the data from this study are in line with those in other recently published studies.
“The problem is that these data were not corrected for further disease activity or drugs,” said Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented.
She said prospective studies that adjusted for factors such as SLE disease activity and medication use will be required to give clinicians a better understanding of how to manage pregnancies in women with SLE.
The study was supported by an award from Weill Cornell Medicine. Dr. Mehta and Dr. Förger reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
AT THE EULAR 2022 CONGRESS
EULAR recommends starting methotrexate and glucocorticoids in RA management
COPENHAGEN – New recommendations for the management of rheumatoid arthritis from the European Alliance of Associations for Rheumatology suggest starting short-term methotrexate and glucocorticoids when starting or changing conventional synthetic disease-modifying antirheumatic drugs (DMARDs), although rapid glucocorticoid dose reduction and discontinuation is also emphasized.
“In this respect we are at odds with the American College of Rheumatology guideline,” said Josef S. Smolen, MD, professor of internal medicine at the Medical University of Vienna, who presented the update at the annual European Congress of Rheumatology.
More evidence supports the recommendation to start methotrexate plus glucocorticoids since this is not surpassed by several biologic DMARDs (bDMARDs) plus methotrexate, said Dr. Smolen, who spoke on behalf of his coauthors, Robert Landewe, MD, PhD, from Amsterdam Rheumatology and Clinical Immunology Center, and the rest of the Global Task Force for the 2022 Update of the EULAR RA-Management Recommendations.
In addition, “JAK [Janus kinase] inhibitors are now only recommended for patients who do not have risk factors for cardiovascular or malignant diseases, but otherwise they remain on the same level [phase 2] as bDMARDS,” he said.
“Registries hitherto do not observe what is reported in the ORAL Surveillance randomized controlled trial [RCT],” but, he added, “RCTs are the decisive studies and we await the baricitinib data on a similar population at risk.”
Dr. Smolen also noted that the ENTRACTE trial comparing tocilizumab with tumor necrosis factor (TNF)–alpha inhibitors did not report similar data as ORAL Surveillance.
“Tapering b/ts [biologic/targeted synthetic] and cs [conventional synthetic] DMARDs in sustained remission have been brought together with the need to discontinue glucocorticoids before other drugs are tapered has been more strongly emphasized,” he explained.
Most of the recommendations from the 2019 update remain unchanged, including all five overarching principles and 6 of the 12 individual items.
Rheumatologist Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, joined the meeting remotely and commented on the working draft of the treatment recommendations. “While much was retained from the previous version, there were several important updates,” he said. “Regarding the use of steroids, it is recommended that when they are used, they should be stopped as soon as possible. Regarding jakinibs, which EULAR considers as a class, they recommended consideration of risk factors for MACE events prior to their utilization,” he said.
Methotrexate plus glucocorticoids (Recommendation 6)
In recent years, many recommendations have suggested combining methotrexate with glucocorticoids as a first-treatment strategy upon diagnosis of RA, said Dr. Smolen, and initially “guidance from the ACR was in agreement.”
In 2021, however, “the ACR published a paper, albeit with a very low level of evidence, that one should not primarily use a combination of methotrexate plus glucocorticoids,” he added, with an emphasis on the “very low level of evidence.”
“Some people on the task force even interpreted it as being in favor of using expensive drugs,” he explained. “This needed to be addressed in the 2022 update.”
The global task force wanted to look further at the benefit-to-risk ratio, despite it being discussed in the 2019 recommendations. “We wanted to check that short-term use of glucocorticoids was not associated with major risks,” said Dr. Smolen. “Glucocorticoids are not used for a long time if used as a bridging therapy. We felt we had to more clearly define what we meant by short term.”
A systematic review of around 7,000 papers, led to consideration of 10 unique studies. “One study published a few years ago in PLOS One, did not find any evidence of increased cardiovascular risk,” Dr. Smolen reported, “however, use of over 1,000 mg of glucocorticoid was associated with a trend for high cardiovascular risk.”
“This trend was confirmed by data from the CorEvitas registry, which shows that up to 1,100 mg of cumulative dose was associated with no increased risk, but above this with increasing dose there was an increased and significant risk,” he added.
When the task force looked at trials that mandated and prespecified a reduction and stopping of glucocorticoids, they found less than 10% persistence of glucocorticoids at 12 months in all trials, some even reduced use to zero.
Dr. Smolen and colleagues also looked at data from the NORD-STAR trial, that compared methotrexate and glucocorticoids with methotrexate and three bDMARDs, namely an anti-TNF inhibitor, certolizumab pegol; anti–co-stimulation, abatacept; and an anti–interleukin-6 receptor, tocilizumab.
“These data prove the validity of the EULAR RA management recommendations regarding the unsurpassed benefit of methotrexate plus glucocorticoids in early RA,” Dr. Smolen said.
“This is confirmation of efficacy and that if you induce tapering and stopping it is not dangerous,” he added. “The level of evidence was very high, and it is the highest level of agreement we have had for any glucocorticoid recommendation over recent years.”
As such, Recommendation 6 says that shortening glucocorticoids should be considered when initiating or changing csDMARDS, in different dose regimens and routes of administration, but should be tapered and discontinued as rapidly as clinically feasible.
JAK inhibitor placed relative to DMARDs (Recommendation 10)
A paper published in the New England Journal of Medicine suggested cardiovascular risks and malignancy risks were higher with the JAK inhibitor, tofacitinib, compared with TNF-alpha inhibitors.
The task force therefore felt the need to evaluate the place of JAK inhibitors next to biologic DMARDs “once phase one with methotrexate plus glucocorticoids has failed,” Dr. Smolen said.
After a systematic literature review of around 4,500 papers, the researchers evaluated 88 safety papers including the ORAL Surveillance study. “This very clearly showed that tocilizumab was not noninferior according to the noninferiority criteria with an upper limit of 1.8 [hazard ratio] and this was independent of dose, compared with TNF-alpha inhibitor,” said Dr. Smolen. “The major adverse cardiovascular events [MACE] were not different, nor were malignancies and overall mortality.”
Dr. Smolen also referred to the ENTRACTE trial that compared etanercept with tocilizumab, and again, there was no evidence of an increased risk of MACE nor mortality for tocilizumab compared with a TNF-alpha inhibitor.
“The increased MACE risk in the ORAL Surveillance trial is unlikely due to inhibition of IL-6 and must be due to some other effects than IL-6 signaling,” he said.
As such, the agreed-on recommendation was that, “if the treatment target is not achieved with the first csDMARD strategy, when poor prognostic factors are present, a bDMARD should be added; JAK inhibitors may be considered but pertinent risk factors must be taken into account [aged over 65 years, history of current or past smoking, either cardiovascular or malignancy risk factors, and risk factors for thromboembolic events].”
There was a high level of agreement by the group for this recommendation.
Switching DMARDs
The task force considered their recommendations on switching DMARDs based on a systematic literature review of 47 papers.
EULAR previously strongly recommended a combination of csDMARDs with bDMARDs (including JAK inhibitors), and this recommendation remains the same except for a note added about risks of tsDMARDs.
Recommendation 10 relates to failure of phase 2 treatment and what to do when the first bDMARD or a tsDMARD has failed (including as per new recommendations, a JAK inhibitor), and if one TNF or IL-6 receptor inhibitor therapy has failed. In this case, patients may receive an agent with another mode of action or a second TNF/IL-6 receptor inhibitor, said Dr. Smolen.
Recommendation 11 has been combined with recommendation 12, he added. “If a patient is in persistent remission after having tapered glucocorticoids, one can consider tapering bDMARDs, or tsDMARDs especially if this treatment is combined with a csDMARD.
“We decided to put more emphasis on the stopping of glucocorticoids, namely not saying ‘tapering’ but ‘discontinued,’ and if the patient is in sustained remission, then consider reduction of DMARDs [biologic, targeted synthetic or conventional synthetic DMARDs],” he explained. “This is left to the discretion of the patient and the physician as to which one should be tapered first. We don’t recommend to taper everything because the patient might be affected by flares but this needs further discussion.”
Dr. Smolen ended his presentation by looking ahead to the next set of recommendations: “With the current rate of evidence development, we expect an update of the recommendations to be necessary in about 3-4 years.”
This article was updated on 6/9/2022.
COPENHAGEN – New recommendations for the management of rheumatoid arthritis from the European Alliance of Associations for Rheumatology suggest starting short-term methotrexate and glucocorticoids when starting or changing conventional synthetic disease-modifying antirheumatic drugs (DMARDs), although rapid glucocorticoid dose reduction and discontinuation is also emphasized.
“In this respect we are at odds with the American College of Rheumatology guideline,” said Josef S. Smolen, MD, professor of internal medicine at the Medical University of Vienna, who presented the update at the annual European Congress of Rheumatology.
More evidence supports the recommendation to start methotrexate plus glucocorticoids since this is not surpassed by several biologic DMARDs (bDMARDs) plus methotrexate, said Dr. Smolen, who spoke on behalf of his coauthors, Robert Landewe, MD, PhD, from Amsterdam Rheumatology and Clinical Immunology Center, and the rest of the Global Task Force for the 2022 Update of the EULAR RA-Management Recommendations.
In addition, “JAK [Janus kinase] inhibitors are now only recommended for patients who do not have risk factors for cardiovascular or malignant diseases, but otherwise they remain on the same level [phase 2] as bDMARDS,” he said.
“Registries hitherto do not observe what is reported in the ORAL Surveillance randomized controlled trial [RCT],” but, he added, “RCTs are the decisive studies and we await the baricitinib data on a similar population at risk.”
Dr. Smolen also noted that the ENTRACTE trial comparing tocilizumab with tumor necrosis factor (TNF)–alpha inhibitors did not report similar data as ORAL Surveillance.
“Tapering b/ts [biologic/targeted synthetic] and cs [conventional synthetic] DMARDs in sustained remission have been brought together with the need to discontinue glucocorticoids before other drugs are tapered has been more strongly emphasized,” he explained.
Most of the recommendations from the 2019 update remain unchanged, including all five overarching principles and 6 of the 12 individual items.
Rheumatologist Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, joined the meeting remotely and commented on the working draft of the treatment recommendations. “While much was retained from the previous version, there were several important updates,” he said. “Regarding the use of steroids, it is recommended that when they are used, they should be stopped as soon as possible. Regarding jakinibs, which EULAR considers as a class, they recommended consideration of risk factors for MACE events prior to their utilization,” he said.
Methotrexate plus glucocorticoids (Recommendation 6)
In recent years, many recommendations have suggested combining methotrexate with glucocorticoids as a first-treatment strategy upon diagnosis of RA, said Dr. Smolen, and initially “guidance from the ACR was in agreement.”
In 2021, however, “the ACR published a paper, albeit with a very low level of evidence, that one should not primarily use a combination of methotrexate plus glucocorticoids,” he added, with an emphasis on the “very low level of evidence.”
“Some people on the task force even interpreted it as being in favor of using expensive drugs,” he explained. “This needed to be addressed in the 2022 update.”
