User login
How Does Telemedicine Compare to Conventional Follow-Up After General Surgery?
Study Overview
Objective. To compare the impact of conventional versus telemedicine follow-up of general surgery patients in outpatient clinics.
Design. Prospective randomized clinical trial.
Setting and participants. Participants were recruited from Hospital Germans Trias i Pujol, a tertiary care university hospital located in the outskirts of Barcelona (Catalonia, Spain). To be included in this study, participants had to have been treated in the general surgery department, have basic computer knowledge (ability to use e-mail or a social network), have a computer with webcam, and be 18 to 75 years of age, or they had to have a partner who met these criteria. Exclusion criteria included any disability making telemedicine follow-up impossible (eg, blindness, deafness, or mental disability; proctologic treatment; difficulty describing and/or showing complications in the surgical area; and clinical complications before discharge more severe than Clavien Dindo II), as well as withdrawal of consent. Patients who met the criteria and had just been discharged from the hospital were offered the opportunity to enroll by the surgeon in charge. Patients who agreed to participate provided informed consent and were assigned using a computerized block randomization list (allocation ratio 1:1).
Intervention. Time to visit was generally between 2 and 4 weeks after discharge (the interval to the follow-up visit was determined at the discretion of the treating surgeon, but always followed the usual schedule). To conduct the telemedicine follow-up through a video call, a medical cloud-based program fulfilling all European Union security and privacy policies was used. Four surgeons were assigned to perform the telemedicine visits and were trained on how to use the program before the study started. Visit format was the same in both groups: clinical and wound condition were assessed and pathology was discussed (the one difference was that physical exploration was not performed in the telemedicine group).
Main outcome measures. The primary outcome was the feasibility of telemedicine follow-up, and this was measured as the percentage of participants who completed follow-up in their corresponding group by the date scheduled at hospital discharge. Secondary outcomes included a comparison of clinical results and patient satisfaction. To assess the clinical results, extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit were collected.
To evaluate patient satisfaction, a questionnaire was sent via email to the participants after the visit and, if they did not respond, a telephone survey was carried out (if there was no contact after 2 telephone calls, the participants was considered a missing value). The questionnaire was informed by the United Kingdom National Health Service outpatients questionnaire and the Telehealth Usability Questionnaire. It included 27 general questions asked of participants in both groups, plus 8 specific questions for participants in the conventional follow-up group and 14 specific questions for participants in the telemedicine group. To summarize all the included fields in the questionnaires (time to visit and visit length, comfort, tests and procedures performed before and during the visit, transport, waiting time, privacy, dealings with staff, platform usability, telemedicine, and satisfaction), participants were asked to provide a global satisfaction score on a scale from 1 to 5.
Analysis. To compare the groups in terms of proportion of outcomes, a chi-square test was used to analyze categorical variables. To compare medians between the groups, ordinal variables were analyzed using the Mann-Whitney U test. Statistical significance was set at P < 0.05.
Main results. Two-hundred patients were randomly allocated to 1 of the 2 groups, with 100 patients in each group. The groups did not differ significantly based on age (P = 0.836), gender (P = 0.393), or American Society of Anesthesiologists (ASA) score (P = 0.232). Time to visit did not differ significantly between the groups (P = 0.169), and while visits were generally shorter in the telemedicine group, the difference was not significant (P = 0.153). Diagnoses and treatments did not differ significantly between the groups (P = 0.853 and P = 0.461, respectively).
The primary outcome (follow-up feasibility) was achieved in 90% of the conventional follow-up group and in 74% of the telemedicine group (P = 0.003). Of the 10 patients in the conventional follow-up group who did not complete the follow-up, 8 did not attend the visit on the scheduled day and 2 were hospitalized for reasons not related to the study. In the telemedicine group, the 2 main reasons for failure to follow-up were technical difficulties (n = 10) and requests by patients to attend a conventional visit after being allocated to the telemedicine group (n = 10). Among the remaining 6 patients in the telemedicine group who did not attend a visit, 3 visited the outpatient clinic because of a known surgical wound infection before the visit, 2 did not respond to the video call and could not be contacted by other means, and 1 had other face-to-face visits scheduled in different departments of the hospital the same day as the telemedicine appointment.
There were no statistically significant differences in the clinical results of the 164 patients meeting the primary endpoint (P = 0.832). Twelve of the 90 (13.3%) patients in the conventional group attended extra visits after the follow-up, while 9 of the 74 patients (12.1%) in the telemedicine group (P = 0.823) attended extra visits after follow-up. The median global patient satisfaction score was 5 in both the conventional group (range, 2-5) and the telemedicine group (range, 1-5), with no statistically significant differences (P = 0.099). When patients in the telemedicine group were asked if they would accept the use of telemedicine as part of their medical treatment on an ongoing basis, they rated the proposition with a median score of 5 (range, 1-5).
Conclusion. Telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. This option produces good satisfaction rates and maintains clinical outcomes.
Commentary
In recent years, telemedicine has gained increased popularity in both medicine and surgery, affording surgeons greater opportunities for patient care, mentoring, collaboration, and teaching, without the limits of geographic boundaries. Telemedicine can be broadly described as a health care service utilizing telecommunication technologies for the purpose of communicating with and diagnosing and treating patients remotely.1-4 To date, literature on telemedicine in surgical care has been limited.
In their systematic review, published in 2018, Asiri et al identified 24 studies published between 1998 and 2018, which included 3 randomized controlled trials, 3 pilot studies, 4 retrospective studies, and 14 prospective observational studies. In these studies, telemedicine protocols were used for preoperative assessment, diagnostic purposes, or consultation with another surgical department (10 studies); postoperative wound assessment (9 studies); and follow-up in place of conventional clinic visits (5 studies).3 In a 2017 systematic review of telemedicine for post-discharge surgical care, Gunter et al identified 21 studies, which included 3 randomized controlled trials, 6 pilot or feasibility studies, 4 retrospective record reviews, 2 case series, and 6 surveys.4 In these studies, telemedicine protocols were used for scheduled follow-up (10 studies), routine and ongoing monitoring (5 studies), or management of issues that arose after surgery (2 studies). These 2 reviews found telemedicine to be feasible, useful, and acceptable for postoperative evaluation and follow-up among both providers and patients.
Additional benefits noted in these studies included savings in patient travel, time, and cost. Perspectives on savings to the health system were mixed—while clinic time slots may open as a result of follow-up visits being done via telemedicine (resulting in potential improvements in access to surgical services and decreased wait times), there are still significant direct costs for purchasing necessary equipment and for educating and training providers on the use of the equipment. Other published reviews have discussed in greater detail the application, benefits, limitations, and barriers to telemedicine and provided insight from the perspectives of patients, providers, and health care systems.1,2
Because studies on the use of telemedicine are limited, particularly in general surgery, and few of these studies have used a randomized clinical trial design, the present study is an important contribution to the literature. The authors found a significant difference between groups in terms of percentage of completed follow-up visits—90% of conventional follow-up group participants completed their visit versus 74% of telemedicine group participants. However, these differences were primarily attributed to technical difficulties experienced by telemedicine group participants, as well requests to have a conventional follow-up visit. In addition, telemedicine capabilities were limited to video calls via computers and webcams, and it is likely that successful completion of the follow-up visit would have been higher in the telemedicine group had the use of video calls via tablets or smartphones been an option. Perhaps more important, no significant differences were found in clinical outcomes (extra visits within 30 days after the follow-up visit) or patient satisfaction.
A key strength of this study is the use of a randomized clinical trial design to evaluate telemedicine as an alternative method for conducting patient visits following general surgery. Inclusion and exclusion criteria did not impose strict limitations on potential participants. Also, the authors evaluated differences in time to visit, length of visit, clinical results, and patient satisfaction between groups, in addition to the primary measure of completion of the follow-up visit.
This study has important limitations that should be noted as well, particularly related to the study design, some of which are acknowledged by the authors. Because this study was implemented in only 1 hospital, specifically, a tertiary care university hospital on the outskirts of an urban European city, the generalizability of the findings is limited. Also, the likelihood of selection bias is high, as enrollment was not offered to all patients who were discharged from the hospital and met inclusion criteria (limited by patient workload). The comparison of clinical results was limited, as the selected measure focused only on extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit. This chosen measure does not account for less severe clinical results that did not require an additional visit, and does not represent a nuanced comparison of specific clinical indicators. In addition, this measure does not account for clinical complications that may have occurred beyond the 30-day period. Recall bias also was likely, given that the patient satisfaction questionnaire was delivered via email to patients at a later time after the follow-up visit, instead of being administered immediately after the visit. Last, group differences at baseline were assessed based only on age, gender, and ASA score, which does not preclude potential differences related to other factors, such as race/ethnicity, household income, comorbidities, insurance, and zip code. Future research with a similar objective would benefit from a randomized clinical trial design that recruits a wider diversity of patients across different clinic settings and incorporates more nuanced measures of primary and secondary outcomes.
Applications for Clinical Practice
With the ongoing COVID-19 pandemic, the integration of telemedicine capabilities into hospital systems is becoming more widespread and is proceeding at an accelerated pace. This study provides evidence that telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. Assuming that the needed technology and appropriate program training are available, telemedicine should be offered to patients, especially to maximize savings in terms of travel, time, and cost. However, the option for conventional (in-person) follow-up should remain, particularly in cases where there may be barriers to successful follow-up visits via telemedicine, including limited digital literacy, lack of access to necessary equipment, language/communication barriers, complex follow-up treatment, and difficulties in describing or showing complications in the surgical area.
–Katrina F. Mateo, PhD, MPH
1. Williams AM, Bhatti UF, Alam HB, Nikolian VC. The role of telemedicine in postoperative care. mHealth. 2018 May;4:11-11.
2. Huang EY, Knight S, Guetter CR et al. Telemedicine and telementoring in the surgical specialties: A narrative review. Am J Surg. 2019;218:760-766.
3. Asiri A, AlBishi S, AlMadani W, et al. The use of telemedicine in surgical care: A systematic review. Acta Informatica Medica. 2018;26:201-206.
4. Gunter RL, Chouinard S, Fernandes-Taylor S, et al. Current use of telemedicine for post-discharge surgical care: a systematic review. J Am College Surg. 2016;222:915-927.
Study Overview
Objective. To compare the impact of conventional versus telemedicine follow-up of general surgery patients in outpatient clinics.
Design. Prospective randomized clinical trial.
Setting and participants. Participants were recruited from Hospital Germans Trias i Pujol, a tertiary care university hospital located in the outskirts of Barcelona (Catalonia, Spain). To be included in this study, participants had to have been treated in the general surgery department, have basic computer knowledge (ability to use e-mail or a social network), have a computer with webcam, and be 18 to 75 years of age, or they had to have a partner who met these criteria. Exclusion criteria included any disability making telemedicine follow-up impossible (eg, blindness, deafness, or mental disability; proctologic treatment; difficulty describing and/or showing complications in the surgical area; and clinical complications before discharge more severe than Clavien Dindo II), as well as withdrawal of consent. Patients who met the criteria and had just been discharged from the hospital were offered the opportunity to enroll by the surgeon in charge. Patients who agreed to participate provided informed consent and were assigned using a computerized block randomization list (allocation ratio 1:1).
Intervention. Time to visit was generally between 2 and 4 weeks after discharge (the interval to the follow-up visit was determined at the discretion of the treating surgeon, but always followed the usual schedule). To conduct the telemedicine follow-up through a video call, a medical cloud-based program fulfilling all European Union security and privacy policies was used. Four surgeons were assigned to perform the telemedicine visits and were trained on how to use the program before the study started. Visit format was the same in both groups: clinical and wound condition were assessed and pathology was discussed (the one difference was that physical exploration was not performed in the telemedicine group).
Main outcome measures. The primary outcome was the feasibility of telemedicine follow-up, and this was measured as the percentage of participants who completed follow-up in their corresponding group by the date scheduled at hospital discharge. Secondary outcomes included a comparison of clinical results and patient satisfaction. To assess the clinical results, extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit were collected.
