User login
New hormonal medical treatment is an important advance for AUB caused by uterine fibroids
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
Action and awareness are needed to increase immunization rates
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Studies gauge role of schools, kids in spread of COVID-19
When officials closed U.S. schools in March to limit the spread of COVID-19, they may have prevented more than 1 million cases over a 26-day period, a new estimate published online July 29 in JAMA suggests.
But school closures also left blind spots in understanding how children and schools affect disease transmission.
“School closures early in pandemic responses thwarted larger-scale investigations of schools as a source of community transmission,” researchers noted in a separate study, published online July 30 in JAMA Pediatrics, that examined levels of viral RNA in children and adults with COVID-19.
“Our analyses suggest children younger than 5 years with mild to moderate COVID-19 have high amounts of SARS-CoV-2 viral RNA in their nasopharynx, compared with older children and adults,” reported Taylor Heald-Sargent, MD, PhD, and colleagues. “Thus, young children can potentially be important drivers of SARS-CoV-2 spread in the general population, as has been demonstrated with respiratory syncytial virus, where children with high viral loads are more likely to transmit.”
Although the study “was not designed to prove that younger children spread COVID-19 as much as adults,” it is a possibility, Dr. Heald-Sargent, a pediatric infectious diseases specialist at Ann and Robert H. Lurie Children’s Hospital and assistant professor of pediatrics at Northwestern University, Chicago, said in a related news release. “We need to take that into account in efforts to reduce transmission as we continue to learn more about this virus.”.
The study included 145 patients with mild or moderate illness who were within 1 week of symptom onset. The researchers used reverse transcriptase–polymerase chain reaction (rt-PCR) on nasopharyngeal swabs collected at inpatient, outpatient, emergency department, or drive-through testing sites to measure SARS-CoV-2 levels. The investigators compared PCR amplification cycle threshold (CT) values for children younger than 5 years (n = 46), children aged 5-17 years (n = 51), and adults aged 18-65 years (n = 48); lower CT values indicate higher amounts of viral nucleic acid.
Median CT values for older children and adults were similar (about 11), whereas the median CT value for young children was significantly lower (6.5). The differences between young children and adults “approximate a 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children,” the researchers wrote.
“Behavioral habits of young children and close quarters in school and day care settings raise concern for SARS-CoV-2 amplification in this population as public health restrictions are eased,” they write.
Modeling the impact of school closures
In the JAMA study, Katherine A. Auger, MD, of Cincinnati Children’s Hospital Medical Center, and colleagues examined at the U.S. population level whether closing schools, as all 50 states did in March, was associated with relative decreases in COVID-19 incidence and mortality.
To isolate the effect of school closures, the researchers used an interrupted time series analysis and included other state-level nonpharmaceutical interventions and variables in their regression models.
“Per week, the incidence was estimated to have been 39% of what it would have been had schools remained open,” Dr. Auger and colleagues wrote. “Extrapolating the absolute differences of 423.9 cases and 12.6 deaths per 100,000 to 322.2 million residents nationally suggests that school closure may have been associated with approximately 1.37 million fewer cases of COVID-19 over a 26-day period and 40,600 fewer deaths over a 16-day period; however, these figures do not account for uncertainty in the model assumptions and the resulting estimates.”
Relative reductions in incidence and mortality were largest in states that closed schools when the incidence of COVID-19 was low, the authors found.
Decisions with high stakes
In an accompanying editorial, Julie M. Donohue, PhD, and Elizabeth Miller, MD, PhD, both affiliated with the University of Pittsburgh, emphasized that the results are estimates. “School closures were enacted in close proximity ... to other physical distancing measures, such as nonessential business closures and stay-at-home orders, making it difficult to disentangle the potential effect of each intervention.”
Although the findings “suggest a role for school closures in virus mitigation, school and health officials must balance this with academic, health, and economic consequences,” Dr. Donohue and Dr. Miller added. “Given the strong connection between education, income, and life expectancy, school closures could have long-term deleterious consequences for child health, likely reaching into adulthood.” Schools provide “meals and nutrition, health care including behavioral health supports, physical activity, social interaction, supports for students with special education needs and disabilities, and other vital resources for healthy development.”
In a viewpoint article also published in JAMA, authors involved in the creation of a National Academies of Sciences, Engineering, and Medicine reported on the reopening of schools recommend that districts “make every effort to prioritize reopening with an emphasis on providing in-person instruction for students in kindergarten through grade 5 as well as those students with special needs who might be best served by in-person instruction.
“To reopen safely, school districts are encouraged to ensure ventilation and air filtration, clean surfaces frequently, provide facilities for regular handwashing, and provide space for physical distancing,” write Kenne A. Dibner, PhD, of the NASEM in Washington, D.C., and coauthors.
Furthermore, districts “need to consider transparent communication of the reality that while measures can be implemented to lower the risk of transmitting COVID-19 when schools reopen, there is no way to eliminate that risk entirely. It is critical to share both the risks and benefits of different scenarios,” they wrote.