The global task force wanted to look further at the benefit-to-risk ratio, despite it being discussed in the 2019 recommendations. “We wanted to check that short-term use of glucocorticoids was not associated with major risks,” said Dr. Smolen. “Glucocorticoids are not used for a long time if used as a bridging therapy. We felt we had to more clearly define what we meant by short term.”
A systematic review of around 7,000 papers, led to consideration of 10 unique studies. “One study published a few years ago in PLOS One, did not find any evidence of increased cardiovascular risk,” Dr. Smolen reported, “however, use of over 1,000 mg of glucocorticoid was associated with a trend for high cardiovascular risk.”
“This trend was confirmed by data from the CorEvitas registry, which shows that up to 1,100 mg of cumulative dose was associated with no increased risk, but above this with increasing dose there was an increased and significant risk,” he added.
When the task force looked at trials that mandated and prespecified a reduction and stopping of glucocorticoids, they found less than 10% persistence of glucocorticoids at 12 months in all trials, some even reduced use to zero.
Dr. Smolen and colleagues also looked at data from the NORD-STAR trial, that compared methotrexate and glucocorticoids with methotrexate and three bDMARDs, namely an anti-TNF inhibitor, certolizumab pegol; anti–co-stimulation, abatacept; and an anti–interleukin-6 receptor, tocilizumab.
“These data prove the validity of the EULAR RA management recommendations regarding the unsurpassed benefit of methotrexate plus glucocorticoids in early RA,” Dr. Smolen said.
“This is confirmation of efficacy and that if you induce tapering and stopping it is not dangerous,” he added. “The level of evidence was very high, and it is the highest level of agreement we have had for any glucocorticoid recommendation over recent years.”
As such, Recommendation 6 says that shortening glucocorticoids should be considered when initiating or changing csDMARDS, in different dose regimens and routes of administration, but should be tapered and discontinued as rapidly as clinically feasible.
JAK inhibitor placed relative to DMARDs (Recommendation 10)
A paper published in the New England Journal of Medicine suggested cardiovascular risks and malignancy risks were higher with the JAK inhibitor, tofacitinib, compared with TNF-alpha inhibitors.
The task force therefore felt the need to evaluate the place of JAK inhibitors next to biologic DMARDs “once phase one with methotrexate plus glucocorticoids has failed,” Dr. Smolen said.
After a systematic literature review of around 4,500 papers, the researchers evaluated 88 safety papers including the ORAL Surveillance study. “This very clearly showed that tocilizumab was not noninferior according to the noninferiority criteria with an upper limit of 1.8 [hazard ratio] and this was independent of dose, compared with TNF-alpha inhibitor,” said Dr. Smolen. “The major adverse cardiovascular events [MACE] were not different, nor were malignancies and overall mortality.”
Dr. Smolen also referred to the ENTRACTE trial that compared etanercept with tocilizumab, and again, there was no evidence of an increased risk of MACE nor mortality for tocilizumab compared with a TNF-alpha inhibitor.
“The increased MACE risk in the ORAL Surveillance trial is unlikely due to inhibition of IL-6 and must be due to some other effects than IL-6 signaling,” he said.
As such, the agreed-on recommendation was that, “if the treatment target is not achieved with the first csDMARD strategy, when poor prognostic factors are present, a bDMARD should be added; JAK inhibitors may be considered but pertinent risk factors must be taken into account [aged over 65 years, history of current or past smoking, either cardiovascular or malignancy risk factors, and risk factors for thromboembolic events].”
There was a high level of agreement by the group for this recommendation.
Switching DMARDs
The task force considered their recommendations on switching DMARDs based on a systematic literature review of 47 papers.
EULAR previously strongly recommended a combination of csDMARDs with bDMARDs (including JAK inhibitors), and this recommendation remains the same except for a note added about risks of tsDMARDs.
Recommendation 10 relates to failure of phase 2 treatment and what to do when the first bDMARD or a tsDMARD has failed (including as per new recommendations, a JAK inhibitor), and if one TNF or IL-6 receptor inhibitor therapy has failed. In this case, patients may receive an agent with another mode of action or a second TNF/IL-6 receptor inhibitor, said Dr. Smolen.
Recommendation 11 has been combined with recommendation 12, he added. “If a patient is in persistent remission after having tapered glucocorticoids, one can consider tapering bDMARDs, or tsDMARDs especially if this treatment is combined with a csDMARD.
“We decided to put more emphasis on the stopping of glucocorticoids, namely not saying ‘tapering’ but ‘discontinued,’ and if the patient is in sustained remission, then consider reduction of DMARDs [biologic, targeted synthetic or conventional synthetic DMARDs],” he explained. “This is left to the discretion of the patient and the physician as to which one should be tapered first. We don’t recommend to taper everything because the patient might be affected by flares but this needs further discussion.”
Dr. Smolen ended his presentation by looking ahead to the next set of recommendations: “With the current rate of evidence development, we expect an update of the recommendations to be necessary in about 3-4 years.”
This article was updated on 6/9/2022.
COPENHAGEN – New recommendations for the management of rheumatoid arthritis from the European Alliance of Associations for Rheumatology suggest starting short-term methotrexate and glucocorticoids when starting or changing conventional synthetic disease-modifying antirheumatic drugs (DMARDs), although rapid glucocorticoid dose reduction and discontinuation is also emphasized.
“In this respect we are at odds with the American College of Rheumatology guideline,” said Josef S. Smolen, MD, professor of internal medicine at the Medical University of Vienna, who presented the update at the annual European Congress of Rheumatology.
More evidence supports the recommendation to start methotrexate plus glucocorticoids since this is not surpassed by several biologic DMARDs (bDMARDs) plus methotrexate, said Dr. Smolen, who spoke on behalf of his coauthors, Robert Landewe, MD, PhD, from Amsterdam Rheumatology and Clinical Immunology Center, and the rest of the Global Task Force for the 2022 Update of the EULAR RA-Management Recommendations.
In addition, “JAK [Janus kinase] inhibitors are now only recommended for patients who do not have risk factors for cardiovascular or malignant diseases, but otherwise they remain on the same level [phase 2] as bDMARDS,” he said.
“Registries hitherto do not observe what is reported in the ORAL Surveillance randomized controlled trial [RCT],” but, he added, “RCTs are the decisive studies and we await the baricitinib data on a similar population at risk.”
Dr. Smolen also noted that the ENTRACTE trial comparing tocilizumab with tumor necrosis factor (TNF)–alpha inhibitors did not report similar data as ORAL Surveillance.
“Tapering b/ts [biologic/targeted synthetic] and cs [conventional synthetic] DMARDs in sustained remission have been brought together with the need to discontinue glucocorticoids before other drugs are tapered has been more strongly emphasized,” he explained.
Most of the recommendations from the 2019 update remain unchanged, including all five overarching principles and 6 of the 12 individual items.
Rheumatologist Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, joined the meeting remotely and commented on the working draft of the treatment recommendations. “While much was retained from the previous version, there were several important updates,” he said. “Regarding the use of steroids, it is recommended that when they are used, they should be stopped as soon as possible. Regarding jakinibs, which EULAR considers as a class, they recommended consideration of risk factors for MACE events prior to their utilization,” he said.
Methotrexate plus glucocorticoids (Recommendation 6)
In recent years, many recommendations have suggested combining methotrexate with glucocorticoids as a first-treatment strategy upon diagnosis of RA, said Dr. Smolen, and initially “guidance from the ACR was in agreement.”
In 2021, however, “the ACR published a paper, albeit with a very low level of evidence, that one should not primarily use a combination of methotrexate plus glucocorticoids,” he added, with an emphasis on the “very low level of evidence.”
“Some people on the task force even interpreted it as being in favor of using expensive drugs,” he explained. “This needed to be addressed in the 2022 update.”
The global task force wanted to look further at the benefit-to-risk ratio, despite it being discussed in the 2019 recommendations. “We wanted to check that short-term use of glucocorticoids was not associated with major risks,” said Dr. Smolen. “Glucocorticoids are not used for a long time if used as a bridging therapy. We felt we had to more clearly define what we meant by short term.”
A systematic review of around 7,000 papers, led to consideration of 10 unique studies. “One study published a few years ago in PLOS One, did not find any evidence of increased cardiovascular risk,” Dr. Smolen reported, “however, use of over 1,000 mg of glucocorticoid was associated with a trend for high cardiovascular risk.”
“This trend was confirmed by data from the CorEvitas registry, which shows that up to 1,100 mg of cumulative dose was associated with no increased risk, but above this with increasing dose there was an increased and significant risk,” he added.
When the task force looked at trials that mandated and prespecified a reduction and stopping of glucocorticoids, they found less than 10% persistence of glucocorticoids at 12 months in all trials, some even reduced use to zero.
Dr. Smolen and colleagues also looked at data from the NORD-STAR trial, that compared methotrexate and glucocorticoids with methotrexate and three bDMARDs, namely an anti-TNF inhibitor, certolizumab pegol; anti–co-stimulation, abatacept; and an anti–interleukin-6 receptor, tocilizumab.
“These data prove the validity of the EULAR RA management recommendations regarding the unsurpassed benefit of methotrexate plus glucocorticoids in early RA,” Dr. Smolen said.
“This is confirmation of efficacy and that if you induce tapering and stopping it is not dangerous,” he added. “The level of evidence was very high, and it is the highest level of agreement we have had for any glucocorticoid recommendation over recent years.”
As such, Recommendation 6 says that shortening glucocorticoids should be considered when initiating or changing csDMARDS, in different dose regimens and routes of administration, but should be tapered and discontinued as rapidly as clinically feasible.
JAK inhibitor placed relative to DMARDs (Recommendation 10)
A paper published in the New England Journal of Medicine suggested cardiovascular risks and malignancy risks were higher with the JAK inhibitor, tofacitinib, compared with TNF-alpha inhibitors.
The task force therefore felt the need to evaluate the place of JAK inhibitors next to biologic DMARDs “once phase one with methotrexate plus glucocorticoids has failed,” Dr. Smolen said.
After a systematic literature review of around 4,500 papers, the researchers evaluated 88 safety papers including the ORAL Surveillance study. “This very clearly showed that tocilizumab was not noninferior according to the noninferiority criteria with an upper limit of 1.8 [hazard ratio] and this was independent of dose, compared with TNF-alpha inhibitor,” said Dr. Smolen. “The major adverse cardiovascular events [MACE] were not different, nor were malignancies and overall mortality.”
Dr. Smolen also referred to the ENTRACTE trial that compared etanercept with tocilizumab, and again, there was no evidence of an increased risk of MACE nor mortality for tocilizumab compared with a TNF-alpha inhibitor.
“The increased MACE risk in the ORAL Surveillance trial is unlikely due to inhibition of IL-6 and must be due to some other effects than IL-6 signaling,” he said.
As such, the agreed-on recommendation was that, “if the treatment target is not achieved with the first csDMARD strategy, when poor prognostic factors are present, a bDMARD should be added; JAK inhibitors may be considered but pertinent risk factors must be taken into account [aged over 65 years, history of current or past smoking, either cardiovascular or malignancy risk factors, and risk factors for thromboembolic events].”
There was a high level of agreement by the group for this recommendation.
Switching DMARDs
The task force considered their recommendations on switching DMARDs based on a systematic literature review of 47 papers.