To evaluate patient satisfaction, a questionnaire was sent via email to the participants after the visit and, if they did not respond, a telephone survey was carried out (if there was no contact after 2 telephone calls, the participants was considered a missing value). The questionnaire was informed by the United Kingdom National Health Service outpatients questionnaire and the Telehealth Usability Questionnaire. It included 27 general questions asked of participants in both groups, plus 8 specific questions for participants in the conventional follow-up group and 14 specific questions for participants in the telemedicine group. To summarize all the included fields in the questionnaires (time to visit and visit length, comfort, tests and procedures performed before and during the visit, transport, waiting time, privacy, dealings with staff, platform usability, telemedicine, and satisfaction), participants were asked to provide a global satisfaction score on a scale from 1 to 5.
Analysis. To compare the groups in terms of proportion of outcomes, a chi-square test was used to analyze categorical variables. To compare medians between the groups, ordinal variables were analyzed using the Mann-Whitney U test. Statistical significance was set at P < 0.05.
Main results. Two-hundred patients were randomly allocated to 1 of the 2 groups, with 100 patients in each group. The groups did not differ significantly based on age (P = 0.836), gender (P = 0.393), or American Society of Anesthesiologists (ASA) score (P = 0.232). Time to visit did not differ significantly between the groups (P = 0.169), and while visits were generally shorter in the telemedicine group, the difference was not significant (P = 0.153). Diagnoses and treatments did not differ significantly between the groups (P = 0.853 and P = 0.461, respectively).
The primary outcome (follow-up feasibility) was achieved in 90% of the conventional follow-up group and in 74% of the telemedicine group (P = 0.003). Of the 10 patients in the conventional follow-up group who did not complete the follow-up, 8 did not attend the visit on the scheduled day and 2 were hospitalized for reasons not related to the study. In the telemedicine group, the 2 main reasons for failure to follow-up were technical difficulties (n = 10) and requests by patients to attend a conventional visit after being allocated to the telemedicine group (n = 10). Among the remaining 6 patients in the telemedicine group who did not attend a visit, 3 visited the outpatient clinic because of a known surgical wound infection before the visit, 2 did not respond to the video call and could not be contacted by other means, and 1 had other face-to-face visits scheduled in different departments of the hospital the same day as the telemedicine appointment.
There were no statistically significant differences in the clinical results of the 164 patients meeting the primary endpoint (P = 0.832). Twelve of the 90 (13.3%) patients in the conventional group attended extra visits after the follow-up, while 9 of the 74 patients (12.1%) in the telemedicine group (P = 0.823) attended extra visits after follow-up. The median global patient satisfaction score was 5 in both the conventional group (range, 2-5) and the telemedicine group (range, 1-5), with no statistically significant differences (P = 0.099). When patients in the telemedicine group were asked if they would accept the use of telemedicine as part of their medical treatment on an ongoing basis, they rated the proposition with a median score of 5 (range, 1-5).
Conclusion. Telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. This option produces good satisfaction rates and maintains clinical outcomes.
Commentary
In recent years, telemedicine has gained increased popularity in both medicine and surgery, affording surgeons greater opportunities for patient care, mentoring, collaboration, and teaching, without the limits of geographic boundaries. Telemedicine can be broadly described as a health care service utilizing telecommunication technologies for the purpose of communicating with and diagnosing and treating patients remotely.1-4 To date, literature on telemedicine in surgical care has been limited.
In their systematic review, published in 2018, Asiri et al identified 24 studies published between 1998 and 2018, which included 3 randomized controlled trials, 3 pilot studies, 4 retrospective studies, and 14 prospective observational studies. In these studies, telemedicine protocols were used for preoperative assessment, diagnostic purposes, or consultation with another surgical department (10 studies); postoperative wound assessment (9 studies); and follow-up in place of conventional clinic visits (5 studies).3 In a 2017 systematic review of telemedicine for post-discharge surgical care, Gunter et al identified 21 studies, which included 3 randomized controlled trials, 6 pilot or feasibility studies, 4 retrospective record reviews, 2 case series, and 6 surveys.4 In these studies, telemedicine protocols were used for scheduled follow-up (10 studies), routine and ongoing monitoring (5 studies), or management of issues that arose after surgery (2 studies). These 2 reviews found telemedicine to be feasible, useful, and acceptable for postoperative evaluation and follow-up among both providers and patients.
Additional benefits noted in these studies included savings in patient travel, time, and cost. Perspectives on savings to the health system were mixed—while clinic time slots may open as a result of follow-up visits being done via telemedicine (resulting in potential improvements in access to surgical services and decreased wait times), there are still significant direct costs for purchasing necessary equipment and for educating and training providers on the use of the equipment. Other published reviews have discussed in greater detail the application, benefits, limitations, and barriers to telemedicine and provided insight from the perspectives of patients, providers, and health care systems.1,2
Because studies on the use of telemedicine are limited, particularly in general surgery, and few of these studies have used a randomized clinical trial design, the present study is an important contribution to the literature. The authors found a significant difference between groups in terms of percentage of completed follow-up visits—90% of conventional follow-up group participants completed their visit versus 74% of telemedicine group participants. However, these differences were primarily attributed to technical difficulties experienced by telemedicine group participants, as well requests to have a conventional follow-up visit. In addition, telemedicine capabilities were limited to video calls via computers and webcams, and it is likely that successful completion of the follow-up visit would have been higher in the telemedicine group had the use of video calls via tablets or smartphones been an option. Perhaps more important, no significant differences were found in clinical outcomes (extra visits within 30 days after the follow-up visit) or patient satisfaction.
A key strength of this study is the use of a randomized clinical trial design to evaluate telemedicine as an alternative method for conducting patient visits following general surgery. Inclusion and exclusion criteria did not impose strict limitations on potential participants. Also, the authors evaluated differences in time to visit, length of visit, clinical results, and patient satisfaction between groups, in addition to the primary measure of completion of the follow-up visit.
This study has important limitations that should be noted as well, particularly related to the study design, some of which are acknowledged by the authors. Because this study was implemented in only 1 hospital, specifically, a tertiary care university hospital on the outskirts of an urban European city, the generalizability of the findings is limited. Also, the likelihood of selection bias is high, as enrollment was not offered to all patients who were discharged from the hospital and met inclusion criteria (limited by patient workload). The comparison of clinical results was limited, as the selected measure focused only on extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit. This chosen measure does not account for less severe clinical results that did not require an additional visit, and does not represent a nuanced comparison of specific clinical indicators. In addition, this measure does not account for clinical complications that may have occurred beyond the 30-day period. Recall bias also was likely, given that the patient satisfaction questionnaire was delivered via email to patients at a later time after the follow-up visit, instead of being administered immediately after the visit. Last, group differences at baseline were assessed based only on age, gender, and ASA score, which does not preclude potential differences related to other factors, such as race/ethnicity, household income, comorbidities, insurance, and zip code. Future research with a similar objective would benefit from a randomized clinical trial design that recruits a wider diversity of patients across different clinic settings and incorporates more nuanced measures of primary and secondary outcomes.
Applications for Clinical Practice
With the ongoing COVID-19 pandemic, the integration of telemedicine capabilities into hospital systems is becoming more widespread and is proceeding at an accelerated pace. This study provides evidence that telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. Assuming that the needed technology and appropriate program training are available, telemedicine should be offered to patients, especially to maximize savings in terms of travel, time, and cost. However, the option for conventional (in-person) follow-up should remain, particularly in cases where there may be barriers to successful follow-up visits via telemedicine, including limited digital literacy, lack of access to necessary equipment, language/communication barriers, complex follow-up treatment, and difficulties in describing or showing complications in the surgical area.
–Katrina F. Mateo, PhD, MPH
Study Overview
Objective. To compare the impact of conventional versus telemedicine follow-up of general surgery patients in outpatient clinics.
Design. Prospective randomized clinical trial.
Setting and participants. Participants were recruited from Hospital Germans Trias i Pujol, a tertiary care university hospital located in the outskirts of Barcelona (Catalonia, Spain). To be included in this study, participants had to have been treated in the general surgery department, have basic computer knowledge (ability to use e-mail or a social network), have a computer with webcam, and be 18 to 75 years of age, or they had to have a partner who met these criteria. Exclusion criteria included any disability making telemedicine follow-up impossible (eg, blindness, deafness, or mental disability; proctologic treatment; difficulty describing and/or showing complications in the surgical area; and clinical complications before discharge more severe than Clavien Dindo II), as well as withdrawal of consent. Patients who met the criteria and had just been discharged from the hospital were offered the opportunity to enroll by the surgeon in charge. Patients who agreed to participate provided informed consent and were assigned using a computerized block randomization list (allocation ratio 1:1).
Intervention. Time to visit was generally between 2 and 4 weeks after discharge (the interval to the follow-up visit was determined at the discretion of the treating surgeon, but always followed the usual schedule). To conduct the telemedicine follow-up through a video call, a medical cloud-based program fulfilling all European Union security and privacy policies was used. Four surgeons were assigned to perform the telemedicine visits and were trained on how to use the program before the study started. Visit format was the same in both groups: clinical and wound condition were assessed and pathology was discussed (the one difference was that physical exploration was not performed in the telemedicine group).
Main outcome measures. The primary outcome was the feasibility of telemedicine follow-up, and this was measured as the percentage of participants who completed follow-up in their corresponding group by the date scheduled at hospital discharge. Secondary outcomes included a comparison of clinical results and patient satisfaction. To assess the clinical results, extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit were collected.
To evaluate patient satisfaction, a questionnaire was sent via email to the participants after the visit and, if they did not respond, a telephone survey was carried out (if there was no contact after 2 telephone calls, the participants was considered a missing value). The questionnaire was informed by the United Kingdom National Health Service outpatients questionnaire and the Telehealth Usability Questionnaire. It included 27 general questions asked of participants in both groups, plus 8 specific questions for participants in the conventional follow-up group and 14 specific questions for participants in the telemedicine group. To summarize all the included fields in the questionnaires (time to visit and visit length, comfort, tests and procedures performed before and during the visit, transport, waiting time, privacy, dealings with staff, platform usability, telemedicine, and satisfaction), participants were asked to provide a global satisfaction score on a scale from 1 to 5.
Analysis. To compare the groups in terms of proportion of outcomes, a chi-square test was used to analyze categorical variables. To compare medians between the groups, ordinal variables were analyzed using the Mann-Whitney U test. Statistical significance was set at P < 0.05.
Main results. Two-hundred patients were randomly allocated to 1 of the 2 groups, with 100 patients in each group. The groups did not differ significantly based on age (P = 0.836), gender (P = 0.393), or American Society of Anesthesiologists (ASA) score (P = 0.232). Time to visit did not differ significantly between the groups (P = 0.169), and while visits were generally shorter in the telemedicine group, the difference was not significant (P = 0.153). Diagnoses and treatments did not differ significantly between the groups (P = 0.853 and P = 0.461, respectively).
The primary outcome (follow-up feasibility) was achieved in 90% of the conventional follow-up group and in 74% of the telemedicine group (P = 0.003). Of the 10 patients in the conventional follow-up group who did not complete the follow-up, 8 did not attend the visit on the scheduled day and 2 were hospitalized for reasons not related to the study. In the telemedicine group, the 2 main reasons for failure to follow-up were technical difficulties (n = 10) and requests by patients to attend a conventional visit after being allocated to the telemedicine group (n = 10). Among the remaining 6 patients in the telemedicine group who did not attend a visit, 3 visited the outpatient clinic because of a known surgical wound infection before the visit, 2 did not respond to the video call and could not be contacted by other means, and 1 had other face-to-face visits scheduled in different departments of the hospital the same day as the telemedicine appointment.
There were no statistically significant differences in the clinical results of the 164 patients meeting the primary endpoint (P = 0.832). Twelve of the 90 (13.3%) patients in the conventional group attended extra visits after the follow-up, while 9 of the 74 patients (12.1%) in the telemedicine group (P = 0.823) attended extra visits after follow-up. The median global patient satisfaction score was 5 in both the conventional group (range, 2-5) and the telemedicine group (range, 1-5), with no statistically significant differences (P = 0.099). When patients in the telemedicine group were asked if they would accept the use of telemedicine as part of their medical treatment on an ongoing basis, they rated the proposition with a median score of 5 (range, 1-5).