The JAMA modeling study received funding from the Agency for Healthcare Research and Quality and the National Institutes of Health. The NASEM report was funded by the Brady Education Foundation and the Spencer Foundation. The authors disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
When officials closed U.S. schools in March to limit the spread of COVID-19, they may have prevented more than 1 million cases over a 26-day period, a new estimate published online July 29 in JAMA suggests.
But school closures also left blind spots in understanding how children and schools affect disease transmission.
“School closures early in pandemic responses thwarted larger-scale investigations of schools as a source of community transmission,” researchers noted in a separate study, published online July 30 in JAMA Pediatrics, that examined levels of viral RNA in children and adults with COVID-19.
“Our analyses suggest children younger than 5 years with mild to moderate COVID-19 have high amounts of SARS-CoV-2 viral RNA in their nasopharynx, compared with older children and adults,” reported Taylor Heald-Sargent, MD, PhD, and colleagues. “Thus, young children can potentially be important drivers of SARS-CoV-2 spread in the general population, as has been demonstrated with respiratory syncytial virus, where children with high viral loads are more likely to transmit.”
Although the study “was not designed to prove that younger children spread COVID-19 as much as adults,” it is a possibility, Dr. Heald-Sargent, a pediatric infectious diseases specialist at Ann and Robert H. Lurie Children’s Hospital and assistant professor of pediatrics at Northwestern University, Chicago, said in a related news release. “We need to take that into account in efforts to reduce transmission as we continue to learn more about this virus.”.
The study included 145 patients with mild or moderate illness who were within 1 week of symptom onset. The researchers used reverse transcriptase–polymerase chain reaction (rt-PCR) on nasopharyngeal swabs collected at inpatient, outpatient, emergency department, or drive-through testing sites to measure SARS-CoV-2 levels. The investigators compared PCR amplification cycle threshold (CT) values for children younger than 5 years (n = 46), children aged 5-17 years (n = 51), and adults aged 18-65 years (n = 48); lower CT values indicate higher amounts of viral nucleic acid.
Median CT values for older children and adults were similar (about 11), whereas the median CT value for young children was significantly lower (6.5). The differences between young children and adults “approximate a 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children,” the researchers wrote.
“Behavioral habits of young children and close quarters in school and day care settings raise concern for SARS-CoV-2 amplification in this population as public health restrictions are eased,” they write.
Modeling the impact of school closures
In the JAMA study, Katherine A. Auger, MD, of Cincinnati Children’s Hospital Medical Center, and colleagues examined at the U.S. population level whether closing schools, as all 50 states did in March, was associated with relative decreases in COVID-19 incidence and mortality.
To isolate the effect of school closures, the researchers used an interrupted time series analysis and included other state-level nonpharmaceutical interventions and variables in their regression models.
“Per week, the incidence was estimated to have been 39% of what it would have been had schools remained open,” Dr. Auger and colleagues wrote. “Extrapolating the absolute differences of 423.9 cases and 12.6 deaths per 100,000 to 322.2 million residents nationally suggests that school closure may have been associated with approximately 1.37 million fewer cases of COVID-19 over a 26-day period and 40,600 fewer deaths over a 16-day period; however, these figures do not account for uncertainty in the model assumptions and the resulting estimates.”
Relative reductions in incidence and mortality were largest in states that closed schools when the incidence of COVID-19 was low, the authors found.
Decisions with high stakes
In an accompanying editorial, Julie M. Donohue, PhD, and Elizabeth Miller, MD, PhD, both affiliated with the University of Pittsburgh, emphasized that the results are estimates. “School closures were enacted in close proximity ... to other physical distancing measures, such as nonessential business closures and stay-at-home orders, making it difficult to disentangle the potential effect of each intervention.”
Although the findings “suggest a role for school closures in virus mitigation, school and health officials must balance this with academic, health, and economic consequences,” Dr. Donohue and Dr. Miller added. “Given the strong connection between education, income, and life expectancy, school closures could have long-term deleterious consequences for child health, likely reaching into adulthood.” Schools provide “meals and nutrition, health care including behavioral health supports, physical activity, social interaction, supports for students with special education needs and disabilities, and other vital resources for healthy development.”
In a viewpoint article also published in JAMA, authors involved in the creation of a National Academies of Sciences, Engineering, and Medicine reported on the reopening of schools recommend that districts “make every effort to prioritize reopening with an emphasis on providing in-person instruction for students in kindergarten through grade 5 as well as those students with special needs who might be best served by in-person instruction.
“To reopen safely, school districts are encouraged to ensure ventilation and air filtration, clean surfaces frequently, provide facilities for regular handwashing, and provide space for physical distancing,” write Kenne A. Dibner, PhD, of the NASEM in Washington, D.C., and coauthors.