EULAR previously strongly recommended a combination of csDMARDs with bDMARDs (including JAK inhibitors), and this recommendation remains the same except for a note added about risks of tsDMARDs.
Recommendation 10 relates to failure of phase 2 treatment and what to do when the first bDMARD or a tsDMARD has failed (including as per new recommendations, a JAK inhibitor), and if one TNF or IL-6 receptor inhibitor therapy has failed. In this case, patients may receive an agent with another mode of action or a second TNF/IL-6 receptor inhibitor, said Dr. Smolen.
Recommendation 11 has been combined with recommendation 12, he added. “If a patient is in persistent remission after having tapered glucocorticoids, one can consider tapering bDMARDs, or tsDMARDs especially if this treatment is combined with a csDMARD.
“We decided to put more emphasis on the stopping of glucocorticoids, namely not saying ‘tapering’ but ‘discontinued,’ and if the patient is in sustained remission, then consider reduction of DMARDs [biologic, targeted synthetic or conventional synthetic DMARDs],” he explained. “This is left to the discretion of the patient and the physician as to which one should be tapered first. We don’t recommend to taper everything because the patient might be affected by flares but this needs further discussion.”
Dr. Smolen ended his presentation by looking ahead to the next set of recommendations: “With the current rate of evidence development, we expect an update of the recommendations to be necessary in about 3-4 years.”
This article was updated on 6/9/2022.
AT THE EULAR 2022 CONGRESS
Updated EULAR recommendations for AAV include new drugs, practices
The
The 2022 revision – which was unveiled at the annual European Congress of Rheumatology – includes guidance on using new drugs, such as avacopan (Tavneos) and mepolizumab (Nucala), as well as revised recommendations on the use of rituximab and glucocorticosteroids.

The overhaul also contains specific recommendations for treating eosinophilic granulomatosis with polyangiitis (EGPA), separating it out from granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) for the first time.
“Until now, EGPA has usually been managed in the same way as [the] other diseases,” Bernhard Hellmich, MD, of the University of Tübingen (Germany) said in an interview ahead of his presentation at the congress.
“But we now have data on each type specifically, so there is good reason to make separate recommendations,” he added.
Indeed, so much new data has become available in the past few years there are only three recommendations that remain unchanged from the previous iteration published in 2016.
Since then, “several high-impact studies in AAV have been published and the results of these studies required an update of the existing recommendations,” Dr. Hellmich said.
Developed in record time – just 7 months from start to finish – the process of updating the recommendations on AAV followed EULAR’s standard operating procedures. An important step in this process is to perform a systemic literature review. Perhaps crucially, and in contrast to the first U.S. vasculitis guidelines published only in 2021, the most recent literature search was able to include data on avacopan, which was approved for use in Europe in January as an adjunctive treatment for adults with severe active GPA and MPA.
The results of the literature review were reported separately at the EULAR 2022 Congress, with separate presentations highlighting the data behind the amended treatment and diagnostic and follow-up procedure recommendations.
Highlights of the changes
A key change is the introduction of four overarching principles, which weren’t included in the previous update, said Dr. Hellmich.
“We moved some of the existing recommendations with low level of evidence to overarching principles,” he added, stating that the first general principle was that all patients should be offered “the best care which must be based on shared decision-making between the patient and the physician considering efficacy, safety, and costs.”
The second principle states that patients should have access to education that covers the prognosis and impact of AAV, including recognizing warning symptoms and treatment options.
The third focuses on screening for adverse effects and comorbidities, recommending that patients are given appropriate prophylaxis and lifestyle advice.
Finally, the fourth general principle recognizes that AAV is a rare group of heterogenous and potentially life-threatening diseases that need multidisciplinary care, with access to specific vasculitis expertise.
New recommendations
Of the 17 recommendations made, 6 are completely new, including one on ANCA testing in patients who are suspected of having AAV.
“We recommend testing for both PR3- and MPO-ANCA using a high-quality antigen-specific assay as the primary method of testing,” Dr. Hellmich said. This is based on strong new evidence that antigen-specific assays have superior diagnostic accuracy, compared with indirect immunofluorescence.
“We also want to emphasize that ANCA testing should be done in patients with signs and symptoms in order to minimize the risk of false-positive results,” Dr. Hellmich said.
Also new is the recommendation to use oral steroids to induce remission in GPA/MPA, followed by a stepwise reduction in the dose, aiming for a dose of not more than 5 mg prednisolone per day by 4-5 months of treatment.
“Glucocorticoids are very effective, but also are the major trigger of infections in AAV,” said Dr. Hellmich. This is important since infections are a major driver of early mortality in AAV.
“Another possibility to reduce glucocorticoid exposure is avacopan,” he said. It’s recommended to be used in combination with rituximab or cyclophosphamide for remission induction in GPA/MPA as a strategy to basically “get rid of steroids.”
Indeed, “for patients who really have a high burden of glucocorticoid-associated adverse effects, especially relapsing patients, I think it would make sense just to give avacopan and no steroids,” Dr. Hellmich said.
Other new recommendations concern remission induction and maintenance therapy in new-onset EGPA. Regarding the latter, the choice of treatment depends on whether there is an organ- or life-threatening situation, with methotrexate, azathioprine, mepolizumab, or rituximab all recommended equally, or if there is no organ- or life-threatening situation, then mepolizumab is preferred.
Revised and unchanged recommendations
Eight of the recommendations have been revised, with rituximab being placed more prominently as a treatment in some. For remission induction in GPA and MPA with organ- or life threatening disease, rituximab is now the preferred option for relapsing disease. Rituximab also replaced methotrexate as the preferred option for maintaining remission, although methotrexate and azathioprine can still be considered as alternatives.
Another changed statement is on the duration of maintenance treatment in GPA and MPA, which now advocates 1-2 years of treatment after achieving remission. Longer therapy might be needed in relapsing cases, but the benefits and risks need to be carefully considered and patient preferences taken into account.
Prophylaxis against pneumonia and other infections is still recommended, with the revised guidance noting that patients receiving cyclophosphamide, rituximab, or high-dose steroids, should be treated with trimethoprim-sulfamethoxazole (co-trimoxazole).
“There are retrospective data in the AAV population that the administration of co-trimoxazole reduces not only the incidence of pneumocystis, but also of other infections. So, this is important recommendation for clinical practice,” Dr. Hellmich said.
Summing up
“For a rare disease group, I think this is very good progress,” said Dr. Hellmich, but “there are still many open questions, so we have a long research agenda.”
There is purposefully no recommendation on COVID-19, however, as “the conditions that impact COVID outcomes change rapidly and any recommendation made now is likely to be outdated soon; the AAV recommendations are intended to last for at least a couple of years.”
In a press release issued by the German Society for Rheumatology, which was unrelated to Dr. Hellmich’s talk, experts commented on vasculitis guidelines generally, noting that there has been a move toward using biologic therapies such as rituximab and mepolizumab as a new standard of therapy.
DGRh President and chief physician at the Immanuel Hospital in Berlin Andreas Krause, MD, observed that “cyclophosphamide, which was used in the past and which inhibits blood formation in the bone marrow and can lead to infertility, can now often be dispensed with.”
Julia Holle, MD, of Rheumazentrum Schleswig-Holstein Mitte in Neumünster, Germany, was also quoted in the press release, saying that, “for patients, the successful use of biologics and the reduction in the glucocorticoid dose is important progress.”
Dr. Holle was involved in the development of revised European guidelines. She is also the lead author of a recent publication on treatment of vasculitis on available evidence. Dr. Hellmich acknowledged having ties to multiple pharma companies, acting as speaker, consultant, or both to Abbvie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, GlaxoSmithKline, InflaRx, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Vifor.
The
The 2022 revision – which was unveiled at the annual European Congress of Rheumatology – includes guidance on using new drugs, such as avacopan (Tavneos) and mepolizumab (Nucala), as well as revised recommendations on the use of rituximab and glucocorticosteroids.

The overhaul also contains specific recommendations for treating eosinophilic granulomatosis with polyangiitis (EGPA), separating it out from granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) for the first time.
“Until now, EGPA has usually been managed in the same way as [the] other diseases,” Bernhard Hellmich, MD, of the University of Tübingen (Germany) said in an interview ahead of his presentation at the congress.
“But we now have data on each type specifically, so there is good reason to make separate recommendations,” he added.
Indeed, so much new data has become available in the past few years there are only three recommendations that remain unchanged from the previous iteration published in 2016.
Since then, “several high-impact studies in AAV have been published and the results of these studies required an update of the existing recommendations,” Dr. Hellmich said.
Developed in record time – just 7 months from start to finish – the process of updating the recommendations on AAV followed EULAR’s standard operating procedures. An important step in this process is to perform a systemic literature review. Perhaps crucially, and in contrast to the first U.S. vasculitis guidelines published only in 2021, the most recent literature search was able to include data on avacopan, which was approved for use in Europe in January as an adjunctive treatment for adults with severe active GPA and MPA.
The results of the literature review were reported separately at the EULAR 2022 Congress, with separate presentations highlighting the data behind the amended treatment and diagnostic and follow-up procedure recommendations.
Highlights of the changes
A key change is the introduction of four overarching principles, which weren’t included in the previous update, said Dr. Hellmich.
“We moved some of the existing recommendations with low level of evidence to overarching principles,” he added, stating that the first general principle was that all patients should be offered “the best care which must be based on shared decision-making between the patient and the physician considering efficacy, safety, and costs.”
The second principle states that patients should have access to education that covers the prognosis and impact of AAV, including recognizing warning symptoms and treatment options.
The third focuses on screening for adverse effects and comorbidities, recommending that patients are given appropriate prophylaxis and lifestyle advice.
Finally, the fourth general principle recognizes that AAV is a rare group of heterogenous and potentially life-threatening diseases that need multidisciplinary care, with access to specific vasculitis expertise.
New recommendations
Of the 17 recommendations made, 6 are completely new, including one on ANCA testing in patients who are suspected of having AAV.
“We recommend testing for both PR3- and MPO-ANCA using a high-quality antigen-specific assay as the primary method of testing,” Dr. Hellmich said. This is based on strong new evidence that antigen-specific assays have superior diagnostic accuracy, compared with indirect immunofluorescence.
“We also want to emphasize that ANCA testing should be done in patients with signs and symptoms in order to minimize the risk of false-positive results,” Dr. Hellmich said.
Also new is the recommendation to use oral steroids to induce remission in GPA/MPA, followed by a stepwise reduction in the dose, aiming for a dose of not more than 5 mg prednisolone per day by 4-5 months of treatment.
“Glucocorticoids are very effective, but also are the major trigger of infections in AAV,” said Dr. Hellmich. This is important since infections are a major driver of early mortality in AAV.
“Another possibility to reduce glucocorticoid exposure is avacopan,” he said. It’s recommended to be used in combination with rituximab or cyclophosphamide for remission induction in GPA/MPA as a strategy to basically “get rid of steroids.”
Indeed, “for patients who really have a high burden of glucocorticoid-associated adverse effects, especially relapsing patients, I think it would make sense just to give avacopan and no steroids,” Dr. Hellmich said.