Conclusion. Telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. This option produces good satisfaction rates and maintains clinical outcomes.
Commentary
In recent years, telemedicine has gained increased popularity in both medicine and surgery, affording surgeons greater opportunities for patient care, mentoring, collaboration, and teaching, without the limits of geographic boundaries. Telemedicine can be broadly described as a health care service utilizing telecommunication technologies for the purpose of communicating with and diagnosing and treating patients remotely.1-4 To date, literature on telemedicine in surgical care has been limited.
In their systematic review, published in 2018, Asiri et al identified 24 studies published between 1998 and 2018, which included 3 randomized controlled trials, 3 pilot studies, 4 retrospective studies, and 14 prospective observational studies. In these studies, telemedicine protocols were used for preoperative assessment, diagnostic purposes, or consultation with another surgical department (10 studies); postoperative wound assessment (9 studies); and follow-up in place of conventional clinic visits (5 studies).3 In a 2017 systematic review of telemedicine for post-discharge surgical care, Gunter et al identified 21 studies, which included 3 randomized controlled trials, 6 pilot or feasibility studies, 4 retrospective record reviews, 2 case series, and 6 surveys.4 In these studies, telemedicine protocols were used for scheduled follow-up (10 studies), routine and ongoing monitoring (5 studies), or management of issues that arose after surgery (2 studies). These 2 reviews found telemedicine to be feasible, useful, and acceptable for postoperative evaluation and follow-up among both providers and patients.
Additional benefits noted in these studies included savings in patient travel, time, and cost. Perspectives on savings to the health system were mixed—while clinic time slots may open as a result of follow-up visits being done via telemedicine (resulting in potential improvements in access to surgical services and decreased wait times), there are still significant direct costs for purchasing necessary equipment and for educating and training providers on the use of the equipment. Other published reviews have discussed in greater detail the application, benefits, limitations, and barriers to telemedicine and provided insight from the perspectives of patients, providers, and health care systems.1,2
Because studies on the use of telemedicine are limited, particularly in general surgery, and few of these studies have used a randomized clinical trial design, the present study is an important contribution to the literature. The authors found a significant difference between groups in terms of percentage of completed follow-up visits—90% of conventional follow-up group participants completed their visit versus 74% of telemedicine group participants. However, these differences were primarily attributed to technical difficulties experienced by telemedicine group participants, as well requests to have a conventional follow-up visit. In addition, telemedicine capabilities were limited to video calls via computers and webcams, and it is likely that successful completion of the follow-up visit would have been higher in the telemedicine group had the use of video calls via tablets or smartphones been an option. Perhaps more important, no significant differences were found in clinical outcomes (extra visits within 30 days after the follow-up visit) or patient satisfaction.
A key strength of this study is the use of a randomized clinical trial design to evaluate telemedicine as an alternative method for conducting patient visits following general surgery. Inclusion and exclusion criteria did not impose strict limitations on potential participants. Also, the authors evaluated differences in time to visit, length of visit, clinical results, and patient satisfaction between groups, in addition to the primary measure of completion of the follow-up visit.
This study has important limitations that should be noted as well, particularly related to the study design, some of which are acknowledged by the authors. Because this study was implemented in only 1 hospital, specifically, a tertiary care university hospital on the outskirts of an urban European city, the generalizability of the findings is limited. Also, the likelihood of selection bias is high, as enrollment was not offered to all patients who were discharged from the hospital and met inclusion criteria (limited by patient workload). The comparison of clinical results was limited, as the selected measure focused only on extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit. This chosen measure does not account for less severe clinical results that did not require an additional visit, and does not represent a nuanced comparison of specific clinical indicators. In addition, this measure does not account for clinical complications that may have occurred beyond the 30-day period. Recall bias also was likely, given that the patient satisfaction questionnaire was delivered via email to patients at a later time after the follow-up visit, instead of being administered immediately after the visit. Last, group differences at baseline were assessed based only on age, gender, and ASA score, which does not preclude potential differences related to other factors, such as race/ethnicity, household income, comorbidities, insurance, and zip code. Future research with a similar objective would benefit from a randomized clinical trial design that recruits a wider diversity of patients across different clinic settings and incorporates more nuanced measures of primary and secondary outcomes.
Applications for Clinical Practice
With the ongoing COVID-19 pandemic, the integration of telemedicine capabilities into hospital systems is becoming more widespread and is proceeding at an accelerated pace. This study provides evidence that telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. Assuming that the needed technology and appropriate program training are available, telemedicine should be offered to patients, especially to maximize savings in terms of travel, time, and cost. However, the option for conventional (in-person) follow-up should remain, particularly in cases where there may be barriers to successful follow-up visits via telemedicine, including limited digital literacy, lack of access to necessary equipment, language/communication barriers, complex follow-up treatment, and difficulties in describing or showing complications in the surgical area.
–Katrina F. Mateo, PhD, MPH
1. Williams AM, Bhatti UF, Alam HB, Nikolian VC. The role of telemedicine in postoperative care. mHealth. 2018 May;4:11-11.
2. Huang EY, Knight S, Guetter CR et al. Telemedicine and telementoring in the surgical specialties: A narrative review. Am J Surg. 2019;218:760-766.
3. Asiri A, AlBishi S, AlMadani W, et al. The use of telemedicine in surgical care: A systematic review. Acta Informatica Medica. 2018;26:201-206.
4. Gunter RL, Chouinard S, Fernandes-Taylor S, et al. Current use of telemedicine for post-discharge surgical care: a systematic review. J Am College Surg. 2016;222:915-927.
1. Williams AM, Bhatti UF, Alam HB, Nikolian VC. The role of telemedicine in postoperative care. mHealth. 2018 May;4:11-11.
2. Huang EY, Knight S, Guetter CR et al. Telemedicine and telementoring in the surgical specialties: A narrative review. Am J Surg. 2019;218:760-766.
3. Asiri A, AlBishi S, AlMadani W, et al. The use of telemedicine in surgical care: A systematic review. Acta Informatica Medica. 2018;26:201-206.
4. Gunter RL, Chouinard S, Fernandes-Taylor S, et al. Current use of telemedicine for post-discharge surgical care: a systematic review. J Am College Surg. 2016;222:915-927.
Chilblain-like lesions reported in children thought to have COVID-19
Two and elsewhere.
These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, from hospitals in Milan and Madrid, published in Pediatric Dermatology.
In the first study, Cristiana Colonna, MD, and colleagues at Hospital Maggiore Polyclinic in Milan described four cases of chilblain-like lesions in children ages 5-11 years with mild COVID-19 symptoms.
In the second, David Andina, MD, and colleagues in the ED and the departments of dermatology and pathology at the Child Jesus University Children’s Hospital in Madrid published a retrospective study of 22 cases in children and adolescents ages 6-17 years who reported to the hospital ED from April 6 to 17, the peak of the pandemic in Madrid.
In all four of the Milan cases, the skin lesions appeared several days after the onset of COVID-19 symptoms, although all four patients initially tested negative for COVID-19. However, Dr. Colonna and colleagues wrote that, “given the fact that the sensitivity and specificity of both nasopharyngeal swabs and antibody tests for COVID-19 (when available) are not 100% reliable, the question of the origin of these strange chilblain-like lesions is still elusive.” Until further studies are available, they emphasized that clinicians should be “alert to the presentation of chilblain-like findings” in children with mild symptoms “as a possible sign of COVID-19 infection.”
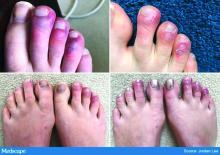
All the patients had lesions on their feet or toes, and a 5-year-old boy also had lesions on the right hand. One patient, an 11-year-old girl, had a biopsy that revealed dense lymphocytic perivascular cuffing and periadnexal infiltration.
“The finding of an elevated d-dimer in one of our patients, along with the clinical features suggestive of a vasoocclusive phenomenon, supports consideration of laboratory evaluation for coagulation defects in asymptomatic or mildly symptomatic children with acrovasculitis-like findings,” Dr. Colonna and colleagues wrote. None of the four cases in Milan required treatment, with three cases resolving within 5 days.
Like the Milan cases, all 22 patients in the Madrid series had foot or toe lesions and three had lesions on the fingers. This larger series also reported more detailed symptoms about the lesions: pruritus in nine patients (41%) and mild pain in seven (32%). A total of 10 patients had systemic symptoms of COVID-19, predominantly cough and rhinorrhea in 9 patients (41%), but 2 (9%) had abdominal pain and diarrhea. These symptoms, the authors said, appeared a median of 14 days (range, 1-28 days) before they developed chilblains.
A total of 19 patients were tested for COVID-19, but only 1 was positive.
This retrospective study also included contact information, with one patient having household contact with a single confirmed case of COVID-19; 12 patients recalled household contact who were considered probable cases of COVID-19, with respiratory symptoms.
Skin biopsies were obtained from the acral lesions in six patients, all showing similar results, although with varying degrees of intensity. All biopsies showed features of lymphocytic vasculopathy. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis described as “mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.”
The only treatments Dr. Andina and colleagues reported were oral analgesics for pain and oral antihistamines for pruritus when needed. One patient was given topical corticosteroids and another a short course of oral steroids, both for erythema multiforme.
Dr. Andina and colleagues wrote that the skin lesions in these patients “were unequivocally categorized as chilblains, both clinically and histopathologically,” and, after 7-10 days, began to fade. None of the patients had complications, and had an “excellent outcome,” they noted.
Dr. Colonna and colleagues had no conflicts of interest to declare. Dr. Andina and colleagues provided no disclosure statement.
SOURCES: Colonna C et al. Ped Derm. 2020 May 6. doi: 10.1111/pde.14210; Andina D et al. Ped Derm. 2020 May 9. doi: 10.1111/pde.14215.
Two and elsewhere.
These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, from hospitals in Milan and Madrid, published in Pediatric Dermatology.
In the first study, Cristiana Colonna, MD, and colleagues at Hospital Maggiore Polyclinic in Milan described four cases of chilblain-like lesions in children ages 5-11 years with mild COVID-19 symptoms.
In the second, David Andina, MD, and colleagues in the ED and the departments of dermatology and pathology at the Child Jesus University Children’s Hospital in Madrid published a retrospective study of 22 cases in children and adolescents ages 6-17 years who reported to the hospital ED from April 6 to 17, the peak of the pandemic in Madrid.
In all four of the Milan cases, the skin lesions appeared several days after the onset of COVID-19 symptoms, although all four patients initially tested negative for COVID-19. However, Dr. Colonna and colleagues wrote that, “given the fact that the sensitivity and specificity of both nasopharyngeal swabs and antibody tests for COVID-19 (when available) are not 100% reliable, the question of the origin of these strange chilblain-like lesions is still elusive.” Until further studies are available, they emphasized that clinicians should be “alert to the presentation of chilblain-like findings” in children with mild symptoms “as a possible sign of COVID-19 infection.”
All the patients had lesions on their feet or toes, and a 5-year-old boy also had lesions on the right hand. One patient, an 11-year-old girl, had a biopsy that revealed dense lymphocytic perivascular cuffing and periadnexal infiltration.
“The finding of an elevated d-dimer in one of our patients, along with the clinical features suggestive of a vasoocclusive phenomenon, supports consideration of laboratory evaluation for coagulation defects in asymptomatic or mildly symptomatic children with acrovasculitis-like findings,” Dr. Colonna and colleagues wrote. None of the four cases in Milan required treatment, with three cases resolving within 5 days.
Like the Milan cases, all 22 patients in the Madrid series had foot or toe lesions and three had lesions on the fingers. This larger series also reported more detailed symptoms about the lesions: pruritus in nine patients (41%) and mild pain in seven (32%). A total of 10 patients had systemic symptoms of COVID-19, predominantly cough and rhinorrhea in 9 patients (41%), but 2 (9%) had abdominal pain and diarrhea. These symptoms, the authors said, appeared a median of 14 days (range, 1-28 days) before they developed chilblains.
A total of 19 patients were tested for COVID-19, but only 1 was positive.
This retrospective study also included contact information, with one patient having household contact with a single confirmed case of COVID-19; 12 patients recalled household contact who were considered probable cases of COVID-19, with respiratory symptoms.