Furthermore, districts “need to consider transparent communication of the reality that while measures can be implemented to lower the risk of transmitting COVID-19 when schools reopen, there is no way to eliminate that risk entirely. It is critical to share both the risks and benefits of different scenarios,” they wrote.
The JAMA modeling study received funding from the Agency for Healthcare Research and Quality and the National Institutes of Health. The NASEM report was funded by the Brady Education Foundation and the Spencer Foundation. The authors disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
When officials closed U.S. schools in March to limit the spread of COVID-19, they may have prevented more than 1 million cases over a 26-day period, a new estimate published online July 29 in JAMA suggests.
But school closures also left blind spots in understanding how children and schools affect disease transmission.
“School closures early in pandemic responses thwarted larger-scale investigations of schools as a source of community transmission,” researchers noted in a separate study, published online July 30 in JAMA Pediatrics, that examined levels of viral RNA in children and adults with COVID-19.
“Our analyses suggest children younger than 5 years with mild to moderate COVID-19 have high amounts of SARS-CoV-2 viral RNA in their nasopharynx, compared with older children and adults,” reported Taylor Heald-Sargent, MD, PhD, and colleagues. “Thus, young children can potentially be important drivers of SARS-CoV-2 spread in the general population, as has been demonstrated with respiratory syncytial virus, where children with high viral loads are more likely to transmit.”
Although the study “was not designed to prove that younger children spread COVID-19 as much as adults,” it is a possibility, Dr. Heald-Sargent, a pediatric infectious diseases specialist at Ann and Robert H. Lurie Children’s Hospital and assistant professor of pediatrics at Northwestern University, Chicago, said in a related news release. “We need to take that into account in efforts to reduce transmission as we continue to learn more about this virus.”.
The study included 145 patients with mild or moderate illness who were within 1 week of symptom onset. The researchers used reverse transcriptase–polymerase chain reaction (rt-PCR) on nasopharyngeal swabs collected at inpatient, outpatient, emergency department, or drive-through testing sites to measure SARS-CoV-2 levels. The investigators compared PCR amplification cycle threshold (CT) values for children younger than 5 years (n = 46), children aged 5-17 years (n = 51), and adults aged 18-65 years (n = 48); lower CT values indicate higher amounts of viral nucleic acid.
Median CT values for older children and adults were similar (about 11), whereas the median CT value for young children was significantly lower (6.5). The differences between young children and adults “approximate a 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children,” the researchers wrote.
“Behavioral habits of young children and close quarters in school and day care settings raise concern for SARS-CoV-2 amplification in this population as public health restrictions are eased,” they write.
Modeling the impact of school closures
In the JAMA study, Katherine A. Auger, MD, of Cincinnati Children’s Hospital Medical Center, and colleagues examined at the U.S. population level whether closing schools, as all 50 states did in March, was associated with relative decreases in COVID-19 incidence and mortality.
To isolate the effect of school closures, the researchers used an interrupted time series analysis and included other state-level nonpharmaceutical interventions and variables in their regression models.
“Per week, the incidence was estimated to have been 39% of what it would have been had schools remained open,” Dr. Auger and colleagues wrote. “Extrapolating the absolute differences of 423.9 cases and 12.6 deaths per 100,000 to 322.2 million residents nationally suggests that school closure may have been associated with approximately 1.37 million fewer cases of COVID-19 over a 26-day period and 40,600 fewer deaths over a 16-day period; however, these figures do not account for uncertainty in the model assumptions and the resulting estimates.”
Relative reductions in incidence and mortality were largest in states that closed schools when the incidence of COVID-19 was low, the authors found.
Decisions with high stakes
In an accompanying editorial, Julie M. Donohue, PhD, and Elizabeth Miller, MD, PhD, both affiliated with the University of Pittsburgh, emphasized that the results are estimates. “School closures were enacted in close proximity ... to other physical distancing measures, such as nonessential business closures and stay-at-home orders, making it difficult to disentangle the potential effect of each intervention.”
Although the findings “suggest a role for school closures in virus mitigation, school and health officials must balance this with academic, health, and economic consequences,” Dr. Donohue and Dr. Miller added. “Given the strong connection between education, income, and life expectancy, school closures could have long-term deleterious consequences for child health, likely reaching into adulthood.” Schools provide “meals and nutrition, health care including behavioral health supports, physical activity, social interaction, supports for students with special education needs and disabilities, and other vital resources for healthy development.”
In a viewpoint article also published in JAMA, authors involved in the creation of a National Academies of Sciences, Engineering, and Medicine reported on the reopening of schools recommend that districts “make every effort to prioritize reopening with an emphasis on providing in-person instruction for students in kindergarten through grade 5 as well as those students with special needs who might be best served by in-person instruction.
“To reopen safely, school districts are encouraged to ensure ventilation and air filtration, clean surfaces frequently, provide facilities for regular handwashing, and provide space for physical distancing,” write Kenne A. Dibner, PhD, of the NASEM in Washington, D.C., and coauthors.