Other new recommendations concern remission induction and maintenance therapy in new-onset EGPA. Regarding the latter, the choice of treatment depends on whether there is an organ- or life-threatening situation, with methotrexate, azathioprine, mepolizumab, or rituximab all recommended equally, or if there is no organ- or life-threatening situation, then mepolizumab is preferred.
Revised and unchanged recommendations
Eight of the recommendations have been revised, with rituximab being placed more prominently as a treatment in some. For remission induction in GPA and MPA with organ- or life threatening disease, rituximab is now the preferred option for relapsing disease. Rituximab also replaced methotrexate as the preferred option for maintaining remission, although methotrexate and azathioprine can still be considered as alternatives.
Another changed statement is on the duration of maintenance treatment in GPA and MPA, which now advocates 1-2 years of treatment after achieving remission. Longer therapy might be needed in relapsing cases, but the benefits and risks need to be carefully considered and patient preferences taken into account.
Prophylaxis against pneumonia and other infections is still recommended, with the revised guidance noting that patients receiving cyclophosphamide, rituximab, or high-dose steroids, should be treated with trimethoprim-sulfamethoxazole (co-trimoxazole).
“There are retrospective data in the AAV population that the administration of co-trimoxazole reduces not only the incidence of pneumocystis, but also of other infections. So, this is important recommendation for clinical practice,” Dr. Hellmich said.
Summing up
“For a rare disease group, I think this is very good progress,” said Dr. Hellmich, but “there are still many open questions, so we have a long research agenda.”
There is purposefully no recommendation on COVID-19, however, as “the conditions that impact COVID outcomes change rapidly and any recommendation made now is likely to be outdated soon; the AAV recommendations are intended to last for at least a couple of years.”
In a press release issued by the German Society for Rheumatology, which was unrelated to Dr. Hellmich’s talk, experts commented on vasculitis guidelines generally, noting that there has been a move toward using biologic therapies such as rituximab and mepolizumab as a new standard of therapy.
DGRh President and chief physician at the Immanuel Hospital in Berlin Andreas Krause, MD, observed that “cyclophosphamide, which was used in the past and which inhibits blood formation in the bone marrow and can lead to infertility, can now often be dispensed with.”
Julia Holle, MD, of Rheumazentrum Schleswig-Holstein Mitte in Neumünster, Germany, was also quoted in the press release, saying that, “for patients, the successful use of biologics and the reduction in the glucocorticoid dose is important progress.”
Dr. Holle was involved in the development of revised European guidelines. She is also the lead author of a recent publication on treatment of vasculitis on available evidence. Dr. Hellmich acknowledged having ties to multiple pharma companies, acting as speaker, consultant, or both to Abbvie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, GlaxoSmithKline, InflaRx, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Vifor.
The
The 2022 revision – which was unveiled at the annual European Congress of Rheumatology – includes guidance on using new drugs, such as avacopan (Tavneos) and mepolizumab (Nucala), as well as revised recommendations on the use of rituximab and glucocorticosteroids.

The overhaul also contains specific recommendations for treating eosinophilic granulomatosis with polyangiitis (EGPA), separating it out from granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) for the first time.
“Until now, EGPA has usually been managed in the same way as [the] other diseases,” Bernhard Hellmich, MD, of the University of Tübingen (Germany) said in an interview ahead of his presentation at the congress.
“But we now have data on each type specifically, so there is good reason to make separate recommendations,” he added.
Indeed, so much new data has become available in the past few years there are only three recommendations that remain unchanged from the previous iteration published in 2016.
Since then, “several high-impact studies in AAV have been published and the results of these studies required an update of the existing recommendations,” Dr. Hellmich said.
Developed in record time – just 7 months from start to finish – the process of updating the recommendations on AAV followed EULAR’s standard operating procedures. An important step in this process is to perform a systemic literature review. Perhaps crucially, and in contrast to the first U.S. vasculitis guidelines published only in 2021, the most recent literature search was able to include data on avacopan, which was approved for use in Europe in January as an adjunctive treatment for adults with severe active GPA and MPA.
The results of the literature review were reported separately at the EULAR 2022 Congress, with separate presentations highlighting the data behind the amended treatment and diagnostic and follow-up procedure recommendations.
Highlights of the changes
A key change is the introduction of four overarching principles, which weren’t included in the previous update, said Dr. Hellmich.
“We moved some of the existing recommendations with low level of evidence to overarching principles,” he added, stating that the first general principle was that all patients should be offered “the best care which must be based on shared decision-making between the patient and the physician considering efficacy, safety, and costs.”
The second principle states that patients should have access to education that covers the prognosis and impact of AAV, including recognizing warning symptoms and treatment options.
The third focuses on screening for adverse effects and comorbidities, recommending that patients are given appropriate prophylaxis and lifestyle advice.
Finally, the fourth general principle recognizes that AAV is a rare group of heterogenous and potentially life-threatening diseases that need multidisciplinary care, with access to specific vasculitis expertise.
New recommendations
Of the 17 recommendations made, 6 are completely new, including one on ANCA testing in patients who are suspected of having AAV.
“We recommend testing for both PR3- and MPO-ANCA using a high-quality antigen-specific assay as the primary method of testing,” Dr. Hellmich said. This is based on strong new evidence that antigen-specific assays have superior diagnostic accuracy, compared with indirect immunofluorescence.
“We also want to emphasize that ANCA testing should be done in patients with signs and symptoms in order to minimize the risk of false-positive results,” Dr. Hellmich said.
Also new is the recommendation to use oral steroids to induce remission in GPA/MPA, followed by a stepwise reduction in the dose, aiming for a dose of not more than 5 mg prednisolone per day by 4-5 months of treatment.
“Glucocorticoids are very effective, but also are the major trigger of infections in AAV,” said Dr. Hellmich. This is important since infections are a major driver of early mortality in AAV.
“Another possibility to reduce glucocorticoid exposure is avacopan,” he said. It’s recommended to be used in combination with rituximab or cyclophosphamide for remission induction in GPA/MPA as a strategy to basically “get rid of steroids.”
Indeed, “for patients who really have a high burden of glucocorticoid-associated adverse effects, especially relapsing patients, I think it would make sense just to give avacopan and no steroids,” Dr. Hellmich said.
Other new recommendations concern remission induction and maintenance therapy in new-onset EGPA. Regarding the latter, the choice of treatment depends on whether there is an organ- or life-threatening situation, with methotrexate, azathioprine, mepolizumab, or rituximab all recommended equally, or if there is no organ- or life-threatening situation, then mepolizumab is preferred.
Revised and unchanged recommendations
Eight of the recommendations have been revised, with rituximab being placed more prominently as a treatment in some. For remission induction in GPA and MPA with organ- or life threatening disease, rituximab is now the preferred option for relapsing disease. Rituximab also replaced methotrexate as the preferred option for maintaining remission, although methotrexate and azathioprine can still be considered as alternatives.
Another changed statement is on the duration of maintenance treatment in GPA and MPA, which now advocates 1-2 years of treatment after achieving remission. Longer therapy might be needed in relapsing cases, but the benefits and risks need to be carefully considered and patient preferences taken into account.
Prophylaxis against pneumonia and other infections is still recommended, with the revised guidance noting that patients receiving cyclophosphamide, rituximab, or high-dose steroids, should be treated with trimethoprim-sulfamethoxazole (co-trimoxazole).
“There are retrospective data in the AAV population that the administration of co-trimoxazole reduces not only the incidence of pneumocystis, but also of other infections. So, this is important recommendation for clinical practice,” Dr. Hellmich said.
Summing up
“For a rare disease group, I think this is very good progress,” said Dr. Hellmich, but “there are still many open questions, so we have a long research agenda.”
There is purposefully no recommendation on COVID-19, however, as “the conditions that impact COVID outcomes change rapidly and any recommendation made now is likely to be outdated soon; the AAV recommendations are intended to last for at least a couple of years.”
In a press release issued by the German Society for Rheumatology, which was unrelated to Dr. Hellmich’s talk, experts commented on vasculitis guidelines generally, noting that there has been a move toward using biologic therapies such as rituximab and mepolizumab as a new standard of therapy.
DGRh President and chief physician at the Immanuel Hospital in Berlin Andreas Krause, MD, observed that “cyclophosphamide, which was used in the past and which inhibits blood formation in the bone marrow and can lead to infertility, can now often be dispensed with.”
Julia Holle, MD, of Rheumazentrum Schleswig-Holstein Mitte in Neumünster, Germany, was also quoted in the press release, saying that, “for patients, the successful use of biologics and the reduction in the glucocorticoid dose is important progress.”
Dr. Holle was involved in the development of revised European guidelines. She is also the lead author of a recent publication on treatment of vasculitis on available evidence. Dr. Hellmich acknowledged having ties to multiple pharma companies, acting as speaker, consultant, or both to Abbvie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, GlaxoSmithKline, InflaRx, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Vifor.
FROM THE EULAR 2022 CONGRESS
Upadacitinib effective against nonradiographic AxSpA
COPENHAGEN – The Janus kinase (JAK) inhibitor upadacitinib (Rinvoq, AbbVie) was associated with significant improvements in disease activity, pain, function, and quality of life, compared with placebo, in patients with nonradiographic axial spondyloarthritis (nr-axSpA), results of the first efficacy analysis of the phase 3, randomized SELECT-AXIS-2 trial showed.
The trial met its primary endpoint of an improvement of Assessment of SpondyloArthritis International Society 40% (ASAS 40) response criteria in the prespecified efficacy analysis at week 14, reported Filip Van den Bosch, MD, PhD, Ghent (Belgium) University.
In all, 45% of patients randomized to receive upadacitinib achieved an ASAS 40, compared with 23% of those assigned to placebo (P < .001).
“This is the first study showing efficacy and showing that the JAK inhibitor upadacitinib might be a therapeutic option in patients with active, nonradiographic spondyloarthritis,” Van den Bosch said at the annual European Congress of Rheumatology.
Although JAK inhibitors have previously been shown to be efficacious and safe for the treatment of ankylosing spondylitis, the SELECT-AXIS-2 trial is the first to evaluate a JAK inhibitor in nonradiographic axSpA, he added.
Study details
Patients 18 years and older with rheumatologist-diagnosed nr-axSpA were eligible for the study if they also met 2009 ASAS classification criteria for axSpA but not the radiologic criterion of modified New York criteria; had objective signs of active inflammation consistent with axSpA on MRI of the sacroiliac joints and/or high-sensitivity C-reactive protein above the upper limit of normal (2.87 mg/L) at screening; and had Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and patient-assessment of total back pain scores of 4 or greater based on a 0-10 numeric rating scale at study entry.
Patients were screened with MRI imaging of the spine and x-rays of the sacroiliac joints and spine, and then randomized to receive either placebo (157 patients) or upadacitinib 15 mg daily (158 patients) for 52 weeks. At the end of 52 weeks, all patients on upadacitinib will continue on the drug at the same dose level, and those assigned to placebo will be switched over to 15 mg upadacitinib daily maintenance.
As well as meeting the primary endpoint at week 14, response rates with the JAK inhibitor were higher at all time points over this initial time period, Dr. Van den Bosch noted.