Skin biopsies were obtained from the acral lesions in six patients, all showing similar results, although with varying degrees of intensity. All biopsies showed features of lymphocytic vasculopathy. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis described as “mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.”
The only treatments Dr. Andina and colleagues reported were oral analgesics for pain and oral antihistamines for pruritus when needed. One patient was given topical corticosteroids and another a short course of oral steroids, both for erythema multiforme.
Dr. Andina and colleagues wrote that the skin lesions in these patients “were unequivocally categorized as chilblains, both clinically and histopathologically,” and, after 7-10 days, began to fade. None of the patients had complications, and had an “excellent outcome,” they noted.
Dr. Colonna and colleagues had no conflicts of interest to declare. Dr. Andina and colleagues provided no disclosure statement.
SOURCES: Colonna C et al. Ped Derm. 2020 May 6. doi: 10.1111/pde.14210; Andina D et al. Ped Derm. 2020 May 9. doi: 10.1111/pde.14215.
Two and elsewhere.
These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, from hospitals in Milan and Madrid, published in Pediatric Dermatology.
In the first study, Cristiana Colonna, MD, and colleagues at Hospital Maggiore Polyclinic in Milan described four cases of chilblain-like lesions in children ages 5-11 years with mild COVID-19 symptoms.
In the second, David Andina, MD, and colleagues in the ED and the departments of dermatology and pathology at the Child Jesus University Children’s Hospital in Madrid published a retrospective study of 22 cases in children and adolescents ages 6-17 years who reported to the hospital ED from April 6 to 17, the peak of the pandemic in Madrid.
In all four of the Milan cases, the skin lesions appeared several days after the onset of COVID-19 symptoms, although all four patients initially tested negative for COVID-19. However, Dr. Colonna and colleagues wrote that, “given the fact that the sensitivity and specificity of both nasopharyngeal swabs and antibody tests for COVID-19 (when available) are not 100% reliable, the question of the origin of these strange chilblain-like lesions is still elusive.” Until further studies are available, they emphasized that clinicians should be “alert to the presentation of chilblain-like findings” in children with mild symptoms “as a possible sign of COVID-19 infection.”
All the patients had lesions on their feet or toes, and a 5-year-old boy also had lesions on the right hand. One patient, an 11-year-old girl, had a biopsy that revealed dense lymphocytic perivascular cuffing and periadnexal infiltration.
“The finding of an elevated d-dimer in one of our patients, along with the clinical features suggestive of a vasoocclusive phenomenon, supports consideration of laboratory evaluation for coagulation defects in asymptomatic or mildly symptomatic children with acrovasculitis-like findings,” Dr. Colonna and colleagues wrote. None of the four cases in Milan required treatment, with three cases resolving within 5 days.
Like the Milan cases, all 22 patients in the Madrid series had foot or toe lesions and three had lesions on the fingers. This larger series also reported more detailed symptoms about the lesions: pruritus in nine patients (41%) and mild pain in seven (32%). A total of 10 patients had systemic symptoms of COVID-19, predominantly cough and rhinorrhea in 9 patients (41%), but 2 (9%) had abdominal pain and diarrhea. These symptoms, the authors said, appeared a median of 14 days (range, 1-28 days) before they developed chilblains.
A total of 19 patients were tested for COVID-19, but only 1 was positive.
This retrospective study also included contact information, with one patient having household contact with a single confirmed case of COVID-19; 12 patients recalled household contact who were considered probable cases of COVID-19, with respiratory symptoms.
Skin biopsies were obtained from the acral lesions in six patients, all showing similar results, although with varying degrees of intensity. All biopsies showed features of lymphocytic vasculopathy. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis described as “mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.”
The only treatments Dr. Andina and colleagues reported were oral analgesics for pain and oral antihistamines for pruritus when needed. One patient was given topical corticosteroids and another a short course of oral steroids, both for erythema multiforme.
Dr. Andina and colleagues wrote that the skin lesions in these patients “were unequivocally categorized as chilblains, both clinically and histopathologically,” and, after 7-10 days, began to fade. None of the patients had complications, and had an “excellent outcome,” they noted.
Dr. Colonna and colleagues had no conflicts of interest to declare. Dr. Andina and colleagues provided no disclosure statement.
SOURCES: Colonna C et al. Ped Derm. 2020 May 6. doi: 10.1111/pde.14210; Andina D et al. Ped Derm. 2020 May 9. doi: 10.1111/pde.14215.
FROM PEDIATRIC DERMATOLOGY
COVID-19 in kids: Severe illness most common in infants, teens
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
FROM THE JOURNAL OF PEDIATRICS
Dermatologic changes with COVID-19: What we know and don’t know
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
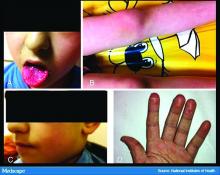
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
COVID-19 triggers new bariatric/metabolic surgery guidance
New recommendations for the management of metabolic and bariatric surgery candidates during and after the COVID-19 pandemic shift the focus from body mass index (BMI) alone to medical conditions most likely to be ameliorated by the procedures.
Meant as a guide for both surgeons and referring clinicians, the document was published online May 7 as a Personal View in Lancet Diabetes & Endocrinology.
“Millions of elective operations have been on hold because of COVID-19. ... In the next few months, we’re going to face a huge backlog of procedures of all types. Even when we resume doing surgery it’s not going to be business as usual for many months. ... Hospital clinicians and managers want to make decisions about who’s going to get those slots first,” lead author of the international 23-member writing panel, Francesco Rubino, MD, told Medscape Medical News.
Rubino is professor of metabolic and bariatric surgery at King’s College Hospital, London, UK.
The recommendations include a guide for prioritizing patients eligible for bariatric or metabolic surgery – the former referring to when it’s performed primarily for obesity and the latter for type 2 diabetes – once the pandemic restrictions on nonessential surgery are lifted.
Rather than prioritizing patients by BMI, the scheme focuses on medical comorbidities to place patients into “expedited” or “standard” access categories.
Historically, bariatric and metabolic surgery have had a low uptake due to factors such as lack of insurance coverage and stigma, with many physicians inappropriately viewing it as risky, ineffective, and/or as a “last resort” treatment, Rubino said.
“They don’t refer for surgery even though we have all the evidence that the benefits for patients are unquestionable,” he added.
Because of that background, “in the situation of limited capacity, patients with obesity and type 2 diabetes are likely to be penalized compared to any other conditions that need elective surgery,” Rubino stressed.
Asked to comment, Scott Kahan, MD, director of the National Center for Weight and Wellness in Washington, D.C., called the document a “really valuable thought piece.”
Noting that only about 1% to 2% of people who are eligible for bariatric or metabolic surgery actually undergo the procedures, Kahan said, “because so few people get the surgery we’ve never really run into a situation of undersupply or overdemand.
“But, as we’re moving forward, one would think that we will run into that scenario. So, better prioritizing and triaging patients likely will be more important down the line, given how effective surgery has been shown to be now, both short term and long term.”
Risks of obesity, shifting away from BMI as the main metric
The new document extensively discusses the risks of obesity – including now as a major COVID-19 risk factor – and the benefits of the procedures and risks of delaying them.
It also addresses ongoing management of patients who had bariatric/metabolic surgery in the past and nonsurgical treatment to mitigate harm until patients can undergo the procedures.
Another important problem the document addresses, Rubino said, is the current BMI-focused bariatric/metabolic surgery criteria (≥ 40 kg/m2 or ≥ 35 kg/m2 with at least one obesity-related comorbidity).
“BMI is an epidemiological measure, not a measure of disease. But we select patients for bariatric surgery by saying who is eligible [without assessing] who has more or less severe disease, and who is at more or less risk for short-term complications from the disease compared to others,” he explained. “We don’t have any mechanism, even in normal times, let alone during a pandemic, to differentiate between patients who need surgery sooner rather than later.”
Indeed, Kahan said, “Traditionally we tend to oversimplify risk stratification in terms of how heavy people are. While that is one factor of importance, it’s far from the only factor and may not be the most important factor.”
In “someone who is relatively lighter but sicker, it would be sensible, in my mind, to prioritize them for a potentially curative procedure compared with someone who is heavier – even much heavier – but is not as sick,” he added.
“Pandemic forces us to do what was long overdue”
The document confirms that bariatric/metabolic surgery should remain suspended during the most intense phase of the COVID-19 pandemic and only resume once overall restrictions on nonessential surgeries are lifted.
Exceptions are limited to emergency endoscopic interventions for complications of prior surgery, such as hemorrhage or leaks.
A section offers guidance for pharmacologic and other nonsurgical options to mitigate harm from delaying the procedures including use of drugs that promote weight loss, such as glucagonlike peptide-1 receptor agonists and/or sodium-glucose cotransporter 2 inhibitors.
Once less-urgent surgeries are allowed to resume, a prioritization scheme addresses which patients should receive “expedited access” (risk of harm if delayed beyond 90 days) versus “standard access” (unlikely to deteriorate within 6 months) within three indication categories: “diabetes (metabolic) surgery,” “obesity (bariatric) surgery,” or “adjuvant bariatric and metabolic surgery.”
Examples of patients who would qualify for “expedited” access in the “diabetes surgery” category include those with an A1c of 8% or greater despite use of two or more oral medications or insulin use, those with a history of cardiovascular disease, and/or those with stage 3-4 chronic kidney disease.
For the “obesity surgery” group, priority patients include those with a BMI of 60 kg/m2 or greater or with severe obesity hypoventilation syndrome or severe sleep apnea.
And for the adjuvant category, those requiring weight loss to allow for other treatments, such as organ transplants, would be expedited.
Individuals with less-severe obesity or chronic conditions could have their surgeries put off until a later date.
The panel also recommends that even though keyhole surgery involves aerosol-generating techniques that could increase the risk for coronavirus infection, laparoscopic approaches are still preferred over open procedures because they carry lower risks for complications and result in shorter hospital stays, thereby lowering infection risk.
Appropriate personal protective equipment is, of course, advised for use by clinicians.
Kahan said of the document: “I think it’s a very sensible piece where they’re thinking through things that haven’t really needed to be thought through all that much. That’s partly with respect to COVID-19, but even beyond that I think this will be a valuable platform going forward.”
Indeed, Rubino said, “The pandemic forces us to do what was long overdue.”
Rubino has reported being on advisory boards for GI Dynamics, Keyron, and Novo Nordisk, has reported receiving consulting fees and research grants from Ethicon Endo-Surgery and Medtronic. Kahan has reported no relevant financial relationships.
This article first appeared on Medscape.com.
New recommendations for the management of metabolic and bariatric surgery candidates during and after the COVID-19 pandemic shift the focus from body mass index (BMI) alone to medical conditions most likely to be ameliorated by the procedures.
Meant as a guide for both surgeons and referring clinicians, the document was published online May 7 as a Personal View in Lancet Diabetes & Endocrinology.
“Millions of elective operations have been on hold because of COVID-19. ... In the next few months, we’re going to face a huge backlog of procedures of all types. Even when we resume doing surgery it’s not going to be business as usual for many months. ... Hospital clinicians and managers want to make decisions about who’s going to get those slots first,” lead author of the international 23-member writing panel, Francesco Rubino, MD, told Medscape Medical News.
Rubino is professor of metabolic and bariatric surgery at King’s College Hospital, London, UK.
The recommendations include a guide for prioritizing patients eligible for bariatric or metabolic surgery – the former referring to when it’s performed primarily for obesity and the latter for type 2 diabetes – once the pandemic restrictions on nonessential surgery are lifted.
Rather than prioritizing patients by BMI, the scheme focuses on medical comorbidities to place patients into “expedited” or “standard” access categories.
Historically, bariatric and metabolic surgery have had a low uptake due to factors such as lack of insurance coverage and stigma, with many physicians inappropriately viewing it as risky, ineffective, and/or as a “last resort” treatment, Rubino said.
“They don’t refer for surgery even though we have all the evidence that the benefits for patients are unquestionable,” he added.
Because of that background, “in the situation of limited capacity, patients with obesity and type 2 diabetes are likely to be penalized compared to any other conditions that need elective surgery,” Rubino stressed.