Furthermore, districts “need to consider transparent communication of the reality that while measures can be implemented to lower the risk of transmitting COVID-19 when schools reopen, there is no way to eliminate that risk entirely. It is critical to share both the risks and benefits of different scenarios,” they wrote.
The JAMA modeling study received funding from the Agency for Healthcare Research and Quality and the National Institutes of Health. The NASEM report was funded by the Brady Education Foundation and the Spencer Foundation. The authors disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
Many children with COVID-19 present without classic symptoms
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
FROM HOSPITAL PEDIATRICS
Appendix may be common site of endometriosis
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
FROM SGS 2020
Does stirrup choice influence vaginal surgery outcome?
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
FROM OBSTETRICS & GYNECOLOGY
Postpartum tubal ligation safe in obese women
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
FROM OBSTETRICS & GYNECOLOGY
Range of interventions reduces likelihood of infection after hysterectomy
Improving hand hygiene, optimizing antibiotic order sets, and removing catheters sooner were among the interventions associated with a decreased risk of infections after hysterectomy, according to research presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
“Implementation of said study author Shitanshu Uppal, MBBS, an ob.gyn. specializing in gynecologic oncology at the University of Michigan in Ann Arbor.
To assess the impact of quality improvement efforts on infectious morbidity after hysterectomy, Dr. Uppal and colleagues analyzed data from 1,867 hysterectomies performed between Oct. 8, 2015, and Oct. 7, 2018. Patients were at least 18 years old, younger than 90 years old, and underwent hysterectomy via any route at the University of Michigan Medical Center Hospital.
Interventions to reduce infections included the use of cefazolin as a preferred antibiotic, the addition of metronidazole to first-generation cephalosporins for antibiotic prophylaxis, subcuticular closure of open incisions, and earlier removal of Foley catheters. In addition, the institution evaluated and shared information about doctors’ hand hygiene, implemented enhanced recovery after surgery (ERAS) protocols, and held periodic meetings with doctors to discuss efforts to reduce infections. Most interventions were implemented by November 2017.
The primary outcome was overall infection rate in the 30 days after surgery for each of the 3 years included the study. Infections included superficial surgical site infections, deep infections, Clostridium difficile infections, and urinary tract infections, among others.
Patients’ baseline clinical characteristics did not differ during the 3 years studied. Length of stay decreased, which may be attributed to increased use of ERAS protocols and institution of same-day discharge in laparoscopic cases, Dr. Uppal said. The rate of malignancy on final pathology decreased from 29% in year 1 to 23% in year 3. The rate of laparoscopic surgery increased from 55% to 64%.
Infectious morbidity rates decreased from 7% in year 1 (47 infections per 644 cases) to 4% in year 3 (22 infections per 616 cases).
“We saw a reduction in infection rate in all categories,” said Dr. Uppal. “However, the reduction in urinary tract infection as well as superficial surgical site infection was most pronounced.”
After adjustment for the route of surgery, body mass index, age, malignancy on final pathology, modality of surgery, and comorbidities, performance of hysterectomy in year 3 was independently predictive of lower rates of infectious morbidity by 56%.
In addition, the standardized incidence ratio for surgical site infection as reported by the Centers for Medicare & Medicaid Services decreased. In December 2016, Michigan Medicine’s ratio was 1.057. At the end of 2018, the projected ratio was 0.243.
The interventions started on different dates, and “we are unable to conclude, from all the implemented interventions, which one worked,” Dr. Uppal noted.
“I hope the elements from this quality improvement initiative will become the standard of care in order to benefit our patients,” said Amy Park, MD, section head of female pelvic medicine and reconstructive surgery at Cleveland Clinic.
In addition, the study “pertains to one of CMS’s major programs to reduce and prevent health care-associated infections,” Dr. Park said at the virtual meeting. Several CMS quality indicators in 2020 relate to gynecologic surgery, including postoperative wound dehiscence rate, catheter-related UTI rate, and surgical site infection related to hysterectomy. The CMS will reduce hospital payments to institutions in the worst performing quartile for hospital-acquired infection scores by 1%.
Dr. Uppal advised that hospitals need four things to achieve similar results: a commitment from the team and leadership to reduce infections; patience; making it easy for doctors to implement the new interventions; and periodic feedback.
The process may be tedious but worth it in the end, he said.
Dr. Uppal disclosed salary support from Blue Cross Blue Shield of Michigan. A coauthor disclosed salary support from the company plus royalties from UpToDate. Dr. Park disclosed speaking for Allergan and royalties from UpToDate.
SOURCE: Uppal S et al. SGS 2020, Abstract 16.
Improving hand hygiene, optimizing antibiotic order sets, and removing catheters sooner were among the interventions associated with a decreased risk of infections after hysterectomy, according to research presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
“Implementation of said study author Shitanshu Uppal, MBBS, an ob.gyn. specializing in gynecologic oncology at the University of Michigan in Ann Arbor.