Most targets hit
Of 14 multiplicity-controlled secondary endpoints, 12 were statistically better with upadacitinib, including change from baseline in patient’s assessment of total back pain, Bath Ankylosing Spondylitis Functional Index, Ankylosing Spondylitis Disease Activity Score, Low Disease Activity, Ankylosing Spondylitis Quality of Life, and MRI Spondyloarthritis Research Consortium of Canada score for sacroiliac joints.
Only the BASDAI and Maastricht Ankylosing Spondylitis Enthesitis Score were not significantly better with the JAK inhibitor.
The safety of upadacitinib in this setting was consistent with its known safety profile, Dr. Van den Bosch said.
Approximately half of all patients in each trial arm had an adverse event. Serious adverse events were reported in four patients assigned to upadacitinib versus two on placebo, and serious adverse events requiring drug discontinuation occurred in two and four patients, respectively.
‘Important’ data
Fabian Proft, MD, head of the clinical trials unit at Charite University Hospital, Berlin, who was not involved in the study, said in an interview that the findings were not surprising.
“We know the efficacy of upadacitinib already in radiographic axial spondyloarthritis, and from all the other drugs that we also know that are effective in radiographic axial spondyloarthritis that are similarly effective in nonradiographic disease,” he said.
“I think it is really important because it is the first data on JAK inhibition also in non-radiographic axial spondyloarthritis – an important step,” said Dr. Proft, who was comoderator of the oral abstract session where Van den Bosch reported the data.
The trial was supported by AbbVie. Dr. Van den Bosch disclosed speaker and consulting fees from AbbVie and others. Dr. Proft disclosed speaker and consulting fees from AbbVie as well.
A version of this article first appeared on Medscape.com.
COPENHAGEN – The Janus kinase (JAK) inhibitor upadacitinib (Rinvoq, AbbVie) was associated with significant improvements in disease activity, pain, function, and quality of life, compared with placebo, in patients with nonradiographic axial spondyloarthritis (nr-axSpA), results of the first efficacy analysis of the phase 3, randomized SELECT-AXIS-2 trial showed.
The trial met its primary endpoint of an improvement of Assessment of SpondyloArthritis International Society 40% (ASAS 40) response criteria in the prespecified efficacy analysis at week 14, reported Filip Van den Bosch, MD, PhD, Ghent (Belgium) University.
In all, 45% of patients randomized to receive upadacitinib achieved an ASAS 40, compared with 23% of those assigned to placebo (P < .001).
“This is the first study showing efficacy and showing that the JAK inhibitor upadacitinib might be a therapeutic option in patients with active, nonradiographic spondyloarthritis,” Van den Bosch said at the annual European Congress of Rheumatology.
Although JAK inhibitors have previously been shown to be efficacious and safe for the treatment of ankylosing spondylitis, the SELECT-AXIS-2 trial is the first to evaluate a JAK inhibitor in nonradiographic axSpA, he added.
Study details
Patients 18 years and older with rheumatologist-diagnosed nr-axSpA were eligible for the study if they also met 2009 ASAS classification criteria for axSpA but not the radiologic criterion of modified New York criteria; had objective signs of active inflammation consistent with axSpA on MRI of the sacroiliac joints and/or high-sensitivity C-reactive protein above the upper limit of normal (2.87 mg/L) at screening; and had Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and patient-assessment of total back pain scores of 4 or greater based on a 0-10 numeric rating scale at study entry.
Patients were screened with MRI imaging of the spine and x-rays of the sacroiliac joints and spine, and then randomized to receive either placebo (157 patients) or upadacitinib 15 mg daily (158 patients) for 52 weeks. At the end of 52 weeks, all patients on upadacitinib will continue on the drug at the same dose level, and those assigned to placebo will be switched over to 15 mg upadacitinib daily maintenance.
As well as meeting the primary endpoint at week 14, response rates with the JAK inhibitor were higher at all time points over this initial time period, Dr. Van den Bosch noted.
Most targets hit
Of 14 multiplicity-controlled secondary endpoints, 12 were statistically better with upadacitinib, including change from baseline in patient’s assessment of total back pain, Bath Ankylosing Spondylitis Functional Index, Ankylosing Spondylitis Disease Activity Score, Low Disease Activity, Ankylosing Spondylitis Quality of Life, and MRI Spondyloarthritis Research Consortium of Canada score for sacroiliac joints.
Only the BASDAI and Maastricht Ankylosing Spondylitis Enthesitis Score were not significantly better with the JAK inhibitor.
The safety of upadacitinib in this setting was consistent with its known safety profile, Dr. Van den Bosch said.
Approximately half of all patients in each trial arm had an adverse event. Serious adverse events were reported in four patients assigned to upadacitinib versus two on placebo, and serious adverse events requiring drug discontinuation occurred in two and four patients, respectively.
‘Important’ data
Fabian Proft, MD, head of the clinical trials unit at Charite University Hospital, Berlin, who was not involved in the study, said in an interview that the findings were not surprising.
“We know the efficacy of upadacitinib already in radiographic axial spondyloarthritis, and from all the other drugs that we also know that are effective in radiographic axial spondyloarthritis that are similarly effective in nonradiographic disease,” he said.
“I think it is really important because it is the first data on JAK inhibition also in non-radiographic axial spondyloarthritis – an important step,” said Dr. Proft, who was comoderator of the oral abstract session where Van den Bosch reported the data.
The trial was supported by AbbVie. Dr. Van den Bosch disclosed speaker and consulting fees from AbbVie and others. Dr. Proft disclosed speaker and consulting fees from AbbVie as well.
A version of this article first appeared on Medscape.com.
COPENHAGEN – The Janus kinase (JAK) inhibitor upadacitinib (Rinvoq, AbbVie) was associated with significant improvements in disease activity, pain, function, and quality of life, compared with placebo, in patients with nonradiographic axial spondyloarthritis (nr-axSpA), results of the first efficacy analysis of the phase 3, randomized SELECT-AXIS-2 trial showed.
The trial met its primary endpoint of an improvement of Assessment of SpondyloArthritis International Society 40% (ASAS 40) response criteria in the prespecified efficacy analysis at week 14, reported Filip Van den Bosch, MD, PhD, Ghent (Belgium) University.
In all, 45% of patients randomized to receive upadacitinib achieved an ASAS 40, compared with 23% of those assigned to placebo (P < .001).
“This is the first study showing efficacy and showing that the JAK inhibitor upadacitinib might be a therapeutic option in patients with active, nonradiographic spondyloarthritis,” Van den Bosch said at the annual European Congress of Rheumatology.
Although JAK inhibitors have previously been shown to be efficacious and safe for the treatment of ankylosing spondylitis, the SELECT-AXIS-2 trial is the first to evaluate a JAK inhibitor in nonradiographic axSpA, he added.
Study details
Patients 18 years and older with rheumatologist-diagnosed nr-axSpA were eligible for the study if they also met 2009 ASAS classification criteria for axSpA but not the radiologic criterion of modified New York criteria; had objective signs of active inflammation consistent with axSpA on MRI of the sacroiliac joints and/or high-sensitivity C-reactive protein above the upper limit of normal (2.87 mg/L) at screening; and had Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and patient-assessment of total back pain scores of 4 or greater based on a 0-10 numeric rating scale at study entry.
Patients were screened with MRI imaging of the spine and x-rays of the sacroiliac joints and spine, and then randomized to receive either placebo (157 patients) or upadacitinib 15 mg daily (158 patients) for 52 weeks. At the end of 52 weeks, all patients on upadacitinib will continue on the drug at the same dose level, and those assigned to placebo will be switched over to 15 mg upadacitinib daily maintenance.
As well as meeting the primary endpoint at week 14, response rates with the JAK inhibitor were higher at all time points over this initial time period, Dr. Van den Bosch noted.
Most targets hit
Of 14 multiplicity-controlled secondary endpoints, 12 were statistically better with upadacitinib, including change from baseline in patient’s assessment of total back pain, Bath Ankylosing Spondylitis Functional Index, Ankylosing Spondylitis Disease Activity Score, Low Disease Activity, Ankylosing Spondylitis Quality of Life, and MRI Spondyloarthritis Research Consortium of Canada score for sacroiliac joints.
Only the BASDAI and Maastricht Ankylosing Spondylitis Enthesitis Score were not significantly better with the JAK inhibitor.
The safety of upadacitinib in this setting was consistent with its known safety profile, Dr. Van den Bosch said.
Approximately half of all patients in each trial arm had an adverse event. Serious adverse events were reported in four patients assigned to upadacitinib versus two on placebo, and serious adverse events requiring drug discontinuation occurred in two and four patients, respectively.
‘Important’ data
Fabian Proft, MD, head of the clinical trials unit at Charite University Hospital, Berlin, who was not involved in the study, said in an interview that the findings were not surprising.
“We know the efficacy of upadacitinib already in radiographic axial spondyloarthritis, and from all the other drugs that we also know that are effective in radiographic axial spondyloarthritis that are similarly effective in nonradiographic disease,” he said.
“I think it is really important because it is the first data on JAK inhibition also in non-radiographic axial spondyloarthritis – an important step,” said Dr. Proft, who was comoderator of the oral abstract session where Van den Bosch reported the data.
The trial was supported by AbbVie. Dr. Van den Bosch disclosed speaker and consulting fees from AbbVie and others. Dr. Proft disclosed speaker and consulting fees from AbbVie as well.
A version of this article first appeared on Medscape.com.
AT THE EULAR 2022 CONGRESS
Gout app improves treat to target, reduces flares
Self-management of gout using a smartphone app to record self-test urate levels and flares, and communicate those results to clinicians, could see more patients reaching target urate levels and even reducing flare frequency, a study has found.
Writing in The Lancet Rheumatology, Philip Riches, PhD, of the rheumatic disease unit at Western General Hospital in Edinburgh, and coauthors presented the findings of their randomized, controlled feasibility study of a new gout self-management approach aimed at helping patients treat to target.
While current rheumatology guidelines stress the importance of keeping urate below target levels to reduce flares and improve clinical outcomes, this isn’t always achieved in clinical practice. A previous trial of a nurse-led treat-to-target intervention did show a reduced incidence of flares and tophaceous disease, but the authors said, despite its cost-effectiveness, this approach has yet to be implemented in the United Kingdom.
Dr. Riches and colleagues developed a self-management strategy in which all 60 patients in the study self-tested their urate levels and were prompted to enter that data into the GoutSMART smartphone app once a month or opportunistically, along with information on disease severity and quality of life. All patients had been recommended for initiation or escalation of urate-lowering therapy, and had a serum urate of 0.36 mmol/L (6 mg/dL) or higher at baseline, and all received a gout management plan at the start of the study.
Patients in the intervention group who recorded a urate level above 0.30 mmol/L (5 mg/dL) via the app during the study were prompted to do a self-test every 2 weeks and given daily reminders in the app. Their urate levels were transmitted securely to the study team who then advised on dose escalation or treatment change. Those in the usual-care group also used the app but it only prompted them to record gout flares, keep quality of life diaries, or message the researchers.
At 24 weeks after the start of the study, 73% of 40 participants in the self-management group had reached the urate target of 0.30 mmol/L or below, compared with 15% of the 20 participants in the usual-care group (P < .0001).