Asked to comment, Scott Kahan, MD, director of the National Center for Weight and Wellness in Washington, D.C., called the document a “really valuable thought piece.”
Noting that only about 1% to 2% of people who are eligible for bariatric or metabolic surgery actually undergo the procedures, Kahan said, “because so few people get the surgery we’ve never really run into a situation of undersupply or overdemand.
“But, as we’re moving forward, one would think that we will run into that scenario. So, better prioritizing and triaging patients likely will be more important down the line, given how effective surgery has been shown to be now, both short term and long term.”
Risks of obesity, shifting away from BMI as the main metric
The new document extensively discusses the risks of obesity – including now as a major COVID-19 risk factor – and the benefits of the procedures and risks of delaying them.
It also addresses ongoing management of patients who had bariatric/metabolic surgery in the past and nonsurgical treatment to mitigate harm until patients can undergo the procedures.
Another important problem the document addresses, Rubino said, is the current BMI-focused bariatric/metabolic surgery criteria (≥ 40 kg/m2 or ≥ 35 kg/m2 with at least one obesity-related comorbidity).
“BMI is an epidemiological measure, not a measure of disease. But we select patients for bariatric surgery by saying who is eligible [without assessing] who has more or less severe disease, and who is at more or less risk for short-term complications from the disease compared to others,” he explained. “We don’t have any mechanism, even in normal times, let alone during a pandemic, to differentiate between patients who need surgery sooner rather than later.”
Indeed, Kahan said, “Traditionally we tend to oversimplify risk stratification in terms of how heavy people are. While that is one factor of importance, it’s far from the only factor and may not be the most important factor.”
In “someone who is relatively lighter but sicker, it would be sensible, in my mind, to prioritize them for a potentially curative procedure compared with someone who is heavier – even much heavier – but is not as sick,” he added.
“Pandemic forces us to do what was long overdue”
The document confirms that bariatric/metabolic surgery should remain suspended during the most intense phase of the COVID-19 pandemic and only resume once overall restrictions on nonessential surgeries are lifted.
Exceptions are limited to emergency endoscopic interventions for complications of prior surgery, such as hemorrhage or leaks.
A section offers guidance for pharmacologic and other nonsurgical options to mitigate harm from delaying the procedures including use of drugs that promote weight loss, such as glucagonlike peptide-1 receptor agonists and/or sodium-glucose cotransporter 2 inhibitors.
Once less-urgent surgeries are allowed to resume, a prioritization scheme addresses which patients should receive “expedited access” (risk of harm if delayed beyond 90 days) versus “standard access” (unlikely to deteriorate within 6 months) within three indication categories: “diabetes (metabolic) surgery,” “obesity (bariatric) surgery,” or “adjuvant bariatric and metabolic surgery.”
Examples of patients who would qualify for “expedited” access in the “diabetes surgery” category include those with an A1c of 8% or greater despite use of two or more oral medications or insulin use, those with a history of cardiovascular disease, and/or those with stage 3-4 chronic kidney disease.
For the “obesity surgery” group, priority patients include those with a BMI of 60 kg/m2 or greater or with severe obesity hypoventilation syndrome or severe sleep apnea.
And for the adjuvant category, those requiring weight loss to allow for other treatments, such as organ transplants, would be expedited.
Individuals with less-severe obesity or chronic conditions could have their surgeries put off until a later date.
The panel also recommends that even though keyhole surgery involves aerosol-generating techniques that could increase the risk for coronavirus infection, laparoscopic approaches are still preferred over open procedures because they carry lower risks for complications and result in shorter hospital stays, thereby lowering infection risk.
Appropriate personal protective equipment is, of course, advised for use by clinicians.
Kahan said of the document: “I think it’s a very sensible piece where they’re thinking through things that haven’t really needed to be thought through all that much. That’s partly with respect to COVID-19, but even beyond that I think this will be a valuable platform going forward.”
Indeed, Rubino said, “The pandemic forces us to do what was long overdue.”
Rubino has reported being on advisory boards for GI Dynamics, Keyron, and Novo Nordisk, has reported receiving consulting fees and research grants from Ethicon Endo-Surgery and Medtronic. Kahan has reported no relevant financial relationships.
This article first appeared on Medscape.com.
New recommendations for the management of metabolic and bariatric surgery candidates during and after the COVID-19 pandemic shift the focus from body mass index (BMI) alone to medical conditions most likely to be ameliorated by the procedures.
Meant as a guide for both surgeons and referring clinicians, the document was published online May 7 as a Personal View in Lancet Diabetes & Endocrinology.
“Millions of elective operations have been on hold because of COVID-19. ... In the next few months, we’re going to face a huge backlog of procedures of all types. Even when we resume doing surgery it’s not going to be business as usual for many months. ... Hospital clinicians and managers want to make decisions about who’s going to get those slots first,” lead author of the international 23-member writing panel, Francesco Rubino, MD, told Medscape Medical News.
Rubino is professor of metabolic and bariatric surgery at King’s College Hospital, London, UK.
The recommendations include a guide for prioritizing patients eligible for bariatric or metabolic surgery – the former referring to when it’s performed primarily for obesity and the latter for type 2 diabetes – once the pandemic restrictions on nonessential surgery are lifted.
Rather than prioritizing patients by BMI, the scheme focuses on medical comorbidities to place patients into “expedited” or “standard” access categories.
Historically, bariatric and metabolic surgery have had a low uptake due to factors such as lack of insurance coverage and stigma, with many physicians inappropriately viewing it as risky, ineffective, and/or as a “last resort” treatment, Rubino said.
“They don’t refer for surgery even though we have all the evidence that the benefits for patients are unquestionable,” he added.
Because of that background, “in the situation of limited capacity, patients with obesity and type 2 diabetes are likely to be penalized compared to any other conditions that need elective surgery,” Rubino stressed.
Asked to comment, Scott Kahan, MD, director of the National Center for Weight and Wellness in Washington, D.C., called the document a “really valuable thought piece.”
Noting that only about 1% to 2% of people who are eligible for bariatric or metabolic surgery actually undergo the procedures, Kahan said, “because so few people get the surgery we’ve never really run into a situation of undersupply or overdemand.
“But, as we’re moving forward, one would think that we will run into that scenario. So, better prioritizing and triaging patients likely will be more important down the line, given how effective surgery has been shown to be now, both short term and long term.”
Risks of obesity, shifting away from BMI as the main metric
The new document extensively discusses the risks of obesity – including now as a major COVID-19 risk factor – and the benefits of the procedures and risks of delaying them.
It also addresses ongoing management of patients who had bariatric/metabolic surgery in the past and nonsurgical treatment to mitigate harm until patients can undergo the procedures.
Another important problem the document addresses, Rubino said, is the current BMI-focused bariatric/metabolic surgery criteria (≥ 40 kg/m2 or ≥ 35 kg/m2 with at least one obesity-related comorbidity).
“BMI is an epidemiological measure, not a measure of disease. But we select patients for bariatric surgery by saying who is eligible [without assessing] who has more or less severe disease, and who is at more or less risk for short-term complications from the disease compared to others,” he explained. “We don’t have any mechanism, even in normal times, let alone during a pandemic, to differentiate between patients who need surgery sooner rather than later.”
Indeed, Kahan said, “Traditionally we tend to oversimplify risk stratification in terms of how heavy people are. While that is one factor of importance, it’s far from the only factor and may not be the most important factor.”
In “someone who is relatively lighter but sicker, it would be sensible, in my mind, to prioritize them for a potentially curative procedure compared with someone who is heavier – even much heavier – but is not as sick,” he added.
“Pandemic forces us to do what was long overdue”
The document confirms that bariatric/metabolic surgery should remain suspended during the most intense phase of the COVID-19 pandemic and only resume once overall restrictions on nonessential surgeries are lifted.
Exceptions are limited to emergency endoscopic interventions for complications of prior surgery, such as hemorrhage or leaks.
A section offers guidance for pharmacologic and other nonsurgical options to mitigate harm from delaying the procedures including use of drugs that promote weight loss, such as glucagonlike peptide-1 receptor agonists and/or sodium-glucose cotransporter 2 inhibitors.
Once less-urgent surgeries are allowed to resume, a prioritization scheme addresses which patients should receive “expedited access” (risk of harm if delayed beyond 90 days) versus “standard access” (unlikely to deteriorate within 6 months) within three indication categories: “diabetes (metabolic) surgery,” “obesity (bariatric) surgery,” or “adjuvant bariatric and metabolic surgery.”
Examples of patients who would qualify for “expedited” access in the “diabetes surgery” category include those with an A1c of 8% or greater despite use of two or more oral medications or insulin use, those with a history of cardiovascular disease, and/or those with stage 3-4 chronic kidney disease.
For the “obesity surgery” group, priority patients include those with a BMI of 60 kg/m2 or greater or with severe obesity hypoventilation syndrome or severe sleep apnea.
And for the adjuvant category, those requiring weight loss to allow for other treatments, such as organ transplants, would be expedited.
Individuals with less-severe obesity or chronic conditions could have their surgeries put off until a later date.
The panel also recommends that even though keyhole surgery involves aerosol-generating techniques that could increase the risk for coronavirus infection, laparoscopic approaches are still preferred over open procedures because they carry lower risks for complications and result in shorter hospital stays, thereby lowering infection risk.
Appropriate personal protective equipment is, of course, advised for use by clinicians.
Kahan said of the document: “I think it’s a very sensible piece where they’re thinking through things that haven’t really needed to be thought through all that much. That’s partly with respect to COVID-19, but even beyond that I think this will be a valuable platform going forward.”
Indeed, Rubino said, “The pandemic forces us to do what was long overdue.”
Rubino has reported being on advisory boards for GI Dynamics, Keyron, and Novo Nordisk, has reported receiving consulting fees and research grants from Ethicon Endo-Surgery and Medtronic. Kahan has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Consider COVID-19–associated multisystem hyperinflammatory syndrome
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
COVID-19 fears tied to dangerous drop in child vaccinations
The social distancing and sheltering in place mandated because of the COVID-19 pandemic are keeping parents and kids out of their doctors’ offices, and that has prompted a steep decline in recommended routine vaccinations for U.S. children, according to Centers for Disease Control and Prevention researchers.
Pediatric vaccinations dropped sharply after the national emergency was declared on March 13, suggesting that some children may be at increased risk for other serious infectious diseases, such as measles.
The researchers compared weekly orders for federally funded vaccines from Jan. 6 to April 19, 2020, with those during the same period in 2019.
They noted that, by the end of the study period, there was a cumulative COVID-19–related decline of 2.5 million doses in orders for routine noninfluenza pediatric childhood vaccines recommended by the Advisory Committee on Immunization Practices, as well as a cumulative decline in orders of 250,000 doses of measles vaccines.
Although the overall decrease in vaccinations during the study period was larger, according to CDC spokesperson Richard Quartarone, the above figures represent declines clearly associated with the pandemic.
The weekly number of measles vaccines ordered for children aged 24 months or older fell dramatically to about 500 during the week beginning March 16, 2020, and fell further to approximately 250 during the week beginning March 23. It stayed at that level until the week beginning April 13. By comparison, more than 2,500 were ordered during the week starting March 2, before the emergency was declared.
The decline was notably less for children younger than 2 years. For those children, orders dropped to about 750 during the week starting March 23 and climbed slightly for 3 weeks. By comparison, during the week of March 2, about 2,000 vaccines were ordered.
The findings, which were published in the CDC’s Morbidity and Mortality Weekly Report, stem from an analysis of ordering data from the federal Vaccines for Children (VFC) Program, as well as from vaccine administration data from the CDC’s Vaccine Tracking System and the collaborative Vaccine Safety Datalink (VSD).
The VFC provides federally purchased vaccines at no cost to about half of persons aged 18 years or younger. The VSD collaborates on vaccine coverage with the CDC’s Immunization Safety Office and eight large health care organizations across the country. Vaccination coverage is the usual metric for assessing vaccine usage; providers’ orders and the number of doses administered are two proxy measures, the authors explained.