To assess the impact of quality improvement efforts on infectious morbidity after hysterectomy, Dr. Uppal and colleagues analyzed data from 1,867 hysterectomies performed between Oct. 8, 2015, and Oct. 7, 2018. Patients were at least 18 years old, younger than 90 years old, and underwent hysterectomy via any route at the University of Michigan Medical Center Hospital.
Interventions to reduce infections included the use of cefazolin as a preferred antibiotic, the addition of metronidazole to first-generation cephalosporins for antibiotic prophylaxis, subcuticular closure of open incisions, and earlier removal of Foley catheters. In addition, the institution evaluated and shared information about doctors’ hand hygiene, implemented enhanced recovery after surgery (ERAS) protocols, and held periodic meetings with doctors to discuss efforts to reduce infections. Most interventions were implemented by November 2017.
The primary outcome was overall infection rate in the 30 days after surgery for each of the 3 years included the study. Infections included superficial surgical site infections, deep infections, Clostridium difficile infections, and urinary tract infections, among others.
Patients’ baseline clinical characteristics did not differ during the 3 years studied. Length of stay decreased, which may be attributed to increased use of ERAS protocols and institution of same-day discharge in laparoscopic cases, Dr. Uppal said. The rate of malignancy on final pathology decreased from 29% in year 1 to 23% in year 3. The rate of laparoscopic surgery increased from 55% to 64%.
Infectious morbidity rates decreased from 7% in year 1 (47 infections per 644 cases) to 4% in year 3 (22 infections per 616 cases).
“We saw a reduction in infection rate in all categories,” said Dr. Uppal. “However, the reduction in urinary tract infection as well as superficial surgical site infection was most pronounced.”
After adjustment for the route of surgery, body mass index, age, malignancy on final pathology, modality of surgery, and comorbidities, performance of hysterectomy in year 3 was independently predictive of lower rates of infectious morbidity by 56%.
In addition, the standardized incidence ratio for surgical site infection as reported by the Centers for Medicare & Medicaid Services decreased. In December 2016, Michigan Medicine’s ratio was 1.057. At the end of 2018, the projected ratio was 0.243.
The interventions started on different dates, and “we are unable to conclude, from all the implemented interventions, which one worked,” Dr. Uppal noted.
“I hope the elements from this quality improvement initiative will become the standard of care in order to benefit our patients,” said Amy Park, MD, section head of female pelvic medicine and reconstructive surgery at Cleveland Clinic.
In addition, the study “pertains to one of CMS’s major programs to reduce and prevent health care-associated infections,” Dr. Park said at the virtual meeting. Several CMS quality indicators in 2020 relate to gynecologic surgery, including postoperative wound dehiscence rate, catheter-related UTI rate, and surgical site infection related to hysterectomy. The CMS will reduce hospital payments to institutions in the worst performing quartile for hospital-acquired infection scores by 1%.
Dr. Uppal advised that hospitals need four things to achieve similar results: a commitment from the team and leadership to reduce infections; patience; making it easy for doctors to implement the new interventions; and periodic feedback.
The process may be tedious but worth it in the end, he said.
Dr. Uppal disclosed salary support from Blue Cross Blue Shield of Michigan. A coauthor disclosed salary support from the company plus royalties from UpToDate. Dr. Park disclosed speaking for Allergan and royalties from UpToDate.
SOURCE: Uppal S et al. SGS 2020, Abstract 16.
Improving hand hygiene, optimizing antibiotic order sets, and removing catheters sooner were among the interventions associated with a decreased risk of infections after hysterectomy, according to research presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
“Implementation of said study author Shitanshu Uppal, MBBS, an ob.gyn. specializing in gynecologic oncology at the University of Michigan in Ann Arbor.
To assess the impact of quality improvement efforts on infectious morbidity after hysterectomy, Dr. Uppal and colleagues analyzed data from 1,867 hysterectomies performed between Oct. 8, 2015, and Oct. 7, 2018. Patients were at least 18 years old, younger than 90 years old, and underwent hysterectomy via any route at the University of Michigan Medical Center Hospital.
Interventions to reduce infections included the use of cefazolin as a preferred antibiotic, the addition of metronidazole to first-generation cephalosporins for antibiotic prophylaxis, subcuticular closure of open incisions, and earlier removal of Foley catheters. In addition, the institution evaluated and shared information about doctors’ hand hygiene, implemented enhanced recovery after surgery (ERAS) protocols, and held periodic meetings with doctors to discuss efforts to reduce infections. Most interventions were implemented by November 2017.
The primary outcome was overall infection rate in the 30 days after surgery for each of the 3 years included the study. Infections included superficial surgical site infections, deep infections, Clostridium difficile infections, and urinary tract infections, among others.