The difference between the two groups was sustained even 1 year after starting the intervention, when 80% of those in the self-management group had reached that target, compared with 45% of those in the usual-care group.
Patients in the intervention group also had fewer flares, experiencing a mean of 2.03 flares in the first 24 weeks, compared with a mean of 3 among the control group, although the study didn’t report any difference in the rates of tophaceous disease.
Those in the self-management group had fewer medical appointments, but were prescribed higher doses of allopurinol at the 24- and 52-week visits.
“Qualitative feedback suggests that the self-monitoring approach was accepted by most participants and was enthusiastically endorsed by many,” the authors wrote. “The approach empowers patients and provides feedback on the effect of medication.”
It will be important to determine if the success of this self-management intervention can be replicated in an even broader patient population, Lisa K. Stamp, MBChB, PhD, of University of Otago, Christchurch, New Zealand, and Angelo L. Gaffo, MD, of University of Alabama at Birmingham, noted in an accompanying editorial. They wrote it was encouraging that only 7% of the 92 people screened for the trial did not have a smartphone and that it the patient sample had a mean age of 53 years. However, the trial did not include people with chronic kidney disease who make up nearly a quarter of all people with gout.
“It remains unknown whether the characteristics of those who did not reach target urate are the same or different as those who did, and a head-to-head comparison of these interventions would be of interest,” Dr. Stamp and Dr. Gaffo wrote. “A key challenge in managing gout is to determine which treatment strategy will be best suited to an individual with gout and to identify those for whom more support might be required.”
This study was supported by the University of Edinburgh and funded by NHS Lothian Health Foundation. No conflicts of interest were declared.
Self-management of gout using a smartphone app to record self-test urate levels and flares, and communicate those results to clinicians, could see more patients reaching target urate levels and even reducing flare frequency, a study has found.
Writing in The Lancet Rheumatology, Philip Riches, PhD, of the rheumatic disease unit at Western General Hospital in Edinburgh, and coauthors presented the findings of their randomized, controlled feasibility study of a new gout self-management approach aimed at helping patients treat to target.
While current rheumatology guidelines stress the importance of keeping urate below target levels to reduce flares and improve clinical outcomes, this isn’t always achieved in clinical practice. A previous trial of a nurse-led treat-to-target intervention did show a reduced incidence of flares and tophaceous disease, but the authors said, despite its cost-effectiveness, this approach has yet to be implemented in the United Kingdom.
Dr. Riches and colleagues developed a self-management strategy in which all 60 patients in the study self-tested their urate levels and were prompted to enter that data into the GoutSMART smartphone app once a month or opportunistically, along with information on disease severity and quality of life. All patients had been recommended for initiation or escalation of urate-lowering therapy, and had a serum urate of 0.36 mmol/L (6 mg/dL) or higher at baseline, and all received a gout management plan at the start of the study.
Patients in the intervention group who recorded a urate level above 0.30 mmol/L (5 mg/dL) via the app during the study were prompted to do a self-test every 2 weeks and given daily reminders in the app. Their urate levels were transmitted securely to the study team who then advised on dose escalation or treatment change. Those in the usual-care group also used the app but it only prompted them to record gout flares, keep quality of life diaries, or message the researchers.
At 24 weeks after the start of the study, 73% of 40 participants in the self-management group had reached the urate target of 0.30 mmol/L or below, compared with 15% of the 20 participants in the usual-care group (P < .0001).
The difference between the two groups was sustained even 1 year after starting the intervention, when 80% of those in the self-management group had reached that target, compared with 45% of those in the usual-care group.
Patients in the intervention group also had fewer flares, experiencing a mean of 2.03 flares in the first 24 weeks, compared with a mean of 3 among the control group, although the study didn’t report any difference in the rates of tophaceous disease.
Those in the self-management group had fewer medical appointments, but were prescribed higher doses of allopurinol at the 24- and 52-week visits.
“Qualitative feedback suggests that the self-monitoring approach was accepted by most participants and was enthusiastically endorsed by many,” the authors wrote. “The approach empowers patients and provides feedback on the effect of medication.”
It will be important to determine if the success of this self-management intervention can be replicated in an even broader patient population, Lisa K. Stamp, MBChB, PhD, of University of Otago, Christchurch, New Zealand, and Angelo L. Gaffo, MD, of University of Alabama at Birmingham, noted in an accompanying editorial. They wrote it was encouraging that only 7% of the 92 people screened for the trial did not have a smartphone and that it the patient sample had a mean age of 53 years. However, the trial did not include people with chronic kidney disease who make up nearly a quarter of all people with gout.
“It remains unknown whether the characteristics of those who did not reach target urate are the same or different as those who did, and a head-to-head comparison of these interventions would be of interest,” Dr. Stamp and Dr. Gaffo wrote. “A key challenge in managing gout is to determine which treatment strategy will be best suited to an individual with gout and to identify those for whom more support might be required.”
This study was supported by the University of Edinburgh and funded by NHS Lothian Health Foundation. No conflicts of interest were declared.
Self-management of gout using a smartphone app to record self-test urate levels and flares, and communicate those results to clinicians, could see more patients reaching target urate levels and even reducing flare frequency, a study has found.
Writing in The Lancet Rheumatology, Philip Riches, PhD, of the rheumatic disease unit at Western General Hospital in Edinburgh, and coauthors presented the findings of their randomized, controlled feasibility study of a new gout self-management approach aimed at helping patients treat to target.
While current rheumatology guidelines stress the importance of keeping urate below target levels to reduce flares and improve clinical outcomes, this isn’t always achieved in clinical practice. A previous trial of a nurse-led treat-to-target intervention did show a reduced incidence of flares and tophaceous disease, but the authors said, despite its cost-effectiveness, this approach has yet to be implemented in the United Kingdom.
Dr. Riches and colleagues developed a self-management strategy in which all 60 patients in the study self-tested their urate levels and were prompted to enter that data into the GoutSMART smartphone app once a month or opportunistically, along with information on disease severity and quality of life. All patients had been recommended for initiation or escalation of urate-lowering therapy, and had a serum urate of 0.36 mmol/L (6 mg/dL) or higher at baseline, and all received a gout management plan at the start of the study.
Patients in the intervention group who recorded a urate level above 0.30 mmol/L (5 mg/dL) via the app during the study were prompted to do a self-test every 2 weeks and given daily reminders in the app. Their urate levels were transmitted securely to the study team who then advised on dose escalation or treatment change. Those in the usual-care group also used the app but it only prompted them to record gout flares, keep quality of life diaries, or message the researchers.
At 24 weeks after the start of the study, 73% of 40 participants in the self-management group had reached the urate target of 0.30 mmol/L or below, compared with 15% of the 20 participants in the usual-care group (P < .0001).
The difference between the two groups was sustained even 1 year after starting the intervention, when 80% of those in the self-management group had reached that target, compared with 45% of those in the usual-care group.
Patients in the intervention group also had fewer flares, experiencing a mean of 2.03 flares in the first 24 weeks, compared with a mean of 3 among the control group, although the study didn’t report any difference in the rates of tophaceous disease.
Those in the self-management group had fewer medical appointments, but were prescribed higher doses of allopurinol at the 24- and 52-week visits.
“Qualitative feedback suggests that the self-monitoring approach was accepted by most participants and was enthusiastically endorsed by many,” the authors wrote. “The approach empowers patients and provides feedback on the effect of medication.”
It will be important to determine if the success of this self-management intervention can be replicated in an even broader patient population, Lisa K. Stamp, MBChB, PhD, of University of Otago, Christchurch, New Zealand, and Angelo L. Gaffo, MD, of University of Alabama at Birmingham, noted in an accompanying editorial. They wrote it was encouraging that only 7% of the 92 people screened for the trial did not have a smartphone and that it the patient sample had a mean age of 53 years. However, the trial did not include people with chronic kidney disease who make up nearly a quarter of all people with gout.
“It remains unknown whether the characteristics of those who did not reach target urate are the same or different as those who did, and a head-to-head comparison of these interventions would be of interest,” Dr. Stamp and Dr. Gaffo wrote. “A key challenge in managing gout is to determine which treatment strategy will be best suited to an individual with gout and to identify those for whom more support might be required.”
This study was supported by the University of Edinburgh and funded by NHS Lothian Health Foundation. No conflicts of interest were declared.
FROM THE LANCET RHEUMATOLOGY
Pfizer COVID vaccine performs well in youth with rheumatic diseases
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CARRA 2022
Lenabasum improved skin symptoms in dermatomyositis, but future is uncertain
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.
Phase 2 findings
The single-center phase 2 trial of lenabasum for DM enrolled 22 adults with minimal muscle involvement as evidenced by normal maximal resistance on muscle testing at entry and throughout the study. Most were taking immunosuppressant medication, and all had CDASI scores of at least 20, with mean scores in the severe range (> 26). Symptoms registered on patient-reported outcome measures were moderate to severe.
Patients received a half-dose of lenabasum (20 mg daily) for 1 month and a full dose (20 mg twice daily) for 2 months, or placebo, and were followed for an additional month without dosing.
Starting at day 43 – approximately 2 weeks after the dose was increased – there was “a trend for the change from baseline CDASI to be greater” in the lenabasum group, compared with those on placebo, Dr. Werth and colleagues reported. The differences reached statistical significance on day 113 (P = .038), a month after patients discontinued lenabasum, “suggesting that the modulation of the inflammatory response by lenabasum continued beyond its last dose.”
Five of the 11 patients treated with lenabasum (45%), and none of those on placebo, achieved at least a 40% reduction in the CDASI activity score by the end of the study.
Patients in the lenabasum group also had greater improvement in the Skindex-29 Symptoms scores – an objective measure of itch – and improvements in other secondary efficacy outcomes, including pain, though these did not reach statistical significance.
Skin biopsies before and after treatment showed significant reductions in inflammatory cytokines relevant to DM pathogenesis. Patients treated with the CB2 agonist had a downward trend in the CD4+ T cell population, which correlated with decreased CDASI activity scores, for instance, and a decrease in IL-31 protein expression, which correlated with decreased Skindex-29 Symptoms scores, the investigators reported.
There were no serious adverse events related to the CB2 agonist, and no treatment discontinuations.
The main part of the phase 2 trial, conducted from 2015 to 2017, was followed by a 3-year, open-label extension, in which 20 of the 22 patients took lenabasum 20 mg twice a day. The drug continued to be safe and well tolerated, and the CDASI activity score and other outcomes improved through year 1 and remained stable thereafter, according to a poster presented by Dr. Werth at the 2021 EULAR meeting.
After 1 year in the open-label extension, 60%-70% of patients had achieved mild skin disease, and 75% had achieved at least a 40% reduction in CDASI activity.
“A lot of patients, even if they weren’t completely cleared, were much happier in terms of their itch,” said Dr. Werth, also chief of dermatology, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia. “It’s been difficult for a lot of them now that they’re off the long-term extension ... a lot of them are flaring.”
The future
In the lab, with funding from the National Institutes of Health, Dr. Werth is continuing to investigate how lenabasum may be working in DM. A paper just published in the open access journal Arthritis Research & Therapy describes CB2 receptor distribution and up-regulation on key immune cells in the skin and blood, and how, in DM skin, its highest expression is on dendritic cells.