“The substantial reduction in VFC-funded pediatric vaccine ordering after the COVID-19 emergency declaration is consistent with changes in vaccine administration among children in the VSD population receiving care through eight large U.S. health care organizations,” wrote Jeanne M. Santoli, MD, and colleagues, of the immunization services division at the National Center for Immunization and Respiratory Diseases. “The smaller decline in measles-containing vaccine administration among children aged ≤24 months suggests that system-level strategies to prioritize well child care and immunization for this age group are being implemented.”
Dr. Santoli, who is an Atlanta-based pediatrician, and associates stressed the importance of maintaining regular vaccinations during the pandemic. “The identified declines in routine pediatric vaccine ordering and doses administered might indicate that U.S. children and their communities face increased risks for outbreaks of vaccine-preventable diseases,” they wrote. “Parental concerns about potentially exposing their children to COVID-19 during well child visits might contribute to the declines observed.” Parents should therefore be reminded of the necessity of protecting their children against vaccine-preventable diseases.
In 2019, a Gallup survey reported that overall support for vaccination continued to decline in the United States.
The researchers predicted that, as social distancing relaxes, unvaccinated children will be more susceptible to other serious diseases. “In response, continued coordinated efforts between health care providers and public health officials at the local, state, and federal levels will be necessary to achieve rapid catch-up vaccination,” they concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The social distancing and sheltering in place mandated because of the COVID-19 pandemic are keeping parents and kids out of their doctors’ offices, and that has prompted a steep decline in recommended routine vaccinations for U.S. children, according to Centers for Disease Control and Prevention researchers.
Pediatric vaccinations dropped sharply after the national emergency was declared on March 13, suggesting that some children may be at increased risk for other serious infectious diseases, such as measles.
The researchers compared weekly orders for federally funded vaccines from Jan. 6 to April 19, 2020, with those during the same period in 2019.
They noted that, by the end of the study period, there was a cumulative COVID-19–related decline of 2.5 million doses in orders for routine noninfluenza pediatric childhood vaccines recommended by the Advisory Committee on Immunization Practices, as well as a cumulative decline in orders of 250,000 doses of measles vaccines.
Although the overall decrease in vaccinations during the study period was larger, according to CDC spokesperson Richard Quartarone, the above figures represent declines clearly associated with the pandemic.
The weekly number of measles vaccines ordered for children aged 24 months or older fell dramatically to about 500 during the week beginning March 16, 2020, and fell further to approximately 250 during the week beginning March 23. It stayed at that level until the week beginning April 13. By comparison, more than 2,500 were ordered during the week starting March 2, before the emergency was declared.
The decline was notably less for children younger than 2 years. For those children, orders dropped to about 750 during the week starting March 23 and climbed slightly for 3 weeks. By comparison, during the week of March 2, about 2,000 vaccines were ordered.
The findings, which were published in the CDC’s Morbidity and Mortality Weekly Report, stem from an analysis of ordering data from the federal Vaccines for Children (VFC) Program, as well as from vaccine administration data from the CDC’s Vaccine Tracking System and the collaborative Vaccine Safety Datalink (VSD).
The VFC provides federally purchased vaccines at no cost to about half of persons aged 18 years or younger. The VSD collaborates on vaccine coverage with the CDC’s Immunization Safety Office and eight large health care organizations across the country. Vaccination coverage is the usual metric for assessing vaccine usage; providers’ orders and the number of doses administered are two proxy measures, the authors explained.
“The substantial reduction in VFC-funded pediatric vaccine ordering after the COVID-19 emergency declaration is consistent with changes in vaccine administration among children in the VSD population receiving care through eight large U.S. health care organizations,” wrote Jeanne M. Santoli, MD, and colleagues, of the immunization services division at the National Center for Immunization and Respiratory Diseases. “The smaller decline in measles-containing vaccine administration among children aged ≤24 months suggests that system-level strategies to prioritize well child care and immunization for this age group are being implemented.”
Dr. Santoli, who is an Atlanta-based pediatrician, and associates stressed the importance of maintaining regular vaccinations during the pandemic. “The identified declines in routine pediatric vaccine ordering and doses administered might indicate that U.S. children and their communities face increased risks for outbreaks of vaccine-preventable diseases,” they wrote. “Parental concerns about potentially exposing their children to COVID-19 during well child visits might contribute to the declines observed.” Parents should therefore be reminded of the necessity of protecting their children against vaccine-preventable diseases.
In 2019, a Gallup survey reported that overall support for vaccination continued to decline in the United States.
The researchers predicted that, as social distancing relaxes, unvaccinated children will be more susceptible to other serious diseases. “In response, continued coordinated efforts between health care providers and public health officials at the local, state, and federal levels will be necessary to achieve rapid catch-up vaccination,” they concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The social distancing and sheltering in place mandated because of the COVID-19 pandemic are keeping parents and kids out of their doctors’ offices, and that has prompted a steep decline in recommended routine vaccinations for U.S. children, according to Centers for Disease Control and Prevention researchers.
Pediatric vaccinations dropped sharply after the national emergency was declared on March 13, suggesting that some children may be at increased risk for other serious infectious diseases, such as measles.
The researchers compared weekly orders for federally funded vaccines from Jan. 6 to April 19, 2020, with those during the same period in 2019.
They noted that, by the end of the study period, there was a cumulative COVID-19–related decline of 2.5 million doses in orders for routine noninfluenza pediatric childhood vaccines recommended by the Advisory Committee on Immunization Practices, as well as a cumulative decline in orders of 250,000 doses of measles vaccines.
Although the overall decrease in vaccinations during the study period was larger, according to CDC spokesperson Richard Quartarone, the above figures represent declines clearly associated with the pandemic.
The weekly number of measles vaccines ordered for children aged 24 months or older fell dramatically to about 500 during the week beginning March 16, 2020, and fell further to approximately 250 during the week beginning March 23. It stayed at that level until the week beginning April 13. By comparison, more than 2,500 were ordered during the week starting March 2, before the emergency was declared.
The decline was notably less for children younger than 2 years. For those children, orders dropped to about 750 during the week starting March 23 and climbed slightly for 3 weeks. By comparison, during the week of March 2, about 2,000 vaccines were ordered.
The findings, which were published in the CDC’s Morbidity and Mortality Weekly Report, stem from an analysis of ordering data from the federal Vaccines for Children (VFC) Program, as well as from vaccine administration data from the CDC’s Vaccine Tracking System and the collaborative Vaccine Safety Datalink (VSD).
The VFC provides federally purchased vaccines at no cost to about half of persons aged 18 years or younger. The VSD collaborates on vaccine coverage with the CDC’s Immunization Safety Office and eight large health care organizations across the country. Vaccination coverage is the usual metric for assessing vaccine usage; providers’ orders and the number of doses administered are two proxy measures, the authors explained.
“The substantial reduction in VFC-funded pediatric vaccine ordering after the COVID-19 emergency declaration is consistent with changes in vaccine administration among children in the VSD population receiving care through eight large U.S. health care organizations,” wrote Jeanne M. Santoli, MD, and colleagues, of the immunization services division at the National Center for Immunization and Respiratory Diseases. “The smaller decline in measles-containing vaccine administration among children aged ≤24 months suggests that system-level strategies to prioritize well child care and immunization for this age group are being implemented.”
Dr. Santoli, who is an Atlanta-based pediatrician, and associates stressed the importance of maintaining regular vaccinations during the pandemic. “The identified declines in routine pediatric vaccine ordering and doses administered might indicate that U.S. children and their communities face increased risks for outbreaks of vaccine-preventable diseases,” they wrote. “Parental concerns about potentially exposing their children to COVID-19 during well child visits might contribute to the declines observed.” Parents should therefore be reminded of the necessity of protecting their children against vaccine-preventable diseases.
In 2019, a Gallup survey reported that overall support for vaccination continued to decline in the United States.
The researchers predicted that, as social distancing relaxes, unvaccinated children will be more susceptible to other serious diseases. “In response, continued coordinated efforts between health care providers and public health officials at the local, state, and federal levels will be necessary to achieve rapid catch-up vaccination,” they concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 experiences from the pediatrician front line
As the COVID-19 pandemic continues to spread across the United States, several members of the Pediatric News Editorial Advisory Board shared how practices have been adapting to the pandemic, especially in terms of immunization.
Karalyn Kinsella, MD, a member of a four-pediatrician private practice in Cheshire, Conn., said in an interview that “we have been seeing only children under age 2 years for their well visits to keep them up to date on their vaccinations” as recommended by infectious disease departments at nearby hospitals such as Connecticut Children’s Medical Center. “We also are seeing the 4- and 5-year-old children for vaccinations.”
Dr. Kinsella explained that, in case parents don’t want to bring their children into the office, her staff is offering to give the vaccinations in the parking lot. But most families are coming into the office.
“We are only seeing well babies and take the parent and child back to a room as soon as they come in the office to avoid having patients sit in the waiting room. At this point, both parents and office staff are wearing masks; we are cleaning the rooms between patients,” Dr. Kinsella said.
“Most of our patients are coming in for their vaccines, so I don’t anticipate a lot of kids being behind. However, we will have a surge of all the physicals that need to be done prior to school in the fall. We have thought about opening up for the weekends for physicals to accommodate this. We also may need to start the day earlier and end later. I have heard some schools may be postponing the date the physicals are due.”
Because of a lack of full personal protective equipment, the practice has not been seeing sick visits in the office, but they have been doing a lot of telehealth visits. “We have been using doxy.me for that, which is free, incredibly easy to use, and Health Insurance Portability and Accountability Act (HIPAA)–compliant,” she said. “I am finding some visits, such as ADHD follow-ups and mental health follow-ups, very amenable to telehealth.”
“The hardest part – as I am sure is for most pediatricians – is the financial strain to a small business,” Dr. Kinsella noted. “We are down about 70% in revenue from this time last year. We have had to lay-off half our staff, and those who are working have much-reduced hours. We did not get the first round of funding for the paycheck protection program loan from the government and are waiting on the second round. We are trying to recoup some business by doing telehealth, but [the insurance companies] are only paying about 75%-80%. We also are charging for phone calls over 5 minutes. It will take a long time once we are up and running to recoup the losses.
“When this is all over, I’m hoping that we will be able to continue to incorporate telehealth into our schedules as I think it is convenient for families. I also am hoping that pediatricians continue to bill for phone calls as we have been giving out a lot of free care prior to this. I hope the American Academy of Pediatrics and all pediatricians work together to advocate for payment of these modalities,” she said.
J. Howard Smart, MD, who is chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego, said in an interview, “We have been bringing all of the infants and toddlers in for checkups and vaccines up to age 18 months.” These visits are scheduled in the morning, and sick patients are scheduled in the afternoon. “Well-child visits for older ages are being done by video, and the kindergarten and adolescent vaccines can be done by quick nurse visits. We will have some catching up to do once restrictions are lifted.”
“A fair amount of discussion went into these decisions. Is a video checkup better than no checkup? There is no clear-cut answer. Important things can be addressed by video: lifestyle, diet, exercise, family coping with stay-at-home orders, maintaining healthy childhood relationships, Internet use, ongoing education, among others. We know that we may miss things that can only be picked up by physical examination: hypertension, heart murmurs, abnormal growth, sexual development, abdominal masses, subtle strabismus. This is why we need to bring these children back for the physical exam later,” Dr. Smart emphasized.
“One possible negative result of doing the ‘well-child check’ by video would be if the parent assumed that the ‘checkup’ was done, never brought the child back for the exam, and something was missed that needed intervention. It will be important to get the message across that the return visit is needed. The American Academy of Pediatrics made this a part of their recommendations. It is going to be important for payers to realize that we need to do both visits – and to pay accordingly,” he concluded.
Francis E. Rushton Jr., MD, of Birmingham, Ala., described in an interview how the pediatricians in his former practice are looking for new ways to encourage shot administration in a timely manner during the COVID-19 pandemic, as well as exploring ways to partner with home visitors in encouraging timely infant and toddler vaccinations.