Patients’ baseline clinical characteristics did not differ during the 3 years studied. Length of stay decreased, which may be attributed to increased use of ERAS protocols and institution of same-day discharge in laparoscopic cases, Dr. Uppal said. The rate of malignancy on final pathology decreased from 29% in year 1 to 23% in year 3. The rate of laparoscopic surgery increased from 55% to 64%.
Infectious morbidity rates decreased from 7% in year 1 (47 infections per 644 cases) to 4% in year 3 (22 infections per 616 cases).
“We saw a reduction in infection rate in all categories,” said Dr. Uppal. “However, the reduction in urinary tract infection as well as superficial surgical site infection was most pronounced.”
After adjustment for the route of surgery, body mass index, age, malignancy on final pathology, modality of surgery, and comorbidities, performance of hysterectomy in year 3 was independently predictive of lower rates of infectious morbidity by 56%.
In addition, the standardized incidence ratio for surgical site infection as reported by the Centers for Medicare & Medicaid Services decreased. In December 2016, Michigan Medicine’s ratio was 1.057. At the end of 2018, the projected ratio was 0.243.
The interventions started on different dates, and “we are unable to conclude, from all the implemented interventions, which one worked,” Dr. Uppal noted.
“I hope the elements from this quality improvement initiative will become the standard of care in order to benefit our patients,” said Amy Park, MD, section head of female pelvic medicine and reconstructive surgery at Cleveland Clinic.
In addition, the study “pertains to one of CMS’s major programs to reduce and prevent health care-associated infections,” Dr. Park said at the virtual meeting. Several CMS quality indicators in 2020 relate to gynecologic surgery, including postoperative wound dehiscence rate, catheter-related UTI rate, and surgical site infection related to hysterectomy. The CMS will reduce hospital payments to institutions in the worst performing quartile for hospital-acquired infection scores by 1%.
Dr. Uppal advised that hospitals need four things to achieve similar results: a commitment from the team and leadership to reduce infections; patience; making it easy for doctors to implement the new interventions; and periodic feedback.
The process may be tedious but worth it in the end, he said.
Dr. Uppal disclosed salary support from Blue Cross Blue Shield of Michigan. A coauthor disclosed salary support from the company plus royalties from UpToDate. Dr. Park disclosed speaking for Allergan and royalties from UpToDate.
SOURCE: Uppal S et al. SGS 2020, Abstract 16.
FROM SGS 2020
Are you SARS-CoV-2 vaccine hesitant?
When the pandemic was just emerging from its infancy and we were just beginning to think about social distancing, I was sitting around enjoying an adult beverage and some gluten free (not my choice) snacks with some friends. A retired nurse who had just celebrated her 80th birthday said, “I can’t wait until they’ve developed a vaccine.” A former electrical engineer sitting just short of 2 meters to her left responded, “Don’t save me a place near the front of the line for something that is being developed in a program called Warp Speed.”
How do you feel about the potential SARS-CoV-2 vaccine? Are you going to roll up your sleeve as soon as the vaccine becomes available in your community? What are you going to suggest to your patients, your children? I suspect many of you will answer, “It depends.”
Will it make any difference to you which biochemical-immune-bending strategy is being used to make the vaccine? All of them will probably be the result of a clever sounding but novel technique, all of them with a track record that is measured in months and not years. Will you be swayed by how large the trials were? Or how long the follow-up lasted? How effective must the vaccine be to convince you that it is worth receiving or recommending? Do you have the tools and experience to make a decision like that? I know I don’t. And should you and I even be put in a position to make that decision?
In the past, you and I may have relied on the Centers for Disease Control and Prevention for advice. But given the somewhat murky and stormy relationship between the CDC and the president, the vaccine recommendation may be issued by the White House and not the CDC.
For those of us who were practicing medicine during the Swine Flu fiasco of 1976, the pace and the politics surrounding the development of a SARS-CoV-2 vaccine has a discomforting déjà vu quality about it. The fact that like this year 1976 was an election year that infused the development process with a sense of urgency above and beyond any of the concerns about the pandemic that never happened. Although causality was never proven, there was a surge in Guillain-Barré syndrome cases that had been linked temporally to the vaccine.
Of course, our pandemic is real, and it would be imprudent to wait a year or more to watch for long-term vaccine sequelae. However, I am more than a little concerned that fast tracking the development process may result in unfortunate consequences in the short term that could have been avoided with a more measured approach to trialing the vaccines.
The sad reality is that as a nation we tend to be impatient. We are drawn to quick fixes that come in a vial or a capsule. We are learning that simple measures like mask wearing and social distancing can make a difference in slowing the spread of the virus. It would be tragic to rush a vaccine into production that at best turns out to simply be an expensive alternative to the measures that we know work or at worst injures more of us than it saves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When the pandemic was just emerging from its infancy and we were just beginning to think about social distancing, I was sitting around enjoying an adult beverage and some gluten free (not my choice) snacks with some friends. A retired nurse who had just celebrated her 80th birthday said, “I can’t wait until they’ve developed a vaccine.” A former electrical engineer sitting just short of 2 meters to her left responded, “Don’t save me a place near the front of the line for something that is being developed in a program called Warp Speed.”