Through both mechanistic and more clinical research, “it’s important to understand the characteristics of the people [lenabasum] worked in or didn’t work in,” she said.
And in clinical trials, it’s important to capture meaningful improvement from the patient perspective. “It may be,” she noted, “that more global, systemic assessments are not the way to go for autoimmune skin disease.”
For dcSSc, Dr. Spiera said, it’s possible that a CB2 agonist may be helpful for patients who have been on immunosuppressants, particularly mycophenolate, for more than 6 months “and are still struggling.”
The phase 2 trial in DM was funded by the National Institutes of Health, the Department of Veterans Affairs, and Corbus Pharmaceuticals. The phase 3 trials in DM and in dcSSc were funded by Corbus. Dr. Werth disclosed grant support from Corbus and several other pharmaceutical companies. Dr. Spiera disclosed that he has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.
Phase 2 findings
The single-center phase 2 trial of lenabasum for DM enrolled 22 adults with minimal muscle involvement as evidenced by normal maximal resistance on muscle testing at entry and throughout the study. Most were taking immunosuppressant medication, and all had CDASI scores of at least 20, with mean scores in the severe range (> 26). Symptoms registered on patient-reported outcome measures were moderate to severe.
Patients received a half-dose of lenabasum (20 mg daily) for 1 month and a full dose (20 mg twice daily) for 2 months, or placebo, and were followed for an additional month without dosing.
Starting at day 43 – approximately 2 weeks after the dose was increased – there was “a trend for the change from baseline CDASI to be greater” in the lenabasum group, compared with those on placebo, Dr. Werth and colleagues reported. The differences reached statistical significance on day 113 (P = .038), a month after patients discontinued lenabasum, “suggesting that the modulation of the inflammatory response by lenabasum continued beyond its last dose.”
Five of the 11 patients treated with lenabasum (45%), and none of those on placebo, achieved at least a 40% reduction in the CDASI activity score by the end of the study.
Patients in the lenabasum group also had greater improvement in the Skindex-29 Symptoms scores – an objective measure of itch – and improvements in other secondary efficacy outcomes, including pain, though these did not reach statistical significance.
Skin biopsies before and after treatment showed significant reductions in inflammatory cytokines relevant to DM pathogenesis. Patients treated with the CB2 agonist had a downward trend in the CD4+ T cell population, which correlated with decreased CDASI activity scores, for instance, and a decrease in IL-31 protein expression, which correlated with decreased Skindex-29 Symptoms scores, the investigators reported.
There were no serious adverse events related to the CB2 agonist, and no treatment discontinuations.
The main part of the phase 2 trial, conducted from 2015 to 2017, was followed by a 3-year, open-label extension, in which 20 of the 22 patients took lenabasum 20 mg twice a day. The drug continued to be safe and well tolerated, and the CDASI activity score and other outcomes improved through year 1 and remained stable thereafter, according to a poster presented by Dr. Werth at the 2021 EULAR meeting.
After 1 year in the open-label extension, 60%-70% of patients had achieved mild skin disease, and 75% had achieved at least a 40% reduction in CDASI activity.
“A lot of patients, even if they weren’t completely cleared, were much happier in terms of their itch,” said Dr. Werth, also chief of dermatology, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia. “It’s been difficult for a lot of them now that they’re off the long-term extension ... a lot of them are flaring.”
The future
In the lab, with funding from the National Institutes of Health, Dr. Werth is continuing to investigate how lenabasum may be working in DM. A paper just published in the open access journal Arthritis Research & Therapy describes CB2 receptor distribution and up-regulation on key immune cells in the skin and blood, and how, in DM skin, its highest expression is on dendritic cells.
Through both mechanistic and more clinical research, “it’s important to understand the characteristics of the people [lenabasum] worked in or didn’t work in,” she said.
And in clinical trials, it’s important to capture meaningful improvement from the patient perspective. “It may be,” she noted, “that more global, systemic assessments are not the way to go for autoimmune skin disease.”
For dcSSc, Dr. Spiera said, it’s possible that a CB2 agonist may be helpful for patients who have been on immunosuppressants, particularly mycophenolate, for more than 6 months “and are still struggling.”
The phase 2 trial in DM was funded by the National Institutes of Health, the Department of Veterans Affairs, and Corbus Pharmaceuticals. The phase 3 trials in DM and in dcSSc were funded by Corbus. Dr. Werth disclosed grant support from Corbus and several other pharmaceutical companies. Dr. Spiera disclosed that he has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.
Phase 2 findings
The single-center phase 2 trial of lenabasum for DM enrolled 22 adults with minimal muscle involvement as evidenced by normal maximal resistance on muscle testing at entry and throughout the study. Most were taking immunosuppressant medication, and all had CDASI scores of at least 20, with mean scores in the severe range (> 26). Symptoms registered on patient-reported outcome measures were moderate to severe.
Patients received a half-dose of lenabasum (20 mg daily) for 1 month and a full dose (20 mg twice daily) for 2 months, or placebo, and were followed for an additional month without dosing.
Starting at day 43 – approximately 2 weeks after the dose was increased – there was “a trend for the change from baseline CDASI to be greater” in the lenabasum group, compared with those on placebo, Dr. Werth and colleagues reported. The differences reached statistical significance on day 113 (P = .038), a month after patients discontinued lenabasum, “suggesting that the modulation of the inflammatory response by lenabasum continued beyond its last dose.”
Five of the 11 patients treated with lenabasum (45%), and none of those on placebo, achieved at least a 40% reduction in the CDASI activity score by the end of the study.
Patients in the lenabasum group also had greater improvement in the Skindex-29 Symptoms scores – an objective measure of itch – and improvements in other secondary efficacy outcomes, including pain, though these did not reach statistical significance.
Skin biopsies before and after treatment showed significant reductions in inflammatory cytokines relevant to DM pathogenesis. Patients treated with the CB2 agonist had a downward trend in the CD4+ T cell population, which correlated with decreased CDASI activity scores, for instance, and a decrease in IL-31 protein expression, which correlated with decreased Skindex-29 Symptoms scores, the investigators reported.
There were no serious adverse events related to the CB2 agonist, and no treatment discontinuations.
The main part of the phase 2 trial, conducted from 2015 to 2017, was followed by a 3-year, open-label extension, in which 20 of the 22 patients took lenabasum 20 mg twice a day. The drug continued to be safe and well tolerated, and the CDASI activity score and other outcomes improved through year 1 and remained stable thereafter, according to a poster presented by Dr. Werth at the 2021 EULAR meeting.
After 1 year in the open-label extension, 60%-70% of patients had achieved mild skin disease, and 75% had achieved at least a 40% reduction in CDASI activity.
“A lot of patients, even if they weren’t completely cleared, were much happier in terms of their itch,” said Dr. Werth, also chief of dermatology, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia. “It’s been difficult for a lot of them now that they’re off the long-term extension ... a lot of them are flaring.”
The future
In the lab, with funding from the National Institutes of Health, Dr. Werth is continuing to investigate how lenabasum may be working in DM. A paper just published in the open access journal Arthritis Research & Therapy describes CB2 receptor distribution and up-regulation on key immune cells in the skin and blood, and how, in DM skin, its highest expression is on dendritic cells.
Through both mechanistic and more clinical research, “it’s important to understand the characteristics of the people [lenabasum] worked in or didn’t work in,” she said.
And in clinical trials, it’s important to capture meaningful improvement from the patient perspective. “It may be,” she noted, “that more global, systemic assessments are not the way to go for autoimmune skin disease.”
For dcSSc, Dr. Spiera said, it’s possible that a CB2 agonist may be helpful for patients who have been on immunosuppressants, particularly mycophenolate, for more than 6 months “and are still struggling.”
The phase 2 trial in DM was funded by the National Institutes of Health, the Department of Veterans Affairs, and Corbus Pharmaceuticals. The phase 3 trials in DM and in dcSSc were funded by Corbus. Dr. Werth disclosed grant support from Corbus and several other pharmaceutical companies. Dr. Spiera disclosed that he has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
‘Shielding’ status provides best indicator of COVID-19 mortality in U.K. arthritis population
Being identified as someone that was advised to stay at home and shield, or keep away from face-to-face interactions with others, during the COVID-19 pandemic was indicative of an increased risk for dying from COVID-19 within 28 days of infection, a U.K. study of inflammatory arthritis patients versus the general population suggests.
In fact, shielding status was the highest ranked of all the risk factors identified for early mortality from COVID-19, with a hazard ratio of 1.52 (95% confidence interval, 1.40-1.64) comparing people with and without inflammatory arthritis (IA) who had tested positive.
The list of risk factors associated with higher mortality in the IA patients versus the general population also included diabetes (HR, 1.38), smoking (HR, 1.27), hypertension (HR, 1.19), glucocorticoid use (HR, 1.17), and cancer (HR, 1.10), as well as increasing age (HR, 1.08) and body mass index (HR, 1.01).
Also important was the person’s prior hospitalization history, with those needing in-hospital care in the year running up to their admission for COVID-19 associated with a 34% higher risk for death, and being hospitalized previously with a serious infection was associated with a 20% higher risk.
This has more to do people’s overall vulnerability than their IA, suggested the team behind the findings, who also found that the risk of catching COVID-19 was significantly lower among patients with IA than the general population (3.5% vs. 6%), presumably because of shielding.
Examining the risks for COVID-19 in real-life practice
“COVID-19 has caused over 10 million deaths,” Roxanne Cooksey, PhD, said at the annual meeting of the British Society for Rheumatology. “It’s greatly affected vulnerable individuals, which includes individuals with IA, this is due to their compromised immune system and increased risk of infection and the medications that they take to manage their conditions.
“Previous studies have had mixed results about whether people with IA have an increased risk of poor outcome,” added Dr. Cooksey, who is a postdoctoral researcher in the division of infection and immunity at Cardiff (Wales) University.
“So, our research question looks to investigate inflammatory arthritis – that’s rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis – to see whether the conditions themselves or indeed their medications predispose individuals to an increased risk of contracting COVID or even more adverse outcomes.”
Dr. Cooksey and colleagues looked specifically at COVID-19 infection rates and outcomes in adults living in Wales during the first year of the pandemic (March 2020 to May 2021). As such they used routinely collected, anonymized health data from the SAIL Databank and performed a retrospective, population-based cohort study. In total, there were 1,966 people with inflammatory arthritis identified as having COVID-19 and 166,602 people without IA but who had COVID-19 in the study population.
As might be expected, people with inflammatory arthritis who tested positive for COVID-19 were older than those testing positive in the general population, at a mean of 62 years versus 46 years. They were also more likely to have been advised to shield (49.4% versus 4.6%), which in the United Kingdom constituted of receiving a letter telling them about the importance of social distancing, wearing a mask when out in public, and quarantining themselves at home whenever possible.