At South Carolina’s Beaufort Pediatrics, Joseph Floyd, MD, described a multipronged initiative. The practice’s well-child visit reminder system is being reprogrammed to check for lapses in vaccinations rather than just well-child visit attendance. For the most part, Dr. Floyd stated parents appreciate the reminders and accept the need for vaccination: “In the absence of immunizations for coronavirus, families seem to be more cognizant of the value of the vaccines we do have.” Beaufort Pediatrics is also partnering with their local hospital on a publicity campaign stressing the importance of staying up to date with currently available and recommended vaccines.
Other child-service organizations are concerned as well. Dr. Francis E. Rushton Jr., as faculty with the Education Development Center’s Health Resources and Services Administration–funded home-visiting quality improvement collaborative (HV CoIIN 2.0), described efforts with home visitors in Alabama and other states. “Home visitors understand the importance of immunizations to the health and welfare of the infants they care for. They’re looking for opportunities to improve compliance with vaccination regimens.” Some of these home-visiting agencies are employing quality improvement technique to improve compliance. One idea they are working on is documenting annual training on updated vaccines for the home visitors. They are working on protocols for linking their clients with primary health care providers, referral relations, and relationship development with local pediatric offices. Motivational interviewing techniques for home visitors focused on immunizations are being considered. For families who are hesitant, home visitors are considering accompanying the family when they come to the doctor’s office while paying attention to COVID-19 social distancing policies at medical facilities.
As the COVID-19 pandemic continues to spread across the United States, several members of the Pediatric News Editorial Advisory Board shared how practices have been adapting to the pandemic, especially in terms of immunization.
Karalyn Kinsella, MD, a member of a four-pediatrician private practice in Cheshire, Conn., said in an interview that “we have been seeing only children under age 2 years for their well visits to keep them up to date on their vaccinations” as recommended by infectious disease departments at nearby hospitals such as Connecticut Children’s Medical Center. “We also are seeing the 4- and 5-year-old children for vaccinations.”
Dr. Kinsella explained that, in case parents don’t want to bring their children into the office, her staff is offering to give the vaccinations in the parking lot. But most families are coming into the office.
“We are only seeing well babies and take the parent and child back to a room as soon as they come in the office to avoid having patients sit in the waiting room. At this point, both parents and office staff are wearing masks; we are cleaning the rooms between patients,” Dr. Kinsella said.
“Most of our patients are coming in for their vaccines, so I don’t anticipate a lot of kids being behind. However, we will have a surge of all the physicals that need to be done prior to school in the fall. We have thought about opening up for the weekends for physicals to accommodate this. We also may need to start the day earlier and end later. I have heard some schools may be postponing the date the physicals are due.”
Because of a lack of full personal protective equipment, the practice has not been seeing sick visits in the office, but they have been doing a lot of telehealth visits. “We have been using doxy.me for that, which is free, incredibly easy to use, and Health Insurance Portability and Accountability Act (HIPAA)–compliant,” she said. “I am finding some visits, such as ADHD follow-ups and mental health follow-ups, very amenable to telehealth.”
“The hardest part – as I am sure is for most pediatricians – is the financial strain to a small business,” Dr. Kinsella noted. “We are down about 70% in revenue from this time last year. We have had to lay-off half our staff, and those who are working have much-reduced hours. We did not get the first round of funding for the paycheck protection program loan from the government and are waiting on the second round. We are trying to recoup some business by doing telehealth, but [the insurance companies] are only paying about 75%-80%. We also are charging for phone calls over 5 minutes. It will take a long time once we are up and running to recoup the losses.
“When this is all over, I’m hoping that we will be able to continue to incorporate telehealth into our schedules as I think it is convenient for families. I also am hoping that pediatricians continue to bill for phone calls as we have been giving out a lot of free care prior to this. I hope the American Academy of Pediatrics and all pediatricians work together to advocate for payment of these modalities,” she said.
J. Howard Smart, MD, who is chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego, said in an interview, “We have been bringing all of the infants and toddlers in for checkups and vaccines up to age 18 months.” These visits are scheduled in the morning, and sick patients are scheduled in the afternoon. “Well-child visits for older ages are being done by video, and the kindergarten and adolescent vaccines can be done by quick nurse visits. We will have some catching up to do once restrictions are lifted.”
“A fair amount of discussion went into these decisions. Is a video checkup better than no checkup? There is no clear-cut answer. Important things can be addressed by video: lifestyle, diet, exercise, family coping with stay-at-home orders, maintaining healthy childhood relationships, Internet use, ongoing education, among others. We know that we may miss things that can only be picked up by physical examination: hypertension, heart murmurs, abnormal growth, sexual development, abdominal masses, subtle strabismus. This is why we need to bring these children back for the physical exam later,” Dr. Smart emphasized.
“One possible negative result of doing the ‘well-child check’ by video would be if the parent assumed that the ‘checkup’ was done, never brought the child back for the exam, and something was missed that needed intervention. It will be important to get the message across that the return visit is needed. The American Academy of Pediatrics made this a part of their recommendations. It is going to be important for payers to realize that we need to do both visits – and to pay accordingly,” he concluded.
Francis E. Rushton Jr., MD, of Birmingham, Ala., described in an interview how the pediatricians in his former practice are looking for new ways to encourage shot administration in a timely manner during the COVID-19 pandemic, as well as exploring ways to partner with home visitors in encouraging timely infant and toddler vaccinations.
At South Carolina’s Beaufort Pediatrics, Joseph Floyd, MD, described a multipronged initiative. The practice’s well-child visit reminder system is being reprogrammed to check for lapses in vaccinations rather than just well-child visit attendance. For the most part, Dr. Floyd stated parents appreciate the reminders and accept the need for vaccination: “In the absence of immunizations for coronavirus, families seem to be more cognizant of the value of the vaccines we do have.” Beaufort Pediatrics is also partnering with their local hospital on a publicity campaign stressing the importance of staying up to date with currently available and recommended vaccines.
Other child-service organizations are concerned as well. Dr. Francis E. Rushton Jr., as faculty with the Education Development Center’s Health Resources and Services Administration–funded home-visiting quality improvement collaborative (HV CoIIN 2.0), described efforts with home visitors in Alabama and other states. “Home visitors understand the importance of immunizations to the health and welfare of the infants they care for. They’re looking for opportunities to improve compliance with vaccination regimens.” Some of these home-visiting agencies are employing quality improvement technique to improve compliance. One idea they are working on is documenting annual training on updated vaccines for the home visitors. They are working on protocols for linking their clients with primary health care providers, referral relations, and relationship development with local pediatric offices. Motivational interviewing techniques for home visitors focused on immunizations are being considered. For families who are hesitant, home visitors are considering accompanying the family when they come to the doctor’s office while paying attention to COVID-19 social distancing policies at medical facilities.
As the COVID-19 pandemic continues to spread across the United States, several members of the Pediatric News Editorial Advisory Board shared how practices have been adapting to the pandemic, especially in terms of immunization.
Karalyn Kinsella, MD, a member of a four-pediatrician private practice in Cheshire, Conn., said in an interview that “we have been seeing only children under age 2 years for their well visits to keep them up to date on their vaccinations” as recommended by infectious disease departments at nearby hospitals such as Connecticut Children’s Medical Center. “We also are seeing the 4- and 5-year-old children for vaccinations.”
Dr. Kinsella explained that, in case parents don’t want to bring their children into the office, her staff is offering to give the vaccinations in the parking lot. But most families are coming into the office.
“We are only seeing well babies and take the parent and child back to a room as soon as they come in the office to avoid having patients sit in the waiting room. At this point, both parents and office staff are wearing masks; we are cleaning the rooms between patients,” Dr. Kinsella said.
“Most of our patients are coming in for their vaccines, so I don’t anticipate a lot of kids being behind. However, we will have a surge of all the physicals that need to be done prior to school in the fall. We have thought about opening up for the weekends for physicals to accommodate this. We also may need to start the day earlier and end later. I have heard some schools may be postponing the date the physicals are due.”
Because of a lack of full personal protective equipment, the practice has not been seeing sick visits in the office, but they have been doing a lot of telehealth visits. “We have been using doxy.me for that, which is free, incredibly easy to use, and Health Insurance Portability and Accountability Act (HIPAA)–compliant,” she said. “I am finding some visits, such as ADHD follow-ups and mental health follow-ups, very amenable to telehealth.”
“The hardest part – as I am sure is for most pediatricians – is the financial strain to a small business,” Dr. Kinsella noted. “We are down about 70% in revenue from this time last year. We have had to lay-off half our staff, and those who are working have much-reduced hours. We did not get the first round of funding for the paycheck protection program loan from the government and are waiting on the second round. We are trying to recoup some business by doing telehealth, but [the insurance companies] are only paying about 75%-80%. We also are charging for phone calls over 5 minutes. It will take a long time once we are up and running to recoup the losses.
“When this is all over, I’m hoping that we will be able to continue to incorporate telehealth into our schedules as I think it is convenient for families. I also am hoping that pediatricians continue to bill for phone calls as we have been giving out a lot of free care prior to this. I hope the American Academy of Pediatrics and all pediatricians work together to advocate for payment of these modalities,” she said.
J. Howard Smart, MD, who is chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego, said in an interview, “We have been bringing all of the infants and toddlers in for checkups and vaccines up to age 18 months.” These visits are scheduled in the morning, and sick patients are scheduled in the afternoon. “Well-child visits for older ages are being done by video, and the kindergarten and adolescent vaccines can be done by quick nurse visits. We will have some catching up to do once restrictions are lifted.”
“A fair amount of discussion went into these decisions. Is a video checkup better than no checkup? There is no clear-cut answer. Important things can be addressed by video: lifestyle, diet, exercise, family coping with stay-at-home orders, maintaining healthy childhood relationships, Internet use, ongoing education, among others. We know that we may miss things that can only be picked up by physical examination: hypertension, heart murmurs, abnormal growth, sexual development, abdominal masses, subtle strabismus. This is why we need to bring these children back for the physical exam later,” Dr. Smart emphasized.
“One possible negative result of doing the ‘well-child check’ by video would be if the parent assumed that the ‘checkup’ was done, never brought the child back for the exam, and something was missed that needed intervention. It will be important to get the message across that the return visit is needed. The American Academy of Pediatrics made this a part of their recommendations. It is going to be important for payers to realize that we need to do both visits – and to pay accordingly,” he concluded.
Francis E. Rushton Jr., MD, of Birmingham, Ala., described in an interview how the pediatricians in his former practice are looking for new ways to encourage shot administration in a timely manner during the COVID-19 pandemic, as well as exploring ways to partner with home visitors in encouraging timely infant and toddler vaccinations.
At South Carolina’s Beaufort Pediatrics, Joseph Floyd, MD, described a multipronged initiative. The practice’s well-child visit reminder system is being reprogrammed to check for lapses in vaccinations rather than just well-child visit attendance. For the most part, Dr. Floyd stated parents appreciate the reminders and accept the need for vaccination: “In the absence of immunizations for coronavirus, families seem to be more cognizant of the value of the vaccines we do have.” Beaufort Pediatrics is also partnering with their local hospital on a publicity campaign stressing the importance of staying up to date with currently available and recommended vaccines.
Other child-service organizations are concerned as well. Dr. Francis E. Rushton Jr., as faculty with the Education Development Center’s Health Resources and Services Administration–funded home-visiting quality improvement collaborative (HV CoIIN 2.0), described efforts with home visitors in Alabama and other states. “Home visitors understand the importance of immunizations to the health and welfare of the infants they care for. They’re looking for opportunities to improve compliance with vaccination regimens.” Some of these home-visiting agencies are employing quality improvement technique to improve compliance. One idea they are working on is documenting annual training on updated vaccines for the home visitors. They are working on protocols for linking their clients with primary health care providers, referral relations, and relationship development with local pediatric offices. Motivational interviewing techniques for home visitors focused on immunizations are being considered. For families who are hesitant, home visitors are considering accompanying the family when they come to the doctor’s office while paying attention to COVID-19 social distancing policies at medical facilities.
Advice on treating rheumatic diseases from a COVID-19 epicenter
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
What does COVID-19 mean for child safety?
In my home county of San Diego, school closure has meant some 800,000 children staying home.1 Parents love and are committed to care for their children, but as these parents struggle with food insecurity and mass unemployment, local pediatricians are joining their national colleagues in worrying about rising rates of child abuse.