How do you feel about the potential SARS-CoV-2 vaccine? Are you going to roll up your sleeve as soon as the vaccine becomes available in your community? What are you going to suggest to your patients, your children? I suspect many of you will answer, “It depends.”
Will it make any difference to you which biochemical-immune-bending strategy is being used to make the vaccine? All of them will probably be the result of a clever sounding but novel technique, all of them with a track record that is measured in months and not years. Will you be swayed by how large the trials were? Or how long the follow-up lasted? How effective must the vaccine be to convince you that it is worth receiving or recommending? Do you have the tools and experience to make a decision like that? I know I don’t. And should you and I even be put in a position to make that decision?
In the past, you and I may have relied on the Centers for Disease Control and Prevention for advice. But given the somewhat murky and stormy relationship between the CDC and the president, the vaccine recommendation may be issued by the White House and not the CDC.
For those of us who were practicing medicine during the Swine Flu fiasco of 1976, the pace and the politics surrounding the development of a SARS-CoV-2 vaccine has a discomforting déjà vu quality about it. The fact that like this year 1976 was an election year that infused the development process with a sense of urgency above and beyond any of the concerns about the pandemic that never happened. Although causality was never proven, there was a surge in Guillain-Barré syndrome cases that had been linked temporally to the vaccine.
Of course, our pandemic is real, and it would be imprudent to wait a year or more to watch for long-term vaccine sequelae. However, I am more than a little concerned that fast tracking the development process may result in unfortunate consequences in the short term that could have been avoided with a more measured approach to trialing the vaccines.
The sad reality is that as a nation we tend to be impatient. We are drawn to quick fixes that come in a vial or a capsule. We are learning that simple measures like mask wearing and social distancing can make a difference in slowing the spread of the virus. It would be tragic to rush a vaccine into production that at best turns out to simply be an expensive alternative to the measures that we know work or at worst injures more of us than it saves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When the pandemic was just emerging from its infancy and we were just beginning to think about social distancing, I was sitting around enjoying an adult beverage and some gluten free (not my choice) snacks with some friends. A retired nurse who had just celebrated her 80th birthday said, “I can’t wait until they’ve developed a vaccine.” A former electrical engineer sitting just short of 2 meters to her left responded, “Don’t save me a place near the front of the line for something that is being developed in a program called Warp Speed.”
How do you feel about the potential SARS-CoV-2 vaccine? Are you going to roll up your sleeve as soon as the vaccine becomes available in your community? What are you going to suggest to your patients, your children? I suspect many of you will answer, “It depends.”
Will it make any difference to you which biochemical-immune-bending strategy is being used to make the vaccine? All of them will probably be the result of a clever sounding but novel technique, all of them with a track record that is measured in months and not years. Will you be swayed by how large the trials were? Or how long the follow-up lasted? How effective must the vaccine be to convince you that it is worth receiving or recommending? Do you have the tools and experience to make a decision like that? I know I don’t. And should you and I even be put in a position to make that decision?
In the past, you and I may have relied on the Centers for Disease Control and Prevention for advice. But given the somewhat murky and stormy relationship between the CDC and the president, the vaccine recommendation may be issued by the White House and not the CDC.
For those of us who were practicing medicine during the Swine Flu fiasco of 1976, the pace and the politics surrounding the development of a SARS-CoV-2 vaccine has a discomforting déjà vu quality about it. The fact that like this year 1976 was an election year that infused the development process with a sense of urgency above and beyond any of the concerns about the pandemic that never happened. Although causality was never proven, there was a surge in Guillain-Barré syndrome cases that had been linked temporally to the vaccine.
Of course, our pandemic is real, and it would be imprudent to wait a year or more to watch for long-term vaccine sequelae. However, I am more than a little concerned that fast tracking the development process may result in unfortunate consequences in the short term that could have been avoided with a more measured approach to trialing the vaccines.
The sad reality is that as a nation we tend to be impatient. We are drawn to quick fixes that come in a vial or a capsule. We are learning that simple measures like mask wearing and social distancing can make a difference in slowing the spread of the virus. It would be tragic to rush a vaccine into production that at best turns out to simply be an expensive alternative to the measures that we know work or at worst injures more of us than it saves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A Multidisciplinary Ambulation Protocol to Reduce Postoperative Venous Thromboembolism After Colorectal Surgery
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.
Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).