The main outcomes were hospitalizations and mortality within 28 days of COVID-19 infection. Considering the overall inflammatory arthritis population, rates of both outcomes were higher versus the general population. And when the researchers analyzed the risks according to the type of inflammatory arthritis, the associations were not statistically significant in a multivariable analysis for people with any of the inflammatory arthritis diagnoses: rheumatoid arthritis (n = 1,283), psoriatic arthritis (n = 514), or ankylosing spondylitis (n = 246). Some patients had more than one inflammatory arthritis diagnosis.
What does this all mean?
Dr. Cooksey conceded that there were lots of limitations to the data collected – from misclassification bias to data possibly not have been recorded completely or missing because of the disruption to health care services during the early stages of the pandemic. Patients may have been told to shield but not actually shielded, she observed, and maybe because a lack of testing COVID-19 cases were missed or people could have been asymptomatic or unable to be tested.
“The study supports the role of shielding in inflammatory arthritis,” Dr. Cooksey said, particularly in those with RA and the risk factors associated with an increased risk in death. However, that may not mean the entire population, she suggested, saying that “refining the criteria for shielding will help mitigate the negative effects of the entire IA population.”
Senior team member Ernest Choy, MD, added his thoughts, saying that, rather than giving generic shielding recommendations to all IA patients, not everyone has the same risk, so maybe not everyone needs to shield to the same level.
“Psoriatic arthritis patients and ankylosing spondylitis patients are younger, so they really don’t have as high a risk like patients with rheumatoid arthritis,” he said.
Dr. Choy, who is professor of rheumatology at the Cardiff Institute of Infection & Immunity, commented that it was not surprising to find that a prior serious infection was a risk for COVID-19 mortality. This risk factor was examined because of the known association between biologic use and the risk for serious infection.
Moreover, he said that, “if you have a serious comorbidity that requires you to get admitted to hospital, that is a reflection of your vulnerability.”
Dr. Cooksey and Dr. Choy had no relevant conflicts of interest to disclose.
Being identified as someone that was advised to stay at home and shield, or keep away from face-to-face interactions with others, during the COVID-19 pandemic was indicative of an increased risk for dying from COVID-19 within 28 days of infection, a U.K. study of inflammatory arthritis patients versus the general population suggests.
In fact, shielding status was the highest ranked of all the risk factors identified for early mortality from COVID-19, with a hazard ratio of 1.52 (95% confidence interval, 1.40-1.64) comparing people with and without inflammatory arthritis (IA) who had tested positive.
The list of risk factors associated with higher mortality in the IA patients versus the general population also included diabetes (HR, 1.38), smoking (HR, 1.27), hypertension (HR, 1.19), glucocorticoid use (HR, 1.17), and cancer (HR, 1.10), as well as increasing age (HR, 1.08) and body mass index (HR, 1.01).
Also important was the person’s prior hospitalization history, with those needing in-hospital care in the year running up to their admission for COVID-19 associated with a 34% higher risk for death, and being hospitalized previously with a serious infection was associated with a 20% higher risk.
This has more to do people’s overall vulnerability than their IA, suggested the team behind the findings, who also found that the risk of catching COVID-19 was significantly lower among patients with IA than the general population (3.5% vs. 6%), presumably because of shielding.
Examining the risks for COVID-19 in real-life practice
“COVID-19 has caused over 10 million deaths,” Roxanne Cooksey, PhD, said at the annual meeting of the British Society for Rheumatology. “It’s greatly affected vulnerable individuals, which includes individuals with IA, this is due to their compromised immune system and increased risk of infection and the medications that they take to manage their conditions.
“Previous studies have had mixed results about whether people with IA have an increased risk of poor outcome,” added Dr. Cooksey, who is a postdoctoral researcher in the division of infection and immunity at Cardiff (Wales) University.
“So, our research question looks to investigate inflammatory arthritis – that’s rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis – to see whether the conditions themselves or indeed their medications predispose individuals to an increased risk of contracting COVID or even more adverse outcomes.”
Dr. Cooksey and colleagues looked specifically at COVID-19 infection rates and outcomes in adults living in Wales during the first year of the pandemic (March 2020 to May 2021). As such they used routinely collected, anonymized health data from the SAIL Databank and performed a retrospective, population-based cohort study. In total, there were 1,966 people with inflammatory arthritis identified as having COVID-19 and 166,602 people without IA but who had COVID-19 in the study population.
As might be expected, people with inflammatory arthritis who tested positive for COVID-19 were older than those testing positive in the general population, at a mean of 62 years versus 46 years. They were also more likely to have been advised to shield (49.4% versus 4.6%), which in the United Kingdom constituted of receiving a letter telling them about the importance of social distancing, wearing a mask when out in public, and quarantining themselves at home whenever possible.
The main outcomes were hospitalizations and mortality within 28 days of COVID-19 infection. Considering the overall inflammatory arthritis population, rates of both outcomes were higher versus the general population. And when the researchers analyzed the risks according to the type of inflammatory arthritis, the associations were not statistically significant in a multivariable analysis for people with any of the inflammatory arthritis diagnoses: rheumatoid arthritis (n = 1,283), psoriatic arthritis (n = 514), or ankylosing spondylitis (n = 246). Some patients had more than one inflammatory arthritis diagnosis.
What does this all mean?
Dr. Cooksey conceded that there were lots of limitations to the data collected – from misclassification bias to data possibly not have been recorded completely or missing because of the disruption to health care services during the early stages of the pandemic. Patients may have been told to shield but not actually shielded, she observed, and maybe because a lack of testing COVID-19 cases were missed or people could have been asymptomatic or unable to be tested.
“The study supports the role of shielding in inflammatory arthritis,” Dr. Cooksey said, particularly in those with RA and the risk factors associated with an increased risk in death. However, that may not mean the entire population, she suggested, saying that “refining the criteria for shielding will help mitigate the negative effects of the entire IA population.”
Senior team member Ernest Choy, MD, added his thoughts, saying that, rather than giving generic shielding recommendations to all IA patients, not everyone has the same risk, so maybe not everyone needs to shield to the same level.
“Psoriatic arthritis patients and ankylosing spondylitis patients are younger, so they really don’t have as high a risk like patients with rheumatoid arthritis,” he said.
Dr. Choy, who is professor of rheumatology at the Cardiff Institute of Infection & Immunity, commented that it was not surprising to find that a prior serious infection was a risk for COVID-19 mortality. This risk factor was examined because of the known association between biologic use and the risk for serious infection.
Moreover, he said that, “if you have a serious comorbidity that requires you to get admitted to hospital, that is a reflection of your vulnerability.”
Dr. Cooksey and Dr. Choy had no relevant conflicts of interest to disclose.
Being identified as someone that was advised to stay at home and shield, or keep away from face-to-face interactions with others, during the COVID-19 pandemic was indicative of an increased risk for dying from COVID-19 within 28 days of infection, a U.K. study of inflammatory arthritis patients versus the general population suggests.
In fact, shielding status was the highest ranked of all the risk factors identified for early mortality from COVID-19, with a hazard ratio of 1.52 (95% confidence interval, 1.40-1.64) comparing people with and without inflammatory arthritis (IA) who had tested positive.
The list of risk factors associated with higher mortality in the IA patients versus the general population also included diabetes (HR, 1.38), smoking (HR, 1.27), hypertension (HR, 1.19), glucocorticoid use (HR, 1.17), and cancer (HR, 1.10), as well as increasing age (HR, 1.08) and body mass index (HR, 1.01).
Also important was the person’s prior hospitalization history, with those needing in-hospital care in the year running up to their admission for COVID-19 associated with a 34% higher risk for death, and being hospitalized previously with a serious infection was associated with a 20% higher risk.
This has more to do people’s overall vulnerability than their IA, suggested the team behind the findings, who also found that the risk of catching COVID-19 was significantly lower among patients with IA than the general population (3.5% vs. 6%), presumably because of shielding.
Examining the risks for COVID-19 in real-life practice
“COVID-19 has caused over 10 million deaths,” Roxanne Cooksey, PhD, said at the annual meeting of the British Society for Rheumatology. “It’s greatly affected vulnerable individuals, which includes individuals with IA, this is due to their compromised immune system and increased risk of infection and the medications that they take to manage their conditions.
“Previous studies have had mixed results about whether people with IA have an increased risk of poor outcome,” added Dr. Cooksey, who is a postdoctoral researcher in the division of infection and immunity at Cardiff (Wales) University.
“So, our research question looks to investigate inflammatory arthritis – that’s rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis – to see whether the conditions themselves or indeed their medications predispose individuals to an increased risk of contracting COVID or even more adverse outcomes.”
Dr. Cooksey and colleagues looked specifically at COVID-19 infection rates and outcomes in adults living in Wales during the first year of the pandemic (March 2020 to May 2021). As such they used routinely collected, anonymized health data from the SAIL Databank and performed a retrospective, population-based cohort study. In total, there were 1,966 people with inflammatory arthritis identified as having COVID-19 and 166,602 people without IA but who had COVID-19 in the study population.
As might be expected, people with inflammatory arthritis who tested positive for COVID-19 were older than those testing positive in the general population, at a mean of 62 years versus 46 years. They were also more likely to have been advised to shield (49.4% versus 4.6%), which in the United Kingdom constituted of receiving a letter telling them about the importance of social distancing, wearing a mask when out in public, and quarantining themselves at home whenever possible.
The main outcomes were hospitalizations and mortality within 28 days of COVID-19 infection. Considering the overall inflammatory arthritis population, rates of both outcomes were higher versus the general population. And when the researchers analyzed the risks according to the type of inflammatory arthritis, the associations were not statistically significant in a multivariable analysis for people with any of the inflammatory arthritis diagnoses: rheumatoid arthritis (n = 1,283), psoriatic arthritis (n = 514), or ankylosing spondylitis (n = 246). Some patients had more than one inflammatory arthritis diagnosis.
What does this all mean?
Dr. Cooksey conceded that there were lots of limitations to the data collected – from misclassification bias to data possibly not have been recorded completely or missing because of the disruption to health care services during the early stages of the pandemic. Patients may have been told to shield but not actually shielded, she observed, and maybe because a lack of testing COVID-19 cases were missed or people could have been asymptomatic or unable to be tested.
“The study supports the role of shielding in inflammatory arthritis,” Dr. Cooksey said, particularly in those with RA and the risk factors associated with an increased risk in death. However, that may not mean the entire population, she suggested, saying that “refining the criteria for shielding will help mitigate the negative effects of the entire IA population.”
Senior team member Ernest Choy, MD, added his thoughts, saying that, rather than giving generic shielding recommendations to all IA patients, not everyone has the same risk, so maybe not everyone needs to shield to the same level.
“Psoriatic arthritis patients and ankylosing spondylitis patients are younger, so they really don’t have as high a risk like patients with rheumatoid arthritis,” he said.
Dr. Choy, who is professor of rheumatology at the Cardiff Institute of Infection & Immunity, commented that it was not surprising to find that a prior serious infection was a risk for COVID-19 mortality. This risk factor was examined because of the known association between biologic use and the risk for serious infection.
Moreover, he said that, “if you have a serious comorbidity that requires you to get admitted to hospital, that is a reflection of your vulnerability.”
Dr. Cooksey and Dr. Choy had no relevant conflicts of interest to disclose.
FROM BSR 2022
Myositis guidelines aim to standardize adult and pediatric care
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
FROM BSR 2022