Dr. Gwendolyn Wright, a local pediatrician at Scripps Coastal Medical Center, San Diego, explains. “Obviously, it’s easy for tempers to flare,” during this stressful time, “so there is increased risk for child abuse. And there’s no one else with eyes on the kids. Usually, there would be teachers at schools and other childcare workers who would have eyes on the kid. And now there is none of that extra protection.”
2018 data from the National Child Abuse and Neglect Data System showed that in 91.7% of child abuse cases, one or more parent perpetrated the abuse.2 Prior reporting in our county showed that calls to the child abuse hotline went down nearly 60% a week after school closure.3 However, this is not necessarily good news. NCANDS data show that educational personnel report 20% of child abuse cases – far more than the number of cases reported by social services, medical professionals, or family members.2
Teachers, childcare workers, law enforcement, and medical professionals all are mandated reporters, meaning that they are legally obligated to report any suspected cases of child abuse to Child Welfare Services. Accordingly, they receive training on how to spot signs of child abuse.
Sometimes, the signs are obvious, sometimes subtle. Subtle injuries are called “sentinel” injuries. In a landmark study published in Pediatrics in 2013, a “sentinel” injury was defined as “a previous injury reported in the medical history that was suspicious for abuse because the infant could not cruise, or the explanation was implausible.” Sentinel injuries can be mild bruising or oral injuries in a young infant. These injuries suggest “there may be escalating and repeated violence toward the infant” that can culminate in death.4,5
In this study, severely abused infants were 4.4 times more likely to initially have come to the doctor with a sentinel injury. Of concern, 42% of parents of definitely abused children reported that a medical provider was aware of the sentinel injury. Of these cases, 56% did not show evidence that a professional was worried about abuse. These data show that medical professionals do miss cases of child abuse.
The cost of child abuse is real and lifelong. According to a policy statement from the American Academy of Pediatrics Council on Child Abuse and Neglect, a quarter of kids who suffer abusive head trauma die. Of the survivors, nearly 70% “have some degree of lasting neurological impairment.”5
Given the potentially disastrous consequences of child abuse, we must stay vigilant about child abuse. In our own profession, we must educate trainees and update experienced pediatricians about suspecting child abuse and reporting. For example, child abuse can be suspected and reported based on telemedicine interactions. The burden of proof for reporting child abuse is only “reasonable suspicion,” not “beyond a reasonable doubt.” In our communities, we must engage with local Child Welfare Services workers and educate them about sentinel injuries. And finally, in our practices, we must build families up with awareness, resources, and coping mechanisms to prevent abuse from happening in the first place.
Dr. Helen C. Wang, associate professor of pediatrics at the University of California, San Diego, talks to parents about managing stress early and often. She says, “I start counseling families at the prenatal visit. I do talk to families about what they liked to do before children. What brought you joy? What communities do you spend time with? And what have you been doing now?”
It can be hard to reconcile prior hobbies with the current recommendations of social distancing. “Now it’s more ‘Do FaceTime’ and ‘Do Zoom’ and spend more time with your extended family,” says Dr. Wang.
By caring for themselves, parents can better protect their children from mistreatment and injury. Healthychildren.org, the parent-facing website of the AAP, offers several tips for parenting in times of stress.
In this unusual time of COVID-19, it is more important than ever to provide parents with suggestions and strategies that will help them – and their children – survive this health crisis. By educating ourselves and our communities about child abuse, we as pediatricians can fulfill our mandate in keeping kids healthy and thriving.
Dr. Parekh is a pediatric resident at University of California, San Diego. She has no financial disclosures. Email Dr. Parekh at [email protected].
References
1. Early childhood age group in California. kidsdata.org.
2. U.S. Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. (2020). Child Maltreatment 2018.
3. Hong Joe. “School closures lead to troubling drop in child abuse reports.” KPBS. 2020 Mar 27.
4. Pediatrics. 2013 Apr;131(4):701-7.
5. Pediatrics. 2020;145(4):e20200203.
In my home county of San Diego, school closure has meant some 800,000 children staying home.1 Parents love and are committed to care for their children, but as these parents struggle with food insecurity and mass unemployment, local pediatricians are joining their national colleagues in worrying about rising rates of child abuse.
Dr. Gwendolyn Wright, a local pediatrician at Scripps Coastal Medical Center, San Diego, explains. “Obviously, it’s easy for tempers to flare,” during this stressful time, “so there is increased risk for child abuse. And there’s no one else with eyes on the kids. Usually, there would be teachers at schools and other childcare workers who would have eyes on the kid. And now there is none of that extra protection.”
2018 data from the National Child Abuse and Neglect Data System showed that in 91.7% of child abuse cases, one or more parent perpetrated the abuse.2 Prior reporting in our county showed that calls to the child abuse hotline went down nearly 60% a week after school closure.3 However, this is not necessarily good news. NCANDS data show that educational personnel report 20% of child abuse cases – far more than the number of cases reported by social services, medical professionals, or family members.2
Teachers, childcare workers, law enforcement, and medical professionals all are mandated reporters, meaning that they are legally obligated to report any suspected cases of child abuse to Child Welfare Services. Accordingly, they receive training on how to spot signs of child abuse.
Sometimes, the signs are obvious, sometimes subtle. Subtle injuries are called “sentinel” injuries. In a landmark study published in Pediatrics in 2013, a “sentinel” injury was defined as “a previous injury reported in the medical history that was suspicious for abuse because the infant could not cruise, or the explanation was implausible.” Sentinel injuries can be mild bruising or oral injuries in a young infant. These injuries suggest “there may be escalating and repeated violence toward the infant” that can culminate in death.4,5
In this study, severely abused infants were 4.4 times more likely to initially have come to the doctor with a sentinel injury. Of concern, 42% of parents of definitely abused children reported that a medical provider was aware of the sentinel injury. Of these cases, 56% did not show evidence that a professional was worried about abuse. These data show that medical professionals do miss cases of child abuse.
The cost of child abuse is real and lifelong. According to a policy statement from the American Academy of Pediatrics Council on Child Abuse and Neglect, a quarter of kids who suffer abusive head trauma die. Of the survivors, nearly 70% “have some degree of lasting neurological impairment.”5
Given the potentially disastrous consequences of child abuse, we must stay vigilant about child abuse. In our own profession, we must educate trainees and update experienced pediatricians about suspecting child abuse and reporting. For example, child abuse can be suspected and reported based on telemedicine interactions. The burden of proof for reporting child abuse is only “reasonable suspicion,” not “beyond a reasonable doubt.” In our communities, we must engage with local Child Welfare Services workers and educate them about sentinel injuries. And finally, in our practices, we must build families up with awareness, resources, and coping mechanisms to prevent abuse from happening in the first place.
Dr. Helen C. Wang, associate professor of pediatrics at the University of California, San Diego, talks to parents about managing stress early and often. She says, “I start counseling families at the prenatal visit. I do talk to families about what they liked to do before children. What brought you joy? What communities do you spend time with? And what have you been doing now?”
It can be hard to reconcile prior hobbies with the current recommendations of social distancing. “Now it’s more ‘Do FaceTime’ and ‘Do Zoom’ and spend more time with your extended family,” says Dr. Wang.
By caring for themselves, parents can better protect their children from mistreatment and injury. Healthychildren.org, the parent-facing website of the AAP, offers several tips for parenting in times of stress.
In this unusual time of COVID-19, it is more important than ever to provide parents with suggestions and strategies that will help them – and their children – survive this health crisis. By educating ourselves and our communities about child abuse, we as pediatricians can fulfill our mandate in keeping kids healthy and thriving.
Dr. Parekh is a pediatric resident at University of California, San Diego. She has no financial disclosures. Email Dr. Parekh at [email protected].
References
1. Early childhood age group in California. kidsdata.org.
2. U.S. Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. (2020). Child Maltreatment 2018.
3. Hong Joe. “School closures lead to troubling drop in child abuse reports.” KPBS. 2020 Mar 27.
4. Pediatrics. 2013 Apr;131(4):701-7.
5. Pediatrics. 2020;145(4):e20200203.
In my home county of San Diego, school closure has meant some 800,000 children staying home.1 Parents love and are committed to care for their children, but as these parents struggle with food insecurity and mass unemployment, local pediatricians are joining their national colleagues in worrying about rising rates of child abuse.
Dr. Gwendolyn Wright, a local pediatrician at Scripps Coastal Medical Center, San Diego, explains. “Obviously, it’s easy for tempers to flare,” during this stressful time, “so there is increased risk for child abuse. And there’s no one else with eyes on the kids. Usually, there would be teachers at schools and other childcare workers who would have eyes on the kid. And now there is none of that extra protection.”
2018 data from the National Child Abuse and Neglect Data System showed that in 91.7% of child abuse cases, one or more parent perpetrated the abuse.2 Prior reporting in our county showed that calls to the child abuse hotline went down nearly 60% a week after school closure.3 However, this is not necessarily good news. NCANDS data show that educational personnel report 20% of child abuse cases – far more than the number of cases reported by social services, medical professionals, or family members.2
Teachers, childcare workers, law enforcement, and medical professionals all are mandated reporters, meaning that they are legally obligated to report any suspected cases of child abuse to Child Welfare Services. Accordingly, they receive training on how to spot signs of child abuse.
Sometimes, the signs are obvious, sometimes subtle. Subtle injuries are called “sentinel” injuries. In a landmark study published in Pediatrics in 2013, a “sentinel” injury was defined as “a previous injury reported in the medical history that was suspicious for abuse because the infant could not cruise, or the explanation was implausible.” Sentinel injuries can be mild bruising or oral injuries in a young infant. These injuries suggest “there may be escalating and repeated violence toward the infant” that can culminate in death.4,5
In this study, severely abused infants were 4.4 times more likely to initially have come to the doctor with a sentinel injury. Of concern, 42% of parents of definitely abused children reported that a medical provider was aware of the sentinel injury. Of these cases, 56% did not show evidence that a professional was worried about abuse. These data show that medical professionals do miss cases of child abuse.
The cost of child abuse is real and lifelong. According to a policy statement from the American Academy of Pediatrics Council on Child Abuse and Neglect, a quarter of kids who suffer abusive head trauma die. Of the survivors, nearly 70% “have some degree of lasting neurological impairment.”5
Given the potentially disastrous consequences of child abuse, we must stay vigilant about child abuse. In our own profession, we must educate trainees and update experienced pediatricians about suspecting child abuse and reporting. For example, child abuse can be suspected and reported based on telemedicine interactions. The burden of proof for reporting child abuse is only “reasonable suspicion,” not “beyond a reasonable doubt.” In our communities, we must engage with local Child Welfare Services workers and educate them about sentinel injuries. And finally, in our practices, we must build families up with awareness, resources, and coping mechanisms to prevent abuse from happening in the first place.
Dr. Helen C. Wang, associate professor of pediatrics at the University of California, San Diego, talks to parents about managing stress early and often. She says, “I start counseling families at the prenatal visit. I do talk to families about what they liked to do before children. What brought you joy? What communities do you spend time with? And what have you been doing now?”
It can be hard to reconcile prior hobbies with the current recommendations of social distancing. “Now it’s more ‘Do FaceTime’ and ‘Do Zoom’ and spend more time with your extended family,” says Dr. Wang.
By caring for themselves, parents can better protect their children from mistreatment and injury. Healthychildren.org, the parent-facing website of the AAP, offers several tips for parenting in times of stress.
In this unusual time of COVID-19, it is more important than ever to provide parents with suggestions and strategies that will help them – and their children – survive this health crisis. By educating ourselves and our communities about child abuse, we as pediatricians can fulfill our mandate in keeping kids healthy and thriving.
Dr. Parekh is a pediatric resident at University of California, San Diego. She has no financial disclosures. Email Dr. Parekh at [email protected].
References
1. Early childhood age group in California. kidsdata.org.
2. U.S. Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. (2020). Child Maltreatment 2018.
3. Hong Joe. “School closures lead to troubling drop in child abuse reports.” KPBS. 2020 Mar 27.
4. Pediatrics. 2013 Apr;131(4):701-7.
5. Pediatrics. 2020;145(4):e20200203.