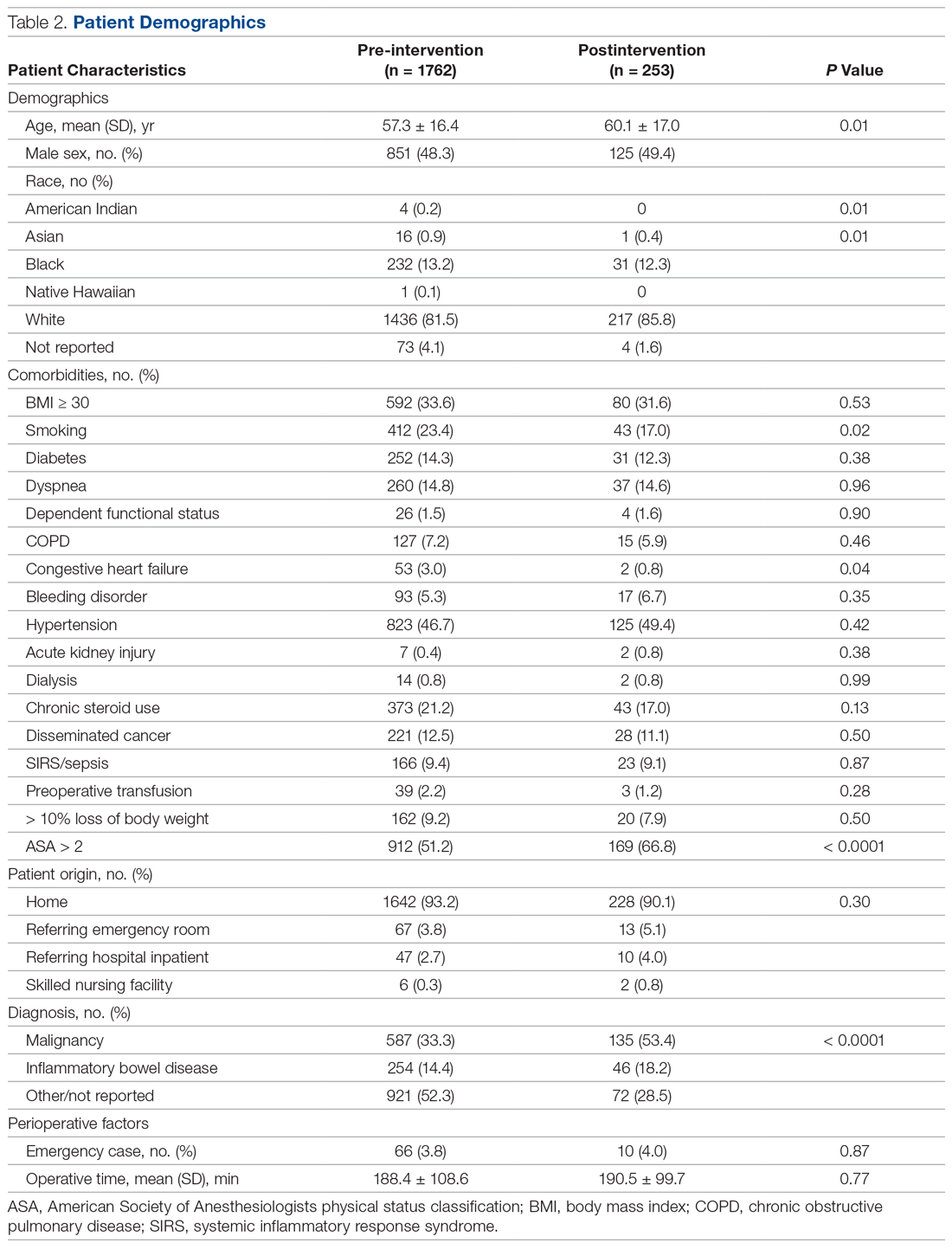
The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.
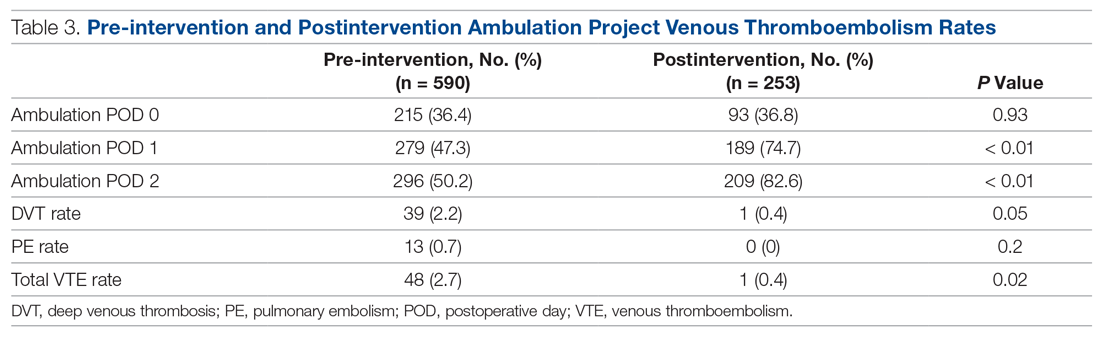
Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.

Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).

The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.

Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.

Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).

The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.

Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.